Citation: Cabezas P, Macpherson E (2014) A new species of Paramunida Baba, 1988 from the Central Pacific Ocean and a new genus to accommodate P. granulata (Henderson, 1885). ZooKeys 425: 15–32. doi: 10.3897/zookeys.425.7882
The genus Paramunida belongs to the most diverse family of galatheoids and it is commonly reported from the continental slope across the Indian and Pacific Oceans. Examination of material collected by the NOAA RV Townsend Cromwell Cruise near Christmas (Kiritimati) Island, Kiribati, revealed the existence of a new species of Paramunida (P. haigae), which represents the fourth record of the genus for the Central Pacific. Furthermore, recent efforts to unravel phylogenetic relationships and diversification patterns in Paramunida revealed P. granulata (Henderson, 1885) to be the most basally diverging taxon within the genus. This species is clearly distinguished from other species of Paramunida by the spinulation of the carapace and the length of the distomesial spine of the second antennal peduncle article, which in combination with a high level of genetic divergence suggest that this species represents a separate monotypic lineage. A new genus, Hendersonida gen. n., is proposed to accommodate this species based on morphological and molecular evidence. An updated dichotomous identification key for all species of Paramunida is presented.
New species, Paramunida, new genus, Hendersonida, Munididae, squat lobster, morphology, phylogeny
Squat lobsters are abundant and highly visible crustaceans in the deep sea (
The genus Paramunida Baba, 1988, recently transferred to the family Munididae (
The genus includes 40 genetically distinct yet morphologically very similar species (
Furthermore, during a recent visit to Los Angeles County Museum of Natural History, some Paramunida specimens previously identified as Munida hawaiiensis (Baba, 1981) were discovered to be an undescribed species. The material examined was collected by the NOAA ship RV Townsend Cromwell in Christmas (Kiritimati) Island, Kiribati, in the Central Pacific Ocean. To date, only the endemic species Paramunida hawaiiensis (Baba, 1981) from Hawaii, Paramunida spatula Macpherson, 2006 from the Austral Archipelago and Paramunida echinata Macpherson, 1999 from the Marquesas Islands are known from Central Pacific waters. Therefore, the new species described here is the fourth record of the genus for the region. Finally, we present an updated dichotomous key to species of Paramunida.
We studied material collected by the NOAA RV Townsend Cromwell Cruise during February–March 1973 in the Central Pacific Ocean. The new described species in this study is deposited in Los Angeles County Museum of Natural History, Los Angeles (LACM). The terminology used mainly follows
(modified from
Paramunida setigera Baba, 1988; by original designation.
The Munida scabra group was recognized by K. Baba in 1981. It included five species – Munida scabra (Henderson, 1885), Munida granulata (Henderson, 1885), Munida proxima (Henderson, 1885), Munida tricarinata (Alcock, 1894) and Munida hawaiiensis (Baba, 1981) – all characterized by having a short rostrum, carapace without transverse ridges covered by spinules and granules, the antennal peduncle with a well-developed anterior prolongation of article 1, and male gonopods absent from first abdominal somite. All these peculiarities suggested that the scabra group represented an independent lineage from Munida, but further investigations were recommended. Later work confirmed the taxonomic significance of this group and the genus Paramunida Baba, 1988 was formally described in a report on the chirostylid and galatheid crustaceans from the “Albatross” Philippine Expedition (
Holotype: Christmas (Kiritimati) Island, Line Islands, Kiribati, 01°51.3'N, 157°30.4'W, February–March 1973, 183 m (NOAA RV Townsend Cromwell Cruise): male, 16.6 mm (LACM–CR1973-3312). Paratypes: collected with holotype: 9 males 11.4–17.2 mm (2 broken), 3 females, 13.5–14.1 mm, 2 ovigerous females, 11.6–14.2 mm (LACM–CR1973-3313).
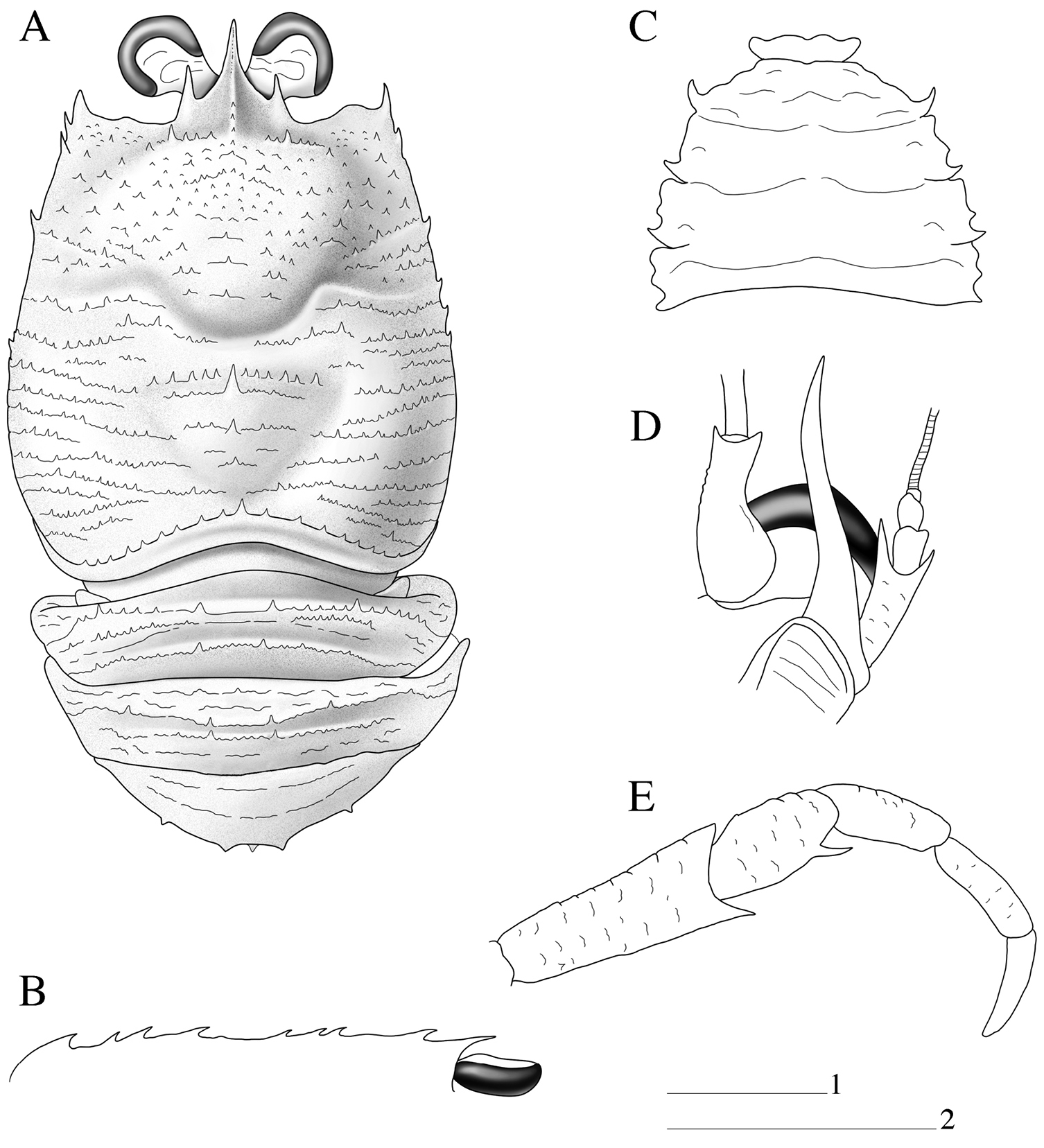
Carapace: As long as broad, dorsal surface covered with spinules; each spinule usually on short arcuate striae, with few short uniramous setae. Epigastric region with 2 spines, each behind supraocular spine; with median row of spinules behind rostral spine. Mesogastric region with median row of 3 small spines. Anterior branch of cervical groove with short setae. Cervical groove distinct. Cardiac and anterior branchial regions slightly circumscribed. Cardiac region with a median row of 3 small spines, first thicker than others. Each branchial region with row of spines near cardiac region. Frontal margin slightly concave. Lateral margins convex, with some spines and iridescent setae on anterior half. Anterolateral spine well developed, reaching sinus between rostral and supraocular spines. Rostral spine spiniform, with thin dorsal longitudinal carina; supraocular spines well developed and slender and shorter than rostrum (Figs 1A, B, 3).
Paramunida haigae sp. n. male holotype, 16.6 mm (LACM–CR1973-3312). Christmas (Kiritimati) Island. A carapace and abdomen, dorsal view B carapace, lateral profile C sternum D left antennule and antenna, ventral view E right maxilliped 3, lateral view. Scale: 5 mm (scale 1 for A–C, E; scale 2 for D).
Sternum: Thoracic sternite 4 with few arcuate striae; sternites 5–7 smooth (Fig. 1C).
Abdomen: Abdominal somites 2–3 each with 4 well-developed spines on anterior ridge, posterior ridge with 2 median spines. Abdominal somite 4 with 4 spines on anterior ridge; posterior ridge with distinct single median spine. Ridges with numerous spinules and a few small spines (Fig. 1A).
Eyes: Maximum corneal diameter more than one-third distance between bases of anterolateral spines.
Antennule: Article 1 slightly exceeding corneae, with distomesial spine small and as long as distolateral; about twice longer than wide and with fringe of long setae along lateral margin; lateral margin with distal slender portion about half as long as proximal convex portion (Fig. 1D).
Antenna: Anterior prolongation of article 1 overreaching antennular peduncle by about one-third of its length. Article 2 about twice length of article 3 and twice longer than wide, ventral surface with scales; distomesial spine spiniform without tuff of setae, overreaching end of article 3, not reaching end of antennal peduncle, reaching mid-length of anterior prolongation of article 1, and clearly not reaching end of basal article of antennule, distolateral spine not reaching end of article 3; article 3 about 1.5 times longer than wide and unarmed (Fig. 1D).
Maxilliped 3: Ischium about twice length of merus measured along extensor margin, flexor margin bearing long distal spine; merus with well-developed median spine on flexor margin; extensor margin unarmed (Fig. 1E).
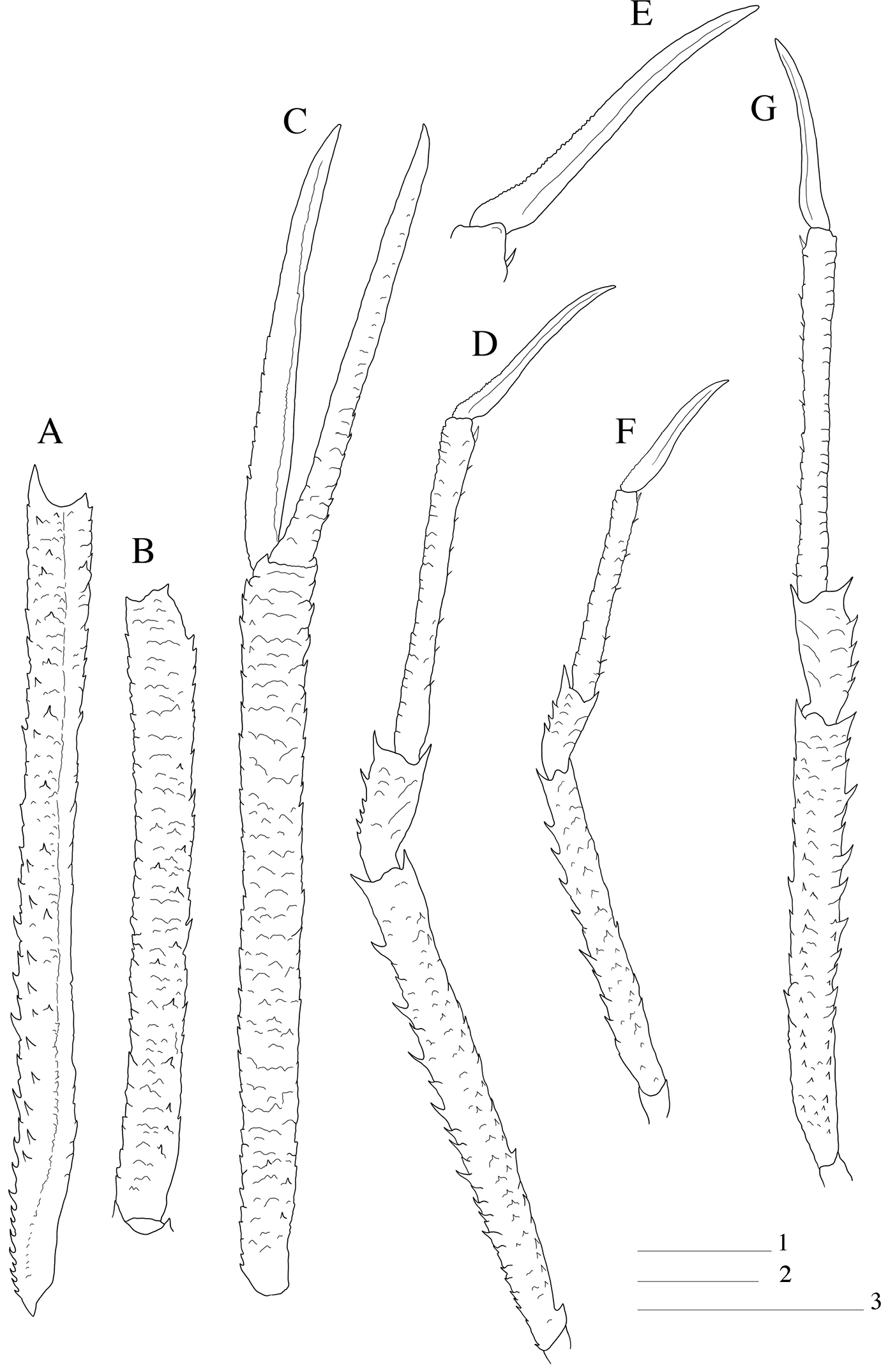
Pereopod 1 (cheliped): Long and slender, squamate, between 6.5–7.5 times carapace length; carpus about as long as palm, and 7–10 times longer than high; palm 1.1–1.5 times fingers length. Base of carpus without bundle of setae (Fig. 2A–C).
Paramunida haigae sp. n. male holotype, 16.6 mm (LACM–CR1973-3312). Christmas (Kiritimati) Island. A left merus P1, dorsal view B left carpus P1, dorsal view C left P1, palm and fingers, dorsal view D right P3, lateral view E right P3 dactylus F male paratype, 11.5 mm (LACM–CR1973-3313), right P2, lateral view. G left P4, lateral view. Scale: 5 mm (scale 1 for A–C, D, G; scale 2 for F; scale 3 for E).
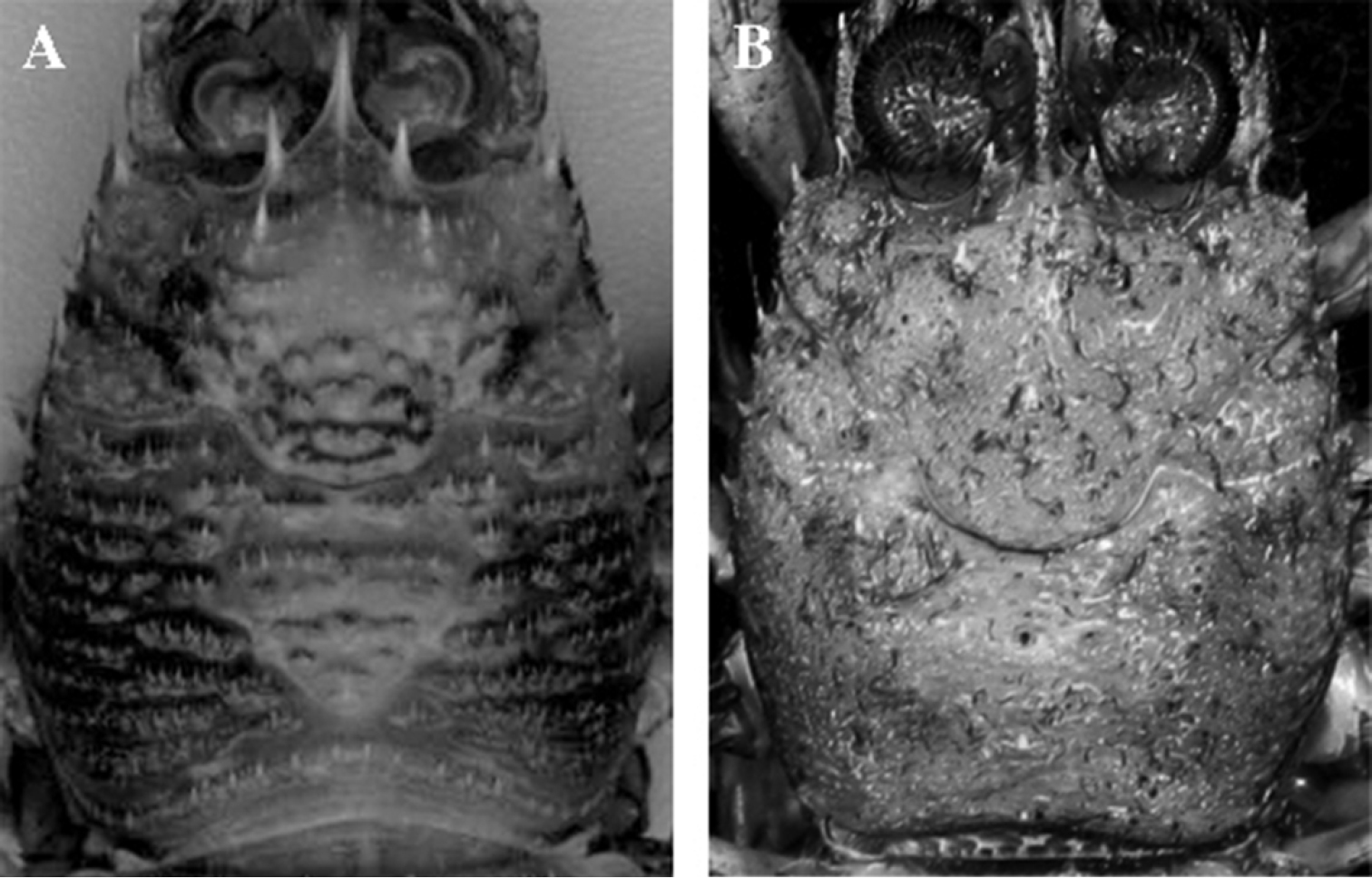
Dorsal surface of the carapace, dorsal view. A Paramunida haigae sp. n., NOAA Townsend Cromwell Cruise, holotype, male 16.6 mm. B Hendersonida granulata, BATHUS 2, Stn CP735, female, 13.7 mm.
Pereopods 2–4 (P2 lacking in holotype): Long and slender, with scales on lateral sides of meri, carpi and propodi; scales with short setae. P2 2.5–3.5 times carapace length, merus 1.1–1.6 times longer than carapace, about 8–10 times as long as high, 4 times as long as carpus and 1.5 times as long as propodus; propodus about 7–10 times as long as high, and 1.4–1.7 times dactylus length. Merus with well-developed spines on extensor margin, increasing in size distally; flexor margin with few spines and one well developed distal spine; row of small spines along flexolateral margin. Carpus with few small extensor spines, small distal spine on extensor and flexor margin. Propodus with small movable flexor spines. Dactylus compressed, slightly curved, with longitudinal carinae along mesial and lateral sides, flexor border unarmed. End of P2 carpus not reaching end of P1 merus. P3 with similar spination and article proportions as P2; propodus slightly longer than P2 propodus, merus and dactylus as long as those of P2. P4 as long as P2; merus 1.1–1.3 times carapace length; propodus and dactylus slightly longer than those of P3; merocarpal articulation clearly exceeding end of anterior prolongation of article1 of antennal peduncle (Fig. 2D–G).
This species is dedicated to the renowned carcinologist Janet Haig (1925–1995) who first classified the material examined.
Paramunida haigae sp. n. closely resembles Paramunida antares Cabezas, Macpherson & Machordom, 2010 from New Caledonia. The new species is readily separated from Paramunida antares in having the rostrum spiniform rather than triangular. Moreover, the mesogastric region in Paramunida antares has 3 well-developed spines, but these spines are very small in Paramunida haigae sp. n. The two species also differ in the article 2 of the antennal peduncle: twice as long as wide in the new species but only 1.5 times in P. antares. Finally, the distomesial spine of antennal article 2 clearly overreaches the end of article 3 in the new species, but this spine only reaches the end of the article 3 in Paramunida antares.
The new species is also very close to Paramunida achernar Cabezas, Macpherson & Machordom, 2010 from Tonga. Paramunida haigae sp. n. can be distinguished from Paramunida achernar by having 3 small mesogastric spines (vs. 3 well-developed spines in Paramunida achernar). Furthermore, the anterior prolongation of antennal article 1 is clearly longer in Paramunida haigae sp. n., overreaching the antennular peduncle by about one-third of its length but only by one-fourth in Paramunida achernar, and the distomesial spine of antennal article 2 overreaching the end of article 3 in the new species (vs. only reaching the end of the article 3 in Paramunida achernar). Finally, the merocarpal articulation of P3 clearly exceeds the anterior prolongation of the antennal article 1 in the new species, only slightly exceeding the anterior prolongation in Paramunida achernar.
Of the regional Central Pacific Paramunida species, Paramunida haigae sp. n. can be easily distinguished from Paramunida hawaiiensis Baba, 1981 from Hawaii in having the rostral spine larger than supraocular spines instead of smaller or at most equal to supraocular spines. Furthermore, the distomesial spine of article 2 reaches end of antennal peduncle in Paramunida hawaiiensis but never reaches it in the new species. The new species can also be easily distinguished from Paramunida echinata Macpherson, 1999 from Marquesas Islands in having the rostral spine spiniform instead of triangular. Finally, Paramunida haigae sp. n. is also easily distinguishable from Paramunida spatula Macpherson, 2006 from the Austral archipelago by the shape of the anterior prolongation of antennal article 1.
Christmas (Kiritimati) Island, Kiribati, at 183 m depth.
Munida granulata Henderson, 1885; here designated and by monotypy.
Carapace as long as wide; dorsal surface granulose, with some scattered spines and small spinules with short uniramous setae and without transverse ridges; few and short setae along anterior branch of cervical groove; posterior margin with some spines; rostrum spiniform, upturned distally, larger and thicker than supraocular spines; supraocular spines small, clearly not reaching midlength of rostrum and falling short the end of corneae; margin between rostral and supraocular spines straight or slightly concave; anterolateral spines well developed situated at front near anterolateral angles, reaching the level between rostrum and supraocular spines; lateral margins with some spines. Eyes large, maximum corneal diameter about half distance between bases of anterolateral spines. Lateral margin of antennular article 1 with distal slender portion about half as long as proximal inflated portion, with 2 distal spines. Antennal peduncle with anterior prolongation of article 1 spiniform; article 2 with distomesial spine long, almost reaching end of anterior prolongation of article 1. P1–P4 long and slender, squamate; P2–P4 dactyli slender, curved and unarmed along flexor margin. Male gonopods only present on the second abdominal somites.
The generic name Hendersonida acknowledges the meaningful contributions of John Robertson Henderson (1863–1925) to the field of crustacean taxonomy. Gender: feminine.
The carapace dorsal surface devoid of distinct transverse ridges or striae, the rostral spine broad at base, the antennal peduncle with a well-developed anterior prolongation of article 1 and the male gonopods absent from the first abdominal link this new genus to Paramunida Baba, 1988. This close relationship has been confirmed by molecular evidence that have rendered this new genus as the sister group of Paramunida (
(modified from
Philippines, Indonesia, Queensland, New Caledonia, Loyalty Islands, Fiji, Tonga, Futuna Island, Vanuatu, Wallis Islands and Bayonnaise Bank, between 395 and 650 m.
Detailed illustrations for Hendersonida granulata are included in
The present study updates the taxonomy of the genus Paramunida Baba, 1988 by describing a new species from the Central Pacific Ocean and transferring one species to a new genus. Deep waters in the Central Pacific Ocean have been poorly sampled and our knowledge on diversity of squat lobster fauna is scarce (
The new genus here described contains only Hendersonida granulata. Although morphologically very similar to Paramunida, recent studies revealed that this species was phylogenetically and genetically very different from the other species of the genus (
Bayesian tree of the combined dataset (16S + ND1) as modified from
Hendersonida granulata is a widespread species distributed from the Philippines to to Northern Australia and the South Western Pacific, including New Caledonia, Vanuatu, Fiji, Tonga and Wallis and Futuna, between 395 and 650 m. This is unusual, since most deep-sea squat lobsters are characterized by having reduced geographic ranges confined to a single archipelago or a biogeographic area (
| 1 | Anterior prolongation of antennal article 1 spatulate | Paramunida spatula Macpherson, 2006 |
| – | Anterior prolongation of antennal article 1 spiniform | 2 |
| 2 | Rostral spine smaller or at most equal to supraocular spines | 3 |
| – | Rostral spine larger than supraocular spines | 8 |
| 3 | Margin between rostral and supraocular spines clearly convex | Paramunida curvata Macpherson, 2004 |
| – | Margin between rostral and supraocular spines straight or slightly concave | 4 |
| 4 | Antennal article 2 with minute distomesial spine | Paramunida microrhina Cabezas, Macpherson & Machordom, 2010 |
| – | Antennal article 2 with well-developed distomesial spine | 5 |
| 5 | Mesogastric region with 3 well-developed spines in midline | Paramunida hawaiiensis (Baba, 1981) |
| – | Mesogastric region with minute spines | 6 |
| 6 | Rostrum triangular | 7 |
| – | Rostrum spiniform | Paramunida aurora Cabezas & Chan, 2014 |
| 7 | Sternal plastron with numerous striae. Bundle of setae at base of carpus of P1 present | Paramunida setigera Baba, 1988 |
| – | Sternal plastron with few striae on each side of sternites 5–7. Bundle of setae at base of carpus of P1 absent | Paramunida tenera Cabezas, Macpherson & Machordom, 2010 |
| 8 | P2–P4 propodi slender, about 20 times as long as broad | Paramunida longior Baba, 1988 |
| – | P2–P4 propodi 7–14 times as long as broad | 9 |
| 9 | Distomesial spine of antennal article 2 mucronated or bluntly produced | 10 |
| – | Distomesial spine of antennal article 2 spiniform | 23 |
| 10 | Mesogastric region with 1 (rarely 2) spine | 11 |
| – | Mesogastric region with a median row of 3 or 4 distinct spines | 14 |
| 11 | Sternal plastron with numerous striae | Paramunida proxima (Henderson, 1885) |
| – | Sternal plastron with few striae on each side of sternites 5–7 | 12 |
| 12 | Distomesial spine of antennal article 2 clearly overreaching antennal peduncle | 13 |
| – | Distomesial spine of antennal article 2 nearly reaching end of antennal peduncle | Paramunida antipodes Ahyong & Poore, 2004 |
| 13 | Distolateral spine of antennal article 2 not reaching end of article 3 | Paramunida akaina Cabezas & Chan, 2014 |
| – | Distolateral spine of antennal article 2 overreaching end of article 3 | Paramunida belone Macpherson, 1993 |
| 14 | Distomesial spine of antennal article 2 slightly or clearly overreaching antennal peduncle | 15 |
| – | Distomesial spine of antennal article 2 never reaching end of antennal peduncle | 20 |
| 15 | Lateral margin of antennular article 1 with distal slender portion as long as proximal inflated portion | Paramunida spica Cabezas, Macpherson & Machordom, 2010 |
| – | Lateral margin of antennular article 1 with distal slender portion about half as long as proximal inflated portion | 16 |
| 16 | Distolateral spine of antennal article 2 exceeding antennal article 3 | Paramunida salai Cabezas, Macpherson & Machordom, 2009 |
| – | Distolateral spine of antennal article 2 not reaching end of antennal article 3 | 17 |
| 17 | Mesial margin of antennal article 2, including distal spine, straight. Rostrum triangular or spiniform | 18 |
| – | Mesial margin of antennal article 2, including distal spine, convex. Rostrum spiniform | 19 |
| 18 | Rostrum triangular | Paramunida ascella Cabezas, Macpherson & Machordom, 2010 |
| – | Rostrum spiniform | Paramunida mozambica Cabezas, Macpherson & Machordom, 2010 |
| 19 | Distomesial spine of antennal article 2 shorter than rest of article 2. Gastric region with short striae. Antennal article 3 about 1.5 times longer than broad | Paramunida stichas Macpherson, 1993 |
| – | Distomesial spine of antennal article 2 as long as rest of article 2. Gastric region with moderate-sized striae. Antennal article 3 about twice longer than broad | Paramunida lophia Cabezas, Macpherson & Machordom, 2009 |
| 20 | Mesogastric region without well-developed spines | Paramunida parvispina Cabezas, Macpherson & Machordom, 2010 |
| – | Mesogastric region with a row of 3 or 4 distinct spines | 21 |
| 21 | Sternal plastron with numerous striae. Article 2 of antennal peduncle bluntly produced distomesially | Paramunida evexa Macpherson, 1993 |
| – | Sternal plastron with few striae, sternites 5–7 with few striae on each side. Article 2 of antennal peduncle produced distomesially ending in distinct spine | 22 |
| 22 | Rostrum triangular. Propodus of walking legs more than 1.5 times dactylus length | Paramunida echinata Macpherson, 1999 |
| – | Rostrum spiniform. Propodus of walking legs slightly longer than dactylus | Paramunida labis Macpherson, 1996 |
| 23 | Rostrum with thick dorsal carina | Paramunida cristata Macpherson, 2004 |
| – | Rostrum with thin dorsal carina | 24 |
| 24 | Distomesial spine of antennal article 2 clearly exceeding antennal peduncle | Paramunida leptotes Macpherson & Baba, 2009 |
| – | Distomesial spine of antennal article 2 at most reaching end of antennal peduncle | 25 |
| 25 | Mesogastric region with 1 (rarely 2) spine | 26 |
| – | Mesogastric region with a row of 3 or 4 distinct spines | 29 |
| 26 | Median cardiac region with 1 spine | Paramunida pronoe Macpherson, 1993 |
| – | Median cardiac region with a row of 3 or 4 spines | 27 |
| 27 | Tufts of long and dense setae along anterior branch of cervical groove | Paramunida crinita Cabezas, Macpherson & Machordom, 2010 |
| – | Few and short setae along anterior branch of cervical groove | 28 |
| 28 | Sternal plastron with few striae, sternites 5–7 only with few striae on each lateral side | Paramunida polita Macpherson, 1993 |
| – | Sternal plastron with numerous striae | Paramunida scabra (Henderson, 1885) |
| 29 | Sternal plastron with numerous striae | 30 |
| – | Sternal plastron with few striae, sternites 5–7 only with few striae on each lateral side | 31 |
| 30 | Antennal article 3 twice as long as broad. Few and short setae along anterior branch of cervical groove | Paramunida thalie Macpherson, 1993 |
| – | Antennal article 3 slightly longer than broad. Tufts of long and dense setae along anterior branch of cervical groove | Paramunida tricarinata (Alcock, 1894) |
| 31 | Distomesial spine of antennal article 2 reaching or slightly exceeding end of antennal peduncle. Distolateral spine of antennal article 2 reaching or slightly exceeding end of antennal article 3 | 32 |
| – | Distomesial spine of antennal article 2 not reaching end of antennal peduncle. Distolateral spine of antennal article 2 not reaching end of antennal article 3 | 33 |
| 32 | Antennal article 3 as long as wide | Paramunida aspera Cabezas & Chan, 2014 |
| – | Antennal article 3 about 1.5 times longer than wide | Paramunida marionis Cabezas, Macpherson & Machordom, 2010 |
| 33 | Antennal article 3 more than twice longer than broad | Paramunida amphitrita Macpherson, 1996 |
| – | Antennal article 3 as long as broad or at most 1.5 times longer than broad | 34 |
| 34 | Antennal article 2 as long as or more than 3 times longer than broad | 35 |
| – | Antennal article 2 at most twice longer than broad | 36 |
| 35 | Distomesial spine of antennal article 2 reaching or slightly overreaching end of antennal article 3. Spinules on gastric and hepatic regions mostly forming groups arising from scale-like striae | Paramunida pictura Macpherson, 1993 |
| – | Distomesial spine of antennal article 2 not reaching end of antennal article 3. Spinules on gastric and hepatic regions mostly not in groups, lacking scaly striae | Paramunida poorei Cabezas, Macpherson & Machordom, 2010 |
| 36 | Antennal article 2 slightly longer than broad | Paramunida cretata Macpherson, 1996 |
| – | Antennal article 2 twice longer than broad | 37 |
| 37 | Row of small epigastric spines behind rostral spine absent | Paramunida luminata Macpherson, 1996 |
| – | Row of small epigastric spines behind rostral spine present | 38 |
| 38 | Rostrum triangular | Paramunida antares Cabezas, Macpherson & Machordom, 2010 |
| – | Rostrum spiniform | 39 |
| 39 | Mesogastric region with 3 small spines. Merocarpal articulation of P3 clearly exceeding end of anterior prolongation of antennal article 1 | Paramunida haigae sp. n. |
| – | Mesogastric region with 3 well-developed spines. Merocarpal articulation of P3 slightly exceeding end of anterior prolongation of antennal article | Paramunida achernar Cabezas, Macpherson & Machordom, 2010 |
We are very grateful to A. Crosnier, P. Bouchet, L. Corbari, and B. Richer de Forges for their support and for making Paramunida material available to us. Thanks are also due to Annie Machordom for her constant support and guidance with previous studies on Paramunida and J. Macpherson for technical assistance with illustrations. Finally, the first author would like to express sincere gratitude to Adam Wall, Regina Wetzer and Dean Pentcheff for the opportunity to study the squat lobster material at Los Angeles County Museum of Natural History and making her visit so delightful. PC was funded by Research and Collections, Natural History Museum of Los Angeles County.