Citation: Darbyshire T (2014) Intertidal and nearshore Nereididae (Annelida) of the Falkland Islands, southwestern Atlantic, including a new species of Gymnonereis. ZooKeys 427: 75–108. doi: 10.3897/zookeys.427.7296
The intertidal and nearshore Nereididae of the Falkland Islands are detailed and a new species of Gymnonereis described. The new species, Gymnonereis tenera sp. n., is the first record of the genus for the Falkland Islands. It is, so far, only known from a few intertidal locations in fine and muddy sands. Main distinguishing characters are: jaw teeth absent (in adults), 3 papillae in Area V–VI, falcigers absent, second ventral cirrus present throughout. Nereis atlantica McIntosh, 1885, known only from the description of a single specimen and one doubtful record from the Falkland Islands, is reviewed and transferred to Perinereis on the basis of the presence of shield-shaped bars in Area VI of the proboscis and the absence of notopodial falcigers. A key to all seven species discussed is provided.
Taxonomy, paragnaths, Polychaeta, Eunereis, Neanthes, Perinereis, Platynereis
The Nereididae is one of the largest polychaete families (
In all, eight species of nereidid, in six genera, have previously been recorded from stations listed as being within the Falkland Islands region. However, two of these species, Platynereis australis (Schmarda, 1861) and Platynereis magalhaensis Kinberg, 1865 have been controversially synonymized (e.g.
Only species that have previously been recorded from Falkland Islands samples taken in less than 30 m (where diving and shallow survey work are most likely to take place) are considered in this paper. For this reason, Nicon maculata Kinberg, 1865 is also excluded as it has not been recorded from less than 129 m in the area (
Most of the nereidids collected were found in mainly coarse or hard habitats, however, a new species of Gymnonereis Horst, 1919, a genus not previously recorded from Falkland Island waters, was identified from a small number of localities where it was almost entirely confined to intertidal, fine and muddy sands. Gymnonereis is a small genus of only six recognized species: Gymnonereis sibogae Horst, 1918 (type locality: Strait of Makassar, Indonesia), Gymnonereis crosslandi Monro, 1933 (type locality: Gorgona Island, Colombian Pacific), Gymnonereis fauveli Hartmann-Schröder, 1962 (type locality: San Julián, Argentina), Gymnonereis phuketensis Hylleberg & Nateewathana, 1988 (type locality: Andaman Sea, Thailand), Gymnonereis minyami Hutchings & Reid, 1990 (type locality: Victoria, South Australia) and Gymnonereis yurieli Hutchings & Reid, 1990 (type locality: Queensland, Australia). All members of the genus lack paragnaths, having only soft papillae on the oral ring and all, except Gymnonereis crosslandi, exhibit highly vascularized dorsal cirrophores on median chaetigers. The new species is distinguishable from the other members of the genus using combinations of characters detailing the presence or absence of jaw teeth, falcigers and enlarged dorsal cirrophores, the number and distribution of the oral ring papillae, the occurrence of accessory dorsal cirri and the relative lengths of the neuropodial lobes.
A key to the seven species of Nereididae recognized from the near shore (< 30 m) waters of the Falkland Islands is provided.
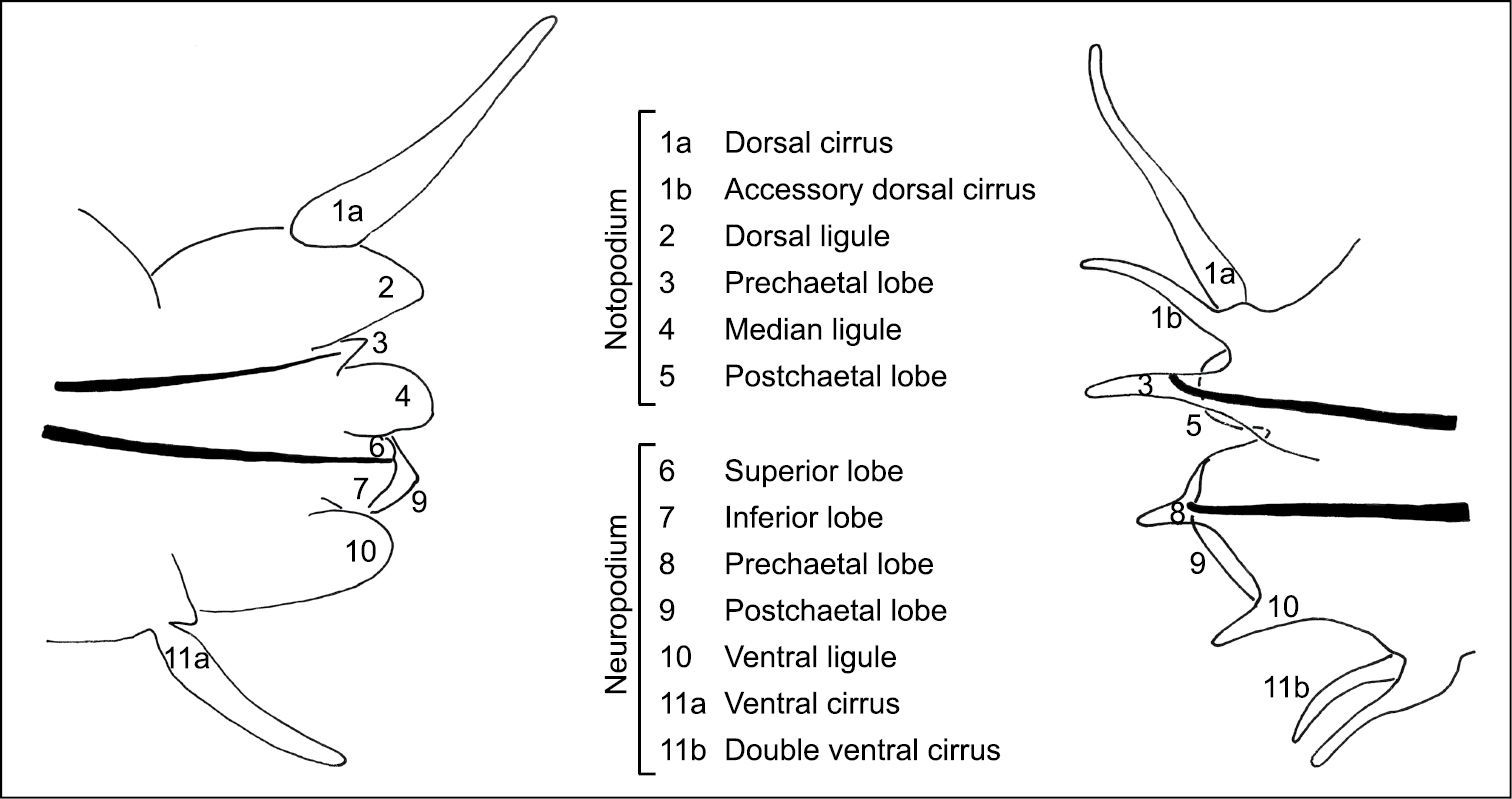
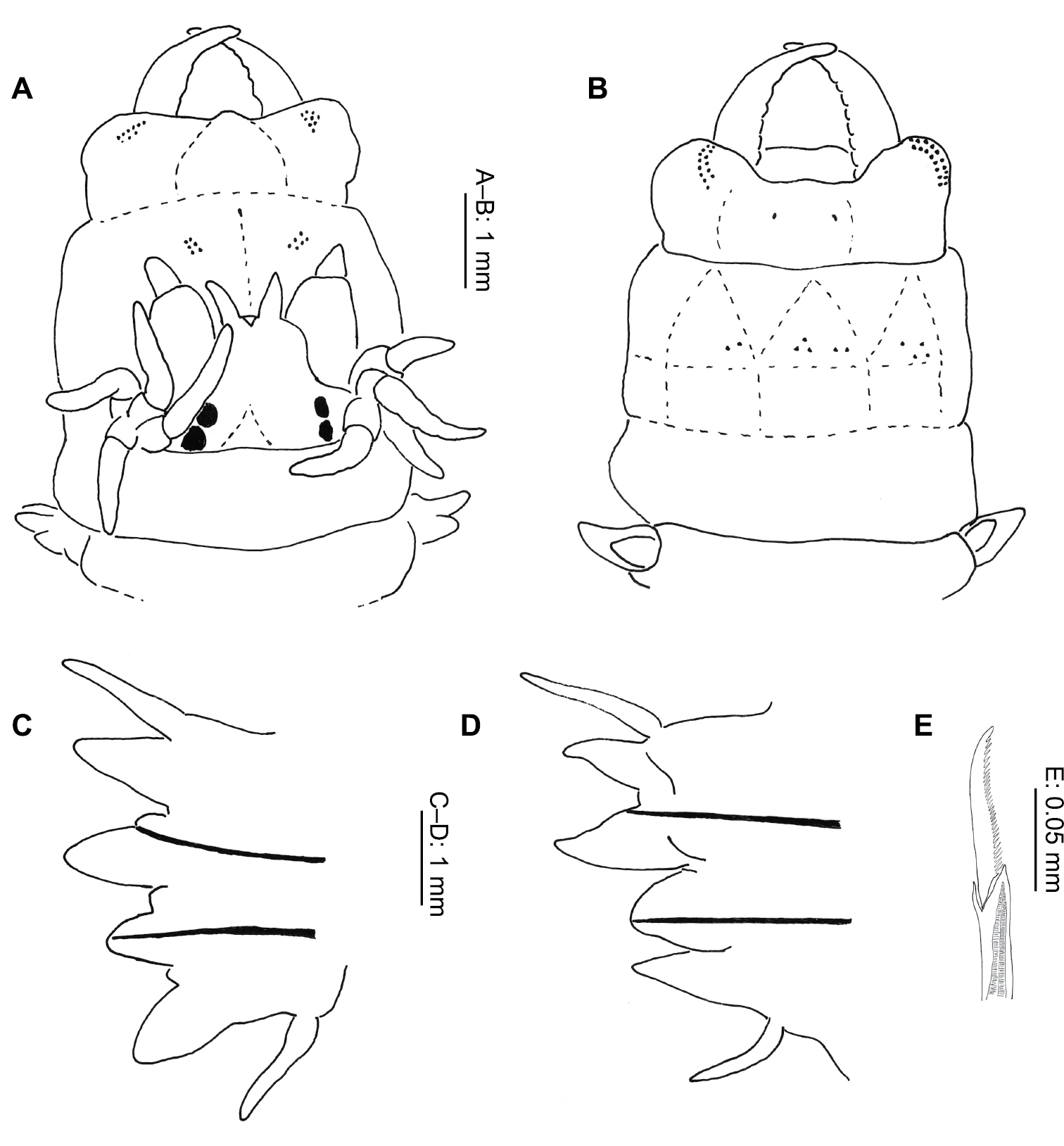
The parapodia of the Gymnonereidinae are more complex than those of the Nereidinae and a diagram is provided in Figure 1 to compare and standardize the terminology used in this paper when describing the different species. In reference to Gymnonereis, the terminology used by
Terminology and diagrammatic representation of A a Nereidinae parapodium (modified from
The terminology used to describe paragnath forms was reviewed by Bakken et al. in
In 2011 and 2013, intertidal and diving survey work was undertaken around the Falkland Islands, covering the two main islands, East and West Falkland, as well as some of the smaller outer islands. Specimens were collected by a variety of methods on the shore and by SCUBA diving. Intertidal habitats were investigated by digging and collecting specimens by hand, by sieving sediment through a 0.5 mm sieve, turning over rocks and removing attached tubes, splitting open rock crevices and by inspecting algal holdfasts. Sampling while diving involved scraping epifaunal turf off rocks, turning over rocks and removing attached tubes, and taking sediment samples that would later be sieved as above.
Specimens were relaxed with a 7% magnesium chloride solution where possible and then fixed with 4% formaldehyde in seawater. After a period of at least 2 days, animals were rinsed with freshwater and preserved in 80% industrial methylated spirits with 2% propylene glycol added.
Morphological examinations and measurements were made using a Nikon Eclipse E400 binocular microscope and a Nikon Labophot-2 compound microscope. Microscope photographs were taken using AutoMontage™ software.
The holotype and most paratypes of Gymnonereis tenera sp. n. are accessioned in the zoological collections of National Museum Wales (NMW.Z). Paratypes are also deposited in the Australian Museum (AM), Natural History Museum, London (NHMUK), National Museum of Natural History, Smithsonian Institution, Washington D.C. (USNM) and the Zoological Museum, Hamburg (ZMH). All other specimens are accessioned in the National Museum Wales collections.
Gymnonereis sibogae (Horst, 1918) by monotypy
(after
Eversible pharynx with jaws having cutting edge smooth or serrated, with papillae on the oral ring. Notopodia with accessory dorsal cirri attached to dorsal cirrophores in anterior region only, with prechaetal lobes and short, rounded postchaetal lobes. Median segments with dorsal cirrophores greatly elongated and highly vascularized (except in Gymnonereis crosslandi) and lacking accessory cirri. Dorsal transverse ridges present or absent. Chaetae homogomph or sesquigomph spinigers and homogomph or sesquigomph falcigers may be present. Chaetae very numerous in anterior chaetigers.
http://zoobank.org/66F36C23-ECF2-4F01-A2CF-BB12F84D1894
East Falkland: Teal Creek, Stn 35d (51°49.248'S, 058°55.561'W), muddy sand, midshore, holotype (NMW.Z.2011.039.0102), 09.12.2011; Sand Bay, Port Harriet, Stn 34d (51°44.231'S, 058°00.585'W), fine sand, mid–low shore, 11 paratypes (9–NMW.Z.2011.039.0093–0095; 1–USNM 1231433; 1–ZMH p-27694), 08.12.2011; Teal Creek, Stn 35b (51°49.231'S, 058°55.573'W), sandy mud, midshore, 18 paratypes (NMW.Z.2011.039.0096), 09.12.2011; Teal Creek, Stn 35c (51°49.236'S, 058°55.563'W), mud, low shore, 22 paratypes (NMW.Z.2011.039.0097–0101), 09.12.2011; West Falkland: Crooked Inlet, Roy Cove, Stn 55b (51°32.546'S, 060°20.562'W), fine sand, high-midshore, 4 paratypes (1–AM W.46477; 1–NHMUK ANEA2014.31; 2– NMW.Z.2012.082.0001), 30.01.2013; Crooked Inlet, Roy Cove, Stn 55c (51°32.595'S, 060°20.367'W), fine sand, midshore, 2 paratypes (NMW.Z.2012.082.0002), 30.01.2013; Crooked Inlet, Roy Cove, Stn 55d (51°32.664'S, 060°20.255'W), fine sand, low shore, 3 paratypes (NMW.Z.2012.082.0003–0004), 30.01.2013; Crooked Inlet, Roy Cove, Stn 55e (51°32.688'S, 060°20.244'W), fine sand, low shore, 2 paratypes (NMW.Z.2012.082.0005), 30.01.2013.
Holotype complete, 98 mm long, 1.5 mm wide (excluding parapodia; measured at widest part of anterior – approximately chaetiger 8), for 160 chaetigers. Complete paratypes 3–143 mm long, 0.15–2.53 mm wide (excluding parapodia) for 28–166 chaetigers. Description based on observations of the holotype and a dissected paratype (NMW.Z.2011.039.0098) used for illustrations. Variation shown by other paratypes described in later section.
Body depressed dorso-ventrally, widest anteriorly on chaetigers 8–10 (more pronounced in smaller specimens), then mostly uniform in width before tapering posteriorly. Colour pink/orange or grey/white in alcohol with black aciculae. Neurochaetae and subacicular notochaetae dark golden in anterior chaetigers, supracicular chaetae pale amber; all chaetae pale amber from chaetiger 14. Live animals bright red on each side of body, including the parapodia, in region of vascularized, enlarged cirrophores; rest of body often with bright white dorsal bands centrally either side of central blood vessel from end of vascularized cirrophore region, fading in posterior. Where white colouration absent, body transparent, coloured only by visible gut and blood vessels. Methyl green staining of preserved animals shows glandular areas on tips of cirri and parapodial lobes but not on cirrophores or main body. Cuticle very soft when animals alive as well as post-fixation, body breaks easily when handled.
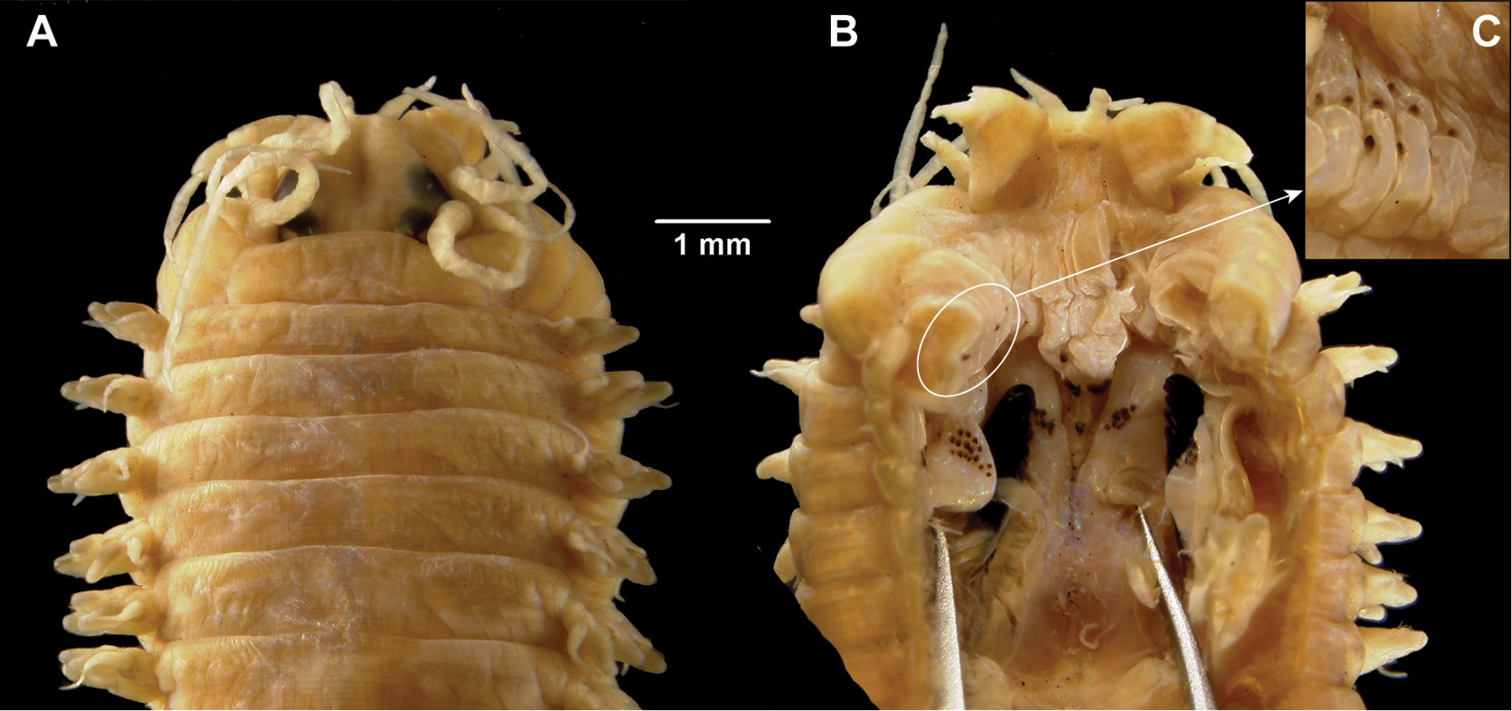
Prostomium with 2 pairs small, black (dark red when alive) eyes, often difficult to discern when preserved (Figs 2A, 9A). Anterior pair smaller, more laterally placed than posterior pair; crescent-shaped with additional small spot in far corners. Posterior pair darker, rounded. Prostomium subrectangular with deep cleft between antennae (Fig. 2A). Palps with large squat palpophores and short triangular palpostyles (0.4 mm long, 0.27 mm wide). Antennae equal length to or slightly longer than palps, more slender in form. Four pairs tentacular cirri, ventral pairs of equal length, 2/3 to 1/2 length of dorsal pairs; 2nd pair dorsal tentacular cirri marginally longer than 1st pair, reaching to chaetiger 4.
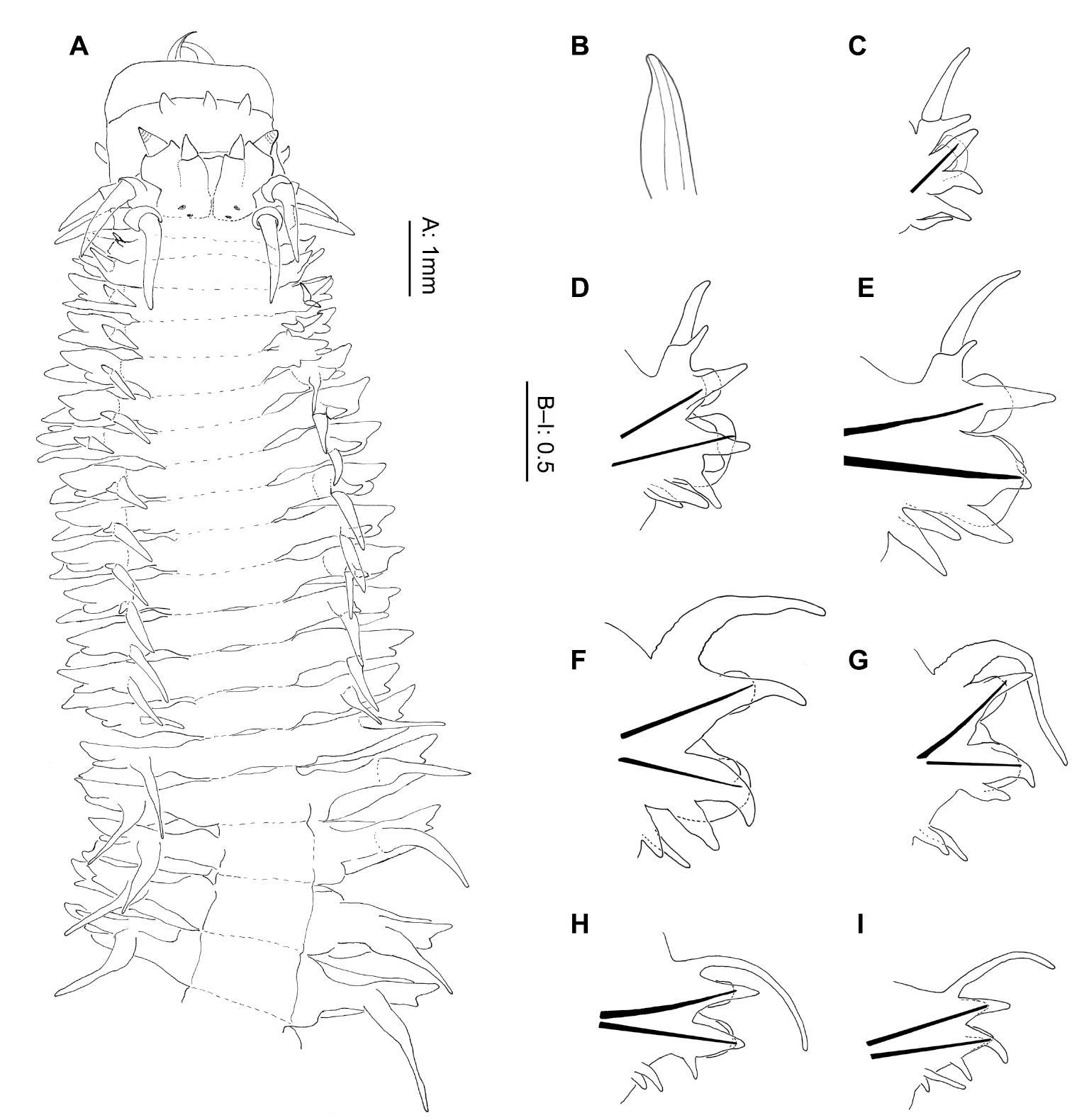
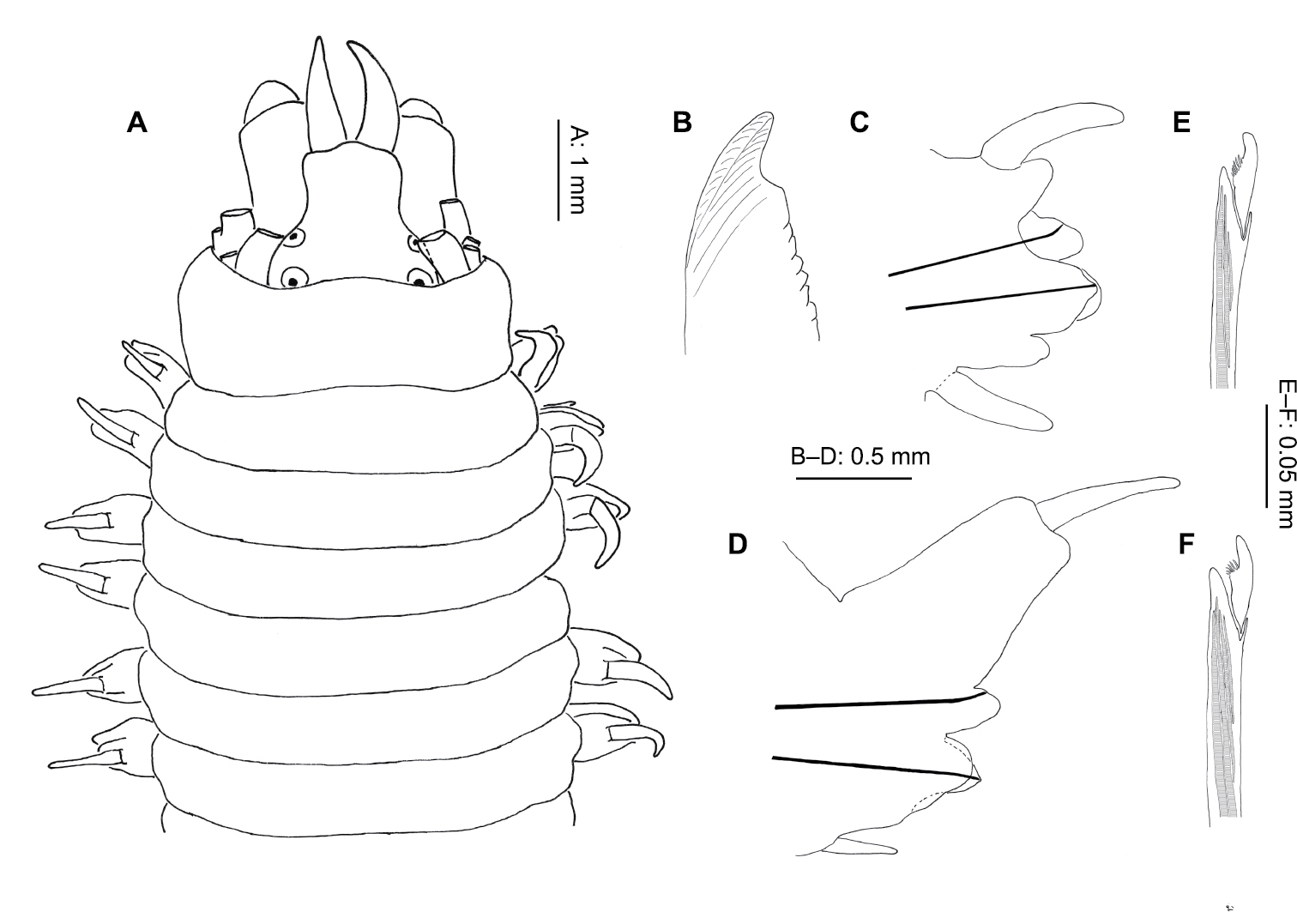
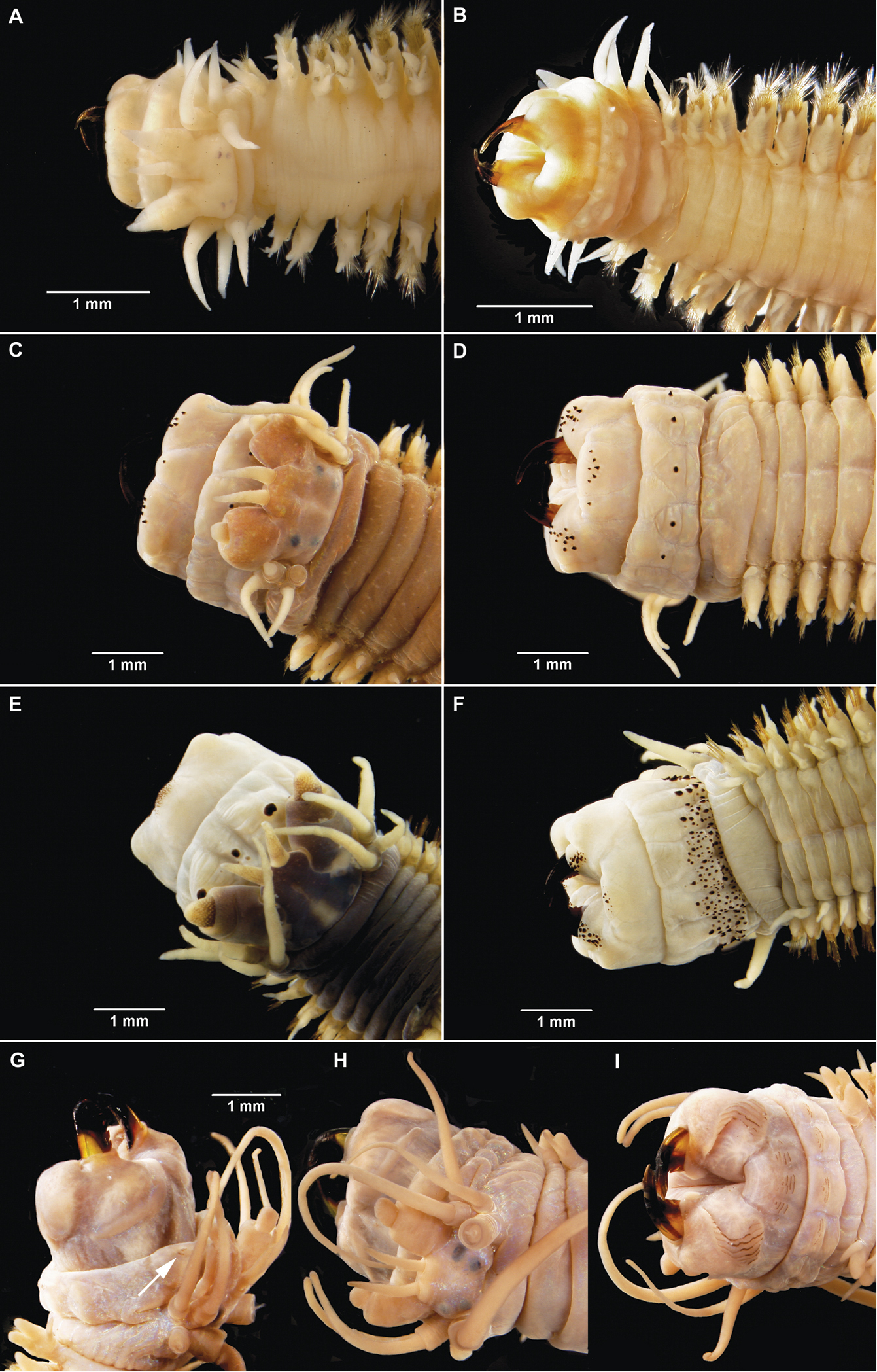
Gymnonereis tenera sp. n. (A NMW.Z.2011.039.0102 B–I NMW.Z.2011.039.0098): A holotype, anterior end, dorsal view (right chaetiger 4 aberrant) B jaw; C: left parapodium, chaetiger 1, anterior view D left parapodium, chaetiger 3, anterior view E left parapodium, chaetiger 9, anterior view F left parapodium, chaetiger 20, anterior view G left parapodium, chaetiger 30, anterior view H left parapodium, chaetiger 50, anterior view I left parapodium, chaetiger 100, anterior view.
Peristomium dorsally more narrow than following segments. Jaws with smooth edges, teeth absent (Fig. 2A–B). Oral ring with triangular papillae arranged as follows (Figs 2A; 9A, B): Area V–VI = 3, VII–VIII = 7; maxillary ring bare.
Chaetigers 1–2 uniramous (Fig. 2C), single black acicula, tip curved, just emergent. Subsequent chaetigers all biramous (Fig. 2D–I), notoacicula not emergent, neuroacicula thicker, emergent anteriorly only up to around chaetiger 50. Dorsal cirri of chaetigers 1–12 with accessory dorsal cirrus (Fig. 2A, C–E), up to 1/3 length of main cirrus, appearing as extension to cirrophore rather than dorsal cirrus. From chaetigers 16–52 (Fig. 2F–G), dorsal cirrophores expanded and vascularized, although start and end of region difficult to define. Remaining chaetigers with dorsal cirrus long, narrow, tapering (Fig. 2H–I).
Double ventral cirri present throughout (Fig. 2C–I), branches unequal, ventral branch reducing in size posteriorly. Dorsal branch 1.5 times as long as ventral branch in anterior region, 4–5 times as long posteriorly.
Chaetiger 1 (Fig. 2C), neuroacicular papilla small, rounded, posterior and slightly dorsal to digitiform prechaetal lobe. Postchaetal lobe broad, rounded, approximately 2/3 length of prechaetal lobe. Acicular lobe similar shape to postchaetal lobe, approximately 1/2 length. Ventral neuropodial ligule of same size and shape as prechaetal lobe.
Chaetiger 3 (Fig. 2D) with basally swollen, digitiform notopodial prechaetal lobe twice as long as broadly rounded notopodial postchaetal lobe; acicular lobe 1/4 length of latter. Notochaetae in 2 unequal bundles, arranged as a smaller semicircle above and larger semicircle below the notopodial prechaetal lobe. Neuropodium as for chaetiger 1, ventral ligule of same size and shape as neuropodial prechaetal lobe. Neurochaetae in 2 semicircular fascicles of greater density than notochaetae. Superior fascicle arranged around neuroacicular papilla with larger, inferior bundle ventral and posterior to neuropodial prechaetal lobe. Arrangement continues to start of vascularized cirrophores then number of chaetae reduces posteriorly, becoming bundles rather than semicircles. Greatest density of chaetae occurs in chaetigers 6–8.
Posteriorly, neuropodial prechaetal lobe reducing in size, ventral ligule even more so. Neuropodial postchaetal lobes also decrease in size proportionately, becoming more conical.
Noto- and neurochaetae consist of both homogomph and sesquigomph spinigers throughout, no falcigers observed. Accurate numbers of chaetae and distribution of homogomph versus sesquigomph chaetae on anterior chaetigers difficult to identify due to density.
Approximate chaetal counts of Gymnonereis tenera sp. n. (paratype, NMW.Z.2011.039.0098).
| Chaetiger | Notochaetae | Neurochaetae |
|---|---|---|
| 1 | 0 | 30 |
| 3 | 20 | 90 |
| 9 | 39 | 108 |
| 20 | 19 | 40 |
| 30 | 20 | 40 |
| 50 | 10 | 12 |
| 100 | 8 | 19 |
No dorsal flaps connecting chaetigers. Transverse, faintly defined ridges present from chaetiger 11–16.
Pygidium with anus terminal; 2 long cirri ventral to anus. Anal cirri of similar shape to dorsal cirri on body, 1.2 mm long.
Eggs found in 2 specimens, spherical, 120–130 µm diameter.
Most characters varied with number of chaetigers and continued to change as the number increased. Accessory dorsal cirri were not observed on animals with less than 95 chaetigers (unless regenerating) although they were absent in one specimen of 103 chaetigers (62 complete specimens examined; 27 with less than 95 chaetigers, 35 with 95 or more chaetigers). As chaetiger number increases, additional anterior dorsal cirri have accessory cirri, with animals of more than 160 chaetigers with accessory dorsal cirri as far as chaetigers 10–14. The variation in this character means that it should not be used as diagnostic for the species on its own but only in conjunction with other characters.
The faint transverse ridges connecting parapodia were mostly visible from chaetiger 11 to 15 or 16 but were occasionally observed as far back as chaetiger 20 on the largest specimens.
Determination of the start and end of the expanded cirrophores was difficult, particularly the former, as the transition was not as abrupt as described for some species. The region generally occurred from around chaetigers 11–18 and continued to chaetigers 22–51 over the range of body sizes observed.
Presence and number of the oral papillae did not vary with size although papillae were occasionally lost and a single specimen was identified with 4 papillae in Area V–VI. Relative length of tentacular cirri was also stable with the longest cirri always reaching to chaetiger 4 in all body sizes.
Although jaw teeth were absent in the majority of specimens, juveniles of less than 80 chaetigers (jaws of 26 specimens were examined including 12 juveniles of 33–80 chaetigers in size) were found to have 4–5 small teeth on each jaw with jaws in larger animals becoming more roughly crenated until the largest jaws appeared almost completely smooth.
The specific name tenera is derived from the latin adjective tener meaning ‘soft, delicate’, referring to the very soft nature of the worm when alive and its fragility when handled.
Found intertidally from mid to low shore in soft, fine, sand or mud sediments.
With 3 papillae in Area V–VI of the oral ring and the absence of jaw teeth, Gymnonereis tenera sp. n. can be distinguished from all other Gymnonereis species except for Gymnonereis sibogae and Gymnonereis phuketensis. Gymnonereis minyami and Gymnonereis yurieli both have jaw teeth and only 1 papilla in each of Areas V and VI. Gymnonereis crosslandi and Gymnonereis fauveli both lack jaw teeth but Gymnonereis crosslandi has only 1 papilla in each of Areas V and VI, accessory dorsal cirri in only chaetigers 1 and 2 (chaetiger 1 to 12 or further in Gymnonereis tenera sp. n.) and no enlarged dorsal cirrophores, whilst Gymnonereis fauveli has 5 papillae in Area V–VI and accessory dorsal cirri from chaetiger 3 (as opposed to chaetiger 1 in the new species).
Gymnonereis tenera sp. n. is most similar to both Gymnonereis sibogae and Gymnonereis phuketensis and can only be distinguished from each of these through combinations of characters. Although
Apart from the character of presence or absence of jaw teeth, the new species is also very similar to Gymnonereis phuketensis, although juveniles of the new species do have a small number of jaw teeth.
Nereis longissima Johnston, 1840
(after
Two pairs of eyes. One apodous anterior segment, greater than length of chaetiger 1. Maxillary ring of pharynx without paragnaths. Oral ring, conical paragnaths: Area V, present or absent; VI, present or absent, smooth bars present or absent; VII−VIII, present or absent. Dorsal notopodial ligule present, similar in size or markedly reduced on posterior chaetigers. Prechaetal notopodial lobe present or absent; when present, restricted to a limited number of anterior chaetigers. Acicular process present or absent. Dorsal cirrus basally attached to dorsal notopodial ligule throughout all chaetigers, lacking basal cirrophore. Neuropodial postchaetal lobe absent or present. Notoaciculae absent from chaetigers 1 and 2. Notochaetae: homogomph spinigers present, homogomph falcigers present or absent. Neurochaetae, superior fascicle: homogomph spinigers and heterogomph falcigers present. Neurochaetae, inferior fascicle: heterogomph spinigers and heterogomph falcigers with long blades present.
Strait of Magellan, stn 313 (52°20'S, 067°39'W), sand, 100.6 m, 2 syntypes (NHMUK 1885.12.1.171) 20.01.1876; South America, off Uruguay, stn 1 (33°00'S, 051°10'W), blackish clay, 80 m, 2 specimens (SMNH 37888), 12.12.1901; south of West Falkland, Burdwood Bank, stn 59 (53°45'S, 061°10'W), gravel & stones, 137–150 m, 13 specimens (9–SMNH 37894; 4–SMNH 37902), 12.09.1902; off Falkland Islands, stn WS 86 (53°53'30"S, 060°34'30"W), 6 syntypes Nereis (Eunereis) hardyi (NHMUK 1930.10.8.841–844), 03.04.1927; Strait of Magellan, stn WS 834 (52°57'45"S, 068°08'15"W), 4 specimens Nereis (Eunereis) hardyi (NHMUK 1936.2.8.1463–1476), 02.02.1932.
Length up to 130 mm, width to 5 mm (excluding parapodia) for up to 85 chaetigers. Eyes present (Fig. 3A–B). Tentacular cirri reaching to chaetiger 6–8 (postero-dorsal pair). Paragnaths absent from maxillary ring; arranged on oral ring as follows (Fig. 3B–C): Area V =1–2; Area VI = 0; Areas VII–VIII = 7–8 in a row. Jaws dark, 5–10 teeth.
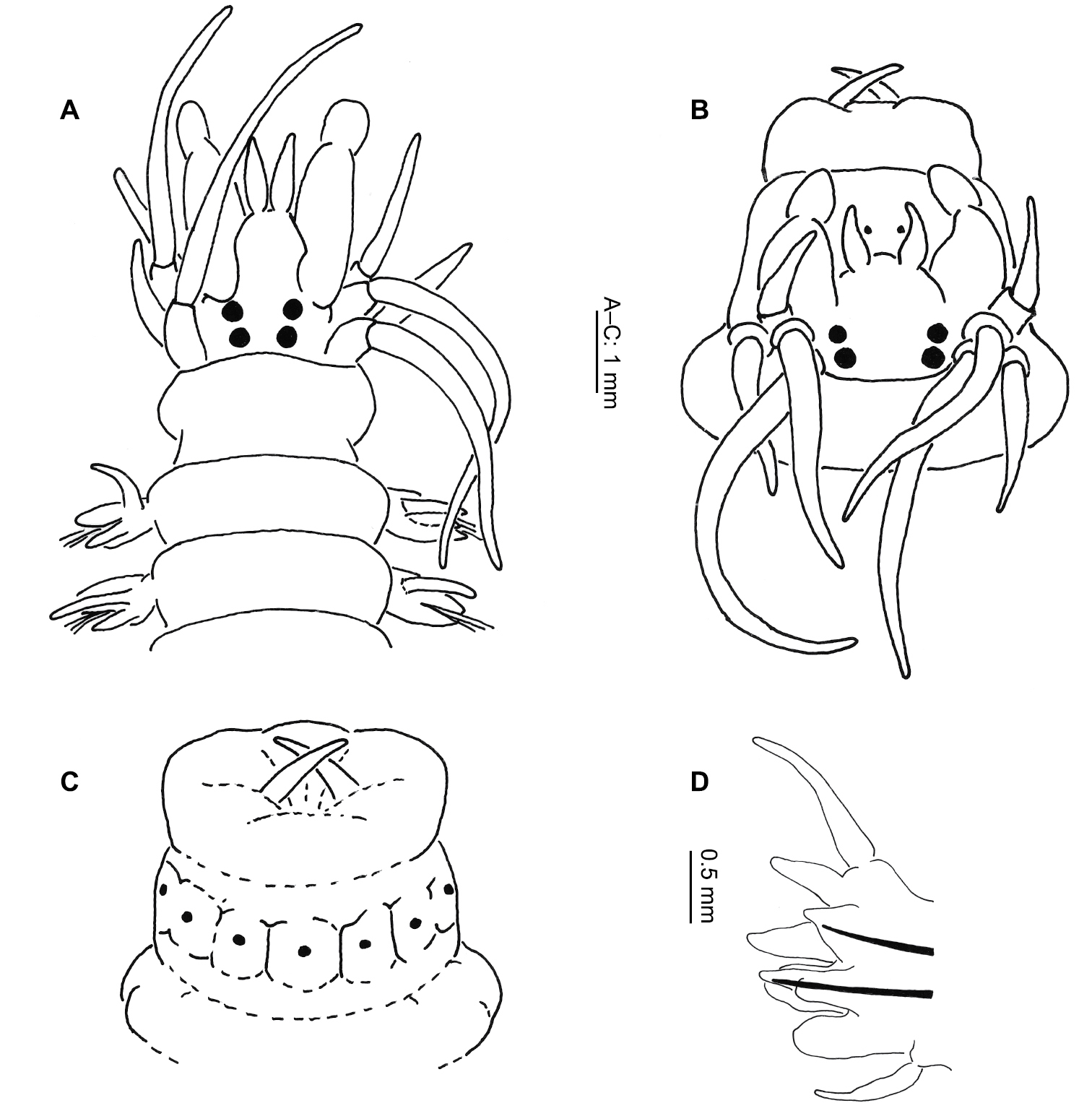
Eunereis patagonica (McIntosh, 1885) (after Monro, 1930, as Nereis (Eunereis) hardyi): A Anterior end, dorsal view B Prostomium and proboscis, dorsal view C Proboscis, ventral view D Parapodium.
Dorsal cirrus longer than notopodia throughout (Fig. 3D), becoming more pronounced posteriorly. Anterior notopodia with dorsal and median ligules conical, median slightly more stout than dorsal. Small, notopodial prechaetal lobe present in anterior chaetigers.
Neuropodia with postchaetal lobe and ventral ligule equal-sized anteriorly; postchaetal lobe conical, reducing in size posteriorly, ventral ligule rounded in the anterior, conical posteriorly.
Notopodia with homogomph spinigers throughout, falcigers absent. Neuropodia with homogomph spinigers and heterogomph falcigers in superior fascicle, inferior fascicle with heterogomph spiniger and falcigers.
Species builds tough-walled tubes coated in sand grains and other coarse particles.
The above description is based on
It is clear that
Eunereis patagonica was first recorded from the Falkland Islands by
Outside of the region, the species was recorded by
The species is here believed unlikely to be found intertidally around the Falkland Islands but with potential to be found in the region’s nearshore (< 30 m) waters;
Sand, shell, stones; 31–1886 m (?3660 m)
Tierra del Fuego, Strait of Magellan, Cape Horn, Falkland Islands, South Shetland Islands, South Orkney Islands, ?Pacific Antarctic Ridge
Nectoneanthes Wilson, 1988: 5.
Neanthes vaalii Kinberg, 1865, by original designation
(after
East Falkland: Stanley foreshore, stn 1a (51°41.454'S, 057°51.870'W), under rocks in coarse sand, midshore, 3 specimens (NMW.Z.2011.039.0120), 15.11.2011; Stanley foreshore, stn 1b (51°41.459'S, 057°51.840'W), under rocks in coarse sand, midshore, 9 specimens (NMW.Z.2011.039.121), 15.1.2011; Stanley foreshore, stn 1c (51°41.459'S, 057°51.823'W), under rocks in coarse sand, low shore, 3 specimens (NMW.Z.2011.039.0122), 15.1.2011; The Canache, east of Stanley, stn 2c (51°41.716'S, 057°47.107'W), under rocks in gravel & coarse sand, mid-low shore, 6 specimens (NMW.Z.2011.039.0123), 16.1.2011; Hookers Point, stn 4 (51°41.994'S, 057°46.747'W), in & under pink encrusting algae, low shore, 3 specimens (NMW.Z.2011.039.0124), 15.1.2011; Hookers Point, stn 6b, (51°41.994'S, 057°46.747'W), algal holdfast scraping, low shore, 1 specimen (NMW.Z.2011.039.0125), 21.11.2011; Sea Lion Island: East Loafers Bay, stn 20a (52°26.306'S, 059°06.229'W), in & under pink encrusting algae, mid-low shore, 4 specimens (NMW.Z.2011.039.0126), 28.11.2011; East Falkland: west Stanley, stn 21 (51°41.402'S, 057°52.580'W), under small stones in coarse sand & gravel, 6 specimens (NMW.Z.2011.039.0127–0128), 01.12.2011; Egg Harbour, Shag Rookery Point, stn 27 (51°49.345'S, 059°26.719'W), under rocks in soft silty sand, 6 m, 2 specimens (NMW.Z.2011.039.0129), 03.12.2011; Kelp Harbour, stn 29a (51°47.715'S, 059°18.400'W), coralline coarse sand, mid-low shore, 15 specimens (NMW.Z.2011.039.0136), 04.12.2011; Stanley marina, stn 32 (51°41.600'S, 057°48.073'W), Macrocystis holdfast, 30 cm, 2 specimens (NMW.Z.2011.039.0132), 05.12.2011; Sand Bay, Port Harriet, stn 34f (51°44.130'S, 058°00.550'W), under rocks within mussel bed, midshore, 7 specimens (NMW.Z.2011.039.0130), 08.12.2011; Teal Creek, east of Darwin, stn 35d (51°49.248'S, 058°55.561'W), under rocks in sand, midshore, 4 specimens (NMW.Z.2011.039.0131), 09.12.2011; Cape Bougainville, stn 38b (51°18.727'S, 058°27.607'W), under rocks in gravel in rock pool, mid-low shore, 1 specimen (NMW.Z.2012.082.0019), 13.01.2013; North Arm, stn 48a (52°07.768'S, 059°22.131'W), mussel bed over silty coarse sand, midshore, 13 specimens (NMW.Z.2013.082.0020), 22.01.2013; West Falkland: Moonlight Bay, Port Stephens, stn 51c (52°06.232'S, 060°50.368'W), in crevices, midshore, 10 specimens (NMW.Z.2012.082.0021), 26.01.2013; The Creek, Hill Cove, stn 56d (51°30.061'S, 060°07.618'W), under algae-covered rocks in fine sand, midshore, 4 specimens (NMW.Z.2012.082.0022), 31.01.2013; Shallow Bay, stn 57e (51°30.032'S, 060°07.726'W), in crevices & under stones, low shore, 3 specimens (NMW.Z.2012.082.0023), 01.02.2013.
Ninety-six entire specimens examined: length 5.9–61.3 mm, width 0.7–3.3 mm (excluding parapodia, measured at 8th chaetiger) for 29–70 chaetigers.
Colour pale cream in alcohol, some with dark brown, uniform shading remaining over anterior chaetigers.
Body depressed dorso-ventrally, of mostly uniform width, tapering in last few chaetigers. Prostomium longer than broad (Fig. 4A), antennae and palps about equal in length, with antennae 1/4 width of palpophores. Palpostyles very short, 1/5 length of palpophores. Four pairs tentacular cirri, postero-dorsal pair extending 2–7 chaetigers, usually 2–3. Two pairs small, equal-sized, black eyes, anterior pair more laterally placed.
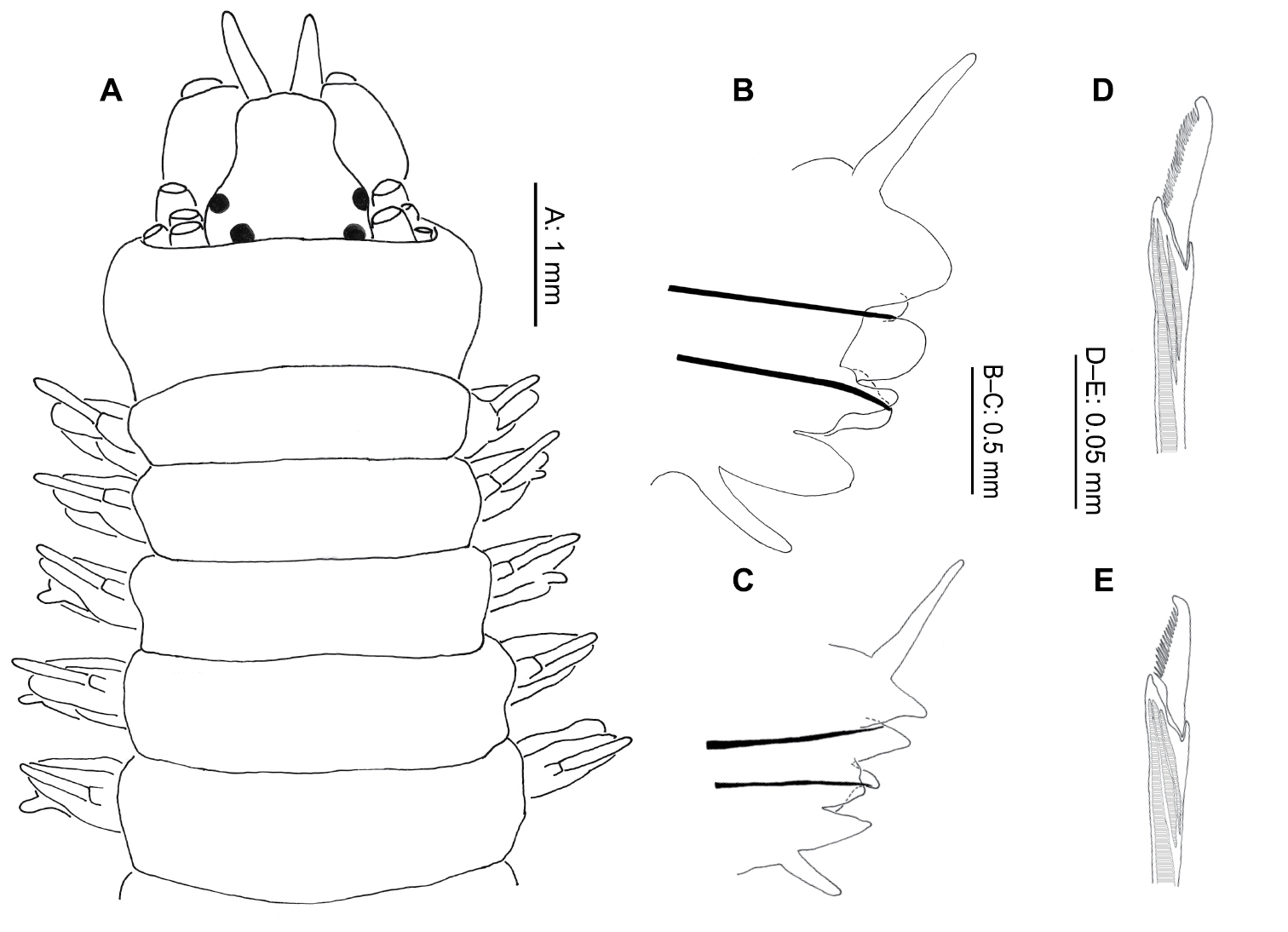
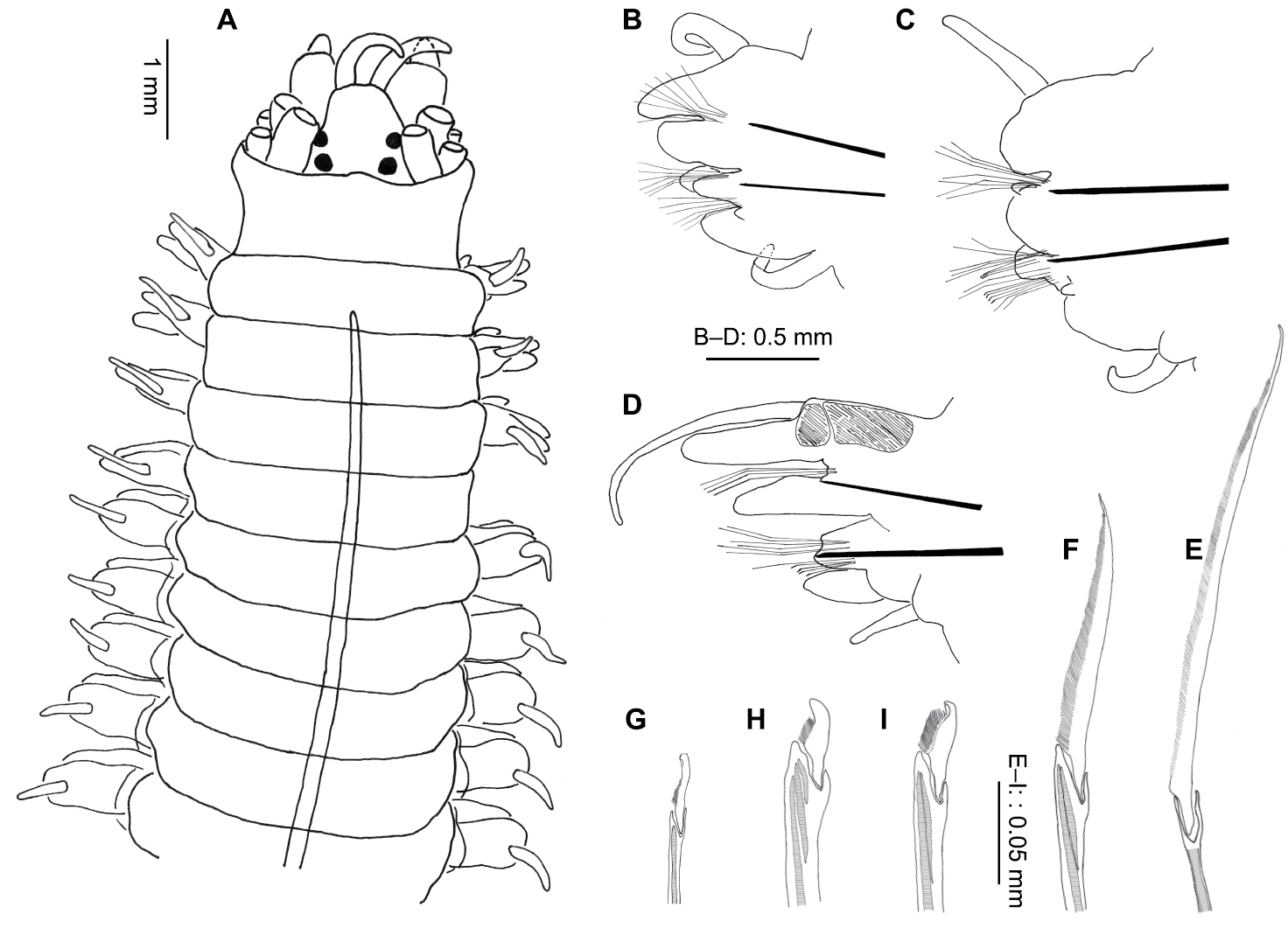
Neanthes kerguelensis (McIntosh, 1885) (NMW.Z.2011.039.0127): A anterior end (tentacular cirri removed), dorsal view B right parapodium, chaetiger 10, posterior view C right parapodium, chaetiger 47, posterior view D neuropodial heterogomph falciger, chaetiger 10 E neuropodial heterogomph falciger, chaetiger 47.
Pharynx with conical paragnaths (Fig. 9C, D), variable in size, sometimes faint, not easily lost. Paragnaths arranged as follows: I = 1 (absent or too small to see in specimens of less than 45 chaetigers); II = 1–8; III = 1–9; IV = 6–17; V = 0; VI = 1 (2 on one specimen only); VII–VIII = 3–8. Jaws dark brown to black, 7–10 teeth.
Notopodia with dorsal and median ligule throughout. Of almost equal size, globular anteriorly (Fig. 4B), dorsal ligule becoming conical, median ligule becoming digitiform, in median chaetigers. Notopodial prechaetal lobe present from chaetigers 5–6 (Fig. 4B), increasingly fused to median ligule, absent posteriorly, difficult to determine more precisely due to the very gradual fusion, generally obvious for at least 10 chaetigers.
Dorsal cirrus 1–1.5 times length of dorsal ligule anteriorly (Fig. 4B), increasing to 2–2.5 times length posteriorly (Fig. 4C).
Neuropodia with postchaetal lobe and ventral ligule throughout; postchaetal lobe rounded anteriorly, reduced in size and digitiform posteriorly, ventral ligule globular anteriorly, conical posteriorly (Fig. 4B, C). Ventral cirrus approximately 3/4 length of ventral ligule, becoming equal in length posteriorly (Fig. 4B, C).
Parapodia biramous from chaetiger 3, sub-biramous on chaetigers 1–2. Notochaetae homogomph spinigers only. Neurochaetae with homogomph spinigers and heterogomph falcigers (Fig. 4D, E) in both superior and inferior (from 3) fascicles throughout. No heterogomph spinigers found.
Pygidium terminal; 2 long, tapering anal cirri inserted ventrally.
In a detailed study of Australian and sub-Antarctic specimens of Neanthes kerguelensis,
The variation in paragnath numbers exhibited by the Falkland Islands specimens is within the boundaries of that described by
There are currently no published genetic sequences for Neanthes kerguelensis. However, a comparison of some of these different populations using molecular techniques may help resolve these discrepancies.
Recorded widely across the southern hemisphere including Australia, New Zealand, Tasmania, Fiji, Taiwan, Antarctic Peninsula, sub-Antarctic Islands (incl. Kerguelen, Macquarie, South Shetlands, South Orkneys), Chile and the Falkland Islands. Previous records from the Falkland Islands exist from
Neanthes kerguelensis is also recorded from the Northern hemisphere from the Mediterranean and Azores (
Nereis pelagica Linnaeus, 1758 (by original designation)
(after
Length up to 170 mm, width up to 3 mm including parapodia for up to 125 chaetigers. Eyes absent or present. Paragnaths arranged as follows (Fig. 5A, B): Area I = 0; Area II = small group (up to 11); Area III = absent or sparse, irregular row (2–6); Area IV = absent or group (0–18); Area V = 0–1; Area VI = small group (3–6); Areas VII–VIII = sparse, irregular row (0–11). Jaws dark, 5–7 teeth.
Nereis eugeniae Kinberg, 1865 (after
Dorsal cirrus longer than notopodia throughout, becoming more pronounced posteriorly. Anterior notopodia (Fig. 5C) with dorsal and median ligules equal in size, dorsal ligule reducing in size posteriorly. Small notopodial prechaetal lobe present in anterior chaetigers.
Neuropodia with postchaetal lobe and ventral ligule conical; postchaetal lobe shorter than notopodial ligules and ventral ligule in anterior chaetigers, becoming more equal in size posteriorly (Fig. 5D).
Anterior notopodia with homogomph spinigers only, 2–3 homogomph falcigers present from median chaetigers on. Neuropodia with homogomph spinigers and heterogomph falcigers in superior fascicle, inferior fascicle with heterogomph spinigers and falcigers (Fig. 5E).
The above description is an amalgamation of the information provided by
Nereis eugeniae was not collected by this survey, however it has been recorded from several offshore locations around the islands from 1–115 m (
Sand, shell, stones, cobbles; intertidal–156 m.
Strait of Magellan, Chile, Falkland Islands, Kerguelen Islands, Patagonia.
Arete Kinberg, 1865; Gnatholycastis Ehlers, 1920.
Perinereis novaehollandiae Kinberg, 1865; by subsequent designation (
(after
St Vincent, Cape Verde Islands (NHMUK.1885.12.1.161), holotype, July 1873.
Examination of the holotype (Fig. 6A–C), shows the description and illustrations by McIntosh to be quite accurate. The only refinements are as follows:
Body dorso-ventrally depressed, mostly of uniform width, gradually tapering in last 20–30 chaetigers to pygidium.
Paragnaths arranged as follows, all conical except for Area VI (Fig. 6B–C): Area I = 1 large, Area II = 6–8, Area III = 8, Area IV = 15–16 arranged in 3–4 rows, Area V = 1 small, Area VI = 1 shield-shaped bar with rounded apex, Area VII–VIII = 3 rows with 6 (distal row), 9 (middle row) & 4 (proximal row) evenly-spaced cones, middle and proximal cones more flattened and blunt than those of the distal row. Jaws robust, dark brown with 4 teeth (Fig. 6B).
Perinereis atlantica (McIntosh, 1885) (NHMUK.1885.12.1.161): A anterior end, dorsal view B anterior end, ventral view C enlarged view of partial Area VII–VIII of proboscis.
Dorsal ligule expanded posteriorly to a greater extent than figured by McIntosh but not as much as Perinereis falklandica.
Notochaetae all homogomph spinigers, neurochaetae homogomph and heterogomph spinigers and heterogomph falcigers (from observations of a limited number of chaetae, most broken so distribution between inferior and superior fascicles unknown). Falciger tips become shorter posteriorly but otherwise do not change in form along the body.
Pygidium terminal; 3 long, thin anal cirri of equivalent length to last 11 chaetigers (1 cirrus apparently lost as McIntosh’s original description states 4 anal cirri, 2 each side of anus). Pygidium and last 3 chaetigers with appearance of regeneration.
This species was described from a single specimen collected at Cape Verde Islands in the southeast Atlantic.
Since its description, the only other record of the species has been by
With the shield-shaped bars now re-described into Area VI, the species would fall into ‘Group 1A’ of
Unknown.
Cape Verde Islands, ?Falkland Islands.
East Falkland: The Canache, east of Stanley, stn 2c (51°41.716'S, 057°47.107'W), under rocks in gravel & coarse sand, mid-low shore, 9 specimens (NMW.Z.2011.039.0108–0109), 16.1.2011; Hookers Point, stn 6a, (51°41.994'S, 057°46.747'W), under pink encrusting algae, low shore, 3 specimens (NMW.Z.2011.039.0110), 21.11.2011; Hookers Point, stn 6c, (51°41.994'S, 057°46.747'W), under pink encrusting algae, low shore, 3 specimens (NMW.Z.2011.039.0111), 21.11.2011; Hookers Point, stn 6d, (51°41.994'S, 057°46.747'W), in silty gravel washings from rock pool, low shore, 1 specimen (NMW.Z.2011.039.0112), 21.11.2011; Egg Harbour, stn 25 (51°50.353'S, 059°27.351'W), rocks & mussel bed in silty coarse sand, mid-low tide, 12 specimens (NMW.Z.2011.039.0114), 03.12.2011; Sea Lion Island: East Loafers Bay, stn 20a (52°26.306'S, 059°06.229'W), in & under pink encrusting algae, mid-low shore, 1 specimen (NMW.Z.2011.039.0113), 28.11.2011; Saunders Island: The Neck, stn 42d (51°18.485'S, 060°14.504'W), under stones on rock ledges, midshore, 3 specimens (NMW.Z.2012.082.0011), 17.01.2013; West Falkland: Shallow Bay, stn 57b (51°30.032'S, 060°07.726'W), in crevices & under stones, high-mid shore, 2 specimens (NMW.Z.2012.082.0012), 01.02.2013; Shallow Bay, stn 57c (51°30.032'S, 060°07.726'W), in crevices & under stones, low shore, 5 specimens (NMW.Z.2012.082.0013), 01.02.2013.
Thirty-nine entire specimens examined; length 19.5–73.6 mm, width (excluding parapodia) 1.5–4.3 mm for 65–89 chaetigers.
Colour in alcohol, dark brown body with pale parapodia, colour becoming paler more posteriorly, variably according to specimen. Head very dark green/brown with pale median line (Fig. 9E). Live colour green-brown with pale markings as described in alcohol.
Body dorso-ventrally depressed, uniform width for most of length, tapering slightly over last few chaetigers. Head with prostomium longer than broad (Fig. 7A), antennae short, stout, 2/3 length of broad palps. Four pairs short, tentacular cirri, pale with dark cirrophores, reaching to chaetiger 2–4. Two pairs small, black eyes, equal size, anterior pair more laterally placed (Fig. 7A). Eyes difficult to discern once preserved due to dark prostomial colour, particularly anterior pair.
Perinereis falklandica Ramsay, 1914 (NMW.Z.2011.039.0108): A anterior end (tentacular cirri & right chaetiger 4 removed), dorsal view B jaw C right parapodium, chaetiger 4, posterior view D right parapodium, chaetiger 71, posterior view E neuropodial heterogomph falciger, chaetiger 4 F neuropodial heterogomph falciger, chaetiger 71.
Proboscis with conical (except for Area VI) paragnaths (Fig. 9E, F), variable in size and number, arranged as follows: Area I = 1 large, central surrounded by triangle of 32–150 small, faint, blunter cones; II = broad triangle of large and small cones, 9–28 each side; III = oval patch of 11–20 medium-sized cones; IV = curved lines of 23–40 small–large cones; V = 1 large, blunt cone (1 aberrant specimen with 1 large & over 20 small cones); VI = 1 large, shield-shaped bar with pointed apex; VII–VIII = 2–3 single, large cones laterally, almost reaching Area VI, becoming a broad swath ventrally of 110–300 large and small blunt cones. Jaws dark black/brown with 5–10 teeth and large distal fang (Fig. 7B).
Anterior notopodia with dorsal and median ligules rounded anteriorly (Fig. 7C), becoming conical in median chaetigers; dorsal ligule swollen and elongated from around chaetiger 50 (Fig. 7D).
Neuropodia with conical postchaetal lobe and ventral ligule anteriorly, ventral ligule smaller, almost absent posteriorly.
Notochaetae homogomph spinigers throughout, figured specimen with 13 on chaetiger 4, reducing posteriorly to 6 on chaetiger 71 (of 89). Neurochaetae with homogomph spinigers in superior fascicle only (chaetiger 4: 5, chaetiger 71: 6), heterogomph falcigers present in both superior (chaetiger 4: 5; chaetiger 71: 3) and inferior (chaetiger 4: 15, chaetiger 71: 8) fascicles throughout, little change in form along body (Fig. 7E, F). Inferior fascicle with falcigers arranged in a C-shape on anterior chaetigers, thereafter in a transverse line.
Pygidium terminal; two short, anal cirri inserted ventrally.
In this study, all specimens were from intertidal, mid-low shore locations, in hard substrates such as coarse sand/gravel, under rocks, in crevices and under pink encrusting algae.
Of the handful of other records in the literature, the species is mostly found intertidally in hard, often exposed habitats.
Falkland Islands, Magellan region (Orange Bay), Tristan da Cunha, Chile
Perinereis falklandica has not been reported very widely in the literature since Ramsay described it from the Falkland Islands in 1914, although it was found to be quite common in coarse, intertidal habitats during this survey. Only one other record exists for the locality, being that of
The validity of the species has not been questioned and it is easily distinguishable from other species. Type material was therefore not examined.
Descriptions of the specimens from the different localities are mostly uniform with the only variation being in the number of paragnaths found in Area V of the proboscis. Most authors have reported a single, large cone in this region with the exception of
Iphinereis Malmgren, 1865; Pisenoë Kinberg, 1865; Leontis Malmgren, 1867; Nectonereis Verrill, 1873; Uncinereis Chamberlin, 1919.
Platynereis magalhaensis Kinberg, 1865, by subsequent designation (
(after
The above description is emended with respect to the paragnath terminology introduced by
East Falkland: Stanley foreshore, stn 1c (51°41.459'S, 057°51.823'W), under rocks in coarse sand, low shore, 1 specimen (NMW.Z.2011.039.0145), 15.1.2011; The Canache, east of Stanley, stn 2e (51°41.731'S, 057°47.001'W), medium sand, low shore, 4 specimens (NMW.Z.2011.039.0146), 16.1.2011; Cochon Island: stn 10 (51°36.287'S, 057°47.684'W), under rocks, 9.5 m, 14 specimens (NMW.Z.2011.039.0147–0149), 24.11.2011; stn 11 (51°36.377'S, 057°489'W), under rocks, 9.6 m, 10 specimens (NMW.Z.2011.039.0150), 24.11.2011; stn 13 (51°36.322'S, 057°47.132'W) epifaunal turf scraping, 13.6 m, 3 specimens (NMW.Z.2011.039.0141), 25.11.2011; stn 15a (51°36.449'S, 057°47.150'W), under rocks, 18.0 m, 1 specimen (NMW.Z.2011.039.0151), 26.11.2011; stn 16b (51°36.366'S, 057°47.082'W), epifaunal turf scraping, 12.5 m, 1 specimen (NMW.Z.2011.039.0142), 26.11.2011; Kidney Island: stn 18b (51°37.517'S, 057°45.301'W), fine-medium sand, 4.6 m, 2 specimens (NMW.Z.2011.039.0152), 27.11.2011; East Falkland: west Stanley, stn 21 (51°41.402'S, 057°52.580'W), under small stones in coarse sand & gravel, 2 specimens (NMW.Z.2011.039.0153), 01.12.2011; Egg Harbour, stn 22 (51°47.471'S, 059°24.360'W), under rocks, 13.9 m, 4 specimens (NMW.Z.2011.039.0157), 02.12.2011; Egg Harbour, stn 23 (51°49.477'S, 059°23.926'W), under rocks, 11.6 m, 5 specimens (NMW.Z.2011.039.0143), 03.12.2011; Egg Harbour, Shag Rookery Point, stn 27 (51°49.345'S, 059°26.719'W), under rocks, 6 m, 1 specimen (NMW.Z.2011.039.0154), 03.12.2011; Kelp Harbour, stn 30 (51°47.021'S, 059°19.848'W), under rocks, 9.3 m, 4 specimens (NMW.Z.2011.039.0144), 04.12.2011; Sand Bay, Port Harriet, stn 34f (51°44.130'S, 058°00.550'W), under rocks within mussel bed, midshore, 4 specimens (NMW.Z.2011.039.0155), 08.12.2011; Teal Creek, east of Darwin, stn 35d (51°49.248'S, 058°55.561'W), under rocks in sand, midshore, 1 specimen (NMW.Z.2011.039.0156), 09.12.2011; Race Point Farm, Port San Carlos, stn 37a (51°30.276'S, 059°00.137'W), in crevices, mid-low shore, 3 specimens (NMWZ.2012.082.0041–0042), 12.01.2013; Race Point Farm, Port San Carlos, stn 37b (51°30.277'S, 059°00.080'W), in crevices, low shore, 2 specimen (NMWZ.2012.082.0043), 12.01.2013; Race Point Farm, Port San Carlos, stn 37c (51°30.276'S, 059°00.137'W), under stones, low shore, 1 specimen (NMWZ.2012.082.0044), 12.01.2013; Race Point Farm, Port San Carlos, stn 37d (51°30.276'S, 059°00.137'W), among rocks & gravel in muddy sand, low shore, 1 specimen (NMWZ.2012.082.0045), 12.01.2013; Cape Bougainville, stn 38a (51°18.720'S, 058°27.603'W), in pink encrusting algae in open crevices, low shore, 2 specimens (NMW.Z.2012.082.0047), 13.01.2013; Cape Bougainville, stn 38b (51°18.727'S, 058°27.607'W), under rocks in gravel in rock pool, mid-low shore, 2 specimens (NMW.Z.2012.082.0048), 13.01.2013; Saunders Island: Sealer Cove harbor, stn 44c (51°21.760'S, 060°04.896'W), under rocks in sandy gravel, low shore, 2 specimens (NMW.Z.2012.082.0049); 18.01.2013; Sealer Cove harbor, stn 44d (51°21.760'S, 060°04.896'W), under rocks in sandy gravel, low shore, 3 specimens (NMW.Z.2012.082.0050); 18.01.2013; East Falkland: North Arm, stn 48a (52°07.768'S, 059°22.131'W), mussel bed over silty coarse sand, midshore, 1 specimen (NMW.Z.2013.082.0051), 22.01.2013; North Arm, stn 48b (52°07.829'S, 059°22.079'W), coarse loose sand, mid-low shore, 1 specimen (NMW.Z.2013.082.0052), 22.01.2013; New Haven, stn 49b (51°43.855'S, 059°12.894'W), under rocks in sandy gravel, mid-low shore, 1 specimen (NMW.Z.2012.082.0054), 24.01.2013; West Falkland: Moonlight Bay, Port Stephens, stn 51d (52°06.266'S, 060°50.334'W), in crevices, mid-low shore, 1 specimen (NMW.Z.2012.082.0055), 26.01.2013; Hot Stone Cove Creek, Dunbar, stn 54g (51°22.883'S, 060°30.886'W), associated with large tunicate attached to rock, low shore, 1 specimen (NMW.Z.2012.082.0056), 29.01.2013; Shallow Bay, stn 57c (51°30.032'S, 060°07.726'W), in crevices & under stones, mid shore, 2 specimens (NMW.Z.2012.082.0057), 01.02.2013.
Eighty-three entire specimens, juveniles to adults, were examined: length 1.9–105.1 mm, width 0.27–4.7 mm (excluding parapodia, measured at chaetiger 4–5) for 16–115 chaetigers. Description based on adult specimens only, defined by the absence of notopodial falcigers.
Colour pale in alcohol.
Body shape depressed dorso-ventrally, mostly of uniform width to posterior, then tapering in last few chaetigers.
Prostomium longer than broad (Fig. 8A), antennae and palps about equal in length; antennae 1/2–1/3 width of palpophores. Four pairs tentacular cirri, postero-dorsal pair longest, reaching to chaetiger 11–14, rarely 16. Two pairs small, dark brown to black eyes, anterior pair marginally smaller, more laterally placed (Fig. 8A). Mid-dorsal nuchal cushion present, projecting forward slightly on to head from apodous peristomial segment (Fig. 8A). Peristomium approximately one third longer than following segments.
Platynereis magalhaensis Kinberg, 1865 (A–F, H–I NMW.Z.2011.039.0159 G NMW.Z.2011.039.0149): A anterior end (tentacular cirri & right chaetiger 4 removed), dorsal view B right parapodium, chaetiger 4, posterior view C right parapodium, chaetiger 10, posterior view D right parapodium, chaetiger 71, posterior view; right parapodium, chaetiger 4, posterior view E notopodial homogomph spiniger, chaetiger 10 F neuropodial heterogomph spiniger, chaetiger 10 G juvenile notopodial heterogomph falciger, chaetiger 20 H neuropodial heterogomph falciger, chaetiger 10 I neuropodial heterogomph falciger, chaetiger 71.
Proboscis with tight lines of rod-like paragnaths in Areas III, IV, VI, VII and VIII, absent in Areas I, II and V. Largest group in area IV with up to 9 long rows, innermost 3–4 rows incomplete. Area III with 3 small groups of up to 4 lines in each. Area VI, the smallest group, often faint, difficult to discern, with up to 3 short lines of rods (Fig. 9G, indicated by arrow). Area VII–VIII with 5 groups of up to 3 curved lines in each (Fig. 9I). Jaws dark brown with up to 12 teeth (Fig. 8G, I).
Images of the paragnaths of the species collected. Gymnonereis tenera sp. n. (NMW.Z.2011.039.0093): A dorsal view B ventral view; Neanthes kerguelensis (NMW.Z.2011.039.0129) C dorsal view D ventral view; Perinereis falklandica (NMW.Z.2011.039.0113) E dorsal view; F. ventral view; Platynereis magalhaensis (NMW.Z.2011.039.0158) G lateral view (arrow indicating Area VI paragnaths) H dorsal view I ventral view.
Parapodia subbiramous on chaetigers 1–2, biramous from chaetiger 3. Parapodial ligules thickened and rounded on chaetigers 5–11, sometimes, to a lesser extent, starting from chaetiger 4 and extending to chaetiger 12, occasionally 13, in larger animals (Fig. 8B, C).
From mid-body dorsal ligule lengthened and glandular (Fig. 8D). Dorsal cirrus longer than dorsal ligule throughout body, minorly so anteriorly, becoming more pronounced and elongate posteriorly (Fig. 8B–D).
Notochaetae homogomph spinigers (Fig. 8E), up to 25–30 per fascicle in mid-body, reduced to around 5 in last few chaetigers. Single heterogomph notopodial falciger, bifid with connecting tendon from tip (Fig. 8G), present in juveniles up to around 60–65 chaetiger stage, absent in adults. First occurrence of notopodial falciger retreats posteriorly as size increases, from around chaetiger 8 (of 16) to chaetiger 62 (of 64).
Neurochaetae homogomph and heterogomph spinigers (Fig. 8F) and heterogomph falcigers (Fig. 8H, I). Superior fascicle spinigers homogomph, up to 8, inferior fascicle spinigers heterogomph, up to 6 (usually 2–3). Falcigers heterogomph, from chaetiger 5 onwards; up to 7 above acicula, up to 17 below; greatest numbers mid-body reducing posteriorly.
Pygidium terminal; two long, thin anal cirri inserted ventrally.
Tube soft, with coarse grains of sand, shell and foraminifera adhered to it.
Platynereis magalhaensis was the most common nereidid collected by diving with most rocks turned over having tubes attached to the underside. It was also widespread intertidally, again in tubes attached to rocks or algal holdfasts.
The original description of Platynereis magalhaensis by
Most recently, a detailed comparison of the Platynereis australis group with Platynereis magalhaensis was published by
Unfortunately, no epitokous forms were among the specimens collected from the Falkland Islands so this aspect cannot be confirmed in this study. However, the few records of epitokes that do exist for this region (
| 1 | Chitinous paragnaths present on pharynx; single ventral cirrus present throughout | 2 |
| – | Chitinous paragnaths absent from pharynx; double ventral cirri present | Gymnonereis tenera sp. n. |
| 2 | Paragnaths present as shield-shaped bars and /or variably-sized cones; chaetiger 5–10 parapodial lobes not noticeably different from lobes on remaining chaetigers | 3 |
| – | Paragnaths present as tight rows of rods; chaetigers 5–10 with globular parapodial lobes | Platynereis magalhaensis Kinberg, 1865 |
| 3 | Area VI with paragnaths as cones, shield-shaped bar with rounded apex or absent; posterior notopodial dorsal lobes not noticeably enlarged | 4 |
| – | Area VI with 1 large, shield-shaped bar with pointed apex; posterior dorsal notopodial lobes greatly enlarged | Perinereis falklandica Ramsay, 1914 |
| 4 | Falcigers absent in notopodia | 5 |
| – | Falcigers present in at least some notopodia | 6 |
| 5 | Paragnaths absent on maxillary ring and Area VI; ventral fascicle of neuropodia includes heterogomph spinigers | Eunereis patagonica (McIntosh, 1885) |
| – | Paragnaths present on maxillary ring and Area VI; all spinigers homogomph, no heterogomph spinigers present | Neanthes kerguelensis (McIntosh, 1885) |
| 6 | Conical paragnaths in Area VI, single sparse row of paragnaths in Area VII–VIII (sometimes absent); falcigers present in dorsal fascicle of neuropodia | Nereis eugeniae (Kinberg, 1865) |
| – | Shield-shaped bar in Area VI, more than 1 row of paragnaths in Area VII–VIII; falcigers absent from dorsal fascicle of neuropodia | Perinereis atlantica McIntosh, 1885, comb. n.* |
* The single record from the Falkland Islands (
This work was funded by both the Shackleton Scholarship Fund (Falkland Islands) and Amgueddfa Cymru–National Museum Wales. Additional logistical support was provided by the Fisheries Department of the Falkland Islands, the Shallow Marine Surveys Group (SMSG) and the South Atlantic Environmental Research Institute (SAERI). I would also like to thank Dr Andy Mackie for his advice and support with the manuscript.