Citation: Ruch J, Riehl T, Michalik P (2014) Re-description of Xysticus bimaculatus L. Koch, 1867 (Araneae, Thomisidae) and characterization of its subsocial lifestyle. ZooKeys 427: 1–19. doi: 10.3897/zookeys.427.7450
Spiders have become an important model to study the evolution of sociality, but a lack of their detailed natural history and taxonomy hinders broader comparative studies. Group-living crab spiders (Thomisidae) provide an excellent contrast to other social spiders since they lack a communal capture web, which was thought to be a critical factor in the evolution of sociality. Only three non-webbuilding crab-spider species are known to be subsocial or social, all of which belong to the genus Diaea Thorell, 1869. The aim of this study is to describe the social lifestyle of Xysticus bimaculatus L. Koch, 1867 for the first time. Furthermore, we present a detailed re-description of this species and discuss its taxonomic implications. Like other subsocial crab spiders, X. bimaculatus builds nests from tree leaves. Nests contain up to 38 spiders and sometimes several adult females, indicating the species may be at a transitory stage between subsociality and permanent sociality.
Social spider, cooperation, female care, micro-CT, palp, taxonomy
The evolution of sociality is puzzling and determining factors that promote the transition towards a social lifestyle is a major challenge in evolutionary biology. Animals living in social groups benefit from cooperation in foraging, brood care and protection from predators (
The generally accepted hypothesis is that sociality in spiders evolved via the ‘subsocial route’, meaning that permanent sociality derived from ancestors with extended maternal care (
To date, all subsocial and social crab spiders are described in the genus Diaea Thorell, 1869 (
We have recently identified another case of subsociality in crab spiders: Xysticus bimaculatus L. Koch, 1867. The discovery of social behavior in a species outside the Diaea genus suggests a possible independent evolutionary event and thus the potential to identify common drivers in the evolution of sociality in spiders. Here, we describe the natural history and subsocial lifestyle for the first time (
We initially discovered nests inhabited by several individuals of Xysticus bimaculatus L. Koch, 1867 in July 2011 on trees along the Enoggera Reservoir, Queensland, Australia (27°26'27.69"S, 152°55'29.03"E). We later surveyed spider nests in November 2011, April 2012 and November 2012 (N = 166) at four locations around Brisbane (Brisbane Forest Park, Toohey Forest, Mt Coot-tha, Mt Tibrogargan). During these surveys, we measured the nests and identified the trees these were built in. We determined the group composition (number, developmental stage and sex) of spiders inhabiting the nests. We used these data to pinpoint the dispersal stage of spiderlings, which is an indicator of the degree of sociality (
For the species re-description, specimens were compared with collection material located at the Australian Museum, Sydney, the Queensland Museum, Brisbane and the Zoological Museum Hamburg and included species from the genera Cymbacha, Diaea, Tharpyna and Xysticus (see Suppl. material 1 (material examined), type Xysticus bimaculatus see Figure 1). The description of the seta pattern was performed using the format described by
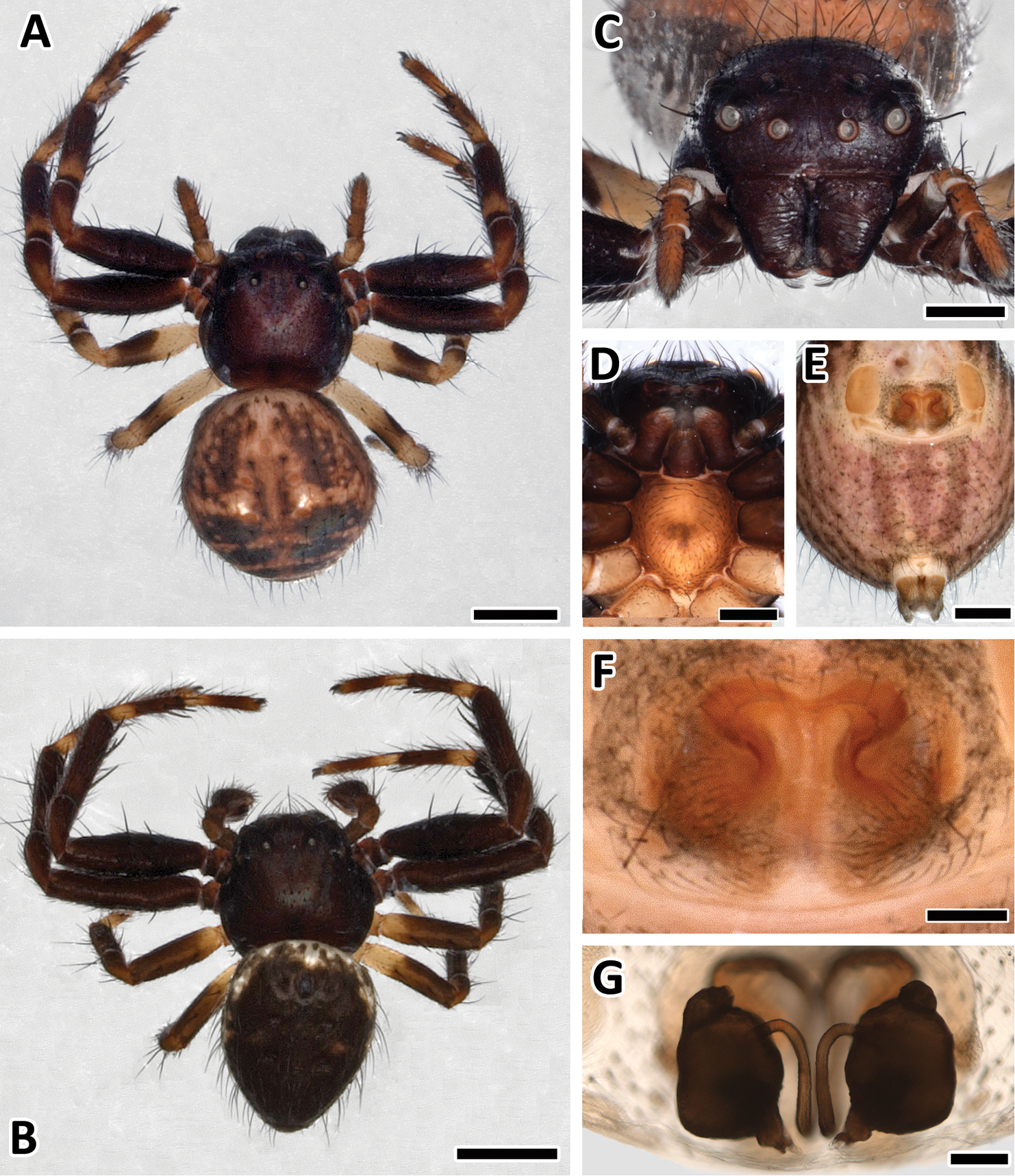
Female holotype of Xysticus bimaculatus, (MG 2260, now ZMH). A Habitus, scale bar 1 mm B Ventral, scale bar = 0.5 mm C Epigyne, scale bar = 0.25 mm.
Since the type locality has not been accurately specified in the original description, the species was re-described from specimens collected in the Enoggera Reservoir in April 2012. Specimens were stored in 70% EtOH and examined using a Zeiss Discovery V20 stereo microscope and imaged with a Zeiss MCr camera and a Leica M205A with a Leica 290 camera as well as with a Keyence Digital Microscope VHX-500 F. The images were edited and plates arranged using Adobe Photoshop CS4.
Female copulatory organs were dissected and macerated using pancreatin (
The left male palpus (sperm transfer organ) was stained with a 1.0% iodine solution overnight and critical point dried for the micro-tomographic analyses. The dry palp was mounted onto an insect pin and scanned with an Xradia MicroXCT-200 X-ray imaging system (Carl Zeiss X-ray Microscopy Inc., Pleasanton, USA) at 30 kV and 6 W (20.0 scintillator-objective lens unit, 6 seconds exposure time, 1.18 µm pixel size). The data were processed using the 3D analysis software AMIRA v5.4.2 (Visage Imaging, Berlin, Germany). Selected parts of the palp were reconstructed by delineation of the contours in each section and surfaces were computed using the surface editor.
Statistical analyses on spider group composition were performed using JMP 9.0 (SAS Institute Inc., USA). Figures were prepared with R version 2.15.3 (R Development Core Team 2013). Continuous data were tested for normal distribution (Shapiro-Wilk-Test) as well as for equal variance. Since data were not normally distributed we used non-parametric tests. Descriptive statistics are given as mean ± standard error (SE).
All measurements in the description are presented in mm unless stated otherwise.
The nests of Xysticus bimaculatus L. Koch, 1867 were constructed from 7.77 ± 0.49 leaves (range = 2–48 leaves, N = 149). The inside of the nests usually consisted of older, brown leaves and spiders subsequently and repeatedly attached fresh green leaves on the outside. The most common host tree across all study sites was Blackwood (Acacia melanoxylon, 68%, Figure 2D). However, the spiders were not restricted to these trees and could also be found on other species, for example Brisbane Golden Wattle (Acacia fimbriata, 7%, Figure 2E) and Soap Trees (Alphitonia excelsa, 20%, Figure 2C).
A Male and female Xysticus bimaculatus B Spiders attach leaves with silk to construct a typical nest C Nest constructed from Alphitonia excelsa D Nest constructed from Acacia melanoxylon E Nest constructed from Acacia fimbriata. Scale bars = 1 cm.
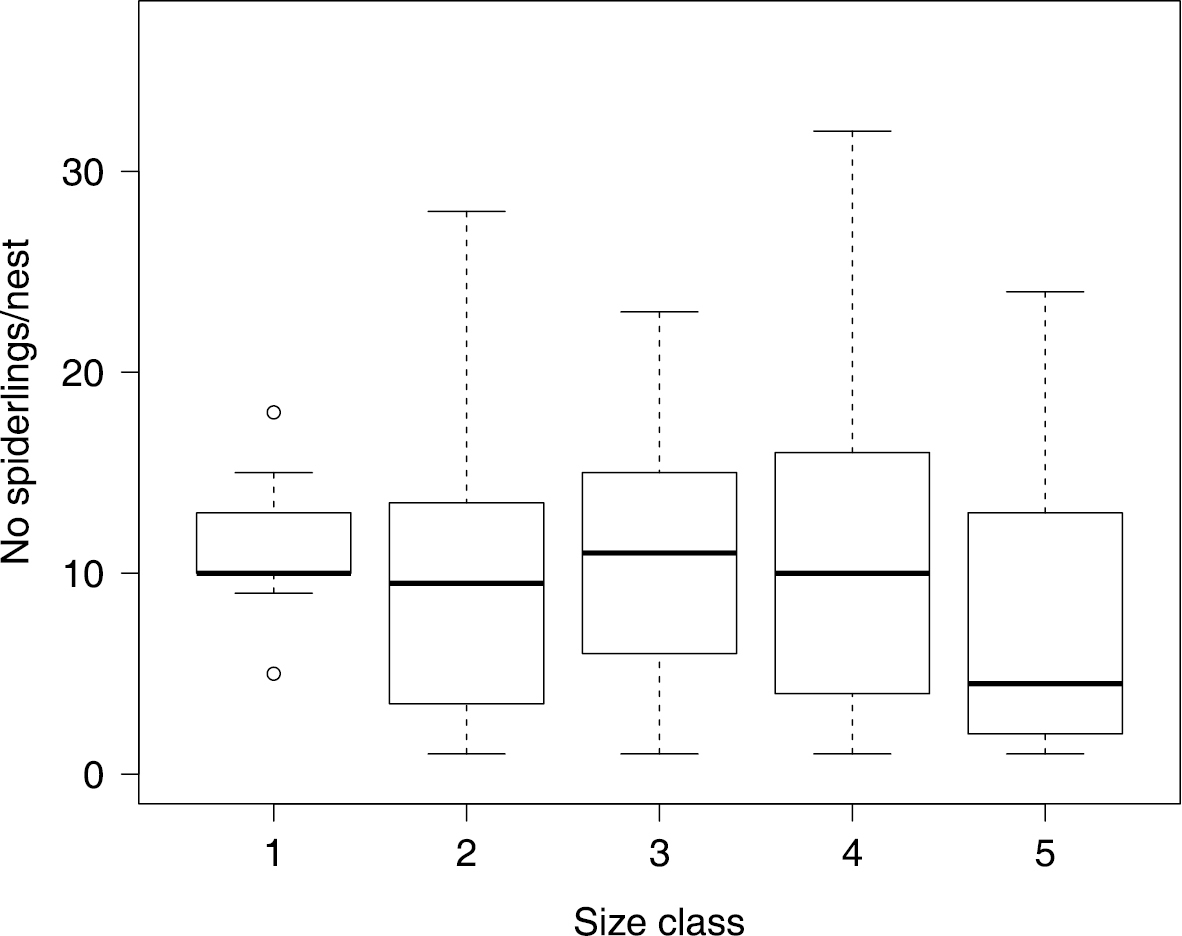
Xysticus bimaculatus has an annual life cycle. Living spiderlings were found in 120 of the 166 surveyed nests. 27 of the 166 nests were old and no longer inhabited by Xysticus bimaculatus. Adult living females were found in 71 nests. Ten of these adult females were found with an egg sac and the others with living spiderlings. On average, we found 10.5 ± 0.3 spiderlings per nest and group size ranged between one and 38 spiderlings (N = 120 nests). We found five size classes of spiderlings and all of these were found with caring adult females present in both seasons of examination (April and November). Usually, all spiderlings within a nest were of approximately the same size. We tested whether there was a certain size class after which group size decreases and found that there was no significant difference between size class (as a factor) and number of spiders inhabiting the nests (Wilcoxon Rank Sums: χ24 = 3.59, P = 0.46, N = 116, Figure 3), although the largest size class was found in smaller groups. This finding indicates that spiders disperse only shortly before maturation. While adult females were alive and present in 85.71% of nests containing small spiderlings (size class 1, Nnests = 14), the presence of an adult female significantly declined when spiderlings were larger (Pearson: χ2 = 9.8, P = 0.04, N = 116). However, the likelihood of an adult female present did not differ between size class 2 with 43.75% (Nnests = 32), size class 3 with 56% (Nnests = 25), size class 4 with 40.74% (Nnests = 27) and size class 5 with 38.89% (Nnests = 18) of the nests containing an adult living female. Subadult and adult males were exclusively found in November with a maximum of six adult males in a single nest.
Average number of spiderlings per nest depending on spiderling size class (which reflects age). We found no significant decline in group size with increasing size class, indicating that spiderlings disperse shortly before maturation. The upper and lower whiskers show 1.5 times interquartile range, the box shows median and upper and lower quartile. Individual dots indicate outliers.
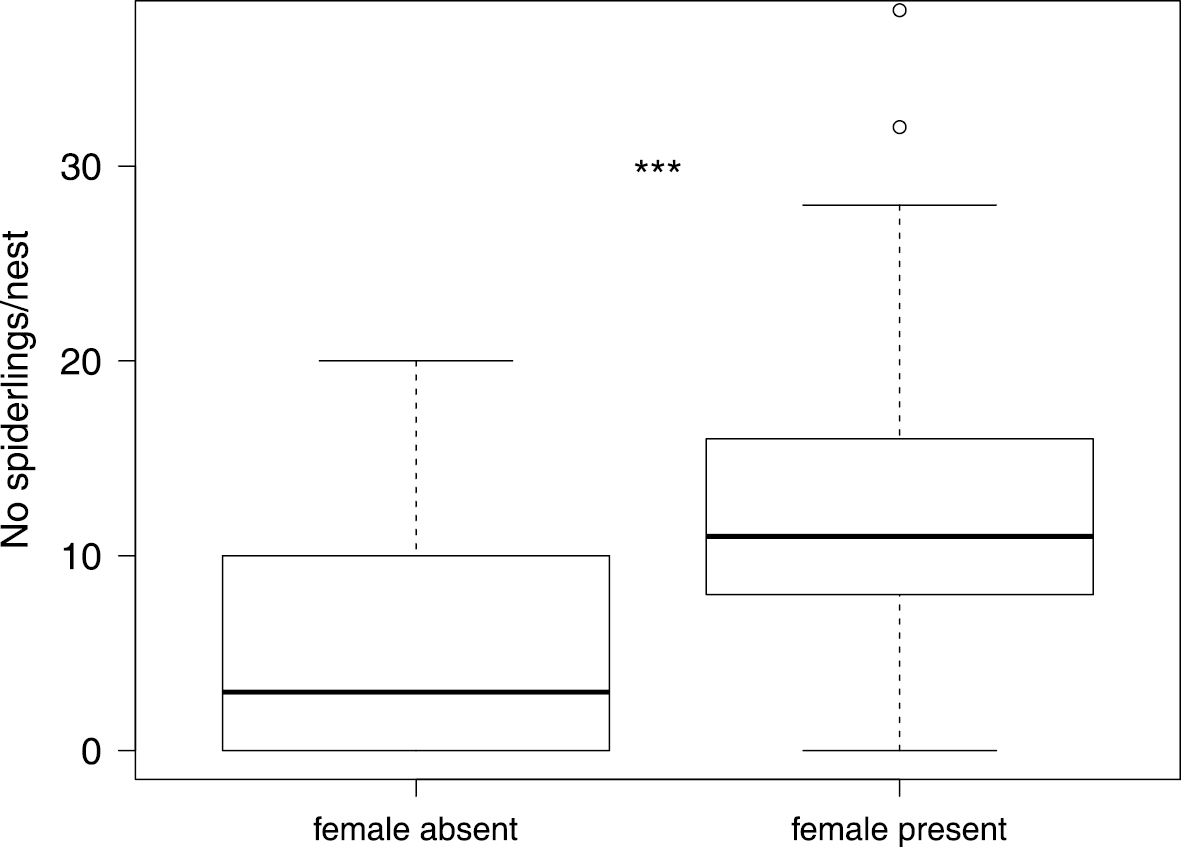
In four nests we found multiple adult females caring for a brood and in four other cases we found two distinct broods within one nest (these were excluded from the analyses of age and number of spiders). The presence of multiple adult females did not overlap with the presence of two distinct broods within one nest. Living adult females were found in April (56.57%) as well as in November (26.79%), meaning that the presence of an adult living female inside the nest was significantly more likely in April (Pearson: χ2= 12.78, P = 0.0004). The number of spiderlings per nest was significantly higher when an adult female was present (Wilcoxon: Z = -4.31, P < 0.0001, N = 120, Figure 4).
Number of spiderlings per nest is positively correlated with the presence of a caring female. The upper and lower whiskers show 1.5 times interquartile range, the box shows median and upper and lower quartile. Individual dots indicate outliers. *** P < 0.0001 indicates a statistically significant difference.
On average, nests contained 2.3 ± 0.25 prey items (N = 131 nests). Main prey types were beetles (Coleoptera, 50%) and ants (Hymenoptera, 36%). In addition, we found wasps (Hymenoptera, 2%), caterpillars (Lepidoptera, 6%) and flies (Diptera, 1%). Most abundant commensals were woolly scale insects (Hemiptera, Coccoidea, 13%) and cockroaches (Blattodea, < 5%). Potential predators present in the nest were other spiders, for example Clubionidae (4%) and Salticidae (1%).
AM Australian Museum, Sydney, Australia
MG Museum Godefroy (now Zoological Museum Hamburg)
ZMH Zoological Museum Hamburg, Germany
ALE anterior lateral eyes
AME anterior median eyes
PLE posterior lateral eyes
PME posterior median eyes
RTA retrolateral tibial apophysis
Based on paratype female KS120583 (AM).
Measurements
Body length: 4.36, carapace length: 1.83, carapace width: 1.83, carapace height: 1.21, carapace length/width ratio: 1, abdomen length: 2.53, abdomen width: 2.34, abdomen height: 2.03, abdomen length/width ratio: 1.08.
Coloration and markings
Carapace and chelicerae colored evenly black-brown. Sternum brown-yellowish with a darker outer frame. Labium and maxillae dark brown with white tips (Figure 5D).
A Female Xysticus bimaculatus (AM, KS120583), habitus, scale bar = 1 mm B Male (AM, KS120583), habitus, scale bar = 1 mm C Female (AM, KS120583), frontal view, scale bar = 0.5 mm D Female (AM, KS120583), sternum and maxillae, scale bar = 0.4 mm E Female (AM, KS120583), ventral view, scale bar = 0.5 mm F Female (AM, KS120583), epigyne, scale bar = 0.25 mm G Female (AM, KS120583), vulva, scale bar = 0.1 mm.
The first two legs (Leg I & II) black-brown with faint orange annulations. Femur of leg I and II black-brown, patella anterior orange and posterior black, tibia anterior black with orange annulation and posterior black-brown, metatarsus and tarsus anterior orange and posterior black-brown.
Leg III and IV with distinct white annulations. Femur of leg III and IV anterior white and posterior black, patella anterior white and posterior black, tibia anterior black with white annulation and posterior black, metatarsus and tarsus anterior more white than black.
Abdomen dark brownish with a dark indented cranial spot and two white spots dorsally in the middle (Figure 5A). Sides of the abdomen with black-brown vertical stripes. Ventral side of the abdomen lighter than the dorsal side with a dark brown section between epigyne and spinnerets (Figure 5E). Surroundings of the epigyne dark, spinnerets brown-yellowish.
Carapace
Carapace shape slightly convex and as long as wide.
Eyes
Lenses on order of diameter: ALE > PLE > AME > PME.
Distance between eyes: AME—AME = 0.45, ALE—ALE = 1.1, AME—ALE = 0.29, ALE—PLE = 0.29, PLE—PLE = 1.39, AME—PME = 0.33, PME—PME = 0.59, PME—ALE = 0.34, PME—PLE = 0.39.
Clypeus width 1.1, height 0.37, surface smooth. One long lateral seta (0.26) next to ALEs.
Chelicerae, maxillae and labium
Chelicerae oval and bulky, length 0.65 and width 1.09, wrinkled surface (Figure 5C). Fangs short (0.17).
Maxillae rounded, arched around labium, length 0.51. Labium shorter (0.36) than maxillae.
Sternum
Shield-shaped and convex, narrower towards leg III and IV, 0.84 long and 0.74 wide. Covered with fine setae (Figure 5D).
Legs
Legs I and II longer than legs III and IV. Surface of the legs evenly covered with setae. Leg setation: I: femur d 0-0-1, p 0-2-2-0; tibia p 0-0-1-0, v 2-2-2; metatarsus r 1(ap), v 2-2-0-2-p1; II: femur d 1-1; tibia v 0-2-0-2-2; metatarsus v 0-2-0-2-2; III: femur d 1-1; tibia v 2(ap); metatarsus p d1, v 2; IV: femur d 0-1-1-0; tibia v 2(ap); metatarsus p 2
Leg I. Fe: 1.73, Pa: 0.71, Ti: 1.15, Me: 0.90, Ta: 0.85, Total: 5.34
Leg II. Fe: 1.69, Pa: 0.77, Ti: 1.19, Me: 0.85, Ta: 0.85, Total: 5.34
Leg III. Fe: 1.21, Pa: 0.49, Ti: 0.76, Me: 0.53, Ta: 0.53, Total: 3.53
Leg IV. Fe: 1.36, Pa: 0.48, Ti: 0.84, Me: 0.59, Ta: 0.56, Total: 3.84
Leg formula: I = II > III < IV
Abdomen
Oval, covering the posterior part of the cephalothorax. Covered with evenly arranged setae. Five obvious indents.
Genitalia
Epigyne slightly wider than long (Figure 5F). Copulatory openings in upper part of epigyne medially to broad heart-shaped sclerotized central hood. Copulatory ducts curved, leading to large ovoid and bipartite spermathecae (Figure 5G).
Based on paratype male KS120583 (AM)
Measurements
Body length: 3.3, carapace length: 1.43, carapace width: 1.50, carapace height: 1.01, carapace length/width ratio: 0.95 abdomen length: 1.87, abdomen width: 1.49, abdomen height: 1.23, abdomen length/width ratio: 1.25
Coloration and markings
Carapace and chelicerae black-brown, sternum brown. Labium and maxillae dark brown with white tips. Palps dark brown.
Leg I & II black-brown with posterior annulations. Femur, patella and tibia of leg I and II black-brown, metatarsus and tarsus anterior white and posterior black-brown.
Leg III and IV with distinct white annulations. Femur and patella of leg III and IV anterior white and posterior black, tibia anterior black with white annulation and posterior black, metatarsus and tarsus anterior more white than black.
Abdomen black with a white anterior frame, an anterior dark indented spot and four median dark indented spots (Figure 5B). Sides of the abdomen black. Ventral side of the abdomen dark brown, spinnerets brown.
Carapace
Carapace slightly convex and as long as wide.
Eyes
Distance between eyes: AME—AME = 0.38, ALE—ALE = 0.90, AME—ALE = 0.29, ALE—PLE = 0.30, PLE—PLE = 0.95, AME—PME = 0.24, PME—PME = 0.46, PME—ALE = 0.27, PME—PLE = 0.31.
Clypeus width 1.14, height 0.39, surface smooth. One long lateral seta (0.31) next to ALEs.
Chelicerae, maxillae and labium
Chelicerae oval and bulky 0.41 long, 0.70 wide, wrinkled surface. Fangs 0.17 long.
Maxillae rounded, arched around labium, 0.43 long. Labium shorter (0.28) than maxillae.
Sternum
Shield-shaped and convex, narrower towards leg III and IV, covered with fine setae. 0.80 long and 0.68 wide.
Legs
Setation of legs: I: femur d 1-1, p 1-1; tibia p 1-1, r 1-1, v 2-2-2; metatarsus p 0-1-1, r 0-1-1, v 2-2; II: femur d 1-1; tibia p1-1, r 1-1, v 0-2-0-2-2; metatarsus p 2-1(ap), r 1-1(ap), v 0-r1; III: femur d 1-1; tibia p 0-1, r 1, v p1-2(ap); metatarsus p 0-2, r 0-1; IV: femur d 1-0-1; tibia r 0-1, v p1-2(ap); metatarsus r 1, v 0-0-p1-p1
Leg I. Fe: 1.47, Pa: 0.61, Ti: 1.02, Me: 0.88, Ta: 0.94, Total: 4.91
Leg II. Fe: 1.47, Pa: 0.53, Ti: 0.92, Me: 0.81, Ta: 0.76, Total: 4.49
Leg III. Fe: 1.01, Pa: 0.41, Ti: 0.56, Me: 0.49, Ta: 0.43, Total: 2.90
Leg IV. Fe: 0.99, Pa: 0.40, Ti: 0.60, Me: 0.57, Ta: 0.44, Total: 3.00
Leg formula: I > II > III < IV
Abdomen
Egg-shaped, covered with evenly arranged setae. Five obvious indents.
Genitalia
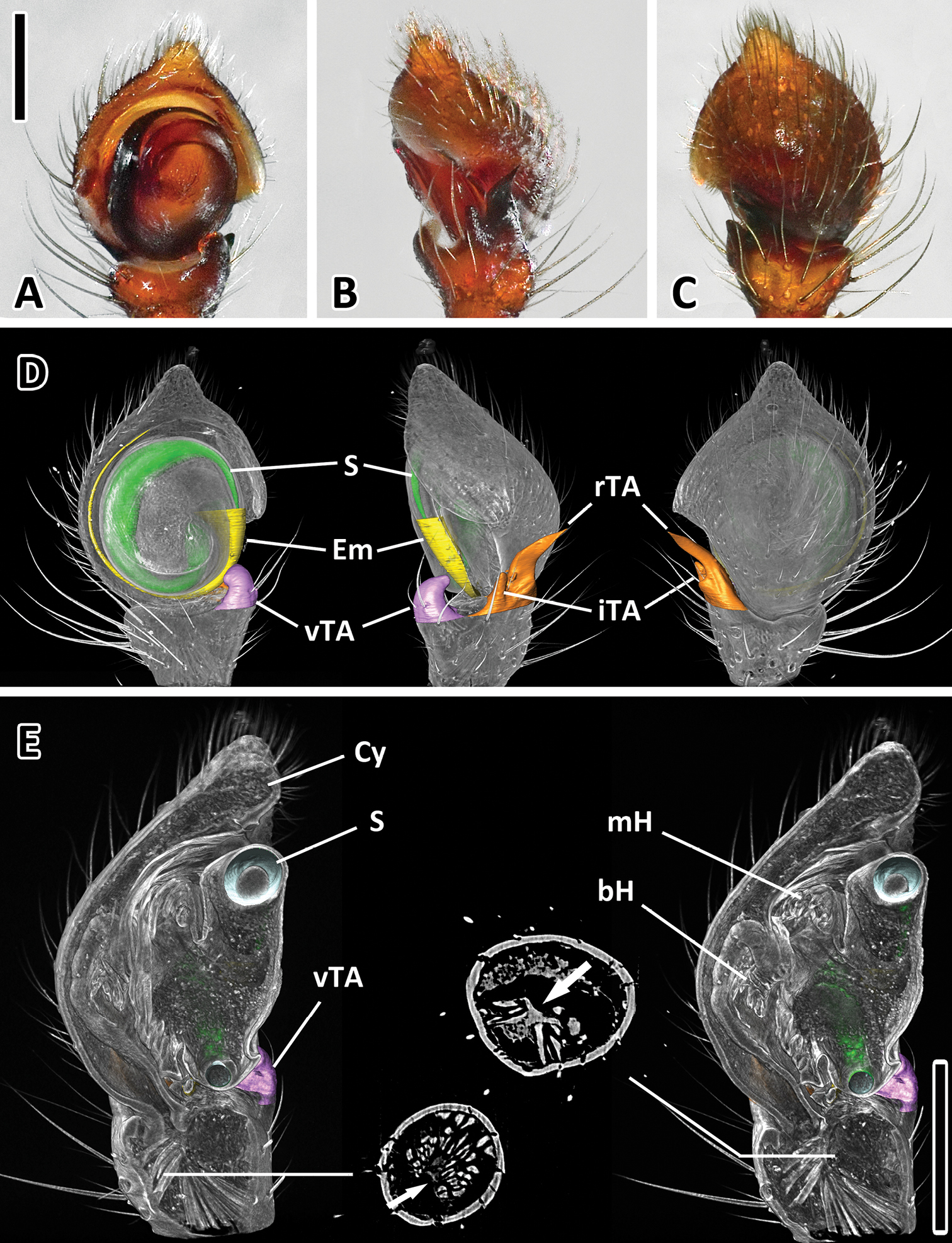
Male pedipalps small with convex cymbium (Figure 6). Embolus short. Tibial apophyses strongly sclerotized. Ventral and intermediate tibial apophyses of similar length and half the size of RTA, RTA curved towards dorsal. No bulbar muscles, well-developed basal hematodocha. Large apodeme in distal part of tibia as attachment for two tibial muscles.
Left male palp of Xysticus bimaculatus (AM, KS120583) A Ventral view B Retro lateral view C Dorsal view D Colored surface models of different parts of the male superimposed on the volume rendering of the male palp (ventral, retrolateral, dorsal) E Longitudinal sections of the volume rendered male palp showing the two prominent hematodochae. Muscles are only present in tibia and attached to a large apodeme (see arrows in cross-sections). Abbreviations: bH basal hematodocha; Cy cymbium; Em embolus; iTA intermediate tibial apophysis; mH median hematodocha; rTA retrolateral tibial apophysis; S spermophor; vTA ventral tibial apophysis. Scale bars = 0.25 mm.
Distribution
Probably widespread in sclerophyll forests around Brisbane, Queensland (Australia).
We report the demographics of Xysticus bimaculatus, a non-webbuilding subsocial crab spider from southern Queensland. Its lifestyle appears to be very similar to the subsocial crab spider Diaea ergandros (Evans, 1995). Like in other subsocial crab spiders, the presence of a caring female seems to be important for offspring survival in Xysticus bimaculatus. We found higher numbers of spiderlings in nests with a caring adult female present and a similar pattern was found in Diaea ergandros (Unglaub et al., 2013). The presence of an adult female is beneficial in Diaea ergandros, but also in the subsocial huntsman spider Delena cancerides, since adult spiders are able to capture prey more efficiently (
Unlike subsocial Diaea, Xysticus bimaculatus builds its nests mostly from Acacia and not from Eucalyptus leaves. This may favor the occurrence of the species in areas that are dominated by Acacia melanoxylon, which is however widely distributed and common along the Australian east coast. We only recorded those trees that were used for nest construction and did not quantify potentially available host trees, but both Acacia and Eucalyptus trees were present in all of our study sites. We never found Diaea ergandros and Xysticus bimaculatus occurring sympatrically. Diaea ergandros seems to be absent along the northern coast of New South Wales and southern coast of Queensland (
Similar to Diaea ergandros, nests of Xysticus bimaculatus serve as foraging areas and major prey types are beetles (Coleoptera), but also wasps and ants (Hymenoptera) (
We found that nests contain on average 10 spiderlings in Xysticus bimaculatus, which is fewer than in Diaea ergandros, where nests contain on average 45 inhabitants (
The taxonomy of Thomisidae is challenging and a revision of most genera is needed (
Although crab spiders have a worldwide distribution (
We would like to thank Robert Whyte for helping with fieldwork and the generous offer to work with his equipment during the fieldtrips as well as Marie E. Herberstein and Melanie Gralow for further assistance with the fieldwork. Robert Raven and Robert Whyte (QM), Graham Milledge (AM), and Sabine Toussaint (ZMH) kindly gave access to the respective museum collections. We thank Pawel Szymkowiak for helpful discussions. Markus Koch and Thure Daalsgard are thanked for granting access to the Keyence microscope. JR was funded by a scholarship from Macquarie University.
List of species examined
Jasmin Ruch, Torben Riehl, Peter Michalik
Data type: specimens data
Explanation note: The table shows a list of all species examined as well as the location of the material. Sex (male, female or juvenile) and whether the material was type material (yes/no) is shown as well.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.