Monograph |
|
Corresponding author: Sydney K. Brannoch ( sydneybrannoch@gmail.com ) Academic editor: Pavel Stoev
© 2017 Sydney K. Brannoch, Frank Wieland, Julio Rivera, Klaus-Dieter Klass, Olivier Béthoux, Gavin J. Svenson.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Brannoch SK, Wieland F, Rivera J, Klass K-D, Béthoux O, Svenson GJ (2017) Manual of praying mantis morphology, nomenclature, and practices (Insecta, Mantodea). ZooKeys 696: 1-100. https://doi.org/10.3897/zookeys.696.12542
|
Abstract
This study provides a comprehensive review of historical morphological nomenclature used for praying mantis (Mantodea) morphology, which includes citations, original use, and assignment of homology. All referenced structures across historical works correspond to a proposed standard term for use in all subsequent works pertaining to praying mantis morphology and systematics. The new standards are presented with a verbal description in a glossary as well as indicated on illustrations and images. In the vast majority of cases, originally used terms were adopted as the new standard. In addition, historical morphological topographical homology conjectures are considered with discussion on modern interpretations. A new standardized formulation to present foreleg femoral and tibial spines is proposed for clarity based on previous works. In addition, descriptions for methods of collection, curation, genital complex dissection, and labeling are provided to aid in the proper preservation and storage of specimens for longevity and ease of study. Due to the lack of consistent linear morphometric measurement practices in the literature, we have proposed a series of measurements for taxonomic and morphological research. These measurements are presented with figures to provide visual aids with homologous landmarks to ensure compatibility and comparability across the Order. Finally, our proposed method of pinning mantises is presented with a photographical example as well as a video tutorial available at http://mantodearesearch.com.
Keywords
Mantodea , measurement, morphology, praying mantis, terminology
1. Introduction
The central motivation for this work is to produce an updated standard for morphological nomenclature, specimen preparation, and measurement data capture. As there is currently a lack of standardization, some level of confusion exists about term use and application to features as well as the optimal method to measure features that retain highly variable or ambiguous boundaries. We believe this to be an important time to propose a set of standards due to a growth in taxonomic interest in Mantodea, the application of new technologies, and to improve lab workflow efficiency. In addition, we outline and propose new methodological standards to improve the ability to research specimens, which includes specimen preparation and pinning, genital dissections, and labeling.
Justification to standardize:
Coding of morphology: Congruence of terminology ensures accurate interpretations and future use of characters and their states in morphological analyses and deposition into morphology databases such as MorphBank. The current lack of a system ensures barriers derived from language and chosen reference material. Morphological terminology is suggested using topographical correspondances, which does not in all cases correspond to a hypothesis of homology.
Formulaic descriptions: Telegraph style descriptions speed taxonomic work, but term standards ensure longevity, direct comparisons with other studies, reuse of descriptions, and extracting coded characters from descriptions.
Imaging: Access to high resolution images of specimens are of great importance to taxonomic work by improving how we gather data, compare specimens, and identify species. However, the way a specimen is dry pinned will have great influence on how many images are needed of the same specimen in order to adequately provide access to the relevant features. Minimizing the number of images captured by standardizing the way a specimen is mounted will greatly increase digitization efficiency and access to feature information. If imaging equipment is not readily available, scientific illustration, when performed with high precision and heeding symmetry in bilaterally symmetric structures, can capture important characters for taxonomic and morphological study.
Morphometric analysis: Capture of measurement data requires standardization for broader future use in other analyses based on phylogenetics or species delimitation. The measurements described for suggested use are for linear morphometrics, to be used for taxonomic or morphological purposes, like distinguishing between closely related species. Advanced geometric morphometric measurement techniques might need to be employed for the purposes of functional morphology, ethology, evaluating evolutionary trends in morphological evolution, among others, but those are not proposed here due to their specificity to the hypothesis being tested.
Data capture: As label data is often obscured on large specimens, standards of specimen preparation will alleviate this by creating a predictable and optimized downstream approach to capture data on mounted specimens.
2. Methods
Imaging: All high resolution images of specimens and features were captured using a Passport Storm system (Visionary Digital, 2012), which includes a Stackshot z-stepper, a Canon 5D SLR, macro lenses (50 mm, 100 mm, and MP-E 65 mm), three Speedlight 580EX II flash units, and an associated computer running Canon utility and Adobe Lightroom 3.6 software. The z-stepper was controlled through Zerene Stacker 1.04 and images were processed using the P-Max protocol. Images were captured over an 18% gray card background for white balance standards. Images were processed in Adobe Photoshop CS6 Extended to add scale bars. Adjustments were made using the stamp tool to correct aberrations. Figures depict distinct morphological features, with structures of interest clearly in focus. Measurement delineations were superimposed on the figures using the line tool.
Illustration: Illustrations of key morphological structures were digitized using either Adobe Illustrator CS6 and Adobe Photoshop CS6 or CorelDraw and CorelPhotopaint. Diagrammatic illustrations were produced by collecting reference images of the specimens using both the Leica M165C stereo-microscope paired with the IC80 HD camera as well as the Passport Storm, Visionary Digital system. Images were imported into Adobe Illustrator and traced using the pen, paintbrush, and smooth tools. Adobe Illustrator was used for all plate layouts. Illustrations were produced by Rebecca Konte, Josh Maxwell, Hiromi Yagui, and authors SKB, FW, KDK, and OB.
Measurements: Standards of measurements were developed using a Leica M165C stereo-microscope and an IC80 HD coaxial video camera using the live measurements module of the Leica Application Suite (LAS).
3. Results
Proposed standardization of morphological nomenclature
In the following sections, historical morphological nomenclature of the praying mantis (Fig.
3.1. Head
General cephalic morphological nomenclature is fairly straightforward and without much discord. The structures of the praying mantis head include a pair of antennae, a pair of compound eyes, three ocelli, a lower frons, a clypeus, a labrum, a pair of mandibles, a pair of maxillae and maxillary palpi, as well as a labium and a pair of labial palpi (Figs
Annotated illustrations of cranial structures. Omomantis Saussure, 1899 ♂ head capsule A anterior view B dorsal view. Generalized mantis head capsule C oblique anterior view demarcating regions where cranial process can arise; colored triangles indicate approximate locations of cuticular projections: light gray triangle = fastigial process; black triangles = juxtaocular processes; blue triangle = vertical process; white triangle = postocellar process; yellow triangle = medial ocellar process; gray triangle = ocellar process. Various compound eye shapes (D–F): D approximately globular in Orthodera Burmeister, 1838 E Heterochaetasp. with a non-visual elongation F anteriorly elongate in Schizocephala bicornis Linné, 1758. Illustrations A–B by Josh Maxwell. Abbreviations: cs = coronal sulcus; ey = compound eye; fp = fastigial process; jop = juxtaocular process; mop = medial ocellar process; nve = non-visual elongation; op = ocellar process; pfs = postfrontal sulcus; pop = postocellar process; vp = vertical process.
Annotated illustrations of cranial structures. Omomantissp. ♂ cranium A anteroventral view; B lateral view. Tenodera sinensis Saussure, 1871 C disarticulated components of mouthparts. Illustrations by Josh Maxwell. Abbreviations: hpx = hypopharynx; lbp = labial palp; mr = molar ridge; mxp = maxillary palp; pg = paraglossa; prmt = prementum.
3.2. Wings and wing venation
The wing venation terminology used within this paper follows the serial wing venation pattern (
As in other neopteran insects, there is no clear elevation shift between branches of the media in mantises. As a consequence we will refer to an undifferentiated media, M. Furthermore, we believe that the occurrence of genuine AP branches in fore- and hindwings of neopteran insects is wanting [with the possible exception of Protophasma dumasii Brongniart, 1879, revised in
Annotated illustrations of fore- and hindwing venation and structures in Metallyticus splendidus Westwood, 1835 (A–C) and Chaeteessasp. (D–F). A, D forewing B, E hindwing (in E, º indicates Iaa1-aa2) C, F detail of the arculus as located on B, E respectively (enlargement 1.5×; black arrow indicates the arculus). Abbreviations: AA = anterior Analis; AA1 = first anterior Analis; AA2 = second anterior Analis; CuA = anterior Cubitus; CuP = posterior Cubitus; M = Media; ppa = plica prima anterior; ppp = plica prima posterior; RA = anterior radius; RP = posterior radius; ScP = posterior subcostal; color coded areas: gray = stigma (sti); purple = preplicatum; turquoise = plicatum; yellow = plicatulum.
Annotated illustrations of fore- and hindwing venation and structures in Mantoida maya Saussure & Zehntner, 1894 (A–C) and Mantis religiosa (D–F). A, D forewing B, E hindwing C, F detail of the arculus as located on B, E respectively (enlargement 1.5×; black arrow indicates the arculus). Abbreviations: AA = anterior Analis; AA1 = first anterior Analis; AA2 = second anterior Analis; CuA = anterior Cubitus; CuP = posterior Cubitus; M = Media; ppa = plica prima anterior; ppp = plica prima posterior; RA = anterior radius; RP = posterior radius; ScP = posterior subcostal; color coded areas: gray = stigma (sti); purple = preplicatum; turquoise = plicatum; yellow = plicatulum.
Sometimes, there can be two rows of cells (delimited by cross-veins) in an area delimited by two veins. It is often the case that the cross-veins delimiting the two rows get aligned and form a vein-like structure. Such structures are referred to as ‘intercalary veins.’ In hindwings they can be aligned with concave folds. They can be referred to using the abbreviations of the surrounding veins written in lowercase (see
Particular wing structures
In insect wings it is generally the case that the anterior wing margin is actually a vein-like structure. However, in Mantodea, it is possible that this structure is preceded by a narrow, membranous area. Therefore we distinguish the anterior veinal margin (avm) from the anterior wing margin (awm). We propose to refer to the area between awm and avm as the ‘visor’ (vs). In the hindwing, the strengthened cross-vein connecting M and CuA is conventionally referred to as the ‘arculus’ (arc).
Wing venation topographic homology conjectures
As usual with polyneopteran insects, a broad array of conjectures of topographical homology (THCs) have been proposed to homologize the wing venation of Mantodea with the serial pattern (or any other pattern). In order to facilitate comparison between publications by proponents of various THCs, we describe and discuss the most relevant ones. Our favored THCs for the forewing follow
There are two major forewing venation patterns among mantises. To ease the following discussion we propose a provisional labelling. The first type, arguably plesiomorphic, encompasses species belonging to Metallyticus Westwood, 1835, Chaeteessa, Mantoida Newman, 1838, and some fossil species (blue shading in Suppl. material
We propose to first address THCs that considered only the MCM-type. They will prove weakly supported and some moreover self-inconsistent.
The contribution by
Second, one of the figured forewings of a single specimen of Chaeteessa valida shows two branches anterior to ‘RP + MA’ (
Third,
The assumed occurrence of an R + MA stem by
Although
Among the more supported accounts, it is argued that vein* belongs to R, the fork observed in the Mantis-type representing the ‘ancestral’ RA-RP fork (
A comparison of the fore- and hindwing venation patterns provides further evidence that a common stem RP + MA occurs in the forewing of the MCM-type. The hindwing venation of mantises generally conforms to that of cockroaches, including the occurrence of arc. The identification of RA, RP, M, and CuA is then straightforward: R and M run alongside until M and then RP, or RP + M, diverge (intraspecific variability can affect this area;
The wing posterior area
Some authors have referred to particular areas of the wing according to the veins delimiting them (e.g., ‘cubital area’). In other cases, function-based names have been proposed, such as ‘vannus,’ a term coined by
The term ‘jugum’ was originally coined by
As mentioned above,
The whole area in the hindwing posterior to the cubital furrow (cf) has also been termed ‘vannus’ (
The area posterior to cf has also been termed in reference to the vein systems supposedly filling it, such as ‘fan-like anal field’ (
This is leading us to coin three new terms for the area posterior to the cf, presumably applicable to polyneopteran insects, at least. This area is divided into three parts. The anterior part is delimited by cf and ppa. It will be referred to as ‘preplicatum’ (a derivate of ‘plicatum,’ defined below). The medial part is anteriorly delimited by ppa and posteriorly delimited by another fold, acting as a concave hinge, herein termed ‘plica prima posterior’ (ppp; beyond this fold the wing is attached to the thorax). We propose to refer to this area as the ‘plicatum.’ This proposition has the substantial advantage of avoiding reference to the nature of the veins filling the corresponding area and the inner folding mechanism of the area (if applicable). Derived from ‘plicatum,’ we propose to refer to the area anteriorly delimited by ppp and posteriorly attached to the thorax, smaller than the plicatum, as ‘plicatulum.’ Although a wider comparative analysis would be necessary for a positive statement, our plicatulum seems to be homologous to
‘Pcu & 1V’ vs. ‘AA1 & Iaa1-aa2’ in hindwings of Chaeteessa spp.
The existence of a ‘Pcu’ vein distinct from CuP and the anal system was proposed by
As for mantises, a ‘Pcu’ was mentioned by
We propose a different interpretation of the vein-like structure located between (our) AA1 and AA2. As in Blattodea and Plecoptera, ppa is located between AA1 and AA2 in mantises. In these insects, as in Phasmatodea (among others), is it very often the case that in the hindwing plicatum, near the posterior wing margin, intercalary veins form along vannal folds. For most of its length this structure does not reach the level of sclerotization observed in the surrounding veins, and it never forms a complete tube. Instead, it is formed of isolated patches of sclerotization, like cross-veins in the plicatum. Moreover, its origin is very faint. All of these features demonstrate that Smart’s ‘1V’ is an intercalary vein (herein ‘Iaa1-aa2’). Among mantises, possessing an intercalary vein along ppa is unique to Chaeteessa, and clearly is a derived condition.
Ongoing research has demonstrated that wing venation THCs proposed by
3.3. Pro-, meso-, and metathoracic legs
Praying mantises have three pairs of legs each consisting of a coxa, trochanter, femur, tibia, and tarsus (Fig.
Annotated illustrations of leg structures. Generalized mantodean foreleg A dorsal view; B ventral view; C interior view. Generalized tarsus D ventral view. Examples of convergent coxal lobes: E Sphodromantissp. ♂; F Paramorphoscelis gondokorensis Werner, 1907 ♀. Example of parallel coxal lobes G Acromantis insularis Giglio-Tos, 1915 ♀. Example of divergent coxal lobes H Gongylus gongylodes Linné, 1758 ♂. Generalized metathoracic leg I ventral view. Examples of carinae that can be present on the meso- and metathoracic femora and tibiae J Stagmatoptera Burmeister, 1838 ♀: ventral view 1 = anterodorsal metafemoral carina; 2 = anteroventral metafemoral carina; 3 = posteroventral metafemoral carina. Example of the cuticular lobes that can project from leg carinae K Alangularis multilobata (Chopard, 1910) ♀: ventral view 1 = anterodorsal metafemoral lobe; 2 = anteroventral metafemoral lobe; 3 = posteroventral metafemoral lobe. Dashed lines demarcate anteroventral carinae (J) and associated lobes (K). Abbreviations: avfs = anteroventral femoral spines; avts = anteroventral tibial spines; cx = coxa; cxl = coxal lobes; ds = discoidal spines; epl = euplantulae; fb = femoral brush; fe = femur; gl = genicular lobe; gs = genicular spur; mepl = medial euplantula; pb = proximal bend in the tibia; pvfs = posteroventral femoral spines; pvts = posteroventral tibial spines; ta5 = tarsomere 5; ti = tibia; tr = trochanter; ts = tibial spur; tsg = tibial spur groove; un = unguis.
Foreleg spination formula
Foreleg spines constitute an important character system in Mantodea taxonomy. Taxonomic description normally includes a verbal description detailing the number and relative arrangement of such spines.
Foreleg annotation
Several different terminologies have been used in the descriptions of spination patterns, (e.g., “internal” and “external” spines, “inner” and “outer” spines, “ventral” and “dorsal” spines).
The following example describes the foreleg spination of a fictive species:
F = 4DS/10–12AvS/4–5PvS; T = 7–8AvS/12–14PvS
This example formulation indicates that this fictive species exhibits 4 discoidal spines. The forefemora carry 10–12 anteroventral spines (thus highlighting observed variation) and 4–5 posteroventral spines (again highlighting observed variation). The foretibiae carry 7–8 anteroventral and 12–14 posteroventral spines.
Sometimes a unique specimen is all that is available (e.g., a holotype) and it might exhibit bilateral asymmetry in the number of spines. When this situation arises, such as when describing a new species based on a single specimen, it is important to make a clear distinction to be as precise as possible. In this case, an additional annotation of “R” (right) or “L” (left) can be added to the number of spines detailed in the spination formula. The following example illustrates this situation:
F = 4DS/10(R)–11(L)AvS/4PvS; T = 7AvS/12(R)–14(L)PvS
This means that this unique specimen exhibits 4 discoidal spines, the right forefemur features 10 anteroventral spines, the left forefemur features 11 anteroventral spines, with 4 posteroventral spines on each forefemora. Similarly, both foretibiae carry 7 anteroventral spines, while 12 and 14 posteroventral spines occur on the right and left foretibia, respectively.
In the form presented above, this formula can be used for the description of the vast majority of forelegs across all mantodean species described so far. There are only a few exceptions with highly modified foreleg morphologies, some of which call for special addenda to the formula. In some species, spines can be reduced up to a state where they can only be detected through scanning electron microscopy (SEM) (
As far as our knowledge allows, the following are the unique cases that we have identified where the spination formula will need special modifications:
1.) Chaeteessa Burmeister, 1838 (Fig.
This enigmatic Neotropical genus is the sister-group of all remaining extant Mantodea according to
Figure
The morphological assignment of several spines is still uncertain. Distally on the forefemur, there is a large spine that may be an enlarged genicular spine (interpreted as such in
The arrangement of anteroventral spines in the proximal region of the forefemur, as well as the number of discoidal spines, are also inconclusive. The row of anteroventral spines splits proximally into two diverging rows. This character has also been observed in other Mantodea, e.g., Mantoida and Acanthops (see
F = 2(3?)DS/19(20?)AvS/7(?)PvS; T = 14AvS/4PvS
2.) Metallyticus Westwood, 1835 (Fig.
F = 1DS/11AvS/4PvS; T = 8AvS/6PvS
3.) Perlamantinae (Fig.
Perlamantis Guérin-Méneville, 1843 and Paramorphoscelis Werner, 1907, the only two genera of this subfamily, have highly specialized forelegs. Perlamantis allibertii was studied by
F = 1DS/4AvS/0PvS; T = 0AvS/0PvS
4.) Amorphoscelinae (Fig.
Foreleg morphology in Amorphoscelinae (e.g., Amorphoscelis Stål, 1871, Gigliotoscelis Roy, 1973, Caudatoscelis Roy, 1973) is unique because all spines except for the tibial spur and one discoidal spine are reduced.
F = 1DS/0AvS/0PvS; T = 0AvS/0PvS
5.) Thespidae and Haaniinae (Fig.
Members of Neotropical and Nearctic Thespidae and Asian Haaniinae exhibit atypical foreleg morphology, where both forefemora and foretibiae have undergone extensive modifications resulting in a remarkable diversity of spination patterns. Despite this, the spination formula can accommodate most of this variation without resorting to additional symbology or annotations. Therefore, members of Thespis Audinet-Serville, 1831 exhibit the following spination formula:
F= 4DS/12–13AvS/4PvS; T=8–11AvS/5PvS
However, special annotation is needed for taxa such as Bantia Stål, 1877, Mantillica Westwood, 1889, Pseudomusonia Werner, 1909, Thesprotia Stål, 1877, Thesprotiella Giglio-Tos, 1915, Thrinaconyx Saussure, 1892, and other related genera. In these genera, one or two of the distal-most spines of the anteroventral foretibial series have shifted from their usual ventral location into either a slightly lateral or decidedly dorsal position (e.g.,
F=4DS/9–10AvS/4PvS; T=4–6[+1]AvS/4–7PvS
Notice that the foretibial AvS exhibit the following count: 4–6[+1]. This means that this part of the foretibia exhibits 4 to 6 spines in the normal, ventral position, whereas the distal-most spine (i.e., [+1]) is displaced from the continuity of the series into a more dorso-lateral location. Therefore, the full foretibial AvS series in Bantia includes 5 to 7 spines as a whole. It is worth mentioning that the number of displaced distal AvS is fixed for each genus, whereas the remaining AvS can (and do) exhibit variation in number. Therefore, the annotation [+1] for Bantia will remain constant for all of its members.
Another, more extreme example of this kind of annotation, is represented by members of Thesprotia (e.g.,
F=3DS/5–6AvS/1PvS; T=[+2]AvS/1PvS
In this example, the foretibial AvS series is reduced to simply [+2], because there are no ventral spines and the only two spines of this series are displaced into a distinctly dorsal position. As in Bantia, the [+2] annotation will remain constant across all species within this genus.
In some taxa, the displacement of the distal-most foretibial AvS is not evident. For example, there has been some controversy on the location of this spine in Galapagia solitaria Scudder, 1893 (see
6.) Compsothespis Saussure, 1872 (Fig.
The foreleg morphology of the African Compsothespis shows a strong tendency of reduction (
F = 3DS/4AvS/4PvS; T = 0AvS/0PvS
7.) Paraoxypilinae (Fig.
The Australian Paraoxypilinae (e.g., Paraoxypilus Saussure, 1870, Gyromantis Giglio-Tos, 1913, Cliomantis Giglio-Tos, 1913) have highly modified forelegs, possibly as an evolutionary response to hunting ants (
F = 3DS/13AvS/4PvS; T = 12AvS/0PvS
For most of the abovementioned taxa larger numbers of specimens have to be studied because the degree of variation is unknown so far. The examination of first instar specimens is likely to shed further light on the evolution of foreleg spination and uncertain cases of morphological assignment (e.g.,
Verbal description of foreleg spines
A good proportion of Mantodea taxa show variation in the way the spines are arranged. For instance, in certain thespids the foretibial AvS may exhibit spines of various sizes, and the size of the gaps between each spine of the series may also vary. All of this information is taxonomically relevant (e.g.,
We suggest representing spine size arrangement by using the letter “I, i” and the underscore symbol, which has been used periodically in the taxonomic descriptions of Mantodea (e.g.,
3.4. Thoracic structures
The thoracic structures of Mantodea include the pronotum (Fig.
Annotated illustrations of thoracic structures. Pronotum in dorsal view A Sphodromantissp. ♂ (illustration by Josh Maxwell) B Theopompella congica Rehn, 1949 ♀ C Idolomantis diabolica (Saussure, 1869) ♀ D Deroplatys indica Roy, 2007 ♂ E Amorphoscelis pulchella ♀. Prothorax in ventral view F Mantoida maya ♂ G Humbertiella Saussure, 1869 ♀ H Phyllocrania paradoxa ♂. Figures F–H reproduced and adapted from
Annotated illustrations of hearing organ forms. Figures reproduced and adapted from
3.5. Abdominal and genitalic structures
At present, praying mantis species are most frequently delimited through observable differences in external morphological and genitalic features (e.g.,
Abdominal structures
The abdomen of Mantodea consists, as primitively in insects, of 11 segments plus a non-segmental apical telson. The segments preceding the genitalia comprise the pregenital segments. Among these, the 1st is strongly modified by forming the transition to the thorax, and the 2nd and 3rd segments also differ to some extent from the ‘typical’ midabdominal segments following them. The segments showing morphological modifications for genital functions are the genital segments, which in Mantodea are segments 7–9 in the female and segment 9 alone in the male (although the male genitalia are probably contributed by segment 10, see below). Segments 10 and 11 are the terminal or postgenital segments, which are even more strongly modified than the genital segments, and partly reduced. The genital and terminal segments comprise the postabdomen.
The morphological literature on the abdomen of Mantodea is quite limited. The exoskeleton and musculature of the entire abdomen, including the genitalia, were treated by Levereault (1936: exoskeleton; 1938: musculature) for Stagmomantis Saussure, 1869 and by
Accordingly, knowledge of the various parts of the abdomen has remained limited to a few species of higher Mantodea; the only exception is the inclusion of “basal” mantodeans with regard to the male genitalia. In addition, though their quality varies, none of the abovementioned works (with the exception of
Regarding male and female genitalia, the limited existing morphological knowledge demonstrates that these highly complicated parts of the mantodean body are a very useful source of characters for taxonomic work. For review of the historical treatment of male and female genitalia in mantodean taxonomy see
In the male and female genital segments and the terminal abdomen, the morphological interpretation regarding the homology and homonomy of many elements has long been strongly disputed, which is partly evident from the heterogeneous terminologies used by the various authors and by some lengthy and complicated discussions in relevant literature. However, most of the issues could be resolved in the coming years by comparative work at the insect level (e.g.,
The following descriptions are predominantly based on
Basics of a new abdominal terminology
In recent years, K.-D. Klass and other researchers have done much comparative morphological work on the cuticular exoskeleton, musculature, and nervous system of the abdomen of various insects, with a focus on male and female genitalia (e.g.,
(1) Sclerotizations: These are areas of the cuticle that are hardened to a varied extent, in contrast to fully flexible membrane. Sclerotizations are usually colored (yellowish to black), but this is not always the case. We view a set of ‘principal sclerotizations’ of the abdominal segments; this essentially comprises sclerites as putatively present in a plesiomorphic insect condition (e.g., Archaeognatha; see
The terminology for the sclerotizations of the male genitalia was established (
(2) Formative elements: These are more or less discrete in- and evaginations of the cuticle or discrete thickenings of the cuticle (upon either membranous or sclerotized cuticle). Abbreviations are composed of 2–4 lowercase letters (e.g., cx, pda, frgp). The terms can have a number in the last position, which specifies the abdominal segment to which the element belongs (e.g., cx8). An appended ‘-o’ denotes the external / internal opening of a strongly invaginated / evaginated formative element (e.g., sp-o is the external opening of the female spermatheca sp). Usually the borders of a formative element cannot be exactly defined, as the body wall gradually bulges inward or outward in its periphery. The body wall areas of different formative elements are not necessarily mutually exclusive; two cases are particularly important: First, a less inclusive formative element can be a part of another, more inclusive formative element. For instance, the lobe vla of the male genitalia can bear a distinct process pda arising from its distal edge (Fig.
Annotated illustrations of male Sphodromantissp. genitalia for morphological use. Male postabdomen, left phallic complex A–D dorsal view. From A (showing intact left complex) to D dorsal and peripheral parts are removed step by step. Thick black lines are (virtual) cutting lines. Continuous thin black lines are freely visible edges (= lines along which the cuticle bends away from the observer’s view). Dashed thin black lines are (parts of) edges hidden beneath other cuticle. Membranous cuticle in very light gray, sclerotized cuticle in darker gray; cuticle shaded darker where it goes underneath other cuticle. For abbreviations see text, glossary, or Suppl. material
The terms used for formative elements of the male genitalia (
For additional discussion regarding this terminology see Suppl. material
Pregenital abdominal segments
The pregenital segments of Mantodea are fairly simple in structure. They bear an undivided ventral sclerotized plate without special differentiations, which is generally called sternite but preferably addressed coxosternite (CS). The coxosternite of the 1st abdominal segment (CS1) is very small and hidden, i.e., the anterior-most freely visible coxosternite belongs to segment 2 (CS2). In addition, each segment bears a large, undivided dorsal plate, the tergite (TG). Its lateral parts are bent downward, usually along an angled longitudinal edge, the laterodorsal carina (ldca). These lateral parts are not separated by membrane from the dorsal main part, but there can be indistinct separation by weaker sclerotization. These lateral parts are best called the paratergal areas (TGπ) and are formally part of the tergite. On segment 1, the paratergal areas are absent. The dorsal remainder of a tergite is here called the centrotergal area (TGκ).
The posterior part of each coxosternite and tergite extends over the anterior portion of the successive coxosternite or tergite, and from its posterior margin a membrane is reflected to the anterior to reach the anterior margin of the overlapped successive sclerite. Both on the dorsal and ventral side, the posterior part of a segment thus forms a transverse fold that covers part of the following segment: the dorsal (df) and ventral (segmental) folds (vf). The reflected membrane is often called the ‘intersegmental membrane,’ but this should be avoided, because with regard to primary segmentation the membrane entirely belongs to the segment in front. The membrane is better called intercoxosternal membrane (ventrally) or intertergal membrane (dorsally), or conjunctival membrane (both sides).
Spiracles (si) and associated spiracle sclerites (SI) are present on abdominal segments 1–8. Each includes a posterior spiracle sclerite (SIk; instead of K) and an anterior spiracle sclerite (SIm; instead of M), both being considered subsets of the spiracle sclerotization SI.
Male genital region
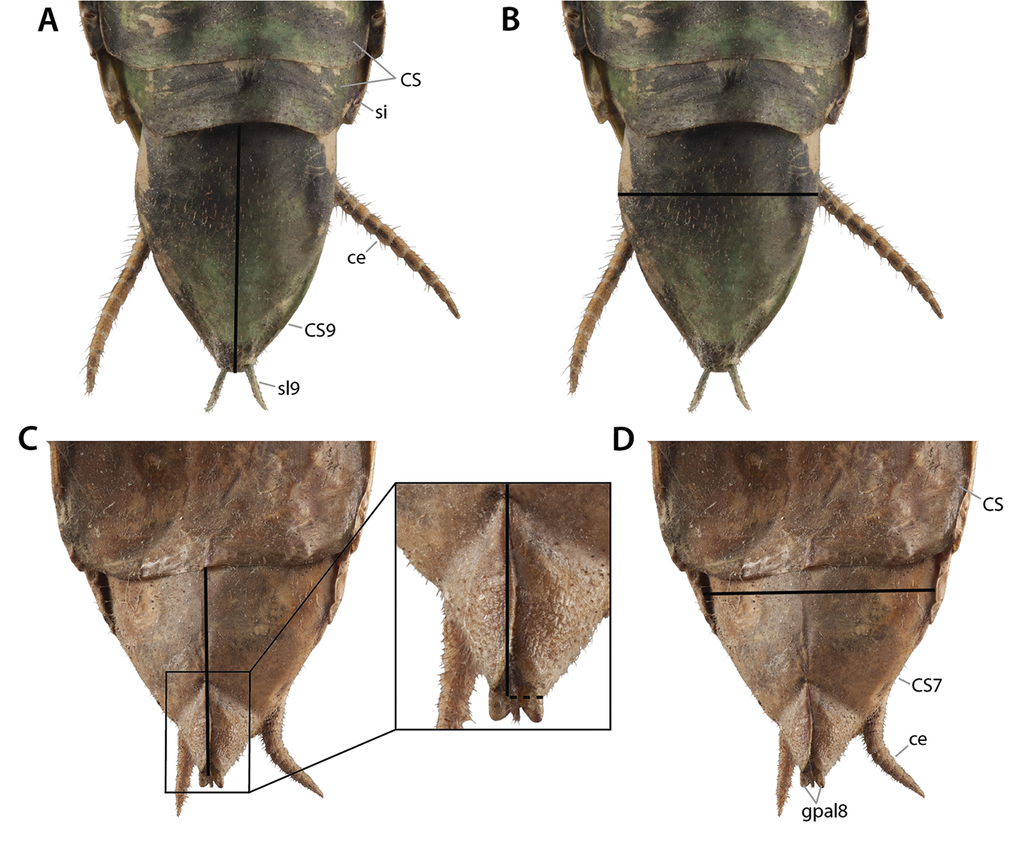
Tergite 9 (TG9) resembles the tergites of the preceding segments. Coxosternite 9 (CS9), however, shows many peculiarities (see
CS9 and vf9 can show asymmetry in various parts: in the anterior apodemes mcsa9, in the hind edge of vf9 (potentially including an asymmetric positioning of the styli), in the dorsocoxal sclerotization CS9δ (as in Fig.
The male genitalia (= phallic organs, Figs
The phallic organs are strongly asymmetrical. Homonomies between the elements of the left and right parts are unresolved –and may not exist. The normal (dextral) orientation of the phallic asymmetry is as in Figs
Annotated illustrations of the right phallomere in male genitalia for morphological use. A–C Male postabdomen, right phallomere of Sphodromantissp. A dorsal view of intact right phallomere B dorsal view of right phallomere with many parts removed C ventral view of intact right phallomere. Chaeteessa caudata Saussure, 1871 right phallomere D ventral view, intact. Thick black lines are (virtual) cutting lines. Continuous thin black lines are freely visible edges (= lines along which the cuticle bends away from the observer’s view). Dashed thin black lines are (parts of) edges hidden beneath other cuticle. Dashed gray line indicating outline of process afa of left phallic complex as it is placed in association with the right phallomere. Membranous cuticle in very light gray, sclerotized cuticle in darker gray; cuticle shaded darker where it goes underneath other cuticle. For abbreviations see text, glossary, or Suppl. material
Annotated illustrations of the male Sphodromantissp. postabdomen for morphological use. Coxosternite CS9 (= subgenital plate) and ventral fold vf9 (“subgenital lobe”) of Sphodromantissp., dorsal view. Thick black lines are (virtual) cutting lines. Continuous thin black lines are freely visible edges (= lines along which the cuticle bends away from the observer’s view). Dashed thin black lines are (parts of) edges hidden beneath other cuticle. Membranous cuticle in very light grаy, sclerotized cuticle in medium to darker grаy (darker = more strongly sclerotized); cuticle shaded darker where it goes underneath other cuticle. For abbreviations see text, glossary, or Suppl. material
The phallic organs of Dictyoptera were often divided in a left phallomere, a right phallomere, and a ventral phallomere. This subdivision, however, is conflicting with regard to the interpretation of the ‘ventral phallomere’ (ventral lobe vla in Fig.
Both the right phallomere and the left phallic complex consist of a variety of processes, pouches, and apodemes, and each bears several sclerites. Internally they are equipped with a rich musculature. In Mantodea the morphology of phallic organs is fairly uniform, especially compared to the much greater structural diversity in Blattodea. Yet, there are some exceptions to this.
Left phallic complex (Fig.
Ventral to the pouch pne, the cuticle is folded outward to form a more or less continuous edge that is drawn out into a maximum of known processes, which from anterior to posterior are (see Fig.
Beneath this process-bearing edge pba, the cuticle is folded inward again to form another deep pouch lve (Fig.
The left phallic complex bears three principal sclerites: L1, L2, and L4 (sclerite L3 only occurs in Blattodea;
The principal sclerite L4 covers much of the dorsal and ventral walls of the left complex. The ventral portion of L4 is usually plate-like (
The principal sclerite L1 extends over parts of the walls of pouch pne and of the edge pba between pouches pne and lve; in the latter area it is limited to the anterior part bearing the processes afa, apa, and loa (Fig.
The principal sclerite L2 takes most of the dorsal wall of pouch lve (Fig.
As far as is known, the sclerites L1, L2, and L4 are separate from each other. The only known example of a fusion is the one between L2 and L4 at the bases of the processes paa and pda in Mantoida (
When examining the phallic organs, one should be aware that additional, smaller sclerites may occur. The only known example is sclerite L5 of Metallyticus, which is located in the otherwise membranous dorsal wall of the ventral lobe vla (
The more or less distinct edge pba bearing the processes afa, apa, loa, and paa and the associated or neighboring parts of sclerites L1 and L2 surely form the most complex and variable part of the left phallic complex of Mantodea. Interesting characters concern the presence and shape of the processes and the extension, the kind of subdivision, and the kind of mutual contact of the sclerites L1 and L2 in this area. An exploitation of the many characters offered by this area requires a very detailed examination.
The right phallomere (Fig.
Annotated illustrations of Sphodromantissp. male genitalia for taxonomic use. Intact genital complex A dorsal view B ventral view. Disarticulated genital complex to isolate the individual phallomeres C–E: C left phallomere (of the left phallic complex), dorsal view D ventral phallomere (of the left phallic complex), dorsal view E right phallomere, ventral view. Blue text refers to major sclerotizations. Abbreviations: afa = anterior process (left phallomere); an = anterior extension of sclerite R3 (anterior apodeme); bm = dextral extension (right phallomere); fda = main posterior lobe (right phallomere); L4A = sclerite extending over the ventral wall (left phallomere); L4B = sclerite extending over the dorsal wall (left phallomere); loa = posteromesal (left phallomere); paa = posterior process (left phallomere); pda = posterior process (ventral phallomere); pia = process posterolateral to pva (right phallomere); pva = process anteromesal to pia (right phallomere); R3 = anteriorly extending sclerite (right phallomere).
Sclerite R1 is a very complex principal sclerite; it takes most or all of the dorsal wall of lobe fda, and along the lateral edge of lobe fda it also bends into its ventral wall to expand over the processes pia and pva and (usually) the area between them (Fig.
The anterior part of the right phallomere consists of the anteriorly expanded ventral wall of the phallomere, which in its larger anterior part is sclerotized by sclerite R3 (Fig.
Female genital region
Tergite 7 (TG7) and spiracles 7 and 8 agree with their counterparts of the preceding segments. Tergite 8 (TG8) and Tergite 9 (TG9) (Fig.
Annotated illustrations of the female Sphodromantissp. postabdomen and genitalia for morphological use. A left gonapophysis 8 with adjoining parts of coxa 8 and laterocoxa 9 in dorsal view B lateral parts of terminal abdomen in ventral view, with focus on base of cercus (enlarged 2.5× from C) C entire genital region, most parts in external view. Posterior parts (segments VIII–XI) are bent 180° dorsally and to the anterior compared to anterior parts (of segment VII), along the axis marked by arrows X. The lower half of the illustration shows the ventral fold 7 (“subgenital lobe”) and the genital fold area in dorsal view; the upper half of the illustration shows the ventral sides of segments VIII–XI in ventral view. Left gonapophysis 8 removed. Thick black lines are (virtual) cutting lines. Continuous thin black lines are freely visible edges (= lines along which the cuticle bends away from the observer’s view). Dashed thin black lines are (parts of) edges hidden beneath other cuticle. Thick gray lines are internal ridges. Dashed gray line next to fold mvtf7 in C giving outline of area where dorsal and ventral walls of ventral fold 7 are firmly connected by columellae. Membranous cuticle in very light gray, sclerotized cuticle in medium to darker gray (darker = more strongly sclerotized); cuticle shaded darker where it goes underneath other cuticle. For abbreviations see text, glossary, or Suppl. material
Coxosternite 7 (CS7) and ventral fold 7 (vf7) show many peculiarities (Figs
The anteriorly reflected cuticle lying above the posterior part of coxosternite CS7 is highly differentiated (Figs
Annotated illustrations of the female Sphodromantissp. postabdomen and genitalia for morphological use. A genital region, most parts in internal view. Posterior parts (segments VIII, IX) are bent 180° dorsally and to the anterior compared to anterior parts (of segment VII), along the axis marked by arrows X. The lower half of the illustration shows part of the dorsal wall of the ventral fold 7 (“subgenital lobe”) and genital fold area in ventral view; the upper half of the picture shows the ventral sides of segments VIII and IX in dorsal view. Right gonoplac 9 cut open B accessory gland with some structures surrounding its opening C gonapophyses 9 and structures around their base in dorsal view, left gonapophysis 9 cut near base (accessory gland not included) D gonapophyses 9 and structures around their base in ventral view, left gonapophysis 9 cut near base, outlet tubes of accessory gland (aglt, agrt) cut near base E base of gonapophyses 9 (right gonapophysis 9 cut open) together with central apodeme and adjacent parts of coxa 9 sclerotizations and gonoplacs 9. Thick black lines are (virtual) cutting lines. Continuous thin black lines are freely visible edges (= lines along which the cuticle bends away from the observer’s view). Dashed thin black lines are (parts of) edges hidden beneath other cuticle. Thick gray lines are internal ridges. Dashed gray line labeled cap+ in (A) (left side only) indicating maximum size of central apodeme cap in other specimens. Membranous cuticle in very light gray, sclerotized cuticle in medium to darker gray (darker = more strongly sclerotized); cuticle shaded darker where it dives beneath other cuticle. For meaning of black arrows and black, white, and gray arrowheads see text. For abbreviations see text, glossary, or Suppl. material
Most of the dorsal wall of ventral fold vf7 is sclerotized by the large vestibular sclerite (VS7), which is not in contact with the coxosternite CS7. VS7 is homonomous with the dorsal sclerotization of ventral fold vf9 in the male, called CSd9 if separated, and could alternatively be termed CSd7. While most of sclerite VS7 is very weak, its anteromedian part is heavier. At the midline the heavier sclerotization reaches further posteriorly along a low ridge-like elevation, the dorsal carina of ventral fold 7. From the upward-bent edge of the anterior part of each ventroterminal lobe, a fold descends to the level of the vestibular sclerite: the descending fold of the ventroterminal lobe. The dorsal walls of the left and right ventroterminal lobes are transversely connected by the transverse fold of the ventroterminal lobes. Beneath this fold the cuticle extends into a large cavity, which presumably is glandular in nature and is here called the gland of ventral fold 7 (vfgl7, seen through window cut into right dorsal wall of ventral fold vf7 in Fig.
The genital fold area bears the languette sclerite LG7 and three lobe-like elements around the midline, gfp7, ppl7, and egl7, which belong to the posterior marginal part of segment 7. The genital fold (gf7) is a very short transverse fold with a very shallow, slit-like cavity beneath it. The fold gf7 is additionally expanded into a pair of paramedian processes of the genital fold (gfp7), which are part of the genital fold and render it strongly bilobate. Sclerite LG7, which in Sphodromantis is a transverse plate while in Mantis it is medially divided, ascends onto the processes gfp7, which are sclerotized by it all around. From the area in front of the margin of sclerite LG7 arises a membranous papilla lobe and in front of this, the epigynal lobe. The cuticulized common oviduct opens into the space between the papilla lobe and the epigynal lobe, and in the dorsal wall of the papilla lobe the opening is extended in a groove. Internally the common oviduct forks into a pair of lateral oviducts, into which the cuticle can extend for some distance or not (only cuticulized parts are retained after KOH maceration). The epigynal lobe bears a weak sclerite in its dorsal wall, the epigyne (EG7). The papilla lobe and the epigynal lobe together form the genital papilla, which thus bears the female genital opening in its center (Fig.
While the aforementioned elements pertain to segment 7, the lateral ends of sclerite LG7 are connected with a pair of weak, finely folded sclerotizations that likely belong to segment 8, representing (part of?) the laterocoxae 8 (LC8). However, this interpretation remains to be tested based on the muscular connections, and there is another candidate sclerite to represent LC8 (see below).
In front of sclerite EG7 and pouches lep7 the body wall is reflected to the posterior (along the transverse line indicated by X→ ←X in Figs
The areas posterolateral to the caudogyne CG8 bear a pair of large, heavy sclerites, which are often sculptured and represent the limb-base sclerotizations of segment 8: the coxae 8 (CX8, Figs
At the posterior margin of each sclerite CX8 a long process originates, the gonapophysis 8 (gp8), the surface of which is mostly sclerotized by the gonapophyseal sclerotization 8 (GP8). The gonapophysis 8 shows a fairly complex structuring, and some of its formative elements have been named or are named herein (Figs
Annotated illustrations of intact female genitalia Tenodera sinensis for taxonomic use. A segments are opened up 180° along the axis marked X, the upper half of the illustration shows the ventral side of segments VIII and IX in dorsal view, the lower half of the illustration shows the dorsal wall of the ventral fold vf7 and genital fold in ventral view B segments VIII and IX in lateral view C dorsal side of segments VIII and IX in dorsal view. Dotted lines indicate regions of membrane, connective tissues, and musculature. Abbreviations: agsl = accessory gland supporting lobe; CG8 = caudogyne; CX8 = coxa 8; cxdl = dorsolateral coxal lobelet; cxvl = ventrolateral coxal lobelet; egl7 = epigynal lobe; gfp7 = process of genital fold; gl9 = gonoplac 9; glbl9 = gonoplac basal lobe 9; gp8 = gonapophysis 8; gp9 = gonapophysis 9; gpal8 = apical lobe of gonapophysis 8; gpmo8 = medial outgrowth of gonapophysis 8; gptm9 = medial tine of gonapophysis 9; ppl7 = papilla lobe; rh = rhachis; sbp = spermathecal bulge; vf7 = ventral segmental fold 7; vfdcp7 = process of dorsal carina of ventral fold 7; VS7 = vestibular sclerite 7.
The last element of segment 8 is the spermatheca (Fig.
The ventrolateral areas of segment 9 bear a pair of heavy sclerites, the laterocoxae 9 (LC9, Figs
The coxae 9 (CX9), originally a pair of sclerites, form an almost ring-shaped sclerite in Dictyoptera; the anterior half is visible in Fig.
The area enclosed by the coxae CX9 and the gonoplac bases bears some further structures, which in anteroventral to posterodorsal succession are the following: a transverse fold whose median part is posteriorly expanded to form a bifid lobe; this is here called the accessory gland supporting lobe (agsl, Figs
Annotated illustrations of digging structures on female genitalia. A–BEremiaphilidae-type digging spine: Eremiaphilasp. terminalia A ventral view B lateral view. C–D Rivetina-type digging spine: Rivetina Berland & Chopard, 1922 terminalia C ventral view D lateral view. E–F Chroicoptera-type digging spine: Chroicoptera longa Giglio-Tos, 1915 terminalia (E) ventral view; Chroicoptera saussurei Giglio-Tos, 1915 F lateral view. G–I Ligaria-type digging spine: Ligaria Stål, 1877 G dorsal view H lateral view I lateral view (left side) of the left gonapophysis 8. Note that in the Ligaria-type, the digging structures are gonapophyses 8. Figures A–E and G–I reproduced and adapted from
Gonapophysis gp9 bears three separate sclerites, which together constitute the gonapophyseal sclerotization 9 (GP9): the mesal gonapophyseal sclerite 9 (GPm9) (Fig.
Terminal abdominal segments
Tergite 10 (TG10, often referred to as the supraanal plate) is a transverse plate resembling the preceding tergites, but its median part is usually more or less strongly expanded to the posterior. This part of tergite TG10 shows much variation in shape, proportions, and the presence of a longitudinal middorsal carina (mdca10) (Fig.
Morphological variation of supraanal plates. A Brunneria Saussure, 1869 ♀ B Coptopteryx Saussure, 1869 ♀ C Acanthopssp. ♀ D Callimantis Stål, 1877 ♂ E Fulcinia Stål, 1877 ♀ F Deroplatys Westwood, 1839 ♂ G Kongobatha diademata Heard, 1920 ♂ juvenile H Metallyticus splendidus ♀ (reproduced and adapted from
The paraprocts PP are a pair of ventral sclerites located posterior to the male or female genitalia (Fig.
The cerci are the limbs of segment 11, the exoskeleton of which consists of a series of cylindrical sclerites (all together: CE) separated by very narrow, more or less distinct annuli of membrane. The sclerotized sections are called cercomeres. When counting cercomeres, particular attention should be paid to the base of the cercus, where the division into sclerite cylinders is often indistinct (
Morphological variation of cerci. A Amorphoscelissp. ♂ in ventral view B Heterochaeta bernadii ♂ in dorsal view C Metallyticus splendidus ♀ in dorsal view D Toxodera maculata ♀ left cercus in dorsal view E Ciulfinasp. ♂ right cercus in lateral view F Acanthopssp. ♀ left cercus in dorsal view. Arrow indicates apical notch in distal cercomere. A–D reproduced and adapted from
The mediotergite 11 (TGm11, the ‘epiproct’) is a small, weak sclerite placed behind tergite 10 at the midline, being more or less strongly overfolded by the dorsal fold df10. It is a median part of the fragmented and reduced tergite 11 (see
3.6 Oothecae
The ootheca, or egg case, is a complex structure female praying mantises form during oviposition to provide support and protection to eggs from environmental conditions and natural enemies (
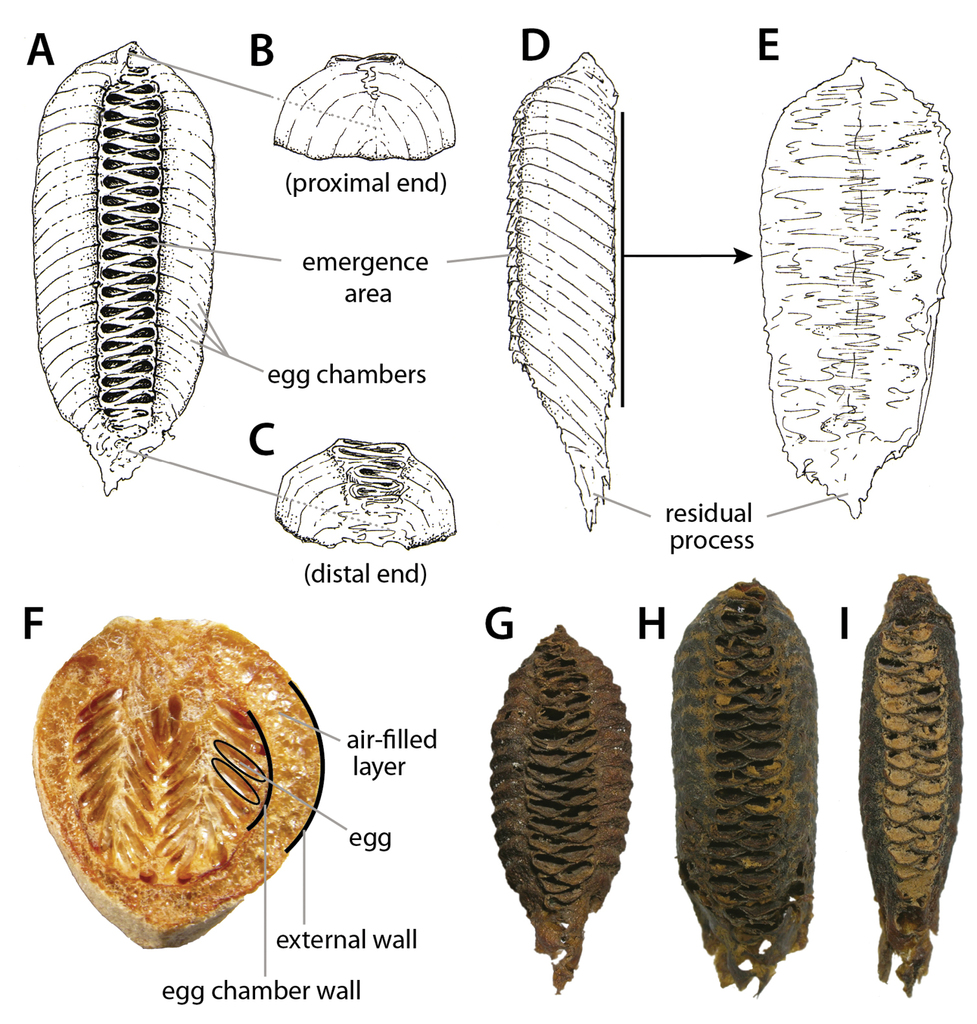
General ootheca morphology. A–E Annotated illustrations of a generic ootheca: A dorsal view B rear view C frontal view D lateral view E ventral view F internal view of a bisected egg case, species unknown. Example of interspecific variation of ootheca morphology (G–I) G Choeradodis rhombicollis Latreille, 1833 H Choeradodis stalii Wood-Mason, 1880 I Choeradodis columbica Beier, 1931. Illustrations and photographs courtesy of Hiromi Yagui.
Oothecae of Stagmatoptera supplicaria Burmeister, 1838 and Callibia diana Stoll, 1813. Stagmatoptera supplicaria oothecae (A–G): A–B dorsal view C lateral view D frontal view E lateral view of ootheca attached to substrate F ventral view G rear view showing attachment site. Callibia diana oothecae (H–N): H–I lateral view; J–K dorsal view L lateral view demonstrating approximate substrate position M–N ventral view. Callibia diana oothecae collected and imaged herein did not retain the attachment substrate. In these situations, we recommend illustrating a line to indicate substrate position. In those oothecae with extensive outer coating, it is suggested to remove part of the coating to reveal relevant details of the external wall, such as color and/or texture; in this case J shows regions where the outer wall is exposed. Asterisks (*) indicate points of attachment to substrate. Photographs courtesy of Hiromi Yagui.
The following are relevant diagnostic features exhibited by all oothecae:
1. Shape and size. Shape and general architecture are relatively well conserved within members of the same family, but often it is difficult to distinguish intrageneric from intraspecific variation; overall, shape is a far better diagnostic character above species level. Oothecae can be fusiform, oblong, rectangular, guttiform, barrel-like, maraca-like, cylindrical, etc., whereas size appears to be associated with the number of eggs contained within the ootheca. Regardless of size, the overall shape of the ootheca is otherwise well conserved and thus a given female will form similarly shaped oothecae. As a result, measurements such as length and width are only partially informative; nevertheless, we suggest including referential measurements to supplement ootheca descriptions. It is also important to note that captive females that are unhealthy, starved, unmated, or lack adequate oviposition substrate within their enclosures, may form abnormal oothecae unsuitable for description and/or identification; otherwise, oothecae obtained under proper artificial breeding conditions differ little if at all from their wild counterparts
2. External wall. The external wall exhibits extensive variation in its mechanical properties, presumably because of variation in the constituent materials that make the ootheca. The external wall can range from smooth and flexible to textured and very strong (Fig.
3. Point of attachment. Depending on the species, females attach their oothecae to specific substrates, such as stems, leaves, bark, rocks, crevices, or even in the ground. The most typical type of ootheca attachment site is along the ventral surface (Fig.
4. Egg chambers. Oothecae basically consist of a series of egg chambers (Fig.
5. Emergence area. Each egg within the egg chamber exhibits a single opening through which each hatchling emerges (Fig.
The following are two examples of how it is suggested oothecae should be described:
Stagmatoptera supplicaria Burmeister, 1838 (Fig.
Callibia diana (Stoll, 1813) (Fig.
3.7. Specimen collection
In temperate climates of the northern hemisphere, praying mantises generally hatch between April and June with their final eclosion occurring 3 to 4 months after hatching, living for roughly 6 to 7 months in the wild (
Praying mantises can be collected using a wide variety of insect collecting techniques. To collect praying mantises via sweep netting, sweep an insect net back and forth over vegetation, such as bushes and shrubs, grasses, and the leaves on tree branches (Fig.
Mantis collecting techniques. A Sweeping vegetation with an insect net for the collection of grass-dwelling mantises B metal halide light trap set up to collect flight-capable mantises C scanning bark with the aid of an insect net for the collection of bark mantises D scanning the ground and leaf litter for ground-dwelling mantises E passive light trap set up to be suspended in tree canopies for the collection of canopy-dwelling mantises F field preparation of collected specimens secured with insect pins inside a Schmitt Box. It is recommended to thoroughly dry out preserved specimens in the sun, being mindful of ants and other scavengers, prior to travel back to home institutions.
To document live specimens with photo- and videography in order to capture information about body position, coloration, and behavior, living specimens can be collected into individual 50 mL vials for temporary storage. The specimens can then be individually placed (to prevent cannibalism) into a pop-up mesh enclosure for long-term housing. For euthanization, place collected specimens into a kill jar activated with cyanide or ethyl acetate. If necessary, the right mesothoracic leg or thoracic muscle tissue can be removed post-euthanization and preserved in 95% ethanol for future DNA tissue vouchering or in RNAlater™ for RNA and mRNA isolation. Lastly, field store specimens in vials of 95% ethanol (especially early instar nymphs), pinned within a Schmitt Box and secured with multiple bracing pins, or individually wrapped in tissue paper or glassine envelopes with chlorocresol crystals added to the storage container to preserve specimens. If field pinning (Fig.
3.8. Specimen preparation
Pinning and spreading insect specimens is a delicate process, which involves some degree of scientific forethought. The joints of specimens become rigid and brittle after death, and with this in mind, specimens need to be properly prepped for mounting. Place dried specimens in a relaxing chamber from 6 hours to two days (depending on specimen size), frozen specimens need to be thawed, and specimens preserved in ethanol need to be rehydrated by transitioning through a sequence of successively lower ethanol concentrations over several days (i.e., from 95% ethanol to 80%, 70%, 50%, 30%). These relaxing techniques should make specimens pliable enough to pin and spread (see specimen mounting section). Large-bodied specimens, such as gravid females, might preserve better if the abdominal cavity is eviscerated and stuffed. To do this, cut along the pleural membrane of one side of the abdomen with a scalpel, between the tergum and sternum. Next, carefully remove the internal contents with forceps, being careful not to destroy or remove the genitalia. To preserve the eviscerated specimen, lightly dust inside the abdominal cavity with a mixture of 3 parts talcum powder to 1 part boracic acid. If desired, the abdominal cavity can be stuffed with cotton to restore shape (
Early instar nymphs should be stored in ethanol, as they tend to desiccate irreparably. Subadults, especially those of large species, can be spread fairly well.
It is recommended that exuviae be stored individually in small cellophane bags, mounted on the pin of the specimen that molted it, if possible. Exuviae can also be stored individually in vials of 95% ethanol.
Oothecae do not require special treatment for preservation, and thus common insect pinning techniques can be used for these structures. Oothecae are better preserved dry, pinned through their proximal end. It is recommended to collect wild oothecae along with the substrate to which they were attached, as this provides useful information for taxonomic identification and description. For embryological research, store oothecae in 95% ethanol, which will adequately preserve the egg case and embryos.
3.9. Specimen mounting
Euthanized praying mantis specimens that are to be mounted for curation or vouchering need to first be relaxed in a humidifying chamber ranging for 6 hours (specimens under 22mm) to 2 days (completely dry, large bodied specimens). To ensure that the specimen does not decay, check the humidifying chamber periodically. Pin and spread specimens on a foam insect pinning board using size 3 insect pins (or size 1–2 insect pins if mounting small and delicate specimens, e.g., species of Amorphoscelis, Hapalopeza, Mantoida, etc.) and a mounting block. Symmetrically arrange and brace the appendages using appropriately sized insect pins. Spread and position the right fore- and hindwings parallel to each other using either size 000 pins or glassine paper secured with pins. Due to the extreme morphological diversity of praying mantises, some slight modifications to the following pinning methods might need to be employed to minimize specimen damage and enhance ease of mounting.
1.) With mantis in hand, gently flex and move the fore-, meso-, and metathoracic legs, ensuring that the specimen can be positioned complanate on the pinning board without sustaining damage.
2.) Place the specimen on the mounting board, dorsal side up. Pin the specimen to the board by inserting a pin to the right of the midline in the mesothorax, just above the forewings (Fig.
3.) To stabilize the specimen during the spreading process, cross two bracing pins anterior to the supracoxal sulcus of the pronotum and another pair above the abdomen (Fig.
4.) Move the forecoxae forward, arranging them symmetrically and perpendicularly to the body. Secure this position with bracing pins (Fig.
5.) Position the forefemora symmetrically and perpendicularly to the forecoxae. Ensure that the foretibiae are directed laterad. Secure this position with bracing pins (Fig.
6.) Position the foretibiae symmetrically and perpendicularly to the forefemora. Secure this position with bracing pins (Fig.
7.) Position the foretarsi along the same axis as the foretibiae, perpendicular to the forefemora. Secure this position with bracing pins (Fig.
8.) Position the left mesofemur anteriorly, at an approximate 60° angle to the mesothorax, with the left mesotibia and mesotarsus positioned approximately parallel to the body (Fig.
9.) Position the metafemora posteriorly at an approximate 120° angle to the metathorax, with the metatibia angled slightly inward and the metatarsi positioned approximately parallel to the body (Fig.
10.) At a point located immediately behind the subcostal vein of the right forewing, slightly more proximal than distal to the body, insert a 000 insect pin and gently sweep the forewing forward to a position perpendicular to the body. Ensure that the pin tip is angled low (i.e., not perpendicular to the complanate specimen) and that the wing is rotated upwards in a consistent arc to minimize damage to the cells of the wing. With the pin still in the wing, gently insert the pin into the mounting board to secure the position of the wing (Fig.
11.) Use the tip of a 000 pin at a point immediately behind the subcostal vein of the hindwing, slightly more proximal than distal to the body, to gently draw the wing forward in a smooth arc. Pin the hindwing just behind, but parallel to, the forewing with no overlap (Fig.
12.) Working with forceps underneath the spread wings, gently position the right mesothoracic leg to achieve relative symmetry with the left mesothoracic leg (Fig.
Position the head symmetrically in relation to its body. This can be done by applying pressure with bracing pins along various points on the head. Secure this position with bracing pins. Position the antennae symmetrically with pins, preferably directed posteriorly to protect the antennae from damage and to save space in collections. For large-bodied specimens, support the abdomen by placing bracing pins underneath to prevent sagging.
Pinning demonstration of a relaxed Sphodromantissp. specimen. Blue arrows indicate approximate locations to arrange and/or pin specimen. A Pin specimen to the right of the midline in the mesothorax, just above the forewings B insert pins across the prozone and abdomen to support the specimen while pinning C position and secure the forecoxae D position and secure the forefemora E position and secure the foretibiae and foretarsi F–I position and secure the left mid- and hindleg as well as the right hindleg; the right midleg needs to be arranged but not secured with pins to accommodate the wings (see J) J position and secure the right forewing K position and secure the right hindwing L if needed, make further adjustments to the position of the head, antennae, and right mesothoracic leg to ensure bilateral symmetry. Due to the morphological diversity of praying mantises, some modifications to this method might need to be employed to minimize specimen damage.
3.10. Male and female genitalia preparation
For viewing and studying the genitalic complex, it is suggested to employ previously optimized methods for musculature or sclerotized and membranous structures (see below). Freshly euthanized specimens or those that have been preserved in ethanol or some other fixative provide the best morphological results. For dry preserved specimens, relax the specimen in a humidifying chamber for 6 hours to 2 days (depending on size) prior to dissecting the terminalia.
To investigate the sclerites and membranes of either the male or female genital complex, one first needs to dissect the terminalia at the apical margin of coxosternite 7, thereby isolating the terminal abdominal elements. A less destructive method for removing male genitalia entails cutting along the abdominal pleura from the abdominal apex to the apical margin of coxosternite 7, as well as to cut the membrane underneath the paraprocts. To remove the genital complex from coxosternite 9 (= subgenital plate), cut tissues between the genitalia and the abdominal wall (
Place the isolated terminalia in a vial of 10% potassium hydroxide (KOH) solution, heating the solution in a water bath ranging from 40°C for small specimens (e.g., Ilomantis Giglio-Tos, 1915) to upwards of 100°C for medium to large specimens (e.g., Sphodromantis) for five minute intervals, until the terminalia are sufficiently cleared of soft tissues (generally between five to thirty minutes). To clear the female genitalia of large bodied species, it might be necessary to gently remove and dislodge some internal tissues within the terminalia at some point during the maceration process in order for the solution to fully penetrate the structures. When the structures are sufficiently cleared, remove the terminalia from the KOH solution, rinse twice with distilled water, and place in ethanol to stop the clearing process. While in ethanol, clean the genitalia of remaining tissues and disarticulate the structures under a microscope with fine forceps and iris scissors (as in
Lactic acid can be used in the place of KOH, as it is less caustic than KOH and is reportedly better at maintaining the shape of the genital structures (
We strongly suggest that prepared male and female genitalia be stored in microvials of glycerin attached to the specimen’s pin for easy association. While slide mounting male genitalia is possible, we find that it creates curatorial issues (e.g., associating genital slides with specimens, storage of slides, etc.) and inhibits future morphological investigations as specimens are mounted flat onto the glass and fixed, making it impossible to study the 3-dimensional shape and subsequent characters of the genitalia. And while it might seem easier to image genitalia that have been slide mounted, characters that are not immediately visible or the shape of structures that are projected outward are lost by the compression of the slide and thus cannot be imaged. To image male and female genitalia that have been preserved in microvials, it is recommended to follow the techniques proposed by
3.11. Specimen labels
Specimen labels should contain relevant collection information for the purposes of scientific utilization and value. The labels should be made of acid-free archival paper. The labels can be made in a spreadsheet using size 4 or 5-point font or written neatly by hand with a fine pen (e.g., 0.25 mm line width). Ink should be of archival quality, and therefore both fade- and waterproof.
The primary label should include at least the following information: country, region, GPS coordinates, elevation, date (presented as either 2.Apr.2013 or 2.iv.2013), and collector’s identity. Other information, such as habitat type and collection method, is valuable to list as well.
The secondary label should include at least the following information: taxon details (e.g., genus and species), taxonomic authority (i.e., the name of the person who described the particular taxon and the year it was formally described), and the name of the person who determined the identification.
Ootheca labels should include details on oviposition substrate, in addition to other relevant collection data (e.g., collection locality; taxonomic determination; number of hatchlings; parasitoids; habitat type; etc.). In general, preservation procedures and labeling must ensure the association of the ootheca with its collection locality, taxon and/or the specific specimen that produced a given ootheca, as well as valuable natural history information (e.g., oviposition sites, habitat use, etc.).
A technique for generationally labeling oothecae and nymphs has been described by
3.12. Standards of measurement
Praying mantis specimens should be mounted complanate and symmetrically in order to maximize access to morphological features, thereby minimizing the needed number of orientations to observe the specimen (see Specimen Mounting). In other words, the more irregularly a specimen is positioned, the greater the number of orientations required to observe all relevant morphological features. With advancements in digital imaging and increased specimen numbers, a standard of orientation will minimize the number of images required for digital observation in addition to streamlining workflow in the lab, thereby reducing time spent imaging specimens. With an optimally mounted specimen, researchers are able to quickly access important morphological information that will help determine sex, species, and other information of interest. Furthermore, measuring specimens that have been mounted in this way is easier and less time consuming.
Prepared specimens. In specimens that are “partially spread,” the wings lay flat against the thorax and abdomen. The mesothoracic and metathoracic legs are arranged symmetrically alongside the body with the forelegs held perpendicularly above the specimen’s head. The “spread” mounting technique is similar to the partially spread method, with one or both sets of wings spread. As the “spread” method best displays the specimen’s morphological features, thereby allowing for an increased ease in data collection and specimen viewing, it is recommended that this technique be employed.
Unprepared specimens. One of the most pervasive approaches for mounting praying mantises is the “unspread” method, that is, pinning the specimen without spreading the legs and wings. This approach allows the specimen to dry with its legs folded naturally underneath its body, making it extremely difficult to obtain morphological data without first undertaking the cumbersome process of relaxing the specimen in order to remount it.
Furthermore, because the “unspread” and “partially spread” methods often require that the specimen be more frequently manipulated for measurement collection, the specimen is at an increased risk of sustaining damage. In the following section, the process for proper measurement collection will be described and pictorially represented on specimens that have been mounted using the “spread” method. Suggestions for how to obtain certain measurements on “unspread” specimens are also included, but it should be noted that with specimens arranged in this manner it might be difficult to obtain consistent measurements.
Head. Arrange head with anterior surface oriented parallel to the scope bed without lateral tilting, which can result in inaccurate measurements. The anterior surface of the head should be arranged parallel to the floor of the stereo-microscope, and in full view. Based on the preservation of the specimen and the angle of the head in relation to the body, it may need to be positioned dorsally or ventrally.
Head width. Measure from the outermost point of the compound eye to the opposing margin at the widest point (Fig.
Head length. Capturing the relative length of the head can be achieved by measuring from the vertex to the clypeo-labral junction. Measure from the vertex midline to the posterior margin of the clypeus, located just above the labrum (Fig.
Head and lower frons measurement standards. Solid black lines indicate approximate location to take length and width measurements. Sphodromantissp. head A head width B head length C lower frons width D lower frons length. Abbreviations: cly = clypeus; ey = compound eye; lb = labrum; lf = lower frons.
Frons width and length. The lower frons is a well-defined area on the head that lies within the arms of the epicranial sulcus extending between the antennal insertions and abutting the upper margin of the clypeus. Measure from the mediolateral margin of the lower frons to the opposing margin for relative width (Fig.
Body. Position the specimen dorsal side up, parallel to the bed of the microscope, without lateral tilting. Total length of specimen is relative to head position, abdominal preservation and wing position (Fig.
Body and pronotal measurement standards. Solid black lines indicate approximate location to take length and width measurements. Sphodromantissp. prepared specimen: A body length measurement method 1; B body length measurement method 2, with measurements designated 1 and 2 indicating the first and second measurements needed for the body length measurement, which, when summed together result in total body length C Sphodromantissp. unprepared specimen D Stagmomantis nahua Saussure, 1869 unprepared brachypterous specimen with the sum of measurements designated 1 and 2 equaling total body length. Sphodromantissp. pronotal measurements E length F width. Asterisks (*) represent potential areas where specimen damage may result in an inability to take an accurate measurement (e.g., wing tip damage). Abbreviations: fw = forewing; h = head; hw = hindwing; pn = pronotum.
Body length – Prepared specimen. For a rough size estimate on partially spread specimens, measure from the central ocellus or medially between the antennal insertion sites as a consistent landmark that accounts for relative head position to the distal terminus of the abdomen, fore-, or hindwing (whichever is longer) (Fig.
Body length – Unprepared specimen. For a rough size estimate, measure from the central ocellus or medially between the antennal insertion sites to the distal terminus of the abdomen, fore-, or hindwing (whichever is longer) (Fig.
For comparable size determinations across taxa, it is recommended to measure from the central ocellus or medially between the antennal insertion sites and conclude at the abdominal terminus (Fig.
Pronotum. To measure the length and width of the pronotum, the specimen should be oriented dorsal side up, the pronotum parallel to the scope bed, without lateral tilting. For measuring the height of the pronotum, position the specimen with the lateral margin facing up (Fig.
Pronotal height, length, and width. To determine pronotal height, measure from the anteromedial point on the lateral pronotal edge to the dorsal surface of the pronotum; the measurement should be parallel to the supracoxal sulcus. For pronotal length, measure along the midline of the pronotum from the anterior margin to the posterior margin; the measurement should be perpendicular to the supracoxal sulcus (Fig.
Pronotal measurements are useful for taxonomic descriptions. If these measurements are to be included in morphological matrices for phylogenetic analysis, however, it may be useful to measure pronotal width without including the lamellar expansions in order to get comparable data throughout taxa (
Wings.Mantodea includes species that can be macropterous, mesopterous, brachypterous, micropterous, and apterous. Wing length will be determined by the classification and condition of the wings. Position the specimen with dorsal side up, parallel to the scope bed, without lateral tilting. To ensure consistency, begin wing measurements from the point where the Analis veins converge on both the fore- and hindwings as this is a landmark feature that can be seen in both extended and folded wings (Fig.
Wing length and width measurement standards. Solid black lines indicate approximate location to take length and width measurements. Sphodromantissp. A prepared specimen fore- and hindwing length B unprepared specimen forewing length. Note: in unprepared specimens, hindwing length and fore- and hindwing width is impossible to measure without first relaxing and re-mounting the specimen C prepared specimen fore- and hindwing width. Asterisks (*) represent potential areas where specimen damage (e.g., wing tip damage) may result in an inability to take an accurate measurement. Sectional sign (§) represents potential area where wing overlap can occur and either great care or re-mounting is needed to take wing width measurements. Black arrows indicate the pterostigma. Abbreviations: fw = forewing; hw = hindwing.
Forewing length and width – Prepared. To determine wing length on spread forewings, measure from the convergence of the Analis veins to the distal terminus of the forewing (Fig.
Hindwing length and width - Prepared. Measure from the convergence of the Analis veins to the distal terminus of the hindwing (Fig.
Forewing length - Unprepared. Measure from the convergence of the Analis veins to the distal terminus of the wing (Fig.
Hindwing length - Unprepared. Without first relaxing and remounting the specimen, hindwing length cannot be accurately obtained.
There are a few exceptional morphologies in mantodean wings, mostly due to cryptic adaptations. Among them are members of Acanthopoidea (e.g., Acanthops or Pseudacanthops Saussure, 1870; see
Prothoracic legs. The raptorial forelegs of the mantis contain extensive morphological information; spines, spurs, and denticulations adorn the forelegs of these hunters. It is important to have access to these features as they are frequently used for specimen identification (Fig.
Prothoracic leg and spine measurement standards. Solid black lines indicate approximate location to take length and width measurements. Sphodromantissp. prepared foreleg measurements (A–D): A forecoxal length B forefemoral length C forefemoral width D foretibial length; line 1 measures foretibial length from the proximal bend (pb) to the foretarsal insertion site; line 2 accounts for tibial spur length, but does not account for total spur length nor its angle; dashed black lines demonstrate approximate measurement endpoints. Sphodromantissp. E unprepared foretibial length measurement (see note). Sphodromantis specimens presented in F–L are positioned for optimal viewing for the reader, not for obtaining accurate measurements. Ensure that structures are arranged appropriately before obtaining measurements. Prepared forefemoral spine length measurements (F–H): F anteroventral femoral spine length G discoidal spine length H posteroventral femoral spine length. Unprepared spine length measurements (I–J) (see note): I posteroventral spine length J anteroventral and posteroventral tibial spine lengths. K Prepared posteroventral tibial spine length measurements L close up view of approximate endpoints to obtain spine length measurements. Note: in unprepared specimens, obtaining certain measurements might be impossible, necessitating the remounting of such specimens. Asterisks (*) represent areas where specimen damage has occurred and sectional signs (§) represents potential areas where overlap can occur and re-mounting is needed to take measurements. Black arrow indicates cuticular margin abutting spine base. Abbreviations: avfs = anteroventral femoral spines; avts = anteroventral tibial spines; ds = discoidal spines; pb = proximal bend in the tibia; pvfs = posteroventral femoral spines; pvts = posteroventral tibial spines.
Forecoxal length – Prepared. Measure from the point of the anterior convergence of the coxal keels to the valley of the coxal lobes, near the coxo-trochanteral hinge (Fig.
Forefemoral length and width – Prepared. Measure from the proximal-most point on the forefemur, where the forefemur abuts the trochanter, to the apex of the genicular lobe (Fig.
Foretibial length – Prepared. Measuring the foretibia involves taking parallel measurements that ultimately account for the total relative length of the structure. As the raptorial and cursorial tibiae serve functionally different roles, it is important to ensure that no information is lost when measuring these seemingly homologous appendages. The success of prey capture being perhaps dependent on the relative length of the foretibia and tibial spur, it is important to attend to both the length from the base of the proximal bend of the foretibia to the tarsal insertion point (Fig.
Foretarsal length – Prepared. The tarsus should be measured using a segmented measuring tool to account for any curvature found in the positioning of the tarsomeres. Measure from the tarsal insertion point on the tibia, concluding at the distal terminus of the final tarsomere, not incorporating the ungues (see Fig.
Meso- and metathoracic leg measurement standards. Solid black lines indicate approximate location to take length and width measurements. Dashed black lines demonstrate approximate locations for measurement endpoints. Prepared Ilomantis ginsburgae Brannoch & Svenson, 2016 meso- and metathoracic leg length measurements, ventral view (A–E): A meso- and metathoracic coxal length B meso- and metathoracic femoral length C meso- and metathoracic tibial length D tarsal length measurements with lines 1–5 indicating contiguous tarsomere measurements E approximate location of endpoints for the meso- and metathoracic femora and tibiae measurements. F Unprepared I. ginsburgae, ventral view, meso- and metathoracic leg length measurements. Note: in unprepared specimens, obtaining certain measurements might be impossible due to appendage overlap, thus necessitating the relaxing and re-mounting of such specimens. Sectional sign (§) represents potential area where overlap can occur and relaxation and re-mounting is needed to take accurate measurements. Abbreviations: cx = coxa; fe = femur; pb = proximal bend in the tibia ta = tarsus; ti = tibia; tr = trochanter.
Foreleg measurements – Unprepared. Depending on the preservation status of the specimen, obtaining accurate measurements might not be possible without first relaxing and remounting specimens. Often, structural overlap will obscure intended measurements (Fig.
Forefemoral spines – Prepared. There are three rows of spines on the forefemur: posteroventral, discoidal, and anteroventral. Posteroventral spines are found on the posteroventral edge of the forefemur; the discoidal spines are found on the ventral surface of the forefemur, between the anteroventral and posteroventral rows of spines; and the anteroventral spines are on the anteroventral edge (some exceptions to this are the posteroventral spines in several species of Paraoxypilus, Hoplocorypha Stål, 1871, Eremiaphila Lefebvre, 1835, and Blepharopsis Rehn, 1902, which are also found along the anteroventral edge). There are some less common phenotypes that lack certain rows of forefemoral spines, such as the posteroventral spines in Perlamantinae and all of the rows of spines except for one persisting discoidal spine in Amorphoscelinae.
Anteroventral forefemoral and foretibial spines – Prepared. Arrange the specimen ventral side up, parallel to the scope bed, and without lateral tilting. Determine the relative length of the spines by measuring from the cuticular margin, the externally visible cuticular rim at the base of the spine, to the distal terminus of the spine (Fig.
Discoidal spines – Prepared. With the specimen positioned ventral side up, parallel to the scope bed, without lateral tilting, measure from the cuticular margin that runs along the base of the spine to the distal terminus of the spine (Fig.
Posteroventral forefemoral and foretibial spines – Prepared. Arrange the specimen dorsal side up, parallel to the scope bed, and without lateral tilting. To determine the relative length of each spine, measure from the cuticular margin of the spine and conclude the measurement at the distal terminus (Fig.
Forefemoral Spines – Unprepared. Accessing the morphological information contained on the forefemur and foretibia of unprepared specimens might be extremely difficult to achieve, if not impossible. Even with visual access to spines, measuring their relative lengths will ultimately lack consistency across taxa because the specimen position needed to view and measure the spines will not be standardized across all specimens. It is highly recommended that the specimen be relaxed and re-mounted with forelegs spread (Fig.
Mesothoracic and metathoracic legs. Arrange the specimen ventral side up, meso- or metathoracic legs parallel to the bed of the microscope, without lateral tilting. The measurements of the meso- and metathoracic legs involve essentially the same methodology as the forelegs, with just a few slight adjustments. With unprepared specimens, inconsistency and inability to take measurements present a challenge and so it is recommended to relax and remount specimens.
Meso-, metacoxa – Prepared. To determine the relative length of the meso- and metacoxa, measure from the anterior convergence of the coxal keels to the distal terminus of the meso- and metacoxa, near the coxo-trochanteral hinge and along the lateral-most side of the segment (Fig.
Meso-, metafemur – Prepared. Measure from the point on the meso- and metafemur most proximal to the body, which abuts the trochanter, to the distal terminus of the femur. This measurement should begin along the lateral margin edge of the femur, concluding at the midline of the femoral terminus (Fig.
Meso-, metatibia – Prepared. Begin the measurement at the proximal bend of the meso- and metatibia (an exoskeletal indentation that serves as an attachment site for the tarsal muscles, see Fig.
Meso-, metatarsus – Prepared. The meso- and metatarsus should be measured using a segmented measuring tool to account for any curvature found in the positioning of the tarsomeres. Measure from the tarsal insertion point on the meso- and metatibia, concluding at the distal terminus of the final tarsomere, not incorporating the ungues (Fig.
Meso- and metathoracic leg measurements - Unprepared. Depending on the preservation status of the specimen, obtaining accurate measurements might not be possible without first relaxing and remounting specimens. Often, overlap of structures will obscure intended measurements (Fig.
Subgenital plates. Position the specimen ventral side up to view the coxosternites, specifically coxosternites 7/9 (CS7/ CS9 = subgenital plate) to determine the sex of the specimen. There are nine coxosternites in male praying mantises, the first of which is strongly reduced and the last being CS9, which is usually rounded along the posterior edge and more or less asymmetrical (Fig.
MaleCS9Length. Measure from the anterior margin of CS9, concluding medially along the terminal edge (Fig.
MaleCS9Width. Measure across the widest point of CS9, concluding at the contralateral margin (Fig.
FemaleCS7Length. Measure from the anterior margin of CS7, concluding medially at the point where the CS7 bifurcation concludes, not including the exposed apical lobes of gpal8 (Fig.
FemaleCS7Width. Measure across the widest point of CS7, concluding at the contralateral margin (Fig.
Coxosternite 7/9 (= ♀/♂ subgenital plate) length and width measurement standard. Solid black lines indicate approximate location to take length and width measurements. Dashed black lines demonstrate approximate locations for measurement endpoints. Omomantissp. ♂ CS9, ventral view (A–B): A length B width. Sphodromantissp. ♀ CS7, ventral view (C–D) C length with inset showing approximate location for length measurement endpoint D width. Abbreviations: ce = cercus; gpal8 = apical lobe of gonapophysis 8; CS = coxosternite; CS7 = ♀ coxosternite 7; CS9 = ♂ coxosternite 9; si = spiracle; sl9 = stylus.
3.13. Glossary
Morphological terms are generally listed in the singular. We indicate whether a term refers to a paired or an unpaired structure, according to the following specifications (note: the following indications refer to the usage of a term rather than to the condition of the structure concerned) – [paired]: term referring to a structure that occurs in two exemplars, one on the left and one on the right side of the body, with clear side-homonomy; [unpaired]: term referring to a structure that occurs in one exemplar that is present at the midline but may to a varied extent extend from there to the sides of the body; [one-sided]: term referring to a structure that occurs in one exemplar either on the left or on the right side of the body and has no or no identified counterpart on the other side; [paired, counterpart: ...]: term referring to a structure that occurs in one exemplar either on the left or on the right side of the body but has a differently named homonomous counterpart on the other side; [unpaired to paired]: term variously referring to an unpaired or a paired structure in different taxa, while it is unclear which is the plesiomorphic condition.
Accessory gland(s) – ag [= colleterial gland(s)] [unpaired]: ♀; a median cuticular invagination of the posterior part of segment 9, opening anteroventrally of the bases of the gonapophyses 9, consisting of a proximal pouch internally dividing in a right and a left branch, which are structurally and functionally asymmetrical.
Accessory gland supporting lobe – agsl [unpaired]: ♀; a posteriorly directed lobe covering the opening of the accessory glands ventrally; apex bifid, membranous or sclerotized by a sclerite AG.
afa – [one-sided]: ♂; left phallic complex (left phallomere), 1st process from anterior upon the edge pba between pouches pne and lve (with L1b or L1B sclerotization) (reference: ‘apofisi falloide’).
age – [one-sided]: ♂; right phallomere, sclerotized groove or deeper infolding along anterior margin of ventral wall, the deeper mesal part often curved (with R3 sclerotization) (reference: ‘anterior groove’).
Antenna – [paired]: sensory appendages inserted near the ocelli on the cranium; generally filiform, sometimes moniliform, pectinate, or serrate (plural: antennae).
Antennal sclerite – [paired]: sclerotized rim around the antennal socket.
Antennifer – [paired]: the point of articulation in the base of the antennae, externally visible as a sulcus within the circumantennal sclerite (
Anterior Analis – AA [paired]: the vein system immediately posterior to CuP; it is composed of a simple first branch, AA1, and of second branch generally branched, AA2.
Anterior Cubitus – CuA [paired]: the fourth major longitudinal wing vein; the anterior branch of the Cubitus; anteriorly (e.g., Metallyticus) or posteriorly (e.g., Chaeteessa) branched in Mantodea (
Anterior Radius – RA [paired]: the second major longitudinal wing vein; the anterior branch of the Radius.
Anterior spermathecal bulge fold – spba [unpaired]: ♀; an external transverse fold bordering the spermathecal bulge to the anterior.
Anterior veinal margin – avm [paired]: vein-like structure along anterior wing margin; area anterior to the avm is the visor.
Anterior wing margin – awm [paired]: membranous area along anterior wing margin; area posterior to the awm is the visor.
Anteroventral forefemoral spine(s) – avfs [paired]: a row of spines on the anteroventral edge of the forefemora.
Anteroventral foretibial spine(s) – avts [paired]: a row of spines on the anteroventral edge of the foretibiae.
Anus – re-o [= opening of rectum (re)] [unpaired]: the posterior opening of the gut (hindgut), forming the morphological posterior tip of the body upon the telson; formed by the inwardly bending central walls of the dorsal fold 11 and the subanal lobes.
apa – [one-sided]: ♂; left phallic complex (left phallomere), 2nd process from anterior upon the edge pba between pouches pne and lve (with L1b or L1B sclerotization) (reference: ‘apophysis, posterior [process]’).
Apex (wing) – [paired]: the distal-most tip of the wing.
Apical cleft of gonoplac 9 – glcf9 [paired]: ♀; a notch in the wide apical edge of the gonoplac gl9, making the gl9 apex slightly bifid.
Apical lobe of gonapophysis 8 – gpal8 [paired]: ♀; the lobe-like apex of gonapophysis 8.
Arculus – arc [paired]: hindwing; a strengthened cross-vein connecting media M and the anterior cubitus CuA.
Aulax – al [paired]: ♀; a longitudinal groove in the middle third of the dorsal wall of gonapophysis 8 that can harbor the rhachis of gonapophysis 9 to form a sliding interlock (a tongue-and-groove system), the olistheter.
Basitarsus – [paired]: the basal segment of the tarsus (plural: basitarsi).
bm – [one-sided]: ♂; right phallomere, a dextral extension (with fda and R1 sclerotization) (reference: ‘braccio mediale del fallomero’).
Cardo – [paired]: proximal subdivision of the maxilla; articulates with the cranium (
Caudogyne – CG8 [unpaired]: ♀; a median sclerite located in the anterior ventral wall of segment 8, between the anterior ends of coxae 8; possibly part of true sternum 8, perhaps the sternite 8.
Cercus – ce [paired]: a long filiform process having its base far laterally posterior to tergite 10 and paraproct; (part of?) the limb of abdominal segment 11.
Cercomere (apical cercomere CEa; basal cercomere CEb) – [paired]: any of the cylindrical sclerites following one after the other along a cercus.
Cervical foramen – an aperture between the posterior surface of the head and the prothorax, a channel between the head and the thorax for various vessels, tracts, and nerves (
Cervix – region between the head and the prothorax, which features sclerotizations (
Chroicoptera-type – ccsp7 [paired]: ♀; in Chroicoptera the coxosternite 7 (= subgenital plate) bears two elongated ventral spines that are distinctly curved laterad; ventromedial ridges present on cs5 and cs6 (
Circumantennal sclerite – [paired]: a sclerite that lies between the base of the antenna and the circumantennal sulcus.
Circumantennal sulcus – [paired]: a sulcus that borders the circumference of each antennal base (plural: circumantennal sulci).
Circumocular sulcus – [paired]: a sulcus that borders the circumference of the compound eyes (plural: circumocular sulci).
Clypeus – [unpaired]: located just below the epistomal sulcus, which isolates the lower frons from the clypeus; the clypeus is separated from the labrum by a cuticular infolding; the clypeus serves as a muscular attachment site and covers the mouthparts of the insect; cuticular expansions (crests, ridges) are present in some species.
Common oviduct – oc [opening = oc-o] [unpaired]: ♀; non-extended, median cuticular outlet duct for eggs, opening on the far posterior ventral wall of segment 7 on the genital papilla, into the space between the papilla lobe and the epigynal lobe.
Common oviduct opening – oc-o [= gonopore] [unpaired]: ♀; opening of the common oviduct.
Compound eyes – ey [paired]: usually globular, surfaced with ommatidia, located laterally on the cranium.
Coronal sulcus – cs [unpaired]: a component of the epicranial sulcus; the coronal sulcus runs dorsomedially from the vertex of the cranium towards the ocelli, where it branches off into the postfrontal sulci; the “stem” of the epicranial sulcus complex.
Coxa – [paired]: the most proximal segment on the leg; articulates with the thorax and trochanter (plural: coxae).
Coxa 8 – CX8 [paired]: ♀; a large sclerite in the lateral ventral wall of segment 8; it represents the main part of the limb-base sclerotization.
Coxa 9 = CX9 [paired, with median fusion]: ♀; originally a pair of sclerites in the posterior ventral wall of segment 9, representing the larger posterior part of the limb-base sclerotization of segment 9; located at the anterior, lateral, and posterior base of the gonoplacs gl9 and also extending into the lateral and mesal walls of the gl9, which represent the limb bases or coxal lobes. The anteromesal parts of the left and right CX9 are medially fused and can be semi-detached by weak sclerotization from the remainder of CX9. CX9 is informally categorized in an unpaired mediocoxal area (CX9μ) and paired basicoxal (CX9β), mesolobocoxal (CX9μλ), and laterolobocoxal (CX9λλ) areas; the left and right basicoxal areas CX9β are narrowly separated at their posterior ends.
Coxosternite – CS [= ‘sternite’] [unpaired, partly fused from paired structures]: the undivided ventral sclerite plate of an abdominal segment; composed (without recognizable borders) of a median (eu)sternum and paired coxae, antelaterocoxae (uncertain), and postlaterocoxae.
Coxosternite 7 – CS7 [= female subgenital plate] [unpaired, partly fused from paired structures]: ♀; the seventh abdominal coxosternite; underlies the genital structures.
Coxosternite 9 – CS9 [= male subgenital plate] [unpaired, partly fused from paired structures]: ♂; the ninth abdominal coxosternite; underlies the genital structures; can be asymmetric, generally bears styli.
Cubital furrow – cf [paired]: forewing; anterior-most fold delimiting the remigulum, parallel to CuP.
Cubitus – Cu [paired]: the stem giving rise to CuA and CuP, visible only at the wing base.
dee – [one-sided]: ♂; left phallic complex (ventral phallomere), a pouch that receives the opening of the ejaculatory duct, ventral to the pouch lve, and more or less formed from part of its ventral wall (membranous) (reference: ‘ductus ejaculatorius [pouch]’).
Digging spines – [paired]: ♀; modified structures on the terminalia of some taxa that aid in the oviposition of oothecae.
Discoidal spine(s) – ds [paired]: a row of spines found mesally on the interior surface of the forefemora (between 1 and 4), between the posteroventral and anteroventral femoral spines.
DK hearing organ – [paired]: ultrasound-sensitive hearing organ; DK denotes morphology with a deep groove with pronounced knobs (
DNK hearing organ – [paired]: higher ultrasonic thresholds than in DK form with reduced sensitivity; DNK denotes morphology with a deep groove and no knobs (
DO hearing organ – [paired]: ultrasound-sensitive hearing organ with reduced sensitivity; DO denotes morphology with a deep open groove with knobs absent or highly reduced (
Dorsal carina of ventral fold 7 – vfdc7 [unpaired]: ♀; a low median longitudinal ridge upon the dorsal wall of ventral fold, sclerotized by part of the vestibular sclerite.
Dorsolateral coxal lobelet – cxdl [paired]: ♀; a posteriorly and laterally directed projection from the posterolateral part of coxa 8, located dorsolateral to the ventrolateral coxal lobelet.
Dorsolateral segmental expansion – dlse [paired]: a flat expansion upon the lateral part of a tergite, likely resulting from a strong elevation of (part of) the laterodorsal carina.
Dorsomedian expansion (of dorsal fold) – dfme [unpaired to paired]: a flat expansion resulting from a local lengthening of the dorsal fold of a segment; the transition between unpaired and paired expansions is fluent, due to the degree of development of a median notch.
Ejaculatory duct – dej [unpaired]: ♂; the partly cuticulized (membranous; ectodermal external part) and partly not cuticulized (mesodermal internal part) duct originating from the nymphal median invagination associated with the male genitalia and then contacting the mesodermal internal genitalia; opening upon the dorsal wall of lobe vla of left phallic complex, occasionally via a pouch (dee) (reference: ductus ejaculatorius).
Epicranial sulcus – es [unpaired]: made up of a coronal sulcus and two postfrontal sulci on the insect cranium; looks similar to an inverted Y (
Epigynal lobe – egl7 [unpaired]: ♀; a lobe or fold on the posterior-most part of the ventral wall of segment 7 (in genital fold area); it covers the opening of the common oviduct, located at its ventral base (= dorsal base of papilla lobe), from above; can be sclerotized dorsally by an epigynal sclerite.
Epigyne – EG7 [unpaired]: ♀; a median sclerite in the posteriormost ventral wall of segment 7, located in the dorsal wall of the epigynal lobe.
Epistomal sulcus – [unpaired]: a sulcus that lies between the lower frons and the clypeus, anteriorly connecting the subgenal sulci (plural: epistomal sulci).
Eremiaphilidae-type – vfme6 [paired]: ♀; in Eremiaphila and Heteronutarsus, coxosternite 6 features two ventral digging spines that are pointed (Eremiaphila) or triangular and more plate-like (Heteronutarsus); coxosternite 6 mostly covers the coxosternite 7 (= subgenital plate) (
Euplantula – epl [paired]: well-developed adhesive pads found on the ventral side of the tarsomeres (plural: euplantulae).
Fastigial process – fp [unpaired]: a process projected from the summit (apex) of the cranial vertex.
fda – [one-sided]: ♂; right phallomere, the main posterior lobe (with R1 sclerotization) (reference: ‘fallomero di destra’).
Femoral brush – fb [paired]: small patch of setae used for grooming; distally located on the ventral surface of the forefemur.
Femur – fe [paired]: the third and generally largest leg segment; fused to the trochanter at its base, articulates with the tibia; forefemora modified with spines for catching prey and a femoral brush for grooming (plural: femora).
Flagellum – the ultimate segment of the antennae; comprised of annuli.
Forewing – fw [paired]: the anterior wings; attached by axillary plates to the mesothorax.
Furcasternite – [unpaired]: a ventral prothoracic plate that includes the furcal invaginations; posterior to the postcervical plate and forecoxal insertions, anterior to the spinasternite (Levereault 1936).
Genicular lobe – gl [paired]: a lobe located antero- and posteroventrally on the distal-most region of the femur; featuring a spine (i.e., genicular spine) in some species.
Genicular spine – gs [paired]: spine that can project from the pro-, meso-, and/or metathoracic genicular lobes of the femora; not considered posteroventral and anteroventral forefemoral spines (
Genital chamber – gc [unpaired]: ♀; the space enclosed above the genital fold area of segment 7 and beneath the ventral walls of segment 8; harboring the genital papilla and the opening of the common oviduct.
Genital pouch – vst+gc [unpaired]: ♀; the vestibulum and the genital chamber together, i.e., the entire space enclosed above the ventral fold and the genital fold area.
Genital fold – gf7 [unpaired]: ♀; a posteriorly directed transverse fold with a very shallow, slit-like cavity beneath it; located between vestibular sclerite VS7 and the languette sclerite, and dorsally sclerotized by the latter; gf7 very short except for its long processes gfp7; forming the ventral posterior border of genital chamber.
Genital papilla – gpp7 [unpaired]: ♀; the papilla lobe and the epigynal lobe together.
Glossa – [paired, with (partial) median division]: the medial, terminal lobes of the labium (plural: glossae).
Gonapophyseal sclerotization 8 – GP8 [paired]: ♀; the sclerotization of gonapophysis 8; it shows a complex distribution over the walls of gonapophysis 8, and its strength varies strongly in different parts.
Gonapophysis 8 – gp8 [paired]: ♀; a long process originating at the posterior margin of sclerite coxa 8; representing a modified coxal vesicle and thus likely an endite of the limb base of segment 8; in Mantodea the bodies of the left and right gonapophyses 8 are free from each other.
Gonapophysis 9 – gp9 [paired, with partial median fusion]: ♀; a long process originating (antero)mesally from the gonoplac 9; representing a modified coxal vesicle and thus likely an endite of the limb base of segment 9. The bodies of the left and right gonapophyses 9 are fused at their very bases to form a short common stem.
Gonoplac 9 – gl9 [= coxal lobe 9 = cx9] [paired]: ♀; a large posteriorly directed projection from the posterior ventral wall of segment 9, in the area sclerotized by coxae 9; representing the projection of the limb base of segment 9.
Gonoplac basal lobe – glbl [paired]: ♀; a membranous anteromesally directed lobe at the dorsal (posterior) base of the gonoplac.
Hindwing – hw [paired]: the posterior wings; attached by an axillary plate to the metathorax.
Hypopharynx – hpx [unpaired]: a medial lobe of the preoral cavity.
Incisor process – [paired]: sharp, toothed processes on the mandible.
Interantennal sulcus – [paired]: a sulcus between the antennal bases.
Intercervical sclerites – ics [paired (occasionally) with median fusion]: two well-sclerotized plates positioned at the base of the cervix, posterior to, and abutting, the lateral cervical sclerites.
Juxtaocular bulges – [paired]: flattened, bulging, or pointed elevations located between each compound eye and the corresponding parietal sulcus (
Labial palpus – lbp [paired]: 3-segmented appendages on the labium (plural: labial palpi).
Labium – [unpaired, with median fusion]: sclerite that forms the base of the insect mouth; bears the labial palpi, the glossae, and the paraglossae.
Labrum – [unpaired]: located just below a cuticular infolding that separates the labrum from the clypeus; the labrum is a preoral structure that covers the mouthparts of the insect.
Languette sclerite 7 – LG7 [unpaired, occasionally with median division]: ♀; a plate in the genital fold area (ventral wall of genital chamber); extending on the processes gfp7 of the genital fold; can be medially divided.
Lateral cervical sclerite – lcs [paired]: two well-sclerotized plates that are positioned laterad on the cervix; they abut the intercervical sclerites at their bases.
Lateral oviduct – ol [paired]: ♀; the pair of outlet ducts for eggs that originate by the internal dichotomy of the common oviduct, usually mesodermal (i.e., removed by maceration via KOH).
Left phallomere – [unpaired]: ♂; the left-dorsal part of the phallic complex, putatively derived from a left-dorsal primary phallic lobe; including the sclerites L1, L2, and L4B, and processes arising from them.
L1 – [one-sided]: ♂; left phallic complex (left phallomere), a sclerite (or its 2 subdivisions) extending over the walls of pouch pne (sclerite area L1a) and the anterior part of the edge pba bearing processes afa, apa, and loa (sclerite area L1b); in case of a subdivision, L1A is the sclerite in pouch pne and L1B is the sclerite on edge pba and its processes.
L2 – [one-sided]: ♂; left phallic complex (left phallomere), a sclerite extending over the walls of pouch lve (mainly dorsal wall; sclerite area L2l) and process paa (sclerite area L2p).
L4 – [one-sided]: ♂; left phallic complex, a sclerite (or its 2 subdivisions) extending over most of the dorsal and ventral walls; in case of subdivision, L4A is the sclerite in the ventral wall, L4B is the sclerite in the dorsal wall.
Laterocoxa 9 – LC9 [= gonangulum] [paired]: ♀; a heavy lateral sclerite in the anteriormost ventral wall of segment 9, forming several distinct articulations with neighboring sclerites (A1, A2, A3, A4); representing a small anterolateral part of the limb-base sclerotization of segment 9.
Left (phallic) complex – [paired, counterpart: right phallomere]: ♂; left phallomere and ventral phallomere together; considered to represent the left part of the phallomere complex.
Ligaria-type – [paired]: ♀; in Ligaria, Ligariella Giglio-Tos, 1915, Parentella Giglio-Tos, 1915, and Entella Stål, 1877 two dorsally-pointing, bipartite sclerotized hooks originate from gonapophyses VIII (gp8) and protrude from the genital chamber (
loa – [one-sided]: ♂; left phallic complex (left phallomere), 3rd process from anterior upon the edge pba between pouches pne and lve (membranous or with L1b or L1B sclerotization) (reference: ‘lobo membranoso’).
Lower frons – [unpaired]: a sclerite located on the head, just below the antennae and in between the compound eyes; can exhibit cuticular depressions or expansions (crests, horns) in some species.
lve – [one-sided]: ♂; left phallic complex (left and ventral phallomere), the ventral pouch invaginated from the right side of the left phallic complex (with L2 sclerotization; dorsal wall belonging to left phallomere, ventral wall belonging to ventral phallomere) (reference: ‘lamina ventrale’).
Mandible – [paired]: strong, gnathal appendages; not bilaterally symmetric.
Maxilla – [paired]: jaw-like appendages; bears the lacinia, the galea, and the maxillary palpi; the cardo, the basal-most segment of the maxilla articulates with the cranium (plural: maxillae).
Maxillary glossa – [paired]: the medial lobe of the maxilla; the stipes bears the muscle attachment site for the maxillary paraglossae (
Maxillary palpus – mxp [paired]: 5-segmented appendages located on the stipes; anterior to the labial palpi (plural: maxillary palpi).
Maxillary paraglossa – [paired]: the lateral lobe of the maxilla; the stipes bears the muscle attachment site for the maxillary paraglossae (
Media – M [paired]: the third major longitudinal wing vein; extant Mantodea feature a composite posterior radius (RP) and media (M) stem in the forewing (
Medial ocellar process – mop [unpaired]: a medially positioned cuticular projection that originates within the limits of the postfrontal sulci, posterior to the ocelli.
Medial tine of gonapophysis 9 – gptm9 [paired]: ♀; a posteriorly directed finger-like process formed by the distal end of the rhachis.
Mediotergite 11 – TGm11 [= ‘epiproct’] [unpaired]: a small, weak median sclerite placed posterior to tergite 10, but more or less overfolded by dorsal fold 10; most likely representing a median fragment of tergite 11.
Medial outgrowth of gonapophysis 8 – gpmo8 [paired]: ♀; a ventromesal membranous lobe near midlength of gonapophysis 8.
Mentum – [unpaired]: the distal plate of the labium, divided into anterior and posterior portions.
Mesal bulge of gonapophysis 8 – gpmb8 [paired]: ♀; a rounded bulge at the mesal base of each gonapophysis 8.
Mesal coxal lobelet – cxml [paired]: ♀; a posteriorly and mesally directed projection from the posteromesal part of a coxa 8.
MESO hearing organ – MESO [paired]: hearing organ in the mesothorax; sensitive to low frequencies but not ultrasound (
Metazone – mz [unpaired]: the posterior division of the pronotum, separated from the prozone by the supracoxal sulcus; generally longer than the prozone, of similar length only in a few Mantodea species (
Middorsal carina – [unpaired]: a longitudinal external ridge (= carina) or keel at the dorsal midline of a segment (on the tergite).
Midventral carina – [unpaired]: a longitudinal external ridge (= carina) or keel at the ventral midline of a segment (on the coxosternite).
Molar ridge – [paired]: a sharp ridge on the mesal surface of the mandible (Levereault 1936).
MSMT “hearing organ” – MSMT [unpaired]: functionless “hearing organ” with both the meso- and metathoracic segments morphologically similar; no auditory chamber present (
Occipital foramen – [unpaired]: an opening on the posterior surface of the head capsule, which allows for the ventral nerve cord, dorsal vessel, and tracheal system to extend from the head into the thorax (
Occipital sulcus – [unpaired]: a transverse sulcus on the posterior surface of the head capsule that terminates on the posterior articulation of the mandibles (
Occiput – [unpaired]: the region between the vertex and the posterior opening (occipital foramen) on the posterior surface of the head capsule (
Ocellar process – [paired]: bilateral cuticular projections that originate within the limits of the postfrontal sulci, posterolateral to the ocelli.
Ocellar tubercle – [unpaired]: an elevated tubercle or knob upon which the ocelli sit; frequently observed in males.
Ocellus – [unpaired]: three simple eyes located in between the compound eyes, posterior to the antennae; maybe be elevated by an ocellar tubercle (plural: ocelli).
Non-visual elongations – nve [paired]: a spine-like elongation present on the compound eyes of some species; presumably non-visual due to a lack of ommatidia on the surface of the structure (
Olistheter – al+rh [paired]: ♀; the sliding tongue-and-groove interlock formed by the dorsal aulax groove on gonapophysis 8 and the ventral rhachis carina on gonapophysis 9.
Ootheca – ♀; egg mass that is encapsulated by a hardened protein matrix that insulates, protects, and camouflages the eggs inside (plural: oothecae).
paa – [one-sided]: ♂; left phallic complex (left phallomere), 4th process from anterior upon the edge pba between pouches pne and lve (with L2p sclerotization) (reference: ‘processo apicale’).
Papilla lobe – ppl7 [unpaired]: ♀; a membranous lobe on the far posterior part of the ventral wall of segment 7 (in genital fold area); supports the opening of the common oviduct, located at its dorsal base, from below; the apex can be bilobate.
Paraglossa – pg [paired, with (partial) median division]: the lateral, terminal lobes on the labium (plural: paraglossae).
Paraproct – PP [paired]: a pair of large ventral sclerites located posterior to the male or female genitalia; their posterior parts are located on the subanal lobes; interpretation unresolved.
Paratergal area – TGπ [paired]: the ventrally bent lateral parts of a tergite.
Parietal sulcus – [paired]: sulcus that runs from the occipital areas toward the frontal sulcus (plural: parietal sulci).
pba – [one-sided]: ♂; left phallic complex (left phallomere), the four processes afa, apa, loa, and paa together plus the edge (between pouches pne and lve) from which they arise (with L1 and L2 sclerotizations) (reference: ‘process-bearing’).
pda – [one-sided]: ♂; left phallic complex (ventral phallomere), a process upon the posterior edge of lobe vla, can be deeply bifurcate (with L4 or L4A sclerotization) (reference: ‘processo distale’).
Pedicel – [paired]: second segment of the antennae.
Phallomere complex – [= phallic organs = male genitalia] [unpaired]: ♂; left phallic complex (including left phallomere and ventral phallomere) and right phallomere together; all elements derived from the nymphal primary phallic lobes around the anlage of the ejaculatory duct (see
pia – [one-sided]: ♂; right phallomere, a process (of varied shape) arising from the midlength to posterior right ventral wall, posterolateral to process pva (with R1 sclerotization, area R1v) (reference: ‘piastra ventrale’).
Planta – small sclerite ventrodistally located on the median flexor plate of the pretarsus (
Plica prima anterior – ppa [paired]: fold in the wing posterior to the cubital fold but anterior to the plica prima posterior; posteriorly delimits the preplicatum; anteriorly delimits the plicatum (reference: ‘anterior primary fold’ in Latin).
Plica prima posterior – ppp [paired]: fold in the wing posterior to the plica prima anterior; posteriorly delimits the plicatum; anteriorly delimits the plicatulum (reference: ‘posterior primary fold’ in Latin).
Plicatulum – [paired]: area posterior to the cubital fold, delimited anteriorly by the plica prima posterior and posteriorly attached to the thorax; positioned posterior to the plicatum and the plicatulum.
Plicatum – [paired]: area posterior to the cubital fold, delimited anteriorly by the plica prima anterior and posteriorly by the plica prima posterior, which acts as a concave hinge; positioned posterior to the plicatum and anterior to the plicatulum.
Prementum – prmt [unpaired]: the distal portion of the labium, delimited posteriorly by the mental sulcus, the anterior portion of the prementum bears the glossae, paraglossae, and palpi of the labium.
Preplicatum – [paired]: area posterior to the cubital fold, delimited anteriorly by the cubital fold and posteriorly by another fold, the plica prima anterior; positioned anterior to the plicatum and the plicatulum.
pne – [one-sided]: ♂; left phallic complex (left phallomere), the dorsal pouch invaginated from the right side of the left phallic complex (with L1a or L1A sclerotization; with opening of phallomere gland, see Suppl. material
Post frontal sulcus – pfs [paired]: a component of the epicranial sulcus; the two frontal sulci branch off from the coronal sulcus at a point above the ocelli and frame the lower frons; the “arms” of the epicranial sulcus complex (plural: post frontal sulci).
Postcervical plate – [unpaired]: a plate on the sternum that exhibits great variability in length between genera; anterior to the forecoxal insertions and the furcasternite (
Posterior Cubitus – CuP [paired]: the fourth major longitudinal wing vein; the posterior branch of the Cubitus.
Posterior Radius – RP [paired]: the second major longitudinal wing vein; the posterior branch of the Radius.
Posterior Subcosta – ScP [paired]: the first major longitudinal wing vein; the posterior branch of the Subcosta.
Posterior spermathecal bulge fold – spbp [unpaired]: ♀; an external transverse fold bordering the spermathecal bulge sbu to the posterior.
Posterior Subcosta – ScP [paired]: the first major longitudinal wing vein; the posterior branch of the Subcosta.
Posteroventral femoral spine(s) – pvfs [paired]: a row of spines on the posteroventral margin on the forefemora.
Posteroventral tibial spine(s) – pvts [paired]: a row of spines on the posteroventral margin of the foretibiae.
Postgena – pge [paired]: the lateral, ventral area located posterior to the gena; flanked by the parietal sulcus, the transverse sulcus, and the postoccipital sulcus (plural: postgenae).
Postocellar process – pop [unpaired]: a cuticular projection arising from the medial region of the cranial vertex.
Prementum – pmtm [paired, with (partial) median division]: the plate on the labium that serves as an insertion point for the muscles of the palpi, glossae, and paraglossae (
Pretarsus – [paired]: the apical, terminal segment of the tarsus; consists of the unguitractor plate, planta, and ungues in nearly all extant insects (otherwise not discernible as a tarsomere) (pleural: pretarsi).
Process of genital fold – gfp7 [paired]: ♀; a process upon the genital fold gf7, being part of gf7; largely sclerotized by the languette sclerite.
Processes on the juxtaocular bulges – jop [paired]: cuticular expansions that can originate from the juxtaocular bulges on some species.
Pronotum – [unpaired]: elongated dorsal plate of the prothorax; with or without lateral expansions, tubercles, denticulations, and conical processes (plural: pronota).
Prozone – pz [unpaired]: the anterior division of the pronotum, separated from the metazone by the supracoxal sulcus; generally shorter than the metazone, of similar length only in a few species (
Pterostigma – sti [paired]: an oft-colored, callused, and ellipsoid region found in forewings. Present in all Mantodea, but not always prominent.
pva – [one-sided]: ♂; right phallomere, a process (of varied shape) arising from the midlength of the ventral wall, anteromesal to process pia (with R1 sclerotization, area R1t) (reference: ‘processo ventrale sclerificato’).
R1 – [one-sided]: ♂; right phallomere, a sclerite (or its 3 subdivisions) extending over the dorsal and right ventral walls of lobe fda, including processes pia and pva in ventral wall; R1 includes the regions R1d (in dorsal wall of lobe fda), R1v (ventrally, on process pia), R1t (ventrally, on process pva), and R1c (centrally between the other areas, bearing articulation R1-R3 labeled A3); in case of a subdivision, R1A is the sclerite in the dorsal wall of lobe fda, R1D is the sclerite on process pva, and R1C is the sclerite on process pia and in the area anterior of it (including the articulation R1-R3 labeled A3); sclerite R1B = R1C+D; sclerite R1F = R1A+C.
R3 – [one-sided]: ♂; right phallomere, a sclerite extending along the anterior ventral wall, also forming apodeme/groove age.
Radius – R [paired]: the stem giving rise to RA and RP; RA and RP are distinct from the base (i.e., there is no visible R) in forewings of Metallyticus, Chaeteessa, and Mantoida spp.
Remigulum – [paired]: area anteriorly delimited by the cubital fold, encompassing the preplicatum, plicatum, and plicatulum.
Rhachis – rh [paired]: ♀; a longitudinal external carina in the proximal half of the ventral wall of gonapophysis 9, with an Ω-like cross section; it fits into the aulax groove of gonapophysis 8 to form a sliding interlock, the olistheter; subapical part of rhachis sclerotized by GPl9 (see Suppl. material
Right phallomere – [paired, counterpart: left phallic complex]: ♂; the right part of the phallomere complex, putatively derived from a right-dorsal primary phallic lobe; including the principal sclerites R1 and R3 as well as various processes.
Rivetina-type – ccsp7 [paired]: ♀; in Rivetina the coxosternite 7 (= subgenital plate) bears two large and strong ventral digging spines (
Scape – [paired]: first segment of the antennae, inserted on the cranium.
Small slender sclerite – sss [paired]: two small sclerites that are underneath the intercervicalia in a cervical fold; articulates with the preepisternite (see
Spermatheca – sp [unpaired]: ♀; a median cuticular invagination of the posterior part of segment 8, opening upon an elevated area (spermathecal bulge) between the coxae 8, consisting of a slender spermathecal duct and a widened internal spermathecal bulb.
Spermathecal bulb – spb [unpaired]: ♀; the bulb-like most internal part of the spermatheca.
Spermathecal bulge – sbu [unpaired]: ♀; a median elevated area between the coxae 8 that bears the spermathecal opening, with the posterior and/or anterior parts overfolding the neighboring areas (folds spbp and spba, respectively).
Spermathecal duct – spd [unpaired]: ♀; the tube-like part of the spermatheca between the external opening and the internal spermathecal bulb.
Spermathecal sclerotization – SP [unpaired]: ♀; a median sclerotization located around the spermathecal opening; as currently known, it occurs either on the anterior (spba) or on the posterior (spbp) spermathecal bulge fold (see Suppl. material
Spiracle – si [paired]: the segmental opening of the tracheal system (in abdominal segments 1–8, located near or behind mid-length upon the paratergal areas).
Spur groove – tsg [paired]: a depression on the posterior edge of the forefemur, which receives the tibial spur (
Stipes – subdivision of the maxillary plate; articulates with the cardo; bears the galea, lacinia, and maxillary palpi (
Stylus – sl9 [paired]: ♂; a basally articulated process (a true stylus) seated upon the hind edge of the ventral fold; representing distal parts of the limbs of segment 9 (plural: styli).
Subantennal sulcus – [paired]: sulcus that runs from the base of the antennal sclerite to the lower frons (plural: subantennal sulci).
Subgena – [unpaired]: a narrow, marginal area below the subgenal sulci; articulation point for gnathal appendages (
Subgenal sulcus – [paired]: a lateral sulcus that corresponds anteriorly with the epistomal sulcus (plural: subgenal sulci).
Supracoxal sulcus – [unpaired]: a dorsal furrow that lies immediately above the forecoxae and divides the pronotum into a pro- and metazone; caused by an internal apodeme that supports the musculature of the forelegs (
T-Shaped sclerite – tss [unpaired]: a sclerite in the shape of a “T” that lies between the forecoxae in species with a relatively short pronotum; comprised of the basisternite and preepisternites (
Tarsus – the fifth segment of the insect leg; articulates with the tibia; made up of five segments (tarsomeres) in Mantodea with Heteronutarsus, an exception, bearing four, (
Telson – [unpaired]: the non-segmental posteroapical part of the body, bearing the anus; in Mantodea not represented by any structure but being a hypothetical membranous area surrounding the anus.
Tergite – TG [unpaired]: the undivided dorsal sclerite plate of an abdominal segment; in contrast to the coxosternite, not a composite sclerite.
Tergite 10 – TG10 [= ‘supraanal plate’] [unpaired]: the undivided dorsal sclerite plate of abdominal segment 10, whose lateral parts bend ventromesally to contact the paraprocts.
Tibia – ti [paired]: the fourth segment of the insect leg; in the foreleg, modified with spines for catching prey between the tibia and the femur; the foretibia terminates in a large, apical spur (plural: tibiae).
Tibial spur – ts [paired]: a frequently curved apical claw that terminates the tibia distally in all mantises except for Chaeteessa in which the spur was possibly secondarily reduced (
Torus intercervicalis – tics [paired]: a small, protruding shelf located on the posterior rim of the intercervical sclerite, which may carry setae (
Transverse anterior part (of T-shaped sclerite) – tap [unpaired]: a transverse sclerotization that forms the anterior part of the T-shaped sclerite; when the transverse anterior part is elongated due to a long prothorax, it forms the postcervical plate (
Transverse carina (of the clypeus) – [unpaired]: dorsal ridge of the clypeus; an outgrowth of the exoskeleton to which muscles attach.
Transverse carina (of the lower frons) – [unpaired]: dorsal protuberances of the lower frons; composed of frontal apodemes; an outgrowth of the exoskeleton to which muscles attach.
Trochanter – tr [paired]: the second and smallest segment of the praying mantis leg, articulating with the coxa at the coxo-trochanteral hinge; fused to the femur; lends insect leg flexibility (
Tubercle – cuticular bulges, bumps, knobs; can be present on the cranium, the pronotum, the forelegs, etc.
Unguis – [paired] claws, generally even in length with the only known exception found on the desert-dwelling Heteronutarsus (
Unguitractor Plate – [paired]: attachment site for the pretarsal depressor muscle; depresses the ungues; located ventroproximally on the pretarsus (
Ventral cervical sclerite – vcs [unpaired, (occasionally) with (partial) median division]: a narrow sclerite that medially traverses the cervical plate, can be divided.
Ventral (segmental) fold 7 – vf7 [unpaired, fused from pair]: ♀; the posteriorly directed and usually strongly posteriorly expanded transverse ventral fold of abdominal segment 7, which is ventrally sclerotized by coxosternite 7 and overlaps the ventral sides of segments 8 and 9 and most of the ovipositor ventrally; representing the projection of the limb bases of segment 7 (medially fused coxal lobes cx7).
Ventral phallomere – [unpaired]: ♂; the left-ventral part of the phallic complex, putatively derived from a mid-ventral or right-ventral primary phallic lobe; including the sclerites L4A and L5, the processes arising from the sclerites, and the opening of the ejaculatory duct.
Ventrolateral coxal lobelet – cxvl [paired]: ♀; a posteriorly and laterally directed projection from the posterolateral part of a coxa 8, located ventromesal to the dorsolateral coxal lobelet.
Ventrolateral segmental expansion – vlse [paired]: a flat expansion upon the lateral part of a coxosternite.
Ventromedian expansion (of ventral fold) – vfme [unpaired to paired]: a flat expansion resulting from a local lengthening of the ventral fold of a segment; the transition between unpaired and paired expansions is fluent, due to the degree of development of a median notch.
Vertex – [unpaired]: a point located equidistant to the compound eyes and above the ocelli; roughly describes the top of the head capsule.
Vertical Process – vp [unpaired]: a cuticular projection arising from the vertex of the cranium; lies posterior to, but not including, the epicranial sulcus.
Vestibulum – vst [unpaired]: ♀; the space enclosed above the ventral fold and beneath the ventral walls of segments 8 (posterior part) and 9.
Vestibular Sclerite – VS7 [unpaired, fused from pair?]: ♀; a sclerite in the dorsal wall of the ventral fold.
Visor – vs [paired]: membranous area between the anterior wing margin and the anterior veinal margin (reference: ‘visor’ in Latin).
4. Conclusion
This work has sought to address a general lack of standardization for mantodean morphological terminology, specimen preparation, and linear morphometric measurements, especially as they pertain to taxonomy and systematics. By developing and implementing a standardized approach for Mantodea research, it is our hope that enthusiasts, students, and researchers alike will find collecting and interpreting taxonomic and morphological information on the charismatic praying mantises more accessible.
5. Acknowledgments
The authors would like to thank Rick Wherley for specimen imaging and image processing. We are grateful to Wendy Wasman for retrieving many of the books and papers necessary for undertaking this project. We thank Rebecca Konte and Joshua Maxwell for providing various morphological illustrations; Hiromi Yagui for providing illustrations and photographs of oothecae; David Yager for use of the hearing organ illustrations; Wendy Moore for posing in the light trapping photograph; Vicki Mercier for providing the insect sweeping photgraph; and Matt Crow for photographing the Mantodea pinning demonstration. The authors would like to thank Henrique M. Rodrigues, Nicole L. Gunter, and Logan J. Mullen for constructive feedback and support. We extend thanks to Roger Roy and Sven Bradler for providing insightful comments and suggestions. This project was supported by the National Science Foundation under the grant DEB-1216309 to Gavin J. Svenson. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or the Cleveland Museum of Natural History. No conflicts of interest exist.
6. References
- Agudelo AA, Rafael JA (2014) Genus Mantillica Westwood, 1889: rediscovery and review of the Amazonian “ant-mantis” (Mantodea: Thespidae: Oligonicinae). Entomological Science 17: 400–408. https://doi.org/10.1111/ens.12072
- Agudelo AA, Rivera J (2015) Some taxonomic and nomenclatural changes in American Mantodea (Insecta, Dictyoptera – Part I. Zootaxa 3936(3): 335–356.
- Anisyutkin LN, Gorochov AV (2004) Haania doroshenkoi, a new species of mantises from Cambodia (Mantina: Mantidae: Thespinae) and a case of mirror symmetry in the structure of the male genitalia of mantises. Russian Entomological Journal 13: 119–122.
- Balderson J (1978) Reversal of the phallic complex in the genera Ciulfina Giglio-Tos and Stenomantis Saussure (Mantodea: Mantidae: Iridopteryginae). Journal of the Australian Entomological Society 17: 235–239. https://doi.org/10.1111/j.1440-6055.1978.tb00150.x
- Beier M (1964) Blattopteroidea. Mantodea. In: Dr. H.G. Bronns Klassen und Ordnungen des Tierreichs, Fünfter Band: Arthropoda III, Abteilung: Insecta, 6. Buch, 5. Lieferung. Geest & Portig K.-G., Leipzig, 849–970.
- Beier M (1965) Die Mantodeen Neu-Guineas. Pacific Insects 7(3): 473–502.
- Beier M (1966) Noona Dan Papers No. 29. Die Mantiden der Noona Dan Expedition nach den Philippinen und Bismarck Inseln. Entomologiske Meddelelser 34: 361–370.
- Beier M (1968) Mantodea (Fangheuschrecken). In: Helmcke J-G, Starck D, Wermuth H (Eds) Handbuch der Zoologie, Walter de Gruyter, Berlin, 1–47.
- Beier M (1970) Dictyoptera (Blattoidae et Mantoidae). In: Tuxen SL (Ed.) Taxonomist’s glossary of genitalia in insects (2nd edn).Copenhagen, 30–41.
- Beier M (1972) 9. Ordnung Saltatoria (Grillen und Heuschrecken). In: Helmcke J-G, Starck D, Wermuth H (Eds) Handbuch der Zoologie, WalterDe Gruyter, Berlin, 4(2), 1–217.
- Berlese A (1882) Ricerche sugli organi genitali degli ortotteri: mantidae, locustidae, gryllidae, gryllotalpidae, truxalidae, acrydiidae.
- Béthoux O (2003) Protophasma dumasii Brongniart 1879, a link between Orthoptera and the ‘dictyopterid’orders?. Journal of Orthoptera Research 12(1): 57–62. https://doi.org/10.1665/1082-6467(2003)012[0057:PDBALB]2.0.CO;2
- Béthoux O (2005) Wing venation pattern of Plecoptera (Insecta: Neoptera). Illiesia 1(9): 52–81.
- Béthoux O (2007) Cladotypic taxonomy applied: titanopterans are orthopterans. Arthropod Systematics & Phylogenetics 65: 135–156.
- Béthoux O (2008) Groundplan, nomenclature, homology, phylogeny, and the question of the insect wing venation pattern. Alavesia 2: 219–232.
- Béthoux O (2012) King crickets, raspy crickets and weta, their wings, their fossil relatives. Journal of Orthoptera Research 21(2): 179–225. https://doi.org/10.1665/034.021.0206
- Béthoux O (2015) The Late Carboniferous Triplosoba pulchella is not a fly in the ointment but a stem‐mayfly. Systematic Entomology 40(2): 342–356. https://doi.org/10.1111/syen.12103
- Béthoux O, Briggs DE (2008) How Gerarus lost its head: stem‐group Orthoptera and Paraneoptera revisited. Systematic Entomology 33(3): 529–547. https://doi.org/10.1111/j.1365-3113.2008.00419.x
- Béthoux O, Nel A (2002) Venation pattern and revision of Orthopterasensu nov. and sister groups. Phylogeny of Palaeozoic and Mesozoic Orthopterasensu nov. Zootaxa 96(1): 1–88.
- Béthoux O, Nel A (2003) Révision de Protagrion audouini Brongniart, 1893 (Insecta: Palaeoptera; Carbonifère supérieur). Bulletin de la Société entomologique de France 108: 237–244.
- Béthoux O, Nel A, Schneider JW, Gand G (2007) Lodetiella magnifica nov. gen. and nov. sp. (Insecta: Palaeodictyoptera; Permian), an extreme situation in wing morphology of palaeopterous insects. Geobios 40(2): 181–189.
- Béthoux O, Wieland F (2009) Evidence for Carboniferous origin of the order Mantodea (Insecta: Dictyoptera) gained from forewing morphology. Zoological Journal of the Linnean Society 156: 79–113. https://doi.org/10.1111/j.1096-3642.2008.00485.x
- Brannoch SK, Svenson GJ (2016a) A new genus and species (Cornucollis gen. n. masoalensis sp. n.) of praying mantis from northern Madagascar (Mantodea, Iridopterygidae, Tropidomantinae). ZooKeys 556: 65–81. https://doi.org/10.3897/zookeys.556.6906
- Brannoch SK, Svenson GJ (2016b) Leveraging female genitalic characters for generic and species delimitation in Nilomantis Werner, 1907 and Ilomantis Giglio-Tos, 1915 (Mantodea, Nilomantinae). Insect Systematics & Evolution 47(3): 1–36. https://doi.org/10.1163/1876312X-47032141
- Breland O, Dobson J (1947) Specificity of mantid oothecae (Orthoptera: Mantidae). Annals of the Entomological Society of America 40(4): 557–575. https://doi.org/10.1093/aesa/40.4.557
- Brongniart C (1893) Recherches pour servir à l’histoire des insectes fossiles des temps primaires précédées d’une étude sur la nervation des ailes des insectes. Bulletin de la Société d’Industrie Minérale de Saint-Etienne 3(7): 124–615. https://doi.org/10.5962/bhl.title.34754
- Bugnion E (1923) Mantes et empuses. Essais d’élevage. Appareil génital de la femelle. Confection de l’oothèque. Eclosion des jeunes larves. Mantis religiosa, Empusa pennata, Gongylus gongylodes. Mémoires de la Société Vaudoise des Sciences Naturelles 1(5): 177–243.
- Cerdá FJ (1993) Valor taxonómico del complejo fálico en mántidos neotropicales (Dictyoptera: Mantodea). Boletín de Entomología Venezolana 8(1): 33–52.
- Chopard L (1920) Recherches sur la Conformation et la Développement des derniers segments abdominaux chez les Orthoptères. Thèse de la Faculté des Sciences de Paris, Rennes, Oberthür, 352 pp.
- Chopard L (1941) Contribution à l’étude des Orthoptéroïdes du nord de l’Afrique. Annales de la Société entomologique de France 110: 25–50.
- Chopard L (1949) Ordre des Dictyoptères, sous-ordre des Mantodea. In: Grassé P (Ed.) Traité de Zoologie.Anatomie, Systématique, Biologie. Masson, Paris, 386–407.
- Comstock JH (1893) Evolution and Taxonomy: an essay on the application of the theory of natural selection in the classification of animals and plants, illustrated by a study of the evolution of the wings of insects and by a contribution to the classification of the Lepidoptera. In: The Wilder quarter-century book. Comstock Publishing co., Ithaca, 37–113.
- Comstock JH (1918) The Wings of Insects. Comstock Publishing Company, New York, 430 pp.
- Courrent A, Quennedy A, Nalepa CA, Robert A, Lenz M, Bordereau C (2008) The fine structure of colleterial glands in two cockroaches and three termites, including a detailed study of Cryptocercus punctulatus (Blattaria, Cryptocercidae) and Mastotermes darwiniensis (Isoptera, Mastotermitidae). Arthropod Structure & Development 37: 55–66. https://doi.org/10.1016/j.asd.2007.03.004
- Crampton GC (1926) A comparison of the neck and prothoracic sclerites throught [sic] the orders of insects from the standpoint of phylogeny. Transactions of the American Entomological Society 52(3): 199–248.
- Crampton GC (1929) The terminal abdominal structures of female insects compared throughout the orders from the standpoint of phylogeny. Journal of the New York Entomological Society 37: 453–511.
- Cui Y, Béthoux O, Klass KD, Ren D (2015) The Jurassic Bajanzhargalanidae (Insecta: Grylloblattida?): new genera and species, and data on postabdominal morphology. Arthropod Structure & Development 44(6): 688–716. https://doi.org/10.1016/j.asd.2015.04.008
- Edmunds M (1972) Defensive behaviour in Ghanaian praying mantids. Zoological Journal of the Linnean Society 51(1): 1–32. https://doi.org/10.1111/j.1096-3642.1972.tb00771.x
- Ehrmann R (2002) Mantodea: Gottesanberinnen der Welt. Münster, Natur und Tier Verlag. Münster, 519 pp.
- Ford N (1923) A comparative study of the abdominal musculature of orthopteroid insects. Transactions of the Royal Canadian Institute 14: 207–319.
- Fuseini BA, Kumar R (1973) The accessory glands of some females mantids. Entomological Monthly Magazine 17(6033): 403–424.
- Giglio-Tos E (1927) Das Tierreich. Orthoptera-Mantidae. Walter de Gruyter, Berlin, 707 pp.
- Gordh G, Headrick DH (2001) A dictionary of entomology. CAB International, Wallingford, 1032 pp.
- Grimaldi D (2003) A revision of the Cretaceous mantises and their relationships, including new taxa (Insecta: Dictyoptera: Mantodea). American Museum Novitates 3412: 1–47. https://doi.org/10.1206/0003-0082(2003)412<0001:AROCMA>2.0.CO;2
- Gullan PJ, Cranston PS (2010) Insects: An Outline of Entomology (4th edn). Wiley-Blackwell, 584 pp.
- Guo Y, Béthoux O, Gu J, Ren D (2013) Wing venation homologies in Pennsylvanian ‘cockroachoids’ (Insecta) clarified thanks to a remarkable specimen from the Pennsylvanian of Ningxia (China). Journal of Systematic Palaeontology 11: 41–46. https://doi.org/10.1080/14772019.2011.637519
- Haas F, Kukalová-Peck J (2001) Dermaptera hindwing structure and folding: New evidence for familial, ordinal and superordinal relationships within Neoptera (Insecta). European Journal of Entomology 98: 445–509. https://doi.org/10.14411/eje.2001.065
- Hackman RH, Goldberg M (1960) Composition of the oothecae of three Orthoptera. Journal of Insect Physiology 5: 73–78. https://doi.org/10.1016/0022-1910(60)90024-X
- Hamilton KGA (1972) The insect wing, part III. Venation of the orders. Journal of Kansas Entomological Society 45: 145–162.
- Hebard M (1920) Expedition of the California Academy of Sciences to the Galapagos Islands, 1905–1906. Dermaptera and Orthoptera. Proceedings of the California Academy of Natural Sciences 2(17/2): 311–318.
- Heitzmann TJ (1959) Genitalia de Parastagmatoptera unipunctata (Burm., 1838) (Mantodea). Papeis Avulsos de Zoologia 13: 329–337.
- Heitzmann-Fontenelle TJ (1964) Estudo Morfólogico de Acanthops erosula Stål, 1877 (Mantodea, Acanthopidae). Papeis Depart. Zool. Sec. Agr.16(23): 229–241.
- Heitzmann-Fontenelle TJ (1965) Acontiothespis concinna (Perty, 1832) (Mantodea, Acontiothespinae): Descrição Morfológica. Papeis Depart. Zool. Sec. Agr.17(22): 277–289.
- Helm C, Treulieb S, Werler K, Bradler S, Klass K-D (2011) The male genitalia of Oxyartes lamellatus – phasmatodeans do have complex phallic organs (Insecta: Phasmatodea). Zoologischer Anzeiger 250: 223–245. https://doi.org/10.1016/j.jcz.2011.04.005
- Hennig W (1981) Insect Phylogeny. Wiley & Sons, New York, 514 pp.
- Hill PJ, Holwell GI, Göth A, Herberstein ME (2004) Preference for habitats with low structural complexity in the praying mantid Ciulfina sp. (Mantidae). Acta Oecologica 26(1): 1–7. https://doi.org/10.1016/j.actao.2004.02.002
- Holwell GI, Ginn S, Herberstein ME (2007) Three new species of Ciulfina (Mantodea: Liturgusidae) from north-eastern Australia. Zootaxa 1583: 23–35.
- Holwell GI, Kazakova O, Evans F, O’Hanlon JC, Barry KL (2015) The functional significance of chiral genitalia: patterns of asymmetry, functional morphology and mating success in the praying mantis Ciulfina baldersoni. PLoS ONE 10: e0128755. https://doi.org/10.1371/journal.pone.0128755
- Hurd LE, Eisenberg RM, Fagan WF, Tilmon KJ, Snyder WE, Vandersall KS, Datz SG, Welch JD (1994) Cannibalism Reverses Male-Biased Sex Ratio in Adult Mantids: Female Strategy against Food Limitation? Oikos 69(2): 193–198. https://doi.org/10.2307/3546137
- Ippolito S, Lombardo F (2012) New data on the genus Chromatophotina Rivera and description of the male of C. cofan Rivera (Mantodea: Mantidae, Photinainae). Boletín de la Sociedad Entomólogica Aragonesa 50: 313–316.
- Kaltenbach AP (1996) Unterlagen für eine Monographie der Mantodea des südlichen Afrika: 1. Artenbestand, geographische Verbreitung und Ausbreitungsgrenzen (Insecta: Mantodea). Annalen des Naturhistorischen Museums in Wien. Serie B für Botanik und Zoologie 98: 193–346.
- Kenchington W, Flower NE (1969a) Studies on insect fibrous proteins: the structural protein of the ootheca in the praying mantis, Sphodromantis centralis Rehn. Journal of Microscopy 89: 263–281. https://doi.org/10.1111/j.1365-2818.1969.tb00673.x
- Kenchington W, Flower NE (1969b) The hatching thread of praying mantids: an unusual chintinous structure. Journal of Morphology 129(3): 307–315. https://doi.org/10.1002/jmor.1051290304
- Klass K-D (1995) Die Phylogenie der Dictyoptera. PhD Thesis, Munich University, Cuvillier, Göttingen, 256 pp.
- Klass K-D (1997) The external male genitalia and the phylogeny of Blattaria and Mantodea. Bonner Zoologische Monographien 42: 1–341.
- Klass K-D (1998) The ovipositor of Dictyoptera (Insecta): homology and ground-plan of the main elements. Zoologischer Anzeiger 236(2–3): 69–101.
- Klass K-D (2001) The female abdomen of the viviparous earwig Hemimerus vosseleri (Insecta: Dermaptera: Hemimeridae), with a discussion of the postgenital abdomen of Insecta. Zoological Journal of the Linnean Society 131: 251–307. https://doi.org/10.1111/j.1096-3642.2001.tb02239.x
- Klass K-D (2003) The female genitalic region in basal earwigs (Insecta: Dermaptera: Pygidicranidae s.l.). Entomologische Abhandlungen 61: 173–225.
- Klass K-D, Meier R (2006) A phylogenetic analysis of Dictyoptera (Insecta) based on morphological characters. Entomologische Abhandlungen 63: 3–50.
- Klass K-D (2008) The female abdomen of ovipositor-bearing Odonata (Insecta: Pterygota). Arthropod Systematics & Phylogeny 66: 45–142.
- Klass K-D, Matushkina NA (2012) The exoskeleton of the female genitalic region in Petrobiellus tokunagae (Insecta: Archaeognatha): insect-wide terminology, homologies, and functional interpretations. Arthropod Structure & Development 41: 575–591. https://doi.org/10.1016/j.asd.2012.06.003
- Klass K-D, Matushkina NA (submitted) The exoskeleton of the male genitalic region in Archaeognatha (Insecta), with considerations on the early evolution of genitalia in insects.
- Klass K-D, Matushkina NA, Kaidel J (2012) The gonangulum: a reassessment of its morphology, homology, and phylogenetic significance. Arthropod Structure & Development 41: 373–394. https://doi.org/10.1016/j.asd.2012.03.001
- Kramer KJ (1973) Oothecal proteins of the oriental praying mantid Tenodera sinensis. Insect Biochemistry 3(11): 297–302. https://doi.org/10.1016/0020-1790(73)90060-7
- Kramer KJ, Bork V, Schaefer J, Morgan TD, Hopkins TL (1989) Solid state C nuclear magnetic resonance and chemical analyses of insect noncuticular sclerotized support structures: mantid oothecae and Cocoon silks. Insect Biochemistry 19(1): 69–77. https://doi.org/10.1016/0020-1790(89)90010-3
- Kukalová-Peck J (1991) Fossil history and the evolution of hexapod structures. In: Naumann ID (Ed.) The insects of Australia, a textbook for students and researchers (2nd edn).Melbourne University Press, 141–179.
- Kukalová-Peck J, Lawrence JF (2004) Relationships among coleopteran suborders and major endoneopteran lineages: evidence from hind wing characters. European Journal of Entomology 101: 95–144. https://doi.org/10.14411/eje.2004.018
- Kumar R, Barnor JL (1974) On some substances produced by the colleterial glands of certain orthopteroid insects. Annals of the Entomological Society of America 67(5): 753–755. https://doi.org/10.1093/aesa/67.5.753
- LaGreca M (1939) Mantidi della Guiana inglese raccolti dalla spedizione Beccari. Bolletino degli Instituti di Zoologia e Anatomia Comparata 29: 1–7.
- LaGreca M (1950) Missione biologica Sagan-Omo. Mantodea. Rivista di Biologia Coloniale 10: 83–102.
- LaGreca M (1954) Sulla struttura morfologica dell’apparato copulatore dei Mantoidei. – Annali dell’Istituto Superiore di Scienze e Lettere “S. Chiara” di Napoli 4: 1–28.
- LaGreca M, Lombardo F (1987) Considerazioni sul genere Chrysomantis G.-T. (Insecta, Mantodea), con descrizione di quattro specie nuove. Animalia 14(1–3): 107–124.
- LaGreca M, Lombardo F (1993) Sul gen. Theopompella Giglio-Tos (Insecta, Mantodea) e descrizione di una nuova specie. Animalia 20(1–3): 47–54.
- LaGreca M, Rainone A (1949) Il dermascheletro e la muscolatura del’addome di Mantis religiosa. Annuario dell’Istituto e Museo di Zoologia della Università di Napoli 1(5): 1–43.
- LaGreca M, Raucci A (1949) Il dermascheletro e la muscolatura del torace di Mantis religiosa. Annuario dell’Istituto e Museo di Zoologia della Università di Napoli 1(3): 1–41.
- Lameere A (1922) Sur la nervation alaire des Insectes. Bulletin de la classe des Sciences de l’Académie Royale de Belgique, Bruxelles 8: 138–149.
- Lameere A (1923) On the wing-venation of insects. Psyche 30: 123–132. https://doi.org/10.1155/1923/16920
- Lawrence SE (1992) Sexual cannibalism in the praying mantid, Mantis religiosa: a field study. Animal Behaviour 43(4): 569–583. https://doi.org/10.1016/S0003-3472(05)81017-6
- Leverault P (1936) The Morphology of the Carolina Mantis, vol. 24. The University of Kansas Science Bulletin 205– 259.
- Levereault P (1938) The morphology of the Carolina mantis (Stagmomantis carolina) II: Musculature University of Kansas Science Bulletin 25: 577–633.
- Lombardo F, Ippolito S, Rivera J (2013) Synopsis of the Neotropical mantid genus Pseudacanthops Saussure, 1870, with the description of three new species (Mantodea: Acanthopidae). Revue Suisse de Zoologie 120: 373–403.
- Marks EP, Lawson FA (1962) A comparative study of the dictyopteran ovipositor. Journal of Morphology 111: 139–172. https://doi.org/10.1002/jmor.1051110203
- Martin RE, Pine RH, DeBlase AF (2001) A Manual of Mammalogy with Keys to Families of the World, 3rd Edition. McGraw-Hill Publishing Co, 333 pp.
- Martynov AV (1925) Über zwei grundtypen der flügel bei den insecten [sic] und ihre evolution. Zeitschrift für Ökologie und Morphologie der Tiere 4: 465–501. https://doi.org/10.1007/BF00408465
- Matsuda R (1976) Morphology and evolution of the insect abdomen, with special reference to developmental patterns and their bearings upon systematics. Pergamon Press Ltd, 534 pp.
- Maxwell MR (1999) Mating behavior. In: Prete FR, Wells H, Wells PH, Hurd LE (Eds) The Praying Mantids.Johns Hopkins University Press, Baltimore, 69–89.
- Nel RI (1929) Studies on the development of genitalia and genital ducts in insects. Quarterly Journal of Microscopical Science 73: 25–85.
- Nel A, Roy R (1996) Revision of the fossil “mantid” and “ephemerid” species described by Piton from the Palaeocene of Menat (France) (Mantodea: Chaeteessidae, Mantidae; Ensifera: Tettigonioidea). European Journal of Entomology 93: 223–234.
- O’Hanlon J (2011) Intraspecific interactions and their effect on habitat utilisation by the praying mantid Ciulfina biseriata (Mantodea: Liturgusidae). Journal of Ethology 29(1): 47–54. https://doi.org/10.1007/s10164-010-0220-6
- Pipa RL (1988) Muscles and nerves of the posterior abdomen and genitalia of male Periplaneta americana (L.) (Dictyoptera: Blattidae). International Journal of Insect Morphology and Embryology 17: 455–471. https://doi.org/10.1016/0020-7322(88)90025-6
- Prenner G (2010) Floral formula updated for routine inclusion in formal taxonomic descriptions. Taxon 59: 241–250.
- Qadri MAH (1940) On the development of the genitalia and their ducts of orthopteroid insects. Transactions of the Royal Entomological Society of London 90: 121–175. https://doi.org/10.1111/j.1365-2311.1940.tb02251.x
- Ragge DR (1955) The wing-venation of the OrthopteraSaltatoria, with notes on Dictyopteran wing-venation. British Museum (Natural History), 160 pp.
- Ramsay GW (1990) Mantodea (Insecta) with a review of aspects of functional morphology and biology. Fauna of New Zealand 19: 1–96.
- Rasnitsyn AP, Novokshonov VG (1997) On the morphology of Uralia maculata (Insecta: Diaphanopterida) from the Early Permian (Kungurian) of Ural (Russia). Scandinavian Entomology 28: 27–38. https://doi.org/10.1163/187631297X00141
- Redtenbacher J (1886) Vergleichende Studien über das Flügelgeäder der Insekten. Annalen des Kaiserlich-Königlichen Naturhistorischen Hofmuseums 1: 153–232.
- Rehn JAG (1935) The Orthoptera of Costa Rica. Part I. –Mantidae. –Proceedings of the Academy of Natural Sciences of Philadelphia, 87: 167–271.
- Rivera J (2010) Chromatophotina, a remarkable new genus of praying mantid from the Neotropical Region and its two new species (Mantodea: Mantidae, Photinainae). Zootaxa 2415: 22–32.
- Rivera J, Yagui H, Ehrmann R (2011) Mantids in the Mist–Taxonomy of the Andean genus Pseudopogonogaster Beier, 1942, a cloud forest specialist, with notes on its biogeography and ecology (Mantodea: Thespidae: Miopteryginae). Insect Systematics & Evolution 42(4): 313–335. https://doi.org/10.1163/187631211X595056
- Rivera J, Svenson GJ (2016) The Neotropical ‘polymorphic earless praying mantises’–Part I: molecular phylogeny and revised higher‐level systematics (Insecta: Mantodea, Acanthopoidea). Systematic Entomology 41(3): 607–649. https://doi.org/10.1111/syen.12178
- Rodrigues HM, Cancello EM (2016) Taxonomic revision of Stagmatoptera Burmeister, 1838 (Mantodea: Mantidae, Stagmatopterinae). Zootaxa 4183(1): 1. https://doi.org/10.11646/zootaxa.4183.1.1
- Roy R (1999) Morphology and taxonomy. In: Prete FR, Wells H, Wells PH, Hurd LE (Eds) The Praying Mantids.Johns Hopkins University Press, Baltimore, 19–40.
- Roy R (2002a) Révision du genere néotropical Macromantis Saussure, 1871 (Dictyoptera: Mantidae). Bulletin de la Société Entomologique de France 107: 403–418.
- Roy R (2002b) Une remarquable espèce nouvelle d’ Acanthops Audinet-Serville, 1831, en Guyane française (Dictyoptera, Mantodea). Bulletin de la Société entomologique de France 107(3): 297–300.
- Roy R, Stiewe MBD (2009) Contribution to the knowledge of Eastern African Amorphoscelis Stål, 1871, with description of two new species (Dictyoptera, Mantodea, Amorphoscelidae). Bulletin de la Société entomologique de France 114(2): 195–210.
- Rudall KM (1956) Protein ribbons and sheets. In: Lectures on the Scientific Basis of Medicine, Athlone Press, London, 217.
- Scudder GGE (1961) The comparative morphology of the insect ovipositor. Transactions of the Royal Entomological Society of London 113(2): 25–40. https://doi.org/10.1111/j.1365-2311.1961.tb00800.x
- Scudder GGE (1971) Comparative morphology of insect genitalia. Annual Review of Entomology 16(1): 379–406. https://doi.org/10.1146/annurev.en.16.010171.002115
- Séguy E (1959) Introduction à l'étude morphologique de l'aile des insectes. Mémoires du Muséum National d'Histoire Naturelle, Paris, Nouvelle série (A) 21: 1–248.
- Sharov AG (1961) Otryad Plecoptera (Order Plecoptera) in Paleozojskoe nasekomye Kuznetskovo bassejna (Paleozoic insects from Kuznetsk basin). Trudy Paleontologicheskogo instituta, Akademiya Nauk SSSR 85: 225–234.
- Sharov AG (1962a) Redescription of Lithophotina floccosa Cock. (mantodea) with some notes on the manteod wing venation. Psyche 69: 102–106. https://doi.org/10.1155/1962/67682
- Sharov AG (1962b) Order Plecoptera. In: Orlov YuA (Ed.) Akademyia Nauk SSSR, Osnovy Palaeontology.Chlenistonogie, Trakheinye i Khelitserovye, 134–138.
- Sharov AG (1968) Filogeniya orthopteroidnykh nasekomykh. Trudy Paleontologicheskogo instituta, Akademiya Nauk SSSR 118: 1–216.
- Sharov AG (1971) Phylogeny of the Orthopteroidea. Israel Program for Scientific Translations, Jerusalem, 251 pp.
- Sharov AG (1991) Order Plecoptera. In Rohdendorf BB (Ed.) Fundamentals of Paleontology. Arthropoda, Tracheata, Chelicerata. Smithsonian Institution Libraries, Washington D. C., 173–179.
- Smart J (1956) On the wing venation of Chaeteessa and other mantids (Insecta: Mantodea). Proceedings of the Zoological Society of London 127: 545–553. https://doi.org/10.1111/j.1096-3642.1956.tb00487.x
- Snodgrass RE (1935) Principles of Insect Morphology. University Press, Cornell Reprint, London, 667 pp.
- Snodgrass RE (1937) The male genitalia of orthopteroid insects. Smithsonian Institution Collections 96(5): 1–107.
- Snodgrass RE (1957) A revised interpretation of the external reproductive organs of male insects. Smithsonian Miscellaneous Collections 135(6): 1–60.
- Su YN (2016) A simple and quick method of displaying liquid-preserved morphological structures for microphotography. Zootaxa 4208(6): 592–593. https://doi.org/10.11646/zootaxa.4208.6.6
- Svenson GJ (2014) Revision of the Neotropical bark mantis genus Liturgusa Saussure, 1869 (Insecta, Mantodea, Liturgusini). ZooKeys 390: 1–214. https://doi.org/10.3897/zookeys.390.6661
- Svenson GJ, Hardy NB, Wightman HMC, Wieland F (2015) Of flowers and twigs: phylogenetic revision of the plant-mimicking praying mantises (Mantodea: Empusidae and Hymenopodidae) with a new suprageneric classification. Systematic Entomology 40(4): 789–834. https://doi.org/10.1111/syen.12134
- Svenson GJ, Medellin C, Sarmiento CE (2016) Re-evolution of a morphological precursor of crypsis investment in the newly revised horned praying mantises (Insecta, Mantodea, Vatinae). Systematic Entomology: 41(1): 229–255. https://doi.org/10.1111/syen.12151
- Svenson GJ, Whiting MF (2009) Reconstructing the origins of praying mantises (Dictyoptera, Mantodea): the roles of Gondwanan vicariance and morphological convergence. Cladistics 25: 468–514. https://doi.org/10.1111/j.1096-0031.2009.00263.x
- Tedrow R, Nathan K, Richard N, Svenson GJ (2014) A new species of Dystacta Saussure, 1871 from Nyungwe National Park, Rwanda (Insecta, Mantodea, Dystactinae). ZooKeys 410: 1–21. https://doi.org/10.3897/zookeys.410.7053
- Travassos Filho L (1945) Tecnicas gerais seguidas no estudo da ordem Mantodea Burmeister, 1938. Arquivos de Zoologia do Estado de São Paulo 4(5): 113–56.
- Triplehorn CA, Johnson NF (2005) Borror and DeLong’s Introduction to the Study of Insects, 7th Edition. Thompson Brooks/Cole. Belmont, California, 864 pp.
- Walker AA, Weisman S, Kameda T, Sutherland TD (2012) Natural templates for coiled-coil biomaterials from praying mantis egg cases. Biomacromolecules 13: 4264–4272. https://doi.org/10.1021/bm301570v
- Walker EM (1919) The terminal abdominal structures of orthopteroid insects: a phylogenetic study. Introduction and Part I The terminal abdominal structures of the female. Annals of the Entomological Society of America 12: 267–316. https://doi.org/10.1093/aesa/12.4.267
- Walker EM (1922) The terminal abdominal structures of orthopteroid insects: a phylogenetic study. Part II The terminal abdominal structures of the male. Annals of the Entomological Society of America 15: 1–76. https://doi.org/10.1093/aesa/15.1.1
- Wieland F (2006) The cervical sclerites of Mantodea discussed in the context of dictyopteran phylogeny (Insecta: Dictyoptera). Entomologische Abhandlungen 63(1–2): 51–76.
- Wieland F (2008) The genus Metallyticus reviewed (Insecta: Mantodea). Species, Phylogeny and Evolution 1(3): 147–170.
- Wieland F (2013) The phylogenetic system of Mantodea (Insecta: Dictyoptera). Species, Phylogeny and Evolution 3: 3–222. https://doi.org/10.17875/gup2013-711
- Wipfler B, Wieland F, Decarlo F, Hörnschemeyer T (2012) Cephalic morphology of Hymenopus coronatus (Insecta: Mantodea) and its phylogenetic implications. Arthropod Structure & Developments 41: 87–100. https://doi.org/10.1016/j.asd.2011.06.005
- Wipfler B (2014) Dictyoptera (Mantodea + Blattodea incl. Isoptera). In Beutel RG, Friedrich F, Ge S-Q, Yang X-K (Eds) Insect Morphology and Phylogeny: A textbook for students of entomology. De Gruyter, Berlin & Boston, 516 pp.
- Wolda H (1988) Insect seasonality: why?. Annual review of ecology and systematics 1–18. https://doi.org/10.1146/annurev.es.19.110188.000245
- Wootton RJ (1979) Function, homology and terminology in insect wings. Systematic Entomology 4: 81–93. https://doi.org/10.1111/j.1365-3113.1979.tb00614.x
- Yager DD, Svenson GJ (2008) Patterns of praying mantis auditory system evolution based on morphological, molecular, neurophysiological, and behavioural data. Biological Journal of the Linnean Society 94(3): 541–568. https://doi.org/10.1111/j.1095-8312.2008.00996.x
Supplementary materials
Head Capsule Terminology
Data type: terminology
Explanation note: Updated standardized terminology of external head capsule morphology with historical usage of topographically homologous terms. Morphological terminology of the external structures on the praying mantis head capsule are organized alphabetically by the updated standardardized terminology. Historical usage of topographically homologous structures are traced in reverse chronological order in subsequent columns. Plural spellings, abbreviations, and figure references for the standardized terminology are listed to aid in the identification of specific structures. † Levereault (1936: 219) referred to the region of the cranial vertex that contains the juxtaocular bulges as the “temporal sclerites,” but did not provide a name for the juxtaocular bulges themselves.
Forewing terminology
Data type: terminology
Explanation note: Updated standardized terminology of forewing venation and structure with historical usage of topographically homologous terms. Wing venation terminology is organized top-down from anterior to posterior-most structures. It is further organized by the two major wing venation patterns observed in species belonging to MCM-type (i.e., Metallyticus, Chaeteessa, Mantoida, and some fossil species; cells shaded blue) and Mantis-type (e.g., Mantis religiosa; cells shaded orange). Red dashed line indicate the delimitation between the Radius R and the Media M. Red frames around cells indicate particularities of an author’s morphological interpretation. When multiple species are considered by an author, the first occurrence of a term in a general context is indicated for the first species.
Hindwing terminology
Data type: terminology
Explanation note: Updated standardized terminology of hindwing venation and structure with historical usage of topographically homologous terms. Wing venation terminology is organized top-down from anterior to posterior-most structures. It is further organized by the two major wing venation patterns observed species belonging to MCM-type (i.e., Metallyticus, Chaeteessa, Mantoida, and some fossil species) and Mantis-type (e.g., Mantis religiosa). Red frames around cells indicated particularities of an author’s morphological interpretation. When multiple species are considered by an author, the first occurrence of a term in a general context is indicated for the first species.
Leg structure terminology
Data type: terminology
Explanation note: Updated standardized terminology of fore-, meso-, and metathoracic leg morphology with historical usage of topographically homologous terms. Morphological terminology of the external structures on the praying mantis fore-, meso-, and metathoracic leg are organized alphabetically by the updated standardardized terminology. Historical usage of topographically homologous structures are traced in reverse chronological order in subsequent columns. Plural spellings, abbreviations, and figure references for the standardized terminology are listed to aid in the identification of specific structures.
Thoracic structure terminology
Data type: terminology
Explanation note: Updated standardized terminology of external thoracic morphology with historical usage of topographically homologous terms. Morphological terminology of the external structures on the praying mantis thorax are organized alphabetically by the updated standardardized terminology. Historical usage of topographically homologous structures are traced in reverse chronological order in subsequent columns. Plural spellings, abbreviations, and figure references for the standardized terminology are listed to aid in the identification of specific structures. † The small slender sclerites (sss) of the cervix can only be seen in fresh or alcohol-preserved specimens.
Abdominal structure terminology
Data type: terminology
Explanation note: Updated standardized terminology of external abdominal structures with historical usage of topographically homologous terms. Morphological terminology of the external structures on the praying mantis abdomen are organized alphabetically by the updated standardardized terminology. Historical usage of topographically homologous structures are traced in reverse chronological order in subsequent columns. Plural spellings, abbreviations, and figure references for the standardized terminology are listed to aid in the identification of specific structures.
Male genital structure terminology
Data type: terminology
Explanation note: Updated standardized terminology of male genital morphology with historical usage of topographically homologous terms. Morphological terminology of the male praying mantis genital complex are organized alphabetically by the updated standardardized terminology. Historical usage of topographically homologous structures are traced in reverse chronological order in subsequent columns. Plural spellings, abbreviations, and figure references for the standardized terminology are listed to aid in the identification of specific structures.
Female genital structure terminology
Data type: terminology
Explanation note: Updated standardized terminology of female genital morphology with historical usage of topographically homologous terms. Morphological terminology of the female praying mantis genital complex are organized alphabetically by the updated standardardized terminology. Historical usage of topographically homologous structures are traced in reverse chronological order in subsequent columns. Plural spellings, abbreviations, and figure references for the standardized terminology are listed to aid in the identification of specific structures.
Abdominal terminology discussion
Data type: discussion
Explanation note: Continued discussion on the abdominal terminology used within the publication.
Extended abdominal glossary
Data type: glossary
Explanation note: Extended glossary of Mantodea abdominal terminology.