Citation: Chandrapatya A, Konvipasruang P, Flechtmann CHW, Moraes GJ (2014) Complementary description of Colomerus novahebridensis Keifer (Acari, Eriophyidae), with a discussion about the constitution of the genus and its economic importance, and a tentative key to Colomerus Newkirk & Keifer species. ZooKeys 434: 17–35. doi: 10.3897/zookeys.434.7308
Colomerus Newkirk & Keifer, 1971 is an eriophyid genus described by Newkirk and Keifer about 43 years ago, that contains species from all continents, except Antarctica. They live mostly on dicotyledonous plants. Colomerus novahebridensis Keifer, 1977 was described from coconut (Cocos nucifera L., Arecaceae) fruits from Vanuatu. A description of a Thai population of this species is given in this paper. A revised characterization of Colomerus and a dichotomous key for the separation of the species presently considered to belong to this genus are provided, and a consideration about the importance of Colomerus species is presented.
Taxonomy, Thailand, Eriophyoidea, Cecidophyinae
Colomerus Newkirk & Keifer is a relatively small genus of eriophyid mites described about 43 years ago by
All Colomerus species have been described from dicotyledonous plants, except Colomerus novahebridensis Keifer, described from coconut (Cocos nucifera L.; Arecaceae) (
The objective of this paper is to present a morphological description of that Thai population (based on adult females and males), to discuss the constitution of the genus, to provide a tentative dichotomous key to Colomerus species worldwide and to summarize the economic importance of this genus.
Specimens used for the complementary description of Colomerus novahebridensis were collected in different coconut fields in the central and southern regions of Thailand. Coconut fruits with symptoms of eriophyid attack similar to that of Aceria guerreronis (whitish to brownish triangular scars starting at the edge of the bracts and progressively enlarging with fruit growth) were collected and taken to the laboratory for examination. The bracts were removed and their undersurfaces as well as the surface of the fruits covered by them were examined, collecting all eriophyid mites found.
The mites were mounted in modified Berlese medium (
All terminology and measurements follow
The revised characterization of the genus and the dichotomous key were prepared by examining the original descriptions of each species, except for Colomerus novahebridensis, collected in this work, Colomerus bucidae (Nalepa), whose characteristics were taken from
Frontal lobe of prodorsal shield rounded, broad-based, short; with parallel microtuberculate lines around lateral margin of ocellar gibbosities; median and admedian lines between anterior shield margin and region slightly anterior to shield center usually broken (indistinct in some specimen), and then continuous to posterior shield margin (broken in some specimens); with several incomplete submedian lines; empodia entire, 5-rayed; opisthosoma with 67–85 microtuberculate annuli; coverflap with longitudinal ridges arranged in two transverse rows. Genital apodeme usually visible as a narrow dark band in ventral view, but sometimes appearing to constitute a pair of subtriangular structures, depending on the position of the focus; spermathecal apparatus moderate distance from apodeme; with 4 coxigenital semiannuli anterior to coverflap, with genital opening somewhat appressed to coxisternum II.
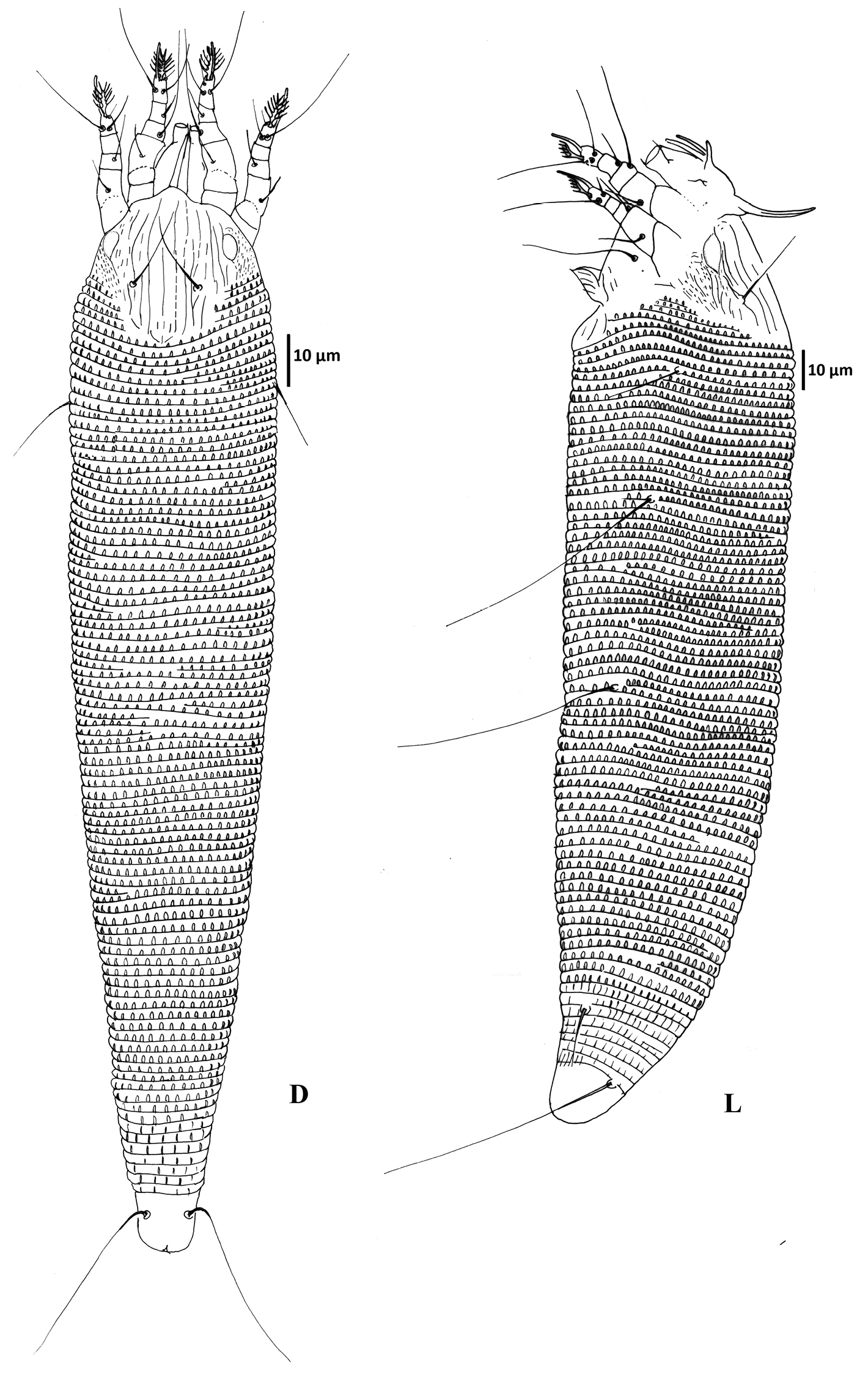
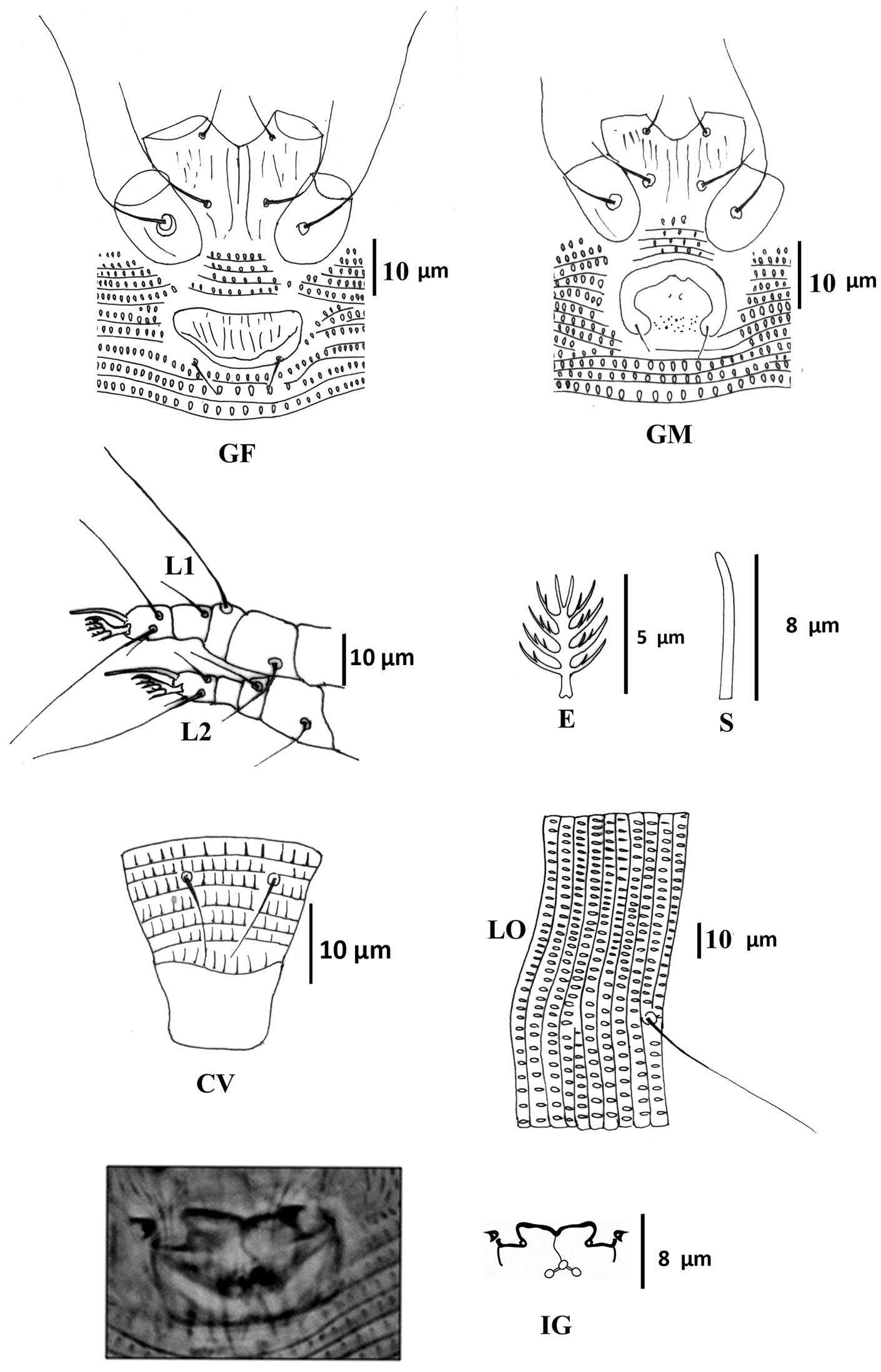
Female (Figs 1–3) (n = 9). Body wormlike, 187–238, 41-47 wide, 47–49 thick, whitish. Gnathosoma (Fig. 1): 16–18, projecting slightly downwards, pedipalp coxal seta (ep) 2–3, dorsal pedipalp genual seta (d) 5–7, subapical pedipalp tarsal seta (v) 2, cheliceral stylets 14–21. Prodorsal shield (Figs 1–3): 28–41, 34–41 wide, semi-oval; prodorsal shield frontal lobe rounded, broad-based, short, 2–3; posterior shield margin convex, interrupting first 4–5 dorsal annuli. Prodorsal shield design with parallel microtuberculate lines around lateral margin of ocellar gibbosities. Line pattern variable (Fig. 3); median and admedian lines usually broken (indistinct in some specimens) between anterior shield margin and region slightly anterior to shield center and then continuous to posterior shield margin (broken in some specimens); some specimens with 1–2 short lines between median and admedian lines near posterior margin of prodorsal shield. Submedian lines variously broken, typically in four pairs running from anterior to posterior margins and four incomplete submedian lines running from anterior to posterior margin; 2 – 3 submedian lines posteriad or mesad of scapular tubercles; ocellar gibbosities prominent. Scapular tubercles situated 7–11 ahead of posterior shield margin, plicate, 12–14 apart, scapular setae (sc) 16–19, directed upward or forward. Coxigenital region: with 4 coxigenital semiannuli, microtuberculate. Coxisternal figs (Fig. 2 GF): coxisternum I with several longitudinal lines, coxisternum II smooth, anterior seta on coxisternum I (1b) 5–6, 9–10 apart; proximal seta on coxisternum I (1a) 15–22, 8–9 apart; proximal seta on coxisternum II (2a) 28–39, 19–21 apart; tubercles of 1b and 1a 8–10 apart. Internal coxisternal apodeme 9–12. Legs (Fig. 2 L1, L2, E, S): with all usual setae. Leg I 23–29, femur 8–10, ventral basifemoral seta (bv) 6–8; genu 4–5, antaxial genual seta (l") 17–22; tibia 4–5, paraxial tibial seta (l') 4–6; tarsus 5–6, antaxial fastigial tarsal seta (ft") 16–18, paraxial fastigial tarsal seta (ft') 10–16, paraxial unguinal tarsal seta (u') 3, tarsal empodium 6–8, entire, 5-rayed, tarsal solenidion (ω) 6–10, slightly curved, blunt. Leg II 22–26, femur 6–10, ventral basifemoral seta (bv) 5; genu 3–4, antaxial genual seta (l") 5–8; tibia 3–4; tarsus 4–6, antaxial fastigial tarsal seta (ft") 18–23, paraxial fastigial tarsal seta (ft') 4–6, paraxial unguinal tarsal seta (u') 2–5, tarsal empodium 6–8, entire, 5-rayed, tarsal solenidion (ω) 8–9, slightly curved, blunt. Opisthosoma (Fig. 1D and L, Fig. 2 ES, CV): dorsum evenly rounded, dorsal annuli 67–83, ventral annuli 71–85, both with elongate, oval microtubercles situated on or near posterior margin of each annulus. Microtubercles more elongate on the last 5–7 ventral annuli and slightly longer, sparser on the last 7-8 dorsal annuli. Seta c2 17–22, 39–46 apart, on ventral annulus 10–12; seta d 43–50, 33–39 apart, on ventral annulus 21–27; seta e 44–64, 19–24 apart, on ventral annulus 37–49; seta f 10–13, 11–13 apart, on ventral annulus 66–80 or annulus 5th from the rear. Seta h1 absent, h2 38–53. Female genitalia (Fig. 2 GF, IG): 8–9, 18–20 wide, coverflap with 8–12 longitudinal ridges in each of two transverse rows, setae 3a 4–6, 11–13 apart. Internal genital apodemes usually visible as a narrow dark band in ventral view (Fig. 2 IG), but sometimes appearing to constitute a pair of subtriangular structures (Fig. 2 IG), depending on the position of the focus; spermathecal apparatus at moderate distance from apodeme.
Colomerus novahebridensis Keifer. Female: D = dorsal view, L = lateral view. Specimens collected in Thailand.
Colomerus novahebridensis Keifer. Female: CV ventral view of caudal region E empodium GF external female genitalia IG internal genitalia L1 leg I L2 leg II LO lateral opisthosoma S solenidion. Male: GM external male genitalia. Specimens collected in Thailand.
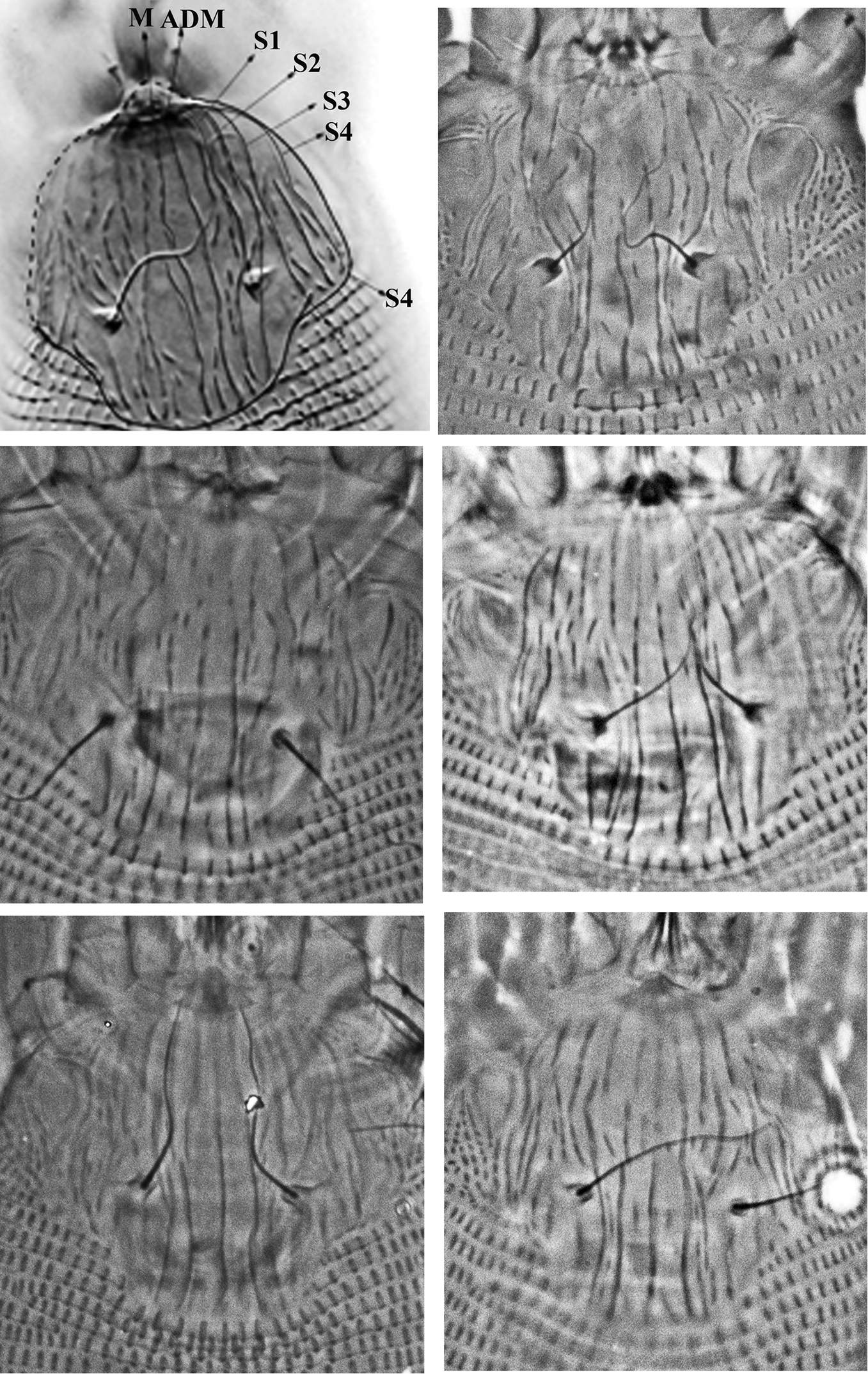
Variation of prodorsal shield sculpture of Colomerus novahebridensis Keifer. The top left figure highlights the prodorsal shield lines: from center to lateral margin, lines running from anterior to posterior margin are interpreted as median (M), admedian (ADM) and submedian lines (S1–S4). Specimens collected in Thailand.
Male (Fig. 2 GM) (n =3): smaller than female, 150–170, 40–48 wide, 44 thick. Gnathosoma: 16–18; pedipalp coxal seta (ep) 2, dorsal pedipalp genual seta (d) 5–6, subapical pedipalp tarsal seta (v) 2, cheliceral stylets 15–17. Prodorsal shield: 30–34, 34–35 wide, prodorsal shield frontal lobe rounded, broad-based, 2–3, shield design similar to that of the female; ocellar gibbosities prominent. Scapular tubercles situated 6–8 ahead of posterior shield margin, plicate, 14–15 apart; scapular setae (sc) 10–12, directed upward or forward. Coxigenital region: with 4 coxigenital semiannuli, microtuberculate. Coxisternal figs (Fig. 2 GM): coxisternum I with several longitudinal lines, coxisternum II smooth, anterior seta on coxisternum I (1b) 5–6, 9–10 apart; proximal seta on coxisternum I (1a) 14–16, 8–9 apart; proximal seta on coxisternum II (2a) 24–26, 18–19 apart, tubercles 1b and 1a 8 apart. Internal coxisternal apodeme 8–11. Legs: with usual setae. Leg I 21–24, femur 7–8, ventral basifemoral seta (bv) 5–8; genu 4–5, antaxial genual seta (l") 15–24; tibia 4–5, paraxial tibial seta (l') 4–5; tarsus 4–5, antaxial fastigial tarsal seta (ft") 14–18, paraxial fastigial tarsal seta (ft') 12–13, paraxial unguinal tarsal seta (u') 2–3, tarsal empodium 5–6, entire, 5-rayed, tarsal solenidion (ω) 6–8, slightly curved, blunt. Leg II 18–20, femur 7, ventral basifemoral seta (bv) 6–7; genu 3, antaxial genual seta (l") 4–5; tibia 3; tarsus 5–6, antaxial fastigial tarsal seta (ft") 16–19, paraxial fastigial tarsal seta (ft') 4–6, paraxial unguinal tarsal seta (u') 2–3, tarsal empodium 5–6, entire, 5-rayed, tarsal solenidion (ω) 10, slightly curved, blunt. Opisthosoma: dorsum evenly rounded, dorsal annuli 59–63 and ventral annuli 63–66. Seta c2 16, 40–47 apart, on annulus 9–10; seta d 30–32, 26–29 apart, on annulus 19–20; seta e 40–45, 16–21 apart, on annulus 34–36; seta f 10, 12–13 apart, on annulus 56–61 or annulus 5th from the rear. Seta h1 absent, h2 28–35. Male genitalia (Fig. 2 GM) 10–14, 18–19 wide, seta 3a 4–6, 10–12 apart.
12 adult females and 5 adult males on 14 slides labeled # 2874, from Mueang Samut Songkhram District, Samut Songkhram Province, 13°24.834'N, 100°0.198'E, 14-II-2011, coll. P. Vichitbandha and G. J. de Moraes; 5 adult females on 5 slides labeled # 2875, from Chumporn Province, 10°15.2'N, 99°5.7'E, 14-II- 2011, coll. P. Vichitbandha and G. J. de Moraes; 3 adult females on 2 slides labeled # 2876, Ban Phaeo District, Samut Sakhon Province, 13°35.433'N, 100°6.466'E, 15-II-2011, coll. P. Vichitbandha and G. J. de Moraes; 7 adult females on 7 slides labeled # 2878 and 5 adult females and 1 adult male on 6 slides labeled # 2879, Kanchanadit District, Surat Thani Province, 9°9.933'N, 99°28.266'E, 15-II-2011, coll. P. Vichitbandha and G. J. de Moraes; 8 adult females on 7 slides labeled # 2883, Kanchanadit District, Surat Thani Province, 9°9.933'N, 99°28.266'E, 23-II-2011, coll. Yingniyom Riyaphan; 3 adult females, 1 adult male and 1 nymph on 5 slides labeled # 2911, Kanchanadit District, Surat Thani Province, 9°9.933'N, 99°28.266'E, 12-IX-2011, coll. Yingniyom Riyaphan; 72 adult females, 6 adult males and 5 nymphs on 23 slides labeled # 2912, Kanchanadit District, SuratThani Province, 9°9.933'N, 99°28.266'E, 28 IX 2011, coll. Yingniyom Riyaphan.
Coconut (Cocos nucifera L. var. nucifera, Ma phrao; Arecaceae)
All specimens were collected from under the bracts of coconut fruit, causing usually the appearance of scanty triangular brown patches of damaged tissue on the fruit surface next to the bracts under which the colonies of the mites developed. In a few occasions damage was slightly more extensive, and the mite apparently caused premature fruit drop.
The morphological characteristics described generally fit the original description of the species, which was much less detailed. Slight differences, subsequently referred to, are considered to represent intraspecific variations. In the original description, admedian lines were mentioned as being complete, which was not the case with the specimens collected in this study. The illustration provided in the original description of the species indicates the presence of a few more submedian lines than observed in the specimens from Thailand. The original description mentioned frontal lobe of prodorsal shield to be truncate. The illustration of prodorsal shield design in the original description shows six partial rings antero-laterally, which is not seen in our specimens; internal coxisternal apodeme is also present in some Thai specimens, but it is not shown in the original description.
Type species: Eriophyes gardeniella Keifer, by original designation.
As stated by
An evaluation of the species assigned to this genus leads to the conclusion that the first of those characteristics (position of genital opening) holds true for all of them. In relation to the second characteristic, the majority of the species placed in this genus has been mentioned to have narrow genital apodemes. However, nothing has been mentioned in the literature about the shape of the genital apodemes of Colomerus oculivitis (
Available illustrations of Colomerus codiaeum Keifer, 1979 and Colomerus trichodesmae Chakrabarti & Pandit, 1997 do not show the typical (narrow) apodemes illustrated by Keifer for the type species of the genus. The inclusion of Colomerus codiaeum in this genus is intriguing, given that it was described by Keifer, just two years after he published the items he considered essential for Colomerus species. Did he make a mistake in accepting that species as Colomerus? Did he then decided that species with different shape of genital apodeme could still be included in that genus, even without explicitly saying so, as could be assumed from his statement in the original description “always shortened in ventral view, but somewhat variable?”. In this publication, we will accept the second option to be the case. This statement by Keifer reflects the assumed variability of the observed shape of these internal structures viewed under phase or interference contrast microscopy. Attempts to determine the real format of these structures could greatly benefit from observations under confocal microscopy, as used by
Nothing has been reported about the shape of the genital apodemes for the following species transferred to or originally described in Colomerus: Colomerus bucidae (Nalepa, 1904), Colomerus lepidaturi (Farkas, 1960), Colomerus pruni Kuang & Luo, 2005 (in
An evaluation of the species referred to Colomerus suggested that it is not convenient to consider the orientation of the scapular seta as characteristic for species to be placed in this genus, given that it may vary when a specimen is slide mounted, although the species referred to this genus in the literature have been rarely mentioned or illustrated as having the scapular seta directed backward [only some Colomerus bucidae, according to
In the original description, Colomerus pruni has been mentioned to have h1 [rarely reported in other Colomerus (see characterization below)]; this species as well as Colomerus robaticus have non-microtuberculate dorsal annuli and genital coverflap without ridges. Thus, they are not considered for the new characterization subsequently proposed for this genus, as they probably belong to a different genus (genera). Conversely, Colomerus trichodesmae, Colomerus bucidae, Colomerus lepidaturi and Colomerus spathodeae are provisionally retained in Colomerus, despite the reportedly non-typical genital apodeme of the first species or the absence of information about the shape of genital apodemes for the others.
A revised characterization of Colomerus could be stated as follows.
Idiosoma: wormlike, with opisthosomal annuli subequal dorsoventrally and microtuberculate; in some species smooth on the few posterior-most opisthosomal annuli (in the original description of Colomerus gardeniella, type species of the genus, microtubercles very faint or absent dorsally on the six posterior-most dorsal annuli); opisthosomal setae h1 absent [except, either reduced or completely absent in Colomerus neopiperis (Wilson, 1970), according to
Prodorsal shield: anterior lobe varying from indistinguishable to distinctly triangular or round and broad-based [absent according to original description of the genus]; scapular tubercles positioned variably from very near posterior shield margin to well anterior to posterior shield margin [slightly anterior to posterior shield margin according to original description of the genus, directing scapular setae diagonally forward or straight ahead (occasionally backward or laterally) [directing setae up and ahead in some degree according to original description of the genus]; gnathosoma short.
Legs: coxae I widely separate, with moderate or short internal coxisternal apodeme (in some species, anterior coxisternal regions totally separated and internal coxisternal apodeme not seen); legs with all usual setae, empodia entire, 4–6 rayed [only species with 5 rayed included in the original description].
Female genitalia: genital opening somewhat appressed to coxisternum II (4 coxigenital semiannuli anterior to genital coverflap); coverflap with longitudinal ridges distinctly arranged in one or two transverse rows, or with some (shorter) ridges in two rows and some (longer) ridges running along most of the length of genital coverflap, constituting a single row [arranged in uneven double rows according to original description of the genus]; genital apodemes usually visible as a narrow dark band in ventral view, but sometimes appearing to constitute a pair of subtriangular structures, depending on the position of the focus.
Eriophyes buceras Trotter, 1929 should not be confused with Eriophyes buceras Cromroy, 1958. As there is no satisfactory description of the first of these species, a confirmation of its generic placement cannot be done. The second species was considered by
In a recent publication,
Eriophyes vitigineusgemmae Mal’chenkova, 1970 may also belong to Colomerus. However it is not included in the subsequent key because, according to the original description, its coverflap does not seem appressed to coxisternum II and because nothing has been mentioned about its genital apodemes.
Colomerus pruni and Colomerus robaticus are also not included in the key because they probably belong to a different genus (genera), as previously discussed in this publication.
| 1 | Without evident ocellar gibbosities; empodia 4-rayed | 2 |
| 1’ | With or without evident ocellar gibbosities; empodia 5- or 6-rayed | 3 |
| 2 | Prodorsal shield without frontal lobe; region between admedian lines with many short lines; on Trichodesma khasianum | Colomerus trichodesmae Chakrabarti & Pandit, 1997 |
| 2’ | Prodorsal shield with frontal lobe; region between admedian lines with few short lines; on Gardenia volkensii subsp. volkensii | Colomerus volkensiae Meyer & Ueckermann, 1990 |
| 3 | With evident ocellar gibbosities; empodia 6-rayed; all opisthosomal annuli microtuberculate | 4 |
| 3’ | With or without ocellar gibbosities; empodia 5-rayed; posterior-most opisthosomal dorsal annuli with or without microtubercles | 6 |
| 4 | Opisthosomal seta e slightly over half as long as opisthosomal seta d and about as long as opisthosomal seta f; on Woodfordia floribunda | Colomerus woodfordis Ghosh & Chakrabarti, 1989 |
| 4’ | Opisthosomal seta e at least 1.2 times as long as opisthosomal seta d and at least 3.5 times as long as opisthosomal seta f | 5 |
| 5 | Scapular seta sc 21 µm; opisthosomal seta d 36 µm; opisthosoma with 70 annuli; microtubercles very narrow (linear); on Vitis vinifera | Colomerus oculivitis (Attiah, 1967) |
| 5’ | Scapular seta sc 10 µm; opisthosomal seta d 25 µm; opisthosoma with 55–62 annuli; microtubercles ovoid to rounded; on Piper jaliscanum | Colomerus neopiperis (Wilson, 1970) |
| 6 | Prodorsal shield smooth, except for few curved broken bases of admedian lines restricted to region between scapular tubercles and a tiny remnant of median line; without evident ocellar gibbosities; most posterior dorsal opisthosomal annuli without microtubercles; on Baloghia inophylla (G.Forst.) P.S. Green (mentioned as Codiaeum inophyllum, junior synonym) | Colomerus codiaeum Keifer, 1979 |
| 6’ | Prodorsal shield with more extensive lines; with or without evident ocellar gibbosities; most posterior dorsal opisthosomal annuli with or without microtubercles; on other hosts | 7 |
| 7 | Median line on prodorsal shield only distinguishable posteriorly, joined by broken arched lines to admedian lines, so as to form a pair of roundish cells at the base of the admedian lines; genital coverflap with longitudinal ridges arranged in two distinct transverse rows, those of the anterior row much shorter, fine and less evident than those of the posterior row; on Gardenia jasminoides | Colomerus gardeniella (Keifer, 1964) |
| 7’ | Median line on prodorsal shield not joined by broken arched lines to admedian lines; longitudinal ridges of genital coverflap not characteristically arranged in two transverse rows or, if so, then anterior row not composed of distinctly shorter, fine and less evident ridges than those of the posterior row | 8 |
| 8 | Prodorsal shield with frontal lobe (sometimes barely distinguishable) | 9 |
| 8’ | Prodorsal shield without frontal lobe | 19 |
| 9 | Prodorsal shield with lateral granulation; without evident ocellar gibbosities | 10 |
| 9’ | Prodorsal shield without lateral granulation; with or without evident ocellar gibbosities | 11 |
| 10 | Opisthosomal setae d and e 30 and 8 µm, respectively; opisthosoma with 48 microtuberculate annuli; on Holodiscus microphyllus | Colomerus holodisci (Keifer, 1970) |
| 10’ | Opisthosomal setae d and e 18–25 and 18–30 µm, respectively; opisthosoma with 55–70 annuli; microtubercles missing on posterior 6–7 dorsal annuli; on Phebalium nudum | Colomerus nudi Manson, 1984 |
| 11 | Opisthosoma with 60–85 annuli | 12 |
| 11’ | Opisthosoma with less than 60 annuli (except Colomerus coplus, with 53–63) | 14 |
| 12 | With evident ocellar gibbosities; with 67–85 microtuberculate annuli; on Cocos nucifera | Colomerus novahebridensis Keifer, 1977 |
| 12’ | Without evident ocellar gibbosities; with 61–75 annuli, all microtuberculate or posterior ten dorsal annuli with few microtubercles | 13 |
| 13 | Opisthosoma with 61–68 annuli; posterior 10 dorsal annuli with few microtubercles; on Tricalysia junodii var. junodii and Sericanthe andongensis | Colomerus tricaseri Meyer & Ueckermann, 1990 |
| 13’ | Opisthosoma with 75 microtuberculate annuli; on Diospyros mespiliformis | Colomerus mespiliformae Meyer & Ueckermann, 1990 |
| 14 | Admedian lines on prodorsal shield well defined and complete; ocellar gibbosities absent; all opisthosomal annuli microtuberculate | 15 |
| 14’ | Admedian lines on prodorsal shield generally not well defined (or broken), may be distinct on posterior half of prodorsal shield; microtubercles may be absent on posterior opisthosomal dorsal annuli | 16 |
| 15 | Median line totally distinct; opisthosoma with 53–63 microtuberculate annuli; opisthosomal setae d and e 19–24 and 14–26 µm, respectively; on Melicope simplex A. Cunn. | Colomerus coplus Manson, 1984 |
| 15’ | Median line distally indistinct; opisthosoma with 48–50 microtuberculate annuli; microtubercles fading dorsally on posterior 10 annuli; opisthosomal setae d and e 36 and 40 µm, respectively; on Vitex wilmsii | Colomerus vitexi Meyer & Ueckermann, 1990 |
| 16 | Without evident ocellar gibbosities; opisthosoma with 50–57 microtuberculate annuli; microtubercles rectangular dorsally, fading on posterior 10 annuli; on Antidesma venosum | Colomerus antidesmae Meyer & Ueckermann, 1990 |
| 16’ | With or without evident ocellar gibbosities; opisthosoma with 50–59 microtuberculate annuli; microtubercles oval dorsally, may be missing on posterior-most annuli; on other hosts | 17 |
| 17 | Frontal lobe of prodorsal shield much broader than long; with ocellar gibbosities (sometimes not well distinct); opisthosoma with 54–59 microtuberculate annuli; microtubercles fading dorsally on posterior 15 annuli; on Tinnea barbata | Colomerus tinneae Meyer & Ueckermann, 1990 |
| 17’ | Frontal lobe of prodorsal shield about as broad as long or slightly broader than long; with or without evident ocellar gibbosities; opisthosoma with 50–55 microtuberculate annuli; posterior-most opisthosomal dorsal annuli with or without microtubercles; on other hosts | 18 |
| 18 | Region between admedian lines on prodorsal shield with many short lines; with prominent ocellar gibbosities; opisthosoma with 55 microtuberculate annuli; on Alangium saviifolium | Colomerus alangii Keifer, 1978 |
| 18’ | Region between admedian lines on prodorsal shield only with median line; without prominent ocellar gibbosities; opisthosoma with 50–55 microtuberculate annuli; posterior dorsal 15 annuli without microtubercles; on Ziziphus mucronata | Colomerus mansus Meyer & Ueckermann, 1990 |
| 19 | Opisthosoma with 70–94 annuli; with evident ocellar gibbosities | 20 |
| 19’ | Opisthosoma with at most 66 annuli; with or without evident ocellar gibbosities | 21 |
| 20 | Opisthosomal setae d and e 40–46 and 38–60 µm, respectively; opisthosoma with 76–89 microtuberculate annuli; posterior 6 dorsal annuli sparsely microtuberculate (all microtuberculate according to |
Colomerus vitis (Pagenstecher, 1857) |
| 20’ | Opisthosomal setae d and e 31 and 27 µm, respectively; opisthosoma with 75–94 microtubertulate annuli; on Ribes nigrum | Colomerus riberini Shi & Boczek, 2002 |
| 21 | Ocellar gibbosities absent; genital coverflap with longitudinal ridges arranged in a single row | Colomerus lepidaturi (Farkas, 1960) |
| 21’ | Ocellar gibbosities well evident, ill-defined or absent; genital coverflap with longitudinal ridges arranged in two transverse rows | 22 |
| 22 | With evident ocellar gibbosities; opisthosoma with about 62 microtuberculate annuli; microtubercles broadly oval; on Spathodea campanulata | Colomerus spathodeae (Carmona, 1967) |
| 22’ | With ill defined ocellar gibbosities; opisthosoma with 49–61 microtuberculate annuli, of which the 8–10 posterior-most without microtubercles; microtubercles elongate dorsally and ventrally, shorter and more rounded laterally; on Terminalia (syn. Buchenavia, Bucida) buceras | Colomerus bucidae (Nalepa, 1904) |
Ectomerus Newkirk & Keifer is the genus that most closely resembles Colomerus morphologically. It was described as a monotypic genus by
Palmiphytoptus Navia & Flechtmann is also similar to this genus. It was described (
The kinds of injury caused by Colomerus species are very diverse, with some species causing more than one type of damage. The main types of damage are mentioned as: disturbance to development of new leaves, by damaging buds (Colomerus oculivitis, Colomerus vitis, Colomerus woodfordis), fruit deformation (Colomerus bucidae), formation of leaf erinea (Colomerus alangii, Colomerus bucidae, Colomerus coplus, Colomerus holodisci, Colomerus mespiliformae, Colomerus nudi, Colomerus riberini, Colomerus spathodeae, Colomerus tricaseri, Colomerus vitexi, Colomerus vitis, Colomerus volkensiae), leaf outgrowth (Colomerus tricaseri), “witch’s broom”, by damaging inflorescences (Colomerus antidesmae), formation of leaf galls (Colomerus bucidae, Colomerus lepidaturi, Colomerus neopiperis, Colomerus tinneae, Colomerus trichodesmae), leaf distortion (Colomerus mansus, Colomerus spathodeae, Colomerus trichodesmae, Colomerus vitis) and fruit necrosis (Colomerus novahebridensis). The following species were not associated with any type of damage on plants from which type specimens were collected: Colomerus codiaeum and Colomerus gardeniella.
While several of these species are known to attack ornamental plants, only 3 species have been reported from major crops: Colomerus oculivitis and Colomerus vitis from grapevine and Colomerus novahebridensis from coconut. Colomerus oculivitis and Colomerus vitis have been mentioned to cause economic damage to their host, especially Colomerus vitis, which has a wide distribution (
We thank Prof. James W. Amrine Jr. and Prof. Philipp Chetverikov for their major collaboration reviewing a former version of this manuscript, for providing invaluable suggestions in the interpretation of the concepts expressed in this paper, and for providing several photographs of specimens from Santo, Vanuatu for comparison with the specimens collected in Thailand. To Dr. Patchanee Vichitbandha, Science Division, Faculty of Liberal Arts and Science, Kasetsart University, Kamphaeng Saen Campus, Thailand and Mrs. Yingniyom Riyaphan of Surat Thani Oil Palm Research Center, Department of Agriculture, Surat Thani Province, Thailand for collecting samples. To Leocadia Sánchez-Ramirez for making specimens of Colomerus buceras collected in the Dominican Republic available for examination in this study.