Citation: Hirose E, Iskandar BH, Wardiatno Y (2014) Photosymbiotic ascidians from Pari Island (Thousand Islands, Indonesia). ZooKeys 422: 1–10. doi: 10.3897/zookeys.422.7431
Photosymbiotic ascidian fauna were surveyed in the subtidal zone off Pari Island in the Thousand Islands (Java Sea, Indonesia). Nine species were recorded: Didemnum molle, Trididemnum miniatum, Lissoclinum patella, L. punctatum, L. timorense, Diplosoma gumavirens, D. simile, D. simileguwa, and D. virens. All of these species have been previously recorded in the Ryukyu Archipelago, Japan. Diplosoma gumavirens and D. simileguwa were originally described from the Ryukyu Archipelago in 2009 and 2005, respectively, and all of the observed species are potentially widely distributed in Indo–West Pacific coral reefs.
Algal symbiosis, Colonial ascidian, Biogeography, Coral reefs, Didemnidae
In tropical waters, some colonial ascidians harbor cyanobacterial symbionts such as Prochloron (reviewed by
The Pulau Pari Technical Management Unit for Human Resources Development on Oceanography Competency is a marine laboratory located on Pari Island (Thousand Islands, Indonesia). This laboratory of the Indonesian Institute of Sciences (LIPI) is one of the key stations for marine science in the Java Sea. Therefore, acquiring biodiversity data in this area is essential. Here, we report the photosymbiotic ascidian fauna observed in the shallow coral reef in the vicinity of this laboratory.
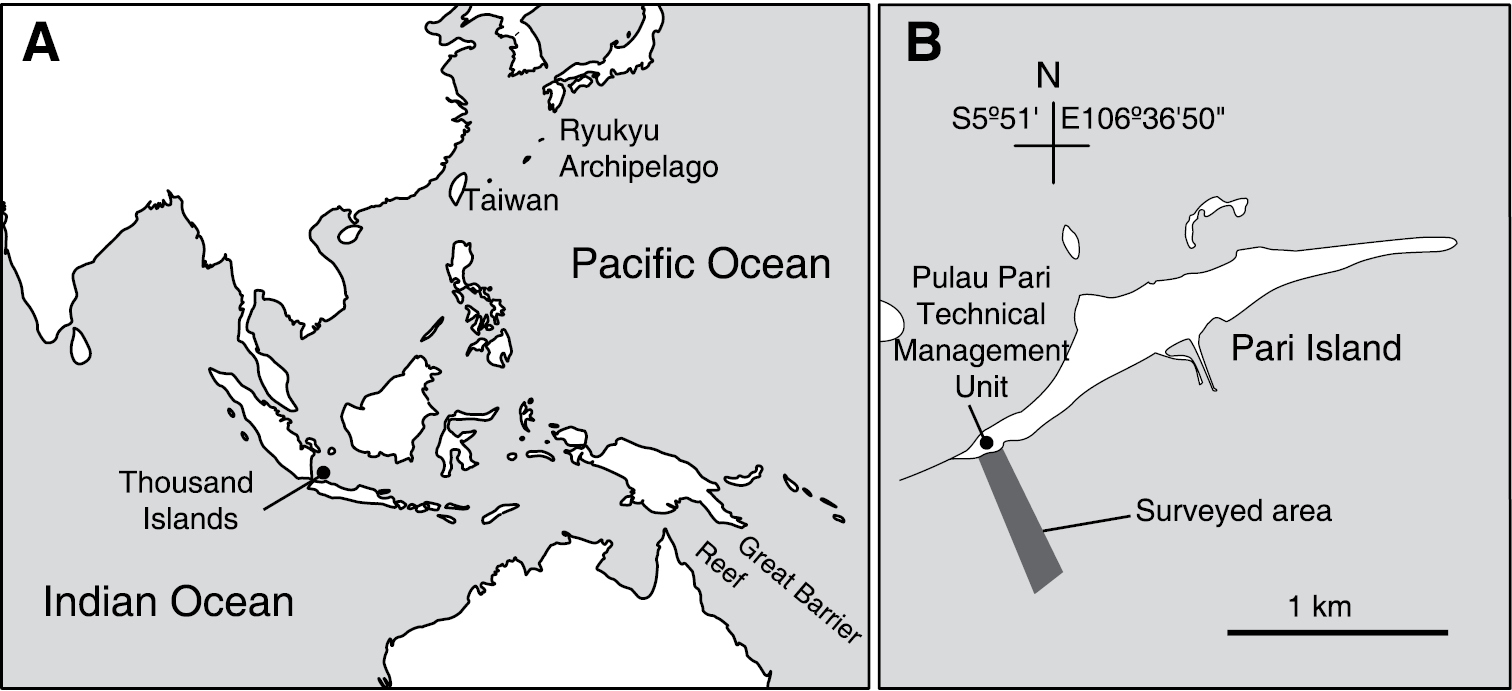
Samples were collected by snorkeling in the shallow subtidal zone down to approximately 2 m or less at low tide in the back reef, reef flat, and reef crest off Pari Island (5°52'S, 106°36'40"E) on 28–30 November 2013 (Fig. 1). Ascidian colonies were photographed in situ prior to collection. Specimens were anesthetized using menthol and 0.37 M MgCl2 for approximately 2 h and then fixed with 10% formalin–seawater. Fixed colonies were dissected under a binocular stereomicroscope. Zooids and spicules were photographed using a microscope equipped with differential interference contrast optics. In some photomicrographs of the thoraxes, several images were combined to increase the depth of field using the post-processing image software Helicon Focus Pro 4.2.8 (Helicon Soft, Ltd., Kharkov, Ukraine). Cyanobacterial symbionts were identified based on the colour in live specimens and the cytomorphology under a light microscope. Ascidian taxa were mainly identified following
Location of Thousand Islands, Indonesia (A) and the surveyed area off Pari Island (B).
Nine photosymbiotic ascidian species were found in the subtidal zone of the coral reef off Pari Island. Symbiont cyanobacteria within all ascidian species were identified as Prochloron didemni that is the only taxonomically valid species. Depending on the host species, Prochloron cells were distributed in the common cloacal cavities, in the tunic, or in both the common cloacal cavity and the tunic. Although the Prochloron cells in the cavity are morphologically different from those in the tunic (
MZB. Asc. 00001
Coral limestone at reef crest.
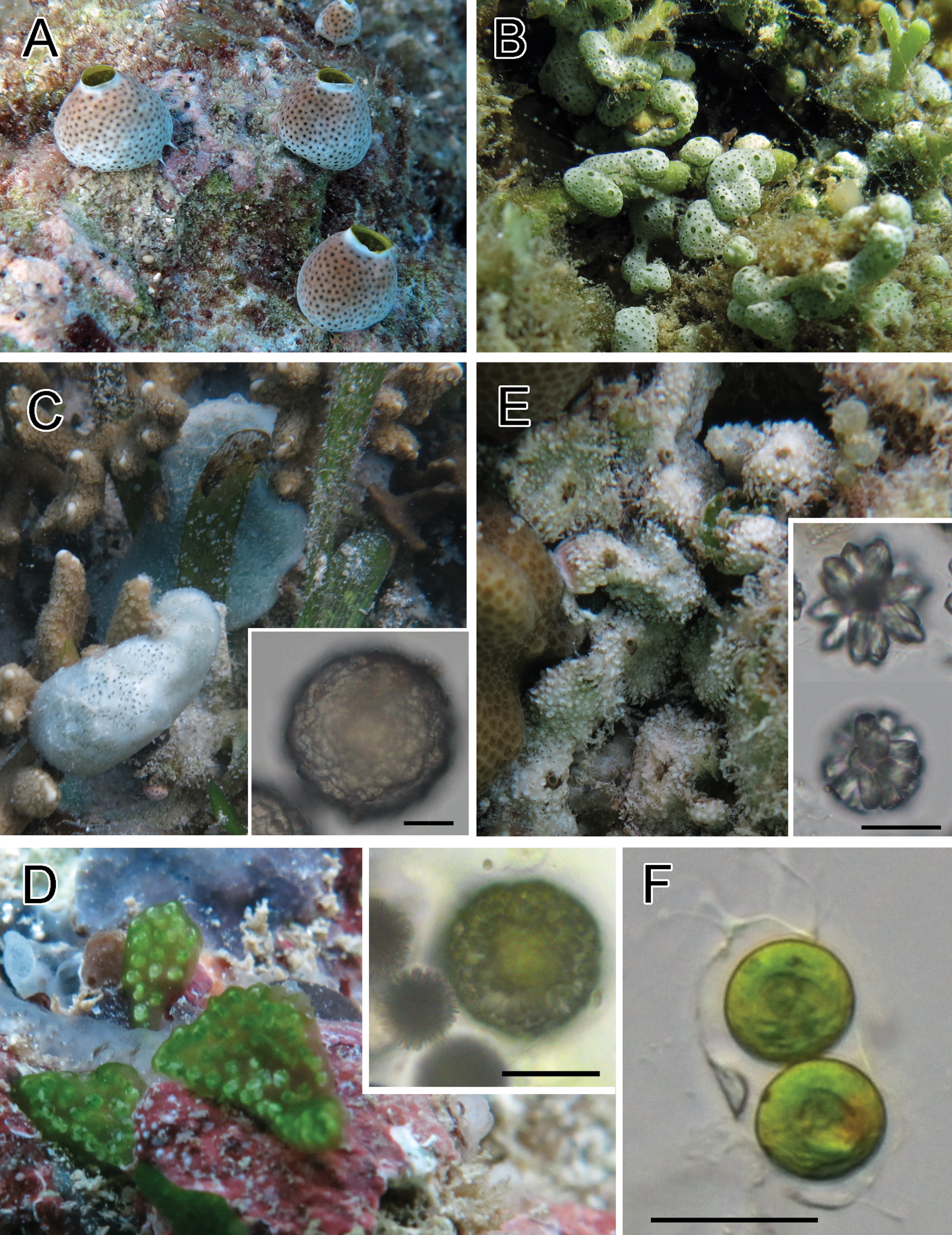
Colonies were dome-shaped. Several morphotypes in colony shape and color exist in this species (i.e., brown, gray, white, large, and small type). These morphotypes can also be distinguished by the partial sequence of the cytochrome oxidase subunit I (COI) gene (
Photosymbiotic ascidians with tunic spicules. Colonies in situ and tunic spicules (inset) of Didemnum molle (A), Trididemnum miniatum (B), Lissoclinum patella (C), Lissoclinum punctatum (D), and Lissoclinum timorense (E). Tunic cells contain Prochloron cells in the tunic of Lissoclinum punctatum (F). Scale bars = 20 µm.
MZB. Asc. 00002
Dead coral skeletons and macroalgae in shallow back reef.
Thin sheets of colonies were white in exposed habitat and pale green in shaded habitat, depending on the amount of calcareous spicules in the tunic. Prochloron cells were distributed within the tunic.
MZB. Asc. 00003
Dead coral skeletons in back reef.
Colonies were thick cushions attaining about 10 mm in thickness. Tunic contains both stellate and globular spicules (Fig. 1C, inset). Prochloron cells were distributed within the common cloacal cavity. Some zooids had testes. Because of the large size, this species has been thoroughly studied for its natural compounds (e.g.,
MZB. Asc. 00004
Shaded side of dead coral skeletons in reef flat.
Colonies were irregularly shaped sheets. Globular spicules (Fig. 1D, inset) form a capsule-like aggregation enveloping each zooid. Prochloron was distributed within the common cloacal cavities and tunic. As reported in
MZB. Asc. 00005
Dead coral skeletons and clefts between coral limestones in back reef and shallow reef flat.
Colonies had linguiform projections of the tunic around the colony periphery and sometimes on the colony surface. Tunic contains both stellate and globular spicules (Fig. 1E, inset). Prochloron cells were distributed within the common cloacal cavity.
Because the zooids of Lissoclinum bistratum and Lissoclinum timorense are very similar in morphology,
MZB. Asc. 00006
Shaded side of dead coral branches in reef flat.
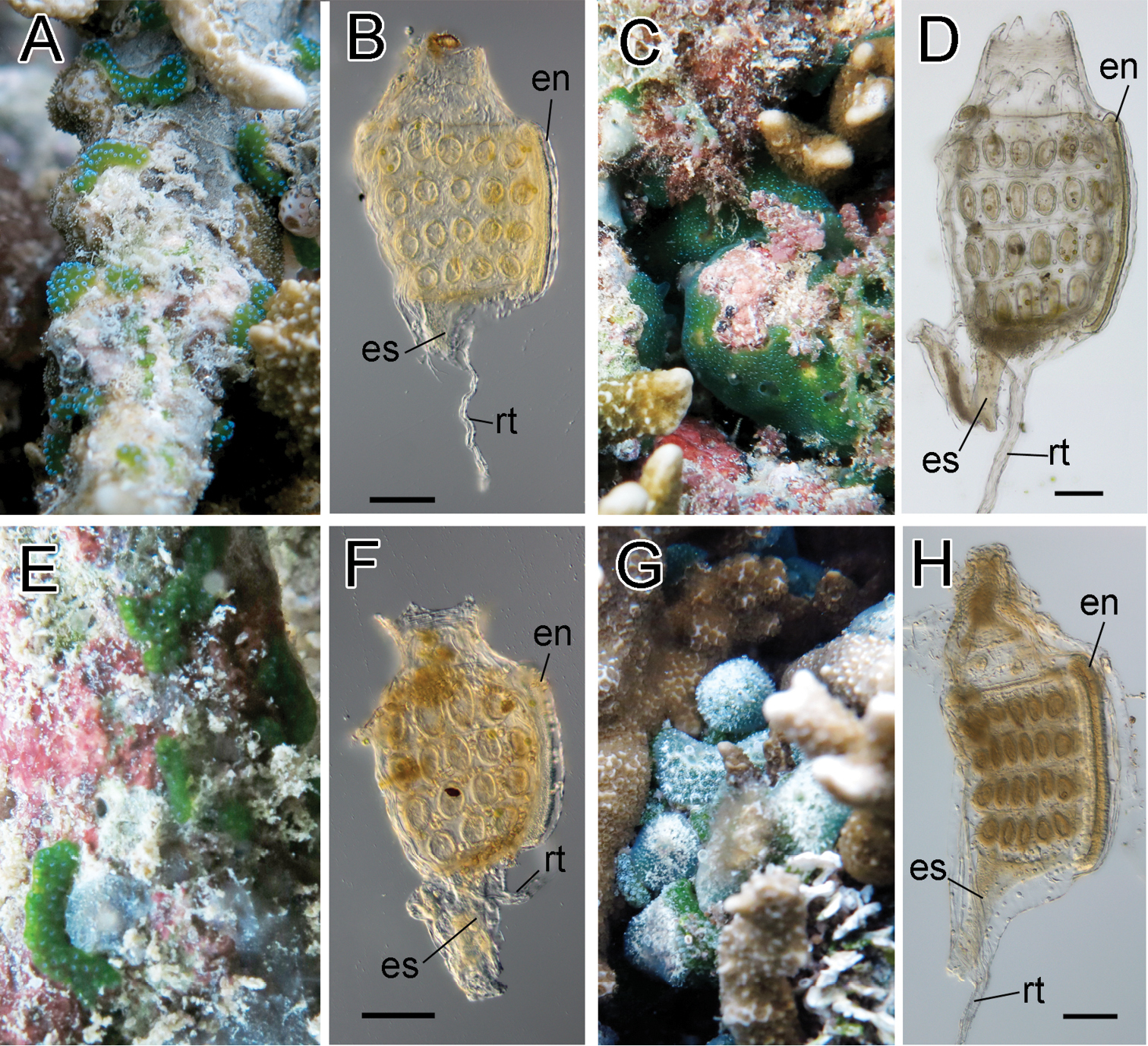
Colonies were oval cushions and entirely green due to Prochloron cells distributed within the common cloacal cavities. A blue ring of structural color encircled each branchial siphon. Retractor muscle emerged from halfway along esophageal neck of a zooid. On each of the right and left halves of the branchial sac, there were five stigmata in the first (top), second, and third stigmatal rows and four stigmata in the fourth row (bottom). Here, we describe the pattern of stigma number as 5–5–5–4. This record is the first of this species from outside of the Taiwan–Ryukyu area.
Photosymbiotic ascidians without tunic spicules (Diplosoma species). Colonies in situ and thorax of zooid of Diplosoma gumavirens (A, B), Diplosoma simile (C, D), Diplosoma simileguwa (E, F), and Diplosoma virens (G, H). en endostyle; es esophagus; rt retractor muscle. Scale bars = 0.1 mm.
MZB. Asc. 00007
Dead coral branch and coral limestone in reef flat and reef crest.
Colonies were irregularly shaped sheets and entirely green due to Prochloron cells distributed within the common cloacal cavities. Retractor muscle emerged from underside of thorax. The numbers of stigmata were 6–6–6–5. Some zooids had testes. Embryos were brooded in some colonies.
MZB. Asc. 00008
Shaded side of dead coral branches in reef flat
Colonies were irregularly shaped sheets and entirely green due to Prochloron cells distributed within common cloacal cavities. Retractor muscle emerged from underside of thorax. The numbers of stigmata were 4–5–4–3. This record is the first of this species from outside of the Taiwan–Ryukyu area.
MZB. Asc. 00009
Basal parts on branching corals in back reef and reef flat.
Colonies were irregularly shaped sheets and entirely green due to Prochloron cells distributed within common cloacal cavities. Retractor muscle emerged from halfway along esophageal neck. The numbers of stigmata were 6–6–6–5. Some zooids had testes.
All photosymbiotic ascidians described here have also been recorded in the Ryukyu Archipelago, Japan (
We thank Ms. Shelly Nur Ekayanti Tutupoho and Mr. Wahyu Muzammil (Bogor Agricultural University) for their kind help during the field survey. The present study was supported by the International Research Hub Project for Climate Change and Coral Reef/Island Dynamics from University of the Ryukyus.