Citation: Luz FA, Gonçalves GL, Moreira GRP, Becker VO (2014) Three new cecidogenous species of Palaeomystella Fletcher (Lepidoptera, Momphidae) from the Brazilian Atlantic Rain Forest. ZooKeys 433: 97–127. doi: 10.3897/zookeys.433.7379
Three new cecidogenous species of Palaeomystella Fletcher (Lepidoptera, Momphidae) from the Brazilian Atlantic Rain Forest are described. Larvae of P. fernandesi Moreira & Becker, sp. n., P. rosaemariae Moreira & Becker, sp. n. and P. tavaresi Becker & Moreira, sp. n. induce galls, respectively, on Tibouchina sellowiana (Cham.) Cogn., T. asperior (Cham.) Cogn. and T. fissinervia (Schrank & Mart. ex DC.) Cogn. (Melastomataceae). Adults, immature stages and galls are illustrated, and data on life history and a preliminary analysis of mitochondrial DNA sequences, including related species, are also provided.
Melastomataceae, melastome, Mompha, momphines, momphid moths, Neotropical region, plant galls, Tibouchina
Cecidogeny has evolved independently in at least 20 microlepidopteran families, mostly within the Gelechioidea. Although they total only a few hundred species, the majority of these moths have been associated with their gall morphotype only, as most are still awaiting taxonomic description at the specific level (
The majority of the gall morphotypes (ca. 30) that are known to be induced by unidentified lepidopteran larvae in Brazil were originally described from the Atlantic Rain Forest (Rio de Janeiro State), mostly on species of Tibouchina (
In the course of an ongoing survey on the diversity of microlepidopterans in the Atlantic Rain Forest, Brazil, three momphid species associated with galls induced on three different species of Tibouchina were found recently: one morphotype in Bahia and two others in Rio Grande do Sul. A comparison between their inducers and type material not only revealed the generic affinity of these microlepidopterans with Palaeomystella, but also indicated that they have diagnosable, stable, distinctive characters. Therefore, three new species are proposed here; their last larval instar, pupal and adult stages are described and illustrated, and their life history, including a general description of their galls, is characterized. A preliminary phylogenetic inference based on mitochondrial DNA sequences, including additional members of the genus, is also presented.
Adults used in the study were reared by the first three authors from galls maintained in small plastic vials under controlled abiotic conditions (14 h light / 10 h dark; 25 ± 2 °C) in the Laboratório de Morfologia e Comportamento de Insetos, Departamento de Zoologia, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre city, Rio Grande do Sul State (RS), Brazil, from March 2012 to October 2013. Galls were field-collected with either late-instar larvae or pupae inside, on shoots of Tibouchina sellowiana (Cham.) Cogn. (São Francisco de Paula, RS), Tibouchina asperior (Cham.) Cogn. (Santo Antônio da Patrulha, RS) and Tibouchina fissinervia (Schrank & Mart. ex DC.) Cogn. (Camacan, Bahia). Immature stages were obtained by dissecting additional galls. Adult specimens were pinned and dry preserved. The immatures were fixed in Kahle-Dietrich´s fluid and preserved in 75% EtOH. For DNA analyses, additional specimens were preserved in 100% EtOH at -20 °C.
For gross morphology studies, the specimens were cleared in a 10% potassium hydroxide (KOH) solution and mounted on slides with either glycerin jelly or Canada balsam. Observations were made with the aid of a Leica® M125 stereomicroscope. Structures selected to be drawn were previously photographed with an attached Sony® Cyber-shot DSC-H10 digital camera. Then, vectorized line drawings were made with the software CorelPhotoPaint® X4, using the corresponding digitalized images as a guide. At least five specimens were used for the descriptions of each life stage. Measurements were made with an attached ocular micrometer; values are presented as mean ± standard deviation unless noted otherwise.
Specimens used in scanning electron microscope (SEM) analyses were dehydrated in a Bal-tec® CPD030 critical-point dryer, mounted with double-sided tape on metal stubs, and coated with gold in a Bal-tec® SCD050 sputter coater. They were examined and photographed in a JEOL® JSM5800 scanning electron microscope at Centro de Microscopia Eletrônica (CME) of UFRGS.
Molecular phylogeny. Total genomic DNA was purified from larval tissue, using a Qiagen DNA Blood and Tissue Kit, to investigate: (i) monophyly of Palaeomystella fernandesi, Palaeomystella rosaemariae and Palaeomystella tavaresi; and (ii) reconstruct phylogenetic relationships within Palaeomystella. For comparison, two pupae of Palaeomystella oligophaga Becker & Adamski, 2008, from a population of Macairea radula (Bonpl.) DC. located in Brasília, Distrito Federal, were also used for DNA extraction (Table 1). Of the mitochondrial gene cytochrome c oxidase subunit I (CO-I) a piece of 660 base pairs (bp) was amplified using the universal primers LCO1490 (5'-ggtcaacaaatcataaagatattgg-3') and HCO2198 (5'-taaacttcagggtgaccaaaaaatca-3') and following PCR conditions proposed by
Specimens used in this study to reconstruct the phylogenetic relationships of the new species of Paleomystella, based on cytochrome oxidase subunit I sequences.
| Genus | Species | Voucher | GenBank accession numbers |
|---|---|---|---|
| Ingroup | |||
| Palaeomystella | Palaeomystella oligophaga | LMCI 234-1A | KJ188233 |
| LMCI 234-1B | KJ188234 | ||
| Palaeomystella sp. 1 | LMCI 211-4A | KJ188235 | |
| LMCI 211-4B | KJ188236 | ||
| Palaeomystella sp. 2 | LMCI 174-25 | KJ188237 | |
| LMCI 174-26 | KJ188238 | ||
| LMCI 174-27 | KJ188239 | ||
| Palaeomystella tavaresi sp. n. | LMCI 209-6A | KJ188240 | |
| LMCI 209-6B | KJ188241 | ||
| Palaeomystella rosaemariae sp. n. | LMCI 211-8A | KJ188242 | |
| LMCI 211-8B | KJ188243 | ||
| Palaeomystella fernandesi sp. n. | LMCI 174-50A | KJ188244 | |
| LMCI 174-50B | KJ188245 | ||
| LMCI 174-56 | KJ188246 | ||
| Outgroup | |||
| Mompha | Mompha conturbatella | 10-JDWBC-1043 | HM862677 |
Phylogenetic reconstructions were based on two methods: Bayesian inference (BI), implemented in BEAST 2.0 (
Abbreviations of the Brazilian states and institutions from which specimens were examined are:
BA Bahia State.
DF Distrito Federal.
DZUP Coll. Padre Jesus S. Moure, Departamento de Zoologia, Universidade Federal do Paraná, Curitiba, Paraná.
LMCI Laboratório de Morfologia e Comportamento de Insetos, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul.
MCTP Museu de Ciências e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul.
RS Rio Grande do Sul State.
VOB Coll. Vitor O. Becker, Reserva Serra Bonita, Camacan, Bahia.
http://zoobank.org/F2B43BAC-11CB-4374-B6C4-17E8CB82C8DC
Although showing congeneric affinity, Palaeomystella fernandesi has morphological features that in conjunction distinguish it from all known Palaeomystella species, as follows: 1) male genitalia with upper section of valve narrowing distally, forming a single process that bends medially; 2) pupa with cremaster short and apically rounded, with four pairs of setae; 3) galls of fusiform type, external surface without conspicuous ornament, bearing a few longitudinal carinae, induced on stem of Tibouchina sellowiana apical branches.
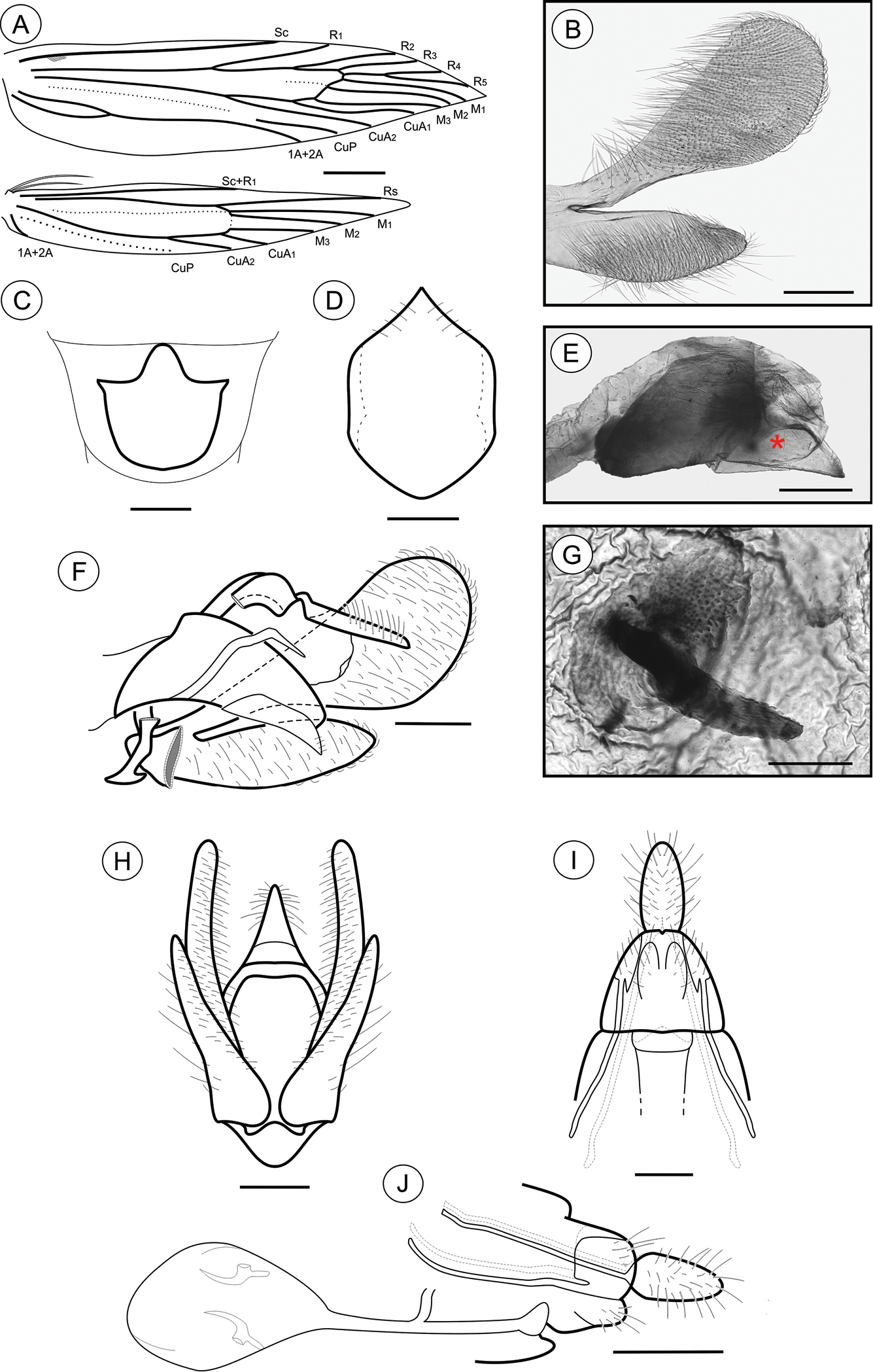
Adult (Figs 1A–B). Sexes similar in size and color; Forewing length 4.68 to 6.11 mm (n = 7). Head (Fig. 1B): Frons and vertex creamy white; labial palpus mostly dark brown, basal segments angled laterally, terminal segment slightly angled upward; antennae dark brown; proboscis yellowish brown. Thorax: Tegula and mesonotum whitish creamy white with pale-brown scales; legs dark brown. Forewing (Figs 1A, 2A): lanceolate, with 13 veins; L/W index ~ 5.1; dorsally covered mostly by dark-brown scales; with three interconnected white areas that form a longitudinal S-like band; one proximal, rounded, in the anal area, made of pale-creamy white scales, followed by a short stripe aligned in the cubital area, made of creamy white scales, and a third, also rounded and faint, in the cell, made of pale-creamy white scales; a tenuous, U-shaped band of pale-gray scales following the contours of the tornus; three raised tufts of pale-gray scales, located posteriorly to cubitus, in anal area, in line with mid-cell, and near tornal area respectively; fringes dark brown; ventrally mostly covered by dark-brown scales; retinaculum subcostal; discal cell closed, ~ 0.8× length of forewing, ending near 1/5 of wing margin; Sc ending ca. middle of anterior margin; R 5-branched; R1 ending near 1/3 of wing margin; R4 and R5 stalked ca. 1/2 distance from the cell apex; M 3-branched; CuA 2-branched; CuP weak proximally and not stalked, with well-developed 1A+2A extending more than 1/2 posterior margin. Hindwing (Figs 1A, 2A): strongly lanceolate, with nine veins; L/W index ~ 7.2, ~ 0.8 forewing in length; scales dark brown on both sides; fringes dark brown; frenulum a single acanthus in male, with two distally directed acanthi in female; Sc+R1 ending ca. 1/2 anterior margin; Rs ending ca. 1/5 anterior margin; M 3-branched, M1 and M2 stalked from remnant chorda of cell, from point beyond base of Rs; CuA 2-branched, with CuA1 stalked to M3; CuP weakly sclerotized, ending 1/3 posterior margin; 1A+2A well developed, ending near basis of posterior margin. Abdomen (not illustrated): pale brown, intermixed with gray scales, with transverse irregular rows of spiniform setae on terga 2–7 in both sexes; eighth sternum (Fig. 2C) anteriorly expanded medially into a short lobe, associated with a subtriangular sternite.
Spread right wings (left column), head and thorax in detail (right column) of pinned Palaeomystella species, dorsal view: A–B Palaeomystella fernandesi C–D Palaeomystella rosaemariae E–F Palaeomystella tavaresi. Scale bars = 2, 0.5, 2, 0.5, 2 and 0.5 mm, respectively.
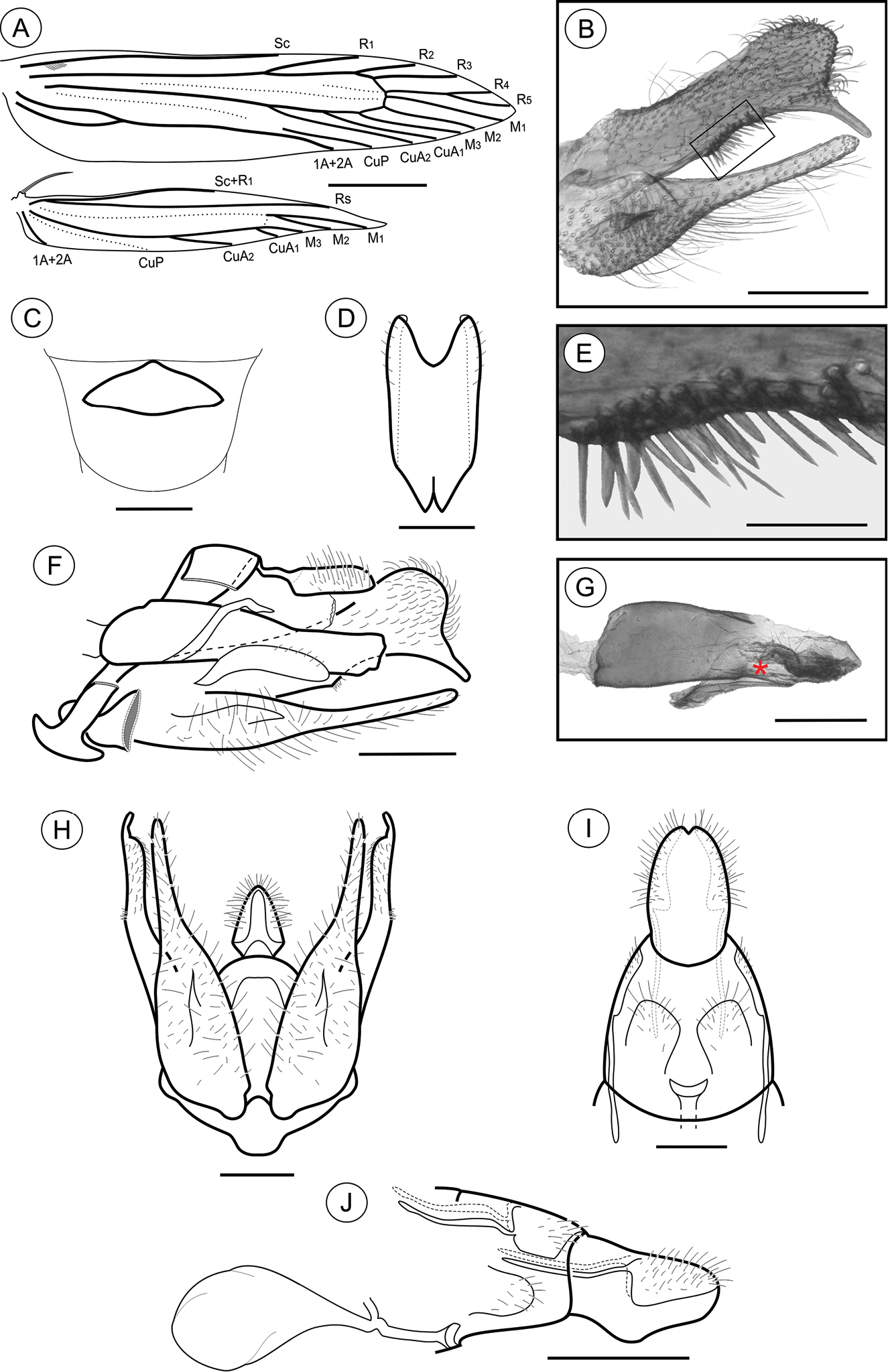
Palaeomystella fernandesi adult morphology: A wings B male valva, mesolateral view C male eighth sternum, ventral view D juxta, ventral E ventral spines of the valva upper section in detail (rectangular area shown in B), mesolateral view F male genitalia, lateral view G aedeagus, lateral view (asterisk indicates attached juxta-lobes) H male genitalia, ventral view (transtilla, aedeagus and juxta not illustrated) I female genitalia, ventral view (corpus bursae not illutrated) J female genitalia, lateral view. Scale bars = 1 mm; 200, 200, 100, 50, 200, 200, 200 and 250µm; 0.5 mm, respectively.
Male genitalia (Figs 2B, D–H). Uncus narrow, separated from tegumen by a narrow membranous area, distally roof like and laterally setose (Figs 2F, H); tegumen narrow; vinculum widened ventrally; transtilla a flat, rounded fig; aedeagus cylindrical, moderately long, slightly wider basally (Fig. 2G); vesica bearing a few short stout cornuti; juxta (Fig. 2D) attached to distal portion of aedeagus (Fig. 2G), longer than wide, with two small, parallel pointed projections mid-anteriorly and deeply concave distally; valva (Figs 2B, F, H) covered with several long setae, divided near 1/3 from the basis, with a long, finger-like sacculus and a wide spatulate costa, bearing a thin ventral finger-like projection near apex, and several stout, medium-sized spines meso-ventrally (Fig. 2E).
Female genitalia (Figs 2I–J). Papillae anales connected dorsally, narrowed distally, setose; anterior apophyses with arms slightly curved, similar in length to posterior apophyses; sterigma divided into a bandlike tergum and a distally bilobed sternum, shallowly and widely emarginate medially; ostium bursae small, wider than long; ductus bursae membranous, shorter than corpus bursae; ductus seminalis inserted distally; corpus bursae an elongate sac, with no sclerotizations on inner wall.
Holotype ♂ Brazil: Centro de Pesquisas e Conservação da Natureza Pró-Mata (CPCN Pró-Mata; 29°29'16"S, 50°10'60"W; 925 m), São Francisco de Paula, RS, Brazil. Dry preserved pinned adults, reared from galls induced on Tibouchina sellowiana (Cham.) Cogn. (Melastomataceae), LMCI 210-56, 7–9.III.2013, by G.R.P. Moreira, F.A. Luz and L.T. Pereira, donated to DZUP (29.409). Paratypes: same data, 26.III.2012, by G.R.P. Moreira, F.A. Luz and P. Pollo; 2♀ (LMCI 174-161 and 162), donated to DZUP (29.410 and 29.411); 1♂ (LMCI 174-157) with genitalia in glycerin (GRPM 50-51) and 1♀ (LMCI 174-158), donated to MCTP (36.225 and 36.226, respectively).
With the same collection data, deposited in LMCI. Adults, dried and pinned: 2♂ (LMCI 174-159 and 210-49), 1♀ (LMCI 174-160), 1♀ (LMCI 174-163) with genitalia in glycerin (GRPM 50-52). Adults, fixed in Kahle-Dietrich’s fluid and preserved in 70% EtOH: 1♂ (LMCI 174-165), 3♀ (LMCI 174-164, 166 and 167). Slide preparations, mounted in Canada balsam: genitalia, 3♂ (GRPM 50-29, 47 and 48), 1♀ (GRPM 50-28); wings, 2 ♂ (GRPM 50-45 and 50), 1♀ (GRPM 50-46); larvae, 2 last instars (GRPM 50-49). Immature stages, fixed in Kahle-Dietrich’s fluid and preserved in 70% EtOH: 8 last-instar larvae (LCMI 174-52); 7 pupae (LMCI 174-168, 169 and 223; and 210-16); 10 galls (LMCI 174-47 to 49, 174-217 to 222, and 210-15). In tissue collection, 9 larvae (LMCI 174-50 and 56) fixed and preserved in 100% EtOH, at -20°C.
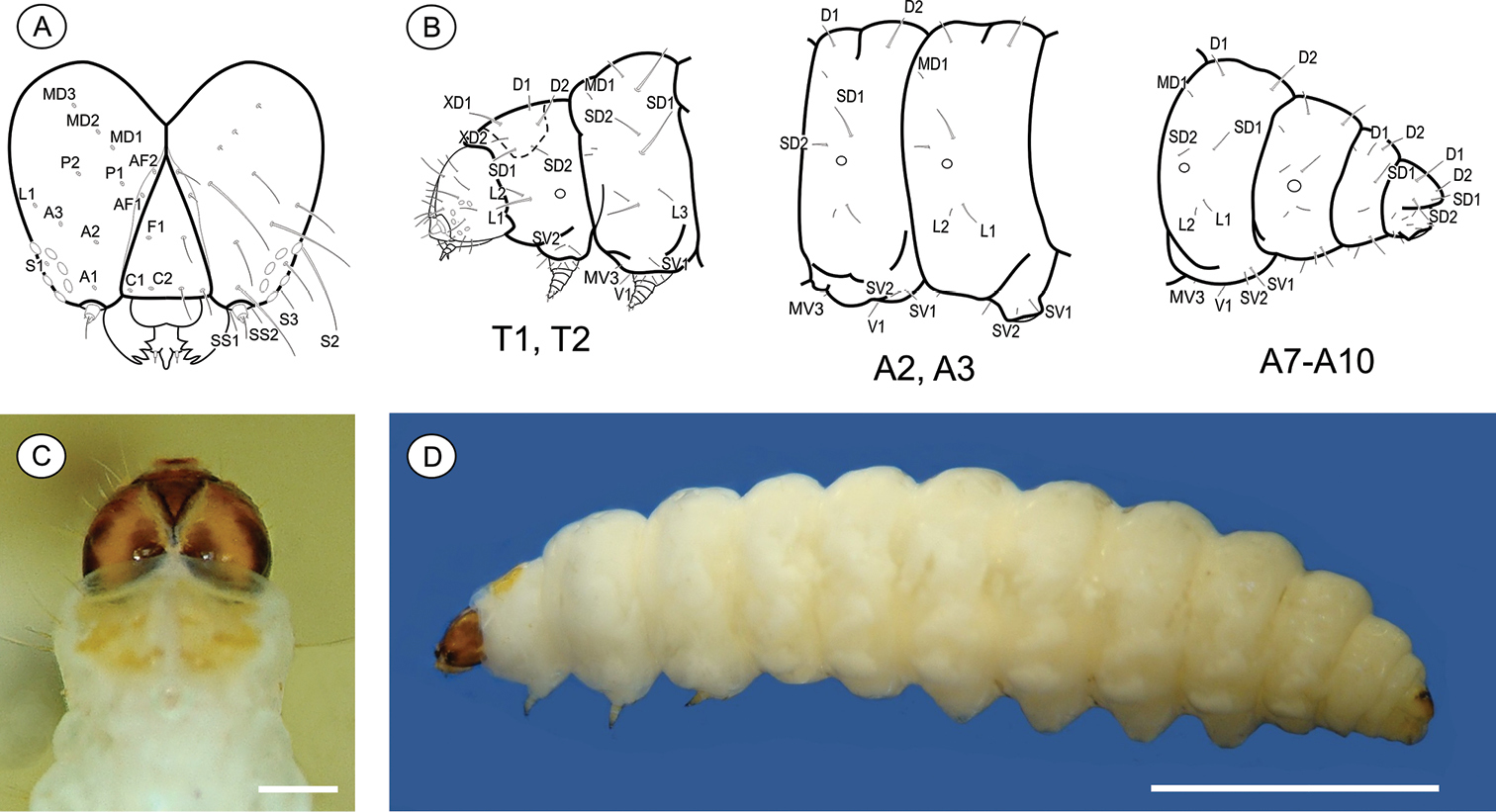
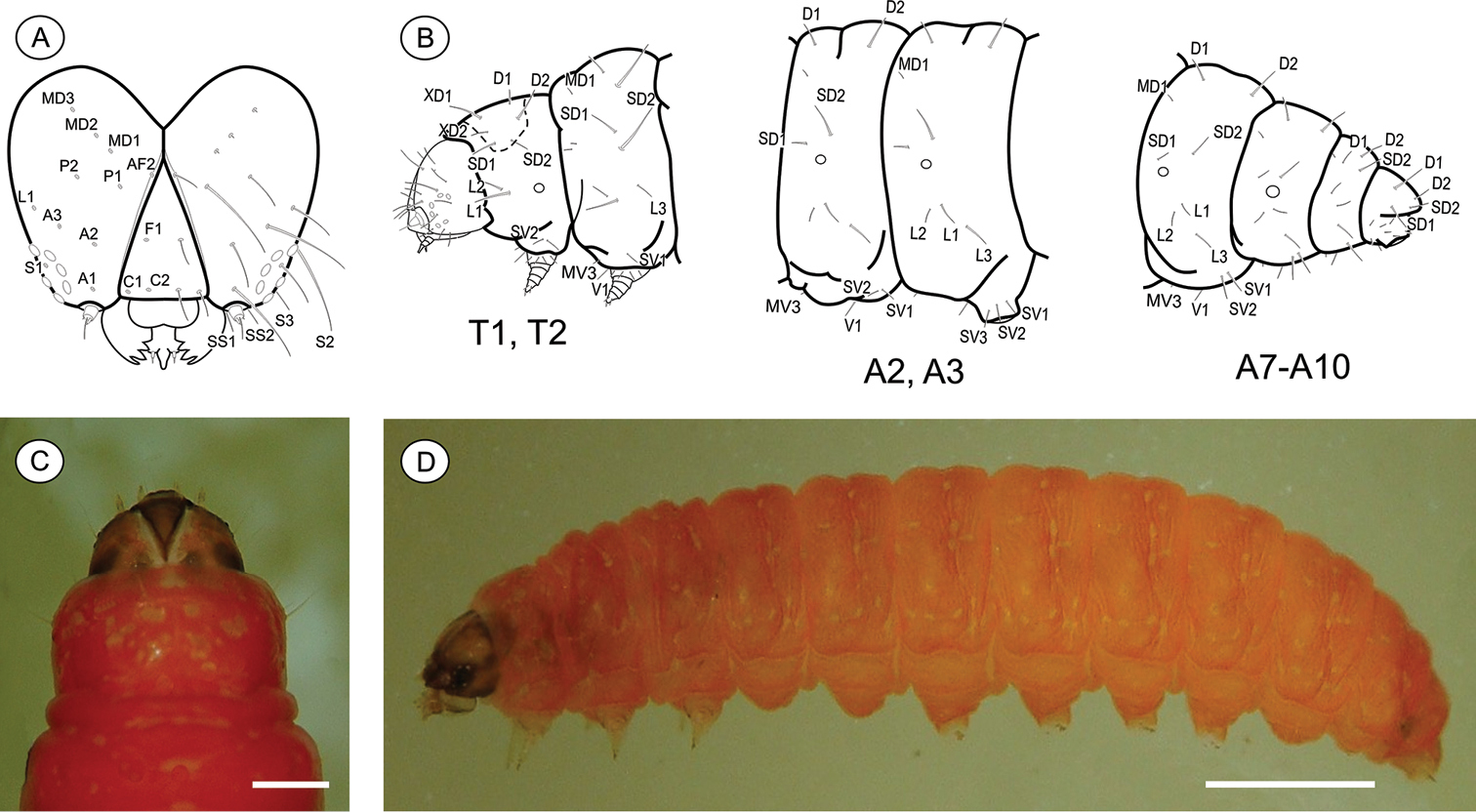
Last instar larva (Figs 3A–D), 3.51 to 7.01 mm (n = 6). Cecidogenous, endophyllous, semiprognathous, and tissue-feeder. Body with setae well developed. Head (Figs 3A, C–D): brown, with two paler mid-dorsal areas; smooth, with shallow ridges; labrum shallowly notched; frons higher than wide, extending ca. 3/4 epicranial notch; six stemmata arranged in C-shape. Chaetotaxy (Fig. 3A): A-group trisetose; L-group unisetose; P-group bisetose; MD trisetose; C-group bisetose; F-group unisetose; AF-group bisetose; S-group trisetose; SS-group trisetose. A1, A3, P1 and S2 about equal in length, longest setae on head; C1, C2, F1, A2, AF2, L1 intermediate in length; AF1 shorter; MD1–3 very reduced and aligned with each other. Antenna two-segmented. Mandibles broad with four teeth, and one seta on outer surface; labium broad, with two-segmented palpus and spinneret parallel-sided; maxilla prominent. Thorax and abdomen (Figs 3B–D): Prothoracic shield light brown, divided longitudinally by indistinctly marked, unpigmented area; anal fig brown. Thoracic legs slightly pigmented. Prolegs on A3–A6 and A10 of equal size; crochets in a circle, uniserial and uniordinal. Thorax chaetotaxy: T1 with D-group bisetose, both located on the dorsal shield, D1 shorter than D2; XD-group bisetose, setae similar in length and both on the dorsal shield; SD bisetose, laterally on the dorsal shield; L-group bisetose, L1 longer than L2; SV-group bisetose, posteroventral to L2, SV1 slightly longer than SV2; V-group unisetose. T2 and T3 with D- and SD-groups bisetose, median-transversely aligned; D2 and SD1 similar in length, and longer than D1 and SD2 respectively; L trisetose, L3 posterior to L1–L2, similar in length to L1; SV unisetose; V unisetose. Abdomen chaetotaxy: D-group bisetose; A1–9 with D2 slightly longer than D1, and A10 with D1 longer than D2; SD-group bisetose, A1–7 with SD1 slightly longer than SD2 and A10 with SD2 longer than SD1, SD2 absent in A9; A1–8 with L-group bisetose, L1 longer than L2, L2 absent in A9; A1–8 with SV-group bisetose, SV1 slightly shorter than SV2, SV1 absent in A9; V-group unisetose.
Palaeomystella fernandesi last larval instar: A cephalic chaetotaxy, frontal view B thoracic and abdominal chaetotaxy, lateral view C head and prothoracic shield in detail, dorsal view D body, lateral view. Scale bars = 50 µm, 1 mm, respectively.
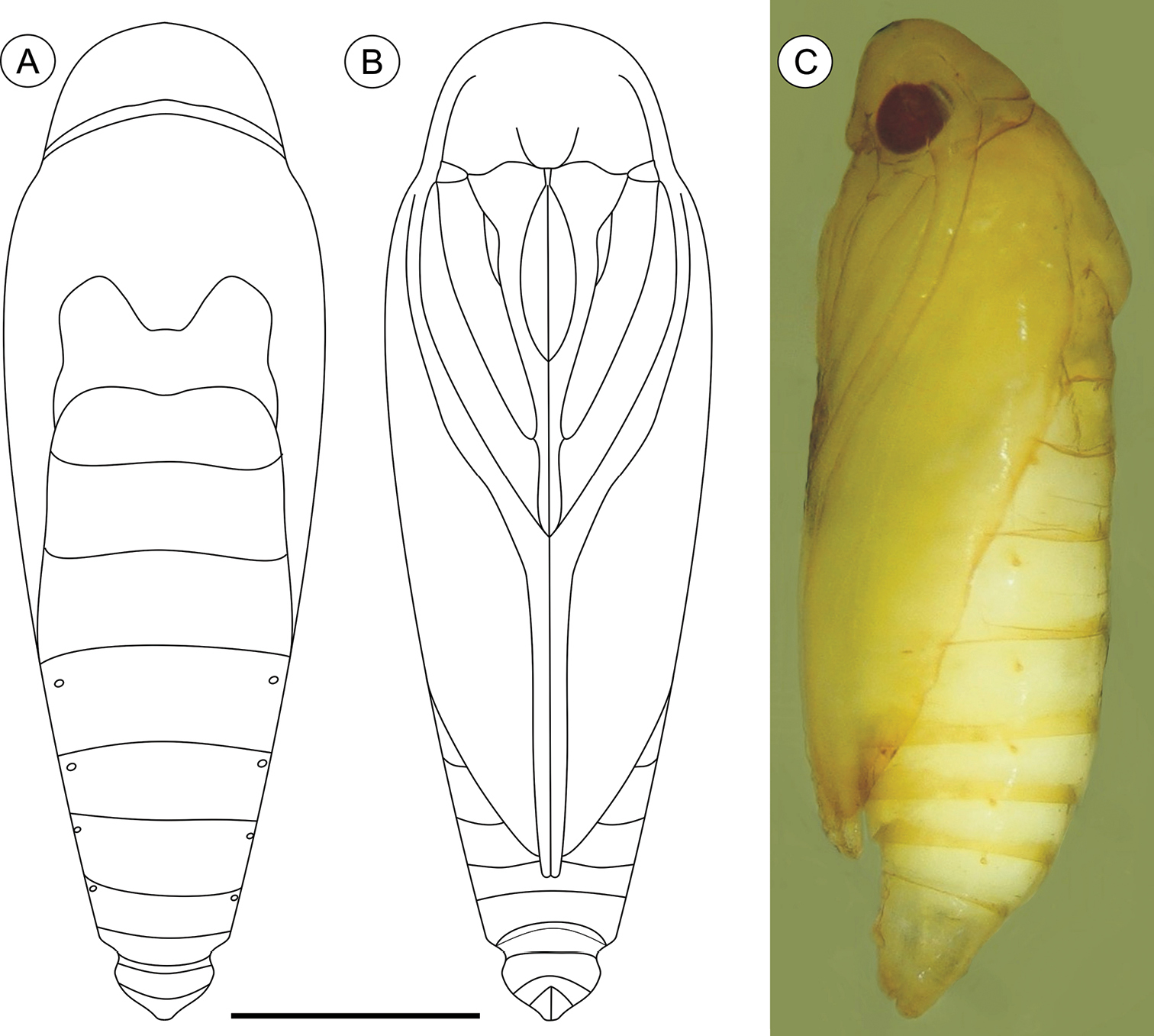
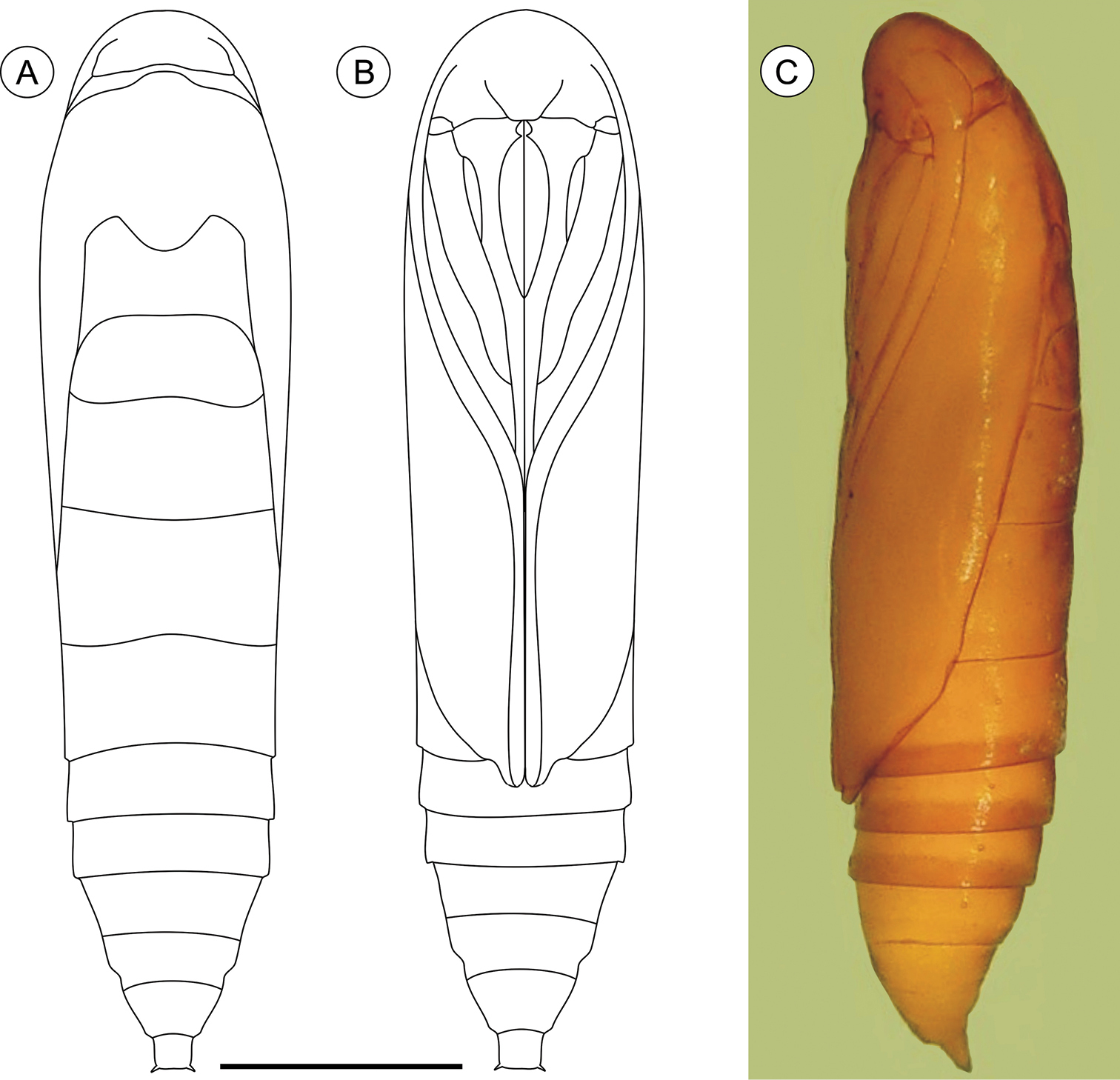
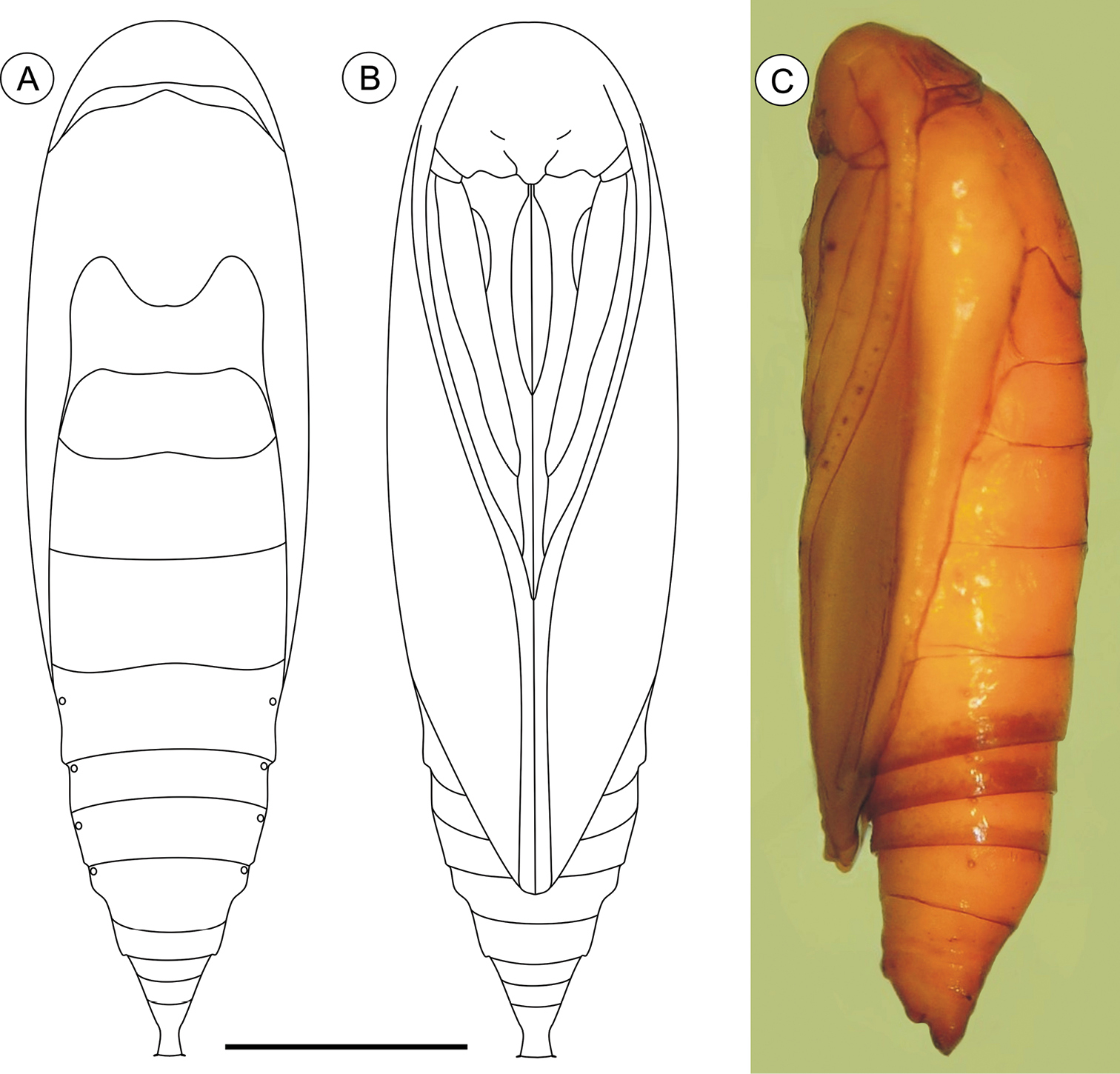
Pupa (Figs 4A–C, 11A–C), 4.42 to 6.11 mm long (n = 5). Body elongate-oval in dorsal and ventral views, widest and dorsally raised in mesothoracic region. Integument weakly melanized, mostly smooth, with a few scattered microsetae dorsally. Frontoclypeal suture not evident. Labrum U-shaped. Labial palpi long; antennae arched anteriorly and separate, approximate and parallel posteriorly to distal margins of maxillae, surpassing apical margin of forewings; maxillae extending distally between sclerites of mid-legs; femora of midleg not fused distally; femora of foreleg extending beyond widest part of labial palpi. Cremaster (Figs 11A–C) short and apically rounded, with four pairs of setae; one latero-basally, another latero-dorsally and two latero-distally.
Palaeomystella fernandesi pupa, in dorsal (A), ventral (B) and lateral (C) views, respectively. Scale bar = 1 mm.
Known only from the type locality, in the Dense Umbrophilous Forest (= Brazilian Atlantic Rain Forest sensu stricto) portions of the CPCN Pró-Mata, São Francisco de Paula, RS, Brazil.
Tibouchina sellowiana (Cham.) Cogn. (Melastomataceae). A small tree (3 to 6 m), endemic to the coastal montane forests of southern Brazil, ranging from Minas Gerais to Rio Grande do Sul, usually flowering in April–May (
(Figs 12A–C). Galls induced by Palaeomystella fernandesi are common on Tibouchina sellowiana at the type locality, during spring (October) and summer (February). They are prosoplasmatic histioid (Küster, in
Named in honor of Prof. Dr. Geraldo Wilson Fernandes, Departamento de Biologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, for his great contributions to the development of cecidology in the Neotropics.
http://zoobank.org/02056832-C637-4552-8A83-2F896EEE4143
Closest to Palaeomystella tavaresi, sharing with this species a valve with a pronounced palmate costa and bladelike signa. These characters distinguish them from all other species of Palaeomystella except Palaeomystella oligophaga. This, however, has the forewings with R4-R5 fused and the hindwing with M1 and M2 stalked from the remnant chorda of the cell (
Adult (Figs 1C–D). Sexes similar, forewing length 4.81 to 5.59 mm (n = 5). Head (Fig. 1D): Frons pale brown; vertex and labial palpus and antenna with pale-brown scales tipped with dark brown; labial palpus with basal segments angled laterally, terminal segment slightly angled upward; proboscis yellowish brown. Thorax: Tegula and mesonotum with pale-brown scales tipped with dark brown, posterior scales having more pale brown; fore and midlegs dark brown; hindlegs pale brown, tibia and tarsus with intermixed dark-brown scales. Forewing (Figs 1C, 5A): lanceolate, with 13 veins; L/W index ~ 4.5; dorsally covered by pale-brown scales intermixed with scattered, pale-brown scales tipped with dark brown, and with longitudinally aligned groups of brown scales; a narrow, ill-defined, dark-brown streak bisecting the wing longitudinally from base to tornus; 3 raised scale tufts located posterior to cubitus, including 1 wider tuft in anal area, 1 in line with midcell, and 1 near tornal area; fringes pale brown, interspersed with a few pale-brown scales tipped with dark brown; tornal area with two bands of pale-brown scales tipped with blackish brown; ventrally, mostly uniformly covered with dark-brown scales; retinaculum subcostal; discal cell closed, ~ 2/3 length of forewing; ending near 1/5 of wing margin; Sc ending ca. middle of anterior margin; R 5-branched; R1 ending near 1/3 of wing margin; R4 and R5 stalked ca. 1/4 distance from the cell apex; M 3-branched; CuA 2-branched; CuP weak proximally and not stalked, with 1A+2A that is well developed, extending more than half length of posterior margin. Hindwing (Fig. 5A) strongly lanceolate, with 9 veins; L/W index ~ 6.4, ~ 0.8 forewing in length; scales pale brown on both sides; fringes pale brown; frenulum with a single acanthus in male, and with two acanthi in female, proximal acanthus anteriorly divergent, and distal acanthus parallel to wing anterior margin; Sc+R1 ending at ca. 1/2 anterior margin; Rs ending at ca. 1/5 anterior margin; M 3-branched; CuA 2-branched, with CuA1 stalked to M3; CuP weakly sclerotized, ending at 1/3 posterior margin; 1A+2A well developed, ending near basis of posterior margin. Abdomen (not illustrated): pale-brown scales intermixed with gray scales, with transverse irregular rows of spiniform setae on terga 2–7 in both sexes; eighth sternum (Fig. 5C) anteriorly expanded medially into a slender, sharply pointed lobe, associated with a subtrapezoidal sternite.
Palaeomystella rosaemariae adult morphology: A wings B male valva, mesolateral view C male eighth sternum, ventral view D juxta, ventral; E aedeagus, lateral view (asterisk indicates attached juxta-lobes) F male genitalia, lateral view G signum, internal view H male genitalia, ventral view (transtilla, aedeagus and juxta not illustrated) I female genitalia, ventral view (corpus bursae not illustrated) J female genitalia, lateral view. Scale bars = 1 mm; 200, 200, 100, 200, 100, 200, 200 and 250 µm; 0.5 mm, respectively.
Male genitalia (Figs 5B, D–F, H). Uncus narrow, separated from tegumen by a narrow membranous area, laterally setose (Fig. 5F); tegumen narrow, widened dorsally; vinculum widened ventrally; transtilla a short, flat fig; aedeagus tubiform, short (twice as long as wide), curved ventrally, slightly wider basally (Figs 5E–F); vesica bearing several stout cornuti; juxta (Fig. 5D) attached to distal portion of aedeagus (Fig. 5E), wider than long, with slightly concave anterior margin and pointed distally; valva (Figs 5B, F, H) covered with several long setae, divided near 1/3 from base, with flat, broad sacculus tapering distad, and long, spatulate costa, rounded distally and gradually constricted toward base.
Female genitalia (Figs 5G, I–J). Papillae anales connected dorsally, setose (Figs 5I–J); anterior apophyses slightly shorter than posterior apophyses; sterigma divided into a bandlike tergum and a distally bilobed sternum, shallowly emarginate medially; ostium bursae large, wider than long; ductus bursae membranous, longer than corpus bursae; ductus seminalis inserted medially; corpus bursae an elongate sac, bearing two narrow and curved, bladelike signa that are connected to transversely elongate, rounded figs located on the wall (Figs 5G, J).
Holotype ♂: Brazil: Private farm belonging to Antonio Malta, Coxilha das Lombas, 30°02'13"S, 50°36'30"W, 17 m, Santo Antônio da Patrulha, RS, Brazil. Dry preserved pinned adults, reared from galls induced on Tibouchina asperior (Cham.) Cogn. (Melastomataceae), LMCI 211, 12.III.2013, by G.R.P. Moreira, F.A. Luz and S. Bordignon, (LMCI 211-12), donated to DZUP (29.412). Paratypes: same data, 1♂, 1♀ (LMCI 211-14 and 06) with genitalia in glycerin (GRPM 50-43 and 44), donated to DZUP (29.413 and 29.414, respectively).
Dry preserved pinned adults, with the same collection data, deposited in LMCI under the following accession numbers: 2♂ (LMCI 211-07 and 10); 1♀ (LMCI 211-11). Slide preparations, mounted in Canada balsam: genitalia, 2♂ (GRPM 50-38 and 39), 1♀ (GRPM 50-40); wings, 1♂ (GRPM 50-36), 1♀ (GRPM 50-37); larvae, 2 last instars (GRPM 50-41 and 42). Immature stages, fixed in Kahle-Dietrich’s fluid and preserved in 70% EtOH: 6 last-instar larvae (LCMI 211-17 to 22); 3 pupae (LMCI 211-5, 9 and 26); 6 mature, intact galls (LMCI 211-25). In tissue collection, 6 larvae (LMCI 211-8), fixed and preserved in 100% ethanol, at -20°C.
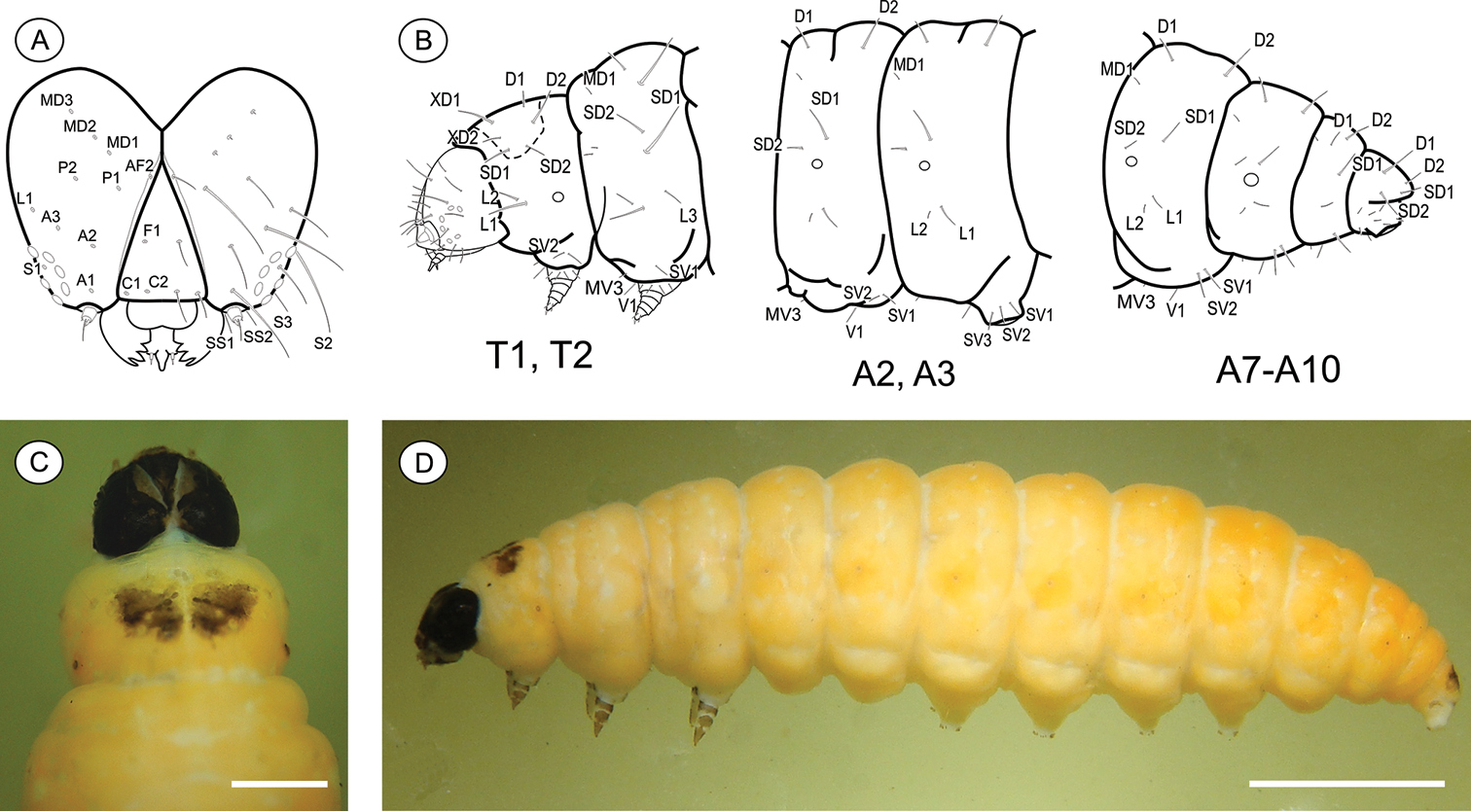
Last-instar larva (Figs 6A–D), 4.94 to 9.88 mm long (n = 5). Cecidogenous, endophyllous except prior to pupation, semiprognathous and tissue-feeder. Body subcylindrical, creamy white, changing to red when mature prior to exit the gall; with setae well developed. Head (Figs 6A, C–D): pale brown, interspersed with two pairs of darker mid-dorsal areas; smooth, with shallow ridges; labrum shallowly notched; frons higher than wide, extending ca. 3/4 epicranial notch; six stemmata arranged in C-shaped configuration. Chaetotaxy (Fig. 6A): A-group trisetose; L-group unisetose; P group bisetose; MD trisetose; C group bisetose; F group unisetose; AF group bisetose; S group trisetose; SS group trisetose. A1, A3, P1 and S2 about equal in length, longest setae on head; C1, C2, F1, A2, AF2, L1 intermediate in length; AF1 absent; MD1–3 very reduced and aligned with each other. Antenna two-segmented. Mandibles broad with four teeth, and one seta on the outer surface; labium broad, with two-segmented palpus, the distal segment minute; spinneret parallel-sided; maxilla prominent. Thorax and Abdomen (Figs 6B–D): Prothoracic shield and anal fig slightly marked by irregularly shaped, small light-brown blots. Thoracic legs also scarcely pigmented. Prolegs on A3-A6 and A10 of equal size; crochets in a semicircle, uniserial and uniordinal. Thorax chaetotaxy: T1 with D group bisetose, both located on dorsal shield, D1 shorter than D2; XD group bisetose, similar in length and both on the dorsal shield; SD bisetose, laterally on the dorsal shield; L group bisetose, L1 longer than L2; SV group bisetose, posteroventral to L2, SV1 slightly longer than SV2; V group unisetose. T2 and T3 with D and SD groups bisetose, median-transversely aligned; D2 and SD1 similar in length, and longer than D1 and SD2 respectively; L trisetose, L3 posteriorly, similar in length to L1; SV unisetose; V unisetose. Abdomen chaetotaxy: D group bisetose; A1–9 with D2 slightly longer than D1, and A10 with D1 longer than D2; SD group bisetose, A1–7 with SD1 slightly longer than SD2 and A10 with SD2 longer than SD1, SD2 absent in A9; A1–8 with L group trisetose, L1 longer than L2, L1 and L2 absent in A9; A1–8 with SV group trisetose, SV3 absent in A7–9; V group unisetose.
Palaeomystella rosaemariae last larval instar: A cephalic chaetotaxy, frontal view B thoracic and abdominal chaetotaxy, lateral view C head and prothoracic shield in detail, dorsal view D body, lateral view. Scale bars = 50 µm, 1 mm, respectively.
Pupa (Figs 7A–C, 11D–F), 5.59 to 6.76 mm long (n = 3), elongate in dorsal and ventral views, slightly wider in thoracic region. Integument light amber-colored, mostly smooth, with a few scattered microsetae dorsally. Frontoclypeal suture not evident. Labrum U-shaped. Labial palpi long; antennae arched anteriorly and separate, approximate and parallel posteriorly to distal margins of maxillae, reaching apical margin of forewings; maxillae extending distally between sclerites of midlegs; femora of midleg not fused distally; femora of foreleg extending beyond widest part of labial palpi. Cremaster (Figs 11D–F) long, tubular, dorsally directed, bearing latero-apically a pair of distally conspicuous, anteriorly curved spines.
Palaeomystella rosaemariae pupa, in dorsal (A), ventral (B) and lateral (C) views, respectively. Scale bar = 1 mm.
Palaeomystella rosaemariae is known only from the type locality, the fragments of lowland Dense Umbrophilous Atlantic Forest of Coxilha das Lombas, Santo Antônio da Patrulha, RS, Brazil.
Tibouchina asperior (Cham.) Cogn. (Melastomataceae), a shrub (0.5 to 1.0 m), in humid grassland areas, endemic to Santa Catarina and Rio Grande do Sul (
(Figs 12D–F). Galls induced by Palaeomystella rosaemariae are located at distal axillary buds of the host. At the type locality, they occur in low numbers per plant. Galls are prosoplasmatic histioid (Küster, in
Named in honor of Prof. Dr. Rosy Mary dos Santos Isaias, an anatomist of the Departamento de Botânica, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, for her great contributions to the development of cecidology in the Neotropics.
http://zoobank.org/D4D1FE46-9C47-4F6B-B4D4-D981BC83F577
Closest to Palaeomystella rosaemariae, sharing with this species a pronounced palmate costa of the valve and a bladelike signa. As already mentioned, these characteristics differentiate them from all other species of Palaeomystella except Palaeomystella oligophaga. This species, however, has the forewings with R4-R5 fused and the hindwing with M1 and M2 stalked from the remnant chorda of the cell (
Adult (Figs 1E–F). Sexes similar, forewing length 7.02 to 9.23 mm (n = 8). Head: Frons pale brown; vertex with pale-brown scales tipped with brown (Fig. 1F); labial palpus pale brown, basal segments angled laterally, terminal segment slightly angled upward; antennae brown; proboscis yellowish brown. Thorax: Tegula and mesonotum (Fig. 1F) with brown scales tipped with dark brown, posterior scales paler brown; fore and midlegs dark brown; hindlegs pale brown, tibia and tarsus with intermixed dark-brown scales. Forewings (Figs 1E, 8A): lanceolate, with 13 veins; L/W index ~ 4.4; dorsally covered with brown scales, intermixed with dark-brown scales tipped with black, and pale-brown scales; a narrow, ill-defined, dark-brown streak bisects the wing longitudinally from base to a brown, subapical, crescentic marking, edged distally with dark-gray scales; 3 raised scale tufts located posterior to cubitus, in anal area, in line with midcell, and near tornal area, respectively; fringes pale brown; ventral side most uniformly covered with dark-brown scales; discal cell closed, ~ 0.7× length of forewing; ending near 1/5 wing margin; Sc ending ca. 1/3 anterior margin; R 5-branched; R1 ending near 1/4 of wing margin; R4 and R5 stalked ca. 1/2 distance from cell apex; M 3-branched; CuA 2-branched; CuP weak proximally and not stalked, with 1A+2A that is well developed, extending more than half length of posterior margin. Hindwing (Figs 1E, 8A): strongly lanceolate, with 9 veins; L/W index ~ 5.4, ~ 0.84 forewing in length; scales light brown on both sides; fringes pale brown; frenulum a single acanthus on male, with two parallel-sided acanthi in female. Sc+R1 ending ca. 1/2 anterior margin; Rs ending near end of anterior margin; M 3-branched, with M1 and M2 stalked near Rs; CuA 2-branched; CuP weakly sclerotized, ending at 1/2 posterior margin; 1A+2A well developed, ending near basis of posterior margin. Abdomen (not illustrated): scales pale brown intermixed with gray scales, with transverse irregular rows of spiniform setae on terga 2–7 in both sexes. Eighth sternum (Fig. 8C) expanded anteromedially into a stout, rounded lobe, associated with a subtrapezoidal sternite.
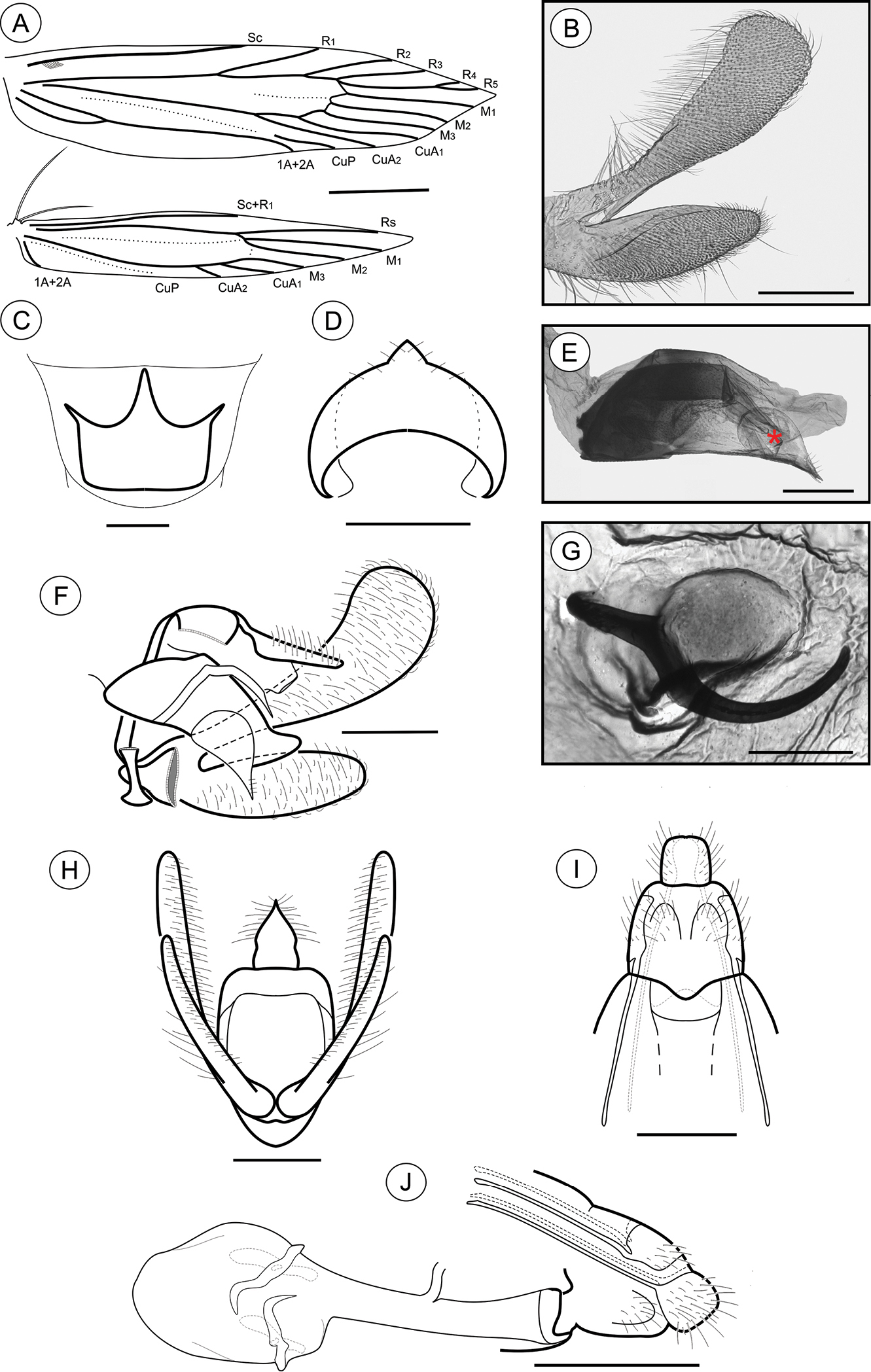
Male genitalia (Figs 8B, D–F, H). Uncus narrow, separated from tegumen by a narrow membranous area, laterally setose (Figs 8F, H); tegumen narrow; vinculum widened ventrally; transtilla a short, flat fig; aedeagus tubiform, curved ventrally, short (2× longer than wide), slightly wider basally (Figs 8E–F); vesica bearing several stout cornuti; juxta (Fig. 8D) attached to distal portion of aedeagus (Fig. 8E), as long as wide, with convex basal margin and pointed distally; valva (Fig. 8B) covered by several long setae, divided near 1/3 from the basis, with sacculus spatulate, tapering distad, and costa long, palmate, gradually constricted basad.
Palaeomystella tavaresi adult morphology: A wings B male valva, mesolateral view C male eighth sternum, ventral view D juxta, ventral; E aedeagus, lateral view (asterisk indicates attached juxta-lobes) F male genitalia, lateral view G signum, internal view H male genitalia, ventral view (transtilla, aedeagus and juxta not illustrated) I female genitalia, ventral view (corpus bursae not illustrated) H female genitalia, lateral view. Scale bars = 1 mm; 200, 250, 50, 200, 100, 200, 200 and 250 µm; 0.5 mm, respectively.
Female genitalia (Figs 8G, I–J). Papillae anales connected dorsally, setose (Figs 8I–J); anterior apophyses similar in length to posterior, slightly curved apophyses; sterigma divided into a bandlike tergum and a distally bilobed sternum, deeply and narrowly emarginate medially; ostium bursae of small size, wider than long; ductus bursae membranous, longer than corpus bursae, with ductus seminalis inserted medially; corpus bursae an elongate sac, bearing two stout, straight, bladelike signa connected to crescentic figs located in the wall (Figs 8G, J).
Holotype ♂: Brazil: Reserva Serra Bonita, 15°23'30"S, 39°33'57"W, 832 m, Camacan, BA, Brazil. Adults preserved dried and pinned, reared from galls induced on Tibouchina fissinervia (Schrank & Mart. ex DC.) Cogn. (Melastomataceae) by G.R.P. Moreira, 15–21.X.2013, LMCI 230-05, donated to DZUP (29.415). Paratypes: same data, 17–23.II.2013, LMCI 209; 1♂ (LMCI 209-31), 1♀ (LMCI 230-20), donated to DZUP (29.416 and 29.417, respectively); 1♂ (LMCI 230-06), 2♀ (LMCI 230-09 and 22) donated to VOB.
Adults dried and pinned, collected in light traps at the type locality, deposited in VOB: 1♂ (VOB 144730), -.VIII.2009, by F.L. Santos; 1♂ (VOB 146783, with genitalia mounted on slide), -.IX.2010, by V.O. Becker. Additional specimens, with the same collection data as the type material, deposited in LMCI: adults dried and pinned, 6♂ (LMCI 230-07, 15, 16, 17 and 21; LMCI 230-08, with genitalia in glycerin GRPM 50-57) and 6♀ (LMCI 230-10, 11, 12, 18 and 19; LMCI 230-23, with genitalia in glycerin GRPM 50-58). Slide preparations, mounted in Canada balsam: adults, 1♂ (GRPM 50-54), 1♀ (GRPM 50-55); wings, 1♂ (GRPM 50-53); larvae, 2 last instars (GRPM 50-56). Immature stages, fixed in Kahle-Dietrich’s fluid and preserved in 70% EtOH: 5 last-instar larvae (LCMI 209-13 and 14, and 230-2); 6 pupae (LMCI 209-7, 11, 18, and 230-1); 12 dissected galls (LMCI 209-21 and 22, 230-3 and 4). In tissue collection, 6 larvae (LMCI 209-06) fixed and preserved in 100% EtOH, at -20°C.
Last larval instar (Fig. 6), 7.28 to 11.7 mm (n = 4). Cecidogenous, endophyllous, semiprognathous and tissue-feeder. Body subcylindrical, creamy white, changing to light yellow before pupation, with setae well developed. Head (Figs 9A, C–D): uniform dark brown, with two conspicuous unpigmented, irregularly shaped areas along ecdysial line; smooth, with shallow ridges; labrum shallowly notched; frons higher than wide, extending ca. 3/4 epicranial notch; six stemmata arranged in C-shaped configuration. Chaetotaxy (Fig. 9A): A-group trisetose; L-group unisetose; P-group bisetose; MD trisetose; C-group bisetose; F-group unisetose; AF-group bisetose; S-group trisetose; SS-group trisetose. A1, A3, P1 and S2 about equal in length, longest setae on head; C1, C2, F1, A2, AF2, L1 intermediate in length; AF1 absent; MD1–3 very reduced and aligned with each other. Antenna two-segmented. Mandibles broad, with four teeth and one seta on the outer surface; labium broad, with two-segmented palpus, the distal segment minute; spinneret parallel-sided; maxilla prominent. Thorax and Abdomen (Figs 9B–D): Prothoracic shield and anal fig irregularly marked with dark brown. Thoracic legs light brown. Prolegs on A3–A6 and A10 of equal size; crochets in a circle, uniserial and uniordinal. Thorax chaetotaxy: T1 with D-group bisetose, both located on the dorsal shield, D1 shorter than D2; XD-group bisetose, setae similar in length and both located on the dorsal shield; SD bisetose, located laterally on the dorsal shield; L group bisetose, L1 longer than L2; SV-group bisetose, posteroventral to L2, SV1 slightly longer than SV2; V-group unisetose. T2 and T3 with D- and SD-groups bisetose, median-transversely aligned; D2 and SD1 similar in length, and longer than D1 and SD2 respectively; L trisetose, L3 posterior to L1 andL2, similar in length to L1; SV unisetose; V unisetose. Abdomen chaetotaxy: D-group bisetose; A1–9 with D2 slightly longer than D1, and A10 with D1 longer than D2; SD-group bisetose, A1–7 with SD1 slightly longer than SD2, A10 with SD2 longer than SD1, SD2 absent in A9; A1–8 with L-group bisetose, L1 longer than L2, L2 absent in A9; A1–8 with SV-group trisetose, SV3 absent in A7–9; V-group unisetose.
Palaeomystella tavaresi last larval instar: A cephalic chaetotaxy, frontal view B thoracic and abdominal chaetotaxy, lateral view C head and prothoracic shield in detail, dorsal view D body, lateral view. Scale bars = 50 µm, 1 mm, respectively.
Pupa (Figs 10A–C, 11G–I), 6.37 to 8.84 mm (n = 5), elongate-oval in dorsal and ventral views, widest in the thoracic region. Integument light amber-colored, mostly smooth, with a few scattered microsetae dorsally. Frontoclypeal suture not evident. Labrum U-shaped, weakly defined. Labial palpi long; antennae arched anteriorly and separate, approximate and parallel posteriorly to distal margins of maxillae, surpassing apical margin of forewings; maxillae extending distally between sclerites of midlegs; femora of midleg not fused distally; femora of foreleg extending beyond widest part of labial palpi. Cremaster (Figs 11G–I) short, slightly bifurcated and posteriorly directed, bearing latero-apical pair of blunt spines.
Palaeomystella tavaresi pupa, in dorsal (A), ventral (B) and lateral (C) views, respectively. Scale bar = 1 mm.
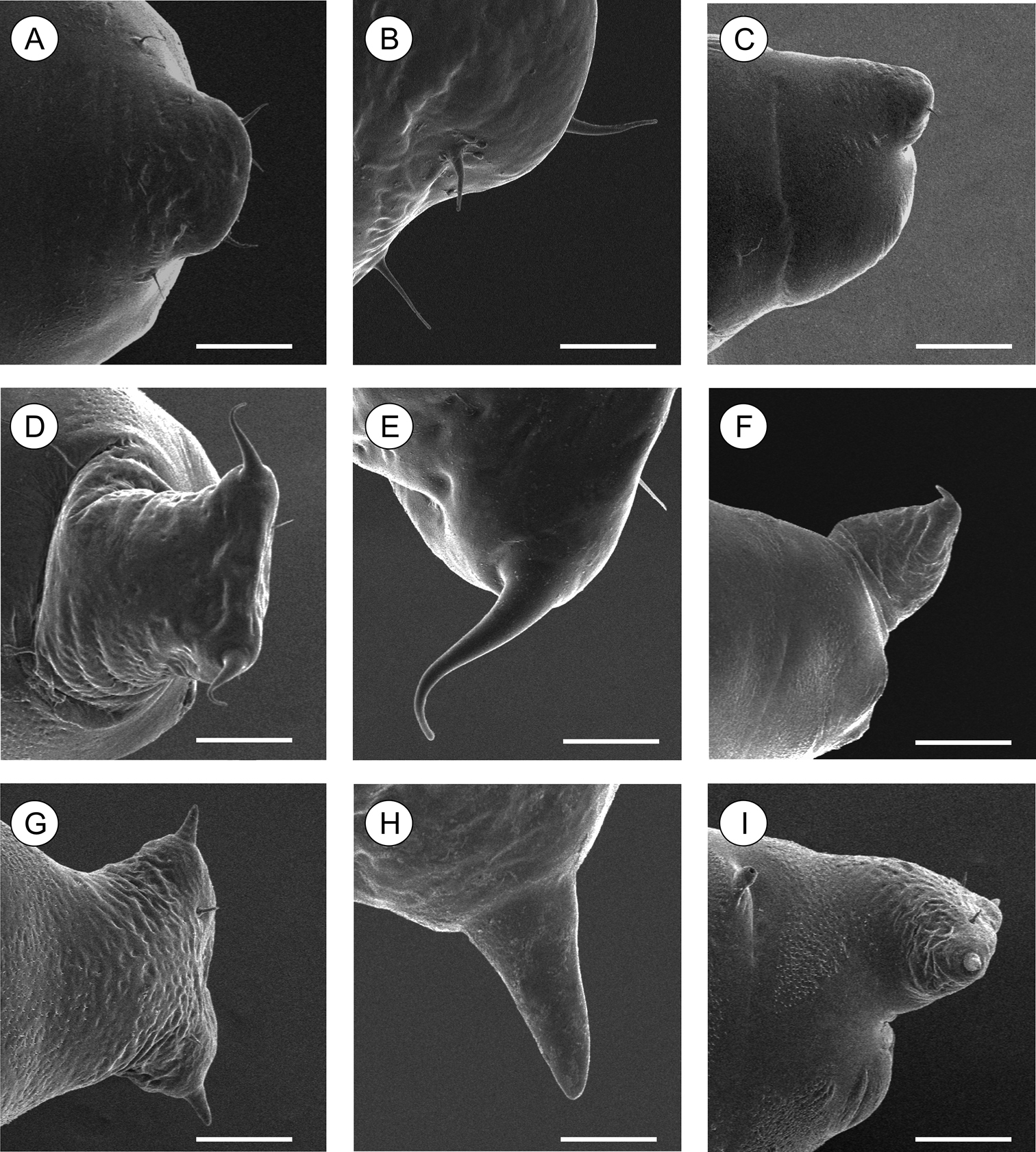
Scanning electron micrographs of Palaeomystella species pupal cremaster, in dorsal view (left column), apical process in detail (central column) and lateral view (right column): A–B Palaeomystella fernandesi C–D Palaeomystella rosaemariae; E–F Palaeomystella tavaresi. Scale bars = 100, 20, 200, 100, 20, 200, 100, 20, 200 µm, respectively.
Palaeomystella tavaresi is known only from the type locality, in preserved fragments of the Atlantic Rain Forest at the Serra Bonita Reserve, Camacan, Bahia, Brazil.
Tibouchina fissinervia (Schrank & Mart. ex DC.) Cogn.(Melastomataceae), a pioneer tree species that grows up to 20 m tall in the Atlantic Rain Forest, where it is endemic, ranging from Bahia to São Paulo (
(Figs 12G–I). Gall prosoplasmatic histioid (Küster, in
Galls induced by Palaeomystella species: A–C Palaeomystella fernandesi D–F Palaeomystella rosaemariae G–I Palaeomystella tavaresi A on Tibouchina sellowiana, general view B operculum made by last-instar larva on gall surface before pupation; C pupal cocoon in a dissected gall (arrow indicates the operculum shown in B) D on Tibouchina asperior, general view E exit hole made by last larval instar on gall surface F pupal cocoon constructed between two leaves, uncovered by pulling them apart (direction indicated by arrows) G on Tibouchina fissinervia, general view H longitudinally dissected gall, showing gall chamber (arrow indicates position of exit orifice on cocoon) I internal chamber in detail, showing the exit orifice on cocoon (asterisk). Scale bars = 10, 2, 4, 10, 5, 4, 10, 10, 2 mm, respectively.
Palaeomystella tavaresi is named in memory of the Jesuit priest Joaquim da Silva Tavares, a Portuguese naturalist and a pioneer in the study and description of Brazilian cecidology (
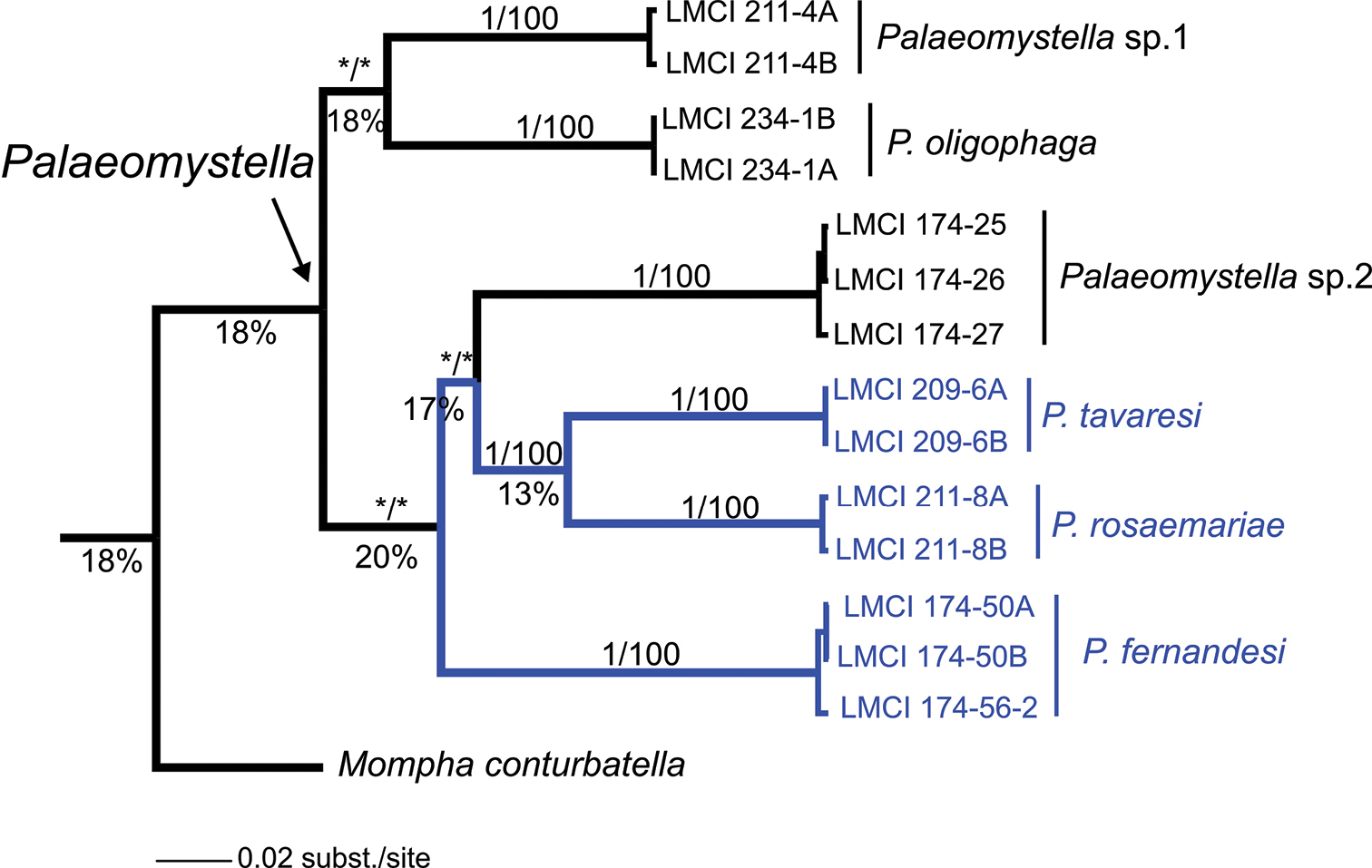
A total of 660 nucleotide sites were analyzed for species of Palaeomystella from different host plants, and 211 (32%) of these were variable. According to the phylogenetic hypothesis proposed here, all species were recovered as monophyletic lineages within the Palaeomystella group of Momphidae, in both methods of inference (BI and ML) with full branch support (Fig. 13). Regarding internal relationships, Palaeomystella rosaemariae was placed as a sister of Palaeomystella tavaresi with strong posterior probability (= 1) and bootstrap (=100). Palaeomystella fernandesi was more distantly related, although with low branch support (< 0.8, posterior probability; < 70, bootstrap). Despite the strong internal statistical branch support of the three new lineages of momphids, the external relationships for Palaeomystella were poorly resolved (i.e., position of clades), and even the monophyly of the genus lacks statistical support. Mompha was used to root the tree, but its position as a sister clade of Palaeomystella was not supported (Fig. 13). The evolutionary divergence observed between comparisons of pairs of species was markedly high, showing greater genetic variation in this group of momphids (Table 2), particularly between clades (Fig. 13). An average of 18% (± 3%) of K2P differences was found between species of Palaeomystella, ranging from 14 (± 2%) to 24% (± 3%). The maximum divergence observed among clades was 20%, found between Palaeomystella fernandesi and the clade formed by Palaeomystella rosaemariae + Palaeomystella tavaresi + Palaeomystella sp. 1 (Fig. 13). The genetic divergence within Palaeomystella (ca. 18%) was greater than between this genus and Mompha (16%).
Bayesian inference tree for Palaeomystella, based on 660 bp of the mitochondrial cytochrome oxidase c subunit I gene (CO-I). Numbers above branches indicate support values > 0.8/60 for Bayesian Posterior Probability (BPP)/Bootstrap - for Maximum Likelihood (ML) (see Material and methods); those located below represent the percentage of evolutionary divergence between clades. Asterisk indicates support < 0.80/60 for BPP and ML, respectively.
Estimates of evolutionary divergence between sequences based on 660 base pairs of the cytochrome oxidase c subunit I (CO-I) gene using the Kimura 2-parameter model. Mean number (± standard error) of base substitutions per site over all sequence pairs between groups, obtained by a bootstrap procedure of 1000 replicates is shown. The analysis involved the three new Palaeomystella species described in this study (marked in bold), two undescribed taxa (sp. 1 and sp. 2), one currently recognized taxa (Palaeomystella oligophaga) and the outgroup (Mompha).
| 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|
| 1. Mompha conturbatella | ||||||
| 2. Palaeomystella sp. 1 | 0.18±0.02 | |||||
| 3. Palaeomystella sp. 2 | 0.20±0.02 | 0.23±0.03 | ||||
| 4. Palaeomystella fernandesi | 0.21±0.02 | 0.23±0.02 | 0.24±0.03 | |||
| 5. Palaeomystella tavaresi | 0.18±0.02 | 0.22±0.03 | 0.20±0.03 | 0.22±0.03 | ||
| 6. Palaeomystella rosaemariae | 0.16±0.02 | 0.24±0.03 | 0.20±0.03 | 0.21±0.03 | 0.14±0.02 | |
| 7. Palaeomystella oligophaga | 0.14±0.02 | 0.18±0.02 | 0.25±0.03 | 0.17±0.02 | 0.19±0.02 | 0.16±0.02 |
As pointed out by
The genetic variability found herein at the generic level (maximum distance among Palaeomystella clades = 20%; average among species = 18%), by using a few putative species, strongly suggests that there are several gaps in diversity in the analysis. This pattern results in part from low collection efforts and the small number of taxonomic studies on this lineage in the area. Alternatively, the result could be associated with the single marker used. Although loci used in DNA barcoding are not the most suitable for making broad inferences on phylogenetic relationships (
As mentioned above, the diversity of moth-induced melastome galls is in fact much greater that presented here (e.g.
This study illustrates further variation in gall morphotypes. Such variation has long been known to exist among Melastomataceae galls (
The present results also demonstrate the existence of considerable variation in life-history styles for the pupal stage of Palaeomystella species, which should be taken into account in future studies. That is, last-instar larvae may remain endophylous until pupation in either sessile (Palaeomystella tavaresi) or dehiscent (Palaeomystella fernandesi) galls, or may leave them to pupate in leaf litter (Palaeomystella rosaemariae). Although varying little in the general integumentary morphology, their pupae show considerable variation in the size and shape of the cremaster which may provide useful characters for future species identifications. Unfortunately, the other known species of Palaeomystella (
Thanks are due to the staff members of the Reserva Serra Bonita, Instituto de Meio Ambiente / PUC-RS (PROMATA) and Antônio Malta (Coxilha das Lombas), for allowing the authors to carry out this study in areas under their care, and for providing assistance with fieldwork. Thanks are also owed to Eduardo Emery (CNPq), for sending samples of Palaeomystella oligophaga used in DNA extraction. Sergio Bordignon (UniLaSalle) kindly assisted in fieldwork at Coxilha das Lombas, and Denis S. Silva (UFRGS) edited some of the figs. Juliana G. Freitas (UEFS) and Sergio Bordignon identified the plants from Bahia and Rio Grande do Sul, respectively. Hector Vargas (UTA), Lucas Kaminski (UNICAMP) and Sandra Hartz (UFRGS) read critically the first version of the manuscript. The final version of the manuscript was substantially improved by comments made by Erik van Nieukerken and Sjaak Koster (Naturalis Biodoversity Center) and Kenji Nishida (Universidad de Costa Rica). The authors are also grateful to the staff members of CME/UFRGS and to Thales O. Freitas (UFRGS) for the use of facilities and assistance with scanning electron microscopy and molecular analyses, respectively. Thanks are also due Janet W. Reid for editing the text. This study was financially supported in part by CNPq (Project numbers 309676/2011-8 and 156153/2011-4, granted to G. R. P. Moreira and G. L. Gonçalves, respectively). F. A. Luz was supported by a CNPq Master’s Program Fellowship.