(C) 2013 Robert Perger. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The genera Parandrocephalus Heller, 1916 and Hexamitodera Heller, 1896 are reviewed and redescribed. Based on the combination of chromatic sexual dimorphism, velvety pubescence on the whole dorsal body and distinctly developed carina on the elytra, Parandrocephalus blairi Bentanachs & Vives, 2009 is transferred to Hexamitodera.
A new subgenus, Sulcognatha Perger, is instituted to accommodate mandible, head and metasternal modifications in Hexamitodera blairi comb. n. that are lacking in the type species of Hexamitodera, Hexamitodera semivelutina. As indicated by fundamental structural differences in the mandibles of Parandrocephalus and Hexamitodera (Sulcognatha) blairi comb. n., the exaggerated secondary sexual traits and open procoxal cavities in both taxa are presumably the result of convergent evolution. Contrary to
Longhorn beetles, Malaysia, Indonesia, Sulawesi, Wallacea, taxonomy, biogeography
The discovery and description of two distinct zoogeographical realms in South East Asia by Alfred Russel Wallace was the birth of biogeography and inspired the naturalist to formulate fundamental principles of the evolution theory, at the same time with Charles Darwin (
A beetle group that has attracted the attention of following generations of collectors and taxonomists and is mainly speciose in the tropical regions of the Austral hemisphere is the heterogeneous tribe Callichromatini (
Taxa with a combination of chromatic sexual dimorphism and velvety pubescence on the whole dorsal body are exceptional for this tribe. Only three Asian Callichromatini species share this combination: Hexamitodera semivelutina Heller, 1896, Parandrocephalus blairi Bentanachs & Vives, 2009 and Niisatochroma celebiana Vives & Bentanachs, 2010, all occurring on Sulawesi.
Particularly Parandrocephalus blairi with the con-generics Parandrocephalus eversor Heller, 1916 and Parandrocephalus drescheri Blair, 1938 on the Sunda Islands represents an interesting case as it belongs to one of the few Cerambycidae genera that occur on both sides of the Wallace line (
In this study I review generic characters to test the hypothesis of whether the genus Parandrocephalus indeed crossed the Wallace line or alternatively, the Sulawesian taxa share characters that rather indicate vicariance processes and support the Wallace line as an effective zoogeographical boundary.
Specimens examined for this study are from the following institutions / private collections:
BMNH Natural History Museum, London, U.K.;
JA Dr. Joachim Adolphi, Private collection, Dresden, Germany;
RMNH National Museum of Natural History in Leiden, The Netherlands;
RP Robert Perger, Private collection, Santa Cruz, Bolivia;
RV Robert Vigneault, Private collection, Quebec, Canada;
SNSDSenckenberg Naturhistorische Sammlungen Dresden, Germany;
TN Tatsuya Niisato, Private collection, Tokyo, Japan;
UN Ulf Nylander, Private collection, Valbo, Sweden.
Morphological characters were examined with a stereomicroscope and specimens were photographed with a Canon 450D reflex camera fitted with macro lenses.
The following specimens were examined:
Parandrocephalus eversor Heller, 1916
Sumatra, Padangsche Bovenlanden: holotype 1 ♂, RMNH, ex coll. Dr. H. J. Veth; Malaysia, Cameron Highlands, Kampong Rajah: 1 ♂, RP, VI-2000; Borneo, Sabah, Mt. Trus Madi: 1 ♀, UN, 19-VI-05, S.Chew coll.
Parandrocephalus drescheri Blair, 1938
Java, G. Tangkoeban Prahoe, 4000-5000 voet [= ft.]: holotype, 1♂, BMNH, VI-1937, F.C. Drescher coll.[G. Tangkoeban Prahoe, 4000-5000 voet [= ft.], Dreanger, Java, VI.1937 / type / Parandroceph. drescheri Blr., ♂ type det. K.G. Blair / Brit.Mus., 1937-662]
Parandrocephalus blairi Bentanachs & Vives, 2009
Indonesia, Sulawesi, Puncak near Palolo: holotype, 1 ♂, TN, II-1990; Indonesia, Sulawesi, Palolo Palu: allotype, 1 ♀, TN, IV-1991; Indonesia, Central Sulawesi, Palolo Palu: 2 ♂ RP, 1 ♂ JA, III-1999; Indonesia, Central Sulawesi, Puncak: 1 ♂, RP, IV-1999.
Hexamitodera semivelutina Heller, 1896
Indonesia, North Sulawesi: holotype, 1 ♀, SNSD [semivelutina Hh. / N.Celebes, 9389 / Typus / Staatl. Museum für Tierkunde, Dresden]; Indonesia, Sulawesi, Minado: 1 ♂, BMNH, 1781, Wallace coll., ex coll. Fry; Indonesia, Sulawesi: 1 ♀, BMNH, 1922; Indonesia, Sulawesi, Pulu Pulu: 1♂, RV, 19-XII-1997, A. Audureau coll.
Parandrocephalus eversor Heller, 1916
Body relatively large, elongated, parallel-sided, flattened, without chromatic sexual dimorphism. Head and mandibles with pronounced sexual dimorphism, abnormally enlarged in male, vertex glabrous. Male mandible sickle-shaped, flattened vertically, with dorsomedian carina, median longitudinal concave. Antennae slightly surpassing the basal elytral half (not the apical third of the elytra as diagnosed by
http://species-id.net/wiki/Parandrocephalus_eversor
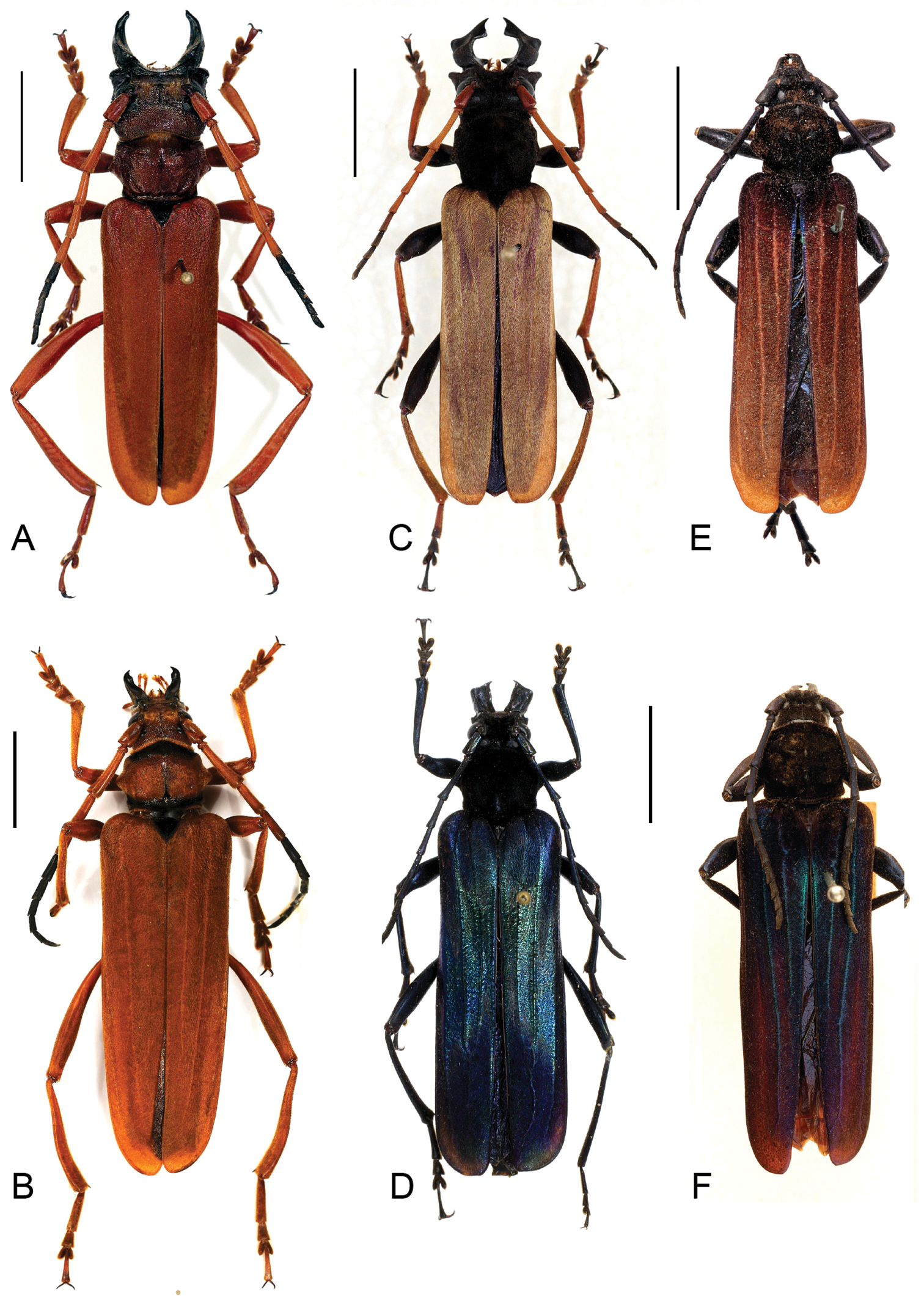
Fig. 1A, BMale. Body 4.2 times as long as wide. Head and pronotum (Fig. 2B) glabrous. Head dorsally polished, coarsely wrinkled, vertex strongly convex, not carinated dorsally; frons strongly concave, with a deep longitudinal furrow. Temple in dorsal view strongly convex; distance between temples wider than anterior border of pronotum and as wide as widest width of pronotum. Gena apically rounded, externally thickened. Width between genae as wide as width of head. Mandible sickle-shaped, curved, forming an elongated ellipse in closed position, with dorsomedian carina; apex conical, unidentate; molar margin without basal tooth. Antenna sparsely pubescent, bicolored. Elytra 2.2 times as long as prothorax and head excluding mandibles.
Female.Vertex and temples straight, distance between temples narrower than anterior pronotal margin and widest pronotal width, vertex setose, with longitudinal furrow reaching anterior pronotal margin. Mandible horizontally flattened, dorsal surface convex, with dorso-lateral carina, externally straight. Pronotal disc setose. Elytra 3.43 times as long as prothorax and head excluding mandibles. Abdomen bordered with yellowish pubescence at the base of all ventrites.
Sumatra, Borneo, Malaysia Peninsula.
Dorsal view habitus, scale bars 10 mm A Parandrocephalus eversor, male, Malaysia, Cameron Highlands, Kampong Rajah B idem, female C Hexamitodera (Sulcognatha) blairi comb. n., male, Indonesia, Central Sulawesi, Puncak D idem, female, allotype, Indonesia, Sulawesi, Palolo Palu E Hexamitodera semivelutina, male, Sulawesi F, idem, female, holotype, Indonesia, North Sulawesi.
Dorsal view head and pronotum, scale bars 5 mm A Hexamitodera (Sulcognatha) blairi comb. n., male, Indonesia, Central Sulawesi, Puncak B Parandrocephalus eversor, male, Malaysia, Cameron Highlands, Kampong Rajah.
Hexamitodera semivelutina Heller, 1896 (monotypic)
Body relatively large, elongated, flattened, dorsally covered with dense velvety pubescence (could be partially abrased, particularly in females), with chromatic sexual dimorphism. Mandible horizontally flattened, dorsal surface straight, without dorsomedian carina, dorso-lateral border, externally straight or with shallow to deep concavity. Antenna relatively short, reaching or slightly projecting above basal half of elytra. Pronotum transverse, obtusely to acutely toothed laterally; apical margin not constricted. Procoxal cavity open posteriorly. Sternum and epimeron of meso- and metathorax densely pubescent. Elytra covering abdomen, parallel-sided, with three distinctly elevated longitudinal costae, the inner two converging in about the apical third of the elytra. First abdominal sternite of female distally bordered with white pubescence. Femur fusiform, comparably short, stout; metatibia moderately flattened and widened, narrower than metafemur.
http://species-id.net/wiki/Hexamitodera_semivelutina
Fig. 1E, FMale. Body 3.7–3.8 times as long as wide. Vertex and temples straight, distance between temples narrower than anterior margin of pronotum and widest pronotal width. Head dorsally uniformly finely punctured, with a fine longitudinal furrow reaching the base. Mandible without inner basal tooth, feebly curved or angulate (Fig. 3A). Mandible as long as one-half of the rest of the head, as long and wide as scape. Gena rounded apically; width between genae distinctly narrower than width between temples, the latter narrower than pronotal width. Antenna sparsely pubescent. Antennomeres 1–2 apically rounded, 3 straight, as 1.9 times as long as scape, 4–11 obtusely toothed. Antenna and legs unicolorous.
Prothorax 1.21 times as wide as long. Procoxal cavity opened posteriorly by a comparable small gap. Apex of mesosternal process not concealed by metasternum.
Elytra 2.9 times as long as prothorax and head excluding mandible and 2.8 times as long as elytra width, brownish.
Female. Head, mandible and pronotum as in male. Elytron 3.3 times as long as prothorax and head excluding mandible, metallic blue with purple brownish stripes and brown apices.
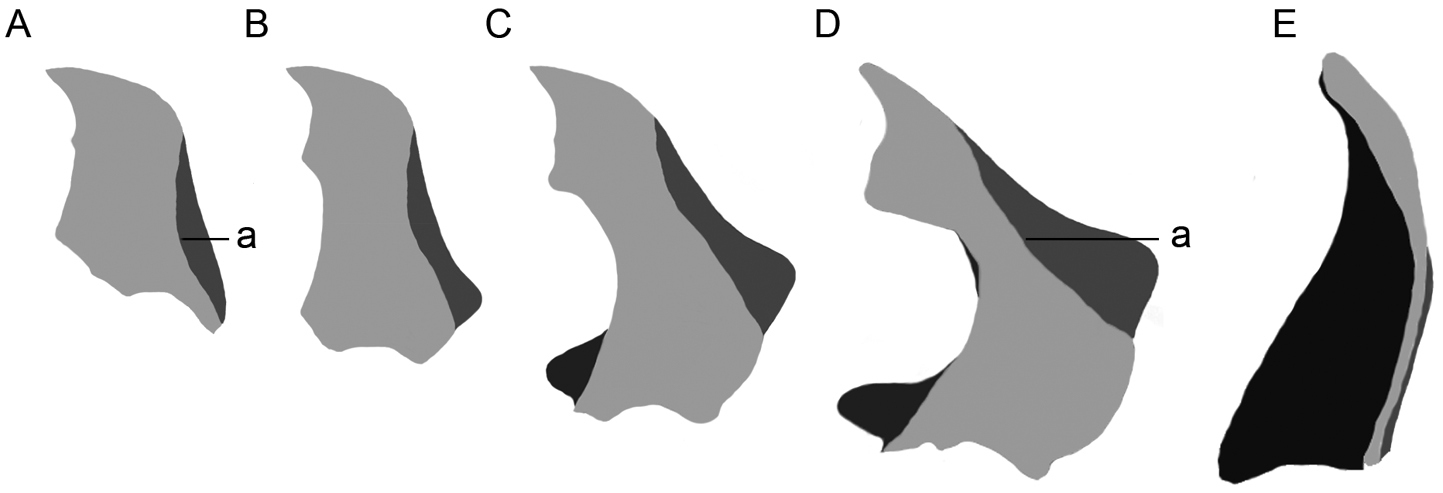
Dorsal view male mandibles; light grey, dorsal surface; dark grey, ventral external concavity; black, interior surface; a, dorso-lateral border; mandibles of A and D are drawn from specimens that were digitally scaled until they had similar body length A Hexamitodera semivelutina B, C hypothetical intermediate forms D Hexamitodera (Sulcognatha) blairi comb. n. E Parandrocephalus eversor.
Sulawesi.
Fig. 1C, D
Hexamitodera (Sulcognatha) blairi (Bentanachs & Vives, 2009) comb. n.
Head and mandible enlarged in both sexes; gena acutely produced; clypeus strongly concave, very short; labrum reduced and not visible from dorsal view; mandible basally broadened, in male conspicuously, and distal half antero-laterally of mandible in both sexes with prominently developed concavity (Fig. 2A; 3D). Apex of mesosternal process concealed by metasternum; antenna and tibia with chromatic sexual dimorphism.
The new subgenus name is a combination of the Latin word sulcus (meaning, “furrow”) and the Greek word gnathus (meaning, “jaw”), and is a reference to the deep antero-lateral furrow in the mandible of both genders. The name is feminine.
This subgenus includes so far only the type species.
http://species-id.net/wiki/Hexamitodera_blairi
Fig. 1C, DMale. Head abnormally developed (Fig. 1C; 2A), dorsally uniformly finely punctured, with a fine longitudinal furrow reaching the base. Temple straight, not protruded under the forehead in dorsal view, distance between temples narrower than anterior pronotal margin. Gena elongate and acutely produced exteriorly, anterolaterally rounded, maximal width distinctly wider than forehead and as wide as pronotum. Mandible as long as head, twice as long as scape, maximal width as long as scape, strongly curved inwards, forming a transverse ellipse in closed position; apex flattened, shovel-shaped, bidentate, internal tooth obtuse; molar margin with strong basal tooth. Antenna slightly surpassing elytral half, articles 1–2 spineless, glabrous, red-brown, 3–6 with small apical spines, fine short pubescence, testaceous to brass-colored, 7–11 spineless, sparsely pubescent, dark-grey/brown, 11 apically rounded. Prosternal process projecting over posterior border of procoxae. Elytra 2.5 times as long as prothorax and head without mandibles, with brass-colored velvety pubescence. Femur dark-brown to black, tibia brass-colored to testaceous.
Female. Mandible as long as scape, flattened, acutely pointed at the apex, between tooth of apex and base continuous, not strongly curved inwards, molar margin without tooth. Gena forming an obtuse angle, their maximal width narrower than width between eyes, nearly as wide as forehead. Antenna dark blue with metallic reflections, sparsely pubescent. Elytron 3.3 times as long as prothorax and head excluding mandibles, metallic blue.
Sulawesi.
In Niisatochroma celebiana, the sole species in this genus, the chromatic gender dimorphism (brass brownish tones in male, bluish-green in female), dorsal body pubescence, prominent developed elytral costae and short limbs (see
Sulawesi.
Contrary to the generic characters provided for Parandrocephalus (
The separate treatment of sexual and non-sexual traits has revealed a suite of apomorphic characters (Table 1) that allows a more coherent interpretation of relationships of Hexamitodera (Sulcognatha) blairi. Actually, the chromatic gender dimorphism (brownish tones in male, blue in female), body pubescence, prominent developed elytral costae, short legs and antennae of Hexamitodera (Sulcognatha) blairi clearly belong to Hexamitodera (Table 1). The relationship becomes already evident in the comparison of the dorsal habitus of the females of Hexamitodera (Sulcognatha) blairi and Hexamitodera semivelutina (Fig. 1D, F).
Generic differences of Parandrocephalus and Hexamitodera
| Character | Parandrocephalus | Hexamitodera |
|---|---|---|
| Body chromatic sexual dimorphism | absent | present |
| Shape male mandibular | vertically flattened, with dorsomedian carina, interiorly concave, distal portion interiorly with deep concavity | horizontally flattened, dorsally straight, dorsomedian carina absent, dorso-lateral border, externally with shallow to deep concavity |
| Pubescence male head + pronotum | absent | distinct |
| Elytral costae + pubescence | weak | distinct |
| Light pubescence at female abdominal sternites | first to fourth | first |
| Metatibia distally flattened and broadened | distinct | moderate |
| Width of metatibia distally | ~ metafemora | <metafemora |
The lacking sexual dimorphism in mandible and head of Hexamitodera semivelutina suggests an ancestral relationship with Hexamitodera (Sulcognatha) blairi, while the enlargement and complex structure of the mandible in Hexamitodera (Sulcognatha) blairi indicate a more derived status. I hypothesise that the grades of differentiation in the mandible in the two Hexamitodera species represent the basal and terminal end of an evolutionary transformation series (Fig. 3A-D) of male adaptations to adjust on the female during the mating. The interior shape of the male mandible in Hexamitodera (Sulcognatha) blairi fits well with the posterior constriction of the female prothorax, while the antero-distal mandibular concavity is perfectly suited to accommodate the female profemora. The large mandibles and their adjustment on the female during the mating are evolutionarily advantageous because they facilitate a successful (possibly prolonged) fertilization and contribution to the gene pool. In this context, the inclusion of Hexamitodera (Sulcognatha) blairi into Hexamitodera provides an interesting hypothesis for testing evolutionary processes that select for large mandibles and co-adaptations.
It might be asked if the modifications in head and mandible of Hexamitodera (Sulcognatha) blairi justify the establishment of a new genus, however, particularly on the basis of morphology it is difficult to assess at which point a phylogenetic distance passes a subgeneric or generic boundary, and additionally there is no operational definition for such boundaries.
To my knowledge, Hexamitodera semivelutina and Hexamitodera (Sulcognatha) blairi are from other taxa of the same rank unambiguously distinguished by apomorphic characters (Table 1) and the lack of intermediate mandible and head forms, indeed representing a certain gap, at the very most justifies the institution of a new subgenus.
The extraordinarily developed mandibles in males of Parandrocephalus and Hexamitodera (Sulcognatha) blairi, evidently showing distinct features (Table 1; Fig. 2; 3D, F), should be interpreted as convergent adaptations, possibly for mate-guarding and the fitting of the male mandible to the posterior constriction of the female prothorax. While the mandible structure might contain useful taxonomical information, the enlargement alone is not a good indicator for relationships since it has also independently evolved in males of other Callichromatini genera (e.g. Aphrodisium niisatoi Vives & Bentanachs, 2007 and Huedepohliana superba (Aurivillius, 1910)), and also in phylogenetically more distant beetle taxa, such as Prioninae (Cerambycidae), Manticorini (Carabidae) and Lucanidae.
Sundaland (including Borneo, Sumatra and Java) is assumed to be zoogeographically separated from ‘Wallacea’ (comprising Sulawesi and the Philippines except for Palawan) (
The distribution of Cerambycidae taxa has been only sporadically treated in respect to the Wallace line and there is no statistical approach identifying geographical patterns.
Actually there are several closely related Cerambycidae taxa that occur on both sides of the Wallace line, e.g. the subspecies of Xixuthrus microcerus White, 1853 (Prioninae) (
The genera Xystrocera Blanchard, 1845 (Xystrocerini) (
However, in both, the South-East Asian taxa that occur on both sides of the Wallace line and pantropical taxa, distributional patterns might also be influenced by transoceanic dispersal of larvae in drifting logs (see
The Wallace line holds for the tribe Tmesisternini (Lamiinae), which is highly diversified in New Guinea and Sulawesi but nearly absent in Sundaland (
There appear to be clear trends in some Cerambycidae tribes supporting the Wallace line, nevertheless, the examples predating such line call for a proper statistical analysis of morphological or biochemical characters to clarify phylogeographical relationships.
According to the current state of knowledge, the genus Parandrocephalus did not cross the Wallace line, supporting the idea that Parandrocephalus and Hexamitodera are indeed examples for convergent evolutionary processes within two zoogeographically distinct realms.
I am grateful to Tatsuya Niisato (Japan), Oliver Jäger (Senckenberg Naturhistorische Sammlungen Dresden, Germany), Sharon Shute, Erica McAlister (Natural History Museum, London, U.K.), Caroline Pepermans (National Museum of Natural History in Leiden, The Netherlands), Ulf Nylander (Sweden), Joachim Adolphi (Germany) and Robert Vigneault (Canada) for providing pictures and the possibility to study the typical specimens mentioned among the examined material.
Grateful thanks are also extended to Steven W. Lingafelter (Systematic Entomology Lab., USDA, National Museum of Natural History, Washington, U.S.A.), Antonio Santos-Silva (Museu de Zoologia, Universidade de São Paulo, Brazil), Eduard Vives (Museu de Ciències Naturals de Barcelona, Terrassa , Spain), Francesco Vitali (Luxembourg) and the anonymous reviewers for providing comments, suggestions and constructive criticism on the manuscript.