Citation: Klishko OK, Lopes-Lima M, Froufe E, Bogan AE (2014) Are Cristaria herculea (Middendorff, 1847) and Cristaria plicata (Leach, 1815) (Bivalvia, Unionidae) separate species? ZooKeys 438: 1–15. doi: 10.3897/zookeys.438.7493
The number of species in the freshwater mussel genus Cristaria Schumacher, 1817 recognized from Far East Russia has varied over the last several decades. While some authors consider the occurrence of only one species, Cristaria plicata (Leach, 1815), widespread in East Asia, others, recognize two separate species Cristaria herculea (Middendorff, 1847) and Cristaria tuberculata Schumacher, 1817 from Far East Russia, distinct from C. plicata. For the present study, freshwater mussels, identified as C. herculea, were collected in the Upper Amur basin (Transbaikalia, Russia). The shell morphology and the whole soft body anatomy were analysed in detail and compared with previously published information on other Cristaria spp.. Additionally, a cytochrome oxidase subunit 1 (CO1) gene fragment was sequenced from foot tissue samples of selected animals, collected from the same region, and compared with published data. Based upon morphological similarities of glochidia and adult morphology and anatomy as well as the mitochondrial DNA sequence analysis, we consider C. herculea as a synonym of C. plicata. Further analysis of Far East Russia C. herculea and C. tuberculata specimens using both molecular and morphological characters should be carried in the future to enhance our knowledge about the taxonomy within the Cristaria genus. Moreover, a comprehensive revision of the genus Cristaria is needed, restricting the type locality and comparing topotypic specimens for both C. plicata and C. tuberculata, and including all recognized Cristaria species.
Bivalvia, Unionidae, Anodontini, CO1, Transbaikalia, Russia
Freshwater bivalves of the Unionidae provide important ecosystem functions and services (
The taxonomy and status of Cristaria species in Far East Russia has not been consistent among malacologists. While some authors, consider the presence of only one species, Cristaria plicata (Leach, 1815) which is widespread in Eastern Asia, from Russia (Amur River basin and Khanka Lake) to Japan and south to South Korea, China, Vietnam, Lao People’s Republic, Thailand and Cambodia (
Cristaria herculea (Middendorff, 1847) known from the Transbaikalia, in Far East Russia, occurs mainly in rivers and reservoirs with slow or no currents, in a variety of substrates, including gravel, sand and mud, being tolerant of silty conditions. The fish-hosts, necessary for glochidia metamorphosis, are still unknown. The conservation status of Cristaria herculea from the rivers of Transbaikalia was considered to be relatively stable during the last century. However, our research over the last ten years showed that the species has become very rare due to pollution and other anthropogenic impacts on rivers and habitats. Under this view, Cristaria herculea was included in the Red Book of Transbaikalsky territory (
To solve taxonomic issues when conchological characters (especially shell convexity) form the basis of species separation, it is relevant to test these uncertainties with molecular DNA sequence analyses. Under these assumptions, the aims of this paper were to study the morphological and anatomical characteristics of Cristaria herculea from the Upper Amur basin in Transbaikalia, to compare them with published Cristaria spp. data and to test the species level status of Cristaria herculea with the use of molecular data.
Specimens of Cristaria herculea were collected in 2008–2012 from the Shilka, the Nercha and the Onon rivers, from the Kharanorsky reservoir, situated in the upper reaches of the Amur (Transbaikalia, Russia), and also from Buir-Nur Lake (Mongolia) (Table 1). Foot tissue samples were collected from living mussels of the Onon River and Kharanorsky reservoir and were preserved in 96% non-denatured ethanol for molecular analyses. The following shell dimensions (mm) were measured in all collected animals: length, width, height at umbo and maximal height. For species identification, the ratio of maximal shell inflation to the distance from the umbo to the posterior end of the posterior tooth was determined, according the identification key by
Shell morphometry characteristics of Cristaria herculea from the upper Amur River basin. L – shell length; H – maximal shell height; h – shell height measured from umbo; h1 – shell height measured from middle lateral tooth to ventral margin; l1 – the distance from umbo to posterior end of the lateral tooth; B – maximal shell inflation (width); R1 = B/l1, R2 = B/h1; n – number of measured shells; * – living mollusks dissected for the anatomical study and (n1) – their number.
| Characteristics | Kharanorsky reservoir | Onon River | Nercha River | Shilka River | Buyr-Nur Lake |
|---|---|---|---|---|---|
| L, mm | 128.7–290.0 258.0* |
147.6–238.0 167.2* |
120.2–174.0 | 152.0 | 148.9–151.0 |
| H, mm | 93.8–212.3 187.5* |
94.9–124.8 102.0* |
73.3–112.9 | 92.1 | 99.9–102.0 |
| h, mm | 65.3–148 130.6* |
66.5–112.0 74.9* |
51.6–80.0 | 66.3 | 69.2–71.1 |
| h1, mm | 75.0–161.2 152.5* |
82.1–134.0 88.9* |
68.7–98.1 | 82.3 | 63.1–64.9 |
| l1 , mm | 52.5–113.0 104.9* |
58.0–99.4 66.9* |
50.0–74.0 | 58.2 | 83.0–84.8 |
| B, mm | 41.2–88.0 79.4* |
44.3–76.1 50.7* |
39.0–53.9 | 47.1 | 46.0–48.0 |
| B/L | 0.316 ± 0.0055 0.308* |
0.294 ± 0.0169 0.289* |
0.317 ± 0.007 | 0.309 | 0.312 ± 0.0065 |
| R1 | 0.760 ± 0.0311 0.756* |
0.763 ± 0.0039 0.758* |
0.768 ± 0.033 | 0.807 | 0.736 ± 0.0053 |
| R2 | 0.540 ± 0.0119 0.521* |
0.552 ± 0.0192 0.570* |
0.561 ±0.0087 | 0.571 | 0.558 ± 0.0052 |
| n (n1) | 5 (1) | 4 (1) | 3 | 1 | 3 |
Whole genomic DNA was extracted from small tissue pieces of two individuals (preserved in 96% ethanol) using a standard high-salt protocol (
List of specimen samples sequenced (CO1) and GenBank accession numbers. *Unpublished
| Species | Locality | Country | Code/GenBank | Study |
|---|---|---|---|---|
| Cristaria herculea | Onon River | Russia | Biv246 | This study |
| Cristaria herculea | Charanorsky Reservoir | Russia | Biv247 | This study |
| Cristaria plicata | Lower Yangtze | China | EU698893; EU698897; EU698913; EU698948 | Jia and Li* |
| Cristaria plicata | Unknown | China | JF700152; JF700153 | Zhang et al.* |
| Cristaria plicata | Zhejiang | China | FJ986302 | |
| Cristaria plicata | Unknown | South Korea | GQ451860 | Park et al.* |
| Cristaria plicata | Unknown | South Korea | GU944476 | |
| Cristaria sp. | Lower Yangtze | China | EU698909; EU698910; EU698940; EU698942 | Jia and Li* |
| Anodonta beringiana | Jo-Jo Lake | Canada | DQ272370 | |
| Sinanodonta woodiana | Unknown | Poland | HQ283347 | Soroka and Burzynski* |
The final data set was then analysed using maximum likelihood (ML) and Bayesian inference (BI) methods. The best-fit model of nucleotide substitution evolution under corrected Akaike Information Criterion was estimated using JModelTest 2.1.4 (
The morphometric characteristics of Cristaria herculea are summarized in Table 1. The shell length of the collected Cristaria individuals ranged from 120 to 290 mm, the maximum shell height from 73 to 212 mm and the shell width was 39–88 mm. The ratio of maximal shell inflation to the distance, measured from umbo to posterior end of the lateral tooth (R1) was 0.76–0.81, and to shell height measured from the middle of the lateral tooth to ventral margin (R2) – 0.52–0.57. This ratio enabled the identification of the collected mussels as Cristaria herculea, according to the published keys (
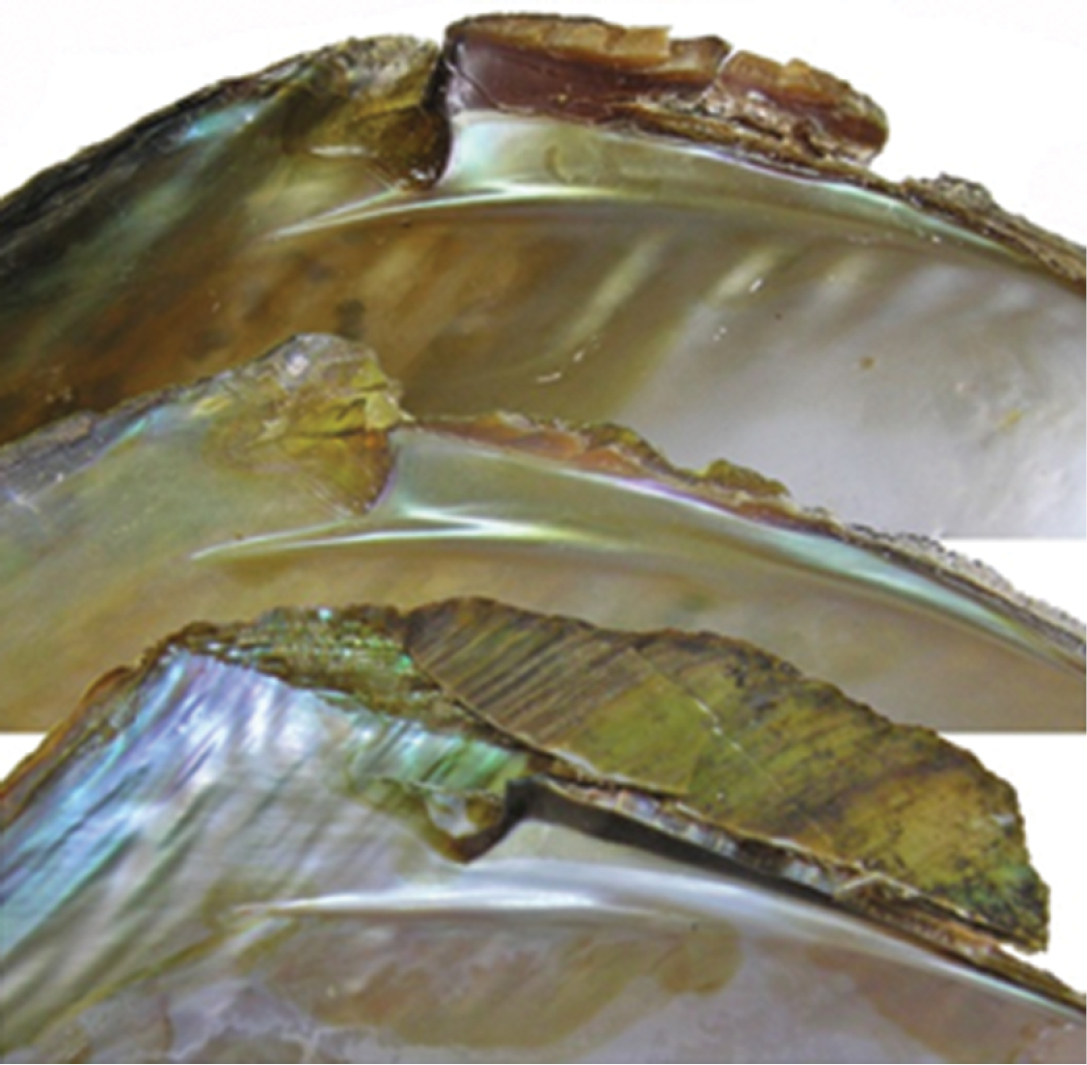
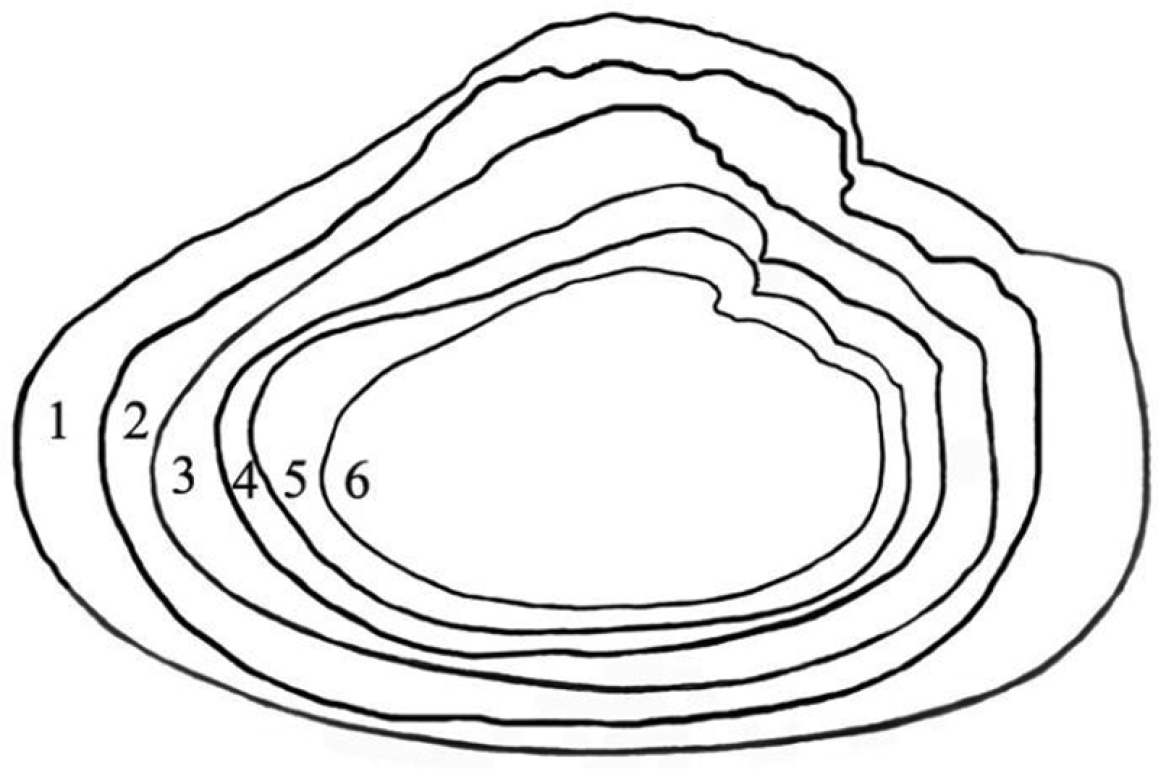
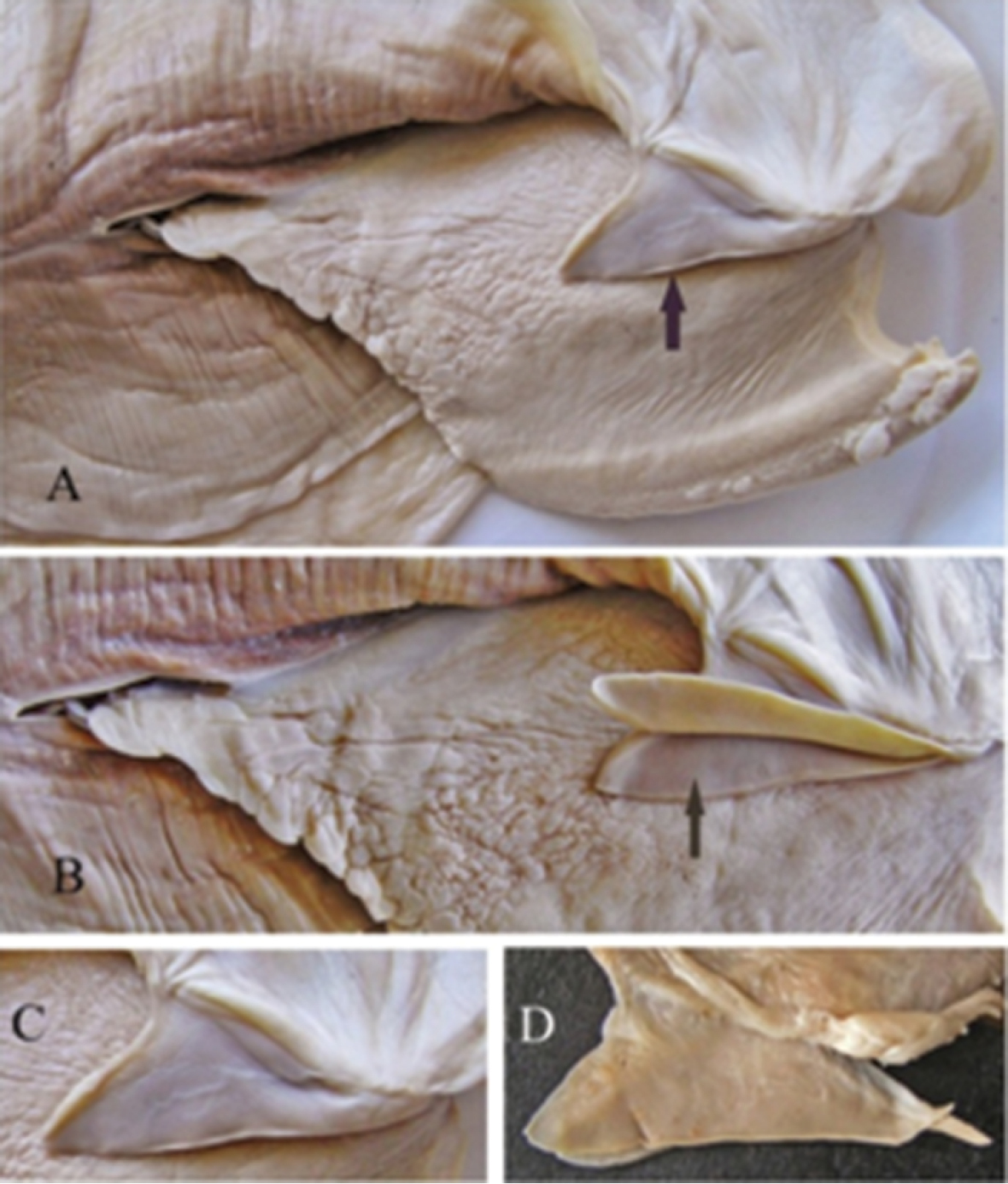
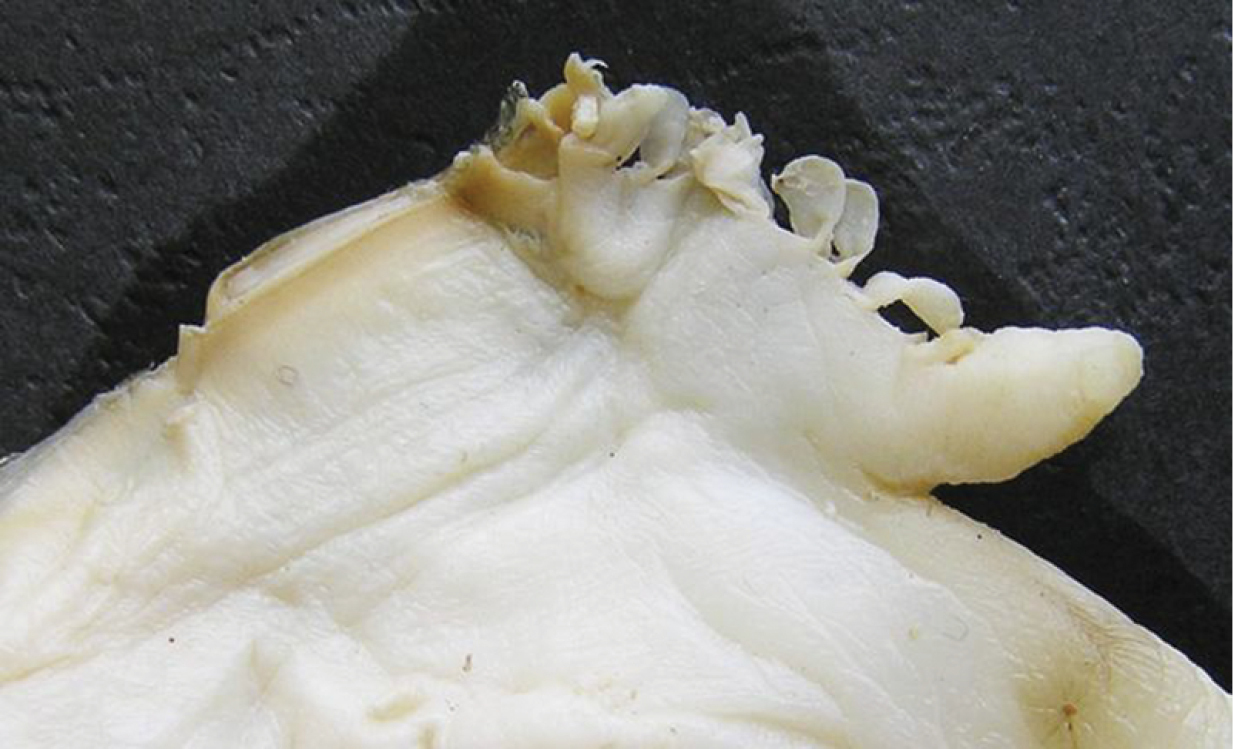
The shells from the reservoir are elongated diamond-shaped with a green-brown or red-brown coloured periostracum, with developed posterior dorsal wing and hardly expressed anterior wing (Fig. 1A, B). On the other hand, the shells from Buir-Nur Lake are oval-triangular with a dark brown or black coloured periostracum and with less developed or broken dorsal wing (Fig. 1C). The shells from the different river biotopes are elongated, oval-triangular, which may become oval when a dorsal wing is eroded or broken, alate, and with a periostracum colour that varies from yellow-brown to dark brown or black; the posterior dorsal wing is underdeveloped and the anterior dorsal wing is absent (Fig. 1D–F). Umbos are broad and slightly elevated above the dorsal margin. The umbo sculpture is presented in the form of a few sub-concentric bars. The dorsal margin behind the umbo turns into the high posterior wing, which is sometimes eroded or broken. There are large undulating folds or ridges on the posterior slope extending onto the posterior dorsal wing that are expressed more clearly in smaller specimens. The shell anterior margin is straight and the ventral margin may vary from slightly convex to straight or even slightly concave. The posterior margin in river shells is evenly rounded, slightly curved or concave when meeting the dorsal margin. The lateral teeth are straight or slightly curved, one in each valve (Fig. 2). While the anterior adductor scar is deep, the posterior is shallow and slightly visible. The nacre is blue, pale-pink, or yellow-pink with large olive spots. The shell outlines of river shells presented, in general, similar anterior margins with those from reservoirs but differ in having a smaller slope of the dorsal margin as seen in Fig. 3 (1–6).
Cristaria herculea from Upper Amur River basin. A, B from Kharanorsky reservoir C from Buir-Nur Lake (Mongolia) D from Onon River E from Shilka River F from Nercha River. Scale bar 1 cm.
Lateral tooth of riverine shells (upper two) and from reservoir (lower).
Shell outlines of Cristaria herculea. 1–3 (reservoir/lakes) 4–6 (rivers).
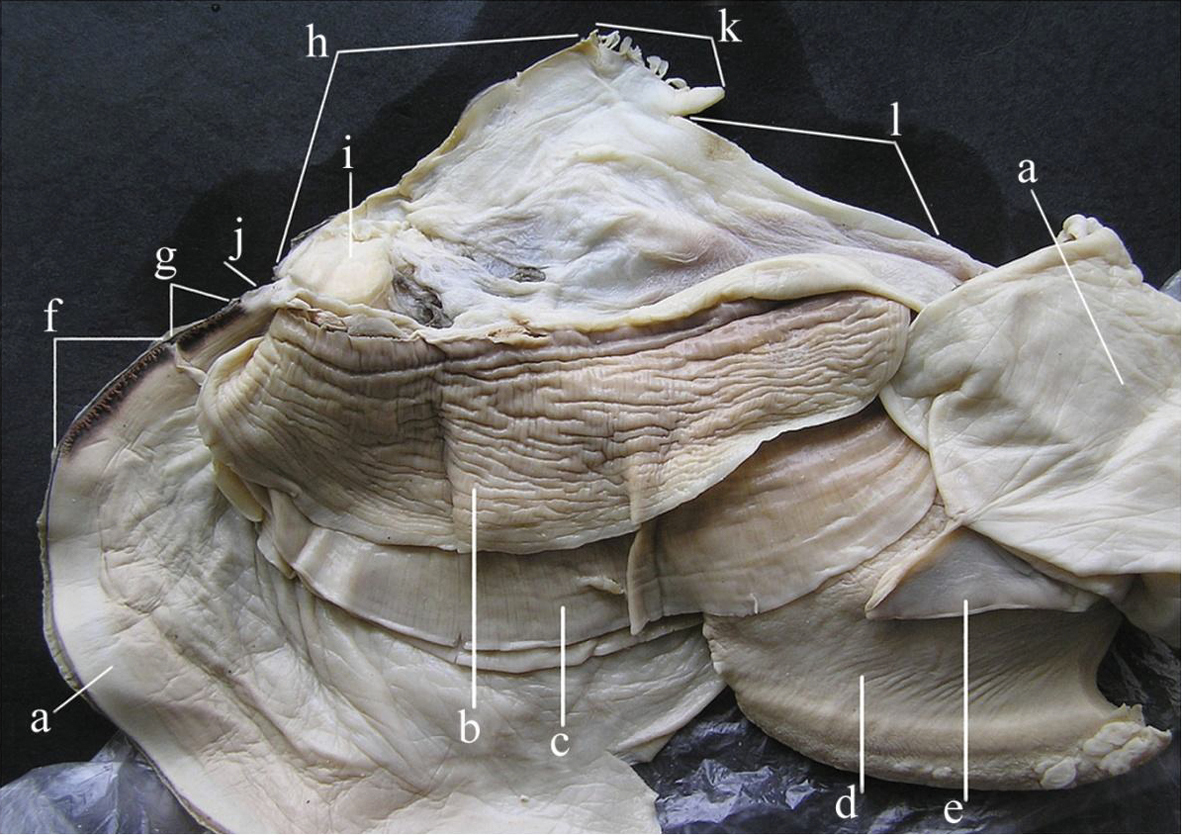
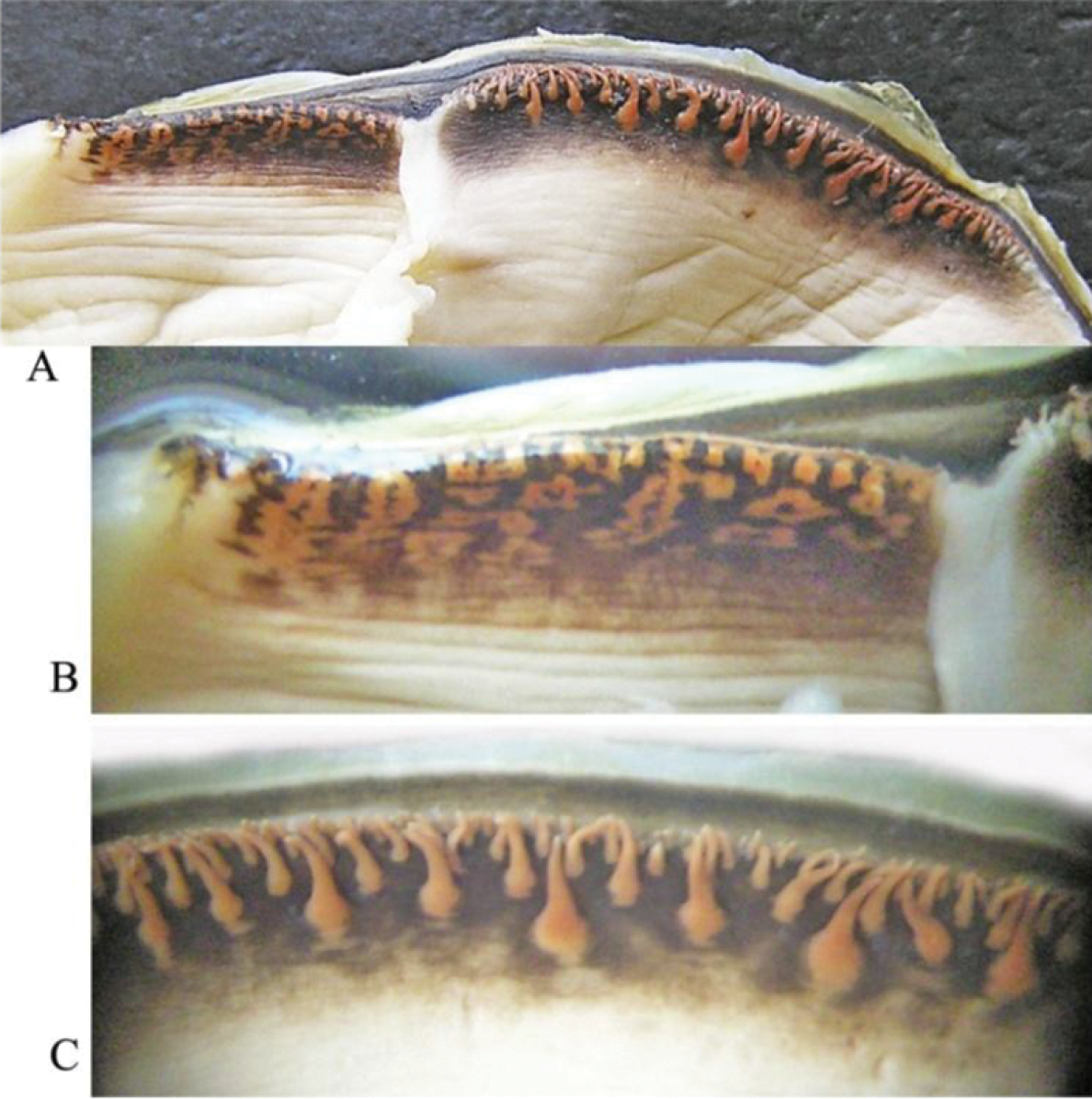
A general view of the whole soft body morphology of Cristaria herculea is shown in Fig. 4. Mantle colour is creamy white, with black or brown edges (Fig. 4a). Dorsal mantle margin presents a well expressed high angle with a comb-shaped projections on the top (Fig. 4k) and a muscular anterior margin (Fig. 4l). Gills are creamy white or light brown; dorsal margin is straight to sinuous and ventral margin is moderately convex. Inner gills are much longer and higher than outer gills (Fig. 4b, c); gill length is 46–54% of shell length, gill height is 25–40% of gill length and outer gill height is 67–75% of the inner gill height. The foot is massive, creamy white and darker distally (Fig. 4d). Labial palps are triangular, creamy white to blue-grey, straight or slightly convex dorsally; straight or gently concave ventrally and bluntly pointed ventrally (Fig. 4e). Labial palp length is 3.9–4.2% of inner gill length and labial palp height 34–35% of labial palp length. Incurrent aperture (Fig. 4f) is longer than the excurrent aperture (Fig. 4g) and shorter than the supra-anal aperture (Fig. 4h). Supra-anal aperture opening is located from the dorsal margin of the posterior adductor muscle (Fig. 4i) to the posterior dorsal edge of the posterior mantle wing. Supra-anal aperture length is 20–25% of the shell length or double the length of the incurrent aperture; it is creamy white to pearly white inside, with a very thin yellow-brown marginal band. Mantle bridge (Fig. 4j) separates the excurrent from the supra-anal aperture and is 8–10% of the supra-anal aperture length. Incurrent aperture length is 11–13% of the shell length, is creamy white to light tan within, with a combination of orange, brown and black basal to the papillae and to the bands margin which may present a reticular pattern. Excurrent aperture length is 46% of incurrent aperture length, colour is creamy white within with black or dark brown edges basally, margin papillate; have irregular mottled pigmentation of some combination of dark brown and orange (Fig. 5A–C). Papillae of the incurrent aperture are located in 3–4 rows, linear-fusiform in shape, mostly simple, with thickening of the papilla basement in the first and second medial rows, dark-orange; papillae of outer or lateral rows are shorter and more numerous (Fig. 5C). Labial palps of Cristaria herculea and Sinanodonta sp. are morphologically distinct (Fig. 6A–D). The anterior acuminate edges of Sinanodonta sp. labial palps are not completely attached to the mantle (Fig. 6D) in contrast with those on Cristaria herculea (Fig. 6A–C). The distinctive feature of the genus Cristaria within the tribe Anodontini is the posterior dorsal mantle wing and projections (Fig. 7). The comb-shaped projections are dorsal extensions of the mantle that penetrate into the cavities of shell wing, to provide for the wing growth.
Morphology of Cristaria herculea soft body: a – mantle, b – outer gill, c – inner gill, d – foot, e – labial palps, f – incurrent aperture, g – excurrent aperture, h – supra-anal aperture, i – dorsal adductor muscle, j – mantle bridge, k – dorsal wing mantle projections, l – muscular anterior margin of dorsal mantle wing.
Cristaria herculea: A excurrent aperture (to the left) and incurrent aperture (to the right) B excurrent aperture (magnification) C shape of papillae in incurrent aperture (magnification).
Labial palps of Cristaria herculea (A, B, C) and of Sinanodonta sp. (D).
Dorsal mantle wing of Cristaria herculea with comb-shaped projections.
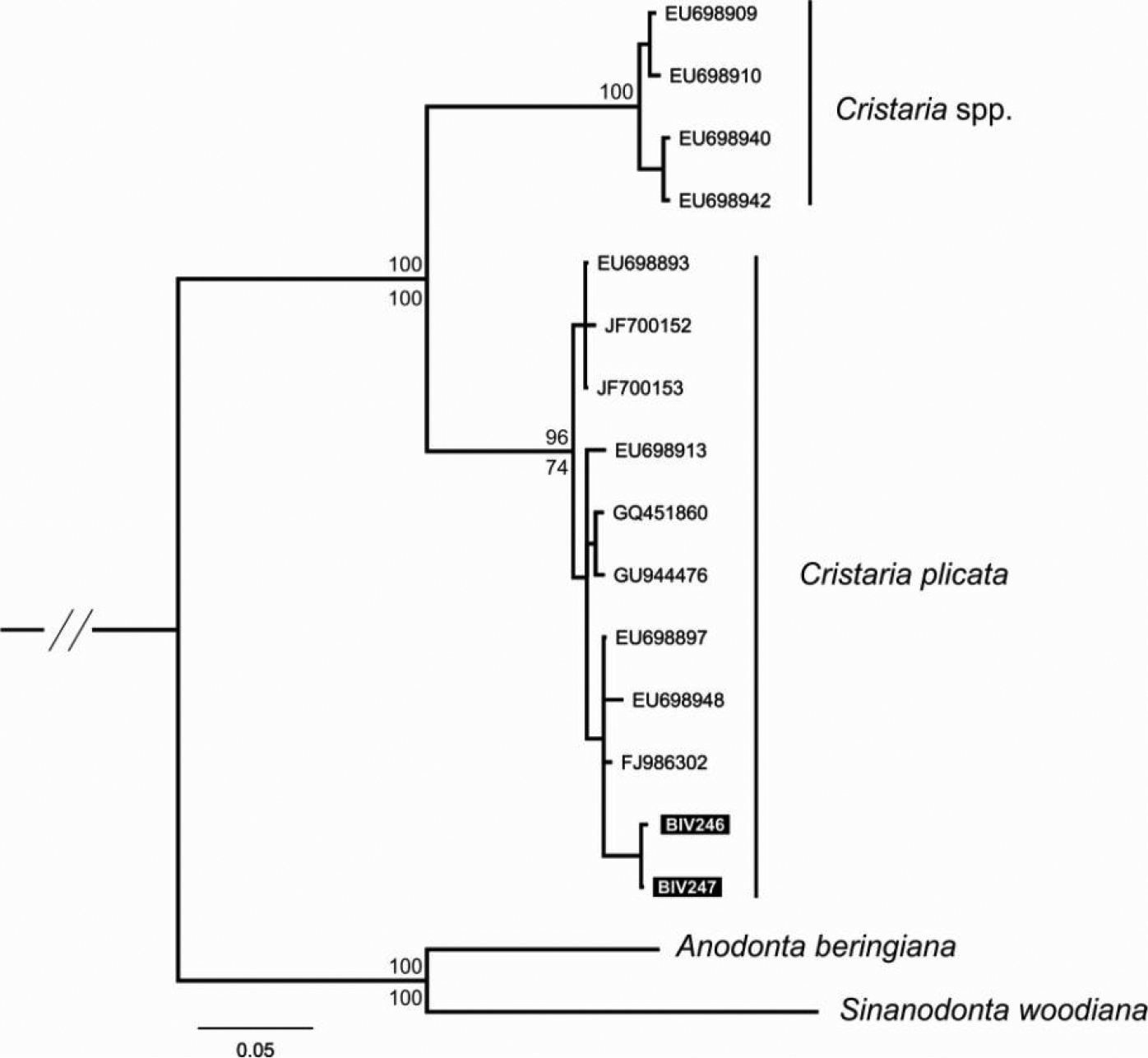
Aligned CO1 sequences had a total length of 620 bp, with 143 polymorphic and 92 parsimony informative sites. No indels and no unexpected stop codons were observed after translating all sequences to amino acids. The tree topologies resulting from the single tree recovered from ML and BI approaches were congruent, and results of both analyses are shown in Figure 8. Two major mtDNA clades were retrieved with strong support: one includes all the individuals from Cristaria plicata, including the new sequences collected for this work (Biv246 and Biv247; Fig. 8) and the other includes six individuals also originally assigned to Cristaria plicata (Jia and Li, Unpublished). However, it is obvious that the phylogeny of the Cristaria genus needs further evaluation, since these individuals are 8.9% (uncorrected p-distance) from the others, strongly indicating the existence of two different Cristaria species in this data set. Thus, this clade is here referred as Cristaria sp.
Phylogenetic tree obtained by Bayesian Inference and Maximum Likelihood analyses, using mtDNA fragments (CO1). Support values are given as Bayesian posterior probability above nodes and as bootstrap support below nodes, except for those within major clades, which have been omitted for clarity. Available sequences downloaded from GenBank and new sequences codes refer to Table 2.
The whole shell morphology of Cristaria herculea from the Upper Amur basin described here is very similar and corresponds to that previously described by
The study of Cristaria herculea and Cristaria tuberculata glochidia has also shown no differences in shell size and proportions or in the disposition of macro spines on the distal end of hooks (
In summary, the shell morphology, anatomy, and known ecological traits of Cristaria herculea from the Upper Amur basin are similar to those described for Cristaria plicata. Additionally, the CO1 molecular analysis confirms Cristaria herculea as a synonym of Cristaria plicata. As for Cristaria tuberculata, while some studies reported similar morphological and molecular characters to Cristaria herculea, other authors reported differences not only in shell shape (inflation) but also in ecological requirements and morphological features of the glochidia, suggesting the occurrence of two distinct species in Far East Russia. Therefore, the distinction of Cristaria tuberculata from Cristaria herculea/Cristaria plicata is still an open question that should be carefully investigated using additional molecular data.
We would like to thank Dr. Richard Willan and Dr. Yulia Bespalaja for all the help in improving the scientific quality of the manuscript. The work was financially supported by the Ministry of Natural Resources and Ecology of Zabaikalsky Territory, Grant No. 20-10/P, and by the Russian Foundation for Basic Research (RFBR), Grant No. 12-04-00594a. Financial support for the genetics work was provided by Portuguese Foundation for Science and Technology (FCT) project PTDC/AAC-AMB/117688/2010.