Citation: Perissinotto R, Miranda NAF, Raw JL, Peer N (2014) Biodiversity census of Lake St Lucia, iSimangaliso Wetland Park (South Africa): Gastropod molluscs. ZooKeys 440: 1–43. doi: 10.3897/zookeys.440.7803
The recent dry phase experienced by the St Lucia estuarine system has led to unprecedented desiccation and hypersaline conditions through most of its surface area. This has changed only recently, at the end of 2011, with the onset of a new wet phase that has already caused a major shift to oligo- and mesohaline conditions. The estuary mouth, however, remains closed to the ocean, making the weak connection recently established between the St Lucia and the Mfolozi estuaries the only conveyance for marine recruitment. As a result, only 10 indigenous and two alien aquatic gastropod species are currently found living in the St Lucia estuarine lake. This is out of a total of 37 species recorded within the system since the earliest survey undertaken in 1924, half of which have not been reported in the literature before. The tick shell, Nassarius kraussianus, which was consistently found in large abundance prior to the recent dry phase, appears to have temporarily disappeared from the system, probably as a result of the extinction of Zostera marine grasses inside the lake. Population explosions of the bubble shell Haminoea natalensis, with its distinct egg masses, were recorded seasonally until 2009, but the species has subsequently not been observed again. A molecular DNA analysis of the various populations previously reported as belonging to the same assimineid species, variably referred to as Assiminea capensis, A. ovata, or A. bifasciata, has revealed that the St Lucia assemblage actually comprises two very distinct taxa, A. cf. capensis and a species provisionally referred to here as “A.” aff. capensis or simply Assimineidae sp. In the mangroves, the climbing whelk Cerithidea decollata is still found in numbers, while ellobiids such as Cassidula labrella, Melampus semiaratus and M. parvulus are present in low abundances and all previously recorded littorinids have disappeared. A number of alien freshwater species have colonized areas of the system that have remained under low salinity. These include the invasive thiarid Tarebia granifera, which can be found in concentrations exceeding 5000 ind.m-2, the lymnaeid Pseudosuccinea columella and the physid Aplexa marmorata.
Mollusca, Gastropoda, biodiversity census, hypersalinity, iSimangaliso Wetland Park, illustrated checklist
Lake St Lucia is a large, complex estuarine lake situated on the South African east coast. It has been extensively investigated since the late 1940s, as it is the largest such system in Africa, the oldest protected estuary in the world and a Ramsar Wetland of International Importance since 1986 (
The rich biodiversity of the St Lucia estuarine lake is one of the main drivers of its special conservation status. Species are the building-blocks of any ecosystem, yet in the St Lucia case there are many misidentifications and several groups of invertebrates remain poorly investigated or completely ignored. A few detailed taxonomic studies of selected invertebrate groups have already been undertaken, starting from 2010, using a combination of traditional morphological analyses and molecular DNA barcoding. These have consistently revealed the occurrence of species that were either previously confused with others or completely unknown to science (e.g.
The class Gastropoda is the most diverse among the molluscs and includes about 55000 extant aquatic species globally (
The purpose of this study is thus to provide a comprehensive review of the diversity of gastropod molluscs in the St Lucia estuarine lake. This includes identifying species that are currently present in the system and comparing them with what was collected in past surveys. Changes in diversity over time are related to shifts in environmental and climatic conditions that have occurred during the past century. The compilation of an annotated and illustrated checklist of all gastropod species recorded so far within the system is designed to aid managers, researchers and visitors in the iSimangaliso Wetland Park with the identification of these important molluscs.
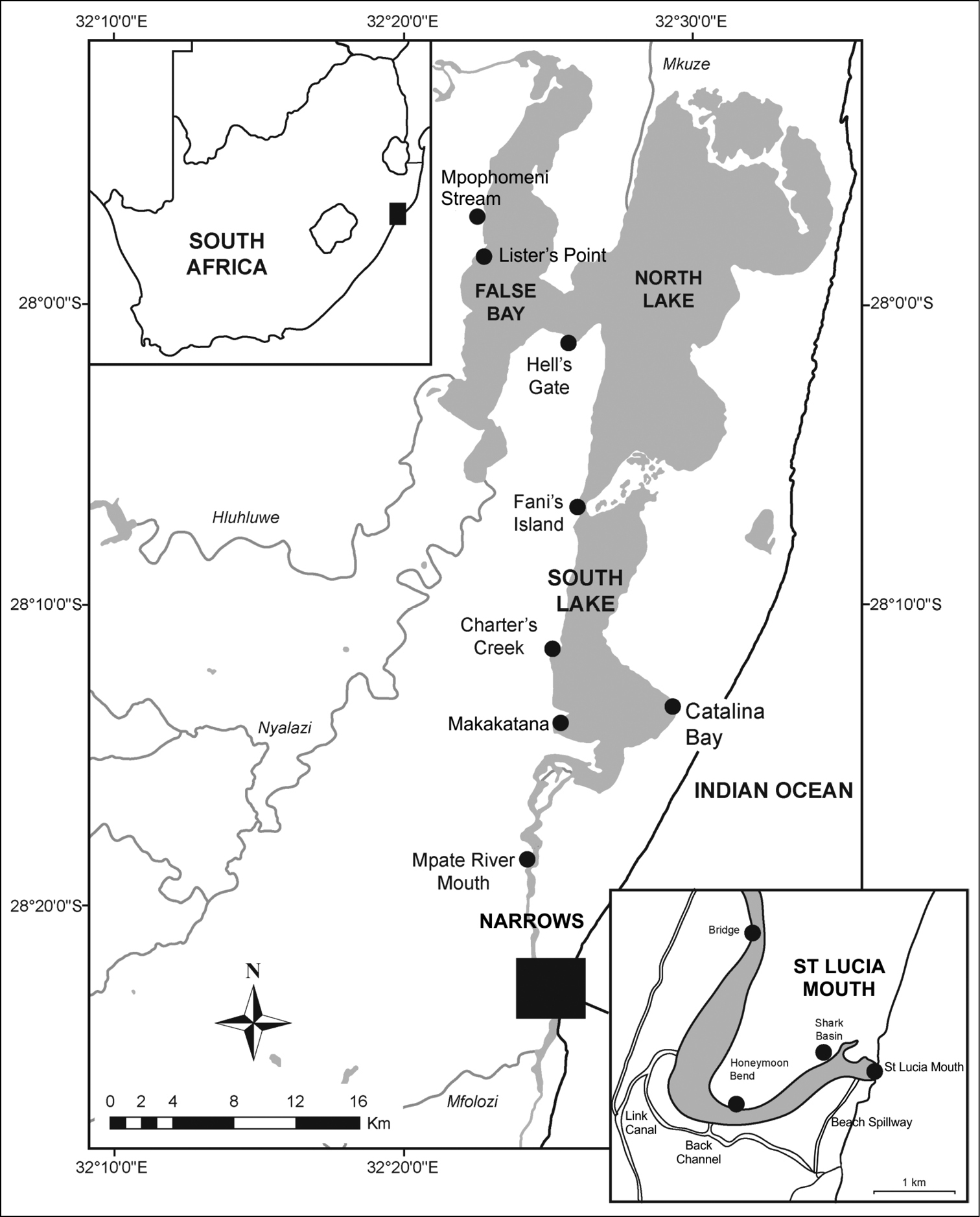
The St Lucia estuarine lake is located on the north coast of KwaZulu-Natal, between 27°52' to 28°24'S and 32°21' to 32°34'E. The system has a surface area of approximately 350 km2 (
Map of the St Lucia estuarine lake, with position of main collection sites used in the study. Adapted from
The first gastropod records from the St Lucia system date back to 1924, with specimens reposited in the KwaZulu-Natal Museum (NMSA), in Pietermaritzburg, ever since. Further collections were later undertaken during the two surveys of the University of Cape Town, in 1948–1949 and 1964–1965. Specimens collected during these surveys are currently reposited at the Iziko South African Museum (ISAM), in Cape Town. In 1982-1983, a dedicated collecting survey was undertaken throughout the lake system by the provincial conservation authority, the Natal Parks Board (NPB). The same authority, renamed Ezemvelo KwaZulu-Natal Wildlife (EKZNW), completed another similar survey during 2005, at the peak of the most recent drought. A number of publications containing gastropod records have also been published since 1954, mainly by researchers operating at the universities of KwaZulu-Natal, Zululand, Rhodes and Cape Town (e.g.
Specimens and data of gastropods collected at St Lucia in past surveys were obtained from the KwaZulu-Natal Museum in Pietermaritzburg and the Iziko South African Museum in Cape Town. Particularly rich collections from St Lucia were undertaken by the NMSA in 1987. Reference to specimens from either museum are here complemented with their accession numbers. Literature involving past macrobenthic surveys undertaken in the St Lucia Estuary (e.g.
Both NPB 1982–1983 and EKZNW 2005 collecting surveys were conducted at the onset of extreme drought conditions, when organisms within the estuarine lake were experiencing mass kills in response to hypersaline conditions and lake fragmentation/desiccation. Dead gastropods were mainly found washed up along the shorelines of St Lucia. The Natal Parks Board surveyed the banks of the whole lake from December 1982 to April 1983, collecting freshly dead specimens. These were later identified to species level by the late R.N. Kilburn. Ezemvelo KZN Wildlife surveyed the St Lucia banks in 2005, taking samples at fixed points along a number of transects in both South and North lakes (Figure 1). On this occasion, gastropods were identified by D. Herbert and R.N. Kilburn. In all cases, no specialized equipment was used and specimens were collected by hand at the surface or within the sediment by using spades and/or mechanical grabs.
Two surveys were conducted in March and July 2012. The survey in March was conducted at Fani’s Island, St Lucia Mouth, Hell’s Gate, Makakatana, Lister’s Point, the Bridge over the Narrows and along a transect from Catalina Bay to Charter’s Creek. The Back Channel, Shark Basin and Mpate Mouth were also visited (Figure 1, Table 1). Macrobenthic samples were taken using a Zabalocki-type Eckman grab with a sampling area of 0.0236 m2 and depth of 15 cm. Each sample was made up of three grabs, with three replicate samples taken at each site. Replicate samples were emptied into 20 L buckets and water was added in each sample. Each sample was stirred vigorously to suspend benthic organisms and the supernatant was passed through a 500 µm sieve. This procedure was repeated five times for each replicate sample. Material that was retained on the sieve was emptied into plastic jars. Sediments that were left in the bucket were washed through a 2 mm sieve. Samples were preserved in 4% phloxin-stained formalin. Qualitative samples were also collected with a D-net. At each site, the net was pushed over the sediment surface for a distance of approximately 5 m. At least one D-net sample was collected from each site. Macrofauna retained on the D-net were emptied into plastic jars and 4% phloxin-stained formalin was added. Both sampling methods (grab and D-net) were used at Fani’s Island, St Lucia Mouth, Hell’s Gate, Makakatana and along a transect from Catalina Bay to Charter's Creek, while only D-net samples were collected from the Bridge and the Back Channel (Figure 1, Table 1).
List of localities mentioned in the study with their coordinates and key biophysical characteristics.
| Region | Site name | Latitude, Longitude | Comments |
|---|---|---|---|
| False Bay | Lister’s Point | -27.9697, 32.3847 | Muddy and fossiliferous coquina substrate; sparse macrophyte cover. |
| Mpophomeni Stream | -27.9519, 32.3771 | Brackish forest stream with muddy sand substrate. | |
| North Lake | Hell’s Gate | -28.0118, 32.4438 | Muddy and fossiliferous coquina substrate; sparse macrophyte cover. |
| South Lake | Catalina Bay | -28.2237, 32.4839 | Limestone flat, muddy sand substrate; freshwater seepage from dune aquifers; exhibiting sedges such as Phragmites, Juncus and Schoenoplectus. |
| Charter’s Creek | -28.1994, 32.4162 | Muddy sand substrate; submerged macrophytes such as Ruppia sp. frequently recorded. | |
| Fani’s Island | -28.1091, 32.4341 | Muddy sand substrate; historic presence of Zostera capensis from this site southwards. | |
| Makakatana | -28.2364, 32.4199 | Sandy substrate, brackish conditions and relatively low turbidity; submerged macrophytes such as Ruppia cirrhosa frequently recorded. | |
| Narrows | Mpate River Mouth | -28.2945, 32.4012 | Muddy substrate, fringed by intertidal reeds Phragmites australis and mangroves. |
| St Lucia Bridge | -28.3689, 32.4096 | Muddy substrate, fringed by mangroves Avicennia marina and Bruguiera gymnorrhiza and some Hibiscus tiliaceus; submerged macrophytes such as Stuckenia pectinata generally present. | |
| St Lucia Mouth | Honeymoon Bend | -28.3871, 32.4032 | Tidal influence; muddy substrate, fringed by Phragmites reeds and mangroves Avicennia marina and Bruguiera gymnorrhiza. |
| Mfolozi Back Channel | -28.3922, 32.4094 | ||
| Mfolozi Link Canal | -28.3945, 32.3943 | ||
| Mfolozi-St Lucia Beach Spillway | -28.3892, 32.4238 | Sandy substrate; recent shallow link between St Lucia Mouth and Mfolozi River; influenced by tide. | |
| Shark Basin | -28.3831, 32.4203 | Sandy mud substrate; fringed by reeds and sedges as well as mangroves; influenced by tides and freshwater draining from adjacent areas to the north. |
The survey also included the manual collection of dead gastropod shells along the shoreline of the lake, within close proximity of the sampling stations. Further collections on an opportunistic basis were undertaken throughout 2013. In the laboratory, each sample was emptied into a sorting tray and gastropods were separated and identified with the aid of specialized literature and, where necessary, external taxonomy experts. Suitable specimens and shells were photographed in a standardized way, so as to show morphological characteristics that aid in their identification in an illustrated checklist.
A total of 20 families and 37 species of gastropods have been found in the St Lucia estuarine system since 1924, with half of the species not previously recorded in the literature. These include Afrolittorina africana, Alaba pinnae, Bulinus natalensis, Cerithium dialeucum, Ergalatax heptagonalis, Jujubinus suarezensis, Littoraria coccinea glabrata, Littoraria intermedia, Littoraria pallescens, Littoraria subvittata, Lymnaea natalensis, Melampus parvulus, Murex brevispinus, Neritina gagates, Neritina natalensis, Phalium areola, Pterotrachea cf. hippocampus and Purpura bufo (Table 2, Appendix).
Gastropod species originally recorded from the St Lucia estuarine lake. Reference codes: B:
| Species (original record) | Current valid name | Record year(s) | Reference(s) |
|---|---|---|---|
| TROCHIDAE | |||
| Jujubinus suarezensis (P. Fischer, 1878) | Idem* | 1987, Jul–Nov 2012 | NMSA (E2145), PMRP 2014 |
| NERITIDAE | |||
| Neritina gagates Lamarck, 1822 | Idem* | 1988 | NPB 1988 |
| Neritina natalensis Reeve, 1855 | Idem* | 1924, 1987 | NMSA (B7378, D5947) |
| THIARIDAE | |||
| Melanoides tuberculata (Müller, 1774) | Idem | Jul 2012–Nov 2013 | RMP 2013 |
| Tarebia granifera (Lamarck, 1822) | Idem | 2006, Apr–Jul 2007, Feb 2007–Mar 2011, 2010 | PP 2008, MPA 2010, MPA 2011, NMSA (W8287) |
| CERITHIIDAE | |||
| Cerithium dialeucum Philippi, 1849 | Idem* | Nov 2013 | PMRP 2014 |
| LITIOPIDAE | |||
| Alaba pinnae (Krauss, 1848) | Idem* | 1967, 1987 | NMSA (8083, E2164) |
| POTAMIDIDAE | |||
| Cerithidea decollata (Linnaeus, 1767) | Idem | Jul 1948–Jul 1951, Jul 1964 & Jan 1965, 2011–2013 | NMSA (A6384), ISAM (STL60), DMB 1954, MB 1970, PMRP 2014 |
| LITTORINIDAE | |||
| Afrolittorina africana (Krauss in Philippi, 1847) | Idem* | 1971 | NMSA (A1635) |
| Littoraria glabrata (Philippi, 1846) | Littoraria coccinea glabrata (Philippi, 1846)* | 1971 | NMSA (A1636) |
| Littoraria intermedia (Philippi, 1846) | Idem* | 1971, 1987, Mar 2012 | NMSA (A1634, D9983, E460), PMRP 2014 |
| Littoraria pallescens (Philippi, 1846) | Idem* | 1987 | NMSA (D9980) |
| Littoraria subvittata (Reid, 1986) | Idem* | Not reported (sine die) | NMSA (7128) |
| Littoraria scabra (Linnaeus, 1758) | Idem | Jul 1948–Jul 1951, Jul 1964 & Jan 1965, Jan–Jul 1972 & Jan 1973 | ISAM (STL50B), DMB 1954, MB 1970, B 1975 |
| PTEROTRACHEIDAE | |||
| Pterotrachea cf. hippocampus Philippi, 1836 | Idem* | Aug 2013 | PMRP 2014 |
| ASSIMINEIDAE | |||
| Assiminea sp. Fleming, 1828 | Probably comprising both Assiminea cf. capensis and Assimineidae sp. | Jul 1948–Jul 1951, Aug 1981–Jul 1982, Oct 2005 | DMB 1954, BKJC 1983, PP 2008 |
| Assiminea bifasciata Nevill, 1880 | Assiminea cf. capensis Bartsch, 1915 | Jul 1964 & Jan 1965, Jan–Jul 1972 & Jan 1973 | ISAM (STL104G), MB 1970, B 1975 |
| Assiminea durbanensis | Assimineidae sp. or “Assiminea” aff. capensis (Sowerby, 1892)* | Jan & May 1992 | W 1993 |
| Assiminea cf. ovata (Krauss, 1848) | Assiminea cf. capensis Bartsch, 1915 | 2007–2009 | MPA2 2011 |
| Assiminea cf. capensis Bartsch, 1915 | Idem | Jan 1927, 1987, 2011-2013 | NMSA (1987), RMP 2013, WPPC 2014 |
| Coriandria durbanensis (Tomlin, 1916) | Assimineidae sp. or “Assiminea” aff. capensis (Sowerby, 1892)* | Jul 1948–Jul 1951, Jul 2012-May 2013 | ISAM (STL18A), DMB 1954, RMP 2013, MET 2014 |
| Syncera sp. Gray, 1821 | Nomen nudum; probably confused with Assiminea sp. | Jul 1948, Jul 1964 & Jan 1965 | ISAM (STL64A), MB 1970 |
| CASSIDAE | |||
| Phalium areola (Linnaeus, 1758) | Idem* | Not reported (sine die) | NMSA (B4786) |
| NASSARIIDAE | |||
| Nassa kraussiana (Dunker, 1846) | Nassarius kraussianus (Dunker, 1846) | Jul 1964 & Jan 1965 | MB 1970 |
| Nassarius kraussianus (Dunker, 1846) | Idem | Jul 1948–Jul 1951, Feb 1971, Dec 1972, Jan–Jul 1972 & Jan 1973, Dec 1981, Aut 2005 & Spr 2006 | NMSA (B5533, W1752, 9144), ISAM (STL6C), DMB 1954, B 1975, MCR 2010 |
| MURICIDAE | |||
| Ergalatax heptagonalis (Reeve, 1846) | Idem* | 1987 | NMSA (D5772) |
| Murex brevispinus Lamarck, 1822 | Idem* | Jul-Nov 2012 | PMRP 2014 |
| Purpura bufo Lamarck, 1822 | Idem* | Jul 2013 | PMRP 2014 |
| HAMINOEIDAE | |||
| Cylichna africana Bartsch, 1915 | Haminoea natalensis (Krauss, 1848) or Cylichna tubulosa Gould, 1859 | Jul 1972 & Jan 1973 | B 1975 |
| Haminea gracilis (Sowerby, III 1897) | Haminoea natalensis (Krauss, 1848) | Jul 1964 & Jan 1965 | MB 1970 |
| Haminoea natalensis (Krauss, 1848) | Idem | Jul 1948–Jul 1951, Dec 1962, Apr 1963, Apr 1965, Jun 1987, 2006, 2007 | NMSA (A2362, A2228, D9971, E478), ISAM (STL6B), DMB 1954, PP 2008, MPA2 2011 |
| Haminea petersi (Martens, 1879) | Haminoea natalensis (Krauss, 1848) | Aug 1981–Jul 1982 | BKJC 1983 |
| APLYSIIDAE | |||
| Barnardaclesia cirrhifera (Quoy & Gaimard, 1832) | Bursatella leachii Blainville, 1817 | Jul 1948-Jul 1951 | DMB 1954 |
| Notarchus cirrhifera (Quoy & Gaimard, 1832) | Bursatella leachii Blainville, 1817 | Jul 1964 & Jan 1965 | MB 1970 |
| Stilocheilus striatus (Quoy & Gaimard, 1832) | Idem | May 2007 | MPA2 2011 |
| SIPHONARIIDAE | |||
| Siphonaria oculus Krauss, 1848 | Idem | Jul 1948–Jul 1951, Jul 1964 & Jan 1965 | ISAM (STL43A), DMB 1954, MB 1970 |
| PLANORBIDAE | |||
| Bulinus natalensis (Krauss in Küster, 1841) | Idem* | Nov 2012 | PMRP 2014 |
| Bulinus tropicus (Krauss, 1848) | Idem | Jul 1948, Jul 1964 & Jan 1965, May 2002–Apr 2003 | ISAM (STL104G), DMB 1954, MB 1970, PM 2013; VMT May 2002-Apr 2003 |
| Bulinus forskalii (Ehrenberg, 1831) | Idem | May 2002-Apr 2003 | V 2004 |
| PHYSIDAE | |||
| Aplexa marmorata (Guilding, 1828) | Idem | Aug 2009, 2009–2010 | MPA1 2010, MPA2 2011 |
| LYMNAEIDAE | |||
| Lymnaea natalensis (Krauss, 1848) | Idem* | 1982–1983 | NPB 1982-1983 |
| Pseudosuccinea columella (Say, 1817) | Idem | Aug 2009 | MPA1 2010 |
| SUCCINEIDAE | |||
| Oxyloma patentissima (Menke in Pfeiffer, 1853) | Idem | May 2002–Apr 2003 | V 2004 |
| ELLOBIIDAE | |||
| Cassidula labrella (Deshayes, 1830) | Idem | Jul 1964 & Jan 1965 | ISAM (STL237M), MB 1970, PMRP 2014 |
| Melampus ordinarius Melvill & Ponsonby, 1901 | Melampus lividus (Deshayes, 1830) | Jul 1964 & Jan 1965 | ISAM (STL237Y), MB 1970 |
| Melampus parvulus Pfeiffer, 1856 | Idem* | 2012–2013 | PMRP 2014 |
| Melampus semiaratus Connolly, 1912 | Idem | Jul 1964 & Jan 1965, Mar 2012 | ISAM (STL237N), MB 1970 |
The earliest records of gastropod specimens collected in the St Lucia system are from the KwaZulu-Natal Museum (NMSA) and date back as far as 1924. Seventeen species originating from St Lucia are currently reposited in its collections, mostly obtained during dedicated surveys conducted in 1987 (Table 2). Of these, 11 are among the new records reported here, as they were never included in previous reports or publications on Lake St Lucia. Another two of the new records were collected during surveys undertaken by the provincial conservation authority, EKZNW, while the other six were only revealed during the latest survey of 2012–2013. Although three among the latter were only recorded as dead shells (i.e. Cerithium dialeucum, Murex brevispinus, Purpura bufo), they were found in sufficient number and deep enough in the upper reaches of the estuarine lake to suggest with reasonable confidence they were once established within the system, rather than accidentally introduced there.
Only 12 species were found alive within the system during the recent 2012–2013 survey. These include Aplexa marmorata, Assiminea cf. capensis, Assimineidae sp., Bulinus natalensis, Cassidula labrella, Cerithidea decollata, Lymnaea natalensis, Melampus parvulus, Melampus semiaratus, Melanoides tuberculata, Pterotrachea cf. hippocampus and Tarebia granifera (Figure 2). Among them, five are freshwater dwellers (Aplexa marmorata, Bulinus natalensis, Lymnaea natalensis, Melanoides tuberculata and Tarebia granifera) that have entered the system only recently, in response to the establishment of a new wet phase after the prolonged drought of 2002–2011. Melanoides tuberculata was found in high abundance at Shark Basin, at shallow depths in permanently submerged channels, as well as in a tributary stream (Mpophomeni) at False Bay (Tables 1–2, Appendix). Two of these freshwater species, i.e. Aplexa marmorata and Tarebia granifera, are actually alien invasives that have spread from colonies initially restricted to the seepage points around Catalina Bay (Appendix). Tarebia granifera was recorded in high abundance at Makakatana in March 2012, spreading subsequently throughout the Narrows to the south and at least as far as Charter’s Creek to the north-west (Figure 1, Table 1).
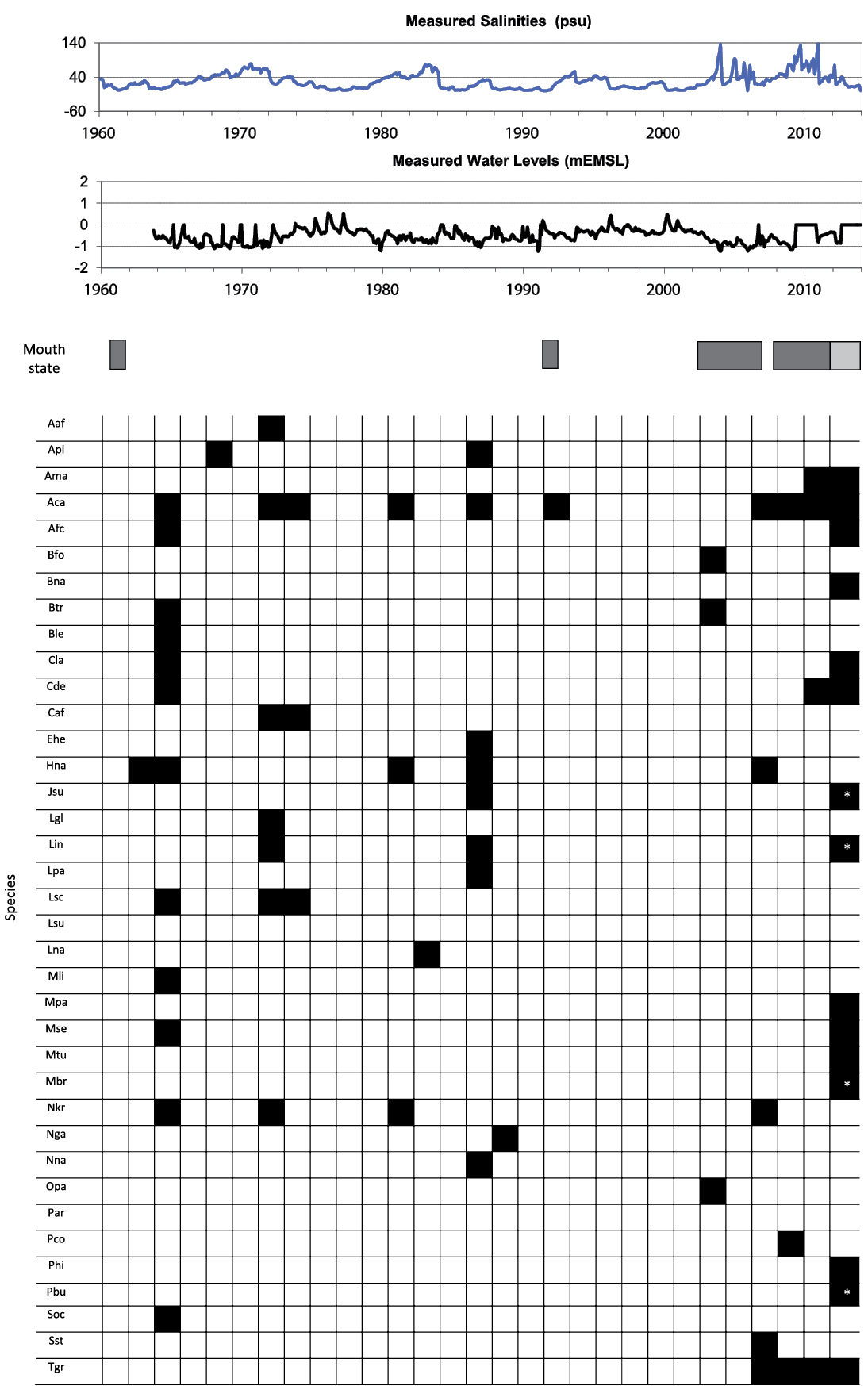
Records of gastropod species collected at Lake St Lucia in relation to changes in salinity, water levels and mouth state during the period 1960-present. Dark gray bar indicates closed mouth, light gray bar indicates intermittent connection with the ocean. No continuous physico-chemical measurements are available for the period prior to 1960. Species codes: Aaf: Afrolittorina africana; Api: Alaba pinnae; Ama: Aplexa marmorata; Aca: Assiminea cf. capensis; Afc: Assimineidae sp. (“Assiminea” aff. capensis); Bfo: Bulinus forskalii; Bna: Bulinus natalensis; Btr: Bulinus tropicus; Ble: Bursatella leachii; Cla: Cassidula labrella; Cde: Cerithidea decollata; Cdi: Cerithium dialeucum; Ehe: Ergalatax heptagonalis; Hna: Haminoea natalensis; Jsu: Jujubinus suarezensis; Lgl: Littoraria coccinea glabrata; Lin: Littoraria intermedia; Lpa: Littoraria pallescens; Lsc: Littoraria scabra; Lsu: Littoraria subvittata; Lna: Lymnaea natalensis; Mli: Melampus lividus; Mpa: Melampus parvulus; Mse: Melampus semiaratus; Mtu: Melanoides tuberculata; Mbr: Murex brevispinus; Nkr: Nassarius kraussianus; Nga: Neritina gagates; Nna: Neritina natalensis; Opa: Oxyloma patentissima; Par: Phalium areola; Pco: Pseudosuccinea columella; Phi: Pterotrachea cf. hippocampus; Pbu: Purpura bufo; Soc: Siphonaria oculus; Sst: Stylocheilus striatus; Tgr: Tarebia granifera.
Cassidula labrella, Cerithidea decollata, Melampus parvulus and Melampus semiaratus are the only mangrove species that have been able to survive within the system, despite the closed mouth conditions that have prevailed since 2002. Cerithidea decollata was the only one among them to be found in abundance at all mangrove sites, including the St Lucia Bridge, Back Channel, Honeymoon Bend (Narrows) and Shark Basin near the St Lucia Mouth (Figure 1, Table 1). On the other hand, Cassidula labrella, Melampus parvulus and Melampus semiaratus were only found in the mangroves at Shark Basin and in very low numbers, on shaded mud surfaces and under fallen wood.
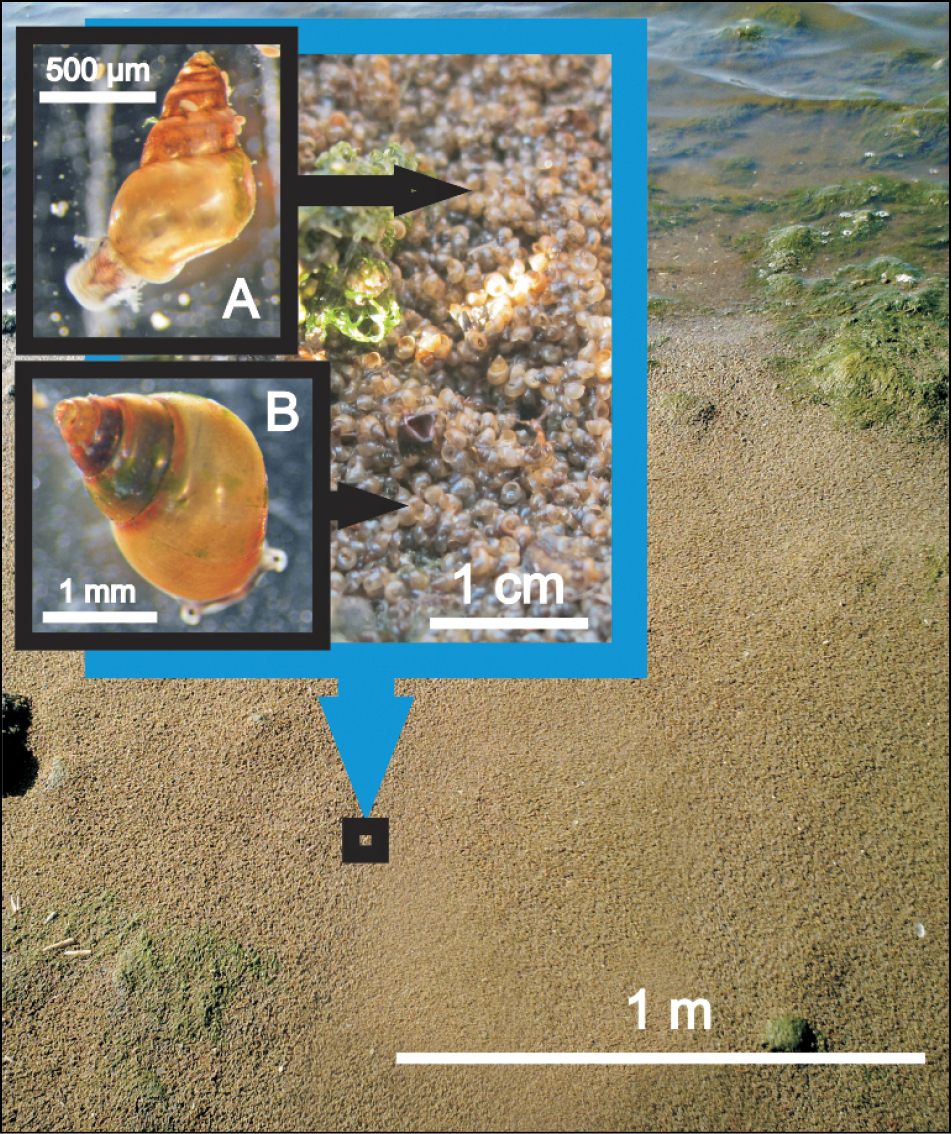
Of the typical estuarine species recorded in all surveys undertaken in the past in Lake St Lucia, only Assiminea cf. capensis and Assimineidae sp. persisted through the latest survey (
Assimineidae sp. (A) and Assiminea cf. capensis (B): thick layer of snails washed up on the shoreline of Lister’s Point at False Bay in July 2012 (Photo: Nelson AF Miranda).
Species that were not found alive during the 2012–2013 survey, but have been previously documented as dominant within the system include Nassarius kraussianus and Haminoea natalensis. Both were only recorded as dead shells during 2012-2013, but in very large numbers and throughout the lake basins, particularly at Charter’s Creek, Catalina Bay and Fani’s Island (South Lake) (Figure 1, Table 1). While the last live record of Nassarius kraussianus during the recent closed mouth phase of the estuary dates back to the spring of 2006 (
Haminoea natalensis: Aggregation of egg masses spawned during September 2006 in the shallows of Catalina Bay, on the Eastern Shores of South Lake (Photo: Lynette Clennell).
Among the mangrove dwellers that were present in the past but have recently disappeared entirely from the system are all the Littoraria species, i.e. Littoraria coccinea glabrata, Littoraria intermedia, Littoraria pallescens, Littoraria subvittata and Littoraria scabra (Table 2, Figure 2). Although the estuarine lake has experienced a large freshwater inflow since late 2011, several freshwater species that were previously found within the system were not recorded alive in 2012–2013. These include both Neritina species, i.e. Neritina gagates and Neritina natalensis, as well as Bulinus tropicus, Bulinus forskalii, Pseudosuccinea columella and Oxyloma patentissima (Figure 2, Appendix).
Typical estuarine and/or coastal marine species that are also among the new records may have entered the system only on sporadic occasions and/or for short periods of time under open mouth conditions. They include Afrolittorina africana, Alaba pinnae, Ergalatax heptagonalis and Phalium areola. All of them are represented in past collections from the KwaZulu-Natal Museum (Table 2). The common coffee-bean snail Melampus lividus, the estuarine limpet Siphonaria oculus and the opisthobranch Bursatella leachii were already reported in the earliest surveys of the University of Cape Town (
Major climatic events and hydrodynamic processes control the gastropod species richness and abundance in the St Lucia estuarine lake (Figure 2). The highest diversity reported so far coincides with the period Jul 1964 – Jan 1965, when the second survey by the University of Cape Town (UCT) was conducted on the system. On that occasion, 12 gastropod species were recorded at a time when the estuarine lake was under tidal influence, with a normal salinity gradient decreasing from the estuary basin to the northern lakes (
During the last decade, St Lucia has undergone some of the most dramatic shifts ever recorded in the region. These have caused an unprecedented crisis and triggered a burst of fresh research activity on the system. It is thus not surprising that of the total 37 species of gastropod recorded within the estuarine lake, 19 are new records arising from the recent escalation in analyses and collecting efforts. During the latest dedicated gastropod survey, undertaken between Jan 2012 and Nov 2013, a total of 15 species were recorded, with only 12 found still alive and four in reasonable abundance, even if intermittently. Among the latter group, two are actually alien invasive species, i.e. Tarebia granifera and Aplexa marmorata (Figure 2).
In 2002, a sand berm closed off the St Lucia Estuary from the ocean, leading to a prolonged period of mouth closure, which still persists currently. The mouth was breached from the seaward side for a brief period of six months, between March and August 2007, by a combination of extreme events linked to Cyclone Gamede (
Since the end of 2011, the system has entered a wet phase, with above average rainfall leading to occasional flooding and the prevalence of oligo- to polyhaline conditions throughout the extent of the system. This was compounded by the excavation of a beach spillway in July 2012, which has since contributed substantial freshwater inflow from the Mfolozi River into the St Lucia system and also partial exchange of water with the open ocean (
Historical collections and surveys have, however, recorded numerous species of euryhaline marine and estuarine species, even in the uppermost reaches of the estuarine lake. For instance, the tick shell Nassarius kraussianus is present in all museum collections from St Lucia and has been recorded as abundant in all previous studies in the area (Table 2, Figure 2). Despite the numerous dead shells retrieved during the past few years throughout the system, it has not been found alive since 2006 (
Apart from causing the disappearance of marine grasses, prolonged mouth closure would also lead to the eventual death of barnacle and oyster beds (
Among the 20 species not previously recorded from the St Lucia estuarine lake are typical mangrove dwellers, such as Littoraria intermedia, Littoraria pallescens, Littoraria subvittata, Littoraria scabra and Melampus parvulus. St Lucia mangroves have undergone significant deterioration since the mouth closed in 2002, as persistent low salinity in the Narrows and near the mouth has favoured the development of reeds at the expense of mangrove vegetation (
Population explosions of the bubble shell Haminoea natalensis with its distinct egg masses were recorded seasonally until 2009 (Figures 2 and 4). The observed trend of population explosions followed by high mortality and dwindling numbers is typical for opisthobranchs. Environmental conditions during the different seasons as well as the recruitment of Haminoea natalensis, are the most important factors driving its population biology (
The taxonomy of assimineids, or sentinel snails, is poorly understood and currently under revision in South Africa. In the St Lucia Estuary, there are inconsistencies in the literature in terms of what species of Assiminea occur in the system. This is not surprising given the morphological and ecological similarities as well as spatial overlap between different assimineids. Earlier literature refers to Assiminea bifasciata as the only species present in the system (
Three of the five predominant alien invasive freshwater gastropods in South Africa have been recently recorded from St Lucia: Aplexa marmorata, Pseudosuccinea columella and Tarebia granifera. Previously, under hypersaline conditions these species were restricted to freshwater seepage areas on the Eastern Shores of the South Lake and along the Narrows (
As the freshwater-dominated phase of St Lucia continues, the potential for alien invasive gastropods to enter and spread within the system increases. The consequences of these expansions vary depending on the species. Pseudosuccinea columella in South Africa is susceptible to the liver flukes (Fasciola spp.) which infect livestock, although it has not been confirmed as an intermediate host (
In addition to the threats from freshwater alien invasive species, estuaries are also threatened by the invasion of marine species from coastal sources (
Throughout its history, the St Lucia estuarine lake has experienced drastic shifts in hydrological states, from extreme dry conditions accompanied by hypersalinity and desiccation, to floods followed by freshwater dominance. The state of the mouth has also varied from an extended open bay joined to the Mfolozi River to extreme constriction and prolonged closure. The latest period of closure has been unprecedented and virtually uninterrupted since 2002. Although the monitoring of gastropod diversity within the system has been erratic until recently, there are clear indications that higher diversity has coincided with periods when the estuarine lake was under tidal influence, with a normal salinity gradient decreasing from the estuary basin to the northern lakes (e.g. 1964–1965). Drastic declines were observed when the system experienced hypersaline (e.g. 1948–1951) or flood conditions (e.g. 1987), with a closed mouth state compounding the problem by preventing any recruitment from the ocean. During the last decade, St Lucia has undergone some of the most dramatic shifts ever recorded in the region. Despite the intense, dedicated gastropod surveys undertaken in 2012-2013, only 12 species were found still alive, with four in reasonable abundance. Among these, unfortunately two are actually alien invasive species, i.e. Tarebia granifera and Aplexa marmorata, with the first spreading at alarming rates as low salinity conditions now prevail throughout the system.
We thank the iSimangaliso Park Authority and Ezemvelo KZN Wildlife for providing permits and logistical support for this project. Special thanks go to Mthobisi S. Ngubane, Ricky H. Taylor and Lynette Clennell for contributing with field collections and laboratory analyses. Elizabeth Hoenson (ISAM, Cape Town), Dai Herbert (NMSA, Pietermaritzburg) and Winston Ponder (Australian Museum, Sydney) are thanked for assisting with specimen identifications and access to museum records. We are also grateful to Frank Wesselingh and an anonymous reviewer for their valuable comments on the draft manuscript. This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology (DST) and National Research Foundation (NRF) of South Africa. Any opinion, finding and conclusion or recommendation expressed in this material is that of the author(s) and the NRF does not accept any liability in this regard.
Annotated and Illustrated Checklist of the Gastropod Molluscs of Lake St Lucia
Common name. Square top –snail.
Size. Maximum shell height 12 mm(
Remarks. Occurs on protected mudflats(
Distribution. East African distribution including Madagascar. Common in Mozambique extending south to Durban in South Africa(
St Lucia records. Not previously reported from St Lucia; collected in 1987 (NMSA). Only dead shells collected in 2012 from Fani’s Island (South Lake).
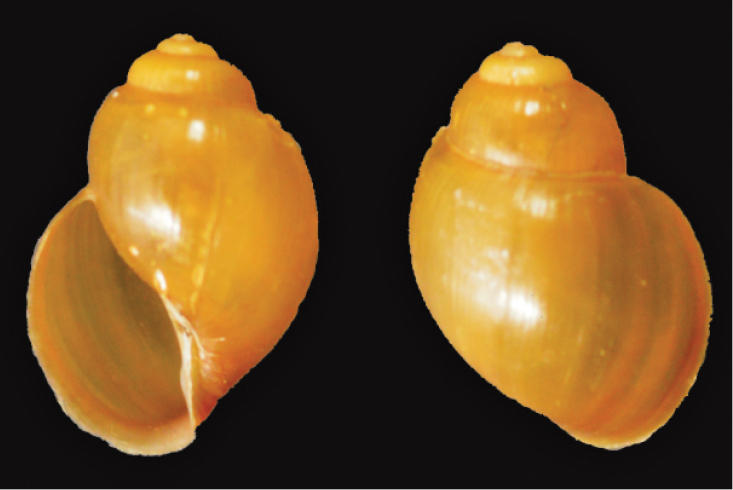
Jujubinus suarezensis (P. Fisher, 1978)
17 mm, Inhaca Island, Jul 1970
RN Kilburn leg.
Common name. Brown nerite or Zebra nerite.
Size. Maximum shell height 22 mm(
Remarks. Occurs on stones in streams that are tidally influenced(
Distribution. Southeastern coast of Africa from Mozambique to Mzamba in the Eastern Cape Province of South Africa(
St Lucia records. Not previously reported from St Lucia; collected in 1988 from the St Lucia Mouth (NPB). Not found during the recent survey.
Neritina gagates Lamarck, 1822
19 mm, Mhlanga Estuary, Feb 2013
NAF Miranda leg.
Common name. Spotted nerite.
Size. Maximum shell height 20 mm(
Remarks. Reportedly occurs within mangroves(
Distribution. East African coast from Somalia extending south to the Umzimkulu River on the KwaZulu-Natal coast of South Africa(
St Lucia records. Not previously reported from St Lucia; collected in 1924 and 1987 (NMSA) from False Bay. Not recorded in the recent survey.
Neritina natalensis Reeve, 1855
23 mm, St Lucia False Bay, Oct 1924
HWB Marley leg.
Common name. Red-rimmed melania.
Size. Maximum shell height 45 mm(
Remarks. Predominantly parthenogenetic species(
Distribution. Natural Indo-Pacific distribution includes much of Africa with a southern limit at Port Elizabeth(
St Lucia records. Reported in 2012(
Melanoides tuberculata (Müller, 1774)
23 mm, St Lucia False Bay, Feb 2013
NAF Miranda leg.
Melanoides tuberculata (Müller, 1774)
14 mm, St Lucia Mouth, Feb 2013
NAF Miranda leg.
Common name. Quilted melania(
Size. Maximum shell height 29.5 mm(
Remarks. Alien invasive species first recorded in northern KwaZulu-Natal in 1999(
St Lucia records. Reported from St Lucia in 2006(
Tarebia granifera (Lamarck, 1822)
21 mm, St Lucia Catalina Bay, Feb 2010
NAF Miranda leg.
Common name. White-studded cerith.
Size. Up to 30.5 mm.
Remarks. Dead shells collected at Lister’s Point in March 2012.
Distribution. Indo-Pacific distribution with southwestern reports from Mozambique(
St Lucia records. Not previously reported.
Cerithium dialeucum Philippi, 1849
30.5 mm, St Lucia False Bay, Mar 2012
R Perissinotto leg.
Common name. Pinnated litiopid.
Size. Maximum shell height 11 mm(
Remarks. Common estuarine species which varies in plumpness(
Distribution. Still Bay to Zululand coast(
St Lucia records. Not previously reported from St Lucia; collected at Charter’s Creek (South Lake) in 1967 and 1987 respectively (NMSA). Not found in the recent survey.
Alaba pinnae (Krauss, 1848)
9.3 mm, St Lucia Charter’s Creek, 1967
R Kilburn leg.
Common name. Truncated mangrove snail(
Size. Maximum shell height 36 mm(
Remarks. Common mangrove climbing whelk also found in salt marshes on mud beneath vegetation(
Distribution. Indo-pacific mangrove species. South African distribution extends from Knysna along southeastern coast into Mozambique(
St Lucia records. Reported in previous surveys from 1948–51(
Cerithidea decollata (Linnaeus, 1767)
36 mm, St Lucia Bridge, Nov 2012
NAF Miranda leg.
Common name. African periwinkle(
Size. Shell height 5–8 mm(
Remarks. Typical high intertidal species which occurs in large numbers usually on exposed rocks(
Distribution. Southwestern Indian Ocean(
St Lucia records. Not previously reported from St Lucia; collected in 1971 from “St Lucia River Estuary” (NMSA). Not recorded in the recent survey.
Afrolittorina africana (Krauss in Philippi, 1847)
7.8 mm, Zululand, Sep 1971
R Kilburn leg.
St Lucia synonyms. Littorina glabrata Philippi, 1846.
Common name. Striped periwinkle(
Size. Maximum shell height 24 mm(
Remarks. High resistance to desiccation(
Distribution. Tropical and subtropical Indian Ocean distribution(
St Lucia records. Not previously reported from St Lucia; collected in 1971 (NMSA) from the St Lucia Estuary. Not found during the recent survey.
Littoraria coccinea glabrata (Philippi, 1846)
14 mm, St Lucia Estuary, Nov 1971
R Kilburn leg.
Common name. Estuarine periwinkle(
Size. Shell height 14–26 mm(
Remarks. Associated with roots and trunks of Rhizophora and occasionally Bruguiera mangrove trees(
Distribution. Tropical and subtropical Indo-Pacific distribution from the east African coast including the Red Sea and east to Polynesia(
St Lucia records. Not previously reported from St Lucia; collected in 1971 and 1987 (NMSA) from St Lucia Estuary and Shark Basin respectively. Only dead shells retrieved recently from Shark Basin (St Lucia Mouth).
Littoraria intermedia (Philippi, 1846)
13 mm, St Lucia River Estuary, Nov 1971
RN Kilburn leg.
Common name. Pale periwinkle
Size. Shell height 15–25 mm(
Remarks. Commonly occurs on the leaves of Rhizophora in mangrove forests(
Distribution. Tropical to sub-tropical Indo-Pacific distribution extending from east African coast to Samoa(
St Lucia records. Not previously reported from St Lucia; collected in 1987 (NMSA) from the St Lucia Estuary. Not found during the recent survey.
Littoraria pallescens (Philippi, 1846)
15 mm, St Lucia Estuary, Aug 1987
RH Taylor leg.
Common name. Aldabra periwinkle.
Size. Shell height 13–34 mm(
Remarks. Occurs in mangroves and marsh grass habitats as well as on sheltered rocks(
Distribution. Coastal Indian Ocean distribution from Aden, Yemen(
St Lucia records. Not previously reported from St Lucia; collected by NMSA at unknown date. Not found during the recent survey.
Littoraria subvittata (Reid, 1986)
18 mm, Lake St Lucia
Burnup Collection.
Common name. Mangrove periwinkle.
Size. Shell height 25–35 mm(
Remarks. Characteristic mangrove and estuarine species found above high water(
Distribution. Tropical and sub-tropical Indo-Pacific distribution extending from the eastern African coast across to Samoa and Hawaii(
St Lucia records. Reported from St Lucia in 1948–51(
Littoraria scabra (Linnaeus, 1758)
14 mm, Kosi Bay Estuary, July 1987
DG Herbert leg.
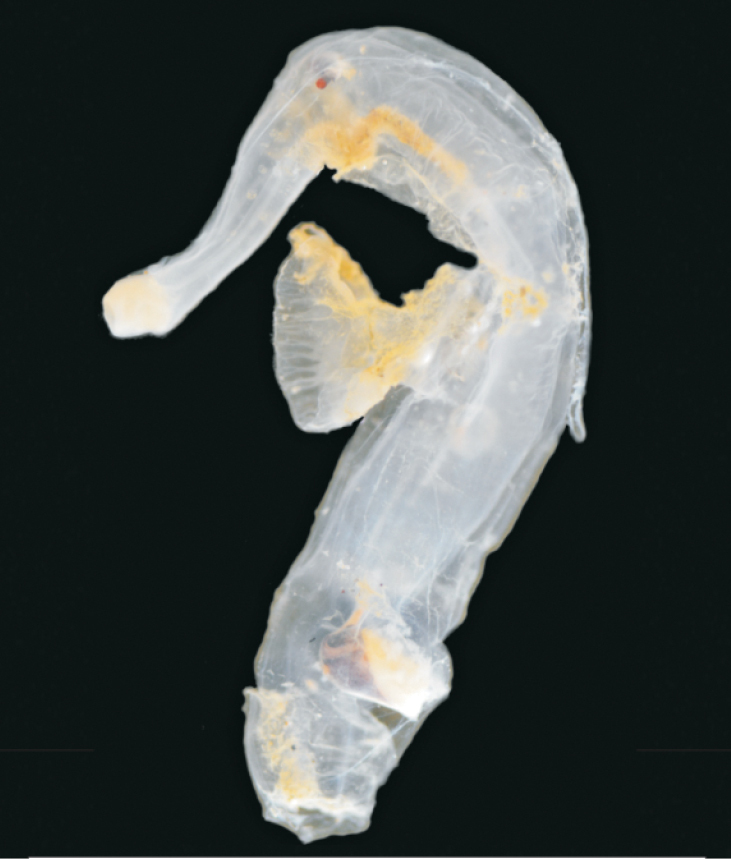
Common name. Sea elephant.
Size. Up to 34 mm.
Remarks. Pelagic marine species related to Charonia spp. (Ranellidae). The species is expected to occur more frequently in the system as the marine connection is maintained.
Distribution. Circumtropical distribution including the Gulf of Mexico(
St Lucia records. Not previously reported from St Lucia; recorded in the recent survey in 2013 from the Mfolozi-St Lucia Beach Spillway (St Lucia Mouth).
Pterotrachea cf. hippocampus Philippi, 1836
34 mm, Mfolozi Channel, Aug 2013
NK Carrasco leg.
St Lucia synonyms. Assiminea bifasciata Nevill, 1880; Coriandria durbanensis (Tomlin, 1916); Rissoa capensis Sowerby, 1892.
Common name. False sentinel.
Size. Up to 2.22 mm.
Remarks. Taxon under revision. Provisional name based on genetics and shell morphometrics(
St Lucia records. Probably combined in the 1948–51(
Assimineidae sp.
0.8 mm, St Lucia False Bay, Jul 2012
NAF Miranda leg.
St Lucia synonyms. Assiminea bifasciata Nevill, 1880; Assiminea cf. ovata (Krauss, 1848); Syncera sp. Gray, 1821;
Common name. Sentinel snail.
Size. Maximum shell height 7 mm(
Remarks. Dominant in estuaries on the east coast(
Distribution. Southern African species extending from Langebaan to Mozambique(
St Lucia records. Previous records in 1948–51(
Assiminea cf. capensis Bartsch, 1915
2 mm, St Lucia Catalina Bay, Nov 2012
NAF Miranda leg.
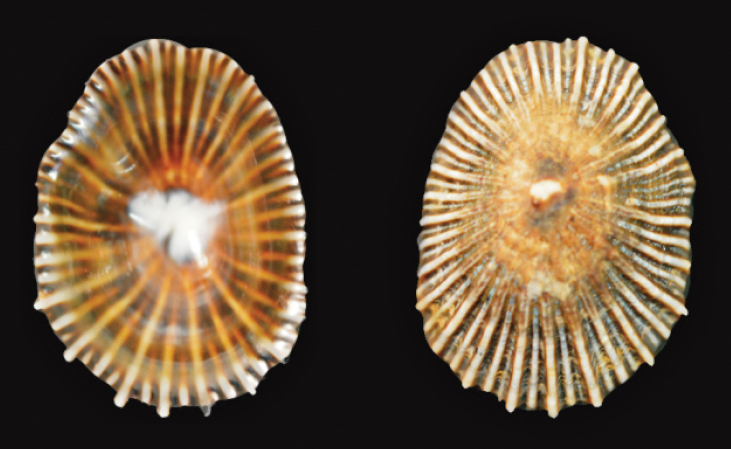
Common name. Checkerboard bonnet.
Size. Maximum shell height 69 mm(
Remarks. Inhabits sandy environments, known to bury itself in the substrate(
Distribution. Indo-Pacific distribution including the KwaZulu-Natal coast(
St Lucia records. Not previously reported from St Lucia; single specimen collected from the St Lucia Mouth by NMSA at an unspecified date. Not reported in the recent survey.
Phalium areola (Linnaeus, 1758)
55 mm, St Lucia Bay, 1978
M Lavoipierre leg.
St Lucia synonyms. Nassa kraussiana (Dunker, 1846).
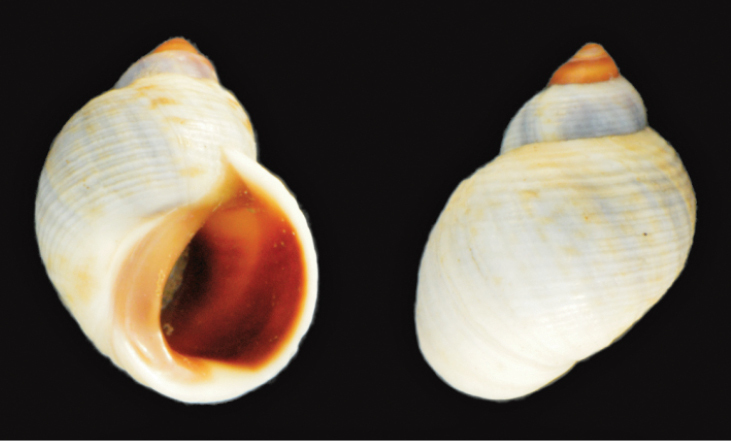
Common name. Tick shell(
Size. Shell height 7.5–10 mm(
Remarks. Characteristic estuarine species found in salt marshes and lagoonal mudbanks(
Distribution. Southern African distribution from the Namaqualand coast(
St Lucia records. Reported in 1948–51(
Nassarius kraussianus (Dunker, 1846)
11 mm, St Lucia Charter’s Creek, Mar 2012
NAF Miranda leg.
Common name. Heptagonal rock snail.
Size. Maximum shell length 30 mm(
Remarks. Common under rocks or logs on muddy sand of estuarine bays(
Distribution. Indo-pacific distribution including the KwaZulu-Natal coast(
St Lucia records. Not previously reported from St Lucia; collected in 1987(NMSA) from the Mfolozi Link Canal (St Lucia Mouth). The specimen collected was probably exposed after excavation of the Link Canal as it is a Pleistocene subfossil. Not found during the recent survey, however the species may re-colonize St Lucia if the marine connection is maintained.
Ergalatax heptagonalis (Reeve, 1846)
25 mm, Mission Rocks, Apr 1987
R Kilburn leg.
Common name. Short spined murex(
Size. Maximum shell height 82 mm(
Remarks. Occurs on protected intertidal sandbanks, often among eelgrass(
Distribution. Indian Ocean distribution along the eastern coast of Africa extending from Kenya to Durban(
St Lucia records. Not previously reported from St Lucia; only dead shells retrieved from Lister’s Point (False Bay) and Fani’s Island (South Lake) in the recent survey.
Murex brevispinus Lamarck, 1822
71 mm, Durban, Aug 1968
BJ Young leg.
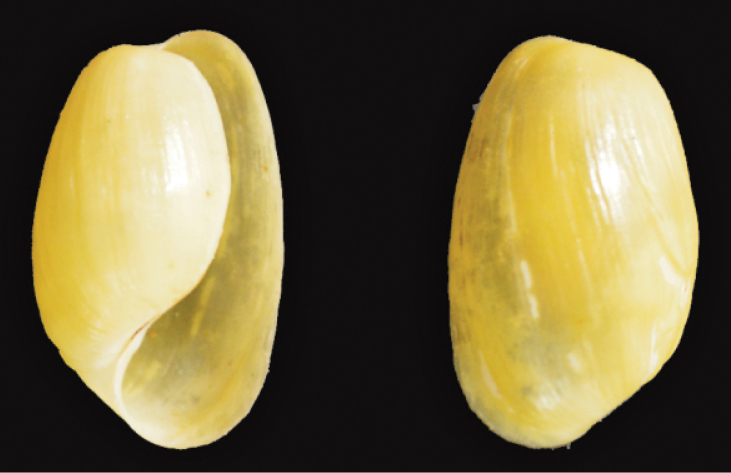
Common name. Toad purpura.
Size. Maximum shell height 70 mm.
Remarks. Common in rock pools on the KwaZulu-Natal coast. Specimen illustrated is a sub-adult.
Distribution. Tropical and sub-tropical Indian Ocean(
St Lucia records. Not previously collected or reported from St Lucia. Only one dead shell was found at False Bay during the recent survey.
Purpura bufo Lamarck, 1822
52 mm, St Lucia False Bay, Jul 2013
NAF Miranda leg.
St Lucia synonyms. Haminoea petersi (Martens, 1879); Haminoea gracilis (Sowerby, 1897); Cylichna africana Bartsch, 1915; Cylichna tubulosa Gould, 1859.
Common name. Natal bubble shell.
Size. Maximum shell length 19 mm(
Remarks. Inhabits shallow water including tidal rock pools(
Distribution. On the South African coast, replaces Haminoea alfredensis from the Transkei into northern KwaZulu-Natal(
St Lucia records. Probably misidentified in 1972–73(
Haminoea natalensis (Krauss, 1848)
18 mm, St Lucia Charter’s Creek, Apr 1963
AC van Bruggen leg.
Haminoea natalensis (Krauss, 1848)
11 mm, St Lucia Catalina Bay, May 2007
L Clennell leg.
St Lucia synonyms. Barnardaclesia cirrhifera (Quoy & Gaimard, 1832); Notarchus cirrhifera (Quoy & Gaimard, 1832).
Common name. Shaggy Sea Hare, Ragged Sea Hare.
Size. Maximum length 130 mm(
Remarks. Common estuarine species which also occurs in tide pools(
Distribution. Cicumtropical species reported from the east coast of Africa(
St Lucia records. Reported as Barnardaclesia cirrhifera in 1948–51(
Bursatella leachii Blainville, 1817
175 mm, Salt Rock, Aug 2009
DG Herbert leg.
Common name. Lined Sea Hare.
Size. 28 mm(
Remarks. Occurs in sheltered pools and estuaries, often sympatric with Bursatella leachii.
Distribution. Circumtropical distribution including Cape Verde(
St Lucia records. Recorded in 2007(
Stylocheilus striatus (Quoy & Gaimard, 1832)
53 mm, St Lucia Estuary, Apr 2007
C Fox leg.
Common name. Eyed false-limpet(
Size. Maximum length 33 mm(
Remarks. Locally common species which is found on sheltered rocks in lagoons and estuaries(
Distribution. Southern African distribution extends along the coast from False Bay in the Western Cape Province to Mozambique(
St Lucia records. Reported in 1948–51(
Siphonaria oculus Krauss, 1848
30 mm, Durban Bay, Sep 1972
BJ Young leg.
Common name. Natal bladder snail
Size. 9.6 × 8.5 mm (depressed form) 9.5 × 6.5 mm (high spired form)(
Remarks. Wide range of habitats including small pools, slow flowing rivers and lakes(
Distribution. East African distribution extending from Ethiopia to the northern coastal region of KwaZulu-Natal(
St Lucia records. Not previously reported from St Lucia; recorded in the recent survey at Catalina Bay (South Lake) in 2012.
Bulinus natalensis (Krauss in Küster, 1841)
14 mm, St Lucia Catalina Bay, Nov 2012
JL Raw leg.
Common name. Tropical bladder snail.
Size. 12.3 × 7.8 mm (slender form), 10.6 × 8.3 mm (more globose form)(
Remarks. Commonly occurs in small earth dams and residual pools of seasonally flowing streams(
Distribution. Eastern and southern Africa, extending from Ethiopia to Namibia and the Western Cape of South Africa. Not commonly found on the eastern coastal region of South Africa(
St Lucia records. Reported in 1948(
Bulinus tropicus (Krauss, 1848)
16 mm, Durban, Mar 2002
D Nadasan leg.
Common name. Forskål’s bladder snail.
Size. 17 × 5.4 mm(
Remarks. Ability to aestivate allows this species to commonly inhabit seasonal pools(
Distribution. Afrotropical distribution extending south from the Egyptian Mediterranean region to Namibia(
St Lucia records. Reported in 2002–03(
Bulinus forskalii (Ehrenberg, 1831)
8.1 mm, Uvongo, Mar 1996
M Coke leg.
Common name. Slender bladder snail(
Size. 15 × 8 mm(
Remarks. Alien species introduced from South America(
Distribution. Occurs in isolated populations in KwaZulu-Natal, Mpumalanga and Limpopo(
St Lucia records. Reported in 2005(
Aplexa marmorata (Guilding, 1828)
12 mm, St Lucia Catalina Bay, Feb 2010
NAF Miranda leg.
Common name. Natal pond snail.
Size. Maximum shell height 25 mm(
Remarks. Occurs in permanent streams(
Distribution. East African distribution including the highlands of Ethiopia(
St Lucia records. Not previously reported from St Lucia; collected in 1982 (NPB). Reported in the recent survey from Catalina Bay (South Lake) in 2012.
Lymnaea natalensis (Krauss, 1848)
12 mm, St Lucia Catalina Bay, Nov 2012
NAF Miranda leg.
Common name. Reticulate pond snail.
Size. 17 × 9 mm(
Remarks. Alien species introduced from North America. Occurs on damp mud at the water-air interface(
Distribution. Widely introduced to many areas, including Puerto Rico, Europe and New Zealand. First reported from Africa in 1944 from the Western Cape Province of South Africa(
St Lucia records. Reported in 2009(
Pseudosuccinea columella (Say, 1817)
11 mm, St Lucia Catalina Bay, Jul 2009
NAF Miranda leg.
Common name. Twisted amber snail(
Size. Maximum shell length 10 mm(
Remarks. Typically occurs on emergent vegetation alongside water(
Distribution. Southern African distribution includes Mozambique, northern Botswana and Zimbabwe(
St Lucia records. Reported in 2002–03(
Oxyloma patentissima (Menke in Pfeiffer, 1853)
8.8 mm, Mhlanga Lagoon, Dec 1995
D Herbert & L Davis leg.
Common name. Keeled coffee-bean snail.
Size. 12 × 7.5 mm(
Remarks. Typically found on the surface of firm mud in mangroves and salt marshes(
Distribution. East African coastal distribution from the Massawa region of the Red Sea to Port Elizabeth in South Africa(
St Lucia records. Reported in 1964–65(
Cassidula labrella (Deshayes, 1830)
12 mm, St Lucia Mouth, Mar 2012
N Peer leg.
Common name. Common coffee-bean snail(
Size. Maximum shell height 18 mm(
Remarks. Reported as Melampus ordinarius Melvill & Ponsonby, 1901 from deep vertical cracks in high level outcrops at Mission Rocks(
Distribution. Tropical to subtropical Indian Ocean distribution extending from East London north along the KwaZulu-Natal coast(
St Lucia records. Reported in 1964–65(
Melampus lividus (Deshayes, 1830)
18 mm, St Lucia Mission Rocks, Apr 1988
D Brink leg.
Common name. Dwarf coffee-bean snail(
Size. Maximum shell height 13 mm(
Remarks. Found on firm mud in lagoons and estuaries where individuals form dense colonies(
Distribution. Tropical Indo-Pacific distribution including Indian Ocean islands. South African distribution extends from KwaZulu-Natal to Port Alfred(
St Lucia records. Not previously reported from St Lucia; recorded from the mangroves at Shark Basin (St Lucia Mouth) in the recent survey.
Melampus parvulus Pfeiffer, 1856
10 mm, St Lucia Mouth, Mar 2013
SM Ngubane leg.
Common name. Half-grooved coffee-bean snail(
Size. Maximum shell height 12 mm(
Remarks. Mangrove species which occurs in the burrows of crabs up to a depth of 150 mm as well as on the surface of the mud(
Distribution. East African distribution ranging from the Giuba River in Tanzania to the Umkomaas River on the southern coast of KwaZulu-Natal in South Africa(
St Lucia records. Reported in St Lucia in 1964–65(
Melampus semiaratus Connolly, 1912
12 mm, St Lucia Mouth, Mar 2013
SM Ngubane leg.