Citation: Kerr PH (2014) The sequoia-loving sprite, a new genus and species of fungus gnat (Diptera, Mycetophilidae) from California. ZooKeys 437: 109–126. doi: 10.3897/zookeys.437.7932
California is one of the most biologically diverse regions of the world, yet the diversity of fungus gnats (Mycetophilidae) remains largely undocumented within the state. A modest survey of these flies has led to the discovery of a new genus and species of gnat that lives alongside one of the most iconic trees in the world, the giant sequoia (Sequoiadendron giganteum). Spritella sequoiaphila gen. et sp. n. is described and illustrated and its status among other mycetophilid genera is analyzed and discussed.
Systematics, fungus gnats, new genus, new species, California Floristic Province, Spritella, giant sequoia tree, Sequoiadendron giganteum
California is home to one of the most biologically diverse regions of the world (
Our understanding of the diversity and distribution of Mycetophilidae and related flies in California will remain limited as long as material available for study is lacking in museums and other curated collections. In an effort to document latent diversity within the California Floristic Province, a modest collecting program has been conducted throughout the state over the last several years. Although woefully incomplete, this effort has generated over 5000 genus-level specimen identifications of fungus gnat specimens from over 170 different collecting events (nearly all Malaise traps) in 12 different California counties.
One of the most iconic symbols of the California Floristic Province is the giant sequoia groves of the Sierra Nevada Mountains. This habitat is special for its natural inhabitants as much as for the emotional reaction it inspires. The giant sequoia is the planet’s largest living organism and found only in California, with some trees towering over 300 feet tall and having trunk diameters of over 55 feet. In 1853, when the ‘Mother of the Forest’ tree was cut down in Calaveras County for speculative commercial exploitation, it set off the first national awakening of environmentalist sentiments that called for the protections of public lands. This awareness, fostered by the popular publications of naturalist John Muir, led to the first protections of these trees in 1864 (Mariposa Grove in Yosemite Valley), and eventually led to the establishment of the United States National Park Service in 1872. Today, the magnificent ‘Big Trees’ bring over 5, 000, 000 people per year to visit parks that contain giant sequoia groves, generating tens of millions of dollars in tourist revenue for local economies every day (
It was within giant sequoia forest habitat that an especially curious fungus gnat was recently discovered. Because of its unusual morphology – particularly, the unique wing venation of the male – the phylogenetic affiliation of this fly was not immediately clear. This paper describes this species, illustrates its morphology, and locates it within a systematic phylogenetic framework among currently known Mycetophilidae.
Terminology for thoracic, wing, and genitalic morphology is consistent with
All measurements are made in millimeters. Ranges are given for body length, wing length, and the mean for each of these values is provided. Measurements of the holotype are given in square brackets. The number of individuals measured is noted in parentheses. All measurements are of critical-point dried specimens.
| 1 | Posterior vein forked, sometimes incomplete at the base; mediotergite setose or not | (Other Sciophilinae) |
| – | Only one posterior wing vein; mediotergite setose | 2 |
| 2 (1) | M2 absent or present in the form of a vein more or less obsolete at the base | 8 |
| – | Anterior fork complete | 3 |
| 3 (2) | Wing macrotrichia oriented toward base of wing; sc-r and R4 present (R4 occasionally absent); metepisternum with fine setae | Monoclona Mik |
| – | Wing macrotrichia oriented toward wing apex; sc-r and R4 present or absent; metepisternum bare | 4 |
| 4 (3) | Sc-r present, R4 present or absent; tibia II without sensory organ | 6 |
| – | Sc-r and R4 absent; tibia II bearing sensory organ | 5 |
| 5 (4) | Subcostal wing vein not exceeding (or just barely exceeding) the apex of the basal cell; second palpal segment strongly dilated; male tergite IX large, lacking modified setae | Cluzobra Edwards |
| – | Subcostal wing vein clearly exceeding the apex of the basal cell; second palpal segment weakly dilated; male tergite IX short, bearing a pair of modified paddle-shaped bristles | Afrocnemia Matile |
| 6 (4) | R4 present; anterior basalare setose; anepisternum bearing short setae; subcostal vein usually less than half length of wing membrane | Parvicellula Marshall |
| – | R4 absent; anterior basalare bare; anepisternum bare or bearing short setae; subcostal vein usually half length of wing membrane or longer | 7 |
| 7 (6) | Anepisternum bare or with small setae; katepisternum bare; sc-r proximal of Rs | Acnemia Winnertz |
| – | Anepisternum with small setae near upper margin; katepisternum with setae; sc-r distad of Rs | Spritella gen. n. |
| 8 (2) | Subcostal very short, free at apex; r-m longitudinal, the basal cell small and rectangular | Azana Walker |
| – | Subcostal ending in C or R, r-m longitudinal or not | 9 |
| 9 (8) | Subcostal long, ending in R; r-m long and longitudinal; anepisternum setose | Neotrizygia Tonnoir & Edwards |
| – | Subcostal short, ending in C; r-m long or short; anepisternum setose or bare | 10 |
| 10 (9) | Sc-r present, anepisternum setose | Trizygia Skuse |
| – | Sc-r absent, anepisternum bare | 11 |
| 11 (10) | Wing macrotrichia directed toward the wing apex; r-m oblique; M1 entire; R4 present (New Zealand) or absent (South America) | Paratrizygia Tonnoir |
| – | Wing macrotrichia directed toward the wing base; r-m longitudinal, the basal cell small rectangular; M1 incomplete at the base; R4 absent | Neoaphelomera Miller |
Spritella sequoiaphila gen. et sp. n., by current designation.
Three ocelli, antennae with 14 cylindrical flagellomeres, maxillary palpus 4-segmented, scutum raised above level of head, upper half of anepisternum with setae, ventro-posterior area of katepisternum with microsetae, tibial spurs 1:2:2. Wing membrane with macrotrichia; costa produced beyond tip of R5; subcosta long, ending at C, approximately at midpoint of wing; sc-r present, arising beyond origin of Rs; r-m missing because Rs and M1 touching or r-m present, short; M2 arising from discal cell basad of origin of Rs or at base of M1; cubital vein unforked, A1 well developed, reaching beyond origin of Rs. Male gonostylus without basal appendages.
Spritella gen. n. resembles Acnemia Winnertz by its lack of a posterior fork and foreshortened medial stem. However, the new genus is readily separated from Acnemia by the presence of setae on the anepisternum and katepisternum; sc-r arising well beyond origin of Rs; and by having male gonostylus without a basal process. Other sciophiline genera that also lack the cubital fork include Afrocnemia Matile, Cluzobra Edwards, Monoclona Mik, and Parvicellula Marshall. In the new genus, crossvein sc-r is clearly present unlike in Afrocnemia and Cluzobra; R4 is absent unlike in Parvicellula; and the macrotrichia of wing membrane are decumbent, directed toward wing apex unlike in Monoclona. The long subcostal vein of Spritella gen. n. (relative to wing length) and position of sc-r relative to Rs is also distinctively different from these genera.
Head shape in anterior view subequal, approximately as long as wide; medial eye margins farther apart dorsally than ventrally; antennal eye notch present, at least two ommatidia deep; interommatidial setulae present between all ommatidia; ocelli three, nearly linear; lateral ocellus between 1× and 1.5× its own diameter from eye margin, between 2.5× and 3× its own distance from median ocellus; all ocelli dorsad of eye margin; occipital suture from median ocellus to occiput absent; frontal suture between median ocellus and ventral margin of frons complete, suture between lateral ocelli and eyes also present; frons with setae; face approximately 2× longer than wide, parallel-sided along most of length, bearing setulae throughout; face and clypeus separated by complete suture; clypeus ovate, approximately one-half length of face, covered with short setae. Antennal scape and pedicel subequal in size; scape with setae approximately 2× scape length; pedicel setae approximate length of pedicel; antennal flagellomeres 14, cylindrical, approximately 3× longer than wide, approximately the same length but thinner distally, densely covered with short setae. Palp with 4 visible segments, none with apparent sensory pit.
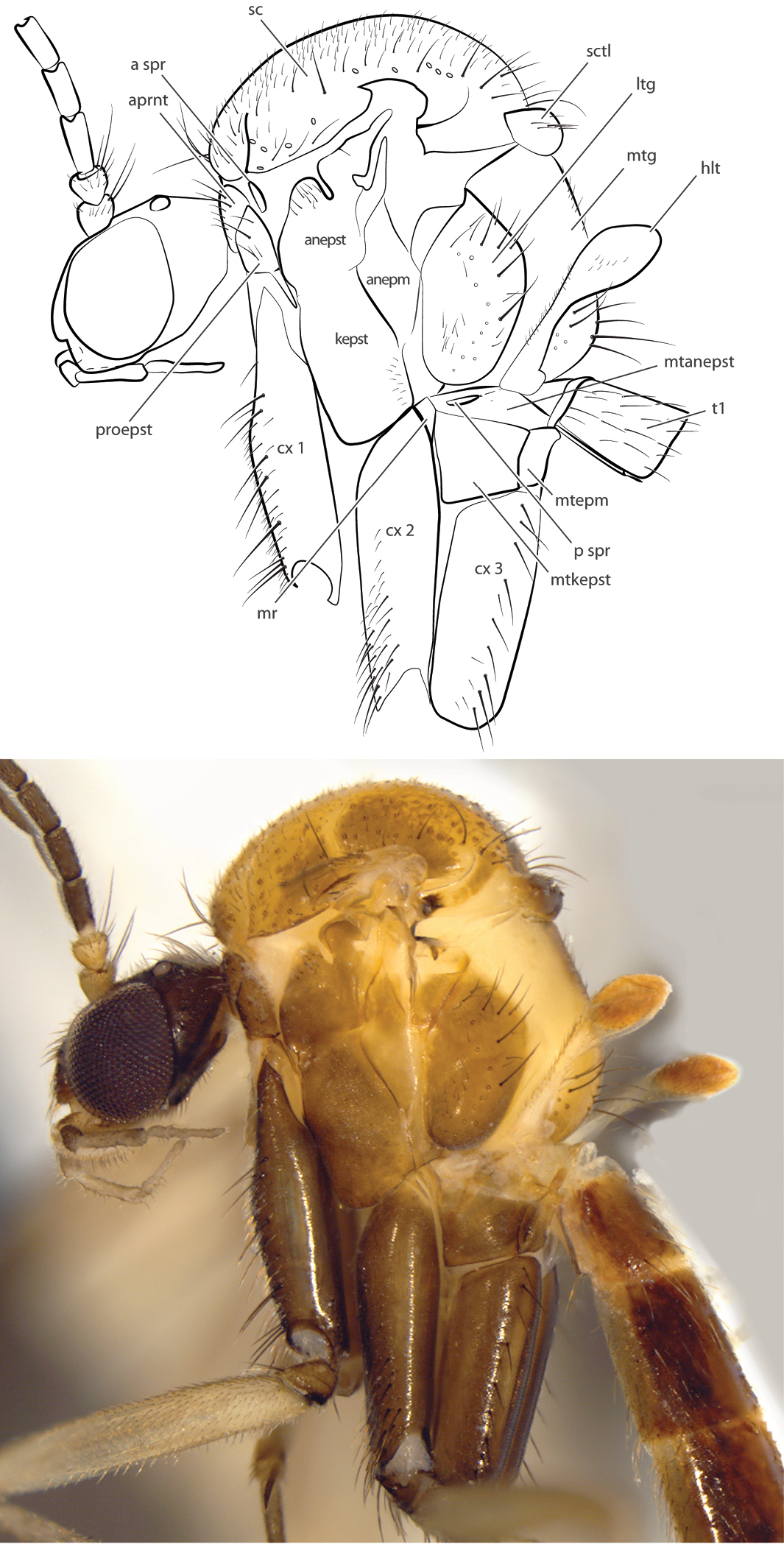
Thorax (Fig. 2) raised, scutum dorsad of head position; short setae distributed throughout scutum, acrostichal setae present, bristles present along lateral margins of scutum; postalar wall and callus separated by carina; scutellum clearly wider than long, narrower than scutum; antepronotum and proepisternum with bristles; anepisternum with setae dorsally; anterior basalare bare; anapleural suture incomplete; katepisternum with setae ventro-posteriorly; anepimeron bare; anepisternum with few inconspicuous setulae; laterotergite raised ventrally, with bristles and shorter setae; metepisternum bare; mediotergite with three bands of bristles ventrally and shorter setae that extend along dorsoventral length, medially. Wing membrane covered with microtrichia and macrotrichia that are arranged irregularly; C ending beyond R5; dorsal surface of humeral vein without setae, ventral surface with setae; subcostal vein setose on both sides, ending in C, approximately at midpoint of wing; sc-r present, arising distad of origin of Rs; R1 setose on dorsal and ventral surfaces, although bare basad of Rs vein ventrally; vestigial M vein within discal cell present or absent; R4 not present, r-m present or absent (R5 joining M1 at junction with Rs); M1 setose above, bare below; M2 setose above, bare below, either arising from bM, from junction of M1 and Rs, or from base of M1; cubital vein unforked, setose above, bare below, ending at wing margin; CuP strong at base, extending apically as weak fold; anal vein strong, setose on both sides (less so ventrally), extending beyond origin of Rs. Legs elongate; coxae with dark, erect setae and lighter, shorter decumbent setae; femora with short, appressed setae and microtrichia; mid tibial organ absent; tibial spur formula 1:2:2; tibiae with short, appressed setulae and short, erect setae that are no longer than half widest width of tibia; tarsal claws small; empodium developed.
Abdomen with segments of subequal width; sternites with two longitudinal fold lines along length; in male, segments 8 modified so that genitalia orient upwards. Male terminalia with enlarged, hood-like epandrial sclerite (tergite IX); cerci and epiproct reduced; hypoproct with lightly sclerotized anterior apodemes; gonocoxites widely separated, joined by narrow medial bridge; gonostyli simple, without subtending appendages, inwardly-directed, and arising from middle area of gonocoxites. Female terminalia with first cerci elongate, second cerci ovoid, sternite 8 clearly larger than tergite 8.
The genus name is feminine, derived from the English “sprite” and the Latin ending “-ella”, as a diminutive.
Holotype: ♂, “USA: CA: USA: CA: Tulare Co.: Whitaker Forest, EshomCrk.Drainage, nr. tree#142, 36.7062°N, -118.9319°W, 1650masl, YPT, 3.vi–16.vii.2010 P.H. Kerr” / “HOLOTYPE 10F761♂ Spritella sequoiaphila Kerr 2014” [red label]. Deposited in CSCA, dissected specimen mounted on gray point, terminalia in glass vial marked “10F761 HT” on pin below specimen. Type locality indicated in Fig. 14.
Paratypes: 1 ♂, USA: CA: Calaveras Co., Calaveras Big Trees SP, S. grove fire rd., nr. Beaver Creek, MT#1, 38°15.41'N, 120°15.25'W 1385masl, 22.v.–11.vi.2007 P.H. Kerr & A.R. Cline 07LOT086” [CSCA]; 7 ♂♂, 8 ♀, “USA: CA: Tulare Co.: Whitaker Forest, E.EshomCrk.Drainage, nr. tree#142, 36.7062°N, 118.9319°W, 1650masl, MT, 3.vi–16.vii.2010 P.H. Kerr CSCA10L174” [CSCA]; 8 ♂♂, 3 ♀, “USA: CA: Tulare Co: Whitaker’s Forest, Ridge S. of Eshom Crk., 1620masl, 36.7011°N, 118.9363°W, MT, 3.vi–16.vii.2010 P.H. Kerr CSCA10L175” [CSCA]; 4 ♂♂, 2 ♀, “USA: CA: Tulare Co.: Whitaker Forest, E.EshomCrk.Drainage, nr. tree#142, 36.7062°N, -118.9319°W, 1650masl, YPT, 3.vi–16.vii.2010 P.H. Kerr CSCA10L258” [CSCA]; 1 ♂, “USA: CA: Glenn Co., Atchison Campsite pine forest, ex: Malaise, elev. 1310m, 20–24.v.2012, colls: K. Will, K. Yao, N. Grady-Grot, 39°45'00"N, 122°55'33"W, CAL2012.v.23.5” [EMEC].
This species may be distinguished by the characters of the genus and by the male genitalia; particularly the gonostylus which has two apical lobes, the ventral one being distinctly sclerotized and darkened.
Male. Body length (n=4): 4.6–6.6, 5.7 [4.6] mm. Wing length: 5.3–5.9, 5.6 [5.9] mm (n=4).
Coloration (Figs 1, 2). Head brown; face and clypeus brown; palpomeres light brown to brown. Antennal scape and pedicel yellowish light brown, base of first flagellomere yellowish light brown, otherwise brown; all remaining flagellomeres brown. Thorax variously yellow, yellowish to orangish brown to brown; scutum yellowish light brown dorsally, with faint brown band postero-medially, orangish brown to brown laterally; scutellum light brown to brown; antepronotum, proepisternum, anepisternum, and katepisternum brown; anepimeron light brown; laterotergite and anepisternum brown; metepimeron and metakatepisternum brown to dark brown; halteres yellowish light brown; mediotergite yellowish light brown. Wing membrane lightly brown infuscated. Coxae dark brown; femora yellowish light brown; tibiae slightly darker yellowish light brown; tarsi brown. Abdominal segments light brown to brown, lighter in color near anterior margin on segments 1–6. Terminalia brown.
Spritella sequoiaphila sp. n., habitus [holotype male, # 10F761]. Scale bar = 1 mm.
Spritella sequoiaphila sp. n., thorax, lateral view [paratype male, #11G040]. Abbreviations: anepm anepimeron anepst anepisternum aprnt antepronotum a spr anterior spiracle cx coxa hlt halter kepst katepisternum ltg laterotergite mr meron mtg mediotergite mtanepst anepisternum mtepm metepimeron mtkepst metakatepisternum p spr posterior spiracle patg paratergite proepm proepimeron proepst proepisternum sc scutum sctl scutellum.
Head. Ocelli slightly raised; middle ocellus smaller than (approx. 0.5× size of) lateral ocelli. Face with golden brown setae laterally, mostly bare medially. Antenna length 2.7–3.0, 2.9 [2.7] mm (n=4); about 2× length of thorax, shorter than abdomen. Palpus with four visible palpomeres, slightly longer than width of head (anterior view); length of palpomeres 1 and 2 nearly subequal (palpomere 2 longer); palpomere 3 approx. 5× longer than wide; palpomere 4 approx. 10× longer than wide, subequal to or shorter than combined length of palpomeres 1–3.
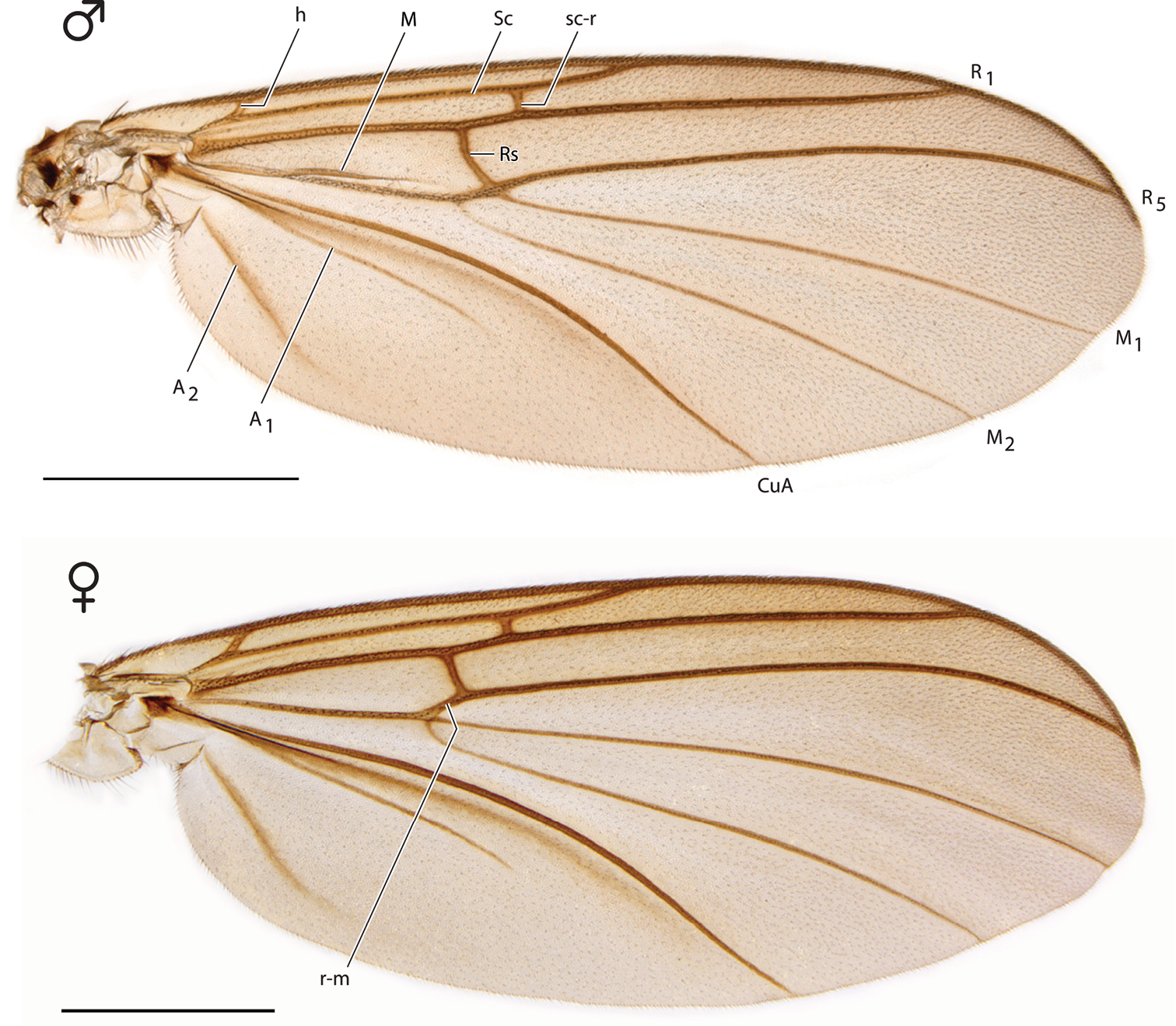
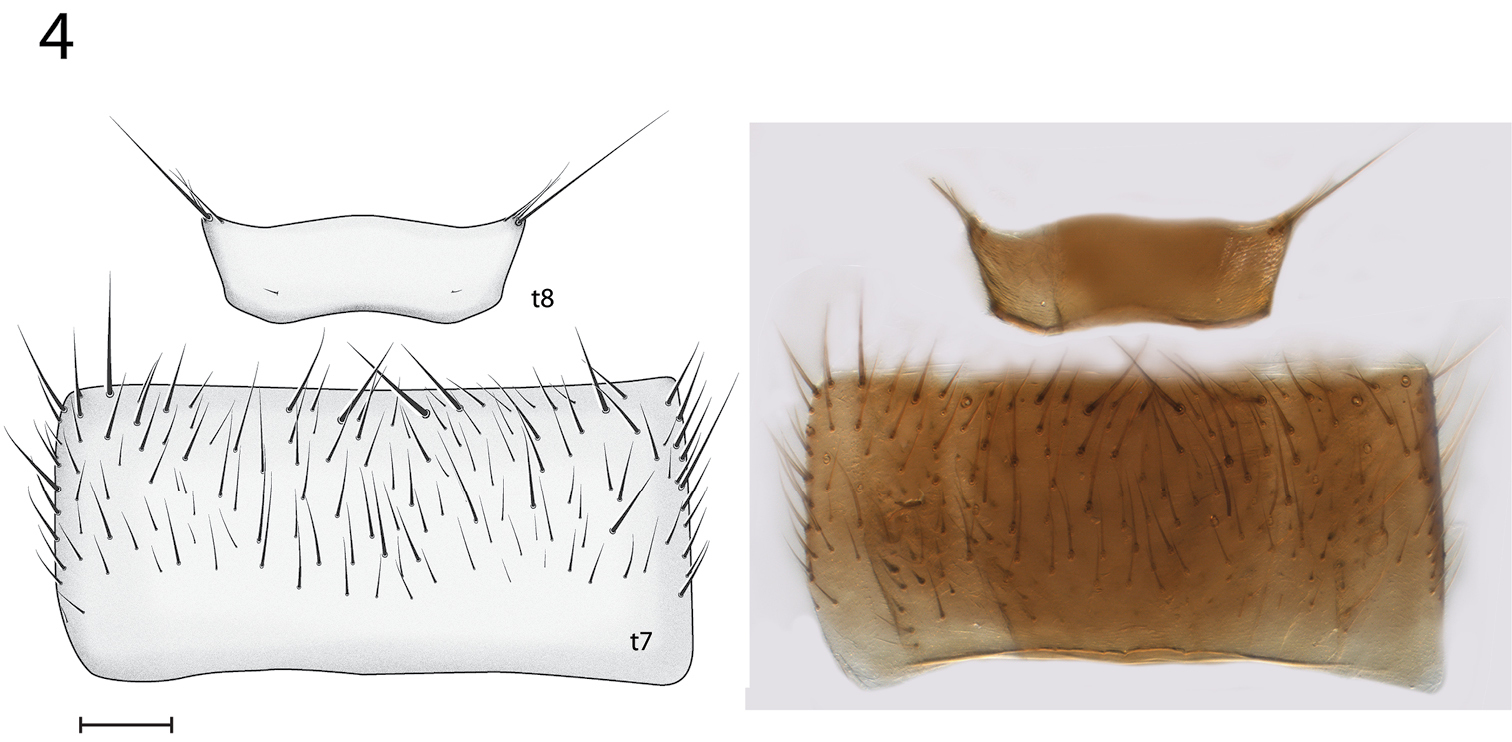
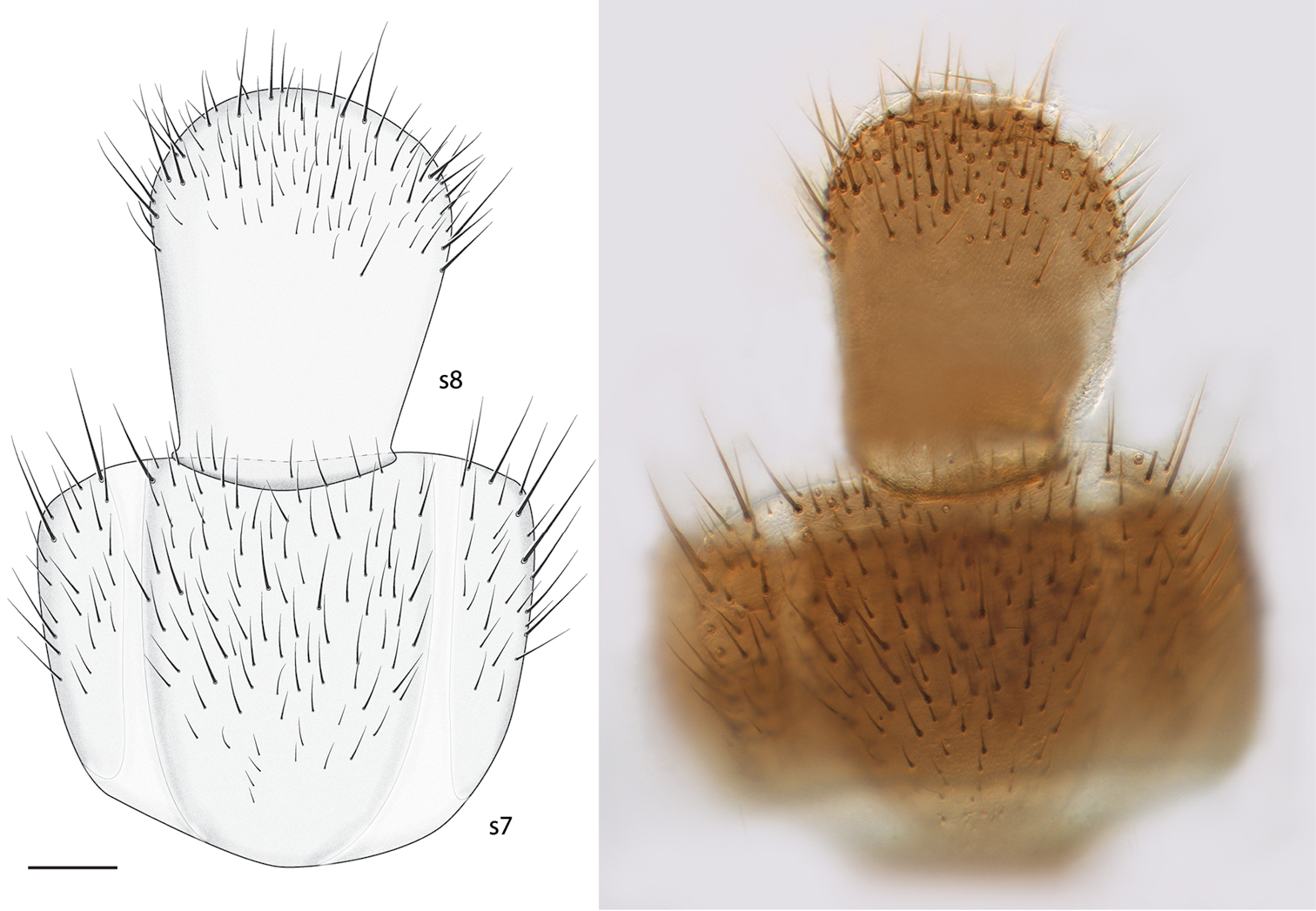
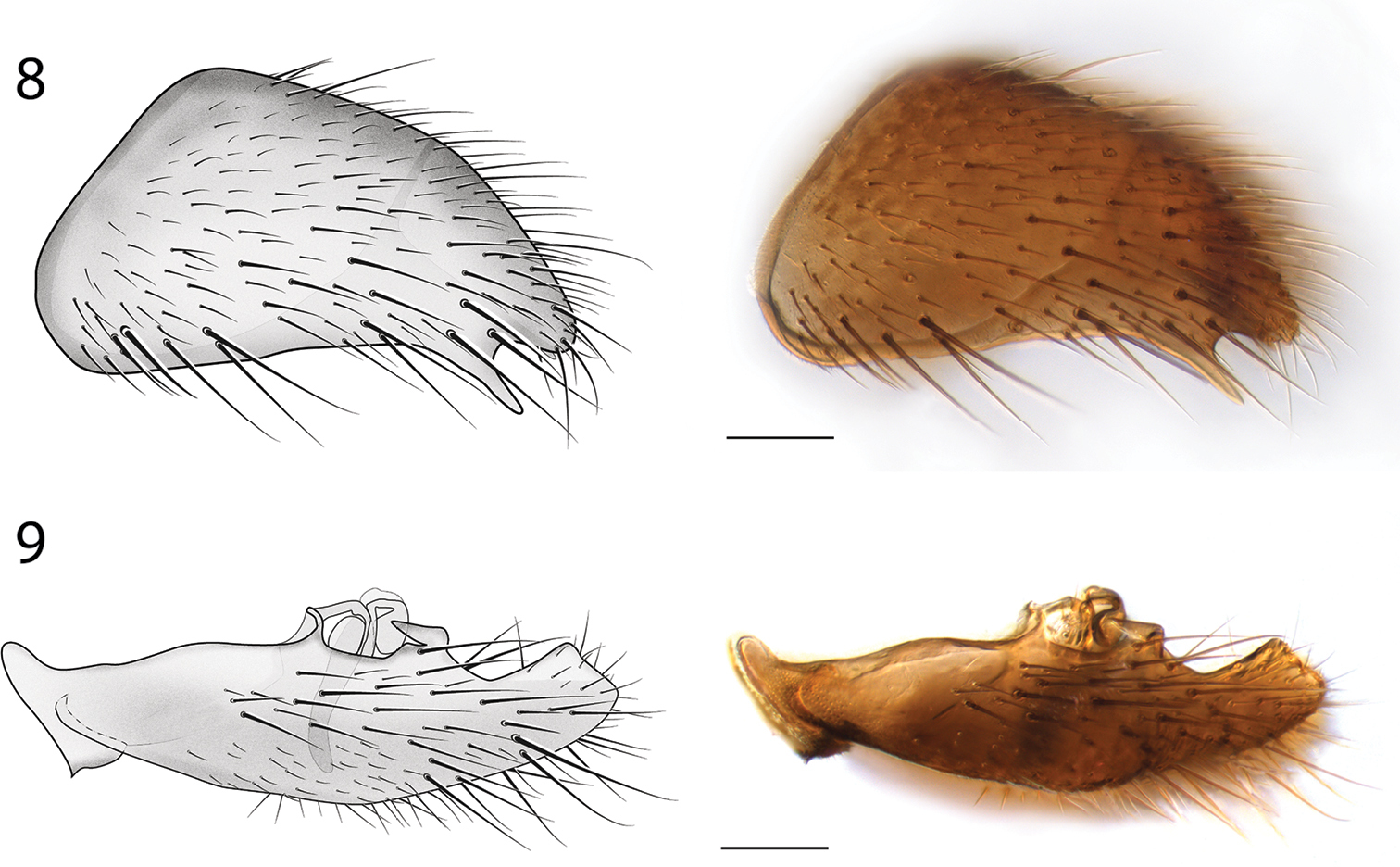
Thorax and Abdomen. Wing as Fig. 3; Rs distinctly elongate; vestigial M vein present within discal cell; M1 arises from R4+5 at origin of Rs so that r-m not present; M2 weak at base. Tergite 8 reduced (Fig. 4), with setae on posterolateral margin, and a pair of setulae sub dorsally; sternite 8 (Fig. 5) elongate, setose on posterior half, approximately half width of sternite 7.
Spritella sequoiaphila sp. n., right wings [paratype male, #10F296; paratype female #14P342]. Scale bar = 1 mm.
Spritella sequoiaphila sp. n., male abdominal tergites 7–8 [holotype, # 10F761]. Sternite 7 slightly visible below. t tergite. Scale bar = 0.1 mm.
Spritella sequoiaphila sp. n., abdominal sternites 7–8 [holotype male, # 10F761] (looking through t7). s sternite. Scale bar = 0.1 mm.
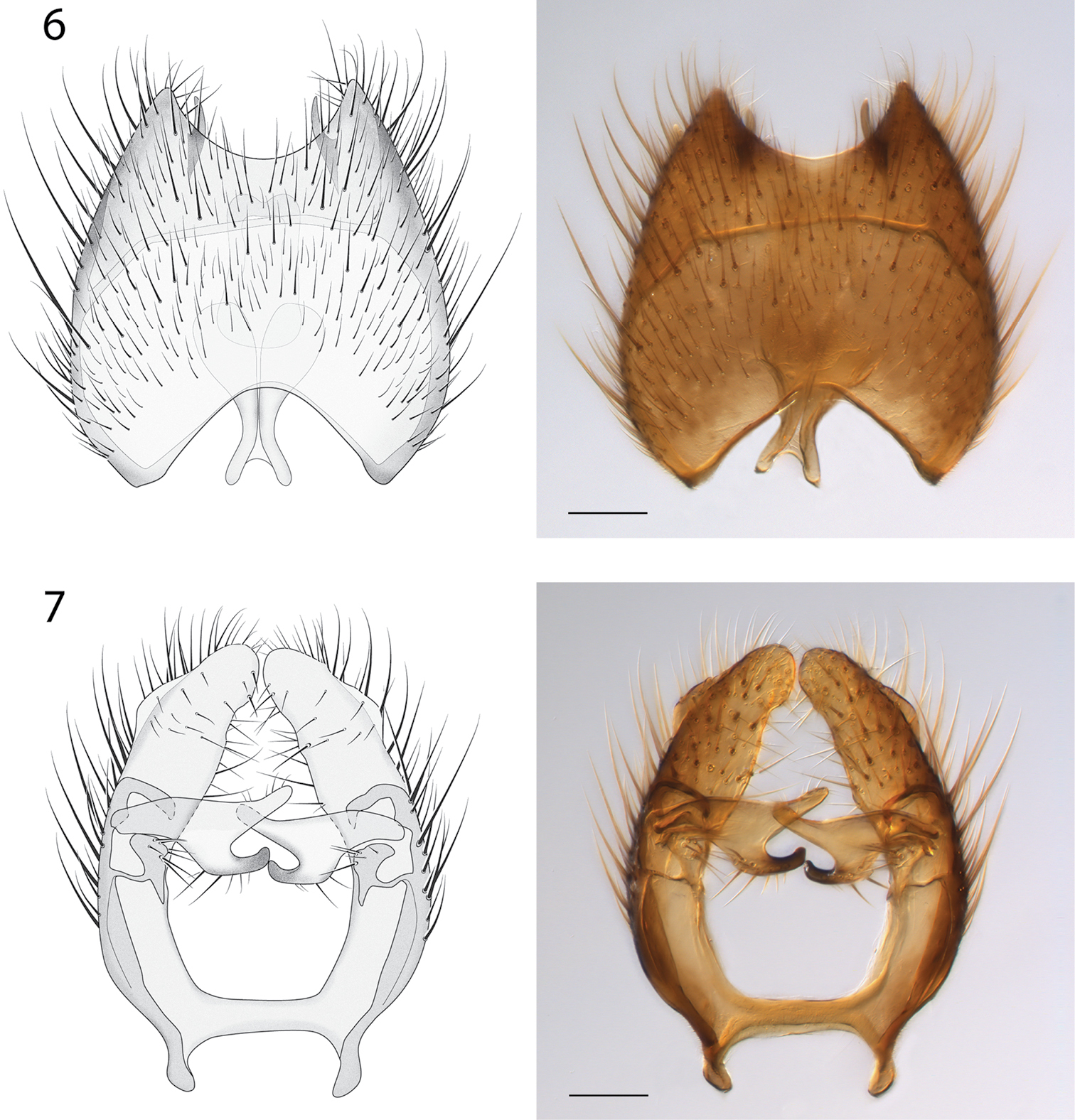
Male Genitalia (Figs 6–9). Epandrium deeply emarginate both anteriorly and posteriorly, with pair of submedial apical fins (Fig. 6) and pair of short lateral processes (Fig. 8). Gonostyli with pair of setose apical lobes, the ventral one with dark sclerotization.
Spritella sequoiaphila sp. n., illustrations and photomicrographs of structures of the male genitalia, dorsal view [holotype male, # 10F761]. 6 epandrium 7 gonocoxites. Images same scale, scale bar = 0.1 mm.
Spritella sequoiaphila sp. n., structures of the male genitalia, lateral view [holotype male, # 10F761]. 8 epandrium 9 gonocoxites. Scale bar = 0.1 mm.
Female. Body length: 4.4–5.5, 5.1 mm (n=4). Wing length: 4.8–5.3, 5.1 mm (n=4). Antenna length 1.9–2.2, 2.1 mm (n=4).
Coloration. Similar to male.
Thorax. Wing as Fig. 3; Rs not as long as in male; vestigial M vein within discal cell absent; r-m present; M2 arises at or near base of M1.
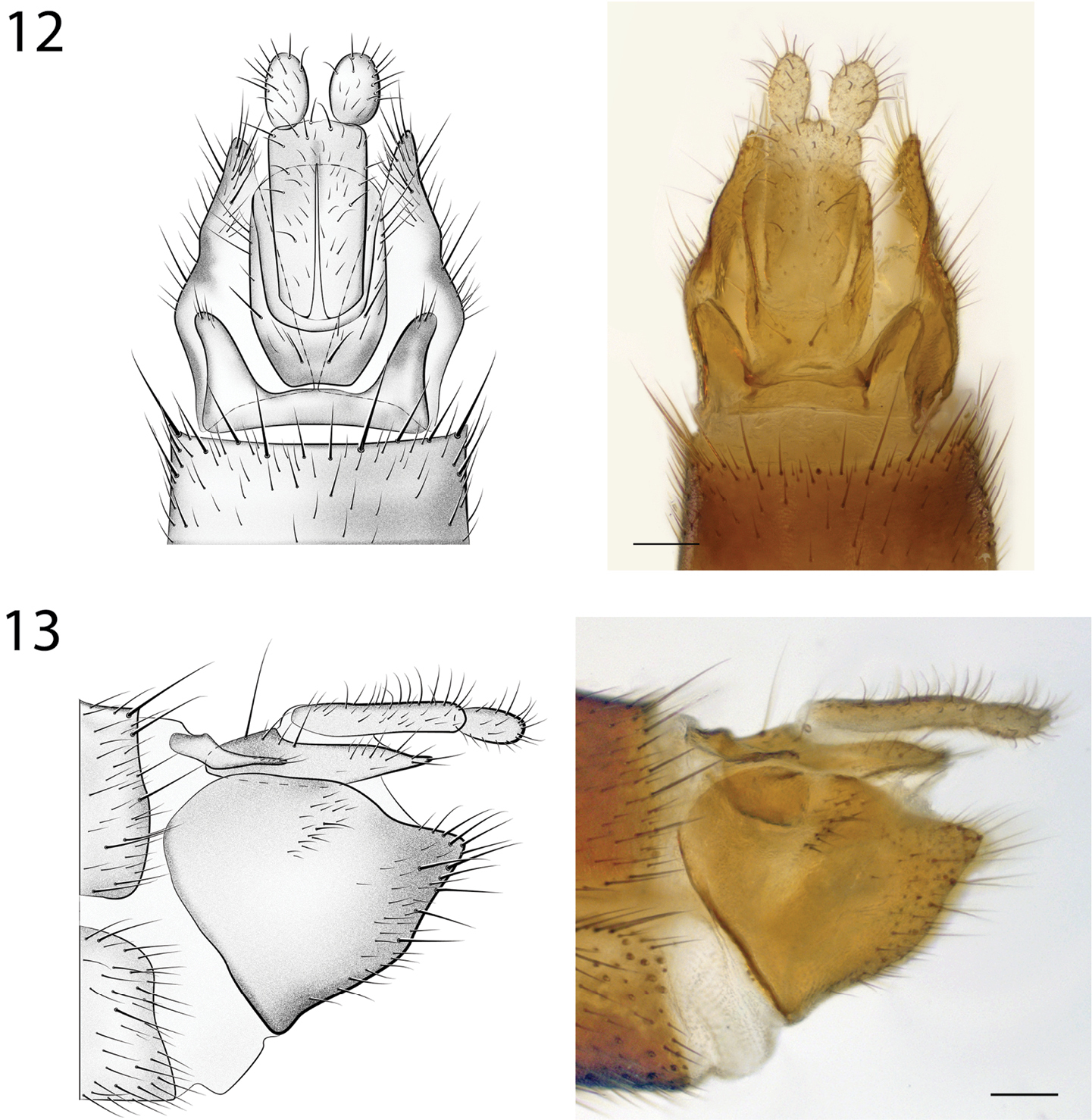
Female Genitalia (Fig. 10). Tergite 8 narrow, expanded laterally; first cerci fused, approx. 2× longer than wide; second cerci ovoid; hypoproct elongate, subtending most of cercus 1; sternite 8 with deep medial cleft reaching anterior margin.
Spritella sequoiaphila sp. n., structures of the male genitalia, posterior view [holotype male, #10F761]. 10 epandrium 11 gonocoxites. Scale bar = 0.1 mm.
12 Spritella sequoiaphila sp. n., structures of the female genitalia, dorsal view [# 14P342]. Scale bar = 0.1 mm 13 Spritella sequoiaphila sp. n., structures of the female genitalia, lateral view [# 14P342]. Scale bar = 0.1 mm
The species epithet is an adjective, referring to its affiliation with giant sequoia groves (“sequoia-loving”).
The phylogenetic analysis was carried out by adding Spritella sequoiaphila gen. et sp. n. to the scored character matrix created by
All characters were scored for Spritella sequoiaphila except the following: 47, humeral vein (oblique or curved); 84, arrangement of vestiture of tibia (irregular, apical portion with parallel lines, or all in parallel lines); and 87, vestiture arrangement on tarsomeres (irregular or in parallel lines). Due to problems of interpretation and reproducibility, these characters were scored as “?” in the final matrix; I could not score these characters confidently for any taxa, including those used in the original
The modified matrix was analyzed using parsimony; 1000 heuristic search replicates and 500 bootstrap replicates were performed using PAUP* 4.0b10 (
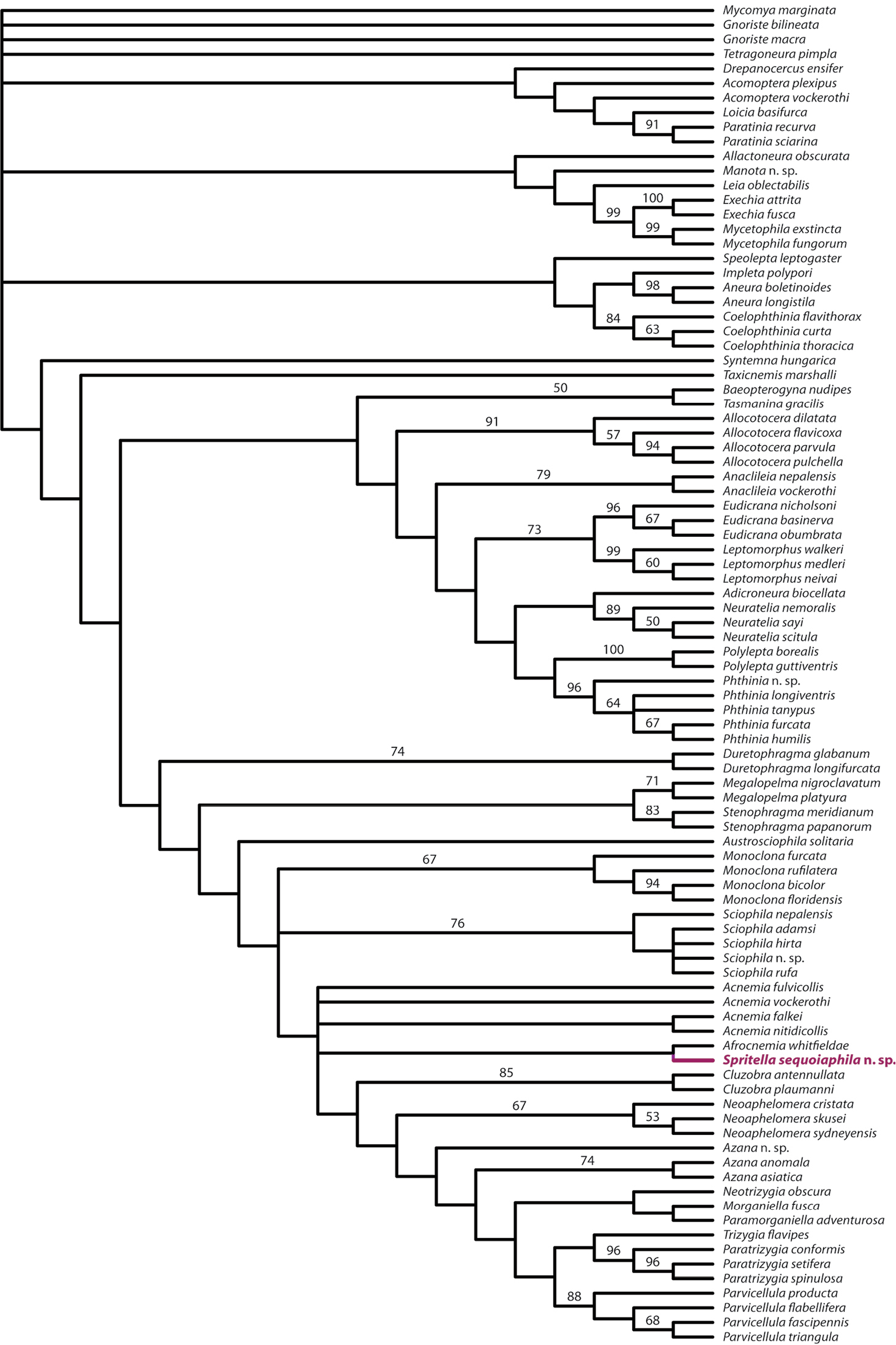
Parsimony heuristic searches found 61 most parsimonious trees (Fig. 14). The strict consensus of these trees differs from the results provided by
Locality of Spritella sequoiaphila sp. n., showing 6-meter Malaise trap; Whitaker Forest (UC Berkeley Center for Forestry), Tulare Co. [CSCA10L258].
The concept of the “Azana group” (
Spritella sequoiaphila is recovered sister to Afrocnemia whitfieldae Matile. The two genera are united by the ocellar arrangement being linear (6: 2) and the first flagellomere being slightly offset (20: 1). Along with most species of Acnemia, Afrocnemia whitfieldae and Spritella sequoiaphila have a distinctively short anterior wing vein fork. This character is difficult to score discretely for phylogenetic analysis (e.g., at what point is it “short” or “long”?) and was not scored by
Acnemia is recovered as a paraphyletic group, which is not especially surprising, since members of this genus can vary widely and in a way that suggests that this group is in need of systematic revision.
Strict consensus tree of 61 equally parsimonious trees from re-analysis of
Many thanks to Stephen Gaimari (CA Dept. Food & Agriculture, Plant Pest Diagnostics Branch), Patricia Raggio (Calaveras Big Trees State Park), and Rob York (UC Berkeley, Center for Forestry, Whitaker Forest) for invaluable field support. Scott Kinnee and Obediah Sage (CA Dept. Food & Agriculture, Plant Pest Diagnostics Branch) provided assistance in critical-point drying specimens. Chris Borkent provided helpful consultation on the interpretation and scoring of characters, in reference to
Data matrix in nexus format (modified from
Authors: Peter Kerr
Data type: NEXUS file with DNA data
Explanation note: Data matrix in nexus format (modified from
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.