Research Article |
|
Corresponding author: Siqin Ge ( gesq@ioz.ac.cn ) Corresponding author: Xingke Yang ( yangxk@ioz.ac.cn ) Academic editor: Duane D. McKenna
© 2020 Yongying Ruan, Alexander S. Konstantinov, Guanya Shi, Yi Tao, You Li, Andrew J. Johnson, Xiaozhu Luo, Xinying Zhang, Mengna Zhang, Jianing Wu, Wenzhu Li, Siqin Ge, Xingke Yang.
This is an open access article distributed under the terms of the CC0 Public Domain Dedication.
Citation:
Ruan Y, Konstantinov AS, Shi G, Tao Y, Li Y, Johnson AJ, Luo X, Zhang X, Zhang M, Wu J, Li W, Ge S, Yang X (2020) The jumping mechanism of flea beetles (Coleoptera, Chrysomelidae, Alticini), its application to bionics and preliminary design for a robotic jumping leg. ZooKeys 915: 87-105. https://doi.org/10.3897/zookeys.915.38348
|
Abstract
Flea beetles (Coleoptera, Chrysomelidae, Galerucinae, Alticini) are a hyperdiverse group of organisms with approximately 9900 species worldwide. In addition to walking as most insects do, nearly all the species of flea beetles have an ability to jump and this ability is commonly understood as one of the key adaptations responsible for its diversity. Our investigation of flea beetle jumping is based on high-speed filming, micro-CT scans and 3D reconstructions, and provides a mechanical description of the jump. We reveal that the flea beetle jumping mechanism is a catapult in nature and is enabled by a small structure in the hind femur called an ‘elastic plate’ which powers the explosive jump and protects other structures from potential injury. The explosive catapult jump of flea beetles involves a unique ‘high-efficiency mechanism’ and ‘positive feedback mechanism’. As this catapult mechanism could inspire the design of bionic jumping limbs, we provide a preliminary design for a robotic jumping leg, which could be a resource for the bionics industry.
Keywords
bionics, catapult, functional morphology, jump, kinematics, metafemoral spring, robotics
Introduction
There are as many as 380,500 described species of recent beetles (Insecta, Coleoptera) in the world (
Apart from jumping beetles, some other arthropods are also well known for having rapid-moving appendages, for example: jumping legs in fleas (
The extraordinary jumping ability of flea beetles mainly depends on the metafemoral spring (
In order to gain comprehensive insights into the mechanics behind the flea beetle jump, we conducted micro-CT scans, 3D reconstructions, high-speed filming and dissection of the metafemur. As a result, the ‘elastic plate’ and its function in the hind legs of flea beetles is revealed; a comprehensive theory of the mechanism involved in flea beetle jumping is given. In addition, and based on our findings, we provide a design diagram for a robotic jumping leg.
Material and methods
Micro-CT scanning analysis
Absolute ethanol-preserved specimens of flea beetles were selected. The meta-femurs were carefully removed and dried at the critical point (hcp-2, Hitachi Inc., Tokyo, Japan), and then glued to the tip of a micropipette using nail polish. Hind legs of seven species [Altica cirsicola Ohno, Clavicornaltica sp., Hespera lomasa Maulik, Nonarthra sp., Asiophrida xanthospilota Baly, Podontia lutea (Olivier, 1790), Psylliodes sp.] were scanned with a MicroXCT-400 scanner (Xradia Inc., California, USA. Beam strength: 60 kV, absorption contrast). Pixel size of images: 0.5~5μm; optical magnification: 4–40× (depending on different specimen size). In most scans, 900–1100 sections of images were obtained, then imported to Amira 5.4.1 (Visage Imaging, San Diego, California, USA) for 3D reconstructions. Autodesk maya 2014 (Autodesk Inc., San Rafael, California, USA) was used to smooth and render the 3D structures.
Hind legs of flea beetles across 13 genera were further dissected and examined (list of species dissected: Agasicles hygrophila Selmen et Vogt, Altica cirsicola, Chaetocnema constricta Ruan, Konstantinov et Yang, Clavicornaltica sp., Hemipyxis sp., Hespera lomasa, Luperomorpha xanthodera (Fairmaire), Nonarthra sp., Asiophrida xanthospilota, Podontia lutea, Psylliodes sp., Stenoluperus sp., Trachytetra obscura (Jacoby)). These genera and species were chosen to represent flea beetles with different body sizes. A conventional optical imaging system consisting of a Zeiss Axiostar plus microscope (Zeiss Inc., Göttingen, Germany), Nikon D300 digital camera (Nikon Inc., Tokyo, Japan) and Helicon Focus 6 software was used to capture and compose 2D images. The figure plates were prepared with Photoshop CS5 (Adobe, San Jose, USA) and Illustrator CS5 (Adobe, San Jose, USA).
The general morphological terminology used throughout this report follows
High-speed filming
Four species of flea beetles (Chaetocnema picipes Stephens, Altica cirsicola, Asiophrida xanthospilota, Psylliodes punctifrons Baly) were collected in the field in Beijing, China from July to October 2015 for high-speed filming. During the study, the flea beetles were reared in the laboratory in plastic containers and fed on their host plants. Videos of their jumps were recorded at 4580–6800 fps using a Phantom M110 high-speed camera (Vision Research Inc., USA). Take-off velocity and acceleration were determined by the recorded videos, which were played frame-by-frame and analyzed using PCC 2.5 software (PHANTOM CAMERA CONTROL 2.5.744.0, Vision Research Inc., USA).
Results
Morphology of the flea beetle hind leg (Figs 1–3)
Our findings on the hind leg musculature are mostly in accord with those described by
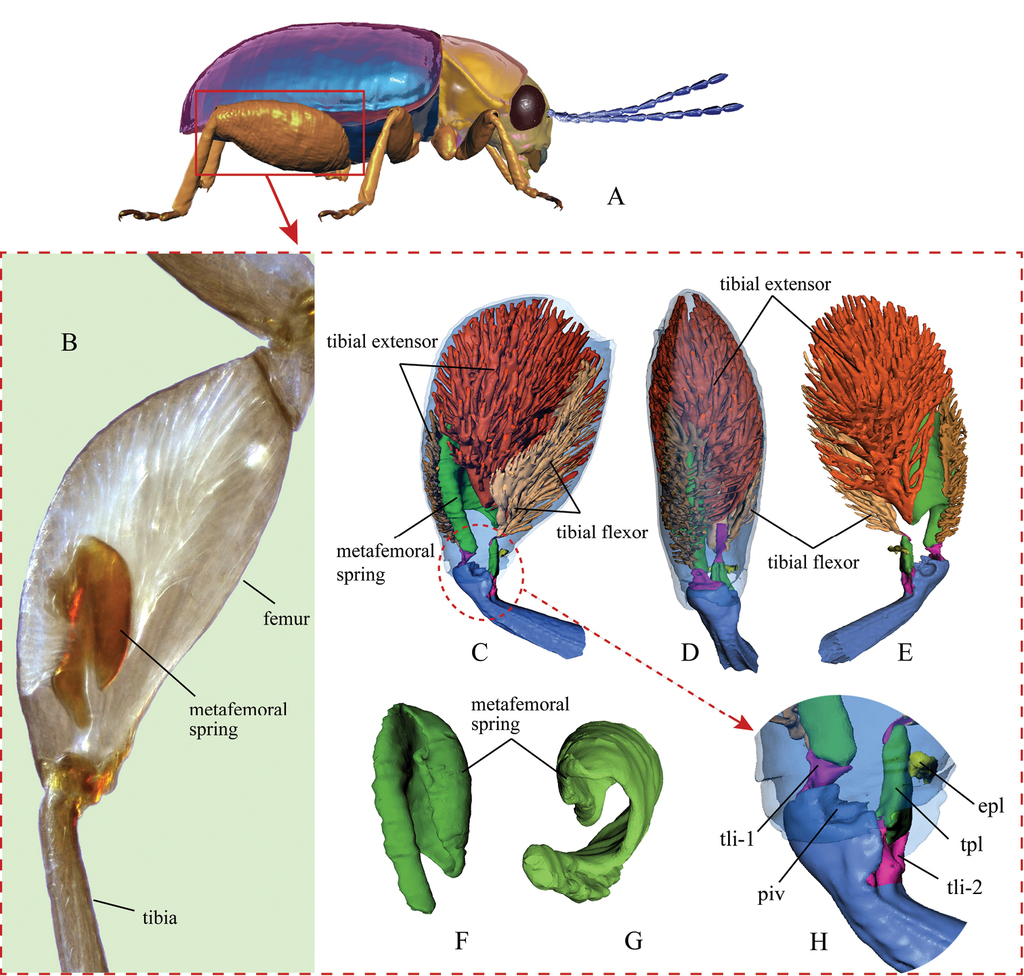
Jumping apparatus in the flea beetle hind leg. A a model of a generic flea beetle (lateral view), indicating the enlarged hind leg B hind leg of Trachytetra obscura under a light microscope (dark-field microscopy) C–H X-ray computer tomography-based 3D reconstructions of the hind leg of the flea beetle Asiophrida xanthospilota C lateral view of the hind leg and internal structures D dorsal view of the hind leg E lateral view of the hind leg (view from an opposite direction of inset C) F lateral view of the metafemoral spring G ventral view of the metafemoral spring H femorotibial joint. Abbreviations: epl: elastic plate; piv: tibial pivot of femorotibial joint; tli-1: primary tibial ligament; tli-2: secondary tibial ligament; tpl: Lever’s triangular plate.
Internal structures of the hind leg of Altica cirsicola. A the flea beetle Altica sp. on foliage B–F X-ray computer tomography 3D reconstructions of the hind leg of A. cirsicola B lateral view of the left hind leg C lateral view of the metafemoral spring D distal view of the metafemoral spring E distal view of the metafemoral spring and tibial extensors F features of the femorotibial joint. Abbreviations: cox: coxa; exa: extended arm of the metafemoral spring; epl: elastic plate; fem: femur; msp: metafemoral spring; ner: nerve; piv: tibial pivot of the femorotibial joint; rfl: recurve flange of the metafemoral spring; tar: tarsi; tex-1: primary tibial extensor; tex-2: secondary tibial extensor; tfl: tibial flexor; tib: tibia; tli-1: primary tibial ligament; tli-2: secondary tibial ligament; tpl: Lever’s triangular plate; tra: trachea; tro: trochanter.
Variation in the metafemoral spring of different flea beetle species (3D reconstructions). A hind femur of Altica cirsicola B hind femur of Podontia lutea C hind femur of Psylliodes sp. D hind femur of Nonarthra sp. E hind femur of Clavicornaltica sp. F hind femur of Hespera lomasa. Scale bar: 0.2 mm.
The elastic plate was found inside the hind femora of all 13 genera of flea beetles that were dissected. In some other jumping insects that we have examined [Galerucines (Chrysomelidae, Galerucini); Scirtes sp. (Coleoptera, Scirtidae); Rhynchaenus sp. (Coleoptera, Curculionidae); Lycorma delicatula (White) (Hemiptera, Fulgoridae); Locusta migratoria (L.) (Orthoptera, Acrididae); Tridactylidae sp. (Orthoptera, Tridactylidae); Brachymeria sp. (Hymenoptera, Chalcididae)], the elastic plate is absent. However, a thin membrane is present in the same area, which may serve to protect the femorotibial joint in locomotion. It is very possible that the elastic plate is developed from this membrane.
The flea beetle jumping process (Fig. 4)
Based on our morphological observations and the high-speed filming, we hypothesize that a typical flea beetle jump can be divided into four major phases (Fig.
Take-off strategy of flea beetles (Asiophrida xanthospilota is shown in the photos at the top of the figure). Acceleration data were calculated based on three typical jumps recorded by a high-speed camera. The three different species were chosen to represent flea beetles with different sizes A Phase I (Crunching): tibial flexor muscles contract, causing flexion of the tibia B Phase II (Co-contraction): tibial extensor muscles and tibial flexor muscles contract simultaneously, catching the triangular plate and hindering the extension of the tibia C Phase III (Triggering and Acceleration), the triangular plate is dislodged, causing the explosive release of energy D Phase IV (Relaxation), the flea beetle is catapulted into the air and the muscles begin to relax.
Phase I entails the preparation for a jump, wherein the flea beetle flexes its hind legs until the femorotibial angle reaches a minimum of approximately 20°. This flexion usually takes no more than 20 ms (Fig.
Phase II is an initiation phase in which elastic strain energy cumulatively builds up inside the femur as the femorotibial angle increases from approximately 20° to 60°, which takes approximately 4–5 ms (Fig.
Phase III is the most dramatic phase, yet it only takes 1–2 ms (Fig.
Finally, in phase IV (Fig.
Furthermore, an experiment was designed to test the four phases of the catapult mechanism identified by the position of the elastic plate during a simulated jump (Supplementary files movie S4).
Mechanical analysis of the flea beetle jump – the ‘Positive Feedback Mechanism’ (Fig. 5)
To understand the explosive manner of the flea beetle jump, we analyzed the mechanical dynamics involved in the jumping process.
For the mechanical analysis, we generated 3D reconstructions of the hind legs during the four different phases of the catapult jump (Fig.
The dynamics of the catapult mechanism of the flea beetle hind leg powered by the elastic plate (Asiophrida xanthospilota, micro-CT 3D reconstructions from four different phases of the jumping leg). (F1 indicates the force generated by tibial extensor muscles responsible for extension of the tibia; F2 indicates the force responsible for flexion of the tibia which is indirectly generated by tibial flexor muscles). A–D 3D reconstructions of four hind legs through each of the four phases of the jump, indicating variable d1 (moment arm of F1) and d2 (moment arm of F2). d1 is at its minimal length at the start of the hind leg extension and at its maximal length during the middle; by contrast, d2 is at its maximal length at the start of the hind leg extension and at its minimal at the end A phase I B phase II C phase III D phase IV E the positive feedback mechanism in the take-off process. F2 comprises F21, which is the constrained force generated by the elastic plate, and F22, which is generated by the tibial flexor muscle F the triangular plate – elastic plate complex (lateral view) G the triangular plate – elastic plate complex (dorsal view) H the elastic plate (dorsal view, under light microscope, dark-field microscopy) I the triangular plate (dorsal view, under light microscope, dark-field microscopy). Abbreviations: epl: elastic plate; tli-2: secondary tibial ligament; tpl: triangular plate; ten: tendon of tibial flexor.
At the beginning of phase II, the total torque M is close to zero, since F1d1≈F2d2. Therefore, in this phase, the tibia extends very slowly, and the metafemoral spring is stretched by F1 and F22, such that the huge energy generated by the antagonistic muscles (tibial extensor and flexor) is stored. In phase III, the continuous extension of the hind leg causes a rapid increase in d1 while d2 decreases; simultaneously, θ decreases and F22 does not change, thereby dramatically decreasing F2=F22/cosθ. This ‘positive feedback mechanism’ leads to the explosive increase in the total torque M=F1d1–F2d2 (at this point, F1d1>>F2d2). When θ decreases to near zero, the triangular plate is dislodged and slips out of the femur. Meanwhile, the enormous elastic strain energy, previously stored in the metafemoral spring, is rapidly converted into kinetic energy, allowing the flea beetle to attain an extraordinarily high acceleration.
Taken together, these results suggest that two structures play a significant role in the catapult mechanism – the elastic plate and the triangular plate, which control the timing of the jumping process, the triggering of the catapult, and the explosive release of energy.
‘High Efficiency Mechanism’ of the flea beetle jump
In order to explore the efficiency of flea beetle jumping, we compared their jumping dynamics with that of humans. Morphologically, the flea beetle hind leg resembles the human leg since both are ‘two-segment systems’ (
High-speed film data demonstrate that the peak acceleration is approximately 10 times as great as that at the start of the jump (in Phase II). As shown by our kinematic data based on high-speed filming, Psylliodes punctifrons jumped with an average acceleration of 3450±10 m/s2 and took 6 ms to complete a jump, yet the main acceleration occurred in approximately 1 ms, peaking at 8650±10 m/s2 at the end of the whole jumping process. This scenario differs from that seen in other jumping insects, which have either a near-constant (
An individual P. punctifrons has an average mass of 1.6 mg, with the hind legs comprising only 17% of the total body mass. The jump pushes individuals to a final velocity of 5.58±0.5 m/s. The peak instantaneous power output (per unit mass) calculated for the hind legs in this species was 2.2±0.1 × 105 W/kg, which is approximately 449 times that of the fastest-known muscle (
In the field, flea beetles conduct contiguous jumps when encountering interference (based on our field observations in this study). When stimulated continuously in the laboratory (tested in this study), they can jump more than 30 times in a row without significant fatigue. Given that the power output of a catapult can be greater than the power input (
Design of a bionic jumping leg (Fig. 6)
Jumping can be a very effective mode of locomotion for small robots (
Bionic design of a jumping limb inspired by the flea beetle leg. A preparation position for jumping B building up elastic strain energy C triggering the jump D acceleration. The required energy for a jump is provided by two ‘motors’ operating simultaneously and generating the forces F1 and F2, respectively. At the beginning (inset B), F1 > F2, the ‘tibia’ rotates clockwise, but the ‘trigger’ blocks the process and leads to the stretching of ‘volute spring’. Thus, the work generated by the ‘motors’ is stored in the ‘volute spring’. At a certain stage (inset C), when the ‘trigger’ can no longer constrain the huge tension built up inside the femur, the ‘latch element’ dislodges suddenly from the ‘trigger’ and the huge amount of energy stored in the ‘volute spring’ is released. This leads to the explosive clockwise movement of the tibia. The leg thereby propels the robot into the air in an explosive manner. The leg can be switched to regular walking mode as required by operating only one ‘motor’ (one to flex the leg and the another to extend it) at a time.
The leg can also be switched to a regular walking mode as required by operating only one ‘motor’ at a time (as the ‘trigger’ will stop working when only one motor is turned on). In the regular walking mode, one motor could be turned on to flex the leg and the other to extend it, respectively.
Discussion
Based on our findings,
The ‘elastic plate’ is not present in other jumping insects other than flea beetles. However, the locust has a similar structure called ‘Heitler’s lump’ (
The mechanism of generating elastic potential energy in the flea beetle leg is like that in many other rapid-moving arthropods (for instance, flea, trap-jaw ants, mantis shrimp, etc.), which requires the ‘co-contraction’ process (extensor and flexor muscles contracting simultaneously). Flea beetles have evolved an enormous independent spring to aid the storage of elastic potential energy. This is significantly different from many other rapid-moving arthropods which usually only rely on exoskeleton or modified exoskeleton to store elastic potential energy, for instance, the semi-lunar process on distal part of hind femur in locusts (
Some arthropod groups (see Table
There were several different modes of locomotion designed in bionic robots previously, such as: flying (
In addition to robotics, designs based on the flea beetle hind leg may be of use in other areas, such as engineering and industrial installations, in which the catapult mechanism and elastic elements could be crucially important.
A comparison of characteristics between flea beetle jump and rapid movements in other arthropods.
| Arthropod species | Acceleration duration (Appr.) · ms | Velocity (Appr.) · m/s | Acceleration (Appr.) · m/s2 | Power output (per unit mass) (Appr.) · W/kg | Movement type |
| Flea beetles | 1.1–7.7 | 0.7–5.6 (max. velocity) | 100–3450 | 2.2×105 (Psylliodes punctifrons) | Jumping |
| Fleas | 1–2 | 0.8–1.9 (max. velocity) | 960–1600 | 6×103–1.4×104 | Jumping |
| Froghoppers | 1–1.5 | 2.5–4.7 (max. velocity) | 1667–5400 | Unknown | Jumping |
| Locusts | 20 | 2.2–3.1 (max. velocity) | 100 | 450 | Jumping |
| Termite soldiers | 0.025 | 56 (average velocity) | Unknown | 1.1×107 | Movement of mandibles |
| Trap-jaw ants | 0.1–0.6 | 17–64 (max. velocity) | 1×105 –1×106 | 2×104– 3×105 | Movement of mandibles |
| Trap-jaw spiders | 0.12 | 8.5 (average velocity) | Unknown | 6.6×104 | Movement of chelicerae |
| Mantis shrimps | 1–6 | 23–31 (max. velocity) | 1×103–1.5×105 | Unknown | Movement of raptorial appendages |
Acknowledgments
We sincerely thank Dr. Norman Woodley (National Museum of Natural History, USA) for reviewing and providing suggestions on the writing of our manuscript, Taina Litwak (National Museum of Natural History, USA) for her help on the illustrations in this paper, and the editor and reviewers for their critical remarks.
This research was funded by Grants from National Science Foundation of China to Yongying Ruan (Grant No. 31802004), the Key Foreign Cooperation Projects of the Bureau of International Cooperation of Chinese Academy of Sciences (152111KYSB20160067) and National Science Foundation of China to Xingke Yang (Grant No. 31372239).
References
- Alexander RM (1995) Leg design and jumping technique for humans, other vertebrates and insects. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences 347(1321): 235–248. https://doi.org/10.1098/rstb.1995.0024
- Alexander RM, Bennet-Clark HC (1977) Storage of elastic strain energy in muscle and other tissues. Nature 265: 114–117. https://doi.org/10.1038/265114a0
- Barth R (1954) O aparelho saltatorio do Halticineo Homophoeta sexnotata Har. (Coleoptera). Memórias do Instituto Oswaldo Cruz 52: 365–376. https://doi.org/10.1590/S0074-02761954000200006
- Bennet-Clark HC (1975) The energetics of the jump of the locust Schistocerca gregaria. Journal of Experimental Biology 63: 53–83.
- Bennet-Clark HC, Lucey ECA (1967) The jump of the flea: a study of the energetics and a model of the mechanism. Journal of Experimental Biology 47: 59–76.
- Bonsignori G, Stefanini C, Scarfogliero U, Mintchev S, Benelli G, Dario P (2013) The green leafhopper, Cicadella viridis (Hemiptera, Auchenorrhyncha, Cicadellidae), jumps with near-constant acceleration. Journal of Experimental Biology 216: 1270–1279. https://doi.org/10.1242/jeb.076083
- Brackenbury J, Wang R (1995) Ballistics and visual targeting in flea-beetles (Alticinae). Journal of Experimental Biology 198: 1931–1942.
- Burrows M (2006) Jumping performance of froghopper insects. Journal of Experimental Biology 209(23): 4607–4621. https://doi.org/10.1242/jeb.02539
- Burrows M, Sutton GP (2012) Locusts use a composite of resilin and hard cuticle as an energy store for jumping and kicking. Journal of Experimental Biology 215(Pt 19): 3501–3512. https://doi.org/10.1242/jeb.071993
- Cai Y, Bi S, Zhang L, Gao J (2009) Design of a robotic fish propelled by oscillating flexible pectoral foils. The 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems 2138–2142. https://doi.org/10.1109/IROS.2009.5354749
- Furth DG (1980) Inter-generic differences in the metafemoral apodeme of flea beetles (Chrysomelidae: Alticinae). Systematic Entomology 5: 263–271. https://doi.org/10.1111/j.1365-3113.1980.tb00413.x
- Furth DG (1982) The metafemoral spring of flea beetles (Chrysomelidae: Alticinae). Spixiana Supplement 7: 11–27.
- Furth DG (1985) Relationships of Palearctic and Nearctic genera of Alticinae. Entomography 3: 375–392.
- Furth DG (1988) The jumping apparatus of flea beetles (Alticinae) – The metafemoral spring. In: Jolivet P, Petitpierre E, Hsiao TH (Eds) Chrysomelidae Biology. Kluwer Academic Publishers Group, Dordrecht, 287–297. https://doi.org/10.1007/978-94-009-3105-3_17
- Furth DG (1989) Metafemoral spring studies of some Neotropical genera of Alticinae. Entomography 6: 497–510.
- Furth DG (1992) The independent evolution of the metafemoral spring in Coleoptera. Systematic Entomology 17: 341–349. https://doi.org/10.1111/j.1365-3113.1992.tb00555.x
- Furth DG, Suzuki K (1994) Character correlation studies of problematic genera of Alticinae in relation to Galerucinae. (Coleoptera: Chrysomelidae). In: Furth DG (Ed.) Proceeding of the Third International Symposium on the Chrysomelidae, Beijing, 1994. Backhuys Publishers, Leiden, 116–135.
- Furth DG, Suzuki K (1998) Studies of Oriental and Australian Alticinae genera based on the comparative morphology of the metafemoral spring, genitalia, and hind wing venation. In: Biondi M, Daccordi M, Furth DG (Eds) Proceeding of the Fourth International Symposium on the Chrysomelidae. Museo Regionale di Scienze Naturali, Tornino, 91–124.
- Furth DG, Traub W, Harpaz I (1983) What makes Blepharida jump? A structural study of the metafemoral spring of a flea beetle. Journal of Experimental Biology 227: 43–47. https://doi.org/10.1002/jez.1402270107
- Ge D, Chesters D, Gomez-Zurita J, Zhang L, Yang X, Vogler AP (2011) Anti-predator defense drives parallel morphological evolution in flea beetles. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences 278: 2133–2141. https://doi.org/10.1098/rspb.2010.1500
- Gronenberg W (1996) Fast actions in small animals: springs and click mechanisms. Journal of Comparative Physiology A 178: 727–734. https://doi.org/10.1007/BF00225821
- Heitler WJ (1974) The locust jump. Journal of Comparative Physiology 89: 93–104. https://doi.org/10.1007/BF00696166
- Kovač M, Fuchs M, Guignard A, Zufferey JC, Floreano D (2008) A miniature 7 g jumping robot. IEEE International Conference on Robotics and Automation 2008: 373–378. https://doi.org/10.1109/ROBOT.2008.4543236
- Kovač M, Schlegel M, Zufferey JC, Floreano D (2010) Steerable miniature jumping robot. Autonomous Robots 28(3): 295–306. https://doi.org/10.1007/s10514-009-9173-4
- Larabee FJ, Gronenberg W, Suarez AV (2017) Performance, morphology and control of power-amplified mandibles in the trap-jaw ant Myrmoteras (Hymenoptera: Formicidae). Journal of Experimental Biology 220(Pt 17): 3062–3071. https://doi.org/10.1242/jeb.156513
- Lever R (1930) A new endoskeletal organ in the hind legs of the Halticinae. Zoologischer Anzeiger Leipzig 92: 287–288.
- Li M, Jiang Z, Wang P, Sun L, Ge SS (2014) Control of a quadruped robot with bionic springy legs in trotting gait. Journal of Bionic Engineering 11(2): 188–198. https://doi.org/10.1016/S1672-6529(14)60043-3
- Lingafelter SW, Konstantinov AS (1998) Systena Chevrolat (Coleoptera: Chrysomelidae: Alticinae): notes on nomenclature, redescription of the genus and a preliminary discussion of characters and phylogenetic relationships. Proceedings of the Entomological Society of Washington 100: 467–483.
- Liu GH, Lin HY, Lin HY, Chen ST, Lin PC (2014) A bio-inspired hopping kangaroo robot with an active tail. Journal of Bionic Engineering 11(4): 541–555. https://doi.org/10.1016/S1672-6529(14)60066-4
- Lohse D, Schmitz B, Versluis M (2001) Snapping shrimp make flashing bubbles. Nature 413: 477–478. https://doi.org/10.1038/35097152
- Martin TP, Stull GA (1969) Effects of various knee angle and foot spacing combinations on performance in the vertical jump. Research Quarterly 40: 324–331. https://doi.org/10.1080/10671188.1969.10614831
- Maulik S (1929) On the structure of the hind femur in Halticine beetles. Journal of Zoology 99: 305–308. https://doi.org/10.1111/j.1469-7998.1929.tb07744.x
- Nadein K, Betz O (2016) Jumping mechanisms and performance in beetles. I. Flea beetles (Coleoptera: Chrysomelidae: Alticini). Journal of Experimental Biology 219: 2015–2027. https://doi.org/10.1242/jeb.140533
- Patek SN (2015) The most powerful movements in biology. American Scientist 103: 330–337. https://doi.org/10.1511/2015.116.330
- Patek SN, Baio JE, Fisher BL, Suarez AV (2006) Multifunctionality and mechanical origins: ballistic jaw propulsion in trap-jaw ants. Proceedings of the National Academy of Sciences 103: 12787–12792. https://doi.org/10.1073/pnas.0604290103
- Patek SN, Korff WL, Caldwell RL (2004) Biomechanics: deadly strike mechanism of a mantis shrimp. Nature 428: 819–820. https://doi.org/10.1038/428819a
- Patek SN, Rosario MV, Taylor JR (2013) Comparative spring mechanics in mantis shrimp. Journal of Experimental Biology 216(Pt 7): 1317–1329. https://doi.org/10.1242/jeb.078998
- Roberts TJ, Azizi E (2011) Flexible mechanisms: the diverse roles of biological springs in vertebrate movement. Journal of Experimental Biology 214: 353–361. https://doi.org/10.1242/jeb.038588
- Scarfogliero U, Stefanini C, Dario P (2007) Design and development of the long-jumping "grillo" mini robot. 2007 IEEE International Conference on Robotics and Automation: 467–472. https://doi.org/10.1109/ROBOT.2007.363830
- Schmitt M (2004) Jumping flea beetles: structure and performance (Insecta, Chrysomelidae, Alticinae). In: Jolivet P, Santiago-Baly JA, Schmitt M (Eds) New developments in the biology of Chrysomelidae. SPB Academic Publications. Hague, Netherlands, 161–169.
- Seid MA, Scheffrahn RH, Niven JE (2008) The rapid mandible strike of a termite soldier. Journal of Experimental Biology 18(22): R1049–R1050. https://doi.org/10.1016/j.cub.2008.09.033
- Shyy W, Berg M, Ljungqvist D (1999) Flapping and flexible wings for biological and micro air vehicles. Progress in Aerospace Sciences 35(5): 455–505. https://doi.org/10.1016/S0376-0421(98)00016-5
- Slipinski SA, Leschen R, Lawrence JF (2011) Order Coleoptera Linnaeus, 1758. In: Zhang ZQ (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148(1), 203–208.https://doi.org/10.11646/zootaxa.3148.1.39
- Song YS, Sitti M (2007) Surface-tension-driven biologically inspired water strider robots: Theory and experiments. IEEE Transactions on Robotics 23(3): 578–589. https://doi.org/10.1109/TRO.2007.895075
- Suhr SH, Song YS, Lee SJ, Sitti M (2005) Biologically Inspired Miniature Water Strider Robot. Robotics: Science and Systems 2005: 319–326.
- Sutton GP, Burrows M (2011) Biomechanics of jumping in the flea. Journal of Experimental Biology 214: 836–847. https://doi.org/10.1242/jeb.052399
- Versluis M, Schmitz B, von der Heydt A, Lohse D (2000) How snapping shrimp snap: through cavitating bubbles. Science 289: 2114–2117. https://doi.org/10.1126/science.289.5487.2114
- Wang M, Zang XZ, Fan JZ, Zhao J (2008) Biological jumping mechanism analysis and modeling for frog robot. Journal of Bionic Engineering 5(3): 181–188. https://doi.org/10.1016/S1672-6529(08)60023-2
- Weis-Fogh T, Alexander RM (1977) The sustained power output from striated muscle. In: Pedley TJ (Ed.) Scale effects in animal locomotion. Academic Press, London, 511–525.
- Wood HM, Parkinson DY, Griswold CE, Gillespie RG, Elias DO (2016) Repeated evolution of power-amplified predatory strikes in trap-jaw spiders. Current Biology 26(8): 1057–1061. https://doi.org/10.1016/j.cub.2016.02.029
- Zaitsev V, Gvirsman O, Hanan UB, Weiss A, Ayali A, Kosa G (2015) A locust-inspired miniature jumping robot. Bioinspiration and Biomimetics 10(6): 066012. https://doi.org/10.1109/IROS.2015.7353426
- Zhao H, Li J (2008) Sports Biomechanics. Higher Education Press, Beijing, 259 pp.
Supplementary materials
Movie S1. A model of the catapult mechanism in the hind legs of flea beetles
Data type: multimedia
Movie S2. The take-off strategy of Asiophrida xanthospilota Baly as filmed by a high-speed camera
Data type: multimedia
Movie S3. Internal structures of the hind leg of Asiophrida xanthospilota Baly
Data type: multimedia
Movie S4. Test and confirmation of the catapult mechanism in the flea beetle hind leg
Data type: multimedia