Citation: Ševčík J, Hippa H, Wahab RA (2014) Diversity of Manota Williston (Diptera, Mycetophilidae) in Ulu Temburong National Park, Brunei. ZooKeys 428: 57–77. doi: 10.3897/zookeys.428.7912
A total of 15 species of Manota Williston, 1896 are recorded from Brunei, based on the investigations in 2013-2014. Thirteen species are recorded from Ulu Temburong National Park and three species from the Universiti Brunei Darussalam Campus in Tungku. Six species are described as new to science: Manota belalongensis sp. n., M. kaspraki sp. n., M. macrothrix sp. n., M. megachaeta sp. n. and M. pileata sp. n. from Ulu Temburong, and M. ricina sp. n. from Tungku. New records of the following species are given: Manota bifida Hippa & Papp, M. bruneiensis Hippa & Ševčík, M. hyboloma Hippa & Ševčík, M. oligochaeta Hippa, M. pappi Hippa, M. perangulata Hippa & Ševčík, M. pollex Hippa, M. procera Hippa and M. simplex Hippa.
Insecta, Sciaroidea, Manotinae, fungus gnats, taxonomy, new species, Borneo, Belalong, Tungku, Oriental region
Fungus gnats (Diptera: Sciaroidea) represent one of the most abundant and diverse groups of insects in forest habitats, in both temperate and tropical regions. Within Sciaroidea, the family Mycetophilidae belongs to the most species rich groups, with some 4500 described species worldwide and possibly the same number of species still awaiting description. The predominantly tropical subfamily Manotinae form a rather uniform and well-defined group that has been proved to be monophyletic in studies based on both morphological (
The specimens of Manota can be easily identifiable among other mycetophilids in collections due to their small size and characteristically reduced wing venation (Fig. 1). On the other hand, the species identification is rather difficult because it is based on the study of male terminalia under a relatively high magnification.
Habitus of Manota kaspraki sp. n. (Photo by D. Kaspřák).
The mycetophilid fauna of Borneo, as well as of the entire Indomalayan Archipelago is still poorly known. Concerning Brunei, only several named species of Mycetophilidae (see
In 2013, collaboration between Universiti Brunei Darussalam and the University of Ostrava was established, resulting in two field trips to Ulu Temburong National Park (February 2013 and January 2014). This contribution is the first from a series of ecological and taxonomic papers devoted to the study of this well preserved and highly diverse lowland rainforest in Brunei. Its aim is to describe 5 new species of Manota from Ulu Temburong National Park, to record 9 additional species and discuss the potential species richness in this area. The opportunity is also taken to describe a new species from the university campus near the capital of Brunei, Bandar Seri Begawan, outside the Temburong District.
Most of the material examined was collected with Malaise traps. A total of six Malaise traps (Fig. 2) were operated in the area in 5–17.ii.2013 and 7–18.i.2014. Additional samples were obtained by sweeping the undergrowth of the rainforest with an entomological net.
Malaise trap in the rainforest around Kuala Belalong Field Studies Centre (Photo by J. Ševčík).
The material was collected into and preserved in ethanol. The abdomen or only the apical part of the abdomen was detached from the specimen and macerated in warm concentrated potassium hydroxide (KOH). We also detached the hypopygium beyond segment 8. After washing in water and dehydration in stages of increasing concentrations of alcohol we mounted them in ‘Euparal’ between two pieces of cover slip, which allows the specimen to be studied from both sides under a compound microscope. These preparations are now attached to normal microscope slides by two strips of adhesive tape across their edges and are easily detached when needed. Other parts of the body were not macerated, but after dehydration we mounted the whole flies as they were in ‘Euparal’, lying on their side. The descriptions of the hypopygium should only be taken as rough guidelines to interpret the drawings. The morphological terminology follows our earlier papers (
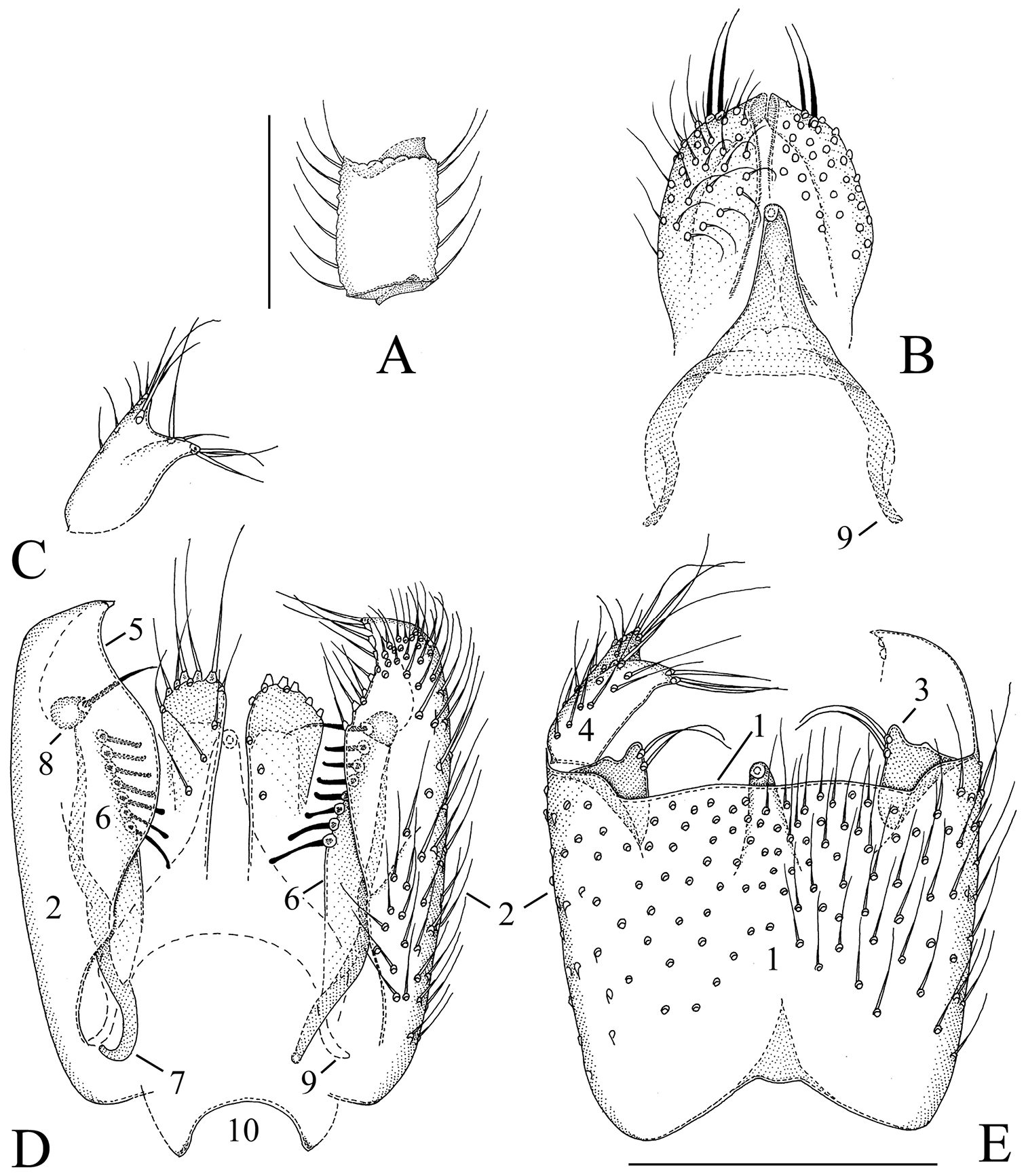
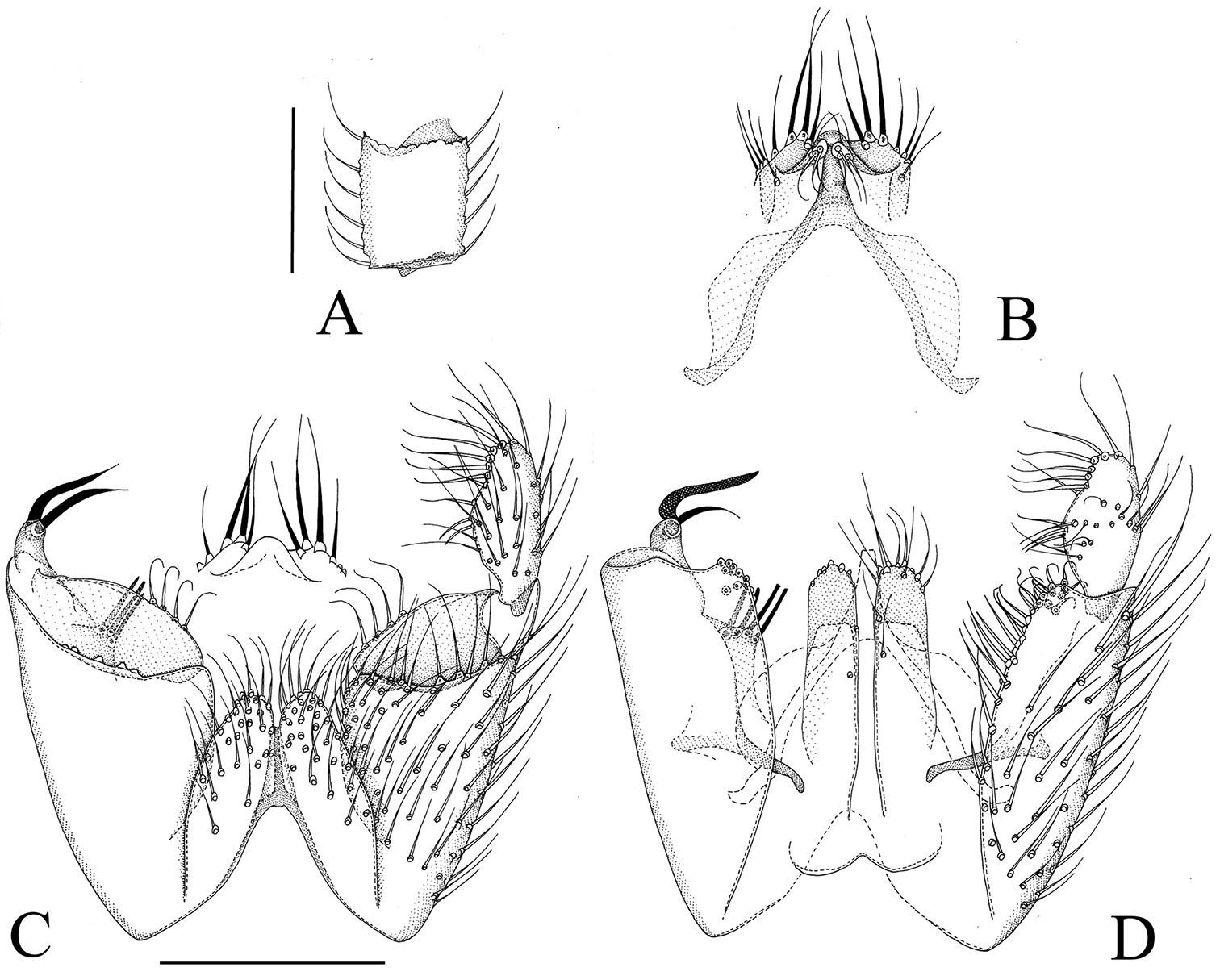
Manota belalongensis sp. n. (holotype). A Antennal flagellomere 4, lateral view B Aedeagus and hypoproct. ventral view C Gonostylus, dorsal view D Hypopygium, dorsal view E Hypopygium, ventral view. Scale 0.1 mm. 1 = sternite 9, 2 = gonocoxa, 3 = parastylar lobe, 4 = gonostylus, 5 = dorsal mesial margin of gonocoxa, 6 = plate-like lobe ventrally from the dorsal mesial margin of gonocoxa, 7 = gonocoxal apodeme, 8 = juxtagonostylar seta, 9 = aedeagal apodeme, 10 = tergite 9.
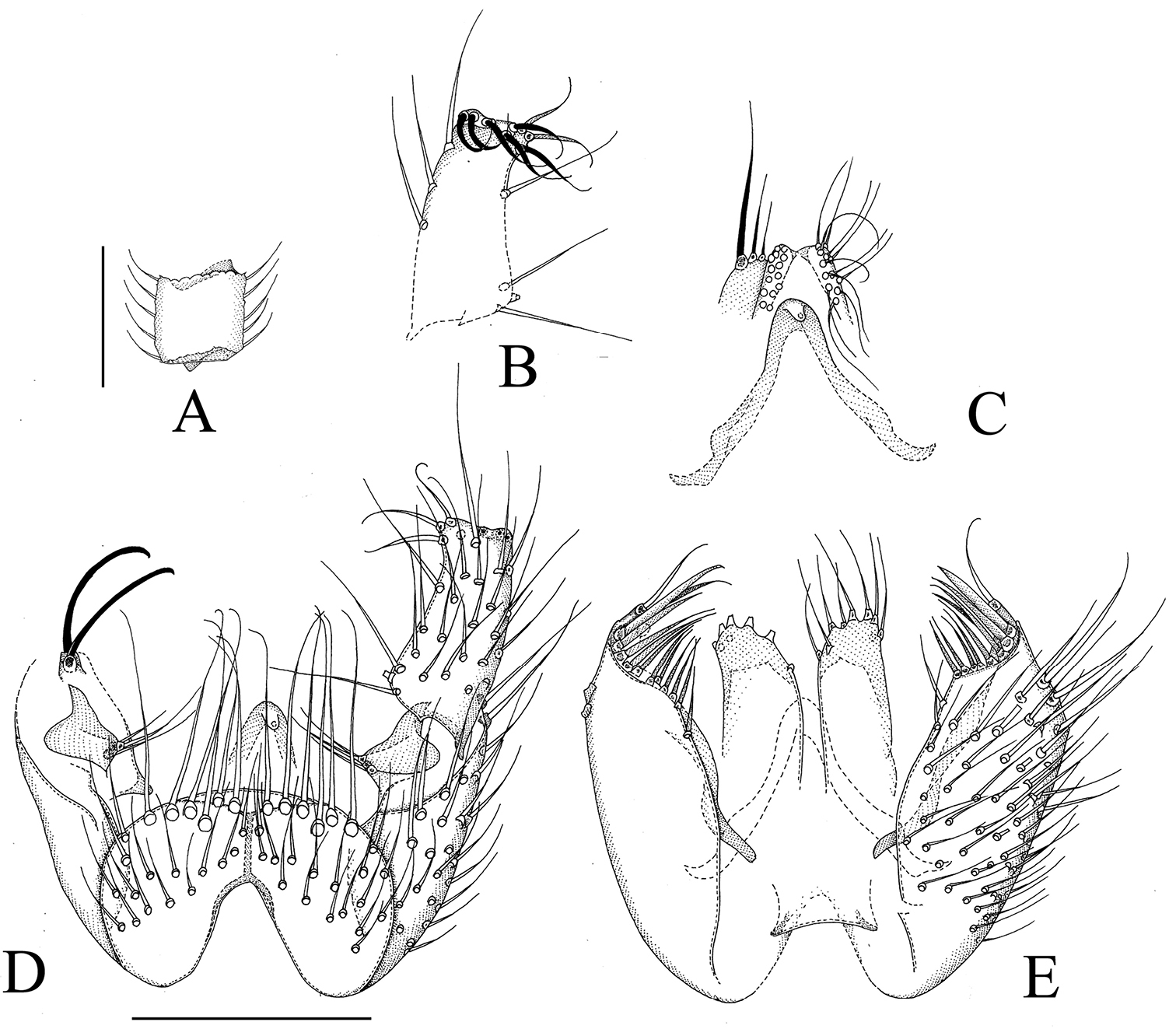
Manota kaspraki sp. n. (holotype). A Antennal flagellomere 4, lateral view B Gonostylus, dorsal view C Aedeagus and hypoproct, ventral view D Hypopygium, ventral view E Hypopygium, dorsal view. Scale 0.1 mm. 1 = sternite 9, 2 = gonocoxa, 3 = ventral mesial margin of gonocoxa, 4 = parastylar lobe, 5 = paraapodemal lobe, 6 = gonostylus, 7 = dorsal mesial margin of gonocoxa, 8 = lobe at dorsal mesial/apical margin of gonocoxa, 9 = gonocoxal apodeme, 10 = juxtagonostylar setae, 11 = aedeagus, 12 = aedeagal apodeme, 13 = membranous lobe, 14 = cerci, 15 = ventral seta of hypoproct (sternite 10), 16 = tergite 9.
The material is deposited in the following collections: Universiti Brunei Darussalam, Brunei (UBDC), University of Ostrava, Czech Republic (UOSC), and Natural History Museum, London, Great Britain (BMNH).
The study area is located at the Kuala Belalong Field Studies Centre (KBFSC), a research field station of the Universiti Brunei Darussalam. It is situated in the Ulu Temburong National Park; geographic coordinates are 4°33'N, 115°10'E, elevations in the KBFSC surroundings range from 60 (the station) to ca 300 m asl. The topography is very rugged, with narrow ridges and steep slopes. The site represents pristine lowland mixed dipterocarp forest, only slightly touched by human activities (
Male. Colour. Head dark brown, face and clypeus paler brown. Antenna brown, scapus and pedicellus paler brown. Mouthparts yellowish. Thorax brown, preepisternum 2 ventrally and episternum 3 paler yellowish. Legs yellowish, apex of coxa 2 and 3, trochanter 3 and basal third of femur 3 infuscated. Wing brownish, halter pale brown with blackish knob. Abdomen brown, sternites paler than tergites. All setosity pale, yellowish or brownish. Head. Antennal flagellomere 4, Fig. 3A. Palpomere 3 of maxillary palpus with apico-mesial thumb-like extension, with 4 apically expanded and curved sensilla; palpomere 4 with parasegment; palpomere 5 is 1.5 times as long as palpomere 4. Number of strong postocular setae 10. Thorax. Anepisternum setose, with 56 setae; anterior basalare setose, with 8 setae; preepisternum 2 non-setose; laterotergite setose, with 37 setae; episternum 3 setose, with 6 setae. Legs. Mid and hind tibial organs absent. Wing. R1 meeting C well on the basal half of the costal margin; the sclerotized part of M2 extending near to the level of the tip of R1; wing length 1.4 (1.5) mm. Hypopygium. Fig. 3B–E: Sternite 9 laterally fused with gonocoxa, posterior margin extending well over the middle of gonocoxa, near to the base of gonostylus, anterior margin incised, the setae similar to ventral setae of the gonocoxa. Ventral mesial margin of gonocoxa short, simple, the ventral setae of gonocoxa unmodified. Parastylar lobe subtriangular, with 2 setae at postero-mesial margin. Paraapodemal lobe not identifiable. Dorsal mesial margin of gonocoxa simple, posteriorly slightly sigmoid in shape, without megasetae or other stronger setae. Gonocoxa without a postero-lateral lobe. The dorsal setae of gonocoxa unmodified, similar to those on the ventral side. One juxtagonostylar seta present; it is a curved acute megaseta arising from oval basal body which is ca. one third of the length of the seta. Anteriorly from the juxtagonostylar megaseta there is a plate-like lobe bearing a row of 7 blunted rather thin megasetae. Gonostylus small, elongate, apically bilobed, the ventral side setose, dorsal side non-setose, the apical lobes with a few long setae which are not much stronger than the other gonostylar setae. Aedeagus elongate subtriangular, with weak lateral shoulders, the apex curved ventrad. Hypoproct large, extending posteriorly to the apices of gonostyli, number of its ventral setae (sternite 10) ca. 30 on each half, these setae widely distributed over the ventral surface. Cerci mesially separate.
Female unknown.
Manota belalongensis belongs to a large group, world-wide in distribution, in which male sternite 9 is long and laterally fused with the gonocoxa. In the key to Oriental and Palaearctic Manota (
The species is named after the river Belalong at which the type locality is situated.
Holotype. Male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 4°32'50"N, 115°09'28"E, 8–18.i.2014, primary lowland rainforest, Malaise trap 4, J. Ševčík & D. Kaspřák leg. (in UBDC).
Male. Colour. Head pale brown, face and clypeus pale yellowish. Antenna brown, scapus and pedicellus paler. Mouthparts yellowish. Thorax pale brown, preepisternum 2 pale yellowish. Legs yellowish. Wing brownish, halter brownish with blackish knob. Abdomen pale brown, sternites paler than tergites. All setosity pale, yellowish or brownish. Head. Antennal flagellomere 4, Fig. 4A. Palpomere 3 of maxillary palpus with apicomesial thumb-like extension, with 3 apically expanded and curved sensilla; palpomere 4 with parasegment; palpomere 5 ca. 1.4 times longer than palpomere 4. Number of strong postocular setae 10–11. Thorax. Anepisternum setose, with 14–31 setae; anterior basalare setose, with 6–10 setae; preepisternum 2 setose, with 8–11 setae, laterotergite non-setose; episternum 3 setose, with 2–3 setae. Legs. Mid and hind tibial organs absent. Wing. R1 meeting C well on the basal half of the costal margin; the sclerotized part of M2 extending near to the level of the tip of R1; wing length 1.3–1.5 (1.1–1.4) mm. Hypopygium. Fig. 4B–E: Sternite 9 about one third of ventral length of gonocoxa, with sharply delimited convex sides, posterior margin transverse with a wide submembranous notch, anterior margin shallowly incised, the setae similar to adjacent ventral setae of gonocoxa. Ventral mesial margin of gonocoxa simple, sigmoid. Parastylar lobe large, almost semicircular, with 5–7 setae scattered on the ventral surface. Paraapodemal lobe oval, at least partly covered by gonocoxa and parastylar lobe. The dorsal mesial margin of gonocoxa simple, convex, contiguous with the simple oblique posterior margin; posteriorly at the dorsal mesial margin there is a finger-like lobe apically bearing a stronger and a weaker seta; this lobe is very similar to the juxtagonostylar setae in appearance. Two juxtagonostylar setae, one an unmodified acute megaseta, the other a usual seta, both arising from a common basal body which is about one half of the length of the setae. Gonostylus elongate, slightly angled, with sub-basal lateral rounded lobe, the setosity confined to the lateral lobe and the apical part, some of the setae shorter but strong (spine-like megasetae). Aedeagus short, with strong lateral shoulders, the apex straight, not curved ventrad; on the ventral side of aedeagus (Fig. 4C–E) there is a membranous lobe, which may belong to aedeagus. Hypoproct posteriorly extending near to the apex of gonostylus, unusually narrow; ventrally with one seta on each side (sternite 10), postero-dorsally with two strong setae on each side, the microtrichia unusually long. Cerci mesially fused.
Female unknown.
Manota kaspraki is not especially similar to any other described Manota. In the key to Oriental and Palaearctic Manota it runs to couplet 38 by the following characters: anepisternum setose, preepisternum 2 setose, laterotergite non-setose, anterior basalare setose, gonostylus unilobed and straight (not geniculate). Couplet 38 leads to a large number of species (18). The acute spine-like megasetae apically on the curiously hump-sided gonostylus and the narrow long microtrichose hypoproct distinguish Manota kaspraki from any hitherto described Manota. The inflated paraapodemal lobe is reminiscent of e.g. Manota vesicaria Hippa, 2009 from Thailand, but otherwise the species are not much similar.
The species is named after Mr. David Kaspřák, a PhD student at the University of Ostrava, who participated in both the expeditions to Brunei, helped with the installation of Malaise traps and with other field activities.
Holotype. Male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 4°32'50"N, 115°09'28"E, 7–18.i.2014, primary lowland rainforest, Malaise trap 1 (night), D. Kaspřák & J. Ševčík leg. (in UBDC).
Paratypes. 2 males with same data as holotype except Malaise trap 1 (ODP) (in UBDC and UOSC); 2 males, the same data except sweeping February 2013 (in BMNH).
Male. Colour. Head brown, face and clypeus paler brown. Antenna brown. Mouthparts yellowish. Thorax pale brown, preepisternum 2 ventrally pale yellowish. Legs yellowish, femur 3 with a very slight indication of infuscation. Wing brownish, halter pale brown with blackish knob. Abdomen pale brown, sternites paler than tergites. All setosity pale, yellowish or brownish. Head. Antennal flagellomere 4, Fig. 5A. Palpomere 3 of maxillary palpus with apico-mesial thumb-like extension, with 5 apically expanded and curved sensilla; palpomere 4 with parasegment; palpomere 5 is 1.3 times as long as palpomere 4. Number of strong postocular setae 10–11. Thorax. Anepisternum setose, with 34–55 setae; anterior basalare non-setose; preepisternum 2 setose, with 15 setae, laterotergite non-setose; episternum 3 setose, with ca. 9 setae. Legs. Mid and hind tibial organs absent. Wing. R1 meeting C well on the basal half of the costal margin; the sclerotized part of M2 extending near to the level of the tip of R1; wing length 1.4–1.6 (1.5–1.7) mm. Hypopygium. Fig. 5B–E: Sternite 9 about one half of ventral length of gonocoxa, with sharply delimited convex sides, posterior margin not well seen in the holotype because the dorsal membranous area extruded, anterior margin deeply incised, the setae similar to adjacent ventral setae of gonocoxa. Ventral mesial margin of gonocoxa simple, sigmoid. Parastylar lobe oblique, subtriangular, with 3 setae antero-mesially (at apex). Paraapodemal lobe well exposed in ventral view. The dorsal mesial margin of gonocoxa simple, convex, posteriorly with a group of tightly placed setae. Gonocoxa posterolaterally with an apically setose apophysis/lobe. Two juxtagonostylar setae, both long apically curved megasetae, both arising from a common basal body, about one third of the length of megasetae. Gonostylus elongate and slightly curved, with moderately long setosity ventrally and dorsally, without megasetae or other setae deviating from the general setosity. Aedeagus short subtriangular, with distinct lateral shoulders, the apex curved ventrad. Hypoproct posteriorly extending to level of middle of gonostylus, the ventral part divided into two elongate oval lobes (sternite 10) covered by ca 30 very long setae on each half, postero-dorsally with a few both fine and strong setae. Cerci mesially separate.
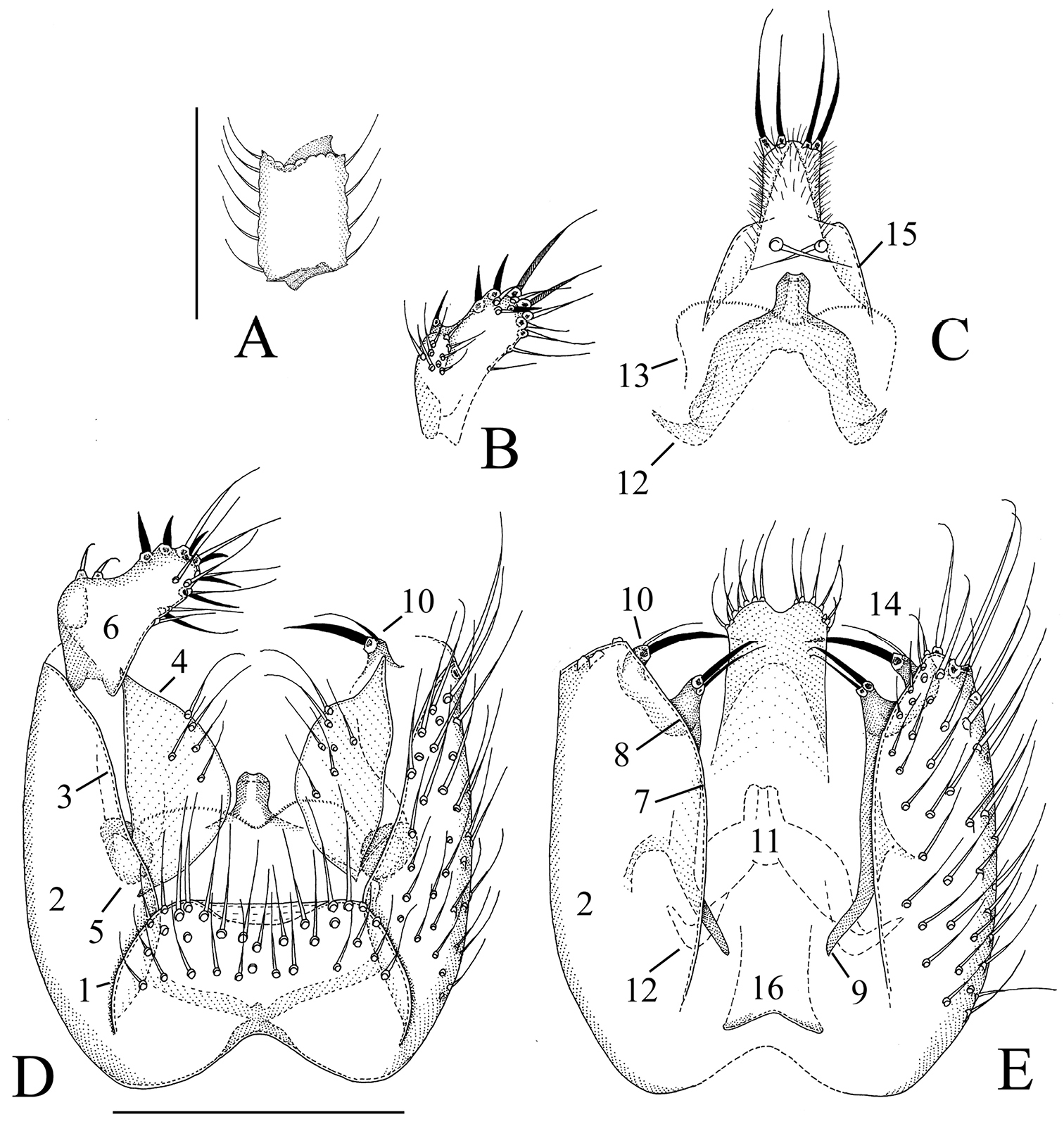
Manota macrothrix sp. n. (holotype). A Antennal flagellomere 4, lateral view B Gonostylus, dorsal view C Aedeagus and hypoproct, ventral view D Hypopygium, ventral view E Hypopygium, dorsal view. Scale 0.1 mm.
Female unknown.
Manota macrothrix belongs to a large group of species, common in the Oriental region, all of which have a well-developed apico-mesial thumb-like extension on palpomere 3, a setose anepisternum and preepisternum 2, a non-setose anterior basalare and laterotergite, a short vein R1, laterally free tergite 9, an oblique sickle-shaped or subtriangular parastylar lobe, well developed paraapodemal lobe, an apically setose apophysis at the apico-dorsal margin of the gonocoxa, two juxtagonostylar megasetae arising from a common basal body and a rather unmodified elongate gonostylus. Within this group Manota macrothrix is similar to Manota dolichothrix Hippa & Ševčík, 2010 from Sabah by having extremely long setae ventrally on the hypoproct (on sternite 10) but differs in having these setae widely scattered on each half of the hypoproct, while they are in a single row on each half in Manota dolichothrix. Further, in Manota macrothrix the subapical setae of gonostylus are shorter, less than twice longer than the medial width of gonostylus, while in Manota dolichothrix they are almost three times as long as the width of gonostylus. There are many minor differences between the two species (Fig. 5B–E,
The name is a Latinized Greek noun, macrothrix, long-hair, referring to the very long setae on the hypoproct.
Holotype. Male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 4°32'50"N, 115°09'28"E, 7–17.i.2014, primary lowland rainforest, Malaise trap 2, J. Ševčík & D. Kaspřák leg. (in UBDC).
Paratype. 1 male, Brunei, Ulu Temburong, 14.ii – 9.iii 1982, Malaise trap, M. C. Day leg. (in BMNH).
Male. Colour. Head brown, face and clypeus paler brown. Antenna brown, scapus and pedicellus paler brown. Mouthparts yellowish. Thorax pale brown, preepisternum 2 ventrally paler yellowish. Legs yellowish. Wing yellowish brown, halter pale brown with blackish knob. Abdomen pale brown, sternites paler than tergites. All setosity pale, yellowish or brownish. Head. Antennal flagellomere 4, Fig. 6A. Palpomere 3 of maxillary palpus with apicomesial thumb-like extension, with 5 apically expanded and curved sensilla; palpomere 4 with parasegment; palpomere 5 is 1.3 times as long as palpomere 4. Number of strong postocular setae ca 9. Thorax. Anepisternum setose, with 33 setae; anterior basalare non-setose; preepisternum 2 setose, with 14 setae, laterotergite non-setose; episternum 3 setose, with ca. 11 setae. Legs. Mid and hind tibial organs absent. Wing. R1 meeting C well on the basal half of the costal margin; the sclerotized part of M2 extending near to the level of the tip of R1; wing length 1.4 (1.5) mm. Hypopygium. Fig. 6B–E: Sternite 9 about one half of ventral length of gonocoxa, with sharply delimited convex sides, posterior margin convex, anterior margin deeply incised, the setae posteriorly stronger than anteriorly. Ventral mesial margin of gonocoxa simple, concave. Parastylar lobe oblique, subtriangular, with 3 setae anteriorly (at apex). Paraapodemal lobe well exposed in ventral view. The dorsal mesial margin of gonocoxa simple, convex, postero-mesially with a weak setose lobe; posterior margin simple, at posterior margin on a more ventral level with a finger-like lobe bearing two apical and one sub-basal acute curved megasetae. Generally the dorsal setae of gonocoxa similar to the ventral ones. Two juxtagonostylar setae, both long curved acute megasetae arising from a common low inconspicuous basal body. Gonostylus oval, with moderately long setosity ventrally, the dorsal side non-setose except for a transverse row of megasetae on apical half, the megasetae acute and increasing in length towards the mesial margin. Aedeagus short subtriangular, with distinct lateral shoulders, the apex curved ventrad; in Fig. 6C the medially visible part is vas deferens. Hypoproct posteriorly extending to level of base of gonostylus, the ventral part divided into two elongate lobes (sternite 10) with 4 setae each, postero-dorsally with a few both fine and strong setae. Cerci mesially separate.
Manota megachaeta sp. n. (holotype). A Antennal flagellomere 4, lateral view B Gonostylus, dorsal view C Aedeagus and hypoproct, ventral view D Hypopygium, ventral view E Hypopygium, dorsal view. Scale 0.1 mm.
Female unknown.
By the following characters Manota megachaeta runs to couplet 65 in the key to Oriental and Palaearctic Manota (
The name is a Latinized Greek noun, megachaeta, large seta, referring to the unusually long megasetae on the gonostylus.
Holotype. Male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 4°32'50"N, 115°09'28"E, 7–18.i.2014, primary lowland rainforest, Malaise trap 1 (DOP), J. Ševčík leg. (in UBDC).
Male. Colour. Head brown, face and clypeus paler brown. Antenna brown. Mouthparts yellowish. Thorax brown, preepisternum 2 and episternum 3 ventrally paler yellowish. Legs yellowish, apical fourth of femur 3 infuscated. Wing brownish, halter pale brownish with blackish knob. Abdomen brown, sternites paler than tergites. All setosity pale, yellowish or brownish. Head. Antennal flagellomere 4, Fig. 7A. Palpomere 3 of maxillary palpus with apico-mesial thumb-like extension, with 4 apically expanded and curved sensilla; palpomere 4 with parasegment; palpomere 5 is 1.7 times as long as palpomere 4. Number of strong postocular setae 11. Thorax. Anepisternum setose, with 33 setae; anterior basalare setose, with 12 setae; preepisternum 2 setose, with ca. 17 setae, laterotergite non-setose; episternum 3 setose, with ca. 12 setae. Legs. Mid tibial organ absent; hind tibial organ present. Wing. R1 meeting C well on the basal half of the costal margin; the sclerotized part of M2 extending near to the level of the tip of R1; wing length 1.5 (1.6) mm. Hypopygium. Fig. 7B–D: Sternite 9 about one half of ventral length of gonocoxa, with sharply delimited convex sides, posterior margin with narrow deep cleft, anterior margin deeply incised, the setae similar to adjacent ventral setae of gonocoxa. Ventral mesial margin of gonocoxa angled. Parastylar lobe large, elongate oval, oblique, with 4 setae at mesial margin. Paraapodemal lobe not identified; at the place where a paraapodemal lobe is usually visible there is a plate-like lobe with three megasetae. The dorsal mesial margin of gonocoxa simple, convex, posteriorly forming a weak lobe with marginal and ventral setae; posterior margin of gonocoxa transverse, simple. The dorsal setae of gonocoxa similar to ventral ones. Two juxtagonostylar setae, both long curved megasetae, the dorsal one stronger than the ventral one, both arising from a common basal body, about one half of the length of the stronger megaseta. Gonostylus elongate oval, with moderately long setosity ventrally and dorsally, fewer and partly weaker setae ventrally, the apico-mesial setae longer than the others, at the middle of mesial margin a few setae which are thick, rather short and which differ from the other setosity. Aedeagus short subtriangular, with distinct lateral shoulders, the apex curved ventrad. Hypoproct posteriorly extending to level of middle of gonostylus, the ventral part (sternite 10) posteriorly with non-setose lobe which have a pair of small oval processes anteriorly bearing three setae each. These processes partly surrounding the apex of aedeagus. Postero-dorsal part of hypoproct with a few both fine and strong setae. Cerci mesially separate.
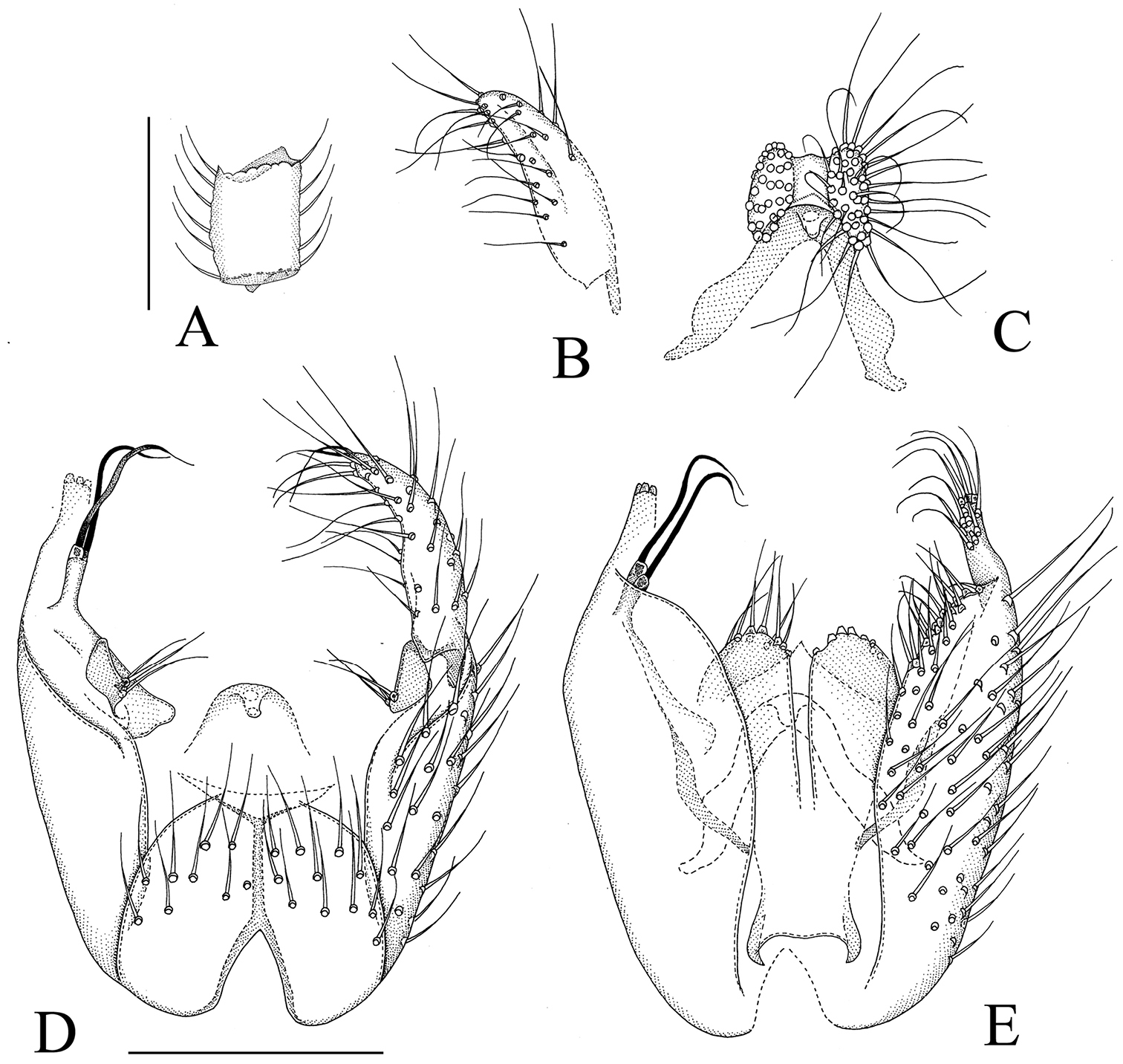
Manota pileata sp. n. A Antennal flagellomere 4, lateral view B Aedeagus and hypoproct, ventral view C Hypopygium, ventral view D Hypopygium, dorsal view. Scale 0.1 mm.
Female unknown.
In the key to Oriental and Palaearctic Manota, Manota pileata runs through couplet 45 to couplet 50 including the Eastern-Palaearctic Manota indahae Hippa & Kjaerandsen, 2010 by the following characters: anepisternum setose, preepisternum 2 setose, laterotergite non-setose, gonostylus one-lobed, parastylar lobe present and cerci medially separate, gonostylus without blunt-ended megasetae on apical half, gonostylus without comb-like row of five setae subbasally at the ventral mesial margin, aedeagus apically narrow, without ear-like apico-lateral lobes, parastylar lobe in anterior–posterior direction short, at most twice as long as broad, aedeagus apically symmetrical, the setae medio-dorsally on gonostylus fine, not deviating from the other gonostylar setosity and the dorsal mesial margin of gonocoxa without a thumb-like lobe posteriorly. The two species are not very similar: Manota pileata is distinguished from Manota indahae e.g. by the following characters: parastylar lobe has only 4 setae (numerous in Manota indahae), dorsally from the parastylar lobe there is a plate-like lobe bearing three megasetae (no such lobe), medio-ventrally on the hypoproct there is a rounded lobe (no lobe), and posterior margin of sternite 9 with a cleft (without). Even if the outline of the posterior part of aedeagus is symmetrical it is seen that there is some asymmetry inside (Fig. 7B).
The name is a Latin adjective, pileatus, -a, -um, capped, referring to the cap- or hood-like lobes enclosing the apex of aedeagus.
Holotype. Male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 4°32'50"N, 115°09'28"E, 7–17.i.2014, primary lowland rainforest, Malaise trap 2, Ševčík & Kaspřák leg. (in UBDC).
Male. Colour. Head brown, face and clypeus paler brown. Antenna brown. Mouthparts yellowish. Thorax brown, preepisternum 2 pale yellowish. Legs yellowish. Wing brownish, halter brownish with blackish knob. Abdomen brown, sternites paler than tergites. All setosity pale, yellowish or brownish. Head. Antennal flagellomere 4, Fig. 8A. Palpomere 3 of maxillary palpus with apico-mesial thumb-like extension, with 5 apically expanded and curved sensilla; palpomere 4 with parasegment; palpomere 5 is 1.4 times as long as palpomere 4. Number of strong postocular setae 9. Thorax. Anepisternum setose, with 50 setae; anterior basalare non-setose; preepisternum 2 setose, with 30 setae, laterotergite non-setose; episternum 3 setose, with ca. 18 setae. Legs. Mid and hind tibial organs absent. Wing. R1 meeting C well on the basal half of the costal margin; the sclerotized part of M2 extending near to the level of the tip of R1; wing length 1.5 (1.6) mm. Hypopygium. Fig. 8B–E: Sternite 9 about half of ventral length of gonocoxa, with sharply delimited convex sides which are contiguous with the convex posterior margin, anterior margin deeply incised, the setae at posterior margin very long and strong, on other parts similar to adjacent ventral setae of gonocoxa. Ventral mesial margin of gonocoxa simple, concave. Parastylar lobe oblique, subtriangular, with 3 setae antero-mesially (at apex). Paraapodemal lobe well exposed in ventral view. The dorsal mesial margin of gonocoxa simple, sigmoid, posteriorly with a row of prominent closely placed setae which become broader and flattened towards the posterior end of the row; posteriorly from the row of setae there is a short finger-like lobe with one apical seta. The general dorsal setosity of gonocoxa similar to the ventral one. Two juxtagonostylar setae present, both long curved megasetae arising from a short common basal body, about one fifth of the length of megasetae. Gonostylus elongate subquadrangular, with moderately long setosity ventrally, dorsally non-setose except for a few very strong setae at apical margin on a slightly tuberculate area. Aedeagus subtriangular, with small lateral shoulders, the apex curved ventrad. Hypoproct posteriorly extending to level of basal third of gonostylus, the ventral part with a pair of elongate lobes (sternite 10) bearing ca. 15 scattered setae each, the posterodorsal part with one strong and a couple of weaker setae on each half. Cerci mesially separate.
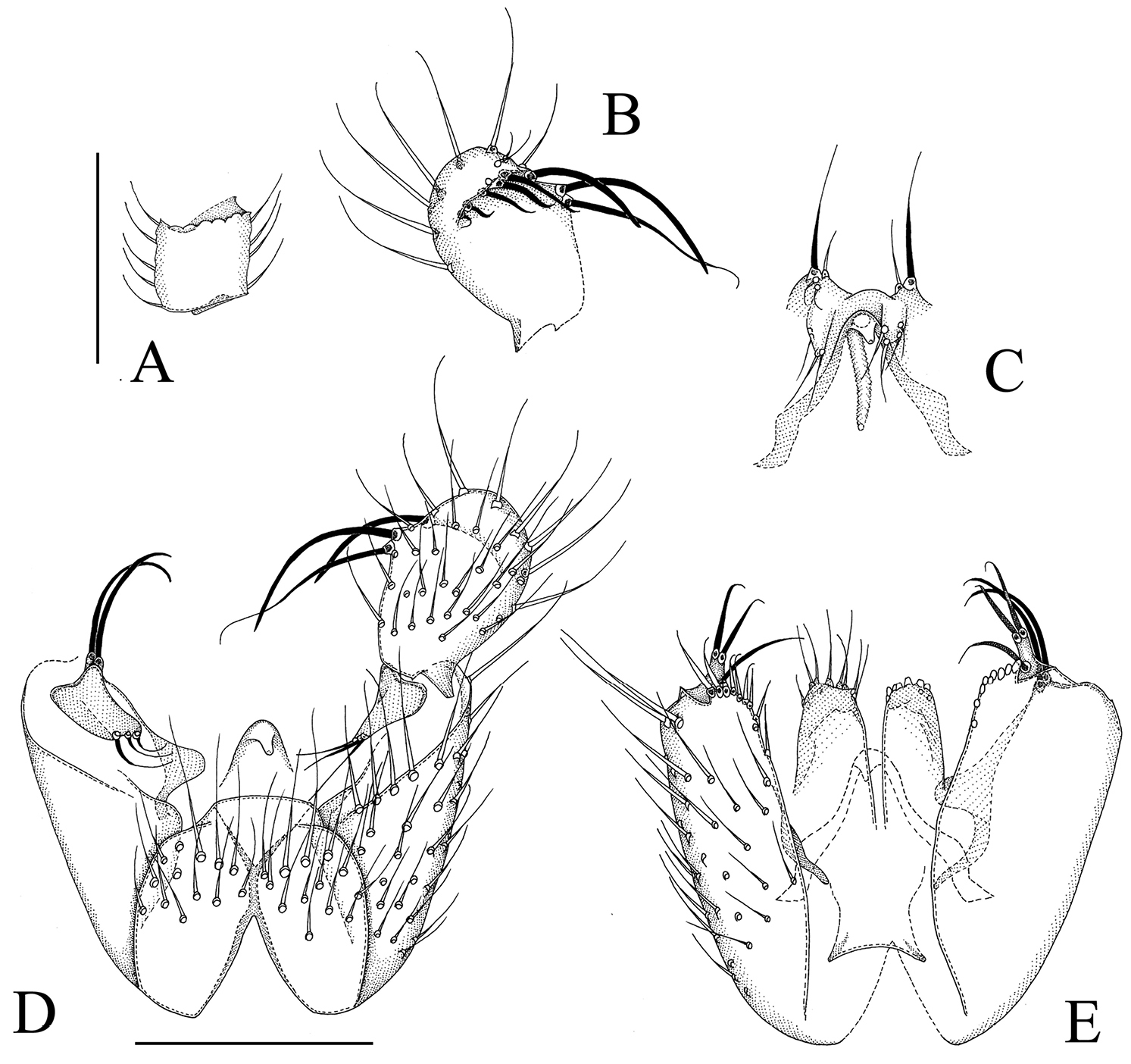
Manota ricina sp. n. (holotype). A Antennal flagellomere 4, lateral view. B Gonostylus, dorsal view C Aedeagus and hypoproct, ventral view D Hypopygium, ventral view E Hypopygium, dorsal view. Scale 0.1 mm.
Female unknown.
Manota ricina resembles Manota curvata Hippa, 2006 from Peninsular Malaysia and Sumatra in many respects and follows it in the key to Oriental and Palaearctic Manota to couplet 70 by the following characters: anepisternum setose, preepisternum 2 setose, laterotergite non-setose, juxtagonostylar megasetae simple, not expanded, gonocoxa with a conspicuous apicodorsal lobe which is fully exposed, this lobe is flattened, not cylindrical. Manota ricina is similar to Manota curvata by having the posteriormost setae at the margin of the above-mentioned lobe flat, blade-like, but the shape of the gonostylus is different: in Manota ricina it is about twice longer than broad, rather straight, in Manota curvata it is more than 4 times longer than broad and curved. We suspect that the small finger-like lobe posteriormost in the row of setae at the mesial margin of the gonocoxa is actually the lobe mentioned above and is not flattened and in this respect the key possibly needs to be revised. Manota ricina also differs from Manota curvata by the chaetotaxy of the gonostylus, the strong apicodorsal setae being lacking in the latter.
The name is a Latin adjective, ricinus, -a, -um, veiled, referring to the veil-like fringe of setae posteriorly on the sternite 9.
Holotype. Male, Brunei, Tungku, UBD Campus, nr KBFSC Headquarters, 4°58'35"N, 114°53'26"E, 19–22.i.2014, secondary forest, Malaise trap, J. Ševčík & D. Kaspřák leg. (in UBDC).
10 males, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 5–17.ii.2013, primary lowland rainforest, Malaise trap 3b, J. Ševčík leg. (5 in UBDC, 5 in UOSC); 5 males with the same data except 7–17.i.2014, Malaise trap 2, J. Ševčík & D. Kaspřák leg. (3 in UBDC, 2 in UOSC); 1 male with the same data except 7–18.i.2014, Malaise traps in gaps, I. H. Tuf leg. (in UOSC).
The species was earlier known from Thailand (
1 male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 5–17.ii.2013, primary lowland rainforest, Malaise trap 1, J. Ševčík leg. (in UBDC); 1 male with the same data except 7–17.i.2014, Malaise trap 2, J. Ševčík & D. Kaspřák leg. (in UOSC).
The species was earlier known only by the type material from Brunei (
1 male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, primary lowland rainforest, sweeping, February 2013, J. Ševčík leg. (in UBDC).
The species was earlier known only by the type material from Brunei (
1 male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 7–17.i.2014, primary lowland rainforest, Malaise trap 2, J. Ševčík & D. Kaspřák leg. (in UOSC); 1 male, Brunei, Tungku, UBD Campus, nr KBFSC Headquarters, 19–22.i.2014, secondary forest, Malaise trap, J. Ševčík & D. Kaspřák leg. (in UBDC).
The species was earlier known from Peninsular Malaysia (
1 male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 7–17.i.2014, primary lowland rainforest, Malaise trap 3, J. Ševčík & D. Kaspřák leg. (in UBDC); 1 male with the same data except 8–18.i.2014, Malaise trap 4 (in UOSC); 1 male with the same data except 15.ii.2013, sweeping (in UOSC).
The species was earlier known from Peninsular Malaysia (
1 male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 7–18.i.2014, primary lowland rainforest, Malaise trap 1 (night), J. Ševčík leg. (in UBDC); 1 male with the same data except 8–18.i.2014, Malaise trap 4, J. Ševčík & D. Kaspřák leg. (in UOSC).
The species was earlier known from Brunei (
1 male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, 5–17.ii.2013, primary lowland rainforest, Malaise trap 3b, J. Ševčík leg. (in UBDC).
The species was earlier known from Peninsular Malaysia (
1 male, Brunei, Bandar Seri Begawan, UBD Campus, nr KBFSC Headquarters, 19–22.i.2014, secondary forest, Malaise trap, J. Ševčík & D. Kaspřák leg. (in UBDC).
The species was earlier known from Peninsular Malaysia (
1 male, Brunei, Ulu Temburong N. P., Kuala Belalong Field Studies Centre, primary lowland rainforest, sweeping, January 2013, J. Ševčík leg. (in UBDC); 1 male with the same data except 7–17.i.2014, Malaise trap 2, J. Ševčík & D. Kaspřák leg. (in UOSC); 1 male with the same data except Malaise trap 2 (in UBDC).
The species was earlier known from Peninsular Malaysia (
A total of 13 species of Manota is reported here from the relatively small area in Ulu Temburong National Park. Two additional species are known from the coastal area of the Universiti Brunei Darussalam campus in Tungku. One species (Manota oligochaeta) is common to both these areas. Out of the total of 15 species, 6 were already reported from Brunei by
If we use the Chao 1 formula (
Altogether, more than 50 species of Mycetophilidae and 16 species of Keroplatidae have so far been collected in Ulu Temburong National Park. The patterns of diversity of these taxocoenoses will be treated in a separate paper (Ševčík et al., in prep.).
One trap was emptied 3 times a day (at 7 am, 1 pm and 7 pm), so that it was possible to acquire also data on diurnal and night activity of several species. This will be subject of a separate paper (Kaspřák et al., in prep.) but concerning Manota, Manota kaspraki and Manota perangulata were collected during the night, Manota bruneiensis and Manota megachaeta in the morning, and Manota bruneiensis in the afternoon, indicating a day-long activity of Manota species in the tropics. These are the first records of diurnal activity of Manota species.
This study was partly supported by the project CZ.1.05/2.1.00/03.0100 ("Innovation of ecological studies by complementary fusion of courses between Palacký University and University of Ostrava") financed by the Structural Funds of the European Union and by the "National Feasibility Program I", project LO1208, of Ministry of Education, Youth and Sports of the Czech Republic. David Kaspřák and other students and staff members of the Department of Biology and Ecology, University of Ostrava, and Department of Ecology, Palacký University, Olomouc (Czech Republic), kindly helped with the field work at the KBFSC station. We are grateful to the Institute for Biodiversity and Environmental Research, Universiti Brunei Darussalam, for allowing us to work in Kuala Belalong Field Studies Centre, and to the Biodiversity and Research Innovation Centre (BioRIC), Ministry of Industry and Primary Resources, for the issuance of our export permits. We also thank Peter Chandler (Melksham, Great Britain) and an anonymous reviewer for their valuable suggestions and linguistic corrections.