Citation: Tauber CA, Garland JA (2014) Kymachrysa, a new genus of Nearctic Green Lacewings (Neuroptera, Chrysopidae, Chrysopini). ZooKeys 437: 87–108. doi: 10.3897/zookeys.437.7984
Two North American species of green lacewings have undergone a number of changes in their generic assignments and are currently classified as incertae sedis. Here we demonstrate that adults (both sexes) and larvae of these species share a set of features that distinguishes them from currently described genera. Thus, to promote nomenclatural stability in Chrysopidae, we describe Kymachrysa, a gen. n. that contains the two species – Kymachrysa intacta (Navás), comb. n. and Kymachrysa placita (Banks), comb. n.. Also, we present modifications for the current generic-level key, illustrations, as well as biological information for identifying the genus and its known species.
New genus, adult, larva, biology
The green lacewing tribe Chrysopini (Neuroptera: Chrysopidae) includes seventeen genera in the New World; ten of these occur in the Nearctic. These New World genera are distinguished from each other on the basis of both adult (male and female), as well as larval, characters (e.g., see
Our procedures were those used in previous publications (e.g.,
Chrysopa placita Banks, 1908: 259.
Kymachrysa adults appear to be typical chrysopine lacewings of medium size and green coloration. Their most distinctive adult features occur in the male and female terminalia; in addition, a few external features are diagnostic of the genus:
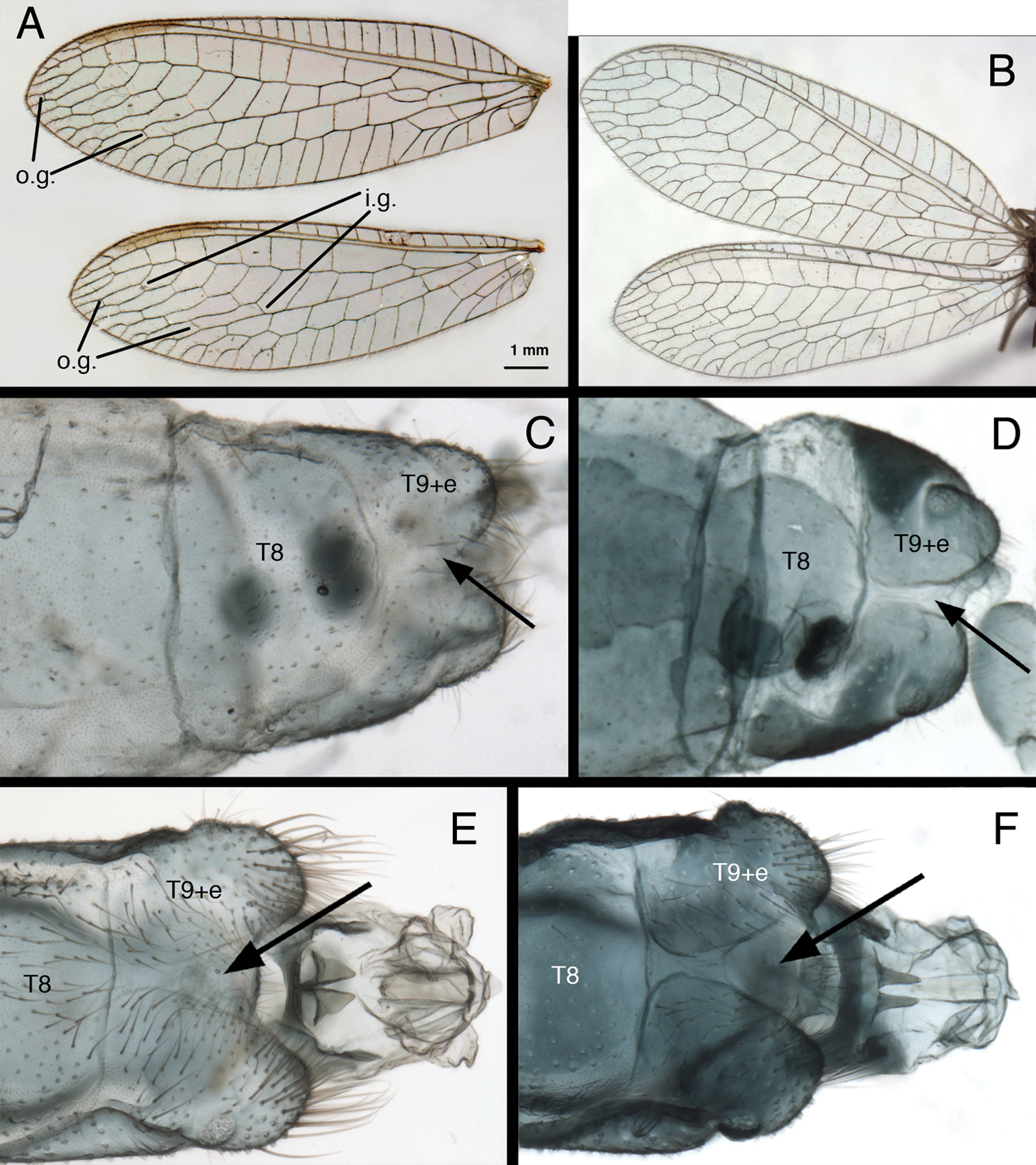
(i) The longitudinal (radial) veins between the first and second rows of gradate veins of the fore and hind wings are sinuous (Fig. 1A, B), whereas in most other chrysopid genera they are relatively straight. (ii) In both males and females, the fused ninth tergite and ectoproct is completely divided by a dorsal invagination, and each side of the terminal abdominal segment is rounded posterolaterally (especially in males) (Fig. 1C–F). Among other New World genera, a complete dorsal invagination of the T9+ect is reported only for Chrysopiella and Parachrysopiella (
Two external features that characterize Kymachrysa adults. A, B Fore and hind wings with sinuate longitudinal veins between the first and second gradate series of crossveins C–F Terminal segments (dorsal) with Tergite 9+ectoproct separated dorsally C, D Female E, F Male A, C, E. Kymachrysa placita A, C Colorado, USNM E Type, Colorado, MCZ B, D, F Kymachrysa intacta B Neotype, Quebec, CNC D New York, TRC F New York, TRC [Males: gonarcal complex not removed]. Abbreviations: i.g. inner gradate veins o.g. outer gradate veins T8 eighth tergite T9+e fused ninth tergite and ectoproct.
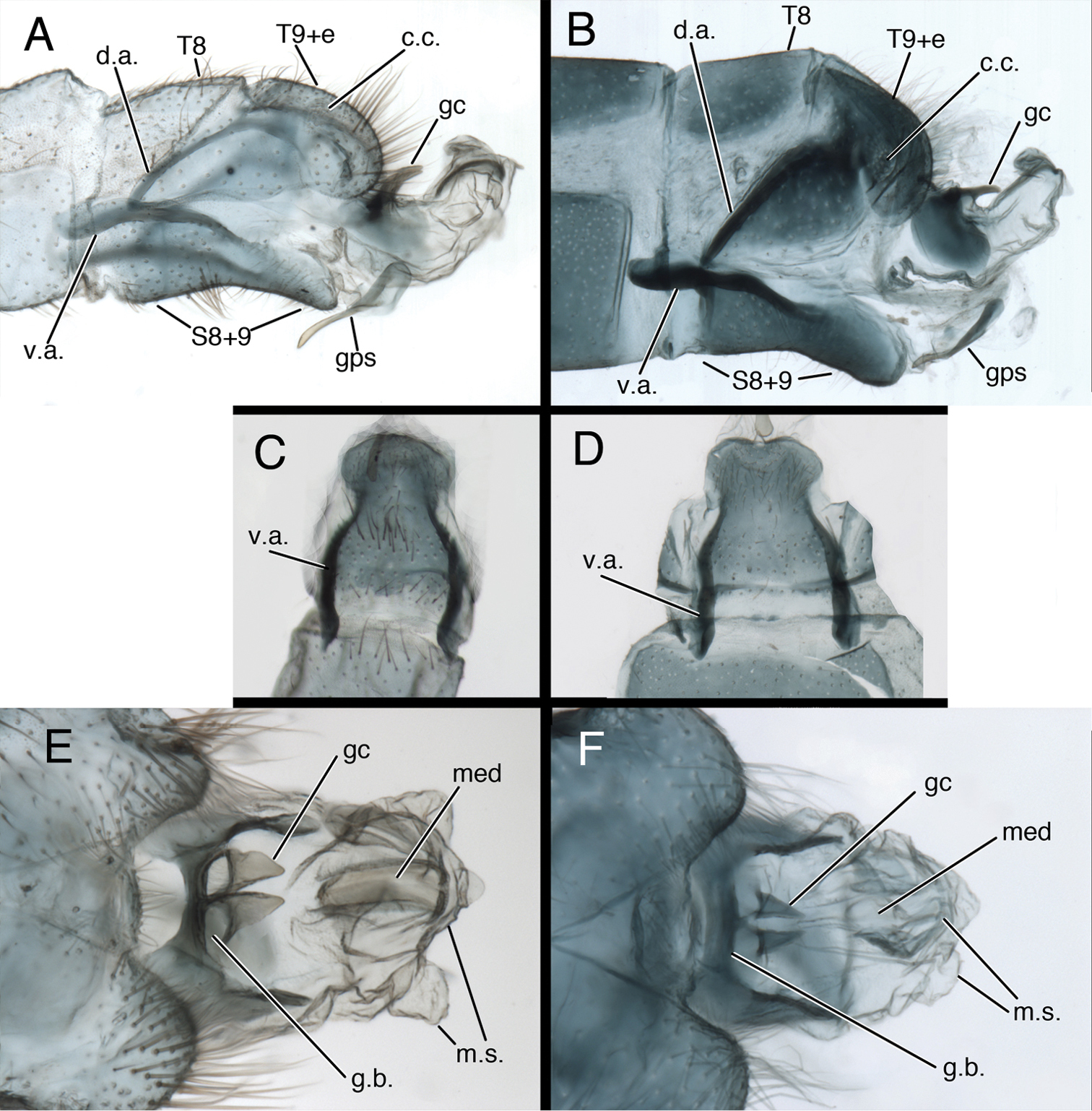
One of the most striking aspects of the Kymachrysa male terminalia is the S8+9, which is entirely fused and well sclerotized as in most chrysopine genera. However, in lateral view, the Kymachrysa S8+9 has an unusual ventral bend, and the ventral apodeme is heavy and elongate – extending anteriorly well beyond the proximal margin of S8 (Fig. 2A, B). In ventral view, S8+9 is constricted mesally and rounded both anteriorly and posteriorly (Fig. 2C, D). These features are unique among New World chrysopids.
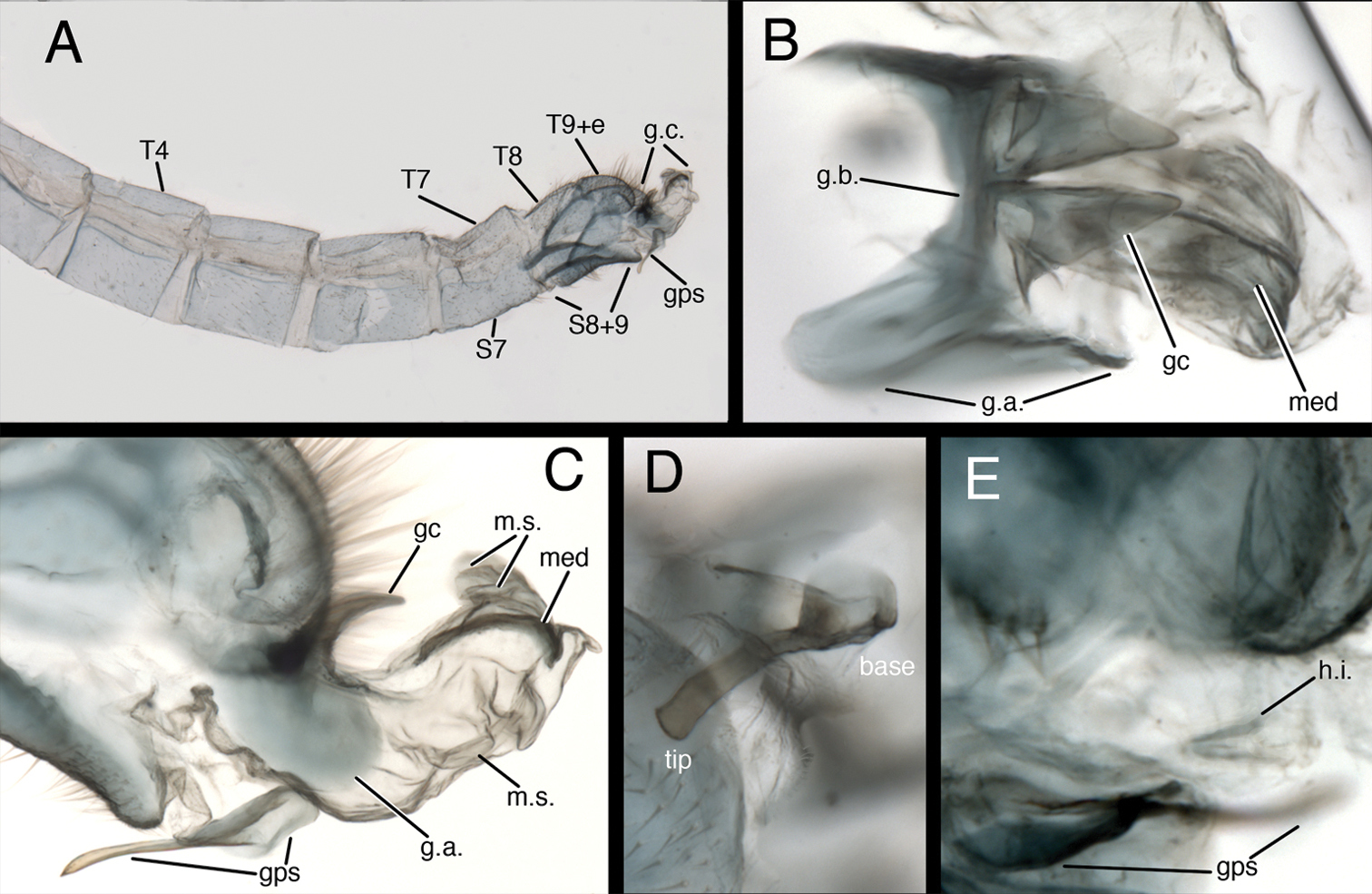
Kymachrysa male terminalia. A, B Abdominal segments 8 and 9 (lateral), with genitalia everted C, D Sternite 8+9 (ventral) E, F Genitalia (everted, dorsal) A, C, E Kymachrysa placita Type, Colorado, MCZ B, D, F Kymachrysa intacta, California, TRC. Abbreviations: c.c. callus cerci d.a. dorsal apodeme (on ninth tergite and ectoproct) gc gonocornu gps gonapsis g.b. gonarcal bridge med mediuncus m.s. membranous sac S8+9 fused eighth and ninth sternites T8 eighth tergite T9+e fused ninth tergite and ectoproct v.a. ventral apodeme (on sternite 8+9).
Gonocornua are present on the gonarcal bridge, as in Ceraeochrysa. In Ceraeochrysa, the gonocornua are usually rounded and unarticulated, and they arise laterally from the gonarcal bridge (see
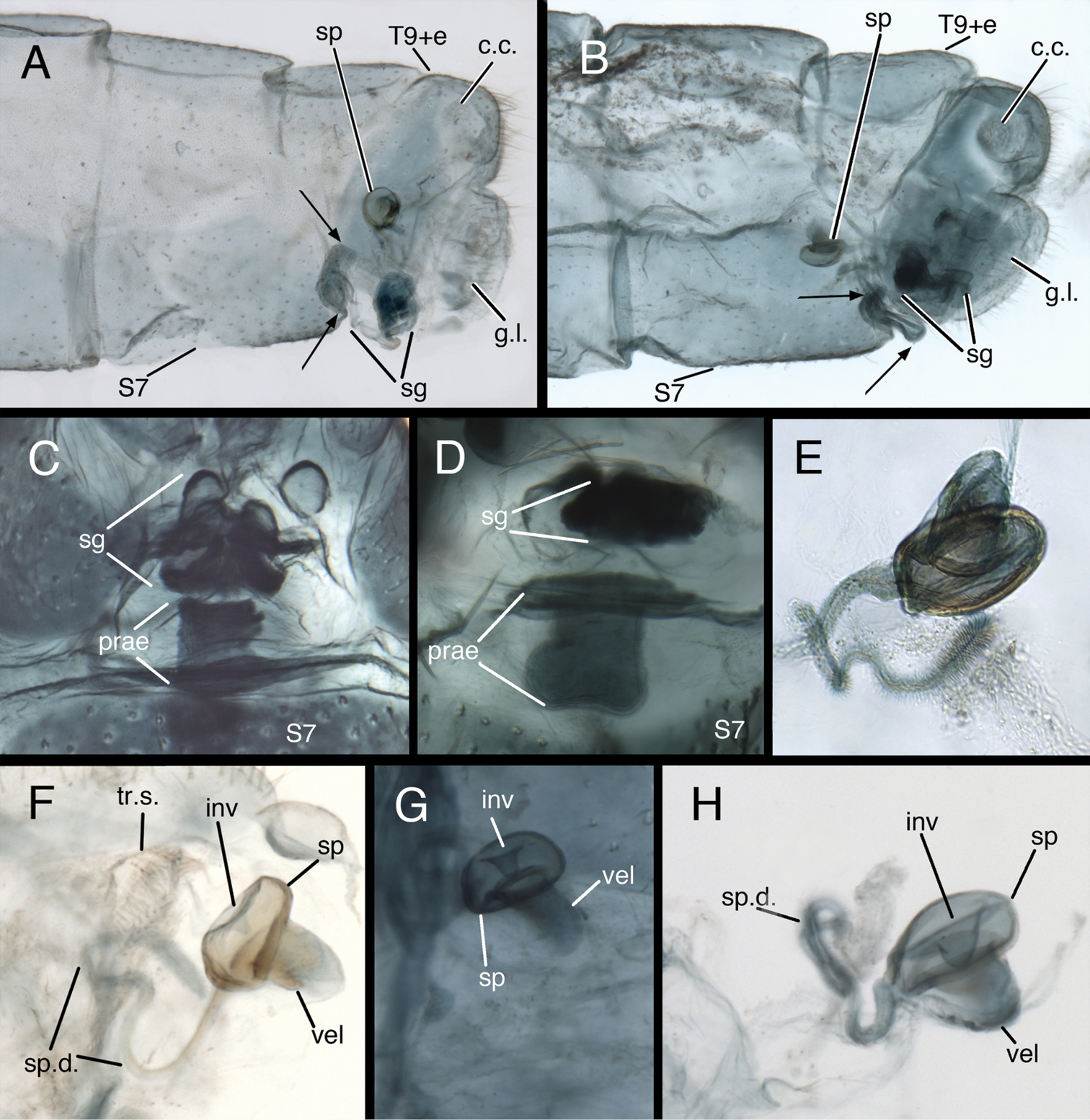
Two notable features distinguish the female genitalia. (i) This chrysopid genus is the only one outside of the tribe Belonopterygini (see
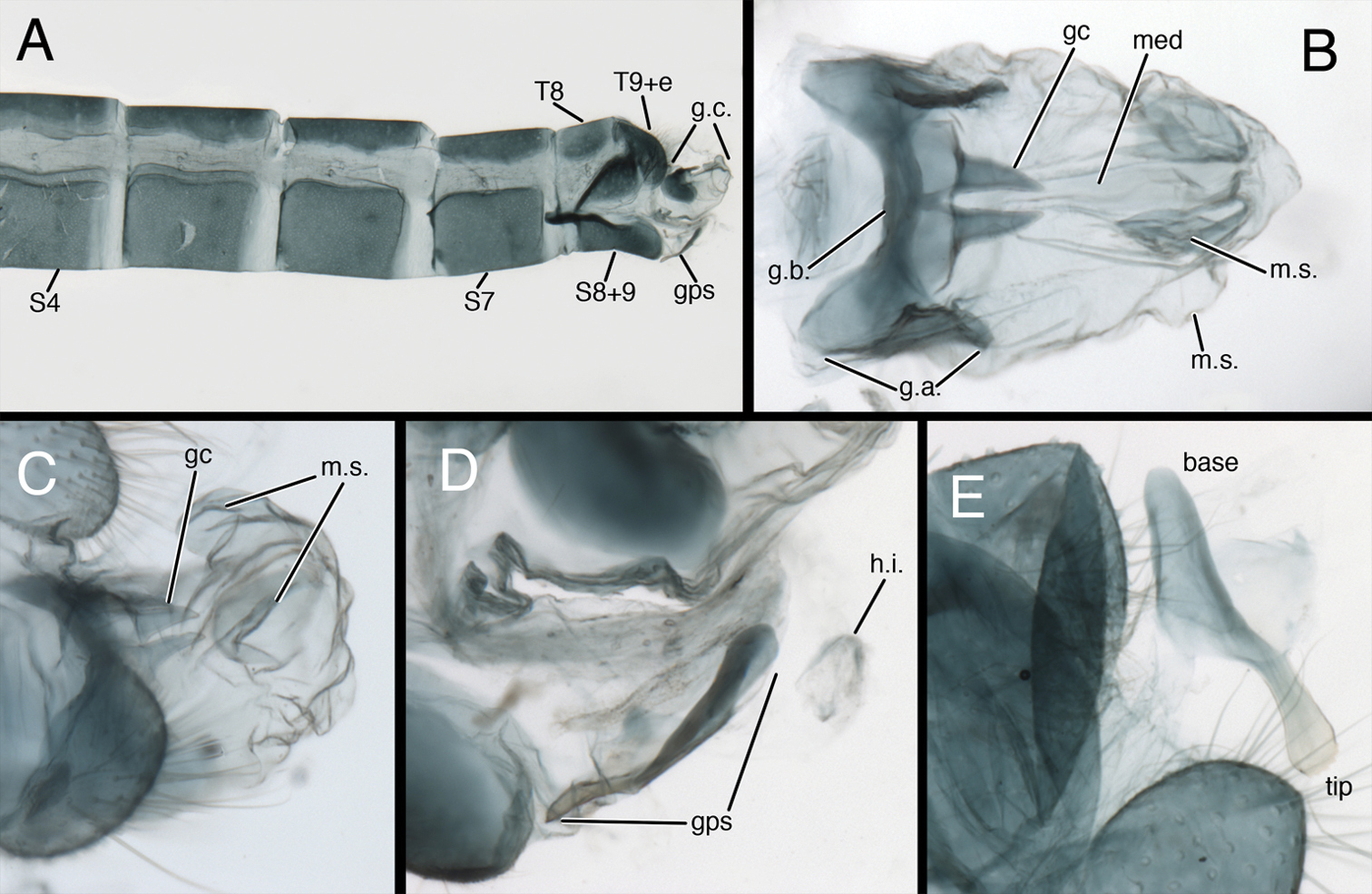
Kymachrysa female terminalia. A, B Abdominal segments 7 and 9 (lateral) [Arrows delineate the praegenitale] C, D Praegenitale at distal margin of S7 (ventral) E–H Spermatheca A, C, F Kymachrysa placita A, F Colorado, USNM C New Mexico, USNM B, D, E, G, H Kymachrysa intacta B, D, E New York, TRC G Colorado, CPG H California, TRC. Abbreviations: c.c. callus cerci g.l. gonapophysis lateralis inv spermathecal invagination prae praegenitale sg subgenitale sp spermatheca sp.d. spermathecal duct S7 seventh sternite tr.s. transverse sclerite T9+e fused ninth tergite and ectoproct vel velum.
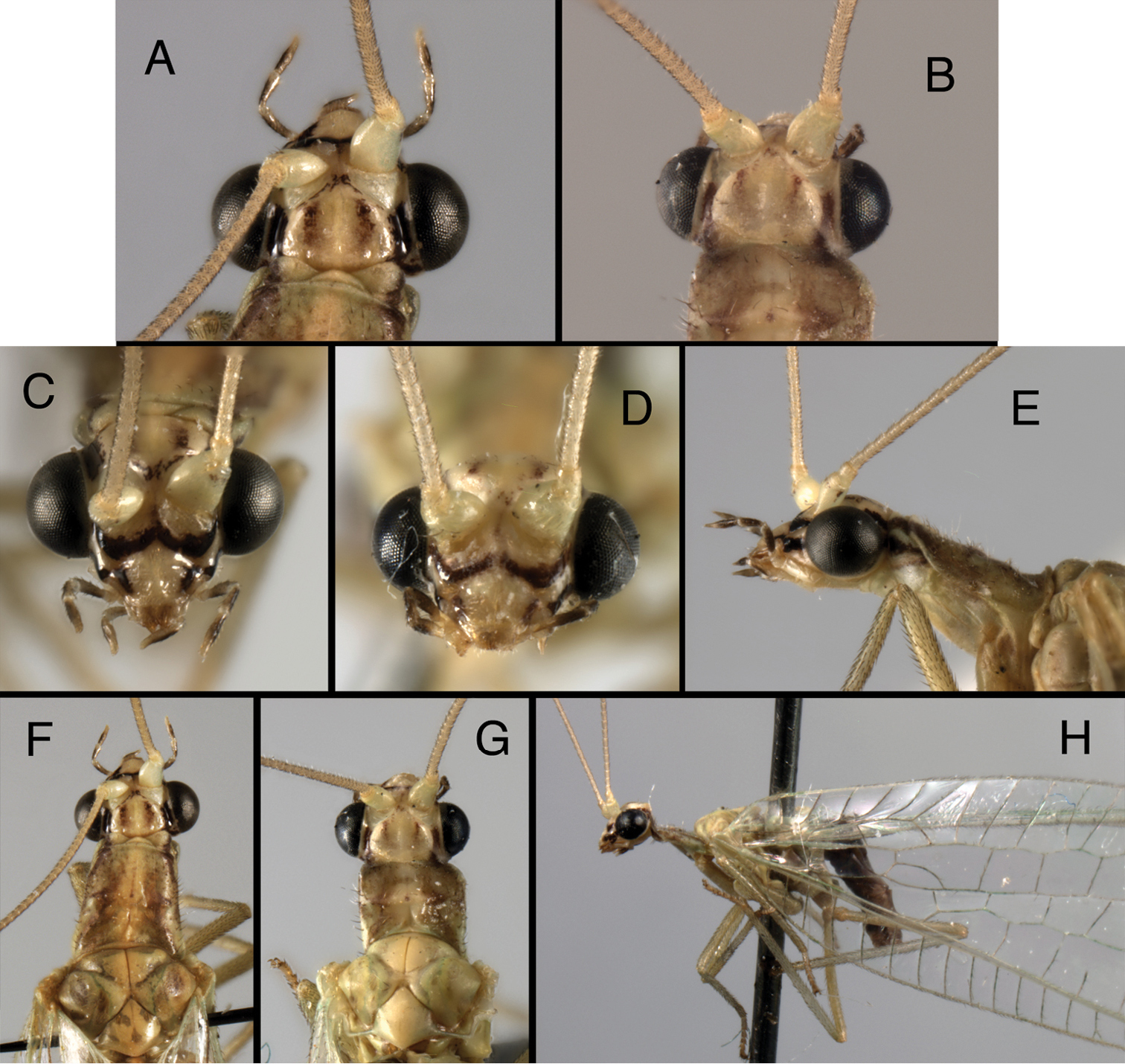
Adult (Figs 4, 5, 8C, D). Delicate, slender, medium sized (forewing length, Kymachrysa placita: 11–13 mm; Kymachrysa intacta 11–15 mm), predominantly green with yellow longitudinal stripe, mesally. Head (Figs 4, 5). Vertex with pair of crescent-shaped, red or brown sublateral marks (sometimes absent or faint), usually with lateral red or brown stripe near margin of eye; frons with or without markings; gena with red or brown longitudinal markings. Distal segments of labial, maxillary palpi with elongate, lateral, black marks. Antenna cream colored, without markings. Thorax (Figs 4E–H, 5F–J, 8C, D). Prothorax variable in length and shape (probably developmental variation), usually long, tapered distally; dorsum without lateral stripes, but usually with irregular, red or brownish, sublateral markings (especially western, southern specimens); mesothorax, metathorax with or without markings. Legs mostly light green, without markings, with numerous dark brown to black setae; tarsal claws with deep U-shaped to V-shaped cleft.
Kymachrysa placita external adult features. Note variation in darkness and size of head markings, prothoracic size and shape. A, B Head (dorsal) C, D Head (frontal) E Head, prothorax (lateral) F, G Head, prothorax, mesothorax (dorsal) H Head, thorax (lateral) [Background with small piece of abdomen visible]. A, C, E, F Wyoming, SDNHM; B, D, G, H Colorado, CPG.
Kymachrysa intacta external adult features. Note variation in the presence, darkness, and size of head and thoracic markings. A–C Head (dorsal) D, E Head (frontal) F Head, prothorax (lateral) G, H Head, prothorax, mesothorax (dorsal) I Thorax (dorsal) J Head, thorax (lateral) A, D, G, I, J California, TRC B, E, F, H Colorado, TRC; C New York, TRC.
Wings (Fig. 1A, B) with costal area slightly enlarged basally; radius straight; im3 cell triangular; forewing, hindwing with two, regular, slightly converging rows of gradate veins; longitudinal veins between inner and outer gradates sinuate; three icu cells, distal one open.
Abdomen (Figs 1C–F, 2A, B, 3A, B, 6A, 7A, 8A, B) with spiracles simple; callus cerci round, located dorsally on T9+ect, with trichobothria stemming from closely spaced sockets; ninth tergite (T9) and ectoproct fused, forming T9+ect; T9+ect completely divided dorsally.
Kymachrysa placita male abdomen. A Abdominal segments 3 to terminus (lateral), with genitalia everted B Gonarcal complex (dorsal) C Genitalia (everted–including gonapsis, lateral) D Gonapsis (ventral) E Hypandrium internum nestled within membrane [Gonapsis not in focus, shown for relative scale]. All Types, Colorado, MCZ Abbreviations: gc gonocornu gps gonapsis g.a. gonarcal apodeme g.b. gonarcal bridge g.c. gonarcal complex h.i. V-shaped hypandrium internum med mediuncus m.s. membranous sac S7 seventh sternite S8+9 fused eighth and ninth sternites Tx tergite, number T9+e fused ninth tergite and ectoproct.
Kymachrysa intacta male abdomen. A Abdominal segments 4 to terminus (lateral), with genitalia everted B Gonarcal complex (dorsal) C Everted genitalia (dorsolateral), showing dorsal membranous sacs D Gonapsis (lateral), hypandrium internum (dorsal) E Gonapsis (ventral). A, D California, TRC B, E New York, TRC; C Colorado, CPG. Abbreviations: gc gonocornu gps gonapsis g.a. gonarcal apodeme g.b. gonarcal bridge g.c. gonarcal complex h.i. V-shaped hypandrium internum med mediuncus m.s. membranous sac Sx sternite, number S8+9 fused eighth and ninth sternites T8 eighth tergite T9+e fused ninth tergite and ectoproct.
Male abdomen (Figs 2, 6–8) slender, with sternites tall (~0.7× length), with or without microtholi; spiracles simple; terminal segments (A8–A9) compact; ectoproct with heavy apodeme along entire dorsal margin, terminus reaching proximal edge of eighth sternite (S8); eighth and ninth sternites fused; S8+9 shallow, with dorsal surface undulating, anterior section of S8+9 with heavy ventral apodeme extending proximally well beyond margin of segment, into S7; ventral surface indented mesally (hour-glass shaped). Gonapsis, hypandrium internum attached to membrane at tip of S9; gonapsis elongate, with basal margin rounded, toothed, distal margin expanded, curved; gonocristae absent. Gonarcus arcuate, with stout bridge, rounded, expanded lateral apodemes; gonocornua triangular, articulated on laterodistal margin of gonarcal bridge; base of gonocornua clear, tip dark, heavy, tapering to angulate terminus; entoprocessus absent. Mediuncus weak, comprising very lightly sclerotized, curved, mesal band distal to gonocornua, with trough-shaped dorsal surface, small, rounded, membranous terminus. Gonosaccus large, expanded dorsally as pair of large, eversible, distal sacs, expanded ventrally with a second pair of large, eversible sacs; gonosetae absent.
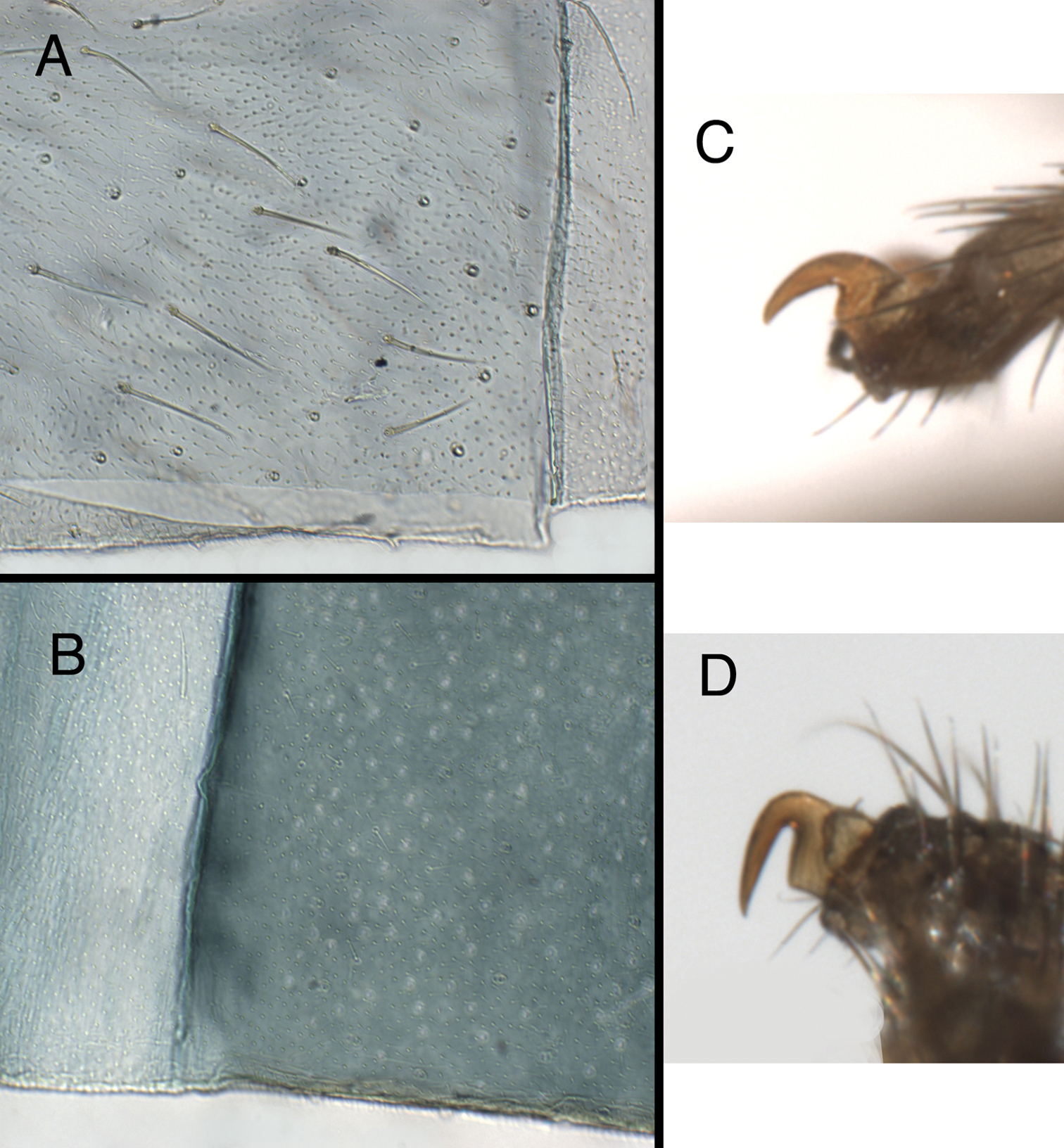
A, B Integument of fifth abdominal sternite. A Kymachrysa placita, without microtholi [typical of Kymachrysa placita and some Kymachrysa intacta populations (see text)] B Kymachrysa intacta, with microtholi (typical of some populations). C, D Prothoracic tarsal claw. C Kymachrysa placita D Kymachrysa intacta. A Type, Colorado, MCZ; B California, TRC; C Wyoming, SDNHM; D Minnesota, CMNH.
Female abdomen (Fig. 3) robust, not slender; terminalia compact. Praegenitale present, extending as truncated lobe from distal margin of S7, with distal, asymmetrical lobe, comb-shaped, with single long seta; lobe extending distally beyond S7 or curving internally. Colleterial gland, reservoir membranous, very delicate, extending proximally well into A7; transverse sclerite relatively large, broad, comb-shaped, with elongate teeth. Spermathecal complex simple; spermatheca small, pillbox shaped with small to moderate, U-shaped invagination, sail-like velum, opening to bursa copulatrix via elongate slit and small, membranous bursal duct; spermathecal duct elongate (>3× width of spermatheca), curvy, covered with fine hairs throughout (mature specimens), most dense, long distally, becoming short, stubby basally; immature specimens with basal ~10–20% of spermathecal duct smooth. Bursa copulatrix small, delicate, membranous, sac-like; bursal glands either absent or very small. Subgenitale large, robust, triangular in ventral view, with pair of distal lobes, extending outward or concealed beneath ectoprocts.
The prefix “Kyma-” comes from the Greek word kýma (κύμα), meaning wave, and refers to the wavy, or sinuate, longitudinal veins between the gradate veins of the forewings that distinguish the two species currently assigned to the genus. The suffix follows the traditional series of chrysopid names ending in “-chrysa” – Greek, feminine, “χρυσα” meaning golden.
The genus, which currently includes only two species, appears to be restricted to North America (Canada, United States and Mexico, as far south as Mexico City) (
The larvae of only one of the Kymachrysa species (Kymachrysa intacta) were described (
Recently, larvae from additional species of Chrysopodes were described in sufficient detail for more robust comparisons than were possible earlier. Now, detailed larval descriptions are available for six of the 47 currently recognized species of Chrysopodes [
Nevertheless, the following features appear most noteworthy:
(a) Setae: Previously, certain setae on the Kymachrysa intacta Semaphorant B were reported to be “serrated” or “thorny”, similar to those on Chrysopodes; these setae include the LS of the thorax and A4-A8, the large LDS on A6 and A7, and some dorsal thoracic setae (“serrated”:
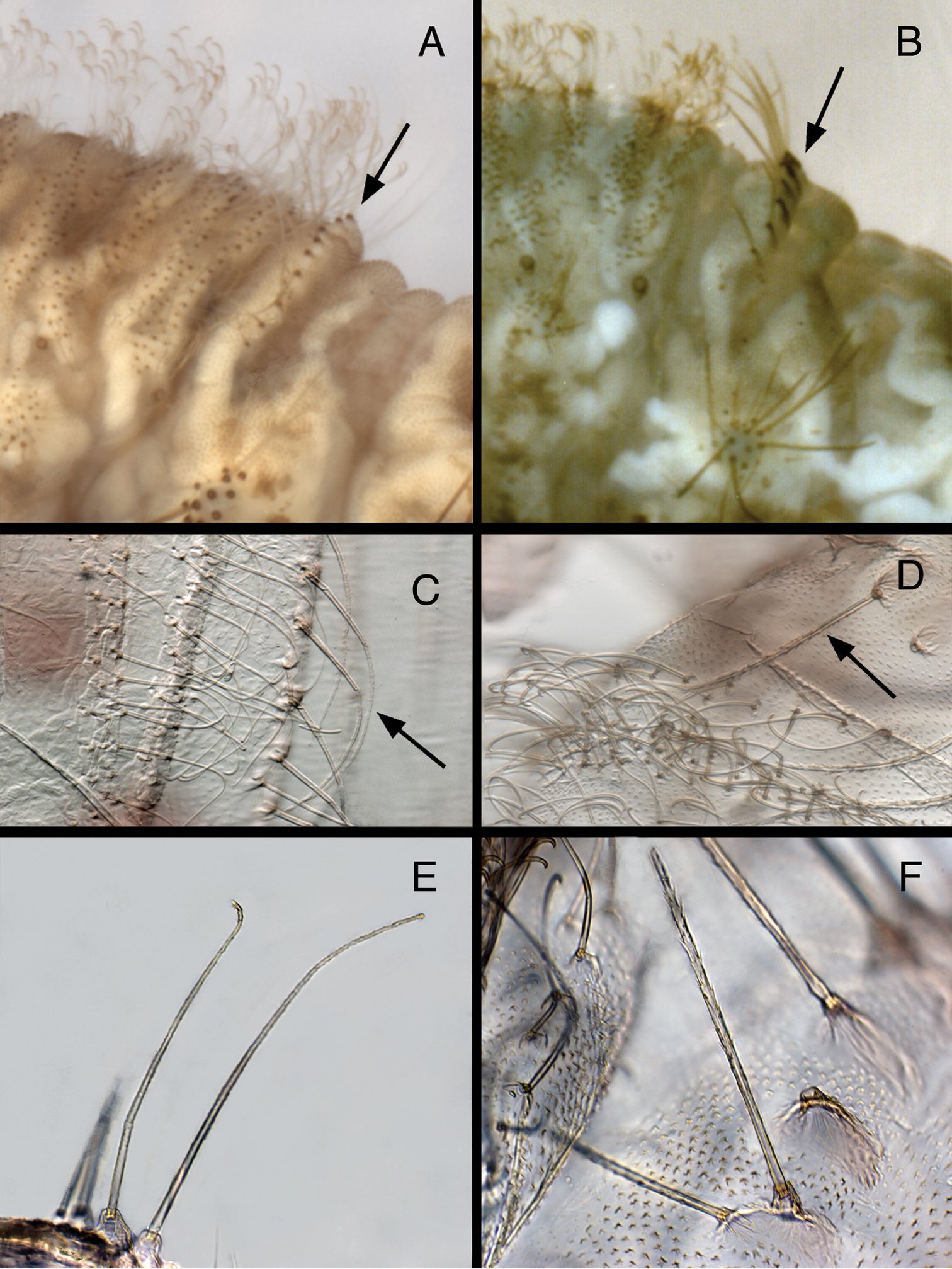
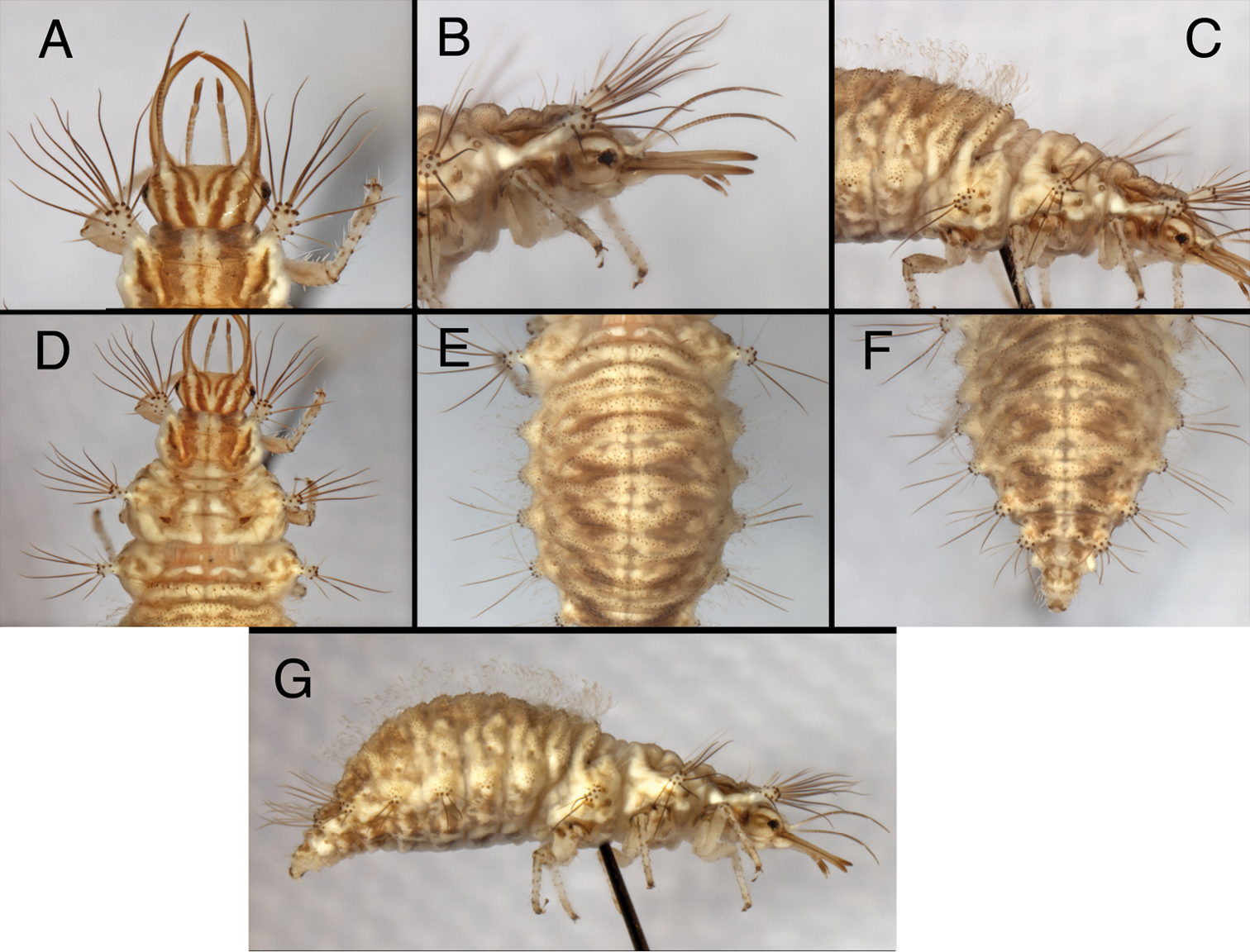
Larval characteristics that distinguish Kymachrysa from Chrysopodes. A, B Raised metathoracic posterior fold [Arrows indicate dark setal bases with distinctive anterior markings] C–F Setae on raised metathoracic fold [Note slender, curved structure, granular surface (Kymachrysa) vs robust, erect structure, thorny surface (Chrysopodes)] A, C, E Kymachrysa intacta (Californa, TRC) B, D, F Chrysopodes geayi (Rio de Janeiro, TRC).
(b) Metathoracic fold: One of the primary reasons for transferring Kymachrysa intacta to Chrysopodes (
First, as discussed above, the surface of the R1 setae on Kymachrysa intacta Semaphorant B is distinctive. It is sabulose (sandy), not thorny as in Chrysopodes.
Second, although the body dimensions of the L3 larvae of Chrysopodes (Chrysopodes) and Kymachrysa intacta that we studied are similar, the length, robustness, and stiffness of the setae differ between the two taxa (Table 1, Fig. 9). For example, in Chrysopodes (Chrysopodes), the R1 setae range in length between 0.28–0.42 mm, and they are thick and erect throughout their entire lengths. In comparison, the Kymachrysa intacta R1 setae range between 0.48–0.62 mm; they are slender throughout, and only the basal section stands erect – the distal section tends to curve. [Note: In the large bodied Chrysopodes (Neosuarius) collaris (Schneider), both the R1 setae and SMS are slightly longer and more slender and flexible than those of Chrysopodes (Chrysopodes) species (Table 1).]
Larval setal lengths (range): comparison between Kymachrysa intacta and Chrysopodes species.
| Species | T3-R1 | SMS |
|---|---|---|
| Kymachrysa intacta | 0.48–0.62 mm | 0.28–0.43 mm |
| Chrysopodes (Chrysopodes) divisus | 0.28–0.36 mm | 0.24–0.36 mm |
| Chrysopodes (Chrysopodes) geayi | 0.39–0.42 mm | 0.21–0.36 mm |
| Chrysopodes (Neosuarius) collaris | 0.43–0.51 mm | 0.31–0.47 mm |
Third, the larvae of Chrysopodes spp. (all instars, including the first) have dark brown markings on the frontal surface of the chalazae in metathoracic R1; these markings are elliptical to ovate and at least as broad as the setal base. In Kymachrysa intacta (second and third instars) they are light brown in color, elongate, and narrower than the setal base (Fig. 9A, B); they are either absent or very light in first instars.
Adult specimens of Kymachrysa placita are not common; those that we have seen were collected during July and August. No larval specimens are reported. Kymachrysa intacta appears to be more abundant; we have seen adult specimens collected from June through mid-October (mostly August), and we have collected larvae during March and April (overwintering second instars) and in September and October (prehibernal first instars).
Biological features have been investigated only for Kymachrysa intacta (
Kymachrysa intacta third instar (darkly marked). Note head markings, debris packet containing woody and dried plant material. (Photo: Stephen A. Marshall).
Developmental stages are relatively prolonged, and they are strongly influenced by photoperiod (
With the addition of Kymachrysa, a total of 17 genera of Chrysopini are now known from the New World. Table 2 lists the eleven that are reported from North America, including Mexico, and it provides references to their distributions.
Genera in the tribe Chrysopini reported from North America, including the new genus, Kymachrysa.
| Ceraeochrysa Adams, 1982 – New World, largely Neotropical; 15 species in North America ( |
| Chrysopa Leach in Brewster, 1815 – Holartic; eleven confirmed species in North America ( |
| Chrysoperla Steinmann, 1964 – Worldwide; ten species from North America ( |
| Chrysopodes (Neosuarius) Adams & Penny, 1985 – Neotropical; largely Central and South America; one species from North America ( |
| Eremochrysa Banks, 1903 – Nearctic, West Indies; largely southwestern USA & northern Mexico: Eremochrysa (Chrysopiella) Banks, 1911, four species; Eremochrysa (Eremochrysa) Banks 1903, thirteen species ( |
| Kymachrysa gen. n. – Nearctic (Canada, USA, and montane Mexico), two species |
| Meleoma Fitch, 1855 – Nearctic, Neotropical, largely USA and Mexico; 28 species ( |
| Nineta Navás, 1912 – Holarctic; two species from North America (USA, Canada) ( |
| Plesiochrysa Adams, 1982 – Neotropical, Oriental, Australasia; ~25 species with two from North America (southern USA & Mexico) ( |
| Pseudomallada Tsukaguchi, 1995 – Holarctic; large genus (~165 species) with five species from North America ( |
| Yumachrysa Banks, 1950 – Western USA, Mexico; four species ( |
Above, we showed that Kymachrysa adults (males and females) express a number of characteristics that provide strong morphological support for a distinct genus. However, its relationship with other chrysopine genera remains perplexing. In general, the male genital structures resemble those of Ceraeochrysa, whereas the female genitalia (apart from the presence of a praegenitale in Kymachrysa) appear similar to those of several other genera (e.g., Ungla, Pseudomallada). Finally, its larval morphology is very close to that of Chrysopodes, and its biological traits (larval habitat, overwintering stage, photoperiodically controlled diapause) resemble those of Pseudomallada. Resolution of the dilemma posed by the above mixture of similarities awaits a broadly based phylogenetic analysis of chysopid genera.
In the most recent taxonomic key for chrysopid genera (
| 45 | Fore wing narrow (length: breadth > 2.8: 1); anal veins not crassate; radial crossveins (between R and Rs) usually straight; median fig [= gonarcal bridge] with dorsal horns [= gonocornua] ( |
45A |
| – | Fore wing broad (length: breadth ≤ 2.8: 1); anal veins crassate; radial crossveins (between R and Rs) usually sinuate; median fig [= gonarcal bridge] without dorsal horns [= gonocornua] ( |
Chrysopodes Navás |
| 45A | Fore wing with veins between gradate veins straight; dorsum of T9+ect invaginated apically, but fused at least basally (male and female); male with ventral apodeme of S8+9 not elongated, extending anteriorly at most to margin of S8, not beyond; spermatheca cylindrical, with U-shaped or J-shaped bend opening to bursa copulatix | Ceraeochrysa Adams |
| – | Fore wing with veins between gradate veins sinuate (wavy); dorsum of T9+ect separate (unfused or divided) dorsally (male and female); male with ventral apodeme of S8+9 elongate, extending anteriorly beyond margin of S8; spermatheca pillbox-shaped, with velum opening to bursa copulatrix via small bursal duct | Kymachrysa gen. n. |
The species was most recently re-described by
We have seen specimens or reliable reports only from the USA (AZ: Chafee, Cochise Co.; CO: Clear Creek, Larimer, Jefferson Co.; NM: Cibola Co.; UT: Uintah Co.; WY: Albany Co.).
In addition to the citations listed above that refer to synonymies and nomenclatural changes,
This species was re-described by
Specimens of Kymachrysa intacta from eastern and western North America appear very similar to each other, but we found some geographic variation of note. First, the male abdominal sternites are densely covered with microtholi on our specimens from California (Alameda, Kern, and Sierra Counties) (Fig. 8B). In contrast, microtholi are very sparse or absent on specimens from Utah (Wasatch County) and Colorado (Larimer County), and absent from specimens from New Hampshire (Belknap County) and New York (Tompkins and Schuyler Counties) (as in Fig. 8 for Kymachrysa placita; also see
The larvae of Kymachrysa intacta have been described (
(a) Semaphorant A (L1): Thorax as figured by
(b) Semaphorant B (L3, L2): Prothorax with S1 and S1Sc1 smooth to very finely sabulose, not thorny. Mesothorax with Sc2 present mesal to lateral tubercles, with two small associated setae (S1Sc2, S2Sc2); S1 present, small; S2 small or absent; S3 of medium-length, slightly granular; S4 small, smooth; but S1Sc3 small, S2Sc3 slightly longer. Metathorax with posterior row of setae (R1) arising from large chalazae.
Kymachrysa intacta third instar. A Head (dorsal) B Head (lateral) C Thorax (lateral) D Thorax (dorsal) E Abdominal segments 1–4 (dorsal) F Abdominal segments 4–10 (dorsal) G Body (lateral). All New York, TRC.
This species occurs broadly throughout North America. We have seen specimens or reliable records from: Canada (ON, QC; see
Kymachrysa placita and Kymachrysa intacta were collected sympatrically in Rustler Park, Cochise Co., AZ, 6-Aug-1991, by R. & J. Robertson (CAS).
Special thanks go to Maurice J. Tauber (Cornell University, MJT) for his careful reading of the manuscript and constructive suggestions, John Rawlins (Carnegie Museum of Natural History) and Oliver S. Flint (Smithsonian Institution) for their earlier comments and suggestions, and Stephen A. Marshall (University of Guelph) for use of his photo (Fig. 10). We thank N. D. Penny (CAS), C. Barr and P. T. Oboyski (EMEC), S. P. Cover & P. Perkins (MCZ), C. Darling and B. Hubley (ROM), O. Flint (USNM), D. Goodger (BMNH), B. Kondartieff (CPG), J. K. Liebherr (CUIC), O. Lonsdale (CNC), J. E. Rawlins (CMNH), and M. Wall (SDNHM) for providing access to types and specimens in their care. The “Lacewing Digital Library” website, developed by J. D. Oswald (Texas A & M University, College Station), was very useful to this study. CAT’s work has benefitted from funding by the National Science Foundation (DEB 0542373, with MJT), USDA/NRI Competitive Grants Programs (9802447, with MJT), and Cornell University.