Data Paper |
|
Corresponding author: Stefano Schiaparelli ( stefano.schiaparelli@unige.it ) Academic editor: Kai Horst George
© 2020 Guido Bonello, Marco Grillo, Matteo Cecchetto, Marina Giallain, Antonia Granata, Letterio Guglielmo, Luigi Pane, Stefano Schiaparelli.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Bonello G, Grillo M, Cecchetto M, Giallain M, Granata A, Guglielmo L, Pane L, Schiaparelli S (2020) Distributional records of Ross Sea (Antarctica) planktic Copepoda from bibliographic data and samples curated at the Italian National Antarctic Museum (MNA): checklist of species collected in the Ross Sea sector from 1987 to 1995. ZooKeys 969: 1-22. https://doi.org/10.3897/zookeys.969.52334
|
Abstract
Distributional data on planktic copepods (Crustacea, Copepoda) collected in the framework of the IIIrd, Vth, and Xth Expeditions of the Italian National Antarctic Program (PNRA) to the Ross Sea sector from 1987 to 1995 are here provided. Sampling was performed with BIONESS and WP2 nets at 94 sampling stations at depths of 0–1,000 m, with a special focus on the Terra Nova Bay area. Altogether, this dataset comprises 6,027 distributional records, out of which 5,306 were obtained by digitizing original data reports and 721 are based on physical museum vouchers curated by the Italian National Antarctic Museum (
Keywords
abundance, biogeography, BIONESS, distribution, museum collection, Terra Nova Bay
Introduction
The study of planktic copepods in the Ross Sea represented one of the earliest scientific efforts and targets of the first oceanographic expeditions of the Italian National Antarctic Research Program (PNRA), which started in 1985. One of the underlying reasons for this dedication was the fact that, at the time of sampling, there was a general lack of exhaustive and accessible literature about Copepoda for the Ross Sea region. Therefore, specific sampling activities were planned to define the copepod community structure and establish a reference baseline for comparisons with future research findings (
Copepods are one of the key groups in marine trophic chains, representing up to 70% of the mesozooplanktic biomass, a condition typically found in all Antarctic seas (
During the first Italian oceanographic expeditions in the Ross Sea, it was therefore natural to focus on copepods, and specifically on their distribution (
The present copepod dataset from the Ross Sea is the eighth
This dataset also represents an Italian contribution to the CCAMLR CONSERVATION MEASURE 91-05 (2016) for the Ross Sea region Marine Protected Area, specifically, addressing Annex 91-05/C (“long-term monitoring of benthic ecosystem functions”).
Project description
Project title: Distributional records of Ross Sea (Antarctica) planktic Copepoda from bibliographic data and samples curated at the Italian National Antarctic Museum (
Curator and promoter: Stefano Schiaparelli.
Personnel: Bonello Guido, Marco Grillo, Matteo Cecchetto, Marina Giallain, Antonia Granata, Letterio Guglielmo, Luigi Pane, Stefano Schiaparelli.
Funding: Data originated in the framework of the first three Italian Antarctic Oceanographic expeditions carried out from 1988 to 1995 within 3 different research projects funded by the PNRA:
– IIIrd Italian Antarctic expedition (1987/1988), Project: “Zooplancton – distribuzione spaziale e verticale delle comunità zooplanctoniche nella Baia di Terra Nova (Mare di Ross) con particolare riferimento al Krill”; Project code 2.1.4.2.; R/V “Polar Queen”; Scientific coordinator: Prof. Letterio Guglielmo.
– Vth Italian Antarctic expedition (1989/1990), Project: “Campagna oceanografica nel mare di Ross”; R/V “Cariboo”; Scientific coordinator: Prof. Letterio Guglielmo.
– Xth Italian Antarctic expedition (1994–1995), Project: “Ecologia zooplancton e micronecton”; Project code (6.9); R/V ”Malippo”; Scientific coordinator: Dr. Riccardo Cattaneo-Vietti
The Italian National Antarctic Museum (
Design description
As the dataset here presented was assembled from data and vouchers collected in the framework of different oceanographic expeditions, which had multiple scientific targets and deployed a variety of sampling gears to investigate the water column physical features and plankton diversity, we briefly introduce the general motivations and scopes of each one of these PNRA expeditions.
The oldest records of the dataset correspond to samples collected during the IIIrd Italian Antarctic expedition in 1987–1988, only two years after the opening of the Italian research station “Mario Zucchelli” (called “Terra Nova” at that time). This was also the first Italian Antarctic oceanographic expedition, and since there was practically no previous information on the study site (Terra Nova Bay, Ross Sea), the objective of this expedition was to define the spatial and temporal variability of physical, chemical and biological characteristics in this area (
The second oceanographic expedition (Vth Italian Antarctic Expedition) took place two years later and investigated a larger geographic area in the Pacific sector of the Southern Ocean. The larger scale of the geographic study site reflected the more ambitious (compared to the first expedition) objectives of the expedition, aiming at achieving a better understanding of the functioning of the Antarctic pelagic ecosystems, through the study of hydrodynamic features (
Finally, the third oceanographic expedition (Xth Italian Antarctic Expedition) was carried out during the austral summer of 1994–1995. Most of the sampling was conducted along the 175° meridian, from the northern continental slope to the Ross Sea Ice Shelf. The main purpose of the expedition was to further investigate the effects of the ice-edge retreat on primary production (
This dataset is not only important for the history of Italian research in Antarctica, but, as it dates back to 1987, it also provides a source of historical data, hence representing a useful baseline to measure possible changes and shifts in copepod abundance and diversity in the Ross Sea area that may have occurred in the meantime. At the same time, as the highly ambitious scopes of those expeditions also lead to the production, for the same sampling stations, of an extensive amount of biological and chemical-physical information, as well as about other taxa (Table
Type of sampling and main bibliographic references about the the IIIrd, Vth, and Xth Expeditions of the Italian National Antarctic Program (PNRA).
| Type of data | References |
|---|---|
| Water column communities | |
| Bacterioplankton and heterotrophic bacteria |
|
| Picoplankton |
|
| Phytoplankton |
|
| Zooplankton |
|
| Microzooplankton | ( |
| Physical variables | |
| Temperature |
|
| Practical salinity, density excess, and potential temperature |
|
| Salinity |
|
| Nutrients, dissolved oxygen, pH, total alkalinity, and total inorganic carbon |
|
| Biological variables | |
| Particulate organic matter |
|
| Total suspended matter, particulate carbohydrates, proteins, and lipids |
|
| Total and fractioned photosynthetic pigments concentration and primary production |
|
Methods
Study extent and sampling description
As the distributional information provided in this data paper (Fig.
Sampling stations for IIIrd (yellow), Vth (blue), and Xth (red) expedition a overview of spatial extent in Antarctica b sampling stations in the Western Ross Sea c focus on Terra Nova Bay sampling stations. This map was produced using the collection of datasets “Quantarctica” (
For the IIIrd PNRA Expedition (first Italian Antarctic Expedition), 32 sampling stations located mainly in Terra Nova Bay between 72°S and 75°S of latitude and 163°E and 173°E of longitude (from 05/01/1988 to 21/02/1988) were investigated (
The majority of mesozooplankton samples from this dataset (i.e., those from the IIIrd and the Vth PNRA Expeditions) were collected using an Eznet-BIONESS multi-net (Fig.
The Eznet-BIONESS was equipped with a KMS II (ME Meerestechnik Elektronik GmbH) multiparametric probe (that recorded temperature, salinity, depth, light attenuation, and oxygen concentration) and two acoustic doppler flowmeters (SM 21H-ME Meerestechnik Elektronik GmbH), put inside and outside the filtering apparatus, that recorded speed, in- and out-flow through the net, filtration efficiency, and net number. Different mesh sizes were used during the sampling activity but, regarding Copepoda, only 500 µm and 250 µm sizes were considered.
Another sampling device employed during these PNRA activities (Xth PNRA Expedition) was a Working Party II (WP2 – UNEP FAO) standardized net (
More details about the sampling methodologies and procedures adopted during the IIIrd and Vth PNRA Expeditions can be found in
All samples collected during the three campaigns were preserved on board in a 4% buffered formaldehyde seawater solution and later dispatched to various experts (see below) for determination. Specimens now present in the collections of the Italian National Antarctic Museum are stored in 96% Ethanol.
Spatial coverage
General geographic description: The study area covers a large portion of the north-western Ross Sea, spanning from the Drygalski Ice Tongue in Terra Nova Bay to the continental slope surrounding the Central Basin. Some sampling stations were located at the Balleny Islands and other northern areas (Fig.
Coordinates: Latitude bounding coordinates: -61.99067 and -75.40556; Longitude bounding coordinates: 161.82867 and -177.74167
Temporal coverage
05 January 1988 to 11 February 1995.
Dataset description and quality control
Title: Distributional records of Ross Sea (Antarctica) planktic Copepoda from bibliographic data and samples curated at the Italian National Antarctic Museum (
Character encoding: UTF-8;
Format name: Darwin Core Archive format;
Distribution: https://doi.org/10.15468/zndaaw
Language: English;
Metadata language: English;
License of use: This dataset [Distributional records of Ross Sea (Antarctica) planktic Copepoda from bibliographic data and samples curated at the Italian National Antarctic Museum (
Date of metadata creation: 10 Feb. 2020;
This dataset comprises a total of 6,027 distributional records, out of which 5,306 were obtained by digitizing original data reports (hereafter “literature records”) and 721 are based on physical museum vouchers (hereafter “
All literature records (defined by the term ‘HumanObservation’ under the column ‘BasisOfRecord’) were manually extracted from five different data reports published in 1990, 1992, and 2002 (
As the two different types of distributional data, i.e.,
All data were then gathered in a single dataset formatted to fulfil the Darwin Core standard protocol (
The Darwin Core elements included in the dataset are: occurrenceID, BasisOfRecord (HumanObservation for the bibliographic records and PreservedSpecimen for the museum specimen records), type (identifying the nature of the resource), scientificName (the name in the lowest taxonomic rank identified and updated according to WoRMS with authorship and date for the records identified at the species level), order, family, genus, specificEpithet, scientificNameAuthorship (corresponding to the updated taxonomy according to WoRMS, together with the previous four elements), originalNameUsage (the original identification as reported in the bibliographic resource), identificationQualifier (the qualifier for the uncertainty of identification, following
Taxonomic coverage
The Copepoda diversity of the dataset is displayed in 6,027 records, among which Calanoida represent the most frequent (80%), followed by Cyclopoida (15.3%) and unidentified Copepoda (4.6%). Only five records belong to Harpacticoida and one to Siphonostomatoida. Regarding the life stages identification, the data set is composed of most adults (52.4%), followed by copepodites (45.5%), nauplii (0.7%), and unreported (1.4%). The three campaigns (IIIrd, Vth, and Xth) data report analysis produced a combined total of 5,306 literature reports divided among 52 morphological units. Among these, 26 species belong to three orders (Calanoida, Cyclopoida, Harpacticoida) and 17 families. Overall, Calanoida were the most frequently found (78.5%), followed by Cyclopoida (16.25%) and Harpacticoida (0.07%). In terms of sampling frequency, among the determined specimens, members of family Metridinidae were the most common (25.56%), followed by Euchaetidae (23.11%), Calanidae (21.19%), and Oithonidae (10.6%); the other 14 families accounted for the remaining 19.53% (Fig.
Taxonomic rank
Kingdom: Animalia
Phylum: Arthropoda
Class: Maxillopoda
Order: Calanoida, Cyclopoida, Harpacticoida, Siphonostomatoida
Families: Acartiidae, Aetideidae, Augaptilidae, Bathypontiidae, Calanidae, Candaciidae, Clausocalanidae, Eucalanidae, Euchaetidae, Harpacticidae, Heterorhabdidae, Lubbockiidae, Lucicutiidae, Metridinidae, Oithonidae, Oncaeidae, Paracalanidae, Phaennidae, Rataniidae, Rhincalanidae, Scolecitrichidae, Spinocalanidae, Stephidae, Tharybidae, Tisbidae
Genera: Aetideopsis, Aetideus, Amallothrix, Calanoides, Calanus, Calocalanus, Candacia, Cephalophanes, Chiridiella, Chiridius, Chirundina, Clausocalanus, Cornucalanus, Ctenocalanus, Euaugaptilus, Eucalanus, Euchirella, Farrania, Gaetanus, Haloptilus, Harpacticus, Heterorhabdus, Lubbockia, Lucicutia, Metridia, Microcalanus, Oithona, Oncaea, Onchocalanus, Paracalanus, Paracomantenna, Paraeuchaeta, Paraheterorhabdus, Paralabidocera, Phaenna, Pleuromamma, Pontoptilus, Pseudeuchaeta, Pseudhaloptilus, Pseudoamallothrix, Pseudochirella, Racovitzanus, Ratania, Rhincalanus, Scaphocalanus, Scolecithricella, Spinocalanus, Stephos, Temorites, Tisbe, Triconia, Undinella
Species: Aetideopsis antarctica, Aetideopsis minor, Aetideus australis, Aetideus pseudarmatus, Amallothrix gracilis, Amallothrix dentipes, Calanoides acutus, Calanoides carinatus, Calanus propinquus, Candacia falcifera, Cornucalanus robustus, Ctenocalanus vanus, Euaugaptilus laticeps, Euchirella rostromagna, Euchirella rostrata, Farrania frigida, Gaetanus tenuispinus, Gaetanus inermis, Gaetanus brevispinus, Gaetanus minor, Haloptilus ocellatus, Harpacticus furcifer, Heterorhabdus austrinus, Heterorhabdus pustulifer, Heterorhabdus tanneri, Lucicutia ovalis, Lucicutia wolfendeni, Lucicutia magna, Lucicutia intermedia, Lucicutia curta, Lucicutia macrocera, Metridia gerlachei, Metridia curticauda, Microcalanus pygmaeus, Oithona frigida, Oithona similis, Oncaea curvata, Onchocalanus magnus, Paraeuchaeta antarctica, Paraeuchaeta similis, Paraeuchaeta exigua, Paraeuchaeta comosa, Paraeuchaeta kurilensis, Paraheterorhabdus farrani, Paralabidocera antarctica, Pleuromamma robusta, Pleuromamma gracilis, Pleuromamma abdominalis, Pontoptilus ovalis, Pseudhaloptilus eurygnathus, Pseudoamallothrix ovata, Pseudochirella hirsuta, Pseudochirella notacantha, Racovitzanus antarcticus, Ratania atlantica, Rhincalanus gigas, Scaphocalanus subbrevicornis, Scaphocalanus magnus, Scaphocalanus vervoorti, Scaphocalanus affinis, Scaphocalanus brevicornis, Scolecithricella minor, Spinocalanus abyssalis, Spinocalanus magnus, Spinocalanus horridus, Spinocalanus brevicaudatus, Stephos longipes, Temorites brevis, Triconia conifera, Triconia antarctica, Undinella simplex
History of the Copepoda collection
The Antarctic copepods sampled during the three expeditions were studied by different research groups and experts in different times. The IIIrd expedition samples were determined and studied by T.Z. Sertorio, P. Salemi Picone, P. Bernat, E. Cattini, C. Ossola, A. M. Carli, L. Pane, and G.L. Mariottini (
Other samples from the Vth expedition were also studied and later published by (
Mimocalanus cultrifer (Farran, 1908); Mimocalanus inflatus (Davis, 1949); Spinocalanus antarcticus (Wolfenden, 1906); Spinocalanus spinipes (Brodsky, 1950); Spinocalanus spinosus (Farran, 1908); Chiridiella megadactyla (Bradford, 1971); Gaetanus antarcticus (Wolfenden, 1905); Pseudochirella elongata (Wolfenden, 1905); Cornucalanus antarcticus (Brodsky & Zvereva, 1950); Lophotrix simplex (Wolfenden, 1911); Mixtocalanus alter (Farran, 1929); Mixtocalanus vervoorti (Park, 1980); Scaphocalanus antarcticus (Park, 1982); Scaphocalanus echinatus (Farran, 1905); Scaphocalanus farrani (Park, 1982); Scaphocalanus parantarcticus (Park, 1982); Scolecithricella cenotelis (Park, 1980); Scolecithricella dentipes (Vervoort, 1951); Scolecithricella emarginata (Farran, 1905); Scolecithricella ovata (Farran, 1905); Temora sp.; Undinella acuta (Vaupel-Klein, 1970); Hemirhabdus sp.; Heterostylites longicornis (Giesbrecht, 1889); Euaugaptilus antarcticus (Wolfenden, 1911); Euaugaptilus nodifrons (Sars, 1905); Haloptilus oxycephalus (Giesbrecht, 1889); Pachyptilus pacificus (Johnson, 1936).
Finally, the whole
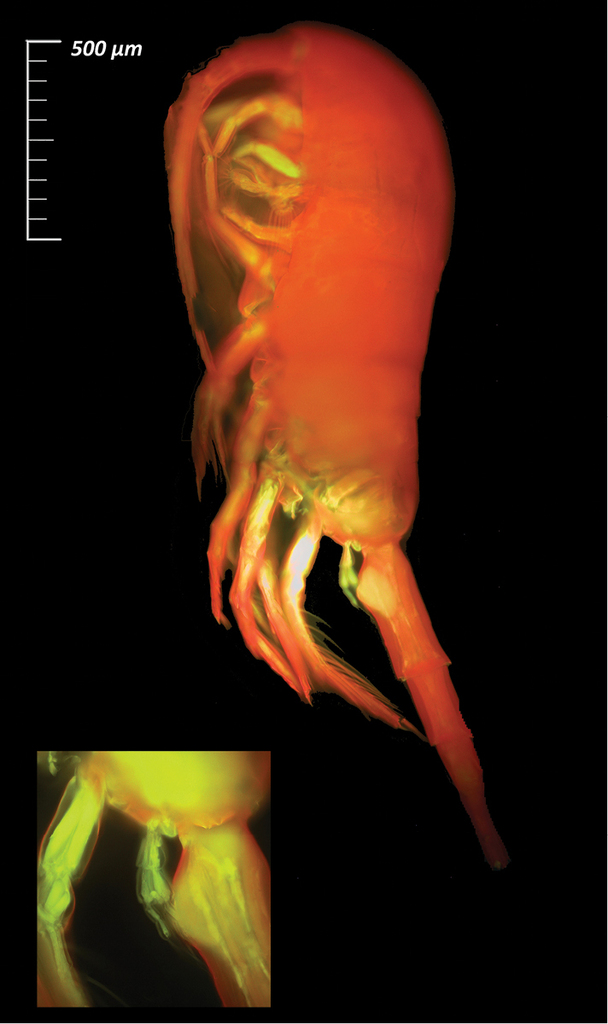
Copepod image acquisition
For all the species listed in this data paper, a selection of complete specimens was prepared to produce high-quality images and highlight taxonomical characters necessary for species identification. For this purpose, different imaging techniques were applied: i) Scanning Electron Microscopy (SEM) after gold coating (e.g., for Paraeuchaeta exigua (Wolfenden, 1911), Fig.
Paraeuchaeta exigua (Copepoda, Calanoida; female,
Geographic data and new distributional records
To evaluate the number of potential new records for a given area, defined as new occurrences in the Global Biodiversity Information Facility repository (GBIF, https://www.gbif.org) for that area, we have used the spocc (version 1.0.8) R package, as well as the online Copepod database provided by the Banyuls sur mer observatory (
Metridia gerlachei (Copepoda, Calanoida; female,
To our knowledge, regarding the Ross Sea area and its boundaries, 62% of the species reported (n = 71) in this data paper represent new records for GBIF for the Western Ross Sea sector and 28% for the whole Antarctic region. It must be considered that some of the sampling stations were close to the northern boundaries of the circumpolar current and the Ross Sea Gyre, hence the presence of pelagic copepods typical for sub-Antarctic areas.
Acknowledgements
We thank Dr Gritta Veit-Köhler (German Centre for Marine Biodiversity Research) and an anonymous reviewer for their precious suggestions and comments that greatly improved the initial manuscript version.
References
- Amato E (1990) Environmental impact assessment at sea. National Scientific Commission for Antarctica, Oceanographic Campaign 1987–1988. In: Cervellati R, Dall’Oglio G, Ramorino MC, Testa M (Eds) Rrapporto preliminare sulla campagna antartica Estate Australe 1987–88. PNRA, Roma, 95–99.
- Benassi G, Ferrari I, Gentile G, Menozzi P, McKenzie KG (1992) Planktonic Ostracoda in the Southern Ocean and in the Ross Sea: 1989–90 Campaign. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part II. National Scientific Commission for Antarctica, Genova, 247–300.
- Boldrin C, Stocchino C (1990) On the hydrological characteristics of Terra Nova Bay (Ross Sea-Antarctica). In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1987–88, Data Report Part I. National Scientific Commission for Antarctica, Genova, 11–57.
- Bruni V, Maugeri TL, Acosta Pomar L, Grasso S, Moio L (1990) Preliminary data on marine microflora in Terra Nova Bay (Antarctica). In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1987–88, Data Report Part I. National Scientific Commission for Antarctica, Genova, 87–104.
- Carli A, Mariottini GL, Pane L (1990) Contribution to the study of copepods collected in Terra Nova Bay (Ross Sea). In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1987–88, Data Report Part II. National Scientific Commission for Antarctica, Genova, 129–167.
- Carli A, Feletti M, Mariottini GL, Pane L (1992) Contribution to the study of copepods collected during the italian oceanographic campaign in Antarctica 1989–90. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part II. National Scientific Commission for Antarctica, Genova, 179–210.
- Carli A, Pane L, Stocchino C (2000) Planktonic copepods in Terra Nova Bay (Ross Sea): distribution and relationship with environmental factors. In: Faranda FM, Guglielmo L, Ianora A (Eds) Ross Sea Ecology. Springer, Berlin, 309–321. https://doi.org/10.1007/978-3-642-59607-0_24
- Carli A, Feletti M, Pane L (2002) Zooplankton biomass and copepod abundance of Terra Nova Bay, Ross Sea Antarctic Campaign 1994/1995. Terra Antarctica Reports B1 51–55.
- Catalano G, Benedetti F (1990) Distributions of nutrients in the Terra Nova Bay and in the Ross Sea. National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1987–88, Data Report Part I. National Scientific Commission for Antarctica, Genova, 61–83.
- Catalano G, Benedetti F, Iorio M (1991a) Coastal oceanography from Cape Russel to Campbell Ice Tongue (Terra Nova Bay). Dissolved oxygen, nutrients, pH, total alkalinity and total inorganic carbon distribution. National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part I. National Scientific Commission for Antarctica, Genova, 25–32.
- Catalano G, Benedetti F, Goffart A, Iorio M (1991b) Distribution of dissolved oxygen, pH, total alkalinity and nutrients in Southern Ocean and Ross Sea (R/V “Cariboo” 1989–90 cruise). In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part I. National Scientific Commission for Antarctica, Genova, 11–23.
- Catalano G (1992) The activity of the Chemical Oceanography Group in the Italian Antarctic Oceanographic Expeditions. Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion, 59–70.
- Cecchetto M, Alvaro MC, Ghiglione C, Guzzi A, Mazzoli C, Piazza P, Schiaparelli S (2017) Distributional records of Antarctic and sub-Antarctic Ophiuroidea from samples curated at the Italian National Antarctic Museum (MNA): check-list update of the group in the terra nova bay area (Ross Sea) and launch of the MNA 3D model “Virtual gallery. ” ZooKeys 705: 61–79. https://doi.org/10.3897/zookeys.705.13712
- Cecchetto M, Lombardi C, Canese S, Cocito S, Kuklinski P, Mazzoli C, Schiaparelli S (2019) The bryozoa collection of the italian national antarctic museum, with an updated check-list from Terra Nova Bay, Ross Sea. ZooKeys 812: 1–22. https://doi.org/10.3897/zookeys.812.26964
- Dearborn JH, Ferrari FD, Edwards KC (2011) Can pelagic aggregations cause benthic satiation? Feeding biology of the Antarctic brittle star Astrotoma agassizii (Echinodermata: Ophiuroidea). Antarctic Research Series 44: 1–28. https://doi.org/10.1029/AR044p0001
- De Broyer C, Clarke A, Koubbi P, Pakhomov E, Scott F, Vanden Berghe E, Danis B (2020) Register of Antarctic Marine Species. http://www.marinespecies.org/rams [accessed on 2020-07-16]
- Fabiano M, Povero P, Medica D, Bruzzone R (1991a) Distribution of particulate organic matter in Antarctic waters. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part I. National Scientific Commission for Antarctica, Genova, 113–137.
- Fabiano M, Povero P, Catalano G, Benedetti F (1991b) Hydrological data collected during the biological, chemical and geological sampling in Terra Nova Bay. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part I. National Scientific Commission for Antarctica, Genova, 35–71.
- Fabiano M, Povero P, Danovaro R, Medica D, Bruzzone R (1991c) Particulate organic matter in Terra Nova Bay. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part I. National Scientific Commission for Antarctica, Genova, 73–112.
- Fabiano M (1992) Observations on the Particulate Organic Matter in Antarctic waters. Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion, 145–148.
- Fabiano M, Povero P, Medica D, Danovaro R (1992) Distribution and composition of the particulate organic matter in Antarctic waters (Oceanographic Expedition 1989–90). Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion: 133–143.
- Faranda FM, Guglielmo L, Ianora A (2000) The Italian Oceanographic Cruises in the Ross Sea (1987–95): strategy, general considerations and description of the sampling sites. In: Faranda FM, Guglielmo L, Ianora A (Eds) Ross Sea Ecology. Springer, Berlin, Heidelberg, 1–13. https://doi.org/10.1007/978-3-642-59607-0_1
- Fonda Umani S, Monti M (1990) Microzooplanktonic populations in Terranova Bay. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1987–88, Data Report Part I. National Scientific Commission for Antarctica, Genova, 241–254.
- Fraser JH (1966) Zooplankton sampling. Nature 211: 915–916. https://doi.org/10.1038/211915a0
- Garlasché G, Karimullah K, Iakovenko N, Velasco-Castrillón A, Janko K, Guidetti R, Rebecchi L, Cecchetto M, Schiaparelli S, Jersabek CD (2020) A data set on the distribution of Rotifera in Antarctica. Biogeographia 35: 17–25. https://doi.org/10.21426/B635044786
- Ghiglione C, Alvaro MC, Griffiths HJ, Linse K, Schiaparelli S (2013) Ross Sea Mollusca from the Latitudinal Gradient Program: R/V Italica 2004 Rauschert dredge samples. ZooKeys 341: 37–48. https://doi.org/10.3897/zookeys.341.6031
- Ghiglione C, Alvaro MC, Cecchetto M, Canese S, Downey R, Guzzi A, Mazzoli C, Piazza P, Rapp HT, Sarà A, Schiaparelli S (2018) Porifera collection of the italian national antarctic museum (MNA), with an updated checklist from Terra Nova Bay (Ross Sea). ZooKeys 758: 137–156. https://doi.org/10.3897/zookeys.758.23485
- Goffart A, Catalano G, Magazzù G, Hecq J (1992) Some Examples of the Influence of Hydrodynamic Constraints on the Phytoplanktonic Biomass Distribution in the Southern Ocean. Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion: 265–271.
- Guglielmo L, Costanzo G, Manganaro A, Zagami G (1990) Spatial and vertical distribution of zooplanktonic communities in the Terra Nova Bay (Ross Sea). In: National Scientific Commission for Antarctica (Ed.) , Data Report Part I. National Scientific Commission for Antarctica, Genova, 257–398.
- Guglielmo L, Costanzo G, Zagami G, Manganaro A, Arena G (1992) Zooplankton ecology in the Southern Ocean. In: National Scientific Commission for Antarctica (Ed.) , Data Report Part II. National Scientific Commission for Antarctica , Genova, 30–468.
- Guglielmo L, Zagami G, Saggiomo V, Catalano G, Granata A (2007) Copepods in spring annual sea ice at Terra Nova Bay (Ross Sea, Antarctica). Polar Biology 30: 747–758. https://doi.org/10.1007/s00300-006-0234-2
- Hagen W, Kattner G, Graeve M (1993) Calanoides acutus and Calanus propinquus, Antarctic copepods with different lipid storage modes via wax esters or triacylglycerols. Marine Ecology Progress Series 97: 135–142. https://doi.org/10.3354/meps097135
- Hagen W, Schnack-Schiel SB (1996) Seasonal lipid dynamics in dominant Antarctic copepods: energy for overwintering or reproduction? Deep-Sea Research Part I: Oceanographic Research Papers 43: 139–158. https://doi.org/10.1016/0967-0637(96)00001-5
- Hecq JH, Guglielmo L (1992) Structure and functioning of the Ross Sea pelagic ecosystem: an interdisciplinary approach. Oceanografía en Antartica. Gallardo VA, O. Ferrati O, Moyano H (Eds) Proyecto Antártica-Italia. Centro EULA, Concepción, Chile, 227–233.
- Hopkins TL (1985) Food web of an Antarctic midwater ecosystem. Marine Biology 89: 197–212. https://doi.org/10.1007/BF00392890
- WoRMS Editorial Board (2020) World Register of Marine Species. http://www.marinespecies.org [accessed on: 2020-07-16]
- ICES (2000) ICES Zooplankton Methodology Manual ICES Zooplankton Methodology Manual. Harris RP, Wiebe PH, Lenz J, Skjoldal HR, Huntley M (Eds). Academic Press. https://doi.org/10.1016/B978-0-12-327645-2.X5000-2
- Innamorati M, Mori G, Lazzara L, Nuccio C, Lici M, Vanucci S (1990) Ecology of phytoplankton. In: National Scientific Commission for Antarctica (Ed.) , Oceanographic Campaign 1987–88, Data Report Part I. National Scientific Commission for Antarctica, Genova, 161–238.
- Innamorati M, Lazzara L, Mori G, Nuccio C, Saggiomo V (1991) Phytoplankton Ecology. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part I. National Scientific Commission for Antarctica, Genova, 141–252.
- Innamorati M, Lazzara L, Massi L, Mori G, Nuccio C, Saggiomo EV (1992) Research on the Phytoplankton Biomass in the Ross Sea in Relation to Environmental Factors. Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion, 235–252.
- Ivanenko VN, Corgosinho PHC, Ferrari F, Sarradin PM, Sarrazin J (2012) Microhabitat distribution of Smacigastes micheli (Copepoda: Harpacticoida: Tegastidae) from deep-sea hydrothermal vents at the Mid-Atlantic Ridge, 37°N (Lucky Strike), with a morphological description of its nauplius. Marine Ecology 33(2): 246–256. https://doi.org/10.1111/j.1439-0485.2011.00484.x
- Kellermann A (1987) Food and feeding ecology of postlarval and juvenile Pleuragramma antarcticum (Pisces; Notothenioidei) in the seasonal pack ice zone off the Antarctic peninsula. Polar Biology 7: 307–315. https://doi.org/10.1007/BF00443949
- Kihara TC, da Rocha CEF (2009) Técnicas para estudos taxonômico de copépodes harpacticóides da meiofauna marinha. Editora Asterisco, Porto Alegre, 94 pp.
- La Ferla R, Acosta Pomar L, Allegra A, Bruni V (1992) Microbial Distribution in Coastal Stations of Terra Nova Bay. Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion, 149–153.
- Loots C, Swadling KM, Koubbi P (2009) Annual cycle of distribution of three ice-associated copepods along the coast near Dumont d’Urville, Terre Adélie (Antarctica). Journal of Marine Systems 78: 599–605. https://doi.org/10.1016/j.jmarsys.2009.01.003
- Magazzù G, Decembrini F (1990) Primary Production and Picoplankton C assimilation in the Ross sea (Antarctica). National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1987–88, Data Report Part I. National Scientific Commission for Antarctica, Genova, 107–157.
- Magazzù G, Decembrini F (1991) Primary Production in the Southern Ocean. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part I. National Scientific Commission for Antarctica, Genova, 255–310.
- Magazzù G, Decembrini F (1992) Results on Primary Production of the Oceanographic Expedition of 1987–88 and 1989–90 of the National Antarctic Research Program. Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion, 273–284.
- Matsuoka K, Skoglund A, Roth G, Tronstad S, Melvær Y (2017) Quantarctica: A unique, open, standalone GIS package for Antarctic Research and Education. In: EGU General Assembly Conference Abstracts (Ed.). Vienna, Austria, April 2017.
- Maugeri T. (1992) Microbiological Research in the Antarctic Oceanographic Expeditions 1987/88 and 1989/90. Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion, 173–186.
- Mazzocchi MG, Zagami G, Ianora A, Guglielmo L, Crescenti N, Hure J (1995) Atlas of Marine Zooplankton Straits of Magellan Atlas of Marine Zooplankton Straits of Magellan. Springer, Berlin, Heidelberg, 245 pp. https://doi.org/10.1007/978-3-642-79139-0
- McKenzie KG, Benassi G, Naldi M, Ferrari I, Menozzi P (1990) Report on planktic Ostracoda from Ross Sea, Antarctica. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1987–88, Data Report Part II, Genova, 171–229.
- Michels J, Büntzow M (2010) Assessment of Congo red as a fluorescence marker for the exoskeleton of small crustaceans and the cuticle of polychaetes. Journal of Microscopy 238(2): 95–101. https://doi.org/10.1111/j.1365-2818.2009.03360.x
- Minutoli R, Brugnano C, Granata A, Zagami G, Guglielmo L (2017) Zooplankton electron transport system activity and biomass in the western Ross Sea (Antarctica) during austral summer 2014. Polar Biology 40: 1197–1209. https://doi.org/10.1007/s00300-016-2043-6
- Nuccio C, Innamorati M, Lazzara L, Mori G (1992) Phytoplankton populations in Terra Nova Bay, Ross Sea. Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion, 253–262.
- Oresland V (1991) Feeding of the carnivorous copepod Euchaeta antarctica in Antarctic waters. Marine Ecology Progress Series 78: 41–47. https://doi.org/10.3354/meps078041
- Oresland V, Ward P (1993) Summer and winter diet of four carnivorous copepod species around South Georgia. Marine Ecology Progress Series 98: 73–78. https://doi.org/10.3354/meps098073
- Oresland V (1995) Winter population structure and feeding of the chaetognath Eukrohnia hamata and the copepod Euchaeta antarctica in Gerlache Strait, Antarctic Peninsula. Marine Ecology Progress Series 19(1–3): 77–86. https://doi.org/10.3354/meps119077
- Pane L, Feletti M, Francomacaro B, Mariottini GL (2004) Summer coastal zooplankton biomass and copepod community structure near the Italian Terra Nova Base (Terra Nova Bay, Ross Sea, Antarctica). Journal of Plankton Research 26(12): 1479–1488. https://doi.org/10.1093/plankt/fbh135
- Piazza P, Błażewicz-Paszkowycz M, Ghiglione C, Alvaro MC, Schnabel K, Schiaparelli S (2014) Distributional records of Ross Sea (Antarctica) Tanaidacea from museum samples stored in the collections of the Italian National Antarctic Museum (MNA) and the New Zealand National Institute of Water and Atmospheric Research (NIWA). ZooKeys 451: 49–60. https://doi.org/10.3897/zookeys.451.8373
- Pusceddu A, Dell’anno A, Vezzulli L, Fabiano M, Saggiomo V, Cozzi S, Catalano G, Guglielmo L (2009) Microbial loop malfunctioning in the annual sea ice at Terra Nova Bay (Antarctica). Polar Biology 32: 337–346. https://doi.org/10.1007/s00300-008-0539-4
- Razouls C, de Bovée F, Kouwenberg J, Desreumaux N (2020) 2005–2020. Diversity and Geographic Distribution of Marine Planktonic Copepods. Sorbonne University, CNRS. http://copepodes.obs-banyuls.fr/en [accessed on: 2020-07-17]
- Reinhardt SB, Van Vleet ES (1986) Lipid composition of twenty-two species of Antarctic midwater zooplankton and fish. Marine Biology 91: 149–159. https://doi.org/10.1007/BF00569431
- Saggiomo V, Massi L, Modigh M, Innamorati M (1992) Size-Fractionated Primary Production in Terra Nova Bay (Ross Sea) During the Austral Summer (1989–90). Oceanografia in Antartide. ENEA-Progetto Antartide, Italia/Centro EULA, Universidad de Concepcion, 289–294.
- Sameoto DD, Jaroszynski LO, Fraser WB (1980) BIONESS, a new design in multiple net zooplankton samplers. Canadian Journal of Fisheries and Aquatic Sciences 37: 722–724. https://doi.org/10.1139/f80-093
- Schnack-schiel SB, Hagen W (1994) Life cycle strategies and seasonal variations in distribution and population structure of four dominant calanoid copepod species in the eastern Weddell Sea, Antarctica. Journal of Plankton Research 16(11): 1543–1566. https://doi.org/10.1093/plankt/16.11.1543
- Schnack-Schiel SB, Thomas D, Dieckmann GS, Eicken H, Gradinger R, Spindler M, Weissenberger J, Mizdalski E, Beyer K (1995) Life cycle strategy of the Antarctic calanoid copepod Stephos longipes. Progress in Oceanography 36: 45–75. https://doi.org/10.1016/0079-6611(95)00014-3
- Schnack-Schiel SB, Thomas DN, Haas C, Dieckmann GS, Alheit R (2001) The occurrence of the copepods Stephos longipes (Calanoida) and Drescheriella glacialis (Harpacticoida) in summer sea ice in the Weddell Sea, Antarctica. Antarctic Science 13: 150–157. https://doi.org/10.1017/S0954102001000232
- Selbmann L, Onofri S, Zucconi L, Isola D, Rottigni M, Ghiglione C, Piazza P, Alvaro MC, Schiaparelli S (2015) Distributional records of Antarctic fungi based on strains preserved in the culture collection of fungi from extreme environments (CCFEE) mycological section associated with the Italian national Antarctic museum (MNA). MycoKeys 10: 57–71. https://doi.org/10.3897/mycokeys.10.5343
- Sigovini M, Keppel E, Tagliapietra D (2016) Open nomenclature in the biodiversity era. Methods in Ecology and Evolution 7(10): 1217–1225. https://doi.org/10.1111/2041-210X.12594
- Swadling K (2001) Population structure of two Antarctic ice-associated copepods, Drescheriella glacialis and Paralabidocera antarctica, in winter sea ice. Marine Biology 139: 597–603. https://doi.org/10.1007/s002270100610
- Tanimura A, Hoshiai T, Fukuchi M (1996) The life cycle strategy of the ice-associated copepod, Paralabidocera antarctica (Calanoida, Copepoda), at Syowa Station, Antarctica. Antarctic Science 8: 257–266. https://doi.org/10.1017/S0954102096000363
- Wiebe PH, Allison D, Kennedy M, Moncoiffé G (2014) A vocabulary for the configuration of net tows for collecting plankton and micronekton. Journal of Plankton Research 37(1): 21–27. https://doi.org/10.1093/plankt/fbu101
- Wieczorek J, Bloom D, Guralnick R, Blum S, Döring M, Giovanni R, Robertson T, Vieglais D (2012) Darwin core: an evolving community-developed biodiversity data standard. PLoS ONE 7(1): e29715. https://doi.org/10.1371/journal.pone.0029715
- Williams R (1985) Trophic relationships between pelagic fish and euphausiids in Antarctic waters. Antarctic nutrient cycles and food webs. In: Siegfried WR, Condy PR, Laws RM (Eds) Antarctic Nutrient Cycles and Food Webs. Springer, Berlin, Heidelberg, 452–459. https://doi.org/10.1007/978-3-642-82275-9_63
- Zmijewska MI (1993) Seasonal and spatial variations in the population structure and life histories of the Antarctic copepod species Calanoides acutus, Calanus propinquus, Rhincalanus gigas, Metridia gerlachei and Euchaeta antarctica (Calanoida) in Croker Passage (Antarctic Peninsula). Oceanologia 35: 73–100.
- Zunini Sertorio T, Salemi Piccone P, Bernat P, Cattini E, Ossola C (1990) Copepods collected in sixteen stations during the Italian Antarctic Expedition 1987–1988. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1987–88, Data Report Part II, Genova, 67–125.
- Zunini Sertorio T, Licandro P, Ricci F, Giallain M (1992) A study on Ross Sea copepods. In: National Scientific Commission for Antarctica (Ed.) Oceanographic Campaign 1989–90, Data Report Part II, Genova, 217–246.
- Zunini Sertorio T, Licandro P, Ossola C, Artegiani A (2000) Copepod Communities in the Pacific Sector of the Southern Ocean in Early Summer. In: Faranda FM, Guglielmo L, Ianora A (Eds) Ross Sea Ecology. Springer, Berlin, 291–307. https://doi.org/10.1007/978-3-642-59607-0_23