Research Article |
|
Corresponding author: David Ross Robertson ( robertsondr@si.edu ) Academic editor: Kyle Piller
© 2020 David Ross Robertson, Carlos J. Estapé, Allison M. Estapé, Ernesto Peña, Luke Tornabene, Carole C. Baldwin.
This is an open access article distributed under the terms of the CC0 Public Domain Dedication.
Citation:
Robertson DR, Estapé CJ, Estapé AM, Peña E, Tornabene L, Baldwin CC (2020) The marine fishes of St Eustatius Island, northeastern Caribbean: an annotated, photographic catalog. ZooKeys 1007: 145-180. https://doi.org/10.3897/zookeys.1007.58515
|
Abstract
Sint Eustatius (Statia) is a 21 km2 island situated in the northeastern Caribbean Sea. The most recent published sources of information on that island’s marine fish fauna is in two non-governmental organization reports from 2015–17 related to the formation of a marine reserve. The species-list in the 2017 report was based on field research in 2013–15 using SCUBA diving surveys, shallow “baited underwater video surveys” (BRUVs), and data from fishery surveys and scientific collections over the preceding century. That checklist comprised 304 species of shallow (mostly) and deep-water fishes. In 2017 the Smithsonian Deep Reef Observation Project surveyed deep-reef fishes at Statia using the crewed submersible Curasub. That effort recorded 120 species, including 59 new occurrences records. In March-May 2020, two experienced citizen scientists completed 62 SCUBA dives there and recorded 244 shallow species, 40 of them new records for Statia. The 2017–2020 research effort increased the number of species known from the island by 33.6% to 406. Here we present an updated catalog of that marine fish fauna, including voucher photographs of 280 species recorded there in 2017 and 2020. The Statia reef-fish fauna likely is incompletely documented as it has few small, shallow, cryptobenthic species, which are a major component of the regional fauna. A lack of targeted sampling is probably the major factor explaining that deficit, although a limited range of benthic marine habitats may also be contributing.
Keywords
biodiversity, checklist, faunal completeness, faunal structure, reef-associated bony fishes, SCUBA surveys, submersible surveys
Introduction
Sint Eustatius island, known locally as Statia, is a 21 km2 island in the northeastern Caribbean, and is one of the Leeward Islands in the Lesser Antilles. Until recently there were very few published accounts relating to the marine-fish fauna of Statia. The most comprehensive are represented by two non-governmental organization (NGO) environmental reports to the Statia government by
Materials and methods
Study area
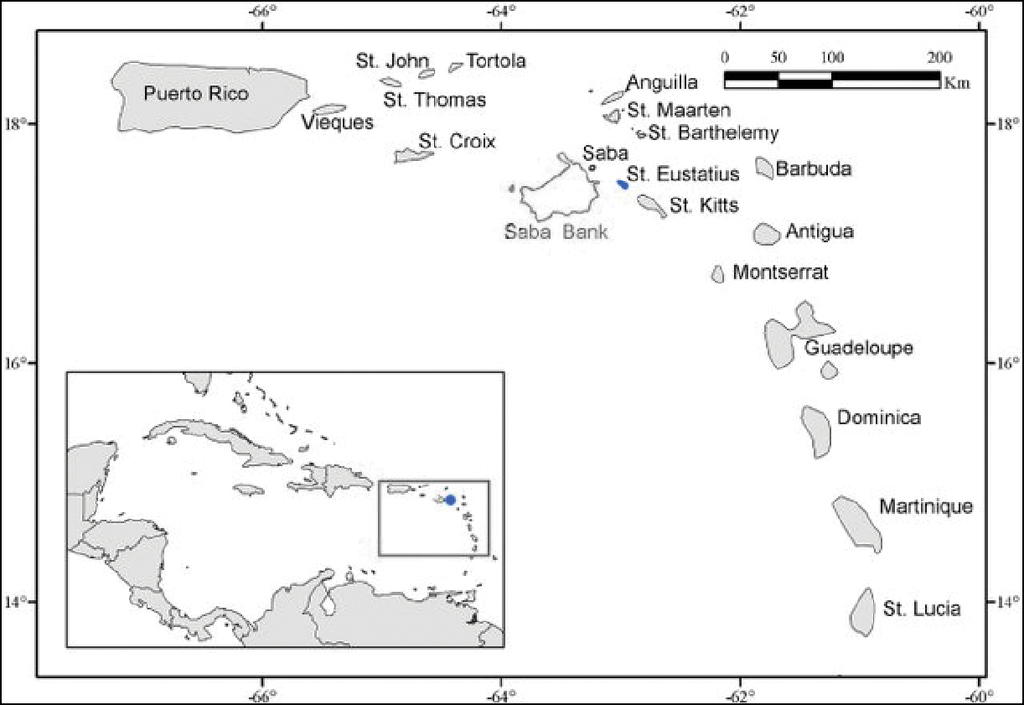
As one of the Dutch Caribbean islands, Statia sits among Saba, Sint Marten and St Kitts and Nevis (Figure
The Caribbean Sea, with the location of Sint Eustatius island indicated in the inset. Source:
Data sources
Published species lists
A comprehensive set of species records came from two NGO studies, which were included in a report by
Research in 2017 and 2020
In 2017 the Smithsonian Institution’s Deep Reef Observation Project (DROP) worked with the crewed submersible Curasub to make collections and observations on deep-reef fishes at Statia, to complement similar prior work at the Antillean islands of Dominica and Curaçao (e.g.,
Study sites at Sint Eustatius Island. Location of dive sites during 2017 and 2020: Black stars indicate submersible dives, blue stars 2017 SCUBA dives, red stars 2020 SCUBA dives (some individual stars indicate multiple dives in very close proximity), purple star an intertidal snorkeling site, and the red outline shows limits of the shore-diving area in 2020. See Suppl. material
Two of the authors, CJE and AME, are citizen scientists with extensive experience photographing reef fishes at various sites in the Greater Caribbean. In 2020 they spent two months (mid-March to mid-May) living at Statia and SCUBA diving daily to obtain photographic vouchers of the fishes they observed. They made 62 dives, each of approximately one-hour duration, at depths between 1–30 m on both hard-reef, sand, rubble and seagrass habitats, as well as on sunken wrecked ships. Half of those dives were nearshore in a restricted area, as, during the second half of their stay at the island, they lacked dive-boat support and were able to dive only from the shoreline (see Figure
Online aggregators
In addition, we also assessed information provided by three major aggregators of online georeferenced location data on marine fishes (GBIF https://www.gbif.org/, OBIS https://obis.org/, and FishNet2 http://www.fishnet2.net/search.aspx, all accessed on 7 May 2020), searching for records in ~ 120-km2 quadrat based on Admiralty Chart 487G that encompassed Statia and the surrounding shelf area: the area bounded by 17.433°N to 17.533°N and – 62.933°W to – 63.033°W. That quadrat contained almost 100 km2 of marine habitat. That area is a little larger than and centered on the area shown in Figure
Location of dive sites during 2017 and 2020: Black stars indicate submersible dives, blue stars 2017 SCUBA dives, red stars 2020 SCUBA dives (some individual stars indicate multiple dives in very close proximity), purple star an intertidal snorkeling site, and the red outline shows limits of the shore-diving area in 2020. See Suppl. material
The structure of the Statia reef-fish fauna
Zoogeography
Members of the entire Statia fauna as currently known (Table
Ecological structure
The research during 2017–2020 was aimed at documenting the reef-associated bony fishes of Statia. For analyses of the structure of the Statia20 fauna we assigned those species to the following ecological groups (following
In the Greater Caribbean region reef-associated bony fishes comprise ~ 900 species from 304 genera in 76 families (
A list of reef-associated fishes known from Alligator Reef was extracted from the list in
Results
Modifications to the list of Davies and Piontek (2017)
We reduced the number of species on the list of
Additions from other sources
The
DROP recorded a total of 120 species, 59 of which were not in any other list, except for two new records it shared with the Estapé 2020 list. Eight of those 59 records are of species that have yet to be described and named. The Estapé 2020 list includes 244 records, 40 of them new, plus two other new additions they share with DROP. Summing the deletions and additions from various sources produced a total of 406 species for the Statia20 checklist (see Table
Updated checklist of marine fishes from Sint Eustatius Island, 2020. Key to column headings and entries: DROP – CP = collected and photographed; C collected only; V = visual observation only; Estapé – P = photographed by CJE and AME; (P) photographed by 3rd parties; V = visual observation only by CJE and AME. New – species is a new record resulting from 2017–20 research, and its source. Other sources of species records are
| Species in families | English common name | New | DROP | Estapé | vK15 | DP17 | GBIF | OBIS | Plate | Zoo | Range | Deep |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACANTHURIDAE | ||||||||||||
| Acanthurus chirurgus (Bloch, 1787) | Doctorfish | V | P | Yes | Yes | Yes | Yes | 1 | GC | |||
| Acanthurus coeruleus Bloch & Schneider, 1801 | Blue Tang | V | P | Yes | Yes | Yes | Yes | 1 | GC | |||
| Acanthurus tractus Poey, 1860 | Northern Ocean Surgeonfish | V | P | Yes | Yes | Yes | Yes | 1 | GC | |||
| ACHIRIDAE | ||||||||||||
| Gymnachirus nudus Kaup, 1858 | Flabby Sole | Estapé | P | 1 | GC | |||||||
| ACROPOMATIDAE | ||||||||||||
| Synagrops bellus (Goode & Bean, 1896) | Blackmouth Bass | Yes | WA | Yes | ||||||||
| AETOBATIDAE | ||||||||||||
| Aetobatus narinari (Euphrasen, 1790) | Spotted Eagle Ray | (P) | Yes | Yes | 1 | WA | ||||||
| ANTENNARIIDAE | ||||||||||||
| Antennarius multiocellatus (Valenciennes, 1837) | Longlure Frogfish | P | Yes | 1 | WA | |||||||
| Histrio histrio (Linnaeus, 1758) | Sargassumfish | (P) | Yes | 1 | PAN | |||||||
| APOGONIDAE | ||||||||||||
| Apogon aurolineatus (Mowbray, 1927) | Bridle Cardinalfish | Yes | GC | |||||||||
| Apogon maculatus (Poey, 1860) | Flamefish | P | Yes | 1 | GC | |||||||
| Apogon pillionatus Bohlke & Randall, 1968 | Broadsaddle Cardinalfish | DROP | V | GC | ||||||||
| Apogon planifrons Longley & Hildebrand, 1940 | Pale Cardinalfish | Estapé | P | 1 | WA | |||||||
| Apogon pseudomaculatus Longley, 1932 | Twospot Cardinalfish | DROP | C | DROP | WA | |||||||
| Apogon quadrisquamatus Longley, 1934 | Sawcheek Cardinalfish | P | Yes | 1 | WA | |||||||
| Apogon townsendi (Breder, 1927) | Belted Cardinalfish | P | Yes | 1 | WA | |||||||
| Astrapogon puncticulatus (Poey, 1867) | Blackfin Cardinalfish | Estapé | V | WA | ||||||||
| Astrapogon stellatus (Cope, 1867) | Conchfish | Yes | WA | |||||||||
| Paroncheilus affinis (Poey, 1875) | Bigtooth Cardinalfish | V | P | Yes | 1 | TA | ||||||
| Phaeoptyx conklini (Silvester, 1915) | Freckled Cardinalfish | Estapé | P | 1 | GC | |||||||
| Phaeoptyx pigmentaria (Poey, 1860) | Dusky Cardinalfish | Yes | 1 | TA | ||||||||
| ARGENTINIDAE | ||||||||||||
| Argentina stewarti Cohen & Atsaides, 1969 | Yes | GC | Yes | |||||||||
| Glossanodon pygmaeus Cohen, 1958 | Pygmy Argentine | DROP | CP | 1 | WA | Yes | ||||||
| ATHERINIDAE | ||||||||||||
| Atherina harringtonensis Goode, 1877 | Reef Silverside | Yes | GC | |||||||||
| Atherinomorus stipes (Müller & Troschel, 1848) | Hardhead Silverside | Estapé | P | 1 | WA | |||||||
| AULOSTOMIDAE | ||||||||||||
| Aulostomus maculatus Valenciennes, 1841 | Atlantic Trumpetfish | P | Yes | Yes | Yes | 1 | GC | |||||
| BALISTIDAE | ||||||||||||
| Balistes capriscus Gmelin, 1789 | Gray Triggerfish | P | Yes | 1 | TA | |||||||
| Balistes vetula Linnaeus, 1758 | Queen Triggerfish | P | Yes | Yes | Yes | 1 | TA | |||||
| Canthidermis sufflamen (Mitchill, 1815) | Ocean Triggerfish | V | Yes | WA | ||||||||
| Melichthys niger (Bloch, 1786) | Black Durgon | P | Yes | Yes | Yes | Yes | 1 | PAN | ||||
| Xanthichthys ringens (Linnaeus, 1758) | Sargassum Triggerfish | DROP | V | WA | ||||||||
| BELONIDAE | ||||||||||||
| Platybelone argalus argalus (Lesueur, 1821) | Keeltail Needlefish | Yes | WA | |||||||||
| Tylosurus crocodilus (Péron & Lesueur, 1821) | Houndfish | P | Yes | 1 | PAN | |||||||
| BLENNIIDAE | ||||||||||||
| Entomacrodus nigricans Gill, 1859 | Pearl Blenny | P | Yes | 1 | GC | |||||||
| Hypleurochilus pseudoaequipinnis Bath, 1994 | Oyster Blenny | Estapé | P | 1 | WA | |||||||
| Hypleurochilus springeri Randall, 1966 | Orangespotted Blenny | Estapé | P | 1 | GC | |||||||
| Hypsoblennius exstochilus Bohlke, 1959 | Longhorn Blenny | (P) | Yes | 2 | GC | |||||||
| Ophioblennius macclurei (Silvester, 1915) | Redlip Blenny | P | Yes | Yes | 2 | GC | ||||||
| Parablennius marmoreus (Poey, 1876) | Seaweed Blenny | P | Yes | 2 | WA | |||||||
| BOTHIDAE | ||||||||||||
| Bothus lunatus (Linnaeus, 1758) | Peacock Flounder | P | Yes | Yes | 2 | TA | ||||||
| Bothus ocellatus (Agassiz, 1831) | Eyed Flounder | P | Yes | 2 | WA | |||||||
| Chascanopsetta lugubris Alcock, 1894 | Pelican Flounder | Yes | TA,IWP | Yes | ||||||||
| CALLIONYMIDAE | ||||||||||||
| Callionymus bairdi (Jordan, 1888) | Lancer Dragonet | P | Yes | 2 | WA | |||||||
| Foetorepus species | DROP | CP | 13 | WA? | ? | Yes | ||||||
| CAPROIDAE | ||||||||||||
| Antigonia capros Lowe, 1843 | Deepbody Boarfish | DROP | V | TA,IWP | Yes | |||||||
| CARANGIDAE | ||||||||||||
| Alectis ciliaris (Bloch, 1787) | African Pompano | Yes | PAN | |||||||||
| Caranx bartholomaei (Cuvier, 1833) | Yellow Jack | P | Yes | 2 | TA | |||||||
| Caranx crysos (Mitchill, 1815) | Blue Runner | P | Yes | 2 | TA | |||||||
| Caranx hippos (Linnaeus, 1766) | Crevalle Jack | Yes | WA | |||||||||
| Caranx latus Agassiz, 1831 | Horse-eye Jack | P | Yes | Yes | 2 | TA | ||||||
| Caranx lugubris Poey, 1860 | Black Jack | V | Yes | Yes | PAN | |||||||
| Caranx ruber (Bloch, 1793) | Bar Jack | V | P | Yes | Yes | Yes | Yes | 2 | WA | |||
| Decapterus macarellus (Cuvier, 1833) | Mackerel Scad | P | Yes | 2 | PAN | |||||||
| Decapterus punctatus (Cuvier, 1829) | Round Scad | P | Yes | 2 | TA | |||||||
| Elagatis bipinnulata (Quoy & Gaimard, 1825) | Rainbow Runner | P | Yes | 2 | PAN | |||||||
| Selar crumenophthalmus (Bloch, 1793) | Bigeye Scad | P | Yes | 2 | PAN | |||||||
| Seriola rivoliana Valenciennes, 1833 | Almaco Jack | P | Yes | Yes | 2 | PAN | ||||||
| Trachinotus falcatus (Linnaeus, 1758) | Permit | P | Yes | 2 | WA | |||||||
| Trachinotus goodei Jordan & Evermann, 1896 | Palometa | P | Yes | 2 | WA | |||||||
| CARCHARHINIDAE | ||||||||||||
| Carcharhinus leucas (Müller & Henle, 1839) | Bull Shark | Yes | PAN | |||||||||
| Carcharhinus limbatus (Müller & Henle, 1839) | Blacktip Shark | Yes | Yes | PAN | ||||||||
| Carcharhinus perezii (Poey, 1876) | Reef Shark | V | Yes | Yes | WA | |||||||
| Galeocerdo cuvier (Peron & Lesueur, 1822) | Tiger Shark | Yes | PAN | |||||||||
| Negaprion brevirostris (Poey, 1868) | Lemon Shark | P | Yes | 2 | TA,EP | |||||||
| CENTROPHORIDAE | ||||||||||||
| Centrophorus granulosus (Bloch & Schneider, 1801) | Large Gulper Shark | Yes | TA,IWP | Yes | ||||||||
| CHAENOPSIDAE | ||||||||||||
| Acanthemblemaria aspera (Longley, 1927) | Roughhead Blenny | P | Yes | 2 | GC | |||||||
| Acanthemblemaria maria Bohlke, 1961 | Secretary Blenny | P | Yes | Yes | 2 | GC | ||||||
| Acanthemblemaria spinosa Metzelaar, 1919 | Spinyhead Blenny | P | Yes | Yes | 2 | GC | ||||||
| Chaenopsis limbaughi Robins & Randall, 1965 | Yellowface Pikeblenny | P | Yes | 2 | GC | |||||||
| Emblemaria pandionis Evermann & Marsh, 1900 | Sailfin Blenny | P | Yes | Yes | 2 | GC | ||||||
| Emblemaria vitta Williams, 2002 | Ribbon Blenny | Estapé | (P) | 2 | GC | |||||||
| Emblemariopsis bahamensis Stephens, 1961 | Blackhead Blenny | Estapé | P | 3 | GC | L | ||||||
| Emblemariopsis carib Victor, 2010 | Carib Blenny | Estapé | P | 3 | GC | L | ||||||
| CHAETODONTIDAE | ||||||||||||
| Chaetodon capistratus Linnaeus, 1758 | Foureye Butterflyfish | V | P | Yes | Yes | Yes | Yes | 3 | GC | |||
| Chaetodon ocellatus Bloch, 1787 | Spotfin Butterflyfish | P | Yes | Yes | Yes | 3 | WA | |||||
| Chaetodon sedentarius Poey, 1860 | Reef Butterflyfish | V | Yes | Yes | Yes | WA | ||||||
| Chaetodon striatus Linnaeus, 1758 | Banded Butterflyfish | V | P | Yes | Yes | Yes | Yes | 3 | WA | |||
| Prognathodes aculeatus (Poey, 1860) | Longsnout Butterflyfish | C | P | Yes | Yes | Yes | Yes | 3 | WA | |||
| Prognathodes guyanensis (Durand, 1960) | Guyana Butterflyfish | DROP | V | GC | Yes | |||||||
| CHAUNACIDAE | ||||||||||||
| Chaunax suttkusi Caruso, 1989 | Pale-cavity Gaper | Yes | TA | Yes | ||||||||
| CHIMAERIDAE | ||||||||||||
| Chimaera cubana Howell Rivero, 1936 | Cuban Chimaera | Yes | GC | Yes | ||||||||
| Hydrolagus alberti Bigelow & Schroeder, 1951 | Gulf Chimaera | Yes | GC | Yes | ||||||||
| CHLOPSIDAE | ||||||||||||
| Chilorhinus suensonii Lutken, 1852 | Seagrass Eel | Yes | WA | |||||||||
| CHLOROPHTHALMIDAE | ||||||||||||
| Chlorophthalmus agassizi Bonaparte, 1840 | Shortnose Greeneye | Yes | TA | Yes | ||||||||
| Parasudis truculenta (Goode & Bean, 1895) | Longnose Greeneye | Yes | WA | Yes | ||||||||
| CIRRHITIDAE | ||||||||||||
| Amblycirrhitus pinos (Mowbray, 1927) | Redspotted Hawkfish | P | Yes | Yes | 3 | WA | ||||||
| CLUPEIDAE | ||||||||||||
| Harengula clupeola (Cuvier, 1829) | False Pilchard | Yes | WA | |||||||||
| Harengula humeralis (Cuvier, 1829) | Redear Sardine | Yes | GC | |||||||||
| Jenkinsia lamprotaenia (Gosse, 1851) | Dwarf Herring | Yes | GC | |||||||||
| Opisthonema oglinum (Lesueur, 1818) | Atlantic Thread Herring | Yes | WA | |||||||||
| Sardinella aurita Valenciennes, 1847 | Spanish Sardine | Yes | TA | |||||||||
| CONGRIDAE | ||||||||||||
| Ariosoma balearicum (Delaroche, 1809) | Bandtooth Conger | Estapé | (P) | 3 | TA | |||||||
| Heteroconger longissimus Gunther, 1870 | Brown Garden Eel | P | Yes | Yes | Yes | 3 | WA | |||||
| Xenomystax bidentatus (Reid, 1940) | Twopatched-teeth Conger | Yes | TA | Yes | ||||||||
| CORYPHAENIDAE | ||||||||||||
| Coryphaena hippurus Linnaeus, 1758 | Dolphinfish | Yes | PAN | |||||||||
| CRURIRAJIDAE | ||||||||||||
| Cruriraja rugosa Bigelow & Schroeder, 1958 | Rough Leg Skate | Yes | GC | Yes | ||||||||
| CYNOGLOSSIDAE | ||||||||||||
| Symphurus marginatus (Goode & Bean, 1886) | Margined Tonguefish | Yes | WA | Yes | ||||||||
| DACTYLOPTERIDAE | ||||||||||||
| Dactylopterus volitans (Linnaeus, 1758) | Flying Gurnard | P | Yes | Yes | Yes | 3 | TA | |||||
| DASYATIDAE | ||||||||||||
| Hypanus americanus Hildebrand & Schroeder, 1928 | Southern Stingray | P | Yes | Yes | Yes | 3 | WA | |||||
| DIODONTIDAE | ||||||||||||
| Chilomycterus antillarum Jordan & Rutter, 1897 | Web Burrfish | P | Yes | Yes | 3 | WA | ||||||
| Chilomycterus schoepfii (Walbaum, 1792) | Striped Burrfish | Yes | NWA | |||||||||
| Diodon holocanthus Linnaeus, 1758 | Balloonfish | P | Yes | 3 | PAN | |||||||
| Diodon hystrix Linnaeus, 1758 | Porcupinefish | P | Yes | Yes | Yes | 3 | PAN | |||||
| DIRETMIDAE | ||||||||||||
| Diretmus argenteus Johnson, 1864 | Silver Spinyfish | Yes | PAN | Yes | ||||||||
| ECHENEIDAE | ||||||||||||
| Echeneis naucrates Linnaeus, 1758 | Sharksucker | P | Yes | Yes | Yes | 3 | PAN | |||||
| Echeneis neucratoides Zuiew, 1786 | Whitefin Sharksucker | Estapé | P | 3 | NWA | |||||||
| Remora remora (Linnaeus, 1758) | Remora | Yes | PAN | |||||||||
| EPHIPPIDAE | ||||||||||||
| Chaetodipterus faber (Broussonet, 1782) | Atlantic Spadefish | Yes | WA | |||||||||
| ETMOPTERIDAE | ||||||||||||
| Etmopterus hillianus (Poey, 1861) | Caribbean Lantern Shark | Yes | NWA | Yes | ||||||||
| Etmopterus robinsi Schofield & Burgess, 1997 | West Indian Lantern Shark | Yes | GC | Yes | ||||||||
| FISTULARIIDAE | ||||||||||||
| Fistularia tabacaria Linnaeus, 1758 | Bluespotted Cornetfish | P | Yes | Yes | 3 | TA | ||||||
| GERREIDAE | ||||||||||||
| Eucinostomus jonesii (Gunther, 1879) | Slender Mojarra | Yes | WA | |||||||||
| Eucinostomus lefroyi (Goode, 1874) | Mottled Mojarra | P | Yes | 3 | WA | |||||||
| Gerres cinereus (Walbaum, 1792) | Yellowfin Mojarra | Yes | WA | |||||||||
| GINGLYMOSTOMATIDAE | ||||||||||||
| Ginglymostoma cirratum (Bonnaterre, 1788) | Nurse Shark | (P) | Yes | Yes | Yes | 3 | TA | |||||
| GOBIESOCIDAE | ||||||||||||
| Derilissus lombardii Sparks & Gruber, 2012 | Tailspot Clingfish | DROP | CP | 3 | GC | Yes | ||||||
| GOBIIDAE | ||||||||||||
| Antilligobius nikkiae Van Tassell & Colin, 2012 | Sabre Goby | DROP | CP | DROP | 3 | GC | Yes | |||||
| Bathygobius antilliensis Tornabene, Baldwin & Pezold, 2010 | Antilles Frillfin | Estapé | P | 3 | GC | |||||||
| Coryphopterus dicrus Bohlke & Robins, 1960 | Colon Goby | P | Yes | 3 | WA | |||||||
| Coryphopterus eidolon Bohlke & Robins, 1960 | Pallid Goby | P | Yes | 3 | GC | |||||||
| Coryphopterus glaucofraenum Gill, 1863 | Bridled Goby | Yes | WA | |||||||||
| Coryphopterus hyalinus Bohlke & Robins, 1962 | Glass Goby | P | Yes | 4 | GC | |||||||
| Coryphopterus kuna Victor, 2007 | Kuna Goby | Estapé | P | 4 | GC | |||||||
| Coryphopterus lipernes Bohlke & Robins, 1962 | Peppermint Goby | P | Yes | 4 | GC | |||||||
| Coryphopterus personatus (Jordan & Thompson, 1905) | Masked Goby | V | P | Yes | 4 | GC | ||||||
| Coryphopterus thrix Bohlke & Robins, 1960 | Bartail Goby | P | Yes | 4 | WA | |||||||
| Coryphopterus tortugae (Jordan, 1904) | Sand Goby | P | Yes | 4 | GC | |||||||
| Coryphopterus venezuelae Cervigon, 1966 | Sand-Canyon Goby | Estapé | P | 4 | GC | |||||||
| Ctenogobius saepepallens (Gilbert & Randall, 1968) | Dash Goby | Estapé | P | 4 | GC | |||||||
| Elacatinus chancei (Beebe & Hollister, 1933) | Shortstripe Goby | C | P | Yes | Yes | 4 | GC | L | ||||
| Elacatinus evelynae (Bohlke & Robins, 1968) | Sharknose Goby | P | Yes | Yes | 4 | GC | ||||||
| Genus 1 species 5 | DROP | CP | 13 | GC? | ? | Yes | ||||||
| Genus 1 species 6 | DROP | CP | 13 | GC? | ? | Yes | ||||||
| Genus 2 species 1 | DROP | CP | 13 | GC? | ? | Yes | ||||||
| Ginsburgellus novemlineatus (Fowler, 1950) | Ninelined Goby | Estapé | P | 4 | GC | |||||||
| Gnatholepis thompsoni Jordan, 1904 | Goldspot Goby | V | P | Yes | Yes | 4 | TA | |||||
| Lythrypnus elasson Bohlke & Robins, 1960 | Dwarf Goby | DROP/ Estapé | C | P | 4 | GC | ||||||
| Microgobius carri Fowler, 1945 | Seminole Goby | Estapé | P | 4 | WA | |||||||
| Nes longus (Nichols, 1914) | Orangespotted Goby | P | Yes | 4 | GC | |||||||
| Palatogobius grandoculus Greenfield, 2002 | Bigeye Goby | DROP | CP | DROP | 4 | GC | Yes | |||||
| Palatogobius incendius Tornabene, Robertson & Baldwin, 2017 | Ember Goby | DROP | C | DROP | GC | Yes | ||||||
| Pinnichthys aimoriensis Van Tassell & Tornabene, 2016 | Thiony’s Goby | DROP | CP | 4 | GC | Yes | ||||||
| Priolepis hipoliti (Metzelaar, 1922) | Rusty Goby | P | Yes | 4 | WA | |||||||
| Ptereleotris helenae (Randall, 1968) | Hovering Dartfish | V | P | Yes | 4 | GC | ||||||
| Risor ruber (Rosen, 1911) | Tusked Goby | C | P | Yes | Yes | 4 | WA | |||||
| Tigrigobius dilepis (Robins & Bohlke, 1964) | Orangesided Goby | P | Yes | 4 | GC | |||||||
| Tigrigobius multifasciatus (Steindachner, 1876) | Greenbanded Goby | Estapé | P | 4 | GC | L | ||||||
| Varicus cephalocellatus Gilmore, Van Tassell & Baldwin, 2016 | Ocellated Split-Fin Goby | DROP | CP | DROP | 4 | GC | L | Yes | ||||
| Varicus veliguttatus Van Tassell, Baldwin & Gilmore, 2016 | Spotted-Sail Goby | DROP | CP | DROP | 4 | GC | Yes | |||||
| GRAMMATIDAE | ||||||||||||
| Gramma linki Starck & Colin, 1978 | Yellowcheek Basslet | DROP | CP | DROP | 5 | GC | ||||||
| Gramma loreto Poey, 1868 | Fairy Basslet | P | Yes | Yes | 5 | GC | ||||||
| Lipogramma evides Robins & Colin, 1979 | Banded Basslet | DROP | CP | DROP | 5 | GC | Yes | |||||
| Lipogramma klayi Randall, 1963 | Bicolor Basslet | DROP | CP | 5 | GC | Yes | ||||||
| Lipogramma levinsoni Baldwin, Nonaka & Robertson, 2016 | Hourglass Basslet | DROP | CP | 5 | GC | Yes | ||||||
| Lipogramma regia Robins & Colin, 1979 | Royal Basslet | DROP | CP | DROP | 5 | GC | Yes | |||||
| Lipogramma trilineata Randall, 1963 | Threeline Basslet | DROP | CP | DROP | 5 | GC | Yes | |||||
| GRAMMICOLEPIDIDAE | ||||||||||||
| Grammicolepis brachiusculus Poey, 1873 | Thorny Tinselfish | Yes | PAN | Yes | ||||||||
| HAEMULIDAE | ||||||||||||
| Anisotremus surinamensis (Bloch, 1791) | Black Margate | P | Yes | Yes | 5 | WA | ||||||
| Brachygenys chrysargyreum (Gunther, 1859) | Smallmouth Grunt | P | Yes | Yes | Yes | 5 | GC | |||||
| Haemulon album Cuvier, 1830 | Margate | P | Yes | Yes | 5 | WA | ||||||
| Haemulon aurolineatum Cuvier, 1830 | Tomtate | P | Yes | Yes | Yes | 5 | WA | |||||
| Haemulon carbonarium Poey, 1860 | Caesar Grunt | P | Yes | Yes | Yes | Yes | 5 | GC | ||||
| Haemulon flavolineatum (Desmarest, 1823) | French Grunt | P | Yes | Yes | Yes | Yes | 5 | GC | ||||
| Haemulon macrostomum Gunther, 1859 | Spanish Grunt | Yes | GC | |||||||||
| Haemulon melanurum (Linnaeus, 1758) | Cottonwick | P | Yes | 5 | WA | |||||||
| Haemulon parra (Desmarest, 1823) | Sailors Choice | Yes | WA | |||||||||
| Haemulon plumierii (Lacepede, 1801) | White Grunt | P | Yes | 5 | WA | |||||||
| Haemulon sciurus (Shaw, 1803) | Bluestriped Grunt | (P) | Yes | Yes | 5 | GC | ||||||
| Haemulon striatum (Linnaeus, 1758) | Striped Grunt | V | V | Yes | WA | |||||||
| Haemulon vittatum (Poey, 1860) | Boga | P | Yes | 5 | GC | |||||||
| HALOSAURIDAE | ||||||||||||
| Halosaurus ovenii Johnson, 1864 | Stripejaw Halosaur | Yes | TA,IWP | Yes | ||||||||
| HEMIRAMPHIDAE | ||||||||||||
| Hemiramphus brasiliensis (Linnaeus, 1758) | Ballyhoo | P | Yes | 5 | WA | |||||||
| HOLOCENTRIDAE | ||||||||||||
| Corniger spinosus Agassiz, 1831 | Spinycheek Soldierfish | DROP | V | TA | Yes | |||||||
| Holocentrus adscensionis (Osbeck, 1765) | Squirrelfish | V | P | Yes | Yes | Yes | 5 | TA | ||||
| Holocentrus rufus (Walbaum, 1792) | Longspine Squirrelfish | V | P | Yes | Yes | Yes | 5 | GC | ||||
| Myripristis jacobus Cuvier, 1829 | Blackbar Soldierfish | V | P | Yes | Yes | 5 | TA | |||||
| Neoniphon coruscum (Poey, 1860) | Reef Squirrelfish | P | Yes | 5 | GC | |||||||
| Neoniphon marianus (Cuvier, 1829) | Longjaw Squirrelfish | C | P | Yes | Yes | 5 | GC | |||||
| Neoniphon vexillarium (Poey, 1860) | Dusky Squirrelfish | P | Yes | 5 | GC | |||||||
| Ostichthys trachypoma (Gunther, 1859) | Bigeye Soldierfish | DROP | CP | DROP | 6 | WA | Yes | |||||
| Plectrypops retrospinis (Guichenot, 1853) | Cardinal Soldierfish | Estapé | P | 6 | WA | |||||||
| ISTIOPHORIDAE | ||||||||||||
| Istiophorus platypterus (Shaw, 1792) | Sailfish | Yes | TA | |||||||||
| Makaira nigricans Lacepede, 1802 | Blue Marlin | Yes | PAN | |||||||||
| KYPHOSIDAE | ||||||||||||
| Kyphosus bigibbus Lacepede, 1801 | Gray Seachub | Estapé | P | 6 | TA/IWP | |||||||
| Kyphosus cinerascens (Forsskal, 1775) | Topsail Seachub | P | Yes | 6 | PAN | |||||||
| Kyphosus sectatrix (Linnaeus, 1766) | Bermuda Chub | P | Yes | 6 | PAN | |||||||
| Kyphosus vaigiensis (Quoy & Gaimard, 1825) | Yellow Chub | V | Yes | PAN | ||||||||
| LABRIDAE | ||||||||||||
| Labrinae | ||||||||||||
| Bodianus rufus (Linnaeus, 1758) | Spanish Hogfish | V | P | Yes | Yes | Yes | 6 | WA | ||||
| Clepticus parrae (Bloch & Schneider, 1801) | Creole Wrasse | V | P | Yes | Yes | Yes | 6 | GC | ||||
| Decodon puellaris (Poey, 1860) | Red Hogfish | DROP | CP | DROP | 6 | WA | Yes | |||||
| Decodon species 2 | DROP | CP | 13 | GC | Yes | |||||||
| Halichoeres bathyphilus (Beebe & Tee-Van,1932) | Greenband Wrasse | DROP | V | GC | Yes | |||||||
| Halichoeres bivittatus (Bloch, 1791) | Slippery Dick | P | Yes | Yes | 6 | WA | ||||||
| Halichoeres cyanocephalus (Bloch, 1791) | Yellowcheek Wrasse | P | Yes | Yes | 6 | GC | ||||||
| Halichoeres garnoti (Valenciennes, 1839) | Yellowhead Wrasse | V | P | Yes | Yes | Yes | 6 | GC | ||||
| Halichoeres maculipinna (Müller & Troschel, 1848) | Clown Wrasse | P | Yes | Yes | 6 | GC | ||||||
| Halichoeres pictus (Poey, 1860) | Rainbow Wrasse | P | Yes | 6 | GC | |||||||
| Halichoeres poeyi (Steindachner, 1867) | Blackear Wrasse | P | Yes | Yes | 6 | WA | ||||||
| Halichoeres radiatus (Linnaeus, 1758) | Puddingwife | P | Yes | Yes | 6 | WA | ||||||
| Thalassoma bifasciatum (Bloch, 1791) | Bluehead | V | P | Yes | Yes | Yes | 6 | GC | ||||
| Xyrichtys martinicensis Valenciennes, 1840 | Rosy Razorfish | P | Yes | Yes | 6 | GC | ||||||
| Xyrichtys novacula (Linnaeus, 1758) | Pearly Razorfish | P | Yes | 6 | WA | |||||||
| Xyrichtys splendens Castelnau, 1855 | Green Razorfish | P | Yes | Yes | Yes | 6 | GC | |||||
| Scarinae | ||||||||||||
| Cryptotomus roseus Cope, 1871 | Bluelip Parrotfish | P | Yes | 6 | WA | |||||||
| Scarus coeruleus (Bloch, 1786) | Blue Parrotfish | Yes | Yes | GC | ||||||||
| Scarus guacamaia Cuvier, 1829 | Rainbow Parrotfish | Yes | GC | |||||||||
| Scarus iseri (Bloch, 1789) | Striped Parrotfish | P | Yes | Yes | Yes | 6 | GC | |||||
| Scarus taeniopterus Desmarest, 1831 | Princess Parrotfish | V | P | Yes | Yes | Yes | Yes | 6 | GC | |||
| Scarus vetula Bloch & Schneider, 1801 | Queen Parrotfish | P | Yes | Yes | Yes | Yes | 6 | GC | ||||
| Sparisoma atomarium (Poey, 1861) | Greenblotch Parrotfish | P | Yes | 6 | GC | |||||||
| Sparisoma aurofrenatum (Valenciennes, 1840) | Redband Parrotfish | V | P | Yes | Yes | Yes | Yes | 7 | GC | |||
| Sparisoma chrysopterum (Bloch & Schneider, 1801) | Redtail Parrotfish | P | Yes | Yes | Yes | 7 | GC | |||||
| Sparisoma radians (Valenciennes, 1840) | Bucktooth Parrotfish | P | Yes | 7 | WA | |||||||
| Sparisoma rubripinne (Valenciennes, 1840) | Yellowtail Parrotfish | P | Yes | Yes | 7 | GC | ||||||
| Sparisoma viride (Bonnaterre, 1788) | Stoplight Parrotfish | V | P | Yes | Yes | Yes | 7 | GC | ||||
| LABRISOMIDAE | ||||||||||||
| Brockius nigricinctus Howell Rivero, 1936 | Spotcheek Blenny | Estapé | P | 7 | GC | |||||||
| Gobioclinus bucciferus Poey, 1868 | Puffcheek Blenny | Estapé | P | 7 | GC | |||||||
| Gobioclinus gobio (Valenciennes, 1836) | Palehead Blenny | Estapé | P | 7 | WA | |||||||
| Gobioclinus guppyi (Norman, 1922) | Mimic Blenny | Estapé | P | 7 | WA | |||||||
| Labrisomus nuchipinnis (Quoy & Gaimard, 1824) | Hairy Blenny | P | Yes | Yes | 7 | TA | ||||||
| Malacoctenus aurolineatus Smith, 1957 | Goldline Blenny | P | Yes | 7 | GC | |||||||
| Malacoctenus boehlkei Springer, 1959 | Diamond Blenny | Yes | GC | |||||||||
| Malacoctenus erdmani Smith, 1957 | Imitator Blenny | Estapé | P | 7 | GC | |||||||
| Malacoctenus macropus (Poey, 1868) | Rosy Blenny | Estapé | P | 7 | GC | |||||||
| Malacoctenus triangulatus Springer, 1959 | Saddled Blenny | P | Yes | 7 | GC | |||||||
| LOBOTIDAE | ||||||||||||
| Lobotes surinamensis (Bloch, 1790) | Atlantic Tripletail | Yes | TA/IWP | |||||||||
| LOPHIIDAE | ||||||||||||
| Lophiodes monodi Le Danois, 1971 | Club-bait Goosefish | Yes | GC | Yes | ||||||||
| LUTJANIDAE | ||||||||||||
| Apsilus dentatus Guichenot, 1853 | Black Snapper | Yes | GC | Yes | ||||||||
| Etelis oculatus (Valenciennes, 1828) | Queen Snapper | Yes | WA | Yes | ||||||||
| Lutjanus analis (Cuvier, 1828) | Mutton Snapper | P | Yes | Yes | 7 | WA | ||||||
| Lutjanus apodus (Walbaum, 1792) | Schoolmaster | V | P | Yes | Yes | Yes | Yes | 7 | GC | |||
| Lutjanus buccanella (Cuvier, 1828) | Blackfin Snapper | V | P | Yes | 7 | WA | ||||||
| Lutjanus cyanopterus (Cuvier, 1828) | Cubera Snapper | P | Yes | 7 | WA | |||||||
| Lutjanus griseus (Linnaeus, 1758) | Gray Snapper | (P) | Yes | Yes | 7 | TA | ||||||
| Lutjanus jocu (Bloch & Schneider, 1801) | Dog Snapper | P | Yes | Yes | 7 | TA | ||||||
| Lutjanus mahogoni (Cuvier, 1828) | Mahogany Snapper | V | P | Yes | Yes | Yes | Yes | 7 | GC | |||
| Lutjanus purpureus (Poey, 1866) | Caribbean Red Snapper | Yes | TA | |||||||||
| Lutjanus synagris (Linnaeus, 1758) | Lane Snapper | P | Yes | Yes | Yes | 7 | TA | |||||
| Lutjanus vivanus (Cuvier, 1828) | Silk Snapper | Yes | TA | Yes | ||||||||
| Ocyurus chrysurus (Bloch, 1791) | Yellowtail Snapper | P | Yes | Yes | Yes | 7 | TA | |||||
| Pristipomoides sp.1 | V | WA? | ? | Yes | ||||||||
| MACROURIDAE | ||||||||||||
| Gadomus arcuatus (Goode & Bean, 1886) | Doublethread Grenadier | Yes | TA | Yes | ||||||||
| Gadomus dispar (Vaillant, 1888) | Onelong Grenadier | Yes | TA | Yes | ||||||||
| Hymenocephalus aterrimus Gilbert, 1905 | Nobeard Grenadier | Yes | WA/PAC | Yes | ||||||||
| Hymenocephalus billsam Marshall & Iwamoto, 1973 | Bigeye Grenadier | Yes | WA | Yes | ||||||||
| Malacocephalus laevis (Lowe, 1843) | Velvet Grenadier | Yes | PAN | Yes | ||||||||
| Nezumia aequalis (Günther, 1878) | Atlantic Blacktip Grenadier | Yes | TA | Yes | ||||||||
| Ventrifossa macropogon Marshall, 1973 | Longbeard Grenadier | Yes | WA/WPAC | Yes | ||||||||
| MALACANTHIDAE | ||||||||||||
| Malacanthus plumieri (Bloch, 1786) | Sand Tilefish | V | P | Yes | Yes | Yes | 7 | WA | ||||
| MEGALOPIDAE | ||||||||||||
| Megalops atlanticus Valenciennes, 1847 | Tarpon | P | Yes | 8 | TA | |||||||
| MERLUCCIIDAE | ||||||||||||
| Steindachneria argentea Goode & Bean, 1896 | Luminous Hake | Yes | GC | Yes | ||||||||
| MONACANTHIDAE | ||||||||||||
| Aluterus scriptus (Osbeck, 1765) | Scrawled Filefish | P | Yes | Yes | 8 | PAN | ||||||
| Cantherhines macrocerus (Hollard, 1853) | Whitespotted Filefish | P | Yes | Yes | Yes | Yes | 8 | WA | ||||
| Cantherhines pullus (Ranzani, 1842) | Orangespotted Filefish | P | Yes | Yes | Yes | 8 | TA | |||||
| Monacanthus ciliatus (Mitchill, 1818) | Fringed Filefish | P | Yes | Yes | 8 | TA | ||||||
| Monacanthus tuckeri Bean, 1906 | Slender Filefish | P | Yes | Yes | 8 | GC | ||||||
| Stephanolepis setifer (Bennett, 1831) | Pygmy Filefish | P | Yes | Yes | 8 | WA | ||||||
| MUGILIDAE | ||||||||||||
| Mugil curema Valenciennes, 1836 | White Mullet | Yes | TA | |||||||||
| MULLIDAE | ||||||||||||
| Mulloidichthys martinicus (Cuvier, 1829) | Yellow Goatfish | V | P | Yes | Yes | Yes | 8 | TA | ||||
| Pseudupeneus maculatus (Bloch, 1793) | Spotted Goatfish | V | P | Yes | Yes | Yes | 8 | WA | ||||
| MURAENIDAE | ||||||||||||
| Echidna catenata (Bloch, 1795) | Chain Moray | P | Yes | 8 | WA | |||||||
| Enchelycore carychroa Bohlke & Bohlke, 1976 | Chestnut Moray | Estapé | (P) | 8 | TA | |||||||
| Enchelycore nigricans (Bonnaterre, 1788) | Viper Moray | Estapé | (P) | 8 | TA | |||||||
| Gymnothorax funebris Ranzani, 1839 | Green Moray | P | Yes | Yes | 8 | TA | ||||||
| Gymnothorax miliaris (Kaup, 1856) | Goldentail Moray | P | Yes | Yes | 8 | TA | ||||||
| Gymnothorax moringa (Cuvier, 1829) | Spotted Moray | P | Yes | Yes | Yes | 8 | TA | |||||
| Gymnothorax vicinus (Castelnau, 1855) | Purplemouth Moray | (P) | Yes | 8 | TA | |||||||
| NARCINIDAE | ||||||||||||
| Narcine bancroftii (Griffith & Smith, 1834) | Lesser Electric Ray | Yes | GC | |||||||||
| OGCOCEPHALIDAE | ||||||||||||
| Dibranchus atlanticus Peters, 1876 | Atlantic Batfish | Yes | TA | Yes | ||||||||
| Ogcocephalus corniger Bradbury, 1980 | Longnose Batfish | DROP | CP | 8 | GC | |||||||
| Zalieutes mcgintyi (Fowler, 1952) | Tricorn Batfish | DROP | CP | 8 | GC | Yes | ||||||
| OPHICHTHIDAE | ||||||||||||
| Myrichthys breviceps (Richardson, 1848) | Sharptail Eel | Yes | WA | |||||||||
| Myrichthys ocellatus (Lesueur, 1825) | Goldspotted Eel | Estapé | P | 8 | WA | |||||||
| Ophichthus ophis (Linnaeus, 1758) | Spotted Snake Eel | Yes | WA | |||||||||
| OPHIDIIDAE | ||||||||||||
| Brotula barbata (Bloch & Schneider, 1801) | Atlantic Bearded Brotula | DROP | CP | DROP | 8 | TA | ||||||
| Neobythites elongatus Nielsen & Retzler, 1994 | Elongate Cusk-eel | Yes | GC | Yes | ||||||||
| Parophidion schmidti (Woods & Kanazawa, 1951) | Dusky Cusk-eel | Estapé | P | 8 | GC | |||||||
| OPISTOGNATHIDAE | ||||||||||||
| Opistognathus aurifrons (Jordan & Thompson, 1905) | Yellowhead Jawfish | P | Yes | Yes | Yes | 8 | WA | |||||
| Opistognathus macrognathus Poey, 1860 | Banded Jawfish | Yes | GC | |||||||||
| Opistognathus maxillosus Poey, 1860 | Mottled Jawfish | Estapé | P | 8 | GC | |||||||
| OSTRACIIDAE | ||||||||||||
| Acanthostracion polygonius Poey, 1876 | Honeycomb Cowfish | V | P | Yes | Yes | Yes | 8 | WA | ||||
| Acanthostracion quadricornis (Linnaeus, 1758) | Scrawled Cowfish | V | P | Yes | 9 | TA | ||||||
| Lactophrys bicaudalis (Linnaeus, 1758) | Spotted Trunkfish | P | Yes | Yes | 9 | TA | ||||||
| Lactophrys trigonus (Linnaeus, 1758) | Trunkfish | P | Yes | Yes | 9 | TA | ||||||
| Lactophrys triqueter (Linnaeus, 1758) | Smooth Trunkfish | P | Yes | Yes | Yes | 9 | WA | |||||
| PARALICHTHYIDAE | ||||||||||||
| Citharichthys cornutus (Gunther, 1880) | Horned Whiff | Yes | WA | Yes | ||||||||
| Gastropsetta frontalis Bean, 1895 | Shrimp Flounder | DROP | CP | DROP | 9 | GC | ||||||
| Syacium micrurum Ranzani, 1842 | Channel Flounder | P | Yes | 9 | WA | |||||||
| PARAZENIDAE | ||||||||||||
| Cyttopsis rosea (Lowe, 1843) | Red Dory | Yes | TA/IWP | Yes | ||||||||
| PEMPHERIDAE | ||||||||||||
| Pempheris schomburgkii Müller & Troschel, 1848 | Glassy Sweeper | P | Yes | 9 | WA | |||||||
| PENTANCHIDAE | ||||||||||||
| Apristurus canutus Springer & Heemstra, 1979 | Hoary Cat Shark | Yes | GC | Yes | ||||||||
| Galeus antillensis Springer, 1979 | Antilles Sawtail Catshark | Yes | GC | L | Yes | |||||||
| PERCOPHIDAE | ||||||||||||
| Bembrops ocellatus Thompson & Suttkus, 1998 | Ocellate Duckbill | Yes | GC | Yes | ||||||||
| Bembrops quadrisella Thompson & Suttkus, 1998 | Saddleback Duckbill | Yes | GC | Yes | ||||||||
| Chrionema squamentum (Ginsburg, 1955) | Scalychin Flathead | DROP | CP | DROP | 9 | GC | Yes | |||||
| PERISTEDIIDAE | ||||||||||||
| Peristedion truncatum (Gunther, 1880) | Black Armored Searobin | Yes | WA | Yes | ||||||||
| POLYMIXIIDAE | ||||||||||||
| Polymixia lowei Gunther, 1859 | Beardfish | Yes | WA | Yes | ||||||||
| POMACANTHIDAE | ||||||||||||
| Centropyge argi Woods & Kanazawa, 1951 | Cherubfish | V | P | Yes | 9 | GC | ||||||
| Holacanthus ciliaris (Linnaeus, 1758) | Queen Angelfish | V | P | Yes | Yes | Yes | 9 | WA | ||||
| Holacanthus tricolor (Bloch, 1795) | Rock Beauty | V | P | Yes | Yes | Yes | Yes | 9 | WA | |||
| Pomacanthus arcuatus (Linnaeus, 1758) | Gray Angelfish | Yes | Yes | Yes | WA | |||||||
| Pomacanthus paru (Bloch, 1787) | French Angelfish | V | P | Yes | Yes | Yes | Yes | 9 | WA | |||
| POMACENTRIDAE | ||||||||||||
| Abudefduf saxatilis (Linnaeus, 1758) | Sergeant Major | P | Yes | Yes | 9 | TA | ||||||
| Abudefduf taurus (Müller & Troschel, 1848) | Night Sergeant | P | Yes | 9 | TA | |||||||
| Chromis cf. enchrysura2 | DROP | CP | DROP | 13 | WA | Yes | ||||||
| Chromis cyanea (Poey, 1860) | Blue Chromis | V | P | Yes | Yes | Yes | 9 | GC | ||||
| Chromis insolata (Cuvier, 1830) | Sunshinefish | V | P | Yes | 9 | GC | ||||||
| Chromis multilineata (Guichenot, 1853) | Brown Chromis | V | P | Yes | Yes | Yes | 9 | TA | ||||
| Chromis scotti Emery, 1968 | Purple Reeffish | DROP | V | WA | ||||||||
| Microspathodon chrysurus (Cuvier, 1830) | Yellowtail Damselfish | P | Yes | Yes | Yes | Yes | 9 | WA | ||||
| Stegastes adustus (Troschel, 1865) | Dusky Damselfish | P | Yes | 9 | GC | |||||||
| Stegastes diencaeus (Jordan & Rutter, 1897) | Longfin Damselfish | P | Yes | 9 | GC | |||||||
| Stegastes leucostictus (Müller & Troschel, 1848) | Beaugregory | Yes | Yes | GC | ||||||||
| Stegastes partitus (Poey, 1868) | Bicolor Damselfish | V | P | Yes | Yes | Yes | 9 | GC | ||||
| Stegastes planifrons (Cuvier, 1830) | Threespot Damselfish | P | Yes | 9 | GC | |||||||
| Stegastes xanthurus (Poey, 1860) | Cocoa Damselfish | P | Yes | 9 | GC | |||||||
| PRIACANTHIDAE | ||||||||||||
| Heteropriacanthus cruentatus (Lacepède, 1801) | Glasseye Snapper | P | Yes | Yes | 9 | TA | ||||||
| Priacanthus arenatus Cuvier, 1829 | Bigeye | V | Yes | TA | ||||||||
| Pristigenys alta (Gill, 1862) | Short Bigeye | DROP | V | WA | ||||||||
| RHINCODONTIDAE | ||||||||||||
| Rhincodon typus Smith, 1828 | Whale Shark | Yes | PAN | |||||||||
| SCIAENIDAE | ||||||||||||
| Equetus lanceolatus (Linnaeus, 1758) | Jackknife-fish | V | P | Yes | Yes | 10 | WA | |||||
| Equetus punctatus (Bloch & Schneider, 1801) | Spotted Drum | P | Yes | Yes | 10 | WA | ||||||
| Pareques acuminatus (Bloch & Schneider, 1801) | High-hat | P | Yes | 10 | WA | |||||||
| Umbrina coroides Cuvier, 1830 | Sand Drum | Yes | WA | |||||||||
| SCOMBRIDAE | ||||||||||||
| Acanthocybium solandri (Cuvier, 1832) | Wahoo | Yes | PAN | |||||||||
| Euthynnus alletteratus (Rafinesque, 1810) | Little Tunny | P | Yes | 10 | TA | |||||||
| Katsuwonus pelamis (Linnaeus, 1758) | Skipjack Tuna | Yes | PAN | |||||||||
| Scomberomorus cavalla (Cuvier, 1829) | King Mackerel | P | Yes | 10 | WA | |||||||
| Scomberomorus regalis (Bloch, 1793) | Cero | V | P | Yes | Yes | 10 | WA | |||||
| Thunnus atlanticus (Lesson, 1831) | Blackfin Tuna | Yes | WA | |||||||||
| SCORPAENIDAE | ||||||||||||
| Pontinus castor Poey, 1860 | Longsnout Scorpionfish | DROP | CP | DROP | 10 | GC | Yes | |||||
| Pontinus nematophthalmus (Gunther, 1860) | Spinythroat Scorpionfish | DROP | CP | 10 | WA | Yes | ||||||
| Pterois volitans (Linnaeus, 1758) | Red Lionfish | V | P | Yes | Yes | 10 | NA | NA | NA | |||
| Scorpaena plumieri Bloch, 1789 | Spotted Scorpionfish | P | Yes | Yes | 10 | TA | ||||||
| Scorpaenodes caribbaeus Meek & Hildebrand, 1928 | Reef Scorpionfish | Estapé | P | 10 | WA | |||||||
| SERRANIDAE | ||||||||||||
| Alphestes afer (Bloch, 1793) | Mutton Hamlet | (P) | Yes | 10 | TA | |||||||
| Baldwinella vivanus (Jordan & Swain, 1885)3 | Red Barbier | DROP | V | WA | Yes | |||||||
| Bathyanthias species A | DROP | CP | 13 | GC | L | Yes | ||||||
| Bullisichthys caribbaeus Rivas, 1971 | Pugnose Bass | DROP | CP | DROP | 10 | GC | Yes | |||||
| Cephalopholis cruentata (Lacepede, 1802) | Graysby | V | P | Yes | Yes | Yes | Yes | 10 | GC | |||
| Cephalopholis fulva (Linnaeus, 1758) | Coney | V | P | Yes | Yes | Yes | Yes | 10 | WA | |||
| Diplectrum bivittatum (Valenciennes, 1828) | Dwarf Sand Perch | Estapé | P | 10 | WA | |||||||
| Epinephelus adscensionis (Osbeck, 1765) | Rock Hind | V | (P) | Yes | 10 | TA | ||||||
| Epinephelus guttatus (Linnaeus, 1758) | Red Hind | V | P | Yes | Yes | Yes | Yes | 10 | WA | |||
| Epinephelus striatus (Bloch, 1792) | Nassau Grouper | Yes | GC | |||||||||
| Gonioplectrus hispanus (Cuvier, 1828) | Spanish Flag | DROP | V | WA | Yes | |||||||
| Hypoplectrus chlorurus (Cuvier, 1828) | Yellowtail Hamlet | P | Yes | Yes | 10 | GC | ||||||
| Hypoplectrus guttavarius (Poey, 1852) | Shy Hamlet | P | Yes | 10 | GC | |||||||
| Hypoplectrus indigo (Poey, 1851) | Indigo Hamlet | DROP | V | GC | ||||||||
| Hypoplectrus nigricans (Poey, 1852) | Black Hamlet | Yes | GC | |||||||||
| Hypoplectrus puella (Cuvier, 1828) | Barred Hamlet | V | P | Yes | Yes | Yes | 10 | GC | ||||
| Hypoplectrus species 1 | Bluelip Hamlet | Estapé | P | 13 | GC | |||||||
| Hypoplectrus unicolor (Walbaum, 1792) | Butter Hamlet | Yes | GC | |||||||||
| Liopropoma carmabi (Randall, 1963) | Candy Basslet | DROP | CP | DROP | 10 | WA | ||||||
| Liopropoma mowbrayi Woods & Kanazawa, 1951 | Cave Basslet | DROP | CP | DROP | 10 | GC | ||||||
| Liopropoma olneyi Baldwin & Johnson, 2014 | Yellow-Spotted Basslet | DROP | CP | DROP | 10 | GC | L | Yes | ||||
| Liopropoma rubre Poey, 1861 | Peppermint Basslet | P | Yes | 11 | GC | |||||||
| Mycteroperca interstitialis (Poey, 1860) | Yellowmouth Grouper | Yes | WA | |||||||||
| Mycteroperca tigris (Valenciennes, 1833) | Tiger Grouper | Yes | WA | |||||||||
| Mycteroperca venenosa (Linnaeus, 1758) | Yellowfin Grouper | P | Yes | Yes | 11 | WA | ||||||
| Paranthias furcifer (Valenciennes, 1828) | Atlantic Creolefish | V | P | Yes | Yes | 11 | TA | |||||
| Plectranthias species A | DROP | CP | 13 | GC | L | Yes | ||||||
| Pronotogrammus martinicensis (Guichenot, 1868) | Roughtongue Bass | DROP | CP | DROP | 11 | WA | Yes | |||||
| Rypticus bistrispinus (Mitchill, 1818) | Freckled Soapfish | Yes | WA | |||||||||
| Rypticus saponaceus (Bloch & Schneider, 1801) | Greater Soapfish | V | P | Yes | Yes | Yes | 11 | TA | ||||
| Serranus annularis (Gunther, 1880) | Orangeback Bass | DROP | V | WA | ||||||||
| Serranus baldwini (Evermann & Marsh, 1899) | Lantern Bass | P | Yes | Yes | Yes | 11 | WA | |||||
| Serranus flaviventris (Cuvier, 1829) | Twinspot Bass | Yes | WA | |||||||||
| Serranus fuscula (Poey, 1861) | Twospot Sea Bass | DROP | CP | DROP | 11 | WA | Yes | |||||
| Serranus luciopercanus Poey, 1852 | Crosshatch Bass | DROP | V | GC | Yes | |||||||
| Serranus notospilus Longley, 1935 | Saddle Bass | DROP | V | GC | ||||||||
| Serranus phoebe Poey, 1851 | Tattler | V | Yes | WA | Yes | |||||||
| Serranus tabacarius (Cuvier, 1829) | Tobaccofish | V | P | Yes | Yes | Yes | 11 | WA | ||||
| Serranus tigrinus (Bloch, 1790) | Harlequin Bass | V | P | Yes | Yes | Yes | 11 | GC | ||||
| Serranus tortugarum Longley, 1935 | Chalk Bass | V | P | Yes | Yes | 11 | GC | |||||
| SETARCHIDAE | ||||||||||||
| Setarches guentheri Johnson, 1862 | Deepwater Scorpionfish | Yes | TA/IWP | Yes | ||||||||
| SPARIDAE | ||||||||||||
| Calamus bajonado (Bloch & Schneider, 1801) | Jolthead Porgy | Yes | Yes | WA | ||||||||
| Calamus calamus (Valenciennes, 1830) | Saucereye Porgy | P | Yes | Yes | 11 | WA | ||||||
| Calamus pennatula Guichenot, 1868 | Pluma Porgy | P | Yes | 11 | WA | |||||||
| SPHYRAENIDAE | ||||||||||||
| Sphyraena barracuda (Edwards, 1771) | Great Barracuda | V | P | Yes | Yes | Yes | Yes | 11 | PAN | |||
| Sphyraena borealis DeKay, 1842 | Sennet | P | Yes | 11 | WA | |||||||
| SPHYRNIDAE | ||||||||||||
| Sphyrna mokarran (Rüppell, 1837) | Great Hammerhead | Yes | PAN | |||||||||
| SQUALIDAE | ||||||||||||
| Squalus clarkae Pfleger, Grubbs, Cotton & Daly-Engel, 2018 | Gulf Dogfish | Yes | GC | Yes | ||||||||
| SYMPHYSANODONTIDAE | ||||||||||||
| Symphysanodon berryi Anderson, 1970 | Slope Bass | DROP | CP | DROP | 11 | TA | Yes | |||||
| Symphysanodon octoactinus Anderson, 1970 | Insular Bunquelovely | DROP | CP | DROP | 11 | GC | Yes | |||||
| SYNGNATHIDAE | ||||||||||||
| Amphelikturus dendriticus (Barbour, 1905) | Seahorse Pipefish | Estapé | P | 11 | WA | |||||||
| Bryx dunckeri (Metzelaar, 1919) | Pugnose Pipefish | Yes | WA | |||||||||
| Cosmocampus albirostris (Kaup, 1856) | Whitenose Pipefish | Yes | WA | |||||||||
| Halicampus crinitus (Jenyns, 1842) | Banded Pipefish | Estapé | V | WA | ||||||||
| Hippocampus erectus Perry, 1810 | Lined Seahorse | P | Yes | 11 | WA | |||||||
| Hippocampus reidi Ginsburg, 1933 | Longsnout Seahorse | P | Yes | 11 | GC | |||||||
| SYNODONTIDAE | ||||||||||||
| Synodus foetens (Linnaeus, 1766) | Inshore Lizardfish | Yes | NWA | |||||||||
| Synodus intermedius (Agassiz, 1829) | Sand Diver | P | Yes | Yes | Yes | 11 | WA | |||||
| Synodus synodus (Linnaeus, 1758) | Red Lizardfish | P | Yes | 11 | TA | |||||||
| Trachinocephalus myops (Forster, 1801) | Snakefish | P | Yes | 11 | TA | |||||||
| TETRAODONTIDAE | ||||||||||||
| Canthigaster jamestyleri Moura & Castro, 2002 | Goldface Toby | DROP | CP | DROP | 11 | GC | ||||||
| Canthigaster rostrata (Bloch, 1786) | Sharpnose Puffer | V | P | Yes | Yes | Yes | 11 | GC | ||||
| Sphoeroides dorsalis Longley, 1934 | Marbled Puffer | DROP/ Estapé | CP | P | DROP | 12 | GC | |||||
| Sphoeroides nephelus (Goode & Bean, 1882) | Southern Puffer | Yes | GC | |||||||||
| Sphoeroides spengleri (Bloch, 1785) | Bandtail Puffer | P | Yes | Yes | 12 | WA | ||||||
| TRACHICHTHYIDAE | ||||||||||||
| Hoplostethus occidentalis Woods, 1973 | Western Roughy | Yes | WA | Yes | ||||||||
| TRIACANTHODIDAE | ||||||||||||
| Hollardia hollardi Poey, 1861 | Reticulate Spikefish | Yes | GC | Yes | ||||||||
| TRIGLIDAE | ||||||||||||
| Bellator egretta (Goode & Bean, 1896) | Streamer Searobin | DROP | CP | 12 | GC | Yes | ||||||
| TRIPTERYGIIDAE | ||||||||||||
| Enneanectes altivelis Rosenblatt, 1960 | Lofty Triplefin | Estapé | P | 12 | GC | |||||||
| Enneanectes boehlkei Rosenblatt, 1960 | Roughhead Triplefin | Estapé | P | 12 | GC | |||||||
| Enneanectes jordani (Evermann & Marsh, 1899) | Mimic Triplefin | p | Yes | 12 | GC | |||||||
| Enneanectes matador Victor, 2013 | Matador Triplefin | Estapé | p | 12 | GC | |||||||
Photographic plates
The 13 photographic plates (Suppl. materials
Structure of the Statia20 reef-associated bony fish fauna
Global geographical ranges
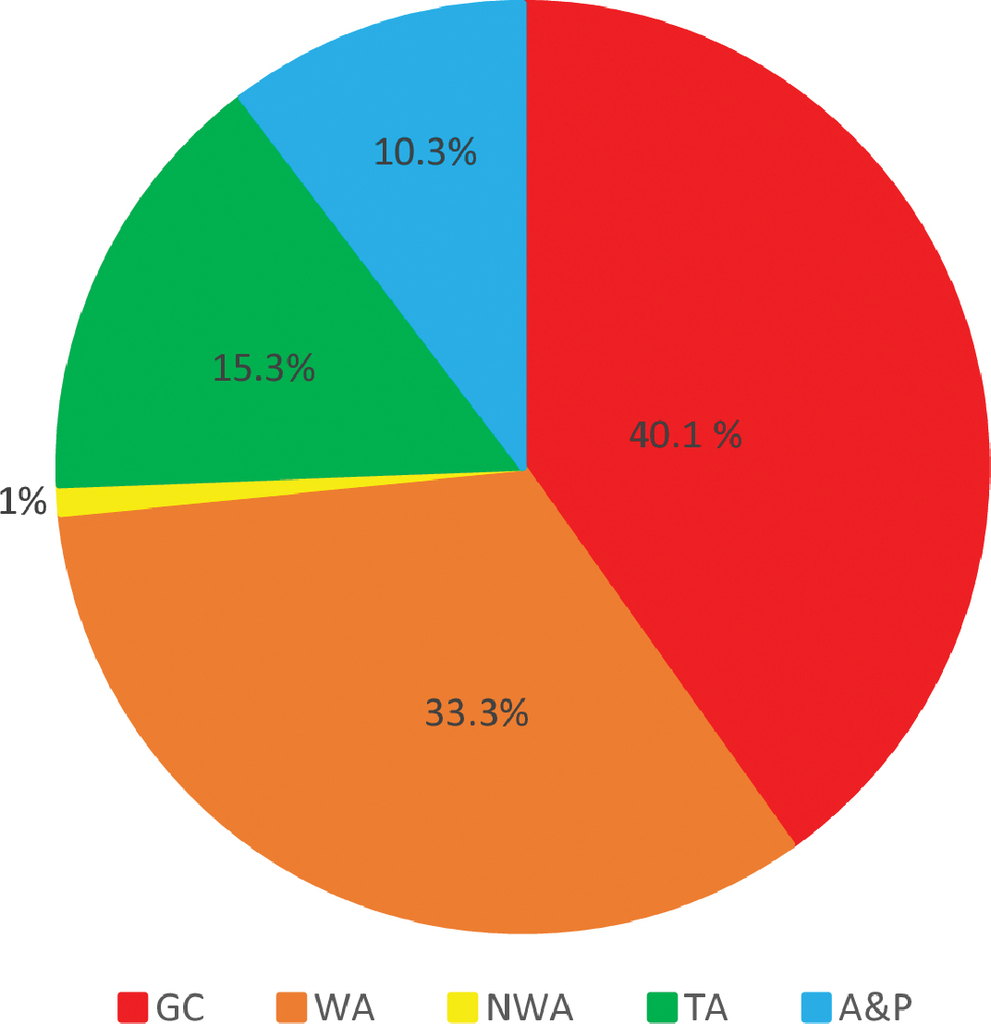
Greater Caribbean endemics represent the largest group of species in the Statia fauna, and, together with more widely ranging western Atlantic endemics, constitute almost three quarters of the species. Trans-Atlantic species and species found outside as well as inside the Atlantic represented only a quarter of the fauna (Table
Percentages of the Sint Eustatius marine fish fauna represented by groups of species with different global geographical ranges. GC = Greater Caribbean endemics; NWA = GC plus temperate eastern USA; WA = GC plus Brazil; TA = WA plus central or East Atlantic; and A&P = species found in both the Atlantic and various parts of the Indo-Pacific.
Extent of geographical ranges within the Greater Caribbean
The vast majority of species are widely distributed within the Greater Caribbean, with only nine (2.25%) of them having ranges limited to a restricted part of the Caribbean. Among those nine, five are deep-living species, and five belong to Core CRF families (Table
Ecology – Depth
The number of deep species increased from 44 on the Statia17 list to 86 in the Statia20 fauna (Table
Characteristics of assemblages of reef fishes at different locations in the Greater Caribbean region. Percentages of ecotypes in the entire regional fauna, the entire faunas from each local area, and within each of two depth subgroups refer to number of species as a % of the entire fauna and of each depth subgroup. Assemblages include those at Statia in 2017 and 2020 (Statia17 and Statia20), in the Saba EEZ in 2017 (Saba17), of species in the Saba17 fauna that are not currently known to occur at Statia (Saba17-Statia20), of the Saba EEZ in 2020 (Statia20 + Saba17), and of Alligator reef in 2020 (Alligator20). Small species are those with ≤ 10 cm maximum total length. Percentage values for individual sites that are greater than the regional value are shown in red, those below the regional value are in blue.
| Region | Statia20 | Statia17 | Saba17 | Saba20 | Alligator20 | Saba17 – Statia20 | |
| ALL SPECIES (n) | 903 | 306 | 220 | 377 | 427 | 427 | 121 |
| Demersal species% | 35.0 | 55.1 | 66.8 | 47.5 | 46.1 | 49.4 | 19.0 |
| Benthic species% | 65.0 | 43.1 | 33.2 | 52.5 | 53.9 | 50.6 | 81.0 |
| Cryptobenthic species% | 59.2 | 40.8 | 30.9 | 49.1 | 50.1 | 46.4 | 73.6 |
| Small cryptobenthic species% | 41.6 | 24.8 | 15.5 | 30.2 | 31.9 | 24.8 | 49.6 |
| Core CRF species% | 45.8 | 28.8 | 20.5 | 33.4 | 35.1 | 27.6 | 48.8 |
| SHALLOW SPECIES% | 85.1 | 87.3 | 97.3 | 93.4 | 88.0 | 95.3 | 90.1 |
| Non-cryptic species% | 40.8 | 59.6 | 68.1 | 50.3 | 47.6 | 58.6 | 23.9 |
| Cryptobenthic species% | 59.2 | 40.4 | 31.3 | 49.7 | 52.4 | 41.1 | 76.1 |
| Small cryptobenthic species% | 41.3 | 23.2 | 15.4 | 30.7 | 31.9 | 25.8 | 53.2 |
| Core CRF species% | 46.2 | 28.5 | 21.0 | 34.1 | 35.4 | 29.0 | 52.3 |
| DEEP SPECIES% | 14.9 | 12.7 | 2.7 | 6.6 | 12.0 | 4.7 | 9.9 |
| Non-cryptic species% | 40.3 | 56.5 | 83.3 | 60.0 | 54.9 | 75 | 50.0 |
| Cryptobenthic species% | 59.7 | 43.5 | 16.7 | 40.0 | 45.1 | 25 | 50.0 |
| Small cryptobenthic species% | 43.3 | 35.6 | 16.7 | 24.0 | 31.4 | 5 | 16.7 |
| Core CRF species% | 44.0 | 38.5 | 0 | 24.0 | 33.3 | 0 | 16.7 |
Ecology – Reef-associated bony fishes
The Statia20 fauna of such species is 38.3% larger than the Statia17 fauna, with numbers of shallow species increasing by 24.8% (from 214 to 267) and of deep species increasing 6.2-fold (from 6 to 39). This led to substantial increases in the relative abundance of deep-reef species, and of benthic, cryptobenthic, small cryptobenthic and core CRFs on both shallow and deep reefs. The Saba17 fauna was 71% larger than that of Statia17, with greater percentages of deep-reef, benthic, cryptobenthic, small cryptobenthic and Core CRFs. The Saba17 fauna was 23% larger than the Statia20 fauna and had a greater proportion of shallow species and fewer deep species, and higher proportions of shallow members of cryptobenthic, small cryptobenthic and Core CRF groups. Thirty-two percent of the Saba17 species were not in the Statia20 fauna. Those 121 species comprised mainly shallow cryptobenthic types, including small-cryptobenthic and Core-CRF species. When those are combined with the Statia20 fauna the resultant Saba20 fauna has substantial increases in the proportions of shallow cryptobenthic, small cryptobenthic and core CRF species compared to the Statia20 fauna. Relative to the regional fauna, however, the faunas of Statia17, Statia20, Saba17, and Saba20 all had deficits of deep species of all types and of shallow cryptobenthic species, including small- and Core-CRF species. The Alligator20 fauna of reef-associated species is the same size as the Saba20 fauna. It has the same characteristics as the Statia17 and Saba17 faunas: a large deficit of deep-reef fishes and deficits of shallow cryptobenthic species, including small- and Core-CRF species. Although there has been some collecting at Alligator reef of shallow cryptobenthic species there has been no submersible-based collecting there.
Discussion
The efforts of
Zoogeographically the two largest groups of species in the Statia20 fauna are Greater Caribbean endemics and western Atlantic endemics, and the smallest group is of species found in the Indo-Pacific as well as the Atlantic. This mixture is fairly representative of the Greater Caribbean fish fauna as a whole (
Most species recorded in the Statia17 fauna are readily visible reef fishes, demersal and non-cryptic benthic species commonly found on wider Caribbean reefs, and the proportions of cryptobenthic (particularly small ones) and deep-reef species were relatively low.
Rotenone is an ichthyocide commonly used in small quantities by researchers to extract cryptobenthic fishes hiding within reef structures or buried in soft bottoms, and is an important tool for elucidating the contribution of such species to reef-fish faunal assessments (
The DROP submersible-based sampling is the only organized research on deep-reef fishes conducted to date at Statia or in the Saba EEZ. It produced more than half the new records in the Statia 2020 fauna, including records of eight recently discovered species that currently lack scientific names. It dramatically increased the numerical and proportional abundance of deep-reef species in the general fauna and in the reef-associated component. A lack of such research at Saba bank and Alligator Reef accounts for the very low abundance of deep-reef fishes at those sites.
The proportional abundances of shallow cryptobenthic species, including small species and core CRFs, are also distinctly lower in the Statia20 fauna than the regional fauna. Even if all 121 reef-associated species in the Saba EEZ that are not known from Statia are assumed to be at Statia those proportions still remain below the regional levels. Some of that difference is probably due to sampling artifacts. However, the proportional abundances of those ecotypes in a local fauna like that of Statia, or Alligator Reef, may always be lower than the regional level. In the Greater Caribbean small cryptobenthic species, particularly Core-CRF species such as blennioids and gobies, often have small geographical ranges (see above), which are scattered in different parts of the region (see
Conclusions
The research reported in the present study substantially increased our knowledge of the size of the marine fish fauna of Statia and resulted in the discovery of a significant number of undescribed deep-reef species. Although that island fauna is now one of the best documented in the Greater Caribbean there is still much to do to provide a thorough assessment of its diversity. Collecting with ichthyocide (or anesthetics) is essential for effective sampling of the fauna of small, shallow cryptobenthic reef fishes present there, and sampling of both deep and shallow reef fishes needs to be more effectively distributed across the range of habitats present at the island. No single site in the Caribbean Sea has been subject to sufficiently thorough sampling to provide a clear understanding of the size of its entire marine fish fauna, the size of its reef-associated fish fauna, or even the size of its shallow, reef-associated fauna, let alone its deep-reef fish fauna.
Permits
Collecting by DROP was performed under Saba/Statia BES Permit No. 120317 to the Foundation Curacao Deep Reef Research Centre.
Animal-Care Permission
DROP collecting was approved by a Smithsonian Institution Animal Care and Use Committee, approval No. 2014-13 to CCB.
Acknowledgements
CJE and AME: We thank Mike Harterink, Marieke van de Wetering, Menno and Ingrid Walther, and the crew of the Scubaqua Dive Center; St. Eustatius National Parks Foundation (STENAPA); Sybolt and Marlise ten Hoopen, The Old Gin House Hotel; and Robert and Marilyn Bentley, Mike Harterink, and Marit Pistor (STENAPA) for photographs they provided of various species of fishes (marit.pistor@statiapark.org; mike@scubaqua.com; bentley.robertn@gmail.com).
LT and CCB: We thank Thomas Devine for organizing fieldwork and assisting with collections. Jenna Barrett helped transcribe data from submersible videos and generate maps of Statia, and Jordan Casey assisted with collections in the field. Barry B. Brown photographed many of the specimens collected by DROP that were brought to the surface alive.The DROP expedition was made possible through the help of Adriaan ‘Dutch’ Schrier, Bruce Brandt, Barbara van Bebber, and the rest of the staff of Substation Curacao and the crew of the R/V Chapman. Ocean Heritage Foundation/Curacao Sea Aquarium/Substation Curacao contribution number OHF/CSA/SC#48
References
- Ackerman JL, Bellwood DR (2000) Reef fish assemblages: a re-evaluation using enclosed rotenone stations. Marine Ecology Progress Series 206: 227–237. https://doi.org/10.3354/meps206227
- Alzate A, Zapata FA, Girald A (2014) A comparison of visual and collection-based methods for assessing community structure of coral reef fishes in the Tropical Eastern Pacific. Revista de Biología Tropical 62: 359–371. https://doi.org/10.15517/rbt.v62i0.16361
- Baldwin CC, Tornabene L, Robertson DR (2018) Below the mesophotic. Scientific Reports 8(4920): 1–13. https://doi.org/10.1038/s41598-018-23067-1
- Brandl SJ, Goatley CHR, Bellwood DR, Tornabene L (2018) The hidden half: ecology and evolution of cryptobenthic fishes on coral reefs. Biological Reviews 93: 1846–1873. https://doi.org/10.1111/brv.12423
- Brandl SJ, Tornabene L, Goatley CHR, Casey JM, Morais RA, Côté IM, Baldwin CC, Parravicini V, Schiettekatte MD, Bellwood DR (2019) Demographic dynamics of the smallest marine vertebrates fuel coral reef ecosystem functioning. Science 364: 1189–1192. https://doi.org/10.1126/science.aav3384
- da Silva R, Pedraza-Marrón CR, Sampaio I, Betancur-R R, Gomes G, Schneider H (2020) New insights about species delimitation in red snappers (Lutjanus purpureus and L. campechanus) using multilocus data. Molecular Phylogenetics and Evolution 147: e106780. https://doi.org/10.1016/j.ympev.2020.106780
- Davies M, Piontek S (2016) Marine fishes of St. Eustatius. In: Hoeksem BW (Ed.) Marine biodiversity survey of St. Eustatius, Dutch Caribbean, 2015. Naturalis Biodiversity Center, Leiden, and ANEMOON Foundation, Bennebroek, 73–82. https://www.persistent-identifier.nl/urn:nbn:nl:ui:19-616970
- Davies M, Piontek S (2017) Marine fishes of St. Eustatius, Dutch Caribbean. Marine Biodiversity 47: 27–35. https://doi.org/10.1007/s12526-016-0575-1
- Ehemann NR, González-González LDV, Tagliafico A, Weigmann S (2019) Updated taxonomic list and conservation status of chondrichthyans from the exclusive economic zone of Venezuela, with first generic and specific records. Journal of Fish Biology 95: 753–771. https://doi.org/10.1111/jfb.14061
- Estapé CJ, Morgan Estapé A, Starck WA (2020) The fishes of Alligator Reef and environs in the Florida Keys: a 2020 update. Journal of the Ocean Science Foundation 36: 16–19. https://doi.org/10.5281/zenodo.4243097
- Hoeksema B [Ed.] (2016) Marine biodiversity survey of St. Eustatius, Dutch Caribbean, 2015. Naturalis Biodiversity Center, Leiden, and ANEMOON Foundation, Bennebroek, 157 pp. https://www.persistent-identifier.nl/urn:nbn:nl:ui:19-616970
- Hoetjes PC, Carpenter KE (2010) Saving Saba Bank: Policy Implications of Biodiversity Studies. PLoS ONE 5: e10769. https://doi.org/10.1371/journal.pone.0010769
- Metzelaar J (1919) Report on the fishes, collected by Dr J Boeke in the Dutch West Indies 1904–1905, with comparative notes on marine fishes of tropical West Africa. F.J. Belanfante, ‘s-Gravenhage, 314 pp.
- Møller PR, Knudsen W, Schwarzhans W, Nielsen JG (2016) A new classification of viviparous brotulas (Bythitidae) – with family status for Dinematichthyidae – based on molecular, morphological and fossil data. Molecular Phylogenetics and Evolution 100: 391–408. https://doi.org/10.1016/j.ympev.2016.04.008
- Robertson DR, Cramer KL (2014) Defining and Dividing the Greater Caribbean: Insights from the biogeography of shorefishes. PLoS ONE 9: 1–16. https://doi.org/10.1371/journal.pone.0102918
- Robertson DR, Smith-Vaniz WF (2008) Rotenone: An Essential but Demonized Tool for Assessing Marine Fish Diversity. BioScience 58: 165–170. https://doi.org/10.1641/B580211
- Robertson DR, Tornabene L (2020) Reef-associated bony fishes of the Greater Caribbean: a checklist (Version 3). Zenodo. https://doi.org/10.5281/zenodo.4279301
- Robertson DR, Van Tassell J (2019) Shorefishes of the Greater Caribbean: online information system. Version 2.0. Smithsonian Tropical Research Institute, Balboa, Panama. https://biogeodb.stri.si.edu/caribbean/en/pages
- Smith-Vaniz WF, Jelks HL (2014) Marine and inland fishes of St. Croix, U. S. Virgin Islands: an annotated checklist. Zootaxa 3903: 1–120. https://doi.org/10.11646/zootaxa.3803.1.1
- Smith-Vaniz WF, Jelks HL, Rocha LA (2006) Relevance of cryptic fishes in biodiversity assessments: A case study at Buck Island National Monument, St. Croix. Bulletin of Marine Science 79: 17–48.
- Starck WA, Estapé CJ, Morgan Estapé A (2017) The fishes of Alligator Reef and environs in the Florida Keys: a half-century update. Journal of the Ocean Science Foundation 27: 74–117. https://doi.org/10.5281/zenodo.851651
- van Kuijk T, de Graaf M, Nagelkerken L, Boman E, Debrot AO (2015) Baseline assessment of the coral reef fish assemblages of St. Eustatius. Technical Report C058/15. IMARES, Wageningen, 49 pp.
- Victor BC (2017) The status of Enneanectes jordani and a new species of triplefin blenny from the Greater Caribbean (Teleostei: Tripterygiidae). Journal of the Ocean Science Foundation 27: 48–73.
- Wilcox CL, Motomura H, Matsunuma M, Bowen BW (2018) Phylogeography of Lionfishes (Pterois) Indicate Taxonomic Over Splitting and Hybrid Origin of the Invasive Pterois volitans. Journal of Heredity 109: 162–175. https://doi.org/10.1093/jhered/esx056
- Williams JT, Carpenter KE, Van Tassell JL, Hoetjes P, Toller W (2010) Biodiversity Assessment of the Fishes of Saba Bank Atoll, Netherlands Antilles. PLoS ONE 5: e10676. https://doi.org/10.1371/journal.pone.0010676
Supplementary materials
Figure S1
Data type: Map of EEZ
Explanation note: Map of Saba EEZ.
Table S1
Data type: Dive site list
Explanation note: List of dive sites with dates and georeferenced coordinates.
Table S2
Data type: Occurences
Explanation note: Fish species occurrences at Saba and Statia.
Plate S1
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S2
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S3
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S4
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S5
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S6
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S7
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S8
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S9
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S10
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S11
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S12
Data type: Photographs
Explanation note: Voucher photographs of fishes.
Plate S13
Data type: Photographs
Explanation note: Voucher photographs of fishes.