Citation: Ermilov SG, Shtanchaeva UYa, Subías LS, Martens J (2014) Two new species of oribatid mites of Lasiobelba (Acari, Oribatida, Oppiidae) from Nepal, including a key to all species of the genus. ZooKeys 424: 1–17. doi: 10.3897/zookeys.424.7990
Two new species of oribatid mites of the genus Lasiobelba (Oribatida, Oppiidae), Lasiobelba (Lasiobelba) daamsae sp. n. and Lasiobelba (Antennoppia) nepalica sp. n., are described from eastern Nepal. Lasiobelba (L.) daamsae sp. n. is most similar to L. (L.) remota Aoki, 1959 and L. (L.) gibbosa (Mahunka, 1985), however, it differs from both by the anterior part of pedotecta I specifically curved, rostrum pointed and exobothridial setae not shorter than bothridial setae. Lasiobelba (Antennoppia) nepalica sp. n. is most similar to L. (A.) granulata (Mahunka, 1986), however, it differs from the latter by the larger body size, exobothridial setae longer than rostral setae and bothridial setae not longer than interlamellar setae. An identification key to known species of Lasiobelba is given.
Oribatid mites, new species, Lasiobelba, key, Nepal
Lasiobelba is a genus of oribatid mites (Oribatida, Oppiidae, Oppiinae) that was proposed by
Currently, Lasiobelba comprises two subgenera (Lasiobelba (Lasiobelba) Aoki, 1959, Lasiobelba (Antennoppia) Mahunka, 1983 – see
In the course of taxonomic identification of oribatid mites from Nepal
Five specimens (holotype: male; four paratypes: all males) of Lasiobelba (Lasiobelba) daamsae sp. n. are from: eastern Nepal, 27°19'N, 87°78'E, Panchthar District, upper course of Mai Majuwa river, pasture Dhorpar Kharka, soil in mixed broadleaved forest, 2770 m a.s.l., 27–28.VIII.1983, collected by J. Martens and B. Daams. Four specimens (holotype: male; three paratypes: two males and one female) of Lasiobelba (Antennoppia) nepalica sp. n. are from: eastern Nepal, 26°99'N, 86°67'E, Ilam District, soil in remnants of broadleaved forest with plantations of Cryptomeria japonica, 2100 m a.s.l., 31.III.–01.IV.1980, collected by J. Martens and A. Ausobsky.
Holotypes and paratypes were mounted in lactic acid on temporary cavity slides for measurement and illustration. The body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the ventral plate. The notogastral width refers to the maximum width in dorsal aspect. Lengths of body setae were measured in lateral aspect. All body measurements are presented in micrometers. Formula for leg setation is given in parentheses according to the sequence trochanter–femur–genu–tibia–tarsus (famulus included). Formula for leg solenidia is given in square brackets according to the sequence genu–tibia–tarsus. General terminology used in this paper follows that of
http://zoobank.org/EE1BC06A-8B49-4004-B3EA-D1FF46336897
Body size: 1278–1310 × 747–863. Rostrum pointed. Prodorsal setae long, barbed; in ≈ le > ss ≈ ex > ro. Bothridial setae spindle-form, with long, thin apex, barbed. Nine pairs of notogastral setae long, barbed (p1–p3 shorter than others). Antero-medial part of rutelli with tooth. Anterior part of pedotecta I specifically curved. Anogenital setae barbed. Dorsal side of leg claws with small teeth.
Measurements. Body length: 1294 (holotype, male), 1278–1310 (four paratypes: males); notogaster width: 796 (holotype), 747–863 (four paratypes).
Integument (Figs 1, 3). Body color light brownish. Body surface smooth, but lateral parts of prodorsum with microgranulate cerotegument (diameter granules less than 1).
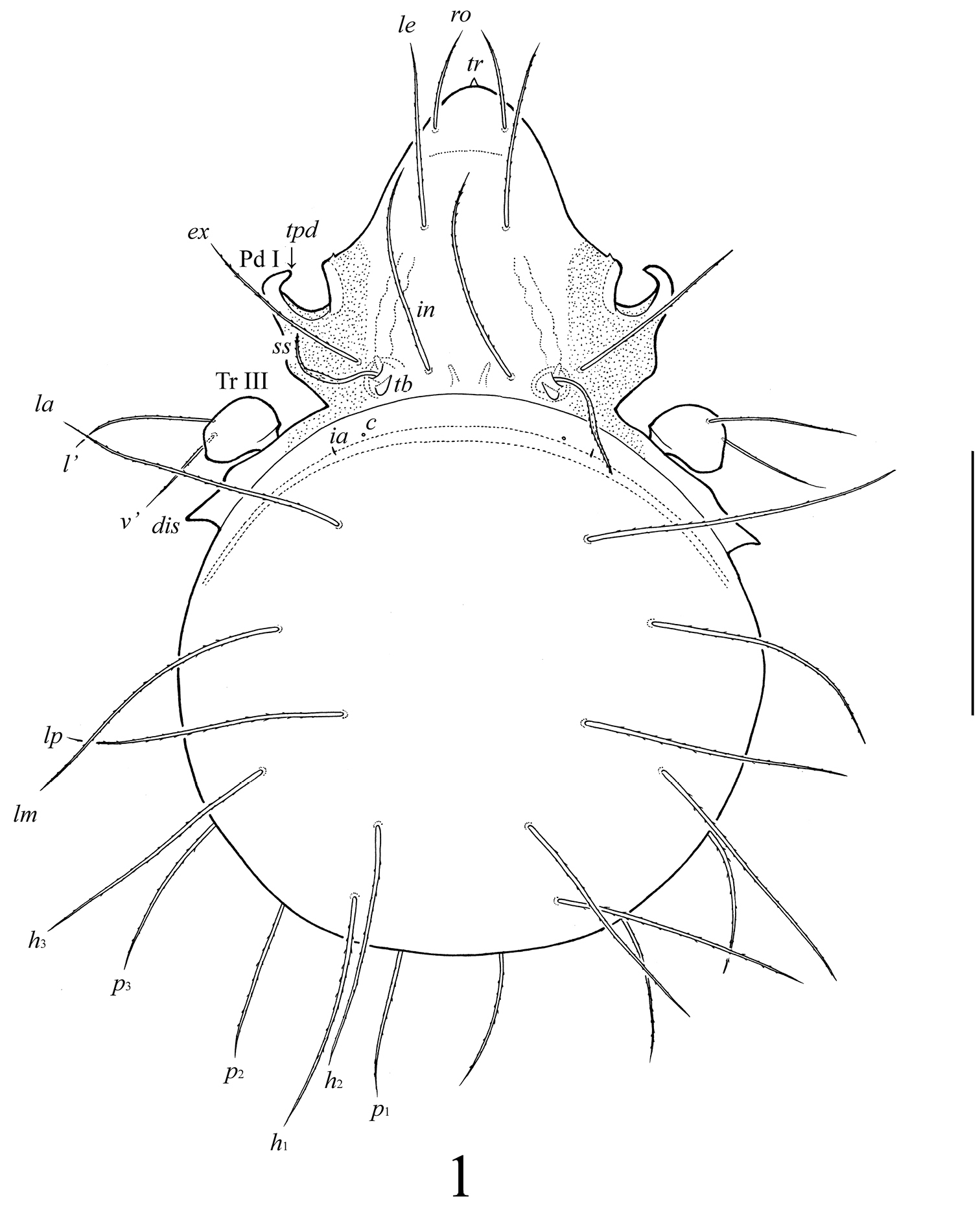
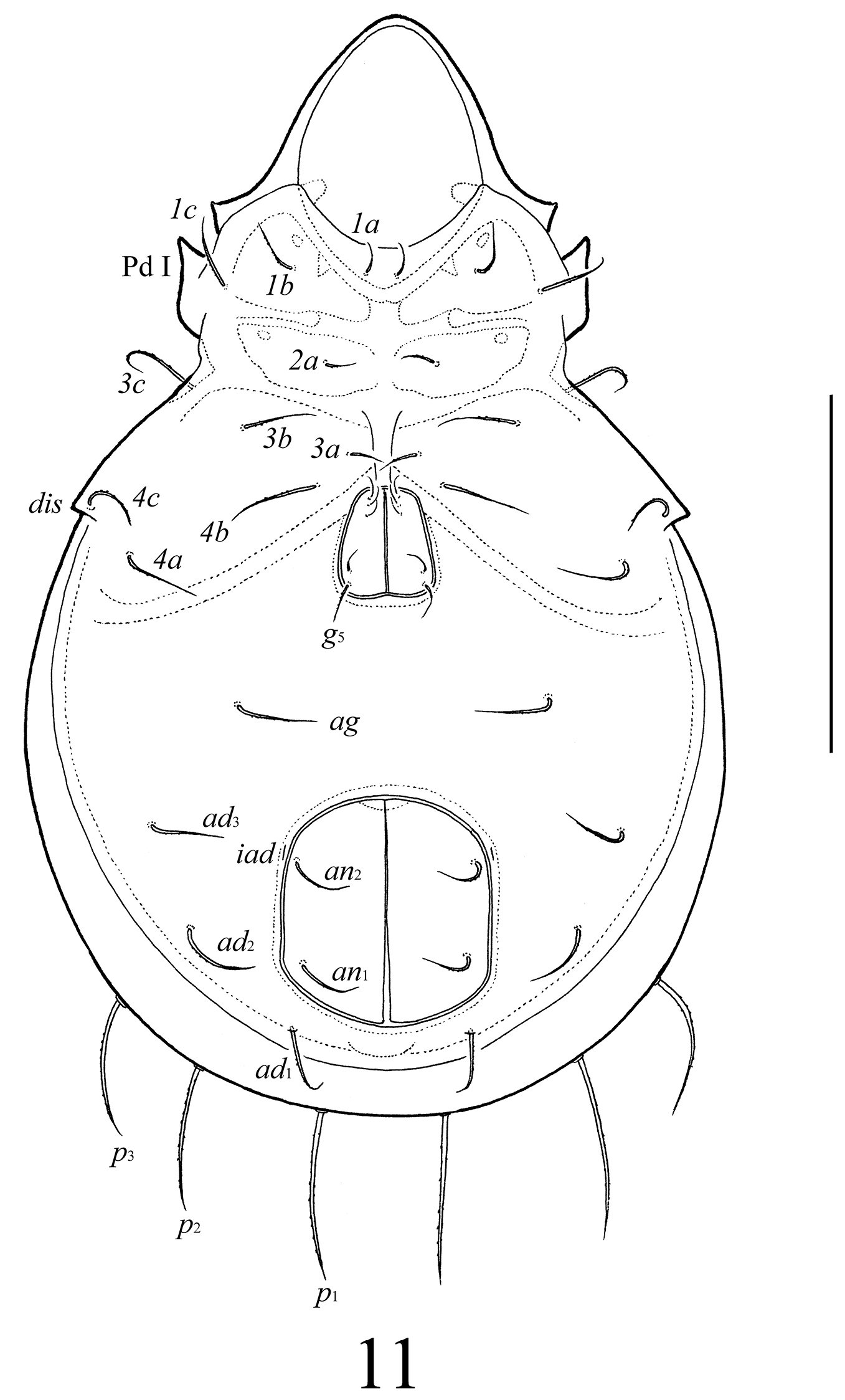
Lasiobelba (Lasiobelba) daamsae sp. n.: dorsal view (legs except trochanters III not illustrated). Scale bar 400 μm.
Prodorsum (Figs 1–3). Rostrum with conical tooth (tr, 12–16). A row, comprising several muscle sigillae, is located in front of the bothridia (usually very poorly visible). Muscle sigilla in interbothridial region absent, but one pair of longitudinal, dark brown structures are present. Rostral (ro, 199–232), lamellar (le, 365–381), interlamellar (in, 365–381) and exobothridial (ex, 265–298) setae well developed, setiform, barbed. Bothridial setae (ss, 265–298) spindle-form, barbed, with weakly developed elongate head and long, thin apex. A pair of triangular tubercles (tb) located posteriorly to bothridia.
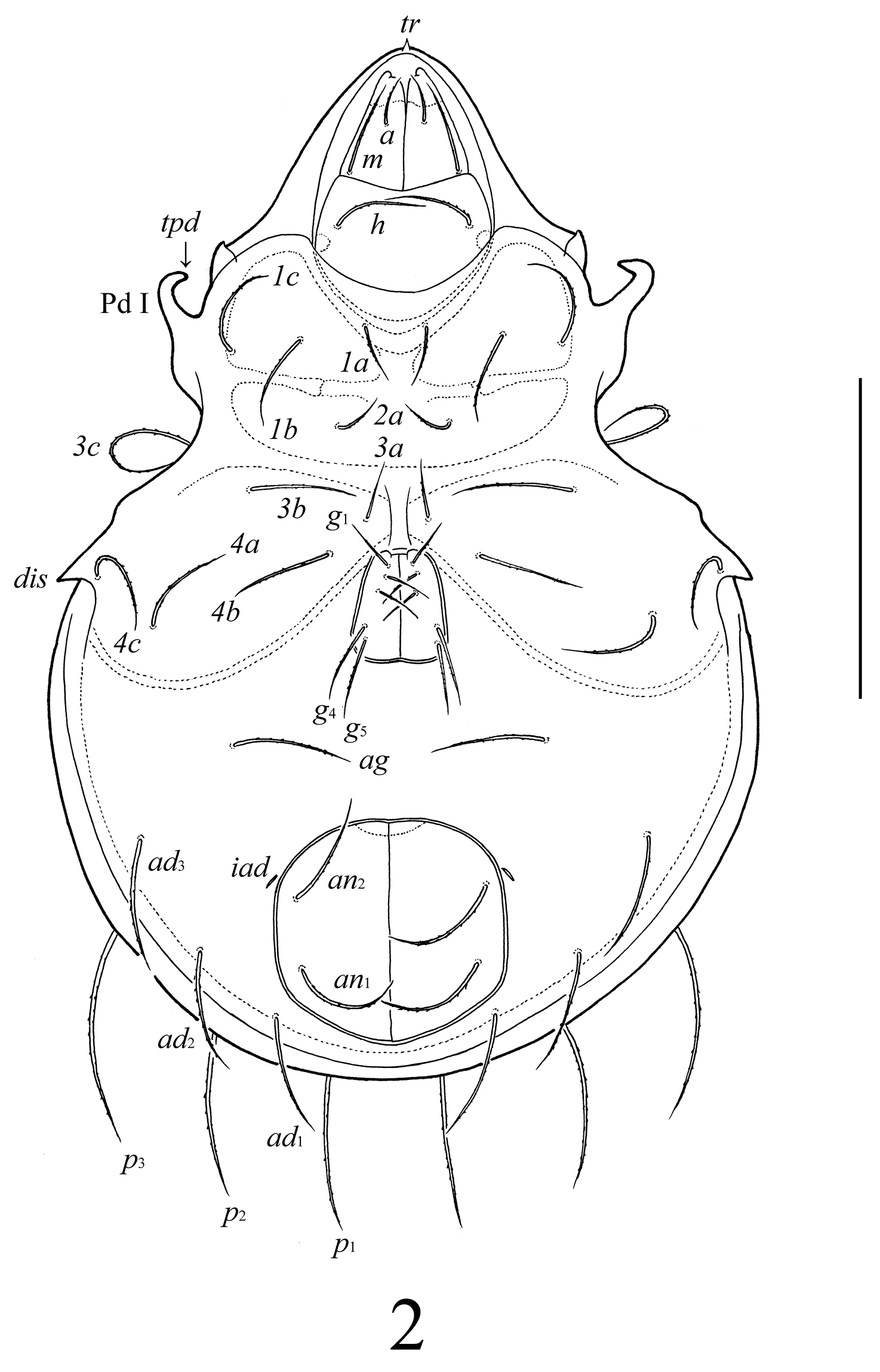
Lasiobelba (Lasiobelba) daamsae sp. n.: ventral view (legs not illustrated). Scale bar 400 μm.
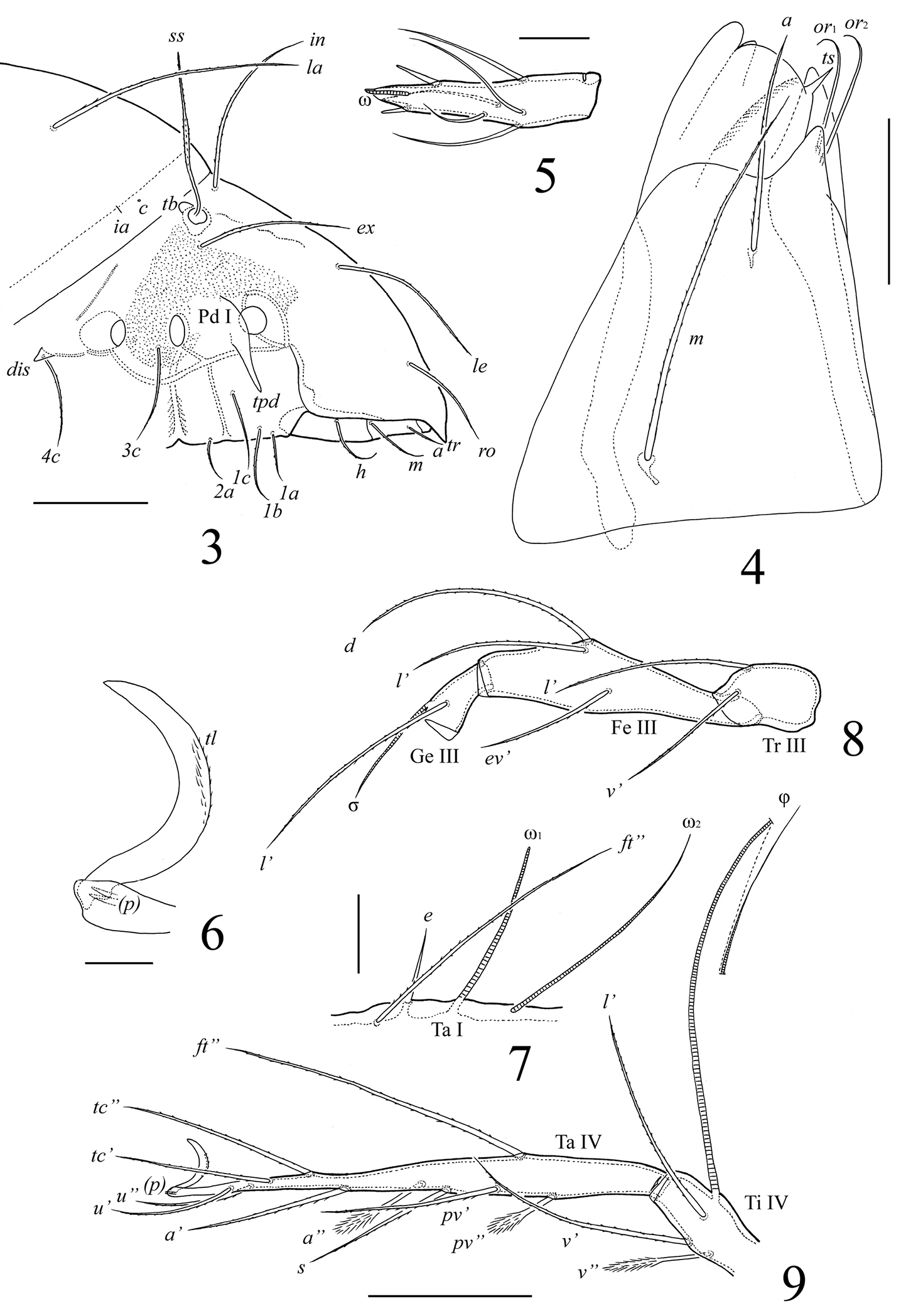
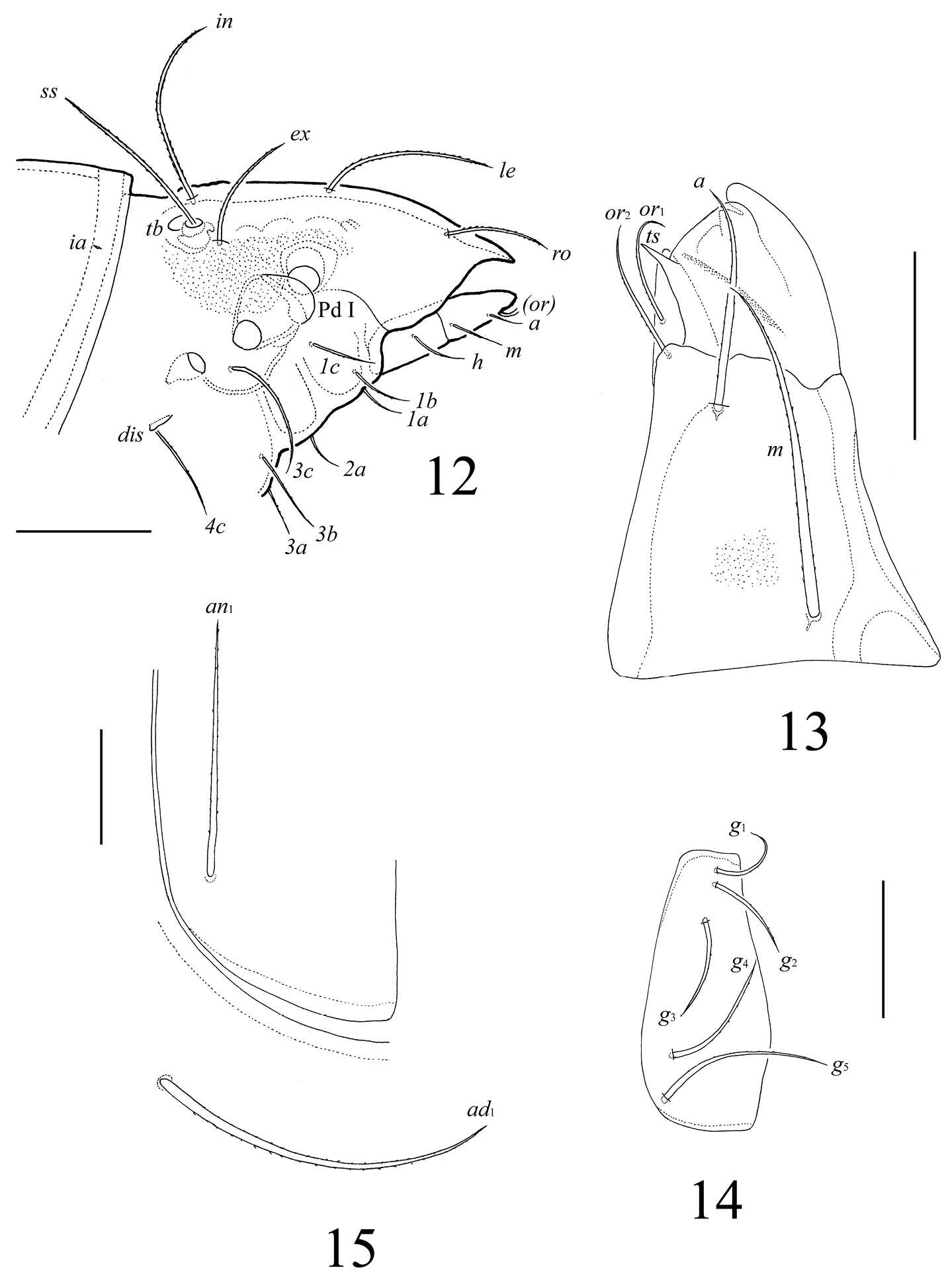
Lasiobelba (Lasiobelba) daamsae sp. n.: 3 lateral view of prodorsum (legs not illustrated) and anterior part of notogaster 4 right rutellum and gena of subcapitulum, ventral view 5 palptarsus 6 leg claw II and setae p 7 localization of solenidia, famulus and seta ft’’ on tarsus I, right, antiaxial view 8 trochanter, femur and genu of leg III, right, antiaxial view 9 tarsus and anterior part of tibia of leg IV, right, antiaxial view. Scale bars 200 μm (3, 8, 9), 50 μm (4, 7), 20 μm (5, 6).
Notogaster (Figs 1–3). Anterior border convex. Notogastral setae c represented by alveolus. Nine other pairs of notogastral setae long, barbed; p1–p3 (215–249) shorter than others (431–481). Lyrifissures ia poorly visible, im, ip, ih, ips and opisthonotal gland openings present, but visible under high magnification in dissected specimens.
Gnathosoma (Figs 2, 4, 5). Subcapitulum longer than wide (298–315 × 199–215). Antero-medial part of rutelli with tooth (ts, 8). Subcapitular setae setiform, barbed; a (66–83) shorter than m and h (both 116–132). Two pairs of adoral setae (or1, or2, 33–49) setiform, hook-like distally, smooth. Palps (199) with setation 0–2–1–3–8(+ω). Solenidion thickened, blunt-ended, pressed to the palptarsus surface in basal part and distal seta in distal part. Chelicerae (298–315) with two barbed setae; cha (99) longer than chb (66). One short tooth (4–6) located posteriorly to seta cha. Trägårdh’s organ distinct.
Epimeral and lateral podosomal regions (Figs 1–3). Apodemes (1, 2, sejugal, 4) weakly developed. Epimeral setae setiform, barbed; setae 1a, 2a, 3a (83–99) shorter than 1b, 1c, 3b, 4a, 4b (149–166) and 3c, 4c (199–232). Anterior part of pedotecta I (Pd I) elongate and specifically curved, forming a tooth (tpd). Discidia (dis) triangular, pointed.
Anogenital region (Figs 2, 3). Five pairs of genital (g1–g3, 74–83; g4, g5, 108–116), one pair of aggenital (ag, 166–199), three pairs of adanal (ad1–ad3, 166–199) and two pairs of anal (an1, an2, 149–166) setae setiform, barbed. Distance between setae ad3–ad3 longer than ad2–ad2 and ad1–ad1. Adanal lyrifissures iad located diagonally, but very close to anal aperture.
Legs (Figs 1, 6–9). Generally, morphology typical for species of Lasiobelba (
Leg setation and solenidia of Lasiobelba (Lasiobelba) daamsae sp. n. (same data for Lasiobelba (Antennoppia) nepalica sp. n.).
| Leg | Trochanter | Femur | Genu | Tibia | Tarsus |
|---|---|---|---|---|---|
| I | v’ | d, (l), bv’’, v’’ | (l), σ | (l), (v), φ1, φ2 | (ft), (tc), (it), (p), (u), (a), s, (pv), v’, (pl), l’’, ε, ω1, ω2 |
| II | v’ | d, (l), bv’’, v’’ | (l), σ | (l), (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv), l’’, ω1, ω2 |
| III | l’, v’ | d, l’, ev’ | l’, σ | l’, (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv) |
| IV | v’ | d, ev’ | d, l’ | l’, (v), φ | ft’’, (tc), (p), (u), (a), s, (pv) |
Roman letters refer to normal setae (ε to famulus), Greek letters to solenidia. Single prime (’) marks setae on anterior and double prime (’’) setae on posterior side of the given leg segment. Parentheses refer to a pair of setae.
The holotype and one paratype are deposited in the collection of the Senckenberg Institution Frankfurt, Germany; three paratypes are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia.
The specific name is dedicated to Mrs. Beate Daams for her assistance in Nepalese scientific researches.
In having the long notogastral setae, large body size and spindle-form bothridial setae, Lasiobelba (Lasiobelba) daamsae sp. n. is most similar to Lasiobelba (Lasiobelba) remota Aoki, 1959 from the Oriental and Palaearctic regions and Lasiobelba (Lasiobelba) gibbosa (Mahunka, 1985) from the Ethiopian region. However, it differs from both by the anterior part of pedotecta I specifically curved (versus straight in Lasiobelba (Lasiobelba) remota and Lasiobelba (Lasiobelba) gibbosa), rostrum pointed (versus rounded in Lasiobelba (Lasiobelba) remota and nasiform in Lasiobelba (Lasiobelba) gibbosa) and exobothridial setae not shorter than bothridial setae (versus shorter in Lasiobelba (Lasiobelba) remota and Lasiobelba (Lasiobelba) gibbosa).
http://zoobank.org/F023D27B-A28D-4A1F-B987-3834F4DF4E97
Body size: 996–1278 × 697–830. Prodorsal setae long, barbed; ss ≈ in > le > ex > ro. Nine pairs of notogastral setae long, barbed (p1–p3 shorter than others). Antero-medial part of rutelli with tooth. Anogenital setae barbed. Dorsal side of leg claws with small teeth.
Measurements. Body length: 1195 (holotype, male), 996–1278 (three paratypes: two males and one female); notogaster width: 730 (holotype), 697–830 (three paratypes).
Integument (Figs 10, 12). Body color light brownish. Body surface smooth, but lateral parts of prodorsum with microgranulate cerotegument (diameter granules up to 1).
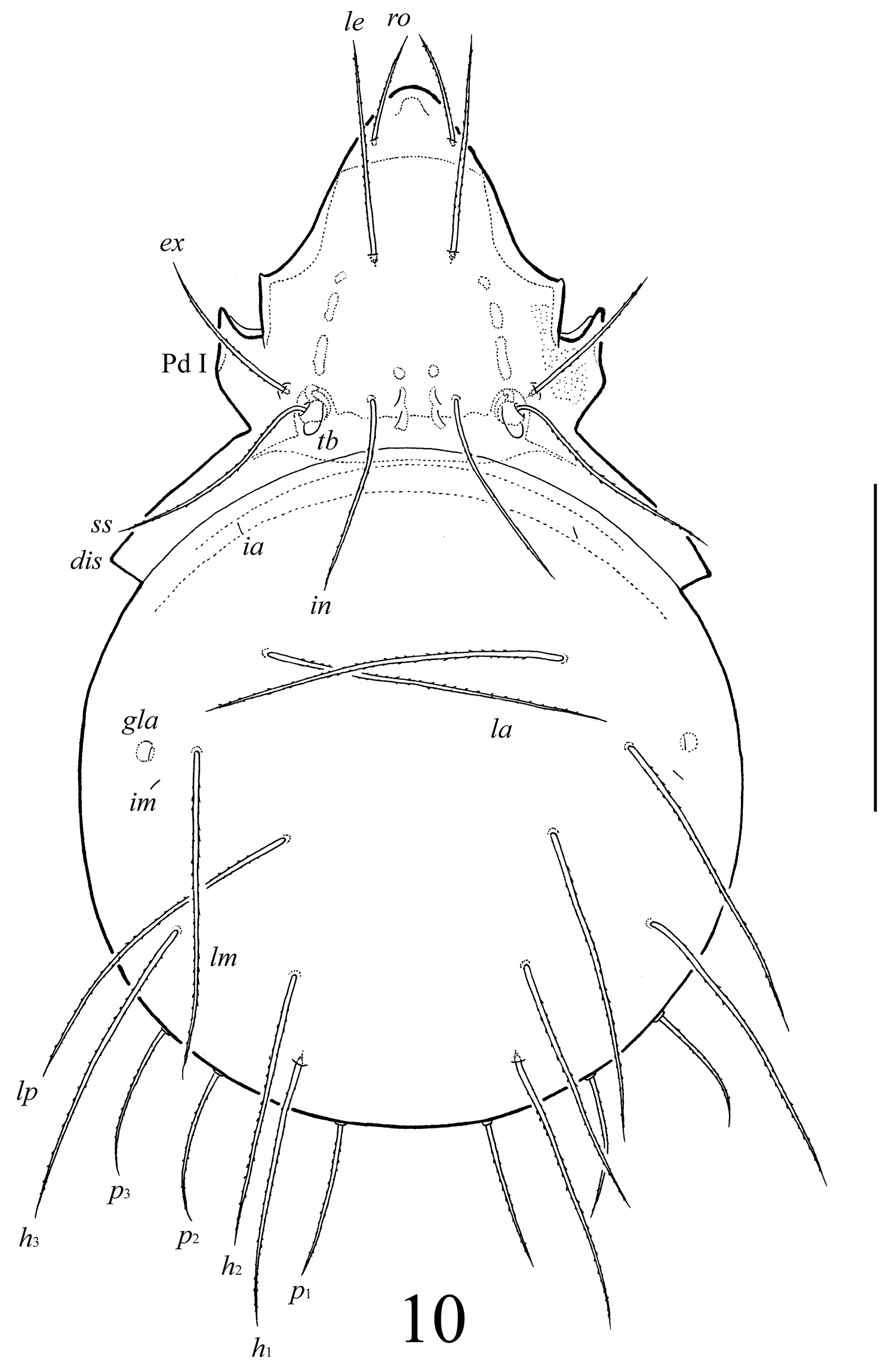
Lasiobelba (Antennoppia) nepalica sp. n.: dorsal view (legs not illustrated). Scale bar 400 μm.
Prodorsum (Figs 10, 12). Rostrum widely or narrowly rounded. A row, comprising several muscle sigillae, is located in front of the bothridia. One pair of muscle sigilla in interbothridial region poorly visible. Rostral (143–164), lamellar (254–287), interlamellar (307–348), exobothridial (205–258) and bothridial (307–348) setae well developed, setiform, barbed. A pair of triangular tubercles located posteriorly to bothridia.
Notogaster (Figs 10–12). Anterior border convex. Notogastral setae c and their alveoli reduced. Nine pairs of notogastral setae long, barbed; p1–p3 (184–192) shorter than h1, h2 (265–332) and others (398–464). Lyrifissures ia, im and opisthonotal gland openings (gla) poorly visible; lyrifissures ip, ih, ips present, but visible under high magnification in dissected specimens.
Lasiobelba (Antennoppia) nepalica sp. n.: ventral view (gnathosoma and legs not illustrated). Scale bar 400 μm.
Lasiobelba (Antennoppia) nepalica sp. n.: 12 lateral view of prodorsum (legs not illustrated) and anterior part of notogaster 13 left rutellum and gena of subcapitulum, ventral view 14 genital plate, right 15 posterior part of anal plate with seta an1 and adanal seta ad1. Scale bar 50 μm.
Gnathosoma (Figs 12, 13). Subcapitulum longer than wide (266 × 199–209). Antero-medial part of rutelli with tooth (8–10). Subcapitular setae setiform, barbed; a (61–65) shorter than m and h (both 98–102). Two pairs of adoral setae (41–45) setiform, indistinctly smooth. Palps (196) with setation 0–2–1–3–8(+ω). Solenidion thickened, blunt-ended, pressed to the palptarsus surface in basal part and distal seta in distal part. Chelicerae (266) with two barbed setae; cha (86) longer than chb (53). One short tooth (4–6) located posteriorly to seta cha. Trägårdh’s organ distinct.
Epimeral and lateral podosomal regions (Figs 10–12). Apodemes (1, 2, sejugal, 4) weakly developed. Epimeral setae setiform, barbed; setae 1a, 2a, 3a (69–86) shorter than 1b, 1c, 3b, 4a, 4b (114–127), 3c (205–209) and 4c (155–164). Pedotecta I normally developed, scale-like. Discidia triangular, pointed.
Anogenital region (Figs 11, 14, 15). Five pairs of genital setae (g1–g3, 41–53; g4, 61–69, g5, 73–82) setiform, indistinctly barbed. One pair of aggenital (123–135), three pairs of adanal (159–172) and two pairs of anal (114–123) setae setiform, barbed. Distance between setae ad3–ad3 longer than ad2–ad2 and ad1–ad1. Adanal lyrifissures iad located longitudinally.
Legs. Generally, similar to Lasiobelba (Lasiobelba) daamsae sp. n. (see also Table 1).
The holotype and one paratype are deposited in the collection of the Senckenberg Institution Frankfurt, Germany; two paratypes are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia.
The specific name “nepalica” refers to the country origin, Nepal.
In having the long prodorsal and notogastral setae and large body size, Lasiobelba (Antennoppia) nepalica sp. n. is most similar to Lasiobelba (Antennoppia) granulata (Mahunka, 1986) from Tanzania. However, it clearly differs from the latter by the larger body size (996–1278 × 697–830 versus 820–861 × 541–574 in Lasiobelba (Antennoppia) granulata), exobothridial setae longer than rostral setae (versus rostral longer in Lasiobelba (Antennoppia) granulata) and bothridial setae not longer than interlamellar setae (versus clearly longer in Lasiobelba (Antennoppia) granulata).
| 1 | Bothridial setae spindle-form | 2, subgenus Lasiobelba (Lasiobelba) |
| – | Bothridial setae setiform | 18, subgenus Lasiobelba (Antennoppia) |
| 2 | Dorsal notogastral setae long, lm reaching the insertions of lp | 3 |
| – | Dorsal notogastral setae of medium size or short, lm not reaching the insertions of lp | 13 |
| 3 | Notogastral setae la, lm, lp longer than bothridial setae | 4 |
| – | Notogastral setae la, lm, lp shorter than bothridial setae | 10 |
| 4 | Rostrum pointed | 5 |
| – | Rostrum widely or narrowly rounded, or truncated | 6 |
| 5 | Anterior part of pedotecta I specifically curved; notogastral setae p1–p3 longer than adanal setae; body size: 1278–1310 × 747–863 | Lasiobelba (Lasiobelba) daamsae sp. n. Distribution: Nepal |
| – | Pedotecta I normally developed; notogastral setae p1–p3 shorter than adanal setae; body size: 772–891 × 410–456 | Lasiobelba (Lasiobelba) gibbosa (Mahunka, 1985). Distribution: Ethiopian region |
| 6 | Interlamellar setae similar in length (little longer or shorter) to bothridial setae | 7 |
| – | Interlamellar setae clearly shorter than bothridial setae | 9 |
| 7 | Rostrum truncated; body size: 794–834 × 492–564 | Lasiobelba (Lasiobelba) insulata Ohkubo, 2001. Distribution: Japan |
| – | Rostrum widely or narrowly rounded | 8 |
| 8 | Rostrum widely rounded; notogastral setae p1–p3 inserted close to each other; body size: 560 × 330 | Lasiobelba (Lasiobelba) subuligera (Berlese, 1916) (see also |
| – | Rostrum with protruding ledge; notogastral setae p1–p3 clearly distanced from each other; body size: 940–1030 × 620–650 | Lasiobelba (Lasiobelba) remota Aoki, 1959. Distribution: Palaearctic and Oriental regions |
| 9 | Bothridial setae with head without long apex; interbothridial region with two pairs of muscle sigilla; body size: 950 × 630 | Lasiobelba (Lasiobelba) suchetae Sanyal, 1992. Distribution: India |
| – | Bothridial setae with long, thin apex; interbothridial region without muscle sigilla; body size: 625–684 × 388–437 | Lasiobelba (Lasiobelba) vietnamica Balogh, 1983 (see |
| 10 | Notogastral setae c short, present | 11 |
| – | Notogastral setae c represented by alveoli | 12 |
| 11 | Anterior part of notogaster smooth; epimeral setae 1а, 2а, 3а thin, almost smooth; body size: 478–522 × 277–315 | Lasiobelba (Lasiobelba) lemurica Mahunka, 1997. Distribution: Madagascar |
| – | Anterior part of notogaster microfoveolate; epimeral setae 1а, 2а, 3а heavily barbed; body size: 566 × 307 | Lasiobelba (Lasiobelba) pontica Vasiliu & Ivan, 2011. Distribution: Romania |
| 12 | Body surface of notogaster with longitudinal ridges; interbothridial region with one pair of tubercles; body size: 693 × 455 | Lasiobelba (Lasiobelba) sculptra Wang, 1993. Distribution: southern China |
| – | Body surface of notogaster granulate; interbothridial region without tubercles; body size: 610–644 × 386–402 | Lasiobelba (Lasiobelba) yunanensis Wen, 1999. Distribution: southern China |
| 13 | Notogastral setae c represented by alveoli | 14 |
| – | Notogastral setae c short, present | 15 |
| 14 | Notogastral setae smooth; body length: 468 | Lasiobelba (Lasiobelba) hespiridiana (Pérez-Íñigo, 1986). Distribution: Mediterranean |
| – | Notogastral setae barbed; body size: 787–825 × 495–539 | Lasiobelba (Lasiobelba) rubida (Wallwork, 1977). Distribution: Santa Helena Islands |
| 15 | Interlamellar setae shorter than lamellar setae; body size: 413–600 × 228–336 | Lasiobelba (Lasiobelba) pori (Vasiliu & Ivan, 1995) (= Lasiobelba arabica Mahunka, 2000, = Lasiobelba (Lasiobelba) neonominata Subías, 2004 (see |
| – | Interlamellar setae longer or similar in length to lamellar setae | 16 |
| 16 | Rostrum tripartite; interbothridial region with three pairs of muscle sigilla; body size: 500–540 × 253 | Lasiobelba (Lasiobelba) decui (Vasiliu & Ivan, 1995). Distribution: Israel |
| – | Rostrum rounded; interbothridial region with two pairs of muscle sigilla | 17 |
| 17 | Bothridial setae with numerous barbs; notogastral setae p3 longer than p1 and p2; body size: 400–530 × 215–280 | Lasiobelba (Lasiobelba) arcidiaconoae ( |
| – | Bothridial setae with several short barbs; notogastral setae p3 similar in length to p1 and p2; body size: 313 × 233 | Lasiobelba (Lasiobelba) kuehnelti (Csiszár, 1961). Distribution: Oriental, Australian and Ethiopian regions |
| 18 | Heterotrichy of dorsal notogastral setae well developed, la and lm considerably longer than lp | 19 |
| – | Heterotrichy of dorsal notogastral setae absent or weakly expressed, la and lm not longer than lp | 20 |
| 19 | Notogastral setae la long, reaching the insertions of lp; lamellar setae longer than rostral setae; body size: 456 × 216 | Lasiobelba (Antennoppia) quadrisetosa Subías, 1989 – see |
| – | Notogastral setae la of medium size, not reaching the insertions of lp; lamellar setae shorter than rostral setae; body size: 498–547 × 298–332 | Lasiobelba (Antennoppia) chistyakovi Ermilov & Kalúz, 2012. Distribution: Ecuador |
| 20 | Dorsal notogastral setae long, lm reaching the insertions of lp | 21 |
| – | Dorsal notogastral setae of medium size or short, lm not reaching the insertions of lp | 29 |
| 21 | Notogastral setae la, lm, lp longer or similar in length to bothridial setae | 22 |
| – | Notogastral setae la, lm, lp shorter than bothridial setae | 25 |
| 22 | Apodemes IV absent; adanal lyrifissures located diagonally to anal aperture; body size: 745 × 510 | Lasiobelba (Antennoppia) insignis Balogh, 1970. Distribution: New Guinea |
| – | Apodemes IV present; adanal lyrifissures located longitudinally to anal aperture | 23 |
| 23 | Bothridial setae smooth; body size: 590 × 330 | Lasiobelba (Antennoppia) subnitida (Sellnick, 1924). Distribution: Brazil |
| – | Bothridial setae barbed | 24 |
| 24 | Exobothridial setae longer than rostral setae; bothridial setae similar in length to interlamellar setae; body size: 996–1278 × 697–830 | Lasiobelba (Antennoppia) nepalica sp. n. Distribution: Nepal |
| – | Exobothridial setae shorter than rostral setae; bothridial setae longer than interlamellar setae; body size: 820–861 × 541–574 | Lasiobelba (Antennoppia) granulata (Mahunka, 1986). Distribution: Tanzania |
| 25 | Rostrum pointed; body size: 715–800 × 448–486 | Lasiobelba (Antennoppia) major (Mahunka, 1983), see |
| – | Rostrum rounded | 26 |
| 26 | Interlamellar setae represented by alveoli; body size: 590–623 × 232–250 | Lasiobelba (Antennoppia) trichoseta (Mahunka, 1983), see |
| – | Interlamellar setae well developed | 27 |
| 27 | Dorsal notogastral setae inserted in four subparallel rows; interbothridial region with one pair of triangular ridges; body size: 810–1180 × 510–526 | Lasiobelba (Antennoppia) yoshii (Mahunka, 1987). Distribution: Borneo |
| – | Dorsal notogastral setae inserted in two parallel rows; interbothridial region without triangular ridges | 28 |
| 28 | Interlamellar setae longer than lamellar setae; interbothridial region with three pairs of muscle sigilla; body size: 740 × 450 | Lasiobelba (Antennoppia) capilligera (Berlese, 1916) (see also |
| – | Interlamellar setae slightly shorter than lamellar setae; interbothridial region without muscle sigilla; body size: 555–652 × 314–367 | Lasiobelba (Antennoppia) minor (Mahunka, 1983), see |
| 29 | Notogastral setae c represented by alveoli; rostrum with protruding ledge; body size: 565–605 × 315–335 | Lasiobelba (Antennoppia) ultraciliata (Jacot, 1934). Distribution: Australian region |
| – | Notogastral setae c short, present; rostrum rounded, without protruding ledge | 30 |
| 30 | Interlamellar setae similar in length to lamellar setae; exobothridial setae similar in length to rostral setae, respectively; body size: 347 × 185 | Lasiobelba (Antennoppia) heterosa (Wallwork, 1964). Distribution: Ethiopian and Palaearctic regions |
| – | Interlamellar setae longer than lamellar setae; exobothridial setae shorter than rostral setae; body size: 525–637 × 288–337 | Lasiobelba (Antennoppia) izquierdoae Arillo, Gil-Martin & Subías, 1994. Distribution: Canary Islands |
The authors are thankful to two anonymous reviewers for valuable comments on the manuscript J. Martens thanks B. Daams and A. Ausobsky for helpful companionship during the Nepalese expeditions, as well as the Feldbausch Foundation and the Wagner foundation at Fachbereich Biologie of Mainz University for over the years many annual grants to carry out field work in Asia.