Citation: Terraneo TI, Berumen ML, Arrigoni R, Waheed Z, Bouwmeester J, Caragnano A, Stefani F, Benzoni F (2014) Pachyseris inattesa sp. n. (Cnidaria, Anthozoa, Scleractinia): a new reef coral species from the Red Sea and its phylogenetic relationships. ZooKeys 433: 1–30. doi: 10.3897/zookeys.433.8036
A new scleractinian coral species, Pachyseris inattesa sp. n., is described from the Red Sea. Despite a superficial resemblance with some species in the agariciid genus Leptoseris with which it has been previously confused, P. inattesa sp. n. has micro-morphological characters typical of the genus Pachyseris. This genus, once part of the Agariciidae, is comprised of five extant species and is widely distributed throughout the tropical Indo-Pacific. It is currently incertae sedis as a result of recent molecular analysis and appears to be closely related to the Euphylliidae. A molecular phylogenetic reconstruction including P. inattesa sp. n., the genus type species P. rugosa, and P. speciosa, all present in the Red Sea, was performed using the mitochondrial intergenic spacer between COI and 16S-rRNA. The results confirm that P. inattesa sp. n. is a monophyletic lineage closely related to the other Pachyseris species examined.
Pachyseris rugosa, Pachyseris speciosa, Leptoseris foliosa, micro-morphology, COI-16S-rRNA intergenic spacer, taxonomy
The zooxanthellate and reef-dwelling hard coral genus Pachyseris Milne-Edwards & Haime, 1849 is widely distributed throughout the Indo-Pacific, from the Red Sea to the Marshall Islands, Samoa, and Tahiti (
Throughout the last two centuries, more than ten nominal species of Pachyseris have been described, probably overestimating the true number of the actual species due to the high intraspecific variability of the species (
Although the Red Sea is known to be an important region of biodiversity and endemism (
Several colonies of Pachyseris were collected at various localities in the Red Sea and in the Indo-Pacific. Digital images of living corals in situ were taken with a Canon G9 in an Ikelite underwater housing or a Nikon Coolpix 7900 in a Nikon WP-CP4 waterproof case. From each coral specimen collected, a 2 cm2 fragment was preserved in either 95% ethanol or CHAOS solution (
Macro and micro-morphological characters of Pachyseris samples were examined using both light microscopy (Zeiss Stemi DV4 stereo-microscope) and scanning electron microscopy (SEM). For SEM, Pachyseris speciosa, Pachyseris rugosa, and Leptoseris foliosa fragments were ground, mounted on stubs using silver glue, sputter-coated with conductive gold film, and examined using Vega Tescan Scanning Electron Microscopy at the SEM Laboratory, University of Milano-Bicocca. Fragments of Pachyseris inattesa sp. n. (specimens KAUST SA492 and KAUST SA1305) were sputter-coated with Au-Pd and imaged using a Quanta 200 FEG SEM at the King Abdullah University of Science and Technology.
Twenty Pachyseris and two Leptoseris foliosa specimens were included in the molecular analyses (Table 1). Total DNA was extracted using DNAeasy® Tissue kit (Qiagen Inc., Valencia, CA, USA) for samples stored in ethanol according to the manufacturer’s protocol and a phenol-chloroform based method for samples in CHAOS (
List of samples used in this study. For each specimen the registration code, identification, collection locality, collector, and EMBL accession number are provided.
| Code | Identification | Locality | Collector | IGR between COI and 16S-rRNA |
|---|---|---|---|---|
| BMRI 62 | Pachyseris rugosa | Sabah, Malaysia | Waheed Z. | LK934487 |
| HS2856 | Pachyseris rugosa | New Caledonia | Benzoni F. | LK934488 |
| HS2594 | Pachyseris rugosa | New Caledonia | Benzoni F. | LK934489 |
| SO 040 | Pachyseris speciosa | Socotra Island, Yemen | Benzoni F. | LK934490 |
| SO 020 | Pachyseris speciosa | Socotra Island, Yemen | Benzoni F. | LK934491 |
| MA 439 | Pachyseris speciosa | Maldives | Benzoni F. | LK934492 |
| MA 476 | Pachyseris speciosa | Maldives | Benzoni F. | LK934493 |
| MA 477 | Pachyseris speciosa | Maldives | Benzoni F. | LK934494 |
| SA 376 | Pachyseris speciosa | Red Sea, Saudi Arabia | Benzoni F. | LK934495 |
| BMRI 06 | Pachyseris speciosa | Sabah, Malaysia | Waheed Z. | LK934496 |
| BMRI 66 | Pachyseris speciosa | Sabah, Malaysia | Waheed Z. | LK934497 |
| BMRI 68 | Pachyseris speciosa | Sabah, Malaysia | Waheed Z. | LK934498 |
| RMNH Coel. 41613 | Pachyseris inattesa sp. n. | Red Sea, Saudi Arabia | Benzoni F. | LK934499 |
| SA 004 | Pachyseris inattesa sp. n. | Red Sea, Saudi Arabia | Benzoni F. | LK934500 |
| SA 1284 | Pachyseris inattesa sp. n. | Red Sea, Saudi Arabia | Benzoni F. | LK934501 |
| SA 1301 | Pachyseris inattesa sp. n. | Red Sea, Saudi Arabia | Benzoni F. | LK934502 |
| SA 1305 | Pachyseris inattesa sp. n. | Red Sea, Saudi Arabia | Benzoni F. | LK934503 |
| SA 887 | Pachyseris inattesa sp. n. | Red Sea, Saudi Arabia | Benzoni F. | LK934504 |
| SA 1300 | Pachyseris inattesa sp. n. | Red Sea, Saudi Arabia | Benzoni F. | LK934505 |
| SA 1293 | Pachyseris inattesa sp. n. | Red Sea, Saudi Arabia | Benzoni F. | LK934506 |
| HS2854 | Leptoseris foliosa | New Caledonia | Benzoni F. | LK934507 |
| HS2873 | Leptoseris foliosa | New Caledonia | Benzoni F. | LK934508 |
Phylogenetic relationships between species were inferred using our sequences and 15 sequences of Agariciidae downloaded from GenBank based on
BIBELOT IRD Biodiversité Benthique dans les iles Loyauté Expedition, Loyalty Islands, New Caledonia, 2014
BMRI Borneo Marine Research Institute, Universiti Malaysia Sabah, Malaysia
IRD Institut de Recherche pour le Développement, Nouméa, New Caledonia
KAUST King Abdullah University of Science and Technology, Thuwal, Saudi Arabia
KBEA KAUST Biodiversity Expedition to the Gulf of Aqaba, 2013
KBEF KAUST Biodiversity Expedition to the Farasan Banks and Farasan Islands, 2013
NIUGINI Niugini Biodiversity Expedition, Papua New Guinea, 2012
RMNH Coel. Rijksmuseum van Natuurlijke Historie, Coelenterate collection, Naturalis Biodiversity Center, Leiden, The Netherlands
SMEE Semporna Marine Ecological Expedition, 2010
TOE Tara Oceans Expedition, 2009-2012
UNIMIB University of Milano-Bicocca, Milan, Italy
USNM United States National Museum of Natural History, Washington DC, USA
(by monotypy). Agaricia rugosa Lamarck, 1801.
BMRI 62, Semporna, Malaysia (MV Celebes, Explorer, SMEE), 04°34'01.8"N, 118°45'27.5"E, 11 December 2010, coll. Z. Waheed; IRD HS2856, Prony Bay, New Caledonia, 22°21.230'S, 166°49.300'E, 10 m, 23 February 2011, coll. F. Benzoni; IRD HS2893, Prony Bay, New Caledonia, 22°21.230'S, 166°49.300'E, 10 m, 22 February 2011, coll. F. Benzoni; IRD HS3383, Maré, Loyalty Islands, New Caledonia (MV Alis, BIBELOT), 16 February 2014, coll. F. Benzoni.
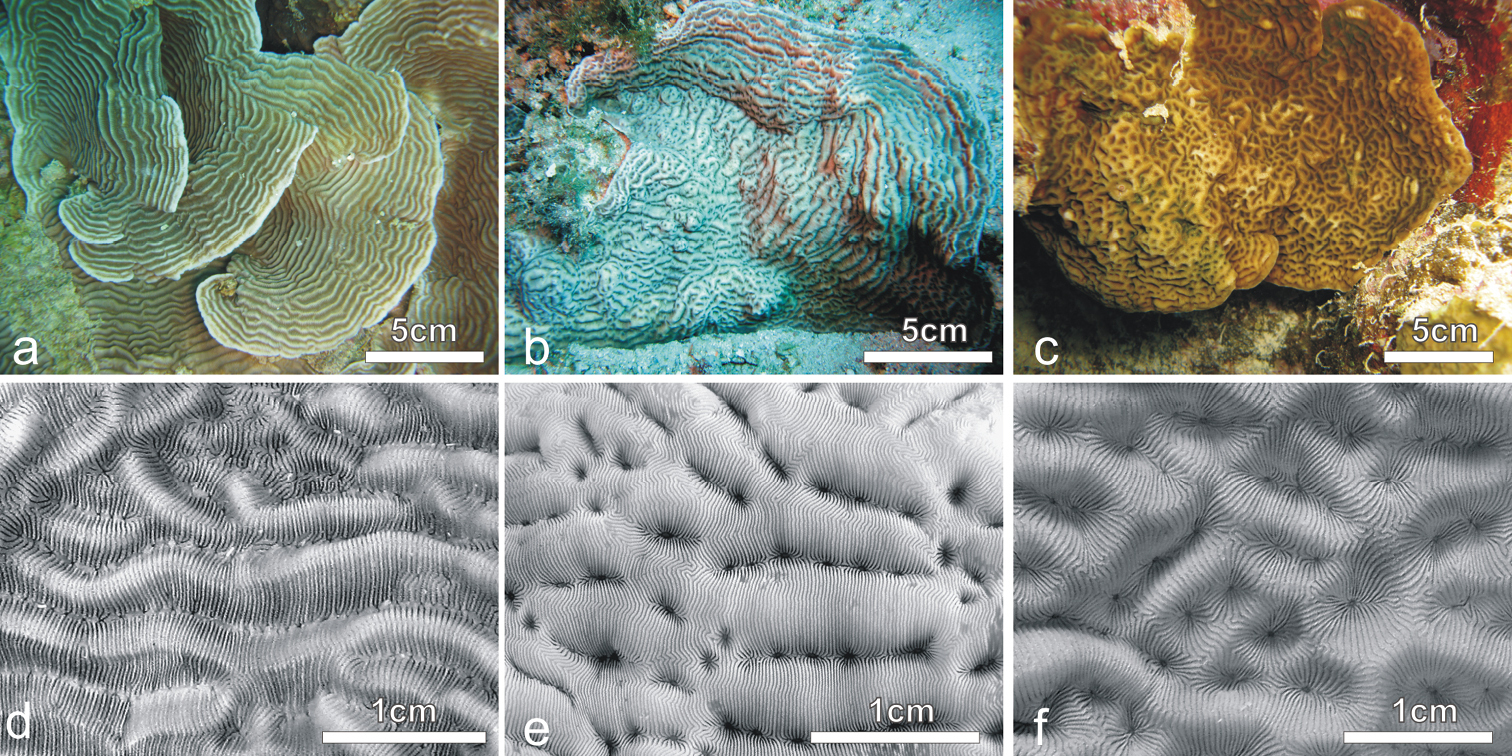
Corallum: Highly variable in shape from encrusting with foliose margins and central knobs (
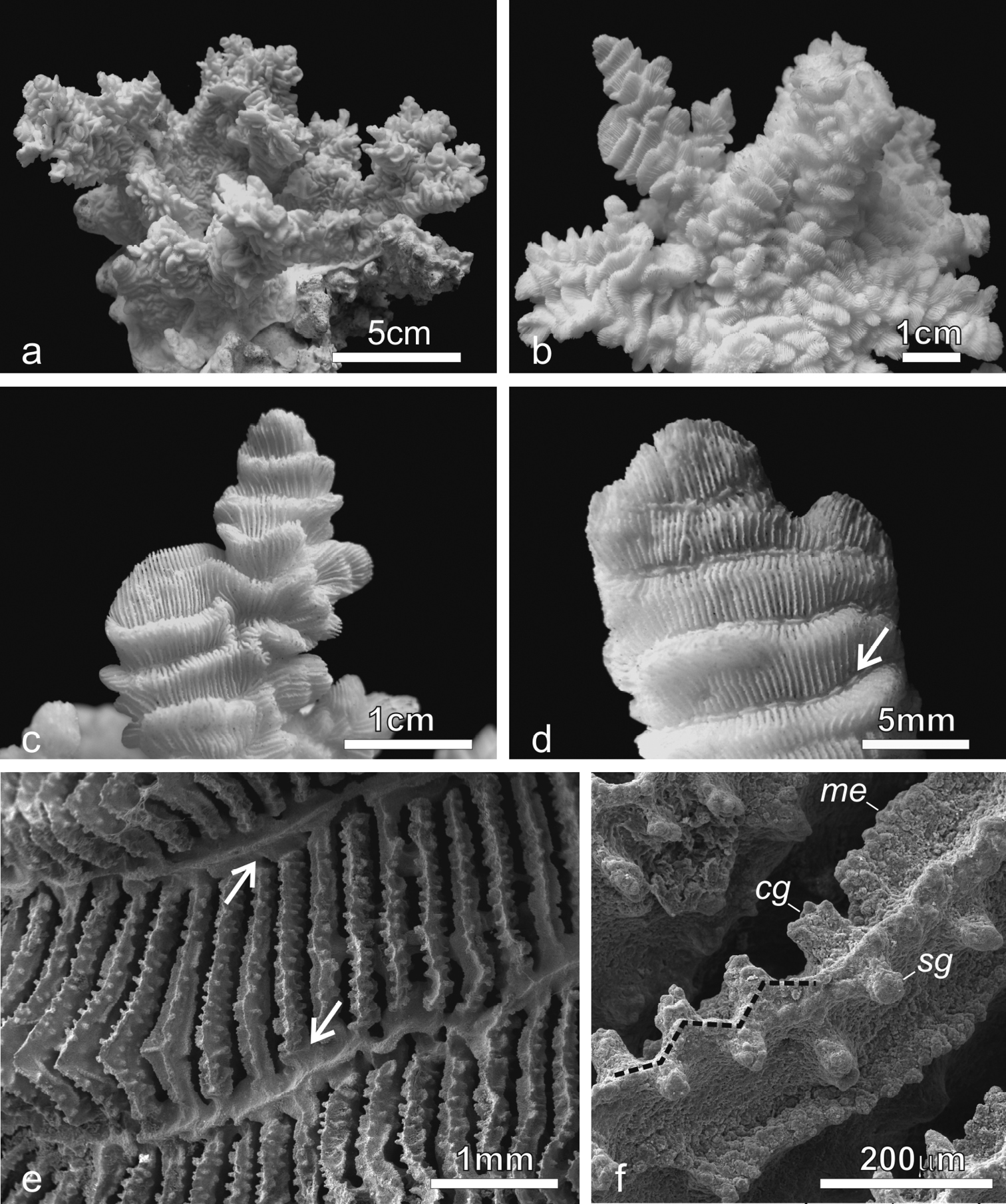
Colonies of Pachyseris rugosa (a–c) and Pachyseris speciosa (d–g) in situ. a Image of the whole colony of specimen IRD HS2893, Prony Bay, New Caledonia b Lateral view of the fronds of specimen IRD HS2594, Prony Bay, New Caledonia c Fronds of specimen IRD HS2856 viewed from above, Prony Bay, New Caledonia d Tiers of foliose projections of a colony from New Caledonia e Image of specimen UNIMIB SO040, Socotra Island f Part of specimen KAUST SA714, Saudi Arabia g Detail of a colony with reduced carinae and brightly colored polyp mouths, growing in very turbid environment, Banc des Japonais, New Caledonia.
Pachyseris rugosa. a Lateral view of colony IRD HS1442 b Lateral view of colony IRD HS152 showing very irregular fronds and carinae c Detail of the fronds of specimen IRD HS152 d Specimen IRD HS221, white arrow points at a dash-like columella e SEM image of IRD HS2594, white arrows point at the fused dissepiments connecting the inner end of the radial elements and the reduced columellae f SEM image of ornamentation on radial elements showing single granules (sg), clumped granules (cg) and menianae (me).
Calices: Arranged in rows, mostly indistinct. Rows can be long and continuous, or short and irregular, especially on the fronds (Figures 2a–d). Series of calices are generally arranged parallel to each other and are concentric in the encrusting or foliose parts of the corallum. Series are separated by carinae with variable vertical development and inclination with respect to the corallum surface (Figures 2b–c). At the base of the fronds, the carinae can be very short and resemble hydnophoroid protuberances (Figure 1c).
Columella: Well-developed, made by a dash-like process rising from a horizontal fig made of dissepiments from the inner ends of the radial elements (Figure 2d;
Radial elements: Radial elements are continuous across the carinae, regularly spaced and equal or slightly alternating (Figures 2c–e). Lateral faces bear regularly distributed, parallel lines of granules or/and ledge-like features called menianae (
Holotype: USNM 119 (Figures 2a, c). Type Locality: East Indies (U.S. Exploring Expedition).
KAUST SA376, Shi'b Nazar, Saudi Arabia, 22°19'60.00"N, 38°51'15.78"E, 16 March 2013, coll. F. Benzoni; KAUST SA714, Ras Al-Ubayd, Saudi Arabia (MV Dream-Master, KBEA), 26°44.167'N, 36°02.659'E, 26 September 2013, coll. F. Benzoni; UNIMIB TO-DJ240, Obock, Djibouti (MV Tara, TOE), 11°57.517'N, 43°18.787'E, 3 February 2010, coll. F. Benzoni; UNIMIB TO-DJ341, Arta region, Djibouti (MV Tara, TOE), 11°35.365'N, 42°52.560'E, 10 February 2010, coll. F. Benzoni; UNIMIB SO020, Roosh, Socotra Island, 12°37.237'N, 54°21.090'E, 12 March 2010, coll. F. Benzoni; UNIMIB SO040, Ras Adho, Socotra Island, 12°38.638'N, 54°16.147'E, 13 March 2010, coll. F. Benzoni; BMRI 66, Semporna, Malaysia (MV Celebes Explorer, SMEE), 04°36'10.0"N, 118°45'53.6"E, 11 December 2010, coll. Z. Waheed; BMRI 68, Semporna, Malaysia, (MV Celebes Explorer, SMEE), 04°37'32.2"N, 118°40'58.0"E, 12 December 2010, coll. Z. Waheed; UNIMIB PFB342, Madang, Papua New Guinea (MV Alis, NIUGINI), 05°05.854'S, 145°48.525'E, 20 November 2012, coll. F. Benzoni; IRD HS2594, Ilot N'do, Nouméa, New Caledonia, 15 May 2009, coll. F. Benzoni.
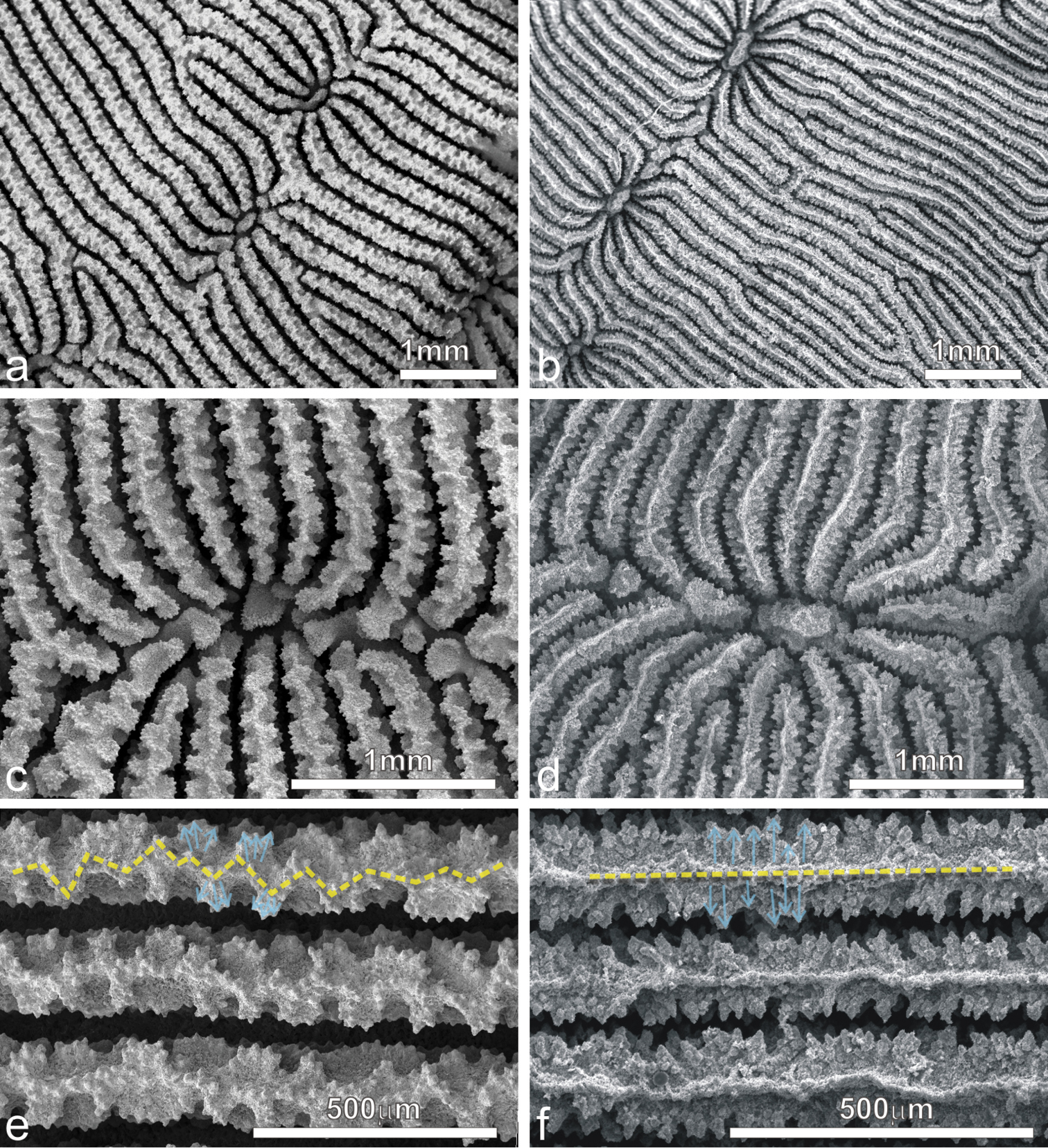
Corallum: Unifacial and encrusting with foliose margins (Figure 1e) to laminar, sometimes forming tiers of laminae (Figure 1d). The corallum surface is corrugated due to the presence of mostly concentric continuous carinae (Figures 1d–g, 3a–d).
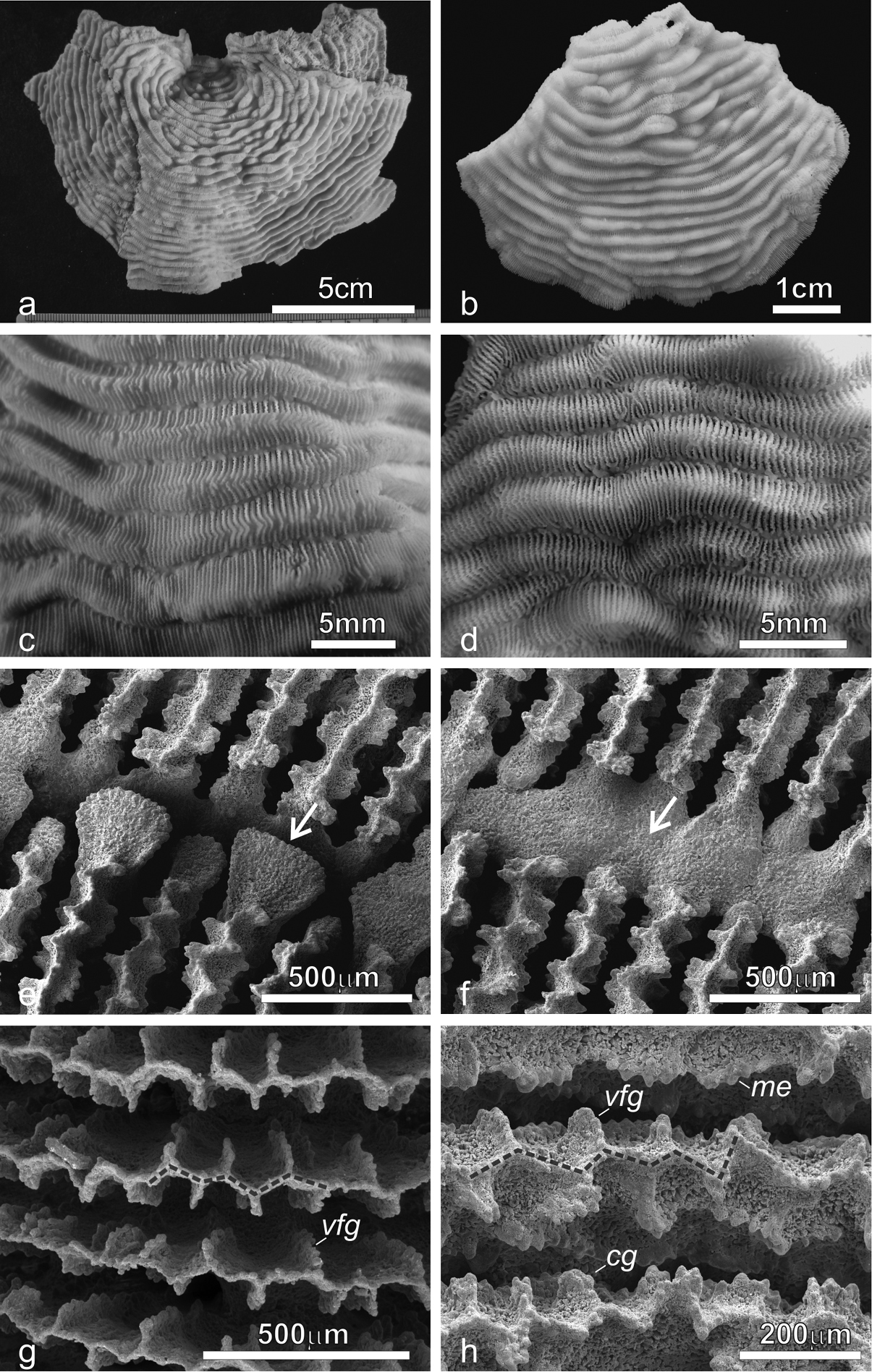
Pachyseris speciosa. a The holotype USNM 119, West Indies b Specimen UNIMIB SO020, Socotra Island c Detail of the holotype showing parallel carinae and alternating radial elements d Detail of the specimen in b e SEM image of IRD HS2673, white arrows point at one of the spatula-shaped processes extending from the inner end of the radial elements and forming the columella f SEM image of the same specimen (Figure 3e) in a portion where columella processes are fused to form a continuous structure (white arrow) g SEM image of IRD HS2263 showing the ornamentation of the radial elements consisting of vertically fused granules (vfg) seen from above and the zigzag pattern of the radial element margin (dashed line) h SEM image of IRD HS2673 showing the radial element zigzag pattern (dashed line) and face ornamentation consisting of clumped granules (cg), vertically fused granules (vfg), and menianae (me).
Calices: Arranged in rows, mostly indistinct although sometimes polyp mouths can have a distinct coloration in vivo (Figure 1g) allowing recognition of the position of the single calice underneath. Rows generally long and continuous (Figures 1d–g, 3a–d). Series of calices are arranged parallel to each other, concentric and separated by carinae with variable vertical development and inclination with respect to the corallum surface (Figures 1d–g, 3a–d). When asymmetrical, carinae are inclined towards the margin of the corallum.
Columella: Well-developed, low-lying in the valleys between carinae formed by the fusion of spatula-shaped processes extending from the inner end of the radial elements (Figure 3e). Radial elements of the higher order form larger processes alternating with the smaller ones from the elements of lower order (Figure 3e). In the same series of calices the processes forming the columella can be separate (Figure 3e) or completely fused (Figure 3f).
Radial elements: Radial elements are continuous across the carinae, regularly spaced and equal or slightly alternating (Figure 3c–d). Lateral faces bear regularly distributed, parallel lines of granules or/and menianae. Such lateral ornamentation is variable and includes groups of clumped granules (Figure 2h), menianae with minutely beaded edges and vertically fused granules forming structures similar to menianae but oriented perpendicularly rather than parallel to the radial element margin (Figure 3g–h). The upper margin of the radial elements is minutely beaded and typically attains a zigzag pattern with lateral ornamentations at the angles (Figure 3g–h). This pattern can be so pronounced in some specimens as to give the radial elements a “wavy or even crenellated” appearance to the naked eye (
http://zoobank.org/4C6008D7-FF14-47CA-B65D-7E65F88C477D
Holotype: RMNH Coel. 41613 (Figures 4d, 6b–d). Type Locality: Al Lith, Saudi Arabia (MV Dream-Master, KAUST Biodiversity Cruise to the Farasan Banks and Farasan Islands), 20°07.690'N, 40°12.513'E, 3 March 2013, coll. F. Benzoni.
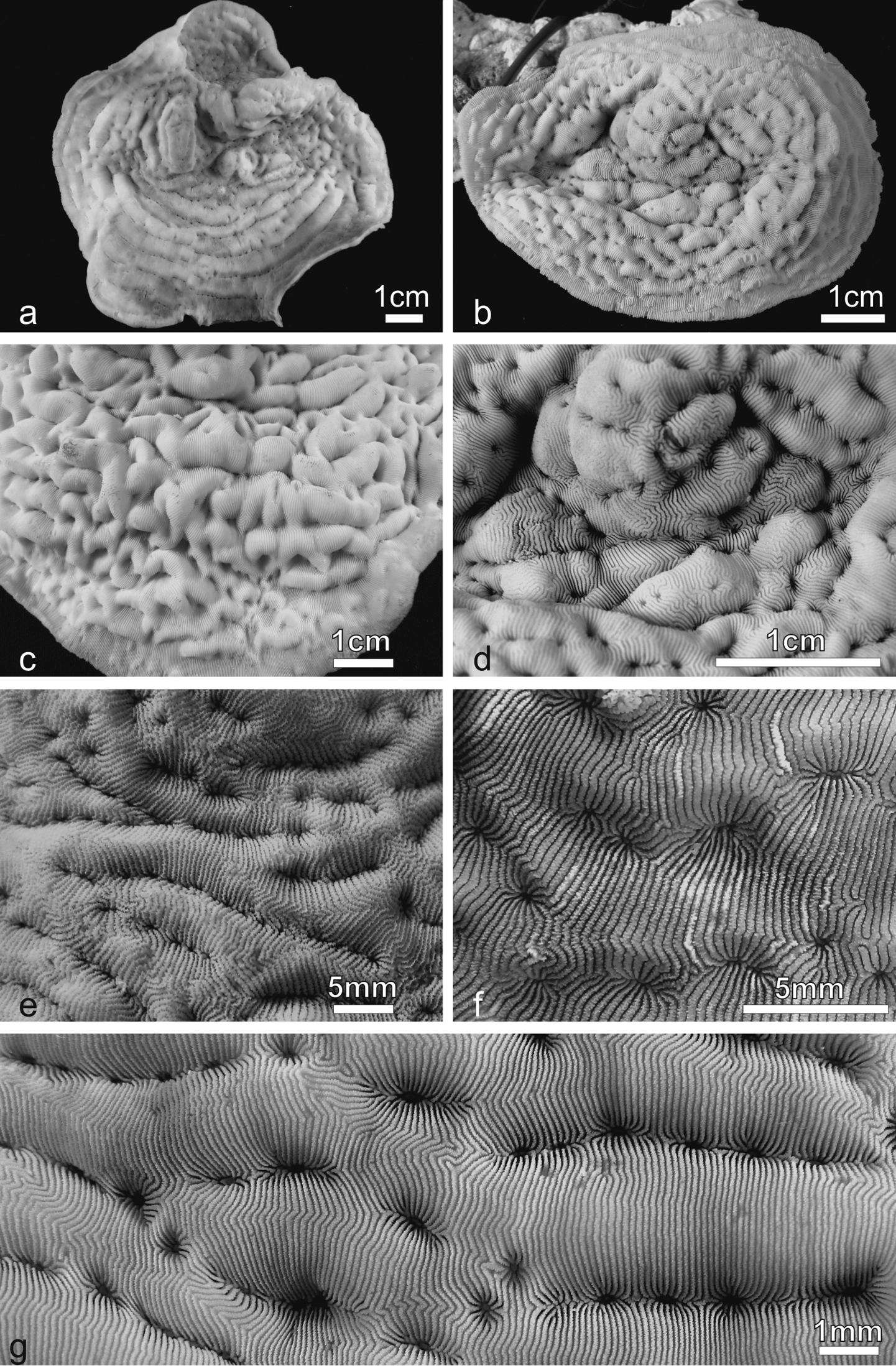
Pachyseris inattesa sp. n. in situ. a KAUST SA1300 b KAUST SA426 c KAUST SA887 d holotype RMNH Coel. 41613 e KAUST SA1284 f KAUST SA004 g KAUST SA1301 h KAUST SA1305.
Development of carinae and series of calices in Pachyseris inattesa sp. n. in situ. a Well-developed carinae and elongated series of calices in specimen KAUST SA429 b Well-developed carinae and short series of calices in specimen KAUST SA887 c Poorly developed carinae and short series of calices in specimen KAUST SA1284 d Poorly developed carinae and long series of calices in specimen KAUST SA1293.
Corallum: The specimen is 1.5 cm high from the base in its original growth position, and 7.5 × 5.2 cm wide. The holotype is oval-shaped, attached at the centre with free margins and sunken in the central part (Figures 4d, 6b). At the opposite ends of its largest diameter the corallum margins bend in different directions with respect to the plane of the central encrusting part (upwards at the left-hand side and downwards at the right-hand side of Figure 6b). The corallum surface is irregularly undulated due to the presence of well-developed carinae, which are symmetrical and thick in the central part (Figure 6d) and become increasingly shorter, lower, and more inclined towards the margins (Figure 6c).
Pachyseris inattesa sp. n. a KAUST SA426 b Holotype RMNH Coel. 41613 c View of the marginal part of the holotype in b d View of the central part of the holotype in b e KAUST SA678 f KAUST SA1301 g KAUST SA429.
Calices: Arranged in short rows, mostly distinct (Figure 6d) especially towards the margins where the series become shorter (Figure 6c). Calices and series of calices are arranged parallel to each other, concentric and separated by wide and rounded carinae with variable vertical development and inclination with respect to the corallum surface (Figures 4d, 6c). Where carinae separate, single calices or short series become distinct. In these cases, short carinae can resemble proximal cushions, the typical features forming in the agariciid genus Leptoseris when the inner or proximal side of an inclined corallite is raised into a cushion-like structure (
Columella: Well-developed, sitting deep in the fossa (Figure 6d) made by one or more processes derived from the inner end of the radial elements.
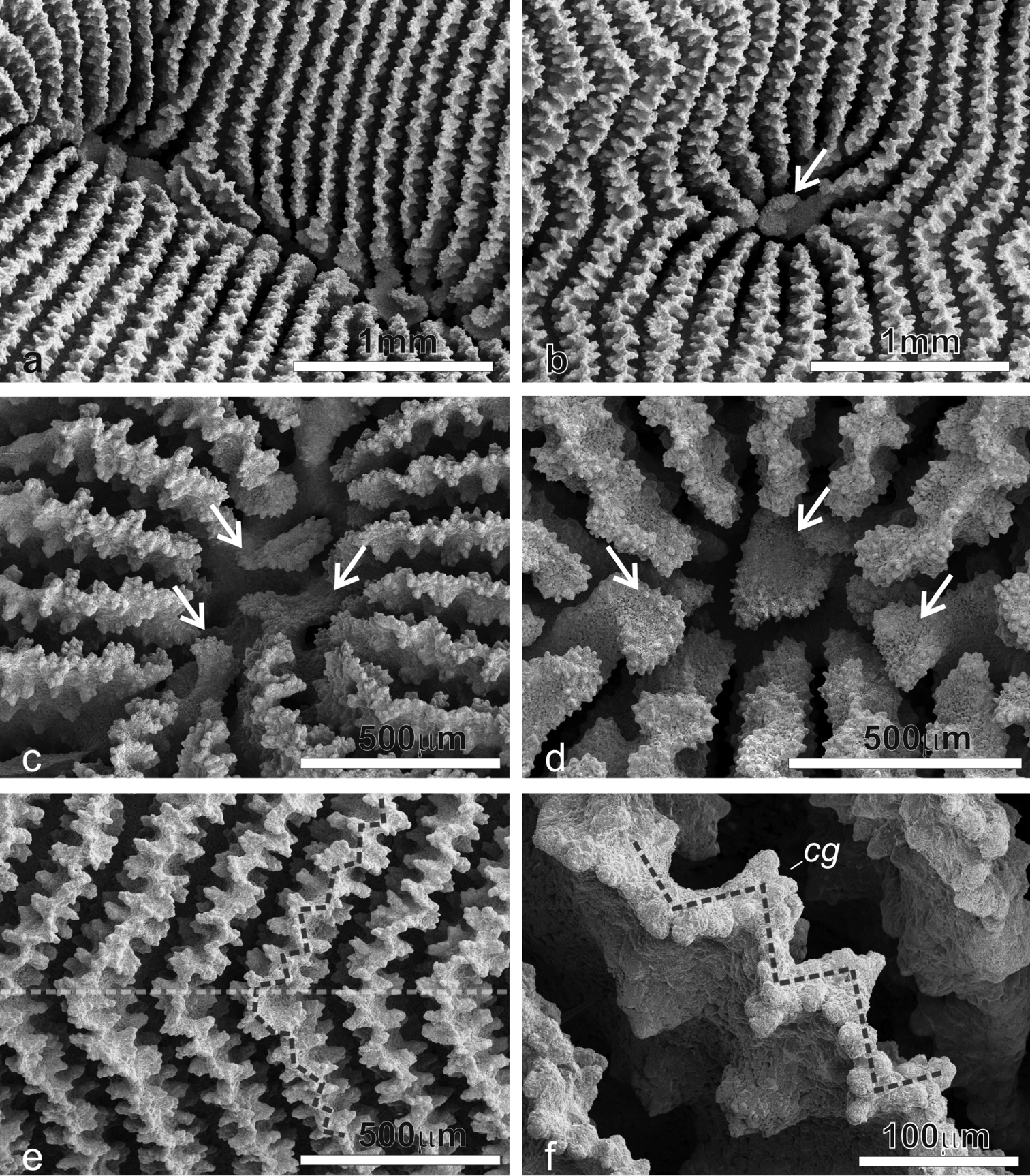
Radial elements: Radial elements are continuous across the carinae, regularly spaced and equal. Lateral faces bear regularly distributed, parallel lines of clumped granules (Figure 7e). The upper margin of the radial elements is minutely beaded and typically attains a zigzag pattern with ornamentations at the angles (Figure 7f).
SEM images of Pachyseris inattesa sp. n. showing micro-morphological details. a Adjacent calices in series in specimen KAUST SA492 b Single calice in the same specimen as in a, the white arrow points at the single columellar process, which extends from the inner end of the radial elements reaching the fossa c Another calice in the same specimen (Figure 7a) with multiple columella processes (white arrows) extending from the inner end of the radial elements d A calice of specimen KAUST SA1305, the white arrows point at the finely ornamented columellar processes extending into the fossa from the inner end of the radial elements e Parallel and equal radial elements across a carina (top of the carina indicated by the dashed transparent white line) presenting the typical zigzag pattern of the margin (dashed grey line) f Detail of radial elements as in Figure 7e, showing the upper margin zigzag pattern (dashed grey line) and face ornamentation consisting of clumped granules (cg).
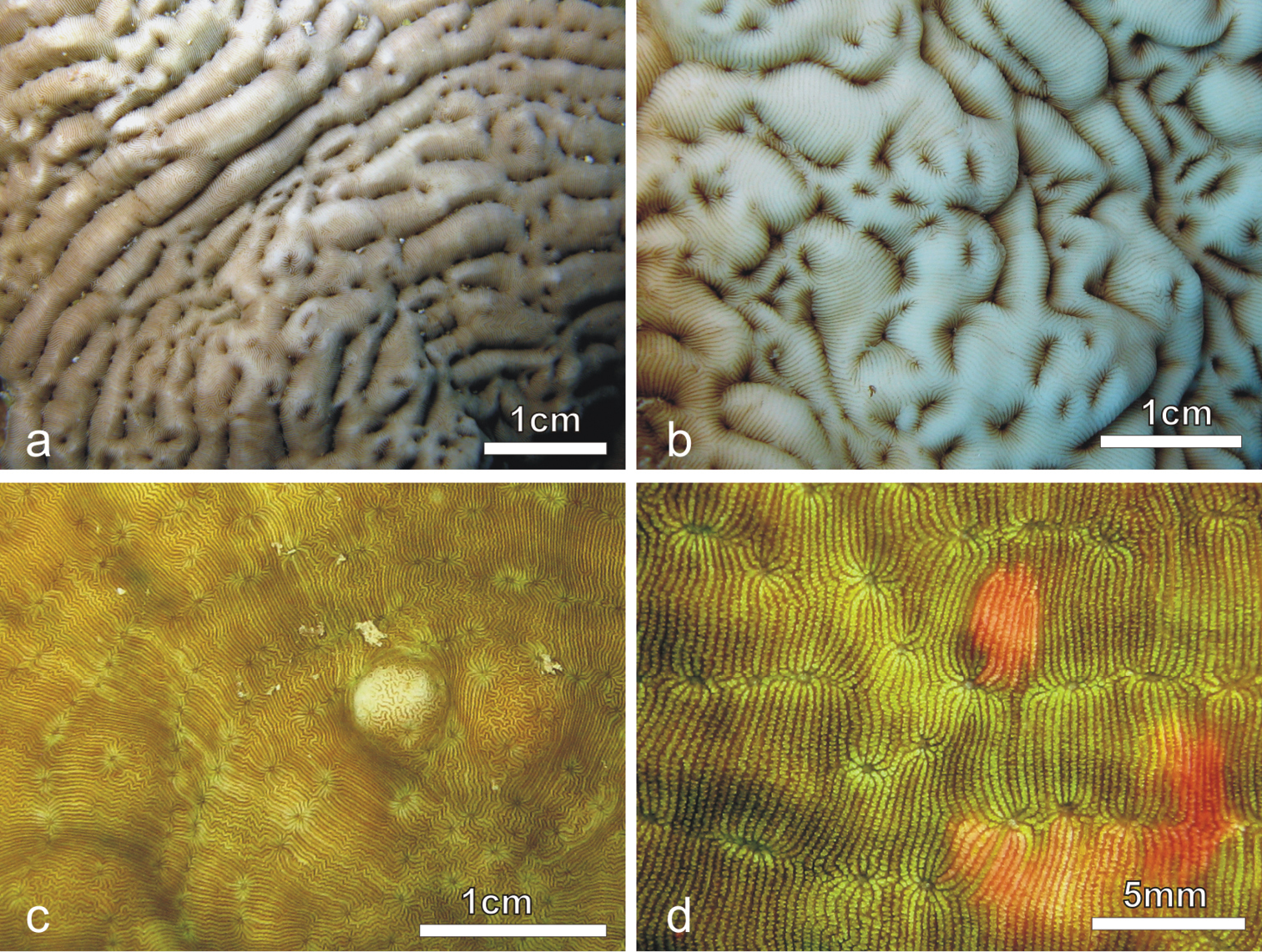
Color: The in vivo color was light brown with the top of the collines and the margins of the colony paler.
(MV Dream-Master, KAUST Biodiversity cruises, Saudi Arabia): KAUST SA004, Al Lith (KBEF), 20°07.690'N, 40°12.513'E, 3 March 2013, coll. F. Benzoni; KAUST SA426, Qita al Kirsh, 22°25.597'N, 38°59.769'E, 18 March 2013, coll. F. Benzoni; KAUST SA429, Qita al Kirsh, 22°25.597'N, 38°59.769'E, 18 March 2013, coll. F. Benzoni; KAUST SA678, Al Wajh (KBEA), 25°23.515'N, 36°41.035'E, 25 September 2013, coll. F. Benzoni; KAUST SA860, Shibb al Hab (KBEA), 27°49.003'N, 35°06.397'E, 28 September 2013, coll. F. Benzoni; KAUST SA887, Jazirat Burcan (KBEA), 27°54.356'N, 35°03.555'E, 28 September 2013, coll. F. Benzoni; KAUST SA1284, Fsar, 22°13.779'N, 39°01.730'E, 20 October 2013, coll. F. Benzoni; KAUST SA1293, Fsar, 22°13.779'N, 39°01.730'E, 20 October 2013, coll. F. Benzoni and J. Bouwmeester; KAUST SA1300, Fsar, 22°13.779'N, 39°01.730'E, 20 October 2013, coll. F. Benzoni and J. Bouwmeester; KAUST SA1301, Fsar, 22°13.779'N, 39°01.730'E, 20 October 2013, coll. F. Benzoni and J. Bouwmeester; KAUST SA1305, Fsar, 22°13.779'N, 39°01.730'E, 20 October 2013, coll. F. Benzoni and J. Bouwmeester.
Average colony size is around 15 cm in diameter (Figure 4). The largest colony observed in the field was 25 cm across (Figure 11b). Corallum generally encrusting at the centre with foliose margins, thicker in colonies grown in well-lit environments and thinner in those from deeper and lower light conditions. Calices always distinct and arranged in series in most specimens although the length of the series can be very variable within and between specimens (Figures 4–6) and single calices can be also observed (Figure 6g). Carinae are always rounded, however they show much variation in height and width (Figure 4). Examples of the two ends of the wide variation range of the development of carinae in this species are provided in Figure 5. Columella always present, sitting low in the fossa, made of one or multiple spatula-shaped processes extending from the inner end of the radial elements (Figures 6a–d). No dissepiments were observed between the inner ends of the radial elements and the processes forming the columella in calices in series or alone (Figures 7a–b). Although radial elements are generally equal (Figures 6f–g, 7a–b) they can be unequal in some specimens (Figure 6e). Their faces’ ornamentation consists of parallel lines of clumped granules (Figures 7, 10a, c, e). Clumps of granules can fuse laterally to form short ledge-like features (Figure 10e), however these never develop into menianae sensu stricto. The upper margin of the radial elements is minutely beaded (Figures 7f, 10e) and typically attains a zigzag pattern with clumps of granules at the angles (Figure 7f).
In well-lit conditions and when growing on a horizontal substrate, this species tends to have a wrinkled appearance due to well-developed carinae. In colonies growing on inclined substrate and shaded conditions the carinae are less developed and the corallum surface can attain a smooth or slightly undulating surface. The coloration ranges from a grayish beige (Figures 4b, c, h, 5a–b) to brown with some areas having a greener tinge (Figures 4a, d–g, 5c–d).
Pachyseris inattesa sp. n. was recorded from different reef habitats between 10 and 35m depth. It grows on exposed reef slopes as well as in underneath overhangs and small caves.
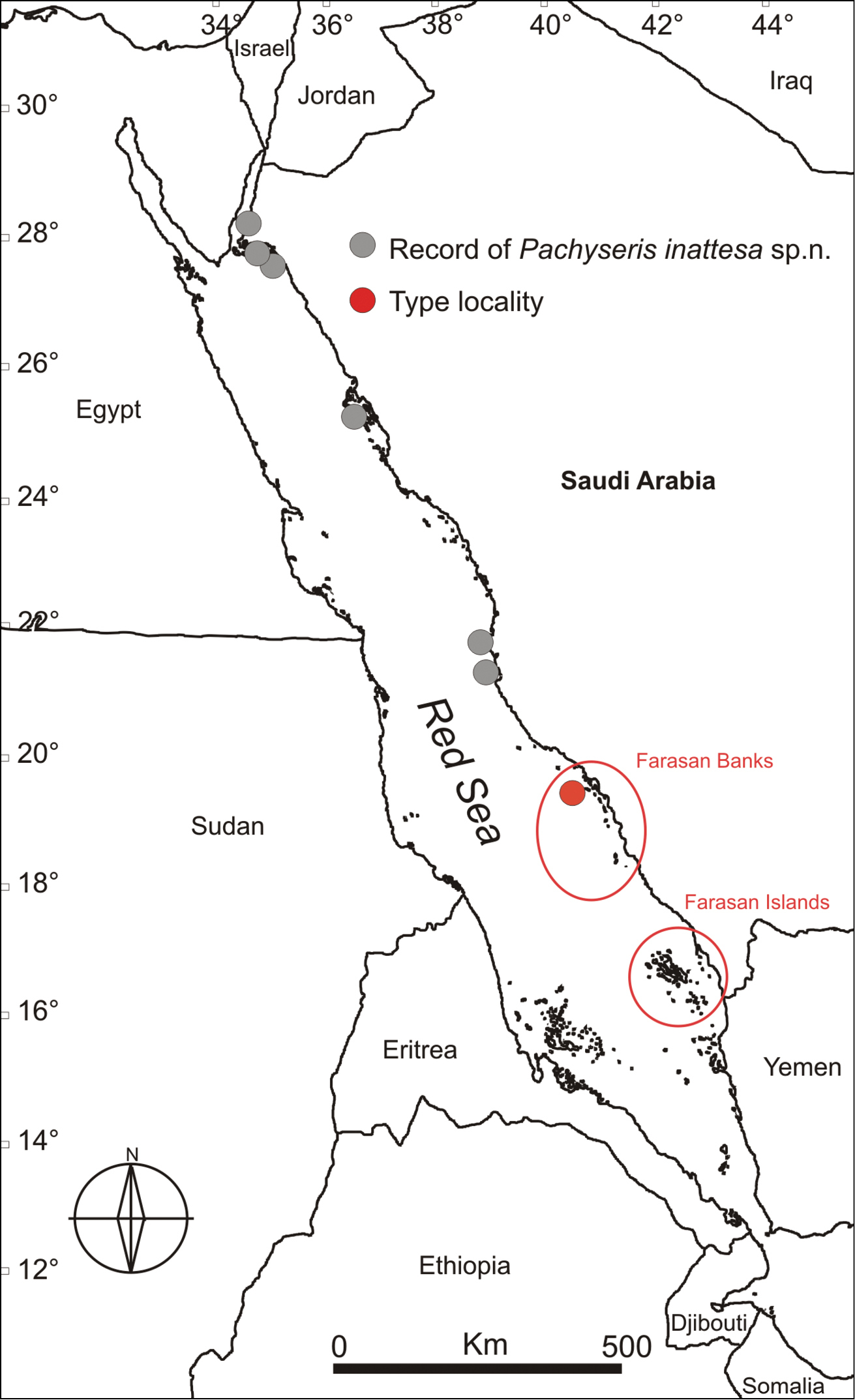
This species has been sampled along the Saudi Arabian coast in the northern and central Red Sea (Figure 8). It was not recorded in the Farasan Islands, nor further south in the Kamaran Islands, Yemen. To date, its distribution appears to be limited to the Red Sea.
Map of the Red Sea showing the sampling sites where Pachyseris inattesa sp. n. was collected, including the type locality. Circled regions indicate approximate distribution of the Farasan Banks (a complex reef network spanning about ~250km of the Saudi Arabian coast) and the Farasan Islands (a system of > 80 islands spanning about 150km of the southernmost Saudi Arabian coast adjacent to the Yemeni border).
Among its congeners, this species bears most resemblance to Pachyseris speciosa. However, with respect to the macro-morphology of the corallum, the corallites, and in corallite arrangement, this species is similar to and has been previously misidentified as Leptoseris foliosa.
Inattesa means “unforeseen” in Italian and stems from the initial bewilderment of the authors once they first examined the skeleton of the new species under a microscope.
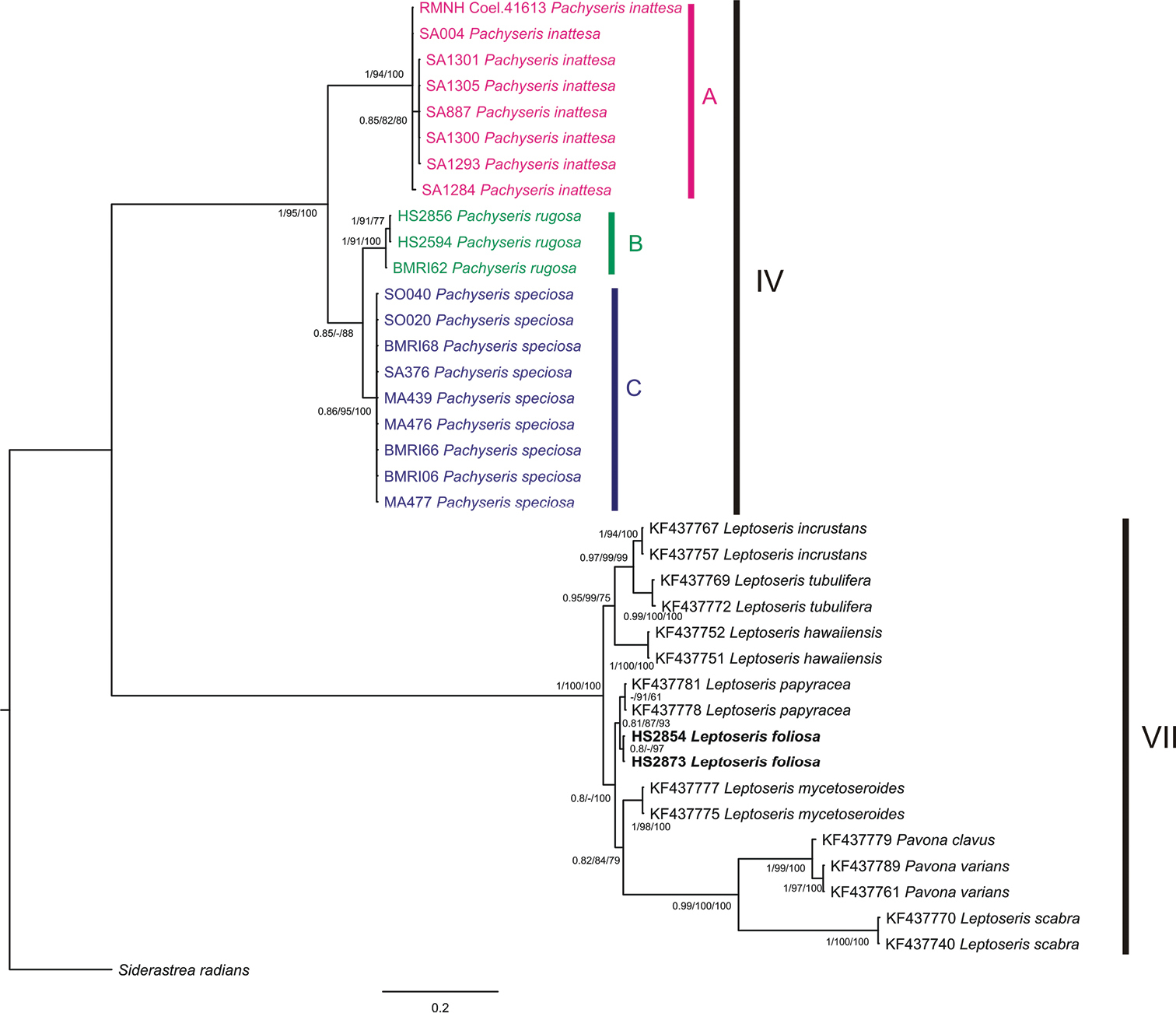
A total of 20 Pachyseris specimens belonging to three different species, namely Pachyseris rugosa, Pachyseris speciosa, and Pachyseris inattesa sp. n., and two Leptoseris foliosa sequences were successfully sequenced and used for phylogenetic reconstruction together with 15 agariciid sequences previously used by
Pachyseris and Agariciidae phylogenetic reconstruction inferred from Bayesian inference analysis of mitochondrial intergenic spacer between COI and 16S-rRNA. Specimens identified as Pachyseris inattesa sp. n., Pachyseris rugosa and, Pachyseris speciosa are highlighted in pink, green and blue, respectively. Specimens of Leptoseris foliosa are indicated in bold. Uppercase letters A, B, and C delineate Pachyseris lineages. Clade numbers IV and VII are as reported by
The phylogenetic reconstruction resolved two main groups congruent with clade IV sensu
In a diagnosis of Pachyseris,
The new species was previously collected in the Red Sea and identified as Leptoseris tenuis by
The genus Leptoseris is characterized, like many other scleractinian taxa, by a great variability in the macro-morphology of the colonies and by a notable interspecific phenotypic variability that led to the identification of several nominal species and an enduring taxonomic confusion (
SEM images of Pachyseris inattesa sp. n. (a, c, e) and Leptoseris foliosa (b, d, f) a and b similar arrangement of adjacent calices in specimen KAUST SA1305 and IRD HS2854, respectively c and d calices the same specimens as in a and b, respectively e and f lateral ornamentation of the radial elements (light blue arrows) and pattern of their upper margin in specimens (yellow dashed lines) as in a and b, respectively.
In situ and corallum images of a and d Pachyseris speciosa b and e Pachyseris inattesa sp. n., c and f Leptoseris mycetoseroides in the Red Sea.
Leptoseris foliosa was not encountered in the Saudi Arabian reefs in 2013, and since, as discussed above, previous records of this species in the Red Sea turned out to be Pachyseris inattesa sp. n., the presence of this species in the region is currently not confirmed. In the Red Sea Pachyseris inattesa sp. n. co-occurs with Pachyseris speciosa (Figures 11a–d) and with the common Leptoseris mycetoseroides. In the original description of this species,
Although the mitochondrial genome of Scleractinia species is usually characterized by a slow evolution rate resulting in low levels of intraspecific variation (
The molecular phylogenetic reconstruction corroborates our micro-morphological results, confirming Pachyseris inattesa sp. n. as a monophyletic lineage belonging to Pachyseris, and not related to the family Agariciidae including the genus Leptoseris. This study confirms the utility of a combined morpho-molecular approach in resolving phylogenetic relationships among scleractinian species (
As researchers increasingly combine traditional morphological with modern molecular taxonomic approaches on reference and museum collections (
The present study indicates that the same pattern may be true for a wider range of taxa including scleractinian corals, i.g., Acropora hemprichii (Ehrenberg, 1834), Acropora variolosa (Kluzinger, 1879), and Cantharellus doederleini (von Marenzeller, 1907) and provides some insight to more general biogeographic trends in the Red Sea (
Moreover, further investigations into the taxonomic status of marine species throughout the wider Red Sea, particularly with reference collections to facilitate morphological and molecular examinations (see
Pachyseris inattesa sp. n. is described from the Saudi Arabian Red Sea based on a combination of morphological and molecular analyses. Although the macro-morphology of this species led to previous misidentifications with Leptoseris tenuis and Leptoseris foliosa, micro-morphological analyses revealed characters consistent with those of Pachyseris such as a concentric arrangement of the carinae, the structure of the columella (formed by extensions from the margins of the radial elements into the fossa), and a zigzag pattern of the upper radial elements. Molecular analyses of the mitochondrial non-coding spacer between COI and 16S-rRNA confirm that Pachyseris inattesa sp. n. is more closely related to other Pachyseris species than to the agariciid genera Leptoseris and Pavona.
We are grateful to P. Saenz-Agudelo, J.D. DiBattista, M.A. Priest, T. Sinclair-Tylor, and E.C. Giles for their assistance at KAUST, and A.H. Baird (ARC) for fruitful discussions. We wish to thank the captain and crew of the MV Dream-Master and the KAUST Coastal and Marine Resources Core Lab for fieldwork logistics. Sampling in New Caledonia was possible thanks to the support of the Institut de Recherche pour le Développement and to C. Payri, J. Butscher, A. Arnaud, and E. Folcher. We are grateful to the Province Sud of New Caledonia for sampling permits in Prony Bay. Sampling in Semporna was done during the Semporna Marine Ecological Expedition 2010 with research permission from the Economic Planning Unit, Malaysia, Sabah Parks and Department of Fisheries Sabah. Grazie to S.E.T. van der Meij for providing the original description and illustration of Leptoseris tenuis by van der Horst. We wish to thank also P. Gentile (UNIMIB) for technical help with SEM specimen preparation, A.R. Behzad for technical help with SEM imaging in KAUST and P. Galli for laboratory support at UNIMIB. This project was supported in part by funding from KAUST (award # CRG-01-BER-2012-002 and baseline research funds to MLB). The paper was improved by comments and corrections from two anonymous reviewers.