Citation: Schmidt BC (2014) Polyphyly of Lichen-cryptic Dagger Moths: synonymy of Agriopodes Hampson and description of a new basal acronictine genus, Chloronycta, gen. n. (Lepidoptera, Noctuidae). In: Schmidt BC, Lafontaine JD (Eds) Contributions to the systematics of New World macro-moths V. ZooKeys 421: 115–137. doi: 10.3897/zookeys.421.7424
The taxonomic composition and systematic position of Agriopodes Hampson is examined through an integrated approach using adult and larval morphology, biology, and molecular sequence data. The type-species of Agriopodes, Moma fallax Herrich-Schäffer is shown to be derived within the Acronicta grisea Walker species-group; accordingly, Agriopodes is relegated to synonymy under Acronicta Ochsenheimer, syn. n. (Acronictinae). Additionally, molecular markers and morphology show that Agriopodes is not monophyletic: Agriopodes tybo (Barnes) is not closely related to A. fallax nor to Acronicta, and is transferred to a new genus, Chloronycta Schmidt & Anweiler, gen. n. The immature stages of Chloronycta tybo comb. n. are described and illustrated for the first time. Although previously treated as a valid species, we show that Agriopodes geminata (Smith) represents the northern terminus of clinal variation in wing pattern of A. fallax and synonymize A. geminata under A. fallax (syn. n.). The history and identity of Agriopodes corticosa (Boisduval), a nomen dubium, is discussed.
Agriopodes fallax, Agriopodes geminata, Agriopodes tybo, dagger moths, Fraxinus, Arizona
The New World genus Agriopodes Hampson (Noctuidae, Acronictinae) has included as many as seven species, united by their striking lichen-mimicking colours of green, white and black. As we show here, their similarity in colour pattern is convergent, and represents a common evolutionary trajectory repeated multiple times across the Noctuoidea. The superficial nature of their shared pattern elements is reflected in the taxonomic history of Agriopodes, with species previously removed from the genus now distributed among three different subfamilies outside the Acronictinae (
As part of an ongoing revision of the North American Acronictinae (Schmidt and Anweiler in prep.), we examined the four remaining species of Agriopodes (fallax, geminata, tybo and corticosa), and it soon became evident that the monophyly of Agriopodes was still problematic.
Morphology. Adult genitalia were prepared using standard methods, described in detail by
Molecular analysis. We compared molecular variation of Agriopodes fallax to other Acronictinae using eight gene regions, namely cytochrome c oxidase subunit 1 (COI) (1477 bp) from the mitochondrial genome and elongation factor-1 α (EF-1α) (1240 bp), ribosomal protein S5 (RpS5) (617 bp), carbamoylphosphate synthase domain protein (CAD) (859 bp), cytosolic malate dehydrogenase (MDH) (407 bp), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (691 bp), isocitrate dehydrogenase (IDH) (716 bp) and wingless (400 bp) genes from the nuclear genome. All genes are single-copy, protein-coding exons and have previously been found to be highly informative in phylogenetic analyses of Lepidoptera at multiple taxonomic levels (
Specimen voucher data and GenBank accession numbers for samples used in phylogenetic analysis. Dash indicates DNA markers that did not amplify.
We also examined molecular variation in Agriopodes fallax, Agriopodes geminata, Agriopodes tybo, and more than 80 species of North American Acronictinae, including exemplars from all recognized genera and species groups, using the barcode region (658 bp) of COI gene (
Phylogenetic analysis. Data matrices (6407 bp total) were analysed by non-model-based (parsimony) with equal weighting and model-based evolutionary methods (Bayesian Inference, BI). Parsimony analyses used New Technology heuristic searches (consisted of Tree Fusion, Ratchet, Tree Drifting and Sectorial searches) implemented in the program TNT v1.1 (
Immature stages. Last instars of Agriopodes fallax from 10 km E of Indian Lakes, Hamilton Co., New York were compared with those of all North American genera of Acronictinae, most species of Nearctic Acronicta and images of both the European (
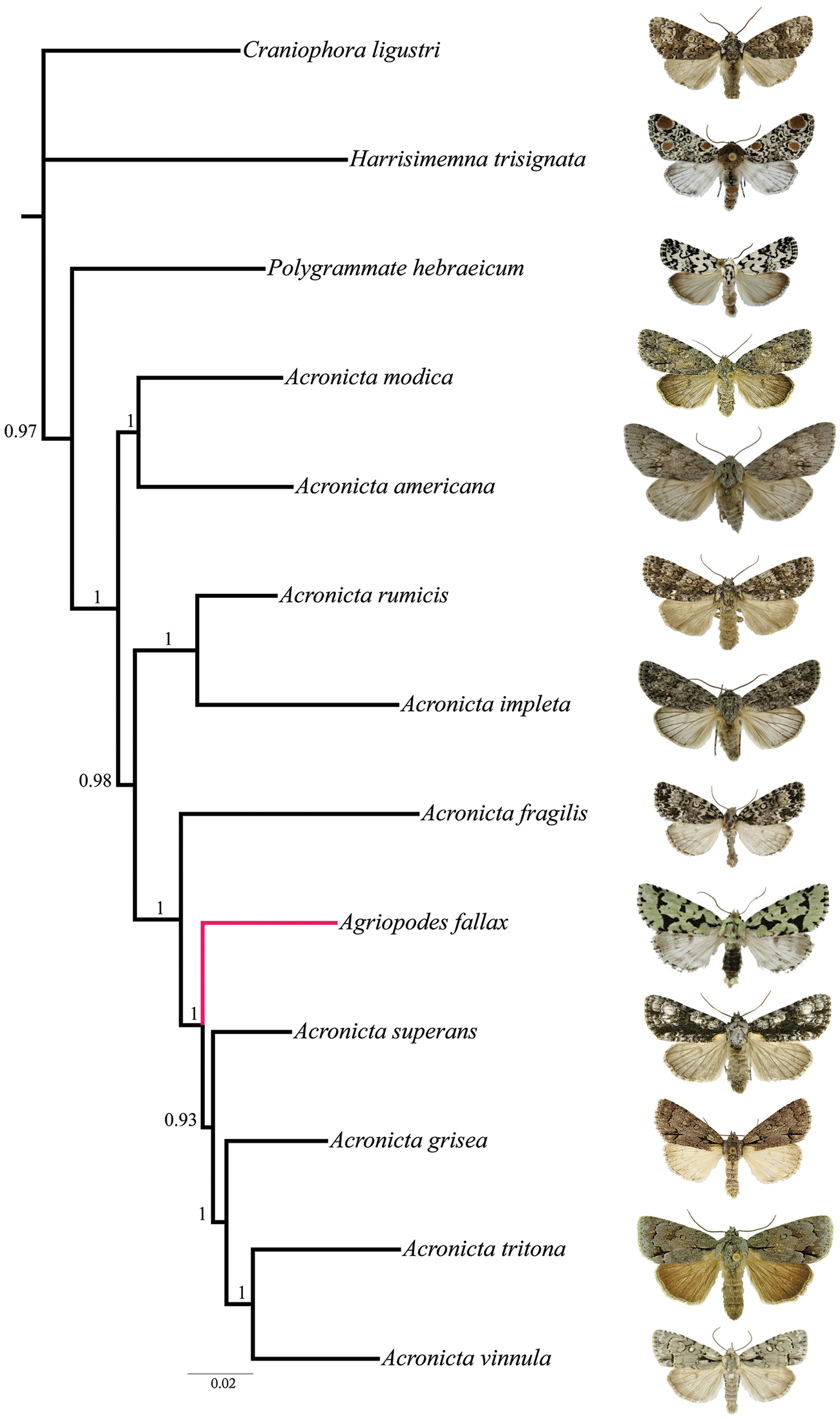
Molecular analysis. Phylogenetic analyses of the multi-gene dataset resolved a well-supported monophyletic Acronicta clade with the inclusion of Acronicta fallax, which placed as the sister species to a group consisting of Acronicta superans Guenée, Acronicta grisea Walker, Acronicta tritona (Hübner) and Acronicta vinnula (Grote) (Fig. 1). As discussed below, these four species represent two structurally delimited groups, with grisea, tritona and vinnula in the tritona-group and superans in the hasta-group (Schmidt and Anweiler unpubl. data).
Phylogenetic placement of Agriopodes fallax (terminal branch in red) relative to 12 additional acronictine taxa, based on Bayesian analysis of eight gene regions. Bootstrap support values >50% are given for internal branches.
Barcode variation showed that the single sample of Agriopodes geminata (641 bp) was very similar to haplotypes of Agriopodes fallax from ON, MI, FL, GA and OK, differing by 2–3 bp; comparison to barcode variation across Agriopodes fallax in the BOLD database (n = 25) indicated intraspecific variation of up to approximately 1.6% (
Agriopodes tybo barcode sequence showed little affinity to any sampled Acronictinae, differing by at least 8% from all other sequences in the BOLD database, which contains approximately 7300 species representing 250 genera of Noctuidae globally (BOLD). To explore a potential relationship of Agriopodes tybo to Amphipyrinae, Psaphidini, we compared Agriopodes tybo to a dataset constrained to Nearctic psaphidine genera (58 species and 29 genera), but minimum divergences were similarly upwards of 7%; as might be expected, nodes of intergeneric relationships were unsupported (data not shown). Barcode sequence of Agriopodes tybo was not found to be phylogenetically informative as to probable subfamily membership. The generic placement of Agriopodes tybo is currently the focus of an expanded study by Wagner et al. (in prep.).
Figs 2, 5, 9, 13
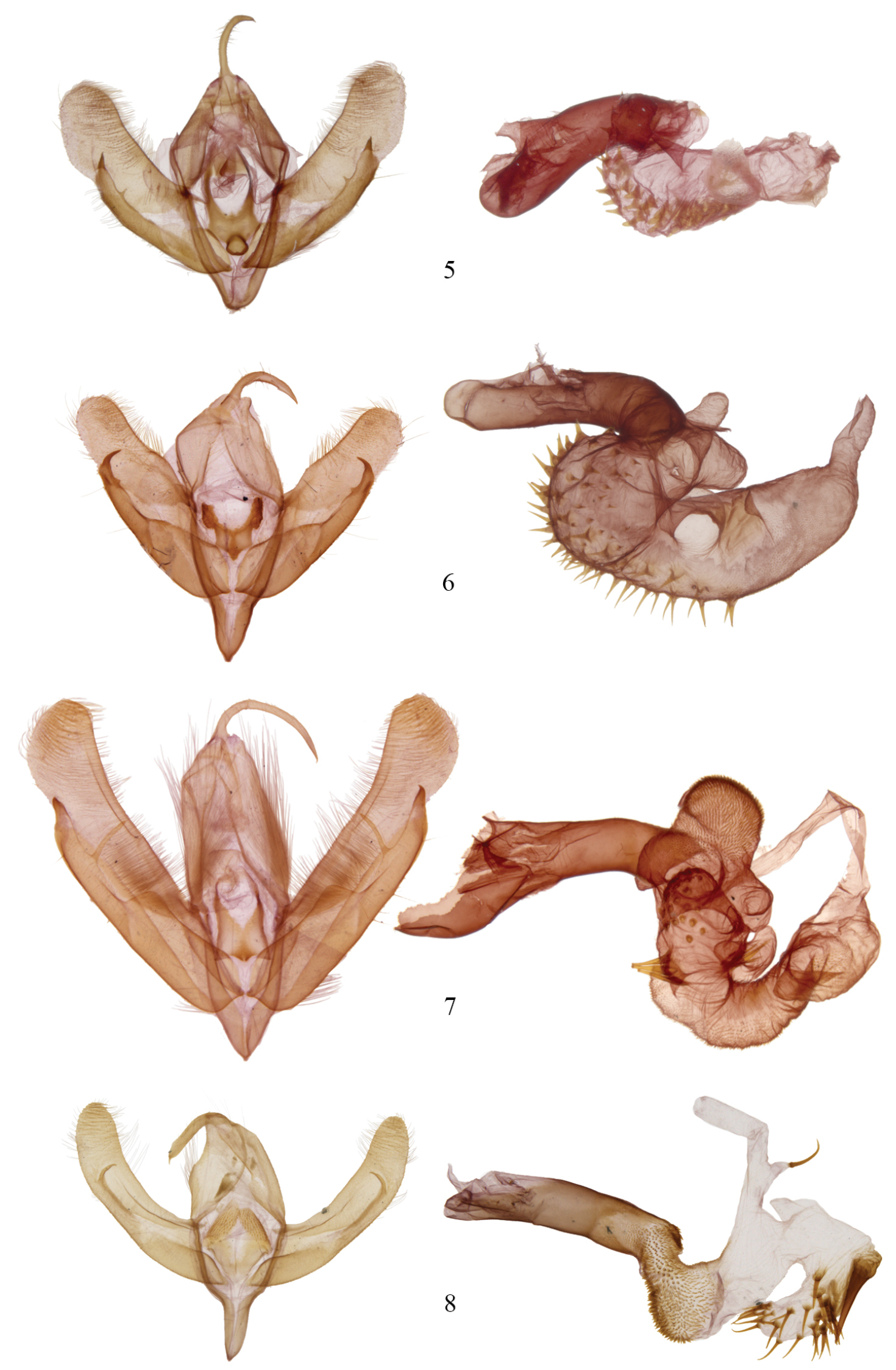
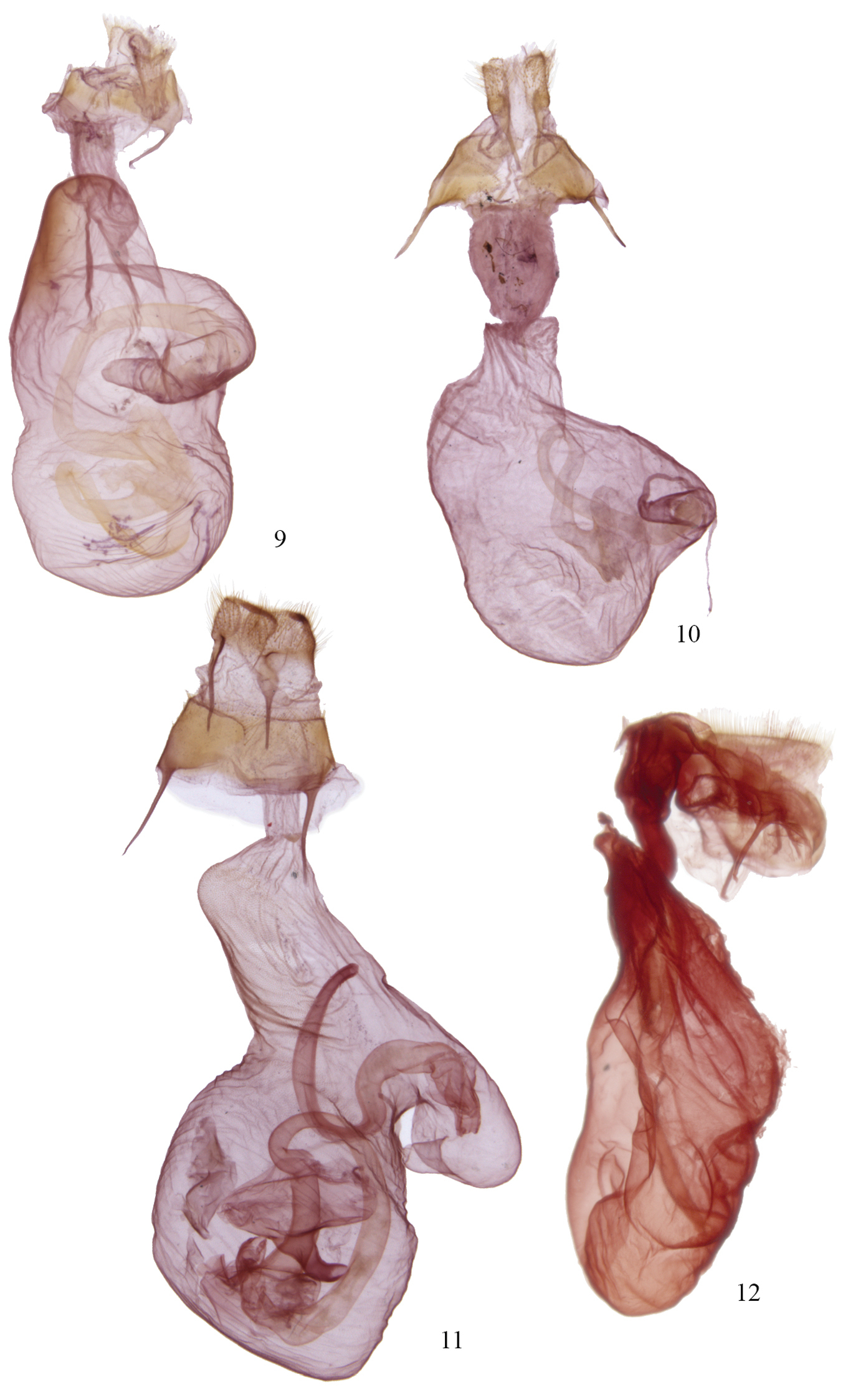
The fate of the genus Agriopodes is anchored to the phylogenetic position of Acronicta fallax, its type-species. Comparison of genitalic structure of Acronicta fallax to all North American and most Eurasian Acronictinae species reveals that genitalic features are most consistent with those found across an endemic North American group of Acronicta species, consisting of Acronicta tritona, Acronicta grisea, Acronicta falcula (Grote), Acronicta lithospila Grote, Acronicta hamamelis Guenée, Acronicta mansueta Smith, Acronicta paralella (Grote) and Acronicta vinnula, here termed the tritona-group. Structural synapomorphies for these species are primarily those of the male genitalia (Fig. 5), including a short, claw-like clasper and a broad shield-like juxta (wider than long), with strap-like dorso-lateral extensions. The male vesica structure is moderately complex and consists of a sausage-shaped main chamber that curves ventrally then right laterally, which is armed with short, spade- to thorn-like spines to longer attenuated spines. The size and position of the vesica diverticula are unique, with thumb-like diverticula consistently present in the basal and sub-basal positions, and smaller diverticula variably present in the medial and apical positions. In females, the corpus bursae is relatively broad and rounded, shaped like a heart or a boxing glove with the appendix bursae forming the ‘thumb’ (Fig. 9). Females of the tritona-group (Figs 10, 11) lack the dense, persistent patch of fine, felt-like hairs between the 8th tergite and sternite that is present in the Acronicta hasta-group. The hasta-group contains at least 14 species, largely corresponding to “Group II” of
Structurally, Acronicta fallax shows clear affinities to Acronicta grisea and Acronicta falcula of the tritona-group; the valve, clasper and uncus are much like those of Acronicta grisea, with the clasper apex slightly less curved. The dorsolateral straps of the juxta are spinulose, and the medioventral portion of the juxta is produced into a rounded knob that is unique to Acronicta fallax, although Acronicta tritona shows a rudimentary form of this. Aedeagus and vesica structure of Acronicta fallax are also similar to those of Acronicta grisea and Acronicta falcula, with two basal, unarmed diverticuli, a spinose main chamber, and a finely spinulose distal portion of the main chamber. The large spine field is composed of short, broad-based spines basally, and rounded, spade-like spines distally, similar to those found in Acronicta tritona. The female genitalic structure of Acronicta fallax is most similar to Acronicta grisea (Fig. 10). Larval morphology does not offer support for a special association among Acronicta fallax and tritona / falcula / grisea, although there is greater similarity of fallax to the tritona group than to larvae of the hasta-group.
Many Acronicta species bear a prominent black basal, anal and apical forewing dash; the basal and anal dashes are sometimes transected by a crescentic line resulting in a dagger-like mark (hence the common name dagger moths). These forewing dashes typical of Acronicta are also present in modified form in Acronicta fallax, with the apical and anal dash (dagger marks) broadly joined to the postmedial line to form two roughly triangular postmedial patches. The basal dash is short and thick; and there is a black rectangular bar connecting the orbicular and reniform spots; the orbicular and reniform spots are occasionally and then only incompletely outlined. Unlike the green psaphidines (Amphipyrinae, Psaphidini: Feralia Grote and Miracavira Franclemont), the green pigment of Acronicta fallax is not sensitive to moisture degradation, where green changes to yellow upon exposure to high humidity (dried specimens of Acronicta fallax can usually be moisture-relaxed without loss of green colouration). This suggests a fundamental biochemical difference in the green pigment of Acronicta (found in Acronicta fallax and Acronicta vinnula) compared to that of psaphidines.
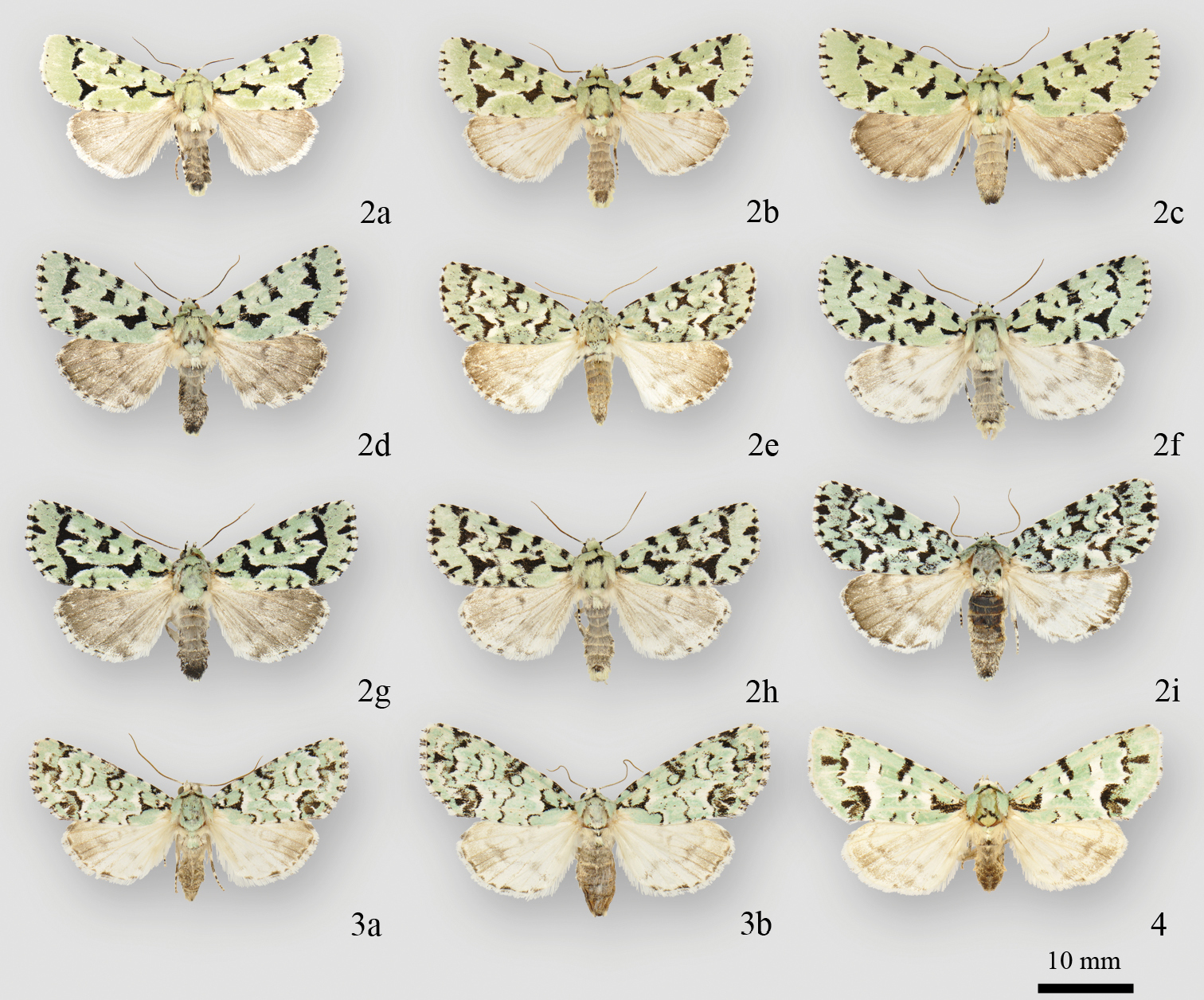
Acronicta and Chloronycta adults. 2a Acronicta fallax male (North Port, FL) 2b Acronicta fallax male (Hillsboro, MO) 2c Acronicta fallax female (Hillsboro, MO) 2d Acronicta fallax female (Backus Woods, ON) 2e Acronicta fallax male (Cartwright, MB) 2f Acronicta fallax male Edmunston, NB) 2g Acronicta fallax female (Ottawa, ON) 2h Acronicta fallax male (La Verendrye Reserve, QC) 2i Acronicta fallax male (Crooked Lake, SK) 3a Chloronycta tybo male (Huachuca Mtns, AZ) 3b Chloronycta tybo female (Cave Ck. Cyn., Chiricahua Mtns, AZ) 4 Chloronycta sp. female (Turundeo, MEX).
Acronicta and Chloronycta male genitalia. 5 Acronicta fallax 6 Acronicta grisea 7 Acronicta tritona 8 Chloronycta tybo. Reproduced to scale.
Acronicta and Chloronycta female genitalia. 9 Acronicta fallax 10 Acronicta grisea 11 Acronicta tritona 12 Chloronycta tybo.
The immature stages of Acronicta fallax were described by
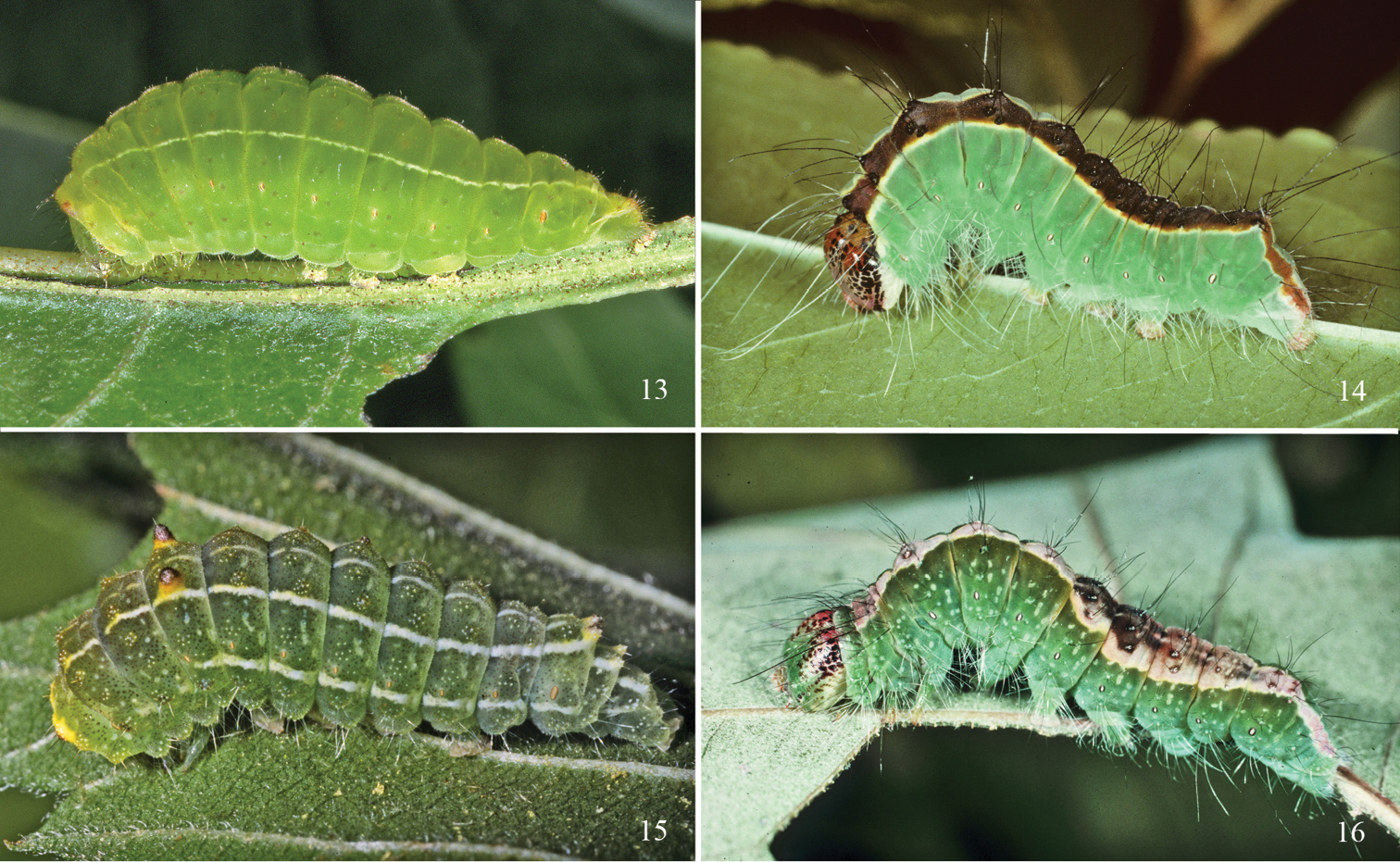
The mature larva is bright lime to yellowish green with a whitish middorsal and somewhat broader subdorsal stripe, with body tapering posteriorly. The integument is covered with abundant, minute secondary setae in the form of spinules that are slightly thickened basally, giving the integument a velvety texture. With the exception of the D1 pinaculum on T1, which is brownish to red and borne from a small wart, the pinacula are nearly obsolete in the last instar, i.e. flattened, faintly brown or concolourous, and with short setae (pinacula are more elevated and reddish brown with longer setae in middle instars). The greenish head sometimes has paired dark spots above the frons and a field of 6–8 darker spots over each lobe, laterad to apex of frons. The head, usually retracted into the thorax, has a rough, granular surface but lacks secondary setae, and is about 4 mm wide when mature. The thoracic shield is lightly sclerotized; prothorax with XD setae longest on body, extending well forward; XD1 and D1 solitary; D2 setal cluster shifted forward and grouping with XD2 seta; SD and L setae grouped, each comprised of 8–11 setae. Nearly all primary setae are replaced with open but defined clusters of 6–12 setae. Abdomen with D, SD, and L setal clusters more or less vertically aligned; D2 in typical position on A7–A10; solitary seta present below L2 group, well forward of spiracle; L3 group a diffuse set of 9–12 setae; numerous setae in each subventral cluster. A8 spiracle approximately 2 × diameter of those on preceding segments. The anal plate and pinacula are ill defined or undifferentiated, with limits defined by clusters of microspinules, which are largest (some tooth-like) over the anal plate. Prolegs with 23–28 crochets. Length of larva at maturity is 28–30 mm. The prepupal larva turns waxy red, and tunnels into soft wood or spins a flimsy cocoon in a crevice. The larva feeds from the leaf underside of Viburnum species, including Viburnum dentatum L. (
Acronicta last instar larvae. 13 Acronicta fallax (Norfolk, CT) 14 Acronicta superans (Norfolk, CT) 15 Acronicta vinnula (Coventry, CT) 16 Acronicta lithospila (Martha’s Vineyard, MA).
Acronicta fallax exhibits a moderate range of geographic variation (Fig. 2), with a gradual increase in size and extent of forewing black markings with increasing latitude. The northwestern-most populations from Manitoba, Saskatchewan and eastern Alberta mark the extreme end of this cline, and have been treated as a separate species, Agriopodes geminata. We can find no consistent differences in genitalic structure between Acronicta fallax and Acronicta geminata, and differences in COI barcodes fall well within the range of variation of Acronicta fallax, as discussed above. We therefore consider geminata to be a geographic form of Acronicta fallax (syn. n.). Acronicta geminata Draudt, 1950 of China is a junior secondary homonym of Acronicta geminata (Smith), but since we treat the latter as a junior subjective synonym of Acronicta fallax, no replacement name for Acronicta geminata Draudt is necessary.
The identity of this taxon remains an enigma. We have been unsuccessful in locating type specimens in collections housing Guenée types (The Natural History Museum, London; National Museum of Natural History, Washington, D.C. ), and the specimens are presumed to be lost. No illustration accompanied the description, a translation of which follows (our comments in square brackets):
“Same size as [Noctua] Glandifera [a junior subjective synonym of Nyctobrya muralis (Forster, 1771; Bryophilinae), diagnosed by Guenée in the account previous to corticosa]. Forewings broad, scaly, variably coloured with greenish white, light brown and black, and with all of the lines black. Basal space also greenish white, with the basal line and one spot at the costal border black. Median space of a grey brown, with the disc [claviform spot] lightly dusted with a fawn color; terminal space spotted with brown. The subterminal line very dark black, very undulated, and more or less parallel to the postmedial line. Fringe whitish, streaked with many fine black lines. Hindwings pearly white, with a blackish shade at the base of the interior [anal] angle and extending nearly halfway along the wing. Underside of the abdomen white. Body rather big. Antennae long.
North America. Boisduval collection. Two specimens.”
Since its description, the identity of this taxon has been uncertain, and it has often been omitted entirely (e.g.,
The description, comparison to Nyctobrya muralis (Forster) (see e.g.,
The handsome green-mottled forewing colour and pattern of Agriopodes have resulted in a century of erroneous systematic placement of the eight species included at one time or another in this genus. Despite the remarkable divergence in wing pattern from other Acronicta species, our genitalic, external morphological, larval, and molecular character evidence confidently places Agriopodes fallax as an Acronicta.
The taxonomic fate of Agriopodes is complicated somewhat by the broad scope of the genus Acronicta.
Feminine.
Moma tybo Barnes, 1904.
Two species are included in Chloronycta, Chloronycta tybo and an undescribed Mexican species near Chloronycta tybo, known to us from only one female specimen (Fig. 4) and therefore it is not formally described here. The only Nearctic species externally similar to Chloronycta is Acronicta fallax, but Chloronycta lacks the black bar between the reniform and orbicular spots, has the reniform and orbicular stigma finely outlined in black, and has various black markings in the subterminal space, which is entirely green in Agriopodes fallax. The two genera do not overlap in range, with Chloronycta essentially a Mexican taxon reaching southeastern Arizona, and Agriopodes fallax restricted to deciduous forests of eastern North America. Genitalic structure of the two genera is very different (Figs 5, 8, 9, 12). The main diagnostic characters for Chloronycta are 1) forewing ground colour pale bluish green and white, the green colouration not degrading to yellowish with exposure to moisture; 2) valve apex with flattened, corona-like setae; 3) vesica with a single long spine isolated at the base of the ductus ejaculatorius; 4) tympanal sclerite consisting of rounded, adjoining nodules, not flange- or scoop-like.
Head. Antenna of male simple-prismatic, such that ventral margin appears slightly serrate when viewed laterally, evenly ciliate laterally and ventrally; female antenna similar but with segments less produced ventrally; antenna with dorsal scales grey, grading to white scales over basal third, with scattered black scales; haustellum normal, approximately equal in length to that of thorax; eye smooth, round; labial palpus with 3rd segment 0.4 × length of 2nd segment; 1st segment clothed with black spatulate scales dorsally and longer, strap-like white scales ventrally; 2nd segment with short, spatulate white scales apically and basally and with black scales forming broad, dark band medially; 3rd segment with short spatulate white scales and scattered black scales; frons with short, appressed, spatulate scales and longer strap-like scales forming a medial crest near ventral margin; scales of frons white, except for a patch of black scales medio-laterally; occiput with longer spatulate white scales, with black scales forming a black medial crest-like line. Thorax. Prothoracic collar with pale bluish-green spatulate scales, bordered dorsally and along eye margin by narrower black scales; mesothorax, metathorax and tegula clothed in bluish-green spatulate scales, margin of tegula with longer hair-like scales; mesothorax with paired patch of subdorsal black scales at posterior margin; tympanal sclerite raised, rounded and spade-like; prothoracic leg with brown-black and white scaling, femur brown black dorsally and greyish white ventrally; tibia black, with a transverse medial and apical band of white scales; epiphysis 0.5 × length of tibia; tarsal segments black scaled, with a distal band of white scales; scaling pattern of meso- and metathoracic legs similar to that of prothoracic leg; tibial spines white scaled, tibial spine formula 0-2-4. Abdomen. Clothed in a mix of brownish-grey and whitish scales, which are short, spatulate and closely appressed; A1 with a dorsal tuft of long spatulate, bluish-green scales; terminal scales white and hair-like; A8 sternites and tergites normal. Male genitalia (Fig. 8). Uncus rod-like, about 5–6 × as long as wide, with a short, curved terminal spine; tegumen roughly rhomboid and broad, 2.3 × longer than wide; vinculum with saccus well developed, base slightly constricted, 2.5 × longer than base width; valves relatively simple, straplike and parallel sided, about 4.5 × longer than wide, evenly curved in a shallow arc; sacculus moderately developed, clasper well developed but thin, slightly curved with a sharp terminus, located 4/5 distance to valve apex, ampulla absent; area between clasper and terminus covered in long fine setae, setae on outer 2/3 thicker and flattened; setae near margin of apex more robust and lance-like, directed towards base of valve; juxta well sclerotized, spinose and rasp-like dorsolaterally, dorsal margin divided; aedeagus 4.5 × longer than wide, nearly straight, slightly decurved ventrally; basal half of vesica angled slightly downward, densely clothed with small but prominent spines, curving dorsad and expanding into a large medioventral diverticulum, this about as wide as long, with 15–20 long prominent spines and two to three massive, partially fused spines at base; vesica narrows abruptly beyond diverticulum, extending anteriorly, poorly differentiated from ductus ejaculatorius; a single prominent, elongate, curved spine arising from small narrow pouch near vesica terminus / base of ductus. Female genitalia (Fig. 12). Corpus bursae elongate globose, 1.25 × longer than wide, with invaginated sclerotized area dorsally at base of ductus bursae; appendix bursae dorsal and to to right of ductus bursae, small and indistinct, tapering abruptly to ductus seminalis; ductus bursae membranous, rugose, 1.5 × longer than wide; ostium bursae moderately sclerotized, with v-shaped ventral notch; antevaginal plate somewhat sclerotized and covered with dense, minute setae, projecting caudad as a somewhat pointed scoop; apophyses short, posterior apophysis 0.8 × and anterior apophysis 0.7 × height of papillae; papillae anales densely setose, margin quadrangular with slightly protuberant ventrocaudal angle.
Chloronycta occurs in the mountainous regions from Mexico to south-eastern Arizona and southwestern New Mexico, where it reaches the northern terminus of its core range in the Sierra Madre Occidental. Chloronycta tybo occurs in canyons and mid-elevation wooded habitats, particularly riparian corridors where the larval host plant, Fraxinus velutina, grows. The larva and host plant of Chloronycta tybo are described here for the first time. The larval description under Agriopodes tybo in
The first two larval instars (Fig. 17) are leaf skeletonizers that remove patches of leaf tissue from the lower leaf surface. Middle (Fig. 18) and late instars feed from a leaf edge, always from the underside of a blade.
Chloronycta tybo (Cave Creek Canyon, AZ) and Polygrammate hebraeicum (Cosby, TN) larvae. 17 Chloronycta tybo early instar 18 Chloronycta tybo penultimate instar 19 Chloronycta tybo ultimate instar 20 Polygrammate hebraeicum ultimate instar. In addition to overall resemblance, note similarities in dorsal pattern elements, as well as head allometry, colouration, and luster (particularly between penultimate instar of Chloronycta tybo and ultimate instar Polygrammate hebraeicum).
Ultimate instar larva (Fig. 19) (total length to 26 mm, n = 3) waxy green, integument translucent, body thickest through A3–A5, strongly tapered rearward. Broken middorsal stripe composed of single lines on T2, T3, and A9, and anterior and posterior lines on A1–A8; broad, creamy subdorsal stripe that gradually widens posteriorly, extending from T1 through to and including anal plate. D1 and D2 pinacula free on all segments. Primary setae only, these fine; D, SD, L, and SV group setae borne from cream-yellow, pimple-like pinacula embedded within a pale yellow spot; longest setae black. One additional yellow subventral spot on T1–T3; four additional yellow lateral spots on A1–A8. D2 on T2 extending well forward of head. D2 on all abdominal segments 1.5 × longer than D1. SD1 and L2 very long on abdominal segments, circa 2 × length of an abdominal segment. D2 setae on A9 and A10 elongate, trailing behind body. Prolegs with 32–35 crochets. Spiracles tan yellow with brown peritreme. Entire integument microspinulose. Head immaculate pale green, shiny, translucent; labrum creamy. Prepupal larva flushed with red.
Prepupae tunnel into punky wood when available to form a pupal crypt, largely free of silk, with the exception of that used to weave the frass-silk cover that renders the pupal chamber essentially invisible to the untrained eye.
Few acronictine groups have appreciable Neotropical representation (there are no confirmed South American Acronictinae). The majority of species are temperate and cold temperate; one exception in the New World is the Acronicta theodora Schaus group, which reaches Costa Rica. Acronictine genera are most diverse in temperate Asia but many genera, subgenera and species-groups are shared between the two realms (e.g. Harrisimemna Grote, Simyra Ochsenheimer and Acronicta subgenera Acronicta, Jocheara Hübner, and Hyboma Hübner). Chloronycta may also be derived from an Asian ancestral group, although there are admittedly no obvious sister taxa—Moma Hübner and Nacna Fletcher are similar in facies, but neither belong to the Acronictinae (Wagner et al., in prep).
Despite the very similar forewing colouration of Chloronycta to Acronicta fallax the two share no uniquely derived structural traits; DNA barcode sequence also does not support an association between with these taxa. Genitalic structure in Chloronycta is unique among Acronictinae, and no close relatives are evident. The simple valve structure, short saccular process along the ventral valve margin, and weakly-developed corona are shared by the Asian acronictine genus Subleuconycta Kozhanchikov (
As there are no unequivocal adult or larval autapomorphies for Acronictinae (
The following adult characters associate Chloronycta with the Acronictinae: 1) a black ‘eye-stripe, ’ which in the natural resting position of the moth is formed by black scaling on the middle of labial palpus segment 2, on the prothoracic collar behind the eye, and extending into the basal dash of the forewing; 2) dorsal tuft of scales on A1 (occurring also in unrelated subfamilies such as Plusiinae, but absent in Psaphidini); 3) legs strongly banded in black and white, shared with other acronictines including Polygrammate, Harrisimemna, and Cerma (
The discovery of a weakly-developed valval corona is noteworthy as all Acronictinae were previously thought to lack this structure (
Examination of morphological and molecular data shows that Acronicta fallax is phylogenetically rooted to the Acronicta tritona/hasta groups, and that Acronicta fallax and Acronicta geminata are conspecific. Accordingly, we place Agriopodes within the current concept of Acronicta as a synonym. In contrast, Chloronycta tybo is not closely related to Acronicta fallax, Acronicta, or, evidently, any other genus of Acronictinae, although available evidence places it in the subfamily in the vicinity of basal genera such as Polygrammate. Additional morphological and molecular studies with greater taxon sampling are needed to determine its phylogenetic position within basal noctuids. Bryophila corticosa, previously included in Agriopodes, cannot currently be associated with any known Noctuidae species (or subfamily), and remains a nomen dubium. The following nomenclatural changes are proposed:
Acronicta Ochsenheimer
Agriopodes Hampson, 1908, Catalogue of the Lepidoptera Phalaenae in the British Museum 7: 16. syn. n.
Type species. Moma fallax Herrich-Schäffer, 1854, by subsequent designation by
Acronicta fallax (Herrich-Schäffer), comb. n.
Diphthera fallax Herrich-Schäffer, [1854], Sammlung neuer oder wenig bekannter aussereuropäische Schmetterlinge 1: pl. 42, f. 211, wrapper.
Type locality. Tennessee, [USA]. [types lost]
Moma geminata Smith, 1903, Journal of the New York Entomological Society 11: 1. syn. n.
Type locality. Cartwright, Manitoba [Canada]. [American Museum of Natural History, New York]
Chloronycta Schmidt & Anweiler, gen. n.
Type species. Moma tybo Barnes, 1904
Chloronycta tybo (Barnes, 1904), comb. n.
Moma tybo Barnes, 1904, Canadian Entomologist, 36: 166.
Type locality. Cochise Co., Arizona. [National Museum of Natural History, Washington, D.C.]
Within the Noctuoidea, green and black lichen patterning has arisen at least nine separate times in North American taxa: Afrida Möschler (Nolidae), Acronicta (Acronictinae), Bryolymnia and “Elaphria” cyanympha (Noctuinae, Elaphriini), Leuconycta (Condicinae), Cryphia (Bryophilinae) and Feralia, Miracavira, Emarginea Guenée (Amphipyrinae, Psaphidini). There are likely also multiple independent derivations in the biochemistry of green-scale pigmentation, since green pigments are moisture sensitive in, for example, psaphidines and geometrines (Geometridae), but not in acronictines. The green pigment may be a novel autapomorphy for the Acronictinae, parallel to the geoverdin pigment present in Geometridae, Geometrinae (
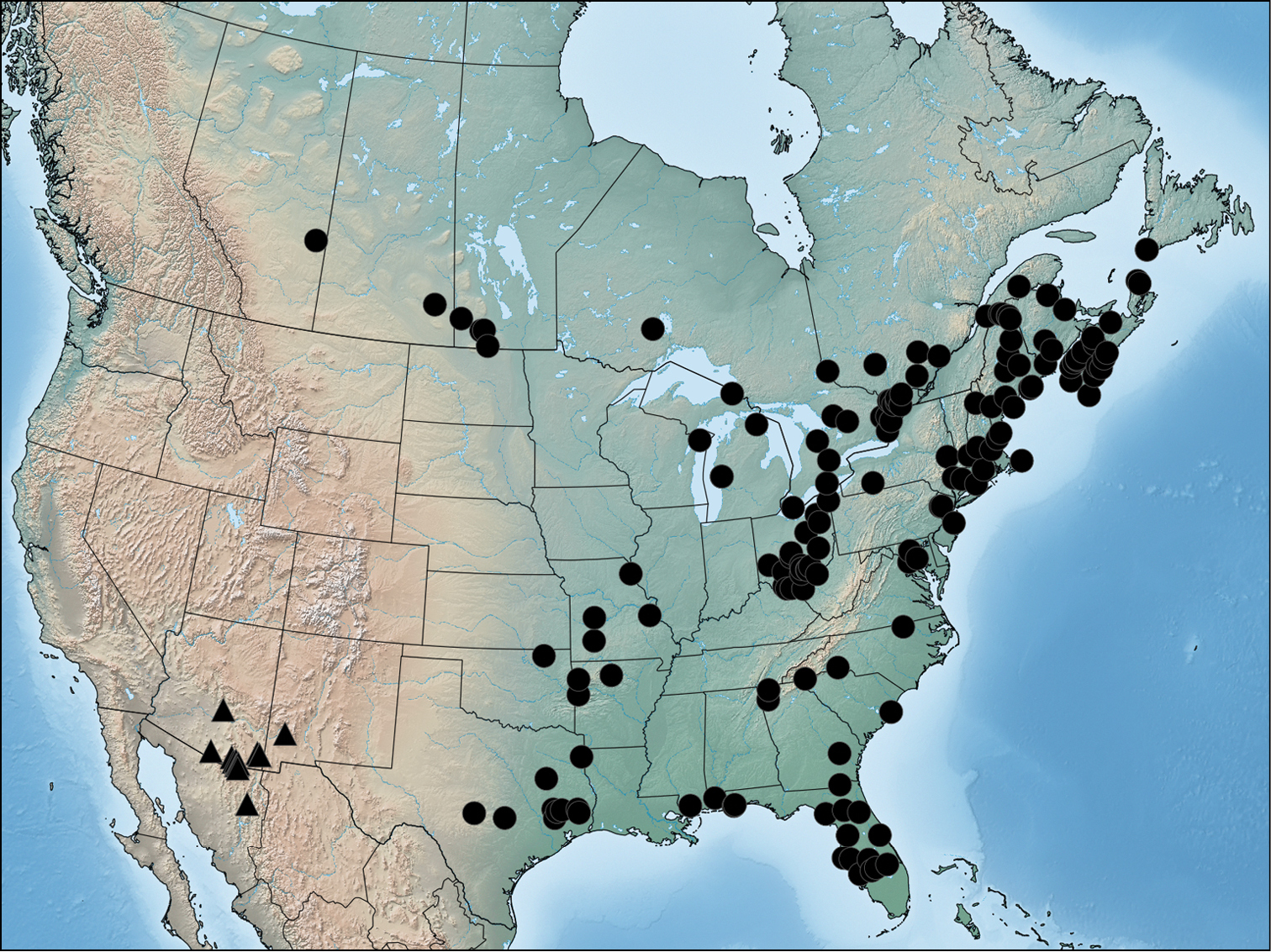
Distribution of Acronicta fallax (circles) and Chloronycta tybo (triangles) based on material examined in this study.
We thank Tim McCabe for the loan of Agriopodes fallax larvae, and Jocelyn Gill for preparing the specimen images. Louis Handfield and Suzanne Fauteux kindly translated French text. Evgeny Zakharov, Paul Hebert and other members of the Barcode of Life Project at the University of Guelph, Ontario, Canada, provided DNA data. Molecular analyses were carried out through grants from the National Science and Engineering Research Council of Canada and Genome Canada through the Ontario Genomics Institute. Jadranka Rota and Niklas Walberg facilitated our efforts during the collection and analysis of the nuclear data reported here. Sam Jaffe was the first to alert us to the presence of Chloronycta tybo larvae on ash at Herb Martyr Campground near the Southwest Research Station, Arizona. Lastly, we thank James Adams and an anonymous reviewer for reviewing the manuscript.