Research Article |
|
Corresponding author: Wayne P. Maddison ( wmaddisn@mail.ubc.ca ) Academic editor: Jeremy Miller
© 2020 Wayne P. Maddison, Imara Beattie, Kiran Marathe, Paul Y. C. Ng, Nilani Kanesharatnam, Suresh P. Benjamin, Krushnamegh Kunte.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Maddison WP, Beattie I, Marathe K, Ng PYC, Kanesharatnam N, Benjamin SP, Kunte K (2020) A phylogenetic and taxonomic review of baviine jumping spiders (Araneae, Salticidae, Baviini). ZooKeys 1004: 27-97. https://doi.org/10.3897/zookeys.1004.57526
|
Abstract
The systematics and taxonomy of the tropical Asian jumping spiders of the tribe Baviini is reviewed, with a molecular phylogenetic study (UCE sequence capture, traditional Sanger sequencing) guiding a reclassification of the group’s genera. The well-studied members of the group are placed into six genera: Bavia Simon, 1877, Indopadilla Caleb & Sankaran, 2019, Padillothorax Simon, 1901, Piranthus Thorell, 1895, Stagetillus Simon, 1885, and one new genus, Maripanthus Maddison, gen. nov. The identity of Padillothorax is clarified, and Bavirecta Kanesharatnam & Benjamin, 2018 synonymized with it. Hyctiota Strand, 1911 is synonymized with Stagetillus. The molecular phylogeny divides the baviines into three clades, the Piranthus clade with a long embolus (Piranthus, Maripanthus), the genus Padillothorax with a flat body and short embolus, and the Bavia clade with a higher body and (usually) short embolus (remaining genera). In general, morphological synapomorphies support or extend the molecularly delimited groups. Eighteen new species are described: Bavia nessagyna, Indopadilla bamilin, I. kodagura, I. nesinor, I. redunca, I. redynis, I. sabivia, I. vimedaba, Maripanthus draconis (type species of Maripanthus), M. jubatus, M. reinholdae, Padillothorax badut, P. mulu, Piranthus api, P. bakau, P. kohi, P. mandai, and Stagetillus irri, all sp. nov., with taxonomic authority W. Maddison. The distinctions between baviines and the astioid Nungia Żabka, 1985 are reviewed, leading to four species being moved into Nungia from Bavia and other genera. Fifteen new combinations are established: Bavia maurerae (Freudenschuss & Seiter, 2016), Indopadilla annamita (Simon, 1903), I. kahariana (Prószyński & Deeleman-Reinhold, 2013), I. sonsorol (Berry, Beatty & Prószyński, 1997), I. suhartoi (Prószyński & Deeleman-Reinhold, 2013), Maripanthus menghaiensis (Cao & Li, 2016), M. smedleyi (Reimoser, 1929), Nungia hatamensis (Thorell, 1881), N. modesta (Keyserling, 1883), N. papakula (Strand, 1911), N. xiaolonghaensis (Cao & Li, 2016), Padillothorax casteti (Simon, 1900), P. exilis (Cao & Li, 2016), P. flavopunctus (Kanesharatnam & Benjamin, 2018), Stagetillus banda (Strand, 1911), all comb. nov. One combination is restored, Bavia capistrata (C. L. Koch, 1846). Five of these new or restored combinations correct previous errors of placing species in genera that have superficially similar palps but extremely different body forms, in fact belonging in distantly related tribes, emphasizing that the general shape of male palps should be used with caution in determining relationships. A little-studied genus, Padillothorus Prószyński, 2018, is tentatively assigned to the Baviini. Ligdus Thorell, 1895 is assigned to the Ballini.
Keywords
Classification, molecular phylogeny, new genus, new species, Salticidae, Salticoida
Introduction
Baviines are tropical Asian jumping spiders with elongate medium to large bodies, living above ground on leaves or branches of vegetation or in suspended litter (
Materials and methods
Material examined
Spider specimens examined for this study are stored in the University of British Columbia Spencer Entomological Collection, Canada (
Morphology
Preserved specimens were examined under both dissecting microscopes and a compound microscope with reflected light. Drawings were made with a drawing tube on a Nikon ME600L compound microscope. Most photographs of living specimens were made with either a Pentax Optio 33WR digital camera with a small lens glued to it for macro capability (2016 and earlier) or an Olympus OM-D E-M10 II camera with 60 mm macro lens (2017 and later). Microscope photographs were made either on a Nikon ME600L compound microscope or an Olympus SZX12 stereoscope and focus stacked using Helicon Focus 4.2.7.
All measurements are given in millimeters. Descriptions of color pattern are based on the alcohol-preserved specimen. Carapace length was measured from the base of the anterior median eyes not including the lenses to the rear margin of the carapace medially; abdomen length to the end of the anal tubercle. The following abbreviations are used:
ALE anterior lateral eyes;
ECP epigynal coupling pocket (also known as a hood or notch);
PLE posterior lateral eyes;
PME posterior median eyes (the “small eyes”);
RTA retrolateral tibial apophysis;
TmA terminal apophysis.
Molecular data
Molecular data was gathered for three gene regions by traditional Sanger PCR methods, and for many genes by Ultra-Conserved Element (UCE) target enrichment sequencing methods (
Specimens from which molecular data were used in phylogenetic analysis of baviines.
| Species | Specimen ID | Sex | Locality | Lat-Long |
|---|---|---|---|---|
| Amycoida | ||||
| Attulus floricola (C. L. Koch, 1837) | d545 | ♂ | Poland: Narew | 52.9, 23.5 |
| d030 | ♂ | Canada: Nova Scotia | 44.4318, -64.6075 | |
| Breda bicruciata (Mello-Leitão, 1943) | d471 | ♀ | Uruguay: Lavalleja | -34.426, -55.195 |
| Colonus hesperus (Richman & Vetter, 2004) | d472 | ♂ | U.S.A.: Arizona | 34.5847, -112.5707 |
| Astioida | ||||
| Helpis minitabunda (L. Koch, 1880) | NZ19-9152 | ♀ | New Zealand | -40.994, 172.994 |
| S194, S195 | New Zealand | |||
| Ligurra latidens (Doleschall, 1859) | AS19.3412 | ♂ | Singapore | 1.4438, 103.7334 |
| d175 | Singapore | |||
| Marpissoida | ||||
| Afromarengo sp. | MRB262 | Gabon | ||
| Phidippus johnsoni (Peckham & Peckham, 1883) | d549 | ♂ | Canada: Iona Beach | 49.222, -123.216 |
| Saltafresia | ||||
| Menemerus bivittatus (Dufour, 1831) | d559 | ♀ | Singapore | 1.4438, 103.7334 |
| S13/S225 | Ecuador | |||
| Salticus scenicus (Clerck, 1757) | NA19-2676 | ♀ | Canada: Iona Beach | 49.222, -123.216 |
| d003, S107 | U.S.A. | |||
| Baviines | ||||
| Bavia aericeps Simon, 1877 | 2008PNG-2407 | ♂ | Papua New Guinea | -5.231, 142.532 |
| Bavia cf. intermedia (Karsch, 1880) | d079 | ♂ | Malaysia: Sabah: Poring Hot Springs | |
| Bavia nessagyna, sp. nov. | SWK12-4087 | ♀ | Malaysia: Lambir Hills | 4.20, 114.037 |
| Bavia sexpunctata (Doleschall, 1859) | AS19.2183 | ♀ | Singapore | 1.36, 103.77 |
| Indopadilla bamilin, sp. nov. | SWK12-1618 | ♂ | Malaysia: Mulu | 4.06, 114.829 |
| Indopadilla kahariana (Prószyński & Deeleman-Reinhold, 2013) | SWK12-1163 | ♂ | Malaysia: Mulu | 4.047, 114.825 |
| SWK12-1876 | ♀ | Malaysia: Mulu | 4.041, 114.817 | |
| Indopadilla kodagura, sp. nov. | AS19.4314 | ♂ | India: Kodagu | 12.22, 75.66 |
| Indopadilla nesinor, sp. nov. | MRB076 | ♀ | Singapore | 1.39, 103.81 |
| Indopadilla redunca, sp. nov. | SWK12-1831 | ♀ | Malaysia: Mulu | 4.040, 114.815 |
| Indopadilla redynis, sp. nov. | SWK12-0080 | ♀ | Malaysia: Kubah | 1.61, 110.19 |
| Indopadilla sabivia, sp. nov. | d107 | ♂ | Malaysia: Sabah: Kiabau | 5.832, 117.225 |
| Indopadilla vimedaba, sp. nov. | SWK12-3620 | ♀ | Malaysia: Mulu | 4.042, 114.814 |
| Stagetillus irri, sp. nov. | S202 | ♀ | Philippines: Luzon | |
| Stagetillus cf. opaciceps Simon, 1885 | MRB079 | ♀ | Malaysia: Ulu Gombak | 3.325, 101.753 |
| Padillothorax badut, sp. nov. | d548 | ♀ | Malaysia: Lambir Hills | 4.200, 114.035 |
| Padillothorax flavopunctus (Kanesharatnam & Benjamin, 2018) | IFS_SAL_1017 | j | Sri Lanka | 7.2833, 80.6303 |
| Padillothorax cf. flavopunctus | IFS_SAL_679 | ♀ | Sri Lanka | 7.3611, 80.8333 |
| Padillothorax mulu, sp. nov. | SWK12-2556 | ♂ | Malaysia: Mulu | 4.049, 114.86 |
| Maripanthus draconis, sp. nov. | d547 | ♀ | Singapore | 1.36, 103.77 |
| d176 | ♂ | Malaysia: Genting Highlands | 3.400, 101.777 | |
| Maripanthus reinholdae, sp. nov. | SWK12-1991 | ♀ | Malaysia: Mulu | 4.023, 114.813 |
| SWK12-1934 | ♀ | Malaysia: Mulu | 4.04, 114.817 | |
| Piranthus bakau, sp. nov. | d424 | ♂ | Malaysia: Bako | 1.722, 110.446 |
| Piranthus cf. kohi, sp. nov. | MRB109 | j | Malaysia: Ulu Gombak | 3.325, 101.753 |
| Piranthus planolancis Malamel, Nafin, Sudhikumar & Sebastian, 2019 | AS19.5940 | ♀ | India: Mysuru | 12.223, 76.627 |
| AS19.5970 | ♂ | India: Mysuru | 12.223, 76.627 | |
Molecular data used for phylogenetics of baviines. UCE probeset was either arachnid (A) or spider (S). SRA indicates sequence read archive accession number. “Reads Pass QC” indicates number of reads retained after quality control and adapter removal via Illumiprocessor. “UCE loci” counts number of loci both as obtained by mixed spider-arachnid file, and after filtering by occupancy and branch length criteria. “Filtered length” is number of non-missing sites, i.e. total base pairs or sequence length. For 28S, mtDNA, 16SND1, and COI, numbers indicate sequence length as obtained by Sanger sequencing (σ) or bycatch from UCE sequencing (β). New Sanger sequences show Genbank accession numbers of the form MW0818##. mtDNA indicates taxa for which entire mitochondrial genome recovered as bycatch. Published sequences indicated by citation (MH03:
| Species | Specimen ID | UCE Probes | SRA | Reads pass QC |
Contigs | UCE loci | filtered UCE loci | filtered UCE length | 28S | mtDNA | 167SND1 | COI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attulus floricola | d545 | A | SRR12832808 | 4519623 | 40309 | 468 | 129 | 78353 | 4797–β | |||
| d030 | 770 –σ (MN06) | 972–σ (MN06) | ||||||||||
| Breda bicruciata | d471 | A | SRX7739885 | (MMDH20) | 38487 | 399 | 87 | 35038 | 6720–β | 14305–β | ||
| Colonus hesperus | d472 | A | SRX7739887 | (MMDH20) | 56975 | 331 | 78 | 28801 | 6688–β | 12586–β | ||
| Helpis minitabunda | NZ19-9152 | S | SRR12832807 | 4037736 | 210843 | 1096 | 1031 | 757228 | 6751–β | 15601–β | ||
| S194, S195 | 956–σ (MH03) | 1047–σ (MH03) | ||||||||||
| Ligurra latidens | AS19.3412 | S | SRR12832798 | 3565247 | 25016 | 1118 | 1076 | 760567 | 5415–β | |||
| d175 | 909–σ (BM12) | |||||||||||
| Afromarengo sp. | MRB262 | 1049–σ (BM12) | 730–σ (BM12) | 946–σ (BM12) | ||||||||
| Phidippus johnsoni | d549 | S | SRR12832797 | 2954316 | 133630 | 1103 | 1038 | 779778 | 6734–β | 14319–β | ||
| Menemerus bivittatus | d559 | S | SRR12832796 | 5327638 | 74123 | 1203 | 1136 | 770717 | 5639–β | |||
| S13/S225 | 956–σ (MH03) | 956–σ (MH03) | ||||||||||
| Salticus scenicus | NA19-2676 | A | SRR12832795 | 2854670 | 25200 | 462 | 122 | 73552 | 6651–β | |||
| d003, S107 | 909–σ (MH03) | 972–σ (BM12) | ||||||||||
| Baviines | ||||||||||||
| Bavia aericeps | 2008PNG-2407 | S | SRR12832794 | 3940528 | 189254 | 1225 | 1162 | 912384 | 6782–β | 14324–β | ||
| Bavia cf. intermedia | d079 | 754–σ (MBN8) | 854–σ (ML14) | 972–σ (MBN8) | ||||||||
| Bavia nessagyna | SWK12-4087 | S | SRR12832793 | 497179 | 5132 | 894 | 860 | 410659 | 2772–β | |||
| Bavia sexpunctata | AS19.2183 | S | SRR12832792 | 1638329 | 77954 | 1236 | 1179 | 911457 | 5677–β | 14346–β | ||
| Indopadilla bamilin | SWK12-1618 | 687–σ MW081881 | ||||||||||
| Indopadilla kahariana | SWK12-1163 | S | SRR12832791 | 2410712 | 16408 | 1187 | 1145 | 820455 | 4759–β | 675–σ MW081883 | 596–β | |
| SWK12-1876 | 1042–σ MW081869 | 632–σ MW081882 | ||||||||||
| Indopadilla kodagura | AS19.4314 | S | SRR12832806 | 542868 | 4171 | 895 | 863 | 525638 | 6758–β | 1136–β | 1083–β | |
| Indopadilla nesinor | MRB076 | 920–σ MW081884 | 960–σ MW081865 | |||||||||
| Indopadilla redunca | SWK12-1831 | 1061–σ MW081871 | 650–σ MW081885 | |||||||||
| Indopadilla redynis | SWK12-0080 | S | SRR12832805 | 307232 | 4791 | 917 | 879 | 483961 | 435–β | |||
| Indopadilla sabivia | d107 | 771–σ MW081872 | 952–σ MW081866 | |||||||||
| Indopadilla vimedaba | SWK12-3620 | 608–σ MW081886 | ||||||||||
| Stagetillus irri | S202 | 656–σ (MH03) | 963–σ (MH03) | 969–σ (MH03) | ||||||||
| Stagetillus cf. opaciceps | MRB079 | S | SRR12832804 | 1033936 | 9649 | 1077 | 1047 | 772184 | 6894–β | 967-βσ (MBN8,β) | 467–β | |
| Padillothorax badut | d548 | S | SRR12832803 | 3504035 | 217449 | 1238 | 1175 | 906962 | 5682–β | 14231–β | ||
| Padillothorax flavopunctus | IFS_SAL_1017 | 729–σ MW081874 | 557–σ MW081867 | |||||||||
| Padillothorax cf. flavopunctus | IFS_SAL_679 | 695–σ MW081875 | 544–σ MW081868 | |||||||||
| Padillothorax mulu | SWK12-2556 | 1033–σ MW081873 | 659–σ MW081887 | |||||||||
| Maripanthus draconis | d547 | S | SRR12832802 | 2632437 | 127899 | 1207 | 1159 | 881761 | 5622–β | 14399–β | ||
| d176 | 821–σ MW081878 | |||||||||||
| Maripanthus reinholdae | SWK12-1991 | S | SRR12832801 | 1554841 | 72255 | 1170 | 1139 | 890576 | 5243–β | 1653–β | ||
| SWK12-1934 | 1043–σ MW081877 | |||||||||||
| Piranthus bakau | d424 | 1044–σ MW081879 | 720–σ MW081888 | |||||||||
| Piranthus cf. kohi | MRB109 | 1067–σ MW081880 | ||||||||||
| Piranthus planolancis | AS19.5940 | S | SRR12832800 | 3050561 | 135553 | 1217 | 1173 | 924020 | 6749–β | 14872–β | ||
| AS19.5970 | S | SRR12832799 | 1235845 | 56562 | 1186 | 1157 | 895555 | 6749–β | 14683–β | |||
Nungia and Capeyorkia specimens sequenced for phylogenetic study, with Genbank accession numbers.
| Species | Specimen ID | Sex | Locality | Lat-Long | 28S | 16SND1 |
|---|---|---|---|---|---|---|
| Nungia hatamensis (Thorell, 1881) | d260 | ♂ | Papua New Guinea: Putuwé | -5.231, 142.532 | MW202326 | |
| Nungia xiaolonghaensis (Cao & Li, 2016) | MRB078 | ♂ | Malaysia: Tanah Rata | 4.46, 101.40 | MW187118 | MW202327 |
| Nungia sp. “NPNGK” | d259 | ♂ | Papua New Guinea: Tualapa | -5.283, 142.498 | MW187119 | |
| Nungia sp. “NSGPQ” | d178 | ♂ | Singapore | 1.44, 103.70 | MW187120 | MW202328 |
| Nungia sp. “NUBWH” | SWK12-3204 | ♀ | Malaysia: Mulu NP | 4.042, 114.814 | MW187121 | MW202329 |
| Nungia sp. “NUMUL” | SWK12-1943 | ♂ | Malaysia: Mulu NP | 4.0405, 114.817 | MW187122 | MW202330 |
| Capeyorkia cf. vulpecula (Thorell, 1881) | MRB087 | ♂ | Papua New Guinea: Bundun | -6.8600, 146.6178 | MW187123 | |
| Capeyorkia sp. “NPNGE” | d261 | ♂ | Papua New Guinea: Varirata NP | -6.07, 145.40 | MW202331 | |
| Capeyorkia sp. “NPNGF” | d258 | ♂ | Papua New Guinea: Goroka | -9.436, 147.364 | MW187124 | MW202332 |
UCE Data
DNA was isolated using the Qiagen DNeasy Blood and Tissue Kit, following the spin-column protocol. Quality of the isolation was estimated using a NanoDrop 2000c Spectrophotometer, and samples were repeated where possible if the 260:280 nm UV absorbance ratio fell outside the range of 1.4 to 2.2. For most taxa 1 to 4 legs were used for DNA extraction, but the entire prosoma was used for Padillothorax badut (specimen d548) and Helpis minitabunda (specimen NZ19-9152). For the target enrichment UCE sequencing, dual-indexed TruSeq-style libraries were prepared following methods previously used in arachnids (e.g.,
From among the contigs thus assembled, those matching particular UCE probes were pulled out using the Phyluce pipeline at default settings. Because some taxa were captured using the arachnid probeset (outgroups Attulus, Breda, Colonus, Salticus), and others using the spider probeset (remaining outgroups, and all baviines), a blended probeset file was needed to best pull out UCE contigs, because each of the arachnid and spider probesets includes loci not included by the other.
Recovered UCE loci were aligned with MAFFT (
Data for 28S and mitochondrial genes
For the new Sanger-sequenced data, specimens were preserved, their DNA extracted, and sequences obtained for the nuclear gene 28S and the mitochondrial gene regions 16SNDI and COI following the protocols using the protocols of
The same three gene regions were also present among the sequence capture genomic contigs as untargeted bycatch. We recovered them by constructing a local BLAST database of the contigs of each taxon, and querying it with 28S, 16SND1 and COI sequences from eight to nine different salticid species (Bavia, Indopadilla, Bathippus, Harmochirus, Idastrandia, Langerra, Phintella, Platycryptus, Salticus, Attulus, Lyssomanes), retaining any contigs matching with an e-value of less than 10-10 and length greater than 200. In a few cases, multiple contigs from a taxon were recovered as matching a single locus, but after alignment against others these could be interpreted as different parts of the target gene, and were thus stitched together to a single sequence.
Phylogenetic analysis
Maximum likelihood phylogenetic analyses were performed with IQ-TREE version 1.6.7.1 (
- “UCEs” – The UCE dataset after filtering of loci, concatenated, unpartitioned.
- “mtDNA+28S” – The concatenated data from 28S and mitochondrial sequences (10 full mtDNA, others Sanger and bycatch 16SND1 aligned against the full mtDNA). Analyzed with 2 partitions, 28S and mtDNA.
- “restricted mtDNA+28S” – A restricted version of 28S and mtDNA with the long bycatch sequences trimmed to put the taxa with legacy Sanger data on almost equal footing (i.e., approximately as much data) with those with bycatch. 28S sites at start and end were trimmed until at least 3 of the shorter legacy Sanger sequences were represented. The same rule for trimming was used for the mtDNA before 16SND1, between 16SND1 and CO1 genes, and after COI. Analyzed with 2 partitions, 28S and mtDNA.
- “UCEs+mtDNA+28S” – The UCE loci (dataset #1) concatenated to the restricted 28S and mtDNA data (dataset #3). Analyzed unpartitioned.
For the partitioned analyses, the options -m MFP -spp were used (extended model selection followed by tree inference, edge-linked partition model, with partition-specific rates); for the unpartitioned analyses, -m MFP (extended model selection followed by tree inference, edge-linked partition model, no partition-specific rates).
Several species of elongate brown Asian and Australasian salticids were initially identified in the field as baviines, but were excluded from the Baviini by preliminary molecular analyses and closer morphological study, which showed them to be near Nungia epigynalis Żabka, 1985. To document this and clarify the limits of the Baviini, we did a small analysis based on 28S and 16SND1, using previously published sequences of baviines, astioids, and other groups from
Raw sequence reads from UCE capture are deposited in the Sequence Read Archive (BioProject PRJNA667925, https://www.ncbi.nlm.nih.gov/sra/PRJNA667925) with accession numbers shown in Table
Taxonomic authority
The taxonomic authority for all nomenclatural acts (synonymies, new combinations, new species) is W. Maddison.
Molecular phylogenetic results
Molecular data obtained
UCE data obtained are outlined in Table
The bycatch 28S sequences were 435–6894 bp long (average 5675.9) and aligned well with the Sanger 28S sequences using MAFFT at default settings. Bycatch 28S were obtained for three specimens for which previous Sanger sequences were available and identical (I. kahariana SWK12-1163, 1021 base pairs; M. reinholdae SWK-01991, 1037 bp; S. cf. opaciceps MRB079, 1067 bp) except for a one base difference at the start of the I. kahariana sequence. An initial alignment of 28S including UCE bycatch and legacy Sanger data was 18646 bp long, but the first 1593 bp and the last 9793 bp of the alignment were poorly aligned and represented by only a few bycatch sequences, and so were trimmed. After addition of a few other taxa and realignment, the final 28S alignment was 7281 bp long.
Among the bycatch contigs for 10 taxa were long sequences containing both the 16SND1 and COI regions, and whose size (12568–15601 bp) suggests they may be the whole or nearly whole mitochondrial genome (marked in the column “mtDNA” in Table
The whole mtDNA sequences aligned well against each other using MAFFT at default settings. The shorter bycatch sequences and Sanger 16SND1 and COI aligned well against the whole mtDNA. The whole mtDNA sequences initially differed in their (arbitrary) starting point on the circular mitochondrial genome, but after a preliminary alignment they were adjusted to all begin at a conserved region in 16S. After adjustments by hand to align ND1 and COI without gaps, the other regions (before ND1, between ND1 and COI, and between COI and the end) were re-aligned using MAFFT.
Trimming of the 28S and mtDNA alignments as explained for dataset #3 (“restricted mtDNA+28S”) yielded a 28S alignment of 1181 base pairs, and a mitochondrial alignment of 2211 bp.
Phylogenetic results
The primary phylogenetic results are shown in Figs
Phylogeny. 1 Maximum Likelihood tree (best of 20 replicates) from combined data set of 1313 UCE loci, plus mitochondrial 16SND1 and COI regions, plus 28S (dataset #4 in Methods). Baviines whose names are in bold, and all outgroups, have UCE data. Numbers are percentage of 500 bootstrap replicates showing the clade 2 Maximum Likelihood tree (best of 50 replicates) from concatenated data from 1313 UCE loci (dataset #1 in Methods). Numbers are percentage of 1000 bootstrap replicates showing the clade 3 Maximum Likelihood tree (best of 50 replicates) from concatenated data from mitochondrial data and 28S (dataset #2 in Methods). Taxa in bold have data from the entire mitochondrial genome, or nearly so. Numbers are percentage of 1000 bootstrap replicates showing the clade for the full dataset (#2), followed by the bootstrap percentage for the restricted mtDNA+28S dataset (#3 in Methods). Spots at nodes show those clades that also appear in the ML tree in the restricted dataset. The branches to B. nessagyna and I. redynis are long, compacted visually by cutting and sliding part of the length over itself; the actual length therefore should be seen as longer by the length of the overlap.
Relationships within each of the three major clades is reasonably stable across datasets. Within the Piranthus clade, the morphologically distinctive Piranthus is monophyletic, as is Maripanthus. The morphologically similar Padillothorax badut and P. mulu are sisters, as are P. flavopunctus and P. cf. flavopunctus. Within the Bavia clade, molecular results more or less match morphological groups: Bavia with relatively large bodies, Indopadilla with ridged chelicerae, thoracic bulges, and exposed clypeal arthrodial membrane, and the elongate yellow-orange Stagetillus. Accordingly, the concepts of genera here come from both morphological and molecular evidence.
Taxonomic results
Tribe Baviini Simon, 1901
Genera included:
Bavia Simon, 1877
Indopadilla Caleb & Sankaran, 2019
Maripanthus Maddison, gen. nov.
Padillothorax Simon, 2001
Padillothorus Prószyński, 2018
Piranthus Thorell, 1895
Stagetillus Simon, 1885
Narrow-bodied medium to large salticids in Asia and Australasia, pluridentate, with an embolus fixed to the tegulum or with some degree of mobility. The abdomen is usually long and the legs (except the first) relatively short. There is no known clearly understood morphological synapomorphy of the group. Nonetheless, molecular data groups together most tropical Asian salticids of this body form as baviines. Some long-bodied ballines (e.g., Mantisatta Warburton, 1900, Copocrossa Simon, 1901), marpissines (Mendoza Peckham & Peckham, 1894), astioids (Holoplatys Simon, 1885 and relatives, Nungia Żabka, 1985), chrysillines (Epocilla Thorell, 1887), and plexippines (Telamonia Thorell, 1887) might be confused for baviines, but most have distinctive features of their own. Most difficult to distinguish from baviines are perhaps the viciriine astioids Nungia and relatives (including Pungalina Richardson, 2013 and Capeyorkia Richardson, 2016). Nungia are generally smaller than baviines, with a more flat-topped carapace; they are quite distinct by molecular data (
Padillothorus (see
Figures
Variation in traits among baviines 4, 5 oblique views of female prosoma 4 Bavia nessagyna (specimen IDWM.20004; note convex front face of chelicerae, and typical thorax) 5 Indopadilla redunca (holotype IDWM.20011; note concave front face of chelicerae, and thoracic bulges) 6–12 left male endite (9 also with chelicera) 6 Bavia nessagyna (holotype IDWM.20005) 7 Indopadilla redunca (specimen SWK12-M0009) 8 Stagetillus opaciceps (specimen JK.08.08.19.0001) 9 Padillothorax semiostrinus (specimen JK.20.06.20.001) 10 Padillothorax badut (specimen SWK12-4688) 11 Maripanthus jubatus (specimen AS19.4373) 12 Piranthus planolancis (specimen AS19.5970) 13–20 carapace (all females except 15) 13 Bavia nessagyna (specimen IDWM.20004) 14 Indopadilla redunca (holotype IDWM.20011) 15 Stagetillus opaciceps male (specimen JK.13.02.26.0017) 16 Stagetillus cf. opaciceps female (specimen MRB079).17 Padillothorax semiostrinus (specimen JK.20.06.20.001) 18 Padillothorax mulu (from Mulu Nat. Pk.) 19 Maripanthus reinholdae (specimen SWK12-1934) 20 Piranthus planolancis (specimen AS19.5940) 21–28 Metatarsus of first leg, retrolateral view. All female except 24 male. All are of left legs except 23, 25, 28 which are of right leg, digitally flipped 21 Bavia nessagyna (specimen IDWM.20004) 22 Indopadilla kahariana (specimen SWK12-1876) 23 Stagetillus cf. opaciceps (specimen MRB079) 24 Padillothorax semiostrinus (specimen SWK12-EP0105) 25 Padillothorax mulu (specimen SWK12-EP0105) 26 Maripanthus reinholdae (specimen SWK12-1934) 27 Maripanthus draconis (from Gunung Belemut, Johor) 28 Piranthus planolancis (specimen AS19.5940) 29–35 prolateral surface of male first leg femur. 29 Bavia cf. capistrata (specimen from Singapore) 30 Indopadilla kahariana (specimen from Lambir Hills Nat. Pk.) 31 Stagetillus opaciceps (specimen JK.08.08.19.0001) 32 Padillothorax semiostrinus (specimen JK.20.06.20.001) 33 Padillothorax badut (specimen SWK12-4688) 34 Maripanthus draconis (specimen from Johor, Gunung Lambak) 35 Piranthus bakau (holotype). Scale bars: 0.1 mm (6–12, 21–35), 1.0 mm (4, 5, 13–20).
Shape of the carapace – flatter (height < 36% of length) in most Piranthus and Padillothorax, higher in others. Indopadilla has distinctive bulges on the thorax sides (Figs
Lateral margin of male endites – Indopadilla and Piranthus males have the endite with a simple rounded margin, but the other genera have varying projections (Figs
Position of the fovea (Figs
Sockets of leg I macrosetae – in the Bavia clade, the sockets of macrosetae extend downward as lateral flange (Figs
Macrosetae of first femur (Figs
In several baviine genera, not each others’ closest relatives, there is a characteristic series of markings consisting of small patches of pale scales on the thorax: one patch medially between the PLE, a short longitudinal stripe at the top of the thoracic slope, and one behind each PLE (e.g., Bavia, Fig.
The taxonomic account below presents in sequence the Bavia clade, Padillothorax, and then the Piranthus clade.
The Bavia Clade (Bavia, Indopadilla, Stagetillus)
Bavia
Bavia Simon 1877. Type species Bavia aericeps Simon, 1877
Acompse L. Koch 1879. Type species Acompse suavis L. Koch, 1879 = B. aericeps.
Species included
Bavia aericeps Simon, 1877
Bavia capistrata (C. L. Koch, 1846), combination restored, removed from synonymy with Evarcha flavocincta (C. L. Koch, 1846)
Bavia fedor Berry, Beatty & Prószyński, 1997
Bavia nessagyna Maddison, sp. nov.
Bavia gabrieli Barrion, 2000
Bavia intermedia (Karsch, 1880)
Bavia maurerae (Freudenschuss & Seiter, 2016), comb. nov., transferred from Epidelaxia
Bavia planiceps (Karsch, 1880)
Bavia sexpunctata (Doleschall, 1859)
Bavia valida (Keyserling, 1882)
Diagnosis
Larger-bodied than most other baviines. Carapace relatively broad and having hexagonal shape, widest at or just behind the PLEs (Fig.
Bavia aericeps 36 male left palp, ventral view (specimen 2008PNG-2407, Papua New Guinea, 5.231°S, 142.532°E) 37 same, retrolateral view of tibia 38 female epigyne, ventral (specimen SMF 60114, Samoa) 39 same, vulva. 40, 41 male (specimen 2008PNG-1517, Papua New Guinea, 5.283°S, 142.498°E). Distance between substrate grooves 10 mm. Scale bars: on genitalia 0.1 mm.
Illustrations are given here of some of the well-known species of Bavia, including B. aericeps (Figs
Bavia cf. capistrata. 42 male left palp, ventral view (specimen AS19.1118, Singapore) 43 same, retrolateral view of tibia 44 epigyne, ventral (specimen AS19.1128, Singapore) 45 vulva, dorsal 46–49 Male AS19.1118 50 Male AS19.2341 (Singapore) 51–53 female AS19.1128 (Singapore). Scale bars: on genitalia 0.1 mm; on bodies 1.0 mm.
A video of the living female B. cf. capistrata (specimen AS19.1128) is available in
In addition to the species below, we have seen an undescribed species near B. nessagyna from Mulu National Park (single female) and a species near B. intermedia (single male, here represented as specimen d079 in the Sanger data).
Bavia nessagyna
Type material
All from Malaysia: Sarawak: Lambir Hills Nat. Pk., and in
Etymology
From the Greek nessa, duck, and gyne, female, referring to the resemblance of the epigyne to a duck’s bill. Other names: In WPM’s lab notebooks the informal code for this species was “BVDUC-S”.
Diagnosis
One of the more delicate Bavia, along with B. capistrata, having legs II–IV very much paler than I, and thus resembling Indopadilla. Its distinction from B. fedor is slight: the embolus of both appears as a curved and narrowing blade with a series of retrolateral teeth. The teeth are short, triangular, and closely spaced in B. nessagyna, but larger in B. fedor, appearing as broad pillars whose bases are well separate (photographs of holotype kindly supplied by J. Boone, Bishop Museum). In B. nessagyna the teeth are not on the embolus proper but on a TmA that parallels the embolus (Fig.
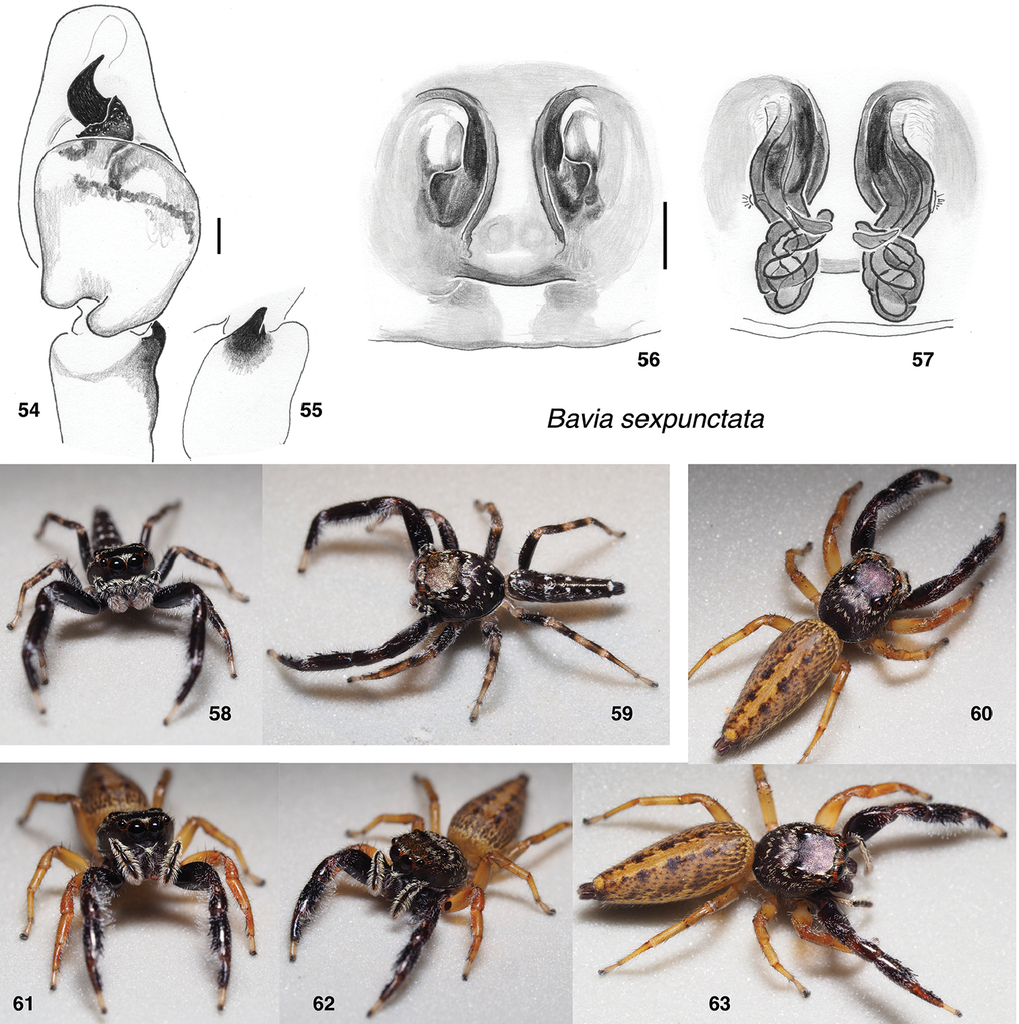
Bavia sexpunctata . 54 male left palp, ventral view (Singapore, Upper Peirce Reservoir) 55 same, retrolateral view of tibia 56 epigyne, ventral (Singapore, Bukit Timah) 57 vulva, dorsal 58, 59 male (specimen AS19.2175, Singapore) 60–63 female (specimen AS19.0230, Singapore). Scale bars: on genitalia 0.1 mm.
Description
Male (based on holotype, specimen IDWM.20005). Carapace length 3.6; abdomen length 4.8. Carapace (Fig.
Bavia nessagyna sp. nov. 64 male left palp, ventral view (holotype IDWM.20005) 65 same, retrolateral view of tibia 66 epigyne, ventral (specimen IDWM.20003) 67 vulva, dorsal 68, 69 male (specimen SWK12-4726) 70–72 male (specimen SWK12-0590) 73–75 female (specimen SWK12-4087). Distance between substrate grooves 10 mm. Scale bars: on genitalia 0.1 mm.
Female (based on paratype IDWM.20003). Carapace length 3.3; abdomen length 5.9. Carapace as in male, but lacking tuft at widest point. Clypeus and Chelicerae as in male. Five retromarginal teeth. Legs similar to those of male, but with first leg not quite so dark, and without the ventral fringe so distinctly developed. Abdomen with only a hint of transverse bands, instead dominated by longitudinal stripes: a narrow central pale band flanked by wide dark bands. Epigyne with ECP beneath a posteriorly-projecting mound (Figs
Natural history
All specimens from Lambir Hills were collected on big-leaved foliage (e.g., palms) except for IDWM.20004 whose collecting record says simply “foliage”.
Additional material examined
One female from Malaysia: Sarawak: Lambir Hills Nat. Pk., Inoue Trail, 4.2002°N, 114.0346°E to 4.2004°N, 114.0342°E, 200 m el. 4 April 2012 Maddison/Piascik/Ang WPM#12-130. One male from Malaysia: Sarawak: Bako Nat. Pk., Mangroves, beach forest, 1.722°N, 110.446°E, 0 m el. 8 March 2012 Maddison/Piascik/Ang/Lee WPM#12-003.
Indopadilla
Indopadilla Caleb & Sankaran, 2019. Type species Indopadilla darjeeling Caleb & Sankaran, 2019.
Species included
Indopadilla annamita (Simon, 1903), comb. nov., transferred from Bavia
Indopadilla bamilin Maddison, sp. nov.
Indopadilla darjeeling Caleb & Sankaran, 2019
Indopadilla kahariana (Prószyński & Deeleman-Reinhold, 2013), comb. nov., transferred from Bavia
Indopadilla kodagura Maddison, sp. nov.
Indopadilla insularis (Malamel, Sankaran & Sebastian, 2015)
Indopadilla nesinor Maddison, sp. nov.
Indopadilla redunca Maddison, sp. nov.
Indopadilla redynis Maddison, sp. nov.
Indopadilla sabivia Maddison, sp. nov.
Indopadilla sonsorol (Berry, Beatty & Prószyński, 1997), comb. nov., transferred from Bavia
Indopadilla suhartoi (Prószyński & Deeleman-Reinhold, 2013), comb. nov., transferred from Bavia
Indopadilla thorelli (Simon, 1901)
Indopadilla vimedaba Maddison, sp. nov.
Diagnosis
Front face of chelicerae concave or flat in both males and females, bordered laterally by a sharp ridge (Fig.
This distinctive group may have many dozens or even hundreds of species, judging from the rate of discovery of new species among the few specimens being collected. We show in Figs
Embolus length varies through Indopadilla, short in many species, in other species (e.g., I. kodagura, I. suhartoi) as long as in some Piranthus and Marapathus. While we might be tempted to split the group into two – Indopadilla with a long embolus in South Asia and southeast Asia, and a new genus with a short embolus in southeast Asia – the most complete molecular data nests the long-embolus I. kodagura among short embolus species (Fig.
The peculiar carapace bulge and exposed arthrodial membrane on the clypeus hint to the possibility that Indopadilla may use unusual biomechanics. The third leg claw tufts appear noticeably larger than the others. Indopadilla are excellent jumpers, difficult to collect even on a beating sheet, from which they can escape in a single decisive bound. They are usually collected from foliage. A video of living males of I. kodagura and the undescribed species “BVBTN” (Figs
Indopadilla bamilin
Type material
Holotype
male (specimen SWK12-1618, in
Etymology
An arbitrary combination of letters, ungendered. Other names: In
Diagnosis
Both embolus and RTA short and simple (Fig.
Description
Male (based on holotype, specimen SWK12-1618). Carapace length 2.3; abdomen length 3.0. Carapace dark brown, slightly paler around fovea, with a few patches of pale scales on thorax (Figs
Indopadilla kahariana , comb. nov.
Bavia kahariana Prószyński & Deeleman-Reinhold, 2013: 115–117, figs 1–9.
Notes
We include illustrations of the described Indopadilla kahariana, to show living specimens and to show the genitalia in detail. Christa Deeleman-Reinhold kindly compared the type specimens to our illustrations and confirmed the match. A photograph of a living female is shown by
Indopadilla kodagura
Type material
Holotype male (NCBS-BN351, also known as AS19.4314), in NCBS collection, from India: Karnataka: Kodagu: Yavakapadi, Honey Valley area, forest & edge, 12.215°N, 75.659°E to 12.216°N, 75.661°E, 1300 m elev. 25 June 2019 W. Maddison & K. Marathe WPM#19-077.
Etymology
In the Kodava language, kodagura means from Kodagu. Other names: In WPM’s field or lab notebooks the informal code for this species was “BVHVW”.
Diagnosis
Very similar to I. insularis, contrastingly marked in dark brown and yellow, with the face appearing white because the clypeus is withdrawn toward the eyes to expose a bright white arthrodial membrane. Like I. insularis, I. darjeeling, I. sonsorol, I. suhartoi, and I. thorelli in having a long thin embolus, but even longer than in those species, arising from the retrolateral basal corner of the bulb.
Description
Male (based on holotype, specimen NCBS-BN351). Carapace length 2.6; abdomen length 4.0. Carapace integument dark brown to black except to either side of fovea and narrow medial stripe on thorax, and with small patches of yellow scales in pattern typical for Indopadilla. Clypeus dark, extremely narrow, exposing white arthrodial membrane. Chelicerae dark, with lateral ridge bearing a tooth (Fig.
Natural history
A video of the living holotype is available in
Indopadilla kahariana . 80 male left palp, ventral view (Mulu Nat. Pk., 4.045°N, 114.816°E) 81 Same, retrolateral view of tibia 82 epigyne, ventral (specimen SWK12-1876) 83 vulva, dorsal 83–88 male (SWK12-1163) 89–92 Female (SWK12-1876). Distance between substrate grooves 10 mm. Scale bars: on genitalia 0.1 mm.
Indopadilla nesinor
Type material
Holotype female (specimen MRB076), in LKCNHM, from Singapore: Nee Soon Swamp Forest, beating vegetation, 1.39°N, 103.81°E, 12 May 2005. W. Maddison, D. Li, I. Agnarsson, J. X. Zhang. WPM#05-015. Paratype female (specimen JK.14.05.19.0015) from Singapore: Central Catchment Nature Reserve Upper Peirce Reservoir 1.3811°N, 103.8156°E, J. K. H. Koh 19 May 2014.
Etymology
An arbitrary combination of letters, ungendered. Other names: In WPM’s lab notebooks the informal code for this species was “BVNES”.
Indopadilla redunca sp. nov. 104 male left palp, ventral view (holotype SWK12-M0009) 105 same, retrolateral view of tibia 106 same, oblique ventral-retrolateral view 107 epigyne, ventral (specimen SWK12-1831) 108 Vulva, dorsal 109 male holotype, carapace 110 same, abdomen 111, 112 Female (SWK12-1831). Distance between substrate grooves 10 mm. Scale bars: on genitalia 0.1 mm.
Diagnosis
As in I. vimedaba, the face appears white because of the exposed arthrodial membrane, whose boundary with the very narrow clypeus is indistinct. Differs from I. vimedaba in having chevroned abdominal markings visible in alcohol and epigynal openings small and copulatory ducts densely tangled and fused (Fig.
Description
Female (based on holotype, specimen MRB076). Carapace length 2.5; abdomen length 3.6. Carapace integument in alcohol dark brown, paler in ocular area and along midline of thorax, with patches of yellow scales (Fig.
Indopadilla redunca
Type material
All from Malaysia: Sarawak: Mulu Nat. Pk. Holotype. Female (specimen IDWM.20011, in
Etymology
Latin, meaning bent backward, referring to both the RTA and the ridge in front of the epigynal openings. Other names: In
Notes
The living paratype male 12.01.22.0024 is shown as figure E on p. 203 of
Diagnosis
Male palp distinctive for bent RTA and thick curled embolus accompanied by TmA (Fig.
Description
Male (based on paratype, specimen SWK12-M0009). Carapace length 2.9; abdomen length 3.6. Carapace in alcohol dark brown except medium brown near fovea, and a small medium brown stripe medially on thoracic slope. Clypeus dark, glabrous, very narrow in middle, exposing a white arthrodial membrane beneath. Chelicerae dark, concave and with lateral ridge. At least four retromarginal teeth, on long sharp ridge. Palp pale except dark gray retrolateral face of femur. Embolus thick and curved, accompanied by similarly curved TmA (Figs
Female (based on holotype, specimen IDWM.20011). Carapace length 3.2; abdomen length 4.1. Carapace dark brown, paler around fovea and in a narrow medial band on thoracic slope. Clypeus narrow, dark, glabrous, exposing white arthrodial membrane. Chelicerae vertical, concave in front. Five retromarginal teeth. Legs pale except for first leg, whose tibia and metatarsus are dark brown in the middle, and the femur which grades to dark brown terminally. Abdomen marked as described under Male-female matching. Epigyne (Figs
Male-female matching
The male and female, both collected at Mulu National Park, are matched primarily on the basis of markings and expected genitalic correlations. They share abdominal markings (Figs
Indopadilla redynis
Type material
Holotype
female (specimen IDWM.20012, in
Etymology
An arbitrary combination of letters, ungendered. Other names: In WPM’s lab notebooks the informal code for this species was “BVMT2”; it was also sometimes confused with I. redunca and labelled “BVMTT”.
Diagnosis
Similar in colour and epigyne to I. redunca. The epigyne differs in having the anterior part of the cavernous ECP with a more sharply defined boundary, the openings not behind a curved ridge, and the edge of the opening clearly sinuate (Fig.
Description
Female (based on holotype, specimen IDWM.20012). Carapace length 3.6; abdomen length 5.7. Carapace integument medium to dark red-brown, except upper part of thorax between and beside eyes, continuing as a broad medial pale area to pedicel. Clypeus dark, extremely narrow, exposing broad white arthrodial membrane. Chelicerae light brown, concave in front. Five teeth on retromargin. Legs pale except dark brown on middle of first tibia and metatarsus, and darker patch at front base of first femur. Abdomen red-brown, with delicate reticulate pale markings. Epigyne (Fig.
Indopadilla sabivia
Type material
Holotype
male (specimen d107, in
Etymology
An arbitrary combination of letters, ungendered. Other names: In WPM’s lab notebooks the informal code for this species was “BVSAB”.
Diagnosis
Palp similar to I. kahariana with a short sharp embolus with a TmA behind it, but the TmA is broader and more retrolaterally placed than in I. kahariana. RTA with sharp point, unlike the broad flat RTA of I. kahariana. These two species are sister groups on the molecular phylogeny (Fig.
Description
Male (based on holotype, specimen d107). Carapace length 2.8; abdomen length 3.8. Overall appearance similar to that of I. kahariana, paler coloured than many male Indopadilla, honey to medium brown. Carapace medium brown to honey coloured, darker on sides, with ocular area and larger portion of dorsal thorax quite pale. Clypeus narrow, exposing white arthrodial membrane. Chelicerae brown, with lateral ridge. Six retromarginal teeth, on long ridge. Palp pale except basal part of femur. Embolus short and sharp, with broad TmA behind (Fig.
Indopadilla vimedaba
Stagetilus semiostrinus: Prószyński, 1987: figs on pages 105–105 (misidentification).
Padillothorax semiostrinus:
Type material
Holotype male (specimen JK 13.09.03.0011), in LKCNHM, from Singapore: Nee Soon Swamp, 2 September 2013. J. K. H. Koh.
Etymology
An arbitrary combination of letters, ungendered. Other names: In
Notes
As noted under Padillothorax, this species was mistaken for Padillothorax semiostrinus, and illustrated by
Diagnosis
Palp with embolus longer that half the length of the bulb, straight and tapering (Fig.
Description
Male (based on holotype, specimen JK 13.09.03.0011). Carapace length 2.4; abdomen length 3.5. Carapace in alcohol dark brown to black, slightly paler around fovea and in narrow medial band along thoracic slope, which also has some white scales; other patches of white scales along borders of ocular area. Clypeus extremely narrow at centre, and beneath it is a broad expanse of white arthrodial membrane; precise boundary between the clypeus and arthrodial membrane indistinct. Chelicerae with strong lateral ridge that extends into a flange near the fang, as drawn by
Female (based on specimen SWK12-3620). Carapace length 3.3; abdomen length 5.4. Carapace integument in alcohol medium red-brown except for narrow medial pale band along thoracic slope. Clypeus extremely narrow, exposing broad white arthrodial membrane (Fig.
Indopadilla vimedaba sp. nov. 121 male left palp, ventral view (holotype JK.13.09.03.0011) 122 same, retrolateral view of tibia 123 epigyne, ventral (specimen SWK12-3620) 124 vulva, dorsal 125 holotype male 126–128 female SWK12-3620. Scale bars: on genitalia 0.1 mm; on bodies 1.0 mm.
Male-female matching
The male and female described above were not co-collected, but they match well the male and female (MNHN 15151, photographs examined) described by Prószyński, which were in the same vial, and thus likely co-collected. These males and females match in markings: the first leg is all dark except the tarsus; the abdominal dorsum is dark with some narrow pale lines, the venter dark; the pale medial band of the integument of the thoracic slope is narrow; there is a narrow band of pale scales on the midline low on the thoracic slope; the triangle of scales near the fovea is narrow. The male has fewer lines in its abdominal markings than the female, but those it has are precise matches to the female. Other Indopadilla differ; e.g., another candidate female, I. nesinor, has the first leg considerably more strongly banded. suggesting her male should have a first patella paler than seen in Fig.
Unidentified or undescribed Indopadilla, all in LKCNHM except 134–136, in UBCZ. 129–131 male “Yellow Long” (specimen JK08.04.29.0029, Brunei, 4.6044°N, 114.6450°E), body, ventral palp, retrolateral palp tibia 132, 133 female “Yellow Long” (specimen JK.12.03.14.0031, Brunei, 4.7036°N, 114.6264°E), epigyne, body 134–136 male “BVBTN” (specimen AS19.2286, Singapore, 1.3562°N, 103.7748°E to 1.3572°N, 103.7734°E), body, ventral palp, retrolateral palp tibia 137, 138 female “Iridescent Ocular” (specimen JK.13.02.16.3005, Brunei 4.5764°N, 115.0731°E), epigyne, body 139, 140 female “Orange Head” (specimen JK.12.03.21.0011, Brunei, 4.5833°N, 114.5047°E), body, epigyne (shown alive as fig. B on p. 203 of
Additional material examined
One female (specimen SWK12-3620, in
Stagetillus
Stagetillus Simon, 1885. Type species S. opaciceps Simon, 1885.
Hyctiota Strand, 1911, syn. nov. Type species H. banda Strand, 1911.
Species included
Stagetillus banda (Strand, 1911), comb. nov.
Stagetillus opaciceps Simon, 1885
Stagetillus irri Maddison, sp. nov.
Notes
Hyctiota banda Strand, 1911 is based on a juvenile (
Diagnosis
Carapace distinctive in shape, widest point toward the posterior, approx. half way between the back eyes and the pedicel, and in colour, orange or yellow with the white digestive diverticulum showing beneath the transparent ocular quadrangle. Palp much like that of Bavia, with short blade-like embolus.
Stagetillus opaciceps
Stagetillus opaciceps Simon, 1885: 32.
Note
Stagetillus opaciceps male, and a female tentatively identified as S. opaciceps. 143 Male left palp, ventral view (specimen JK.13.02.26.0017) 144 same, retrolateral view of tibia 145 living male from Belait, Brunei (photograph Joseph Koh 2019) 146, 147 male (specimen JK.08.08.19.0001) 148 female epigyne (specimen MRB079), ventral 149 vulva, dorsal 150 living female. Scale bars: on genitalia 0.1 mm; on bodies 1.0 mm.
Material examined
Male (specimen JK.13.02.26.0017, in LKCNHM) Malaysia: Negeri Sembilan, Hutan Lipur Ulu Bendul, 2.73°N, 102.0789°E, J. K. H. Koh 26 February 2013; Male (JK.08.08.19.0001, in LKCNHM) Brunei: Belait, Disturbed forest off Labi Road, 4.5858°N, 114.5067°E, J. K. H. Koh 19 August 2008. Female (specimen MRB079, in
Stagetillus irri
Type material
Holotype
male (specimen IDWM.20023, in
Etymology
From the acronym for the type locality. Other names: This species was referred to by
Notes
The
Diagnosis
Similar in overall appearance to S. opaciceps, from which it differs in palp (narrower and longer embolus) and epigyne (shorter openings and having a central mound, presumably bearing the ECP). Male lacking the dense fringe beneath the first leg (Figs
Description
Male (based on holotype, specimen IDWM.20023). Carapace length 3.3; abdomen length 4.6. Carapace yellow-orange with two darker stripes passing along PME, PLE, and to posterior margin, with transparent ocular areas showing bright white digestive diverticular beneath. Shape as in S. opaciceps (Fig.
Stagetillus irri sp. nov. 151 male paratype IDWM.20022, left palp, ventral view 152 same, retrolateral view 153 female paratype S202, epigyne, ventral 154 same, vulva, dorsal. 155–156 male holotype IDWM.20023 157 female paratype IDWM.20014. Scale bars: on epigyne 0.1 mm; on body 1.0 mm.
Female (based on paratype, specimen IDWM.20014). Carapace length 2.9; abdomen length 5.2. Entirely light in colour, from white to medium orange, except for the black of eyes, and small black patch at the front distal tip of the first leg femur. Carapace pale orange, with transparent ocular area showing bright white digestive diverticular beneath. Shape as in S. opaciceps (Fig.
Genus Padillothorax
Padillothorax
Padillothorax Simon, 1901. Type species Padillothorax semiostrinus Simon, 1901.
Bavirecta Kanesharatnam & Benjamin, 2018, syn. nov. Type species Bavirecta flavopuncta Kanesharatnam & Benjamin, 2018.
Species included
Padillothorax badut Maddison, sp. nov.
Padillothorax casteti (Simon, 1900), comb. nov., transferred from Bavirecta
Padillothorax exilis (Cao & Li, 2016), comb. nov., transferred from Bavirecta
Padillothorax flavopunctus (Kanesharatnam & Benjamin, 2018), comb. nov., transferred from Bavirecta
Padillothorax mulu Maddison, sp. nov.
Padillothorax semiostrinus Simon, 1901
Padillothorax taprobanicus Simon, 1902
Notes
The synonymy of Bavirecta with Padillothorax can be established now that the identity of the type species of the latter has been clarified (see below). The synapomorphies uniting them include the position of macrosetae on the first femur, flattened carapace, placement of fovea, pale thoracic “window” (
Diagnosis
Distinctive for the macroseta(e) in the middle of the front surface of the first leg femur, the palp with narrow distally-pointing embolus, the pale trapezoidal “window” dorsally on the thorax (
Padillothorax semiostrinus
Padillothorax semiostrinus Simon, 1901: 71.
Notes
There has been confusion about the identity of P. semiostrinus.
As to what is Padillothorax semiostrinus, we have not been able to examine the type specimens, as they have not yet been located in the MNHN Paris. However, specimens found recently in Singapore and Taiwan match well
"♂. Length 7.5 mm. Cephalothorax low, long and oval, red-brown, darker towards the border; texture very wormy-coriaceous except for the middle of the thoracic part which is smoother. Cephalic area in front and at both sides, [and?] near the eyes, decorated with white-silver hairs. Two wide medial thoracic bands, nearly contiguous; a thin marginal line decorated with white-silver hairs. Few white hairs around the eyes. Clypeus very narrow, bald. Abdomen narrow and very long, decorated above with dark violet, a medial band that is wide, entire, and yellow-brick red, bordered in front with lines and behind with a series of spots covered with silver-white hairs, marked on each side with a straight line in front and two white oblique [or crosswise?] lines behind. Venter reddish-yellow. Spinnerets dark. Chelicerae shiny black, short and diverging, convex outside, inside somewhat ribbed, inferior margin having a sunken furrow, then very raised and armed with a series of contiguous teeth, the middle larger. Mouth area black. Laminae truncate at the tip, convex, but with a compressed corner that is slightly extended. Sternum yellow. First pair of legs much longer and thicker than the others, femur clavate, tibia long and ovate, dark brown, coxa and femur black, tarsus yellow, tibia and metatarsus with fringe of reasonably long but not very dense black hairs. Remaining legs pale yellow, armed by a few small spines, as in Bavia. Palps reasonably small, pale yellow, thick with white hairs; tibia and patella rather short, equipped at the outside with a [long?] apophysis with a straight front and a black and acute tip."
There are two apparent or possible conflicts between this description and the specimen seen in Figs
We therefore provisionally identify the specimen of Figs
A juvenile found in Singapore (Fig.
Natural history. The male in Singapore was found in the open on a simpoh air leaf (Dillenia suffruticosa). A video of the living juvenile (specimen AS19.2448) is available in
Material examined
Adult male (specimen JK.20.06.20.001), in LKCNHM, from Singapore: Mandai Road, 1.4106°N, 103.7631°E. Y. Ng 20 June 2020. Juvenile (specimen AS19.2448), in
Padillothorax semiostrinus 158–164 male JK.20.06.20.001 from Singapore 158 male left palp, ventral view 159 same, retrolateral view of tibia 160 oblique dorsal-lateral view just below posterior eye, showing sculpturing 161 carapace, dorsal view 162–164 living male (photographs Yongi Ng 2020) 165 juvenile (specimen AS19.2448, Singapore) 166 male (from Taichung, Taiwan, photograph Liu Shu Fen 2020) 167 female (from Taichung, Taiwan, photograph Otto Lee 2020). Scale bars: 0.1 mm (158–160); 1.0 mm (161, 165).
Padillothorax flavopunctus , comb. nov.
Bavirecta flavopuncta Kanesharatnam & Benjamin, 2018: 4–8. figs 1–3.
Notes
This species shares the diagnostic features of the genus, including macrosetae on the middle of the front face of the first femur. P. flavopunctus has two such macrosetae on each femur, as in P. badut and P. mulu, but larger and placed even more proximally. We have concerns that the paratype female (IFS_SAL_679) is not conspecific with the male holotype, but if not conspecific, it is a closely related species, both by its strong morphological similarity (body form, markings) to the male and by its molecular proximity to a juvenile (IFS_SAL_1017) collected alongside the male at the type locality.
Padillothorax badut
Type material
All from Malaysia: Sarawak: Lambir Hills Nat. Pk., and in
Etymology
From the Malay word badut, meaning clown. In the field we called these (and P. mulu) the “banded clowns”. Other names: In
Diagnosis
Very similar to P. mulu, differing most notably in details of genitalia. The embolus of P. badut lacks the prolateral basal ridges and has a longer terminal part; the epigyne has the openings hidden beneath a fold.
Notes
This and the other new Malaysian species (P. mulu) are very similar in appearance, thin and banded; together we consider them as the P. badut species group. When the first legs are held forward in life (e.g., Fig.
Padillothorax badut sp. nov. 168 male left palp, ventral view (holotype IDWM.20007) 169 same, retrolateral view of tibia 170 epigyne, ventral (specimen IDWM.20008) 171 vulva, dorsal 172, 173 Male (SWK12-4688) 174, 175 female (SWK12-4350). Scale bars: on genitalia 0.1 mm; on bodies 1.0 mm.
Description
Male (holotype, specimen IDWM.20007). Carapace length 2.3; abdomen length 3.1. Carapace (Fig.
Female (paratype, specimen IDWM.20008). Carapace length 2.1; abdomen length 3.1. Colour and structure matches that of male in nearly all aspects, with the most distinct difference being the slightly shorter first legs. Cheliceral teeth not examined in this specimen, but another female from Lambir Hills has four retromarginal teeth, together in a mound. Epigyne (Fig.
Natural history
On large-leaved understory plants such as palms. In life, they often hold the front legs out or to the front.
Additional material examined
All from Malaysia: Sarawak: Lambir Hills Nat. Pk., collected 4–6 April 2012 by Maddison/Piascik/Ang, in
Padillothorax mulu
Type material
All from Malaysia: Sarawak: Mulu Nat. Pk., in
Padillothorax mulu sp. nov. 176 male left palp, ventral view (holotype IDWM.20009) 177 same, retrolateral view of tibia 178 epigyne, ventral (specimen SWK12-EP0105) 179 vulva, dorsal 180–183 Male (SWK-2556) 184–187 female (SWK12-2574). Scale bars: on genitalia 0.1 mm; on bodies 1.0 mm.
Etymology
From the name of the type locality. Other names: In
Diagnosis
Very similar to P. badut, differing most notably in details of genitalia. The embolus of P. mulu is shorter, and has a toothed ridge on the prolateral base; the epigyne has the openings exposed on a more or less flat surface.
Description
Male (holotype, specimen IDWM.20009). Carapace length 2.5; abdomen length 3.5. Colour and structure matches that of P. badut as described above. Cheliceral teeth not examined. Palp pale except for base of femur. Base of embolus with various fine teeth; embolus shorter than in P. badut (Fig.
Female (paratype, specimen SWK12-EP0105). Carapace length 2.1; abdomen length 3.3. Colour and structure matches that of P. badut as described above. Cheliceral teeth not examined in this specimen, but another female from Mulu has five retromarginal teeth. Epigyne (Fig.
Natural history
As for P. badut, on large-leaved understory plants. They often hold the front legs out or to the front.
Additional material examined
All from Malaysia: Sarawak: Mulu Nat. Pk., in
The Piranthus Clade (Maripanthus, Piranthus)
Maripanthus , gen. nov.
Type species
Maripanthus draconis Maddison, sp. nov.
Species included
Maripanthus draconis Maddison, sp. nov.
Maripanthus jubatus Maddison, sp. nov.
Maripanthus menghaiensis (Cao & Li, 2016), comb. nov. (transferred from Nannenus)
Maripanthus reinholdae Maddison, sp. nov.
Maripanthus smedleyi (Reimoser, 1929), comb. nov., transferred from Bavia
Etymology
An arbitrary combination of letters, reminiscent of Marpissa (as the females resemble) and Piranthus (to which it is closely related). To be treated grammatically as masculine.
Diagnosis
Epigynal atria long and gaping, anteriorly placed. Embolus long and beginning on the basal side of the tegulum, apparently freely articulated from the tegulum (as in the related Piranthus). Retromarginal cheliceral teeth close together, forming a single short ridge. Male endite with sharp corner (Fig.
Maripanthus draconis
Type material
Holotype male (specimen AS19.2232), two paratype females (specimens AS19.2250 and d547), all in LKCNHM, from Singapore: Bukit Timah Nature Reserve, stream at Jungle Falls Path. 1.3562°N, 103.7748°E to 1.3572°N, 103.7734°E 110–150 m elev. 12 June 2019 Maddison, Morehouse, & Marathe WPM#19-051. Paratype male (specimen WSG018) from Singapore: Nee Soon Swamp Forest. Beating vegetation. 1.39°N, 103.81°E 12 May 2005. W. Maddison, D. Li, I. Agnarsson, J. X. Zhang. WPM#05-015.
Etymology
Greek, δράκων, referring to the fiery colours of the male. Other names: In
Diagnosis
Most similar to M. smedleyi, of which only the female is known; M. draconis differs in having longer epigynal atria (greater than half of epigynal length) and less distinct atrial cliff (“ac”, Fig.
Description
Male (based on holotype, specimen AS19.2232). Carapace length 4.0; abdomen length 5.0. Carapace (Figs
Female (paratype, specimen AS19.2250). Carapace length 4.2; abdomen length 6.1. Carapace (Fig.
Natural history
In Singapore, beating vegetation in forest understory.
Additional material examined
In
Maripanthus jubatus
Type material
Holotype male (specimen NCBS-BN352, also known as AS19.4373) and paratype female (specimen NCBS-BN353, also known as AS19.4996), in NCBS collection, from India: Karnataka: Kodagu: Yavakapadi, Honey Valley area, buildings and roadside, 12.22°N, 75.66°E, 1100 m elev. 23–28 June 2019 W. Maddison & K. Marathe WPM#19-069.
Etymology
Latin, meaning maned or crested, referring to the field of short black setae on the male ocular area. Other names: In WPM’s field or lab notebooks the informal code for this species was “CFMA2”.
Diagnosis
Similar size and body form to M. draconis, but differs most notably in solid dark integument of the carapace and face. Male palp with dramatically long embolus and RTA.
Description
Male (based on holotype, NCBS-BN352). Carapace length 3.9; abdomen length 4.8. Carapace (Figs
Female (based on paratype, NCBS-BN353). Carapace length 4.2; abdomen length 5.1. Carapace black except medium brown areas (yellow in alcohol) around fovea and along margin, covered with yellow cream scales in band along ventral margin, and dorsally on ocular area and anterior part of thorax (Figs
Natural history
Found in dry hanging banana leaves.
Additional material examined
All in NCBS collection. One male (specimen NCBS-BN354, also known as AS19.4403) from India: Karnataka: Kodagu: Yavakapadi, on top of car, 12.2408°N, 75.6547°E, 23 June 2019 K. Marathe WPM#19-068. One male one female (specimens NCBS-BN355 and NCBS-BN355) from India: Karnataka: Kodagu: near Madikeri, Rainforest Retreat, banana plantation, 12.480°N, 75.709°E, 30 June 2019 K. Marathe WPM#19-103.
Maripanthus menghaiensis , comb. nov.
Nannenus menghaiensis Cao & Li, 2016: 82–85, figs 28–29.
Note
Nannenus menghaiensis is here transferred to Maripanthus (and thus to the Baviini) based on its many close similarities with M. reinholdae, which itself is placed in Maripanthus by both morphological and molecular data. M. menghaiensis has an elongate body and pattern of thoracic and abdominal markings very much like those of other baviines (and unlike Nannenus, which is a compact-bodied ground dweller). See Diagnosis of M. reinholdae for distinctions therefrom.
A male (specimen IDWM.20013) that is either M. menghaiensis or a very closely related species is shown in Figs
Maripanthus reinholdae sp. nov. and M. cf. menghaiensis215–222 Maripanthus reinholdae215 male left palp, prolateral view (holotype JK.11.12.24.0006) 216 same, ventral view 217 same, retrolateral view 218 epigyne, ventral (specimen SWK12-1934) 219 vulva, dorsal 220, 221 female SWK12-1934 222 holotype male 223, 224 male M. cf. menghaiensis (specimen IDWM.20013). Scale bars on genitalia 0.1 mm; on body 1.0 mm.
Maripanthus reinholdae
Type material
Holotype
male (specimen JK 11.12.24.0006), in LKCNHM, from Brunei: Ulu Temburong National Park, Canopy Walk Trail, 4.5522°N, 115.1578°E, J. K. H. Koh 24 Dec. 2011. Paratype female (specimen SWK12-1934, in
Etymology
Named in honour of Christa Deeleman-Reinhold, whose extensive work on southeast Asian spiders has greatly increased our knowledge of the area’s fauna. She has discovered and described over 350 new species, including 54 new salticids. Other names: In
Diagnosis
Very similar to M. menghaiensis, and like it smaller and more Indopadilla-like in body form than M. draconis and M. jubatus (shorter first legs, more elongate abdomen). Differs from M. menghaiensis in the longer epigynal openings, and in details of the palp’s bulb. In view from the retrolateral, the embolus is first directed to the distal then quickly turns dorsally (M. menghaiensis, embolus begins toward the dorsal). When the embolus comes out from behind the tegulum it is directed slightly proximally (slightly distally in M. menghaiensis). The embolus is thinner near the tip than in M. menghaiensis.
Description
Male (based on holotype, specimen JK.11.12.24.0006; living holotype shown on p. 208 of
Female (based on paratype, specimen SWK12-1934). Carapace length 3.7; abdomen length 5.0. Carapace as in male, but slightly paler in integument. Chelicerae with three promarginal and five retromarginal teeth. Legs as in male but with first legs only slightly darker than the others. Abdomen brown with central pale chevroned band, and thin white streaks as in male. Epigyne (Fig.
Additional material examined
One female (specimen JK.12.02.04.0010, in LKCNHM) from Brunei: Belait, Trail To Wasai Teraja Secondary Forest, 4.2911°N, 114.4231°E, J. K. H. Koh 4 February 2012.
Maripanthus smedleyi , comb. nov.
Bavia smedleyi
Reimoser, 1929: 130–132, fig. 4, holotype female
Notes
This species is close to M. draconis, with similar body form and markings (Figs
Piranthus
Piranthus Thorell, 1895. Type species Piranthus decorus Thorell, 1895.
Species included
Piranthus api Maddison, sp. nov.
Piranthus bakau Maddison, sp. nov.
Piranthus decorus Thorell, 1895
Piranthus kohi Maddison, sp. nov.
Piranthus mandai Maddison, sp. nov.
Piranthus planolancis Malamel, Nafin, Sudhikumar & Sebastian, 2019
Diagnosis
Carapace surface rugose, with a coarse reticulate sculpturing throughout. Carapace flat (height well less than half the length), with ocular area and front part of thorax on a plane, and fovea well back of PLE, 1.3–1.5 × further from front of carapace than is the back of the PLE. Legs robust, especially the first pair. Embolus begins at prolateral basal corner of bulb; epigyne with central septum. Tip of abdomen black.
Two of the species (P. bakau and P. kohi) are distinctive for their black-and-white banding, three others (P. decorus, P. mandai, and P. planolancis) are more simply marked with brown and black, while the last (P. api) is a red-orange-black ember.
The four new species described here extend the range of Piranthus eastward as far as Borneo. The two previously described species, P. decorus (Thorell 1895;
Piranthus api
Type material
Holotype female (specimen AS19.3205), in LKCNHM, from Singapore: Sungei Buloh Wetland Reserve, near Visitor Centre, 1.440°N, 103.734°E, 19 June 2019 Maddison/Marathe/Morehouse/et al. WPM#19-064.
Etymology
From the Malay word, “api”, meaning “fire”, referring to the colour. Other names: In WPM’s field or lab notebooks the informal code for this species was “PIORG”.
Diagnosis
A distinctively narrow species with bright red-orange legs.
Description
Female (holotype, specimen AS19.3205). Carapace length 3.0; abdomen length 3.5. Carapace narrow and low, orange-red-brown with central black area covering ocular area and medial part of thorax (Figs
Natural history
The two specimens were both found deep within large grass tussocks overhanging a moist ditch. A video of the living holotype is available in
Additional material examined
A second female (specimen AS19.3217, in
Piranthus bakau
Type material
Holotype
male (specimen SWK12-0561, also known as d424), in
Etymology
Referring to the type locality and to the holotype’s habitat, mangroves (Malay, bakau = mangrove). Other names: In
Piranthus bakau sp. nov. 237 male left palp, prolateral view (holotype SWK12-0561) 238 same, ventral view 239 same, retrolateral view 240 epigyne, ventral (specimen AS19.2895) 241 vulva, dorsal 242 male JK.11.04.17.0040 (photograph Joseph K. H. Koh) 243–245 male holotype (SWK12-0561) 246 male holotype left fourth patella and tibia, dorsal view (right leg digitally flipped to appear as left) 247–254 female specimen AS19.2895 247 left fourth patella and tibia, dorsal view 248 left first leg, prolateral view 249 adult female prosoma 250 adult female abdomen 251–254 same specimen while immature. Scale bars on genitalia 0.1 mm; on bodies and legs 1.0 mm.
Diagnosis
This and the closely similar P. kohi differ from other known Piranthus in having white transverse banding on the body and legs, and the posterior legs striped with black and translucent white. P. bakau (Figs
- Second white transverse band on the dorsum of the abdomen (i.e., the first behind the basal band) complete or broken by only a slight space;
- Sides of thorax lacking the three distinct narrow vertical lines seen in P. kohi (at most only a hint of two);
- Carapace lateral to the PLE with a bare patch, lacking golden scales, extending from PME back to behind PLE, and lateral to the bare patch is a stripe of denser golden scales (Figs
245 ,249 ); - First and second tibia bicoloured (black with white tip, arrow in Fig.
248 ); - Second femur bicoloured (white basally, black terminally, Fig.
249 ); - Black dorsal band on the fourth tibia incomplete, beginning mid-segment and reaching to the end (Figs
246 ,247 ). - Carapace slightly flatter than in P. kohi.
- Embolus (Fig.
237 ) notably longer than in P. kohi (Fig.255 ), closely resembling that of P. planolancis (Nafin et al. 2020); - Epigyne with cavernous atria framed by a curved medial ridge, and relatively long copulatory ducts leading to a posterior tangle of tubes and spermathecae.
Juveniles have markings consistent with those of adults, and thus can be distinguished by the non-genitalic features above.
Description
Male (based on holotype, SWK12-0561). Carapace length 2.7; abdomen length 3.0. Carapace with rugose surface, black on ocular area, dark brown otherwise, covered thinly with narrow golden to white scales except bare patch lateral to PLE, and on posterior slope. Clypeus narrow, dark, with a few white setae. Chelicerae small and vertical, dark, with a few pale setae. Palp (Figs
Female (based on specimen AS19.2895). Carapace length 3.0; abdomen length 4.1. The specimen was collected and photographed as a small juvenile (Figs
Male-female matching
P. bakau and P. kohi are similar in general appearance, have overlapping geographical ranges, and have been collected to date with only adult males or adult females at a locality, not both. This leads to a question of which male matches which female. Unless there are additional closely similar species in the same areas, the inferred matching is well supported by the differences in markings, carapace shape, and lengths of embolus/copulatory ducts. The male of P. bakau and the female from Tengkorak inferred to match it share the diagnostic traits mentioned above. Doubt might arise because of one difference in their markings: the female has the second transverse abdominal band more oblique, with its two sides meeting at a central peak, while in the holotype the band is straight across. However, the second male, from Brunei, shows a peak (Fig.
Natural history
Holotype male collected from mangroves; female from Tengkorak collected by shaking vines and understory trees near waterfall.
Additional material examined
Two juveniles with same data as holotype. Also, one male (specimen JK.11.04.17.0040), in LKCNHM, from Brunei: Tutong, Tasek Merimbun, Zone C2, Palau Luba, Sungai Melunchur, 4.5817°N, 114.6872°E, J. K. H. Koh 17 Apr. 2011. One female (specimen AS19.2895), in
Piranthus kohi
Type material
Holotype male (specimen AS19.1813), in LKCNHM, from Singapore: Sungei Buloh Wetland Reserve, 1.440–1.447°N, 103.730–103.735°E, 10 June 2019 Maddison/Morehouse/et al. WPM#19-045. Paratype female (specimen JK 19.07.19.0001), in LKCNHM, from Singapore: Pulau Ubin, Balai Quarry Trail, 1.4178°N, 103.9850°E, leg. P. Ng 19 July 2019.
Piranthus kohi sp. nov. 255 male left palp, prolateral view (holotype AS19.1813) 256 same, ventral view 257 same, retrolateral view 258 epigyne, ventral (specimen JK.12.04.11.0032) 259 Vulva, dorsal 260–265 holotype 266 male holotype left fourth patella and tibia, dorsal view 267 female left fourth patella and tibia, dorsal view (specimen JK.19.07.19.0001) 268 female JK.19.07.19.0001 269 Female JK.12.04.11.0032 (photograph Joseph K. H. Koh). Scale bars: on genitalia 0.1 mm; on bodies and legs 1.0 mm.
Etymology
This elegant species is named in honour of Joseph Koh Kok Hong, arachnologist, conservationist, and diplomat. Koh has worked tirelessly to build peace with nature. Through his collecting and excellent books (
Diagnosis
P. kohi (Figs
- Three distinct vertical lines on each side of the thorax;
- Carapace lateral to the PLE more or less uniformly covered in white to gold setae;
- Second transverse dorsal band of the abdomen well broken at the middle;
- First and second tibiae and femora solid dark, not bicoloured;
- Black dorsal stripe extends the full length of tibia 4 (Figs
266 ,267 ); - Shorter embolus and copulatory ducts.
Juveniles can be distinguished by the non-genitalic features above.
Description
Male (based on holotype, AS19.1813). Carapace length 2.5; abdomen length 2.5. Carapace with rugose surface, black, dusted above with narrow golden scales. Sides and back of thorax bare except for three narrow and distinct vertical lines of pale setae. Clypeus black. Chelicera vertical and black. Palp black except for white cymbium. Embolus arising on prolateral basal corner, proceeding ventrally then curving distally (Figs
Female (based on specimen, 12.04.11.0032). Carapace length 3.4; abdomen length 4.0. Structure and markings as in male, but generally more reddish, especially first and second legs, which are red-orange-brown in the femur and patella (and tibia of the second pair). Epigyne (Fig.
Male-female matching
See comments under P. bakau. Male and female P. kohi share the diagnostic traits mentioned above. The matching is supported by both males and females occurring in Singapore and in similar mangrove habitats – eight specimens from Sungei Buloh including 4 males; 6 specimens from Palau Ubin including 2 females.
Natural history
Specimens in Singapore were found beating trees and vines in a mangrove area. It appeared that our greatest success in finding them was when shaking woody vines. Their motion when alive has a different sense than other baviines; rather than the sharply-jumping Indopadilla, or the frequently waving Padillothorax badut group, or the more sedate Piranthus planolancis and P. api, P. kohi is constantly flicking up and down the first legs, palps, and abdomen, somewhat like ant mimicking salticids. A video of the living holotype is available in
Additional material examined
Singapore: Sungei Buloh Wetland Reserve, 1.440–1.447°N, 103.730–103.735°E, 10 June 2019 Maddison/Morehouse/et al. WPM#19-045 (3 additional males raised in captivity, 2 juveniles,
Piranthus mandai
Type material
Holotype male (specimen JK.91.05.31.0001), in LKCNHM, from Singapore: Mandai Track 15 Trail, 1.4106°N, 103.7783°E, J K H Koh 31 May 1991.
Etymology
Named for the type locality. Other names: In lab notebooks the informal code for this species was “SGOMG”.
Diagnosis
In colouration similar to P. planolancis, browns and blacks, but with body more compact and robust, as in P. bakau and P. kohi. Embolus with only a small loop before proceeding distally, and thus P. mandai is second in sequence from least to most rotated embolic bases: P. kohi (Fig.
Description
Male (holotype, specimen JK.91.05.31.0001). Carapace length 2.7; abdomen length 2.5. Carapace with rugose surface, black and dark brown, with a sparse covering of narrow scales that is more or less uniform: there is no bare patch beside the PLE, and no distinct three vertical thoracic lines, but there is a slight condensation of scales into a single vertical thoracic line, similar to that in P. bakau. Clypeus narrow and dark. Chelicerae vertical, dark, with a few pale scales basally. Palp brown. Palp similar to that of P. planolancis, but RTA much shorter (Figs
Tribe Viciriini Simon, 1901
Nungia
Nungia Zabka, 1985. Type species Nungia epigynalis Żabka, 1985.
Note
At least 16 species of elongate dull brown salticids collected in field work in Asia and New Guinea were initially thought to be baviines (example photographs, Figs
Nungia hatamensis (Thorell, 1881), comb. nov., transferred from Diplocanthopoda Abraham, 1925. See Fig.
Nungia modesta (Keyserling, 1883), comb. nov., transferred from Bavia. Based on
Nungia papakula (Strand, 1911), comb. nov., transferred from Bavia. Male and female syntypes in
Nungia xiaolonghaensis (Cao & Li, 2016), comb. nov., transferred from Cosmophasis Simon, 1901. See Fig.
Some of these new combinations, and others made above in baviines, correct mistaken placements that may have resulted from convergence in the general form of the male palp. The palps of Nungia hatamensis, Nungia xiaolonghaensis, Maripanthus menghaiensis, Bavia capistrata, and Bavia maurerae do in fact resemble those of the genera in which they had been placed, respectively, Diplocanthopoda (a hasariine), Cosmophasis (a chrysilline), Nannenus (a nannenine), Evarcha (a plexippine), and Epidelaxia (a nannenine). Those genera are all in the Saltafresia, a group phylogenetically distinct from the astioids and baviines. The resemblance is largely restricted to the palps, as the remainder of the body is quite distinct in each case. Convergent evolution of the basic form of the palp is widespread in salticids, insofar as the palps are simple and variation in a single dimension (embolus length) can generate palps that look superficially quite similar. The general shape of the palp should be used with caution in determining relationships.
Species incertae sedis
The following species are too poorly known to assign to a known baviine genus, or for that matter to confirm their placement in the Baviini:
Bavia albolineata Peckham & Peckham, 1885
Bavia decorata (Thorell, 1890)
Bavia hians (Thorell, 1890)
Bavia sinoamerica Lei & Peng, 2011
Of these, B. sinoamerica is almost certainly not a baviine by body shape. Based on the form of the palp, and its being unident with a compact body, it may be a hasariine. B. albolineata has a palp that is credibly baviine, but its location in Madagascar and diverging chelicerae suggest it is not baviine.
Nungia and Capeyorkia species and their phylogenetic placement, using informal species code names; all males except 277, female. 275 Nungia sp. “NUMUL”, Sarawak (specimen SWK12-1943) 276 Nungia sp. “NSGPQ”, Singapore (specimen d178) 277 Nungia sp. “NUBWH”, Sarawak (specimen SWK12-3204) 278 Nungia sp. “NPNGK”, Papua New Guinea (specimen d259) 279 Nungia xiaolonghaensis, Pahang (specimen MRB078) 280 Nungia hatamensis, Papua New Guinea (specimen d260) 281 Nungia papakula, Papua New Guinea, 9.436°S, 147.364°E (specimen 2008PNG-3538) 282 Capeyorkia sp. “NPNGE”, Papua New Guinea (specimen d261) 283 molecular phylogeny placing Nungia species and related (in bold) within the viciriine astioids, based on IQ-TREE maximum likelihood analysis (50 replicates, partitioned) of 28S and 16SND1 sequences, with bootstrap percentages from 500 replicates (shown only within the Astioida).
Acknowledgements
This work has been greatly aided by many who have offered help over many years and much field work. Joseph Koh offered loan of material, use of photographs, advice, and assistance in the field. Daiqin Li organized and participated in field work in Singapore. The staff and facilities of NCBS in Bengaluru assisted with specimen processing and data gathering. For assistance with collecting and logistics we thank in addition Edyta Piascik, Ch'ien Lee, N. Morehouse, D. Outomuro Priede, J. Sung, Peifen Koh, Chris Ang, Liew Qi, and Emmanuel Goh. For permission to collect we thank Jayasri Lakshminarayanan and NPARKS (Singapore), the Sarawak forestry department, Suresh Chengappa of Honey Valley (Yavakapadi), and Anurag and Sujata Goel of the Rainforest Retreat (Madikeri). Acknowledgements for older field work are given by
References
- Ali PAA, Maddison WP, Zahid M, Butt A (2018) New chrysilline and aelurilline jumping spiders from Pakistan (Araneae: Salticidae). ZooKeys 783: 1–15. https://doi.org/10.3897/zookeys.783.21985
- Andriamalala D (2007) Revision of the genus Padilla Peckham & Peckham, 1894 (Araneae: Salticidae) – convergent evolution of secondary sexual characters due to sexual selection and rates of molecular evolution in jumping spiders. Proceedings of the California Academy of Sciences 58: 243–330.
- Berry JW, Beatty JA, Prószyński J (1997) Salticidae of the Pacific Islands. II. Distribution of nine genera, with descriptions of eleven new species. Journal of Arachnology 25: 109–136.
- Bodner MR, Maddison WP (2012) The biogeography and age of salticid spider radiations (Araneae: Salticidae). Molecular Phylogenetics and Evolution 65: 213–240. https://doi.org/10.1016/j.ympev.2012.06.005
- Caleb JTD, Sankaran PM, Nafin KS, Acharya S (2019) Indopadilla, a new jumping spider genus from India (Araneae: Salticidae). Arthropoda Selecta 28: 567–574. https://doi.org/10.15298/arthsel.28.4.10
- Caleb JTD, Sanap RV (2017) Rediscovery of Piranthus decorus Thorell 1895 (Araneae: Salticidae) after 122 years since the original description. Acta Arachnologica 66: 25–29. https://doi.org/10.2476/asjaa.66.25
- Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology Evolution 17: 540–552. https://doi.org/10.1093/oxfordjournals.molbev.a026334
- Derkarabetian S, Starrett J, Tsurusaki N, Ubick D, Castillo S, Hedin M (2018) A stable phylogenomic classification of Travunioidea (Arachnida, Opiliones, Laniatores) based on sequence capture of ultraconserved elements. ZooKeys 760: 1–36. https://doi.org/10.3897/zookeys.760.24937
- Edwards GB (2006) A review of described Metacyrba, the status of Parkella, and notes on Platycryptus and Balmaceda, with a comparison of the genera (Araneae: Salticidae: Marpissinae). Insecta Mundi 19: 193–226.
- Faircloth BC (2013) illumiprocessor: a trimmomatic wrapper for parallel adapter and quality trimming.
- Faircloth BC (2016) PHYLUCE is a software package for the analysis of conserved genomic loci. Bioinformatics 32: 786–788. https://doi.org/10.1093/bioinformatics/btv646
- Faircloth BC (2017) Identifying conserved genomic elements and designing universal probe sets to enrich them. Methods in Ecology & Evolution 8: 1103–1112. https://doi.org/10.1111/2041-210X.12754
- Hedin M, Derkarabetian S, Ramírez M, Vink C, Bond J (2018) Phylogenomic reclassification of the world’s most venomous spiders (Mygalomorphae, Atracinae), with implications for venom evolution. Scientific Reports 8(1636D): 1–7. https://doi.org/10.1038/s41598-018-19946-2
- Kanesharatnam N, Benjamin SP (2018) A new genus and three new species of jumping spiders (Araneae: Salticidae) from Sri Lanka. European Journal of Taxonomy 444: 1–24. https://doi.org/10.5852/ejt.2018.444
- Katoh D, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. https://doi.org/10.1093/molbev/mst010
- Keyserling E (1883) Die Arachniden Australiens, nach der Natur beschrieben und abgebildet [Erster Theil, Lieferung 31]. Bauer & Raspe, Nürnberg, 1421–1489. [pls 120–123]
- Koh JKH (1989) A Guide to Common Singapore Spiders, Singapore Science Centre.
- Koh JKH, Bay N (2019) Borneo Spiders: A photographic field guide. Sabah Forestry Department, city, 498 pp.
- Koh JKH, Leong TM (2013) Spiders of Brunei Darussalam. Natural History Publications (Borneo), Kota Kinabalu, 358 pp.
- Kulkarni S, Wood H, Lloyd M, Hormiga G (2019) Spider-specific probe set for ultraconserved elements offers new perspectives on the evolutionary history of spiders (Arachnida, Araneae). Molecular Ecology Resources 20: 185–203. https://doi.org/10.1111/1755-0998.13099
- Maddison WP (2009) New cocalodine jumping spiders from Papua New Guinea (Araneae: Salticidae: Cocalodinae). Zootaxa. 2021: 1–22. https://doi.org/10.11646/zootaxa.2021.1.1
- Maddison WP (2015a) A phylogenetic classification of jumping spiders (Araneae: Salticidae). Journal of Arachnology 43: 231–292. https://doi.org/10.1636/arac-43-03-231-292
- Maddison WP (2015b) Salticid images. http://salticidae.org/salticidImages/ [Accessed 28 July 2020]
- Maddison WP (2020) Baviine jumping spider videos. https://www.youtube.com/watch?v=Q0pI-0vCBvg [Accessed 10 August 2020]
- Maddison WP, Bodner MR, Needham K (2008) Salticid spider phylogeny revisited, with the discovery of a large Australasian clade (Araneae: Salticidae). Zootaxa. 1893: 49–64. https://doi.org/10.11646/zootaxa.1893.1.3
- Maddison WP, Hedin MC (2003) Jumping spider phylogeny (Araneae: Salticidae). Invertebrate Systematics 17: 529–549. https://doi.org/10.1071/IS02044
- Maddison DR, Maddison WP (2020) Zephyr: a Mesquite package for interacting with external phylogeny inference programs. Version 3.1.
- Maddison WP, Maddison DR (2019) Mesquite: a modular system for evolutionary analysis. Version 3.61 http://www.mesquiteproject.org
- Maddison WP, Needham K (2006) Lapsiines and hisponines as phylogenetically basal salticid spiders (Araneae: Salticidae). Zootaxa 1255: 37–55.
- Maddison WP, Li DQ, Bodner MR, Zhang JX, Xu X, Liu QQ (2014) The deep phylogeny of jumping spiders (Araneae, Salticidae). ZooKeys 440: 57–87. https://doi.org/10.3897/zookeys.440.7891
- Maddison WP, Maddison DR, Derkarabetian S, Hedin M (2020) Sitticine jumping spiders: phylogeny, classification, and chromosomes (Araneae, Salticidae, Sitticini). ZooKeys 925: 1–54. https://doi.org/10.3897/zookeys.925.39691
- Maddison WP, Zhang JX (2011) Salticid spiders of Papua New Guinea. In: Richards SJ, Gamui BG (Eds) Rapid Biological Assessments of the Nakanai Mountains and the upper Strickland Basin: surveying the biodiversity of Papua New Guinea’s sublime karst environments. RAP Bulletin of Biological Assessment 60. Conservation International. Arlington, VA, 186–189.
- Malamel JJ, Sankaran PM, Sebastian PA (2015) First record of the jumping spider genus Bavia Simon, 1877 from India, with the description of a new species. Zootaxa 4007: 596–599. https://doi.org/10.11646/zootaxa.4007.4.11
- Malamel JJ, Nafin NS, Sudhikumar AV, Sebastian PA (2019) Two new species of the jumping spiders (Araneae: Salticidae) from the genera Epeus Peckham et Peckham, 1886 and Piranthus Thorell, 1895 from India. Arthropoda Selecta 28: 267–276. https://doi.org/10.15298/arthsel.28.2.10
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. https://doi.org/10.1093/molbev/msu300
- Nurk S, Bankevich A, Antipov D, Gurevich A, Korobeynikov A, Lapidus A, Prjibelsky A, Pyshkin A, Sirotkin A, Sirotkin Y, Stepanauskas R, McLean J, Lasken R, Clingenpeel SR, Woyke T, Tesler G, Alekseyev MA, Pevzner PA (2013) Assembling Genomes and Mini-metagenomes from Highly Chimeric Reads. In: Deng M, Jiang R, Sun F, Zhang X (Eds) Research in Computational Molecular Biology. RECOMB 2013. Lecture Notes in Computer Science, vol 7821. Springer, Berlin/Heidelberg, 344 pp. https://doi.org/10.1007/978-3-642-37195-0_13
- Prószyński J (1984) Atlas rysunków diagnostycznych mniej znanych Salticidae (Araneae). Zeszyty Naukowe Wyższej Szkoły Rolniczo-Pedagogicznej w Siedlcach 2: 1–177.
- Prószyński J (1987) Atlas rysunków diagnostycznych mniej znanych Salticidae 2. Zeszyty Naukowe Wyższej Szkoly Rolniczo-Pedagogicznej, Siedlcach, 172 pp.
- Prószyński J, Deeleman-Reinhold CL (2013) Description of some Salticidae (Araneae) from the Malay Archipelago. III. Salticidae of Borneo, with comments on adjacent territories. Arthropoda Selecta 22(2): 113–144. https://doi.org/10.15298/arthsel.22.2.02
- Prószyński J (2018) Review of genera Evarcha and Nigorella, with comments on Emertonius, Padilothorax [sic], Stagetillus, and description of five new genera and two new species (Araneae: Salticidae). Ecologica Montenegrina 16: 130–179. https://doi.org/10.37828/em.2018.16.12
- Reimoser E (1927) Spinnen von Sumatras Ostküste. Miscellanea Zoologica Sumatrana 13: 1–6.
- Simon E (1901a) On the Arachnida collected during the Skeat expedition to the Malay Peninsula. Proceedings of the Zoological Society of London 71(1): 45–84. https://doi.org/10.1111/j.1469-7998.1901.tb08164.x
- Simon E (1901b) Histoire naturelle des araignées. Deuxième édition, tome second. Roret, Paris, 381–668.
- Stamatakis A (2014) RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
- Starrett J, Derkarabetian S, Hedin M, Bryson Jr RW, McCormack JE, Faircloth BC (2017) High phylogenetic utility of an Ultraconserved element probe set designed for Arachnida. Molecular Ecology Resources 17: 812–823. https://doi.org/10.1111/1755-0998.12621
- Strand E (1911) Araneae von den Aru- und Kei-Inseln. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 34: 127–199.
- Talavera G, Castresana J (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology 56: 564–577. https://doi.org/10.1080/10635150701472164
- Żabka M (1988) Salticidae (Araneae) of Oriental, Australian and Pacific regions, III. Annales Zoologici (Warszawa) 41: 421–479.
- Zhang JX, Maddison WP (2013) Molecular phylogeny, divergence times and biogeography of spiders of the subfamily Euophryinae (Araneae: Salticidae). Molecular Phylogenetics and Evolution 68: 81–92. https://doi.org/10.1016/j.ympev.2013.03.017
- Zhang JX, Maddison WP (2014) Tisaniba, a new genus of marpissoid jumping spiders from Borneo (Araneae: Salticidae). Zootaxa 3852: 252–272. https://doi.org/10.11646/zootaxa.3852.2.5
Supplementary material
Blended spider and arachnid UCE probe set
Data type: UCE probes
Explanation note: File blending the