Research Article |
|
Corresponding author: Natapot Warrit ( ich108@hotmail.com ) Academic editor: Thorleif Dörfel
© 2023 Pakorn Nalinrachatakan, John S. Ascher, Max Kasparek, Prapun Traiyasut, Chawatat Thanoosing, Natapot Warrit.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Nalinrachatakan P, Ascher JS, Kasparek M, Traiyasut P, Thanoosing C, Warrit N (2023) A review of the anthidiine bees (Apoidea, Megachilidae) in Thailand. ZooKeys 1186: 235-284. https://doi.org/10.3897/zookeys.1186.95203
|
Abstract
Bees of the tribe Anthidiini (Apoidea: Megachilidae) are notable pollinators consisting of resin bees, wool-carder bees, and cleptoparasitic bees. Twelve anthidiine species were historically reported in Thailand, though the taxonomic information of the group was needed revising. In this study, 165 (97♀, 68♂) anthidiine bee specimens deposited at the Chulalongkorn University Natural History Museum, Thailand, were examined with material obtained from various museum collections. Specimens were principally collected in Thailand with some from Laos and Myanmar. Here, at least eight genera and 15 species of anthidiine bees are recognized: Anthidiellum (5), Bathanthidium (1), Eoanthidium (1), Euaspis (4), Pachyanthidium (1), Pseudoanthidium (1), Stelis (1), and Trachusa (1). Dianthidium chinensis Wu, 1962, Eoanthidium chinensis (Wu, 1962), Eoanthidium semicarinatum Pasteels, 1972, and Eoanthidium punjabensis Gupta & Sharma, 1953 are relegated as junior synonyms of Eoanthidium (Hemidiellum) riparium (Cockerell, 1929), stat. nov. Both Anthidiellum (Pycnanthidium) latipes (Bingham, 1897) from Phang Nga and Euaspis aff. wegneri Baker, 1995 from Chumphon were identified as new records for Thailand. Trachusa aff. vietnamensis Flaminio & Quaranta, 2021 from Phitsanulok is a new record for the genus found in Thailand, whereas Pseudoanthidium (Pseudoanthidium) orientale (Bingham, 1897) is a new record for Laos. Annotated comments are provided for some taxa and identification keys for the Thai anthidiine bees is provided.
Key words
Pollinator, resin bees, Southeast Asia, taxonomy, wool carder bees
Introduction
Megachilid bees in tribe Anthidiini are robust, usually with yellow maculation and sparse pubescence on the body. The diagnostic characters for the Anthidiini include a short pterostigma (length less than twice of its width), the absence of a median spine on the metanotum, and in many species also by an absence of long hairs on the hind tibial surface (
The tribe Anthidiini is classified into three groups, based on their nesting material usages: resin users, plant fiber users, and cleptoparasitic species (
Anthidiine bees have been scarcely collected in Thailand, except for the cleptoparasitic Euaspis polynesia Vachal, 1903 since its preferred host, Megachile (Callomegachile) disjuncta (Fabricius, 1781), is common. Only 12 species of Anthidiini have been previously recorded in Thailand (
There are persistent taxonomic difficulties for Thai anthidiines which need revision since many species were only recorded once. For example, the rare endemic resin bee genus Anthidiellum subgenus Ranthidiellum, of which four species were recorded, two were only known from females (see
Material and methods
One hundred and sixty-five anthidiine specimens (97♀, 68♂) were examined in this study. Most of the specimens were collected after 2003 and deposited at the Chulalongkorn University Natural History Museum (
Specimens were photographed with two photographic systems. The first system used the Canon 7D Mark II digital camera attached to a Zeiss Stemi 508 stereomicroscope, with a T2-T2 1.6× SLR long-distance microscope lens, controlled via Canon EOS Utility software. The second system used the identical digital camera but was mounted into the Cognisys Stackshot Macro Rail Package system and attached with Canon MP-E 65 mm f/2.8 1-5× macro lens. These sets of photographs were calibrated using AXIOVISION SE64 Rel. 4.9.1 software, for the measurement of the morphological characters of the specimens. All images taken were then post-processed using Adobe Photoshop CC 2018 and Adobe Lightroom CC 2018 software. Other software, including Adobe Illustrator CC 2018, ImageJ, Google Earth Pro, and QGIS (3.16.0) were also used to produce the illustrations, examining small and often overlooked characters, and ascertaining the localities of the samples through mapping.
Male bee specimens were dissected for their genitalia and terminalia examination: i.e., using 3M KOH to clear out muscular artifacts and later preserved in glycerin (adapted from
In addition to records obtained through specimen examinations, the five Thai Anthidiini taxa were consulted in
Results
Taxonomic account
Anthidiellum
Anthidium (Anthidiellum) Cockerell, 1904: 3. Type species: Trachusa strigata Panzer, 1805, by original designation.
Note
Anthidiellum is a small-robust genus that has an arcuate subantennal suture (Fig.
Anthidiellum (Pycnanthidium) smithii
Anthidium smithii Ritsema, 1874: 111. (♂) Male holotype from Ambarawan, Java (NBC, not examined).
Anthidium minutissimum
Bingham, 1903: 6. (♂) Male holotype from “Biserat, Jalor, Siam” [= Yala province, Thailand] (
Anthidium javanicum Friese, 1909: 257. (♂) Two syntypes from Buitenzorg [= Bogor, Java], collected by Schmiedeknecht.
Anthidiellum smithii smithii
(Ritsema):
Material examined
11 (3♀, 8♂). Myanmar (new record): 1♀, Dawei city, Dawei Hospital (13°59.117'N, 98°7.479'E, alt. 4 m), 3 May 2018, N. Warrit et al. (
Distribution
Indonesia (Bangka, East Kalimantan, Java, Sumba, Maluku Island), LAOS (Houaphanh), Malaysia (Negiri Sembilan, Penang), Myanmar (Dawei, new record), Philippines (Palawan), Singapore, Thailand (Chaiyaphum, Chiang Mai:
This species can be rarely found in the Southeast Asian region. A similar species, Anthidiellum (Pycnanthidium) carinatum (Wu, 1962), is known from China (Hainan, Yunnan) and India (Tripura) (see
Diagnosis
Within subgenus Pycnanthidium, this species has a small black body (3.9–5.0 mm) with yellow maculations on all tagmata. It differs from other congeners by its metasomal coloration, i.e., T1 with yellow markings laterally, T2 entirely black, T3–T6 with broad yellow bands, mostly interrupted medially on T3; axilla yellow; broad yellow marginal band on scutellum, medially interrupted; outer surface of the hind tibia and hind basitarsus with longitudinal carinae; black apical comb of S5 in male interrupted medially resembles small notch; gonostylus bifid. However, this species is similar to A. carinatum (Wu, 1962) from China, although
Floral association
Bidens pilosa L. (Asteraceae), Muntingia calabura L. (Muntingiaceae) (
Remarks
Anthidiellum smithii was originally reported in Thailand from Yala province as A. minutissimum Bingham, 1903. More than a hundred years later,
In this study, four males collected at the same time and place in Chumpon Province display varying color patterns. Yellow teardrop markings on the frons are small in two specimens (Fig.
Anthidiellum (Pycnanthidium) latipes
Anthidium latipes
Bingham, 1897: 495 (♀). Holotype from “Rangoon” [= Yangon], Myanmar (
Paraanthidium latipes
Bingham:
Trachusa (Paraanthidium) latipes
(Bingham, 1897):
Anthidiellum (Pycnanthidium) latipes
(Bingham, 1897):
Material examined
3 (3♀). Myanmar: 1♀ holotype, Rangoon [= Yangon, Myanmar], 1–87 Bingham coll., Anthidium latipes ♀ Bingh Type B.M. TYPE HYM. 17a.1873, Col. C.T. Bingham. 96–30. (
Records from iNaturalist
(2023). Myanmar: Yangon, Yangon District, (16°47'12.3"N, 96°08'38.1"E) observed by ‘chimik’ on 25 Apr. 2022. (observation id: 113229246). Thailand: Chiang Mai, Mueang District, Suthep-Pui (18°49'00.5"N, 98°55'26.8"E, accuracy 240 m) observed by ‘jackychiangmai’ on 9 Apr. 2023 (observation id: 154207614), and on 13 Apr. 2023 (observation id: 154700134 and 154702585).
Distribution
China (Yunnan), Myanmar (Yangon), Thailand (Chiang Mai (new record from
Diagnosis
Anthidiellum latipes can be assigned to a group of Asian Pycnanthidium which includes A. butarsis Griswold, 2001, A. ramakrishnae (Cockerell, 1919), A. rasorium (Smith, 1875), A. coronum (Wu, 2004), and A. cornu Tran & Engel, 2023. The group contains medium-sized bees without carina on their hindlegs (
Floral association
Marigold (Tagetes erecta L., Asteraceae, see Fig.
Female of Anthidiellum latipes (Bingham, 1897) from Phang Nga, Thailand (BSRU-AB-0162) (A–E), and the female holotype of A. latipes from Yangon, Myanmar (
Remarks
The knowledge on the Asian Pycnanthidium is relatively scant due to the limited material and the damages in type specimens such as in Anthidiellum ramakrishnae (Griswold, 2001), and with the materials of A. coronum (discussed below). The female holotype of A. latipes from Myanmar is also not in good condition, the head and most of the legs were missing while the mesosoma and metasoma were glued together.
There is a possibility that A. coronum (Wu, 2004) is a junior synonym of A. latipes, as the color patterns on the supraclypeal area (Fig.
Anthidiellum (Ranthidiellum) apicepilosum
Dianthidium apicepilosum
Dover, 1929: 55 (♀). Holotype from “Khao Ram, Siam” [= Nakhon Si Thammarat, Thailand] (
Dianthidium apicepilosum
Dover:
Anthidiellum (Ranthidiellum) apicepilosum
(Dover):
Anthidiellum
(Rhanthidiellum [sic!]) apicepilosum (Dover):
Material examined
1♀. Khao Ram, Siam [= Thailand: Nakhon Si Thammarat: Ronpibun, Khao Ramrome], 750–1200 [possibly altitude], 24 Feb. 1922, Anthidium apicepilosum Dover, 1926 (holotype
Distribution
Malaysia (Negeri Sembilan, Penang, Selangor), Thailand (Nakhon Si Thammarat). The species is rarely found, hence the records are based on the original designation (
Diagnosis
Anthidiellum apicepilosum has a black body with distinct brownish coloration disrupted. Since only the female is known, the most comparable characters include the following: clypeus, scape, lower paraocular area, tegula, axilla, and the margin of scutellum brownish; scutum black; wing base conspicuously dark brown, clearly contrasting to apical hyaline parts on 1st submarginal cells; T1–T5 with reddish to brownish ferruginous apical band that becomes wider on the rear segments; T6 black; the rear of the metasoma covered with yellowish hairs; foreleg light brownish, generally brighter than in midleg and hindleg, which are almost black on their tibia and basitarsus. According to
Remarks
This is the first species of Ranthidiellum that has been documented for its nesting biology (
Anthidiellum (Ranthidiellum) ignotum
Anthidiellum ignotum Engel, 2009: 30–34, figs 1–3. (♀) Holotype from Sakaerat Environmental Research Area, Nakhon Ratchasima Province, Thailand (SEMC, not examined).
Anthidiellum ignotum
Engel:
Material examined
(6♀, 1♂). Same specimens as in
Record from iNaturalist
(2023). Thailand: Chiang Mai, Mueang District, Suthep Subdistrict (18°49'0.47"N, 98°55'26.81"E) uploaded by ‘jackychiangmai’ on 27 Oct. 2022 (observation id: 140223648).
Distribution
Thailand (Chiang Mai, Nakhon Ratchasima, Phayao). The species is rare and appears to be endemic.
Floral association
Plant family Amaranthaceae (possibly Achyranthes aspera L., commonly known as devil’s horsewhip), shown in the iNaturalist observation noted above. Also, the bee must mobilize plant resin as do other Ranthidiellum species (Pagden 1932;
Remarks
Anthidiellum ignotum has distinct sexual dimorphism in which the male particularly had its base coloration brighter, with a noticeable black facial mark. More details on the presence of Ranthidiellum species in Thailand and its variation were discussed in
Comparing specimens of Anthidiellum (Ranthidiellum) recently collected from Thailand, and its cleptoparasite A female Anthidiellum ignotum Engel, 2009 (BSRU-AA-1249) B female paratype of Anthidiellum phuchongense Nalinrachatakan & Warrit, 2021 (BSRU-AB-0159) C male paratype of cleptoparasite Stelis flavofuscinular Nalinrachatakan & Warrit, 2021 (BSRU-AB-0156). Scale bar: 2 mm.
Anthidiellum (Ranthidiellum) phuchongense
Anthidiellum (Ranthidiellum) phuchongensis
Nalinrachatakan & Warrit in
Material examined
(5♀, 1♂). Same specimens as in
Distribution
Thailand (Ubon Ratchathani). From a survey in other adjacent national parks in Ubon Ratchathani, there is evidence that Ranthidiellum is present through an abandoned nest with collapsed structures (i.e., resin became opaque, whitish, and the entrance apically fractured).
Floral association
Mobilizing the resin of plants in the family Dipterocarpaceae, possibly Dipterocarpus obtusifolius Teijsm. ex Miq., which is broadly distributed along their nesting habitats.
Bee cleptoparasites
Stelis flavofuscinular Nalinrachatakan & Warrit, 2021.
Remarks
The species-group name phuchongensis is changed to phuchongense following a mandatory change for gender agreement under ICZN article 34.2. The species was discovered to build its nest in a dipterocarp forest in Phu Chong Na Yoy National Park, in a preexisting hole near water stream. Their nesting structures are unique, with a distinct downwardly curved, resinous, translucent tube. Further details of its nesting biology and morphology variations were discussed in
Bathanthidium
Bathanthidium Mavromoustakis, 1953: 837. Type species: Dianthidium bifoveolatum Alfken, 1937, by original designation.
Note
Bathanthidium is an Asiatic genus consisting of small to medium-sized species that are mostly found in China. They come with almost black body and distinct yellow maculation (see Fig.
Type specimen of Stelis siamensis Friese, 1904, male, from Nan province, Thailand, which was recently synonymized with Bathanthidium (Manthidium) binghami (Friese, 1901) A lateral view B dorsal view C posterior angle of metasoma D original label E face F dorsal of metasoma. Scale bars: 1 mm.
Bathanthidium (Manthidium) binghami
Anthidium fraternum
Bingham, 1897 (nec
Anthidium binghami Friese, 1901: 224, replacement name for Anthidium fraternum Bingham, 1897.
Manthidium binghami
(Friese, 1901):
Stelis siamensis
Friese, 1925: 40 (♂). Holotype from “Siam bei Hinlap” [= Nan province, Thailand] (
Paraanthidium concavum Wu, 1962: 164 (♂). Holotype from China, Yunnan, Xishuangbanna (IZCAS: Institute of Zoology, Chinese Academy of Sciences, images examined).
Trachusa (Paraanthidium) concavum
(Wu, 1962):
Bathanthidium (Manthidium) binghami
(Friese, 1901):
Material examined
(2♂). India: 1♂, West Bengal, Buxa Tiger Reserve, 22 miles, East Damanpur (26°37.067'N, 89°33.633'E), 27 Mar. 2019, A. Rameshkumar (ZSI) as in Sadar et al. (2022). Thailand: 1♂, Siam [= Thailand], Hinlap [= Nan province, “Hinlap” must refer to the area of “Baan Hinlap”, or “Huai Hinlap reservoir” as currently named (not in Chaiyaphum province) in Pua district, Sila lang Subdistrict], Januar [= January], H. Fruhstorfer, Stelis siamensis, ♂, 1904, Friese det., Type (
Records from iNaturalist
(2023). Thailand: Chiang Mai Province, Chiang Dao District, (19°24'44.3"N, 98°55'17.3"E) observed by ‘charlieglasser’ on 23 Mar 2023 (observation id: 160344574 and 160340826).
Distribution
China (Yunnan), India (Sikkim, West Bengal), Myanmar (Tenasserim), Thailand (Chiang Mai (new record from
Diagnosis
Bathanthidium binghami has a robust, small to medium-sized body with black integument disrupted by striking yellow markings. The species is distinctly separated from its congeners by the combination of the follows: yellow on its mandible, clypeus, and paraocular area that do not exceed beyond the antennal socket plane; narrow yellow stripe laterally on T2–T5, while tending to abut together on the rear segment; yellow stripe on T6 and also T7 in male; rounded omaulus; T6 (also in the smaller male T7) sub-truncate, with distinct median elevation that extends its apical margin (Fig.
Remarks
Bathanthidium was revised by
Through personal communication with Mr. Charles H. Glasser, who provided the iNaturalist records, we know that the bee inhabits farmland cultivated by the indigenous people of Lisu tribe.
Eoanthidium
Dianthidium (Eoanthidium) Popov, 1950: 316. Type species: Anthidium insulare Morawitz, 1873, by original designation.
Eoanthidium (Eoanthidiellum) Pasteels, 1969: 51. Type species: Anthidium elongatum Friese, 1897 = Anthidium clypeare Morawitz, 1873, by original designation.
Note
An old-world genus which is mostly discernable from other genera by its more slender, striking black-yellow body, with a distinct juxta-antennal carina (Fig.
Eoanthidium (Hemidiellum) riparium (Cockerell, 1929). Female holotype of Dianthidium riparium Cockerell, 1929 (syn.) from Thailand (A, B). Male from Thailand (BSRU-AB-4360) (C, D). Female from Laos (BMNH-ENT-2017-196 (ACQ)) (E), Female from Laos (BSRU-AA-1224) (F), male from Laos (BSRU-AA-1236) (G–K), and male from Thailand (BSRU-AB-4358) (L) H–J male genitalia in dorsal, ventral, and lateral view K male S8 L apical sterna of male in ventral view. Scale bars: 2 mm (D); 1 mm (A–C, E–G); 0.5 mm (H–L).
Eoanthidium (Hemidiellum) riparium , comb. nov.
Dianthidium riparium
Cockerell, 1929: 204 (♀). Holotype from Nan, Thailand (
Dianthidium chinensis Wu, 1962: 167–168, figs 22–26 (♂) (syn. nov.). Type from Yunnan, Xishuangbanna, 9 Apr 1955.
Eoanthidium (Hemidiellum) semicarinatum Pasteels, 1972: 112–116 (♀, ♂) (syn. nov.). Female holotype and male paratypes from Pondicherry State, Karikal, India (NBC, examined).
Eoanthidium (Hemidiellum) punjabensis
Gupta & Sharma in
Eoanthidium (Hemidiellum) punjabense
Gupta & Sharma in
Eoanthidium (Eoanthidium s. str.) chinensis (Wu, 1962): Wu, 2006: 134, fig. 66 (syn. nov.).
Material examined
39 (16♀, 23♂). India: Karnataka: Bangalore, GKVK, 1♀, 2 Apr. 1982, Ghorepade, 1♂, 15 Apr. 2013, Girish, (UAS); Mysore, 1♀, 19 Apr. 2009, 2♀, 16 Apr. 2009, 1♂, 5 Apr. 2009, Dhanyavathi. (UAS); 1♂, Mandya, 1 May 2014, Veereshkumar (UAS; same specimens as
Distribution
China (Yunnan, new record), India (Haryana: Hisar, Karnataka: Bangalore, Koppala, Mysore, Mandya, Tamil Nadu: Karikal, Coimbatore, Punjab: Pathankot), Laos (Champasak, new record), Myanmar (Dawei, new record), Pakistan (Punjab: Lahore), Thailand (Chiang Mai (new record), Lampang (new record), Nan (new record)).
Diagnosis
The species exhibits pale yellow maculation, remarkably on supraclypeal area (which is reduced medially into a unique shape or absent (Fig.
Floral associations
The record of Chinese element (
Remarks
Although the specimens from China, Laos, Myanmar, and Thailand are different in their coloration compared to the type bearing the name Eoanthidium (Hemidiellum) semicarinatum, some characters and male genitalia are unique among the genus, and obviously comparable (see also the figures in
When compared with “Eo. semicarinatum” specimens from India and Pakistan, it is evident that the individuals from Southeast Asia and China (Yunnan) are larger and darker, and tend to come with a reduction in pale yellow facial maculation in supraclypeal area, frons, and on mesepisternum, scutum, and scutellum (Fig.
Illustration of Eoanthidium (Hemidiellum) riparium (Cockerell, 1929) facial maculation mapped according to their geographic locations (blue boxes indicate Indian-Pakistani morphs, reddish boxes indicate Indochina morphs; a morph marked with asterisk is illustrated based on
Individuals from the eastern part of the distribution (China, Laos, Myanmar, and Thailand) have a black background color of the integument with yellow markings (Fig.
Male of Eoanthidium riparium (Cockerell, 1929) from different regions A–C dorsal view of individuals from South India (Karnakata), Pakistan (Panjab), and North India (Hisar, Haryana) respectively D face of a male Eoanthidium riparium from Pakistan (SEMC27). Note the change in the ground color from black (A) across reddish brown on terga and black on scutum (B) to entirely reddish brown (C). Also, note the shape of the reddish apical margin of the clypeus (black arrows) which is similar to the drawing of
Additionally, specimens from India and Pakistan have a much larger paramedian mark on the scutum, often extending to connect with the anterolateral mark, and generally they display more extensive maculations. The female almost has a fully yellow hindleg, sometimes with the black left on the tarsi and parts of basitarsus. Such individuals with richer yellow maculation are typical for Pakistan. Some females from southern India (including those shown by
For some West Palaearctic Eoanthidium and Rhodanthidium species,
In addition, Eoanthidium punjabense Gupta & Sharma, 1993 is established here as a new synonym of Eo. riparium.
Euaspis
Euaspis Gerstaecker, 1858: 460. Type species: Thynnus abdominalis Fabricius, 1793, by original designation.
Dilobopeltis Fairmaire, 1858: 266. Type species: Dilobopeltis fuscipennis Fairmaire, 1858 = Thynnus abdominalis Fabricius, 1793, by original designation.
Parevaspis
Ritsema, 1874: 71. Type species: Parevaspis basalis Ritsema, 1874, by designation of
Note
As a cleptoparasitic bee, Euaspis has a distinct median longitudinal carina (Fig.
Euaspis aequicarinata
Euaspis aequicarinata
Pasteels, 1980: 78 (♀, ♂). Female holotype from Kalabankan, Sabah, Malaysia (image in
Euaspis aequicarinata
Pasteels:
Material examined
(1♀, 2♂). Thailand: 1♀, Chiang Mai (new record), Chom Thong District, Ban Luang Subdistrict, Doi Inthanon National Park, Ban Mae Klang Luang. (18°32'17.9"N, 98°32'49.6"E, alt. 1,057 m), 30 Aug. 2021, on Coleus scutellarioides (L.) Benth. [Lamiaceae], T. Srimaneeyanon et al. (
Distribution
China (Yunnan), Indonesia (Java), Laos (Vientiane), Malaysia (Negeri Sembilan, Borneo: Sabah, Sarawak), Thailand (Chiang Mai (new record), Nakhon Ratchasima, Phayao (new record), Surat Thani)), Vietnam (Kon Tum, Hoa Binh).
Diagnosis
Typically for Euaspis, Eu. aequicarinata has a black body with a reddish metasoma, and a median carina and a juxta-antennal carina are present on its face. This is the only species that has a distinct longitudinal carina on the clypeus, while the sculptures are confluent. Pale yellow patches are found on the lateral margin of scutellum and posterior margin of axilla (absent on axilla for female in this study, in contrast to the monochrome pictures in
Females of Euaspis polynesia Vachal, 1904 (BSRU-AA-4453) (A–D) and Euaspis strandi Meyer, 1922 (BSRU-AA-4470) (E–H) A, E lateral habitus B, F face C, G mesosoma including the scutellum D, H S6, with a white dash line indicating boundary of the median elevated area in the left. Scale bars: 2 mm (A, C, E, G); 1 mm (B, D, F, H).
Floral associations
Coleus scutellarioides (L.) Benth. (Lamiaceae).
Remarks
As mentioned in
Euaspis polynesia
Stelis abdominalis
Smith, 1858 (nec
Euaspis polyesia
Vachal, 1903a: 97. (♀ nov., ♂), incorrectly labeled (
Euaspis polynesia Vachal, 1903b: 173, justified emendation.
Euaspis smithii Friese, 1904: 137, unnecessary replacement name.
Parevapis impressus Vierick, 1924: 745. (♀, ♂) Male holotype and female allotype from Surigao, Mindanao (USNM: United States National Museum, not examined).
Euaspis basalis chinensis
Cockerell, 1930: 50. (♀, ♂). Female type and male cotype from Foochow, China (
Euaspis (Parevapis) polynesia
Vachal:
Euaspis (Parevapis) polyesia
Vachal:
Euaspis polynesia
Vachal:
Material examined
43 (20♀, 23♂). Thailand: 1♀, Chainat (new record) [with obscured label] (KKIC); 1♀, Chanthaburi, Makam District, 25 May 2015, N. Chattanabun (
Distribution
China (Anhui, Fujian, Gansu, Guangdong, Hebei, Hunan, Jiangsu, Jiangxi, Shangdong, Xizang, Yunnan, Zhejiang), Hong Kong, Indonesia (Bali, Bangka Island, Engano Island, Java, Maluku Islands [Ambon, Buru, Kai islands], Sebesi Island, Sumatra, Sulawesi), India (Arunachal Pradesh), Japan (Okinawa Prefecture), Laos (Xiengkhouang), Malaysia (Kedah, Kelantan, Melaka, Penang, Perak, Selangor), Myanmar (Shan State, Tenasserim, Yangon), Nepal (Kathmandu), Philippines (Luzon, Mindanao), Singapore, Taiwan (Pingtung), Thailand (Chiang Mai, Chainat (new record), Kanchanaburi (new record), Loei, Mukdahan (new record), Nakhon Pathom (new record), Pattani, Phayao (new record), Phetchabun (new record), Phetchaburi (new record), Ratchaburi (new record), Saraburi (new record), Satun, Songkhla (new record), Surat Thani, Trang (new record), Ubon Ratchathani (new record)), Vietnam (Bak Kan, Dak Lak, Dak Nong, Dien Bien, Hoa Binh, Phu Tho, Son La, Thanh Hoa, Vinh Phuc).
Most of the previous records were documented by
Diagnosis
This Euaspis species has an entirely reddish metasoma, while the prosoma and mesosoma are all black; face with longitudinal carina and a median longitudinal ridge; clypeus with uniform punctation; punctures on the scutellum looser and coarser than on the scutum; scutellum large, strongly produced posteriorly, apicomedially with a depressed area; female S6 acute, with a median carina, without a distinct basal area (Fig.
Floral associations
A female collected from Chiang Mai was wandering on the inflorescences of “Tropical whiteweed” Ageratum conyzoides L. (Asteraceae), “Black-Jack” Bidens pilosa (L.) Benth. (Asteraceae), and “Mexican heather” Cuphea hyssopifolia K. (Lythraceae). For Singapore,
Host-parasite relationship
Remarks
Euaspis polynesia is the most common anthidiine bees in Thailand, exhibiting a size range, with the females ranging 9.0–13.1 mm and the males 6.2–12.1 mm. As a cleptoparasitic bee, its occurrence seems to follow the distribution of its hosts, especially Megachile disjuncta (see
A probable new species of Euaspis from Singapore (
Euaspis strandi
Euaspis (Parevaspis) strandi
Meyer, 1922: 236, 239 (♀, ♂, syntypes, male selected as lectotype by
Parevaspis bakeri Vierick, 1924: 745 (♂). Holotype from Kolambugan, Mindanao, Philippines (USNM: United States National Museum, not examined).
Euaspis strandi
(Meyer):
Material examined
(2♀). Thailand: Phayao (new record), Mueang District, Maeka Subdistrict, Phayao University (19°1'31.45"N, 99°53'24.17"E, alt. 558 m), 1 Jun. 2012, W. Suwannarak et al. (
Distribution
China (Yunnan, “Kinpin”:
Diagnosis
Euaspis strandi has a reddish metasoma, whereas the rest of the body is black, with a remarkable pale yellow stripe on the mesonotum (i.e., axilla and scutellum with pale yellow marginal band); clypeus with coarse, somewhat irregular punctures (Fig.
Floral associations
Sindora siamensis Teijsm. ex Miq. (Fabaceae) is associated with the female collected from Nakhon Ratchasima, Thailand (
Remarks
In Thailand, Eu. strandi was reported from Sakaerat, Nakhon Ratchasima province in 1995 (
The female individual was not observed in this study. Previously, two male specimens had been designated, the first one by
Euaspis aff. wegneri
Euaspis wegneri Baker, 1995: 290, figs 24, 31 (♀). Holotype from “BATJAN” [= Bacan, north Maluku, Indonesia] (NBC, not examined).
Material examined
1♀. Thailand: Chumporn (new record?), Sawi District, Na Sak Subdistrict (10°10'10.7"N, 98°56'50.5"E), 1 Jun. 2021, Suntaree Kanchananiyom. (
Distribution
Indonesia (Bacan province in north Maluku [= Batjan (in Dutch) in
Diagnosis
This female Euaspis aff. wegneri has a typical black body and reddish metasoma, with a pale yellow stripe on the mesonotum (Fig.
Floral associations
Unknown.
Remarks
Euaspis wegneri has been described on the basis of a single female and has never been reported after that. The holotype of Eu. wegneri represented with monochrome digitization in
Pachyanthidium
Anthidium (Pachyanthidium)
Friese, 1905: 66–75. Type species: Anthidium bicolor Lepeletier, 1841 designated by
Pachyanthidium
Friese:
Note
This genus can be easily distinguished by its explicit robust body, closed scutoscutellar suture (Fig.
Pachyanthidium (Trichanthidium) lachrymosum
Anthidium lachrymosum
Smith, 1879: 463 (♀, ♂, syntype). from Bombay [Mumbai, Maharashtra, India] (
Anthidium lachrymosum Smith: Bingham, 1897: 492.
Anthidium serapiforme
Friese, 1914: 322 (♂). Holotype from Perak [Perak, Malaysia] (
Pachyanthidium lachrymosum
(Smith):
Pachyanthidium lachrymosum
(Smith):
Material examined
(24♀, 3♂). India: 1♀, Bombay Dist. [= Mumbai, Maharashtra], B.M. TYPE HYM.17a 1866, (syntype) (
Distribution
India (Karnataka (Bangalore, Mysore), Malabar (as per
The records from
Diagnosis
Pachyanthidium lachrymosum can be distinguished from other congeneric species by its black body with a white lateral band of short white hairs on the metasoma; lamellate parts are often translucent reddish brown to black; eyes with sparse short hairs; mandibles with four teeth; arolia absent; male similar to females but mostly differs in the presence of the arolia, three mandibular teeth, lateral spines on T3–T6, and a tridentate T7 (Fig.
Floral associations
Bidens pilosa (L.) (Asteraceae) (this study), Leucas aspera (Willd.) Link (Lamiaceae) (
Remarks
The other four species of Pachyanthidium (Trichanthidium) were revised by
All specimens in
Pseudoanthidium
Anthidium (Pseudoanthidium)
Friese, 1898: 101. Type species: Anthidium alpinum Morawitz, 1873, designated by Sandhouse, 1943: 593. See
Paranthidiellum Michener, 1948: 25. Type species: Anthidium cribratum Morawitz, 1875, by original designation.
Pseudoanthidium (Paraanthidiellum) Pasteels, 1969: 79, unnecessary emendation of Paranthidiellum Michener.
Pseudoanthidium (Carinellum) Pasteels, 1969a: 80. Type species: Anthidium ochrognathum Alfken, 1932, by original designation.
Trachusa (Orientotrachusa) Gupta, 1993: 50. Type species: Anthidium orientale Bingham, 1897, by original designation.
Pseudoanthidium
Friese:
Note
Pseudoanthidium commonly has a tentorial pit placed below the connection of the subantennal suture and the epistomal suture (
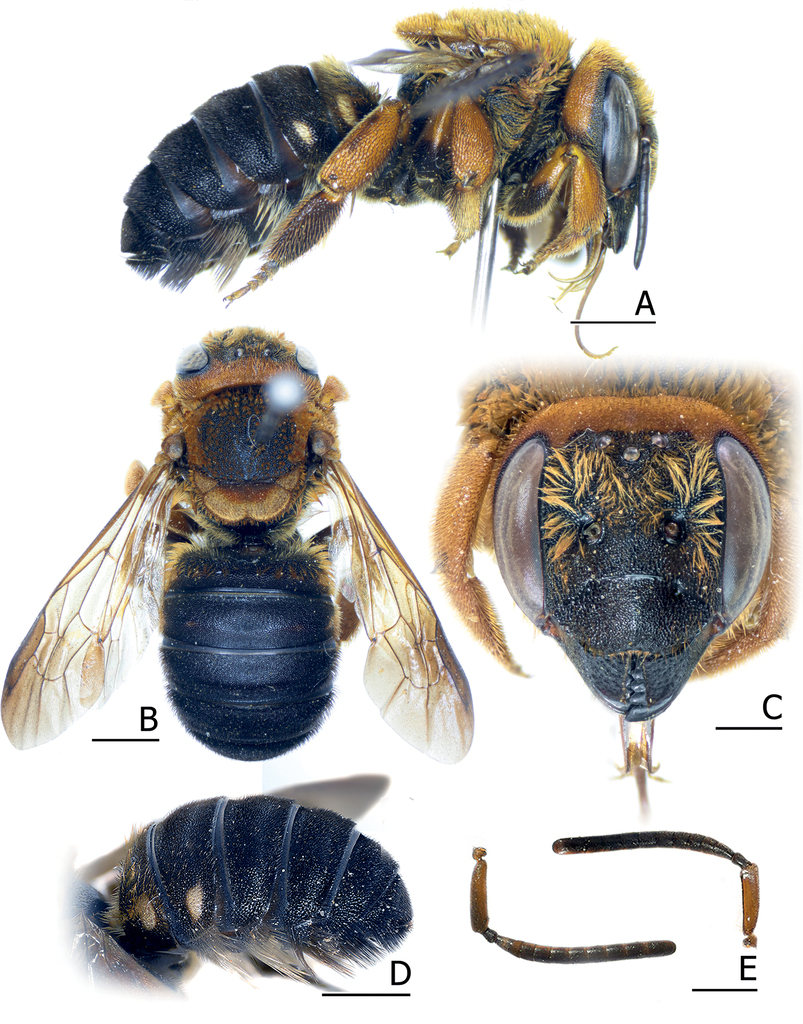
Pseudoanthidium orientale (Bingham, 1897) [showing Mae Hong Son female (BSRU-AA-1240) (A–D), and male (BSRU-AA-1241) (E–K)] A, H dorsal habitus B, G lateral habitus C, F face D female T6 E male T7 I male S5 J S8 K male genitalia in lateral (left), dorsal (middle) and ventral (right). Scale bars: 2 mm (A, B, G, H); 1 mm (C–F); 0.5 mm (I–K).
Pseudoanthidium (Pseudoanthidium) orientale
Anthidium orientale
Bingham, 1897: 496 (♀). Holotype from Tenasserim, Myanmar, image examined in
Anthidium kryzhanovskii Wu, 1962: 167 (♀). Holotype from Jinping Xian, Yunnan, China (IZCAS: Institute of Zoology, Chinese Academy of Sciences, not examined).
Pseudoanthidium (Paraanthidiellum) orientale
(Bingham):
Trachusa (Orientotrachusa) orientale
(Bingham, 1897):
Anthidium (s. str.) kryzhanovskii
Wu, 1962:
Pseudoanthidium (Pseudoanthidium) orientale
(Bingham):
Pseudoanthidium (Pseudoanthidium) orientale
(Bingham):
Material examined
(10♀, 3♂). Laos (new record): 3♀, Champasak, Si Phan Don, Don Det, 20 Jan. 2015, N. Warrit et al., (
Records from iNaturalist
(2023). Thailand: Chiang Mai, San Sai District, San Sai Noi Subdistrict (18°49'08.6"N, 99°01'15.1"E) uploaded by ‘jackychiangmai’ on 14 Jan. 2022 (observation id:104911660); Chiang Rai, Chiang Saen Lake, Viang Yonok Hotel (20°15'42.5"N, 100°02'59.5"E), uploaded by ‘pam-pilombino’ on 27 Jan 2020.
Distribution
Cambodia (Mondulkiri:
Diagnosis
Pseudoanthidium orientale is a medium-sized bee (6–8 mm) and usually has a black integument with yellow maculations in all tagmata. It has a remarkably pale yellow mark on the paraocular area reaching close to the top of eyes, female mandibles with five or six teeth, rounded scutellum with broad marginal maculation which is medially disrupted, tibia and tarsi yellow except black on the venter, female terga with yellow paramedian maculation on T1–T5 in female, which is more laterally extended on T1 and T2 and nearly rectangular in T3–T5. The male looks superficially similar to the female but has a different dentition of the mandible (i.e., distinctly tridentate, broader especially the inner tooth), and maculation on T6 and T7. Male genitalia broad.
Floral associations
Plants with hairy surfaces (see iNaturalist observation from Lamphun) must be the resources for the nesting material. Also, from iNaturalist image from Chiang Mai, the photographs clearly show the bee wandering on the inflorescence of Antigonon leptopus Hook. & Arn. (Polygonaceae), which is a hairy plant, although there is no direct evidence to this claim.
Remarks
This species was described by
Pseudoanthidium orientale is superficially similar to Ps. rotundiventre (Pasteels, 1987) from Sri Lanka and India.
As the male genitalia of Ps. orientale illustrated in
Finally, there is an observation of wool-collecting behavior of a female of Ps. orientale from Chiang Saen Lake, Chiang Rai, Thailand, reported and observed by Ms. Pamela Piombino [user: ‘pam-pilombino’] on 27 January 2020, and published on iNaturalis.org (
Stelis
Trachusa
Jurine, 1801: 164 (nec
Stelis
Panzer, 1806: 246. Type species: Apis aterrima Panzer, 1798 (nec
Gyrodroma
Klug in
Gymnus
Spinola, 1808: 9. Type species: Apis aterrima Panzer, 1798 (nec
Ceraplastes
Gistel, 1848: x [10], unjustified replacement for Stelis Panzer, 1806. Type species: Apis aterrima Panzer, 1798 (nec
Chelynia
Provancher, 1888: 322. Type species: Chelynia labiata Provancher, 1888, monobasic [see
Melanostelis Ashmead, 1898: 283. Type species: Melanostelis betheli Ashmead, 1898 = Stelis rubi Cockerell, 1898, by original designation.
Stelidium
Robertson, 1902: 323. Type species: Stelidium trypetinum Robertson, 1902, monobasic [see
Microstelis Robertson, 1903: 170, 175. Type species: Stelis lateralis Cresson, 1864, by original designation.
Stelis (Pavostelis) Sladen, 1916: 313. Type species: Stelis montana Cresson, 1864, monobasic.
Stelis (Stelidina) Timberlake, 1941: 131. Type species: Stelis hemirhoda Linsley, 1939, by original designation.
Stelis (Stelidiella) Timberlake, 1941: 133. Lapsus for Stelidina Timberlake, 1941.
Stelis (Leucostelis) Noskiewicz, 1961: 126, 132. Type species: Gyrodroma ornatula Klug, 1807, by original designation.
Note
Most of the cleptoparasitic bees of the Anthidiini are attributed to the genus Stelis due to the very diverse morphs. The recent works by
Stelis (Malanthidium) flavofuscinular
Stelis (Malanthidium) flavofuscinular
Nalinrachatakan & Warrit in
Material examined
(2♂). Same specimens as in
Distribution
Thailand (Ubon Ratchathani: Phu Chong Na Yoy National Park).
Since Stelis is a cleptoparasitic bee, its distribution must be in accordance with its host. Noteworthy, the other known species of the subgenus Malanthidium, S. macaccensis (Friese, 1914) is known from Malaysia; thus, Malanthidium is the only subgenus of Stelis present in South East Asian region.
Bee host
Anthidiellum phuchongense Nalinrachatakan & Warrit, 2021.
Floral association
Unknown.
Remarks
With only two males known, some differences between both specimens and their biology were mentioned and discussed in
Trachusa
Trachusa
Panzer, 1804: 14–15. Type species: Trachusa serratulae Panzer, 1804 = Apis byssina Panzer, 1798, by designation of
Diphysis Lepeletier, 1841: 307. Type species: Diphysis pyrenaica Lepeletier, 1841 = Apis byssina Panzer, 1798, monobasic.
Megachileoides Radoszkowski, 1874: 132. Type species: Trachusa serratulae Panzer, 1804 = Apis byssina Panzer, 1798, by designation of Michener 1995: 375.
Megachiloides Saussure, 1890: 35, incorrect spelling of Megachileoides Radoszkowski, 1874; see Michener 1995.
Note
A medium to large, robust, round-edged species, genus Trachusa appears to be sister to the remainder of the tribe Anthidiini (
Trachusa aff. vietnamensis
Trachusa vietnamensis
Flaminio & Quaranta in
Material examined
(5♀). Thailand: Phitsanulok, Nakhon Thai District, 27 May 2014, N. Warrit et al. (
Distribution
Thailand (Phitsanulok) and Vietnam (Quang Nam).
Diagnosis
The species is very close to, or maybe identical to Trachusa vietnamensis. Only the female is known: body large, robust, and black. Bands with yellowish, orangish, or light-brown coloration on the vertex, preoccipital area, anterolateral of scutum, and scutellum, while mesosoma covered in orangish pubescence. The Thai specimens are distinguished from the Vietnamese material by a unique elongate metasoma making it more chalicodomiform, and more limited maculation on the metasoma (fully striped on T1 and T2 of T. vietnamensis, small pale marks on the side of T1 and T2 and minute or absent on T3 and T4 for Thai specimens). The species is also close to T. ovata, from which it differs by the combination of five mandibular teeth, clypeus black with ill-defined shiny median longitudinal line, conspicuous rounded light-brown scutellum which seems darker basally, and head with orangish to light-brown maculations running continuously from the vertex to genal area.
Description
Female: Body length 13.4, 13.3, 13.2, 13.0, 13.3 mm, head width 4.3, 4.3, 4.2, 4.3, 4.1 mm, intertegular distance 3.6, 3.8, 3.8, 3.7, 3.5 mm, respectively. Wingspan 25.0, 25.6, 25.0, 24.7, 24.9 mm.
Head largely black, with light-brown band on vertex running continuously to genal area, lighter on occipital ridge, but not abutting margins of eyes and ocelli. Clypeus (see Fig.
Mesosoma black, covered with fulvous hair except on pronotum with exposed shiny black median area with coarse punctures. Pronotal lobe strongly carinated, light brown. Mesepisternum black. Omaulus carinated, extending to ventral part of thorax. Scutum laterally carinate, punctures uniform, dense, with light-brown color on anterolateral band, not abutting together in middle. Axilla rounded laterally, entirely light brown. Tegula fulvous with dark patch lining medio-posterior. Scutellum broad, apically round with median emargination, light brown, darker on median triangular basal area.
Wing subhyaline, fuscate, forewing darker at apical margin and marginal cell. Pterostigma brown. Veins dark brown to black; 2nd recurrent vein abutted to 2nd submarginal crossvein distally.
Legs covered with short fulvous hairs. Coxae, trochanters, and basal parts of femora dark reddish brown to black; legs otherwise light brown except dark brown on inner surfaces of basitarsus and tarsi, slightly subtle on outer surfaces of hind basitarsus and hind tarsal segments. Apical tarsal segments with apical dark spot. Claw with inner tooth, light brown, apically black. Arolium present, dark brown to black.
Metasoma black. Discs of all terga swollen, with fine dense punctures. Terga covered with short black hairs, lighter to fulvous hairs on T1–T3 lateral surfaces, longer fulvous hairs covering frontal carina of T1. T1 and T2 with small pale lateral patches (Figs
Remarks
Trachusa species have been reported from upper and lower Indochina but with limited materials (
Since the species is very close to T. vietnamensis from Vietnam, here we propose that the Thai specimens belong to the same species. The differences in tergal bands on the metasoma may be considered as variation; however, the Thai specimens exhibit a more elongate metasoma. To confirm that both species are indeed the same, DNA barcoding would be useful since the barcode of T. vietnamensis was provided by
Subgeneric placement of Trachusa vietnamensis is still uncertain.
Trachusa vietnamensis seems to not be congruent with any of these groups, but is closely related to the ovata group by its face, especially in its clypeal shape, and the reduced maculation on the metasoma. Also, the superficially color pattern and almost parallel-sided body form are not congruent with the robust-megachiliform that occurs in all described females of Paraanthidium ; from this, it more resembles the subgenus Orthanthidium from mainland China and Taiwan for which two fairly different species are known: Trachusa formosana (Friese, 1917) and T. cornopes Wu, 2004. Orthanthidium was designated by
The astonishing record of another Trachusa species that is completely different from the aforementioned T. aff. vietnamensis has been retrieved from the citizen science database platform iNaturalist (
Since the identification is restricted to the available photographs, we cannot identify the bee definitively. These observations show multiple Trachusa bees (25+) grouping on a semi-limestone concrete surface (Fig.
Keys to the species of Anthidiine bees in Thailand
Two keys are provided below, one for females and one for males. The keys are modified from
Key to females of anthidiine bees in Thailand
Excluding Stelis flavofuscinular as the female is unknown but must presumably be identifiable to genus due to the absence of metasomal scopa and juxta-antennal carina.
| 1 | Mandible with ≥ 4 teeth. Terga without depressed apical zone (genus Pseudoanthidium); body black with distinct yellow maculation, especially lateral yellow patch on all terga except T6 | Pseudoanthidium orientale |
| – | Mandible teeth < 4 teeth. Terga with apical zone either depressed or not depressed. Terga without yellow maculation but, if present, the pattern will differ from above | 2 |
| 2 | Face with both longitudinal median carina and juxta-antennal carina (Figs |
3 |
| – | Face without carinae as described above. Metasomal scopa present | 6 |
| 3 | S6 acute with median carina and lateral tooth (Fig. |
Euaspis polynesia |
| – | S6 broad, obtuse, or subacute, with a basal platform. Scutellum black with pale maculations on the margin | 4 |
| 4 | S6 with distinct basal platform (Fig. |
Euaspis aequicarinata |
| – | Basal platform of S6 not distinct but can be noticed at median area. Clypeus without median carina while punctation somewhat irregular | 5 |
| 5 | S6 apical margin obtuse, basal platform arise as a bulge on the median area (Fig. |
Euaspis strandi |
| – | S6 apical margin subacute, basal platform smaller (Fig. |
Euaspis aff. wegneri |
| 6 | Face with a pair of juxta-antennal carinae but without longitudinal median carina (Fig. |
Eoanthidium riparium |
| – | Face without any distinct carina. Subantennal suture arcuate | 7 |
| 7 | Large species (length > 11 mm). Cu-V of hindwing usually ≥ half of 2nd M+Cu. (genus Trachusa) | Trachusa aff. vietnamensis |
| – | Smaller (< 11 mm long). Cu-V of hindwing < half of 2nd M+Cu | 8 |
| 8 | Omaular carina not extending down to the venter of thorax (genus Bathanthidium); Paraocular area black. T6 with median raised platform (similar to Fig. |
Bathanthidium binghami |
| – | Omaulus with a distinct carina, extended to the venter of thorax. T6 without raised platform | 9 |
| 9 | Preoccipital ridge and omaulus lamellate (Fig. |
Pachyanthidium lachrymosum |
| – | Omaulus carinated but not lamellated (genus Anthidiellum). Body black with yellow maculations, or reddish to fulvous. Metasoma without clumping white hair patches; arolia present | 10 |
| 10 | Body black with distinct yellow maculations scattered in most parts. The apex of mandible little wider than its base. T1 with obvious anterior carina which separates frontal and dorsal surfaces | 11 |
| – | Body somewhat orangish to fulvous, or black. If black, without distinct yellow maculations on metasoma. Apex of mandible ~ 1.5× wider than its base. T1 without distinct carina | 12 |
| 11 | Small species (length ~ 4–5 mm). Hind tibia and basitarsus simple without any distinct swollen parts. T1 with lateral yellow patches. T2 black while T3–T6 with yellow transverse band which is often medially disrupted on T3 (see Fig. |
Anthidiellum smithii |
| – | Moderate species (length ~ 7 mm). Hind tibia and basitarsus distinctly enlarged. Yellow marks present on each tergum, medially disrupted on T1–T3, and becoming full stripes on T4–T6 (see Fig. |
Anthidiellum aff. latipes |
| 12 | Head extensively black, brownish on clypeus and lower part of paraocular area. Scutum black. Metasoma dark brown to black, with metallic red infused especially on T2 and T3 | Anthidiellum apicepilosum |
| – | Head orange or fuscous, without extensive black maculation; if present, only on frons. Scutum reddish or fulvous, with extensive black marks. Metasoma reddish or fulvous, sometimes with black marks | 13 |
| 13 | Body largely ferruginous. T6 black, covered with golden-white hairs. T1–T5 sometimes with scattered black maculations (Fig. |
Anthidiellum ignotum |
| – | Body appears reddish to orange. T6 orange while T1–T5 have a black apical band (Fig. |
Anthidiellum phuchongense |
Key to males of anthidiine bees in Thailand
Excluding the males of Anthidiellum aff. latipes, Euaspis aff. wegneri, and Trachusa aff. vietnamensis, as they are unknown. Also note that the status of male Euaspis aequicarinata and Eu. strandi is still ambiguous.
| 1 | Arolia absent. Preoccipital ridge and omaulus not carinate. Terga without depressed apical zone (genus Pseudoanthidium). Body black with yellow maculation. S3 with apical extended lobe, lined with a series of yellow hair fringes (Fig. |
Pseudoanthidium orientale |
| – | Arolia present. Preoccipital ridge smooth or carinated, omaulus carinated, or at least in the dorsal part. Body black with yellow maculation, or different. S3 without extended apical lobe | 2 |
| 2 | Face with both longitudinal median carina and juxta-antennal carinae (as Figs |
3 |
| – | Face without combination of carinae as in above | 5 |
| 3 | Scutellum extended with distinct medial shallow depression, black. Apical lamina of gonoforceps with length < 2× its width | Euaspis polynesia |
| – | Scutellum apically with small median notch, black, with or without pale maculation on the margin. Apical lamina of gonoforceps length > 2× its width | 4 |
| 4 | Clypeal punctation irregular, with a strong median carina. Scutellum black with pale maculation on the margin |
Euaspis aequicarinata sensu |
| – | Clypeus without median carina while the punctures only somewhat irregular. Scutellum black without pale maculation on the margin |
Euaspis strandi sensu |
| 5 | Face with a pair of juxta-antennal carinae but without longitudinal median carina (Fig. |
Eoanthidium riparium |
| – | Face without distinct carina. Subantennal suture arcuate | 6 |
| 6 | Front and middle tibia with two apical spines (genus Stelis). Body elongate. Axilla with yellow posterolateral hook | Stelis flavofuscinular |
| – | Front and middle tibia with one apical spine. Body robust, not elongate. Axilla without posterolateral hook | 7 |
| 7 | Omaular carina incomplete, not extending down to the venter of thorax (genus Bathanthidium); Paraocular area black. T6 and T7 with median raised platform (Fig. |
Bathanthidium binghami |
| – | Omaulus distinctly with complete carina. Terga without median raised platform | 8 |
| 8 | Preoccipital ridge and omaulus carinate or lamellate (Fig. |
Pachyanthidium lachrymosum |
| – | Omaulus carinate but not lamellate (genus Anthidiellum). Body black with yellow maculations, or reddish to fulvous. Metasoma without basolateral white hair patches | 9 |
| 9 | Small species (< 6 mm). Body black with yellow maculation. T1 with obvious anterior carina and lateral yellow maculation. T2 black while T3–T7 with yellow transverse band often medially disrupted on T3 | Anthidiellum smithii |
| – | Larger species (usually > 6 mm). Body somewhat orangish to fulvous. T1 without frontal carina | 10 |
| 10 | Scutum black. Metanotum black to dark ferruginous, brighter in T5–T7 | Anthidiellum apicepilosum |
| – | Scutum and metanotum reddish or fulvous, with black marks infused | 11 |
| 11 | Body integument largely ferruginous. Face with extensive black marks. S4 gradulus incomplete. Apical lamina of gonoforceps without inner apical angulation | Anthidiellum ignotum |
| – | Body integument appears orangish. Facial black mark restricted to the frons. S4 gradulus complete. Apical lamina of gonoforceps with inner apical angulation | Anthidiellum phuchongense |
Acknowledgments
We sincerely thank David Notton, Joseph Monks and Natalie Dale-Skey for the opportunity to access type specimens at the Natural History Museum, London (
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
No ethical statement was reported.
Funding
This work is supported by the Center of Excellence in Biodiversity, Office of Higher Education Commission (Thailand) (PERDO-BDC: BDCPG2-159009/1) to NW, the Research Potential Development Program for Graduate Student, Faculty of Science, Chulalongkorn University to PN, the 90th Anniversary of Chulalongkorn University Scholarship (GCUGR1125631073M) to PN and NW, and the Asahi Glass Foundation Grant, Japan to NW.
Author contributions
Conceptualization: NW. Data curation: NW. Formal analysis: NW, PN. Funding acquisition: NW. Investigation: PT, PN, NW. Methodology: NW, PN. Project administration: NW. Resources: CT, JSA, NW, MK, PT. Supervision: NW. Validation: NW, JSA, PN, MK. Visualization: PN, NW. Writing – original draft: PN, NW. Writing – review and editing: CT, NW, JSA, PT, MK, PN.
Author ORCIDs
Pakorn Nalinrachatakan https://orcid.org/0000-0001-7962-5844
John S. Ascher https://orcid.org/0000-0002-7887-2461
Max Kasparek https://orcid.org/0000-0002-5604-6791
Prapun Traiyasut https://orcid.org/0000-0002-7114-0890
Chawatat Thanoosing https://orcid.org/0000-0002-4228-748X
Natapot Warrit https://orcid.org/0000-0002-6338-1782
Data availability
All of the data that support the findings of this study are available in the main text.
References
- Ascher JS, Pickering J (2022) Discover Life Bee Species Guide and World Checklist (Hymenoptera: Apoidea: Anthophila). http://www.discoverlife.org/mp/20q?guide=Apoidea_species [accessed 21 September 2022]
- Ascher JS, Phallin H, Sokha K, Kang L, Sokchan L, Shao C, Greef SD, Chartier G, Sophany P (2016) A report on the bees (Hymenoptera: Apoidea: Anthophila) of Cambodia. Cambodian Journal of Natural History 2016: 23–39.
- Ashmead WH (1898) Some new genera of bees. Psyche 8(271): 282–285. https://doi.org/10.1155/1898/70320
- Baker DB (1995) A review of Asian species of genus Euaspis Gerstäcker (Hymenoptera: Apoidea: Megachilidae). Zoölogische Mededeelingen 69(22): 281–302.
- Bingham CT (1897) Hymenoptera. – Vol. I. Wasps and bees. In: Blanford WT (Ed.) Fauna of British India, including Ceylon and Burma. Taylor and Francis, London, 414–567. https://doi.org/10.5962/bhl.title.10364
- Bingham CT (1903) Diagnoses of aculeate Hymenoptera. In: Annadale N, Robinsons HC (Eds) Fasciculi Malayenses Zoology (Vol. 1: App. 6). University Press of Liverpool, London, 16–60.
- Christ JL (1791) Naturgeschichte, Klassification und Nomenclatur der Insekten vom Bienen, Wespen und Ameisengeschlecht: als der fünften Klasse fünfte Ordnung des Linneischen Natursystems von den Insekten, Hymenoptera: mit häutigen Flügeln. Hermannischen Buchhandlung, Frankfurt, 576 pp. [In German] https://doi.org/10.5962/bhl.title.87724
- Cockerell TDA (1904) The bees of southern California 1. Bulletin of the Southern California Academy of Sciences 3: 3–6.
- Cockerell TDA (1920) XXV. On South African bees, chiefly collected in Natal. Annals of the Durban Museum 2: 286–318.
- Cockerell TDA (1929) XXVII. Descriptions and records of bees. CXIV. Annals & Magazine of Natural History 10(14): 195–204. https://doi.org/10.1080/00222932908672959
- Cockerell TDA (1930) Descriptions and records of bees CXXIV. Annals & Magazine of Natural History 10(6): 48–57. https://doi.org/10.1080/00222933008673186
- Dover C (1929) Wasps and bees of the Raffles Museum, Singapore. Bulletin of Raffles Museum, Singapore 2: 43–70.
- Eardley C, Griswold T (2017) Taxonomic revision of the Afrotropical species of Pachyanthidium Friese (Hymenoptera: Megachilidae: Anthidiini). Zootaxa 4237(3): 401–453. https://doi.org/10.11646/zootaxa.4237.3.1
- Engel MS (2009) A new species of Ranthidiellum from Thailand, with a key to species (Hymenoptera: Megachilidae). Acta Entomologica Slovenica 17(1): 29–35.
- Fabre JH (1891) Souvenirs Entomologiques. Quatrième Série. Delagrave, Paris, 327 pp.
- Fabricius JC (1793) Entomologia systematica emendata et aucta. Secundum classes, ordines, genera, species adjectis synonymis, locis, observationibus, descriptionibus. 4 vols. 1792–1794. Christ. Gottl. Proft, Copenhagen, 519 pp. https://doi.org/10.5962/bhl.title.125869
- Fairmaire LMH (1858) Ordre Hyménoptères. In: Thompson J (Ed.) Archives Entomologiques, ou, Recueil Contenant des Illustrations D’insectes Nouveaux ou Rares. rue Hauterfeuille (Vol. 2. 19), Paris, 263–267.
- Flaminio S, Nalinrachatakan P, Warrit N, Cilla G, Quaranta M (2021) Trachusa vietnamensis, a new species from Vietnam (Hymenoptera Apoidea Megachilidae). Bulletin of Insectology 74(2): 307–310.
- Friese H (1898) Die Bienen Europa‘s (Apidae europaeae) nach ihren Gattungen, Arten und Varietäten auf vergleichend morphologisch-biologischer Grundlage. Theil IV: Solitäre Apiden: Genus Eriades. Genus Trachusa. Genus Anthidium. C. Lampe, Innsbruck & Imst (A), Berlin, 303 pp. https://doi.org/10.5962/bhl.title.160173
- Friese H (1901) Die Bienen Europa‘s (Apidae europaeae) nach ihren Gattungen, Arten und Varietaten auf vergleichend morphologisch-biologischer Grundlage. Theil VI. Solitäre Apiden. C. Lampe, Innsbruck, 284 pp.
- Friese H (1904) Über die Bienengattung Euaspis Gerst. Allgemeine Zeitschrift für Entomologie 9: 137–138.
- Friese H (1905) Die Wollbienen Afrikas. Genus Anthidium (Hym.). Zeitschrift für systematische Hymenopterologie und Dipterologie 5: 65–75.
- Friese H (1909) Die Bienenfauna von Neu-Guinea. Annales Historico-Naturales Musei Nationalis Hungarici 7: 179–288.
- Friese H (1914) Neue Bienenarten der orientalischen Region. Deutsche Entomologische Zeitschrift 1914: 320–324. https://doi.org/10.1002/mmnd.48019140311
- Friese H (1925) Neue Formen von Schmarotzerbienen, besonders aus dem paläarktischen Gebiet. Konowia 4: 27–42.
- Gerstäcker CEA (1858) Bearbeitung der von Peters aus Mossambique mitgebrachten Hymenopteren. Monatsberichte der Königlichen Preussische Akademie des Wissenschaften zu Berlin 1857: 460–464.
- Ghosh D, Saini J, Subramanian KA, Chandra K (2023) Euaspis polynesia Vachal, 1903 (Hymenoptera: Apoidea: Megachilidae): A New Addition to Bee Fauna of India with Comments on Natural History. National Academy Science Letters 46(3): 193–197. https://doi.org/10.1007/s40009-023-01234-x
- Gistel J (1848) Naturgeschichte des Thierreichs: Für höhere Schulen. R. Hoffmann, Stuttgart, 216 pp.
- Gonzalez VH, Griswold T, Praz CJ, Danforth BN (2012) Phylogeny of the bee family Megachilidae (Hymenoptera: Apoidea) based on adult morphology. Systematic Entomology 37(2): 261–286. https://doi.org/10.1111/j.1365-3113.2012.00620.x
- Gorain M, Charan SK, Ahmed SI (2012) Role of insect bees in the pollination of Prosopis cineraria (L.) Druce (Leguminosae, Subfamily Mimosoideae) in Rajasthan. Advances in Applied Science Research 3(6): 3448–3451.
- Griswold T (2001) Two new species of trap-nesting Anthidiini (Hymenoptera: Megachilidae) from Sri Lanka. Proceedings of the Entomological Society of Washington 103(2): 269–273.
- Gupta RK (1993) Taxonomic Studies on the Megachilidae of North-Western India (Insecta, Hymenoptera, Apoidea). Scientific Publishers, Jodphur, 294 pp.
- Huang J, Xu YJ, Zeng L, Lu YY, Liang GW (2011) Changes to the spatial distribution of Ageratum conyzoides (Asterales: Asteraceae) due to red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae) in China. Journal of Insect Behavior 24(4): 307–316. https://doi.org/10.1007/s10905-011-9258-8
- ICZN (1999) International Code of Zoological Nomenclature (4th Edn.). International Trust for Zoological Nomenclature, London, xxix + 306 pp.
- Illiger K (1807) Vergleichung der Gattungen der Hautflügler. Piezata Fabr. Hymenoptera Linn. Jur. Magazin für Insektenkunde 6: 189–199.
- iNaturalist (2023) iNaturalist. https://www.inaturalist.org [Accessed 20 Mar 2023]
- ITIS (2008) Retrieved from the Integrated Taxonomic Information System on-line database. http://www.itis.gov [Accessed 20 Nov 2019]
- Jurine (1801) Nachricht von einen neuen entomologischen Werke des Hrn. Prof. Jurine in Geneve. Intelligenzblatt der Literatur-Zeitung 21: 160–165.
- Kasparek M (2015) The Cuckoo Bees of the Genus Stelis Panzer, 1806 in Europe, North Africa and the Middle East. Entomofauna (Supplement 18): 1–144.
- Kasparek M (2017) Resin bees of the anthidiine genus Trachusa: Identification, taxonomy, distribution and biology of the Old World species. Entomofauna (Supplement 21): 1–152.
- Kasparek M (2019a) A new species in the Trachusa ovata species group (Hymenoptera: Apoidea) from Peninsular Malaysia with an overview of the old-world species within the genus Trachusa. Journal of Natural History 53(17–18): 1079–1094. https://doi.org/10.1080/00222933.2019.1632953
- Kasparek M (2019b) Eoanthidium nasicum (Apoidea: Anthidiini) in the Middle East: from microevolution towards speciation? Oriental Insects 54(4): 1–18. https://doi.org/10.1080/00305316.2019.1693438
- Kasparek M (2020) Variation in Eoanthidium judaeense (Mavromoustakis, 1945) and E. clypeare (Morawitz, 1874) (Apoidea: Megachilidae: Anthidiini) in the Middle East: semispecies or cases of geographic dimorphism? Zoology in the Middle East 66(2): 145–166. https://doi.org/10.1080/09397140.2020.1729563
- Kasparek M (2021) So different but nonetheless belonging to the same species: Multiple geographic clines explain the diverse forms of the anthidiine bee Rhodanthidum caturigense s.l. (Apoidea: Megachilidae: Anthidiini). Organisms, Diversity & Evolution 21(4): 719–735. https://doi.org/10.1007/s13127-021-00510-2
- Kasparek M, Ebmer AW (2023) The wool carder bee Pseudoanthidium alpinum (Morawitz, 1874): Identity of the enigmatic type species of the genus Pseudoanthidium. Osmia 11: 39–50. https://doi.org/10.47446/OSMIA11.7
- Kasparek M, Griswold T (2021) New species of the genus Eoanthidium (Apoidea: Megachilidae: Anthidiini) from the Middle East link the Afrotropical with the Palaearctic realm, with a key to the Palaearctic taxa. Journal of Natural History 55(33–34): 2083–2110. https://doi.org/10.1080/00222933.2021.1977406
- Kasparek M, Leins P, Erbar C (2022) Clypeal pollen accumulation in a new species of bee from Syria – a hitherto unknown phenomenon in megachilid bees (Megachilidae: Anthidiini). Zoology in the Middle East 68(1): 1–14. https://doi.org/10.1080/09397140.2022.2030527
- Klug JCF (1807) Kritische Revision der Bienengattungen in Fabricius neuem Piezatensysteme mit Berücksichtigung der Kirbyschen Bienefamilien und Illiger’s Bemerkunde zu Kirbys Monographie. Magazin für Insektenkunde 6: 200–228.
- Kumar V, Griswold T, Belavadi VV (2017) The Resin and Carder bees of south India (Hymenoptera: Megachilidae: Anthidiini). Zootaxa 4317(3): 436–468. https://doi.org/10.11646/zootaxa.4317.3.2
- Lepeletier de Saint-Fargeau ALM (1841) Histoire Naturelle des Insectes (Vol. 2). Hyménoptères. Roret, Paris, 680 pp.
- Litman JR (2019) Under the radar: Detection avoidance in brood parasitic bees. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 374(1769): e20180196. https://doi.org/10.1098/rstb.2018.0196
- Litman JR, Griswold T, Danforth BN (2016) Phylogenetic systematics and a revised generic classification of anthidiine bees (Hymenoptera: Megachilidae). Molecular Phylogenetics and Evolution 100: 183–198. https://doi.org/10.1016/j.ympev.2016.03.018
- Mavromoustakis GA (1953) LXXXIV.—New and little-known bees of the subfamily Anthidiinae (Apoidea).—Part VI. Annals & Magazine of Natural History 6(71): 834–840. https://doi.org/10.1080/00222935308654492
- Meyer R (1922) Apidae-Stelidinae II. gatt. Euaspis Gerst. Archiv für Naturgeschichte 12: 233–247.
- Michener CD (1995 [“1994”]) Some genus-group names of bees (Hymenoptera, Apoidea). Journal of the Kansas Entomological Society 67(4): 373–377.
- Michener CD (1997) Genus-group names of bees and supplemental family-group names. Scientific Papers of the Natural History Museum. The University of Kansas 1: 1–81. https://doi.org/10.5962/bhl.title.4052
- Michener CD (2000) The bees of the world. Johns Hopkins University Press, Baltimore, London, 913 pp. [16 pls.]
- Michener CD (2007) The bees of the world (2nd edn.). Johns Hopkins University Press, Baltimore, 953 pp. [20 pls.]
- Michener CD, Griswold TL (1994) The classification of old world Anthidiini (Hymenoptera, Megachilidae). The University of Kansas Science Bulletin 55: 299–327.
- Michener CD, McGinley RJ, Danforth BN (1994) The bee genera of North and Central America (Hymenoptera: Apoidea). Smithsonian Institution Press, Washington D.C., 209 pp.
- Morice FD, Durrant JH (1915 [“1914”]) The authorship and first publication of the “Jurinean” genera of Hymenoptera: Being a reprint of a long-lost work by Panzer, with a translation into English, an introduction, and bibliographical and critical notes. The Transactions of the Entomological Society of London 1914(3–4): 339–436. https://doi.org/10.1111/j.1365-2311.1915.tb02984.x
- Nalinrachatakan P, Chatthanabun N, Thanoosing C, Warrit N (2021a) Database and digitization of bees in Thailand. Version 1.07. Chulalongkorn University, Department of Biology. https://doi.org/10.15468/tf4ejd
- Nalinrachatakan P, Traiyasut P, Khongnak A, Muangkam M, Ascher JS, Warrit N (2021b) The resin bee subgenus Ranthidiellum in Thailand (Megachilidae, Anthidiini): Nesting biology, cleptoparasitism by Stelis, and new species. ZooKeys 1031: 161–182. https://doi.org/10.3897/zookeys.1031.57836
- Niu Z-Q, Ascher JS, Luo A-R, Griswold T, Zhu C-D (2016) Revision of the Anthidiellum Cockerell, 1904 of China (Hymenoptera, Apoidea, Megachilidae, Anthidiini). Zootaxa 4127(2): 327–344. https://doi.org/10.11646/zootaxa.4127.2.5
- Niu Z-Q, Ascher JS, Griswold T, Zhu C-D (2019) Revision of the bee genus Bathanthidium Mavromoustakis (Hymenoptera: Apoidea: Megachilidae: Anthidiini) with description of a new species from China. Zootaxa 4657(1): 97–116. https://doi.org/10.11646/zootaxa.4657.1.3
- Niu Z-Q, Luo A-R, Griswold T, Zhu C-D (2021) Review of the bee genus Pseudoanthidium Friese, 1898 (Hymenoptera: Apoidea: Megachilidae: Anthidiini) of China with descriptions of three new species. Zootaxa 4996(1): 133–152. https://doi.org/10.11646/zootaxa.4996.1.5
- Noskiewicz J (1961) Beiträge zur Kenntnis der paläarktischen Arten der Gattung Stelis Panz. (Hym., Apidae). Polskie Pismo Entomologiczne 31(12): 113–133.
- Pagden HT (1934) Biological notes on some Malayan aculeate Hymenoptera III. Journal of the Federated Malay States Museums 17(3): 487–492.
- Panzer GWF (1804) Systematische Nomenclatur über Weiland Herrn Dr. Jacob Christian Schaeffers Natürlich Ausgemalte Abbildungen Regensburgisch. Insecten. Johann Jakob Palm, Erlangen, 260 pp.
- Panzer GWF (1806) Kritische Revision der Insektenfaune Deutschlands nach den System bearbeitet. T. 2. Felssecker, Nürnberg, 271 pp. [2 pls.] https://doi.org/10.5962/bhl.title.6539
- Pasteels JJ (1969) La systématique générique et subgénérique des Anthidiinae (Hymenoptera, Apoidea, Megachilidae) de l’Ancien Monde. Mémoires de la Société royale d’entomologie de Belgique 31: 1–148.
- Pasteels JJ (1972) Revision des Anthidiinae (Hymenoptera Apoidea) de la région Indo-Malaise. Bulletin et Annales de la Société Royale Belge d’Entomologie 108: 72–128.
- Pasteels JJ (1980) Revision du Genre Euaspis Gerstaecker (Hymenoptera, Apoidea, Megachilidae). Bulletin et Annales de la Société Royale Belge d’Entomologie 116: 73–89.
- Pérez J (1895) Espèces Nouvelles de Méllifères de Barbarie (Diagnoses préliminaires). Gounouilhou, Bordeaux, 64 pp.
- Popov VB (1933) Palaearctic forms of the tribe Stelidini Roberts. (Hymenoptera, Megachilidae). Proceedings of the Zoological Institute of the Academy of Sciences of the USSR 1(3/4): 375–414. [In Russian]
- Popov VB (1950) Generic groupings of the Middle Asian bees of the subfamily Anthidiinae (Hymenoptera, Megachilidae). Doklady Akademii Nauk SSSR 70(2): 315–318.
- Portman ZM, Orr MC, Griswold T (2019) A review and updated classification of pollen gathering behavior in bees (Hymenoptera, Apoidea). Journal of Hymenoptera Research 71: 171–208. https://doi.org/10.3897/jhr.71.32671
- Provancher LL (1889) Additions et Corrections au Volume II de la Faune Entomologique du Canada, Traitant des Hyménoptères. C. Darveau, Québec, 475 pp. https://doi.org/10.5962/bhl.title.46411
- Radoszkowski O (1874) Supplément indispensable a l’article publié par M. Gerstaecker en 1869, sur quelques genres d’Hyménoptères. (Suite et Fin). Bulletin de la Société Impériale des Naturalistes de Moscou 46(2/3): 132–151.
- Rasmussen C, Ascher JS (2008) Heinrich Friese (1860–1948): Names proposed and notes on a pioneer melittologist (Hymenoptera, Anthophila). Zootaxa 1833(1): 1–118. https://doi.org/10.11646/zootaxa.1833.1.1
- Ritsema C (1874) Description et figures de deux espéces nouvelles du genre Anthidium, Fab., provenant de l’Archipel des Indes-Orientales. Revue et Magasin de Zoologie Pure et Appliqué 3(2): 111–115.
- Robertson C (1902) Some new or little-known bees. IV. Canadian Entomologist 34(12): 321–325. https://doi.org/10.4039/S0008347X0017779X
- Robertson C (1903) Synopsis of Megachilidae and Bombinae. Transactions of the American Entomological Society 29: 163–178.
- Sandhouse GA (1943) The type species of the genera and subgenera of bees. Proceedings of the United States National Museum 92(3156): 519–619. https://doi.org/10.5479/si.00963801.3156.519
- Sardar S, Kasparek M, Rameshkumar A, Kazmi S (2022) Four species of anthidiine bees (Apoidea: Megachilidae) new to India. Canadian Entomologist 154(1): e7. https://doi.org/10.4039/tce.2021.55
- Sladen FWL (1916) Canadian species of the bee genus Stelis Panz. Canadian Entomologist 48(9): 312–314. https://doi.org/10.4039/Ent48312-9
- Smith F (1858) Catalogue of the hymenopterous insects collected at Sarawak, Borneo; Mount Ophir, Malacca; and at Singapore by A. R. Wallace. Journal of the Proceedings of the Linnean Society of London. Zoology 2(7): 89–130. https://doi.org/10.1111/j.1096-3642.1858.tb02548.x
- Smith F (1879) Descriptions of New Species of Hymenoptera in the Collection of the British Museum. British Museum, London, 240 pp.
- Soh EJ, Soh ZW, Chui S-X, Ascher JS (2016) The bee tribe Anthidiini in Singapore (Anthophila: Megachilidae: Anthidiini) with notes on the regional fauna. Nature in Singapore 9: 49–62.
- Spinola M (1808) Insectorum Liguriae Species Novae aut Rariores, Quas in Agro Ligustico Nuper Detexit, Descripsit, et Iconibus Illustratavit. Tom II. Yves Gravier, Genoa, 262 pp. https://doi.org/10.5962/bhl.title.65985
- Tadauchi O, Tasen W (2009) Bees of Natural Forests, Teak Plantations and Agricultural Fields in Thailand. Esakia 49: 7–13. https://doi.org/10.5109/16144
- Timberlake PH (1941) Ten new species of Stelis from California. Journal of the New York Entomological Society 49: 123–137.
- Tran NT, Khuat LD, Nguyen LPT (2016) Taxonomic notes on the genus Euaspis Gerstäcker (Hymenoptera: Apoidea: Megachilidae) from Vietnam. TAP CHI SINH HOC 38(4): 515–520. https://doi.org/10.15625/0866-7160/v38n4.8948
- Tran NT, Engel MS, Nguyen CQ, Tran DD, Nguyen LTP (2023) The bee genus Anthidiellum in Vietnam: Descriptions of five new species and the first male of Anthidiellum coronum (Hymenoptera, Megachilidae). ZooKeys 1144: 171–196. https://doi.org/10.3897/zookeys.1144.98644
- Urban D, Moure JS (2012) Anthidiini Ashmead, 1899. In: Moure JS, Urban D, Melo GAR (Eds) Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region – online version. http://www.moure.cria.org.br/catalogue [Accessed 30 Dec 2019]
- Vachal J (1903a) Note sur Euaspis Gerst, et Ctenoplectra Sm., deux genres d’Hymenoptera mellifera peu ou mal connus. Bulletin de la Société Entomologique de France 8(4): 95–100. https://doi.org/10.3406/bsef.1903.23223
- Vachal J (1903b) Note complémentaire et rectificative sur Euaspis et Ctenoplectra. Bulletin de la Société Entomologique de France 8(9): 173–174. https://doi.org/10.3406/bsef.1903.23276 [Hym.]
- Viereck HL (1924) The Philippine species of Parevaspis, a genus of bees. Philippine Journal of Science 24: 745–746. https://doi.org/10.4039/Ent5619-1
- Warncke K (1980) Die Bienengattung Anthidium Fabricius, 1804, in der Westpaläarktis und im turkestanischen Becken. Entomofauna 1(10): 119–210.
- Wu Y-R (1962) Yunnan biological investigation report. Apoidea II, Megachilidae, Anthidiini. Acta Entomologica Sinica 11(Suppl.): 161–171. [Results of the zoological-botanical expedition in 1955–1957 to south-west China. Bees (Apoidea) II., Anthidiini (Megachilidae)] [in Chinese with Russian summary]
- Wu Y-R (2006) Fauna Sinica Insecta (Vol. 44). Hymenoptera. Megachilidae. Science Press, Beijing, 474 pp. [In Chinese]
- Wu Y-R, He W, Wang S-F (1988) Bee Fauna of Yunnan. Yunnan Science & Technology Press, Kunming, 131 pp. [pls. i–vi.] [In Chinese]