Citation: Sun X, Wu D (2014) Onychiurid species from Wanda Mountains in China, with descriptions of two new species (Collembola: Onychiuridae). ZooKeys 425: 99–111. doi: 10.3897/zookeys.425.7724
A checklist of onychiurid species from the Wanda Mountains in China is presented. Eighteen species belonging to twelve genera have been found, including two new species. Bionychiurus qinglongensis sp. n. can be easily distinguished from other known species of the genus by the absence of pseudocelli on Th. I tergum and fewer number of vesicles in postantennal organ. Onychiurus heilongjiangensis sp. n. is diagnosed by pseudocellar formulae as 32/133/33352 dorsally and 3/011/31120 ventrally, parapseudocellar formula as 0/000/111001+1m, ratio of anal spine/unguis as 0.6, unguiculus without inner basal lamella, and male ventral organ absent.
Taxonomy, new species, Bionychiurus qinglongensis sp. n. , Onychiurus heilongjiangensis sp. n.
Heilongjiang Province is located in the northeast China, at the highest latitudes and the northernmost end of the country. It neighbors Russia across the Heilongjiang and Wusuli rivers running in its north and east respectively; in the west, it adjoins the Inner Mongolian Autonomous Region; and to its south is Jilin Province. It covers an area of 454, 000 km2, accounting for 4.7% of the nation’s total. However, the fauna of Onychiuridae has been ignored in this area for many years. From 2011, we have investigated this group in Heilongjiang Province and reported several species gradually, including eleven species new to science and five new record species (
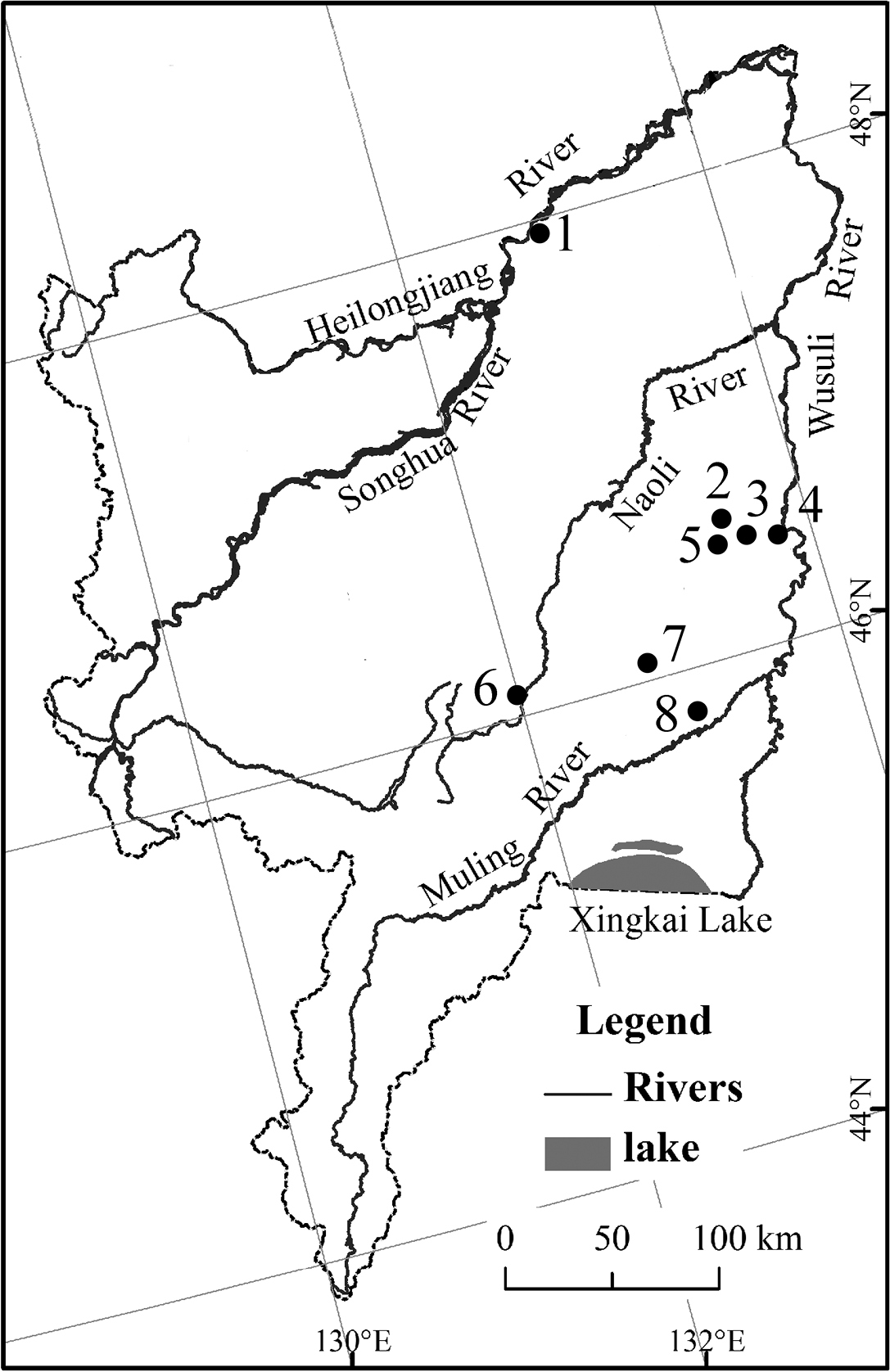
Eight sampling sites of Wanda Mountains have been studied, which are all planted forest with Quercus mongolica Fisch. ex Ledeb., Pinus koraiensis Sieb. et Zucc., Betula costata Trautv. or Populus davidiana Dode (Table 1, Fig. 5). Specimens were collected by Berlese extraction, cleared in lactic acid and then mounted in Marc André II solution. They were studied using a Nikon Eclipse 80i microscope. The material is deposited in the Key Laboratory of Wetland Ecology and Environment, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun.
List of the sampling sites of Wanda Mountains in China.
| No. | Name of the site | City | County | Coordinates | Altitude | Habitats | Date | Collector |
|---|---|---|---|---|---|---|---|---|
| 1 | Jiejinshan | Jiamusi | Tongjiang | 47.9185°N, 132.8503°E | 95 m | Litter and soil under Quercus mongolica Fisch. ex Ledeb. | 7 Aug. 2010 | Donghui Wu et al. |
| 2 | Qiyuan | Shuangyashan | Raohe | 46.6295°N, 133.4319°E | 155 m | Litter and soil under Pinus koraiensis Sieb. et Zucc. | 16 Aug. 2010 | Donghui Wu et al. |
| 3 | Wulindong | Shuangyashan | Raohe | 46.5650°N, 133.6690°E | 207 m | Litter and soil under Pinus koraiensis Sieb. et Zucc. | 18 Sept. 2011 | Haitao Wu and Lihong Song |
| 4 | Zhenbaodao | Shuangyashan | Raohe | 46.4882°N, 133.8454°E | 75 m | Litter and soil under Pinus koraiensis Sieb. et Zucc. | 16 Aug. 2010 | Donghui Wu et al. |
| 5 | Shendingshan | Shuangyashan | Raohe | 46.4760°N, 133.3031°E | 159 m | Litter and soil under Pinus koraiensis Sieb. et Zucc. | 16 Aug. 2010 | Donghui Wu et al. |
| 6 | Qinglongshan | Shuangyashan | Baoqing | 46.1504°N, 131.9591°E | 259 m | Litter and soil under Betula costata Trautv. | 14 Aug. 2010 | Donghui Wu et al. |
| 7 | Bawu’er | Shuangyashan | Baoqing | 46.1370°N, 132.8580°E | 149 m | Litter and soil under Populus davidiana Dode | 15 Aug. 2010 | Donghui Wu et al. |
| 8 | Hulin | Jixi | Hulin | 45.7633°N, 133.0453°E | 91 m | Litter and soil under Quercus mongolica Fisch. ex Ledeb. | 15 Aug. 2010 | Donghui Wu et al. |
Labial types are determined after
Ant. – antennal segments, PAO – postantennal organ, Th. – thoracic segments, Abd. – abdominal segments, ms – microsensillum, pso – pseudocellus, psx – parapseudocellus, Sp – posterior S-chaeta on Abd. V tergum, m – unpaired pseudopore or parapseudocellus.
The pseudocelli, parapseudocelli and pseudopores formula are the number of pseudocelli, parapseudocelli, or pseudopores by half-tergum (dorsally) or half-sternum (ventrally) as follows: head anterior, head posterior/Th. I, II, III/Abd. I, II, III, IV, V (for instance: 32/033/33343).
Allonychiurus songi Sun & Wu, 2012
Locality. Bawu’er, Hulin, Jiejinshan, Qiyuan, Qinglongshan, Shendingshan, Zhenbaodao.
Bionychiurus qinglongensis sp. n.
Locality. Qinglongshan.
Bionychiurus changbaiensis Sun & Wu, 2012
Locality. Zhenbaodao.
Dimorphaphorura sanjiangensis Sun & Wu, 2012
Locality. Bawu’er, Qinglongshan, Shendingshan.
Heteraphorura seolagensis (Lee, 1974)
Locality. Hulin, Jiejinshan, Qiyuan, Qinglongshan, Zhenbaodao.
Hymenaphorura maoerensis Sun, 2014
Locality. Jiejinshan.
Hymenaphorura wusuliensis Sun & Wu, 2011
Locality. Wulindong.
Oligaphorura chankaensis Sun & Wu, 2012
Locality. Shendingshan.
Oligaphorura koreana (Weiner, 1994)
Locality. Jiejinshan, Qiyuan, Zhenbaodao.
Oligaphorura ursi Fjellberg, 1984
Locality. Hulin, Shendingshan.
Onychiurus heilongjiangensis sp. n.
Locality. Qinglongshan, Jiejinshan.
Protaphorura bicampata (Gsin, 1956)
Locality. Hulin.
Protaphorura minima Sun, Zhang & Wu, 2013
Locality. Jiejinshan, Qiyuan, Qinglongshan.
Protaphorura nutak (Yosii, 1972)
Locality. Zhenbaodao.
Psyllaphorura raoheensis Sun & Wu, 2011
Locality. Bawu’er, Qinglongshan, Shendingshan, Wulindong, Zhenbaodao.
Sensillonychiurus changchunensis Sun & Wu, 2012
Locality. Shendingshan.
Supraphorura furcifera (Börner, 1901) new record
Locality. Zhenbaodao.
Thalassaphorura macrospinata Sun & Wu, 2012
Locality. Hulin, Jiejinshan, Qinglongshan, Zhenbaodao.
Thalassaphorura problematica Sun, Deharveng & Wu, 2013
Locality. Wulindong, Zhenbaodao.
http://zoobank.org/2811C6C2-4DCD-43EA-8A52-CF3210DB03F0
Holotype male, two female paratypes on slides – China, Heilongjiang, Shuangyashan, Baoqing, Qinglongshan (46.1504°N, 131.9591°E), 14.Aug.2010, litter and soil under Betula costata Trautv., Berlese extraction, Wu Donghui et al. leg. (LD-10-344).
Pso formulae as 32/033/33343 dorsally and 11/000/01(0)010 ventrally. Subcoxae 1 of legs I, II and III with 1, 1 and 1 pso respectively. Psx formula as 00/000/100001m ventrally. PAO with 14–17 compound vesicles. Th. I–III sterna with 0+0, 1+1, 1+1 chaetae respectively. Ventral tube with 1+1 basal chaetae. Subcoxae 1 of legs I, II and III with 5, 7 and 6 chaetae respectively.
Body length: females 1.30–1.37 mm, male holotype 1.08 mm. Shape of body typical of the genus: cylindrical with strong anal spines on papillae. Color in alcohol white.
Pso formulae: 32/033/33343 dorsally and 11/000/01(0)010 ventrally (Figs 1A, B, C). Subcoxae 1 of legs I–III with 1 pso each. Parapseudocellar formulae: 00/000/100001m ventrally, dorsally psx absent (Figs 1A, B, C). Pseudopore formulae: 00/011/11110 dorsally and 00/111/001m1m 0 ventrally (Figs 1A, B, C).
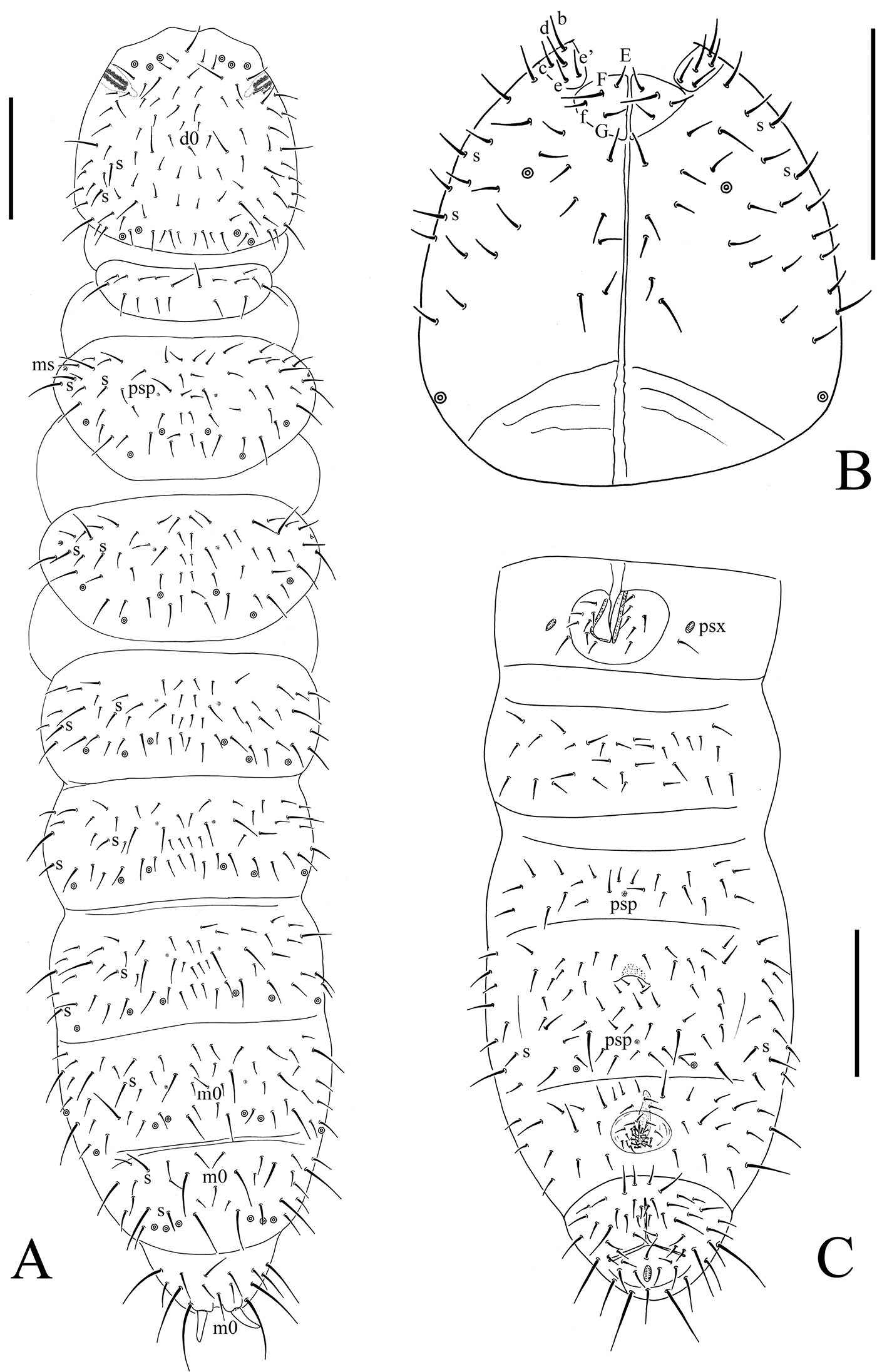
Bionychiurus qinglongensis sp. n. A dorsal side of body B ventral side of head C general view of Abd. I–VI sterna. Scale bars: 0.1 mm (A–C).
Head. Antennae short, 1.2 times as long as head. Length ratio of antennal segments I : II : III : IV = 1 : 1.5 : 1.5 : 2. Subapical organite on Ant. IV with globular apex; basolateral ms above the second proximal row of chaetae. Ant. III sensory organ consisting of 5 papillae, 5 guard chaetae, 2 small sensory rods, 2 granulated sensory clubs and a later ms (Fig. 2A). Ant. II with 15 chaetae. Ant. I with 10 chaetae. Antennal base weakly marked. PAO with 14–17 compound vesicles arranged in two rows along axis of organ (Fig. 2B). Dorsal cephalic chaeta d0 present. 4+4 p-chaetae between posterior a-pso on head, p1 in line with others (Fig. 1A). Mandible with strong molar plate and 4 apical teeth. Maxilla bearing 3 teeth and 6 lamellae. Maxillary palp simple with 1 basal chaeta and 2 sublobal hairs. Labral chaetal formula 4/342. Labium with 6 proximal, 4 basomedian (E, F, G and f), and 5 basolateral chaetae (b, c, d, e, e’) (Fig. 1B); labial palp of AC type, labial papillae A–E with 1, 4, 0, 3, and 3 guard chaetae respectively. 5+5 postlabial chaetae along ventral groove (Fig. 1B).
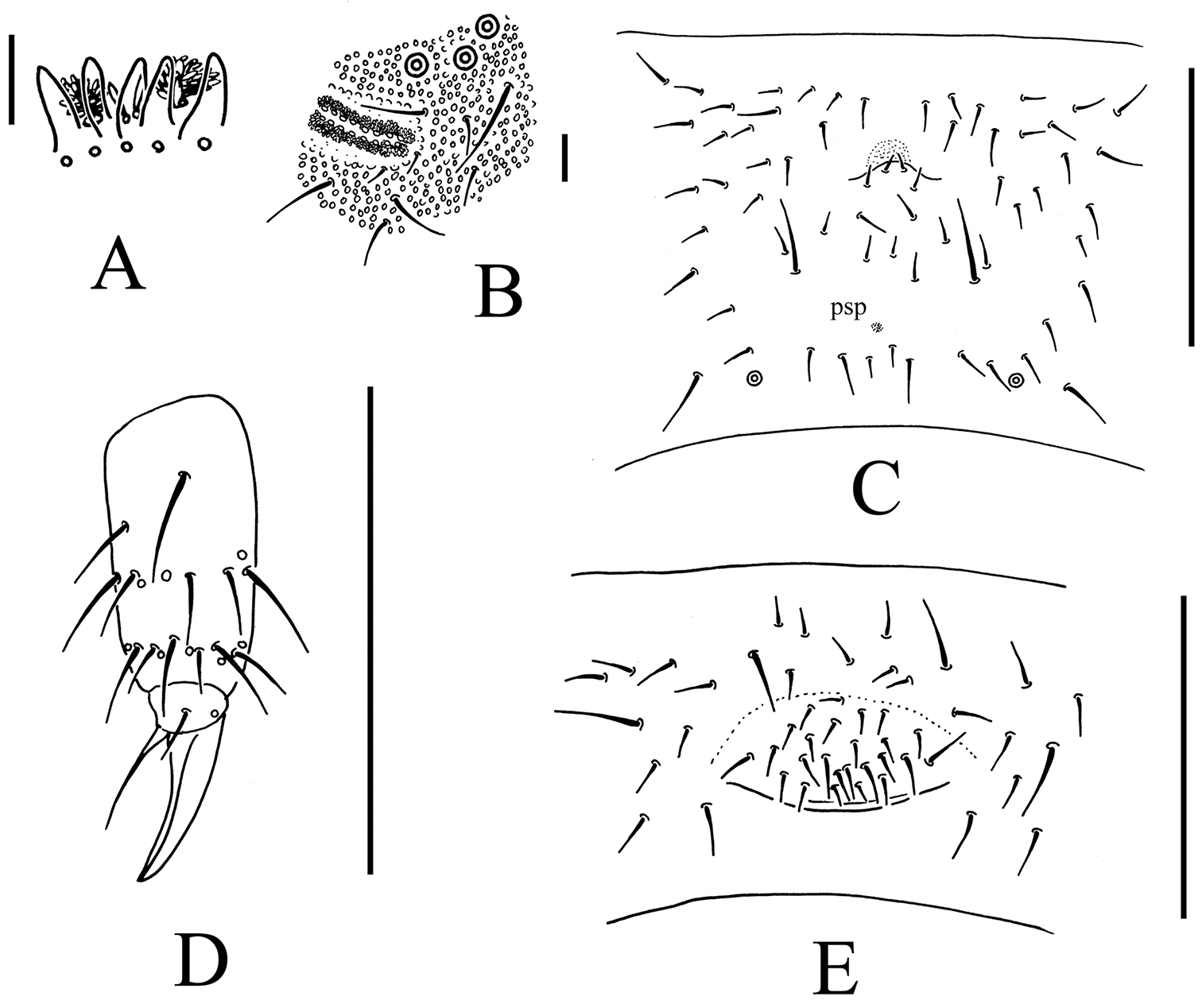
Bionychiurus qinglongensis sp. n. A Ant. III sensory organ B PAO C Abd. IV sternum D distal part of leg III E female genital plate. Scale bars: 0.1 mm (C–E), 0.01 mm (A–B).
Body chaetotaxy. S-chaetae formulae as 11/022/222120 dorsally and 11/000/000100 ventrally (Figs 1A, B, C). Tiny and blunt ms, present on Th. II–III dorsal-laterally (Fig. 1A). Dorsal ordinary chaetae differentiated, Sp : m1 : p1 ratio on Abd. V = 1 : 0.8 : 2.5 (Fig. 1A). Th. I tergum with 9–12 chaetae. Th. II–III terga with 5+5 and Abd. I–III terga with 3–4+3–4 chaetae on both side of axial line respectively and no unpaired axial chaetae (Fig. 1A). On each other abdominal terga from Abd. IV to Abd. VI tergum axial chaetae m0 present (Fig. 1A). Th. I–III sterna with 0+0, 1+1, 1+1 chaetae between legs respectively.
Appendages. Subcoxae 1 of legs I, II and III with 5, 7 and 6 chaetae, subcoxae 2 with 1, 5 and 5 chaetae respectively. Coxae of legs I, II and III with 4, 11 and 14 chaetae, trochanters with 10 chaetae each and femora with 15, 17 and 17 chaetae. Tibiotarsi of legs I, II and III with 22 (3, 8, 11), 20 (1, 8, 11) and 21 (2, 8, 11) chaetae (Fig. 2C). Unguis without teeth. Unguiculus slender and pointed, 0.8–0.9 times as long as inner edge of unguis, without inner basal lamella (Fig. 2C). Ventral tube with 6–9+6–9 distal chaetae and 1+1 basal chaetae, without anterior chaetae (Fig. 1C). Furca reduced to cuticular fold with 4 small dental chaetae posteriorly and two manubrial rows of chaetae (Fig. 2B)
Genital plate with 16–25 chaetae in females (Fig. 2D), 26 in male. Male ventral organ absent. Anal valves with numerous acuminate chaetae; each lateral valve with a0, 2a1 and 2a2; upper valve with chaetae a0, 2a1, 2b1, 2b2, c0, 2c1 and 2c2 (Fig. 1C). Anal spines set on distinct papillae, as long as inner edge of hind unguis (Fig. 1A).
Named for the name of mountain (Qinglongshan, and -shan means mountain in Chinese) where the species was found.
Until now, there are four known species belonging to the genus Bionychiurus: Bionychiurus changbaiensis Sun & Wu, 2012 from China, Bionychiurus normalis (Gisin, 1949) from Europe, Bionychiurus oblongatus (Lee & Park, 1986), and Bionychiurus yongyeonensis (Yosii, 1966) from South Korea (
http://zoobank.org/540034F0-0A0F-4599-9E38-91BDC042C420
Holotype male, 7 female and 6 male paratypes on slides – China, Heilongjiang, Shuangyashan, Baoqing, Qinglongshan (46.1504°N, 131.9591°E), 14.Aug.2010, litter and soil under Betula costata Trautv., Berlese extraction, Wu Donghui et al. leg. (LD-10-444, LD-10-445); 12 female and 3 male paratypes on slides – China, Heilongjiang, Jiamusi, Tongjiang, Jiejinshan (47.9185°N, 132.8503°E), 7.Aug.2010, litter and soil under Quercus mongolica Fisch. ex Ledeb., Berlese extraction, Wu Donghui et al. leg. (LD-10-484, LD-10-485, LD-10-486, LD-10-488).
Diagnosis. Pso formulae as 32/133/33352 dorsally and 3/011/31120 ventrally. Psx formula as 0/000/111001+1m ventrally.. Ratio of AS/unguis as 0.6. Unguiculus without inner basal lamella. Male ventral organ absent.
Body length: females 1.6–1.8 mm, males 1.4–1.6 mm; holotype 1.6 mm. Shape of body typical of the genus: cylindrical with anal spines on papillae, Abd. III–IV more or less broadened. Color in alcohol white.
Pso formulae: 32/133/33352 dorsally and 3/011/31120 ventrally (Figs 3A, H, 4A). Subcoxae 1 of legs I–III with 2 pso each. Parapseudocellar formulae: 0/000/111001+1m ventrally, dorsally psx absent (Figs 3A, H, 4A). Pseudopore formulae as 00/011/11110 dorsally and 00/111/0001m00 ventrally (Figs 3A, H, 4A).
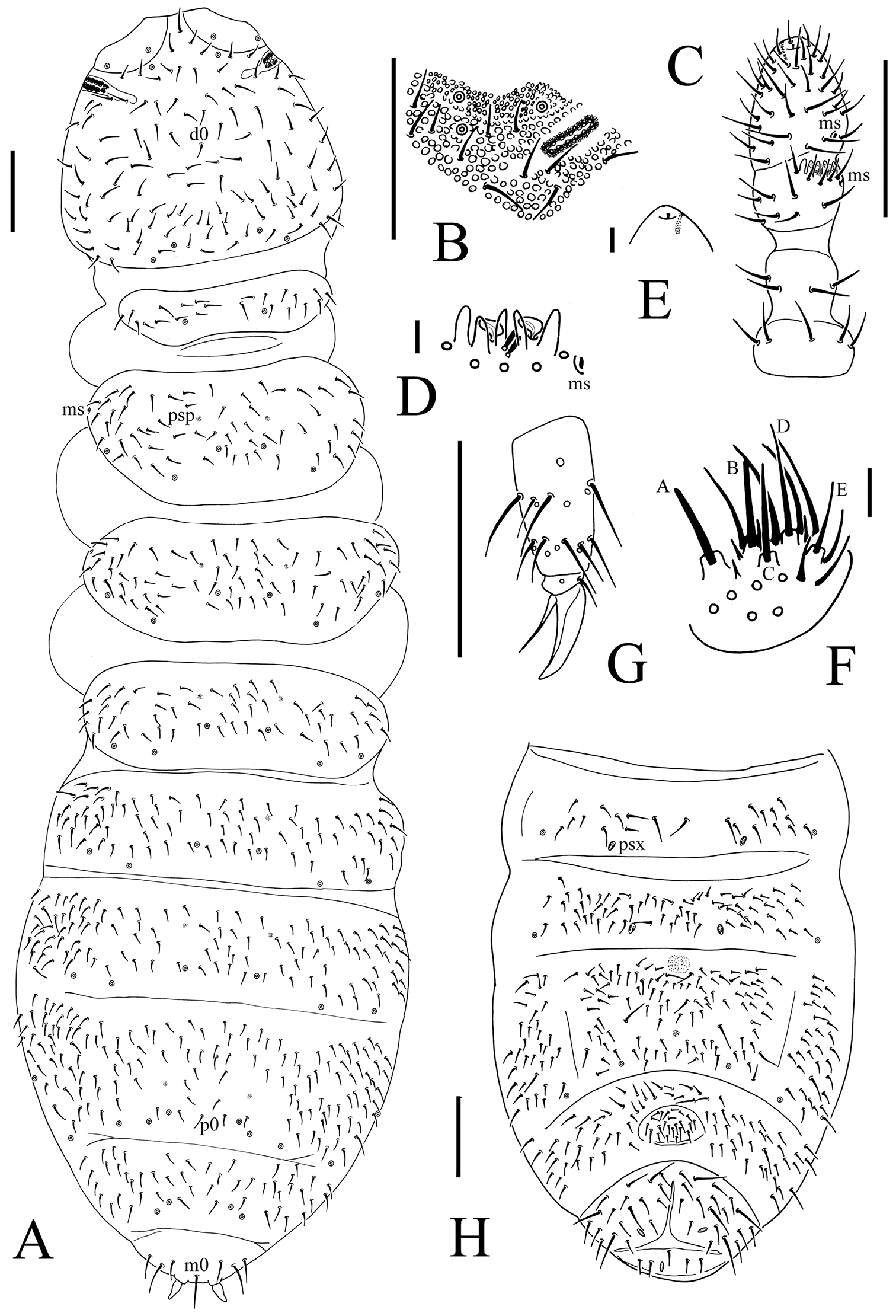
Onychiurus heilongjiangensis sp. n. A dorsal side of body B PAO C Ant. I–IV D Ant. III sensory organ E antennal tip F labium G distal part of leg III H Abd. II–VI sterna. Scale bars: 0.1 mm (A, C, G–H), 0.01 mm (B, D–F).
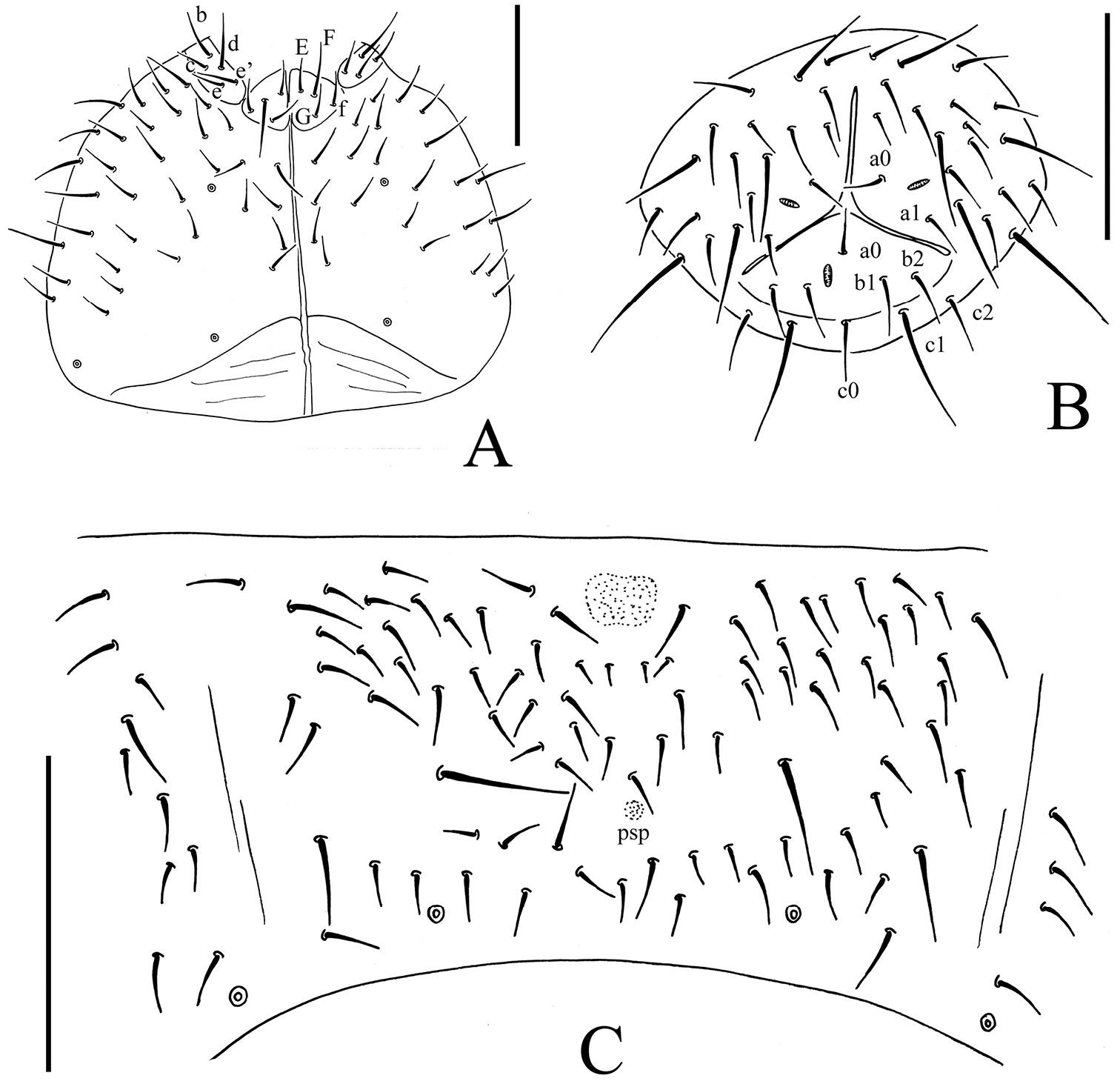
Onychiurus heilongjiangensis sp. n. A ventral side of head B anal valves C Abd. IV sternum. Scale bars: 0.1 mm (A–C).
Head. Antennae short, as long as head. Length ratio of antennal segments I : II : III : IV = 1 : 1.5 : 1.5 : 2.0. Subapical organite on Ant. IV with globular apex (Fig. 3C); invaginated apical bulb present (Fig. 3E); basolateral ms above the first proximal row of chaetae (Fig. 3C). Ant. III sensory organ composed of 5 papillae, 5 guard chaetae, 2 small rods, and 2 smooth sensory clubs (Fig. 3D); lateral ms just behind sensory organ (Fig. 3C). Ant. II with 14 chaetae. Ant. I with 8 chaetae. Antennal base well marked. PAO with 14–16 compound vesicles arranged in two rows along axis of organ (Fig. 3B). Dorsal cephalic chaeta d0 present. 3+3 p-chaetae between two inner posterior pso on head, p1 anterior to others (Fig. 3A). Mandible with strong molar plate and 4 apical teeth. Maxilla bearing 3 teeth and 6 lamellae. Maxillary palp simple with 1 basal chaeta and 2 sublobal hairs. Labral chaetae 4/142. Labium with 6 proximal, 4 basomedian (E, F, G, and f) and 5 basolateral (b, c, d, e, e’) chaetae (Fig. 4A). Labial palp of AB type, labial papillae A–E with 1, 4, 0, 3 and 3 guard chaetae respectively (Fig. 3F). Postlabial chaetae 4–5+4–5 along ventral groove (Fig. 4A).
Body chaetotaxy. S-chaetae not distinguishable from ordinary chaetae. Tiny and blunt ms, present on Th. II and III dorso-laterally (Fig. 3A). Dorsal ordinary chaetae poorly differentiated, usually coarse and short. Th. I tergum with 8–14+8–14 chaetae. Th. II–Abd. III terga with 4–5+4–5 chaetae along axial line respectively, usually with asymmetrical chaetae (Fig. 3A). Abd. IV tergum with p0 chaeta; Abd. V tergum without axial chaeta; Abd. VI tergum with m0 chaeta (Fig. 3A). Th. I–III sterna without chaetae between legs.
Appendages. Subcoxae 1 of legs I–III with 5, 5 and 5 chaetae, subcoxae 2 with 1, 4 and 4 chaetae respectively. Coxae of legs I, II and III with 3, 9–11 and 9–12 chaetae, trochanters with 9 chaetae each and femora with 15, 16 and 16 chaetae. Tibiotarsi of legs I, II and III with 17 (9, 7, 1), 18 (9, 7, 2) and 17 (9, 7, 1) chaetae, M-chaeta absent. Unguis without teeth. Unguiculus slender and pointed, 0.8 times as long as inner edge of unguis, without inner basal lamella (Fig. 3G). Ventral tube with 6–8+6–8 distal chaetae, without anterior or basal chaetae. Furca reduced to finely granulated area, with 4 small dental chaetae in one row posteriorly; three manubrial rows of chaetae present (Figs 3H, 4C).
Genital plate with 17–23 chaetae in females, 40–63 chaetae in males. Male ventral organ absent. Anal valves with numerous acuminate chaetae; each lateral valve with chaetae a0 and 2a1; upper valve with chaetae a0, 2b1, 2b2, c0, 2c1 and 2c2 (Fig. 4B). Anal spines set on distinct papillae, 0.6 times as long as inner edge of hind unguis (Fig. 3A).
Named for the province of the type locality.
The new specie shares the same dorsal pso formula from head to Abd. IV (32/133/3335), ventral pso formula from head to Abd. I (3/011/3) and number of pso on subcoxae 1 of legs I–III (2, 2, 2 respectively) with a number of the known European species, i.e. Onychiurus ambulans (Linnaeus, 1758) sensu Pomorski, 1998, Onychiurus arans Gisin, 1952, Onychiurus circulans Gisin, 1952, Onychiurus insinuans Gisin, 1952, Onychiurus subcirculans Gisin, 1962, and Onychiurus sublegans Gisin, 1960, but it can be distinguished easily from all these species as having only 2 pso on each side of Abd. V (3 or 4 in other species) and no male ventral organ (present in other species).
Sampling localities in Wanda Mountains.
Thanks should be given to the two reviewers for their useful comments. The present study was supported by the National Natural Sciences Foundation of China (31301862, 31311130106, 31370532), the Fund for Excellent Young Scholars of Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences (DLSYQ13003), and the Chinese Academy of Sciences Visiting Professorships for Senior International Scientists.