Research Article |
|
Corresponding author: Thomas G. Dahlgren ( thda@mac.com ) Academic editor: Christopher Glasby
© 2019 Helena Wiklund, Lenka Neal, Adrian G. Glover, Regan Drennan, Muriel Rabone, Thomas G. Dahlgren.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Wiklund H, Neal L, Glover AG, Drennan R, Rabone M, Dahlgren TG (2019) Abyssal fauna of polymetallic nodule exploration areas, eastern Clarion-Clipperton Zone, central Pacific Ocean: Annelida: Capitellidae, Opheliidae, Scalibregmatidae and Travisiidae. ZooKeys 883: 1-82. https://doi.org/10.3897/zookeys.883.36193
|
Abstract
We present DNA taxonomy of abyssal polychaete worms from the eastern Clarion-Clipperton Zone (CCZ), central Pacific Ocean, using material collected as part of the Abyssal Baseline (ABYSSLINE) environmental survey cruises ‘AB01’ and ‘AB02’ to the UK Seabed Resources Ltd (UKSRL) polymetallic nodule exploration contract area ‘UK-1’, the Ocean Mineral Singapore exploration contract area ‘OMS-1’ and an Area of Particular Environmental Interest, ‘APEI-6’. This is the fourth paper in a series to provide regional taxonomic data with previous papers reporting on Cnidaria, Echinodermata and Mollusca. Taxonomic data are presented for 23 species from 85 records within four polychaete families: Capitellidae, Opheliidae, Scalibregmatidae and Travisiidae, identified by a combination of morphological and genetic data, including molecular phylogenetic analyses. Two taxa (genetically separated from one another) morphologically matched the same known cosmopolitan species, Ophelina abranchiata that has a type locality in a different ocean basin and depth from where no genetic data was available. These two species were assigned the open nomenclature ‘cf.’ as a precautionary approach in taxon assignments to avoid over-estimating species ranges. Twelve (12) taxa are here described as new species, Ammotrypanella keenani sp. nov., Ammotrypanella kersteni sp. nov., Ophelina curli sp. nov., Ophelina ganae sp. nov., Ophelina juhazi sp. nov., Ophelina martinezarbizui sp. nov., Ophelina meyerae sp. nov., Ophelina nunnallyi sp. nov., Oligobregma brasierae sp. nov., Oligobregma tani sp. nov., Oligobregma whaleyi sp. nov. and Travisia zieglerae sp. nov. For the remaining nine taxa, we have determined them to be potentially new species, for which we make the raw data, imagery and vouchers available for future taxonomic study. The CCZ is a region undergoing intense exploration for potential deep-sea mineral extraction from polymetallic nodules. We present these data to facilitate future taxonomic and environmental impact study by making both data and voucher materials available through curated and accessible biological collections.
Keywords
CCZ, deep-sea mining, molecular phylogeny, new species, Polychaeta, Scolecida, 18S, 16S, COI
Introduction
In the last decades there has been rapid growth in the commercial exploration of the abyssal deep sea for mineral resources (
Annelida is one of the most abundant macrofauna groups on soft bottoms at abyssal depths (e.g.
In terms of recent molecular studies,
The DNA taxonomy part of the UK Seabed Resources (UKSR) program aims to fill some gaps in our knowledge and make data publicly available that will eventually allow for a complete taxonomic synthesis of the CCZ supported by openly available molecular and morphological data. We present results from a DNA taxonomy survey of abyssal benthic annelids collected as part of the UKSR ABYSSLINE cruises ‘AB01’ and ‘AB02’ to the UK Seabed Resources Ltd (UKSRL) polymetallic nodule exploration contract area ‘UK-1’, Ocean Mineral Singapore contract area ‘OMS-1’, and an Area of Particular Environmental Interest, ‘APEI-6’, (Fig.
Map over sampling sites. A UK-1 Stratum-A B UK-1 Stratum-B study areas, both within the UK Seabed Resources UK-1 exploration contract area C OMS Stratum-A study area, in the Ocean Mineral Singapore (OMS) polymetallic nodule exploration contract area D Area of Particular Interest APEI-6. Inset map showing location of Clarion-Clipperton Fracture Zone. Bathymetric survey and sample localities from the AB01 2013 RV Melville survey cruise and AB02 2015 Thomas G. Thompson survey cruise, data courtesy Craig R. Smith (University of Hawaii), UK Seabed Resources Ltd and Seafloor Investigations, LLC.
Materials and methods
Fieldwork
The first UKSR ABYSSLINE cruise (AB01), sampling the UK-1 exploration contract area, took place in October 2013 aboard the RV Melville, and the second cruise (AB02), sampling the UK-1 and OMS-1 exploration contract areas and APEI-6, took place in February-March 2015 onboard RV Thomas G. Thompson.
A comprehensive description of our DNA taxonomy pipeline is provided in
Laboratory work
In the laboratory, specimens were re-examined using stereo and compound microscopes, identified and described to the best possible taxonomic level with key morphological features photographed with digital cameras and a small tissue-sample taken for DNA extraction. Shirlastain A and Methyl Green stain were used during the morphological examination on some specimens, in order to better observe certain characters. Methyl Green stain was limited to Capitellidae, as the staining pattern is considered of value in capitellid taxonomy (e.g.
Extraction of DNA was done with DNeasy Blood and Tissue Kit (Qiagen) using a Hamilton Microlab STAR Robotic Workstation. About 1800 bp of 18S, 450 bp of 16S, and 650 bp of cytochrome c oxidase subunit I (COI) were amplified using primers listed in Table
| Primer | Sequence 5’-3’ | Reference |
|---|---|---|
| 18S | ||
| 18SA | AYCTGGTTGATCCTGCCAGT |
|
| 18SB | ACCTTGTTACGACTTTTACTTCCTC |
|
| 620F | TAAAGYTGYTGCAGTTAAA |
|
| 1324R | CGGCCATGCACCACC |
|
| COI | ||
| LCO1490 | GGTCAACAAATCATAAAGATATTGG |
|
| HCO2198 | TAAACTTCAGGGTGACCAAAAAATCA |
|
| COI-E | TATACTTCTGGGTGTCCGAAGAATCA |
|
| polyLCO | GAYTATWTTCAACAAATCATAAAGATATTGG |
|
| polyHCO | TAMACTTCWGGGTGACCAAARAATCA |
|
| 16S | ||
| ann16SF | GCGGTATCCTGACCGTRCWAAGGTA |
|
| 16SbrH | CCGGTCTGAACTCAGATCACGT |
|
Data handling
The field and laboratory work created a series of databases and sample sets that are integrated into a data-management pipeline. This includes the transfer and management of data and samples between a central collections database, a molecular collections database and external repositories (GenBank, WoRMS, OBIS, GBIF, GGBN, ZooBank) through DarwinCore archives and usage of the GGBN data standard (
Taxonomic assignments
Future studies of biogeographic and bathymetric ranges, gene-flow, extinction risks, natural history, reproductive ecology, functional ecology and geochemical interactions of CCZ species are dependent on accurate taxonomic identifications. This taxonomy is dependent on a sound theoretical underpinning—a species concept—coupled with the availability of both raw data and voucher samples. Here we use a phylogenetic species concept sensu
Taxon treatments presented in this paper. Includes family, DNA taxonomy ID (a species-level identification based on combined DNA and morphological evidence), GUID (Global Unique Identifier link to data record at http://data.nhm.ac.uk), ABYSSLINE record number, NHM accession number, NHM Molecular Collection Facility (MCF) sample ID number (NHMUK_MCF#) and NCBI GenBank accession number (Genbank#) for successfully sequenced genetic markers. GenBank numbers for phylogenetic analysis data downloaded from GenBank are presented in Supplementary material 1.
| Family | DNA taxonomy ID | ABYSSLINE record | GUID | NHMUK Record no. | NHMUK MCF no. | Genbank no. |
|---|---|---|---|---|---|---|
| Capitellidae | Capitellidae sp. (NHM_1486) | NHM_613 | be34eb86-fc0c-411c-8eb9-e86774c6515a | 2019.7105 | 0109494702 | MN217415 |
| MN217495 | ||||||
| NHM_1486 | 05d46c60-8b4d-4623-b7ff-2e8089453d7e | 2019.7106 | 0109494649 | MN217416 | ||
| MN217496 | ||||||
| Notomastus sp. (NHM_162) | NHM_162 | f34e2921-7b6d-4c14-b217-690d9315f073 | 2019.7100 | 0109494624 | MN217417 | |
| MN217497 | ||||||
| NHM_915B | 98291a2a-c89a-4f62-bde5-91171368c749 | 2019.7101 | 0109494712 | MN217418 | ||
| NHM_1025D | 3e34e783-a51b-4a84-bc2e-6a19bac82b4e | 2019.7102 | 0109494679 | MN217419 | ||
| NHM_1200 | 2612dd53-cce5-47b9-aeed-c321bda6c3d8 | 2019.7103 | 0109494687 | MN217420 | ||
| NHM_1948J | 24374a21-17b6-4de5-8436-18c160aa5c8d | 2019.7104 | 0109494636 | MN217421 | ||
| Opheliidae | Ammotrypanella keenani sp. nov. | NHM_1166C | 483c6faa-0338-4cf5-a21d-6f448c72f4aa | 2019.7109 | 0109494704 | MN217408 |
| MN217513 | ||||||
| NHM_1250 (holotype) | 1514d25d-b485-4b90-8981-3e84381bf250 | 2019.7110 | 0109494644 | MN217409 | ||
| MN217491 | ||||||
| MN217514 | ||||||
| NHM_1871 (paratype) | d93680b5-a3a3-4623-afc4-17062e1a1a58 | 2019.7111 | 0109494707 | MN217410 | ||
| NHM_1949 | cff2696d-06ab-42a1-843e-6ef894872f32 | 2019.7112 | 0109494683 | MN217411 | ||
| Ammotrypanella kersteni sp. nov. | NHM_254 (holotype) | 3441cd68-7432-4dee-8415-966b104c3077 | 2019.7107 | 0109494672 | MN217412 | |
| MN217492 | ||||||
| MN217515 | ||||||
| Ammotrypanella sp. (NHM_1653) | NHM_1653 | a2f7ed04-7275-4a57-a058-bd750cacc715 | 2019.7108 | 0109494685 | MN217413 | |
| MN217493 | ||||||
| MN217516 | ||||||
| Ammotrypanella sp. (NHM_2114) | NHM_2114 | f4492dd1-8088-47c6-97d9-32e43ae99552 | 2019.7113 | 0109494699 | MN217414 | |
| MN217494 | ||||||
| Ophelina curli sp. nov. | NHM_2112 (holotype) | c1554f01-2324-4d8d-b775-dca42f5918e7 | 2019.7131 | 0109494716 | MN217435 | |
| MN217502 | ||||||
| Ophelina ganae sp. nov. | NHM_245 | 3a34b9cb-504b-48a3-a8e9-93077ec69520 | 2019.7140 | 0109494696 | MN217436 | |
| MN217503 | ||||||
| MN217521 | ||||||
| NHM_248 | e67f7724-8c9f-4463-943e-7cda20441728 | 2019.7141 | 0109494197 | MN217437 | ||
| NHM_473 | 79dcab18-936b-430e-b770-6aab60d285c5 | 2019.7142 | 0109494671 | MN217438 | ||
| MN217522 | ||||||
| NHM_598 (paratype) | 2fa20a59-8bb3-4ef8-b2e9-efccbe2c9414 | 2019.7143 | 0109494713 | MN217439 | ||
| NHM_708 | 2077c6c6-0e3e-4dfa-97a0-16d6c386ff07 | 2019.7144 | 0109494665 | MN217440 | ||
| NHM_1098 | 5661fb64-83a2-4e9a-b3c3-a8405705ed1a | 2019.7145 | 0109494631 | MN217441 | ||
| NHM_1137 (holotype) | 11616c16-bdb5-4813-9d17-7170bb62702b | 2019.7146 | 0109494711 | MN217442 | ||
| NHM_1309 (paratype) | 1ff41b52-9801-4b2e-8e01-ea34597b708d | 2019.7147 | 0109494639 | MN217523 | ||
| Opheliidae | Ophelina juhazi sp. nov. | NHM_1073 (holotype) | f7330230-224b-49e7-aa80-41e8654ea087 | 2019.7132 | 0109494655 | MN217443 |
| MN217504 | ||||||
| Ophelina martinezarbizui sp. nov. | NHM_681 (holotype) | 0de17415-a8bf-4461-a663-dea9a3e6a2b9 | 2019.7116 | 0109494717 | MN217444 | |
| MN217505 | ||||||
| MN217524 | ||||||
| NHM_718 | f6e2fa9b-a479-4e0d-aec6-57efff6987b2 | 2019.7117 | 0109494693 | MN217445 | ||
| MN217525 | ||||||
| NHM_883 | d9a3a3b3-c16e-4359-8eb0-f09deed98401 | 2019.7118 | 0109494627 | MN217446 | ||
| MN217526 | ||||||
| NHM_994 | 4f6d2b7a-169f-46a9-8b3b-5d91a021aa34 | 2019.7119 | 0109494626 | MN217447 | ||
| NHM_1066 | 972f9cb1-79d7-4296-a6d6-e04543c9105c | 2019.7120 | 0109494664 | MN217448 | ||
| NHM_1766 (paratype) | dc754b1c-e66b-4a58-a93e-796ebfd32f6a | 2019.7121 | 0109494658 | MN217449 | ||
| MN217527 | ||||||
| NHM_1870 | b6247e8d-d155-4646-87d7-e5358ada5352 | 2019.7122 | 0109494708 | MN217450 | ||
| NHM_2088 | 7dd04f2c-435b-44b1-a85f-3b05dd3014d7 | 2019.7123 | 0109494669 | MN217451 | ||
| NHM_2092 | 1a095836-fa97-48b8-ad4c-07ed28356ecb | 2019.7124 | 0109494650 | MN217528 | ||
| NHM_2102 | 93e91313-61a3-4cd7-8221-66bf20232f14 | 2019.7125 | 0109494645 | MN217452 | ||
| MN217529 | ||||||
| NHM_2116 (paratype) | d439156e-657d-4dd5-8bb5-3531e150961e | 2019.7126 | 0109494692 | MN217453 | ||
| MN217530 | ||||||
| NHM_2144 | 79767cab-eb56-4ef1-acd0-5067ec3736de | 2019.7127 | 0109494668 | MN217454 | ||
| NHM_2149 | 1caa9eb3-3281-4ed6-8424-dfaebcf1e20b | 2019.7128 | 0109494675 | MN217455 | ||
| NHM_2150 | 993f577c-ee86-4660-b2d9-af0146606f92 | 2019.7129 | 0109494651 | MN217456 | ||
| MN217531 | ||||||
| Ophelina meyerae sp. nov. | NHM_1241 (holotype) | 920d8670-507e-4126-a42b-6e208bbe66d3 | 2019.7130 | 0109494220 | MN217457 | |
| MN217506 | ||||||
| Ophelina nunnallyi sp. nov. | NHM_683 (holotype) | 220fa671-4576-45b7-930d-efde148f223f | 2019.7133 | 0109494235 | MN217458 | |
| MN217507 | ||||||
| MN217532 | ||||||
| NHM_700 (paratype) | 63115f48-bcf1-4b3b-9c2e-c339b97845bd | 2019.7134 | 0109494678 | MN217459 | ||
| NHM_783F | 376a42db-0497-4b4a-851b-c1d5e07bd2b6 | 2019.7135 | 0109494630 | MN217460 | ||
| NHM_1273 (paratype) | a3540563-8a0c-475b-96b5-12969fb8c2ba | 2019.7136 | 0109494663 | MN217461 MN217533 | ||
| NHM_1309A | 25066e63-ecc9-439a-9907-eaeaeb72e78c | 2019.7137 | 0109494656 | MN217462 | ||
| MN217534 | ||||||
| Ophelina cf. abranchiata sp. (NHM_1769) | NHM_1769 | 8a2cbe4f-277d-4355-a34f-0b53c797bef0 | 2019.7148 | 0109494637 | MN217433 | |
| MN217501 | ||||||
| MN217520 | ||||||
| Ophelina cf. abranchiata sp. (NHM_2017) | NHM_2017 | 9ebcd947-c53b-4616-81d4-da42afaeca03 | 2019.7149 | 0109494660 | MN217434 | |
| Ophelina sp. (NHM_689) | NHM_689 | 6755d584-a20a-4ce5-a4f1-32ce0965128e | 2019.7114 | 0109494689 | MN217463 | |
| MN217508 | ||||||
| Ophelina sp. (NHM_1068) | NHM_1068 | b28fd52f-5717-45e3-b0cc-369172a690e5 | 2019.7138 | 0109494646 | MN217464 | |
| MN217509 | ||||||
| NHM_1874 | c3ffe5f4-6ca3-4816-966c-25ec98bbb003 | 2019.7139 | 0109494684 | MN217466 | ||
| Ophelina sp. (NHM_1331) | NHM_1331 | 06d48d7f-7339-4cc5-8445-b51a980e4e0f | 2019.7115 | 0109494710 | MN217465 | |
| MN217510 | ||||||
| Scalibregmatidae | Oligobregma brasierae sp. nov. | NHM_032 | 43545746-b8ad-43a8-92b7-53637dd131d6 | 2019.7150 | 0109494647 | MN217422 |
| NHM_404 | 5fda0cac-0a77-4ec7-a2fa-5cd529548a19 | 2019.7151 | 0109494694 | MN217423 | ||
| NHM_684 (paratype) | d84c37ed-138e-4064-a11d-a11a2470dfdf | 2019.7152 | 0109494698 | MN217424 | ||
| MN217498 | ||||||
| MN217517 | ||||||
| NHM_823 (holotype) | 74781dbb-1f65-4839-a766-24d6cde63ed0 | 2019.7153 | 0109494676 | MN217425 | ||
| NHM_1423 (paratype) | d949e987-6e03-4092-8492-c51dd7fcf4d7 | 2019.7154 | 0109494681 | MN217426 | ||
| NHM_1895 | 02aaa9c0-837a-4836-8b34-5e68296c958e | 2019.7155 | 0109494643 | MN217427 | ||
| Oligobregma tani sp. nov. | NHM_773A (paratype) | 4b673a6a-9090-4c24-a4eb-231190507b60 | 2019.7156 | 0109494629 | MN217428 | |
| MN217499 | ||||||
| MN217518 | ||||||
| NHM_1454 (holotype) | 67d3f58a-9c13-423e-93b7-3ddcf98a361e | 2019.7157 | 0109494662 | MN217429 | ||
| NHM_1480J | d47f17aa-c0c1-44f0-a448-d3f3c395fc47 | 2019.7158 | 0109494633 | MN217430 | ||
| NHM_1665 (paratype) | eca166ae-3fe0-4367-860f-08c7410165dd | 2019.7159 | 0109494661 | MN217431 | ||
| MN217519 | ||||||
| Oligobregma whaleyi sp. nov. | NHM_822 (holotype) | dde1c8f9-f87a-430b-be9d-5e34685772bb | 2019.7160 | 0109494667 | MN217432 | |
| MN217500 | ||||||
| Scalibregmatidae sp. (NHM_2308) | NHM_2308 | 7b9d4ab8-4b7b-45c4-9cf4-6fd6b1229f48 | 2019.7161 | 0109494623 | MN217467 | |
| Travisiidae | Travisia zieglerae sp. nov. | NHM_140 (paratype) | ed10356b-32a0-4b45-9fe3-c56fbc696e87 | 2019.7162 | 0109494719 | MN217470 |
| MN217512 | ||||||
| NHM_188 | c8a0ef70-e7f7-4605-bf78-dc54ed9151eb | 2019.7170 | 0109494718 | MN217471 | ||
| NHM_241 | 5c0ac0b7-60cc-473e-a23b-2f49a40540f4 | 2019.7163 | 0109494648 | MN217472 | ||
| NHM_356 | 8d2cbf0e-6522-403d-a58a-905fb13c70d6 | 2019.7164 | 0109494697 | MN217473 | ||
| NHM_364 | ef6e520f-7ef5-4ff9-87b5-985b8576271f | 2019.7165 | 0109494673 | MN217474 | ||
| NHM_748B (paratype) | db527676-1030-4bf0-b28d-2382825bc6bf | 2019.7166 | 0109494654 | MN217475 | ||
| NHM_753 | 393203b1-cb80-4185-9e40-fca6e1b6fe34 | 2019.7167 | 0109494715 | MN217476 | ||
| NHM_760 | d3e8ec3c-d7f3-4908-b315-84f3758aecc1 | 2019.7168 | 0109494691 | MN217477 | ||
| NHM_792 | 5d30a61b-5894-484f-b79a-df1cd4268ec1 | 2019.7169 | 0109494641 | MN217478 | ||
| NHM_909 (paratype) | 5f570dab-4b56-4f74-b126-ed6ceab344e3 | 2019.7171 | 0109494670 | MN217479 | ||
| NHM_970 | 4ccb364c-35f4-458c-9c71-6f77e71493ca | 2019.7172 | 0109494703 | MN217480 | ||
| NHM_1097 | 939ba16d-b844-49ca-a740-bb42f039cc11 | 2019.7173 | 0109494625 | MN217481 | ||
| NHM_1310 | 16844478-de27-448c-9acb-057835026447 | 2019.7174 | 0109494690 | MN217482 | ||
| NHM_1311 | 192cbbb3-680b-4bcd-9cc4-a420f42af578 | 2019.7175 | 0109494700 | MN217483 | ||
| NHM_1431 (holotype) | fd6bab0e-0cda-4b42-808f-a6006d409535 | 2019.7176 | 0109494211 | MN217484 | ||
| NHM_1543 (paratype) | c78cc5fd-ca98-43b0-a0fb-8804fb606c71 | 2019.7177 | 0109494628 | MN217485 | ||
| Travisiidae | Travisia zieglerae sp. nov. | NHM_1873 | 24409a12-2a50-4689-80dc-902cdeb5af69 | 2019.7178 | 0109494642 | MN217486 |
| NHM_1883 | 9e8c22f7-a94b-45ed-a1d0-cae287a7ac2d | 2019.7179 | 0109494666 | MN217487 | ||
| NHM_1911 | 489dd5a6-2c68-416b-9a06-ed773d4791d6 | 2019.7180 | 0109494632 | MN217488 | ||
| NHM_2019 | 2684a5f8-b4d4-4bcb-b386-65775506cf87 | 2019.7181 | 0109494659 | MN217489 | ||
| NHM_2024 | cf54f81e-5836-4684-94dc-151f589ebab4 | 2019.7182 | 0109494653 | MN217490 | ||
| Travisia sp. (NHM_1244) | NHM_1244 | f6906eae-67ec-4d37-83c6-590f3c53df76 | 2019.7183 | 0109494714 | MN217468 | |
| NHM_1863 | fa708aca-6dd1-4b53-8d54-c76a93f43363 | 2019.7184 | 0109494652 | MN217469 | ||
| MN217511 |
Systematics
Annelida
Capitellidae
Notes
Capitellidae represent an important group of polychaetes owing to their use as indicators of environmental health (e.g.,
At least two species were recognised in the UKSR material, five poorly preserved representatives of a species in the diverse genus Notomastus, and two specimens of a species representing a new genus based on morphological and genetic data. However, given the caveats of generic definitions given above, we choose not to provide the formal description of this species and genus, and make these data and vouchers available for future revision.
Capitellidae
Material examined
NHM_613 NHMUK ANEA 2019.7105, coll. 17 Feb. 2015, 12°23.175N, 116°32.92W, 4202 m http://data.nhm.ac.uk/object/be34eb86-fc0c-411c-8eb9-e86774c6515a; NHM_1486 NHMUK ANEA 2019.7106, coll. 04 Mar. 2015, 12°29.70N, 116°39.01W, 4260 m http://data.nhm.ac.uk/object/05d46c60-8b4d-4623-b7ff-2e8089453d7e.
Description
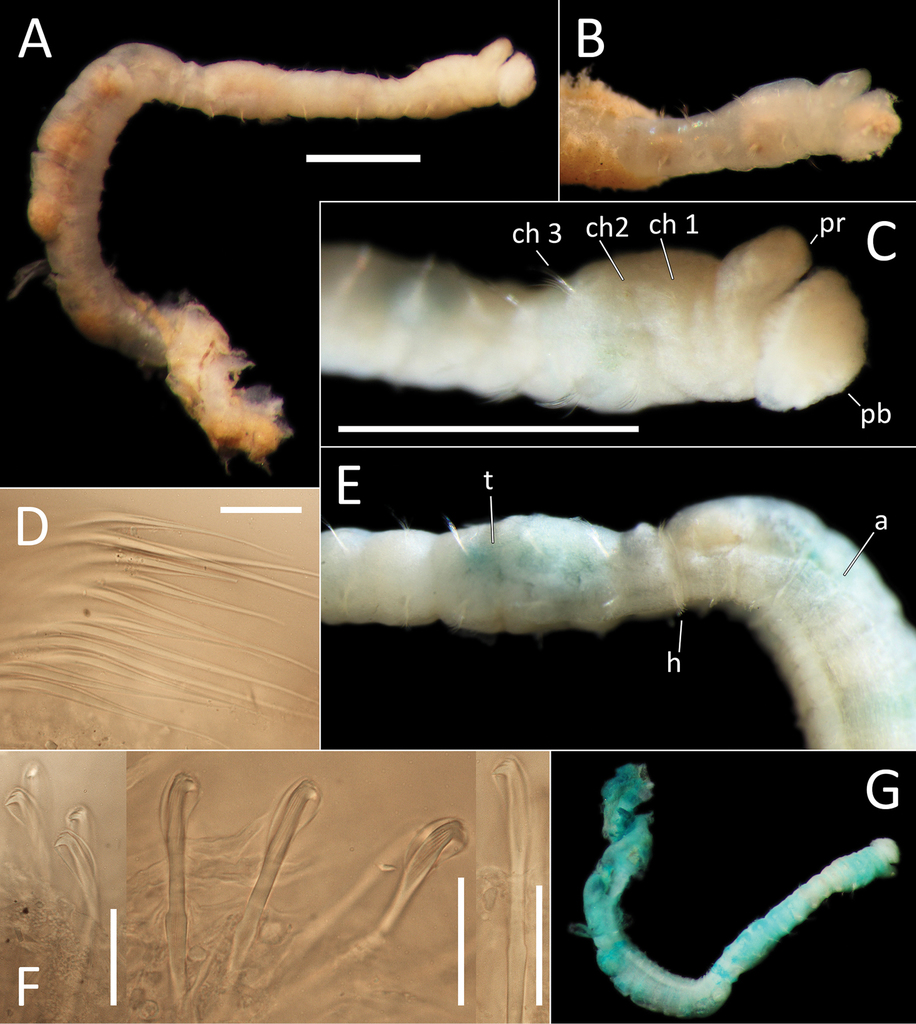
Species represented by one anterior fragment and one body fragment only. Specimen NHM_1486 posteriorly incomplete, 8 mm long and 0.6 mm wide for about 22 chaetigers (posterior part of fragment is damaged). Preserved specimens creamy white in ethanol (Fig.
Capitellidae sp. NHM_1486 (specimen NHM_1486). A Lab image, whole specimen B Live image, anterior C Lab image, anterior, highlighting first three chaetigers (faded stain, pr = prostomium, pb = proboscis) D Lab image, thoracic capillary chaetae E Lab image, transitionary chaetigers between thorax and abdomen (faded stain) (t = thorax, h = hooks, a = abdomen) F Lab image, hooks G Lab image, whole specimen (methyl green stain). Scale bars: 1 mm (A, C); 25 μm (D, F).
Prostomium conical, anteriorly broadly rounded, slightly longer than wide (Fig.
Chaetigers 1–10 (= thorax) with capillaries only. First chaetiger with chaetae in notopodia only, subsequent nine chaetigers with chaetae in both noto- and neuropodia. All thoracic chaetae slender, bilimbate capillaries (Fig.
Chaetigers 11–12 are considered transitional between thorax and abdomen marked by appearance of hooded hooks only in neuropodia, but segments are of similar thickness to those in anterior part of body (Fig.
Abdominal segments enlarged (inflated), without lobe (Fig.
Methyl green stain
Prostomium, chaetigers 4–6 and abdominal chaetigers do not stain (or at best stain very lightly). Peristomium, chaetigers 1–3 and 7–12/13 stain more strongly (Fig.
Genetic data
GenBank MN217415-MN217416 for 16S and MN217495-MN217496 for 18S. COI was unsuccessful for this species, no identical matches on GenBank for 16S or 18S. The species is distinct from all other specimens in this study and in our phylogenetic analyses forms an unresolved clade with Barantolla lepte Hutchings, 1974, three Notomastus M. Sars, 1851 and one Heteromastus Eisig, 1887 species (Fig.
Remarks
This species is unusual amongst Capitellidae due to its large number of mixed segments (with capillaries only in notopodia and hooded hooks only in neuropodia). Usually, the abdominal chaetigers in Capitellidae bear hooded hooks only, or there are a small number of posterior thoracic and/or anterior abdominal segments that bear both capillaries and hooks. Of the known genera, only Promastobranchus Gallardo, 1968 shows such chaetal distribution. However, it can be distinguished from the other UKSR-collected species in having the first chaetiger with both noto- and neurochaetae and possessing 12 to 13 instead of 10 chaetigers with capillary chaetae in both rami. Therefore, this material represents not only a new species, but based on current morphological criteria supported also by genetic data, a new genus as well. Because the material is not complete (morphology for the posterior end is missing) the species is here not formally described.
Ecology
Found in the eastern polymetallic nodule province of the CCZ.
Notomastus M. Sars, 1851
Notomastus
Material examined
NHM_162 NHMUK ANEA 2019.7100, coll. 13 Oct. 2013, 13°57.794N, 116°34.093W, 4084 m http://data.nhm.ac.uk/object/f34e2921-7b6d-4c14-b217-690d9315f073; NHM_915B NHMUK ANEA 2019.7101, coll. 23 Feb. 2015, 12°34.28N, 116°36.63W, 4198 m http://data.nhm.ac.uk/object/98291a2a-c89a-4f62-bde5-91171368c749; NHM_1025D NHMUK ANEA 2019.7102, coll. 24 Feb. 2015, 12°08.02N, 117°17.52W, 4122 m http://data.nhm.ac.uk/object/3e34e783-a51b-4a84-bc2e-6a19bac82b4e; NHM_1200 NHMUK ANEA 2019.7103 coll. 27 Feb. 2015, 12°00.567N, 117°10.687W, 4144 m http://data.nhm.ac.uk/object/2612dd53-cce5-47b9-aeed-c321bda6c3d8; NHM_1948J NHMUK ANEA 2019.7104, coll. 13 Mar. 2015, 12°02.49N, 117°13.03W, 4094 m http://data.nhm.ac.uk/object/24374a21-17b6-4de5-8436-18c160aa5c8d.
Description
All specimens short anterior fragments, posteriorly incomplete with thorax only or thorax and 2–5 abdominal segments only. Small species, 2–4 mm long and 0.3–0.8 mm wide for 11–16 chaetigers. Preserved specimens creamy white in ethanol; live specimens white to light brown semi-opaque/translucent. Epithelium of peristomium and first two chaetigers smooth or at best weakly annulated, on chaetigers 3–11 epithelium tessellated, distinctly bi-annulated (Fig.
Notomastus sp. NHM_162. A Lab image, whole specimen (specimen NHM_162) B Live image, whole specimen (specimen NHM_162) C Lab image, biannulated chaetigers (specimen NHM_162) D Lab image, prostomium (specimen NHM_162, pr = prostomium) E Lab image, whole specimen, dorsal (specimen NHM_915B, no = notopodial lobe, nu = neuropodial lobe) F Lab image, whole specimen, ventral (specimen NHM_915B, nu = neuropodial lobe) G Lab image, thoracic chaetae, (specimen NHM_162) H Lab image, thoracic hooks (specimen NHM_162) I Lab image, whole specimen (specimen NHM_162, methyl green stain). Scale bars: 1 mm (A, E, F, I); 50 μm (G, H).
Prostomium low, rounded mound (Fig.
Thorax with 11 chaetigers, first with notochaetae only. All thoracic chaetae long, slender, bilimbate capillaries (Fig.
Abdominal segments with hooded hooks only in both rami. Noto- and neuropodia free laterally, notopodia widely separated dorsally. Abdominal notopodia coalesce into lobe, which protrudes from dorsum (Fig.
Methyl green stain
Anterior fragment with 13 chaetigers staining more or less uniformly; more pronounced in chaetigers 6–11 (Fig.
Genetic data
GenBank MN217417-MN217421 for 16S, MN217497 for 18S. COI was unsuccessful for this species, no identical matches on GenBank for 16S or 18S. In our phylogenetic tree Notomastus sp. (NHM_162) is sister to Notomastus latericeus M. Sars, 1851, but the genus Notomastus is not monophyletic in our tree (Fig.
Phylogenetic analysis of Capitellidae. 50% majority rule tree from the Bayesian analyses using 18S and 16S, with posterior probability values on nodes. Twenty-four taxa from GenBank were included, and Echiura was chosen as outgroup following the annelid phylogeny of
Remarks
This species is consistent with the genus Notomastus in possessing 11 chaetigers with notochaetae only, followed by abdominal chaetigers with hooded hooks only. As the specimens are poorly preserved with the thorax only or with 2–5 abdominal segments observed, this species cannot be meaningfully compared with other known species in this genus and is therefore not formally described.
Ecology
Found in the eastern polymetallic nodule province of the CCZ.
Opheliidae
Notes
Due to their simple morphology, there is much confusion in the taxonomic literature dealing with Opheliidae, and many species and genera are currently considered invalid (Read and Fauchald 2018c). Useful recent studies clarifying some of the confusion are
Unfortunately, the ABYSSLINE material provides very few specimens (often just one) per species, which complicates the morphological interpretation. Nevertheless, in combination with DNA data, we believe it is important to provide the currently best possible morphology, which can be amended as more and better-preserved examples become available in the future. As a result, only 8 out of 15 opheliid species found in the ABYSSLINE material are here formally described. Morphologically, the ABYSSLINE material can be assigned to two known genera, Ammotrypanella McIntosh, 1878 and Ophelina Örsted, 1843.
Ammotrypanella
Notes
The confused taxonomic history of Ammotrypanella and its type species, Ammotrypanella arctica McIntosh, 1878 has been summarized by
As a taxonomic revision is beyond the scope of this study, we follow the definition of Ammotrypanella given by
Diagnosis
Body long and thin, with ventral groove along whole length of body. Prostomium bluntly rounded to conical with small palpode, peristomium indistinct. Eyes absent. Parapodia embedded into lateral groove in median region, becoming more distinct in posterior region. Parapodia with branchiae in third quarter of body. All chaetae simple. Branchiae flat, wide at base, tapering to top. Anal tube present.
Several morphotypes with branchiae restricted to the posterior part of the body were encountered in the UKSR material, which is consistent with genus Ammotrypanella as discussed above. The UKSR-collected species can be distinguished from four known species assigned to this genus mainly by the form of anal tube:
Ammotrypanella arctica McIntosh, 1878 has an elongated anal tube about same length as posterior abranchiate region and provided with a deciduous anal cirrus and terminal anus (see Schüller et al. 2008;
Ammotrypanella cirrosa Schüller, 2008 has an elongated anal tube, its length equals to length of last 5–8 chaetigers, posterior margin with numerous cirri.
Ammotrypanella mcintoshi Schüller, 2008 lacks an anal tube. Although the absence of an anal tube was considered real and a distinguishing feature of this species by
Ammotrypanella princessa Schüller, 2008 has a prostomium which mimics the shape of a royal crown (
Additionally, Ophelina opisthobranchiata Wirén, 1901 described from the deep sea of Spitsbergen, also has a posterior distribution of branchiae. In his recent re-description
Our molecular analysis revealed the presence of four distinct CCZ species, forming a well-supported clade. Three of those species (Ammotrypanella keenani sp. nov., Ammotrypanella kersteni sp. nov. and Ammotrypanella sp. NHM_1653) are represented by reasonably well-preserved specimens. Unfortunately, species NHM_2114 is represented by a single specimen with all branchiae now lost and it is therefore assigned to this genus only based on molecular data.
Ammotrypanella keenani sp. nov.
Material examined
NHM_1166C NHMUK ANEA 2019.7109, coll. 26 Feb. 2015, 12°06.93N, 117°09.87W, 4100 m http://data.nhm.ac.uk/object/483c6faa-0338-4cf5-a21d-6f448c72f4aa; NHM_1250 (holotype) NHMUK ANEA 2019.7110, coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object1514d25d-b485-4b90-8981-3e84381bf250; NHM_1871 (paratype) NHMUK ANEA 2019.7111, coll. 13 Mar. 2015, 12°02.49N, 117°13.03W, 4094 m http://data.nhm.ac.uk/object/d93680b5-a3a3-4623-afc4-17062e1a1a58; NHM_1949 NHMUK ANEA 2019.7112, coll. 14 Mar. 2015, 12°11.406N, 117°22.282W, 4182 m http://data.nhm.ac.uk/object/cff2696d-06ab-42a1-843e-6ef894872f32.
Type locality
Pacific Ocean, CCZ, 12°15.44N, 117°18.13W, depth 4302 m, in mud between polymetallic nodules.
Description
This is a small to medium-sized species (6–16 mm long), represented by four specimens. NHM_1250 and NHM_1871 are complete specimens in good condition 12 mm long and 0.8 mm wide for 38 chaetigers and 16 mm long and about 1 mm wide respectively for 38 chaetigers. NHM_1166C and NHM_1949 are complete, but much smaller specimens in poor condition, 6–7mm long, with mid-body region twisted and damaged, therefore the exact number of chaetigers cannot be established, but at least 34 chaetigers observed in both specimens.
Body cylindrical, iridescent and smooth, no annulation detectable. Ventral groove along the entire body length. Preserved specimen pale yellow in ethanol (Fig.
Ammotrypanella keenani sp. nov. A Lab images, whole specimens (holotype [specimen NHM_1250], post-staining, faded stain [left], paratype NHM_1871, unstained [right]) B Live images, whole specimens (holotype [left], paratype NHM_1871 [right]) C Lab image, prostomium (paratype NHM_1871, damaged, no = everted nuchal organ) D Lab image, prostomium (holotype, p = palpode) E Lab image, detail of palpode (holotype) F Lab image, posterior branchiae (paratype NHM_1871, br = branchiae) G Lab image, posterior branchiae (holotype, br = branchiae) H Live image, anterior (paratype NHM_1871, pp = parapodia, no = nuchal organ) I Lab image, detail of capillary chaetae (holotype) J Lab image, detail of anal funnel, ventro-distal (left) and latero-distal (right) views (holotype, stained, cp = cushioned pad, vc = ventral cirrus). Morphological features in plates C–H, J have been outlined with a fine white line to improve clarity of those features. Scale bars: 1 mm (A, F,G); 100 μm (E, I).
Prostomium conical with distinct, slightly elongated palpode (NHM_1871, NHM_1166C) (Fig.
Branchiae present, but limited to posterior region only, where at least 10 or 11 pairs present in chaetigers 22(23)–32, but only seven pairs were observed in smaller specimens (NHM_1166C and NHM_1949). All branchiae cirriform; first two pairs observed in NHM_1781 reduced in size, with the first pair (ch. 22) smallest (Fig.
Parapodia distinct, biramous; observed as broad lobes in chaetigers 1–5 (Fig.
Anal tube the length of about half of the length of abranchiate posterior region, elongated, cylindrical; distal end with circlet of about four tightly packed cushion-like pads and thickened ventral pad observed in specimen NHM_1250 (damaged in other specimens) (Fig.
Genetic data
GenBank MN217408-MN217411 for 16S, MN217491 for 18S and MN217513–MN217514 for COI. This species is genetically identical or very similar to COI sequences attributed to “Opheliidae sp. 2” in
Remarks
Posterior distribution of branchiae and variously preserved cylindrical tube was observed in all specimens examined, irrespective of their size. These specimens represent one of several species consistent with genus Ammotrypanella recognized from the UKSR material. This species is most similar to Ammotrypanella sp. NHM_1653 in the relatively small body size and possession of an elongated (cylindrical) anal tube. This species can be distinguished from other Ammotrypanella material in this study and known Ammotrypanella species in having an anal tube distally with 4 or 5 cushion-like pads rather than distinct cirri, although this observation is based on only single specimen.
Ecology
Found in the eastern part of polymetallic nodule province in the CCZ.
Etymology
Named in honor of Edward Keenan, boatswain onboard RV Melville on the AB01 ABYSSLINE cruise in 2013.
Ammotrypanella kersteni sp. nov.
Material examined
NHM_254 (holotype) NHMUK ANEA 2019.7107, coll. 17 Oct. 2013, 13°45.21N, 116°29.12W, 4128 m http://data.nhm.ac.uk/object/3441cd68-7432-4dee-8415-966b104c3077.
Type locality
Pacific Ocean, CCZ, 13°45.21N, 116°29.12W, depth 4128 m, in mud between polymetallic nodules.
Description
This species is represented by a single specimen in very good condition, although now split into two fragments, following tissues sampling for molecular analysis. Specimen (when complete) 31 mm long and 1.5 mm wide for 36 chaetigers. Body cylindrical, iridescent and smooth, no annulation detectable (Fig.
Prostomium of preserved specimen oval and broad (about as long as wide) and anteriorly blunt, somewhat truncated and bearing very distinct short, button-like palpode (Fig.
Ammotrypanella kersteni sp. nov. holotype (specimen NHM_254). A Lab images, whole specimens, dorsal (upper) and ventral (lower) B Lab image, anterior C Lab image, detail of palpode D Lab image, mid-body parapodia (p = parapodium) E Lab image, detail of parapodium F Lab image, posterior and anal funnel. Morphological features in plates B, D, F have been outlined with a fine white or black line to improve clarity of those features. Scale bars: 2 mm (A); 1 mm (B, D, F); 100 μm (C, E).
Branchiae present, but limited to posterior region only, where present in chaetigers 22–28, seven pairs. All branchiae cirriform, of similar length, with red pigment in live specimen (Fig.
Parapodia distinct, biramous; observed as a broad lobe in chaetigers 1–7 (Fig.
Posterior achateous end (it is unclear if it represents anal tube) the length of two posterior chaetigers, a funnel-shaped structure with broad distal opening, distal margin smooth (Fig.
Reproductive information
Ovigerous specimen with eggs of 200–250 µm in size clearly observed in mid through to posterior part of the body (Fig.
Genetic data
GenBank MN217412 for 16S, MN217492 for 18S and MN217515 for COI. No identical matches on GenBank for COI, 16S or 18S. This taxon does not match any previous COI sequences, and we only have one specimen from the current study, which may indicate that this represents a rare species. In our phylogenetic analyses it forms a monophyletic clade with Ammotrypanella keenani sp. nov., Ammotrypanella sp. (NHM_2114) and Ammotrypanella sp. (NHM_1653) (Fig.
Remarks
Ammotrypanella princessa Schüller, 2008 is most similar to our species because of the shape of prostomium; however, this may be a preservation artefact (see earlier comments), which mimics the shape of a royal crown (
The anal tube commonly becomes detached in opheliids and when short anal tubes have been described in the past, it is important to be mindful that the anal tube may in fact be missing. The posterior achateous end in UKSR species is rather short, but it appears to have a distinct form, and therefore we suggest it may possibly represent anal tube rather than damaged posterior end. However, other Ammotrypanella species possess an elongated cylindrical anal tube, which could suggest that the anal tube in A. kersteni sp. nov. is in fact missing. At the same time, the anal tubes of Opheliidae species show a variety of forms, and it is not impossible to speculate that similar variability can be found in Ammotrypanella as more species are discovered. With the current evidence based on single specimen we cannot clarify if the funnel-shaped posterior end represents the anal tube.
Ecology
Found in the eastern polymetallic nodule province of the CCZ.
Etymology
Named in honor of Oliver Kersten, member of the science party of both ABYSSLINE cruises.
Ammotrypanella
Material examined
NHM_1653 NHMUK ANEA 2019.7108, coll. 10 Mar. 2015, 12°21.81N, 116°40.86W, 4233 m http://data.nhm.ac.uk/object/a2f7ed04-7275-4a57-a058-bd750cacc715.
Description
This small species is represented by a single complete specimen in reasonable condition, except for some damage to anal tube (Fig.
Ammotrypanella sp. NHM_1653 (specimen NHM_1653). A Lab image, whole specimen (faded stain B Live image, whole specimen C Lab image, anterior (faded stain) D Lab image, detail of palpode E Lab image, posterior (faded stain, br = branchiae) F Lab image, anterior-midbody (faded stain, pp = parapodia) G Lab image, detail of capillary chaetae H Lab image, detail of posterior and anal tube. Morphological features in plates C, E, F, G have been outlined with a very fine white to improve clarity of those features. Scale bars: 1 mm (A); 100 μm (D, G, H).
Prostomium conical (longer than wide) anteriorly tapering into blunt tip and bearing very distinct, round palpode (Fig.
Branchiae present, but limited to posterior region only, where present in chaetigers 22–28, seven pairs. All branchiae cirriform; large, of similar length except for the last branchial pair, which is reduced (Fig.
Parapodia distinct, biramous; observed as a small lobe in chaetigers 1–7, becoming smaller in subsequent chaetigers; parapodia embedded in distinct lateral grooves (Fig.
Anal tube the length of three posterior chaetigers (Fig.
Genetic data
GenBank MN217413 for 16S, MN217493 for 18S and MN217516 for COI. This species is genetically identical or very similar to COI sequences collected in the German and French exploration contract areas and published in
Remarks
This is another species with branchiae limited to the posterior end consistent with the genus Ammotrypanella. While this species is similar to Ammotrypanella kersteni sp. nov., it can be clearly distinguished from it by a much smaller body size, shape of prostomium and bearing narrow, elongated, cylindrical anal tube. This form of tube is however similar to other known anal tube-bearing species of Ammotrypanella and due to some damage to this feature in the UKSR specimen, its form cannot be established with certainty.
Ecology
Found in polymetallic nodule province.
Ammotrypanella
Material examined
NHM_2114 NHMUK ANEA 2019.7113, coll. 20 Mar. 2015, 19°27.874N, 120°01.525W, 4026 m, http://data.nhm.ac.uk/object/f4492dd1-8088-47c6-97d9-32e43ae99552.
Description
Single, minute, damaged specimen; now with posterior part of the body removed for molecular analysis. Anterior fragment 1.6 mm long 0.2 mm wide for about 16 chaetigers (chaetae observed on only 11 of these, the rest of the fragment damaged with chaetae missing). Ventral groove along the entire length of the fragment. First six chaetigers crowded. Preserved specimens pale yellow in ethanol; live specimen translucent with orange gut (Fig.
Ammotrypanella sp. NHM_2114 (specimen NHM_2114). A Live images, whole specimen with detail of anterior B Lab images, fragmented whole specimen, post-staining, (faded stain, anterior fragment [left], posterior fragment [right],) C Lab image, anterior fragment, post-staining, faded stain D Lab image, detail of anterior and palpode E Lab image, detail of capillary chaetae. Scale bars: 1 mm (B); 250 μm (C) 50 μm (D); 25 μm (E).
Additional morphological observations from live specimen
Upon collection the live specimen was imaged and appears to be complete. Presence and distribution of branchiae cannot be established from the image. The anal tube was probably missing upon collection of the specimen.
Genetic data
GenBank MN217414 for 16S and MN217494 for 18S. COI was unsuccessful for this specimen, no identical GenBank matches for 16S or 18S. Ammotrypanella sp. (NHM_2114) cluster with the Ammotrypanella keenani sp. nov., Ammotrypanella sp. (NHM_1653) and Ammotrypanella kersteni sp. nov. in our phylogenetic analyses (Fig.
Remarks
Although important diagnostic features cannot be fully confirmed in this specimen, in the phylogenetic tree it falls into a well-supported clade containing Ammotrypanella species and likely represents another species in this genus reported from UKSR material. At present, morphological information obtained from this single representative is limited which prevent us from providing a formal description.
Ophelina
Notes
The diagnosis of Ophelina presented here follows that given by
Diagnosis
Body elongate, with deep ventral groove and two lateral grooves along entire length of body. Prostomium conical, sometimes with terminal palpode; eyes present or absent. Branchiae present or absent; if present, beginning on chaetiger 2, continuing to posterior end, sometimes absent from middle or far posterior chaetigers; branchiae single, cirriform. Segmental lateral eyes absent. Noto- and neuropodia with small fascicles of capillary chaetae; small ventral cirrus present. Pygidium with anal funnel sometimes bearing long unpaired cirrus and additional lateral cirri.
Ophelina curli sp. nov.
Material examined
NHM_2112 (holotype) NHMUK ANEA 2019.7131, coll. 20 Mar. 2015, 19°27.874N, 120°01.525W, 4026 m, http://data.nhm.ac.uk/object/c1554f01-2324-4d8d-b775-dca42f5918e7.
Type locality
Pacific Ocean, CCZ, 19°27.874N, 120°01.525W, depth 4026 m, in mud between polymetallic nodules.
Description
This species is represented by a single specimen 30 mm long and 1 mm wide for 28 chaetigers.
Ventral and lateral grooves distinct along whole length of body. Colour in alcohol yellow to light tan (Fig.
Ophelina curli sp. nov. holotype (specimen NHM_2112). A Lab image, whole specimen (post-staining, very faded stain) B Live image, with detail of anterior C Lab image, anterior and proboscis, lateral view, stained D Lab image, detail of palpode E Lab image, anterior and proboscis, ventral view (stained, pb = proboscis) F Lab image, mid-body parapodium (stained, pp = parapodia) G Lab image, detail of capillary chaeta H Lab image, anal funnel. Scale bars: 1 mm (A, E); 100 μm (D, G)
Prostomium conical (longer than wide), with distinct, tear-shaped terminal palpode (Fig.
Branchiae absent. Parapodia biramous, embedded in lateral grooves; parapodia small conical lobes, best observed on anterior seven chaetigers; no distinct pre- or postchaetal lobes observed (Fig.
Chaetae all slender, smooth capillaries (Fig.
Anal tube attached; narrow and smooth; no cirri observed (Fig.
Genetic data
Remarks
Morphologically this species is very similar to Ophelina nematoides (Ehlers, 1913) and to UKSR Ophelina juhazi sp. nov. in being abranchiate and having 28–30 chaetigers. Two other abranchiate species that are morphologically similar to Ophelina abranchiata Støp-Bowitz, 1948 are also reported in this material, but these differ in having much smaller body size (4.5–8 mm) and having only 17 or 18 chaetigers.
The main difference between O. nematoides and Ophelina curli sp. nov. is the number of chaetigers, 30 in the former versus 28 in the latter. The shape of anal tube appears to be similar, but without drawing or access to Ehlers’ type specimen, this structure cannot be meaningfully compared using Ehlers’ description alone.
The morphologically similar species Ophelina juhazi sp. nov. also found in the UKSR material can be distinguished by its smaller size, 17 mm compared to the 30 mm O. curli sp. nov., and the shape of anal tube, which in O. juhazi sp. nov. is cylindrical throughout, entire (no ventral slit), distally slightly narrowing and symmetrical.
Ecology
Found in polymetallic nodule province of the eastern CCZ.
Etymology
Named in honor of Cassidy Curl, Ordinary Seaman onboard RV Melville on the AB01 ABYSSLINE cruise in 2013.
Ophelina ganae sp. nov.
Material examined
NHM_245 NHMUK ANEA 2019.7140, coll. 16 Oct. 2013, 13°48.70N, 116°42.60W, 4076 m http://data.nhm.ac.uk/object/3a34b9cb-504b-48a3-a8e9-93077ec69520; NHM_248 NHMUK ANEA 2019.7141, coll. 16 Oct. 2013, 13°48.70N, 116°42.60W, 4076 m http://data.nhm.ac.uk/object/e67f7724-8c9f-4463-943e-7cda20441728; NHM_473 NHMUK ANEA 2019.7142, coll. 22 Oct. 2013, 13°43.597N, 116°40.20W, 4160 m http://data.nhm.ac.uk/object/79dcab18-936b-430e-b770-6aab60d285c5; NHM_598 (SEM) (paratype) NHMUK ANEA 2019.7143, coll. 17 Feb. 2015, 12°23.174N, 116°32.92W, 4202 m http://data.nhm.ac.uk/object/2fa20a59-8bb3-4ef8-b2e9-efccbe2c9414; NHM_708 NHMUK ANEA 2019.7144, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/2077c6c6-0e3e-4dfa-97a0-16d6c386ff07; NHM_1098 NHMUK ANEA 2019.7145, coll. 26 Feb. 2015, 12°06.93N, 117°09.87W, 4100 m http://data.nhm.ac.uk/object/5661fb64-83a2-4e9a-b3c3-a8405705ed1a; NHM_1137 (holotype) NHMUK ANEA 2019.7146, coll. 26 Feb. 2015, 12°06.93N, 117°09.87W, 4100 m http://data.nhm.ac.uk/object/11616c16-bdb5-4813-9d17-7170bb62702b; NHM_1309 (paratype) (SEM) NHMUK ANEA 2019.7147, coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object/1ff41b52-9801-4b2e-8e01-ea34597b708d.
Type locality
Pacific Ocean, CCZ, 12°06.93N, 117°09.87W, depth 4100 m, in mud between polymetallic nodules.
Description
Small species (4.5–8 mm long without anal tube), represented by eight specimens; all preserved specimens without anal tube (likely missing due to damage). Holotype is 7 mm long and 0.3 mm wide for 17 chaetigers. Paratypes 7–8 mm long and 0.33–0.35 mm wide for 17 chaetigers. Body cylindrical and smooth without distinct annulation. Preserved specimen yellow in ethanol (Fig.
Ophelina ganae sp. nov. A Lab image, whole specimen (holotype [specimen NHM_1137] B Live images, whole specimens (holotype [bottom], paratype NHM_598 [middle], paratype NHM_1309 [top]) C Lab image, detail of palpode (holotype) D SEM image, second chaetiger with lateral organ (paratype NHM_1309, lo = lateral organ) E SEM images, lateral organs, (a) pre-chaetigerous segment, (b) chaetiger 1, (c) chaetiger 2, (d) chaetiger 17 (last chaetiger) (paratype NHM_1309) F Lab image, detail of capillary chaetae (holotype) G Live image, with detail of potential anal funnel (specimen NHM_473, vc = potential ventral cirrus). H Lab image, detail of posterior (holotype) I Lab image, whole specimen (paratype NHM_598, stained). Morphological features in plates G, H have been outlined with a fine white or black line to improve clarity of those features. Scale bars: 1 mm (A); 100 μm (C); 20 μm (D); 5 μm (E); 50 μm (F); 100 μm (H); 1 mm (I).
Prostomium elongate, conical with small acute terminal palpode (Fig.
Chaetae all slender, smooth capillaries (Fig.
Branchiae absent. Anal tube not observed in preserved specimens (e.g. Fig.
Genetic data
GenBank MN217436-MN217442 for 16S, MN217503 for 18S and MN217521-MN217523 for COI. In our phylogenetic analyses, Ophelina ganae sp. nov. is sister to Ophelina cf. abranchiata (NHM_1769) and form a well-supported clade with this species and Ophelina cf. abranchiata (NHM_2017) together with at least two other abranchiate species (Fig.
Remarks
Molecular analysis of the UKSR-collected material revealed presence of three distinct small abranchiate species that morphologically resemble Ophelina abranchiata. Given the taxonomic problems of this species, the challenge is not only to morphologically distinguish these species from each other, but also from O. abranchiata. Here, we restrict the definition of O. abranchiata to that provided by
Ecology
Found in polymetallic nodule province of the eastern CCZ.
Etymology
Named in honor of Bin Qi Gan, member of the science party of the ABYSSLINE AB02 cruise onboard the RV Thomas G. Thompson.
Ophelina juhazi sp. nov.
Material examined
NHM_1073 (holotype) NHMUK ANEA 2019.7132, coll. 26 Feb. 2015, 12°06.93N, 117°09.87W, 4100 m http://data.nhm.ac.uk/object/f7330230-224b-49e7-aa80-41e8654ea087.
Type locality
Pacific Ocean, CCZ, 12°06.93N, 117°09.87W, depth 4100 m, in mud between polymetallic nodules.
Description
This species is represented by a single specimen 17 mm long and 0.5 mm wide for 29 chaetigers. Ventral and lateral grooves distinct along whole length of body. Live specimens translucent, with orange-red gut (Fig.
Ophelina juhazi sp. nov. holotype (specimen NHM_1073). A Live images, whole specimen (center) with detail of anterior (left) and posterior (right) B Lab image, whole specimen C Lab image, anterior and prostomium D Lab image, detail of palpode E Lab image, anterior and parapodia (stained, pp = parapodia) F Lab image, detail of capillary chaetae G Lab image, posterior and anal tube (stained). Morphological features in plates C, E, G have been outlined with a very fine white line to improve clarity of those features. Scale bars: 1 mm (B); 100 μm (D); 50 μm (F); 1 mm (E).
Prostomium conical (longer than wide), with distinct, tear-shaped terminal palpode (Fig.
Branchiae absent. Parapodia biramous, embedded in lateral grooves; parapodia small conical lobes, best observed on anterior seven chaetigers (Fig.
Anal tube attached; narrow, cylindrical structure, symmetrical, smooth (no cirri observed) and distally slightly narrowing (Fig.
Genetic data
Remarks
Morphologically similar to Ophelina curli sp. nov. and to Ophelina nematoides; see Remarks under Ophelina curli sp. nov. for details.
Ecology
Found in polymetallic nodule province of the eastern CCZ.
Etymology
Named in honor of Bob Juhazi, Oiler onboard RV Melville on the AB01 ABYSSLINE cruise in 2013.
Ophelina martinezarbizui sp. nov.
Material examined
NHM_681 (holotype) NHMUK ANEA 2019.7116, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/0de17415-a8bf-4461-a663-dea9a3e6a2b9; NHM_718 NHMUK ANEA 2019.7117, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/f6e2fa9b-a479-4e0d-aec6-57efff6987b2; NHM_883 NHMUK ANEA 2019.7118, coll. 20 Feb. 2015, 12°34.28N, 116°36.63W, 4198 m http://data.nhm.ac.uk/object/d9a3a3b3-c16e-4359-8eb0-f09deed98401; NHM_994 NHMUK ANEA 2019.7119, coll. 24 Feb. 2015, 12°08.02N, 117°17.52W, 4122 m http://data.nhm.ac.uk/object/4f6d2b7a-169f-46a9-8b3b-5d91a021aa34; NHM_1066 NHMUK ANEA 2019.7120, coll. 26 Feb. 2015, 12°06.93N, 117°09.87W, 4100 m http://data.nhm.ac.uk/object/972f9cb1-79d7-4296-a6d6-e04543c9105c; NHM_1766 (paratype) NHMUK ANEA 2019.7121, coll. 11 Mar. 2015, 12°10.43N, 117°11.57W, 4045 m http://data.nhm.ac.uk/object/dc754b1c-e66b-4a58-a93e-796ebfd32f6a; NHM_1870 NHMUK ANEA 2019.7122, coll. 13 Mar. 2015, 12°02.49N, 117°13.03W, 4094 m, http://data.nhm.ac.uk/object/b6247e8d-d155-4646-87d7-e5358ada5352; NHM_2088 (SEM specimen) NHMUK ANEA 2019.7123, coll. 20 Mar. 2015-03-20, 19°27.874N, 120°01.525W, 4026 m, http://data.nhm.ac.uk/object/7dd04f2c-435b-44b1-a85f-3b05dd3014d7; NHM_2092 NHMUK ANEA 2019.7124, coll. 20 Mar. 2015, 19°27.874N, 120°01.525W, 4026 m, http://data.nhm.ac.uk/object/1a095836-fa97-48b8-ad4c-07ed28356ecb; NHM_2102 NHMUK ANEA 2019.7125, coll. 20 Mar. 2015, 19°27.874N, 120°01.525W, 4026 m, http://data.nhm.ac.uk/object/93e91313-61a3-4cd7-8221-66bf20232f14; NHM_2116 (paratype) NHMUK ANEA 2019.7126, coll. 20 Mar. 2015, 19°27.874N, 120°01.525W, 4026 m, http://data.nhm.ac.uk/object/d439156e-657d-4dd5-8bb5-3531e150961e; NHM_2144 NHMUK ANEA 2019.7127, coll. 20 Mar. 2015, 19°27.874N, 120°01.525W, 4026 m, http://data.nhm.ac.uk/object/79767cab-eb56-4ef1-acd0-5067ec3736de; NHM_2149 NHMUK ANEA 2019.7128, coll. 20 Mar. 2015, 19°27.874N, 120°01.525W, 4026 m, http://data.nhm.ac.uk/object/1caa9eb3-3281-4ed6-8424-dfaebcf1e20b; NHM_2150 NHMUK ANEA 2019.7129, coll. 20 Mar. 2015, 19°27.874N, 120°01.525W, 4026 m, http://data.nhm.ac.uk/object/993f577c-ee86-4660-b2d9-af0146606f92.
Type locality
Pacific Ocean, CCZ, 12°32.23N, 116°36.25W, depth 4425 m, in mud between polymetallic nodules.
Description
This is a medium-sized species (8–14 mm long), represented by 14 specimens.
Body cylindrical, iridescent, some annulation detectable in first five to eight and last eight chaetigers, rest of body very smooth, no annulation detectable (Fig.
Ophelina martinezarbizui sp. nov. A Live images, whole specimen (center) with detail of anterior (left) and anal funnel (right) (holotype [specimen NHM_681], e = eggs, vc = enlarged ventral cirrus, c = cirri) B Lab image, whole specimen (holotype). C Lab image, detail of palpode (paratype NHM_2116) D Live image, prostomium, “royal crown” palpode (specimen NHM_2092) E Lab image, ventral posterior and branchiae (paratype NHM_1766, br = branchiae) F Lab image, lateral posterior and branchiae (paratype NHM_1766, br = branchiae) G Lab image, detail of anal funnel (paratype NHM_1766, vc = enlarged ventral cirri [folded over], c = cirri, br = branchiae H Lab image, detail of capillary chaetae (holotype). Morphological features in plates A, E, G have been outlined with a fine white or black line to improve clarity of those features. Scale bars: 1 mm (B, E, F); 100 μm (C, H).
Prostomium of all preserved specimens oval and broad (about as long as wide) and anteriorly bluntly rounded, somewhat truncated; bearing very distinct palpode, mostly short button-like sometimes distinctly bi-articulated with distal article oval in specimen NHM_2116 (Figs
Ophelina martinezarbizui sp. nov. (specimen NHM_2088). A Live image, whole specimen B SEM image, dorsal anterior C SEM image, lateral anterior D SEM image, dorsal posterior, br = branchiae) E SEM image, detail of mid-body lateral organ (lo = lateral organ). Scale bars: 300 μm (B, C, D); 40 μm (E).
Branchiae present, with disjointed distribution in anterior and posterior chaetigers only, absent in mid-body chaetigers. Six very small (easily overlooked) branchial pairs observed consistently in chaetigers 2–7, with those on chaetigers 3–5 slightly longest. The number of attached posterior branchial pairs observed varied from one to eight pairs, with the most complete set observed in NHM_883 and NHM_1766, where eight pairs present in chaetigers 24–31 (the last chaetiger); first posterior pair small (1/2 the length of the subsequent pairs), others very long and robust in NHM_883, but all branchiae large in NHM_1766. All branchiae cirriform (Figs
Parapodia distinct, biramous; well developed in anterior part of the body, then becoming smaller in subsequent chaetigers. Parapodia with short rounded dorsal cirrus present; provided with a tongue-shaped lobe bearing lateral organs (observable under SEM) (Fig.
Anal tube best preserved in specimen NHM_1766; anal tube relatively short (about the length of two posterior chaetigers) and thick distally asymmetrical with dorsal margin slightly longer than ventral one; distally with several short cirri, particularly on dorsal margin (Fig.
Reproductive information
Holotype ovigerous, with eggs of roughly 100 mm size clearly observed in mid through to posterior part of the body (Fig.
Morphological variation
This species is represented by the greatest number of specimens (n = 13) of Opheliidae species found in UKSR material. The features observed consistently are: the “royal crown”-like shape of prostomium (even in live specimens, Fig.
Genetic data
GenBank MN217444–MN217456 for 16S, MN217505 for 18S and MN217524–MN217531 for COI. This species is genetically identical or very similar to “Ophelina sp. 2” (
Remarks
This species superficially resembles Ammotrypanella species due to the presence of large branchiae in the posterior part of the body, but very small and easily overlooked branchiae are present in anterior chaetigers 2–7 in Ophelina martinezarbizui sp. nov. The presence of these very small branchiae easily distinguish this species from other Ophelina species encountered in UKSR-collected material, which are either abranchiate or branchiae are large (or at least easy to observe) in anterior chaetigers. Ophelina martinezarbizui sp. nov. represents a form with disjointed branchial distribution (see also comments under Ophelina sp. NHM_689 and NHM_1331), but it can be distinguished from these by the size of anterior branchiae, number of segments and form of anal funnel. Ophelina martinezarbizui sp. nov. also appears to have contrasting annulated and smooth body regions (Figs
Of the known Ophelina species, O. ammotrypanella Schüller, 2008 from the abyssal Southern Ocean shares the presence of small branchiae in anterior chaetigers and its “Ammotrypanella-like look” as the name suggests. However, in O. ammotrypanella the branchiae have a continuous distribution, being absent only in posterior quarter of the body.
Ecology
Found in polymetallic nodule province of the eastern CCZ. This species is represented by 13 sequenced specimens, with potentially another 28 specimens available in material that has not been sequenced yet, making it the most abundant opheliid species in the UKSR samples.
Etymology
Named in honor of Pedro Martinez Arbizu, member of the science party of the first ABYSSLINE cruise.
Ophelina meyerae sp. nov.
Material examined
NHM_1241(holotype) NHMUK ANEA 2019.7130, coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object/920d8670-507e-4126-a42b-6e208bbe66d3.
Type locality
Pacific Ocean, CCZ, 12°15.44N, 117°18.13W, depth 4302 m, in mud between polymetallic nodules.
Description
This is a medium-sized species represented by a single specimen. Body cylindrical, iridescent, some annulation detectable in first five and few posterior chaetigers, the rest of body smooth, no annulation detectable (Fig.
Ophelina meyerae sp. nov. holotype (specimen NHM_1241). A Live images, whole specimen with detail of anterior (p = palpode) B Lab image, whole specimen, lateral view (faded stain) C Lab image, anterior and branchiae (faded stain) (br = branchiae) D Lab image, mid-body branchiae (faded stain, br = branchiae) E Lab image, anterior parapodia (faded stain, pp = parapodia) F Lab image, detail of capillary chaetae (br = branchiae) G Lab image, detail of capillary chaetae (br = branchiae). Morphological features in plates C–F have been outlined with a fine white line to improve clarity of those features. Scale bars: 1 mm (B–E); 100 μm (F, G).
Prostomium of preserved specimen oval and broad (about as long as wide) and anteriorly bluntly rounded, somewhat truncated; bearing very distinct oval palpode (Fig.
Branchiae present in all chaetigers, except for first chaetiger; branchiae remain attached in most chaetigers, including ch. 29, but are occasionally missing (lost) in some chaetigers. Branchiae easy to detect, although rather slender, best observed in anterior chaetigers (Fig.
Parapodia distinct, biramous; with a broad lobe in chaetigers 2–10, becoming smaller in subsequent chaetigers; parapodia embedded in distinct lateral grooves (Fig.
Anal tube well preserved; relatively short (about the length of two posterior chaetigers) and thick; distally symmetrical; distal opening with circlet of about 20 short, slender cirri with the exception of ventral part of the margin, which is smooth; ventral cirrus not observed (Fig.
Genetic data
Remarks
Similar to Ophelina martinezarbizui sp. nov. in overall look and form of anal tube, but slender branchiae are present in all chaetigers, midbody and posterior branchiae are smaller than those of the anterior region.
Ecology
Found in polymetallic nodule province of the eastern CCZ.
Etymology
Named in honor of Kirstin Meyer-Kaiser, member of the science party onboard RV Thomas G. Thompson on the AB02 ABYSSLINE cruise in 2015.
Ophelina nunnallyi sp. nov.
Material examined
NHM_683 (holotype) NHMUK ANEA 2019.7133, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/220fa671-4576-45b7-930d-efde148f223f; NHM_700 (paratype) NHMUK ANEA 2019.7134, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/63115f48-bcf1-4b3b-9c2e-c339b97845bd; NHM_783F NHMUK ANEA 2019.7135, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/376a42db-0497-4b4a-851b-c1d5e07bd2b6; NHM_1273 (paratype) NHMUK ANEA 2019.7136, coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object/a3540563-8a0c-475b-96b5-12969fb8c2ba; NHM_1309A (SEM specimen) NHMUK ANEA 2019.7137, coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object/25066e63-ecc9-439a-9907-eaeaeb72e78c.
Type locality
Pacific Ocean, CCZ, 12°32.23N, 116°36.25W, depth 4425 m, in mud between polymetallic nodules.
Description
This species is represented by five complete specimens, none in an excellent condition, anal tube damaged and not clearly observed. However, images of live specimens are available to observe some now missing or damaged features such as anal tube.
Small to medium-sized species 9–14 mm long and 0.25–0.8 mm wide, for 33 chaetigers. Body cylindrical and smooth without distinct annulation. Preserved specimen yellow semi-translucent, iridescent in ethanol (Fig.
Ophelina nunnallyi sp. nov. A Lab image, whole specimen (paratype NHM_700) B Live images, whole specimens (holotype [specimen NHM_683] [left], paratype NHM_700 [right]) C SEM image, anterior and palpode (specimen NHM_1309A, m = mouth) D Lab image, detail of palpode, (paratype NHM_1273) E Lab image, anterior and branchiae (paratype NHM_1273, br = branchiae) F Lab image, parapodia (holotype, pp = parapodia) G Lab image, capillary chaeta (holotype) H Live image, anal funnel (holotype, c = cirri) I Lab image, detail of anal funnel (holotype, c = cirri). Morphological features in plates E, F, H, I have been outlined with a fine white line to improve clarity of those features. Scale bars: 1 mm (A, E, F); 200 μm (C, D); 100 μm (G, I).
Prostomium of preserved specimen conical (longer than wide), anteriorly pointed and extending into very long, thick palpode (Fig.
Branchiae observed in anterior chaetigers only, some missing (broken off), present from chaetigers 4–9 (Fig.
Parapodia biramous, embedded in lateral grooves; observed as distinct conical lobes throughout the body (Fig.
Anal tube attached (in holotype, NHM_783F and NHM_1309A), but now poorly preserved and variably damaged, always separated from the rest of the body by a shallow constriction (Fig.
Additional morphological observations
In addition to the material examined here, several more specimens consistent with this species have been found, but no DNA sequencing has been carried out on these and thus they are not included in this manuscript. Where the anal tube was observed, it is scoop-shaped, but the preservation of cirri is variable. In some specimens, short slender cirri can be detected on the lateral margins of the anal tube. Chaetiger counts consistent with 33 chaetigers. Branchiae were consistently observed on chaetigers 4–9. However, in the absence of DNA data we are reluctant to ascribe these specimens formally to O. nunnallyi sp. nov. until further analyses has been done.
Genetic data
GenBank MN217458-MN217462 for 16S, MN217507 for 18S and MN217532–MN217534 for COI. This species is genetically identical to or very similar to “Ophelina sp. 1” (
Remarks
Other than sp. NHM_1068 (see Remarks under sp. NHM_1068), the DNA suggest similarity of Ophelina nunnallyi sp. nov. to O. acuminata, originally described from the shallow coast of Denmark, but frequently reported in all oceans (see references in
The first occurrence of branchiae from chaetiger 4 is very unusual in Ophelina, where branchiae appear from chaetiger 2. Branchiae are fragile and easily lost structures; therefore, we cannot exclude a possibility that branchiae prior to chaetiger 4 are present but lost in our specimens. Nevertheless, this distribution has been observed in all material examined (including additional specimens, no DNA available) as well as in very similar species Ophelina sp. (NHM_1068).
Ecology
Found in polymetallic nodule province of the eastern CCZ.
Etymology
Named in honor of Clifton Nunnally, member of the science party of both the ABYSSLINE cruises.
Ophelina cf. abranchiata
General comments on Ophelina abranchiata and similar morphotypes
Three small, abranchiate morphospecies found in the UKSR material, Ophelina ganae sp. nov., O. cf. abranchiata NHM_1769 and O. cf. abranchiata NHM_2017, are very similar to Ophelina abranchiata Støp-Bowitz, 1948. This species has its type locality as East Greenland, 200 m depth, but has subsequently been reported worldwide, from predominantly deep waters [see references in
Such confused taxonomic history is further complicated by the fact that published (
The anal tube, an important feature upon which opheliid species have been differentiated in the past is mostly missing in these morphotypes even where hundreds of specimens are available (Neal pers. obs.). Where the anal tube has been observed (
Clearly, additional morphological characters are needed to distinguish small abranchiate species currently lumped under O. abranchiata, P. translucens and O. farallonensis.
Ophelina cf. abranchiata
Material examined
NHM_1769 NHMUK ANEA 2019.7148, coll. 11 Mar. 2015, 12°10.43N, 117°11.57W, 4045 m http://data.nhm.ac.uk/object/8a2cbe4f-277d-4355-a34f-0b53c797bef0.
Description
This species is represented by a single specimen, in reasonable condition, but anal tube is missing. Small species, 5.3 mm long and 0.3 mm wide; the exact number of chaetigers is difficult to establish, but at least 16 counted, although 17 may be present.
Body cylindrical and smooth without distinct annulation (Fig.
Ophelina cf. abranchiata sp. NHM_1769 (specimen NHM_1769). A Live image, whole specimen B Lab image, whole specimen (very faded post-stain,) C Lab image, detail of prostomium and palpode D Lab image, detail of chaetae E Lab image, whole specimen (stained). Scale bars: 1 mm (B, E); 50 μm (C, D).
Prostomium elongate, conical with small acute terminal palpode (Fig.
Chaetae all slender, smooth capillaries (Fig.
Branchiae absent. Anal tube not observed. Shirlastained specimens without distinct pattern (Fig.
Genetic data
Remarks
Please refer to section “General comments on Ophelina abranchiata and similar morphotypes” above and remarks for Ophelina ganae sp. nov.
Ophelina cf. abranchiata
Material examined
NHM_2017 NHMUK ANEA 2019.7149, coll. 16 Mar. 2015, 12°03.03N, 117°24.28W, 4235 m http://data.nhm.ac.uk/object/9ebcd947-c53b-4616-81d4-da42afaeca03.
Description
This species is represented by a single specimen, in reasonable condition, but anal tube is missing. Small species, 4 mm long and 0.35 mm wide; exact number of chaetigers difficult to establish, but at least 17 counted, although 18 may be present.
Body cylindrical and smooth without distinct annulation (Fig.
Ophelina cf. abranchiata sp. NHM_2017 (specimen NHM_2017). A Live image, whole specimen B Lab image, whole specimen (stained,) C Lab image, whole specimen (faded stain) D Lab image, anterior (stained, arrow highlighting dark banding) E Lab image, detail of anterior F Lab image, detail of capillary chaetae. Scale bars: 1 mm (B, C); 100 μm (E, F).
Prostomium elongate, conical with small acute terminal palpode (Fig.
Chaetae all slender, smooth capillaries (Fig.
Branchiae absent. Anal tube not observed. Shirlastained specimens with wide, dark red, strongly stained stripe on the dorsum (Fig.
Genetic data
GenBank MN217434 for 16S. In our phylogenetic analyses, Ophelina cf. abranchiata sp. (NHM_2017) is part of a well-supported clade including Ophelina ganae sp. nov., Ophelina cf. abranchiata (NHM_1769) and at least two other abranchiate opheliid species (Fig.
Remarks
Please refer to section “General comments on Ophelina abranchiata and similar morphotypes” above and remarks for Ophelina ganae sp. nov.
Ophelina
Material examined
NHM_689 NHMUK ANEA 2019.7114, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/6755d584-a20a-4ce5-a4f1-32ce0965128e.
Description
This species is represented by a single specimen in poor condition, with anal tube and most of branchiae missing. Posteriorly incomplete specimen 4.7 mm long and 0.35 mm wide for at least 22 chaetigers (exact number of chaetigers is difficult to count in places). Body cylindrical and smooth without distinct annulation (Fig.
Ophelina sp. NHM_689 (specimen NHM_689). A Lab image, whole specimen, lateral view (pre-stain) B Lab image, whole specimen, ventral view (faded stain, vg = ventral groove) C Live image, whole specimen D Lab image, damaged anterior (stained, br = branchiae, pr = prostomium) E Lab image, detail of anterior branchiae (br = branchiae) F Lab image, posterior branchiae (stained, br = branchiae) G Lab image, mid-body parapodia (stained, pp = parapodia) H Lab image, detail of capillary chaetae. Morphological features in plates B, D–G have been outlined with a fine white line to improve clarity of those features. Scale bars: 1 mm (A, B); 0.5 mm (D); 50 μm (E); 0.25 (F, H).
Prostomium of preserved specimen conical, broad (only slightly longer than wide), anteriorly bluntly rounded (but prostomium appears damaged) (Fig.
Branchiae present, but many are likely missing. Branchiae observed in chaetigers 2–4 (Fig.
Parapodia distinct, biramous; embedded in lateral grooves (Fig.
Genetic data
Remarks
Due to the condition of the single specimen representing this morphospecies, important diagnostic characters such as the structure of the anal tube and distribution of the branchiae cannot be determined. See Remarks under Ophelina sp. (NHM_1331) for more details.
Ophelina
Material examined
NHM_1068 NHMUK ANEA 2019.7138, coll. 26 Feb. 2015, 12°06.93N, 117°09.87W, 4100 m http://data.nhm.ac.uk/object/b28fd52f-5717-45e3-b0cc-369172a690e5; NHM_1874 NHMUK ANEA 2019.7139, coll. 13 Mar. 2015, 12°02.49N, 117°13.03W, 4094 m, http://data.nhm.ac.uk/object/c3ffe5f4-6ca3-4816-966c-25ec98bbb003.
Description
This species is represented by two specimens, both in poor condition; specimen NHM_1874 posteriorly incomplete, specimen NHM_1068 mostly complete, but anal tube damaged. Large species 25–30 mm long and 0.8 mm wide, for minimum of 30 chaetigers (exact number of chaetigers cannot be established). Body cylindrical and smooth without distinct annulation (Fig.
Ophelina sp. NHM_1068. A Lab image, whole specimen (specimen NHM_1068,) B Lab image, whole specimen (specimen NHM_1874, faded stain) C Live image, whole specimen (specimen NHM_1874) D Live image, anterior, with branchiae outlined in a fine black line (specimen NHM_1068, br = branchiae) E Lab image, detail of palpode (specimen NHM_1068) F Lab image, detail of anterior parapodia and branchiae (specimen NHM_1874, stained) G Lab image, posterior (specimen NHM_1068) H Lab image, detail of anal funnel (specimen NHM_1068). Scale bars: 1 mm (A, B); 100 μm (E).
Prostomium of preserved specimen conical (longer than wide), anteriorly pointed and extending into very large and long thick palpode (Fig.
Parapodia biramous, embedded in lateral grooves; parapodia small conical lobes, no distinct pre- or postchaetal lobes observed (Fig.
Anal tube missing in specimen NHM_1874; damaged in NHM_1068, but probably scooped-shaped (Fig.
Genetic data
Remarks
According to our molecular results, this species forms a clade with Ophelina nunnallyi sp. nov., which is sister to O. acuminata (Fig.
Ophelina
Material examined
NHM_1331 NHMUK ANEA 2019.7115, coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object/06d48d7f-7339-4cc5-8445-b51a980e4e0f.
Description
This species is represented by a single complete specimen in relatively good condition. Specimen about 4.5 mm long and 0.5 mm wide for about 28 chaetigers. Body cylindrical and smooth with some annulation detectable (Fig.
Ophelina sp. NHM_1331 (specimen NHM_1331). A Lab image, whole specimen, dorsal view (faded stain) B Lab image, whole specimen, lateral view (faded stain) C Live image, whole specimen D Lab image, anterior (faded stain, br = branchiae, no = nuchal organ) E Lab image, detail of anterior (br = branchiae, no = nuchal organ) F Lab image, posterior, anal funnel (br = branchiae) G Lab image, detail of posterior and anal funnel (br = branchiae). Morphological features in plates B, D–G have been outlined with a fine white line to improve clarity of those features. Scale bars: 1 mm(A, B); 100 μm (E, G).
Prostomium of preserved specimen conical, broad (only slightly longer than wide) and anteriorly bluntly rounded; palpode not observed (Fig.
Branchiae present; with disjointed distribution, with three pairs on chaetigers 2–4 (Fig.
Parapodia distinct, biramous; embedded in lateral grooves on chaetigers 1–24; no distinct pre- or postchaetal lobes. Chaetae are capillaries only; all very long but longest on chaetiger 1 where they are nearly twice the length of chaetae of subsequent chaetigers.
Anal tube attached, but not well preserved; cylindrical; appears distally asymmetrical with dorsal lobe overlapping the ventral lobe (but this may be an artefact of poor preservation) (Fig.
Genetic data
GenBank MN217465 for 16S and MN217510 for 18S. COI was unsuccessful for this specimen, no identical GenBank matches for 16S or 18S. In our phylogenetic tree, Ophelina sp. (NHM_1331) is sister to “Ophelina sp. F14588” forming a clade with the taxa Ophelina cylindricaudata from the Atlantic (New England) and Ophelina sp. (NHM_689) (Fig.
Phylogenetic analysis of Opheliidae. 50% majority rule tree from the Bayesian analyses using 18S and 16S, with posterior probability values on nodes. Twenty-nine taxa from GenBank were included, and Capitellidae and Echiura was chosen as outgroup following the annelid phylogeny of
Remarks
Morphologically, Ophelina sp. (NHM_1331) is similar to Ophelina sp. (NHM_689) in having a broad prostomium and very long chaetae on chaetiger 1. Their branchiae appear to be arranged in a similar pattern (three pairs are present in chaetigers 2–5 and then few pairs present in posterior chaetigers). Nuchal organs are not everted in O. sp. (NHM_689). They may differ in number of chaetigers, although this is difficult to establish due to damage of O. sp. (NHM_689), anal tube has not been observed in O. sp. (NHM_689) (assumed missing) and cannot be compared.
Of known species of Ophelina, O. sp. (NHM_1331) is similar to a group with 28 chaetigers and four posterior parapodia crowded: O. cylindricaudata, O. breviata (Ehlers, 1913) and O. brattegardi
The lack of branchiae in midbody has been described in some of these species, but for O. cylindricaudata this has been clarified as a mistake in the original description.
Scalibregmatidae
Notes
The family Scalibregmatidae was established by
The characters used to differentiate genera are the prostomial shape, presence and development of branchiae, presence of spines in anterior notopodia (and sometimes also in neuropodia), presence and development of branchiae and development of dorsal and ventral cirri, particularly in posterior part of the body (e.g.
Although Scalibregmatidae range from the intertidal to the deep sea, most species occur below 1000 m (
The diagnosis of Oligobregma presented here is amended from that given by Blake (2017), mainly to take into account a more posterior appearance of furcate chaetae, which Blake (2017) considered to appear prior to chaetigers 2–4.
Diagnosis
Body elongate and arenicoliform. Prostomium T-shaped with two prominent frontal horns. Eyes present or absent, nuchal organs present. Peristomium achaetous, surrounding prostomium dorsally and forming upper and lower lips of mouth ventrally. Branchiae absent. Parapodia well developed, with dorsal and ventral cirri on posterior chaetigers; interramal papillae present or absent. Large acicular spines present on anterior chaetigers. Capillaries present in all parapodia; lyrate chaeta present. Some species with short, slender, blunt or pointed spinous chaetae anterior to capillaries of chaetigers 1, 2 or 3, representing homologues of lyrate chaetae. Pygidium with anal cirri.
Oligobregma brasierae sp. nov.
Material examined
NHM_032 NHMUK ANEA 2019.7150, coll. 09 Oct. 2013, 13°50.232N, 116°33.506W, 4336 m http://data.nhm.ac.uk/object/43545746-b8ad-43a8-92b7-53637dd131d6; NHM_404 NHMUK ANEA 2019.7151, coll. 20 Oct. 2013, 13°51.797N, 116°32.931W, 4050 m http://data.nhm.ac.uk/object/5fda0cac-0a77-4ec7-a2fa-5cd529548a19; NHM_684 (paratype) NHMUK ANEA 2019.7152, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/d84c37ed-138e-4064-a11d-a11a2470dfdf; NHM_823 (holotype) NHMUK ANEA 2019.7153, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/74781dbb-1f65-4839-a766-24d6cde63ed0; NHM_1423 (paratype) NHMUK ANEA 2019.7154, coll. 03 Mar. 2015, 12°27.26N, 116°36.77W, 4137 m http://data.nhm.ac.uk/object/d949e987-6e03-4092-8492-c51dd7fcf4d7; NHM_1895 NHMUK ANEA 2019.7155, coll. 13 Mar. 2015, 12°02.49N, 117°13.03W, 4094 m, http://data.nhm.ac.uk/object/02aaa9c0-837a-4836-8b34-5e68296c958e.
Type locality
Pacific Ocean, CCZ, 12°32.23N, 116°36.25W, depth 4425 m, in mud between polymetallic nodules.
Description
Small species, represented by six specimens. Holotype posteriorly incomplete, but otherwise in good condition, 9 mm long and 1 mm wide at the widest point for 24 chaetigers; paratypes complete, 6.0–6.5 mm long and 0.5–0.7 mm wide for 26 chaetigers. Body most expanded (inflated) through chaetigers 5–9, thereafter narrowing to posterior end. Colour in alcohol creamy white, without body pigment (Fig.
Oligobregma brasierae sp. nov. A Lab image, whole specimen (holotype [specimen NHM_823], pre-staining) B Lab image, dorsal anterior (holotype, faded stain, h = prostomial horns, pe = peristomial ring) C Lab image, dorsal segments, quadriannulate chaetigers (holotype, faded stain) D Lab image, ventral segments (holotype, faded stain, vm = ventral midline) E Lab image, ventral anterior, (holotype, pre-staining) F Lab image, mid-body parapodia (holotype, shirlastained, dc = dorsal cirrus, vc = ventral cirrus, ip = interramal papilla) G Lab image, detail of dorsal cirrus (paratype NHM_684, ig = internal gland) H Lab image, detail of hirstute notopodial spines on chaetiger 1 (paratype NHM_684, ig = internal gland) I Lab image, detail of capillary and lyrate chaetae (paratype NHM_684) J Lab images, posterior (paratype NHM_684, [bottom-left panel], ac = anal cirri, ig = internal gland). Morphological features in plates B–D, F, G, H have been outlined with a fine white or black line to improve clarity of those features. Scale bars: 1 mm (A, E); 50 μm (G); 25 μm (H, I); 100 μm (J).
Oligobregma brasierae sp. nov. A Live image, whole specimen, ventral view (holotype [specimen NHM_823]) B Live image, whole specimen, dorsal view (paratype NHM_684) C Live image, dorsal anterior, with prostomial features outlined in a fine white line(paratype NHM_684, h = prostomial horns, pe = peristomial ring).
Anterior body segments smooth, no obvious annulation of raised pads detected (even after staining) (Fig.
Prostomium broadly rounded anteriorly, weakly expanded laterally, narrowing posteriorly; with two short, rounded lobes (horns) emerging anterolaterally from anterior prostomial margin (Figs
Parapodia biramous; inconspicuous in chaetigers 1–7, becoming longer posteriorly and prominent from around chaetiger 14. Tiny dorsal cirri detectable from chaetigers 14 in holotype, whereas ventral cirri occur from chaetiger 15 where well developed; both cirri large on subsequent segments; conical with broad base (Fig.
Curved acicular spines present in notopodia and neuropodia on chaetigers 1–4 (Fig.
Single achaetigerous ring subsequent to the last chaetiger. Pygidium missing in holotype, but observed in paratypes; broad, triannulated, distally broadly rounded lobe; with few terminal, short anal cirri still attached in paratype NHM_684 (Fig.
Morphological variation: Some variability was noticed between different sized specimens. In the slightly bigger holotype (NHM_823) the spines can be observed on chaetigers 1–4 in both rami, and the dorsal cirri can be detected from chaetiger 14. In the smaller paratype (NHM_684), the spines cannot be unambiguously confirmed in ch. 4, particularly in neuropodia and dorsal cirrus can be detected from chaetiger 13.
Genetic data
GenBank MN217422-MN217427 for 16S, MN217498 for 18S and MN217517 for COI. This species is genetically identical or very similar to sequences published in
Remarks
Currently, there are nine valid species assigned to the genus Oligobregma (
More specifically, Oligobregma simplex Kudenov & Blake, 1978, O. lonchochaeta Detinova, 1985 and O. quadrispinosa Schüller & Hilbig, 2007 share the presence of spines in chaetigers 1–4 with O. brasieri sp. nov., as well as having relatively large posterior dorsal and ventral cirri. Oligobregma simplex is a shallow water species (Western Port, Victoria, Australia, 11 m) and, while similar in size (5 mm long), it has a greater number of chaetigers (43 versus 26 in UKSR species) and more posterior appearance of dorsal and ventral cirri (on ch. 20–22 versus ch. 13–15). Oligobregma lonchochaeta has been described from a single, incomplete specimen from the abyssal North Atlantic, but its description is brief, not including the observation on the appearance of dorsal and ventral cirri, and there are no DNA data.
Ecology
Found in polymetallic nodule province of the eastern CCZ.
Etymology
Named in honor of Madeleine Brasier, member of the science party of the ABYSSLINE AB02 cruise onboard the RV Thomas G. Thompson.
Oligobregma tani sp. nov.
Material examined
NHM_773A (paratype) NHMUK ANEA 2019.7156, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/4b673a6a-9090-4c24-a4eb-231190507b60; NHM_1454 (holotype) NHMUK ANEA 2019.7157, coll. 03 Mar. 2015, 12°27.26N, 116°36.77W, 4137 m http://data.nhm.ac.uk/object/67d3f58a-9c13-423e-93b7-3ddcf98a361e; NHM_1480J NHMUK ANEA 2019.7158, coll. 03 Mar. 2015, 12°27.26N, 116°36.77W, 4137 m http://data.nhm.ac.uk/object/d47f17aa-c0c1-44f0-a448-d3f3c395fc47; NHM_1665 (paratype) NHMUK ANEA 2019.7159, coll. 10 Mar. 2015, 12°21.81N, 116°40.86W, 4233 m http://data.nhm.ac.uk/object/eca166ae-3fe0-4367-860f-08c7410165dd.
Type locality
Pacific Ocean, CCZ, 12°27.26N, 116°36.77W, depth 4137 m, in mud between polymetallic nodules.
Description
Small species, represented by four posteriorly incomplete specimens, 4–4.5 mm long and 0.4–0.7 mm wide. Holotype posteriorly incomplete, but otherwise in good condition, 4.5 mm long and 0.7 mm wide at the widest point for 18 chaetigers long fragment. Colour in alcohol creamy white, without body pigment (Fig.
Oligobregma tani sp. nov. holotype (specimen NHM_1454). A Lab image, whole specimen (pre-stain,) B Live image, whole specimen C Lab image, dorsal anterior, (stained, h = prostomial horns, pe = peristomial ring) D Lab image, lateral anterior, (stained, h = prostomial horns, pe = peristomial ring) E Lab image, ventral anterior, (stained, vm = ventral midline) F Lab image, mid-body parapodia (faded stain, dc = dorsal cirrus, vc = ventral cirrus, ip = interramal papilla) G Lab image, detail of dorsal cirrus, with no internal gland visible (dc = dorsal cirrus) H Lab image, detail of hirsute spines on notopodia on chaetiger 1 ([left panel]) I Lab image, detail of capillary and lyrate chaetae on chaetiger 12 J Lab image, detail lyrate chaetae. Morphological features in plates F, G have been outlined with a fine white line to improve clarity of those features. Scale bars: 0.5 mm (A, C, E); 50 μm (G–I).
Prostomium broad (wider than long), nearly oval; with two very prominent, distinctly rounded lobes (horns) emerging from anterior prostomial margin (Fig.
Parapodia biramous; inconspicuous in chaetigers 1–14, becoming conical and prominent from around chaetiger 15. Tiny dorsal cirri detectable from chaetiger 13 in holotype, whereas ventral cirri occur from chaetiger 15; both cirri best developed from chaetigers 16 and 17, remaining small and conical (less than 1/2 the size of corresponding podial lobes) (Fig.
Curved acicular spines present in notopodia only on chaetigers 1‒4 (Fig.
Genetic data
GenBank MN217428–MN217431 for 16S, MN217499 for 18S and MN217518–MN217519 for COI. This species is genetically very similar to one sequence published in
Remarks
The UKSR-collected species is most similar to Oligobregma quadrispinosa described from abyssal Southern Ocean (
Comparison of Oligobregma species with spines in chaetigers 1–4, including the new UKSR-collected species. Information collected from the literature.
| Distribution of spines in chaetigers 1–4 | Annulation of chaetigers | Appearance of podial cirri | No. of chaetigers | Presence of furcate chaetae | |
|---|---|---|---|---|---|
| O. simplex | In both rami | 1–2 uni-; 3–4 bi-; 5–12 or 15 quadriannulate | Chaetigers 20–22 | 43 (complete) | From chaetiger 6 |
| O. lonchochaeta | In both rami | 1–4 triannulate | In posterior chaetigers, detail not given | 22+ (incomplete) | In mid and posterior chaetigers |
| O. quadrispinosa | In notopodia only | Anterior quadriannulate; posterior with 5 annuli | Chaetigers 13–14 | 28 (complete) | From chaetiger 5 |
| O. brasierae sp. nov. | In both rami, spines hirsute | Anterior smooth, posterior quadriannulate | Chaetigers 13–15 | 26 (complete) | From chaetiger 5 |
| O. tani sp. nov. | In notopodia only, spines hirsute, transitional in ch. 4 | Not observed | Chaetigers 13–15 | 18 (incomplete) | From chaetiger 5 |
| O. whaleyi sp. nov. | In both rami | Anterior smooth, midbody quadriannulate | Chaetiger 14 | 26 (incomplete) | First observed from chaetiger 11 |
Ecology
Found in polymetallic nodule province of the eastern CCZ.
Etymology
Named in honor of Koh Siang Tan, member of the science party of the ABYSSLINE AB02 cruise onboard the RV Thomas G. Thompson.
Oligobregma whaleyi sp. nov.
Material examined
NHM_822 (holotype) NHMUK ANEA 2019.7160, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/dde1c8f9-f87a-430b-be9d-5e34685772bb.
Type locality
Pacific Ocean, CCZ, 12°32.23N, 116°36.25W, depth 4425 m, in mud between polymetallic nodules.
Description
Large species, represented by a single, posteriorly incomplete specimen, with 26 chaetigers, 16 mm long and about 2 mm wide at widest (inflated) region (first eight chaetigers, particularly chaetigers 3–8), with another widening of the body in chaetigers 23–26, likely due to sediment ingestion. Colour in alcohol creamy white, without body pigment, live specimens semi-translucent (Fig.
Oligobregma whaleyi sp. nov. holotype (specimen NHM_822). A Lab (upper) and live (lower) images, whole specimens [lab image] B Lab image, mid-body segments and annulation C Lab image, ventral midbody (vm = ventral midline) D Live (left) and lab (right) images of prostomium (pe = peristomial ring, h = prostomial horns) E Lab images, midbody and posterior parapodia, chaetigers 15 (left) and 24 (right) (dc = dorsal cirrus, vc = ventral cirrus, ip = interramal papilla) F Lab image, detail of dorsal cirrus and internal gland (dc = dorsal cirrus, ig = internal gland) G Lab image, detail of notopodial spines on chaetiger 1 H Lab image, detail of capillary chaetae (lc = lyrate chaetae) I Lab image, detail of lyrate chaetae. Morphological features in plates B–D, F, G, H have been outlined with a fine white or black line to improve clarity of those features. Scale bars: 1 mm (A); 100 μm (F–H); 50 μm (I).
Prostomium broadly rounded anteriorly, weakly expanded laterally, narrowing posteriorly; with two well-developed, anterior rounded lobes (horns) emerging from anterior prostomial margin (Fig.
Parapodia biramous; conspicuous even in anterior-most segments (Fig.
Curved acicular spines present in notopodia and neuropodia on chaetigers 1‒4. Notopodia with about 15 spines arranged in irregular row, accompanied posteriorly by single row of capillaries; neuropodial spines fewer in numbers arranged irregularly. Spines slightly curved, narrowing to slender elongated tip (Fig.
Genetic data
Remarks
The UKSR-collected species O. whaleyi sp. nov. differs from other Oligobregma species bearing spines on the first four chaetigers in having a peristomial ring forming a figure-of-8 loops laterally to prostomium and in furcate chaetae appearing more posteriorly (first observed on chaetiger 11 although due to its large size the specimen was difficult to manipulate and removal of several parapodia would have damaged the single specimen significantly), while in other species the furcate chaetae are present from chaetiger 6. In O. lonchochaeta
Ecology
Found in polymetallic nodule province of the eastern CCZ.
Etymology
Named in honor of Jeremy Whaley, Able Seaman onboard RV Melville on the ABYSSLINE cruise AB01 in 2013.
Scalibregmatidae
Material examined
NHM_2308 NHMUK ANEA 2019.7161, coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object/7b9d4ab8-4b7b-45c4-9cf4-6fd6b1229f48.
Description
This species is represented by a single, small, posteriorly incomplete specimen, 2.5 mm long and 0.4 mm wide for about 12 chaetigers, in poor condition. Colour of preserved specimen creamy yellow (Fig.
Scalibregmatidae sp. NHM_2308 (specimen NHM_2308). A Lab image, whole specimen, dorsal view (pre-stain) B Lab image, dorsal anterior (stained) C Lab image, ventral anterior, (shirla stained, vm = ventral midline) D Lab image, detail of hirsute spines on chaetigers 1 and 2 E Lab image, detail of hirsute spine tips F Lab image, detail of lyrate chaeta. Scale bars: 1 mm (A–C); 100 μm (D); 50 μm (E); 10 μm (F).
Prostomium small, broadly rounded anteriorly, weakly expanded laterally, narrowing posteriorly; with two short, rounded lobes (horns) emerging anterolaterally from anterior prostomial margin (Fig.
Heavily curved acicular spines present in notopodia only on chaetigers 1 and 2 (Fig.
Parapodia biramous, podial lobes not developed in 12 chaetigers long fragment. Dorsal and ventral cirri not observed in 12 chaetiger long fragment. Rest of the body unknown.
Genetic data
GenBank MN217467 for 16S. Scalibregmatidae (NHM_2308) does not cluster convincingly with any other Scalibregmatidae species available on GenBank (Fig.
Remarks
Poor preservation of mid body and missing posterior part prevents reliable identification to genus level. Observations from the anterior part (the absence of branchiae and presence of acicular spines) suggest that this may be yet another representative of genus Oligobregma in the UKSR-collected material. It can be distinguished from other scalibregmatid species in this study by having spines in notopodia of chaetigers 1 and 2 only.
Travisiidae Hartmann-Schröder, 1971
Travisia
Notes
These distinctive, grub-like polychaetes with rugose epidermis were first described by
The higher taxonomic position of Travisia has been in dispute for some time. While usually placed in Opheliidae, its relationship with Scalibregmatidae has also been long suggested (
Travisia species have predominantly deep-water distribution (
Travisia zieglerae sp. nov.
Material examined
NHM_140 (paratype) NHMUK ANEA 2019.7162, coll. 11 Oct. 2013, 13°45.50N, 116°41.91W, 4080 m http://data.nhm.ac.uk/object/ed10356b-32a0-4b45-9fe3-c56fbc696e87; NHM_188 NHMUK ANEA 2019.7170, coll. 14 Oct. 2013, 13°57.43N, 116°30.10W, 4130 m http://data.nhm.ac.uk/object/c8a0ef70-e7f7-4605-bf78-dc54ed9151eb; NHM_241 NHMUK ANEA 2019.7163, coll. 16 Oct. 2013, 13°48.70N, 116°42.60W, 4076 m http://data.nhm.ac.uk/object/5c0ac0b7-60cc-473e-a23b-2f49a40540f4; NHM_356 NHMUK ANEA 2019.7164, coll. 17 Oct. 2013, 13°45.21N, 116°29.12W, 4128 m http://data.nhm.ac.uk/object/8d2cbf0e-6522-403d-a58a-905fb13c70d6; NHM_364 NHMUK ANEA 2019.7165, coll. 19 Oct. 2013, 13°55.98N, 116°42.977W, 4182 m http://data.nhm.ac.uk/object/ef6e520f-7ef5-4ff9-87b5-985b8576271f; NHM_748B (paratype) NHMUK ANEA 2019.7166, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/db527676-1030-4bf0-b28d-2382825bc6bf; NHM_753 NHMUK ANEA 2019.7167, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/393203b1-cb80-4185-9e40-fca6e1b6fe34; NHM_760 NHMUK ANEA 2019.7168, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/d3e8ec3c-d7f3-4908-b315-84f3758aecc1; NHM_792 NHMUK ANEA 2019.7169, coll. 20 Feb. 2015, 12°32.23N, 116°36.25W, 4425 m http://data.nhm.ac.uk/object/5d30a61b-5894-484f-b79a-df1cd4268ec1; NHM_909 (paratype) NHMUK ANEA 2019.7171, coll. 23 Feb. 2015, 12°34.28N, 116°36.63W, 4198 m http://data.nhm.ac.uk/object/5f570dab-4b56-4f74-b126-ed6ceab344e3; NHM_970 NHMUK ANEA 2019.7172, coll. 23 Feb. 2015, 12°34.28N, 116°36.63W, 4198 m http://data.nhm.ac.uk/object/4ccb364c-35f4-458c-9c71-6f77e71493ca; NHM_1097 NHMUK ANEA 2019.7173, coll. 26 Feb. 2015, 12°06.93N, 117°09.87W, 4100 m, http://data.nhm.ac.uk/object/939ba16d-b844-49ca-a740-bb42f039cc11; NHM_1310 NHMUK ANEA 2019.71745,coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object/16844478-de27-448c-9acb-057835026447; NHM_1311 NHMUK ANEA 2019.7175, coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object/192cbbb3-680b-4bcd-9cc4-a420f42af578; NHM_1431 (holotype) NHMUK ANEA 2019.7176, coll. 03 Mar. 2015, 12°27.26N, 116°36.77W, 4137 m http://data.nhm.ac.uk/object/fd6bab0e-0cda-4b42-808f-a6006d409535; NHM_1543 (paratype) NHMUK ANEA 2019.7177, coll. 06 Mar. 2015, 12°30.38N, 116°29.07W, 4244 m http://data.nhm.ac.uk/object/c78cc5fd-ca98-43b0-a0fb-8804fb606c71; NHM_1873 NHMUK ANEA 2019.7178, coll. 13 Mar. 2015, 12°02.496N, 117°13.03W, 4094m, http://data.nhm.ac.uk/object/24409a12-2a50-4689-80dc-902cdeb5af69; NHM_1883 NHMUK ANEA 2019.7179, coll. 13 Mar. 2015, 12°02.49N, 117°13.03W, 4094 m, http://data.nhm.ac.uk/object/9e8c22f7-a94b-45ed-a1d0-cae287a7ac2d; NHM_1911 NHMUK ANEA 2019.7180, coll. 13 Mar. 2015 12°02.49N, 117°13.03W, 4094 m http://data.nhm.ac.uk/object/489dd5a6-2c68-416b-9a06-ed773d4791d6; NHM_2019 NHMUK ANEA 2019.7181, coll. 16 Mar. 2015, 12°03.03N, 117°24.28W, 4235 m http://data.nhm.ac.uk/object/2684a5f8-b4d4-4bcb-b386-65775506cf87; NHM_2024 NHMUK ANEA 2019.7182, coll. 16 Mar. 2015, 12°03.03N, 117°24.28W, 4235 m http://data.nhm.ac.uk/object/cf54f81e-5836-4684-94dc-151f589ebab4.
Type locality
Pacific Ocean, CCZ, 12°27.26’N 116°36.77’W, depth 4137 m, in mud between polymetallic nodules.
Additional material examined
Travisia glandulosa McIntosh, 1879, holotype BMNH 1921.5.1.2431 and specimen of
Description
This species is represented by 21 specimens. It is a small species 1.2–7.5 mm long and 0.25–0.8 mm wide for 21–24 segments, 19 or 20 of which chaetigerous and 2–4 posterior-most achaetigerous. Preserved specimens pale yellow (Fig.
Travisia zieglerae sp. nov. A Lab image, whole specimen, pre-stain (holotype [specimen NHM_1431]) B Live images, whole specimens (specimen NHM_1911 [left], specimen NHM_188 [right]) C Lab image, lateral anterior, (holotype, stained, pr = prostomium, pp = parapodial lappets) D Lab image, distal anterior, (holotype, stained, m = mouth) E Lab image, lateral posterior, (holotype, stained, pp = parapodia, io = interramal organs) F Detail of capillary chaeta (paratype NHM_140) G Lab image, pygidium, distal view (left) and lateral view (right), with pygidial features outlined in a fine white line (holotype, stained, vl = ventral lobe). Scale bar: 1 mm (A).
Holotype in good condition, 6 mm long and 0.8 mm wide (at the widest point). Body robust, compact, grub like, anteriorly (commonly on chaetigers 1–7) somewhat enlarged then tapering posteriorly and relatively slender. Body surface rugose, with transverse rows of small squarish lobes.
Prostomium short, smooth, conical (Fig.
Branchiae absent. Parapodia biramous, located on row with largest lobes, both rami well separated (Fig.
Pygidium short, thick (only slightly longer wide), ventrally with keel-like very thick lobe. In distal view (Fig.
Shirlastain pattern
Prostomium stains strongly and stain is retained even after one week. Interramal sense organs observed as darkly red stained spots (Fig.
Morphological variation
Number of segments is slightly variably and appears to be linked to size, with the smallest specimens possessing 21 segments (19 of which chaetigerous), while the largest specimen possessed 24 segments (20 of which chaetigerous). Body shape remains mainly consistent, although some specimens were slightly thinner or thicker. Thick, keel-like ventral lobe on pygidium observed consistently, but the detection of slenderer lobes differs (probably an artefact of preservation) and some occasionally appear inflated (Fig.
Remarks
Differences between the known Travisia species and the species delineated herein are discussed in the Remarks section for Travisia sp. (NHM_1244), see below.
Genetic data
GenBank MN217470–MN217490 for 16S and MN217512 for 18S. Travisia zieglerae sp. nov. fall within a clade consisting of the other Travisia species in this study as well as other Travisia species on GenBank and the taxon Neolipobranchus sp., a result similar to Martinez et al. (2014), suggesting a paraphyletic genus Travisia (Fig.
Ecology
Found in polymetallic nodule province of the eastern CCZ.
Etymology
Named in honor of Amanda Ziegler, member of the science party of the ABYSSLINE AB02 cruise onboard the RV Thomas G. Thompson.
Travisia
Material examined
NHM_1244 NHMUK ANEA 2019.7183, coll. 01 Mar. 2015, 12°15.44N, 117°18.13W, 4302 m http://data.nhm.ac.uk/object/f6906eae-67ec-4d37-83c6-590f3c53df76; NHM_1863 NHMUK ANEA 2019.7184, coll. 13 Mar. 2015, 12°02.49N, 117°13.03W, 4094 m, http://data.nhm.ac.uk/object/fa708aca-6dd1-4b53-8d54-c76a93f43363.
Description
This species is represented by two specimens only. It is a small species 5.5–6 mm long and 0.7 mm wide for 24 segments, 20 of which chaetigerous and four posterior-most achaetigerous. Preserved specimens pale yellow (Fig.
Travisia sp. NHM_1244. A Lab images, whole specimens (specimen NHM_1244, unstained [top], specimen NHM_1863, faded stain [bottom]) B Live image, whole specimen (specimen NHM_1863) C Lab image, dorsal anterior (specimen NHM_1863, stained, pr = prostomium) D Lab image, ventral anterior, (stained) (specimen NHM_1863, m = mouth) E Lab image, lateral posterior (specimen NHM_1863, stained, pp = parapodia, io = interramal organs) F Lab image, detail of capillary chaetae (specimen NHM_1244) G Lab image, pygidium, distal view (lower left) and lateral view (upper right), with pygidial features outlined in a fine white line (specimen NHM_1863, stained, vl = ventral lobe). Scale bars: 1 mm (A); 50 μm (F).
Prostomium short, smooth, conical (Fig.
Branchiae absent. Parapodia biramous, located on row with largest lobes, both rami well separated (Fig.
Pygidium very short, thick (slightly longer wide); in distal view with a tightly packed circlet of around 11 lobes (Fig.
Shirlastain pattern
Stain retained uniformly. Interramal sense organs observed as darkly red stained spots (Fig.
Genetic data
Remarks
Both UKSR-collected species are morphologically very similar, in having a similar number of segments and in being abranchiate. They can be distinguished by a suite of subtle characters, which in case of Travisia sp. NHM_1224 is represented by only two specimens, so caution is needed. The two species differ somewhat in body shape as Travisia zieglerae sp. nov. is more slender in the posterior half, while Travisia sp. NHM_1224 is thicker (Fig.
Comparison between Travisia sp. NHM_1244 and Travisia zieglerae sp. nov., and holotypes of Travisia glandulosa (BMNH 1921.5.1.2431) and Travisia gravieri (BMNH 1921.5.1.2429). A Lab images, whole specimens (Travisia sp. NHM_1244 specimen NHM_1863 [left], Travisia zieglerae sp. nov. holotype [specimen NHM_1431] [right], stained,) B Lab images, comparison of prostomia (Travisia sp. NHM_1244 specimen NHM_1863 [left], Travisia zieglerae sp. nov. holotype [right], stained) C Lab images, comparison of pygidia (Travisia sp. NHM_1244, specimen NHM_1863 [left], Travisia zieglerae sp. nov. holotype [right], stained) D Travisia glandulosa holotype, with detail of pygidium E Travisia gravieri holotype, with detail of pygidium. Morphological features in plates C–E have been outlined with a very fine white line to improve clarity of those features. Scale bars: 1 mm (A, D, E).
Of the known species of Travisia, only five were described as completely abranchiate, with four of these currently valid: T. glandulosa McIntosh, 1879; T. gravieri McIntosh, 1908; T. nigrocincta Ehlers, 1913 and T. fusus (Chamberlin, 1919) with T. abyssorum (Monro, 1930) considered a subjective synonym of T. glandulosa. Type specimens of McIntosh (1879;
T. fusus: has a larger body size of 14 mm and 28 chaetigers. Pygidium is divided into 12–14 inconspicuous lobes. Type locality: Pacific Ocean, towards the Marquesas Islands, 0°60’N, 137°54’W, 4504 m.
T. glandulosa (Fig.
T. gravieri (Fig.
T. nigrocincta: much larger species, up to 34 mm long and 6 mm wide for 25 segments (the smallest specimen reported by Ehlers was 6 mm long and about 2 mm wide for 17 chaetigers), with dark transverse bands; pygidium not described in detail. Type locality: Southern Ocean, Wilhelm II Coast, 2725 m.
Phylogenetic analysis of Scalibregmatidae and Travisiidae. 50% majority rule tree from the Bayesian analyses using 18S and 16S, with posterior probability values on nodes. Twenty taxa from GenBank were included, and Opheliidae was chosen as outgroup following the annelid phylogeny of
Discussion
We have added 23 annelid species and 85 records to the total available knowledge of the benthic macrofauna of the CCZ. While this is certainly less than 10% of the estimated annelid diversity (based on the around 350 DNA-delineated species that are present in the UKSR collections), it represents a substantial increase in the published taxonomic knowledge linked to accessible voucher material, online genetic data and imagery of morphological features. Several of the taxa we report on are likely to be common and may have wide distributions across at least the eastern CCZ.
In terms of comparison to other studies, there are few sequences from just a few benthic faunal groups from the CCZ available on GenBank, for example echinoderms (Glover et al. 2016), cnidarians (
Acknowledgements
The UKSR ABYSSLINE (ABYSSal baseLINE) environmental surveys were supported by a collaborative partnership between six non-profit global academic research institutes (University of Hawaii at Manoa, Natural History Museum, NORCE Norwegian Research Centre, National Oceanography Centre, Senckenberg Institute) and through an arrangement with UKSRL. Additional support was provided by the Swedish research council FORMAS (TGD). We acknowledge Magdalena Georgieva and Madeleine Brasier from the Natural History Museum team and Swee Cheng Lim from National University of Singapore for support with sampling on board ship, and Chief Scientist Craig R. Smith for organizing. We also acknowledge the expert support from the Senckenberg Institute team in the deployment and recovery of successful Brenke Epibenthic Sledge samples. Comments from the editor and one reviewer greatly improved this manuscript. This study was made possible only by the dedicated help from the entire scientific party, the Masters and Crew of the RV Melville during the first cruise of the ABYSSLINE project in October 2013 and the Masters and Crew of the RV Thomas G. Thompson during the second ABYSSLINE cruise in February and March 2015. Thanks are also due to Emma Sherlock, Senior Curator of Annelida collection, Jackie Mackenzie-Dodds at the Molecular Collection Facility and Harry Rousham, Consultancy Manager, all at Natural History Museum.
References
- Amon DJ, Ziegler AF, Drazen JC, Grischenko AV, Leitner AB, Lindsay DJ, Voight JR, Wicksten MK, Young CM, Smith CR (2017) Megafauna of the UKSRL exploration contract area and eastern Clarion-Clipperton Zone in the Pacific Ocean: Annelida, Arthropoda, Bryozoa, Chordata, Ctenophora, Mollusca. Biodiversity Data Journal (5): e14598. https://doi.org/10.3897/BDJ.5.e14598
- Ashworth JH (1902) The anatomy of Scalibregma inflatum Rathke. Quarterly Journal of Microscopical Science, London 45: 237–309.
- Baker E, Beaudoin Y (2013) Deep Sea Minerals: Manganese Nodules, a physical, biological, environmental, and technical review. Vol. 1B. Secretariat of the Pacific Community. ISBN: 978-82-7701-119-6
- Barroso R, Paiva PC (2013) Deep sea Ophelina (Polychaeta: Opheliidae) from southern Brazil. Marine Biodiversity Records 6: e51. https://doi.org/10.1017/S1755267213000201
- Bely AE, Wray GA (2004) Molecular phylogeny of naidid worms (Annelida: Clitellata) based on cytochrome oxidase I. Molecular Phylogenetics and Evolution 30(1): 50–63. https://doi.org/10.1016/S1055-7903(03)00180-5
- Blake JA (1981) The Scalibregmatidae (Annelida: Polychaeta) from South America and Antarctica collected chiefly during the cruises of the R/V Anton Bruun, R.V. Hero and USNS Eltanin. Proceedings of the Biological Society of Washington 94(4): 1131–1162.
- Blake JA (2000a) Family Capitellidae Grube, 1862. Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and the Western Santa Barbara Channel. Santa Barbara Museum of Natural History, Santa Barbara, California 7: 47–96.
- Blake JA (2000b) Family Opheliidae Malmgren, 1867. Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and the Western Santa Barbara Channel. Santa Barbara Museum of Natural History, Santa Barbara, California 7: 145–168.
- Blake JA (2000c) Family Scalibregmatidae Malmgren 1867. Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and the Western Santa Barbara Channel. Santa Barbara Museum of Natural History, Santa Barbara, California 7: 129–144.
- Blake J (2009) Redescription of Capitella capitata (Fabricius) from West Greenland and designation of a neotype (Polychaeta, Capitellidae). Zoosymposia 2(1): 55–80.
- Blake JA (2015) New species of Scalibregmatidae (Annelida, Polychaeta) from the East Antarctic Peninsula including a description of the ecology and post-larval development of species of Scalibregma and Oligobregma. Zootaxa 4033: 57–93. https://doi.org/10.11646/zootaxa.4033.1.3
- Blake JA (2016) Kirkegaardia (Polychaeta, Cirratulidae), new name for Monticellina Laubier, preoccupied in the Rhabdocoela, together with new records and descriptions of eight previously known and sixteen new species from the Atlantic, Pacific, and Southern Oceans. Zootaxa 4166(1): 1–93. http://doi.org/10.11646/zootaxa.4166.1.1
- Blake JA, Maciolek NJ (2016) Travisiidae Hartmann-Schröder, 1971, new family status. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology. De Gruyter, Berlin.
- Bonifácio P, Menot L (2018) New genera and species from the Equatorial Pacific provide phylogenetic insights into deep-sea Polynoidae (Annelida). Zoological Journal of the Linnean Society. [Version of Record, published online 14 November 2018] https://doi.org/10.1093/zoolinnean/zly063
- Carr CM, Hardy SM, Brown TM, Macdonald TA, Hebert PD (2011) A tri-oceanic perspective: DNA barcoding reveals geographic structure and cryptic diversity in Canadian polychaetes. PLoS ONE 6: e22232. https://doi.org/10.1371/journal.pone.0022232
- Chamberlin R (1919) The Annelida Polychaeta. Memories of the Museum of Comparative Zoology at Harvard College 48: 1–514.
- Cohen BL, Gawthrop A, Cavalier-Smith T (1998) Molecular phylogeny of brachiopods and phoronids based on nuclear-encoded small subunit ribosomal RNA gene sequences. Philosophical Transactions of the Royal Society of London B: Biological Sciences 353: 2039–2061. https://doi.org/10.1098/rstb.1998.0351
- Dahlgren TG, Wiklund H, Rabone M, Amon DJ, Ikebe C, Watling L, Smith CR, Glover AG (2016) Abyssal fauna of the UK-1 polymetallic nodule exploration area, Clarion-Clipperton Zone, central Pacific Ocean: Cnidaria. Biodiversity Data Journal: e9277. https://doi.org/10.3897/BDJ.4.e9277
- Dauvin J-C, Bellan G (1994) Systematics, ecology and biogeographical relationships in the sub-family Travisiinae (Polychaeta, Opheliidae). Mémoires du Muséum national d'histoire naturelle 162: 169–184.
- Detinova NN (1985) Многощетинковые черви хребта Рейкъянес (Северная Атлантика) [Polychaetous worms from the Reykjanes Ridge (the North Atlantic). Bottom Fauna from Mid-Ocean Rises in the North Atlantic. Donnayafauna Otkryto. Okeanicheskikh. Podnyatij. Severnaya. Atlantika]. Trudy Instituta Okeanologii im. P.P. Shirshova 120: 96–136. [in Russian]
- Donoghue MJ (1985) A critique of the biological species concept and recommendations for a phylogenetic alternative. Bryologist 88(3): 172–181. https://doi.org/10.2307/3243026
- Droege G, Barker K, Astrin JJ, Bartels P, Butler C, Cantrill D, Cottington J, Forest F, Gemeinholzer B, Hobern D, Mackenzie-Dodds J, Ó Tuama É, Petersen G, Sanjur O, Schindel D, Seberg O (2014) The Global Genome Biodiversity Network (GGBN) Data Portal, Nucleic Acids Research 42: D607–D612. https://doi.org/10.1093/nar/gkt928
- Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. https://doi.org/10.1093/nar/gkh340
- Eisig H (1887) Monographie der Capitelliden des Golfes von Neapel und der angrenzenden meeres-abschnitte nebst untersuchungen zur vergleichenden anatomie und physiologie. Fauna und Flora des Golfes von Neapel und der angrenzenden Meeres-Abschnitte 16, 906 pp. [37 pls.] https://doi.org/10.5962/bhl.title.11542
- Ehlers E (1887) Florida-Anneliden No. 31. Memoirs of the Museum of Comparative Zoology (Harvard College) 15: 1–335.
- Ehlers E (1913) Die Polychaeten-Sammlungen der deutschen Südpolar-Expedition, 1901–1903. Deutsche Südpolar-Expedition 1901–1903 im Auftrage des Reichsamtes des innern herausgegeben von Erich von Drygalski Leiter Expedition 13(4): 397–598. [pls XXVI–XLVI]
- Ewing RM (1982) A partial revision of the genus Notomastus (Polychaeta: Capitellidae) with a description of a new species from the Gulf of Mexico. Proceedings of the Biological Society of Washington 95: 232–237.
- Ewing RM (1984) Capitellidae, Vol. 2. In: Uebelacker JM, Johnson PG (Eds) Taxonomic Guide to the Polychaetes of the Northern Gulf of Mexico. Final Report to the Minerals Management Service, contract. Barry A. Vinor and Associates Inc., Mobile, Alabama, 14.1–14.47
- Ewing RM (1991) Review of monospecific genera of the family Capitellidae (Polychaeta) (abstract). In: Petersen ME, Kirkegaard JB (Eds) Systematics, Biology, and Morphology of World Polychaeta. Proceedings of the 2nd International Polychaete Conference (Copenhagen), August 1986. Ophelia Supplement, Denmark, 692–693.
- Fauchald K (1972) Benthic polychaetous annelids from deep water off western Mexico and adjacent areas in the eastern Pacific Ocean. Allan Hancock Monographs in Marine Biology 7: 1–575.
- Fauchald K (1977) The polychaete worms. Definitions and keys to the orders, families and genera. Natural History Museum of Los Angeles Country, Science Series 28: 1–190.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3(5): 294–299.
- Gallardo VA (1968) Polychaeta from the Bay of Nha Trang, South Viet Nam. Naga Report 4(3): 35–279.
- Glover AG, Smith CR, Paterson GLJ, Wilson GDF, Hawkins L, Sheader M (2002) Polychaete species diversity in the central Pacific abyss: local and regional patterns, and relationships with productivity. Marine Ecology Progress Series 240: 157–170. https://doi.org/10.3354/meps240157
- Glover AG, Dahlgren TG, Wiklund H, Mohrbeck I, Smith CR (2016a) An end-to-end DNA taxonomy methodology for benthic biodiversity survey in the Clarion-Clipperton Zone, Central Pacific Abyss. Journal of Marine Science and Engineering 4: 2. https://doi.org/10.3390/jmse4010002
- Glover AG, Wiklund H, Rabone M, Amon DJ, Smith CR, O’Hara T, Mah CL, Dahlgren TG (2016b) Abyssal fauna of the UK-1 polymetallic nodule exploration claim, Clarion-Clipperton Zone, central Pacific Ocean: Echinodermata. Biodiversity data journal: e7251. https://doi.org/10.3897/BDJ.4.e7251
- Glover AG, Wiklund H, Chen C, Dahlgren TG (2018) Managing a sustainable deep-sea ‘blue economy’ requires knowledge of what actually lives there. eLife 7: e41319. https://doi.org/10.7554/eLife.41319
- Gollner S, Kaiser S, Menzel L, Jones DO, Brown A, Mestre NC, Van Oevelen D, Menot L, Colaço A, Canals M, Cuvelier D (2017) Resilience of benthic deep-sea fauna to mining activities. Marine Environmental Research 129: 76–101. https://doi.org/10.1016/j.marenvres.2017.04.010
- Green KD (2002) Capitellidae (Polychaeta) from the Andaman Sea. Phuket Marine Biological Center Special Publication 24: 249–343.
- Grube AE (1862) Noch ein Wort über die Capitellen und ihre Stelle im Systeme der Anneliden. Archiv für Naturgeschichte, Berlin 28(1): 366–378.
- Hansen GA (1879) Annelider fra den norske Nordhavsexpedition i 1876. Nyt Magazin for Naturvidenskaberne, Christiania 24(1): 1–17.
- Hartman O (1960) Systematic account of some marine invertebrate animals from the deep basins off southern California. Allan Hancock Pacific Expeditions 22(2): 69–216.
- Hartman O (1965) Deep-water benthic polychaetous annelids off New England to Bermuda and other North Atlantic areas. Occasional Papers of the Allan Hancock Foundation 28: 1–384.
- Hartman O, Fauchald K (1971) Deep-water benthic polychaetous annelids off New England to Bermuda and other North Atlantic Areas. Part II. Allan Hancock Monographs in Marine Biology 6: 1–327.
- Hartmann-Schröder G (1971) Annelida, borstenwurmer, polychaeta. Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise 58: 1–594. https://doi.org/10.1086/407180
- Hein JR, Mizell K, Koschinsky A, Conrad TA (2013) Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: comparison with land-based resources. Ore Geology Reviews 51: 1–4. https://doi.org/10.1016/j.oregeorev.2012.12.001
- Hessler RR, Jumars PA (1974) Abyssal community analysis from replicate cores in the central North Pacific. Deep Sea Research and Oceanographic Abstracts 21: 185–209. https://doi.org/10.1016/0011-7471(74)90058-8
- Hutchings PA (1974) Polychaeta of Wallis Lake, New South Wales. Proceedings of the Linnean Society of New South Wales 98(4): 175–195.
- Janssen A, Kaiser S, Meissner K, Brenke N, Menot L, Martínez Arbizu P (2015) A reverse taxonomic approach to assess macrofaunal distribution patterns in abyssal Pacific polymetallic nodule fields. PLoS ONE 10: e0117790. https://doi.org/10.1371/journal.pone.0117790
- Johnston G (1840) Miscellanea Zoologica. British Annelids. Annals and Magazine for Natural History London 1(4): 368–375. https://doi.org/10.1080/00222934009512507
- Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. https://doi.org/10.1093/nar/gkf436
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
- Kinberg JGH (1867) Annulata nova. Öfversigt af Kungliga Vetenskaps Akademiens Förhandlingar 23: 97–103.
- Kongsrud JA, Bakken T, Oug E (2011) Deep-water species of the genus Ophelina (Annelida, Opheliidae) in the Nordic Seas, with the description of Ophelina brattegardi sp. nov. Italian Journal of Zoology 78: 95–111. https://doi.org/10.1080/11250003.2011.606658
- Kudenov JD, Blake JA (1978) A review of the genera and species of the Scalibregmidae (Polychaeta) with descriptions of one new genus and three new species from Australia. Journal of Natural History 12: 427–444. https://doi.org/10.1080/00222937800770291
- Law CJ, Dorgan KM, Rouse GW (2014) Relating divergence in polychaete musculature to different burrowing behaviors: A study using Opheliidae (Annelida). Journal of Morphology 42: 548–571. https://doi.org/10.1002/jmor.20237
- Levenstein RY (1975) The polychaetous annelids of the deep-sea trenches of the Atlantic sector of the Antarctic Ocean. Transactions of the P.P. Shirov Institute of Oceanology Academy. Trudy Instituta Okeanologia, Akademia nauk SSSR 103: 119–142. [in Russian]
- Lim SC, Wiklund H, Glover AG, Dahlgren TG, Tan KS (2017) A new genus and species of abyssal sponge commonly encrusting polymetallic nodules in the Clarion-Clipperton Zone, East Pacific Ocean. Systematics and Biodiversity 15(6): 507–519. https://doi.org/10.1080/14772000.2017.1358218
- McIntosh WC (1878) On the Annelida obtained during the Cruise of H.M.S. ‘Valorous’ to Davis Strait in 1875. Transactions of the Linnean Society of London. Second Series: Zoology 1(7): 499–511. [pl. LXV]
- McIntosh WC (1908) Notes from the Gatty Marine Laboratory, St. Andrews. Annals and Magazine of Natural History 1(8): 373–387. https://doi.org/10.1080/00222930808692422
- Maciolek NJ, Blake JA (2006) Opheliidae (Polychaeta) collected by the R/V Hero and the USNS Eltanin cruises from the Southern Ocean and South America. Scientia Marina 70: 101–113. https://doi.org/10.3989/scimar.2006.70s3101
- Malmgren AJ (1867) Annulata Polychaeta: Spetsbergiae, Groenlandiae, Islandiae et Scandinaviae. Hactenus cognita. Öfversigt af Kungliga Vetenskaps Akademiens Förhandlingar 24: 127–255. https://doi.org/10.5962/bhl.title.13358
- Martínez A, Di Domenico M, Worsaae K (2014) Gain of palps within a lineage of ancestrally burrowing annelids (Scalibregmatidae). Acta Zoologica 95: 421–429. https://doi.org/10.1111/azo.12039
- Medlin L, Elwood H, Stickel S, Sogin M (1988) The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71: 491–499. https://doi.org/10.1016/0378-1119(88)90066-2
- Monro CCA (1930) Polychaete worms. Discovery Reports 2: 1–222.
- Neal L, Hardy SM, Smith CR, Glover AG (2011) Polychaete species diversity on the West Antarctic Peninsula deep continental shelf. Marine Ecology Progress Series 428: 119–134. https://doi.org/10.3354/meps09012
- Neal L, Taboada S, Woodall LC (2018) Slope-shelf faunal link and unreported diversity off Nova Scotia: evidence from polychaete data. Deep Sea Research Part I: Oceanographic Research Papers 138: 72–84. https://doi.org/10.1016/j.dsr.2018.07.003
- Nygren A, Sundberg P (2003) Phylogeny and evolution of reproductive modes in Autolytinae (Syllidae, Annelida). Molecular Phylogenetics and Evolution 29: 235–249. https://doi.org/10.1016/s1055-7903(03)00095-2
- OBIS (2018) Global biodiversity indices from the Ocean Biogeographic Information System. http://www.iobis.org [Accessed on: 2018-11-13]
- Ørsted AS (1843) Annulatorum danicorum conspectus. Volume Fasc. 1 Maricolae (Quaestio ab universitate Hafniensi ad solvendum proposita et proemio ornata). Librariae Wahlianae, Hafniae, 52 pp. https://doi.org/10.5962/bhl.title.11849
- Paul C, Halanych KM, Tiedemann R, Bleidorn C (2010) Molecules reject an opheliid affinity for Travisia (Annelida). Systematics and Biodiversity 8(4): 507–512. https://doi.org/10.1080/14772000.2010.517810
- Palumbi SR (1996) Nucleic acid. II. The polymerase chain reaction. In: Hillis DM, Moritz G, Mable BK (Eds) Molecular Сystematics. Sinauer Associates, Sunderland, 205–247.
- Parapar J, Moreira J (2008) Sobre la presencia del género Ophelina Ørsted, 1843 (Polychaeta, Opheliidae) en el litoral de la península Ibérica. NACC: Nova Acta Científica Compostelana. Bioloxía 117–134.
- Parapar J, Moreira J, Helgason GV (2011) Distribution and diversity of the Opheliidae (Annelida, Polychaeta) on the continental shelf and slope of Iceland, with a review of the genus Ophelina in northeast Atlantic waters and description of two new species. Organisms Diversity and Evolution 11: 83. https://doi.org/10.1007/s13127-011-0046-2
- Paterson GLJ, Glover AG, Froján CRSB, Whitaker A, Budaeva N, Chimonides J, Doner S (2009) A census of abyssal polychaetes. Deep-Sea Research Part II 56: 1739–1746. https://doi.org/10.1016/j.dsr2.2009.05.018
- Paterson GLJ, Neal L, Altamira I, Soto EH, Smith CR, Menot L, Billett DSM, Cunha MR, Marchais-Laguionie C, Glover AG (2016) New Prionospio and Aurospio species from the deep sea (Annelida: Polychaeta). Zootaxa 4092(1): 1–32. http://doi.org/10.11646/zootaxa.4092.1.1
- Persson J, Pleijel F (2005) On the phylogenetic relationships of Axiokebuita, Travisia and Scalibregmatidae (Polychaeta). Zootaxa 998: 1–14. https://doi.org/10.11646/zootaxa.998.1.1
- Purschke G, Hausen H (2007) Lateral organs in sedentary polychaetes (Annelida) – Ultrastructure and phylogenetic significance of an insufficiently known sense organ. Acta Zoologica 88: 23–39. https://doi.org/10.1111/j.1463-6395.2007.00247.x
- Rathke H (1843) Beiträge zur Fauna Norwegens. Nova Acta Academiae Caesareae Leopoldino-Carolinae Naturae Curiosorum 20: 1–264. https://doi.org/10.5962/bhl.title.120119
- Read G, Fauchald K (2018a) World Polychaeta database. Scalibregmatidae Malmgren, 1867. World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=925 [Accessed on: 2018-5-9]
- Read G, Fauchald K (2018b) World Polychaeta database. Oligobregma Kudenov & Blake, 1978. World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=324770 [Accessed on: 2018-5-10]
- Read G (2018c) World Polychaeta database. Opheliidae Malmgren, 1867. World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=924 [Accessed on: 2018-5-10]
- Read G, Fauchald K (2019) World Polychaeta database. Ophelina Örsted, 1843. World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=129414 [Accessed on: 2019-2-19]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
- Sardá R, Gil J, Taboada S, Gili JM (2009) Polychaete species captured in sediment traps moored in northwestern Mediterranean submarine canyons. Zoological Journal of the Linnean Society 155: 1–21. https://doi.org/10.1111/j.1096-3642.2008.00442.x
- Sars M (1851) Beretning om en i Sommeren 1849 foretagen zoologisk Reise i Lofoten og Finmarken. Nyt Magazin for Naturvidenskaberne 6: 121–211.
- Schüller M (2008) New polychaete species collected during the expeditions ANDEEP I, II, and III to the deep Atlantic sector of the Southern Ocean in the austral summers 2002 and 2005 – Ampharetidae, Opheliidae, and Scalibregmatidae. Zootaxa 1705: 51–68. https://doi.org/10.11646/zootaxa.1705.1.4
- Schüller M, Hilbig B (2007) Three new species of the genus Oligobregma (Polychaeta, Scalibregmatidae) from the Scotia and Weddell Seas (Antarctica). Zootaxa 1391: 35–45. https://doi.org/10.11646/zootaxa.1391.1.2
- Sene-Silva G (2007) Filogenia de Opheliidae (Annelida: Polychaeta). Unpublished Thesis presented for the degree, Doctor of Sciences, in Zoology, Universidade Federal do Paraná, Curitiba, Brazil, xii + 95 pp.
- Sjölin E, Erséus C, Källersjö M (2005) Phylogeny of Tubificidae (Annelida, Clitellata) based on mitochondrial and nuclear sequence data. Molecular Phylogenetics and Evolution 35: 431–441. https://doi.org/10.1016/j.ympev.2004.12.018
- Smith CR, Dahlgren TG, Drazen J, Gooday A, Glover AG, Kurras G, Martinez-Arbizu P, Shulse C, Spickermann R, Sweetman AK, Vetter E (2013) Abyssal Baseline Study (ABYSSLINE) Cruise Report. Seafloor Investigations Report 2013-1304-051J-SRDL-AB01.
- Smith CR, Church M, Chow J, Dahlgren TG, Drazen J, Glover AG, Gooday A, Kaylan B, Lui B, Kurras G, Martinez-Arbizu P, Sweetman AK, Tan KS, Vetter E (2015) Abyssal Baseline Study (ABYSSLINE) Cruise Report. Seafloor Investigations Report 2015-1408-061J-SRDL-AB02.
- Støp-Bowitz C (1945) Les ophéliens norvégiens. Meddelelser fra det Zoologiske Museum, Oslo 52: 21–61.
- Støp-Bowitz C (1948) Sur les polychètes arctiques des famillies des glycériens des ophéliens, des scalibregmiens et des flabelligériens. Tromso Museums Årshefter 66(2): 1–58.
- Tomassetti P, Porrello S (2005) Polychaetes as indicators of marine fish farm organic enrichment. Aquaculture International 13(1–2): 109–128. https://doi.org/10.1007/s10499-004-9026-2
- Tzetlin A, Zhadan A (2009) Morphological variation of axial non-muscular proboscis types in the Polychaeta. Zoosymposia 2: 415–427.
- Weigert A, Bleidorn C (2016) Current status of annelid phylogeny. Organisms, Diversity and Evolution 16(2) 345–362. https://doi.org/10.1007/s13127-016-0265-7
- Wiklund H, Taylor JD, Dahlgren TG, Todt C, Ikebe C, Rabone M, Glover AG (2017) Abyssal fauna of the UK-1 polymetallic nodule exploration area, Clarion-Clipperton Zone, central Pacific Ocean: Mollusca. ZooKeys 707: 1–46. https://doi.org/10.3897/zookeys.707.13042
- Wirén A (1901) Ueber die waehrend der schwedischen arktischen Expedition von 1898 und 1900 eingesammelten Anneliden. Zoologischer Anzeiger 24: 253.