Citation: Lotfollahi P, de Lillo E, Irani-Nejad KH (2014) Three new species from the subfamily Phyllocoptinae (Acari, Trombidiformes, Eriophyidae) in Iran. ZooKeys 426: 17–27. doi: 10.3897/zookeys.426.8087
Three new eriophyid species (Phyllocoptinae), Shevtchenkella denticulata sp. n., Notallus pestehae sp. n. and Echinacrus ruthenicus sp. n., were described from Eryngium thyrsoideum Boiss. (Apiaceae), Pistacia vera L. (Anacardiaceae) and Lycium ruthenicum Murray (Solanaceae), respectively. All the three new species were collected from southwest of the East Azerbaijan province, Iran in 2011. It is the first record of an eriophyoid mite collected from E. thyrsoideum and L. ruthenicum and the first record of Notallus from Anacardiaceae plant family.
East Azerbaijan, Notallus, Echinacrus, Shevtchenkella, Eriophyoidea, washing method
As far as known concerning Iranian fauna, no eriophyoid species has been recorded from Apiaceae. Four eriophyoid species (Aceria mangiferae Sayed, 1946, Aceria pistaciae (Nalepa, 1899), Aceria stefanii (Nalepa, 1898) and Calacarus citrifolii Keifer, 1955) have been recorded from Anacardiaceae (
Considering the relevance of this subject and the scientific importance of the evaluation of the mite fauna in scarcely known areas (
The eriophyoid mite fauna of Eryngium thyrsoideum, Pistacia vera and Lycium ruthenicum was surveyed in the southwest of East Azerbaijan, Iran, during 2011. Mites were recovered from plant materials according to the modified washing method based on the protocol developed by
Type materials are deposited in the collection of the Acarology Laboratory, Department of Plant Protection, Faculty of Agriculture, University of Tabriz, Tabriz (Iran) and of the Department of Soil, Plant and Food Sciences (Di.S.S.P.A.), section of Entomology and Zoology, University of Bari Aldo Moro (Italy).
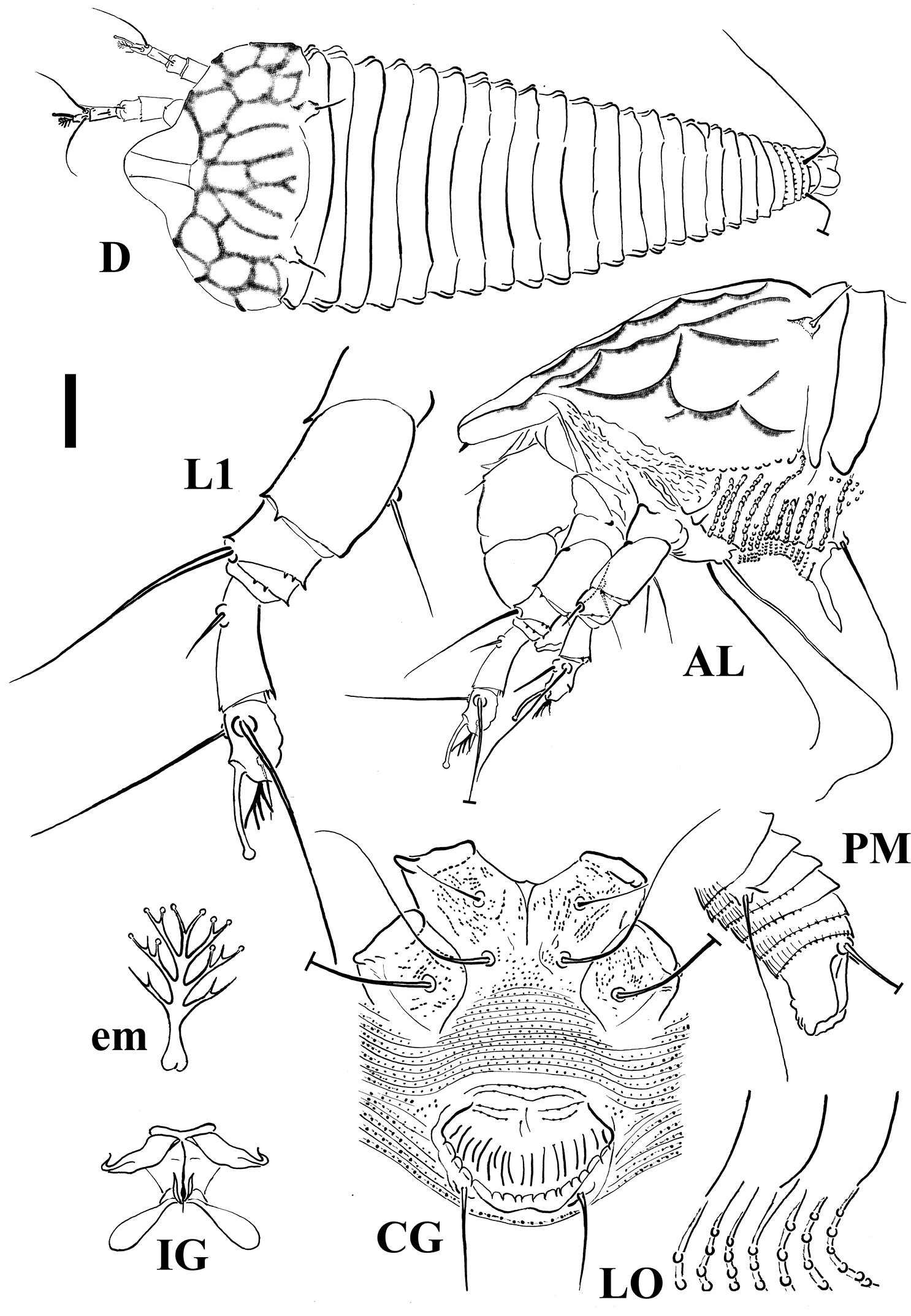
FEMALE. Body dorso-ventrally depressed, 205 (186–226, n = 10), 38 thick, 71 (68–77) wide. Gnathosoma 35 (31–38) projecting obliquely downwards, chelicerae 23 (23–32), setae d 6 (5–7) and unbranched. Prodorsal shield 44 (44–52) included the frontal lobe, 73 (68–77) wide, semicircular in anterior shape with a broad, semicircular frontal lobe, 13 (12–16), over gnathosomal base provided with a spine on the lateral view. Shield pattern distinct and including 26 depressed cells; tubercles of setae sc on the rear shield margin 32 (32–37) apart, setae sc 8 (7–9), projecting posteriorly. Leg I 35 (32–37), femur 10, genu 4 (4–5), tibia 9 (8–10), tarsus 8 (7–8), ω 7 (6–7) and knobbed, empodium simple, 4 (4–4.5), 4-rayed; setae bv 10 (9–11), setae l" 17 (15–20), setae l' 4 (4–5), setae ft' 17 (15–20), setae ft" 20 (18–22). Leg II 32 (30–34), femur 10 (9–10), genu 4 (4–5), tibia 7 (6–7), tarsus 7 (7–8), ω 6 (6–7) and knobbed, empodium simple, 4, 4-rayed; setae bv 10 (8–12), setae l" 5 (5–7), setae ft' 5 (4–5), setae ft" 17 (17–20). Coxae with microgranules sometimes lined; setae 1b 8 (7–10), tubercles 1b 12 (11–19) apart, setae 1a 27 (26–31), tubercles 1a 8 (8–9) apart, setae 2a 45 (44–53), tubercles 2a 26 (23–26) apart. Prosternal apodeme 9 (8–10). Opisthosoma dorsally flat, with a large furrow and small lobes, 21 (21–24) broad and smooth dorsal semiannuli with the exception of the last two provided with spiny microtubercles protruding from the posterior margin of the annuli; 67 (67–81) narrow microtuberculated ventral semiannuli (counted since the first annulus after the coxae II); 9 (9–13) semiannuli between coxae and genital area plus 4–5 transversal rows of lined granules at the base of the genital coverflap. Small and circular microtubercles, closer to the posterior part of ventral semiannuli. Setae c2 25 (20–26) on ventral semiannulus 13 (12–17), setae d 59 (59–70) on ventral semiannulus 27 (27–35); setae e 15 (14–16) on ventral semiannulus 44 (44–57); setae f 28 (26–30) on ventral semiannulus 63 (63–77). Last 4 ventral semiannuli with elongated linear microtubercles protruding from the posterior margin of the annuli. Setae h2 62 (62–78) very thin at the apex, h1 1–2. Genital coverflap 15 (13–18), 23 (23–27) wide, with 14 (13–15) striae and denticulate margin; setae 3a 20 (15–20) apart, 15 (14–17).
MALE. Similar in shape and prodorsal shield arrangement to female, 192 (n = 1). Prodorsal shield 48; setae sc 9, 34 apart; opisthosoma with 21 dorsal semiannuli and 68 ventral semiannuli; male genitalia 20 wide.
Schematic drawings of Shevtchenkella denticulata sp. n.: AL Lateral view of anterior body region CG Female coxigenital region D Dorsal view em Empodium IG Internal female genitalia LO Lateral view of annuli L1 Leg I PM Lateral view of posterior opisthosoma. Scale bar: 17.5 μm for D; 10 μm for AL, CG, IG, PM; 5 μm for LO, L1; 2.5 μm for em.
Eryngium thyrsoideum Boiss. (Apiaceae), Eringo or Sea Holly.
Vagrant on leaves; no apparent damage was observed.
Amir dizaj village, Azarshahr, Iran (37°40'17”N, 46°01'58”E), 1, 950 m above sea level; late July 2011, coll. P. Lotfollahi.
Holotype: single female on a microscope slide (ET-IEA-AJ11L-1) (deposited at the Acarology Laboratory, Department of Plant Protection, Faculty of Agriculture, University of Tabriz, Tabriz, Iran). Paratypes: 12 females, 1 male and 2 nymphs mounted on separate microscope slides.
Mites preserved in Oudemans’ fluid and extracted from the sample collected in the same locality on the same date above mentioned.
This species is named based on the denticulate shape of the female genital coverflap.
This is the first record of a species belonging to the genus Shevtchenkella collected on a plant of the family Apiaceae and the first record of an eriophyoid mite on Eryngium thyrsoideum.
The new species herein described does not show any similarity with any known Shevtchenkella spp. whereas shows some similarities with Aculus pimpinellae (Liro, 1941) collected from Pimpinella saxifraga L. (Apiaceae) in Hollola, Hatsina, Tavastia australis Natural Province, Finland. Differences between these two species, other than those related to the fact they belong to two different genera, are: the ratio between the prodorsal shield length and the length of sc setae (5.5 in Iranian species versus 2 in Liro’s species); number of dorsal annuli (21–24 in Iranian species versus 28 in Liro’s species); size and shape of the female genital coverflap (15×23 with denticulate rear margin in Iranian species versus 15×16 with smooth margin in Liro’s species).
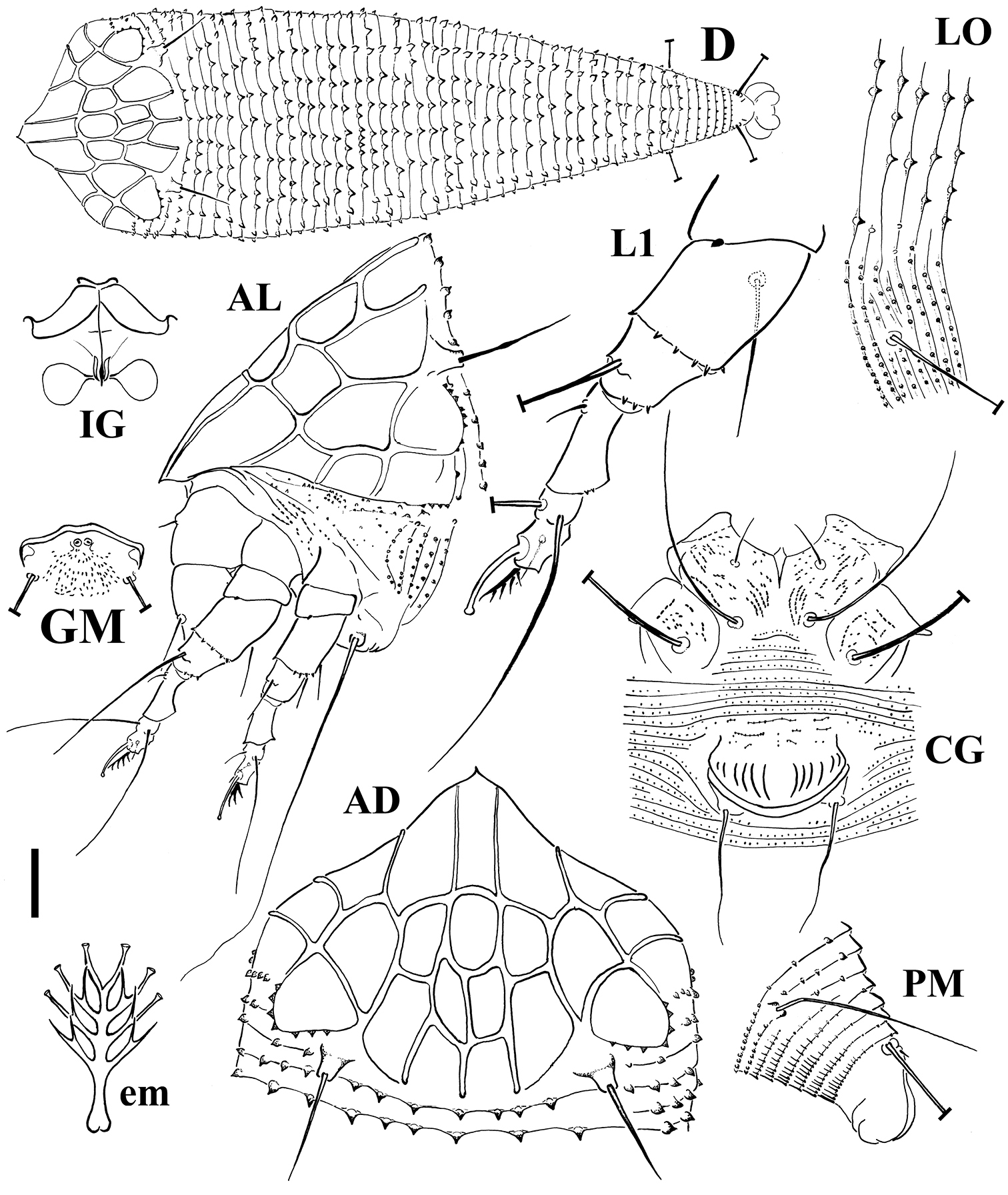
FEMALE (n=10). Body spindle shaped, 195 (195–255, including gnathosoma), 73 thick, 68 (68–79) wide. Gnathosoma 26 (25–37) projecting obliquely downwards, chelicerae 26 (22–30), setae d 7 (7–9), unbranched. Prodorsal shield 47 (47–54) included the frontal lobe, 70 (60–74) wide, sub-triangular with a broad based and distally pointed frontal lobe, 10 (8–11) over gnathosomal base (starting from the distal motivator end). Shield pattern reticulated, composed of 22 cells resulted of connecting distinct median, admedian, submedian and lateral lines with transverse lines. Tubercles of setae sc on the rear shield margin, 33 (28–35) apart, setae sc 16 (15–19), directing backward. Leg I 37 (35–38), femur 11 (10–12), genu 6 (5–6), tibia 10 (8–10), tarsus 9 (8–9), ω 6.5 (6–7) distally knobbed, empodium simple, 4 (4–5), 4-rayed, rays distally funnel shaped; setae bv 13 (11–15), setae l" 24 (22–26), setae l' 4 (3–5), setae ft' 20 (19–20), setae ft" 22 (22–23). Leg II 36 (32–36), femur 11 (10–11), genu 5 (5–6), tibia 8 (7–8), tarsus 8 (8–9), ω 6.5 (6–7) distally knobbed, empodium simple, 4 (4–5), 4-rayed; setae bv 10 (9–11), setae l" 5 (4–7), setae ft' 4, setae ft" 21 (19–22). Coxae with lined dashes; setae 1b 7 (5–8), tubercles 1b 10 (9–12) apart, setae 1a 38 (27–38), tubercles 1a 7 (7–8) apart, setae 2a 60 (60–73), tubercles 2a 21 (21–26) apart. Prosternal apodeme 5 (5–6). Opisthosoma dorsally arched, with 44 (41–49) broad dorsal semiannuli, 76 (70–86) narrow ventral semiannuli (counted from the first annulus after the coxae II) and 11 semiannuli between coxae and genital coverflap plus 2–3 broken transversal rows of lined granules at the base of the coverflap. Triangular broad based microtubercles on the posterior margin of dorsal semiannuli with a lined longitudinal distribution; circular microtubercles, finely spiny, on the middle of ventral semiannuli; last 6 ventral semiannuli with elongated and linear microtubercles. Setae c2 45 (36–45) on ventral semiannulus 15 (12–17), setae d 70 (65–85) on ventral semiannulus 29 (25–34); setae e 58 (43–64) on ventral semiannulus 49 (44–57); setae f 29 (24–33) on ventral semiannulus 70 (64–80). 6 annuli after setae f. Setae h2 102 (92–112) very thin at the apex, h1 2 (2–3). Genital coverflap 14 (11–16), 22 (20–25) wide, with 12 (11–13) striae; setae 3a 18 (18–23), 15 (15–17) apart.
MALE (n=2). Similar in shape and prodorsal shield arrangement to female, 170–205. Prodorsal shield 45–50; setae sc 13–14, 23–32 apart. Opisthosoma with 39–44 dorsal semiannuli and 56–69 ventral semiannuli.
Schematic drawings of Echinacrus ruthenicus sp. n.: AD Dorsal view of anterior body region AL Lateral view of anterior body region CG Female coxigenital region D Dorsal view em Empodium GM Male genital region IG Internal female genitalia LO Lateral view of annuli L1 Leg I PM Lateral view of posterior opisthosoma. Scale bar: 20 μm for D; 10 μm for AD, AL, CG, IG, GM, PM; 5 μm for LO, L1; 2.5 μm for em.
Lycium ruthenicum Murray (Solanaceae), Russian Box Thorn.
Vagrant on leaves; no apparent damage was observed.
Ilkhchi, Iran (37°57'02"N, 45°58'40"E), 1, 300 m above sea level; late July 2011, coll. P. Lotfollahi.
Holotype: single female on a microscope slide (LR-IEA-II11L-1) (at the Acarology Laboratory, Department of Plant Protection, Faculty of Agriculture, University of Tabriz, Tabriz, Iran). Paratypes: 9 females, 2 males and 1 nymph mounted on separate microscope slides.
Mites preserved in Oudemans’ fluid as extracted from the same sample as the type specimens.
The specific epithet is coming from the host plant name ruthenicum, deleting “m” and adding “s” as suffix.
This is the first record of the genus Echinacrus on plants of family Solanaceae, first record of this genus in Iran and the first record of eriophyoid mites on Lycium ruthenicum.
The new species herein described was compared with all Echinacrus species and similarities along with Echinacrus septemcarinatus (Liro, 1941), collected on Frangula dodonei Ard. (the synonym Rhamnus frangula L. was originally listed by Liro) in Lintula, Isthmus karelicus, Finland, were observed. The empodial rays (4 of the Iranian species versus 5 of Liro’s species), shape, number and density of dorsal microtubercles (denser and more numerous in the Iranian species than those of Liro’s description) and prodorsal shield pattern (22 cells in the Iranian species versus a lower number of cells in part differently arranged) are the main differences between the two species.
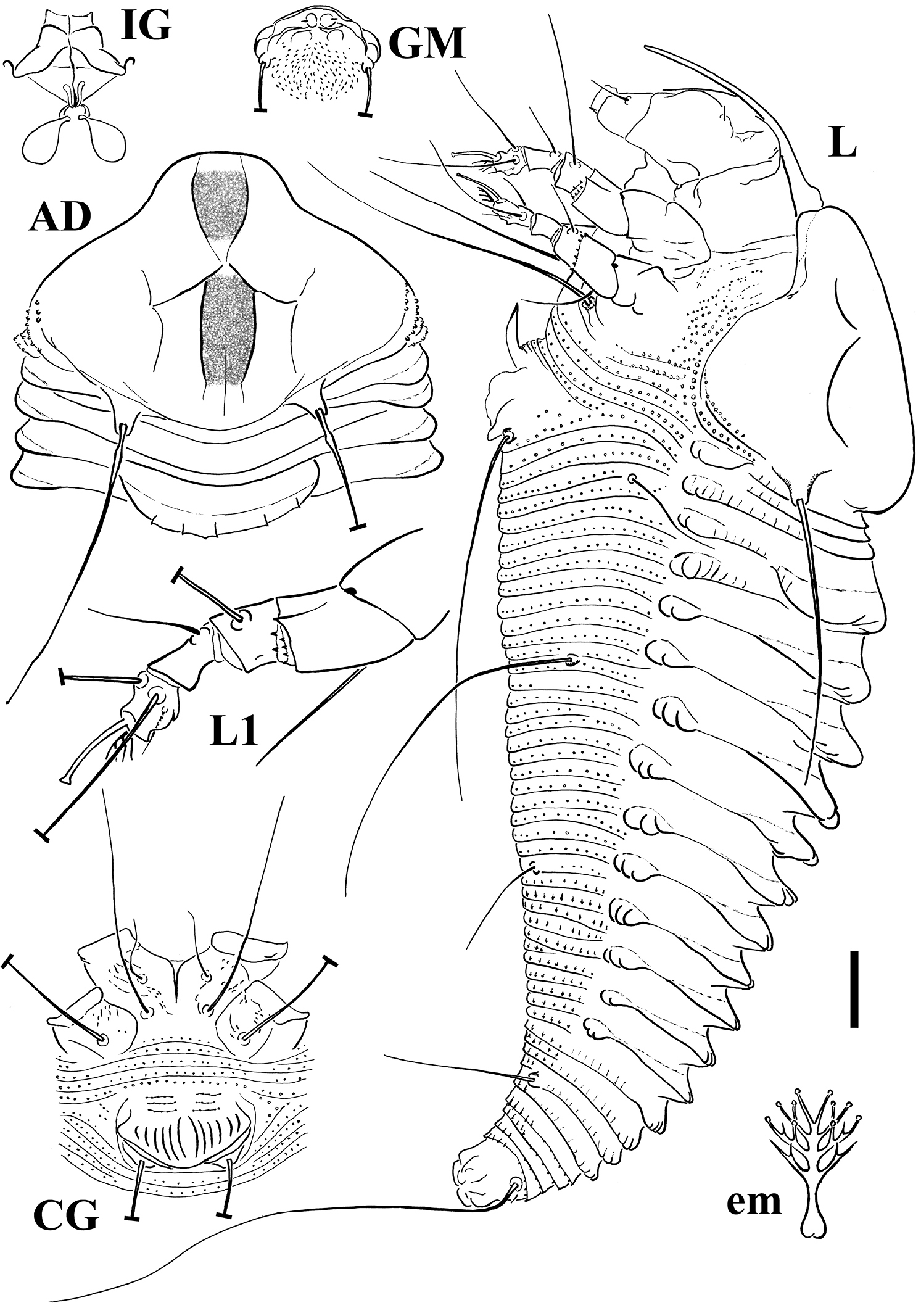
FEMALE (n=11). Body spindle shaped, 165 (156–185, including gnathosoma), 53 (48–57) thick, 52 (49–52) wide. Gnathosoma 41 (38–43) projecting obliquely downwards, chelicerae 37 (35–41), setae d 5 (4–5), unbranched. Prodorsal shield 39 (38–44) included the frontal lobe, 50 (46–50) wide, broad oval, with a broad based and distally truncated frontal lobe, 8 (7–11) over gnathosomal base. Shield pattern composed of a faint short median line on posterior ¼ of prodorsal shield, complete admedian lines close together in the middle of the prodorsal shield, and short first submedian lines on posterior 2/3 of the prodorsal shield, connected to admedian lines with a pair of transverse lines. Admedian lines delimit a median obscure strip (Fig. 3-AD). Tubercles of setae sc on the rear shield margin, 25 (24–26) apart, setae sc 42 (37–45), directing backward. Leg I 26 (25–28), femur 9 (7–9), genu 5 (4–5), tibia 5 (5–6), tarsus 6 (6–8), ω 7 (6.5–8) distally knobbed, empodium simple, 3.5 (3–4), 4-rayed; setae bv 11 (9–13), setae l" 19 (18–20), setae l' 7 (5–7), setae ft' 15 (12–16), setae ft" 17 (17–19). Leg II 20 (20–23), femur 7, genu 3 (3–4), tibia 4 (3–4), tarsus 6 (6–7), ω 7.5 (6.5–8) distally knobbed, empodium simple, 3.5 (3–4), 4-rayed; setae bv 11 (10–12), setae l" 6 (6–7), setae ft' 6 (4–6), setae ft" 16 (15–18). Coxae with sparse dashes in part lined; setae 1b 7 (7–9), tubercles 1b 8 apart, setae 1a 28 (27–33), tubercles 1a 6 (6–7) apart, setae 2a 45 (37–55), tubercles 2a 17 (17–18) apart. Prosternal apodeme 6 (6–6.5). Opisthosoma with 22 (21–23) broad dorsal semiannuli provided with three dorsal ridges; median ridge from forth dorsal semiannulus extended up to 16 (16–17) semiannulus, lateral ridges from first dorsal semiannulus extended up to 16 semiannulus; faint elongated microtubercles on the ridges; 59 (53–59) narrow microtuberculated ventral semiannuli (counted from the first annulus after the coxae II) and 5 semiannuli between coxae and genital coverflap plus 3 transversal rows of lined granules at the base of the coverflap. Setae c2 13 (11–15) on ventral semiannulus 11 (9–11), setae d 50 (43–51) on ventral semiannulus 22 (20–22); setae e 13 (13–15) on ventral semiannulus 39 (33–39); setae f 20 (15–23) on ventral semiannulus 54 (48–54). 5 annuli after setae f. Setae h2 53 (40–70) very thin at the apex, h1 very minute about 1. Genital coverflap 8 (8–11), 18 (18–19) wide, with 14 (12–14) striae; setae 3a 52 (43–52), 11 (10–13) apart.
MALE (n=2). Similar in shape and prodorsal shield arrangement to female, 160–168. Prodorsal shield 37–41; setae sc 24–31, 23 apart. Opisthosoma with 22 dorsal semiannuli and 49–51 ventral semiannuli; genital region 17 wide; setae 3a 41.
Schematic drawings of Notallus pesthae sp. n.: AD Dorsal view of anterior body region CG Female coxigenital region em Empodium GM Male genital region IG Internal female genitalia L Lateral view L1 Leg I. Scale bar: 10 μm for AD, CG, IG, GM, L, PM; 5 μm for L1; 2.5 μm for em.
Pistacia vera L. (Anacardiaceae), Pistachio.
Vagrant on leaves; no apparent damage was observed.
Akhijahan village, Gogan, Iran (37°47'14"N, 45°57'03"E), 1, 346 m above sea level; late July 2011, coll. P. Lotfollahi.
Holotype: single female on a microscope slide (PV-IEA-AN11L-1) (deposited at the Acarology Laboratory, Department of Plant Protection, Faculty of Agriculture, University of Tabriz, Tabriz, Iran). Paratypes: 11 females and 4 males mounted on separate microscope slides.
Mites preserved in Oudemans’ fluid as extracted from the same sample as the type specimens.
The specific epithet is coming from the Persian common name pesteh given to pistachio.
This is the first record of a species belonging to the genus Notallus on plants of the Anacardiaceae family.
The genus Notallus is characterized by both lateral and middorsal ridges beginning on the forth dorsal semiannulus (
The authors are grateful to Prof. Radmila Petanović (Department of Entomology and Agricultural Zoology, Faculty of Agriculture, University of Belgrade, Serbia) for her critical review of this manuscript. This research was partially supported by the University of Tabriz, Iran, and University of Bari Aldo Moro, Italy.