Citation: Doorenweerd C, van Haren MM, Schermer M, Pieterse S, van Nieukerken EJ (2014) A Linnaeus NGTM interactive key to the Lithocolletinae of North-West Europe aimed at accelerating the accumulation of reliable biodiversity data (Lepidoptera, Gracillariidae). ZooKeys 422: 87–101. doi: 10.3897/zookeys.422.7446
We present an interactive key that is available online through any web browser without the need to install any additional software, making it an easily accessible tool for the larger public. The key can be found at http://identify.naturalis.nl/lithocolletinae. The key includes all 86 North-West European Lithocolletinae, a subfamily of smaller moths (“micro-moths”) that is commonly not treated in field guides. The user can input data on several external morphological character systems in addition to distribution, host plant and even characteristics of the larval feeding traces to reach an identification. We expect that this will enable more people to contribute with reliable observation data on this group of moths and alleviate the workload of taxonomic specialists, allowing them to focus on other new keys or taxonomic work.
Cameraria, Phyllonorycter, Macrosaccus, Triberta, identification, monitoring, conservation, biodiversity, leafminers
Taxonomic identification is the key to aggregating knowledge about species. We are increasingly aware that we live in a changing world and that different species respond differently to changes (
For the past two centuries identifications of organisms have usually been carried out with dichotomous keys. One of the governing issues with dichotomous keys is that they “are compiled by those who do not need them for those who cannot use them” (http://www.zin.ru/Animalia/Coleoptera/eng/syst8.htm). Although useful dichotomous keys certainly exist, they always have the disadvantage of being static and cannot be adjusted easily with new taxonomic insights. Furthermore, it is impossible to skip certain couplets when a required character is missing or not visible. Often, several keys are needed for a single group to target different developmental stages, sexes or character sets (e.g. external characters, genitalia). The total amount of couplets in a key is the amount of taxa included, minus one. The more questions a key contains, the less likely there will be an accurate identification (
Butterflies are often used as biodiversity indicators, but only represent a small proportion of the order Lepidoptera (
The 86 species included in the key in alphabetical order.
| Cameraria ohridella Deschka & Dimić, 1986 | Phyllonorycter lantanella (Schrank, 1802) |
| Macrosaccus robiniella (Clemens, 1859) | Phyllonorycter lautella (Zeller, 1846) |
| Phyllonorycter abrasella (Zeller, 1846) | Phyllonorycter leucographella (Zeller, 1850) |
| Phyllonorycter acaciella (Duponchel, 1843) | Phyllonorycter maestingella (Müller, 1764) |
| Phyllonorycter acerifoliella (Zeller, 1839) | Phyllonorycter mannii (Zeller, 1846) |
| Phyllonorycter aemula Triberti, Deschka & Huemer, 1997 | Phyllonorycter medicaginella (Gerasimov, 1930) |
| Phyllonorycter agilella (Zeller, 1846) | Phyllonorycter mespilella (Hübner, 1805) |
| Phyllonorycter alpina (Frey, 1856) | Phyllonorycter messaniella (Zeller, 1846) |
| Phyllonorycter anderidae (W. Fletcher, 1885) | Phyllonorycter millierella (Staudinger, 1871) |
| Phyllonorycter apparella (Herrich-Schäffer, 1855) | Phyllonorycter monspessulanella (Fuchs, 1897) |
| Phyllonorycter blancardella (Fabricius, 1781) | Phyllonorycter muelleriella (Zeller, 1839) |
| Phyllonorycter brevilineatella (Benander, 1944) | Phyllonorycter nicellii (Stainton, 1851) |
| Phyllonorycter cavella (Zeller, 1846) | Phyllonorycter nigrescentella (Logan, 1851) |
| Phyllonorycter cerasicolella (Herrich-Schäffer, 1855) | Phyllonorycter oxyacanthae (Frey, 1855) |
| Phyllonorycter cerasinella (Reutti, 1853) | Phyllonorycter parisiella (Wocke, 1848) |
| Phyllonorycter comparella (Duponchel, 1843) | Phyllonorycter pastorella (Zeller, 1846) |
| Phyllonorycter connexella (Zeller, 1846) | Phyllonorycter platani (Staudinger, 1870) |
| Phyllonorycter coryli (Nicelli, 1851) | Phyllonorycter populifoliella (Treitschke, 1833) |
| Phyllonorycter corylifoliella (Hübner, 1796) | Phyllonorycter quercifoliella (Zeller, 1839) |
| Phyllonorycter cydoniella (Denis & Schiffermüller, 1775) | Phyllonorycter quinqueguttella (Stainton, 1851) |
| Phyllonorycter delitella (Duponchel, 1843) | Phyllonorycter rajella (Linnaeus, 1758) |
| Phyllonorycter deschkai Triberti, 2007 | Phyllonorycter roboris (Zeller, 1839) |
| Phyllonorycter distentella (Zeller, 1846) | Phyllonorycter rolandi (Svensson, 1966) |
| Phyllonorycter dubitella (Herrich-Schäffer, 1855) | Phyllonorycter sagitella (Bjerkander, 1790) |
| Phyllonorycter emberizaepenela (Bouché, 1834) | Phyllonorycter salicicolella (Sircom, 1848) |
| Phyllonorycter esperella (Goeze, 1783) | Phyllonorycter salictella (Zeller, 1846) |
| Phyllonorycter eugregori Laštůvka & Laštůvka, 2006 | Phyllonorycter scabiosella (Douglas, 1853) |
| Phyllonorycter fraxinella (Zeller, 1846) | Phyllonorycter schreberella (Fabricius, 1781) |
| Phyllonorycter froelichiella (Herrich-Schäffer, 1855) | Phyllonorycter scitulella (Duponchel, 1843) |
| Phyllonorycter geniculella (Ragonot, 1874) | Phyllonorycter scopariella (Zeller, 1846) |
| Phyllonorycter gerasimowi (Hering, 1930) | Phyllonorycter sorbi (Frey, 1855) |
| Phyllonorycter harrisella (Linnaeus, 1761) | Phyllonorycter spinicolella (Zeller, 1846) |
| Phyllonorycter heegeriella (Zeller, 1846) | Phyllonorycter staintoniella (Nicelli, 1853) |
| Phyllonorycter heringiella (Grønlien, 1932) | Phyllonorycter stettinensis (Nicelli, 1852) |
| Phyllonorycter hilarella (Zetterstedt, 1839) | Phyllonorycter strigulatella (Zeller, 1846) |
| Phyllonorycter hostis Triberti, 2007 | Phyllonorycter tenerella (de Joannis, 1915) |
| Phyllonorycter ilicifoliella (Duponchel, 1843) | Phyllonorycter trifasciella (Haworth, 1828) |
| Phyllonorycter insignitella (Zeller, 1846) | Phyllonorycter trifoliella (Gerasimov, 1933) |
| Phyllonorycter issikii (Kumata, 1963) | Phyllonorycter tristrigella (Haworth, 1828) |
| Phyllonorycter joannisi (Le Marchand, 1936) | Phyllonorycter ulicicolella (Stainton, 1851) |
| Phyllonorycter junoniella (Zeller, 1846) | Phyllonorycter ulmifoliella (Hübner, 1817) |
| Phyllonorycter klemannella (Fabricius, 1781) | Phyllonorycter viminetorum (Stainton, 1854) |
| Phyllonorycter kuhlweiniella (Zeller, 1839) | Triberta helianthemella (Herrich-Schäffer, 1860) |
Keys that currently exist for Lithocolletinae or Phyllonorycter treat restricted geographic regions and are mostly for adults only. Examples include a key for the British Isles (
This paper is formatted following the guidelines for interactive keys (
Linnaeus NG is a web-based species information management system. Linnaeus NG comprises several modules, such as a species description module, a module for plotting distribution, and two types of keys. For this study, the multi-entry key was employed. The data underlying the key are managed in a spreadsheet and can be uploaded to a Linnaeus NG project as comma separated value (.CSV) files by project administrators. Two different files need to be uploaded. One contains a matrix with species data, characters, states and the relation between species and states. A second file contains image links for all character states. Alternatively, these values can be added and edited directly through the web-based multi-entry key management interface. This interface also contains an upload facility for supplying images for states and, optionally, species. Linnaeus NG is developed using open source techniques (PHP, MySQL) and is hosted in a Linux environment. On the client-side, project administrators interact with the program solely through a web browser. Recent versions of all major browsers are supported, for regular platforms and tablets. Currently, Linnaeus NG is proprietary software; updates and changes can only be made in agreement with Naturalis Biodiversity Center. However, access to Linnaeus NG is not limited to employees or associates of Naturalis Biodiversity Center and can be granted on request.
Users can access the key at: http://identify.naturalis.nl/lithocolletinae and fully use the key online through any web browser. No additional software is required. The interface was designed to be intuitive and graphic, using detail images to explain different character states and directly showing the effects of each choice by only displaying images of the remaining possible outcomes. This combination of character state selection and general visual recognition of candidate species prevents users from having to select character states until only a single option remains.
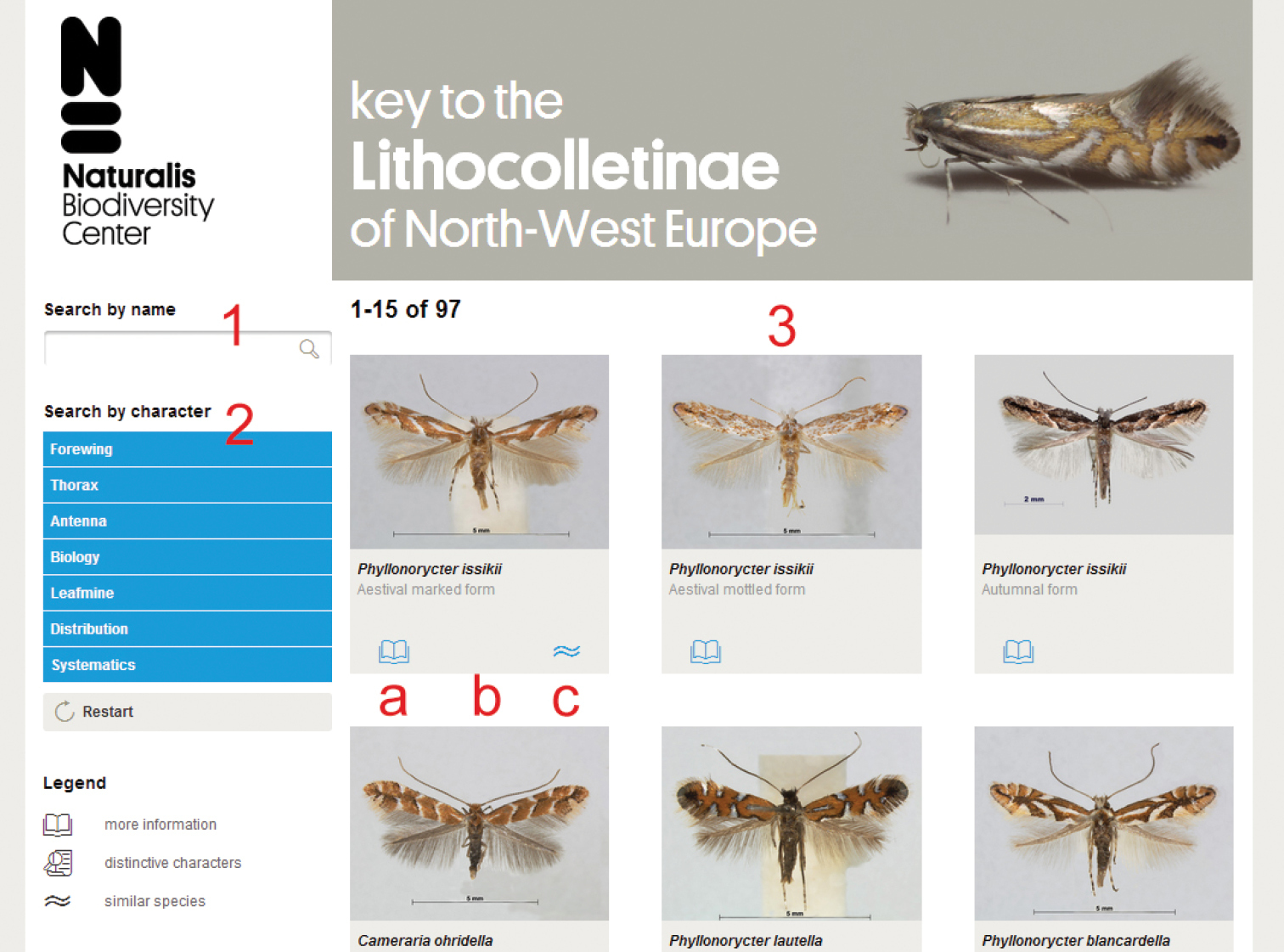
Each possible outcome of the key is represented by a thumbnail photograph of a mounted adult specimen in the main section (Figure 1: 3). Photographs were taken using stacking photography with a motorized Zeiss V20 with MRc5 camera and Axiovision software. During post-processing, photographs were sharpened, reduced in size to 800×600 pixels and backgrounds were homogenized in Adobe Photoshop CS5®. The main section of the key starts with all 96 possible outcomes, of which only the first 15 will be shown initially to prevent long loading times. Scrolling down and clicking the “show more results” button will show more options with increments of 15. Photographs can be enlarged by clicking on the thumbnail. By using the forward and previous buttons in the image overlay, the user can navigate through all remaining search results. There are three buttons below each thumbnail. The left symbol (Figure 1: a) provides additional information through an external link to the corresponding species page on Lepiforum.de. The centre symbol (Figure 1: b) is activated when less than eight options remain and lists the differences in character states. The symbol on the right (Figure 1: c) shows which outcomes are highly similar, when applicable. The selection of highly likely outcomes is based on indications in literature or personal experience of the authors, there is no automated algorithm involved.
Screenshot of the user interface with different sections indicated. 1 Search by name 2 search by character 3 main window with resulting selection, a more information, links to respective species page on fauna europaea b distinctive characters, becomes visible with a selection of 8 or less c displays species with a similar appearance.
A user can directly search for a species they suspect they might be trying to identify by using the search box (Figure 1: 1). Alternatively, identification may be reached by selecting character states. The section beneath the search option (Figure 1: 2) lists the character groups, which will expand upon clicking and show all characters within that group. As with any interactive key the user is free to choose which character to begin with. There are three morphological character groups for adult specimens: forewing, thorax and antenna. Of these, only the forewing is further subdivided, into 11 characters. Once a character has been selected, a pop-up will appear on the screen with a brief description and 200×150 pixel detail images representing the different character states. After making a choice the resulting selection is shown in the main section (Figure 1: 3). Instead of just focussing on adults, the key can also be used to identify larvae or leafmines. The characters in the sections biology, distribution and systematics are not morphological, but may help with the identification nonetheless. Alternatively, these options may be used to quickly get a graphic overview of a given species group in a certain country, or all species found on a particular family of host plants.
There are 97 possible outcomes of the key, representing 86 species (Table 1). The majority of outcomes coincide with species, but some species that exhibit intraspecific variation are subdivided. There are species with clearly different forms, such as the aestival and autumnal form of Phyllonorycter issikii (
The 86 species (Table 1) that are included make the key complete for all species found in North-West Europe, more specifically those reported as present in Fauna Europaea (
The selection of morphological characters (Table 2) was initially based on existing dichotomous keys and we follow the terminology used therein. The white ground colour of the forewings for example is the starting point in both the NationalNyckeln (
Morphology characters and states.
| Character group | Character | States |
|---|---|---|
| Forewing | Forewing ground colour | White; other |
| Forewing | Forewing mottling | Mottled; no mottling |
| Forewing | Forewing basal streak | Present; absent |
| Forewing | Forewing basal streak contour | None; costal; bilateral |
| Forewing | Forewing fascia | 0; 1; 2; 3 |
| Forewing | Forewing costals | 0; 1; 2; 3; 4 |
| Forewing | Forewing dorsals | 0; 1; 2; 3; 4 |
| Forewing | Forewing markings contour | Unilateral basal; unilateral proximal; bilateral; absent |
| Forewing | Forewing apical marking | Dot; stripe; mottled; absent |
| Forewing | Forewing cilia line | Present; absent |
| Forewing | Forewing apical fringe | With markings; uninterrupted |
| Thorax | Thorax pattern | Striped; uniform; silver or golden |
| Antenna | Antenna colour pattern | Even or chequered; black tip; white tip |
| Leafmine | Leafmine orientation | Tentiform underside; Tentiform upperside; Full depth blotch; Epidermal upperside blotch; Stem-mine |
| Leafmine | Leafmine location | Along secondary veins; Along main vein; Leaf base; Leaf margin; Leaf lobe; Whole leaf; Stem; Rachis wings |
| Leafmine | Leafmine ribs | None; 1; Several; Many |
| Leafmine | Leafmine frass | Linear; Aggregated; Scattered; Attached to cocoon |
Lithocolletinae are all herbivores in their larval life stage with commonly a high degree of monophagy. The host plant is therefore often important for the identification. The key includes data on: host family, host genus and host genus/species (Table 3). Host genus and host genus/species partly overlap. The list ‘host genus/species’ includes all 303 known host species for the 86 Lithocolletinae (
Non-morphological characters.
| Character group | Character |
|---|---|
| Biology | Host family |
| Biology | Host genus |
| Biology | Host genus/species |
| Distribution | Country |
| Systematics | Species group |
The key presented here for the Lithocolletinae of North-West Europe includes more species, covers a larger area and can be used for more life stages than any existing key for this subfamily. As such, it enables a large potential user group to identify Lithocolletinae through different approaches with minimized specialist effort involved, including future effort regarding updates. However, accomplishing this has not been without challenges. Most existing keys treat the species of a relatively restricted region, often a single country. A larger area holds more species and thus more candidate species that have to be ruled out. This can be circumvented by first selecting a country under distribution. However, inherent to an interactive key, the user has to make this choice actively. On the other hand, having more species in the key than just those already known and published for a country may enable recognition of introduced, migrating or previously overlooked species. A second challenge posed by covering North-West Europe involves the differences in voltinism at different altitudes. Species that are strictly univoltine in northern Sweden may be bivoltine in a warmer climate in Belgium. Adult flight period(s) or larval feeding period(s) could therefore not be included in our key. An advantage that dichotomous keys have over interactive keys in general is that they can include characters that are specific for a selection of taxa. An interactive key is based on a character matrix where for each taxon all character states need to be filled out; in a dichotomous key, there may be certain species pairs or groups that have distinguishing characters that are lacking in all other taxa (e.g.
Aside from the challenges, this key also contains many advantages over existing keys. Perhaps one of the most crucial is the ability to combine quantitative characters with the human brain’s ability to recognize subjective visual patterns (
Data on the larval life stage, host plant and distribution were included to take further advantage of all the benefits an interactive key has to offer. Several cautionary notes need to be taken into consideration with these characters. The list of host species is not cross-referenced with distribution. For example, a species may be recorded to feed on Acer pseudoplatanus in Germany, but not in Great Britain. If a user in Great Britain thus selects Acer pseudoplatanus as host species and Great Britain as country, they may end up with a selection of Lithocolletinae that includes false positives. On the other hand it can broaden the view of the user and allow for earlier recognition of new host records for a country, similar to how new species records for a country can be enabled by not narrowing down to a country first. When using the key, it should be advised to consult regional literature or websites on the resulting identification to see if this may be the case. Using a combination of several fairly easy characters of the larval feeding traces and an identified host should in most cases provide a reliable identification. The key can thus be used to record Lithocolletinae not only during their flight period, but also during the larval feeding period and greatly expand on the amount of faunistic data.
The target audience for this key is limited by the requirement that the insect first has to be recognised or identified as belonging to the Lithocolletinae. However, Lithocolletinae is a species rich subfamily with between 33 (Luxembourg) and 84 species (Austria) per country with distinct adult and larval features that separate them from other Lepidoptera. This makes them generally recognizable by professional lepidopterists and enthusiasts alike. The connection of the 86 Lithocolletinae in this key with 303 host plant species further indicates that the subfamily is an important component of most ecosystems in North-West Europe. Collecting faunistic data on Lithocolletinae has potential to contribute to biodiversity studies, and hopefully more interactive keys with this objective for other Lepidoptera groups will be created by taxonomic specialists to enable more enthusiasts to contribute their data to databases.
We expect that the key presented here for Lithocolletinae of North-West Europe enables more people to contribute faunistic data with reliable identifications. The key has been designed to allow easy access for inexperienced users, yet still be an efficient tool for advanced users. This publication marks the release of version 1.0. Future changes will be noted under the version history at the website. We will greatly appreciate feedback from users and we hope to further expand and improve the key. Ultimately, we hope to include all European Lithocolletinae, and develop databases with reliable faunistic knowledge that can be useful for biodiversity estimates.
The manuscript was improved after suggestions from Jeremy Miller on grammar and content, for which we are grateful. We are grateful for reviews by Lauri Kaila and Paolo Triberti that further improved the manuscript. The authors would like to thank Zdeněk Laštůvka, Bengt Åke Bengtsson, David Lees, Carlos Lopez-Vaamonde and Marko Mutanen for their willingness to share photographs of specimens with verified identifications. Zdeněk Laštůvka is further thanked for clarifying the difference in wing morphology between Phyllonorycter mannii and Phyllonorycter distentella and pointing out the likely synonymy of Phyllonorycter pyrifoliella with Phyllonorycter gerasimowi. Tymo Muus, Sifra Corver, Jean-Yves Baugnee and Willem Ellis are thanked for providing photographs of leafmines. A large group of people composed of professionals and both inexperienced and more advanced hobbyists is thanked for beta-testing a preliminary version of the key. Sjaak Koster is acknowledged for sorting material in the Naturalis Biodiversity Center RMNH collection for the past decade, which allowed us easy access to all species. We thank collection manager Rob de Vos for his overall helpfulness and for indicating interesting specimens in the Naturalis Biodiversity Center ZMA collection.