Monograph |
|
Corresponding author: Mary L. Jameson ( maryliz.jameson@gmail.com ) Academic editor: Frank Krell
© 2017 Matthew R. Moore, Mary L. Jameson, Beulah H. Garner, Cédric Audibert, Andrew B. T. Smith, Matthias Seidel.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Moore MR, Jameson ML, Garner BH, Audibert C, Smith ABT, Seidel M (2017) Synopsis of the pelidnotine scarabs (Coleoptera: Scarabaeidae: Rutelinae: Rutelini) and annotated catalog of the species and subspecies. ZooKeys 666: 1-349. https://doi.org/10.3897/zookeys.666.9191
|
Abstract
The pelidnotine scarabs (Scarabaeidae: Rutelinae: Rutelini) are a speciose, paraphyletic assemblage of beetles that includes spectacular metallic species (“jewel scarabs”) as well as species that are ecologically important as herbivores, pollinators, and bioindicators. These beetles suffer from a complicated nomenclatural history, due primarily to 20th century taxonomic and nomenclatural errors. We review the taxonomic history of the pelidnotine scarabs, present a provisional key to genera with overviews of all genera, and synthesize a catalog of all taxa with synonyms, distributional data, type specimen information, and 107 images of exemplar species. As a result of our research, the pelidnotine leaf chafers (a paraphyletic group) include 27 (26 extant and 1 extinct) genera and 420 valid species and subspecies (419 extant and 1 extinct). Our research makes biodiversity research on this group tractable and accessible, thus setting the stage for future studies that address evolutionary and ecological trends. Based on our research, 1 new species is described, 1 new generic synonym and 12 new species synonyms are proposed, 11 new lectotypes and 1 new neotype are designated, many new or revised nomenclatural combinations, and many unavailable names are presented. The following taxonomic changes are made:
New generic synonym: The genus Heteropelidnota Ohaus, 1912 is a new junior synonym of Pelidnota MacLeay, 1819.
New species synonyms: Plusiotis adelaida pavonacea Casey, 1915 is a syn. n. of Chrysina adelaida (Hope, 1841); Odontognathus gounellei Ohaus, 1908 is a revised synonym of Pelidnota ebenina (Blanchard, 1842); Pelidnota francoisgenieri Moore & Jameson, 2013 is a syn. n. of Pelidnota punctata (Linnaeus, 1758); Pelidnota genieri Soula, 2009 is a syn. n. of Pelidnota punctata (Linnaeus, 1758); Pelidnota lutea (Olivier, 1758) is a revised synonym of Pelidnota punctata (Linnaeus, 1758); Pelidnota (Pelidnota) texensis Casey, 1915 is a revised synonym of Pelidnota punctata (Linnaeus, 1758); Pelidnota (Strigidia) zikani (Ohaus, 1922) is a revised synonym of Pelidnota tibialis tibialis Burmeister, 1844; Pelidnota ludovici Ohaus, 1905 is a syn. n. of Pelidnota burmeisteri tricolor Nonfried, 1894; Rutela fulvipennis Germar, 1824 is syn. n. of Pelidnota cuprea (Germar, 1824); Pelidnota pulchella blanda Burmeister, 1844 is a syn. n. of Pelidnota pulchella pulchella (Kirby, 1819); Pelidnota pulchella scapularis Burmeister, 1844 is a syn. n. of Pelidnota pulchella pulchella (Kirby, 1819); Pelidnota xanthogramma Perty, 1830 is a syn. n. of Pelidnota pulchella pulchella (Kirby, 1819).
New or revised statuses: Pelidnota fabricelavalettei Soula, 2009, revised status, is considered a species; Pelidnota rioensis Soula, 2009, stat. n., is considered a species; Pelidnota semiaurata semiaurata Burmeister, 1844, stat. rev., is considered a subspecies.
New or comb. rev. and revised status: Plusiotis guaymi Curoe, 2001 is formally transferred to the genus Chrysina (C. guaymi (Curoe, 2001), comb. n.); Plusiotis transvolcanica Morón & Nogueira, 2016 is transferred to the genus Chrysina (C. transvolcanica (Morón & Nogueira, 2016), comb. n.). Heteropelidnota kuhnti Ohaus, 1912 is transferred to the genus Pelidnota (P. kuhnti (Ohaus, 1912), comb. n.); Odontognathus riedeli Ohaus, 1905 is considered a subspecies of Pelidnota rubripennis Burmeister, 1844 (Pelidnota rubripennis riedeli (Ohaus, 1905), revised status and comb. rev.); Pelidnota (Strigidia) acutipennis (F. Bates, 1904) is transferred to the genus Sorocha (Sorocha acutipennis (F. Bates, 1904), comb. rev.); Pelidnota (Odontognathus) nadiae Martínez, 1978 is transferred to the genus Sorocha (Sorocha nadiae (Martínez, 1978), comb. rev.); Pelidnota (Ganonota) plicipennis Ohaus, 1934 is transferred to the genus Sorocha (Sorocha plicipennis (Ohaus, 1934), comb. rev.); Pelidnota similis Ohaus, 1908 is transferred to the genus Sorocha (Sorocha similis (Ohaus, 1908), comb. rev.); Pelidnota (Ganonota) yungana Ohaus, 1934 is transferred to Sorocha (Sorocha yungana (Ohaus, 1934), comb. rev.); Pelidnota malyi Soula, 2010: 58, revised status; Xenopelidnota anomala porioni Chalumeau, 1985, revised subspecies status.
To stabilize the classification of the group, a neotype is designated for the following species: Pelidnota thiliezi Soula, 2009. Lectotypes are designated for the following names (given in their original combinations): Pelidnota brevicollis Casey, 1915, Pelidnota brevis Casey, 1915, Pelidnota debiliceps Casey, 1915, Pelidnota hudsonica Casey, 1915, Pelidnota oblonga Casey, 1915, Pelidnota pallidipes Casey, 1915, Pelidnota ponderella Casey, 1915, Pelidnota strenua Casey, 1915, Pelidnota tarsalis Casey, 1915, Pelidnota texensis Casey, 1915, and Scarabaeus punctatus Linnaeus, 1758.
The following published infrasubspecific names are unavailable per ICZN Article 45.6.1: Pelidnota (Odontognathus) cuprea var. coerulea Ohaus, 1913; Pelidnota (Odontognathus) cuprea var. rufoviolacea Ohaus, 1913; Pelidnota (Odontognathus) cuprea var. nigrocoerulea Ohaus, 1913; Pelidnota pulchella var. fulvopunctata Ohaus, 1913; Pelidnota pulchella var. sellata Ohaus, 1913; Pelidnota pulchella var. reducta Ohaus, 1913; Pelidnota unicolor var. infuscata Ohaus, 1913.
The following published species name is unavailable per ICZN Article 11.5: Neopatatra synonyma Moore & Jameson, 2013.
The following published species name is unavailable per application of ICZN Article 16.1: Parhoplognathus rubripennis Soula, 2008.
The following published species name is unavailable per application of ICZN Article 16.4.1: Strigidia testaceovirens argentinica Soula, 2006, Pelidnota (Strigidia) testaceovirens argentinica (Soula, 2006), and Pelidnota testaceovirens argentinica (Soula, 2006).
The following published species names are unavailable per application of ICZN Article 16.4.2: Homonyx digennaroi Soula, 2010; Homonyx lecourti Soula, 2010; Homonyx mulliei Soula, 2010; Homonyx simoensi Soula, 2010; Homonyx wagneri Soula, 2010; Homonyx zovii Demez & Soula, 2011; Pelidnota arnaudi Soula, 2009; Pelidnota brusteli Soula, 2010; Pelidnota chalcothorax septentrionalis Soula, 2009; Pelidnota degallieri Soula, 2010; Pelidnota lavalettei Soula, 2008; Pelidnota lavalettei Soula, 2009; Pelidnota dieteri Soula, 2011; Strigidia gracilis decaensi Soula, 2008, Pelidnota (Strigidia) gracilis decaensi (Soula, 2008), and Pelidnota gracilis decaensi (Soula, 2008); Pelidnota halleri Demez & Soula, 2011; Pelidnota injantepalominoi Demez & Soula, 2011; Pelidnota kucerai Soula, 2009; Pelidnota malyi Soula, 2010: 36-37; Pelidnota mezai Soula, 2009; Pelidnota polita darienensis Soula, 2009; Pelidnota polita orozcoi Soula, 2009; Pelidnota polita pittieri Soula, 2009; Pelidnota punctulata decolombia Soula, 2009; Pelidnota punctulata venezolana Soula, 2009; Pelidnota raingeardi Soula, 2009; Pelidnota schneideri Soula, 2010; Pelidnota simoensi Soula, 2009; Pelidnota unicolor subandina Soula, 2009; Sorocha carloti Demez & Soula, 2011; Sorocha castroi Soula, 2008; Sorocha fravali Soula, 2011; Sorocha jeanmaurettei Demez & Soula, 2011; Sorocha yelamosi Soula, 2011; Xenopelidnota bolivari Soula, 2009; Xenopelidnota pittieri pittieri Soula, 2009.
Due to unavailability of the name Pseudogeniates cordobaensis
Keywords
leaf chafers, jewel beetles, New World, taxonomy
Introduction
The pelidnotine leaf chafers (Rutelinae: Rutelini) include the brilliantly metallic jewel scarabs (Chrysina spp.; e.g., Fig.
Pelidnotine leaf chafers are a poorly studied group with a great need for systematics research. The lack of a taxonomic and phylogenetic framework remains an impediment to the circumscription of natural, monophyletic groups within the Rutelini. Lacking this essential foundation, we cannot understand the evolution of characters such as circular polarization of light in the cuticle of these beetles, the broad context of ecological services such as pollination that the species may provide, and we cannot reconstruct biogeographic patterns nor predict future distributional changes of genera and species of Rutelini.
The objective of this paper is to provide a foundation for understanding the taxonomy of 27 (26 extant and 1 extinct) genera and over 400 species of pelidnotine beetles, assist in stabilizing the classification and nomenclature of the genera, enable identification of genera, and provide a foundation for continued biodiversity research on leaf chafer scarabs. This work synthesizes the taxonomic and biodiversity literature for the pelidnotine scarabs, also encapsulating work that assisted in clarifying the nomenclature for the group (
Legacy and history.
Fredrick Bates (1829–1903, collection at BMNH), the younger brother of the well-known tropical biologist and coleopterist Henry Walter Bates (see
Friedrich Ohaus (1864–1946, collection at ZMHB) was a student of coleopterist Edgar von Harold and a practicing medical doctor, which allowed him to travel to South America as a ship’s doctor. Ohaus provided the most comprehensive body of literature on world Rutelinae, and he developed the classification of the subfamily Rutelinae that is still used today. His work synthesized the body of knowledge on this highly biodiverse group, providing catalogs of species and their distributions, keys to higher-level groups, natural history, illustrations, and interpretation of characters. Ohaus’s classification of subtribes and genera is largely artificial, but this was a reflection of the state of systematics at the time. The Genera Insectorum on the Rutelini (
Johann W. Machatschke (1912–1975, collection at NHMB) continued Ohaus’s work, completing the Genera Insectorum volumes on Orthochilous Rutelinae (Anoplognathini, Adoretini, Anatistini) as well as Anomalini. He was the curator of the Coleoptera at Deutsche Entomologische Institut (DEI) in Berlin and later at the Museum G. Frey in Tutzing near Munich.
Marc Soula (1947–
Higher-level nomenclature.
Many of Soula’s descriptions of new genera within the Rutelini lack information regarding higher-level classification (e.g., Patatra Soula, Pachacama Soula, and Homeochlorota Soula were not clearly assigned to a subtribe of Rutelini at the time of their description). Because his work was published in parts, they included a mix of many genera from formerly accepted subtribes (Pelidnotina, Anticheirina) or accepted subtribes (Areodina, Lasiocalina), and they were not organized in a systematic manner. Thus, Soula’s tribal and subtribal classification within the Rutelinae was not clear. Soula recognized that his classification was not based on monophyletic groups (“La plupart des taxons supragénériques, n’étant pas monophylétiques...” [
Nomenclature.
Pelidnota was placed on the Official List of Generic Names in Zoology (ICZN 2003) and included in the subtribe Rutelina (tribe Rutelini) by
The type species of Pelidnota is Scarabaeus punctatus Linnaeus, 1758. To ensure nomenclatural stability, the name was conserved due to homonymy with Scarabaeus punctatus Villers, 1789 (the dynastine scarab Pentodon bidens punctatus [Villers]) (ICZN 1999,
The name “Pelidnota” (from which the subtribe Pelidnotina takes its name) is derived from the blackish markings (“pelidnos” or “pelios” = Greek for black; “nota”=Latin for markings) that are common on the elytra of North American Pelidnota species.
Life history and biology.
Immature life stages are known for only a handful of the pelidnotine genera including Homonyx Guérin-Méneville (
Human cultural uses.
The beauty, large size, and ease of collecting of many pelidnotine leaf chafers have promoted the cultural use of many species. For example, in Ecuador and Peru, the Jivaro and Sequoia Indians use the brilliant, metallic green elytra, pronota, or entire bodies of Chrysophora chrysochlora (Latreille) to make necklaces and headdresses (
Fossil pelidnotines.
Fossil organisms provide important information on ancestral character states, habitats, ecosystems, and adaptations. The only known leaf chafer fossil sets the minimum age of the subfamily Rutelinae at 50-42 mya (
Identification of pelidnotine scarabs.
Keys to the genera of “Pelidnotina” were created by F.
Classification and phylogeny.
The leaf chafers (Rutelini) are members of the phytophagous scarab beetle clade (Melolonthinae, Cetoniinae, Dynastinae, Rutelinae, and a few minor subfamilies), a group that has been widely accepted as monophyletic for about 150 years and corroborated by molecular and morphological phylogenetic studies (
Relationships among the pelidnotine scarabs need to be addressed by phylogenetic analyses. Pelidnotine genera, especially Pelidnota and the generic-level synonyms of Pelidnota (e.g., Strigidia Burmeister), should be re-structured into monophyletic groups with clear hypothesized synapomorphies. Based on phylogenetic analyses (
Research on specific pelidnotine genera has led to classification changes that affected the composition of pelidnotine scarabs. For example, Plusiotis and Chrysina were historically separate genera. As new species were described, our understanding of characters that circumscribe these groups was broadened and, as stated by
We reiterate that Pelidnotina, as historically defined, is a paraphyletic grouping of disparate genera and species, and it should not be considered a valid taxon. We consider the subtribe a synonym of the subtribe Rutelina (
Materials and methods
Specimens and taxonomic material.
Specimens examined for this study were provided by many institutions and private collections. We include information on type specimens to provide a foundation for continued research in the leaf chafers. Type specimens are international standards for scientific names (
BIOG Biodiversity Institute of Ontario, University of Guelph, Guelph, Ontario, Canada (Renee Labbee)
BMNH The Natural History Museum, London, United Kingdom (Max Barclay, Beulah Garner)
CAS California Academy of Sciences, San Francisco, California, USA (Norman Penny)
CCECL Musée des Confluences, Lyon, France (Cédric Audibert)
CMNC Canadian Museum of Nature Collection, Ottawa, Canada (Robert Anderson, François Génier)
CNC Canadian National Collection of Insects, Arachnids, and Nematodes, Ottawa, Canada (Pat Bouchard)
CNIN Colección Nacional de Insectos, Instituto de Biología, Universidad Nacional Autónoma de México (UNAM), México D. F., México (Harry Alperowitz, Santiago Zaragoza Caballero)
DBPC Denis Bouchard Personal Collection, Autouillet, France
DEPC David Ebrard Personal Collection, Velars sur Ouche, France
DJCC Daniel Curoe Personal Collection, Palo Alto, California, USA
EAPZ Escuela Agrícola Panamericana, Tegucigalpa, Honduras (Ron Cave, Jesús Orozco)
EMEC Essig Museum of Entomology, University of California, Berkeley, California, USA (Cheryl Barr, Peter Oboyski)
FMNH Field Museum of Natural History, Chicago, Illinois, USA (Alfred Newton, Crystal Maier)
FSCA Florida State Collection of Arthropods, Gainesville, Florida, USA (Paul Skelley)
HNHM Hungarian Natural History Museum, Budapest, Hungary (Ottó Merkl)
IAZA Instituto Argentino de Investigaciones de las Zonas Áridas, Mendoza, Argentina (Adriana Marvaldi)
IEXA Colección Entomológica, Instituto de Ecología, A.C., Xalapa, México (Miguel Ángel Morón)
IFML Instituto Fundación Miguel Lillo, Tucumán, Argentina (Dominga Carolina Berta)
IRSNB Institute Royal des Sciences Naturelles de Belgique, Brussels (Alain Drumont)
INBC Instituto Nacional de Biodiversidad, San José, Costa Rica (Ángel Solís)
INPA National Institute for Amazonian Research, Manaus, Brazil (Marcio Luiz de Oliveira)
IREC Institut de Recherches Entomologique de la Caribe, Pointe-a-Pitre, Guadeloupe (also known as Centre de Recherches Agronomiques Antilles Guyana, Duclos, Petit-Bourg [CRAAG] (Girard Chovet, Fortuné Chalumeau)
JEMC José Mondaca E. Personal Collection, Peñaflor, Chile
JPBC Jean-Pierre Beraud Personal Collection, Cuernavaca, Morelos, México
LACM Los Angeles County Museum of Natural History, Los Angeles, California, USA (Brian Brown, Weiping Xie)
MACN Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina (Arturo Roig)
MCZ Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA (Brian Farrell, Naomi Pierce)
MHNN Muséum d’Histoire Naturelle, Geneva, Switzerland (Peter Schwendinger)
MHNP Museo de Historia Natural, Universidad Nacional de San Antonio Abad, Cusco, Perú (Percy Yangue Yucra)
MIUP Museo de Invertebrados “G.B. Fairchild”, Universidad de Panamá, Panamá (Diomedes Quintero Arias)
MIZA Museo del Instituto de Zoología Agrícola, Maracay, Venezuela (José Clavijo)
MLJC Mary Liz Jameson Personal Collection, Wichita, Kansas, USA
MLPA Museo de la Plata, Universidad Nacional de la Plata, La Plata, Argentina (Analía Lanteri, Nora Cabrera)
MLUH Zentralmagazin Naturwissenschaftlicher Sammlungen, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany (Karla Scheider, Joachim Händel)
MNHN Muséum National d’Histoire Naturelle, Paris, France (Olivier Montreuil)
MNNC Coleccion Nacional de Insectos, Museo Nacional de Historia Natural, Santiago, Chile (Mario Elgueta)
MNCR Museo Nacional de Costa Rica, San José, Costa Rica (formerly INBC, Ángel Solís)
MSPC Matthias Seidel Personal Collection, Prague, Czech Republic
MTD Museum für Tierkunde, Dresden, Germany (Klaus-Dieter Klass, Olaf Jäger)
MXAL Miguel Ángel Morón Collection, Xalapa, México
NHMB Naturhistorisches Museum, Basel, Switzerland (Daniel H. Burckhardt)
NMPC Department of Entomology, National Museum (Natural History), Prague, Czech Republic (Jiří Hájek)
OUMNH University Museum of Natural History, Oxford, United Kingdom (Darren Mann, Amoret Spooner)
PAPC Patrick Arnaud Personal Collection, Saintry sur Seine, France
PMNH Peabody Museum of Natural History, Yale University, New Haven, Connecticut, USA (Leonard Munstermann)
PVGH Pedro Vidal Personal Collection, Santiago, Chile
SDEI Senckenberg Deutsches Entomologisches Institut, Müncheberg, Germany (Lothar Zerche, Konstantin Nadein)
STRI Smithsonian Tropical Research Institute, Balboa, Panama (Annette Aiello)
UAEH Universidad Autónoma del Estado Hidalgo, Pachuca, Hidalgo, México (Juan Marquez Luna)
UAG Escuela de Biología de la Universidad Autónoma de Guadalajara, México (Jose Luis Navarette)
UCCC Museo de Zoología, Universidad de Concepción, Concepción, Chile (Jorge Artigas)
UCRC Entomology Research Museum, Department of Entomology, University of California, Riverside, California, USA (Doug Yanega)
UFRJ Museu Nacional, São Cristóvão, Rio de Janeiro, Brazil (Miguel Monné, Marcela Monné)
UVGC Colección de Artrópodos, Universidad del Valle de Guatemala, Guatemala City, Guatemala (Jack Schuster, Enio Cano)
UNSM University of Nebraska State Museum, Lincoln, Nebraska, USA (Brett Ratcliffe, M. J. Paulsen)
USNM U.S. National Museum, Washington, D.C. (currently housed at the University of Nebraska State Museum for off-site enhancement) (Brett Ratcliffe, Floyd Shockley)
UUZM Zoological Institute, Uppsala University, Uppsala, Sweden (Hans Mejlon)
WBWC William B. Warner Personal Collection, Chandler, Arizona, USA
WSU Maurice T. James Entomological Collection, Washington State University, Pullman, Washington, USA (Richard Zack)
ZMHB Museum für Naturkunde der Humboldt-Universität, Berlin, Germany (Manfred Uhlig, Joachim Willers, Johannes Frisch)
ZSMC Zoologische Staatssammlung des Bayerischen Staates, Munich, Germany (Martin Baehr)
Images and terminology
. Digital images of type specimens were taken over a 10-year period and were captured using several imaging applications including the Leica Application Suite V3.8. Images were edited in Adobe Photoshop CS2 (background removed, contrast manipulated). Figure legends for type specimens provide the valid, accepted name (as in the catalog) and the original combination of the species. We provide images of type specimen labels, but we largely defer from designating specimens as lectotypes. In our view, this is incumbent upon future revisionary scientists who will observe the best practices of systematics (ICZN 2003) and properly assign type status to specimens based on thorough review of all literature. Morphological terminology follows
Literature reviewed.
In compiling this work, we reviewed all available literature including major catalogs (
Soula’s descriptions of new taxa were often vague about the number, sex, and associated label data of type specimens. To rectify this lack of basic information, we report the verbatim label data of every pelidnotine scarab type specimen deposited in the Soula collection now housed at CCECL and CMNC. Due to the number of taxa that Soula named and described, both collections are rich in Rutelini type specimens. Among the pelidnotine scarabs, Soula’s material contains over 80 primary types (holotypes and neotypes) and nearly 700 secondary types (allotypes, paratypes, and paralectotypes) that are now all curated at CCECL. Additionally, examination of the CCECL collection revealed Soula specimens with type labels that had not been validly described. These “manuscript names” and the associated specimen data are listed in the appropriate genera (see “Annotated Catalog” below) as in litteris, and they are unavailable names. In a few instances, it appears that Soula omitted paratype data from the original published description or added paratype specimens to a type series after publication of a species description. For example, he added 17 “paratypes” collected in 2011 to Pseudochlorota peruana lecourti (described by Soula in 2005). Soula clearly knew that this violated nomenclatural rules, because he stated: “Répétons que de nombreux “cotypes” de cette Collection ne sont pas de “bons” types car ils ont été désignés et étiquetés après la description originale” (
During our study of the Soula collection at CCECL, we were able to return some primary type material to the institutions that had loaned specimens to Marc Soula (
Annotated Catalog. We list the author and date of the description of the species and genera, type species of genera (indicated with an asterisk), subspecies and forms, and transfer of species to other genera. This catalog builds on the work of
Rules of zoological nomenclature.
Numerous nomenclatural changes within the pelidnotine scarabs are necessary due to misspellings, invalid type designations, and unavailability of infrasubspecific names (
Infrasubspecific names such as varieties and forms were widely used by authors such as Friedrich Ohaus and occasionally Marc Soula. These names are used to indicate unique color variants. Many of these names were treated as forms (forma; infrasubspecific entities) in catalogs (
Species for which Soula designated type material but for which specimens are missing and presumed lost resulted in our neotype designations. The International Code of Zoological Nomenclature (ICZN 1999) requires that a neotype “is validly designated when there is an exceptional need and only when that need is stated expressly” (75.3). To be validly designated, Article 75.3.7 (ICZN 1999) requires a statement regarding the accessibility of the specimen. Upon publication, the specimen must be “the property of a recognized scientific or educational institution, cited by name.” Thus, some neotypes were invalidly designated by Soula. Designation of some neotype specimens was necessary for names proposed by Soula.
The lack of synthesis and attention to detail in Soula’s works resulted in some names that were not validly described (see
For groups that have dramatic sexual dimorphism, some taxonomists refer to the “alloréférent” or the “neallotype” specimen for the first specimen of the opposite sex that is described in a publication subsequent to the original description (
Poor editing and many misspellings compromise the scientific value of Soula’s works (e.g., scientific names, localities, descriptive characters, figure legends, indices, and identification keys). These errors pose problems because they can be propagated by future researchers. And, in some cases, the error confuses or obscures Soula’s intended species name. We include these misspellings to limit confusion and promote future research.
Type specimens and lectotype designation.
For purposes of nomenclatural stability, we designate lectotypes for some species (ICZN Art. 74). In these cases, a specimen was selected among a group of syntype specimens or cotype specimens. During this research (initiated by MLJ in the late 1990s) many type series were studied, lectotype labels added, and specimens returned to museum collections. However, when research on the group became intractable due to Soula’s concurrent work on the group, these lectotypes were not published. Soula also designated lectotypes. In some cases, he removed previous lectotype labels and he changed the collection depository. In other cases, we have reason to reject Soula’s attempted lectotypification. For example, Soula designated paralectotypes without first designating a lectotype (see P. laevissima Burmeister in
Concepts for genera and species.
In this work, we do not generally assess the validity of species, subspecies, or genera. In our view, this is best conducted as part of comprehensive, revisionary studies. Instead, we provide a taxonomic and nomenclatural framework for future research. Although we do not name new species within this work, we adhere to the phylogenetic species concept (
Hardy’s standards for species circumscription provide a solid basis for ruteline systematics.
In our view,
In contrast to
In the pelidnotine scarabs,
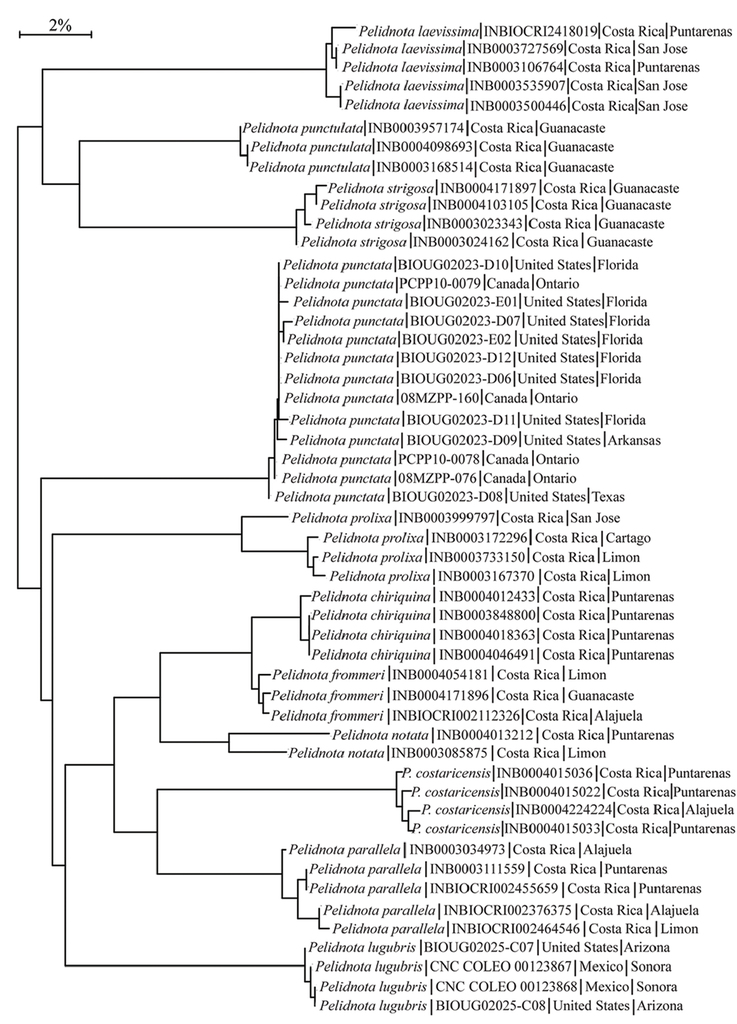
DNA barcode analysis for Pelidnota punctata. Cytochrome Oxidase 1 (CO1) DNA data were used to address genetic variation in Pelidnota punctata across the distributional range of the species. Using the Barcode of Life Database (BOLD: http://www.boldsystems.org), CO1 data were gathered for P. punctata (13 specimens) and 10 other species of Pelidnota (38 specimens). The distance model used the Kimura 2 parameter with a neighbor-joining tree building method in BOLD. Nodes are labeled by species name, BOLD ID number, and country and state/province where the specimen was collected (Fig.
Overviews of genera. Biological and natural history data in the “Generic Overviews” were synthesized from the literature, specimens, and specimen labels from many institutions. Overviews do not summarize all literature and all specimens. Instead, they highlight: 1) potential complications such as paraphyly and nomenclatural issues, 2) potential synapomorphic characters and discuss possible sister-group relationships, 3) basic distribution and habitat affiliations, and 4) known larvae and natural history information.
Overview of the pelidnotine genera
Diagnosis.
Pelidnotine scarabs are members of the tribe Rutelini (for a key to tribes of Rutelinae, see
Males and females are generally separated based on the inner protarsal claw that is wider than the outer claw and may or may not possess a small, inner tubercle. Protarsal claws of the females, in comparison, are more similar in width and lack a small, inner tubercle.
Identification key. We provide a provisional key to the pelidnotine scarabs that should be used with caution. First, the subtribe Pelidnotina is paraphyletic and users should not be misled into thinking that the key circumscribes a natural group. Second, some genera are also very likely paraphyletic, thus causing complications for circumscription and identification. Third, the key will not be useful for both males and females for some genera (e.g., Mesomerodon Ohaus, Hoplopelidnota F. Bates) due to use of sexually dimorphic characters. Fourth, owing to likely paraphyly, the genera Microogenius and Eremophygus could not be separated in the key. Fifth, two genera are keyed twice (Chalcoplethis, Epichalcoplethis). These complications in key construction are indicators of the complexity of the group and need for further systematics studies.
Key to the genera of pelidnotine scarabs (Coleoptera, Scarabaeidae, Rutelinae, Rutelini)
Males: inner protarsal claw wider than the outer claw; may or may not possess a small, inner tubercle; sternites usually concave. Females: protarsal claws similar in width; lack a small, inner tubercle; sternites usually convex.
| 1 | Claws on all legs simple and of similar size; protarsal claw (male) lacking apical or subapical tubercle, lacking apical split | Neogutierrezia Martínez |
| – | Claws on all legs with the inner claw different than the outer claw (wider or split apically); protarsal claw (male) wider than outer claw, with or without small, inner tubercle, and with or without apical incision | 2 |
| 2 | Labrum and clypeus fused anteriorly | Peruquime Mondaca and Valencia |
| – | Labrum and clypeus not fused anteriorly, free | 3 |
| 3 | Lateral edge of mandible lobe-like and flattened, without reflexed teeth (e.g., Fig. |
4 |
| – | Lateral edge of mandible not flattened, with 1 or 2 reflexed teeth (Fig. |
8 |
| 4 | Apex of labrum extends beyond clypeal apex, visible from dorsal view | 5 |
| – | Apex of labrum does not extend beyond clypeal apex, not visible from dorsal view | 6 |
| 5 | Metatarsomere 4 at apex with 4-6 spinules, medial spinules thickened | Oogenius Solier |
| – | Metatarsomere 4 at apex with 4-6 spinules, medial spinules seta-like (not thickened) | Microogenius Gutiérrez and Eremophygus Ohaus |
| 6 | Clypeus with apex quadrate or subquadrate (Fig. |
7 |
| – | Clypeus with apex rounded, parabolic, or trapezoidal; apex with or without emargination (Fig. |
8 |
| 7 | Lateral edge of protibia with 2 teeth | Chipita Soula |
| – | Lateral edge of protibia with 3 teeth | Parhoplognathus Ohaus |
| 8 | Apex of elytra in males with acute, spiniform projections (Fig. |
9 |
| – | Apex of elytra in males rounded | 10 |
| 9 | Males without acute process on posterior margin of mesofemur. Females with 2 deep emarginations near the apex of the terminal sternite; pygidial disc with a concavity. Dorsal color metallic green | Hoplopelidnota F. Bates |
| – | Males with acute process on posterior margin of mesofemur (Fig. |
Mesomerodon Ohaus |
| 10 | Pronotum with apical bead obsolete or lacking medially (Fig. |
Chrysina Kirby |
| – | Pronotum with apical bead complete medially (Fig. |
11 |
| 11 | Males with metatibia enlarged, curved, produced posteriorly at apex | Chrysophora Dejean |
| – | Males without metatibia enlarged, curved, produced posteriorly at apex | 12 |
| 12 | Metatarsomeres 1–5 longer than metatibia | Chalcoplethis Burmeister |
| – | Metatarsomeres 1–5 subequal to metatibia | 13 |
| 13 | Metatibia somewhat laterally flattened (Fig. |
Epichalcoplethis F. Bates |
| – | Metatibia not laterally flattened (Fig. |
14 |
| 14 | Prosternal projection (between procoxae) produced to level of procoxae | 15 |
| – | Prosternal projection (between procoxae) shorter, not produced to level of procoxae Xenopelidnota F. Bates | |
| 15 | Base of metatibia with semicircular notch (Fig. |
Mecopelidnota F. Bates |
| – | Base of metatibia lacking semicircular notch, straight (Fig. |
16 |
| 16 | Apex of metatibia straight and with numerous spinules | Ectinoplectron Ohaus |
| – | Apex of metatibia not straight (biemarginate or with external apex produced), with 0–8 spinules | 17 |
| 17 | Metatibia lacking produced external apex, lacking apical spinules | 18 |
| – | Metatibia with external apex produced posteriorly and with apical spinules | 26 |
| 18 | Disc of frons with weak V-shaped depression (Fig. |
Sorocha Soula |
| – | Disc of frons planar, smooth, lacking a V-shaped depression (Fig. |
19 |
| 19 | Metatibia laterally flattened | 20 |
| – | Metatibia not laterally flattened | 21 |
| 20 | Metatarsomeres 1-5 subequal to metatibia | Epichalcoplethis F. Bates |
| – | Metatarsomeres 1-5 longer than metatibia | Chalcoplethis Burmeister |
| 21 | Elytral shoulder rounded (not flat in ventral view), lacking bead | Homothermon Ohaus |
| – | Elytral shoulder flat in ventral view, with bead | 22 |
| 22 | Mesometasternal keel surpassing mesocoxae (Fig. |
Pelidnota MacLeay |
| – | Mesometasternal keel not surpassing mesocoxae (Fig. |
23 |
| 23 | Lateral edge of mandible with two reflexed teeth (Fig. |
24 |
| – | Lateral edge of mandible with one reflexed tooth (Fig. |
25 |
| 24 | Metatarsomere 3 with apical setae (externally) of unequal length and width; color castaneous to black | Homonyx Guérin-Méneville |
| – | Metatarsomere 3 with apical setae (externally) of equal length and width; color metallic green | Catoclastus Solier |
| 25 | Fifth meso- and metatarsomeres without internal teeth, tarsomeres simple | Pseudogeniates Ohaus |
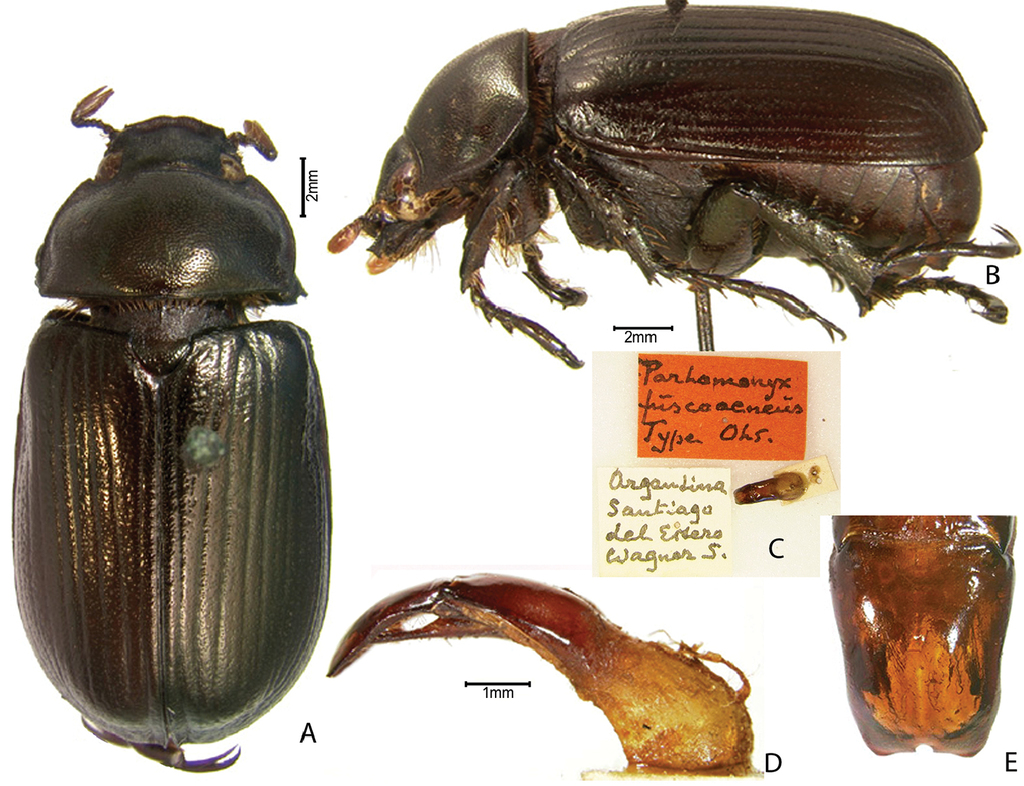
| – | Fifth meso- and metatarsomeres with one or two internal teeth (may be rounded) | Parhomonyx Ohaus |
| 26 | Protibia with 2 external teeth | Pachacama Soula |
| – | Protibia with 3 external teeth | 27 |
| 27 | Labrum with apex bilobed | Patatra Soula |
| – | Labrum with apex projecting anteriorly at middle, not bilobed | Homeochlorota Soula |
Clypeal shape varying from rounded, parabolic, trapezoidal, subquadrate (A–D), and emarginate (E–F). Lateral edge of mandibles with no reflexed teeth (lacking teeth in A, but the mandible is reflexed rather than flattened in A, two reflexed teeth (B, E, F), or one reflexed tooth (C)). Apical bead of pronotum varying from obsolete (A) to complete medially (B, C, D, F). Disc of frons with V-shaped depression (E) or frons planar, smooth, lacking V-shaped depression (A–D, F) A Chrysina beyeri Skinner B Epichalcoplethis velutipes velutipes (Arrow) C Parhomonyx fuscoaeneus (Ohaus) D Chipita mexicana (Ohaus) E Sorocha sp. F Homonyx elongatus (Blanchard).
Characters of the mesofemora and metatibiae in pelidnotine genera. A Epichalcoplethis aciculata (F. Bates), metatibia somewhat flattened (dorsal view) B Pelidnota virescens, metatibia not flattened (dorsal view) C Mecopelidnota sp., metatibia at base with a semicircular notch D Pseudogeniates cordobaensis Moore et al., metatibia simple at base E Mesomerodon gilletti Soula male, acute production of posterior margin of mesotibia (ventral view).
Characters of the thorax (ventral view) and elytral apex (dorsal and lateral views). Mesosternal keel not surpassing base of mesocoxae (A) or keel surpassing base of mesocoxae (B). Acute, spiniform projections at apex of elytra (C, D). A Homonyx elongatus B Pelidnota dobleri Frey C Mesomerodon gilletti, dorsal view D Mesomerodon gilletti, lateral view.
Catoclastus
Type species
Catoclastus chevrolatii Solier, 1851.
Species
3 species; length 14–23 mm.
Three species are included in this genus and are distributed in western Peru. Species are elongate-oval, metallic green with dark red appendages, and similar in overall appearance to species of Mecopelidnota and Homonyx.
Chalcoplethis
Type species
Chrysophora kirbii Gray, 1832.
Species
2 subspecies; length 22–27 mm.
As circumscribed by
Chalcoplethis kirbii is diagnosed by its metallic green color, striate elytra, elytral epipleuron shelf-like (not rounded), pronotum with bead incomplete apically (complete laterally and basally), metatibia of the male that is strongly compressed (less so in females) and lacking apical spinules, meso- and metatarsomere 5 lacking an internomedial tooth; mandibles that are bidentate externally, prosternal process well developed, and mesometasternal keel surpassing the mesocoxae. The species is distributed in the Atlantic Coastal Forest of Brazil from Bahia in the north to Rio Grande do Sul in the south. Larvae are not described.
Chipita
Type species
Byrsopolis mexicana Ohaus, 1905.
Species
1 species; length 14–18 mm.
The monotypic genus Chipita was proposed by
Chipita mexicana is diagnosed by the following characters: profemur produced anteriorly and widest at middle (autapomorphic for the genus); protibia with 2 external teeth (shared with Platyrutela); mandibular palp with deep, horizontal sulcus (shared with Platyrutela); clypeus quadrate and apex reflexed (shared with Platyrutela); clypeus greatly declivous with respect to plane of frons; pronotum broadest at base (shared with Platyrutela); elytra striate; elytral epipleuron rounded; claws simple on all legs (male and female; shared with Platyrutela); male protarsal and mesotarsal claws with inner, apical tubercle; meso- and metatarsomere 5 with internomedial tooth; apex of metatibia with short spines (versus long setae in Platyrutela); color gray or castaneous with or without metallic green sheen.
Adult C. mexicana inhabit tropical deciduous and sub-deciduous forests at elevations between sea level and 200 m (
Chrysina
Type species
Chrysina peruviana Kirby, 1828.
Species
113 species; length 19–40 mm.
Species in the genus Chrysina are commonly known as the “jewel scarabs” for their spectacular metallic and iridescent coloration and large size. Species range from metallic green, pink, purple, gold, and silver, and their elytra may be adorned with metallic gold or silver pin stripes or polka dots. The males of some species have enlarged metafemora (e.g., Chrysina macropus [Francillon]).
The genera Plusiotis and Chrysina were historically separate genera.
Species in the genus are distributed from the southwestern United States to Ecuador with the greatest diversity of species occurring between 1000–2000 m elevation (
Chrysophora
Type species
Melolontha chrysochlora Latreille, 1812.
Species
1 species; length 25–42 mm.
The dazzling, metallic green Chrysophora chrysochlora is a distinctive species and the only member of its genus. The large size, conspicuously rugose elytra, and elongate legs of the male are distinguishing characteristics. Additional characters include the metatibia of the male that is prolonged and acuminate at the apex, the 5th tarsomere with an internal tooth (all legs), the mandibles that are broadly rounded externally, the pronotum with a complete bead, and the mesosternum that is not appreciably produced beyond mesometasternal suture. Research is needed to examine sister-group relationships of this monotypic genus.
The species is distributed in Colombia, Ecuador, and Peru where the Jivaro, Shuar, and Sequoia Indians use the elytra, pronota, legs, or entire body for adornment (
Ectinoplectron
Type species
Homonyx oryctoides Ohaus, 1905.
Species
1 species; length 21–23 mm.
This monotypic genus is endemic to northwestern Mexico. Adults have a rufous dorsal coloration without metallic reflections, and are similar to Pelidnota (Pelidnota) in overall appearance. Adults in the genus Ectinoplectron are diagnosed by the disc of the prosternal peg that is weakly concave with reflexed margins (an autapomorph). Additional diagnostic characters include: lateral edge of mandibles with two reflexed teeth; apex of metatibia straight (not biemarginate) and lacking spinules or setae; meso- and metatarsomere 5 lacking an internomedial tooth; mesosternum not appreciably produced beyond the mesometasternal suture; pronotum with bead complete apically, basally, and laterally; lateral edge of protibia with three rounded teeth; and, apex of clypeus subtrapezoidal to subtriangular.
Ectinoplectron oryctoides is known from Pacific coastal states of Mexico (Durango, Jalisco, Michoacán, Nayarit, Sinaloa and Sonora), northern Chihuahua (
Epichalcoplethis
Type species
Pelidnota velutipes Arrow, 1900.
Species
16 species and subspecies; length 15–19 mm.
Previously considered a subgenus of Pelidnota, Epichalcoplethis was circumscribed by
Species in the genus Epichalcoplethis can be diagnosed, in part, based on the following characters: metatibia weakly compressed (strongly compressed in C. kirbii) and apex lacking spinules; meso- and metatarsomere 5 lacking internomedial tooth; punctate-striate elytra; elytral epipleuron shelf-like (not rounded); pronotum with bead incomplete apically (complete laterally and basally); mandibles that are bidentate externally; prosternal process well-developed; and, mesometasternal keel surpassing the mesocoxae. Epichalcoplethis chamaeleon (Herbst) differs from other species in the genus based on the form of the male parameres, form of the metatibia in the male (not compressed laterally and apex with a well-developed corbel). For many years, this large and conspicuous species was misidentified as Pelidnota rostrata Burmeister.
Species in the genus are distributed from Guatemala and Belize, St. Vincent and the Grenadines, Trinidad and Tobago, and south to Argentina, Uruguay, and Paraguay. In Grenada, E. velutipes is common in the temperate zone from April to May (
Eremophygus
Type species
Eremophygus philippii Ohaus, 1910.
Species
6 species; length 14–15 mm.
Rarity of specimens in collections as well as possible paraphyly with the genera Oogenius, Microogenius, Peruquime, and Lasiocala hampers our understanding of the biodiversity of this group. Species in the genus Eremophygus are distributed in the altiplano of Bolivia, Argentina, Peru, and Chile. Gutiérrez described two species in the genus (
Some species in the genus lack the independently movable claws that are diagnostic of Rutelinae (that is, the apex of meso- and metatarsomere 5 lack a longitudinal slit, a character suite shared with cyclocephaline rhinoceros beetles [Dynastinae: Cyclocephalini]). One species, Eremophygus pereirai Martínez (from Jujuy, Argentina), was transferred to the dynastine tribe Cyclocephalini and the genus Cyclocephala by
Diagnostic characters have greatly diminished reliability because of overlap with Lasiocala, Oogenius, Peruquime, Microogenius, and Cyclocephala and should be used with great caution: dorsal surface often with long, tawny setae; apex of labrum extends beyond clypeal apex, visible in dorsal view; antenna 9- or 10-segmented (9-segmented according to
Homeochlorota
Type species
Pseudochlorota chiriquina Ohaus, 1905.
Species
1 species; length 18–20 mm.
The monotypic genus Homeochlorota is rarely encountered in collections and is narrowly distributed in Costa Rica and Panama. As the generic name implies, the genus shares similarities with the genus Chlorota (an anticheirine leaf chafer) including the form of the metatibia (with emargination at apex and with external apex posteriorly produced), form of the claws (widely toothed), and metamesosternal peg that is produced ventrally. In general appearance, it could be confused with Chlorota flavicollis Bates. Analyses should closely examine relationships with Chlorota and other anticheirine leaf chafers in combination with lasiocaline and pelidnotine chafers.
The ruteline genera Pseudochlorota and Lasiocala comprise the subtribe Lasiocalina (
The taxon is characterized by the following features: pronotum with apical bead lacking or obsolete medially; mesosternum not appreciably produced beyond metamesosternal suture; metamesosternal peg produced ventrally; lateral edge of mandible with one reflexed tooth; labrum extends beyond apex of the clypeus; apical margin of the labrum arcuate and with a small tooth at the middle; frontoclypeal suture obsolete; metatibia with emargination at apex and with external apex posteriorly produced, and; larger claw on all legs widely cleft (shared with Lasiocala). Natural history and larvae are not known, and sister-group relationships have not been examined.
Homonyx
Type species
Homonyx cupreus Guérin-Méneville, 1839.
Species
14 species and subspecies; length 12–19 mm.
Species in the genus Homonyx are elongate, parallel-sided, subcylindrical, and dark-colored beetles. They strongly resemble the allied genus Parhomonyx but can be separated based on the form of the mandibles (bidentate in Homonyx and broadly rounded with one apical tooth in Parhomonyx), the apex of the metatibia (with many spinules in Parhomonyx and biemarginate in Homonyx), and the feathery fringe of setae at the apex of the elytra (exposed in Parhomonyx; hidden in Homonyx). These genera share additional characters: prosternal process short (well-developed in H. planicostatus); mesosternum not produced beyond the mesometasternal suture; pronotum with bead complete apically, laterally, and basally; claws simple; lateral set of setae on apical edge of 3rd metatarsomere of unequal length and width (versus equal in length and width in Catoclastus).
Species in the genus are distributed in Argentina, Brazil, Bolivia, Ecuador, Uruguay, and Peru.
Homothermon
Type species
Homothermon bugre Ohaus, 1898.
Species
4 species; length 9–19 mm.
The genus Homothermon includes four uncommon species that are distributed in the Paulista center of endemism in Brazil and Argentina (Rio de Janeiro in the north to Santa Catarina, Rio Grande do Sul, and Misiones in the south) (
Classification and nomenclatural history of members of the genus has been confused.
Homothermon serrano Ohaus and H. bugre are apparently sympatric. Based on our examination of specimens (including type specimens), the two species differ only in color but are conspecific in all other respects.
Natural history for the genus is unknown. Homothermon serrano is known from the forested mountains near Theresopolis, Santa Catarina, Brazil (
Hoplopelidnota
Type species
Hoplopelidnota candezei F. Bates, 1904.
Species
1 species; length 19–24 mm.
The monotypic genus Hoplopelidnota is rarely found in collections. Similar to species of Chalcoplethis and Catoclastus, it possesses metallic green, rugose elytra. Prior to this work, females were not associated with males. The elytral callus of the male possesses a well-developed spine (shared with the pelidnotine genus Mesomerodon; lacking in females of both Mesomerodon and Hoplopelidnota). In addition to the spinose elytra, several unusual characters serve to diagnose the genus: fringe of setae produced beyond apex of elytra; metatibial apex straight (lacking a corbel); mesosternum produced beyond the mesometasternal suture; metasternum with two parallel, longitudinal furrows; pygidium of female with a well-developed horizontal ridge and weak discal concavity; terminal sternite in the female with two deep emarginations on either side of the apex.
The genus includes one species, Hoplopelidnota metallica (Laporte), which has a turbulent nomenclatural history (see “Annotated Catalog”;
Mecopelidnota
Type species
Mecopelidnota arrowi F. Bates, 1904.
Species
8 species; length 17–26 mm.
Species in the genus Mecopelidnota are distinctive for their dark metallic green coloration, large size, elongate body form, and emargination at the base of the metatibia in the male. As currently constituted, the genus includes eight species (
The form of the male metatibia (base with an emargination) serves as a synapomorph for the group. Based on our analyses of external morphological characters, the genus includes two lineages: one to the west side of the Andes and one on the east of the Andes. Species on the west of the Andes (M. arrowi, M. cylindrica (Waterhouse), M. marxi Soula, and M. obscura [Taschenberg]) share the form of the male parameres (with enlarged “thumb” in lateral view) and greatly enlarged female gonocoxites. Species on the east side of the Andes (M. witti Ohaus, M. gerardi Soula, M. mezai Soula, and M. dewynteri Soula) share the form of the male parameres (lacking the enlarged “thumb” in lateral view) and reduced female gonocoxites. Both lineages exhibit north-south clinal variation in the form of the male parameres, and
Species in the genus are recorded from less than 10 m elevation (M. cylindrica and M. obscura;
Mesomerodon
Type species
Mesomerodon spinipenne Ohaus, 1905.
Species
2 species; length 17–24 mm.
The genus Mesomerodon includes two species that are distributed in Colombia, Ecuador, Peru, and Bolivia (
The biology of Mesomerodon species is unknown and larvae are not described; sister-group relationships have not been examined. Adults are collected at lights at elevations between 300–750 m.
Microogenius
Type species
Oogenius martinezi
Species
4 species; length 10–13 mm.
The classification and nomenclatural history of this genus are quite complicated due to two impediments: lack of robust circumscription of ruteline groups and access to literature. Historically, the genus Microogenius was considered a member of the subtribe Lasiocalina and closely related to Lasiocala Blanchard (
Although the validity of the genus requires evaluation, a few characters can be used with caution for diagnosis: apex of labrum extends beyond clypeal apex and visible in dorsal view (shared with Eremophygus and Oogenius); metatarsomere 4 at apex with 4-6 long setae that are subequal in length and thickness; mandible on external margin rounded (shared with Eremophygus); pronotal basal bead complete (shared with Eremophygus); terminal tergite of female rounded at apex (shared with Eremophygus).
As currently composed, species in the genus are distributed in the altiplano of Bolivia and Argentina. Larvae, natural history, and sister group relationships are not known.
Neogutierrezia
Type species
Neogutierrezia mirabilis Martínez, 1953.
Species
10 species; length 6–9 mm.
Similar to Peruquime, the genus Neogutierrezia is a difficult-to-place taxon with affinities to both Melolonthinae and Rutelinae. Molecular and morphological phylogenetic analyses provided strong evidence that the genus is closely related to members of the Rutelinae, thus Neogutierrezia was transferred from Melolonthinae to Rutelinae (
The genus Neogutierrezia is endemic to the Monte biogeographic province in Argentina (Mendoza, Río Negro, Neuquén, Chubut), a shrub steppe region and that coincides with the distribution of Larrea spp., Bulnesia spp., and Plectocarpha spp. (all Zygophyllaceae) (
The genus is diagnosed by the following characters: antennal club longer than stem, and club 3- or 4-segmented (3-segmented in Peruquime); labrum kidney-shaped; pygidial apex “recumbent towards metacoxae” in males; parameres with dorsal and ventral plates fused. Other characters include: frontoclypeal suture complete or obsolete at middle; pronotal apical bead obsolete at middle, complete laterally and basally; and all claws simple.
Species are associated with sandy habitats (sea shores, dunes), and females of one species (N. araucana Martínez) are known to be flightless, probably living underground and only coming to the surface to mate (
Oogenius
Type species
Oogenius virens Solier, 1851.
Species
7 species; length 12–23 mm.
Species in the genus Oogenius are egg-shaped (from which the generic name was derived) and distributed in Chile and Argentina. Based on prevailing usage of the name,
Circumscription of the genus and phylogenetic analyses that include Microogenius, Eremophygus, and Lasiocala are necessary to better understand the composition of the genus and sister group relationships. Although
The genus Oogenius can provisionally be identified based on the following characters: pronotum with basal bead obsolete or complete medially, complete laterally and apically; clypeus broadly rounded apically, reflexed; labrum produced beyond apex of clypeus; mandibles broadly rounded externally; inner claw enlarged and weakly split in male; unguitractor plate subcylindrical; 5th meso- and metatarsomeres lacking medial tooth; mesosternum not appreciably produced beyond mesometasternal suture; and ventral surface densely setose.
The immature stages of Oogenius have not been described, but
Pachacama
Type species
Pachacama ocampoi Soula, 2006.
Species
2 subspecies; length 15–17 mm.
As noted by
Pachacama can be diagnosed based on the following characters: dorsal surface smooth (lacking striae, obvious punctures or rugosity); clypeus elongate with parabolic apex (subequal in length and width); external margin of mandible bisinuate with apical tooth reflexed; pronotum with apical bead incomplete at middle (bead complete medially and basally); protibia with 2 external teeth; apex of metatibia produced on external margin; metatarsus 1 short (half the length of metatarsus 2); metacoxal corner produced, acute; mesosternum appreciably produced beyond metamesosternal suture; protarsal claws of male with internal claw enlarged, split (female split); meso- and metatarsal claws simple in male (widely split in female); 5th tarsomeres (all legs) with medial tooth; uncus subcylindrical, tapering at apex.
Pachacama ocampoi is endemic to Ecuador where it is recorded between 500 to 1650 m elevation in the provinces of Cañar and Pichincha. Natural history and larvae are unknown.
Parhomonyx
Type species
Homonyx fuscoaeneus Ohaus, 1905.
Species
1 species; length 17–22 mm.
The monotypic genus Parhomonyx is endemic to northern Argentina.
Parhomonyx fuscoaeneus is castaneous-bronze in color and is diagnostic for its rounded mandibular apex with apical tooth (shared with Pseudogeniates; bidentate in Homonyx); metatibial apex with many spinules (biemarginate in Homonyx); elytral apex with a fringe of setae (shared with Pseudogeniates; hidden in Homonyx); protibia lacking weak, basal notch; all claws simple; fifth meso- and metatarsomeres with one or two internal teeth (lacking in Pseudogeniates, shared with Homonyx); lateral set of setae on apical edge of 3rd metatarsomere of equal length and width (versus unequal length and width in Pseudogeniates); pronotum with bead complete apically, laterally, and basally; prosternal process short; mesosternal peg lacking (shared with Homonyx and Pseudogeniates); elytra striate (shared with Pseudogeniates and Homonyx); and body form elongate and parallel-sided (shared with Homonyx and Pseudogeniates). Larvae are not described. Label data indicate that specimens are collected at blacklight.
Parhoplognathus
Type species
Areoda maculata Gory, 1833.
Species
4 species; length 12–16 mm.
On first glance, the Brazilian Atlantic Coastal forest endemic genus Parhoplognathus appears similar to areodine leaf chafers such as Areoda MacLeay or Byrsopolis Burmeister due to their strongly convex form (in lateral view) and the apex of the metatibia that possesses many spinules. However, whereas areodine chafers possess a complete frontoclypeal suture, species in the genus Parhoplognathus have an obsolete frontoclypeal suture.
Species in the genus Parhoplognathus are diagnosed by the following characters: pronotum with apical bead obsolete or lacking medially; clypeal apex quadrate, reflexed, with or without emargination; external edge of protibia with 3 teeth; all claws simple (shared with Chipita and Platyrutela); mandibular palp with horizontal/longitudinal sulcus.
A synopsis of the species in the genus was provided by
Patatra
Type species
Patatra mathani Soula, 2008.
Species
1 species; length 15.5 mm.
Patatra mathani is metallic green, the internal protarsal claw is widely toothed and other claws are simple, and the parameres share some similarity to species of Chlorota Burmeister or Pseudothyridium Soula (anticheirine scarabs).
Pelidnota
Type species
Scarabaeus punctatus Linnaeus, 1758.
Species
195 species and subspecies; length 11–37 mm.
From southeastern Canada to Argentina and the Caribbean, members of the genus Pelidnota are obvious members of the entomofauna with diverse forms (some with enlarged metafemora such as P. burmeisteri), diverse colors (from metallic silver in P. teocuitlamayatli Delgado-Castillo, Deloya, and Morón to shiny red and blue in P. rubripennis riedeli [Ohaus]), and diverse maculations (striped green and tan in P. liturella [Kirby] or colorfully spotted in P. xanthospila [Germar]). Their large size, abundance, and beauty make them fairly recognizable. Some species are recognized as pests: P. filippiniae Soula, which defoliates plantations (
Research on Pelidnota and its allies was initiated by one of us in the 1990s (MLJ). This research, however, became intractable when Soula began describing many pelidnotine taxa and depositing type specimens in his private collection where they were not accessible to other scientists. Additionally, Soula created many new species for North American morphotypes of P. punctata (see “Pelidnota punctata (Linnaeus) species hypothesis and synonyms” below). A comprehensive revision of the genus and its allies is needed, including identification resources for all species.
Molecular and morphological phylogenetic analyses are necessary to unravel the evolutionary and ecological patterns within this interesting group. For over a century, taxonomy and nomenclature of the genus has been mired with several genus-level nomenclatural and classification conflicts (F.
Due to possible paraphyly, diagnosis of the genus is difficult. For most species of Pelidnota, the pronotal basal bead is complete (obsolete in some); external margin of the mandible is bidentate; mesosternum with a transverse suture that separates the metasternum; prosternal projection more or less prominent and beaded; scutellum as wide as long; mesosternal projection not well-developed, not strongly produced anteriorly; elytral shoulder with a bead; metatrochanters sometimes protruding; claws simple in both sexes; male protarsal claw with or without inner tubercle; metatibia simple, gradually widening from base or corbeled.
Peruquime
Type species
Peruquime arequipensis Mondaca & Valencia, 2016.
Species
1 species; length 8.3–10.5 mm.
Peruquime arequipensis is a small, setose scarab that inhabits high elevation (3,800–4,000 m), arid regions in southern Peru. The monotypic taxon possesses several unusual characters that are not typically observed in the Rutelinae: labrum projects anteriorly beyond the clypeal apex and fused to the clypeus (similar to some Melolonthinae: Tanyproctini or “pachydemine” scarabs), labrum horizontally produced with respect to the clypeus, antennal club is greatly enlarged (
Peruquime arequipensis is endemic to the Puna biogeographic region of the Andes, an area known for high endemism. Adult Peruquime arequipensis are diurnal and are active during the rainy season where they were collected in traps (flight intercept, pan, and pitfall). Larvae and sister-group relationships are not known.
Pseudogeniates
Type species
Pseudogeniates richterianus Ohaus, 1910.
Species
3 species; length 12–19 mm.
The genus Pseudogeniates is endemic to Argentina, and species are associated with arid habitats in the Chaco, Pampa, Espinal, and Monte ecoregions (
Species in the genus are reviewed and an identification key is available (
Sorocha
Type species
Pelidnota acutipennis F. Bates, 1904.
Species
16 species and subspecies; length 16–19 mm.
This taxon requires phylogenetic analysis because we think some species are probably more appropriately placed in Pelidnota. Sorocha can be characterized, in part, by the following characters: disc of the frons with a V-shaped depressed region (shared with Pseudochlorota); all claws simple; male protarsal claw with inner tubercle; bidentate mandibles; pronotum with bead complete or incomplete apically (complete laterally and basally); elytral base with a median “dimple” lateral of scutellum; elytral epipleuron shelf-like (not rounded); protibia with basal external tooth slightly removed from apical teeth; clypeal length shorter than length of frons; eyes large; apex of the metatibia biemarginate and lacking apical spinules; meso- and metatarsomere 5 lacking internomedial tooth; mesometasternal keel not surpassing mesocoxae; metasternum with dense pilosity.
Species in the genus are superficially similar, and identification is hampered due to lack of a key. Females cannot currently be identified due to similarity among species. Species in the genus are distributed at high elevations from Colombia and Venezuela to Ecuador, Bolivia, and Peru. Larvae are not known.
Xenopelidnota
Type species
Plusiotis anomala Burmeister, 1844.
Species
3 species and subspecies; length 19–27 mm.
Species in the genus Xenopelidnota resemble castaneous-colored Pelidnota, but the taxon is easily diagnosed by its dark-brown color and parabolic clypeus. The apices of the mandibles are quite variable (weakly bidentate, unidentate, rounded), perhaps due to wear and age. Additional characters that diagnose the genus are as follows: claws simple; male protarsal claw with inner tubercle; pronotum with bead complete apically, laterally and basally; elytral epipleuron shelf-like (not rounded); elytral apex with dense, short tawny setae; fifth meso- and metatarsomeres lacking internomedial tooth; apex of metatibia expanded, straight (lacking corbel or emarginations), and with many spinules; prosternal keel short (not produced to level of procoxae); and mesosternum not appreciably produced beyond mesometasternal suture.
Species in the genus are distributed in northern South America (Colombia, Venezuela, Trinidad, St. Vincent and the Grenadines). As typical of rutelines in this region, species are externally quite similar but male parameres possess a great deal of variability. Phylogenomic analyses of the Xenopelidnota lineage may reveal a greater understanding of the biogeography of the region. Larvae, natural history, and sister-group relationships of the group are not known.
Pelidnota punctata (Linnaeus) species hypothesis and synonyms
Pelidnota punctata (Linnaeus) is a widespread species in North America occurring from Ontario and Quebec to Florida west to South Dakota and Texas. The host plant of this species is grape (Vitis Linnaeus; Vitaceae) foliage and fruit and the larvae develop in rotting stumps and logs of various deciduous trees.
The taxonomic history of this species dates back to the very beginning of zoological binomial nomenclature with a brief description by
Melolontha lutea Olivier, 1789 was later described, but it has since been recognized that
Pelidnota genieri Soula was described as a purported species endemic to Ottawa, Ontario, Canada (
Neighbor-joining tree for individuals of P. punctata across the species’ distribution based on CO1 data. Between-species divergence is typically above 10% (e.g., P. punctulata and P. strigosa), whereas within species divergence is typically less than 1% (e.g., P. punctata and P. lugubris).
In the genus Pelidnota, analysis of the CO1 barcode data (Fig.
Annotated Catalog of the Pelidnotine Scarabs (Coleoptera: Scarabaeidae: Rutelinae: Rutelini)
Tribe RUTELINI MacLeay, Subtribe RUTELINA MacLeayGroup Pelidnotine scarabs (paraphyletic)
27 genera (26 extant and 1 extinct) and 420 species and subspecies (419 extant and 1 extinct).
CATOCLASTUS
Catoclastus Solier, 1851: 95–96.
Type species
Catoclastus chevrolatii Solier, 1851: 96-97, by monotypy.
Gender
Masculine.
Species
3 species.
Catoclastus chevrolatii
Catoclastus chevrolatii Solier, 1851: 96–97 [original combination].
Catoclastus chevrolati
Solier [incorrect subsequent spelling by
Distribution
PERU: Ayacucho (
Types
Remarks
Catoclastus jaumesi
Catoclastus jaumesi Soula, 2010a: 6 [original combination].
Distribution
PERU (
Types
The following specimens are deposited at CCECL. 1 ♂ Holotype (Fig.
Catoclastus rabinovichi
Catoclastus rabinovichi Martínez, 1971: 79–81[original combination].
Distribution
PERU: Cusco (
CHALCOPLETHIS
Chalcoplethis Burmeister, 1844: 410.
Pelidnota (Chalcoplethis)
Burmeister [new subgenus status by
Chalcoplethis
Burmeister [revised genus status by
Type species
Chrysophora kirbii Gray, 1832: 516, by monotypy.
Gender
Feminine.
Species
2 subspecies.
Remarks
Chalcoplethis kirbii kirbii
Chrysophora kirbii Gray, 1832: 516 [original combination].
Chalcoplethis kirbii
(Gray) [new combination by
Chalcoplethis kirbyi
(Gray) [incorrect subsequent spelling by
Pelidnota (Chalcoplethis) kirbyi
(Gray) [new subgeneric combination by
Chalcoplethis kirbyi
(Gray) [revised combination by
Distribution
BRAZIL: Bahia, Paraná, Espírito Santo, Rio Grande do Sul (
Chalcoplethis kirbii misionesensis
Chalcoplethis kirbyi misionesensis Soula, 2010a: 46–47 [original combination].
Chalcoplethis kirbii misionesensis
Soula [see suggested correct spelling by
Distribution
ARGENTINA: Misiones (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype (Fig.
Chipita
Chipita Soula, 2008: 10.
Type species
Byrsopolis mexicana
Gender
Feminine.
Species
1 species.
Chipita mexicana
Byrsopolis mexicana Ohaus, 1905: 324–325 [original combination].
Parhoplognathus mexicanus
(Ohaus) [new combination by
Chipita mexicana
(Ohaus) [new combination by
Distribution
MEXICO: Sinaloa (FSCA), Guerrero, Jalisco, Nayarit, Oaxaca (
CHRYSINA
Chrysina Kirby, 1828: 522.
synonym. Plusiotis Burmeister, 1844
Plusiotis Burmeister, 1844: 417. [Type species. Pelidnota victorina Hope, 1841, by original designation].
Chrysina
Kirby [syn. by
synonym. Plusiotina Casey, 1915
Plusiotina
Casey, 1915: 84. [Type species. Plusiotina aeruginis Casey, 1915 by subsequent designation (
Plusiotis
Burmeister [syn. by
synonym. Pelidnotopsis Ohaus, 1915b
Pelidnotopsis Ohaus, 1915b: 257. [Type species. Pelidnota plusiotina Ohaus, 1912, by monotypy].
Chrysina
Kirby [syn. by
Pelidnotopsis
Ohaus [revised genus status by
Chrysina
Kirby [syn. by
Type species
Chrysina peruviana
Gender
Feminine.
Species
113 species.
Remarks
Chrysina adelaida
Pelidnota adelaida Hope, 1841: 147 [original combination].
Plusiotis adelaida
(Hope) [new combination by
Plusiotis adelaidae
(Hope) [incorrect subsequent spelling by
Chrysina adelaida
(Hope) [new combination by
synonym. Pelidnota ornatissima Sturm, 1843
Pelidnota ornatissima Sturm, 1843: 341-342 [original combination].
Plusiotis adelaida
(Hope) [syn. by
synonym. Plusiotis adelaida pavonacea Casey, 1915
Plusiotis adelaida pavonacea Casey, 1915: 84.
Chrysina adelaida (Hope) [syn. n.].
Distribution
MEXICO: Chiapas, Chihuahua, Coahuila, Colima, Durango, Guerrero, Hidalgo, Jalisco, México, Michoacán, Morelos, Oaxaca, Puebla, Queretaro, San Luis Potosí, Tamaulipas, Tlaxcala, Veracruz (
Remarks
Chrysina adolphi
Chrysina adolphi Chevrolat, 1859: 481 [original combination].
Chrysina macropus var. adolphi Chevrolat [new infrasubspecific status by H. W. Bates 1888: 285].
Chrysina macropus adolphi
Chevrolat [new subspecific status by
Chrysina macropus
(Francillon) [syn. by
Chrysina adolphi
Chevrolat [revised species status by
Distribution
MEXICO: Guerrero, Oaxaca (
Types
1 ♀ lectotype of Chrysina adolphi at BMNH (
Chrysina aenigmatica
Plusiotis aenigmatica
Chrysina aenigmatica
(Morón) [new combination by
Distribution
MEXICO: México, Morelos (
Types
1 ♂ holotype, 1 ♀ allotype and 3 paratypes of Plusiotis aenigmatica at MXAL (
Chrysina alfredolaui
Plusiotis alfredolaui Hawks, 1995: 273–275 [original combination].
Chrysina alfredolaui
(Hawks) [new combination by
Distribution
GUATEMALA (Thomas et al. 2006,
Types
1 ♂ holotype of Plusiotis alfredolaui at CAS (
Chrysina alphabarrerai
Plusiotis alphabarrerai Morón, 1981: 57-63 [original combination].
Chrysina alphabarrerai
(Morón) [new combination by
Distribution
MEXICO: Veracruz (
Types
1 ♂ holotype and 1 ♀ allotype at MXAL (
Chrysina arellanoi
Chrysina arellanoi Monzón, 2012: 1–4 [original combination].
Distribution
MEXICO: Oaxaca (
Types
1 ♂ holotype and 1 ♀ allotype at CNIN (UNAM) (
Remarks
Chrysina argenteola
Plusiotis argenteola H. W. Bates, 1888: 277 [original combination].
Chrysina argenteola
(H. W. Bates) [new combination by
Distribution
COLOMBIA: Antioquia, Cauca, Chocó, Nariño, Putumayo, Valle del Cauca (H. W. Bates 1888,
Types
1 ♂ neotype at MNHN (
Chrysina aurigans
Plusiotis aurigans Rothschild & Jordan, 1894: 504–505 [original combination].
Chrysina aurigans
(Rothschild and Jordan) [new combination by
synonym. Plusiotis keithi Linell, 1895
Plusiotis keithi Linell, 1895: 1–2 [original combination].
Plusiotis aurigans
Rothschild and Jordan [syn. by
Distribution
COSTA RICA: Alajuela, Cartago, San José (
Chrysina aurilisternum
Chrysina aurilisternum Pérez-Flores, Villagomez, & Galindo, 2016: 607–610 [original combination].
Distribution
MEXICO: Guanajuato (
Types
1 ♂ holotype, 1 ♀ allotype and 17 paratypes at CNIN (UNAM) (
Chrysina auripes
Chrysina auripes Gray, 1832: 517 [original combination].
Plusiotis auripes
(Gray) [new combination by
Chrysina auripes
Gray [revised combination and revised application by
synonym. Pelidnota auripes Hope, 1841
Pelidnota auripes
Plusiotis auripes
(Gray) [syn. by
Chrysina auripes
Gray [syn. by
synonym. Plusiotis chalchihuitli Morón, 1990
Plusiotis chalchihuitli Morón, 1990: 16, 36-37 [original combination].
Chrysina auripes
Gray [syn. by
Distribution
MEXICO: Nuevo León, Oaxaca, San Luis Potosi, Tamaulipas (
Types
1 ♂ lectotype at OUMNH (
Remarks
Chrysina aurofoveata
Plusiotis aurofoveata Morón, 1981: 50–57 [original combination].
Chrysina aurofoveata
(Morón) [new combination by
Distribution
MEXICO: Hidalgo, Puebla (
Types
1 ♂ holotype, 1 ♀ allotype and paratypes at MXAL (
Chrysina auropunctata
Plusiotis auropunctata Ohaus, 1913: 491 [original combination].
Chrysina auropunctata
(Ohaus) [new combination by
Distribution
GUATEMALA: San Marcos (
Chrysina aurora
Plusiotis aurora Boucard, 1875: 119 [original combination].
Chrysina aurora
(Boucard) [new combination by
Distribution
COSTA RICA: Alajuela, San José (
Chrysina badeni
Plusiotis badeni Boucard, 1878: 298–295 [original combination].
Chrysina badeni
(Boucard) [new combination by
Distribution
MEXICO: Hidalgo, Puebla, San Luis Potosí, Veracruz (
Types
1 ♀ lectotype at ZMHB (
Chrysina baileyana
Chrysina baileyana Monzón, 2010: 7–10 [original combination].
Distribution
GUATEMALA: Huehuetenango (
Types
1 ♂ holotype and 1 ♀ allotype at UVGC (
Chrysina batesi
Plusiotis batesi Boucard, 1875: 119–120 [original combination].
Chrysina batesi
(Boucard) [new combination by
Distribution
COSTA RICA: Cartago, San José (
Chrysina beckeri
Chrysina beckeri H. W. Bates, 1889: 411 [original combination].
Distribution
MEXICO: Durango (H. W. Bates 1889,
Types
Holotype of Chrysina beckeri at MNHN.
Chrysina benesi
Chrysina benesi Pokorný & Curoe, 2012: 111–116 [original combination].
Distribution
MEXICO: Chiapas (
Types
1 ♂ holotype (Fig.
Chrysina beraudi
Plusiotis beraudi Warner, Hawks, & Bruyea, 1992: 99–100 [original combination].
Chrysina beraudi
(Warner, Hawks, and Bruyea) [new combination by
Distribution
COSTA RICA: San José (
Types
1 ♂ holotype at CAS (
Chrysina beyeri
Plusiotis beyeri Skinner, 1905: 289-290 [original combination].
Chrysina beyeri
(Skinner) [new combination by
synonym. Plusiotis ampliata Casey, 1915
Plusiotis ampliata Casey, 1915: 82 [original combination].
Plusiotis beyeri
Skinner [syn. by
synonym. Plusiotis beyeri ocularis Casey, 1915
Plusiotis beyeri ocularis Casey, 1915: 83 [original combination].
Plusiotis beyeri
Skinner [syn. by
Distribution
MEXICO: Chihuahua, Sinaloa, Sonora (
Chrysina blackalleri
Chrysina blackalleri Monzón & García, 2011: 1–4 [original combination]
Distribution
MEXICO: Oaxaca (
Types
1 ♂ holotype and 1 ♀ allotype at CNIN (UNAM) (
Chrysina boucardi
Plusiotis boucardi Sallé, 1878: 21 [original combination].
Chrysina boucardi
(Sallé) [new combination by
synonym. Plusiotis magnificus Arrow, 1919
Plusiotis magnificus Arrow, 1919: 380 [original combination].
Plusiotis boucardi
Arrow [syn. by
Distribution
COSTA RICA: Puntarenas, San José (
Chrysina brevis
Plusiotis brevis Rothschild & Jordan, 1894: 507 [original combination].
Chrysina brevis
(Rothschild and Jordan) [new combination by
Distribution
MEXICO: Durango, Sinaloa (
Chrysina bruyeai
Plusiotis bruyeai Hawks, 1999: 22–24 [original combination].
Chrysina bruyeai
(Hawks) [new combination by
Distribution
COSTA RICA: Alajuela, Cartago, Guanacaste, Heredia (
Types
1 ♂ holotype, 1 ♀ allotype and 31 paratypes at MNCR (
Chrysina cavei
Chrysina cavei Hawks & Bruyea, 1999: 16–18 [original combination].
Distribution
HONDURAS: Olancho, Yoro (
Types
4 paratypes at BMNH (Natural History Museum 2014, BHG pers. obs. Aug. 2016); 3 ♂ paratypes at CMNC.
Chrysina centralis
Plusiotis centralis
Chrysina centralis
(
Distribution
GUATEMALA: Quetzaltenango, San Marcos (
Types
1 ♂ holotype at MXAL (
Remarks
Chrysina chalcothea
Plusiotis chalcothea H. W. Bates, 1888: 284 [original combination].
Chrysina chalcothea
(H. W. Bates) [new combination by
Distribution
COSTA RICA: Cartago, San José (H. W. Bates 1888,
Types
1 ♂ lectotype at BMNH (Natural History Museum 2014).
Chrysina chloreis
Plusiotis chloreis H. W. Bates, 1888: 282 [original combination].
Chrysina chloreis
(H. W. Bates) [new combination by
Distribution
MEXICO: Chiapas, Oaxaca, Veracruz (H. W. Bates 1888,
Chrysina chrysargyrea
Pelidnota chrysargyrea Sallé, 1874: 362 [original combination].
Plusiotis chrysargyrea
(Sallé) [new combination by
Chrysina chrysargyrea
(Sallé) [new combination by
Distribution
COSTA RICA: Puntarenas, San José (
Chrysina chrysopedila
Plusiotis aurora var. chrysopedila H. W. Bates, 1888: 277 [original combination].
Plusiotis chrysopedila
H.W. Bates [new species status by
Chrysina chrysopedila
(H. W. Bates) [new combination by
Distribution
COSTA RICA (
Types
1 ♀ lectotype and 8 paralectotypes of Plusiotis aurora chrysopedila at BMNH (
Chrysina citlaltepetlamayatli
Plusiotis citlaltepetlamayatli Blackaller-Bages & Delgado, 1994: 79–83 [original combination].
Chrysina citlaltepetlamayatli
(Blackaller-Bages and Delgado) [new combination by
Distribution
MEXICO: Querétaro, Veracruz (
Types
1 ♂ holotype and 1 ♀ allotype at MXAL (declaration by authors of final deposition at CNIN [UNAM]) (
Remarks
Chrysina clypealis
Plusiotis clypealis Rothschild & Jordan, 1894: 505–506 [original combination].
Chrysina clypealis
(Rothschild and Jordan) [new combination by
Distribution
COSTA RICA: Cartago, Limón (
Chrysina colima
Plusiotis colima Morón, 1992: 60-62 [original combination].
Chrysina colima
(Morón) [new combination by
Distribution
MEXICO: Colima, Jalisco (
Types
1 ♂ holotype, 1 ♀ allotype and paratypes at MXAL (
Chrysina confusa
Plusiotis confusa Ohaus, 1913: 487–488 [original combination].
Chrysina confusa
(Ohaus) [new combination by
Distribution
COSTA RICA (
Chrysina costata
Plusiotis costata Blanchard, 1851: 210 [original combination].
Plusiotis psittacina var. costata Blanchard [new infrasubspecific status by
Plusiotis costata
Blanchard [revised species status by
Chrysina costata
(Blanchard) [new combination by
Distribution
MEXICO: México, Oaxaca, Puebla, Veracruz (
Types
Holotype of Chrysina costata at MNHN.
Chrysina crassimargo
Plusiotis crassimargo Rothschild & Jordan, 1894: 506 [original combination].
Chrysina crassimargo
(Rothschild and Jordan) [new combination by
Distribution
MEXICO: Colima, Guerrero, Jalisco, México, Michoacán (
Chrysina cunninghami
Plusiotis cunninghami Curoe, 1999: 1–4 [original combination].
Chrysina cunninghami
(Curoe) [new combination by
Distribution
PANAMA: Bocas del Toro (
Types
1 ♂ holotype at MIUP (
Chrysina cupreomarginata
Plusiotis cupreomarginata F. Bates, 1904: 272 [original combination].
Chrysina cupreomarginata
(F. Bates) [new combination by
Distribution
COSTA RICA: Cartago, San José (F.
Types
1 ♂ lectotype and 1 ♀ paralectotype at BMNH (Natural History Museum 2014, BHG pers. obs. Aug. 2016).
Chrysina curoei
Plusiotis curoei Warner, LeBlanc, Hawks, & Bruyea, 1992: 96–99 [original combination].
Chrysina curoei
(Warner, LeBlanc, Hawks, and Bruyea) [new combination by
Distribution
COSTA RICA: San José (
Types
1 ♂ holotype at CAS (
Chrysina cusuquensis
Plusiotis cusuquensis Curoe, 1994: 35–37, 38 [original combination].
Chrysina cusuquensis
(Curoe) [new combination by
Distribution
HONDURAS: Cortés (
Types
1 ♂ holotype and 1 ♀ allotype at CAS (
Chrysina dianae
Plusiotis dianae Ratcliffe & Taylor, 1992: 62–63 [original combination].
Chrysina dianae
(Ratcliffe and Taylor) [new combination by
Distribution
MEXICO: Veracruz, Oaxaca (
Types
1 ♂ holotype at MXAL (
Chrysina difficilis
Plusiotis difficilis Morón, 1990: 19–20 [original combination].
Chrysina difficilis
(Morón) [new combination by
Distribution
MEXICO: Hidalgo, Querétaro, Tlaxcala (
Types
1 ♀ holotype and 1 paratype at MXAL (
Remarks
Chrysina diversa
Plusiotis diversa Ohaus, 1912: 306–307 [original combination].
Chrysina diversa
(Ohaus) [new combination by
synonym. Chalcochlamys nobilis Ohaus, 1935
Chalcochlamys nobilis Ohaus, 1935: 125 [original combination].
Chrysina diversa
(Ohaus) [syn.
Distribution
BELIZE: Cayo (
Chrysina donthomasi
Chrysina donthomasi Monzón & García, 2011: 5–8 [original combination].
Distribution
MEXICO: Nuevo León (
Types
1 ♂ holotype and 1 ♀ allotype at CNIN (UNAM) (
Chrysina dzidorhum
Plusiotis dzidorhum Arnaud, 1994: 36–37 [original combination].
Chrysina dzidorhum
(Arnaud) [new combination by
Distribution
ECUADOR: Cañar, Pichincha (
Types
1 ♂ holotype and 1 ♀ allotype at PAPC (
Chrysina ericsmithi
Plusiotis ericsmithi Monzón & Cano, 1999: 213–214 [original combination].
Chrysina ericsmithi
(Monzón and Cano) [new combination by
Distribution
GUATEMALA: Izabal (
Types
1 ♂ holotype and 1 ♀ allotype at UVGC (
Chrysina erubescens
Chrysina erubescens H. W. Bates, 1889: 411 [original combination].
Distribution
MEXICO: Chihuahua, Durango, Nayarit, Sinaloa (H.W. Bates 1889,
Types
Holotype of Chrysina erubescens at MNHN.
Chrysina expansa
Plusiotis expansa Ohaus, 1913: 489–490 [original combination].
Chrysina expansa
(Ohaus) [new combination by
Distribution
MEXICO: Guerrero, Oaxaca (
Chrysina eyai
Chrysina eyai Curoe, 2012: 9–15 [original combination].
Distribution
PANAMA: Darien (
Types
1 ♂ holotype at EMEC (
Chrysina flohri
Plusiotis flohri Ohaus, 1905: 321 [original combination].
Chrysina flohri
(Ohaus) [new combination by
Distribution
MEXICO: Durango, Nayarit (
Chrysina gaellae
Chrysina gaellae Ebrard & Soula, 2010: 7–9 [original combination].
synonym. Chrysina hawksi Monzón, 2010
Chrysina hawksi Monzón, 2010: 4-7 [original combination].
Chrysina gaellae
Ebrard and Soula [syn. by
Distribution
GUATEMALA: Baja Verapáz, Huehuetenango, Zacapa (
Types
1 ♂ holotype of Chrysina gaellae at DEPC (Soula and Ebrard 2010).
Remarks
Possibly with ill intentions, the names Plusiotis hawksi (
Chrysina gaitalica
Chrysina gaitalica
Curoe & Hawks, 2012: 9–15 [original combination in
Distribution
PANAMA: Coclé (
Types
1 ♂ holotype at UCRC (
Chrysina giesberti
Chrysina giesberti Monzón, 2010: 1 [original combination].
Distribution
GUATEMALA: Huehuetenango, Quiché (
Types
1 ♂ holotype and 1 ♀ allotype at UVGC (
Chrysina gloriosa
Plusiotis gloriosa LeConte, 1854: 221–222 [original combination].
Chrysina gloriosa
(LeConte) [new combination by
Distribution
MEXICO: Chihuahua, Coahuila, Sonora (
Chrysina gorda
Chrysina gorda Delgado, 2003: 319–321 [original combination].
Distribution
MEXICO: Hidalgo, Querétaro, Veracruz (Delgado 2003, Thomas et al. 2006,
Types
1 ♂ holotype and 1 ♀ allotype at IEXA (Delgado 2003); paratypes at UAEH (Delgado 2003).
Chrysina guatemalensis
Plusiotis guatemalensis Monzón, Cano, & Bailey, 1999: 183–184 [original combination].
Chrysina guatemalensis
(Monzón, Cano, and Bailey) [new combination by
Distribution
GUATEMALA: San Marcos (
Types
1 ♂ holotype and 1 ♀ allotype at UVGC (
Chrysina guaymi
Plusiotis guaymi Curoe, 2001: 46–49 [original combination].
Chrysina guaymi (Curoe) [comb. n.].
Distribution
PANAMA: Chiriquí (
Types
1 ♂ holotype and 1 ♀ allotype at MIUP (
Remarks
The genus Plusiotis was synonymized with Chrysina in 2001 (
Chrysina halffteri
Plusiotis halffteri Morón, 1990: 28 [original combination].
Chrysina halffteri
(Morón) [new combination by
Distribution
GUATEMALA: Huehuetenango (Thomas et al. 2006). MEXICO: Chiapas (
Types
1 ♂ holotype and 1 ♀ allotype MXAL (
Chrysina howdenorum
Plusiotis howdenorum Morón, 1990: 31–32 [original combination].
Chrysina howdenorum
(Morón) [new combination by
Distribution
MEXICO: Oaxaca (
Types
The following specimens are deposited at CMNC. 1 ♂ holotype, 1 ♀ allotype, and 1 ♂ paratype: “7000’, 32mi. S. Valle Nacional Oax. Mex. V.21-24, 1971 H. Howden//H. & A. Howden Collection//HOLOTIPO//Plusiotis ♂ howdenorum Morón M. A. Morón, det. 1987//[barcode matrix] Canadian Museum of Musée canadien de la NATURE CMNEN 00011917”, allotype with identical collecting data label and database number CMNEN 00011918; 1 paratype at MXAL (
Chrysina intermedia
Plusiotis intermedia Ohaus, 1913: 488–489 [original combination].
Chrysina intermedia
(Ohaus) [new combination by
Distribution
MEXICO: Oaxaca (
Chrysina karschi
Plusiotis karschi Nonfried, 1891: 306 [original combination].
Chrysina karschi
(Nonfried) [new combination by
Distribution
GUATEMALA: Baja Verapaz, Zacapa (
Chrysina lacordairei
Plusiotis lacordairei Boucard, 1875: 122 [original combination].
Chrysina lacordairei
(Boucard) [new combination by
Distribution
MEXICO: Guerrero, Oaxaca (
Chrysina laniventris
Pelidnota laniventris Sturm, 1843: 339–340 [original combination].
Plusiotis laniventris
(Sturm) [new combination by
Chrysina laniventris
(Sturm) [new combination by
synonym. Pelidnota latipennis Sturm, 1843
Pelidnota latipennis Sturm, 1843: 338-339 [original combination].
Pelidnota laniventris
Sturm [syn. by
synonym. Plusiotis mnizechii Boucard, 1875
Plusiotis mnizechii Boucard, 1875: 124 [original combination].
Plusiotis mniszechii
Boucard [incorrect subsequent spelling by
Plusiotis mniszechi
Boucard [incorrect subsequent spelling by
Plusiotis laniventris
Sturm [syn. by
Distribution
MEXICO: Distrito Federal, Guerrero, Hidalgo, Jalisco, México, Michoacán, Morelos, Veracruz (
Types
1 ♂ lectotype of Pelidnota laniventris at BMNH (Natural History Museum 2014).
Remarks
Chrysina lecontei
Plusiotis lecontei Horn, 1882: 120–121 [original combination].
Plusiotina lecontei
(Horn) [new combination by
Plusiotis lecontei
Horn [revised combination by
Chrysina lecontei
(Horn) [new combination by
synonym. Plusiotina aeruginis Casey, 1915
Plusiotina aeruginis Casey, 1915: 85 [original combination].
Plusiotis aeruginis
(Casey) [new combination by
Plusiotis lecontei
Horn [syn. by
synonym. Plusiotina angusta Casey, 1915
Plusiotina angusta Casey, 1915: 86 [original combination].
Plusiotis angustata
(Casey) [new combination and incorrect subsequent spelling by
Plusiotis lecontei
Horn [syn. by
synonym. Plusiotina sonorica Casey, 1915
Plusiotina sonorica Casey, 1915: 87 [original combination].
Plusiotis sonorica
(Casey) [new combination by
Plusiotis lecontei
Horn [syn. by
synonym. Plusiotina subenodis Casey, 1915
Plusiotina subenodis Casey, 1915: 86 [original combination].
Plusiotis subenodis
(Casey) [new combination by
Plusiotis lecontei
Horn [syn. by
Distribution
MEXICO: Chihuahua, Durango, Sinaloa, Sonora (H. W. Bates 1888,
Remarks
Chrysina limbata
Plusiotis limbata Rothschild & Jordan, 1894: 505 [original combination].
Chrysina limbata
(Rothschild and Jordan) [new combination by
Distribution
COSTA RICA: Cartago, Limón, San José (
Chrysina luteomarginata
Plusiotis luteomarginata Ohaus, 1913: 492–493 [original combination].
Chrysina luteomarginata
(Ohaus) [new combination by
Distribution
COSTA RICA: Alajuela, Cartago (
Types
1 ♂ lectotype at ZMHB (
Chrysina macropus
Scarabaeus macropus Francillon, 1795: 1–4 [original combination].
Trichius macropus
(Francillon) [new combination by
Chrysina macropus
(Francillon) [new combination by
synonym. Chrysina mexicana Gray, 1832
Chrysina mexicana Gray, 1832: 516–517 [original combination].
Chrysina macropus
(Francillon) [syn. by
synonym. Chrysina macropus mniszechi H. W. Bates, 1888
Chrysina macropus var. mniszechi H. W. Bates, 1888: 285 [original combination].
Chrysina macropus mniszechi
H. W. Bates [new subspecific status by
Chrysina macropus
(Francillon) [syn. by
Distribution
MEXICO: Guerrero, Hidalgo, Oaxaca, Puebla, Querétaro, San Luis Potosí, Veracruz (
Remarks
The name “Chrysina henrybatesi” is cited as a synonym of C. macropus on the website checklist of Chrysina species (
The names C. macropus mniszechi H. W. Bates and C. mnizechii Boucard have created some confusion (see “Remarks” under C. laniventris). The spelling of these names differs, but revalidation of species status would risk homonymy.
Chrysina magnistriata
Plusiotis magnistriata
Chrysina magnistriata
(Morón) [new combination by
Distribution
PANAMA: Chiriquí (
Types
1 ♂ holotype and 1 ♀ allotype at MNHN (
Chrysina marginata
Plusiotis marginata Waterhouse, 1871: 5–6 [original combination].
Chrysina marginata
(Waterhouse) [new combination by
Distribution
COSTA RICA: Cartago, Limón, Puntarenas (
Types
1 ♀ holotype at BMNH (Natural History Museum 2014).
Chrysina miguelangeli
Chrysina miguelangeli Nogueira & Curoe, 2012: 2–5 [original combination].
Distribution
MEXICO: Oaxaca (
Types
1 ♂ holotype and 1 ♀ allotype at MXAL (
Chrysina misteca
Plusiotis misteca Morón, 1990: 37 [original combination].
Chrysina misteca
(Morón) [new combination by
Distribution
MEXICO: Oaxaca (
Types
The following specimens are deposited at CMNC. 1 ♂ holotype: “MEXICO: Oaxaca Disto. de Yautepec Juquila Mixes VI. 1973 W. Miller//H. & A. Howden Collection//HOLOTIPO//Plusiotis ♂ misteca Morón M. A. Morón, det. 1989//[barcode matrix] Canadian Museum of Musée canadien de la NATURE CMNEN 00011919”. 2 paratypes at MXAL (
Chrysina modesta
Pelidnota modesta Sturm, 1843: 338 [original combination].
Chrysina macropus var. modesta (Sturm) [new combination and new infrasubspecific status by H. W. Bates 1888: 285].
Chrysina modesta
(Sturm) [revised species status by
Distribution
MEXICO: México, Michoacán (
Chrysina moroni
Plusiotis moroni Curoe & Beraud, 1994: 31–33 [original combination].
Chrysina moroni
(Curoe and Beraud) [new combination by
Distribution
GUATEMALA: San Marcos (Thomas et al. 2006). MEXICO: Chiapas (
Types
1 ♂ holotype and 1 ♀ allotype at CNIN (UNAM) (
Chrysina nogueirai
Plusiotis nogueirai Morón, 1992: 62-66 [original combination].
Chrysina nogueirai
(Morón) [new combination by
Distribution
MEXICO: Aguascalientes, Jalisco (
Types
1 ♂ holotype, 1 ♀ allotype and paratypes at MXAL (
Chrysina ofidiodontophallica
Chrysina ofidiodontophallica Curoe, 2011: 2–4 [original combination].
Distribution
PANAMA: Darien (
Types
1 ♂ holotype at MIUP (
Chrysina ohausi
Plusiotis ohausi Franz, 1928: 3–5 [original combination].
Chrysina ohausi
(Franz) [new combination by
Distribution
PANAMA (
Remarks
Chrysina ohausi (Franz) was known only from a single holotype female described from “Panama” and the holotype is apparently lost (
Chrysina optima
Plusiotis optima H. W. Bates, 1888: 279 [original combination].
Chrysina optima
(H. W. Bates) [new combination by
synonym. Plusiotis melior Rothschild & Jordan, 1894
Plusiotis melior Rothschild & Jordan, 1894: 506 [original combination].
Plusiotis optima var. melior Rothschild and Jordan [new infrasubspecific status by
Plusiotis optima melior
(Rothschild and Jordan) [new subspecific status by
Plusiotis optima
H. W. Bates [syn. by
Distribution
COSTA RICA: Cartago (H. W. Bates 1888,
Types
1 ♂ holotype of Plusiotis optima at BMNH (Natural History Museum 2014).
Chrysina oreicola
Plusiotis oreicola Morón, 1992: 72–73 [original combination].
Chrysina oreicola
(Morón) [new combination by
Distribution
COSTA RICA: Limón (
Types
1 ♂ holotype at MNCR (
Remarks
Chrysina orizabae
Plusiotis orizabae H. W. Bates, 1889: 410 [original combination].
Chrysina orizabae
(H. W. Bates) [new combination by
synonym. Plusiotis alticola H. W. Bates, 1889
Plusiotis alticola H. W. Bates, 1889: 409–410 [original combination].
Chrysina orizabae
(H. W. Bates) [syn. by
Distribution
MEXICO: Colima, Distrito Federal, Hidalgo, Jalisco, México, Morelos, Puebla, Tlaxcala, Veracruz (H. W. Bates 1889,
Types
1 ♀ holotype of Plusiotis orizabae at BMNH (Fig.
Remarks
Chrysina pastori
Plusiotis pastori Curoe, 1994: 37–39 [original combination].
Chrysina pastori
(Curoe) [new combination by
Distribution
HONDURAS: Cortés (
Types
1 ♂ holotype and 1 ♀ allotype at CAS (
Chrysina pehlkei
Plusiotis pehlkei Ohaus, 1930b: 265–266 [original combination].
Chrysina pehlkei
(Ohaus) [new combination by
Distribution
GUATEMALA: Chimaltenango, El Quiché, Quetzaltenango, Sacatepéquez, San Marcos, Sololá (
Remarks
Chrysina pehlkei was reported from Chiapas and Oaxaca, Mexico (
Chrysina peruviana
Chrysina peruviana
Chrysina macropus
(Francillon) [syn. by
Chrysina peruviana
Kirby [revised species status by
synonym. Pelidnota aeruginosa Sturm, 1843
Pelidnota aeruginosa Sturm, 1843: 337 [original combination].
Chrysina amoena
(Sturm) [syn. by
synonym. Plusiotis hoegei Boucard, 1895
Plusiotis högei Boucard, 1895: 4–5 [original combination].
Chrysina hoegei
Boucard [emendation by
Chrysina amoena
(Sturm) [syn. by
synonym. Pelidnota amoena Sturm, 1843
Pelidnota amoena Sturm, 1843: 337–338 [original combination].
Chrysina amoena
(Sturm) [new combination by
Chrysina peruviana
Kirby [syn. by
Distribution
MEXICO: Hidalgo, Puebla, Querétaro, San Luis Potosí, Veracruz (
Types
1 ♀ holotype at OUMNH (
Remarks
Chrysina plusiotina
Pelidnota plusiotina Ohaus, 1912: 304–305 [original combination].
Pelidnotopsis plusiotina
(Ohaus) [new combination by
Chrysina plusiotina
(Ohaus) [new combination by
Pelidnotopsis plusiotina
(Ohaus) [revised combination by
Chrysina plusiotina
(Ohaus) [revised combination by
Distribution
MEXICO: Coahuila, Nuevo León (
Types
1 ♀ lectotype and 1 paralectotype at ZMHB (
Remarks
Chrysina prasina
Plusiotis prasina Boucard, 1878: 295 [original combination].
Chrysina prasina
(Boucard) [new combination by
Distribution
MEXICO: Guerrero, Hidalgo, Oaxaca, Puebla, Veracruz (
Chrysina prototelica
Plusiotis prototelica Morón & Howden, 1992: 205–209 [original combination].
Chrysina prototelica
(Morón and Howden) [new combination by
Distribution
GUATEMALA: Baja Verapáz, Guatemala, Sacatepéquez (
Types
The following specimens are deposited at CMNC. 1 ♂ holotype: “GUATML. B. Verapaz 5 km S Sn Jeronimo May 24-31 1989 4500’ J. E. Wappes//No match at BM 1990//SEM//HOLOTIPO//Plusiotis ♂ prototelica Morón-Howden M. A. Morón, det. 1990//[barcode matrix] Canadian Museum of Musée canadien de la NATURE CMNEN 00000040”, 4 ♂ and 5 ♀ paratypes at CMNC. 1 ♀ allotype at MXAL (
Chrysina psittacina
Pelidnota psittacina Sturm, 1843: 340 [original combination].
Plusiotis auripes
(Gray) [syn. by
Plusiotis psittacina
(Sturm) [revised species status by
Chrysina psittacina
(Sturm) [new combination and revised application by
synonym. Plusiotis amalia Burmeister, 1844
Plusiotis amalia Burmeister, 1844: 422 [original combination].
Plusiotis laeta
Sturm [syn. by
Plusiotis psittacina Sturm [syn. by H. W. Bates 1888: 281].
synonym. Pelidnota laeta Sturm, 1843
Pelidnota laeta Sturm, 1843: 341 [original combination].
Plusiotis psittacina
(Sturm) [syn. by
Plusiotis adelaidae
(Hope) [syn. by
Plusiotis psittacina var. laeta (Sturm) [syn. by
Distribution
MEXICO: Chiapas, Hidalgo, Nuevo León, San Luis Potosi, Tamaulipas (
Types
1 ♀ lectotype of Pelidnota psittacina at BMNH (
Remarks
In reference to species groups,
Chrysina purpurata
Plusiotis purpurata Morón, 1990: 20–21 [original combination].
Chrysina purpurata
(Morón) [new combination by
Distribution
MEXICO: Guerrero (
Types
1 ♂ holotype and 1 ♀ allotype at MXAL (
Remarks
Chrysina purulhensis
Plusiotis purulhensis Warner & Monzón, 1993: 211–213 [original combination].
Chrysina purulhensis
(Warner & Monzón, 1993) [new combination
Distribution
BELIZE: Cayo (
Types
1 ♂ holotype at FSCA (
Chrysina quetzalcoatli
Plusiotis quetzalcoatli Morón, 1990: 22 [original combination].
Chrysina quetzalcoatli
(Morón) [new combination by
Distribution
GUATEMALA: Alta Verapaz, El Quiché, Huehuetenango, Jutiapa, Sacatepéquez, San Marcos (
Types
1 ♂ holotype, 1 ♀ allotype and 5 paratypes at MXAL (
Chrysina quiche
Plusiotis quiche Morón, 1990: 41 [original combination].
Chrysina quiche
(Morón) [new combination by
Distribution
GUATEMALA: Quetzaltenango, San Marco, Zacapa (
Types
1 ♂ holotype at MXAL (
Chrysina ratcliffei
Plusiotis ratcliffei Morón, 1990: 44–45 [original combination].
Chrysina ratcliffei
(Morón) [new combination by
Distribution
COSTA RICA: Limón, Puntarenas (
Types
The following specimen is deposited at CMNC. 1 ♂ holotype “Panamá: Canal Zone Barro Colorado Is. 9°10'N 79°50'W//5. vi. 1977 H. A. Hespenheide//H. & A. Howden Collection//HOLOTIPO//Plusiotis ♂ ratcliffei Morón M. A. Morón, det. 1988//[barcode matrix] Canadian Museum of Musée canadien de la NATURE CMNEN 00011920”; 1 ♂ paratype at CMNC (
Chrysina resplendens
Plusiotis resplendens Boucard, 1875: 119 [original combination].
Chrysina resplendens
(Boucard) [new combination by
Distribution
COSTA RICA: Puntarenas, San José (
Chrysina rodriguezi
Plusiotis rodriguezi Boucard, 1878: 295 [original combination].
Chrysina rodriguezi
(Boucard) [new combination by
Distribution
GUATEMALA: Alta Verapaz, Baja Verapaz, Guatemala, Huehuetenango, Quetzaltenango, Quiché (
Chrysina rutelidedundeei
Chrysina rutelidedundeei Soula, 2012: 5–6 [original combination].
Distribution
MEXICO: Chiapas, Oaxaca (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype: “Mexique Chiapas San Cristobal VII. 2011//Holotype 2012 Chrysina ebrardi S. Soula//Chrysina rutelidedundeei M. Soula det. 2012 Holotype ♂” (47030025); “Mexique Chiapas San Cristobal VII. 2011// Chrysina rutelidedundeei M. Soula det. 2012 Allotype ♀” (47030026). Genitalia card-mounted underneath the male holotype and female allotype. Chrysina ebrardi is a manuscript name and an invalid label on the male holotype. Box 4618645 SOULA.
Remarks
The unavailable manuscript name Chrysina ebrardi appears on a label underneath the male holotype specimen of C. rutelidedundeei. The distributional data from
Chrysina sallaei
Plusiotis sallaei Boucard, 1875: 123–124 [original combination].
Plusiotis sallei
Boucard [incorrect subsequent spelling by
Chrysina sallaei
(Boucard) [new combination by
Distribution
MEXICO: Hidalgo, Puebla, Veracruz (
Chrysina schusteri
Plusiotis schusteri Monzón, Cano, & Bailey, 1999: 183, 184–185 [original combination].
Chrysina schusteri
(Monzón, Cano, and Bailey) [new combination by
Distribution
GUATEMALA: San Marcos (
Types
1 ♂ holotype and 1 ♀ allotype at UVGC (
Chrysina sirenicola
Plusiotis sirenicola Solís & Morón, 1994: 31, 37–40 [original combination].
Chrysina sirenicola
(Solís and Morón) [new combination by
Distribution
COSTA RICA: Puntarenas (
Types
1 ♂ holotype, 1 ♀ allotype and 4 paratypes at MNCR (
Chrysina spectabilis
Plusiotis spectabilis Ratcliffe & Jameson, 1992: 59–61 [original combination].
Chrysina spectabilis
(Ratcliffe and Jameson) [new combination by
Distribution
HONDURAS: Cortés (
Types
1 ♀ holotype at FMNH (
Chrysina strasseni
Plusiotis strasseni Ohaus, 1924: 185–186 [original combination].
Chrysina strasseni
(Ohaus) [new combination by
Distribution
GUATEMALA: Zacapa (
Chrysina tapantina
Plusiotis tapantina Morón, 1992: 70–72 [original combination].
Chrysina tapantina
(Morón) [new combination by
Distribution
COSTA RICA: Cartago (
Types
1 ♀ holotype at MNCR (
Chrysina taylori
Plusiotis taylori Morón, 1990: 31 [original combination].
Chrysina taylori
(Morón) [new combination by
Distribution
MEXICO: Hidalgo, Veracruz (
Types
1 ♂ holotype, 1 ♀ allotype and 1 paratype at MXAL (
Chrysina tecunumani
Plusiotis tecunumani Cano & Morón, 1994: 2–8 [original combination].
Chrysina tecunumani
(Cano and Morón) [new combination by
Distribution
GUATEMALA: El Progreso, Izabal (
Types
1 ♂ holotype at UVGC (
Chrysina terroni
Plusiotis terroni Morón, 1990: 35 [original combination].
Chrysina terroni
(Morón) [new combination by
Distribution
MEXICO: Hidalgo (
Types
1 ♂ holotype and 1 ♀ allotype at MXAL (
Chrysina transvolcanica
Plusiotis transvolcanica Morón & Nogueira, 2016: 13–15 [original combination].
Chrysina transvolcanica (Morón and Nogueira) [comb. n.].
Distribution
MEXICO: Jalisco, Méxcio, Michoacán, Morelos, Puebla, Tlaxcala (
Types
1 ♂ holotype and 1 ♀ allotype and paratypes at MXAL (
Remarks
The genus Plusiotis was synonymized with Chrysina (
Chrysina tricolor
Plusiotis tricolor Ohaus, 1922: 323 [original combination].
Chrysina tricolor
(Ohaus) [new combination by
Distribution
COSTA RICA: Cartago, San José (
Chrysina triumphalis
Chrysina triumphalis Morón, 1990: 54–55 [original combination].
Distribution
GUATEMALA: San Marcos (
Types
1 ♂ holotype, 1 ♀ allotype and 1 paratype at MXAL (
Chrysina tuerckheimi
Plusiotis türckheimi Ohaus, 1913: 491–492 [original combination].
Plusiotis tuerckheimi
Ohaus [justified emendation by
Chrysina turckheimi
(Ohaus) [new combination and incorrect subsequent spelling by
Distribution
GUATEMALA: San Marcos (
Chrysina veraguana
Plusiotis veraguana Ohaus, 1922: 324 [original combination].
Chrysina veraguana
(Ohaus) [new combination by
Distribution
PANAMA: Veraguas (
Remarks
In reference to species groups,
Chrysina victorina
Pelidnota victorina Hope, 1841: 147 [original combination].
Plusiotis victorina
(Hope) [new combination by
Chrysina victorina
(Hope) [new combination by
Distribution
MEXICO: Chiapas, Oaxaca, Veracruz (
Chrysina wolfi
Plusiotis wolfi Ohaus, 1912: 305 [original combination].
Chrysina wolfi
(Ohaus) [new combination by
Distribution
ECUADOR: Manabí, Pichincha (
Chrysina woodi
Plusiotis woodi Horn, 1884: xxxi [original combination].
Plusiotis woodii
Horn [incorrect subsequent spelling by
Chrysina woodii
(Horn) [new combination by
Chrysina woodi
(Horn) [suggested correct spelling by
Distribution
MEXICO: Chihuahua (H. W. Bates 1888,
Chrysina xalixteca
Plusiotis xalixteca Morón, 1992: 66–70 [original combination].
Chrysina xalixteca
(Morón) [new combination by
Distribution
MEXICO: Jalisco (
Types
1 ♂ holotype, 1 ♀ allotype and paratypes at MXAL (
Chrysina zapoteca
Plusiotis zapoteca Morón, 1990: 39–40 [original combination].
Chrysina zapoteca
(Morón) [new combination by
Distribution
MEXICO: Oaxaca (
Types
1 ♂ holotype and 1 paratype at MXAL (
CHRYSOPHORA
Chrysophora Dejean, 1821: 60.
Type species
Melolontha chrysochlora Latreille, 1812: 131, by monotypy.
Gender
Feminine.
Chrysophora chrysochlora
Melolontha chrysochlora Latreille, 1812: 131 [original combination].
Chrysophora chrysochlora
(Latreille) [new combination by
Distribution
COLOMBIA: Antioquia, Boyacá, Caquetá, Cauca, Cesar, Chocó, Cundinamarca, Huila, Meta, Nariño, Norte de Santander, Tolima, Valle del Cauca (
ECTINOPLECTRON
Ectinoplectron Ohaus, 1915b: 257.
Type species
Homonyx oryctoides Ohaus, 1905: 314–315, by monotypy.
Gender
Neuter.
Ectinoplectron oryctoides
Homonyx oryctoides Ohaus, 1905: 314–315 [original combination].
Ectinoplectron oryctoides
(Ohaus) [new combination by
synonym. Pelidnota howdeni Hardy, 1975
Pelidnota howdeni Hardy, 1975: 6, 14-15 [original combination].
Ectinoplectron oryctoides
(Ohaus) [syn. by
Distribution
MEXICO: Chihuahua, Durango, Jalisco, Michoacán, Nayarit, Sinaloa, Sonora (
Types
1 ♂ lectotype of Homonyx oryctoides at ZMHB (
EPICHALCOPLETHIS
Epichalcoplethis F. Bates, 1904: 253, 272–273.
Chalcoplethis
Burmeister, 1844 [new synonym by
Epichalcoplethis
F. Bates [revised genus status by
Type species
Pelidnota velutipes Arrow, 1900: 179, by monotypy.
Gender
Feminine.
Species
16 species and subspecies.
Remarks
Epichalcoplethis aciculata
Pelidnota aciculata F. Bates, 1904: 254, 261 [original combination].
Pelidnota (Chalcoplethis) aciculata
F. Bates [new subgeneric combination by
Epichalcoplethis aciculata
(F. Bates) [new combination by
Distribution
BOLIVIA: Santa Cruz (WBWC). BRAZIL: Amazonas, Pará (INPA). FRENCH GUIANA: Cayenne, St.-Laurent du Maroni (F.
Epichalcoplethis benjamini
Epichalcoplethis benjamini Bouchard & Soula, 2006: 102, 107 [original combination].
Distribution
BOLIVIA: La Paz (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♂ paratype: “Coll. G. Lecourt Ixiamas 11/91 310 m. Bolivie//Holotype Epichalcoplethis benjamini 2006 S. Soula” (47030043); “Coll. G. Lecourt Ixiamas 11/91 310 m. Bolivie//Allotype Epichalcoplethis benjamini 2006 S. Soula” (47030044); “Coll. G. Lecourt Ixiamas 11/91 310 m. Bolivie//Paratype Epichalcoplethis benjamini 2006 S. Soula” (47030045). Genitalia card-mounted underneath male specimens. Box 4618648 SOULA.
Epichalcoplethis blancoi
Epichalcoplethis blancoi Soula, 2006: 102, 109 [original combination].
Distribution
VENEZUELA: Bolívar, Miranda (
Types
The following specimens are deposited at CCECL. 1 ♀ holotype, 2 ♀ paratypes: “Camp. Minero Payapal Rio Yuruan//Exp. Instituto Zool. Agricola//Venezuela Bolivar//El Dorado 190 m 23-30-V-87//Holotype Epichalcoplethis blancoi S. 2006 Soula” (47030055); “En la luz//VENEZUELA: Bolívar Guri 200 m 27-vi- al 6-vii-1998 L. J. Joly; J. L. García; Y. Zavala//Paratype Epichalcoplethis blancoi S. 2006 Soula” (47030056); “VENEZUELA: Miranda Tacarigua de Manporal 10°22’32”N 66°12’10”W 23-v-1998 Col. O. Hernández S.//Paratype 2006 Epichalcoplethis blancoi S. Soula Soula” (47030057). This is the entire series and it is noted in
Epichalcoplethis chamaeleon
Scarabaeus chamaeleon Herbst, 1789: 247–248 [original combination].
Pelidnota chamaeleon
(Herbst) [new combination by
Pelidnota ignita var. chamaeleon (Herbst) [new infrasubspecific status by F.
Pelidnota (Chalcoplethis) chamaeleon
(Herbst) [new subgeneric combination and revised species status by
Epichalcoplethis chamaeleon
(Herbst) [new combination by
synonym. Pelidnota equestris Laporte, 1840
Pelidnota equestris Laporte, 1840: 122 [original combination].
Pelidnota ignita
(Olivier) [syn. by
Chalcoplethis chamaeleon
(Herbst) [syn. by
Epichalcoplethis chamaeleon
(Herbst) [syn. by
synonym. Cetonia ignita Olivier, 1789
Cetonia ignita Olivier, 1789: 69–70 [original combination].
Rutela ignita
(Olivier) [new combination by
Pelidnota ignita
(Olivier) [new combination by
Chalcoplethis chamaeleon var. ignita (Olivier) [new combination and new infrasubspecific status by
Chalcoplethis chamaeleon forma ignita (Olivier) [revised infrasubspecific status by
Epichalcoplethis chamaeleon
(Herbst) [syn. by
Distribution
BRAZIL: Amazonas, Roraima (INPA) (
Remarks
Voet (1769) is often cited as the author for this species. However, names in Voet’s Catalogus Systematicus Coleopterorum (1778 and subsequent editions) are not consistently binomial (ICZN, Art. 11.4). As such, all of Voet’s names are rejected (Löbl and Smetana 2011).
Epichalcoplethis gilletti
Epichalcoplethis gilletti Soula, 2010a: 48–49 [original combination].
Distribution
ECUADOR: Pastaza (
Remarks
According to
Epichalcoplethis ledezmaae
Epichalcoplethis ledezmaae Bouchard & Soula, 2006: 102, 104–105 [original combination].
Distribution
BOLIVIA: Santa Cruz (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Rte de Camiri à Sta. Cruz Bol. coll. – SOULA //Holotype Epichalcoplethis ledezmaae S. 2006 Soula” (47030042). The genitalia are card-mounted underneath this male specimen. Box 4618648 SOULA.
Epichalcoplethis monzoni
Epichalcoplethis monzoni Soula, 2006: 102, 112 [original combination].
Distribution
BELIZE: Cayo (
Types
The holotype of Epichalcoplethis monzoni is deposited at UVGC (
Epichalcoplethis navarropoloi
Epichalcoplethis navarropoloi Soula, 2011: 73 [original combination].
Distribution
ECUADOR: Pastaza (
Types
The holotype ♂ is deposited at the Malý collection (
Epichalcoplethis porioni
Epichalcoplethis porioni Soula, 2010a: 48 [original combination].
Distribution
HONDURAS: Lempira (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “HONDURAS-LEMPIRA Montana de Celaque AOUT 1995 Thierry PORION Leg//Coll. TH. PORION//Holotype 2009 Epichalcoplethis porioni S. Soula” (47030955). Genitalia card-mounted underneath the male holotype. Box 4616345 PORION.
Epichalcoplethis richteri
Pelidnota richteri Ohaus, 1910a: 186 [original combination].
Pelidnota (Chalcoplethis) richteri
Ohaus [new subgeneric combination by
Epichalcoplethis richteri
(Ohaus) [new combination by
Distribution
BRAZIL: Mato Gross do Sul (
Types
1 type specimen of Pelidnota richteri at MLPA.
Epichalcoplethis santistebani
Epichalcoplethis santistebani Bouchard & Soula, 2006: 102, 105 [original combination].
Distribution
PERU: Huánuco (
Types
The following specimen is deposited at CCECL (Fig.
Epichalcoplethis sanctijacobi
Pelidnota sanctijacobi Ohaus, 1905: 318 [original combination].
Pelidnota (Chalcoplethis) sanctijacobi
Ohaus [new subgeneric combination by
Epichalcoplethis sanctijacobi
(Ohaus) [new combination by
Distribution
ARGENTINA: Córdoba, Salta, Santiago del Estero, Tucumán (
Types
1 ♂ type specimen of Pelidnota sanctijacobi at ZMHB (Fig.
Epichalcoplethis schiffleri
Epichalcoplethis schiffleri Bouchard & Soula, 2006: 102, 107–108 [original combination].
Distribution
PERU: Loreto, Piura (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 4 ♂ paratypes: “Iquitos, Loreto Pérou; VIII/2003//Holotype 2006 Epichalcoplethis schiffleri S. Soula” (47030027); “Iquitos, Loreto Pérou; VIII/2003//Paratype 2005 Epichalcoplethis schiffleri S. Soula” (47030028); “Iquitos 100 m 9.03 Loreto/PERU//Paratype 2005 Epichalcoplethis schiffleri S. Soula” (47030030); “Yamamono River Iquitos (P) 6/88// Paratype 2005 Epichalcoplethis schiffleri S. Soula” (47030031); “Carbajal, Rio Itaya Piura, Pérou, IX/2005// Paratype 2005 Epichalcoplethis schiffleri S. Soula” (47030029). The Yamamono River locality does not appear in the
Epichalcoplethis seriatopunctata
Pelidnota seriatopunctata Ohaus, 1912: 304 [original combination].
Pelidnota (Chalcoplethis) seriatopunctata
Ohaus [new subgeneric combination by
Epichalcoplethis seriatopunctata
(Ohaus) [new combination by
Distribution
BRAZIL (
Epichalcoplethis velutipes romeroi
Epichalcoplethis velutipes romeroi Soula, 2006: 111–112 [original combination].
Distribution
VENEZUELA: Bolívar (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype (Fig.
Remarks
The male holotype specimen labeled from “N. Venezuela” is in fact not the true holotype specimen according to
Epichalcoplethis velutipes velutipes
Pelidnota velutipes Arrow, 1900: 179 [original combination].
Epichalcoplethis velutipes
(Arrow) [new combination by F.
Pelidnota (Chalcoplethis) velutipes
Arrow [revised combination and new subgeneric combination by
Epichalcoplethis velutipes
(Arrow) [new combination by
Distribution
GRENADA (
Types
1 ♂ type at at BMNH (
Eremophygus
Eremophygus Ohaus, 1910c: 21–22.
synonym. Heterocallichloris Gutíerrez, 1951
Heterocallichloris Gutíerrez 1951: 112–114. [Type species. Heterocallichloris bicolor Gutiérrez, 1951 by original designation].
Platycoelia
Dejean [syn. by
Eremophygus
Ohaus [syn. by
Type species
Eremophygus philippii Ohaus, 1910c: 22, by monotypy.
Gender
Masculine.
Species
6 species.
Eremophygus bicolor
Heterocallichloris bicolor Gutiérrez, 1951: 112, 114 [original combination].
Platycoelia bicolor
(Gutiérrez) [new combination by
Eremophygus bicolor
(Gutiérrez) [new combination by
Distribution
BOLIVIA (
Remarks
Heterocallichloris bicolor was originally described in the ruteline tribe Anoplognathini (subtribe Platycoeliina). As a result of a broad analysis of the Platycoeliina (
Eremophygus calvus
Eremophygus calvus Gutiérrez, 1952: 223–224 [original combination].
Distribution
BOLIVIA: La Paz (
Eremophygus lasiocalinus
Eremophygus lasiocalinus Ohaus, 1915a: 76–77 [original combination].
Distribution
BOLIVIA: La Paz (
Eremophygus leo
Eremophygus leo Gutiérrez, 1951: 106 [original combination].
Distribution
ARGENTINA: Jujuy (
Eremophygus pachyloides
Eremophygus pachyloides Ohaus, 1925: 76 [original combination].
Distribution
BOLIVIA (
Eremophygus philippii
Eremophygus philippii Ohaus, 1910c: 22 [original combination].
Distribution
CHILE: Arica and Parinacota; Tarapacá (
HOMEOCHLOROTA
Homeochlorota Soula, 2006: 148–149.
Type species
Pseudochlorota chiriquina Ohaus, 1905: 306-307, by monotypy.
Gender
Species
1 species.
Remarks
Homeochlorota chiriquina
Pseudochlorota chiriquina Ohaus, 1905: 306–307 [original combination].
Homeochlorota chiriquina
(Ohaus) [new combination by
Distribution
COSTA RICA: Guanacaste (
Types
Lectotype male of Pseudochlorota chiriquina at ZMHB labeled: “Panama, V.d. Chiriqui”; “typus!” (red label, typed); male genitalia card mounted; “Pseudochlorota chiriquina Ohaus” (red label, handwritten). Paralectotype female at ZMHB labeled as lectotype with mouthparts card mounted. An exemplar specimen is shown in Fig.
Remarks
HOMONYX
Homonyx Guérin-Méneville, 1839: 299–300.
Type species
Homonyx cupreus Guérin-Méneville, 1839: 300, by monotypy.
Gender
Masculine.
Species
14 species and subspecies.
Homonyx argentinus
Homonyx planicostatus argentinus Gutiérrez, 1952: 224, 225 [original combination].
Homonyx argentinus
Gutiérrez [new species status by
Distribution
ARGENTINA: Jujuy, Mendoza, Salta, Tucumán (
Types
1 ♀ paratype at MNNC. 1 ♂ and 5 ♀ paratypes at CMNC.
Remarks
Homonyx chalceus bahianus
Homonyx bahianus Ohaus, 1913: 495–496 [original combination].
Homonyx chalceus bahianus
Ohaus [new species status by
Distribution
BRAZIL: Bahia (
Types
1 ♂ lectotype and 1 paralectotype at ZMHB (
Remarks
Homonyx chalceus chalceus
Homonyx chalceus Blanchard, 1851: 214 [original combination].
Distribution
ARGENTINA: Corrientes, Mendoza, Salta, San Luis (
Types
1 ♂ holotype at MNHN (
Homonyx cupreus
Homonyx cupreus Guérin-Méneville, 1839: 300 [original combination].
Distribution
ARGENTINA: Corrientes, Salta (
Remarks
Homonyx cupreus Guérin-Méneville was erroneously reported from the extreme southern Chilean Magallanes Province and later from the specific locality of Port Famine (modern Puerto del Hambre) (
Homonyx demezi
Homonyx demezi Soula, 2010a: 23 [original combination].
Distribution
BRAZIL: Mato Grosso (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 3 ♂ paratypes, 1 ♀ paratype: “Mato Grosso Brésil coll. – SOULA//Holotype 2010 Homonyx demezi S. Soula (47030999); “Matto Grosso Brésil coll. – SOULA//Allotype 2010 Homonyx demezi S. Soula (47031000); “Rosario Matto Grosso M. SOULA det 19 [obverse] 10/61//Paratype 2010 Homonyx demezi S. Soula (47031001); “Corumba Matt.//Paratype 2010 Homonyx demezi S. Soula (47031002); “Rosario Oeste Matto Grosso 01/72 coll. – Soula [obverse] Rosario Oeste//Paratype 2010 Homonyx demezi S. Soula (47031003); “Gob. de Los Andes//Paratype 2009 Homonyx demezi S. Soula (47031004). Genitalia card-mounted underneath the male holotype and the three male paratypes. Box 4618689 SOULA. The following specimen is deposited at CMNC. 1 ♀ paratype: “BRASIL Mato Grosso Rosario Oeste A. Maller-leg. Coll. Martínez Oct.-968// H. & A. HOWDEN COLLECTION ex. A. Martinez coll.//Paratype 2010 Homonyx demezi S. Soula”.
Homonyx elongatus
Rutela elongata Blanchard, 1842: plate 11 [original combination].
Homonyx elongatus
(Blanchard) [new combination by
Distribution
ARGENTINA (
Types
1 ♀ holotype at MNHN (
Homonyx feyeri
Homonyx feyeri Ohaus, 1913: 496–497 [original combination].
Distribution
ECUADOR: Morona-Santiago (
Types
1 ♂ holotype specimen of Homonyx feyeri Ohaus at ZMHB (Fig.
Homonyx fuscocupreus
Homonyx chalceus var. fuscocupreus Ohaus, 1913: 494 [original combination].
Homonyx chalceus fuscocupreus
Ohaus [new subspecific status by
Homonyx fuscocupreus
Ohaus [new species status by
Distribution
ARGENTINA: Catamarca, Tucumán (
Types
1 lectotype and 1 paralectotype at ZMHB (
Remarks
Homonyx holligeri
Homonyx holligeri Soula, 2010a: 19–20 [original combination].
Distribution
BOLIVIA: Santa Cruz (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♂ paratype: “Coroïco à Caranavi 850 m 10/88//Holotype 2010 Homonyx holligeri S. Soula (47030993); “Bolivia-Dept. Santa Cruz-800 m 25.X.1960-Zischka//Paratype 2010 Homonyx holligeri S. Soula (47030994). Genitalia card-mounted underneath the male holotype and the male paratype. Box 4618689 SOULA.
Homonyx maurettei
Homonyx maurettei Soula, 2010a: 18–19 [original combination].
Distribution
PERU: Piura (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Abra Porculla, Dt. Piura N-W Pérou; 1800m II/2007//Holotype Homonyx maurettei S. 2010 Soula (47030998). Genitalia card-mounted underneath the male holotype. Box 4618689 SOULA.
Homonyx peruanus
Homonyx planicostatus peruanus Ohaus, 1913: 496 [original combination].
Homonyx elongatus peruanus
Ohaus [revised subspecies status by
Homonyx planicostatus peruanus
Ohaus [revised subspecies status by
Homonyx elongatus peruanus
Ohaus [revised subspecies status by
Homonyx peruanus
Ohaus [new species status by
Distribution
PERU (
Types
1 ♀ syntype specimen of Homonyx planicostatus peruanus Ohaus at ZMHB (Fig.
Homonyx planicostatus
Homonyx planicostatus Blanchard, 1851: 214 [original combination].
Distribution
ARGENTINA: Mendoza, Tucumán (
Types
1 ♀ syntype at MNHN (
Remarks
CCECL contains a H. planicostatus specimen labeled as a male ♂ alloréférent with the following data: “Vaccaguzman [arrow] Camiri coll. – SOULA [obverse] 1615m//Alloréférent ♂ de Homonyx planicostatus Bl. M. SOULA det 19 (47030995). Genitalia card-mounted underneath specimen. Box 4618689 SOULA.
Homonyx santiagensis
Homonyx chalceus santiagensis Ohaus, 1913: 494 [original combination].
Homonyx santiagensis
Ohaus [new species status by
Distribution
ARGENTINA: Córdoba, Jujuy, Santiago del Estero (
Types
1 ♂ lectotype and 1 paralectotype at ZMHB (Fig.
Remarks
Homonyx uruguayanus
Homonyx chalceus uruguayanus Ohaus, 1913: 494 [original combination].
Homonyx uruguayensis
Ohaus [new species status and incorrect subsequent spelling by
Distribution
ARGENTINA: Córdoba, Entre Ríos (
Types
1 ♂ syntype of Homonyx chalceus uruguayanus at ZMHB (called a holotype by
Remarks
Unavailable names in Homonyx(application of ICZN Article 16.4.2)
We consider the following names proposed by Soula in Homonyx as unavailable per ICZN Article 16.4.2 which states that fixation of holotype specimens for new names must be accompanied by the following information, “where the holotype or syntypes are extant specimens, by a statement of intent that they will be (or are) deposited in a collection and a statement indicating the name and location of that collection”. The names below were proposed by Soula (2010,
Homonyx digennaroi
Homonyx digennaroi Soula, 2010a: 19, 21-22 [original combination, unavailable, invalid name].
Distribution
BOLIVIA (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype: “Rte de Camiri à Sta Cruz Bol. coll. – SOULA//Holotype 2010 Homonyx digennaroi S. Soula (47031008). Genitalia card-mounted underneath the male holotype. Box 4618689 SOULA.
Homonyx lecourti
Homonyx lecourti Soula, 2010a: 19, 20 [original combination, unavailable, invalid name].
Distribution
BOLIVIA: La Paz (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype and 1 invalid ♀ allotype: “Yocumo 920 m 26/10/2000 M. SOULA det 19//Holotype 2010 Homonyx lecourti S. Soula (47031006); “Yocumo 920 m 26/X/2000 M. SOULA det 19//Allotype 2010 Homonyx lecourti S. Soula (47031007). Genitalia card-mounted underneath the male holotype. Box 4618689 SOULA.
Homonyx mulliei
Homonyx mulliei Soula, 2010a: 23, 24 [original combination, unavailable, invalid name].
Distribution
BOLIVIA (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Camiri à Sta Cruz coll. – Soula//Holotype 2010 Homonyx mulliei S. Soula (47031005). Genitalia card-mounted underneath the male holotype. Box 4618689 SOULA.
Homonyx simoensi
Homonyx simoensi Soula, 2010a: 22, 23 [original combination, unavailable, invalid name].
Distribution
BOLIVIA (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Camiri à Sta Cruz coll. – SOULA//Holotype 2010 Homonyx simoensi S. Soula (47031009). Genitalia card-mounted underneath the male holotype. Box 4618689 SOULA.
Homonyx wagneri
Homonyx wagneri Soula, 2010a: 23, 25 [original combination, unavailable, invalid name].
Distribution
ARGENTINA: Salta (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Salta Argentine XI/2006 M. SOULA det 19//Holotype 2010 Homonyx wagneri S. Soula (47030997). Genitalia card-mounted underneath the male holotype. Box 4618689 SOULA.
Homonyx zovii
Homonyx zovii Demez & Soula, 2011: 74 [original combination, unavailable, invalid name].
Distribution
PERU: San Martín (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Janjui San Martin IX/2010 M. SOULA det 19//Holotype 2011 Homonyx zovii S. 2011 Soula (47030996). Genitalia card-mounted underneath the male holotype. Box 4618689 SOULA.
HOMOTHERMON
Homothermon Ohaus, 1898: 59-60.
Type species
Homothermon bugre Ohaus, 1898: 60, original designation by
Gender
Neuter.
Species
4 species.
Homothermon bugre
Homothermon bugre Ohaus, 1898: 60 [original combination].
Distribution
ARGENTINA: Misiones (
Types
1 ♂ lectotype and paralectotypes at ZMHB (
Homothermon drumonti
Homothermon drumonti Soula, 2008: 33 [original combination].
Distribution
BRAZIL: São Paulo (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Brasil OL. Guillot//Det Dr. Ohaus Homothermon paulista Ohaus//Holotype Homothermon drumonti S. 2007 Soula” (47031074). Genitalia card-mounted underneath the male holotype. Box 4618691 SOULA.
Homothermon praemorsus
Odontognathus praemorsus Burmeister, 1855: 521 [original combination].
Homothermon praemorsus
(Burmeister) [new combination by
synonym. Homothermon paulista Ohaus, 1898
Homothermon paulista Ohaus, 1898: 61 [original combination].
Homothermon praemorsus
(Burmeister) [syn. by
Distribution
BRAZIL: São Paulo (
Types
1 ♀ syntype of Odontognathus praemorsus at ZMHB (
Homothermon serrano
Homothermon serrano Ohaus, 1898: 60 [original combination].
Distribution
ARGENTINA: Misiones (
HOPLOPELIDNOTA
Hoplopelidnota Bates, 1904: 253, 274–275.
Type species
Hoplopelidnota candezei F. Bates, 1904: 274–275, by monotypy.
Gender
Feminine.
Species
1 species.
Hoplopelidnota metallica
Pelidnota metallica Laporte, 1840: 122 [original combination].
Hoplopelidnota candezei
F. Bates [syn. by
Hoplopelidnota metallica
(Laporte) [revised species status by
synonym. Hoplopelidnota armata Ohaus, 1912
Hoplopelidnota armata Ohaus, 1912: 309 [original combination; sometimes erroneously referred to as H. armata F. Bates].
Hoplopelidnota metallica
(Laporte) [syn. by
synonym. Hoplopelidnota candezei F. Bates, 1904
Hoplopelidnota candezei F. Bates, 1904: 274–275 [original combination].
Hoplopelidnota metallica
(Laporte) [syn. by
Distribution
BRAZIL: Territorio de Amapa (Serra Navia). FRENCH GUIANA: Cayenne (
Types
The following specimen is deposited at CCECL. 1 invalid ♂ neotype (Fig.
Remarks
The classification of Hoplopelidnota metallica (Figs
Hoplopelidnota metallica is distributed in northern South America. Prior to this work, H. metallica was only recorded from French Guiana. In addition to French Guiana, we record the species from Guyana (Moraballi Creek, Essequibo River), Venezuela (Amazonas Dept., Rio Negro) and Brazil (Territorio de Amapa, Serra Navia). The species is rare in collections, and is apparently much more wide spread in northern South America than previous data would indicate. Specimens are recorded from 140 m elevation in March, April, July, August, and November.
Hoplopelidnota metallica (Laporte) invalid neotype male from CCECL. A Dorsal habitus B Male parameres, caudal view C Specimen labels.
MECOPELIDINOTA
Mecopelidnota F. Bates, 1904: 252, 270–271.
Type species
Mecopelidnota arrowi F. Bates, 1904: 271–272, by monotypy.
Gender
Feminine.
Species
8 species.
Mecopelidnota arrowi
Mecopelidnota arrowi F. Bates, 1904: 271–272 [original combination].
synonym. Pelidnota egregia Frey, 1967
Pelidnota egregia Frey, 1967: 374–375 [original combination].
Pelidnota (Pelidnota) egregia
Frey [new subgeneric combination by
Mecopelidnota arrowi
F. Bates [syn. by
Distribution
ECUADOR: Azuay, Guayas (F.
Types
1 ♂ holotype specimen of Mecopelidnota arrowi at BMNH (
Mecopelidnota cylindrica
Pelidnota cylindrica Waterhouse, 1876: 24 [original combination].
Mecopelidnota cylindrica
(Waterhouse) [new combination by F.
Distribution
ECUADOR: Guayas (
Types
1 ♂ lectotype at BMNH (
Remarks
Mecopelidnota cylindrica (Waterhouse) has been reported from Guatemala without further details (
Mecopelidnota dewynteri
Mecopelidnota dewynteri Soula, 2008: 26 [original combination].
Distribution
PERU: Cajamarca (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 2 ♂ paratypes, 7 ♀ paratypes: “Limon, Cajamarca N-Pérou, 1800m XII/2000//Holotype Mecopelidnota dewinteri (sic) S. 2007 Soula” (47031098); “Limon, Cajamarca N-Pérou, 1800m XII/2000//Allotype Mecopelidnota dewynteri S. 2007 Soula” (47031099); Nine paratypes with identical label data: “Limon, Cajamarca N-Pérou, 1800m XII/2000//Paratype Mecopelidnota dewynteri S. 2007 Soula” (47031100 to 47031106). Genitalia card-mounted underneath the male holotype and the male paratype. Box 4618692 SOULA.
Mecopelidnota gerardi
Mecopelidnota gerardi Soula, 2008: 25–26 [original combination].
Distribution
ECUADOR: Azuay (
Types
Mecopelidnota marxi
Mecopelidnota marxi Soula, 2008: 25 [original combination].
Distribution
ECUADOR: Loja (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 5 ♂ paratypes, 13 ♀ paratypes: “Abra Porculla, Dt. Piura N-W Pérou; 1800m II/2007//Holotype Mecopelidnota marxi S. 2007 Soula” (47031109); “Abra Porculla, Dt. Piura N-W Pérou; 1800m II/2007//Allotype Mecopelidnota marxi S. 2007 Soula” (47031110); Eleven paratypes with identical label data: “Abra Porculla, Dt. Piura N-W Pérou; 1800m II/2007//Paratype Mecopelidnota marxi S. 2007 Soula” (47031111 to 47031120, exch61); “PERU Sullana Hda. Mallares 9. III.58 W. MARKL//Paratype Mecopelidnota marxi S. 2007 Soula” (47031121); “PERU Sullana Hda. Mallares 28. II.57 W. MARKL//Paratype Mecopelidnota marxi S. 2007 Soula” (47031122); Two paratypes with label data “Catamayo Loja (Eq.) coll. – SOULA [obverse] 7/III/98 (1300m)//Paratype Mecopelidnota marxi S. 2007 Soula” (47031123). Genitalia card-mounted underneath the male holotype and three female paratypes. Box 4618692 SOULA.
Mecopelidnota mezai
Mecopelidnota mezai Soula, 2008: 28 [original combination].
Distribution
PERU: Lambayeque, Piura (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 9 ♂ paratypes, 12 ♀ paratypes: “Abra Porculla, Dt. Piura N-W Pérou ; 1800m II/2007//Holotype 2007 Mecopelidnota mezai S. Soula” (47031076); “Abra Porculla, Dt. Piura N-W Pérou ; 1800m II/2007//Allotype 2007 Mecopelidnota mezai S. Soula” (47031077); Six paratypes with identical label data: “Abra Porculla, Dt. Piura N-W Pérou ; 1800m II/2007//Paratype 2007 Mecopelidnota mezai S. Soula” (47031078 to 47031083); “Abra Porculla Piura - Pérou coll. – SOULA [obverse] XI/2000 ~1000m//Paratype 2007 Mecopelidnota mezai S. Soula” (47031084); Eight paratypes with identical label data: “Penachi, 1800m Dto. Lambayeque N.W. Pérou, III/2007//Paratype 2007 Mecopelidnota mezai S. Soula” (47031085 to 47031091, exch60); “Penachi Dt Lambayeque Pérou M. SOULA det 19 [obverse] 2000 III/2007//Paratype 2007 Mecopelidnota mezai S. Soula” (47031093); “Huasmaca Piura 1700 m M. SOULA det 19 [obverse] N-W-Pérou 5-6/2006//Paratype Mecopelidnota mezai S. 2007 Soula” (47031094). Two paratypes with identical label data “Penachi, 1800m Dto. Lambayeque N. W. Pérou, III/2007//Paratype 2007 Mecopelidnota mezai S. Soula” (47031095 and 47031096). Genitalia card-mounted underneath the male holotype, the female allotype, one female paratype and four male paratypes. Box 4618691 SOULA.
Mecopelidnota obscura
Pelidnota obscura Taschenberg, 1870: 184–185 [original combination].
Mecopelidnota obscura
(Taschenberg) [new combination by
Distribution
COLOMBIA (
Mecopelidnota witti
Mecopelidnota witti Ohaus, 1913: 497–498 [original combination].
Distribution
ECUADOR: Azuay, Loja (
Types
1 ♂ lectotype and 2 paralectotypes at ZMHB (
Invalid, unavailable names in Mecopelidnota
Mecopelidnota willersi
Types
The following specimens are deposited at CCECL. 1 ♂ invalid holotype, 1 ♀ invalid allotype, 3 ♂ invalid paratypes, 2 ♀ invalid paratypes: “Oña, Equat. 2500 m II/2001 M. SOULA det 19//Holotype Mecopelidnota willersi S. 2007 Soula//Invalid Holotype det. MR Moore ‘15” (47031128); “Oña, Equat. 2500 m II/2001 M. SOULA det 19//Allotype Mecopelidnota willersi S. 2007 Soula//Invalid Allotype det. MR Moore ‘15” (47031129); Two invalid paratypes with identical label data: “Equateur M. SOULA det 19//Paratype Mecopelidnota willersi S. 2007 Soula//Invalid Paratype det. MR Moore ‘15” (47031130 and 47031131); Three invalid paratyes with identical label data “Oña - Equ. II/2001 M. SOULA det 19 [obverse] 2500 m//Paratype Mecopelidnota willersi S. 2007 Soula//Invalid Paratype det. MR Moore ‘15” (47031132). Genitalia card-mounted underneath the invalid male holotype and the two invalid male paratypes. Box 4618692 SOULA.
Remarks
The name Mecopelidnota willersi Soula has never been associated with a species description or holotype designation in the literature. These type specimens of Mecopelidnota willersi are thus invalid.
Mecopelidnota bondili
Types
The following specimens are deposited at CCECL. 1 invalid ♂ paratype: “Oña, Equateur 2500m II/2001 M. SOULA det 19//Paratype Mecopelidnota bondili Sou. 2007 Soula” (47031097)// Invalid paratype det. M. R. Moore ‘15”. Genitalia card-mounted (only apex of aedeagus) underneath the invalid male paratype. Box 4618691 SOULA.
Remarks
The name Mecopelidnota bondili Soula has never been associated with a species description or holotype designation in the literature. This type specimen of Mecopelidnota bondili is thus invalid.
MESOMERODON
Mesomerodon Ohaus, 1905: 319.
Type species
Mesomerodon spinipenne Ohaus, 1905: 320–321, by monotypy.
Gender
Neuter.
Species
2 species.
Mesomerodon gilletti
Mesomerodon gilletti Soula, 2008: 21 [original combination].
Distribution
ECUADOR: Napo (
Types
The following specimens are deposited at CCECL (Fig.
Mesomerodon gilletti Soula holotype male and allotype female from CCECL. A Dorsal habitus holotype B Specimen labels, holotype C Male genitalia, lateral view D Specimen labels, allotype E Dorsal habitus, allotype.
Mesomerodon spinipenne
Mesomerodon spinipenne Ohaus, 1905: 320–321 [original combination].
Distribution
BOLIVIA: Cochabamba (WBWC), Santa Cruz (
Types
1 ♂ lectotype and 2 paralaectotypes at ZMHB (
MICROOGENIUS
Oogenius (Microogenius)
Lasiocala
Blanchard [syn. by
Minilasiocala
Soula [syn. by
Microogenius
Gutiérrez [new genus status by
synonym. Minilasiocala Soula, 2006
Minilasiocala Soula, 2006: 116, 139. [Type species. Lasiocala arrowi Ohaus, 1910b, by original designation].
Microogenius
Gutiérrez [syn. by
Type species
Oogenius (Microogenius) martinezi
Gender
Masculine.
Species
4 species.
Remarks
Microogenius arrowi
Lasiocala arrowi Ohaus, 1910b: 221–222 [original combination].
Minilasiocala arrowi
(Ohaus) [new combination by
Microogenius arrowi
(Ohaus) [new combination by
Distribution
BOLIVIA: La Paz (
Types
1 ♂ lectotype and 2 paralectotypes of Microogenius arrowi at ZMHB; 1 ♂ paralectotype at BMNH (Fig.
Remarks
Microogenius gutierrezi
Oogenius (Microogenius) gutierrezi Martínez, 1953: 81–86 [original combination].
Lasiocala gutierrezi
(Martínez) [new combination by
Oogenius (Microogenius) gutierrezi
Martínez [revised combination and revised subgeneric combination by
Minilasiocala gutierrezi
(Martínez) [new combination by
Microogenius gutierrezi
(Martínez) [new combination by
Distribution
BOLIVIA: Cochabamba (
Types
Holotype ♂ of Oogenius (Microogenius) gutierrezi Martínez at MACN (Fig.
Microogenius lanterii
Minilasiocala lanterii Soula, 2006: 143 [original combination].
Microogenius lanterii
(Soula) [new combination by
Distribution
ARGENTINA: Jujuy (
Types
Remarks
Microogenius martinezi
Oogenius (Microogenius) martinezi Gutiérrez, 1951: 107 [original combination].
Lasiocala martinezi
(Gutiérrez) [new combination by
Oogenius (Microogenius) martinezi
Gutiérrez [revised combination and revised subgeneric combination by
Minilasiocala martinezi
(Gutiérrez) [new combination by
Microogenius martinezi
(Gutiérrez) [new combination by
Distribution
BOLIVIA: Cochabamba (
Types
Holotype specimen of O. (M.) martinezi at MACN; 1 ♂ paratype specimen of O. (M.) martinezi at UCCC (Fig.
NEOGUTIERREZIA
Neogutierrezia Martínez, 1953: 2.
Type species
Neogutierrezia mirabilis Martínez, 1953: 2, by original designation.
Gender
Feminine.
Species
10 species.
Neogutierrezia affinis
Neogutierrezia mirabilis affinis Martínez, 1973: 35 [original combination].
Neogutierrezia affinis
Martínez [new species status by
Distribution
ARGENTINA: Río Negro (
Types
1 ♂ holotype at MACN (
Neogutierrezia araucana
Neogutierrezia araucana Martínez, 1973: 36–41 [original combination].
Distribution
ARGENTINA: Neuquén (
Types
1 ♂ holotype and 1 ♀ allotype at MACN (
Neogutierrezia bicolor
Neogutierrezia bicolor Ocampo & Ruiz-Manzanos, 2010: 95–98 [original combination].
Distribution
ARGENTINA: Neuquén (
Neogutierrezia chelii
Neogutierrezia chelii Ocampo & Ruiz-Manzanos, 2010: 98 [original combination].
Distribution
ARGENTINA: Chubut (
Types
1 ♂ holotype and 3 ♂ paratypes at IAZA (
Neogutierrezia galileoi
Neogutierrezia galileoi Ocampo & Ruiz-Manzanos, 2010: 98–99 [original combination].
Distribution
ARGENTINA: Mendoza (
Types
1 ♂ holotype and 1 ♂ paratype at IAZA (
Neogutierrezia lagosae
Neogutierrezia lagosae Ocampo & Ruiz-Manzanos, 2010: 99–100 [original combination].
Distribution
ARGENTINA: Mendoza (
Types
1 ♂ holotype and 11 ♂ paratypes at IAZA (
Neogutierrezia mirabilis
Neogutierrezia mirabilis Martínez, 1953: 2 [original combination].
Neogutierrezia mirabilis mirabilis
Martínez [new subspecific status by
Neogutierrezia mirablis mirablis
Martínez [incorrect spelling by
Neogutierrezia mirabilis
Martínez [revised species status by
Distribution
ARGENTINA: Río Negro (
Types
1 ♂ holotype at MACN (
Neogutierrezia payuniensis
Neogutierrezia payuniensis Ocampo & Ruiz-Manzanos, 2010: 101–102 [original combination].
Distribution
ARGENTINA: Mendoza (
Types
1 ♂ holotype at IAZA (
Neogutierrezia scutata
Neogutierrezia scutata Ocampo & Ruiz-Manzanos, 2010: 102–103 [original combination].
Distribution
ARGENTINA: Mendoza (
Types
1 ♂ holotype and 13 ♂ paratypes at IAZA (
Neogutierrezia variabilis
Neogutierrezia variabilis Ocampo & Ruiz-Manzanos, 2010: 103 [original combination].
Distribution
ARGENTINA: Mendoza (
Types
1 ♂ holotype and 13 ♂ paratypes at IAZA (
OOGENIUS
Oogenius Solier, 1851: 97-98.
Type species
Oogenius virens Solier, 1851: 98, by monotypy.
Gender
Masculine.
Species
7 species.
Oogenius arrowi
Oogenius arrowi Gutiérrez, 1949: 27–29 [original combination].
Oogenius (Oogenius) arrowi
Gutiérrez [new subgeneric combination by
Oogenius arrowi
Gutiérrez [revised combination by
Distribution
ARGENTINA: Mendoza (
Types
1 ♂ holotype specimen of Oogenius arrowi Gutiérrez at BMNH (Fig.
Remarks
Oogenius castilloi
Oogenius castilloi Martínez & Peña, 1990: 9–11 [original combination].
Oogenius (Oogenius) castilloi
Martínez and Peña [new subgeneric combination by
Oogenius castilloi
Martínez and Peña [revised combination by
Distribution
CHILE: Coquimbo (
Oogenius chilensis
Oogenius chilensis Ohaus, 1905: 326–327 [original combination]
Oogenius (Oogenius) chilensis
Ohaus [new subgeneric combination by
Oogenius chilensis
Ohaus [revised combination by
synonym. Oogenius chilensis barrosi Gutiérrez, 1949
Oogenius chilensis var. barrosi Gutiérrez, 1949: 27 [original combination, available name per ICZN Article 45.6.4].
Oogenius chilensis forma barrosi Gutiérrez [revised infrasubspecific status by
Oogenius chilensis barrosi
Gutiérrez [new subspecific status by
Oogenius chilensis
Ohaus [syn. by
Distribution
CHILE: Atacama, Coquimbo (CMNC), O’Higgins, Valparaíso, Metropolitan Region (FSCA), Maule (CMNC), Bío Bío (
Types
Lectotype ♂ of Oogenius chilensis at ZMHB (
Remarks
Oogenius chilensis barrosi Gutiérrez was proposed originally as a “variety” of O. chilensis Ohaus (
Oogenius kuscheli
Oogenius kuscheli Gutiérrez, 1949: 29–30 [original combination].
Oogenius (Oogenius) kuscheli
Gutiérrez [new subgeneric combination by
Oogenius kuscheli
Gutiérrez [revised combination by
Distribution
CHILE: Bío Bío (
Types
Holotype ♂ of Oogenius kuscheli at UCCC (
Oogenius lariosae
Oogenius (Oogenius) lariosae Martínez, 1953: 76, 77–81 [original combination].
Oogenius lariosae
Martínez [removal of subgeneric classification by
Distribution
ARGENTINA: Chubut (CMNC), Mendoza, Río Negro (
Oogenius penai
Oogenius gutierrezi Martínez & Peña, 1994 [original combination].
Oogenius (Oogenius) penai
Mondaca [new replacement name by
Oogenius penai
Mondaca [revised combination by
Distribution
CHILE: Metropolitan Region (
Types
Holotype ♂ of Oogenius gutierrezi at MNNC (
Oogenius virens
Oogenius virens Solier, 1851: 98 [original combination].
Oogenius (Oogenius) virens
Solier [new subgeneric combination by
Oogenius virens
Solier [revised combination by
Distribution
CHILE: Coquimbo, Valparaíso (
Types
Lectotype ♂ of Oogenius virens at MNHN (
PACHACAMA
Pachacama Soula, 2006: 113–114.
Type species
Pachacama ocampoi ocampoi Soula, 2006: 113–114, by original designation.
Gender
Feminine.
Species
2 subspecies.
Pachacama ocampoi cagnarensis
Pachacama ocampoi cagnarensis Soula, 2006: 115 [original combination].
Distribution
ECUADOR: Cañar (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “El Triumfo (500m) (E) Prov. de Cañar Cañar 2/90//Holotype 2006 Pachacama ocampoi caniarensis (sic) Soula det. S.” (47031072). Box 4618686 SOULA.
Pachacama ocampoi ocampoi
Pachacama ocampoi Soula, 2006: 113–114 [original combination].
Distribution
ECUADOR: Pichincha (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype: “ECUADOR OCCIDENTE PINCHINCHA ancienne rte. QUITO/SANTO DOMINGO Km 78 (1650 m) 25 dec. 1978 Rec. Th. PORION//COLL. TH. PORION//Holotype 2006. Pachacama ocampoi S. Soula det.” (47030951); “Pacto Pichincha Equateur M. SOULA det 19//Allotype 2006 Pachacama ocampoi ocampoi S. Soula” (47031071). Genitalia card-mounted underneath the male holotype. Box 4618686 SOULA and 4616344 PORION. An exemplar specimen is figured (Fig.
PARHOMONYX
Parhomonyx Ohaus, 1915b: 257–258.
Type species
Homonyx fuscoaeneus Ohaus, 1905: 313–314, by monotypy.
Gender
Masculine.
Species
1 species.
Parhomonyx fuscoaeneus
Homonyx fuscoaeneus Ohaus, 1905: 313–314 [original combination].
Parhomonyx fuscoaeneus
(Ohaus) [new combination by
Distribution
ARGENTINA: Catamarca (FSCA), San Luis (FSCA), Córdoba, Santa Fe, Santiago del Estero (
Types
1 ♂ lectotype specimen of Homonyx fuscoaeneus and 3 paralectotypes at ZMHB (
Remarks
One paralectotype of P. fuscoaeneus, labeled “R. d. JANEIRO Therespolis” (=Theresopolis, Rio de Janeiro, Brazil), is disjunct from all other known localities. Other than this specimen, we have not examined any specimens outside of northern Argentina, and we believe that the data on this label are in error.
Homonyx fuscoaeneus Ohaus (valid name Parhomonyx fuscoaeneus [Ohaus]) male type (see “Type specimens and lectotype designation” in Methods) from ZMHB. A Dorsal habitus B Lateral habitus C Specimen labels, mouthparts, and male genitalia D Male genitalia, lateral view E Male parameres, dorsal view.
PARHOPLOGNATHUS
Parhoplognathus Ohaus, 1915b: 257.
Type species
Areoda maculata Gory, 1833b, by original designation.
Gender
Masculine.
Species
4 species.
Parhoplognathus bousqueti
Parhoplognathus bousqueti Soula, 2008: 9 [original combination].
Distribution
BRAZIL (
Types
1 ♂ holotype at ZMHB (
Remarks
This species was described based on one male specimen from “Brazil”.
Parhoplognathus limbatipennis
Hoplognathus limbatipennis Ohaus, 1905: 323–324 [original combination].
Parhoplognathus limbatipennis
(Ohaus) [new combination by
Distribution
BRAZIL: Minas Gerais, Rio de Janeiro, (
Parhoplognathus maculatus
Areoda maculata Gory, 1833b: new taxon N°13 [original combination].
Hoplognathus maculatus
(Gory) [new combination by
Parhoplognathus maculatus
(Gory) [new combination by
synonym. Pelidnota bimaculata Laporte, 1840
Pelidnota bimaculata Laporte, 1840: 122–123 [original combination].
Hoplognathus maculatus
(Gory) [syn. by F.
Distribution
BRAZIL (
Types
1 ♂ syntype of Areoda maculata at IRSNB (described as a holotype by
Parhoplognathus parvulus
Hoplognathus parvulus Ohaus, 1905: 323 [original combination].
Parhoplognathus parvulus
(Ohaus) [new combination by
Distribution
BRAZIL: Santa Catarina (
Types
1 ♂ lectotype and 1 paralectotype at ZMHB (
Remarks
Kracjik (2008) considered “P. parvulus var. rubripennis Ohaus” to be a synonym of P. parvulus (Ohaus). However, because the name P. parvulus var. rubripennis Ohaus is infrasubspecific (see “Parhoplognathus rubripennis Soula, 2008”) and therefore unavailable, this nomenclatural act was not valid.
Unavailable names in Parhoplognathus (application of ICZN Articles 45.6 and 16.1)
Parhoplognathus rubripennis
Parhoplognathus parvulus var. rubripennis Ohaus, 1930a: 138 [original combination, unavailable, invalid name per ICZN Articles 45.6.1; 1.3.4].
Parhoplognathus parvulus forma rubripennis Machatschke, 1972: 9 [original combination, unavailable, invalid name per ICZN Article 45.6.3].
Parhoplognathus rubripennis Soula, 2008: 7 [original combination, unavailable name per ICZN Article 16.1].
Distribution
BRAZIL: Bahia (
Types
1 invalid ♀ holotype probably at ZMHB (
PATATRA
Patatra Soula, 2008: 40.
Type species
Patatra mathani Soula, 2008: 40, by monotypy.
Gender
Feminine.
Species
1 species.
Patatra mathani
Patatra mathani Soula, 2008: 40 [original combination].
Distribution
BRAZIL: Pará (
Types
According to
Remarks
This species was described based on one male specimen (at MNHN).
PELIDNOTA
Pelidnota MacLeay, 1819: 157–158.
synonym. Aglycoptera Sharp, 1885
Aglycoptera Sharp, 1885: xxiii–xxiv. [Type species. Aglycoptera lacerdae Sharp, 1885 by monotypy (= Pelidnota burmeisteri burmeisteri Burmeister, 1844)].
Pelidnota
MacLeay [syn. by
synonym. Pelidnota (Ganonota) Ohaus, 1915b
Pelidnota (Ganonota)
Ohaus, 1915b: 259. [Type species. Rutela cuprea Germar, 1824, by subsequent designation (
Pelidnota (Strigidia)
Burmeister [syn. by
Pelidnota
MacLeay [syn. by
synonym. Pelidnota (Delipnia) Casey, 1915
Pelidnota (Delipnia) Casey, 1915: 80. [Type species. Pelidnota belti Sharp, 1877 by monotypy].
Pelidnota (Ganonota)
Ohaus [syn. by
synonym. Pelidnota (Pelidnotidia) Casey, 1915
Pelidnota (Pelidnotidia) Casey, 1915: 77. [Type species. Pelidnota strigosa Laporte, 1840, by original designation].
Pelidnota (Pelidnota)
MacLeay [syn. by
synonym. Strigidia Burmeister, 1844
Strigidia
Burmeister, 1844: 388–389. [Type species. Rutela cuprea Germar, 1824, by original designation (
Pelidnota (Ganonota)
Ohaus [syn. by
Pelidnota (Strigidia)
Burmeister [syn. by
Pelidnota (Odontognathus)
Laporte [syn. by
Pelidnota (Strigidia)
Burmeister [syn. by
Pelidnota
MacLeay [syn. by
synonym. Odontognathus Laporte, 1840
Odontognathus Laporte, 1840: 137. [Type species. Odontognathus unicolor Laporte, 1840, by monotypy (= Pelidnota cuprea (Germar, 1824))].
Pelidnota (Odontognathus)
Laporte [new subgeneric status by
Pelidnota (Ganonota)
Ohaus [syn. by
Pelidnota (Strigidia)
Burmeister [syn. by
Pelidnota (Odontognathus)
Laporte [revised subgeneric status by
Pelidnota (Strigidia)
Burmeister [syn. by
Pelidnota
MacLeay [syn. by
synonym. Heteropelidnota Ohaus, 1912.
Heteropelidnota Ohaus, 1912: 309–310. [Type species. Heteropelidnota kuhnti Ohaus, 1912, by monotypy].
Pelidnota MacLeay [syn.].
Type species
Scarabaeus punctatus Linnaeus, 1758: 350, by monotypy (
Gender
Feminine.
Species
194 species and subspecies.
Pelidnota abracadabra
Pelidnota abracadabra Soula, 2009: 31, 62–63 [original combination].
Distribution
MEXICO: Colima, Guerrero, Jalisco (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 probable ♂ paratype: “Manzillo Mexique XII 86 Dr F. GARNIER//Holotype 2008 Pelidnota abracadabra S. Soula det.” (47030490); “MEXIQUE CHAMELA (JAL) STATION U N A M 6 7-IX-1984 D&B SIGWALT REC//[unwritten red label]//Probable paratype Pelidnota abracadabra S. det. MR Moore ‘15” (47030491). Genitalia card-mounted underneath the male holotype. Box 4618666 SOULA.
Remarks
In his description,
Pelidnota acconciai
Pelidnota acconciai Soula, 2009: 30, 49 [original combination].
Distribution
VENEZUELA: Apure (
Types
The ♂ holotype of Pelidnota acconciai is at MNHN. The following specimen is deposited at CCECL. 1 ♂ paratype: 87//MUSEUM PARIS, RIVES DE L’ORÉNOQUE, CHAFFANJON 1887//Ohaus determ. Pelidnota lucida Brm.//Paratype 2008 Pelidnota acconciai S. Soula” (47030497). Genitalia are card-mounted underneath the male paratype. Box 4618668 SOULA.
Pelidnota agnesae
Pelidnota agnesae Soula, 2010a: 56 [original combination].
Distribution
BRAZIL: Mato Grosso (
Types
The following specimen is deposited at CCECL. 1 probable ♂ holotype: “Mato Grosso BRESIL 2-1980 Coll. Th. PORION//Holotype [blank] Soula//Probable holotype Pelidnota agnesae Soula det. MR Moore ‘15” (47030948). Genitalia card-mounted underneath the probable male holotype. Box 4616343 PORION.
Pelidnota alliacea
Rutela alliacea Germar, 1824: 117 [original combination].
Pelidnota glauca
(Olivier) [syn. by
Pelidnota aeruginosa
L. [syn. by
Pelidnota alliacea
(Germar) [revised species status and new combination by
Pelidnota (Pelidnota) alliacea
(Germar) [new subgeneric combination by
Pelidnota alliacea
(Germar) [revised combination by
synonym. Melolontha americana Herbst, 1790
Melolontha americana Herbst, 1790: 66 [original combination].
Pelidnota glauca
(Olivier) [syn. by
Pelidnota aeruginosa
(Linnaeus) [syn. by
Pelidnota alliacea
(Germar) [syn. by
synonym. Melolontha glauca Olivier, 1789
Melolontha glauca Olivier, 1789: 21 [original combination].
Pelidnota glauca
(Olivier) [new combination by
Pelidnota aeruginosa
(Linnaeus) [syn. by
Pelidnota alliacea
(Germar) [syn. by
synonym. Rutela prasina Germar, 1824
Rutela prasina Germar, 1824: 117–118 [original combination].
Pelidnota glauca
(Olivier) [syn. by
Pelidnota aeruginosa
(Linnaeus) [syn. by
Pelidnota alliacea
(Germar) [syn. by
Distribution
BRAZIL: Espírito Santo, Santa Catarina (
Types
1 ♂ lectotype and 3 paralectotypes of Rutela alliacea at ZMHB (
Remarks
The species Melolontha americana Herbst, Melolontha glauca Olivier, and Rutela prasina Germar were previously treated as synonyms of Pelidnota aeruginosa (Linnaeus) (nomen dubium) in catalogs of Rutelini (
Pelidnota aeruginosa (Linnaeus) was designated as a “nomen nullum” by
Pelidnota alutacea
Pelidnota strigosa var. alutacea H. W. Bates, 1888: 276 [original combination].
Pelidnota (Pelidnota) strigosa
Laporte [syn. by
Pelidnota alutacea
H. W. Bates [removal of subgeneric classification and new species status by
Distribution
COSTA RICA (H. W. Bates 1888,
Types
1 ♂ lectotype at BMNH (
Pelidnota ancilla
Pelidnota ancilla F. Bates, 1904: 258, 267–268 [original combination].
Pelidnota (Pelidnota) ancilla
F. Bates [new subgeneric combination by
Pelidnota ancilla
F. Bates [removal of subgeneric classificationby
Distribution
BRAZIL: Espírito Santo, Goiás, Santa Catarina (F.
Pelidnota angiae
Pelidnota angiae Demez & Soula, 2010a: 56–57 [original combination].
Distribution
PERU: Junín (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 2 ♂ paratypes: “Atalaya, Ucayali Pérou, V/2010//Holotype 2010 Pelidnota angiae S. Soula” (47030232); “Atalaya, Ucayali Pérou, V/2010//Paratype 2010 Pelidnota angiae S. Soula” (47030233); “Satipo Junin XI/2007 M. SOULA det 19//Paratype 2010 Pelidnota angiae S. Soula” (47030234). Genitalia card-mounted underneath male holotype and one male paratype. Box 4618657 SOULA.
Pelidnota aurescens
Pelidnota virescens var. aurescens H. W. Bates, 1888: 274 [original combination].
Pelidnota aurescens
H. W. Bates [new species status by
Pelidnota (Pelidnota) aurescens
H. W. Bates [new subgeneric combination by
Pelidnota aurescens
H. W. Bates [removal of subgeneric classification by
Distribution
GUATEMALA: Escuintla, Quetzaltenango, San Marcos, Sololá (H. W. Bates 1888,
Types
1 ♂ lectotype at BMNH (
Pelidnota bahiana adriani
Pelidnota (Odontognathus) adriani Martínez, 1982: 65–68 [original combination].
Strigidia bahiana adriani
(Martínez) [new combination and new subspecific status by
Pelidnota (Strigidia) adrianae
Martínez [revised combination, revised species status, and new subgeneric classification by
Pelidnota bahiana adriani
Martínez [removal of subgeneric classification and revised subspecific status by
Distribution
BRAZIL: Espírito Santo (
Types
Holotype and allotype specimens of Pelidnota (Odontognathus) adriani at MACN; 1 ♂ (Fig.
Pelidnota bahiana bahiana
Pelidnota bahiana Ohaus, 1905: 315–316 [original combination].
Pelidnota (Chalcoplethis) bahiana
Ohaus [new subgeneric combination by
Strigidia bahiana
(Ohaus) [new combination by
Pelidnota bahiana
Ohaus [revised combination by
Distribution
BRAZIL: Bahia (
Types
1 ♂ syntype at ZMHB (
Pelidnota belti belti
Pelidnota belti Sharp, 1877: 132 [original combination].
Pelidnota (Delipnia) belti
Sharp [new subgeneric combination by
Pelidnota (Ganonota) belti
Sharp [new subgeneric combination by
Pelidnota (Strigidia) belti
Sharp [new subgeneric combination by
Pelidnota (Odontognathus) belti
Sharp [new subgeneric combination by
Strigidia belti
(Sharp) [new combination by
Pelidnota (Strigidia) belti
Sharp [revised subgeneric combination by
Pelidnota belti belti
Sharp [removal of subgeneric classification and revised subspecific status by
Distribution
COLOMBIA: Boyacá (
Types
1 ♀ lectotype specimen of Pelidnota belti at BMNH (
Pelidnota belti boyacaensis
Strigidia belti boyacaensis Soula, 2006: 73 [original combination].
Pelidnota (Strigidia) belti boyacaensis
(Soula) [new combination and new subgeneric combination by
Pelidnota belti boyacaensis
(Soula) [removal of subgeneric classification by
Distribution
COLOMBIA: Boyacá (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 7 ♂ paratypes, 4 ♀ paratypes: “Strigidia belti Otanche (C) 4/89//Holotype 2006 Strigidia belti boyacaensis Soula” (47030320); “Otanche COLOMBIE 1987 Coll. A. Hayoz//Allotype 2006 Strigidia belti boyacaensis Soula” (47030321); “Otanche COLOMBIE 1987 Coll. A. Hayoz//Paratype 2006 Strigidia belti boyacaensis Soula” (47030322); “Otanche Colombie 1983 A. Hayoz//Paratype 2006 Strigidia belti boyacaensis Soula” (47030323); “Otanche Colombie 1987//Paratype 2006 Strigidia belti boyacaensis S. Soula” (47030324); “OTANCHE IV-1986//Paratype 2006 Strigidia belti boyacaensis S. Soula” (47030325); “Otanché Colombie V/86//Paratype 2006 Strigidia belti boyacaensis S. Soula” (47030326); “Otanche (Col.)//Paratype 2006 Strigidia belti boyacaensis S. Soula” (47030327); “Otanche. C. IX/85 Coll. Megard.//Paratype 2006 Strigidia belti boyacaensis S. Soula” (47030328); “Strigidia belti Otanche. C. IX/85 IX Coll. Megard.//Paratype 2006 Strigidia belti boyacaensis S. Soula” (47030329); “Nouvelle Grenade Etat Cundinamarca Cananche M.de Mathan 1er Sem. 1900//Paratype 2006 Strigidia belti boyacaensis S. Soula” (47030330); “Muzo - Colombie 06/93 coll. – SOULA//Paratype 2006 Strigidia belti boyacaensis S. Soula” (47030331). “Muzo - Colombie//COLL. TH. PORION//Paratype 2006 Strigidia belti boyacaensis S. Soula” (47030942). Genitalia card-mounted underneath the male holotype and four male paratypes. Box 4618661 SOULA and 4616343 PORION. Two additional paratype specimens are deposited at BMNH.
Pelidnota belti guatemalaensis
Strigidia belti guatemalaensis Soula, 2006: 72 [original combination].
Pelidnota (Strigidia) belti guatemalaensis
(Soula) [new combination and new subgeneric combination by
Pelidnota belti guatemalaensis
(Soula) [removal of subgeneric classification by
Distribution
GUATEMALA: Izabal (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 3 ♂ paratypes: “Finca Firmeza, Sierra de Caral, Morales, Izabal, Guatemala, 450m, 20/V/2006//Holotype 2006 Strigidia belti guatemalensis S. Soula” (47030342); “Finca Firmeza, Sierra de Caral, Morales, Izabal, Guatemala, 450m, 20/V/2006//Allotype 2006 Strigidia belti guatemalensis S. Soula” (47030343); Two paratypes with identical label data: “Finca Firmeza, Sierra de Caral, Morales, Izabal, Guatemala, 450m, 20/V/2006//Paratype 2006 Strigidia belti guatemalensis S. Soula” (47030344 and 47030345); “GUATEMALA, Izabal Morales, Junio 2000 600 m90m//COLL. TH. PORION// Strigidia belti ssp ? M. SOULA det 19//Paratype 2006 Strigidia belti guatemalensis S. Soula” (47030346). Genitalia card-mounted underneath the male holotype and the three male paratypes. Box 4618661 SOULA. One additional paratype specimen is deposited at BMNH.
Pelidnota belti panamaensis
Strigidia belti panamaensis Soula, 2006: 72–73 [original combination].
Pelidnota (Strigidia) belti panamaensis
(Soula) [new combination and new subgeneric combination by
Pelidnota belti panamaensis
(Soula) [removal of subgeneric classification by
Distribution
PANAMA: Chiriquí (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 3 ♂ paratypes, 5 ♀ paratypes: “Chiriqui//H.W.Bates Biol.Cent.Amer. Muséum Paris ex Coll. R. Oberthür 1952//Holotype 2006 Strigidia belti panamensis Soula” (47030332); “V. de Chiriqui M.de Mathan 1901//Allotype 2006 Strigidia belti panamensis Soula” (47030333); “V. de Chiriqui M.de Mathan 1901//Muséum Paris ex Coll. R. Oberthür 1952//Paratype 2006 Strigidia belti panamensis S. Soula” (47030334); “Chiriqui//Muséum Paris ex Coll. R. Oberthür 1952//Paratype 2006 Strigidia belti panamensis S. Soula” (47030335); “Panama Chiriqui IV.86 col. DURANTON//Paratype 2006 Strigidia belti panamensis S. Soula” (47030336); “PANAMA CHIRIQUI prov. Santa Clara env. - 1440 m 18. 5. - 15. 6. 2003 Vlad. Malý lgt. P - 2//Pelidnota (Strigidia) belti Sharp. Det. V. Malý 2003//Paratype 2006 Strigidia belti panamensis S. Soula” (47030337); “PANAMA CHIRIQUI prov. Santa Clara env. - 1440 m 28. 5. - 23. 6. 2002 Vlad. Malý lgt. P - 1//Pelidnota (Strigidia) belti Sharp Det. V. Malý 200//Paratype 2006 Strigidia belti panamensis S. Soula” (47030338); “PANAMA CHIRIQUI Santa Clara env. - 1546 m 08°51'42.2"N:082°44'36.5"W 17.6.-4.7.06;V.Malý lgt. P7//Pelidnota (Strigidia) belti Sharp Det. Vl. Malý 2006//Paratype 2006 Strigidia belti panamensis S. Soula” (47030339); “Serro (sic!) Campana Panama M. SOULA det [obverse] 700 m V/07//Paratype 2006 Strigidia belti panamensis S. Soula” (47030340); Cerro Campana, 3000’, Panama. July 31, 1970, H. & A. Howden//Paratype 2006 Strigidia belti panamensis S. Soula” (47030341). Genitalia card-mounted underneath the male holotype and one male paratype. Box 4618661 SOULA. 1 ♂ and 3 ♀ paratypes at CMNC: 1 ♂ “Cerro Campana, 3000’ Panama. July 31, 1970, H. & A, Howden//H.&A. Howden Collection// H. & A. HOWDEN COLLECTION ex. A. Martinez coll.// Pelidnota ♂ belti Sharp det.A.R.Hardy 1970//Paratype Strigidia belti panamensis S. Soula”, 1 ♀ “Cerro Campana, 3000’ Panama. July 31, 1970, H. & A, Howden//H.&A. Howden Collection//Pelidnota ♀ belti Sharp det.A.R.Hardy 1970//Paratype 2006 Strigidia belti panamensis S. Soula”, 1 ♀ “Panama Chiriqui Prov Santa Clara 4000’ Col: R.Hartmann 3 May 197// Paratype 2006. Strigidia belti panamensis S. Soula”, 1 ♀ “Panamá: Panamá Pr. Cerro Campana, 850M 8°40’N 79°56’W//24 iv. 1970 H. A. Hespenheide//ON PALM//Paratype 2006 Strigidia belti panamensis S. Soula//Pelidnota belti Sharp DET. H.F. HOWDEN 70”.
Pelidnota beniouioui
Pelidnota beniouioui Soula, 2010a: 41–42 [original combination].
Distribution
BOLIVIA: Beni (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 6 ♂ paratypes: “Beni 1000 m. Bol. coll. – SOULA [obverse] Bolivie VIII/96.//Holotype 2010 Pelidnota beniouioui S. Soula” (47030224); “Beni (1000 m) Bolivie coll. - SOULA [obverse] VIII/96//Allotype Pelidnota beniouioui S. 2010 Soula” (47030225); “Beni, La Paz [arrow] coll. – SOULA [obverse] Rurrenabaque pk 298 VIII/94 1000 m//Paratype 2010 Pelidnota beniouioui Soula” (47030226); Five paratypes with identical labels “Beni, La Paz [arrow] coll. – SOULA [obverse] Rurrenabaque pk 298 1000 m 1/VIII/94//Paratype 2010 Pelidnota beniouioui Soula” (47030227 to 47030231). Genitalia card-mounted underneath the holotype, allotype and 5 paratypes. Box 4618656 SOULA.
Pelidnota beraudi
Pelidnota beraudi Soula, 2009: 33, 105 [original combination].
Distribution
COLOMBIA: Caldas (
Types
The holotype, allotype, and some paratypes are deposited at MNHN (
Pelidnota bertrandi
Pelidnota bertrandi Soula, 2009: 31, 59 [original combination].
Distribution
NICARAGUA: Rivas (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 7 ♂ paratypes, 5 ♀ paratypes: “Route MANAGUA/RIVAS Km 14,5 (NICARAGUA) IX-1969 [7 crossed out] Chasses M. DARGE//Holotype 2007 Pelidnota bertrandi S. Soula” (47030473); “Route MANAGUA/RIVAS Km 14,5 (NICARAGUA) IX-1969 [7 crossed out] Chasses M. DARGE//Allotype 2007 Pelidnota bertrandi S. Soula” (47030474); “Route MANAGUA/RIVAS Km 14,5 (NICARAGUA) IX-1969 [7 crossed out] Chasses M. DARGE//Paratype 2007 Pelidnota bertrandi S. Soula” (47030475); Eleven paratypes with identical labels “Route MANAGUA/RIVAS Km 14,5 (NICARAGUA) IX-1969 [7 crossed out] Chasses M. DARGE//Paratype 2008 Pelidnota bertrandi S. Soula” (47030476 to 47030484, exch26 and exch27). Genitalia card-mounted underneath the holotype and two male paratypes. Box 4618665 SOULA.
Pelidnota bivittata
Scarabaeus bivattatus Swederus, 1787: 189 [original combination].
Rutela bivittata
(Swederus) [new combination by
Pelidnota bivittata
(Swederus) [new combination by
Pelidnota (Ganonota) bivittata
(Swederus) [new subgeneric combination by
Pelidnota (Strigidia) bivittata
(Swederus) [new subgeneric combination by
Pelidnota (Odontognathus) bivittata
(Swederus) [new subgeneric combination by
Strigidia bivittata
(Swederus) [new combination by
Pelidnota (Strigidia) bivittata
(Swederus) [revised subgeneric combination by
Pelidnota bivittata
(Swederus) [removal of subgeneric classification by
Distribution
BRAZIL: Espírito Santo (
Pelidnota bleuzeni
Chalcoplethis bleuzeni Bouchard, 2003: 103, 105–107 [original combination].
Strigidia bleuzeni
(Bouchard) [new combination by
Pelidnota bleuzeni
(Bouchard) [new combination by
Distribution
FRENCH GUIANA (
Types
The following specimens are deposited at CCECL. 2 ♂ paratypes, 12 ♀ paratypes: “Mgne de Kaw G. F. 08/92//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030155); Three paratypes with identical label data “KAW. PK 40 27/8/84 [obverse] P. L.//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030156 and 47030157, exch08); “KAW. PK 40 23/8/84 [obverse] P. L.//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030158); “Guyane f. Kourou VIII 90//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030164); “GUYANE Française Roura I 85 J. P. MARECHAL//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030166); “08/1997 P.K. 39-Rte de KAW GUYANE FRANCAISE FRENCH GUIANA//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030162); Two paratypes with identical label data “Petit Saut G. F. 07/92//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030167 and 47030168); “M de Kaw Guyane fr. 8.90//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030163); “KAW. KAW. PK34 21/9/84 [obverse] P. L.//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030160); “Kaw PK 34 P. L. 28/7/84 Kaw//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030159); “Coll P. BLEUZEN Mgne de Kaw PK 31 GUYANE FR. 17 IX 1985//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030165); “GUYANE FRANCAISE Piste de Kaw pK 38 11-VII-1996 H. de Toulgoët & J. Navatte réc//Chalcoplethis bleuzeni sp. n. PARATYPE” (47030161). Genitalia card-mounted underneath two male paratypes and two female paratypes. Box 4618655 SOULA.
Pelidnota bondili
Strigidia bondili Soula, 2006: 10, 38–39 [original combination].
Pelidnota bondili
(Soula) [new combination by
Distribution
PERU: Amazonas (
Types
The holotype ♂ of P. bondili should be at CCECL (
Pelidnota boulangeri
Pelidnota boulangeri Soula, 2009: 33, 96–97 [original combination].
Distribution
VENZUELA: Aragua, Distrito Federal, Merida (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 7 ♂ paratypes, 7 ♀ paratypes: “P. N. Henri Pittier Choroni; Venezuela V-VI/2005//Holotype 2008 Pelidnota boulangeri S. Soula” (47030625); “P. N. Henri Pittier Choroni; Venezuela V-VI/2005//Allotype 2008 Pelidnota boulangeri S. Soula” (47030626); six paratypes with identical label data: “P. N. Henri Pittier Choroni; Venezuela V-VI/2005//Paratype 2008 Pelidnota boulangeri Soula” (47030627 to 47030631, exch36); “VENEZUELA Rancho Grande 1150m 3-VII-1986//Paratype 2008 Pelidnota boulangeri Soula” (47030632); “Caracas (V) 9/87//Paratype 2008 Pelidnota boulangeri Soula” (47030633); “Caracas Venezuela IX/87//Paratype 2008 Pelidnota boulangeri Soula” (47030634); “Caracas et environs//Paratype 2008 Pelidnota boulangeri Soula” (47030635); two paratypes with identical label data: “N. Venezuela S. Klages 1904//Paratype 2008 Pelidnota boulangeri Soula” (47030636 and 47030637); two paratypes with identical label data: “N. Venezuela S. Klages 1904//Paratype Pelidnota boulangeri S. 2008-2009” (47030638 and 47030639). Genitalia card-mounted underneath the male holotype and five male paratypes. Box 4618676 SOULA.
Pelidnota boyi
Pelidnota (Ganonota) boyi Ohaus, 1929: 389–390 [original combination].
Pelidnota (Strigidia) boyi
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) boyi
Ohaus [new subgeneric combination by
Strigidia boyi
(Ohaus) [new combination by
Pelidnota (Strigidia) boyi
Ohaus [revised subgeneric combination by
Pelidnota boyi
Ohaus [removal of subgeneric classification by
Distribution
BRAZIL: Amazonas (
Pelidnota burmeisteri burmeisteri
Pelidnota burmeisteri Burmeister, 1844: 409 [original combination].
Pelidnota (Pelidnota) burmeisteri
Burmeister [new subgeneric combination by
Pelidnota burmeisteri
Burmeister [removal of subgeneric classification by
synonym. Aglycoptera lacerdae Sharp, 1885
Aglycoptera lacerdae Sharp, 1885: 23–24 [original combination].
Pelidnota (Pelidnota) burmeisteri
Burmeister [syn. by
Distribution
BRAZIL: Bahia, Minas Gerais (
Pelidnota burmeisteri tricolor
Pelidnota tricolor Nonfried, 1894: 123–124 [original combination].
Pelidnota sumptuosa var. tricolor Nonfried [new infrasubspecific status by F.
Pelidnota burmeisteri var. tricolor Nonfried [revised infrasubspecific status by
Pelidnota (Pelidnota) burmeisteri var. tricolor Nonfried [new subgeneric combination by
Pelidnota burmeisteri tricolor
Nonfried [new combination and new subpecific status by
synonym. Pelidnota ludovici Ohaus, 1905
Pelidnota ludovici Ohaus, 1905: 317 [original combination].
Pelidnota (Pelidnota) ludovici
Ohaus [new subgeneric combination by
Pelidnota ludovici
Ohaus [removal of subgeneric classification by
Pelidnota burmeisteri tricolor Nonfried [syn. n.].
Distribution
BRAZIL: Bahia, Espírito Santo, Mato Grosso (
Types
1 ♂ syntype specimen of Pelidnota tricolor Nonfried at ZMHB (Fig.
Remarks
Pelidnota carlettii
Pelidnota carlettii Soula, 2009: 32, 77–78 [original combination].
Distribution
ARGENTINA: Misiones (
Types
The following specimens are deposited in CCECL. 1 ♂ holotype, 1 ♀ allotype, 9 ♂ paratypes, 3 ♀ paratypes, 1 ♂ invalid paratype, 4 ♀ invalid paratypes: “Oberá Misiones Ar I/99 M. SOULA det 19//Holotype 2008 Pelidnota carlettii S. Soula” (47030588); “Eldorado - Misiones ARGENTINE (I/93)//Allotype 2008 Pelidnota carlettii S. Soula” (47030589); Three paratypes with identical label data: “Eldorado - Misiones ARGENTINE (I/93)//Paratype 2008 Pelidnota carlettii S. Soula” (47030590 to 47030592); Three paratypes with identical label data: “Puerto Iguazu-ARG XII/88.//Paratype 2008 Pelidnota carlettii S. Soula” (47030593 to 47030595); “Puerto Iguazu ARGENTINE (I/93)//Paratype 2008 Pelidnota carlettii S. Soula” (47030596); “Oberá - Misiones ARGENTINA-I/99 Col. Andrés Varga//Paratype 2008 Pelidnota carlettii S. Soula” (47030597); Two paratypes with identical label data: Iguazu Misiones (Ar.) coll. – SOULA//Paratype 2008 Pelidnota carlettii S. Soula” (47030598 and 47030599); “Pelidnota sordida ♀ Puerto Iguazu Misiones Argentina 22-DIC 1987 det. Forster” (47030600); “Argentina//Paratype 2008 Pelidnota carlettii S. Soula” (47030601); Two invalid paratypes with identical label data: “Puerto Iguazu Misiones, Argentine II/1995//Paratype Pelidnota carlettii S. 2006 Soula//Invalid paratype Pelidnota carlettii Soula det. MR Moore ‘15” (47030602 and 47030603)”; “Puerto Iguazu Misiones, Argentine II/1995//Paratype 2006 Pelidnota carlettii S. Soula//Invalid paratype Pelidnota carlettii Soula det. MR Moore ‘15” (47030604)”; Two invalid paratypes with identical label data: “Dos de Mayo Misiones M. SOULA det 19 [obverse] 1/II/89//Paratype Pelidnota carlettii S. 2007 Soula//Invalid paratype Pelidnota carlettii Soula det. MR MOORE ‘15” (47030605 and 47030606). Genitalia card-mounted underneath the male holotype, nine male paratypes and one female paratype. Box 4618671 SOULA.
Remarks
Five specimens labeled as Pelidnota carlettii Soula paratypes in CCECL are considered invalid. These specimens have label data that are not reported in
Pelidnota cayennensis
Pelidnota cayennensis F. Bates, 1904: 258, 269–270 [original combination].
Pelidnota laevissima cayennensis
F. Bates [new subspecific status by
Pelidnota (Pelidnota) laevissima cayennensis
F. Bates [new subgeneric combination by
Pelidnota cayennensis
F. Bates [removal of subgeneric classification and revised species status by
Distribution
FRENCH GUIANA: St.-Laurent du Maroni (F.
Types
1 ♂ lectotype and 1 paralectotype of Pelidnota cayennensis at BMNH (
Remarks
Pelidnota centroamericana
Pelidnota punctata centroamericana Ohaus, 1913: 499–500 [original combination].
Pelidnota (Pelidnota) punctata centroamericana
Ohaus [new subgeneric combination by
Pelidnota (Pelidnota) lutea centroamericana
Ohaus [revised subspecific status by
Pelidnota (Pelidnota) centroamericana
Ohaus [new species status by
Pelidnota centroamericana
Ohaus [removal of subgeneric classification by
Distribution
BELIZE: Corozal (
Types
1 ♂ lectotype of Pelidnota punctata centroamericana at ZMHB (
Pelidnota cerdai
Strigidia cerdai Soula, 2006: 11, 48–49 [original combination].
Pelidnota cerdai
(Soula) [new combination by
Distribution
FRENCH GUIANA: Cayenne (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♂ paratype: “FRG PK12 PL. 18/2/85//Holotype Strigidia cerdai S. 2005. Soula” (47030421). “Cayenne//So named in Reiches Collection. C.W.//67.45//nitidula Reiche Cayenne//Pelidnota sp. ♀. Belti mit falsch. Fündort //534// Allotype Strigidia cerdai S. 2005 Soula” (47030422). “Kaw PK 37 PL. 24/12/84//Paratype Strigidia cerdai S. 2005 Soula” (47030423). Male genitalia card-mounted underneath male holotype and paratype. Box 4618663 SOULA.
Pelidnota chalcopus
Pelidnota virescens var. chalcopus H. W. Bates, 1888: 275 [original combination].
Pelidnota (Pelidnota) virescens var. chalcopus H. W. Bates [new subgeneric combination by
Pelidnota (Pelidnota) virescens chalcopus
H. W. Bates [new subspecific status by
Pelidnota (Pelidnota) aurescens
H. W. Bates [syn. by
Pelidnota chalcopus
H. W. Bates [removal of subgeneric classification and new species status by
Distribution
BELIZE: Cayo (H. W. Bates 1888,
Types
1 ♂ lectotype and 3 paralectotypes of Pelidnota virescens var. chalcopus at BMNH (
Pelidnota chalcothorax
Pelidnota chalcothorax Perty, 1830: 48 [original combination].
Pelidnota (Pelidnota) chalcothorax
Perty [new subgeneric combination by
Pelidnota chalcothorax
Perty [removal of subgeneric classification by
Distribution
BRAZIL: Mato Grosso (WBWC), Espírito Santo, Minas Gerais, Rio de Janeiro, São Paulo (
Pelidnota championi
Pelidnota championi F. Bates, 1904: 258, 267 [original combination].
Pelidnota (Pelidnota) fulva championi
F. Bates [new subgeneric combination and new subspecific status by
Pelidnota championi
F. Bates [removal of subgeneric classification and revised species status by
Distribution
ARGENTINA: Córdoba, Misiones, Tucumán (F.
Types
1 ♂ lectotype of Pelidnota championi F. Bates at BMNH (
Remarks
Pelidnota chiapasensis
Pelidnota chiapasensis Soula, 2009: 31, 65–66 [original combination].
Distribution
MEXICO: Chiapas (
Types
The following specimens are deposited at CMNC. 1 ♂ holotype, 1 ♀ allotype, 2 ♀ paratypes: “MEXICO, Chiapas, Cinco Cerros, Km30 on Hwy 190 1500m 8. VI.1989. H.Howden//at light//Holotype 2008 Pelidnota chiapasensis Sou Soula//[barcode matrix] Canadian Museum of Musée canadien de la NATURE CMNEN 00010903”, allotype with identical collecting data label and database number CMNEN 00010904, paratypes with identical collecting data label and database numbers CMNEN 00010905 and CMNEN 00010906. The following specimens are deposited at CCECL. 2 ♀ paratypes, 1 invalid ♂ paratype: “MEXICO. Chiapas, Cinco Cerros 860m. 9. VI. 1990 H. & A. Howden//at light//Paratype 2008 Pelidnota chiapasensis S. Soula det.” (47030492); “MEXICO. Chiapas ElAguacero, 16 km W Ocozocoautla. 680m 5. VI. 1990 H. & A. Howden//Paratype 2008 Pelidnota chiapasensis S. Soula det.” (47030493); “MEXICO. Chiapas ElAguacero, 16 km W Ocozocoautla. 680m 10. VI. 1990 H. & A. Howden//at light//Paratype 2008 Pelidnota chiapasensis S. Soula//Invalid Paratype Pelidnota chiapasensis S. det. M.R. Moore ‘15” (47030494). Genitalia card-mounted underneath the invalid male paratype. Box 4618666 SOULA.
Remarks
Box 4618666 SOULA contains a male P. chiapasensis Soula from Mexico (Chiapas, El Aguacero) labeled as a paratype. This specimen is an invalid paratype as this specific specimen was not reported in
Pelidnota chibchana
Pelidnota chibchana Ohaus, 1922: 324–325 [original combination].
Pelidnota (Chalcoplethis) chibchana
Ohaus [new subgeneric combination by
Strigidia chibchana
(Ohaus) [new combination by
Pelidnota chibchana
Ohaus [removal of subgeneric classification by
Distribution
COLOMBIA: Cundinamarca, Distrito Capital, Santander (
Types
Pelidnota chimborazoensis
Pelidnota chimborazoensis Soula, 2009: 32, 87 [original combination].
Distribution
ECUADOR: Bolívar, Chimborazo (
Types
The holotype ♂ of Pelidnota chimborazoensis is at MNHN. The following specimens are deposited at CCECL. 4 ♂ paratypes, 3 ♀ paratypes: “Equateur La Chima M.de Mathan 1er Semestre 1893//Muséum Paris Coll. R. Öberthür//Paratype 2008 Pelidnota chimborazoensis S. Soula det.” (47030454); “Chimbo Equateur M.de Mathan 1897//Paratype 2008 Pelidnota chimborazoensis S. Soula det.” (47030455); Two paratypes with identical label data “Equateur Chimbo M.de Mathan 1er Semestre 1892//Paratype 2008 Pelidnota chimborazoensis S. Soula det.” (47030456 and 47030457); “La Chima (Equateur) IV 93 coll. – SOULA//Paratype 2008 Pelidnota chimborazoensis S. Soula det.” (47030458); “Equateur//Paratype 2008 Pelidnota chimborazoensis S. Soula” (47030459); “Balzar mountains Ecuador. Illingworth 1879//Ex-Musæo D.Sharp 1890//Paratype 2009 Pelidnota chimborazoensis S. Soula” (47030460). Genitalia card-mounted underneath four male and one female paratypes. Box 4618664 SOULA.
Remarks
“Chimbo 1897” is recorded as 1891 in the original description (
Pelidnota chiriquicola
Pelidnota laevissima chiriquicola Ohaus, 1913: 499 [original combination].
Pelidnota (Pelidnota) laevissima chiriquicola
Ohaus [new subgeneric combination by
Pelidnota chiriquicola
Ohaus [removal of subgeneric classification and new species status by
Distribution
COSTA RICA: Puntarenas (
Remarks
Pelidnota chiriquina
Pelidnota chiriquina F. Bates, 1904: 257, 265–266 [original combination].
Pelidnota (Pelidnota) chiriquina
F. Bates [new subgeneric combination by
Pelidnota chiriquina
F. Bates [removal of subgeneric classification by
Distribution
COLOMBIA: Chocó (
Types
1 ♂ lectotype of Pelidnota chiriquina at BMNH (
Pelidnota chlorana
Pelidnota chlorana Erichson, 1847: 99 [original combination].
Pelidnota (Pelidnota) chlorana
Erichson [new subgeneric combination by
Pelidnota chlorana
Erichson [removal of subgeneric classification by
Distribution
BOLIVIA: La Paz, Santa Cruz (
Types
1 ♀ syntype of Pelidnota chlorana at ZMHB (
Pelidnota costaricensis
Pelidnota costaricensis H. W. Bates, 1888: 274 [original combination].
Pelidnota (Pelidnota) costaricensis
H. W. Bates [new subgeneric combination by
Pelidnota costaricensis
H. W. Bates [removal of subgeneric classification by
Distribution
COSTA RICA: Alajuela, Guanacaste, Heredia, Puntarenas, San José (H. W. Bates 1888,
Types
1 ♀ lectotype of Pelidnota costaricensis at BMNH (
Pelidnota courtini
Pelidnota courtini Soula, 2009: 111–112 [original combination].
Distribution
BRAZIL: Bahia, Minas Gerais (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♂ paratype, 4 ♀ paratypes: “Facenda Baxinha - 450 m Amargosa - Bahia - Brésil 28. III.89 COLL - B. COURTIN//Holotype 2008 Pelidnota courtini S. Soula” (47030779); “Facenda Baxinha - 450 m Amargosa (Bahia - Brésil) 15. III.89 - B. Courtin//Allotype 2008 Pelidnota courtini S. Soula” (47030780); “Pelichnota (sic) palidipennis Bahia Brésil Amargosa - 15. III-89 COLL - B. COURTIN//Paratype 2008 Pelidnota courtini S. Soula” (47030781); “Pelichnota (sic) palidipennis Bahia Brésil Amargosa - 15. III-89 COLL - B. COURTIN//Paratype 2008 Pelidnota courtini S. Soula” (47030781); “Pelichnota (sic) palidipennis Facenda Baxinha - 400m Amargosa (Bahia - Brésil) 15-III-89 - B. Courtin//Paratype 2008 Pelidnota courtini S. Soula” (47030782); “Facenda Baxinha - 450 m Amargosa - Bahia - Brésil 15. III.89 COLL - B. COURTIN//Paratype 2008 Pelidnota courtini S. Soula” (47030783); “Facenda Baxinha Amargosa - 400 m Bahia - Brésil 15. III.89 B. Courtin//Paratype 2008 Pelidnota courtini S. Soula” (47030784); “MUSEUM PARIS BRÉSIL BAHIA P. SERRE 1913//Ohaus determ. Pelidnota palidipeñis F. Bates//Paratype 2009 Pelidnota courtini S. Soula” (47030785). Genitalia card-mounted underneath the male holotype and the male paratype. Box 4618680 SOULA. The following specimens are deposited at CMNC. 1 ♂ paratype “BRASIL Eo de BAHIA Santa Ana Bondar-coleg. Coll. Martínez Jul.-927// H. & A. HOWDEN COLLECTION ex. A. Martinez coll.//Paratype 2008 Pelidnota courtini S. Soula”.
Pelidnota crassipes
Pelidnota crassipes Ohaus, 1905: 319 [original combination].
Pelidnota (Ganonota) crassipes
Ohaus [new subgeneric combination by
Pelidnota (Strigidia) crassipes
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) crassipes
Ohaus [new subgeneric combination by
Pelidnota (Ganonota) crassipes
Ohaus [revised subgeneric combination by
Strigidia crassipes
(Ohaus) [new combination by
Pelidnota (Strigidia) crassipes
Ohaus [revised combination and revised subgeneric combination by
Pelidnota crassipes
Ohaus [removal of subgeneric classification by
Distribution
ARGENTINA: Misiones (
Types
1 ♂ lectotype and 2 paralectotypes of Pelidnota crassipes at ZMHB (
Pelidnota cribrata
Heteropelidnota cribrata Ohaus, 1913: 506 [original combination].
Pelidnota cribrata
(Ohaus) [new combination by
Distribution
BRAZIL: Amazonas (INPA), Rondônia (INPA), Pará (
Types
1 ♂ lectotype of Heteropelidnota cribrata from Para, Brazil at ZHMB (
Remarks
According to
Pelidnota cuprea
Rutela cuprea Germar, 1824: 120–121 [original combination].
Pelidnota cuprea
(Germar) [new combination by
Strigidia cuprea
(Germar) [new combination by
Odontognathus cupreus
(Germar) [new combination by
Strigidia cuprea
(Germar) [new combination by
Odontognathus cupreus
(Germar) [revised combination by
Pelidnota (Odontognathus) cuprea
(Germar) [revised combination and new subgeneric combination by
Pelidnota (Ganonota) cuprea
(Germar) [new subgeneric combination by
Pelidnota (Strigidia) cuprea
(Germar) [new subgeneric combination by
Pelidnota (Odontognathus) cuprea
(Germar) [revised subgeneric combination by
Pelidnota (Ganonota) cuprea
(Germar) [revised subgeneric combination by
Strigidia cuprea
(Germar) [revised combination by
Pelidnota (Strigidia) cuprea
(Germar) [revised combination and revised subgeneric combination by
Pelidnota cuprea
(Germar) [removal of subgeneric classification by
synonym. Odontognathus unicolor Laporte, 1840
Odontognathus unicolor Laporte, 1840: 137 [original combination].
Strigidia fulvipennis
(Germar) [syn. by
Odontognathus fulvipennis var. unicolor Germar [revised combination and new infrasubspecific status by
Pelidnota (Ganonota) cuprea
(Germar) [syn. by
synonym. Rutela fulvipennis Germar, 1824
Rutela fulvipennis Germar, 1824: 121 [original combination].
Strigidia fulvipennis
(Germar) [new combination by
Odontognathus cupreus var. fulvipennis (Germar) [new combination and new infrasubspecific status by
Pelidnota (Odontognathus) cuprea var. fulvipennis (Germar) [new combination and new subgeneric combination by
Pelidnota (Ganonota) cuprea var. fulvipennis (Germar) [new subgeneric combination by
Pelidnota (Strigidia) cuprea var. fulvipennis (Germar) [new subgeneric combination by
Pelidnota (Strigidia) cuprea forma fulvipennis (Germar) [revised infrasubspecific status by
Pelidnota (Odontognathus) cuprea forma fulvipennis (Germar) [revised subgeneric combination by
Pelidnota (Ganonota) cuprea forma fulvipennis (Germar) [revised subgeneric combination by
Strigidia cuprea var. fulvipennis (Germar) [revised combination and revised infrasubspecific status by
Pelidnota (Strigidia) cuprea var. fulvipennis (Germar) [revised combination and revised subgeneric combination by
Pelidnota cuprea var. fulvipennis (Germar) [removal of subgeneric classification by
Pelidnota cuprea (Germar) [syn. n.].
Distribution
ARGENTINA (
Remarks
Color variation in this species is found within populations. At least 80 specimens were collected in a single collecting event and single collecting locality (Rio Je Janeiro, Brazil). From this collecting event, Ohaus’s determinations refer to P. cuprea var. cuprea (blackish and shiny cupreous reflections), P. cuprea var. coerulea (black with shiny green reflections), and P. cuprea var. fulvipennis (castaneous with shiny green reflections). Research should examine if this variation is intraspecific or, instead, indicative of interspecific variation in several sympatric species. Relationships of the species in the Pelidnota cuprea complex require analysis. Species in the group have bounced to and from the genera Pelidnota, Odontognathus, Ganonota, and Strigidia, demonstrating historical classification difficulties and illustrating the need for phylogenetic analysis within the broader context of the Rutelini. Male species in the “cuprea complex” (P. rubripennis, P. riedeli, P. cuprea, P. ebenina) share a concavity on the disc of the sternites.
Pelidnota cuprea var. coerulea Ohaus (unavailable name) (valid name Pelidnota cuprea [Germar]) invalid type female from ZMHB. A Dorsal habitus B Lateral habitus C Specimen labels.
Pelidnota cuprea var. nigrocoerulea Ohaus (unavailable name) (valid name Pelidnota cuprea [Germar]) invalid type female from ZMHB. A Dorsal habitus B Lateral habitus C Specimen labels.
Pelidnota cupripes cupripes
Pelidnota cupripes Perty, 1830: 48 [original combination].
Pelidnota (Ganonota) cupripes
Perty [new subgeneric combination by
Pelidnota (Strigidia) cupripes
Perty [new subgeneric combination by
Pelidnota (Odontognathus) cupripes
Perty [new subgeneric combination by
Strigidia cupripes
(Perty) [new combination by
Pelidnota (Strigidia) cupripes
Perty [revised combination and revised subgeneric combination by
Pelidnota cupripes
Perty [removal of subgeneric classification by
Distribution
BRAZIL: Espírito Santo, Rio de Janeiro, São Paulo, Santa Catarina (
Pelidnota cupripes goyasensis
Strigidia cupripes goyasensis Soula, 2006: 53 [original combination].
Pelidnota (Strigidia) cupripes goyasensis
(Soula) [new combination and new subgeneric combination by
Pelidnota cupripes goyasensis
(Soula) [removal of subgeneric classification by
Distribution
BRAZIL: Goiás (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Goyaz, Bresil//Pelidnota viridana//MUSÉUM PARIS 1930 COLL SICARD//Holotype 2005 Strigidia cupripes goyasensis Sou. Soula det.” (47030397). Genitalia card-mounted underneath holotype. Box 4618663 SOULA.
Pelidnota cupripes surinamensis
Strigidia cupripes surinamensis Soula, 2006: 53 [original combination].
Pelidnota (Strigidia) cupripes surinamensis
(Soula) [new combination and new subgeneric combination by
Pelidnota cupripes surinamensis
(Soula) [removal of subgeneric classification by
Distribution
SURINAME (
Types
The following specimens are deposited at CCECL. 1 ♀ holotype, 1 ♀ paratype: “Surinam//Rutela glabrata// MUSÉUM PARIS 1930 COLL SICARD//Holotype 2005 Strigidia cupripes surinamensis Sou. Soula det.” (47030398); “Surinam//Rutela glabrata//MUSÉUM PARIS 1930 COLL SICARD//Paratype 2005 Strigidia cupripes surinamensis Sou. Soula det.” (47030399). Box 4618663 SOULA.
Pelidnota cyanipes
Rutela cyanipes Kirby, 1819: 406–407 [original combination].
Pelidnota cyanipes
(Kirby) [new combination by
Pelidnota (Chalcoplethis) cyanipes
(Kirby) [new subgeneric combination by
Pelidnota cyanipes
(Kirby) [removal of subgeneric classification by
Distribution
ARGENTINA: Misiones (
Types
Most of Kirby’s type specimens are located at BMNH. A search for the type specimen of P. cyanipes did not locate the specimen in the collection.
Pelidnota cyanitarsis
Rutela cyanitarsis Gory, 1833a: 67–68 [original combination].
Pelidnota cyanitarsis
(Gory) [new combination by
Pelidnota (Pelidnota) cyanitarsis
(Gory) [new subgeneric combination by
Pelidnota cyanitarsis
(Gory) [removal of subgeneric classification by
synonym. Rutela nitidissima Guérin-Méneville, 1834
Rutela nitidissima Guérin-Méneville, 1834: 91 [original combination].
Pelidnota (Pelidnota) cyanitarsis
(Gory) [syn. by
Distribution
BRAZIL: Bahia, Minas Gerais, Pará (
Remarks
Two spectacular species, P. cyanitarsis and P. sumptuosa Vigors, have been confused in collections and the literature. Both species are brilliant metallic blue, green, or blue-green with enlarged metatibia. Several characters serve to separate these species (P. cyanitarsis with well-developed fovea on pronotal margin whereas P. sumptuosa has a strigate patch on the pronotal margins; P. cyanitarsis male with well developed medial tooth on foreclaw and P. sumptuosa male with well-developed subapical tooth on foreclaw), and male parameres are also diagnostic (see
Pelidnota discicollis
Pelidnota discicollis Ohaus, 1912: 303 [original combination].
Pelidnota (Ganonota) discicollis
Ohaus [new subgeneric combination by
Pelidnota (Strigidia) discicollis
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) discicollis
Ohaus [new subgeneric combination by
Strigidia discicollis
(Ohaus) [new combination by
Pelidnota (Strigidia) discicollis
Ohaus [revised combination and revised subgeneric combination by
Pelidnota discicollis
Ohaus [removal of subgeneric classification by
Distribution
BRAZIL: Pará (
Types
1 ♀ holotype specimen of Pelidnota discicollis Ohaus at ZMHB (Fig.
Remarks
CCECL contains a specimen of P. discicollis labeled as a male alloréférent with the following data: 1 ♂ alloréférent: “Para (Brésil) de Mathan//Alloréférent de Strigidia discicollis Oh. M. SOULA det 19 2006” (47030430). Genitalia card-mounted underneath the alloréférent. Box 4618663 SOULA. The male specimen from Venezuela represents a new country record.
Pelidnota dobleri
Pelidnota dobleri Frey, 1967: 375–376 [original combination].
Pelidnota (Pelidnota) doblerae
Frey [new subgeneric combination and incorrect subsequent spelling by
Strigidia doblerae
(Frey) [new combination by
Pelidnota doblerae
Frey [revised combination by
Distribution
BOLIVIA (
Types
1 ♂ holotype of Pelidnota dobleri at NHMB (
Pelidnota drumonti
Pelidnota drumonti Soula, 2009: 34, 113–114 [original combination].
Distribution
BRAZIL: São Paulo (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 12 ♂ paratypes, 5 ♀ paratypes: “Fazenda Rhodia Paulinia, São Paulo 19/01/92//Holotype 2008 Pelidnota drumonti S.Soula” (47030762); “Fazenda Rhodia Paulinia, São Paulo 19/01/92/Allotype 2008 Pelidnota drumonti S.Soula” (47030763); Six paratypes with identical label data: “Fazenda Rhodia Paulinia, São Paulo 19/01/92/Paratype 2008 Pelidnota drumonti S. Soula” (47030764 to 47030769); Nine paratypes with identical label data: “Fazenda Rhodia Paulinia, São Paulo 19/01/92/Paratype Pelidnota drumonti S. 2008-2009” (47030770 to 47030778). Genitalia card-mounted underneath the male holotype and two male paratypes. Box 4618680 SOULA. The following specimens are deposited at CMNC: 26 ♂ paratypes, 27 ♀ paratypes.
Pelidnota dubia
Pelidnota dubia F. Bates, 1904: 254, 262–263 [original combination].
Pelidnota (Ganonota) dubia
F. Bates [new subgeneric combination by
Pelidnota (Strigidia) dubia
F. Bates [new subgeneric combination by
Pelidnota (Odontognathus) dubia
F. Bates [new subgeneric combination by
Strigidia dubia
(F. Bates) [new combination by
Pelidnota (Strigidia) dubia
F. Bates [revised combination and revised subgeneric combination by
Pelidnota dubia
F. Bates [removal of subgeneric classification by
Distribution
COLOMBIA: Caldas, Cauca (F.
Types
1 ♂ lectotype specimen of Pelidnota dubia F. Bates at BMNH (Fig.
Remarks
F.
Pelidnota durantonorum
Pelidnota durantonorum Soula, 2009: 33, 106–107 [original combination].
Distribution
FRENCH GUIANA: Iracoubo, Kourou (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 34 ♂ paratypes, 25 ♀ paratypes, 1 ♂ invalid paratype: “Patagaïe G. F. 08/2001 M. SOULA det 19//Holotype 2008 Pelidnota durantonorum S. Soula” (47030671); “Patagaïe (G. F.) 08/2001 M. SOULA det 19//Allotype 2008 Pelidnota durantonorum S. Soula” (47030672); Three paratypes with identical label data: “GUYANE FRANÇAISE Piste de Nancibo pK 1,5 11-VIII-1996 H. de Toulgoët & J. Navatte réc.//Paratype Pelidnota durantonorum S. 2008-2009” (47030673 and 47030674, exch38); “GUYANE FRANÇAISE Piste de Kaw pK 39 8-VII-1996 H. de Toulgoët & J. Navatte réc.//Paratype Pelidnota durantonorum S. 2008-2009” (47030675); “GUYANE FRANÇAISE Route de Coralie pK 2,2 15-VII-1996 H. de Toulgoët & J. Navatte réc.//Paratype Pelidnota durantonorum S. 2008-2009” (47030676); Two male paratypes with identical label data: “GUYANE FRANÇAISE Route de Régina pK 79 18-VII-1996 H. de Toulgoët & J. Navatte réc.//Paratype Pelidnota durantonorum S. 2008-2009” (47030677 and 47030678); “GUYANE FRANÇAISE Piste de Kaw pK 36 6-VIII-1996 H. de Toulgoët & J. Navatte réc.//Paratype Pelidnota durantonorum S. 2008-2009” (47030679); Three paratypes with identical label data: “Guyane fr. (Est) M. SOULA det 19//Paratype Pelidnota durantonorum S. 2008-2009” (47030680, 47030683, exch39); Two paratypes with identical label data: “Patagaïe Guyane fr. M. SOULA det 19 [obverse] VIII 2001//Paratype Pelidnota durantonorum S. 2008-2009” (47030681 and 47030682); “Guyane fr. Bélizon M. SOULA det 19//Paratype Pelidnota durantonorum S. 2008-2009” (47030684); “Nancibo PK 6 P.L. 20/7/85//Paratype Pelidnota durantonorum S. 2008-2009” (47030685); “K [Kaw] - PK 40 25/8/84 P.L. //Paratype Pelidnota durantonorum S. 2008-2009” (47030686); “Mgne de Kaw G. F. 8/92//Paratype Pelidnota durantonorum S. 2008-2009” (47030687); “Piste de Kaw 8/92//Paratype Pelidnota durantonorum S. 2008-2009” (47030688); “Kaw 7/87//Paratype Pelidnota durantonorum S. 2008-2009” (47030689); Two paratypes with identical label data: “KAW PK 34 29/8/84 [obverse] P.L.//Paratype Pelidnota durantonorum S. 2008-2009” (47030690, exch40); “KAW PK 34 28/7/84//Paratype Pelidnota durantonorum S. 2008-2009” (47030691); Two paratypes with identical label data: “Rocoucova P.L. PK4 23/7/84//Paratype Pelidnota durantonorum S. 2008-2009” (47030692 and 47030694); “Rocoucova PK4 23/7/84 P.L.//Paratype Pelidnota durantonorum S. 2008-2009” (47030693); Five paratypes with identical label data: “Rocoucova 26/6/85 P.L.//Paratype Pelidnota durantonorum S. 2008-2009” (47030695 to 47030698, exch41); “St Jean Laurent du Maroni 1980 - 82//Paratype Pelidnota durantonorum S. 2008-2009” (47030699); “Coll. BLEUZEN Mgne de KAW PK 10 Guyane Fr. 12 Juillet 1983//Paratype Pelidnota durantonorum S. 2008-2009” (47030700); “St Georges VIII/87//Paratype Pelidnota durantonorum S. 2008-2009” (47030701); “Saül Mt la Fumée G.F.//Paratype Pelidnota durantonorum S. 2008-2009” (47030702); “Saül Mt la Fumée G.F. 08/92//Paratype Pelidnota durantonorum S. 2008-2009” (47030703); “Rio Juruti Obidos (Para) coll. – SOULA//Paratype Pelidnota durantonorum S. 2008-2009” (47030704); “Saül G.F. 8/92//Paratype Pelidnota durantonorum S. 2008-2009” (exch42); “Les 2 flots G.G. VIII/01 M. SOULA det 2002//Paratype Pelidnota durantonorum S. 2008-2009” (47030705); “Kaw, pk 37 IX/1998 M. SOULA det 19//Paratype Pelidnota durantonorum S. 2008-2009” (47030706); “Degrad Saramaca G.F. 7/92//Paratype Pelidnota durantonorum S. 2008-2009” (47030707); “P. pallidipennis coll. – SOULA//Guyane F. coll. – SOULA//Paratype Pelidnota durantonorum S. 2008-2009” (47030708); “P. laevissima coll. – SOULA// Maroni Guyane F. coll. – SOULA//Paratype Pelidnota durantonorum S. 2008-2009” (47030709); “Le Chateau Cacao G.F. M. Soula det. 20 [obverse] 5/09/2008//Paratype Pelidnota durantonorum S. 2008-2009” (47030710); “Région de Cacao Guyane Franç.//Paratype Pelidnota durantonorum S. 2008-2009” (47030711); “15-août-07 [Guyane française] RN2 ; PK 72//Paratype Pelidnota durantonorum S. 2008-2009” (47030712); “15 km au S de Kourou G-F 20/7/83//Paratype Pelidnota durantonorum S. 2008-2009” (47030713); Two female paratypes with identical label data: “NANCIBO GUYANE FR 25-26 VIII 84//Paratype Pelidnota durantonorum S. 2008-2009” (47030714 and 47030725); “Saint Laurent Guyane Fse VI 1984 M.Duranton Recolt//Paratype Pelidnota durantonorum S. 2008-2009” (47030715); “Rte Paul Isnard PK 19//Saint Laurent Guyane Fse VI 1984 M.Duranton Recolt//Paratype Pelidnota durantonorum S. 2008-2009” (47030716); Two paratypes with identical label data: “494 64//MUSEUM PARIS GUYANE FR. LA MANA MÉLINON 1864//Paratype Pelidnota durantonorum S. 2008-2009” (47030717 and 47030718); “Guyana Cayensis Deyr.//ZOOL. MUSEUM DK COPENHAGEN//Paratype Pelidnota durantonorum S. 2008-2009” (47030719); “GUYANE Française Mission M. Boulard et P. Pompanon Muséum PARIS//KOUROU FORÊT 3-7-VIII-1975//Paratype Pelidnota durantonorum S. 2008-2009” (47030720); “Paratype Pelidnota durantonorum S. 2008-2009” (exch43); “PELIDNOTA MADRONA 300 m. Panama 15. III.90 Coll. B. COURTIN//Paratype Pelidnota durantonorum S. 2008-2009//Invalid Paratype Pelidnota durantonorum Soula det. Moore ‘15” (47030721); “Patagaïe G. F. 08/2001 M. SOULA det 19//Paratype Pelidnota durantonorum S. 2008-2009” (47030722); “Kaw PK 40 25/8/84 P.L. //Paratype Pelidnota durantonorum S. 2008-2009” (47030723); “Piste de Kaw 8/92 G. F. coll. – SOULA//Paratype Pelidnota durantonorum S. 2008-2009” (47030724); “Coll. P. BLEUZEN Gonbolo [sic pro Gonfolo] Kourou Guyane Fr. 8 Août 1983//Paratype Pelidnota durantonorum S. 2008-2009” (47030726). Genitalia card-mounted underneath the male holotype, 27 male paratypes and the invalid male paratype. Box 4618679 SOULA.
Pelidnota ebenina
Anomala ebenina Blanchard, 1842: plate 11 [original combination].
Odontognathus ebeninus
(Blanchard) [new combination by
Strigidia ebenina
(Blanchard) [new combination by
Odontognathus ebeninus
(Blanchard) [revised combination by
Pelidnota (Ganonota) ebenina
(Blanchard) [new combination and new subgeneric combination by
Pelidnota (Strigidia) ebenina
(Blanchard) [new subgeneric combination by
Pelidnota (Odontognathus) ebenina
(Blanchard) [new subgeneric combination by
Strigidia ebenina
(Blanchard) [revised combination by
Pelidnota (Strigidia) ebenina
(Blanchard) [revised combination and revised subgeneric combination by
Pelidnota ebenina
(Blanchard) [removal of subgeneric classification by
synonym. Odontognathus gounellei Ohaus, 1908c
Odontognathus gounellei Ohaus, 1908c: 307 [original combination].
Pelidnota (Ganonota) gounellei
(Ohaus) [new combination and new subgeneric combination by
Pelidnota (Strigidia) gounellei
(Ohaus) [new subgeneric combination by
Pelidnota (Odontognathus) gounellei
(Ohaus) [new subgeneric combination by
Strigidia ebenina
(Blanchard) [syn. by
Pelidnota (Strigidia) gounellei
(Ohaus) [revised subgeneric combination and revised species status by
Pelidnota ebenina (Blanchard) [revised synonymy].
Distribution
ARGENTINA (
Types
1 ♀ syntype (lacking head) at MNHN (
Remarks
CCECL contains a P. ebenina specimen labeled as a male ♂ alloréférent with the following data: “Camiri [arrow] Sta Cruz 650 m coll. – SOULA/Alloreferent ♂ de Strigidia ebenina (Bl.) M. SOULA det. 19” (47030122). Genitalia card-mounted underneath specimen. Box 4618652 SOULA.
Pelidnota egana
Pelidnota egana Ohaus, 1912: 298, 300 [original combination].
Pelidnota (Chalcoplethis) egana
Ohaus [new subgeneric combination by
Strigidia egana
(Ohaus) [new combination by
Pelidnota egana
Ohaus [revised combination by
Distribution
BRAZIL: Amazonas (
Pelidnota equatoriana
Pelidnota equatoriana Soula, 2009: 32, 86–87 [original combination].
Distribution
ECUADOR: Esmeraldas, Napo, Pichincha, Santo Domingo (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 7 ♂ paratypes, 10 ♀ paratypes: “Pacto Equateur 4/2001 M. SOULA det 19//Holotype 2007 Pelidnota equatoriana S. Soula” (47030439); “Pacto Equateur 4/2001 M. SOULA det 19//Paratype 2007 Pelidnota equatoriana S. Soula” (47030440); “Tena (E) 9/90 [0 crossed out] 1//Paratype 2007 Pelidnota equatoriana S. Soula” (47030441); “Tena (E) 9/91//Paratype 2007 Pelidnota equatoriana S. Soula” (47030442); “Celica Pichincha Eq. III/97 M. SOULA det 19//Paratype 2007 Pelidnota equatoriana S. Soula” (47030443); Two paratypes with identical label data “Malimpia (Esmereldas [sic]) Equateur M. SOULA det 19 [obverse] II/2008//Paratype 2008 Pelidnota equatoriana S. Soula” (47030444 and 47030445); “Lita [arrow] San Lorenzo coll. – SOULA [obverse] pk 7,5 770m 15/08/93//Paratype 2007 Pelidnota equatoriana S. Soula” (47030446); “Ecuador St Domingo Avril 1982//Paratype 2007 Pelidnota equatoriana S. Soula” (47030447); “Santo Domingo Equateur M. SOULA det 19 [obverse] V/2007//Paratype 2008 Pelidnota equatoriana S. Soula” (47030448); Two paratypes with identical label data “Alluriquin – 800m. VI/2000 Equateur M. SOULA det 19//Paratype 2008 Pelidnota equatoriana S. Soula” (47030449 and 47030450); “San José Quinindé Equateur M. SOULA det 19 [obverse] 15/4/76 (Dzido)//Paratype 2008 Pelidnota equatoriana S. Soula” (47030451); “Equateur M. SOULA det 19//Paratype 2007 Pelidnota equatoriana S. Soula” (exch24); “Equateur M. SOULA det 19//Paratype 2008 Pelidnota equatoriana S. Soula” (exch25);. “Quinindé Equateur XII/91 M. SOULA det 19//Paratype 2008 Pelidnota equatoriana S. Soula” (47030452); “P. notata coll. – SOULA” // Sto Domingo (Equateur) coll. – SOULA//Paratype 2007 Pelidnota equatoriana S. Soula” (47030453). Genitalia card-mounted underneath the male holotype and seven male paratypes. Box 4618664 SOULA. The following specimen is deposited at CMNC. 1 ♀ paratype: “ECUADOR, 700’ RioPalenque 47 km S. St. Domingo Feb 22-27 1976 H. & A. Howden//Paratype 2007 Pelidnota equatoriana S. Soula”.
Remarks
Soula mentions an allotype in the original description but no specimen is labeled as such in CCECL. The allotype should have label data “Pacto, Pichincha, Equateur 4/2001.” These data are recorded only for the holotype and one paratype (“Pichincha” from original description). However, the number of specimens correlates with the number in the original description. Soula cites three labels with the locality “Malimpia”, but there are only two at CCECL. No paratypes were found with the locality “Palenque Howden” as cited in the original description, but there are two non-type ♀’s with locality “Rio Palenque”. The specimen labeled as “P. notata” was not included in original description.
Pelidnota estebanabadiei
Pelidnota estebanabadiei Soula, 2009: 34, 112 [original combination].
Distribution
BRAZIL: Rio de Janeiro (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 2 ♂ paratypes, 1 ♀ paratype: “Boca do Mato Cochoeiras de Macaçu-II/1995 Rio de Janeiro//Holotype 2008 Pelidnota estebanabadiei Soula” (47030786); “Boca do Mato Cochoeiras de Macaçu-II/1995 Rio de Janeiro//Allotype 2008 Pelidnota estebanabadiei Soula” (47030787); Two male paratypes with identical label data: “Boca do Mato Cochoeiras de Macaçu-II/1995 Rio de Janeiro//Paratype Pelidnota estebanabadiei Soula” (47030788 and 47030789); “Ex-Musæo H.W. BATES 1892//Rio J.//Paratype 2009 Pelidnota estebanabadiei S. Soula” (47030790). Genitalia card-mounted underneath the male holotype and the two male paratypes. Box 4618680 SOULA.
Pelidnota estebandurani ecuatoriana
Strigidia estebandurani ecuatoriana Soula, 2006: 25 [original combination].
Pelidnota estebandurani ecuatoriana
(Soula) [new combination by
Distribution
ECUADOR: Napo (
Types
The following specimen is deposited at CCECL. 1 ♀ allotype: “ECUADOR NAPO SC STATION YASUNI PUCE 400m 27NOV 1995 ITapia//Allotype 2005 Strigidia estebandurani ecuatoriana Sou. Soula det.” (47030297). Box 4618652 SOULA.
Pelidnota estebandurani estebandurani
Strigidia estebandurani estebandurani Soula, 2006: 12, 24–25 [original combination].
Pelidnota estebandurani estebandurani
(Soula) [new combination by
Distribution
COLOMBIA: Huila (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♂ paratype, 1 ♀ paratype: “Albania, Colombie 20-31/VII/1975//Holotype 2005 Strigidia estebandurani Sou. Soula det.” (47030293); “Albania, Colombie 20-31/VII/1975//Allotype 2005 Strigidia estebandurani Sou. Soula det.” (47030294); “Colombia Gigante Huila (parte alta cordillera)//Zona n˚3//Col. O. Rojas//Paratype 2005 Strigidia estebandurani Sou. Soula det.” (47030296); “Gazon (sic); 900 m 20/VII/1975 Colombie M. SOULA det 19//Paratype 2005 Strigidia estebandurani Soula Soula det.” (47030295). Genitalia card-mounted underneath the male holotype, female allotype, and one male paratype. Box 4618658 SOULA.
Pelidnota fabricelavalettei
Pelidnota fabricelavalettei Soula, 2009: 131–132 [original combination].
Pelidnota lavalettei
Soula [syn. by
Pelidnota fabricelavalettei Soula [stat. rev.].
Distribution
FRENCH GUIANA (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Guyane fr. Est. du dép. M. SOULA det. 20//Holotype 2008 Pelidnota lavalettei S. Soula//Holotype of P. fabricelavalettei
Remarks
The same holotype specimen was described twice, resulting in a case synonymy created by
Pelidnota filippiniae
Pelidnota filippiniae Soula, 2009: 108–109 [original combination].
Distribution
BRAZIL: Pará (
Types
Holotype ♂ at MNHN (
Pelidnota flavovittata
Rutela flavovittata Perty, 1830: 49 [original combination].
Pelidnota liturella var. flavovittata (Perty) [new combination and new infrasubspecific status by
Pelidnota (Ganonota) liturella var. flavovittata (Perty) [new subgeneric combination by
Pelidnota (Strigidia) flavovittata
(Perty) [new subgeneric combination by
Pelidnota (Odontognathus) flavovittata
(Perty) [new subgeneric combination by
Strigidia flavovittata
(Perty) [new combination by
Pelidnota (Strigidia) flavovittata
(Perty) [revised combination and revised subgeneric combination by
Pelidnota flavovittata
(Perty) [removal of subgeneric classification by
Distribution
BRAZIL: Minas Gerais (
Pelidnota fracida
Pelidnota fracida F. Bates, 1904: 258, 269 [original combination].
Pelidnota (Pelidnota) fracida
F. Bates [new subgeneric combination by
Pelidnota fracida
F. Bates [removal of subgeneric classification by
synonym. Pelidnota (Pelidnota) testaceipes Casey, 1915
Pelidnota (Pelidnota) testaceipes Casey, 1915: 75 [original combination].
Pelidnota (Pelidnota) fracida
F. Bates [syn. by
Distribution
BRAZIL: Amazonas, Pará (F.
Pelidnota frommeri
Pelidnota (Pelidnota) frommeri Hardy, 1975: 7, 25–26 [original combination].
Pelidnota frommeri
Hardy [removal of subgeneric classification by
Distribution
COSTA RICA: Alajuela, Cartago, Guanacaste, Heredia, Limón, San José (
Types
1 ♂ holotype and 1 ♀ allotype at USNM (
Pelidnota fulva
Pelidnota fulva Blanchard, 1851: 211 [original combination].
Pelidnota (Pelidnota) fulva
Blanchard [new subgeneric combination by
Pelidnota fulva
Blanchard [removal of subgeneric classification by
Distribution
BOLIVIA: Chuquisaca (
Types
1 ♂ lectotype and 1 paralectotype at MNHN (
Pelidnota fusciventris columbica
Strigidia fusciventris columbica Soula, 2006: 24 [original combination].
Pelidnota (Strigidia) fusciventris columbica
(Soula) [new combination and new subgeneric combination by
Pelidnota fusciventris columbica
(Soula) [removal of subgeneric classification by
Distribution
COLOMBIA: Cundinamarca, Valle del Cauca (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Columbia Cumaral 400 m [obverse] RU 137 ♂ 2.59.//Holotype 2005 Strigidia fusciventris columbica Sou. Soula det.” (47030284). Genitalia card-mounted underneath the male holotype. Box 4618658 SOULA.
Pelidnota fusciventris fusciventris
Pelidnota fusciventris Ohaus, 1905: 318–319 [original combination].
Pelidnota (Ganonota) fusciventris
Ohaus [new subgeneric combination by
Pelidnota (Strigidia) fusciventris
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) fusciventris
Ohaus [new subgeneric combination by
Pelidnota (Ganonota) fusciventris
Ohaus [revised subgeneric combination by
Strigidia fusciventris
(Ohaus) [new combination by
Pelidnota (Strigidia) fusciventris
Ohaus [revised combination and revised subgeneric combination by
Pelidnota fusciventris
Ohaus [removal of subgeneric classification by
Distribution
PERU: Junín, Pasco (
Pelidnota fusciventris lecourti
Strigidia fusciventris lecourti Soula, 2006: 23–24 [original combination].
Pelidnota (Strigidia) fusciventris lecourti
(Soula) [new combination and new subgeneric combination by
Pelidnota fusciventris lecourti
(Soula) [removal of subgeneric classification by
Distribution
BOLIVIA: La Paz, Cochabamba (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 4 ♂ paratypes, 3 ♀ paratypes: “Caranavi 1000 m III/2002 M. SOULA det 19//Holotype 2005 Strigidia fusciventris lecourti Sou. Soula det.” (47030275); “Caranavi 1000 m ? III/2002 M. SOULA det 19//Allotype Strigidia fusciventris lecourti Sou. Soula det.” (47030276); “Cristal Mayu Chaparé (B) 8/87//Paratype 2005 Strigidia fusciventris lecourti Sou. Soula det.” (47030278); Two paratypes with identical label data “Cristal Mayu Chapare (B) 10/87//Paratype 2005 Strigidia fusciventris lecourti Sou. Soula det.” (47030279 and 47030280); “Caranavi 800 m 21/X/2000 M. SOULA det 19//Paratype 2006 Strigidia fusciventris lecourti Sou. Soula det.” (47030277); “Cristal Mayu Chaparé (B) 10/88//Paratype 2005 Strigidia fusciventris lecourti Sou. Soula det.” (47030281); “Bolivie M. SOULA det 19//Paratype 2005 Strigidia fusciventris lecourti Sou. Soula det.” (47030283); “Ron 1531//Paratype 2005 Strigidia fusciventris lecourti Sou. Soula det.” (47030282). Genitalia card-mounted underneath the male holotype and four male paratypes. Box 4618658 SOULA.
Pelidnota fuscoviridis
Pelidnota fuscoviridis Ohaus, 1913: 500–501 [original combination].
Pelidnota (Pelidnota) fuscoviridis
Ohaus [new subgeneric combination by
Pelidnota fuscoviridis
Ohaus [removal of subgeneric classification by
Distribution
VENEZUELA (
Types
1 ♀ syntype of Pelidnota fuscoviridis at ZMHB (
Pelidnota gabrielae
Pelidnota (Odontognathus) gabrielae Martínez, 1979: 1–3 [original combination].
Strigidia gabrielae
(Martínez) [new combination by
Pelidnota (Strigidia) gabrielae
Martínez [revised combination and new subgeneric combination by
Pelidnota gabrielae
Martínez [removal of subgeneric classification by
Distribution
VENEZUELA: Amazonas, Bolívar (
Types
Allotype specimen (♀) of Pelidnota (Odontognathus) gabrielae at MACN (Fig.
Remarks
Based on examination of specimens including type specimens, it is possible that P. labyrinthophallica is a junior synonym of P. gabrielae. Soula’s illustration of the male genitalia of P. gabrielae (
Pelidnota genieri
Strigidia genieri Soula, 2006: 76 [original combination].
Pelidnota genieri
Soula [new combination by
Distribution
VENEZUELA: Tachira Betania (
Types
The following specimen is deposited at CMNC. 1 ♂ holotype: “Venezuela-Tachi-na. 2425 m. 16-20-III-1983//Exp. Instituto Zoologia Agricola Fac. Agronomia//Betania via Paramo El Tama//Collection François Génier//Holotype Strigidia genieri S. 2006 Soula”.
Remarks
Strigidia genieri Soula, 2006 was transferred to Pelidnota by
Pelidnota gilletti
Pelidnota gilletti Soula, 2009: 31, 55–56 [original combination].
Distribution
MEXICO: Chiapas, Veracruz (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♂ paratype, 1 ♀ paratype, 2 ♂ invalid paratypes, 3 ♀ invalid paratypes: “P. centro-americana Sta Rosa Chiapas 8/90//Holotype Pelidnota gilleti S. Soula det. 2006” (47030463); “P. centro-americana Sta Rosa Chiapas 8/90//Allotype Pelidnota gilleti S. Soula det. 2006” (47030464); Two paratypes with identical label data “Sta Rosa Chiapas 8/90 (Mex.)//Paratype 2006 Pelidnota gilleti S. Soula” (47030465 and 47030466); Three invalid paratypes with identical label data “San Pedro de Soteapan VERACRUZ – 500 m. MEXIQUE – Sept. 1987 Thierry PORION Leg.//Paratype 2006 Pelidnota gilleti S. Soula//Invalid Paratype det. M. R. Moore 2014” (47030467 to 47030469); “San Pedro de Soteapan VERACRUZ – 500 m. MEXIQUE – Sept. 1987 Thierry PORION Leg.//Paratype Pelidnota gilleti Sou. 2006 Soula//Invalid Paratype det. M. R. Moore 2014” (47030470); “METATEZ OAXACA MEXIQUE IX. 85//METATEZ OAXACA MEXIQUE IX. 85//Paratype 2006 Pelidnota gilleti S. Soula//Invalid Paratype det. M. R. Moore 2014” (47030471). Genitalia card-mounted underneath the male holotype. Box 4618665 SOULA.
Remarks
We designated five paratypes as invalid because these specimens do not have label data that matches
Pelidnota girardi
Chalcoplethis girardi Bouchard, 2003: 103–108 [original combination].
Strigidia girardi
(Bouchard) [new combination by
Pelidnota girardi
(Bouchard) [new combination by
Distribution
FRENCH GUIANA (
Types
The following specimens are deposited at CCECL. 4 ♂ paratypes, 15 ♀ paratypes: Two paratypes with identical label data “Piste de Belizon (G. F.) coll. – SOULA//Chalcoplethis girardi sp. n. PARATYPE” (47030264 and 47030265); Two paratypes with identical label data “M. Kaw I. 89 guyane F.//Chalcoplethis girardi sp. n. PARATYPE” (47030270 and 47030271); “Dd Saramaca PK. 6 Kourou/Guyane Fse 16.17 XII 1987 M.Duranton Recolt.//Chalcoplethis girardi sp. n. PARATYPE” (47030274); Two paratypes with identical label data “Guyane f. Kourou VIII 1990//Chalcoplethis girardi sp. n. PARATYPE” (47030258, exch17); “Tingo Maria (Pé) 9/93 coll. – SOULA//Chalcoplethis girardi sp. n. PARATYPE” (47030267); “Rocoucova PK 3 P.L. 25/1/85//Chalcoplethis girardi sp. n. PARATYPE” (47030269); “P. de Kaw G. Franç. coll. – SOULA//Chalcoplethis girardi sp. n. PARATYPE” (47030263); Two paratypes with identical label data “M. de Kaw guyane Fr. 8. 90//Chalcoplethis girardi sp. n. PARATYPE” (47030272 and 47030273); “Coralie G. F. 15/12/92//Chalcoplethis girardi sp. n. PARATYPE” (47030268); Three paratypes with identical label data “Saut Dalles G. F. 7/03/92//Chalcoplethis girardi sp. n. PARATYPE” (47030260, 47030261, exch16); “Piste de Kaw G. F. 02/93 coll. – SOULA//Chalcoplethis girardi sp. n. PARATYPE” (47030262); “ et 2/ 90 [obverse] Piste de Belizon coll. – SOULA//Piste de Belizon G. F. coll. – SOULA//Chalcoplethis girardi sp. n. PARATYPE” (47030266); “Petit Saut G. F. 07/92//Chalcoplethis girardi sp. n. PARATYPE” (47030259). Genitalia card-mounted underneath three male paratypes and three female paratypes. Box 4618657 SOULA.
Pelidnota glaberrima glaberrima
Pelidnota glaberrima Blanchard, 1851: 213–214 [original combination].
Pelidnota (Ganonota) glaberrima
Blanchard [new subgeneric combination by
Pelidnota (Strigidia) glaberrima
Blanchard [new subgeneric combination by
Pelidnota (Odontognathus) glaberrima
(Blanchard) [new subgeneric combination by
Strigidia glaberrima
(Blanchard) [new combination by
Pelidnota (Strigidia) glaberrima
Blanchard [revised combination and revised subgeneric combination by
Pelidnota glaberrima glaberrima
Blanchard [removal of subgeneric classification by
Distribution
ARGENTINA: Misiones (MLJC). BRAZIL: Espírito Santo, Minas Gerais, Rio de Janeiro, São Paulo (
Types
1 ♂ syntype of Pelidnota glaberrima at MNHN (
Remarks
CCECL contains a specimen of P. glaberrima glaberrima that is labeled as a female alloréférent with the following data: 1 ♀ alloréférent: “Jtatiaya R.d Janeiro//Ganonota glaberrima Bl Burgeon L. 1930 [0 crossed out] 1 det://R. I. Sc. N. B. 16.117 L. Burgeon, coll. et det. ://Alloréférent ♀ de Strigidia glaberrima (Bl.) M. SOULA det 19 2005” (47030396). Box 4618663 SOULA.
Pelidnota glaberrima meridionalis
Strigidia glaberrima meridionalis Soula, 2006: 37 [original combination].
Pelidnota (Strigidia) glaberrima meridionalis
(Soula) [new combination and new subgeneric combination by
Pelidnota glaberrima meridionalis
(Soula) [removal of subgeneric classification by
Distribution
ARGENTINA: Misiones (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 3 ♂ paratypes, 15 ♀ paratypes: “Puerto Iguazu ARGENTINE (I/93)//Holotype 2004 Strigidia glaberrima meridionalis Sou. Soula det.” (47030400); “Puerto Iguazu, ARGENTINE (I/93)//Allotype 2004 Strigidia glaberrima meridionalis Soula det. Sou.” (47030401); Eight paratypes with identical label data “Puerto Iguazu ARGENTINE (I/93)//Paratype 2005 Strigidia glaberrima meridionalis Sou. Soula det.” (47030402 to 47030407, exch22 and exch23); Three paratypes with identical label data “Tayao Aeguazu coll. – SOULA [obverse] Paraguay, 10/10/98//Paratype 2005 Strigidia glaberrima meridionalis Sou. Soula det.” (47030411 to 47030413); “Iguazu Misiones (Arg.) coll. – SOULA//Paratype 2005 Strigidia glaberrima meridionalis Sou. Soula det.” (47030415); “Puerto Iguazu-ARG-XII/88//Paratype 2005 Strigidia glaberrima meridionalis Sou Soula det..” (47030414); Three paratypes with identical label data “Eldorado – Misiones, ARGENTINE (I/93)//Paratype 2005 Strigidia glaberrima meridionalis Sou. Soula det.” (47030408 to 47030410); “ARGENTINE: Iguazu, Misiones, XII-88//Paratype 2005 Strigidia glaberrima meridionalis Sou. Soula det.” (47030416); “Nova Friborgo (sic for Firburgo) – R.J. XII/92 – BRESIL//Paratype 2005 Strigidia glaberrima meridionalis Sou. Soula det.” (47030417). Genitalia card-mounted underneath the male holotype and paratypes. Box 4618663 SOULA.
Pelidnota glaberrima septentrionalis
Strigidia glaberrima septentrionalis Soula, 2006: 37–38 [original combination].
Pelidnota (Strigidia) glaberrima septentrionalis
(Soula) [new combination and new subgeneric combination by
Pelidnota glaberrima septentrionalis
(Soula) [removal of subgeneric classification by
Distribution
BRAZIL: Bahia (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Cachimbo Prov.de.Bahia Ch Pujol 1890//Muséum Paris ex Coll. R. Oberthür 1952//Holotype 2005 Strigidia glaberrima septentrionalis Sou. Soula det.” (47030418). Genitalia card-mounted underneath the specimen. Box 4618663 SOULA.
Pelidnota glabra audureaui
Pelidnota glabra audureaui Soula, 2009: 130–131 [original combination].
Distribution
NICARAGUA: Granada (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♂ paratype, 6 ♀ paratypes: “Reserva Silvestre de Domitila Granada prov. PL NICARAGUA 09-21.06.2007 Alain Audureau leg.//Holotype 2008 Pelidnota glabra audureaui S. Soula” (47030347); “Reserve sylvestre de Domitila M. SOULA det 19 [obverse] Granada Prov Nicaragua 09-21/6/2007 P.L.//Allotype 2008 Pelidnota glabra audureaui S. Soula” (47030348); “Reserve sylv. de Domitila Granada Prov. M. SOULA det 19 [obverse] Nicaragua 09-21/6/2007//Paratype 2008 Pelidnota glabra audureaui S. Soula” (47030349); “Reserve sylv. de Domitila Granada Prov. M. SOULA det 19 [obverse] Nicaragua P. L. 1-5/6/2005//Paratype 2008 Pelidnota glabra audureaui S. Soula” (47030350); “Reserve syl. de Domitila Granada Prov. M. SOULA det 19 [obverse] Nicaragua P. L. 1-5/6/2005//Paratype 2008 Pelidnota glabra audureaui Soula” (47030351); “Reserva silvestra de Domitila PL Granade prov. Nicaragua 01-05.06.2005 Alain Audureau leg.//Paratype 2008 Pelidnota glabra audureaui S. Soula” (47030352); “Reserva silvestra privada de Domitila PL Granada prov. NICARAGUA 13-16/06/2004 Alain Audureau legit//Paratype 2008 Pelidnota glabra audureaui S. Soula” (47030353); “Bartola lodge PL Rio San Juan Nicaragua 06-13. VI.2005 Alain & Sylvaine Audureau//Paratype 2008 Pelidnota glabra audureaui S. Soula” (47030354); “Bartola lodge PL Rio San Juan Nicaragua 06-13. VI.2005 Alain & Sylvaine Audureau leg.//Paratype 2008 Pelidnota glabra audureaui S. Soula” (47030355). Genitalia card-mounted underneath the male holotype and the three male paratypes. Box 4618661 SOULA.
Pelidnota glabra glabra
Pelidnota glabra Ohaus, 1922: 324 [original combination].
Pelidnota (Chalcoplethis) glabra
Ohaus [new subgeneric combination by
Strigidia glabra
(Ohaus) [new combination by
Pelidnota glabra
Ohaus [revised combination by
Distribution
COSTA RICA: Cartago, Guanacaste, Limón (
Types
1 ♂ syntype of Pelidnota glabra at ZMHB (
Pelidnota gracilis debahia
Strigidia gracilis debahia Soula, 2006: 30 [original combination].
Pelidnota (Strigidia) gracilis debahia
(Soula) [new combination and new subgeneric combination by
Pelidnota gracilis debahia
(Soula) [removal of subgeneric classification by
Distribution
BRAZIL: Bahia (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 3 ♀ paratypes: “Bahia Bresil//Pelidnota gracilis//Holotype 2006 Strigidia gracilis debahia Sou. Soula” (47030299); “Cachimbo Prov. de Bahia Ch. Pujol 1890//Museum Paris ex. Coll. R. Oberthur//Allotype 2006 Strigidia gracilis debahia Sou. Soula” (47030300); Three paratypes with identical label data “Brésil//Allotype 2006 Strigidia gracilis debahia Sou. Soula” (47030301 to 47030303). Genitalia card-mounted underneath the male holotype. Box 4618659 SOULA.
Pelidnota gracilis gracilis
Rutela gracilis Gory, 1834: 111 [original combination].
Pelidnota gracilis
(Gory) [new combination by
Pelidnota (Ganonota) gracilis
(Gory) [new subgeneric combination by
Pelidnota (Strigidia) gracilis
(Gory) [new subgeneric combination by
Pelidnota (Odontognathus) gracilis
(Gory) [new subgeneric combination by
Strigidia gracilis
(Gory) [new combination by
Pelidnota (Strigidia) gracilis
(Gory) [revised combination and revised subgeneric combination by
Pelidnota gracilis gracilis
(Gory) [removal of subgeneric classification by
Distribution
BRAZIL: Espírito Santo, Minas Gerais, Rio de Janeiro (
Pelidnota gracilis wagneri
Strigidia gracilis wagneri Soula, 2006: 30 [original combination].
Pelidnota (Strigidia) gracilis wagneri
(Soula) [new combination and new subgeneric combination by
Pelidnota gracilis wagneri
(Soula) [removal of subgeneric classification by
Distribution
ARGENTINA: Misiones (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 4 ♂ paratypes, 1 ♀ paratype, 1 invalid ♀ allotype: “Iguazu Misiones (Arg.) coll. – SOULA//Holotype 2006 Strigidia gracilis wagneri Sou. Soula” (47030304); “Puerto Iguazu-ARG XII/88//Allotype 2006 Strigidia gracilis wagneri Sou. Soula” (47030305); “Puerto Iguazu (Arg.) coll. – SOULA [obverse] 11/89//Invalid ♀ Allotype probable paratype of P. gracilis wagneri Soula det. M. R. 2014//Allotype 2006 Strigidia gracilis wagneri S. Soula” (47030306); Two paratypes with identical label data “Puerto Iguazu (Arg.) coll. – SOULA [obverse] 11/87//Paratype 2006 Strigidia gracilis wagneri Sou. Soula” (47030310 and 47030311); Two paratypes with identical label data “Iguazu Misiones (Arg.) coll. – SOULA//Paratype 2006 Strigidia gracilis wagneri Sou. Soula” (47030307 and 47030308); “Iguazu Misiones (Ar.) coll. – SOULA//Paratype 2006 Strigidia gracilis wagneri Sou. Soula” (47030309). Genitalia card-mounted underneath the male holotype and four male paratypes. Box 4618659 SOULA.
Remarks
The female allotype specimen labeled from “Puerto Iguazu (Arg.)” is not the valid allotype specimen.
Pelidnota grangesi
Strigidia grangesi Soula, 2006: 10, 39 [original combination].
Pelidnota grangesi
(Soula) [new combination by
Distribution
BOLIVIA: Cochabamba, La Paz (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 3 ♂ paratypes, 2 ♀ paratypes: “Coroïco à Caranavi 850 m (B) 10/90//Holotype 2005 Strigidia grangesi Sou. Soula det.” (47030286); “De Coroïco à Caranavi 850 m 10/88//Allotype 2005 Strigidia grangesi Sou. Soula det.” (47030287); Two paratypes with identical labels “Coroïco à Caranavi 850 m (B) 10/90//Paratype 2006 Strigidia grangesi Sou. Soula det.” (47030289 and 47030290); “De Coroïco à Caranavi 850 m 10/88//Paratype 2006 Strigidia grangesi Sou. Soula” (47030288); “N. Yungas (Bo.) coll. – SOULA//Paratype 2006 Strigidia grangesi Sou. Soula” (47030291); “Cochabamba a Villa Tunasi (sic for Tunari) pk 102 (2000 m) [obverse] 10/88 (B)//Paratype 2006 Strigidia grangesi Sou. Soula” (47030292). Genitalia card-mounted underneath the male holotype and three male paratypes. Box 4618658 SOULA.
Pelidnota granulata
Rutela granulata Gory, 1834: 112 [original combination].
Pelidnota granulata
(Gory) [new combination by
Pelidnota (Chalcoplethis) granulata
(Gory) [new subgeneric combination by
Strigidia granulata
(Gory) [new combination
Pelidnota granulata
(Gory) [revised combination by
Distribution
BRAZIL: Amazonas (INPA). FRENCH GUIANA: Cayenne, St.-Laurent du Maroni (
Types
1 ♂ neotype of Rutela granulata at MNHN (
Pelidnota grossiorum
Pelidnota grossiorum Soula, 2009: 34, 110–111 [original combination].
Distribution
BRAZIL: Minas Gerais (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 3 ♂ paratypes, 2 ♀ paratypes, 1 ♂ invalid paratype: “Ipatinga M. G. XI/992 (sic) - BRESIL//Holotype 2008 Pelidnota grossiorum S. Soula” (47030799); “BRASIL: MG Cordisburgo Faz. Pontinha XII/1993 F. Z. Vaz de Mello//Allotype 2008 Pelidnota grossiorum S. Soula” (47030800); Three paratypes with identical label data: “Ipatinga M. G. XI/992 (sic) - BRESIL//Paratype 2008 Pelidnota grossiorum Soula” (47030801 to 47030803); “BRASIL: MG Cordisburgo Faz. Pontinha XII/1993 F. Z. Vaz de Mello//Paratype 2009 Pelidnota grossiorum S. Soula” (47030804); “Vale Rio Doce Minas Geraes 10/86 [obverse] Minas Geraes//Paratype 2009 Pelidnota grossiorum S. Soula” (47030805); “Ipatinga M.G. 11/94 coll. – SOULA//Paratype 2008 Pelidnota grossiorum S. Soula//Invalid Paratype Pelidnota grossiorum Soula det. MR Moore ‘15” (47030806). Genitalia card-mounted underneath the male holotype, the female allotype and the invalid male paratype. Box 4618681 SOULA.
Remarks
One male specimen labeled as a paratype bears a collecting date that was not reported in
Pelidnota guatemalensis
Pelidnota costaricensis var. guatemalensis H. W. Bates, 1888: 274 [original combination].
Pelidnota (Pelidnota) costaricensis guatemalensis
H. W. Bates [new subgeneric combination and new subspecific status by
Pelidnota (Pelidnota) guatemalensis
H. W. Bates [new species status by
Pelidnota guatemalensis
H. W. Bates [removal of subgeneric classification by
synonym. Pelidnota (Pelidnota) composita Casey, 1915
Pelidnota (Pelidnota) composita Casey, 1915: 71 [original combination].
Pelidnota (Pelidnota) guatemalensis
H. W. Bates [syn. by
Distribution
BELIZE: Toledo (H. W. Bates 1888,
Types
1 ♂ lectotype of Pelidnota costaricensis var. guatemalensis at BMNH (
Pelidnota gwendolinae
Strigidia gwendolinae Soula, 2006: 10, 81 [original combination].
Pelidnota gwendolinae
(Soula) [new combination by
Distribution
BOLIVIA: Cochabamba, La Paz (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 7 ♂ paratypes, 7 ♀ paratypes: “Inca Huara 1450 m. 11/95 coll. – SOULA//Holotype 2006 Strigidia gwendolinae S. Soula det” (47030058); “Carasco 1450 m M. SOULA det. 19 [obverse] La Paz Prov. 22/10/97//Allotype 2006 Strigidia gwendolinae Soula det. S.” (47030059); Three paratype males with identical labels “Inca Huara 1450 m. 11/95 coll. – SOULA//Paratype 2006 Strigidia gwendolinae S. Soula” (47030060 and 47030061, exch01); “Cochabamba à Villa Tunasi (sic) pk 102 (B) (2000 m) [obverse] 10/88 //Paratype 2006 Strigidia gwendolinae S. Soula” (47030062); “Cochabamba à Villa Tunari pk 102 (B) (2000 m) 10/88//Chalc. hoefigi coll. – SOULA//Paratype 2006 Strigidia gwendolinae S. Soula” (47030063); “ Route de Coroico à Coranavi [pro Caranavi] (Bolivie)//[retranscription of faded label]//Paratype 2006 Strigidia gwendolinae S. Soula” (47030064); “Region des Yungas Bolivie//Paratype 2006 Strigidia gwendolinae S. Soula” (47030066); “Bolivie M. SOULA det 19//Paratype 2006 Strigidia gwendolinae S. Soula” (47030067); “5 km de Chuspipata coll. – SOULA [obverse] 2007 m la Paz Prov. 4/10/1996//Paratype 2006 Strigidia gwendolinae S. Soula” (47030065); One male and one female paratype with identical label data “Col. G LECOURT Rte de La Paz. Yocumo 1000m. Km 301 31/03/98 Prov. Alto Beni. Bolivie//Paratype 2006 Strigidia gwendolinae S. Soula” (47030068 to 47030069); “BOLIVIE – CARANAVI NOR YUNGAS – ALT.900m DU 16 AU 30/11/89 COLLECTION LECOURT//Paratype 2006 Strigidia gwendolinae S. Soula” (47030072); “Coll. P. BLEUZEN Nor - Yungas Bolivie XI 1990//Paratype 2006 Strigidia gwendolinae S. Soula” (47030071); “Col G. LECOURT Yungus (sic) Coroïco 1 700 m BOLIVIE [the date 03/1986 is crossed out] [obverse] 12 - 90//Paratype 2006 Strigidia gwendolinae S. Soula” (47030070)”. Genitalia card-mounted underneath the male holotype and 6 male paratypes. Box 4618650 SOULA.
Pelidnota herbacea
Pelidnota herbacea Blanchard, 1851: 212 [original combination].
Pelidnota (Pelidnota) chlorana
Erichson [syn. by
Pelidnota herbacea
Blanchard [revised combination and revised species status by
Distribution
BOLIVIA (
Types
1 ♀ syntype of Pelidnota herbacea at MNHN (
Pelidnota hernanlequericai
Strigidia hernanlequericai Soula, 2006: 10, 40 [original combination].
Pelidnota hernanlequericai
(Soula) [new combination by
Distribution
PERU: Loreto (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ invalid allotype: “Iquitos, Loreto Pérou, I-II/2005//Holotype 2006 Strigidia hernanlequericai Sou. Soula det.” (47030419); “Iquitos, Loreto Pérou; VIII/2003//Allotype 2006 Strigidia hernanlequericai Sou. Soula det.//Invalid Allotype ♀, see
Remarks
Because there was no description or mention of an allotype specimen or paratype series of P. hernanlequericai (
Pelidnota hirsutiphallica
Pelidnota hirsutiphallica Ratcliffe & Jameson, 1989: 259–261 [original combination].
Strigidia santidomini
(sic) (Ohaus) [syn. by
Pelidnota hirsutiphallica
Ratcliffe and Jameson [revised combination and revised species status by
Distribution
COSTA RICA (
Types
1 ♂ holotype and 1 ♀ allotype of Pelidnota hirsutiphallica at UNSM (
Remarks
Pelidnota hoefigi
Pelidnota hoefigi Ohaus, 1912: 318 [original combination].
Pelidnota (Chalcoplethis) hoefigi
Ohaus [new subgeneric combination by
Strigidia hoefigi
(Ohaus) [new combination by
Pelidnota hoefigi
Ohaus [revised combination by
Distribution
FRENCH GUIANA: Saint-Georges (
Pelidnota huetheri
Pelidnota (Pelidnota) huetheri Howden, 1998: 171–173 [original combination].
Pelidnota huetheri
Howden [removal of subgeneric classification by
Distribution
PANAMA: Chiriquí (
Types
The following specimens are deposited at CMNC. 1 ♂ holotype and 1 ♀ allotype: “PANAMA Chiriqui Prv vic Hornito 4200’ 14-18 May 1996 Wappes Huether & Morris//HOLOTYPE Pelidnota huetheri H. Howden//Holotype Pelidnota huetheri How. Soula det. 2009//[barcode matrix] Canadian Museum of Musée canadien de la NATURE CMNEN 00011035”, allotype with identical collecting data label and database number CMNEN 00010902.
Pelidnota impressicollis
Pelidnota (Ganonota) impressicollis Ohaus, 1925: 76–77 [original combination].
Pelidnota (Strigidia) impressicollis
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) impressicollis
Ohaus [new subgeneric combination by
Strigidia impressicollis
(Ohaus) [new combination by
Pelidnota (Strigidia) impressicollis
Ohaus [revised combination and revised subgeneric combination by
Pelidnota impressicollis
Ohaus [removal of subgeneric classification by
Distribution
BRAZIL: Mato Grosso (
Types
1 ♂ holotype of Pelidnota (Ganonota) impressicollis at ZMHB (
Pelidnota incerta
Strigidia incerta Soula, 2006: 9, 20 [original combination].
Pelidnota incerta
(Soula) [new combination by
Distribution
PERU: Huánuco (
Types
The following specimen is deposited at CCECL (Fig.
Pelidnota instabilis
Pelidnota instabilis Ohaus, 1912: 302–303 [original combination].
Pelidnota (Chalcoplethis) instabilis
Ohaus [new subgeneric combination by
Strigidia instabilis
(Ohaus) [new combination by
Pelidnota instabilis
Ohaus [removal of subgeneric classification by
Distribution
BRAZIL: Espírito Santo, Rio de Janeiro, São Paulo (
Types
1 ♂ syntype of Pelidnota instabilis is possibly at ZMHB, but this is unclear (
Pelidnota jalapensis
Pelidnota virescens var. jalapensis H. W. Bates, 1888: 275 [original combination].
Pelidnota (Pelidnota) virescens var. jalapensis H. W. Bates [new subgeneric combination by
Pelidnota (Pelidnota) virescens jalapensis
H. W. Bates [new subspecific status by
Pelidnota (Pelidnota) jalapensis
H. W. Bates [new species status by
Pelidnota jalapensis
H. W. Bates [removal of subgeneric classification by
Distribution
MEXICO: Guerrero, Oaxaca, Veracruz (H. W. Bates 1888,
Types
1 ♂ lectotype at BMNH (
Pelidnota jolyi
Pelidnota (Chalcoplethis) jolyi Martínez, 1982: 61–65 [original combination].
Strigidia jolyi
(Martínez) [new combination by
Pelidnota jolyi
Martínez [new combination by
Distribution
BRAZIL: Acre (
Types
Holotype and allotype specimens of Pelidnota (Chalcoplethis) jolyi at MACN; 1 ♂ (Fig.
Pelidnota kirschi kirschi
Pelidnota kirschi F. Bates, 1904: 254, 261–262 [original combination].
Pelidnota (Chalcoplethis) kirschi
F. Bates [new subgeneric combination by
Strigidia kirschi
(F. Bates) [new combination by
Pelidnota kirschi
F. Bates [revised combination by
Distribution
COLOMBIA: Caldas, Cauca (F.
Types
1 ♂ lectotype and 1 paralectotype of Pelidnota kirschi at BMNH (
Pelidnota kirschi tenuistriata
Pelidnota kirschi var. tenuistriata F. Bates, 1904: 254, 262 [original combination].
Pelidnota (Chalcoplethis) kirschi var. tenuistriata F. Bates [new subgeneric combination by
Pelidnota (Chalcoplethis) kirschi forma tenuistriata F. Bates [revised infrasubspecific status by
Strigidia kirschi tenuistriata
(F. Bates) [new combination and new subspecific status by
Pelidnota kirschi tenuistriata
F. Bates [revised combination by
Distribution
VENEZUELA (F.
Pelidnota kuhnti
Heteropelidnota kuhnti Ohaus, 1912: 310–311 [original combination].
Pelidnota kuhnti (Ohaus) [comb. n.].
Distribution
PARAGUAY: Paraguarí (
Types
Holotype ♂ at ZMHB (Fig.
Remarks
The specimen on which the species is named appears to be a teratological deviant. The base of the pronotum is weakly triemarginate and surface sculpturing of pronotum appears weakly protuberant anterior to the emarginations. Additionally, the inner apices of the elytra are rounded, the apices of the meta- and mesotibia are eroded, the metacoxae and metafemur are quite gracile (about ½ the width of any pelidnotine scarab). The male genitalia are quite similar to P. semiaurata citripennis as well as the coloration, head, protibia, and protarsal claws. The apices of the elytra are poorly developed, thus exposing dense setae (one of the characters for which the genus was proposed). Other than the holotype specimen, no additional specimens are identified as H. kuhnti. It is possible that this specimen is a teratological deviant of P. semiaurata citripennis. Indeed,
Pelidnota labyrinthophallica
Pelidnota (Odontognathus) labyrinthophallica Solís & Morón, 1994: 31–35 [original combination].
Strigidia labyrinthophallica
(Solís and Morón) [new combination by
Pelidnota (Strigidia) labyrinthophallica
Solís and Morón [revised combination and new subgeneric combination by
Pelidnota labyrinthophallica
Solís and Morón [removal of subgeneric classfication by
Distribution
COSTA RICA: Puntarenas (
Types
1 ♂ holotype, 1 ♀ allotype and 2 paratypes of Pelidnota (Odontognathus) labyrinthophallica at MNCR (
Remarks
Pelidnota lacazei
Pelidnota lacazei Soula, 2010a: 38 [original combination].
Distribution
PERU: Junín (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 8 ♂ paratypes, 1 ♀ paratype: “Satipo, Junin Pérou, X/2003//Holotype 2010 Pelidnota lacazei S. Soula” (47030179); “Satipo, Junin Pérou, X/2003//Allotype 2010 Pelidnota lacazei S. Soula” (47030180); Seven paratypes with identical labels “Satipo, Junin Pérou, X/2003 M. SOULA det 19//Paratype 2010 Pelidnota lacazei Soula” (47030181 to 47030186, exch09); “Satipo Pérou X-XI/2002//Paratype 2010 Pelidnota lacazei Soula” (47030188); “PERU Satipo VI.1989 Pres. by. [illegible] Perry B. M. 1989-258//Paratype 2010 Pelidnota lacazei Soula” (47030187). Genitalia card-mounted underneath the holotype and five male paratypes. Box 4618656 SOULA.
Pelidnota laevissima
Pelidnota laevissima Burmeister, 1855: 522 [original combination].
Pelidnota (Pelidnota) laevissima
Burmeister [new subgeneric combination by
Pelidnota laevissima
Burmeister [removal of subgeneric classification by
Distribution
COLOMBIA: Atlántico, Caldas, Valle del Cauca (
Types
1 paralectotype of Pelidnota laevissima at MLUH (
Remarks
Pelidnota lagoi
Pelidnota lagoi Soula, 2011: 78–79 [original combination].
Distribution
BRAZIL: Goiás (
Types
The holotype ♂ and allotype ♀ are deposited at the Malý collection (
Pelidnota langsdorffi
Rutela langsdorffi Mannerheim, 1829: 48–49 [original combination].
Pelidnota langsdorffi
(Mannerheim) [new combination by
Pelidnota (Pelidnota) langsdorffi
(Mannerheim) [new subgeneric combination by
Pelidnota langsdorffi
(Mannerheim) [removal of subgeneric classification by
Distribution
BRAZIL (
Pelidnota liturella assumpta
Pelidnota assumpta Ohaus, 1929: 388-389 [original combination].
Pelidnota (Ganonota) assumpta
Ohaus [new subgeneric combination by
Pelidnota (Strigidia) assumpta
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) assumpta
Ohaus [new subgeneric combination by
Strigidia liturella assumpta
(Ohaus) [new combination and new subspecific status by
Pelidnota (Strigidia) assumpta
Ohaus [revised combination, revised species status, and revised subgeneric status by
Pelidnota liturella assumpta
Ohaus [removal of subgeneric classification and revised status by
Distribution
BRAZIL: Minas Gerais (
Pelidnota liturella liturella
Rutela liturella Kirby, 1819: 406 [original combination].
Pelidnota liturella
(Kirby) [new combination by
Pelidnota (Ganonota) liturella
(Kirby) [new subgeneric combination by
Pelidnota (Strigidia) liturella
(Kirby) [new subgeneric combination by
Pelidnota (Odontognathus) liturella
(Kirby) [new subgeneric combination by
Strigidia liturella
(Kirby) [new combination by
Pelidnota (Strigidia) liturella
(Kirby) [revised combination and revised subgeneric combination by
Pelidnota liturella liturella
(Kirby) [removal of subgeneric classification by
Distribution
ARGENTINA: Misiones (
Types
Most of Kirby’s type specimens are located at the BMNH. A search for the type specimen of P. liturella did not locate the specimen in the collection.
Pelidnota louzadai
Strigidia louzadai Soula, 2006: 12, 55–56 [original combination].
Pelidnota louzadai
(Soula) [new combination by
Distribution
BRAZIL: Mato Grosso (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype: “BRASIL – MT Faz.Sao Tiago 12.35S-56.20W XI-81//Holotype 2006 Strigidia louzadai S. Soula” (47030437); “BRASIL – MT Faz.Sao Tiago 12.35S-56.20W XI-81//Allotype 2006 Strigidia louzadai S. Soula” (47030438). The genitalia are card-mounted underneath the male holotype. Box 4618663 SOULA.
Pelidnota lucae
Pelidnota lucae LeConte, 1863: 78 [original combination].
Pelidnota (Pelidnota) lucae
LeConte [new subgeneric combination by
Pelidnota lucae
LeConte [removal of subgeneric classification by
Distribution
MEXICO: Baja California Norte, Baja California Sur (
Types
Syntype of Pelidnota lucae at MCZ (
Pelidnota lucida
Pelidnota lucida Burmeister, 1844: 401 [original combination].
Pelidnota (Pelidnota) lucida
Burmeister [new subgeneric combination by
Pelidnota lucida
Burmeister [removal of subgeneric classification by
Distribution
COLOMBIA (
Types
1 ♂ lectotype and 2 paralectotypes of Pelidnota lucida at MLUH (
Pelidnota lugubris
Pelidnota lugubris LeConte, 1874: 54 [original combination].
Pelidnota (Pelidnota) lugubris
LeConte [new subgeneric combination by
Pelidnota lugubris
LeConte [removal of subgeneric classification by
Distribution
MEXICO: Sinaloa, Sonora (
Types
1 syntype of Pelidnota lugubris at MCZ (
Pelidnota luridipes
Pelidnota luridipes Blanchard, 1851: 212 [original combination].
Pelidnota (Pelidnota) luridipes
Blanchard [new subgeneric combination by
Pelidnota luridipes
Blanchard [removal of subgeneric classification by
Distribution
BRAZIL: Mato Grosso (
Types
1 ♀ syntype at MNHN (
Remarks
CCECL contains a P. luridipes specimen labeled as a male alloréférent with the following data: 1 ♂ alloréférent: “Corumba Matt. Grosso//P. luridipes coll. – SOULA//Alloréférent ♂ de Pelidnota luridipes Bl. M. SOULA det 19” (47030640). Genitalia card-mounted underneath the male alloréférent. Box 4618678 SOULA.
Pelidnota malyi
Pelidnota malyi
Pelidnota vladimalyi
[new replacement name by
Pelidnota malyi Soula [revised status].
Distribution
ECUADOR: Imbabura (
Types
The holotype ♂ is deposited at the Malý collection (
Remarks
The species name P. vladimalyi was a replacement name for a homonym that Soula created by using the specific epithet “malyi” twice for two separate, distinct taxa in the genus Pelidnota (
Pelidnota mantillerii
Pelidnota mantillerii Soula, 2009: 132 [original combination].
Distribution
BRAZIL: Amazonas (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Taffé Amaz. Brésil M. Soula det. 20//Holotype Pelidnota mantillerii S. Soula” (47030128). Genitalia card-mounted underneath the holotype. Box 4618654 SOULA.
Pelidnota matogrossensis
Pelidnota (Ganonota) matogrossensis Frey, 1976: 346 [original combination].
Strigidia matogrossensis
(Frey) [new combination by
Pelidnota (Strigidia) matogrossensis
Frey [revised combination and new subgeneric combination
Pelidnota matogrossensis
Frey [removal of subgeneric classification by
Distribution
BOLIVIA: Santa Cruz (BMNH). BRAZIL: Mato Grosso (
Types
1 ♂ holotype and paratypes Pelidnota (Ganonota) matogrossensis at NHMB (
Pelidnota micobalaguerae micobalaguerae
Strigidia micobalaguerae Soula, 2006: 10, 82 [original combination].
Pelidnota micobalaguerae micobalaguerae
(Soula) [new combination by
Distribution
ECUADOR: Guayas, Napo (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 5 ♂ paratypes, 1 ♀ paratype: “Cosanga Napo Ecuador 12/2005//Holotype 2006 Strigidia micobalaguerae S. Soula” (47030073); “Guacamayos Equateur M. SOULA det. 19//Paratype 2006 Strigidia micobalaguerae S. Soula” (47030074); “Antizana Guacamayos Napo Eq. M. SOULA det. 19 [obverse] V/2006//Paratype 2006 Strigidia micobalaguerae S. Soula” (47030075); “Lila [arrow] San Lorenzo coll. – SOULA [obverse] pk 7,5 770 m 15/08/93//Paratype 2006 Strigidia micobalaguerae S. Soula” (47030076); “ECUADOR OCCIDENTE GUAYAS Rte Machala-Guayaquil env. Naranjal niv. mer 29 janv. 79 Rec. Th. PORION//COLL. TH. PORION//Paratype 2006 Strigidia micobalaguerae S. Soula” (47030939); Two paratypes with identical label data: “ECUADOR OCCIDENTE GUAYAS Rte Machala-Guayaquil env. Naranjal niv. mer 29 janv. 79 Rec. Th. PORION//COLL. TH. PORION//Paratype Pelidnota micobalaguerae” (47030940 and 47030941). Genitalia card-mounted underneath the male holotype and four male paratypes. Box 4618650 SOULA and 4616343 PORION.
Pelidnota micobalaguerae occidentalis
Pelidnota micobalaguerae occidentalis Soula, 2009: 133 [original combination].
Distribution
ECUADOR: Cañar (
Types
The holotype male of Pelidnota micobalaguerae occidentalis is deposited in the Chichery Collection (
Pelidnota neitamorenoi neitamorenoi
Strigidia neitamorenoi Soula, 2006: 11, 50 [original combination].
Pelidnota neitamorenoi
(Soula) [new combination by
Distribution
BOLIVIA: La Paz (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype: “Caranavi 800m, 21/X/2000, M. SOULA det 19//Holotype 2005 Strigidia neitmorenoi (sic) Sou. Soula det.” (47030432); “Caranavi 800m, 21/X/2000, M. SOULA det 19//Allotype 2005 Strigidia neitmorenoi (sic) Sou. Soula det.” (47030433). The genitalia are card-mounted underneath the male holotype. Box 4618663 SOULA.
Pelidnota neitamorenoi rodriguezdemendozaensis
Pelidnota neitamorenoi rodriguezdemendozaensis Soula, 2010a: 59 [original combination].
Distribution
PERU: Amazonas (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Rodriguez de Mendosa 1600m Col.Galic 07.76//Holotype 2010 Pelidnota neitamorenoi S. rodriguezdemendozaensis Soula” (47030427). Genitalia are card-mounted underneath the male holotype. Box 4618663 SOULA.
Pelidnota nitescens
Rutela nitescens Vigors, 1825: 417 [original combination].
Pelidnota nitescens
(Vigors) [new combination by
Pelidnota (Ganonota) nitescens
(Vigors) [new subgeneric combination by
Pelidnota (Strigidia) nitescens
(Vigors) [new subgeneric combination by
Pelidnota (Odontognathus) nitescens
(Vigors) [new subgeneric combination by
Strigidia nitescens
(Vigors) [new combination by
Pelidnota (Strigidia) nitescens
(Vigors) [revised combination and revised subgeneric combination by
Pelidnota nitescens
(Vigors) [removal of subgeneric classification by
synonym. Rutela strigata (Mannerheim, 1829)
Rutela strigata Mannerheim, 1829: 50 [original combination].
Pelidnota nitescens
(Vigors) [syn. by
Distribution
BRAZIL: Minas Gerais, Paraná, São Paulo (
Types
Holotype ♀ at BMNH with following labels: a) “Type” (round, white label with red circle, typeset), b) “5957 Vigors’ Coll.” (typeset), c) “Brazil” (typeset), d) “Rutela nitescens, Vigors type” (handwritten) and reverse side “identified from descriptions as Vigors’ type. GRA” (handwritten by Gilbert Arrow), e) “Rutela nitescens Vigors [female symbol] Det. Jameson 2000 Holotype (red label, printed and handwritten), f) “Holotype Rutela nitescens Vig. 2006 Soula” (red label, printed and handwritten), g) “Strigidia nitescens (Vig.) M. SOULA det. 19 2006” (white label, printed and handwritten).
Remarks
This distinctive species possesses striate, reddish-brown and black-striped elytra. Adults have been recorded feeding on leaves of Psidium granifolium Mart. ex DC. (Myrtaceae).
Pelidnota notata
Pelidnota notata Blanchard, 1851: 212 [original combination].
Pelidnota (Pelidnota) notata
Blanchard [new subgeneric combination by
Pelidnota notata
Blanchard [removal of subgeneric classification by
Distribution
BELIZE: Toledo (
Types
1 ♀ syntype at MNHN (
Remarks
CCECL contains a P. notata specimen labeled as a male alloréférent with the following data: 1 ♂ alloréférent: “Oaxaca (M) 9/69 //Alloreferent ♂ de Pelidnota notata (Bl.) M. SOULA det. 19 2007”. Genitalia card-mounted underneath specimen. Box 4618664 SOULA.
Pelidnota ohausi ohausi
Pelidnota (Ganonota) ohausi Frey, 1976: 345 [original combination].
Strigidia ohausi
(Frey) [new combination by
Pelidnota (Strigidia) ohausi
Frey [revised combination and revised subgeneric combination by
Pelidnota ohausi ohausi
Frey [removal of subgeneric classification by
Distribution
BRAZIL: Mato Grosso (
Pelidnota ohausi piurensis
Strigidia ohausi piurensis Soula, 2006: 22 [original combination].
Pelidnota (Strigidia) ohausi piurensis
(Soula) [new combination and new subgeneric combination by
Pelidnota ohausi piurensis
(Soula) [removal of subgeneric classification by
Distribution
PERU: Piura (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Carabal, Rio Itaya Piura, Pérou, IX/2005//Holotype 2006 Strigidia ohausi piurensis Sou. Soula” (47030285). Genitalia card-mounted underneath male holotype. Box 4618658 SOULA.
Pelidnota osculatii
Pelidnota osculatii Guérin-Méneville, 1855: 585 [original combination].
Pelidnota (Chalcoplethis) osculatii
Guérin-Méneville [new subgeneric combination by
Strigidia osculatii
(Guérin-Méneville) [new combination by
Pelidnota osculatii
Guérin-Méneville [revised combination by
Distribution
COLOMBIA: Boyacá, Cundinamarca (
Types
The following specimen is deposited at CCECL. 1 invalid ♂ neotype: “Iquitos, V/2002, M. SOULA det 19//Neotype Pelidnota osculatii Gué. 2010 Soula det.” (47030356). Genitalia card-mounted underneath invalid male neotype. Box 4618662 SOULA.
Remarks
The original description of P. osculatii indicated that there were two specimens in the type series.
Pelidnota pallidipennis
Pelidnota pallidipennis F. Bates, 1904: 258, 268–269 [original combination].
Pelidnota (Pelidnota) pallidipennis
F. Bates [new subgeneric combination by
Pelidnota pallidipennis
F. Bates [removal of subgeneric classification by
Distribution
BRAZIL: Bahia, Goiás, Mato Grosso, Minas Gerais, Pernambuco, São Paulo (F.
Types
1 ♂ lectotype of Pelidnota pallidipennis at BMNH (
Pelidnota paraguayensis
Pelidnota paraguayensis F. Bates, 1904: 258, 266–267 [original combination].
Pelidnota (Pelidnota) fulva
Blanchard [syn. by
Pelidnota paraguayensis
F. Bates [revised species status by
Distribution
PARAGUAY: Asunción (F.
Types
1 ♂ lectotype of Pelidnota paraguayensis at BMNH (
Remarks
Pelidnota parallela
Pelidnota (Pelidnota) parallela Hardy, 1975: 6, 28–30 [original combination].
Pelidnota parallela
Hardy [removal of subgeneric classification by
Distribution
COLOMBIA: Chocó, Santander, Valle del Cauca (
Types
1 ♂ holotype and 1 ♀ allotype of Pelidnota (Pelidnota) parallela at CNC (
Pelidnota parvasedmagnifica
Strigidia parvasedmagnifica Soula & Moragues, 2006: 12, 74–75 [original combination].
Pelidnota parvasedmagnifica
(Soula and Moragues) [new combination by
Distribution
FRENCH GUIANA (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 11 ♂ paratypes, 15 ♀ paratypes: “Petit Saut pk 9 coll. – SOULA [obverse] 12/08/99//Holotype 2005 Strigidia parvasedmagnifica Sou. Soula det.” (47030235); “Piste DANGER Dd SARAMACA GUYANE FR 12 VIII 1988 [M. Duranton]//Allotype 2005 Strigidia parvasedmagnifica Sou. Soula det.” (47030236); Two paratypes with identical label data “GUYANE FRANCAISE Forêt de Tamanoir Pk 47 PL 7/8 IX 2002 M. DURANTON Coll.//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030251, exch13); “GUYANE FRANCAISE Ft de Tamanoir Pk 49 PL 7/8 IX 2004 M & S DURANTON Coll.//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030250); “GUYANE FRANCAISE Forêt de Tamanoir Pk 51 PL 23/24 VIII 2003 M & S DURANTON Coll.//Paratype 2006 Strigidia parvasedmagnifica Sou. Soula” (47030249); “GUYANE FRANCAISE Forêt de Patagaïe Pk 10 PL 10/11 VIII 2002 M. DURANTON Coll.//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030252); “GUYANE FRANCAISE Forêt de Patagaïe Pk 10 PL 10/11 IX 2004 M & S DURANTON Coll.//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030253); Three paratypes with identical label data “GUYANE FRANCAISE Saut Ananas Haute Mana 20/27 IX 1995 M DURANTON Coll.//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030254, 47030255, exch14); “Guyane française M. SOULA det 19//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030256); “Pte. de Kaw pk 37,5 3/III/98 M. SOULA det 19//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030257); “Mgne de Singes Dd Saramaca Guyane Fr. 19 VII 1990//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030243); “Mgne de Singes Dd Saramaca Guyane Fr. 19 VII 1990//Paratype 2006 Strigidia parvasedmagnifica Soul. Soula” (47030244); “Mgne des Singes Dd Saramaca GUYANE Fr. 19 VII 1990//Paratype Soula Strigidia parvasedmagnifica Sou. Soula” (exch15); “Dg. Saramaca G. F. 12/08/88 coll. – SOULA//Paratype 2005Strigidia parvasedmagnifica Sou. Soula det.” (47030248); “Dd Saramaca PK. Rte des Compagnons Guyane Fse 11.12 VIII 1990 M.Duranton Recolt.//Paratype 2005 Strigidia parvasedmagnifica S. Soula det.” (47030247); “Piste CORALIE RN2 GUYANE Fr. VIII 1990//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030246); “Dd Saramaca PK. Rte des Compagnons Guyane Fse 26.27 IX 1984 M.Duranton Recolt.//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030245); “CORALIE GUYANE Fr. 16.17 VIII 1990//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030242); “Dd Saramaca Mgne des Singes 13 IX 1989 M.Duranton Recolt.//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030241); “8.90 Piste St Elie Guyane//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030240); “Petit Saut pk 9 12/08/99 coll. – SOULA//Paratype 2005 Strigidia parvasedmagnifica Sou. Soula det.” (47030239); “Dd Saramaca PK. Rte des Compagnons Guyane Fse 16.17 VIII 1985 M.Duranton Recolt.//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030238); “Piste de BELIZON GUYANE FRSE VII 1994 D. CAMUS Leg//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030237); “GUYANE F. St-Jean/Maroni 5.2.78 PORION//COLL. TH. PORION//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030943); “GUYANE F. St-Jean/Maroni 2.I.78 PORION//COLL. TH. PORION//Paratype 2006 Strigidia parvasedmagnifica S. Soula” (47030944). Genitalia card-mounted underneath the male holotype and nine male paratypes. Box 4618657 SOULA and 4616343 PORION.
Pelidnota pennata
Pelidnota pennata Ohaus, 1912: 298, 299–300 [original combination].
Pelidnota (Chalcoplethis) pennata
Ohaus [new subgeneric combination by
Strigidia pennata
(Ohaus) [new combination by
Pelidnota pennata
Ohaus [revised combination by
Distribution
BRAZIL: Amazonas, Pará (
Types
1 ♂ lectotype of Pelidnota pennata at ZMHB (
Pelidnota perplexa
Pelidnota (Pelidnota) perplexa Hardy, 1975: 7, 15–16 [original combination].
Pelidnota perplexa
Hardy [removal of subgeneric classification by
Distribution
MEXICO: Hidalgo, Nuevo León, San Luis Potosí, Veracruz (
Types
1 ♂ holotype of Pelidnota (Pelidnota) perplexa at USNM (
Pelidnota peslieri
Pelidnota peslieri Soula, 2009: 133 [original combination]
Distribution
PERU: Loreto (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Iquitos; Loreto Pérou ; XI-XII/2004//Holotype 2008 Strigidia peslieri S. Soula” (47030127). Genitalia mounted underneath the holotype. Box 4618654 SOULA.
Pelidnota polita cupritarsis
Pelidnota cupritarsis H. W. Bates, 1888: 275 [original combination].
Pelidnota lucida
Burmeister [syn. by F.
Pelidnota (Pelidnota) polita
(Latreille) [syn. by
Pelidnota polita cupritarsis
H. W. Bates [removal of subgeneric classification and new subspecific status by
Distribution
COLOMBIA (H. W. Bates 1888,
Types
1 ♀ lectotype and 1 paralectotype of Pelidnota cupritarsis at MNHN (
Remarks
This subspecies is apparently sympatric with the nominal subspecies. The validity of the taxon should be addressed in future studies.
Pelidnota polita polita
Rutela polita Latreille, 1812: 134 [original combination].
Pelidnota polita
(Latreille) [new combination by
Pelidnota (Pelidnota) polita
(Latreille) [new subgeneric combination by
Pelidnota polita polita
(Latreille) [removal of subgeneric classification and new subspecific status by
Distribution
BRAZIL (
Types
1 ♂ neotype of Rutela polita at MNHN (
Pelidnota porioni
Pelidnota porioni Soula, 2010a: 59-60 [original combination].
Distribution
PERU: Cusco (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♀ paratype: “Pérou Cuzco Rte Cuzco Manu K 171 700 m 12/14-XII-79 T. Porion leg.//Holotype Pelidnota porioni S. 2009 Soula” (47030945). “Pérou Cuzco Rte Cuzco Manu K 160 1200 m 9/12-XII-79 T. Porion leg.//Allotype Pelidnota porioni S. 2009 Soula” (47030946); “Pérou Cuzco Rte Cuzco Manu K 151 1650 m 15/18-XII-79 T. Porion leg.//Paratype Pelidnota porioni S. 2009 Soula” (47030947). Genitalia card-mounted underneath the male holotype. Box 4616343 PORION.
Pelidnota prasina
Pelidnota prasina Burmeister, 1844: 402–403 [original combination].
Pelidnota (Pelidnota) prasina
Burmeister [new subgeneric combination by
Pelidnota prasina
Burmeister [removal of subgeneric classification by
Distribution
BRAZIL: Rondônia (WBWC). COLOMBIA: Antioquia, Boyacá, Cauca, Caquetá, Casanare, Cundinamarca, Meta, Risaralda, Santander, Tolima, Valle del Cauca (
Types
1 ♂ lectotype and 2 paralectotypes of Pelidnota prasina at MLUH (
Pelidnota prolixa
Pelidnota prolixa Sharp, 1877: 132–133 [original combination].
Pelidnota (Pelidnota) prolixa
Sharp [new subgeneric combination by
Pelidnota prolixa
Sharp [removal of subgeneric classification by
Distribution
COLOMBIA: Chocó, Valle del Cauca (
Types
1 ♀ lectotype of Pelidnota prolixa at BMNH (
Pelidnota pulchella altoparanaensis
Strigidia pulchella altoparanaensis Soula, 2006: 29 [original combination].
Pelidnota (Strigidia) pulchella altoparanaensis
(Soula) [new combination and new subgeneric combination by
Pelidnota pulchella altoparanaensis
(Soula) [removal of subgeneric classification by
Distribution
PARAGUAY (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 3 ♀ paratypes: “Ht Parana Paraguay III 2005 M. Soula det 19//Holotype 2006 Strigidia pulchella altoparanaensis S. Soula.” (47030313); “Ht Parana Paraguay III/2005 M. Soula det 19//Allotype 2006 Strigidia pulchella altoparanaensis S. Soula.” (47030314); Three paratypes with identical label data “Ht Parana Paraguay III/2005 M. Soula det 19//Paratype 2006 Strigidia pulchella altoparanaensis S. Soula.” (47030315 to 47030317). Genitalia card-mounted underneath the male holotype and the female allotype. Box 4618660 SOULA.
Pelidnota pulchella pulchella
Rutela pulchella Kirby, 1819: 405–406 [original combination].
Pelidnota pulchella
(Kirby) [new combination by
Rutela pulchella
(Kirby) [new combination by
Pelidnota pulchella
(Kirby) [revised combination by
Pelidnota (Ganonota) pulchella
(Kirby) [new subgeneric combination by
Pelidnota (Strigidia) pulchella
(Kirby) [new subgeneric combination by
Pelidnota (Odontognathus) pulchella
(Kirby) [new subgeneric combination by
Strigidia pulchella
(Kirby) [new combination by
Pelidnota (Strigidia) pulchella
(Kirby) [revised combination and revised subgeneric combination by
Pelidnota pulchella pulchella
(Kirby) [removal of subgeneric classification by
synonym. Pelidnota pulchella blanda Burmeister, 1844
Pelidnota pulchella var. blanda Burmeister, 1844: 394 [original combination, name is available as a subspecies per ICZN Article 45.6.4].
Pelidnota pulchella forma blanda Burmeister [revised infrasubspecific status by
Pelidnota pulchella var. blanda Burmeister [revised infrasubspecific status by
Pelidnota pulchella pulchella (Kirby) [syn. n.].
synonym. Pelidnota pulchella scapularis Burmeister, 1844
Pelidnota pulchella var. scapularis Burmeister, 1844: 394 [original combination, name is available as a subspecies per ICZN Article 45.6.4].
Pelidnota pulchella forma scapularis Burmeister [revised infrasubspecific status by
Pelidnota pulchella var. scapularis Burmeister [revised infrasubspecific status by
Pelidnota pulchella pulchella (Kirby) [syn. n.].
synonym. Pelidnota xanthogramma Perty, 1830
Pelidnota xanthogramma Perty, 1830: 49 [original combination].
Pelidnota pulchella var. xanthogramma Perty [new infrasubspecific status by
Pelidnota pulchella forma xanthogramma Perty [revised infrasubspecific status by
Pelidnota pulchella var. xanthogramma Perty [revised infrasubspecific status by
Pelidnota pulchella pulchella (Kirby) [syn. n.].
Distribution
ARGENTINA (
Types
Remarks
Three names were proposed by
Two “varieties” of P. pulchella pulchella were described by
Pelidnota punctata
Scarabaeus punctatus Linnaeus, 1758: 350 [original combination].
Melolontha punctata
(Linnaeus) [new combination by
Rutela punctata (Linnaeus) [new combination by Latreille 1802: 151].
Pelidnota punctata
(Linnaeus) [new combination by
Pelidnota (Pelidnota) punctata
(Linnaeus) [new subgeneric combination by
Pelidnota punctata
(Linnaeus) [removal of subgeneric classification by
synonym. Pelidnota francoisgenieri Moore & Jameson, 2013 [syn. n.].
synonym.
Pelidnota genieri
Pelidnota francoisgenieri
[replacement name by
Pelidnota punctata (Linnaeus) [syn. n.].
synonym. Melolontha lutea Olivier, 1789
Melolontha lutea Olivier, 1789: 23 [original combination].
Pelidnota punctata
(Linnaeus) [syn. by
Pelidnota (Pelidnota) lutea
(Olivier) [new subgeneric combination and revised species status by
Pelidnota (Pelidnota) punctata lutea
(Olivier) [new subspecific status by
Pelidnota (Pelidnota) lutea
(Olivier) [revised species status by
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
Pelidnota lutea
(Olivier) [removal of subgeneric classification and revised species status by
Pelidnota punctata (Linnaeus) [revised synonymy].
synonym. Pelidnota (Pelidnota) lutea brevicollis Casey, 1915
Pelidnota (Pelidnota) lutea brevicollis Casey, 1915: 74 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
synonym. Pelidnota (Pelidnota) lutea hudsonica Casey, 1915
Pelidnota (Pelidnota) lutea hudsonica Casey, 1915: 74 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
synonym. Pelidnota (Pelidnota) lutea pallidipes Casey, 1915
Pelidnota (Pelidnota) lutea pallidipes Casey, 1915: 74 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
synonym. Pelidnota (Pelidnota) oblonga debiliceps Casey, 1915
Pelidnota (Pelidnota) oblonga debiliceps Casey, 1915: 73 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
synonym. Pelidnota (Pelidnota) oblonga oblonga Casey, 1915
Pelidnota (Pelidnota) oblonga oblonga Casey, 1915: 72-73 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
synonym. Pelidnota (Pelidnota) oblonga ponderella Casey, 1915
Pelidnota (Pelidnota) oblonga ponderella Casey, 1915: 73 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
synonym. Pelidnota (Pelidnota) punctata brevis Casey, 1915
Pelidnota (Pelidnota) punctata brevis Casey, 1915: 72 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
synonym. Pelidnota (Pelidnota) punctata strenua Casey, 1915
Pelidnota (Pelidnota) punctata strenua Casey, 1915: 72 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
synonym. Pelidnota (Pelidnota) tarsalis Casey, 1915
Pelidnota (Pelidnota) tarsalis Casey, 1915: 74 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
synonym. Pelidnota (Pelidnota) lutea texensis Casey, 1915
Pelidnota (Pelidnota) lutea texensis Casey, 1915: 74 [original combination].
Pelidnota (Pelidnota) punctata
(Linnaeus) [syn. by
Pelidnota texensis
Casey [removal of subgeneric classification and new species status by
Pelidnota punctata (Linnaeus) [revised synonymy].
Distribution
CANADA: Manitoba, Ontario, Quebec (
USA: Alabama, Arkansas, Arizona, Connecticut, Delaware, District of Columbia, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Virginia, West Virginia, Wisconsin (
Types
The Scarabaeus punctatus Linnaeus, 1758 lectotype ♀ (Fig.
The Melolontha lutea Olivier, 1789 neotype ♂ is deposited at CMNC labeled a) “FLORIDA: Monroe Co. / Big Pine Key / Watsons Hammock / 6–30. VII.81 S. Peck / for. intercept-mal” (typeset), b) “Neotype 2009 / Melolontha / lutea Ol / Soula det.” (red label, typeset and handwritten), c) “Pelidnota / lutea (Ol.) / M Soula det 20” (handwritten and typeset), d) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂”.
The Pelidnota brevicollis Casey, 1915 lectotype ♂ is deposited at USNM labeled a) “Jacksnvle / 8.02 Fla.” (typeset and handwritten), b) “♂” (typeset) c) “CASEY / bequest / 1925” (typeset), d) “TYPE USNM / 48537” (red label, typeset and handwritten), e) “brevicollis / Csy” (handwritten), f) “PELIDNOTA / BREVICOLLIS / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♂” (red label, handwritten and typeset), g) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂” (typeset). Lectotype here designated.
The Pelidnota brevis Casey, 1915 lectotype ♀ is deposited at USNM labeled a) “Brooklyn” (light blue label, handwritten), b) “CASEY / bequest / 1925” (typeset), c) “TYPE USNM / 48529” (red label, typeset and handwritten), d) “brevis / Csy.” (handwritten), e) “PELIDNOTA BREVIS / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♀” (red label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). Lectotype here designated.
The Pelidnota debiliceps Casey, 1915 lectotype ♂ is deposited at USNM labeled a) “Atlantic City, / N.J.” (handwritten), b) “CASEY / bequest / 1925” (typeset), c) “TYPE USNM / 48533” (red label, typeset and handwritten), d) “debiliceps / Csy.” (handwritten), e) “PELIDNOTA DEBILICEPS / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♀” (red label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). Lectotype here designated.
The Pelidnota hudsonica Casey, 1915 lectotype ♂ is deposited at USNM labeled a) “CASEY / bequest / 1925” (typeset), b) “hudsonica- 2 / PARATYPE USNM / 48536” (red label, typeset and handwritten), c) “PELIDNOTA HUDSONICA / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♂” (red label, handwritten and typeset), d) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂” (typeset). Lectotype here designated. One paralectotype ♀ is deposited at USNM labeled a) “Peekskill / NY” (typeset), b) “CASEY / bequest / 1925” (typeset), c) “TYPE USNM / 48536” (red label, handwritten and typeset), d) “hudsonica / Csy.” (handwritten), e) “PELIDNOTA HUDSONICA / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). One paralectotype ♀ is deposited at USNM labeled a) (round white label), b) “CASEY / bequest / 1925” (typeset), c) “CASEY determ. / hudsonica-3” (typeset and handwritten), d) “PELIDNOTA HUDSONICA / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), e) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset).
The Pelidnota oblonga Casey, 1915 lectotype ♂ is deposited at USNM labeled a) “La.” (typeset), b) “CASEY / bequest / 1925” (typeset), c) “TYPE USNM / 48532” (red label, typeset and handwritten), d) “oblonga / Csy.” (handwritten), e) “PELIDNOTA / OBLONGA / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♂” (red label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂” (typeset). Lectotype here designated. One paralectotype ♀ is deposited at USNM labeled a) “La.” (typeset), b) “CASEY / bequest / 1925” (typeset), c) “CASEY determ. / ponderella 2” (typeset and handwritten), d) “Paratype of / oblonga” (red label, handwritten), e) “PELIDNOTA / OBLONGA / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset).
The Pelidnota pallidipes Casey, 1915 lectotype ♂ is deposited at USNM labeled a) “Newport News, Va / 6/25/89” (typeset and handwritten), b) “♂” (typeset), c) “CASEY / bequest / 1925” (typeset), d) “TYPE USNM / 48535” (red label, typeset and handwritten), e) “pallidipes / Csy.” (handwritten), f) “PELIDNOTA / PALLIDIPES / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♂” (red label, handwritten and typeset), g) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂” (typeset). Lectotype here designated. One paralectotype ♂ is deposited at USNM labeled a) “Southern Pines / VI-26 N.C 01 / A. H. Maneo” (typeset), b) “CASEY / bequest / 1925” (typeset), c) “CASEY determ. / pallidipes-9” (typeset and handwritten), d) “PELIDNOTA / PALLIDIPES / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♂” (yellow label, handwritten and typeset), e) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂” (typeset). One paralectotype ♂ is deposited at USNM labeled a) “Del” (handwritten), b) “♂” (typeset), c) “CASEY / bequest / 1925” (typeset), d) “CASEY determ. / pallidipes-2” (typeset and handwritten), e) “PELIDNOTA / PALLIDIPES / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♂” (yellow label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂” (typeset). One paralectotype ♂ is deposited at USNM labeled a) “Va” (typeset), b) “♂” (typeset), c) “CASEY / bequest / 1925” (typeset), d) “pallidipes. 3 / PARATYPE USNM / 48535” (red label, typeset and handwritten), e) “PELIDNOTA / PALLIDIPES / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♂” (yellow label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂” (typeset). One paralectotype ♀ is deposited at USNM labeled a) “Newport News, Va / 6/10/89” (typeset and handwritten), b) “♀” (typeset), c) “CASEY / bequest / 1925” (typeset), d) “pallidipes. 4 / PARATYPE USNM / 48535” (red label, typeset and handwritten), e) “PELIDNOTA / PALLIDIPES / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). One paralectotype ♀ is deposited at USNM labeled a) “Miss” (typeset), b) “CASEY / bequest / 1925” (typeset), c) “pallidipes. 6 / PARATYPE USNM / 48535” (red label, typeset and handwritten), d) “PELIDNOTA / PALLIDIPES / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), e) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). One paralectotype ♀ is deposited at USNM labeled a) “Miss” (typeset), b) “CASEY / bequest / 1925” (typeset), c) “pallidipes. 7 / PARATYPE USNM / 48535” (red label, typeset and handwritten), d) “PELIDNOTA / PALLIDIPES / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), e) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). One paralectotype ♀ is deposited at USNM labeled a) “Miss” (typeset), b) “♀” (handwritten), c) “CASEY / bequest / 1925” (typeset), d) “pallidipes. 8 / PARATYPE USNM / 48535” (red label, typeset and handwritten), e) “PELIDNOTA / PALLIDIPES / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). One paralectotype female at USNM labeled a) “Jacksnvle / Fla” (typeset), b) “♀” (handwritten), c) “CASEY / bequest / 1925” (typeset), d) “CASEY determ. / pallidipes-5” (typeset and handwritten), e) “PELIDNOTA / PALLIDIPES / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset).
The Pelidnota ponderella Casey, 1915 lectotype ♀ is deposited at USNM labeled a) “NE / U.S.” (handwritten), b) “CASEY / bequest / 1925” (typeset), c) “TYPE USNM / 48534” (red label, typeset and handwritten), d) “ponderella / Csy.” (handwritten), e) “PELIDNOTA / PONDERELLA / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♀” (red label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). Lectotype here designated.
The Pelidnota strenua Casey, 1915 lectotype ♀ is deposited at USNM labeled a) (pink disc with no text), b) “CASEY / bequest / 1925” (typeset), c) “TYPE USNM / 48528” (red label, typeset and handwritten), d) “strenua / Csy” (handwritten), e) “PELIDNOTA / STRENUA / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♀” (red label, handwritten and typeset), f) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). Lectotype here designated.
The Pelidnota tarsalis Casey, 1915 lectotype ♀ is deposited at USNM labeled a) “Peekskill / NY” (handwritten), b) “♀” (handwritten), c) “CASEY / bequest / 1925” (typeset), d) “TYPE USNM / 48539” (red label, typeset and handwritten), e) “tarsalis / Csy.” (handwritten), f) “PELIDNOTA / TARSALIS / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♀” (red label, handwritten and typeset), g) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). Lectotype here designated.
The Pelidnota texensis Casey, 1915 lectotype ♂ is deposited at USNM labeled a) “Horristo / Tex.” (handwritten), b) “CASEY / bequest / 1925” (typeset), c) “texensis- 3 / PARATYPE USNM / 48538” (red label, typeset and handwritten), d) “PELIDNOTA / TEXENSIS / CASEY, 1915 / LECTOTYPE / A.B.T. SMITH ♂” (red label, handwritten and typeset), e) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂” (typeset). Lectotype here designated. One paralectotype ♂ is deposited at USNM labeled a) “Horristo / Tex.” (handwritten), b) “CASEY / bequest / 1925” (typeset), c) “texensis- 4 / PARATYPE USNM / 48538” (red label, handwritten and typeset), d) “PELIDNOTA / TEXENSIS / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♂” (yellow label, handwritten and typeset), e) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♂” (typeset). One paralectotype ♀ is deposited at USNM labeled a) “Horristo / Tex.” (handwritten), b) “♀” (handwritten), c) “CASEY / bequest / 1925” (typeset), d) “TYPE USNM / 48538” (red label, handwritten and typeset), e) “texensis / Csy” (handwritten), f) “PELIDNOTA / TEXENSIS / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), g) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset). One paralectotype ♀ is deposited at USNM labeled a) “Horristo / Tex.” (handwritten), b) “CASEY / bequest / 1925” (typeset), c) “texensis- 2 / PARATYPE USNM / 48538” (red label, handwritten and typeset), d) “PELIDNOTA / TEXENSIS / CASEY, 1915 / PARALECTOTYPE / A.B.T. SMITH ♀” (yellow label, handwritten and typeset), e) “Pelidnota / punctata (Linnaeus) / Det: A.B.T. Smith 2015 ♀” (typeset).
The Pelidnota genieri Soula, 2009 holotype ♂ is deposited at CMNC labeled a) “Ottawa, ONT. / 5. VIII.1971 / J. E. H. Martin” (typeset), b) “C4269” (typeset), c) “CANADIAN / SCARAB / DATABASE / CSD014159” (typeset with two-dimensional barcode), d) “Holotype 2009 / Pelidnota / genieri S. / Soula” (red label, typeset and handwritten), e) “PELIDNOTA PUNCTATA (LINNAEUS) ♂ / Det:A.B.T.Smith 2015”. Allotype ♀ is deposited at CMNC labeled a) “Ottawa, ONT. / 5. VIII.1971 / J. E. H. Martin” (typeset), b) “C4280” (typeset), c) “Pelidnota / punctata / “(L.) / Det. J. McNamara 1974” (handwritten and typeset), d) “Allotype 2009 / Pelidnota / genieri S. / Soula” (red label, typeset and handwritten), e) “Canadian Museum of / Musée canadien de la / NATURE / CMNEN 00028000” (typeset with two-dimensional barcode), “PELIDNOTA PUNCTATA (LINNAEUS) ♀ / Det:A.B.T.Smith 2015”. 3 male paratypes, and 3 female paratypes at CMNC; 1 ♂ paratype labeled a) “Ottawa, ONT. / 5. VIII.1971 / J. E. H. Martin” (typeset), b) “C4302” (typeset), c) “CANADIAN / SCARAB / DATABASE / CSD014160” (typeset with two-dimensional barcode), d) “PELIDNOTA PUNCTATA (LINNAEUS) ♂ / Det:A.B.T.Smith 2015” e) “Paratype 2009 / Pelidnota / genieri S. / Soula” (red label, typeset and handwritten); 1 ♂ paratype labeled a) “ONT.: Ottawa / 14. VII.1988 / I. Dworakowska” (typeset), b) “CANADIAN / SCARAB / DATABASE / CSD014162” (typeset with two-dimensional barcode), c) “PELIDNOTA PUNCTATA (LINNAEUS) ♂ / Det:A.B.T.Smith 2015” d) “Paratype 2009 / Pelidnota / genieri S. / Soula” (red label, typeset and handwritten); 1 ♂ paratype labeled a) “OTTAWA, ONT. / VI. 1980 / H. & A. Howden / reared from elm / stump, 2 years as larva” (typeset and handwritten), b) “CANADIAN / SCARAB / DATABASE / CSD014163” (typeset with two-dimensional barcode), c) “PELIDNOTA PUNCTATA (LINNAEUS) ♂ / Det:A.B.T.Smith 2015” d) “Paratype 2009 / Pelidnota / genieri S. / Soula” (red label, typeset and handwritten); 1 ♀ paratype labeled a) “Ottawa, ONT. / 5. VIII.1971 / J. E. H. Martin” (typeset), b) “C4277” (typeset), c) “CANADIAN / SCARAB / DATABASE / CSD014165” (typeset with two-dimensional barcode), d) “PELIDNOTA PUNCTATA (LINNAEUS) ♀ / Det:A.B.T.Smith 2015” e) “Paratype 2008 / Pelidnota / genieri S. / Soula” (red label, typeset and handwritten); 1 ♀ paratype labeled a) “CANADA: Ont. / Ottawa / 16. VIII.1993 / H. & A. Howden” (typeset and handwritten), b) “CANADIAN / SCARAB / DATABASE / CSD014166” (typeset with two-dimensional barcode), c) “PELIDNOTA PUNCTATA (LINNAEUS) ♀ / Det:A.B.T.Smith 2015” e) “Paratype 2008 / Pelidnota / genieri S. / Soula” (red label, typeset and handwritten); 1 ♀ paratype labeled a) “Ottawa , Ont. / 14. VII 72 / H. F. HOWDEN” (handwritten), b) “CANADIAN / SCARAB / DATABASE / CSD014167” (typeset with two-dimensional barcode), c) “PELIDNOTA PUNCTATA (LINNAEUS) ♀ / Det:A.B.T.Smith 2015” e) “Paratype 2009 / Pelidnota / genieri S. / Soula” (red label, typeset and handwritten). The following specimens are deposited at CCECL. 2 ♂ paratypes, 3 ♀ paratypes: “OTTAWA, ONT. VII.5.76 M. SANBORNE//[matrix barcode] CANADIAN SCARAB DATABASE CSD014161//PELIDNOTA PUNCTATA (LINNAEUS) ♂ det. A.B.T.Smith 2015//Paratype 2009 Pelidnota genieri S. Soula” (47030610); “ONT. RUSSEL CO. Cumberland Village VII.31.72 L. Ling//[matrix barcode] CANADIAN SCARAB DATABASE CSD014164//PELIDNOTA PUNCTATA (LINNAEUS) ♂ det. A.B.T.Smith 2015//Paratype 2009 Pelidnota genieri S. Soula” (47030611); “CANADA: Ont. Ottawa 10. VIII.1992 N. House//[matrix barcode] CANADIAN SCARAB DATABASE CSD014168//PELIDNOTA PUNCTATA (LINNAEUS) ♀ det. A.B.T.Smith 2015//Paratype 2009 Pelidnota genieri S. Soula” (47030612); “Constance Bay Carieton Co. ONT VII.18.77//[matrix barcode] CANADIAN SCARAB DATABASE CSD014169//PELIDNOTA PUNCTATA (LINNAEUS) ♀ det. A.B.T.Smith 2015//Paratype 2009 Pelidnota genieri S. Soula” (47030613); “Ottawa, Ont. 12. VII.1977 A.T. Howden//Paratype 2009 Pelidnota genieri S. Soula//Pelidnota francoisgenieri Moore + Jameson 2013 det. MR Moore ‘15” (47030614). Genitalia card-mounted underneath one male paratype (47030614). Box 4618674 SOULA.
Pelidnota punctulata
Pelidnota punctulata H. W. Bates, 1888: 276 [original combination].
Pelidnota (Pelidnotidia) punctulata
H. W. Bates [new subgeneric combination by
Pelidnota (Pelidnota) punctulata
H. W. Bates [new subgeneric combination by
Pelidnota punctulata
H. W. Bates [removal of subgeneric classification by
Distribution
BELIZE: Toledo (H. W. Bates 1888,
Types
1 ♀ lectotype of Pelidnota punctulata at BMNH (
Pelidnota purpurea esperitosantensis
Strigidia purpurea esperitosantensis Soula, 2006: 34-35 [original combination].
Pelidnota (Strigidia) purpurea esperitosantensis
(Soula) [new combination and new subgeneric classification by
Pelidnota purpurea esperitosantensis
(Soula) [removal of subgeneric classification by
Distribution
BRAZIL: Espírito Santo (
Types
The following specimens are deposited at CMNC. 1 ♂ holotype, 1 ♀ allotype: “BRASIL E. SANTO. Linhares Sooretama NOV.62 A. Martínez//H. & A. HOWDEN COLLECTION ex. A. Martinez coll.//Pelidnota purpurea ♂ Burm. A. MARTINEZ-DET.1965//Holotype Pelid? Strigidia purpurea esperitosanten-sis Soula Soula”. Allotype with same labels except “♀” on determination label.
Remarks
Pelidnota purpurea purpurea
Pelidnota purpurea Burmeister, 1844: 394 [original combination].
Pelidnota (Ganonota) purpurea
Burmeister [new subgeneric combination by
Pelidnota (Strigidia) purpurea
Burmeister [new subgeneric combination by
Pelidnota (Odontognathus) purpurea
Burmeister [new subgeneric combination by
Strigidia purpurea
(Burmeister) [new combination by
Pelidnota (Strigidia) purpurea
Burmeister [revised combination and revised subgeneric combination by
Pelidnota purpurea purpurea
Burmeister [removal of subgeneric classification by
Distribution
BRAZIL: Rio de Janeiro (
Types
1 ♀ lectotype and 1 paralectotype of Pelidnota purpurea at MLUH (
Remarks
CCECL contains a P. purpurea purpurea specimen labeled as a male alloréférent with the following data: 1 ♂ alloréférent: “MUSÉUM PARIS Rio de Castelnau 1844//Alloréférent de Strigidia purpurea Burm. M. SOULA det 19” (47030312). Genitalia card-mounted underneath the male alloréférent. Box 4618659 SOULA.
Pelidnota quadripunctata
Pelidnota quadripunctata F. Bates, 1904: 253, 260 [original combination].
Pelidnota (Ganonota) quadripunctata
F. Bates [new subgeneric combination by
Pelidnota (Strigidia) quadripunctata
F. Bates [new subgeneric combination by
Pelidnota (Odontognathus) quadripunctata
F. Bates [new subgeneric combination by
Strigidia quadripunctata
(F. Bates) [new combination by
Pelidnota (Strigidia) quadripunctata
F. Bates [revised combination and revised subgeneric combination by
Pelidnota quadripunctata
F. Bates [removal of subgeneric classification by
Distribution
FRENCH GUIANA: Cayenne (F.
Types
1 ♀ holotype of Pelidnota quadripunctata at IRSNB (
Pelidnota recondita
Pelidnota (Pelidnota) recondita Delgado-Castillo, Deloya, & Morón, 1988: 132, 133–139 [original combination].
Pelidnota (Pelidnota) jalapensis
H. W. Bates [syn. by
Pelidnota recondita
Delgado-Castillo, Deloya, & Morón, 1988 [removal of subgeneric classification and revised species status by
Distribution
MEXICO: Guerrero (
Types
1 ♂ holotype, 1 ♀ allotype of Pelidnota (Pelidnota) recondita in MXAL; additional paratypes at ZMHB and IEXA (
Remarks
Pelidnota rioensis
Pelidnota arnaudi rioensis Soula, 2009: 73–74 [original combination].
Pelidnota rioensis Soula [new status].
Distribution
BRAZIL: Rio de Janeiro (
Types
Male holotype, female allotype, and three paratypes at MNHN (
Remarks
Because Pelidnota arnaudi arnaudi Soula is an unavailable name and P. arnaudi rioensis is available, it is given herein new status as P. rioensis.
Pelidnota rivascanteroi
Strigidia rivascanteroi Soula, 2006: 12, 54–55 [original combination].
Pelidnota rivascanteroi
(Soula) [new combination by
Distribution
BRAZIL: Ceará (
Types
The following specimens are deposited at CCECL. 1 ♀ holotype, 2 ♀ paratypes: “UBAJARA mt. 800 CEARÁ-BRASILE, GEN. 95 MIGLIOLI//Holotype 2006 Strigidia rivascanteroi Sou Soula” (47030424); “UBAJARA mt. 800, CEARÁ-BRASILE, GEN. 95 MIGLIOLI//Paratype 2006 Strigidia rivascanteroi Sou. Soula” (47030425); “Cametá//Paratype 2006 Strigidia rivascanteroi S. Soula” (47030426). Genitalia are card-mounted underneath the female holotype and a female paratype. Box 4618663 SOULA.
Remarks
Pelidnota rostrata
Pelidnota rostrata Burmeister, 1844: 406 [original combination].
Heteropelidnota rostrata
(Burmeister) [new combination by
Pelidnota rostrata
Burmeister [revised combination by
synonym. Pelidnota viridana Blanchard, 1851
Pelidnota viridana Blanchard, 1851: 213 [original combination].
Heteropelidnota rostrata
(Burmeister, 1844) [syn. by
Distribution
BRAZIL: Minas Gerais, Rio de Janeiro, Santa Catarina, São Paulo (
Types
1 ♀ lectotype and 1 paralectotype of Pelidnota rostrata at MLUH (
Remarks
Females of P. rostrata possess a longitudinal carina at the apex of the pygidium. Soula provided an image of the male parameres of P. rostrata (
Pelidnota rostrata Burmeister (male specimen compared with Burmeister’s syntype by Ohaus from the Weber Collection). A Dorsal habitus B Lateral habitus C Specimen labels and male genitalia D Male genitalia, lateral view E Male parameres, dorsal view.
Pelidnota rouchei
Strigidia rouchei Soula, 2006: 11, 76–77 [original combination].
Pelidnota rouchei
(Soula) [new combination by
Distribution
VENEZUELA: Merida (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♀ paratype: “VENEZUELA Edo. Merida NP Sierra Nevada, La Mucuy 2400m 13. IV.1995 leg. Hornburg, Krause//Holotype 2006 Strigidia rouchei Sou. Soula” (47030135); “Merida; 1850 m V/1999; Venez.//Allotype 2006 Strigidia rouchei Sou. Soula” (47030136); “Merida; 1850 m V/1999; Venez.//Paratype 2006 Strigidia rouchei Sou. Soula” (47030137). Genitalia card-mounted underneath the holotype. Box 4618654 SOULA.
Pelidnota rubripennis riedeli
Odontognathus riedeli Ohaus, 1905: 312–313 [original combination].
Pelidnota (Ganonota) riedeli
(Ohaus) [new combination and new subgeneric combination by
Pelidnota (Strigidia) riedeli
(Ohaus) [new subgeneric combination by
Pelidnota (Odontognathus) riedeli
(Ohaus) [new subgeneric combination by
Strigidia riedeli
(Ohaus) [new combination by
Strigidia rubripennis riedeli
(Ohaus) [new subspecific status by
Pelidnota (Strigidia) riedeli
(Ohaus) [revised combination and revised species status by
Pelidnota rubripennis riedeli (Ohaus) [revised status and revised combination].
Distribution
BRAZIL: São Paulo (
Types
1 ♂ syntype of Odontognathus riedeli was recorded by
Remarks
Pelidnota rubripennis rubripennis
Strigidia rubripennis Burmeister, 1844: 390 [original combination].
Odontognathus rubripennis
(Burmeister) [new combination by
Pelidnota (Ganonota) rubripennis
(Burmeister) [new combination and new subgeneric combination by
Pelidnota (Strigidia) rubripennis
(Burmeister) [new subgeneric combination by
Pelidnota (Odontognathus) rubripennis
(Burmeister) [new subgeneric combination by
Strigidia rubripennis
Burmeister [revised combination by
Pelidnota (Strigidia) rubripennis
(Burmeister) [revised combination and revised subgeneric combination by
Pelidnota rubripennis rubripennis
(Burmeister) [removal of subgeneric classification by
synonym. Pelidnota rufipennis Waterhouse, 1876
Pelidnota rufipennis Waterhouse, 1876: 23 [original combination].
Pelidnota (Ganonota) rubripennis
(Burmeister) [syn. by
Pelidnota (Strigidia) rubripennis forma rufipennis (Waterhouse) [new infrasubspecific status and new subgeneric combination by
Pelidnota (Odontognathus) rubripennis forma rufipennis (Waterhouse) [new subgeneric combination by
Strigidia rubripennis
Burmeister [syn. by
Distribution
BRAZIL: Pernambuco, Rio de Janeiro (
Types
1 ♂ syntype specimen of Strigidia rubripennis Burmeister is deposited at MLUH; 1 ♀ type specimen of Strigidia rufipennis (Waterhouse) is deposited at BMNH (Fig.
Pelidnota rubripennis rubripennis (Burmeister) (male specimen compared with Burmeister’s syntype by Ohaus from MLUH). A Dorsal habitus B Lateral habitus C Specimen labels and male genitalia D Male genitalia, lateral view E Male parameres, dorsal view.
Pelidnota rubriventris
Pelidnota rubriventris Blanchard, 1851: 213 [original combination].
Pelidnota (Chalcoplethis) rubriventris
Blanchard [new subgeneric combination by
Strigidia rubriventris
(Blanchard) [new combination by
Pelidnota rubriventris
Blanchard [revised combination by
Distribution
COLOMBIA: Antioquia, Boyacá, Valle del Cauca (
Types
Pelidnota rugulosa rugulosa
Pelidnota rugulosa Burmeister, 1844: 398–399 [original combination].
Pelidnota (Chalcoplethis) rugulosa
Burmeister [new subgeneric combination by
Strigidia rugulosa
(Burmeister) [new combination by
Pelidnota rugulosa rugulosa
Burmeister [revised combination by
Distribution
BRAZIL: Rio de Janeiro, Sáo Paulo (
Pelidnota rugulosa santacatarinensis
Strigidia rugulosa santacatarinensis Soula, 2006: 66 [original combination].
Pelidnota rugulosa santacatarinensis
(Soula) [new combination by
Distribution
BRAZIL: Santa Catarina (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 13 ♂ paratypes, 4 ♀ paratypes: “Sao Bento do Sul. 11/94 S.C. coll. – SOULA//Holotype Sou. Chalcoplethis rugulosa santacatarinensis Soula det. [obverse] 2005” (47030138); “Sao Bento do Sul (S. C.) 11/94 coll. – SOULA//Allotype Sou. 2005 Chalcoplethis rugulosa santacatarinensis Sou. Soula det.” (47030139); “Sao Bento do Sul S. C. 11/94 coll. – SOULA//Paratype 2005 Chalcoplethis rugulosa santacatarinensis S. Soula det.” (47030140); eight paratypes with identical label data “São Bento do Sul Santa Catarina Brésil//Paratype 2005 Chalcoplethis rugulosa santacatarinensis S. Soula det.” (47030141 to 47030146, exch06 and exch07); “Sao Bento S. C. coll. – SOULA//Paratype 2005 Chalcoplethis rugulosa santacatarinensis S. Soula det.” (47030147); “São Bento do Sul S. C. M. SOULA det 19//Nevinson Coll. 1918-14.//Paratype 2005 Chalcoplethis rugulosa santacatarinensis S. Soula det.” (47030148); two paratypes with identical label data “Rio Vermelho Santa Catarina Brésil M. SOULA det 19 [obverse] 12/89//Paratype 2005 Chalcoplethis rugulosa santacatarinensis S. Soula det.” (47030149 and 47030150); “Rio Vermelho Santa Catarina 12/89 Brasil M. SOULA det 19//Paratype 2005 Chalcoplethis rugulosa santacatarinensis S. Soula det.” (47030151); “Rio Vermelho Santa Catarina 12/89 M. SOULA det 19//Paratype Chalcoplethis rugulosa santacatarinensis S. Soula det.” (47030152); “Caripo alegre Santa Catarina BRESIL Fev. 1991 Coll. Th. Porion//Paratype 2005 Chalcoplethis rugulosa santacatarinensis S. Soula det.” (47030153). “JOINVILLE SANTA CATARINA DECEMBRE 1986 BRESIL T. PORION LEG.//Chalcoplethis rugulosa rugulosa//Paratype 2005 Chalcoplethis rugulosa santacatarinensis S. Soula det.” (47030154). Genitalia card-mounted underneath the holotype male, allotype female, thirteen male paratypes, and two female paratypes. Box 4618655 SOULA. The following specimen is deposited at CMNC. 1 ♀ paratypes: “BRASIL Sta. Catarina Sao Bento A. Maller-leg. Coll. Martínez Oct.-967//H. & A. HOWDEN COLLECTION ex. A. Martinez coll.//Pelidnota rugulosa ♂ Burm. A. MARTINEZ-DET.1969//Paratype 2006 Strigidia rugulosa santacatarinen-sis Soula Soula”.
Pelidnota sanctidomini caliensis
Strigidia santidomini (sic) caliensis Soula, 2006: 79 [original combination].
Pelidnota (Strigidia) sanctidomini caliensis
(Soula) [new combination and new subgeneric combination by
Pelidnota sanctidomini caliensis
(Soula) [removal of subgeneric classification by
Distribution
COLOMBIA: Valle del Cauca (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “(Colombie) Cali 06/92//Holotype 2006 Strigidia santidomini caliensis Soula Soula” (47030126). Genitalia card-mounted underneath the holotype. Box 4618654 SOULA.
Remarks
Pelidnota sanctidomini sanctidomini
Pelidnota sanctidomini Ohaus, 1905: 317 [original combination].
Pelidnota (Ganonota) sanctidomini
Ohaus [new subgeneric combination by
Pelidnota (Strigidia) sanctidomini
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) sanctidomini
Ohaus [new subgeneric combination by
Pelidnota (Strigidia) sanctidomini
Ohaus [revised subgeneric combination by
Strigidia sanctidomini
(Ohaus) [new combination by
Pelidnota (Strigidia) santidomini
(sic) Ohaus [revised combination and revised subgeneric combination by
Pelidnota santidomini santidomini
(sic) Ohaus [removal of subgeneric classification by
Pelidnota sanctidomini sanctidomini Ohaus [valid name].
synonym. Pelidnota pubes Ohaus, 1913: 502–503 [original combination].
Pelidnota (Ganonota) pubes
Ohaus [new subgeneric combination by
Pelidnota (Strigidia)
pubes
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) pubes
Ohaus [new subgeneric combination by
Strigidia santidomini
(sic) (Ohaus) [syn. by
Distribution
CENTRAL AMERICA (
Types
1 ♀ holoype specimen of Pelidnota sanctidomini sanctidomini Ohaus is deposited at ZMHB (Fig.
Remarks
Pelidnota pubes Ohaus syntype male from ZMHB (valid name Pelidnota sanctidomini sanctidomini Ohaus). A Dorsal habitus B Lateral habitus C Specimen labels, mouthparts, and male genitalia D Male parameres, dorsal view E Male genitalia, lateral view.
Pelidnota satipoensis
Pelidnota satipoensis Demez & Soula, 2010: 60–61 [original combination].
Distribution
PERU: Junín (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♂ paratype: “Rio Tambo Val Paraiso Tuncana M. SOULA det. 19 [obverse] XI/2007//Holotype Pelidnota satipoensis S. 2010 Soula” (47030077); “Rio Tambo Val Paraison (sic!) Tuncana M. SOULA det. 19 [obverse] Junin XI/2007//Allotype Pelidnota satipoensis S. 2010 Soula” (47030078); “Satipo, Junin Rio Negro, 1-15/IV/2010//Paratype Pelidnota satipoensis S. 2010 Soula” (47030079). Genitalia card-mounted underneath the male holotype and paratype specimens. Box 4618650 SOULA.
Pelidnota semiaurata citripennis
Pelidnota aeuruginosa citripennis Ohaus, 1900: 185 [original combination].
Pelidnota (Pelidnota) aeruginosa citripennis
Ohaus [new subgeneric combination by
Pelidnota semiaurata var. citripennis Ohaus [removal of subgeneric classification and new infrasubspecific status by
Pelidnota semiaurata citripennis
Ohaus [revised subspecific status by
Distribution
BRAZIL: Minas Gerais, Rio Grande do Sol (
Remarks
Both subspecific and infrasubspecific names (e.g., varieties) are used by
Pelidnota semiaurata semiaurata
Pelidnota glauca var. semiaurata Burmeister, 1844: 402 [original combination].
Pelidnota (Pelidnota) aeruginosa semiaurata
Burmeister [new subgeneric combination and new subspecific status by
Pelidnota semiaurata
Burmeister [removal of subgeneric classification and new species status by
Pelidnota semiaurata semiaurata Burmeister [revised status].
Distribution
BRAZIL: Rio de Janeiro (INPA), Rio Grande do Sol, Santa Catarina (
Types
1 ♂ lectotype and 1 paralectotype at ZMHB (
Pelidnota sericeicollis
Pelidnota (Ganonota) sericeicollis Frey, 1976: 344 [original combination].
Strigidia sericeicollis
(Frey) [new combination by
Pelidnota (Strigidia) sericeicollis
Frey [revised combination and new subspecific combination by
Pelidnota sericeicollis
Frey [removal of subgeneric classification by
Distribution
BRAZIL: Bahia (
Types
Pelidnota sikorskii
Strigidia sikorskii Soula, 2006: 10, 20-21 [original combination].
Pelidnota sikorskii
(Soula) [new combination by
Distribution
BRAZIL: Bahia (
Types
The following specimen is deposited at CCECL. 1 ♂ paratype: “Cachimbo Prov.de.Bahia Ch Pujol 1890//Museum Paris ex Coll. R. Oberthur//Paratype Strigidia sikorskii S. 2006 Soula” (47030431). Genitalia mounted underneath the male paratype. Box 4618663 SOULA.
Pelidnota soederstroemi
Pelidnota söderströmi Ohaus, 1908b: 402–403 [original combination].
Pelidnota (Ganonota) söderströmi Ohaus [new subgeneric combination by
Pelidnota (Strigidia) söderströmi Ohaus [new subgeneric combination by
Pelidnota (Strigidia) soederstroemi
Ohaus [justified emendation by
Pelidnota (Odontognathus) soederstroemi
Ohaus [new subgeneric combination by
Strigidia soederstroemi
(Ohaus) [new combination by
Pelidnota (Strigidia) soederstroemi
Ohaus [revised combination and revised subgeneric combination by
Pelidnota soederstroemi
Ohaus [removal of subgeneric classification by
Distribution
ECUADOR: Cotopaxi, Pichincha (
Types
Holotype ♀ of Pelidnota (Strigidia) soederstroemi at ZMHB (Fig.
Remarks
Pelidnota sordida
Rutela sordida Germar, 1824: 118–119 [original combination].
Pelidnota sordida
(Germar) [new combination by
Pelidnota (Pelidnota) sordida
(Germar) [new subgeneric combination by
Pelidnota sordida
(Germar) [removal of subgeneric classification by
Distribution
ARGENTINA (
Types
1 ♂ lectotype and 2 paralectotypes at ZMHB (
Pelidnota striatopunctata
Odontognathus striatopunctatus Kirsch, 1885: 222 [original combination].
Pelidnota (Ganonota) striatopunctata
(Kirsch) [new combination and new subgeneric combination
Pelidnota (Strigidia) striatopunctata
(Kirsch) [new subgeneric combination by
Pelidnota (Odontognathus) striatopunctata
(Kirsch) [new subgeneric combination by
Strigidia striatopunctata
(Kirsch) [new combination by
Pelidnota (Strigidia) striatopunctata
(Kirsch) [revised combination and revised subgeneric combination by
Pelidnota striatopunctata
(Kirsch) [removal of subgeneric classification by
Distribution
BOLIVIA: La Paz (
Types
1 ♀ syntype of Odontognathus striatopunctatus at MTD (
Remarks
CCECL contains a P. striatopunctata specimen labeled as a male alloréférent with the following data: 1 ♂ alloréférent: “Prov. de La Paz XI/2001 M. SOULA det 19 [obverse] Bolivie//Alloréferent ♂ de Strigidia striatopunctata (K.) M. SOULA det 19” (47030121). Genitalia card-mounted underneath the alloréférent male. Box 1418652 SOULA.
Pelidnota strigosa
Pelidnota strigosa Laporte, 1840: 122 [original combination].
Pelidnota (Pelidnotidia) strigosa
Laporte [new subgeneric combination by
Pelidnota (Pelidnota) strigosa
Laporte [new subgeneric combination by
Pelidnota strigosa
Laporte [removal of subgeneric classification by
synonym. Pelidnota (Pelidnotidia) cuprascens Casey, 1915
Pelidnota (Pelidnotidia) cuprascens Casey, 1915: 78 [original combination].
Pelidnota (Pelidnota) cuprascens
Casey [new subgeneric combination by
Pelidnota (Pelidnota) strigosa
Laporte [syn. by
synonym. Pelidnota (Pelidnotidia) obscurella Casey, 1915
Pelidnota (Pelidnotidia) obscurella Casey, 1915: 79 [original combination].
Pelidnota (Pelidnota) obscurella
Casey [new subgeneric combination by
Pelidnota (Pelidnota) strigosa
Laporte [syn. by
synonym. Pelidnota (Pelidnotidia) refulgens Casey, 1915
Pelidnota (Pelidnotidia) refulgens Casey, 1915: 79 [original combination].
Pelidnota (Pelidnota) refulgens
Casey [new subgeneric combination by
Pelidnota (Pelidnota) strigosa
Laporte [syn. by
Distribution
BELIZE (
Types
1 ♂ neotype of Pelidnota strigosa at MNHN (
Remarks
CCECL contains a specimen of P. strigosa that is labeled as a female alloréférent with the following data: 1 ♀ alloréférent: “Soteapan 500 m Vera Cruz, Mex M. SOULA det 19 [obverse] VIII/2006//Alloréferent ♀ de Strigidia strigosa (Lap.) M. SOULA det 19” (47030472). Genitalia card-mounted underneath the alloréférent female. Box 1418665 SOULA.
Pelidnota subandina orellanai
Strigidia subandina orellanai Soula, 2006: 58-59 [original combination].
Pelidnota subandina orellanai
(Soula) [new combination by
Distribution
VENEZUELA: Barinas, Táchira (
Types
1 ♂ holotype, 1 ♀ allotype and paratypes of Strigidia subandina orellanai at MIZA (
Pelidnota subandina subandina
Pelidnota subandina Ohaus, 1905: 316 [original combination].
Pelidnota (Chalcoplethis) subandina
Ohaus [new subgeneric combination by
Strigidia subandina
(Ohaus) [new combination by
Pelidnota subandina subandina
Ohaus [removal of subgeneric classification and new subspecific status by
Distribution
BRAZIL: Amazonas (
Types
1 ♂ syntype specimen of Pelidnota subandina Ohaus at ZMHB (Fig.
Pelidnota sumptuosa
Rutela sumptuosa Vigors, 1825: 542 [original combination].
Pelidnota sumtuosa
(Vigors) (sic) [new combination by
Pelidnota (Pelidnota) sumptuosa
(Vigors) [new subgeneric combination by
Pelidnota (Pelidnota) ludovici
Ohaus [syn. by
Pelidnota sumptuosa
(Vigors) [removal of subgeneric classification and revised species status by
synonym. Rutela smaragdina Perty, 1830
Rutela smaragdina Perty, 1830: 50 [original combination].
Pelidnota sumtuosa
(Vigors) (sic) [syn. by
Pelidnota (Pelidnota) luxuriosa
Blackwelder [syn. by
Pelidnota sumptuosa
(Vigors) [syn. by
synonym. Rutela smaragdina var. plicata Gory, 1846
Rutela smaragdina var. plicata Gory, 1846: 192 [original combination].
Pelidnota sumptuosa
(Vigors) [syn. by
Pelidnota (Pelidnota) luxuriosa
Blackwelder [syn. by
Pelidnota sumptuosa
(Vigors) [syn. by
Distribution
BRAZIL: Bahia, Goiás, Mato Grosso, Minas Gerais, Pará, São Paulo (
Types
1 ♀ holotype of Rutela sumptuosa at BMNH (
Remarks
Pelidnota sumptuosa and P. cyanitarsis are superficially similar and have been confused in collections and the literature. Both species are bright metallic blue, green, or blue-green with enlarged metatibia in male specimens. Several characters serve to separate these species (see “Remarks” for P. cyanitarsis), and male parameres are also diagnostic (see
Pelidnota teocuitlamayatli
Pelidnota (Pelidnota) teocuitlamayatli Delgado-Castillo, Deloya, & Morón, 1988: 132, 139–141 [original combination].
Pelidnota teocuitlamayatli
Delgado-Castillo, Deloya, and Morón [removal of subgeneric classification by
Distribution
MEXICO: Guerrero (
Types
The following specimen is deposited at CMNC. 1 ♂ holotype: “24 mi. south Iguala Gro. MEXICO VII 18 1963//H. & A. Howden Collection//HOLOTIPO//Pelidnota ♂ teocuitlamayatli Delgado, Deloya, Morón 1988. L.L. Delgado det. 1988.//CMNEN 1999-0383”.
Remarks
This species is metallic silver and thus it strongly resembles species in the genus Chrysina.
Pelidnota testaceovirens felipemezai
Strigidia testaceovirens felipemezai Soula, 2006: 62 [original combination].
Pelidnota (Strigidia) testaceovirens felipemezai
(Soula) [new combination and new subgeneric combination by
Pelidnota testaceovirens felipemezai
(Soula) [removal of subgeneric classification by
Distribution
PERU: Junín (
Types
The following specimens are deposited at CCECL. 10 ♂ paratypes, 4 ♀ paratypes: six paratypes with identical label data “Satipo, Junin Pérou, X/2003//Paratype 2006 Strigidia testaceovirens felipemezai S. Soula” (47030080 to 47030084, exch02); “Satipo, Junin Pérou, X/XI/2002//Paratype 2006 Strigidia testaceovirens felipemezai S. Soula” (47030085); “Satipo Pérou IX/2003 M. SOULA det. 19//Paratype Pelidnota testaceovirens mezai S. Soula” (47030086); “Satipo Junin Pérou M. SOULA det. 19//Paratype 2006 Strigidia testaceovirens felipemezai S. Soula” (47030087); “Satipo XI/2007 M. SOULA det. 19//Paratype 2004 Strigidia testaceovirens felipemezai S. Soula” (47030088); “Satipo (P) 10/88//Paratype 2006 Strigidia testaceovirens felipemezai S. Soula” (47030089); “Satipo E. Peru Dec. 2002 //Paratype 2006 Strigidia testaceovirens felipemezai S. Soula” (47030090); two paratypes with identical label data “Pérou Chanchamayo La Merced C. O. Schunke Recu Novembre 1904//Paratype 2006 Strigidia testaceovirens felipemezai S. Soula” (47030091 and 47030092). Genitalia card-mounted underneath five male paratypes and one female paratype. Box 4618651 SOULA.
Pelidnota testaceovirens noaensis
Pelidnota testaceovirens noaensis Soula, 2009: 134 [original combination].
Distribution
ARGENTINA: Jujuy (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 6 ♂ paratypes, 2 ♀ paratypes: “Calilegua, 1110m NOA, 26/01/06 Leg. P. Schmit//Holotype 2008 Pelidnota testaceovirens noaensis Soula” (47030111); “Calilegua, 1110m NOA, 26/01/06 Leg. P. Schmit//Allotype 2008 Pelidnota testaceovirens noaensis S. Soula” (47030112); eight paratypes with identical label data “Calilegua, 1110m NOA, 26/01/06 Leg. P. Schmit//Paratype 2008 Pelidnota testaceovirens noaensis Soula” (47030113 to 47030119, exch05). Genitalia card-mounted underneath male holotype. Box 4618651 SOULA.
Pelidnota testaceovirens testaceovirens
Pelidnota testaceovirens Blanchard, 1851: 213 [original combination].
Pelidnota (Ganonota) testaceovirens
Blanchard [new subgeneric combination by
Pelidnota (Strigidia) testaceovirens
Blanchard [new subgeneric combination by
Pelidnota (Odontognathus) testaceovirens
Blanchard [new subgeneric combination by
Strigidia testaceovirens
(Blanchard) [new combination by
Pelidnota (Strigidia) testaceovirens
Blanchard [revised combination and revised subgeneric combination by
Pelidnota testaceovirens testaceovirens
Blanchard [removal of subgeneric classification and new subspecies status by
Distribution
BOLIVIA: La Paz, Santa Cruz (
Types
1 ♀ lectotype at MNHN (
Pelidnota testaceovirens vittipennis
Pelidnota vittipennis F. Bates, 1904: 256, 264 [original combination].
Pelidnota testaceovirens
Blanchard [syn. by
Strigidia testaceovirens vittipennis
(F. Bates) [new combination and new subspecies status by
Pelidnota (Strigidia) testaceovirens vittipennis
F. Bates [revised combination and new subgeneric combination by
Pelidnota testaceovirens vittipennis
F. Bates [removal of subgeneric classification by
Distribution
ARGENTINA (
Pelidnota testaceovirens xinguensis
Strigidia testaceovirens xinguensis Soula, 2006: 62-63 [original combination].
Pelidnota (Strigidia) testaceovirens xinguensis
(Soula) [new combination and new subgeneric combination by
Pelidnota testaceovirens xinguensis
(Soula) [removal of subgeneric classification by
Distribution
BRAZIL: Pará (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “SAO FELIX DO XINGU 29-30-IX-1975//Holotype 2006 Strigidia testaceovirens xinguensis S. Soula” (47030120). Genitalia card-mounted underneath male holotype. Box 4618651 SOULA.
Pelidnota thiliezi
Pelidnota thiliezi Soula, 2009: 34, 112–113 [original combination].
Distribution
BRAZIL: Goiás (
Types
The following specimens are deposited at CCECL. 1 ♂ neotype, 25 ♂ paratypes, 9 ♀ paratypes, 2 probable ♂ paratypes: 21 paratypes with identical label data: “Goiana, Goyas Brésil, IX-X/95//Paratype Pelidnota thiliezi S. 2008-2009” (47030732 to 47030748, exch44 to exch47); “Goiana, Goyas Brésil, IX-X/95//Paratype Pelidnota grossiorum S. 2008-2009//Probable Pelidnota thiliezi C. Audibert 2016” (47030749); “Goias Goiañia coll. – SOULA//Paratype Pelidnota grossiorum S. 2008-2009//Probable Pelidnota thiliezi C. Audibert 2016” (47030750); five paratypes with identical label data: “Goias Goiañia coll. – SOULA//Paratype Pelidnota thiliezi S. 2008-2009” (47030751 to 47030755); three paratypes with identical label data: “Goias Goiañia 11/93 coll. – SOULA//Paratype Pelidnota thiliezi S. 2008-2009” (47030756 to 47030758); “Goiar K.P. Klausen 30/11 15//ZOOL. MUSEUM DK COPENHAGEN//Paratype Pelidnota thiliezi S. 2008-2009” (47030759); two paratypes with identical label data: “Goiar K.P. Klausen 30/11 1915//ZOOL. MUSEUM DK COPENHAGEN//Paratype Pelidnota thiliezi S. 2008-2009” (47030760 to 47030761). Neotype here designated and deposited at CCECL: “Goiana, Goyas Brésil, IX-X/95//Paratype Pelidnota thiliezi S. 2008-2009//Neotype Pelidnota thiliezi
Diagnosis.
Remarks
Pelidnota tibialis aenigmatica
Strigidia tibialis aenigmatica Soula, 2006: 47 [original combination].
Pelidnota (Strigidia) tibialis aenigmatica
(Soula) [new combination and new subgeneric combination by
Pelidnota tibialisaenigmatica
(sic) (Soula) [removal of subgeneric classification by
Distribution
BRAZIL (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Brésil. coll. – SOULA//Holotype 2006 Strigidia tibialis incerta S. Soula.//Holotype ♂ Strigidia tibialis aenigmatica
Remarks
The holotype specimen is labeled “Pelidnota tibialis incerta”, a name that is not found in the literature. We compared the holotype specimen (labeled “Pelidnota tibialis incerta”), description, and image (
Pelidnota tibialis pernambucoensis
Strigidia tibialis pernambucoensis Soula, 2006: 47 [original combination].
Pelidnota (Strigidia) tibialis pernambucoensis
(Soula) [new combination and new subgeneric combination by
Pelidnota tibialis pernambucoensis
(Soula) [removal of subgeneric classification by
Distribution
BRAZIL: Pernambuco (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Pernambuco Brésil M. SOULA det 19//Holotype 2006 Strigidia tibialis pernambucoensis Sou. Soula det.” (47030319). Genitalia card-mounted underneath the male holotype. Box 4618660 SOULA.
Pelidnota tibialis tibialis
Pelidnota tibialis Burmeister, 1844: 396–397 [original combination].
Pelidnota (Ganonota) tibialis
Burmeister [new subgeneric combination by
Pelidnota (Strigidia) tibialis
Burmeister [new subgeneric combination by
Pelidnota (Odontognathus) tibialis
Burmeister [new subgeneric combination by
Strigidia tibialis
(Burmeister) [new combination by
Pelidnota (Strigidia) tibialis
Burmeister [revised combination and revised subgeneric combination by
Pelidnota tibialis tibialis
Burmeister [removal of subgeneric classification and new subspecies status by
synonym. Pelidnota zikani Ohaus, 1922
Pelidnota zikani Ohaus, 1922: 324 [original combination].
Pelidnota (Ganonota) zikani
Ohaus [new subgeneric combination
Pelidnota (Strigidia) zikani
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) zikani
Ohaus [new subgeneric combination by
Pelidnota (Ganonota) zikani
Ohaus [revised subgeneric combination by
Strigidia tibialis
(Ohaus) [syn. by
Pelidnota (Strigidia) zikani
Ohaus [revised combination and revised subgeneric combination by
Pelidnota tibialis tibialis Burmeister [revised synonymy].
Distribution
BRAZIL: Minas Gerais, Rio de Janeiro (
Types
1 ♂ lectotype of P. tibialis tibialis Burmeister at MLUH (
Remarks
Pelidnota toulgoeti
Strigidia toulgoeti Soula, 2006: 11, 50–51 [original combination].
Pelidnota toulgoeti
(Soula) [new combination by
Distribution
PERU: Huánuco, Piura (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♀ paratype: “Carbajal, Rio Itaya, Piura Pérou 9/2005 M. SOULA det 19//Holotype 2006 Strigidia toulgoueti (sic) Sou. Soula” (47030434); “Carbajal, Rio Itaya Piura, Pérou, IX/2005//Allotype 2006 Strigidia toulgoueti (sic) Sou. Soula” (47030435); “Tingo Maria, Huanuco, Pérou 800m, III/2004//Paratype Strigidia toulgoeti Sou. Soula” (47030436). The genitalia are card-mounted underneath the male holotype and female paratype. Box 4618663 SOULA.
Pelidnota touroulti
Pelidnota touroulti Soula, 2008: 37–38 [original combination].
Distribution
FRENCH GUIANA (
Types
2 ♂ paratypes (= paralectotypes of Pelidnota cribrata [Ohaus]) at ZMHB (Fig.
Remarks
According to
Pelidnota ulianai
Pelidnota ulianai Soula, 2010a: 40-41 [original combination].
Distribution
BOLIVIA: La Paz (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 3 ♂ invalid paratypes, 4 ♀ invalid paratypes: “Inca Huara 1450 m. (Bo.) 11/94 coll. - SOULA//Holotype 2010 Pelidnota ulianai S. Soula” (47030215); “N. Yungas Bolivie coll. - SOULA//Allotype 2010 Pelidnota ulianai S. Soula” (47030216); “Route de Coroico à Coranavi [pro Caranavi] (Bolivie)//Paratype 2010 Pelidnota ulianai Soula//Invalid paratype see Soula 2010 det. M. R. Moore 2014” (47030217); “Région des Yungas Bolivie//Paratype 2010 Pelidnota ulianai Soula//Invalid paratype see Soula 2010 det. M. R. Moore 2014 ” (47030218); two paratypes with identical labels “Inca Huara 1400 m (BO.) coll. – SOULA//Paratype 2010 Pelidnota ulianai Soula//Invalid paratype see Soula 2010 det. M. R. Moore 2014” (47030219 and 47030220); two paratypes with identical labels “Caranavi [arrow] Tocumo [pro Yucumo ?] (860 m) coll. – SOULA//Paratype 2010 Pelidnota ulianai Soula//Invalid paratype See Soula 2010 det. M. R. Moore 2014” (47030221 and 47030222); “Yungas 1600 m 2/2003 M. SOULA det 19//Paratype 2010 Pelidnota ulianai Soula//Invalid Paratype See Soula 2010 det. M. R. Moore 2014” (47030223). Genitalia card-mounted underneath the holotype, allotype and 5 invalid paratypes. Box 4618656 SOULA.
Remarks
There is no mention of a paratype series of P. ulianai in
Pelidnota uncinata
Pelidnota uncinata Ohaus, 1930a: 139–140 [original combination].
Pelidnota (Ganonota) uncinata
Ohaus [new subgeneric combination by
Pelidnota (Strigidia) uncinata
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) uncinata
Ohaus [new subgeneric combination by
Strigidia uncinata
(Ohaus) [new combination by
Pelidnota (Strigidia) uncinata
(Ohaus) [revised combination and revised subgeneric combination by
Pelidnota uncinata
Ohaus [removal of subgeneric classification by
Distribution
BOLIVIA: Cochabamba, Santa Cruz (
Types
1 ♂ type specimen of Pelidnota uncinata Ohaus at ZMHB (Fig.
Pelidnota unicolor bonariensis
Pelidnota bonariensis Burmeister, 1855: 522 [original combination].
Pelidnota unicolor bonariensis
Burmeister [new subspecific status by
Pelidnota (Pelidnota) unicolor bonariensis
Burmeister [new subgeneric combination by
Pelidnota unicolor bonariensis
Burmeister [removal of subgeneric classification by
Distribution
ARGENTINA: Buenos Aires (
Pelidnota unicolor unicolor
Scarabeus unicolor Drury, 1782: 61 [original combination].
Pelidnota unicolor
(Drury) [new combination by
Pelidnota (Pelidnota) unicolor
(Drury) [new subgeneric combination by
Pelidnota unicolor
(Drury) [removal of subgeneric combination by
synonym. Melolontha druryana Herbst, 1790
Melolontha druryana Herbst, 1790: 163 [original combination].
Pelidnota unicolor
(Drury) [syn. by
synonym. Pelidnota testacea Laporte, 1840
Pelidnota testacea Laporte, 1840: 122 [original combination].
Pelidnota druryana
(Drury) [syn. by
Distribution
BRAZIL: Espírito Santo, Minas Gerais, Pernambuco, Rio de Janeiro, São Paulo, Santa Catarina (
Types
The following specimen is at CCECL. 1 invalid ♂ neotype: “Salesopolis São Paulo Brésil M. SOULA det 19 [obverse] II/2000//Pelidnota unicolor (Dr.) M. SOULA det 2008//Néotype 2008 Scarabeus unicolor Dr Soula det.” (47030861). Genitalia card-mounted underneath the invalid neotype. Box 4618683 SOULA.
Remarks
We treat Pelidnota unicolor var. infuscata Ohaus (Fig.
Pelidnota ustarani
Heteropelidnota ustarani Martínez, 1967: 147–152 [original combination].
Pelidnota ustarani
(Martínez) [new combination by
Distribution
BRAZIL: Espírito Santo (
Types
1 ♂ holotype of Heteropelidnota ustarani at MACN (Fig.
Pelidnota vanderberghi
Pelidnota vanderberghi Soula, 2010a: 39-40 [original combination].
Distribution
BOLIVIA: La Paz (
Types
The following specimens are at CCECL. 1 ♂ holotype, 1 ♀ allotype, 18 ♂ paratypes, 9 ♀ paratypes: “Région des Yungas Bolivie//Holotype 2010 Pelidnota vanderberghi S. Soula” (47030189); “COLL. LECOURT G. INCA-HUARA, 1450 m. NOR-YUNGAS XI.1995. BOLIVIE//Allotype 2010 Pelidnota vanderberghi S. Soula” (47030190); seven paratypes with identical labels “Région des Yungas Bolivie//Paratype 2010 Pelidnota vanderberghi Soula” (47030191 to 47030194, exch10 to exch12); two paratypes with identical labels “Nord-Yungas, 1500-1800m Bolivie//Paratype 2010 Pelidnota vanderberghi S. Soula” (47030195 and 47030196); three paratypes with identical labels “Yungas Bolivie M. SOULA det. 20 [obverse] XI/2010//Paratype 2010 Pelidnota vanderberghi S. Soula” (47030197 to 47030199); “Yungas (Bo) 520 m coll. – SOULA//Paratype 2010 Pelidnota vanderberghi Soula” (47030200); “Caranavi [arrow] Tocumo [pro Yucumo ?] (850 m) coll. – SOULA//Paratype 2010 Pelidnota vanderberghi Soula” (47030201); “Appolo [arrow] Guanay (Bo.) coll. – SOULA [obverse] (Bol.)//Paratype 2010 Pelidnota vanderberghi Soula” (47030202); “N. Yungas Bolivie (en 90)//Paratype 2010 Pelidnota vanderberghi Soula” (47030203); “Route de Coroico à Coranavi [pro Caranavi] (Bolivie)//Paratype 2010 Pelidnota vanderberghi Soula” (47030204); “Inca Huara 1450 m. 11/94 coll. – SOULA//Paratype 2010 Pelidnota vanderberghi Soula” (47030205); two paratypes with identical labels “Inca Huara 1450 m (Bo.) 11/94 coll. – SOULA//Paratype 2010 Pelidnota vanderberghi Soula” (47030206 and 47030207); three paratypes with identical labels “Inca Huara 1400 m (Bo.) coll. – SOULA//Paratype 2010 Pelidnota vanderberghi Soula” (47030208 to 47030210); “Inca-Huara (1450m) N. Yungas-Bolivie XI/95-Lecourt leg.//Paratype 2010 Pelidnota vanderberghi Soula” (47030211); “Col G. LECOURT Chappare km 95 1900 m/[the date 08.1984 is crossed out] BOLIVIE [obverse] 10.88//Paratype 2010 Pelidnota vanderberghi Soula” (47030212); “BOLIVIE - CARANAVI NOR YUNGAS - ALT.900m Du 15 AU 30/11/89 COLLECTION LECOURT//Paratype 2010 Pelidnota vanderberghi Soula” (47030213); “Caranavi 1000m Nor Yungas BOLIVIA 1.90 [fade] coll. M. Büche//Paratype 2010 Pelidnota vanderberghi Soula” (47030214). Genitalia card-mounted underneath the holotype and 16 paratypes. Box 4618656 SOULA.
Pelidnota vazdemelloi
Strigidia vazdemelloi Soula, 2006: 12, 55 [original combination].
Pelidnota vazdemelloi
(Soula) [new combination by
Distribution
BRAZIL: Mato Grosso, Mato Grosso do Sul (
Types
The following specimens are at CCECL. 1 ♂ holotype, 1 ♀ allotype: “Mato Grosso, Brasil leg Alvarenga, XI 63 [crossed out]//Holotype 2006 Strigidia vazdemelloi Sou. Soula” (47030428); “Sinop//Allotype 2006 Strigidia vazdemelloi Sou. Soula” (47030429). Genitalia are card-mounted underneath the male holotype. Box 4618663 SOULA.
Remarks
Pelidnota villavicencioensis
Pelidnota villavicencioensis Soula, 2010a: 61 [original combination].
Distribution
COLOMBIA: Meta (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Colombie coll. – SOULA [obverse] Villavicencio//Holotype 2010 Pelidnota villaviciencoensis (sic) S. Soula” (47030495). Genitalia card-mounted underneath the male holotype. Box 4618666 SOULA.
Pelidnota virescens
Pelidnota virescens Burmeister, 1844: 403 [original combination].
Pelidnota (Pelidnota) virescens
Burmeister [new subgeneric combination by
Pelidnota virescens
Burmeister [removal of subgeneric classification by
synonym. Pelidnota (Pelidnotidia) permicans Casey, 1915
Pelidnota (Pelidnotidia) permicans Casey, 1915: 77 [original combination].
Pelidnota (Pelidnota) permicans
Casey [new subgeneric combination by
Pelidnota (Pelidnota) virescens
Burmeister [syn. by
synonym. Pelidnota (Pelidnota) virescens planipennis Ohaus, 1918
Pelidnota (Pelidnota) virescens var. planipennis Ohaus, 1918: 24 [original combination].
Pelidnota (Pelidnota) virescens planipennis
Ohaus [new subspecific status by
Pelidnota permicans
Casey [syn. by
Distribution
COSTA RICA: San José (
Types
1 ♂ neotype of Pelidnota virescens at MNHN (
Remarks
Pelidnota viridicuprea
Pelidnota viridicuprea Ohaus, 1908b: 401–402 [original combination].
Pelidnota (Chalcoplethis) viridicuprea
Ohaus [new subgeneric combination by
Strigidia viridicuprea
(Ohaus) [new combination by
Pelidnota viridicuprea
Ohaus [revised combination by
Distribution
ECUADOR: Napo, Pastaza (
Pelidnota vitalisi
Pelidnota (Ganonota) vitalisi Ohaus, 1925: 77–78 [original combination].
Pelidnota (Strigidia) vitalisi
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) vitalisi
Ohaus [new subgeneric combination by
Pelidnota (Ganonota) vitalisi
Ohaus [revised subgeneric combination by
Strigidia vitalisi
(Ohaus) [new combination by
Pelidnota (Strigidia) vitalisi
Ohaus [revised combination and revised subgeneric combination by
Pelidnota vitalisi
Ohaus [removal of subgeneric classification by
Distribution
BRAZIL: Mato Grosso (
Types
Lectotype of Pelidnota (Ganonota) vitalisi in ZMHB and an unknown number of paralectotypes should be at MNHN, but were not recorded in Soula (
Remarks
Pelidnota vitticollis
Pelidnota vitticollis Burmeister, 1844: 396 [original combination].
Pelidnota bivittata
(Swederus) [syn. by F.
Pelidnota vitticollis
Burmeister [revised species status by
Pelidnota (Ganonota) vitticollis
Burmeister [new subgeneric combination by
Pelidnota (Strigidia) vitticollis
Burmeister [new subgeneric combination by
Pelidnota (Odontognathus) vitticollis
Burmeister [new subgeneric combination by
Strigidia vitticollis
(Burmeister) [new combination by
Pelidnota (Strigidia) vitticollis
Burmeister [revised combination and revised subgeneric combination by
Pelidnota vitticollis
Burmeister [removal of subgeneric classification by
Distribution
BRAZIL: Espírito Santo, Rio de Janeiro, Santa Catarina (
Types
Types of Pelidnota vitticollis at MHNN (
Pelidnota werneri
Strigidia werneri Soula, 2006: 10, 85 [original combination].
Pelidnota werneri
(Soula, 2006) [new combination by
Distribution
PERU: Loreto (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♀ paratype: “Iquitos, Loreto Pérou; XI/2003//Holotype 2006 Strigidia werneri S. Soula” (47030129); “Iquitos, Loreto Pérou; XI/2003//Allotype Strigidia werneri S. 2006 Soula” (47030130); “Iquitos, Loreto Pérou; VIII/2003//Paratype 2006 Strigidia werneri S. Soula” (47030131). Genitalia card-mounted underneath holotype. Box 4618654 SOULA.
Pelidnota xanthopyga
Pelidnota (Odontognathus) xanthopyga Hardy, 1975: 6, 12 [original combination].
Strigidia xanthopyga
(Hardy) [new combination by
Pelidnota (Strigidia) xanthopyga
Hardy [revised combination and new subgeneric combination by
Pelidnota xanthospyga
(sic) Hardy [removal of subgeneric classification by
Distribution
COLOMBIA: Santander (
Types
1 ♂ holotype and 1 ♀ allotype of Pelidnota (Odontognathus) xanthopyga at USNM (
Pelidnota xanthospila
Rutela xanthospila Germar, 1824: 119 [original combination].
Pelidnota xanthospila
(Germar) [new combination by
Pelidnota (Ganonota) xanthospila
(Germar) [new subgeneric combination by
Pelidnota (Strigidia) xanthospila
(Germar) [new subgeneric combination by
Pelidnota (Odontognathus) xanthospila
(Germar) [new subgeneric combination by
Strigidia xanthospila
(Germar) [new combination by
Pelidnota (Strigidia) xanthospila
(Germar) [revised combination and revised subgeneric combination by
Pelidnota xanthospila
(Germar) [removal of subgeneric classification by
synonym. Rutela ornata Perty, 1830
Rutela ornata Perty, 1830: 49 [original combination].
Pelidnota xanthospila
(Germar) [syn. by
synonym. Rutela rubiginosa Laporte, 1840
Rutela rubiginosa Laporte, 1840: 120 [original combination].
Pelidnota xanthospila var. rubiginosa (Laporte) [new combination and new infrasubspecific status by
Pelidnota xanthospila forma rubiginosa (Laporte) [revised infrasubspecific status by
Pelidnota xanthospila
(Germar) [syn. by
Distribution
BRAZIL: Bahia, Espírito Santo, Minas Gerais, Santa Catarina, São Paulo, Rio de Janeiro (
Remarks
This species has a great deal of color variation and variation in elytral maculae (broad, yellow to narrow and confined to near the base). The species is distributed in the Brazilian coastal states.
Pelidnota yungasensis
Pelidnota yungasensis Soula, 2009: 32, 89–90 [original combination].
Distribution
BOLIVIA: La Paz (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 9 ♂ paratypes: “N. Yungas (Bo.) coll. – SOULA//Holotype 2008 Pelidnota fulva yungasensis S. Soula//Pelidnota yungasensis Soula det. MR Moore ‘15” (47030615); “N. Yungas (Bo.) coll. – SOULA//Allotype 2008 Pelidnota fulva yungasensis S. Soula//Pelidnota yungasensis Soula det. MR Moore ‘15” (47030616); nine paratypes with identical label data: “N. Yungas (Bo.) coll. – SOULA//Paratype 2008 Pelidnota fulva yungasensis S. Soula//Pelidnota yungasensis Soula det. MR Moore ‘15” (47030617 to 47030624, exch35). Genitalia card-mounted underneath the male holotype and eight male paratypes. Box 4618675 SOULA.
Remarks
Pelidnota zovii
Pelidnota zovii Soula, 2010a: 39 [original combination].
Distribution
PERU: Huánuco, Junín (
Types
Pelidnotanames nomen dubium
Pelidnota aeruginosa , nomen dubium
Scarabaeus aeruginosus Linnaeus, 1767: 558 [original combination].
Pelidnota aeruginosa
(Linnaeus) [new combination by
Remarks
This name is widely used in collections and the literature, but the identity of the species is uncertain (F.
Pelidnota species incertae sedis
Pelidnota emerita , incertae sedis
Cetonia emerita Olivier, 1789: 71 [original combination].
Rutela emerita
(Olivier) [new combination by
Pelidnota emerita
(Olivier) [new combination by
Distribution
SOUTH AMERICA (
Remarks
Cetonia emerita Olivier was described based on a specimen from “Amérique méridionale” (
Pelidnota fallax
Pelidnota fallax Gistel, 1857: 80 [original combination].
Distribution
BRAZIL (
Remarks
Pelidnota sybarita
Pelidnota sumptuosa Laporte, 1840: 123 [original combination, junior homonym of Pelidnota sumptuosa (Vigors, 1825)].
Pelidnota sybarita Harold, 1869a: 124 [original combination, new replacement name for Pelidnota sumptuosa Laporte].
synonym. Pelidnota luxuriosa Blackwelder, 1944
Pelidnota luxuriosa Blackwelder, 1944: 237 [original combination, new replacement name for Pelidnota sumptuosa Laporte].
Pelidnota sumptuosa
(Vigors) [syn. by
Pelidnota (Pelidnota) luxuriosa
Blackwelder [new subgeneric combination and revised species status by
Pelidnota luxuriosa
Blackwelder [removal of subgeneric classification by
Pelidnota sybarita Harold [objective synonymy].
Distribution
BRAZIL (
Remarks
Pelidnota luxuriosa Blackwelder is the name for this species in prevailing usage and has been cited as such since
Pelidnota versicolor , incertae sedis
Rutela versicolor Billberg, 1820: 384 [original combination].
Pelidnota versicolor
(Billberg) [new combination by
Distribution
BRAZIL (
Remarks
Rutela caesarea
Rutela caesarea Gistel, 1857: 29 [original combination].
Distribution
COLOMBIA (
Remarks
Rutela runica
Rutela runica Gistel, 1850: 381 [original combination].
Distribution
FRENCH GUIANA: Cayenne (
Remarks
Rutela tristis
Rutela tristis Gistel, 1850: 381 [original combination].
Distribution
BRAZIL (
Remarks
Unavailable, invalid names in Pelidnota
Pelidnota auripes in litt.; Unavailable, invalid name
Remarks
Pelidnota demergesi in litt.; Unavailable, invalid name
Types
The following specimens are deposited at CCECL. 1 invalid ♂ holotype, 3 invalid ♂ paratypes: “Tingo Maria Pérou, X/2005//Holotype 2010 Pelidnota demergesi S. Soula//Invalid Holotype name in litt. det. M. R. Moore 2014” (47030175); two invalid paratypes with identical label data “Tingo Maria Las cuevas de las pavas M. SOULA det 19 [obverse] 15/IV/2010//Paratype 2010 Pelidnota demergesi S. Soula//Invalid Paratype name in litt. det. M. R. Moore 2014” (47030176 and 47030177); “Rio Ucayali Pérou M. SOULA det 19//Paratype 2010 Pelidnota demergesi S. Soula//Invalid Paratype name in litt. det. M. R. Moore 2014” (47030178). Genitalia card-mounted underneath the invalid holotype and 2 invalid paratypes. Box 4618656 SOULA.
Remarks
The name Pelidnota demergesi has never been associated with a species description or type designation. The name appears only in a figure legend in
Pelidnota desantacatarina in litt.; Unavailable, invalid name
Types
The following specimens are deposited at CCECL. 1 ♂ invalid holotype, 1 ♀ invalid allotype, 4 ♂ invalid paratypes: “São Bento do Sul. S. C. I/94 M. SOULA det 19//Holotype 2008 Pelidnota desantacatarina S. Soula”//Invalid holotype det. Moore ’15” (47030862); “Corupa. S.C. II/1938 M. SOULA det 19//Allotype 2008 Pelidnota desantacatarina S. Soula”//Invalid Allotype det. Moore ’15” (47030863); “Santa Catarina II/92 M. SOULA det 19//Paratype 2008 Pelidnota desantacatarina S. Soula”//Invalid Paratype det. Moore ’15” (47030864); “Déc. 1963 Corupa, S.C. Brazil//Paratype 2008 Pelidnota desantacatarina S. Soula”//Invalid Paratype det. Moore ’15” (47030865); “06.01.1985 Corupa Santa Caterina Brasilien//Paratype 2008 Pelidnota desantacatarina S. Soula”//Invalid Paratype det. Moore ’15” (47030866); “Pelidnota unicolor//Brésil Hansa. S.C.//Ru 22.//1 1932//Paratype 2008 Pelidnota desantacatarina S. Soula”//Invalid Paratype det. Moore ’15” (47030867). Genitalia card-mounted underneath the invalid male holotype and two invalid male paratypes. Box 4618683 SOULA.
Remarks
The name Pelidnota desantacatarina Soula does not appear in the literature nor has the name been associated with a species description. These type specimens are considered invalid and the name Pelidnota desantacatarina is unavailable.
Pelidnota rectificata in litt.; Unavailable, invalid name
Types
The following specimen is deposited at CCECL. 1 ♂ invalid holotype: “Faz. Aceiro Jatai, Goiás - Brasil X.1962//Holotype 2008 Pelidnota rectificata S. Soula//Invalid Holotype det. MR Moore ‘15” (47030808). Genitalia card-mounted underneath the invalid male holotype. Box 4618681 SOULA. The following specimen is deposited at CMNC. 1 ♀ invalid allotype: “BRASIL GOIAZ Jatai J. Guetin-leg. Coll. Martinez Oct. 953//H. & A. HOWDEN COLLECTION ex. A. Martinez coll.//Allotype 2008 Pelidnota rectificata S. Soula”.
Remarks
The name Pelidnota rectificata Soula does not appear in the literature nor has the name been associated with a species description. This specimen is considered an invalid type and the name Pelidnota rectificata is unavailable.
Pelidnota unicolor desantacatarina in litt.; Unavailable, invalid name
Types
The following specimens are deposited at CCECL. 3 ♂ invalid paratypes: “Santa Catarina Br. I/97 M. SOULA det 19//Paratype 2006 Pelidnota unicolor desantacatarina Soula”//Invalid Paratype det. Moore ’15” (47030868); “Bré. Santa Catarina I/97 M. SOULA det 19//Paratype 2006 Pelidnota unicolor desantacatarina S. Soula”//Invalid Paratype det. Moore ’15” (47030869); “Santa Catarina Brés. I/97 M. SOULA det 19//Paratype 2006 Pelidnota unicolor S. desantacatarina Soula”//Invalid Paratype det. Moore ’15” (47030870). Box 4618683 SOULA.
Remarks
The name Pelidnota unicolor desantacatarina Soula does not appear in the literature nor has the name been associated with a species description. These type specimens are considered invalid and the name Pelidnota unicolor desantacatarina is unavailable.
Pelidnota unicolor occidentalis in litt.; Unavailable, invalid name
Types
The following specimen is deposited at CCECL. 1 ♂ invalid holotype: “PÉROU CHANCHAMAYO 2000 M//Muséum Paris ex Coll. R. Oberthür 1952//Holotype 2008 Pelidnota unicolor occidentalis S. Soula//Invalid Holotype det. MR Moore ‘15” (47030807). Genitalia card-mounted underneath the invalid male holotype. Box 4618681 SOULA.
Remarks
The name Pelidnota unicolor occidentalis Soula does not appear in the literature nor has the name been associated with a species description. This specimen is considered an invalid holotype and the name Pelidnota unicolor occidentalis is unavailable.
Unavailable names in Pelidnota (application of ICZN Article 16.4.1)
The name Strigidia testaceovirens argentinica Soula was proposed for a subspecies from Misiones, Argentina (
Pelidnota testaceovirens argentinica(Soula, 2006) Unavailable, invalid name
Strigidia testaceovirens argentinica Soula, 2006: 62 [original combination, unavailable, invalid name].
Pelidnota (Strigidia) testaceovirens argentinica
(Soula) [new combination and new subgeneric combination by
Pelidnota testaceovirens argentinica
(Soula) [removal of subgeneric classification by
Distribution
ARGENTINA: Misiones (
Types
The following invalid type specimens are deposited at CCECL. 9 invalid ♂ paratypes, 11 invalid ♀ paratypes: six invalid paratypes with identical label data “Puerto Iguazu ARGENTINE (I/93)//Paratype 2006 Strigidia testaceovirens argentinica S. Soula” (47030093 to 47030096, exch03 and exch04); three invalid paratypes with identical label data “Puerto Iguazu Arg. M. SOULA det 19//Paratype 2006 Strigidia testaceovirens argentinica S. Soula” (47030097 to 47030099); two invalid paratypes with identical label data “Oberá – Misiones ARGENTINA-I/99 Col. Andrés Varga//Paratype 2006 Strigidia testaceovirens argentinica S. Soula” (47030100 and 47030101); three invalid paratypes with identical label data “Puerto Iguazu Misiones, Argentine II/1995//Paratype 2006 Strigidia testaceovirens argentinica S. Soula” (47030102 to 47030104); “Puerto Iguazu-ARG XII/88.//Paratype 2006 Strigidia testaceovirens argentinica S. Soula” (47030110); “ARGENTINA Iguazu Misiones 1996 Coll. M. DURANTON//Paratype 2006 Strigidia testaceovirens argentinica S. Soula” (47030105); “ARGENTINE Misiones Iguazu 1997 Coll. M. DURANTON//Paratype 2006 Strigidia testaceovirens argentinica S. Soula” (47030106); “Puerto Iguazu 22/11/87 coll. – SOULA [obverse] Misiones (Arg.)//Paratype 2006 Strigidia testaceovirens argentinica S. Soula” (47030107); “Puerto Iguazu (Ar.) coll. – SOULA [obverse] Misiones (Arg.) 22/11/87//2006 Strigidia testaceovirens argentinica S. Soula” (47030108); “Calilegua NOA 1110 m, 26/01/06 M. SOULA det. 19//2006 Strigidia testaceovirens argentinica S. Soula” (47030109). Genitalia card-mounted underneath six of the invalid male paratypes. Box 4618651 SOULA.
Remarks
There is no mention of holotype or allotype specimens of Pelidnota testaceovirens argentinica in
Unavailable names in Pelidnota (application of ICZN Article 16.4.2)
We consider the following names proposed by Soula in Pelidnota and Strigidia as unavailable per ICZN Article 16.4.2. which states that fixation of holotype specimens for new names must be accompanied by the following information, “where the holotype or syntypes are extant specimens, by a statement of intent that they will be (or are) deposited in a collection and a statement indicating the name and location of that collection”. The names below were proposed by
Pelidnota arnaudi
Pelidnota arnaudi Soula, 2009: 32, 72-73 [original combination, unavailable, invalid name].
Distribution
BRAZIL: Espírito Santo, São Paulo (
Types
The following invalid type specimens are deposited at CCECL. 1 ♂ invalid holotype, 1 ♀ invalid allotype, 17 ♂ invalid paratypes, 1 probable ♂ invalid paratype, 1 ♀ invalid paratype: “Paulinia, São Paulo 12/95 M. SOULA det 19//Holotype 2008 Pelidnota arnaudi S. Soula” (47030564); “Paulinia, Sao Paulo M. SOULA det 19 [obverse] 12/95//Allotype 2008 Pelidnota arnaudi S. Soula” (47030565); four paratypes with identical label data (once São is spelled Sao): “Paulinia, São Paulo 12/95 M. SOULA det 19//Paratype 2008 Pelidnota arnaudi S. Soula” (47030566 to 47030569); “Paulinia 12/95 Sao Paulo M. SOULA det 19//Paratype 2008 Pelidnota arnaudi S. Soula” (47030570); nine paratypes with identical label data: “Esp. Santo 7/III/97 coll. – SOULA//Paratype 2008 Pelidnota arnaudi S. Soula” (47030571 to 47030577, exch33 and exch34); “Esp. Santo 7/III/97 coll. – SOULA//Probable paratype Pelidnota arnaudi Soula det. MR Moore ‘15//Pelidnota arnaudi Soula Paratype probable” (47030578); three invalid paratypes with identical label data: “Nova Friburgo Rio, XI/2008//Paratype 2009 Pelidnota arnaudi S. Soula//Invalid Paratype Pelidnota arnaudi Soula det. MR. Moore ‘15” (47030579 and 47030581); “Pelidnota glauca Oliv. Brésil//Paratype Soula//Invalid paratype See
Remarks
Pelidnota brusteli
Pelidnota brusteli Soula, 2010a: 33 [original combination, unavailable, invalid name].
Distribution
PERU: (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Tingo Maria Pérou M. SOULA det. 20 [obverse] XII/2005//Holotype 2010 Pelidnota brusteli S. Soula” (47030125). Genitalia card-mounted underneath the invalid holotype. Box 4618653 SOULA.
Pelidnota chalcothorax septentrionalis
Pelidnota chalcothorax septentrionalis Soula, 2009: 94 [original combination, unavailable, invalid name].
Distribution
BRAZIL: Bahia, Minas Gerais (
Types
The following invalid type specimens are deposited at CCECL. 2 invalid ♂ paratypes, 1 invalid ♀ paratype: “S. Antonio da Barra Prov. de Bahia Ch. Pujol 1890//Paratype 2008 Pelidnota chalcothorax septentrionalis S. Soula” (47030607); “Villa Victoria Prov. de Bahia Ch. Pujol 1890//Paratype 2008 Pelidnota chalcothorax septentrionalis S. Soula” (47030608); “Aguas Vermillas [pro Vermelhas] (B) M. Geraes 3/92 coll. – SOULA//Paratype 2008 Pelidnota chalcothorax septentrionalis S. Soula” (47030609). Genitalia card-mounted underneath the invalid male paratypes. Box 4618673 SOULA.
Pelidnota degallieri
Pelidnota degallieri Soula, 2010a: 32-33 [original combination, unavailable, invalid name].
Distribution
VENEZUELA: Bolívar (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Le 15 VII 1986 Route de SANTA ELENA P. K. 35 U. V. Etat du BOLIVAR VENEZUELA J. HAXAIRE & P. BLEUZEN Leg.//Holotype 2010 Pelidnota degallieri S. Soula” (47030124). Genitalia card-mounted underneath the invalid holotype. Box 4618653 SOULA.
Pelidnota dieteri
Pelidnota lavalettei Soula, 2009: 110 [original combination and secondary junior homonym, unavailable, invalid name].
Pelidnota dieteri
[new replacement name by
Distribution
BRAZIL: Mato Grosso (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype, 1 invalid ♀ paratype: “Matto Grosso Brésil M. SOULA det 19//Holotype 2008 Pelidnota lavalettei Soula//Pelidnota dieteri Soula det. MR MOORE ‘15” (47030727). “Matto Grosso//Allotype 2008 Pelidnota lavalettei Soula//Pelidnota dieteri Soula det. MR MOORE ‘15” (47030728). “Ipatinga (Minais G) 12/89 M. Soula det. 20//[blank]” (47030729). Genitalia card-mounted underneath the invalid male holotype. Box 4618680 SOULA.
Pelidnota gracilis decaensi (Soula, 2008) Unavailable, invalid name
Strigidia gracilis decaensi Soula, 2008: 35 [original combination, unavailable, invalid name].
Pelidnota (Strigidia) gracilis decaensi
(Soula) [new combination and new subgeneric combination by
Pelidnota gracilis decaensi
(Soula) [removal of subgeneric classification by
Distribution
BRAZIL: Paraná (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Londrina Paraná 600 m, X/2005 M. SOULA det 19//Holotype Strigidia gracilis decaensi S. Soula” (47030298). Genitalia card-mounted underneath the invalid male holotype. Box 4618659 SOULA.
Pelidnota halleri
Pelidnota halleri Demez & Soula, 2011: 77 [original combination, unavailable, invalid name].
Pelidnota helleri
Demez and Soula [incorrect subsequent spelling by
Distribution
PERU: Loreto (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Iquitos Loreto VI/2011 M. SOULA det. 19//Holotype 2011 Pelidnota halleri D. et S. Soula” (47030133). Genitalia card-mounted underneath the invalid holotype. Box 4618654 SOULA.
Remarks
Pelidnota injantepalominoi
Pelidnota injantepalominoi Demez & Soula, 2011: 77–78 [original combination, unavailable, invalid name].
Distribution
PERU: Loreto (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Iquitos VI/2011 M. SOULA det. 19//Holotype Pelidnota injantepalominoi D. et S. Soula” (47030134). Genitalia card-mounted underneath the invalid holotype. Box 4618654 SOULA.
Pelidnota kucerai
Pelidnota kucerai Soula, 2009: 31, 55 [original combination, unavailable, invalid name].
Distribution
COLOMBIA: Valle del Cauca (
Types
The following invalid type specimen was deposited at CCECL. 1 invalid ♂ holotype: “Anchicaya, Valle del Cauca, Colombie, V/93//Holotype 2008 Pelidnota bucerai S. Soula” (47030496). Genitalia card-mounted underneath the invalid holotype. Box 4618667 SOULA.
Pelidnota lavalettei
Pelidnota lavalettei Soula, 2008: 39 [original combination, unavailable, invalid name].
Distribution
FRENCH GUIANA (
Types
The following type specimen is deposited at CCECL. 1 ♂ holotype: “Guyane fr. Est. du dép. M. SOULA det. 20//Holotype 2008 Pelidnota lavalettei S. Soula//Holotype of P. fabricelavalettei
Remarks
This specimen was the holotype specimen for two species: P. lavalettei
Pelidnota malyi
Pelidnota malyi Soula, 2010a: 36–37 [original combination, unavailable, invalid name].
Distribution
ECUADOR: Napo (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype, 2 invalid ♂ paratypes, 2 invalid ♀ paratypes: “Misahuali Oriente Ecuador M. SOULA det 19 [obverse] II/2006//Holotype 2010 Pelidnota malyi S. Soula” (47030169); “Misahuali Oriente Ecuador M. SOULA det 19 [obverse] II/2006//Allotype 2010 Pelidnota malyi S. Soula” (47030170); “Misahuali Oriente Ecuador M. SOULA det 19 [obverse] II/2006//Paratype Pelidnota malyi Soula 2010” (47030171); “PERU - NAPO Misahualli Tijuana 2.06 coll. V. Malý//2,-€//STRIGIDIA Sp. sp. n. ?//coll. V. Malý CZ - Praha//Paratype Pelidnota malyi Soula 2010” (47030172); “ECUADOR-Napo Misahualli 450 m 17-21. 4. 93 L. & T. Racheli leg. [obverse] Chalco.//Paratype Pelidnota malyi Soula 2010” (47030173); “ECUADOR-Napo Misahualli 450 m 18-20. 10. 1993 L. & T. Racheli leg.//Paratype Pelidnota malyi Soula 2010” (47030174). Box 4618656 SOULA.
Remarks
The specific epithet “malyi” was used for two separate, distinct species of Pelidnota in the same publication (
Pelidnota mezai
Pelidnota mezai Soula, 2009: 33, 101–102 [original combination, unavailable, invalid name].
Distribution
PERU (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Tingo Maria Pérou, X-XI/2005//Holotype 2008 Pelidnota mezai S. Soula” (47030730). Genitalia card-mounted underneath the invalid male holotype. Box 4618680 SOULA.
Pelidnota polita darienensis
Pelidnota polita darienensis Soula, 2009: 46 [original combination, unavailable, invalid name].
Distribution
PANAMA: Darien (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype, 9 invalid ♂ paratypes, 26 invalid ♀ paratypes: Meteti Darien 12/XI/2003 M. SOULA det 19 [obverse] Panama//Holotype 2007 Pelidnota polita darienensis S. Soula” (47030498); “Meteti Darien 12/XI/2003 M. SOULA det 19 [obverse] 12/XI/2003//Allotype 2007 Pelidnota polita darienensis S. Soula” (47030499); four invalid paratypes with identical label data “Meteti Darien Panama M. SOULA det 19 [obverse] 12/XI/2003//Paratype 2007 Pelidnota polita darienensis S. Soula” (47030524 to 47030527); “Meteti Darien Panama [obverse] 12/XI/2003//Paratype 2007 Pelidnota polita darienensis S. Soula” (47030528); “Meteti Darien Panama M. SOULA det 19 [obverse] II/2004//Paratype 2007 Pelidnota polita darienensis S. Soula” (47030529); “Panama Darien M. SOULA det 19//Paratype 2007 Pelidnota polita darienensis S. Soula” (47030530); twelve invalid paratypes with identical label data “Meteti, Darien Panama, 12/XI/2003//Paratype 2007 Pelidnota polita darienensis S. Soula” (47030515 to 47030523, exch28 to exch30); six invalid paratypes with identical label data “Rio Iglesia, Darien Panama, 20/XI/03//Paratype 2007 Pelidnota polita darienensis S. Soula” (47030500 to 47030505); “Rio Iglesia, Darien Panama, 20/XI/03//Paratype 2008 Pelidnota polita darienensis S. Soula”//Invalid paratype see
Pelidnota polita orozcoi
Pelidnota polita orozcoi Soula, 2009: 45 [original combination, unavailable, invalid name].
Distribution
COLOMBIA: Meta (
Types
The invalid holotype ♂ of Pelidnota polita orozcoi is at MNHN. The following invalid type specimens are deposited at CCECL. 1 invalid ♀ allotype, 2 invalid ♂ paratypes: “Coll. Nonfried. Columbia.//Allotype 2007 Pelidnota polita orozcoi S. Soula” (47030549); “Carimagua; Meta; Colombie; 175m VII/VIII 1999//Paratype 2008 Pelidnota polita orozcoi S. Soula det.” (47030550); San Juan de Cordova M. SOULA det 19 [obverse] Cianaga Colombie //Paratype 2008 Pelidnota polita orozcoi S. Soula det. (47030551)”. Genitalia card-mounted underneath the two invalid male paratypes. Box 4618669 SOULA.
Pelidnota polita pittieri
Pelidnota polita pittieri Soula, 2009: 46 [original combination, unavailable, invalid name].
Distribution
VENEZUELA: Aragua (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype, 14 invalid ♂ paratypes, 2 invalid ♀ paratypes, 1 probable invalid ♀ paratype: “P.N. Henri Pittier Choroni; Venezuela V-VI/2005//Holotype 2007 Pelidnota polita pittieri S. Soula” (47030532); “P.N. Henri Pittier Choroni; Venezuela V-VI/2005//Allotype 2007 Pelidnota polita pittieri S. Soula” (47030533); twelve paratypes with identical label data “P.N. Henri Pittier Choroni ; Venezuela V-VI/2005//Paratype 2007 Pelidnota polita pittieri S. Soula” (47030534 to 47030543, exch31 and exch32); “P.N. Henri Pittier Choroni; Venezuela V-VI/2005//Paratype 2007 Pelidnota polita darienensis S. Soula//Pelidnota polita pittieri Soula probable paratype det. MR Moore ’15” (47030544); “Caracas. Mus: Drews.//ZOOL. MUSEUM DK COPENHAGEN//Paratype 2008 Pelidnota polita darienensis S. Soula” (47030545); “Caracas//ZOOL. MUSEUM DK COPENHAGEN//Paratype 2008 Pelidnota polita darienensis S. Soula” (47030546); two paratypes with identical label data “N. Venezuela S. Klages 1904//Paratype 2008 Pelidnota polita pittieri S. Soula” (47030547 and 47030548). Genitalia card-mounted underneath the invalid male holotype, 5 invalid male paratypes, and 1 probable invalid female paratype. Box 4618668 SOULA.
Remarks
The probable female paratype from P. N. Henri Pittier has a paratype label indicating that this specimen was determined as Pelidnota polita darienensis Soula.
Pelidnota punctulata decolombia
Pelidnota punctulata decolombia Soula, 2009: 80 [original combination, unavailable, invalid name].
Distribution
COLOMBIA (
Types
The invalid holotype ♂ of Pelidnota punctulata decolombia is at MNHN.
Pelidnota punctulata venezolana
Pelidnota punctulata venezolana Soula, 2009: 80 [original combination, unavailable, invalid name].
Distribution
VENEZUELA (
Types
The invalid holotype ♂ of Pelidnota punctulata venezolana is at MNHN.
Pelidnota raingeardi
Pelidnota raingeardi Soula, 2009: 33, 97 [original combination, unavailable, invalid name].
Distribution
ECUADOR: Napo (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype, 13 invalid ♂ paratypes, 4 invalid ♀ paratypes: “Napo - Coca Ecuador VIII-1982 Onoré leg.//Holotype 2008 Pelidnota raingeardi Soula” (47030651); “Napo - Coca Ecuador VIII-1982 Onoré leg.//Allotype 2008 Pelidnota raingeardi Soula” (47030652); three invalid female paratypes with identical label data: “Napo - Coca Ecuador VIII-1982 Onoré leg.//Paratype 2008 Pelidnota raingeardi S. Soula” (47030653 to 47030655); three invalid paratypes with identical label data: “Baeza (Eq.) 08/91 coll. – SOULA//Paratype 2008 Pelidnota raingeardi S. Soula” (47030656 to 47030658); “Tena [arrow] Loreto (E) pk 30 8/90//Paratype 2008 Pelidnota raingeardi Soula” (47030659); “Tena [arrow] Loreto (E) 7/90//Paratype 2008 Pelidnota raingeardi Soula” (47030660); “Tena (Equateur) 05/91//Paratype 2008 Pelidnota raingeardi Soula” (47030661);“Tena (E) 9/90 pk 30 //Paratype 2008 Pelidnota raingeardi Soula” (47030662); “[arrow] Loreto (E) 8/90//Paratype 2008 Pelidnota raingeardi Soula” (exch37); “Loreto (E) 9/90//Paratype 2008 Pelidnota raingeardi Soula” (47030663); “San Jorge ? (E) 8/90 [obverse] Equateur !//Paratype 2008 Pelidnota raingeardi Soula” (47030664); “Misahuali 9/91//Paratype 2008 Pelidnota raingeardi Soula” (47030665); “Misahuali 9/91//Paratype 2008 Pelidnota raingeardi Soula” (47030665); “Piste Puyo Macas P.L. Rio Pastaza (800m) 30/7/88 Pastaza (E) [obverse] 30/7/88 Pastaza (E)//Paratype 2008 Pelidnota raingeardi Soula” (47030666); “Route de Baños E 30/7/88 Macas Rio Pastaza [obverse] Rio Pastaza//Paratype 2008 Pelidnota raingeardi Soula” (47030667); “Rurrerabaque (sic) Bolivie M. SOULA det 19 [obverse] II/98//Paratype 2008 Pelidnota raingeardi Soula//Invalid Paratype Pelidnota raingeardi Soula det. MR Moore ‘15” (47030668). Genitalia mounted underneath the invalid male holotype and ten invalid male paratypes. Box 4618678 SOULA.
Remarks
Box 4618678 SOULA contains a male specimen from Ruzzezbaque, Bolivia labeled as a paratype of Pelidnota raingeardi Soula.
Pelidnota schneideri
Pelidnota schneideri Soula, 2010a: 35 [original combination, unavailable, invalid name].
Distribution
PERU: Loreto (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype, 18 invalid ♂ paratypes, 23 invalid ♀ paratypes: “Iquitos, Loreto, Pérou, II/2010//Holotype 2010 Pelidnota schneideri S. Soula” (47030357); “Iquitos; Loreto, Pérou; 200m X/2002//Allotype Pelidnota schneideri S. 2010 Soula” (47030358); two specimens with identical label data “Iquitos; Loreto, Pérou; X/2002//Paratype 2010 Pelidnota schneideri Soula” (47030359 and 47030360); “Iquitos; Loreto Pérou; 200m X/2002//Paratype 2010 Pelidnota schneideri Soula” (47030375); three invalid paratypes with identical label data “Iquitos, Loreto Pérou, II/2010//Paratype 2010 Pelidnota schneideri Soula” (47030361 to 47030363); three invalid paratypes with identical label data “Iquitos ; Loreto Pérou; XI-XII/2004//Paratype 2010 Pelidnota schneideri Soula” (47030364 to 47030366); three invalid paratypes with identical label data “Iquitos, Loreto, Pérou, I-II/2005//Paratype 2010 Pelidnota schneideri Soula” (47030367 and 47030368, exch21); “Iquitos, Loreto Pérou, VI/2010 [crossed out] [obverse] XI/2009//Paratype 2010 Pelidnota schneideri Soula” (47030369); “Iquitos, Loreto Pérou, VI [crossed out] XI/2010 [obverse] XI/2009//Paratype 2010 Pelidnota schneideri Soula” (47030370); three invalid paratypes with identical label data “Iquitos, Loreto Pérou, X/XI/2004//Paratype 2010 Pelidnota schneideri Soula” (47030371 to 47030373); “Iquitos, Loreto Pérou, II/2005//Paratype 2010 Pelidnota schneideri Soula” (47030374); “Iquitos Loreto Pérou M. SOULA det 19//Paratype 2010 Pelidnota schneideri Soula” (47030376); “Iquitos, Loreto Pérou; VIII/2003//Paratype 2010 Pelidnota schneideri Soula” (47030377); “Iquitos V/2002 M. SOULA det 19//Paratype 2010 Pelidnota schneideri Soula” (47030378); “Iquitos Pérou II-III/2004 M. SOULA det 19//Paratype 2010 Pelidnota schneideri Soula” (47030379); four invalid paratypes with identical label data “Iquitos, Loreto Pérou, VI/2010//Paratype 2010 Pelidnota schneideri Soula” (47030380 and 47030381, exch19 and exch20); two invalid paratypes with identical label data “San Pablo Loreto Pérou M. Soula det 19 [obverse] X/2003//Paratype 2010 Pelidnota schneideri Soula” (47030382 and 47030383); “Colombie Caqueta M. SOULA det 19//Paratype 2010 Pelidnota schneideri Soula” (47030384); “C. osculatii? 1/9/91 Tena (E)//Paratype 2010 Pelidnota schneideri Soula” (47030385); four invalid paratypes with identical label data “San Jorge (Equateur) 8/90//Paratype 2010 Pelidnota schneideri Soula” (47030386 to 47030388, exch18); three invalid paratypes with identical label data “San Jorge (E) 8/90//Paratype 2010 Pelidnota schneideri Soula” (47030389 to 47030391); three invalid paratypes with identical label data “San Jorge 8/90 (Eq.) coll. – SOULA//Paratype 2010 Pelidnota schneideri Soula”(47030392 to 47030394); “Macas Ecuador or.//Paratype 2010 Pelidnota schneideri Soula” (47030395). Genitalia card-mounted underneath the invalid male holotype and invalid paratype specimens. Box 4618662 SOULA. There are probably more invalid paratypes of Pelidnota schneideri at ZMHB (
Pelidnota simoensi
Pelidnota simoensi Soula, 2009: 33, 99–100 [original combination, unavailable, invalid name].
Distribution
BOLIVIA: La Paz (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype, 5 invalid ♂ paratypes, 3 invalid ♀ paratypes: “Région des Yungas Bolivie//Holotype 2009 Pelidnota simoensi S. Soula” (47030641); “Nord-Yungas 1800m; Bolivie//Allotype 2008 Pelidnota simoensi S. Soula” (47030642); “P. brevissima Caranavi N. Yungas 10/90 (B)//Paratype 2008 Pelidnota simoensi S. Soula” (47030643); “Yungas (Bol.) coll. – SOULA//Paratype 2008 Pelidnota simoensi S. Soula” (47030644); “Inca-Huara (1450m) - Bolivie XI/95 Lecourt leg.//Paratype 2008 Pelidnota simoensi S. Soula” (47030645); “Pointe Villa 1500 m coll. – SOULA [obverse] 11/10/96 La Paz Prov.//Paratype 2008 Pelidnota simoensi S. Soula” (47030646); “Pointe Villa 1500 m 11/10/96 coll. – SOULA [obverse] 11/10/96 La Paz Prov.//Paratype 2008 Pelidnota simoensi S. Soula” (47030647); “Pointe Villa (1500 m) coll. – SOULA [obverse] La Paz Prov.//Paratype 2009 Pelidnota simoensi S. Soula” (47030648); “N. Venezuela S. Klages 1904//Pelidnota prasina Burm.//Paratype 2008 Pelidnota simoensi S. Soula//Invalid Paratype Pelidnota simoensi Soula det. MR Moore ‘15” (47030649); “P.N. Henri Pittier Choroni; Venezuela V-VI/2005//Paratype 2008 Pelidnota simoensi S. Soula//Invalid Paratype Pelidnota simoensi Soula det. MR Moore ‘15” (47030650). Genitalia mounted underneath the invalid male holotype and five invalid male paratypes. Box 4618678 SOULA.
Remarks
Two male specimens labeled as paratypes of P. simoensi Soula are considered invalid paratypes because their localities are not reported in
Pelidnota unicolor subandina
Pelidnota unicolor subandina Soula, 2009: 93 [original combination, unavailable, invalid name].
Distribution
PERU: Junín (
Remarks
†Pelidnotites
Pelidnotites Cockerell, 1920: 462–463.
Type species
Pelidnotites atavus Cockerell, 1920: 463, by monotypy.
Gender
Masculine.
Species
1 species.
†Pelidnotites atavus
Pelidnotites atavus Cockerell, 1920: 462–463 [original combination].
Distribution
ENGLAND [EOCENE] (
Remarks
The true identity of this species is uncertain.
PERUQUIME
Peruquime Mondaca & Valencia, 2016: 3–4.
Type species
Peruquime arequipensis Mondaca & Valencia, 2016: 4–6, by monotypy.
Gender
Feminine.
Species
1 species.
Peruquime arequipensis
Peruquime arequipensis Mondaca & Valencia, 2016: 4–6 [original combination].
Distribution
PERU: Arequipa (
Types
Holotype ♂ at MHNP. Male paratypes (40) distributed at several institutions including CMNC, IEXA, MHNP, and UNSM (
PSEUDOGENIATES
Pseudogeniates Ohaus, 1910a: 179–180.
Type species
Pseudogeniates richterianus Ohaus, 1910a: 180, by monotypy.
Gender
Masculine.
Species
3 species.
Pseudogeniates cordobaensis
Pseudogeniates cordobaensis Soula, 2009: 122 [original combination, unavailable name].
Pseudogeniates cordobaensis Soula [in Jameson & Ocampo, 2012, unavailable name].
Pseudogeniates cordobaensis Moore, Jameson, Garner, Audibert, Smith, and Seidel, sp. n.
Distribution
ARGENTINA: Catamarca, Córdoba (
Types
Holotype and 7 paratypes. 1 ♂ holotype at ZMHB (= paralectotype of Pseudogeniates intermedius) (see
Remarks
For all new species-group names, the holotype and the type depository must be explicitly stated for the name to be deemed available (ICZN Art. 16.4). Because
Description of Pseudogeniates cordobaensis, new species
A full redescription of the species was provided in (
Pseudogeniates intermedius
Pseudogeniates intermedius Ohaus, 1914: 303 [original combination].
Distribution
ARGENTINA: Santiago del Estero (
Types
1 ♂ lectotype and 3 paralectotypes at ZMHB (
Pseudogeniates richterianus
Pseudogeniates richterianus Ohaus, 1910a: 180 [original combination].
Pseudogeniates richteri Ohaus, 1934b: T. 2, f. 6 [lapsus]
Distribution
ARGENTINA: Buenos Aires, Mendoza, Neuquén, Río Negro, San Juan, Santa Fe (
Types
1 ♀ lectotype and 1 paralectotype at ZMHB (
SOROCHA
Sorocha Soula, 2006: 86–87.
Type species
Pelidnota acutipennis F. Bates, 1904: 255, 263–264, original designation.
Gender
Feminine.
Species
16 species and subspecies.
Remarks
Sorocha acutipennis
Pelidnota acutipennis F. Bates, 1904: 255, 263–264 [original combination].
Pelidnota (Ganonota) acutipennis
F. Bates [new subgeneric combination by
Pelidnota (Strigidia) acutipennis
F. Bates [new subgeneric combination by
Pelidnota (Odontognathus) acutipennis
F. Bates [new subgeneric combination by
Sorocha acutipennis
(F. Bates) [new combination by
Pelidnota (Strigidia) acutipennis
F. Bates [revised combination and revised subgeneric combination by
Sorocha acutipennis (F. Bates) [revised combination].
Distribution
VENEZUELA: Merida, Tachira (F.
Types
Soula designated 1 ♀ syntype, but he did not provide the depository (
Remarks
CCECL contains a specimen of S. acutipennis labeled as a male alloréférent with the following data: 1 ♂ alloréférent: “Zea-Mérida I/2002 VENEZUELA Col. Andrés Varga//Alloréférent ♂ de Strigidia acutipennis (Oh.) M. SOULA det 2005” (47030980). Genitalia card-mounted underneath the male alloréférent. Box 4618687 SOULA. While clarifying the subgeneric classification of Pelidnota (due to homonymy of the genus-group name Odontognathus Laporte),
Sorocha bousqueti
Sorocha bousqueti Soula, 2006: 88–89 [original combination].
Distribution
PERU: San Martin (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Pérou Moyobamba M. de Mathan 1888//Holotype Sorocha bousqueti Sou. Soula” (47030964). Genitalia card-mounted underneath the male holotype. Box 4618687 SOULA.
Sorocha champenoisi
Sorocha champenoisi Soula, 2006: 94 [original combination].
Distribution
PERU: Huánuco (
Types
The following specimen is deposited at CMNC. 1 ♂ holotype: “PERU Huanuco Tingo Maria Universidad Coll. Martínez Dic.-974//H. & A. HOWDEN COLLECTION ex. A. Martinez coll.//Holotype 2006 Sorocha champenoisi S. Soula”.
Sorocha chapellei
Sorocha chapellei Demez & Soula, 2011: 79–80 [original combination].
Distribution
PERU: Ucayali, Junín (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♂ paratype: “Atalaya Pérou VIII/2010 M. SOULA det 19//Holotype 2010 Sorocha chapellei S. Soula” (47030967); “Satipo Rio Tambo M. SOULA det 19 [obverse] IX/2010//Allotype 2010 Sorocha chapellei S. Soula” (47030968); “Satipo Rio Tambo M. SOULA det 19 [obverse] IX/2010//Paratype 2010 Sorocha chapellei S. Soula” (47030969). Genitalia card-mounted underneath the male holotype. Box 4618687 SOULA.
Sorocha damasoi
Sorocha damasoi Soula, 2006: 92 [original combination].
Distribution
PERU: Huánuco (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 2 ♂ paratypes: “Chinchao//Holotype 2005 Sorocha damasoi Sou. Soula” (47030977); “Tingo Maria Pérou, X/2005//Paratype 2005 Sorocha damasoi Sou. Soula det.” (47030978); “Tingo Maria Pérou, X/2005//Paratype Sorocha damasoi Sou. Soula det. 2005” (47030979). Genitalia card-mounted underneath the male holotype and the two male paratypes. Box 4618687 SOULA.
Sorocha lamasi lamasi
Sorocha lamasi lamasi Soula, 2006: 91–92 [original combination].
Distribution
PERU: Pasco (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype, 1 ♂ paratype, 2 ♀ paratypes: “Oxapampa (1800m) 10/88//Holotype Sorocha lamasi Sou. Soula det. 2005” (47030970); “Oxapampa (1800m) 10/88//Allotype Sorocha lamasi Sou. Soula det. 2005” (47030971); three paratypes with identical label data: “Oxapampa (1800m) 10/88//Paratype Sorocha lamasi Sou. Soula det. 2005” (47030972 to 47030974). Genitalia card-mounted underneath the male holotype. Box 4618687 SOULA.
Sorocha lamasi satipoensis
Sorocha lamasi satipoensis Soula, 2006: 92 [original combination].
Distribution
PERU: Junín (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♀ allotype: “Satipo Junin Pérou, II/III/2004//Holotype 2006 Sorocha lamasi satipoensis Soula det. Sou.” (47030975); “Satipo Junin Pérou, II/III/2004//Allotype 2006 Sorocha lamasi satipoensis Soula det. Sou.” (47030976). Genitalia card-mounted underneath the male holotype. Box 4618687 SOULA.
Sorocha martinezi
Sorocha martinezi Soula, 2006: 93–94 [original combination].
Distribution
BOLIVIA: Cochabamba (
Types
The following specimen is deposited at CCECL. 1 ♂ paratype: “BOLIVIA D° Cochabamba Pcia. Chapare Yungas del Palmas LOCOTAL, 1200 m Coll. Martínez Marz. 952//Pelidnota ♂ [plicipennis crossed out] Ohs A. MARTINEZ-DET.1967//H. & A. HOWDEN COLLECTION ex A. Martinez coll.//Paratype Sorocha martinezi S. 2006 Soula” (47030981). Box 4618688 SOULA. The following specimens are deposited at CMNC: 5 ♂ and 4 ♀ paratypes all with the same locality label as the paratype above, 1 ♂ paratype “BOLIVIA D° Cochabamba Pcia. Chapare Km. 145.-1200 mts. Locotal Coll. Martínez. Feb.-952//H. & A. HOWDEN COLLECTION ex A. Martinez coll.//Pelidnota ♂ plicipennis Ohs. A. MARTINEZ-DET.1967//Paratype Sorocha martinezi S. 2006 Soula”.
Remarks
The holotype of Sorocha martinezi is deposited at CMNC (
Sorocha marxi
Sorocha marxi Soula, 2006: 90 [original combination].
Distribution
ECUADOR: Napo (
Types
The following specimens are deposited at CCECL. 1 ♂ holotype, 1 ♂ probable paratype, 1 ♀ paratype: “ECUADOR NAPO SUMACO 10-20 NOV 1995 ABrarrágan//Holotype 2005 Sorocha marxi Sou. Soula” (47030965); “ECUADOR OCCIDENTE CANAR Rte Gun El Triumfo parroquia Chontamarca (500 m) 14 mars 1980 Rec. PORION-BERTRAND//Paratype Sorocha marxi S. 2005 Soula” (47030966); “ECUADOR OCCIDENTE CANAR Rte Gun El Triumfo parroquia Chontamarca (500 m) 09 mars 1980 Rec. PORION-BERTRAND//Holotype 2006 Sorocha marxi occidentalis S. Soula//Probable paratype Sorocha marxi Soula det. M. R. Moore ‘15” (47030952). Genitalia card-mounted underneath the male holotype. Box 4618687 SOULA and 4616344 PORION. An exemplar specimen is figured (Fig.
Sorocha maylini
Sorocha maylini Soula, 2006: 90–91 [original combination].
Distribution
BOLIVIA: Santa Cruz (DJCC). PERU: Puno (
Types
According to
Sorocha nadiae
Pelidnota (Odontognathus) nadiae Martínez, 1978: 125–129 [original combination].
Sorocha nadiae
(Martínez) [new combination by
Pelidnota (Strigidia) nadiae
Martínez [revised combination and new subgeneric combination by
Sorocha nadiae (Martínez) [revised combination].
Distribution
ECUADOR: Pichincha (
Types
Holotype specimen of Pelidnota (Odontognathus) nadiae at MACN; 1 ♂ paratype at CMNC.
Remarks
CCECL contains a S. nadiae specimen labeled as a female alloréférent with the following data: 1 ♀ alloréférent: “Pacto Pichincha Equa. M. SOULA det 19/Sorocha nadiae (Mar.) M. SOULA det [19 crossed out] 2006//Alloreferent ♀ Sorocha nadiae det. M. R. Moore 2014 (M)” (47030992). Box 4618688 SOULA. While clarifying the subgeneric classification of Pelidnota (due to homonymy of the genus-group name Odontognathus Laporte),
Sorocha plicipennis
Pelidnota (Ganonota) plicipennis Ohaus, 1934a: 10–11 [original combination].
Pelidnota (Strigidia) plicipennis
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) plicipennis
Ohaus [new subgeneric combination by
Sorocha plicipennis
(Ohaus) [new combination by
Pelidnota (Strigidia) plicipennis
(Ohaus) [revised combination and revised subgeneric combination by
Sorocha plicipennis (Ohaus) [revised combination].
Distribution
BOLIVIA: La Paz (
Types
1 ♂ type specimen of Pelidnota (Ganonota) plicipennis Ohaus at ZMHB (Fig.
Remarks
While clarifying the subgeneric classification of Pelidnota (due to homonymy of the genus-group name Odontognathus Laporte),
Sorocha similis
Pelidnota similis Ohaus, 1908b: 400–401 [original combination].
Pelidnota (Ganonota) similis
Ohaus [new subgeneric combination by
Pelidnota (Strigidia) similis
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) similis
Ohaus [new subgeneric combination by
Sorocha similis
(Ohaus) [new combination by
Pelidnota (Strigidia) similis
Ohaus [revised combination and revised subgeneric combination by
Sorocha similis (Ohaus) [revised combination].
Distribution
ECUADOR: Morona-Santiago, Zamora Chinchipe (1908b, 1918, 1925, 1934b,
Types
1 possible ♂ holotype of Pelidnota similis Ohaus at ZMHB (Fig.
Remarks
While clarifying the subgeneric classification of Pelidnota (due to homonymy of the genus-group name Odontognathus Laporte),
Sorocha tolimana
Pelidnota tolimana Ohaus, 1935: 121–122 [original combination].
Pelidnota (Pelidnota) tolimana
Ohaus [new subgeneric combination by
Sorocha tolimana
(Ohaus) [new combination by
Distribution
COLOMBIA: Tolima (
Types
1 ♂ syntype specimen of Pelidnota tolimana Ohaus at ZMHB (Fig.
Sorocha touroulti
Sorocha touroulti Soula, 2009: 135 [original combination].
Distribution
BOLIVIA (
Types
The following specimen is deposited at CCECL. 1 ♂ holotype: “Rte de Chaparé pk 96. BO. 2000 m M. SOULA det 19 [obverse] 4/10/07//Holotype 2009 Sorocha touroulti S. Soula” (47030982). Genitalia card-mounted underneath the male holotype. Box 4618688 SOULA.
Sorocha yungana
Pelidnota (Ganonota) yungana Ohaus, 1934a: 11 [original combination].
Pelidnota (Strigidia) yungana
Ohaus [new subgeneric combination by
Pelidnota (Odontognathus) yungana
Ohaus [new subgeneric combination by
Sorocha yungana
(Ohaus) [new combination by
Pelidnota (Strigidia) yungana
Ohaus [revised combination and revised subgeneric combination by
Sorocha yungana (Ohaus) [revised combination].
Distribution
BOLIVIA: La Paz (
Types
Remarks
While clarifying the subgeneric classification of Pelidnota (due to homonymy of the genus-group name Odontognathus Laporte),
Unavailable, invalid names in Sorocha
Sorocha ferrandi in litt.; Unavailable, invalid name
Types
The following specimen is deposited at CCECL. 1 invalid ♂ holotype: “Route de Loreto à Coca P.L. pk 11 - Napo. (1450 m) (E) [obverse] 13/8/88 P.W.//Holotype 2006 Sorocha ferrandi Sou. Soula//Invalid type not described det. M. R. Moore 2014” (47030983). Genitalia card-mounted underneath the invalid male holotype. Box 4618688 SOULA.
Remarks
We regard the name “Sorocha ferrandi” as an unavailable name because it was not associated with a species description and because its continued use may lead to further nomenclatural instability. The male specimen in CCECL is an invalid holotype specimen because the species name is not associated with a species description or type designation in the published literature.
Unavailable names in Sorocha (application of ICZN Article 16.4.2)
We consider the following names proposed by Soula in Sorocha as unavailable per ICZN Article 16.4.2. which states that fixation of holotype specimens for new names must be accompanied by the following information, “where the holotype or syntypes are extant specimens, by a statement of intent that they will be (or are) deposited in a collection and a statement indicating the name and location of that collection”. The names below were proposed by
Sorocha carloti
Sorocha carloti Demez & Soula, 2011: 79 [original combination, unavailable, invalid name].
Distribution
PERU: Pasco (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype: “Oxapampa Pérou I/2011 M. SOULA det 19//Holotype 2011 Sorocha carloti S. Soula det.” (47030986); “Oxapampa Pérou I/2011 M. SOULA det 19//Allotype 2011 Sorocha carloti S. Soula det.” (47030987). Genitalia card-mounted underneath the invalid male holotype. Box 4618688 SOULA.
Sorocha castroi
Sorocha castroi Soula, 2008: 35–36 [original combination, unavailable, invalid name].
Distribution
PERU: San Martin (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “San Martin Pérou 10/2006 M. SOULA det 19//Holotype Sorocha castroi S. Soula det. 2007” (47030984). Genitalia card-mounted underneath the invalid male holotype. Box 4618688 SOULA.
Sorocha fravali
Sorocha fravali Soula, 2011: 80–81 [original combination, unavailable, invalid name].
Distribution
VENEZUELA: Yaracuy (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “VENEZUELA Yaracuy Via Cocorote - El Candelo 10,36889°N - 68,82689°W 1650m 15-21-x-2001//R. Briceño; J. Clavijo; L.J. Joly; F. Díaz; E. Arcaya; R. Paz Proyecto S1-2000000479//Holotype 2011 Sorocha fravali S. Soula” (47030963). Genitalia card-mounted underneath the invalid male holotype. Box 4618687 SOULA.
Sorocha jeanmaurettei
Sorocha jeanmaurettei Demez & Soula, 2011: 81 [original combination, unavailable, invalid name].
Distribution
PERU: Cusco (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype, 1 invalid ♂ paratype, 1 invalid ♀ paratype: “Valle Cosnipata 2600 m Paucartambo M. SOULA det 19 [obverse] Cusco III/2011//Holotype 2011 Sorocha jeanmaurettei D. et S. Soula” (47030988); “Valle Cosnipa Paucartambo 2800 m M. Soula det. 20 [obverse] III/2011 Cusco//Allotype 2011 Sorocha jeanmaurettei D. et S. Soula” (47030989); “Valle Cosnipa Paucartambo 2800 m M. Soula det. 20 [obverse] III/2011//Paratype Sorocha jeanmaurettei D. et S. Soula” (47030990); “Valle Cosnipa Paucartambo 2800 m M. Soula det. 20 [obverse] III/2011 Cusco//Paratype 2011 Sorocha jeanmaurettei D. et S. Soula” (47030991). Genitalia card-mounted underneath the invalid male holotype and the invalid male paratype. Box 4618688 SOULA.
Sorocha yelamosi
Sorocha yelamosi Soula, 2011: 82 [original combination, unavailable, invalid name].
Distribution
PERU: Junín (
Types
The following invalid type specimen is deposited at CCECL. 1 invalid ♂ holotype: “Satipo Pérou IX/2003 M. SOULA det 19//Holotype 2011 Sorocha yelamosi S. Soula det.” (47030985). Genitalia card-mounted underneath the invalid male holotype. Box 4618688 SOULA.
XENOPELIDNOTA
Xenopelidnota F. Bates, 1904: 253, 275–276.
Type species
Plusiotis anomala Burmeister, 1844: 422-423, subsequent designation (monotypy) by F.
Gender
Feminine.
Species
3 species and subspecies.
Xenopelidnota anomala anomala
Plusiotis anomala Burmeister, 1844: 422–423 [original combination].
Pelidnota anomala
(Burmeister) [new combination by
Xenopelidnota anomala
(Burmeister) [new combination by F.
Distribution
BOLIVIA (
Types
1 ♂ neotype of Plusiotis anomala at MNHN (
Remarks
CCECL contains a X. anomala specimen labeled as a female alloréférent with the following data: 1 ♀ alloréférent: “Colüm-bia//Alloréferent ♀ de Plusiotis anomala Burm M. SOULA det 19” (47030552). Box 4618669 SOULA.
Xenopelidnota anomala porioni
Xenopelidnota anomala porioni Chalumeau, 1985: 236–237 [original combination].
Xenopelidnota pittieri porioni
Chalumeau [new combination by
Xenopelidnota anomala porioni Chalumeau [revised subspecific status].
Distribution
GRENADA (FSCA) (
Types
1 ♂ holotype at IREC. Paratypes at IREC, MNHN (Fig.
Remarks
Xenopelidnota fuscoaenea
Pelidnota fuscoaenea Blanchard, 1851: 211 [original combination].
Xenopelidnota anomala
(Burmeister) [syn. by
Xenopelidnota fuscoaenea
(Blanchard) [revised species status by
Distribution
COLOMBIA (
Types
1 ♀ syntype of Pelidnota fuscoaenea at MNHN (
Remarks
Specific locality data reported for this species in the literature is highly uncertain. Per
Unavailable names in Xenopelidnota (application of ICZN Article 16.4.2)
We consider the following names proposed by Soula in Xenopelidnota as unavailable per ICZN Article 16.4.2 which states that fixation of holotype specimens for new names must be accompanied by the following information, “where the holotype or syntypes are extant specimens, by a statement of intent that they will be (or are) deposited in a collection and a statement indicating the name and location of that collection”. The names below were proposed by
Xenopelidnota bolivari
Xenopelidnota bolivari Soula, 2009: 125 [original combination, unavailable, invalid name].
Distribution
VENEZUELA: Bolívar (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype: “Jabillal Bolivar Venez. 7/89 coll. – SOULA//Holotype 2009 Xenopelidnota bolivari S. Soula” (47030553); “Jabillal Bolivar Venez. 7/89 M. SOULA det. 19//Allotype 2009 Xenopelidnota bolivari S. Soula” (47030554). Box 4618669 SOULA.
Xenopelidnota pittieri pittieri
Xenopelidnota pittieri pittieri Soula, 2009: 126–127 [original combination, unavailable, invalid name].
Distribution
VENEZUELA: Aragua, Distrito Federal (
Types
The following invalid type specimens are deposited at CCECL. 1 invalid ♂ holotype, 1 invalid ♀ allotype, 2 invalid ♂ paratypes, 5 invalid ♀ paratypes: “P. N. Henri Pittier Choroni, Venezuela V-VI/2005//Holotype 2009 Xenopelidnota pittieri S. Soula” (47030555); “P. N. Henri Pittier Choroni, Venezuela V-VI/2005//Allotype 2009 Xenopelidnota pittieri S. Soula” (47030556); Five paratypes with identical label data “P. N. Henri Pittier Choroni, Venezuela V-VI/2005//Paratype 2009 Xenopelidnota pittieri S. Soula” (47030557 to 47030561); “Caracas M. SOULA det. 19//Paratype 2009 Xenopelidnota pittieri S. Soula” (47030562); “Guiana M. SOULA det. 19//Paratype 2009 Xenopelidnota pittieri S. Soula” (47030563). Genitalia card-mounted underneath the invalid male holotype, invalid female allotype, and two invalid male paratypes. Box 4618669 SOULA.
Remarks
Acknowledgments
Complicated nomenclatural research of this nature is possible only with the assistance of many colleagues, to whom we are extremely grateful. We deeply thank the experts who assisted with interpretation of nomenclature: David Notton (NHM and ICZN), Max Barclay (NHM), J. Howard Frank (Univ. of Florida), and Francisco Welter-Schultes (Universität Göttingen). François Génier (CNMC) is gratefully acknowledged for his translations and interpretations of Soula’s works. Oliver Keller (Univ. of Florida) is thanked for his translation of the difficult German in Gistel and his editorial comments on the manuscript. Katherine M. Arguez (Univ. of Florida) and Keita Matsumoto (NHM) are thanked for help in imaging and preparing figures for publication. J. Howard Frank (Univ. of Florida) is thanked for his help in determining gender of generic names. Hitoshi Takano (NHM) and Andreas Reichenbach (Leipzig, Germany) are thanked for their comments on the key to genera. We are grateful for the assistance of curators, collections managers, and museum personnel who kindly loaned us specimens for this study or provided us specimen data (listed in “Specimens and taxonomic material”). For assistance in locating the vast literature for this research, we thank Leccinum J. Garciá Morales (Herbarium of the Museo de Historia Natural de Tamaulipas, Ciudad Victoria, Tamaulipas, México) and Andrés Ramírez Ponce (Laboratorio Regional de Biodiversidad y Cultivo de Tejidos Vegetales, Instituto de Biologia, UNAM, Tlaxcala, México). For assistance with the Burmeister collection, we thank Karla Schneider and Joachim Händel (MLUH). We are very grateful for images of rare pelidnotines that were provided by Denis Bouchard (Autouillet, France), José Monzon (Santiago, Chile), and Federico Ocampo (Pergamino, Argentina). We thank Ángel Solís (MNCR) for access to the Pelidnota DNA barcode data from Costa Rica. This research was supported by The Natural History Museum Departmental Investment Fund (Insects Division, Life Sciences) to Garner, Jameson, and Moore (with special thanks to Theresa Howard); SVV 260 434/2017 (Department of Zoology, Charles University, Prague) to Seidel; Entomology and Nematalogy Student Organization Travel Grant (Department of Entomology and Nematology, University of Florida) to Moore. Funds for publication were provided by Wichita State University, Wichita, Kansas. Author contributions (in order of effort): MLJ, MRM conceived and designed research; MRM, MLJ, MS, BHG, CA, ABTS wrote the paper and analyzed data; MRM, MLJ, MS, ABTS identified specimens; MS, MRM, MLJ, CA, BHG processed images; BHG, MRM secured funding for travel to CCECL; MLJ secured funding for publication.
References
- Ahrens D, Scott M, Vogler AP (2011) The phylogeny of monkey beetles based on mitochondrial and ribosomal RNA genes (Coleoptera: Scarabaeidae: Hopliini). Molecular Phylogenetics and Evolution 60: 408–415. https://doi.org/10.1016/j.ympev.2011.04.011
- Alcázar-Ruiz JA., Morón-Rios A, Morón MA (2003) Fauna de Coleoptera Melolonthidae de Villa las Rosas, Chiapas, México. Acta Zoológica Mexicana (N. S.) 88: 59–86.
- Aragón-García A, Morón MA, Damián-Huato MA, López-Olguín JF, Pinson-Rincón EP, Pérez-Quintanilla JN (2012) Fauna de Coleoptera Lamellicornia de la zona cañera del ingenio de Atencingo, Puebla, México. Acta Zoológica Mexicana (N. S.) 28: 161–171.
- Arnaud P (1994) Description d’un Plusiotis nouveau (Coleoptera, Rutelinae). Bulletin de la Société Sciences Nat 82: 36–37.
- Arrow GJ (1900) On Pleurostict Lamellicorns from Grenada and St. Vincent (West Indies). Transactions of the Royal Entomological Society of London 48: 175–182. https://doi.org/10.1111/j.1365-2311.1900.tb00999.x
- Arrow GJ (1919) Notes on ruteline Coleoptera and descriptions of a few new species in the British Museum. The Annals and Magazine of Natural History, Ninth Series, 4: 379–385. https://doi.org/10.1080/00222931908673907
- Bates F (1904) A revision of the sub-family Pelidnotinae of the coleopterous family Rutelidae, with descriptions of new genera and species. Transactions of the Entomological Society of London 1904: 249–276. https://doi.org/10.1111/j.1365-2311.1904.tb02746.x
- Bates HW (1888–1889) Insecta. Coleoptera. Vol. II. Part 2. Pectinicornia and Lamellicornia. In: Salvin O, Godman F du Cane (Eds) Biologia Centrali-Americana. R. H. Porter, London, 1–432.
- Bernardi O, Silveira Garcia M, Ely e Silva EJ, Zazycki LCF, Bernardi D, Miorelli D, Ramiro GA, Finkenauer É (2010) Coleópteros coletados com armadilhas luminosas e etanólicas em plantio de Eucalyptus spp. no sul do Rio Grande do Sul. Ciência Florestal, Santa Maria 20: 579–588. https://doi.org/10.5902/198050982416
- Biederman CR (1907) Notes on Plusiotis beyeri Skinner. Entomological News 18: 7–9.
- Billberg GJ (1820) Novae insectorum species, descriptae. Memoires de L’Académie Impériale des Sciences de St. Pétersbourg 7: 381–395.
- Blackaller-Bages J, Delgado L (1994) Plusiotis citlaltepetlamayatli, a new species of the lecontei group from Mexico (Coleoptera: Melolonthidae; Rutelinae). The Journal of the New York Entomological Society 102: 79–85.
- Blackwelder RE (1939) Fourth supplement 1933 to 1938 (inclusive) to the Leng catalogue of Coleoptera of America, north of Mexico. John D. Sherman, Jr., Mount Vernon, 146 pp.
- Blackwelder RE (1944) Checklist of the Coleopterous Insects of Mexico, Central America, the West Indies, and South America. United States National Museum Bulletin 185: 189–265.
- Blackwelder RE (1952) Preprints of Proc. U.S. National Museum, 1890-1897. Systematic Zoology 1: 86–89. https://doi.org/10.2307/2411369
- Blanchard CÉ (1842) Famille des Scarabéiens, plate 11. In: Bertrand P (Ed.) Voyage dans l’Amérique Méridionale: (Le Brésil, la République Orientale de l’Uruguay, la République Argentine, la Patagonie, la République du Chile, la République de Bolivia, la République du Pérou), exécuté pendant les années 1826, 1827, 1828, 1829, 1830, 1831, 1832, et 1833 par Alcide d’Orbigny, volume 6, part 2: Insectes. V. Levrault, Strasbourg, plate 11.
- Blanchard CÉ (1851) Ordre des Coleoptera. In: Milne-Edwards H, Blanchard CÉ, Lucas H (Eds) Muséum d’Histoire Naturelle de Paris. Catalogue de la Collection Entomologique. Classe des Insectes. Volume 1. Part 1. Gide and Baudry, Paris, 129–240.
- Boucard A (1875) Monographic list of the Coleoptera of the genus Plusiotis of America, north of Panama, with descriptions of several new species. Proceedings of the Zoological Society of London 1875: 117–125.
- Boucard A (1878) Notes on some Coleoptera of the genus Plusiotis, with descriptions of three new species from Mexico and Central America. Proceedings of the Zoological Society of London 1878: 293–296. https://doi.org/10.1111/j.1469-7998.1878.tb07957.x
- Boucard A (1895) Description of a supposed new species of Plusiotis, from Mexico. The Humming Bird 5: 4–5.
- Bouchard D (2003) Description de deux nouvelles espèces de Chalcoplethis Burmeister, 1844 (Coleoptera, Rutelidae). Coléoptères 9: 103–108.
- Bouchard P, Bousquet Y, Davies AE, Alonso-Zarazaga MA, Lawrence JF, Lyal CH, Newton AF, Reid CA, Schmitt M, Ślipiński SA, Smith ABT (2011) Family-group names in Coleoptera (Insecta). ZooKeys 88: 1–972. https://doi.org/10.3897/zookeys.88.807
- Bubna M (1902) Coleoptera of Cuyahoga County, Ohio. The Ohio Naturalist 2: 193–197.
- Burmeister HCC (1844) Handbuch der Entomologie (Coleoptera Lamellicornia Anthobia et Phyllophaga Systellochela). Volume 4, Part 1. T. C. F. Enslin, Berlin, 588 pp.
- Burmeister HCC (1855) Handbuch der Entomologie (Coleoptera Lamellicornia Phyllophaga chaenochela). Volume 4, Part 2. T. C. F. Enslin, Berlin, 570 pp.
- Buss EA (2006) Flight activity and relative abundance of phytophagous scarabs (Coleoptera: Scarabaeoidea) from two locations in Florida. Florida Entomologist 89: 32–40. https://doi.org/10.1653/0015-4040(2006)89[32:FAARAO]2.0.CO;2
- Bustos-Santana HF, Rivera-Cervantes LE (2003) Abundancia estacional de los coleópteros nocturnos de la familia Melolonthidae (Insecta: Lamelicornia), asociados a un bosque de pino-encino en la Sierra de Quila, Jalisco, México. In: Aragón GA, Morón MA, Marín Jarillo A (Eds) Estudios sobre coleópteros del suelo en América. Publicación especial de la Benemérita Universidad Autónoma de Puebla, Puebla, 137–148.
- Brady P, Cummings M (2010) Differential response to circularly polarized light by the jewel scarab beetle Chrysina gloriosa. The American Naturalist 175: 614–620. https://doi.org/10.1086/651593
- Camacho Cárdenas LF (2015) Vulnerability of a jewel scarab (Coleoptera, Scarabaeidae, Rutelinae) in a highly fragmented light-polluted landscape in Ecuador. Masters Thesis, Quito, Ecuador: Pontificia Universidad Católica del Ecuador, Facultad de Ciencias Exactas y Naturales, Escuela de Ciencias Biológicas.
- Cano EB, Morón MA (1994) Una nueva especie Guatemalteca de Plusiotis Burmeister del grupo lacordairei (Coleoptera: Melolonthidae, Rutelinae). Folia Entomológica Mexicana 91: 1–8.
- Carpenter FM (1992) Treatise on Invertebrate Paleontology, Part R, Arthropoda 4, Volume 3 and 4: Superclass Hexapoda. Geological Society of America and University of Kansas, Boulder, Colorado and Lawrence, Kansas, 655 pp.
- Carrillo-Ruiz H, Morón MA (2003) Fauna de Coleoptera Scarabaeoidea de Cuetzalan del Progreso, Puebla, México. Acta Zoológica Mexicana (N. S. ) 88: 87–121.
- Carrillo JL, Ortega-C A Gibson WW (1966) Lista de insectos en la coleccion entomologica del Instituto Nacional de Investigaciones Agricolas. Primer suplemento a la “Lista de Insectos de la colección entomológica de la Oficina de Estudios Especiales, S. A. G.”. Instituto Nacional de Investigaciones Agricola, S. A. G. Mexico, Folleto Misceláneo 14: 1–133.
- Casey TL (1915) A review of the American species of Rutelinae, Dynastinae, and Cetoniinae. Memoires on the Coleoptera, Volume 6: 1–394.
- Castañeda-Osorio R, Carrillo-Ruiz H, Rivas-Arancibia SP, Sánchez-Carrillo M (2015) Melolonthidae y Cetoniidae (Coleoptera: Scarabaeoidea) en el Rancho El Salado, Jolalpan, Puebla, México. Dugesiana 22: 227–241. https://doi.org/10.1093/jee/tov209
- Cazier MA (1951) The genera Chrysina and Plusiotis of north central Mexico (Coleoptera, Scarabaeidae). American Museum Novitates 1516: 1–8.
- Chalumeau F (1985) Les Rutelinae (Coleoptera: Scarabaeidae) des Antilles. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 58: 231–260.
- Chevrolat A (1859) Description d’une nouvelle espèce de Lamellicorne du genre Chrysine (sic) de Kirby. Revue et Magasin de Zoologie Pure et Appliquée, Serie 2(11): 481–483.
- Chong J-H, Hinson KR (2015) A comparison of trap types for assessing diversity of Scarabaeoidea on South Carolina golf courses. Journal of Economic Entomology 108: 2383–2396.
- Cockerell TDA (1920) LXI. Fossil arthropods in the British Museum. II. Annals and Magazine of Natural History, Series 9(5): 455–463.
- Coolidge KR (1911) Plusiotis beyeri Skinner. Entomological News 22: 326–327.
- Cuate VA, Aragón A, Morón MA (2013) Capítulo 14. Región de Chiautla. In: Morón MA, Aragón A, Carrillo-Ruiz H (Eds) Fauna de escarabajos del estado de Puebla. M. A. Morón, Coatepec, 297–322.
- Curoe DJ (1994) Two new Plusiotis Burmeister from Honduras (Coleoptera: Scarabaeidae). Giornale Italiano di Entomologia 7: 35–39.
- Curoe DJ (1999) A new Plusiotis Burmeister of the victorina species group (Coleoptera: Scarabaeidae). Occasional Papers of the Consortium Coleopterorum 3: 1–4.
- Curoe DJ (2001) Description of a new Plusiotis Burmeister with notes on Plusiotis ohausi Franz (Coleoptera: Scarabaeidae: Rutelinae). Occasional Papers of the Consortium Coleopterorum 4: 45–49.
- Curoe DJ (2011) A new species of Chrysina of the marginata complex (Coleoptera: Scarabaeidae: Rutelinae). Besoiro: Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale 20: 2–4.
- Curoe DJ (2012) Two new species of Chrysina Kirby of the aurora group (Coleptera: Scarabaeidae: Rutelinae). Giornale Italiano di Entomologia 13: 9–15.
- Curoe DJ, Beraud JP (1994) A new Plusiotis Burmeister from Mexico (Chiapas) and Guatemala (Coleoptera: Scarabaeidae). Giornale Italiano di Entomologia 36(7): 31–33.
- Dechambre R-P (2001) Néallotype ou alloréférent: de l’utilité de ce concept et de son bon usage. Le Coléoptériste 43: 163–164.
- Dejean PFMA (1821) Catalogue de la Collection de Coléoptères. Crevot, Paris, 136 pp.
- Delgado-Castillo L (2003) A new Mexican species of Chrysina Kirby (Coleoptera, Melolonthidae, Rutelinae). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 76: 319–321.
- Delgado-Castillo L, Deloya C, Morón MA (1988) Descripcion de dos nuevas especies mexicanas de Pelidnota (Coleoptera: Melolonthidae; Rutelinae). Folia Entomológica Mexicana 74: 131–144.
- Delgado-Castillo L, Márquez J (2006) Estado del conocimiento y conservación de los coleópteros Scarabaeoidea (Insecta) del estado de Hidalgo, México. Acta Zoológica Mexicana (N. S. ) 22: 57–108.
- Delgado-Castillo L, Mora-Aguilar EF, Escobar-Hernandez F (2012) Scarabaeoidea (Coleoptera) of the municipality of Xalapa, Veracruz, Mexico: Inventory and Analysis. The Coleopterists Bulletin 66: 319–332. https://doi.org/10.1649/072.066.0405
- Deloya C, Burgos A, Blackaller J, Lobo JM (1993) Los coleopteros lamelicornios de Cuernavaca, Morelos, México (Passalidae, Trogidae, Scarabaeidae y Melolonthidae). Boletín Sociedad Veracruzana de Zoología 3: 15–55.
- Deloya C, Calvo-Gatica H, García-Díaz OJ, Rendón-Sosa M, González-Hilario S, Aguirre-León G (2014) Familia Scarabaeidae Latreille, 1802. In: Deloya C, Melgar DC (Eds) Escarabajos del Estado de Guerrero (Coleoptera: Scarabaeoidea). S y G Editores, Mexico City, 69–116.
- Demez P, Soula M (2010) [new taxa]. In: Soula M (2010a) Les Coléoptères du Nouveau Monde. Volume 4: Rutelini 4. Révision des Pelidnotina 4. Une révision des genres Catoclastus, Homonyx. Retour sur quelques espèces ou groupe d’espèces de Pelidnota. Addenda 2010. (Coleoptera: Scarabaeidae: Rutelinae: Rutelini: Pelidnotina). Besoiro: Supplément au Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale. AECFT, Saintry, 66 pp.
- Demez P, Soula M (2011) [new taxa]. In: Soula M (2011) Les Coléoptères du Nouveau Monde. Volume 5: Geniatini 1. Révision du genre Bolax (Coleoptera: Scarabaeidae: Rutelini: Geniatini). Besoiro: Supplément au Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale. AECFT, Saintry, 85 pp.
- Dohrn CA (1883) Exotisches. Entomologische Zeitung 44: 495–500.
- Dreschsel U (2014) A first glance to “Reserva Natural Privada Morombi”. Paraguay Biodiversidad 1: 26–32.
- Drury D (1773) Index to the First Volume. Names of the insects, according to the System of Linnaeus. London, 2 pp.
- Drury D (1782) Illustrations of Natural History. Wherein are exhibited upwards of two hundred figures of exotic insects. According to their different genera; Very few of which have hithero been figured by any author, being engraved and coloured from nature, with the greatest accuracy, and under the author’s own inspection, on fifty copper-plates. With a particular description of each insect: interpreted with remarks and reflections on the nature and properties of many of them. Volume 3. London, 76 pp. [+ plates]
- Ebrard D, Soula M (2010) Description d’une nouvelle Chrysina. Besoiro: Supplément au Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale. AECFT (Saintry) 19: 7–9.
- Endrödi S (1977) Alissonotum piceum besucheti subsp. n. (Col. Melolonthidae, Dynastinae). Revue Suisse de Zoologie 84: 319–321. https://doi.org/10.5962/bhl.part.91390
- Endrödi S (1985) The Dynastinae of the World. Dr. W. Junk Publisher, Dordrecht, 800 pp. [+ 46 plates]
- Erichson GF (1847) Conspectus insectorum coleopterorum, quae in Republica Peruana observata sunt. Archiv für Naturgeschichte 1847: 67–185.
- Evans AV (2003) A checklist of the New World chafers (Coleoptera: Scarabaeidae: Melolonthinae). Zootaxa 211: 1–458. https://doi.org/10.11646/zootaxa.211.1.1
- Evans AV, Smith ABT (2009) An electronic checklist of the New World chafers (Coleoptera: Scarabaeidae: Melolonthinae). Version 30. http://www.museum.unl.edu/research/entomology/SSSA/nwmelos.htm
- Evenhuis NL (2016) The insect and spider collections of the world website. http://hbs.bishopmuseum.org/codens/
- Fabricius JC (1775) Systemat entomologiae, sistens insectorum classes, ordines, genera, sepcies adiectis synonymis, locis, desciptionibus, observationibus. Kortii, Flensburgi et Lipsiae [=Flensburg & Leipzig]. 8 vol. [32] + 832 p.
- Ferrú MA, Elgueta M (2011) Lista de coleópteros (Insecta: Coleoptera) de las regiones de Arica y Parinacota y de Tarapacá, Chile. Boletín del Museo Nacional de Historia Natural, Chile 60: 9–61.
- Francillon J (1795) Description of a rare Scarabaeus from Potosi, in South America; with engraved representations of the same, coloured from nature. C. Whittingham, London, 2 pp. [+ 1 plate]
- Franz E (1928) Plusiotis ohausi sp. n. (Coleopt.). Senckenbergiana 10: 3–5.
- Freitas FA de, Zanuncio TV, Lacerda MC, Zanuncio JC (2002) Fauna de Coleoptera coletada com armadilhas luminosas em plantio de Eucalyptus grandis em Santa Bárbara, Minas Gerais. Revista Árvore 26: 505–511. https://doi.org/10.1590/S0100-67622002000400014
- Frey G (1967) Neue Ruteliden aus dem Museum G. Frey (Col.). Entomologische Arbeiten aus dem Museum G. Frey Tutzing bei Muenchen 18: 374–383.
- Frey G (1976) Neue südamerikanische Ruteliden. Entomologische Arbeiten aus dem Museum G. Frey Tutzing bei Muenchen 27: 344–356.
- García AA, García GAL, Olivas AR, Álvarez PC, Cota JRV, Morón MA (2009) Huéspedes vegetales de adultos de Coleoptera Scarabaeoidea en el Valle del Carrizo, Sinaloa, México. Southwestern Entomologist 35: 99–108. https://doi.org/10.3958/059.035.0111
- Garcia FP, Rodrigues SR, Bagnara CAC, de Oliveira DS (2013) Survey of saproxylophagous Melolonthidae (Coleoptera) and some biological aspects in Aquidauana, MS. Biota Neotropica 13(3): 38–43. https://doi.org/10.1590/S1676-06032013000300004
- García-Atencia S, Martínez-Hernández N (2015) Escarabajos fitófagos (Coleoptera: Scarabaeidae) del departamento del Atlántico, Colombia. Acta Zoológica Mexicana (N. S. ) 31: 89–96. https://doi.org/10.1016/j.rmb.2015.07.009
- García-Atencia S, Martínez-Hernández N, Pardo-Locarno C (2015) Escarabajos fitófagos (Coleoptera: Scarabaeidae) en un fragmento de bosque seco tropical del Atlántico, Colombia. Revista Mexicana de Biodiversidad 86: 754–763. https://doi.org/10.1016/j.rmb.2015.07.009
- García-López A, Micó E, Numa C, Galante E (2010) Spatiotemporal variation of scarab beetle assemblages (Coleoptera: Scarabaeidae: Dynastinae, Melolonthinae, Rutelinae) in the premontane rain forest in Costa Rica: A question of scale. Conservation Biology and Biodiversity 103: 956–964. https://doi.org/10.1603/an10078
- García-López A, Micó E, Múrria C, Galante E, Vogler AP (2013) Beta diversity at multiple hierarchical levels: explaining the high diversity of scarab beetles in tropical montane forests. Journal of Biogeography 40: 2134–2145. https://doi.org/10.1111/jbi.12148
- García-Montiel JC, Rivera-Cervantes LE, Morón MA (2003) Composición y abundancia estacional de los Melolonthidae nocturnos (Insecta: Coleoptera) asociados a un bosque mesofilo de Montaña, en el municipio de Miniatitlán, Colima, México. In: Aragón GA, Morón MA, Marín Jarillo A (Eds) Estudios sobre coleópteros del suelo en América. Publicación especial de la Benemérita Universidad Autónoma de Puebla, Puebla, 115–127.
- Garner B, Audibert C (2015) The Musée des Confluences, Lyon (France) – research and repatriation of specimens from the Soula collection of Rutelini. Scarabs 78: 1–11.
- Germar EF (1815) Südamerikanische Insekten, gesammelt von v. Humbolt und Bonpland, auf ihrer Reise im südlichen America; beschrieben von P. A. Latreille. Magazin der Entomologie 1(2): 104–121.
- Germar EF (1824) Insectorum Species. Novae aut Minus cognitae, descriptionibus illustratae. Volume 1. Coleoptera. J. C. Hendelii et Filii, Halle, 624 pp. [+ 2 plates]
- Gibson WW, Carrillo SJ (1959) Lista de insectos en la coleccion entomologica de la Oficina de Estudios Especiales, S.A.G. Folleto Misceláneo 9: 1–254.
- Gillett CPDT (2009) A new country record for Chrysina diversa (Ohaus, 1912) (Coleoptera: Scarabaeidae: Rutelinae) in Central America. Insecta Mundi 108: 1–3.
- Gistel JNFX (1850) Handbuch der Naturgeschichte aller drei Reiche, für Lehrer und Lernende, für Schule und Haus. Hoffmann, Stuttgart, 1037 pp. https://doi.org/10.5962/bhl.title.37040
- Gistel JNFX (1857) Achthundert und zwanzig neue oder unbeschriebene wirbellose Thiere. Schorner, Straubing, 94 pp.
- Gory HL (1833a) Description de deux Coléoptères nouveaux des genres Rutela et Buprestis. Annales de la Société Entomologique de France 2: 67–68.
- Gory HL (1833b) [new taxa N°13, Areoda maculata Gory]. Revue Entomologique (Silbermann) 1: N°13. [unpaginated]
- Gory HL (1834) Deux Rutela nouvelles. Annals de la Société Entomologique de France 3: 111–112.
- Gory HL (1846) [new taxa]. In: Bertrand P (Ed.) Voyage dans L’Amérique Méridionale: (Le Brésil, la République Orientale de l’Uruguay, la République Argentine, la Patagonie, la République du Chile, la République de Bolivia, la République du Pérou), exécuté pendant les années 1826, 1827, 1828, 1829, 1830, 1831, 1832, et 1833 par Alcide d’Orbigny, volume 6, part 2: Insectes. Chez V. Levrault, Strasbourg, 192.
- Gottsberger G, Silberbauer-Gottsberger I (2006) Life in the Cerrado: A South American tropical seasonal ecosystem. Volume II. Pollination and seed dispersal. Reta Verlag, Ulm, 385 pp.
- Gray GR (1832) Supplement on the Lamellicornes. In: Griffith E, Pidegon E (Eds) The animal kingdom arranged in conformity with its organization by the Baron Cuvier. Volume 14. The Class Insecta (Part I). Whittaker, Teacher & Co., London, 504–537.
- Gruner L (1971) Scarabaeidae Melolonthidae, Dynastinae, Rutelinae, Cetoniinae [Coleoptera] récoltés en Guyane Française par la mission du Muséum National d’Histoire Naturelle (1). Société Entomologique de France (N. S. ) 7: 843–848.
- Guérin-Méneville FÉ (1834) Iconographie du Règne Animal de G. Cuvier, ou Représentation d’Après Nature de L’une des Espèces les Plus Remarquables, et Souvent non Encore Figurées, de Chaque Genre d’Animaux. Avec un texte descriptif mis au courant de la science. Ouvrage Pouvant Servir d’Atlas à Tous les Traités de Zoologie. Insectes. Vol. 7. J. B. Baillière, Paris, 576 pp. [+ 110 plates]
- Guérin-Méneville FÉ (1839) Description de quelques Coléoptères des côtes du détroit de Magellan. Revue Zoologique, par la Société Cuvierienne 1839: 295–305.
- Guérin-Méneville FÉ (1855) Catalogue des Insectes Coléoptères, recueillis par M. Gaetano Osculati, pendant son exploration de la région équatoriale, sur les bords du Napo et l’Amazone. Verhandlungen des zoologisch-botanischen Vereins in Wien 5: 573–612.
- Guimarães LR (1944) Rutelidae, Cetonidae, Melolonthidae e Dynastidae de Monte Alegre. Papéis Avulsos do Departamento de Zoologia (São Paulo) 6: 93–102.
- Gutiérrez AR (1949) Notas sobre Scarabaeidae Neotropicos (Coleoptera Lamellicornia). Anales de la Sociedad Científica Argentina 148: 9–35.
- Gutiérrez AR (1950) Scarabaeidae del Norte de Chile. Anales de la Sociedad Científica Argentina 149(2): 52–75.
- Gutiérrez AR (1951) Notas sobre Scarabaeidae neotrópicos II (Coleopt. Lamellic.). Anales de la Sociedad Científica Argentina 151: 106–125.
- Gutiérrez AR (1952) Notas sobre Scarabaeidae neotrópicos (III) (Coleoptera). Revista Chilena de Entomología 2: 207–227.
- Gyllenhaal L (1817) [new taxa]. In: Schönherr CJ (Ed.) Appendix ad C. J. Schönherr synonymiam insectorum, Tom 1. Part. 3. Sistens descriptiones novarum specierum. F. Leverentz, Skara, 1–266.
- Hardy AR (1974) New synonymies in North American Pelidnota. Proceedings of the Entomological Society of Washington 76: 89.
- Hardy AR (1975) A revision of the genus Pelidnota of America north of Panama (Coleoptera: Scarabaeidae: Rutelinae). University of California Publications in Entomology 78: 1–43.
- Hardy AR (1985) Pelidnota punctulata in Costa Rica: A correction (Coleoptera: Scarabaeidae). The Coleopterists Bulletin 39: 199.
- Hardy AR (1991) A catalog of the Coleoptera of America north of Mexico. Family: Scarabaeidae, Subfamilies: Rutelinae and Dynastinae. United States Department of Agriculture, Agriculture Handbook 529-34b, 56 pp.
- Harold E (1869a) [new names] Abänderungen vergebener Namen. In: Harold E (Ed.) Coleopterologische Hefte, Vol. 1, Part 5. Carl Merhoff’s Verlag, Munich, 122–125.
- Harold E (1869b) Scarabaeidae. In: Gemminger M, Harold E (Eds) Catalogus Coleopterorum Hucusque Descriptorum Synonymicus et Systematicus, Vol. 4. E. H. Gummi, Munich, 979–1346.
- Harpootlian PJ (2001) Scarab beetles (Coleoptera: Scarabaeidae) of South Carolina. Biota of South Carolina. Vol. 2. Clemson University, Clemson, 157 pp.
- Hawks DC (1995) Plusiotis alfredolaui, a new sibling species of P. badeni Boucard (Coleoptera: Scarabaeidae: Rutelinae). Insecta Mundi 9: 273–276.
- Hawks DC (1999) A review of the Plusiotis marginata complex including the description of a new species (Coleoptera: Scarabaeidae: Rutelinae). Occasional Papers of the Consortium Coleopterorum 3: 21–29.
- Hawks DC (2001) Taxonomic and nomenclatural changes in Chrysina and synonymic checklist of species (Scarabaeidae: Rutelinae). Occasional Papers of the Consortium Coleopterorum 4: 1–8.
- Hawks DC (2006) Checklist to Chrysina species (Scarabaeidae: Rutelinae: Rutelini) (http://www.museum.unl.edu/research/entomology/Guide/Scarabaeoidea/Scarabaeidae/Rutelinae/Rutelinae-Tribes/Rutelini/Chrysina/Chrysina-Catalog/ChrysinaC.html). In: Ratcliffe BC, Jameson ML (Eds) Generic Guide to New World Scarab Beetles. http://www-museum.unl.edu/research/entomology/Guide/index4.htm [Accessed on: August 20, 2016]
- Hawks DC, Bruyea GP (1999) Chrysina cavei, a new species from Honduras (Coleoptera: Scarabaeidae: Rutelinae). Occasional Papers of the Consortium Coleopterorum 3: 15–20.
- Hawksworth DL (2010) Terms used in bionomenclature: the naming of organisms and plant communities: including terms used in botanical, cultivated plant, phylogenetic, phytosociological, prokaryote (bacteriological), virus, and zoological nomenclature. Global Biodiversity Information Facility, Copenhagen, 215 pp.
- Hayes WP (1928) The epipharynx of lamellicorn larvae (Coleop.), with a key to common genera. Annals of the Entomological Society of America 21: 282–306. https://doi.org/10.1093/aesa/21.2.282
- Hayes WP (1929) Morphology, taxonomy, and biology of larval Scarabaeoidea. Illinois Biological Monographs 12: 1–119.
- Hayes WP, McColloch JW (1928) Ecological studies of Kansas scarabaeid larvae (Coleop.). Journal of Economic Entomology 21: 249–260. https://doi.org/10.1093/jee/21.2.249
- Herbst JFW (1789) Natursystem aller bekannten in- und ausländlischen Insekten, als eine Fortsetzung der von Büffonschen Naturgeschichte. Nach dem System des Ritters von Linné und Fabricius zu bearbeiten angefangen von Carl Gustav Jablonsky. Der Käfer zweyter Theil. Pauli, Berlin, 336 pp.
- Herbst JFW (1790) Natursystem aller bekannten in- und ausländlischen Insekten, als eine Fortsetzung der von Büffonschen Naturgeschichte. Der Käfer dritter Theil. Pauli, Berlin, 325 pp.
- Hicks SD (1965) The northern limits of several species of Coleoptera with special reference to their occurrence in the Ottawa district, Ontario. The Coleopterists Bulletin 19: 37–42.
- Hilje L (1996) Estacionalidad de adultos de Scarabaeidae (Coleoptera) en Barva, Costa Rica. Revista de Biología Tropical 44: 719–729.
- Hoffmann CH (1926) Additions to our knowledge of the biology of Pelidnota punctata Linn. (Scarabaeidae-Coleoptera). Journal of the Kansas Entomological Society 9: 103–105.
- Hope FW (1837) The coleopterists manual, containing the lamellicorn insects of Linneus and Fabricius. Henry G. Bohn, London, 121 pp. [+ 3 plates]
- Hope FW (1841) On a new species of Dynastes and other Coleoptera. The Annals and Magazine of Natural History 7: 147.
- Horn GH (1883) Notes on some little known genera and species of Coleoptera. Transactions of the American Entomological Society 10: 113–126.
- Horn GH (1884) Vice-Director Dr. Horn in the chair. Proceedings of the Monthly Meetings of the Entomological Section of the Academy of Natural Sciences, Philadelphia (November 9, 1883), 31–32.
- Horn GH (1883) Notes on some little known genera and species of Coleoptera. Transactions of the American Entomological Society 10: 113–126.
- Horn GH (1884) November 9, 1884. Vice-Director Dr. Horn in the chair. Proceedings of the Monthly Meetings of the Entomological Section of the Academy of Natural Sciences, Philadelphia (November 9, 1883): 31–32.
- Horn GH (1885) Descriptions of new North American Scarabaeidae. Transactions of the American Entomological Society 12: 117–128. https://doi.org/10.2307/25076453
- Howden HF (1998) A new species of Pelidnota MacLeay from Chiriqui province, Panama (Scarabaeidae, Rutelinae). The Coleopterists Bulletin 52: 171–173.
- International Commission on Zoological Nomenclature (ICZN) (1999) International Code of Zoological Nomenclature, Fourth Edition. International Trust for Zoological Nomenclature, London, 306 pp.
- International Commission on Zoological Nomenclature (ICZN) (2003) Opinion 2054 (Case 3201). Scarabaeus punctatus Villers, 1789 (currently Pentodon bidens punctatus; Insecta, Coleoptera): specific name conserved. Bulletin of Zoological Nomenclature 60: 247–248.
- Jameson ML (1990) Revision, phylogeny and biogeography of the genera Parabyrsopolis Ohaus and Viridimicus, new genus (Coleoptera: Scarabaeidae: Rutelinae). The Coleopterists Bulletin 44: 377–422.
- Jameson ML (1998) Phylogenetic analysis of the subtribe Rutelina and revision of the Rutela generic groups (Coleoptera: Scarabaeidae: Rutelinae: Rutelini). Bulletin of the University of Nebraska State Museum 14: 1–184. [Dated 1997]
- Jameson ML (2005) Key to tribes of Rutelinae (Scarabaeidae). http://museum.unl.edu/research/entomology/Guide/Scarabaeoidea/Scarabaeidae/Rutelinae/Rutelinae-Key/RutelinaeK.html
- Jameson ML, Drumont A (2013) Aroid scarabs in the genus Peltonotus Burmeister (Coleoptera, Scarabaeidae, Dynastinae): key to species and new distributional data. ZooKeys 320: 63–95. https://doi.org/10.3897/zookeys.320.5352
- Jameson ML, Ocampo F (2012) Synopsis of the Argentinian scarab genus Pseudogeniates Ohaus (Coleoptera, Scarabaeidae, Rutelinae). ZooKeys 241: 33–53. https://doi.org/10.3897/zookeys.241.3802
- Jameson ML, Ratcliffe BC (2011) The Neotropical scarab beetle tribe Anatistini (Coleoptera: Scarabaeidae: Rutelinae). Bulletin of the University of Nebraska State Museum 26: i–iv, 1–100.
- Jameson ML, Wada K (2004) Revision of the genus Peltonotus Burmeister (Coleoptera: Scarabaeidae: Dynastinae) from Southeastern Asia. Zootaxa 502: 1–66. https://doi.org/10.11646/zootaxa.502.1.1
- Jameson ML, Wada K (2009) Five new species of Peltonotus Burmeister (Scarabaeidae: Dynastinae: Cyclocephalini) from Southeast Asia. Insecta Mundi 102: 1–16.
- Jelínek J, Audisio P (2009) The Kateretidae, Nitidulidae and Monotomidae (Coleoptera: Cucojoidea) described by Gistel (1856, 1857): new synonymies and type designations. Acta Entomologica Musei Nationalis Pragae 49: 225–238.
- Jocque M, Vanhove MPM, Creedy TJ, Burdekin O, Nuñez-Miño JM, Casteels J (2013) Jewel scarabs (Chrysina sp. ) in Honduras: Key species for cloud forest conservation monitoring? Journal of Insect Science 13(21): 1–18. https://doi.org/10.1673/031.013.2101
- King IN (1914) The Coleoptera of Henry County, Iowa. Proceedings of the Iowa Academy of Science 21: 317–340.
- Kirby W (1819) A century of insects, including several new genera described from his cabinet. The Transactions of the Linnean Society of London 12(2): 291–598. https://doi.org/10.1111/j.1095-8339.1817.tb00239.x
- Kirby W (1824) Hundert Kerfe, worunter mehrere neue Sippen beschrieben. Isis von Oken 14: 112–134.
- Kirby W (1828) A description of some coleopterous insects in the collection of Rev. F. W. Hope. The Zoological Journal 3, 1827 (1828): 520–525.
- Kirk VM, Balsbaugh Jr. EU (1975) A list of the beetles of South Dakota. South Dakota State University Agricultural Experiment Station Technical Bulletin 42: 1–139.
- Kirsch T (1885) Neue südamerikanische Käfer. Berliner Entomologische Zeitschrift 29: 207–224. https://doi.org/10.1002/mmnd.18850290206
- Knapp S, Lamas G, Nic Lughadha E, Novarino G (2004) Stability or stasis in the names of organisms: the evolving codes of nomenclature. Philosophical Transactions of the Royal Society B: 611–622. https://doi.org/10.1098/rstb.2003.1445
- Krajcik M (2008) Animma. X. Supplement 4. Checklist of the Scarabaeoidea of the World. 2. Rutelinae (Coleoptera: Scarabaeidae: Rutelinae). Second Edition. Plzen, 142 pp.
- Krajcik M (2012) Checklist of the world Scarabaeoidea. Animma. X Supplement 5, Plzen, 278 pp.
- Krajcik M (2013) Addenda to the checklist of world Scarabaeoidea 2012. Animma.X. No. 49–54, Plzen, 299 pp.
- Krell F-T (2006) Fossil record and evolution of Scarabaeoidea (Coleoptera: Polyphaga). Coleopterists Society Monograph 5: 120–143.
- Krell F-T, Rey A, Micó E, Dutto M (2012) On nomenclature and identity of Scarabaeus aeruginosus Linnaeus, S. aeruginosus Drury and S. speciosissimus Scopoli (Coleoptera: Scarabaeoidea: Cetoniinae and Rutelinae). Revue Suisse de Zoologie 119: 99–110.
- Kriska NL, Young DK (2002) An annotated checklist of Wisconsin Scarabaeoidea (Coleoptera). Insecta Mundi 16: 31–48.
- Lacordaire JT (1856) Histoire Naturelle de Insectes. Genera des Coléoptères ou Exposé Méthodique et Critique de Tous les Genres Proposés Jusqu’ici dans cet Ordre d’Insectes. Contenant les Familles de Pectinicornes et Lamellicornes, Volume 3. Librairie Encyclopédique de Roret, Paris, 594 pp.
- Landin B-O (1956) The Linnean species of Lamellicornia described in “Systema Naturae”, Ed. X (1758). (Col.). Entomologisk Tidskrift 77(1): 1–18.
- Laporte [=Castelnau] FLNCde (1840) Histoire naturelle des insectes Coléoptères; avec une introduction renfermant l’anatomie et la physiologie des animaux articulés, par M. Brullé. Tome premier. Histoire naturelle des animaux articulés, annélides, crustacés, arachnides, myriapodes et insectes Tome troisième. P. Duménil, Paris, 324 pp. [+ 19 plates]
- Latreille PA (1812) Insectes de l’Amérique équinoxiale recueillis pendant le voyage de M. M. de Humboldt et Bonpland. In: Cuvier G, Humboldt A, Bonpland A (Eds) Voyage de Humboldt et Bonpland, deuxième partie. Observations de zoologie et d’anatomie comparée 1. F. Schoell et G. el Dufour, De L’Imprimerie de J. H. Stône, Paris, 127–252.
- LeConte JL (1854) Descriptions of new Coleoptera collected by Thos. H. Webb, M. D., in the years 1850–1851 and 52, while Secretary to the U. S. and Mexican Boundary Commission. Proceedings of the Academy of Natural Sciences of Philadelphia 7: 220–225.
- LeConte JL (1863) New species of North American Coleoptera. Part 1. Smithsonian Miscellaneous Collections 167: 1–177.
- LeConte JL (1874) Descriptions of new Coleoptera chiefly from the Pacific Slope of North America. Transactions of the American Entomological Society 5: 43–72. https://doi.org/10.2307/25076287
- Leng CW (1920) Catalogue of the Coleoptera of America, north of Mexico. John D. Sherman, Jr., Mount Vernon, 470 pp.
- Leng CW, Mutchler AJ (1914) A preliminary list of the Coleoptera of the West Indies as recorded to January 1, 1914. Bulletin of the American Museum of Natural History 33: 391–493.
- LePeletier ALM, Serville JGA (1828) Chrysophore, Chrysophora. In: Latreille PA, LePeletier ALM, Serville JGA, Guérin-Méneville FE (Eds) , Encyclopédie Méthodique. Histoire Naturelle. Entomologie, ou Histoire Naturelle des Crustacés, des Arachnides et des Insectes, Volume 10, Part 2. Mme veuve Agasse, Paris, 806–807.
- Le Tirant S, Limoges R (2016) Notes on Chrysophora chrysochlora (Coleoptera: Scarabaeidae: Rutelinae: Rutelini). Scarabs 82: 1–4.
- Linell ML (1895) Description of a new species of golden beetle from Costa Rica. SHEET No. 10: 1–2.
- Lingafelter SW, Nearns EH (2013) Elucidating Article 45.6 of the International Code of Zoological Nomenclature: A dichotomous key for the determination of subspecific or infrasubspecific rank. Zootaxa 3709(6): 597–600. https://doi.org/10.11646/zootaxa.3709.6.9
- Linnaeus C (1758) Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis (10 edn), volume 1. Laurentii Salvii, Stockholm, 823 pp.
- Linnaeus C (1764) Museum S:ae R:ae Ludovicae Ulricae Reginae. Svecorum, Gothorum, Vandalorumque &c. &c. &c. In quo Animalis rariora, exotica, imprimis Insecta & conchilia describuntur & determinantur. Prodromus instar editum. Laurentii Salvii, Stockholm, vi + 720 + [2]. https://doi.org/10.5962/bhl.title.119811
- Linnaeus C (1767) Systema Naturae per regna tria naturae secundum classes, ordines, genera, species, cum characteribus, differentis, synonymis, locis edito dudecima. reformata (12th edn). Vol. 1, part 2. Laurentii Salvii, Stockholm, 553–1327.
- Löbl I, Smetana A (Eds) (2006) Catalogue of Palearctic Coleoptera. Vol. 3. Scarabaeoidea – Scirtoidea – Dascilloidea – Buprestoidea – Byrrhoidea. Apollo Books, Stenstrup, 690 pp.
- Lobo JM, Morón MA (1993) La modificación de las comunidades de coleópteros Melolonthidae y Scarabaeidae en dos áreas protegidas mexicanas tras dos décadas de estudios faunísticos. Giornale Italiano de Entomologia 6: 391–406.
- López-García MM, García-Atencia S, Amat-García G (2015) Escarabajos fitófagos (Coleoptera: Scarabaeidae “Pleurosticti”) de los Andes orientales de Colombia (Departamentos de Santander, Boyacá y Cundinamarca). Boletín Científico Centro de Museos: Museo de Historia Natural 19: 322–358.
- Lucas MH (1865) Note sur les Plusiotis adelaida et costata, coléoptères de la famille des Lamellicornes et de la tribu des Rutélides. Annales de la Société Entomologique de France, Série 4, Volume 5: 203–205.
- Lugo GA, Morón MA, Aragón-Garcia A, Ortega-Arenas LD (2011) Especies fotófilas de Coleoptera Lamellicornia en la region de Los Tascates, Sinaloa y Chihuahua (México). Boletín de la Sociedad Entomológica Aragonesa 49: 179–188.
- Lugo GA, Morón MA, Aragón A, Ortega-Arenas LD, Reyes-Olivas A, Sánchez BH (2013) Especies nocturnas de Scarabaeoidea (Coleoptera: Polyphaga) en el norte de Sinaloa, México. Revista Colombiana de Entomología 39: 95–104.
- Lugo GA, Morón MA, Aragón-García A, Ortega-Arenas LD, Reyes-Olivas A, Sánchez-Soto H (2014) Coleoptera Scarabaeoidea capturados con trampa de luz en bosque tropical caducifolio del Norte de Sinaloa, México. Southwestern Entomologist 39: 355–366.
- Lugo GA, Ortega-Arenas LD, Aragón-García A, González-Hernández H, Romero-Nápoles J, Reyes-Olivas Á, Morón MA (2012) Especies de gallina ciega (Coleoptera: Scarabaeoidea) asociadas al cultivo de maíz en Ahome, Sinaloa, México. Agrociencia 46: 307–320.
- Lunz MA, Vaz-de-Mello FZ, Monteiro OM (2011) Pelidnota filippiniae Soula, 2009 (Coleoptera: Melolonthidae: Rutelinae): a new defoliator of Australian wattle, Racosperma mangium (Willd.) (Fabaceae), in the eastern Amazon, Brazil. Acta Amazonica 41(3): 439–422. https://doi.org/10.1590/S0044-59672011000300017
- Machatschke JW (1965) Coleoptera Lamellicornia. Fam. Scarabaeidae, Subfam. Rutelinae, Section Rutelinae Orthochilidae. Genera Insectorum 199(c): 1–145.
- Machatschke JW (1970) Rutelinae (Col., Scarabaeidae): Synonymische Bemerkungen. The Entomologist’s Monthly Magazine 105: 157–158.
- Machatschke JW (1972) Scarabaeoidea: Melolonthidae, Rutelinae. Coleopterorum Catalogus Supplementa 66: 1–361.
- Machatschke JW (1974) Scarabaeoidea: Melolonthidae, Rutelinae. Coleopterorum Catalogus Supplementa 66: 363–429.
- MacLeay WS (1819) Horae Entomologicae: or essays on the annulose animals. Volume 1. Part 1. Containing: General observations on the geography, manners, and natural affinities of the insects which compose the genus Scarabaeus of Linnaeus; To which are added a few incidental remarks on the genera Lucanus and Hister of the same author. With an appendix and plates. S. Bagster, London, 524 pp.
- McNamara J (1991) Family Scarabaeidae (Scarab beetles). Checklist of the Beetles of Canada and Alaska (online version). The CanaColl Foundation http://canacoll.org/Coleo/Checklist/checklist.htm
- Maes J-M (1987) Catalogo de los Scarabaeidae (Coleoptera) de Nicaragua. Revista Nicaragüense de Entomología 1: 27–60.
- Maes J-M, Ratcliffe BC, Jameson ML (1997) Fauna entomologica de la Reserva Natural Bosawas, Nicaragua. XI. Escarabajos (Coleoptera: Scarabaeidae) nuevos para la fauna de Nicaragua. Revista Nicaragüense de Entomología 39: 41–45.
- Mannerheim CG (1829) Description de quarante nouvelles espèces de Scarabeides du Brésil. Nouveaux Mémoires de la Société Impériale des Naturalistes de Moscou 1: 29–80.
- Marley C (2008) Pheromone: The Insect Artwork of Christopher Marley. Pomegranate Publishers, Petaluma, 256 pp.
- Márquez J (2008) Escarabajos gema del estado de Hidalgo (Coleoptera: Scarabaeidae, Rutelinae). Herreriana: Revista de Divulgación de la Ciencia 4(1): 1–4.
- Márquez J, Asiain J, Morón MA, Hornung-Leoni CT (2013) Grado de conservación de los bosques del Estado de Hidalgo, México. Interciencia 38: 410–417.
- Márquez J, Sierra-Martínez S (2008) Teratología y nuevo registro de Chrysina adelaida (Hope, 1840) (Coleoptera: Scarabaeidae: Rutelinae). Dugesiana 15: 39–40.
- Márquez J, Sierra-Martínez S (2009) Nuevos datos de distribución geográfica de Chrysina peruviana Kirby (Coleoptera: Scarabaeidae: Rutelinae) en Hidalgo, México. Acta Zoológica Mexicana (N. S. ) 25: 191–193.
- Martínez A (1953) Nuevas especies de Oogenius Solier Coleoptera, Scarabaeidae, Rutelinae. Revista Chilena de Entomología 3: 75–86.
- Martínez A (1960) Una nueva especie de Eremophygus (Col., Scarab., Rutelin.). Ciencia 20: 131–133.
- Martínez A (1965) Notas Coleopterologicas X. Dos nuevas especies de Cyclocephalini Neotropicales (Dynastinae). Anales de la Sociedad Científica Argentina 179: 63–74.
- Martínez A (1967) Notas coleopterologicas XI: Comentarios sobre el genero Heteropelidnota Ohaus, con descripcion de una especie nueva (Col. Scarabaeidae, Rutelinae). Anales de la Sociedad Científica Argentina 183: 145–154.
- Martínez A (1971) Una nueva especie de Catoclastus Solier (Col. Scarab. Rutel. Pelidnotina). Neotropica XVII: 79–82.
- Martínez A (1973) El genero Neogutierrezia Martínez 1953 (Col. Scarab. Melolonth. Pachydemini). Anales de la Sociedad Científica Argentina 32: 25–41.
- Martínez A (1974) Notas sobre el género Lasiocala Blanchard, 1850 (Col., Scarabaeidae: Rutelinae). Studia Entomologica, Revista Internacional de Entomología 17: 303–312.
- Martínez A (1975a) Contribución al conocimiento de los Pachydemini neotropicales (Col. Scarabaeidae, Melolonthinae). Entomologische Arbeiten aus dem Museum G. Frey 26: 227–251.
- Martínez A (1975b) Cyclocephala sudamericanas nuevas o poco conocidas (Col. Scarabaeidae Dynastinae). Entomologische Arbeiten aus dem Museum G. Frey Tutzing bei München 26: 263–274.
- Martínez A (1978) Una nueva especie de Pelidnota de Ecuador (Col. Scarab. Rutel. Rutelini, Pelidnotina). Revista de la Sociedad Entomológica Argentina 37: 125–129.
- Martínez A (1979) Una nueva especie de Pelidnota de Venezolana (Col. Scarabaeidae, Rutelinae, Rutelini, Pelidnotina). Acta Scientifica, Serie Entomologia 13: 1–7.
- Martínez A (1982) Dos nuevas especies de Pelidnota MacLeay, 1819 (Coleoptera: Scarabaeidae: Rutelinae). Boletín de Entomología Venezolana 2: 61–68.
- Martínez A, Peña-Guzmán LE (1990) Oogenius castilloi sp. nov. (Coleoptera: Scarabaeidae). Revista Chilena de Entomología 18: 9–11.
- Martínez A, Peña-Guzmán LE (1994) Una nueva especie de Oogenius Solier, de Chile (Coleoptera: Scarabaeidae). Revista Chilena de Entomología 21: 117–120.
- McKenna DD, Farrell BD, Caterino MS, Farnum CW, Hawks DC, Maddison DR, Seago AE, Short AEZ, Newton AF, Thayer MK (2014) Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: forest litter as a stepping stone for diversification of nonphytophagous beetles. Systematic Entomology 40: 835–880. https://doi.org/10.1111/syen.12132
- Mondaca J (2005) . Utilización de Oogenius Solier, 1851 nuevo nombre para Oogenius gutierrezi Martínez y Peña, 1994 (Coleoptera: Scarabaeidae) de Chile, incluido listado de especies. Revista Chilena de Entomología 30: 17–20. [Dated 2004].
- Mondaca J (2016) Revisión del género Oogenius Solier, 1851 (Coleoptera: Scarabaeidae: Rutelinae: Rutelini). Insecta Mundi 0515: 1–24.
- Mondaca J, Valencia G (2016) Nuevo género y especie de Rutelini (Coleoptera: Scarabaeidae: Rutelinae) de los Andes peruanos. Insecta Mundi 473: 1–10.
- Montgomery BE, Amos JM (1940) Contributions to a list of the Coleoptera of the Clark County State Forest. Proceedings of the Indiana Academy of Science 50: 251–258.
- Monzón J (1995) Guatemalan Plusiotis and Chrysina (Coleoptera: Scarabaeidae: Rutelinae): new records. Insecta Mundi 9: 347–350.
- Monzón J (1996) Guía para los escarabajos (Coleoptera: Scarabaeoidea) de Guatemala. Universidad del Valle de Guatemala, Guatemala City.
- Monzón J (2010) Three new species of Chrysina Kirby (Coleoptera: Scarabaeidae; Rutelinae) from Guatemala and Mexico. Insecta Mundi 143: 1–12.
- Monzón J (2012) A new species of Chrysina Kirby (Coleoptera: Scarabaeidae: Rutelinae) from Oaxaca, Mexico. Insecta Mundi 215: 1–4.
- Monzón J, Cano EB (1999) Plusiotis ericsmithi (Coleoptera: Scarabaeidae): a new metallic species from eastern Guatemala. Insecta Mundi 13: 213–215.
- Monzón J, Cano EB, Bailey AC (1999) Notes on Guatemalan Plusiotis (Coleoptera: Scarabaeidae; Rutelinae). Insecta Mundi 13: 183–188.
- Monzón J, García LJ (2011) Two new species of Chrysina Kirby (Coleoptera: Scarabaeidae: Rutelinae) from Mexico. Insecta Mundi 195: 1–8.
- Moore MR, Jameson ML (2013) Taxonomic and nomenclatural changes in the pelidnotine scarabs (Coleoptera: Scarabaeidae: Rutelinae: Rutelini). The Coleopterists Bulletin 67: 377–387. https://doi.org/10.1649/0010-065X-67.3.377
- Moore MR, Jameson ML, Paucar-Cabrera A (2014) Taxonomic and nomenclatural changes in the anticheirine scarabs (Coleoptera: Scarabaeidae: Rutelinae: Rutelini). Insecta Mundi 392: 1–20.
- Morelli E (1996) Descripción de la larva y de la pupa de Homonyx chalcea Blanchard, 1850 (Coleoptera: Scarabaeidae, Rutelinae). Acta Zoológica Mexicana (N. S. ) 68: 53–60.
- (1976) Descripción de las larvas de tres especies mexicanas de pelidnotinos (Coleoptera, Melolonthidae, Rutelinae) y algunas observaciones sobre su biología. Anales Instituto de Biología UNAM, serie Zoología 74: 7–18.
- Morón MA (1979) Fauna de Coleópteros Lamelicornios de la estación de biología, “Los Tuxtlas”, Veracruz, Unam. México. Anales del Instituto de Biología de la Universidad Nacional Autónoma de México, Series Zoología 50: 375–454.
- Morón MA (1981) Descripción de dos especies nuevas de Plusiotis Burmeister, 1844 y discussion de algunos aspectos zoogeográficos del grupo de especies costata (Coleoptera; Melolonthidae, Rutelinae). Folia Entomológica Mexicana 49: 49–69.
- Morón MA (1985) Observaciones sobre la biologia de dos especies de rutelinos saproxilofagos en la Sierra de Hidalgo, Mexico. (Coleoptera: Melolonthidae: Rutelinae). Folia Entomológica Mexicana 64 (supplement): 41–53.
- Morón MA (1990) The Beetles of the World. Volume 10: Rutelini 1. Sciences Nat, Venette, 145 pp. [+ 32 plates]
- Morón MA (1991) Estudio biogeográfico-ecológico preliminar del género Plusiotis Burmeister (Coleoptera: Melolonthidae, Rutelinae). Giornale Italiano di Entomologia 5: 309–323.
- Morón MA (1992) Adiciones al género Plusiotis Burmeister, 1844 (Coleoptera: Melolonthidae, Rutelinae). Giornale Italiano di Entomologica 6: 59–78.
- Morón MA (1993) Los lamelicornios (Insecta: Coleoptera) de las Sierras Húmedas del estado de Hidalgo, México: Una síntesis taxonómica y ecológica. In: Villavicencio MA, Marmolejo Y, Pérez Escandon BE (Eds) Investigaciones recientes sobre Flora y Fauna de Hidalgo, México. Universidad Autónoma de Hidalgo, Pachuca, 181–211.
- Morón MA (1994) Fauna de Coleoptera Lamellicornia en las montañas del noreste de Hidalgo, México. Acta Zoológica Mexicana (N. S. ) 63: 7–59.
- Morón MA (2010) Observaciones sobre la reproducción y el ciclo vital de Plusiotis costata Blanchard, 1851 (Coleoptera: Melolonthidae: Rutelinae). Acta Zoológica Mexicana (N. S. ) 26: 705–720.
- Morón MA, Deloya C. (1991) Los coleopteros lamelicornios de la Reserva de la Biosfera “La Michilia”, Durango, Mexico. Folia Entomológica Mexicana 81: 209–283.
- Morón MA, Deloya C (2002) Observaciones sobre el ciclo de vida de Pelidnota (Pelidnota) virescens Burmeister, 1844 (Coleoptera: Melolonthidae; Rutelinae). Acta Zoológica Mexicana (N. S. ) 85: 109–118.
- Morón MA, Deloya C, Delgado-Castillo L (1988) Fauna de coleopteros Melolonthidae, Scarabaeidae y Trogidae de la region de Chamela, Jalisco, México. Folia Entomológica Mexicana 77: 313–378.
- Morón MA, Deloya C, Ramírez-Campos A, Hernández-Rodriquez S (1998) Fauna de Coleoptera Lamellicornia de la region de Tepic, Nayarit, Mexico. Acta Zoológica Mexicana (N. S. ) 75: 73–116.
- Morón MA, Howden HF (1992) A new species of Plusiotis Burmeister (Coleoptera: Scarabaeidae, Rutelinae) from Guatemala, C.A. The Canadian Entomologist 124: 205–210. https://doi.org/10.4039/Ent124205-2
- Morón MA, Márquez J (2012) Nuevos registros estatales y nacionales de escarabajos (Coleoptera: Scarabaeoidea) y comentarios sobre su distribución. Revista Mexicana de Biodiversidad 83: 698–711. https://doi.org/10.7550/rmb.28386
- Morón MA, Nogueira G (2016) Revisión del grupo de especies “lecontei” de Plusiotis (s. str.) (Coleoptera: Melolonthidae : Rutelinae). Boletín de la Sociedad Entomológica Aragonesa (S. E. A. ) 58: 6–27.
- Morón MA, Ratcliffe BC, Deloya C (1997) Atlas de los Escarabajos de México. Coleoptera: Lamellicornia. Volume 1. Familia Melolonthidae. Subfamilias Rutelinae, Dynastinae, Cetoniinae, Trichiinae, Valginae y Melolonthinae. Sociedad Mexicana de Entomología, Mexico City, 280 pp. [+ 32 plates]
- Morón MA, Villalobos FJ, Deloya C (1985) Fauna de coleopteros lamelicornios de Boca del Chajul, Chiapas, México. Folia Entomológica Mexicana 66: 57–118.
- Morón MA, Zaragoza S (1976) Coleópteros Melolonthidae y Scarabaeidae de Vill de Allende, Estado de México. Anales del Instituto de Biología, Universidad Nacional Autónoma de México. Serie Zoología 47: 83–118.
- Morón-Ríos A, Morón MA (2001) La fauna de Coleoptera Melolonthidae de la reserva de la biósfera “El Triunfo”, Chiapas, México. Acta Zoológica Mexicana (N. S. ) 84: 1–25.
- Morón-Ríos A, Morón MA (2016) Evaluación de la fauna de Coleoptera Scarabaeoidea en la Reserva de la Biósfera de Calakmul, Campeche, México. Southwestern Entomologist 41: 469–484. https://doi.org/10.3958/059.041.0217
- Morrill WL (1979) Coloration and temporal flight activity of some scarabiids. Journal of the Georgia Entomological Society 14: 255–258.
- Muñoz-Hernández A, Morón MA, Aragón A (2008) Coleoptera Scarabaeoidea de la región de Teziutlán, Puebla, México. Acta Zoológica Mexicana (N. S. ) 24(3): 55–78.
- Müller P (1973) The dispersal centres of terrestrial vertebrates in the Neotropical realm. Dr. W. Junk, The Hague, 244 pp.
- Natural History Museum (2014) Dataset: Index Lot collection. Resource: Index Lots. http://dx.doi.org/10.5519/0073880
- Neita-Moreno JC (2011) Escarabajos (Coleoptera: Scarabaeoidea) del departamento del Chocó, Colombia. Revista Biodiversidad Neotropical 1: 17–27. https://doi.org/10.18636/bioneotropical.v1i1.25
- Neita-Moreno JC, Orozco-Araujo J, Ratcliffe BC (2006) Escarabajos (Scarabaeidae: Pleurosticti) de la selva baja del bosque pluvial tropical “BP-T”, Chocó, Colombia. Acta Zoológica Mexicana (N. S. ) 22: 1–32.
- Nogueira G, Curoe D (2012) Description of new species of Chrysina (Coleoptera, Melolonthidae, Rutelinae). Besoiro 22: 2–5.
- Nonfried AF (1891) Beitrag zu einer Monographie der Gattung Plusiotis Burm. Wiener Entomologische Zeitung 10: 300–306.
- Nonfried AF (1892) Beitrag zu einer Monographie der Gattung Plusiotis Burm. Wiener Entomologische Zeitung 11: 127–130. https://doi.org/10.5962/bhl.part.27703
- Nonfried AF (1894) Beschreibungen neuer Lamellicornier, Buprestiden und Cerambyciden aus Central- und Süd-Amerika. Entomologische Nachrichten 20: 113–142. https://doi.org/10.5962/bhl.part.19849
- Ocampo FC, Ruiz-Manzanos E, Marvaldi AE (2010) Systematic revision, cladistics and biogeography of the genus Neogutierrezia Martínez (Coleoptera: Scarabaeidae) and its phylogenetic placement in Rutelinae based on structural alignment of 28S rDNA sequences. Invertebrate Systematics 24: 81–111. https://doi.org/10.1071/IS09035
- O’Hara JE (1995) Henry Walter Bates–his life and contributions to biology. Archives of Natural History 22: 195–219. https://doi.org/10.3366/anh.1995.22.2.195
- Ohaus F (1898) Ruteliden der neuen Welt. Stettiner Entomologische Zeitung 59: 42–63.
- Ohaus F (1900) Bericht über eine entomologische Reise nach Centralbrasilien. Stettiner Entomologische Zeitung 61: 164–191.
- Ohaus F (1903) Verzeichnis der von Herrn Richard Haensch in Ecuador gesammelten Ruteliden (Coleoptera lamellicornia). Berliner Entomologische Zeitschrift 48: 215–239. https://doi.org/10.1002/mmnd.19030480306
- Ohaus F (1905) Beiträge zur Kenntniss der amerikanischen Ruteliden. Stettiner Entomologische Zeitung 66: 283–329.
- Ohaus F (1908a) Die Ruteliden meiner Sammelreisen in Südamerika (Col.). Deutsche Entomologische Zeitschrift 1908: 239–262.
- Ohaus F (1908b) Die Ruteliden meiner Sammelreisen in Südamerika (Col.). Deutsche Entomologische Zeitschrift 1908: 383–408.
- Ohaus F (1908c) Beitrag zur Kenntnis der amerikanischen Ruteliden. Annales de la Société Entomologique de Belgique 52: 299–308.
- Ohaus F (1910a) Neue Coleoptera lamellicornia aus Argentinien. Deutsche Entomologische Zeitschrift 1910: 173–186.
- Ohaus F (1910b) Beiträge sur Kenntnis der Ruteliden. VII. Annales de la Société Entomologique de Belgique 54: 213–227. https://doi.org/10.5962/bhl.part.21454
- Ohaus F (1910c) Nachträge und Berichtigungen zu meiner Revision der Brachysterniden (Coleopt. Lamellicornia). Entomologische Zeitung 71: 3–26.
- Ohaus F (1912) Beiträge zur Kenntnis der Ruteliden, X. Stettiner Entomologische Zeitung 73: 273–319.
- Ohaus F (1913) XI. Beitrag zur Kenntnis der Ruteliden. (Col.). Deutsche Entomologische Zeitschrift 1913: 487–511.
- Ohaus F (1914) Neue Coleoptera lamellicornia aus Argentinien. IV. Deutsche Entomologische Zeitschrift 1914: 299–304.
- Ohaus F (1915a) Eremophygus lasiocalinus sp. n. (Col. lamell. Rutelin.). Deutsche Entomologische Zeitschrift 1915: 76–77.
- Ohaus F (1915b) XVI. Beitrag zur Kenntnis der Ruteliden. (Col. lamell.). Deutsche Entomologische Zeitschrift 1915: 256–260.
- Ohaus F (1918) Scarabaeidae: Euchirinae, Phaenomerinae, Rutelinae. Coleopterorum Catalogus 20: 1–241.
- Ohaus F (1922) XIX. Beitrag zur Kenntnis der Ruteliden (Col. Lamell.). Deutsche Entomologische Zeitschrift 1922: 323–331. https://doi.org/10.1002/mmnd.192219220310
- Ohaus F (1924) Plusiotis strasseni new sp. Senckenbergiana 6: 185–186.
- Ohaus F (1925) XXI. Beitrag zur Kenntnis der Ruteliden. (Col. lamell.). Deutsche Entomologische Zeitschrift 1925: 75–83. https://doi.org/10.1002/mmnd.192519250106
- Ohaus F (1929) XXV. Beitrag zur Kenntnis der Rutelin. (Col. lamell.). Deutsche Entomologische Zeitschrift 1928: 385–406.
- Ohaus F (1930a) XXVI. Beitrag zur Kenntnis der Rutelinen (Col. lamell.). Deutsche Entomologische Zeitschrift 1930: 138–158. https://doi.org/10.1002/mmnd.193019300205
- Ohaus F (1930b) Eine neue Plusiotis aus Guatemala. Stettiner Entomologische Zeitung 91: 265–266.
- Ohaus F (1934a) XXVII. Beitrag zur Kenntnis der Ruteliden (Col. Scarabaeidae). Mitteilungen der Deutschen Entomologischen Gesellschaft 5: 9–15. https://doi.org/10.1002/mmnz.19340050104
- Ohaus F (1934b) Coleoptera lamellicornia, Fam. Scarabaeidae, Subfam. Rutelinae. T.1: Tribus Rutelini. Genera Insectorum Fasc. 199A: 1–172.
- Ohaus F (1935) XXVIII. Beitrag zur Kenntnis der Rutelinae (Col. Scarab.). Deutsche Entomologische Zeitschrift 1935: 121–130. https://doi.org/10.1002/mmnd.193519350107
- Ohaus F (1952) Rutelinae (Col. Scarab.). In: Titschack E (Ed.) Beiträge zur Fauna Perus. Gustav Fischer, Jena, Germany, 1–10.
- Oliveira DS, Faria TAC, Gomes ES, Rodrigues SR (2016) Biodiversidade de Scarabaeidae saproxilófagos (Coleoptera: Scarabaeidae) em fragmento de Cerrado em Corumbá, Mato Grosso do Sul, Brasil. Entomotropica 31: 248–255.
- Olivier AG (1789) Entomologie, ou Histoire Naturelle des Insectes, avec leurs Caractères Génériques et Spécifiques, leur Description, leur Synonymie, et leur Figure Enluminée. Coléoptères, Tome Premier (genera separately paged). Baudouin, Paris, 432 pp. [xix + 2 pp]
- Olivier AG (1802) Entomologie oder Naturgeschichte der Insekten mit ihren Gattungs- und Art-Mekmalen, ihrer Beschreibung und Synonymie. Käfer. Zweiter Theil. Mit Kupfern. Karl Reichard, Braunschweig, 266 pp.
- Ortenburger AI, Hatch MH (1926) Notes on Coleoptera from southeastern Oklahoma with a few records from adjacent portions of Texas and Arkansas, including a new species. Proceedings of the Oklahoma Academy of Science 6(1926): 142–148.
- Özdikmen H (2009) Nomenclatural changes for five preoccupied scarab beetle genus group names (Coleoptera: Scarabaeidae). Munis Entomology and Zoology 4: 139–147.
- Pacheco Flores C, Castro Ramírez AE, Morón MA, Gómez y Gómez B (2008) Fauna de escarabajos melolóntidos (Coleoptera: Scarabaeoidea) en el municipio de Villaflores, Chiapas, Mexico. Acta Zoológica Mexicana (N. S. ) 24: 139–168.
- Pacheco Flores C, Deloya C, Cortés-G P (2006) Phytophagous scarab beetles from the Central Region of Guerrero, Mexico (Coleoptera: Scarabaeidae: Melolonthinae, Rutelinae, Dynastinae, Cetoniinae). Revista Colombiana de Entomología 32: 191–199.
- Palacios-Rios M, Rico-Gray V, Fuentes E (1990) Inventario preliminar de los Coleoptera Lamellicornia de la zona de Yaxchilan, Chiapas, México. Folia Entomológica Mexicana 78: 49–60.
- Paoletti MG, Buscardo E, Dufour DL (2000) Edible invertebrates among Amazonian Indians: A critical review of disappearing knowledge. Environment, Development and Sustainability 2: 195–225. https://doi.org/10.1023/A:1011461907591
- Paoletti MG, Dufour DL (2005) Edible invertebrates among Amazonian Indians: A critical review of disappearing knowledge. In: Paoletti MG (Ed.) Ecological implications of minilivestock: potential of insects, rodents, frogs, and snails. CRC Press, Boca Raton, 293–342.
- Pardo-Locarno LC (2013) Escarabajos (Coleoptera: Melolonthidae) del plan aluvial del Río Cauca, Colombia I. Ensamblaje, fichas bioecológicas, extinciones locales y clave para adultos. Dugesiana 20: 1–15.
- Pardo-Locarno LC, González JC, Pérez CR, Yepes F, Fernández C (2012) Escarabajos de importancia agrícola (Coleoptera: Melolonthidae) en la region Caribe Colombiana: registros y propuestas de Manejo. Boletín del Museo Entomológico Francisco Luís Gallego 4(4): 7–23.
- Pardo-Locarno LS, Morón MA (2007) Larva and pupa of Chrysophora chrysochlora (Coleoptera: Scarabaeidae: Rutelinae: Rutelini). The Canadian Entomologist 139: 80–86. https://doi.org/10.4039/n06-002
- Pardo-Locarno LC, Montoya-Lerma J, Bellotti AC, Van Schoonhoven A (2005) Structure and composition of the white grub complex (Coleoptera: Scarabaeidae) in agroecological systems of northern Cauca, Colombia. Florida Entomologist 88: 355–363. https://doi.org/10.1653/0015-4040(2005)88[355:SACOTW]2.0.CO;2
- Pardo-Locarno LC, Ramírez-Pava B, Villota H, Villanueva O, Bahamón W (2011) Ensamblaje de escarabajos Melolonthidae (Coleoptera: Scarabaeoidea) asociados con pasturas en el departamento del Caquetá y su posible relación con la salubridad edáfica. Acta Agronómica 60(3): 1–12.
- Paucar-Cabrera A (2005) A catalog and distributional analysis of the Rutelinae (Coleoptera: Scarabaeidae) of Ecuador. Zootaxa 948: 1–92.
- Peck SB (2010) The beetles of the island of St. Vincent, Lesser Antilles (Insecta: Coleoptera); diversity and distributions. Insecta Mundi 144: 1–77.
- Peck SB (2016) The beetles of the Lesser Antilles (Insecta, Coleoptera): diversity and distributions. Insecta Mundi 460: 1–360.
- Peck SB, Cook J, Hardy Jr. JD (2002) Beetle fauna of the island of Tobago, Trinidad and Tobago, West Indies. Insecta Mundi 16: 9–23.
- Peck SB, Thomas MC (1998) A distributional checklist of the beetles (Coleoptera) of Florida. Arthropods of Florida and Neighboring Land Areas 16: i-viii, 1–180.
- Pérez-Flores O, Villagomez F, Galindo OR (2016) A new species of Chrysina Kirby (Coleoptera: Scarabaeidae: Rutelini) from Mexico based on morphological and molecular data. Journal of Entomology and Zoology Studies 4: 606–610.
- Pérez-Torres CB, Aragón A, Tapia AM (2013) Capítulo 3. El valle de Puebla. In: Morón MA, Aragón A, Carrillo-Ruiz H (Eds) Fauna de escarabajos del estado de Puebla. M. A. Morón, Coatepec, 55–82.
- Perty JAM (1830) Insecta Brasiliensia, In: Perty, J.A.M. (1830–1833). Delectus animalium articulatorum, quae in itinere per Brasiliam annis MDCCCXVII-MDCCCXX jussu et auspiciis Maximiliani Josephi I. Bavariae regis augustissimi peracto collegerunt Dr. J. B. de Spix et Dr. C. F. Ph. de Martius. Impensis Editoris, Munich, Germany, 1–60.
- Philippi F (1861) Observaciones sobre los lamelicornios de Chile, descitos en la obra del Señor Gay, con descripcíon de algunas especies nuevas. Anales de la Universidad de Chile 18: 735–744.
- Philippi F (1887) Catálogo de los coleópteros de Chile. Anales de la Universidad de Chile 71: 619–806.
- Pokorný S, Curoe DJ (2012) A new species of Chrysina Kirby (Coleoptera: Scarabaeidae: Rutelinae) of the quiche group. Folia Heyrovskyana, series A 20: 111–116.
- Pye JD (2010) The distribution of circularly polarized light reflection in the Scarabaeoidea (Coleoptera). Biological Journal of the Linnean Society 100(3): 585–596. https://doi.org/10.1111/j.1095-8312.2010.01449.x
- Raphael S (1970) The publication dates of the Transactions of the Linnean Society of London, Series I, 1791–1875. Biological Journal of the Linnean Society 2: 61–76. https://doi.org/10.1111/j.1095-8312.1970.tb01688.x
- Ratcliffe BC (2002) A checklist of the Scarabaeoidea (Coleoptera) of Panama. Zootaxa 32: 1–48. https://doi.org/10.11646/zootaxa.32.1.1
- Ratcliffe BC (2006) Scarab beetles in human culture. The Coleopterists Bulletin 60, Special Issue 5: 85–101. https://doi.org/10.1649/0010-065X(2006)60[85:SBIHC]2.0.CO;2
- Ratcliffe BC, Jameson ML (1989) A new species of Pelidnota (Coleoptera: Scarabaeidae: Rutelinae) from Panama. The Coleopterists Bulletin 43: 259–262.
- Ratcliffe BC, Jameson ML, Taylor T (1992) Two remarkable new species of Plusiotis (Coleoptera: Scarabaeidae: Rutelinae) from Mexico and Central America. Insecta Mundi 6: 59–63.
- Ratcliffe BC, Jameson ML, Figueroa L, Cave RD, Paulsen MJ, Cano EB, Beza-Beza C, Jimenez-Ferbans L, Reyes-Castillo R (2015) Beetles (Coleoptera) of Peru: A survey of families. Scarabaeoidea. The Journal of the Kansas Entomological Society 88: 186–207. https://doi.org/10.2317/kent-88-02-186-207.1
- Ratcliffe BC, Paulsen MJ (2008) The scarabaeoid beetles of Nebraska. Bulletin of the Nebraska State Museum 22: 1–570.
- Reed EC (1876) Catálogo de los coleópteros de Chile (segunda parte). Anales de la Universidad de Chile 48: 274–295.
- Restrepo-Giraldo H, Morón MA, Vallejo F, Pardo-Locarno LC, López-Avila A (2003) Catálogo de Coleoptera Melolonthidae (Scarabaeidae Pleurosticti) de Colombia. Folia Entomológica Mexicana 42: 239–263.
- Reyes Novelo E, Morón MA (2005) Fauna de Coleoptera Melolonthidae y Passalidae de Tzucacab y Conkal, Yucatán, México. Acta Zoológica Mexicana (N. S. ) 21: 15–49.
- Ritcher PO (1945) Rutelinae of eastern North America with descriptions of the larvae of Strigodermella pygmaea (Fab.) and three species of the tribe Rutelini (Coleoptera: Scarabaeidae). Bulletin of the Kentucky Agricultural Experiment Station Lexington 471: 3–19.
- Ritcher PO (1966) White grubs and their allies. Oregon State University Press, Corvallis, 219 pp.
- Rivera-Gasperín SL, Carrillo-Ruiz H, Morón MA, Yanes-Gómez G (2013) Fauna de Coleoptera Melolonthidae (Scarabaeoidea) en el Rancho Canaletas, Pas del Macho, Veracruz, México. Acta Zoológica Mexicana (N. S. ) 29: 194–208.
- Roberts A (1946) Récente découverte du Pelidnota punctata dans la Province de Québec. Rapport annuel de la Société de Québec pour la Protection des Plantes contre les Insectes et les Maladies Fongueuses 30: 189–191.
- Roberts A (1962) Acclimatation de certains insectes au climat du Québec. Annales de la Société Entomologique du Québec 7: 93–95.
- Rodrigues SR, da Silva Falco J (2011) Aspectos biológicos de Pelidnota fulva Blanchard, 1850 (Coleoptera, Scarabaeidae, Rutelinae). Biota Neotropica 11: 157–160. https://doi.org/10.1590/S1676-06032011000100015
- Rodrigues SR, Morón MA, Nogueira GAL (2012) Description of the third instar of Pelidnota fulva Blanchard, 1850 (Coleoptera: Scarabaeidae: Rutelinae). The Coleopterists Bulletin 66(3): 266–270. https://doi.org/10.1649/072.066.0314
- Rodríguez-Palafox A, Corona AM (2002) Lista de artrópodas de la region de Chamela, Jalisco, México. In: Noguera FA, Vega Rivera JH, García Aldrete AN, Avendaño MQ (Eds) Historia natural de Chamela. Universidad Nacional Autónoma de México, Mexico City, 203–232.
- Rothschild W, Jordan K (1894) Six new species of Plusiotis and one new Anoplostethus. Novitates Zoologicae 1: 504–507. https://doi.org/10.5962/bhl.part.24570
- Sallé A (1874) Diagnose d’une nouvelle espèce de Pelidnota (P. chrysargyrea). Annales de la Société Entomologique de France, Série 5, Tome 4: 362.
- Sallé A (1878) Séance du 13 Février 1878: Communications. Bulletin des Séances de la Société Entomologique de France 1878: xvii–xviii.
- Sánchez-Soto S (1997) Nuevos registros de Melolonthidae (Coleoptera) para el estado de Tabasco, México. Folia Entomológica Mexicana 100: 67–70.
- Schlein O (2011) Inventario preliminar de filo Arthropoda (Inventario de familias, géneros y especies identificadas. In: Moreno R, Vásquez S, Palacios A (Eds) Segundo studio de biodiversidad en la Reserva Biológica “El Chile”. Oficina Local, Guaimaca, 18–34.
- Schönherr CJ (1817) Synonymia Insectorum, oder: Versuch einer Synonymie aller bisher bekannten Insecten nach Fabricii Systema Eleutheratorum etc. geordnet, mit Berichtigungen und Anmerkungen, wie auch Beschreibungen neuer Arten und illuminierten Kupfren. Erster Band. Eleutherata oder Käfer. Dritter Theil. Hispa-Molorchus, Skara, Lewerentzischen Buchdruckerey, Uppsala, xi + 507 pp.
- Schülke M (2004) Zur Taxonomie der Tachyporinae (Coleoptera: Staphylinidae) Typenrevision, Typendesignation, Neukombinationen, Untergattungszuordnungen, Nomina nova und neue Synonymien. Linzer Biologische Beiträge 36(2): 919–1000.
- Sharma V, Crne M, Park JO, Srinivasarao M (2009) Structural origin of circularly polarized iridescence in jeweled beetles. Science 325: 449–451. https://doi.org/10.1126/science.1172051
- Sharp D (1877) Descriptions of some new species of beetles (Scarabaeidae) from Central America. The Journal of the Linnean Society of London 13: 129–138. https://doi.org/10.1111/j.1096-3642.1877.tb02376.x
- Sharp D (1885) Descriptions of two new Coleoptera sent from M. de Lacerda from Bahia. Bulletin de la Société Entomologique de Belgique 1885: 21–24.
- Skinner H (1905) Descriptions of new Coleoptera from Arizona with notes on some other species. Entomological News 16: 289–292.
- Skinner H (1911) Two rare species of Coleoptera. Entomological News 22: 354–357.
- Smith ABT (2003) A monographic revision of the genus Platycoelia Dejean (Coleoptera: Scarabaeidae: Rutelinae: Anoplognathini). Bulletin of the University of Nebraska State Museum 15: 1–202.
- Smith ABT (2006) A review of the family-group names for the superfamily Scarabaeoidea (Coleoptera) with corrections to nomenclature and a current classification. The Coleopterists Bulletin 60, Special Issue 5: 144–204. https://doi.org/10.1649/0010-065X(2006)60[144:AROTFN]2.0.CO;2
- Smith ABT (2009) Checklist and nomenclatural authority file of the Scarabaeoidea of the Neartic Realm including Canada, the continental United States, and the northern Mexican states of Baja California, Baja California Sur, Chihuahua, Coahuila de Zaragoza, Nuevo Leon, Sinaloa, Senora, Tamaulipas, and Zacatecas. Version 4. Electronically published, Ottawa, 97 pp.
- Smith ABT, Hawks DC, Heraty JM (2006) An overview of the classification and evolution of the major scarab beetle clades (Coleoptera: Scarabaeoidea) based on preliminary molecular analyses. The Coleopterists Bulletin 60, Special Issue 5: 35–46. https://doi.org/10.1649/0010-065X(2006)60[35:AOOTCA]2.0.CO;2
- Smith ABT, Jameson ML (2001) Eremophygus bicolor (Gutiérrez) (Coleoptera: Scarabaeidae: Rutelinae: Rutelini): a new tribal and generic placement for the Bolivian scarab Platycoelia bicolor (Gutiérrez) (Anoplognathini). The Coleopterists Bulletin 55: 103–106. https://doi.org/10.1649/0010-065X(2001)055[0103:EBGRCS]2.0.CO;2
- Solier AJJ (1851) Zoologia: Coleopteros. In: Gay C (Ed. ) Historia fisica y politica de Chile segun documentos adquiridos en esta republica durante doce anos de residencia en ella Paris, Zoologia 5: 1–285.
- Solís A, Morón MA (1994) Nuevas especies de Rutelini (Coleoptera: Melolonthidae: Rutelinae) del sureste Costa Rica. Folia Entomologica Mexicana 92: 31–41.
- Soula M (1998) Les Coléoptères du Monde, 26.1: Rutelini 2. Révision des Anthicheirina 1. Hillside Books, Canterbury, 116 pp.
- Soula M (2002a) Les Coléoptères du Monde, 26.2: Rutelini 2. Révision des Anthicheirina 2. Hillside Books, Canterbury, 296 pp.
- Soula M (2002b) Les Coléoptères du Monde, 26: Rutelini 2. Crathoplus – Telaugis – Platyrutela – Pseudohypaspidius – Badiasis – Chlorota – Exothyridium – Thyriochlorota – Parathyridium – Hypaspidius – Mucama – Xenochlorota – Parachlorota – Pichica – Tipicha – Heterochlorota – Thyridium – Pseudothyridium – Exochlorota – Aequatoria – Acraspedon – Paratelaugis – Exanticheira – Chalcentis – Vayana – Minidorysthetus – Pseudomacraspis – Maripa. Hillside Books, Canterbury, 102 pp.
- Soula M (2003) Les Coléoptères du Monde, 29: Rutelini 3. Pseudoptenomela – Paraptenomela – Exoptenomela – Ptenomela – Calomacraspis – Paramacraspis – Paradorysthetus – Macraspis – Pseudorysthetus – Dorysthetus – Anticheiroides – Anticheira – Pseudoanthicheiroides. Hillside Books, Canterbury, 79 pp.
- Soula M (2005) Les Coléoptères du Monde, 26.3: Rutelini 2. Révision des Anticheirina 3. Hillside Books, Canterbury, 297–409.
- Soula M (2006) Les Coléoptères du Nouveau Monde. Volume 1: Rutelini 1. Révision des Pelidnotina 1 et des Lasiocalina. Une révision des genres Strigidia, Chalcoplethis, Epichalcoplethis, Sorocha, Lasiocala, Minilasiocala, Pseudochlorota, Homeochlorota, Pachacama (Coleoptera: Scarabaeidae: Rutelinae: Rutelini: Pelidnotina et Lasiocalina). Besoiro: Supplément au Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale. AECFT, Saintry, 176 pp.
- Soula M (2008) Les Coléoptères du Nouveau Monde. Volume 2: Rutelini 2. Révision des Pelidnotina 2. Révision des genres: Parhoplognathus, Chipita n. gen., Heteropelidnota, Homothermon, Hoplopelidnota, Mesomerodon, Mecopelidnota, Patatra n. gen. (Coleoptera: Scarabaeidae, Rutelinae, Rutelini). Besoiro: Supplément au Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale. AECFT, Saintry, 40 pp.
- Soula M (2009) Les Coléoptères du Nouveau Monde. Volume 3: Rutelini 3. Révision des Pelidnotina 3. Photos de toutes les espèces de Lagochile. Une révision des genres Pelidnota, Ectinoplectron, Pseudogeniates, Xenopelidnota (Coleoptera: Scarabaeidae: Rutelinae: Rutelini: “Pelidnotina”). Besoiro: Supplément au Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale. AECFT, Saintry, 137 pp.
- Soula M (2010a) Les Coléoptères du Nouveau Monde. Volume 4: Rutelini 4. Révision des Pelidnotina 4. Une révision des genres Catoclastus, Homonyx. Retour sur quelques espèces ou groupe d’espèces de Pelidnota. Addenda 2010. (Coleoptera: Scarabaeidae: Rutelinae: Rutelini: “Pelidnotina”). Besoiro: Supplément au Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale. AECFT, Saintry, 66 pp.
- Soula M (2010b) Pelidnotopsis Ohaus, revalidation du genre. Besoiro 19: 11–12.
- Soula M (2010c) Les Rutelinae: présentation des tribus et genres de Guyane (Coleoptera, Scarabaeidae). In: Touroult J (Ed.) Contribution à l’Étude des Coléoptères de Guyane. Tome II. Supplément au Bulletin de liaison d’ACOREP-France “Le Coléoptèriste”, Paris, 50–61.
- Soula M (2011) Les Coléoptères du Nouveau Monde. Volume 5: Geniatini 1. Révision du genre Bolax (Coleoptera: Scarabaeidae: Rutelini: Geniatini). Besoiro: Supplément au Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale. AECFT, Saintry, 85 pp.
- Soula M (2012) Description d’une nouvelle espece de Chrysina (Coleoptera, Melolonthidae, Rutelinae). Besoiro 21: 5–6.
- Soula M, Moragues G (2006) [new taxa]. In: Soula M (2006) Les Coléoptères du Nouveau Monde. Volume 1: Rutelini 1. Révision des Pelidnotina 1 et des Lasiocalina. Une révision des genres Strigidia, Chalcoplethis, Epichalcoplethis, Sorocha, Lasiocala, Minilasiocala, Pseudochlorota, Homeochlorota, Pachacama (Coleoptera: Scarabaeidae: Rutelinae: Rutelini: Pelidnotina et Lasiocalina). Besoiro: Supplément au Bulletin de liaison de l’Association Entomologique pour la Connaissance de la Faune Tropicale. AECFT, Saintry, 176 pp.
- Steinheil E (1874) Symbolae ad historiam Coleopterorum Argentiniae meridionalis. II. Centuria. Atti della Società Italiana di Scienze Naturali 15: 554–578.
- Sturm J (1843) Catalog der Kaefer-Sammlung, mit 6 ausgemalten Kupfertafeln. Sebald, Nuremberg, 386 pp. [+ 6 plates] https://doi.org/10.5962/bhl.title.37837
- Swederus NS (1787) Et nytt Genus och Femtio nya Species af Insecter, beskrifne. Kongliga Vetenskaps Academiens Nya Handlingar 8: 181–201.
- Taschenberg EL (1870) Neue Käfer aus Colombien und Ecuadór. Zeitschrift für die Gesammten Naturwissenschaften 35: 177–199.
- Thomas DB (1993) Scarabaeidae (Coleoptera) of the Chiapanecan forests: A faunal survey and chorographic analysis. The Coleopterists Bulletin 47: 363–408.
- Thomas DB, Robacker D, Hawks DC (2006–2016) [Chrysina species pages]. In: Jameson ML (Ed.) Chrysina overview (http://www.museum.unl.edu/research/entomology/Guide/Scarabaeoidea/Scarabaeidae/Rutelinae/Rutelinae-Tribes/Rutelini/Chrysina/Chrysina-Catalog/ChrysinaC.html). In: Ratcliffe BC, Jameson ML (Eds) Generic Guide to New World Scarab Beetles. http://www.museum.unl.edu/research/entomology/Guide/Guide-introduction/Guideintro.html [Accessed August 20, 2016]
- Torres Martínez A, Guevara Correal Á (2012) Escarabajos Rutelinae colectados en la vereda guanacas del municipio El Peñón, Cundinamarca, Colombia (Coleoptera: Melolonthidae). Universidad Pedagógica Nacional, Facultad de Ciencia y Tecnología, Departamento de Biología, Bogotá, 103 pp.
- Vigors NA (1825) Descriptions of some rare, interesting, or hitherto uncharacterized subjects of zoology. The Zoological Journal 1: 526–542.
- Voet JE (1766–1806) Catalogus systematicus Coleopterorum. 2 vols. Bakhuysen, Haag.
- Warner WB, Hawks DC, Bruyea GP, Le Blanc R (1992) Two new Costa Rican Plusiotis Burmeister (Coleoptera: Scarabaeidae). The Coleopterists Bulletin 46: 95–101.
- Warner WB, Monzón J (1993) A new Plusiotis from Guatemala and Belize (Coleoptera: Scarabaeidae). Insecta Mundi 7: 211–213.
- Waterhouse CO (1871) Description of a new species of Rutelidae. The Entomologist’s Monthly Magazine 8: 5–6.
- Waterhouse CO (1876) On various new genera and species of coleoptera. Transactions of the Royal Entomological Society of London 24: 11–25. https://doi.org/10.1111/j.1365-2311.1876.tb01122.x
- Wheeler QD (2004) Taxonomic triage and the poverty of phylogeny. Philosophical Transactions of the Royal Society of London B 359: 571–583. https://doi.org/10.1098/rstb.2003.1452
- Wheeler QD, Platnick NI (2000) The phylogenetic species concept (sensu Wheeler and Platnick). In: Wheeler QD, Meier R (Eds) Species Concepts and Phylogenetic Theory: A Debate. Columbia University Press, New York, 17–29.
- Wickham HF (1894) The Coleoptera of Canada. IV. The pleurostict Scarabaeidae of Onatario and Quebec. The Canadian Entomologist 26: 259–264. https://doi.org/10.4039/Ent26259-9
- Woodruff RE (2009) Scarab jewels. Scarabs 41: 1–12.
- Yanes-Gómez G, Morón MA (2010) Fauna de coleópteros Scarabaeoidea de Santo Domingo Huehuetlán, Puebla, México. Su Potencial como indicadores ecológicos. Acta Zoológica Mexicana (N. S. ) 26: 123–145.
- Yanes-Gómez G, Morón MA (2013) Capítulo 13. Región de Huehuetlán. In: Morón MA, Aragón A, Carrillo-Ruiz H (Eds) Fauna de escarabajos del estado de Puebla. M.A. Morón, Coatepec, 275–296.
- Young RM (1999) A new country record for Chrysina karschi Nonfried (Scarabaeidae: Rutelinae). The Coleopterists Bulletin 53: 126.
- Young RM (2002) New country records for Neotropical and Oriental Rutelinae (Scarabaeidae: Rutelinae). The Coleopterists Bulletin 56: 540. https://doi.org/10.1649/0010-065X(2002)056[0540:NCRFNA]2.0.CO;2
- Zamora-Vuelvas MC, Ponce-Saavedra J, Deloya-López AC (2014) Scarabaeoidea (Insecta: Coleoptera) capturados con trampas en el cerro “El Águila”, municipio de Morella, Michoacán. Entomología Mexicana 1: 519–524.