Citation: Hadfield KA, Sikkel PC, Smit NJ (2014) New records of fish parasitic isopods of the gill-attaching genus Mothocya Costa, in Hope, 1851 from the Virgin Islands, Caribbean, with description of a new species. ZooKeys 439: 109–125. doi: 10.3897/zookeys.439.8093
Two species of Mothocya Costa, in Hope, 1851 are reported from the Virgin Islands. Mothocya xenobranchia Bruce, 1986 was collected from St. John Island from the gills of the Atlantic needlefish, Strongylura marina, which is a new locality record and also confirms a previously uncertain host identity. Mothocya bertlucy sp. n. is described from St. Thomas, St John and Guana Islands, from the gills of the redlip blenny, Ophioblennius macclurei, the first record of a blenny as host for any Mothocya. The distinguishing characters of Mothocya bertlucy sp. n. include its small size (< 9 mm) and eyes, the slender pleotelson with a narrowly rounded caudomedial point, extended uropod peduncle and uropods which do not extend past the pleotelson posterior margin, and the narrow pleon which is only slightly overlapped by pereonite 7.
Cymothoidae, Mothocya, gill chamber, fish parasite, Caribbean Sea, St. Thomas, St. John, Guana Island
Cymothoid isopods are one of the most recognisable groups of isopods to fisherman and anglers (
In some cases, these parasites cause gill and branchial filament damage (
One of these gill-attaching cymothoid genera is Mothocya Costa, in Hope, 1851. Historically the systematics and biology of this genus had not been considered problematic, but
There are six known species of Mothocya in the Caribbean Sea. These are Mothocya argenosa Bruce, 1986 (Bermuda; Florida and Georgia, USA; Cuba; and the British Virgin Islands); Mothocya bermudensis Bruce, 1986 (Bermuda; Haiti; Saint Barthélemy, Leeward Islands); Mothocya bohlkeorum Williams & Williams, 1982 (Florida, USA; Bahamas; Saint Eustatius, Leeward Islands; and Puerto Rico); Mothocya nana (Schioedte & Meinert, 1884) (Florida, Georgia and Maryland USA; Saint Barthélemy, Leeward Islands; and Panama), Mothocya omidaptria Bruce, 1986 (Brazil and West Indies), and Mothocya xenobranchia Bruce, 1986 (Florida, USA; and Venezuela). To date there are no known species recorded from the US Virgin Islands and only one species known from the British Virgin Islands (Mothocya argenosa). The new species described here increases the number of species known from the Caribbean to seven.
Collections were made from the Virgin Islands, specifically St. John, and St. Thomas, US Virgin Islands, and Guana Island, British Virgin Islands, in the Caribbean Sea during 2013 as part of a study on blood parasites of Caribbean reef fishes. Atlantic needlefish (Strongylura marina) were collected near the surface at night by snorkelers using hand nets, while redlip blennies (Ophioblennius macclurei) were collected by hand nets during the day from reef habitat in shallow bays by snorkelers or divers. Isopods were removed from the gills of their infected hosts using forceps, preserved in 70% ethanol, and processed according to techniques described in
Abbreviations. AMNH – American Museum of Natural History, New York, NY, USA; TL – total length; USNM – National Museum of Natural History, Smithsonian Institution, Washington, DC, USA; W – width.
Body not vaulted, widest at pereonite 5, usually twisted to one side. Cephalon with rostrum folded back, anterior margin rounded. Antennae widely separated, antennula longer and more stout than antenna. Eyes distinct. Maxilliped article 3 with 3–5 recurved robust setae; without oostegite lobe. Maxilla mesial lobe partly fused to lateral lobe. Maxillula simple. Pereonite 1 anterolateral angles slightly extended around cephalon. Pleon subequal to pereon. Pleonite 1 partly concealed by pereonite 7. Coxae 5–7 dorsally visible, projecting posteriorly past respective somite; large, and rounded, reniform. Brood pouch formed from coxae 2–4 and 6. Pereopods without carina, never enlarged or with protrusions. Pleopods simple, without setae. Pleopods 3–5 with lamellar proximomedial lobe, frequently with peduncle lobe. Uropod peduncle without retinaculae, exopod longer than endopod.
Mothocya epimerica Costa, in Hope, 1851; by subsequent designation (
Female Mothocya are often twisted to one side due to the confines of the gill chamber. Mothocya can be identified by the asymmetrical body shape, antennula longer than the antenna, a maxilliped with an oostegite lobe and the brood pouch from coxae 2–4 and 6. Males are smaller and not twisted, with appendix masculina on pleopod 2.
A detailed diagnosis of Mothocya was given by
When looking at individual characters, Mothocya can be distinguished from other gill-inhabiting genera. Elthusa Schioedte & Meinert, 1884 is similar to Mothocya and can be distinguished by the antennula being shorter than the antenna (longer in Mothocya), maxilliped article 3 is slender with setae (robust and without setae in Mothocya), and the pereopod dactyli are relatively short whereas they are long and robust in Mothocya (
Mothocya occurs in all oceans and is predominantly tropical and subtropical in its distribution. Currently 29 species names are valid (Mothocya contracta Costa, in Hope, 1851 designated as nomen dubium), with four species described since
♀ (15.0 mm TL; 10.0 mm W), ♂ (9.0 mm TL; 4.0 mm W) collected from Lameshur Bay, 18°18'59"N, 64°43'25"W, St. John Island, US Virgin Islands, from the gills of the Atlantic needlefish (34 mm TL), Strongylura marina, 18 May 2013, coll. Nico J. Smit (AMNH_IZC 00197448).
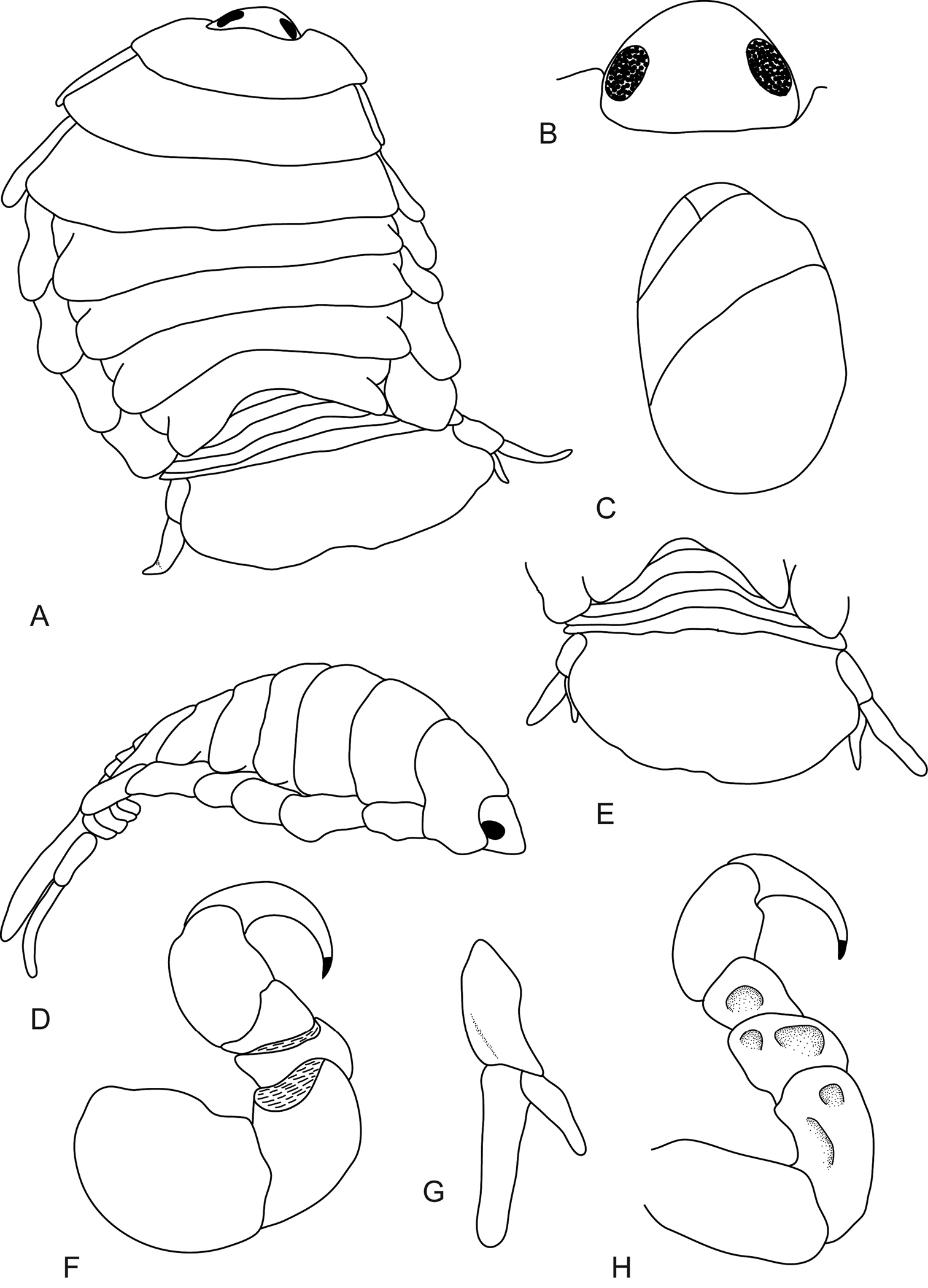
Body moderately twisted, 1.4 times as long as greatest width, strongly arched longitudinally, widest at pereonite 3, most narrow at pereonite 1, lateral margins slightly convex. Cephalon 0.7 times longer than wide, visible from dorsal view, subtriangular. Eyes oval with distinct margins, 0.2 times width of cephalon, 0.4 times length of cephalon. Coxae 2–3 narrow; 4–7 large, rounded and produced, slightly produced past pereonite margin. Pereonites 1–4 increasing in length and width; 5–7 decreasing in length and width; becoming more progressively rounded posteriorly. Pleon with pleonite 1 largely concealed by pereonite 7; pleonites posterior margin smooth, mostly concave; pleonites 2–5 partially overlapped by pereonite 7; pleonite 5 posterior margin straight. Pleotelson 0.6 times as long as anterior width, dorsal surface smooth, anterolateral margin recessed, lateral margins widen slightly then curve inwards, posterior margin broadly truncate, without median point.
Antennula comprised of 7 articles; articles 1 and 2 distinct and articulated; article 2 0.8 times as long as article 1; article 3 as long as wide, 0.5 times as long as combined lengths of articles 1 and 2; last article terminating in 4–7 short simple setae. Antenna comprised of 7 articles; article 3 1.2 times as long as article 2, 2.1 times as long as wide; article 4 2.3 times as long as wide, 0.9 times as long as article 3; article 5 0.7 times as long as article 4, 1.7 times as long as wide; last article terminating in 6–7 short simple setae.
Molar process present, mandible palp without setae. Maxillula with 4 terminal robust setae. Maxilla lateral lobe with 2 recurved robust setae; mesial lobe with 2 large recurved robust setae. Maxilliped weakly segmented, palp article 2 with no simple setae, article 3 with 4 recurved robust setae and no simple setae.
Pereopod 1 basis 1.2 times as long as greatest width; ischium 0.9 times as long as basis; merus proximal margin with slight bulbous protrusion; carpus with straight proximal margin; propodus 1.3 times as long as wide; dactylus slender, 1.1 times as long as propodus, 2.3 times as long as basal width. Pereopod 7 basis 1.9 times as long as greatest width; ischium 0.9 as long as basis, without protrusions; merus proximal margin without bulbous protrusion, 0.5 as long as ischium, 0.9 times as long as wide; carpus 0.9 as long as ischium, without bulbous protrusion, 1.1 times as long as wide; propodus 0.8 as long as ischium, 1.7 times as long as wide; dactylus slender, 0.9 as long as propodus, 2.4 times as long as basal width. Pereopod 7 with small indentations on the inner side of the ischium, merus and carpus.
Pleopod 1 exopod as long as wide, lateral margin strongly convex, distally broadly rounded, mesial margin strongly convex; endopod 1.2 times as long as wide, lateral margin weakly convex, distally narrowly rounded, mesial margin straight, peduncle 0.7 times as wide as long. Pleopods 2–5 similar in structure to pleopod 1. Large medial lobes present and increasing in size from pleopods 1 to 5. Peduncle lobes increasing in size from pleopods 2 to 5.
Uropod longer than pleotelson; peduncle 0.7 times longer than exopod, lateral margin without setae; rami extending beyond pleotelson, marginal setae absent, apices broadly rounded. Endopod apically slightly pointed, 3.6 times as long as greatest width, lateral margin weakly convex, mesial margin weakly convex, terminating without setae. Exopod extending beyond endopod, 1.9 times longer than endopod, 3.8 times as long as greatest width, apically rounded, lateral margin straight, mesial margin straight, terminating without setae.
Holotype (16.2 mm TL) from the gill cavity of Tylosurus crocodilis crocodilis from Bahia Mochima, Venezuela (USNM 216274); Paratypes (USNM 216275–216278) (
Off the coast of Florida, Florida Keys (USA); Cumaná, Venezuela (
Known from the hound needlefish, Tylosurus crocodilis crocodilis (Péron & Lesueur, 1821) (
Mothocya xenobranchia is known from Belonidae fish hosts and distinguished by the broad body which is arched in lateral view, the invaginations on the inner portion of pereopod 7, antenna with seven articles, and the shape of the pleotelson which is tapered anteriorly, then widens before bluntly rounding off.
When comparing Mothocya xenobranchia from the present study to the description given by
The other Caribbean species differ from Mothocya xenobranchia in that Mothocya bermudensis is smaller overall, with smaller eyes and less produced coxae; Mothocya argenosa has larger eyes, a larger and rounder pleotelson and smaller coxae; and Mothocya nana has a narrower body shape and is not arched longitudinally. Mothocya bohlkeorum has a narrow strongly produced rostrum; antennula and antenna bases closer together; larger and rounder coxae; and less developed proximomedial and peduncle lobes on the pleopods. Lastly, Mothocya omidaptria has much longer uropods, is not arched in lateral view, acute coxae on pereonite 7, and a narrowly produced rostrum. Furthermore, these species all have different hosts to Mothocya xenobranchia and thus there is no overlap of this isopod species on its host species in the Caribbean.
This record of Mothocya xenobranchia in the US Virgin Islands is a new locality record and also confirms the previously uncertain host record of Strongylura marina (
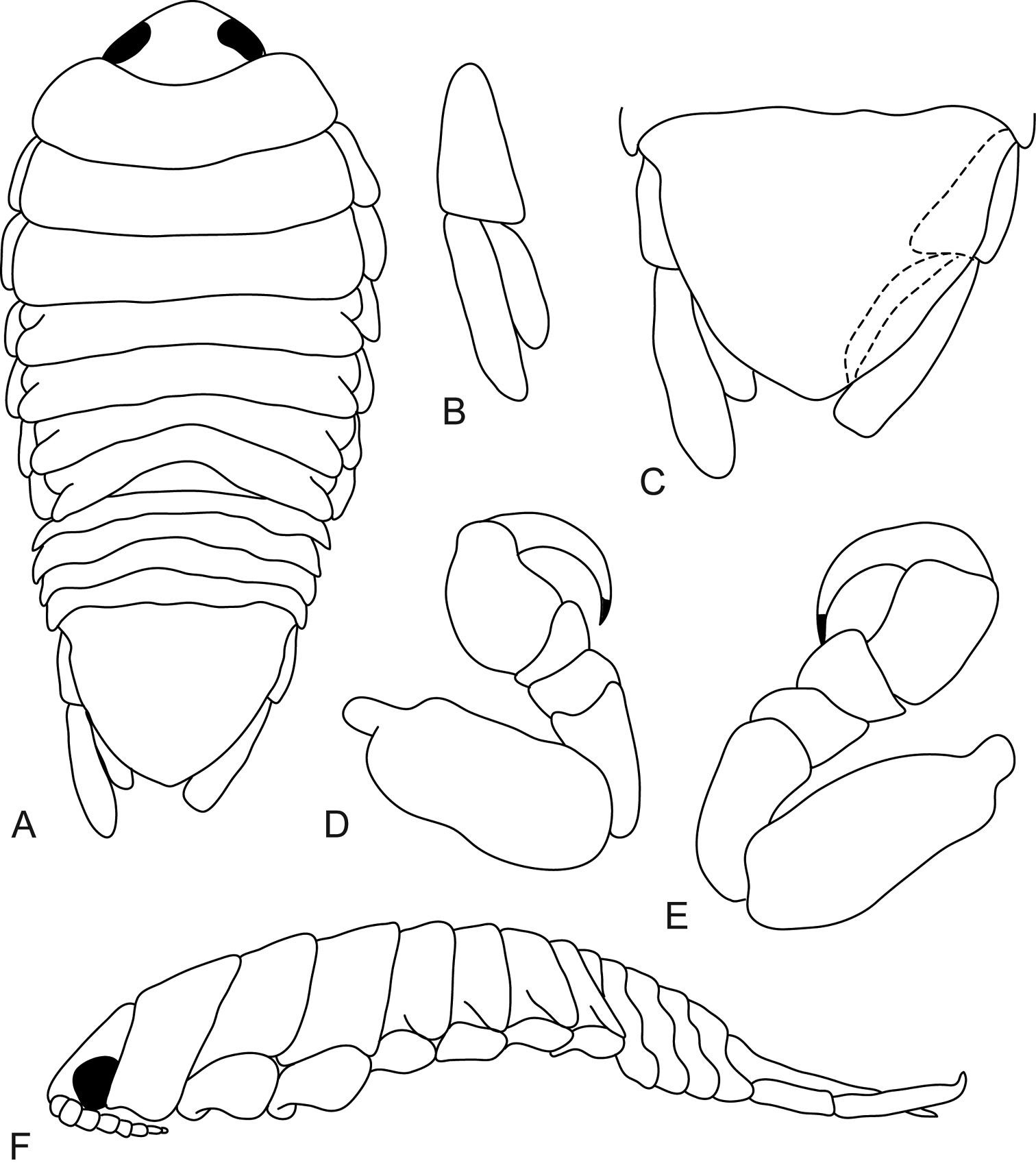
Mothocya xenobranchia Bruce, 1986 (15 mm) (AMNH_IZC 00197448): A dorsal view B dorsal view of cephalon C oostegites D lateral view E dorsal view of pleotelson F pereopod 1 G uropod H pereopod 7 showing indentations.
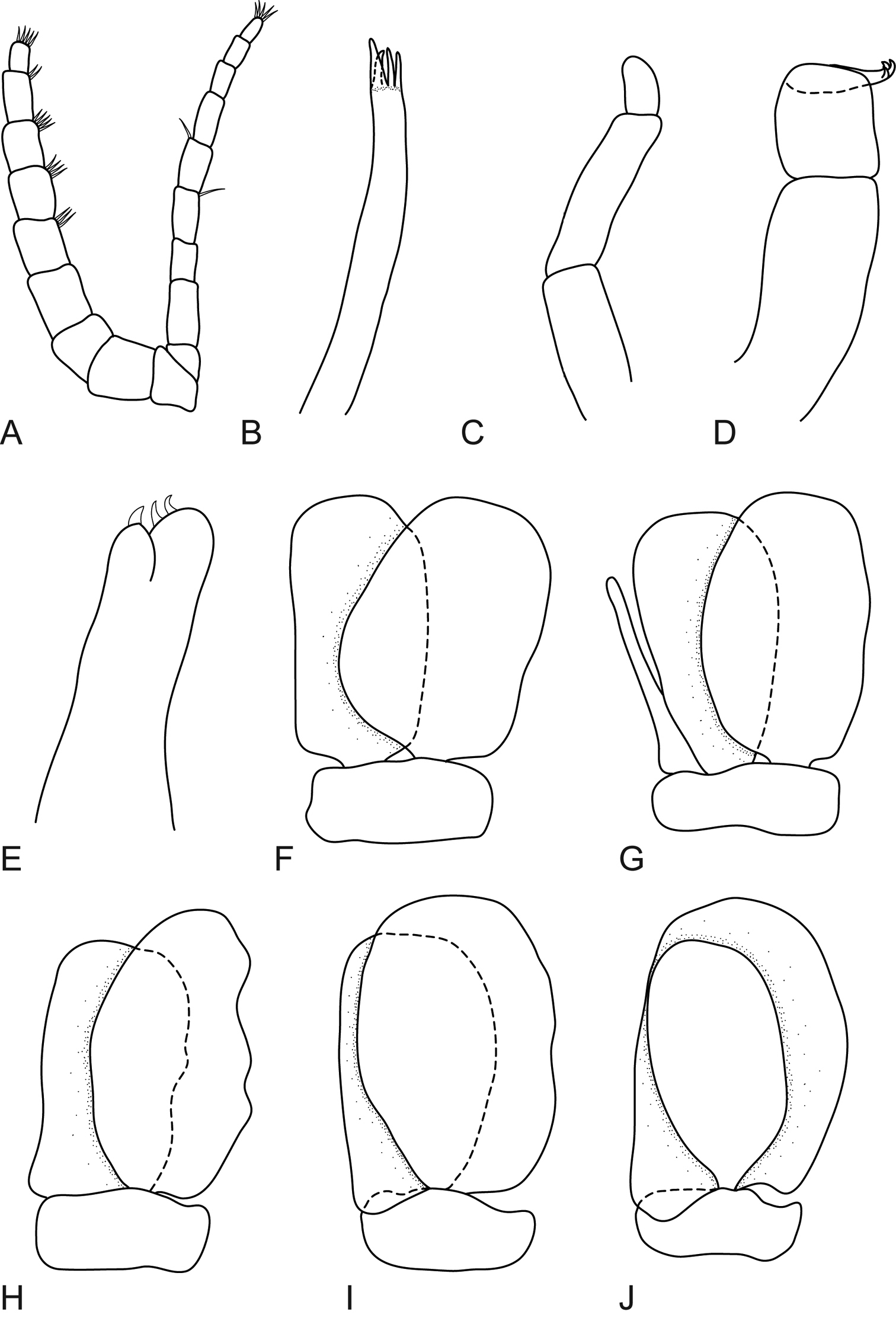
Mothocya xenobranchia Bruce, 1986 (15 mm) (AMNH_IZC 00197448): A antennula B antenna C tip of maxillula D tip of maxilla E tip of maxilliped article 3 F pleopod 1 G pleopod 2 H pleopod 3 I pleopod 4 J pleopod 5.
All material from the gills of the redlip blenny, Ophioblennius macclurei.
Holotype. Ovigerous ♀ (8.0 mm TL; 4.5 mm W), collected from Lameshur Bay, 18°18'59"N, 64°43'25"W, St. John Island, US Virgin Islands, July 2013, coll. L. Renoux & J. Sellers (AMNH_IZC 00197449).
Paratypes. ♀ dissected (7.0 mm TL; 3.5 mm W), three immature ♂♂, one dissected (5.5–6.0 mm TL; 2.0–2.5 mm W), collected from Brewers Bay, 18°20'24"N, 64°58'44"W, St. Thomas Island, Caribbean Sea), 19 May 2013, coll. J. A. Barry & A. McCammon (AMNH_IZC 00197450). Ovigerous ♀ (9.0 mm TL; 5.0 mm W), collected from Lameshur Bay, 18°18'59"N, 64°43'25"W, St. John Island, US Virgin Islands, July 2013, coll, L. Renoux & J. Sellers (AMNH_IZC 00197451). Ovigerous ♀ (7.5 mm TL; 4.0 mm W), mature ♂ (6.0 mm TL; 4.0 mm W), collected from Guana Island, 18°28'0"N, 64°33'59"W, British Virgin Islands, 07 July 2013, coll: R. Ditter & J. Barry (AMNH_IZC 00197452).
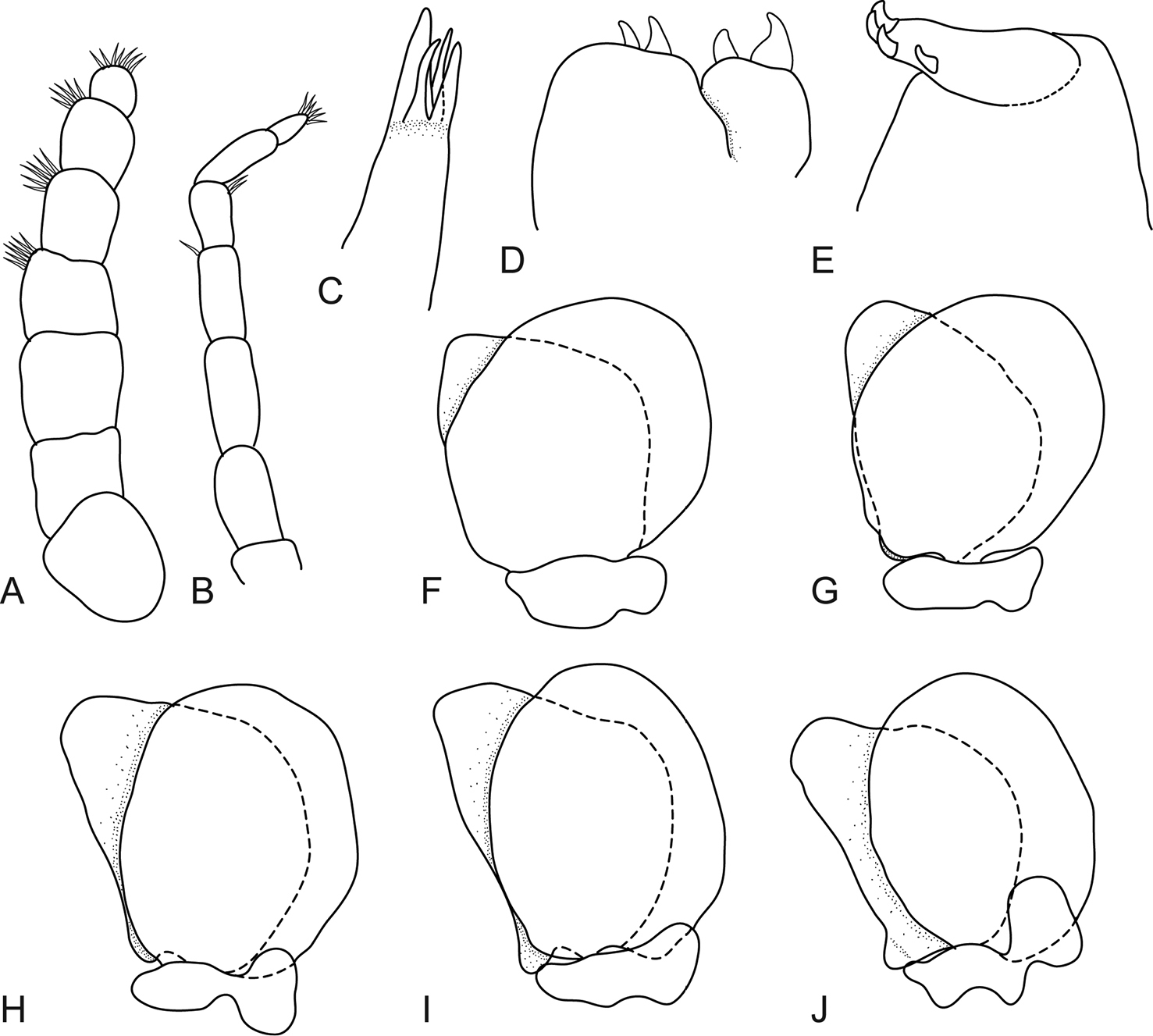
Body oval and moderately twisted, 1.9 times as long as greatest width, widest at pereonite 3, most narrow at pereonite 1, lateral margins slightly convex. Cephalon 0.7 times longer than wide, visible from dorsal view. Eyes oval with distinct margins, 0.2 times width of cephalon, 0.4 times length of cephalon. Pereonite 1 smooth, anterolateral angle rounded. Posterior margins of pereonites smooth and slightly curved laterally. Coxae narrow with rounded point, shorter or same length as pereonite. Pereonites 1–3 increasing in length and width; 4–7 decreasing in length and width, becoming progressively rounded posteriorly. Pleon with pleonite 1 largely concealed by pereonite 7, visible in dorsal view; pleonites posterior margin smooth, mostly concave; pleonite 2 partially overlapped by pereonite 7; pleonite 5 posterior margin slightly concave. Pleotelson 0.6 times as long as anterior width, dorsal surface smooth, lateral margins weakly concave, posterior margin converging to blunt caudomedial point.
Antennula comprised of 8 articles; articles 1 and 2 distinct and articulated with plumose setae; article 2 0.9 times as long as article 1; article 3 1.2 times as long as wide, 0.5 times as long as combined lengths of articles 1 and 2 with plumose seta; short simple setae present on last four articles, last article terminating in 4–8 short simple setae. Antenna comprised of 9 articles; article 3 1.3 times as long as article 2, 1.3 times as long as wide; article 4 1.4 times as long as wide, 1.1 times as long as article 3; article 5 as long as article 4, 1.4 times as long as wide; short simple setae on last three articles, last article terminating in 6–7 short simple setae.
Molar process present, mandible palp without setae. Maxillula with 4 terminal robust setae. Mxilla lateral lobe with 2 recurved robust setae; mesial lobe with 2 large recurved robust setae. Maxilliped comprised of 3 articles, palp article 2 without simple setae, article 3 with 3 recurved robust setae, and no simple setae.
Pereopods without robust or simple setae. Pereopod 1 basis 1.8 times as long as greatest width; ischium 0.6 times as long as basis; merus proximal margin without bulbous protrusion; carpus with straight proximal margin; propodus 1.4 times as long as wide; dactylus slender, 1.3 times as long as propodus, 2.6 times as long as basal width. Pereopod 2 propodus 1.3 as long as wide; dactylus 1.3 as long as propodus. Pereopod 7 basis 1.7 times as long as greatest width; ischium 0.7 as long as basis, without protrusions; merus proximal margin with slight bulbous protrusion, 0.4 as long as ischium, 0.6 times as long as wide; carpus 0.9 as long as ischium, without bulbous protrusion, 0.6 times as long as wide; propodus 0.9 as long as ischium, 1.3 times as long as wide; dactylus slender, 1.7 as long as propodus, 2.7 times as long as basal width.
Pleopod 1 exopod 1.3 times as long as wide, lateral margin weakly convex, distally narrowly rounded, medial margin weakly oblique, mesial margin strongly convex; endopod 1.8 times as long as wide, lateral margin weakly convex, distally narrowly rounded, mesial margin straight, peduncle 0.4 times as wide as long. Pleopods 2–5 similar to pleopod 1. Proximomedial lobes present and increasing in size from pleopod 1 to 5. Peduncle lobes absent.
Uropod more than half the length of pleotelson, peduncle 1.2 times longer than rami, peduncle lateral margin without setae; rami not extending beyond pleotelson, marginal setae absent, apices broadly rounded. Endopod apically rounded, 2.8 times as long as greatest width, lateral margin straight, mesial margin straight, terminating without setae. Exopod extending beyond endopod, 1.7 times longer than endopod, 4.2 times as long as greatest width, apically rounded, lateral margin straight, mesial margin straight, terminating without setae.
Mothocya bertlucy sp. n. ovigerous female holotype (7 mm) (AMNH_IZC 00197449): A dorsal view B anterodorsal view of pereonite 1 and cephalon C dorsal view of pleotelson D uropod E lateral view.
Mothocya bertlucy sp. n. female paratype (7 mm) (AMNH_IZC 00197450): A antennula B antenna C maxillula D molar process E maxilliped F maxilla G pereopod 1 H pereopod 2 I pereopod 7.
Mothocya bertlucy sp. n. female paratype (7 mm) (AMNH_IZC 00197450): A–E dorsal pleopod 1–5 respectively F–J ventral pleopod 1–5 respectively.
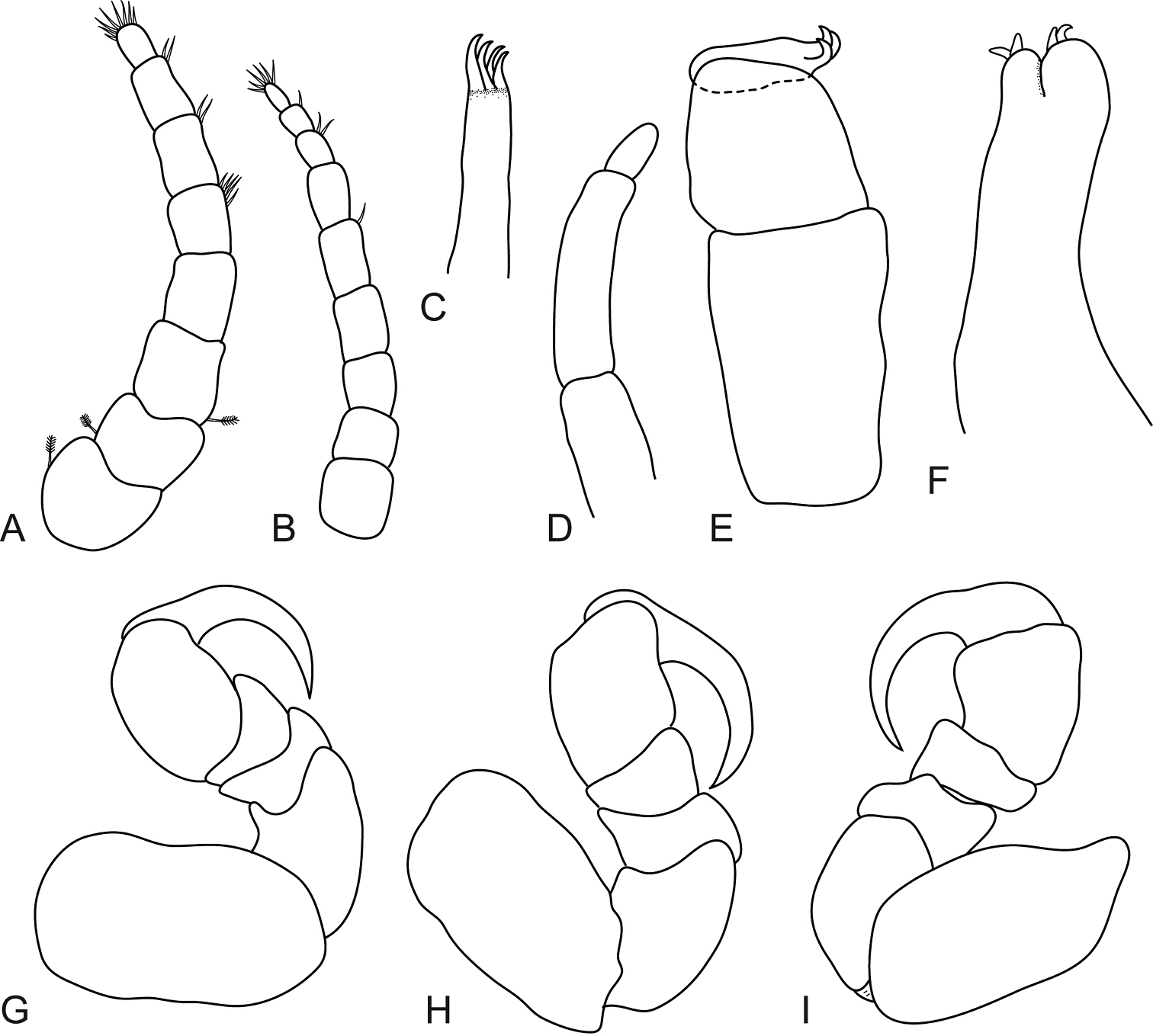
Males similar to females but smaller. Body more oval and not twisted, 2.1 times as long as wide. Maxilliped article three with three recurved robust setae. Maxilla with one recurved robust seta on the medial lobe and two on the lateral lobe. Penis set close together, medially united. Pleopod 2 appendix masculina basally swollen, 0.8 times as long as endopod, distally bluntly rounded. Pleotelson triangular converging to a sharp caudal point. Uropods extend past posterior margin of pleotelson and endopod is longer, exopod 1.5 times as long as endopod.
Mothocya bertlucy sp. n. male paratype (5.5 mm) (AMNH_IZC 00197450): A dorsal view B uropod C dorsal view of pleotelson D pereopod 1 E pereopod 7 F lateral view.
Mothocya bertlucy sp. n. male paratype (5.5 mm) (AMNH_IZC 00197450): A antennula and antenna B maxillula C molar process D maxilliped E maxilla F–J pleopod 1–5 respectively.
Ovigerous females (7.0–9.0 mm TL; 3.5–5.0 mm W), non-ovigerous females (7.0 mm TL; 3.0 mm W); mature male (6.0 mm TL; 4.0 mm W), immature males (5.5–6.0 mm TL; 2.0–2.5 mm W).
This species is named in honour of Ernest H. (“Bert”) Williams Jr. and Lucy Bunkley-Williams on the occasion of their retirement and in recognition of their contribution to Caribbean marine parasitology; noun in apposition.
Known from St. John, St. Thomas, and Guana Islands, Caribbean Sea.
Only known from the redlip blenny, Ophioblennius macclurei (Silvester, 1915).
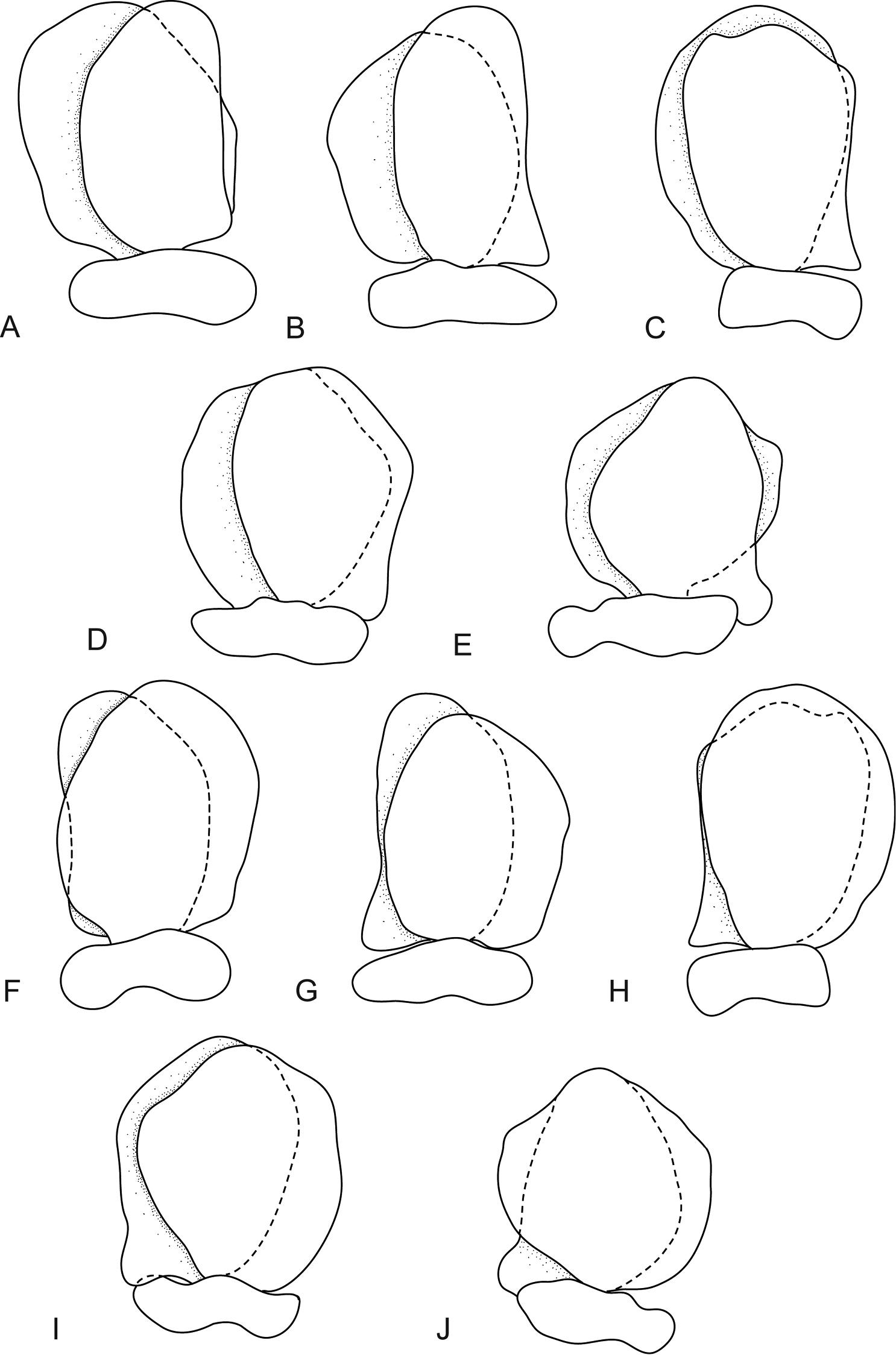
Mothocya bertlucy sp. n. can be identified by its unique host (redlip blenny), small size (like those reported from atherinids), relatively small eyes, the small pleotelson with a narrowly rounded caudomedial point, large uropod peduncle with short rami, uropods which do not extend past the pleotelson posterior margin, and the narrow pleon which is only slightly overlapped by pereonite 7.
The species most similar to Mothocya bertlucy sp. n. is Mothocya rosea Bruce, 1986 found on the Mexican and Californian coasts. In comparison to Mothocya bertlucy, Mothocya rosea has more produced proximomedial lobes on pleopods 3–5, larger eyes, broad truncate pleotelson, and four setae on the maxilliped article 3.
The three small Mothocya species from atherinids (Mothocya argenosa; Mothocya epimerica; and Mothocya waminda Bruce, 1986) were all compared to the current species. Mothocya argenosa from the western Atlantic measures 5.6–9.8 mm, but has larger eyes, longer uropods, the pleotelson is more rounded and the posterolateral margins of pereonite 7 are acute. Mothocya epimerica from the Mediterranean has a more pointed rostrum, rounded pleotelson, larger eyes and four setae on the maxilliped. Mothocya waminda from the Indo-Pacific has an appendix masculina on pereopod 2 in the female and longer uropods.
Mothocya bertlucy sp. n. differs from all the other known Caribbean species in that Mothocya bohlkeorum has much larger and more produced coxae and a larger truncate pleotelson; Mothocya nana has a wider pleotelson, truncate rostrum and larger coxae; Mothocya bermudensis has an antennula with only seven articles, large eyes and an arched body; and Mothocya omidaptria has longer uropods extending past the pleotelson, a strongly produced rostrum and acute coxae as well as posterolateral angles of pereonite 7.
This is the first account of a Mothocya species from the US Virgin Islands and is also the first record on a blenny, which helps establish its status as a new species as
The financial assistance of the South African National Research Foundation (NRF project IFR2011040100022, N.J. Smit, PI and SFP12091012541, K.A. Hadfield, PI), the US National Science Foundation (NSF OCE-121615, P. Sikkel, PI), and the Falconwood Corporation towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the funders. We thank J.A. Barry, R. Ditter, A. Mc Cammon, L. Renoux, J. Sellers, and J. Wagner for assistance with collection of fish specimens. We are also grateful to the staff of the MacLean Marine Science Center, Virgin Islands Environmental Resource Station, and Guana Island for logistic support. This is contribution number 116 from the University of the Virgin Islands Center for Marine and Environmental Studies. The authors would like to thank Dr Niel Bruce (Museum of Tropical Queensland, Australia) for valuable comments on the draft manuscript.