Research Article |
|
Corresponding author: Adrian G. Glover ( a.glover@nhm.ac.uk ) Academic editor: Andrew Davinack
© 2022 Lenka Neal, Helena Wiklund, Laetitia M. Gunton, Muriel Rabone, Guadalupe Bribiesca-Contreras, Thomas G. Dahlgren, Adrian G. Glover.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Neal L, Wiklund H, Gunton LM, Rabone M, Bribiesca-Contreras G, Dahlgren TG, Glover AG (2022) Abyssal fauna of polymetallic nodule exploration areas, eastern Clarion-Clipperton Zone, central Pacific Ocean: Amphinomidae and Euphrosinidae (Annelida, Amphinomida). ZooKeys 1137: 33-74. https://doi.org/10.3897/zookeys.1137.86150
|
Abstract
This is a contribution in a series of taxonomic publications on benthic fauna of polymetallic nodule fields in the eastern abyssal Clarion-Clipperton Zone (CCZ). The material was collected during environmental surveys targeting exploration contract areas ‘UK-1’, ‘OMS’ and ‘NORI-D’, as well as an Area of Particular Environmental Interest, ‘APEI-6’. The annelid families Amphinomidae and Euphrosinidae are investigated here. Taxonomic data are presented for six species from 41 CCZ-collected specimens as identified by a combination of morphological and genetic approaches; of the six species, three are here described as new, one species is likely to be new but in too poor condition to be formalised and the two others likely belong to known species. Description of three new species Euphrosinella georgievae sp. nov., Euphrosinopsis ahearni sp. nov., and Euphrosinopsis halli sp. nov. increases the number of formally described new annelid species from the targeted areas to 21 and CCZ-wide to 52. Molecular data suggest that four of the species reported here are known from CCZ only, but within CCZ they have a wide distribution. In contrast, the species identified as Bathychloeia cf. sibogae Horst, 1910 was found to have a wide distribution within the Pacific based on both morphological and molecular data, using comparative material from the abyssal South Pacific. Bathychloeia cf. balloniformis Böggemann, 2009 was found to be restricted to APEI-6 based on DNA data available from CCZ specimens only, but morphological data from other locations suggest potentially a wide abyssal distribution. The genus Euphrosinopsis was previously known only from Antarctic waters, and Euphrosinella georgievae sp. nov. was recovered as a sister taxon to the Antarctic specimens of Euphrosinella cf. cirratoformis in our molecular phylogenetic analysis, strengthening the hypothesised link between the deep-sea and Antarctic benthic fauna.
Keywords
Amphinomida, CCZ, COI, deep-sea mining, molecular phylogeny, species distribution, taxonomic novelty, 18S, 16S
Introduction
The Clarion-Clipperton Zone (CCZ) polymetallic nodule region, a vast area (ca. 6 million km2) of the central abyssal Pacific, has been explored in recent decades for its deep-sea mineral resources and their potential for commercial mining (e.g.,
The knowledge of the biodiversity and distribution of benthic taxa found within areas of potential mining operations is paramount to informed environmental impact assessments and conservation efforts (
Our main objective has been to provide taxonomic hypotheses on macrofaunal annelids collected from the targeted areas within the CCZ based on morphology and molecular data. These data build up on previous taxonomic work on annelids from the target areas (
Annelids of order Amphinomida are commonly known as fire worms due to the skin burning sensation upon contact with their chaetae caused by a complex mixture of defensive toxins (
In terms of their systematics, Amphinomida were regarded as part of the Errantia (e.g.,
Materials and methods
Fieldwork
The first UKSR ABYSSLINE cruise (AB01) took place in October 2013 onboard the RV ‘Melville’ and targeted the UK-1 exploration contract area (Fig.
For a comprehensive description of the methodological pipeline, see
Morphological laboratory work
In the laboratory, preserved specimens were re-examined using stereo and compound microscopes. They were identified to morphospecies, and the best-preserved examples (voucher specimens) were then used to provide informal descriptions with key morphological features photographed with digital camera. Shirlastain A was used during the morphological examination on some specimens, in order to better observe certain characters. Scanning electron microscopy (SEM) using a SEM FEI Quanta 650 was conducted on selected specimens, following graded ethanol dehydration, critical point drying, and gold coating. Figures were assembled using Adobe Photoshop CS6 software. In some instances, a fine line was used to outline and highlight particular morphological features where such features were unclear from images alone. Line drawings were made using camera lucida system.
Additionally, Amphinomidae specimens recently collected from the abyssal South Pacific (ca. 4000 m) as part of the RV ‘Investigator’ voyage ‘Sampling the Abyss’ were made available for examination (see also
Molecular laboratory work
Extraction of DNA was done with DNeasy Blood and Tissue Kit (Qiagen) using a Hamilton Microlab STAR Robotic Workstation. Approximately 1800 bp of 18S were amplified using the primers 18SA 5’-AYCTGGTTGATCCTGCCAGT-3’(
Molecular data were used to place species covered in this study within the Amphinomida phylogenetic relationships. The phylogenetic analyses were done in two parts, producing one tree for Euphrosinidae with a taxon from Amphinomidae as root, and one for Amphinomidae with a taxon from Euphrosinidae as root. Sequences added from GenBank are listed in Supplementary data with taxon names and sequence accession numbers. The program jModelTest (
Taxonomic assignments
Here we use a phylogenetic species concept sensu
List of taxa presented in this paper – family, DNA taxonomy ID (a species-level identification based on combined DNA and morphological evidence), cruise record number, GUID (Global Unique Identifier link to data record at http://data.nhm.ac.uk),
| DNA taxonomy ID | NHM no. | GUID | Reg no. |
MCf no. | COI AK no. | 16S AK no. | 18S AK no. |
|---|---|---|---|---|---|---|---|
| Family Amphinomidae | |||||||
| Bathychloeia cf. balloniformis | NHM_2107 | c79b4600-e8e9-4484-b06a-e18330a1421d | ANEA 2022.630 | 0118302190 |
ON903198 |
ON900088 | ON905671 |
| Bathychloeia cf. balloniformis | NHM_2109 | ac3dd714-64ac-44ea-9168-22437dc3cfba | ANEA 2022.631 | 0118302189 | ON900113 | ||
| Bathychloeia cf. sibogae juvenile | NHM_6880_HW01 | 06f82805-e608-4715-af62-ab1d44df2a79 | ANEA 2022.632 | 0118302159 | ON903200 | ON900089 | |
| Bathychloeia cf. sibogae | NHM_0821 | 73a7200a-ae19-4c0c-8381-8d4509a318cf | ANEA 2022.633 | 0118302202 | ON903197 | ON900100 | ON905670 |
| Bathychloeia cf. sibogae | NHM_2906 | d3848fcf-4cb2-49fd-b49c-e09422419a70 | ANEA 2022.634 | 0118302177 | ON900116 | ||
| Bathychloeia cf. sibogae juvenile | NHM_2115 | 2cbc0d92-247c-4197-bd7a-4715adb5e8f4 | ANEA 2022.635 | 0118302188 | ON900114 | ||
| Bathychloeia cf. sibogae | NHM_3539 | 083df63d-60e7-48ae-95c4-6a11a61b01e8 | ANEA 2022.636 | 0118302158 | ON903199 | ON900118 | |
| Bathychloeia cf. sibogae | NHM_8922 | 805f34aa-ec4f-4318-b18b-46447350aa1e | ANEA 2022.637 | 0118302156 | ON903201 | ||
| Paramphinome sp. NHM_6022E | NHM_1167D | fd4902df-aef2-44cf-991f-31905434c2a1 | ANEA 2022.638 | ||||
| Paramphinome sp. NHM_6022E | NHM_4044 | 56235559-3f2c-426e-b4cd-37462593a4ba | ANEA 2022.639 | 0118302160 | |||
| Paramphinome sp. NHM_6022E | NHM_6022E | bd4b405d-3e56-4671-909e-fdf9c3e7fbcf | ANEA 2022.640 | 0118302162 | ON900125 | ON905673 | |
| Family Euphrosinidae | |||||||
| Euphrosinopsis halli sp. nov. | NHM_0779 | 1a683870-d904-4c2c-bf1a-a34ead0a42fc | ANEA 2022.641 | 0118302182 | ON900099 | ||
| Euphrosinopsis halli sp. nov. | NHM_4339 (holotype) | 670dfd34-338d-4edc-8856-b0a9a728efc9 | ANEA 2022.642 | 0118302157 | ON900119 | ON905672 | |
| Euphrosinopsis halli sp. nov. | NHM_6018 (paratype) | ab26e2ea-ab87-4013-8106-e817c0485cc9 | ANEA 2022.643 | 0118302167 | ON900124 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_0095 | a351cb41-736c-4390-8ad8-02c0358b73e0 | ANEA 2022.644 | 0118302201 | ON900092 | ON905668 | |
| Euphrosinopsis ahearni sp. nov. | NHM_0888 | 4d76b4e2-569d-4a17-9276-3ce721cbdf72 | ANEA 2022.645 | 0118302187 | ON900101 | ||
| Euphrosinopsis ahearni sp. nov | NHM_0551 (paratype, SEM) | 241b828d-a574-47f2-995d-0bdef239c427 | ANEA 2022.646 | 0118302186 | ON900094 | ||
| Euphrosinopsis ahearni sp. nov | NHM_5042 | 1662fd8b-54a5-4f97-9083-02dbb2df7e39 | ANEA 2022.647 | 0118302178 | ON900121 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_1737A | 4f372c07-c466-4b6c-91a9-229cd7c7a17d | ANEA 2022.648 | 0118302171 | ON900107 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_1876 | 6ad5c2b3-ece8-4195-a19f-3913de511e71 | ANEA 2022.649 | 0118302175 | ON900112 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_0550 | 92791783-35c2-4fbf-80b0-2b074ef70828 | ANEA 2022.650 | 0118302203 | ON900093 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_1302 | 7aabe644-2ec6-4671-8c1a-f826eeeb0b46 | ANEA 2022.651 | 0118302168 | ON900105 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_1302A (holotype) | 479933d3-9943-4d87-a1b8-ea120bd8f4ee | ANEA 2022.652 | 0118302169 | ON900104 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_1737 | 2ca3e584-a68d-4ea5-98d2-75ce10515386 | ANEA 2022.653 | 0118302173 | ON900110 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_1737C (paratype) | efe95a8c-fc88-4849-ad26-1df3d292ef20 | ANEA 2022.654 | 0118302172 | ON900109 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_ 0616 | 4758bf19-c6d0-42e0-b5ba-e83e203d2e18 | ANEA 2022.655 | 0118302185 | ON900096 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_0759 | b0f9162f-a861-4eb2-89a1-ce25c2bd09c4 | ANEA 2022.656 | 0118302184 | ON900097 | ||
| Euphrosinopsis ahearni sp. nov. | NHM_1839 | 02a5ace7-841e-4f50-bf03-57ba21f02f7c | ANEA 2022.657 | 0118302174 | ON900111 | ||
| Euphrosinella georgievae sp. nov. | NHM_0587 | b7a0bf33-0dc4-4f61-90de-35865647a99f | ANEA 2022.658 | 0118302191 | ON900095 | ON905669 | |
| Euphrosinella georgievae sp. nov. | NHM_0777 | a8f0e776-d7b6-4ec6-a549-78f40f17d89b | ANEA 2022.659 | 0118302183 | ON900098 | ||
| Euphrosinella georgievae sp. nov. | NHM_1737B | 2784df45-eec0-4151-b12d-11d955985faa | ANEA 2022.660 | ON900108 | |||
| Euphrosinella georgievae sp. nov. | NHM_0910 | 05dfb32c-fc3a-4028-bf09-3eb840175661 | ANEA 2022.661 | 0118302181 | ON900102 | ||
| Euphrosinella georgievae sp. nov. | NHM_1134 (paratype) | 00590d2b-f952-4c69-8bc2-ac2a408da17a | ANEA 2022.662 | 0118302180 | ON900103 | ||
| Euphrosinella georgievae sp. nov. | NHM_1514 | 96cb7b69-c0ea-4559-9b57-3abe6af4a4c7 | ANEA 2022.663 | 0118302170 | ON900106 | ||
| Euphrosinella georgievae sp. nov. | NHM_2391 (holotype) | 1ce8325f-74de-47de-a776-2dc50b8d69ae | ANEA 2022.664 | 0118302176 | ON900115 | ||
| Euphrosinella georgievae sp. nov. | NHM_4975 | 677b7d67-d9cc-4ebd-8d79-cf5da5dc40da | ANEA 2022.665 | 0118302165 | ON900120 | ||
| Euphrosinella georgievae sp. nov. | NHM_6087 | eebfaecd-5ee2-49d6-be73-51eb91678487 | ANEA 2022.666 | 0118302166 | ON900126 | ||
| Euphrosinella georgievae sp. nov. | NHM_5802 | c0e408e3-91e7-408f-aaef-3be86507105a | ANEA 2022.667 | 0118302164 | ON900123 | ||
| Euphrosinella georgievae sp. nov. | NHM_5057 | d92b1574-eccb-443c-a15d-b79357360b59 | ANEA 2022.668 | 0118302179 | ON900122 | ||
| Euphrosinella georgievae sp. nov. | NHM_7235 | 55637dc0-f9b9-4586-9bfb-7a821c785279 | ANEA 2022.669 | 0118302163 | ON900127 | ||
| Euphrosinella georgievae sp. nov. | NHM_2908 | fba3fab7-ae4b-4415-a73c-a2ba6cd44601 | ANEA 2022.670 | 0118302161 | ON900117 | ||
Data handling
The field and laboratory work led to a series of databases and sample sets that were integrated into a ‘data-management pipeline’. This included the transfer and management of data and samples between a central collections database, a molecular collections database and external repositories (GenBank, WoRMS, OBIS, GBIF, GGBN, ZooBank) through DarwinCore archives (Suppl. material
Systematics section
Amphinomidae Lamarck, 1818
Archinominae Kudenov, 1991
Bathychloeia
Type species
Bathychloeia sibogae Horst, 1910.
Diagnosis
(modified from
Remarks
As the name Bathychloeia suggests, this genus was established for deep-water representatives similar to forms in predominantly shallow water genus Chloeia Lamarck, 1818. Chloeia was established by
Bathychloeia cf. balloniformis
Material examined
NHM_2107,
Comparative material
Amphinomidae spp.; AM. W.52607; 3 specimens; IN2017; sta. V03_110; 4005 m; South Pacific, Australia, off Fraser Island (-25.220, 154.160); col. 11/06/2017; EBS.
Diagnosis
This very small species is represented by two specimens, up to 2.9 mm long and 0.75 mm wide for ten chaetigers. Body compact, spindle-shaped, of bloated appearance (Figs
Bathychloeia cf. balloniformis (specimen,
Bathychloeia cf. balloniformis (specimen
Prostomium rounded, longer than wide; anterior lobe broadly rounded, bearing a pair of cirriform lateral antennae (Figs
Parapodia biramous. Parapodial appendages often broken off, where attached dorsal, lateral and ventral cirri observed, including on chaetiger 1 (Fig.
Molecular information
Specimen,
Majority-rule consensus trees from the Bayesian analyses with posterior probability values on nodes. Taxon names highlighted in blue are news species or new sequences for already known species. A Amphinomidae phylogenetic tree using a combined datasets for COI, 16S, and 18S with 26 terminal taxa of which Euphrosine foliosa (Euphrosinidae) was used as a root B Euphrosinidae phylogenetic tree using a combined datasets for 16S and 18S with nine terminal taxa of which Paramphinome jeffreysii (Amphinomidae) was used as a root.
Remarks
The CCZ-collected specimens correspond morphologically to another abyssal species Bathychloeia balloniformis Böggemann, 2009 described from Cape and Guinea Basins in SE Atlantic, 5048–5144 m depth. The specimens agree in small, spindle-shaped body, having ca. 10 chaetigers, the form of greatly folded and crenulated caruncle and the form and distribution of branchiae (see comparative Fig.
Distribution
Central Pacific Ocean, Eastern CCZ, in the Area of Particular Environmental Interest, ‘APEI-6’ only (Fig.
Bathychloeia cf. sibogae
Material examined
NHM_6880HW,
Comparative material
Bathychloeia cf. sibogae;
Diagnosis
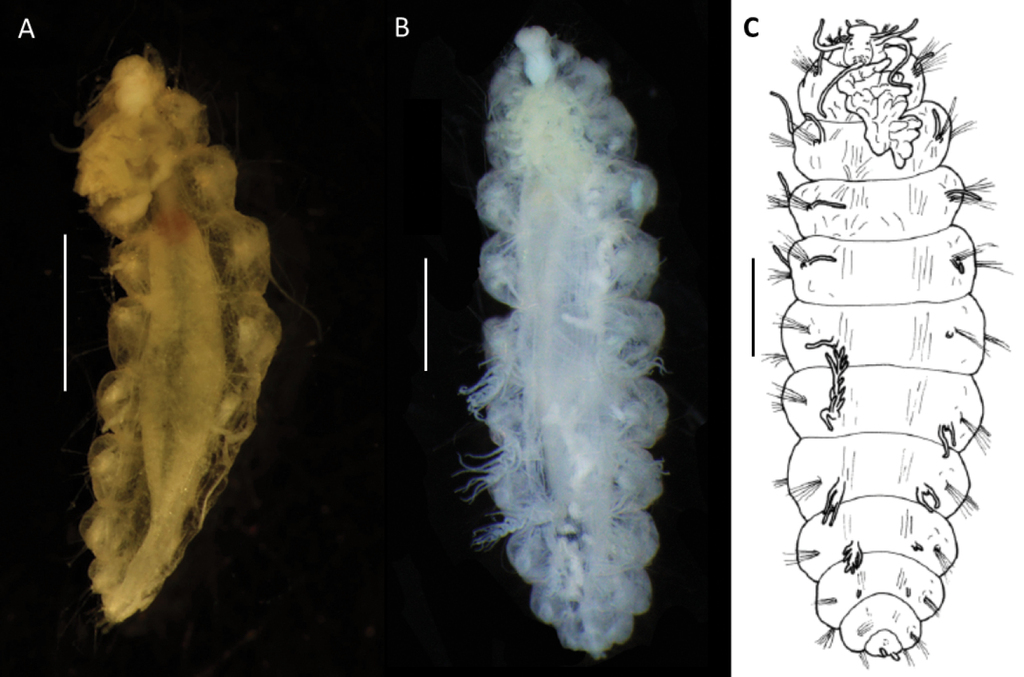
Body size variable, up to 18 mm long and 6 mm wide for larger specimens with 15 or 16 chaetigers (Figs
Bathychloeia cf. sibogae (specimen
Bathychloeia cf. sibogae (specimen
Bathychloeia cf. sibogae CCZ-collected specimens A large live specimen (
Prostomium indistinctly divided into an anterior and a posterior lobe; tightly surrounded by reduced first chaetigerous segment. Anterior lobe rounded, bearing a pair of lateral cirriform antennae plus a pair of slightly shorter ventrolateral palps. Posterior lobe bell-shaped, ca. as long as wide. One pair of tiny red eyes present (Fig.
Parapodia biramous with distinctly separated rami, bearing cirri that are easily detached. Dorsal and lateral cirri slender, filiform, and long, present in notopodia; dorsal cirrus inserted dorsolaterally to notochaetae, lateral cirrus, inserted medially behind notopodial chaetae. Ventral cirri also filiform and elongated (particularly in chaetiger 1, Fig.
Notopodia with chaetae much larger and usually thicker than those of neuropodia, almost forming a “cage” over dorsum, obscuring the branchiae in some specimens, but very fragile and easily lost in most specimens, best preserved in juvenile specimens (Fig.
Bathychloeia cf. sibogae (specimen
Variation
Molecular analysis suggests that smaller and larger specimens that differ predominantly in the form of caruncle and form of branchiae as described above, represent the same species. Therefore, the size difference likely represents different developmental changes.
Molecular information
Only one CCZ specimen of B. cf. sibogae, specimen
Remarks
The enlarged branchiae of chaetiger 5 suggest close affiliation of CCZ specimens to Bathychloeia sibogae Horst, 1910 described from the Banda Sea, depth of 1100 m. Since its original description and subsequent re-description (
Although the original definition of B. sibogae given by
Comparative figure of form of enlarged branchiae from chaetiger 5 showing variation in development of long branchial filaments, Ms – Main stalk, Lb – lateral branches, Lf – long filaments A Bathychloeia cf. sibogae CCZ specimen,
Distribution
Central Pacific Ocean, Eastern CCZ, in the exploration areas UK-1, OMS, NORI-D (Fig.
Ecology
It is of interest that a closely related form to the CCZ species known as Cholenopsis atlantica (McIntosh, 1885) has been described in association with a sponge growing on a dead coral coated with manganese of peroxide (
Paramphinome
Type species
Paramphinome pulchella M. Sars in G. Sars, 1872.
Diagnosis
Small but long long-bodied forms. Prostomium posteriorly with Y-shaped or elongated caruncle. Branchiae comb-shaped, limited to the anterior chaetigers. First chaetiger with curved hooks in notopodia.
Paramphinome
Material examined
NHM_1167D,
Diagnosis
(after
Paramphinome sp. NHM_6022E (specimen,
Parapodia biramous. Dorsal cirri small and ovoid (Fig.
Molecular information
Only one specimen,
Remarks
Three very small posteriorly incomplete specimens were collected in CCZ samples. They differ from known species by its very small size and low number of branchial pairs (only two pairs) and undeveloped prostomial appendages, which are tiny and globular. While body size, number of segments and number of branchial pairs were previously linked to developmental stages (e.g.,
Of the known deep-sea Paramphinome species, none were described from the abyssal depths. Paramphinome pacifica Fauchald & Hancock, 1981 has been described from NE Pacific Ocean: off central Oregon (USA), 1800–2900 m; (type locality: Cascadia Abyssal Plain, 2860 m). Paramphinome australis Monro, 1930 has type locality off Signy Island, South Orkney Islands, Southern Ocean in depths between 244–344 m, although it has been widely reported from the Southern Ocean (
It is likely that the CCZ-collected specimens represent a new species; however, their tiny size and poor morphological preservation prevent its formal description, therefore the specimens are assigned to morphospecies only.
Distribution
Central Pacific Ocean, Eastern CCZ, the exploration contract areas UK-1, OMS, and NORI-D (Fig.
Euphrosinidae Williams, 1852
Euphrosinella
Type species
Euphrosine cirratoformis Averincev, 1972.
Diagnosis
(modified from
Remarks
Genus Euphrosinella was established by
Euphrosinella georgievae sp. nov.
Material examined
NHM_0587,
Diagnosis
Holotype
(
Euphrosinella georgievae sp. nov. (holotype,
Euphrosinella georgievae sp. nov. (paratype
Parapodia biramous, two rami well separated. Parapodia of chaetiger 1 well developed, not reduced, with dorsal, lateral, and ventral cirri (Fig.
Diagrammatic representation of prostomial (A–C) and parapodial appendages from mid-body chaetigers (D, E) of CCZ-collected Euphrosinidae species (relative lengths preserved, but not drawn to scale) A Euphrosinella georgievae sp. nov. B Euphrosinopsis ahearni sp. nov. C Euphrosinopsis halli sp. nov. D Euphrosinella georgievae sp. nov. E Euphrosinopsis ahearni sp. nov. F Euphrosinopsis halli sp. nov. Abbreviations: P – palps, La – lateral antennae, Ma – median antenna, Car – caruncle, E – eyes, DC – dorsal cirrus, LC – lateral cirrus, VC – ventral cirrus, Br – branchiae.
All chaetae well developed, but prone to breakage, all bifurcate (Fig.
Euphrosinella georgievae sp. nov. (specimen
Molecular information
One specimen,
Remarks
Euphrosinella georgievae sp. nov. is consistent with the genus Euphrosinella in having five prostomial appendages, caruncle mostly free from body wall and absence of ringent chaetae. Only two valid species in Euphrosinella are currently known as mentioned earlier. A known Pacific species Euphrosinella paucibranchiata can be distinguished by having some branchiae branched, as well as much shallower depth distribution of 1737 m in Santa Cruz Basin. Euphrosinella georgievae sp. nov. is more similar to the Antarctic species E. cirratoformis in having simple unbranched branchiae. The species also share a similar form and length of caruncle and median antenna. However, the two species differ in the following characters: 1. The presence of two pairs of eyes in the Antarctic species, while CCZ specimens are eyeless; 2. Notochaetae arranged in 3 tiers in new species, rather than 2 tiers in the known species and 3. Branchiae are not developed on first chaetiger in E. georgievae sp. nov., whilst they are present in E. cirratoformis. As further evidence, the molecular data suggest that Antarctic specimens identified in a previous study as Euphrosinella cf. cirratoformis (see
Distribution
Central Pacific Ocean, Eastern CCZ, the exploration areas UK-1, OMS, and NORI-D (Fig.
Etymology
This species is named for Dr. Magdalena Georgieva, who took part in ABYSSLINE expeditions to CCZ. She also collected Bathychloeia cf. sibogae specimens from CCZ used in this study as well as samples from the South Pacific during the RV Investigator cruise used here as a comparative material.
Euphrosinopsis
Type species
Euphrosinopsis antipoda Kudenov, 1993.
Diagnosis
(after
Remarks
The genus Euphrosinopsis is currently endemic to Antarctica and has been established to accommodate three known Antarctic species (
Euphrosinopsis ahearni sp. nov.
Material examined
NHM_0095,
NHM_1737C (paratype),
Diagnosis
Holotype
(
Euphrosinopsis ahearni sp. nov. (holotype
Parapodia biramous, two rami well separated. Parapodia of chaetiger 1 well developed, not reduced, parapodial cirri, branchiae or ringent chaetae not observed. In subsequent parapodia, parapodial appendages in the following dorsoventral order: dorsal cirrus, 1st branchia, lateral cirrus, 2nd branchia, ventral cirrus (Fig.
Euphrosinopsis ahearni sp. nov. (paratype
Chaetae fragile, prone to breakage, of two main types: 1. Numerous, bifurcate chaetae arranged in three rows in notopodia; their shafts of various length and thickness (Fig.
Euphrosinopsis ahearni sp. nov. (paratype
Molecular Information
Specimen (
Remarks
The CCZ species agrees well with the genus Euphrosinopsis in having five prostomial appendages, caruncle partially free from the body wall and the presence of large, deeply embedded eyes lateral to median antenna and caruncle. However, this species shows differences from all known species in this genus, suggesting it belongs to a new species. Euphrosinopsis crassiseta (type locality: Weddell Sea, 3697 m) can be easily distinguished by having only small, cirriform branchia per segment rather than two pairs of branched branchiae, by the absence of ringent chaetae and presence of coarsely serrated neurochaetae. Euphrosinopsis horsti (type locality: Pacific Antarctic Ridge, 3219–3255 m) also has only one very small, cirriform branchia per segment. Ringent chaetae are present in the known species, but they possess a distal tooth in the gap, which is absent in the new species. Finally, the most similar species, Euphrosinopsis antarctica can be distinguished by having up to three branchiae per segment, the first branched, but the others cirriform and style of median antenna of similar length to caruncle, rather than much longer as in the new species.
Thus, Euphrosinopsis ahearni sp. nov. can be distinguished mainly by having two pairs of branched branchiae in midbody chaetigers, both with very long thin branches. That is also the main distinguishing character from its congener from the CCZ, E. halli sp. nov. also described in this study, which possess only single cirriform branchia in each parapodium. Both new species possess ringent notochaetae, that can be distinguished as follow: 1. They are numerous (ca. 20 per notopodium) and easily observed in E. ahearni sp. nov., whilst only few (ca. 5 per notopodium) can be found in E. halli sp. nov.; 2. The serration of inner margin is more pronounced in E. ahearni sp. nov. and 3. The distal tip is shorter and stubbier in E. ahearni sp. nov. Further, the caruncle is more developed in E. ahearni sp. nov. reaching to chaetiger four, not two as in E. halli sp. nov., and style of median antenna is much longer than caruncle in the former species, whilst they are ca. the same length in the latter.
Distribution
Central Pacific Ocean, Eastern CCZ, the exploration areas UK-1, OMS, and NORI-D (Fig.
Etymology
This species is named for Patrick A’Hearn, technician from the University of Washington onboard the RV Thomas G Thompson.
Euphrosinopsis halli sp. nov.
Material examined
NHM_0779,
Diagnosis
Holotype
(
Euphrosinopsis halli sp. nov. A preserved holotype
Parapodia biramous, two rami well separated. With parapodial appendages observed dorso-ventrally as follow (Figs
Chaetae fragile, prone to breakage, of two main types: 1. Numerous, bifurcate chaetae arranged in approximately three rows in notopodia; their shafts of various length and thickness (Fig.
Euphrosinopsis halli sp. nov. (holotype
Molecular information
Specimen
Remarks
CCZ species agrees well with the genus Euphrosinopsis in having five prostomial appendages, caruncle partially free from the body wall and the presence of large, deeply embedded eyes lateral to median antenna and caruncle. However, this species shows differences from all known species in this genus, suggesting it belongs to a new species. The presence of single, small, unbranched cirriform branchia per parapodium suggest affiliation with E. crassiseta and E. horsti, which share the same character. However, the new species differs from E. crassiseta in possessing the ringent chaetae and lacking the coarse serration on neurochaetae. The most similar species, E. horsti can be easily separated by having anal cirri fused instead of typical cylindrical tube feet as in the new species. For comparison with another new Euphrosinopsis species also described in this study see the remarks section for E. ahearni sp. nov.
Distribution
Central Pacific Ocean, Eastern CCZ, the exploration areas UK-1 and NORI-D (Fig.
Etymology
This species is named for Preben Hall, the captain onboard the ship Maersk Launcher that was used in NORI-D expeditions in 2020 and 2021.
Discussion
This study has added six annelid species, three of those formally described and one likely new, and 41 records to the knowledge of the benthic annelid macrofauna of the CCZ, bringing a published record from the targeted areas (Fig.
Unlike other annelid taxa, each Amphinomida species is represented by several specimens (no singletons), most with wide CCZ-distribution. More importantly molecular data confirmed a wide abyssal distribution for one species identified as Bathychloeia cf. sibogae. This species has been found in CCZ (Central Pacific) and off Australia (South Pacific) with the sampling sites separated by the distance of ca. 7500 km. However, both sampling areas were at similar depths of ca. 4000 m, providing further evidence that genetic connectivity over large geographic areas is more likely to be maintained at similar depths (
Molecular phylogeny of the family Euphrosinidae has not been undertaken hitherto, as the number of taxa available on GenBank is very low. The difficulties of getting COI from members of Euphrosinidae further complicates the analyses, and more data (both in terms of number of genetic markers and taxa,) is needed to resolve the relationships within this family. Our phylogenetic results (Fig.
The phylogenetic analyses of the family Amphinomidae resulted in a tree similar to that of
To summarise, the number of DNA sequences for benthic faunal groups from the CCZ available on GenBank are growing, representing echinoderms (e.g.
Acknowledgements
We thank the masters, crew, and technical staff on the RV ‘Melville’, RV ‘Thomas G Thompson’, M/V #Pacific Constructor’, and Maersk Launcher for their outstanding support. We acknowledge the expert leadership of the research cruises and ABYSSLINE project by Prof Craig R Smith, University of Hawaii. We received help sorting and sieving samples at sea from science teams in successful deep-sea coring operations on all cruises. Joke Bleeker communicated information about type material held at Naturalis Biodiversity Centre, Leiden. We would also like thank Eva Stewart for providing the map in Fig.
References
- Ahrens JB, Borda E, Barroso R, Paiva PC, Campbell AM, Wolf A, Nugues MM, Rouse GW, Schulze A (2013) The curious case of Hermodice carunculata (Annelida: Amphinomidae): evidence for genetic homogeneity throughout the Atlantic Ocean and adjacent basins. Molecular Ecology 22(8): 2280–2291. https://doi.org/10.1111/mec.12263
- Averincev VG (1972) Benthic polychaetes Errantia from the Antarctic and Subantarctic collected by the Soviet Antarctic Expedition]. Issledovaniya fauny morei. Zoologicheskii Institut Akademii Nauk USSR 11(19): 88–292. [Biological Results of the Soviet Antarctic Expeditions, 5]
- Barroso R, Paiva PC (2008) A new deep-sea species of Paramphinome (Polychaeta: Amphinomidae) from southern Brazil. Journal of the Marine Biological Association of the United Kingdom 88(4): 743–746. https://doi.org/10.1017/S0025315408001549
- Barroso R, Paiva PC (2011) A new deep-sea species of Chloeia (Polychaeta: Amphinomidae) from southern Brazil. Journal of the Marine Biological Association of the United Kingdom 91(2): 419–423. https://doi.org/10.1017/S0025315410001499
- Barroso R, Klautau M, Solé-Cava AM, Paiva PC (2010) Eurythoe complanata (Polychaeta: Amphinomidae), thecosmopolitan’ fireworm, consists of at least three cryptic species. Marine Biology 157(1): 69–80. https://doi.org/10.1007/s00227-009-1296-9
- Barroso R, Kudenov JD, Halanych KM, Saeedi H, Sumida PY, Bernardino AF (2018) A new species of xylophilic fireworm (Annelida: Amphinomidae: Cryptonome) from deep-sea wood falls in the SW Atlantic. Deep-sea Research. Part I, Oceanographic Research Papers 137: 66–75. https://doi.org/10.1016/j.dsr.2018.05.005
- Barroso R, Kudenov JD, Shimabukuro M, Carrerette O, Sumida PY, Paiva PC, Seixas VC (2021) Morphological, molecular and phylogenetic characterization of a new Chloeia (Annelida: Amphinomidae) from a pockmark field. Deep-sea Research. Part I, Oceanographic Research Papers 171: 103499. https://doi.org/10.1016/j.dsr.2021.103499
- Beckers P, Tilic E (2021) Fine structure of the brain in Amphinomida (Annelida). Acta Zoologica 102(4): 483–495. https://doi.org/10.1111/azo.12383
- Bely AE, Wray GA (2004) Molecular phylogeny of naidid worms (Annelida: Clitellata) based on cytochrome oxidase I. Molecular Phylogenetics and Evolution 30(1): 50–63. https://doi.org/10.1016/S1055-7903(03)00180-5
- Blake JA (2016) Kirkegaardia (Polychaeta, Cirratulidae), new name for Monticellina Laubier, preoccupied in the Rhabdocoela, together with new records and descriptions of eight previously known and sixteen new species from the Atlantic, Pacific, and Southern Oceans. Zootaxa 4166(1): 1–93. https://doi.org/10.11646/zootaxa.4166.1.1
- Blake JA (2017) Polychaeta Orbiniidae from Antarctica, the Southern Ocean, the abyssal Pacific Ocean, and off South America. Zootaxa 4218(1): 1–45. https://doi.org/10.11646/zootaxa.4218.1.1
- Blake JA (2019) New species of Cirratulidae (Annelida, Polychaeta) from abyssal depths of the Clarion-Clipperton Fracture Zone, North Equatorial Pacific Ocean. Zootaxa 4629(2): 151–187. https://doi.org/10.11646/zootaxa.4629.2.1
- Blake JA (2020) New species and records of deep-water Orbiniidae (Annelida, Polychaeta) from the Eastern Pacific continental slope, abyssal Pacific Ocean, and the South China Sea. Zootaxa 4730(1): 1–61. https://doi.org/10.11646/zootaxa.4730.1.1
- Böggemann M (2009) Polychaetes (Annelida) of the abyssal SE Atlantic. Organisms, Diversity & Evolution 9(4–5): 251–428.
- Bonifácio P, Menot L (2019) New genera and species from the Equatorial Pacific provide phylogenetic insights into deep-sea Polynoidae (Annelida). Zoological Journal of the Linnean Society 185(3): 555–635. https://doi.org/10.1093/zoolinnean/zly063
- Borda E, Kudenov JD (2014) Euphrosinidae (Annelida: Amphinomida) collected from Antarctica (R/V Polarstern, 1984, 1986) with comments on the generic placement of Euphrosine magellanica Ehlers, 1900. Proceedings of the Biological Society of Washington 126(4): 299–311. https://doi.org/10.2988/0006-324X-126.4.299
- Borda E, Kudenov JD, Chevaldonné P, Blake JA, Desbruyères D, Fabri MC, Hourdez S, Pleijel F, Shank TM, Wilson NG, Schulze A (2013) Cryptic species of Archinome (Annelida: Amphinomida) from vents and seeps. Proceedings of the Royal Society B: Biological Sciences 280(1770): 20131876. https://doi.org/10.1098/rspb.2013.1876
- Borda E, Yáñez‐Rivera B, Ochoa GM, Kudenov JD, Sanchez‐Ortiz C, Schulze A, Rouse GW (2015) Revamping Amphinomidae (Annelida: Amphinomida), with the inclusion of Notopygos. Zoologica Scripta 44(3): 324–333. https://doi.org/10.1111/zsc.12099
- Brandt A, Gooday AJ, Brandao SN, Brix S, Brökeland W, Cedhagen T, Choudhury M, Cornelius N, Danis B, De Mesel I, Diaz RJ, Gillan DC, Ebbe B, Howe JA, Janussen D, Kaiser S, Linse K, Malyutina M, Pawlowski J, Raupach M, Vanreusel A (2007) First insights into the biodiversity and biogeography of the Southern Ocean deep-sea. Nature 447(7142): 307–311. https://doi.org/10.1038/nature05827
- Brasier MJ, Wiklund H, Neal L, Jeffreys R, Linse K, Ruhl H, Glover AG (2016) DNA barcoding uncovers cryptic diversity in 50% of deep-sea Antarctic polychaetes. Royal Society Open Science 3(11): 160432. https://doi.org/10.1098/rsos.160432
- Brenke N (2005) An epibenthic sledge for operations on marine soft bottom and bedrock. Marine Technology Society Journal 39(2): 10–21. https://doi.org/10.4031/002533205787444015
- Bribiesca-Contreras G, Dahlgren TG, Drazen JC, Drennan R, Horton T, Jones DO, Leitner AB, McQuaid K, Smith CR, Taboada S, Wiklund H (2021) Biogeography and connectivity across habitat types and geographical scales in Pacific abyssal scavenging amphipods. Frontiers in Marine Science 8: 1028. https://doi.org/10.3389/fmars.2021.705237
- Briggs JC (2003) Marine centres of origin as evolutionary engines. Journal of Biogeography 30(1): 1–18. https://doi.org/10.1046/j.1365-2699.2003.00810.x
- Christodoulou M, O’Hara T, Hugall AF, Khodami S, Rodrigues CF, Hilario A, Vink A, Martinez Arbizu P (2020) Unexpected high abyssal ophiuroid diversity in polymetallic nodule fields of the northeast Pacific Ocean and implications for conservation. Biogeosciences 17(7): 1845–1876. https://doi.org/10.5194/bg-17-1845-2020
- Clarke A, Barnes DK, Hodgson DA (2005) How isolated is Antarctica? Trends in Ecology & Evolution 1(1): 1–3. https://doi.org/10.1016/j.tree.2004.10.004
- Cohen BL, Gawthrop A, Cavalier–Smith T (1998) Molecular phylogeny of brachiopods and phoronids based on nuclear–encoded small subunit ribosomal RNA gene sequences. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 353(1378): 2039–2061. https://doi.org/10.1098/rstb.1998.0351
- Dahlgren TG, Wiklund H, Rabone M, Amon DJ, Ikebe C, Watling L, Smith CR, Glover AG (2016) Abyssal fauna of the UK-1 polymetallic nodule exploration area, Clarion-Clipperton Zone, central Pacific Ocean: Cnidaria. Biodiversity Data Journal 9277: e9277. https://doi.org/10.3897/BDJ.4.e9277
- Dean HK (2008) The use of polychaetes (Annelida) as indicator species of marine pollution: A review. Revista de Biología Tropical 56(4): 11–38.
- Detinova NN (1985) Polychaetous worms from the Reykjanes Ridge (the North AtlantiC). Bottom Fauna from Mid-Ocean Rises in the North Atlantic. Trudy Instituta okeanologii im. P.P. Shirshova 120: 96–136.
- Donoghue MJ (1985) A critique of the biological species concept and recommendations for a phylogenetic alternative. The Bryologist 88(3): 172–181. https://doi.org/10.2307/3243026
- Drennan R, Wiklund H, Rabone M, Georgieva MN, Dahlgren TG, Glover AG (2021) Neanthes goodayi sp. nov. (Annelida, Nereididae), a remarkable new annelid species living inside deep-sea polymetallic nodules. European Journal of Taxonomy 760: 160–185. https://doi.org/10.5852/ejt.2021.760.1447
- Edgar RC (2004) MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32(5): 1792–1797. https://doi.org/10.1093/nar/gkh340
- Eilertsen MH, Georgieva MN, Kongsrud JA, Linse K, Wiklund H, Glover AG, Rapp HT (2018) Genetic connectivity from the Arctic to the Antarctic: Sclerolinum contortum and Nicomache lokii (Annelida) are both widespread in reducing environments. Scientific Reports 8(4810): 4810. https://doi.org/10.1038/s41598-018-23076-0
- Emson RH, Young CM, Paterson GLJ (1993) A fire worm with a sheltered life: Studies of Benthoscolex cubanus Hartman (Amphinomidae), an internal associate of the bathyal sea urchin Archeopneustes hystrix (A. Agassiz, 1880). Journal of Natural History 27(5): 1013–1028. https://doi.org/10.1080/00222939300770641
- Fauchald K (1977) The polychaete worms, definitions and keys to the orders, families and genera. Natural History Museum of Los Angeles County: Los Angeles, CA (USA), Science Series 28: 1–188
- Fauchald K, Jumars PA (1979) The diet of worms: A study of polychaete feeding guilds. Oceanography and Marine Biology – an Annual Review 17: 193–284.
- Fiege D, Bock G (2009) A new species of Archinome (Polychaeta: Archinomidae) from hydrothermal vents on the Pacific-Antarctic Ridge 37 S. Marine Biological Association of the United Kingdom. Journal of the Marine Biological Association of the United Kingdom 89(4): 689–696. https://doi.org/10.1017/S0025315409000174
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates 3: 294–299.
- Gage JD (2004) Diversity in deep-sea benthic macrofauna: The importance of local ecology, the larger scale, history and the Antarctic. Deep-sea Research. Part II, Topical Studies in Oceanography 51(14–16): 1689–1708. https://doi.org/10.1016/j.dsr2.2004.07.013
- Georgieva MN, Wiklund H, Bell JB, Eilertsen MH, Mills RA, Little CT, Glover AG (2015) A chemosynthetic weed: The tubeworm Sclerolinum contortum is a bipolar, cosmopolitan species. BMC Evolutionary Biology 15(1): 1–7. https://doi.org/10.1186/s12862-015-0559-y
- Glover AG, Smith CR, Paterson GL, Wilson GD, Hawkins L, Sheader M (2002) Polychaete species diversity in the central Pacific abyss: Local and regional patterns, and relationships with productivity. Marine Ecology Progress Series 240: 157–170. https://doi.org/10.3354/meps240157
- Glover AG, Wiklund H, Rabone M, Amon DJ, Smith CR, O’Hara T, Mah CL, Dahlgren TG (2016a) Abyssal fauna of the UK-1 polymetallic nodule exploration claim, Clarion-Clipperton Zone, central Pacific Ocean: Echinodermata. Biodiversity Data Journal (4).
- Glover AG, Dahlgren TG, Wiklund H, Mohrbeck I, Smith CR (2016b) An end-to-end DNA taxonomy methodology for benthic biodiversity survey in the Clarion-Clipperton Zone, central Pacific abyss. Journal of Marine Science and Engineering 4(1): 2. https://doi.org/10.3390/jmse4010002
- Glover AG, Wiklund H, Chen C, Dahlgren TG (2018) Point of view: Managing a sustainable deep-sea ‘blue economy’requires knowledge of what actually lives there. eLife 7: e41319. https://doi.org/10.7554/eLife.41319
- Gollner S, Kaiser S, Menzel L, Jones DO, Brown A, Mestre NC, Van Oevelen D, Menot L, Colaço A, Canals M, Cuvelier D, Durden JM, Gebruk A, Egho GA, Haeckel M, Marcon Y, Mevenkamp L, Morato T, Pham CK, Purser A, Sanchez-Vidal A, Vanreusel A, Vink A, Martinez Arbizu P (2017) Resilience of benthic deep-sea fauna to mining activities. Marine Environmental Research 129: 76–101. https://doi.org/10.1016/j.marenvres.2017.04.010
- Guggolz T, Meißner K, Schwentner M, Dahlgren TG, Wiklund H, Bonifácio P, Brandt A (2020) High diversity and pan-oceanic distribution of deep-sea polychaetes: Prionospio and Aurospio (Annelida: Spionidae) in the Atlantic and Pacific Ocean. Organisms, Diversity & Evolution 18(2): 1–7. https://doi.org/10.1007/s13127-020-00430-7
- Gunton LM, Neal L, Gooday AJ, Bett BJ, Glover AG (2015) Benthic polychaete diversity patterns and community structure in the Whittard Canyon system and adjacent slope (NE Atlantic). Deep-sea Research. Part I, Oceanographic Research Papers 106: 42–54. https://doi.org/10.1016/j.dsr.2015.07.004
- Gunton LM, Kupriyanova EK, Alvestad T, Avery L, Blake JA, Biriukova O, Böggemann M, Borisova P, Budaeva N, Burghardt I, Capa M, Georgieva MN, Glasby CJ, Hsueh P-W, Hutchings P, Jimi N, Kongsrud JA, Langeneck J, Meißner K, Murray A, Nikolic M, Paxton H, Ramos D, Schulze A, Sobczyk R, Watson C, Wiklund H, Wilson RS, Zhadan A, Zhang J (2021) Annelids of the eastern Australian abyss collected by the 2017 RV ‘Investigator’voyage. ZooKeys 1020: 1–198. https://doi.org/10.3897/zookeys.1020.57921
- Hartman O (1959) Catalogue of the polychaetous annelids of the world. Part I. Allan Hancock Foundation Publications. Occasional Paper 23: 1–353.
- Hartman O (1960) Systematic account of some marine invertebrate animals from the deep basins off southern California. Allan Hancock Pacific Expeditions 22(2): 69–216. [plates 1–19]
- Hartmann-Schröder G, Rosenfeldt P (1992) Die Polychaeten der “Polarstern”-Reise ANT V/1 in die Antarktis 1986. Teil 1: Euphrosinidae bis Iphitimidae. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 89: 85–124. [page(s): 87]
- Held C (2000) Phylogeny and biogeography of Serolid Isopods (Crustacea, Isopoda, Serolidae) and the use of ribosomal expansion segments in molecular systematics. Molecular Phylogenetics and Evolution 15(2): 165–178. https://doi.org/10.1006/mpev.1999.0739
- Horst R (1910) On the genus Chloeia with some new species from the Malay Archipelago, partly collected by the Siboga-Expedition. Notes from the Leyden Museum 32: 169–175.
- Horst R (1912) Polychaeta errantia of the Siboga Expedition. Part 1, Amphinomidae. Siboga-Expeditie Uitkomsten op Zoologisch, Botanisch, Oceanographisch en Geologisch gebied verzameld in Nederlandsch Oost-Indië 1899-1900, 24a: 1–43 [10 plates] https://biodiversitylibrary.org/page/2187401 [page(s): 25]
- Janssen A, Kaiser S, Meissner K, Brenke N, Menot L, Martínez Arbizu P (2015) A Reverse Taxonomic Approach to Assess Macrofaunal Distribution Patterns in Abyssal Pacific Polymetallic Nodule Fields. PLoS ONE 10(2): e0117790. https://doi.org/10.1371/journal.pone.0117790
- Jeffreys RM, Levin LA, Lamont PA, Woulds C, Whitcraft CR, Mendoza GF, Wolff GA, Cowie GL (2012) Living on the edge: Single-species dominance at the Pakistan oxygen minimum zone boundary. Marine Ecology Progress Series 470: 79–99. https://doi.org/10.3354/meps10019
- Jumars PA, Dorgan KM, Lindsay SM (2015) Diet of worms emended: An update of polychaete feeding guilds. Annual Review of Marine Science 7(1): 497–520. https://doi.org/10.1146/annurev-marine-010814-020007
- Kaiser S, Brix S, Kihara TC, Janssen A, Jennings RM (2018) Integrative species delimitation in the deep-sea genus Thaumastosoma Hessler, 1970 (Isopoda, Asellota, Nannoniscidae) reveals a new genus and species from the Atlantic and central Pacific abyss. Deep-sea Research. Part II, Topical Studies in Oceanography 148: 151–179. https://doi.org/10.1016/j.dsr2.2017.05.006
- Katoh K, Misawa K, Kuma KI, Miyata T (2002) MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30(14): 3059–3066. https://doi.org/10.1093/nar/gkf436
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12): 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
- Kirkegaard JB (1995) Bathyal and abyssal polychaetes (errant species). Galathea Report 17: 7–56.
- Kobayashi G, Mukai R, Alalykina I, Miura T, Kojima S (2018) Phylogeography of benthic invertebrates in deep waters: a case study of Sternaspis cf. williamsae (Annelida: Sternaspidae) from the northwestern Pacific Ocean. Deep-sea Research. Part II, Topical Studies in Oceanography 154: 159–166. https://doi.org/10.1016/j.dsr2.2017.12.016
- Kudenov JD (1991) A new family and genus of the order Amphinomida (Polychaeta) from the Galapagos Hydrothermal vents. Ophelia, supplement 5 (Systematics, Biology and Morphology of World Polychaeta): 111–120.
- Kudenov JD (1993) Amphinomidae and Euphrosinidae (Annelida: Polychaeta) principally from Antarctica, the Southern Ocean, and Subantarctic regions. Antarctic Research Series, Biology of the Antarctic Seas XXII 58: 93–150. https://doi.org/10.1029/AR058p0093
- Lamarck JB (1818) [volume 5 of] Histoire naturelle des Animaux sans Vertèbres, préséntant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s’y rapportent; precedes d’une Introduction offrant la determination des caracteres essentiels de l’Animal, sa distinction du vegetal et desautres corps naturels, enfin, l’Exposition des Principes fondamentaux de la Zoologie. Paris, Deterville. Vol 5, 612 pp. http://biodiversitylibrary.org/page/12886879 [page(s): 327 [as ‘Amphinomae’]]
- Lim SC, Wiklund H, Glover AG, Dahlgren TG, Tan KS (2017) A new genus and species of abyssal sponge commonly encrusting polymetallic nodules in the Clarion-Clipperton Zone, East Pacific Ocean. Systematics and Biodiversity 15(6): 507–519. https://doi.org/10.1080/14772000.2017.1358218
- Lodge M, Johnson D, Le Gurun G, Wengler M, Weaver P, Gunn V (2014) Seabed mining: International Seabed Authority environmental management plan for the Clarion–Clipperton Zone. A partnership approach. Marine Policy 49: 66–72. https://doi.org/10.1016/j.marpol.2014.04.006
- Maciolek NJ (2020) Anguillosyllis (Annelida: Syllidae) from multiple deep-water locations in the northern and southern hemispheres. Zootaxa 4793(1): 1–73. https://doi.org/10.11646/zootaxa.4793.1.1
- Maddison WP, Maddison DR (2021) Mesquite: a modular system for evolutionary analysis. Version 3.70. http://www.mesquiteproject.org
- McIntosh WC (1885) Report on the Annelida Polychaeta collected by H.M.S. Challenger during the years 1873–1876. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873–76. Zoology (Jena, Germany) 12(part 34).
- Medlin L, Elwood H, Stickel S, Sogin M (1988) The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71(2): 491–499. https://doi.org/10.1016/0378-1119(88)90066-2
- Mohrbeck I, Horton T, Jażdżewska AM, Arbizu PM (2021) DNA barcoding and cryptic diversity of deep-sea scavenging amphipods in the Clarion-Clipperton Zone (Eastern Equatorial Pacific). Marine Biodiversity 51(2): 1–5. https://doi.org/10.1007/s12526-021-01170-3
- Neal L, Barnich R, Wiklund H, Glover AG (2012) A new genus and species of Polynoidae (Annelida, Polychaeta) from Pine Island Bay, Amundsen Sea, Southern Ocean – a region of high taxonomic novelty. Zootaxa 3542(1): 80–88. https://doi.org/10.11646/zootaxa.3542.1.4
- Neal L, Linse K, Brasier MJ, Sherlock E, Glover AG (2017) Comparative marine biodiversity and depth zonation in the Southern Ocean: Evidence from a new large polychaete dataset from Scotia and Amundsen seas. Marine Biodiversity 48(1): 581–601. https://doi.org/10.1007/s12526-017-0735-y
- Neal L, Taboada S, Woodall LC (2018) Slope-shelf faunal link and unreported diversity off Nova Scotia: Evidence from polychaete data. Deep-sea Research. Part I, Oceanographic Research Papers 138: 72–84. https://doi.org/10.1016/j.dsr.2018.07.003
- Neal L, Wiklund H, Rabone M, Dahlgren T, Glover AG (2022) Abyssal fauna of polymetallic nodule exploration areas, eastern Clarion-Clipperton Zone, central Pacific Ocean: Annelida: Spionidae and Poecilochaetidae. Marine Biodiversity 52(51): 51. https://doi.org/10.1007/s12526-022-01277-1
- Nygren A (2014) Cryptic polychaete diversity: A review. Zoologica Scripta 43(2): 172–183. https://doi.org/10.1111/zsc.12044
- Nygren A, Sundberg P (2003) Phylogeny and evolution of reproductive modes in Autolytinae (Syllidae, Annelida). Molecular Phylogenetics and Evolution 29(2): 235–249. https://doi.org/10.1016/S1055-7903(03)00095-2
- Palumbi SR (1996) Nucleic acids II: the polymerase chain reaction. Molecular Systematics, 205–247.
- Paterson GL, Neal L, Altamira I, Soto EH, Smith CR, Menot L, Billett DS, Cunha MR, Marchais-Laguionie C, Glover AG (2016) New Prionospio and Aurospio species from the deep sea (Annelida: Polychaeta). Zootaxa 4092(1): 1–32. https://doi.org/10.11646/zootaxa.4092.1.1
- Posada D (2008) jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution 25(7): 1253–1256. https://doi.org/10.1093/molbev/msn083
- Read G, Fauchald K [Eds] (2021) World Polychaeta Database. Amphinomida. [Accessed through: World Register of Marine Species at:] http://www.marinespecies.org/aphia.php?p=taxdetails&id=893 [2021-05-14]
- Righi S, Savioli M, Prevedelli D, Simonini R, Malferrari D (2021a) Unravelling the ultrastructure and mineralogical composition of fireworm stinging bristles. Zoology 144: 125851. https://doi.org/10.1016/j.zool.2020.125851
- Righi S, Savioli M, Prevedelli D, Simonini R, Malferrari D (2021b) Response to Tilic and Bartolomaeus’s Commentary on the original Research Paper “Unravelling the ultrastructure and mineralogical composition of fireworm stinging bristles”(Zoology, 144). Zoology 144: 125889. https://doi.org/10.1016/j.zool.2020.125889
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. https://doi.org/10.1093/sysbio/sys029
- Rouse GW, Fauchald K (1997) Cladistics and polychaetes. Zoologica Scripta 26(2): 139–204. https://doi.org/10.1111/j.1463-6409.1997.tb00412.x
- Sars GO (1872) On some remarkable forms of animal life from the great deeps off the Norwegian coast. Part 1, partly from posthumous manuscripts of the late prof. Mich. Sars. University Program for the 1st half-year 1869. Brøgger & Christie, Christiania viii + 82 pp. [pls 1–6] http://biodiversitylibrary.org/page/11677777 [page(s): 45–49, pl. 4]
- Sjölin E, Erséus C, Källersjö M (2005) Phylogeny of Tubificidae (Annelida, Clitellata) based on mitochondrial and nuclear sequence data. Molecular Phylogenetics and Evolution 35(2): 431–441. https://doi.org/10.1016/j.ympev.2004.12.018
- Smith CR, De Leo FC, Bernardino AF, Sweetman AK, Arbizu PM (2008) Abyssal food limitation, ecosystem structure and climate change. Trends in Ecology & Evolution 23(9): 518–528. https://doi.org/10.1016/j.tree.2008.05.002
- Smith CR, Paterson G, Lambshead J, Glover A, Rogers A, Gooday A, Kitazato H, Sibuet M, Galeron J, Menot L (2011) Biodiversity, species ranges, and gene flow in the abyssal Pacific nodule province: predicting and managing the impacts of deep seabed mining. ISA Technical Study No. 3, International Seabed Authority, Kingston, Jamaica. [ISBN: 978-976-95217-2-841]
- Smith CR, Clark MR, Goetze E, Glover AG, Howell KL (2021) Biodiversity, Connectivity and Ecosystem Function Across the Clarion-Clipperton Zone: A Regional Synthesis for an Area Targeted for Nodule Mining. Frontiers in Marine Science 8: e797516. https://doi.org/10.3389/fmars.2021.797516
- Strugnell JM, Rogers AD, Prodöhl PA, Collins MA, Allcock AL (2008) The thermohaline expressway: The Southern Ocean as a centre of origin for deep-sea octopuses. Cladistics 24(6): 1–8. https://doi.org/10.1111/j.1096-0031.2008.00234.x
- Taylor ML, Roterman CN (2017) Invertebrate population genetics across Earth’s largest habitat: The deep‐sea floor. Molecular Ecology 26(19): 4872–4896. https://doi.org/10.1111/mec.14237
- Tilic E, Bartolomaeus T (2021) Commentary on:“Unravelling the ultrastructure and mineralogical composition of fireworm stinging bristles” by Righi et al. 2020. Zoology 144: 125890. https://doi.org/10.1016/j.zool.2020.125890
- Verdes A, Simpson D, Holford M (2018) Are fireworms venomous? Evidence for the convergent evolution of toxin homologs in three species of fireworms (Annelida, Amphinomidae). Genome Biology and Evolution 10(1): 249–268. https://doi.org/10.1093/gbe/evx279
- Washburn TW, Jones DO, Wei CL, Smith CR (2021) Environmental heterogeneity throughout the Clarion-Clipperton zone and the potential representativity of the APEI network. Frontiers in Marine Science 8: e319. https://doi.org/10.3389/fmars.2021.661685
- Wedding LM, Friedlander AM, Kittinger JN, Watling L, Gaines SD, Bennett M, Hardy SM, Smith CR (2013) From principles to practice: a spatial approach to systematic conservation planning in the deep sea. Proceedings of the Royal Society B: Biological Sciences 280(1773): 20131684. https://doi.org/10.1098/rspb.2013.1684
- Weigert A, Helm C, Meyer M, Nickel B, Arendt D, Hausdorf B, Santos SR, Halanych KM, Purschke G, Bleidorn C, Struck TH (2014) Illuminating the base of the annelid tree using transcriptomics. Molecular Biology and Evolution 31(6): 1391–1401. https://doi.org/10.1093/molbev/msu080
- Wiklund H, Nygren A, Pleijel F, Sundberg P (2008) The phylogenetic relationships between Amphinomidae, Archinomidae and Euphrosinidae (Amphinomida: Aciculata: Polychaeta), inferred from molecular data. Marine Biological Association of the United Kingdom. Journal of the Marine Biological Association of the United Kingdom 88(3): 509–513. https://doi.org/10.1017/S0025315408000982
- Wiklund H, Taylor JD, Dahlgren TG, Todt C, Ikebe C, Rabone M, Glover AG (2017) Abyssal fauna of the UK-1 polymetallic nodule exploration area, Clarion-Clipperton Zone, central Pacific Ocean: Mollusca. ZooKeys 1(707): 1–46. https://doi.org/10.3897/zookeys.707.13042
- Wiklund H, Neal L, Glover AG, Drennan R, Rabone M, Dahlgren TG (2019) Abyssal fauna of polymetallic nodule exploration areas, eastern Clarion-Clipperton Zone, central Pacific Ocean: Annelida: Capitellidae, Opheliidae, Scalibregmatidae, and Travisiidae. ZooKeys 883: 1–82. https://doi.org/10.3897/zookeys.883.36193
Supplementary material
DarwinCore database of CCZ Amphinomida
Data type: excel file
Explanation note: DarwinCore database.