Research Article |
|
Corresponding author: Ashley P. G. Dowling ( adowling@uark.edu ) Academic editor: Vladimir Pesic
© 2016 Joseph C. O’Neill, J. Ray Fisher, Whitney A. Nelson, Micheal J. Skvarla, Danielle M. Fisher, Ashley P. G. Dowling.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
O’Neill JC, Fisher JR, Nelson WA, Skvarla MJ, Fisher DM, Dowling APG (2016) Systematics of testudacarine torrent mites (Acari, Hydrachnidia, Torrenticolidae) with descriptions of 13 new species from North America. ZooKeys 582: 13-110. https://doi.org/10.3897/zookeys.582.7684
|
Abstract
Thirteen new species of North American Testudacarus (Torrenticolidae: Testudacarinae) are described: T. deceptivus O’Neill & Dowling, sp. n., T. hitchensi O’Neill & Dowling, sp. n., T. harrisi O’Neill & Dowling, sp. n., T. dennetti O’Neill & Dowling, sp. n., T. dawkinsi O’Neill & Dowling, sp. n., T. radwellae O’Neill & Dowling, sp. n., T. kirkwoodae O’Neill & Dowling, sp. n., T. hyporhynchus O’Neill & Dowling, sp. n., T. smithi O’Neill & Dowling, sp. n., T. rollerae O’Neill & Dowling, sp. n., T. elongatus O’Neill & Dowling, sp. n., T. rectangulatus O’Neill & Dowling, sp. n., and T. oblongatus O’Neill & Dowling, sp. n. Testudacarus vulgaris Habeeb, 1954 is resurrected from synonymy with T. minimus and redescribed. Debsacarus (Habeeb, 1961), Testudacarus americanus Marshall, 1943, and T. minimus Marshall, 1943 are redescribed. All redescriptions are from original types. Species delimination was accomplished through examination of morphology, biogeography, and molecular phylogenetics of the barcoding region of COI. Other species are addressed and a key to world species is presented. For Testudacarinae, this represents the first published: 1) descriptions from multiple specimens (i.e. intraspecific variation); 2) colored photographs; 3) explicit illustrations and discussion of sexual dimorphism within the subfamily; 4) genetic data. A comprehensive testudacarine reference list is also included.
Keywords
Hydrachnidiae, Hydrachnidia , water mites, Testudacarinae , Testudacarus , Debsacarus
Introduction
Torrenticolidae Piersig, 1902 are ubiquitous and diverse in North America, but the majority of species remain undescribed. This study is the second in a series of descriptions of North American torrenticolids. The goal of this ongoing taxonomic project is to explore the family and make these mites amenable to other researchers.
Testudacarinae Cook, 1974 are found abundantly in riffles of fast flowing streams throughout most of North America and sporadically in Asia. Typical of lotic-dwelling water mites, testudacarines are dorso-ventrally flattened, heavily sclerotized, and possess robust legs with large tarsal claws used for crawling. Most testudacarines are less than 1 mm in size and can exhibit striking coloration. Larvae are reported to be ectoparasites of chironomid adults (
Despite their abundance, few testudacarines are described worldwide and in North America the most recent description is over fifty years old. Limited morphological and distributional data have been presented, and no genetic data has ever been published on Testudacarinae. Minimalistic and incomplete descriptions have led to considerable confusion throughout testudacarine taxonomic history. There is a need describe new species with modern methods and to redescribe older species with the same thoroughness.
Thirteen descriptions and four redescriptions of North American Testudacarus Walter, 1928 are included within. Following
Taxonomic history
There are currently nine testudacarines described worldwide: Testudacarus tripeltatus Walter, 1928 from India; T. japonicus Imamura, 1955 and T. okadai Imamura, 1976 from Japan; T. binodipalpis Guo and Jin, 2005 from China; and T. americanus Marshall, 1943, T. minimus Marshall, 1943, T. minimus vulgaris (Habeeb, 1954), T. americanus galloi Habeeb, 1969, and Debsacarus oribatoides (Habeeb, 1961) from the United States. However, the status of several of these testudacarines remains unclear.
Testudacarus americanus and T. minimus were described by
Only two authors,
Methods
Sampling and curation
Mites were collected and preserved using protocols detailed in
Morphological terminology
Terminology used in this study is detailed in Figs
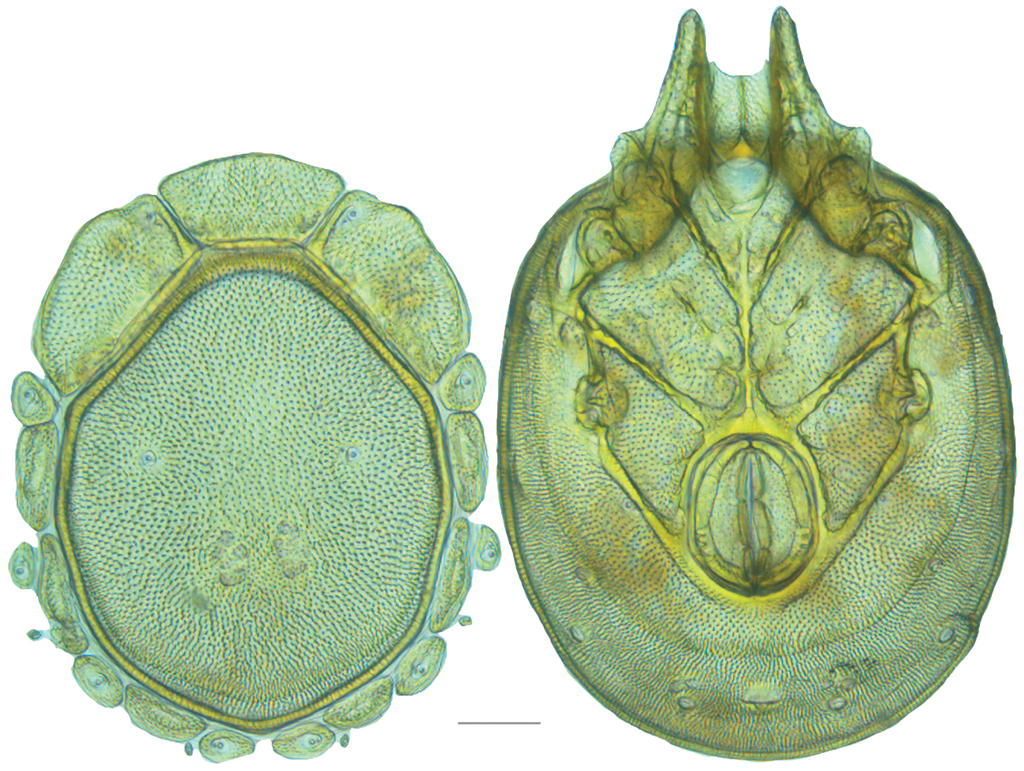
Testudacarine male dorsum (generalized): (Left) – anterio-medial platelet (amp); anterio-lateral platelet (alp); dorsal plate (dp); lateral platelets (lp); (Right) – dorso-glandularia (dg); post-ocularial setae (pos); dorsal membrane (dm); lyriffisures (l); muscle scars (ms); latero-glandularia (lg).
Testudacarine male venter (generalized): Left – coxo-glandularia (cg); latero-glandularia (lg); ventro-glandularia (vg); Middle – coxae (c). Right – gnathosomal bay (gb); coxae-II+III midline (ml); genital field (gf); acetabula (a); line of secondary sclerotization (ss); excretory pore (ep).
Testudacarine male venter (SEM): coxo-glandularia (cg); latero-glandularia (lg); ventro-glandularia (vg); coxae (c); coxae-II+III midline (ml); genital field (gf); acetabula (a); line of secondary sclerotization (ss); excretory pore (ep). Scale: 100 µm. Photo Michelle Hoppner and Ian Smith (used with permission).
Images and measurements
Images were produced and measurements taken following the protocol detailed in
Material deposition of Nearctic types
All holotypes, allotypes, and some paratypes have been deposited in the
Morphological and distributional examinations
Material from the
Molecular examination
The “barcoding” region of COI was used as an independent test of morphological species hypotheses. COI was used to determine if any morphological characters, conservative or ambiguous, indicated species boundaries by sorting into distinct genetic lineages. COI was also used in the same way to test distributional hypotheses. Taxon sampling included roughly 300 specimens spanning “morphotypes” from across North America. Unfortunately, ethanol collections were limited from Mexico, northern Canada, and the eastern United States and therefore do not fully represent the ranges of species from these regions. Later, twenty specimens were included for phylogenetic analysis of 28S (D1-3) to investigate interspecific relationships. Genbank accession numbers of specimens for which sequences were obtained and used in this study are located in Table
Genbank accession numbers and GenSeq nomenclature for each specimen sequenced for this study.
| Species | Genbank Accession # | Specimen Catalog # |
GenSeq Nomenclature |
|
|---|---|---|---|---|
| COI | 28S | |||
| T. vulgaris | KU243701 | ACUA135545 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243702 | KU243846 | ACUA135544 (non-type voucher) | genseq-4 COI, 28S |
| T. harrisi | KU243703 | ACUA135543 (paratype) | genseq-2 COI | |
| T. harrisi | KU243704 | ACUA146756 (paratype) | genseq-2 COI | |
| T. harrisi | KU243705 | ACUA138471 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243706 | ACUA141898 (holotype) | genseq-1 COI | |
| T. hitchensi | KU243707 | ACUA138472 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243708 | ACUA138473 (paratype) | genseq-2 COI | |
| T. dennetti | KU243709 | ACUA138469 (paratype) | genseq-2 COI | |
| T. dennetti | KU243710 | ACUA144021 (paratype) | genseq-2 COI | |
| T. vulgaris | KU243711 | ACUA138476 (non-type voucher) | genseq-4 COI | |
| T. dawkinsi | KU243712 | ACUA141897 (holotype) | genseq-1 COI | |
| T. vulgaris | KU243713 | ACUA138476 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243714 | ACUA138478 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243715 | ACUA141903 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243716 | ACUA138479 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243717 | ACUA141904 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243718 | ACUA138480 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243719 | ACUA138481 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243720 | ACUA138482 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243721 | ACUA141901 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243722 | ACUA141902 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243723 | ACUA141900 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243724 | ACUA138484 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243725 | KU243847 | ACUA138487 (non-type voucher) | genseq-4 COI, 28S |
| T. vulgaris | KU243726 | ACUA141899 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243727 | ACUA141905 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243728 | ACUA138486 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243729 | ACUA138488 (non-type voucher) | genseq-4 COI | |
| T. rectangulatus | KU243730 | ACUA138494 (holotype) | genseq-1 COI | |
| T. elongatus | KU243731 | ACUA138495 (holotype) | genseq-1 COI | |
| T. minimus | KU243732 | ACUA138489 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243733 | ACUA141906 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243734 | ACUA138490 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243735 | ACUA138491 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243736 | ACUA138492 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243737 | ACUA138493 (non-type voucher) | genseq-4 COI | |
| T. dennetti | KU243738 | ACUA143634 (paratype) | genseq-2 COI | |
| T. dennetti | KU243739 | ACUA141892 (paratype) | genseq-2 COI | |
| T. dennetti | KU243740 | ACUA141893 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243741 | KU243848 | ACUA141894 (paratype) | genseq-2 COI, 28S |
| T. vulgaris | KU243742 | KU243850 | ACUA142194 (non-type voucher) | genseq-4 COI, 28S |
| T. harrisi | KU243743 | ACUA141896 (paratype) | genseq-2 COI | |
| T. harrisi | KU243744 | ACUA143618 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243745 | ACUA143629 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243746 | ACUA143633 (non-type voucher) | genseq-4 COI | |
| T. hitchensi | KU243747 | ACUA141895 (paratype) | genseq-2 COI | |
| T. harrisi | KU243748 | ACUA143619 (paratype) | genseq-2 COI | |
| T. harrisi | KU243749 | ACUA143623 (paratype) | genseq-2 COI | |
| T. kirkwoodae | KU243750 | ACUA141885 (holotype) | genseq-1 COI | |
| T. americanus | KU243751 | ACUA141886 (non-type voucher) | genseq-4 COI | |
| T. americanus | KU243752 | KU243849 | ACUA141887 (non-type voucher) | genseq-4 COI, 28S |
| T. americanus | KU243753 | ACUA142195 (non-type voucher) | genseq-4 COI | |
| T. elongatus | KU243754 | ACUA141888 (paratype) | genseq-2 COI | |
| T. elongatus | KU243755 | KU243851 | ACUA141889 (paratype) | genseq-2 COI, 28S |
| T. elongatus | KU243756 | ACUA142196 (paratype) | genseq-2 COI | |
| T. elongatus | KU243757 | ACUA142197 (paratype) | genseq-2 COI | |
| T. minimus | KU243758 | ACUA141890 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243759 | ACUA142198 (non-type voucher) | genseq-4 COI | |
| T. elongatus | KU243760 | ACUA142199 (paratype) | genseq-2 COI | |
| T. kirkwoodae | KU243761 | KU243852 | ACUA142200 (non-type voucher) | genseq-4 COI, 28S |
| T. minimus | KU243762 | ACUA141891 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243763 | ACUA143643 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243764 | ACUA143644 (non-type voucher) | genseq-4 COI | |
| T. dennetti | KU243765 | ACUA143645 (holotype) | genseq-1 COI | |
| T. vulgaris | KU243766 | ACUA143646 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243767 | ACUA143647 (non-type voucher) | genseq-4 COI | |
| T. harrisi | KU243768 | KU243853 | ACUA143648 (paratype) | genseq-2 COI, 28S |
| T. dennetti | KU243769 | ACUA143649 (paratype) | genseq-2 COI | |
| T. deceptivus | KU243770 | KU243854 | ACUA143652 (holotype) | genseq-1 COI, 28S |
| T. oribatoides | KU243771 | KU243855 | ACUA143654 (non-type voucher) | genseq-4 COI, 28S |
| T. vulgaris | KU243772 | ACUA143655 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243773 | ACUA143657 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243774 | ACUA143658 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243775 | ACUA143659 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243776 | ACUA143661 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243777 | ACUA143664 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243778 | KU243856 | ACUA143665 (non-type voucher) | genseq-4 COI, 28S |
| T. deceptivus | KU243779 | ACUA143666 (paratype) | genseq-2 COI | |
| T. vulgaris | KU243780 | KU243857 | ACUA143667 (non-type voucher) | genseq-4 COI, 28S |
| T. vulgaris | KU243781 | ACUA143669 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243782 | ACUA143671 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243783 | ACUA146717 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243784 | ACUA146718 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243785 | ACUA146719 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243786 | ACUA146720 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243787 | ACUA146721 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243788 | ACUA146722 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243789 | ACUA146723 (non-type voucher) | genseq-4 COI | |
| T. rollerae | KU243790 | ACUA146727 (paratype) | genseq-2 COI | |
| T. rollerae | KU243791 | KU243858 | ACUA146724 (paratype) | genseq-2 COI, 28S |
| T. rollerae | KU243792 | ACUA146725 (holotype) | genseq-1 COI | |
| T. oblongatus | KU243793 | ACUA146726 (paratype) | genseq-2 COI | |
| T. oblongatus | KU243794 | ACUA146728 (holotype) | genseq-1 COI | |
| T. minimus | KU243795 | ACUA146729 (non-type voucher) | genseq-4 COI | |
| T. dennetti | KU243796 | ACUA146732 (paratype) | genseq-2 COI | |
| T. minimus | KU243797 | ACUA146733 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243798 | ACUA146734 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243799 | ACUA146735 (non-type voucher) | genseq-4 COI | |
| T. dawkinsi | KU243800 | ACUA146736 (paratype) | genseq-2 COI | |
| T. harrisi | KU243801 | ACUA146738 (paratype) | genseq-2 COI | |
| T. harrisi | KU243802 | ACUA146737 (paratype) | genseq-2 COI | |
| T. minimus | KU243803 | ACUA146739 (non-type voucher) | genseq-4 COI | |
| T. harrisi | KU243804 | ACUA146740 (paratype) | genseq-2 COI | |
| T. dawkinsi | KU243805 | ACUA146742 (paratype) | genseq-2 COI | |
| T. dawkinsi | KU243806 | KU243859 | ACUA146743 (paratype) | genseq-2 COI, 28S |
| T. dawkinsi | KU243807 | ACUA146744 (paratype) | genseq-2 COI | |
| T. dawkinsi | KU243808 | ACUA146745 (paratype) | genseq-2 COI | |
| T. dennetti | KU243809 | ACUA146746 (paratype) | genseq-2 COI | |
| T. harrisi | KU243810 | ACUA146747 (paratype) | genseq-2 COI | |
| T. harrisi | KU243811 | ACUA146748 (paratype) | genseq-2 COI | |
| T. minimus | KU243812 | ACUA146749 (non-type voucher) | genseq-4 COI | |
| T. harrisi | KU243813 | ACUA146750 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243814 | ACUA146751 (paratype) | genseq-2 COI | |
| T. harrisi | KU243815 | ACUA146752 (holotype) | genseq-1 COI | |
| T. harrisi | KU243816 | ACUA146753 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243817 | ACUA146754 (non-type voucher) | genseq-4 COI | |
| T. hitchensi | KU243818 | ACUA146755 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243819 | ACUA146756 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243820 | ACUA146757 (paratype) | genseq-2 COI | |
| T. hitchensi | KU243821 | ACUA146758 (non-type voucher) | genseq-4 COI | |
| T. dawkinsi | KU243822 | ACUA146759 (paratype) | genseq-2 COI | |
| T. minimus | KU243823 | ACUA146760 (non-type voucher) | genseq-4 COI | |
| T. hyporhynchus | KU243824 | ACUA146762 (holotype) | genseq-1 COI | |
| T. hyporhynchus | KU243825 | KU243860 | ACUA146763 (paratype) | genseq-2 COI, 28S |
| T. hyporhynchus | KU243826 | ACUA146764 (paratype) | genseq-2 COI | |
| T. americanus | KU243827 | ACUA146768 (non-type voucher) | genseq-4 COI | |
| T. smithi | KU243828 | KU243861 | ACUA146769 (holotype) | genseq-1 COI, 28S |
| T. smithi | KU243829 | ACUA146770 (paratype) | genseq-2 COI | |
| T. smithi | KU243830 | ACUA146772 (paratype) | genseq-2 COI | |
| T. oblongatus | KU243831 | ACUA146774 (paratype) | genseq-2 COI | |
| T. oblongatus | KU243832 | ACUA146775 (paratype) | genseq-2 COI | |
| T. oblongatus | KU243833 | ACUA146776 (paratype) | genseq-2 COI | |
| T. oblongatus | KU243834 | ACUA146777 (paratype) | genseq-2 COI | |
| D. oribatoides | KU243835 | ACUA146778 (non-type voucher) | genseq-4 COI | |
| D. oribatoides | KU243836 | ACUA146779 (non-type voucher) | genseq-4 COI | |
| D. oribatoides | KU243837 | ACUA146780 (non-type voucher) | genseq-4 COI | |
| D. oribatoides | KU243838 | KU243862 | ACUA146781 (non-type voucher) | genseq-4 COI, 28S |
| D. oribatoides | KU243839 | ACUA146778 (non-type voucher) | genseq-4 COI | |
| T. oblongatus | KU243840 | KU243863 | ACUA146782 (paratype) | genseq-2 COI, 28S |
| T. oblongatus | KU243841 | ACUA146783 (paratype) | genseq-2 COI | |
| T. dennetti | KU243842 | KU243864 | ACUA146784 (paratype) | genseq-2 COI, 28S |
| T. minimus | KU243843 | ACUA146785 (non-type voucher) | genseq-4 COI | |
| T. minimus | KU243844 | ACUA146786 (non-type voucher) | genseq-4 COI | |
| T. vulgaris | KU243845 | No voucher | No classification | |
Genomic DNA extraction was completed with Qiagen DNeasy Tissue Kits (Qiagen Inc.,Valencia, California). Amplifications of the target region of COI were performed with LCO1490 and HCO2198 (
Species delimitation results
Phylogenetic analysis of COI and 28S resulted in five well-supported (posterior probability greater than 95%) clades; however, analyses did not produce resolution at the base of Testudacarinae, resulting in a five-branched polytomy (Fig.
Testudacarinae molecular phylogeny and species complexes: (Left) combined 28S and COI Bayesian analysis resulting in a five branched soft polytomy (●: >95% posterior probability); monophyly tested across Torrenticolidae but not depicted; (A–E represent tree continuation in Figs
Three morphotypes (Testudacarus minimus, T. hitchensi, and T. elongatus) exhibited more intraspecific variation than expected, suggesting potential cryptic species. Further investigation of specimens identified morphological and biogeographic differences suggesting three Testudacarus minimus-like species, four T. hitchensi-like species, and three T. elongatus-like species. However, some of these “species” exhibit high intraspecies COI divergence with restricted geographic ranges and no diagnosable morphological variability, and should be the target of further research.
In summary, we find strong support through a combination of morphology, biogeography, and phylogenetic analysis of COI and 28S for 17 species sorted into four robustly supported species complexes. The following species complexes are proposed to better organize the subfamily: Testudacarus minimus complex, T. hitchensi complex, T. americanus complex, and T. elongatus complex. Each complex is treated below within the taxonomic descriptions.
Key to Testudacarinae species complexes
| 1 | Pedipalp four-segmented, anterior tips of coxae-I with projections | D. oribatoides |
| – | Pedipalp five-segmented, anterior tips of coxae-I without projections | 2 |
| 2 | Body elongate to rectangular | T. elongatus complex |
| – | Body oval | 3 |
| 3 | Body large (>700 µm female and >650 male dorsal length), dull coloration common; within and west of the Rocky Mountains | T. americanus complex (except T. rollerae) |
| – | Body small (<700 µm female and <650 male dorsal length), bright coloration (orange, red, violet, blue) common; present throughout North America | (T. minimus complex, T. hitchensi complex, T. rollerae)...4 |
| 4 | Anterio-medial platelet wide (>140 µm) and more than or nearly twice as wide as long | T. rollerae |
| – | Anterio-medial platelet unmodified (<140 µm) and far less than twice as wide as long | 5 |
| 5 | Anterio-medial and anterio-lateral platelets with consistent coloration (either colored or colorless across) | T. minimus complex |
| – | Anterio-lateral platelets with coloration and anterio-medial platelet colorless | T. hitchensi complex |
Taxonomy
Torrenticolidae
Note
See
Testudacarinae
Subfamilial diagnosis
For larval diagnosis see
Distribution
Testudacarines have been reported on many occasions outside of their original descriptions. Furthermore, the Canadian National Collection in Ottawa, Canada includes thousands of testudacarines collected from across most of North America (
Remarks
The three pairs of acetabula, coxae-IV condyles, and “generalized” pedipalps are plesiomorhphic states that clearly show testudacarines as retaining ancestral torrenticolid characteristics (
Testudacarine sexual dimorphism: female dorsal shield (A) and ventral shield (C) differing from male (B, D) by the following characters: 1) dorso-glandularia-4 positioned far closer to muscle scares; 2) area of secondary sclerotization always present (males rarely present; very small if present); 3) with shorter coxae-II+III midline; 4) genital field enveloped by coxal field; 5) larger and rounder body (males around 80% of female size); 6) excretory pore well separated from ventral line of secondary sclerotization.
Debsacarus
Type species
Debsacarus oribatoides (Habeeb, 1961).
Generic diagnosis
Debsacarus differ from all other Testudacarinae in having four-segmented pedipalps (instead of five) and projections on the anterio-tips of coxae-I. With the exception of Testudacarus hyporhynchus, Debsacarus differ from all other Testudacarinae in having an elongate gnathosoma and an extremely narrow gnathosomal bay that is covered dorsally and ends anterior to the leg-I insertion ventrally.
Distribution
Known from only two counties (Los Angeles and Monterey) in California.
Debsacarus oribatoides
Debsacarus oribatoides:
Testudacarus oribatoides:
Type series
Lectotype (1♀): California, USA: 1♀ from Los Angeles County, Coldbrook Guard Station, North Fork of San Gabriel River, 25 June 1961, by H Habeeb, HH610024; Paralectotype (1♂): California, USA: 1♂ from Los Angeles County, Coldbrook Guard Station, North Fork of San Gabriel River, 25 June 1961, by H Habeeb, HH610024.
Other material examined
Other (10♀, 8♂): California, USA: 1♂ from Monterey County, Salmon Falls Creek, beside Route 1 12.5 km south of Gorda (35°48'56.00"N, 121°21'30.00"W), 2 June 2010, by IM Smith, IMS100045; 5♀ and 3♂ from Monterey County, Los Padres National Forest, Lucia beside Ferguson-Nacimiento Road 5.6 km east of Route 1 (36°0'3.00"N, 121°28'31.00"W), 3 June 2010, by IM Smith, IMS100048; 1♀ and 3♂ from Monterey County, Los Padres National Forest, Lucia beside Nacimiento-Ferguson Road 11.3 km west of Nacimiento Campground (36°1'N, 121°27'W), 30 July 1987, by IM Smith, IMS8700119; 1♀ and 1♂ from Monterey County, Los Padres National Forest, Salmon Creek, beside Route 1 south of Gorda (35°49'N, 121°22'W), 29 July 1987, by IM Smith, IMS870118; 1♀ from Monterey County, Limekiln State Park, Hare Canyon Creek, near campground (36°0'41.00"N, 121°31'1.00"W), 6 September 2013, by JR Fisher, JRF13-0906-001; 1♀ from Monterey County, Salmon Creek, beside Route 1 south of Gorda (35°49'N, 121°22'W), 28 July 1987, by IM Smith, IMS870114A; 1♀ from Los Angeles County, Angeles National Forest, North Fork of San Gabriel River, off Route 39 (34°16'16.00"N, 117°50'46.00"W), 8 September 2013, by JR Fisher, JRF13-0908-001.
Type deposition
Lectotype (1♀), and paralectotype (1♂) deposited at
Redescription
Female (n=11) with characteristics of the genus with following specifications.
Gnathosoma (Fig.
Dorsum (Fig.
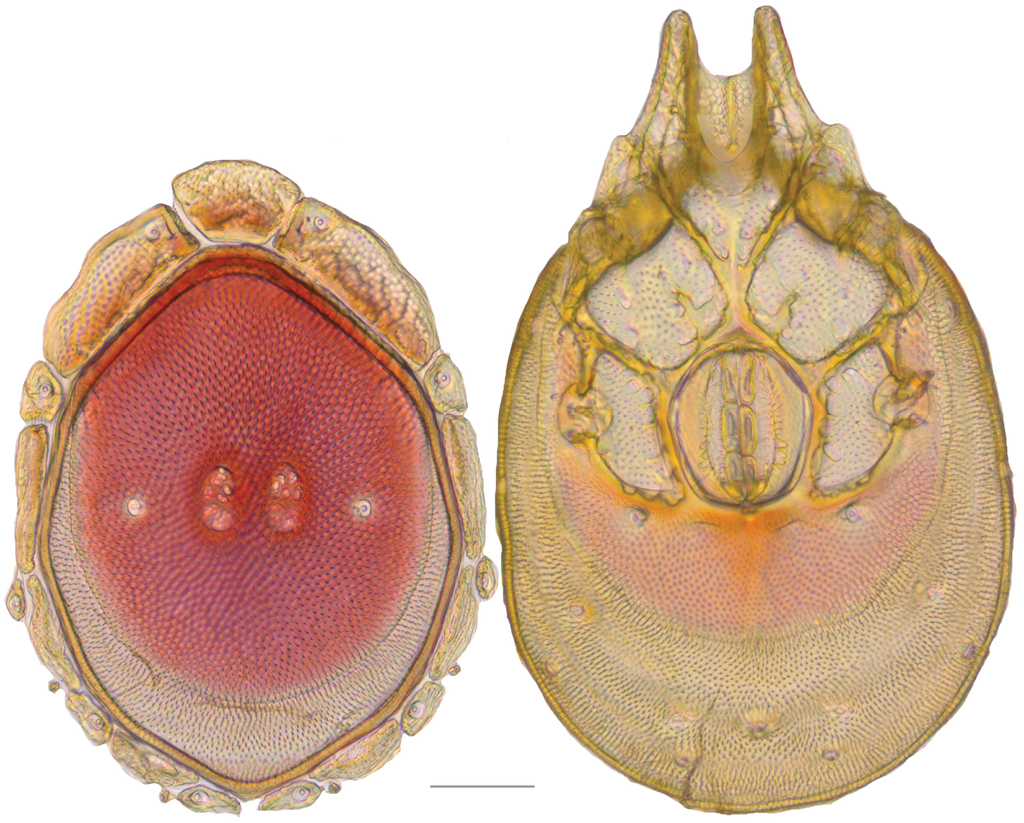
Debsacarus oribatoides molecular phylogeny: 28S and COI Bayesian analysis showing strong support single distinct clade (●: >95% posterior probability); clade exhibits <.6% divergence in COI within and >15% divergence between any other clade (not pictured); continuation of (E) lineage from Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [459–505 total; trochanter 54–62; basifemur 81–91; telofemur 63–68; genu 81–91; tibia 86–100; tarsus 84–95]. Leg-II [516–554 total; trochanter 62–65; basifemur 85–100; telofemur 63–71; genu 84–96; tibia 100–114; tarsus 106–115]. Leg-III [593–644 total; trochanter 63–69; basifemur 97–105; telofemur 70–78; genu 104–118; tibia 125–143; tarsus 130–142]. Leg-IV [779–862 total; trochanter 84–96; basifemur 118–127; telofemur 115–129; genu 141–166; tibia 160–181; tarsus 148–170].
Male (n=9) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma (Fig.
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [447–476 total; trochanter 59–63; basifemur 80–88; telofemur 61–68; genu 79–88; tibia 85–95; tarsus 80–92]. Leg-II [479–526 total; trochanter 54–67; basifemur 82–89; telofemur 60–72; genu 80–90; tibia 94–105; tarsus 105–114]. Leg-III [544–624 total; trochanter 56–66; basifemur 79–102; telofemur 65–74; genu 95–110; tibia 119–138; tarsus 120–137]. Leg-IV [743–857 total; trochanter 85–110; basifemur 107–125; telofemur 113–130; genu 134–160; tibia 152–177; tarsus 145–158].
Diagnosis
Same as genus.
Distribution
Same as genus.
Remarks
Debsacarus oribatoides show at least 15% COI divergence from all other Testudacarinae and less than .6% divergence from one another (Fig.
Testudacarus
*
Type species
Testudacarus tripeltatus Walter, 1928
Generic diagnosis
Members of this genus, unlike Debsacarus, lack projections on the anterior tips of coxae-I and have five-segmented pedipalps (instead of four). Furthermore, with the exception of Testudacarus hyporhynchus, they differ from Debsacarus in having a rounded gnathosoma (rather than elongate) and a wide gnathosomal bay that is uncovered dorsally and ventrally ends posterior to the leg-I insertion.
Distribution
Same as subfamily.
Testudacarus minimus complex
Species complex diagnosis.These species can be distinguished from most other testudacarines by their small size (female and male dorsal length less than 700 and 600 µm, respectively), highly variable coloration (red, orange, blue, violet, and rarely colorless), and small (<140 µm), rounded anterio-medial platelet (differing from Testudacarus rollerae, which has a large (>140 µm) anterio-medial platelet more than or nearly twice as wide as long). Additionally, only this complex and the T. hitchensi complex are present east of the Great Plains. These two complexes resemble each other morphologically in many respects, but can be easily distinguished because members of this complex have uniform coloration across all three anterior platelets while T. hitchensi-like mites have a colorless anterio-medial platelet and colored anterio-lateral platelets. With the exception of T. radwellae, males of this complex differ from T. hitchensi-like mites in having dorso-glandularia-4 positioned less anterior to and more lateral to the muscle scars. This complex is abundant and present across most of North America and comprises the following species: T. deceptivus, T. minimus, T. radwellae, and T. vulgaris.
Remarks. Molecular data show strong support for three distinct clades (Fig.
Testudacarus minimus complex molecular phylogeny: 28S and COI Bayesian analysis showing strong support for a soft polytomy with three distinct clades (●: >95% posterior probability); colored clades exhibit <2.5% divergence in COI within and >6.5% divergence between; continuation of (A) lineage from Fig.
Testudacarus minimus
Testudacarus minimus:
Testudacarus americanus:
Testudacarus americanus minimus:
Type series
Holotype (1♂): California, USA: 1♂ from Santa Cruz County, Waddell Creek, 30–31 August 1933, by PR Needham, RM330016.
Other material examined
Other (15♀, 15♂): Montana, USA: 2♂ from Ravalli County, Bitterroot National Forest, Lost Horse River, downstream of confluence of North Lost Horse (45°7'7.00"N, 114°18'0.00"W), 3 August 2012, by JR Fisher and WA Nelson, ROW12-0803-006; 1♂ from Powell County, Monture Creek, at fishing access off Highway 200 west of Ovando (47°2'15.00"N, 112°13'12.00"W), 9 August 2012, by AJ Radwell and JA Hinsey, AJR12-0809-415A; Washington, USA: 2♂ from Snohomish County, Mount Baker National Forest, Clean Creek, (48°13'8.00"N, 121°34'7.00"W), 28 July 2013, by JC O’Neill and WA Nelson, JNOW13-0728-007; 2♀ from Jefferson County, Olympic National Forest, Snow Creek, (47°56'11.00"N, 122°56'53.00"W), 22 July 2013, by WA Nelson and JC O’Neill, JNOW13-0722-001; 2♀ from Grays Harbor County, Capitol State Forest, Porter Creek, (46°58'13.00"N, 123°16'2.00"W), 25 July 2013, JC O’Neill and WA Nelson, JNOW13-0725-005; 1♀ from Skamania County, Gifford Pinchot National Forest, Lewis Creek, (46°7'40.00"N, 121°59'24.00"W), 1 August 2013, by JC O’Neill and WA Nelson, JNOW13-0801-004; California, USA: 1♂ from Inyo County, Inyo National Forest, Bishop Creek, downstream of campground (37°17'23.00"N, 118°33'14.00"W), 2 September 2013, by JR Fisher, JRF13-0902-003; 2♀ from Nevada County, Tahoe National Forest, Sagehen Creek, off Route 89 (39°26'2.00"N, 120°12'17.00"W), 26 August 2013, by JR Fisher, JRF13-0826-006; 1♀ from Siskiyou County, Klamath National Forest, Shadow Creek, off Cecilville Road, (41°12'13.00"N, 123°4'18.00"W), 17 August 2013, by JR Fisher, JRF13-0817-002; Wyoming, USA: 1♂ from Albany County, North Fork of Little Laramie River, at bridge on Highway 130 (41°19'42.00"N, 106°9'42.00"W), 3 August 2012, by AJ Radwell and JA Hinsey, AJR12-0803-406; 2♂ from Albany County, South Clear Creek, across from Southfork Campground on Highway 16 (44°16'36.00"N, 106°57'4.00"W), 14 August 2012, by AJ Radwell and JA Hinsey, AJR12-0814-419; 1♀ from Fremont County, Wind River, off County Road 773 30 miles east of Moran on Highway 26/287 (43°43'5.00"N, 110°48'0.00"W), 5 August 2012, by AJ Radwell and JA Hinsey, AJR12-0805-410; Utah, USA: 2♂ from Cache County, Wasatch-Cache National Forest, Jordan River, (41°44'33.00"N, 111°45'57.00"W), 24 July 2012, by JR Fisher and WA Nelson, ROW12-0724-004; Idaho, USA: 2♂ from Blaine County, Sawtooth National Forest, Baker Creek, (43°45'28.00"N, 114°33'44.00"W), 28 July 2012, by JR Fisher and WA Nelson, ROW12-0728-001; 2♂ from Lemhi County, Salmon National Forest, Niapas Creek at confluence with Panther Creek, (45°8'15.00"N, 114°13'4.00"W), 2 August 2012, by JR Fisher and WA Nelson, ROW12-0802-003; Colorado, USA: 1♀ from Gunnison County, Quartz Creek, north of Ohio City on County Road 76 mile marker 11 (38°34'2.00"N, 106°34'6.00"W), 1 August 2012, by AJ Radwell and JA Hinsey, AJR12-0801-403A; Oregon, USA: 1♀ from Tillamook County, Siuslaw National Forest, Alder Creek, (45°9'27.00"N, 123°47'60.00"W), 6 August 2013, by JC O’Neill, JNOW13-0806-002; 1♀ from Lane County, Gate Creek, (44°8'48.00"N, 122°34'20.00"W), 11 August 2013, by JC O’Neill and WA Nelson, JNOW 13-0811-001; 1♀ from Curry County, Rogue River National Forest, Elk River, off National Forest Road 5325 (42°42'46.00"N, 124°18'41.00"W), 13 August 2013, by JR Fisher, JRF13-0813-003; Arizona, USA: 1♀ from Cochise County, Chirichua Mountains west of Portal, East Turkey Creek, off Forest Road 42 above junction with Forest Road 42B (31°54'32.00"N, 109°15'11.00"W), 15 May 2011, by IM Smith, IMS110003; 1♀ from Cochise County, Chiricahua Mountains west of Portal, East Turkey Creek, off Forest Road 42 just above junction with Forest Road 42B (31°54'32.00"N, 109°15'11.00"W), 15 May 2011, by IM Smith, IMS110004.
Type deposition
Holotype (1♂) deposited at the
Diagnosis
Testudacarus minimus most resemble T. vulgaris and T. deceptivus. Throughout the majority of their shared range in the west, T. minimus are orange to red and T. vulgaris are violet to blue. While these two species have overlapping size ranges, T. minimus are generally larger. Testudacarus vulgaris females rarely exhibit a dorsal length over 600 µm and males rarely exceed 500 µm while T. minimus females and males are usually larger than 600 and 500 µm, respectively. Testudacarus deceptivus have only been found in two counties in California and cannot be distinguished from either T. minimus or T. vulgaris using morphology. Testudacarus minimus are the only members of their complex that have been found in Washington and northern Oregon.
Redescription
Female (n=14) with characteristics of the genus with following specifications.
Gnathosoma — Subcapitulum [154–173 ventral length; 96–108 dorsal length; 90–105 tall] elliptic to ovoid with short rostrum. Chelicerae [133–152 long] unmodified with slightly curved fangs [29–32 long]. Pedipalp [181–202 long] unmodified. Trochanter [25–30 long; 30–35 wide]. Femur [49–58 long; 38–42 wide]. Genu [38–42 long; 32–35 wide]. Tibia [45–52 long; 22–25 wide]. Tarsus [19–23 long; 9–12 wide].
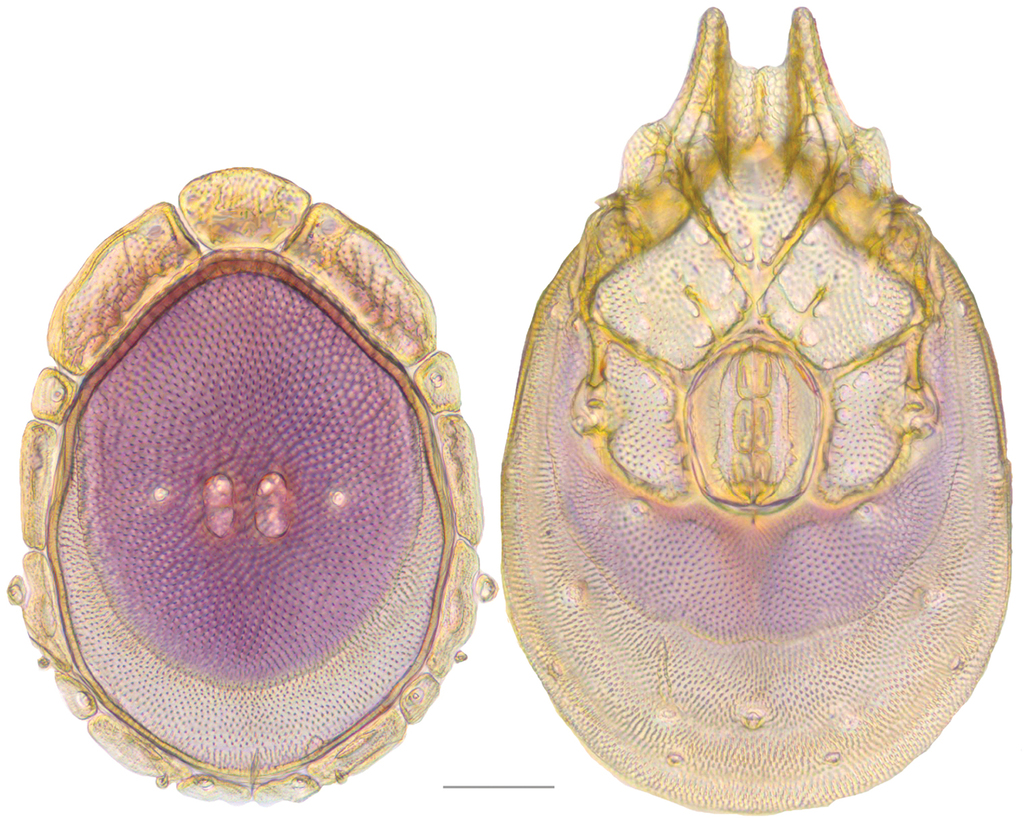
Dorsum (Fig.
Venter (Fig.
Legs — colorless, or with same color as dorsal plate. Total leg and podomere lengths as follows: Leg-I [428–477 total; trochanter 48–55; basifemur 72–85; telofemur 60–69; genu 78–90; tibia 83–95; tarsus 79–92]. Leg-II [453–530 total; trochanter 54–62; basifemur 74–87; telofemur 58–68; genu 83–96; tibia 96–110; tarsus 99–113]. Leg-III [440–625 total; trochanter 55–65; basifemur 76–88; telofemur 64–76; genu 106–117; tibia 120–137; tarsus 131–148]. Leg-IV [677–843 total; trochanter 87–97; basifemur 106–120; telofemur 111–122; genu 146–160; tibia 160–173; tarsus 147–180].
Male (n=16) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [138–164 ventral length; 88–105 dorsal length; 83–93 tall]. Chelicerae [120–145 long]. Fangs [27–30 long]. Pedipalp [181–206 long]. Trochanter [24–32 long; 28–33 wide]. Femur [48–59 long; 35–40 wide]. Genu [38–46 long; 29–34 wide]. Tibia [43–54 long; 19–25 wide]. Tarsus [16–22 long; 9–12 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [435–483 total; trochanter 53–63; basifemur 75–84; telofemur 57–69; genu 78–89; tibia 82–93; tarsus 80–90]. Leg-II [458–518 total; trochanter 52–64; basifemur 75–87; telofemur 59–69; genu 79–90; tibia 92–104; tarsus 96–109]. Leg-III [530–599 total; trochanter 54–62; basifemur 75–88; telofemur 63–72; genu 97–111; tibia 114–133; tarsus 124–137]. Leg-IV [722–813 total; trochanter 81–95; basifemur 102–122; telofemur 103–118; genu 130–159; tibia 150–167; tarsus 145–158].
Distribution
Abundant throughout North America, ranging from the Pacific Northwest to the southwestern United States (and potentially into northern Mexico), and east into the western Great Plains.
Remarks
Commonly colorless or orange in the southwestern United States; red, pink, or orange–red in the northwest, Rocky Mountains, and western Great Plains; and uncommonly red–violent in the northwest, Rocky Mountains, and western Great Plains.
Testudacarus vulgaris
Testudacarus vulgaris:
Testudacarus american vulgaris:
Testudacarus minimus vulgaris:
Type series
Syntypes (1♀, 1♂): New Brunswick, Canada: from Victoria County, Salmon River, 21 June 1953, by H. Habeeb, 87-53
Other material examined
Other (18♀, 19♂): Ontario, Canada: 1♀ and 1♂ from Lennox and Addington County, Hydes Creek, beside Highway 41 23.7km north of Highway 28 at Denbigh (45°11'22.00"N, 77°13'38.00"W), 29 April 2010, by IM Smith, IMS100023; 1♀ from Hastings County, Maple Leaf and Papineau Creek, east of Davis Road before Highway 62, 18 August 2011, by IM Smith, IMS110053; New Brunswick, Canada: 2♀ and 1♂ from Victoria County, Little Wapske River, Plaster Rock beside Highway108 20.5km east of Highway109, 5 September 2011, by IM Smith, IMS110061; Nova Scotia, Canada: 1♂ from Inervess County, Cheticamp River, 10 September 2011, by IM Smith, IMS110071; Tennessee, USA: 1♀ and 1♂ from Monroe County, Turkey Creek, beside Forest Road #210 just east of Forest Road #35 7.1km southeast of Route 165 (35°20'28.00"N, 84°11'30.00"W), 12 September 2009, by IM Smith, IMS090110; 2♂ from Sevier County, Great Smoky Mountains Nation Park, Rhododendron Creek, beside Greenbrier Road 2.2 km south of Route 321 (35°43'32.00"N, 83°24'2.00"W), 2 September 2009, by IM Smith, IMS090093; North Carolina, USA: 2♀ and 1♂ from Haywood County, Great Smoky Mountains National Park, Big Creek, Waterville Big Creek Picnic Area (35°44'59.00"N, 83°6'42.00"W), 16 September 2010, by IM Smith, IMS100138; 1♀ and 1♂ from Haywood County, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road near bridge 1.7km north of road to campground (35°38'45.00"N, 83°4'34.00"W), 6 September 2009, by IM Smith, IMS090099; 1♀ from Haywood County, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road near bridge 1.7km north of road to campground (35°38'45.00"N, 83°4'32.00"W), 20 September 2010, by IM Smith, IMS100150; South Dakota, USA: 1♀ and 1♂ from Lawrence County, Jim Creek, south of Nemo Road on Goodhope Road behind cab at Green Mountain Black Hills (44°9'9.00"N, 103°28'51.00"W), 15 August 2012, by AJ Radwell and JA Hinsey, AJR12-0815-421; Colorado, USA: 1♂ from San Miguel County, San Miguel River, beside Route 145 12.5km northwest of junction with road to Telluride (37°59'17.00"N, 107°59'34.00"W), 31 July 2012, by AJ Radwell and JA Hinsey, AJR12-0731-400; Pennsylvania, USA: 1♂ from Fayette County, Ohiophyle State Park, Laurel Run, fishing access #2 off T798 (Meadow Run Road) (39°50'58.00"N, 79°30'51.00"W), 10 August 2014, by MJ Skvarla, MS14-0810-005; 2♀ and 2♂ from Fayette County, State Game Lands #51, Dunbar Creek, off Furnace Hill Road East of Dunbar (39°56'16.10"N, 79°35'3.70"W), 10 August 2014, by MJ Skvarla, MS14-0810-002; California, USA: 1♂ from Monterey County, Andrew Molera State Park, Big Sur River, off Route 1 (36°16'31.00"N, 121°49'14.00"W), 4 September 2013, by JR Fisher, JRF13-0904-003; 1♂ from Inyo County, Inyo National Forest, Bishop Creek, downstream of campground (37°17'23.00"N, 118°33'14.00"W), 2 September 2013, by JR Fisher, JRF13-0902-003; 1♂ from Alpine County, Markleeville Creek, off Route 89 downstream of bridge (38°41'39.00"N, 119°46'41.00"W), 30 August 2013, by JR Fisher, JRF13-0830-001; 1♂ from Mendocino County, Jackson Demonstration State Park, North Fork of Big River, (39°20'46.00"N, 123°30'35.00"W), 22 August 2013, by JR Fisher, JRF13-0822-002; 1♀ from Mono County, Humboldt-Toiyabe National Forest, Little Walker River, off Route 108 downstream of tunnel (38°20'57.00"N, 119°27'15.00"W), 31 August 2013, by JR Fisher, JRF13-0831-002; 1♀ from Trinity County, Shasta-Trinity National Forest, North Fork of Trinity River, (40°46'47.00"N, 123°7'46.00"W), 18 August 2013, JRF13-0818-005; Oregon, USA: 2♂ from Douglas County, Umpqua National Forest, Calf Creek, (43°17'28.00"N, 122°37'12.00"W), 12 August 2013, by JC O’Neill and WA Nelson, JNOW13-0812-006; Utah, USA: 2♀ from Utah County, Uinta National Forest, Hobble Creek, just upstream on right fork Hobble Creek Road from Cherry Campground (40°10'9.00"N, 111°28'26.00"W), 22 July 2012, by JR Fisher and WA Nelson, ROW12-0722-001; Idaho, USA: 1♀ from Fremont County, Targhee National Forest, Rock Creek, downstream of tributary (44°6'44.00"N, 111°15'4.00"W), 25 July 2012, by JR Fisher and WA Nelson, ROW12-0725-001; Arkansas, USA: 1♀ from Searcy County, Tomahawk Creek, (36°1'20.00"N, 92°40'43.00"W), 20 July 2009, by AJ Radwell, AJR090101.
Type deposition
Syntypes (1♀, 1♂) deposited at the
Diagnosis
Testudacarus vulgaris most resemble T. minimus and T. deceptivus. Throughout the majority of their shared range in the west, T. minimus are orange to red and T. vulgaris are violet to blue. While these two species have overlapping size ranges, T. minimus are generally larger. Testudacarus vulgaris females rarely exhibit a dorsal length over 600 µm and males rarely exceed 500 µm while T. minimus females and males are usually larger than 600 and 500 µm, respectively. Testudacarus deceptivus have only been found in two counties in California and cannot be distinguished from either T. minimus or T. vulgaris using morphology. Testudacarus vulgaris are the only members of their complex that have been found east of the Great Plains.
Redescription
Female (n=18) with characteristics of the genus with following specifications.
Gnathosoma — Subcapitulum [151–190 ventral length; 90–114 dorsal length; 84–115 tall] elliptical to ovoid with short rostrum. Chelicerae [133–170 long] unmodified with lightly curved fangs [28–35 long]. Pedipalp [169–211 long] unmodified. Trochanter [23–32 long; 28–37 wide]. Femur [46–62 long; 33–45 wide]. Genu [33–42 long; 28–36 wide]. Tibia [42–53 long; 19–26 wide]. Tarsus [18–23 long; 9–12 wide].
Dorsum (Fig.
Venter (Fig.
Legs — colorless, or with same color as dorsal plate. Total leg and podomere lengths as follows: Leg-I [401–497 total; trochanter 50–61; basifemur 74–85; telofemur 55–72; genu 72–96; tibia 75–97; tarsus 78–97]. Leg-II [417–564 total; trochanter 51–63; basifemur 71–92; telofemur 57–72; genu 75–100; tibia 92–118; tarsus 96–120]. Leg-III [513–664 total; trochanter 55–68; basifemur 71–96; telofemur 58–82; genu 91–124; tibia 112–147; tarsus 124–157]. Leg-IV [726–911 total; trochanter 85–105; basifemur 103–132; telofemur 99–138; genu 134–174; tibia 145–177; tarsus 148–185].
Male (n=17) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [128–155 ventral length; 83–96 dorsal length; 78–95 tall]. Chelicerae [115–145 long]. Fangs [25–29 long]. Pedipalp [156–190 long]. Trochanter [22–28 long; 28–33 wide]. Femur [42–55 long; 32–42 wide]. Genu [32–41 long; width 25–32 wide]. Tibia [43–52 long; 19–23 wide]. Tarsus [16–21 long; 9–11 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [402–452 total; trochanter 49–59; basifemur 67–80; telofemur 53–63; genu 70–82; tibia 75–88; tarsus 78–88]. Leg-II [421–488 total; trochanter 51–61; basifemur 68–81; telofemur 51–63; genu 73–86; tibia 84–96; tarsus 91–105]. Leg-III [501–552 total; trochanter 52–61; basifemur 72–82; telofemur 59–68; genu 89–100; tibia 105–119; tarsus 118–130]. Leg-IV [664–746 total; trochanter 79–90; basifemur 95–106; telofemur 92–108; genu 124–144; tibia 130–155; tarsus 129–150].
Distribution
Abundant throughout the majority of North America. Unreported in Washington and northern Oregon.
Remarks
Commonly orange and uncommonly violet in the southwestern United States; commonly violet or blue and uncommonly red–violet in the Rocky Mountains and Great Plains; commonly violet or blue east of the Great Plains.
Testudacarus deceptivus , sp. n.
Type series
Holotype (1♀): California, USA: 1♀ from Los Angeles County, Angeles National Forest, North Fork of San Gabriel River, off Route 39 (34°16'16.00"N, 117°50'46.00"W), 8 September 2013, by JR Fisher, JRF13-0908-001 (Specimen 143652 – DNA#2078); Paratype (1♂): California, USA: (allotype) 1♂ from Sierra County, Tahoe National Forest, Milton Creek near confluence of North Yuba River, (39°34'4.00"N, 120°36'54.00"W), 25 August 2013, by JR Fisher, JRF13-0825-004 (Specimen 143666 – DNA#2091)
Type deposition
Holotype (1♀) and allotype (1♂) deposited at the
Diagnosis
Testudacarus deceptivus have only been found in two counties (Los Angeles and Sierra) in California and cannot be distinguished from either T. minimus or T. vulgaris using morphology.
Description. Female (n=1) with characteristics of the genus with following specifications.
Gnathosoma — Subcapitulum [174 ventral length; 104 dorsal length; 90 tall] elliptical to ovoid with short rostrum and colorless. Chelicerae [144 long] unmodified with lightly curved fangs [32 long]. Pedipalp [190 long] unmodified. Trochanter [28 long; 29 wide]. Femur [53 long; 42 wide]. Genu [39 long; 32 wide]. Tibia [50 long; 23 wide]. Tarsus [19 long; 10 wide].
Dorsum (Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [480 total; trochanter 62; basifemur 80; telofemur 64; genu 91; tibia 92; tarsus 90]. Leg-II [519 total; trochanter 63; basifemur 83; telofemur 69; genu 94; tibia 104; tarsus 106]. Leg-III [615 total; trochanter 63; basifemur 85; telofemur 72; genu 115; tibia 133; tarsus 145]. Leg-IV [821 total; trochanter 93; basifemur 112; telofemur 122; genu 161; tibia 178; tarsus 155].
Male (n=1) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [139 ventral length; 90 dorsal length; 83 tall]. Chelicerae [125 long]. Fangs [29 long]. Pedipalp [179 long]. Trochanter [26 long; 29 wide]. Femur [48 long; 35 wide]. Genu [40 long; width 29 wide]. Tibia [44 long; 23 wide]. Tarsus [20 long; 10 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [413 total; trochanter 51; basifemur 69; telofemur 61; genu 73; tibia 79; tarsus 78]. Leg-II [462 total; trochanter 60; basifemur 75; telofemur 59; genu 80; tibia 94; tarsus 93]. Leg-III [517 total; trochanter 56; basifemur 73; telofemur 65; genu 95; tibia 111; tarsus 116]. Leg-IV [688 total; trochanter 76; basifemur 97; telofemur 97; genu 132; tibia 146; tarsus 138].
Etymology
Specific epithet deceptivus (decept-, L. deceptive) refers to the lack of morphological characters differentiating this species from related species.
Distribution
Known from only two counties (Los Angeles and Sierra) in California.
Testudacarus radwellae , sp. n.
Type series
Holotype (1♀): Arkansas, USA: 1♀ from Montgomery County, Ouachita National Forest, Collier Springs, at spring structure picnic area (34°29'7.04"N, 93°35'38.12"W), 11 November 2009, by AJ Radwell, AJR090317C (Specimen 144016); Paratypes (1♀, 7♂): Arkansas, USA: (allotype) 1♂ from Montgomery County, Ouachita National Forest, Collier Springs, at spring structure picnic area (34°29'7.04"N, 93°35'38.12"W), 29 July 2011, by AJ Radwell and B Crump, AJR110301 (Specimen 144011); 4♂ from Montgomery County, Ouachita National Forest, Collier Springs, at spring structure picnic area (34°29'7.04"N, 93°35'38.12"W), 29 July 2011, by AJ Radwell and B Crump, AJR110301; 1♂ from Polk County, Ouachita National Forest, upper small pond on stream running along trail (34°27'36.73"N, 93°59'52.38"W), 21 July 2008, by AJ Radwell, AJR080303A; 1♂ from Montgomery County, Ouachita National Forest, Collier Springs, picnic area beside Forest Road 177 (34°29'3.00"N, 93°35'35.00"W), 19 September 2008, by IM Smith, IMS080061A; 1♀ from Montgomery County, Ouachita National Forest, Collier Springs, at spring structure picnic area (34°29'7.04"N, 93°35'38.12"W), 11 November 2009, by AJ Radwell, AJR090317C.
Type deposition
Holotype (1♀), allotype (1♂), and three paratypes (3♂) deposited at the
Diagnosis
Testudacarus radwellae and T. vulgaris are the only testudacarines known to occur in Arkansas. Testudacarus radwellae are conspicuously violet over the entirety of their body, whereas the violet coloration of T. vulgaris is less vibrant and often absent, particularly on the platelets, legs, and secondary sclerotization of the venter. Males of T. radwellae also have dorsal-glandularia-4 located far lateral to the muscle scars, unlike others in the complex.
Description
Female (n=2) with characteristics of the genus with following specifications.
Gnathosoma — Subcapitulum [153–155 ventral length; 117–133 dorsal length; 88–97 tall] ovoid with short rostrum. Chelicerae [148–156 long] unmodified with lightly curved fangs [28–29 long]. Pedipalp [177–187 long] unmodified and violet. Trochanter [27–30 long; 26–29 wide]. Femur [46–51 long; 35–38 wide]. Genu [38–42 long; 27–28 wide]. Tibia [44–49 long; 19–20 wide]. Tarsus [18–19 long; 10–11 wide].
Dorsum (Fig.
Venter (Fig.
Legs — violet. Total leg and podomere lengths as follows: Leg-I [464–466 total; trochanter 57–58; basifemur 81–82; telofemur 65–68; genu 83–84; tibia 88–90; tarsus 86–87]. Leg-II [489–490 total; trochanter 54–55; basifemur 81–83; telofemur 64–66; genu 86–87; tibia 97–101; tarsus 102–105]. Leg-III [559–564 total; trochanter 57–58; basifemur 77–85; telofemur 73–76; genu 102–105; tibia 116–117; tarsus 126–130]. Leg-IV [760–767 total; trochanter 86–87; basifemur 107–108; telofemur 108–109; genu 145–146; tibia 158–159; tarsus 152–159].
Male (n=7) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [132–143 ventral length; 85–90 dorsal length; 81–86 tall]. Chelicerae [107–115 long]. Fangs [25–28 long]. Pedipalp [170–181 long]. Trochanter [25–27 long; 28–30 wide]. Femur [45–52 long; 33–35 wide]. Genu [38–39 long; width 27–29 wide]. Tibia [45–50 long; 18–21 wide]. Tarsus [14–17 long; 8–11 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [440–454 total; trochanter 53–58; basifemur 76–80; telofemur 58–67; genu 75–80; tibia 84–89; tarsus 82–90]. Leg-II [464–478 total; trochanter 52–57; basifemur 75–80; telofemur 58–62; genu 78–86; tibia 94–97; tarsus 99–103]. Leg-III [512–535 total; trochanter 49–55; basifemur 74–83; telofemur 62–69; genu 93–96; tibia 106–116; tarsus 110–125]. Leg-IV [699–726 total; trochanter 77–87; basifemur 101–110; telofemur 99–108; genu 130–133; tibia 144–148; tarsus 133–147].
Etymology
Specific epithet radwellae after the late Dr Andrea J. Radwell, the American water mite researcher who collected the specimens needed for this description. Dr Radwell collaborated with us on the larger torrenticolid project as a whole, giving us invaluable advice and mentorship. Without her, large portions of this project would not have been possible. She is dearly missed.
Distribution
Reported from only two counties (Polk and Montgomery) in Arkansas.
Testudacarus hitchensi complex
Species complex diagnosis. Only this complex and the T. minimus complex are present east of the Great Plains. These two complexes resemble each other morphologically in many respects, but can be easily distinguished because members of this complex have non-uniform coloration across all three anterior platelets (colorless anterio-medial platelet and colored anterio-lateral platelets) while T. minimus-like mites possess uniform coloration across all three platelets. Males of this complex differ from T. minimus-like mites in having dorso-glandularia-4 positioned more anterior to and less lateral to the muscle scars. These mites are common in eastern United States and rare in eastern Canada and Florida, small (female and male dorsal length less than 700 and 600 µm, respectively), and violet to blue in color. This complex comprises the following species: T. harrisi, T. dennetti, T. dawkinsi, and T. hitchensi.
Remarks. Distinguishable morphological characters can be found for four lineages while genetic data indicates more diversity (Fig.
Testudacarus hitchensi complex molecular phylogeny: 28S and COI Bayesian analysis showing strong support for at least four distinct clades, but suggesting more (●: >95% posterior probability); excepting green clade, clades exhibit <1.5% divergence in COI within and >6% between; green clade exhibits <4.5% within and >9.5% between other clades; specimens in red constitute additional suspected species based on genetic data, but lack morphological or distributional variation from green clade; continuation of (B) lineage from Fig.
Testudacarus hitchensi , sp. n.
Type series
Holotype (1♀): North Carolina, USA: 1♀ from Haywood, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road at Hannah Hoglen Cemetery site (35°38'29.00"N, 83°3'22.00"W), 22 September 2010, by IM Smith, IMS100154 (Specimen 141898 – DNA#1493); Paratypes (9♀, 10♂): North Carolina, USA: (allotype) 1♂ from Haywood, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road at Hannah Hoglen Cemetery site (35°38'29.00"N, 83°3'22.00"W), 22 September 2010, by IM Smith, IMS100154 (Specimen 146756 – DNA#2171); 1♀ and 2♂ from Haywood, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road at Hannah Hoglen Cemetery site (35°38'29.00"N, 83°3'22.00"W), 22 September 2010, by IM Smith, IMS100154; 2♀ and 1♂ from Haywood County, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road near bridge 1.7km north of road to campground (35°38'45.00"N, 83°4'32.00"W), 20 September 2010, by IM Smith, IMS100150; 2♂ from Macon County, Rainbow Springs, beside Forest Road 67 4.4 km south of Standing Indian Campground (35°3'6.00"N, 83°30'45.00"W), 1 July 2006, by IM Smith, IMS060040; 2♂ from Yancey County, Pisgah National Forest, South Toe River, Lost Cove beside Toe River Road (Forest Road 472) 0.4km east of Forest Road 2074 (35°45'0.00"N, 82°12'53.00"W), 9 September 2007, IM Smith, IMS070059; 1♀ from Yancey County, Pisgah National Forest, South Toe River, Lost Cove Picnic Area beside Toe River Road (Forest Road 472) 2.8 km east of Route 80 (35°45'13.00"N, 82°12'42.00"W), 27 September 2009, by IM Smith, IMS090127; Tennessee, USA: 1♂ from Monroe, beside Forest Route #35 2.3km northeast of road from Route 165 to Miller Chapel Baptist Church (35°21'47.00"N, 84°9'47.00"W), 12 September 2009, by IM Smith, IMS090112; 3♀ and 1♂ from Sevier County, Great Smoky Mountains National Park, Bullhead Branch, Sugarlands Nature Trail off Route 441/71 (35°40'47.00"N, 83°31'52.00"W), 7 September 2009, by IM Smith, IMS090101; 1♀ from Sevier County, Great Smoky Mountains National Park, Bullhead Branch, Sugarlands Nature Trail off Route 441/71 (35°40'48.00"N, 83°31'53.00"W), 3 September 2009, by IM Smith, IMS090095; Georgia, USA: 1♀ from Floyd County, beside road from Everrett Springs to Villanow 1.4 km south of The Pocket Recreation Area, 4 July 1990, by IM Smith, IMS900077.
Paratypes examined but measurements not included
(1♀, 2♂): North Carolina, USA: 1♀ from Haywood County, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road near bridge 1.7km north of road to campground (35°38'45.00"N, 83°4'32.00"W), 20 September 2010, by IM Smith, IMS100150; 1♂ from Haywood County, Great Smoky Mountains National Park, tributary of Hemphill Creek, Appalachian Highlands Science Learning Center near Ferguson Cabin site, (35°34'56.00"N, 83°4'30.00"W), 21 September 2010, by IM Smith, IMS100153; Tennessee, USA: 1♀ from Sevier County, Great Smoky Mountains National Park, Catron Branch, Elkmont Road off Little River Road (35°39'51.00"N, 83°35'19.00"W), 24 September 2010, IMS100156.
Type deposition
Holotype (1♀), allotype (1♂), and eight paratypes (4♀, 4♂) deposited at
Diagnosis
These mites differ from all others in the complex in having large medial pores on the dorsal plate surrounded by a ring of smaller pores (all pores uniform in other species). Males also have a “bleached” or colorless area posterior to the coxal plate that is colored in other members of the complex.
Description
Female (n=10) with characteristics of genus with following specifications.
Gnathosoma — Subcapitulum [165–175 ventral length; 99–106 dorsal length; 90–100 tall] ovoid with short rostrum. Chelicerae [139–150 long] unmodified with lightly curved fangs [29–32 long]. Pedipalp [192–205 long] unmodified. Trochanter [25–28 long; 29–32 wide]. Femur [54–57 long; 37–40 wide]. Genu [40–46 long; 29–33 wide]. Tibia [51–55 long; 20–23 wide]. Tarsus [19–21 long; 10–11 wide].
Dorsum (Fig.
Venter (Fig.
Legs — orange and restricted violet to blue. Total leg and podomere lengths as follows: Leg-I [473–524 total; trochanter 60–62; basifemur 83–93; telofemur 65–76; genu 86–95; tibia 92–105; tarsus 83–95]. Leg-II [501–552 total; trochanter 54–63; basifemur 83–93; telofemur 65–72; genu 88–99; tibia 101–111; tarsus 102–115]. Leg-III [586–635 total; trochanter 61–65; basifemur 89–100; telofemur 70–80; genu 105–113; tibia 122–137; tarsus 132–144]. Leg-IV [805–876 total; trochanter 93–109; basifemur 115–132; telofemur 115–125; genu 151–167; tibia 167–180; tarsus 158–177].
Male (n=10) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [150–160 ventral length; 95–106 dorsal length; 86–95 tall]. Chelicerae 127–139 long]. Fangs [26–29 long]. Pedipalp [180–195long]. Trochanter [25–27 long; 27–30 wide]. Femur [50–55 long; 34–37 wide]. Genu [38–41 long; width 26–29 wide]. Tibia [49–52 long; 19–22 wide]. Tarsus [17–20 long; 9–11 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [444–508 total; trochanter 55–62; basifemur 75–89; telofemur 63–73; genu 80–91; tibia 85–99; tarsus 84–96]. Leg-II [474–533 total; trochanter 60–64; basifemur 77–90; telofemur 61–71; genu 82–93; tibia 92–106; tarsus 99–113]. Leg-III [537–598 total; trochanter 57–64; basifemur 80–92; telofemur 65–73; genu 96–108; tibia 113–128; tarsus 121–137]. Leg-IV [721–778 total; trochanter 88–99; basifemur 96–117; telofemur 102–113; genu 135–151; tibia 147–168; tarsus 142–156].
Etymology
Specific epithet hitchensi after the late Christopher Eric Hitchens, the English author, journalist, and literary critic. As Sam Harris’ wife, Annaka, said: “Nothing Hitchens does is ever boring.” Hitchens has inspired thousands of free-thinkers to remain clever and engaged in our attempts to understand the world around us.
Distribution
Eastern United States east of the Mississippi River with southern limits in Florida.
Remarks
As it is likely that this species represents a cryptic species complex, measurements were only included from specimens exhibiting less than 2% COI divergence within the clade (those highlighted in red in Fig.
Testudacarus harrisi , sp. n.
Type series
Holotype (1♀): North Carolina, USA: 1♀ from Haywood County, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road near bridge 1.7km north of road to campground (35°38'45.00"N, 83°4'32.00"W), 20 September 2010, by IM Smith, IMS100150 (Specimen 146752 – DNA#2166); Paratypes (12♀, 10♂): North Carolina, USA: (allotype) 1♂ from Haywood County, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road near bridge 1.7km north of road to campground (35°38'45.00"N, 83°4'32.00"W), 20 September 2010, by IM Smith, IMS100150 (Specimen 146750 – DNA#2164); Tennessee, USA: 1♂ from Sevier County, Great Smoky Mountains National Park, Crosby Creek, Cosby Recreation Area beside Cosby Campground Road 0.3km from Route 321 (35°46'54.00"N, 83°13'2.00"W), 16 September 2010, by IM Smith, IMS100140; 2♀ and 2♂ from Sevier County, Great Smoky Mountains National Park, Bullhead Branch, Sugarlands Nature Trail off Route 441/71 (35°40'47.00"N, 83°31'52.00"W), 7 September 2009, by IM Smith, IMS090101; 1♀ from Sevier County, Great Smoky Mountains National Park, Bullhead Branch, Sugarlands Nature Trail off Route 441/71 (35°40'48.00"N, 83°31'53.00"W), 3 September 2009, by IM Smith, IMS090095; 1♂ from Blount County, Great Smoky Mountains National Park, Cades Cove, near parking lot for Abrams Falls Trail (35°35'26.00"N, 83°51'10.00"W), 17 September 2010, by IM Smith, IMS100143; 2♀ from Sevier Co, Great Smoky Mountains National Park, Bullhead Branch, Sugarlands Nature Trail off Route 441/71 (35°40'47.00"N, 83°31'51.00"W), 10 September 2010, by IM Smith, IMS100125; North Carolina, USA: 2♀ and 2♂ from Haywood County, Great Smoky Mountains National Park, Cataloochee Creek, beside Cataloochee Road 0.3km north of Cataloochee Campground (35°38'1.00"N, 83°5'2.00"W), 6 September 2009, IMS090097; 2♀ and 1♂ from Haywood County, Great Smoky Mountains National Park, Big Creek, Waterville Big Creek Picnic Area (35°44'59.00"N, 83°6'42.00"W), 16 September 2010, by IM Smith, IMS100138; 1♀ and 1♂ from Haywood County, Great Smoky Mountains National Park, Cataloochee Creek, beside Mount Sterling Road near bridge 1.7km north of road to campground (35°38'45.00"N, 83°4'32.00"W), 20 September 2010, by IM Smith, IMS100150; 1♂ from Yancey County, Pisgah National Forest, South Toe River, Lost Cove beside Toe River Road (Forest Road 472) 0.4km east of Forest Road 2074 (35°45'0.00"N, 82°12'53.00"W), 9 September 2007, IM Smith, IMS070059; 1♀ from Haywood County, Great Smoky Mountains National Park, Rough Fork Creek, beside road to Nellie 0.3 km west of Pretty Hollow Gap Trailhead (35°37'31.00"N, 83°6'46.00"W), 20 September 2010, by IM Smith, IMS100148; Pennsylvania, USA: 1♀ from Fayette County, State Game Lands #51, Dunbar Creek, off Furnace Hill Road east of Dunbar (39°57'50.00"N, 79°35'8.70"W), 10 August 2014, MJ Skvarla, MS14-0810-001.
Type deposition
Holotype (1♀), allotype (1♂) and ten paratypes (5♀, 5♂) deposited at Canadian National Collection; eleven paratypes (7♀, 4♂) at ACUA.
Diagnosis
These mites have violet to blue coloration over the majority of their anterio-lateral platelets while the rest of the complex have coloration restricted to the posterior half of the platelet.
Description
Female (n=13) with characteristics of the genus with following specifications.
Gnathosoma — Subcapitulum [143–165 ventral length; 90–105 dorsal length; 84–95 tall] ovoid with short rostrum. Chelicerae [119–136 long] unmodified with lightly curved fangs [24–32 long]. Pedipalp [167–191 long] unmodified. Trochanter [23–30 long; 29–31 wide]. Femur [47–53 long; 33–38 wide]. Genu [37–42 long; 27–30 wide]. Tibia [40–53 long; 17–22 wide]. Tarsus [15–20 long; 9–12 wide].
Dorsum (Fig.
Venter (Fig.
Legs — violet to blue and orange. Total leg and podomere lengths as follows: Leg-I [449–485 total; trochanter 54–62; basifemur 77–83; telofemur 62–70; genu 80–90; tibia 89–99; tarsus 80–90]. Leg-II [471–510 total; trochanter 54–60; basifemur 74–84; telofemur 61–66; genu 82–94; tibia 98–107; tarsus 87–109]. Leg-III [548–612 total; trochanter 55–64; basifemur 79–91; telofemur 66–74; genu 96–114; tibia 116–137; tarsus 119–141]. Leg-IV [737–825 total; trochanter 79–99; basifemur 103–123; telofemur 103–121; genu 138–154; tibia 154–169; tarsus 147–167].
Male (n=10) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [133–144 ventral length; 83–90 dorsal length; 72–84 tall]. Chelicerae [110–120 long]. Fangs [25–30 long]. Pedipalp [168–183 long]. Trochanter [22–25 long; 25–29 wide]. Femur [45–50 long; 32–36 wide]. Genu [36–40 long; width 24–30 wide]. Tibia [44–52 long; 18–20 wide]. Tarsus [16–20 long; 8–11 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [419–451 total; trochanter 45–56; basifemur 70–77; telofemur 58–68; genu 74–82; tibia 81–90; tarsus 79–84]. Leg-II [429–472 total; trochanter 47–52; basifemur 69–77; telofemur 56–63; genu 76–86; tibia 86–98; tarsus 93–99]. Leg-III [491–540 total; trochanter 49–53; basifemur 70–86; telofemur 59–66; genu 89–98; tibia 107–120; tarsus 114–124]. Leg-IV [665–739 total; trochanter 74–90; basifemur 95–109; telofemur 95–108; genu 128–138; tibia 139–150; tarsus 131–145].
Etymology
Specific epithet after Samuel Benjamin Harris, the American author, philosopher, and co-founder of Project Reason. Sam Harris, more than any speaker and author, has challenged my (JCO) views and assumptions and kept me on my toes.
Distribution
Eastern United States east of the Mississippi river, with southern limits in Florida.
Testudacarus dennetti , sp. n.
Type series
Holotype (1♀): Pennsylvania, USA: 1♀ from Fayette County, Ohiopyle State Park, Laurel Run, fishing access #2 off T798 (Meadow Run Rd) Ohiopyle State Park (39°50'58.00"N, 79°30'51.00"W), 10 August 2014, by MJ Skvarla, MS14-0810-005 (Specimen 143645 – DNA#2071); Paratypes (8♀, 7♂): Mississippi, USA: (allotype) 1♂ from Tishomingo County, Tishomingo State Park, Rocky Quarry Branch, beside road just outside park entrance (34°36'43.00"N, 88°12'4.00"W), 20 September 2009, by IM Smith, IMS090115 (Specimen 146784 – DNA#2202); 3♀ and 4♂ from Tishomingo County, Tishomingo State Park, Rocky Quarry Branch, beside road just outside park entrance (34°36'43.00"N, 88°12'4.00"W), 20 September 2009, by IM Smith, IMS090115; 2♀ and 2♂ from Tishomingo County, Tishomingo State Park, Rocky Quarry Branch, (34°36’” N, 88°11'W), 18 September 1991, by IM Smith, IMS910049; Pennsylvania, USA: 2♀ from Fayette County, State Game Lands #51, Dunbar Creek, off Furnace Hill Road east of Dunbar (39°57'50.00"N, 79°35'8.70"W), 10 August 2014, MJ Skvarla, MS14-0810-001; Alabama, USA: 1♀ from DeKalb County, Desoto State Park, beside Trail Y (Yellow) (34°29'N, 85°32'W), 26 September 1992, by IM Smith, IMS920053A.
Type deposition
Holotype (1♀), allotype (1♂), and six paratypes (3♀, 3♂) deposited at
Diagnosis
Both Testudacarus dennetti and T. dawkinsi have dorsal plates with uniform pores (unlike T. hitchensi) and anterio-lateral platelets with color restricted to the posterior half (unlike T. harrisi). However, they can be distinguished based on size. Testudacarus dennetti females and males have dorsal lengths less than 575 and 450 µm, respectively. Testudacarus dawkinsi females and males have dorsal lengths greater than 600 and 475 µm, respectively.
Description
Female (n=9) with characteristics of the genus with following specifications.
Gnathosoma — Subcapitulum [139–152 ventral length; 85–97 dorsal length; 85–91 tall] ovoid with short rostrum. Chelicerae [117–126 long] unmodified with lightly curved fangs [24–28 long]. Pedipalp [168–189 long] unmodified. Trochanter [23–27 long; 28–31 wide]. Femur [42–52 long; 33–35 wide]. Genu [35–41 long; 25–32 wide]. Tibia [45–52 long; 17–22 wide]. Tarsus [18–20 long; 8–12 wide].
Dorsum (Fig.
Venter (Fig.
Legs — violet to blue and orange. Total leg and podomere lengths as follows: Leg-I [431–463 total; trochanter 48–58; basifemur 70–78; telofemur 59–65; genu 77–84; tibia 85–93; tarsus 81–88]. Leg-II [455–487 total; trochanter 51–56; basifemur 72–79; telofemur 57–64; genu 80–88; tibia 92–102; tarsus 98–109]. Leg-III [538–572 total; trochanter 54–59; basifemur 73–83; telofemur 62–67; genu 95–106; tibia 114–127; tarsus 128–134]. Leg-IV [641–768 total; trochanter 84–89; basifemur 96–115; telofemur 102–110; genu 137–144; tibia 147–163; tarsus 142–158].
Male (n=7) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [125–134 ventral length; 80–86 dorsal length; 74–83 tall]. Chelicerae [100–115 long]. Fangs [24–28 long]. Pedipalp [164–179 long]. Trochanter [22–24 long; 26–29 wide]. Femur [44–50 long; 30–35 wide]. Genu [36–43 long; width 25–28 wide]. Tibia [44–49 long; 17–20 wide]. Tarsus [17–19 long; 9–10 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [414–434 total; trochanter 47–54; basifemur 67–73; telofemur 55–62; genu 72–79; tibia 81–85; tarsus 79–85]. Leg-II [432–450 total; trochanter 48–54; basifemur 66–72; telofemur 54–61; genu 73–80; tibia 88–91; tarsus 96–99]. Leg-III [478–525 total; trochanter 49–58; basifemur 66–76; telofemur 58–64; genu 83–93; tibia 102–114; tarsus 114–126]. Leg-IV [658–685 total; trochanter 76–86; basifemur 85–103; telofemur 90–100; genu 124–130; tibia 140–141; tarsus 133–140].
Etymology
Specific epithet dennetti after Daniel Clement Dennett III, the American philosopher, writer, and cognitive scientist. Dennett’s work has been the focus of many late night debates in close social circles just as he adds the necessary philosophical spice to the New Athiests.
Distribution
Eastern United States east of the Mississippi River, with southern limits in Florida.
Testudacarus dawkinsi , sp. n.
Type series
Holotype (1♀): New York, USA: 1♀ from Franklin County, Little Aldo Creek, Little Aldo Creek trail from Keese Mill Rd (44°25'32.00"N, 74°20'43.00"W), 19 July 2013, by AJ Radwell and C Milewski, AJR13-0719-205 (Specimen 141897 – DNA#1501); Paratypes (5♀, 9♂): Tennessee, USA: (allotype) 1♂ from Sevier County, Great Smoky Mountains National Park, Crosby Creek, Cosby Recreation Area beside Cosby Campground Road 0.3km from Route 321 (35°46'54.00"N, 83°13'2.00"W), 16 September 2010, by IM Smith, IMS100140 (Specimen 146744 – DNA#2156); 1♂ from Sevier County, Great Smoky Mountains National Park, Bullhead Branch, Sugarlands Nature Trail off Route 441/71 (35°40'47.00"N, 83°31'51.00"W), 10 September 2010, by IM Smith, IMS100125; 2♀ and 1♂ from Sevier County, Great Smoky Mountains National Park, Crosby Creek, Cosby Recreation Area beside Cosby Campground Road 0.3km from Route 321 (35°46'54.00"N, 83°13'2.00"W), 16 September 2010, by IM Smith, IMS100140; 1♂ from Sevier County, Great Smoky Mountains National Park, Bullhead Branch, Sugarlands Nature Trail off Route 441/71 (35°40'47.00"N, 83°31'52.00"W), 24 September 2010, by IM Smith, IMS100158; 1♂ from Sevier County, Great Smoky Mountains National Park, Bullhead Branch, Sugarlands Nature Trail off Route 441/71 (35°40'48.00"N, 83°31'53.00"W), 3 September 2009, by IM Smith, IMS090095; 1♀ and 1♂ from Sevier County, Great Smoky Mountains National Park, Bullhead Branch, Sugarlands Nature Trail off Route 441/71 (35°40'47.00"N, 83°31'52.00"W), 7 September 2009, by IM Smith, IMS090101; 1♀ and 2♂ from Sevier County, Great Smoky Mountains National Park, Cosby Creek, beside road to Cosby Campground at Gabes Mountain Trailhead (35°45'27.00"N, 83°12'36.00"W), 19 September, by IM Smith, IMS050093A; Virginia, USA: 1♂ from Alleghany County, Simpson Creek, Longdale Furnace beside Route 850 2.2 km northeast of I-64 overpass (37°49'41.00"N, 79°39'30.00"W), 14 August 2008, by IM Smith, IMS080044; North Carolina, USA: 1♀ from Macon County, Rainbow Springs, beside Forest Road 67 4.4 km south of Standing Indian Campground (35°3'6.00"N, 83°30'45.00"W), 1 July 2006, by IM Smith, IMS060040.
Type deposition
Holotype (1♀), allotype (1♂), and six paratypes (3♀, 3♂) deposited at
Diagnosis
Both Testudacarus dennetti and T. dawkinsi have dorsal plates with uniform pores (unlike T. hitchensi) and anterio-lateral platelets with color restricted to the posterior half (unlike T. harrisi). However, they can be distinguished based on size. Testudacarus dennetti females and males have dorsal lengths less than 575 and 450 µm, respectively. Testudacarus dawkinsi females and males have dorsal lengths greater than 600 and 475 µm, respectively.
Description
Female (n=6) with characteristics of the genus with following specifications.
Gnathosoma — Subcapitulum [160–168 ventral length; 102–105 dorsal length; 91–95 tall] ovoid with short rostrum. Chelicerae [136–141 long] unmodified with lightly curved fangs [29–30 long]. Pedipalp [188–193 long] unmodified. Trochanter [26–29 long; 28–32 wide]. Femur [50–54 long; 35–37 wide]. Genu [39–41 long; 30–33 wide]. Tibia [51–52 long; 19–22 wide]. Tarsus [19–20(–21) long; 9–11 wide].
Dorsum (Fig.
Venter (Fig.
Legs — violet to blue. Total leg and podomere lengths as follows: Leg-I [487–500 total; trochanter 57–63; basifemur 84–86; telofemur 67–73; genu 90–94; tibia 94–99; tarsus 87–94]. Leg-II [532–548 total; trochanter 58–65; basifemur 84–89; telofemur 67–72; genu 94–99; tibia 107–113; tarsus 110–116]. Leg-III [599–629 total; trochanter 63–68; basifemur 88–97; telofemur 73–76; genu 107–117; tibia 128–138; tarsus 134–140]. Leg-IV [830–861 total; trochanter 83–104; basifemur 113–130; telofemur 119–130; genu 156–164; tibia 172–181; tarsus 164–175].
Male (n=9) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [143–156 ventral length; 89–97 dorsal length; 81–91 tall]. Chelicerae [113–129 long]. Fangs [26–30 long]. Pedipalp [180–195 long]. Trochanter [24–30 long; 26–29 wide]. Femur [50–53 long; 33–36 wide]. Genu [38–43 long; width 27–29 wide]. Tibia [47–52 long; 19–22 wide]. Tarsus [17–20 long; 9–10 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [452–497 total; trochanter 52–63; basifemur 78–87; telofemur 63–72; genu 84–92; tibia 89–99; tarsus 84–93]. Leg-II [486–519 total; trochanter 53–61; basifemur 77–85; telofemur 59–70; genu 87–91; tibia 102–107; tarsus 101–112]. Leg-III [551–588 total; trochanter 54–60; basifemur 81–90; telofemur 66–73; genu 100–107; tibia 118–129; tarsus 126–134]. Leg-IV [752–796 total; trochanter 85–94; basifemur 99–120; telofemur 107–117; genu 143–146; tibia 158–163; tarsus 148–158].
Distribution
Eastern United States east of the Mississippi River, with southern limits in Florida.
Etymology
Specific epithet dawkinsi after Clinton Richard Dawkins, the English evolutionary biologist and writer. Dawkins has proven repeatedly that one can change the world as a biologist by day and keep going as a free-thinker by night.
Testudacarus americanus complex
Complex diagnosis. These mites lack the four-segmented pedipalp of the Debsacarus oribatoides-like mites, the elongate body of the Testudacarus elongatus-like mites, and with the exception of T. rollerae, are much larger (female and male dorsal length usually more than 700 and 600 µm, respectively) than mites of the T. minimus and T. hitchensi complexes. Testudacarus rollerae have a larger (>140 µm) anterio-medial platelet that is more than or nearly twice as wide as long, while T. minimus-like mites have a smaller (<140 µm), more rounded anterio-medial platelet. These mites are present in western North America within and west of the Rocky Mountains, have very light to no coloration, have a large rectangular anterio-medial platelet, and comprise the following species: T. kirkwoodae, T. americanus, T. hyporhynchus, T. smithi, and T. rollerae.
Remarks. Molecular data show strong support for five distinct clades (Fig.
Testudacarus americanus complex molecular phylogeny: 28S and COI Bayesian analysis showing strong support for five distinct clades (●: >95% posterior probability); excluding pink clade, colored clades exhibit <1.3% divergence in COI within and >9% divergence between; pink exhibits 4.5% variation within; red specimen is a suspected species based on genetic data, but specimen is teneral and too badly damaged to diagnose; continuation of (C) lineage from Fig.
Testudacarus americanus
Testudacarus americanus:
Testudacarus american galloi:
Type series
Holotype (1♀): California, USA: 1♀ from Santa Cruz County, Waddell Creek, 29-30 June 1933, by PR Needham, RM330008
Other material examined
Other (9♀, 8♂): Oregon, USA: 1♀ and 1♂ from Lincoln County, Siuslaw National Forest, Lord Creek, (44°14'24.00"N, 123°46'11.00"W), 8 August 2013, by JC O’Neill and WA Nelson, JNOW13-0808-002; 3♀ and 4♂ from Lane County, Cape Perpetua, Cape Perpetua Campground (44°16'51.00"N, 124°5'38.00"W), 15 September 2004, by IM Smith, IMS040077; 1♀ and 1♂ from Lane County, Rock Creek, Rock Creek Campground off Route 101 between Heceta Head and Yachats (44°11'6.00"N, 124°6'34.00"W), 14 September 2004, by IM Smith, IMS040076; 1♀ from Lane County, Cape Creek, Cape Perpetua, Cape Perpetua Campground (44°16'51.00"N, 124°5'38.00"W), 24 June 2010, by IM Smith, IMS100083; 1♀ and 1♂ from Curry County, Port Orford, beside road from Humbug Mountain State Park to McGribble Campground (Forest Road 5002) 5.3 km from Route 101 (42°42'11.00"N, 124°23'54.00"W), 25 June 1976, by IM Smith, IMS760161; 1♀ from Curry County, Port Orford, beside road from Humbug Mountain State Pk to McGribble Campground (Forest Road 5002) 4.6 km from Route 101 (42°42'3.00"N, 124°24'21.00"W), 17 June 2010, by IM Smith , IMS100070; 1♀ from Curry County, Siskiyou National Forest, North Fork of Foster Creek, beside Road #33 between Powers and Agness (42°39'N, 124°4'W), 2 July 1983, IMS 830019; Washington, USA: 1♂ from Kittitas County, Wenatchee National Forest, Squawk Creek, (47°16'51.00"N, 120°41'53.00"W), 31 July 2013, by JC O’Neill, WA Nelson, JNOW13-0731-002.
Type deposition
Holotype (1♀) deposited at
Diagnosis
Resembling most Testudacarus smithi, these mites differ by shape, color, and several other characters. Most notably, T. americanus are elliptical and colorless to peach and have a small cheliceral fang (<33 µm) while T. smithi are rounded and are grey to colorless with large cheliceral fangs (>40 µm).
Redescription
Female (n=10) with characteristics of the genus with following specifications.
Gnathosoma — Subcapitulum [180–199 ventral length; 108–121 dorsal length; 110–124 tall] ovoid with short rostrum and colorless. Chelicerae [148–173 long] unmodified with lightly curved fangs [30–33 long]. Pedipalp [209–236 long] unmodified. Trochanter [29–37 long; 31–33 wide]. Femur [54–59 long; 38–45 wide]. Genu [47–60 long; 32–36 wide]. Tibia [53–59 long; 21–24 wide]. Tarsus [19–23 long; 9–13 wide].
Dorsum (Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [542–604 total; trochanter 68–75; basifemur 91–108; telofemur 75–83; genu 95–111; tibia 108–122; tarsus 101–112]. Leg-II [548–611 total; trochanter 63–72; basifemur 95–108; telofemur 70–83; genu 93–103; tibia 107–123; tarsus 110–127]. Leg-III [595–683 total; trochanter 66–73; basifemur 99–108; telofemur 76–84; genu 103–124; tibia 127–150; tarsus 124–153]. Leg-IV [854–987 total; trochanter 94–112; basifemur 125–152; telofemur 134–154; genu 170–192; tibia 171–216; tarsus 154–181].
Male (n=8) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [164–178 ventral length; 97–109 dorsal length; 96–114 tall]. Chelicerae [132–152 long]. Fangs [28–33 long]. Pedipalp [202–222 long]. Trochanter [28–33 long; 28–33 wide]. Femur [50–58 long; 38–42 wide]. Genu [50–55 long; width 30–35 wide]. Tibia [53–58 long; 20–22 wide]. Tarsus [18–23 long; 11–13 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [501–560 total; trochanter 57–64; basifemur 85–99; telofemur 70–80; genu 90–102; tibia 101–113; tarsus 95–108]. Leg-II [508–567 total; trochanter 58–67; basifemur 88–96; telofemur 67–74; genu 83–99; tibia 101–117; tarsus 105–119]. Leg-III [554–615 total; trochanter 59–63; basifemur 83–98; telofemur 70–76; genu 97–115; tibia 117–138; tarsus 119–132]. Leg-IV [526–882 total; trochanter 79–103; basifemur 116–130; telofemur 121–135; genu 157–175; tibia 171–197; tarsus 149–166].
Distribution
Western North America within and west of the Rocky Mountains. California (
Remarks
Having examined the type material, we suggest that T. americanus galloi Habeeb, 1969 is simply a teneral T. americanus that Habeeb confused for an “a-typical” T. americanus.
Testudacarus kirkwoodae , sp. n.
Type series
Holotype (1♀) Oregon, USA: 1♀ from Douglas County, Rouge River National Forest, Muir Creek, (43°2'53.00"N, 122°21'4.00"W), 12 August 2013, by JC O’Neill and WA Nelson, JNOW13-0812-004 (Specimen 141885 – DNA#1791); Other examined but not measured (1♀): Washington, USA: 1♀ from Snohomish County, Mount Baker National Forest, tributary of South Fork of Sauk River, (48°1'40.00"N, 121°26'24.00"W), 28 July 2013, JC O’Neill and WA Nelson, JNOW13-0728-003
Type deposition
Holotype (1♀) deposited at
Diagnosis
These are the largest known testudacarines and also differ further from their complex in having a smaller, more rounded anterio-medial platelet.
Description
Female (n=1) with characteristics of genus with following specifications.
Gnathosoma — Subcapitulum [245 ventral length; 133 dorsal length; 130 tall] ovoid with short rostrum. Chelicerae [200 long] unmodified with lightly curved fangs [40 long]. Pedipalp [259 long] unmodified. Trochanter [38 long; 43 wide]. Femur [70 long; 50 wide]. Genu [63 long; 40 wide]. Tibia [63 long; 28 wide]. Tarsus [25 long; 15 wide].
Dorsum (Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [620 total; trochanter 70; basifemur 119; telofemur 89; genu 101; tibia 119; tarsus 121]. Leg-II [681 total; trochanter 77; basifemur 113; telofemur 90; genu 117; tibia 145; tarsus 140]. Leg-III [756 total; trochanter 70; basifemur 116; telofemur 93; genu 144; tibia 165; tarsus 167]. Leg-IV [1081 total; trochanter 136; basifemur 148; telofemur 159; genu 207; tibia 224; tarsus 208].
Male (n=0) unknown.
Etymology
Specific epithet kirkwoodae after my (JCO) mother’s maiden name. It was her bringing me to the leafcutter ant exhibit at our local science center that helped me become interested in biology as a child, and her endless support and advice that helped me finish my education.
Remarks
As it is likely that this species represents a cryptic species complex (with 4.5% COI divergence between the two available specimens), measurements were included from only one specimen (the other highlighted in red in Fig.
Distribution
Only one specimen know from Douglas County, Oregon.
Testudacarus hyporhynchus , sp. n.
Type series
Holotype (1♀): California, USA: 1♀ Humboldt County, Willow Creek, Willow Creek Campground off Rt. 299 (40°54'17.00"N, 123°42'21.00"W), 14 June 2010, by IM Smith, IMS100065 (Specimen 146762 – DNA#2177); Paratype (1♀, 2♂): California, USA: (allotype) 1♂ Humboldt County, Willow Creek, Willow Creek Campground off Rt. 299 (40°54'17.00"N, 123°42'21.00"W), 14 June 2010, by IM Smith, IMS100065 (Specimen 146763 – DNA#2178); 1♂ Humboldt County, Willow Creek, Willow Creek Campground off Rt. 299 (40°54'17.00"N, 123°42'21.00"W), 14 June 2010, by IM Smith, IMS100065; Oregon, USA: 1♀ from Curry County, Port Orford, beside Elk River Road 9.0 km east of Elk River Fish Hatchery (42°42'22.00"N, 124°20'28.00"W), 22 June 2010, by IM Smith, IMS100080.
Type deposition
Holotype (1♀) and allotype (1♂) deposited at
Diagnosis
These mites differ from the rest of the complex in having a dorsally “covered” gnathosomal bay (short doral gnathosomal bay length) and an elongate gnathosoma with a long rostrum that exteneds below the gnathosoma ventral surface.
Description
Female (n=2) with characteristics of genus with following specifications.
Gnathosoma (Fig.
Dorsum (Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [566–594 total; trochanter 68–74; basifemur 104–106; telofemur 81–85; genu 108–112; tibia 109–117; tarsus 95–102]. Leg-II [614–645 total; trochanter 75–78; basifemur 102–108; telofemur 77–79; genu 111–116; tibia 124–130; tarsus 125–135]. Leg-III [714–753 total; trochanter 74–79; basifemur 109–119; telofemur 88–94; genu 136–139; tibia 154–160; tarsus 152–161]. Leg-IV [952–961 total; trochanter 105–109; basifemur 142–144; telofemur 138–139; genu 191–192; tibia 199–201; tarsus 175–178].
Male (n=2) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma (Fig.
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [593–606 total; trochanter 73–79; basifemur 111–114; telofemur 78–83; genu 107–111; tibia 113–118; tarsus 105–106]. Leg-II [635–645 total; trochanter 74–79; basifemur 102–104; telofemur 84–85; genu 115–120; tibia 129–132; tarsus 124–130]. Leg-III [724–726 total; trochanter 77–78; basifemur 109–116; telofemur 87–91; genu 136–137; tibia 155–156; tarsus 152–155]. Leg-IV [905–964 total; trochanter 107–118; basifemur 139–140; telofemur 129–142; genu 183–191; tibia 179–198; tarsus 167–176].
Etymology
Specific epithet hyporhynchus (hypo-, G. under; rhynchus, G. snout) refers to the long rostrum that extends below the ventral surface of the gnathosoma.
Distribution
Humbolt County, California and Curry County, Oregon.
Testudacarus smithi , sp. n.
Type series
Holotype (1♀): British Columbia, Canada: 1♀ from Vancouver Island, spring run, Lake Cowichan, beside North Shore Road 1.7 km north of town (48°49'29.00"N, 124°4'2.00"W), 1 July 2010, by IM Smith, IMS100091 (Specimen 146769 – DNA#2184); Paratypes (10♀, 12♂): British Columbia, Canada: (allotype) 1♂ from Vancouver Island, spring run, Lake Cowichan, beside North Shore Road 1.7 km north of town (48°49'29.00"N, 124°4'2.00"W), 1 July 2010, by IM Smith, IMS100091 (Specimen 146770 – DNA#2185); 2♂ from Vancouver Island, spring run, Lake Cowichan, beside North Shore Road 1.7 km north of town (48°49'29.00"N, 124°4'2.00"W), 1 July 2010, by IM Smith, IMS100091; 3♀ and 2♂ from Vancouver Island, Lake Cowichan, spring run, beside North Shore Road 1.7 km north of town (48°49'29.00"N, 124°4'13.00"W), 11 June 1979, by IM Smith, IMS790013A; 3♀ and 3♂ from Vancouver Island, Lake Cowichan, spring run, beside South Shore Road 2.3 km north of town (48°48'25.00"N, 124°5'13.00"W), 7 July 1976, by IM Smith, IMS760194; 3♀ and 2♂ from Vancouver Island, Port Alberni, beside road to Mount Arrowsmith Ski Area 11.6 km from Highway 4 (49°12'50.00"N, 124°36'18.00"W), 19 September 2004, by IM Smith, IMS040084A; 1♀ and 1♂ from Vancouver Island, Lake Cowichan, spring run, beside South Shore Road 2.3 km north of town (48°48'25.00"N, 124°5'13.00"W), 31 July 1979, by IM Smith, IMS790056; 1♂ from Vancouver Island, Lake Cowichan, spring run, beside South Shore Road 2.3 km north of town (48°48'25.00"N, 124°5'13.00"W), 26 July 1985, by IM Smith, IMS850122A.
Type deposition
Holotype (1♀), allotype (1♂), and eight paratypes (4♀, 4♂) deposited at
Diagnosis
Resembling most Testudacarus americanus, these mites differ by shape, color, and several other characters. Most notably, T. americanus are elliptical and colorless to peach and have a small cheliceral fang (<33 µm) while T. smithi are rounded and are grey to colorless with large cheliceral fangs (>40 µm).
Description
Female (n=10) with characteristics of genus with following specifications.
Gnathosoma — Subcapitulum [217–245 ventral length; 137–145 dorsal length; 125–152 tall] elliptical to ovoid with short rostrum. Chelicerae [190–205 long] unmodified with lightly curved fangs [40–45 long]; fangs characteristically large. Pedipalp [250–272 long] unmodified. Trochanter [37–45 long; 39–46 wide]. Femur [72–80 long; 52–61 wide]. Genu [55–61 long; 41–49 wide]. Tibia [57–66 long; 23–26 wide]. Tarsus [21–26 long; 11–13 wide].
Dorsum (Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [624–660 total; trochanter 74–81; basifemur 110–121; telofemur 84–95; genu 117–125; tibia 123–130; tarsus 108–116]. Leg-II [658–718 total; trochanter 75–87; basifemur 111–123; telofemur 83–95; genu 115–130; tibia 133–149; tarsus 133–145]. Leg-III [755–820 total; trochanter 78–89; basifemur 112–131; telofemur 88–102; genu 139–155; tibia 162–184; tarsus 165–182]. Leg-IV [1017–1058 total; trochanter 117–132; basifemur 139–150; telofemur 140–153; genu 197–210; tibia 212–228; tarsus 194–205].
Male (n=12) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [205–232 ventral length; 125–145 dorsal length; 124–133 tall]. Chelicerae [178–202 long]. Fangs [39–42 long]. Pedipalp [250–279 long]. Trochanter [37–42 long; 38–43 wide]. Femur [71–81 long; 52–60 wide]. Genu [55–65 long; width 40–47 wide]. Tibia [59–69 long; 23–25 wide]. Tarsus [21–27 long; 11–15 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [617–679 total; trochanter 73–80; basifemur 110–122; telofemur 84–97; genu 116–129; tibia 118–138; tarsus 107–123]. Leg-II [664–743 total; trochanter 74–84; basifemur 110–125; telofemur 85–103; genu 118–137; tibia 134–157; tarsus 131–148]. Leg-III [753–841 total; trochanter 76–83; basifemur 111–133; telofemur 89–106; genu 138–158; tibia 163–186; tarsus 155–186]. Leg-IV [952–1098 total; trochanter 111–131; basifemur 136–154; telofemur 139–161; genu 189–217; tibia 189–238; tarsus 176–210].
Etymology
Specific epithet smithi after Dr Ian Smith, the Canadian water mite researcher who collected the specimens needed for this description. Dr Smith has advanced water mite research in North America more than anyone else and without him, this work would have been impossible.
Distribution
British Columbia, Canada.
Testudacarus rollerae , sp. n.
Type series
Holotype (1♀): California, USA: 1♀ from Nevada County, Tahoe National Forest, Bear River, at Sierra Discovery day use area upstream from bridge (39°18'35.00"N, 120°39'56.00"W), 26 August 2013, by JR Fisher, JRF13-0826-001 (Specimen 146725 – DNA# 2135); Paratypes (2♀, 2♂): California, USA: (allotype) 1♂ (allotype)from Nevada County, Tahoe National Forest, Bear River, at Sierra Discovery day use area upstream from bridge (39°18'35.00"N, 120°39'56.00"W), 26 August 2013, by JR Fisher, JRF13-0826-001 (Specimen 146724 – DNA# 2134); 1♀ from Nevada County, Tahoe National Forest, Bear River, at Sierra Discovery day use area upstream from bridge (39°18'35.00"N, 120°39'56.00"W), 26 August 2013, by JR Fisher, JRF13-0826-001; 1♀ and 1♂ from Calaveras County, Calaveras Big Trees State Park, Big Trees River, (38°16'N, 120°16'W), 12 June 1976, by IM Smith, IMS760099; 1♂ from Mendocino County, Navarro River, Paul M. Dimmick Recreation Area beside Route 128 (39°`10'N, 123°38'W), 29 September 1993, by IM Smith, IMS9300026A.
Type deposition
Holotype (1♀) and allotype (1♂) deposited at
Diagnosis
These mites are smaller and more colorful than other species in the complex and therefore resemble most the T. minimus-like mites; however, mites of the T. minimus complex are even smaller and have a smaller (<140 µm, and far less than twice as wide as long), more rounded anterio-medial platelet, while these mites have a larger (>140 µm, and more than or nearly twice as wide as long) more rectangular anterio-medial platelet.
Description
Female (n=3) with characteristics of genus with following specifications.
Gnathosoma — Subcapitulum [176–188 ventral length; 105–107 dorsal length; 100–103 tall] elliptical ovoid with short rostrum and colorless. Chelicerae [145–152 long] unmodified with lightly curved fangs [28–30 long]. Pedipalp [202–212 long] unmodified. Trochanter [30–31 long; 28–30 wide]. Femur [56–58 long; 40–41 wide]. Genu [43–47 long; 33–35 wide]. Tibia [52–55 long; 20–23 wide]. Tarsus [20–22 long; 9–10 wide].
Dorsum (Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [503–542 total; trochanter 65–68; basifemur 88–93; telofemur 70–81; genu 93–103; tibia 96–102; tarsus 89–97]. Leg-II [510–577 total; trochanter 52–74; basifemur 82–94; telofemur 66–78; genu 94–102; tibia 103–118; tarsus 106–112]. Leg-III [610–657 total; trochanter 64–71; basifemur 92–99; telofemur 73–82; genu 110–122; tibia 127–146; tarsus 137–141]. Leg-IV [843–914 total; trochanter 96–101; basifemur 116–125; telofemur 117–133; genu 166–185; tibia 181–195; tarsus 168–178].
Male (n=3) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [160–170 ventral length; 98–109 dorsal length; 81–92 tall]. Chelicerae [132–140 long]. Fangs [27–29 long]. Pedipalp [184–190 long]. Trochanter [23–26 long; 29–30 wide]. Femur [50–51 long; 36–38 wide]. Genu [41–42 long; width 29–30 wide]. Tibia [49–52 long; 19–20 wide]. Tarsus [17–21 long; 8–9 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [472 total; trochanter 59–60; basifemur 83–91; telofemur 66–71; genu 88–91; tibia 91–97; tarsus 83–88]. Leg-II [496–515 total; trochanter 61–66; basifemur 84–87; telofemur 65–69; genu 85–97; tibia 96–108; tarsus 102–107]. Leg-III [554–593 total; trochanter 62–69; basifemur 84–89; telofemur 65–74; genu 100–110; tibia 117–126; tarsus 125–136]. Leg-IV [784–822 total; trochanter 80–95; basifemur 109–116; telofemur 114–115; genu 155–162; tibia 164–174; tarsus 155–162].
Etymology
Specific epithet rollerae after Elizabeth Ashley Roller, my (JCO) life partner.
Distribution
Reported from only two counties (Mendocino and Nevada) in California.
Testudacarus elongatus complex
Complex diagnosis. Unlike all other Testudacarinae, members of this complex have an elongate idiosoma. In contrast to most T. minimus- and T. hitchensi-like mites, these mites are colorless and much larger (female and male dorsal length greater than 700 and 600 µm, respectively). These mites are found in western North America west of the Rocky Mountains. This complex comprises three species: T. elongatus, T. oblongatus, and T. rectangulatus.
Remarks.
Combined molecular, distributional, and morphological data support three distinct clades within the T. elongatus complex (Fig.
Testudacarus elongatus complex molecular phylogeny: 28S and COI Bayesian analysis showing strong support at least three distinct clades (●: >95% posterior probability); colored clades exhibit <2.4% divergence in COI within and >3.3% divergence between; divergence of the two basal clades >9%; continuation of (D) lineage from Fig.
Testudacarus elongatus , sp. n.
Type series
Holotype (1♀): Washington, USA: 1♀ from Okanogan County, Okanogan National Forest, Early Winters Creek, (48°35'55.00"N, 120°35'20.00"W), 29 July 2013, by JC O’Neill and WA Nelson, JNOW13-0729-004 (Specimen 138495 – DNA#1522); Paratypes (7♀, 4♂): Washington, USA: (allotype) 1♂ from Okanogan County, Okanogan National Forest, Early Winters Creek, (48°35'55.00"N, 120°35'20.00"W), 29 July 2013, by JC O’Neill and WA Nelson, JNOW13-0729-004 (Specimen 141889 – DNA#1797); 1♂ from Whatcom County, Mount Baker National Forest, Porcupine Creek, (48°31'51.00"N, 120°44'42.00"W), 29 July 2013, by JC O’Neill and WA Nelson, JNOW13-0729-003; 2♀ and 2♂ from Okanogan County, Okanogan National Forest, Early Winters Creek, (48°35'55.00"N, 120°35'20.00"W), 29 July 2013, by JC O’Neill and WA Nelson, JNOW13-0729-004; 1♀ from Snohomish County, Mount Baker National Forest, tributary of South Fork of Sauk River, (48°1'40.00"N, 121°26'24.00"W), 28 July 2013, JC O’Neill and WA Nelson, JNOW13-0728-003; 1♀ from Okanogan County, Okanogan National Forest, North Fork of Twentymile Creek, (48°43'7.00"N, 119°56'14.00"W), 29 July 2013, by JC O’Neill and WA Nelson, JNOW13-0729-007; 3♀ from Okanagan County, North Fork of Salmon Creek, (48°37'48.00"N, 119°48'52.00"W), 29 July 2013, by JC O’Neill and WA Nelson, JNOW13-0729-008.
Type deposition
Holotype (1♀), allotype (1♂), and four paratypes (3♀, 1♂) deposited at
Diagnosis
Since morphological variation is limited, a combination of morphology and distribution is best used to diagnose members of the complex. These mites occur in Washington within and east of the Cascade Mountains, while T. rectangulatus occur in the Olympic Mountains, and T. oblongatus occur along the western Coast of Washington, Oregon, California, and British Columbia. Additionally, both T. rectangulatus and these mites differ from T. oblongatus in having more robust lateral platelets; most notably, lateral-platelet-4 tends to be larger in these two species than T. oblongatus, and is in direct or near direct contact with lateral-platelet-2. Reversely, T. oblongatus generally have less robust platelets and a smaller lateral-platelet-4 that has a noticeable gap between it and lateral-platelet-2. Limited specimens were found of T. elongatus and T. rectangulatus, but T. rectangulatus appear to have leg and pedipalp measurements roughly 10% larger than T. elongatus even between individuals of similar idiosoma size. More data is needed to better diagnose these species.
Description
Female (n=8) with characteristics of genus with following specifications.
Gnathosoma — Subcapitulum [174–175 ventral length; 110–118 dorsal length; 102–122 tall] ovoid with short rostrum. Chelicerae [140–163 long] unmodified with lightly curved fangs [33–37 long]. Pedipalp [221–248 long] unmodified. Trochanter [28–38 long; 32–36 wide]. Femur [60–64 long; 45–50 wide]. Genu [51–59 long; 37–43 wide]. Tibia [61–69 long; 24–28 wide]. Tarsus [20–25 long; 10–12 wide].
Dorsum (Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [561–614 total; trochanter 63–66; basifemur 101–114; telofemur 79–93; genu 103–116; tibia 110–127; tarsus 96–109]. Leg-II [559–623 total; trochanter 56–65; basifemur 96–120; telofemur 75–88; genu 103–116; tibia 120–128; tarsus 107–120]. Leg-III [630–703 total; trochanter 60–80; basifemur 97–116; telofemur 79–96; genu 116–139; tibia 136–152; tarsus 129–140]. Leg-IV [863–920 total; trochanter 98–109; basifemur 126–140; telofemur 130–140; genu 172–189; tibia 183–194; tarsus 152–167].
Male (n=4) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [148–160 ventral length; 98–108 dorsal length; 95–100 tall]. Chelicerae [135–140 long]. Fangs [30–31 long]. Pedipalp [208–215 long]. Trochanter [30–31 long; 28–33 wide]. Femur [53–60 long; 40–44 wide]. Genu [47–52 long; width 33–35 wide]. Tibia [55–61 long; 23–25 wide]. Tarsus [20–21 long; 10–12 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [531–558 total; trochanter 54–65; basifemur 95–99; telofemur 75–80; genu 98–104; tibia 105–111; tarsus 100–105]. Leg-II [549–572 total; trochanter 59–65; basifemur 93–101; telofemur 73–79; genu 98–105; tibia 112–120; tarsus 100–109]. Leg-III [577–610 total; trochanter 64–69; basifemur 93–105; telofemur 75–84; genu 106–119; tibia 122–135; tarsus 117–131]. Leg-IV [765–812 total; trochanter 91–100; basifemur 110–120; telofemur 115–121; genu 153–171; tibia 152–168; tarsus 140–152].
Etymology. Specific epithet elongatus (elong-, L. extend) refers to the elongate idiosoma of adults.
Distribution
Within and east of the Cascade Mountains, Washington.
Testudacarus rectangulatus , sp. n.
Type series
Holotype (1♂): Washington, USA: 1♂ from Mason County, Olympic National Forest, Cabin Creek, by Hamma Hamma River (47°35'44.00"N, 123°7'39.00"W), 22 July 2013, by JC O’Neill and WA Nelson, JNOW13-0722-004 (Specimen 138494 – DNA#1521)
Type deposition
Holotype (1♂) deposited at
Diagnosis
Since morphological variation is limited, a combination of morphology and distribution is best used to diagnosed members of the complex. These mites occur in the Olympic Mountains, while T. elongatus occur in Washington within and east of the Cascade Mountains, and T. oblongatus occur along the western Coast of Washington, Oregon, California, and British Columbia. Additionally, both T. elongatus and these mites differ from T. oblongatus in having more robust lateral platelets; most notably, lateral-platelet-4 tends to be larger in these two species than T. oblongatus, and is in direct or near direct contact with lateral-platelet-2. Reversely, T. oblongatus generally have less robust platelets and a smaller lateral-platelet-4 that has a noticeable gap between it and lateral-platelet-2. Limited specimens were found of T. elongatus and T. rectangulatus, but T. rectangulatus appear to have leg and pedipalp measurements roughly 10% larger than T. elongatus even between individuals of similar idiosoma size. More data is needed to better diagnose these species.
Description
Female (n=0) unknown.
Male (n=1) with characteristics of genus with following specifications.
Gnathosoma — Subcapitulum [173 ventral length; 108 dorsal length; 105 tall] ovoid with short rostrum. Chelicerae [150 long] unmodified with lightly curved fangs [33-37 long]. Pedipalp [249 long] unmodified. Trochanter [35 long; 34 wide]. Femur [60 long; 48 wide]. Genu [56 long; 40 wide]. Tibia [75 long; 25 wide]. Tarsus [23 long; 12 wide].
Dorsum (Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [603 total; trochanter 61; basifemur 103; telofemur 89; genu 116; tibia 124; tarsus 108]. Leg-II [610 total; trochanter 63; basifemur 101; telofemur 85; genu 115; tibia 126; tarsus 120]. Leg-III [674 total; trochanter 63; basifemur 110; telofemur 86; genu 130; tibia 145; tarsus 137]. Leg-IV [870 total; trochanter 87; basifemur 123; telofemur 130; genu 179; tibia 189; tarsus 160].
Etymology
Specific epithet rectangulatus (rectangulum, L. straight angle) refers to the boxy, elongate idiosoma of adults.
Distribution
One specimen found in Mason County in the Olympic Mountains, Washington.
Testudacarus oblongatus , sp. n.
Type series
Holotype (1♀): Oregon, USA: 1♀ from Curry County, Siskiyou National Forest, confluence of tributary and Wheeler Creek, off NF 1205 (42°4'42.00"N, 124°8'53.00"W), by JR Fisher, JRF13-0814-004 (Specimen 146728 – DNA#2138); Paratypes (11♀, 9♂): British Columbia, Canada: (allotype) 1♂ from Vancouver Island, beside Harris Creek Mainline 5 km west of Old Hillcrest Gate (26 km west of Mesachie Lake) (48°40'7.00"N, 124°13'20.00"W), 3 July 2010, by IM Smith, IMS100095 (Specimen 146776 – DNA#2192); 2♀ and 1♂ from Vancouver Island, beside Harris Creek Mainline 5 km west of Old Hillcrest Gate (26 km west of Mesachie Lake) (48°40'7.00"N, 124°13'20.00"W), 3 July 2010, by IM Smith, IMS100095; 3♀ and 1♂ from Vancouver Island, beside Highway 4 16.6 km east of road to Ucluelet (Pacific Rim Road) (49°9'N, 125°54'W), 18-19 July 1979, by IM Smith, IMS790047; 1♀ and 3♂ from Bonanza Pass Walker Creek Picnic Area beside Highway 3 between Grand Forks and Castlegar (49°10'N, 118°5'W), 20 July 1988, by IM Smith, IMS880034; 1♂ from Vancouver Island, Honeymoon Bay Wildflower Reserve, (48°49'38.00"N, 124°12'10.00"W), 19 June 1979, by IM Smith, IMS790023A; 1♀ from Vancouver Island, beside Harris Creek Mainline 5 km west of Old Hillcrest Gate (26 km west of Mesachie Lake) (48°40'6.00"N, 124°13'19.00"W), 3 July 2010, by IM Smith, IMS100097; 2♀ from Vancouver Island, beside Harris Creek Mainline 5 km west of Old Hillcrest Gate (26 km west of Mesachie Lake) (48°40'6.00"N, 124°13'16.00"W), 10 July 1988, by IM Smith, IMS880007; California, USA: 1♂ from Monterey County, Los Padres National Forest, Lucia, beside Ferguson-Nacimiento Road 5.6 km east of Route 1 (36°0'3.00"N, 121°28'31.00"W), 3 June 2010, by IM Smith, IMS100048; 1♂ from Trinity County, Shasta-Trinity National Forest, beside Route 36 6.2 km west of Forest Glen Station Campground (40°22'57.00"N, 123°23'26.00"W), 11 June 2010, by IM Smith, IMS100061; 1♀ from Trinity County, Shasta-Trinity National Forest, beside Route 36 7 km west of Forest Glen Station Campground (40°23'5.00"N, 123°23'57.00"W), 11 June 2010, by IM Smith, IMS100062; Oregon, USA: 1♀ from Curry County, Siskiyou National Forest, confluence of tributary and Wheeler Creek, off NF 1205 (42°4'42.00"N, 124°8'53.00"W), by JR Fisher, JRF13-0814-004.
Type deposition
Holotype (1♀), allotype (1♂), and ten paratypes (6♀, 4♂) deposited at
Diagnosis
Since morphological variation is limited, a combination of morphology and distribution is best used to diagnosed members of the complex. These mites occur along the western coast of Washington, Oregon, California, and British Columbia, while T. elongatus occur in Washington within and east of the Cascade Mountains, and T. rectangulatus occur in the Olympic Mountains. These mites differ from others in the complex in having less robust platelets and a smaller lateral-platelet-4 that has a noticeable gap between it and lateral-platelet-2. Reversely lateral-platelet-4 tends to be larger in the other two species of the complex than T. oblongatus, and is in direct or near direct contact with lateral-platelet-2.
Description
Female (n=11) with characteristics of genus with following specifications.
Gnathosoma — Subcapitulum [192–208 ventral length; 116–132 dorsal length; 120–134 tall] ovoid with short rostrum. Chelicerae [149–166 long] unmodified with lightly curved fangs [33–36 long]. Pedipalp [231–242 long] unmodified. Trochanter [31–36 long; 30–33 wide]. Femur [58–63 long; 45–49 wide]. Genu [53–59 long; 36–38 wide]. Tibia [64–69 long; 22–25 wide]. Tarsus [20–25 long; 11–12 wide].
Dorsum (Fig.
Venter (Fig.
Legs — colorless. Total leg and podomere lengths as follows: Leg-I [623–676 total; trochanter 72–85; basifemur 106–115; telofemur 85–94; genu 114–129; tibia 123–137; tarsus 109–120]. Leg-II [642–689 total; trochanter 75–80; basifemur 108–117; telofemur 88–94; genu 115–135; tibia 131–152; tarsus 119–133]. Leg-III [710–777 total; trochanter 70–80; basifemur 106–126; telofemur 92–100; genu 129–151; tibia 151–172; tarsus 146–161]. Leg-IV [941–1001 total; trochanter 106–125; basifemur 135–150; telofemur 139–146; genu 188–199; tibia 197–215; tarsus 160–178].
Male (n=9) similar to female except for sexually dimorphic characters previously discussed and with following specifications.
Gnathosoma — Subcapitulum [153–177 ventral length; 99–118 dorsal length; 98–110 tall]. Chelicerae [123–148 long]. Fangs [28–32 long]. Pedipalp [201–231 long]. Trochanter [29–33 long; 27–30 wide]. Femur [51–55 long; 37–46 wide]. Genu [44–55 long; width 32–37 wide]. Tibia [54–66 long; 20–23 wide]. Tarsus [19–22 long; 10–12 wide].
Dorsum (Fig.
Venter (Fig.
Legs — total leg and podomere lengths as follows: Leg-I [526–617 total; trochanter 57–69; basifemur 90–103; telofemur 75–90; genu 100–116; tibia 105–124; tarsus 97–116]. Leg-II [536–629 total; trochanter 59–70; basifemur 87–106; telofemur 73–86; genu 100–117; tibia 106–134; tarsus 104–123]. Leg-III [589–691 total; trochanter 60–73; basifemur 89–111; telofemur 71–90; genu 115–130; tibia 123–151; tarsus 128–147]. Leg-IV [810–878 total; trochanter 92–101; basifemur 112–125; telofemur 114–127; genu 144–177; tibia 158–188; tarsus 143–168]
Etymology
Specific epithet oblongatus (oblong-, L. rather long) referring to the oblong idiosoma.
Distribution
West coast of British Columbia, Washington, Oregon, and California.
Asian species
Testudacarus tripeltatus
Testudacarus tripeltatus:
Type series
Holotype (1♀): Himachal Pradesh, India: (1♀) from Kangra Valley, Upper Dharamsala, Bhagsunath, June 4th 1926, by Dr Hora.
Type deposition
Holotype (1♀) at Naturhistorisches Museum Basel, Switzerland.
Diagnosis
Testudacarus tripeltatus can be differentiated from all other Asian species by distribution (India, Java, and Bhutan) and large size (dorsal length >700 µm). More research and updated descriptions are needed for a better diagnosis.
Distribution
India (
Testudacarus japonicus
Testudacarus japonicus:
Type series
Holotype (1♂): Shizuoka, Japan: brook connected with a stream in Takékura, Mishima, Shizuoka, Japan, 15 May 1953, by T. Imamura.
The types were not examined for this publication.
Type deposition
Holotype (1♂) at Taiji Imamura Collection at Ibaraki Nature Museum, Japan.
Holotype loans are not available from Ibaraki Nature Museum. The museum provided a low-magnification photograph through e-mail, though permission to print the photograph was not obtained.
Diagnosis
These mites differ from all other Testudacarinae by distribution (Japan), and from T. tripeltatus by small size (dorsal length <700 µm). Testudacarus japonicus may be conspecific with T. okadai. More research and updated descriptions are needed for a better diagnosis.
Distribution
Takékura, Japan (
Remarks
It is reasonable to assume
Testudacarus okadai
Testudacarus okadai:
Type series
Holotype (1♀): Tichigi, Japan: Onisawa, Shôbuga-Hama, Nikkô National Park, 13 May 1974, by Y. Okada.; Allotype (1♂?): Tichigi, Japan: Onisawa, Shôbuga-Hama, Nikkô National Park, 13 May 1974, by Y. Okada.
The types were not examined for this publication.
Type deposition
Holotype (1♀) and allotype (1♂?) at Taiji Imamura Collection at Ibaraki Nature Museum, Japan.
Holotype loans are not available from Ibaraki Nature Museum. The museum provided a low-magnification photograph through e-mail, though permission to print the photograph was not obtained.
Diagnosis
These mites differ from all other Asian Testudacarinae by distribution (Japan), and from T. tripeltatus by small size (dorsal length <700 µm). Testudacarus okadai may be conspecific with T. japonicus. More research and updated descriptions are needed for a better diagnosis.
Distribution
Throughout Honshu, Japan (
Discussion
A drawing of the “male” dorsum is left out of the T. okadai description. This is of the utmost importance because the sex of the “male” specimen is in question. The positioning of the genital field in relation to coxae-IV and the short coxae-II+III midline is typical of female testudacarines, but the coxal field size in relation to the venter is typical of males (Fig.
Testudacarus binodipalpis
Testudacarus binodipalpis:
Type series
Holotype (1♀): Guizhou, China: Mt. Fanjing (27°49'–28.01'N, 108°46'–108°49'E), 29 July 2001, by Guo Jian-Jun, 2001-VII-291; Paratype (1♀): Guizhou, China: Mt. Fanjing (27°49'–28.01'N, 108°46'–108°49'E), 4 Aug 2001, by Guo Jian-Jun, 2001-VII-292.
The types were not examined for this publication; contact with the authors was attempted but unsuccessful and the the types were not examined.
Type deposition
Institute of Entomology, Guizhou University.
Diagnosis
These mites can differ from all other Testudacarinae by distribution (China) and from T. tripeltatus by their small size (dorsal length <700 µm). More research and updated descriptions are needed for a better diagnosis.
Distribution
Mt. Fanjing (
Remarks
Testudacarus binodipalpis was described from one female and one “male.” The described “male” is almost certainly a female as it exhibits all female sexual characters and no ejaculatory complex is noted in the description. However, these two females differ in some noteworthy respects. From illustrations it appears that the smaller female seems to have undergone tertiary sclerotization, while the larger female seems to have only undergone primary and secondary sclerotization. The size and positioning of lateral platelets are also quite different in each specimen. For these reasons the specimens should be re-examined as they might represent two species diagnosable by size.
Key to world species of Testudacarinae
| 1 | Pedipalp four-segmented, anterior tip of coxa-I with projection; California | D. oribatoides |
| – | Pedipalp five-segmented, anterior tip of coxa-I without projection | 2 |
| 2 | Body elongate to rectangular | (T. elongatus complex)...3 |
| – | Body oval | 5 |
| 3 | Lateral platelets robust; lateral-platelet-4 large and in direct or near direct contact with lateral-platelet-2 | 4 |
| – | Lateral-platelet-4 small, gap present between it and lateral-platlet-2; widespread along west coast of N. America | T. oblongatus |
| 4 | Distribution restricted to the Olympic Mountains | T. rectangulatus |
| – | Distribution within and east of the Cascade Mountains | T. elongatus |
| 5 | Body large (>700 µm female and >650 male dorsal length; if smaller, found in Asia), dull coloration common; within and west of the Rocky Mountains or Asia | (Asian species and T. americanus complex except T. rollerae)...6 |
| – | Body small (<700 µm female and <650 male dorsal length), bright coloration (orange, red, violet, blue) common; present throughout North America | (T. minimus complex, T. hitchensi complex, T. rollerae)...12 |
| 6 | Gnathosomal bay “covered” (short dorsal gnathosomal bay length), gnathosoma elongate with long rostrum that extends below ventral surface of gnathosoma | T. hyporhynchus |
| – | Without these characters | 7 |
| 7 | Body very large (>900 µm dorsal length, female) with small, square (unusual for complex) anterio-medial platelet (male unknown) | T. kirkwoodae |
| – | Body not this large (<900 µm dorsal length, female and male) often with wide, rectangular or trapezoidal anterio-medial platelet | 8 |
| 8 | Anterior-medial platelet compact and pentagonal, suture lines between second and third coxae absent; India, Java, Bhutan | T. tripeltatus |
| – | Anterior-medial platelet wider, more trapezoidal in shape, suture lines between second and third coxae present, but incomplete | 9 |
| 9 | Body small (<650 µm dorsal length female and <450 µm dorsal length male); Japan | T. japonicas or T. okadai |
| – | Body larger (>700 µm dorsal length female and >500 µm dorsal length male) | 10 |
| 10 | Body <770 µm dorsal length female and <660 µm dorsal length male, dg-4 in line with middle of dorsal muscle scars; China | T. binodipalpis |
| – | Body >780 µm dorsal length female and >670 µm dorsal length male, dg-4 anterior to dorsal muscle scars | 11 |
| 11 | Body elliptical, colorless to peach, small cheliceral fang (<33 µm) | T. americanus |
| – | Body rounded, grey to colorless, large cheliceral fang (>40 µm) | T. smithi |
| 12 | Anterio-medial platelet wide (>140 µm) and more than or nearly twice as wide as long | T. rollerae |
| – | Anterio-medial platelet unmodified (<140 µm) and less than twice as wide as long | (T. minimus and T. hithensi complex)...13 |
| 13 | Anterio-medial and anterio-lateral platelets with consistent coloration (either colorless or colorled across) | (T. minimus complex)...14 |
| – | Anterio-lateral platelets with coloration and anterio-medial platelet colorless | 18 |
| 14 | Body in entirety conspicuously violet; currently known only from Arkansas | T. radwellae |
| – | Body orange, red, pink, blue, or violet but not covering the majority of body | 15 |
| 15 | Distribution east of the Great Plains, color violet to blue | T. vulgaris |
| – | Distribution within and west of the Great Plains | 16 |
| 16 | Distribution in Washington or northern Oregon, orange to red | T. minimus |
| – | Distribution in and west of Great Plains excluding Washington and northern Oregon | 17 |
| 17 | Violet to blue, females and males smaller than 600 and 500 µm, respectively | T. vulgaris (most likely) |
| – | Collected outside of California, orange to red to clear, females and males larger than 600 and 500 µm, respectively | T. minimus (most likely) |
| – | Collected in California and orange to clear | T. minimus, vulgaris, or deceptivus (no morphological characters for reliable identification available within California) |
| 18 | Dorsal plate with large medial pores surrounded by distal ring of smaller pores (all pores uniform in other species), area posterior to coxal plate “bleached” in males | T. hitchensi |
| – | Dorsal plate with uniform pores, males without “bleached” area | 19 |
| 19 | Anterio-lateral platelets with violet to blue coloration covering most of platelet | T. harrisi |
| – | Anterio-lateral platelets with coloration restricted to the posterior half of platelet | 20 |
| 20 | Females and males with dorsal lengths less than 575 and 450 µm, respectively | T. dennetti |
| – | Females and males with dorsal lengths greater than 600 and 475 µm, respectively | T. dawkinsi |
Acknowledgements
Special thanks to Ian M. Smith (
References
- Abé H (2005) Annotated checklist of Japanese water mites (Acari: Prostigmata: Hydracarina). Acta Arachnologica 54: 111–145. doi: 10.2476/asjaa.54.111
- Abé H (2006) A catalogue of Japanese water mites (Acari: Prostigmata: Hydracarina). The Acarological Society of Japan 15: 1–16. doi: 10.2300/acari.15.1[In Japanese]
- Abé H, Imamura T, Kikuchi Y (2006) Catalogue of type specimens of aquatic mites (Acari, Hydrachnellae and Halacaridae) in the Taiji Imamura collection of Ibaraki Nature Museum, Ibaraki, Japan. Bulletin of Ibaraki Nature Museum 9: 1–18.
- Bader C (1988) Die Torrenticolidae (Acari, Hydrachnaellae). Eine abklärende studie über eine schwierige Wassermilben-Familie. Revue Suisse de Zoologie 95: 87–98. doi: 10.5962/bhl.part.79640
- Baker EW, Wharton GW (1952) An Introduction to Acarology. The Macmillan Company, New York, 465 pp.
- Barr DW (1972) The ejaculatory complex in water mites (Acari: Parasitengona): morphology and potential value for systematics. Life Sciences Contributions of the Royal Ontario Museum 81: 1–87.
- Barr DW (1977) A new water mite genus from western Canada (Acari: Parasitengona: Anisitsiellidae). Canadian Journal of Zoology 55: 877–881. doi: 10.1139/z77-114
- Barr DW (1982) Comparative morphology of the genital acetabula of aquatic mites (Acari, Prostigmata): Hydrachnoidea, Eylaoidea, Hydryphantoidea and Lebertioidea. Journal of Natural History 16: 147–160. doi: 10.1080/00222938200770111
- Bergstrom D (1953) Hydracarina from the Rocky Mountain Region. Transactions of the American Microscoicpal Society 72: 157–162. doi: 10.2307/3223514
- Boyaci YÖ, Özkan M (2008) The species of the genus Monatractides Viets, 1926 (Acari, Hydrachnidia, Torrenticolidae) in Turkey. Turkish Journal of Zoology 32: 363–366.
- Chakrabarty P, Warren M, Page LM, Baldwin CC (2013) GenSeq: An updated nomenclature and ranking for genetic sequences from type and non-type sources. ZooKeys 346: 29–41. doi: 10.3897/zookeys.346.5753
- Conroy JC (1968) The water-mites of western Canada. Bulletin of the National Museum of Canada 223: 23–42.
- Conroy JC, Scudder GGE (1975) An annotated checklist of the water mites (Acari) of British Columbia. Syesis 8: 305–310.
- Cook DR (1967) Water mites from India. Memoirs of the American Entomological Institute 9: 1–411.
- Cook DR (1974) Water mite genera and subgenera. Memoirs of the American Entomological Institute 21: 1–841.
- Cramer C (1992) Estudios sobre hidracáridos Mexicanos, familia Torrenticolidae. I. Cinco especies nuevas de Neoatractides y Torrenticola y primer registro de Testudacarus para México. Anales Instituto de Biologia, Universidad Nacionale Autónoma de México, Seria Zoologia 63: 13–27. [In Spanish]
- Cramer C, Cook DR (2000) Water mites of the genera Neoatractides Lundblad and Pseudotorrenticola Walter (Acari: Hydrachnida: Torrenticolidae) from Mexico. International Journal of Acarology 26: 51–61. doi: 10.1080/01647950008683635
- Crowell RM (1961) Catalogue of the distribution and ecological relationship of North American Hydracarina. Canadian Entomologist 93: 321–359. doi: 10.4039/Ent93321-5
- Cuellar D, Underwood DLA (2012) Final report wadeable streams bioassessment region 8 sites samples: May-July 2009. Prepared by California State University Long Beach Stream Ecology and Assessment Laboratory for Santa Ana Regional Water Quality Control Board, 73 pp.
- Davids C, di Sabatino A, Gerecke R, Gledhill T, Van der Hammen H, Smit H (2007) Acari: Hydrachnidia. In: Gerecke R (Ed.) Chelicerata: Araraneae, Acari I. Süßwasserfauna von Mitteleuropa, vol. 7/2–1. Elsevier Spektrum Akademischer Verlag, München, Germany, 241–376.
- Esen Y, Erman O (2014) Kahramanmaraş İli Monatractides K. Viets, 1926 ve Torrenticola Piersig, 1896 (Acari: Hydrachnidia: Torrenticolidae) Türleri ve Türkiye faunası için İki yeni kayıt. Firat University Journal of Science 26: 49–44. [In Turkish]
- Fernández HR, Reid B (2012) Invertebrate distribution on a macroalgae/macrophyte mixed mat in flowing water. Fundamental and Applied Limnology 181: 289–299. doi: 10.1127/1863-9135/2012/0373
- Fisher JR, Fisher DM, Nelson WA, O’Neill JC, Skvarla MJ, Radwell AJ, Ochoa R, Bauchan G, Dowling APG (2015) An integrative description of Torrenticola trimaculata sp. nov. (Parasitengona: Torrenticolidae), a three-spotted, riffle- dwelling mite from eastern North America: morphology, phylogenetics, and taxonomic history of the genus. Acarologia 55: 71–116. doi: 10.1051/acarologia/20152155
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
- Fuste LA (1980) Effects of the Mount St. Helens eruption on the benthic fauna of the Toutle River, Muddy River, and Pine Creek Drainage Basins, Washington. Geological Survey Circular 850-H, 19 pp.
- GEI (2008) Evaluation of potential site-specific zinc, cadmium, and copper standards for the Eagle River, segment 5 and Cross Creek, segment 7b: technical memorandum. Prepared by GEI Consultants, Inc. Ecological Devision, Littleton, Colorado for CBS Operations, 101 pp.
- Goldschmidt T (2007) Studies on Latin American water mites of the genus Torrenticola Piersig, 1896 (Torrenticolidae, Hydrachnidia, Acari). Zoological Journal of the Linnean Society 150: 443–678. doi: 10.1111/j.1096-3642.2007.00305.x
- Guo J-J, Jin D-C (2005) Description of a new species Testudacarus in the subfamily Testudacarinae newly recorded from China (Acari, Lebertioidea, Torrenticolidae). Acta Zootaxonomica Sinica 30: 70–72.
- Guo J-J, Zhang P (2011) Cladistics and phylogeny of genus (subgenus) relationships within Torrenticolidae. Journal of Southwest University (Natural Science Edition) 33: 46–49. [In Chinese]
- Habeeb H (1954) North American Hydrachnellae, Acari – IX-XVI. Leaflets of Acadian Biology 2: 1–14.
- Habeeb H (1956) Notes on water-mites – I. Leaflets of Acadian Biology 11: 1–2.
- Habeeb H (1959a) List of North American water-mites. Le Naturaliste Canadien 86: 19–25.
- Habeeb H (1959b) New Hydrachnellae chiefly from California. Leaflets of Acadian Biology 19: 1–6.
- Habeeb H (1961) Walter Vincent Powers, noble fellow, 1895-1954. Leaflets of Acadian Biology 22: 1–6.
- Habeeb H (1967) A check list of North American water-mites. Leaflets of Acadian Biology 43: 1–8.
- Habeeb H (1969) Notes on water-mites – II. (erratum: III). Leaflets of Acadian Biology 51: 1–2.
- Habeeb H (1974a) New genera of water-mites. Leaflets of Acadian Biology 61: 1–2.
- Habeeb H (1974b) Notes on water-mites. VIII. Leaflets of Acadian Biology 59: 1–4.
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium 41: 95–98.
- Harvey MS (1998) The Australian Water Mites: A Guide to Families and Genera. CSIRO Publishing, Collingwood, 150 pp.
- Hawkins CP (2009) Revised invertebrate RIVPACS model and O/E index for assessing the biological condition of Colorado streams. Prepared by Western Center for Monitoring and Assesment of Freshwater Ecosystems, Department of Watershed Sciences, Utah State University for Colorado Department of Public Health and Environment, Water Quality Control Division–Monitoring Unit, 42 pp.
- Herbst DB, Medhurst RB, Bell ID (2013) Evaluating recovery of stream invertebrate communities following removal of introduced trout in Kings Canyon National Park: baseline biological stream surveys and contrasts after fish removal. Sierra Nevada Aquatic Research Laboratory, University of California, Mammoth Lakes, California, 31 pp.
- Herbst DB, Medhurst RB, Roberts SW (2011) Development of biological criteria for sediment TMDLs: the relation of sediment deposition to benthic invertebrate communities of streams exposed to varied land use disturbances in the Sierra Nevada and Coast Range mountains of California. Prepared by Sierra Nevada Aquatic Research Laboratory for California State Water Resources Control Board, 31 pp.
- Herbst DB, Roberts SW, Medhurst RB, Hayden NG (2011) Sediment TMDL guidance for Central Coast Region of California and the San Lorenzo River: physical habitat and biological criteria for deposited sediments in streams, 24 pp.
- Herbst DB, Silldorff EL (2009) Development of a benthic macroinvertebrate index of biological integrity (IBI) for stream assessments in the Eastern Sierra Nevada of California. Prepared by Sierra Nevada Aquatic Research Laboratory and Delaware River Basin Commission, 89 pp.
- Herbst D, Roberts S, Medhurst B, Bogan M (2010) A Sentinel monitoring network for detecting the hydrologic effects of climate change on Sierra Nevada headwaters stream ecosystems and biological indicators. Sierra Nevada Aquatic Research Labratory, University of California, 26 pp.
- Imamura T (1955) Crenophilous and rheophilous water-mites from Mishima and its vicinity. Bulletin of the Biogeographical Society of Japan 16-19: 181–192.
- Imamura T (1965) Hydrachnellae. In: Sasa M (Ed.) Mites, an introduction to classification, bionomics and control of Acarina.University of Tokyo Press, Tokyo, Japan, 216–251. [In Japanese]
- Imamura T (1976) Two new species of water-mites from Nikko National Park, Japan. Annotationes Zoologicae Japonenses 49: 279–284.
- Imamura T (1980) Hydrachnellae. In: Ehara S (Ed.) Illustrations of the mites and ticks of Japan.Zenokoku Noson Kyoiku Kyokai, Tokyo, 330–379. [In Japanese]
- Imamura T (1986) Acarina: Hydrachnellae. In: Ueno M (Ed.) Freshwater Biology of Japan. Hokuryukan, Tokyo, 368–395.
- Jin D-C, Yi T-C, Guo J-J (2010) A review of progress in taxonomy of water mites from China (Acari: Hydrachnidia). In: Zhang Z-Q, Hong X-Y, Fan Q-H (Eds) Xin J-L, Centenary: Progress in Chinese Acarology.Zoosymposia 4: 106–119.
- Laubitz DR, Sutherland I, Sharma N, Antoine W (1983) 1 Bibliographia invertebratorum aquaticorum canadensium, vol. 1. Invertebrate Zoology Division National Museum of Natural Sciences, Ottawa, 56 pp.
- Lewis WM, McCutchan JH (2005) Environmental thresholds for nutrients in streams and rivers of the Colorado mountains and foothills. USEPA Project number: X7-97805801, 87 pp.
- Lundblad CO (1967) Wassermilben von Hinterindien. Arkiv för Zoologi 19: 391–419. [In German]
- Lundblad O (1941) Eine übersicht des Hydrachnellensystems und der bis jetzt bekannten Verbreitung der Gattungen dieser Gruppe. Zoologiska Bidrag från Uppsala 20: 359–379. [In German]
- Marshall R (1943) Hydracarina from California. Part I. Transactions of the American Microscopical Society 62: 306–324. doi: 10.2307/3223036
- ME Inc. (2011) Evaluation of study design alternatives for benthic invertebrate community assessment at United Keno Hill Mines (DRAFT). Prepared by Minnow Environmental Inc., Georgetown, Ontario, Canada for Elsa Recplamation and Development Company Ltd., Whitehorse, 25 pp.
- Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylo- genetic trees. Proceedings of the Gateway Computing Environments Workshop. New Orleans, Louisiana, 1–8.
- Mitchell RD (1954) Check list of North American water-mites. Fieldiana: Zoology 35: 27–70.
- Mitchell RD (1962) The structure and evolution of water-mite mouthparts. Journal of Morphology 110: 41–59.
- MMWD (2008) Final report: consolidated concept proposal for nonpoint source projects, Greater San Pablo Bay Area grant agreement no. 04-155-552-2. Prepared by Marin Municipal Water District, Corte Madera, 71 pp.
- Morimoto S (2012) Jewelry in the water. Symbiosis 7: 85–87. [In Japanese]
- Newell IM (1959) Acari. In: Edmonson WT (Ed.) Fresh-Water Biology (2nd Ed). John Wiley & Sons Inc, New York, 1080–1116.
- O’Neill JC (2015) Systematics of testudacarine torrent mites (Parasitengona, Hydrachnidiae, Torrenticolidae). Master’s Thesis, University of Arkansas.
- Park J, O’Foighill D (2000) Sphaeriid and corbiculid clams represent separate heterodont bivalve radiations into freshwater environments. Molecular Phylogenetics and Evolution 14: 75–88. doi: 10.1006/mpev.1999.0691
- Peckarsky BI, Fraissinet PR, Penton MA, Conklin DJ (1990) Freshwater macroinvertebrates of Northeastern North America. Cornell University Press, Ithica, 456 pp.
- Pennak RW (1953) Fresh-water invertebrates of the United States. The Ronald Press Company, New York, 769 pp.
- Pennak RW (1978) Fresh-water invertebrates of the United States. John Wiley and Sons Inc, New York, 803 pp.
- Pennak RW (1989) Fresh-water invertebrates of the United States: Protozoa to Mollusca. John Wiley and Sons Inc, New York, 628 pp.
- Pernot CP, Underwood DLA (2010) Final report: wadeable streams bioassessment region 8 sites sampled: May – June 2008. Prepared by California State University Long Beach Stream Ecology and Assessment Laboratory, 73 pp.
- Perrin CJ (2001) Trophic Structure and function in the Cheakamus River for water use planning. Prepared by Limnotek Research and Development Inc., Vancouver, Canada, for BC Hydro and The Resort Municipality of Whistler, 67 pp.
- Perrin CJ (2006) Perophyton and benthic incertebrate monitoring for water use planning in the Coquitlam River, 2006. Prepared by Limnotek Research and Development Inc., Vancouver, 51 pp.
- Perrin CJ, Bennett SA (2011) Coquitlam River periphyton and benthic invertebrate monitoring: Coquitlam River Monitoring Program #5. Prepared by Limnotek Research and Development Inc. for BC Hydro, 73 pp.
- Pešić V, Chatterjee T, Bordoloi S (2010) A checklist of the water mites (Acari: Hydrachnidia) of India, with new records and description of one new species. Zootaxa 2617: 1–54.
- Pešić V, Smit H (2007) First records of water mites (Acari: Hydrachnidia) from Bhutan, with descriptions of two new species. Zootaxa 1613: 45–56.
- Prasad V (1974) A Catalogue of Mites of India. Indira Acarology Publishing House, Ludhiana, 320 pp.
- Proctor HC (1992) The Evolution of Sperm Transfer Behaviour in Water Mites (Acari: Parasitengona).PhD Dissertation, University of Toronto, Toronto, 279 pp.
- Proctor HC (2006) Key to Aquatic Mites Known from Alberta, 13 pp.
- Proctor HC, Smith IM, Cook DR, Smith BP (2015) Subphylum Chelicerata, Class Arachnida. In: Thorp JH, Rogers DC (Eds) Thorp and Covich’s Freshwater Invertebrates: Ecology and General Biology. Academic Press, London, 599–660. doi: 10.1016/b978-0-12-385026-3.00025-5
- De Queiroz K (1998) The general lineage concept of species, species criteria, and the process of speciation: A conceptual unification and terminological recommendations. In: Howard DJ, Berlocher SH (Eds) Endless forms: species and speciation. Oxford University Press, Oxford, 57–75.
- De Queiroz K (1999) The general lineage concept of species and the defining properties of the species category. In: Wilson RA (Ed.) Species: New Interdisciplinary Essays. MIT Press, Cambridge, Massachusetts, 49–89.
- De Queiroz K (2005) A unified species concept and its consequences for the future of taxonomy. Proceedings of the California Academy of Sciences 56: 196–215.
- De Queiroz K (2007) Species Concepts and Species Delimitation. Systematic Biology 56: 879–886. doi: 10.1080/10635150701701083
- Radford CD (1950) Systematic check list of mite genera and type species. Union Internationale des Sciences Biologiques. Série C, Section Entomologique 1: 1–232.
- Richards AB, Rogers DC (2006) List of Freshwater Macroinvertebrate Taxa from California and Adjacent States Including Standard Taxonomic Effort Levels. Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT), 215 pp.
- Richards AB, Rogers DC (2011) List of Freshwater Macroinvertebrate Taxa from California and Adjacent States Including Standard Taxonomic Effort Levels. Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT), 266 pp.
- Smith IM (1982) Larvae of water mites of the genera of the superfamily Lebertioidea (Prostigmata: Parasitengona) in North America with comments on phylogeny and higher classification of the superfamily. Canadian Entomologist 114: 901–990. doi: 10.4039/Ent114901-10
- Smith IM (1987) Order Acariformes, suborder Actinedida (or Prostigmata). In: Lafontaine JD, Allyson S, Behan-Pelletier VM, Borkent A, Campbell JM, Hamilton KGA, Martin JEH, Masner L (Eds) The Insects, Spiders and Mites of Cape Breton Highlands National Park.Biosystematics Research Centre Report 1, 41–46.
- Smith IM (1991a) Descriptions of new species representing new or unreported genera of Lebertioidea (Acari: Hydrachnida) from North America. Canadian Entomologist 123: 811–825. doi: 10.4039/Ent123811-4
- Smith IM (1991b) Water mites (Acari: Parasitengona: Hydrachnida) of spring habitats in Canada. Memoirs of the Entomological Society of Canada 155: 141–167. doi: 10.4039/entm123155141-1
- Smith IM (2010) Water mites (Acarina: Hydrachnidiae) of the Atlantic Maritime Ecozone. In: McAlpine DF, Smith IM (Eds) Assessment of Species Diversity in the Atlantic Maritime Ecozone.Canadian Science Publishing (NRC Research Press), Ottawa, 283–311.
- Smith IM, Cook DR (1991) Water mites (Hydrachnidiae) and other arachnids. In: Thorp JH, Covich AP (Eds) Ecology and Classification of North American Freshwater Invertebrates. Academic Press, San Diego, 523–592.
- Smith IM, Cook DR (1999) An assessment of global distribution patterns in water mites (Acari: Hydrachnida). In: Needham GR, Mitchell RD, Horn DJ, Welbourn WC (Eds) Acarology IX (Vol. 2. Symposia). Ohio Biological Survey, Columbus, 109–124.
- Smith IM, Cook DR, Smith BP (2010) Water mites (Hydrachnidiae) and other arachnids. In: Thorp JH, Covich AP (Eds) Ecology and Classification of North American Freshwater Invertebrates. Academic Press, San Diego, 485–586. doi: 10.1016/B978-0-12-374855-3.00015-7
- Smith IM, Lindquist EE, Behan-Pelletier V (2011) Mites (Acari). In: Scudder GGE, Smith IM (Eds) Assessment of Species Diversity in the Montane Cordillera Ecozone, 193–268. Available at http://www.royalbcmuseum.bc.ca/assets/Montane-Cordillera-Ecozone.pdf
- Smith IM, Smith BP, Cook DR (2001) Water mites (Hydrachnida) and other arachnids. In: Covich JH, Thorp AP (Eds) Ecology and Classification of North American Freshwater Invertebrates. Academic Press, San Diego, 551–659. http://www.sciencedirect.com/science/article/pii/B978012690647950017X [April 10, 2015]
- Stalingo DM (2009) Assessment of aquatic macroinvertebrates on USFS/BLM lands of the Crooked and Sage Creek watersheds. Prepared by the Montana Natural Heritage Program for Darin Watschke, USFS Custer National Forest, Billings, 26 pp.
- Thompson JD, Gibson TJ, Pewniak F, Jeanmougin F, Higgins DG (1997) The Clustal X windows interface: flexible strategies for multiple sequence alignments aided by quality analysis tools. Nucleic Acids Research 24: 4876–4882. doi: 10.1093/nar/25.24.4876
- Viets K (1936) Wassermilben oder Hydracarina (Hydrachnellae und Halacaridae). In: Dahl F (Ed.) Die Tierwelt Deutschlands und der umgrenzenden Meeresteile (31/32). G. Fischer Verlag, Stuttgart, 1–574.
- Viets K (1956) Die milben des süßwassers und des meeres: Hydrachnellae und Halacaridae (Acari). Zweiter un dritter teil. Katalog und nomenklator. Gustav Fischer, Jena, 870 pp.
- Viets K (1935) Die wassermilben von Sumatra, Java und Bali nach den Ergebnissen der Deutschen Limnologischen Sunda-Expedition. II. Teil. Archiv für Hydrobiologie, Supplement13: 595–738.
- Viets KO (1987) Die milben des Süßwassers (Hydrachnellae und Halacaridae [part.], Acari). 2: Katalog. Sonderbände des Naturwissenschaftlichen Vereins in Hamburg 8: 1–1012. [In German]
- Vitzthum H (1942) Acarina. In: Bronn HG (Ed.) Dr H.G. Bronn’s klassen und ordnungen des thierreichs, wissenschaftlich dargestellt in wort und bild. Bd. 5, Abt. 4, Buch 5. C.F. Winter (Akademie Verlag), Leipzig und Heidelberg. [In German]
- Walter C (1928) Zur kenntnis der mikrofauna von Britisch Indien. II. Hydracarina. Records of the Indian Museum 30: 57–108.
- Walter C (1929) Hydracarinen aus Java. Treubia 11: 211–273.
- Walter DE, Lindquist EE, Smith IM, Cook DR, Krantz GW (2009) Order Trombidiformes. In: Krantz GW, Walter DE (Eds) A Manual of Acarology. Texas Tech University Press, Lubbock, 233–420.
- Wiles PR (1997a) Asian and Oriental Torrenticolidae Piersig, 1902 (Acari: Hydrachnidia: Lebertioidea): a revision of the family and descriptions of ne species of Torrenticola Piersig and Pseudotorrenticola Walter, from Southeast Asia. Journal of Natural History 31: 191–236. doi: 10.1080/00222939700770121
- Wiles PR (1997b) The homology of glands and glandularia in the water mites (Acari: Hydrachnidia). Journal of Natural History 31: 1237–1251. doi: 10.1080/00222939700770671
- Williams DD, Mundie JH, Mounce DE (1977) Some aspects of benthic production in a salmonid rearing channel. Journal of the Fisheries Research Board of Canada 34: 2133–2141. doi: 10.1139/f77-280
- Williams D, Hogg ID (1988) Ecology and production of invertebrates in a Canadian coldwater spring-springbrook system. Holarctic Ecology 11: 41–54. doi: 10.1111/j.1600-0587.1988.tb00779.x
- Winger PV, Peters EJ, Donahoo MJ, Barnes JR, White DA (1972) A checklist of the macroinvertebrates of the Provo River, Utah. Great Basin Naturalist 32: 211–219.
- Young WC (1969) Ecological distribution of Hydracarina in north central Colorado. American Midland Naturalist 82: 367–401. doi: 10.2307/2423785
- Zhang P, Guo J-J (2010) Research progress on phylogeny of Torrenticolidae (Acari, Lebertioidea). Guizhou Agricultural Sciences 38: 116–118, 122.