Research Article |
|
Corresponding author: Laetitia M. Gunton ( laetitia.gunton@austmus.gov.au ) Academic editor: Greg Rouse
© 2021 Laetitia M. Gunton, Elena K. Kupriyanova, Tom Alvestad, Lynda Avery, James A. Blake, Olga Biriukova, Markus Böggemann, Polina Borisova, Nataliya Budaeva, Ingo Burghardt, Maria Capa, Magdalena N. Georgieva, Christopher J. Glasby, Pan-Wen Hsueh, Pat Hutchings, Naoto Jimi, Jon A. Kongsrud, Joachim Langeneck, Karin Meißner, Anna Murray, Mark Nikolic, Hannelore Paxton, Dino Ramos, Anja Schulze, Robert Sobczyk, Charlotte Watson, Helena Wiklund, Robin S. Wilson, Anna Zhadan, Jinghuai Zhang.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Gunton LM, Kupriyanova EK, Alvestad T, Avery L, Blake JA, Biriukova O, Böggemann M, Borisova P, Budaeva N, Burghardt I, Capa M, Georgieva MN, Glasby CJ, Hsueh P-W, Hutchings P, Jimi N, Kongsrud JA, Langeneck J, Meißner K, Murray A, Nikolic M, Paxton H, Ramos D, Schulze A, Sobczyk R, Watson C, Wiklund H, Wilson RS, Zhadan A, Zhang J (2021) Annelids of the eastern Australian abyss collected by the 2017 RV ‘Investigator’ voyage. ZooKeys 1020: 1-198. https://doi.org/10.3897/zookeys.1020.57921
|
Abstract
In Australia, the deep-water (bathyal and abyssal) benthic invertebrate fauna is poorly known in comparison with that of shallow (subtidal and shelf) habitats. Benthic fauna from the deep eastern Australian margin was sampled systematically for the first time during 2017 RV ‘Investigator’ voyage ‘Sampling the Abyss’. Box core, Brenke sledge, and beam trawl samples were collected at one-degree intervals from Tasmania, 42°S, to southern Queensland, 24°S, from 900 to 4800 m depth. Annelids collected were identified by taxonomic experts on individual families around the world. A complete list of all identified species is presented, accompanied with brief morphological diagnoses, taxonomic remarks, and colour images. A total of more than 6000 annelid specimens consisting of 50 families (47 Polychaeta, one Echiura, two Sipuncula) and 214 species were recovered. Twenty-seven species were given valid names, 45 were assigned the qualifier cf., 87 the qualifier sp., and 55 species were considered new to science. Geographical ranges of 16 morphospecies extended along the eastern Australian margin to the Great Australian Bight, South Australia; however, these ranges need to be confirmed with genetic data. This work providing critical baseline biodiversity data on an important group of benthic invertebrates from a virtually unknown region of the world’s ocean will act as a springboard for future taxonomic and biogeographic studies in the area.
Keywords
Biodiversity, Biogeography, deep sea, Echiura, lower-bathyal, Marine Parks, Polychaeta, Sipuncula, Tasman Sea
Introduction
The deep sea (> 200 m depth) is the least explored environment on our planet, where most species have not been sampled and remain undiscovered. The vast sediments of the deep sea cover approximately 65% of the Earth’s surface, and it is a unique environment characterised by darkness, low temperatures and low currents, high hydrostatic pressure, and well oxygenated oligotrophic waters (
In Australia, the abyssal plain (3000 to 6000 m depth) and deep ocean floor covers ~ 2.8 million km2, or 30% of Australia’s marine territory (
Earlier sampling of the eastern Australian abyss was performed as part of research expeditions to the area organised by non-Australian institutions. These include expeditions dating back to the H.M.S. ‘Challenger’ expedition (1874, the UK), the ‘Galathea’ expedition (1951–52, Denmark), the research vessel (RV) ‘Dmitry Mendeleev’ (1975–76, USSR), and RV ‘Tangaroa’ voyages (1982, New Zealand) (reviewed in
A new era for deep-sea biological exploration in Australia began in 2014 with the launch of the Marine National Facility’s RV ‘Investigator’, the first Australian research vessel equipped to routinely perform biological sampling to depths of 5000 m. The systematic biological study of abyssal depths in Australia on board RV ‘Investigator’ started with the Great Australian Bight (GAB) Research Program. This programme conducted six surveys off the southern coastline of Australia during 2013, 2015, and 2017, sampling epifauna from soft substrates, rocky outcrops in canyons and seamounts from depths of 200–5000 m (
The significant gap in knowledge about the eastern abyss was addressed by the 2017 ‘Sampling the Abyss’ research project supported by the Marine National Facility, the Commonwealth Scientific and Industrial Research Organisation (
Annelids occur in all marine environments and they are typically a dominant macrofauna (> 300 µm) taxon in terms of abundance and species diversity in deep-sea soft sediments (
This study reports an illustrated and annotated preliminary species-level checklist of the annelid fauna collected during the 2017 ‘Sampling the Abyss’ survey along with species diversity and distribution data. Morphospecies are compared with those collected from the GAB sampling programme where possible.
Annelid species described below 1000 m in Australian waters (roughly corresponding to Exclusive Economic Zone, 12 nautical miles from the coast). Bold font indicates species from eastern Australian margin.
| Family | Species | Depth (m) | Type Locality |
|---|---|---|---|
| Polynoidae | Lepidasthenia australiensis (Augener, 1927) | 1000 | Off eastern Victoria |
| Sabellidae | Potaspina australiensis Capa, 2007 | 1000 | South of Point Hicks, Victoria |
| Polynoidae | Brychionoe karenae Hanley & Burke, 1991 | 1100 | Cascade Plateau off Tasmania |
| Onuphidae | Paradiopatra imajimai Paxton & Budaeva, 2013 | 1277 | Off eastern Victoria |
| Polynoidae | Lagisca torbeni Kirkegaard, 1995 | 1320–1340 | Great Australian Bight, south of Adelaide |
| Polynoidae | Harmothoe australis Kirkegaard, 1995 | 1340 | Great Australian Bight, south of Adelaide |
| Spionidae | Laonice pectinata Greaves, Meißner & Wilson, 2011 | 1440 | Indian Ocean, west of Perth |
| Onuphidae | Paradiopatra spinosa Paxton & Budaeva, 2013 | 1600 | Bass Canyon |
| Polynoidae | Eunoe ivantsovi Averincev, 1978 | 1640 | Lord Howe Island Rise |
| Polynoidae | Eunoe papillaris Averincev, 1978 | 1800 | Off southwestern Tasmania |
| Nephtyidae | Aglaophamus profundus Rainer & Hutchings, 1977 | 2195 | Off northeastern Tasmania |
| Polynoidae | Parapolyeunoa flynni (Benham, 1921) | 2379 | Off Maria Island, Tasmania |
| Fauveliopsidae | Fauveliopsis challengeriae McIntosh, 1922 | 3566 | South Indian Ocean, midway between Australia and Antarctica |
| Polynoidae | Eunoe abyssorum McIntosh, 1885 | 4755 | South of Australia |
| Polynoidae | Polynoe ascidioides McIntosh, 1885 (now considered a nomen dubium) | 4755 | South of Australia |
Materials and methods
Sampling area
The eastern Australian continental shelf is relatively narrow compared with the rest of the continent. The shelf break occurs ~ 15 km from the coast and the foot-of-slope and beginning of the abyssal plain can be as close as 60 km from the coast (
The East Australian Current (EAC) is an important shallow water current carrying ~ 22–27 Sverdrups from north to south along the east coast of Australia. This counter-clockwise southern Pacific gyre circulates shallow water from the Coral Sea along the continental margin until 32–35°S before heading eastward to New Zealand. Part of the EAC is deflected offshore ~ 30°S along the Tasman Front, this divides the warm waters of the Coral Sea and the cooler waters of the Tasman Sea (
Field collection and processing
Biological samples were collected from 13 sites at one-degree intervals of latitude from 42°S to 24°S along the east coast of Australia from Tasmania to Southern Queensland (Fig.
Sample Sites. Beam trawl, Brenke sledge, and box core deployments on RV ‘Investigator’ cruise IN2017_V03 from the Australian eastern lower bathyal and abyssal environment. Abbreviations: Op., operation, BT,
| Op. | Location | Gear | Date | Start latitude and longitude | End latitude and longitude | Trawling distance (km) | Start depth (m) | End depth (m) |
|---|---|---|---|---|---|---|---|---|
| 004 | Freycinet MP | BT | 18/05/17 | -41.731, 149.120 | -41.791, 149.156 | 7.3 | 2820 | 2751 |
| 005 | Freycinet MP | BS | 18/05/17 | -41.730, 149.135 | -41.753, 149.147 | 2.8 | 2789 | 2779 |
| 006 | Freycinet MP | BT | 18/05/17 | -41.626, 149.552 | -41.689, 149.584 | 7.5 | 4022 | 4052 |
| 007 | Freycinet MP | BC | 18/05/17 | -41.647, 149.570 | 4030 | |||
| 008 | Freycinet MP | BC | 19/05/17 | -41.647, 149.569 | 4012 | |||
| 009 | Freycinet MP | BS | 19/05/17 | -41.626, 149.560 | -41.662, 149.574 | 4.2 | 4021 | 4035 |
| 011 | Freycinet MP | BC | 19/05/17 | -41.721, 149.125 | 2793 | |||
| 013 | Flinders MP | BT | 20/05/17 | -40.386, 148.928 | -40.383, 148.951 | 2.0 | 932 | 1151 |
| 014 | Flinders MP | BT | 20/05/17 | -40.464, 149.102 | -40.461, 149.147 | 3.8 | 2298 | 2486 |
| 015 | Flinders MP | BT | 20/05/17 | -40.473, 149.397 | -40.464, 149.426 | 2.6 | 4114 | 4139 |
| 016 | Flinders MP | BS | 21/05/17 | -40.463, 149.415 | -40.461, 149.364 | 4.3 | 4129 | 4131 |
| 017 | Flinders MP | BC | 21/05/17 | -40.460, 149.109 | s | 2331 | ||
| 022 | Bass Strait | BT | 22/05/17 | -39.462, 149.276 | -39.465, 149.242 | 2.9 | 2760 | 2692 |
| 023 | Bass Strait | BS | 22/05/17 | -39.462, 149.277 | -39.465, 149.246 | 2.7 | 2774 | 2694 |
| 027 | Bass Strait | BC | 22/05/17 | -39.462, 149.271 | 2741 | |||
| 028 | Bass Strait | BC | 22/05/17 | -39.500, 149.535 | 4147 | |||
| 030 | Bass Strait | BT | 23/05/17 | -39.552, 149.553 | -39.496, 149.598 | 7.3 | 4197 | 4133 |
| 031 | Bass Strait | BS | 23/05/17 | -39.422, 149.604 | -39.391, 149.597 | 3.5 | 4150 | 4170 |
| 032 | East Gippsland MP | BT | 24/05/17 | -38.479, 150.185 | -38.453, 150.186 | 2.9 | 3850 | 3853 |
| 033 | East Gippsland MP | BS | 24/05/17 | -38.521, 150.213 | -38.498, 150.207 | 2.6 | 4107 | 4064 |
| 035 | East Gippsland MP | BT | 25/05/17 | -37.792, 150.382 | -37.818, 150.353 | 3.9 | 2338 | 2581 |
| 040 | East Gippsland MP | BS | 25/05/17 | -37.815, 150.373 | -37.818, 150.356 | 1.5 | 2746 | 2600 |
| 041 | off Bermagui | BT | 26/05/17 | -36.418, 150.800 | 3980 | |||
| 042 | off Bermagui | BS | 26/05/17 | -36.385, 150.863 | -36.434, 150.863 | 5.4 | 4744 | 4716 |
| 043 | off Bermagui | BT | 27/05/17 | -36.351, 150.914 | -36.384, 150.913 | 3.7 | 4800 | 4800 |
| 044 | off Bermagui | BT | 27/05/17 | -36.355, 150.644 | -36.315, 150.651 | 4.5 | 2821 | 2687 |
| 045 | off Bermagui | BS | 27/05/17 | -36.360, 150.644 | -36.323, 150.650 | 4.1 | 2835 | 2739 |
| 046 | off Bermagui | BC | 27/05/17 | -36.284, 150.658 | 2643 | |||
| 053 | Jervis MP | BT | 28/05/17 | -35.114, 151.469 | -35.084, 151.441 | 4.2 | 3952 | 4011 |
| 054 | Jervis MP | BS | 28/05/17 | -35.117, 151.473 | -35.099, 151.455 | 2.6 | 4026 | 3881 |
| 055 | Jervis MP | BS | 28/05/17 | -35.335, 151.259 | -35.334, 151.219 | 3.6 | 2667 | 2665 |
| 056 | Jervis MP | BT | 29/05/17 | -35.333, 151.258 | -35.332, 151.214 | 4.0 | 2650 | 2636 |
| 57* | Jervis MP | BC | ||||||
| 065 | off Newcastle | BT | 30/05/17 | -33.441, 152.702 | -33.435, 152.665 | 3.5 | 4280 | 4173 |
| 066 | off Newcastle | BS | 30/05/17 | -33.448, 152.733 | -33.437, 152.674 | 5.6 | 4378 | 4195 |
| 067 | off Newcastle | BT | 31/05/17 | -32.985, 152.952 | -33.015, 152.913 | 4.9 | 2704 | 2902 |
| 068 | off Newcastle | BS | 31/05/17 | -32.993, 152.957 | -33.023, 152.943 | 3.6 | 2745 | 2963 |
| 069 | Hunter MP | BT | 03/06/17 | -32.479, 152.994 | -32.507, 152.991 | 3.1 | 1006 | 1036 |
| 070 | Hunter MP | BT | 03/06/17 | -32.575, 153.162 | -32.632, 153.142 | 6.6 | 2595 | 2474 |
| 076 | Hunter MP | BS | 03/06/17 | -32.577, 153.161 | -32.613, 153.149 | 4.2 | 2534 | 2480 |
| 078 | Hunter MP | BT | 04/06/17 | -32.138, 153.527 | -32.182, 153.524 | 4.9 | 3980 | 4029 |
| 079 | Hunter MP | BS | 04/06/17 | -32.131, 153.527 | -32.163, 153.524 | 3.6 | 4031 | |
| 080 | Central Eastern MP | BT | 05/06/17 | -30.099, 153.596 | -30.128, 153.571 | 4.0 | 1257 | 1194 |
| 086 | Central Eastern MP | BT | 05/06/17 | -30.098, 153.899 | -30.119, 153.875 | 3.3 | 2429 | 2518 |
| 087 | Central Eastern MP | BS | 06/06/17 | -30.113, 153.898 | -30.116, 153.867 | 3.0 | 2634 | 2324 |
| 088 | Central Eastern MP | BT | 06/06/17 | -30.264, 153.870 | -30.287, 153.830 | 4.6 | 4481 | 4401 |
| 089 | Central Eastern MP | BS | 06/06/17 | -30.263, 153.859 | -30.289, 153.844 | 3.2 | 4436 | 4414 |
| 090 | off Byron Bay | BT | 07/06/17 | -28.677, 154.203 | -28.709, 154.190 | 3.8 | 2587 | 2562 |
| 096 | off Byron Bay | BS | 07/06/17 | -28.678, 154.204 | -28.716, 154.189 | 4.5 | 2591 | 2566 |
| 097 | off Byron Bay | BT | 08/06/17 | -28.355, 154.636 | -28.414, 154.615 | 6.9 | 3762 | 3803 |
| 098 | off Byron Bay | BS | 08/06/17 | -28.371, 154.647 | -28.389, 154.612 | 4.0 | 3811 | 3754 |
| 099 | off Byron Bay | BT | 09/06/17 | -28.371, 154.649 | -28.388, 154.617 | 3.7 | 3825 | 3754 |
| 100 | off Byron Bay | BT | 09/06/17 | -28.054, 154.083 | -28.097, 154.081 | 4.8 | 999 | 1013 |
| 101 | off Moreton Bay | BT | 09/06/17 | -26.946, 153.945 | -26.971, 153.951 | 2.8 | 2520 | 2576 |
| 102 | off Moreton Bay | BT | 10/06/17 | -27.008, 154.223 | -27.049, 154.224 | 4.6 | 4274 | 4264 |
| 103 | off Moreton Bay | BS | 10/06/17 | -27.000, 154.223 | -27.061, 154.223 | 6.8 | 4260 | 4280 |
| 104 | off Moreton Bay | BT | 10/06/17 | -26.961, 153.848 | -26.991, 153.847 | 3.3 | 1071 | 1138 |
| 109 | off Fraser Island | BT | 11/06/17 | -25.221, 154.164 | -25.253, 154.192 | 4.5 | 4006 | 4005 |
| 110 | off Fraser Island | BS | 11/06/17 | -25.220, 154.160 | -25.261, 154.200 | 6.1 | 4005 | 4010 |
| 115 | off Fraser Island | BT | 11/06/17 | -25.325, 154.068 | -25.351, 154.076 | 3.0 | 2350 | 2342 |
| 118** | off Fraser Island | BS | ||||||
| 119 | off Fraser Island | BS | 12/06/17 | -25.206, 153.991 | -25.178, 153.979 | 3.3 | 2247 | 2369 |
| 121 | Coral Sea MP | BT | 13/06/17 | -23.587, 154.194 | -23.617, 154.195 | 3.3 | 1013 | 1093 |
| 122 | Coral Sea MP | BT | 13/06/17 | -23.751, 154.639 | -23.773, 154.616 | 3.4 | 2369 | 2329 |
| 123 | Coral Sea MP | BS | 13/06/17 | -23.749, 154.641 | -23.774, 154.617 | 3.7 | 2271 | 2339 |
| 128 | Coral Sea MP | BT | 13/06/17 | -23.631, 154.660 | -23.659, 154.644 | 3.5 | 1770 | 1761 |
| 131 | Coral Sea MP | BS | 14/06/17 | -23.748, 154.643 | -23.778, 154.613 | 4.5 | 2297 | 2358 |
| 132 | Coral Sea MP | BS | 14/06/17 | -23.756, 154.568 | -23.780, 154.540 | 3.9 | 2181 | 2132 |
| 134 | Coral Sea MP | BS | 14/06/17 | -23.750, 154.572 | -23.774, 154.546 | 3.8 | 2093 | 2156 |
| 135 | Coral Sea MP | BT | 15/06/17 | -24.352, 154.291 | -24.384, 154.325 | 5.0 | 3968 | 4034 |
The
The Brenke sledge (mesh size 1 mm) was used to collect microbenthic infauna living near the sediment-water interface and more mobile epibenthic fauna (
Prior to fixation, all specimens were weighed and registered on board and assigned labels with operation (op) and accession numbers (acc).
Annelid specimens collected during the voyage were shipped to the Australian Museum, Sydney (
Laboratory identification of annelids
At the respective institutions, annelids fixed in formalin were soaked in water, preserved with 80% ethanol and sorted in 80% ethanol, while ethanol-fixed annelids were sorted in 95% ethanol. Mixed lots of annelids were sorted to families at the
All beam trawl specimens were identified. Brenke sledge and box core material was identified past family level when specimens were large enough (considered adult) and/or complete. Annelids were assigned Latin binomial names where possible or determined in open nomenclature following
The matrix of all annelid species-level abundance and presence data (including beam trawl, box core, and Brenke sledge material) from voyage IN2017_V03 was constructed in MS Excel in standardised Darwin Core format.
Results
Taxonomic overview
Family Acoetidae Kinberg, 1856
A. Murray
This family of scale worms is characterised by the presence of internal ‘spinning’ glands which produce fibres used to construct their tough fibrous permanent tubes. These fibres often appear as golden strings emerging from the notopodia. Acoetidae are active carnivores and predators, and most frequently collected by fishers on baited lines, in shallow to deep waters (1–200 m). There are currently nine valid genera with 58 nominal species worldwide (
Panthalis
Diagnosis
One damaged specimen, with 24 anterior segments measuring 1.2 cm long, 0.6 mm wide. Head region badly damaged, but some features recognisable: low rounded ommatophores without necks and colourless, a single long median antenna attached mid-prostomium, longer than prostomium length; lateral antennae and palps missing, however; tentaculophores with a few chaetae, styles missing; elytra present on segments 2, 4, 5, 7 and alternating segments thereafter, delicate, transparent. All chaetae simple. Acicular neurochaetae starting from chaetiger 3, notochaetae absent from chaetiger 4 and on all parapodia thereafter. Notopodia with notoaciculum and spinning glands internally, golden ‘spinning’ fibres emergent from the inner surface of the notopodial bract. Superior group of neurochaetae from chaetiger 9 onwards, of two types: long, with plumose (brush) tips, and shorter chaetae with few whorls of short widely spaced hairs along shafts; middle group of neurochaetae stout, acicular chaetae with hairy aristate tips; inferior group of neurochaetae curved, lanceolate, with many transverse rows of overlapping spines along shaft.
Remarks
This specimen possesses brush-tipped neurochaetae typical of the genera Acoetes and Panthalis, but lacks notochaetae in all middle segments, a feature which distinguishes it as a species of Panthalis. The genus Panthalis has not yet been reported from Australian waters; however, specimens have been collected previously from deep water in the Arafura Sea off Western Australia and Northern Territory (
Records
1 specimen. Suppl. material
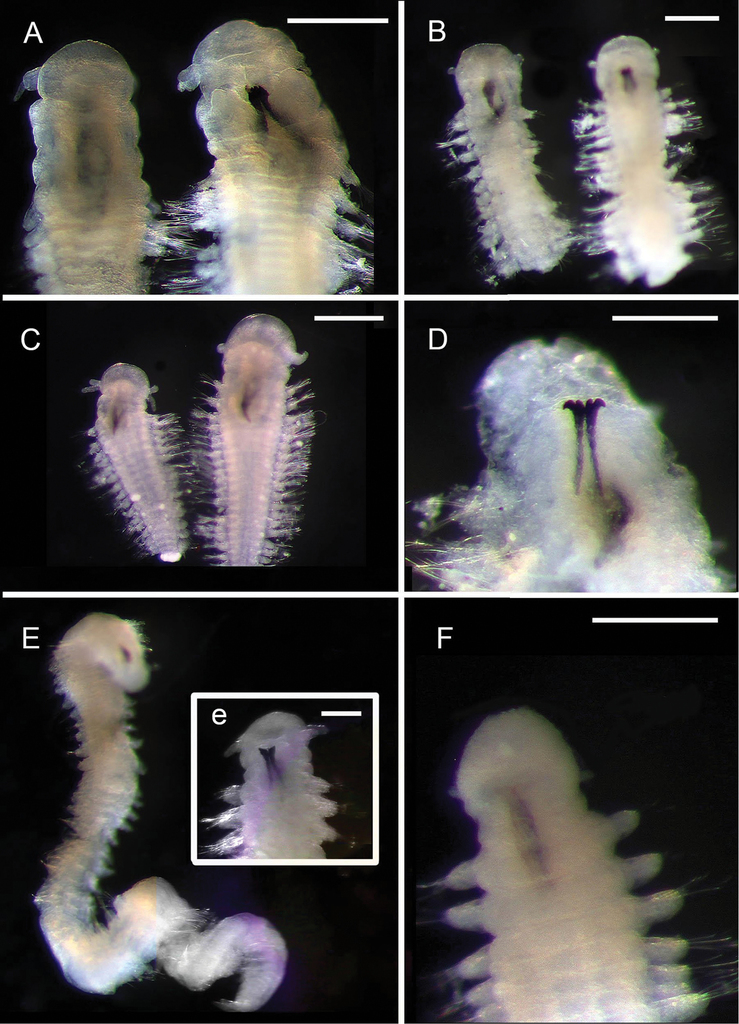
Acoetidae, Chrysopetalidae, Nephtyidae, Oweniidae A Acoetidae, Panthalis sp., dorsal view (
Family Acrocirridae Banse, 1969
N. Jimi
Acrocirridae are generally small, thread-like or maggot-shaped worms, which are predominantly benthic. There are currently nine valid genera with 43 nominal species (
Chauvinelia
Diagnosis
Length 1.5 mm, width 0.4 mm, 19 chaetigers, two pairs of branchiae, palps lost. Large ventral papillae present in anterior achaetous segments. Notochaetae elongated, simple, spinous in the tip. Neurochaetae elongated, compound, spinous in the tip.
Records
5 specimens. Suppl. material
Flabelligella
Records
1 specimen: Suppl. material
Flabelligena
Diagnosis
Length ~ 15 mm, width 1–2 mm, 31–35 chaetigers, prostomium subpentagonal, three pairs of branchiae, two or three spinous notochaetae, one or two composite neurochaetae, short lateral cirri. Body papillae short, with sediment particles. Large ventral papillae absent.
Records
12 specimens. Suppl. material
Flabelligena
Diagnosis
Incomplete, length ~ 7 mm, width 0.5 mm, ~ 18 chaetigers, prostomium subpentagonal, three pairs of branchiae, one or two spinous notochaetae, one composite neurochaetae, long lateral cirri in posterior chaetigers. Body papillae short, without attached sediment particles. Large ventral papillae present.
Records
5 specimens. Suppl. material
Flabelligena
Diagnosis
Length ~ 7 mm, width 0.5 mm, 13 chaetigers, prostomium subpentagonal, two pairs of branchiae, one or two spinous notochaetae, one or two composite neurochaetae, short lateral cirri. Body papillae very short, without attached sediment particles. Large ventral papillae absent.
Records
4 specimens. Suppl. material
Flabelligena
Diagnosis
Length ~ 7 mm, width 0.5 mm, 40 chaetigers, prostomium subpentagonal, two pairs of branchiae, three or four spinous notochaetae, 2–4 composite neurochaetae, pair of short lateral cirri. Body papillae very short, with attached sediment particles. Large ventral papillae present.
Records
6 specimens. Suppl. material
Flabelligena spp.
Records
6 specimens. Suppl. material
Swima
Records
2 specimens. Suppl. material
Acrocirridae
Diagnosis
Incomplete, length ~ 7 mm, width 0.4 mm, ~ 17 chaetigers, prostomium subpentagonal, ~ four pairs of branchiae, two or three notochaetae, three or four composite neurochaetae. Body papillae short, without sediment particles. Large ventral papillae absent.
Records
14 specimens. Suppl. material
Acrocirridae
Diagnosis
Incomplete (posterior fragment), length ~ 10 mm, width 0.7 mm, 25 chaetigers, 1–2 spinous notochaetae, one composite short neurochaetae. Body papillae short, with sediment particles. Large ventral papillae absent. Similar to Flabelligena sp. 1, but different in neurochaetal shape.
Records
1 specimen. Suppl. material
Acrocirridae
Diagnosis
Incomplete, length ~ 4 mm, width 0.4 mm, 12 chaetigers, 2–3 notochaetae, 2–3 composite neurochaetae. Body papillae short, without sediment particles. Large ventral papillae absent.
Records
1 specimen. Suppl. material
Acrocirridae gen. spp.
Remarks
Samples were identified to family level only or individuals were too fragmented for further analysis.
Records
16 specimens. Suppl. material
Family Ampharetidae Malmgren, 1866
T. Alvestad, L. M. Gunton
Ampharetidae are tubicolous annelids, with a body divided into a distinct thorax and abdomen, unlike the closely related Terebellidae, species of Ampharetidae are able to fully retract buccal tentacles into the mouth. The family Ampharetidae is composed of 64 accepted genera and > 300 species (
Amage
Diagnosis
Length 12 mm, width 4 mm. Body short, thick, with a short abdomen. Prostomium complex; central part drawn out into two lateral horns, lateral parts form large lobes while front part forms a ‘lip’. No glandular ridges or eyes. Approximately three pairs of branchiae in a transverse line in two widely separate groups. No paleae. Fourteen thoracic segments with notopodia with chaetae. First three pairs of notopodia and chaetae small. Thoracic uncini from segment VI. Eleven thoracic uncinigers. Nine abdominal uncinigers. Abdomen with rudimentary notopodia. Pygidium without lateral cirri.
Records
1 specimen. Suppl. material
Ampharetidae and Melinnidae. Ampharetidae A Amage sp. nov. 1 B Anobothrus sp. nov. 1, anterior end methyl blue staining C Anobothrus sp. nov. 2 anterior end methyl blue staining. Melinnidae D Melinna cf. armandi and tube (
Amage tasmanensis
Diagnosis
Length 16–30 mm, width 3–5 mm. Widest at branchial region. Thorax long and cylindrical, not tapering towards abdomen. Abdomen short; half length of thorax tapering towards pygidium. Prostomium without glandular ridges or eyes. Distal part of prostomium with longitudinal folds. Ventral surface of buccal segment with longitudinal folds. Four pairs of branchiae arranged as three middle pairs, almost in a transverse line, and one outer pair behind the outermost of the inner branchiae. Right and left branchial group separated by a space more or less equal to width of one branchia. Large lateral lobes on segment II. No paleae. Third segment with rudimentary notopodia with a few extremely small chaetae. Fourth and fifth segment with small notopodia with a few very short chaetae. Sixth to 16th segment with normal sized notopodia and notochaetae. Fourteen thoracic segments with notochaetae. Thoracic uncini from segment VI. Eleven thoracic uncinigers. Approximately 12 abdominal uncinigers. Abdomen with rudimentary notopodia. Pygidium with a pair of lateral cirri with thick bases and slender tips.
Remarks
The holotype of Amage tasmanensis was collected from 3830 m in the Tasman Sea. Due to the matching morphology and close proximity of the specimens from this study to the collection location of the holotype, we assign the name Amage tasmanensis.
Records
29 specimens. Suppl. material
Amphicteis sp. nov.
Diagnosis
Length 20–30 mm, 3 mm at widest section. Paired longitudinal glandular ridges curving slightly sidewise anteriorly. Paired transverse nuchal ridges separated by median gap, ridges at right angle to each other. Buccal tentacles and branchiae missing on both specimens. Chaetae on segment II modified to golden paleae extending past prostomium. Seventeen thoracic chaetigers including paleae, 15 abdominal chaetigers including pygidium. Anal cirri absent.
Records
2 specimens. Suppl. material
Anobothrus
Diagnosis
Length 14 mm, width 1 mm. Prostomium trilobed. Median lobe, narrow and protruding, delimited by deep lateral grooves. Eye spots present. Three pairs of branchiae. Branchiae arranged in transverse row without median gap. Branchiophores fused at base, forming a characteristic and well-marked edge/fold above head. Long filiform paleae. Thorax and abdomen of similar length. Fifteen thoracic segments with notopodia and capillary chaetae. Last 12 chaetigers of thorax with neuropodia and uncini. Notopodia on thoracic unciniger 8 slightly elevated and connected with a ciliated band. Tube a thin layer of secretion loosely incrusted with mud and foraminifera.
Records
1 specimen. Suppl. material
Anobothrus
Diagnosis
Incomplete, 9 mm length, 1 mm width. Specimens not in a good condition. Not possible to discern characters on the prostomium or count segments. Conical prostomium. Long filiform paleae. Space between the two groups of branchiae similar to width of one branchia. Tube a thin layer of secretion loosely incrusted with mud and foraminifera.
Records
2 specimens. Suppl. material
Jugamphicteis galatheae
Diagnosis
Length 25–40 mm, width 2–3 mm. Prostomium with four curved nuchal arches. Body long tapering towards pygidium. Four pairs of branchiae. Paleae present, long golden extend past rim of prostomium. First abdominal segment with dorsal fan with large median notch.
Remarks
The holotype of Jugamphicteis galatheae was collected from Kermadec Trench in the South Pacific Ocean ~ 4500 m; however, paratypes were recovered from both the Kermadec Trench and off the east coast of South Africa between Cape Town and Durban ~ 5000 m. The species is reported to have a wide distribution, which may indicate a species complex. Due to the matching morphology and close proximity of the specimens from this study to collection location of the holotype, we assign the name Jugamphicteis galatheae.
Records
40 specimens. Suppl. material
Ampharetidae gen. spp.
Remarks
Beam trawl specimens were incomplete which does not allow further identification, while Brenke sledge samples were identified to family level.
Records
260 specimens. Suppl. material
Family Amphinomidae Lamarck, 1818
L. M. Gunton, D. Ramos, R. S. Wilson
The family Amphinomidae is characterised by simple calcareous chaetae, in some species these chaetae are very fragile breaking off if touched and causing a burning sensation giving them the common name, ‘fireworms’. The family is divided in to two subfamilies, Archinominae Kudenov, 1991 and Amphinominae Lamarck, 1818 based on the presence of accessory dorsal cirrus in the former and absence in the latter. Currently, there are 23 genera containing 148 valid species (
Bathychloeia cf. sibogae
Diagnosis
Body short, ovate, ~ 8 mm in length. 17–18 chaetigers bearing long (3–44 mm) furcate chaetae. Body pale colour, pair of purple spots dorsal on chaetiger 6, visible under skin. Dark blue-black colouration visible under skin on chaetigers 10–13, dorsal and ventral. Caruncle lobed, extending to chaetiger 3. Branchiae branched, only found on chaetiger 5. Parapodia short, but neuro-and notochaetae well separated, neurochaetae lateral. Notochaetae dorsal (remaining tuft on chaetiger 7). Parapodial cirri on all (?) chaetigers, longer on final five. Chaetae long, bifurcate. No serrations or harpoon chaetae. Neurochaetae shorter than notochaetae. Faint membrane/covering visible over the furcate tips of some chaetae. Pygidium with thick anal cirrus, may be part of a pair.
Remarks
The type locality of Bathychloeia sibogae is in the Banda Sea, Malay Archipelago 1158 m depth.
Records
4 specimens: Suppl. material
Linopherus
Diagnosis
Prostomium divided into two. Posterior portion pentagonal with medial antennae on posterior edge, flanked laterally by the first chaetiger. Anterior section round with antennae and palps reduced to small bumps anterolaterally and laterally respectively. Body small, slightly wider anteriorly and tapering posteriorly. Eyes absent. First chaetiger reduced, not continuous dorsally. Papilliform notopodial postchaetal lobe present throughout. Bipinnate branchiae present on chaetigers 3–5.
Remarks
Linopherus sp. 1 differs from a second species of Linopherus known from the GAB (
Records
1 specimen. Suppl. material
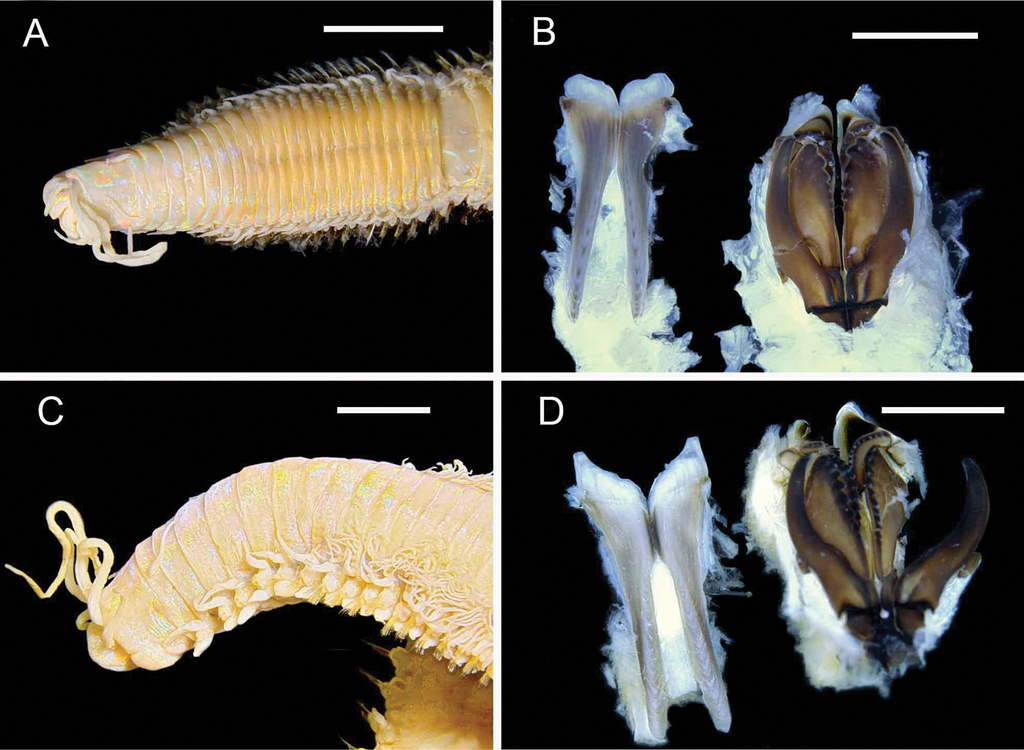
Amphinomidae A Linopherus sp. 1, bipinnate branchiae B Linopherus sp. 1, antennae and palps C Paramphinome cf. australis D Paramphinome cf. australis, hooks E Pareurythoe sp. (
Paramphinome cf. australis
Diagnosis
Body shape elongate ~ 3 mm length. Eyes absent. Prostomium rounded. One or two strongly curved hooks on chaetiger 1 (Fig.
Remarks
A redescription of Paramphinome australis is given in
Records
40 specimens. Suppl. material
Pareurythoe
Diagnosis
Body shape elongate (with parallel sides). Notochaetae in dorsal tufts. Caruncle inconspicuous. Caruncle median ridge absent. Branchiae as tufts from chaetiger 3.
Remarks
Also known from six stations (189–2867 m) in the GAB (
Records
2 specimens. Suppl. material
Amphinomidae gen. spp.
Remarks
Samples identified to family level only as individuals too damaged for further analysis, or juveniles (Fig.
Records
5 specimens. ops. 16, 110 (
Family Aphroditidae Malmgren, 1867
A. Murray, R. S. Wilson
Aphroditidae is a family of scale-worms commonly referred to as ‘sea mice’ due to their hairy appearance. Currently, there are seven genera containing 104 species (
Aphrodita cf. talpa
Diagnosis
Body shape ovate, length less than twice maximum width. Specimens with dorsal felt of fine notochaetae covering and obscuring elytra; 15 pairs elytra, elytral surface with micropapillae. Prostomium rounded, without ocular peduncles, eye pigment absent (may be present), nuchal flaps absent; facial tubercle well-developed, ~ same length as prostomium, papillate. Median antenna long, thin, as long as prostomium, with ceratophore ~ one third the length of style; palps long, minute papillae present. Notochaetae of three kinds: capillary chaetae forming matted dorsal felt; iridescent capillary chaetae projecting laterally; and stout, golden acicular spines with fine tubercles and hairs and with fine curved/hooked tips. Neurochaetae stout, superior tier thicker, brown with pilose margin and smooth slightly curved naked tip, inferior tier similar but golden brown and thinner than upper neurochaetae, with thickly pilose margin and slightly curved naked tips.
Remarks
This species may be undescribed; it differs from Aphrodita talpa Quatrefages, 1866 (described from New Zealand) in having an elongate median antenna, hirsute notochaetae, iridescent capillary notochaetae, and lacking hastate neurochaetae. It has previously been reported from a number of locations around Australia at depths of 17–171 m as Aphrodita talpa by
Records
10 specimens. Suppl. material
Aphroditidae A Aphrodita cf. talpa (NMV F.293294) dorsal view B Aphrodita cf. talpa (
Aphrodita goolmarris
Diagnosis
Large-bodied specimens, body shape ovate, length less than twice maximum width. Thin dorsal felt of fine notochaetae covering elytra; 15 pairs elytra, elytral surface with micropapillae. Prostomium rounded, without ocular peduncles, with raised ocular areas, pigment absent; facial tubercle well-developed, inflated, with small papillae. Palps extending to segment 11 with minutely papillated margins. Median antenna rod-shaped, fifth length of prostomium. Notochaetae of three kinds: capillary chaetae forming matted dorsal felt; stout, golden-brown, smooth chaetae curving over dorsum; and lateral short tuft of faintly iridescent capillary notochaetae, not forming a fringe. Neuropodia with three tiers of chaetae: stout, superior tier with two stout dark, pilose-tipped acicular neurochaetae, middle tier with 4–9 similar chaetae, inferior tier with 8–15 similar chaetae. Some anterior segments with non-pilose acicular neurochaetae with smooth margins and tips. Numerous golden-yellow, bipinnate neurochaetae present in chaetigers 2 and 3 in inferior position.
Remarks
This species is recorded from Western Australia (WA), New South Wales (NSW) and Queensland (QLD) (Cape York) in depths of 353–3058 m (
Records
4 specimens. Suppl. material
Laetmonice benthaliana
Diagnosis
Body shape elongate, > twice as long as maximum width. Number of chaetigers 33–34. Dorsal felt of fine notochaetae absent. Fifteen pairs of elytra, elytral surface smooth. Elytra comments: some inconspicuous brown pigmented spots in the middle. Prostomium rounded, facial tubercle large and visible dorsally. Facial tubercle papillate. Eye pigmentation absent. Eyes located on elongate ocular peduncles, longer than wide. Median antenna bi-articulate with basal ceratophore and elongate style. Median antenna ceratophore length ~ as long as prostomium. Median antenna ceratostyle length much longer than prostomium (3–6 × as long). Palp surface smooth, palps extending to segment 15. Ventrum with sparse cover of papillae, appearing almost smooth. Ventral cirri of mid-body chaetigers short, not reaching base of neurochaetae. Dorsal notochaetae harpoon-like, with several barb-like tips. Harpoon notochaetae (stout with barbed tips) present. Harpoon notochaetae shaft with fine granulations. Prominent basal spur on neurochaetae present. Spur-neurochaetae of (mid-body segments) subdistal teeth absent. Spur-neurochaetae subdistal hairs present. Marginal hairs not reaching the tip of the spur but leaving a significant basal gap.
Remarks
In this study the species was recorded from 2751–2820 m; other records from the Australian region include RV ‘Dmitry Mendeleev’ voyage 16 stations 1372 and 1373 in the GAB (700–1976 m) by
Records
4 specimens. Suppl. material
Laetmonice yarramba
Diagnosis
Specimens dorsally with debris entangled in felted notochaetae, sometimes obscuring elytra, but dorsal felt not covering elytra, elytra 12–15 pairs. Prostomium with short ocular peduncles, eye pigment absent; facial tubercle well-developed, papillate; nuchal flaps absent. Palps extending to segments 13 and 14, margins finely papillate. Median antenna with ceratophore half length of prostomium; antennal style long thin, > 3 × length of prostomium. Notochaetae of three kinds: golden, curved, smooth, acicular chaetae arched over dorsum; stout, long harpoon chaetae with three recurved fangs below tips, shafts tuberculate on some specimens; tuft of fine mud-covered chaetae ventrally. Neurochaetae in two tiers: superior tier of 2–4 yellow acicular chaetae with basal spur and subdistal fringe of hairs, inferior tier with numerous yellow bipinnate chaetae. Ventrum with small papillae present, or may be absent.
Remarks
Specimens range from 0.2 mm to 7 cm in length. Some individuals are badly damaged, with chaetae missing, and other intact specimens show some morphological variability from the original description by
Records
354 specimens. Suppl. material
Laetmonice cf. producta
Diagnosis
Large-bodied specimen, body shape elongate, > twice as long as maximum width. Dorsal felt of fine notochaetae absent, 18 pairs elytra with purple colouration on inner halves. Prostomium with pair of large ocular peduncles, without eye pigment; facial tubercle well-developed, with long conical papillae; small nuchal flaps present. Palps extending to segment 11, margins finely papillate. Median antenna with ceratophore half the length of the prostomium; antennal ceratostyle longer than prostomium, slender, clavate-tipped, 3 × length of prostomium. Notochaetae of three kinds: ~ 15 smooth, golden, unidentate acicular chaetae; ~ ten long, stout, yellow brown harpoon-like chaetae with 3–5 recurved fangs below tips, shafts smooth or tuberculate; tuft of short, fine mud-covered capillary chaetae ventrally. Neurochaetae in two tiers: superior tier of yellow acicular chaetae with basal spur and subdistal fringe of long hairs and bare tips, inferior tier with numerous golden bipinnate neurochaetae. Ventrum covered with small papillae.
Remarks
This specimen agrees well with the description of other Australian specimens assigned to Laetmonice producta Grube, 1877 by Hutchings & McRae (1993), who stated that the species displayed much morphological variability and had a broad distribution. Grube originally described L. producta from the area of the Kerguelen Islands in the Southern Ocean.
Records
1 specimen. Suppl. material
Family Capitellidae Grube, 1862
L. M. Gunton
Capitellids resemble terrestrial earthworms due to their simple cylindrical body shape, lack of head appendages and often reduced parapodia. The family contains 44 genera and ~ 186 species (
Capitella spp.
Records
7 specimens. Suppl. material
Notomastus spp.
Remarks
A single OTU provisionally referred to Notomastus was recorded from six stations (437–3771 m) in the GAB (
Records
2 specimens. Suppl. material
Capitellidae gen. spp.
Remarks
Specimens not identified beyond family level.
Records
19 specimens. Suppl. material
Family Chaetopteridae Audouin & Milne Edwards, 1833
L. M. Gunton, J. Zhang
Chaetopterids are characterised by a pair of long palps and a body divided into three distinct regions. Some species produce bright blue luminescent mucus. Currently there are five genera and 73 accepted species (
Phyllochaetopterus spp.
Records
7 specimens. Suppl. material
Chaetopteridae gen. spp.
Remarks
The specimens were too fragmented to be identified further.
Records
2 specimens. Suppl. material
Family Chrysopetalidae Ehlers, 1864
C. Watson
Chrysopetalids are distinguished by broad notochaetal, leaf-like paleae and/or notochaetal spines in fans covering the dorsum. Chrysopetalidae currently contains 29 genera and ~ 110 species (
Dysponetus cf. caecus
Diagnosis
Prostomium truncate, with three short antennae and two short palps. No eyes. Large mouth cirrus and pair of rod-like stylets. Two pairs of cirriform tentacular cirri on segments 1 and 2; segments one and two fused; dorsal cirri segment 1 very long, notopodia 2 with notochaetae. Mid-body notopodia with two lengths of semi-erect, golden-coloured notochaetal fascicles: short, broad and long, slender, spines with two rows of spinelets; elongate cirrophores and long dorsal styles. Neuropodia with fascicle of very slender falcigerous neurochaetae with very long shafts; ventral cirri longer than neuropodia.
Remarks
Similar to, but differs from abyssal East Atlantic Dysponetus cf. caecus (sensu
Records
48 specimens. Suppl. material
Family Cirratulidae Ryckholt, 1851
J. A. Blake
Cirratulids possess many anterior tentacular filaments and numerous long filamentous branchiae along their body which gives them a frilly appearance. Currently there are 11 genera and ~ 277 accepted species (
Cirratulids are divided into (1) bitentaculate genera, having a narrow head consisting of a distinct prostomium and peristomium, a pair of long dorsal tentacles and branchiae along most of the body, and (2) multitentaculate genera having a wedge-shaped head and numerous dorsal tentacles arising from anterior segments (
Aphelochaeta spp. nov.
Remarks
At least three new species of Aphelochaeta are present among these samples. Four OTUs of the genus Aphelochaeta were recorded from the GAB (189–2867 m) (
Records
16 specimens. Suppl. material
Chaetocirratulus sp. nov.
Remarks
A single specimen, believed to be a new species, is similar to Chaetocirratulus pinguis (Hartman, 1978) from Weddell Sea, Antarctica re-described by
Records
1 specimen. Suppl. material
Chaetozone spp. nov.
Remarks
One distinct new species is similar to Chaetozone brunnea described by
Records
13 specimens. Suppl. material
Kirkegaardia sp. nov.
Remarks
At least one new species of Kirkegaardia is present. The specimens have a long smooth peristomium, typical of several species of this genus (
Records
3 specimens. Suppl. material
Cirratulidae gen. spp.
Remarks
Brenke sledge samples were identified to family level.
Records
7 specimens. Suppl. material
Family Dorvilleidae Chamberlin, 1919
H. Wiklund
Dorvilleids contain some of the smallest described annelids and are the only extant group with ctenognath jaws. The family Dorvilleidae consists of ~ 200 species arranged in 32 genera (
Remarks. Species were preliminary separated on the basis of the forms of mandibles, shape of head and appendages, and shape of chaetae. The species vary slightly in size, with the smallest species being just 1 mm long (Ophryotrocha sp. 2) and the largest being 3.2 mm long (Ophryotrocha sp. 5).
Ophryotrocha
Ophryotrocha
Records
137 specimens. Suppl. material
Ophryotrocha
Records
136 specimens. Suppl. material
Ophryotrocha
Records
29 specimens. Suppl. material
Ophryotrocha
Records
17 specimens. Suppl. material
Ophryotrocha
Records
17 specimens. Suppl. material
Ophryotrocha
Records
1 specimen. Suppl. material
Ophryotrocha
Records
3 specimens. Suppl. material
Dorvilleidae gen. spp.
Remarks
Specimens from whale fall (op. 100) were too damaged or dried out to be identified. A single specimen provisionally referred to Schistomeringos was recorded from 932 m in the GAB (
Records
75 specimens. Table
Family Eunicidae Berthold, 1827
R. S. Wilson
Along with other members of the order Eunicida, species of Eunicidae possess a ventral muscular pharynx with mineralized or sclerotized jaws. Eunicidae are recognisable by possessing a prostomium with one to three antennae which lack ringed ceratophores. The family consists of 11 extant genera and 453 currently accepted species (
Eunice sp. nov.
Diagnosis
No pigmentation on preserved specimens. Prostomium bilobed, slightly notched. Eyes present, behind bases of palps. Prostomial appendages with widest gap separating palps from lateral antennae. Palpostyles, antennal styles, and peristomial cirri with irregular articulations. Peristomial rings distinct dorsally and ventrally but continuous laterally. Maxillae dentition: Mx I left 1, right 1. Mx II left 7, right 6. Mx III left 6. Mx IV left 5, right 9. Mx V left 1, right 1.
Branchiae absent. Lateral black dot between posterior parapodia absent. Dorsal cirri length short, at most as long as two body segments. Dorsal cirri of anterior chaetigers tapering, median chaetigers tapering, posterior chaetigers tapering, smooth, without articulations. Digitiform ventral cirri, basally inflated, commence chaetiger 3.
Pectinate chaetae absent. Compound falcigers present, appendages distally bidentate, hoods without mucros (rounded). Compound spinigers absent. Aciculae dark honey-coloured to black, distally bluntly pointed. Subacicular hooks dark honey-coloured to black, bidentate, distal tooth directed distally, subdistal tooth directed laterally. Subacicular hooks first present from chaetiger 24–27.
Remarks
Although referred to here as ‘Eunice sp.’, the above combination of characters cannot be accommodated in any currently known eunicid genus. The species is here treated as a member of Eunice since that genus remains poorly defined and already contains species of uncertain relationships (
Leodice sp. nov.
Diagnosis
Prostomial lobes frontally rounded, bilobed, slightly notched. Eyes behind bases of palps. Prostomial appendages evenly spaced, with palps slightly thinner than antennae. Antennal styles and palpostyles without articulations. Peristomial rings distinct dorsally and ventrally, continuous laterally. Peristomial (tentacular) cirri present, reach anterior region of peristomium, styles tapering, without articulations.
Maxillae dentition: Mx I left 1, right 1. Mx II left 7, right 9. Mx III left 12 (right absent). Mx IV left 6, right 11. Mx V left 1, right 1.
Branchiae present from chaetiger 4. Branchiae distinctly longer than dorsal cirri. Maximum number of branchial filaments 12–13 (at ~ chaetiger 10–12); five or six anterior chaetigers with single branchial filaments, no posterior chaetigers with single branchial filaments. Branchiae continuing until chaetiger 32–34. Ventral cirri of anterior segments digitiform.
Compound falcigers present, appendages bidentate, hoods without mucros (rounded). Aciculae light yellow, translucent. Subacicular hooks colour light yellow, translucent, bidentate.
Variation
A number of very small specimens (< ~ 1.5 mm maximum width) differ from the above description in having at most three or four branchial filaments, and a single specimen much larger than the remaining material has < 18 branchial filaments. These specimens apparently do not differ in other respects and all are assumed to represent a single species.
Remarks
This species clearly belongs in the genus Leodice following the generic concept of
Records
28 specimens. Suppl. material
Family Euphrosinidae Williams, 1852
D. Ramos
Euphrosinidae have short, wide bodies bearing many chaetae. There are ~ 62 species in four genera now considered valid (
Euphrosinopsis cf. horsti
Diagnosis
Narrow prostomium, flanked by first three chaetigers, with a broader ventral pad. Pair of large eyes dorsally. Oval body dorsoventrally flattened. Abundant chaetae densely covering the dorsum. Notochaetae in five tiers: first and fifth tiers with small furcate chaetae, second and fourth tiers with large furcate chaetae, and third tier with category IIB ringent chaetae. Neurochaetae furcate. Paired inflated anal cirri with unfused bases present.
Remarks
Observed specimens differ from described Euphrosinopsis species in having five tiers of notochaetae instead of two in E. antarctica, three in E. crassiseta, and four in E. horsti (
Records
3 specimens. Suppl. material
Euphrosinidae
Records
1 specimen. op. 9 (
Family Fabriciidae Rioja, 1923
A. Murray
Fabriciids are small (0.85–10 mm long) fanworms. Approximately 80 species in 17 valid genera are now considered to be in the family Fabriciidae (
Fabriciidae
Diagnosis
One small complete specimen, ~ 2 mm long. Branchial crown with three pairs of radioles with long pinnules terminating at same height as radioles. Eight thoracic and three abdominal chaetigers. Peristomial collar low, membranous, entire ventrally, mid-dorsally incised, anterior margin with shallow lateral notches (ventral conical flap or lobe absent). Dorsal lips as low rounded structures, ventral filamentous appendages absent. Conical structure above mouth absent. Peristomial glandular patches present. All thoracic notochaetae of two lengths: superior elongate, narrowly hooded; inferior short, narrowly hooded. Notochaetae of segments 3–8 similar to those of 1 and 2, pseudospatulate notochaetae absent. Thoracic uncini acicular with few rows of similar-sized teeth above main fang, hood present. Abdominal uncini with rasp-shaped teeth and long manubrium. Pigmented pygidial eyespots absent.
Remarks
This single specimen has been preserved in 95% ethanol and may have lost pigmentation of the peristomial and pygidial eyes. There are many similarities with the genus Fabriciola but the absence of ventral filamentous appendages is not typical for that genus, so the identification is tentative. More complete and well-preserved specimens would be required to provide a positive identification. This specimen also does not match the diagnosis for Pseudofabriciola, with which it is also similar. This specimen is also similar to that found in the deep GAB surveys of 2015 and 2017, recorded as ‘? Fabriciola sp.’ (
Records
1 specimen. Suppl. material
Fabriciidae
Records
1 specimen. Suppl. material
Family Fauveliopsidae Hartman, 1971
A. Murray, D. Ramos
Fauveliopsids may be cylindrical or have swellings along the body, they can be free-living or occupy gastropod shells, foraminifera tests, or tubes (
Fauveliopsis cf. challengeriae
Diagnosis
Specimens complete, 14.5 mm long, 1.5 mm wide at widest point. Body integument rugose and opaque, with scattered small papillae, tapered, posteriorly swollen, with segments in posterior region short (2–4 × wider than long), and 33 chaetigers. Anterior chaetigers with 2–4 chaetae per ramus, capillary and acicular (sigmoid or falcate) chaetae; middle and posterior chaetigers with 2–3 chaetae per notopodium and 3–5 chaetae per neuropodium, including falcate acicular chaetae and capillary chaetae. Interramal papillae distinct, somewhat stalked. Genital papillae not seen. Living in cemented sediment foraminifera tubes.
Remarks
The type locality for this species is in the Southern Ocean between Antarctica and Australia in 3510 m depth. It has not previously been recorded in Australian waters, but was recently redescribed from specimens from Antarctic waters and Eastern Pacific Ocean (
Records
3 specimens. Suppl. material
Laubieriopsis hartmanae
Diagnosis
Prostomium retracted. Body linear, blunt on both ends with 16 chaetigers. Chaetigers 1–4 shorter with 2–3 large acicular chaetae and 2–3 small acicular chaetae per parapodia. Chaetigers 5–16 with one acicular and one capillary per ramus, longest on chaetiger 16. Granular genital papillae on boundary of chaetigers 6 and 7.
Remarks
Similar in appearance to L. brevis from the Atlantic Ocean, but differs in the tips of the aciculars (bidentate in L. brevis) and genital papilla (smooth in L. brevis) (
Records
22 specimens. Suppl. material
Riseriopsis cf. santosae
Diagnosis
Prostomium retracted. Body linear with annulations, slightly inflated terminally with 27 chaetigers. Chaetigers 1–3 shorter, parapodia with one acicular and one capillary per ramus and an interramal papillae. Following chaetigers with one acicular and one capillary in the neuropodia and one larger acicular in the notopodia.
Remarks
Riseriopsis santosae resembles this specimen but differs in having more chaetigers (37–88). Riseriopsis santosae is known from shallower depths (410–415 m), while the only described deep-sea species in the genus, R. confusa (Thiel, Purschke & Böggemann, 2011), has a similar number of chaetigers as this specimen, but with an acicular and capillary in the medial notopodia. Both of these species are recorded from the South Atlantic.
Records
1 specimen. Suppl. material
Fauveliopsidae gen. spp.
Remarks
Brenke sledge samples identified to family level.
Records
60 specimens. Suppl. material
Family Flabelligeridae de Saint-Joseph, 1894
N. Jimi
The family Flabelligeridae is a group of sedentary annelids living in soft sediments or on hard substrates, except for two pelagic genera (
Bradabyssa cf. kirkegaardi
Diagnosis
Length 3 mm, width 0.5 mm, body papillae very long, thin, abundant. Cephalic cage not developed. One capillary notochaeta, one anchylosed neurochaeta. Sediment particles present on base of papillae.
Remarks
Records
1 specimen. Suppl. material
Flabelligeridae A Bradabyssa cf. kirkegaardi (
Bradabyssa
Diagnosis
Incomplete, length 9 mm, width 1 mm, 26 chaetigers, body papillae long, thin, abundant, with mud. Cephalic cage not developed. 2–4 capillary notochaetae, 3–4 anchylosed neurochaetae. Sediment particles present on body surface.
Remarks
This species belongs to group ‘villosa’ described in
Records
2 specimens. Suppl. material
Bradabyssa
Diagnosis
Incomplete, length 25 mm, width 3 mm, 28 chaetigers, body tubercles short, without sediment particles, abundant. Cephalic cage not developed. 4–5 capillary notochaetae, 3–4 anchylosed neurochaetae. Sediment particles present on body surface.
Remarks
This species belongs to group ‘verrucosa’ described in
Records
1 specimen. Suppl. material
Diplocirrus
Diagnosis
Incomplete, length 5.4 mm, width 1 mm, 16 chaetigers. Body with first ten chaetigers swollen, thereafter cylindrical. Cephalic cage (chaetiger 1) developed. Lateral papillae and chaetae well developed in posterior chaetigers. Sand particles restricted on body wall. Body papillae few, short, thin.
Records
7 specimens. Suppl. material
Diplocirrus
Diagnosis
Incomplete, length ~ 5–8 mm, width 1 mm, ~ 17 chaetigers. Body with first nine chaetigers swollen, thereafter cylindrical. Cephalic cage (chaetigers 1 and 2) developed. Lateral papillae and chaetae well developed in posterior chaetigers. Sand particles on body wall, absent on body papillae. Body papillae abundant, short, thin.
Records
2 specimens. Suppl. material
Diplocirrus
Diagnosis
Incomplete. Length 4 mm, width 0.5 mm, ~ 16 chaetigers. Body with first eight chaetigers swollen, thereafter cylindrical. Cephalic cage (chaetigers 1–3) developed. Lateral papillae not developed in posterior chaetigers, chaetae developed in posterior chaetigers. Attached sand particles absent. Body papillae scarce, very short, thin.
Records
2 specimens. Suppl. material
Diplocirrus
Diagnosis
Incomplete. Length 5 mm, width 1.5 mm, ~ 13 chaetigers. Body with first eight chaetigers swollen, thereafter cylindrical. Cephalic cage (chaetigers 1–4) developed. Lateral papillae not developed in posterior chaetigers, chaetae developed along entire body. Sand particles present on body surface. Body papillae abundant, very short, thin, without sand particles.
Records
5 specimens. Suppl. material
Diplocirrus
Diagnosis
Incomplete, only anterior fragments. Cephalic cage (chaetiger 1) developed. Large sand particles present on body surface. Body papillae few, short, thin, with large sediment particles on the base.
Records
3 specimens. Suppl. material
Ilyphagus
Diagnosis
Incomplete, damaged. Only anterior chaetigers. Body papillae very long, thin, abundant, with sediment particles at base of papillae. Cephalic cage developed. 1–2 capillary notochaetae, 4–5 anchylosed neurochaetae.
Records
4 specimens. Suppl. material
Flabelligeridae gen. spp.
Remarks
Specimens were too damaged to identify further and Brenke sledge samples were identified only to family level.
Records
1 specimen. Suppl. material
Family Glyceridae Grube, 1850
M. Böggemann, R. Sobczyk
Cylindrical and long-bodied worms, widely distributed in soft bottom sediments from intertidal zone to abyssal depths (
Glycera cf. capitata
Diagnosis
Specimens < 7 mm long, 1.2 mm wide. Prostomium with ~ ten rings. Parapodia of mid-body with longer neuropodial than notopodial prechaetal lobes and one rounded postchaetal lobe, dorsal cirri inserted on body wall far above parapodial base. Proboscideal papillae of two types, long digitiform (Fig.
Remarks
The identification to species level is tentative because the type locality of Glycera capitata is in the Atlantic Ocean off Greenland and there are no molecular data.
Records
4 specimens. Suppl. material
Glyceridae and Goniadidae. A Glyceridae, Glycera sp. anterior body with everted pharynx, ventrolateral view (
Glycera cf. russa
Diagnosis
Specimen 74 mm long, 6 mm wide. Prostomium with only nine rings. Postchaetal lobes of mid-parapodia both triangular and similar in length along most of the body, posteriorly more acute. Proboscideal papillae of two types, long conical ones (Fig.
Remarks
This specimen most resembles Glycera russa Grube, 1870. However, the prostomium consists of nine rings and the proboscideal papillae have only up to ten ridges, therefore, this may be a new species.
Records
1 specimen. Suppl. material
Glycera spp.
Diagnosis
Specimens with various lengths of the body and number of segments. Specimens dried up or missing the anterior parts of body. Prostomium when present prolonged, distinctly annulated with two pairs of appendages situated on anterior margin; eyes absent. Cylindrical proboscis, if present, covered by papillae; terminal part with four ailerons. Parapodia biramous.
Records
4 specimens. Suppl. material
Glyceridae gen. spp.
Remarks
Brenke sledge samples were identified to family level.
In addition to the taxa recorded from this study, Glycera lapidum Quatrefages, 1866 (13 stations, 212–2063 m) and Glycerella magellanica (McIntosh, 1885) (a single specimen, 2503 m) were recorded from the GAB (
Records
17 specimens. Suppl. material
Family Goniadidae Kinberg, 1865
M. Böggemann, R. Sobczyk
As the sister group to glycerids, goniadids are cylindrical, long-bodied annelids. The family is easily distinguished from glycerids by presence of usually one pair of macrognaths and variable number of ventral and dorsal micrognaths instead of two pairs of jaws on the anterior end of pharynx (
Bathyglycinde profunda
Diagnosis
Up to 24 mm length and 69 segments; anterior 36–37 parapodia uniramous; prostomium indistinctly annulated with bi-articulated terminal appendages; eyes absent; chevrons not present; papillae on pharynx arranged in rows (Fig.
Remarks
A Depth range of 350–5500 m has been reported (
Records
7 specimens. Suppl. material
Goniadidae gen. spp.
Remarks
Brenke sledge samples were identified to family level. Goniada antipoda (a single specimen, 2366 m), Progoniada regularis (a single specimen, 1486 m) and Progoniada sp. MoV7077 (5 stations, 932–4068 m) were recorded from the GAB (
Records
1 specimen. Suppl. material
Family Hesionidae Grube, 1850
C.J. Glasby, D. Ramos
Hesionids are a reasonably common and widespread group of polychaetes that have affinities with other nereidiforms, especially nereidids and syllids (
Microphthalmus
Diagnosis
Prostomium round, anteriorly cleft, broader than long, with three antennae and two palps. Antennae twice the length of the palps. No eyes. Six pairs of cirriform tentacular cirri on segments 1–3, longer than dorsal cirri. Uniramous parapodia. Dorsal cirri shorter on segment 4 than those on segment 5 onwards. Neuropodia with a pointed prechaetal lobe longer than the blunt postchaetal lobe. Neurochaetae heterogomph falcigers with serrated edge. Pygidium with two anal cirri and a ventral anal plate. Colour in ethanol pale yellow.
Records
10 specimens. Suppl. material
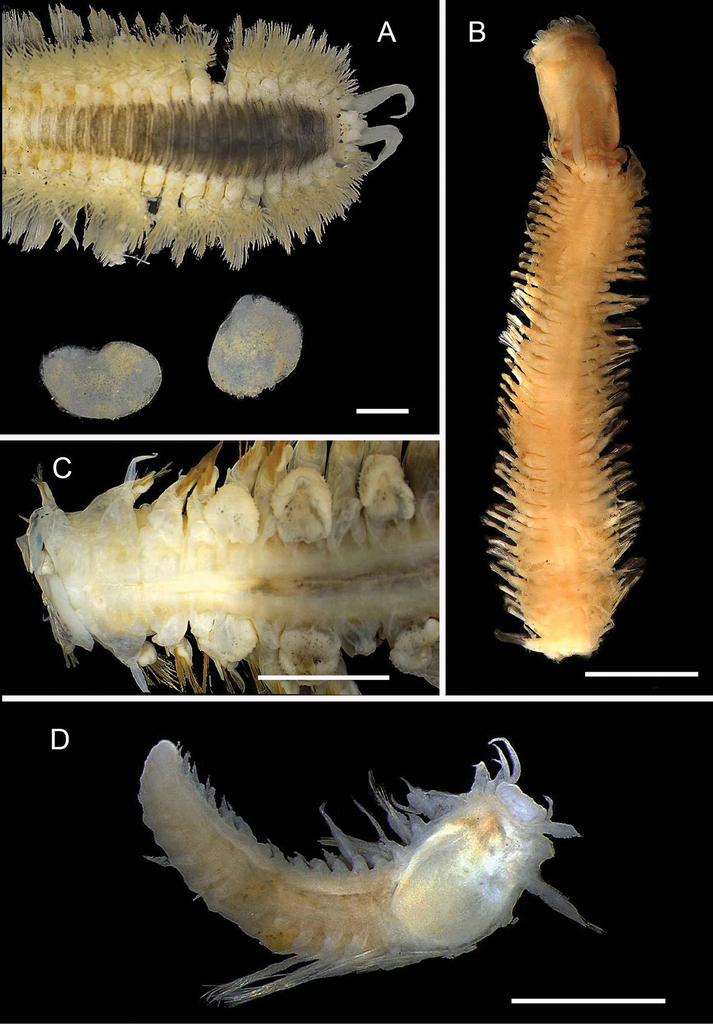
Hesionidae A Microphthalmus sp. B Microphthalmus sp., anterior end C Microphthalmus sp., pygidium D Neogyptis sp. nov. 1 (
Neogyptis
Diagnosis
Specimens all incomplete. Prostomium subrectangular (broader than long), posteriorly with deep mid-dorsal incision; one pair lateral antennae, tips well defined, extending two thirds to just short of length of palps; one short mid-prostomial antenna. Eyespots not visible. Palps bi-articulate, palpostyle cylindrical, twice length palpophore. Proboscis with many cylindrical papillae two or three rows deep, each papilla cilia-tipped. Nuchal organs very conspicuous, light brown, extending from anterolateral prostomium and coalescing mid-posterodorsally. First four segments tentacular, achaetous, bearing eight pairs tentacular cirri, longest extending back to chaetigers 5 or 6. Parapodia biramous throughout, dorsal cirri slender tapered, ~ two thirds length parapodial lobes anteriorly, half length in mid body; ventral cirri slender distally attached, tapered, extending less than half length parapodial lobes. Notopodia bearing capillaries only. Neuropodia compound spinigers. Colour in ethanol yellow-white base, with dark brown band dorsally and laterally on all tentacular segments, and lighter brown nuchal organs.
Remarks
The present material clearly falls within the concept of the tribe Amphidurini Pleijel, Rouse, Sundkvist & Nygren, 2012, which includes Amphiduros Hartman, 1959, Amphiduropsis Pleijel, 2001, Neogyptis Pleijel, Rouse, Sundkvist & Nygren, 2012, and questionably Parahesione Pettibone, 1956. Of these three genera the present specimens are closest to Neogyptis because of the terminal ring of proboscideal papillae and presence of a median antenna. The fine-tipped lateral cirri and cilia-tipped cylindrical papillae were clearly visible only in the formalin-fixed specimen (
Of the 12 species in the genus the only species currently known from the West Pacific is Neogyptis hinehina Pleijel, Rouse, Sundkvist & Nygren, 2012 from the Lau Basin, south of Tonga. However, this species has twisted noto- and neurochaetae, which were not observed in the present material. Therefore, the present material probably represents an undescribed species.
Records
11 specimens. Suppl. material
Neogyptis
Diagnosis
Specimens incomplete. Prostomium subrectangular (broader than long), posteriorly with slight mid-dorsal incision; antennae, presence implied from scars as follows: two lateral and one median antenna situated mid-posteriorly. Eyespots not visible. Palps bi-articulate, palpostyle cylindrical to conical, approximately equal in length to palpophore. Everted proboscis with many cushion-shaped distal papillae. First four segments tentacular, achaetous, bearing eight pairs tentacular cirri, slightly shorter than dorsal cirri of chaetiger 1. Nuchal organs, unpigmented, coalescing mid-dorsally. Parapodia biramous throughout, dorsal cirri slender tapered, ~ equal in length to parapodial lobes, except those on first chaetiger which are many times longer than parapodial lobe; ventral cirri distally attached, slender tapered, ~ half-length parapodial lobes throughout. Notopodia bearing serrated capillaries and one or two smooth spines. Neurochaetae all compound spinigers. Colour in ethanol yellow-white, unpigmented.
Remarks
See above for discussion justifying placement of this material into Neogyptis, tribe Amphidurini Pleijel, Rouse, Sundkvist & Nygren, 2012, and reasons for considering it represents an undescribed species. Neogyptis sp. 2 differs from Neogyptis sp. 1 in having palpostyles approximately equal in length to the palpophore, and lacking brown pigmentation on tentacular segments and the nuchal organs.
Records
2 specimens. Suppl. material
Parahesiocaeca
Diagnosis
Specimens in poor condition, incomplete. Prostomium sub-rectangular, with three antennae, all tapering rapidly and broader at base, extending just beyond tip of palps. Bi-articulated palps, palpostyle cylindrical. Eyes absent. Proboscis with marginal papillae. First two segments tentacular, achaetous, bearing four pairs of tentacular cirri (inferred from stubs). Tentacular and dorsal cirri appearing to be articulated. Parapodia sub-biramous. Neuropodia with long prechaetal lobe, short postchaetal lobe, and short ventral cirrus; all neurochaetae with long-bladed heterogomph falcigers with very fine tips.
Remarks
The present specimens fit the description of the genus Parahesiocaeca Uchida, 2004, although it differs from the description of the only species, P. japonica Uchida, 2004, in lacking eyes, having brown-pigmented nuchal organs, and the heterogomph falcigers having very long blades with very fine tips. The specimens probably represent an undescribed species, but their condition is too poor to describe as a new species.
Records
2 specimens. Suppl. material
Pleijelius cf. longae
Diagnosis
Prostomium ovoid, broader than long, with three short antennae and two short palps. No eyes. Six pairs of cirriform tentacular cirri on segments 1–3, longer than dorsal cirri. Dorsal cirri from segment 4 onwards multiarticulate, longer than body width. Notopodia low mounds with notochaetae spread out in a fan-like arrangement dorsally. Neuropodia elongated, with straight acicula and long acicular lobes. Neurochaetae heterogomph falcigers with serrated edge. Ventral cirri cirriform, much smaller than dorsal cirri. Three cirriform anal cirri on pygidium. Colour in ethanol white.
Remarks
We use the cf. designation here in recognition that Pleijelius longae, the only representative of the genus and originally described from the Northwestern Atlantic Ocean at 3500 m, is unlikely to be the same species as ours. Pleijelius is represented by a single species, P. longae Salazar-Vallejo & Orensanz, 2006. Observed specimens differ from P. longae in having notochaetal capillaries with denticles along the entire length instead of just near the tip, as well as possessing three anal cirri instead of six. This is the first report of this genus in the Pacific Ocean, occurring on whale fall, and at a depth of 1000 m.
Records
2 specimens. Suppl. material
Vrijenhoekia ketea
Diagnosis
Prostomium rectangular, with two lateral antennae, a very small median antenna, two palps, and a facial tubercle. Palpophores thicker than palpostyles, similar lengths. No eyes. Everted proboscis lacking papilla. Three fused anterior segments. Parapodia uniramous. Dorsal cirri long, especially in segments 1–5. Ventral cirri the same length as neuropodia after segments 1–3, digitiform, and inserted subterminally. Colour in ethanol pale yellow.
Remarks
The specimen differs from other specimens of the V. ketea species complex in having a larger body, being closer to the size range observed for V. balaenophila (
Records
1 specimen. Suppl. material
Vrijenhoekia
Diagnosis
Two complete specimens, 22 chaetigers (i.e., appears to be fixed growth of maximum 22 chaetigers). Prostomium subrectangular (broader than long), one pair lateral antennae, long, tapered, extending to tip of palps or 2 × longer; median antenna absent. Small red eyespots present, two or three pairs. Palps bi-articulate, palpostyle oval to globulose, slightly longer than palpophore. Proboscis with ten digitate terminal papillae and micro-papillae on surface; jaws absent. Facial tubercle absent. First three segments tentacular, achaetous, bearing six pairs tentacular cirri, slender, longest almost half length of body. Parapodia uniramous throughout, bearing digitate prechaetal lobe; dorsal and ventral cirri slender, tapered, similar in length throughout: ~ 0.5–1 × length of parapodial lobes, except for first and second pairs which are many times longer than parapodial lobe (similar in length to tentacular cirri). Ventral cirri inserted subterminally. Fan-like supra- and sub-neuropodial fascicles, bearing compound spinigers only, all with similar-length blades. Colour in ethanol yellow-white, unpigmented.
Remarks
The present specimens are closer to Vrijenhoekia than any other described hesionid genus, but differ from its type species, Vrijenhoekia balaenophila, and from V. cf. ketea as described above, as follows: a mediodorsal prostomial process (tubercle or antenna) was not observed (present in other members of the genus though minute and probably only observable clearly with scanning electron microscopy); palpostyles are globulose in the present material vs. tapered; compound chaetae blades are relatively longer in the present material; and the present specimens appear to have a maximum of 22 chaetigers, whereas there are 35 in the type species (this character is not reported in other species of the genus). On the other hand, the present material resembles more closely the type species than V. cf. ketea in having ten digitate proboscideal papillae (absent in the latter). The globulose (= ovoid) palpostyles are the most distinctive feature of the species.
Records
13 specimens. Suppl. material
Hesionidae gen. spp.
Remarks
Twelve specimens of Hesionidae could not be identified beyond family because key features were lacking or the specimens were damaged (missing posterior segments, tentacles, antennae etc.).
Material provisionally referred to Leocrates cf. chinensis (four stations, 987–1402 m), Hesiolyra sp. (one specimen, 996 m), Nereimyra sp. (one specimen, 1256 m), and Parahesione sp. MoV6858 (three stations, 203–236 m) was recorded from the GAB (
Records
11 specimens. Suppl. material
Family Lacydoniidae Bergström, 1914
A. Murray
Lacydoniidae are an uncommon, but widespread group of polychaetes that have affinities with phyllodocids (
Lacydonia cf. laureci
Diagnosis
Specimen incomplete, ~ 2 mm wide excluding chaetae, 3 mm long for head plus 12 anterior segments. Body dorsoventrally flattened. Prostomium approximately as wide as long, somewhat indented anteriorly, with conspicuous lateral lobes present on posterior margin of prostomium, giving the appearance of a much wider than long prostomium. Eyes absent, median antenna missing. Pair of short digitiform to filiform lateral antennae located in slight incisions mid-prostomium; pair of similar-sized/shaped palps arising ventral to prostomial anterior margin. Faded pale brown pigment present on prostomium, dorsally and ventrally, and dorsally as transverse bands on tentacular segment and some other segments, and as spots on dorsal cirri and parapodia. Tentacular segment short, achaetous, with pair of ventrolateral cirri. Chaetigers 1–3 uniramous, with compound spinigerous chaetae, subsequent parapodia biramous, rami elongate and widely separated, with elongate supracicular lobes. Notochaetae simple capillary chaetae, finely spinulose distally; neurochaetae compound spinigers with heterogomph shaft-heads and long, finely spinulose blades. Dorsal cirri short, thick, digitiform, glandular, inserted basally on first three chaetigers, thereafter medially to distally on notopodia. Ventral cirri of similar size and shape, inserted distally on neuropodia. Posterior segments, pygidium and pygidial cirri, all missing and therefore unknown.
Remarks
Lacydonia laureci Laubier, 1975 is the only currently described species that possesses conspicuous lateral lobes on the posterior margin of the prostomium. This specimen bears some similarity to L. laureci, because of these lobes, as well as the absence of eyes, but there are a few differences also apparent:
Records
1 specimen (incomplete). Suppl. material
Family Lumbrineridae Schmarda, 1861
P. Borisova, N. Budaeva, D. Ramos
Lumbrineridae is a family of jaw-bearing annelids from the large monophyletic group Eunicida. Lumbrinerids have a simple external morphology, with uniform elongated body, simple uniramous parapodia, and conical prostomium lacking distinct appendages or eyes. No appendages are present on the peristomium consisting of two rings, and only few species have branchiae associated with parapodia. In contrast, the diversity of jaw morphology is remarkable, and morphology of maxillary plates is used as diagnostic characters at genus and species levels. The family comprises 19 genera and ~ 300 species and has world-wide distribution (
Cenogenus sp. nov.
Diagnosis
Body width: 1–2 mm. Prostomium conical, elongated, shorter than peristomium. Nuchal antenna not observed. All parapodia well developed, first four pairs of parapodia smaller than remaining parapodia. Parapodia with inconspicuous prechaetal and postchaetal lobes equal in size. Anterior parapodia with simple digitate elongate (length to width ratio near 2:3) branchia attached dorsally and posteriorly to parapodial lobes, in middle parapodia branchiae decrease in length becoming more rounded.
Anterior parapodia with long fragile limbate chaetae only, middle parapodia with limbate chaetae and simple multidentate hooded hooks with six to seven small teeth and long blade. All chaetae dark, black or dark brown in colour, becoming translucent near tip. Acicula dark, two in median parapodia.
Maxillary apparatus dark, stout, with four pairs of maxillae. Maxillary carriers shorter than MI. MI forceps-like with attachment lamellae, without connecting plates. MII as long as MI, with two large teeth. MIII unidentate, completely pigmented, dark in colour. MIV large, unidentate round-square plates, completely pigmented.
Remarks
The specimen
Records
4 specimens. Suppl. material
Lumbrineridae A Cenogenus sp. nov. (
Eranno sp. nov.
Diagnosis
Prostomium conical, as long as wide, shorter than peristomium. Nuchal antenna absent. All parapodia well developed, first four to five pairs of parapodia smaller than remaining parapodia. Prechaetal lobes inconspicuous in all parapodia, postchaetal lobes auricular in anterior parapodia (1–40), becoming small and rounded posteriorly, always longer than prechaetal lobes.
Anterior parapodia with limbate chaetae and limbate simple hooded hooks. Clear simple multidentate hooded hooks present after chaetiger 25, with seven to eight teeth and long blades, all of similar size. In median chaetigers (27–40) part of limbate chaetae exceedingly longer than in the remaining chaetigers and ~ twice as long as hooded hooks.
Maxillary apparatus dark, with five pairs of maxillae, elongated. Maxillary carriers shorter than MI. MI forceps-like with attachment lamellae, with narrow connecting plates. MII shorter than MI, with five teeth. MIII unidentate, completely pigmented, dark in colour. MIV unidentate, large, completely pigmented. MV reduced to attachment lamella, partly fused with MIV.
Remarks
Limbate simple hooded hooks are not typical for Eranno and similar to those reported for Abyssoninoe, however, maxillary apparatus is of typical Eranno shape with narrow connecting plates and MII significantly shorter than MI. There is not enough information to consider this a new genus, but we suggest it is a new species.
Records
1 specimen. Suppl. material
Lumbrineris
Diagnosis
Bluntly conical prostomium without appendages. Two peristomial rings of similar sizes. Parapodia uniramous, neuropodia bearing yellow acicula, limbate chaetae compound (until chaetiger 13) and simple (chaetiger 14 onwards) hooded hooks with up to nine teeth. Maxillary apparatus: MII quadridentate, almost as long as MI; MIII and MIV unidentate. Colour in ethanol pale yellow.
Records
1 specimen. op. 4 (
Lumbrineridae gen. spp.
Remarks
Brenke sledge samples were identified to family level.
Records
5 specimens: Suppl. material
Family Maldanidae Malmgren, 1867
J.A. Kongsrud
The family Maldanidae, commonly known as bamboo worms, are infaunal burrowers inhabiting tubes made of sediments consolidated by mucus. The family comprises ~ 240 valid species in 38 genera and five subfamilies (
Boguea sp. nov.
Diagnosis
Complete specimens with ~ 30 chaetigers, < 20 mm long and 0.2 mm wide. Head rounded without a cephalic plate. Cephalic keel well developed. Nuchal slits curved, parallel on each side of the cephalic keel. Neuropodia with avicular uncini present from chaetiger 5. Avicular uncini in single row in chaetiger 5–8, in double rows from chaetiger 9 and onwards. Pygidium simple, without papillae. Anus terminal. Tube cylindrical, straight, thick and solid, consisting of a thin inner organic layer incrusted with a thick layer of densely packed mud. Ventral glandular pads on anterior part of chaetigers 4–6 with reddish-brown pigmentation.
Remarks
At present, only two species of Boguea have been described, B. enigmatica Hartman, 1945 from North Carolina, USA and B. panwaensis Meyer & Westheide, 1997 from Phuket, Thailand. This is the first record of the genus in the deep sea.
Records
10 specimens. Suppl. material
Chirimia sp. nov.
Diagnosis
Largest specimen available, anterior fragment with 14 chaetigers, 43 mm long and 3 mm wide. Head with cephalic plate bordered with well-developed rim. Cephalic rim divided in lateral and posterior lobes by deep lateral incisions; lateral lobes with five elongated, triangular cirri. Posterior lobe with eight triangular cirri. Nuchal slits long, U-shaped. No visible cephalic keel between nuchal slits. Palpode wide, rounded. Chaetiger 1 with distinct collar with deep lateral notches. Neurochaeta as rostrate hooks, starting on chaetiger 2. Posterior part of body and pygidium unknown. Tube unknown. Specimen in alcohol uniformly pale.
Remarks
In general, this species is similar to C. fauchaldi Light, 1991, described from 2070 m depth in the East Pacific, off Panama, but differs in the development of the cephalic rim.
Records
2 specimens. Suppl. material
Lumbriclymene
Diagnosis
Incomplete, anterior fragment with 11 chaetigers, 90 mm long and 1.5 mm wide. Head well defined, rounded, ~ as long as wide, with distinct cephalic keel. Nuchal slits long and curved on each side of the cephalic keel. All chaetigers elongated: chaetiger 1 approximately as long as wide, chaetiger 2 and chaetiger 3 ~ 4 × longer than wide, chaetigers 4–11 ~ 6 × longer than wide. Anterior four chaetigers with a single straight acicular spine per neuropodium. Neuropodia of remaining chaetigers with single row of rostrate hooks. Posterior part of body and pygidium unknown. Tube unknown. Specimen in alcohol uniformly pale.
Records
1 specimen. op. 70 (
Maldane
Diagnosis
Incomplete, anterior fragment with ten chaetigers, 50 mm long and 4 mm wide. Head with oval cephalic plate and a wide, rounded palpode. Cephalic rim divided in lateral and posterior lobes by distinct lateral incisions; posterior rim forming small pocket covering posterior part of the cephalic plate. Cephalic keel prominent; nuchal slits long and parallel, on each side of the cephalic keel. Anterior four chaetigers distinctly biannulate, with parapodia placed on anterior annulus. Epidermal glands distinct in anterior chaetigers. Neurochaetae as rostrate hooks, starting on chaetiger 2.
Records
1 specimen. Suppl. material
Maldanella
Diagnosis
Single complete specimen with 19 chaetigers and three preanal achaetous segments, 80 mm long and 4 mm wide. Additional numerous anterior fragments and a few posterior fragments available. Head with oval cephalic plate. Cephalic rim well developed with minute lateral incisions. Cephalic palpode very small, rounded. Cephalic keel not visible. Nuchal slits straight and parallel, not outward-curved anteriorly, on anterior one third of the cephalic plate. Neurochaetae as rostrate hooks, in single rows from chaetiger two onwards. Notochaetae as simple capillaries in two rows in all chaetigers. Posterior achaetous region with three achaetous segments with rudimentary parapodia, and a well-developed pygidial funnel. Posterior rim of anal funnel rimmed with triangular cirri. Anus on small cone inside anal funnel. Tube greyish, thin and flexible, loosely incrusted by fine sand particles. Specimens in alcohol uniformly pale.
Remarks
Species of Maldanella (including Abyssoclymene as synonym) are common members of abyssal soft bottom fauna (
Records
107 specimens. Suppl. material
Maldanella
Diagnosis
Incomplete, anterior fragment with 14 chaetigers, 65 mm long and 5 mm wide. In general, similar to Maldanella sp.1, but differing in details of the head. The cephalic rim is comparatively low and the cephalic keel is distinct. Two pigmented lines run parallel on each side of the cephalic keel. Nuchal slits straight, parallel on each side of the cephalic keel. Palpode minute. Neurochaetae as rostrate hooks, in single rows starting on chaetiger 2. Notochaetae as simple capillaries in two rows. Tube not known. Colour in alcohol: body uniformly pale, with distinct pigmented parallel lines along cephalic keel.
Records
2 specimens: Suppl. material
Notoproctus oculatus antarcticus
Diagnosis
Complete specimens with 19 chaetigers and two preanal achaetous segments, < 27 mm long and 1 mm wide. Cephalic plate with wide anterior palpode and bordered by a thickened low rim with distinct postero-lateral incisions; nuchal slits strongly curved, located centrally on cephalic plate, transversely oriented. No visible cephalic keel between nuchal organs. Ocelli not observed. Head and anterior four chaetigers biannulate. Anterior four chaetigers with single, straight acicular spine per neuropodium. Neuropodia of remaining chaetigers with single row of rostrate hooks. Notochaetae as simple capillaries. Anal plate slightly oval without distinct rim.
Records
15 specimens. Suppl. material
Notoproctus
Diagnosis
Complete specimens with 19 chaetigers and three achaetous preanal segments, < 29 mm long and 1.5 mm wide. Head rounded, cephalic plate without distinct border. Anterior part of cephalic plate (palpode) distinctly set-off in an angle from the plate. Cephalic keel indistinct. Nuchal slits slightly curved, transversally oriented. Ocelli as numerous small reddish dots in two groups located antero-laterally on palpode. Anterior four chaetigers with single, straight acicular spine per neuropodium. Neuropodia of remaining chaetigers with single row of rostrate hooks. Anal plate more or less circular, slightly pointed dorsally, with thickened rim.
Records
3 specimens. Suppl. material
Notoproctus cf. scutiferus
Diagnosis
Only two posterior fragments available, largest 26 mm long and 0.8 mm wide for 13 chaetigers and two achaetous preanal segments. Anal plate oval, without distinct rim. A characteristic quadrangular pad/ridge present ventrally on preanal chaetigers, slightly overhanging ventral part of anal plate. Tube relatively robust, incrusted with small stones and shell fragments. Brown/black pigmentation on tori.
Remarks
The species is similar to N. scutiferus Wesenberg-Lund, 1948, described from abyssal depths in the NW Atlantic, in the presence of a quadrangular pad/ridge ventrally on preanal achaetigerous segments.
Records
2 specimens. Suppl. material
Notoproctus
Diagnosis
Several anterior and posterior fragments present. Largest anterior fragment with ten chaetigers, 15 mm long and 0.4 mm wide. Cephalic plate more or less circular. Nuchal slits slightly curved, transversely oriented. No cephalic keel visible between nuchal slits. Ocelli absent. Anterior four chaetigers with one or two straight acicular spine(s) per neuropodium. Neuropodia of remaining chaetigers with a single row of rostrate hooks. Two preanal achaetigerous segments. Anal plate more or less circular with distinct lateral incisions. Anal opening dorsally to plate.
Remarks
Presence of an anal plate with distinct lateral incisions, similar to what is seen in species of Maldane, is unique within the genus Notoproctus.
Records
14 specimens, Suppl. material
Maldanidae gen. spp.
Remarks
Brenke sledge samples were identified to family level or material was too damaged to identify further.
Records
19 specimens. Suppl. material
Family Melinnidae Chamberlin, 1919
T. Alvestad, L. M. Gunton
Melinnidae are tubicolous annelids that often have dorsal hooks. Recently the subfamily Melinninae Chamberlin, 1919 within Ampharetidae was raised to the family Melinnidae (
Melinna cf. armandi
Diagnosis
No complete specimens, large, robust worm > 30 mm length, 5 mm width. Abdominal segments very badly preserved and/or missing on all specimens. Body long, widest in postbranchial region. Thorax with 18 chaetigers; neurochaetae as small acicular spines on first four chaetigers and uncini on remaining 14 chaetigers. Prostomium with well-defined anterior and posterior parts, separated by a pair of deep transverse nuchal that almost meeting mid-dorsally. Anterior part distally trilobed. No eyespots. Many smooth buccal tentacles. Chaetiger 1 collar-like, laterally and ventrally encompassing head region; anterior margin not crenulated. Branchiae in two basally fused groups of four. Inner and anterior most branchia of each group only fused at base. Branchiae all long, circular in cross section, tapering evenly to narrow tips. Postbranchial hooks (Fig.
Remarks
Melinna armandi was originally described from west of North Island, New Zealand. Specimens here have 14 lanceolate projections on transverse dorsal membrane whereas M. armandi have eight.
Records
21 specimens. Suppl. material
Melinnopsis chadwicki
Diagnosis
Neurochaetae small acicular spines with lanceolate tips on segments 2–5. Neuropodial uncini from chaetiger 5 (segment VI), present in 12 thoracic uncinigers. Postbranchial dorsal membrane low inconspicuous, located on chaetiger 4. Branchiae emerging together on dorsal branchial ridge at level of segments II and III, arranged in two basally fused groups of four. Uncini of thoracic uncinigers with two teeth in one vertical row over rostral tooth, subrostral process and basal prow.
Remarks
Type locality is eastern Australia at 1006–1257 m. For detailed description see Melinnopsis chadwicki
Records
27 specimens: Suppl. material
Melinnopsis gardelli
Diagnosis
Neurochaetae small acicular spines with lanceolate tips on segment II–V. Neuropodial uncini from chaetiger 5 (segment VI), present in 12 thoracic uncinigers. Postbranchial dorsal membrane low inconspicuous, located on chaetiger 4. Branchiae emerging together on dorsal branchial ridge at level of segments II–III, arranged in two basally fused groups of four. Conspicuous stained band immediately behind dorsal fold ending between chaetigers 9 and 10. Uncini of thoracic uncinigers with three teeth in one vertical row over rostral tooth, subrostral process and basal prow.
Remarks
Type locality is eastern Australia at 2520–2821 m. For detailed description see Melinnopsis gardelli
Records
62 specimens. Suppl. material
Melinnopsis spp. nov.
Diagnosis
Minute acicular chaetae present on segments II–V. One long buccal tentacle present, diagnostic of genus. Four pairs of branchiae. Colour in ethanol pale yellow. Many specimens, but usually in poor shape.
Remarks
At least two species of Melinnopsis are present. Further molecular investigation is required to delineate species. Some specimens are near to Melinnopsis tetradentata (Imajima, 2001) described from 621–622 m depth in Tosa Bay, Japan, but further investigation of type material is required to confirm their identity.
Records
112 specimens. Suppl. material
Melinnidae gen. spp.
Remarks
Specimens were incomplete which does not allow further identification.
Records
2 specimens. Suppl. material
Family Nephtyidae Grube, 1850
A. Murray, D. Ramos
The family Nephtyidae is distinguished by the presence of an interramal branchia attached to the ventral notopodial margin and a single median pygidial cirrus. The family is composed of > 140 species in four genera (
Aglaophamus spp.
Diagnosis
Prostomium rectangular with anteriorly-projecting lateral antennae and ventro-laterally-projecting palps. Nuchal glands present in posterior corners of prostomium. No eyes observed. Body tapering posteriorly. Parapodia of first chaetiger directed anteriorly, neuropodia projecting forward beside the prostomium while the notopodia shorter and without dorsal cirri. Following parapodia distinctly biramous, with dorsal and ventral cirri. Involute interramal cirri present from chaetiger 8 or 12–15, emerging from notopodia only. Preacicular chaetae barred (Fig.
Remarks
Preliminary molecular work using 16S gene on these specimens has separated them into three clades, each clade restricted to either lower bathyal or abyssal depths (
Records
17 specimens. Suppl. material
Micronephthys
Diagnosis
Small bodied specimens, 4–8 mm long for < 30 chaetigers. Branchiae (or interramal cirri) absent. Prostomium with straight to slightly convex anterior margin, subpentagonal to round in shape; short, conical antennae inserted on distal margin slightly medial to anterolateral corners; palps single, short, conical, inserted and directed ventrally. Subdermal eyespots not visible. First chaetiger similar in size to following chaetigers, not reduced. Parapodial acicular lobes conical, aciculae with curved tips. Neuropodial superior lobes absent. Parapodial rami with four types of chaetae: barred chaetae present in preacicular position, spinose chaetae present in postacicular position; capillary chaetae present in neuropodia of chaetiger 1; and some finely spinulose (almost smooth) long capillary-like chaetae in postacicular position of notopodia on following chaetigers. Furcate (lyrate) chaetae absent; thick dentate chaetae absent from chaetiger 1. Some specimens with very long spinulose chaetae in mid and posterior chaetigers, so specimens perhaps in swimming phase. Small papilla-like dorsal cirrus present on inner posteroventral face of all notopodia. Dissected pharynx with pair of conical jaws, 20 bifid terminal papillae (none enlarged more than others), plus 20–22 longitudinal rows of subterminal papillae with more than eight long papillae per row, diminishing in size proximally, single elongate middorsal and midventral papillae absent.
Remarks
These specimens agree with the emended diagnosis for the genus by
Records
71 specimens. Suppl. material
Nephtys cf. paradoxa
Diagnosis
Body stout, wider anteriorly and tapering from middle chaetigers to posterior. Prostomium subrectangular, eyes not visible, pharynx when everted with ten pairs of terminal bifid papillae, and 22 rows of subterminal papillae (rows with 4–6 similar conical papillae) extending only one third of pharyngeal length, median dorsal and ventral papillae not elongated, proximal region smooth. Antennae and palps conical, short, nuchal organs conspicuous, rounded. Parapodia biramous with well-separated rami. Interramal branchiae present, somewhat recurved, from chaetiger 11, becoming foliaceous from ~ chaetiger 16 and appearing membranous from chaetiger 14–16, ciliated and fully developed with membranous expansions (or ‘foliaceous lamellae’) ~ chaetiger 20, becoming small and rudimentary from ~ chaetiger 38 to posterior chaetigers. Acicular lobes obliquely rounded, notopodia with rudimentary preacicular and low postacicular lobes; neuropodia with rudimentary preacicular lobe, postacicular lamella longer than acicular lobe. Chaetae short and ‘spiky’, of three kinds: barred chaetae in preacicular position, spinulose chaetae in postacicular position and capillary chaetae present in neuropodia of chaetiger 1.
Remarks
This species has previously been recorded from off the east Australian coast by
Records
2 specimens Suppl. material
Nephtyidae gen. spp.
Remarks
Brenke sledge samples were identified to family level.
Records
7 specimens. ops. 16, 31, 40, 54 (
Family Nereididae Blainville, 1818
D. Ramos, R. S. Wilson
Nereididae are commonly found in intertidal areas worldwide, as a result the family has been extensively studied and used by physiologists for laboratory experiments and as bait by fishermen. They possess an eversible pharynx with one pair of jaws and often have accessory papillae or denticles in a regular pattern. There are 48 currently accepted genera and ~ 708 extant marine species (
Ceratocephale
Diagnosis
Eyes absent; tentacular cirri all very short, longest extending just beyond peristomium; cirrophore of dorsal cirri not significantly expanded; double ventral cirri from chaetiger 3, posterior one twice length of anterior one. Specimens too small to dissect to observe paragnath/papillae arrangement.
Remarks
Specimens do not fit descriptions of any named species. Members of the genus are typically common in deep-sea samples, however, in this study they were rare.
Records
3 specimens. Suppl. material
Neanthes cf. bassi
Diagnosis
Area I = 0–4; II = 6–27; III = 1–14; IV = 1–18 conical paragnaths + 2–7 smooth bars; V = 0–1; VI = 2–16; VII–VIII = 5–30. Dorsal notopodial ligule similar size to acicular ligule throughout (not markedly reduced or expanded on posterior chaetigers). Prechaetal notopodial lobe absent. Neuropodial postchaetal lobe present on chaetigers 1– ~ 12. Ventral neuropodial ligule on posterior chaetigers reduced, up to half length of acicular neuropodial ligule. Notochaetae homogomph spinigers only (homogomph falcigers absent). Neuropodial dorsal fascicle fused falcigers absent.
Remarks
Prior to the collection of the first abyssal samples in Australian waters, Neanthes bassi was only known from 0–147 m. The most similar species is Neanthes tasmani Bakken, 2002 (known from slightly deeper locations, 75–220 m). However, Neanthes specimens from this study and recent GAB voyages are very close to N. bassi, yet they have been recorded from 200–4800 m. This taxon is referred to here as Neanthes cf. bassi, a hypothesis to be tested when molecular data are available. Two species other than N. tasmani are similar enough to be confused with N. bassi (until the pharynx is dissected), Neanthes kerguelensis (which has fewer maxillary ring paragnaths and lacks oral ring paragnaths or has at most VI = 1 and VII–VIII = 8) and Nicon maculata (which is also described here and lacks paragnaths completely yet is otherwise strikingly similar to N. kerguelensis).
This species was also recorded from eight stations (199–4518 m) in the GAB (
Records
15 specimens. Suppl. material
Neanthes cricognatha
Diagnosis
Prostomium with entire anterior margin. Longest tentacular cirri extend back to chaetiger 4. Maxillary ring of pharynx without papillae. Area I = 9–16; II = 22–45; III = 23–45 paragnaths; IV = 29–54; V, VI, VII–VIII forming a dorsally and ventrally continuous ring.
Notopodial prechaetal lobe present, well developed, so that notopodium made up of two ligules and one lobe similar and triangular. Dorsal cirrus length ~ 1 × ventral notopodial ligule at chaetiger 10–20. Neuropodial postchaetal lobe present, at least on some anterior chaetigers. Ventral neuropodial ligule on posterior chaetigers similar to length of acicular neuropodial ligule. Ventral cirri single.
Notopodial homogomph spinigers present; sesquigomph spinigers absent. Notopodial homogomph falcigers absent. Neuropodial dorsal fascicle fused falcigers absent.
Remarks
At 1194–1257 m, this is the deepest record of this species which is widely distributed in Australia and New Zealand from the intertidal to 253 m, suggesting this may be a species complex.
Records
1 specimen. Suppl. material
Nereididae A Neanthes cricognatha (op. 80) B Neanthes heteroculata, prostomium C Neanthes sp. 1, live specimen D Neanthes sp. 1, prostomium E Neanthes sp. 2, live specimen F Neanthes sp. 2, prostomium G Nereis sp. 1 H Nereis sp. 1, prostomium. Scale bars: 5 mm (A); 1 mm (B, D, F); 10 mm (C, E); 5 mm (G); 0.5 mm (H).
Neanthes heteroculata
Diagnosis
Prostomium slightly wider than long, one anterior pair of very large eyes and one very small pair posteriorly. Jaws with dentate cutting edge, translucent yellow to brown with six teeth. Maxillary ring of pharynx with paragnaths as follows: Area I absent; II absent; III absent; IV 1–3 conical paragnaths; V absent; VI 2 conical paragnaths; VII–VIII 7 conical paragnaths in a ventral band.
Longest tentacular cirri extend back to chaetiger 6. Dorsal notopodial ligule of posterior chaetigers similar to those on anterior chaetigers. Prechaetal notopodial lobe present; smaller than dorsal notopodial ligule on anterior chaetigers, reduced and ultimately absent posteriorly. Acicular process absent. Dorsal cirrus basally attached throughout, ~ 1 × acicular notopodial ligule at chaetiger 10–20. Neuropodial prechaetal and postchaetal lobes absent. Ventral neuropodial ligule of anterior chaetigers present, ~ as long as acicular neuropodial ligule throughout.
Notoaciculae absent from segments 1 and 2. Notopodial homogomph spinigers present. Neurochaetae: dorsal fascicle heterogomph spinigers absent. Neuropodial dorsal fascicle homogomph spinigers present. Neuropodial dorsal fascicle with heterogomph falcigers and homogomph falcigers on anterior chaetigers present. Neurochaetae, ventral fascicle: homogomph spinigers; ventral fascicle heterogomph falcigers.
Remarks
Although previously only known from the type material in the North Atlantic, Bay of Biscay, 4700 m, the present material from 3980–4280 m is indistinguishable on morphological characters. This is the first record from Australia and the first record since the original description.
Records
4 specimens. Suppl. material
Neanthes
Diagnosis
Antennae ~ one quarter length of prostomium. Longest tentacular cirri extending back to chaetiger 5–6. Area I = 13; II ≥ 30; III ≥ 30; IV > 30; V absent; VI = 6; VII–VIII = 51. Prechaetal notopodial lobe present; approximately equal to length of dorsal notopodial ligule at least on anterior chaetigers (thus notopodium of three similar sized ligules/lobes); present throughout all chaetigers. Dorsal cirrus length ~ 1 × acicular notopodial ligule at chaetiger 10–20. Neuropodial prechaetal and postchaetal lobes absent. Neuropodial postchaetal lobe present, at least on some anterior chaetigers; projecting strongly beyond end of acicular ligule throughout all chaetigers. Ventral neuropodial ligule of anterior chaetigers ~ as long as acicular neuropodial ligule. Notopodia larger than neuropodia, dorsal notopodial ligule prominent triangular, largest structure in the parapodia.
Notopodial homogomph spinigers present. Neurochaetae: dorsal fascicle homogomph spinigers only (heterogomph falcigers absent). Neurochaetae, ventral fascicle: heterogomph spinigers absent. Homogomph spinigers present, heterogomph falcigers absent.
Records
1 specimen. Suppl. material
Neanthes
Diagnosis
Eyes absent. Antennae ~ half length of prostomium. Longest tentacular cirri extend back to chaetiger 3 and 4.
Area I = 3; II = 16–17; III = > 30; IV = > 60; V absent; VI = 7–9; VII–VIII = > 120. Dorsal cirrus length ~ 1.5 × acicular notopodial ligule at chaetiger 10–20.
Neuropodial prechaetal lobe absent. Neuropodial postchaetal lobe absent. Ventral neuropodial ligule of anterior chaetigers ~ as long as acicular neuropodial ligule. Notopodia larger than neuropodia, dorsal notopodial ligule triangular, pointed.
Notopodial homogomph spinigers present. Neurochaetae: dorsal fascicle homogomph spinigers and heterogomph falcigers. Neurochaetae, ventral fascicle: heterogomph spinigers and heterogomph falcigers.
Records
1 specimen: Suppl. material
Nereis
Diagnosis
Eyes absent. Paragnath counts: Area I = 2; II = 14–16; III = 0–3 (unclear, possibly damaged during dissection); IV = 9; V absent; VI = 6; VII–VIII = 30–40. Dorsal notopodial ligule markedly broader and elongate on posterior chaetigers. Prechaetal notopodial lobe absent. Dorsal cirrus length ~ twice acicular notopodial ligule at chaetiger 10–20. Dorsal cirrus on posterior chaetigers terminally attached to dorsal notopodial ligule. Neuropodial prechaetal and postchaetal lobes absent. Ventral neuropodial ligule ~ as long as acicular neuropodial ligule. Notopodial homogomph spinigers present. Notopodial homogomph falcigers present. Notopodial homogomph falciger blades very long. Notopodial homogomph falcigers multidentate, with two or more small lateral teeth, first and subsequent lateral teeth much smaller than terminal tooth. Neurochaetae: dorsal fascicle heterogomph spinigers absent. Neuropodial dorsal fascicle homogomph spinigers and heterogomph falcigers. Neurochaetae, ventral fascicle: heterogomph spinigers and heterogomph falcigers.
Records
1 specimen. Suppl. material
Nicon maculata
Diagnosis
Longest tentacular cirri extending back to chaetiger 5–9. Maxillary and oral rings of pharynx entirely bare of papillae and paragnaths. Dorsal notopodial ligule similar size to acicular ligule throughout (not markedly reduced or expanded on posterior chaetigers). Prechaetal notopodial lobe absent. Neuropodial postchaetal lobe present on chaetigers 1– ~ 12. Ventral neuropodial ligule on posterior chaetigers reduced, up to half length of acicular neuropodial ligule. Notochaetae homogomph spinigers only (homogomph falcigers absent). Neuropodial dorsal fascicle fused falcigers absent.
Remarks
Until the pharynx is dissected and found to be bare, this species is strikingly similar to Neanthes bassi and N. kerguelensis (McIntosh, 1885). Although Nicon maculata does have slightly longer tentacular cirri and the postchaetal neuropodial lobe (which is present in all three species) seems slightly longer and appears articulated or constricted in Nicon maculata where it meets the neuropodial lobe (see further comments for N. bassi, above). These taxa are otherwise very similar, and it is difficult to sustain the placement of Nicon maculata in a different genus as no such revision has yet been undertaken.
This record here at 2687–2821 m is the deepest record of this species that we are aware of. The species is also known from a single specimen (1391 m) in the GAB (
Records
1 specimen. Suppl. material
Nereididae gen. spp.
Remarks
Specimens were too small to dissect to observe paragnath arrangement. Brenke sledge samples were identified to family level.
Records
3 specimens. Suppl. material
Family Onuphidae Kinberg, 1865
H. Paxton, N. Budaeva
The family Onuphidae, a member of the jaw-bearing order Eunicida, consists of the subfamilies Onuphinae comprising 18 genera and Hyalinoeciinae Paxton, 1986 comprising five genera. Most onuphids are tubicolous; while the Onuphinae are sediment dwellers, well represented in intertidal to shelf depths, the Hyalinoeciinae are often found as epibenthic crawlers in deeper environments, making the onuphids the fourth most diverse polychaete family in the deep sea (
Anchinothria cf. pycnobranchiata
Diagnosis
Peristomial cirri present. Parapodia 1 enlarged; parapodia 1–3 with bi- to trilobed prechaetal lobes; subulate ventral cirri on chaetiger 1 and 2. Bidentate simple to pseudocompound hooks on first three parapodia; pectinate chaetae scoop-shaped. Simple branchiae from chaetiger 16–19. Round tubes of inner parchment-like lining and outer muddy layer with embedded foreign objects such as spines or spicules.
Remarks
The species is widely distributed in great depths of southern oceans, perhaps a species complex. It is new to Australian waters, also collected from six stations at the GAB (990–1790 m depth) reported as Anchinothria sp. 1 (
Records
6 specimens. Suppl. material
Onuphidae A Anchinothria cf. pycnobranchiata (op. 56) B Nothria cf. paxtonae (op. 56) C Nothria sp. nov. 1 (
Hyalinoecia abranchiata
Diagnosis
Frontal lips fused; palps subulate, with brown median patch. Eyes absent; peristomial cirri absent. Parapodium 1 enlarged; chaetigers 1 and 2 with bidentate simple hooks; limbate and scoop-shaped pectinate chaetae from chaetiger 2. Branchiae absent. Clear, quill-like tubes.
Remarks
The species was originally described from abyssal zones off New Caledonia. It is new to Australian waters.
Records
7 specimens. Suppl. material
Hyalinoecia
Diagnosis
Frontal lips fused; palps subulate, with brown median patch. Eyes absent; peristomial cirri absent. Parapodium 1 enlarged. Only chaetiger 1 with bidentate simple hooks; limbate and scoop-shaped pectinate chaetae from chaetiger 2. Branchiae absent. Clear, quill-like tubes.
Records
43 specimens. Suppl. material
Hyalinoecia
Diagnosis
Frontal lips subulate; palps cirriform. Eyes absent; peristomial cirri absent. Parapodium 1 enlarged, with weakly bidentate simple hooks. Scoop-shaped pectinate chaetae and limbate chaetae from chaetiger 2. Single branchial filament from chaetiger 26. Clear, quill-like tubes.
Remarks
Specimen is perhaps a juvenile Hyalinoecia longibranchiata (McIntosh, 1885).
Records
1 specimen. Suppl. material
Leptoecia ultraabyssalis
Diagnosis
Frontal lips, peristomial cirri and branchiae absent. Chaetiger 1 enlarged. Parapodia 1 and 2 with bidentate simple and pseudocompound hooks. Limbate and pectinate chaetae from chaetiger 2. Subacicular hooks from chaetiger 13. Tube transparent, very delicate, quill-like.
Remarks
The species was originally described from Philippine Trench in 6290–6330 m. It is new to Australian waters.
Records
5 specimens. Suppl. material
Nothria cf. paxtonae
Diagnosis
No eyes visible; peristomial cirri present. Chaetiger 1 enlarged; with auricular prechaetal lobes. Branchiae absent. Bidentate pseudocompound hooks on chaetigers 1 and 2. Chaetiger 3 with limbate chaetae only. Flat pectinate chaetae present from chaetiger 8–9. Subacicular hooks from chaetiger 8–9. Flattened tube, thin lining, covered with foraminifera.
Remarks
This species was originally described from Japan, in 150 m depth. Japanese species is with eight papillae surrounding the anus, which cannot be confirmed for Australian specimens as all are incomplete. This species was also collected from the GAB reported as Nothria sp. (
Records
2 specimens. Suppl. material
Nothria
Diagnosis
Eyes absent; peristomial cirri present. Chaetiger 1 greatly enlarged; with auricular prechaetal lobes. Branchiae absent. Dorsal cirri absent from ~ chaetiger 30. Bidentate simple and pseudocompound hooks on chaetiger 1; bidentate compound hooks on chaetiger 2 and 3. ‘Scoop-shaped’ pectinate and limbate chaetae from chaetiger 2. Subacicular hooks from chaetiger 11–13. Flattened tube, clear inner layer, covered with pieces of shells and pebbles, elongate fragments placed transversely.
Remarks
This species was also collected from the GAB reported as Nothria sp. (
Records
3 specimens. Suppl. material
Nothria
Diagnosis
Eyes absent; peristomial cirri present. Chaetiger 1 greatly enlarged; with auricular prechaetal lobes. Simple, short branchiae from chaetiger 10–12. Uni- to bidentate simple and pseudocompound hooks on chaetiger 1 and 2. Bidentate compound hooks on chaetiger 3. ‘Scoop-shaped’ pectinate and limbate chaetae from chaetiger 2. Subacicular hooks from chaetiger 11–14. Flattened tube, transparent lining, covered with pieces of shells and foraminifera.
Remarks
This species was also collected from the GAB reported as Nothria sp. (
Records
2 specimens. Suppl. material
Nothria
Diagnosis
Eyes present; peristomial cirri present. Chaetiger 1 greatly enlarged; with auricular prechaetal lobes. Simple, short branchiae from chaetiger 11–14. Bidentate simple and pseudocompound hooks on chaetiger 1 and 2. Bidentate compound hooks on chaetiger 3. ‘Scooped-shaped’ pectinate and limbate chaetae from chaetiger 3. Subacicular hooks from chaetiger 9–12. Flattened tube with transparent inner layer, covered on outside with shell pieces, some larger than diameter of tube, spaces filled in with small particles.
Records
40 specimens. Suppl. material
Nothria
Diagnosis
Eyes absent; peristomial cirri present. Chaetiger 1 enlarged; with auricular prechaetal lobes. Simple branchiae from chaetiger 10. Bidentate simple and pseudocompound hooks on chaetiger 1 and 2. Chaetiger 3 with limbate chaetae and ‘scoop-shaped’ pectinate chaetae only. Subacicular hooks from chaetiger 14–16. Flattened tube with transparent inner layer, covered on outside with foraminifera and small shell pieces.
Records
3 specimens. Suppl. material
Paradiopatra ehlersi
Diagnosis
Ceratophores without lateral projections. Peristomial cirri present. Almost unidentate and bidentate pseudocompound hooks with long pointed hoods on first three chaetigers. Subacicular hooks from chaetiger 10. Branchiae with single filaments from chaetiger 17–22, becoming pectinate. Tube with tough lining, outside muddy.
Remarks
This species was previously reported NE of Sydney, collected from 4530 m during RV ‘Galathea’ expedition (
Records
3 specimens. Suppl. material
Paradiopatra
Diagnosis
Ceratophores without lateral projections. Peristomial cirri present. Bidentate pseudocompound hooks with long pointed hoods on first three chaetigers. Subacicular hooks from chaetiger 9. Branchiae with single filaments throughout, starting on chaetiger 14–16, filaments becoming very long. Thick mud tube.
Records
11 specimens. Suppl. material
Paradiopatra
Diagnosis
Ceratophores with lateral projections. Almost unidentate pseudocompound hooks with long pointed hoods on first three chaetigers. Subacicular hooks from chaetiger 14. Branchiae absent. Mud tube.
Remarks
This species was also recorded from 3794 m at the GAB as Paradiopatra sp. nov.
Records
29 specimens. Suppl. material
Family Opheliidae Malmgren, 1867
D. Ramos
Opheliids are usually elongate, tapering at both ends, with ventral grooves and reduced parapodial lobes (
Ophelina
Diagnosis
Following synonymy of Ammotrypanella and Ophelina (
Records
15 specimens. Suppl. material
Ophelina cf. cirrosa
Diagnosis
Bluntly conical prostomium with slit-like nuchal organs. Eyes absent. Chaetigers 1–9 compressed, having more abundant chaetae. Midbody chaetigers longer than anterior and posterior chaetigers. Ventral and lateral grooves present along entire body. Fan-shaped parapodia. Branchiae from chaetiger 23 to chaetiger 30. Abranchiate chaetigers compressed, with the long chaetae directed dorsally. Anal funnel as long as last six posterior chaetigers, directed dorsally. Colour in ethanol white.
Remarks
Observed specimens resemble Ammotrypanella cirrosa described from the Weddell Sea at 3050 m depth. These also possess an anal funnel with small cirri on the posterior margin but differ in having a large terminal ventral papilla instead of a ventral cirrus in the anal funnel. We followed the synonymy of Ammotrypanella and Ophelina as suggested by
Records
76 specimens. Suppl. material
Opheliidae A Ophelina cf. cirrosa B Ophelina cf. cirrosa, posterior end C Ophelina cf. cirrosa, anterior end D Ophelina cf. helgolandiae E Ophelina cf. helgolandiae, prostomium F Ophelina cf. bowitzi, branchiae with blister-shaped bumps G Ophelina cf. bowitzi, anal funnel. Scale bars: 3 mm (A); 0.5 mm (B, C); 5 mm (D); 0.25 mm (E, F); 1 mm (G).
Ophelina cf. meyerae
Diagnosis
Bluntly conical prostomium with oval palpode. Eyes absent. 30 chaetigers. Midbody chaetigers longer than anterior and posterior chaetigers. Ventral and lateral grooves present along entire body. Wider space between parapodial rami on chaetigers 1–8. Chaetae all capillaries. Branchiae from chaetiger 2 to 29, largest posteriorly and smallest on midbody chaetigers. Anal funnel as long as last two posterior chaetigers, with thickened ventral keel and small terminal cirri. Colour in ethanol white.
Remarks
Current specimens mostly conform to the description of Ophelina meyerae, the species was described from a single specimen from the Clarion-Clipperton Zone, Central Pacific at 4300 m depth. These specimens have their largest branchiae on the posterior end of the body unlike O. meyerae. They were inferred as the sister group to the latter using the 16S marker, with K2P distances of 2.15–2.40% (
Records
138 specimens. Suppl. material
Ophelina cf. helgolandiae
Diagnosis
Prostomium triangular, longer than wide with oval palpode. Four annuli on prostomium revealed using Shirlastain. Eyes absent. 32 chaetigers. Chaetigers 2–7 having more abundant chaetae. Chaetigers 25–32 ventrally located and compressed. Branchiae on chaetigers 2–5, then on chaetigers 25–32. Anal funnel slightly longer than the last two posterior chaetigers, directed dorsally, with thickened ventral keel and small terminal cirri.
Remarks
Observed specimens closely resemble Ophelina helgolandiae Augener, 1912, which is recorded from the Nordic Seas at depths of 600–1300 m (
Records
8 specimens. Suppl. material
Ophelina cf. bowitzi
Diagnosis
Prostomium bluntly conical with distinct nuchal organs and an oval palpode. Eyes absent. Posterior chaetigers compressed. Deep ventral and lateral grooves. Branchiae starting from chaetiger 2. Chaetae all capillaries. Longest branchiae found posteriorly, the largest ones with blister-shaped bumps. Anal funnel bent dorsally, as long as last ten posterior chaetigers, becoming narrower from base to tip, and with short terminal cirri. Three specimens having a more rectangular anal tube with a thickened lip instead of terminal cirri. Colour in ethanol pale yellow.
Remarks
Initial observations of these specimens show that they match the description of Ophelina bowitzi, which has previously been recorded only from the North Atlantic Ocean (
Records
14 specimens. Suppl. material
Ophelina cf. juhazi
Diagnosis
Prostomium conical (sunken in specimen) with teardrop-shaped palpode. Eyes absent. Deep ventral and lateral grooves. Parapodia small lobes with few chaetae. Chaetae all capillaries. Branchiae absent. Anal funnel cylindrical, length of last four chaetigers. Colour in ethanol pale yellow.
Remarks
The specimen is morphologically similar to O. juhaz, but is found as a sister clade to it in initial COI and 16S phylogenies (
Records
1 specimen. Suppl. material
Family Orbiniidae Hartman, 1942
A. Zhadan
Orbiniidae are deposit feeders burrowing in sediments, they range in size from a few millimetres to few centimetres and inhabit all depths from intertidal to abyssal. The body of the larger orbiniids is usually separated into a muscular dorsally flattened thorax and a more cylindrical abdomen; abdominal parapodia are shifted dorsally. Smaller species do not show such a separation of body regions. The parapodia are biramous; many genera bear notopodial and/or neuropodial postchaetal lobes. An autapomorphic character for Orbiniidae is the presence of camerated capillary chaetae with characteristic crenulations (
Berkeleyia
Diagnosis
Posteriorly incomplete fragment ~ 4 mm long, 0.35 mm wide. Prostomium short, conical; one peristomial ring. Postchaetal lobes inconspicuous in anterior segments, becoming elongate digitiform in chaetiger 7. First seven chaetigers with tufts of long thin crenulated capillaries in both rami. Following chaetigers with smaller number of capillaries, neuropodia also bearing long thin slightly curved acicular spines with bidentate tips. Forked chaetae not observed.
Records
1 specimen. Suppl. material
Leitoscoloplos cf. abranchiatus
Diagnosis
All specimens incomplete posteriorly. Longest fragment ~ 15 mm long, 1.3 mm wide, consisting of 41 chaetigerous segments. Thorax slightly flattened, abdomen cylindrical. Prostomium conical; one peristomial ring. Eleven thoracic chaetigers. Abdominal parapodia lateral in anterior abdomen, shifted dorsally on posteriorward segments. Branchiae from chaetiger 24, first short triangle, then becoming longer, strap-like. Thoracic postchaetal lobes conical in both rami; short in anterior thorax, becoming longer in middle and posterior parts; notopodial lobes longer. No subpodial or stomach papillae. Abdominal notopodia digitiform, same length as branchiae or shorter; abdominal neuropodia weakly bilobed, with short round lobes, inner slightly larger. Subpodial flange not developed, no flange papillae. All chaetae crenulated capillaries; forked chaetae not observed, probably broken. Colour in ethanol whitish-brown, with pigment spots on prostomium and in intersegmental furrows, or completely white.
Remarks
Leitoscoloplos abranchiatus was described as entirely lacking branchiae, but all specimens studied were posteriorly incomplete (
Records
4 specimens. Suppl. material
Leitoscoloplos cf. kerguelensis
Diagnosis
Incomplete specimen, 5 mm long, 0.4 mm wide, consisting of 21 chaetigers. Body cylindrical, thoracic segments short, abdominal segments long. Prostomium conical with round tip, one peristomium ring. Nine thoracic chaetigers. Branchiae from chaetiger 17–19, exact position unknown. Thoracic postchaetal lobes short conical in anterior thorax, becoming long and oval in middle and posterior parts; notopodial lobes longer. No subpodial or stomach papillae. Abdominal parapodia small, with short lobes. Notopodia oval, shorter than in thorax; neuropodia weakly bilobed, with short subequal lobes. Chaetae supposedly all crenulated capillaries, but mostly broken; presence of uncini or forked chaetae unknown. Colour in ethanol white.
Remarks
Leitoscoloplos kerguelensis is widespread in Antarctic and subantarctic seas, intertidal to 1400 m; it has 8–10 thoracic chaetigers and branchiae from chaetigers 13–17 (
Records
1 specimen. Suppl. material
Leitoscoloplos cf. simplex
Remarks
Specimens resemble Leitoscoloplos simplex Blake, 2017 from Clarion-Clipperton Fracture Zone.
Records
4 specimens. Suppl. material
Leitoscoloplos
Diagnosis
Incomplete specimen, 17 mm long, 1.9 mm wide. Thorax slightly flattened, abdomen cylindrical. First nine segments short, then becoming longer. Fourteen thoracic chaetigers, last one intermediate. Prostomial sharp conical; one peristomial ring. All thoracic segments and at least first three abdominal segments without branchiae. Thoracic postchaetal lobes short conical in both rami, notopodial lobes slightly longer. No subpodial or stomach papillae. Thoracic noto- and neurochaetae long crenulated capillaries. Abdominal region macerated; chaetae broken. Shape of abdominal parapodia and chaetae unknown. Colour in ethanol brown-yellowish.
Remarks
This specimen is similar to L. abranchiatus but has more thoracic chaetigers and shorter thoracic postchaetal lobes. Two OTUs assigned to Leitoscoloplos (four stations, 486–2224 m) were recorded in the GAB (
Records
1 specimen. Suppl. material
Leitoscoloplos spp.
Remarks
Brenke sledge specimens were incomplete and poorly preserved which does not allow further identification.
Records
3 specimens. Suppl. material
Orbiniella cf. aciculata
Diagnosis
All specimens represented by short anterior fragments 1.2–2.5 mm long, 0.6–1 mm wide. Prostomium rounded, wider than long; two peristomial rings. No branchiae. Parapodial rami conical, prominent without postchaetal lobes. Both rami with thin crenulated capillaries and one or two short thick acicular spines. Colour in ethanol white with brown pigmentation on dorsal side of prostomium and peristomium, and on ventral side of anterior segments.
Records
4 specimens. Suppl. material
Orbiniella sp. nov.
Records
2 specimens. Suppl. material
Protoaricinae gen. spp.
Diagnosis
Body long and thin, < 95 chaetigers, < 15 mm long and 0.5 mm wide; without clear division on thorax and abdomen; parapodia not shifting dorsally in posterior segments. Prostomium short, bluntly conical with round tip; two peristomial rings. Branchiae from chaetiger 15–30 as short lobes, becoming very long and prominent on posterior segments. Parapodia with widely arranged rami, chaetal tufts emerging from low tubercles. Anterior neuropodia without postchaetal lobes, short conical lobes appearing ~ chaetiger 7 or beyond, becoming long posteriorward, disappearing in posterior segments; some specimens without neuropodial lobes. No notopodial postchaetal lobes. Notopodia and neuropodia with crenulated capillaries and long thin acicular spines in all segments; spines absent in juveniles. Pygidium with two lobes. Colour in ethanol white.
Remarks
Genus is uncertain, probably new. Two morphospecies: one with neuropodial postchaetal lobes and another without.
Records
127 specimens. Suppl. material
Orbiniidae
Remarks
Beam trawl specimens were incomplete and poorly preserved which does not allow further identification. Brenke sledge samples were identified to family level.
Records
6 specimens. Suppl. material
Family Oweniidae Rioja, 1917
P. Hutchings
Oweniids are slender, fragile annelids which have a cylindrical body composed of relatively few segments and reduced parapodia. They live inside tightly fitting tubes made of cemented sand grains, shell fragments, or Foraminifera tests. The family is composed of four genera and ~ 60 species (
Myriowenia spp.
Diagnosis
Head with large grooved palps and bilobed prostomium, mouth anteroventral, with ventral pharyngeal organ. First three segments uniramous, with capillary notochaetae. Subsequent segments biramous with capillary notochaetae and neuropodial uncini with teeth arranged in a vertical position.
Remarks
At least two species are present based on tubes, one very substantial tube, other fine, difficult to extract entire animal from tube but all are characterised by a pair of large grooved palps. The genus Myriowenia is represented by four species, one from California, two from the Gulf of Mexico, and an undescribed species from Australia (
Records
14 specimens. Suppl. material
Oweniidae gen. spp.
Remarks
Material is too damaged to be identified further or Brenke sledge material was identified to family level only.
Records
18 specimens. Suppl. material
Family Paraonidae Cerruti, 1909
J. Langeneck, D. Ramos
Paraonids are small, elongate worms. They have a well-defined prostomium on which some genera have one distinct median antenna. The family Paraonidae includes ~ 140 described species (
Aricidea
Diagnosis
Three-lobed prostomium with deep nuchal grooves flanking a cirriform median antenna reaching chaetiger 3. Narrow elongated body. Notopodial postchaetal lobe becoming distinctly cirriform on chaetiger 8. Papilliform neuropodial postchaetal lobe. Cirriform branchiae from chaetigers 4–7. Notopodia with simple capillaries, neuropodia with hooks and capillaries with long arista.
Records
6 specimens. Suppl. material
Paraonidae A Aricidea sp., prostomium with median antennae B same, capillary with arista C Aricidea cf. simplex, dorsal view anterior (
Aricidea cf. simplex
Diagnosis
All specimens consist of anterior fragments; most complete with 56 chaetigers, 12.7 mm total length, 1.45 mm maximum width. Prostomium sub-trapezoidal, with large, conspicuous nuchal organs showing dark brown pigmentation. Antenna very short, clavate, reaching the anterior margin of the first chaetiger (absent, likely broken, in one individual). Three pre-branchial chaetigers, 14–17 pairs of acute, relatively short branchiae; last five or six pairs of branchiae gradually decreasing in size. Notopodial lobes tubercular in the first two chaetigers, spindle-shaped in chaetigers 3–15, gradually thinner and thread-like from chaetiger 16 to the end of the fragment. Neuropodial post-chaetal lobes inconspicuous. Notopodial modified chaetae absent. Neuropodial modified chaetae from chaetiger 32, first one or two, then up to five (possibly more in the posterior part of the body, missing in all examined specimens), strong, slightly reddish hooks with bent tip.
Remarks
The examined specimens are very similar to each other and are clearly similar to ‘Aricidea’ simplex as described by
Records
3 specimens. Suppl. material
Levinsenia uncinata
Diagnosis
Body thread-like, posteriorly incomplete, ~ 12 mm for 35 chaetigers, 0.33 mm maximum width. Prostomium triangular, with apical organ, without eyes, without prostomial antenna, nuchal organs as thin slits on the posterior part of the prostomium. Branchiae absent. Notopodial post-chaetal lobes inconspicuous in the anterior part of the body, posterior part of the body too damaged to determine. Modified neuropodial chaetae after chaetiger 18, first two, then up to four or five, strong, thick and slightly curved hooks with well-developed sub-distal dorsal sheath. Methyl green staining pattern: a ventral median rectangular dot on chaetigers 1–5; ventrally complete bands (dorsally open) from chaetiger 6 to chaetiger 17; nuchal slits pigmented.
Remarks
The examined specimen corresponds well to the original description by
Records
1 specimen. Suppl. material
Paraonella
Diagnosis
Complete specimen with 72 chaetigers, 0.3 mm maximum width, 8 mm total length. Prostomium oval, without apical organ, without eyes, two large nuchal organs, often with rusty pigmentation. Branchiae absent, notopodial post-chaetal lobes finger-like from chaetiger 1 to chaetiger 9, triangular, short afterwards, increasing in length for last 15 chaetigers. Pygidium rounded with three cirri approximately of the same length. Chaetae all capillaries. Methyl green staining: no pattern. The only complete specimen partially in a brittle, mucous tube.
Remarks
The absence of modified chaetae and prostomial antenna allows the assignation of these specimens to the genus Paraonella Strelzov, 1973. Currently the genus includes eight species, three of which are abranchiate, namely Paraonella monilaris (Hartman & Fauchald, 1971), Paraonella myriamae (Laubier & Ramos, 1974) and Paraonella abranchiata Fauchald & Hancock, 1981. Both P. myriamae and P. abranchiata are characterised by triangular prostomium, and can be readily distinguished from Paraonella sp. 1; P. monilaris, instead, has a rounded prostomium and a similar pattern of notopodial lobes, and might be closer to this species, even though the structure of nuchal organs is not clear from the original drawings. However, P. monilaris has moniliform segments, with clear constrictions in between, while Paraonella sp. has less pronounced constrictions and shorter segments. Moreover, although the size of the specimens examined by
Paraonella as currently described is most likely polyphyletic, including species close to Paradoneis Hartman, 1965 and to Paraonis Grube, 1873. The pattern of notopodial lobes observed in this species clearly resembles that occurring in Paradoneis, as in the majority of the known Paraonella species. However, there is the possibility that Paradoneis-like Paraonella also represent separate lineages that independently lost the modified notochaetae.
Records
11 specimens. Suppl. material
Paraonis cf. quadrilobata
Diagnosis
All specimens anterior fragments; most complete specimen with 47 chaetigers (into two pieces), ~ 10 mm length, 1.2 mm maximum width. Prostomium sub-triangular, wider than long, with two large, conspicuous nuchal organs, showing traces of dark pigmentation. Antenna slender, thread-like, reaching chaetiger 3–7 backwards (tip often broken). Three pre-branchial chaetigers, five to 12 pairs of flattened branchiae. Branchial region wider and slightly flattened. Notopodial post-chaetal lobes tubercular in the first three chaetigers, then slender, elongated, with bulbous base in chaetigers 4–15, thread-like from chaetiger 16 to the end of the body. Neuropodial post-chaetal lobes conical, well-developed, in the first 15–17 chaetigers. Notopodial modified chaetae absent. Neuropodial modified chaetae occuring after chaetiger 25 thickened capillaries, with abruptly tapered tips. Remains of thin, dark transverse bars on the dorsal side of the branchial region. The largest specimen showing oocytes (140 × 110 μm) in the coelom of the post-branchial region.
Remarks
These specimens correspond well to material sampled in the sub-arctic Atlantic Ocean (Norway) in regard to size and number of branchiae. However, P. quadrilobata has been reported from all over the world and from different depths, and most likely represents a species complex (unpublished molecular data point at a separation at least between North Atlantic and Mediterranean specimens, the latter described as Aricidea annae Laubier, 1967 which is now Paraonis annae).
Records
8 specimens. Suppl. material
Paraonidae gen. spp.
Remarks
Brenke sledge samples were identified to family level. Seven OTUs not yet confidently assigned to genus (23 stations, 416–2850 m) were recorded in the GAB (
Records
1 specimen. Suppl. material
Family Pectinariidae Quatrefages, 1866
E. K. Kupriyanova, J. Zhang
Pectinariids are easily recognisable by their stout golden paleae, and distinctive tubes made of cemented sand grains that resemble an ice-cream cone. The family Pectinariidae is composed of five genera and 63 accepted species (
Petta investigatoris
Diagnosis
Cephalic veil completely free from operculum, with smooth or bearing several lappets (slightly raised mounds) anterior margin. Operculum semi-circular with smooth dorsal and lateral margins. Ventral margin of operculum with a transverse row of numerous stout notopodial paleae on each side. Two pairs of comb-like branchiae on segments 3 and 4, consisting of large basal hump and series of well separated free lamellae. Pair of dorso-lateral pads on segment 5. Ventro-lateral lobes with continuous row of papillae on segment 3. Notopodia with paleae on segment 1 and with notochaetae on segments 5–21 (17 pairs). Neuropodia present on segments 8–21, > 14 pairs with transverse tori, each with a row of uncini. Scaphe indistinctly separated from posterior segments.
Remarks
Type locality is Jervis Marine Park, eastern Australia, 2650–2636 m.
Petta williamsonae
Diagnosis
Cephalic veil completely free from operculum, with smooth or bearing several lappets (slightly raised mounds) anterior margin. Operculum semi-circular with smooth dorsal and lateral margins. Ventral margin of operculum with a transverse row of numerous stout notopodial paleae on each side. Two pairs of comb-like branchiae on segments 3 and 4, consisting of large basal hump and series of well separated free lamellae. Pair of dorso-lateral pads on segment 5. Ventro-lateral lobes smooth on segment 3. Notopodia with paleae on segment 1 and with notochaetae on segments 5–21 (17 pairs). Neuropodia present on segments 8–21, > 14 pairs with transverse tori, each with a row of uncini. Scaphe distinctly separated from posterior segments.
Remarks
Type locality is Bass Strait, eastern Australia, 2760–2692 m.
Records
2 specimens. Suppl. material
Pectinariidae gen. spp.
Remarks
Material is too damaged, no further identification is possible.
Records
2 specimens. Suppl. material
Family Phyllodocidae Örsted, 1843
D. Ramos, R. S. Wilson
Phyllodocids are commonly known as ‘paddle-worms’ due to their large leaf-like dorsal cirri. There are currently 31 extant genera and 497 accepted species (
Clavadoce
Records
1 specimen. Suppl. material
Eumida
Records
36 specimens. Suppl. material
Eumida cf. angolensis
Diagnosis
Prostomium wider than long, with three antennae and two palps. Antennae and palps digitiform. Eyes absent. Tentacular cirri with broad base tapering to a fine tip, four pairs on anterior three segments (1–2–1 arrangement). First tentacular segment dorsally reduced. Everted proboscis barrel-shaped with round terminal papilla. Parapodia uniramous. Chaetae present from segment 2. Dorsal cirri lanceolate, ventral cirri conical, both approximately as long as the neuropodia. Colour in ethanol pale yellow.
Remarks
This specimen closely resembles Eumida angolensis Böggemann, 2009, described from the Angola Basin at depths of 3950–5443 m.
Records
1 specimen, anterior fragment only. Suppl. material
Eumida cf. longicirrata
Diagnosis
Broadly triangular prostomium (Fig.
Remarks
Current specimens closely resemble the redescription of Eumida longicirrata (
Records
23 specimens. Suppl. material
Pseudomystides
Diagnosis
Prostomium broadly triangular and cleft anteriorly, with three antennae and two palps. Antennae and palps digitiform, ~ as long as prostomium. Short bodies with few segments (11–14). Tentacular cirri with broad base tapering to a fine tip, three pairs on anterior two segments (1–2 arrangement). Dorsal cirri absent on segment 3. Parapodia uniramous. Dorsal cirri lanceolate, slightly shorter than neuropodia. Ventral cirri digitiform. A pair of tear-drop-shaped anal cirri and a small median papilla. Colour in ethanol pale yellow to brown.
Records
13 specimens. Suppl. material
Phyllodocidae gen. spp.
Remarks
Brenke sledge material is not identified past family level. Phyllodoce duplex (5 stations, 410–1836 m) and three specimens belonging to Protomystides and Pseudomystides (3 stations, 995–1154 m) were recorded in the GAB (
Records
20 specimens. Suppl. material
Family Pilargidae Saint-Joseph, 1899
C.J. Glasby
Pilargidae are free-living sediment dwellers with similarities to the nereidiforms (
Ancistrosyllis
Diagnosis
Small specimen, incomplete. Median antenna short, approximately same length as laterals. Dorsal cirri of chaetiger 1 ~ 1 × longer than following. Dorsal hooks starting from chaetiger 6. Ventral cirri starting on chaetiger 3. Verrucae present on body surface, short and sparse. Colour in ethanol white.
Remarks
The genus is only known in Australia from a single named species, A. cf. hartmanae Pettibone, 1966 (
Records
1 specimen. Suppl. material
Sigambra
Diagnosis
Small specimen, complete, 47 chaetigers. Pharynx with eight or nine terminal papillae. Dorsal cirri (except for first) slightly longer than ventral cirri. Dorsal hooks starting from chaetiger 3, extending to within a few segments from pygidium; accompanied by one or two small capillary chaetae. Neurochaetae smooth, broad-bladed capillaries of varying lengths. Chaetiger 2 with ventral cirri. Median antenna much longer than laterals, extending back to chaetiger 6; first dorsal cirri cirriform, several times longer than following ones which are slender, foliose. Colour in ethanol white.
Remarks
The specimen is similar to S. magnuncus Paterson & Glover, 2000. It probably represents a new species.
Records
1 specimen. Suppl. material
Pilargidae gen. spp.
Records
1 specimen. Suppl. material
Family Polynoidae Kinberg, 1856
A. Murray, R. S. Wilson
The Polynoidae is the most species-rich of the seven families of Aphroditiformia, commonly known as scale-worms (
From recent sampling voyages by RV ‘Investigator’ to the GAB in 2013–2017, 16 polynoid taxa were distinguished to species level OTUs (
In this study we report at least 11 genera and 15 species, some of which are likely undescribed.
Admetella cf. longipedata
Diagnosis
Four long-bodied specimens with < ~ 62 segments, at least 24 pairs of elytrophores, all elytrae missing. Pharynx dark purple, with four plate-like falcigerous jaws, and ringed with 21 or 22 pairs of papillae. Prostomiums all badly degraded. Antennal styles all missing, median antenna ceratophore present, inserted in medial notch on prostomium, dorsal to scars of small lateral antennae inserted anteroterminally, antennal scales missing. Cephalic peaks absent. Palps long and robust, with longitudinal rows of very fine papillae. Facial tubercle present, rounded, located dorsal to ridged upper lip, between palp bases. Eyes absent. Low transverse nuchal fold present between first pair of elytrophores. Tentaculophores with chaetae. Parapodia subbiramous, well developed, flattened transversely, elongated (neuropodium 2 × longer than notopodium), with elongated acicular lobes and long cirriform subacicular processes, neuropodia not deeply split dorsally and ventrally, short notopodia arising from anterodorsal faces of parapodia. Neurochaetae flattened iridescent chaetae with pointed bare tips (often split) with rows of fine serrations along one side. Notochaetae all missing or absent.
Remarks
Because of the poor state of prostomiums, it was not possible to determine the presence of antennal scales or sheaths between ceratophores of median and lateral antenna, which A. longipedata possesses. All specimens lacked notochaetae which were assumed to be broken off. In all other features, such as elongate parapodia with long acicular lobes and long cirriform subacicular processes, the position of antennae on prostomium, presence of a nuchal fold, numerous, long, flattened, transparent neurochaetae, > 20 pairs of elytra and > 50 body segments, the specimens resemble Admetella and are closer to A. longipedata than to A. brevis Levenstein, 1978, due to the presence of chaetae on the tentaculophore (see
Records
4 specimens. Suppl. material
Polynoidae A Admetella cf. longipedata anterior end, dorsal view (
Austrolaenilla
Diagnosis
Two complete specimens (
Remarks
The small size of the specimens (30–35 segments, ~ 40+ segments for Austrolaenilla species) indicates that they may be juvenile, but the identification is based on the diagnostic feature for the genus: the presence of neurochaetae much slenderer than notochaetae, and with capillary tips that terminate distally in tufts of fine hairs, which these specimens possess. There are ten currently valid Austrolaenilla species (
Records
2 specimens. Suppl. material
Bathyedithia
Diagnosis
Specimen small-bodied, short, with 23 segments and 10 pairs of elytrophores. All elytra missing. Prostomium bilobed, median and lateral antennae absent, frontal filaments absent, facial tubercle absent. Jaws with lateral serrations, pharynx not everted, thus number of terminal papillae not observed. Palps with small palpophores, palps of similar length to tentacular cirri. Tentaculophores achaetous. Notopodia shorter than neuropodia, with elongate acicular lobes. Notochaetae slenderer than neurochaetae; notochaetae with subdistal rows of fine spines, neurochaetae flattened to concave, serrated along both margins. Ventral cirri from segment 3 inserted medially on neuropodia. Nephridial papillae not observed. Ventral keel absent posteriorly.
Remarks
The specimen is damaged and fragile, and most notochaetae and all elytra are missing. Two genera share the characters which distinguish our specimen (10 pairs of elytra, median and lateral antennae absent): Bathyedithia and Polaruschakov. Both genera also have similar neurochaetae, which are flattened and serrated on both margins. Bathyedithia has seven or nine pairs of terminal papillae on the pharynx compared with Polaruschakov which has 14 pairs (
Records
1 specimen. Suppl. material
Bathyeliasona nigra
Diagnosis
Specimens short-bodied, complete ones with 18 segments, eight pairs of elytra. Body colouration dark purple-black. Bilobed prostomium tapering anteriorly to subulate frontal filaments (lateral antennae absent). Median antenna on long ceratophore inserted mid-dorsally on prostomium, posterior to frontal filaments. Palps long, tapering, smooth. Tentaculophores with chaetae. Facial tubercle absent. Eyes absent. Dorsal tubercles indistinct/absent, dorsal cirri with papillated styles. Nephridial papillae large, wide, on segments 10–12, but small, cylindrical, on segments 5–9 and 13–18. Parapodia with distally elongate pre-acicular neuro- and notopodial lobes, neuropodial supra-acicular process absent. Notochaetae present, as thick as neurochaetae, stout, with numerous transverse spinous rows and blunt bare tips. Neurochaetae wide, flattened, with serrated lateral margins, tips bluntly pointed, unidentate.
Remarks
Of the four species of Bathyeliasona (
Bathyeliasona nigra was also recorded from the GAB surveys 2013/2015, albeit as ‘Lepidonotinae sp. 2’ (
Records
22 specimens. Suppl. material
Bathypolaria magnicirrata
Diagnosis
Specimens with short bodies, 18–19 segments, nine pairs of small, reduced elytrophores (all elytra missing). Everted pharynx light brown, with seven pairs of similar-sized terminal papillae, two pairs of smooth amber-coloured jaws. Prostomium wider than long, bilobed, eyes absent, median antenna present on small cylindrical ceratophore, style long, tapering. Lateral antennae and frontal filaments absent. Long smooth palps present, inserted ventrolaterally. Tentacular segment fused to prostomium, tentaculophores achaetous, styles filiform, long, ventral style longer than dorsal style. Cirrophores on non-elytrigerous segments more prominent than elytrophores, large, cylindrical anteriorly. Parapodia biramous, elongate with notopodia almost as long as neuropodia, aciculae penetrating epidermis. Ventral cirri inserted subdistally from segment 3. Notopodia with long flattened, wide chaetae with fine serrations along one side, tips pointed, unidentate. Neurochaetae slenderer than notochaetae, flattened and with fine serrations along both sides of each chaeta. Posterior ventral keel present.
Remarks
Austropolaria was originally described as a monotypic genus, based on A. magnicirrata described by
Records
8 specimens. Suppl. material
Bruunilla
Diagnosis
Specimens small-bodied with 17 segments. All elytra missing, eight pairs of elytrophores present. Prostomium bilobed with small median antennal ceratophore (style missing), frontal filaments present, lateral antennae, eyes and facial tubercle absent. Palps smooth, short. Pair of large lamellate wing-like structures with blunt tips present ventrally, emergent from the lower lip. Tentaculophores achaetous, tentacular styles long. Notopodia reduced, much shorter than neuropodia, both neuropodia and notopodia with elongate acicular lobes. Notochaetae present, slenderer than neurochaetae, both distally flattened to concave with serrations along both sides. Ventral cirri from segment 3 inserted medially on neuropodia; ventral cirri on segment 2 longer than those on following segments.
Remarks
These specimens possess a pair of large wing-like structures on the ventral surface of the lower lip (Bonifácio and Menot 2018: fig. 11B, G), a character so far unique to the genus Bruunilla, and specimens most resemble Bruunilla nealae Bonifácio & Menot, 2018 because of the blunt tips of these structures. However, because the posterior ends are all somewhat damaged, the presence of cirriform papillae on neuropodia 12–17, a character differentiating this species from B. natalensis Hartman, 1971, could not be confirmed. This former species is only known from a single specimen in the equatorial eastern Pacific Ocean, from 2979 m depth.
Records
4 specimens. Suppl. material
Eunoe cf. abyssorum
Diagnosis
Short-bodied, 35–40 segments, 15 pairs of elytra. Elytra pale, with minute conical microtubercles around edges and on posterior half, margins without papillae or fimbriae. Prostomium (violet-coloured when newly preserved, but fading in ethanol) with small cephalic peaks. Eyes absent from most specimens, a few with small subdermal ones. Palps long, smooth. Short lateral antennae ventrally attached (sensu
Remarks
The type locality for Eunoe abyssorum McIntosh, 1885 is the GAB, from 4750 m depth. The only other records are by
Eunoe includes 46 accepted species, of which at least 8 are known from southern Australia, New Zealand and adjacent regions of the Southern Ocean (
Records
9 specimens. Suppl. material
Eunoe cf. opalina
Diagnosis
Specimens with 38–42 segments. Elytra 15 pairs, far posterior part not covered by elytra, present on 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 29, 32; margins with short fine papillae, also longer ones present internally on posterior section, microtubercles cylindrical with truncated flattened tips and few larger macrotubercles (soft, globular and bell-shaped) with similar truncated tips. Some faint small brown pigment spots on prostomium and dorsum. Prostomium ovate, wider than long. Cephalic peaks present, two pairs of eyes with anterior pair laterally at widest part of prostomium, posterior pair lateral, but closer together than anterior pair. Lateral antennae inserted ventrally, bases separate, antennal styles papillate and longer than prostomial width. Tentaculophores with notochaetae present, styles papillated. Dorsal cirri papillated. Noto- and neuropodia with elongate acicular lobes, supra-acicular digitiform process present on neuropodia. Aciculae penetrating epidermis. Notochaetae all spinous, with many rows of spines right up to the blunt tip, neurochaetae thinner with fine spinous rows, all unidentate with bare falcigerous tips. Notochaetal fascicles held erect dorsally but not orientated over dorsum.
Remarks
These specimens most resemble Eunoe opalina McIntosh, 1885, because of the combination of unidentate neurochaetae, the form of spination of noto- and neurochaetae, presence of papillae on lateral antennae, presence of notochaetae on tentaculophores, and the forms of elytral ornamentation (papillae, small microtubercles and a few larger soft globular macrotubercles). However, because there are some differences to previous descriptions of Eunoe opalina (e.g., presence of papillae on dorsal cirri and presence of chaetae on tentaculophores), the identification remains tentative. Eunoe opalina has previously been recorded from the Southern Ocean at depths of 100–500 m.
Records
2 specimens. Suppl. material
Eunoe
Diagnosis
Specimens with 25–38 segments, 15 pairs of elytra. Some brown pigment present on prostomium and spots on anterior dorsum and ventrum. Elytra with small fine papillae marginally and sub-marginally scattered on surface, conical microtubercles also present, some curved distally, macrotubercles absent. Two pairs of eyes present, sometimes not visible, anterior pair at widest part of prostomium, oriented laterally, posterior pair located more dorsally. Cephalic peaks present. Facial tubercle present. Tentaculophores with several stout curved chaetae. Lateral antennae inserted ventrally, short, approximately half as long as prostomial width, styles papillate, bases almost touching, not fused. Median antenna ceratophore large, style long, papillate. Palps long, at least as long as eight anterior chaetigers, with minute papillae in rows along length. Dorsal cirri long, 1–2 × length of parapodia with chaetae, sparsely papillate. Parapodia long, as long as body width. Notochaetal fascicles held dorsally erect, but not joining mid-dorsally. Neuropodia with preacicular elongate lobe. Notochaetae slightly thicker than neurochaetae, with numerous spinous rows along chaetae, tapering to pointed tip. Neurochaetae of two types: superior ones elongate with numerous rows of small spines alternating along length, tapering to conical (broad) unidentate tips (not hooked); inferior ones shorter, with 6–10 rows short spines starting mid-length, somewhat curved and tapering to fine pointed unidentate tips.
Remarks
These specimens do not exactly agree with any descriptions of the 46 valid species of Eunoe, particularly most of those that have been reported from southern Australian, New Zealand and Antarctic waters, i.e., E. opalina, E. abyssorum, E. leiotentaculata Averincev, 1978, E. papillaris Averincev, 1978, E. ivantsovi Averincev, 1978, E. iphionoides McIntosh, 1885, and E. campbellica Averincev, 1978. There are differences such as long papillate palps, elytral ornamentation, and the two distinctive types of neurochaetae. The most similar species is E. etheridgei Benham, 1915, with which our specimens share features such as type of elytral ornamentation, papillate antennal and dorsal cirri styles, ornamentation of chaetae, and notochaetae thicker than neurochaetae, but which differs from descriptions of E. etheridgei by the presence of two types of neurochaetae, and the presence of small papillae on long palps. Eunoe etheridgei was recorded from Bass Strait at 360 m. Polynoinae sp. 5 from the GAB surveys in 426–1027 m depth may be the same as Eunoe sp. 3 (
Records
31 specimens. Suppl. material
Harmothoe
Diagnosis
Short-bodied, brown pigment on anterior dorsum, < 32 segments, 15 pairs of elytra (elytra to posterior end). Elytra with microtubercles with numerous points like a crown, and large inflated cylindrical to globular macrotubercles with small mounds, present on lateral and posterior sections of elytra, short papillae present on posterior surface and lateral edges. Prostomium with cephalic peaks, brown spots present on posterior prostomium; two pairs of large eyes on prostomium, anterior pair dorsolateral on widest part of prostomium, posterior pair also lateral. Palps with minute papillae in short rows; lateral antennae short and papillate, attached ventrally with bases slightly separate; median antenna with large ceratophore. Frontal ridge of upper lip without papillae; facial tubercle absent; nuchal flap absent. Tentacular segment with notochaetae. Dorsal cirri styles papillate, with filiform tips, not subdistally inflated. Neuropodia with extended prechaetal acicular lobe and small cirriform supra-acicular lobe. Notopodial lobes low, not extended visibly. Notochaetae in spiky fascicles held vertically but not meeting dorsally, long, thicker than but not longer than neurochaetae, with rows of serrations along one side. Neurochaetae slenderer than notochaetae, bipinnate with rows of teeth and with fine bidentate tips. Single terminal pygidial cirrus present.
Remarks
This species is different to all the GAB Harmothoe spp. 1–4, and other Harmothoe species reported from Australian waters.
Records
16 specimens. Suppl. material
Harmothoe
Diagnosis
Single specimen, broken, with 37 segments, missing elytrae. Fifteen pairs of elytrophores on 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 29, 32. Palps smooth. Prostomium with prominent cephalic peaks. Two pairs widely spaced large eyes, anterior ones laterally located, posterior ones also laterally placed. Median antenna with large ceratophore, style papillated and longer than palps, lateral antenna short, inserted ventrally, bases separated. Lateral antennae styles papillated, short, approximately half length of prostomium. Prostomium pigmented (brown). Tentacular segment with notochaetae. Dorsal cirri styles papillate, tapering. Notochaetae slightly thicker than neurochaetae. Notochaetal bundles held erect but not meeting dorsally. Notochaetae with rows of spines to tip. Neurochaetae slenderer, with rows of spines, medially inflated, and fine bidentate bare tips (secondary tooth small). Neuropodial acicular lobe with small distal digitiform lobe. Notopodial lobe with elongate acicular lobe.
Remarks
As elytra are all missing, this specimen could not be identified to species. It does not resemble Harmothoe sp. 5 of this survey or any of the Harmothoe species found in the GAB surveys.
Records
1 specimen. Suppl. material
Lepidasthenia
Diagnosis
Single specimen incomplete with at least 46 segments and 19 pairs of elytra. Elytra thin, colourless, fragile, without macrotubercles, on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 29, 32, 36, 39, 42, 45+. Nuchal flap absent. Facial tubercle present as small round flap ventral to antennae. Lateral antennae and median antenna terminally inserted. Two pairs of eyes present. Notochaetae absent, notopodia reduced to acicular lobe on neuropodia. Neurochaetae slender and of two types: long, thinner ones with spinous rows and fine blunt tips; additional wider, falcigerous ones with spinous rows subdistally on inflated region and larger tooth below distal tooth (tip) which is bifid or bidentate. Neuropodial prechaetal lobe slightly longer than postchaetal lobes. Papillae present on surface of neuropodium ventral to ventral cirrus.
Remarks
There are 42 species of Lepidasthenia worldwide (
Records
1 specimen. Suppl. material
Macellicephala
Diagnosis
Small-bodied, < 10 mm in length, with 18 segments, nine pairs elytrophores, on 2, 4, 5, 7, 9, 11, 13, 15, 17. Elytra all missing. Prostomium bilobed, lateral antennae and frontal filaments absent, median antenna elongate with large ceratophore; palps smooth, long, reaching to at least segment 6. Eyes absent, facial tubercle absent. Tentaculophores achaetous, tentacular styles long, smooth. Parapodia sub-biramous; notopodia reduced with elongate acicular lobe and few blunt-tipped notochaetae with many faint rows of low teeth (many specimens with notochaetae missing); neuropodia with elongated acicular lobe and long flattened neurochaetae with acutely pointed straight tips and serrations along both sides. Notochaetae slightly slenderer than neurochaetae. Dorsal tubercles indistinct. Dorsal cirrophores elongate. Ventral cirri attached mid-parapodium from segment 3. Body smooth, without papillae. Posteriorly, ventral keel absent; anus opens dorsally. Pharynx often dark purple, seen through the body wall anteriorly, with two pairs of smooth jaws and nine pairs of terminal papillae.
Remarks
These specimens most resemble M. laubieri Reyss, 1971, described from the Mediterranean in 2665 m, because of the combination of species characters such as the long length of palps and tentacular cirri, lack of frontal filaments and facial tubercle, inconspicuous dorsal tubercles, the form of the noto- and neurochaetae, and lack of papillae on the body. However, because of the geographical distance of these specimens from the type locality of M. laubieri, we do not assign the name.
Records
14 specimens. Suppl. material
Macellicephalinae
Diagnosis
Small specimens with < 17 segments and eight pairs of large elytrophores, elytra all missing. Palps long, smooth; median antenna present, long, with short ceratophore, inserted posteriorly on prostomium; lateral antennae and frontal filaments absent. Facial tubercle absent, upper lip trilobed. Tentaculophores with strongly projecting acicular lobes, chaetae missing or absent, and long styles. Dorsal tubercles with large branchial-like cirriform processes, dorsal cirri styles elongate, attached subdistally on notopodia. Ventral cirri inserted medially on neuropodia from segment 3. Parapodia biramous, notopodia subequal to neuropodia. Notochaetae stout, curved and serrate on convex side, neurochaetae slenderer, with distal part flattened and serrated along both lateral margins. Posterior end without ventral keel, rounded. Pharynx not everted, not dissected due to fragility of specimens, thus unknown.
Remarks
There are five genera within the Macellicephalinae that possess branchial-like cirriform dorsal tubercles: Bathyfauvelia Pettibone, 1976, Bathycatalina Pettibone, 1976, Bathybahamas Pettibone, 1985, Vampiropolynoe Marcus & Hourdez, 2002, and Yodanoe Bonifácio & Menot, 2018. However, only Bathybahamas and Yodanoe have only eight pairs of elytra. Yodanoe possesses notopodia much shorter than neuropodia, whereas these specimens have noto- and neuropodia subequal in length. Bathybahamas is a monotypic genus, with B. charleneae Pettibone, 1985 described from off the Bahamas at a depth of 2066 m, but it possesses two types of neurochaetae, and 18 body segments, which these specimens do not.
Records
6 specimens. Suppl. material
Macellicephalinae
Diagnosis
Large bodied specimen, complete, 33 mm long, 13 mm wide (including parapodia) for 26 segments. Facial tubercle present. Pharynx not everted, but jaws observed via dissection: two pairs of triangular jaws with four or five teeth per jaw; unknown number of pharyngeal papillae. Prostomium bilobed, lobes rounded anteriorly, and posteriorly, median antenna missing or absent, lateral antennae and frontal filaments absent, eyes absent. Palps smooth, short, with reduced palpophores. Tentacular segment fused to prostomium, with tentaculophores situated lateral to palps, both dorsal and ventral styles similar in length and form to palps, achaetous. Large dorsal papillated swollen structures present on (non-elytrigerous) segments 6, 8, 10, located between dorsum and base of cirrophores, possibly reproductive. Dorsal cirri long, longer than parapodia; ventral cirri on segment 2 larger than following segments, becoming small and filiform from segment 3 to posterior segments, inserted subdistally on neuropodia. Parapodia with notopodia reduced to small elongate acicular lobe on anterior face, with slender notochaetae emerging basally, chaetae with rows of fine spines and filiform tips; neuropodia large with elongate pre-chaetal lobe and rounded postchaetal bract-like lobe, neurochaetae much more stout than notochaetae, golden, lanceolate with rows of spines along two sides. Only six pairs of elytrophores distinct, on segments 2, 4, 5, 7, 9, and 11; thereafter difficult to discern due to swollen dorsal bases of parapodia. Pair of thick elytra present on first chaetiger (segment 2), elongate-reniform, covering dorsum, with some dark pigment spots and small marginal papillae; a single large elytra present on segment 11, thin, translucent, round to oval, without pigment or marginal papillae, not covering dorsum. Dorsal tubercles large, and from segment 12, basal swellings present dorsally on every parapodium, with two pairs of ridges running anteriorly and posteriorly along parapodium from dorsum towards dorsal cirrus; these swollen bases also papillated from segment 21. Ventral keel absent. Pygidium rounded, anus dorsal.
Remarks
Due to the lack of lateral antennae, this specimen is assigned to Macellicephalinae, but does not appear to bear resemblance to any of the 37 currently valid Macellicephalinae genera (
Records
1 specimen. Suppl. material
Macellicephalinae
Diagnosis
Small specimens from 3–8 mm in length, with 12–23 segments, and 6–11 pairs of elytrophores. Most somewhat damaged, thus difficult to identify. Possibly seven morphologically different species. Median antenna present or absent, lateral antennae absent, frontal filaments present or absent, facial tubercle absent or present, palps long or short (one specimen with thick, leaf-shaped palps), tentaculophores achaetous. Parapodia with subequal noto- and neuropodial lobes, or notopodial lobes reduced; notochaetae missing, present or absent, neurochaetae either similar thickness to, more robust than, or thinner than, notochaetae; most neurochaetae distally flattened, concave with rows of serrations along both sides. Dorsal tubercles large, or indistinct. Jaws present; pharynx with terminal papillae. Branchial and reproductive structures absent. Ventral keel absent.
Remarks
Numerous specimens were from Brenke sledge samples, most damaged. Approximately seven species are present.
Records
30 specimens. Suppl. material
Polaruschakov
Diagnosis
Several small specimens, ~ 5 mm long, 1.5 mm wide, some damaged posteriorly, some with palps and many neuropodia missing, with < 21 segments. All elytra missing, nine pairs of small elytrophores present on segments 2, 4, 5, 7, 9, 11, 13, 15, 17. Pharynx with two pairs of smooth jaws (denticles absent), seven pairs of distal papillae present, none larger than others. Prostomium bilobed, median antenna, lateral antennae and frontal filaments all absent, eyes absent (or unpigmented), tentacular segment with long tentacular cirri, achaetous. Palps short, smooth, reaching only to chaetiger 3 or chaetiger 4. Segment 2 with long ventral (buccal) cirrus similar in length to tentacular cirri. Segment 3 with long dorsal cirrus, subsequent ones (mostly) missing. Ventral cirri inserted medially on neuropodia, shorter than neuroacicular lobes. Parapodia sub-biramous, with elongate preacicular neuropodial lobe, notopodia inserted on anterodorsal face of neuropodia, conical and much shorter than neuropodia, aciculae penetrating epidermis. Notochaetae long, slender with transverse rows of fine spines along shaft and with blunt tips. Neurochaetae all flattened, coarsely serrated along both margins, tips pointed. Last three or four posteriormost chaetigers reduced. Swollen dorsal structures may be present on some specimens.
Remarks
These specimens agree with the emended genus diagnosis by Bonifácio and Menot (2018) for Polaruschakov Pettibone, 1976, because of the absence of all antennae combined with smooth jaws (or with a single small secondary marginal tooth) and the absence of flattened scale-like structures on segment 6, but, because of their small size, damaged bodies and posterior ends and missing elytra, they could not be identified to species. There are five species in this genus which has only been reported from deep Arctic waters, off the Mediterranean (in
Records
58 specimens. Suppl. material
Polynoidae
Remarks
Specimens were identified to family level only, and some others were unidentifiable due to damage, from Brenke sledge samples.
Records
12 specimens. Suppl. material
Family Protodrilidae Hatschek, 1888
D. Ramos
Protodrilids are interstitial annelids that possess two anterior palps, but lack parapodia, chaetae and other appendages. The family is composed of 38 species in six genera (
Protodrilus cf. puniceus
Diagnosis
Round prostomium with two terminal palps. No eyes. Thick bands of cilia around mouth, continuing ventrally along length of body. Slender filiform body, less than 5 mm long when preserved. Pygidium with two lateral lobes and a median cluster of cilia. Colour in ethanol white.
Remarks
Protodrilus puniceus is the only species of Protodrilus reported from whale fall communities, all other species are distributed in sandy, intertidal areas (
Family Sabellariidae Johnston, 1865
E. K. Kupriyanova, J. Zhang
Sabellariids are filter feeding annelids, which inhabit tubes made of sand and shell fragments cemented together. The family Sabellariidae is composed of 12 genera and 132 species (
Gesaia csiro
Diagnosis
Operculum completely divided into two elongate free lobes. Twenty-two pairs of outer paleae, their blades with frayed thecae and rolled inward tips. Two pairs of inner opercular paleae on dorsal margin of opercular lobes, with straight cylindrical blades with smooth margins. Six pairs of long conical papillae spirally arranged around opercular lobes. One pair of nuchal hooks without limbation. Three pairs of tentacular filaments along margins of buccal cavity. Buccal flaps absent. One pair of long palps extending beyond operculum. Thoracic segment 1 with neuropodial cirri. Thoracic segment 2 with one pair of triangular lateral lobe. Eleven pairs of dorsal branchiae on chaetigers 2–12. Four parathoracic chaetigers (3–6) bearing notopodia with robust lanceolate chaetae interspersed with fine capillaries and neuropodia bearing thin lanceolate chaetae interspersed with fine capillaries. Cauda long and smooth with three pairs of anal appendages.
Remarks
Type locality is Central Eastern MP, eastern Australia, 4414–4436 m. The genus Gesaia is recorded from eastern Australian waters for the first time. Seven specimens of Gesaia sp. 1 were recorded from three stations in the GAB (932–1836 m) (
Records
208 specimens. Suppl. material
Sabellariidae A Gesaia csiro holotype (
Gesaia
Remarks
The specimen is identified to genus, further investigation required to determine if it is the same species as Gesaia csiro.
Records
1 specimen. Suppl. material
Phalacrostemma timoharai
Diagnosis
Opercular lobes completely fused to each other. 18–22 pairs of outer paleae, their blades straight with ornamented thecae. Two pairs of inner opercular paleae on dorsal margin of opercular lobes, with straight cylindrical or slightly flattened blades with smooth margins. Eight pairs of robust long conical papillae around operculum. Four pairs of nuchal hooks with limbs on concave margin. Tentacular filaments absent along margins of buccal cavity. Pair of buccal flaps present. One pair of palps similar in length to operculum. Thoracic segment 1 with two (or one) pair of neuropodial cirri. Thoracic segment 2 (chaetiger 2) with one pair of triangular lateral lobes. Nine pairs of dorsal branchiae on chaetigers 2–10. Four parathoracic chaetigers (3–6) bearing notopodia with robust and retractile lanceolate chaetae interspersed with capillaries and neuropodia with fine lanceolate chaetae interspersed with fine capillaries. Cauda smooth, anal appendages absent.
Remarks
Type locality is Coral Sea MP, eastern Australia, 1013–1093 m. Phalacrostemma timoharai is characterized by having 18–22 pairs of outer paleae, two pairs of neuropodial cirri on thoracic segment 1 and one pair of lateral lobes on thoracic segment 2.
Records
4 specimens. Suppl. material
Phalacrostemma sp. nov.
Diagnosis
Opercular lobes completely fused to each other. 12 pairs of (broken) outer golden paleae with pointed tips and compact thecae with straight margins. One pair of inner paleae, their blades smooth, amber-coloured with tapering tips. Eight pairs of robust and tapering opercular papillae, not extending to tip of outer paleae. Two pairs of flattened nuchal hooks, with poorly developed limbs on concave side. Tentacular filaments absent. Pair of buccal flaps present. One pair of short and robust palps, not extending to operculum. Thoracic segment 1 with one pair of long and tapering neuropodial cirri. Thoracic segment 2 with one pair of broad triangular lateral lobes. Eight pairs of dorsal branchiae on chaetigers 2–9. Four parathoracic chaetigers (3–6) bearing notopodia with robust non-retractile lanceolate chaetae interspersed with fine capillaries and neuropodia with thin lanceolate chaetae interspersed with fine short capillaries. Cauda lost.
Remarks
This single specimen from 1761–1770 m is different from the specimens of P. timoharai as it has only 12 pairs of outer paleae (but many are broken), only one pair of inner paleae, two pairs of nuchal hooks and non-retractile lanceolate notopodial chaetae on parathoracic segments 3–6 (see
Records
1 specimen. Suppl. material
Family Sabellidae Latreille, 1825
A. Murray
Sabellids are sedentary filter-feeding annelids inhabiting tubes composed of pure mucus or agglutinated sand grains. The family Sabellidae is composed of ~ 40 genera, and > 400 species (
Sabellidae
Diagnosis
Small-bodied species. Several very small specimens (1.5 mm long including crown). Eight thoracic and 8–14 abdominal chaetigers, branchial crown with three pairs of radioles. Radioles with flanges, long pinnules and at least one pair of long ventral radiolar appendages present, dorsal lips present. Posterior peristomial ring collar present, with entire dorsal margin (no mid-dorsal gap), ventrally slightly higher with short mid-ventral incision. Glandular ridge present on chaetiger 2, inconspicuous. Thoracic notochaetae include superior elongate narrowly-hooded chaetae and an inferior row of bayonet chaetae only (paleate chaetae absent). Thoracic uncini acicular with long curving shaft and slight subdistal swelling, with rows of different-sized teeth above main fang, companion chaetae absent. Abdominal neurochaetae narrowly-hooded; abdominal uncini avicular with rasp-shaped teeth above main fang, short neck, quadrangular breast, handle absent. Pygidium without eyespots due to preservation. Pygidial cirrus absent. Anal flanges and depressions absent.
Remarks
Due to small size of specimens, examination of uncinal teeth was difficult, and a positive identification to genus was not able to be confirmed. Specimens were stained with methyl blue and photographed to show staining pattern. Some characters of the genus Amphicorina could be observed: eight thoracic and at least eight abdominal chaetigers; three pairs of radioles with few pinnules, long ventral pinnular appendages present; glandular girdle on chaetiger 2; thoracic notochaetae either elongate and narrowly-hooded or small thin bayonet chaetae (with paleate chaetae absent entirely), thoracic uncini acicular with rows of teeth over main fang (though presence of one larger proximal tooth was not observable); abdominal neurochaetae narrowly-hooded, uncini rasp-shaped; posterior anal depression absent. The collar features are, however, more consistent with Chone or Jasmineira, though the chaetal features are more consistent with Amphicorina. However, Amphicorina is more typically recorded from shallow and nearshore waters than deep and has not been reported previously from abyssal depths.
Records
6 specimens. Suppl. material
Sabellidae, Syllidae, Terebellidae. Sabellidae A Sabellidae sp. 1 (
Sabellidae
Diagnosis
One small gravid specimen, body 0.8 mm long including crown, damaged by poor preservation (plus one half branchial crown without a body). Eight thoracic chaetigers, and seven abdominal chaetigers, 3 (4?) pairs radioles with long pinnules. Branchial crown membrane present, dorsal lip with radiolar appendage, dorsal pinnular appendages present, ventral pinnular appendages present. Anterior peristomial ring dorsally exposed, posterior ring somewhat developed into a higher ventral lobe, with wide mid-dorsal gap. Glandular ridge present on chaetiger 2. Paleate thoracic chaetae apparently absent in segments where chaetae still intact, narrowly-hooded chaetae present (bayonet chaetae not seen). Thoracic uncini acicular, with long curved handles and rows of teeth (sizes not determinable) above main fang. Abdominal uncini avicular with rows of teeth above main fang, breast square or rectangular, handles absent. Posterior anal depression and flanges all absent.
Remarks
This specimen displays some features of the genus Chone such as the number of radiole pairs, presence of branchial membrane and pinnular appendages, collar lobation, and the form of the uncini. However, the diagnostic paleate thoracic chaetae are apparently lacking, thus, this is a tentative identification, and may be due to the poor condition and size of the specimen. Specimen was stained with methyl blue and photographed to show staining pattern. Further specimens are required for confirmation.
Records
1 specimen. Suppl. material
Jasmineira
Diagnosis
Medium to large body size. Branchial crown with 21 pairs of radioles, lobes involute ventrally. Collar without lateral incisions (not four-lobed), dorsally high with pockets and fused to faecal groove, ventrally high with small midventral incision, lappets small. Dorsal lips with long radiolar appendages, length of ~ 2–3 thoracic segments. Glandular girdle present on chaetiger 2. Thoracic superior notochaetae narrowly-hooded, inferior chaetae paleate with long mucro, bayonet chaetae present. Thoracic uncini long-handled, acicular with sub-distal swelling on shaft, companion chaetae absent. Abdominal neurochaetae elongate, narrowly-hooded; uncini avicular with elongate neck, small breast, short-handled.
Remarks
One large specimen is in three pieces with most radioles split off at abscission zone, but present in sample; another large specimen is too degraded by poor preservation to distinguish anything other than chaetae. These are different to the species found in the 2017 GAB survey (recorded as ‘Jasmineira sp.’;
Records
2 specimens. Suppl. material
Jasmineira
Diagnosis
One of two specimens badly damaged, thus identification difficult, tube stuck to branchial crown and body which broke during examination. Other specimen very small with all radioles broken off at abscission zone, mouth features undistinguishable, collar low dorsally with deep pockets, fused to faecal groove mid-dorsally, laterally entire, ventrally high with short mid-ventral incision (lappets small). Both specimens with Jasmineira features: eight thoracic chaetigers, thoracic uncini acicular and long-handled, companion chaetae absent; thoracic inferior chaetae paleate (though more broadly-hooded type A - but with long mucro of
Remarks
These specimens are different to ‘Jasmineira sp. 2’ from op. 115, which has paleate inferior thoracic chaetae with shorter mucro. They are somewhat similar to specimen (also damaged) found in the 2017 GAB survey, recorded as ‘Jasmineira sp.’ (
Records
2 specimens. Suppl. material
Perkinsiana
Diagnosis
Single small incomplete specimen, only thorax and one abdominal segment remaining. Eight thoracic segments, six pairs of radioles with long pinnules, radiolar eyes absent. Basal flanges absent. Ventral lamellae present and external. First segment enlarged. Ventral shields prominent, in contact with thoracic neuropodial tori. Dorsal lips with short radiolar appendage. Thoracic inferior chaetae two rows of paleate chaetae, bayonet chaetae absent. Thoracic uncini avicular with long necks, medium-length handles and many rows of teeth above main fang. Companion chaetae present, with long, roughly symmetrical hoods. Chaetigers 7–9 with uncini and companion chaetae, spines absent. Abdominal uncini avicular with long necks and short handles, similar to thoracic uncini.
Remarks
Specimen is poorly preserved, the collar region is damaged, and its details are unclear. The specimen conforms most to the genus Perkinsiana because of the types of chaetae and the elongation of the first segment but identification to species level is difficult due to lack of abdomen and poor preservation of collar. It is possibly the same species as the larger of two specimens recorded as ‘Sabellidae sp. 3’ in the 2015 GAB survey (
Records
1 specimen. Suppl. material
Potamethus cf. scotiae
Diagnosis
Eight thoracic and numerous abdominal segments. Branchial crown with 6–9 pairs radioles. Wide ventral ‘flange’ on ventralmost radioles; ventral sacs large, external to crown; ventral shields present on body. Collar with dorsal lamellae and dorsal pockets, prolonged ventrally with large ventral lappets, oblique laterally. Peristomial ring elongate, exposed above collar. Thoracic notochaetae including superior narrowly-hooded chaetae and two inferior rows of paleate chaetae; thoracic uncini avicular with extremely long handles (> 10 × distance of main fang to breast); companion chaetae present, with similarly long handles. Abdominal neurochaetae of two types: short, broadly-hooded with long tips and longer elongate narrowly-hooded chaetae; abdominal uncini avicular with long handles/shafts, but with breast reduced to narrow swelling at curvature. Ventral surface glandular, thoracic shields prominent. Tubes muddy with fine transverse striations.
Remarks
Four large fragmented specimens were removed from tubes. There are currently no Potamethus species reported from Australia, however, there are museum records of Potamethus collected from deep water east of Tasmania in 1986 (Murray, pers. obs.), and more recently from the GAB surveys in 2015 and 2017 (
Records
4 specimens. Suppl. material
Potamethus
Remarks
Identification is to genus only, based on presence of dorsal lamellae joining dorsal collar, and presence of extremely long-handled thoracic uncini and companion chaetae, paleate inferior thoracic chaetae, and broadly-hooded superior thoracic chaetae. Possibly is the same as above specimens of Potamethus cf. scotiae, but specimens were small, incomplete and/or degraded too much from poor preservation whilst in their tubes.
Records
2 specimens. Suppl. material
Family Scalibregmatidae Malmgren, 1867
J.A. Blake
Scalibregmatids, sometimes called maggot worms, are characterized by anteriorly swollen, short bodies. They are active burrowers and subsurface deposit feeders, which never form tubes. The family is composed of 16 genera and ~ 72 species (
Asclerocheilus
Diagnosis
Large specimens, heavy yellow spines in noto- and neuropodia of chaetiger 3.
Records
5 specimens. Suppl. material
Oligobregma
Diagnosis
The most abundant species in the collections. Similar to Oligobregma mucronata Blake, 2015, from upper slope depths, Antarctica.
Records
193 specimens. Suppl. material
Scalibregmatidae and Siboglinidae. Siboglinidae A Frenulate tube (op. 11) B Osedax sp. nov., preserved female specimen inside tube C Osedax sp. nov., detail of palps of preserved female specimen. Scalibregmatidae D Oligobregma sp. nov. 1 (
Oligobregma spp.
Records
3 specimens. Suppl. material
Pseudoscalibregma
Records
5 specimens. Suppl. material
Scalibregmides
Remarks
Both previously known species of the genus Scalibregmides were described from shallow water off South America: Scalibregmides chilensis Hartmann-Schröder, 1965 and Scalibregmides peruanus Blake, 1981. The current specimen represents the third known species from the genus and the first from deep water.
Records
3 specimens. Suppl. material
Scalibregmatidae gen. spp.
Remarks
Brenke sledge samples were identified to family level only.
Records
27 specimens. Suppl. material
Family Serpulidae Rafinesque, 1815
E. K. Kupriyanova
The family Serpulidae (including Spirorbinae) is a group of sedentary annelids inhabiting self-secreted calcareous tubes. The family is composed of ~ 70 genera and > 500 species (
Bathyvermilia challengeri
Diagnosis
Tubes with characteristic sculpture of numerous transverse ridges close to each other.
Remarks
Only empty tubes were collected. The original records of this species came from three RV ‘Challenger’ stations in the North and South Pacific Ocean taken at 4246–5719 m (
Records
2 tubes. Suppl. material
Serpulidae A Bathyvermilia challengeri, tube only (
Bathyvermilia cf. kupriyanovae
Diagnosis
Large white tubes with numerous wide peristomes.
Remarks
Only empty tubes were collected. Bathyvermilia kupriyanovae Bastida-Zavala, 2008 and B. zibrowiusi Kupriyanova, 1993b both have large tubes with wide flaring peristomes. The other characters separating these two species are structures of thoracic membranes and the opercula. The tubes collected from the Australian eastern abyss are tentatively attributed here to B. kupriyanovae as they appear to have wider peristomes than those of B. zibrowiusi.
Records
4 tubes. Suppl. material
Bathyvermilia
Diagnosis
White tubes with shiny smooth surface, surface with slight keel made of small denticles and small peristomes. Six thoracic chaetigerous segments plus simple collar chaetae. Opercular peduncle slightly annulated, constriction separates from operculum conical covered with white calcareous endplate.
Remarks
Tubes were mostly found attached to deep-sea corals. SEM and molecular data are needed to confirm this preliminary identification.
Records
21 specimens. Suppl. material
Bathyvermilia
Diagnosis
White tubes attached to substrate throughout their length, triangular in cross-section with high smooth keels. Five thoracic chaetigerous segments plus simple collar chaetae. Peduncle smooth thin, slightly thickened distally, separated by a distinct constriction from elongated conical operculum, covered with distinct brownish flat chitinous endplate. Apomatus chaetae present. Abdominal chaetae short, with flat triangular denticulate blade. Uncini with pointed anterior fang.
Remarks
This species differs from other species of Bathyvermilia by having only six thoracic chaetigerous segments (which is typical for representatives of the genus Hyalopomatus) and triangular in cross-section tube without peristomes. It was the most abundant serpulid collected during the voyage.
Records
661 specimens. Suppl. material
Bathyvermilia
Diagnosis
Pinkish tubes with rugose surface, with poorly developed transverse ridges. Operculum unknown. Six thoracic chaetigerous segments plus simple collar chaetae. Thoracic membranes ending at chaetiger 3. Apomatus chaetae present. Thoracic uncini saw-shaped with pointed anterior fang.
Remarks
Species is distinct because of its pinkish tube.
Records
6 specimens. Suppl. material
Hyalopomatus dieteri
Diagnosis
Very typical straight thick-walled quadrangular in cross-section tube with rouned edges.
Remarks
Only one empty tube was collected. The species was originally described off New Caledonia, 1820–1980 m.
Records
1 tube. Suppl. material
Hyalopomatus
Diagnosis
White smooth tubes completely attached to substrate, without external sculpture, except for several indistinct transverse ridges. Nearly globular, only slightly elongated semi-transparent, undifferentiated operculum. Peduncle of the same width as radioles, smooth, without distinct constriction. Thoracic membranes with rounded edges ending right after chaetiger 2. Collar trilobed, ventral lobe larger than lateral ones, covering radiolar lobes and half of radioles. Five thoracic chaetigers plus collar chaetae bundle including special fin-and-blade chaetae. Uncini rasp-shaped with very characteristic for the genus anterior peg made of two (thoracic) or three or four (abdominal) rounded lobes with shallow incision(s) in between. Apomatus chaetae absent. Abdominal chaetae unknown.
Records
1 specimen. Suppl. material
Hyalopomatus
Diagnosis
Smooth white tube. Elongated operculum with distinctly differentiated endplate. Peduncle smooth, of same thickness as radioles, annulated distally, separated from operculum by a constriction. Five thoracic chaetigerous segments plus minute simple collar chaetae. Thoracic membranes short, ending at chaetiger 3. Collar high, trilobed, ventral lobe larger than lateral ones. Uncini rasp-shaped with very characteristic for the genus anterior peg made of two or more rounded lobes with shallow incision(s) in between. Apomatus chaetae absent. Abdominal chaetae in posterior chaetigers only, capillary with tip made of two rows of denticles.
Records
3 specimens. Suppl. material
Protis
Diagnosis
Smooth white relatively thick tubes lacking sculpture. Six thoracic chaetigerous segments plus collar chaetae. Operculum, if present, globular transparent on normal pinnulated radioles.
Remarks
SEM and molecular data are needed to confirm this preliminary identification.
Records
48 specimens. Suppl. material
Protis
Diagnosis
Smooth white relatively thin tubes lacking sculpture. Six thoracic chaetigerous segments plus collar chaetae. Operculum, if present, elongated transparent undifferentiated vesicle on normal pinnulated radioles.
Remarks
SEM and molecular data are needed to confirm this preliminary identification.
Records
22 specimens. Suppl. material
Protis
Diagnosis
Rugose greyish, relatively thick-walled tubes. Six thoracic chaetigerous segments plus collar chaetae. Operculum, if present, globular transparent vesicle on normal pinnulated radioles.
Remarks
One specimen is with two elongated egg sacks attached to the abdomen. SEM and molecular data are needed to confirm this preliminary identification.
Records
10 specimens. Suppl. material
Spirodiscus sp. nov.
Diagnosis
Tube less than 1 cm long, very characteristic, thin tusk-shaped and unattached. Tubes fluted with eight ridges (octagonal in cross-section) anteriorly, but tetragonal in cross-section posteriorly. Pinnulated peduncles, thick relative to normal radioles. Opercula cup-shaped with concave chitinous endplates. Five thoracic chaetigerous segments, including simple collar chaetae. Apomatus chaetae absent. Thoracic uncini saw-to-rasp-shaped with wide pegs divided into two lobes, abdominal uncini rasp-shaped. Abdominal chaetae short, with flat triangular denticulate blade.
Remarks
The species is morphologically similar to Spirodiscus groenlandicus (McIntosh, 1877) known from the North Atlantic Ocean and Southern Indian Ocean but differs by the tube morphology. Specimens were reasonable common in samples collected by Brenke sledge.
Records
126 specimens. Suppl. material
Serpulidae
Diagnosis
Tubes with 4–5 keels and typical transverse sculpture making honey-comb appearance.
Remarks
Tube sculpture slightly resemble that of Metavermilia arctica Kupriyanova, 1993c.
Records
3 tubes. Suppl. material
Family Siboglinidae Caullery, 1914
M. Georgieva
The siboglinids are highly modified in comparison to other annelids, as they do not have a mouth, gut, or anus, but instead host symbiotic bacteria within a specialised organ known as the trophosome. All siboglinids are also tube-dwelling as adults, with the robustness of the tube made by each species varying among the family. They comprise 32 genera that form four monophyletic lineages, namely the vestimentiferans (21 currently described species), Sclerolinum (seven species), Osedax (26 species) and the frenulates (143 species) (
Siboglinidae gen. spp.
Remarks
Frenulate tubes, possibly including live-collected worms, were also collected from seven stations (437–4013 m) in the GAB (
Records
11 specimens of frenulate tube pieces. Suppl. material
Osedax sp. nov.
Diagnosis
Siboglinid found colonising fin whale fall. Female living within tube and with ‘root’ structures embedded into bones. Females with crown of four palps fused for much of their length, without obvious pinnules but with distinct blood vessels in live specimens. Trunk short in relation to the length of the palps, ovisac was not observed. Tube: anterior thin, semi-transparent and appearing closed at the tip, posterior tougher and creased. Colour in ethanol pale yellow.
Remarks
Genetic data confirm that these specimens are a new species that falls within the same clade as other nude palp Osedax species (
Records
More than 20 specimens. Suppl. material
Family Sigalionidae Kinberg, 1856
A. Murray
Sigalionids are a family of scale worms with elongate, narrow bodies and usually with a larger number of segments than in the Polynoidae. There are currently considered to be five subfamilies: Sigalioninae, Pelogeniinae, Pholoinae, Pisioninae, and Sthenelanellinae (
Leanira sp. nov.
Diagnosis
Mostly incomplete specimens, at least 28 mm long, 3 mm wide, for 70 segments (or 26 mm long, 4.5 mm wide for 40 segments). Prostomium sometimes with dark pigmentation or a few spots. Elytra smooth on surface, small and round anteriorly, becoming larger and more ovate (kidney-shaped) posteriorly, almost covering dorsum, lacking lateral indentations. Median antenna short, subulate, without auricles, lateral antennae located on inner dorsal side of tentacular segment. Eyes absent. Palps long, palpal sheaths present. Labial lobes present on lateral lips, bulbous. Dorsal cirri absent from segment 3. Neurochaetae all compound spinigers with entire tips, canaliculate. Clavate stylodes present on parapodia. Branchiae starting at ~ chaetiger 30.
Remarks
This species has also been collected from depths of < 920 m along the east Australia coast during cruises by the FRV ‘Kapala’ (1980) and RV ‘Franklin’ (1988) (
Records
17 specimens. Suppl. material
Neoleanira sp. nov.
Diagnosis
Eyes absent. Elytra with smooth margins (except for first elytra bearing row of small papillae on anterior margin), lateral ‘pockets’ present. Lateral antennae long, inner tentacular sheath present next to palpal sheath; long dorsal cirri present on segment 3; small auricles on median antenna ceratophore. Neurochaetae all compound canaliculate spinigers. Stylodes present on noto- and neuropodia.
Remarks
This is the same species that has been previously recorded off the east coast of Australia in depths of 815–1075 m, during cruises by the RV ‘Franklin’ (1988) and RV ‘Tangaroa’ (1982) (
Records
7 specimens. Suppl. material
Pholoe
Diagnosis
Many small specimens ≤ 4 mm in length and 1 mm in width, easily fragmented, < 32 chaetigers. Body compressed dorsolaterally. Dorsal cirri and branchiae absent. Prostomium oval, bilobed anteriorly, eyes absent. Median antenna present on ceratophore in anterior notch, small lateral antennae present between median antenna and tentaculophores, slightly ventral to median antenna ceratophore (often hidden). Palps stout, ventrolateral to tentaculophores, at least 2.5 × longer than tentaculophore cirri. Tentaculophores achaetous, with dorsal and ventral cirri, with styles similar to median antenna, digitiform, tapering. Parapodia biramous, notopodia much shorter than neuropodia. Neuropodia with stylodes. Notochaetae thin, capillary, spinulose, curved. Neurochaetae all falcigers with medium-length finely-spinulose blades and with fine serrations subdistally on shafts. Elytra delicate, without concentric rings, with few simple elongate papillae submarginally and on surface, becoming longer on more posterior elytra, elytra covering dorsum.
Remarks
There are currently five described species of Pholoe lacking eyes. This species resembles P. petersenae Ravara & Cunha, 2016, though that species was described from the NE Atlantic (Gulf of Cadiz) in depths of 1000–2000 m, and also P. courtneyae Blake, 1995, which was described from the Californian continental slope in depths of 900–1880 m; these three species are anoculate, and lateral antennae are present, though small and often not visible dorsally.
Records
312 specimens. Suppl. material
Pholoides
Diagnosis
Specimen small, incomplete, 24 chaetigers and only seven pairs of elytra remaining. Elytra with concentric rings, simple marginal and submarginal papillae, and sand grains attached to surface. Single median antenna attached anteriorly on prostomium. Lateral antennae absent. Tentacular segment with single tentacular cirrus, similar to antenna, chaetae present. Two minute pairs of prostomial eyes, each pair very close, but not overlapping. Notochaetae spinulose capillaries; neurochaetae short-bladed compound falcigers with unidentate tips, most with smooth blades and some with smooth shafts, some in anterior chaetigers with faintly serrated longer blades and subdistally serrated shafts. Ventrum papillate.
Remarks
Pholoides mendeleevi Averincev, 1978 was described from southern Australian waters in depths of < 730 m, and that author’s illustrations bear some resemblance to this specimen. Other Pholoides specimens have also been collected from eastern Australia, mostly in Bass Strait at a depth of 120 m during a cruise by the RV ‘Tangaroa’ in 1981, which may represent a new species (
Records
1 specimen. Suppl. material
Sigalioninae
Diagnosis
Most specimens incomplete and missing elytra. Largest complete specimen 13 mm long, 1 mm wide, for 68 chaetigers. Prostomium with single long median antenna with short ceratophore, auricles absent. Eyes absent. Lateral antennae absent from all specimens (or missing). Palps long, smooth, reaching to at least chaetiger 14, with short inner palpal sheaths. Tentacular segment fused to prostomium, with two pairs of tapering cirri, dorsal cirri as long as median antenna, ventral ones short; tentaculophores with spinulose capillary chaetae. Labial lobes on lateral lips not observed. Parapodia without long bracts or stylodes. Dorsal cirri absent from chaetiger 3. Branchiae not observed. Notochaetae all capillary, smooth or spinulose, very long posteriorly. Neurochaetae mostly all compound spinigers with canaliculate blades, ventralmost chaetae shorter, canaliculate, and with blunt tips. Most elytra missing, remaining ones small, round, thin, translucent, without marginal or other papillae, not overlapping dorsally. Ventral cirri subulate.
Remarks
These specimens appear to lack lateral antennae completely, and have canaliculate spinigerous compound neurochaetae only, though some in the ventralmost position possess blunt tips. There are few genera of Sigalionidae that lack lateral antennae altogether: Mayella Hartmann-Schröder, 1959, known from a single specimen collected intertidally in El Salvador, which
Records
70 specimens. Suppl. material
Sigalionidae gen. spp.
Remarks
Specimens from Brenke sledge were identified to family only, specimen from beam trawl were too damaged to identify (op. 80).
Records
5 specimens. Suppl. material
Family Sphaerodoridae Malmgren, 1867
M. Capa
Sphaerodorids are typically benthic annelids that are characterised by the presence of conspicuous epithelial tubercles arranged in more or less distinct rows (longitudinal and/or transverse) and a thick cuticle without collagen (e.g.,
Clavodorum cf. longipes
Diagnosis
Body short and ovoid (~ 2 mm, 22 chaetigers), dorsum strongly convex. Head with seven appendages; palps and lateral antennae digitiform, ~ 6–7 × as long as wide, with two or three digitiform basal papillae each. Median antenna as long as paired appendages, without basal papillae. Tentacular cirri similar in shape to digitiform head additional papillae. Antenniform papillae absent. Macrotubercles stalked, smooth, without terminal papilla; arranged in more or less clear longitudinal rows, one transverse row per segment, with six macrotubercles each. Additional dorsal papillae absent. Ventrum with 4–6 papillae per segment, arranged in two longitudinal bands near the base of parapodia. Parapodia with conical ventral cirri, not surpassing the tip of acicular lobe; lacking parapodial papillae. All chaetae compound, with blades 5–9 × as long as wide in mid-body segments.
Records
3 specimens. Suppl. material
Geminofilum
Diagnosis
Body short (1.5 mm), sub-cylindrical, strongly converse dorsally (sub-circular in cross section), with a dark purple-brown pigment in preserved specimen, and whitish macrotubercles. Head invaginated, appendices not observed. Macrotubercles sessile, hemispherical, arranged in two transverse rows per segment, with eight and nine tubercles each. Additional dorsal epithelial papillae absent. Scarce ventral papillae in mid-body segments, but not clearly observed. Parapodia with cylindrical ventral cirri, reaching the tip of acicular lobe. One spherical parapodial papillae, at the base of parapodia (?). All chaetae compound (five or six per parapodium), with blades 4–5 × as long as wide in mid-body segments.
Remarks
This is a possible new species, but material is too damaged to confirm.
Records
1 specimen. Suppl. material
Geminofilum
Diagnosis
Body elongated (6 mm, 30 chaetigers), sub-cylindrical, strongly converse dorsally (sub-circular in cross section); lacking any pigmentation pattern (preserved specimen). Head with at least four digitiform appendages, ~ 6 × as long as wide, without spurs or basal papillae; antenniform papillae not distinguished. Small spherical, sessile and smooth tubercles, scattered over body surface (in four irregular transverse rows, and > 40 per segment, transverse rows above parapodia with ~ 14 larger tubercles). Ventrum with ~ four irregular transverse rows of papillae. Parapodia with ventral cirri, surpassing the tip of acicular lobe and ~ eight papillae. Approximately ten compound chaetae per parapodium, with blades 5–6 × as long as wide in mid-body segments.
Records
1 specimen. Suppl. material
Sphaerephesia
Diagnosis
Body ellipsoid (~ 3–5 mm, 15–20 chaetigers), flattened dorsoventrally wider than high; some preserved specimens with yellowish macrotubercles. Head with seven appendages, smooth, lacking basal papillae or spurs; paired appendages ~ 3 × as long as wide, bottle-shaped; median antenna slightly smaller. Antenniform papillae absent. Four longitudinal rows of dorsal macrotubercles, lateral rows closer to each other, one transverse row per segment. Macrotubercles sessile, hemispherical, and with a pointy distal end. Additional dorsal papillae hemispherical, ~ 15 per segment, arranged in four irregular transverse rows. Ventral papillae ~ 20 in mid-segments, arranged in four transverse rows. Parapodia with digitiform ventral cirri, reaching the tip of acicular lobe. Two or three spherical parapodial papillae. All chaetae compound, with blades 4–5 × as long as wide in mid-body segments.
Records
51 specimens. Suppl. material
Sphaerephesia
Diagnosis
Body ellipsoid (~ 1–5 mm, 20–28 chaetigers), flattened dorsoventrally wider than high. Some preserved specimens with small dark pigment spots in dorsal macrotubercles. Head with seven appendages, smooth, lacking basal papillae or spurs; ~ 5 × as long as wide, bottle shaped; median antenna slightly smaller. Antenniform papillae present, shorter and thinner than median antennae. Four longitudinal rows of dorsal macrotubercles, one transverse row per segment. Macrotubercles sessile, pear-shaped. Additional dorsal papillae hemispherical, ~ 40 per segment, arranged in ~ four irregular transverse rows in mid-body segments. Ventral papillae ~ 20–30 in mid-body segments, arranged in more or less clear transverse rows. Parapodia stout, with prominent acicular lobe and bottle-shaped ventral cirri, reaching the tip of acicular lobe. more than ten parapodial papillae, spherical. All chaetae compound (> ten), with blades ~ 7–10 × as long as wide in mid-body segments.
Records
30 specimens. Suppl. material
Sphaerephesia
Diagnosis
Body ellipsoid (3 mm, 23 chaetigers), with convex dorsum. Head with seven appendages, conical, smooth, lacking basal papillae or spurs; ~ 3–4 × as long as wide; median antenna shorter, digitiform. Antenniform papillae present; additional digitiform papillae covering the head. Four longitudinal rows of dorsal macrotubercles, one transverse row per segment; lateral rows closer to each other. Macrotubercles sessile, pear-shaped and with terminal papillae. Additional ellipsoid dorsal papillae, ~ 20 per between dorsal most macrotubercles, arranged in four or five irregular transverse rows. Ventral papillae, ~ 40 in mid-body segments, arranged in four or five irregular transverse rows. Parapodia with conical ventral cirri, not surpassing the tip of acicular lobe; and > 20 spherical parapodial papillae. All chaetae (> ten) compound, with blades > 15 × longer than wide in mid-body segments.
Remarks
Identification as ‘sp. nov.’ is tentative, further analysis is needed to confirm.
Records
2 specimens. Suppl. material
Sphaerodorum
Diagnosis
Body long and slender, subquadrangular in cross section. Head with seven appendages, smooth, lacking basal papillae or spurs; ~ 3 × as long as wide; median antenna and tentacular cirri shorter. Antenniform papillae absent. Two longitudinal rows of dorsal macrotubercles, one pair per segment; sessile, with terminal papillae. Two longitudinal rows of microtubercles, one pair per segment, running parallel between macrotubercles. Additional dorsal papillae faint in studied material. Ventral papillae not observed. Parapodia with less than six spherical papillae. All chaetae semi-compound with blades ~ 5 × as long as wide, in mid-body chaetigers; hooks in first chaetiger not observed.
Records
2 specimens. Suppl. material
Sphaerodoridae gen. spp.
Remarks
Brenke sledge samples were identified to family level.
Records
3 specimens. Suppl. material
Family Spionidae Grube, 1850
K. Meißner
Spionidae are benthic annelids which possess a pair of elongate, prehensile grooved palps extending from the head. Spionidae is a large group of ~ 600 species grouped into 38 genera (
Aurospio cf. dibranchiata
Diagnosis
Prostomium round anteriorly, elongated posteriorly (keel), extending to middle or the end of chaetiger 1, without appendages. Prostomial peaks and eyes absent. Peristomium fused to first chaetiger, with golden pigments dorsally along posterior margin of the prostomium. Dorsal crests and interparapodial pouches absent. Cirriform branchiae on chaetigers 3 and 4, small, often partially hidden by parapodial dorsal lamellae to which they are fused basally. Dorsal lamellae large and foliaceous from chaetigers 2–6, smaller and round thereafter. Particularly long capillaries present in anterior chaetigers. Multidentate long-shafted hooded hooks present in noto- and neuropodia, in neuropodia starting on chaetigers 10, much later in notopodia according to original description. Sabre chaetae from chaetiger 10.
Remarks
Diagnostic characters are not consistently observable in all specimens due to their poor condition (all anterior fragments, longest anterior fragment with 19 chaetigers). Notopodial hooks were not present in examined specimens (all short anterior fragments). In some specimens a few branchiae were still present and the start of sabre chaetae and neuropodial hooks on chaetigers 10 could be observed. We here tentatively identify the examined specimens as Aurospio cf. dibranchiata Maciolek, 1981. The species is known to occur in deep waters of the Atlantic and central Pacific Oceans, but has not been reported yet from near Australia.
Records
8 specimens. Suppl. material
Spionidae A Aurospio cf. dibranchiata: anterior end, dorsal view (
Aurospio
Diagnosis
Prostomium round anteriorly, extending into a short caruncle to the end of chaetiger 1, without appendages. Eyes absent. Peristomium moderately developed and separated from first segment. First chaetiger dorsally with yellowish pigment lateral to the caruncle. Branchiae present on chaetigers (2, potential branchial scars) 3 and 4, club-shaped to cirriform, smaller than notopodial lamellae and not fused to it. Dorsal crests and interparapodial pouches absent. Parapodial lamellae on chaetiger 1 small, tapered in notopodia, rounded in neuropodia; from chaetigers 2–6 notopodial lamellae large, subtriangular and foliaceous, smaller and rounded thereafter; neuropodial lamellae at same chaetigers wide and foliaceous, thereafter low, wider than long, rounded. Long capillaries present in anterior chaetigers; multidentate long-shafted hooded hooks from chaetigers 15 in neuropodia, apical teeth in a row above main fang. Notopodial hooks not present. Sabre chaetae first present together with neuropodial hooks from chaetiger 15.
Remarks
The specimen is an anterior fragment in rather poor condition. It might belong to an undescribed species of Aurospio with neuropodial hooks and sabre chaetae from chaetiger 15.
Records
1 specimen. Suppl. material
Prionospio spp.
Remarks
Specimens are identified as Prionospio based on the shape of the prostomium (anteriorly rounded, posteriorly extending into a short caruncle), presence of branchiae or branchial scars not earlier than chaetiger 2, and the presence of low dorsal crests. Moreover, sabre chaetae and hooded hooks were usually present. However, most specimens are in poor condition and characters essential for their identification to species level are lost, e.g., mostly anterior fragments, only few branchiae preserved. Other characters observed are very variable. Several species are present according to observed characters (see Suppl. material
Records
8 specimens. Suppl. material
Prionospio cf. amarsupiata
Diagnosis
Prostomium longer than wide, inverse bottle-shaped, slightly rounded anteriorly, elongated posteriorly into short caruncle extending to the end of chaetiger 1, prostomium without appendages. Eyes absent. Peristomium moderately developed and separated from first chaetiger; with yellow pigment lateral to the caruncle as semi-circular ridges (possibly position of nuchal organs). Branchiae mostly lost but scars of lost branchiae seem apparent on chaetigers 2–4, potentially also chaetigers 5 with branchial scars; branchiae on chaetigers 3 and 4 cirriform, shorter than notopodial lamellae and not fused to it. Interparapodial pouches not observed. Notopodial lamellae on chaetigers 1–5 lanceolate, small on chaetigers 1, afterwards increasing in size until chaetiger 4, smaller again on chaetigers 5, largest usually on chaetigers 3 and 4. Neuropodial lamellae small and rounded on first chaetiger, from chaetiger 2 semi-circular, in hook-bearing chaetigers reduced in size and not well preserved in examined material. Chaetae of three types: capillaries, hooded hooks, sabre chaetae. Anterior chaetae until chaetiger 18–20 all capillaries with thin sheaths, in notopodia arranged in up to three rows, in two rows in neuropodia. From chaetiger 18–20 stout granulated sabre chaetae in inferior position. From chaetigers 19 or 20 capillaries without sheaths, and neuropodial hooks; hooks long-shafted, hooded, stout, with ~ seven apical teeth above main fang, sometimes appearing acicular with distally bent tip. Notopodial hooks not present in examined material (all anterior fragments of fewer than 30 chaetigers).
Remarks
We refer to our specimens as P. cf. amarsupiata since branchial scars on chaetigers 2–5 seem to be present. Specimens with long branchiae on 2nd and 5th chaetigers, as shown in the original description, could not be found.
Records
4 specimens. Suppl. material
Dipolydora notialis
Diagnosis
Specimens all short anterior fragments, moderately preserved. Prostomium narrow, rounded anteriorly, caruncle not well preserved in present material; occipital tentacle not observed. Chaetiger 1 with capillaries in noto- and neuropodia. Chaetiger 5 moderately modified; modified heavy spines of one type arranged in a curved row, heavy spines with bent tip and crest of bristles on convex side, arranged together with thin companion chaetae; dorsal fascicle of geniculate chaetae and of neuropodial capillaries present. Bidentate hooded hooks with smooth, curved shafts without constriction start in neuropodia of chaetiger 7. Branchiae from chaetiger 7, continuing to the end of fragments. Gizzard-like structure in anterior part of the digestive tract not very distinct.
Remarks
The morphology of specimens examined is generally in good accordance with the original description of Polydora notialis by
Records
7 specimens. Suppl. material
Laonice cf. blakei
Diagnosis
Specimens all anterior fragments, almost all in very poor condition, usually very short, only two specimens with > 20 chaetigers. Prostomium bell-shaped, anteriorly broadly rounded; small cirriform occipital tentacle present at posterior end; eyes absent. Nuchal organ if discernible with yellow pigment, extending to ~ chaetiger 8 in best preserved specimens. Peristomium moderately developed and not fused to prostomium. Branchiae from chaetiger 2, cirriform, separate from dorsal lamellae (mostly lost in present material). Interparapodial pouches present from between chaetigers 3 and 4. Parapodial lamellae broad, particularly foliaceous in notopodia of the anterior mid-body. Capillaries arranged in two or three rows in anterior notopodia, in neuropodia in two rows. Sabre chaetae first present from chaetigers 10–13, appearing first as up to five capillaries in inferiormost position, in hook-bearing chaetigers usually as one or two stout granulated chaetae. Hooded hooks first observed in neuropodia of chaetigers 17–19, numerous (numbering 15), with four apical teeth above main fang; in notopodia hooks absent. Dorsal crests not observed. Pygidium unknown.
Remarks
The morphology of specimens examined is in accordance with diagnostic characters for L. blakei. Important characters are the start of interparapodial pouches between chaetigers 3 and 4, prostomium not fused to the peristomium, the start of sabre chaetae not before chaetigers 10, and of neuropodial multidentate hooded hooks from about chaetigers 20. However, most of the specimens from IN2017_V03 were in very poor condition and not all diagnostic characters could be observed in each specimen. Laonice blakei is known from deep waters of the Atlantic Ocean and Nordic Seas but has not been reported before from Australian waters or the Pacific Ocean in general. Considering this we refer to our specimens as L. cf. blakei.
Records
11 specimens. Suppl. material
Spiophanes anoculata
Diagnosis
Prostomium broad anteriorly, bell-shaped, with short but distinct anterolateral horns, posteriorly short straight extension with papilliform occipital antenna. Eyes absent in material from the present study but four minute, deeply embedded, red eyes sometimes present in material from the east Pacific. Dorsal ciliated organs as continuous ciliated grooves to the end of chaetiger 2, thereafter as segmental dorsal ciliated grooves interrupted by segmental furrows, after chaetiger 18 or later changing again to continuous double lines (missing in IN2017_V03 specimens); ciliated grooves bordered by pigment of dark orange or ochre colour. Chaetal spreader of ‘0+1’ type, present on chaetigers 5–8, opening of glandular organs on chaetigers 9–14 as simple vertical slits. Parapodial lamellae not well preserved in most specimens. Chaetiger 1 bearing stout, crook-like chaeta in neuropodium. Notochaetae mostly simple capillaries and capillaries with narrow sheath arranged in a tuft, neurochaetae capillaries with sheaths arranged in two or three rows, stout capillaries in anterior and middle body region; from chaetigers 15 neuropodia with quadridentate hooded hooks; stout granulated sabre chaetae starting on chaetigers 4, very long in anterior chaetigers. Ventrolateral intersegmental pouches absent.
Remarks
Specimens are in good agreement with former descriptions, with the most conspicuous character being the metameric dorsal ciliated organs. See
Records
5 specimens. Suppl. material
Spiophanes cf. viriosus
Remarks
The specimen is only a middle fragment in poor condition, without prostomium and posterior end. Due to this we abstain from a more detailed description. However, based on pigment observable in parapodia of the middle body region, chaetal spreaders which are not of the ‘0+1’ type but possibly of the ‘2+3 type’ present in chaetigers 5–7, and glandular openings in chaetiger 8 being absent the fragment might be tentatively referred to Spiophanes viriosus Meißner & Hutchings, 2003.
Spiophanes viriosus was originally described from coastal waters in Queensland, Australia.
Records
1 specimen, middle fragment. Suppl. material
Spionidae gen. spp.
Remarks
Specimens from Brenke sledge samples were incomplete and could not be identified.
Records
24 specimens. Suppl. material
Family Sternaspidae Carus, 1863
M. Georgieva
Commonly known as mud owls, Sternaspidae are distinctive round-bodied or peanut-shaped worms are easily recognized by their characteristic and often colourful ventro-caudal shield (
Sternaspis
Diagnosis
Body ~ 5 mm long and < 2 mm wide. Segments between introvert and rest of body highly cinched, with body 0.7 mm wide at narrowest point. Differing colouration between introvert and abdomen apparent. Ventro-caudal shield a bright orange colour, ribbed and concentrically ringed.
Remarks
These specimens may also represent Sternaspis cf. annenkovae, but further investigation is required to confirm this.
Records
3 specimens. Suppl. material
Sternaspis cf. annenkovae
Diagnosis
Body ~ 11.5 mm long and 5 mm wide. Segments between introvert and rest of body appearing cinched. Body covered in fine papillae largest and densest on segments 7 and 8. Ventro-caudal shield ribbed and concentrically ringed.
Remarks
For further details see
Records
2 specimens. Suppl. material
Family Syllidae Grube, 1850
A. Murray
Syllidae is a family of small to medium-sized (2–3 mm to 14 cm) annelids distinguished by the presence of a muscular region of the anterior digestive tract known as the proventricle, which may be seen through the dorsal body wall. Syllids are a diverse and abundant group, with currently, 74 genera and ~ 700 species (San Martin and Aguado 2019), that inhabit most marine environments, but they are more scarce in the deep sea, with ~ 90 species recorded from this environment, though greater numbers are expected to be discovered with the increasing exploration of deep and abyssal depths (
Anguillosyllis
Diagnosis
Specimens small, < 3.5 mm in length, 11 chaetigers, prostomium short, wider than long, with pair of oval pigmented nuchal organs posteriorly, eyes absent. Palps narrow, elongate, longer than prostomial length, fused for almost full length, with tip distally notched. Lateral antennae short, cirriform, wrinkled (not ovate), median antenna missing. Pharyngeal tooth absent, nine or ten terminal papillae around pharynx rim. Proventricle extending through segments 3–4, with an indistinct number of muscle bands (12–15?). Single pair of papilla-like tentacular cirri on peristomium. Dorsal cirri long, filiform, wrinkled, coiling; few remaining, absent or missing from chaetiger 2. Ventral cirri digitiform, short, inserted somewhat distally (more than midway) on parapodia. Parapodia elongate with distally rounded posterior lobes, retractile elongate postchaetal lobes not obvious (all retracted?), but presumably represented by a small dorsal papilla-like protuberance. Parapodial glands not evident. Chaetae all compound, long-bladed spinigerous chaetae and shorter-bladed falcigers with finely spinulose blades and unidentate tips. Emergent aciculae and simple chaetae not observed. Posterior end truncated, damaged on most specimens, with at least one pair of long lateral pygidial cirri present, but ventromedial pygidial cirri missing on all specimens.
Remarks
Recently, a revision of the Anguillosyllis species from deep-water locations was published by
For these Australian specimens, because we cannot determine the relative extent of the posterior parapodial lobes which are retracted completely (presumably, or are absent entirely), and because all dorsal cirri are missing from chaetiger 2 (or may be completely absent), it is not possible to determine whether these specimens are the same as one of the two species (described as one) of
Records
16 specimens. Suppl. material
Exogone cf. heterosetosa
Diagnosis
Specimen incomplete, 3 mm long, 0.25 mm wide for 29 segments. Palps fused for full length, curled ventrally. Two pairs eyes. Three antennae, median antenna longer than combined length of prostomium and palps, lateral antennae shorter than palps. Proventricle through 3–4 chaetigers. Single pair of papillae-like tentacular cirri; dorsal cirri similar to tentacular cirri, slightly longer, absent on chaetiger 2. Parapodia uniramous with compound chaetae and a single dorsal simple chaeta per parapodium from chaetiger 1; compound chaetae mostly short-bladed heterogomph bidentate falcigers with secondary tooth larger than distal one, and short marginal spines, plus a single spiniger-like compound chaeta per parapodium, shafts distally spinose, blades elongate, enlarged basally (triangular) and tapering to fine indistinctly bidentate tips; single aciculum per parapodium, distally rounded.
Remarks
This specimen most resembles E. heterosetosa McIntosh, 1885, according to the original description and the subsequent redescription by San Martin (2005) and comments by
Records
1 specimen. Suppl. material
Syllis
Diagnosis
Palps free to base. Two pairs of eyes, prostomium broad. Pharynx everted, single anterior tooth present, ten soft papillae around rim. Pharynx extending to chaetiger 8, proventriculus extends through another 9–10 segments. Body large anteriorly, tapering posteriorly. Parapodia all short, ventral cirri short, dorsal cirri all articulate, long, thin, some curled, alternating lengths after ~ chaetiger 10, some articles longer in posterior dorsal cirri; dorsal cirri present on chaetiger 2. Chaetae in anterior segments with at least one large aciculum per chaetiger, projecting tip curved in anterior chaetigers, tip straight in mid-body and curved in posterior chaetigers, plus a few other thinner, straight aciculae (tapering tips) in mid- and posterior body chaetigers; chaetae all compound falcigers, at least ten per chaetiger: very short, finely serrated blades with bidentate tips; anteriorly, chaetal blades fine with small subdistal tooth, almost unidentate; posteriorly, teeth almost subequal; some shafts of falcigers with acute asymmetric extension; pseudocompound falcigers absent; simple dorsal chaetae present in posterior chaetigers, slender, slightly curving and distally minutely bifid.
Remarks
Specimen were in sponge collected with echinoderms. It is not S. sclerolaema Ehlers, 1901, which has pseudocompound chaetae.
Records
1 specimen. Suppl. material
Syllis
Diagnosis
Body pigment absent. Palps free to base. Pharyngeal tooth present, ten soft papillae around rim of pharynx; two pairs of eyes. Dorsal cirri alternating long and short with > 20 articles. Parapodia with compound falcigers only, with medium to short serrated bidentate blades; aciculae pointed. Pseudocompound and spiniger-like chaetae absent. Dorsal simple chaetae only present posteriorly, with minute bifid tips.
Remarks
This species is not the same as those collected in the GAB samples e.g., NMV F242523 – RE2017_C01, which has very short dorsal cirri, and NMV F242524 – RE2017_C01, which lacks eyes and has larger dorsal cirri which are straight, not curled.
Records
1 specimen. Suppl. material
Syllinae
Diagnosis
Specimen incomplete, damaged, ~ 10 mm long, 0.8 mm wide for 69 chaetigers, all antennae and chaetal blades missing. Palps free to base, two pairs of eyes, plus a minute pair of anterior eyespots. Pharynx everted with a single dorsal tooth just below papillated rim. A few anterior dorsal cirri remaining, with short thin articles. Parapodia uniramous, with heterogomph compound chaetae, blades unknown.
Remarks
This specimen is too damaged to be identified. It does not appear to be the same as Syllis sp. 2, which has longer dorsal cirri and which was collected at the same operation.
Records
1 specimen. Suppl. material
Family Terebellidae Johnston, 1846 emended Nogueira, Fitzhugh & Hutchings, 2013
P. Hutchings
Terebellids have multiple long grooved palps which extend out from the worm, thus giving them the name spaghetti worms. The body has a distinct thorax and abdomen defined by the distribution of noto- and neuropodia, and usually two or three pairs of branched or tufted branchiae. The family currently contains 73 genera and > 675 species (
Amphitrite
Diagnosis
One pair (?) of poorly branched branchiae and lateral lobes.
Records
5 specimens. Suppl. material
Loimia
Diagnosis
Lateral lobes present on segment 1, and also present on segment 3 and sometimes on segment 4, three pairs of arborescent branchiae on segments 2–17, pairs of notopodia with smooth tipped winged capillaries from segment 4. Neuropodia from segment 5, short handled uncini with high, pectinate crests, partially intercalated to completely separated double rows back to back from segment 11 until termination of notopodia. Abdominal neuropodia with uncini arranged in single rows.
Records
1 damaged specimen. Suppl. material
Pista
Diagnosis
Glandular lobes on segments 2–4 of variable sizes and positions, and segment 1 reduced dorsally with pair of glandular lobes. Branchiae arborescent, pectinate or plumose from segment 2, typically two pairs on segments 2 and 3, rarely a single pair or three pairs. Seventeen pairs of smooth tipped winged notochaetae from segment 4. Neuropodia from segment 5, as long handled avicular uncini at least on anterior segments arranged in single rows, then arranged in double rows until end of thorax, reverting to single rows to pygidium.
Remarks
Specimens of Pista were in poor condition. They probably represent at least two species, but branchiae and lateral lobes, critical characters to distinguish species, are damaged and in many cases incomplete, including posterior thorax. Molecular data may help in distinguishing between species.
Records
23 specimens. Suppl. material
Terebellidae
Diagnosis
Fourteen pairs of notopodia, neuropodia begining before end of notopodia, abranchiate genus.
Remarks
Potentially new genus and species.
Records
1 specimen. Suppl. material
Terebellidae gen. spp.
Remarks
Specimens were too damaged for further identification.
Records
16 specimens Suppl. material
Family Travisiidae Hartmann-Schröder, 1971
L. Avery, R. S. Wilson
The family Travisiidae is characterised by a short, thick, grub-like body tapered at both ends. Travisiidae contains a single genus, Travisia, with three accepted species. Travisia specimens are not usually numerous in benthic samples, but the genus is well represented in abyssal and bathyal environments. Twenty species are recorded from depths of 250 m or greater (
Travisia
Diagnosis
Body of 22–25 chaetigers. Prostomium conical, longer than maximum width. Chaetae present from segment 2, one achaetous posterior segment (smallest specimens with chaetae only visible on anterior segments 2–5). Mouth located between chaetigers 1 and 2. Segment 1 uniannulate; anterior and posterior segments, starting at segment 2 triannulate (no obvious differentiation between anterior and posterior regions). Branchiae present, first on chaetiger 3–6, continue for 8–11 chaetigers. Branchiae much shorter than body diameter. Branchiae absent on specimens less than ~ 9.5 mm long, but present on an increasing number of segments on the largest specimens collected. Epidermal papillae are low and sparse at the anterior margin of each segment, becoming larger towards the posterior margin of each segment. Notopodial and neuropodial lobes commencing on chaetiger 3 (in small specimens either absent or difficult to distinguish from adjacent epidermal papillae). Parapodial lobes continuous with an encircling row of papillae, remaining epidermis of each segment low tessellation. Interramal pores first present chaetiger 1, last on chaetiger 20. Pre-pygidial 8–12 segments forming deep lateral grooves within which parapodia and chaetae located (only on the largest specimens). Pygidial tube with six or seven blunt lobes equal in length to the last two chaetigers. The last six dorsal posterior chaetigers crenulated.
Remarks
Initially the smallest specimens were treated as a distinct OTU (in these the chaetae are sparse, papillae are less distinct and branchiae and parapodial lappets are not observable) but it seems more likely that this represents size-related variation. Other than having branchiae, Travisia sp. 1 is strikingly similar to abranchiate species Travisia glandulosa McIntosh, 1879 (e.g., see
Among species with branchiae, only four other species along with Travisia sp. 1 have branchiae commencing at chaetiger 3 (Travisia carnea Verrill, 1873; Travisia filamentosa León-González, 1998; Travisia hobsonae Santos, 1977 and Travisia profundi Chamberlin, 1919) but none of these have all chaetigers triannulate. T. profundi is similar in having 12 chaetigers with branchiae (Travisia sp. 1 has 8–11 chaetigers with branchiae), but in T. profundi there is a transition to biannulate and uniannulate posterior chaetigers, and ten or 11 anal lobes compared with six or seven in Travisia sp. 1. This species differs from the two Travisia OTUs reported from 141–375 m in the GAB (
Records
6 specimens. Suppl. material
Travisia
Remarks
Material is represented by immature unidentifiable specimens.
Records
1 specimen. Suppl. material
Subclass Echiura Sedgwick, 1898
Order Echiuroidea
Suborder Bonelliida
Family Bonelliidae Lacaze-Duthiers, 1858
P.-W. Hsueh
The family is characterised by the presence of sexual dimorphism which is not seen in all other families of Echiura. The female is small to medium in size with sac-like trunk and with truncate or bifid proboscis. The male is usually small, planarian-like or nematiform, often parasitic in or on the female (
Alomasoma
Diagnosis
Specimen 56 mm in length, body cylindrical, with trace of proboscis, body wall thin (Fig.
Records
1 specimen. Suppl. material
Echiura Alomasoma sp. nov. 1 (
Alomasoma
Diagnosis
Specimen 47 mm in length, body pear-shaped, with trace of proboscis, body wall thin (Fig.
Records
1 specimen. Suppl. material
Maxmuelleria sp. nov.
Diagnosis
Specimens medium in size, ranging from 31 to 40 mm in length. Specimens with either none, one or two ventral chaetae remaining. Present description based on one specimen (
Records
3 specimens. Suppl. material
Bonelliidae gen. spp.
Class Sipuncula
A. Schulze
Formerly considered a distinct phylum, Sipuncula are now regarded as a branch within the annelid radiation (
In contrast to the ‘typical’ annelid body plan, the sipunculan body is unsegmented. It consists of a trunk region and a retractable introvert, generally with a crown of tentacles at its anterior end. Recurved, proteinaceous hooks are often present along the introvert. The anus is usually located dorsally at the anterior end of the trunk. Nephridiopores (usually two) open at a similar level as the anus on the ventral side. Internally, one two four introvert retractor muscles present.
Sipunculans are generally cryptic in their lifestyle, but can reach high densities in some habitats. They range from the intertidal zone to depths of > 7000 m (Saiz Salinas et al. 2018).
Sipuncula , gen. spp.
Remarks
Many specimens not identified beyond phylum level.
Records
101 specimens. Suppl. material
Sipuncula A Sipunculus sp. (
Family Sipunculidae
Relatively large sipunculans (usually > 5 cm), with an introvert shorter than the trunk; no introvert hooks. Tentacles arranged in a circle surrounding the mouth. Body wall with externally visible bands of longitudinal and circular musculature crossing each other, giving the impression of rectangular ‘mini-pillows’. One genus and four species are known from the deep sea (> 2000 m) (Saiz Salinas et al. 2018). In Australian waters 11 species from two genera (Siphonosoma and Sipunculus) have been reported (http://www.ala.org.au). We report at least one species from Sipunculus.
Sipunculus spp.
Diagnosis
Four stem-like tentacles, with the ventral pair smaller than the dorsal pair. Nephridiopores located slightly anterior to the anus.
Records
25 specimens. Suppl. material
Family Golfingiidae Stephen & Edmonds, 1972
Small to large-sized worms (max 200 mm); introvert length similar to trunk length or shorter. Tentacles encircling mouth. Hooks, if present, simple, scattered, not sharply curved and often deciduous. Trunk wall externally smooth or covered with small papillae. The family is well represented in the deep sea (> 2000 m) with 36 species and six genera (Saiz Salinas et al. 2018). In Australian waters six species from two genera (Golfingia and Nephasoma) have been reported (http://www.ala.org.au). We report at least seven species from four genera.
Golfingiidae
Records
1 specimen. Suppl. material
Golfingia
Diagnosis
Small to medium worms (usually < 30 mm). Introvert hooks, if present, small (< 40 μm); nephridial pores anterior to anus. Body wall smooth.
Remarks
Further identification is uncertain.
Records
6 specimens. Suppl. material
Golfingia (Golfingia) muricaudata
Diagnosis
Presence of a distinctive caudal appendage (< ~ 30% of the trunk length); introvert shorter than trunk.
Records
34 specimens. Suppl. material
Phascolion spp.
Diagnosis
Small to medium-sized worms (< ~ 50 mm), commonly inhabiting abandoned gastropod or scaphopod shells, polychaete tubes or foraminiferan tests. Trunk usually with unique holdfast papillae. Single nephridium, usually located posterior to the anus.
Remarks
Specimens are not identified to species.
Records
25 specimens. Suppl. material
Phascolion (Montuga) lutense
Diagnosis
Trunk smooth, except for densely packed papillae at the anterior end. No holdfast papillae. Trunk with characteristic grey anterior ‘cap’. Inhabiting soft clay tubes instead of hard shells.
Remarks
This is the first report of this species from Australian waters.
Records
4 specimens. Suppl. material
Phascolion (Isomya) cf. hedraeum
Diagnosis
Inhabiting sediment tube; round holdfast papillae with hardened borders anteriorly.
Records
1 specimen. Suppl. material
Onchnesoma
Diagnosis
Small worms (< 10 mm). Introvert always longer than trunk. Anus on distal end of introvert. No introvert hooks. Single nephridium.
Remarks
Specimens are not identified to species. This is the first report of this genus from Australian waters.
Records
4 specimens. Suppl. material
Thysanocardia cf. catharinae
Diagnosis
Up to 70 mm trunk length. Complex tentacular arrangement in ‘festoons’ (double rows extending along the distal introvert) and around the nuchal organ. Tentacles arranged in 14–16 festoons, each with < 40 tentacles.
Remarks
This is the first record of this genus from Australian waters.
Records
9 specimens. Suppl. material
Family Phascolosomatidae Stephen & Edmonds, 1972
Small to medium-sized worms. Tentacles surrounding the nuchal organ in a semi-circle. Introvert hooks usually present, recurved and organized in circles, typically with internal structures visible under transmitted light. Four introvert retractor muscles. Two genera and eight species are known from the deep sea (> 2000 m) (Saiz Salinas et al. 2018). In Australian waters, 15 species from two genera (Apionsoma and Phascolosoma) have been reported.
Apionsoma
Diagnosis
Small specimens (usually > 20 mm) with an introvert much longer than trunk. Introvert hooks arranged in rings with accessory basal spinelets. Nephridia bilobed.
Remarks
Identification is uncertain.
Records
15 specimens. Suppl. material
Analysis of annelid biodiversity
We report > 6000 annelid specimens (box core – 57 specimens, Brenke sledge – 2481, beam trawl – 3470) (Suppl. material
Sixteen of the species collected from the eastern abyss were also reported from stations at the GAB. Of these 16, seven (Nereididae: Nicon maculata, Polynoidae: Bathyeliasona nigra, Eunoe abyssorum, Aphroditidae: Aphrodita goolmarris, Laetmonice benthaliana, Laetmonice yarramba, Goniadidae: Bathyglycinde profunda) were named species. The remaining nine undescribed species were confirmed by specialists who had compared both sets of material (restricted to the families Ampharetidae, Aphroditidae, Fabriciidae, Nereididae, Onuphidae, Polynoidae, and Sabellidae).
The most species-rich family was the Polynoidae (> 17 species) followed by Onuphidae (13 species), Serpulidae (12 species), Acrocirridae (10 species) and Maldanidae (10 species). Total species richness of annelids was similar between lower bathyal and abyssal depths (163 and 160 species respectively) and decreased to mid-bathyal depths (70 species). Total species richness was highest at Bass Strait (90 species), Byron Bay (82 species) and Jervis MP (81 species) whilst the Moreton Bay (30 species) and off Fraser Island had the lowest richness (41 species).
Discussion
This is the first comprehensive report of annelids from the eastern Australian lower bathyal and abyssal region. Our results indicate a higher number of families and species (50 and 214 respectively) than was reported from the recent deep-water survey of the Great Australian Bight (GAB) at 200 to 5000 m depth (42 annelid families, 179 species) (
Prior to the 2017 survey, only eight annelid species were described from the eastern continental margin of Australia from below 1000 m and only two of them (Parapolyeunoa flynni and Aglaophamus profundus) from the targeted depths in this study, below 2000 m (Table
While so far only six species have been formally described from this survey material (pectinariids Petta investigatoris and P. williamsonae, sabellariids Gesaia csiro and Phalacrostemma timoharai and melinnids Melinnopsis gardelli and Melinnopsis chadwicki) taxonomic studies are ongoing. The 24 new species currently being described by co-authors of this study include one acoetid of the genus Panthalis (Murray), one glycerid (Böggemann), two lumbrinerids (Borisova and Budaeva), four maldanids of the genera Boguea, Chirimia and Notoproctus (Kongsrud), four onuphids of the genus Nothria (Paxton and Budaeva), four serpulids of the genera Bathyvermilia, Hyalopomatus and Spirodiscus (Kupriyanova), four sphaerodorids of the genera Geminofilum and Sphaerephesia (Capa), and four scalibregmatids of the genera Asclerocheilus, Oligobregma, Pseudoscalibregma and Scalibregmides (Blake). At least one new species of Siboglinidae (genus Osedax) and several species of Dorvilleidae (genus Ophryotrocha) were recovered from a whale carcass collected from op. 100 (Georgieva). It is anticipated that the large international effort in species identification by taxonomic specialists in each annelid group will eventually result in the description of all the new annelids from the expedition. Once this taxonomic work is complete, we can test relevant biogeographical hypotheses, in particular we will be able to address the question of species connectivity along the eastern continental margin across to the deep the GAB.
Sixteen morphospecies reported from this study were also recorded from the GAB in southern Australia (
Existing molecular data on the annelid material collected during ‘Sampling the Abyss’ voyage support annelid species connectivity along the eastern Australian margin from southern Queensland to Tasmania. Analysis on DNA sequence data confirmed that specimens of pectinariid Petta investigatoris were collected < 491 km apart from Bass Strait to Jervis MP, NSW (
From 16 morphospecies reported from the present study and the GAB, the two nominal morphospecies with the widest reported distributions in our data were the aphroditid Laetmonice yarramba Hutchings & McRae, 1993 and the goniadid Bathyglycinde profunda (
Laetmonice yarramba, originally described from 60–102 m off the coast of NSW, was reported across a distance of more than 1800 km from Freycinet MP (op. 4) to off Fraser Island (op. 115) with a total depth range of 3868 m (Suppl. material
Bathyglycinde profunda (Hartman & Fauchald, 1971) was originally described from the equatorial region off northeast South America (4825 m). This taxon in the present study was recorded from Flinders MP to Coral Sea MP (ops. 16 to 134), a distance of 1920 km and depth range 2093–4280 m. The same morphospecies has been also reported from northwest Atlantic Ocean (2862–5023 m) by
Once more data on the extent of annelid species range sizes from this study become available, we will be able to not only address the degree of genetic connectivity between the GAB and eastern Australian margin, but also to examine underlying biogeographical the patterns across annelids. In particular, we will be able to address the question whether the tropical to temperate transition between 30–40°S reported for deep-sea ophiuroids (
Conclusions
We report 214 annelid species from the eastern Australian margin, at least 55 of which are new to science. Prior to 2017, only two annelid species were described from the region below 2000 m, consequently this work vastly increases our knowledge of deep-water annelids and provides critical baseline data on an important group of benthic invertebrates from a virtually unknown region of the world’s ocean. This is important as comprehensive taxonomically-consistent deep-water datasets that cover large areas in Australia are rare (
The strong feature of this study is that specific annelids groups were sent to taxonomic specialists around the world giving us more confidence in the species identifications. Furthermore, because all material from this study is deposited or will soon be in properly curated museum collections (
Acknowledgements
The authors wish to thank the
References
- Aguirrezabalaga F, Ceberio A (2006) Flabelligena gascognensis sp. nov. (Polychaeta: Acrocirridae), a new species from the Capbreton Canyon (Bay of Biscay, NE Atlantic). Scientia Marina 70: 14–147. https://doi.org/10.3989/scimar.2006.70s1141
- Aguirrezabalaga F, Gil J (2009) Paraonidae (Polychaeta) from the Capbreton Canyon (Bay of Biscay, NE Atlantic) with the description of eight new species. Scientia Marina 73: 631–666. https://doi.org/10.3989/scimar.2009.73n4631
- Aguirrezabalaga F, Parapar J (2014) Deep-sea Ampharetidae (Polychaeta) from Capbreton Canyon (north-east Atlantic) with the description of a new species. Journal of the Marine Biological Association of the United Kingdom 94(5): 947–967. https://doi.org/10.1017/S0025315413001422
- Alalykina IL (2018) Composition of deep-sea polychaetes from the SokhoBio expedition with a description of a new species of Labioleanira (Annelida: Sigalionidae) from the Sea of Okhotsk. Deep Sea Research Part II – Topical Studies in Oceanography 154: 140–158. https://doi.org/10.1016/j.dsr2.2018.04.004
- Alalykina IL (2020) Polychaeta: a review on the deep-sea benthic polychaetes along the NW Pacific. In: Saeedi H, Brandt A (Eds) Biogeographic Atlas of the Deep NW Pacific Fauna. Advanced Books. Pensoft Publishers, Sofia, 527 pp. https://doi.org/10.3897/ab.e51315
- Alderslade P, Althaus F, McEnnulty F, Gowlett-Holmes K, Williams A (2014) Australia’s deep-water octocoral fauna: historical account and checklist, distributions and regional affinities of recent collections. Zootaxa 3796(3): 435–452. https://doi.org/10.11646/zootaxa.3796.3.2
- Allen JA, Sanders HL (1996) The zoogeography, diversity and origin of the deep-sea protobranch bivalves of the Atlantic: the epilogue. Progress in Oceanography 38: 95–153. https://doi.org/10.1016/S0079-6611(96)00011-0
- Althaus F, Williams A, Alderslade P, Schlacher TA (2017) Conservation of marine biodiversity on a very large deep continental margin: how representative is a very large offshore reserve network for deep-water octocorals? Diversity and Distributions 23(1): 90–103. https://doi.org/10.1111/ddi.12501
- Alvestad T, Budaeva N (2015) Neosabellides lizae, a new species of Ampharetidae (Annelida) from Lizard Island, Great Barrier Reef, Australia. Zootaxa 4019(1): 61–69. https://doi.org/10.11646/zootaxa.4019.1.6
- Annenkova NP (1934) Paraoniden der Meeren des Fernen Osten der USSR. Doklady Akademii Nauk SSSR 3(8–9): 656–658.
- Arwidsson I (1911) Die Maldaniden. Wissenschaftliche Ergebnisse der Schwedischen Südpolar Expedition 1901–1903 Stockholm Zoologie II, Band 6, Lieferung 6, 44 pp. https://doi.org/10.5962/bhl.title.6756
- Audouin JV, Milne Edwards H (1833) [Part 5.] Classification des Annélides et description de celles qui habitent les côtes de la France. Annales des Sciences Naturelles, Paris. sér. 1, 30: 411–425. https://www.biodiversitylibrary.org/page/6096524
- Augener H (1912) Beitrag zur Kenntnis verschiedener Anneliden und Bemerkungen über die nordischen Nephthys-Arten und deren epitoken Formen. Archiv für Naturgeschichte, Berlin 78A: 162–212. https://www.biodiversitylibrary.org/page/13317436
- Augener H (1913) Polychaeta I. Errantia. 65–304. In: Michaelsen W, Hartmeyer R (Eds) Die Fauna Südwest-Australiens. Ergebnisse der Hamburger Südwest-Australischen Forschungsreise 1905. Gustav Fischer, Jena, 65–304.
- Augener H (1914) Polychaeta II: Sedentaria. In: Michaelsen W, Hartmeyer R (Eds) Die Fauna Südwest-Australiens. Ergebnisse der Hamburger Südwest-australischen Forschungsreise 1905, ed., volume 5. Gustav Fischer, Jena, 72 pp.
- Augener H (1918) Polychaeta. Beitrage zur Kenntnis der Meeresfauna Westafrikas 2(2): 67–625. https://biodiversitylibrary.org/page/7172280
- Augener H (1927) Papers from Dr. Th. Mortensen’s Pacific Expedition 1914–16. 38. Polychaeten von Südost- und Süd-Australien. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i Köbenhavn 83: 71–275.
- Averincev VG (1978) The polychaetous annelids of the Aphroditiformia of the shelf and upper bathyal of Australian and New Zealand region and of Macquarie Island (on the base data of 16th cruise of R/V Dmitri Mendeleev). Trudy Instituta Okeanologii AN SSR 113: 51–72.
- Bailey DM, Collins MA, Gordon JDM, Zuur AF, Priede IG (2009) Long-term changes in deep-water fish populations in the northeast Atlantic: a deeper reaching effect of fisheries? Proceedings of the Royal Society B – Biological Sciences 276(1664): 1965–1969. https://doi.org/10.1098/rspb.2009.0098
- Bakken T (2002) A new species of Neanthes (Polychaeta: Nereididae) from southern Australia. Memoirs of the Museum of Victoria 59(2): 327–331. https://doi.org/10.24199/j.mmv.2002.59.4
- Banse K (1969) Acrocirridae n. fam. (Polychaeta Sedentaria). Journal of the Fisheries Research Board of Canada 26(10): 2595–2620. https://doi.org/10.1139/f69-253
- Barnich R, Fiege D (2009) Revision of the genus Harmothoe Kinberg, 1856 (Polychaeta: Polynoidae) in the Northeast Atlantic. Zootaxa 2104: 1–76. https://doi.org/10.11646/zootaxa.2104.1.1
- Barnich R, Fiege D (2010) On the distinction of Harmothoe globifera (G.O. Sars, 1873) and some other easily confused polynoids in the NE Atlantic, with the description of a new species of Acanthicolepis Norman in McIntosh, 1900 (Polychaeta, Polynoidae). Zootaxa 2525: 1–18. https://doi.org/10.11646/zootaxa.2525.1.1
- Barroso R, De Paiva PC, Nogueira JM, Fukuda MV (2017) Deep sea Syllidae (Annelida, Phyllodocida) from Southwestern Atlantic. Zootaxa 4221(4): 401–430. https://doi.org/10.11646/zootaxa.4221.4.1
- Bastida-Zavala JR (2008) Serpulids (Annelida: Polychaeta) from the Eastern Pacific, including a brief mention of Hawaiian serpulids. Zootaxa 1722: 1–61.
- Benham WB (1915) Report on the Polychaeta obtained by the F.I.S. ‘Endeavour’ on the coasts of New South Wales, Victoria, Tasmania and South Australia, Part 1. Biological Results of the Fisheries Expedition F.I.S. “Endeavour” 1909–14, Volume 3(4): 171–237. [H.C. Dannevig, Sydney] https://biodiversitylibrary.org/page/953505
- Benham WB (1916) Report on the Polychaeta obtained by the F.I.S. “Endeavour’’ on the coasts of New South Wales, Victoria, Tasmania and South Australia. Part II. Fisheries: Biological Results of the Fishing Experiments carried on by the F.I.S. ‘’Endeavour’’, 1909–1914 4(2): 127–162. [plates XLVI–XLVIII] [H.C. Dannevig, Sydney] https://biodiversitylibrary.org/page/5916746
- Benham WB (1921) Polychaeta. Australian Antarctic Expedition 1911–1914, Scientific Reports, Series C. Zoology and Botany 6(3): 1–128. https://doi.org/10.5962/bhl.title.16201
- Benham WB (1927) Polychaeta [Terra Nova]. British Antarctic ‘Terra Nova’ Expedition Natural History Reports. Zoology 7(2): 47–182.
- Bergström E (1914) Zur Systematik der Polychaetenfamilie der Phyllodociden. Zoologiska Bidrag från Uppsala 3: 37–224. http://biodiversitylibrary.org/page/36924250
- Berthold AA (1827) Latreille’s Natürliche Familien des Thierreichs. Aus dem Franzoesischen, mit Anmerkungen und Zusätzen.Verlage Landes-Industrie-Comptoirs, Weimar, 227–228. https://doi.org/10.5962/bhl.title.11652
- Blainville HMD de (1818) Mémoire sur la classe des Sétipodes, partie des Vers à sang rouge de M. Cuvier, et des Annélides de M. de Lamarck. Bulletin des Sciences, par la Société Philomatique de Paris 1818: 78–85.
- Bick A (2020) Fabriciidae Rioja, 1923. In: Westheide W, Purschke G, Böggemann M (Eds) Handbook of Zoology. A Natural History of the Phyla of the Animal Kingdom. Annelida: Polychaetes, Volume 3 Sedentaria 111, Errantia 1. De Gruyter, Osnabrück, 1–33. https://www.degruyter.com/view/Zoology/bp_029147-6_86 [April 4, 2019]
- Blake JA (1981) The Scalibregmatidae (Annelida: Polychaeta) from South America and Antarctica collected chiefly during the cruises of the R/V Anton Bruun, R/V Hero and USNS Eltanin. Proceedings of the Biological Society of Washington 94(4): 1131–1162. https://www.biodiversitylibrary.org/page/34607646
- Blake JA (1985) Polychaeta from the vicinity of deep-sea geothermal vents in the eastern Pacific. I: Euphrosinidae, Phyllodocidae, Hesionidae, Nereididae, Glyceridae, Dorvilleidae, Orbiniidae and Maldanidae. Bulletin of the Biological Society of Washington 6: 67–101. https://bit.ly/31jOeWn
- Blake JA (1995) Family Pholoidae Kinberg, 1858. In: Blake JA, Hilbig B, Scott PH (Eds) Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel. 5 – The Annelida Part 2. Polychaeta: Phyllodocida (Syllidae and scale-bearing families), Amphinomida, and Eunicida. Santa Barbara Museum of Natural History, Santa Barbara, 175–188.
- Blake JA (1996a) Family Paraonidae Cerruti, 1909. In: Blake JA, Hilbig B, Scott PH (Eds) Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel. 6 – The Annelida Part 3. Polychaeta: Orbiniidae to Cossuridae. Santa Barbara Museum of Natural History, Santa Barbara, 27–70.
- Blake JA (1996b) Family Spionidae Grube, 1850. In: Blake JA, Hilbig B, Scott PH (Eds) Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel. 6 – The Annelida Part 3. Polychaeta: Orbiniidae to Cossuridae. Santa Barbara Museum of Natural History, Santa Barbara, 81–223.
- Blake JA (1997) Family Phyllodocidae Örsted, 1843. In: Blake JA, Hilbig B, Scott PH (Eds) Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and the Western Santa Barbara Channel. The Annelida Part 2. Oligochaeta and Polychaeta: Phyllodocida (Phyllodocidae to Paralacydoniidae). Santa Barbara Museum of Natural History, Santa Barbara, 109–178.
- Blake JA (2000) Family Oweniidae Rioja, 1917. In: Blake JA, Hilbig B, Scott PH (Eds) Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and the Western Santa Barbara Channel (Vol. 7). The Annelida Part 4. Polychaeta: Flabelligeridae to Sternaspidae. Santa Barbara Museum of Natural History, Santa Barbara, 97–127.
- Blake JA (2006) New species and records of deep-water Cirratulidae (Polychaeta) from off Northern California. Scientia Marina 70 (Supplement 3): 45–57. https://doi.org/10.3989/scimar.2006.70s345
- Blake JA (2015) New species of Scalibregmatidae (Annelida, Polychaeta) from the East Antarctic Peninsula including a description of the ecology and post-larval development of species of Scalibregma and Oligobregma. Zootaxa 4033(1): 57–93. https://doi.org/10.11646/zootaxa.4033.1.3
- Blake JA (2016) Kirkegaardia (Polychaeta, Cirratulidae), new name for Monticellina Laubier, preoccupied in the Rhabdocoela, together with new records and descriptions of eight previously known and sixteen new species from the Atlantic, Pacific, and Southern Oceans. Zootaxa 4166(1): 1–93. https://doi.org/10.11646/zootaxa.4166.1.1
- Blake JA (2017) Polychaeta Orbiniidae from Antarctica, the Southern Ocean, the abyssal Pacific Ocean, and off South America. Zootaxa 4218: 1–145. https://doi.org/10.11646/zootaxa.4218.1.1
- Blake JA (2018) Bitentaculate Cirratulidae (Annelida, Polychaeta) collected chiefly during cruises of the R/V Anton Bruun, USNS Eltanin, R/V Hero, RVIB Nathaniel B. Palmer, and R/V Polarstern from the Southern Ocean, Antarctica, and off Western South America. Zootaxa 4537: 1–130. https://doi.org/10.11646/zootaxa.4537.1.1
- Blake JA (2019a) New species of Cirratulidae (Annelida, Polychaeta) from abyssal depths of the Clarion-Clipperton Fracture Zone, North Equatorial Pacific Ocean. Zootaxa 4629(2): 151–187. https://doi.org/10.11646/zootaxa.4629.2.1
- Blake JA (2019b) Paraonidae Cerruti, 1909. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 1: Annelida Basal Groups and Pleistoannelida, Sedentaria I. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 281–308.
- Blake JA (2019c) Scalibregmatidae Malmgren, 1867. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 2: Pleistoannelida, Sedentaria II. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 312–349. https://doi.org/10.1515/9783110291681-010
- Blake JA (2020) New species and records of deep-water Orbiniidae (Annelida, Polychaeta) from the Eastern Pacific continental slope, abyssal Pacific Ocean, and the South China Sea. Zootaxa 4730(1): 1–61. https://doi.org/10.11646/zootaxa.4730.1.1
- Blake JA, Kudenov JD (1978) The Spionidae (Polychaeta) from southeastern Australia and adjacent areas with a revision of the genera. Memoirs of the Museum Victoria 39: 171–280. https://doi.org/10.24199/j.mmv.1978.39.11
- Blake JA, Maciolek NJ, Ota AY, Williams IP (2009) Long-term benthic infaunal monitoring at a deep-ocean dredged material disposal site off Northern California. Deep-Sea Research II, 56: 1775–1803. https://doi.org/10.1016/j.dsr2.2009.05.021
- Blake JA, Maciolek NJ (2019a) Opheliidae Malmgren, 1867. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 2: Pleistoannelida, Sedentaria II. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 285–302. https://doi.org/10.1515/9783110291681-008
- Blake JA, Maciolek NJ (2019b) Travisiidae Hartmann-Schröder, 1971, New Family Status. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 2: Pleistoannelida, Sedentaria II. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 302–311. https://doi.org/10.1515/9783110291681-009
- Blake JA, Maciolek NJ, Meißner K (2019) Spionidae Grube, 1850. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 2: Pleistoannelida, Sedentaria II. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 103 pp. https://doi.org/10.1515/9783110291681-001
- Blake JA, Magalhães W (2019) Cirratulidae Ryckholt, 1851. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 1: Annelida Basal groups and Pleistoannelida, Sedentaria I. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 339–397.
- Bleidorn C, Helm C (2019) Orbiniidae Hartman, 1942. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 1: Annelida Basal groups and Pleistoannelida, Sedentaria I. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 251–268.
- Bock S (1942) On the structure and affinities of “Thalassema” lankesteri Herdman and the classification of the group Echiuroidea. Göteborgs Kungliga Vetenskaps- och Vitterhets-Samhälles Handlingar, Sjätte följden – Series B 2(6): 1–94.
- Böggemann M (2002) Revision of the Glyceridae Grube, 1850 (Annelida: Polychaeta). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 555: 1–249. http://www.schweizerbart.de/publications/list/series/abh_senck
- Böggemann M (2005) Revision of the Goniadidae (Annelida, Polychaeta). Abhandlungen des Naturwissenschaftlichen Vereins in Hamburg 39: 1–354.
- Böggemann M (2009) Polychaetes (Annelida) of the abyssal SE Atlantic. Organisms Diversity and Evolution 9(4): 251–428.
- Böggemann M (2014a [Online]) Glyceridae Grube, 1850. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Walter de Gruyter GmbH & Co. KG, Berlin/Boston.
- Böggemann M (2014b [Online]) Goniadidae Kinberg, 1865. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Walter de Gruyter GmbH & Co. KG, Berlin/Boston.
- Böggemann M (2015) Glyceriformia Fauchald, 1977 (Annelida: “Polychaeta”) from Lizard Island (Australia, Queensland) and adjacent areas. Zootaxa 4019(1): 70–97. https://doi.org/10.11646/zootaxa.4019.1.7
- Böggemann M, Wilson RS (2003) Glyceridae (Polychaeta) – A DELTA database of genera, and Australian species. In: Wilson RS, Hutchings PA, Glasby CJ (Eds) Polychaetes: An Interactive Identification Guide. CSIRO Publishing, Melbourne.
- Böggemann M, Purschke G (2005) Abyssal benthic Syllidae (Annelida: Polychaeta) from the Angola Basin. Organisms Diversity and Evolution 5 (Supplement 1): 221–226. https://doi.org/10.1016/j.ode.2004.11.006
- Bonifácio P, Lavesque N, Bachelet G, Parapar J (2015) Anobothrus amourouxi sp. nov., a new species of Ampharetidae (Polychaeta) from the Capbreton Canyon (Bay of Biscay, NE Atlantic Ocean). Journal of the Marine Biological Association of the United Kingdom 95(5): 961–969. https://doi.org/10.1017/S0025315414002094
- Bonifácio P, Menot L (2019 [online first 2018]) New genera and species from the Equatorial Pacific provide phylogenetic insights into deep-sea Polynoidae (Annelida). Zoological Journal of the Linnean Society 185(3): 555–635. [published online 14 November 2018; printed publication 27 February 2019] https://doi.org/10.1093/zoolinnean/zly063
- Borda E, Kudenov JD, Bienhold C, Rouse GW (2012) Towards a revised Amphinomidae (Annelida, Amphinomida): description and affinities of a new genus and species from the Nile deep‐sea Fan, Mediterranean Sea. Zoologica Scripta 41: 307–325. https://doi.org/10.1111/j.1463-6409.2012.00529.x
- Borda E, Kudenov JD, Chevaldonne P, Blake JA, Desbruyères D, Fabri M-C, Hourdez S, Pleijel F, Shank TM, Wilson NG, Schulze A, Rouse GW (2013) Cryptic species of Archinome (Annelida: Amphinomida) from vents and seeps. Proceedings of the Royal Society B 280(1770): 20131876. https://doi.org/10.1098/rspb.2013.1876
- Brasier MJ, Harle J, Wiklund H, Jeffreys RM, Linse K, Ruhl HA, Glover AG (2017) Distributional patterns of polychaetes across the West Antarctic based on DNA barcoding and particle tracking analyses. Frontiers in Marine Science 4: e356. https://doi.org/10.3389/fmars.2017.00356
- Brasier MJ, Wiklund H, Neal L, Jeffreys R, Linse K, Ruhl H, Glover AG (2016) DNA barcoding uncovers cryptic diversity in 50% of deep-sea Antarctic polychaetes. Royal Society Open Science 3: e160432. https://doi.org/10.1098/rsos.160432
- Brenke N (2005) An epibenthic sledge for operations on marine soft bottom and bedrock. Marine Technology Society Journal 39(2): 10–21. https://doi.org/10.4031/002533205787444015
- Brown A, Thatje S (2014) Explaining bathymetric diversity patterns in marine benthic invertebrates and demersal fishes: physiological contributions to adaptation of life at depth. Biological Reviews of the Cambridge Philosophical Society 89(2): 406–426. https://doi.org/10.1111/brv.12061
- Capa M (2007) Taxonomic revision and phylogenetic relationships of apomorphic sabellids (Sabellidae: Polychaeta) from Australia. Invertebrate Systematics 21: 537–567. https://doi.org/10.1071/IS07002
- Capa M, Aguado MT, Bakken T (2016) Phylogenetic hypothesis of Sphaerodoridae Malmgren, 1867 (Annelida) and its position within Phyllodocida. Cladistics 32: 335–350. https://doi.org/10.1111/cla.12134
- Capa M, Bakken T (2015) Revision of the Australian Sphaerodoridae (Annelida) including the description of four new species. Zootaxa 4000(2): 227–267. https://doi.org/10.11646/zootaxa.4000.2.3
- Capa M, Bakken T, Meißner K, Nygren A (2018) Three, two, one! Revision of the long-bodied sphaerodorids (Sphaerodoridae, Annelida) and synonymization of Ephesiella, Ephesiopsis and Sphaerodorum. PeerJ 6: e5783. https://doi.org/10.7717/peerj.5783
- Capa M, Bakken T, Purschke G (2014 [Online]). Sphaerodoridae Malmgren, 1867. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Walter de Gruyter GmbH & Co. KG, Berlin/Boston.
- Capa M, Hutchings P, Aguado MT, Bott N (2010) Phylogeny of Sabellidae (Annelida) and relationships with other taxa inferred from morphology and multiple genes. Cladistics 26: 449–469. https://doi.org/10.1111/j.1096-0031.2010.00341.x
- Capa M, Murray A (2015) Integrative taxonomy of Parasabella and Sabellomma (Sabellidae: Annelida) from Australia: description of new species, indication of cryptic diversity, translocation of some species out of their natural distribution range. Zoological Journal of the Linnean Society 175: 764–811. https://doi.org/10.1111/zoj.12308
- Capa M, Parapar J, Hutchings P (2012) Phylogenetic analyses of Oweniidae (Polychaeta) and taxonomic revision of Australian fauna reveals relationships and undocumented diversity within this enigmatic group of marine worms. Zoological Journal of the Linnean Society 166: 236–278. https://doi.org/10.1111/j.1096-3642.2012.00850.x
- Capa M, Nishi E, Katsuhiko T, Katsunori F (2013) First record of a Bispira species (Sabellidae: Polychaeta) from a hydrothermal vent. Marine Biodiversity Records 6: 1–6. https://doi.org/10.1017/S1755267213000468
- Capa M, Parapar J, Hutchings P (2019a) Oweniidae Rioja, 1917. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 1: Annelida Basal Groups and Pleistoannelida, Sedentaria I. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 91–112. https://doi.org/10.1515/9783110291582-004
- Capa M, Giangrande A, Nogueira JMM, Tovar-Hernández M (2019b) Sabellidae. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 2: Pleistoannelida, Sedentaria II. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 164–212.
- Capa M, Nygren A, Parapar J, Bakken T, Meißner K, Moreira J (2019c) Systematic re-structure and new species of Sphaerodoridae (Annelida) after morphological revision and molecular phylogenetic analyses of the North East Atlantic fauna. ZooKeys 845: 1–97. https://doi.org/10.3897/zookeys.845.32428
- Carr CM, Hardy SM, Brown TM, Macdonald TA, Hebert PDN (2011) A Tri-Oceanic perspective: DNA barcoding reveals geographic structure and cryptic diversity in Canadian polychaetes. PLoS ONE 6(7): e22232. https://doi.org/10.1371/journal.pone.0022232
- Carrera-Parra LF (2006) Phylogenetic analysis of Lumbrineridae Schmarda, 1861 (Annelida: Polychaeta). Zootaxa 1332: 1–36. https://doi.org/10.11646/zootaxa.1332.1.1
- Carus JV (1863) Vermes. In: Peters WCH, Carus JV, Gerstäcker CEA (Eds) Handbuch der Zoologie. Wilhelm Engelmann, Leipzig, 422–484. https://doi.org/10.5962/bhl.title.1399
- Carney RS (2005) Zonation of deep biota on continental margins. Oceanography and Marine Biology: an Annual Review 43: 211–278. https://doi.org/10.1201/9781420037449.ch6
- Caullery M (1914) Sur les Siboglinidae, type nouveau d’invertébrés receuillis par l’expédition du Siboga. Comptes rendus hebdomadaires des séances de l’Académie des sciences 158: 2014–2017. http://biodiversitylibrary.org/page/7159983
- Cerruti A (1909) Contributo all’anatomia: biologia e sistematica delle Paraonidae (Levinsenidae) con particolare riguardo alle specie del golfo di Napoli. Mittheilungen aus der Zoologischen Station zu Neapel 19(3): 459–512. https://biodiversitylibrary.org/page/47419517
- Chamberlin RV (1919) The Annelida Polychaeta [Albatross Expeditions]. Memoirs of the Museum of Comparative Zoology at Harvard College 48: 1–514. http://www.biodiversitylibrary.org/ia/memoirsofmuseumo4801harv
- Claparède É, Mecznikow E (1869) Beiträge zur Kenntnis der Entwicklungsgeschichte der Chaetopoden. Zeitschrift für wissenschaftliche Zoologie 19: 163–205. https://www.biodiversitylibrary.org/page/45006862
- Cutler EB (1977) The bathyal and abyssal Sipuncula. Galathea Report 14: 135–156. https://doi.org/10.11646/zootaxa.525.1.1
- Cutler EB (1994) The Sipuncula. Their systematics, biology and evolution. Cornell University Press, Ithaca, 453 pp. https://doi.org/10.7591/9781501723643
- Dahlgren TG, Lundberg J, Pleijel F, Sundberg P (2002) Morphological and molecular evidence of the phylogeny of Nereidiform polychaetes (Annelida). Journal of Zoological Systematics and Evolutionary Research 38(4): 249–254. https://doi.org/10.1046/j.1439-0469.2000.384150.x
- Day JH (1963) The polychaete fauna of South Africa. Part 8: New species and records from grab samples and dredgings. Bulletin of the British Museum (Natural History), Series Zoology 10(7): 381–445. https://doi.org/10.5962/bhl.part.20530
- Day JH (1977) A review of the Australian and New Zealand Orbiniidae (Annelida: Polychaeta). In: Reish DJ, Fauchald K (Eds) Essays on Polychaetous Annelids in Memory of Dr. Olga Hartman. Allan Hancock Foundation, Los Angeles, 217–243.
- Day JH, Hutchings PA (1979) An annotated check-list of Australian and New Zealand Polychaeta, Archiannelida and Myzostomida. Records of the Australian Museum 32: 80–161. https://doi.org/10.3853/j.0067-1975.32.1979.203
- Desbruyères D, Segonzac M (1997) Handbook of deep-sea hydrothermal vent fauna. IFRÉMER, Brest, 279 pp.
- Detinova NN (1982) Deep-water Maldanidae (Polychaeta) of the Pacific Ocean 1. The genus Maldanella. Trudy Instituta Okeanologii AN SSR 117: 63–75.
- Dixon-Bridges K, Gladstone W, Hutchings PA (2014) One new species of Micronephthys Friedrich, 1939 and one new species of Nephtys Cuvier, 1817 (Polychaeta: Phyllodocida: Nephtyidae) from eastern Australia with notes on Aglaophamus australiensis (Fauchald, 1965) and a key to all Australian species. Zootaxa 3872(5): 513–540. https://doi.org/10.11646/zootaxa.3872.5.5
- Dnestrovskaya NY, Jirkov IA (2010) Micronephthys (Polychaeta: Nephtyidae) of Northern Europe and Arctic. Invertebrate Zoology 7(2): 107–121. https://doi.org/10.15298/invertzool.07.2.03
- Dnestrovskaya NY, Jirkov IA (2019) Redescription of Micronephthys longicornis (Perejaslavtseva, 1891) (Annelida: Nephtyidae). Zootaxa 4550(3): 391–400. https://doi.org/10.11646/zootaxa.4550.3.6
- Drennan R, Wiklund H, Rouse GW, Georgieva MN, Wu X, Kobayashi G, Yoshino K, Glover AG (2019) Taxonomy and phylogeny of mud owls (Annelida: Sternaspidae), including a new synonymy and new records from the Southern Ocean, North East Atlantic Ocean and Pacific Ocean: challenges in morphological delimitation. Marine Biodiversity 49: 2659–2697. https://doi.org/10.1007/s12526-019-00998-0
- Ebbe B, Purschke G (2021) Ampharetidae Malmgren, 1866. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Volume 3: Sedentaria III, Errantia I. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 50–65.
- Edmonds SJ (1987) Echiurans of Australia (Echiura). Records of the South Australian Museum 32: 119–138. https://www.biodiversitylibrary.org/page/40781430
- Ehlers E (1864) Die Borstenwürmer, nach systematischen und anatomischen Untersuchungen dargestellt. Wilhelm Engelmann, Leipzig, 748 pp. https://doi.org/10.5962/bhl.title.2081
- Ehlers E (1874) Annulata nova vel minus cognita in Expeditione ‘Porcupine’ capta. Annals and Magazine of Natural History, Series 4, 13: 292–298. https://doi.org/10.1080/00222937408680863
- Ehlers E (1901) Die Polychaeten des magellanischen und chilenischen Strandes. Ein faunistischer Versuch. Festschrift zur Feier des Hundertfünfzigjährigen Bestehens der Königlichen Gesellschaft der Wissenschaften zu Göttingen, Abhandlungen der Mathematisch-Physikalischen Klasse. Weidmannsche Buchhandlung, Berlin, 232 pp. http://www.annelida.net/docs/Ehlers1901_PolychaetenMagellanischenChilenischenStrandes.pdf
- Ehlers E (1904) Neuseeländische Anneliden. Abhandlungen der Königlichen Gesellschaft der Wissenschaften zu Göttingen Mathematisch-Physikalische Klasse. Neue Folge 3(1): 1–80. http://biodiversitylibrary.org/page/46755553
- Ehlers E (1913) Die Polychaeten-Sammlungen der deutschen Südpolar-Expedition, 1901–1903. Deutsche Südpolar-Expedition 1901–1903 im Auftrage des Reichsamtes des innern herausgegeben von Erich von Drygalski Leiter Expedition 13(4): 397–598. https://www.biodiversitylibrary.org/page/2139283
- Eibye-Jacobsen D (1991) A revision of Eumida Malmgren, 1865 (Polychaeta: Phyllodocidae). Steenstrupia 17: 81–140.
- Eibye-Jacobsen D, Aungtonya C, Gonzalez BC (2020 [Online]). Sigalionidae Kinberg, 1856. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Walter de Gruyter GmbH & Co. KG, Berlin/Boston.
- Eilertsen MH, Kongsrud JA, Alvestad T, Stiller J, Rouse GW, Rapp HT (2017) Do ampharetids take sedimented steps between vents and seeps? Phylogeny and habitat-use of Ampharetidae (Annelida Terebelliformia) in chemosynthesis-based ecosystems. BMC Evolutionary Biology 17(1): e222. https://doi.org/10.1186/s12862-017-1065-1
- Eilertsen MH, Georgieva MN, Kongsrud JA, Linse K, Wiklund H, Glover AG, Rapp HT (2018) Genetic connectivity from the Arctic to the Antarctic: Sclerolinum contortum and Nicomache lokii (Annelida) are both widespread in reducing environments. Scientific Reports 8: e4810. https://doi.org/10.1038/s41598-018-23076-0
- Ekins M, Erpenbeck D, Hooper J (2020) Carnivorous sponges from the Australian Bathyal and Abyssal zones collected during the RV Investigator 2017 Expedition. Zootaxa 4774: 1–159. https://doi.org/10.11646/zootaxa.4774.1.1
- Ekman S (1953) Zoogeography of the Sea. Sidgwick and Jackson, London, 417 pp. https://doi.org/10.2307/1439946
- Etter RJ, Rex MA (1990) Population differentiation decreases with depth in deep-sea gastropods. Deep Sea Research Part A. Oceanographic Research Papers 37(8): 1251–1261. https://doi.org/10.1016/0198-0149(90)90041-S
- Farrelly CA, Ahyong ST (2019) Deepwater decapod, stomatopod and lophogastrid Crustacea from Eastern Australia and the Great Australian Bight collected in 2015–2017: preliminary identifications of 191 species. Museum Victoria Science Reports 21: 1–97. https://doi.org/10.24199/j.mvsr.2019.21
- Fauchald K (1963) Nephtyidae (Polychaeta) from Norwegian waters. Sarsia 13: 1–32. https://doi.org/10.1080/00364827.1963.10409514
- Fauchald K (1972) Benthic polychaetous annelids from deep water off western Mexico and adjacent areas in the Eastern Pacific Ocean. Allan Hancock Monographs in Marine Biology 7: 1–575. https://repository.si.edu/handle/10088/6207
- Fauchald K (1974) Sphaerodoridae (Polychaeta: Errantia) from world-wide areas. Journal of Natural History 8(3): 257–289. https://doi.org/10.1080/00222937400770241
- Fauchald K, Hancock DR (1981) Deep-water polychaetes from a transect off central Oregon. Allan Hancock Monographs in Marine Biology 11: 1–73. http://hdl.handle.net/10088/3445
- Fauchald K, Jumars P (1979) The diet of worms: A study of polychaete feeding guilds. Oceanography and Marine Biology – an Annual Review 17: 193–284.
- Filippova A, Purschke G, Tzetlin AB, Müller MCM (2010) Musculature in polychaetes: comparison of Myrianida prolifera (Syllidae) and Sphaerodoropsis sp. (Sphaerodoridae). Invertebrate Biology 129: 184–198. https://doi.org/10.1111/j.1744-7410.2010.00191.x
- Fischer W (1919) Gephyreen der Südwestküste Australiens. Zoologischer Anzeiger 50: 277–285. https://www.biodiversitylibrary.org/page/29993777
- Fitzhugh K (1990) A revision of the fabriciin genus Augeneriella Banse, 1957 (Polychaeta: Sabellidae). Journal of Natural History 24: 195–218. https://doi.org/10.1080/00222939000770131
- Fitzhugh K (1992) On the systematic position of Monroika africana (Monro) (Polychaeta: Sabellidae: Fabriciinae) and a description of a new fabriciin genus and species from Australia. Proceedings of the Biological Society of Washington 105(1): 116–131. https://www.biodiversitylibrary.org/page/35607285
- Fitzhugh K (1996) New fanworm species (Polychaeta: Sabellidae: Fabriciinae) in the genus Pseudofabriciola Fitzhugh. Journal of Natural History 30: 1267–1286. https://doi.org/10.1080/00222939600771211
- Fitzhugh K (2001) A new deep-water genus and species of Fabriciinae fanworm (Polychaeta: Sabellidae) from Antarctica. Contributions in Science, Natural History Museum of Los Angeles County 491: 1–8.
- Fitzhugh K (2002) New species of Fabricinuda Fitzhugh and Pseudofabriciola Fitzhugh (Polychaeta: Sabellidae: Fabriciinae), with an emendation of Pseudofabriciola australiensis (Hartmann-Schröder). Journal of Natural History 36: 893–925. https://doi.org/10.1080/00222930110034580
- Fitzhugh K, Wolf PS (1990) Gross morphology of the brain of pilargid polychaetes: taxonomic and systematic implications. American Museum Novitates 2992: 1–16. http://hdl.handle.net/2246/5090
- France SC, Kocher TD (1996) Geographic and bathymetric patterns of mitochondrial 16S rRNA sequence divergence among deep-sea amphipods, Eurythenes gryllus. Marine Biology 126: 633–643. https://doi.org/10.1007/BF00351330
- Gage JD, Tyler PA (1991) Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor. Cambridge University Press, Cambridge, 504 pp. https://doi.org/10.1017/CBO9781139163637
- Georgieva MN, Wiklund H, Bell JB, Eilertsen MH, Mills RA, Little CTS, Adrian AG (2015) A chemosynthetic weed: the tubeworm Sclerolinum contortum is a bipolar, cosmopolitan species. BMC Evolutionary Biology 15: e280. https://doi.org/10.1186/s12862-015-0559-y
- Georgieva MN, Gunton LM, Wiklund H, Watson C, Ramos DA, Glover AG (in prep) The annelid community associated with a natural deep-sea whale fall from Australia’s east coast.
- Glasby CJ (1993) Family revision and cladistic analysis of the Nereidoidea (Polychaeta: Phyllodocida). Invertebrate Taxonomy 7: 1551–1573. https://doi.org/10.1071/IT9931551
- Glasby CJ (2000a) Family Orbiniidae. In: Beesley PL, Ross GJB, Glasby CJ (Eds) Polychaetes and Allies: The Southern Synthesis. Fauna of Australia (Vol. 4A). Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing, Melbourne, 79–82. https://environment.gov.au/science/abrs/publications/fauna-of-australia/fauna-4a
- Glasby CJ (2000b) Family Paraonidae. In: Beesley PL, Ross GJB, Glasby CJ (Eds) Polychaetes and Allies: The Southern Synthesis. Fauna of Australia. Vol. 4A. Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing, Melbourne, 82–84. https://environment.gov.au/science/abrs/publications/fauna-of-australia/fauna-4a
- Glazier AE, Etter RJ (2014) Cryptic speciation along a bathymetric gradient. Biological Journal of the Linnean Society 113: 897–913. https://doi.org/10.1111/bij.12389
- Glover AG, Smith CR, Paterson GLJ, Wilson GDF, Hawkins L, Sheader M (2002) Polychaete species diversity in the central Pacific abyss: local and regional patterns, and relationships with productivity. Marine Ecology Progress Series 240: 157–170. https://doi.org/10.3354/meps240157
- Glover AG, Smith CR (2003) The deep-sea floor ecosystem: current status and prospects of anthropogenic change by the year 2025. Environmental Conservation 30(3): 219–241. https://doi.org/10.1017/S0376892903000225
- Glover AG, Dahlgren TG, Wiklund H, Mohrbeck I, Smith CR (2016) An end-to-end DNA taxonomy methodology for benthic biodiversity survey in the Clarion-Clipperton Zone, central Pacific abyss. Journal of Marine Science and Engineering 4(1): 1–2. https://doi.org/10.3390/jmse4010002
- Goffredi SK, Johnson SB, Vrijenhoek RC (2007) Genetic diversity and potential function of microbial symbionts associated with newly discovered species of Osedax polychaete worms. Applied and Environmental Microbiology 73(7): 2314–2323. https://doi.org/10.1128/AEM.01986-06
- Gonzalez BC, Martínez A, Borda E, Iliffe T, Eibye-Jacobsen D, Worsaae K (2018) Phylogeny and systematics of Aphroditiformia. Cladistics 34: 225–259. https://doi.org/10.1111/cla.12202
- Greaves E, Meißner K, Wilson R (2011) New Laonice species (Polychaeta: Spionidae) from western and northern Australia. Zootaxa 2903: 1–20. https://doi.org/10.11646/zootaxa.2903.1.1
- Grube AE (1850) Die Familien der Anneliden. Archiv für Naturgeschichte, Berlin 16(1): 249–364. https://www.biodiversitylibrary.org/page/6958350
- Grube AE (1862) Noch ein Wort über die Capitellen und ihre Stelle im Systeme der Anneliden. Archiv für Naturgeschichte, Berlin 28(1): 366–378. https://www.biodiversitylibrary.org/page/7160797
- Grube AE (1868) Naturwissenschaftliche Section mit einigen Sipunkuloiden bekannt und sprach namentlich über Loxosiphon, Cloeosiphon, und einige Phascolosomen. Jahres-Bericht der Schlesischen Gesellschaft für Vaterländische Kultur 45: 47–49. https://www.biodiversitylibrary.org/page/46568003
- Grube AE (1870) Bemerkungen über die familie der Glycereen. Jahresbericht der Schlesischen Gesellschaft für vaterländische Cultur Breslau 47: 56–68. https://archive.org/details/jahresberichtder4718schl/page/n7/mode/2up
- Grube AE (1873) Über ein paar neue Anneliden aus der Familie der Spiodeen. Jahres-Bericht der Schlesischen Gesellschaft für Vaterländische Kultur 50: 57–58. https://www.biodiversitylibrary.org/page/38491131
- Grube AE (1877) Anneliden – Ausbeute S.M.S. Gazelle. Monatsbericht der Koniglich Preussischen Akademie der Wissenschaften zu Berlin, 509–554. https://www.biodiversitylibrary.org/page/35723828
- Gunton LM, Kupriyanova EK, Alvestad T (2020) Two new deep-water species of Ampharetidae (Annelida: Polychaeta) from the eastern Australian continental margin. Records of the Australian Museum 72(4): 101–121. https://doi.org/10.3853/j.2201-4349.72.2020.1763
- Hanley JR, Burke M (1991) A new genus and species of scaleworm (Polychaeta: Polynoidae) from the Cascade Plateau, Tasman Sea. The Beagle, Records of the Northern Territory Museum of Arts and Sciences 8(1): 97–102.
- Hartman O (1942) A review of the types of polychaetous annelids at the Peabody Museum of Natural History, Yale University. Bulletin of the Bingham Oceanographic Collection, Yale University 8(1): 1–98.
- Hartman O (1945) The marine annelids of North Carolina. Duke University Marine Station Bulletin 2: 1–54.
- Hartman O (1959) Catalogue of the Polychaetous Annelids of the World. Parts 1 and 2. Allan Hancock Foundation Occasional Paper. 23: 1–628. http://digitallibrary.usc.edu/cdm/ref/collection/p15799coll82/id/19573/
- Hartman O (1960) Systematic account of some marine invertebrate animals from the deep basins off southern California. Allan Hancock Pacific Expeditions 22: 69–216. https://www.biodiversitylibrary.org/page/4682240
- Hartman O (1964) Polychaeta Errantia of Antarctica. Antarctic Research Series 3: 1–131. https://doi.org/10.1002/iroh.19660510124
- Hartman O (1965) Deep-water benthic polychaetous annelids off New England to Bermuda and other North Atlantic areas. Occasional Papers of the Allan Hancock Foundation 28: 1–378. http://digitallibrary.usc.edu/cdm/ref/collection/p15799coll82/id/20299/
- Hartman O (1966a) New records of some little known Australian Polychaetous annelids. Records of the Australian Museum 26: 361–365. https://doi.org/10.3853/j.0067-1975.26.1966.685
- Hartman O (1966b) Polychaeta Myzostomidae and Sedentaria of Antarctica. Antarctic Research Series 7: 1–158. https://doi.org/10.1029/AR007
- Hartman O (1967a) Polychaetous annelids collected by the USNS Eltanin and Staten Island cruises, chiefly from Antarctic Seas. Allan Hancock Monographs in Marine Biology 2: 1–387.
- Hartman O (1967b) Annelida, Polychaeta, Archiannelida (Vol. 10). McGraw-Hill Book Co., Inc., New York, 461–465.
- Hartman O (1969) Atlas of the sedentariate polychaetous annelids from California. Allan Hancock Foundation, University of Southern California, Los Angeles, 812 pp.
- Hartman O (1971) Abyssal polychaetous annelids from the Mozambique Basin off southeast Africa, with a compendium of abyssal polychaetous annelids from world-wide areas. Journal of the Fisheries Research Board of Canada 28(10): 1407–1428. https://doi.org/10.1139/f71-219
- Hartman O (1978) Polychaeta from the Weddell Sea quadrant, Antarctica. Antarctic Research Series 26(4): 125–223. https://doi.org/10.1029/AR026p0125
- Hartman O, Fauchald K (1971) Deep-water benthic polychaetous annelids off New England to Bermuda and other North Atlantic Areas. Part II. Allan Hancock Monographs in Marine Biology 6: 1–327. https://repository.si.edu/handle/10088/3458
- Hartmann-Schröder G (1959) Zur Ökologie der Polychaeten des Mangrove‐Estero‐Gebietes von El Salvador. Beiträge zur Neotropischen Fauna 1(2): 69–183. https://doi.org/10.1080/01650525909380612
- Hartmann-Schröder G (1965) Zur Kenntnis des Sublitorals der chilenischen Küste unter besonderer Berücksichtigung der Polychaeten und Ostracoden. II Die Polychaeten des Sublitorals. Mitteilungen aus dem Hamburgischen zoologischen Museum und Institut. 62 supplement: 59–305 [231–233].
- Hartmann-Schröder G (1971) Annelida, Borstenwürmer, Polychaeta. Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise 58, 594 pp. https://doi.org/10.1086/407180
- Hartmann-Schröder G (1975) Polychaeten der Iberischen Tiefsee, gesammelt auf der 3. Reise der Meteor im Jahre 1966. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 72: 47–73.
- Hartmann-Schröder G (1979) Die Polychaeten der tropischen Nordwestküste Australiens (zwischen Derby im Norden und Port Hedland im Süden). Teil 2. In: Hartmann-Schröder G, Hartmann G (Eds) Zur Kenntnis des Eulitorals der australischen Küsten unter besonderer Berücksichtigung der Polychaeten und Ostracoden. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 76: 77–218.
- Hartmann-Schröder G (1980) Die Polychaeten der tropischen Nordwestküste Australiens (zwischen Port Samson im Norden und Exmouth im Süden). Teil 4. In: Hartmann-Schröder G, Hartmann G (Eds) Zur Kenntnis des Eulitorals der australischen Küsten unter besonderer Berücksichtigung der Polychaeten und Ostracoden. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 77: 41–110.
- Hartmann-Schröder G (1981) Polychaeten der tropisch-subtropischen Westküste Australiens (zwischen Exmouth im Norden und Cervantes im Süden). Teil 6. In: Hartmann-Schröder G, Hartmann G (Eds) Zur Kenntnis des Eulitorals der australischen Küsten unter besonderer Berücksichtigung der Polychaeten und Ostracoden (Teil 6 und Teil 7). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 78: 19–96.
- Hartmann-Schröder G (1982) Die Polychaeten der subtropischen-antiborealen Westküste Australiens (zwischen Cervantes im Norden und Cape Naturaliste im Süden). Teil 8. In: Hartmann-Schröder G, Hartmann G (Eds) Zur Kenntnis des Eulitorals der australischen Küsten unter besonderer Berücksichtigung der Polychaeten und Ostracoden (Teil 6 und Teil 7). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 79: 51–118.
- Hartmann-Schröder G (1983) Die Polychaeten der antiborealen Südwestküste Australiens (zwischen Dunsborough im Norden und Denmark im Süden). Teil 9. In: Hartmann-Schröder G, Hartmann G (Eds) Zur Kenntnis des Eulitorals der australischen Küsten unter besonderer Berücksichtigung der Polychaeten und Ostracoden. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 80: 123–167.
- Hartmann-Schröder G (1986) Die Polychaeten der antiborealen Südküste Australiens (zwischen Wallaroo im Westen und Port MacDonnell im Osten). Teil 12. In: Hartmann-Schröder G, Hartmann G (Eds) Zur Kenntnis des Eulitorals der australischen Küsten unter besonderer Berücksichtigung der Polychaeten und Ostracoden. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 83: 31–70.
- Hartmann-Schröder G (1987) Die Polychaeten der antiborealen Küste von Victoria (Australien) (Zwischen Warrnambool im Westen und Port Welshpool im Osten). Teil 13. In: Hartmann-Schröder G, Hartmann G (Eds) Zur Kenntnis des Eulitorals der australischen Küsten unter besonderer Berücksichtigung der Polychaeten und Ostracoden. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 84: 27–66.
- Hartmann-Schröder G (1991) Die Polychaeten der subtropisch-tropischen bis tropischen Ostküste Australiens zwischen Maclean (New South Wales) und Gladstone (Queensland) sowie von Heron Island (Grosses Barriere-Riff). Teil 16. In: Hartmann-Schröder G, Hartmann G (Eds) Zur Kenntnis des Eulitorals der australischen Küsten unter besonderer Berücksichtigung der Polychaeten und Ostracoden. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 88: 17–71.
- Hartmann-Schröder G (1993) Die Polychaeten der “Polarstern” – Reise ANTX/1b zur Antarktischen Halbinsel und Isla de los Estados (Feuerland, Argentinien) 1991. Teil 1: Polynoidae bis Iphitimidae. Mitteilungen aus den Hamburgischen Zoologischen Museum und Institut 90: 127–150.
- Hartmann-Schröder G, Parker SA (1995) Four new species of the family Opheliidae (Polychaeta) from southern Australia. Records of the South Australian Museum 28(1): 1–12. https://archive.org/details/RecordsSouthAus28Sout/page/1/mode/2up
- Hartmann-Schröder G, Rosenfeldt P (1988) Die Polychaeten der “Polarstern” – Reise ANT III/2 in die Antarktis 1984. Teil 1: Euphrosinidae bis Chaetopteridae. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 85: 25–72.
- Hartmann-Schröder G, Rosenfeldt P (1989) Die Polychaeten der “Polarstern” – Reise 68/1 nach Elephant Island (Antarktis) 1985. Teil 2: Cirratulidae bis Serpulidae. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 86: 65–106.
- Hartmann-Schröder G, Rosenfeldt P (1991) Die Polychaeten der “Walter Herwig” – Reise ANT III/2 in die Antarktis 1984. Teil 2: Acrocirridae bis Serpulidae. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 88: 73–96.
- Haswell WA (1886) Observations on some Australian Polychaeta. Proceedings of the Linnean Society of New South Wales 10: 733–756. https://doi.org/10.5962/bhl.part.17962
- Haswell WA (1892) Observations on the Chloraemidae, with special reference to certain Australian forms. Proceedings of the Linnean Society of New South Wales Series 2, 6: 329–356. https://doi.org/10.5962/bhl.part.29896
- Hatschek B (1888) Lehrbuch der Zoologie, eine morphologische Übersicht des Thierreiches zur Einführung in das Studium dieser Wissenschaft. Vol. 1. Gustav Fischer, Jena, 144 pp. https://doi.org/10.5962/bhl.title.1381
- Hausen H (2005) Chaetae and chaetogenesis in polychaetes (Annelida). In: Purschke G, Bartolomaeus T (Eds) Morphology, Molecules, Evolution and Phylogeny in Polychaeta and Related Taxa, Vol. 535/536, Hydrobiologia, 199–225.
- Hausen H (2005) Chaetae and chaetogenesis in polychaetes (Annelida). In: Purschke G, Bartolomaeus T (Eds) Morphology, Molecules, Evolution and Phylogeny in Polychaeta and Related Taxa, Vol. 535/536, Hydrobiologia, 199–225. https://doi.org/10.1007/1-4020-3240-4_4
- Heap AD, Harris PT (2008) Geomorphology of the Australian margin and adjacent seafloor. Australian Journal of Earth Sciences 55(4): 555–585. https://doi.org/10.1080/08120090801888669
- Herring P (2010) The Biology of the Deep Ocean. Oxford University Press, Oxford, 314 pp.
- Hessler RR, Jumars PA (1974) Abyssal community analysis from replicate box cores in central north Pacific. Deep-Sea Research 21(3): 185–209. https://doi.org/10.1016/0011-7471(74)90058-8
- Hocknull SA, Glasby CJ (2009) Diversity and ecology of Pilargidae (Annelida: Polychaeta) from the Gulf of Carpentaria and Arafura Sea, northern Australia. Zoosymposia 2: 537–550. https://doi.org/10.11646/zoosymposia.2.1.37
- Higgs ND, Attrill MJ (2015) Biases in biodiversity: wide-ranging species are discovered first in the deep sea. Frontiers in Marine Science 2: 1–8. https://doi.org/10.3389/fmars.2015.00061
- Hilbig B (1997) Family Nereididae Johnston, 1845. In: Blake JA, Hilbig B, Scott PH (Eds) The Annelida Part 2 Oligochaeta and Polychaeta: Phyllodocida (Phyllodocidae to Paralacydoniidae). Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and the Western Santa Barbara Channel. Santa Barbara Museum of Natural History, Santa Barbara, California, 291–316.
- Holthe T (2000) Bathyal and abyssal Ampharetidae (Annelida: Polychaeta) (sedentary species II). Galathea Report 18: 57–68. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.613.2272&rep=rep1&type=pdf
- Horst R (1903) New species of the Euphrosyne from the Siboga Expedition, with a table of the species hitherto known. Notes from the Leyden Museum 23: 213–222. https://www.biodiversitylibrary.org/page/9658066
- Horst R (1910) On the genus Chloeia with some new species from the Malay Archipelago, partly collected by the Siboga Expedition. Notes from the Leyden Museum 32: 169–175. https://www.biodiversitylibrary.org/page/13217595
- Horst R (1923) On three remarkable Annelida Polychaeta. Zoologische Mededeelingen Leiden 7: 221–224. https://www.repository.naturalis.nl/document/150120
- Hove ten HA, Zibrowius H (1986) Laminatubus alvini gen. et sp. n. and Protis hydrothermica sp. n. (Polychaeta, Serpulidae) from the bathyal hydrothermal vent communities in the eastern Pacific. Zoologica Scripta 15(1): 21–31. https://doi.org/10.1111/j.1463-6409.1986.tb00205.x
- Huang D, Fitzhugh K, Rouse GW (2011) Inference of phylogenetic relationships within Fabriciidae (Sabellidae, Annelida) using molecular and morphological data. Cladistics 27: 356–379. https://doi.org/10.1111/j.1096-0031.2010.00343.x
- Hutchings P, Reid A (1991) The Nereididae (Polychaeta) from Australia-Leonnates, Platynereis and Solomononereis. Records of the Australian Museum 43(1): 47–62. https://doi.org/10.3853/j.0067-1975.43.1991.40
- Hutchings P (1998) Biodiversity and functioning of polychaetes in benthic sediments. Biodiversity and Conservation 7: 1133–1145. https://doi.org/10.1023/A:1008871430178
- Hutchings PA (2000a) Family Acoetidae. In: Beesley PL, Ross GJB, Glasby CJ (Eds) Polychaetes and Allies: The Southern Synthesis. Fauna of Australia. Volume 4A, Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing, Melbourne, 112–115. https://environment.gov.au/system/files/resources/d603deda-71f4-4647-a3a8-0fe7945f5f32/files/4a-polychaetes-01-polychaeta-02.pdf
- Hutchings PA (2000b) Family Capitellidae. In: Beesley PL, Ross GJB, Glasby CJ (Eds) Polychaetes and Allies: The Southern Synthesis. Fauna of Australia. Volume 4A, Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing, Melbourne, 67–72. https://environment.gov.au/system/files/resources/d603deda-71f4-4647-a3a8-0fe7945f5f32/files/4a-polychaetes-01-polychaeta-02.pdf
- Hutchings PA (2000c) Family Opheliidae. In: Beesley PL, Ross GJB, Glasby CJ (Eds) Polychaetes and Allies: The Southern Synthesis. Fauna of Australia. Volume 4A, Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing, Melbourne, 99–103. https://environment.gov.au/system/files/resources/d603deda-71f4-4647-a3a8-0fe7945f5f32/files/4a-polychaetes-01-polychaeta-02.pdf
- Hutchings PA (2000d) Family Polynoidae. In: Beesley PL, Ross GJB, Glasby CJ (Eds) Polychaetes and Allies: The Southern Synthesis. Fauna of Australia. Volume 4A, Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing, Melbourne, 152–157. https://environment.gov.au/system/files/resources/d603deda-71f4-4647-a3a8-0fe7945f5f32/files/4a-polychaetes-01-polychaeta-02.pdf
- Hutchings PA (2007) New species of Terebellidae and Trichobranchidae from the deep sea. Galathea Report 21: 75–90.
- Hutchings PA, DeDeckker P, Geddes MC (1981) A new species of Manayunkia (Polychaeta) from ephemeral lakes near the Coorang, South Australia. Transactions of the Royal Society of South Australia 105(1): 25–28. https://www.biodiversitylibrary.org/page/41071515
- Hutchings P, Rainer S (1979) The polychaete fauna of Careel Bay, Pittwater, New South Wales, Australia. Journal of Natural History 13: 745–796. https://doi.org/10.1080/00222937900770561
- Hutchings PA, McRae J (1993) The Aphroditidae (Polychaeta) from Australia, together with a redescription of the Aphroditidae collected during the Siboga Expedition. Records of the Australian Museum 45(3): 279–363. https://doi.org/10.3853/j.0067-1975.45.1993.24
- Hutchings P, McRae J, Murray A (in prep.) The Pholoidae and selected genera of the Sigalionidae (Annelida) from Australia. Zootaxa.
- Hutchings P, Murray A (1984) Taxonomy of polychaetes from Hawkesbury River and the southern estuaries of New South Wales, Australia. Records of the Australian Museum, Supplement 3: 1–119. https://doi.org/10.3853/j.0812-7387.3.1984.101
- Hutchings P, Peart R (2002) A review of the genera of Pectinariidae (Polychaeta) together with a description of the Australian Fauna. Records of the Australian Museum 54: 99–127. https://doi.org/10.3853/j.0067-1975.54.2002.1356
- Hutchings P, Capa M, Peart R (2012) Revision of the Australian Sabellariidae (Polychaeta) and description of eight new species. Zootaxa 3306: 1–60. https://doi.org/10.11646/zootaxa.3306.1.1
- Hutchings P, Carrerette O, Nogueira JMM (2021) Pectinariidae de Quatrefages, 1866. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Volume 3: Sedentaria III, Errantia I. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 68–137.
- Imajima M (1973) Paraonidae (Polychaeta) from Japan. Bulletin of the National Science Museum, Tokyo 16(2): 253–292.
- Imajima M (1991) Spionidae (Annelida, Polychaeta) from Japan. VII. The genus Spiophanes. Bulletin of National Science Museum, Tokyo, Series A 17: 115–137.
- Imajima M (1999) Onuphidae (Annelida, Polychaeta) from Japan, excluding the genus Onuphis. National Science Museum Monographs 16: 1–115.
- Imajima M (2001) Deep-sea benthic polychaetous annelids of Tosa Bay, southwestern Japan. National Science Museum Monographs 20: 31–100.
- Imajima M (2009) Deep-sea benthic polychaetes off Pacific Coast of the northern Honshu, Japan. National Museum of Nature and Science Monographs 39: 39–192. https://www.kahaku.go.jp/english/research/publication/monograph/v39.html
- Janssen A, Stuckas H, Vink A, Arbizu PM (2019) Biogeography and population structure of predominant macrofaunal taxa (Annelida and Isopoda) in abyssal polymetallic nodule fields: implications for conservation and management. Marine Biodiversity 49: 2641–2658. https://doi.org/10.1007/s12526-019-00997-1
- Jenkins CJ (1984) Erosion and Deposition at Abyssal Depths in the Tasman Sea. A Seismic Stratigraphic Study of the Bottom-current Patterns. Ocean Science Institute, University of Sydney. Report 4.
- Johnston G (1846) An index to the British Annelides. Annals and Magazine of Natural History 1(16): 433–462. https://doi.org/10.1080/037454809495980
- Johnston G (1865) A catalogue of the British non-parasitical worms in the collection of the British Museum. Trustees of the British Museum. Taylor & Francis, London, 366 pp. https://doi.org/10.5962/bhl.title.12291
- Johnston TH, Tiegs OW (1919) Pseudobonellia, a new echiuroid from the Great Barrier Reef. Proceedings of the Linnean Society of New South Wales 44: 213–239. https://www.biodiversitylibrary.org/page/3765309
- Johnston TH, Tiegs OW (1920) A new species of Bonellia from Port Jackson. Records of the Australian Museum 13(2): 73–76. https://doi.org/10.3853/j.0067-1975.13.1920.856
- Jumars PA, Dorgan KM, Lindsay SM (2015) Diet of worms emended: An update of polychaete feeding guilds. Annual Review of Marine Science 7(1): 497–520. https://doi.org/10.1146/annurev-marine-010814-020007
- Kawauchi GY, Sharma PP, Giribet G (2012) Sipunculan phylogeny based on six genes, with a new classification and the descriptions of two new families. Zoologica Scripta 41(2): 186–210. https://doi.org/10.1111/j.1463-6409.2011.00507.x
- Kinberg JGH (1856) Nya slägten och arter af Annelider, Öfversigt af Kongl. Vetenskaps-Akademiens Förhhandlingar Stockholm 12(9–10): 381–388. [read 1855, printed 1856] https://www.biodiversitylibrary.org/page/15970133
- Kinberg JGH (1865) Annulata nova. [Continuatio.]. Öfversigt af Königlich Vetenskapsakademiens Förhandlingar, Stockholm 22(2): 167–179. https://biodiversitylibrary.org/page/32339443
- Kirkegaard JB (1956) Benthic Polychaeta from depths exceeding 6000 meters. Galathea Report 2: 63–78.
- Kirkegaard JB (1994) The biogeography of some abyssal polychaetes. Mémoires Museum National d’Histoire Naturelle 162: 471–477. https://archive.org/details/memoiresdumuseu162muse/page/470/mode/2up
- Kirkegaard JB (1995) Bathyal and abyssal polychaetes (errant species). Galathea Report 17: 7–56.
- Kirkegaard JB (1996) Bathyal and abyssal polychaetes (sedentary species I). Galathea Report 17: 57–77.
- Kirtley DW (1994) A review and taxonomic revision of the family Sabellariidae Johnston, 1865 (Annelida; Polychaeta). Sabecon Press Science Series 1: 1–223.
- Knox GA, Cameron DB (1998) The Marine fauna of the Ross Sea: Polychaeta. NIWA Biodiversity Memoir 108: 1–125. http://docs.niwa.co.nz/library/public/Memoir%20108_Marine%20Fauna%20of%20Ross%20Sea_Polychaeta%20-%201998.pdf
- Kolbasova G, Kosobokova K, Neretina T (2020) Bathy- and mesopelagic annelida from the Arctic Ocean: Description of new, redescription of known and notes on some “cosmopolitan” species. Deep Sea Research Part I: Oceanographic Research Papers 165: 103327. https://doi.org/10.1016/j.dsr.2020.103327
- Kongsrud JA, Bakken T, Oug E (2011) Deep-water species of the genus Ophelina (Annelida, Opheliidae) in the Nordic Seas, with description of Ophelina brattegardi sp. nov. Italian Journal of Zoology 78(sup1): 95–111. https://doi.org/10.1080/11250003.2011.606658
- Kongsrud JA, Budaeva N, Barnich R, Oug E, Bakken T (2013) Benthic polychaetes from the northern Mid-Atlantic Ridge between the Azores and the Reykjanes Ridge. Marine Biology Research 9: 516–546. https://doi.org/10.1080/17451000.2012.749997
- Kucheruk NV (1977) On the specific composition and distribution of the deep-sea genus Paraonuphis [sic for Paronuphis] (Polychaeta, Eunicidae). Trudy Instituta Okeanologii AN SSR 108: 44–51.
- Kudenov JD (1991) A new family and genus of the order Amphinomida (Polychaeta) from the Galapagos hydrothermal vents. Ophelia, supplement 5 (Systematics, Biology and Morphology of World Polychaeta): 111–120.
- Kudenov JD (1993) Amphinomidae and Euphrosinidae (Annelida: Polychaeta) principally from Antarctica, the Southern Ocean, and Subantarctic regions. Antarctic Research Series, Ser. Biology of the Antarctic Seas XXII 58: 93–150. https://doi.org/10.1029/AR058p0093
- Kudenov JD (1976) Polychaeta from Southeastern Australia 1. Acrocirridae Banse, 1969, from Victoria and New South Wales. Records of the Australian Museum 30(9): 137–149. https://doi.org/10.3853/j.0067-1975.30.1976.395
- Kupriyanova EK (1993a) Deep-water Serpulidae (Annelida, Polychaeta) from the Kurile-Kamchatka Trench: 1. Genus Hyalopomatus. Zoologichesky Zhurnal 72: 145–152.
- Kupriyanova EK (1993b) Deep-water Serpulidae (Annelida, Polychaeta) from Kurile-Kamchatka Trench. 2. Genera Bathyditrupa, Bathyvermilia, and Protis. Zoologichesky Zhurnal 72(3): 21–28.
- Kupriyanova EK (1993c) A new species, Metavermilia arctica (Polychaeta, Serpulidae), from the Arctic Ocean. Sarsia 78(2): 155–157. https://doi.org/10.1080/00364827.1993.10413532
- Kupriyanova EK, Rouse GW (2008) Yet another example of paraphyly in Annelida: molecular evidence that Sabellidae contains Serpulidae. Molecular Phylogenetics and Evolution 46: 1174–1181. https://doi.org/10.1016/j.ympev.2007.10.025
- Kupriyanova EK, Ippolitov AP (2015) Deep-sea serpulids (Annelida: Polychaeta) in tetragonal tubes: on a tube convergence path from the Mesozoic to Recent. Zootaxa 4044: 151–200. https://doi.org/10.11646/zootaxa.4044.2.1
- Kupriyanova EK, Bailey-Brock J, Nishi E (2011) New records of Serpulidae (Annelida, Polychaeta) collected by R/V “Vityaz” from bathyal and abyssal depths of the Pacific Ocean. Zootaxa 2871: 43–60. https://doi.org/10.11646/zootaxa.2871.1.3
- Kupriyanova EK, Nishi E, Kawato M, Fujiwara T (2010) New records of Serpulidae (Annelida, Polychaeta) from hydrothermal vents of North Fiji, Pacific Ocean. Zootaxa 2389: 57–68. https://doi.org/10.11646/zootaxa.2389.1.3
- Kupriyanova EK, Vinn O, Taylor PD, Schopf JW, Kudryavtsev AB, Bailey-Brock J (2014) Serpulids living deep: calcareous tubeworms beyond the abyss. Deep-Sea Research Part I: Oceanographic Research Papers 90: 91–104. https://doi.org/10.1016/j.dsr.2014.04.006
- Lacaze-Duthiers H (1858) Recherches sur la Bonellie (Bonellia viridis) (1). Annales des Sciences Naturelles 10: 49–110 [plates 1–4]. https://www.biodiversitylibrary.org/page/13469606
- Lamarck JB de (1818) Histoire naturelle des Animaux sans Vertèbres, préséntant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s’y rapportent; précédés d’une Introduction offrant la détermination des caractères essentiels de l`Animal, sa distinction du végétal et desautres corps naturels, enfin, l’Exposition des Principes fondamentaux de la Zoologie. Deterville, Paris, 612 pp. http://www.biodiversitylibrary.org/item/46337
- Langeneck J, Barbieri M, Maltagliati F, Castelli A (2019a) Molecular phylogeny of Paraonidae (Annelida). Molecular Phylogenetics and Evolution 136: 1–13. https://doi.org/10.1016/j.ympev.2019.03.023
- Langeneck J, Busoni G, Aliani S, Lardicci C, Castelli A (2019b) Distribution and diversity of polychaetes along a bathyal escarpment in the western Mediterranean Sea. Deep-Sea Research, Part I 144: 85–94. https://doi.org/10.1016/j.dsr.2019.01.006
- Langerhans P (1880) Die Wurmfauna Madeiras. 11. Zeitschrift für Wissenschafiliche Zoologie 33: 271–316. https://www.biodiversitylibrary.org/page/45632727
- Latreille PA (1825) Familles naturelles du règne animal, exposé succinctement et dans un ordre analytique avec l’indication de leurs genres. J B. Baillière, Paris, 570 pp. https://doi.org/10.5962/bhl.title.16094
- Laubier L (1967) Sur quelques Aricidea (Polychètes, Paraonidae) de Banyuls-sur-Mer. Vie et Milieu, Série A 18(1): 99–132. https://wwwphp.obs-banyuls.fr/Viemilieu/index.php/archives.html
- Laubier L (1975) Lacydonia laureci sp. n., annelide polychete nouvelle de l’étage abyssal de Méditerranée orientale. Vie et Milieu, Série A 25(1): 75–82. https://wwwphp.obs-banyuls.fr/Viemilieu/index.php/archives.html
- Laubier L, Ramos J (1974) Paraonidae (Polychètes sédentaires) de Méditerranée. Bulletin du Muséum d’Histoire Naturelle, Paris, 3e Série 168 (Zoologie 113): 1097–1148. https://archimer.ifremer.fr/doc/1973/publication-5122.pdf
- Lechapt JP, Kirtley DW (1996) Bathysabellaria spinifera (Polychaeta: Sabellariidae), a new species from deep water off New Caledonia, Southwest Pacific Ocean. Proceedings of the Biological Society of Washington 109: 560–574. https://www.biodiversitylibrary.org/page/34645167
- Lechapt JP (1997) Two new species of Hyalinoecia (Polychaeta, Onuphidae) from the deep zones off New Caledonia (southwest Pacific Ocean). Bulletin of Marine Science 60(2): 306–312. https://www.ingentaconnect.com/contentone/umrsmas/bullmar/1997/00000060/00000002/art00012
- Lemer S, Kawauchi GY, Andrade SCS, González VL, Boyle MJ, Giribet G (2015) Re-evaluating the phylogeny of Sipuncula through transcriptomics. Molecular Phylogenetics and Evolution 83: 174–183. https://doi.org/10.1016/j.ympev.2014.10.019
- de Leon-Gonzalez JA (1998) Spionidae and Opheliidae (Annelida: Polychaeta) from the western coast of Baja California, Mexico. Bulletin of Marine Science 62(1): 7–16. https://www.ingentaconnect.com/contentone/umrsmas/bullmar/1998/00000062/00000001/art00002
- Levenstein RY (1970) New and rare species of the abyssal genus Fauveliopsis McIntosh (Polychaeta, Annelida) and the peculiarities of its distribution. Trudy Instituta Okeanologii AN SSR 88: 227–235.
- Levenstein RY (1978) Annelida (Polychaeta) from the deep waters of the Pacific region of Antarctic. Trudy Instituta Okeanologii AN SSR 113: 73–87.
- Lewis M (2010) The CSIRO 4 m beam trawl. CSIRO Marine and Atmospheric Research Paper 033 (CMAR CSIRO: Hobart). http://www.cmar.csiro.au/publications/cmarseries/Internal%20Report_BeamTrawl.pdf
- Light WJH (1991) Systematic revision of the genera of the polychaete subfamily Maldaninae Arwidsson. Ophelia, Supplement 5: 133–146.
- Lützen J (1961) Sur une nouvelle espèce de polychète Sphaerodoridium commensalis n. g., n. sp. (Polychaeta Errantia, famille des Sphaerodoridae) vivant en commensal de Terebellides stroemii Sars. Cahiers de Biologie Marine 2: 409–416. https://doi.org/10.21411/CBM.A.635247C6
- MacIntosh H, Althaus F, Williams A, Tanner JE, Alderslade P, Ahyong ST, Bax N, Criscione F, Crowther AL, Farrelly CA, Finn JK, Goudie L, Gowlett-Holmes K, Hosie AM, Kupriyanova E, Mah C, McCallum AW, Merrin KL, Miskelly A, Mitchell ML, Molodtsova T, Murray A, O’Hara TD, O’Loughlin PM, Paxton H, Reid AL, Sorokin SJ, Staples D, Walker-Smith G, Whitfield E, Wilson RS (2018) Invertebrate diversity in the deep Great Australian Bight (200–5000 m). Marine Biodiversity Records 11(1): e23. https://doi.org/10.1186/s41200-018-0158-x
- Maciolek NJ (1981) A new genus and species of Spionidae (Annelida: Polychaeta) from the North and South Atlantic. Proceedings of the Biological Society of Washington 94: 228–239. https://www.biodiversitylibrary.org/page/34608079
- Maciolek NJ (2020) Anguillosyllis (Annelida: Syllidae) from multiple deep-water locations in the northern and southern hemispheres. Zootaxa 4793: 1–73. https://doi.org/10.11646/zootaxa.4793.1.1
- Mackie ASY (1987) A review of species currently assigned to the genus Leitoscoloplos Day, 1977 (Polychaeta: Orbiniidae), with descriptions of species newly referred to Scoloplos Blainville, 1828. Sarsia 72: 1–28. https://doi.org/10.1080/00364827.1987.10419701
- Magalhães WF, Blake JA (2019) Capitellidae Grube, 1862. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 2: Pleistoannelida, Sedentaria II. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 349–403. https://doi.org/10.1515/9783110291681-011
- Magalhães WF, Rizzo AE, Bailey-Brock JH (2019) Opheliidae (Annelida: Polychaeta) from the western Pacific islands, including five new species. Zootaxa 4555(2): 209–234. https://doi.org/10.11646/zootaxa.4555.2.3
- Malm AW (1874) Annulata i hafvet utmed Sveriges westkust och omkring Göteborg. Göteborgs Königlich vetenskaps – och vitterhetssamhälles handlingar. [Zoologiska observationer. VII.]. Göteborgs Königlich vetenskaps – och vitterhetssamhälles handlingar 14: 67–105.
- Malmgren AJ (1866) Nordiska Hafs-Annulater. Öfversigt af Königlich Vetenskapsakademiens förhandlingar, Stockholm 22(5): 355–410. https://www.biodiversitylibrary.org/page/32339631
- Malmgren AJ (1867) Annulata Polychaeta Spetsbergiæ, Grœnlandiæ, Islandiæ et Scandinaviæ hactenus cognita. Öfversigt af Kungliga Vetenskaps-Akademiens Förhandligar 24: 127–235. [in Swedish] https://doi.org/10.5962/bhl.title.13358
- Marcus J, Hourdez S (2002) A new species of scale-worm (Polychaeta: Polynoidae) from Axial Volcano, Juan de Fuca Ridge, northeast Pacific. Proceedings of the Biological Society of Washington 115(2): 341–349. https://www.biodiversitylibrary.org/page/35518704
- Marenzeller E von (1879) Südjapanische Anneliden. I. (Amphinomea, Aphroditea, Lycoridea, Phyllodocea, Hesionea, Syllidea, Eunicea, Glycerea, Sternaspidea, Chaetopterea, Cirratulea, Amphictenea). Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch-naturwissenschaftliche Classe, Wien 41(2): 109–154 [plates I–VI]. https://www.biodiversitylibrary.org/page/7215498
- Martinez A, Domenico MD, Rouse GW, Worsaae K (2015) Phylogeny and systematics of Protodrilidae (Annelida) inferred with total evidence analyses. Cladistics 31: 250–276. https://doi.org/10.1111/cla.12089
- Martínez A, Worsaae K, Núñez J (2019) Acrocirridae Banse, 1968. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 1: Annelida Basal Groups and Pleistoannelida, Sedentaria I. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 422–439.
- McClain CR, Hardy SM (2010) The dynamics of biogeographic ranges in the deep sea. Proceedings of the Royal Society B 277: 3533–3546. https://doi.org/10.1098/rspb.2010.1057
- McCowin MF, Rowden AA, Rouse GW (2019) A new record of Lamellibrachia columna (Siboglinidae, Annelida) from cold seeps off New Zealand, and an assessment of its presence in the western Pacific Ocean. Marine Biodiversity Records 12(1): e10. https://doi.org/10.1186/s41200-019-0169-2
- McEnnulty FR, Gowlett-Holmes KL, Williams A, Althaus F, Fromont J, Poore GCB, O’Hara TD, Marsh L, Kott P, Slack-Smith S, Alderslade P, Kitahara MV (2011) The deepwater megabenthic invertebrates on the continental margin of Australia (100–1100 m depths): composition, distribution and novelty. Records of the Western Australian Museum, Supplement 80: 1–191. https://doi.org/10.18195/issn.0313-122x.80.2011.001-191
- McIntosh WC (1879) On the Annelida obtained during the Cruise of H.M.S. ‘Valorous’ to Davis Strait in 1875. Transactions of the Linnean Society of London. Second Series: Zoology 1(7): 499–511 [plate LXV]. https://doi.org/10.1111/j.1096-3642.1878.tb00663b.x
- McIntosh WC (1885) Report on the Annelida Polychaeta collected by H.M.S. Challenger during the years 1873–1876. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873–76. Zoology 12: 1–554. https://www.biodiversitylibrary.org/page/50688426
- McIntosh WC (1877) Annelida. In: Jeffreys JG (Ed.) Preliminary report of the biological results of a cruise in H.M.S. “Valorous” to Davis Strait in 1875. Proceedings of the Royal Society of London 25: 215–222. https://www.biodiversitylibrary.org/page/43410688
- McIntosh WC (1900) A monograph of the British Annelids. Polychaeta. Amphinomidae to Sigalionidae. Royal Society London 1(2): 215–442. https://www.biodiversitylibrary.org/page/38577949
- McIntosh WC (1908) Notes from the Gatty Marine Laboratory, St. Andrews. Annals and Magazine of Natural History 1(8): 373–387. https://doi.org/10.1080/00222930808692422
- McIntosh WC (1922) Notes from the Gatty Marine Laboratory, St. Andrews, 44: 1. On new and rare Polychaeta, Gephyrea, etc., from various regions; 2. Recent additions to the British marine Polychaeta (continued). Annals and Magazine of Natural History Series 9 9(49): 1–30. https://doi.org/10.1080/00222932208632638
- Meißner K, Hutchings PA (2003) Spiophanes species (Polychaeta: Spionidae) from Eastern Australia – with descriptions of new species, new records and an emended generic diagnosis. Records of the Australian Museum 55: 117–140. https://doi.org/10.3853/j.0067-1975.55.2003.1379
- Meißner K (2005) Revision of the genus Spiophanes (Polychaeta, Spionidae): with new synonymies, new records and descriptions of new species. Mitteilungen aus dem Museum für Naturkunde in Berlin, Zoologische Reihe 81: 3–66. https://doi.org/10.1002/mmnz.200310001
- Méndez N (2006) Deep-water polychaetes (Annelida) from the southeastern gulf of California, Mexico. Revista de Biologia Tropical 54(3): 773–785. https://doi.org/10.15517/rbt.v54i3.12776
- Mengerink KJ, Van Dover CL, Ardron J, Baker M, Escobar-Briones E, Gjerde K, Koslow JA, Ramirez-Llodra E, Lara-Lopez A, Squires D, Sutton T, Sweetman AK, Levin LA (2014) A call for deep-ocean stewardship. Science 344(6185): 696–698. https://doi.org/10.1126/science.1251458
- Meyer C, Westheide W (1997) Boguea panwaensis, a new species from Thailand: the first member of the Bogueinae (Polychaeta: Maldanidae) to be found outside northeast America. Proceedings of the Biological Society of Washington 110(2): 203–220. https://www.biodiversitylibrary.org/page/35458229
- Miura T, Laubier L (1989) Nautilina calyptogenicola, a new genus and species of parasitic polychaete on a vesicomyid bivalve from the Japan Trench, representative of a new family Nautilinidae. Zoological Science 6: 387–390. https://www.biodiversitylibrary.org/page/40504548
- Moreira J, Parapar J (2017) New data on the Opheliidae (Annelida) from Lizard Island (Great Barrier Reef, Australia): five new species of the genus Armandia Filippi, 1861. Zootaxa 4290(3): 483–502. https://doi.org/10.11646/zootaxa.4290.3.4
- Monro CCA (1930) Polychaete worms. Discovery Reports, Cambridge 2: 1–222. http://biodiversitylibrary.org/page/15904801
- Müller F (1858) Einiges über die Annelidenfauna der Insel Santa Catharina an der brasilianischen Küste. Archiv für Naturgeschichte, Berlin. 24(1): 211–220. https://www.biodiversitylibrary.org/page/7460059
- Murina G-WW (1978) New and rare echiurids of the family Bonelliidae. Trudy Instituta Okeanologii AN SSR 113: 107–119.
- Murray A, Hutchings P (in prep.) Revision of species of Acoetidae (Annelida) from the Australasian region.
- Murray A, Wong E, Hutchings P (2015) Nephtyidae (Annelida: Phyllodocida) of Lizard Island, Great Barrier Reef, Australia. Zootaxa 4019(1): 414–436. https://doi.org/10.11646/zootaxa.4019.1.16
- Neal L, Barnich R, Wiklund H, Glover AG (2012) A new genus and species of Polynoidae (Annelida, Polychaeta) from Pine Island Bay, Amundsen Sea, Southern Ocean – a region of high taxonomic novelty. Zootaxa 3542: 80–88. https://doi.org/10.11646/zootaxa.3542.1.4
- Neal L, Linse K, Brasier MJ, Sherlock E, Glover AG (2018) Comparative marine biodiversity and depth zonation in the Southern Ocean: evidence from a new large polychaete dataset from Scotia and Amundsen seas. Marine Biodiversity 48: 581–601. https://doi.org/10.1007/s12526-017-0735-y
- Neal L, Paterson GLJ, Blockley D, Scott B, Sherlock E, Huque C, Glover AG (2020) Biodiversity data and new species descriptions of polychaetes from offshore waters of the Falkland Islands, an area undergoing hydrocarbon exploration. ZooKeys 938: 1–86. https://doi.org/10.3897/zookeys.938.49349
- Neave MJ, Glasby CJ (2013) New species of Ophelina (Annelida: Opheliidae: Ophelininae) from northern Australia. Organisms Diversity and Evolution 13: 331–347. https://doi.org/10.1007/s13127-013-0130-x
- Nogueira JMM, Fitzhugh K, Hutchings P (2013) The continuing challenge of phylogenetic relationships in Terebelliformia (Annelida: Polychaeta). Invertebrate Systematics 27(2): 186–238. https://doi.org/10.1071/IS12062
- Norlinder E, Nygren A, Wiklund H, Pleijel F (2012) Phylogeny of scale-worms (Aphroditiformia, Annelida), assessed from 18SrRNA, 28SrRNA, 16SrRNA, mitochondrial cytochrome c oxidase subunit I (COI), and morphology. Molecular Phylogenetics and Evolution 65: 490–500. https://doi.org/10.1016/j.ympev.2012.07.002
- O’Hara TD (2008) Bioregionalisation of the waters around Lord Howe and Norfolk Islands using brittle stars (Echinodermata: Ophiuroidea). Department of the Environment, Water, Heritage and the Arts, Australia. https://parksaustralia.gov.au/marine/pub/scientific-publications/archive/brittlestars-lord-howe.pdf
- O’Hara TD, Rowden AA, Bax NJ (2011) A Southern Hemisphere bathyal fauna is distributed in latitudinal bands. Current Biology 21: 226–230. https://doi.org/10.1016/j.cub.2011.01.002
- O'Hara TD, England PR, Gunasekera RM, Naughton KM (2014) Limited phylogeographic structure for five bathyal ophiuroids at continental scales. Deep Sea Research Part I: Oceanographic Research Papers 84: 18–28. https://doi.org/10.1016/j.dsr.2013.09.009
- O’Hara T (2017) IN2017_V03 Voyage Summary. https://www.cmar.csiro.au/data/trawler/survey_details.cfm?survey=IN2017%5FV03
- O’Hara T (2019) The Eastern Australian Marine Parks: Biodiversity, assemblage structure, diversity and origin. Report to Parks Australia from the National Environmental Science Program Marine Biodiversity Hub. Museums Victoria. https://www.nespmarine.edu.au/document/eastern-australian-marine-parks-biodiversity-assemblage-structure-diversity-and-origin
- O’Hara TD, Williams A, Althaus F, Ross AS, Bax NJ (2020a) Regional‐scale patterns of deep seafloor biodiversity for conservation assessment. Diversity and Distributions 26: 479–494. https://doi.org/10.1111/ddi.13034
- O’Hara TD, Williams A, Woolley SNC, Nau AW, Bax NJ (2020b) Deep-sea temperate-tropical faunal transition across uniform environmental gradients. Deep Sea Research Part I: Oceanographic Research Papers 161: 103283. https://doi.org/10.1016/j.dsr.2020.103283
- O’Hara TD, Williams A, Ahyong ST, Alderslade P, Alvestad T, Bray D, Burghardt I, Budaeva N, Criscione F, Crowther AL, Ekins M, Eléaume M, Farrelly CA, Finn JK, Georgieva MN, Graham A, Gomon M, Gowlett-Holmes L, Gunton LM, Hallan A, Hosie AM, Hutchings P, Kise H, Köhler F, Konsgrud JA, Kupriyanova E, Lu CC, Mackenzie M, Mah C, MacIntosh H, Merrin KL, Miskelly A, Mitchell ML, Moore K, Murray A, O’Loughlin PM, Paxton H, Pogonoski JJ, Staples D, Watson JE, Wilson RS, Zhang J, Bax NJ (2020c) The lower bathyal and abyssal seafloor fauna of eastern Australia. Marine Biodiversity Records 13: e11. https://doi.org/10.1186/s41200-020-00194-1
- Örsted AS (1842) Udtog af en Beskrivelse af Grönlands Annulata dorsibranchiata. Naturhistorisk Tidsskrift, Köbenhavn 4: 109–127. https://biodiversitylibrary.org/page/2322860
- Örsted AS (1843) Annulatorum danicorum conspectus. I. Maricolae, Hafniae Copenhagen: Sumtibus Librariae Wahlianae, 52 pp. http://www.biodiversitylibrary.org/bibliography/11849
- Otto AG (1820) De Sternaspide thalassemoideo et Siphostomate diplochaito vermibus duobus marinis. [Epistola Gratulatoria quam ad celebrandum diem laetissimum VI Marti MDCCCXX (etc, etc)]. Vratislaviae, 16 pp. [2 plates] https://www.marinespecies.org/polychaeta/aphia.php?p=sourcedetails&id=177296
- Parapar J, Besteiro C, Moreira J (2004) Familia Pilargidae Saint-Joseph, 1899. In: Ramos MA (Ed.) Fauna Iberica Vol. 25. Annelida Polychaeta I. Museo Nacional de Ciencias Naturales, Madrid, 267–293. http://www.fauna-iberica.mncn.csic.es/english/publicaciones/fi25.php
- Parapar J, Moreira J, Helgason GV (2011) Distribution and diversity of the Opheliidae (Annelida, Polychaeta) on the continental shelf and slope of Iceland, with a review of the genus Ophelina in northeast Atlantic waters and description of two new species. Organisms Diversity and Evolution 11: 83–105. https://doi.org/10.1007/s13127-011-0046-2
- Parapar J, Moreira J (2015) Six new species of the genus Armandia Filippi, 1861 (Polychaeta, Opheliidae) from Lizard Island (Great Barrier Reef, Australia). Zootaxa 4019: 577–603. https://doi.org/10.11646/zootaxa.4019.1.19
- Paterson GLJ, Glover AG (2000) A new species of Sigambra (Polychaeta, Pilargidae) from the abyssal plains of the NE Atlantic. Bulletin of the Natural History Museum, London (Zoology) 66: 167–170. http://www.biodiversitylibrary.org/page/41005372
- Paterson GLJ, Glover AG, Barrio Froján CRS, Whitaker A, Budaeva N, Chimonides J, Doner S (2009) A census of abyssal polychaetes. Deep Research Part II: Tropical Studies Oceanography 56: 1739–1746. https://doi.org/10.1016/j.dsr2.2009.05.018
- Paterson GLJ, Neal L, Altamira I, Soto EH, Smith CR, Menot L, Billett DSM, Cunha MR, Marchais-Laguionie C, Glover AG (2016) New Prionospio and Aurospio species from the deep-sea (Annelida: Polychaeta). Zootaxa 4092: 1–32. https://doi.org/10.11646/zootaxa.4092.1.1
- Paul C, Halanych KM, Tiedemann R, Bleidorn C (2010) Molecules reject an opheliid affinity for Travisia (Annelida). Systematics and Biodiversity 8: 507–512. https://doi.org/10.1080/14772000.2010.517810
- Paxton H (1974) Contribution to the study of the Australian Nephtyidae (Polychaeta). Records of the Australian Museum 29: 197–208. https://doi.org/10.3853/j.0067-1975.29.1974.226
- Paxton H (1986) Generic revision and relationships of the family Onuphidae (Annelida: Polychaeta). Records of the Australian Museum 38: 1–74. https://doi.org/10.3853/j.0067-1975.38.1986.175
- Paxton H (2000) Family Onuphidae. In: Beesley PL, Ross GJB, Glasby CJ (Eds) Polychaetes and Allies: The Southern Synthesis. Fauna of Australia: Volume 4A. Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing, Collingwood, 99–104. https://environment.gov.au/science/abrs/publications/fauna-of-australia/fauna-4a
- Paxton H, Davey A (2010) A new species of Ophryotrocha (Annelida: Dorvilleidae) associated with fish farming at Macquarie Harbour, Tasmania, Australia. Zootaxa 2509: 53–61. https://doi.org/10.11646/zootaxa.2509.1.4
- Paxton H, Budaeva N (2013) Paradiopatra (Annelida: Onuphidae) from eastern Australian waters, with the description of six new species. Zootaxa 3686(2): 140–164. https://doi.org/10.11646/zootaxa.3686.2.2
- Pettibone MH (1956) Some polychaete worms of the families Hesionidae, Syllidae and Nereidae from the east coast of North America, West Indies, and Gulf of Mexico. Journal of the Washington Academy of Sciences 46: 281–294. https://www.biodiversitylibrary.org/page/39693975
- Pettibone MH (1962) New species of polychaete worms (Spionidae: Spiophanes) from the east and west coast of North America. Proceedings of the Biological Society of Washington 75: 77–88. https://www.biodiversitylibrary.org/page/34571529
- Pettibone MH (1966) Revision of the Pilargidae (Annelida: Polychaeta), including descriptions of new species, and redescription of the pelagic Podarmus ploa Chamberlain (Polynoidae). Proceedings of the United States National Museum 118: 155–207. https://doi.org/10.5479/si.00963801.118-3525.155
- Pettibone MH (1967) Some bathyal polynoids from central and northeastern Pacific (Polychaeta: Polynoids). Proceedings of the United States National Museum 121: 1–15. https://doi.org/10.5479/si.00963801.121-3575.1
- Pettibone MH (1970) Two new genera of Sigalionidae (Polychaeta). Proceedings of the Biological Society of Washington 83: 365–386. https://repository.si.edu/handle/10088/3407
- Pettibone MH (1976) Revision of the genus Macellicephala McIntosh and the subfamily Macellicephalinae Hartmann-Schröder (Polychaeta: Polynoidae). Smithsonian Contributions to Zoology 229: 1–71. https://doi.org/10.5479/si.00810282.229
- Pettibone MH (1985) Polychaete worms from a cave in the Bahamas and from experimental wood panels in deep water of the North Atlantic (Polynoidae, Macellicephalinae, Harmothoinae). Proceedings of the Biological Society of Washington 98: 127–149. http://biodiversitylibrary.org/page/34648400
- Pettibone MH (1989) Polynoidae and Sigalionidae (Polychaeta) from the Guaymas Basin, with descriptions of two new species, and additional records from hydrothermal vents of the Galapagos Rift, 21°N, and seep-sites in the Gulf of Mexico (Florida and Lousiana). Proceedings of the Biological Society of Washington 102: 154–168. https://www.biodiversitylibrary.org/page/34606745
- Pettibone MH (1992) Contribution to the Polychaete Family Pholoidae Kinberg. Smithsonian Contributions to Zoology 532: 1–24. https://doi.org/10.5479/si.00810282.532
- Pixell HLM (1913) Polychaeta of the families Serpulidae and Sabellidae, collected by the Scottish National Antarctic Expedition. Transactions of the Royal Society of Edinburgh 49: 347–358. https://doi.org/10.1017/S0080456800003987
- Pleijel F (2001) Revision of Amphiduros Hartman, 1959 (Polychaeta, Hesionidae, Gyptini). Ophelia 54: 15–27. https://doi.org/10.1080/00785326.2001.10409453
- Pleijel F, Rouse GW, Ruta C, Wiklund H, Nygren A (2008) Vrijenhoekia balaenophila, a new hesionid polychaete from a whale fall off California. Zoological Journal of the Linnean Society 152: 625–634. https://doi.org/10.1111/j.1096-3642.2007.00360.x
- Pleijel F, Rouse GW, Sundkvist T, Nygren A (2012) A partial revision of Gyptis (Gyptini, Ophiodrominae, Hesionidae, Aciculata, Annelida), with descriptions of a new tribe, a new genus and five new species. Zoological Journal of the Linnean Society 165: 471–494. https://doi.org/10.1111/j.1096-3642.2012.00819.x
- Poore GCB, Just J, Cohen B (1994) Composition and diversity of Crustacea Isopoda of the southeastern Australian continental slope. Deep Sea Research Part I: Oceanographic Research Papers 41: 677–693. https://doi.org/10.1016/0967-0637(94)90049-3
- Poore GCB, Avery L, Błażewicz-Paszkowycz M, Browne J, Bruce NL, Gerken S, Glasby C, Greaves E, McCallum AW, Staples D, Syme A, Taylor J, Walker-Smith G, Warne M, Watson C, Williams A, Wilson RS, Woolley S (2015) Invertebrate diversity of the unexplored marine western margin of Australia: taxonomy and implications for global biodiversity. Marine Biodiversity 45: 271–286. https://doi.org/10.1007/s12526-014-0255-y
- Ponder W, Hutchings P, Chapman R (2002) Overview of the conservation of Australian marine invertebrates. A Report for Environment Australia. Australian Museum, Sydney. http://malsocaus.org/marine_invert/
- Przeslawski R, Glasby CJ, Nichol S (2018) Polychaetes (Annelida) of the Oceanic Shoals region, northern Australia: considering small macrofauna in marine management. Marine and Freshwater Research 70: 307–321. https://doi.org/10.1071/MF18060
- Quatrefages A de (1866) Histoire naturelle des Annelés marins et d’eau douce. Annélides et Géphyriens. Librarie Encyclopédique de Roret, Paris 1, 588 pp. https://doi.org/10.5962/bhl.title.122818
- Rafinesque CS (1815) Analyse de la nature ou Tableau de l’univers et des corps organisés. Palerme, 224 pp. https://doi.org/10.5962/bhl.title.106607
- Rainer SF, Hutchings PA (1977) Nephtyidae (Polychaeta: Errantia) from Australia. Records of the Australian Museum 31: 307–347. https://doi.org/10.3853/j.0067-1975.31.1977.216
- Ramirez-Llodra E, Tyler PA, Baker MC, Bergstad OA, Clark MR, Escobar E, Levin LA, Menot L, Rowden AA, Smith CR, Van Dover CL (2011) Man and the last great wilderness: human impact on the deep sea. PLoS ONE 6: e22588. https://doi.org/10.1371/journal.pone.0022588
- Ramos D (2019) Diversity and connectivity of deep-sea Annelids off the Eastern Australian coast: a preliminary assessment. Master’s thesis. Imperial College, London.
- Ravara A, Rizzo AE, Lana P (2017a [Online]) Family Nephtyidae Grube, 1850. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Walter de Gruyter GmbH & Co. KG, Berlin/Boston.
- Ravara A, Ramos D, Teixeira MAL, Costa FO, Cunha MR (2017b) Taxonomy, distribution and ecology of the order Phyllodocida (Annelida, Polychaeta) in deep-sea habitats around the Iberian margin. Deep Sea Research Part II: Tropical Studies in Oceanography 137: 207–231. https://doi.org/10.1016/j.dsr2.2016.08.008
- Ravara A, Cunha MR, Pleijel F (2010a) Nephtyidae (Annelida, Polychaeta) from southern Europe. Zootaxa 2682: 1–68. https://doi.org/10.11646/zootaxa.2682.1.1
- Ravara A, Wiklund H, Cunha MR, Pleijel F (2010b) Phylogenetic relationships within Nephtyidae (Polychaeta: Annelida). Zoologica Scripta 39: 394–405. https://doi.org/10.1111/j.1463-6409.2010.00424.x
- Ravara A, Cunha MR (2016) Two new species of scale worms (Polychaeta: Aphroditiformia) from deep-sea habitats in the Gulf of Cadiz (NE Atlantic). Zootaxa 4097: 442–450. https://doi.org/10.11646/zootaxa.4097.3.12
- Read G, Fauchald K [Eds] (2020) World Polychaeta database. Accessed through: World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=920 [on 2020–04–15]
- Reimers H (1933) Morphologie der Polychaetengattung Sphaerodorum. Monographie. Zoologische Jahrbücher, Abteilung für Systematik, Ökologie und Geographie der Tiere 64: 41–110. https://www.biodiversitylibrary.org/bibliography/8980#/summary
- Reuscher M, Fiege D, Wehe T (2009) Four new species of Ampharetidae (Annelida: Polychaeta) from Pacific hot vents and cold seeps, with a key and synoptic table of characters for all genera. Zootaxa 2191: 1–40.
- Reyss D (1971) Résultats scientifiques de la Campagne Polymède. II. – Polychètes Aphroditidae de profondeur en Méditerranée. Remarques systématiques et biogéographiques. Vie et Milieu, Série A 22: 243–258. https://archimer.ifremer.fr/doc/1971/publication-5029.pdf
- Rex MA, Etter RJ (2010) Deep-Sea Biodiversity: Pattern and Scale. Harvard University Press, 354 pp.
- Rintoul S, Feng M, Hardman-Mountford N, Raes E (2017) Australia’s ocean currents. In: Mapston BD (Ed.) Oceans: Science and Solutions for Australia. CSIRO, 13–24. http://hdl.handle.net/102.100.100/87597?index=1
- Rioja E (1917) Datos para le conocimiento de la fauna de Anélidos Poliquetos del Cantábrico. Trabajos del Museo Nacional de Ciencias Naturales. Serie Zoológica 29: 1–111.
- Rioja E (1923) Estudio sistemático de las Especies Ibéricas del suborden Sabelliformia. Trabajos del Museo Nacional de Ciencias Naturales 48: 5–144.
- Rizzo AE, Amaral ACZ (2004) Bathyglycinde profunda (Hartman and Fauchald) (Polychaeta, Goniadidae): new combination. Revista Brasileira de Zoologia 21: 937–942. https://doi.org/10.1590/S0101-81752004000400031
- Rizzo AE, Magalhães WF, Santos CSG (2016) Lacydoniidae Bergström, 1914 (Polychaeta) in the South Atlantic: morphology, three new species and five new records. Journal of the Marine Biological Association of the United Kingdom 96: 1265–1285. https://doi.org/10.1017/S0025315415001381
- Rizzo AE, Magalhães WF (2019 [Online]) Lacydoniidae Bergström, 1914. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Walter de Gruyter GmbH & Co. KG, Berlin/Boston.
- Rouse GW, Fauchald K (1997) Cladistics and polychaetes. Zoologica Scripta 26: 139–204. https://doi.org/10.1111/j.1463-6409.1997.tb00412.x
- Rouse GW, Pleijel F (2001) Polychaetes. Oxford University Press, Oxford, 354 pp.
- Ruderman L (1911) Recherches sur Ephesia gracilis Rathke, Annélide polychète de la famille des sphaerodorides; morphologie, anatomie, histology. Mémoires de la Société Zoologique de France 24: 1–96. https://www.biodiversitylibrary.org/part/39992#/summary
- Ruff RE, Brown B (1989) A new species of Euchone (Polychaeta: Sabellidae) from the northwest Atlantic with comments on ontogenetic variability. Proceedings of the Biological Society of Washington 102: 753–760. https://archive.org/details/biostor-74631
- Ryckholt P de (1851) Mélanges paléontologiques. Part 1. Memoires Couronnes et Memoires des Savants Etrangers de l’Academie Royale des Sciences, des Lettres et des Beaux-Arts de Belgique 24: 1–176. https://biodiversitylibrary.org/page/2718124
- Saint-Joseph AA de (1894) Les Annélides polychètes des côtes de Dinard. Troisième Partie. Annales des Sciences Naturelles, Paris, Série 7, 17: 1–395. https://biodiversitylibrary.org/page/35662416
- Saint-Joseph AA de (1899) Note sur une nouvelle famille d’Annélides Polychètes. Bulletin du Muséum d’Histoire Naturelle, Paris 5: 41–42. https://www.biodiversitylibrary.org/page/5029420
- Saiz Salinas JI, Bustamante M, Tajadura J (2018) A census of deep-water sipunculans (Sipuncula). Marine Biodiversity 48: 449–464. https://doi.org/10.1007/s12526-016-0568-0
- Salazar-Vallejo SI (2008) Revision of Poeobius meseres Heath, 1930 (Polychaeta: Flabelligeridae). Cahiers de Biologie Marine 49: 191–120. https://dx.doi.org/10.21411/CBM.A.6FB6F3A2
- Salazar-Vallejo SI (2011a) Revision of Piromis Kinberg, 1867 and Pycnoderma Grube, 1877 (Polychaeta: Flabelligeridae). Zootaxa 2819: 1–50. https://doi.org/10.11646/zootaxa.2819.1.1
- Salazar-Vallejo SI (2011b) Revision of Stylarioides delle Chiaje, 1831 (Annelida: Flabelligeridae). Italian Journal of Zoology 78: 163–200. https://doi.org/10.1080/11250003.2011.606985
- Salazar-Vallejo SI (2012a) Revision of Semiodera Chamberlin, 1919 (Polychaeta: Flabelligeridae). Zootaxa 3562: 1–62. https://doi.org/10.11646/zootaxa.3562.1.1
- Salazar-Vallejo SI (2012b) Revision of Trophoniella Hartman, 1959 (Polychaeta, Flabelligeridae). Zoosystema 34: 453–519. https://doi.org/10.5252/z2012n3a1
- Salazar-Vallejo SI (2012c) Revision of Flabelligera Sars, 1829 (Polychaeta: Flabelligeridae). Zootaxa 3203: 1–64. https://doi.org/10.11646/zootaxa.3203.1.1
- Salazar-Vallejo SI (2017) Revision of Brada Stimpson, 1853, and Bradabyssa Hartman, 1967 (Annelida, Flabelligeridae). Zootaxa 4343: 1–98. https://doi.org/10.11646/zootaxa.4343.1.1
- Salazar-Vallejo SI, Orensanz JM (2006) Pleijelius longae n. gen, n. sp., a remarkable deep water polychaete from the Northwestern Atlantic (Polychaeta: Hesionidae). Scientia Marina 70: 157–166. https://doi.org/10.3989/scimar.2006.70s3157
- Salazar-Vallejo SI, Gillet P (2007) Revision of Chauvinelia, redescriptions of Flabelliseta incrusta, and Helmetophorus rankini, and their recognition as acrocirrids (Polychaeta: Acrocirridae) Journal of the Marine Biological Association of the United Kingdom 87: 465–477. https://doi.org/10.1017/S0025315407054501
- Salazar-Vallejo SI, Zhadan AE (2007) Revision of Buskiella McIntosh, 1885 (including Flota Hartman, 1967), and description of its trifid organ (Polychaeta: Flotidae). Invertebrate Zoology 4: 65–82. https://doi.org/10.15298/invertzool.04.1.06
- Salazar-Vallejo SI, Buzhinskaja G (2011) Revision of Diplocirrus Haase, 1915, including Bradiella Rullier, 1965, and Diversibranchius Buzhinskaja, 1993 (Polychaeta, Flabelligeridae). ZooKeys 106: 1–45. https://doi.org/10.3897/zookeys.106.795
- Salazar-Vallejo S, Buzhinskaja G (2013) Six new deep-water sternaspid species (Annelida, Sternaspidae) from the Pacific Ocean. ZooKeys 348: 1–27. https://doi.org/10.3897/zookeys.348.5449
- Salazar-Vallejo SI, Angel de León J, Carrera-Parra LF (2019a) Phylogeny of Microphthalminae Hartmann-Schröder, 1971, and revision of Hesionella Hartman, 1939, and Struwela Hartmann-Schröder, 1959 (Annelida, Errantia). PeerJ 7: e7723 [1–35]. https://doi.org/10.7717/peerj.7723
- Salazar-Vallejo SI, Zhadan AE, Rizzo AE (2019b) Revision of Fauveliopsidae Hartman, 1971 (Annelida, Sedentaria). Zootaxa 4637: 1–67. https://doi.org/10.11646/zootaxa.4637.1.1
- San Martín G (2005) Exogoninae (Polychaeta, Syllidae) from Australia with the description of a new genus and twenty-two new species. Records of the Australian Museum 57: 39–152. https://doi.org/10.3853/j.0067-1975.57.2005.1438
- San Martín G, SAguado MT (2019 [Online]) Family Syllidae Grube. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Walter de Gruyter GmbH & Co. KG, Berlin/Boston.
- Santos SL (1977) A new species of Travisia (Polychaeta, Opheliidae) from Tampa Bay, Florida. Proceedings of the Biological Society of Washington 89: 559–564. https://www.biodiversitylibrary.org/page/34554019
- Sardá-Borroy R (1987) Sphaerodoridae (Annelida, Polychaeta) from the region of the Gibraltar Strait with description of Euritmia hamulisetosa gen. et sp. n. Zoologica Scripta 16(1): 47–50. https://doi.org/10.1111/j.1463-6409.1987.tb00051.x
- Sato-Okoshi W, Okoshi K, Fujiwara Y (2015) A new species of Protodrilus (Annelida: Protodrilidae), covering bone surfaces bright red, in whale-fall ecosystems in the northwest Pacific. Biological Bulletin 229: 209–219. https://doi.org/10.1086/BBLv229n2p209
- Savigny J-C (1822) Système des annélides, principalement de celles des côtes de l’Égypte et de la Syrie, offrant les caractères tant distinctifs que naturels des Ordres, Familles et Genres, avec la Description des Espèces. Description de l’Égypte ou Recueil des Observations et des Recherches qui ont été faites en Égypte pendant l’Expédition de l’Armée Française, publié par les Ordres de sa Majesté l’Empereur Napoléon le Grand, Histoire Naturelle, Paris 1: 1–128. http://biodiversitylibrary.org/page/41329897
- Schmarda LK (1861) Neue Wirbellose Thiere: Beobachtet und gesammelt auf einer Reise um die Erde 1853 bis 1857. In: Turbellarien, Rotatorien und Anneliden. Erster Band, Zweite Hälfte. Verlag von Wilhelm Engelmann, Leipzig, 66 pp. https://doi.org/10.5962/bhl.title.14426
- Schüller M (2008) New polychaete species collected during the expeditions ANDEEP I, II, and III to the deep Atlantic sector of the Southern Ocean in the austral summers 2002 and 2005 – Ampharetidae, Opheliidae, and Scalibregmatidae. Zootaxa 1705: 51–68. https://doi.org/10.11646/zootaxa.1705.1.4
- Schüller M, Hutchings PA (2012) New species of Terebellides (Polychaeta: Trichobranchidae) indicate long-distance dispersal between western South Atlantic deep-sea basins. Zootaxa 3254: 1–31. https://doi.org/10.11646/zootaxa.3254.1.1
- Schulze A, Boyle MJ, Kawauchi GY (2019) Sipuncula. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Annelida. Volume 1: Annelida Basal Groups and Pleistoannelida, Sedentaria I. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, 177–201. https://doi.org/10.1515/9783110291582-006
- Schulze A, Maiorova A, Timm LE, Rice ME (2012) Sipunculan larvae and “cosmopolitan” species. Integrative and Comparative Biology 52: 497–510. https://doi.org/10.1093/icb/ics082
- Sedgwick A (1898) A Student’s Textbook of Zoology. Swan Sonnenschein & Co. Ltd., London, 600 pp.
- Selenka E (1885) Report on the Gephyrea collected by HMS Challenger during the years 1873–76. Report on the Scientific Results of the Voyage of HMS Challenger, London. https://doi.org/10.5962/bhl.title.6513
- Selenka E, de Man JG, Bülow C (1883) Die Sipunculiden, eine systematische Monographie. Semper Reisen im Archipel der Philippinen 2, 4: 1–131.
- Sendall K, Salazar-Vallejo S (2013) Revision of Sternaspis Otto, 1821 (Polychaeta, Sternaspidae). ZooKeys 286: 1–74. https://doi.org/10.3897/zookeys.286.4438
- Sigovini M, Keppel E, Tagliapietra D (2016) Open Nomenclature in the biodiversity era. Methods in Ecology and Evolution 7: 1217–1225. https://doi.org/10.1111/2041-210X.12594
- Sikorski AV, Jirkov IA, Tzetlin AB (1988) The genus Laonice (Polychaeta, Spionidae) in the Arctic Ocean: weighing the taxonomic characters and species composition. Zoologichesky Zhurnal 67: 826–838.
- Solis-Weiss V (1993) Grassleia hydrothermalis, a new genus and species of Ampharetidae (Annelida: Polychaeta) from the hydrothermal vents off the Oregon coast (U.S.A.), at Gorda Ridge. Proceedings of the Biological Society of Washington 106: 661–665.
- Southern R (1913) Gephyrea of the coasts of Ireland. Scientific Investigations. Department of Agriculture and Technical Instruction for Ireland, Fisheries Branch 1912, 46 pp.
- Southward EC (1962) A new species of Galathealinum (Pogonophora) from the Canadian Arctic. Canadian Journal of Zoology 40: 385–389. https://doi.org/10.1139/z62-035
- Southward EC (2000) Class Pogonophora. In: Beesley PL, Ross GJB, Glasby CJ (Eds) Polychaetes and Allies: The Southern Synthesis. Fauna of Australia: Volume 4A. Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing, Collingwood, 331–351.
- Stephen AC, Edmonds SJ (1972) The Phyla Sipuncula and Echiura. Trustees of the British Museum (Natural History), London.
- Stiller J, Rousset V, Pleijel F, Chevaldonné P, Vrijenhoek RC, Rouse GW (2013) Phylogeny, biogeography and systematics of hydrothermal vent and methane seep Amphisamytha (Ampharetidae, Annelida), with descriptions of three new species. Systematics and Biodiversity 11(1): 35–65. https://doi.org/10.1080/14772000.2013.772925
- Stiller J, Tilic E, Rousset V, Pleijel F, Rouse GW (2020) Spaghetti to a tree: a robust phylogeny for Terebelliformia (Annelida) based on transcriptomes, molecular and morphological data. Biology 9(4): e73. https://doi.org/10.3390/biology9040073
- Strelzov VE (1973) Polychaete worms of the family Paraonidae Cerruti, 1909 (Polychaeta, Sedentaria). Akademia Nauk SSSR, Moscow, 170 pp. http://herba.msu.ru/shipunov/school/books/streltsov1973_paraonidae.pdf
- Struck TH, Halanych KM (2010) Origins of holopelagic Typhloscolecidae and Lopadorhynchidae within Phyllodocide (Phyllodocida, Annelida). Zoologica Scripta 39: 269–275. https://doi.org/10.1111/j.1463-6409.2010.00418.x
- Summers M, Pleijel F, Rouse GW (2015) Whale falls, multiple colonisations of the deep, and the phylogeny of Hesionidae (Annelida). Invertebrate Systematics 29: 105–123. https://doi.org/10.1071/IS14055
- Thiel D, Purschke G, Boggemann M (2011) Abyssal Fauveliopsidae (Annelida) from the South East Atlantic. Journal of Natural History 45(15–16): 923–937. https://doi.org/10.1080/00222933.2010.540046
- Tovar-Hernández MA (2008) Phylogeny of Chone Kröyer, 1856 (Polychaeta: Sabellidae) and related genera. Journal of Natural History 42: 2193–2226. https://doi.org/10.1080/00222930802254714
- Tovar-Hernández MA, Yáñez-Rivera B, Giangrande A, Gambi MC (2012) Notes on the species of Perkinsiana (Polychaeta: Sabellidae) from Antarctica with the description of P. brigittae sp. nov. Zootaxa 3485: 56–68. https://doi.org/10.11646/zootaxa.3485.1.4
- Uchida H (2004) Hesionidae (Annelida, Polichaeta [sic]) from Japan. I. Kuroshio Biosphere 1: 27–92.
- UNESCO (2009) Global Open Oceans and Deep Seabed (GOODS) – Biogeographic Classification. Paris, UNESCO, IOC Technical Series No. 84.
- Verrill AE (1873) XVIII. Report upon the invertebrate animals of Vineyard Sound and the adjacent waters, with an account of the physical characters of the region. Report on the condition of the sea fisheries of the south coast of New England 1: 295–778. https://doi.org/10.5962/bhl.title.11688
- von Nordheim H (1989) Six new species of Protodrilus (Annelida, Polychaeta) from Europe and New Zealand, with a concise presentation of the genus. Zoologica Scripta 18: 245–268. https://doi.org/10.1111/j.1463-6409.1989.tb00450.x
- Walker-Smith GK, Wilson RS (2003) Goniadidae (Polychaeta) – A DELTA database of genera, and Australian species. In: Wilson RS, Hutchings PA, Glasby CJ (Eds) Polychaetes: An Interactive Identification Guide. CSIRO Publishing, Melbourne.
- Watling L, Guinotte J, Clark MR, Smith CR (2013) A proposed biogeography of the deep ocean floor. Progress in Oceanography 111: 91–112. https://doi.org/10.1016/j.pocean.2012.11.003
- Watson Russell C (1991) Strepternos didymopyton Watson Russell in Bhaud and Cazaux, 1987 (Polychaeta: Chrysopetalidae) from experimental wooden panels in deep waters of the western Atlantic. Ophelia Supplement 5: 283–294.
- Watson C (2001) New genus and species of Chrysopetalidae (Polychaeta) from hydrothermal vents (south-western Pacific). The Beagle, Records of the Museums and Art Galleries of the Northern Territory 17: 57–66.
- Watson C (2020 [Online]) Chrysopetalidae Ehlers, 1864. In: Purschke G, Böggemann M, Westheide W (Eds) Handbook of Zoology, a Natural History of the Phyla of the Animal Kingdom. Walter de Gruyter GmbH & Co. KG, Berlin/Boston.
- Watson C (in prep.) Cryptic species complex Chrysopetalum multisetosa (sensu Hartmannn-Schröder, 1981) (Chrysopetalidae: Chrysopetalinae: Annelida) comprises six new species: three new species, pan-Australia, littoral depths; three new species, Bass Strait to New Zealand, shelf to slope depths (100–1000 m).
- Watson C, Chivers AJ, Narayanaswamy BE, Lamont P, Turnewitsch R (2014) Chrysopetalidae (Annelida: Phyllodocida) from the Senghor Seamount, north-east Atlantic: taxa with deep-sea affinities and morphological adaptations. Memoirs of Museum Victoria 71: 311–325. https://doi.org/10.24199/j.mmv.2014.71.24
- Watson C, Carvajal I, Sergeeva NG, Pleijel F, Rouse GW (2016) Free-living, calamyzin chrysopetalids (Annelida) from methane seeps, anoxic basins and whale falls. Zoological Journal of the Linnean Society 177: 700–719. https://doi.org/10.1111/zoj.12390
- Webster HE, Benedict JE (1887) The Annelida Chaetopoda from Eastport, Maine. U.S. Commission of Fish and Fisheries. Report of the United States Commissioner of Fisheries 13: 707–758. http://biodiversitylibrary.org/page/15839855
- Wehe T (2007) Revision of the scale worms (Polychaeta: Aphroditidae) occurring in the seas surrounding the Arabian Peninsula. Part II. Sigalionidae. Fauna of Arabia 23: 41–124.
- Weigert A, Golombek A, Gerth M, Schwarz F, Struck TH, Bleidorn C (2016) Evolution of mitochondrial gene order in Annelida. Molecular Phylogenetics and Evolution 94: 196–206. https://doi.org/10.1016/j.ympev.2015.08.008
- Weigert A, Helm C, Meyer M, Nickel B, Arendt D, Hausdorf B, Santos SR, Halanych KM, Purschke G, Bleidorn C, Struck TH (2014) Illuminating the base of the annelid tree using transcriptomics. Molecular Biology and Evolution 31: 1391–1401. https://doi.org/10.1093/molbev/msu080
- Wesenberg-Lund E (1948) Maldanidae (Polychaeta) from west Greenland waters. Meddelelser om Grønland 134(9): 1–58.
- Wiklund H, Neal L, Glover AG, Drennan R, Rabone M, Dahlgren TG (2019) Abyssal fauna of polymetallic nodule exploration areas, eastern Clarion-Clipperton Zone, central Pacific Ocean: Annelida: Capitellidae, Opheliidae, Scalibregmatidae, and Travisiidae. ZooKeys 883: 1–82. https://doi.org/10.3897/zookeys.883.36193
- Wiklund H, Nygren A, Pleijel F, Sundberg P (2005) Phylogeny of Aphroditiformia (Polychaeta) based on molecular and morphological data. Molecular Phylogenetics and Evolution 37: 494–502. https://doi.org/10.1016/j.ympev.2005.07.005
- Williams T (1852) Report on the British Annelida. Report of the British Association for the Advancement of Science 1851: 159–272. http://www.biodiversitylibrary.org/page/13846822
- Williams A, Althaus F, MacIntosh H, Loo M, Gowlett-Holmes K, Tanner JE, Sorokin SJ, Green M (2018) Characterising the invertebrate megafaunal assemblages of a deep-sea (200–3000 m) frontier region for oil and gas exploration: the Great Australian Bight, Australia. Deep Sea Research Part II: Topical Studies in Oceanography 157–158: 78–91. https://doi.org/10.1016/j.dsr2.2018.07.015
- Wilson RS (1984) Neanthes (Polychaeta: Nereididae) from Victoria with descriptions of two new species. Proceedings of the Royal Society of Victoria 96: 209–226.
- Wilson RS (1990) Prionospio and Paraprionospio (Polychaeta: Spionidae) from Southern Australia. Memoirs of the Museum Victoria 50: 243–274. https://doi.org/10.24199/j.mmv.1990.50.02
- Wilson RS (2000) Family Glyceridae. In: Beesley PL, Ross GJB, Glasby CJ (Eds) Polychaetes and Allies: The Southern Synthesis. Fauna of Australia: Volume 4A. Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing, Collingwood, 127–129.
- Wong E, Hutchings P (2015) New records of Pectinariidae (Polychaeta) from Lizard Island, Great Barrier Reef, Australia and the description of two new species. Zootaxa 4019: 733–744. https://doi.org/10.11646/zootaxa.4019.1.25
- Worsaae K, Nygren A, Rouse GW, Giribet G, Persson J, Sundberg P, Pleijel F (2005) Phylogenetic position of Nerillidae and Aberranta (Polychaeta, Annelida), analysed by direct optimization of combined molecular and morphological data. Zoologica Scripta 34: 313–328. https://doi.org/10.1111/j.1463-6409.2005.00190.x
- Yokoyama H (2007) A revision of the genus Paraprionospio Caullery (Polychaeta: Spionidae). Zoological Journal of the Linnean Society 151: 253–284. https://doi.org/10.1111/j.1096-3642.2007.00323.x
- Zanol J, Hutchings PA, Fauchald K (2020) Eunice sensu latu (Annelida: Eunicidae) from Australia: description of seven new species and comments on previously reported species of the genera Eunice, Leodice and Nicidion. Zootaxa 4748: 1–43. https://doi.org/10.11646/zootaxa.4748.1.1
- Zardus JD, Etter RJ, Chase MR, Rex MA, Boyle EE (2006) Bathymetric and geographic population structure in the Pan-Atlantic deep-sea bivalve Deminuculaatacellana (Schenck, 1939). Molecular Ecology 15: 639–651. https://doi.org/10.1111/j.1365-294X.2005.02832.x
- Zenkevitch LA (1958) The deep sea echiurids of the north-western part of the Pacific Ocean. Trudy Instituta Okeanologii AN SSR 27: 192–203.
- Zhang J, Hutchings P (2019) A revision of Australian Pectinariidae (Polychaeta), with new species and new records. Zootaxa 4611: 1–70. https://doi.org/10.11646/zootaxa.4611.1.1
- Zhang J, Hutchings P, Kupriyanova E (2019) A revision of the genus Petta (Polychaeta Pectinariidae), with two new species from the abyss of south-eastern Australia, and comments on the phylogeny of the family. Zootaxa 4614: 303–333. https://doi.org/10.11646/zootaxa.4614.2.3
- Zhang J, Hutchings P, Burghardt I, Kupriyanova E (2020) Two new species of Sabellariidae (Annelida, Polychaeta) from the abyss of eastern Australia. Zootaxa 4821(3): 487–510. https://doi.org/10.11646/zootaxa.4821.3.4
- Zibrowius H (1973) Revision of some Serpulidae (Annelida, Polychaeta) from abyssal depths in the Atlantic and Pacific, collected by the “Challenger” and Prince of Monaco Expedition. Bulletin of British Museum of Natural History (Zoology) 24: 427–439.
- Zrzavý J, Říha P, Piálek L, Janouškovec J (2009) Phylogeny of Annelida (Lophotrochozoa): total evidence analysis of morphology and six genes. BMC Evolutionary Biology 9: e189. https://doi.org/10.1186/1471-2148-9-189
Appendix 1
Note added in proofs: Microphthalmidae Hartmann-Schröder, 1971 was accepted as a valid family in the World Register of Marine Species on 27 June 2020. [Read G, Fauchald K [Ed.] (2021) World Polychaeta database. Microphthalmidae Hartmann-Schröder, 1971. Accessed through: World Register of Marine Species at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=322549 on 2021-01-25].
Supplementary materials
Annelid identifications from IN2017_V03_Darwin Core format
Data type: occurrences
Explanation note: species occurrences along with museum registration numbers.
Spionidae characters
Data type: morphological
Explanation note: Comparison of morphological characters of Prionospio species from IN2017_V03.