Monograph |
|
Corresponding author: Jean-Jacques Jaeger ( jean-jaques.jaeger@univ-poitiers.fr ) Corresponding author: Somsak Panha ( somsak.pan@chula.ac.th ) Academic editor: Raquel López-Antoñanzas
© 2016 Kantapon Suraprasit, Jean-Jacques Jaeger, Yaowalak Chaimanee, Olivier Chavasseau, Chotima Yamee, Pannipa Tian, Somsak Panha.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Suraprasit K, Jaeger J-J, Chaimanee Y, Chavasseau O, Yamee C, Tian P, Panha S (2016) The Middle Pleistocene vertebrate fauna from Khok Sung (Nakhon Ratchasima, Thailand): biochronological and paleobiogeographical implications. ZooKeys 613: 1-157. https://doi.org/10.3897/zookeys.613.8309
|
Abstract
The fluviatile terrace deposits of Khok Sung, Nakhon Ratchasima province, have yielded more than one thousand fossils, making this the richest Pleistocene vertebrate fauna of Thailand. The excellent preservation of the specimens allows precise characterization of the faunal composition. The mammalian fauna consists of fifteen species in thirteen genera, including a primate, a canid, a hyaenid, proboscideans, rhinoceroses, a suid, cervids, and bovids. Most species correspond to living taxa but globally (Stegodon cf. orientalis) and locally (Crocuta crocuta ultima, Rhinoceros unicornis, Sus barbatus, and Axis axis) extinct taxa were also present. The identification of Axis axis in Khok Sung, a chital currently restricted to the Indian Subcontinent, represents the first record of the species in Southeast Asia. Three reptilian taxa: Crocodylus cf. siamensis, Python sp., and Varanus sp., are also identified. Faunal correlations with other Southeast Asian sites suggest a late Middle to early Late Pleistocene age for the Khok Sung assemblage. However, the Khok Sung mammalian fauna is most similar to that of Thum Wiman Nakin, dated to older than 169 ka. The Khok Sung large mammal assemblage mostly comprises mainland Southeast Asian taxa that migrated to Java during the latest Middle Pleistocene, supporting the hypothesis that Thailand was a biogeographic pathway for the Sino-Malayan migration event from South China to Java.
Keywords
Large mammals, taxonomy, Ailuropoda–Stegodon assemblage, paleobiogeography, late Middle Pleistocene, Quaternary, northeastern Thailand, mainland Southeast Asia
Introduction
In the Pleistocene, mammalian faunas in mainland Southeast Asia as well as in South China are characterized by the occurrence of Ailuropoda (giant panda) and/or Stegodon (extinct proboscidean), also called “Ailuropoda–Stegodon faunal complex”. This faunal association is a characteristic of the long period ranging from the Early to Late Pleistocene (
Map of Southeast Asia showing A the Sundaland boundaries and the migration route hypothesis: Siva-Malayan route (black), Sino-Malayan route (red), and Taiwan-Philippine Archipelago route (blue) and B the location of the Khok Sung sand pit (star) and other Middle (red circle) and Late (yellow circle) Pleistocene sites. The Sunda shelf boundaries at the sea level about 120 m lower than the present day are compiled from
Dating from the Middle to Late Pleistocene, there are numerous paleontological and archaeological sites with mammalian fossil faunas discovered in Southeast Asian mainland (Indochinese) and islands (Sundaic) (Fig.
An ongoing survey of Pleistocene deposits in Thailand has led to the discovery of numerous mammalian fossils by the Thai-French paleontological team in limestone caves from the northern to southern part of the country. Several fissure-filling and cave deposits: Thum Wiman Nakin (
In 2005, the Khok Sung sand pit (Nakhon Ratchasima province, northeastern Thailand) was excavated (Fig.
Material and methods
Fossil collecting and material
The sand pit was open for the pond construction (approximately 50 m long × 50 m wide × 8 m deep) (Fig.
All fossil specimens are housed at the Khok Sung local museum (Nakhon Ratchasima) and at the
iPHEP
Institut International de Paléoprimatologie et de Paléontologie Humaine: Evolution et Paléoenvironnements,
NHMP
THNHM-M
Mammal collection, Thailand
Dental terminology and taxonomic nomenclature
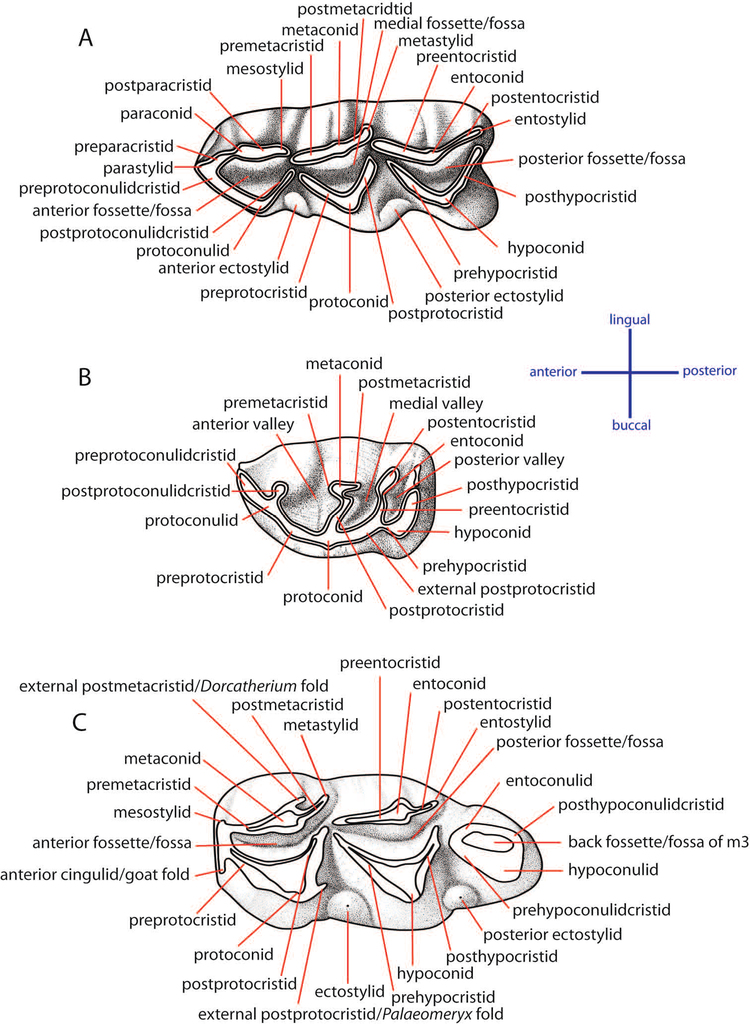
The dental nomenclature follows
Measurements
All specimens were measured using digital callipers to the nearest 0.01 mm. The tooth dimensions for all mammals were measured at the base of the crown along the anterior-posterior margins for the maximum length (L) and from the labial (incisors and canines)/buccal (premolars/molars) to lingual margins for the maximum width (W). In the case of measurements of stegodontid cheek teeth, the methods and parameters used for molar and ridge dimensions were given in Fig.
Parameters and measurement methods used for the lower third molar of Stegodon. Lengths and widths of the molar ridges are abbreviated as “LR” and “WR”, respectively. An illustration of two right m3 (lateral and occlusal views) of Stegodon orientalis is duplicated from the specimen
| Scapula | |
| HS | Height along the spine |
| DHA | Diagonal height from the most distal point of the scapula to the thoracic angle |
| Ld | Greatest dorsal length |
| SLC | Smallest length of the Collum scapulae (neck of the scapula) |
| GLP | Greatest length of the Processus articularis (glenoid process) |
| LG | Length of the glenoid cavity |
| BG | Breadth of the glenoid cavity |
| Long bones | |
| GL | Greatest length |
| GLl | Greatest length of the lateral part |
| GLC | Greatest length from the caput (head) |
| PL | Physiological length (for radius only) |
| Ll | Length of the lateral part |
| Bp | Greatest breadth of the proximal end |
| BFp | Greatest breadth of the Facies articularis proximalis (for radius only) |
| BPC | Greatest breadth across the coronoid process (=greatest breadth of the proximal articular surface) (for ulna only) |
| SD | Smallest breadth of diaphysis |
| Dp | Depth of the proximal end |
| Bd | Greatest breadth of the distal end |
| BFd | Greatest breadth of the Facies articularis distalis (for radius only) |
| Dd | Greatest breadth of the distal end |
| DC | Greatest depth of the Caput femoris |
| DD | Smallest depth of the diaphysis (for metapodials only) |
| BT | Greatest breadth of the trochlea (for humerus only) |
| LO | Length of the olecranon (for ulna only) |
| DPA | Depth across the Processus anconaeus (for ulna only) |
| SDO | Smallest depth of the olecranon (for ulna only) |
| Pelvis | |
| GL | Greatest length of one half |
| LA | Length of the acetabulum including the lip |
| LS | Length of the symphysis |
| SH | Smallest height of the shaft of ilium |
| SB | Smallest breadth of the shaft of ilium |
| SC | Smallest circumference of the shaft of ilium |
| LFo | Inner length of the foramen obturatum |
| GBTc | Greatest breadth across the Tubera coxarum–greatest breadth across the lateral angle |
| GBA | Greatest breadth across the acetabula |
| GBTi | Greatest breadth across the Tubera ischiadica |
| SBI | Smallest breadth across the bodies of the Ischia |
Body mass estimation
The body mass of ruminants was estimated using the equations of
Faunal similarity measures and cluster analysis
We compared differences in species compostion of Southeast Asian large mammal fauna during the Middle Pleistocene, using an analysis of the faunal similarity. According to unequal sampling conditions for our data, we applied two criteria for undertaking this analysis: localities are disqualified when they have fewer than 10 taxa identified at the species level and taxa are excluded when their appearances are still doubtful (i.e. poor taxonomic description or identification). We therefore selected Simpson’s Faunal Resemblance Index (FRI) because it has the smallest influence of sample sizes and emphasizes faunal resemblances (
Systematic paleontology
Class MAMMALIA Linnaeus, 1758
Order PRIMATES Linnaeus, 1758
Suborder HAPLORRHINI Pocock, 1918
Family CERCOPITHECIDAE Gray, 1821
Genus Macaca Lacépède, 1799
Macaca sp.
Referred material
A right tibia, DMR-KS-05-04-04-1.
Material description
The right tibia is complete (Fig.
Postcranial remains of Macaca sp. A–D and Cuon sp. E–H from Khok Sung: A–D DMR-KS-05-04-04-1, a right tibia in anterior (A), medial (B), proximal (C), and distal (D) views E–F DMR-KS-05-04-11-34, a right ulna in medial (E) and anterior (F) views G–H DMR-KS-05-04-28-13, a right femur in proximal (G) and posterior (H) views.
Taxonomic remarks and comparisons
Tibial morphology is relatively conservative within and among primates. Particularly, the morphological differences of tibiae among cercopithecoids are minimal (
Ratios of the greatest lengths of tibiae (GL) to the lengths and widths of proximal and distal tibiae (Bp, Dp, Bd, and Dd) of Khok Sung macaques compared to recent Southeast Asian primates.
| DMR-KS-05-04-04-1 | Presbytis (N = 30) | Hylobates (N = 24) | Macaca (N = 71) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | ||
| GL/Bp | 6.09 | 7.70 | 6.76 | 7.29 | 7.52 | 6.06 | 7.01 | 6.56 | 4.55 | 5.61 |
| GL/Dp | 7.81 | 9.89 | 8.15 | 9.07 | 9.95 | 7.96 | 9.43 | 9.62 | 6.36 | 7.67 |
| GL/Bd | 9.25 | 12.38 | 10.26 | 11.37 | 14.49 | 9.01 | 11.31 | 10.84 | 7.20 | 8.79 |
| GL/Dd | 12.94 | 16.21 | 12.75 | 14.13 | 16.79 | 10.94 | 14.50 | 12.77 | 7.69 | 10.78 |
Order CARNIVORA Bowdich, 1821
Family CANIDAE Fischer de Waldheim, 1817
Genus Cuon Hodgson, 1838
Cuon sp.
Referred material
A right ulna, DMR-KS-05-04-11-34; a right femur, DMR-KS-05-04-28-13.
Material description
DMR-KS-05-04-11-34 is a half proximal ulna preserving complete parts from the olecranon to the midshaft (Fig.
The right femur preserves a complete proximal part and broken shaft (Fig.
Taxonomic remarks and comparisons
The proximal ulna of canids is characterized by a bilobed and laterally compressed olecranon process, well-developed anconeal and lateral coronoid processes, and a laterally compressed shaft. The proximal crest of the olecranon is grooved anteriorly, but enlarged and rounded posteriorly (
Measurements (in millimetres) of ulnae and femurs of Khok Sung and other extant and fossil canids. * indicates a subadult individual. Metrical data of fossil canids are from
| Ulna | |||||||
| Specimen no. | Taxa | Age | Locality | LO | DPA | SDO | BPC |
| DMR-KS-05-04-11-34 | Cuon sp. | late Middle Pleistocene | Khok Sung, northeastern Thailand | 15.16 | 18.51 | 15.21 | 11.65 |
|
|
Canis lupus | Recent | Eastern India | 29.91 | 24.11 | 18.38 | 15.65 |
| 29.29 | 24.43 | 18.43 | 15.33 | ||||
|
|
Canis lupus | Late Pleistocene | Geographical Society Cave, Russia | – | 32.30 | 27.80 | – |
| NHMP R5387 | Canis lupus | Late Pleistocene | Srbsko Chlum-Komín Cave, Czech Republic | – | 34.80 | 27.60 | – |
|
|
Cuon alpinus | Recent | Java, Indonesia | 19.23 | 19.37 | 16.36 | 14.43 |
| 19.74 | 19.29 | 16.33 | 14.07 | ||||
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 1 Cave, Southern Ossetia, Caucasus | – | – | – | 18.30 |
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 1 Cave, Southern Ossetia, Caucasus | – | 32.20 | – | 17.20 |
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 3 Cave, Southern Ossetia, Caucasus | – | 28.70 | 24.50 | 18.90 |
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 3 Cave, Southern Ossetia, Caucasus | – | 34.00 | 29.50 | 21.50 |
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 3 Cave, Southern Ossetia, Caucasus | – | 33.60 | 28.60 | 21.70 |
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 3 Cave, Southern Ossetia, Caucasus | – | 30.30 | 26.50 | 17.00 |
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 3 Cave, Southern Ossetia, Caucasus | – | 28.80 | – | 18.50 |
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 3 Cave, Southern Ossetia, Caucasus | – | – | – | 15.00 |
| Femur | |||||||
| Specimen no. | Taxa | Age | Locality | Bp | Dp | DC | SD |
| DMR-KS-05-04-28-13 | Cuon sp. | late Middle Pleistocene | Khok Sung, northeastern Thailand | 35.69 | 17.90 | 16.58 | 11.34 |
|
|
Canis lupus | Recent | Eastern India | 35.05 | 16.70 | 16.75 | 10.43 |
| 35.57 | 16.82 | 16.73 | 10.52 | ||||
|
|
Cuon alpinus | Recent | Java, Indonesia | 31.03 | 15.95 | 16.62 | 11.66 |
| 31.58 | 16.08 | 16.38 | 11.79 | ||||
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 3 Cave, Southern Ossetia, Caucasus | 48.70 | – | 22.70 | – |
|
|
Cuon alpinus caucasicus | Late Pleistocene | Kudaro 3 Cave, Southern Ossetia, Caucasus | – | – | 21.70 | 15.20 |
Living canids generally show a typical morphology of the proximal femur, characterized by their relatively vertical intertrochanteric crests, prominent lesser trochanter with the sharp crest extending downward along the shaft, moderately-sized greater trochanter, and slender shaft (
Because the Khok Sung ulna and femur morphologically match better Cuon alpinus than Canis lupus, we identify these two postcranial specimens as belonging to Cuon sp.
Order PROBOSCIDEA Illiger, 1811
Family STEGODONTIDAE Osborn, 1918
Genus Stegodon Falconer and Cautley, 1857
Stegodon cf. orientalis
Referred material
A right DP4 (posterior part), DMR-KS-05-03-28-14; a left DP4 (anterior part), DMR-KS-05-03-19-7; a left M2, DMR-KS-05-03-29-1 (posterior part); a right M3, DMR-KS-05-03-22-19 (posterior part); a fragmentary tusk, DMR-KS-05-03-15-2; a left dp3 (anterior part), DMR-KS-05-04-01-8; two mandibles with m3—DMR-KS-05-03-08-1 (right) and DMR-KS-05-03-08-2 (left); a right humerus fragment (proximal part), DMR-KS-05-03-10-5; a left humerus, DMR-KS-05-03-10-6; two ulna fragments (proximal parts)—DMR-KS-05-03-09-7 and DMR-KS-05-03-10-2; a femoral head fragment, DMR-KS-05-03-10-3; a right femur, DMR-KS-05-03-10-4; a right tibia fragment (distal part), DMR-KS-05-03-10-3; a right fibula, DMR-KS-05-03-00-124; two pelvis fragments—DMR-KS-05-03-10-11 (right) and DMR-KS-05-03-10-12 (left); five vertebrae—DMR-KS-05-03-17-11, DMR-KS-05-03-10-7, DMR-KS-05-03-09-18, DMR-KS-05-03-10-1, and DMR-KS-05-03-28-20; a sacrum fragment, DMR-KS-05-03-10-8; two ribs—DMR-KS-05-03-10-13 and DMR-KS-05-03-10-14; three rib fragments—DMR-KS-05-03-09-6 (body), DMR-KS-05-03-09-45 (body), and DMR-KS-05-03-09-4 (head and neck).
Material description
Upper dentition: both fragments of DP4 (DMR-KS-05-03-28-14: Fig.
Dental remains of Stegodon cf. orientalis from Khok Sung: A–B DMR-KS-05-03-28-14, a right DP4 in occlusal (A) and buccal (B) views C DMR-KS-05-03-19-7, an anterior lobe of DP4 in occlusal view D DMR-KS-05-04-01-8, a left dp3 in occlusal view E–F DMR-KS-05-03-29-1, a left posterior fragment of M2 in occlusal (E) and buccal (F) views G–H DMR-KS-05-03-22-19, a right posterior fragment of M3 in occlusal (G) and buccal (H) views I–K DMR-KS-05-03-15-2, a fragmentary upper tusk in proximal (I–J) and dorsal (K) views L DMR-KS-05-03-08-1, a left mandible with m3 in occlusal view M DMR-KS-05-03-08-2, a right mandible with m3 in occlusal view.
Measurements (in millimeters) of cheek teeth of Khok Sung proboscideans, including a number of preserved ridges (NR), lengths (L), widths (W), heights (H), enamel thickness (ET), H/W indices (100 × H/W), and laminar frequencies (LF). The laminar frequencies are expressed as the following formula: LF=n*100/dl + n*100/db / 2, where “dl” and “db” are referred to distances at the lingual and buccal side of the tooth, respectively, and “n” is equivalent to the number of ridges between two measuring points (
| Specimen no. | NR | L | W | H | ET | H/W index | LF | |
|---|---|---|---|---|---|---|---|---|
| Stegodon cf. orientalis | ||||||||
| DMR-KS-05-03-28-14 | DP4 | 4 | 60.08 | 50.04 | 26.71 | 0.69–1.21 | 53.38–58.23 | 7.99 |
| DMR-KS-05-03-19-7 | DP4 | 1 | 18.65 | 49.89* | 26.71 | 2.06 | 53.53 | – |
| DMR-KS-05-03-29-1 | M2 | 3 | 70.14* | 78.83 | 55.18 | 1.62–3.06 | 70.00–73.34 | 4.61 |
| DMR-KS-05-03-22-19 | M3 | 3 | 90.43* | 84.66 | 46.14 | 3.77–4.35 | 57.33–62.71 | 3.86 |
| DMR-KS-05-04-01-8 | dp3 | 3 | 26.68* | 26.09 | 12.08 | 1.82 | 46.30–47.76 | 10.41 |
| DMR-KS-05-03-08-1 | m3 | 8 | 245.86* | 95.66 | 41.50 | 3.41–6.87 | 43.38–51.77 | 3.91 |
| DMR-KS-05-03-08-2 | m3 | 8 | 247.78* | 95.57 | 42.56 | 3.39–6.54 | 44.53–52.21 | 3.94 |
| Elephas sp. | ||||||||
| DMR-KS-05-03-17-12 | Lower molar | 2 | 41.04* | 66.77* | 108.94 | 2.48–3.30 | 163.16–165.18 | 10.61 |
DMR-KS-05-03-29-1 (M2) preserves three posterior ridges with a small cingulum (Fig.
DMR-KS-05-03-22-19 (M3) preserves only three posterior ridges with a cingulum (Fig.
A fragmentary tusk (DMR-KS-05-03-15-2) contains dentine (outer and inner layers), cementum, and a pulp cavity (Fig.
Lower dentition: DMR-KS-05-04-01-8 (dp3) is heavily worn and comprises three preserved ridges and an anterior cingulum (Fig.
Two hemi-mandibles of the same individual (DMR-KS-05-03-08-1 and DMR-KS-05-03-08-2) are moderately well-preserved (Tab.
Postcranial remains: postcranial elements include two humeri (Fig.
Postcranial remains of Stegodon cf. orientalis from Khok Sung: A–B DMR-KS-05-03-10-6, a left distal humerus in anterior (A) and distal (B) views C–D DMR-KS-05-03-10-4, a right femur posterior (C) and distal (D) views E DMR-KS-05-03-00-124, a right fibula in posterior view F DMR-KS-05-03-10-11, a right pelvis in dorsal view G DMR-KS-05-03-10-12, a left pelvis in lateral view H DMR-KS-05-03-09-18 and I DMR-KS-05-03-10-7, vertebrae in anterior view J DMR-KS-05-03-10-8, a sacrum in ventral view K DMR-KS-05-03-10-14 and L DMR-KS-05-03-10-13, ribs in anterior view.
Taxonomic remarks and comparisons
The proboscidean cheek teeth from Khok Sung are assigned to Stegodon because there are more than five ridges or loph(id)s on molars, V-shaped valleys between ridges on molars , and step-like worn surface reliefs on the enamel layer (
The morphologies and ridge sizes of upper molars from Khok Sung are congruent with Chinese S. orientalis (Tabs
Ridge dimensions (lengths and widths in millimeters) of upper fourth deciduous premolars between Khok Sung Stegodon and Stegodon orientalis.
| DP4 | Ridge (from anterior to posterior) | |||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | |
| Stegodon cf. orientalis (Khok Sung) | ||||||
| Length | 15.7 | – | > 10.7 | 12.4 | 13.5 | 13.5 |
| Width | 49.9 | – | 49.9 | 50.0 | 49.7 | 48.4 |
| Specimen measurements: DMR-KS-05-03-28-14 and DMR-KS-05-03-19-7 | ||||||
| Stegodon orientalis (×6×) | ||||||
| N | 3 | 3 | 3 | 3 | 3 | 3 |
| Length | 12.3–16.2 | 15.3–19.7 | 14.3–20.4 | 13.3–18.4 | 12.6–16.2 | 11.1–16.5 |
| Mean | 14.1 | 17.0 | 17.4 | 16.1 | 15.0 | 13.6 |
| N | 3 | 3 | 2 | 3 | 3 | 3 |
| Width | 43.7–54.1 | 49.2–63.1 | 51.8–63.3 | 51.2–60.2 | 50.0–57.2 | 45.8–52.2 |
| Mean | 49.0 | 54.6 | 57.5 | 54.4 | 53.0 | 48.9 |
| Specimen measurements: |
||||||
Ridge dimensions (lengths and widths in millimeters) of lower third deciduous premolars between Khok Sung Stegodon and Stegodon orientalis.
| dp3 | Ridge (from anterior to posterior) | |||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | |
| Stegodon cf. orientalis (Khok Sung) | ||||||
| Length | 9.3 | – | – | – | – | – |
| Width | 25.5 | > 26.1 | – | – | – | – |
| Stegodon orientalis (×5×) | ||||||
| N | 7 | 7 | 7 | 7 | 7 | – |
| Length | 7.2–10.8 | 6.5–10.3 | 9.6–12.9 | 10.9–12.0 | 10.0–14.0 | – |
| Mean | 8.5 | 9.0 | 11.0 | 11.5 | 12.6 | – |
| N | 7 | 7 | 7 | 7 | 7 | – |
| Width | 19.5–32.3 | 24.8–27.9 | 27.3–31.9 | 32.6–37.8 | 36.2–42.3 | – |
| Mean | 25.0 | 26.6 | 29.9 | 34.9 | 39.2 | – |
| Specimen measurements: |
||||||
| Stegodon orientalis (×6×) | ||||||
| N | 5 | 5 | 5 | 5 | 5 | 5 |
| Length | 8.6–13.1 | 7.0–11.8 | 10.1–12.8 | 10.6–13.0 | 10.6–13.4 | 8.5–12.5 |
| Mean | 10.5 | 8.6 | 11.5 | 11.7 | 11.7 | 10.0 |
| N | 4 | 5 | 5 | 5 | 5 | 5 |
| Width | 23.7–31.1 | 26.8–32.1 | 29.1–34.7 | 33.1–41.1 | 36.7–47.3 | 36.0–52.4 |
| Mean | 27.3 | 28.9 | 31.5 | 36.8 | 41.3 | 40.4 |
| Specimen measurements: IVPP1799, |
||||||
Ridge dimensions (lengths and widths in millimeters) of upper second and third molars between Khok Sung Stegodon and Stegodon orientalis. The total ridge number of upper molars of Khok Sung stegodontids used for our comparisons follows that of Stegodon orientalis.
| M2 and M3 | Ridge (from anterior to posterior) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | Posterior halfridge | |
| Stegodon cf. orientalis (Khok Sung) | |||||||||||
| DMR-KS-05-03-29-1 (M2) | |||||||||||
| Length | – | – | – | – | – | 28.2 | 23.9 | 19.7 | – | – | – |
| Width | – | – | – | – | – | 78.8 | 76.9 | 63.3 | – | – | – |
| DMR-KS-05-03-22-19 (M3) | |||||||||||
| Length | – | – | – | – | – | – | – | 29.3 | 24.2 | 21.8 | 12.9 |
| Width | – | – | – | – | – | – | – | 80.5 | 77.6 | 70.1 | 49.7 |
| Stegodon orientalis (M2) (×8×) | |||||||||||
| N | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | – | – | – |
| Length | 23.4 | 25.0 | 30.7 | 25.4 | 22.1 | 20.3–22.5 | 20.5–22.0 | 15.4–17.7 | – | – | – |
| Mean | – | – | – | – | – | 21.4 | 21.2 | 16.6 | – | – | – |
| N | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | – | – | – |
| Width | 77.8 | 80.4 | 83.1 | 83.0 | 81.6 | 76.4–78.4 | 73.0–73.8 | 63.2–69.1 | – | – | – |
| Mean | – | – | – | – | – | 77.4 | 73.4 | 66.1 | – | – | – |
| Specimen measurements: |
|||||||||||
| Stegodon orientalis (M3) (×10×) | |||||||||||
| N | 3 | 3 | 3 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 |
| Length | 22.4–25.3 | 22.2–27.4 | 22.3–27.1 | 24.6–24.8 | 25.5 | 22.5 | 21.1 | 19.9–26.3 | 17.8–24.1 | 16.4–22.4 | 7.2–15.8 |
| Mean | 23.7 | 25.6 | 24.5 | 24.7 | – | – | – | 23.1 | 20.9 | 19.4 | 11.5 |
| N | 2 | 2 | 2 | 1 | – | 2 | 2 | 2 | 2 | 2 | 2 |
| Width | 85.4–91.5 | 88.3–96.7 | 84.9–101.3 | 100.4 | – | 81.4–87.1 | 83.3–85.7 | 75.0–87.9 | 65.4–89.5 | 57.4–81.8 | 38.6–54.6 |
| Mean | 88.4 | 92.5 | 93.1 | – | – | 84.3 | 84.5 | 81.4 | 77.5 | 69.6 | 46.6 |
| Specimen measurements: |
|||||||||||
Ridge dimensions (lengths and widths in millimeters) of lower third molars of derived Stegodon in Southeast Asia. The ridge formula of each taxon follows
| Lower third molar | Ridge (from anterior to posterior) | ||||||||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | |||
| Stegodon cf. orientalis (Khok Sung) ((×?2)9×) | |||||||||||||
| N | – | – | – | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| Length | – | – | – | 29.8–32.4 | 28.8–30.6 | 31.8–32.8 | 28.2–34.1 | 28.9–32.3 | 24.5–30.7 | 23.3–26.9 | 16.6–22.4 | ||
| Mean | – | – | – | 31.1 | 29.7 | 32.3 | 31.2 | 30.6 | 27.6 | 25.1 | 19.5 | ||
| N | – | – | – | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| Width | – | – | – | 95.7–97.6 | 94.4–95.8 | 92.7–94.0 | 83.1–83.3 | 72.3–76.3 | 67.7–68.9 | 61.6–65.9 | 55.4–58.6 | ||
| Mean | – | – | – | 96.7 | 95.1 | 93.4 | 83.2 | 74.3 | 68.3 | 63.8 | 57.0 | ||
| Stegodon cf. orientalis (×11×) | |||||||||||||
| N | – | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| Length | – | 26.0–31.7 | 26.4–33.8 | 23.3–34.4 | 25.5–33.1 | 28.6–31.7 | 24.8–39.4 | 26.8–36.4 | 25.6–31.0 | 21.1–24.3 | 15.4–15.9 | ||
| Mean | – | 28.9 | 30.1 | 28.9 | 29.3 | 30.2 | 32.1 | 31.6 | 31.3 | 22.7 | 15.7 | ||
| N | – | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| Width | – | 82.43 | 68.0–86.1 | 88.34 | 90.11 | 72.4–93.1 | 72.0–91.9 | 71.2–88.3 | 64.6–80.1 | 54.8–63.6 | 41.8–43.1 | ||
| Mean | – | – | 77.1 | – | – | 82.8 | 82.0 | 79.8 | 72.4 | 59.2 | 42.5 | ||
| Specimen measurements: |
|||||||||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 12th | ||
| Stegodon insignis (×12×) | |||||||||||||
| N | – | 1 | 2 | 3 | 2 | 2 | 2 | 3 | 3 | 2 | 2 | 2 | |
| Length | – | 27.7 | 26.4–29.2 | 20.7–23.7 | 24.2 | 21.6–23.8 | 20.9–22.8 | 23.0–25.6 | 23.4–30.9 | 22.5–25.3 | 21.5–23.5 | 19.5–23.7 | |
| Mean | – | – | 27.8 | 22.1 | 24.2 | 22.7 | 21.9 | 24.6 | 26 | 23.9 | 22.5 | 21.6 | |
| N | – | 1 | 3 | 3 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | |
| Width | – | 79.3 | 83.9–92.6 | 81.7–91.2 | 92.7–94.8 | 90.7–98.5 | 89.5–89.9 | 84.6–88.0 | 73.7–77.7 | 66.6–68.8 | 61.6–64.0 | 47.1–52.5 | |
| Mean | – | – | 87.4 | 88.0 | 93.8 | 94.6 | 89.7 | 85.9 | 75.7 | 67.7 | 62.8 | 49.8 | |
| Specimen measurements: |
|||||||||||||
| Stegodon orientalis (×12×) | |||||||||||||
| N | 4 | 1 | 4 | 4 | 4 | 4 | 4 | 4 | 5 | 8 | 8 | 8 | |
| Length | 17.4–25.1 | 20.4 | 25.5–31.7 | 25.9–33.8 | 23.3–34.42 | 25.5–35.0 | 28.6–35.3 | 24.8–39.4 | 26.8–36.4 | 25.6–37.0 | 16.2–31.5 | 13.4–22.6 | |
| Mean | 21.1 | – | 28.0 | 28.8 | 30.3 | 30.3 | 31.1 | 32.3 | 31.1 | 29.4 | 23.0 | 16.8 | |
| N | 4 | 1 | 2 | 3 | 3 | 3 | 4 | 4 | 4 | 6 | 8 | 8 | |
| Width | 71.6–81.0 | 75.7 | 81.2–82.4 | 68.0–86.1 | 85.6–88.3 | 84.9–90.1 | 72.4–93.1 | 72.0–91.9 | 71.2–88.3 | 64.6–82.7 | 54.8–78.3 | 28.7–59.1 | |
| Mean | 74.7 | – | 81.8 | 79.5 | 87.1 | 87.6 | 84.7 | 84.2 | 82.2 | 75.2 | 66.0 | 46.0 | |
| Specimen measurements: |
|||||||||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 12th | 13th | |
| Stegodon trigonocephalus trigonocephalus (×13×) | |||||||||||||
| N | – | – | – | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | – |
| Length | – | – | – | 16.8 | 20.4–25.0 | 22.1–26.0 | 24.4–26.0 | 23.7–24.9 | 21.4–24.5 | 21.1–24.0 | 16.6–24.2 | 18.4–19.4 | – |
| Mean | – | – | – | – | 23.4 | 24.0 | 25.3 | 24.1 | 22.9 | 22.5 | 21.1 | 19.0 | – |
| N | – | – | – | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | – |
| Width | – | – | – | 71.8 | 71.4–87.3 | 71.4–86.8 | 75.0–87.1 | 76.8–83.3 | 72.6–81.1 | 70.5–76.4 | 66.6–69.6 | 53.9–63.1 | – |
| Mean | – | – | – | – | 80.2 | 80.4 | 80.8 | 80.4 | 77.0 | 72.6 | 68.0 | 58.4 | – |
| Specimen measurements: |
|||||||||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 12th | 13th | |
| Stegodon florensis (×13×) | |||||||||||||
| N | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | – | – |
| Length | 23.1–25.8 | 20.2–20.9 | 19.2–21.6 | 18.4–23.8 | 17.6–22.7 | 18.9–20.9 | 18.3–20.3 | 21.16 | 21.83 | 26.48 | 26.97 | – | – |
| Mean | 24.5 | 20.6 | 20.4 | 21.1 | 20.2 | 19.9 | 19.3 | – | – | – | – | – | – |
| N | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | – | – |
| Width | 63.1 | 66.0 | 67.2–68.4 | 69.3–69.9 | 69.0–69.9 | 67.5–69.9 | 68.3–68.6 | 66.95 | 60.47 | 65.75 | 58.71 | – | – |
| Mean | – | 66.0 | 67.8 | 69.6 | 69.5 | 68.7 | 68.5 | – | – | – | – | – | |
| Specimen measurements: |
|||||||||||||
Family ELEPHANTIDAE Gray, 1821
Genus Elephas Linnaeus, 1758
Elephas sp.
Referred material
A fragmentary tusk, DMR-KS-05-03-22-1; a posterior fragment of a right lower molar, DMR-KS-05-03-17-12.
Material description
Upper tusk: DMR-KS-05-03-22-1 is a short fragmentary tusk. The dorsal side is partially broken away (Fig.
Lower molar: DMR-KS-05-03-17-12 preserves only two adjoining worn plates of a high-crowned molar, distinctly more hypsodont than that of Stegodon (Tab.
Taxonomic remarks and comparisons
The fragmentary tusk (DMR-KS-05-03-22-1) is distinguished from DMR-KS-05-03-15-2 (S. orientalis) by a more rounded cross-section, a larger diameter, and a radiate fracture pattern with the development of concentric incremental lines (Fig.
Order PERISSODACTYLA Owen, 1848
Family RHINOCEROTIDAE Owen, 1840
Subfamily RHINOCEROTINAE Owen, 1845
Genus Rhinoceros Linnaeus, 1758
Rhinoceros sondaicus
Referred material
A left P2, DMR-KS-05-03-00-128; a left P3, DMR-KS-05-03-22-17; a left M1, DMR-KS-05-03-00-129; a left M3, DMR-KS-05-03-00-127; a mandible with right (i2 and p2–m3) and left (p3–m3) tooth rows, DMR-KS-05-03-00-126; a partial mandible, DMR-KS-05-03-31-28; a fragmentary nasal bone, DMR-KS-05-03-00-56; a left scapula, DMR-KS-05-03-00-58; a left humerus, DMR-KS-05-03-31-3; a right metacarpus II, DMR-KS-05-03-28-29; a metacarpus III, DMR-KS-05-03-22-49; a right metacarpus IV, DMR-KS-05-04-05-15; a left tibia, DMR-KS-05-03-00-52; a right calcaneus, DMR-KS-05-04-27-19; a left astragalus, DMR-KS-05-03-26-23.
Material description
Upper dentition: P2 (DMR-KS-05-03-00-128: Fig.
Cranial, mandibular, dental remains of Rhinoceros sondaicus from Khok Sung: ADMR-05-03-00-128, a left P2 in occlusal view B DMR-KS-05-03-22-17, a left P3 in occlusal view C DMR-KS-05-03-00-129, a left M1 in occlusal view D DMR-KS-05-03-00-127, a left M3 in occlusal view E–G DMR-KS-05-03-00-126, a mandible in lateral (E), occlusal (F) and ventral (G) views H–I DMR-KS-05-03-31-28, a fragmentary mandible in occlusal (H) and lateral (I) views J–K DMR-KS-05-03-00-56, a nasal in dorsal (J) and lateral (K) views.
Measurements (in millimeters) of cheek teeth of Khok Sung rhinoceroses, Rhinoceros sondaicus and Rhinoceros unicornis, compared to recent specimens (data from
| Rhinoceros sondaicus | Rhinoceros unicornis | ||||||
|---|---|---|---|---|---|---|---|
| Khok Sung | Recent | Khok Sung | Recent | ||||
| Anterior | Posterior | Range | Anterior | Posterior | Range | ||
| Upper cheek teeth | |||||||
| P2 | L | 35.57 (i) | 30–38.5 | – | 37–45.5 | ||
| W | 42.34 (i) | 41.24 (i) | 34.5–44 | – | – | 43–48 | |
| P3 | L | 42.00 (i) | 36.5–50 | – | 43–50 | ||
| W | 55.36 (i) | 53.70 (i) | 42–55 | – | – | 55.5–60.5 | |
| P4 | L | – | 41–47.5 | – | 42–51 | ||
| W | – | – | 52–59 | – | – | 59–69.5 | |
| M1 | L | 51.38 (i) | 46–51 | 47.95 (i) | 48–58 | ||
| W | 63.53 (i) | 58.67 (i) | 52.5–60 | 70.48 (i) | 58.80 (i) | 62–72.5 | |
| M2 | L | – | 44.5–55 | – | 53–62 | ||
| W | – | – | 53–62.5 | – | – | 64.5–76 | |
| M3 | L | 55.65 (i) | 44.5–61.5 | – | 59–65 | ||
| W | 55.92 (i) | 43.5–57 | – | – | 56–68.5 | ||
| Lower cheek teeth | |||||||
| p2 | L | – | 25–29.5 | > 30.80 (i) | 31–32 | ||
| W | – | – | 15.5–21 | 18.15 (i) | 22.39 (i) | 21.5–24.5 | |
| p3 | L | 42.83 (m) | 33–39 | 40.24 (m) | 38–42 | ||
| W | 26.58 (m) | 29.92 (m) | 22–27.5 | – | – | 27–32 | |
| p4 | L | 43.03 (m) | 36.5–42.5 | 48.13 (m) | 41–46 | ||
| W | 27.71 (m) | 33.42 (m) | 24–29 | – | – | 29–34 | |
| m1 | L | 41.45 (m) | 41–46.5 | 42.57 (m) | 46–48 | ||
| W | 28.8–29.67 (m) | 30.88 (m) | 26–32 | – | – | 28–32.5 | |
| m2 | L | 44.83–48.87 (m) | 40.5–51 | 50.74 (m) | 52–56.5 | ||
| W | 29.65 (m) | 30.78–31.79 (m) | 27–32.5 | – | – | 31–36 | |
| m3 | L | 54.90 (m) | 41–53 | 55.48 (m) | 49.5–60 | ||
| W | 32.54 (m) | 25.11* (m) | 24.5–29.5 | – | – | 29–35 | |
| Lower tooth rows | |||||||
| DMR–KS–05–03–00–126 | Recent | DMR-KS–05–03–17–13 | Recent | ||||
| Molar row length | 133 (right) | 126.5–147 | 158 | 147.5–161 | |||
| Tooth row length | > 238 | 211.5–257 | – | 242–276 | |||
Measurements (lengths and widths in millimeters) of cheek teeth of Khok Sung Sus barbatus compared to the recent and fossil species. The number of specimens is given within the parentheses. The measured specimens of recent Sus scrofa include three subspecies: S. s. scrofa, S. s. vittatus, and S. s. attila.
| Khok Sung | Recent | Java (Pleistocene) | ||||||
|---|---|---|---|---|---|---|---|---|
| S. barbatus | S. scrofa | S. barbatus | S. verrucosus | S. celebensis | S. brachygnathus | S. macrognathus | ||
| P3 | L | 16.75 | 12.33–14.41 (16) | 13.17–14.98 (12) | 11.87–13.77 (8) | 9.37 | 11.09–12.29 (7) | 12.47–13.86 (2) |
| W | 14.42 | 10.12–12.19 (16) | 10.06–13.22 (12) | 9.71–12.64 (8) | 7.35 | 9.60–11.54 (7) | 10.75–13.43 (2) | |
| P4 | L | 15.06 | 11.41–14.61 (16) | 12.56–14.81 (12) | 11.85–13.97 (8) | 8.96–9.44 (3) | 10.35–11.61 (7) | 11.89–12.39 (3) |
| W | 18.59 | 12.77–15.23 (16) | 13.51–16.00 (12) | 13.23–14.82 (8) | 10.68–11.01 (3) | 11.05–13.80 (7) | 13.72–15.68 (3) | |
| M1 | L | 20.68 | 14.01–17.88 (16) | 16.71–19.24 (12) | 14.36–16.13 (8) | 13.44–13.76 (3) | 13.89–14.94 (6) | 13.62–17.17 (3) |
| W | 17.17 | 13.59–17.57 (16) | 13.59–15.85 (12) | 13.32–15.78 (8) | 10.59–11.49 (3) | 12.68–14.36 (6) | 12.57–15.54 (3) | |
| M2 | L | 29.35–29.49 (2) | 20.08–24.78 (16) | 22.60–24.60 (12) | 20.53–22.39 (8) | 16.89–17.98 (4) | 19.81–24.26 (7) | 17.17–24.38 (4) |
| W | 21.37–23.40 (3) | 16.43–20.82 (16) | 17.45–19.89 (12) | 16.82–19.74 (8) | 13.33–14.96 (4) | 16.09–17.97 (7) | 15.54–21.06 (4) | |
| M3 | L | 37.36 | 29.09–39.01 (16) | 30.31–36.50 (12) | 31.75–37.13 (8) | 21.59–24.81 (3) | 27.27–33.26 (8) | 31.44–40.89 (60 |
| W | 21.46–24.97 (2) | 19.68–23.76 (16) | 17.44–24.94 (12) | 18.73–20.59 (8) | 14.88–16.18 (3) | 18.08–20.37 (8) | 19.95–24.30 (6) | |
| p1 | L | 7.32–7.71 (2) | 7.03–9.13 (8) | 7.25–9.51 (8) | 5.42–7.81 (3) | ? | 6.32–9.98 (6) | ? |
| W | 4.16–4.35 (2) | 3.56–4.17 (8) | 3.33–4.09 (8) | 3.22–3.88 (3) | ? | 3.50–5.15 (6) | ? | |
| p2 | L | 11.71–13.17 (4) | 10.42–13.21 (16) | 12.10–14.80 (12) | 10.77–11.89 (8) | ? | 9.96–12.02 (6) | ? |
| W | 5.55–6.66 (4) | 4.48–6.49 (16) | 4.78–6.61 (12) | 5.84–6.43 (8) | ? | 4.87–5.46 (6) | ? | |
| p3 | L | 13.17–14.31 (4) | 13.09–15.75 (16) | 14.01–16.07 (12) | 12.91–14.85 (8) | 10.31 | 11.94–14.59 (7) | 12.14–13.84 (2) |
| W | 7.84–8.60 (4) | 6.32–9.10 (16) | 6.51–8.53 (12) | 6.49–7.80 (8) | 6.57 | 6.56–7.38 (7) | 7.44–7.46 (2) | |
| p4 | L | 13.87–15.01 (3) | 13.40–16.05 (16) | 14.57–17.29 (12) | 14.44–16.10 (8) | 10.11–10.22 (2) | 12.75–14.30 (8) | 15.41–15.75 (2) |
| W | 10.13–11.68 (3) | 8.78–11.44 (16) | 9.18–10.60 (12) | 8.79–11.28 (8) | 7.46–8.34 (2) | 8.84–10.60 (8) | 9.56–10.48 (2) | |
| m1 | L | 14.32–18.47 (2) | 14.64–18.75 (16) | 15.94–19.60 (12) | 12.90–14.95 (8) | 12.34–12.61 (3) | 13.77–14.83 (8) | 15.81–17.94 (2) |
| W | 13.11–13.8 (2) | 11.55–13.94 (16) | 10.84–13.22 (12) | 11.04–13.56 (8) | 8.55–9.92 (3) | 10.80–12.07 (8) | 11.79–12.11 (2) | |
| m2 | L | 19.96–23.38 (2) | 19.66–24.24 (16) | 21.84–23.97 (12) | 19.88–21.22 (8) | 15.35–16.01 (4) | 17.19–20.84 (8) | 21.31–25.00 (3) |
| W | 17.65–18.06 (2) | 14.61–17.39 (16) | 14.61–16.56 (12) | 14.14–15.95 (8) | 10.77–13.25 (4) | 12.96–14.45 (8) | 14.15–16.30 (3) | |
| m3 | L | 40.92 | 32.92–41.27 (16) | 35.60–43.02 (12) | 37.45–40.27 (8) | 21.68–24.44 (3) | 30.56–39.84 (7) | 40.72–46.37 (4) |
| W | 19.89 | 16.71–19.32 (16) | 16.24–19.74 (12) | 15.92–17.84 (8) | 12.16–13.38 (3) | 16.06–21.44 (7) | 15.84–18.15 (4) | |
Mandibles and lower dentition: a mandible (DMR-KS-05-03-00-126) preserves both sides of cheek tooth rows (right p2–m3 and left p3–m3), but most of its symphysis and entire ramus are broken off (Fig.
Nasal: a nasal bone (DMR-KS-05-03-00-56) is short and robust, bending downward and narrowing anteriorly towards the tip (Fig.
Postcranial remains: postcranial elements include a scapula (Fig.
Postcranial remains of Rhinoceros sondaicus from Khok Sung: A–B DMR-KS-05-03-00-58, a left scapula in lateral (A) and distal (B) views C–E DMR-KS-05-03-31-3, a left humerus in anterior (C), proximal (D), and distal (E) views F–H DMR-KS-05-04-05-15, a right metacarpus IV in posterior (F), proximal (G), and distal (H) views I DMR-KS-05-04-27-19, a right calcaneus in lateral view J DMR-KS-05-03-26-23, a left astragalus in dorsal view.
Taxonomic remarks and comparisons
Four isolated cheek teeth (P2, P3, M1, and M3) assigned to R. sondaicus are characterized by the following morphological features: a presence of the moderately developed crochet, sinuosity of the ectoloph, distinct parastyle fold, and deeper median valley compared to the posterior valley, and the absences of an antecrochet, protocone fold, and metacone bulge on M3. All of these characters coincide with the upper molars of R. sondaicus (
Large tusk-like incisors (i2) are notably typical of Asian rhinoceroses. The two small alveoli corresponding to the lost central incisors are autapomorphic of Rhinoceros (
Isolated lower molars of rhinoceroses from Khok Sung are difficult to assign to either R. unicornis or R. sondaicus due to heavy wear. In addition, there is a significant size overlap between these two species (
Rhinoceros unicornis
Referred material
A left mandible with p3–m3, DMR-KS-05-03-17-13; a left p2, DMR-KS-05-03-19-4; a right M1, KS-05-03-18-X; a left femur, DMR-KS-05-03-00-63; a left astragalus, DMR-KS-05-03-00-67.
Material description
Upper dentition: a relatively worn M1 (DMR-KS-05-03-18-X) is nearly square in outline and displays a flattened ectoloph and a well developed crochet, medifossette, and posterior fossette (Fig.
Remains of Rhinoceros unicornis from Khok Sung: A DMR-KS-05-03-18-X, a right M1 in occlusal view B DMR-KS-05-03-19-4, a left p2 in occlusal view C–E DMR-KS-05-03-17-13, a left mandible in occlusal (C), medial (D), and lateral (E) views F–G DMR-KS-05-03-00-63, a left femur in posterior (F) and distal (G) views.
Mandible and lower dentition: a hemi-mandible (DMR-KS-05-03-17-13) is strongly compressed laterally and preserves a partial mandibular ramus and body with worn cheek teeth, except for the m3 which is unbroken (Fig.
Postcranial remains: an isolated femur (Fig.
Taxonomic remarks and comparisons
We assign the M1 (DMR-KS-05-03-18-X) to R. unicornis according to the presence of the flattened ectoloph and enclosed medifossette (on a worn specimen), as well as its larger size than that of R. sondaicus. These upper molar features are characteristic of R. unicornis (
Order ARTIODACTYLA Owen, 1848
Family SUIDAE Gray, 1821
Genus Sus Linnaeus, 1758
Sus barbatus
Referred material
A left maxillary fragment with P3–M2, DMR-KS-05-04-19-2; two left M2—DMR-KS-05-04-19-5 and DMR-KS-05-03-18-23 (posterior portion); two right M3—DMR-KS-05-04-03-4 and DMR-KS-05-04-19-4 (anterior portion); two mandible with two tooth rows—DMR-KS-05-03-15-1 (right: i1, i2, c1, p2, and p3 and left: i1, i2, c1, and p2–m2) and DMR-KS-05-04-19-1 (right: i1, i2, c1, and p1–m3 and left: i1, i2, c1, and p1–p4); a left posterior fragment of m3, DMR-KS-05-04-19-3; a right humerus, DMR-KS-05-03-26-8.
Material description
Upper dentition: DMR-KS-05-04-19-2 is a maxillary tooth row preserving a slightly worn P3 to M2 (Fig.
Remains of Sus barbatus from Khok Sung: A DMR-KS-05-04-19-2, a left upper cheek tooth row in occlusal view B DMR-KS-05-04-19-5, a left M2 C DMR-KS-05-03-18-23, a left fragmentary M2 D DMR-KS-05-04-03-4, a right M3; (E) DMR-KS-05-04-19-4, a right M3 F–G DMR-KS-05-03-15-1, a mandible in occlusal (F) and lateral (G) views H–I DMR-KS-05-04-19-1, a mandible in occlusal (H) and lateral (I) views J DMR-KS-05-04-19-3, a left fragmentary m3 K–N DMR-KS-05-03-26-8, a right humerus in proximal (K), posterior (L), anterior (M), and distal (N) views. Cross-sections of canines are given. All isolated teeth are shown in occlusal view.
Mandible and lower dentition: DMR-KS-05-03-15-1 is incomplete, lacking the body and ascending ramus, broken posterior to the right p3 and to the left m2 (Fig.
Lower incisors show a chisel-like appearance with long roots. The i2 is larger than the i1. Lower canines are slender and pointed, and curve backward. The lower canines of the mandible DMR-KS-05-03-15-1 belong to a male individual because of a more sharply triangular section (
Postcranial bone: DMR-KS-05-03-26-8 is a complete humerus (Fig.
Taxonomic remarks and comparisons
We compare our material to some Pleistocene Southeast Asian suid species, although only two distinct suid species, S. scrofa and S. barbatus, are known from many Pleistocene localities of mainland Southeast Asia. The sizes of the Khok Sung material are obviously larger than those of Pleistocene and extant Indonesian suids (S. brachygnathus, S. macrognathus, S. verrucosus, and S. celebensis) (Tab.
However, it is difficult to distinguish S. scrofa from S. barbatus only based on the cheek teeth because both species overlap in size (Tab.
Measurements (in millimeters) of lower canines of Khok Sung Sus barbatus. The canine index is expressed by the following formula: labial surface*100/posterior surface (
| Specimen no. | Widths | Canine index | |||
|---|---|---|---|---|---|
| anterolingual surface | posterior surface | labial surface | |||
| DMR-KS-05-03-15-1 (male) | right c1 | 13.61 | 8.54 | 11.46 | 134.2 |
| left c1 | 13.88 | 9.05 | 11.57 | 127.8 | |
| DMR-KS-05-04-19-1 (female) | right c1 | 13.18 | 11.92 | 10.32 | 86.6 |
| left c1 | 13.30 | 12.21 | 10.54 | 86.3 | |
The female mandible (DMR-KS-05-04-19-1) and other isolated teeth are assigned to S. barbatus according to those described features. We also suggest that Pleistocene Sus barbatus probably shows evidence of sexual size dimorphism because the female specimen DMR-KS-05-04-19-1 is markedly smaller than the male specimen DMR-KS-05-03-15-1, as seen in the recent population.
Family CERVIDAE Gray, 1821
Genus Axis Hamilton-Smith, 1827
Axis axis
Referred material
Four crania—DMR-KS-05-04-18-50 (with two antlers), DMR-KS-05-03-00-30 (with left partial and right broken antlers), DMR-KS-05-03-18-X9 (with pedicles), and DMR-KS-05-03-27-1 (with pedicles); two right complete antlers—DMR-KS-05-03-31-30 and DMR-KS-05-03-22-4; a nearly complete left antler, DMR-KS-05-04-4-1; five right fragmentary antlers—DMR-KS-05-03-18-21, DMR-KS-05-03-19-82, DMR-KS-05-03-28-22, DMR-KS-05-06-22-2, and DMR-KS-05-03-28-1; eight left fragmentary antlers—DMR-KS-05-03-00-12, DMR-KS-05-03-19-81, DMR-KS-05-03-22-2, DMR-KS-05-03-24-1, DMR-KS-05-04-09-1, DMR-KS-05-03-19-13, DMR-KS-05-03-26-21, and DMR-KS-05-03-08-17; two left fragmentary maxilla—DMR-KS-05-03-28-6 (with M1–M3) and DMR-KS-05-03-08-31 (with P3, P4, and M1 root); a right P4, DMR-KS-05-04-01-3; a left M1, DMR-KS-05-04-28-5; a left M2, DMR-KS-05-03-14-5; thirteen right mandibles—DMR-KS-05-03-14-2 (with m3), DMR-KS-05-03-20-1 (with p4–m3), DMR-KS-05-03-20-2 (with m2 and m3), DMR-KS-05-03-22-7 (with m2 and m3), DMR-KS-05-04-03-1 (with p2–m3), and DMR-KS-05-03-27-3 (with m2 and m3), DMR-KS-05-03-19-1 (with p2–m3), DMR-KS-05-03-22-8 (with m2 and m3), DMR-KS-05-04-01-1 (with p2–m3), DMR-KS-05-03-24-4 (with m2), DMR-KS-05-03-26-12 (with m2 and m3), DMR-KS-05-04-7-10 (with p3, m1, and m2), and DMR-KS-05-03-26-10 (with p2–m1); eight left mandibles—DMR-KS-05-03-18-22 (with p2), DMR-KS-05-03-22-6 (with m1–m3), DMR-KS-05-03-27-22 (with p3-m2 sockets and broken m3), DMR-KS-05-04-09-2 (with p3, p4, m1 and m2 sockets, and m3), DMR-KS-05-03-00-102 (with p4 and m1), DMR-KS-05-03-19-2 (with m1–m3), DMR-KS-05-03-23-1 (with p2 and p3 roots and p4–m3), and DMR-KS-05-03-29-1 (with p2-m3); a left m1, DMR-KS-05-04-28-6; three m2—DMR-KS-05-03-25-4 (right), DMR-KS-05-03-00-104 (left), and DMR-KS-05-03-22-11 (left); four left m3—DMR-KS-05-04-9-4, DMR-KS-05-03-22-9, DMR-KS-05-04-01-2, and DMR-KS-05-03-08-33; three right fragmentary humeri (distal part)—DMR-KS-05-03-13-4, DMR-KS-05-04-11-32, and DMR-KS-05-03-17-17; six metacarpi—DMR-KS-05-03-18-2 (right), DMR-KS-05-03-19-3 (right), DMR-KS-05-03-22-28 (right), DMR-KS-05-03-08-2 (right), DMR-KS-05-04-30-20 (right proximal fragment), and DMR-KS-05-03-19-37 (left); a right fragmentary femur, DMR-KS-05-03-27-4 (distal part); three metatarsi—DMR-KS-05-03-26-3 (right), DMR-KS-05-03-29-30 (left), and DMR-KS-05-03-15-14 (left).
Material description
Crania and upper dentition: four crania are almost complete, lacking only the anterior portions (e.g., nasal, jugal, palatine, and maxilla) (Fig.
Cranial remains of Axis axis from Khok Sung: A–B DMR-KS-05-04-18-50, a cranium with nearly complete antlers in dorsal (A) and ventral (B) views C–D DMR-KS-05-03-00-30, a cranium in lateral (C) amd ventral (D) views E DMR-KS-05-03-18-X9, a cranium in anterior view F–G DMR-KS-05-03-27-1 a cranium in dorsal (F) and ventral (G) views H DMR-KS-05-03-31-30, a right antler in anterior view; (I) DMR-KS-05-03-22-4, a right antler in lateral view J DMR-KS-05-03-18-21, a left antler fragment in lateral view KDMR-05-03-22-2, a left antler fragment in lateral view L DMR-KS-05-03-19-81, a left antler fragment in medial view.
P3 and P4 are similar to recent Axis, characterized by well-developed styles, medial cristae (more distinct on the P4), and posterolingual fossettes (Fig.
Dental remains of Axis axis from Khok Sung: A DMR-KS-05-03-08-31, an upper left P3 and P4 in occlusal view B DMR-KS-05-03-28-6, a left upper molar row in occlusal view C DMR-KS-05-04-01-3, a right P4 in occlusal view D DMR-KS-05-04-28-5, a left M1 in occlusal view E DMR-KS-05-03-14-5, a left M2 in occlusal view F–G DMR-KS-05-03-29-1, a left mandible in occlusal (F) and lateral (G) views H–I DMR-KS-05-03-26-10, a right mandibular fragment in occlusal (H) and medial (I) views J–K DMR-KS-05-04-03-1, a right mandible in occlusal (J) and lateral (K) views L–M DMR-KS-05-03-20-1, a right mandible in occlusal (L) and lateral (M) views N–O DMR-KS-05-03-22-7, a right mandible in occlusal (N) and lateral (O) views P–Q DMR-KS-05-03-08-33, a left m3 in occlusal (P) and buccal (Q) views.
Measurements (lengths and widths in millimeters) of cervid teeth from Khok Sung. N=number of specimens.
| Length | Width | |||||
|---|---|---|---|---|---|---|
| N | Range | Mean | N | Range | Mean | |
| Axis axis | ||||||
| P3 | 1 | 12.40 | – | 1 | 13.60 | – |
| P4 | 2 | 10.04–11.29 | 10.67 | 2 | 12.19–14.28 | 13.24 |
| M1 | 2 | 13.32–15.19 | 14.26 | 2 | 15.60–15.93 | 15.77 |
| M2 | 2 | 18.07–18.08 | 18.08 | 2 | 17.41–17.84 | 17.63 |
| M3 | 1 | 17.53 | – | 1 | 16.42 | – |
| p2 | 6 | 7.93–9.54 | 8.72 | 6 | 5.44–6.89 | 5.93 |
| p3 | 7 | 9.17–12.11 | 10.67 | 7 | 6.53–7.14 | 6.88 |
| p4 | 8 | 10.64–13.62 | 11.65 | 10 | 6.77–8.13 | 7.39 |
| m1 | 9 | 11.81–18.20 | 14.2 | 13 | 8.27–10.29 | 9.59 |
| m2 | 18 | 15.94–21.42 | 17.91 | 19 | 8.56–11.67 | 10.56 |
| m3 | 18 | 21.69–25.78 | 24.1 | 20 | 8.87–11.89 | 10.74 |
| Panolia eldii | ||||||
| P2 | 1 | 11.09 | – | 1 | 13.97 | – |
| M1 | 2 | 12.07–14.95 | 13.51 | 2 | 16.52–17.77 | 17.15 |
| M2 | 5 | 16.67–20.48 | 19.35 | 6 | 17.85–19.35 | 18.56 |
| M3 | 5 | 18.80–21.39 | 19.96 | 5 | 16.99–19.50 | 18.30 |
| i1 | 1 | 12.86 | – | 1 | 6.31 | – |
| p2 | 2 | 9.97–11.33 | 10.65 | 2 | 7.03–7.44 | 7.24 |
| p3 | 2 | 13.04–13.67 | 13.36 | 2 | 8.33–8.56 | 8.45 |
| p4 | 2 | 13.65–14.05 | 13.85 | 2 | 8.94–9.33 | 9.14 |
| m1 | 2 | 14.67–15.67 | 15.17 | 2 | 11.23–12.25 | 11.74 |
| m2 | 2 | 17.73–19.36 | 18.55 | 2 | 12.63–13.26 | 12.95 |
| m3 | 1 | 23.61 | 1 | 12.84 | ||
| Rusa unicolor | ||||||
| M1 | 1 | 17.15 | – | 1 | 20.10 | – |
| M2 | 2 | 20.67–22.88 | 21.78 | 2 | 23.06–27.07 | 25.07 |
| M3 | 1 | 25.37 | – | 1 | 24.97 | – |
| p3 | 1 | 17.29 | – | 1 | 9.26 | – |
| p4 | 1 | 17.71 | – | 2 | 10.34–13.35 | 11.85 |
| m1 | 2 | 18.64–20.84 | 19.74 | 2 | 14.39–14.59 | 14.49 |
| m2 | 3 | 22.77–23.82 | 23.33 | 3 | 15.37–15.61 | 15.46 |
| m3 | 3 | 30.78–34.57 | 32.67 | 3 | 15.49–17.85 | 16.79 |
Mandibles and lower dentition: twenty one mandibles range from fragmentary (preserving only the broken corpus) to nearly complete (lacking only the ascending ramus and coronoid process) individuals (Fig.
Lower third and fourth premolars exhibit a well developed metaconid which projects obliquely in occlusal view, posterior to the entoconid (Fig.
Postcranial remains: postcranial bones include isolated humeri (Fig.
Postcranial remains of Axis axis from Khok Sung: A–B DMR-KS-05-04-11-32, a right distal humerus in anterior (A) and distal (B) views C–E DMR-KS-05-03-18-2, a right metacarpus in proximal (C), anterior (D), and distal (E) views F–H DMR-KS-05-03-19-37, a left metacarpus in proximal (F), anterior (G), and distal (H) views I–J DMR-KS-05-03-27-4, a right distal femur in posterior (I) and distal (J) views K–M DMR-KS-05-03-26-3, a right metatarsus in proximal (K), anterior (L), and distal (M) views.
Proportional indices of postcranial remains of identified ruminant taxa from Khok Sung.
| Scapula | ||||||||||||
| Specimen | Taxa | HS/Ld | DHA/Ld | Ld/SLC | LG/BG | GLP/LG | SLC/BG | |||||
| DMR-KS-05-03-26-2 | Bubalus arnee | 1.50 | 1.28 | 3.89 | 1.20 | 1.30 | 1.12 | |||||
| DMR-KS-05-02-20-4 | Bubalus arnee | 1.39 | 1.42 | 4.09 | 1.23 | 1.26 | 0.96 | |||||
| DMR-KS-05-06-24-4 | Panolia eldii | 1.95 | 1.90 | 4.62 | 1.10 | 1.27 | 0.74 | |||||
| Humerus | ||||||||||||
| Specimen | Taxa | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dp/Dd | Bp/Dp | Bd/Dd | Bd/BT | ||
| DMR-KS-05-03-20-2(1) | Bos sauveli | – | – | – | – | – | – | – | 0.99 | 1.04 | ||
| DMR-KS-05-03-00-62 | Bos gaurus | – | – | 3.41 | 3.66 | – | – | – | 1.07 | 1.06 | ||
| DMR-KS-05-05-1-1 | Bos gaurus | 2.91 | 2.74 | 3.44 | 3.67 | 1.18 | 1.34 | 0.94 | 1.07 | 1.05 | ||
| DMR-KS-05-03-31-1 | Bubalus arnee | 3.57 | 3.25 | 4.30 | 4.77 | 1.21 | 1.47 | 0.91 | 1.11 | 1.05 | ||
| DMR-KS-05-03-31-8 | Bubalus arnee | 3.54 | 3.29 | 4.25 | 4.74 | 1.20 | 1.44 | 0.93 | 1.11 | 1.03 | ||
| DMR-KS-05-03-13-4 | Axis axis | – | – | – | – | – | – | – | 1.02 | 1.09 | ||
| DMR-KS-05-04-11-32 | Axis axis | – | – | – | – | – | – | – | 1.06 | 1.07 | ||
| DMR-KS-05-03-17-17 | Axis axis | – | – | – | – | – | – | – | 1.12 | 1.04 | ||
| DMR-KS-05-04-11-35 | Panolia eldii | – | – | – | – | – | – | – | 1.12 | 1.13 | ||
| DMR-KS-05-03-18-1 | Panolia eldii | – | – | – | – | – | – | 0.82 | – | – | ||
| DMR-KS-05-03-15-43 | Rusa unicolor | – | – | – | – | – | – | – | 1.14 | 1.12 | ||
| Ulna and radius | ||||||||||||
| Specimen | Taxa | PL/Bp | PL/Dp | PL/Bd | PL/Dd | Bp/Bd | Dd/Dp | Bp/Dp | Bd/Dd | Bp/BFp | Bd/BFd | GL/LO |
| DMR-KS-05-03-00-61 | Bubalus arnee | 2.87 | 5.76 | 3.04 | 4.63 | 1.06 | 1.24 | 2.00 | 1.52 | 1.15 | 1.11 | 3.86 |
| DMR-KS-05-03-31-2 | Bubalus arnee | 3.15 | 5.85 | 3.25 | 4.61 | 1.03 | 1.27 | 1.86 | 1.42 | 1.09 | 1.12 | 3.48 |
| DMR-KS-05-03-31-9 | Bubalus arnee | 3.09 | 5.88 | 3.24 | 4.55 | 1.05 | 1.29 | 1.90 | 1.40 | 1.10 | 1.12 | 3.45 |
| DMR-KS-05-03-31-10 | Panolia eldii | 5.06 | 9.51 | 5.35 | 9.32 | 1.06 | 1.02 | 1.88 | 1.74 | 1.07 | 1.14 | – |
| DMR-KS-05-04-11-3 | Panolia eldii | 4.83 | 9.09 | 5.54 | 8.70 | 1.15 | 1.04 | 1.88 | 1.57 | 1.11 | 1.06 | – |
| DMR-KS-05-03-19-16 | Panolia eldii | 4.93 | 8.93 | 4.87 | 6.62 | 0.99 | 1.35 | 1.81 | 1.36 | 1.22 | 1.04 | – |
| DMR-KS-05-03-25-9 | Rusa unicolor | – | – | – | – | – | – | 1.90 | – | 1.03 | – | – |
| DMR-KS-05-03-19-14 | Rusa unicolor | – | – | – | – | – | – | 1.70 | – | 1.04 | – | – |
| DMR-KS-05-03-26-19 | Rusa unicolor | – | – | – | – | – | – | – | 1.34 | – | 1.05 | – |
| Femur | ||||||||||||
| Specimen | Taxa | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dd/Dp | Bp/Dp | Dd/Bd | |||
| DMR-KS-05-03-9-2 | Bos gaurus | 3.37 | 6.29 | 3.92 | 3.03 | 1.17 | 2.07 | 1.87 | 1.29 | |||
| DMR-KS-05-04-1-1 | Bubalus arnee | 2.79 | 5.54 | 3.48 | 2.85 | 1.25 | 1.95 | 1.99 | 1.22 | |||
| DMR-KS-05-04-1-2 | Bubalus arnee | 2.67 | 5.26 | 3.38 | 2.82 | 1.27 | 1.86 | 1.97 | 1.20 | |||
| DMR-KS-05-03-20-8 | Bubalus arnee | – | – | – | – | – | – | – | 1.46 | |||
| DMR-KS-05-03-27-4 | Axis axis | – | – | – | – | – | – | – | 1.37 | |||
| DMR-KS-05-03-27-11 | Panolia eldii | – | – | – | – | 1.26 | 2.23 | 2.11 | 1.33 | |||
| DMR-KS-05-03-17-36 | Panolia eldii | – | – | – | – | 1.21 | 2.06 | 1.93 | 1.29 | |||
| DMR-KS-05-03-28-20 | Panolia eldii | – | – | – | – | – | – | – | 1.34 | |||
| DMR-KS-05-04-05-38 | Panolia eldii | – | – | – | – | – | – | 1.92 | – | |||
| DMR-KS-05-03-00-119 | Panolia eldii | – | – | – | – | – | – | – | 1.38 | |||
| DMR-KS-05-03-19-2 | Panolia eldii | – | – | – | – | – | – | – | 1.41 | |||
| DMR-KS-05-08-16-1 | Panolia eldii | – | – | – | – | – | – | 1.84 | – | |||
| DMR-KS-05-04-11-2 | Rusa unicolor | – | – | – | – | – | – | – | 1.27 | |||
| DMR-KS-05-03-19-7 | Rusa unicolor | – | – | – | – | – | – | 1.51 | – | |||
| DMR-KS-05-03-12-2* | Rusa unicolor | – | – | – | – | – | – | 1.52 | – | |||
| DMR-KS-05-04-30-9 | Rusa unicolor | – | – | – | – | – | – | – | 1.27 | |||
| DMR-KS-05-04-19-10 | Rusa unicolor | – | – | – | – | – | – | – | 1.11 | |||
| Tibia | ||||||||||||
| Specimen | Taxa | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dp/Dd | Bp/Dp | Bd/Dd | |||
| DMR-KS-05-04-1-11 | Bubalus arnee | 3.24 | 3.43 | 4.82 | 6.03 | 1.49 | 1.76 | 1.06 | 1.25 | |||
| DMR-KS-05-04-1-3 | Bubalus arnee | 3.31 | 3.50 | 5.01 | 6.29 | 1.51 | 1.80 | 1.06 | 1.25 | |||
| DMR-KS-05-03-20-9 | Bubalus arnee | 3.21 | 3.83 | 4.60 | 6.29 | 1.43 | 1.64 | 1.19 | 1.37 | |||
| DMR-KS-05-03-28-16 | Rusa unicolor | 4.00 | 4.38 | 6.68 | 8.48 | 1.67 | 1.94 | 1.10 | 1.27 | |||
| Metacarpus | ||||||||||||
| Specimen | Taxa | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dp/Dd | Bp/Dp | Bd/Dd | |||
| DMR-KS-05-03-26-27 | Bos gaurus | 3.66 | 5.57 | 3.96 | 7.66 | 1.08 | 1.37 | 1.52 | 1.93 | |||
| DMR-KS-05-03-26-3(1) | Bubalus arnee | 2.68 | 4.17 | 2.64 | 4.87 | 0.98 | 1.17 | 1.55 | 1.85 | |||
| DMR-KS-05-03-18-2 | Axis axis | 6.50 | 9.99 | 6.69 | 10.55 | 1.03 | 1.06 | 1.54 | 1.58 | |||
| DMR-KS-05-03-22-28 | Axis axis | – | 9.59 | 6.81 | 10.36 | – | 1.08 | – | 1.52 | |||
| DMR-KS-05-03-08-2 | Axis axis | 6.36 | 8.79 | 6.18 | 10.18 | 0.97 | 1.16 | 1.38 | 1.65 | |||
| DMR-KS-05-03-19-3 | Axis axis | 6.58 | 9.06 | 6.30 | 10.42 | 0.96 | 1.15 | 1.38 | 1.65 | |||
| DMR-KS-05-03-19-37 | Axis axis | 7.14 | 11.05 | 6.84 | 10.75 | 0.96 | 0.97 | 1.55 | 1.57 | |||
| DMR-KS-05-04-30-20 | Axis axis | 6.87 | 10.36 | – | – | – | – | 1.51 | – | |||
| DMR-KS-05-03-24-2 | Panolia eldii | 6.39 | 8.99 | 6.57 | 10.41 | 1.03 | 1.16 | 1.41 | 1.58 | |||
| DMR-KS-05-03-17-26 | Rusa unicolor | 5.97 | 7.57 | 6.06 | 9.10 | 1.02 | 1.20 | 1.27 | 1.50 | |||
| Metatarsus | ||||||||||||
| Specimen | Taxa | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dp/Dd | Bp/Dp | Bd/Dd | |||
| DMR-KS-05-04-1-8 | Bubalus arnee | 3.80 | 4.59 | 3.17 | 5.54 | 0.83 | 1.21 | 1.21 | 1.75 | |||
| DMR-KS-05-04-1-6 | Bubalus arnee | 3.88 | 4.39 | 3.11 | 5.67 | 0.80 | 1.29 | 1.13 | 1.82 | |||
| DMR-KS-05-03-28-30 | Bubalus arnee | 4.25 | 4.28 | 3.40 | 6.38 | 0.80 | 1.49 | 1.01 | 1.88 | |||
| DMR-KS-05-03-26-3 | Axis axis | 7.21 | 6.91 | 6.99 | 9.16 | 0.97 | 1.33 | 0.96 | 1.31 | |||
| DMR-KS-05-03-15-14 | Axis axis | 6.84 | 7.37 | 6.15 | 9.22 | 0.90 | 1.25 | 1.08 | 1.50 | |||
| DMR-KS-05-03-29-30 | Axis axis | 6.91 | 6.82 | 6.52 | 8.58 | 0.94 | 1.26 | 0.99 | 1.32 | |||
| DMR-KS-05-03-28-17 | Panolia eldii | 8.05 | 7.71 | 7.73 | 11.69 | 0.96 | 1.52 | 0.96 | 1.51 | |||
| DMR-KS-05-03-25-8 | Panolia eldii | 7.81 | 7.47 | 7.22 | 11.57 | 0.92 | 1.55 | 0.96 | 1.60 | |||
| DMR-KS-05-03-15-15 | Panolia eldii | 8.08 | 7.44 | 7.37 | 11.29 | 0.91 | 1.52 | 0.92 | 1.53 | |||
| DMR-KS-05-03-19-11 | Rusa unicolor | 6.64 | 6.86 | 6.49 | 9.20 | 0.98 | 1.34 | 1.03 | 1.42 | |||
Taxonomic remarks and comparisons
The antlers are useful to distinguish among the cervids, whereas the morphologies of lower cheek teeth are identical among Axis. The skulls, antlers, and teeth from Khok Sung are morphologically similar to those observed from recent A. axis. This suggests a morphological stasis in the evolution of antlers and teeth for this species.
Based on our observation on the extant comparative material of A. axis (e.g., the specimens
Body mass prediction of Khok Sung ruminants using second molar variables, compared to relative sizes of the recent population (
| Body mass (kg) | ||||
|---|---|---|---|---|
| Cervidae | Khok Sung | Recent | ||
| Taxa | N | Range | Mean | Range |
| Axis axis | 17 | 67.6–127.6 | 90.8 | 75–100 |
| Panolia eldii | 7 | 99.1–157.6 | 133.5 | 95–150 |
| Rusa unicolor | 5 | 215.6–332.3 | 255.4 | 100–350 |
| Bovidae | Khok Sung | Recent | ||
| Taxa | N | Range | Mean | Range |
| Bos sauveli | 3 | 660.8–756.0 | 720.5 | 700–900 |
| Bos gaurus | 3 | 808.5–940.8 | 873.2 | 700–1000 |
| Bubalus arnee | 12 | 694.5–1243.0 | 944.7 | 700–1200 |
Compared to other Pleistocene cervid species, the cheek teeth of A. axis from Khok Sung are smaller than those of A. shansius from Anhui and Yunnan (China) and of A. javanicus from Ngandong and Buitenzorg in Java and Carnul Cave in India, but are larger than those of A. lydekkeri from Trinil H. K. (Java) (Figs
Genus Panolia Gray, 1843
Panolia eldii
Referred material
A cranium with a right partial antler, DMR-KS-05-04-20-4; a right P2, DMR-KS-05-03-15-11; two left M1—DMR-KS-05-03-00-24 and DMR-KS-05-03-00-25; six M2—DMR-KS-05-03-00-23 (right), DMR-KS-05-03-30-5 (right), DMR-KS-05-04-3-4 (right), DMR-KS-05-03-30-6 (left posterior lobe), DMR-KS-05-03-27-7 (left), and DMR-KS-05-04-3-5 (left); five M3—DMR-KS-05-03-27-6 (right), DMR-KS-05-04-9-1 (right), DMR-KS-05-04-8-3 (right), DMR-KS-05-03-00-22 (left), and DMR-KS-05-04-9-2 (left); two left mandibles—DMR-KS-05-03-27-2 (with p2–m3) and DMR-KS-05-04-9-5 (with p2–m2); a right i1, DMR-KS-05-03-29-2; a right scapula, DMR-KS-05-06-24-4; a left humerus, DMR-KS-05-04-11-35; a right fragmentary humerus, DMR-KS-05-03-18-1 (proximal part); three radii—DMR-KS-05-03-31-10 (right), DMR-KS-05-04-11-3 (right), and DMR-KS-05-03-19-16 (left); a right metacarpus, DMR-KS-05-03-24-2; two right femora—DMR-KS-05-03-27-11 and DMR-KS-05-03-17-36; five fragmentary femora—DMR-KS-05-04-05-38 (right proximal part), DMR-KS-05-03-28-20 (right distal part), DMR-KS-05-03-00-119 (right distal part), DMR-KS-05-03-19-2 (right distal part), and DMR-KS-05-08-16-1 (left proximal part); three left metatarsi—DMR-KS-05-03-25-8, DMR-KS-05-03-28-17, and DMR-KS-05-03-15-15.
Material description
Cranium and upper dentition: DMR-KS-05-04-20-4 is an incomplete cranium, lacking the whole anterior parts (nasal, jugal, palatine, and maxilla) (Fig.
Remains of Panolia eldii from Khok Sung: A–C DMR-KS-05-04-20-4, a cranium in dorsal (A), lateral (B), and ventral (C) views D DMR-KS-05-03-15-11, a right P2 E DMR-KS-05-03-00-24, a left M1 F DMR-KS-05-03-00-23, a right M2 G DMR-KS-05-03-27-6, a left M3 H DMR-KS-05-04-9-2, a left M3 I DMR-KS-05-03-29-2, a right i1 in lingual view J–K DMR-KS-05-03-27-2, a left mandible in lateral (J) and occlusal (K) views L–M DMR-KS-05-04-9-5, a left mandible in occlusal (L) and lateral (M) views. All teeth are shown in occlusal view.
P2 exhibits a prominent medial crista which divides the fossette into two islands (Fig.
Mandibles and lower dentition: Two mandibles (DMR-KS-05-03-27-2: Fig.
An isolated i1 is spatulate (Fig.
Postcranial remains: postcranial bones include a scapula (Fig.
Postcranial remains of Panolia eldii from Khok Sung: A–B DMR-KS-05-06-24-4, a right scapula in lateral (A) and distal (B) views C–E DMR-KS-05-03-18-1, a right proximal humerus in proximal (C), anterior (D), and posterior (E) views F–H DMR-KS-05-03-31-10, a right radius in proximal (F), anterior (G), distal (H) views I–K DMR-KS-05-03-24-2, a right metacarpus in proximal (I), anterior (J), and distal (K) views L–N DMR-KS-05-03-25-8, a left metatarsus in proximal (L), anterior (M), distal (N) views O–Q DMR-KS-05-03-17-36, a right femur in proximal (O), posterior (P), distal (Q) views.
Taxonomic remarks and comparisons
Several authors consider Eld’s deer as belonging to either the genus Cervus (e.g.,
The shed antler of the Eld’s deer, Panolia eldii, is characterized by bow- or lyre-like shapes, long, noticeable, and laterally bending-main beams with a distal portion curving medially, and small ornamented branches of brow tines. The cheek teeth of P. eldii differ from those of A. axis in having a larger size, a more complex wear pattern of the mesolingual conids on the p3, more developed anterior cingulids on the lower molars, and a posterior ectostylid on the m3. The Khok Sung specimens assigned to P. eldii are similar in morphology to the extant specimens. As demonstrated by the body mass estimation (Tab.
Scatter diagrams of upper cheek tooth (P2, M1, M2, and M3) lengths and widths of some recent and fossil large cervids. The measurements of fossil cervids from the caves of Phnom Loang, Thum Wiman Nakin, and Ma U’Oi are obtained from
Scatter diagrams of lower cheek tooth (p2–m3) lengths and widths of some recent and fossil large cervids. The measurements of fossil cervids from the caves of Thum Wiman Nakin, Thum Prakai Phet, and Ma U’Oi are obtained from
Genus Rusa Hamilton-Smith, 1827
Rusa unicolor
Referred material
Three right antlers—DMR-KS-05-03-20-11 (nearly complete specimen), DMR-KS-05-03-26-2 (fragment), and DMR-KS-05-03-28-23 (fragment); a right M1, DMR-KS-05-03-22-10; two left M2—DMR-KS-05-04-9-3 and DMR-KS-05-04-3-3; a left M3, DMR-KS-05-03-31-1; two right mandibles—DMR-KS-05-03-31-2 (with m2) and DMR-KS-05-03-13 (with p4–m3); two left mandibles—DMR-KS-05-03-00-101 (with p3–m3) and DMR-KS-05-03-27-4 (with m3); a right m1, DMR-KS-05-03-00-5; a left fragmentary humerus, DMR-KS-05-03-15-43 (distal part); three right fragmentary radii—DMR-KS-05-03-25-9 (proximal part), DMR-KS-05-03-19-14 (proximal part), and DMR-KS-05-03-26-19 (distal part); a left metacarpus, DMR-KS-05-03-17-26; six fragmentary femora—DMR-KS-05-03-19-7 (right proximal part), DMR-KS-05-03-12-2 (right proximal part), DMR-KS-05-04-11-2 (right distal part), DMR-KS-05-03-26-5 (left proximal part), DMR-KS-05-04-30-9 (left distal part), and DMR-KS-05-04-19-10 (left distal part); a right tibia, DMR-KS-05-03-28-16; a right metatarsus, DMR-KS-05-03-19-11
Material description
Antlers: DMR-KS-05-03-20-11 is a nearly complete antler, slightly broken at the middle part of the main beam (Fig.
Remains of Rusa unicolor from Khok Sung: A DMR-KS-05-03-20-11, a right antler in lateral view B DMR-KS-05-03-26-2, a right antler fragment in lateral view C DMR-KS-05-03-28-23, a right antler fragment in medial view D DMR-KS-05-04-9-3, a left M2 E DMR-KS-05-03-31-1, a left M3 F–G DMR-KS-05-03-13, a right mandible in lateral (F) and occlusal (G) views H–I DMR-KS-05-03-00-101, a left mandible in lateral (H) and occlusal (I) views J DMR-KS-05-03-00-5, a right m1 K DMR-KS-05-03-27-4, a left m3. All isolated teeth are shown in occlusal view.
Upper dentition: upper molars are robust (Tab.
Mandibles and lower dentition: four mandibles are incomplete (for measurements, see Appendix
Postcranial remains: postcranial elements include a humerus (Fig.
Postcranial remains of Rusa unicolor from Khok Sung: A–C DMR-KS-05-03-15-43, a left humerus in anterior (A), posterior (B), and distal (C) views D–E DMR-KS-05-03-19-14, a right proximal radius in proximal (D) and anterior (E) views F–G DMR-KS-05-03-26-19, a right distal radius in anterior (F) and distal (G) views H–J DMR-KS-05-03-17-26, a left metacarpus in proximal (H), anterior (I), and distal (J) views K–L DMR-KS-05-03-19-7, a right proximal femur in proximal (K) and anterior (L) views M–N DMR-KS-05-04-30-9, a left distal femur in posterior (M) and distal (N) views O–Q DMR-KS-05-03-28-16, a right tibia in proximal (O), anterior (P), and distal (Q) views R–T DMR-KS-05-03-19-11, a right metatarsus in proximal (R), anterior (S), and distal (T) views.
Taxonomic remarks and comparisons
According to Leslie (2011), we regard here Rusa as a separate genus within the family Cervidae. Four species are currently recognized: R. unicolor (sambar), R. marianna (Philippine deer), R. timorensis (rusa), and R. alfredi (Prince Alfred’s deer).
Antlers of R. unicolor are characterized by its typical three tines and forked beams at the tip, similar in shape to those of Axis porcinus but much more robust. The sambar deer shares a similar dental morphology with the Eld’s deer. But it differs from P. eldii as well as A. axis in being larger-sized and in having more developed anterior cingulids on lower molars. The sambar deer is much larger than A. axis (Figs
Family BOVIDAE Gray, 1821
Genus Bos Linnaeus, 1758
Bos sauveli
Referred material
A left DP3, DMR-KS-05-03-29-8; a left P3, DMR-KS-05-04-01-4; a left fragmentary M1 or M2 (posterior portion), DMR-KS-05-03-23-2; a right M3, DMR-KS-05-03-29-6; a right mandible with m1–m3, DMR-KS-05-03-9-1; two left mandibles—DMR-KS-05-04-9-1 (with p2, p4, and m1–m3) and DMR-KS-05-04-29-1 (with m3); a left i2, DMR-KS-05-03-15-12; a right i3, DMR-KS-05-03-23-4; a right p2, DMR-KS-05-04-01-6; a right m1, DMR-KS-05-03-15-10; a right m2, DMR-KS-05-03-29-7; two m3—DMR-KS-05-04-28-4 (right broken posterior lobe) and DMR-KS-05-03-24-5 (left); a left humerus, DMR-KS-05-03-20-2(1).
Material description
Upper dentition: DP3 (DMR-KS-05-03-29-8) is molariform and elongated, characterized by well-developed anterior and posterior cingula, buccal styles, and medial fossettes, a slightly-developed entostyle, and a reduction of the anterior lobe width and height compared to the posterior lobe (Fig.
Remains of Bos sauveli from Khok Sung: A DMR-KS-05-03-29-8, a left DP3 B DMR-KS-05-04-01-4, a left P3 C–D DMR-KS-05-03-23-2, a left M1 or M2 in occlusal (C) and lingual (D) views E DMR-KS-05-03-29-6, a right M3 F DMR-KS-05-04-01-6, a right p2 G DMR-KS-05-04-28-4, a broken right m3 H–I DMR-KS-05-04-9-1, a left mandible in occlusal (H) and lateral (I) views J–K DMR-KS-05-03-9-1, a right mandible in occlusal (J) and lateral (K) views L–N DMR-KS-05-03-20-2(1), a left humerus in anterior (L), posterior (M), and distal (N) views. All isolated teeth are shown in occlusal view.
Measurements (lengths and widths in millimeters) of large bovid teeth from Khok Sung. N=number of specimens.
| Length | Width | |||||
|---|---|---|---|---|---|---|
| N | Range | Mean | N | Range | Mean | |
| Bos sauveli | ||||||
| DP3 | 1 | 27.39 | – | 1 | 14.91 | – |
| P3 | 1 | 17.57 | – | 1 | 19.71 | – |
| M1 or M2 | – | – | – | 1 | 25.63 | – |
| M3 | 1 | 35.46 | – | 1 | 23.55 | – |
| i2 | 1 | 13.67 | – | 1 | 11.53 | – |
| i3 | 1 | 13.68 | – | 1 | 8.68 | – |
| p2 | 2 | 14.13–14.77 | 14.45 | 2 | 8.52–10.39 | 9.46 |
| p4 | 1 | 23.39 | – | 1 | 12.91 | – |
| m1 | 3 | 27.24–27.96 | 27.72 | 3 | 17.21–18.26 | 17.73 |
| m2 | 3 | 29.70–32.47 | 30.11 | 3 | 17.87–18.79 | 18.29 |
| m3 | 3 | 40.60–47.60 | 43.78 | 5 | 17.09–19.91 | 18.37 |
| Bos gaurus | ||||||
| DP2 | 1 | 22.28 | – | 1 | 10.67 | – |
| P2 | 2 | 19.42–20.79 | 20.11 | 2 | 13.55–15.58 | 14.57 |
| DP3 | 1 | 28.73 | – | 1 | 18.97 | – |
| DP4 | 1 | 29.75 | – | 1 | 22.55 | – |
| M1 | 1 | 26.33 | – | 1 | 29.95 | – |
| M3 | 1 | 36.96 | – | 1 | 26.94 | – |
| i1 | 1 | 20.30 | – | 1 | 11.35 | – |
| p2 | 1 | 13.77 | – | 1 | 8.56 | – |
| p3 | 1 | 21.58 | – | 1 | 11.92 | – |
| p4 | 1 | 21.11 | – | 1 | 12.72 | – |
| m1 | 2 | 25.29–28.67 | 26.98 | 2 | 18.25–19.28 | 18.77 |
| m2 | 3 | 30.36–35.09 | 32.82 | 3 | 19.00–20.07 | 19.45 |
| m3 | 2 | 42.56–46.23 | 44.40 | 2 | 18.72–18.79 | 18.76 |
| Bubalus arnee | ||||||
| P2 | 3 | 22.30–26.78 | 24.04 | 3 | 14.47–17.26 | 15.76 |
| DP3 | 1 | 31.92 | – | 1 | 19.75 | – |
| P3 | 7 | 17.85–25.03 | 21.58 | 7 | 15.56–21.93 | 20.32 |
| DP4 | 1 | 31.60 | – | 1 | 23.36 | – |
| P4 | 7 | 17.76–23.55 | 20.46 | 7 | 21.01–23.20 | 22.34 |
| M1 | 9 | 25.73–33.16 | 28.61 | 8 | 26.01–29.79 | 27.30 |
| M2 | 8 | 30.45–36.18 | 33.11 | 7 | 26.09–29.23 | 27.23 |
| M3 | 6 | 33.74–37.40 | 36.07 | 6 | 25.26–27.30 | 26.29 |
| i1 | 1 | 21.21 | – | 1 | 10.31 | – |
| i2 | 1 | 16.17 | – | 1 | 11.94 | – |
| i3 | 1 | 16.61 | – | 1 | 11.63 | – |
| i4 | 1 | 15.82 | – | 1 | 8.80 | – |
| p2 | 4 | 13.56–16.24 | 15.05 | 4 | 8.01–9.80 | 8.87 |
| dp3 | 2 | 21.59–23.20 | 22.40 | 2 | 8.65–9.90 | 9.28 |
| p3 | 3 | 21.88–23.09 | 22.30 | 3 | 10.23–13.09 | 11.80 |
| dp4 | 3 | 37.25–42.59 | 40.74 | 3 | 13.34–15.24 | 14.39 |
| p4 | 2 | 23.81–24.97 | 24.39 | 3 | 11.93–13.26 | 12.76 |
| m1 | 9 | 30.49–36.77 | 32.66 | 6 | 17.67–20.36 | 18.94 |
| m2 | 6 | 32.13–39.20 | 36.03 | 5 | 19.00–21.22 | 20.18 |
| m3 | 3 | 46.52–48.33 | 47.29 | 4 | 17.64–20.72 | 19.66 |
Mandible and lower dentition: two mandibles, DMR-KS-05-03-9-1 (Fig.
The i2 (DMR-KS-05-03-22-15) and i3 (DMR-KS-05-03-23-4) are spatulate and small, compared to other species of Bos (for measurements, see Tab.
Postcranial remains: a humerus, DMR-KS-05-03-20-2(1), preserves the shaft and distal part (Fig. L–N). We attribute this humerus to B. sauveli according to the proportional correlation with the extant specimens (Tab.
Taxonomic remarks and comparisons
Southeast Asian large bovids are accurately identified by differences in cranial features (especially horn cores), although they show sexual and ontogenetic variation in morphology. Lacking the cranial remains, it is difficult to make a distinction within the species of Bos. Due to the lack of cranial remains of koupreys (B. sauveli) collected from Khok Sung, we identify these fossils on the basis of dental features.
Based on our comparisons with some extant specimens (
Scatter diagrams of lower cheek tooth (p2–m3) widths of recent and fossil large bovids. Fossil data from Phnom Loang, Lang Trang, Thum Wiman Nakin, Thum Prakai Phet, Duoi U’Oi, and Tam Hang South are from
According to the molecular phylogenetic analyses, the kouprey may have been domesticated in Cambodia (
Bos gaurus
Referred material
A left horn core, DMR-KS-05-03-26-22; a right DP2, DMR-KS-05-03-20-4; two right P2—DMR-KS-05-03-19-27 and DMR-KS-05-04-03-3; a right DP3, DMR-KS-05-03-20-3; a right DP4, DMR-KS-05-03-17-3; a right M1, DMR-KS-05-03-00-20; a right M3, DMR-KS-05-03-17-1; a right mandible with m1–m3, DMR-KS-05-03-00-1; a left mandible with p2–m3, DMR-KS-05-04-3-1; a left i1, DMR-KS-05-03-00-27; two left m2—DMR-KS-05-03-19-26 and DMR-KS-05-03-16-1; two humeri—DMR-KS-05-05-1-1 (right) and DMR-KS-05-03-00-62 (left); a right metacarpus, DMR-KS-05-03-26-27; two left femora—DMR-KS-05-03-9-2 and DMR-KS-05-04-30-1 (proximal part).
Material description
Horn core: a single horn core (DMR-KS-05-03-26-22) is small, curved upward (Fig.
Remains of Bos gaurus from Khok Sung: A–B DMR-KS-05-03-26-22, a left horn core in posterior (A) and anterior (B) view C DMR-KS-05-03-20-1, a right DP2 D DMR-KS-05-03-19-27, a right P2 E DMR-KS-05-04-03-03, a right P2 F DMR-KS-05-03-20-3, a right DP3 G–HDMR-05-03-17-3, a right DP4 in occlusal (G) and lingual (H) views IDMR-05-03-17-1, a right M3 JDMR-05-03-19-26, a left m2 K–L DMR-KS-05-04-3-1, a left mandible in and occlusal (K) and lateral (L) views M DMR-KS-05-03-00-1, a fragmentary mandible in occlusal view. All isolated teeth are shown in occlusal view.
Upper dentition: DP2 (DMR-KS-05-03-20-4) is small and elongated, characterized by three main cones (anterior cone, paracone, and metacone) and a well-developed metastyle (Fig.
Mandibles and lower dentition: DMR-KS-05-04-3-1 is complete, posterior to the p2, with the exception of a small part of the angular region (Fig.
Postcranial remains: postcranial elements include humeri (Fig.
Postcranial remains of Bos gaurus from Khok Sung: A–D DMR-KS-05-05-1-1, a right humerus in proximal (A), posterior (B), anterior (C), and distal (D) views E–G DMR-KS-05-03-26-27, a right metacarpus in proximal (E), anterior (F), and distal (G) views H–J DMR-KS-05-03-9-2, a left femur in proximal (H), distal (I), and anterior (J) views.
Taxonomic remarks and comparisons
According to
We assign the juvenile horn core (DMR-KS-05-03-26-22) to B. gaurus because the horn cores of gaurs are different from all other Bos species. They grow outward and curve upward, similar to those of Bubalus arnee, but their apical portion curves inward and slightly forward (
Mandibles and isolated teeth of B. gaurus are also observed. The cheek teeth of B. gaurus are distinguished from B. sauveli and B. javanicus by having two separate fossettes on the P2, more developed metaconids and more anteroposteriorly constricted postprotocristids on the p3 and p4, and more robust cheek teeth (Figs
Genus Bubalus Hamilton-Smith, 1827
Bubalus arnee
Referred material
A nearly complete cranium associated with a right mandible, DMR-KS-05-03-20-1; a cranium with a right tooth row (P3–M3), DMR-KS-05-03-21-1; a partial cranium with two tooth rows (P3–M1), DMR-KS-05-03-16-3; a partial cranium with a right tooth row (P3–M3), DMR-KS-05-03-11-1; three horn cores—DMR-KS-05-03-16-2 (right), DMR-KS-05-03-31-6 (right), and DMR-KS-05-03-19-28 (left); a left P2, DMR-KS-05-03-18-14; a left DP3, DMR-KS-05-03-00-103; two right P3—DMR-KS-05-03-22-14 and DMR-KS-05-04-05-3; a right DP4, DMR-KS-05-04-29-8 (broken anterior lobe); two P4—DMR-KS-05-03-18-13 (right) and DMR-KS-05-03-18-9 (left); four M1—DMR-KS-05-03-31-5 (right), DMR-KS-05-03-18-12 (right), DMR-KS-05-03-18-6 (left), and DMR-KS-05-03-22-13 (left); five M2—DMR-KS-05-03-00-2 (right), DMR-KS-05-03-25-21 (right), DMR-KS-05-03-18-5 (right), DMR-KS-05-03-16-2(1) (left), and DMR-KS-05-03-18-7 (left); four M3—DMR-KS-05-03-00-7 (right), DMR-KS-05-03-22-12 (left), DMR-KS-05-03-14-1 (left), and DMR-KS-05-03-18-10 (left); a right mandible with p2–m1, DMR-KS-05-03-20-2; three left mandibles—DMR-KS-05-03-10-3 (with p2–m3), DMR-KS-05-03-20-10 (with p2–m1), and DMR-KS-05-03-20-20 (with m1 and m2); a right i1, DMR-KS-05-03-18-8; a right i2, DMR-KS-05-03-22-15; a left i3, DMR-KS-05-03-00-106; a right i4, DMR-KS-05-03-16-3; a right p3, DMR-KS-05-03-14-4; a left dp4, DMR-KS-05-03-00-4; a right p4, DMR-KS-05-03-19-6; four m1—DMR-KS-05-03-25-3 (right), DMR-KS-05-03-18-18 (right), DMR-KS-05-03-00-105 (left), and DMR-KS-05-03-00-3 (left); two m2—DMR-KS-05-03-27-12 (right) and DMR-KS-05-03-25-2 (left); two m3—DMR-KS-05-03-18-11 and DMR-KS-05-04-29-2 (left posterior lobe); eleven thoracic vertebrae—DMR-KS-05-04-1-11 (T3), DMR-KS-05-04-1-26 (T4), DMR-KS-05-04-1-13 (T5), DMR-KS-05-04-1-14 (T6), DMR-KS-05-04-1-15 (T7), DMR-KS-05-04-1-16 (T8), DMR-KS-05-04-1-12 (T9), DMR-KS-05-04-1-17 (T10), DMR-KS-05-04-1-18 (T11), DMR-KS-05-04-1-19 (T12), and DMR-KS-05-04-1-20 (T13); four lumbar vertebrae—DMR-KS-05-04-1-24 (L1), DMR-KS-05-04-1-23 (L2), DMR-KS-05-04-1-22 (L3), and DMR-KS-05-04-1-21 (L4); two humeri—DMR-KS-05-03-31-1 (right) and DMR-KS-05-03-31-8 (left); two scapulae—DMR-KS-05-03-26-2 (right) and DMR-KS-05-02-20-4 (left); three ulnae and radii—DMR-KS-05-03-00-61 (right), DMR-KS-05-03-31-2 (right) and DMR-KS-05-03-31-9 (left); a right metacarpus, DMR-KS-05-03-26-3(1); a pelvis, DMR-KS-05-04-1-25; two femora—DMR-KS-05-04-1-1 (right) and DMR-KS-05-04-1-2 (left); a right fragmentary femur, DMR-KS-05-03-20-8 (distal part); three tibiae—DMR-KS-05-4-1-11 (right), DMR-KS-05-04-1-3 (left), and DMR-KS-05-03-20-9 (left); two fourth tarsal bones—DMR-KS-05-04-1-7 (right) and DMR-KS-05-04-1-5 (left); three metatarsi—DMR-KS-05-04-1-8 (right), DMR-KS-05-04-1-6 (left), and DMR-KS-05-03-28-30 (left); a left astragalus, DMR-KS-05-04-1-4; a left phalanx I, DMR-KS-05-04-1-9; a left phalanx II, DMR-KS-05-04-1-10.
Material description
Crania and upper dentition: DMR-KS-05-03-20-1 is undeformed and nearly complete (for measurements, see Appendix
Cranial and upper dental remains of Bubalus arnee from Khok Sung: A–C DMR-KS-05-03-20-1, a cranium in dorsal (A), ventral (B), and lateral (C) views and D–E DMR-KS-05-03-21-1, a cranium in dorsal (D) and ventral (E) views F–G DMR-KS-05-03-11-1, a right upper jaw in lateral (F) and occlusal (G) views H–I DMR-KS-05-03-16-3, a partial cranium in ventral view (H) with a right tooth row (I) J DMR-KS-05-03-16-2, a right horn core in dorsal view K DMR-KS-05-03-18-14, a left P2 L DMR-KS-05-03-00-103, a left DP3 M DMR-KS-05-04-29-8, a right DP4 N DMR-KS-05-03-00-7, a right M3. Cross-sections of basal horn cores are given. All isolated teeth are shown in occlusal view.
DMR-KS-05-03-21-1, a juvenile cranium, is incomplete but slightly deformed. The posterior part of the skull is almost complete but the anterior part is broken (Fig.
DMR-KS-05-03-11-1 preserves the right zygomatic bone and the premaxilla and maxilla with a nearly complete tooth row (P3–M3) (Fig.
Three isolated horn cores (DMR-KS-05-03-16-2: Fig.
Upper cheek teeth of Bubalus arnee are more robust, compared to those of Bos. P2 (DMR-KS-05-03-18-14: Fig.
Upper molars display Bos-like patterns (e.g., the degree of the hypsodonty and selenodonty and the presence of distinct styles) but are more robust than most species of Bos (e.g., B. sauveli and B. javanicus) (Tab.
Mandibles and lower dentition: five mandibles: DMR-KS-05-03-20-1 (Fig.
Mandibular and lower dental remains of Bubalus arnee from Khok Sung: A–B DMR-KS-05-03-20-1, a right mandible in lateral (A) and occlusal (B) views C–D DMR-KS-05-03-10-3, a left mandible in mesial (C) and occlusal (D) views E–FDMR-05-03-20-2, a right mandible in lateral (E) and occlusal (F) views G–HDMR-05-03-20-10, a left mandible in lateral (G) and occlusal (H) views I DMR-KS-05-03-20-20, a left fragmentary mandible with m1 and m2 in occlusal view J DMR-KS-05-03-18-8, a right i1 in lingual view; (K) DMR-KS-05-03-00-106, a left i3 in lingual view L DMR-KS-05-03-16-3, a right i4 M DMR-KS-05-03-00-4, a left dp4 in occlusal view N–O DMR-KS-05-03-00-105, a left m1 in occlusal (N) and buccal (O) views.
All lower cheek teeth are robust. All lingual stylids are distinct. The p2 has a well-developed postentocristid and posthypocristid (Fig.
Lower molars have well-developed stylids and conids. The metastylid is most developed on the unworn to slightly worn specimens (Fig.
Postcranial remains: postcranial elements include scapulae (Fig.
Articulated postcranial skeletons of Bubalus arnee from Khok Sung: A thoracic (abbreviated as “T”) vertebrae in lateral view: DMR-KS-05-04-1-11 (T3), DMR-KS-05-04-1-26 (T4), DMR-KS-05-04-1-13 (T5), DMR-KS-05-04-1-14 (T6), DMR-KS-05-04-1-15 (T7), DMR-KS-05-04-1-16 (T8), DMR-KS-05-04-1-12 (T9), DMR-KS-05-04-1-17 (T10), DMR-KS-05-04-1-18 (T11), DMR-KS-05-04-1-19 (T12), and DMR-KS-05-04-1-20, (T13) B lumbar (L) vertebrae in dorsal view: DMR-KS-05-04-1-24 (L1), DMR-KS-05-04-1-23 (L2), DMR-KS-05-04-1-22 (L3), and DMR-KS-05-04-1-21 (L4) C–E a left forelimb in anterior view: (C) DMR-KS-05-02-20-4, a scapula in lateral and distal views D DMR-KS-05-03-31-8, a humerus in proximal and distal views E DMR-KS-05-03-31-9, an ulna and a radius in proximal and distal views F DMR-KS-05-03-26-3(1), a right metacarpus in proximal, anterior, and distal views G DMR-KS-05-04-1-25, a pelvis in ventral view H–R hindlimbs in anterior view: H DMR-KS-05-04-1-1, a right femur in proximal and distal views I DMR-KS-05-4-1-11, a right tibia in proximal and distal views; (J) DMR-KS-05-04-1-7, a right 4th tarsal bone in dorsal view K DMR-KS-05-04-1-8, a right metatarsus in proximal and distal views L DMR-KS-05-04-1-2, a left femur M DMR-KS-05-04-1-3, a left tibia N DMR-KS-05-04-1-4, a left astragalus in plantar view O DMR-KS-05-04-1-5, a left 4th tarsal bone P DMR-KS-05-04-1-6, a left metatarsus Q DMR-KS-05-04-1-9, a left phalanx I in lateral view R DMR-KS-05-04-1-10, a left phalanx II in lateral view.
Taxonomic remarks and comparisons
According to
Although the cheek teeth of Bos and Bubalus are almost morphologically identical and often show highly variable occlusal morphologies in relation to the wear stages, they are distinguishable based on the dental morphology.
As demonstrated by the scatter diagrams (Figs
Genus Capricornis Ogilby, 1836
Capricornis sumatraensis
Referred material
A left M2, DMR-KS-05-03-18-16; three m3—DMR-KS-05-04-05-4 (right), DMR-KS-05-03-27-5 (left), and DMR-KS-05-03-28-10 (left posterior fragment).
Material description
Isolated teeth are almost complete (for measurements, see Tab.
Dental remains of Capricornis sumatraensis from Khok Sung: A–B DMR-KS-05-03-18-16, a left M2 in occlusal (A) and lingual (B) views C–D DMR-KS-05-04-05-4, a right m3 in occlusal (C) and buccal (D) views E–F DMR-KS-05-03-27-5, a left m3 in occlusal (E) and buccal (F) views G–H DMR-KS-05-03-28-10 in occlusal (G) and buccal (H) views.
Taxonomic remarks and comparisons
We assign these isolated teeth from Khok Sung to Capricornis sumatraensis (Sumatran serow) because they are comparable in size and morphology to the extant specimens (Fig.
Scatter diagrams of M2 and m3 lengths and widths of recent and fossil serows and gorals. The measurements of fossil specimens from Lang Trang, Thum Wiman Nakin, Thum Prakai Phet, and Tam Hang South are from
Compared to other fossil records, C. sumatraensis from Khok Sung is smaller than that from the Late Pleistocene of Lang Trang in Vietnam (
Class REPTILIA Laurenti, 1768
Order CROCODILIA Owen, 1842
Family CROCODYLIDAE Laurenti, 1768
Genus Crocodylus Laurenti, 1768
Crocodylus cf. siamensis
Referred material
A fragmentary cranium, DMR-KS-05-03-30-30; a dentary fragment with one tooth, DMR-KS-05-03-21-1; five isolated teeth—DMR-KS-05-03-00-19, DMR-KS-05-03-14-3, DMR-KS-05-03-22-22, DMR-KS-05-04-06-3, and DMR-KS-05-04-29-10; three osteoderms—DMR-KS-05-03-29-57, DMR-KS-05-03-29-58, and DMR-KS-05-03-27-25.
Material description
Skull and dentition: DMR-KS-05-03-30-30 is a slightly deformed skull preserving a nearly complete premaxilla, maxilla, nasal, and palatine process (Fig.
Remains of non-mammalian vertebrates from Khok Sung: Crocodylus cf. siamensis— A–B DMR-KS-05-03-30-30, a cranium in dorsal (A) and ventral (B) views C DMR-KS-05-03-21-1, a tooth in lingual view D–E DMR-KS-05-03-29-57 and F–G DMR-KS-05-03-27-25, osteoderms in dorsal (D, F) and ventral (E, G) views; Python sp.— H–I DMR-KS-05-03-00-16, a trunk vertebra in anterior (H) and ventral (I) views; Varanus sp. J–K DMR-KS-05-03-08-36, a trunk vertebra in anterior (J) and ventral (K) views; Galliformes indet.— L–M DMR-KS-05-04-05-40, a cervical vertebra fragment in dorsal (L) and ventral (M) views; Siluridae indet.— N–O DMR-KS-05-03-22-76, a vertebra in anterior (N) and lateral (O) views P DMR-KS-05-04-11-20, a pectoral spine in dorsal view Q DMR-KS-05-04-05-25, a pectoral spine in medial view. Anatomical abbreviations: al, alveolus; pmx, premaxilla; en, external naris; n, nasal; mx, maxilla; pal, palatine; palp, palatine process.
Osteoderms: two nearly complete specimens (Fig.
Taxonomic remarks and comparisons
The specimen DMR-KS-05-03-30-30 is a crocodilian cranium with a possible maximum length up to 50 cm. All morphological characters of the Khok Sung crocodiles are congruent with the extant fresh water crocodile, Crocodylus siamensis, as well as with its fossils recovered from the Early and Middle Pleistocene of Java (Trinil H. K., Kedung Brubus, and Kedung Lumbu) (
Order SQUAMATA Oppel, 1811
Suborder SERPENTES Linnaeus, 1758
Family BOIDAE Gray, 1825
Genus Python Daudin, 1803
Python sp.
Referred material
Four trunk vertebrae—DMR-KS-05-03-00-21, DMR-KS-05-03-00-16 (two attached vertebrae), and DMR-KS-05-04-28-12.
Material description
Vertebrae are almost complete and represent a large-sized snake (for measurements, see Tab.
Measurements (in millimeters) of vertebrae of Python and Varanus from Khok Sung. Abbreviations: CL, centrum length (measured at the ventral midline); H, maximum height (measured from the tip of the neural spine to the ventral rim of the cotyle); WPP, width between pre- and postzygapophyseal processes; Wpre, width across zygapophyseal processes; Wpost, width across postzygapophyseal processes; Wcd, width of the condyle; Hcd, height of the condyle (measured from the dorsal to ventral rim); Wct, width of the cotyle; Hct, height of the cotyle. + refers to the measurement of two attached vertebrae and * indicates an incomplete preservation.
| CL | H | WPP | Wpre | Wpost | WCd | Hcd | WCd | Hct | |
|---|---|---|---|---|---|---|---|---|---|
| Python sp. | |||||||||
| DMR-KS-05-03-00-21 | 20.85 | 40.36 | 22.47 | 36.48 | 15.27 | 14.03 | 13.95 | 12.87 | 15.12 |
| DMR-KS-05-03-00-16 | 28.75+ | 26.35* | 23.82 | 35.23 | 13.73 | – | 12.04 | – | 16.44 |
| DMR-KS-05-04-28-12 | 14.06 | 17.69* | 17.18 | 20.46 | 23.64 | 6.50 | 6.80 | 6.62 | 7.82 |
| Varanus sp. | |||||||||
| DMR-KS-05-03-29-36 | 24.98 | 25.39* | 27.90 | 21.91 | 34.98 | 7.18 | 9.21 | 18.96 | 22.09 |
| DMR-KS-05-03-08-36 | 31.73 | 28.56 | 34.11 | 36.21 | 35.60 | 7.82 | 12.68 | 18.27 | 21.91 |
Taxonomic remarks and comparisons
These four vertebrae are attributed to the family Boidae because of the following characters: a short, wide, and massive vertebral body (i.e., the widths of the centra are greater than the lengths, sensu
Suborder LACERTILIA Günther, 1867
Family VARANIDAE Merrem, 1820
Genus Varanus Merrem, 1820
Varanus sp.
Referred material
Two trunk vertebrae—DMR-KS-05-03-08-36 and DMR-KS-05-03-29-36.
Material description
The vertebra DMR-KS-05-03-08-36 is more complete than the specimen DMR-KS-05-03-29-36 (for measurements, see Tab.
Taxonomic remarks and comparisons
We assign these two vertebrae to the the family Varanidae due to the following morphological characters: a centrum tapering posteriorly, a precondylar constriction, a ventrally facing cotyle, and a large and flared condyle (
Faunal composition of Khok Sung vertebrate assemblage
Nine taxa: seven Testudines, an extinct gharial (Gavialisbengawanicus), and a spotted hyaena (Crocuta crocuta ultima), have been previously described from Khok Sung by
| Mammalia | ||
| Proboscidea | ||
| Stegodontidae | ||
| Stegodon cf. orientalis | ||
| Elephantidae | ||
| Elephas sp. | ||
| Perissodactyla | ||
| Rhinocerotidae | ||
| Rhinoceros sondaicus | ||
| Rhinoceros unicornis | ||
| Artiodactyla | ||
| Bovidae | ||
| Bos sauveli | ||
| Bos gaurus | ||
| Bubalus arnee | ||
| Capricornis sumatraensis | ||
| Cervidae | ||
| Axis axis | ||
| Panolia eldii | ||
| Rusa unicolor | ||
| Suidae | ||
| Sus barbatus | ||
| Primates | ||
| Cercopithecidae | ||
| Macaca sp. | ||
| Carnivora | ||
| Hyaenidae | ||
|
Crocuta crocuta ultima (identified by |
||
| Canidae | ||
| Cuon sp. | ||
| Reptilia | ||
|
Testudines (identified by |
||
| Geoemydidae | ||
| Batagur cf. trivittata | ||
| Heosemys annandalii | ||
| Heosemys cf. grandis | ||
| Malayemys sp. | ||
| Trionychidae | ||
| Chitra sp. | ||
| cf. Amyda sp. | ||
| Crocodilia | ||
| Gavialidae | ||
|
Gavialis cf. bengawanicus (identified by |
||
| Crocodylidae | ||
| Crocodylus cf. siamensis | ||
| Squamata | ||
| Varanidae | ||
| Varanus sp. | ||
| Boidae | ||
| Python sp. | ||
| Actinopterygii | ||
| Siluridae indet. | ||
| Aves | ||
| Galliformes indet. | ||
According to the fact that Khok Sung yields only large mammals (> 8 kg), the absence of medium- and small-sized mammal remains is likely due to taphonomic conditions and/or fossil collecting methods. Similarly to most of the Middle and Late Pleistocene fossil sites in Southeast Asia, the biodiversity of Khok Sung large mammals is likely greater than that of present-day faunas (see Appendices
Individual species distribution patterns
Past records and recent distribution patterns of large mammalian species present in Khok Sung are revealed in this work. Paleontological sites in Southeast Asia as well as South China are examined for the Early, Middle, and Late Pleistocene, compared with the modern distribution patterns. We only focus on mammalian taxa assigned to the species-level, including Stegodon orientalis and its co-occuring species, Rhinoceros sondaicus, Rhinoceros unicornis, Sus barbatus, Axis axis, Panolia eldii, Rusa unicolor, Bos sauveli, Bos gaurus, Bubalus arnee, and Capricornis sumatraensis.
Stegodontids and elephantids
The earliest records of derived Stegodon (e.g., Stegodon orientalis from Dayakou (
A fossil species of Palaeoloxodon is reported from several Middle Pleistocene localities in mainland Southeast Asia (Fig.
The Middle (red) and Late (yellow) Pleistocene records of stegodontids and relative fossil elephants, and the current distribution (green) of Elephas maximus (Indian elephant). Stars indicate the co-occurrence of sympatric proboscideans. The current distribution of Indian elephants is compiled from
Elephas maximus is known from the late Middle Pleistocene of Thum Wiman Nakin (northeastern Thailand) (
Javan and Indian rhinoceroses
The Early Pleistocene records of Asian rhinoceroses are poorly documented in Southeast Asia. Only R. sondaicus is reported from the upper part of the Irrawaddy Formation, near Pauk Township in central Myanmar (
The Early (blue circle), Middle (red circle), and Late (yellow circle) Pleistocene records and the current distribution (green) of Rhinoceros sondaicus (Javan rhinoceros). The current distribution of the species is compiled from
The Middle Pleistocene record, especially the late Middle Pleistocene, includes numerous reports of Asian rhinoceroses (Figs
The Middle (red circle) and Late (yellow circle) Pleistocene records and the current distribution (green) of Rhinoceros unicornis (Indian rhinoceros). “?” indicates the possible record of R. unicornis according to
During the late Pleistocene, Javan and Indian rhinoceroses were widespread in Indochinese subregion (Figs
Nowadays, the Indian rhinoceros is locally extinct from the Thai territory and several other countries in Southeast Asia. The species is restricted to Nepal and India and some parts of northernmost Myanmar (
Bearded pigs
During the Middle Pleistocene, Sus barbatus (bearded pig) is known from the caves of Thum Wiman Nakin and Thum Prakai Phet (
In the late Pleistocene, S. barbatus is well-documented from many localities, extending its geographic distribution across Sumatra, Borneo, and Java. This species is likely more widespread in the late Pleistocene than the Middle Pleistocene (Fig.
Today S. barbatus is restricted to Peninsular Malaysia, Sumatra, and Borneo (
Chitals
Fossils of Axis axis (chital) have never been previously recorded from Thailand but were present in mainland Southeast Asia, at least in Khok Sung, during the late Middle Pleistocene (Fig.
Nowadays Axis axis is restricted to the Indian Subcontinent (India, Nepal, Sikkim, and Sri Lanka) (Fig.
Eld’s and sambar deer
The Eld’s deer is known from the Middle Pleistocene of Thailand. Fossils of P. eldii are collected from the caves of Thum Wiman Nakin and Kao Pah Nam (
During the Late Pleistocene, the Eld’s and sambar deer co-occurred in the Cave of the Monk (Ban Fa Suai), northern Thailand (
Nowadays, Panolia eldii is restricted to the Indochinese province (Fig.
Koupreys, gaurs, and wild water buffaloes
Large bovids in Southeast Asia currently comprise four wild species: Bos sauveli (kouprey), Bos javanicus (banteng), Bos gaurus (gaur), and Bubalus arnee (wild water buffalo). Bantengs, gaurs, and koupreys presumably shared a common ancestor at 2.6 Ma (Plio-Pleistocene) and their lineages split in a short period of time (i.e., between 200 and 300 ka) based on the molecular estimations of divergence times (
During the Late Pleistocene, the locality of the Cave of the Monk (Ban Fa Suai) yielded remains of these bovid species (cf.) (
The historical distribution of koupreys during the last century is restricted to Cambodia, southern Laos, southeastern Thailand, and western Vietnam (
Overall, the Pleistocene large bovid species in Southeast Asia is more widespread than the modern population. The anthropogenic impacts on the environments and landscapes seem to have caused the reduction of large bovid population in several areas during the past decade. The koupreys is more widely distributed during the Pleistocene than today (Fig.
Sumatran serows
The possible earliest records of Capricornis sumatraensis are from the middle Early Pleistocene site of Gongwangling (
The Sumatran serow is a widespread species, native to mountain forests on the Himalayan range (northern India, Sikkim, and Nepal) of the Indochinese subregion (Southern China, Myanmar, Thailand, Laos, Cambodia, Vietnam, and Peninsular Malaysia) and on the island of Sumatra (
Faunal comparisons of the assemblage with other penecontemporaneous assemblages
For the comparisons of vertebrate faunas between Khok Sung and other Pleistocene sites, we focus only on large mammals (for the mammalian fauna lists of the Middle to Late Pleistocene of Southeast Asian sites, see Appendices
The Khok Sung assemblage shares at least one similar archaic mammal taxon such as Crocuta crocuta ultima and Stegodon orientalis, with these faunas. Crocuta crocuta is also recorded from Thum Wiman Nakin, Thum Prakai Phet, and Nam Lot, whereas Stegodon orientalis is reported from two Laotian sites: Nam Lot and Tam Hang South. By the way, most of forest dwelling and carnivorous taxa that are representatives of Middle Pleistocene mammalian assemblages such as Ailuropoda melanoleuca (giant panda), Ursus thibetanus (Asiatic black bear), Pongo pygmaeus (orang-utan), Muntiacus muntjak (Southern red muntjac), and Tapirus indicus (Malayan tapir) are absent in Khok Sung. The paleoenvironments of Khok Sung corresponded to a floodplain near the river channel (
The degree of the faunal similarity also depends on the number of identified taxa for each site. We further analyse the relationships between the geographic regions and faunas in Southeast Asia, using the Simpson coefficient of faunal similarity (Tab.
Similarity matrix based on the Simpson coefficients. Locality abbreviations: YCK, Yenchingkou; KLS, Koloshan; DX, Daxin; HJ, Hejiang; GX, Ganxian; PXDD, Panxian Dadong; WY, Wuyun; MB, Maba; HST, Hoshantung; KS, Khok Sung; TWN ; Thum Wiman Nakin; TPKP, Thum Phra Khai Phet; TK, Tham Khuyen; TO, Tham Om; BDB, Boh Dambang; KDBB, Kedung Brubus; TNHK, Trinil Hauptknochenschicht; ND, Ngandong.
| YCK | KLS | DX | HJ | GX | PXDD | WY | MB | HST | KS | TWN | TPKP | TK | TO | BDB | KDBB | TNHK | ND | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YCK | 1.00 | |||||||||||||||||
| KLS | 0.38 | 1.00 | ||||||||||||||||
| DX | 0.54 | 0.31 | 1.00 | |||||||||||||||
| HJ | 0.55 | 0.27 | 0.45 | 1.00 | ||||||||||||||
| GX | 0.50 | 0.20 | 0.40 | 0.40 | 1.00 | |||||||||||||
| PXDD | 0.53 | 0.46 | 0.46 | 0.55 | 0.30 | 1.00 | ||||||||||||
| WY | 0.53 | 0.23 | 0.38 | 0.45 | 0.70 | 0.33 | 1.00 | |||||||||||
| MB | 0.94 | 0.38 | 0.62 | 0.55 | 0.50 | 0.44 | 0.47 | 1.00 | ||||||||||
| HST | 0.50 | 0.30 | 0.20 | 0.30 | 0.20 | 0.40 | 0.40 | 0.50 | 1.00 | |||||||||
| KS | 0.50 | 0.00 | 0.08 | 0.18 | 0.10 | 0.25 | 0.17 | 0.25 | 0.10 | 1.00 | ||||||||
| TWN | 0.48 | 0.15 | 0.31 | 0.27 | 0.60 | 0.24 | 0.40 | 0.50 | 0.30 | 0.83 | 1.00 | |||||||
| TPKP | 0.58 | 0.17 | 0.17 | 0.36 | 0.30 | 0.33 | 0.33 | 0.50 | 0.30 | 0.50 | 0.92 | 1.00 | ||||||
| TK | 0.63 | 0.31 | 0.54 | 0.55 | 0.60 | 0.41 | 0.40 | 0.63 | 0.40 | 0.33 | 0.53 | 0.42 | 1.00 | |||||
| TO | 0.94 | 0.38 | 0.54 | 0.55 | 0.50 | 0.50 | 0.47 | 0.81 | 0.40 | 0.42 | 0.63 | 0.50 | 0.75 | 1.00 | ||||
| BDB | 0.80 | 0.10 | 0.20 | 0.20 | 0.30 | 0.40 | 0.50 | 0.60 | 0.20 | 0.40 | 0.80 | 0.60 | 0.60 | 0.60 | 1.00 | |||
| KDBB | 0.11 | 0.08 | 0.00 | 0.09 | 0.00 | 0.06 | 0.07 | 0.13 | 0.10 | 0.17 | 0.22 | 0.17 | 0.06 | 0.13 | 0.10 | 1.00 | ||
| TNHK | 0.21 | 0.08 | 0.08 | 0.09 | 0.00 | 0.14 | 0.14 | 0.21 | 0.10 | 0.08 | 0.14 | 0.17 | 0.07 | 0.14 | 0.30 | 0.64 | 1.00 | |
| ND | 0.10 | 0.10 | 0.00 | 0.10 | 0.00 | 0.10 | 0.10 | 0.10 | 0.10 | 0.00 | 0.10 | 0.00 | 0.00 | 0.10 | 0.00 | 0.90 | 0.60 | 1.00 |
As a result, the Middle Pleistocene Southeast Asian taxa reveal two distinct associations (Javanese and mainland Southeast Asian faunas) (Fig.
Overall, this analysis suggests initially that the differences in species composition and distribution do not follow a trend of the latitudinal gradient north to south, but show spatial and time variability of large mammalian fauna in Southeast Asia. The main problems of mammalian fauna comparisons in Southeast Asia are likely due to the poorly-known species diversity and/or the imprecisely chronological determination in several localities.
Discussion
Biochronology of Khok Sung fauna
According to the similarity analysis of the fauna, the mammalian fauna composition of Khok Sung is considerably different from the Early to early Middle Pleistocene assemblage of Java. This suggests an inconsistent age of the Early Pleistocene for Khok Sung. The Khok Sung assemblage is highly comparable in composition to three late Middle Pleistocene faunas: Thum Wiman Nakin (> 169 ka,
On the basis of the previous paleomagnetic data analysed by
Evolutionary and biogeographic affinities of Khok Sung fauna
Relationships of the Khok Sung vertebrate fauna for dispersal events from India to Java has been first proposed by
However, in terms of faunal age, this scenario is no longer consistent because the Khok Sung fauna is recently attributed to a late Middle Pleistocene age (
Based on the occurrence of mammalian taxa in Khok Sung, we suggest an alternative relevance of this fauna for the “Sino-malayan” dispersal events from mainland Southeast Asia to Java (Fig.
On the other hand, the Khok Sung fauna lacks any evidence of taxa originating from Java. But the possible presence of Duboisia santeng in Tambun site (Peninsular Malaysia) may indicate the faunal exchange from Indonesia to the mainland Southeast Asia (
The Khok Sung mammalian assemblage supports that Thailand was a biogeographic gateway of the Sino-Malayan migration event as the mainland forested faunal association replaced the earlier Siva-Malayan fauna (Stegodon-Homo erectus complex) subsequently in Java (
Acknowledgments
We would like to thank Joséphine Lesur (
References
- Antoine PO (2012) Pleistocene and Holocene rhinocerotids (Mammalia, Perissodactyla) from the Indochinese Peninsula. Comptes Rendus Palevol 11: 159–168. doi: 10.1016/j.crpv.2011.03.002
- Averianov AO, Danilov IG (1997) A varanid lizard (Squamata: Varanidae) from the Early Eocene of Kirghizia. Russian Journal of Herpetology 4: 143–147.
- Bacon AM, Demeter F, Schuster M, Long VT, Thuy NTK, Antoine PO, Sen S, Nga HH, Huong NTM (2004) The Pleistocene Ma U’Oi cave, northern Vietnam: palaeontology, sedimentology and palaeoenvironments. Geobios 37: 305–314. doi: 10.1016/j.geobios.2003.03.010
- Bacon AM, Demeter F, Roussé S, Long VT, Duringer P, Antoine PO, Thuy NTK, Mai BT, Huong NTM, Dodo Y, Matsumura H, Schuster M, Anezaki T (2006) New palaeontological assemblage, sedimentological and chronological data from the Pleistocene Ma U’Oi cave (Northern Vietnam). Palaeogeography, Palaeoclimatology, Palaeoecology 230: 280–298. doi: 10.1016/j.palaeo.2005.07.023
- Bacon AM, Demeter F, Tougard C, de Vos J, Sayavongkhamdy T, Antoine PO, Bouasisengpaseuth B, Sichanthongtip P (2008a) Redécouverte d’une faune pléistocène dans les remplissages karstiques de Tam Hang au Laos: premiers résultats. Comptes Rendus Palevol 7: 277–288. doi: 10.1016/j.crpv.2008.03.009
- Bacon AM, Demeter F, Duringer P, Helm C, Bano M, Long VT, Thuy NTK, Antoine PO, Mai BT, Huong NTM, Dodo Y, Chabaux F, Rihs S (2008b) The Late Pleistocene Duoi U’Oi cave in northern Vietnam: palaeontology, sedimentology, taphonomy and palaeoenvironments. Quaternary Science Reviews 27: 1627–1654. doi: 10.1016/j.quascirev.2008.04.017
- Bacon AM, Duringer P, Antoine PO, Demeter F, Shackelford L, Sayavongkhamdy T, Sichanthongtip P, Khamdalavong P, Nokhamaomphu S, Sysuphanh V, Patole-Edoumba E, Chabaux F, Pelt E (2011) The Middle Pleistocene mammalian fauna from Tam Hang karstic deposit, northern Laos: new data and evolutionary hypothesis. Quaternary International 245: 315–332. doi: 10.1016/j.quaint.2010.11.024
- Bacon AM, Demeter F, Duringer P, Patole-Edoumba E, Sayavongkhamdy T, Coupey AS, Shackelford L, Westaway KE, Ponche JL, Antoine PO, Sichanthongtip P (2012) Les sites de Tam Hang, Nam Lot et Tam Pà Ling au nord du Laos: Des gisements à vertébrés du Pléistocène aux origines des Hommes modernes. CNRS Editions, 149 pp.
- Bacon AM, Westaway KE, Antoine PO, Duringer P, Blin A, Demeter F, Ponche JL, Zhao JX, Barnes LM, Sayavonkhamdy T, Thuy NTK, Long VT, Patole-Edoumba E, Shackelford L (2015) Late Pleistocene mammalian assemblages of Southeast Asia: New dating, mortality profiles and evolution of the predator–prey relationships in an environmental context. Palaeogeography, Palaeoclimatology, Palaeoecology 422: 101–127. doi: 10.1016/j.palaeo.2015.01.011
- Badoux DM (1959) Fossil mammals from two fissure deposits at Punung (Java). Uitgeversmij v/h Kemink & Zoon NV, Utrecht, 151 pp.
- Barker G, Barton H, Bird M, Daly P, Datan I, Dykes A, Farr L, Gilbertson D, Harrisson B, Hunt C, Higham T, Kealhofer L, Krigbaum J, Lewis H, McLaren S, Paz V, Pike A, Piper P, Pyatt B, Rabett R, Reynolds T, Rose J, Rushworth G, Stephens M, Stringer C, Thompson J, Turney C (2007) The “human revolution” in lowland tropical Southeast Asia: the antiquity and behavior of anatomically modern humans at Niah Cave (Sarawak, Borneo). Journal of Human Evolution 52: 243–261. doi: 10.1016/j.jhevol.2006.08.011
- Bärmann EV, Rössner GE (2011) Dental nomenclature in Ruminantia: towards a standard terminological framework. Mammalian Biology 76: 762–768. doi: 10.1016/j.mambio.2011.07.002
- Baryshnikov GF (2012) Pleistocene Canidae (Mammalia, Carnivora) from the Paleolithic Kurado caves in the Caucasus. Russian Journal of Theriology 11: 77–120. doi: 10.15298/rusjtheriol.11.2.01
- Baryshnikov GF (2015) Late Pleistocene Canidae remains from Geographical Society Cave in Russian Far East. Russian Journal of Theriology 14: 65–83.
- Beden M, Guérin C (1973) Le gisement de vertébrés du Phnom Loang (Province de Kampot, Cambodge): Faune du Pléistocène moyen terminal (Loangien). Travaux et Documents de l’ORSTOM, vol. 27, Paris, 97 pp.
- Bekken D, Schepartz LA, Miller-Antonio S, Yamei H, Weiwen H (2004) Taxonomic Abundance at Panxian Dadong, a Middle Pleistocene Cave in South China. Asian Perspectives 43: 333–359. doi: 10.1353/asi.2004.0019
- Bocherens H, Schrenk F, Chaimanee Y, Kullmer O, Mörike D, Pushkina D, Jaeger JJ (in press) Flexibility of diet and habitat in Pleistocene South Asian mammals: Implications for the fate of the giant fossil ape Gigantopithecus. Quaternary International. doi: 10.1016/j.quaint.2015.11.059
- Brown CL, Gustafson CE (2000) A key to postcranial Skeletal Remains of Cattle/Bison, Elk, and Horse. Reports of Investigations, No. 57, Department of Anthropology, Washington State University, 199 pp.
- Bulbeck F (2003) Hunter-gatherer occupation of the Malay Peninsula from the ice age to the iron age. In: Mercader J (Ed.) Under the Canopy: The Archaeology of Tropical Rain Forests. Rutgers University Press, New Brunswick, 119–160.
- Bulbeck F (2014) The chronometric Holocene archaeological record of the southern Thai-Malay Peninsula. International Journal of Asia Pacific Studies 10: 111–162.
- Chaimanee Y (1998) Plio-Pleistocene rodents of Thailand. Thai Studies in Biodiversity 3: 1–303.
- Chaimanee Y, Jaeger JJ (1993) Pleistocene mammals of Thailand and their use in the reconstruction of the palaeoenvironments of Southeast Asia. SPAFA Journal 3: 4–10.
- Chaimanee Y, Yamee C, Tian P, Khaowiset K (2005) Fossils and Their Managements at Ban Khok Sung, Muang District, Nakhon Ratchasima Province, NE Thailand. Academic report no. DMR 25/2005, Department of Mineral Resources, Bangkok, Thailand, 60 pp. [In Thai]
- Chen D, Qi G (1978) Fossil human and associated mammalian fauna from Xizhou, Yunnan. Vertebrata PalAsiatica 16: 33–46. [In Chinese with English summary]
- Chen Q, Li Q (1994) A brief report on Xianrendong cave site, Jilin province. Acta Anthropologica 13: 12–19. [In Chinese with English summary]
- Chen GJ, Wang W, Mo JY, Huang ZT, Tian F, Huang WW (2002) Pleistocene vertebrate fauna from Wuyun Cave of Tiandong County, Guangxi. Vertebrata PalAsiatica 40: 42–51. [In Chinese with English summary]
- Chen SK, Pang LB, He CD, Wei GB, Huang WB, Yue ZY, Zhang H, Qin L (2013) New discoveries from the classic Quaternary mammalian fossil area of Yanjinggou, Chingqing, and their chronological explanations. Chinese Science Bulletin 58: 3780–3787. doi: 10.1007/s11434-013-5839-6
- Ciochon RL (2009) The mystery ape of Pleistocene Asia. Nature 459: 910–911. doi: 10.1038/459910a
- Ciochon RL, Long VT, Larick R, González L, Grün R, de Vos J, Yonge C, Taylor L, Yoshida H, Reagan M (1996) Dated co-occurrence of Homo erectus and Gigantopithecus from Tham Khuyen Cave, Vietnam. Proceedings of the National Academy of Sciences 93: 3016–3020. doi: 10.1073/pnas.93.7.3016
- Claude J, Naksri W, Boonchai N, Buffetaut E, Duangkrayom J, Laojumpon C, Jintasakul P, Lauprasert K, Martin J, Suteethorn V, Tong H (2011) Neogene reptiles of northeastern Thailand and their paleogeographical significance. Annales de Paléontologie 97: 113–131. doi: 10.1016/j.annpal.2011.08.002
- Colbert EH (1938) Fossil mammals from Burma in the American Museum of Natural History. Bulletin of the American Museum of Natural History 74: 255–436.
- Colbert EH (1942) Notes on the lesser one-horned rhinoceros, Rhinoceros sondaicus: 2. the position of the Rhinoceros sondaicus in the phylogeny of the genus Rhinoceros. American Museum Novitates 1207: 1–6.
- Colbert EH (1943) Pleistocene vertebrates collected in Burma by the American Southeast Asiatic expedition. Transactions of the American Philosophical Society 32: 395–429.
- Colbert EH, Hooijer DA (1953) Pleistocene mammals from the limestone fissures of Szechwan, China. Bulletin of the American Museum of Natural History 102: 1–134.
- Corbet GB, Hill JE (1992) Mammals of the Indomalayan Region: a Systematic Review. Oxford University Press, Oxford, 488 pp.
- Cranbrook EO (2000) Northern Borneo environments of the past 40,000 years: archaeozoological evidence. The Sarawak Museum Journal 55: 61–109.
- Cranbrook EO, Piper PJ (2007) The Javan rhinoceros Rhinoceros sondaicus in Borneo. The Raffles Bulletin of Zoology 55: 217–220.
- Cranbrook EO, Currant AP, Davison GW (2000) Quaternary mammal fossils from Borneo: Stegodon and Hippopotamus. The Sarawak Museum Journal 55: 215–233.
- Cuong NL (1985) Fossile Menschenfunde aus Nordvietnam. In: Herrmann J, Ullrich H (Eds) Menschwerdung–Biotischer und gesellschaftlicher Entwicklungsprozess. Akademieverlag, Berlin, 96–102.
- Davidson GWH (1994) Some remarks on vertebrate remains from the excavation of Gua Gunung Runtuh, Perak. In: Zuraina M (Ed.) The Excavation of Gua Gunung Runtuh. Department of Museums and Antiquity, 141–148.
- Delfino M, Segid A, Yosief D, Shoshani J, Rook L, Libsekal Y (2004) Fossil reptiles from the Pleistocene Homo-bearing locality of Buia (Eritrea, Northern Danakil depression). Rivista Italiana di Paleontologia e Stratigrafia 110: 51–60.
- Delfino M, de Vos J (2010) A Revision of the Dubois Crocodylians, Gavialis bengawanicus and Crocodylus ossifragus, from the Pleistocene Homo erectus beds of Java. Journal of Vertebrate Paleontology 30: 427–441. doi: 10.1080/02724631003617910
- Demeter F, Bacon AM, Sytha P (2013) État des connaissances actuelles sur le Cambodge. In: Patole-Edoumba E, Duringer P, Pottier C (Eds) Premiers peuplements d’Asie du sud-est. UNESCO, Phnom Penh, 1–74.
- de Terra H (1943) Pleistocene geology and early man in Java. Transactions of the American Philosophical Society 32: 437–464.
- de Vos J (1983) The Pongo faunas from Java and Sumatra and their significance for biostratigraphical and paleoecological interpretations. Proceedings of the Koninklijke Nederlandse Akadademie van Wetenschappen, Serie B86: 417–425.
- de Vos J (1995) The migration of Homo erectus and Homo sapiens in South East Asia and the Indonesian Archipelago. In: Bower JRF, Sartono S (Eds) Human Evolution on the Ecological Context, Volume I, Evolution and Ecology of Homo erectus. Pithecanthropus Centennial Foundation, Leiden University, Netherlands, 239–260.
- de Vos J (2007) Vertebrate records | Mid-Pleistocene of Southern Asia. In: Elias SA (Ed.) Encyclopedia of Quaternary Science. Elsevier, Oxford, 3232–3249. doi: 10.1016/B0-44-452747-8/00256-8
- de Vos J, Long VT (1993) Systematic discussion of the Lang Trang fauna. Unpublished report.
- de Vos J, Long VT (2001) First settlements: relations between continental and insular Southeast Asia. In: Sémah F, Falguères C, Grimaud-Hervé D, Sémah AM (Eds) Origine des peuplements et chronologie des cultures paléolithiques dans le Sud-Est Asiatique Semenanjung-Artcom. Semenanjung, Paris, 225–249.
- de Vos J, Sondaar PY, van den Bergh GD, Aziz F (1994) The Homo-bearing deposits of Java and its ecological context. Courier Forschungs-Institut Senckenberg 171: 129–140.
- Dong W, Liu J, Zhang L, Xu Q (2014) The Early Pleistocene water buffalo associated with Gigantopithecus from Chongzuo in southern China. Quaternary International 354: 86–93. doi: 10.1016/j.quaint.2013.12.054
- Duangkrayom J, Ratanasthien B, Jintasakul P, Carling PA (2014) Sedimentary facies and paleoenvironment of a Pleistocene fossil site in Nakhon Ratchasima province, northeastern Thailand. Quaternary International 325: 220–238. doi: 10.1016/j.quaint.2013.07.048
- Duckworth JW, Kumar NS, Anwarul Islam Md, Hem Sagar Baral, Timmins RJ (2008a) Axis axis. The IUCN Red List of Threatened Species, version 2015.2http://www.iucnredlist.org [downloaded on 31 August 2015]
- Duckworth JW, Steinmetz R, Timmins RJ, Pattanavibool A, Than Zaw, Do Tuoc, Hedges S (2008b) Bos gaurus. The IUCN Red List of Threatened Species, version 2015.2http://www.iucnredlist.org [downloaded on 31 August 2015]
- Esposito M, Chaimanee Y, Jaeger JJ, Reyss JL (1998) Datation des concrétions carbonatées de la ‘’Grotte du Serpent’’ (Thaïlande) par la méthode Th/U. Comptes Rendus de l’Académie des Sciences, Série IIA326: 603–608. doi: 10.1016/S1251-8050(98)80250-4
- Esposito M, Reyss JL, Chaimanee Y, Jaeger JJ (2002) U-series dating of fossil teeth and carbonates from snake cave, Thailand. Journal of Archaeological Science 29: 341–349. doi: 10.1006/jasc.2002.0718
- Filoux A, Wattanapituksakul A, Lespes C, Thongcharoenchaikit C (2015) A Pleistocene mammal assemblage containing Ailuropoda and Pongo from Tham Prakai Phet cave, Chaiyaphum Province, Thailand. Geobios 48: 341–349. doi: 10.1016/j.geobios.2015.07.003
- France D (2009) Human and nonhuman bone identification: A color atlas. CRC Press, Boca Raton, FL, 584 pp.
- Galbreath GJ, Mordacq JC, Weiler FH (2006) Genetically solving a zoological mystery: was the kouprey (Bos sauveli) a feral hybrid? Journal of Zoology 270: 561–564. doi: 10.1111/j.1469-7998.2006.00188.x
- Gentry AW, Rössner GE, Heizmann EPJ (1999) Suborder Ruminantia. In: Rössner GE, Heissig K (Eds) The Miocene Land Mammals of Europe. Verlag Dr. Friedrich Pfeil, München, 225–258.
- Gentry A, Clutton-Brock J, Groves CP (2004) The naming of wild animal species and their domestic derivatives. Journal of Archaeological Science 31: 645–651. doi: 10.1016/j.jas.2003.10.006
- Ginsburg L, Ingavat R, Sen S (1982) A Middle Pleistocene (Loangian) cave fauna in northern Thailand. Comptes Rendus de l’Académie des Sciences, Paris, Série III294: 295–297.
- Grossman A, Liutkus-Pierce C, Kyongo B, M’Kirera F (2014) New Fauna from Loperot Contributes to the Understanding of Early Miocene Catarrhine Communities. International Journal of Primatology 6: 12531274. doi: 10.1007/s10764-014-9799-8
- Grote P (2007) Studies of fruits and seeds from the Pleistocene of northeastern Thailand. Courier Forschunginstitut Senckenberg 258: 171–181.
- Groves CP (1967) On the rhinoceroses of south-east Asia. Säugetierkundliche Mitteilungen 15: 221–237.
- Groves CP (1981) Ancestors for the pigs: taxonomy and phylogeny of the genus Sus. Technical Bulletin No. 3, Department of Prehistory, Research School of Pacific Studies, Australian National University, Canberra, 96 pp.
- Groves CP (1985) Plio-Pleistocene mammals in Island Southeast Asia. Modern Quaternary Research SE Asia 9: 43–54.
- Groves CP (1997) Taxonomy of wild pigs (Sus) of the Philippines. Zoological Journal of the Linnean Society 120: 163–191. doi: 10.1111/j.1096-3642.1997.tb01277.x
- Groves CP, Leslie DM (2011) Rhinoceros sondaicus (Perissodactyla : Rhinocerotidae). Mammalian Species 43: 190–208. doi: 10.1644/887.1
- Groves CP, Grubb P (2011) Ungulate Taxonomy. Johns Hopkins University Press, Baltimore, Maryland, 317 pp.
- Grubb P (2005) Artiodactyla. In: Wilson DE, Reeder DM (Eds) Mammal Species of the World. A Taxonomic and Geographic Reference (3rd edition). Johns Hopkins University Press, Baltimore, 637–722.
- Gruwier B, de Vos J, Kovarovic K (2015) Exploration of the taxonomy of some Pleistocene Cervini (Mammalia, Artiodactyla, Cervidae) from Java and Sumatra (Indonesia): a geometric- and linear morphometric approach. Quaternary Science Reviews 119: 35–53. doi: 10.1016/j.quascirev.2015.04.012
- Grzimek B (1975) Grzimek’s Animal Life Encyclopedia. Vol 2, 642 pp.
- Guérin C (1980) Les rhinocéros (Mammalia, Perissodactyla) du Miocène terminal au Pléistocène supérieur en Europe occidentale. Documents des Laboratoires de Géologie de Lyon, Vol. 79, 1185 pp.
- Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1): 9 pp.
- Han D, Xu C (1985) Pleistocene mammalian faunas of China. In: Wu R, Olsen J (Eds) Palaeoanthropology and Palaeolithic Archaeology in the People’s Republic of China. Academic Press, Orlando, 267–289.
- Hardjasasmita HS (1987) Taxonomy and phylogeny of the Suidae (Mammalia) in Indonesia. Scripta geologica 85: 1–68.
- Harrison T (1996) The palaeoecological context at Niah Cave, Sarawak: evidence from the primate fauna. Indo-Pacific Prehistory Association Bulletin 14: 90–100. doi: 10.7152/bippa.v14i0.11592
- Hassanin A, Ropiquet A (2004) Molecular phylogeny of the tribe Bovini (Bovidae, Bovinae) and the taxonomic status of the Kouprey, Bos sauveli Urbain 1937. Molecular Phylogenetics and Evolution 33: 896–907. doi: 10.1016/j.ympev.2004.08.009
- Hassanin A, Ropiquet A (2007) What is the taxonomic status of the Cambodian banteng and does it have close genetic links with the kouprey? Journal of Zoology 271: 246–252. doi: 10.1111/j.1469-7998.2006.00272.x
- Hassanin A, Ropiquet A, Cornette R, Tranier M, Pfeffer P, Candegabe P, Lemaire M (2006) Has the kouprey (Bos sauveli Urbain, 1937) been domesticated in Cambodia? Comptes Rendus Biologies 329: 124–135. doi: 10.1016/j.crvi.2005.11.003
- Head JJ (2005) Snakes of the Siwalik Group (Miocene of Pakistan): systematics and relationship to environmental change. Palaeontologia Electronica 8: 1–33.
- Heaney LR (1985) Zoogeographic evidence for middle and late Pleistocene land bridges to the Philippine Islands. Modern Quaternary Research in Southeast Asia 9: 127–144.
- Heaney LR (1991) A synopsis of climatic and vegetational change in Southeast Asia. Climatic Change 19: 53–61. doi: 10.1007/BF00142213
- Hedges S, Sagar Baral H, Timmins RJ, Duckworth JW (2008) Bubalus arnee. The IUCN Red List of Threatened Species, version 2015.2http://www.iucnredlist.org [downloaded on 31 August 2015]
- Heintz E (1970) Les Cervidés villafranchiens de France et d’Espagne, Volume 2: figures et tableaux. Mémoires du Muséum National d’Histoire Naturelle, Série C, Sciences de la Terre, Edition du Muséum, 206 pp.
- Hillson S (2005) Teeth (2nd edition)–Cambridge Manuals in Archaeology. Cambridge University Press, 388 pp.
- Hocknull SA, Piper PJ, van den Bergh GD, Due RA, Morwood MJ, Kurniawan I (2009) Dragon’s Paradise Lost: Palaeobiogeography, Evolution and Extinction of the Largest-Ever Terrestrial Lizards (Varanidae). PLoS ONE 4: e7241. doi: 10.1371/journal.pone.0007241
- Hoffstetter R (1964) Les serpents du Néogène du Pakistan (couches des Siwaliks). Bulletin de la Société Géologique de France, Série 76: 467–474.
- Hooijer DA (1946) Prehistoric and fossil rhinoceros from the Malay Archipelago and India. Zoologische Mededeelingen, Leiden 26: 1–138.
- Hooijer DA (1958) Fossil Bovidae from the Malay Archipelago and the Punjab. Zoologische Verhandelingen, Leiden 38: 1–112.
- Hooijer DA (1962) Report upon a collection of Pleistocene mammals from Tin-bearing deposits in a limestone cave near Ipoh, Kinta Valley, Perak. Federation Museum Journal 7: 1–5.
- Hooijer DA (1964) New records of mammals from the Middle Pleistocene of Sangiran, Central Java. Zoologische Mededelingen 40: 73–87.
- Hu CK, Qi T (1978) Gongwangling Pleistocene mammalian fauna of Lantian, Shaanxi. Palaeontologica Sinica 155: 1–64. [In Chinese with English abstract]
- Ibrahim YK, Tshen LT, Westaway KE, Cranbrook EO, Humphrey L, Muhammad RF, Zhao J, Peng LC (2013) First discovery of Pleistocene orangutan (Pongo sp.) fossils in Peninsular Malaysia: Biogeographic and paleoenvironmental implications. Journal of Human Evolution 65: 770–797. doi: 10.1016/j.jhevol.2013.09.005
- Indriati E, Swisher III CC, Lepre C, Quinn RL, Suriyanto RA, Hascaryo AT, Grün R, Feibel CS, Pobiner BL, Aubert M, Lees W, Antón SC (2011) The Age of the 20 Meter Solo River Terrace, Java, Indonesia and the Survival of Homo erectus in Asia. PLoS ONE 6: e21562. doi: 10.1371/journal.pone.0021562
- IUCN (2015) The IUCN Red List of Threatened Species, version 2015.1http://www.iucnredlist.org [downloaded on 7 August 2015]
- Janis CM (1990) Correlation of cranial and dental variables with body size in ungulates and macropodoids. In: Damuth J, MacFadden BJ (Eds) Body Size in Mammalian Paleobiology: Estimation and Biological Implications. Cambridge University Press, Cambridge, 255–299.
- Joordens JCA, d’Errico F, Wesselingh FP, Munro S, de Vos J, Wallinga J, Ankjæergaard C, Reimann T, Wijbrans JR, Kuiper KF, Mücher HJ, Coqueugniot H, Prié V, Joosten I, van Os B, Schulp AS, Panuel M, van der Haas V, Lustenhouwer W, Reijmer JJG, Roebroeks W (2014) Homo erectus at Trinil on Java used shells for tool production and engraving. Nature 518: 228–231. doi: 10.1038/nature13962
- Kahlke HD (1961) On the complex of the Stegodon-Ailuropoda fauna of Southern China and the chronological position of Gigantopithecus blacki v. Koenigswald. Vertebrata PalAsiatica 6: 83–108.
- Kha LT (1976) First remarks on the Quaternary fossil fauna of northern Vietnam. Vietnamese Studies 46: 107–126.
- Laurie WA, Lang EM, Grove CP (1983) Rhinoceros unicornis. Mammalian Species 211: 1–6. doi: 10.2307/3504002
- Lee MSY (2005) Squamate phylogeny, taxon sampling, and data congruence. Organisms Diversity & Evolution 5: 25–45. doi: 10.1016/j.ode.2004.05.003
- Lekagul B, McNeely JA (1988) Mammals of Thailand. Association for the Conservation of Wildlife, Bangkok, 758 pp.
- Leslie DM (2001) Rusa unicolor (Artiodactyla: Cervidae). Mammalian Species 43: 1–30. doi: 10.1644/871.1
- Leslie AJ, Taplin LE (2001) Recent developments in osmoregulation of crocodilians. In: Grigg G, Seebacher F, Franklin CE (Eds) Crocodilian Biology and Evolution. Surrey Beatty, Chipping Norton, New South Wales, 265–279.
- Li Y, Wen B (1986) Guanyindong: A lower Paleolithic site at Qianxi County, Guizhou Province. Cultural Relics Publishing House, Beijing, 181 pp. [In Chinese with English summary]
- Long VT, de Vos J, Ciochon RS (1996) The fossil mammal fauna of the Lang Trang caves, Vietnam, compared with Southeast Asian fossil and recent mammal faunas: the geographical implications. Bulletin of the Indo-Pacific Prehistory Association 14: 101–109.
- Louys J, Curnoe D, Tong H (2007) Characteristics of Pleistocene megafauna extinctions in Southeast Asia. Palaeogeography, Palaeoclimatology, Palaeoecology 243: 152–173. doi: 10.1016/j.palaeo.2006.07.011
- Lund SP, Williams T, Acton GD, Clement B, Okada M (2001) Brunhes Chron magnetic field excursions recovered from Leg 172 sediments. In: Keigwin LD, Rio D, Acton GD, Arnold E (Eds) Proceedings of the Ocean Drilling Program-Scientific Results, vol. 172, 1–18 (online). doi: 10.2973/odp.proc.sr.172.216.2001
- Lydekker R (1880) Indian Tertiary and Post-Tertiary Vertebrata-Siwaliks and Narbada Proboscidea. Palaeontologia Indica, Series 101: 182–294.
- Ma A, Tang H (1992) On discovery and significance of a Holocene Ailuropoda–Stegodon fauna from Jinhua, Zhejinag. Vertebrata PalAsiatica 30: 295–312. [In Chinese with English Abstract]
- Maglio VJ (1973) Origin and evolution of the Elephantidae. Transactions of the American Philosophical Society New Series 63: 1–149. doi: 10.2307/1006229
- Martin JE, Buffetaut E, Naksri W, Lauprasert K, Claude J (2012) Gavialis from the Pleistocene of Thailand and its Relevance for drainage connections from India to Java. PLoS ONE 7: e44541. doi: 10.1371/journal.pone.0044541
- Marwick B (2009) Biogeography of Middle Pleistocene hominins in mainland Southeast Asia: A review of current evidence. Quaternary International 202: 51–58. doi: 10.1016/j.quaint.2008.01.012
- Medway L (1960) The Malay Tapir in late Quaternary Borneo. The Sarawak Museum Journal 9: 356–360.
- Medway L (1972) The Quaternary era in Malesia. In: Ashton PS, Ashton M (Eds) Miscellaneous series; Aberdeen, Scotland. University of Hull, University of Aberdeen, 63–83.
- Meijaard E (2003) Mammals of south-east Asian islands and their Late Pleistocene environments. Journal of Biogeography 30: 1245–1257. doi: 10.1046/j.1365-2699.2003.00890.x
- Meijaard E, Groves CP (2004) Morphometrical relationships between South-east Asian deer (Cervidae, tribe Cervini): evolutionary and biogeographic implications. Journal of Zoology 263: 179–196. doi: 10.1017/S0952836904005011
- Moigne AM, Awe RD, Sémah F, Sémah AM (2004) The cervids from the Ngebung site (‘Kabuh’ series, Sangiran Dome, Central Java) and their biostratigraphical significance. In: Keates SG, Pasveer JM (Eds) Quaternary Research in Indonesia. Balkema, Leiden, 45–62.
- Morley RJ, Flenley JR (1987) Late Cainozoic vegetational and environmental changes in the Malay archipelago. In: Whitmore TC (Ed.) Biogeographical evolution of the Malay archipelago. Clarendon Press, Oxford, 50–59.
- Nowak RM (1999) Walker’s Mammals of the World. The John Hopkins University Press, London, 1936 pp.
- Olsen JW, Ciochon RL (1990) A review of evidence for postulated Middle Pleistocene occupations in Viet Nam. Journal of Human Evolution 19: 761–788. doi: 10.1016/0047-2484(90)90020-C
- Osborn HF (1942) Proboscidea, volume II: Stegodontoidea, Elephantoidea. American Museum of Natural History, New York, 805–1675.
- Palombo MR, Villa P (2001) Schreger lines as support in the Elephantinae identification. In: Cavaretta G, Gioia P, Mussi M, Palombo MR (Eds) The World of Elephants. Consiglio Nazionale Richerche, Roma, 656–660.
- Pionnier-Capitan M, Bemilli C, Bodu P, Célérier G, Ferrié JG, Fosse P, Garcià M, Vigne JD (2011) New evidence for Upper Palaeolithic small domestic dogs in South-Western Europe. Journal of Archaeological Science 38: 2123–2140. doi: 10.1016/j.jas.2011.02.028
- Pitra C, Fickel J, Meijaard E, Groves CP (2004) Evolution and phylogeny of old world deer. Molecular Phylogenetics and Evolution 33: 880–895. doi: 10.1016/j.ympev.2004.07.013
- Pocock RI (1945) Some cranial and dental characters of the existing species of Asiatic rhinoceroses. Proceeding of Zoological Society of London 14: 437–450. doi: 10.1111/j.1096-3642.1945.tb00235.x
- Pope GG, Frayer DW, Liangchareon M, Kulasing P, Nakabanlang S (1981) Palaeoanthropological investigations of the Thai-American expeditions in Northern Thailand (1978-1980): an interim report. Asian Perspectives 21: 147–163.
- Pramankij S, Subhavan V (2001) Preliminary report on the discovery of evidence of the oldest hominids (2 million to 200000 years old) in Thailand. Silpa Wattanatham 23: 38–47. [In Thai]
- Prentice ML, Denton GH (1988) The deep-sea oxygen isotope record, the global ice sheet system, and hominid evolution. In: Grine FE (Ed.) The Evolutionary History of the Robust Australopithecines. Aldine de Gruyter, New York, 383–403.
- Pushkina D, Bocherens H, Chaimanee Y, Jaeger JJ (2010) Stable carbon isotope reconstructions of diet and paleoenvironment from the late Middle Pleistocene Snake Cave in Northeastern Thailand. Naturwissenschaften 97: 299–309. doi: 10.1007/s00114-009-0642-6
- Rage JC (2001) Fossil snakes from the Paleocene of São José de Itaboraí, Brazil. Part II. Boidae. Palaeovertebrata 30: 111–150.
- Raup DM, Crick RE (1979) Measurement of faunal similarity in paleontology. Journal of Paleontology 53: 1213–1227.
- Rink WJ, Wei W, Bekken D, Jones HL (2008) Geochronology of Ailuropoda-Stegodon fauna and Gigantopithecus in Guangxi province, southern China. Quaternary Research 69: 377–387. doi: 10.1016/j.yqres.2008.02.008
- Ripoll MP, Morales Pérez JV, Sanchis Serra A, Aura Tortosa JE, Montañana IS (2010) Presence of the genus Cuon in upper Pleistocene and initial Holocene sites of the Iberian Peninsula: new remains identified in archaeological contexts of the Mediterranean region. Journal of Archaeological Science 37: 437–450. doi: 10.1016/j.jas.2009.10.008
- Romer AS (1956) Osteology of the reptiles. University of Chicago Press, Chicago, 772 pp.
- Rookmaker LC (1980) The distribution of the rhinoceroes in eastern India, Bangladesh, China, and the Indo-Chinese region. Zoologischer Anzeiger 205: 253–268.
- Saegusa H (1996) Stegodontidae: Evolutionary relationships. In: Shoshani J, Tassy P (Eds) The Proboscidea: Evolution and Palaeoecology of Elephants and Their Relatives. Oxford University Press, Oxford, 178–192.
- Saegusa H, Thasod Y, Ratanasthien B (2005) Notes on Asian stegodontids. Quaternary International 126–128: 31–48. doi: 10.1016/j.quaint.2004.04.013
- Santa Luca AP (1980) The Ngandong Fossil Hominids. Yale University Publications in Anthropology 78: 1–175.
- Scanlon JD, Mackness BS (2001) A new giant python from the Pliocene Bluff Downs Local Fauna of northeastern Queensland. Alcheringa: An Australasian Journal of Palaeontology 25: 425–437. doi: 10.1080/03115510108619232
- Schepartz LA, Stoutamire S, Bekken DA (2005) Stegodon orientalis from Panxian Dadong, a Middle Pleistocene archaeological site in Guizhou, South China: taphonomy, population structure and evidence for human interactions. Quaternary International 126–128: 271–282. doi: 10.1016/j.quaint.2004.04.026
- Shen G, Tu H, Xiao D, Qiu L, Feng YX, Zhao JX (2014) Age of Maba hominin site in southern China: Evidence from U-series dating of Southern Branch Cave. Quaternary Geochronology 23: 56–62. doi: 10.1016/j.quageo.2014.06.004
- Simpson GG (1943) Mammals and the nature of continents. American Journal of Science 241: 1–31. doi: 10.2475/ajs.241.1.1
- Simpson GG (1960) Notes on the measurement of faunal resemblance. American Journal of Science 258A: 300–311.
- Sondaar PY (1984) Faunal evolution and the mammalian biostratigraphy of Java. Courrier Forschungsinstitut Senckenberg 69: 219–235.
- Sondaar PY, van der Geer AAE, Dermitzakis MD (2006) The unique postcranial of the Old World monkey Paradolichopithecus: more similar to Australopithecus than to baboons. Hellenic Journal of Geosciences 41: 19–28.
- Storm P, de Vos J (2006) Rediscovery of the Late Pleistocene Punung hominid sites and the discovery of a new site Gunung Dawung in East Java. Senckenbergiana Lethaea 86: 121–131. doi: 10.1007/BF03043494
- Storm P, Aziz F, de Vos J, Kosasih D, Baskoro S, Ngaliman van den Hoek Ostende LW (2005) Late Pleistocene Homo sapiens in a tropical rainforest fauna in East Java. Journal of Human Evolution 49: 536–545. doi: 10.1016/j.jhevol.2005.06.003
- Storm P, Wood R, Stringer C, Bartsiokas A, de Vos J, Aubert M, Kinsley L, Grün R (2013) U-series and radiocarbon analyses of human and faunal remains from Wajak, Indonesia. Journal of Human Evolution 64: 356–365. doi: 10.1016/j.jhevol.2012.11.002
- Suraprasit K, Jaeger JJ, Chaimanee Y, Benammi M, Chavasseau O, Yamee C, Tian P, Panha S (2015) A complete skull of Crocuta crocuta ultima indicates a late Middle Pleistocene age for the Khok Sung (northeastern Thailand) vertebrate fauna. Quaternary International 374: 34–45. doi: 10.1016/j.quaint.2014.12.062
- Szyndlar Z, Böhme W (1996) Redescription of Tropidonotus atavus von Meyer, 1855 from the upper Oligocene of Rott (Germany) and its allocation to Rottophis gen. nov. (Serpentes, Boidae). Palaeontographica Abteilungen A 240: 145–161.
- Szyndlar Z, Rage JC (2003) Non-erycine Booidea from the Oligocene and Miocene of Europe. Institute of Systematics and Evolution of Animals, Polish Academy of Sciences Kraków, 109 pp.
- Takai M, Saegusa H, Thaung-Htike Zin-Maung-Maung-Thein (2006) Neogene mammalian fauna in Myanmar. Asian paleoprimatology 4: 143–172.
- Tallman M, Almécija S, Reber SL, Alba DM, Moyà-Solà S (2013) The distal tibia of Hispanopithecus laietanus: More evidence for mosaic evolution in Miocene apes. Journal of Human Evolution 64: 319–327. doi: 10.1016/j.jhevol.2012.07.009
- Taplin LE, Grigg GC, Beard L (1985) Salt gland function in fresh water crocodiles: evidence for a marine phase in eusuchian evolution? In: Grigg G, Shine R, Ehmann H (Eds) Biology of Australasian Frogs and Reptiles.Royal Zoological Society of New South Wales, 403–410.
- Thein T (1974) La faune néolithique du Phnom Loang (Cambodge) (Ruminants). Doctorat de 3ème cycle de l’Université Paris VI, 159 pp.
- Timmins RJ, Hedges S, Duckworth JW (2008) Bos sauveli. The IUCN Red List of Threatened Species, version 2015.2http://www.iucnredlist.org [downloaded on 31 August 2015]
- Tong H, Guérin C (2009) Early Pleistocene Dicerorhinus sumatrensis remains from the Liucheng Gigantopithecus Cave, Guangxi, China. Geobios 42: 525–539. doi: 10.1016/j.geobios.2009.02.001
- Tong H, Patou-Mathis M (2003) Mammoth and other proboscideans in China during the Late Pleistocene. In: Reumer JWF, de Vos J, Mol D (Eds) Advances in Mammoth Research.Proceedings of the Second International Conference, Rotterdam, May 1999. Deinsea, vol. 9, 421–428.
- Tong H, Liu J (2004) The Pleistocene–Holocene extinctions of mammals in China. In: Dong W (Ed.) Proceedings of the Ninth Annual Symposium of the Chinese Society of Vertebrate Paleontology. China Ocean Press, Beijing, 111–119. [In Chinese with English abstract]
- Tong H, Hu N, Wang XM (2012) New remains of Canis chiliensis (Mammalia, Carnivora) from Shanshenmiaozui, a Lower Pleistocene site in Yangyuan, Hebei. Vertebrata PalAsiatica 50: 335–360.
- Tougard C (1998) Les faunes de grands mammifères du Pléistocène moyen terminal de Thaïlande dans leur cadre phylogénétique, paléoécologique et biochronologique.PhD thesis, University of Montpellier II, Montpellier, France, 175 pp.
- Tougard C (2001) Biogeography and migration routes of large mammal faunas in South-East Asia during the Late Middle Pleistocene: focus on the fossil and extant faunas from Thailand. Palaeogeography, Palaeoclimatology, Palaeoecology 168: 337–358. doi: 10.1016/S0031-0182(00)00243-1
- Tougard C, Montuire S (2006) Pleistocene paleoenvironmental reconstructions and mammalian evolution in South-East Asia: focus on fossil faunas from Thailand. Quaternary Science Reviews 25: 126–141. doi: 10.1016/j.quascirev.2005.04.010
- Tougard C, Jaeger JJ, Chaimanee Y, Suteethorn V, Triamwichanon S (1998) Discovery of a Homo sp. tooth associated with a mammalian cave fauna of Late Middle Pleistocene age, Northern Thailand. Journal of Human Evolution 35: 47–54. doi: 10.1006/jhev.1998.0221
- Travouillon KJ, Archer M, Hand SJ, Godthelp H (2006) Multivariate analyses of Cenozoic mammalian faunas from Riversleigh, north-western Queensland. Alcheringa Special Issue 1: 323–349. doi: 10.1080/03115510609506871
- Tshen LT (2013) Quaternary Elephas fossils from Peninsular Malaysia: historical overview and new material. The Raffles Bulletin of Zoology 29: 139–153.
- Tsubamoto T, Takai M, Egi N (2004) Quantitative analyses of biogeography and faunal evolution of middle to late Eocene mammals in East Asia. Journal of Vertebrate Paleontology 24: 657–667. doi: 10.1671/0272-4634(2004)024[0657:QAOBAF]2.0.CO;2
- Turley K, Guthrie EH, Frost SR (2011) Geometric morphometric analysis of tibial shape and presentation among Catarrhine taxa. The Anatomical Record 294: 217–230. doi: 10.1002/ar.21307
- van den Bergh GD (1999) The late Neogene elephantoid-bearing faunas of Indonesia and their palaeozoogeographic implications: a study of the terrestrial faunal succession of Sulawesi, Flores and Java, including evidence for early hominid dispersal east of Wallace’s Line. Scripta Geologica 117: 1–419.
- van den Bergh GD, de Vos J, Sondaar P (2001) The Late Quaternary palaeogeography of mammal evolution in the Indonesian archipelago. Palaeogeography, Palaeoclimatology, Palaeoecology 171: 385–408. doi: 10.1016/S0031-0182(01)00255-3
- van den Bergh GD, Due RA, Morwood MJ, Sutikna T, Jatmiko P, Wahyu Saptomo E (2008) The youngest Stegodon remains in Southeast Asia from the Late Pleistocene archaeological site Liang Bua, Flores, Indonesia. Quaternary International 182: 16–48. doi: 10.1016/j.quaint.2007.02.001
- van den Brink LM (1982) On the mammal fauna of the Wajak Cave, Java (Indonesia). Modern Quaternary Research Southeast Asia 7: 177–193.
- van der Geer A, Lyras G, de Vos J, Dermitzakis M (2010) Evolution of island mammals: adaptation and extinction of placental mammals on islands. Wiley Blackwell, Oxford, 479 pp. doi: 10.1002/9781444323986
- van der Kaars WA (1991) Palynology of Eastern Indonesian marine piston-cores: a Late Quaternary vegetational and climatic record for Australasia. Palaeogeography, Palaeoclimatology, Palaeoecology 85: 239–302. doi: 10.1016/0031-0182(91)90163-L
- van der Kaars WA, Dam MAC (1995) A 135,000-year record of vegetational and climatic change from the Bandung area, West-Java, Indonesia. Palaeogeography, Palaeoclimatology, Palaeoecology 117: 55–72. doi: 10.1016/0031-0182(94)00121-N
- van der Made J (1996) Listriodontinae (Suidae, Mammalia), their evolution, systematic, and distribution in time and space. Contributions to Tertiary and Quaternary Geology 33: 3–254.
- von den Driesch A (1976) A guide to the measurement of animal bones from archaeological sites.Peabody Museum Bulletin, vol. 1, Peabody Museum of Archaeology and Ethnology, Harvard University, 137 pp.
- von Koenigswald GHR (1933) Beitrag zur Kenntnis der fossilen Wirbeltiere Javas, I. Teil. Wetenschappelijke Mededeelingen (Dienst van den Mijnbouw in Nederlandsch Indië) Vol. 23, 184 pp.
- von Koenigswald GHR (1935) Die fossilen Saugertier Fauna Javas. Proceeding Koninklijke Nederlandsche Akademie van Wetenschappen 38: 188–198.
- von Koenigswald GHR (1938) The relationship between the fossil mammalian faunae of Java and China, with special reference to early man. Peking Natural. History Bulletin 13: 293–298.
- Voris HK (2000) Maps of Pleistocene sea levels in South East Asia: Shorelines, river systems, time durations. Journal of Biogeography 27: 1153–1167. doi: 10.1046/j.1365-2699.2000.00489.x
- Wang W, Potts R, Baoyin Y, Huang W, Cheng H, Edwards RL, Ditchfield P (2007) Sequence of mammalian fossils, including hominoid teeth, from the Bubing Basin caves, South China. Journal of Human Evolution 52: 370–379. doi: 10.1016/j.jhevol.2006.10.003
- Wang W, Liao W, Li D, Tian F (2014) Early Pleistocene large-mammal fauna associated with Gigantopithecus at Mohui Cave, Bubing Basin, South China. Quaternary International 354: 122–130. doi: 10.1016/j.quaint.2014.06.036
- Westaway KE, Morwood MJ, Roberts RG, Rokus AD, Zhao Jx, Storm P, Aziz F, van den Bergh GD, Hadi P, Jatmiko de Vos J (2007) Age and biostratigraphic significance of the Punung Rainforest Fauna, East Java, Indonesia, and implications for Pongo and Homo. Journal of Human Evolution 53: 709–717. doi: 10.1016/j.jhevol.2007.06.002
- Woodruff D (2010) Biogeography and conservation in Southeast Asia: How 2.7 million years of repeated environmental fluctuations affect today’s patterns and the future of the remaining refugial-phrase biodiversity. Biodiversity and Conservation 19: 919–941. doi: 10.1007/s10531-010-9783-3
- Wu XJ, Schepartz LA, Liu W, Trinkaus E (2011) Antemortem trauma and survival in the late Middle Pleistocene human cranium from Maba, South China. Proceedings of the National Academy of Sciences 108: 19558–19562. doi: 10.1073/pnas.1117113108
- Yamee C, Chaimanee Y (2005) Fossils of a Hyaenid (Crocuta crocuta) and its Associated Fauna from Thum Phedan, Thung Yai District, Nakhon Sri Thammarat. Academic report no. DMR 11/2005. Department of Mineral Resources, Bangkok, Thailand, 40 pp. [In Thai]
- Yan Y, Wang Y, Jin C, Mead JI (2014) New remains of Rhinoceros (Rhinocerotidae, Perissodactyla, Mammalia) associated with Gigantopithecus blacki from the Early Pleistocene Yanliang Cave, Fusui, South China. Quaternary International 354: 110–121. doi: 10.1016/j.quaint.2014.01.004
- Zeitoun V, Seveau A, Forestier H, Thomas H, Lenoble A, Laudet F, Antoine PO, Debruyne R, Ginsburg L, Mein P, Winayalai C, Chumdee N, Doyasa T, Kijngam A, Nakbunlung S (2005) Découverte d’un assemblage faunique à Stegodon–Ailuropoda dans une grotte du Nord de la Thaïlande (Ban Fa Suai, Chiang Dao). Comptes Rendus Palevol 4: 255–264. doi: 10.1016/j.crpv.2004.11.013
- Zeitoun V, Lenoble A, Laudet F, Thompson J, Rink WJ, Mallye JB, Chinnawut W (2010) The Cave of the Monk (Ban fa Suai, Chiang Dao wildlife sanctuary, northern Thailand). Quaternary International 220: 160–173. doi: 10.1016/j.quaint.2009.11.022
- Zhang Y, Jin C, Cai Y, Kono R, Wang W, Wang Y, Zhu M, Yan Y (2014) New 400–320 ka Gigantopithecus blacki remains from Hejiang Cave, Chongzuo City, Guangxi, South China. Quaternary International 354: 35–45. doi: 10.1016/j.quaint.2013.12.008
- Zheng Z, Lei ZQ (1999) A 400,000 year record of vegetational and climatic changes from a volcanic basin, Leizhou Peninsula, southern China. Palaeogeography, Palaeoclimatology, Palaeoecology 145: 339–362. doi: 10.1016/S0031-0182(98)00107-2
- Zhou MZ, Zhang YP (1974) Fossil Elephants of China. Science Press, Beijing, 74 pp. [In Chinese with English abstract]
- Zhu ZY, Dennell R, Huang WW, Wu Y, Rao ZG, Qiu SF, Xie JB, Liu W, Fu SQ, Han JW, Zhou HY, Ou Yang TP, Li HM (2015) New dating of the Homo erectus cranium from Lantian (Gongwangling), China. Journal of Human Evolution 78: 144–157. doi: 10.1016/j.jhevol.2014.10.001
- Zin-Maung-Maung-Thein Thaung-Htike, Tsubamoto T, Takai M, Egi N (2006) Early Pleistocene Javan rhinoceros from the Irrawaddy Formation, Myanmar. Asian Paleoprimatology 4: 197–204.
Appendices
Measurements (in millimeters) of postcranial remains of identified mammal taxa from Khok Sung. * indicates a juvenile individual.
| Scapula | |||||||||||||||
| Specimen | Taxa | GLP | LG | SLC | BG | Ld | DHA | HS | |||||||
| DMRKS-05-03-00-58 | Rhinoceros sondaicus | 87.49 | 78.81 | 62.97 | 55.33 | 144.77 | – | 325.57 | |||||||
| DMR-KS-05-03-26-2 | Bubalus arnee | 106.72 | 81.99 | 76.45 | 68.40 | 297.28 | 381.27 | 445.28 | |||||||
| DMR-KS-05-02-20-4 | Bubalus arnee | 84.00 | 66.89 | 52.13 | 54.50 | 213.22 | 301.79 | 296.17 | |||||||
| DMR-KS-05-06-24-4 | Panolia eldii | 44.00 | 34.56 | 23.41 | 31.54 | 108.17 | 206.05 | 211.37 | |||||||
| Humerus | |||||||||||||||
| Specimen | Taxa | Bp | Dp | Bd | BT | Dd | SD | GLC | GLl | GL | |||||
| DMR-KS-05-03-10-6 | Stegodon cf. orientalis | – | – | 195.27 | 173.98 | 162.73 | – | – | – | – | |||||
| DMR-KS-05-03-31-3 | Rhinoceros sondaicus | 174.58 | 142.51 | 155.86 | 94.39 | 115.77 | 66.09 | 377.04 | 422.18 | 437.23 | |||||
| DMR-KS-05-03-26-8 | Sus barbatus | 65.67 | 76.44 | 57.39 | 47.06 | 45.11 | 20.72 | 224.71 | 236.13 | 238.28 | |||||
| DMR-KS-05-03-20-2(1) | Bos sauveli | > 99.80 | – | 88.14 | 84.92 | 88.80 | 38.86 | – | – | – | |||||
| DMR-KS-05-03-00-62 | Bos gaurus | > 99.45 | > 98.85 | 103.69 | 98.14 | 96.59 | 50.36 | 328.18 | 347.28 | 353.87 | |||||
| DMR-KS-05-05-1-1 | Bos gaurus | 122.37 | 130.12 | 103.54 | 98.77 | 97.16 | 56.37 | 317.26 | 354.28 | 356.28 | |||||
| DMR-KS-05-03-31-1 | Bubalus arnee | 125.53 | 135.07 | 104.40 | 101.70 | 93.72 | 52.29 | 343.74 | 413.22 | 444.12 | |||||
| DMR-KS-05-03-31-8 | Bubalus arnee | 125.79 | 138.19 | 104.38 | 99.09 | 94.07 | 52.92 | 347.41 | 424.77 | 449.11 | |||||
| DMR-KS-05-03-13-4 | Axis axis | – | – | 34.75 | 31.96 | 34.12 | < 17.02 | – | – | – | |||||
| DMR-KS-05-04-11-32 | Axis axis | – | – | 35.98 | 33.77 | 34.05 | 17.37 | – | – | – | |||||
| DMR-KS-05-03-17-17 | Axis axis | – | – | 36.86 | 35.57 | 32.77 | 16.10 | – | – | – | |||||
| DMR-KS-05-04-11-35 | Panolia eldii | – | > 59.10 | 46.10 | 40.85 | 41.18 | 23.3 | 187.53 | – | – | |||||
| DMR-KS-05-03-18-1 | Panolia eldii | 53.34 | 65.34 | – | – | – | 21.45 | – | – | – | |||||
| DMR-KS-05-03-15-43 | Rusa unicolor | – | – | 54.89 | 49.04 | 48.30 | < 27.70 | – | – | – | |||||
| Ulna and radius | |||||||||||||||
| Specimen | Taxa | Bp/BPC | BFp | Dp | Bd | BFd | Dd | LO | DPA | SDO | SD | PL | Ll | GLl | GL |
| DMR-KS-05-03-00-61 | Bubalus arnee | 106.16 | 92.17 | 52.99 | 100.36 | 90.17 | 65.85 | 125.53 | 93.64 | 72.61 | 55.54 | 305.18 | 308.75 | 476.61 | 484.72 |
| DMR-KS-05-03-31-2 | Bubalus arnee | 108.45 | 98.91 | 57.03 | 103.37 | 92.25 | 73.75 | 131.15 | 98.37 | 76.03 | 50.74 | 335.29 | 343.51 | 427.78 | 452.17 |
| DMR-KS-05-03-31-9 | Bubalus arnee | 106.69 | 97.99 | 57.44 | 103.49 | 92.06 | 72.92 | 128.89 | 99.00 | 76.54 | 47.66 | 335.93 | 344.77 | 424.77 | 449.11 |
| DMR-KS-05-03-31-10 | Panolia eldii | 39.40 | 36.72 | 20.95 | 37.22 | 32.67 | 21.37 | – | – | – | 22.52 | 199.23 | 198.21 | – | 197.52 |
| DMR-KS-05-04-11-3 | Panolia eldii | 42.30 | 38.22 | 22.47 | 36.84 | 34.64 | 23.46 | – | – | – | 22.61 | 204.18 | 203.71 | – | 215.70 |
| DMR-KS-05-03-19-16 | Panolia eldii | 40.16 | 33.03 | 22.16 | 40.62 | 39.21 | 29.89 | – | – | – | 23.58 | 197.89 | 193.72 | – | 204.53 |
| DMR-KS-05-03-25-9 | Rusa unicolor | 55.06 | 53.58 | 28.92 | – | – | – | – | – | – | – | – | – | – | – |
| DMR-KS-05-03-19-14 | Rusa unicolor | 52.37 | 50.16 | 30.83 | – | – | – | – | – | – | 28.12 | – | – | – | – |
| DMR-KS-05-03-26-19 | Rusa unicolor | – | – | – | 43.12 | 41.08 | 32.20 | – | – | – | 24.83 | – | – | – | – |
| Pelvis | |||||||||||||||
| Specimen | Taxa | GL | LA | LS | SH | SB | SC | LFo | GBTc | GBA | GBTi | SBI | |||
| DMR-KS-05-03-10-11 | Stegodon cf. orientalis | > 855.79 | 148.93 | – | 139.18 | 66.29 | 399.46 | 206.77 | – | – | – | – | |||
| DMR-KS-05-03-10-12 | Stegodon cf. orientalis | > 496.75 | 140.75 | – | 145.17 | 59.60 | 389.92 | 201.25 | – | – | – | – | |||
| DMR-KS-05-04-1-25 | Bubalus arnee | 494.85 | 96.57 | 149.23 | 70.55 | 35.81 | 256.67 | 103.24 | 517.48 | 303.98 | 319.25 | 204.37 | |||
| Femur | |||||||||||||||
| Specimen | Taxa | Bp | Dp | DC | Bd | Dd | SD | GLC | GL | ||||||
| DMR-KS-05-03-10-4 | Stegodon cf. orientalis | – | – | 125.14 | 178.16 | 211.72 | 126.18 | – | – | ||||||
| DMR-KS-05-03-00-63 | Rhinoceros unicornis | – | – | – | 140.58 | 183.17 | 46.21 | – | – | ||||||
| DMR-KS-05-03-9-2 | Bos gaurus | 124.57 | 66.64 | 52.31 | 106.89 | 138.24 | 46.15 | 405.57 | 419.28 | ||||||
| DMR-KS-05-04-30-1 | Bos gaurus | 150.06 | > 65.51 | 59.63 | – | – | – | – | – | ||||||
| DMR-KS-05-04-1-1 | Bubalus arnee | 160.51 | 80.69 | 66.76 | 128.54 | 156.98 | 54.05 | 420.18 | 447.15 | ||||||
| DMR-KS-05-04-1-2 | Bubalus arnee | 165.98 | 84.20 | 67.30 | 130.92 | 156.78 | 55.12 | 425.66 | 442.68 | ||||||
| DMR-KS-05-03-20-8 | Bubalus arnee | – | – | – | 124.57 | 181.53 | – | – | – | ||||||
| DMR-KS-05-03-27-4 | Axis axis | – | – | – | 50.49 | 69.42 | – | – | – | ||||||
| DMR-KS-05-03-27-11 | Panolia eldii | 66.29 | 31.42 | 28.4 | 52.67 | 70.12 | 21.97 | – | – | ||||||
| DMR-KS-05-03-17-36 | Panolia eldii | 68.57 | 35.49 | 28.73 | 56.56 | 73.06 | 21.97 | 251.58 | – | ||||||
| DMR-KS-05-03-28-20 | Panolia eldii | – | – | – | 53.72 | 72.00 | – | – | – | ||||||
| DMR-KS-05-04-05-38 | Panolia eldii | 67.59 | 35.21 | 27.74 | – | – | – | – | – | ||||||
| DMR-KS-05-03-00-119 | Panolia eldii | – | – | – | 51.60 | 71.30 | < 22.94 | – | – | ||||||
| DMR-KS-05-03-19-2 | Panolia eldii | – | – | – | 51.85 | 73.23 | < 26.51 | – | – | ||||||
| DMR-KS-05-08-16-1 | Panolia eldii | 59.40 | 32.32 | 26.14 | – | – | – | – | – | ||||||
| DMR-KS-05-04-11-2 | Rusa unicolor | – | – | – | 55.20 | 69.90 | 22.54 | – | – | ||||||
| DMR-KS-05-03-19-7 | Rusa unicolor | 67.01 | 44.50 | 28.58 | – | – | 21.72 | – | – | ||||||
| DMR-KS-05-03-12-2* | Rusa unicolor | 48.57 | 31.96 | – | – | – | 18.91 | – | – | ||||||
| DMR-KS-05-03-26-5 | Rusa unicolor | > 77.29 | 42.06 | 37.82 | – | – | 30.98 | – | – | ||||||
| DMR-KS-05-04-30-9 | Rusa unicolor | – | – | – | 54.46 | 69.12 | 21.85 | – | – | ||||||
| DMR-KS-05-04-19-10 | Rusa unicolor | – | – | – | 48.39 | 53.48 | 23.49 | – | – | ||||||
| Tibia | |||||||||||||||
| Specimen | Taxa | Bp | Dp | Bd | Dd | SD | Ll | GL | |||||||
| DMR-KS-05-03-00-52 | Rhinoceros sondaicus | – | – | 94.95 | > 74.11 | – | – | – | |||||||
| DMR-KS-05-04-1-11 | Bubalus arnee | 132.04 | 125.08 | 87.26 | 69.54 | 55.84 | 363.36 | 437.17 | |||||||
| DMR-KS-05-04-1-3 | Bubalus arnee | 128.45 | 121.16 | 86.15 | 68.95 | 52.64 | 385.14 | 415.56 | |||||||
| DMR-KS-05-03-20-9 | Bubalus arnee | 126.74 | 106.22 | 88.37 | 64.62 | 53.42 | 354.45 | 406.77 | |||||||
| DMR-KS-05-03-28-16 | Rusa unicolor | 79.37 | 72.45 | 47.53 | 37.44 | 30.61 | 300.01 | 317.52 | |||||||
| DMR-KS-05-04-04-1 | Macaca sp. | 27.50 | 21.46 | 18.12 | 12.95 | 8.77 | 158.71 | 167.57 | |||||||
| Fibula | |||||||||||||||
| Specimen | Taxa | GL | |||||||||||||
| DMR-KS-05-03-00-124 | Stegodon cf. orientalis | > 354.56 | |||||||||||||
| Metacarpus | |||||||||||||||
| Specimen | Taxa | Bp | Dp | Bd | Dd | DD | SD | Ll | GLl | GL | |||||
| DMR-KS-05-03-28-29 | Rhinoceros sondaicus | 53.45 | 42.75 | 48.53 | 39.70 | – | 40.32 | – | – | 152.50 | |||||
| DMR-KS-05-03-22-49 | Rhinoceros sondaicus | – | – | 51.80 | 34.10 | 22.26 | – | – | – | – | |||||
| DMR-KS-05-04-05-15 | Rhinoceros sondaicus | 49.18 | 41.15 | 37.64 | 36.51 | 25.58 | 31.19 | 136.96 | 138.35 | 141.26 | |||||
| DMR-KS-05-03-26-27 | Bos gaurus | 70.18 | 46.08 | 64.78 | 33.53 | 30.97 | 43.42 | 247.28 | 252.75 | 256.78 | |||||
| DMR-KS-05-03-26-3(1) | Bubalus arnee | 77.07 | 49.58 | 78.33 | 42.45 | 30.17 | 52.02 | 189.78 | 197.23 | 206.75 | |||||
| DMR-KS-05-03-18-2 | Axis axis | 25.80 | 16.79 | 25.06 | 15.89 | 11.69 | 14.50 | 160.18 | 162.37 | 167.66 | |||||
| DMR-KS-05-03-22-28 | Axis axis | – | 19.82 | 27.91 | 18.35 | 14.76 | 17.61 | 186.70 | 187.90 | 190.10 | |||||
| DMR-KS-05-03-08-2 | Axis axis | 29.68 | 21.47 | 30.56 | 18.54 | 16.46 | 16.38 | 186.68 | 187.11 | 188.81 | |||||
| DMR-KS-05-03-19-3 | Axis axis | 29.27 | 21.25 | 30.59 | 18.49 | 15.60 | 19.37 | 188.77 | 191.11 | 192.58 | |||||
| DMR-KS-05-03-19-37 | Axis axis | 23.45 | 15.14 | 24.46 | 15.56 | 12.74 | 12.03 | 163.43 | 165.54 | 167.32 | |||||
| DMR-KS-05-04-30-20 | Axis axis | 28.80 | 19.11 | – | – | – | 14.22 | 195.55 | 196.17 | 197.89 | |||||
| DMR-KS-05-03-24-2 | Panolia eldii | 30.95 | 22.01 | 30.11 | 19.01 | 14.96 | 17.63 | 192.33 | 194.14 | 197.83 | |||||
| DMR-KS-05-03-17-26 | Rusa unicolor | 37.58 | 29.64 | 37.00 | 24.64 | 19.31 | 24.38 | 216.98 | 217.35 | 224.25 | |||||
| Metatarsus | |||||||||||||||
| Specimen | Taxa | Bp | Dp | Bd | Dd | DD | SD | Ll | GLl | GL | |||||
| DMR-KS-05-04-1-8 | Bubalus arnee | 66.22 | 54.89 | 79.59 | 45.49 | 38.42 | 44.58 | 237.44 | 241.11 | 251.92 | |||||
| DMR-KS-05-04-1-6 | Bubalus arnee | 65.79 | 58.19 | 82.22 | 45.07 | 38.88 | 44.44 | 239.66 | 241.52 | 255.33 | |||||
| DMR-KS-05-03-28-30 | Bubalus arnee | 55.89 | 55.48 | 69.85 | 37.24 | 35.40 | 39.31 | 225.71 | 229.18 | 237.54 | |||||
| DMR-KS-05-03-26-3 | Axis axis | 25.54 | 26.65 | 26.37 | 20.11 | 16.67 | 15.61 | 176.37 | 180.01 | 184.21 | |||||
| DMR-KS-05-03-15-14 | Axis axis | 32.87 | 30.52 | 36.54 | 24.38 | 20.48 | 21.48 | 219.75 | 220.21 | 224.81 | |||||
| DMR-KS-05-03-29-30 | Axis axis | 27.10 | 27.45 | 28.73 | 21.81 | 16.14 | 16.88 | 183.77 | 184.96 | 187.21 | |||||
| DMR-KS-05-03-28-17 | Panolia eldii | 27.78 | 29.00 | 28.93 | 19.12 | 17.32 | 17.75 | 217.28 | 219.89 | 223.54 | |||||
| DMR-KS-05-03-25-8 | Panolia eldii | 28.90 | 30.23 | 31.26 | 19.51 | 18.51 | 20.04 | 221.45 | 224.13 | 225.71 | |||||
| DMR-KS-05-03-15-15 | Panolia eldii | 28.12 | 30.52 | 30.82 | 20.11 | 17.01 | 17.80 | 218.79 | 221.74 | 227.14 | |||||
| DMR-KS-05-03-19-11 | Rusa unicolor | 36.89 | 35.69 | 37.75 | 26.63 | 22.36 | 24.08 | 233.75 | 238.89 | 244.97 | |||||
Measurements (in millimeters) of mandibles of rhinoceroses from Khok Sung.
| Taxon | Rhinoceros sondaicus | Rhinoceros unicornis | |
|---|---|---|---|
| Mandible no. | DMR-KS-05-03-00-126 | DMR-KS-05-03-31-28 | DMR-KS-05-03-17-13 |
| Metrical parameters (mm) | |||
| Length of the mandible | > 311.0 | > 198.3 | > 404.1 |
| Length of the mandibular symphysis | > 108.1 | 124.2 | – |
| Width of the mandibular symphysis | – | 59.5 | – |
| Length of the diastema | – | 50.5 | – |
| Height of the mandibular corpus below the p2 | 51.7 (right) | 44.5 (right) | – |
| – | 41.0 (left) | – | |
| Height of the mandibular corpus below the p3 | 56.1 (right) | – | – |
| 50.1 (left) | 46.8 (left) | – | |
| Height of the mandibular corpus below the p4 | 76.2 (right) | – | – |
| 74.5 (left) | – | – | |
| Height of the mandibular corpus below the m1 | 80.7 (right) | – | – |
| 84.2 (leftt) | – | – | |
| Height of the mandibular corpus below the m2 | 94.0 (right) | – | – |
| 91.9 (leftt) | – | – | |
| Height of the mandibular corpus below the m3 | 97.6 (right) | – | – |
| 92.4 (left) | – | 126.6 (left) | |
| Width of the mandibular corpus below the m1 | 55.3 (right) | – | – |
| 54.2 (left) | – | – | |
| Width of the mandibular corpus below the m2 | 57.7 (right) | – | – |
| 57.5 (left) | – | – | |
| Width of the mandibular corpus below the m3 | 57.3 (right) | – | – |
| 56.4 (left) | – | – | |
Measurements (in millimeters) of mandible of Sus barbatus from Khok Sung. Numbers within the parentheses refer to the numbers used in von den Driesch’s metrical methods (1976: fig. 22b).
| Taxon | Sus barbatus | |
|---|---|---|
| Mandible no. | DMR-KS-05-03-15-1 | DMR-KS-05-04-19-1 |
| Metrical parameters (mm) | male | female |
| Minimum length of the mandible | 189.0 | 207.0 |
| Diastema between c1 and p1 | 8.6 | 5.8 |
| Diastema between p1 and p2 | 13.9 | 6.5 |
| (9) Length of the premolar row, p1–p4 | 58.2 (left) | 53.4 (right) 60.1 (left) |
| (9a) Length of the premolar row, p2–p4 | 41.4 (left) | 38.4 (right) 39.0 (left) |
| (8) Length of the molar row | – | 75.3 (right) |
| (7a) Length of the cheek tooth row, p2–m3 | – | 112.4 (right) |
| (7) Length of the cheek tooth row, p1–m3 | – | 126.3 (right) |
| (4) Length of the horizontal ramus: aboral border of the alveolus of m3 to infradentale | – | 163.4 (right) |
| (6) Length: aboral border of the alveolus of m3 to aboral border of the canine alveolus | – | 133.2 (right) |
| (11) Length: oral border of the alveolus of p2 to aboral border of the alveolus of i3 | 45.1 (right) 45.9 (left) | 39.4 (right) 39.2 (left) |
| (12) Length of the median section of the body of mandible: from the mental prominence to infradentale | 64,9 (right) 64.7 (left) | 58.8 (right) |
| (16c) Height of the mandible in front of p2 | 50.9 (right) 50.4 (left) | 41.5 (right) |
| (16b) Height of the mandible in front of m1 | 48.5 (left) | 43.3 (right) |
| (16a) Height of the mandible in front of m3 | – | 45.1 (right) |
Measurements (in millimeters) of crania of cervids from Khok Sung. Numbers within the parentheses refer to the numbers used in von den Driesch’s metrical methods (1976: fig.11). * indicates measurements of the maximum length of the preservation according to incomplete specimens. “es” refers to an estimated value of the full length due to incomplete specimens.
| Taxon | Axis axis | Panolia eldii | |||
|---|---|---|---|---|---|
| Cranium no. | DMR-KS-05-03-00-30 | DMR-KS-05-04-18-50 | DMR-KS-05-03-18-X9 | DMR-KS-05-03-27-1 | DMR-KS-05-04-20-4 |
| Metrical parameters (mm) | |||||
| (6) Basicranial axis: Basion–Synsphenion | 68.11 | 76.94 | 70.95 | 58.65* | 93.34 |
| (8) Neurocranium length: Basion–Nasion | – | – | – | 143.64* | – |
| (10) Median frontal length: Akrokranion–Nasion | – | – | – | 148.51* | – |
| (23) Greatest inner length of the orbit: Ectorbitale–Entorbitale | – | – | 48.76 (right) | 47.26 (left) | – |
| (25) Greatest mastoid breadth: Otion–Otion | 100.94 | 92.35 | 93.29 | 96.29 | 98.28 |
| (26) Greatest breadth of the occipital condyles | 55.28 | 50.66 | 51.42 | 52.35 | 57.13 |
| (27) Greatest breadth at the bases of the paraoccipital processes | 83.89 | 78.94 | 80.24 | 88.51* | 83.49 |
| (28) Greatest breadth of the foramen magnum | 22.53 | 25.15 | 22.65 | 24.83 | 26.20 |
| (31) Least frontal breadth = least breadth of the forehead aboral of the orbits | 88.42 | 99.22 | – | 99.06 | 81.08 |
| (32) Greatest breadth across the orbits = greatest frontal breadth = nearly greatest breadth of skull: Ectorbitale–Ectorbitale | – | – | – | 124.03* | – |
| (38) Basion–the highest point of the superior nuchal crest | 62.62 | 61.91 | 62.65 | 63.52 | 66.97 |
| (40) Proximal circumference of the burr = circumference of the distal end of the pedicle | 116.2 (right), 114.50 (left) | 104.56 (right), 104.81 (left) | 111.87 (right), 103.59 (left) | 100 (es) (right and left) | 85.13 (right) |
| (41) Distal circumference of the burr | 129.80 (left) | 146.53 (right), 144.53 (left) | – | – | 88.51* (right) |
Measurements (in millimeters) of mandibles of cervids from Khok Sung. Numbers within the parentheses refer to the numbers used in von den Driesch’s metrical methods (1976: fig. 21).
| Taxon | Axis axis | Panolia eldii | ||||||||
| Mandible no. | DMR-KS-05-03-19-2 | DMR-KS-05-03-19-1 | DMR-KS-05-03-22-8 | DMR-KS-05-04-01-1 | DMR-KS-05-03-23-1 | DMR-KS-05-03-29-1 | DMR-KS-05-04-7-10 | DMR-KS-05-03-26-10 | DMR-KS-05-03-27-2 | DMR-KS-05-04-9-5 |
| Metrical parameters (mm) | Juvenile | |||||||||
| (3) Length: Gonion caudale–aboral border of the alveolus of m3 | – | 59.47 | – | – | – | – | – | – | – | – |
| (5) Length: Gonion caudale–oral border of the alveolus of p2 | – | 149.42 | – | – | – | – | – | – | – | – |
| (7) Length of the cheek tooth row, measured along the alveoli on the buccal side | – | – | – | 80.53 | 88.11 | 84.49 | – | – | 89.82 | – |
| (8) Length of the molar row, measured along the alveoli on the buccal side | – | 56.09 | – | 54.29 | 54.22 | 54.20 | – | – | 54.83 | – |
| (9) Length of the premolar row, measured along the alveoli on the buccal side | – | 31.24 | – | 28.59 | 34.18 | 30.34 | – | 28.85 | 34.91 | 39.01 |
| (11) Length of the diastema: oral border of the alveolus of p2–aboral border of the alveolus of i4 | – | – | – | – | 58.40 | 60.02 | – | 68.60 | – | – |
| (12) Aboral height of the vertical ramus: Gonion ventrale–highest point of the condyle process | – | 85.87 | – | – | – | – | – | – | – | – |
| (13) Middle height of the vertical ramus: Gonion ventrale–deepest point of the mandibular notch | – | 79.22 | – | – | – | – | – | – | – | – |
| (15a) Height of the mandible behind m3 from the most aboral point of the alveolus on the buccal side | 31.99 | 33.60 | 34.35 | 29.03 | 36.02 | 35.60 | 31.14 | – | 38.84 | – |
| (15b) Height of the mandible in front of m1 | – | 26.72 | – | – | 29.74 | 26.01 | – | 28.85 | 31.13 | – |
| (15c) Height of the mandible in front of p2 | – | 22.13 | – | 24.11 | 21.43 | 21.57 | 16.28 | 23.02 | 27.10 | – |
| Taxon | Axis axis | Rusa unicolor | ||||||||
| Mandible no. | DMR-KS–05–03–18–22 | DMR-KS–05–03–20–1 | DMR-KS–05–03–22–7 | DMR-KS–05–03–22–6 | DMR-KS–05–04–03–1 | DMR-KS–05–03–27–22 | DMR-KS–05–03–27–3 | DMR-KS–05–04–09–2 | DMR-KS–05–03–00–101 | DMR-KS–05–03–13 |
| (3) Length: Gonion caudale–aboral border of the alveolus of m3 | – | – | – | – | – | – | – | 59.53 | – | – |
| (7) Length of the cheek tooth row, measured along the alveoli on the buccal side | – | – | – | – | 95.21 | – | – | – | – | – |
| (8) Length of the molar row, measured along the alveoli on the buccal side | – | 53.17 | – | – | 61.52 | – | – | 51.78 | 68.51 | 78.11 |
| (9) Length of the premolar row, measured along the alveoli on the buccal side | – | – | – | – | 35.88 | – | – | – | – | – |
| (11) Length of the diastema: oral border of the alveolus of p2–aboral border of the alveolus of i4 | – | – | – | – | 58.91 | – | – | – | – | – |
| (12) Aboral height of the vertical ramus: Gonion ventrale–highest point of the condyle process | – | – | – | – | – | – | – | 88.50 | – | – |
| (13) Middle height of the vertical ramus: Gonion ventrale–deepest point of the mandibular notch | – | – | – | – | – | – | – | 84.79 | – | – |
| (14) Oral height of the vertical ramus: Gonion ventrale–Coronion | – | – | – | – | – | – | – | 125.12 | – | – |
| (15a) Height of the mandible behind m3 from the most aboral point of the alveolus on the buccal side | – | – | 36.92 | – | 38.71 | 36.22 | 38.50 | 37.30 | 51.76 | – |
| (15b) Height of the mandible in front of m1 | – | – | – | 28.58 | 24.35 | 32.29 | – | 31.76 | – | – |
| (15c) Height of the mandible in front of p2 | 23.21 | – | – | – | 19.72 | – | – | – | – | – |
Measurements (in millimeters) of scapulae of extant ruminants from Southeast Asia.
| HS | DHA | Ld | SLC | GLP | LG | BG | HS/Ld | DHA/Ld | Ld/SLC | LG/BG | GLP/LG | SLC/BG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Axis axis (N=6) | |||||||||||||
| Max | 176.10 | 164.20 | 98.30 | 21.42 | 38.84 | 30.11 | 25.46 | 1.82 | 1.72 | 4.70 | 1.25 | 1.42 | 0.85 |
| Min | 157.80 | 152.10 | 91.20 | 19.39 | 34.32 | 25.50 | 23.66 | 1.73 | 1.58 | 4.51 | 1.02 | 1.19 | 0.82 |
| Mean | 168.62 | 157.47 | 95.18 | 20.75 | 36.60 | 27.83 | 24.69 | 1.77 | 1.65 | 4.59 | 1.13 | 1.32 | 0.84 |
| Axis porcinus (N=2) | |||||||||||||
| Max | 145.40 | 134.10 | 80.90 | 17.73 | 31.45 | 23.58 | 23.82 | 1.80 | 1.66 | 4.61 | 1.05 | 1.35 | 0.79 |
| Min | 143.60 | 133.20 | 80.80 | 17.54 | 30.20 | 22.41 | 22.55 | 1.78 | 1.65 | 4.56 | 0.94 | 1.33 | 0.74 |
| Mean | 144.50 | 133.65 | 80.85 | 17.64 | 30.83 | 23.00 | 23.19 | 1.79 | 1.65 | 4.58 | 0.99 | 1.34 | 0.76 |
| Panolia eldii (N=4) | |||||||||||||
| Max | 228.90 | 210.10 | 118.50 | 28.07 | 42.45 | 34.93 | 32.25 | 1.93 | 1.77 | 4.86 | 1.15 | 1.28 | 0.87 |
| Min | 200.20 | 178.90 | 109.31 | 22.63 | 39.75 | 31.07 | 27.75 | 1.82 | 1.63 | 4.22 | 1.07 | 1.22 | 0.82 |
| Mean | 214.65 | 194.53 | 114.04 | 25.38 | 41.16 | 33.07 | 29.99 | 1.88 | 1.70 | 4.53 | 1.10 | 1.25 | 0.84 |
| Rusa unicolor (N=4) | |||||||||||||
| Max | 269.40 | 274.70 | 167.40 | 38.43 | 61.87 | 47.23 | 45.60 | 1.62 | 1.66 | 4.43 | 1.08 | 1.32 | 0.93 |
| Min | 233.10 | 235.10 | 152.70 | 34.69 | 52.37 | 40.50 | 37.66 | 1.52 | 1.54 | 4.36 | 1.01 | 1.29 | 0.83 |
| Mean | 250.93 | 254.73 | 159.78 | 36.38 | 56.94 | 43.64 | 41.50 | 1.57 | 1.59 | 4.39 | 1.05 | 1.30 | 0.88 |
| Bos sauveli (N=4) | |||||||||||||
| Max | 389.00 | 359.70 | 216.50 | 66.42 | 73.22 | 62.72 | 56.78 | 2.25 | 2.12 | 3.28 | 1.13 | 1.17 | 1.18 |
| Min | 381.20 | 357.80 | 169.60 | 52.38 | 65.48 | 56.54 | 50.25 | 1.80 | 1.65 | 3.23 | 1.10 | 1.15 | 1.03 |
| Mean | 385.08 | 358.85 | 193.08 | 59.31 | 69.41 | 59.59 | 53.55 | 2.02 | 1.89 | 3.25 | 1.11 | 1.16 | 1.10 |
| Bos javanicus (N=6) | |||||||||||||
| Max | 441.90 | 381.60 | 226.80 | 62.95 | 77.54 | 65.88 | 61.83 | 1.95 | 1.71 | 3.69 | 1.18 | 1.19 | 1.03 |
| Min | 384.30 | 339.10 | 198.20 | 53.91 | 71.11 | 61.66 | 52.24 | 1.83 | 1.65 | 3.60 | 1.07 | 1.15 | 1.01 |
| Mean | 403.53 | 355.58 | 211.47 | 57.94 | 74.20 | 63.41 | 56.79 | 1.91 | 1.68 | 3.65 | 1.12 | 1.17 | 1.02 |
| Bos gaurus (N=6) | |||||||||||||
| Max | 536.10 | 491.20 | 258.70 | 71.23 | 90.16 | 80.07 | 70.45 | 2.07 | 1.90 | 3.72 | 1.19 | 1.20 | 1.20 |
| Min | 393.70 | 356.70 | 191.30 | 54.34 | 79.07 | 66.08 | 55.65 | 1.87 | 1.86 | 3.52 | 1.14 | 1.13 | 0.91 |
| Mean | 462.95 | 434.45 | 230.78 | 63.76 | 83.68 | 72.37 | 62.47 | 2.01 | 1.88 | 3.61 | 1.16 | 1.16 | 1.02 |
| Bubalus arnee (N=6) | |||||||||||||
| Max | 374.00 | 362.80 | 238.10 | 71.55 | 81.72 | 65.77 | 55.71 | 1.66 | 1.52 | 4.29 | 1.39 | 1.31 | 1.50 |
| Min | 320.90 | 303.70 | 207.60 | 53.11 | 74.22 | 62.38 | 45.02 | 1.41 | 1.34 | 3.07 | 1.17 | 1.16 | 1.04 |
| Mean | 346.50 | 327.02 | 223.60 | 64.02 | 79.49 | 64.20 | 50.60 | 1.55 | 1.46 | 3.55 | 1.28 | 1.24 | 1.27 |
Measurements (in millimeters) of humeri of extant ruminants from Southeast Asia.
| Bp | Dp | Bd | Dd | SD | GL | BT | GLC | GLl | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dp/Dd | Bp/Dp | Bd/Dd | Bd/BT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Axis axis (N=8) | ||||||||||||||||||
| Max | 48.01 | 55.42 | 41.82 | 36.58 | 18.48 | 200.30 | 36.44 | 177.10 | 196.80 | 4.20 | 3.77 | 5.13 | 5.67 | 1.30 | 1.68 | 0.91 | 1.23 | 1.17 |
| Min | 42.12 | 47.92 | 33.98 | 30.63 | 15.21 | 172.40 | 31.87 | 153.10 | 167.30 | 3.84 | 3.16 | 4.59 | 5.16 | 1.11 | 1.46 | 0.80 | 1.04 | 1.07 |
| Mean | 48.16 | 44.50 | 35.57 | 24.98 | 98.52 | 107.03 | 98.24 | 169.66 | 90.14 | 3.77 | 4.16 | 5.10 | 3.29 | 1.37 | 1.20 | 1.00 | 1.12 | 1.12 |
| Axis porcinus (N=2) | ||||||||||||||||||
| Max | 35.42 | 51.11 | 32.42 | 27.11 | 14.67 | 153.20 | 27.65 | 125.10 | 142.30 | 4.40 | 3.02 | 4.87 | 5.78 | 1.12 | 1.91 | 0.69 | 1.23 | 1.19 |
| Min | 34.74 | 50.53 | 31.49 | 26.43 | 14.55 | 152.80 | 27.34 | 124.30 | 141.80 | 4.33 | 3.00 | 4.71 | 5.65 | 1.07 | 1.89 | 0.69 | 1.16 | 1.14 |
| Mean | 35.08 | 50.82 | 31.96 | 26.77 | 14.61 | 153.00 | 27.50 | 124.70 | 142.05 | 4.36 | 3.01 | 4.79 | 5.72 | 1.10 | 1.90 | 0.69 | 1.19 | 1.16 |
| Panolia eldii (N=4) | ||||||||||||||||||
| Max | 54.56 | 62.98 | 43.48 | 38.15 | 20.40 | 211.70 | 36.81 | 185.80 | 206.30 | 4.08 | 3.40 | 4.87 | 5.57 | 1.26 | 1.66 | 0.87 | 1.19 | 1.19 |
| Min | 47.17 | 56.65 | 42.52 | 35.75 | 18.52 | 192.30 | 36.02 | 171.30 | 190.40 | 3.88 | 3.35 | 4.51 | 5.35 | 1.11 | 1.57 | 0.83 | 1.14 | 1.16 |
| Mean | 50.94 | 59.78 | 43.06 | 36.97 | 19.46 | 202.00 | 36.53 | 178.53 | 198.45 | 3.97 | 3.38 | 4.69 | 5.46 | 1.18 | 1.62 | 0.85 | 1.17 | 1.18 |
| Rusa unicolor (N=4) | ||||||||||||||||||
| Max | 73.14 | 85.10 | 66.12 | 46.33 | 27.62 | 277.80 | 61.23 | 252.40 | 267.90 | 3.98 | 3.54 | 4.81 | 6.22 | 1.21 | 1.91 | 0.89 | 1.48 | 1.14 |
| Min | 64.44 | 72.40 | 53.35 | 44.66 | 24.82 | 256.40 | 47.29 | 224.10 | 254.30 | 3.80 | 3.26 | 4.19 | 5.54 | 1.10 | 1.56 | 0.86 | 1.16 | 1.08 |
| Mean | 68.75 | 78.66 | 59.81 | 45.61 | 26.06 | 266.83 | 54.21 | 238.05 | 261.23 | 3.89 | 3.40 | 4.49 | 5.85 | 1.15 | 1.73 | 0.88 | 1.31 | 1.11 |
| Bos sauveli (N=4) | ||||||||||||||||||
| Max | 105.62 | 104.67 | 90.28 | 84.60 | 43.08 | 338.10 | 86.30 | 306.10 | 333.70 | 3.28 | 3.23 | 3.86 | 4.17 | 1.18 | 1.41 | 1.01 | 1.08 | 1.07 |
| Min | 90.62 | 100.44 | 76.98 | 71.24 | 32.67 | 296.80 | 71.78 | 276.10 | 291.20 | 3.19 | 2.94 | 3.74 | 3.99 | 1.17 | 1.23 | 0.90 | 1.07 | 1.05 |
| Mean | 98.12 | 102.63 | 83.64 | 77.67 | 37.89 | 317.35 | 79.09 | 290.95 | 312.63 | 3.24 | 3.09 | 3.80 | 4.09 | 1.17 | 1.33 | 0.95 | 1.08 | 1.06 |
| Bos javanicus (N=6) | ||||||||||||||||||
| Max | 111.54 | 137.34 | 102.41 | 79.42 | 43.75 | 354.40 | 83.12 | 291.30 | 318.40 | 3.48 | 3.00 | 3.82 | 4.48 | 1.12 | 1.74 | 0.95 | 1.29 | 1.23 |
| Min | 94.97 | 109.87 | 86.67 | 76.08 | 40.06 | 326.10 | 77.15 | 278.80 | 302.50 | 3.13 | 2.58 | 3.46 | 4.25 | 1.08 | 1.43 | 0.80 | 1.14 | 1.12 |
| Mean | 103.36 | 119.39 | 94.08 | 77.42 | 42.71 | 337.57 | 79.41 | 285.97 | 312.97 | 3.27 | 2.85 | 3.60 | 4.36 | 1.10 | 1.54 | 0.87 | 1.21 | 1.18 |
| Bos gaurus (N=6) | ||||||||||||||||||
| Max | 135.46 | 154.89 | 110.89 | 96.12 | 56.49 | 395.40 | 99.32 | 353.80 | 391.50 | 2.99 | 3.46 | 3.82 | 4.20 | 1.28 | 1.61 | 1.22 | 1.15 | 1.13 |
| Min | 117.31 | 96.43 | 94.21 | 85.01 | 43.22 | 333.20 | 87.12 | 302.10 | 321.50 | 2.79 | 2.55 | 3.49 | 3.64 | 1.21 | 1.05 | 0.86 | 1.04 | 1.06 |
| Mean | 124.31 | 123.64 | 99.99 | 91.03 | 49.09 | 362.02 | 91.55 | 325.98 | 355.32 | 2.91 | 3.00 | 3.62 | 3.98 | 1.24 | 1.35 | 1.03 | 1.10 | 1.09 |
| Bubalus arnee (N=6) | ||||||||||||||||||
| Max | 102.11 | 106.03 | 87.29 | 77.75 | 50.59 | 319.60 | 80.69 | 271.70 | 317.80 | 3.41 | 3.16 | 3.85 | 4.28 | 1.24 | 1.46 | 0.97 | 1.15 | 1.11 |
| Min | 92.71 | 101.13 | 82.33 | 72.58 | 38.44 | 310.50 | 73.89 | 262.10 | 305.40 | 3.04 | 2.93 | 3.66 | 4.07 | 1.10 | 1.33 | 0.88 | 1.06 | 1.04 |
| Mean | 97.08 | 103.83 | 83.98 | 75.75 | 42.93 | 315.92 | 77.86 | 266.88 | 312.07 | 3.26 | 3.04 | 3.76 | 4.17 | 1.16 | 1.37 | 0.94 | 1.11 | 1.08 |
Measurements (in millimeters) of radii and ulnae of extant ruminants from Southeast Asia.
| Bp | Dp | Bd | Dd | SD | GL | GLl | BFd | BFp | BPC | PL | Ll | SDO | DPA | LO | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Axis axis (N=8) | |||||||||||||||
| Max | 37.72 | 18.84 | 34.78 | 26.20 | 23.88 | 241.10 | 236.80 | 29.93 | 33.07 | 20.72 | 171.20 | 177.70 | 30.96 | 34.01 | 49.28 |
| Min | 34.16 | 16.11 | 30.00 | 21.74 | 19.46 | 203.70 | 198.90 | 26.97 | 30.12 | 16.33 | 139.40 | 160.30 | 24.87 | 28.49 | 41.44 |
| Mean | 35.43 | 17.78 | 32.68 | 24.28 | 21.06 | 222.60 | 218.74 | 28.37 | 31.30 | 18.93 | 162.54 | 165.38 | 27.89 | 30.82 | 45.17 |
| Axis porcinus (N=4) | |||||||||||||||
| Max | 31.15 | 15.78 | 25.09 | 24.81 | 16.45 | 165.70 | 162.80 | 24.02 | 27.11 | 15.44 | 126.03 | 132.09 | 25.17 | 26.16 | 40.22 |
| Min | 28.45 | 13.37 | 24.11 | 16.43 | 12.81 | 164.10 | 158.40 | 22.73 | 25.85 | 13.66 | 122.60 | 119.42 | 20.45 | 23.94 | 36.03 |
| Mean | 29.80 | 14.36 | 24.65 | 20.72 | 14.71 | 165.08 | 160.58 | 23.29 | 26.30 | 14.64 | 124.52 | 125.71 | 22.97 | 25.17 | 37.99 |
| Panolia eldii (N=3) | |||||||||||||||
| Max | 42.22 | 20.72 | 39.16 | 26.63 | 23.31 | 276.60 | 271.80 | 36.15 | 37.87 | 24.74 | 212.80 | 217.50 | 33.98 | 34.91 | 54.59 |
| Min | 38.88 | 19.02 | 35.18 | 25.16 | 19.93 | 254.80 | 249.20 | 33.17 | 35.14 | 20.32 | 196.70 | 200.10 | 30.69 | 33.48 | 46.71 |
| Mean | 40.04 | 19.85 | 36.68 | 25.80 | 21.08 | 262.30 | 256.90 | 34.20 | 36.07 | 21.84 | 202.20 | 206.07 | 31.82 | 34.09 | 49.43 |
| Rusa unicolor (N=4) | |||||||||||||||
| Max | 59.02 | 31.24 | 54.29 | 40.62 | 32.49 | 322.50 | 320.50 | 46.82 | 54.38 | 29.77 | 239.80 | 243.60 | 46.89 | 50.60 | 70.12 |
| Min | 51.89 | 24.21 | 44.25 | 34.29 | 29.10 | 296.30 | 291.30 | 41.82 | 46.02 | 23.81 | 217.80 | 222.20 | 39.89 | 42.37 | 57.71 |
| Mean | 55.35 | 27.73 | 49.20 | 37.52 | 30.66 | 309.45 | 305.83 | 44.26 | 50.07 | 26.34 | 228.53 | 232.90 | 43.18 | 46.18 | 63.96 |
| Bos sauveli (N=4) | |||||||||||||||
| Max | 89.11 | 38.72 | 83.52 | 58.24 | 42.13 | 372.30 | 364.50 | 74.01 | 78.29 | 45.63 | 316.50 | 314.20 | 51.33 | 64.95 | 109.67 |
| Min | 78.88 | 35.44 | 68.52 | 56.47 | 36.54 | 329.40 | 324.80 | 66.14 | 72.13 | 44.24 | 285.40 | 274.10 | 46.46 | 64.32 | 93.75 |
| Mean | 83.99 | 37.14 | 76.06 | 57.41 | 39.35 | 350.90 | 344.63 | 70.21 | 75.22 | 44.99 | 300.95 | 294.05 | 48.89 | 64.59 | 101.67 |
| Bos javanicus (N=6) | |||||||||||||||
| Max | 93.83 | 43.24 | 90.23 | 57.23 | 50.34 | 420.90 | 421.30 | 70.11 | 81.49 | 47.56 | 293.50 | 283.40 | 61.20 | 73.76 | 114.89 |
| Min | 82.22 | 39.12 | 75.30 | 47.26 | 43.74 | 389.20 | 372.40 | 65.35 | 76.32 | 44.59 | 285.10 | 281.30 | 50.30 | 71.04 | 103.98 |
| Mean | 88.05 | 41.32 | 82.89 | 52.20 | 46.17 | 402.75 | 393.83 | 68.04 | 79.14 | 45.96 | 288.23 | 282.52 | 54.78 | 71.99 | 109.80 |
| Bos gaurus (N=6) | |||||||||||||||
| Max | 107.54 | 56.43 | 103.42 | 69.94 | 60.98 | 470.60 | 461.70 | 88.76 | 96.43 | 58.43 | 345.60 | 328.60 | 67.68 | 90.12 | 137.40 |
| Min | 90.31 | 46.45 | 80.33 | 53.26 | 48.24 | 414.85 | 415.70 | 72.96 | 84.93 | 49.64 | 291.30 | 281.40 | 56.42 | 71.45 | 86.43 |
| Mean | 97.23 | 50.39 | 92.17 | 59.82 | 53.57 | 439.98 | 432.00 | 80.35 | 88.83 | 53.83 | 320.88 | 310.42 | 60.96 | 79.86 | 114.21 |
| Bubalus arnee (N=6) | |||||||||||||||
| Max | 86.89 | 45.55 | 81.67 | 64.24 | 51.84 | 411.20 | 385.40 | 78.73 | 76.52 | 50.66 | 293.50 | 292.60 | 60.43 | 78.24 | 112.34 |
| Min | 77.01 | 41.45 | 73.23 | 53.10 | 43.07 | 377.40 | 372.30 | 70.68 | 70.91 | 46.32 | 283.10 | 281.20 | 48.44 | 68.09 | 95.47 |
| Mean | 81.86 | 43.36 | 77.95 | 58.93 | 46.51 | 393.25 | 380.40 | 74.37 | 73.89 | 48.47 | 288.32 | 287.23 | 54.81 | 72.12 | 104.12 |
| PL/Bp | PL/Dp | PL/Bd | PL/Dd | Bp/Bd | Dd/Dp | Bp/Dp | Bd/Dd | Bp/BFp | Bd/BFd | GL/LO | |||||
| Axis axis (N=8) | |||||||||||||||
| Max | 4.87 | 9.86 | 5.33 | 7.65 | 1.15 | 1.48 | 2.14 | 1.52 | 1.18 | 1.22 | 5.31 | ||||
| Min | 3.93 | 7.81 | 4.30 | 5.45 | 1.04 | 1.27 | 1.91 | 1.22 | 1.10 | 1.08 | 4.68 | ||||
| Mean | 4.59 | 9.16 | 4.98 | 6.73 | 1.09 | 1.37 | 2.00 | 1.35 | 1.13 | 1.15 | 4.93 | ||||
| Axis porcinus (N=4) | |||||||||||||||
| Max | 4.42 | 9.43 | 5.13 | 7.53 | 1.29 | 1.86 | 2.17 | 1.47 | 1.16 | 1.09 | 4.59 | ||||
| Min | 3.97 | 7.77 | 4.89 | 5.08 | 1.16 | 1.08 | 1.94 | 1.00 | 1.10 | 1.00 | 4.11 | ||||
| Mean | 4.19 | 8.72 | 5.05 | 6.23 | 1.21 | 1.46 | 2.08 | 1.23 | 1.13 | 1.06 | 4.36 | ||||
| Panolia eldii (N=3) | |||||||||||||||
| Max | 5.06 | 10.34 | 5.59 | 7.99 | 1.11 | 1.32 | 2.04 | 1.47 | 1.11 | 1.08 | 5.47 | ||||
| Min | 5.04 | 9.95 | 5.43 | 7.70 | 1.08 | 1.29 | 1.97 | 1.39 | 1.11 | 1.06 | 5.07 | ||||
| Mean | 5.05 | 10.19 | 5.51 | 7.84 | 1.09 | 1.30 | 2.02 | 1.42 | 1.11 | 1.07 | 5.32 | ||||
| Rusa unicolor (N=4) | |||||||||||||||
| Max | 4.20 | 9.01 | 4.92 | 6.35 | 1.18 | 1.43 | 2.14 | 1.34 | 1.14 | 1.17 | 5.15 | ||||
| Min | 4.04 | 7.63 | 4.42 | 5.87 | 1.07 | 1.30 | 1.88 | 1.28 | 1.08 | 1.06 | 4.60 | ||||
| Mean | 4.13 | 8.32 | 4.67 | 6.11 | 1.13 | 1.36 | 2.01 | 1.31 | 1.11 | 1.11 | 4.86 | ||||
| Bos sauveli (N=4) | |||||||||||||||
| Max | 3.62 | 8.93 | 4.17 | 5.60 | 1.15 | 1.59 | 2.51 | 1.48 | 1.14 | 1.13 | 3.97 | ||||
| Min | 3.55 | 7.38 | 3.79 | 4.90 | 1.06 | 1.50 | 2.04 | 1.18 | 1.09 | 1.03 | 3.01 | ||||
| Mean | 3.59 | 8.13 | 3.97 | 5.25 | 1.11 | 1.55 | 2.27 | 1.33 | 1.12 | 1.08 | 3.49 | ||||
| Bos javanicus (N=6) | |||||||||||||||
| Max | 3.49 | 7.32 | 3.81 | 6.16 | 1.11 | 1.46 | 2.17 | 1.78 | 1.15 | 1.29 | 3.74 | ||||
| Min | 3.13 | 6.75 | 3.18 | 5.01 | 0.99 | 1.10 | 2.10 | 1.32 | 1.07 | 1.15 | 3.59 | ||||
| Mean | 3.28 | 6.99 | 3.49 | 5.56 | 1.06 | 1.27 | 2.13 | 1.60 | 1.11 | 1.22 | 3.67 | ||||
| Bos gaurus (N=6) | |||||||||||||||
| Max | 3.58 | 6.97 | 4.03 | 6.08 | 1.12 | 1.25 | 1.96 | 1.64 | 1.12 | 1.17 | 4.80 | ||||
| Min | 3.13 | 5.99 | 3.18 | 4.94 | 1.01 | 1.14 | 1.90 | 1.48 | 1.06 | 1.09 | 3.42 | ||||
| Mean | 3.31 | 6.38 | 3.51 | 5.40 | 1.06 | 1.18 | 1.93 | 1.54 | 1.09 | 1.15 | 3.96 | ||||
| Bubalus arnee (N=6) | |||||||||||||||
| Max | 3.68 | 6.84 | 3.87 | 5.39 | 1.06 | 1.55 | 1.95 | 1.48 | 1.17 | 1.07 | 3.96 | ||||
| Min | 3.38 | 6.44 | 3.59 | 4.41 | 1.03 | 1.21 | 1.84 | 1.14 | 1.07 | 1.04 | 3.50 | ||||
| Mean | 3.53 | 6.66 | 3.70 | 4.92 | 1.05 | 1.36 | 1.89 | 1.33 | 1.11 | 1.05 | 3.79 | ||||
Measurements (in millimeters) of metacarpi of extant ruminants from Southeast Asia.
| Bp | Dp | Bd | Dd | SD | GL | GLl | Ll | DD | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dp/Dd | Bp/Dp | Bd/Dd | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Axis axis (N=8) | |||||||||||||||||
| Max | 28.75 | 19.36 | 28.03 | 17.75 | 18.20 | 174.30 | 172.80 | 169.00 | 17.55 | 6.52 | 9.77 | 7.69 | 12.69 | 1.26 | 1.36 | 1.59 | 1.88 |
| Min | 25.13 | 17.23 | 22.56 | 13.59 | 14.18 | 162.10 | 159.50 | 156.70 | 12.33 | 6.06 | 8.74 | 6.22 | 9.52 | 1.03 | 1.02 | 1.35 | 1.30 |
| Mean | 26.94 | 18.25 | 24.86 | 16.24 | 15.66 | 169.30 | 167.04 | 164.05 | 14.58 | 6.29 | 9.28 | 6.83 | 10.52 | 1.09 | 1.13 | 1.48 | 1.54 |
| Axis porcinus (N=4) | |||||||||||||||||
| Max | 23.14 | 15.71 | 23.60 | 14.61 | 14.18 | 120.80 | 116.88 | 118.82 | 11.76 | 5.68 | 7.92 | 5.81 | 8.82 | 1.06 | 1.14 | 1.59 | 1.76 |
| Min | 21.28 | 14.43 | 20.79 | 13.38 | 12.63 | 111.00 | 109.20 | 107.80 | 9.42 | 4.83 | 7.45 | 4.70 | 7.66 | 0.97 | 1.03 | 1.36 | 1.50 |
| Mean | 22.18 | 15.11 | 21.77 | 13.86 | 13.39 | 115.99 | 113.35 | 113.52 | 10.62 | 5.25 | 7.68 | 5.35 | 8.38 | 1.02 | 1.09 | 1.47 | 1.57 |
| Panolia eldii (N=4) | |||||||||||||||||
| Max | 34.01 | 20.89 | 29.98 | 20.66 | 18.08 | 224.80 | 222.50 | 221.30 | 17.58 | 7.18 | 11.33 | 7.82 | 11.20 | 1.18 | 1.06 | 1.72 | 1.49 |
| Min | 30.61 | 19.81 | 28.11 | 19.64 | 17.60 | 219.80 | 216.20 | 214.40 | 15.06 | 6.60 | 10.52 | 7.50 | 10.87 | 1.08 | 0.96 | 1.47 | 1.40 |
| Mean | 32.22 | 20.32 | 28.83 | 20.04 | 17.82 | 222.25 | 219.48 | 217.63 | 16.45 | 6.91 | 10.95 | 7.71 | 11.09 | 1.12 | 1.01 | 1.59 | 1.44 |
| Rusa unicolor (N=4) | |||||||||||||||||
| Max | 42.02 | 28.45 | 41.13 | 27.81 | 24.99 | 234.30 | 230.40 | 226.70 | 22.07 | 5.77 | 8.24 | 5.96 | 8.84 | 1.03 | 1.11 | 1.48 | 1.54 |
| Min | 36.43 | 26.66 | 35.35 | 24.08 | 22.19 | 210.20 | 205.10 | 203.50 | 19.54 | 5.55 | 7.83 | 5.70 | 8.43 | 1.02 | 1.02 | 1.36 | 1.46 |
| Mean | 39.22 | 27.58 | 38.11 | 25.60 | 23.42 | 221.95 | 217.60 | 214.95 | 20.69 | 5.67 | 8.04 | 5.83 | 8.68 | 1.03 | 1.08 | 1.42 | 1.49 |
| Bos sauveli (N=2) | |||||||||||||||||
| Max | 65.72 | 36.61 | 57.43 | 32.87 | 36.44 | 249.10 | 246.40 | 241.50 | 29.43 | 3.81 | 6.82 | 4.35 | 7.68 | 1.15 | 1.13 | 1.80 | 1.77 |
| Min | 65.44 | 36.54 | 57.09 | 32.45 | 36.23 | 248.40 | 245.30 | 241.20 | 29.22 | 3.78 | 6.79 | 4.34 | 7.56 | 1.14 | 1.11 | 1.79 | 1.74 |
| Mean | 65.58 | 36.58 | 57.26 | 32.66 | 36.34 | 248.75 | 245.85 | 241.35 | 29.33 | 3.79 | 6.80 | 4.34 | 7.62 | 1.15 | 1.12 | 1.79 | 1.75 |
| Bos javanicus (N=6) | |||||||||||||||||
| Max | 66.76 | 40.44 | 60.38 | 39.84 | 39.86 | 225.20 | 214.30 | 215.10 | 27.71 | 3.76 | 6.46 | 4.09 | 6.86 | 1.11 | 1.16 | 1.74 | 1.69 |
| Min | 59.28 | 34.48 | 54.45 | 32.45 | 34.21 | 215.30 | 206.10 | 205.70 | 26.03 | 3.37 | 5.53 | 3.73 | 5.65 | 1.09 | 1.01 | 1.58 | 1.51 |
| Mean | 62.88 | 37.73 | 57.30 | 35.67 | 37.25 | 222.10 | 212.44 | 211.66 | 26.84 | 3.54 | 5.92 | 3.88 | 6.27 | 1.10 | 1.06 | 1.67 | 1.61 |
| Bos gaurus (N=6) | |||||||||||||||||
| Max | 75.52 | 43.88 | 69.11 | 40.81 | 51.55 | 251.50 | 247.20 | 239.10 | 33.49 | 3.78 | 6.24 | 4.14 | 6.61 | 1.12 | 1.11 | 1.76 | 1.75 |
| Min | 66.25 | 39.22 | 60.68 | 37.86 | 39.04 | 226.50 | 218.20 | 215.10 | 28.44 | 3.28 | 5.73 | 3.35 | 5.87 | 1.02 | 1.02 | 1.60 | 1.59 |
| Mean | 70.36 | 41.52 | 65.83 | 39.08 | 44.16 | 242.95 | 235.85 | 230.37 | 31.13 | 3.46 | 5.85 | 3.70 | 6.22 | 1.07 | 1.06 | 1.70 | 1.68 |
| Bubalus arnee (N=6) | |||||||||||||||||
| Max | 68.11 | 42.38 | 74.01 | 38.81 | 45.12 | 195.20 | 186.60 | 180.60 | 28.92 | 2.97 | 6.08 | 2.79 | 5.43 | 0.97 | 1.10 | 2.12 | 1.98 |
| Min | 61.63 | 31.14 | 66.52 | 33.77 | 30.97 | 182.80 | 170.70 | 172.40 | 24.32 | 2.87 | 4.57 | 2.61 | 4.99 | 0.91 | 0.85 | 1.58 | 1.91 |
| Mean | 65.18 | 33.89 | 69.73 | 35.89 | 36.91 | 189.60 | 178.42 | 176.14 | 25.74 | 2.91 | 5.66 | 2.72 | 5.29 | 0.93 | 0.94 | 1.94 | 1.94 |
Measurements (in millimeters) of femora of extant ruminants from Southeast Asia.
| Bp | Dp | Bd | Dd | SD | GL | GLC | DC | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dd/Dp | Bp/Dp | Dd/Bd | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Axis axis (N=6) | ||||||||||||||||
| Max | 55.93 | 27.30 | 47.01 | 65.81 | 18.53 | 219.70 | 209.20 | 24.02 | 4.24 | 8.93 | 4.94 | 3.52 | 1.26 | 2.56 | 2.15 | 1.49 |
| Min | 49.76 | 23.63 | 43.72 | 59.88 | 17.31 | 210.90 | 198.20 | 22.35 | 3.90 | 8.05 | 4.63 | 3.32 | 1.11 | 2.35 | 2.02 | 1.33 |
| Mean | 53.26 | 25.66 | 45.37 | 63.15 | 18.09 | 215.97 | 204.03 | 23.32 | 4.06 | 8.43 | 4.76 | 3.42 | 1.17 | 2.46 | 2.08 | 1.39 |
| Axis porcinus (N=2) | ||||||||||||||||
| Max | 47.14 | 23.25 | 38.75 | 56.33 | 16.43 | 183.60 | 173.40 | 19.77 | 3.89 | 8.14 | 4.84 | 3.26 | 1.24 | 2.50 | 2.10 | 1.49 |
| Min | 46.71 | 22.26 | 37.91 | 55.71 | 16.04 | 181.20 | 172.70 | 19.36 | 3.88 | 7.90 | 4.68 | 3.25 | 1.21 | 2.42 | 2.03 | 1.44 |
| Mean | 46.93 | 22.76 | 38.33 | 56.02 | 16.24 | 182.40 | 173.05 | 19.57 | 3.89 | 8.02 | 4.76 | 3.26 | 1.22 | 2.46 | 2.06 | 1.46 |
| Panolia eldii (N=4) | ||||||||||||||||
| Max | 66.06 | 30.81 | 52.98 | 73.65 | 22.92 | 267.70 | 253.10 | 26.77 | 4.05 | 8.83 | 5.06 | 3.63 | 1.25 | 2.43 | 2.18 | 1.44 |
| Min | 60.54 | 30.30 | 49.74 | 71.12 | 21.50 | 241.40 | 225.30 | 24.54 | 3.99 | 7.85 | 4.85 | 3.39 | 1.22 | 2.31 | 1.97 | 1.39 |
| Mean | 63.29 | 30.55 | 51.36 | 72.45 | 22.19 | 254.63 | 239.35 | 25.63 | 4.02 | 8.34 | 4.96 | 3.51 | 1.23 | 2.37 | 2.07 | 1.41 |
| Rusa unicolor (N=4) | ||||||||||||||||
| Max | 91.36 | 44.39 | 76.39 | 102.41 | 32.56 | 354.80 | 336.20 | 38.43 | 4.27 | 8.15 | 4.75 | 4.18 | 1.21 | 2.35 | 2.08 | 1.34 |
| Min | 74.13 | 42.25 | 66.99 | 76.12 | 26.90 | 316.30 | 296.70 | 32.08 | 3.87 | 7.45 | 4.64 | 3.46 | 1.09 | 1.78 | 1.75 | 1.13 |
| Mean | 82.68 | 43.23 | 71.67 | 88.78 | 29.58 | 335.80 | 316.10 | 35.29 | 4.08 | 7.76 | 4.69 | 3.83 | 1.15 | 2.05 | 1.91 | 1.23 |
| Bos sauveli (N=4) | ||||||||||||||||
| Max | 131.83 | 69.02 | 99.92 | 132.22 | 46.31 | 408.20 | 389.50 | 48.12 | 3.44 | 5.92 | 4.09 | 3.11 | 1.32 | 2.04 | 1.91 | 1.33 |
| Min | 106.60 | 62.94 | 96.44 | 128.62 | 31.30 | 366.20 | 347.10 | 46.27 | 3.10 | 5.79 | 3.80 | 2.85 | 1.11 | 1.90 | 1.69 | 1.31 |
| Mean | 118.88 | 66.04 | 98.18 | 130.18 | 38.61 | 387.23 | 368.30 | 47.09 | 3.27 | 5.86 | 3.94 | 2.97 | 1.21 | 1.97 | 1.80 | 1.33 |
| Bos javanicus (N=6) | ||||||||||||||||
| Max | 134.88 | 72.44 | 105.98 | 135.67 | 48.37 | 427.30 | 395.10 | 55.28 | 3.30 | 6.12 | 4.09 | 3.17 | 1.32 | 1.94 | 1.98 | 1.30 |
| Min | 124.52 | 63.45 | 94.81 | 122.95 | 38.89 | 387.10 | 363.70 | 51.97 | 3.09 | 5.76 | 3.94 | 3.10 | 1.22 | 1.86 | 1.85 | 1.25 |
| Mean | 128.18 | 67.43 | 100.88 | 129.08 | 43.23 | 406.38 | 381.20 | 53.46 | 3.17 | 6.03 | 4.03 | 3.15 | 1.27 | 1.92 | 1.90 | 1.28 |
| Bos gaurus (N=6) | ||||||||||||||||
| Max | 151.32 | 93.76 | 124.94 | 156.78 | 49.63 | 483.20 | 458.70 | 59.27 | 3.68 | 6.69 | 4.19 | 3.18 | 1.31 | 2.26 | 1.90 | 1.32 |
| Min | 115.19 | 63.46 | 108.35 | 142.42 | 43.55 | 423.70 | 428.70 | 37.88 | 3.19 | 5.15 | 3.77 | 2.96 | 1.04 | 1.67 | 1.61 | 1.25 |
| Mean | 135.93 | 77.41 | 115.22 | 147.53 | 45.77 | 452.82 | 440.95 | 54.42 | 3.35 | 5.94 | 3.93 | 3.07 | 1.18 | 1.94 | 1.77 | 1.28 |
| Bubalus arnee (N=6) | ||||||||||||||||
| Max | 131.65 | 69.14 | 105.25 | 134.59 | 47.78 | 386.20 | 378.10 | 51.44 | 2.95 | 5.73 | 3.68 | 3.04 | 1.25 | 1.98 | 1.95 | 1.29 |
| Min | 123.63 | 67.17 | 101.98 | 125.25 | 39.52 | 353.70 | 336.50 | 49.23 | 2.86 | 5.22 | 3.46 | 2.81 | 1.21 | 1.86 | 1.82 | 1.21 |
| Mean | 128.81 | 67.82 | 104.06 | 129.11 | 42.99 | 374.52 | 361.50 | 50.72 | 2.91 | 5.52 | 3.60 | 2.90 | 1.24 | 1.90 | 1.90 | 1.24 |
Measurements (in millimeters) of tibiae of extant ruminants from Southeast Asia.
| Bp | Dp | Bd | Dd | SD | GL | Ll | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dp/Dd | Bp/Dp | Bd/Dd | |
| Axis axis (N=6) | |||||||||||||||
| Max | 52.61 | 49.43 | 34.89 | 24.24 | 19.97 | 222.20 | 240.10 | 4.51 | 4.97 | 7.07 | 9.61 | 1.65 | 2.16 | 1.14 | 1.56 |
| Min | 48.92 | 42.77 | 30.03 | 22.11 | 17.98 | 209.60 | 226.40 | 4.09 | 4.44 | 6.17 | 9.14 | 1.45 | 1.89 | 0.99 | 1.33 |
| Mean | 50.12 | 46.99 | 32.48 | 23.06 | 18.62 | 216.52 | 232.87 | 4.32 | 4.62 | 6.68 | 9.39 | 1.55 | 2.04 | 1.07 | 1.41 |
| Axis porcinus (N=4) | |||||||||||||||
| Max | 43.79 | 43.73 | 28.22 | 22.21 | 17.11 | 207.90 | 198.30 | 4.77 | 5.18 | 7.88 | 9.42 | 1.66 | 2.08 | 1.08 | 1.35 |
| Min | 41.22 | 40.05 | 26.36 | 20.53 | 16.80 | 182.70 | 192.30 | 4.41 | 4.18 | 6.47 | 8.65 | 1.46 | 1.82 | 0.94 | 1.19 |
| Mean | 42.51 | 41.84 | 27.15 | 21.47 | 16.98 | 195.33 | 195.55 | 4.59 | 4.68 | 7.21 | 9.09 | 1.57 | 1.95 | 1.02 | 1.27 |
| Panolia eldii (N=4) | |||||||||||||||
| Max | 58.99 | 59.38 | 37.56 | 29.35 | 21.62 | 294.80 | 285.40 | 5.02 | 5.14 | 7.86 | 10.35 | 1.68 | 2.03 | 1.03 | 1.32 |
| Min | 58.01 | 57.40 | 34.96 | 28.46 | 20.65 | 274.30 | 258.00 | 4.66 | 4.62 | 7.83 | 9.36 | 1.56 | 2.01 | 0.98 | 1.19 |
| Mean | 58.63 | 58.29 | 36.27 | 28.88 | 21.09 | 284.65 | 271.75 | 4.85 | 4.89 | 7.85 | 9.86 | 1.62 | 2.02 | 1.01 | 1.26 |
| Rusa unicolor (N=4) | |||||||||||||||
| Max | 84.53 | 83.90 | 51.92 | 43.54 | 30.12 | 334.60 | 354.40 | 4.00 | 4.47 | 6.66 | 8.22 | 1.68 | 1.97 | 1.12 | 1.35 |
| Min | 73.20 | 65.43 | 47.48 | 35.53 | 28.83 | 291.90 | 316.80 | 3.96 | 3.97 | 6.06 | 7.68 | 1.53 | 1.83 | 0.99 | 1.15 |
| Mean | 78.67 | 74.36 | 49.45 | 39.37 | 29.44 | 313.13 | 335.33 | 3.98 | 4.24 | 6.33 | 7.97 | 1.59 | 1.88 | 1.06 | 1.26 |
| Bos sauveli (N=4) | |||||||||||||||
| Max | 102.24 | 92.12 | 73.59 | 49.56 | 44.92 | 398.30 | 364.90 | 3.90 | 4.55 | 5.82 | 8.20 | 1.55 | 1.86 | 1.17 | 1.51 |
| Min | 98.75 | 87.54 | 63.92 | 48.58 | 38.54 | 372.20 | 311.60 | 3.76 | 4.04 | 5.40 | 7.51 | 1.39 | 1.80 | 1.07 | 1.29 |
| Mean | 100.51 | 89.86 | 68.80 | 49.12 | 41.72 | 384.98 | 338.15 | 3.83 | 4.29 | 5.61 | 7.84 | 1.47 | 1.83 | 1.12 | 1.40 |
| Bos javanicus (N=6) | |||||||||||||||
| Max | 106.11 | 97.35 | 69.35 | 56.17 | 46.06 | 414.50 | 364.70 | 3.91 | 4.28 | 6.23 | 7.63 | 1.61 | 1.81 | 1.10 | 1.24 |
| Min | 100.05 | 96.02 | 64.88 | 53.01 | 38.60 | 385.30 | 354.80 | 3.83 | 3.96 | 5.80 | 6.86 | 1.51 | 1.73 | 1.03 | 1.18 |
| Mean | 103.61 | 96.78 | 66.86 | 55.09 | 42.05 | 401.23 | 360.73 | 3.87 | 4.15 | 6.00 | 7.29 | 1.55 | 1.76 | 1.07 | 1.21 |
| Bos gaurus (N=6) | |||||||||||||||
| Max | 130.17 | 107.70 | 75.51 | 60.10 | 57.06 | 449.70 | 396.30 | 3.66 | 4.22 | 5.99 | 7.81 | 1.73 | 1.87 | 1.21 | 1.31 |
| Min | 113.50 | 98.40 | 72.50 | 57.60 | 46.00 | 415.40 | 351.70 | 3.45 | 4.18 | 5.69 | 7.10 | 1.56 | 1.69 | 1.15 | 1.23 |
| Mean | 120.97 | 103.27 | 74.01 | 58.39 | 50.25 | 432.45 | 373.75 | 3.58 | 4.19 | 5.84 | 7.41 | 1.63 | 1.77 | 1.17 | 1.27 |
| Bubalus arnee (N=6) | |||||||||||||||
| Max | 107.80 | 103.58 | 71.90 | 53.37 | 48.92 | 367.20 | 340.50 | 3.53 | 4.40 | 5.30 | 7.07 | 1.56 | 1.99 | 1.25 | 1.44 |
| Min | 101.53 | 82.14 | 67.68 | 47.10 | 40.71 | 324.10 | 294.70 | 3.11 | 3.45 | 4.77 | 6.78 | 1.41 | 1.58 | 1.04 | 1.30 |
| Mean | 104.67 | 93.55 | 69.57 | 50.67 | 45.08 | 350.18 | 316.90 | 3.35 | 3.77 | 5.03 | 6.91 | 1.51 | 1.85 | 1.13 | 1.38 |
Measurements (in millimeters) of metatarsi of extant ruminants from Southeast Asia.
| Bp | Dp | Bd | Dd | SD | GL | GLl | Ll | DD | GL/Bp | GL/Dp | GL/Bd | GL/Dd | Bp/Bd | Dp/Dd | Bp/Dp | Bd/Dd | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Axis axis (N=6) | |||||||||||||||||
| Max | 24.36 | 26.28 | 26.56 | 18.15 | 17.76 | 187.80 | 185.40 | 182.50 | 15.25 | 8.00 | 7.74 | 7.53 | 12.97 | 0.96 | 1.80 | 0.98 | 1.72 |
| Min | 23.38 | 24.26 | 24.86 | 14.43 | 13.97 | 180.10 | 179.60 | 175.50 | 13.48 | 7.50 | 6.95 | 6.91 | 10.06 | 0.90 | 1.42 | 0.90 | 1.40 |
| Mean | 23.81 | 25.46 | 25.78 | 16.72 | 15.32 | 184.22 | 182.05 | 179.68 | 14.31 | 7.74 | 7.24 | 7.15 | 11.12 | 0.92 | 1.53 | 0.94 | 1.55 |
| Axis porcinus (N=4) | |||||||||||||||||
| Max | 23.95 | 22.96 | 24.00 | 15.63 | 13.45 | 143.46 | 139.93 | 139.08 | 13.56 | 6.69 | 6.74 | 6.25 | 9.32 | 1.02 | 1.53 | 1.08 | 1.55 |
| Min | 21.44 | 21.27 | 22.94 | 15.01 | 12.37 | 130.90 | 129.40 | 128.30 | 12.56 | 5.48 | 5.70 | 5.52 | 8.47 | 0.93 | 1.37 | 1.01 | 1.48 |
| Mean | 22.70 | 21.96 | 23.32 | 15.41 | 13.08 | 137.47 | 134.71 | 133.73 | 13.15 | 6.09 | 6.28 | 5.90 | 8.92 | 0.97 | 1.43 | 1.03 | 1.51 |
| Panolia eldii (N=4) | |||||||||||||||||
| Max | 30.76 | 29.92 | 29.58 | 19.81 | 17.68 | 237.80 | 235.30 | 230.80 | 18.82 | 8.39 | 8.22 | 8.06 | 12.18 | 1.04 | 1.53 | 1.04 | 1.52 |
| Min | 27.27 | 27.84 | 28.52 | 19.49 | 16.88 | 228.10 | 224.80 | 218.80 | 17.02 | 7.72 | 7.95 | 7.99 | 11.51 | 0.96 | 1.41 | 0.98 | 1.44 |
| Mean | 29.01 | 28.80 | 29.04 | 19.60 | 17.28 | 232.95 | 229.80 | 224.73 | 17.80 | 8.05 | 8.09 | 8.02 | 11.89 | 1.00 | 1.47 | 1.01 | 1.48 |
| Rusa unicolor (N=4) | |||||||||||||||||
| Max | 40.63 | 36.93 | 42.15 | 29.40 | 24.01 | 258.20 | 254.60 | 246.60 | 28.47 | 6.52 | 6.99 | 6.19 | 8.83 | 0.96 | 1.38 | 1.11 | 1.44 |
| Min | 34.82 | 35.34 | 36.63 | 25.66 | 23.56 | 226.70 | 223.50 | 220.10 | 24.56 | 6.32 | 6.34 | 6.08 | 8.64 | 0.95 | 1.25 | 0.97 | 1.40 |
| Mean | 37.68 | 36.16 | 39.38 | 27.66 | 23.69 | 241.93 | 238.58 | 233.05 | 26.40 | 6.43 | 6.69 | 6.15 | 8.75 | 0.96 | 1.31 | 1.04 | 1.42 |
| Bos sauveli (N=2) | |||||||||||||||||
| Max | 53.65 | 51.21 | 52.82 | 33.45 | 31.96 | 282.60 | 273.50 | 263.70 | 32.34 | 5.28 | 5.53 | 5.36 | 8.47 | 1.02 | 1.53 | 1.05 | 1.58 |
| Min | 53.44 | 51.07 | 52.67 | 33.36 | 31.85 | 282.40 | 273.10 | 263.40 | 32.20 | 5.27 | 5.51 | 5.35 | 8.44 | 1.01 | 1.53 | 1.04 | 1.57 |
| Mean | 53.55 | 51.14 | 52.75 | 33.41 | 31.91 | 282.50 | 273.30 | 263.55 | 32.27 | 5.28 | 5.52 | 5.36 | 8.46 | 1.02 | 1.53 | 1.05 | 1.58 |
| Bos javanicus (N=6) | |||||||||||||||||
| Max | 54.98 | 51.89 | 55.74 | 37.28 | 35.52 | 257.80 | 246.40 | 246.90 | 32.40 | 5.14 | 5.56 | 5.12 | 7.58 | 1.00 | 1.49 | 1.08 | 1.55 |
| Min | 48.30 | 46.33 | 50.31 | 33.94 | 30.30 | 243.00 | 234.00 | 230.80 | 29.05 | 4.59 | 4.78 | 4.53 | 6.77 | 0.90 | 1.35 | 0.95 | 1.46 |
| Mean | 51.13 | 49.68 | 53.05 | 35.21 | 33.34 | 250.98 | 238.20 | 236.65 | 30.41 | 4.92 | 5.07 | 4.74 | 7.14 | 0.96 | 1.41 | 1.03 | 1.51 |
| Bos gaurus (N=6) | |||||||||||||||||
| Max | 60.25 | 59.22 | 64.81 | 41.66 | 42.06 | 273.10 | 265.70 | 261.20 | 36.04 | 4.85 | 5.33 | 4.34 | 7.18 | 0.94 | 1.50 | 1.14 | 1.66 |
| Min | 52.35 | 50.30 | 61.89 | 37.36 | 35.48 | 254.00 | 245.70 | 239.10 | 32.84 | 4.43 | 4.61 | 3.92 | 6.10 | 0.81 | 1.21 | 1.02 | 1.56 |
| Mean | 57.31 | 53.27 | 63.77 | 39.75 | 38.00 | 265.57 | 257.70 | 251.92 | 34.65 | 4.64 | 5.00 | 4.17 | 6.70 | 0.90 | 1.34 | 1.08 | 1.61 |
| Bubalus arnee (N=6) | |||||||||||||||||
| Max | 57.42 | 49.56 | 66.93 | 37.74 | 37.03 | 226.40 | 213.50 | 206.80 | 33.13 | 4.05 | 4.67 | 3.41 | 6.47 | 0.86 | 1.41 | 1.20 | 1.91 |
| Min | 52.56 | 46.69 | 62.34 | 34.87 | 32.31 | 212.80 | 198.20 | 200.80 | 30.30 | 3.91 | 4.54 | 3.34 | 5.92 | 0.84 | 1.27 | 1.13 | 1.76 |
| Mean | 55.45 | 48.13 | 65.28 | 36.01 | 34.84 | 221.17 | 208.05 | 203.58 | 31.39 | 3.99 | 4.59 | 3.39 | 6.15 | 0.85 | 1.34 | 1.15 | 1.81 |
Measurements (in millimeters) of mandibles of large bovids from Khok Sung. Numbers within the parentheses refer to the numbers used in von den Driesch’s metrical methods (1976: fig. 21). * indicates measurements of the maximum length of the preservation according to incomplete specimens. “es” refers to an estimated value of the full length due to incomplete specimens.
| Taxon | Bos sauveli | Bos gaurus | Bubalus arnee | ||||||
| Mandible no. | DMR-KS-05-03-9-1 | DMR-KS-05-04-9-1 | DMR-KS-05-03-00-1 | DMR-KS-05-04-3-1 | DMR-KS-05-03-20-10 | DMR-KS-05-03-20-20 | DMR-KS-05-03-20-2 | DMR-KS-05-03-10-3 | DMR-KS-05-03-20-1 |
| Metrical parameters (mm) | Juvenile | Juvenile | Juvenile | ||||||
| (1) Length from the angle: Gonion caudale–Infradentale | 450* | 446.21 | – | – | 377* | – | – | 461.14 | 464.54 |
| (2) Length from the condyle: aboral border of the condyle process–Infradentale | 483.55 | 478.08 | – | – | 371.28* | – | – | 490.82 | 493.34 |
| (3) Length: Gonion caudale–aboral border of the alveolus of m3 | 132.51 | 122.31 | – | 137.11 | 83.57 | – | 94.92 | 126.23 | 127.48 |
| (4) Length of the horizontal ramus: aboral border of the alveolus of m3–Infradentale | 313.60 | 312.1 | – | – | 278.91* | – | – | 324.52 | 325.67 |
| (5) Length: Gonion caudale–oral border of the alveolus of p2 | 307.52 | 289.11 | – | 305.44 | 245.18 | – | – | 301.04 | 299.77 |
| (6) Length: Gonion caudale–the most aboral indentation of the mental foramen | 397.32 | 356.52 | – | – | 311.78 | – | – | 375.44 | 374.89 |
| (7) Length of the cheek tooth row, measured along the alveoli on the buccal side | 169.58 | 165.66 | – | 171.58 | – | – | – | 173.72 | 174.52 |
| (8) Length of the molar row, measured along the alveoli on the buccal side | 107.01 | 103.86 | 101.01 | 109.10 | – | – | – | 111.57 | 113.69 |
| (9) Length of the premolar row, measured along the alveoli on the buccal side | 65.31 | 56.54 | – | 60.26 | 78.22 (p2–dp4) | – | 79.09 (p2–dp4) | 60.31 | 59.02 |
| (11) Length of the diastema: oral border of the alveolus of p2–aboral border of the alveolus of i4 | 110.41 | 116.57 | – | – | 106.53 | – | – | 116.41 | 118.35 |
| (12) Aboral height of the vertical ramus: Gonion ventrale–highest point of the condyle process | 189.21 | 175.03 | – | 186.53 | 139.48 | – | 143.18 | 181.59 | 183.21 |
| (13) Middle height of the vertical ramus: Gonion ventrale–deepest point of the mandibular notch | 168.52 | 165.07 | – | 181.08 | 139.97 | – | 145.30 | 177.52 | 169.18 |
| (14) Oral height of the vertical ramus: Gonion ventrale–Coronion | 254.16 | 249.58 | – | 251.12 | 205.47 | – | – | 236.58 | 246.55 |
| (15a) Height of the mandible behind m3 from the most aboral point of the alveolus on the buccal side | 90.59 | 90.40 | – | 89.86 | – | – | 80.84 | 94.89 | 96.63 |
| (15b) Height of the mandible in front of m1 | 64.88 | 68.83 | 70.33 | 65.79 | 66.84 | 64.28 | 68.61 | 68.48 | 69.63 |
| (15c) Height of the mandible in front of p2 | 54.99 | 55.04 | – | 57.77 (es) | 41.63 | – | 42.38 | 55.57 | 56.33 |
Measurements (in millimeters) of crania of Bubalus arnee from Khok Sung. Numbers within the parentheses refer to the numbers used in von den Driesch’s metrical methods (1976: fig. 8). * indicates measurements of the maximum length of the preservation according to incomplete specimens. “es” refers to an estimated value of the full length due to incomplete specimens.
| Taxon | Bubalus arnee | |||
|---|---|---|---|---|
| Cranium no. | DMR-KS-05-03-16-3 | DMR-KS-05-03-21-1 | DMR-KS-05-03-11-1 | DMR-KS-05-03-20-1 |
| Metrical parameters (mm) | ||||
| (1) Total length: Akrokranion–Prosthion | – | – | – | 568.97 |
| (2) Condylobasal length: aboral border of the occipital condyles–Prosthion | – | – | – | 565.98* |
| (3) Basal length: Basion–Prosthion | – | – | – | 553.31 |
| (4) Short skull length: Basion–Premolare | – | 426.18 | – | 381.21 |
| (5) Premolar–Prosthion | – | – | – | 185.07 |
| (6) Neurocranium length: Basion–Nasion | – | 230 (es) | – | 246.53 |
| (7) Viscerocranium length: Nasion–Prosthion | – | – | – | 344.25 |
| (8) Median frontal length: Akrokranion–Nasion | – | – | – | 206.61 |
| (9) Greatest frontal length: Akrokranion–the median point of intersection of the line joining the oral points of the frontals | – | – | – | 248.82 |
| (10) Short upper cranium length: Akrokranion–Rhinion | – | – | – | 440.31 |
| (11) Akrokranion–Infraorbitale of one side | – | – | – | 385.55 |
| (12) Greatest length of the nasals: Nasion–Rhinion | – | – | – | 230.87 |
| (13) From the aboral border of one occipital condyle to the Entorbitale of the same side | – | 263.18 | – | 243.75 |
| (14) Lateral facial length: Entorbitale–Prosthion | – | – | – | 389.11 |
| (15) From the aboral border of one occipital condyle to the Infraorbitale of the same side | – | – | – | 390.16 |
| (16) Infraorbitale–Prosthion | 168.66 | – | – | 181.36 |
| (17) Dental length: Postdentale–Prosthion | – | – | – | 341.43 |
| (18) Oral palatal length: Palatinoorale–Prosthion | – | – | – | 272.03 |
| (19) Lateral length of the premaxilla: Nasointermaxillare–Prosthion | – | – | – | 197.68 |
| (20) Length of the cheek tooth row (measured along the alveoli) | – | – | 169.58 (right) | 165.56 (left) |
| (21) Length of the molar row (measured along the alveoli on the buccal side) | – | 110 (es) (right) | 98.06 (right) | 102.63 (left) |
| (22) Length of the premolar row (measured along the alveoli on the buccal side) | 62.56 (right), 62.09 (left) | – | 70.34 (right) | 65.76 (right), 68.70 (left) |
| (23) Greatest inner length of the orbit: Ectorbitale–Entorbitale | – | – | – | 67.73 (right), 68.26 (left) |
| (24) Greatest inner height of the orbit | – | – | – | 55.51 (right), 61.44 (left) |
| (25) Greatest mastoid breadth: Otion–Otion | – | 188.71 | – | – |
| (26) Greatest breadth of the occipital condyles | – | 106.59 | – | – |
| (27) Greatest breadth at the bases of the paraoccipital processes | – | 154.23 | – | – |
| (28) Greatest breadth of the foramen magnum | – | 47.38 | – | – |
| (29) Height of the foramen magnum: Basion–Opisthion | – | 40.96 | – | – |
| (30) Least occipital breasdth: the distance between the most medial points of the aboral borders of the temporal grooves | – | 117.35 | – | 93.06 |
| (31) Least breadth between the bases of the horn cores | – | 129.32 | – | 151.45 |
| (32) Least frontal breadth: breadth of the narrowest part of the frontal aboral of the orbits | – | 210 (es) | – | 222.23 |
| (33) Greatest breadth across the orbits = Greatest frontal breadth = greatest breadth of skull: Ectorbitale–Ectorbitale | – | – | – | 231.78 |
| (34) Least breadth between the orbits: Entorbitale–Entorbitale | – | – | – | 165.52 |
| (35) Facial breadth: across the facial tuberosities | – | – | – | 167.42 |
| (36) Greatest breadth across the nasals | – | – | – | 64.01 |
| (37) Breadth across the premaxillae on the oral protuberances | – | – | – | 112.89 |
| (38) Greatest palatal breadth: measured across the outer borders of the alveoli | – | – | – | 148.14 |
| (39) Least inner height of the temporal groove, roughly from the middle of one bone edge to the other | – | 40.08 | – | 24.57 |
| (40) Greatest height of the occipital region: Basion–highest point of the intercornual ridge in the median plane | – | 139.25 | – | – |
| (41) Least height of the occipital region: Opisthion–highest point of the intercornual ridge in the median plane | – | 115.68 | – | 138.74 |
| (42) Distance between the horn core tips | – | 540 (es) | – | 607.18 |
| (43) Greatest tangential distance between the outer curves of the horn cores | – | – | – | 341.38 (right), 333.17 (left) |
| (44) Horn core basal circumference | – | 199.91 (left) | – | 341.38 (right) |
| (45) Greatest (oro–aboral) diameter of the horn core base | – | 72.03 (right), 70.77 (left) | – | 132.71 (right), 131.53 (left) |
| (46) Least (dorso–basal) diameter of the horn core base | – | 44.67 (right), 49.49 (left) | – | 56.05 (right), 53.45 (left) |
| (47) Length of the outer curvature of the horn core | – | 102.21 (right), 258.17 (left) | – | 164* (right), 215* (left) |
Fauna lists of large mammalian species from the Middle Pleistocene of Central eastern and South China. Locality abbreviations: YCK, Yenchingkou; KLS ; Koloshan; WM, Wuming; DX, Daxin; HJ, Hejiang; GX, Ganxian; PXDD, Panxian Dadong; WY, Wuyun; MB, Maba; HST, Hoshantung; HG, Hsingan. The fauna lists and ages follow
| Region | Central eastern China | South China | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Locality | YCK | KLS | WM | DX | HJ | GX | PXDD | WY | MB | HST | HG |
| Approximate age (ka) | 745 to 481 | 380 to 308 | 400 to 320 | > 350 | 300 to 130 | 279 to 76 | > 230 | ||||
| Taxa | |||||||||||
| PRIMATES | |||||||||||
| Macaca sp. | + | + | + | + | + | + | |||||
| Macaca assamensis | cf. | ||||||||||
| Macaca robustus | + | ||||||||||
| Macaca arctoides | + | ||||||||||
| Colobinae indet. | + | ||||||||||
| Presbytis sp. | + | ||||||||||
| Rhinopithecus sp. | + | ||||||||||
| Rhinopithecus roxellana | + | + | |||||||||
| Trachypithecus sp. | + | ||||||||||
| Nomascus sp. | + | ||||||||||
| Hylobates sp. | + | ||||||||||
| Bunopithecus sericus | + | ||||||||||
| Szechuanopithecus yangtsensis | + | ||||||||||
| Pongo sp. | + | + | + | ||||||||
| Pongo pygmaeus | + | + | + | + | |||||||
| Gigantopithecus blacki | + | + | + | ||||||||
| Homo sp. | + | + | |||||||||
| CARNIVORA | |||||||||||
| Canidae indet. | + | + | |||||||||
| Canis lupus | + | ||||||||||
| Cuon simplicidens | + | ||||||||||
| Cuon alpinus (=javanicus) | + | + | cf. | + | |||||||
| Vulpes vulpes | + | ||||||||||
| Ursus sp. | + | + | |||||||||
| Ursus angustidens | + | ||||||||||
| Ursus thibetanus | + | + | + | + | + | + | + | + | |||
| Helarctos malayanus | |||||||||||
| Ailuropoda melanoleuca | + | + | + | + | + | + | + | + | + | + | |
| Ailuropoda microta | + | ||||||||||
| Ailurus fulgens | + | ||||||||||
| Mustela sibirica | + | + | |||||||||
| Martes flavigula | + | ||||||||||
| Martes sinensis | + | ||||||||||
| Meles sp. | + | ||||||||||
| Melogale sp. | + | ||||||||||
| Parameles simplicidens | + | ||||||||||
| Arctonyx sp. | + | ||||||||||
| Arctonyx collaris | + | + | + | + | + | + | |||||
| Viverra sp. | + | ||||||||||
| Viverra zibetha | + | + | |||||||||
| Viverricula malaccensis | + | ||||||||||
| Lutra sp. | + | ||||||||||
| Felis sp. | + | + | + | + | |||||||
| Felis teilhardi | + | ||||||||||
| Lynx lynx | + | + | |||||||||
| Panthera pardus | + | + | + | ||||||||
| Panthera tigris | + | + | + | + | + | cf. | + | + | |||
| Paradoxurus sp. | + | ||||||||||
| Prionailurus bengalensis | + | ||||||||||
| Paguma larvata | + | + | |||||||||
| Hyaena sp. | + | + | + | ||||||||
| Crocuta crocuta | + | + | + | + | |||||||
| PROBOSCIDEA | |||||||||||
| Stegodon sp. | + | + | |||||||||
| Stegodon orientalis | + | + | + | + | + | + | + | + | |||
| Elephas maximus | + | + | |||||||||
| Palaeoloxodon sp. | + | ||||||||||
| Palaeoloxodon namadicus | + | + | + | ||||||||
| PERISSODACTYLA | |||||||||||
| Tapirus sp. | + | ||||||||||
| Tapirus indicus | + | ||||||||||
| Tapirus sinensis | + | + | |||||||||
| Megatapirus augustus | + | + | + | + | + | + | + | ||||
| Rhinocerotidae indet. | |||||||||||
| Rhinoceros sp. | + | ||||||||||
| Rhinoceros unicornis | ? | ||||||||||
| Rhinoceros sinensis | + | + | + | + | + | + | + | + | |||
| Rhinoceros fusuiensis | + | + | |||||||||
| ARTIODACTYLA | |||||||||||
| Sus sp. | + | + | + | + | + | + | + | ||||
| Sus scrofa | + | + | + | + | + | + | |||||
| Sus xiaozhu | + | + | |||||||||
| Sus bijiashanensis | + | + | |||||||||
| Dicoryphochoerus ultimus | + | ||||||||||
| Cervidae indet. | + | + | + | + | |||||||
| Cervus sp. or Rusa sp. | + | + | + | ||||||||
| Cervus yunnanensis | + | ||||||||||
| Rusa unicolor | + | cf. | + | + | |||||||
| Elaphodus cephalophus | + | ||||||||||
| Muntiacus sp. | + | + | + | + | + | + | + | ||||
| Muntiacus muntjak | + | + | |||||||||
| Muntiacus szechuanensis | + | ||||||||||
| Hydropotes sp. | + | ||||||||||
| Mochus sp. | + | ||||||||||
| Mochus moschiferus | + | ||||||||||
| Bovidae indet. | + | + | + | + | + | ||||||
| Bos sp. or Bubalus sp. | + | + | + | + | |||||||
| Bos gaurus | + | ||||||||||
| Bubalus arnee | + | ||||||||||
| Bubalus brevicornis | + | ||||||||||
| Caprinae indet. | + | + | + | ||||||||
| Capricornis sumatraensis | + | cf. | + | + | |||||||
| Naemorhedus goral | + | + | |||||||||
| Megalovis guangxiensis | + | + | + | ||||||||
Fauna lists of large mammalian species from the Middle Pleistocene of Southeast Asia. Locality abbreviations: KS, Khok Sung; TWN ; Thum Wiman Nakin; TPKP, Thum Phra Khai Phet; KPN, Kao Pah Nam; TPD, Thum Phedan; TK, Tham Khuyen; TH, Tham Hai; TO, Tham Om; MG, Mogok Caves; PNL, Phnom Loang; BDB, Boh Dambang; BDC, Badak Cave; TB, Tambun (Kinta Valley); KDBB, Kedung Brubus; TNHK, Trinil Hauptknochenschicht; ND, Ngandong. The fauna lists and ages follow
| Country | Thailand | Vietnam | Myanmar | Cambodia | Malaysia | Indonesia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locality | KS | TWN | TPKP | KPN | TPD | TK | TH | TO | MG | PNL | BDB | BDC | TB | KDBB | TNHK | ND |
| Approximate age (ka) | 213 to 120 | > 169 ± 11 | ≈TWN | ≈690 | 475 ± 125 | ≈TK | 250 to 140 | > TWN | ≈PNL | 274 to 208 | 800 to 700 | 540 to 430 | > 143 | |||
| Taxa | ||||||||||||||||
| PRIMATES | ||||||||||||||||
| Macaca sp. | + | + | + | + | + | |||||||||||
| Macaca assamensis | cf. | |||||||||||||||
| Macaca nemestrina | cf. | + | ||||||||||||||
| Macaca fascicularis | + | + | ||||||||||||||
| Trachypithecus sp. | + | |||||||||||||||
| Trachypithecus cristatus | + | |||||||||||||||
| Hominoidea indet. | + | + | ||||||||||||||
| Nomascus concolor | cf. | |||||||||||||||
| Hylobates sp. | + | |||||||||||||||
| Pongo sp. | + | ? | + | |||||||||||||
| Pongo pygmaeus | + | + | + | + | + | |||||||||||
| Gigantopithecus blacki | + | ? | ||||||||||||||
| Homo sp. | + | + | ||||||||||||||
| Homo erectus | + | + | + | |||||||||||||
| CARNIVORA | ||||||||||||||||
| Cuon sp. | + | + | + | |||||||||||||
| Cuon alpines (=javanicus) | + | + | ||||||||||||||
| Ursus thibetanus | + | + | + | + | + | + | + | |||||||||
| Helarctos malayanus | + | + | ||||||||||||||
| Ailuropoda melanoleuca | + | + | + | + | + | |||||||||||
| Martes sp. | + | |||||||||||||||
| Martes flavigula | + | |||||||||||||||
| Arctonyx collaris | + | + | + | |||||||||||||
| Viverra zibetha | cf. | |||||||||||||||
| Lutrogale palaeoleptonyx | + | |||||||||||||||
| Cynogale sp. | + | |||||||||||||||
| Felis sp. | + | |||||||||||||||
| Panthera pardus | cf. | + | ||||||||||||||
| Panthera tigris | + | + | + | + | + | + | + | |||||||||
| Paradoxurus hermaphroditus | + | cf. | ||||||||||||||
| Prionailurus bengalensis | + | + | ||||||||||||||
| Paguma larvata | + | cf. | ||||||||||||||
| Hyaena brevirostris | + | |||||||||||||||
| Crocuta sp. | + | |||||||||||||||
| Crocuta crocuta | + | + | + | + | + | + | ||||||||||
| PHOLIDOTA | ||||||||||||||||
| Manis palaeojavanica | + | |||||||||||||||
| PROBOSCIDEA | ||||||||||||||||
| Stegodon orientalis | cf. | + | + | + | + | |||||||||||
| Stegodon trigonocephalus | + | + | + | |||||||||||||
| Elaphas sp. | + | + | ||||||||||||||
| Elephas maximus | cf. | |||||||||||||||
| Elephas celebensis | ? | |||||||||||||||
| Elephas hysudrindicus | + | + | ||||||||||||||
| Palaeoloxodon namadicus | + | cf. | ? | + | ||||||||||||
| PERISSODACTYLA | ||||||||||||||||
| Tapirus sp. | + | + | + | |||||||||||||
| Tapirus indicus | + | + | + | + | ||||||||||||
| Megatapirus augustus | + | + | ||||||||||||||
| Rhinocerotidae indet. | + | |||||||||||||||
| Rhinoceros sp. | + | + | + | + | ||||||||||||
| Rhinoceros sondaicus | + | + | + | + | + | + | + | |||||||||
| Rhinoceros unicornis | + | + | + | |||||||||||||
| Rhinoceros sinensis | + | + | + | |||||||||||||
| ARTIODACTYLA | ||||||||||||||||
| Sus sp. | + | + | + | + | ||||||||||||
| Sus barbatus | + | cf. | cf. | |||||||||||||
| Sus scrofa | + | + | + | + | + | + | + | + | + | |||||||
| Sus lydekkeri | cf. | |||||||||||||||
| Sus brachygnathus | + | ? | ||||||||||||||
| Sus macrognathus | + | + | ||||||||||||||
| Cervidae indet. | + | + | + | + | ||||||||||||
| Cervus sp. or Rusa sp. | + | + | + | + | + | + | + | |||||||||
| Panolia eldii | + | + | + | |||||||||||||
| Rusa unicolor | + | + | + | + | cf. | + | + | + | + | |||||||
| Rusa leptodus | cf. | |||||||||||||||
| Elaphodus sp. | + | |||||||||||||||
| Axis porcinus | ? | ? | ? | |||||||||||||
| Axis axis | + | |||||||||||||||
| Axis lydekkeri | + | + | ||||||||||||||
| Muntiacus sp. | + | + | + | |||||||||||||
| Muntiacus muntjak | + | + | + | + | + | + | + | + | ||||||||
| Bovidae indet. | + | |||||||||||||||
| Bos sp. or Bubalus sp. | + | + | + | + | + | |||||||||||
| Bos sauveli | + | + | + | |||||||||||||
| Bos javanicus | + | + | ||||||||||||||
| Bos gaurus | + | + | cf. | + | + | |||||||||||
| Bibos palaeosondaicus | + | + | + | |||||||||||||
| Bubalus arnee | + | + | + | + | cf. | + | ||||||||||
| Bubalus palaeokerabau | + | + | + | |||||||||||||
| Duboisia santeng | + | + | + | |||||||||||||
| Epileptobos groeneveldtii | + | |||||||||||||||
| Caprinae indet. | + | |||||||||||||||
| Capricornis sumatraensis | + | + | + | + | + | + | + | |||||||||
| Naemorhedus sp. | + | |||||||||||||||
| Hippopotamus sp. | + | |||||||||||||||
| Hexaprotodon sp. | + | |||||||||||||||
| Hexaprotodon sivalensis | + | + | ||||||||||||||
Fauna lists of large mammalian species from the Late Pleistocene of Southeast Asia and South China. Locality abbreviations: CMBFS, the Cave of the Monk (Ban Fa Suai); LPC, Lower Pubu Cave; LN, Luna Cave; NL, Nam Lot; THS, Tam Hang South; HH, Hang Hum; LT, Lang Trang; DUO, Duoi U’Oi; MUO, Ma U’Oi; KL, Keo Leng; GGR, Gua Gunung Runtuh; GC, Gua Cha; BTC, Batu Caves; N, Niah Caves; PN, Punung; GD, Gunung Dawung; WJ, Wajak; LA, Lida Ajer; SBB, Sibrambang. The fauna lists and ages follow
| Country | Thailand | South | Laos | Vietnam | Malaysia | Indonesia | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Java | Sumatra | ||||||||||||||||||
| Locality | CMBFS | LPC | LN | NL | THS | HH | LT | DUO | MUO | KL | BTC | GGR | GC | N | PN | GD | WJ | LA | SBB |
| Approximate age (ka) | > 32–19 | ≈70 | 86 to 72 | 94 to 60 | 140 to 80 | 100 to 80 | 66 ± 3 | > 49 | 30 to 20 | 66 to 33 | 13.6 to 2.6 | 6.3 to 0.8 | < 45 | 128 to 118 | ≈PN | 37 to 29 | ≈PN | ≈PN | |
| Taxa | |||||||||||||||||||
| PRIMATES | |||||||||||||||||||
| Nycticebus coucang | + | ||||||||||||||||||
| Macaca sp. | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Macaca assamensis | cf. | cf. | |||||||||||||||||
| Macaca nemestrina | cf. | + | + | + | |||||||||||||||
| Macaca anderssoni | cf. | ||||||||||||||||||
| Macaca mulatta | cf. | ||||||||||||||||||
| Macaca fascicularis | + | + | + | ||||||||||||||||
| Colobinae indet. | + | ||||||||||||||||||
| Presbytis sp. | ? | ? | + | + | |||||||||||||||
| Presbytis melalophos | + | ||||||||||||||||||
| Presbytis rubicunda | + | ||||||||||||||||||
| Trachypithecus sp. | ? | ? | + | + | + | ||||||||||||||
| Trachypithecus cristatus | + | + | + | + | |||||||||||||||
| Pygatrix nemaeus | cf. | ||||||||||||||||||
| Hylobates sp. | + | + | + | + | + | + | + | + | |||||||||||
| Hylobates lar | + | ||||||||||||||||||
| Hylobates muelleri | + | ||||||||||||||||||
| Hylobates moloch | cf. | ||||||||||||||||||
| Symphalangus syndactylus | + | + | + | ||||||||||||||||
| Semnopithecus sp. | + | ||||||||||||||||||
| Pongo sp. | + | ||||||||||||||||||
| Pongo pygmaeus | cf. | + | + | + | + | + | + | + | + | + | + | + | |||||||
| Homo sp. | + | + | + | + | + | ||||||||||||||
| Homo sapiens | + | + | + | + | + | ||||||||||||||
| CARNIVORA | |||||||||||||||||||
| Nyctereutes sp. | + | ||||||||||||||||||
| Cuon sp. | + | + | + | + | |||||||||||||||
| Cuon alpinus | cf. | + | + | + | + | + | + | ||||||||||||
| Canidae indet. | + | + | |||||||||||||||||
| Ursidae indet. | + | ||||||||||||||||||
| Ursus thibetanus | cf. | + | + | + | + | + | + | + | + | ||||||||||
| Helarctos malayanus | cf. | + | + | + | + | + | + | + | + | + | + | + | |||||||
| Ailuropoda melanoleuca | + | + | + | + | + | + | |||||||||||||
| Martes flavigula | + | ? or cf. | |||||||||||||||||
| Arctonyx sp. | + | ||||||||||||||||||
| Arctonyx collaris | + | + | + | + | + | + | + | ||||||||||||
| Meles meles | + | + | |||||||||||||||||
| Mustela nupides | + | ||||||||||||||||||
| Melogale personata | + | ||||||||||||||||||
| Melogale everetti | + | ||||||||||||||||||
| Viverridae indet. | + | ||||||||||||||||||
| Viverra zibetha | + | + | + | cf. | |||||||||||||||
| Viverra megaspila | cf. | ||||||||||||||||||
| Viverra tangalunga | + | + | |||||||||||||||||
| Lutra perspicillata | + | ||||||||||||||||||
| Lutrogale sumatrana | + | ||||||||||||||||||
| Herpestes sp. | + | ||||||||||||||||||
| Catopuma temminckii | cf. | + | + | ||||||||||||||||
| Neofelis nebulosa | + | + | + | + | |||||||||||||||
| Panthera pardus | + | + | + | + | |||||||||||||||
| Panthera tigris | cf. | + | + | + | + | + | ? | + | + | + | + | ||||||||
| Paradoxurus sp. | + | ||||||||||||||||||
| Paradoxurus hermaphroditus | + | cf. | + | cf. | |||||||||||||||
| Prionailurus bengalensis | cf. | ||||||||||||||||||
| Paguma sp. | + | ||||||||||||||||||
| Paguma larvata | + | ||||||||||||||||||
| Arctictis binturong | + | + | |||||||||||||||||
| Hemigalus derbyanus | + | ||||||||||||||||||
| Hyaenidae indet. | + | ||||||||||||||||||
| Crocuta crocuta | + | ||||||||||||||||||
| PHOLIDOTA | |||||||||||||||||||
| Manis javanica | + | ||||||||||||||||||
| Manis palaeojavanica | + | ||||||||||||||||||
| PROBOSCIDEA | |||||||||||||||||||
| Stegodon sp. | + | ||||||||||||||||||
| Stegodon orientalis | + | cf. | + | + | + | + | |||||||||||||
| Elaphas sp. | + | + | + | + | + | + | |||||||||||||
| Elephas maximus | + | + | + | + | + | ||||||||||||||
| Palaeoloxodon namadicus | + | + | cf. | ||||||||||||||||
| PERISSODACTYLA | |||||||||||||||||||
| Tapirus sp. | + | + | |||||||||||||||||
| Tapirus indicus | + | + | + | + | + | + | + | + | + | + | + | + | |||||||
| Tapirus sinensis | + | ||||||||||||||||||
| Megatapirus augustus | + | + | + | ||||||||||||||||
| Rhinocerotidae indet. | + | + | + | + | + | ||||||||||||||
| Rhinoceros sp. | + | + | + | ||||||||||||||||
| Rhinoceros sondaicus | cf. | + | + | + | cf. | + | + | + | + | + | |||||||||
| Rhinoceros unicornis | cf. | + | + | + | + | cf. | + | ||||||||||||
| Rhinoceros sinensis | cf. | + | |||||||||||||||||
| Dicerorhinus sumatrensis | cf. | + | + | + | + | + | + | + | |||||||||||
| Equus hemionus | + | ||||||||||||||||||
| ARTIODACTYLA | |||||||||||||||||||
| Sus sp. | + | + | + | + | |||||||||||||||
| Sus barbatus | cf. | cf. | + | + | + | + | + | + | + | ||||||||||
| Sus scrofa | cf. | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Sus officinalis | cf. | ||||||||||||||||||
| Sus xiaozhu | + | ||||||||||||||||||
| Sus lydekkeri | cf. | cf. | |||||||||||||||||
| Cervidae indet. | + | + | + | ||||||||||||||||
| Cervus sp. or Rusa sp. | + | + | + | + | ? | + | + | ||||||||||||
| Panolia eldii | cf. | ||||||||||||||||||
| Rusa unicolor | cf. | + | + | + | + | + | cf. | + | + | + | + | + | |||||||
| Rusa timorensis | + | ||||||||||||||||||
| Cervus nippon | cf. | ||||||||||||||||||
| Axis porcinus | cf. | ||||||||||||||||||
| Muntiacus sp. | + | + | + | + | |||||||||||||||
| Muntiacus muntjak | cf. | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||
| Muntiacus vuquangensis | cf. | ||||||||||||||||||
| Tragulus javanicus | + | ||||||||||||||||||
| Tragulus napu | + | + | |||||||||||||||||
| Bovidae indet. | + | + | + | + | + | + | |||||||||||||
| Bos sp. | + | + | + | + | |||||||||||||||
| Bos sauveli | cf. | cf. | |||||||||||||||||
| Bos javanicus | cf. | + | + | + | |||||||||||||||
| Bos gaurus | cf. | + | + | ||||||||||||||||
| Bubalus sp. | + | ||||||||||||||||||
| Bubalus arnee | cf. | + | + | + | cf. | + | + | + | |||||||||||
| Pseudoryx sp. | + | ||||||||||||||||||
| Caprinae indet. | + | ||||||||||||||||||
| Capricornis sumatraensis | cf. | + | + | + | + | + | + | + | + | + | + | ||||||||
| Naemorhedus sp. | cf. | ||||||||||||||||||
| Naemorhedus caudatus | cf. | ||||||||||||||||||
| Naemorhedus goral | cf. | ||||||||||||||||||
Fauna lists of extant large mammalian species from Southeast Asia and South China. The biogeographic affinities of the mammalian species are given and abbreviated as (I) for Indochinese taxa, (S) for Sundaic taxa, (O) for other geographic taxa (e.g., Palearctic and Indian regions), and (W) for widespread taxa. Data are compiled from
| Biogeographic province | Indochinese province | Thailand (Kra Isthmus) | Sundaic province | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | South China | Myanmar | Laos | Cambodia | Vietnam | North | South | Malaysia | Sumatra | Java | Borneo |
| Taxa | |||||||||||
| PRIMATES | |||||||||||
| Nycticebus coucang (I, S) | + | + | + | + | + | + | + | + | + | + | + |
| Nycticebus pygmaeus (I) | + | + | + | + | |||||||
| Tarsius bancanus (S) | + | + | |||||||||
| Macaca arctoides (I) | + | + | + | + | + | + | + | ||||
| Macaca assamensis (I, O) | + | + | + | + | + | ||||||
| Macaca fascicularis (S) | + | + | + | + | + | + | + | + | + | + | |
| Macaca mulatta (I, O) | + | + | + | + | + | ||||||
| Macaca nemestrina (I, S) | + | + | + | + | + | + | + | + | + | ||
| Macaca thibetana (I) | + | ||||||||||
| Presbytis comata (S) | + | ||||||||||
| Presbytis femoralis (S) | + | + | + | + | + | ||||||
| Presbytis frontana (S) | + | ||||||||||
| Presbytis hosei (S) | + | ||||||||||
| Presbytis melalophos (S) | + | ||||||||||
| Presbytis potenziani (S) | + | ||||||||||
| Presbytis rubicunda (S) | + | ||||||||||
| Presbytis thomasi (S) | + | ||||||||||
| Trachypithecus auratus (S) | + | ||||||||||
| Trachypithecus cristatus (S) | + | + | + | + | + | + | + | + | + | ||
| Trachypithecus francoisi (I) | + | + | + | ||||||||
| Trachypithecus obscurus (S) | + | + | + | + | |||||||
| Trachypithecus phayrei (I) | + | + | + | + | + | ||||||
| Trachypithecus pileatus (I) | + | ||||||||||
| Pygathrix nemaeus (I) | + | + | + | ||||||||
| Rhinopithecus avunculus (I) | + | ||||||||||
| Rhinopithecus bieti (I) | + | ||||||||||
| Rhinopithecus brelichi (I) | + | ||||||||||
| Nasalis larvatus (S) | + | ||||||||||
| Simias concolor (S) | + | ||||||||||
| Nomascus concolor (I) | + | + | + | ||||||||
| Nomascus gabriellae (I) | + | + | |||||||||
| Nomascus leucogenys (I) | + | + | + | ||||||||
| Hoolock hoolock (I) | + | + | |||||||||
| Hylobates agilis (S) | + | + | + | + | |||||||
| Hylobates klossii (S) | + | ||||||||||
| Hylobates lar (I, S) | + | + | + | + | + | + | |||||
| Hylobates moloch (S) | + | ||||||||||
| Hylobates muelleri (S) | + | ||||||||||
| Hylobates pileatus (I) | + | + | + | ||||||||
| Symphalangus syndactylus (S) | + | + | |||||||||
| Pongo pygmaeus (S) | + | + | |||||||||
| CARNIVORA | |||||||||||
| Canis aureus (I, O) | + | + | |||||||||
| Canis lupus (W) | + | ||||||||||
| Vulpes vulpes (I, O) | + | + | |||||||||
| Nyctereutes procyonoides (I, O) | + | + | |||||||||
| Cuon alpinus (W) | + | + | + | + | + | + | + | + | + | + | |
| Ursus thibetanus (I) | + | + | + | + | + | ||||||
| Helarctos malayanus (I, S) | + | + | + | + | + | + | + | + | + | + | |
| Ailuropoda melanoleuca | + | ||||||||||
| Ailurus fulgens (I) | + | + | |||||||||
| Mustela kathiah (I, O) | + | + | + | + | |||||||
| Mustela lutreolina (S) | + | + | |||||||||
| Mustela nivalis (I, O) | + | + | |||||||||
| Mustela nudipes (S) | + | + | + | + | |||||||
| Mustela sibirica (I, O) | + | + | + | + | + | ||||||
| Mustela strigidorsa (I) | + | + | + | + | + | ||||||
| Martes flavigula (I, S, O) | + | + | + | + | + | + | + | + | + | + | + |
| Meles meles (I, O) | + | + | + | ||||||||
| Arctonyx collaris (I, O) | + | + | + | + | + | + | + | + | |||
| Melogale everetti (S) | + | ||||||||||
| Melogale moschata (I) | + | + | + | ||||||||
| Melogale orientalis (S) | + | ||||||||||
| Melogale personata (I) | + | + | + | ||||||||
| Mydaus javanensis (S) | + | + | + | ||||||||
| Lutra lutra (W) | + | + | + | + | + | + | + | ||||
| Lutra sumatrana (S) | + | + | + | + | + | + | + | ||||
| Lutrogale perspicillata (W) | + | + | + | + | + | + | + | + | + | + | + |
| Amblonyx cinereus (W) | + | + | + | + | + | + | + | + | + | + | + |
| Viverra megaspila (I) | + | + | + | + | + | + | + | + | |||
| Viverra tangalunga (S) | + | + | + | ||||||||
| Viverra zibetha (I) | + | + | + | + | + | + | + | + | |||
| Viverricula indica (W) | + | + | + | + | + | + | + | + | + | + | |
| Prionodon linsang (S) | + | + | + | + | + | + | + | ||||
| Prionodon pardicolor (I) | + | + | + | + | + | ||||||
| Paradoxurus hermaphroditus (W) | + | + | + | + | + | + | + | + | + | + | + |
| Paguma larvata (I, S, O) | + | + | + | + | + | + | + | + | + | + | |
| Arctictis binturong (I, S) | + | + | + | + | + | + | + | + | + | + | + |
| Arctogalidia trivirgata (I, S) | + | + | + | + | + | + | + | + | + | + | + |
| Hemigalus derbyanus (S) | + | + | + | + | + | ||||||
| Chrotogale owstoni (I) | + | + | + | ||||||||
| Diplogale hosei (S) | + | ||||||||||
| Cynogale bennettii (S) | + | + | + | ||||||||
| Herpestes brachyurus (S) | + | + | + | ||||||||
| Herpestes javanicus (I, S, O) | + | + | + | + | + | + | + | + | + | ||
| Herpestes semitorquatus (S) | + | + | |||||||||
| Herpestes urva (I) | + | + | + | + | + | + | + | + | |||
| Felis chaus (W) | + | + | + | + | + | + | |||||
| Prionailurus bengalensis (W) | + | + | + | + | + | + | + | + | + | + | + |
| Prionailurus planiceps (S) | + | + | + | + | |||||||
| Prionailurus viverrinus (W) | + | + | + | + | + | + | + | + | |||
| Catopuma badia (S) | + | ||||||||||
| Catopuma temminckii (I, S) | + | + | + | + | + | + | + | + | + | ||
| Pardofelis marmorata (I, S) | + | + | + | + | + | + | + | ||||
| Neofelis nebulosa (I, S) | + | + | + | + | + | + | + | + | + | + | |
| Panthera pardus (W) | + | + | + | + | + | + | + | + | + | ||
| Panthera tigris (W) | + | + | + | + | + | + | + | + | + | + | |
| PHOLIDOTA | |||||||||||
| Manis javanica (S) | + | + | + | + | + | + | |||||
| Manis pentadactyla (I) | + | + | + | + | + | ||||||
| PROBOSCIDEA | |||||||||||
| Elephas maximus (I, O) | + | + | + | + | + | + | + | + | + | ||
| PERISSODACTYLA | |||||||||||
| Tapirus indicus (S) | + | + | + | + | + | + | |||||
| Rhinoceros sondaicus (I, S) | + | + | + | + | + | + | + | + | + | ||
| Dicerorhinus sumatrensis (I, S) | + | + | + | + | + | + | + | + | + | + | |
| ARTIODACTYLA | |||||||||||
| Sus barbatus (S) | + | + | + | ||||||||
| Sus scrofa (W) | + | + | + | + | + | + | + | + | + | + | |
| Sus verrucosus (S) | + | ||||||||||
| Tragulus javanicus (S) | + | + | + | + | + | + | + | + | + | + | |
| Tragulus napu (S) | + | + | + | + | + | + | + | + | |||
| Moschus berezovskii (I) | + | + | |||||||||
| Panolia eldii (I) | + | + | + | + | |||||||
| Cervus nippon (I) | + | ||||||||||
| Rusa unicolor (W) | + | + | + | + | + | + | + | + | + | + | |
| Rusa timorensis (S, O) | + | ||||||||||
| Axis porcinus (I, O) | + | + | + | + | + | + | |||||
| Axis kuhlii (S) | + | ||||||||||
| Muntiacus atherodes (S) | + | ||||||||||
| Muntiacus feae (I) | + | + | |||||||||
| Muntiacus gongshanensis (I) | + | + | |||||||||
| Muntiacus muntjak (W) | + | + | + | + | + | + | + | + | + | + | + |
| Muntiacus reevesi (I) | + | + | |||||||||
| Muntiacus rooseveltorum (I) | + | ||||||||||
| Elaphodus cephalophus (I) | + | + | |||||||||
| Hydropotes inermis (I) | + | ||||||||||
| Bos sauveli (I) | + | + | + | + | |||||||
| Bos javanicus (I, S) | + | + | + | + | + | + | + | + | |||
| Bos gaurus (I) | + | + | + | + | + | + | + | + | |||
| Bubalus arnee (I, O) | + | + | + | ||||||||
| Budorcas taxicolor (I) | + | + | |||||||||
| Capricornis sumatraensis (I, S, O) | + | + | + | + | + | + | + | + | + | ||
| Naemorhedus baileyi (I) | + | + | |||||||||
| Naemorhedus caudatus (I) | + | + | |||||||||