Monograph |
|
Corresponding author: Sérgio N. Stampar ( sergiostampar@gmail.com ) Academic editor: Bert W. Hoeksema
© 2020 Sérgio N. Stampar, James D. Reimer, Maximiliano M. Maronna, Celine S. S. Lopes, Hellen Ceriello, Thais B. Santos, Fabián H. Acuña, André C. Morandini.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Stampar SN, Reimer JD, Maronna MM, Lopes CSS, Ceriello H, Santos TB, Acuña FH, Morandini AC (2020) Ceriantharia (Cnidaria) of the World: an annotated catalogue and key to species. ZooKeys 952: 1-63. https://doi.org/10.3897/zookeys.952.50617
|
Abstract
The diversity of Ceriantharia is known from studies formally describing species from the late 18th Century onwards. However, no nomenclators including a list and discussion of all valid species have been produced since a list discussed by Carlgren in 1912. The present nomenclator presents a complete list of adult species of Ceriantharia of the World, including a discussion on each species. It includes the three families (Arachnactidae, Botrucnidiferidae, Cerianthidae) and the currently accepted 54 species based on their adult form. This study serves as a presentation of the “state-of-the-art” list of species of Ceriantharia, and includes a species identification key to support taxonomic identification. Additional in-depth species-by-species investigations for almost all cerianthid species is still needed, as the information available for most of these species is quite superficial.
Keywords
Cnidaria, families, genera, identification key, tube-dwelling anemones
Introduction
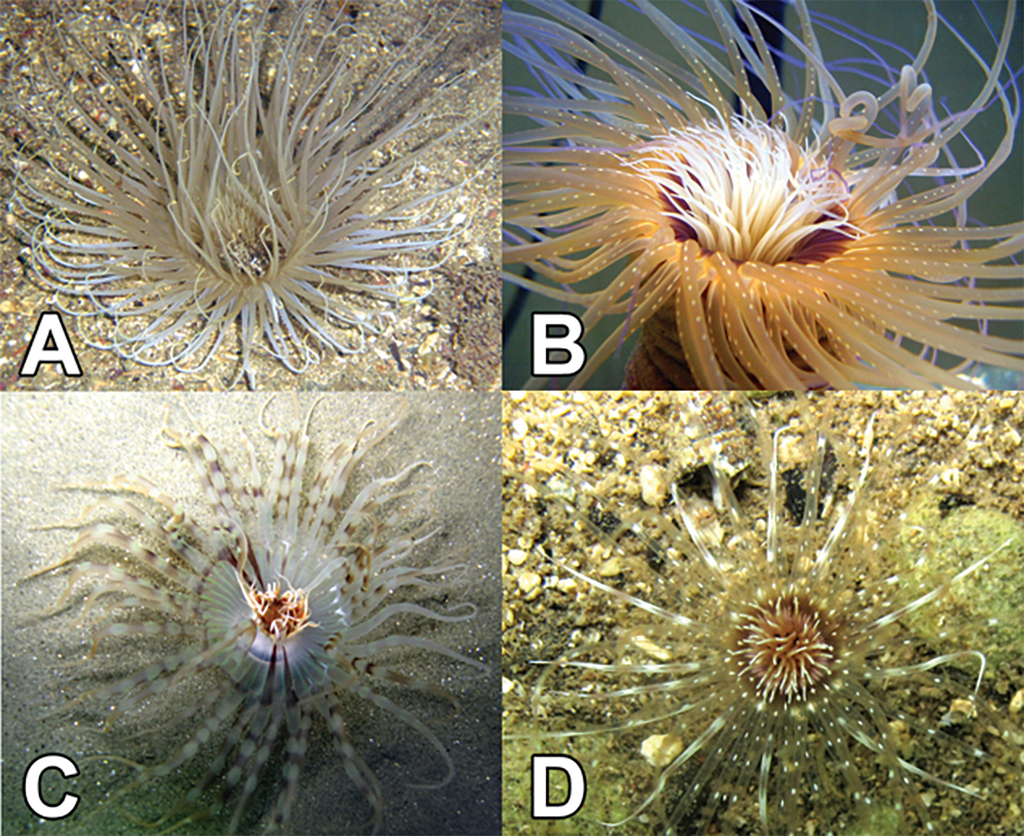
The subclass Ceriantharia Perrier, 1893 (Fig.
Knowledge on Ceriantharia dates back from the late 1700s, with the description by
Material and methods
The checklist’s classification follows
Results
The compilation of species resulted in a list of 54 valid species (Fig.
Checklist
Phylum Cnidaria Hatschek, 1888
Class Anthozoa Ehrenberg, 1834
Subclass Ceriantharia Perrier, 1893
Order Spirularia den Hartog, 1977
Number of valid taxa: two families, six genera, and 45 species
Family Cerianthidae Milne Edwards & Haime, 1851
Number of valid taxa: four genera and 41 species
Ceriantheomorphe
Type species
Ceriantheomorphe brasiliensis by original designation (
Number of valid species: 3
Comparison of anatomical features of Ceriantheomorphe species (after
| C. ambonensis | C. brasiliensis | C. adelita | |
|---|---|---|---|
| Marginal tentacles | More than 100 | Up to 392 | Up to 352 |
| Directive labial tentacle | Absent | Present | (?) |
| Arrangement of labial tentacles | (0)112.2112 | (1)123.1324.3124.3124 | (?)112.211 |
| Actinopharynx | 1/8 to 1/10 of gastric cavity | 1/4–1/5 of gastric cavity | 1/7 to 1/8 of gastric cavity |
| Oral disc | ~ 4 cm | ~5 cm | ~3 cm |
| Siphonoglyph | Long, 6 mesenteries attached | Rather wide, 4 mesenteries attached | Long, 6 mesenteries attached |
| Directive mesenteries | >Actinopharynx | >Actinopharynx | >Actinopharynx |
| P2 | Almost to aboral pole | To aboral pole | Almost to aboral pole |
| P3 | Short, ≅ directives | Short, < directives | Short, > directives |
| M | ± 2B | ± 2B | ± 2B |
| M3 | ± M2 | =M2 | ± M2 |
| Cnido-glandular tract at fertile mesenteries of first quartets | Present | Present | Present |
| Craspedion tract at fertile mesenteries | 2/4 | 2/5 – 3/5 | 2/5 – 3/5 |
Ceriantheomorphe ambonensis
Cerianthus ambonensis
Kwietniewski, 1898: 426;
(?) Cerianthus sulcatus McMurrich, 1910: 28–30
Ceriantheomorphe ambonensis:
Type locality
Moluccas (Maluku) Islands, Indonesia, shallow waters.
Distribution
Only known from shallow water at the type locality.
Remarks
The original description made by
Type material
Not found in this study.
Ceriantheomorphe brasiliensis
(?) Cerianthus americanus: Hertwig, 1882: 110 116
Cerianthus brasiliensis Mello-Leitão, 1919: 38–39
Ceriantheomorphe brasiliensis:
(?) Cerianthromorphe brasiliensis:
Type locality
Baía de Guanabara, Rio de Janeiro, Brazil.
Distribution
Brazil (Espírito Santo (20.5°S) to Rio Grande do Sul (33.7°S) states); Uruguay (35°S), and Gulf of Mexico (dubious record, 24–29°N), shallow waters (at < 40 m depth).
Remarks
This species was first described as Cerianthus brasiliensis by
Type material
Museu Nacional do Rio de Janeiro – MNRJ 100 (Holotype).
Ceriantheomorphe adelita
Ceriantheomorphe adelita
Cerianthromorphe brasiliensis:
(?) Cerianthromorphe brasiliensis:
Type locality
off Port Aransas, 32 km south off Corpus Christi, Texas, United States of America.
Distribution
Gulf of Mexico (Northern Mexico) to North Atlantic (North Carolina, United States of America), shallow waters.
Remarks
A very large species, which for many years was considered to be synonymous with C. brasiliensis even without biogeographic justification. Recently,
Type material
Smithsonian National Museum of Natural History NMNH 50015 (holotype).
Ceriantheopsis
Type species
Ceriantheopsis americana by original designation (
Number of valid species: 4
Comparison of anatomical features of Ceriantheopsis species (after
| C. americana | C. nikitai | C. austroafricana | C. lineata | |
|---|---|---|---|---|
| Marginal tentacles | Up to 100–120 | Up to 70 | Up to 70 | Up to 60 |
| Directive labial tentacle | Present | Present | Present | Absent |
| Arrangement of labial tentacles | (2)413.4232.4312* (4)413.4231.4312.4312 | (3)423.4232.4312.4312 | (2)313.4343.4324.3124 | 4231.4231.4231.4231 |
| Actinopharynx | 1/12–1/8 of gastric cavity | 1/5–1/4 of gastric cavity | 1/10–1/8 of gastric cavity | 1/6-1/5 of gastric cavity |
| Oral disc | 0.7–1.0 cm | ~0.6–0.7 cm | Wide, ~1.5 cm in preserved | 1.0 – 1.5 cm in preserved |
| Siphonoglyph | Narrow, 4 mesenteries attached | Wide, 4 mesenteries attached | Wide, 4 mesenteries attached | Narrow, 2 mesenteries attached |
| Directive mesenteries | <Actinopharynx | ~Actinopharynx | ~Actinopharynx | <Actinopharynx |
| P2 | To aboral pole | To aboral pole | To aboral pole | Almost to aboral pole |
| P3 | =B | =B | =B | =B |
| M | >>B | <2B | >B | ≥B |
| M3 | ≤M2 | >M2 | ≤M2 | <Half M2 |
| Cnido-glandular tract at fertile mesenteries of first quartets | Present | Not present | Present | Present |
| Craspedion tract at fertile mesenteries | 6/7–8/9 | 3/5 | 6/7 | ~6/7-8/9 |
| Cnido-glandular tract at B | <<b | =b | <b | <b |
| Craspedonemes of craspedion at fertile mesenteries | Sometimes present | Absent | Absent | Absent |
Ceriantheopsis americana
Cerianthus
sp.
Cerianthus americanus
Agassiz in Verrill, 1864b: 32–33;
Ceriantheopsis americanus:
Cerianthiopsis americanus:
Ceriantheopsis americana:
Type locality
Off Charleston, South Carolina; Beaufort, North Carolina, United States of America (not specified).
Distribution
Atlantic coast of United States and Canada, Gulf of Mexico, and Caribbean Sea, at 2–250 m depth.
Remarks
This species is probably the most extensively studied among the Ceriantharia. There are appropriate descriptions of specimens (
Type material
Museum of Comparative Zoology (Harvard) – Invertebrate Zoology 243 and SCOR-1245 and Peabody Museum of Natural History (Yale) – YPM IZ 000977.CN (syntype).
Ceriantheopsis austroafricanus
Ceriantheopsis austroafricanus
Type locality
Off Cape Town, South Africa.
Distribution
Only known from shallow waters at the type locality (8–15 m depth).
Remarks
This species was recently described and therefore little is known about it beyond a detailed morphological description. One of the most interesting features of the species is the wide range of colors (
Type material
Zoological Museum of Moscow State University – ZMMU No. Ec-105 (holotype).
Ceriantheopsis lineata
Ceriantheopsis lineata
Type locality
off Quequén, Buenos Aires, Argentina.
Distribution
Warm temperate south-western Atlantic, from Argentina (Buenos Aires State) to Brazil, Laje de Santos (São Paulo State), at 5–130 m depth.
Remarks
This species was recently described, and little is known beyond a detailed morphological description. Similar to Ceriantheopsis austroafricanus, this species shows considerable variation in color pattern (
Type material
Museu de Zoologia da Universidade de São Paulo – MZUSP 2686 (Holotype).
Ceriantheopsis nikitai
Ceriantheopsis nikitai
Molodtsova, 2001a: 773–780;
Type locality
Benguela Upwelling System, Namibia.
Distribution
Only known from deep water at the type locality (145–240 m depth).
Remarks
This species was recently described and has not been the subject of any study since the original description of the species by
Type material
Zoological Museum of Moscow University – ZMMU UE-97 (Holotype).
Cerianthus
Type species
Cerianthus membranaceus (Spallanzani, 1784)
Number of valid species: 18
| Species | Directive mesenteries length | Directive labial tentacle | M-mesentery (M1) length | M-mesentery (M2) length | M-mesentery (m1) length | M-mesentery (m2) length | Mesenteries attached to siphonoglyph | Siphonoglyph shape | Number of marginal tentacles |
|---|---|---|---|---|---|---|---|---|---|
| C. andamanensis | – | – | Reach aboral pore | – | – | – | – | – | ~160 |
| C. bathymetricus | – | – | Reach aboral pore | 1/2 of M-1 | – | – | – | Wide? | 28 |
| C. filiformis | > stomodeum | Present | Reach aboral pore | 6/8 of M-1 | 7/8 of M-1 | 5/8 of M-1 | 6 | Wide? | ~70 |
| C. incertus | – | – | – | – | – | – | – | – | 38-42 |
| C. japonicus | > stomodeum | Present | Almost reach aboral pore | ≅ M-1 | 3/4 of M-1 | ~1/2 of M-1 | 4? | Wide | 65 |
| C. lloydii | > stomodeum | Present | Almost reach aboral pore | Longer than M-1 | 1/5 of M-1 | 1/6 of M-1 | 4 | Narrow | Up to 70 |
| C. malakhovi | ? | Half column (?) | > M-1 | ? | ? | ? | ? | ~160 | |
| C. medusula | ? | ? | ? | ? | ? | ? | ? | ? | Few |
| C. membranaceus | > stomodeum | Present | Almost reach aboral pore | ≅M-1 | ≅P2 | ≅m-1 | 6 | Narrow | 140 |
| C. mortenseni | >stomodeum | Present | Short, almost half of gastrovascular cavity | ≅ M-1 | 3/4 of M-1 | 1/2 of M-1 | 8 | Wide | 125 |
| C. punctatus | > stomodeum | Present | Almost reach aboral pore | =M-1 | 2/3 of M-1 | 1/4 of M-1 | 6 | Rather wide | 80-90 |
| C. roulei | ? | ? | Long? | ? | ? | ? | ? | ? | ~40 |
| C. stimpsonii | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| C. sulcatus | > stomodeum | Present | Reach aboral pore | ≅M-1 | ? | ? | ? | Narrow | ~180 |
| C. taedus | > stomodeum | Present | Short? | Short | ? | ? | ? | Narrow | 55 |
| C. valdiviae | ≅stomodeum | Absent | Short? | =M-1 | ≅M-1 | 1/2 of M-1 | 4 | Narrow | 35 |
| C. vas | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| C. vogti | > stomodeum | Present | (?)Almost reach aboral pore | (?)Longer than M-1 | (?)1/5 of M-1 | (?)1/6 of M-1 | ? | Narrow | 30-40 |
Cerianthus andamanensis
Cerianthus andamanensis
Alcock, 1893: 153;
(?) Cerianthus andamanensis:
Type locality
off Port Blair, Andaman and Nicobar Islands, India.
Distribution
Only known from shallow water at the type locality.
Remarks
The species description is based on three specimens from Port Blair in the Andaman Sea (
Type material
(?) Indian Museum.
Cerianthus bathymetricus
Cerianthus bathymetricus
Moseley, 1877: 302–305;
Type locality
Deep sea, North Atlantic (35° 26’N 50° 53’W), at 5000 m depth.
Distribution
Only known from deep water at the type locality.
Remarks
This species is one of the smallest tube-dwelling anemone species known. The described specimens are only 2.5 cm long and lived in a very long membranous tube of more than 11 cm in length. The description is not detailed but provides some information on the anatomy, indicating a very long hyposulcus (especially in figure 17,
Type material
Not found in this study, but the original description provided a graphic representation.
Cerianthus filiformis
In part Cerianthus orientalis
Cerianthus
sp. 1
Cerianthus
sp. 2
Cerianthus filiformis
Carlgren, 1924: 169–173;
Cerianthus misakiensis Nakamoto, 1923: 167
Type locality
Aburatsubo Bay, Miura, Japan.
Distribution
South Japan, South Korea, Korea (East China Sea), and China (Yellow Sea), at 1–50 m depth.
Remarks
There are some detailed descriptions about this species (e.g.,
Type material
Lund Museum of Zoology – MZLU L930/3095b (Syntype).
Cerianthus incertus
Cerianthus danielsseni
Levinsen, 1893: 398;
Cerianthus incertus
Carlgren, 1932: 255;
Type locality
North Sea (not specified).
Distribution
Arctic Ocean, Norway, and Iceland, at 650–1185 m depth.
Remarks
Cerianthus incertus has a complicated taxonomic history and was originally described as C. danielsseni by
Type material
Not found in this study.
Cerianthus japonicus
Cerianthus japonicus
Carlgren, 1924: 173–175,
Type locality
Aburatsubo, Misaki (Sagami Bay), Japan.
Distribution
Sagami Bay and Miyazaki, Kyushu Island, Japan; North Hamgyong Province, North Korea, at 10–100 m depth.
Remarks
The original species description was based on two small specimens, one from North Korea (North Hamgyong Province) and the other one from Japan, Aburatsubo, Misaki (Sagami Bay). The description is quite adequate and presents the most important characteristics of the species. However, as noted by
Type material
Museum of Evolution- Evolutionsmuseet (Uppsala University – ZTY 2516) (Holotype).
Cerianthus lloydii
Edwardsia vestita Gosse, 1856a: 74–75
Cerianthus membranaceus Gosse, 1858: 419
Cerianthus lloydii
Gosse, 1859: 50;
(?) Cerianthus borealis Danielssen, 1860: 251;
Cerianthus vermicularis Lütken, 1860: 199–200
Cerianthus lutkenii Andres, 1883: 353
Arachnactis bournei Fowler, 1897: 805–807 (larval stage)
Cereanthus lloydii Goette, 1897: 293
Cerianthus lloydii borealis Grieg, 1913: 142
Synarachnactis bournei Leloup, 1962: 2–4, 6–7 (larval stage)
Cerianthus septentrionalis
van Beneden, 1924: 120: 126–131;
(?) Cerianthus sp.
(?) Cerianthus lloydii:
Type locality
Menai Strait, Irish Sea, United Kingdom.
Distribution
North Sea, Norwegian Sea, Barents Sea, Greenland Sea, Bay of Biscay, and (?) Sea of Okhotsk; (?) depths from 2 m to the deep sea,
Remarks
This species has been the subject of many studies. There have been several morphological descriptions (e.g.,
Type material
Not found in this study.
Cerianthus malakhovi
Cerianthus malakhovi
Molodtsova, 2001a: 909–913;
Type locality
Close to Torra Bay and Mowe Bay, Skeleton Coast Park, Namibia; at 300–350 m depth.
Distribution
Only known from deep water at the type locality.
Remarks
This species has been described in detail relatively recently based on five collected specimens. The original description, in Russian, contains no information on living animals because the material examined was already fixed at the time of diagnosis. This is a species that requires attention, as it can occur in deeper waters and may contain very important evolutionary information.
Type material
Zoological Museum of Moscow University, ZMMU EC-102 (Holotype).
Cerianthus medusula
Paractis medusula Klunzinger, 1877: 71–72
Cerianthus medusula
Andres, 1883: 353–354;
(?) Pachycerianthus maua:
(?) Pachycerianthus mana:
Type locality
Al-Qusair (Red Sea), Egypt.
Distribution
Only known from shallow water (at < 5 m depth) at the type locality.
Remarks
This is another species with only little available data, and these are quite contradictory. This species was described as a sea anemone (order Actiniaria) by
Type material
Not found in this study, but the original description provided a graphic representation.
Cerianthus membranaceus
Tubularie Spallanzani, 1784: 627–628
(?) Tubularie: Rapp 1829: 656–658
Tubularia membranosa Gmelin, 1791: 3836
Actinia cylindrica Renier, 1807: 23
Actinia vestita Renier, 1807: 23–24
Moschata rhododactyla
Renier in de Blainville, 1830: 284;
Cereus cupreus
Ilmoni, 1830: 698–699;
In part Actinia elongata Grube, 1840: 11–12;
Cerianthus cornucopia
Delle Chiaje, 1841: 136;
Cerianthus breae Delle Chiaje, 1841: 136
Cerianthus actiniodeus Delle Chiaje, 1841: 136
Cerianthus membranaceus:
(?) Edwardsia vestita Gosse, 1856a: 74–75;
(?) Cerianthus membranaceus:
Cerianthus cylindricus
Milne-Edwards, 1857: 309;
Saccanthus purpurescens Milne-Edwards, 1857: 310
Cerianthis membranaceus:
(?) Cerianthus lloydii Gosse, 1859: 419;
Cerianthus membranaceus nigricans Andres, 1881: 332
Cerianthus membranaceus violaceus Andres, 1881: 332
Cerianthus membranaceus viridis Andres, 1881: 332
Cerianthus membranaceus roseus Andres, 1881: 332
Cerianthus nans Andres, 1881: 333
Saccanthus purpurascens Andres, 1883: 351
Saccanthus purpurensis van Beneden, 1897: 142
Pachycerianthus multiplicatus:
Type locality
Mediterranean Sea, Italy (not specified).
Distribution
Mediterranean Sea (Italian coast), shallow waters.
Remarks
This is one of the most well-known cerianthid species, but at the same time there are many questions about the taxonomic consistency of past works. This was the first species of Ceriantharia described (type locality Italy); however, this has caused many records from the Mediterranean Sea being incorrectly attributed to this species. For example,
Type material
Not found in this study.
Cerianthus mortenseni
Cerianthus mortenseni
Carlgren, 1924: 175–182, 195;
Type locality
Paniquian Island, Mindoro, Philippines.
Distribution
Only known from shallow water at the type locality.
Remarks
This is a very intriguing species as the two specimens described in the original description are very different in shape. The organization of the mesenteries is not coincident on both sides of the body, indicating a considerable difference in the development of mesenteries (
Type material
Department of Zoology, University of Stockholm, Sweden (holotype) (?).
Cerianthus punctatus
Cerianthus punctatus
Uchida, 1979: 189–195;
Type locality
Suruga Bay (Numazu), Japan.
Distribution
Only known from shallow water at the type locality.
Remarks
The available information on this species is amongst the most complete from before the advent of detailed descriptions in the 2000s.
Type material
Saibura Marine Park Research Station (lost?), but the original description provided a graphic representation.
Cerianthus roulei
Cerianthus lloydii
Gosse, 1859: 50;
Cerianthus roulei
Carlgren, 1912a: 3–5;
Type locality
close to Svalbard, Norway, Greenland Sea.
Distribution
Svalbard, Norway, Greenland Sea; depth unknown.
Remarks
This species has a very deficient description and is represented by very few museum specimens for comparison. The description of C. lloydii by
Type material
Not found in this study.
Cerianthus stimpsonii
Cerianthus stimpsonii
Verrill, 1868: 317–318;
Type locality
Port Lloyd, Bonin Islands (Ogasawara Islands), Japan.
Distribution
Only known from shallow water (18 m depth) at the type locality.
Remarks
Based on the description by
Type material
Not found in this study.
Cerianthus sulcatus
Cerianthus sulcatus
Kwietniewski, 1898: 427;
(?) Cerianthus sulcatus:
Type locality
Raha, Ambon, Moluccas, Indonesia.
Distribution
Only known from shallow water at the type locality.
Remarks
This species was described by
Type material
Not found in this study.
Cerianthus taedus
Cerianthus taedus
McMurrich, 1910: 30–31;
Type locality
Makassar Strait, Central Sulawesi, Indonesia.
Distribution
Only known from deep water (at 724 m depth) at the type locality.
Remarks
This species was described based on only one damaged specimen, which was 6 cm long, with 55 marginal and labial tentacles arranged in two and four cycles, respectively. The organization of the mesenteries was not described in detail by
Type material
Possibly lost (Zoological Museum of Amsterdam, now Naturalis Biodiversity Center, Leiden).
Cerianthus valdiviae
Cerianthus valdiviae
Carlgren, 1912a: 44–47;
Type locality
Between Keeling and south Sumatra, Indian Ocean.
Distribution
Only known from deep water (at 5000 m depth) at the type locality.
Remarks
This species was initially described in a table by
Type material
Not found in this study.
Cerianthus vas
Cerianthus vas
McMurrich, 1893: 202–203, 206;
Type locality
Cedros Island, Mexico (Pacific coast).
Distribution
Only known from shallow to deep water (at 80 m depth) at the type locality.
Remarks
This is a doubtful species, as the original description is very incomplete, and some characters are incongruent.
Type material
Not found in this study, but the original description provided a graphic representation.
Cerianthus vogti
Cerianthus vogti
Danielssen, 1890: 137–142;
Cerianthus abyssorum
Danielssen, 1890: 143;
Type locality
Norwegian Sea (not specified).
Distribution
Only known from deep water (at 900–1400 m depth) at the type locality.
Remarks
This species is well known, even though it is a species from deeper areas. The description by
Type material
Not found in this study.
Pachycerianthus
Type species
Pachycerianthus multiplicatus Carlgren, 1912a (proposed by
Number of valid species: 16
Comparison of anatomical features of Pachycerianthus species (after Stampar et al. 2015).
| Species | Directive mesenteries length | Directive labial tentacle | M-mesentery (M1) length | M-mesentery (M2) length | M-mesentery (m1) length | M-mesentery (m2) length | Mesenteries attached to siphonoglyph | Siphonoglyph shape | Number of marginal tentacles |
|---|---|---|---|---|---|---|---|---|---|
| P. aestuari | > stomodeum | ? | Reach aboral pore | ≅ M-1 | 1/5 of M-1 | = m-1 | 16 | Wide | 30–34 |
| P. benedeni | < stomodeum | ? | Reach aboral pore | ? | ? | ? | 6? | Wide? | ~125 |
| P. borealis | > stomodeum | ? | Reach aboral pore | = M-1 | 3/4 of M-1 | ~1/3 of M-1 | 8 | Wide | 139–155 |
| P. curacaoensis | > stomodeum | Absent | Reach aboral pore | 1/2 of M-1 | 1/4 of M-1 | 2/3 of m-1 | 4 | Short and narrow | 74–105 |
| P. delwynae | > stomodeum | Present | Almost reach aboral pore | Larger than M-1 | 1/3 of M-1 | 1/2 of M-1 | 6 | Narrow | 89–114 |
| P. dohrni | ? | Half column (?) | > M-1 | ? | ? | ? | ? | ~160 | |
| P. fimbriatus | > stomodeum | Present | Reach aboral pore | 3/4 of M-1 | 1/3 of M-1 | 1/3 of M-1 | 8 | Wide and long | <60 |
| P. insignis | < stomodeum | Present | Almost reach aboral pore | ≅M-1 | ≅M-1 | ≅M-2 | 8 | ? | ~100 |
| P. johnsoni | < stomodeum | ? | Reach aboral pore | ≅3/4 of M-1 | 3/4 of M-1 | 1/2 of M-1 | 8 | Wide | ~108 |
| P. longistriatus | > stomodeum | Present | Reach aboral pore | =M-1 | 1/3 of M-1 | 1/4 of M-1 | 6 | Wide | 138–140 |
| P. magnus | > stomodeum | Present | Almost reach aboral pore | 3/4 of M-1 | 1/3 of M-1 | 1/2 of M-1 | 6 | Short and narrow | ~120 |
| P. maua | < stomodeum | Absent | Reach aboral pore | 1/4 of M-1? | 1/3 of M-1? | 1/3 of M-1? | 6 | Narrow | ~150 |
| P. monostichus | > stomodeum | Present | Reach aboral pore | ≅M-1 | 1/2 of M-1 | ≅m-1 | 8 | Narrow and long | ~47 |
| P. multiplicatus | > stomodeum | Absent | Reach aboral pore | =M-1 | 1/3 of M-1 | 1/3 of M-1 | 6 | Narrow | 175 |
| P. nobilis | ? | ? | ? | ? | ? | ? | ? | ? | 160–170 |
| P. schlenzae | > stomodeum | Present | Reach aboral pore | 3/4 of M-1 | 1/2 of M-1 | 1/3 of M-1 | 6 | Long and narrow | 60–85 |
| P. solitarius | > stomodeum | Present | Reach aboral pore | ≅ M-1 | 1/4 of M-1 | 1/5 of M-1 | 6 | Narrow | ~64 |
Pachycerianthus aestuarii
Cerianthus aestuarii: Child, 1908: 27–53; Torrey and Kleeburger 1909: 115–119, 121, 123;
Pachycerianthus aestuari: McMurrich, 1910: 11;
Pachycerianthus aestuarii:
Type locality
Mission Bay, East Pacific, California, United States of America.
Distribution
East Pacific, California, USA, shallow waters.
Remarks
This species was described by Torrey and Kleeburger (1909) based on specimens obtained from Mission Bay, California. This description is not very detailed but relevant information about its morphology is available.
Type material
Not found in this study, but the original description provided a graphic representation.
Pachycerianthus borealis
Cerianthus borealis:
Cerianthus verrillii McMurrich, 1910: 10–11
Pachycerianthus borealis:
Type locality
Georges Bank, Massachusetts, United States/Nova Scotia, Canada (not specified).
Distribution
Northwestern Atlantic (Arctic Sea to North Carolina, USA), at depths of 10–500 m.
Remarks. This species was described by
Type material
Peabody Museum of Natural History (Yale – YPM 9830, 9831, 9832 (Syntype).
Pachycerianthus curacaoensis
Pachycerianthus curacaoensis
den Hartog, 1977: 215–221, 237;
Type locality
Curaçao, Dutch Caribbean.
Distribution
Caribbean Sea (Curaçao), at 65–75 m depth.
Remarks
This species was described by
Type material
Naturalis Biodiversity Center (former Rijksmuseum van Natuurlijke Historie, Leiden – RMNH.COEL.11359 (holotype).
Pachycerianthus delwynae
Pachycerianthus delwynae
Carter, 1995: 2–3;
Type locality
off Port Jackson, Sydney harbor, Australia.
Distribution
Sydney harbor, Australia, at 5–15 m depth.
Remarks
This is one of two species of this genus described from Australia by
Type material
Australian Museum; AMG15399 (holotype).
Pachycerianthus dohrni
Cerianthus membranaceus viridis Andres, 1881: 332
Cerianthus membranaceus Andres, 1883: 347–349
Cerianthus dohrni:
In part Cerianthus viridis Torelli, 1932: 1–15
Pachycerianthus dohrni:
Type locality
Naples, Tyrrhenian Sea, Italy.
Distribution
Tyrrhenian Sea, Italy and Aegean Sea, Greece, shallow waters.
Remarks
This species was initially described from the Italian coast (Naples region) as a variation of Cerianthus membranaceus (
Type material
Not designated (several specimens mentioned, which can be considered syntypes).
Pachycerianthus fimbriatus
(?) Cerianthus elongatus Kwietniewski, 1898: 426–427;
Pachycerianthus fimbriatus
McMurrich, 1910: 35–38;
Pachycerianthus plicatus
Carlgren, 1924: 182–186, 195;
(?) Pachycerianthus torreyi Arai, 1965: 205–210;
Type locality
Cebu, Philippines.
Distribution
Sulu Sea and Celebes Sea, Philippines, and Indonesia, (?) Pacific Coast of US and Canada; shallow waters.
Remarks
This species forms part of a taxonomic problem. The description of P. fimbriatus was based on a study of 15 specimens collected mainly from the Celebes Sea, Philippines, by
Type material
The provenance data of a specimen in the Natural History Museum at London, NHMUK 1889.11.25.64, is coherent with the locality and dates in the original description, but it is impossible to make an exact connection between the materials.
Pachycerianthus insignis
Pachycerianthus insignis
Carlgren, 1951: 435–436;
Type locality
El Mogote, Baja California, Mexico.
Distribution
Gulf of California, Mexico; shallow waters.
Remarks
Although this species occurs in an area with a long history of marine research, it is still little known, and the only study focused on this species is the original description by
Type material
Smithsonian National Museum of Natural History – USNM 49454 (Holotype).
Pachycerianthus johnsoni
Cerianthus johnsoni
Torrey and Kleeburger, 1909: 116, 119, 123–125;
Pachycerianthus johnsoni:
Type locality
Los Angeles, East Pacific, United States of America.
Distribution
Only known from shallow water at the type locality.
Remarks
This is another species described from the United States’ Pacific Coast by Torrey and Kleeburger (1909) with a relatively good amount of detail; like P. insignis, there have been no more subsequent detailed or comparative studies.
Type material
Not found in this study, but the original description provided a graphic representation.
Pachycerianthus longistriatus
Pachycerianthus longistriatus
Carter, 1995: 3–5;
Type locality
off Port Jackson, Sydney harbor, Australia.
Distribution
Sydney Harbor, Australia; 5–10 m depth.
Remarks
As mentioned for P. delwynae, the taxonomic status between the two Australian species, P. delwynae and P. longistriatus, is not clear. Both were described from a very restricted area and the morphological variation between them is very subtle. There is a need for a more detailed study approach to understand the differences between these two currently valid species.
Type material
Australian Museum – AM G15402 (Holotype).
Pachycerianthus magnus
Cerianthus magnus Nakamoto, 1919: 118–120
Pachycerianthus magnus:
Type locality
south of Jogashima, Sagami Bay, Miura, Kanagawa, Japan (at 1100 m depth).
Distribution
Japan and China, shallow to deep waters.
Remarks
The description of Cerianthus magnus by
Type material
Not found in this study, but the original description provided a graphic representation.
Pachycerianthus maua
Cerianthus maua
Carlgren, 1900: 27–29;
Cerianthus mana
Pachycerianthus maua:
Type locality
Mkokotoni, Zanzibar, Tanzania.
Distribution
Indian Ocean (Mozambique, Madagascar, and Tanzania) and Aden Gulf (Djibouti) and Red Sea (Egypt and Saudi Arabia), shallow waters.
Remarks
This species was described by
Type material
Not found in this study, but the original description provided a graphic representation.
Pachycerianthus monostichus
Pachycerianthus monostichus
McMurrich, 1910: 38–39;
Type locality
Ambon, Maluku, Indonesia.
Distribution
Only known from shallow water at the type locality.
Remarks
This species was described by
Type material
Not found in this study, but the original description provided a graphic representation.
Pachycerianthus multiplicatus
Cerianthus membranaceus:
Cerianthus danielssen:
Pachycerianthus multiplicatus
Carlgren, 1912a: 5–11;
(?) Pachycerianthus multiplicatus:
Type locality
Two areas are mentioned – Kattegat Strait and Trondheim, Norway (not specified)
Distribution
North, Inner, Celtic, Irish and Norwegian Seas, Gulf of Biscay, at < 130 m depth.
Remarks.
Type material
(?) Lund Museum of Zoology (MZLU) - 6570 (syntype), but not formally designated in description.
Pachycerianthus nobilis
Cerianthus nobilis
Haddon and Shackleton, 1893: 116, 118;
Pachycerianthus nobilis:
Type locality
Thursday Island, Queensland, Australia.
Distribution
Queensland and Northern Territory, Australia, New Caledonia, shallow waters.
Remarks
A large species originally described from northeastern Australia as Cerianthus nobilis. This description is very simple and was based only on external characters and there have been no further studies based on specimens from this area.
Type material
Museum of Zoology (University of Cambridge) – I.33575.A-B (holotype).
Pachycerianthus schlenzae
Pachycerianthus
sp.
Pachycerianthus schlenzae
Type locality
off Guarapari, Espírito Santo state, Brazil.
Distribution
Brazil, from Bahia to Espírito Santo states (Abrolhos Bank and Royal Charlotte Bank), at 5–10 m depth.
Remarks
This species was recently described based on a study of several specimens from the central area of the Brazilian coast, where it is an endemic occurring along a coastline of approximately 500 km length. Some aspects of external morphology are similar to those of P. curacaoensis and may reflect a correlated evolutionary history between the two species. Although
Type material
Museu de Zoologia, Universidade de São Paulo (MZSP) – 1949 (Holotype).
Pachycerianthus solitarius
Tubularia solitaria
Rapp, 1829a: 656–658;
(?) Cereus cupreus Ilmoni, 1830: 689–699;
Cerianthus brerae Delle Chiaje, 1841: 136
Edwardsia vestita
Forbes, 1843: 42;
Cerianthus membranaceus:
Cerianthus solitarius:
Pachycerianthus solitarius:
(?) Pachycerianthus solitarius: Kisseleva 1975: 1595–1596;
Cerianthus bicyclus Torelli, 1961: 17–28
Type locality
off Languedoc coast, France.
Distribution
Mediterranean Sea, Azores, and (?) Black Sea; shallow waters.
Remarks
After Cerianthus membranaceus, this was the second species to be formally described in Ceriantharia. It was first described as an unclassified polyp with some similarities with Hydrozoa and Anthozoa (
Type material
Not found in this study.
Family Botrucnidiferidae Carlgren, 1912
Number of valid taxa: two genera and four species.
Botruanthus
Type species
Botruanthus benedeni (Torrey & Kleeberger, 1909)
Number of valid species: 2
Comparison of anatomical features of Botruanthus species (after
| B. benedeni | B. mexicanus | |
|---|---|---|
| Marginal tentacles | Up to 90–100 | Up to 40–60 |
| Directive labial tentacle | Present | Present |
| Arrangement of labial tentacles | (1)321.3213.3213 | (2)314.2314.2314.2314 |
| Actinopharynx | 1/3 – 1/4 of gastric cavity | 1/5–1/4 of gastric cavity |
| Oral disc | 1.1–1.3 cm | 0.5–0.7 cm |
| Siphonoglyph | Broad, 8 mesenteries attached | Narrow, 2 mesenteries attached |
| Directive mesenteries | >Actinopharynx (= size of Actinopharynx) | > Actinopharynx |
| P2 | Long, almost to aboral pole (> 2/3 of gastric cavity) | Short (<1/3 of gastric cavity) |
| P3 | Short (1/3 of P2) | Short (~P2) |
| M1 | To aboral pore | Almost to aboral pore |
| M3 | Almost to aboral pore | Short, 1/2 of M1 |
| Cnido-glandular tract at fertile mesenteries of first quartets | Present | Present |
| Craspedion tract at fertile mesenteries | 5/7–8/9 | 8/9 |
| Cnido-glandular tract at B | < ½ | 3/4 |
|
Craspedonemes of craspedion at fertile mesenteries |
Sometimes present | Sometimes present |
| Botrucnidae | Rare in m and B, absent in M and b mesenteries | Very abundant (4-5 groups) in M and m, absent in B and b mesenteries |
Botruanthus benedeni
Pachycerianthus benedeni Roule, 1904: 708–710
Cerianthus benedeni:
Botryanthus benedeni:
Botruanthus benedeni:
Type locality
San Diego Bay, California, United States of America.
Distribution
California (United States of America), Baja California (Mexico) and Galapagos Islands (Ecuador), shallow waters.
Remarks
This species was described based on a study of a single specimen. This species (and genus) is characterized by possessing wart-like structures (cnidorages) organized in bunches (botrucnids) in the mesenterial filaments. Except for these structures, the anatomy is very similar to species of the genus Pachycerianthus. The holotype is not available, and we therefore here designate a neotype collected from the same region by Charles Cutress in 1955 (NMNH 49400). This specimen was studied by
Type material
Smithsonian National Museum of Natural History (USNM) – 49400 (neotype).
Botruanthus mexicanus
Botruanthus mexicanus
Type locality
off Veracruz, Mexico.
Distribution
Gulf of Mexico, intertidal to shallow waters.
Remarks
This species was recently described by specimens from the intertidal zone in reefs of Central Mexico in the Gulf of Mexico. Morphological characterization is quite easy, as the number of anatomical characters allow its distinction in relation to B. benedeni. There have been no studies on ecological or biological aspects of this species.
Type material
Museu de Zoologia da Universidade de São Paulo; MZUSP 002757 (Holotype).
Botrucnidifer
Type species
Botrucnidifer norvegicus Carlgren, 1912
Number of valid species: two
| B. novergicus | B. shtokmani | |
|---|---|---|
| Marginal tentacles | Up to 17 | 72 |
| Directive labial tentacle | Present | Absent |
| Arrangement of labial tentacles | (1)431.3231.3231 | (0)230.2024.3123.3142 |
| Actinopharynx | 1/4 – 1/5 of gastric cavity | 1/3 of gastric cavity |
| Oral disc | 0.3 cm | 1.5 cm |
| Siphonoglyph | Narrow, 2 mesenteries attached | Narrow, 4 mesenteries attached |
| Directive mesenteries | >Actinopharynx | = Actinopharynx |
| P2 | Long, almost to aboral pole (> 4/5 of gastric cavity) | Regular (<2/3 of gastric cavity) |
| P3 | Long (2/3 of P2) | Short, 1/2 of P2 |
| M1 | Almost to aboral pore | = P2 |
| M3 | Almost to aboral pore (3/4 of M1) | Long, 3/4 of P2 |
| Cnido-glandular tract at fertile mesenteries of first quartets | Present | Present |
| Craspedion tract at fertile mesenteries | ¾ | 1/2 – 3/4 |
| Cnido-glandular tract at B | Present | Present |
| Botrucnidae | Only in M mesenteries | Only in B/b mesenteries |
Botrucnidifer norvegicus
Botrucnidifer novergicus
Carlgren, 1912a: 30–34;
Type locality
Trondheimfjord, Trondheim, Norway.
Distribution
Norwegian Sea, at 50–700 m depth.
Remarks
This species was described by
Type material
Lund Museum of Zoology (MZLU) – L898/3051 and Marine invertebrate collection Norwegian University of Science and Technology University Museum (NTNU) – 40499 (syntype).
Botrucnidifer shtokmani
Botrucnidifer shtokmani
Molodtsova, 2001a: 773;
Type locality
off Namibia coast (southeast Atlantic), at 130–350 m depth.
Distribution
Only known from deep water at the type locality.
Remarks
This species was described based on dredged specimens from off the Namibian coast. This is the second species of this genus that has been sampled beyond conventional SCUBA diving depths. The description of this species (in Russian) is very detailed and addresses all the necessary characters. As discussed by
Type material
Zoological Museum of Moscow University – ZMMU EC-100 (holotype).
Order Penicillaria den Hartog, 1977
Number of valid taxa: one family, two genera, and nine species
Family Arachnactidae McMurrich, 1910
Number of valid taxa: two genera, and nine species
Arachnanthus
Type species
Arachnanthus oligopodus (Cerfontaine, 1891)
Number of valid species: Five
Comparison of anatomical features of Arachnanthus species (after
| A. australiae | A. bockii | A. oligopodus | A. sarsii | A. lilith | |
|---|---|---|---|---|---|
| Marginal tentacles | Up to 40 | Up to 30 | ~20 | Up to 35 | Up to 24 |
| Arrangement of labial tentacles | (0)1.11.11.11.11 | (0)1.11.11.11.11(?) | (0)1.11.11.11.11 | (0)1.11.11.11.11 | (0)3.12.31.23.23.12 |
| Length of actinopharynx | ~2/3 of gastric cavity | ~1/2 of gastric cavity | ~1/2 of gastric cavity | ~1/2 of gastric cavity | >1/2 of gastric cavity |
| Hyposulcus | ~1/2 size of stomodeum | ~1/2 size of stomodeum | ~2X size of stomodeum | < size of stomodeum | = size of stomodeum |
| Oral disc diameter | ~0.7 cm | – | – | ~1 cm | 0.5 cm |
| Mesentery attachment to actinopharynx | Broad, 12 mesenteries attached | Broad, 12 mesenteries attached | Narrow, 4 mesenteries attached | Broad, 6 mesenteries attached | Broad, 8 mesenteries attached |
| Directive mesenteries | = length of Actinopharynx | < length of Actinopharynx | > length of Actinopharynx | < length of Actinopharynx | < length of Actinopharynx |
| P(C)2 | Short, 1/2 of gastric cavity | Very short, 1/4 of gastric cavity | Short, 1/2 of gastric cavity | Long, 3/4 of gastric cavity | Long, 6/7 of gastric cavity, almost to aboral pole |
| P(C)3 | Very short, <1/4 of gastric cavity | Very short, <1/4 of gastric cavity | Short, ~1/2 of gastric cavity | Short, ~1/3 of gastric cavity | Short, 1/3 of gastric cavity |
| M1 | Almost to aboral pore | Almost to aboral pore | To aboral pore | Almost to aboral pore | To aboral pore |
| M3 | 4/5 of gastric cavity | Almost to aboral pore | 1/5 of gastric cavity | Almost to aboral pore | 3/4 of gastric cavity |
| Cnido-glandular tract of fertile mesenteries | Present (short?) | Present (short?) | Present | Present | Present |
| Cnido-glandular tract of B | Present (short?) | Present (short?) | Present (short?) | Present (short) | Present (short) |
| Acontioids | Only in M1, M2 and M3 | Only in M1, M2 and M3 | Only in M1 | Only in M1, M2 and M3 | Only in M3 and M4 |
Arachnanthus australiae
Arachnanthus australiae
Carlgren, 1937: 177–180;
Type locality
Low Isles, Queensland, Australia.
Distribution
Queensland, Australia, shallow waters.
Remarks
Type material
Natural History Museum (London); NHMUK – 1954.6.25.47 (holotype).
Arachnanthus bockii
Arachnanthus bockii
Carlgren, 1924: 193–195;
Type locality
Viti Levu, Fiji.
Distribution
Only known from shallow water at the type locality.
Remarks
This is another species with little information, except for the morphological description. There are some characters in
Type material
Not found in this study, but the original description provided a graphic representation.
Arachnanthus oligopodus
Cerianthus oligopodus
Cerfontaine, 1891a: 32–38;
Pachycerianthus oligopodus:
Arachnanthus oligopodus:
Type locality
Italian Coast, Mediterranean Sea (not specified in detail).
Distribution
Mediterranean Sea, shallow waters and caves.
Remarks
Arachnanthus oligopodus was initially described as a species of the genus Cerianthus by
Type material
Not found in this study.
Arachnanthus lilith
Arachnanthus lilith
Stampar and El Didi in
Type locality
island near Jaz’air Sila, Saudi Arabia.
Distribution
Red Sea, shallow waters.
Remarks
This species was recently described from shallow Saudi Arabian waters of the Red Sea. Morphological characterization was based on internal anatomy and there have been no studies on ecological or biological aspects of this species yet.
Type material
Florida Museum of Natural History – FLMNH UF9168 (holotype).
Arachnanthus sarsi
Arachnanthus sarsi
Carlgren, 1912a: 27–30;
Arachnanthus sarsii:
Type locality
Röberg Indalbay, Trondheim, Norway.
Distribution
North Sea, at 10–200 m depth.
Remarks
This species is rather common in some areas of Great Britain and Scotland and there are two detailed descriptions; the original (
Type material
Swedish Museum of Natural History (Naturhistoriska riksmuseet) – NRM 134778 (Holotype).
Isarachnanthus
Type species
Isarachnanthus maderensis (Johnson, 1861)
Number of valid species: 4
| I. bandanensis | I. maderensis | I. nocturnus | I. panamensis | |
|---|---|---|---|---|
| Marginal tentacles | Up to 40 | Up to 42 | Up to 60 | Up to 32 |
| Arrangement of labial tentacles | (3)413.4242.4312.4312 | (1)1.11.11.11.11 | (1)2.12.12.12.12 | (2)431.4231.4231 |
| Length of actinopharynx | ~1/4 of gastric cavity | ~2/5 of gastric cavity | ~2/5 of gastric cavity | ~1/2 to 1/3 of gastric cavity |
| Hyposulcus | ~2/3 size of stomodeum | = size of stomodeum | = size of stomodeum | = size of stomodeum |
| Oral disc diameter | ~2cm | 2 cm | 3.5 cm | ~0.5 cm |
| Mesentery attached to siphonoglyph | Broad, 18 mesenteries attached | Broad, 10 mesenteries attached | Broad, 12-14 mesenteries attached | Broad, 16 mesenteries attached |
| Directive mesenteries | = length of Actinopharynx | > length of Actinopharynx | > length of Actinopharynx | >length of Actinopharynx |
| P(C)2 | Long, 3/4 of gastric cavity | Short, 1/3 of gastric cavity | Short, 1/3 of gastric cavity | Long, 3/4 of gastric cavity |
| P(C)3 | Very short, <1/8 of gastric cavity | Very short, ~1/6 of gastric cavity | Very short, ~1/5 of gastric cavity | Short, ~1/5 of gastric cavity |
| M1 | Almost to aboral pore | Almost to aboral pore | Almost to aboral pore | Reach aboral pore |
| M3 | Almost to aboral pore | Almost to aboral pore | Almost to aboral pore | Reach aboral pore |
| Cnido-glandular tract of fertile mesenteries | Present (short?) | Present | Present | Present (short?) |
| Cnido-glandular tract of B | Present (short?) | Present (short) | Present (short) | Present (short?) |
| Acontioids | Only in M1- M4 | Only in M1-M6 | M1-M3 (sometimes in M4 and M5) | Only in M1- M5 or absent |
Isarachnanthus bandanensis
Isarachnanthus bandanensis
Carlgren, 1924: 187–190, 195;
Type locality
Neira, Banda Island, Indonesia.
Distribution
Indonesia, French Polynesia, and Hawaii (USA), shallow waters.
Remarks
This species was described based on two specimens from the Banda Islands, Indonesia. The diagram of mesenteries, part of cnidome, and tentacle organization are present in the original description, however, there are some evident similarities in relation to Isarachnanthus panamensis. Furthermore, unpublished molecular data indicate similarity between these two species and studies on this clade should be prioritized.
Type material
Zoological Museum of Amsterdam (now Naturalis Biodiversity Center, Leiden) – (ZMA.COEL.000209 – Lectotype/ ZMA.COEL.000210 – Paralectotype).
Isarachnanthus maderensis
Saccanthus maderensis
Johnson, 1861: 305–306;
Cerianthus maderensis:
In part Cerianthus membranaceus
Arachnanthus nocturnus:
Isarachnanthus cruzi Brito, 1986: 174–181
? Cerianthus sp.
Isarachnanthus maderensis:
Type locality
Madeira Island, Portugal.
Distribution
Madeira Island (Portugal), Ascension Island, Rocas Atoll (Brazil), Caribbean Sea, (?) Mediterranean Sea; at 2–30 m depth.
Remarks
This species was described by
Type material
Not found in this study.
Isarachnanthus nocturnus
Cerianthus natans:
Ceriantheopsis
sp.
Arachnanthus nocturnus
den Hartog, 1977: 221–230;
Isarachnanthus nocturnus:
Isarachnanthus
sp.
Tessera gemmaria
Goy, 1979: 288–289;
Type locality
Piscadera Bay, Curaçao, Dutch Caribbean.
Distribution
Caribbean Sea, South Atlantic (Argentina; Brazil), at 1–20 m depth.
Remarks
This species was described by
Type material
Naturalis Biodiversity Center, Leiden (former Rijksmuseum van Natuurlijke Historie) – RMNH.COEL.11364 (Holotype).
Isarachnanthus panamensis
Isarachnanthus panamensis
Carlgren, 1924: 190–193, 195;
Type locality
Taboga, Panama (Pacific coast).
Distribution
Only known from shallow water at the type locality
Remarks
This species was described from the Panama coast based on three specimens. The description is also detailed, including two mesentery diagrams. Thus, variation in mesenterial organization is quite evident, especially in relation to the size of directive mesenteries. As discussed above, regarding Isarachnanthus bandanensis, these two species are very similar in terms of both morphological and molecular data and further studies are needed.
Type material
Zoological Museum of Amsterdam (now Naturalis Biodiversity Center, Leiden) – ZMA.COEL .000211) (holotype).
Key to species
* Species with limited information on their anatomy, therefore key must be used with caution.
| 1a | Ceriantharia with mesenteries organized in doublets (Spirularia) | 2 |
| 1b | Ceriantharia with mesenteries organized in quartets (Penicillaria) | 16 |
| 2a | Ceriantharia with cnidorage (botrucnidae) | 3 |
| 2b | Ceriantharia without cnidorage (botrucnidae) | 6 |
| 3a | Cnidorage on appendages united as botrucnidae | 4 |
| 3b | Cnidorage over mesenteries | 5 |
| 4a | P-mesenteries (P2) and M-mesenteries (M3) long, almost to aboral pore | Botruanthus benedeni (Torrey & Kleeburger, 1909) |
| 4b | P-mesenteries (P2) and M-mesenteries (M3) short, 1/2 to 1/3 of gastric cavity | Botruanthus mexicanus Stampar, González-Muñoz & Morandini, 2016 |
| 5a | Directive mesenteries much longer than hyposulcus | Botrucnidifer novergicus Carlgren, 1912 |
| 5b | Directive mesenteries shorter or equal than hyposulcus | Botrucnidifer shtokmani Molodtsova, 2001 |
| 6a | Ceriantharia with all mesenteries except directives fertile | 7 |
| 6b | Ceriantharia with second couple of protomesenteries (P) short and sterile | 8 |
| 6c | Ceriantharia with second couple of protomesenteries (P) long and fertile, mesenteries in quartets m, B, M, b | 11 |
| 6d | Ceriantharia with second couple of protomesenteries (P) long and fertile, mesenteries in quartets M, B, m, b | 12 |
| 7a | Directive mesenteries of the same length as protomesenteries 3 (P3) | Ceriantheomorphe brasiliensis (Mello-Leitão, 1919) |
| 7b | Directive mesenteries shorter than protomesenteries 3 (P3) | Ceriantheomorphe ambonensis (Kwietniewski, 1898) |
| 7c | Directive mesenteries longer than protomesenteries 3 (P3) |
Ceriantheomorphe adelita Lopes, Morandini & Stampar in |
| 8a | Number of marginal tentacles – less than 90 | 9 |
| 8b | Number of marginal tentacles – more than 115 | 10 |
| 9a | Metamesenteries 2 (M2) longer than ¾ of metamesenteries 1 (M1) and 6 mesenteries attached to siphonoglyph | Pachycerianthus schlenzae Stampar, Silveira & Morandini, 2014 |
| 9b | Metamesenteries 2 (M2) longer than ¾ of metamesenteries 1 (M1) and more than 90 marginal tentacles | Pachycerianthus johnsoni (Torrey & Kleeburger, 1909) |
| 9c | Metamesenteries 2 (M2) longer than ¾ of metamesenteries 1 (M1) and less than 70 marginal tentacles | Pachycerianthus fimbriatus (Kwietniewski, 1898) |
| 9d | Metamesenteries 2 (M2) longer than half of metamesenteries 1 (M1) and 4 mesenteries attached to siphonoglyph | Pachycerianthus curacaoensis den Hartog, 1977 |
| 9e | Metamesenteries 2 (M2) longer than metamesenteries 1 (M1), 6 mesenteries attached to siphonoglyph and directive labial tentacle present | Pachycerianthus delwynae Carter, 1995 |
| 9f | Metamesenteries 2 (M2) longer than Metamesenteries 1 (M1) and 16 mesenteries attached to siphonoglyph | Pachycerianthus aestuarii (Torrey & Kleeburger, 1909) |
| 9g | Metamesenteries 2 (M2) and metamesenteries 1 (m1) longer than metamesenteries 1 (M1) and 8 mesenteries attached to siphonoglyph | Pachycerianthus insignis Carlgren, 1951 |
| 9h | Metamesenteries 2 (M2) and metamesenteries 2 (m2) longer than metamesenteries 1 (M1) and 8 mesenteries attached to siphonoglyph | Pachycerianthus monostichus McMurrich, 1910 |
| 9i | Metamesenteries 1 (m1) longer than ¼ of Metamesenteries 1 (M1) and 6 mesenteries attached to siphonoglyph | Pachycerianthus solitarius van Beneden, 1924 |
| 10a | Metamesenteries 2 (M2) longer than metamesenteries 1 (M1) and metamesenteries 1 (m1) longer than than ¾ of M1 | Pachycerianthus borealis Kingsley, 1904 |
| 10b | Metamesenteries 2 (M2) longer than metamesenteries 1 (M1) and metamesenteries 1 (m1) longer than 1/3 of M1, labial directive tentacle present | Pachycerianthus longistriatus Carter, 1995 |
| 10c | Metamesenteries 2 (M2) longer than ¾ of metamesenteries 1 (M1) and metamesenteries 1 (m1) longer than 1/3 of M1 | Pachycerianthus magnus Uchida, 1979 |
| 10d | Metamesenteries 2 (M2) longer than 1/4 of metamesenteries 1 (M1) and metamesenteries 1 (m1) longer than 1/3 of M1 | Pachycerianthus maua Carlgren, 1900 |
| 10e | Metamesenteries 2 (M2) longer than metamesenteries 1 (M1) and metamesenteries 1 (m1) longer than 1/3 of M1, labial directive tentacle absent | Pachycerianthus multiplicatus Carlgren, 1912 |
| 10f | Polyp with more than 160 tentacles from Australia | Pachycerianthus nobilis (Haddon & Shackleton, 1894) |
| 10g | Polyp with more than 160 tentacles from Mediterranean Sea | Pachycerianthus dohrni van Beneden, 1924 |
| 11a | Polyp with up to 60 marginal tentacles and directive labial tentacle absent | Ceriantheopsis lineata Stampar, Scarabino, Pastorino & Morandini, 2015 |
| 11b | Polyp with up to 70 marginal tentacles and cnido-glandular tract at fertile mesenteries present | Ceriantheopsis austroafricana Molodtsova, Griffiths & Acuña, 2011 |
| 11c | Polyp with up to 70 marginal tentacles and cnido-glandular tract at fertile mesenteries absent | Ceriantheopsis nikitai Molodtsova, 2001 |
| 11d | Polyp with more than 90 marginal tentacles and short directive mesenteries | Ceriantheopsis americana (Agassiz in Verrill, 1864) |
| 12a | Polyp from India (shallow waters) with more than 150 marginal tentacles | Cerianthus andamanensis Alcock, 1893* |
| 12b | Polyp from India (deep sea ~ 5000 m) with up to 40 marginal tentacles and directive labial tentacle absent | Cerianthus valdiviae Carlgren, 1912* |
| 12c | Polyp from North Atlantic (deep sea ~ 5000 m) with up to 30 marginal tentacles | Cerianthus bathymetricus Moseley, 1877* |
| 12d | Polyp from Red Sea (shallow waters) with up to 20 marginal tentacles | Cerianthus medusula (Klunzinger, 1877)* |
| 12e | Description with information about mesentery organization and tentacle distribution | 13 |
| 13a | Species from Pacific Ocean | 14 |
| 13b | Species from Atlantic Ocean | 15 |
| 14a | Protomesenteries 2 (P2) short, sterile and metamesenteries 1 (M1) reach or almost reach the aboral pore, marginal/ labial tentacles in 4 pseudocycles | Cerianthus (?) mortenseni Carlgren, 1924 |
| 14b | Polyp from Japan, Korea or China, marginal tentacles in 4 pseudocycles and directive in position 2, labial tentacles in 4 pseudocycles and directive in position 3 | Cerianthus filiformis Carlgren, 1924 |
| 14c | Polyp from Japan, marginal tentacles in 3 pseudocycles and directive in position 2, labial tentacles in 4 pseudocycles and directive in position 2 | Cerianthus japonicus Carlgren, 1924 |
| 14d | Polyp from Japan, marginal tentacles in 4 pseudocycles and directive in position 2, labial tentacles in 4 pseudocycles and directive in position 2 | Cerianthus punctatus Uchida, 1979 |
| 14e | Polyp from Indonesia, marginal tentacles in 4 pseudocycles and directive in position 2, labial tentacles in 4 pseudocycles and directive in position 2 | Cerianthus sulcatus Kwietniewski, 1898 |
| 14f | Polyp from Indonesia, marginal tentacles in 2 pseudocycles and directive in position 1, labial tentacles in 4 pseudocycles and directive in position 2 | Cerianthus taedus McMurrich, 1910 |
| 15a | Polyp from North Sea/North Atlantic, directive labial tentacle absent, 4 mesenteries attached to siphonoglyph | Cerianthus lloydii Gosse, 1859 |
| 15b | Polyp from Mediterranean Sea and Central Atlantic, directive labial tentacle present, 6 mesenteries attached to siphonoglyph | Cerianthus membranaceus (Gmelin, 1791) |
| 15c | Polyp from Norwegian Sea, directive labial tentacle present, 4 mesenteries attached to siphonoglyph | Cerianthus vogti Danielssen, 1890 |
| 15d | Polyp from Namibia, mesenteries type M and m and P2 are almost of the same size | Cerianthus malakhovi Molodtsova, 2001 |
| 16a | Directive labial tentacle present | 17 |
| 16b | Directive labial tentacle absent | 18 |
| 17a | Polyp from Atlantic Ocean, microbasic P-mastigophore absent in column | Isarachnanthus nocturnus (den Hartog, 1977) |
| 17b | Polyp from Atlantic Ocean, microbasic P-mastigophore present in column | Isarachnanthus maderensis (Johnson, 1861) |
| 17c | Polyp from Pacific Ocean, directive labial tentacle in position 2 | Isarachnanthus panamensis Carlgren, 1924 |
| 17d | Polyp from Pacific Ocean, directive labial tentacle in position 3 | Isarachnanthus bandanensis Carlgren, 1924 |
| 18a | Polyp with 6 mesenteries attached to actinopharynx, protomesenteries 2 (P2) long (3/4 of gastric cavity) | Arachnanthus sarsi Carlgren, 1912 |
| 18b | Polyp with 4 mesenteries attached to actinopharynx, protomesenteries 2 (P2) short (1/2 of gastric cavity) | Arachnanthus oligopodus (Cerfontaine, 1891) |
| 18c | Polyp with 12 mesenteries attached to actinopharynx, protomesenteries 2 (P2) very short (1/4 of gastric cavity) | Arachnanthus bockii Carlgren, 1924 |
| 18d | Polyp with 12 mesenteries attached to actinopharynx, protomesenteries 2 (P2) short (1/2 of gastric cavity) | Arachnanthus australiae Carlgren, 1937 |
| 18e | Polyp with 8 mesenteries attached to actinopharynx, protomesenteries 2 (P2) long (almost to aboral pole) |
Arachnanthus lilith Stampar & El Didi in |
The species Cerianthus incertus, Cerianthus roulei, Cerianthus vas and Cerianthus stimpsonii are not included in key due to absence of characters.
Acknowledgements
This work was supported by São Paulo Research Foundation FAPESP 2015/24408-4, 2016/50389-0, 2019/03552-0, CNPq (PROTAX) 440539/2015-3 and CNPq (Research Productivity Scholarship) 301293/2019-8 to SNS, FAPESP 2016/04560-9 to MMM, FAPESP 2015/21007-9 and CNPq 309440/2019-0 to ACM, and FAPESP 2018/07622-0, 2019/14236-2 to TBS. SNS, HC and CL were supported by CAPES/CNPQ; PROTAX II 88887.301759/2018-00. SNS was also supported by Australian Museum and Research Institute (AMRI) Visiting Collection Fellowship. We are grateful to several curators and researchers who helped with information or providing access to materials (Dr Stephen Keable (Australian Museum), Dr Sadie Mills (NIWA Invertebrate Collection), Dr Bert Hoeksema (Naturalis Biodiversity Center), Dr Torkild Bakken (NTNU University Museum), Dr Kensuke Yanagi (Natural History Museum and Institute Chiba), Dr So Ishida (Osaka Museum of Natural History), Dr Takuma Fujii (Kagoshima University), Dr Danwei Huang, Dr Neo Mei Lin and Dr Nicholas Yap (National University of Singapore), Dr Priscila Grohmann (Universidade Federal do Rio de Janeiro), Dr Débora Pires (Museu Nacional – UFRJ), Dr Marymegan Daly (Ohio State University), Dr Stephen Cairns and Dr Allen Collins (Smithsonian National Museum of Natural History), Dr Gustav Paulay (Florida Museum Science), Dr Fabrizio Scarabino (Centro Universitario Regional Este UDELAR), Dr Kennet Lundin (Göteborgs Naturhistoriska Museum), Dr Antonio Carlos Marques and Dr Alvaro E Migotto (Universidade de São Paulo), Dr Marcelo V Kitahara (Universidade Federal de São Paulo), Dr Victor Quintino and Dr Ana Rodriguez (Universidade de Avero), Dr Mark Vermeij (Caribbean Research and Management of Biodiversity), Dr Mark Gibbons (University of the Western Cape), Dr Adam Reitzel (University of North Carolina at Charlotte), Dr Jason Macrander (Florida Southern College), Dr Shin Kubota (Seto Marine Biological Laboratory), and Dr Andreja Ramsak (National Institute of Biology, Slovenia)). We are also grateful to the two reviewers and Dr Bert Hoeksema (Subject Editor) who provided constructive comments on an earlier version of this manuscript. This is a publication of NP-BioMar-USP.
References
- Agassiz L (1859) On some new actinoid polyps of the coast of the United States. Proceedings of the Boston Society of natural History 7: 23–24.
- Agassiz A (1863) On Arachnactis brachiolata, a species of floating Actinia found at Nahant, Massachusetts. Journal of the Boston Society of Natural History 7: 525–531.
- Alcock A (1893) On some Actiniaria from the Indian seas. Journal of the Asiatic Society of Bengal 62: 151–153.
- Andres A (1881) Prodromus neapolitanae actiniarum faunae addito generalis actiniarum bibliographiae catalogo. Mitteilungen aus der Zoologischen Station zu Neapel 2: 305–371.
- Andres A (1883) Le Attinie. Coi Tipi der Salviucci, Roma, 460 pp.
- Arai MN (1965) A new species of Pachycerianthus, with discussion of the genus and an appended glossary. Pacific Science 19: 205–218.
- Arai MN (1971) Pachycerianthus (Ceriantharia) from British Columbia and Washington. Journal of the Fisheries Research Board of Canada 28: 1677–1680. https://doi.org/10.1139/f71-250
- Arai MN (1972) The muscular system of Pachycerianthus fimbriatus. Canadian Journal of Zoology 50: 311–317. https://doi.org/10.1139/z72-042
- Arai MN (1985) Electrical activity associated with withdrawal and feeding of Pachycerianthus fimbriatus (Anthozoa, Ceriantharia). Marine Behaviour and Physiology 12: 47–56. https://doi.org/10.1080/10236248509378632
- Arai MN, Karakashian S (1973) The fine structure of the mesogloea of the column of Pachycerianthus fimbriatus (Anthozoa). Publications of the Seto Marine Biological Laboratory 20: 719–729. https://doi.org/10.5134/175747
- Arai MN, Walder GL (1973) The feeding response of Pachycerianthus fimbriatus (Ceriantharia). Comparative Biochemistry and Physiology Part A: Physiology 44: 1085–1092. https://doi.org/10.1016/0300-9629(73)90246-6
- Ates RML (1982) De Viltkokeranemoon; Cerianthus lloydii. Het Aquarium Maandbl voor Aquarium, Terr en Insektariumkd 52: 80–84.
- Ates RML (1985) Kokeranemonen (Ceriantharia) … geen aanbeveling nodig! Het Zee-Aquarium Maandblad voor Zee-Aquarium Liefhebbers 35: 228–232.
- Ates RML (1997) Bloemdieren: De Zeeanemonen En Hun Verwanten Van De Nederlandse Kust. C. Moerman, Zeeanjer, 31 pp.
- Blanco FR (1987) Antozoos nuevos para el litoral ibérico, recolectados en Galicia. Boletin de la Real Sociedad Española de Historia Natural 83: 197–204.
- Bolam SG, Barry J, Bolam T, Mason C, Rumney HS, Thain JE, Law RJ (2011) Impacts of maintenance dredged material disposal on macrobenthic structure and secondary productivity. Marine Pollution Bulletin 62: 2230–2245. https://doi.org/10.1016/j.marpolbul.2011.04.012
- Braber L, Borghouts CH (1977) Distribution and ecology of Anthozoa in the estuarine region of the rivers Rhine, Meuse and Scheldt. Hydrobiologia 52: 15–21. https://doi.org/10.1007/BF02658077
- Brito A (1986) Descripcion de Isarachnanthus cruzi, una nueva specie de cerianthario (Cnidaria: Anthozoa: Ceriantharia) de las Islas Canarias. Vieraea 16: 173–181.
- Brown CJ, Collier JS (2007) Mapping benthic habitat in regions of gradational substrata: An automated approach utilizing geophysical, geological, and biological relationships. Estuarine, Coastal and Shelf Science 78: 203–214. https://doi.org/10.1016/j.ecss.2007.11.026
- Cairns S, Hartog C, Arneson C (1986) Class Anthozoa (Corals, anemones). In: Sterrer W, Schoepfer-Sterrer C (Eds) Marine Fauna and Flora of Bermuda. A Systematic Guide to the Identification of Marine Organisms. John Wiley & Sons Ltd., New York, 159–194.
- Calado R (2006) Marine ornamental species from European waters: a valuable overlooked resource or a future threat for the conservation of marine ecosystems? Scientia Marina 70: 389–398. https://doi.org/10.3989/scimar.2006.70n3389
- Carlgren O (1893) Studien über nordische Actinien. Kungliga Svenska Vetenskapsakademiens Handlingar 25: 1–148.
- Carlgren O (1895) Jahresberichte für 1889, 1890, und 1891 über die Anthozoen. Archiv für Naturgeschichte 61: 235–298.
- Carlgren O (1896) Jahresbericht über die Anthozoen für die Jahre 1892 und 1893. Archiv für Naturgeschichte 62: 145–180.
- Carlgren O (1900) Ostafrikanische Actinien. Gesammelt von Herrn Dr. F. Stuhlmann 1888 und 1889. Mittheilungen aus dem Naturhistorischen Museum 17: 21–144.
- Carlgren O (1906) Die Actinien-Larven. In: Brandt K, Apstein C (Eds) Nordisches Plankton. Verlag von Lipsius & Tischer, Leipzig, 65–89.
- Carlgren O (1912a) Ceriantharia. Danish Ingolf-Expedition 5: 1–79.
- Carlgren O (1912b) Über Ceriantharien des Mittelmeers. Mitteilungen aus der Zoologischen Station zu Neapel 20: 356–391.
- Carlgren O (1923) Ceriantharia und Zoantharia der Deutschen Tiefsee-Expedition. Deutsche Tiefsee-Expedition 1898–1899 19: 243–337.
- Carlgren O (1924) Papers from Dr. Th. Mortensen’s Pacific expedition 1914-16. XVI. Ceriantharia. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening 75: 169–195.
- Carlgren O (1927) Report on the Actiniaria and Ceriantharia. Transactions of the Zoological Society of London 22: 443–445. https://doi.org/10.1111/j.1096-3642.1927.tb00205.x
- Carlgren O (1928) Ceriantharier, Zoantharier och Actiniarier. Meddelelser om Grønland 23 suppl: 253–308.
- Carlgren O (1931) On some Ceriantharia. Arkiv för Zoologi 23: 1–10.
- Carlgren O (1932) Die Ceriantharien, Zoantharien und Actiniarien des arktischen Gebietes. In Eine Zusammenstellung der arktischen Tierformen mit besonderer Berücksichtigung des Spitzbergen-Gebietes auf Grund der Ergebnisse der Deutschen Expedition in das Nördliche Eismeer im Jahre 1898. Gustav Fischer, Jena, 255–266.
- Carlgren O (1937) Ceriantharia and Zoantharia. Scientific Reports of the Great Barrier Reef Expedition 1928–29 5(5): 177–207.
- Carlgren O (1940) A contribution to the knowledge of the structure and distribution of the cnidae in the Anthozoa. Kungliga Fysiografiska Sällskapets Handlingar 51: 1–62.
- Carlgren O (1942) Actiniaria Part II. Danish Ingolf-Expedition 5: 1–92.
- Carlgren O (1945) Polypdyr (Coelenterata) III. Koraldyr. Danmarks Fauna Udgivet af Dansk Naturhistorisk Forening 51: 3–167.
- Carlgren O (1951) The actinian fauna of the Gulf of California. Proceedings of the United States National Museum 101: 415–449. https://doi.org/10.5479/si.00963801.101-3282.415
- Carlgren O, Hedgpeth JW (1952) Actiniaria, Zoantharia and Ceriantharia from shallow water in the northwestern Gulf of Mexico. Publications of the Institute of Marine Science (University of Texas) 2: 143–172.
- Carter S (1995) Pachycerianthus (Anthozoa: Cerianthidea), two newly described species from Port Jackson, Australia. Records of the Australian Museum 47: 1–6. https://doi.org/10.3853/j.0067-1975.47.1995.3
- Casellato S, Masiero L, Sichirollo E, Soresi S (2007) Hidden secrets of the Northern Adriatic: “Tegnúe”, peculiar reefs. Central European Journal of Biology 2: 122–136. https://doi.org/10.2478/s11535-007-0004-3
- Cerfontaine P (1891a) Notes préliminaires sur l’organisation et le développement de différentes formes d’Anthozoaires. IV. Sur un nouveau cerianthe du golfe de Naples, Cerianthus oligopodus (n. sp.). Bulletin de l’Académie Royale des Sciences, des Lettres et des Beaux-Arts de Belgique (3me série) 21: 32–39.
- Cerfontaine P (1891b) Notes préliminaires sur l’organisation et le développement de différentes formes d’Anthozoaires. Deuxième communication. Bulletin de l’Académie Royale des Sciences, des Lettres et des Beaux-Arts de Belgique (3me série) 22: 128–148.
- Cerfontaine P (1909) Contribution à l’études des Cerianthides. Archives de Biologie 24: 653–707.
- Ceriello H, Lopes CS, Dias GM, Stampar SN (2019) A different manner to share a house: is a colonial species possible in Ceriantharia (Cnidaria; Anthozoa)? Marine Biodiversity 49(4): 2017–2020. https://doi.org/10.1007/s12526-019-00942-2
- Child CM (1903a) Form regulation in Cerianthus. 1. The typical course of regeneration. Biological Bulletin 5: 239–260. https://doi.org/10.2307/1535783
- Child CM (1903b) Form regulation in Cerianthus. 2. The effect of position, size and other factors upon regeneration. Biological Bulletin 6: 1–11. https://doi.org/10.2307/1535808
- Child CM (1904a) Form regulation in Cerianthus. 3. The initiation of regeneration. Biological Bulletin 6: 55–74. https://doi.org/10.2307/1535589
- Child CM (1904b) Form regulation in Cerianthus. 4. The role of water-pressure in regeneration. Biological Bulletin 6: 266–286. https://doi.org/10.2307/1535820
- Child CM (1908) Form regulation in Cerianthus æstuarii. Biological Bulletin 15: 27–53. https://doi.org/10.2307/1535562
- Chintiroglou C, den Hartog JC (1995) Additional records of Actiniaria (Anthozoa) from Greece. Zoologische Mededelingen 69: 353–364.
- Chintiroglou C, Koukouras A (1991) Observations on the feeding habits of Calliactis parasitica (Couch, 1842), Anthozoa, Cnidaria. Oceanologica Acta 14: 389–396.
- Çinar ME, Yokes MB, Açik S, Bakir AK (2014) Checklist of Cnidaria and Ctenophora from the coasts of Turkey. Turkish Journal of Zoology 38: 677–697. https://doi.org/10.3906/zoo-1405-68
- Coolen JWP, Bos OG, Glorius S, Lengkeek W, Cuperus J, van der Weide B, Agüera A (2015) Reefs, sand and reef-like sand: A comparison of the benthic biodiversity of habitats in the Dutch Borkum Reef Grounds. Journal of Sea Research 103: 84–92. https://doi.org/10.1016/j.seares.2015.06.010
- Cosentino A, Potoschi S, Giacobbe A (2011) The presence of Phoronis australis (Phoronida) in southern Italian waters. Biogeographia 30: 409–415. https://doi.org/10.21426/B630110602
- Cutress CE (1961) Habrosanthus bathamae, n. gen., n. sp. (Actiniaria: Sagartiidae) from New Zealand. Transactions of the Royal Society of New Zealand 1: 95–101.
- Cutress CE (1977) Corallimorpharia, Actiniaria, Ceriantharia. In: Devaney DM, Eldredge LG (Eds) Reef and Shore Fauna of Hawaii. Bishop Museum Press, Honolulu, 130–147.
- Cutress CE, Arneson CA (1987) Sea anemones of Enewetak Atoll. In: Devaney DM, Reese ES, Burch BL, Helfrich P (Eds) The Natural History of Enewetak Atoll. Office of Scientific and Technical Information, US Department of Energy, Honolulu, 53–62.
- Daly M, Brugler MR, Cartwright P, Collins AG, Dawson MN, Fautin DG, France SC, McFadden CS, Opresko DM, Rodriguez E, Romano SL, Stake JL (2007) The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa, 127–182. https://doi.org/10.11646/zootaxa.1668.1.11
- Danielssen DC (1860) Forhandlinger i Videnskabs-Selskabet i Christiania On Cerianthus borealis n. sp. Trykt hos Brøgger & Christie, Christiania, 251 pp.
- Danielssen DC (1888) Cerianthus borealis. Bergens Museums Aarsberetning 1: 1–12.
- Danielssen DC (1890) Actinida. In: Den Norske Nordhavs-Expedition 1876–1878. Zoologi. Grøndahl and Søn, Christiania, 184.
- de Blainville HMD (1830) Dictionnaire des Sciences Naturelles. FG Levrault, Paris, 631 pp.
- de Blainville HMD (1834) Manuel d’Actinologie ou de Zoophytologie. FG Levrault, Paris, 644 pp. https://doi.org/10.5962/bhl.title.8768
- Delle Chiaje S (1841) Descrizione e anotomia degli animali invertebrati della Sicilia Citeriore osservati vivi negli anni 1822–1830. C. Batelli e Comp., Napoli, 420 pp. https://doi.org/10.5962/bhl.title.10031
- den Hartog JC (1977) Descriptions of two new Ceriantharia from the Caribbean region, Pachycerianthus curancaoensis n. sp. and Arachnanthus nocturnus n. sp. with a discussion of the Cnidom and of the classification of the Ceriantharia. Zoologische Mededelingen 51: 211–242.
- den Hartog JC (1997) The sea anemone fauna of Indonesian coral reefs. In: Tomascik T, Mah AJ, Nontji A, Moosa MK (Eds) The ecology of the Indonesian seas 1. Periplus Editions, Singapore, 351–370.
- Dons C (1945) Norges strandfauna XXXII hexakoraller. Kongelige Norske Videnskabers Selskab Forhandlinger 18: 17–20.
- Duerden JE (1902a) Report on the actinians of Porto Rico. Bulletin of the U.S. Fisheries Commission 20: 323–374.
- Duerden JE (1902b) On the actinian Bunodeopsis globulifera, Verrill. Transactions of the Linnean Society 8: 297–317. https://doi.org/10.1111/j.1096-3642.1902.tb00478.x
- Eleftheriou A, Basford DJ (1983) The general behaviour and feeding of Cerianthus lloydii Gosse. Cahiers de Biologie Marine 24: 147–158.
- Emig CC, Herberts C, Thomassin BA (1972) Sur l’association de Phoronis australis avec Cerianthus maua dans les zones récifales de Madagascar. Marine Biology 15: 304–315. https://doi.org/10.1007/BF00401390
- Faurot L (1895) Études sur l’anatomie, l’histologie et le développement des actinies. Archives de Zoologie Expérimentale et Générale 3: 43–262.
- Fautin DG (1998) Class Anthozoa: Orders Actiniaria, Ceriantharia, and Zoantharia. In: Scott PV, Blake JV (Eds) , Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel. Santa Barbara Museum of Natural History, Santa Barbara, 113–139.
- Fautin DG (2013) Hexacorallians of the World. http://geoportal.kgs.ku.edu/hexacoral/anemone2/index.cfm
- Fautin DG, Hickman Jr. CP, Daly M, Molodtsova TN (2007) Shallow-Water Sea Anemones (Cnidaria: Anthozoa: Actiniaria) and Tube Anemones (Cnidaria: Anthozoa: Ceriantharia) of the Galápagos Islands. Pacific Science 61: 549–573. https://doi.org/10.2984/1534-6188(2007)61[549:SSACAA]2.0.CO;2
- Field LR (1949) Sea Anemones and Corals of Beaufort, North Carolina. Duke University Press, Durham, 39 pp.
- Fischer P (1874) Recherches sur les actinies des cotes océaniques de France. Nouvelles Archives du Muséum d’Histoire Naturelle de Paris 10: 193–244.
- Fischer P (1875) Anthozoaires du département de la Gironde et des côtes du sud-ouest de la France. Actes de la Société Linnéenne de Bordeaux 30: 183–192.
- Fischer P (1887) Contribution a l’actinologie française. Archives de Zoologie Expérimentale et Générale 5: 381–442.
- Fischer P (1889) Nouvelle contribution à l’actinologie française. Première Partie. Actinies d’Arcachon (Gironde). Actes de la Société Linnéenne de Bordeaux 43: 252–309.
- Fishelson L (1970) Littoral fauna of the Red Sea: the population of non-scleractinian anthozoans of shallow waters of the Red Sea (Eilat). Marine Biology 6: 106–116. https://doi.org/10.1007/BF00347239
- Forbes E (1843) Retrospective comments. Annals and Magazine of Natural History 12: 40–42. https://doi.org/10.1080/03745484309442482
- Mejia ACF, Molodtsova T, Östman C, Bavestrello G, Rouse GW (2019) Molecular phylogeny of Ceriantharia (Cnidaria: Anthozoa) reveals non-monophyly of traditionally accepted families. Zoological Journal of the Linnean Society https://doi.org/10.1093/zoolinnean/zlz158
- Fowler GH (1897) Contributions to our knowledge of the plankton of the Faroe Channel. III. The latter development of Arachnactis albida (M. Sars) with notes on Arachnactis bournei (sp.n.). Proceedings of the Zoological Society of London 65: 803–808. https://doi.org/10.1111/j.1096-3642.1897.tb03123.x
- Frey RW (1970) The Lebensspuren of some common marine invertebrates near Beaufort, North Carolina. II. Anemone burrows. Journal of Paleontology 44: 308–211.
- Füller H (1957) Exkursionsfauna von Deutschland. Wirbellose I. Volk und Wissen Volkseigener, Berlin, 485 pp.
- Gili J-M (1982) Fauna de Cnidaris de les Illes Medes. KETRES, Barcelona, 175 pp.
- Gmelin JF (1791) Caroli a Linné. Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. 1 (6). Beer, Leipzig, 3021–3909.
- Goette A (1897) Einiges über die Entwickelung der Scyphopolypen. Zeitschrift für Wissenschaftliche Zoologie 63: 292–378.
- González-Muñoz R, Simões N, Guerra-Castro EJ, Hernández-Ortíz C, Carrasquel G, Mendez E, Lira C, Rada M, Hernández I, Pauls SM, Croquer A, Cruz-Motta JJ (2016) Sea anemones (Cnidaria: Actiniaria, Corallimorpharia, Ceriantharia, Zoanthidea) from marine shallow-water environments in Venezuela: new records and an updated inventory. Marine Biodiversity Records 9: 1–35. https://doi.org/10.1186/s41200-016-0016-7
- Gosse PH (1856a) Edwardsia vestita (Forbes). Annals and Magazine of Natural History, Series 2, 18: 73–74. https://doi.org/10.1080/00222935608697585
- Gosse PH (1856b) On Edwardsia carnea, a new British Zoophyte. Annals and Magazine of Natural History, Series 2, 18: 219–211. https://doi.org/10.1080/00222935608697624
- Gosse PH (1858) Synopsis of the families, genera, and species of the British Actiniae. Annals and Magazine of Natural History, Series 3, 1: 414–419. https://doi.org/10.1080/00222935808696950
- Gosse PH (1859) Characters and descriptions of some new British sea-anemones. Annals and Magazine of Natural History, Series 3, 3: 46–50.
- Gosse PH (1860) A History of the British Sea-Anemones and Corals. Van Voorts, London, 362 pp. https://doi.org/10.5962/bhl.title.27151
- Goy J (1979) Campagne de la Calypso au large des côtes atlantiques de l’Amérique du Sud (1961–1962); 35. Méduses. Résultats scientifiques des campagnes de la Calypso 11: 263–296.
- Graeffe E (1884) Seethierfauna des Golfes von Triest. Arbeiten aus dem Zoologischen Instituten Universität Wien 5: 333–362.
- Gravier C (1902) Sur un cérianthaire pélagique adulte. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 135: 591–593.
- Gravier C (1904) Recherches sur un cérianthaire pélagique du Golfe de Californie (Dactilactis benedeni, n. sp.). Annales des Sciences Naturelles 20: 253–294.
- Gravier C (1922) Hexactinidés provenant des campagnes des yachts Hirondelle I et II et Princesse-Alice I et II (1888–1913). In: Résultats des Campagnes Scientifiques Accomplies sur son Yacht par Albert Ier Prince Souverain de Monaco Publiés sous sa Direction avec le Concours de M. Jules Richard. Imprimerie de Monaco, Monaco, 3–104.
- Gray JE (1867) Notes on Zoanthinæ, with the descriptions of some new genera. Proceedings of the Zoological Society of London 15: 233–240.
- Grebel’nyi SD (2001) Hexacorallia. Explorations of the Fauna of the Seas 51: 36–38.
- Grieg JA (1913) Bidrag til knudskapen om Hardangerfjordens fauna. Bergens Museums Aarbok 1: 3–147.
- Grube AE (1840) Actinien, Echinodermen und Würmer des Adriatischen und Mittelmeers [nach eigenen Sammlungen beschrieben von Dr. Adolph Eduard Grube]. J. H. Bon, Königsberg, 92 pp.
- Haddon AC (1898) The Actiniaria of Torres Straits. Scientific Transactions of the Royal Dublin Society 6: 393–520.
- Haddon AC, Shackleton AM (1893) Description of some new species of Actiniaria from Torres Straits. Scientific Proceedings of the Royal Dublin Society 8: 116–131.
- Haime J (1854) Memoire sur le cérianthe (Cerianthus membranaceus). Annales des Sciences Naturelles 1: 341–389.
- Haldar BP (1981) On the climatology of the Beyt Island, western India, with special reference to the systematics of Phoronis australis Haswell (Phoronida). Indian Journal of Zootomy 22: 59–63.
- Haque MM (1977) Some littoral coelenterates of Bangladesh and Pakistan coasts. Bangladesh Journal of Zoology 1: 33–40.
- Harger O, Smith SI (1876) Report on the dredgings in the region of St. George’s Banks, in 1872. Transactions of the Connecticut Academy of Arts and Sciences 3: 1–57.
- Hargitt CW (1912) The Anthozoa of the Woods Hole region. Bulletin of the Bureau of Fisheries 32: 221–254.
- Harms J (1993) Check list of species (algae, invertebrates and vertebrates) found in the vicinity of the island of Helgoland (North Sea, German Bight); a review of recent records. Helgoländer Meeresuntersuchungen 47: 1–34. https://doi.org/10.1007/BF02366182
- Haurtlaub C (1884) Die Coelenteraten Helgolands. Vorläufiger Bericht. Wissenschaftliche Meeresuntersuchungen 1: 161–206.
- Hedgpeth JW (1954) Anthozoa: the anemones. Fisheries Bulletin of the Fish and Wildlife Service (US) 55: 285–290.
- Heider AV (1879) Cerianthus membranaceus Haime. Ein Beitrag zur Anatomie der Actinien. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien, Mathematisch-Naturwissenschaftliche 79: 204–254.
- Heller C (1868) Die Zoophyten und Echinodermen des adriatischen Meeres. Druck von Carl Ueberreuter, Wien, 86 pp. https://doi.org/10.5962/bhl.title.11393
- Hertwig R (1882) Die Actinien der Challenger Expedition. Gustav Fischer, Jena, 119 pp.
- Hertwig R (1888) Report on the Actiniaria dredged by HMS Challenger during the years 1873–1876 [Supplement]. Report on the Scientific Results of the Voyage of the H.M.S. Challenger during the years 1873–76 (Zoology) 26: 1–56. https://doi.org/10.5962/bhl.title.11290
- Hickson SJ (1889) Coelenterata. Zoological Record 26: 1–24.
- Holohan BA, Klos EG, Oviatt CA (1998) Population density, prey selection, and predator avoidance of the burrowing anemone (Ceriantheopsis americanus) in Narragansett Bay, Rhode Island. Estuaries and Coasts 21: 466–469. https://doi.org/10.2307/1352844
- Ilmoni I (1830) Dr. Ilmoni aus Finnland schickte folgende Beiträge zur Naturgeschichte der Actinien ein. Isis von Oken 23: 694–699.
- Ilmoni I (1831) Sur deux espèces nouvelles de la famille des actinies. Bulletin des Sciences Naturelles 24: 123.
- Jensen P (1992) Cerianthus vogti Danielssen, 1890 (Anthozoa: Ceriantharia). A species inhabiting an extended tube system deeply buried in deep-sea sediments off Norway. Sarsia 77: 75–80. https://doi.org/10.1080/00364827.1992.10413494
- Johnson JY (1861) Notes on the sea-anemones of Madeira, with descriptions of new species. Proceedings of the Zoological Society of London 1861: 298–306.
- Jonsson LG, Lundälv T, Johannesson K (2001) Symbiotic associations between anthozoans and crustaceans in a temperate coastal area. Marine Ecology Progress Series 209: 189–195. https://doi.org/10.3354/meps209189
- Jourdan E (1880) Recherches zoologiques et histologiques sur les zoanthaires du Golfe de Marseille. Annales des Sciences Naturelles 10: 1–154. https://doi.org/10.5962/bhl.title.4956
- Kayal E, Roure B, Philippe H, Collins AG, Lavrov DV. (2013) Cnidarian phylogenetic relationships as revealed by mitogenomics. BMC Evolutionary Biology 13: 5. https://doi.org/10.1186/1471-2148-13-5
- Keegan BF, Könnecker G (1973) In situ quantitative sampling of benthic organisms. Helgoländer Wissenschaftliche Meeresuntersuchungen 24(1–4): 256–263. https://doi.org/10.1007/BF01609516
- Kelly E, Keegan BF (2000) Case 3111. Pachycerianthus Roule, 1904 (Cnidaria, Anthozoa): Proposed Designation of P. multiplicatus Carlgren, 1912 as the Type Species. Bulletin of Zoological Nomenclature 57: 11–13. https://doi.org/10.5962/bhl.part.20659
- Kingsley JS (1904) A description of Cerianthus borealis Verrill. Tufts College Studies 1: 345–361.
- Kiseleva MI (1975) Food spectra of some benthic invertebrates in the Black Sea. Zoologischeskii Zhurnal1 54: 1596–1601.
- Klunzinger CB (1877) Die Korallthiere des Rothen Meeres. 1: Die Alcyonarien und Malacodermen, 1st ed. Gutmann’schen Buchhandlung, Berlin, 98 pp, 8 pls.
- Koren J, Danielssen DC (1877) Beskrivelse over nogle nye norske Coelenterater [Description of some new Norwegian coelenterates]. In: Koren J, Danielssen DC (Eds) Fauna Littoralis Norvegiae. JD Beyer, Bergen, 77–81.
- Krempf A (1905) Liste des Hexanthides rapportés de l’Océan Indien (Golfe de Tadjourah) par M. Ch. Gravier. Bulletin du Muséum national d’Histoire naturelle 11: 191–196.
- Kristensen E, Aller RC, Aller JY (1991) Oxic and anoxic decomposition of tubes from the burrowing sea anemone Ceriantheopsis americanus: Implications for bulk sediment carbon and nitrogen balance. Journal of Marine Research 49: 589–617. https://doi.org/10.1357/002224091784995774
- Kussakin G, Kostina EE (1996) The Intertidal Biota of Volcanic Yankich Island (Middle Kuril Islands). Publications of the Seto Marine Biological Laboratory 37: 201–225. https://doi.org/10.5134/176267
- Kwietniewski CR (1898) Actiniaria von Ambon und Thursday Island. In: Zoologische Forschungsreisen in Australien und dem Malayischen Archipel von Richard Semon. Gustav Fischer, Jena, 385–430.
- Laverack MS, Blackler M (1974) Fauna and Flora of St. Andrews Bay. Scottish Academic Press, Edinburgh, 310 pp.
- Leloup E (1931) Contribution a la repartition des cerianthaires dans le sud de la Mer du Nord. Bulletin du Musée Royal d’Histoire Naturelle de Belgique 7: 1–10.
- Leloup E (1932) Cerianthaires de l´Ocean Atlantique. Bulletin du Musée Royal d’Histoire Naturelle de Belgique 8: 1–19.
- Leloup E (1960) Larves de Cérianthaires de Monaco et de Villefranche-sur-Mer. Bulletin de l’Institut Océanographique 1185: 1–19.
- Leloup E (1962) Anthozoa Ceriantharia: larvae. Conseil International pour l’Exploration de la mer 93: 1–7.
- Leloup E (1964) Larves de cerianthaires. Discovery Reports 33: 251–307.
- Levinsen GMR (1893) Annulata, Hydroidae, Anthozoa, Porifera. In: Petersen CGJ (Ed.) , Videnskabelige Udbytte af Kanonbaaden “Hauchs” togter i de Danske Have indenfor Skagen i Aarene 1883–1886. Host, Copenhagen, 307–464.
- Lo Bianco S (1909) Notizie biologiche riguardanti specialmente il periodo di maturità sessuale degli animali del golfo di Napoli. Mitteilungen aus der Zoologischen Station zu Neapel 19: 513–716.
- Lo Iacono C, Orejas C, Gori A, Gili JM, Requena S, Puig P, Ribó M (2012) Habitats of the Cap de Creus continental shelf and Cap de Creus canyon, northwestern Mediterranean. In: Harris PT, Baker EK (Eds) , Seafloor Geomorphology as Benthic Habitat-GeoHAB Atlas of Seafloor Geomorphic Features and Benthic Habitats. Elsevier, Amsterdam, 457–469. https://doi.org/10.1016/B978-0-12-385140-6.00032-3
- Lopes CSS, Ceriello H, Morandini AC, Stampar SN (2019) Revision of the Genus Ceriantheomorphe (Cnidaria, Anthozoa, Ceriantharia) with description of a new species from the Gulf of Mexico and northwestern Atlantic. ZooKeys 874: 127–148. https://doi.org/10.3897/zookeys.874.35835
- Lütken C (1861) Nogle Bémærkninger om de ved de danske Kyster iagttagne Arter af Aktiniernes Gruppe. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening 14: 184–200.
- Lütken C (1889) Nogle temmelig uventede Forøgelser af den norske Havfauna. Videnskabelige meddelelser fra den Naturhistoriske forening i Kjöbenhavn 7: 358–362.
- MacGinitie GE (1955) Distribution and ecology of the marine invertebrates of Point Barrow, Alaska. Smithsonian Miscellaneous Collections 128: 1–120.
- Manuel RL (1977) A redescription of Edwardsia beautempsi and E. timida (Actiniaria: Edwardsidae). Cahiers de Biologie Marine 18: 483–497.
- Manuel RL (1981) British Anthozoa keys and notes for the identification of the species. Academic Press, London, 241 pp.
- Mariscal RN, Conklin EJ, Bigger CH (1977) The Ptychocyst, a major new category of cnida used in tube construction by a cerianthid anemone. Biological Bulletin 152: 392–405. https://doi.org/10.2307/1540427
- Mark EL (1884) Selections from embryological monographs. III. Polyps. Memoirs of the Museum of Comparative Zoology at Harvard College 9: 5–52.
- Mata SA, Corsetti CL, Corsetti FA, Awramik SM, Bottjer DJ (2012) Lower Cambrian anemone burrows from the upper member of the Wood Canyon Formation, Death Valley region, United States. Palaios 27: 594–606. https://doi.org/10.2110/palo.2012.p12-016r
- McFarlane ID (1988) Variability in the startle response of Pachycerianthus multiplicatus (Anthozoa: Ceriantharia). Comparative Biochemistry and Physiology Part A: Physiology 89: 365–370. https://doi.org/10.1016/0300-9629(88)91041-9
- McMurrich JP (1887) Notes on the fauna of Beaufort, North Carolina. Studies at the Biological Laboratory of the Johns Hopkins University 4: 55–63.
- McMurrich JP (1893) Report on the Actiniæ collected by the United States Fish Commission Steamer Albatross during the winter of 1887–1888. Proceedings of the United States National Museum 16: 119–216. https://doi.org/10.5479/si.00963801.16-930.119
- McMurrich JP (1910) Actiniaria of the Siboga expedition, Part I. Ceriantharia. Siboga-Expeditie Monographes 10: 1–48.
- Mello-Leitão CF (1919) Cerianthus Brasiliensis; um novo cerianthoide americano. Archivos da Escola Superior de Agricultura e Medicina Veterinaria 3: 35–39.
- Menon KR (1927) Subclass Zoantharia (except Scleractiniae). Bulletin of Madras Government Museum 1: 31–40.
- Milne Edwards H (1857) Histoire Naturelle des Coralliaires ou Polypes Proprement Dits, vol. 1, 1st ed. Librairie Encyclopédique de Roret, Paris, 326 pp. https://doi.org/10.5962/bhl.title.11574
- Milne Edwards H, Haime J (1851) Archives du Muséum d’Histoire Naturelle. 5 Monographie des Polypiers fossiles des terrains paléozoïques, précédée d’un tableau général de la classification des Polypes. Gide et J Baudry, Paris, 502 pp.
- Miranda LS, Marques AC (2016) Hidden impacts of the Samarco mining waste dam collapse to Brazilian marine fauna-an example from the staurozoans (Cnidaria). Biota Neotropica 16 (2): e20160169 https://doi.org/10.1590/1676-0611-BN-2016-0169
- M’Intosh WC (1875) The Marine Invertebrates and Fishes of St. Andrews. Taylor and Francis, London, 186 pp. https://doi.org/10.5962/bhl.title.6247
- Molodtsova TN (2000) Fauna ceriantariy atlanticheskogo okeana I sostav roda Cerianthus mirovoy fauny. Aftoreferat na soiskanie uchenoy stepeni kandidata biologicheskih nauk. Dialog-MGU, Moscow, 21 pp.
- Molodtsova TN (2001a) Cerianthids (Anthozoa, Cnidaria) of the region of Bengual upwelling 1. Ceriantheopsis nikitai n.sp. Zoologicheskii Zhurnal 80: 773–780. [original in Russian]
- Molodtsova TN (2001b) Cerianthids (Anthozoa, Cnidaria) of the region of Bengual upwelling 2. Cerianthus malakhovi n. sp. and composition of the genus Cerianthus. Zoologicheskii Zhurnal 80: 909–920. [original in Russian]
- Molodtsova TN (2001c) Cerianthids (Anthozoa, Cnidaria) from Bengual upwelling region. 3. Botrucnidifer shtockmani. Zoologicheskii Zhurnal 80: 1027–1037.
- Molodtsova TN (2001d) On the taxonomic status of Cerianthus septentrionalis van Beneden, 1923 (Cnidaria: Anthozoa: Ceriantharia). Zoosystematica Rossica 10: 9–10.
- Molodtsova TN (2003) On Isarachnanthus from Central Atlantic and Caribbean region with notes on Isarachnactis lobiancoi (Carlgren, 1912). Zoologische Verhandelingen 345: 249–255.
- Molodtsova TN (2004a) Ceriantharia (Cnidaria: Anthozoa) from the Faroe Islands. Fróðskaparrit 51: 292–297.
- Molodtsova TN (2004b) On the taxonomy and presumable evolutionary pathways of planktonic larvae of Ceriantharia (Anthozoa, Cnidaria). Hydrobiologia 530–531: 261–266. https://doi.org/10.1007/s10750-004-2671-7
- Molodtsova TN (2007) Tube anemones (Ceriantharia Anthozoa) of New Caledonia. Compendium of marine species of New Caledonia. Documents scientifiques et techniques, IRD, II7: 133.
- Molodtsova TN (2009) Ceriantharia (Cnidaria) of the Gulf of Mexico. In: Gulf of Mexico Origin, Waters, and Biota: Volume I, Biodiversity. Texas A&M University Press, College Station, 365–367.
- Molodtsova TN (2014) Deep-sea fauna of European seas: An annotated species check-list of benthic invertebrates living deeper than 2000 m in the seas bordering Europe. Ceriantharia. Invertebrate Zoology 11(1): 99–100. https://doi.org/10.15298/invertzool.11.1.09
- Molodtsova TN, Malakhov VV (1995a) Cerianthus lloydii (Anthozoa, Ceriantharia) from the volcanic ecosystem of Kraternaya Bay. 1. Morphology and anatomy of adult polyps, geographic distribution. Zoologicheskii Zhurnal 74(10): 5–17.
- Molodtsova TN, Malakhov VV (1995b) Cerianthus lloydii (Anthozoa, Ceriantharia) from a volcanic ecosystem of Kraternaya Bay. II. Larval development. Zoologicheskii Zhurnal 74(11): 4–11.
- Molodtsova TN, Griffiths CLCF, Acuña FH (2011) A new species of shallow-water cerianthid (Cnidaria: Anthozoa) from South Africa, with remarks on the genus Ceriantheopsis. African Natural History 7: 1–8.
- Molodtsova T (2020) World List of Ceriantharia. Ceriantharia. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=1361 on 2020-04-16
- Moore PG, Cameron KS (1999) A note on a hitherto unreported association between Photis longicaudata (Crustacea: Amphipoda) and Cerianthus lloydii (Anthozoa: Hexacorallia). Journal of the Marine Biological Association of the United Kingdom 79: 369–370. https://doi.org/10.1017/S0025315498000447
- Morri C, Bavestrello G, Bianchi CN (1991) Faunal and ecological notes on some benthic cnidarian species from the Tuscan Archipelago and eastern Ligurian sea (Western Mediterranian). Bollettino dei musei e degli istituti biologici dell’Università di Genova 54/55: 27–47.
- Moseley HN (1877) On new forms of Actiniaria dredged in the deep sea; with a description of certain pelagic surface-swimming species. Transactions of the Linnean Society 1: 295–305. https://doi.org/10.1111/j.1096-3642.1877.tb00444.x
- Müllegger S (1938) Die Aktinien: Beschreibung der vornehmlich im Aquarium gehaltenen Aktinien. Wochenschrift für Aquarien und Terrarienkunde 39–44: 1–16.
- Nair RV (1949) On two new ceriantharian larvae from the Madras plankton. Records of the Indian Museum 47: 239–251.
- Nakamoto D (1919) A new species of Cerianthus. Zoological Magazine (Tokyo) 31: 118–120.
- Nakamoto D (1923) Cerianthus misakiensis n. sp. Zoological Magazine (Tokyo) 35: 167–172.
- Naumov D V (1968) Coelenterata. In: Opredelitel fauny Chernogo I Azovskogo. Naukova Dumka, Kiev, 56–74.
- Nienhaus K, Renzi F, Vallone B, Wiedenmann J, Nienhaus GU (2006) Exploring chromophore − protein interactions in fluorescent protein cmFP512 from Cerianthus membranaceus: X-ray structure analysis and optical spectroscopy. Biochemistry 45(43): 12942–12953. https://doi.org/10.1021/bi060885c
- Nyholm K-G (1943) Zur Entwicklung und Entwicklungsbiologie der Ceriantharien und Aktinien. Zoologiska Bidrag från Uppsala 22: 87–248.
- Ocaña MA, Tocino LS, López González S, Viciana Martín JF (2000a) Guía submarina de invertebrados no artrópodos. Albolote, Granada, 417 pp.
- Ocaña O, Tocino LS, Lopéz-González PJ (2000b) Consideraciones faunística y biogeográficas de los antozoos (Cnidaria: Anthozoa) de la costa de Granada (Mar de Alborán). Zoologica Baetica 11: 51–65.
- Panikkar NK (1936) On Apiactis bengalensis, species nova, a new pelagic larval ceriantharian from the Madras plankton. Zoologischer Anzeiger 140: 250–260.
- Panikkar NK (1947) Observations on the structure and developmental stages of a new species of Arachnactis from the Madras plankton. Annales des Sciences Naturelles 9: 227–250.
- Parker GH (1900) Synopses of North American invertebrates. XIII. The Actiniaria. American Naturalist 34: 747–758. https://doi.org/10.1086/277763
- Pax F (1908) Anthozoa. Die Aktinienfauna Westafrikas. Denkschriften der Medizinisch-Naturwissenschaftlichen Gesellschaft 13: 261–302.
- Pax F (1909) Die Aktinien der ostafrikanischen Inseln. In: Voeltzkow A (Ed.) , Reise in Ostafrika in den Jahren 1903–1905. E Schweizerbart Verlagsbuchhandlung, Stuttgart, 399–418.
- Pax F (1910) Studien an westindischen Actinien. Zoologische jahrbucher. Abteilung fur allgemeine zoologie und physiologie der tiere suppl 11: 157–330.
- Pax F (1924) Actiniarien, Zoantharien und Ceriantharien von Curaçao. Kungliga Zoologisch Genootschap Natura Artis Magistra 23: 93–122.
- Pax F (1928) Anthozoa. In: Dahl F (Ed.) , Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise. Gustav Fischer, Jena, 190–237.
- Pax F, Müller I (1955) Gli Antozoi del Museo Civico di Storia Naturale di Trieste Parte I: Antipatharia, Ceriantharia, Zoantharia, Actiniaria, Alcyonaria e Pennatularia. Atti del Museo Civico di Storia Naturale Trieste 20: 103–129.
- Pax F, Müller I (1962) Fauna antozoa Jadrana. Fauna et Flora Adriatica 3: 1–343.
- Peckett FJ, Glegg GA, Rodwell LD (2014) Assessing the quality of data required to identify effective marine protected areas. Marine Policy 45: 333–341. https://doi.org/10.1016/j.marpol.2013.09.013
- Pei Z (1998) Coelenterata Actiniaria Ceriantharia Zoanthidea. Science Press, Beijing, 286 pp.
- Perrier E (1893) Traité de Zoologie. Librairie S. Savy, Paris 1(2): 1–864.
- Peteya DJ (1973a) A light and electron microscope study of the nervous system of Ceriantheopsis americanus (Cnidaria, Ceriantharia). Zeitschrift für Zellforschung und Mikroskopische Anatomie 141: 301–317. https://doi.org/10.1007/BF00307408
- Peteya DJ (1973b) A possible proprioceptor in Ceriantheopsis americanus (Cnidaria, Ceriantharia). Zeitschrift für Zellforschung und mikroskopische Anatomie 144: 1–10. https://doi.org/10.1007/BF00306682
- Picton BE (1985) Anthozoans (Coelenterata: Anthozoa) new to Ireland and new records of some rarely recorded species. Irish Naturalists Journal 21: 484–488.
- Picton BE, Manuel RL (1985) Arachnanthus sarsi Carlgren, 1912: a redescription of a cerianthid anemone new to the British Isles. Zoological Journal of the Linnean Society 83: 343–349. https://doi.org/10.1111/j.1096-3642.1985.tb01180.x
- Pirtle JL, Ibarra SN, Eckert GL (2012) Nearshore subtidal community structure compared between inner coast and outer coast sites in Southeast Alaska. Polar Biology 35: 1889–1910. https://doi.org/10.1007/s00300-012-1231-2
- Rapp W (1829a) Ueber den Bau einiger Polypen des mittelländichen Meeres. Nova Acta Academiae Caesareae Leopoldino-Coralinae Naturae Curiosorum 14: 653–658.
- Rapp W (1829b) Ueber die Polypen im Allgemeinen und die Actinien. Grolsherzogl. Sdch, Weimar, 62 pp.
- Rastorgueff PA, Bellan-Santini D, Bianchi CN, Bussotti S, Chevaldonn P, Guidetti P, Harmelin JG, Montefalcone M, Morri C, Perez T, Ruitton S, Vacelet J, Personnic S (2015) An ecosystem-based approach to evaluate the ecological quality of Mediterranean undersea caves. Ecological Indicators 54: 137–152. https://doi.org/10.1016/j.ecolind.2015.02.014
- Reft AJ, Daly M (2012) Morphology, distribution, and evolution of apical structure of nematocysts in Hexacorallia. Journal of morphology 273: 121–136. https://doi.org/10.1002/jmor.11014
- Rehm P, Rachor E (2007) Benthic macrofauna communities of the submersed Pleistocene Elbe valley in the southern North Sea. Helgoland Marine Research 61: 127–134. https://doi.org/10.1007/s10152-007-0060-0
- Reiner SA (1807) Prospetto della Classe dei Vermi. In: Prodromus osserv. Venezia 1804–1807. Unavailable publication, ICZN Opinion 316, Padua, 15–27.
- Riemann-Zürneck K (1969) Sagartia troglodytes (Anthozoa). Biologie und Morphologie einer schlickbewohnenden Aktinie. Veröffentlichungen des Institutes für Meeresforschung Bremerhaven 12: 169–230.
- Rioja Y, Martin J (1906) Datos para el conocimiento de la fauna marina de España. Boletin de la Real Sociedad Española de Historia Natural 6: 275–281.
- Ritzmann O, Jokat W, Czuba W, Guterch A, Mjelde R, Nishimura Y (2004) A deep seismic transect from Hovgård Ridge to northwestern Svalbard across the continental-ocean transition: A sheared margin study. Geophysical Journal International 157(2): 683–702. https://doi.org/10.1111/j.1365-246X.2004.02204.x
- Robertson D (1876) On the sea anemones of the shores of the Cumbraes. Proceedings of the Natural History Society of Glasgow 2: 24–30.
- Robins MW (1969) The marine flora and fauna of the Isles of Scilly. Cnidaria and Ctenophora. Journal of Natural History 3: 329–343. https://doi.org/10.1080/00222936900770291
- Rodriguez C, Marques AC, Stampar SN, Morandini AC, Christiansen E, Genzano G, Mianzan H (2011) The taxonomic position of the pelagic “staurozoan” Tessera gemmaria as a ceriantharian larva. Zootaxa 2971: 49–58. https://doi.org/10.11646/zootaxa.2971.1.5
- Roule L (1904) Note préliminaire sur quelques formes nouvelles de cerianthaires. Comptes Rendus de l’Association Française pour l’Avancement des Sciences 32: 791–793.
- Roule L (1905) Description des antipathaires et cerianthaires recueillis par S.A.S. le Prince de Monaco dans l’Atlantique nord (1886–1902). Résultats des Campagnes Scientifiques Accomplies sur son Yacht par Albert I Prince Souverain de Monaco 30: 1–96. https://doi.org/10.5962/bhl.title.59328
- Sars M (1857) Bidrag til Kundskaben om Middelhavets Littoral-Fauna, Reisebemærkninger fra Italien. Johan Dahls-Forlag, Christiania, 55 pp.
- Schmidt H (1972) Die Nesselkapseln der Anthozoen und ihre Bedeutung fur die phylogenetische Systematik. Helgoländer Wissenschaftliche Meeresuntersuchungen 23: 422–458. https://doi.org/10.1007/BF01625294
- Schmidt H (1974) On evolution in the Anthozoa. Proceedings of the Second International Coral Reef Symposium 1: 533–560.
- Schückel U, Siegfried E, Kröncke I (2010) Temporal variability of three different macrofauna communities in the northern North Sea. Estuarine, Coastal and Shelf Science 89: 1–11. https://doi.org/10.1016/j.ecss.2010.04.006
- Sciberras M, Hinz H, Bennell JD, Jenkins SR, Hawkins SH, Kaiser MJ (2013) Benthic community response to a scallop dredging closure within a dynamic seabed habitat. Marine Ecology Progress Series 480: 83–98. https://doi.org/10.3354/meps10198
- Sebens KP (1998) Anthozoa: Actiniaria, Zoanthidea, Corallimorpharia, and Ceriantharia. In: Pearce JB (Ed.) , Marine Flora and Fauna of the Eastern United States. National Marine Fisheries Service, Seattle, 1–67.
- Shepard AN, Theroux RB, Cooper RA, Uzmann JR (1986) Ecology of Ceriantharia (Coelenterata, Anthozoa) of the northwest Atlantic from Cape Hatteras to Nova Scotia. Fishery Bulletin 84: 625–646.
- Silveira FL, Morandini AC (2011) Checklist dos Cnidaria do Estado de São Paulo, Brasil. Biota Neotropica 11: 1–10. https://doi.org/10.1590/S1676-06032011000500016
- Song J-I (1986) First report of a tube anemone, Cerianthus filiformis Carlgren, from Korean waters, including comparison of cnidae in adults and planulae. Korean Journal of Systematic Zoology 2: 79–87. https://doi.org/10.1080/12265071.1998.9647407
- Song J-I (1998) Taxonomy of Cerianthus filiformis (Ceriantharia, Anthozoa) and its phoronid associate, Phoronis australis in Korea. Korean Journal of Biological Science 2: 195–201.
- Song J-I (2000) Cnidaria 2: Anthozoa. Korea Inst. Daejeon, 332 pp.
- Song J-I, Lee IS (1998) Fauna of the anthozoans from adjacent waters of Geojedo Island in Korea. Korean Journal of Systematic Zoology 14: 229–242.
- Spallanzani L (1784) Diversi animali nuovi. Memorie della Società Italiana di Verona 2: 627–629.
- Spier D, Stampar SN, Prantoni AL (2012) New record of the endangered cerianthid Ceriantheomorphe brasiliensis (Cnidaria: Hexacorallia) in Paranaguá Bay, southern Brazil. Marine Biodiversity Records 5: e119. https://doi.org/10.1017/S1755267212001078
- Stampar SN, Silveira FLD (2006) Espécies sem glamour: lista de animais brasileiros ameaçados de extinção negligencia invertebrados. Scientific American Brazil (52): 10–11.
- Stampar SN, Emig C, Morandini AC, Kodja G, Balboni AP, Silveira FL (2010) Is there any risk in a symbiotic species associating with an endangered one? A case of a phoronid worm growing on a Ceriantheomorphe tube. Cahiers de Biologie Marine 51: 207–211. Available from: http://cbm-online.sb-roscoff.fr/pdf/cb51-2-205-211.pdf
- Stampar SN, Maronna MM, Vermeij MJA, Silveira FLD, Morandini AC (2012) Evolutionary diversification of banded tube-dwelling anemones (Cnidaria; Ceriantharia; Isarachnanthus) in the Atlantic Ocean. PLoS ONE 7: e4109. https://doi.org/10.1371/journal.pone.0041091
- Stampar SN, Maronna MM, Kitahara M V., Reimer JD, Morandini AC (2014a) Fast-Evolving mitochondrial DNA in Ceriantharia: A reflection of Hexacorallia paraphyly? PLoS ONE 9: e86612. https://doi.org/10.1371/journal.pone.0086612
- Stampar SN, Morandini AC, Da Silveira FL (2014b) A new species of Pachycerianthus (Cnidaria, Anthozoa, Ceriantharia) from tropical southwestern Atlantic. Zootaxa 3827: 343–354. https://doi.org/10.11646/zootaxa.3827.3.4
- Stampar SN, Beneti JS, Acuña FH, Morandini AC (2015a) Ultrastructure and tube formation in Ceriantharia (Cnidaria, Anthozoa). Zoologischer Anzeiger 254: 67–71. https://doi.org/10.1016/j.jcz.2014.11.004
- Stampar SN, Morandini AC, Branco LC, Da Silveira FL, Migotto AE (2015b) Drifting in the oceans: Isarachnanthus nocturnus (Cnidaria, Ceriantharia, Arachnactidae), an anthozoan with an extended planktonic stage. Marine Biology 162: 2161–2169. https://doi.org/10.1007/s00227-015-2747-0
- Stampar SN, Scarabino F, Pastorino G, Morandini AC (2015c) A new species of tube-dwelling anemone (Cnidaria, Anthozoa, Ceriantharia, Ceriantheopsis) from the Warm Temperate South-western Atlantic. Journal of the Marine Biological Association of the United Kingdom: 96(7): 1475–1481. https://doi.org/10.1017/S0025315415001745
- Stampar SN, Maronna MM, Kitahara M V, Reimer JD, Beneti JS, Morandini AC (2016) Ceriantharia in Current Systematics: Life Cycles, Morphology and Genetics. In: Goffredo S, Dubinsky Z (Eds) , The Cnidaria, Past, Present and Future: The world of Medusa and her sisters. Springer International Publishing, Cham, 61–72. https://doi.org/10.1007/978-3-319-31305-4_5
- Stampar SN, González-Muñoz R, Morandini AC (2017) Botruanthus mexicanus (Cnidaria: Ceriantharia), a new species of tube-dwelling anemone from the Gulf of Mexico. Marine Biodiversity 47: 113–118. https://doi.org/10.1007/s12526-016-0521-2
- Stampar SN, Morandini AC (2017) Occurrence of Isarachnanthus (Cnidaria: Anthozoa: Ceriantharia) at Ascension Island: A test of hypothesis. Journal of the Marine Biological Association of the United Kingdom 97: 689–693. https://doi.org/10.1017/S0025315414000423
- Stampar SN, El Didi SO, Paulay G, Berumen ML (2018) A new species of Arachnanthus from the Red Sea (Cnidaria, Ceriantharia). ZooKeys 748: 1–10. https://doi.org/10.3897/zookeys.748.22914
- Stampar SN, Broe MB, Macrander J, Reitzel AM, Brugler MR, Daly M (2019) Linear mitochondrial genome in Anthozoa (Cnidaria): A case study in Ceriantharia. Scientific Reports 9: 6094. https://doi.org/10.1038/s41598-019-42621-z
- Stephenson TA (1928) The British Sea Anemones. Volume I. The Ray Society, London, 148 pp.
- Strain EMA, Allcock AL, Goodwin CE, Maggs CA, Picton BE, Roberts D (2012) The long-term impacts of fisheries on epifaunal assemblage function and structure, in a Special Area of Conservation. Journal of Sea Research 67: 58–68. https://doi.org/10.1016/j.seares.2011.10.001
- Tarasov VG, Propp M V, Propp LN, Zhirmunsky A V, Namsakakv BB, Gorlenko VM, Starynin DA (1990) Shallow water gasohydrothermal vents of Ushishir Volcano and the ecosystem of Kraternaya Bight (the Kurile Islands). Marine Ecology 11: 1–23. https://doi.org/10.1111/j.1439-0485.1990.tb00225.x
- Teissier G (1950) Inventaire de la faune marine de Roscoff Cnidaires et Cténaires. Station Biologique de Roscoff, Roscoff, 42 pp.
- Tiffon Y, Hugon JS (1977) Ultrastructural localization of acid and alkaline phosphatases in sterile septae of the Anthozoa Pachycerianthus fimbriatus. Histochemistry 54: 289–297. https://doi.org/10.1007/BF00508272
- Torelli B (1932) Nuova specie di Cerianthus del Golfo di Napoli (Cerianthus viridis). Pubblicazioni della Stazione Zoologica di Napoli 12: 1–15.
- Torelli B (1961) Un Cerianthus del Golfo di Napoli: C. bicyclus n. sp. (Anthozoa). Pubblicazioni della Stazione Zoologica di Napoli 32: 17–28.
- Torelli B (1963) Due larve di Ceriantharia del Golfo di Napoli (Anthozoa). Pubblicazioni della Stazione Zoologica di Napoli 33: 169–177.
- Torrey HB, Kleeberger FL (1909) Contributions from the laboratory of the Marine Biological Association of San Diego. XXVII. Three species of Cerianthus from southern California. University of California Publications in Zoology 6: 115–125.
- Tur JM, Godall P (1982) Consideraciones preliminares sobre la ecología de los antozoos del litoral sur de la Costa Brava. Oecologia Aquatica: 175–183.
- Uchida H (1979) Cerianthids (Anthozoa, Coelenterata) from Kii Region, Middle Japan. Memoirs of the National Science Museum 12: 185–199.
- Uchida H, Soyama I (2001) Sea Anemones in Japanese Waters. TBS, Tokyo, 157 pp.
- Vafidis D, Koukouras A (1998) Antipatharia, Ceriantharia, and Zoantharia (Hexacorallia, Anthozoa) of the Aegean Sea with a check list of the Mediterranean and Black Sea species. Annales de l’Institute Oceanographique 74: 115–126.
- van Beneden E (1897) Les Anthozoaires de la “Plankton-Expedition” (Die Anthozoen der Plankton-Expedition). Ergebnisse der Plankton-Expedition der Humboldt-Stiftung 2K: 1–222.
- van Beneden E (1924) Travaux posthumes d’ Edouard van Beneden sur les cérianthaires collationnés par Paul Cerfontaine. Archives de Biologie (hors série): 1–242.
- van Soest RWM (1979) A catalogue of the coelenterate type specimens of the Zoological Museum of Amsterdam. IV. Gorgonacea, Actiniaria, Scleractinia. Beaufortia 29: 81–126.
- Verrill AE (1864a) List of the polyps and corals sent by the Museum of Comparative Zoölogy to other institutions in exchange, with annotations. Bulletin of the Museum of Comparative Zoology 1: 29–60.
- Verrill AE (1864b) Revision of the *Polypi* of the eastern coast of the United States. Memoirs of the Boston Society of Natural History 1: 1–45. https://doi.org/10.5962/bhl.title.78052
- Verrill AE (1865) Classification of polyps (Extract condensed from a Synopsis of the Polypi of the North Pacific Exploring Expedition, under Captains Ringgold and Rodgers, U.S.N.). Proceedings of the Essex Institute 4: 145–152. https://doi.org/10.1080/00222936508679407
- Verrill AE (1868) Synopsis of the polyps and corals of the North Pacific Exploring Expedition under Commodore C. Ringgold and Captain John Rodgers, USN, from 1853–1856. Collected by Dr. Wm. Stimpson, naturalist to the Expedition. With descriptions of some additional species from the west coast of North America. Part III. Madreporaria. Proceedings of the Essex Institute 5: 315–330.
- Verrill AE (1870) Synopsis of the polyps and corals of the North Pacific Exploring Expedition, under Commodore C. Ringgold and Capt. John Rodgers, USN, from 1853 to 1856. Collected by Dr. Wm. Stimpson, Naturalist to the Expedition. Part IV. Actiniaria. Proceedings of the Essex Institute 5: 51–104.
- Verrill AE (1872) On Radiata from the coast of North Carolina. American Journal of Science and Arts 3: 432–438. https://doi.org/10.2475/ajs.s3-3.18.432
- Verrill AE (1873a) Explorations of Casco Bay by the U.S. Fish Commission, in 1873. Proceedings of the American Association for the Advancement of Science 22: 340–395.
- Verrill AE (1873b) Results of recent dredging expeditions on the coast of New England. No. 3. American Journal of Science and Arts 5: 1–15. https://doi.org/10.2475/ajs.s3-5.26.98
- Verrill AE (1873c) Results of recent dredging expeditions on the coast of New England. No. 3. American Journal of Science and Arts 5: 435–441. https://doi.org/10.2475/ajs.s3-5.26.98
- Verrill AE (1874) Results of recent dredging expeditions on the coast of New England. No. 6. American Journal of Science and Arts 7: 405–413. https://doi.org/10.2475/ajs.s3-7.40.405
- Verrill AE (1879) Preliminary check-list of the marine Invertebrata of the Atlantic Coast, from Cape Cod to the Gulf of St. Lawrence. Tuttle, Morehouse & Taylor, Printers, New Haven, 32 pp.
- Verrill AE (1901) Additions to the fauna of the Bermudas from the Yale Expedition of 1901, with notes on other species. Transactions of the Connecticut Academy of Arts and Sciences 11: 15–62.
- Verrill AE (1922) The Actiniaria of the Canadian Arctic Expeditions, with notes on interesting species from Hudson Bay and other Canadian localities. Report on the Canadian Arctic Expedition 1913–1918 8: 89–164.
- Vieira LM, Stampar SN (2014) A new Fenestrulina (Bryozoa, Cheilostomata) commensal with tube-dwelling anemones (Cnidaria, Ceriantharia) in the tropical southwestern Atlantic. Zootaxa 3780: 365–374. https://doi.org/10.11646/zootaxa.3780.2.8
- Walton CL (1908) Actiniae collected by the S.S. “Huxley” in the North Sea during the summer of 1907. Journal of the Marine Biological Association of the United Kingdom 8: 215–226. https://doi.org/10.1017/S0025315400072532
- Wassilieff A (1908) Japanische Actinien. Abhandlungen des Mathematischen-Physikalischen Institutes der Kaiserlichen Bayerischen Akademie der Wissenschaften Suppl 1: 1–49.
- Widersten B (1976) Ceriantharia, Zoanthidea, Corallimorpharia, and Actiniaria from the continental shelf and slope off the eastern coast of the United States. Fishery Bulletin 74: 857–878.
- Wiedenmann J, Ivanchenko S, Oswald F, Nienhaus GU (2004) Identification of GFP-like Proteins in Nonbioluminescent, Azooxanthellate Anthozoa Opens New Perspectives for Bioprospecting. Marine Biotechnology 6: 270–277. https://doi.org/10.1007/s10126-004-3006-4
- Wieking G, Kröncke I (2005) Is benthic trophic structure affected by food quality? The Dogger Bank example. Marine Biology 146: 387–400. https://doi.org/10.1007/s00227-004-1443-2
- Williams G (1954) Fauna of Strangford Lough and neighbouring coasts. Proceedings of the Royal Irish Academy 56: 29–123.
- Williams RM (1981) A sea anemone, Edwardsia meridionalis sp. nov., from Antarctica and a preliminary revision of the genus Edwardsia De Quatrefages, 1841 (Coelenterata: Actiniaria). Records of the Australian Museum 33: 325–360. https://doi.org/10.3853/j.0067-1975.33.1981.271
- Wirtz P, Ocaña O, Molodtsova TN (2003) Actiniaria and Ceriantharia of the Azores (Cnidaria Anthozoa). Helgoland Marine Research 57: 114–117. https://doi.org/10.1007/s10152-003-0146-2
- WoRMS (2020) Anthozoa. http://marinespecies.org/aphia.php?p=taxdetails&id=1292