(C) 2013 Paul Z. Goldstein. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Neotropical genus Schacontia
Schacontia, Crambidae, Glaphyriinae, Brassicales, secondary sexual characters
Schacontia Dyar, 1914: 400 represents a small cluster of species recently transferred to the Glaphyriinae (
With respect to their actual biology, Schacontia larvae have been variously associated with Capparaceae (Brassicales) and have been recently reported as parasites of cassidine chrysomelid beetles (
In the present work, we treat newly assembled historical and recent material from the Western Hemisphere. Our purpose is to refine the circumscription and composition of Schacontia by identifying and describing new species and presenting a phylogenetic analysis of their relationships. Recent collecting and rearing work, including the efforts in Costa Rica by D. Janzen and W. Hallwachs, have generated life history information, most importantly the association of Schacontia with capparaceous plants. Those potentially allied with Schacontia on the basis of wing venation and features of the gnathos and tegumen, comprise eight undescribed species ranging from Mexico through Central and South America and the Caribbean, some narrowly endemic, others widespread.
Pinned specimens were examined with an incandescent light source (reflected light). Male and female genitalic preparations varied, some of those pre-dating this study having accumulated from several sources. Most were prepared following
This work drew in part from an effort to treat taxa with taxonomic and nomenclatural problems identified during preliminary surveys of pyraloids in the extensive Costa Rican collection of D. Janzen and W. Hallwachs. Because the genitalic characters of Schacontia species had not been adequately explored, specimens of all known Costa Rican species were initially dissected to survey diagnostic characters of each species and putative synapomorphies for the genus. In order to determine the nomenclatural status of Costa Rican species, types of all neotropical species at the Zoologische Staatssammlung München, Munich, Germany (ZSM), Naturhistoriches Museum, Vienna (NHMV), The Natural History Museum, London (BMNH), and the National Museum of Natural History, Smithsonian Institution, Washington, DC (USNM) were examined. Following this preliminary work, a more expansive series of material (all the specimens of Schacontia we could locate) was examined, most recently including Bolivian and Puerto Rican material housed at the Carnegie Museum of Natural History (CMNH) as well as at Cornell University Insect Collection (CUIC), and all the available material at USNM, including material from the V.O. Becker collection (VOB). Specimens are listed for each species with all attendant label data, including genitalic dissection slide numbers and record numbers from the database of Janzen and Hallwachs (http//Janzen.sas.upenn.edu). Primary types are deposited at the USNM (Washington, DC) and the CMNH (Pittsburgh, PA).
The following abbreviations refer to collections from which specimen material forms the basis of this work:
BMNH The Natural History Museum [statutorialy: British Museum (Natural History)], London, UK
CMNH Carnegie Museum of Natural History, Pittsburgh, PA, USA
CNC Canadian National Collection of Insects, Arachnids, and Nematodes, Ottawa, Ontario, Canada
CUIC Cornell University Insect Collection, Ithaca, NY, USA
INBio Instituto Nacional de Biodiversidad (INBio), Santo Domingo de Heredia, Costa Rica
MGCL McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL, USA
NHMV Naturhistoriches Museum, Vienna, Austria
USNM National Museum of Natural History [formerly, United States National Museum], Washington, District of Columbia, USA
ZMHB Museum für Naturkunde der Humboldt-Universität, Berlin, Germany
ZSM Zoologische Staatssammlung, Munich, Germany
Following the identification of a suite of male secondary sexual characters suspected of diagnosing multiple species pairs, we undertook a preliminary DNA barcoding exploration of two putative species for which relatively recent (~20 year old) material existed. Sequencing was done using standard protocols at the Biodiversity Institute of Ontario (
All characters were equally weighted and coded as unordered. Preference was given to combinations of binary and inapplicable coding schemes over multistate characters. Conspecificity of male and female specimens was inferred by locality when other biological information was unavailable. Preliminary phylogenetic analysis involved parsimony searches via the ratchet routine (island-hopper, 1000 iterations per rep) in Windada/Winclada (1000 iterations per rep;
The scope of our treatment of described Schacontia is based on
Initial examination of specimens tentatively identified as Schacontia revealed, first, a cohesive group of species comparable to the type species [Schacontia medalba (Schaus, 1904)] unified by a uniquely hood-like or mucronate uncus, reduced male valvae, a divided tegumen with a prominent medial sulcus, and a gnathos with a unique, four-armed configuration. A somewhat more variable group, including Schacontia ysticalis (Dyar, 1925) and several undescribed species, appeared to bear similarities to Schacontia in forewing pattern and, in modified form, features of the male valva, tegumen, and gnathos. Bearing in mind that member species of Schacontia have been placed in several subfamilies prior to the genus’ transfer to Glaphyriinae by
A total of 64 characters (56 binary, 8 multistate; 5 head, 13 thoracic, 13 abdominal and tympanal, and 25 male genitalic, and 8 female genitalic) were adduced and coded (below; see Appendix I for the full matrix). Inapplicable and missing data were coded with “-” and “?”, respectively.
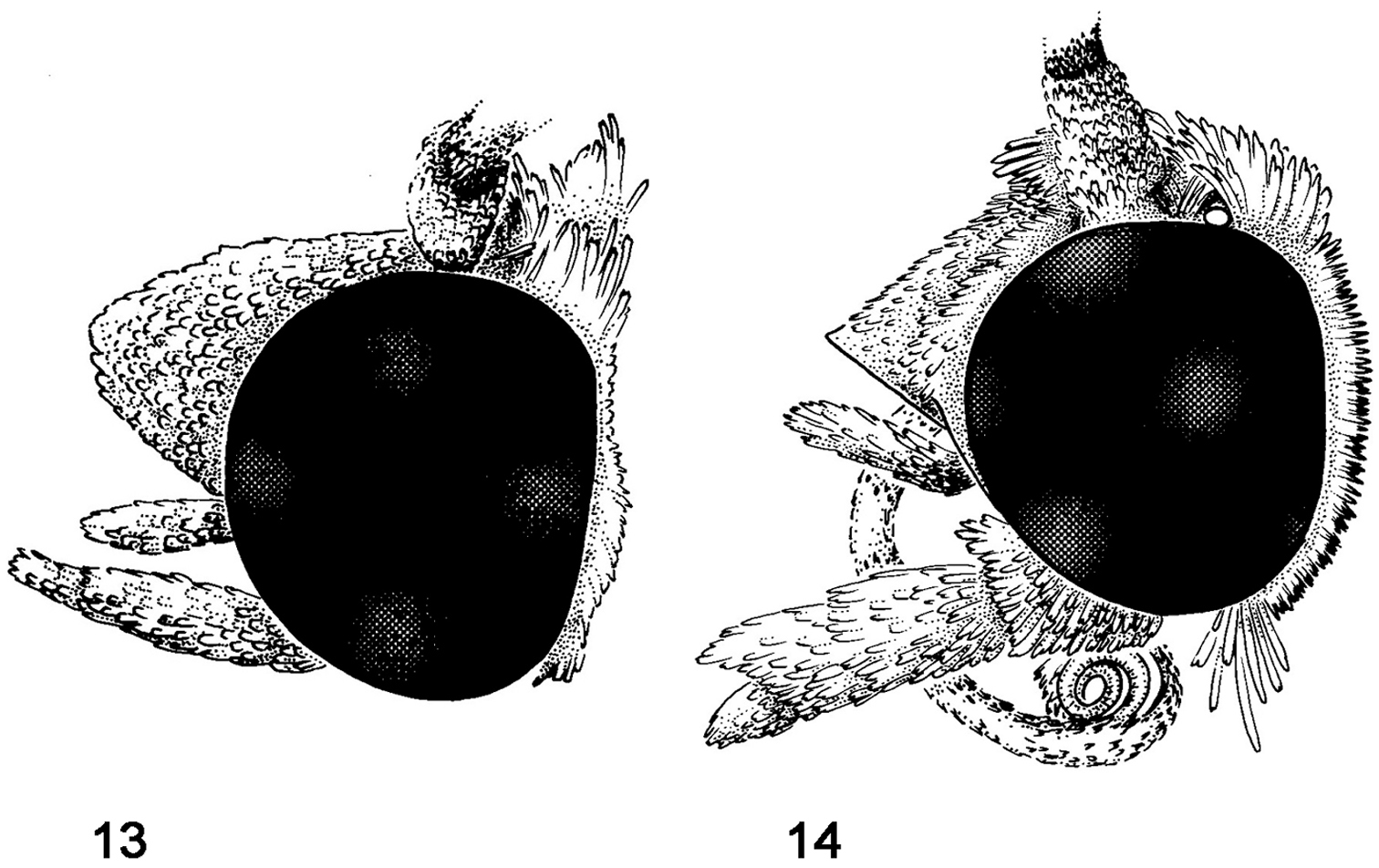
Head [Figs 13, 14]
0. Ocelli: (0) absent; (1) present
1. Proboscis: (0) reduced, inconspicuous; (1) conspicuous (Figs 13, 14)
2. Frons: (0) of normal contour, evenly rounded; (1) conical or expressed as a small hump; (2) carinate or otherwise modified (Figs 13, 14)
3. Length of labial palpus: (0) extending beyond clypeus; (1) not extending beyond clypeus
4. Maxillary palpi: (0) extending anteriorly beyond frons; (1) not reaching anterior margin of frons
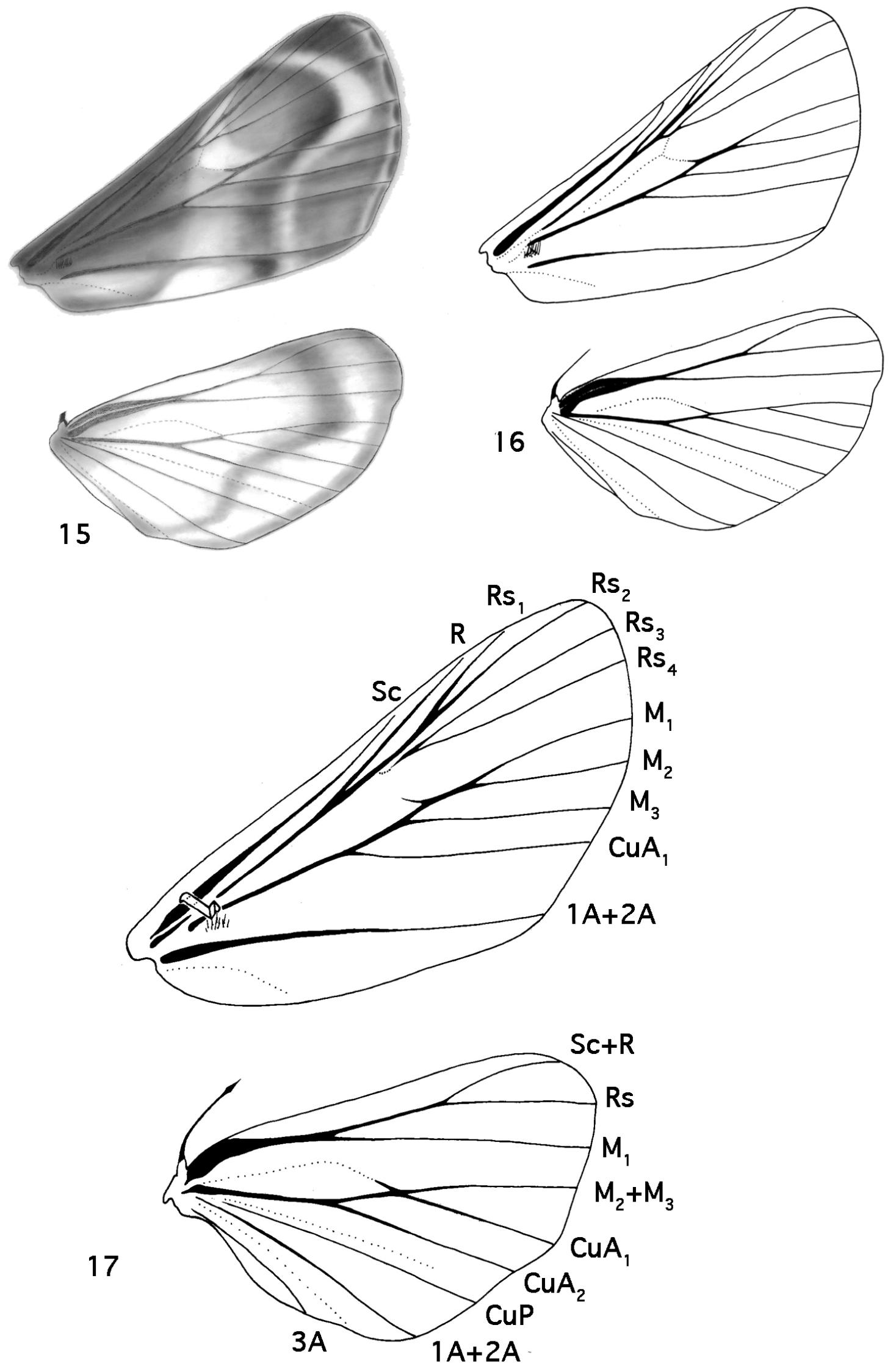
Thorax [Figs 15–20]
5. Forewing (Rs3, Rs4): (0) bases separate; (1) stalked (Figs 16, 17)
6. Forewing (M1, M2): (0) bases separate; (1) stalked (Figs 16, 17)
7. Medial area: (0) contrast with basal and postmedial areas subtle, almost unicolorous except for lines, spot; (1) contrast between medial area and basal and postmedial areas sharp
8. Forewing coloration: (0) compound, ground color not uniform in any given area (antemedial, medial, postmedial; Fig. 15); (1) two-toned, medial area contrasts with basal and apical area in ground color
9. Concentration of white scales apical to antemedial line: (0) absent, or if present then only diffusely; (1) present (Fig. 15)
10. Hindwing (HW) postmedial line: (0) not conspicuous or nearing inner margin; (1) distinct, approaching or reaching inner margin (Fig. 15)
11. Distance between postmedial line and wing terminus: (0) narrow (Fig. 15); (1) wide
12. Wing lines: (0) dark on light ground; (1) light on dark ground (Fig. 15)
13. HW (M2M3+CuA1): (0) bases separate; (1) stalked (Figs 16, 17)
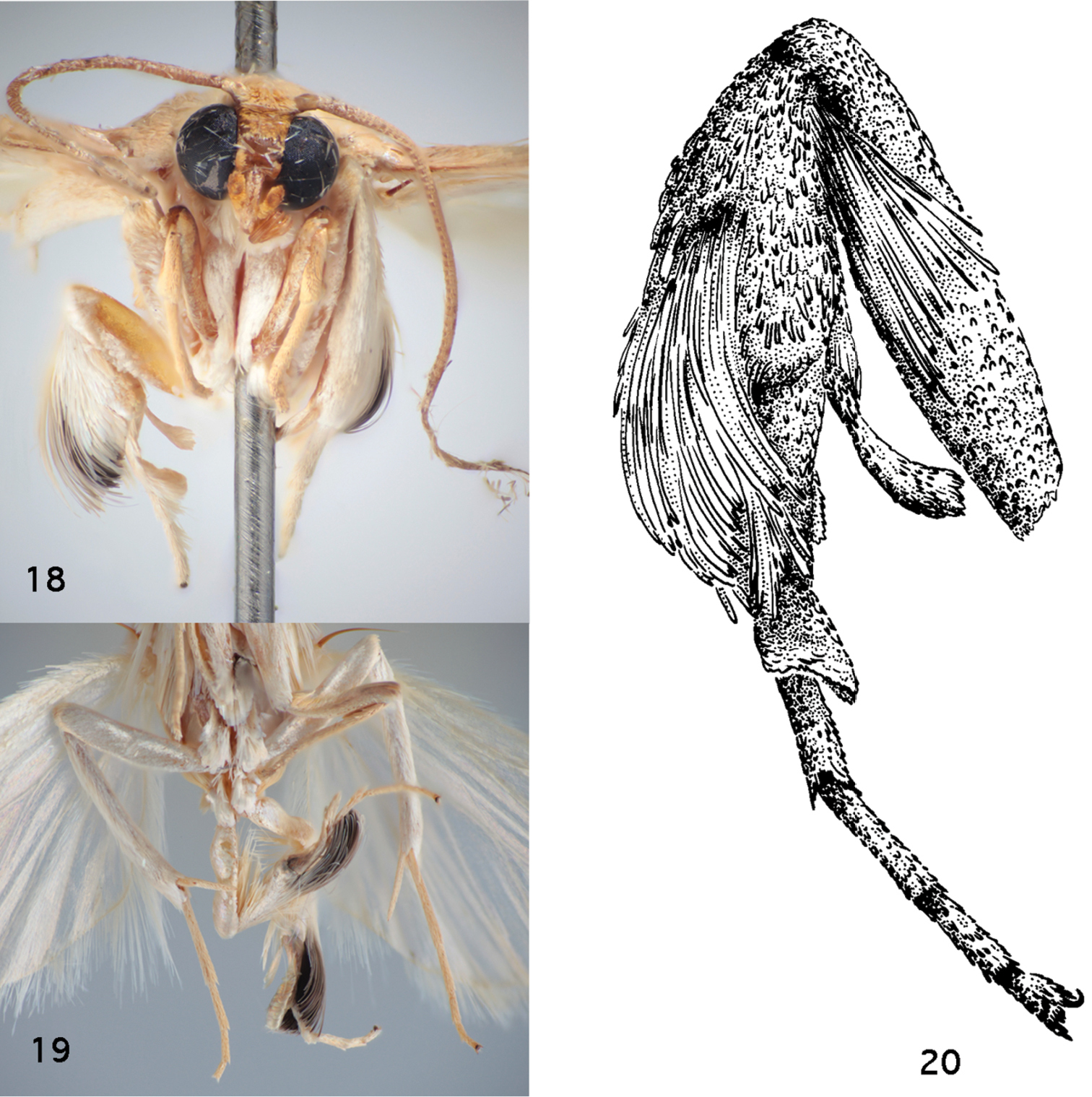
14. Male hind leg secondary sexual complex consisting of a flattened hind tibial spur with flattened scales and basal tarsus with concave spoon-like modification: (0) absent; (1) present (Figs 18–20)
15. Dark patch amidst hind tibial scales: (0) absent; (1) present (Figs 18, 19)
16. Tufts of epipleural setae: (0) absent; (1) present
17. Female medial hind tibial spurs: (0) one pair (medial pair absent); (1) two pair (medial pair present)
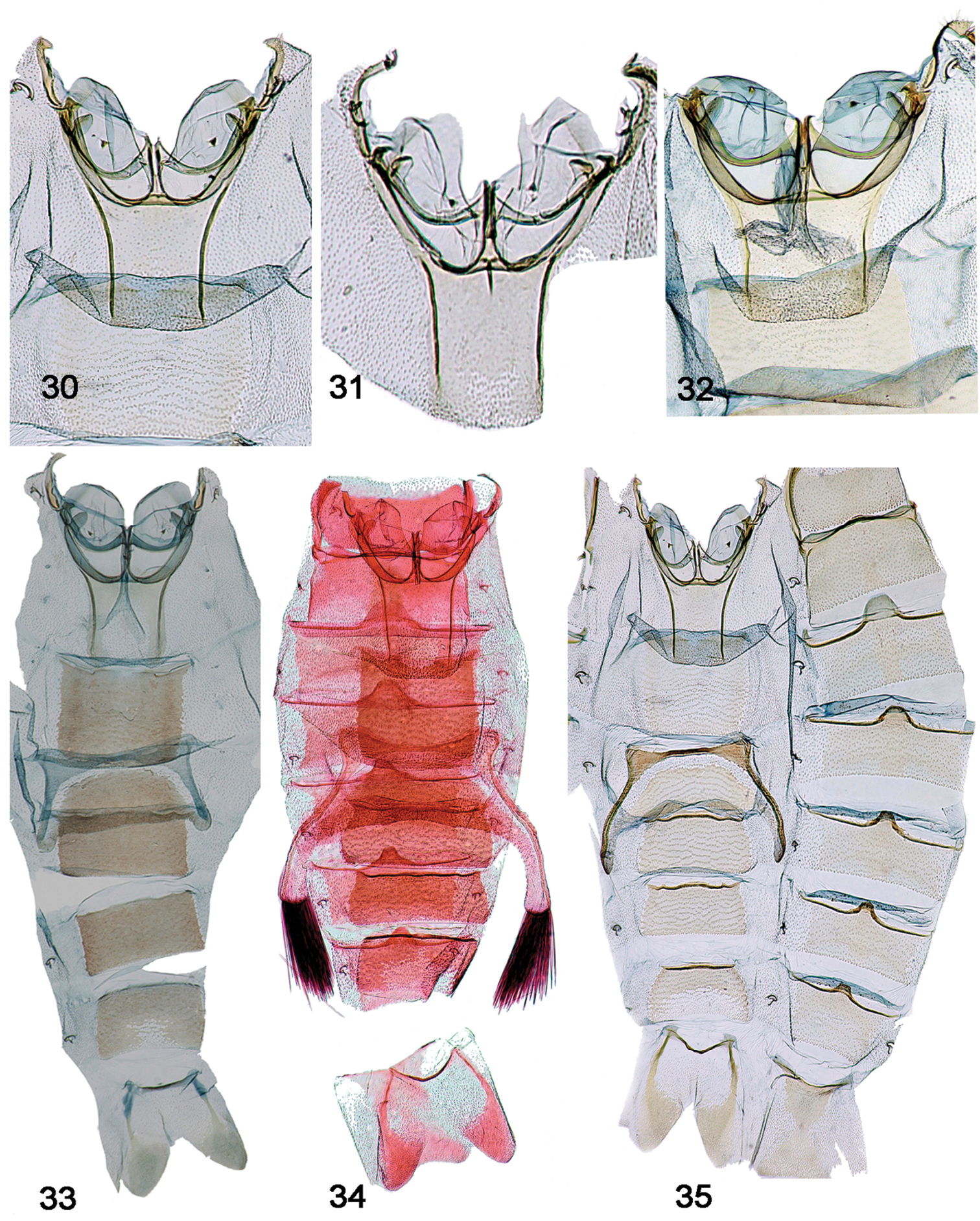
Abdomen - Tympanal characters [Figs 21–32]
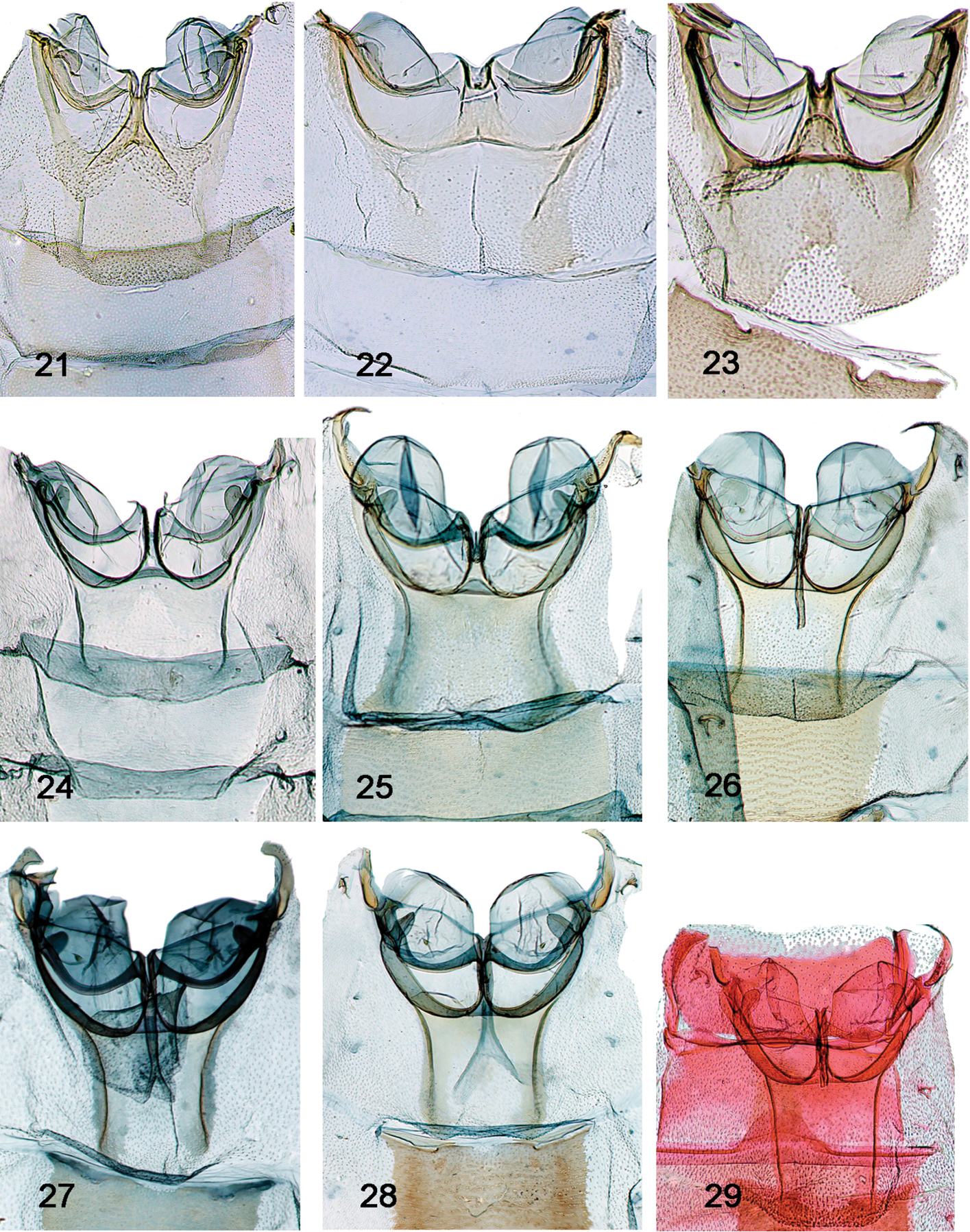
18. Bullae tympani invaginated in Sternum 2: (0) not (all ingroup taxa; Figs 21–32); (1) slightly to strongly
19. Saccus tympani invagination: (0) short, not beyond puteolus; (1) deep, with posterior ridge, but not prominently invaginated posteriad (Figs 21–23); (2) capacious, ovate chamber; conspicuous broad lip (Figs 24–32)
20. Saccus: (0) not prominent (Figs 21–23); (1) prominent (Figs 24–32)
21. Mesal extent of saccus (@ pons): (0) short (Figs 21–23); (1) intermediate (Figs 24–32);
22. Puteoli: (0) absent or indistinguishable from saccus tympani (all ingroup taxa; Figs 21–32); (1) present, if small
23. Processus tympani: (0) inconspicuous; (1) approximately semi-circular (Figs 21–23); (2) thumblike, lobulate (Figs 24–32)
24. Fornix, protrusion over venula prima: (0) protruded over slightly, flat; (1) far removed from edge (all ingroup taxa; Figs 21–32)
25. Fornical ulna: (0) > 90 degrees or low arc (all ingroup taxa; Figs 21–32); (1) < 90 degrees
26. Sclerotization of fornix: (0) light to moderate; (1) heavy (all ingroup taxa; Figs 21–32)
27. Fornix: (0) robust, broad (Figs 21–24); (1) narrow, ribbonlike (Figs 24–32)
28. Venulae secundae: (0) wide, gently tapered (Figs 21–24); (1) more sharply tapered, elongate, forming a neck (Figs 24–32)
29. Tergo-sternal sclerite: (0) present, not elongate (Figs 21–24); (1) prominent, elongate, roughly equivalent in length to that of bulla tympani (Figs 24–32)
Abdomen - Post-tympanal characters [Figs 33–35]
30. Coremata on 4th abdominal segment: (0) absent; (1) present (Figs 33–35)
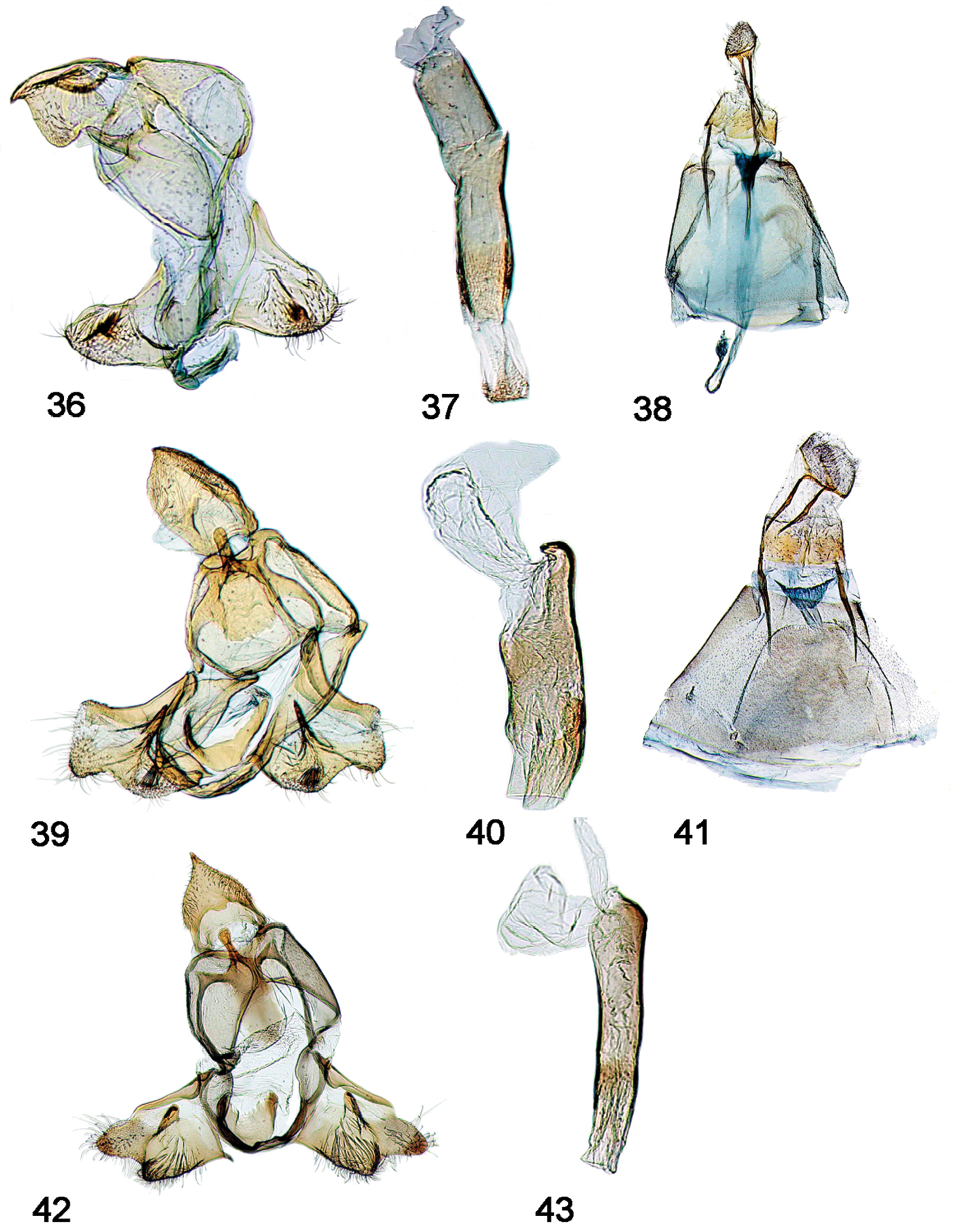
Male genitalia [Figs 36–65, part]
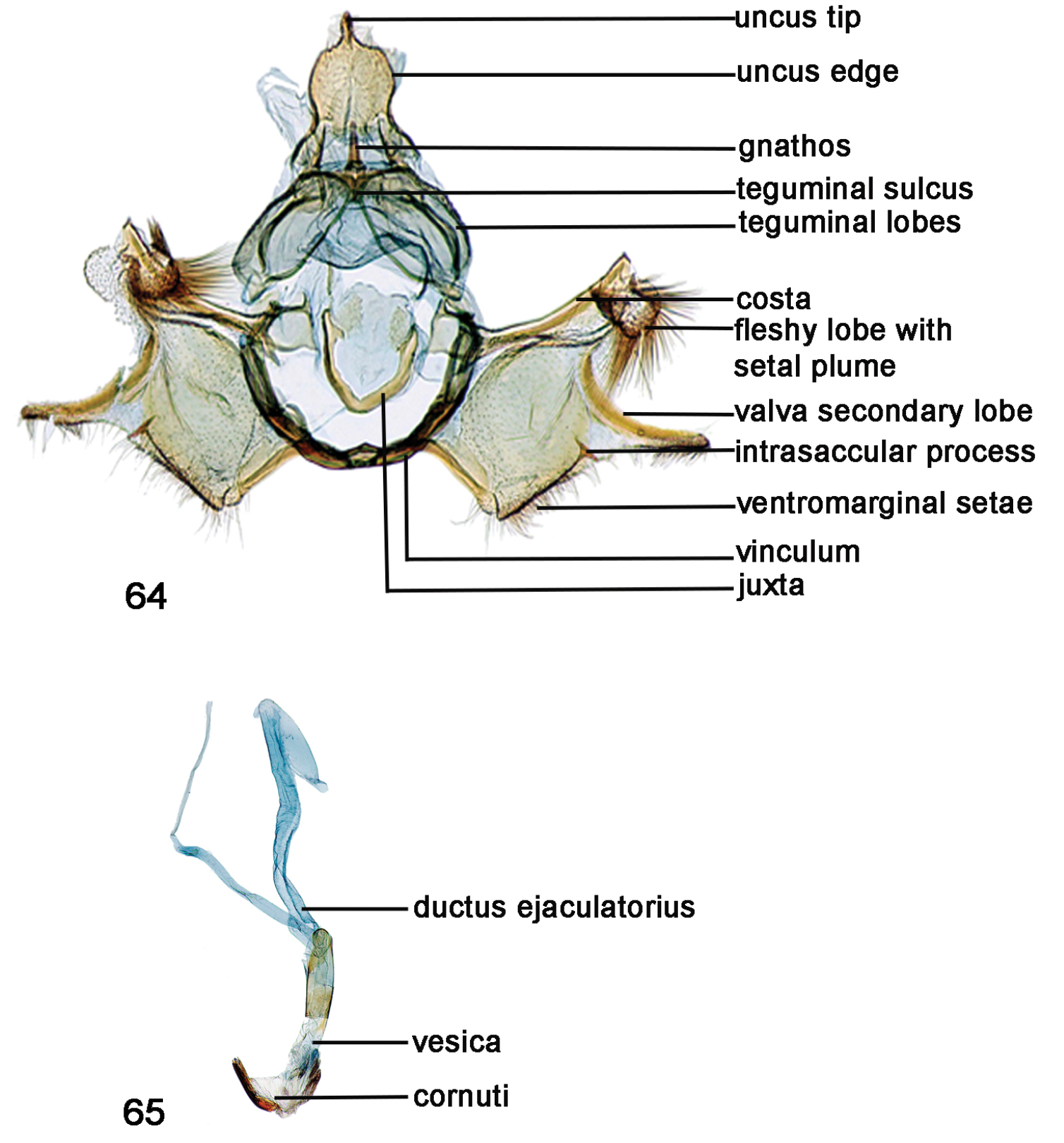
31. Gnathos-ventrotergal rods complex: (0) absent; (1) present (all ingroup taxa; Figs 36–65, in part)
32. Gnathos, middle process: (0) absent; (1) present (all ingroup taxa; Figs 36–65, in part)
33. Dorsal ridges of tegumen: (0) absent, split to uncus, or inverted U; (1) cruciate, crossing near uncus (Figs 46, 49, 52, 55, 58, 61, 64); (2) inverted upsilon with medial ridge (Figs 36, 39, 42, 44)
34. Teguminal sulcus: (0) absent; (1) present (all ingroup taxa; Figs 36–65, in part)
35. Uncus tip trefoil shaped: (0) absent (Figs 36, 39, 42, 44, 46); (1) present (Figs 49, 52, 55, 58, 61, 64)
36. Shape of trefoil, if present: (0) reduced, rhomboid (Figs 55, 58, 61, 64); (1) expanded, hastate (Figs 49, 52)
37. Uncus edges: (0) simple, undifferentiated (Figs 36, 39, 42, 44, 46, 55, 58, 64); (1) modified, swollen (Figs 49, 52, 61)
38. Uncus, interior (under-surface: (0) clear, without relief (Figs 36, 39, 42, 44, 46, 55, 58, 64); (1) with elongate central development appearing as a raised ridge (Figs 49, 52)
39. Valva - outer margin: (0) entire or emarginate, but continuous, such that trajectory of valval membrane continues apically (Figs 36, 39, 42, 44); (1) trajectory of valval membrane recurves such that upper and lower extensions are evident, a fleshy lobe bearing a setal tuft associated with end of costa, and the subcostal projection sclerotized dorsally (Figs 46, 49, 52, 55, 58, 61, 64)
40. Glabrous central area of valva: (0) Absent [valva elongate, setose]; (1) truncate - squared or subquadrate (emarginate) (Figs 36, 39, 42); (2) ~subtriangular or subrectangular, ~1.5 x long as wide (Figs 44, 46, 49, 52); (3) about as long as wide, sub-symmetrical (Figs 55, 58, 61, 64)
41. Intra-saccular process: (0) absent; (1) slightly raised bump, flange, or paddle centrally located on inner face of valva (Figs 36, 39, 42, 44, 46); (2) trigger-like extension at outer margin of lower lobe of valva (Figs 49, 52, 55, 58, 61, 64)
42. Intra-saccular process, adornment: (0) denticled or rugose (Figs 39, 44, 46, 49, 52, 55, 58, 61, 64); (1) naked (Figs 36, 42)
43. Saccular bend: (0) absent (Figs 36, 39, 42, 44); (1) present (Figs 46, 49, 52, 55, 58, 61, 64)
44. Saccular margin: (0) angled close to vinculum (Figs 46, 49, 52); (1) angled or rounded with apex at saccular midpoint (Figs 55, 58, 61, 64)
45. Saccular bend angled versus rounded: (0) angled, 90 degrees (Figs 61, 64); (1) rounded (Figs 55, 58)
46. Ventro-medial setal comb: (0) absent; (1) present (Figs 55, 58)
47. Localized patch or cluster of ventral, saccular spine-like setae: (0) absent; (1) present (Figs 36, 39, 42, 46, 49, 52, 55, 58, 61, 64)
48. Ventro-marginal setae: (0) absent or rudimentary (Figs 36, 39, 42, 44); (1) distributed along length of outer margin of sacculus (Figs 61, 64); (2) localized or concentrated at ventral bend (Figs 46, 49, 52, 55, 58)
49. Isolation of costal bar: (0) <75% along length of costa (Figs 46, 49, 52); (1) > 75% along length of costa (Figs 55, 58, 61, 64)
50. Secondary lobe of valva: (0) absent (Figs 46, 49, 52); (1) present, extending not beyond distal end of costa; (2) pronounced, finger-like (Figs 55, 58, 61, 64)
51. Recurved or decumbent setal plume associated with end of costa: (0) absent (Figs 36, 39, 42); (1) present (Figs 44, 46, 49, 52, 55, 58, 61, 64)
52. Setae arranged in recurved hook-shaped cluster: (0) absent; (1) present (Figs 58, 61, 64)
53. Scales arranged in terminal black dots on male abdomen: (0) absent; (1) present, conspicuous (all ingroup taxa)
54. Phallus - cornuti: (0) absent (Figs 37, 40, 43, 44); (1) present (Figs 47, 50, 53, 56, 59, 62, 65)
55. Number of cornuti: (0) one (Fig. 47); (1) two (Figs 50, 53, 56, 59, 62, 65)
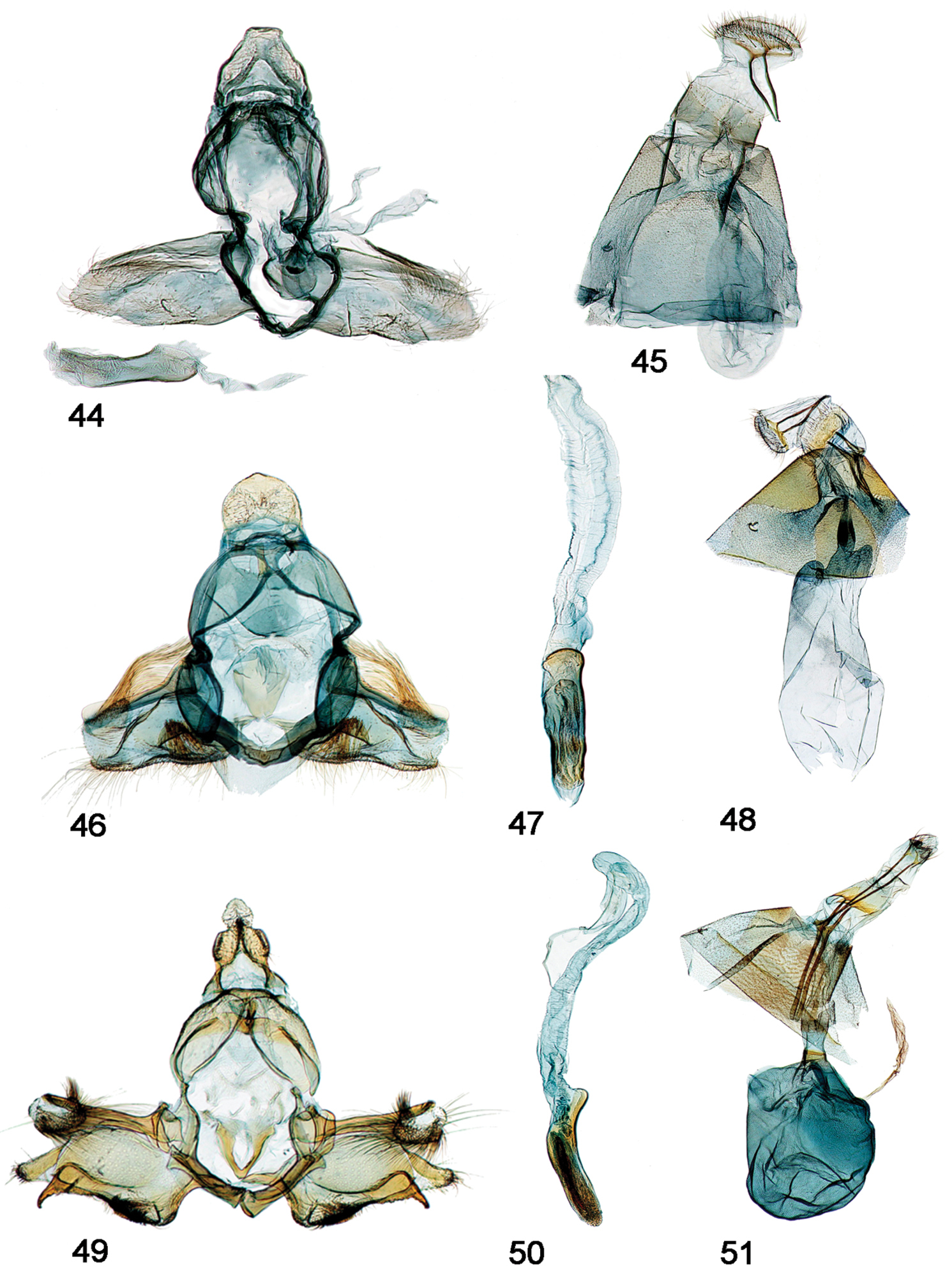
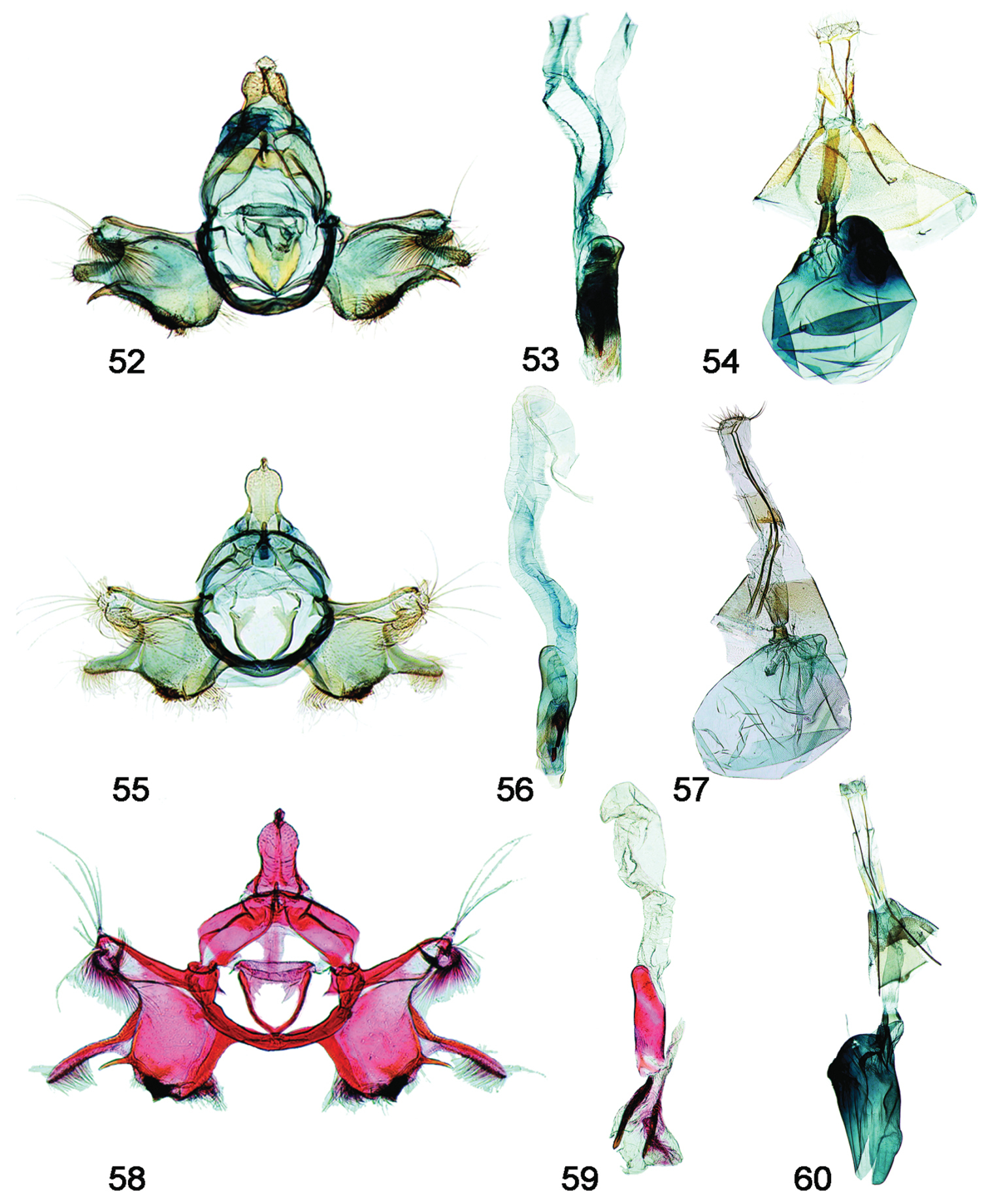
Female Genitalia [Figs 38-63, part]
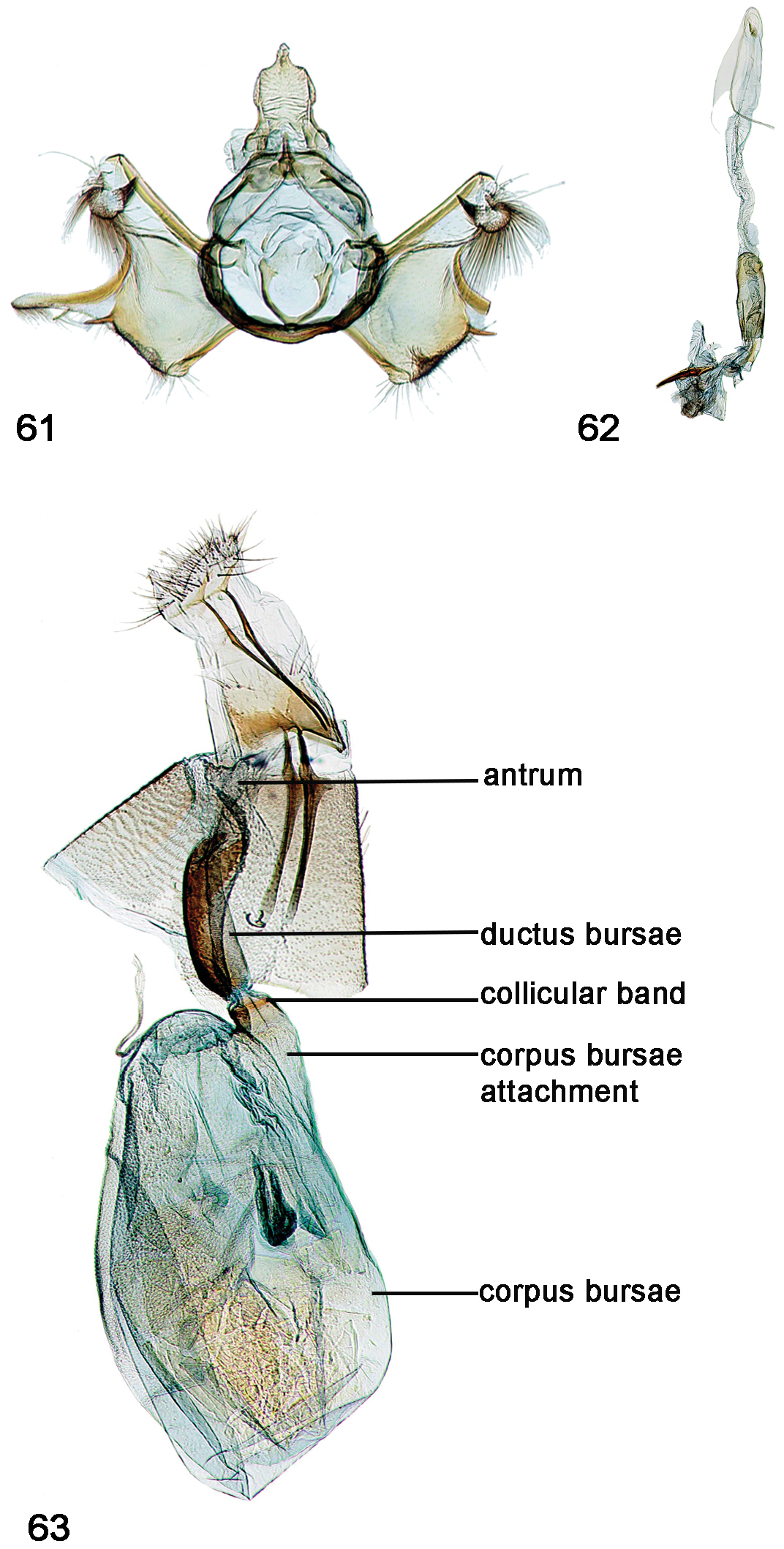
56. Antrum: (0) present, chalice-like (Figs 38, 41); (1) elongate, diffuse/indistinct from ductus bursae (Figs 45, 48, 51, 54, 57, 60, 63)
57. Colliculum: (0) absent (Fig. 38); (1) present, even if inconspicuous as in chanesalis (Figs 41, 45, 48, 51, 54, 57, 60, 63)
58. Narrow, differentially sclerotized band around center of colliculum: (0) absent (Figs 41, 45, 48, 54, 60); (1) present (Figs 51, 57, 63)
59. Ductus bursae: (0) effectively absent or inconspicuously short (Figs 38, 41); (1) present, variously sclerotized anterior to colliculum (Figs 45, 48, 51, 54, 57, 60, 63)
60. Sclerotization on floor of ductus bursae: (0) absent, indiscernable (Figs 45, 48); (1) present, either weak and diffuse or strong and conspicuous (Figs 51, 54, 57, 60, 63)
61. Corpus bursae: (0) elongate (Figs 38, 41, 45, 48); (1) globular (Figs 51, 54, 57, 60, 63)
62. Attachment of ductus bursae to corpus bursae: (0) basal (Figs 38, 41, 45); (1) sub-basal, creating shoulders on corpus bursae, associated with migration of point of attachment of ductus seminalis to sub-basal position of corpus bursae (Figs 48, 51, 54, 57, 60, 63)
63. Modification of Sternum 8: (0) absent; (1) present (all ingroup taxa)
After surveying all the described Schacontia species and determining there was insufficient evidence to retain Schacontia replica and Schacontia pfeifferi, these characters were scored (Appendix I) for three described Schacontia species (medalba, chanesalis and ysticalis), eight undescribed species, and three outgroups (Eustixia pupula, Glaphyria sesquistrialis, and Hellula undalis).
Habitus of adults. 1 Schacontia medalba male, Peru, 1883, “Boniti P., Peru, Jan. 7. 83” 2 Schacontia chanesalis male, Mexico, Becker 68741, Tam El Ensino 250 m, 4–13.viii.1988, V.O.Becker Col. 3 Schacontia umbra male paratype, “Col. Becker 100503, Ecuador: Past. Mera, 1300 m, xii 1992, V.O. Becker Col, Schacontia n. sp. #3 det. M.A. Solis” 4 Schacontia speciosa male holotype, “Col. Becker 65271, Brasil, RJ Marica, 5 m, 11.x.1985, V.O. Becker Col. 5 Schacontia ysticalis Sirena, Corcovado Nat. Pk., Osa Penin., Costa Rica, 19–27 Mar 1981, DH Janzen, W. Hallwachs 6 Schacontia themis Venezuela, Guarico, Hato Masaguaral, 45 km S Calabozo, 8.57N, 67.58W, Galry For #20, 75 m, 13–16 May 1988, uv lt., M. Epstein & R. Blahnik 7 Schacontia rasa male holotype, Col. Becker 110514, Mexico, Tam San Fernando, 50 m, 28.vi.1997, V. O. Becker Col. 8 Schacontia nyx “Venezuela: Guarico, Hato Masaquaral, 45 km S Calabozo, 8.57N, 67.58W, Galry Forest #20, 75 m, 13–16 May 1988, uv lt., M. Epstein & R. Blahnik 9 Schacontia clotho male holotype, Col. Becker 102660, Ecuador, Loja Catamayo 1300 m, 20.xii.1992, V.O. Becker Col., Genitalia 1287 10 Schacontia lachesis male, “Col. Becker 55439, Brasil, RJ Arrai al do Cabo, 50 m, 29.i.1985, V.O. Becker Col.” 11 Schacontia lachesis male, “Bolivia, Santa Cruz, Puerto Suarez, 150 m, Nov 1908, J. Steinbach, CMNH Acc. 3758” 12 Schacontia atropos male holotype, “San Estaban, Carabobo, Venez., Dec. 1–20 1939, Pablo J. Anduze.”
Head, lateral view. 13 Schacontia chanesalis; frons “normal” 14 Schacontia ysticalis; frons carinate.
Wings. 15 Schacontia chanesalis female underside 16 Schacontia chanesalis female underside 17 Schacontia chanesalis male underside.
Thoracic and leg structures in Schacontia clotho and Schacontia themis. 18 Schacontia themis, hind leg, frontal view, illustrating secondary sexual characters: flattened hind tibial spur, scales with dark patch, and flattened concave metatarsal modification, and epipleural setae (data Fig. 6) 19 Schacontia clotho, ventral view, illustrating darkened hind tibial scales. Ecuador: Loja Catamayo, 1300 m, 20.xii.1992, V.O. Becker Col; Col. Becker 102660 20 Schacontia themis, hind leg, lateral view.
Tympanal organs. Collection and/or dissection numbers follow country of origin; when label data presented elsewhere, annotated as such. 21 Schacontia medalba, Brazil, “Bnito Prov., Pernmbuco, Brazil 83; Collection C.V. Riley; USNM genitalia slide 107887 22 Schacontia chanesalis male, Guatemala: Quirigua Guat; Schaus and Barnes coll; Genitalia slide by DA ♂ USNM 107, 891 23 Schacontia umbra male Holotype, Ecuador, Past. Mera: 1300 m, xii.1992, V.O. Becker Col; Col. Becker 100504 24 Schacontia speciosa male, Paratype, Brazil (data Fig. 4) 25 Schacontia ysticalis male 108100; Venezuela Guarico, Huato Masaguaral , 45 km S Calabozo, 8.57°N, 67.58°W, Galry For#4 75 m, 13–16 May 1988, uv light, M. Epstein R. Blahnik 26 Schacontia themis, male, Costa Rica, Guanacaste, Santa Rosa Nat’l Pk., 97 SRNP 2354.1 JAL, 2 May 2003, #5 27 Schacontia rasa male Holotype, Mexico, Col. Becker 110514, Mexico, Tam San Fernando, 50 m, 28.vi.1997, V. O. Becker Col., [green USNM genitalia slide label “JAL 18”] 28 Schacontia nyx male, Venezuela, Lara, 4 km NW of La Pastora, 2–3.III.1978, riparian forest, blacklight, J.B. Heppner, Genitalia slide by DA ♂ USNM 108, 101 29 Schacontia clotho Cf Fig. 9.
Tympanal organs and male abdominal segments. 30–32. Tympanal organs. 30 Schacontia lachesis male, Bolivia, Cf. Fig. 11 31 Schacontia lachesis female, Bolivia, Cf. Fig. 11 32 Schacontia atropos male, Venezuela, Cf. Fig. 12 33–35. Male abdominal segments, illustrating coremata. 33 Schacontia nyx Venezuela, Cf. Fig. 22 34 Schacontia clotho holotype, Catamayo, Ecuador, 1287, Cf. Fig. 9 35 Schacontia lachesis Bolivia, Cf. Fig. 24.
Male, female genitalia. 36 Schacontia medalba male, Brazil 107887 37 Schacontia medalba phallus, data as above 38 Schacontia medalba female, Brazil, Castro, Parana, Collection Wm Schaus, Genitalia slide by MAS ♀ USNM 107, 011 39 Schacontia chanesalis male, Guatemala USNM 107, 891m, Cf. Fig. 22 40 Schacontia chanesalis phallus, data as above 41 Schacontia chanesalis female, Guatemala, Quirigua Guat., Schaus and Barnes coll, Genitalia slide by DA ♀ USNM 107, 892 42 Schacontia umbra male, Ecuador VOB 100504, Cf. Fig. 3 43 Schacontia umbra phallus, data as above.
Male, female genitalia. 44 Schacontia speciosa male, paratype, Brasil, VOB 65271, Cf. Fig. 4 zzz45 Female, Brasil: BA Jequié, 600–750m; Col. Becker 105714 46 Schacontia ysticalis (a) male, Venezuela: Guarico, Huato Masaguaral , 45km S Calabozo 8.57°N, 67.58°W, Galry For#4 75m, 13-16May1988, uv light M. Epstein R. Blahnik; green label Genitalic Slide by DA ♂ USNM 108, 100 47 Phallus, data as above 48 Female, Venezuela Guarico, Huato Masaguaral , 45km S Calabozo Slide by DA ♀ USNM 107, 896 49 Schacontia themis (a) male, Costa Rica, Cf. Fig. 20 50 Phallus, data as above 51 Female, Costa Rica, Guanacaste: Santa Rosa Nat’l Pk. 97 SRNP 2354.2 JAL 2 May 2003 #6.
Male, female genitalia. 52 Schacontia rasa (a) male, Mexico; f. Fig. 21 53 Phallus, data as above 54 Female, Mexico JAL 19 (same data) 55 Schacontia nyx (a) male, Venezuela, Cf. Fig. 22 56 Phallus, data as above 57 Female, Venezuela; Cf. Fig. 8 58 Schacontia clotho (a) male, HOLOTYPE, Ecuador; Cf. Fig. 9 59 Phallus, data as above 60 Female, Ecuador: Loja, Catamayo, 1300 m, 20 XII 1992, V.O. Becker JAL 5 May 2003 #24.
Male, female genitalia. 61 Schacontia lachesis (a) male, HOLOTYPE, Bolivia, CMNH; Cf. Fig. 11 62 Phallus, data as above 63 Female, Brasil: MT 60 km S Pocone. Pantanal 100m 1-7.xii. 1997; V.O. Becker Col.; Col Becker 111257.
64 Schacontia atropos, male, Venezuela; Cf. Fig. 12 65 Phallus, data as above.
We were unable to discern consistently different characters among Schacontia chanesalis, Schacontia pfeifferi, and Schacontia replica, but in view of there being extremely limited material of Schacontia pfeifferi in particular, and despite Amsel’s description’s being the only detailed and well-figured one to date, we elected to synonymize Schacontia replica and Schacontia pfeifferi with Schacontia chanesalis based in large part on a lack of discernable discrete variation in the male genitalia.
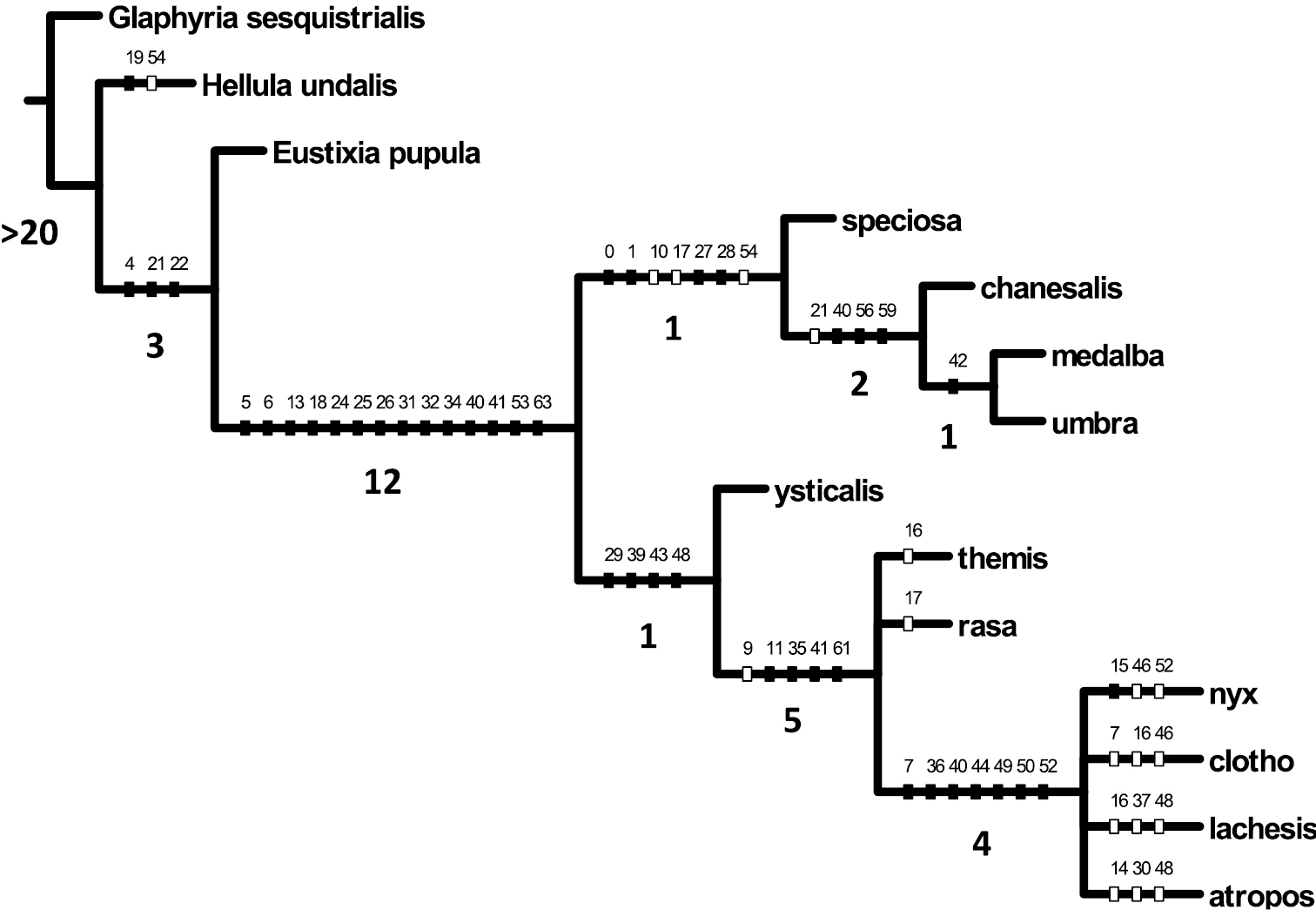
From cladistic analysis eight most parsimonious trees obtain (L=102, CI=71, RI=84), the strict consensus of which (L=108, CI=67, RI=81) is presented (Fig. 66) with the topology: Glaphyria sesquistrialis [root] + [Hellula undalis + [Eustixia pupula + [[Schacontia speciosa + [Schacontia chanesalis + [Schacontia medalba + Schacontia umbra]]] + [Schacontia ysticalis + [Schacontia rasa + Schacontia themis + [Schacontia lachesis + Schacontia atropos + Schacontia nyx + Schacontia clotho]]]]]].
Strict consensus (L=108, CI=67, RI=81) of eight most parsimonious trees (L=102, CI=71, RI=84) obtained from a cladistic analysis of morphological data with unambiguous character state transformations indicated. Numbered hatch marks on nodes refer to characters undergoing forward changes (solid=unreversed; hollow=reversed). Bremer values are indicated below relevant branches.
The monophyly of Schacontia is supported by synapomorphies enumerated in the diagnosis of the genus (below). Two primary groupings appear in the strict consensus (character numbers given parenthetically). The first comprises the type Schacontia medalba, Schacontia chanesalis and the newly described species Schacontia umbra and Schacontia speciosa; these are united by the absence of ocelli (0); reduced proboscis (1); a compound, non-uniform ground color that does not sharply delineate the medial area (8); a distinct hindwing postmedial line approaching or reaching the inner margin (10); robust, broad fornix (27); and wide, gently tapered venulae secundae (28). In these species the outer margin of the valva is also continuous, the valva highly reduced in all but Schacontia speciosa (40), and without a conspicuous saccular bend; phallus simple, without cornuti (55); attachment of the ductus bursae basal (63). Three of these species (excluding Schacontia speciosa) are characterized by having the saccus tympani deep, with a posterior ridge, but not invaginated posteriad (19) and a tipped, mucronate uncus that is not conspicuously obovoid and is longer than its width at the base. As will be discussed, 4 of 5 described species - all but one of which fall within this group - were described on the basis of female types. The morphology of the female genitalia is rather uniform among the species in this group; their putative association with males is based on a combination of wing pattern and geographical proximity.
The second major intrageneric grouping, the ysticalis-themis group comprises Schacontia ysticalis and six newly described species, whose association with Schacontia had been hypothesized initially. This group is united by a forewing pattern that is either essentially unicolorous excepting the antemedial and postmedial lines and orbicular spot, or two toned, but with the basal area unbroken and the medial area contrasting with the basal and apical areas (8); an inconspicuous hindwing postmedial line not nearing the inner margin (10); the saccus tympani a capacious ovate chamber with a conspicuous broad lip, comparable to that of Cybalomia Lederer (19); the dorsal ridge of the tegumen cruciate, crossing near the base of the uncus (33); the uncus either gently tapered and bluntly rounded, wider at the base than long, or variously nippled, trefoiled, and/or conspicuously obovoid (35, 36); the outer margin of the valva with upper and lower lobes, not with a continuous edge (39), but with a saccular bend or elbow either at its midpoint or proximal to the vinculum (44, 45); ventro-marginal setae present and well-developed (48); costal setae present, sometimes arranged in a recurved, fish-hook-shaped cluster (52); phallus with cornuti (54); attachment of the ductus bursae sub-basally, creating the shouldered appearance on the corpus bursae (62).
Morphologically, this second, perhaps more enigmatic species-group, is less homogeneous than that surrounding the type species of Schacontia. Its most basal member (Schacontia ysticalis) retains numerous features common to the latter group, viz. concentration of white scales apical to the postmedial line (character 9), the narrow distance between the postmedial line and the wing terminus (character 11), the light wing lines in contrast with the dark ground color (character 12), the undifferentiated uncus (character 35), the configuration of the intra-saccular process (character 41), and the elongate corpus bursae (character 61). The remaining species, all hitherto undescribed, form a complex of species exhibiting a heterogeneous collection of male secondary sexual characters, including unusual metatarsal structures, tibial scales and spurs, and abdominal coremata. These appear somewhat homoplastically, such that their downweighting or removal results in a more decisively resolved topology, but we retain them in analysis to emphasize their relevance to future work.
Acontia medalba Schaus, 1904: 163, by subsequent designation.
Brazil.
“Schacontia” seems to be Dyar’s contraction of Schaus and Acontia, the noctuid genus in which Schaus mistakenly attributed medalba and subsequently designated by Dyar as the type species of Schacontia.
Schacontia species may be recognized by (character numbers listed parenthetically): Foreweing Rs3 and Rs4 stalked (5); M1 and M2 stalked (6); hindwing M2M3 + CuA1 stalked (13); bullae tympani invaginated in S2 (18); absence of puteoli (22); fornix heavily sclerotized and far removed from the edge of Ve1 (24); fornical angle a low arc > 90 degrees (25); presence of gnathos-ventrotergal rods complex (31), bearing a finger-like middle process (32); presence of teguminal sulcus (34); intrasaccular process a bump or flange towards base of valve or as a trigger-like process at margin of lower lobe of valve (41); pair of terminal black dots on abdominal dorsum of male (53); uncus hood-like, mucronate, or obovoid, with variously modified terminal nipple (35, 36). In addition, the costal bulge in the FW postmedial line is frequently coupled with a color contrast between the FW medial area and the basal and terminal areas, often involving white scaling. Unlike the medalba group (for present purposes including Schacontia speciosa), the proboscis is not reduced in the ysticalis-themis group, the labial palpi droop, the tympanal fornix is narrow, ribbonlike; venulae secundae tapered to form a “neck.”
In the species most readily identifiable as Schacontia (by virtue of their similarity to the type species Schacontia medalba), hereafter referred to as the medalba group, the forewings are gray with a metallic sheen and the antemedial and postmedial lines variously suffused with white, the exception being Schacontia umbra, which may be almost uniformly shaded dark brown. Towards the costa, the postmedial line bulges outward; the hind wings are by and large nondescript in pattern beyond the presence of a faint postmedial line. The ysticalis-themis group including the Schacontia themis-rasa sister pair and the Schacontia nyx complex [Schacontia nyx+Schacontia clotho+Schacontia lachesis+Schacontia atropos], are distinguished from these in having ocelli present; frons with normal, convex contour, except in Schacontia ysticalis; and labial palps porrect, extending beyond the clypeus.
All Schacontia bear a modification of the intrasaccular region of the valva. In the case of those species surrounding the type species of Schacontia, this comprises a naked or denticled flange; the valvae are characteristically reduced, if not truncate, and the uncus prominent but unadorned, mucronate. The valvae become progressively more complex in the ysticalis-themis group, with the intrasaccular feature transposed laterally to form a sclerotized trigger-like structure. Also in the ysticalis-themis group: the dorsal ridges of the tegumen are cruciate, meeting near the uncus; the tegumen is much wider than the uncus such that the lateral edges of the tegumen appear to taper/fall away from the uncus gradually; the outer margin of the valva is complex, including a variously adorned subcostal process, the costa associated with a fleshy lobe at its terminus and at least one setal tuft; the sacculus bears a localized patch or cluster of setae ventrad; and a membranous area exists between the costa and the subcostal process.
Head - In medalba group, ocelli and chaetosemata absent; proboscis reduced; frons conical; labial and maxillary palpi straight. In ysticalis-themis group, ocelli present; frons of normal, convex contour except in Schacontia ysticalis; labial palps porrect, extending beyond clypeus. Thorax - In medalba group, pronotum, mesonotum, legs gray; hind leg of female with 1 pair of tibial spurs. Males of several members of ysticalis-themis group bear a flattened, hind tibial spur, specialized hind tibial scales, a shallow concave spoon-like metatarsal modification, and coremata on 4th abdominal segment (on Schacontia themis, Schacontia nyx, Schacontia clotho, Schacontia lachesis, and Schacontia atropos); in addition, epipleural setae may be present (in Schacontia rasa, Schacontia clotho, Schacontia lachesis, and Schacontia atropos); and female hind tibia usually bear two pair of spurs (a medial pair present) except in Schacontia ysticalis and Schacontia rasa. Forewing (FW) - Schacontia exhibit a characteristic curvature of postmedial line, outwardly bulging towards costa. In medalba group FW medial area partially suffused with white; in ysticalis-themis group, FW either unicolorous with basal and postmedial areas or polymorphic, with some specimens more darkly shaded. Rs3 and Rs4 stalked; M1 and M2 stalked. Hindwing - In medalba group, HW generally pale with few contrasting markings; female frenulum with a single seta; postmedial line sometimes present, conspicuous, but never in ysticalis-themis group. [M2M3]+CuA1 stalked. Abdomen - Scales arranged in two terminal black dorsal spots in males, more conspicuous in ysticalis-themis group. Tergites gray with dark-gray scaling in medalba group. Tympanal organs crambiform (tympanum and conjunctivum not co-planar, praecinctorium present, bullae tympani open anteromedially), but somewhat variable. In medalba group, bullae tympani broad, tympanal assemblage wider than long (cf.
Species variation. Individual species variation with respect to wing polymorphism is especially acute in the Schacontia nyx complex; of particular interest here are the male secondary sexual characteristics, which covary imperfectly across species and are discussed below. Schacontia species may vary greatly in size (>100% wingspan).
Distribution. Collectively, Schacontia species are distributed across Mexico, south to Central America (Guatemala, Costa Rica, Panama) and South America (Bolivia, Brazil, Ecuador, Venezuela) and the Caribbean (Puerto Rico, Cuba, Hispaniola). A single North American record of Schacontia themis is reported here from Sanibel Island, Florida (USA: Lee Co.).
Larvae are internal feeders that may induce galls, and pupate within the host. The only known host plant records are in Capparaceae: in Costa Rica, larvae have been reared from Podangrogyne decipiens (Triana & Planch.) Woodson (Solis, Nishida and Metz, in preparation); Cleome spinosa Jacq. has been reported as host for Schacontia chanesalis; Capparis frondosa Jacq., and Capparis verrucosa Jacq. are reported for other Schacontia species.
Schacontia was described by
Key to species of Schacontia: Male Genitalia + Habitus + Female genitalia (part)
| 1 | Forewing generally silvery gray or gray brown with white shading in vicinity of antemedial and postmedial lines, particularly in medial area and at outermost edge of postmedial line; or dark brown with poorly contrasted markings except for postmedial line. Hindwing postmedial line conspicuous, nearing inner margin. Frons conical. Valva simple or reduced, sub-quadrate or emarginate/mildly bilobed; lacking a straight, prominent coastal arm; medial projection or flange arising from within sacculus; apex of costa lacking a tuft or plume, or a fleshy, setose subcostal lobe. Uncus mucronate, hoodlike. Tegumen dividedwherein the two tablet-shaped, bubble-like sections meet centrally for some or most of their length. Juxta more or less horseshoe shaped. Tympanal apparatus with saccus indistinct, posterior ridge lightly if at all sclerotized, grading into second sternite; venulae secundae not sharply tapering inward caudally; fornix broad, robust | 2 |
| 1’ | Forewing ground color straw or light gray, uniform or with a contrasting gray medial area suffused with white; or with outer margin and basal areas rust colored (Schacontia ysticalis) or yellowish brown (Schacontia lachesis and Schacontia atropos); antemedial and postmedial lines conspicuous; HW postmedial line faint, without secondary postmedial shading, not reaching inner margin. Frons with normal undifferentiated convex contour. Valva broad with a distinct costal bar or boom and either a faint, rudimentary hump appearing in the ventro-saccular region or as a more prominent, lateral sclerotized process at ventro-marginal edge of sacculus; a tuft or plume associated with costal terminus, each of which may also bear a fleshy, setose subcostal lobe. Uncus, either gently tapering to a wide rounded tip, or obovoid or squared, bearing a trefoil-like tip, in the last case appearing nearly tridentate, with a raised central ridge resulting in a webbed appearance (this feature may vary in prominence). Tegumen deeply divided such that two ovular sections meet obliquely towards base of uncus. Juxta robust, V- or broadly V-shaped. Tympanal saccus distinct, with posterior ridge or lip heavily sclerotized; venulae secundae tapered inward caudally; fornix narrow, ribbon-like. | 4 |
| 2(1) | Forewing medial area variously but diffusely shaded, generally without sharp contrast or orbicular spot; basal area not traversed by a white band. Uncus tapering to a distinct, ventrally directed squared tip. Tegumen invaginate such that sulcus joining two teguminal hemispheres extends less than 40% of length of tegumen. Intra-saccular process smooth, not conspicuously denticled | 3 |
| 2’ | Forewing medial area variously shaded, but often with contrast, an orbicular spot varyingly distinct or inconspicuous, if present; the basal area usually traversed by a white band. Uncus broadly tapered with a simple rounded nipple. Teguminal sulcus extends most of length of tegumen. Intra-saccular process rugose or denticled | Schacontia chanesalis |
| 3(2) | Forewing color variously shaded with white scaling; lines or variously shaded regions conspicuous. Orbicular spot faint, if present. Hind wing slate gray. Valva truncate, rounded, entire | Schacontia medalba |
| 3’ | Forewing shaded chocolate brown, markings not obvious. Hind wings dark gray. Valva slightly emarginate | Schacontia umbra |
| 4(1’) | FW medial area suffused with white basad; postmedial line with broad, gentle costal bulge. Uncus dorsoventrally flattened, edges nearly carinate; uncus tip broad, neither acutely sharp nor sculpted with trefoil shape. Valva lacking a trigger-like process below costa; costa with mane-like tuft of elongate setae, recurved medially. Phallus simple, cornuti absent. Corpus bursae elongate, without signa | 5 |
| 4’ | FW shading either unicolorous or with medial area more darkly shaded than both basal and postmedial areas. Uncus tip swollen, either obovate or squared, in latter case with lateral edges thickened. Valva with a trigger-like process arising from within sacculus along ventral edge, and a conspicuous fleshy subcostal lobe and setose plume; costa lacking tuft of elongate setae medially recurved. Phallus with two prominent cornuti. Corpus bursae more or less globular, rarely with signa | 6 |
| 5(4) | Basal area of FW with a brown ovoid spot, delineated by white bands crossing from wing base to antemedial line. Uncus tapered towards blunt squarish tip at a roughly 60 degree angle. Valva entire, not emarginate, without distinct upper and lower extensions; center of valva unadorned; intra-saccular structures indistinct. Phallus simple, naked, without cornuti | Schacontia speciosa |
| 5’ | Basal area of FW rust colored, mottled. Uncus broad, lateral edges parallel, tapering to a wide, gently rounded tip at a roughly 45 degree angle. Valva with upper and lower extensions, the lower sclerotized dorsad; intrasaccular flange conspicuous, adorned with both surficial and adjacent setal clusters. Phallus with a single cornutus. | Schacontia ysticalis |
| 6(4’) | FW uniform mouse gray or mottled, in latter case with medial area more darkly shaded. Uncus rounded, obovate with distinct, rhomboid nipple; uncal edges not reinforced or swollen. Valva gently rounded ventrally with moderate to elongate lateral process, ventro-medial edge with a distinct comb of elongate setae | 7 |
| 6’ | FW ground color gray or straw colored, contrasting gray medial area in some specimens. Uncus squared or scooplike in appearance with lateral edges swollen, sometimes conspicuously so, with or without a pronounced central ridge, tip hastate or trefoil-like; valva either elbowed or sharply angled towards mid-point, but not gently rounded, lacking an elongate process distally, ventro-medial edge without a distinct comb of elongate setae | 8 |
| 7(6) | FW mottled in appearance, medial area slightly darker than basal and postmedial areas; orbicular spot pronounced. Epipleural setae absent. Ventral trigger-like process on valva rudimentary, if present; subcostal lobe robust, squat, <=3x longer than wide | Schacontia nyx |
| 7’ | FW gray, unicolorous; orbicular spot faint. Epipleural setae present. Ventral trigger-like process pronounced; subcostal lobe elongate and narrow, ~5x longer than wide | Schacontia clotho |
| 8 (6’) | Uncus with conspicuous, prominent central ridge. Elongate lateral lobe of valva absent; subcostal lobe not elongate; ventral edge of valva not conspicuously elbowed close to vinculum; central membranous area of valva conspicuously longer than wide | 9 |
| 8’ | Uncus with a uniformly smooth contour. Subcostal lobe pronounced, finger-like; ventral edge of valva angled or elbowed sharply (not rounded) approximately mid-way between vinculum and lateral edge of valva; central membranous area of valva not conspicuously longer than wide | 10 |
| 9(8) | Female with two pairs of hind tibial spurs; male with coremata present on 4th abdominal segment, flattened hind tibial spur, specialized hind tibial scales with embedded dark patch, cuplike metatarsal modification, epipleural setae | Schacontia themis |
| 9’ | Female with single pair of hind tibial spurs; male secondary sexual features above absent | Schacontia rasa |
| 10(8’) | Male secondary sexual characters (including coremata on 4th abdominal segment, cf. 10) all present | Schacontia lachesis |
| 10’ | Male secondary sexual characters (cf. 10) absent | Schacontia atropos |
http://species-id.net/wiki/Schacontia_medalba
Figs 1, 21, 36–38(19♂, 10♀, 1 sex undet.).
Holotype (♀, USNM): Castro, Parana; Collection Wm Schaus; [red type label] type 10575; Acontia? medalbi [sic] sp. Schs; Pyralie Schoenobiana gen. nov.; USNM Genitalia Slide by DA ♀ 107, 899.
Brazil: (19♂, 8♀, 1 sex undet.): Bnito Prov. Pernmbuco Brazl 83, unknown [illegible] 84 W.S. 165, genitalia slide by DA ♂ USNM 107, 909 (1♂); Bnito Prov., Pernmbuco Brazl 83, [illeg.], Collection C.V. Riley, Genitalia slide by DA ♂ USNM 107, 887 (1♂); Bnito Prov., Pernmbuco Brazl 83, [illeg.], Collection C.V. Riley, Genitalia slide by DA ♀ USNM 107, 888 (1♀); Pernambuco, Brazil, coll. Pickel, 17 II 929 2065, Genitalia slide by DA ♀ 107, 910 (1♀); Pernambuco Tapera, 1934.VIII.24, 2087♀ (1♀); Bnito Prov., Pernmbuco, Brazl. 6/1 83, Not known [illeg.], Collection of C.V. Riley ♂ (1♂); Pernambuco, Brazil, Coll. Pickel ♂ (1♂); 28, Bnito Prov., Pernmbuco, Brazl 8 3, 2, Fernald ♂ (1♂); 73, Not in BM 1925, W Schaus ♂ (1♂); 2♂ 1♀, as previous; Castro, Parana, Collection Wm Schaus, incl. 1: Genitalia slide by MAS ♀ USNM 107, 011 (1♀); 1♂ as previous; Brazil: Nova Teutonia, F. Plaumann (1♂); Col. Becker No. 4601, Rio Brilbante, Mato Grosso, Brasil, 25.I.1971, V. O. Becker Col., Schacontia medalba det. M.A. Solis [on one only] (2♀); Col. Becker No. 9164, Rio Brilbante, Mato Grosso, Brasil, 24.X.1970, Becker leg. (1♀); Nova Teutonia, 27°11’S, 52°23’W, Brazil, 300–500 m, 4-IV-1954, Fritz Plaumann [CNC] (4♂); Nova Teutonia, 27°11'S, 52°23'W, Brazil, 300–500 m, 10-IV-1954 Fritz Plaumann [CNC] (4♂); as above, “Slide No. 3645M.S.” (1 sex undet.); Nova Teutonia, 27°11'S, 52°23'W, Brazil, 300–500 m, 3-III-1954, Fritz Plaumann, Schacontia n. sp. 7, Det. E.G. Munroe 1998 [CNC], Genitalia slide by JAL ♂ (1♂). Peru (1♀): Boniti P, Peru, Jan 7. 83.
Specimens of Schacontia medalba are most readily diagnosed from those of Schacontia chanesalis by male genitalia, specifically the reduced, unlobed valvae and the naked intrasaccular process, features they share with Schacontia umbra.
(Fig. 1). Forewing length 6.5–1.0 mm. Head - Frons conical; labial palpi straight, extending as far as clypeus. Thorax - Female with one pair of hind tibial spurs (medial pair absent); legs uniform gray brown. Forewing. Basal area primarily gray brown, undivided; antemedial (am) line meets anal margin. Subterminal line interrupted by wing veins; medial area partially suffused with white, especially basad; white postmedial line appears shaded basally, interrupts/traverses dark shading between apical area and distal region of medial area; this “double” line faintly common to HW; FW fringe gray-brown. Hindwing. Postmedial line present, conspicuous (see above); terminal area lightly shaded, fringe white. Abdomen - Apical bands of pale scales on abdominal segments; terminal dots grayish brown, faint if present. Tympanal organs (Fig. 21). As for the medalba group, vide supra. Male genitalia (Figs 36, 37). Teguminal sulcus short, such that anterior margin of tegumen appears deeply invaginate; juxta U-shaped; valvae simple, reduced, rounded, not bilobed or emarginate; intrasaccular process a simple flange; intrasaccular process naked; phallus simple, cornuti absent. Female genitalia (Fig. 38). Antrum wider than deep, chalice-like; ductus bursae inconspicuous, no colliculum apparent; corpus bursae indistinct, weakly sclerotized, elongate.
Unknown.
Variation. Variable in size; forewings vary with respect to obfuscation in medial area.
Unknown.
Brazil, Peru.
http://species-id.net/wiki/Schacontia_chanesalis
Figs 2, 13, 15–17, 22, 39, 41Below we summarize material examined for Schacontia chanesalis. We include material previously determined as its new synonyms, Schacontia replica and Schacontia pfeifferi, and list them accordingly. We acknowledge that cryptic species may yet be identified pending the accumulation of molecular data.
Holotype (♀, BMNH): Holotype [round white label w/ red border]; El Tumbador, Guatemala, Champion; Godman-Salvin, Coll. 1904-1., B.C.A. Lep.-Het., Pionea chanesalis Druce; Pionea chanesalis Druce, type [hand written]; Genitalia Slide by DA, ♀. [Holotype of Schacontia replica]: March 1912, Orizaba, Mex, 3414, R Muller Collector, [red type label] Type 15484, Schacontia replica Dyar Type, [green label] USNM Genitalia Slide by DA ♀ 107, 898, left FW missing (1♀). [Holotype; ZSM, Munich]; Typus ♀ leg. H. Amsel; Venezuela Maracay leg. P. Vogl.; Genitalia slide by DA 108, 040 ♀, det. Amsel 1953 Schacontia pfeifferi Ams. [“Allotype”, ZSM, Munich].
Costa Rica (8♂, 11♀, 4 sex undet.): Santa Rosa National Park Guanacaste Prov. Costa Rica 2–4 May 1980 DH Janzen & W. Hallwachs, Genitalia Slide by DA ♂ USNM 107, 903, INBio Barcode # CR1001 115186 (1♂) as previous [no slide label], #CR1001 115190 (1♀); Estac. Quebrada Bonita, 50m R.B. Carara Puntarenas Pr. Costa Rica Nov 1989. R. Zuniga. 194500, 469850, Genitalia Slide by DA ♂ USNM 105, 819, head illustrated; INBio Barcode # CR1000 120043 (1♂); Estac. Quebrada Bonita, 50m R.B. Carara Puntarenas Pr. Costa Rica April 1989. R. Zuniga. 194500, 469850, Genitalia Slide by DA ♂ USNM 106, 418 [v. poor specimen], INBio Barcode # CR1001 103073 (1♂); Estac. Quebrada Bonita, 50m R.B. Carara Puntarenas Pr. Costa Rica Oct 1989. R. Zuniga. 194500, 469850, Genitalia Slide by DA ♀USNM 105, 820, INBio Barcode # CR1000 160925 (1♀); Estac. Quebrada Bonita, 50m R.B. Carara Puntarenas Pr. Costa Rica Set 1989. R. Zuniga. 194500, 469850, INBio Barcode # CR1001 103076 (1♀[?]); Est. Sta. Rosa, 800m, P.N. Guanacaste, Prov. Guan. Costa Rica, I. Curso Microlepidopt., Jul 1990 L-N-313000, 359800, INBio Barcode # CR1000 182323 (1♀); Est. Maritza, 600 m, Lado oeste del Volcan Orosi I curso Microlepidopt., July 1990 L-N-326900, 373000, INBio Barcode # CR1000 181312 (1♂); Santa Rosa National Park Guanacaste Prov. Costa Rica 7–9 July 1980 DH Janzen & W. Hallwachs, INBio Barcode # CR1001 115188 (1♀); Santa Rosa Nat. Pk., Prov Guanacaste, Costa Rica 10–12 Nov 1979 D.H. Janzen, INBio Barcode # CR1001 115187 (1♂); 97-SRNP-320, 8, Genitalia Slide by JAL ♀ (1♀); 97-SRNP-320, [right FW detached] (1♀); Prov. Guanacaste, Z.P. Nosara, Sector of Mirador, 800 m 2–8 Nov 2002. H. Mendez. Tp. De Laz. L N 220750 383450 #72175, INB0003554509 (1♀); Estac. Quebrada Bonita 50m R.B. Carara Puntarenas Pr. Costa Rica Oct 1989. R. Zuniga. 194500, 469850, INBio Barcode # CR1000 196823 (1♂); Fca. Cafrosa, Est. Las Mellizas, P.N. Amistad, 1300m Prov. Punt. COSTA RICA M. Ramirez & G. Mora, Nov. 1990 L-S-316100-596100, INBio Barcode # CR1000 278769 (1♀); Estac. Quebrada Bonita, 50m R.B. Carara Puntarenas Pr. Costa Rica Set 1989. R. Zuniga. 194500, 4698500 (1 sex undet.); Est. Sta. Rosa, 800m, P.N. Guanacaste, Prov. Guan. Costa Rica, I. Carso Microlepidopt., Jul 1990 L-N-313000, 359800 (1 sex undet.); Schacontia sp. Crambidae, Costa Rica, Cartago prov. Parque National Tapanti near the ranger station 1250m, 25-V-2005 (adult emergence) Col/rear: Kenji NISHIDA Host Plant: Podandrogyne decipiens (Capparidaceae), gall inducer on the stem unknown family, female (1♀); Schacontia sp. Crambidae, Costa Rica, Cartago prov. Parque National Tapanti near the ranger station 1250m, 25-V-2005 (adult emergence) Col/rear: Kenji NISHIDA Host Plant: Podandrogyne decipiens (Capparidaceae), gall inducer on the stem, Schacontia n. sp. 2/06 det. M.A. Solis (1 sex undet.); Schacontia sp. Crambidae, Costa Rica, Cartago prov. Parque National Tapanti near the ranger station 1250m, 25-V-2005 (adult emergence) Col/rear: Kenji NISHIDA Host Plant: Podandrogyne decipiens (Capparidaceae), gall inducer on the stem Deformed adult caught in its pupal shell x1 Pupated 15-VI-2005 (pupal stage 1 month) (1♂); Schacontia sp. Crambidae, Costa Rica, Cartago prov. Parque National Tapanti near the ranger station 1250m, 25-V-2005 (adult emergence) Col/rear: Kenji NISHIDA Host Plant: Podandrogyne decipiens (Capparidaceae), gall inducer on the stem unknown family, female (1♀); Schacontia sp. Crambidae, Costa Rica, Cartago prov. Parque National Tapanti near the ranger station, 1250m, 25-V-2005 (adult emergence) Col/rear: Kenji NISHIDA Host Plant: Podandrogyne decipiens (Capparidaceae), gall inducer on the stem unknown family, male (1♂); Costa Rica: Estac. Biol. Las Cruces 6 km SE San Vito Rio Jaba 1150m X-20/21/1993, blacklight in secondary forest J. Powell coll. (1sex undet.); Voucher: D.H. Janzen & W. Hallwachs DB: http//Janzen.sas.upenn.edu Area de Conservacion Guanacaste Costa Rica 97-SRNP-320.1, “legs away for DNA” (1 sex undet.); same as previous, 97-SRNP-320.2, 97-SRNP-320.3, and 11-SRNP-12677 (1♂, 1♀, 1 sex undet., respectively). Guatemala: Cayuga Guat, Dec, Schaus and Barnes coll, Genitalia slide by DA ♂ USNM 108, 097 (1♂); Quirigua Guat, Schaus and Barnes coll, Genitalia slide by DA ♀ USNM 107, 892, FW in capsule (1♀); Grutas de San Pedro Martir, Guatemala Escuintla VIII-10-1965 P.J. Spangler 1♀[?]. Honduras: El Hatillo Honduras Black light 3-VIII-1972 Robert D. Lehman (1 sex undet., obscured by mold). Mexico (22♂, 3♀, 3 sex undet.): Col. Becker 44006, Mexico: Veracruz Huatusco 1300m 19–23. Viii. 1981 V.O. Becker col., Comp. c/tipo USNM 1981 V.O. Becker (1♂); Nov ’11, Orizaba Mex, R Muller collector, 3414, Chanesalis or [illeg.] desc. as Pionea [illeg.] Schoenobiinae, ♀ USNM 197, 890 Genitalia slide by DA (1♀); Mexico: 2 mi. N. Tamazunchale, S.L.P. 400’, July 16–18, 1963, Duckworth & Davis, Genitalia slide by DA ♂ USNM 108, 099 (1♂); Mexico: 2 mi. N. Tamazunchale, S.L.P. 400’, July 16–18 1963, Duckworth & Davis, Genitalia slide by DA ♂ USNM 108, 889 (1♂); Mexico: El Salto Falls, 26 mi W. Antiguo Morelos, Tamps., 2000’, July 11–14 1963, Duckworth & Davis (1♀); Mexico: .2 mi. N. Tamazunchale, S.L.P., 400’, Aug. 2 1963, Duckworth & Davis (1♂); (17♂, 1♀, 1 sex undet.[genitalic slide unavailable, “1291”]): Col. Becker 68741, Mexico, Tam El Ensino, 250 m, 4–13.viii.1988, V.O.Becker Col., Genitalia Slide ♂ by JAL [one specimen only]; Col. Becker 108733, Mexico: Tam El Encino, 250 m, 21–31.v. 1997, V. O. Becker Col. (1♂); Mex: Ver., 7 km NNW Huatusco, 1300 m, VIII-15-1987, J.T. Doyen (1 sex undet.). Venezuela (3♂): Venezuela: Guarico, Hato Masaguaral, 45 km S Calabozo, 8.57N, 67.58W, Galry Forest #20, 75 m, 3–5 June 1988, uv light, M. Epstein Genitalia slide ♂ by JAL USNM 108, 083] (1♂); VENEZUELA: San Esteban Carabobo, Venez., Dec. 1–20 1939, Pablo J. Anduse, Illustration of wing pattern (1♂); Venezuela Maracay leg. P. Vogl., Jan.–Febr.35, Typus ♂ leg. H. Amsel, Genitalia slide by DA 108, 039 ♂ (1♂).
Specimens of Schacontia chanesalis are best distinguished from those of Schacontia medalba by the male genitalia, specifically a more sinuate valva and more denticled or rugose (as opposed to naked) intrasaccular process. The valvae are less conspicuously lobate than in Schacontia umbra (below). Forewing pattern somewhat variable, as in Schacontia medalba, but antemedial area more often traversed by white bar originating at scapula, enhancing the baso-costal patch.
(Fig. 2). Forewing length: 4.5–9.0 mm. Head - Ocelli and chaetosemata absent; proboscis reduced. Labial palpi porrect, extending slightly beyond clypeus. Frons conical; vertex and frons grayish brown, intermixed with white scales medially and along anterior bases of antennae. Thorax - Prothoracic collar light gray intermixed with gray-brown and white scales. Tegula and mesoscutum mostly gray, intermixed with light-gray and/or grayish-brown scales, the posterior apex of tegulae pale gray. Legs predominantly white, gray shading throughout foreleg; female with one pair of hind tibial spurs (medial pair absent). Forewing. Baso-costal triangle flanked by white scaling towards inner margin and in medial area, which is outwardly shaded brown (suffused with white basad). Postmedial area (between postmedial line and subterminal line) grayish brown. Subterminal line white; terminal line black, interrupted. Marginal scales brown. Basal area grayish brown traversed by a white band. Fringe scales light gray. Hindwing. Ground color white/very light gray, darker postmedially; postmedial lines grayish brown, white distally, conspicuous and common with FW. Subterminal area shaded darker brown; fringe white. Sc+R1 and Rs anastomosed slightly beyond dilated base of former. Male and female acanthae of frenulum fused from near base to apex to form one bristle. Abdomen - Ground color mostly dark gray intermixed with gray and light-gray scales above, white on undersurface. Tympanal organs (Fig. 22). As above for medalba group, vide supra. Male Genitalia (Figs 39, 40) - Tegumen divided dorsally into two dihedral or hemi-spherical “bubbles” that meet at a central sulcus, which divides anterior to base of uncus and forms a Y-shaped strut. Teguminal sulcus long, extending length of two teguminal lobes, such that anterior margin of tegumen appears emarginate, but not deeply invaginated. Uncus oblong, mucronate or miter-like, culminating in a distinct tip; concave or spatulate, setose on inner (ventral) surface. A membranous, more or less circular region at base of uncus positioned directly above (dorsal to) finger-like projection of gnathos, which also comprises a floating sub-tegumenal plate with four arms. Finger-like process arises from center of gnathos; dorsal pair of arms, which meet at juncture of uncus and tegumen, appearing to fulfill traditional description of gnathos by enveloping the anal tube, and the anterior pair extending ventrolaterally towards the vinculum, resembling a wishbone. Gnathos thus appears as a subtegumental (ventrad) suspension. Valvae reduced, broadly emarginate, bilobed; intrasaccular process a simple flange, denticled or rugose; subapical setal cluster near saccular margin. Costa robust, curved, appearing to arise near the respective vincular terminus. Juxta horseshoe-shaped, the base wider than the lateral “arms.” Phallus simple, moderately sclerotized throughout; cornuti absent. Female Genitalia (Fig. 41) - Papillae anales appressed; antrum apparent, chalice-like; ductus bursae short; corpus bursae elongate, without signa, caeca, or appendix bursae; ductus seminalis originating from posterior portion of corpus bursae. Ostium bursae with membrane between seventh and eighth segments.
Larvae have been reared from Podandrogyne decipiense (Capparaceae) (D. Janzen, pers. comm.).
Mexico, Guatemala; Costa Rica; Venezuela.
Unknown.
In size, with Mexican specimens appearing smaller in wingspan (FW length 4.5–7.0 mm) than Central American specimens.
It is with some trepidation that we synonymize both replica and especially pfeifferi with chanesalis. Pfeifferi in particular was, until this work, the only Schacontia for which a detailed description had been published, and its continental separation from the type locality and primary distribution of chanesalis might suggest the potential for as yet unrecognized diagnosable species.
urn:lsid:zoobank.org:act:524F3C52-1E37-4E82-B0F8-AF4485B49E2C
http://species-id.net/wiki/Schacontia_umbra
Figs 3, 23, 42, 43Type material. Ecuador: Holotype (♂, USNM): Ecuador, Past. Mera: 1300 m xii. 1992 V.O. Becker Col; Col. Becker 100503, (1♂). Paratypes (4♂), USNM. Ecuador, Past. Mera: 1300 m xii. 1992 V.O. Becker Col, Col. Becker 100503, (♂); Ibidem, 100504, (1♂); Ecuador, Past. Puyo: (22 km W) 5 February 1976 Blacklite [sic] Spangler, et al., Ecuador, Peace Corps. Smithsonian Institution Aquatic Insect Survey, Genitalic slide by DA ♂108, 095, (1♂); (27 km N) Est. Fluv. Metrica 4 February 1976 Spangler, et al. (1♂).
Habitus, male genitalia (Figs 3, 42). This species is most readily diagnosed by the darkly shaded forewings and by the male genitalia, which have the following features in common with medalba: anterior margin of tegumen deeply invaginate, outer margin of valva entire, intra-saccular process naked.
(Fig. 3). Forewing length: 7.5–8.0mm (n=5). Head - Ocelli absent; proboscis reduced; frons conical; labial and maxillary palpi straight, not extending beyond clypeus. Thorax - Female with one pair of hind tibial spurs (medial pair absent). Forewing. Shaded gray brown, hind wings dark gray brown; postmedial line, when evident, characteristic of genus, outwardly bulged towards costa, sinuous towards inner margin, but entire forewing more darkly shaded than in other species. Medial area partially suffused with white in some but not all specimens. Subterminal line pale, unbroken; fringe gray. Hindwing. Uniformly brown gray; subterminal line pale tawny, unbroken; fringe gray. Abdomen - Uniformly covered in gray-brown scales. Tympanal organs. (Fig. 23). As for medalba group, vide supra. Male genitalia (Figs 42, 43) - Teguminal sulcus short, such that anterior margin of tegumen appears deeply invaginate; juxta a U-shaped plate; valvae simple, reduced, rounded, not bilobed or emarginate; intrasaccular process a simple flange, naked; phallus simple, cornuti absent. Female genitalia (Fig. 44) - Unknown.
Unknown.
Markings may be obscured in some specimens, rendering them more or less uniformly gray brown.
The specific epithet refers to the dark wing shading of this species and is treated as a noun in apposition.
Unknown.
Central Ecuador.
urn:lsid:zoobank.org:act:77C2883E-34CB-4725-8AEC-A6C530C487F9
http://species-id.net/wiki/Schacontia_speciosa
Figs 4, 24, 44, 45Type material. Brazil: Holotypemale, USNM (Fig. 4): Col. Becker 65271; Brasil: RJ Marica 5m, 11. x.1985, V.O.Becker Col. Paratypes 10♂, 1♀, 2 sex undet., USNM. Brazil: Same data as holotype (9♂, with additional label “Genitalia 1290”); BRAZIL: BA Jequié, 600–750 m; Col. Becker 105714 (1♀); BRAZIL: Rio Jan. 10 km SW Maricá “restinga” sand dune, 11–12-X-85 Scott E. Miller (1♂, 2 sex undet, ex abd.).
Habitus, male genitalia(Figs 4, 44). The forewing pattern of this species makes it unmistakable; readily diagnosed by a combination of the frosted medial area common to other Schacontia and the interruption of the brown basal area to render a medio-basal patch encircled in white. Male genitalia diagnosed from those of other Schacontia species by the combination of expanded (not truncate) but inornate valvae, and reduced features associated with them, such as the inconspicuous intrasaccular flange; and a blunt, squarish, barely-tapering uncus.
Male (Fig. 4). Forewing length: 7.5–8.0 mm, (n=14). Head - Ocelli absent; proboscis normal; frons expressed as a small hump, but not conspicuously conical; labial palpi porrect, extending beyond clypeus. Thorax - Vertical scales mocha; female with one pair of hind tibial spurs (medial pair absent). Forewing. Medial area gray, partially suffused with white basad; postmedial line shaded white outwardly, brown inwardly; basal and submarginal areas primarily mocha brown; basal area interrupted by oblong basal patch surrounded by white. Subterminal line dark, unbroken; fringe scale gray, darkest at termini. Hindwing. Brownish white, no contrasting markings, postmedial line inconspicuous if present; subterminal line dark, unbroken; fringe scales brown, pale gray at margin. Abdomen - Scales arranged in two terminal black dorsal spots in males. Tympanal organs (Fig. 24). Tergo-sternal sclerite robust, conspicuous; bullae tympani longer than wide, saccus or rim of bullae sclerotized at base; processi tympani present, lamellate, thumblike, towards anterolateral end of fornix; processus spiniformis present; fornix tympani sclerotized; angle of fornical ulna obtuse; pons of intermediate length, roughly half the depth of saccus, component rods broad, separate along entire length, diverging at anterior termini; posteromedial margin of saccus extends and remains parallel to pons for most of its length, pons extending towards bottom of saccus; saccus pronounced; venulae secundae prominent, tapering slightly at base of tympanal case such that “partie libre” (sensu Minet) of second sternite forms a neck; puteoli absent. Male genitalia (Fig. 44) - Teguminal sulcus short, not apparent; juxta U-shaped, lateral arms modesty recurved; valvae simple, broad, not truncate; intra-saccular process rudimentary, if present; costa with recurved, elongate tufts of setae but no conspicuous fleshy lobe; phallus simple, cornuti absent. Female genitalia (Fig. 45) - Papillae anales separate, round, swollen; colliculum present as faintly sclerotized collar embedded in ductus bursae, which is short, inconspicuously delimited and unsclerotized anterior to colliculum; corpus bursae elongate, membranus, without signa or appendix bursae; ductus seminalis inserted between antrum and ductus bursae.
Unknown.
The specific epithet is from the Latin for showy or handsome.
Unknown. Adults active in October.
Southeastern Brazil (Bahia, Rio de Janeiro).
http://species-id.net/wiki/Schacontia_ysticalis
Figs 5, 25, 46–48(16♂, 16♀, 1 sex undet.).
Mexico: Holotype (♀, USNM).
Bolivia: Puerto Suarez, Bolivia, 150 m, Dec. 1908, J. Steinbach C.M. Acc. 3758 (1♂) [CMNH]. Costa Rica (4♂, 6♀):Santa Rosa National Park, Guanacaste Prov., Costa Rica, D.H. Janzen, 12 Dec 1978–10 Jan 1979, Genitalia Slide by DA ♂ USNM 107, 905, INBio Barcode # CR 1001 115170 (1♂); Santa Rosa National Park, Guanacaste Prov., Costa Rica, D.H. Janzen, 12 Dec 1978–10 Jan 1979, Genitalia Slide by DA ♂ USNM 107, 904, INBio Barcode # CR 1001 115169 (1♂); Playa Naranjo, Sta Rosa P.N., Guanacaste Prov., Costa Rica, E. Alcazar, Ene 1991 L-N-309300-353300, INBio Barcode # CR 1000 640648 (1♂); Santa Rosa National Park, Guanacaste Prov., Costa Rica, D.H. Janzen, 12 Dec 1978–10 Jan 1979, Genitalia Slide by DA ♀ USNM 107, 906, INBio Barcode # CR 1001 115171 (1♀); Est. Queb. Bonita, 50 m, Res. Biol. Carara, Prov. Punt., Costa Rica, R. Zuniga, Jun 1991, L-N-194500, 469850, INBio Barcode # CR 1000 343579 (1♀); Estac. Quebrada Bonita, 50 m, R.B. Carara, Puntarenas Pr., Costa Rica, R. Zuniga, April 1989, 194500, 469850 INBio Barcode # CR 1000 017910 (1♀); Sirena, Corcovado Nat. Pk., Osa Penin., Costa Rica, 19–27 Mar1981, DH Janzen, W. Hallwachs, INBio Barcode # CR 1001 115172 (1♀); Est. Quebrada Bonita, R.B. Carara, Prov. Punta, Costa Rica, 50 m, Mar 1994, R. Guzman, L N 194500_469850 #2803, INBio Barcode # CR1001 754072 (1♀); Est. Quebrada Bonita, R.B. Carara, Prov. Punta, Costa Rica, 100 m, ENE 1995, R. Guzman, L_N_195250_469850 #4433, INBio Barcode # CR1002 243527 (1♀); Costa Rica, Prov. Limon, Sector Cedrales de la Rita, 3 km N del Puente Rio Suerte, Ruta Puerto Lindo, 10 m, Feb 1997, E. Rojas, L_N_278600_566500 #45311, INBio Barcode # CR1002 499299 (1♂). Mexico (2♂, 4♀):Col. Becker 42358, Mexico: Veracruz Est. Biol. Tuxtlas, 11–16.vi.1981, V. O. Becker Col., Schacontia ysticalis Dyar, Det. M.A. Solis (1♀); Ibid. x4 exc. Col. Becker 42297 (3♀, 1♂); Venadio, Sinaloa, Mex, B P Clark donor, Not in BM 1925 W Schaus (1♂). Nicaragua [CMNH]: Managua Dist., Laguna de Xiloa, 23 April 1996, E. van den Berghe (3♂, 1♀); Managua Dist., Laguna de Xiloa, 14 April 1996, E. van den Berghe (2♂);Managua Dist., Laguna de Xiloa, 8 March 1997, E. van den Berghe (1♂) [CMNH]. Venezuela (7♂, 5♀): Venezuela, Guarico, Huato Masaguaral, 45 km S Calabozo, 8.57°N, 67.58°W, Galry For#4, 75 m, 12–13 Apr 1988, uv light, M. Epstein, R. Blahnik, Genitalic Slide by DA ♀ USNM 107, 896 (1♀); same as previous (2♂, 4♀, 1 sex undet.), “hind leg used for illustration” [Fig. 20]); Venezuela, Guarico, Huato Masaguaral , 45 km S Calabozo, 8.57°N, 67.58°W, Galry For#4, 75 m, 13–16 May 1988, uv light, M. Epstein, R. Blahnik [3♂, incl. 1w/ label Genitalic Slide by DA ♂ USNM 108, 100]; Venezuela, Guarico, Huato Masaguaral, 45 km S Calabozo, 8.57°N, 67.58°W, Galry For#4, 75 m, 23–24 Apr 1988, uv light, M. Epstein, R. Blahnik, Genitalic Slide by DA ♂ USNM 107, 895, “Head illustrated” (1♂); Venezuela, Guarico, Huato Masaguaral, 45 km S Calabozo, 8.57°N, 67.58°W, Galry For#4, 75 m, 25 May, uv light M. Epstein & C. Canaday (1♂).
Habitus, male and female genitalia (Figs 5, 46–48). Distinct by virtue of orange cast to basal and postmedial areas of forewing. Male genitalia distinct by virtue of wide, rounded uncus without modifications; heavy, long recurved setal tufts at costa of valva; and intrasaccular patch of heavy setae. Female genitalia distinct by virtue of elongate, robust bursa combined with conspicuous appendices bursae.
Male (Fig. 5). Forewing length: 7.0–11.0 mm (n=14). Head (Fig. 14) - Ocelli present; proboscis normal; frons conical or expressed as a small hump; labial palpi porrect, extending beyond clypeus. Thorax - Legs white, forelegs cupreous dorsally, as basal tarsomeres on all legs. Female with two pair of hind tibial spurs (medial pair present). Forewing. Basal, antemedial, and subterminal fasciae brownish orange, shaded distally with white (dark basad). Both antemedial and postmedial lines shaded distally with white (dark basad); antemedial and postmedial areas rust colored/cupreous; medial area sparsely suffused with white. FW fringe brown. Hindwing. postmedial line faint if present; HW yellowed at margin, subterminal line interrupted; fringe whitish beige. Abdomen - Cupreous sheen; white abdominal bands on all segments. Scales arranged in two terminal black dorsal spots in males. Tympanal organs (Fig. 25). As for ysticalis-themis group, vide supra. Male genitalia (Figs 46, 47) - Teguminal sulcus short, such that anterior margin of tegumen appears deeply invaginate, the two oblong teguminal lobes joined obliquely. Uncus wider than long; terminal edge of uncus entire. Gnathos quadrate. Juxta an inverted triangular plate or robust “V”, less sclerotized at center. Valvae complex; costa robust with recurved, elongate tufts of setae; subcostal lobe with petiolate scales, most arched towards dorsal articulation of valva; with secondary outer, oblong lobe or process below costa; with fleshy setose lobe associated with terminus of costa and located between distal portion of costa and lower portion. Intrasaccular process a simple flange, the inner surface of which bears chisel-shaped setae; with robust, spine-like setae at base; submarginal area of sacculus setose. Saccular margin angled close to vinculum, not at saccular mid-point; ventro-marginal setae concentrated at saccular ulna. Phallus moderately sclerotized; vesica with a small cornutus. Female genitalia (Fig. 48) - Papillae anales separate, more or less round, flat, and swollen; ostium bursae with membrane between seventh and eighth segment; antrum membranous; colliculum present as a sclerotized collar of intermediate length embedded within ductus bursae; two conspicuous appendices bursae located at posterior end of corpus bursae; corpus elongate, membranous, without signa; ductus seminalis near posterior end of corpus bursae.
Unknown.
Unremarkable.
Unknown. Recorded adult activity mid-June (Mexico), January–June (Costa Rica), 8 March–23 April (Nicaragua), 12 April–25 May (Venezuela), December (Bolivia).
Mexico, Costa Rica, Nicaragua, Venezuela, Bolivia.
urn:lsid:zoobank.org:act:81B6D7E0-F6F0-44C8-B4B7-BB8424FE4B9F
http://species-id.net/wiki/Schacontia_themis
Figs 6, 19, 26, 49–51Type material. Holotype (♂, CMNH). Dominican Republic: La Altagracia. 2 km N Bayahibe, 18-23N, 68-51W, 10 m, 3 July 1992, C. Young, R. Davidson, S. Thompson, J. Rawlins, Dry seasonal forest on limestone, USNM ENT 00808538, DNA 2012 (1♂) [GenBank Accession #KC789515]. Paratypes (41♂, 19♀) USNM, except where otherwise designated. Costa Rican paratypes with an INBio barcode label deposited at INBio. Brazil (5♂, 1♀, 1 sex undet.): Unit. Amaz. Taperinha b. Santarem 1–10 VI ’27, Zerny (1♂) [NHMV]; Unit. Amaz. Taperinha b. Santarem, 1–10 VIII ‘27, Zerny (1♂) [NHMV]; Unit. Amaz. Taperinha b. Santarem, 21–31 VII ’27, Zerny (1♂) [NHMV]; Unit. Amaz. Taperinha b Santarem 1–10 VII ’27, Zerny, 86 (1♂) [NHMV]; Col. Becker 105713, Brasil: BA Jequié, 600–750 m, 11–22 xi 1995, V.O. Becker Col. [1 sex undet.]; Unit. Amaz. Taperinha b. Santarem 1–7 IX ’27, Zerny, 43, genitalia slide by JAL ♀ USNM 108, 870 (1♀); Unit. Amaz. Taperinha b. Santarem 1–10 VII ’27, Zerny, 43, Genitalia slide by JAL ♂ USNM 108, 880 (1♂). Cayman Islands (7♂, 1♀): N.B. Certain Cayman Islands specimens have multiple data labels with conflicting dates. 17 iv–26.viii 1938, Oxf. Un. Cayman Is. Biol. Exped., Coll. by C.B., G.H. Thompson, 18 v 1938, Cayman Brac., N. coast of Stakes Bay, Light trap A, [yellow tag PARATYPE, in errato], Proboscontia amica Munroe, CNC (2♂); 17 iv –26 viii 1938, Oxf. Un., Cayman Is. Biol. Exped., Coll. by C.B. Lewis, G.H. Thompson, 4 v 1938, Grand Cayman, N coast of Rum Point, Light trap (1♂) [BMNH] [red Holotype label, in errato]; 17 iv –26 viii 1938, Oxf. Un. Cayman Is. Biol. Exped., Coll. by C.B. Lewis, G.H. Thompson, 1 vii 1938, Grand Cayman, East end of Interior, The Cliff, Light trap, ♀ Pyralidae Brit. Mus. Slide No. 19801, Slide No. 3663 MS (1♀) [BMNH]; 17 iv –26 viii 1938, Oxf. Un. Cayman Is. Biol. Exped., Coll. by C.B. Lewis, G.H. Thompson, 14 v 1938, Grand Cayman, East End light trap B (1♂) [BMNH]; 17 iv –26 viii 1938, Oxf. Un. Cayman Is. Biol. Exped., Coll. by C.B. Lewis, G.H. Thompson, 7 v 1938, Grand Cayman, N coast of, Rum Point, Light trap (1♂) [BMNH]; 17 iv –26 viii 1938, Oxf. Un. Cayman Is. Biol. Exped., Coll. by C.B. Lewis, G.H. Thompson, 22 v 1938, Cayman Brac., N coast of Stakes Bay, Light trap A, ♂ Pyralidae Brit. Mus. Slide No. 19802 (1♂) [BMNH]; 17 iv –26 viii 1938, Oxf. Un. Cayman Is. Biol. Exped., Coll. by C.B. Lewis, G.H. Thompson, 11 vii 1938, Grand Cayman, N coast of North Side, Light trap B (1♂) [BMNH]. Costa Rica (5♂, 6♀, 1 sex undet.): Guanacaste. Voucher INBio data base Costa Rica 97-SRNP-2346 Testigo Base de datos INBio Costa Rica (1♂); Voucher INBio data base Costa Rica 97-SRNP-2354.2 Testigo Base de datos INBio Costa Rica, Genitalia slide ♀ by JAL(1♀); Voucher INBio data base Costa Rica 97-SRNP-2349 Testigo Base de datos INBio Costa Rica (1♀); Sirena, Corcovado Nat. Pk. Osa Penin. Costa Rica 10–12 Aug. 1980, D.H. Janzen & W. Hallwachs, Genitalia slide by DA ♀ USNM 107, 907, INBio Barcode #CR1001 115167, “Head illustrated” (1♀); Est. Cacao, 1000–1400 m, Lado SO Vol. Cacao, P.N.G. Prov. Guan, Costa Rica, C. Chaves, Abr 1991, L-N-323300, 375700 (1♀); INBio Barcode # CR1000 700522, Est. Santa Rosa, Prov. Guana, Costa Rica, 300 m, 25 Feb–5 MAR 1995, B. Gamboa, L N 313300 #4730 (1♀); INBio Barcode # CR1000 187409, Est. Sirena, 0–100 m, P.N. Corcovado, Prov. Punt., Costa Rica, G. Fonseca, May 1991, L-S-270500, 508800, INBio Barcode # CR1000 563282 (1♀); Voucher INBio data base Costa Rica 97-SRNP-2352 Testigo Base de datos INBio Costa Rica, “S. mootii #4” (1♂); Voucher INBio data base Costa Rica 97-SRNP-2354.1 Testigo Base de datos INBio Costa Rica, genitalia slide ♂ by JAL (1♂); Sirena, Corcovado Nat. Pk., Osa Penin., Costa Rica 5–11 Jan 1981, D.H. Janzen & W. Hallwachs, Genitalia slide by DA ♂ USNM 107, 908, INBio Barcode #CR1001 115168 (1♂); 80.SRNP.47, Santa Rosa National Park, Guanacaste Province, Costa Rica, D.H. Janzen, genitalia slide ♂ by JAL (1♂); 1sex undet.: Santa Rosa National Park, Guanacaste Prov., Costa Rica, 1 May 1980, D.J. Janzen & W. Hallwachs, INBio Barcode # CR1002 506841. Cuba (2♂):Col. Becker 72733, Cuba: Gtnmo Imias, 10 m, 17 vii 1990, V.O. Becker, 17, Genitalia slide by JAL ♂, USNM ENT 00808541, DNA 2012 (1♂); Col. Becker 73068, Cuba: Stgo. Siboney, 20 m, 23 vii 1990, V.O. Becker, 15, genitalia slide by JAL ♂, USNM ENT 00808540, DNA 2012 (1♂). Dominican Republic (1♂, CMNH): Same data as holotype, USNM ENT 00808539 [GenBank Accession # KC789516] (1♂). Jamaica (9♂, 1♀): Jamaica: Clar. Par., Mason River Station 4 mi NW Kellits , DNA 2012, 2200 ft, 16–19 Apr ’73, Don & Mignon Davis (1♂); Jamaica: Clar. Par., Portland Ridge nr. Jackson Bay Cave, 40 ft, 4 May 1973, Don & Mignon Davis (1♂); Jamaica: Ann Par., nr. Runaway Bay Cave, 50 ft, 1–2 May 1973, Don & Mignon Davis (1♂); Jamaica: Clar. Par., nr. Jackson Bay Cave, 1.5 mi SE Jack. Beach, 50 ft, 4 May 1973, Don & Mignon Davis (6♂, 1♀) [incl. 1♂ genitalia slide ♂ by JAL USNM 108, 879, + 1♀ genitalia slide ♀ by JAL USNM 108, 868]. Panama (5♂, 2♀males): Panama: Canal Zone, Barro Colorado Isl., 21 Mar 1979, Silberglied/Aiello, at light, 35, Genitalia slide by JAL USNM 108, 874 (1♂); Panama: Canal Zone, Barro Colorado Isl., 7 Mar 1979, Silberglied/Aiello, at light (1♂); Panama: Canal Zone, Barro Colorado Isl., 31 Mar 1979, Silberglied/Aiello, at light (1♂); Panama: Canal Zone, Barro Colorado Isl., 19 Mar 1979, Silberglied/Aiello, at light (1♂); Panama: Canal Zone, Barro Colorado Isl., 18 Mar 1979, Silberglied/Aiello, at light (1♂); Panama: Canal Zone, Barro Colorado Isl., 12 Mar 1979, Silberglied/Aiello, at light, 34, Genitalia slide ♀ by JAL USNM 108, 873 (1♀); Panama: Canal Zone, Barro Colorado Isl., 1 Apr 1979, Silberglied/Aiello, at light (1♀). Puerto Rico (4♂, 2♀): Puerto Rico, Guanica, Bosque Estatal de Guanica, 3.6 km E Guanica, 17-58-11N, 66-52-28W, Thornscrub, 100 m, 12 June 1996, J. Rawlins, R. Davidson, C. Young, M. Klingler, W. Zanol, S. Thompson, Carnegie Museum Specimen Number 65, 312 (1♂) [CMNH]; Puerto Rico, Guanica, Bosque Estatal de Guanica, 3.6 km E Guanica, 17-58-11N, 66-52-28W, thornscrub, 100 m, 12 June 1996, J. Rawlins, R. Davidson, C. Young, M. Klingler, W. Zanol, S. Thompson, Carnegie Museum Specimen Number 65, 695 (1♂) [CMNH]; Col. Becker 67784, Puerto Rico, Guanica, 170 m, 20 viii 1987, V.O. Becker (2♂, 1♀ ) [incl. 1 genitalia slide ♂ by JAL]; Col. Becker 67782, Puerto Rico, Guanica, 170 m, 20 viii 1987, V.O. Becker (1♀). Venezuela (3♂):Venezuela, Guarico, Hato Masaguaral, 45 km S Calabozo 8.57N, 67.58W, Galry For #10, 75 m, 23–24 Apr 1988, uv lt., M. Epstein & R. Blahnik (2♂); Venezuela, Guarico, Hato Masaguaral, 45 km S Calabozo 8.57N, 67.58W, Galry Forest #20, 75 m, 13–16 May 1988, uv lt., M. Epstein & R. Blahnik [x2 incl. 1w/Genitalia slide ♂ by JAL USNM 108, 869] (1♂) [Fig. 6].
British Virgin Islands (BVI) (29♂, 16♀): Brit. Virgin Isl., Guana Island, 0–80 m, 5–23 July 1985, S.E. & P.M. Miller, Clubhouse 60 m, U.V. light trap, 9–15 July 1985 (1♂); same as previous, 36, VOB ♀ USNM 108, 875 (1♀); Brit. Virgin Isl., Guana Island, 0–80 m, 5–23 July 1985, S.E. & P.M. Miller (5♂); same as previous, VOB ♂ USNM 95900 (1♂); Col. Becker 66651, Brit. Virgin Isl., Guana I., 0–80 m, 9–23.vii.1987, V.O. Becker & S.E. Miller, Genitalia 1260 (1 sex undet.); Col. Becker 70821, Brit. Virgin Isl., Guana X. 1989 V.O. Becker (10♂, 3♀) [incl. ♂ “slide 21”, hair pencils and abdominal coremata present]; same as previous, Genitalia slide by JAL ♂ (1♂, 1♀); same as previous, Genitalia 1261 and 1262 (2♀); Col. Becker 66649, Brit. Virgin Isl., Guana I., 0–80 m, 9–23 vii 1987, V.O. Becker & S.E. Miller, 11, Genitalia Slide ♂ by JAL (1♂) [hair pencils and abdominal coremata present]; Col. Becker 66651, Brit. Virgin Isl., Guana I, 0–80 m, 9–23 vii 1987, V.O. Becker & S.E. Miller (1♂, 2♀); Col. Becker 66650, Brit. Virgin Isl., Guana I., 0–80 m, 9–23 vii 1987, V.O. Becker & S.E. Miller (3♂, 1♀); Col. Becker 66649, Brit. Virgin Isl., Guana I, 0–80 m, 9–23 vii 1987, V.O. Becker & S.E. Miller (2♂, 5♀); same as previous, Genitalia 1296 [Abdomen remains on specimen (!)] (1♂); Brit. Virgin Isl., Guana Island, 0–80m, 13–26 July 1986, S.E. Miller & M.G. Pogue, North Bay, Coccoloba forest, U.V. light trap. sea level, 15–25 July 1986, [note label dates contradict], #25 (1♂); Brit. Virgin Isl., Guana I, 0–80 m, 24–31.x. 1990, S.E. Miller & T.M. Kuklenski, Collectors Bishop Museum, n. gen. + sp, Schacontiinae det. E.G. Munroe, 1991, Schacontia n. sp. det. M.A. Solis (1♂); Brit. Virgin Isl., Guana I, 0–80 m, 10–25.vii.1988, S.E. Miller & C. O’Connell, Colls. Bishop Museum, 89: 13 Apr #1 EGM (1♂); Brit. Virgin Isl., Guana Island, 1–14 July 1984, S.E. & P.M. Miller, 39, Genitalia Slide by JAL ♀ USNM 108, 878 (1♀); Anomalous BVI specimens with tibial hair pencils but no abdominal coremata (3♂): Col. Becker 70821, Brit. Virgin Isl., Guana X. 1989 V.O. Becker, 23, Genitalia slide ♂ by JAL (1♂); Col. Becker 66650, Brit. Virgin Isl., Guana I., 0–80 m 9–23.vii.1987 V.O. Becker & S.E. Miller, 13, Genitalia slide ♂ by JAL (1♂); Brit. Virgin Isl., Guana Island, 0–80 m, 5–23 July 1985, S.E. & P.M. Miller, Clubhouse, 60 m, U.V. light trap, 9–15 July 1985, 38, VOB ♂ USNM 108, 877 (1♂). Mexico (4♀). Chichen Itza. Yucatan, Mexico. EC Welling Coll. 5.III.1956 (1♀) [CNC]; Chichen Itza, Yucatan, Mexico, EC Welling Coll., 2.III.956 (1♀) [CNC]; Mexico, Mazatlan, July 10, 1969, El 50’, James H. Baker collection 1978 (1♀); Taboga Isl Pan, Febr 12 August, Busck (1♀). United States (1♀): Florida. “Sanibel Island, Lee Co., FLA.”, “30-VI-1984 Leg. W.L. Adair”, MGCL slide no. 687 [MGCL].
Habitus (Fig. 6). Unlike many Schacontia, there is little to no contrast between the medial area and the rest of the forewing; although this holds for both Schacontia rasa and Schacontia clotho as well, those two are readily distinguished on other grounds. Male Schacontia themis exhibit the full range of secondary sexual features known from the genus (flattened hind tibial spur, elongate hind tibial scales with embedded dark patch, epipleural setae, and concave metatarsal structure) as well as long abdominal coremata (Fig. 19; Table 1); Schacontia rasa (below), the putative sister species of Schacontia themis, has none of these (but see remarks). Two other Schacontia species (Schacontia clotho and Schacontia lachesis) do share these features in part, but not the genitalic configuration that characterizes Schacontia themis and Schacontia rasa (see below). Male and female genitalia (Figs 49–51). As with the remaining Schacontia, males of this species are most readily diagnosed by a combination of genitalic and external secondary sexual characteristics. The male genitalia best distinguish this species and its putative sister, Schacontia rasa (below), from other Schacontia: The uncus has a characteristic, expansive trefoil-shaped tip and lateral edges that appear swollen or re-enforced (as Schacontia lachesis and Schacontia atropos do), and a raised, pronounced medial ridge (as they do not). The intrasaccular flange is robust and forms a trigger-shaped process at the latero-ventral edge of the valva.
Species diagnoses for the Schacontia nyx complex based on male genitalia and secondary sexual characters. Format inspired by
| Thorax | Abdomen | Genitalia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flattened hind tibial spur | Elongatehind tibial scales | Dark patch embedded within tibial scales | Epipleural setal tufts | Concavemeta-tarsal structure | Coremata | Saccular bend or ulna | Ventro-medial setal comb | Distribution of ventro-marginal setae | Hook-shaped decumbent costal setal cluster | |
| themis | + | 0/+ | 0/+ | + | + | Short | NA | 0 | 0 | 0 |
| rasa | 0 | 0 | NA | 0 | 0 | NA | NA | 0 | 0 | 0 |
| nyx | + | + | 0 | 0 | + | Short | Round | + | Localized | 0 |
| clotho | + | + | + | + | + | Long | Round | + | Localized | + |
| lachesis | + | + | 0/+ | + | + | Short | Angled | 0 | Even | + |
| atropos | 0 | 0 | NA | 0 | 0 | NA | Angled | 0 | Even | + |
Male. (Fig. 6). Forewing length: 5.3–10.0 mm. Head - Ocelli present; proboscis with pale basal scales in males, light brown basal scales in females. Vertex and frons yellowish white in males, intermixed with brownish-gray scales in females; frons of normal (convex) contour; maxillary palpi uniformly grayish brown; labial palpi grayish brown in males, fading to gray apically in females; labial and maxillary palpi porrect, extending well beyond clypeus; antennal scape and pedicel yellowish white, flagellomeres grayish brown. Thorax - Thoracic collar, tegula and mesoscutum yellowish white in males, brownish gray in females. Males with flattened hind tibial apical spur, hind tibial tuft of long black scales intermixed with pale yellowish-white scales, epipleural setae, and shallow concave metatarsal modification. Females with two pair of hind tibial spurs (medial pair present). Forewingground color straw, with few contrasting markings other than jagged chocolate-brown antemedial and postmedial lines, postmedial line outwardly bulging only slightly, towards costa. Medial area unicolorous with basal area and postmedial areas. Fringe darkened apically. Hindwing. Almost uniformly pale, shaded brown at subterminal area. Abdomen - Dorsal surface straw brown. Scales arranged in two terminal black dorsal spots in males. Lobe-like extensions resembling rudimentary coremata on 4th abdominal sternum. Tympanal organs. (Fig. 26). As for ysticalis-themis group, vide supra. Male genitalia (Figs 49, 50) - Tegumen divided into two obliquely opposed oval sections meeting caudally near base of uncus and diverging widely anteriorly towards valvae. Teguminal sulcus short, such that anterior margin of tegumen appears deeply invaginate, two oblong teguminal lobes joined obliquely. Uncus with prominent medial ridge; uncal tip hastate, trefoil-like; lateral edges of uncus swollen, appearing re-enforced and lending a shovel- or scoop-like appearance to uncus. Gnathal plate narrowed to a transverse band with arms at each corner and a small, nub-like process arising centrally; dorsal arms wrap around anal tube while a ventral pair extend to termini of vinculum. Vinculum variously U-shaped or horseshoe shaped with pronounced pockets or eyelets at each terminus. Juxta robust, V-shaped; valvae complex, robust costa arising near vincular terminus, extending almost length of valva, and tapering to a point. A serrate, trigger-like ventral spine arises from ventral margin of sacculus; robust, spine-like setae at base of valva; saccular margin angled close to vinculum, not at saccular mid-point; ventro-marginal setae concentrated at venter or saccular ulna; valva with secondary fleshy subcostal setose lobe, setal plume recurved/decumbent. Elongate saccular process at outer saccular margin; saccular margin with several stout setae. Inner surface of valvae dorsal to saccular area with a small, circular setal cluster. Phallus moderately sclerotized; vesica with two large cornuti. Female genitalia (Fig. 51) - Papillae anales separate, not especially swollen; anterior and posterior apophyses threadlike, approximately equivalent in length; antrum/ductus bursae conspicuously sclerotized with a partial ventral collar or sleeve ventrally anterior to colliculum; a sharp constriction between ductus bursae and colliculum; colliculum a short sclerotized ring immediately posterior to corpus bursae with a narrow differentially sclerotized band around its center; corpus bursae membranous, more or less round, without signa or conspicuous appendices except single posterio-dorsal lobe; ductus seminalis appears to originate from antrum.
Unknown.
This species varies most obviously in size (5.3 mm–10.0 mm in male forewing length), and based on the examination of several anomalous specimens from the British Virgin Islands, the presence of male secondary sexual characteristics (tibial hair pencils and abdominal coremata) do not perfectly covary: Hair-penciled males with and without coremata are noted from Guana Island, examples annotated and/or segregated in “Material examined” section above; see also Discussion.
The specific epithet refers to the Greek Titaness and embodiment of divine order and is treated as a noun in apposition.
Possibly associated with Capparaceae. (http://janzen.sas.upenn.edu/caterpillars/database.lasso). Phenology, based on examined material: January–April, August in Costa Rica; March–April in Panama; April–May in Jamaica & Cayman Islands; July in Dominican Republic; June–July in Cuba; June, August in Puerto Rico; April–May in Venezuela; July–August in Brazil.
Brazil, Cayman Islands, Costa Rica, Cuba, Dominican Republic, Florida (USA), Jamaica, Mexico, Panama, Puerto Rico, Venezuela. The Sanibel Island, Florida record represents the only known United States occurrence of any Schacontia.
Schacontia themis is among the more widespread and collected species of Schacontia. It seems likely that more cryptic species (like Schacontia rasa, below) exist.
urn:lsid:zoobank.org:act:FDC54534-0A71-424E-9CFC-C48604E452F5
http://species-id.net/wiki/Schacontia_rasa
Figs 7, 27, 52–54(22♂, 32♀).
(3♂, 13♀), USNM. Holotype ♂ (Fig. 7). Mexico: Col. Becker 110514; Mexico: Tam San Fernando, 50 m, 28. vi. 1997, V. O. Becker Col.; USNM genitalia slide “JAL 18” Paratypes (2♂, 13♀, 4 sex undet.). Same data as holotype (2♂, 4 sex undet., 13♀ incl.1 with green USNM genitalia slide label “JAL 19.”
Cuba (3♂, 2♀):Col. Becker 72733, Cuba Gtnmo. Imias, 10 m, 17. vii. 1990, V.O. Becker, USNM ENT 00808543, DNA 2012 (1♂); Col. Becker 72409, Cuba: Holquin Mayari, 400 m, 12.vii.1990, V.O. Becker, USNM ENT 00808544, DNA 2012 (1♀); Santiago, Cuba, Collection Wm Schaus, Genitalia slide by DA ♂ USNM 108, 096 (1♂); Col. Becker 73068, Cuba: Stgo. Siboney, 20 m, 23.vii.1990, V.O. Becker, genitalia slide by JAL, 16 (1♂, 1♀). Dominican Republic (15♂, 13♀) [CMNH]: Dominican Republic: Pedernales, 14.5 km N Cabo Rojo, 18-03N, 71-39W, 165 m, 19 July 1990, J. Rawlins, C.W. Young, S.A. Thompson (1♂, 7♀); Dominican Republic: Pedernales, 9.5 km N Cabo Rojo, 18-02N, 71-39W, 35 m, 19 July 1990, J. Rawlins, C.W. Young, S.A. Thompson (5♂, 3♀); Dominican Republic: Pedernales, 14.5 km N Cabo Rojo, 10 m, 17-55N, 71-39W 26–27 September 1991, C. Young, B. Thompson, R. Davidson, J. Rawlins Coastal desert (2♂, 1♀); Dominican Republic: Pedernales, 14.5 km N Cabo Rojo, 18-03N, 71-39W, 165 m, 26–27 September 1991, C. Young, S. Thompson, R. Davidson, J. Rawlins, Arid thornscrub (1♂); Dominican Republic: Pedernales, 1 km W Cabo Rojo, 17-55N, 71-39W, 10 m, 30 July 1990, C.W. Young, J.E. Rawlins, S. Thompson (4♂, 1♀); Dominican Republic: Pedernales, Cabo Rojo, Sea level, 17-55N 71-39W, 21 Oct 1991, J. Rawlins, R. Davidson, C. Young, S. Thompson, Edge of salt marsh (1♂); Dominican Republic: La Altagracia, Parque del Este, Caseta Guaraguao, 4.4 km SE Bayahibe, 18-19-59N, 68-48-42W, 3 m, 26–27 May 2004, C. Young, J. Rawlins, J. Fetzner, C. Nunez, semi-humid forest near sea, limestone, UV light. Sample 51114 (1♀); Dominican Republic: La Altagracia, 2 km N Bayahibe, 10-23N, 68-51W, 10 m, 3 July 1992, C. Young, R. Davidson, S. Thompson, J. Rawlins, Dry seasonal forest on limestone, USNM ENT 00808542, DNA 2012 [GenBank Accession #KC789514] (1♂). British Virgin Islands (1♂, 2♀):Virgin Gorda BVI Prickley Pear Id, Vixen Pt, 14.IV.56, J.F.G. Clarke, 33, Genitalia Slide ♂ by JALUSNM 108, 872 (1♂); Col. Becker 66649, British Virgin Is., Guana I., 0–80 m. 9–23 vii 1987, V.O. Becker & S.E. Miller, #20, Genitalia Slide ♀ by JAL (1♀); British Virgin Is., Virgin Gorda Island, V. Gorda Peak, Ca. 400 m, 17–19 July 1986, S.E. Miller & M.G. Pogue, Black light trap in secondary moist forest, 37, Genitalia slide by JAL ♀ USNM 108, 876 (1♀).
(Figs 7, 52–54). Very similar to Schacontia themis (above), particularly with respect to the male genitalia, but lacking the secondary sexual characters enumerated above and the forewing ground color usually mousegray instead of straw colored. Female hind tibia with a single pair of spurs (medial pair absent - diagnostic within the ysticalis-themis group).
Male (Fig. 7). Forewing length: 7.0 mm–8.0 mm. Head - Ocelli present; proboscis normal; frons unmodified; labial palpi porrect, extending beyond clypeus. Thorax - Forewing. Medial area gray, unicolorous with basal area and postmedial areas; antemedial and postmedial lines jagged, darker gray. Hindwing. Postmedial line faint if present; outwardly tinged with bronze. Abdomen - Terga unicolorous with wings and thorax; scales arranged in two terminal black dorsal spots in males. Tympanal organs. (Fig. 27). As for ysticalis-themis group, vide supra. Male genitalia (Figs 52, 53) - Teguminal sulcus short, anterior margin of tegumen appears deeply invaginate, two oblong teguminal lobes joined obliquely; uncus trefoil shaped, outermost tip hastate; lateral edges of uncus swollen, appearing re-enforced; uncus with raised, pronounced medial ridge; juxta robust, V-shaped; valvae complex, intrasaccular flange at latero-ventral edge and sclerotized to form a trigger-shaped process; robust, spine-like setae at base of valva; saccular margin angled close to vinculum, not at saccular mid-point; ventro-marginal setae concentrated at saccular ulna; valva with secondary outer, oblong lobe or process below costa; fleshy setose lobe and recurved/decumbent setal plume associated with terminus of costa. Phallus moderately sclerotized; vesica with two large cornuti. Female genitalia (Fig. 54) - Very similar to Schacontia themis but posterior lobe of corpus bursae more pronounced, superficially rugose; both colliculum and sclerotized channel or sleeve of ductus more elongate than in Schacontia themis.
Unknown.
The specific epithet refers to the absence of male hind tibial and metatarsal structures and epipleural setal tufts (presumably secondary sexual characters) present in other Schacontia species (Table 1).
Unknown. Adults active June (Mexico), July (Cuba), and July, September (Dominican Republic).
Mexico, Cuba, Dominican Republic (essentially, vic. Gulf of Mexico).
Schacontia rasa is evidently the sister species of Schacontia themis. Were it not for the characters associated with the forewing ground color, female hind tibia, and male genitalia and given the homoplastic nature of certain male secondary sexual characters comparable to the system described by
It was suggested by V.O. Becker (pers. comm., following the submission of this work) that the name Dichogama fernaldi Möschler, 1890, the type material of which has apparently been lost from MNHU, might refer to this species (see Becker & Miller, in prep., for discussion) and that it should be placed in the now monotypic genus Dichochroma, whose description is, in turn, based on a single female (and only known specimen) of the type species, Dichochroma muralis Forbes, 1944. This attribution of the specimens we consider to fall within Schacontia themis (or Schacontia rasa, below) to Dichochroma fernaldi is, however, not well corroborated by any character identified in the original description by Möschler, but only by process of elimination and the report of its being reared on Capparis by
The Schacontia nyx complex: Some of these species are not readily diagnosed by a single character; each, rather, is characterized by either an absence of characters (as in Schacontia atropos) or by combinations of characters, all of them male, both genitalic and external, the latter presumably secondary sexual features.
urn:lsid:zoobank.org:act:B7C1FDB3-9A23-44BB-9CD4-8E1D8B8DBE78
http://species-id.net/wiki/Schacontia_nyx
Figs 8, 28, 33, 55–57Type material. Holotype (♂, USNM): Venezuela: Guarico, Hato Masaguaral, 45 km S Calabozo 8.57N, 67.58W, Galry For #10, 75 m, 23–24 April 1988 uv lt. M. Epstein & R. Blahnik. Paratypes (4♂, 2♀), USNM: Venezuela: Same data as holotype (1♂, 1♀); Guarico, Hato Masaguaral, 45 km S Calabozo 8.57N, 67.58W, Galry Forest #20, 75 m, 13–16 May 1988 uv lt. M. Epstein & R. Blahnik (2♂, 1♀); Lara, 4 km NW of La Pastora 2–3 III 1978 riparian forest blacklight, J.B. Heppner, Genitalia slide by DA ♂ USNM 108, 101 (1♂)
Habitus (Fig. 8). Although more readily diagnosed by the male genitalia, nyx can be differentiated on the basis of wing pattern. Nyx shares with other members of the genus (and in particular of the complex of sibling species to which it belongs) the configuration of the medial area, with its outward subcostal bulge, but its more mottled appearance and less uniformly contrasting ground coloration between the medial and both the antemedial and postmedial areas. Male Schacontia nyx does not bear the epipleural setae shared by most other members of the complex; nor do they exhibit a dark patch embedded within the elongate hind tibial scales (Table 1).Genitalia (Figs 55, 56). Male specimens of Schacontia nyx are most readily diagnosed by the obovate uncus, which is without pronounced medial ridges or lateral swellings. The subcostal processes are more conspicuous and elongate than in Schacontia themis or Schacontia rasa, but less narrow than in other species in the complex (Schacontia clotho, Schacontia lachesis, Schacontia atropos, or Schacontia androgyne).
Male (Fig. 8). Forewing length: 6.0–7.0 mm (n=3) (Female 7.3–7.7 mm; n=2). Head - Ocelli present; proboscis normal; frons of normal contour; labial and maxillary palpi drooping, extending beyond clypeus; vertex gray. Thorax - Tegulae uniformly gray brown; flattened hind tibial spur, specialized hind tibial scales, and shallow concave metatarsal modification all present; female with two pair of hind tibial spurs (medial pair present). Forewing. Antemedial and postmedial lines darkened at medial area, white towards basal and postmedial areas; white scales suffused both in basal area near anal margin and medial area, especially surrounding orbicular spot; patchy white scales in postmedial area; subterminal line unbroken; FW fringe dark brown, scales paler basad. Hindwing. White, shaded grayish brown towards margin; postmedial line absent; subterminal line unbroken; HW fringe gray, scales paler basad. Abdomen - Scales arranged in two terminal black dorsal spots in males. Short coremata on 4th abdominal segment (Fig. 33). Tympanal organs (Fig. 28). As for ysticalis-themis group, vide supra. Male genitalia (Figs 55, 56) - Teguminal sulcus short, anterior margin of tegumen appears deeply invaginate, two oblong teguminal lobes joined obliquely; uncus trefoil-shaped tip reduced to a small, more or less rhomboid nipple, edges simple, undifferentiated; juxta U-shaped, tapered ventrally; valva complex, intrasaccular flange at latero-ventral edge and sclerotized to form a trigger-shaped process; robust, spine-like setae at base of valva; saccular margin rounded at saccular mid-point; prominent setal comb at ventro-medial margin of valva; ventro-marginal setae concentrated at saccular ulna; valva with pronounced, elongate secondary outer lobe or process below costa; recurved/decumbent setal plume associated with terminus of costa. Phallus moderately sclerotized; vesica with two large cornuti. Female genitalia (Fig. 57) - Papillae anales separate, not especially swollen; antrum/ductus bursae present, diffusely sclerotized ventrally anterior to colliculum; colliculum present, short, sclerotized, immediately posterior to corpus bursae, with narrow differentially sclerotized band around center; ductus seminalis attached posterior of corpus bursae; corpus bursae round; small accessory bursa present; ductus seminalis originates at posterior end of corpus.
Unknown
Nyx, the primordial goddess of the night who according to myth stood at the beginning of creation, refers to the first of five closely related species in this complex. The specific epithet is treated as a noun in apposition.
Unknown. Adults March–May.
Northern Venezuela.
urn:lsid:zoobank.org:act:55C68C29-D157-4838-8F52-F277B9E1CA66
http://species-id.net/wiki/Schacontia_clotho
Figs 9, 29, 34, 58–60Type material. Holotype (♂, USNM) (Fig. 9): Ecuador: Loja Catamayo, 1300 m, 20. xii 1992, V.O. Becker Col., Col. Becker 102660, Genitalia 1287. Paratypes (1♂, 1♀), USNM. Ecuador: Same data as holotype (1♂, 1♀), the latter accompanied by “Genitalia Slide ♀ by JAL.”
Habitus (Fig. 9). This species superficially resembles Schacontia rasa in coloration and maculation; it is smaller and the male bears all of the secondary sexual characters, including coremata, known to occur within Schacontia (Table 1).Genitalia(Figs 58–60). The male genitalia of Schacontia clotho place it unambiguously within the Schacontia nyx complex as opposed to with Schacontia themis or Schacontia rasa. Moreover the subcostal lobe of the valva is elongate.
Male. (Fig. 9). Forewing length: 6.9–7.0 mm (n=3) (Female 6.8 mm). Head - Ocelli present; proboscis normal; frons of normal contour; labial palpi porrect, extending beyond clypeus. Thorax - Prothoracic collar and tegulae an admixture of brown and mouse-gray scales. Flattened hind tibial spur, specialized hind tibial scales, epipleural setae present, and dark patch amidst male hind tibial scales all present. Female with two pairs of hind tibial spurs (medial pair present); shallow concave metatarsal modification present. Forewing. More lanceolate than in other Schacontia species. More or less uniform mouse gray, with very light dusting of very pale gray in medial and postmedial areas; medial area more darkly shaded than basal area and postmedial areas; FW fringe brown; subterminal line unbroken. Hindwing. Nearly translucent; postmedial line absent; fringe pale yellowish; subterminal line unbroken. Abdomen - Scales arranged in two terminal black dorsal spots in males; elongate coremata on 4th abdominal segment (Fig. 34). Tympanal organs (Fig. 29). As for ysticalis-themis group, vide supra. Male genitalia (Figs 58, 59) - Uncus trefoil-shaped tip reduced to a small, more or less rhomboid nipple; lateral edges of uncus simple, undifferentiated; juxta broadly V-shaped, comparable in shape to an avian furcula, arms not robust; valvae complex, intrasaccular flange at latero-ventral edge and sclerotized to form a trigger-shaped process; robust, spine-like setae at base of valva; saccular margin rounded at mid-point; prominent setal comb at ventro-medial margin of valva; ventro-marginal setae concentrated at saccular ulna; costal bar diverges from subcostal lobe towards base of costa (isolation of costa >75% along length, character 49); valva with pronounced, elongate secondary outer lobe or process below costa; recurved/decumbent setal plume associated with terminus of costa; sharply hooked setal cluster prominent. Phallus moderately sclerotized; vesica with two large cornuti. Female genitalia (Fig. 60) - Papillae anales separate, not swollen; antrum/ductus bursae elongate (not chalice-like), faintly sclerotized posterior to colliculum, separated from colliculum by a sharp constriction; colliculum a short ring (not an elongate collar), with faintly sclerotized band, immediately posterior to corpus bursae; corpus more or less globular, surface complex; ductus seminalis originates at posterior end of corpus bursae.
Unknown.
The specific epithet refers to the youngest of the three fates in Greek mythology, responsible for spinning the thread of human life, and is treated as a noun in apposition.
Unknown. Adults December.
Southern Ecuador.
urn:lsid:zoobank.org:act:886A4F51-EA8A-4D43-9C58-FA7A1743BA1A
http://species-id.net/wiki/Schacontia_lachesis
Figs 10, 11, 30, 35, 61–63Type material. Holotype (♂, USNM) (Fig. 10): Brazil: Col. Becker 55439, Brasil: RJ Arrai al do Cabo, 50 m, 29.i.1985, V.O. Becker col. Paratypes 14 males, 11 females, USNM: Brazil: Same data as holotype (1♂, 1♀);Col. Becker 91635, Brasil: CE Pacatuba, 250 m, 6. iv. 1994, V.O. Becker Col. (1♂);Col. Becker 111257, Brasil: MT 60 km, S Poconé, 1–7.xii.1997, V.O. Becker Col. (1♀);Col. Becker 88540, Brasil: RO Cacaulãndia, 140 m, 15–18.x.1993, V. O. Becker Col. (1♀);Col. Becker 93888, Brasil: MT Chapada dos Guimarães, 800 m, 20.xi.1994, V. O. Becker Col. (1♀);Col. Becker 105713, Brasil: BA Jequié, 600–750 m, 11–22. xi.1995, V.O. Becker Col. (4♂, 1 ♀ and a disassociated male genitalic slide); Col. Becker 54553 Brasil: RJ Maricá, 5 m, 12–15.i.1995, V.O. Becker Col. (3♂, incl. 1 male w/white tag “Genitália 1259”, 1♀). Bolivia: Santa Cruz, Puerto Suarez, 150 m, Nov 1908, J. Steinbach, CMNH Acc. 3758 (3♂, 1 abd. detached, prob. ♀); Santa Cruz, Provincia del Sara, 350 m, October 1911, Jose Steinbach, CMNH Acc. 5038 (2♀); Santa Cruz, Provincia del Sara, 350 m, Dec 1912, Jose Steinbach, CMNH Acc. 5038 (1♀); Santa Cruz, Puerto Suarez, 150 m, Dec 1908, J. Steinbach, CMNH Acc. 3758 (2♂, 1♀, dissection).
Habitus (Figs 10–11). A polymorphic species, some specimens resembling Schacontia rasa and Schacontia clotho in showing little to no ground color contrast between the variably mouse-gray or straw-colored medial area and the rest of the forewing; other specimens display a sharper contrast, with the medial area primarily gray and the antemedial and postmedial areas straw colored, very similar to some specimens of Schacontia atropos (below). Like most members of the genus (other than Schacontia rasa; Table 1), Schacontia lachesis males bear specialized hind tibial scales, but like Schacontia nyx some specimens lack the dark patch embedded within them. Genitalia (Figs 61–63).Like those of Schacontia themis, Schacontia rasa, and Schacontia atropos, male genitalia of Schacontia lachesis have the uncus with raised or swollen edges, but do not share the other synapomorphies of the Schacontia themis-rasa pair and strongly resemble other members of the Schacontia nyx complex in bearing more elongate subcostal processes and ornate costae. Males of Schacontia lachesis are distinguished from those of Schacontia atropos (below), which they most closely resemble, by a combination of short coremata (Fig. 35), angled as opposed to rounded saccular bend (ulna), absence of a ventro-medial setal comb, and a more even distribution of ventro-marginal setae (Table 1).
(Figs 10, 11). Male. Forewing length: 5.0–7.5 mm. Head - Ocelli present; proboscis normal; frons of normal convex contour; labial palpi porrect, extending beyond clypeus. Thorax - Flattened hind tibial spur, specialized hind tibial scales, and epipleural setae present; female with two pair of hind tibial spurs (medial pair present); shallow concave metatarsal modification. Forewing. Prothoracic scaling tan gray, straw or yellowish. Forewing coloration equally variable, medial area polymorphic, exhibiting a range of contrast not known from other Schacontia, ranging from light to dark brown in both sexes, dusted with white; basal and postmedial areas straw colored; antemedial and postmedial lines jagged; orbicular spot faint but apparent; fringe scales darker at base; subterminal line unbroken. Hindwing. Postmedial line absent; fringe scales darker at base; subterminal line unbroken. Abdomen - Coremata on 4th abdominal segment (Fig. 35); scales arranged in two terminal black dorsal spots in males. Tympanal organs (Figs 30, 31). As for ysticalis-themis group, vide supra. Male genitalia (Figs 61, 62) - Teguminal sulcus short, such that anterior margin of tegumen appears deeply invaginate, two oblong teguminal lobes joined obliquely; lateral edges of uncus swollen, appearing reinforced; uncus trefoil-shaped tip reduced to a small, more or less rhomboid nipple; juxta U-shaped, tapered ventrally; valvae complex, intrasaccular flange transposed towards latero-ventral edge and sclerotized to form a trigger-shaped process; robust, spine-like setae at base of valva; saccular margin sharply angled at saccular mid-point; ventro-marginal setae distributed along length of outer margin of sacculus; valva with pronounced, elongate secondary outer lobe or process below costa; fleshy setose lobe and recurved/decumbent setal plume associated with terminus of costa; sharply hooked setal cluster prominent. Phallus moderately sclerotized; vesica with two large cornuti. Female genitalia (Fig. 63) - Papillae anales separate, unswollen; colliculum present, short, sclerotized, immediately posterior to corpus bursae, with narrow sclerotized band around center, sometimes separated from bursa by a sharp constriction; ductus bursae present, conspicuously sclerotized ventrally, entering corpus bursae dorsally; appendix bursae ventral, superficially complex; ductus seminalis attached at posterior end of ventral corpular out-pocketing.
Unknown.
In Greek mythology, Lachesis is the middle sister of the three fates, the personification of destiny responsible for measuring the duration of human life. The specific epithet is treated as a noun in apposition.
Unknown. Adults in Brazil active January, April, November, December; adults in Bolivia active October–December.
Central Brazil (Rondonia east to Bahia, Ceara and Rio de Janeiro), Bolivia (Santa Cruz).
urn:lsid:zoobank.org:act:D7D8AE98-0E23-4C74-8813-79F0B16CBF62
http://species-id.net/wiki/Schacontia_atropos
Figs 12, 32, 64, 65Type material. Holotype (♂, USNM) (Fig. 12): Venezuela: San Estaban Carabobo, Venez., Dec. 1–20 1939, Pablo J. Anduse. Paratypes (2♂), USNM. Venezuela: Same data as holotype (1♂); Lara, 4 km NW of La Pastora, 2–3 III 1978 riparian forest blacklight, J.B. Heppner (1♂).
Habitus (Fig. 12). Overlaps in appearance with Schacontia lachesis, but males lack all secondary sexual features; hindwing uniformly pale throughout. Genitalia (Figs 64, 65). Male genitalic features place it squarely in the nyx complex, from whose other member species it may be distinguished by the combination of the angled ventral edge of the saccus (ulna) and unmodified edges of the uncus.
(Fig. 12). Male. Forewing length: 5.4–5.5 mm (n= 2). Head - Ocelli present; proboscis normal; frons of normal contour; labial palpi porrect, extending beyond clypeus. Thorax - Prothoracic scaling light straw yellow. Female with two pair of hind tibial spurs (medial pair present). Forewing. Ground color staw yellow; medial area brown gray, heavily suffused with white scales, orbicular spot present. Hindwing. Pale overall, nearly translucent, not more darkly tinged subterminally as in other members of Schacontia nyx complex; postmedial line faint if present. Abdomen - Scales arranged in two terminal black dorsal spots in males. Tympanal organs (Fig. 32). As for ysticalis-themis group, vide supra. Male genitalia (Figs 64, 65) - Teguminal sulcus short, such that anterior margin of tegumen appears deeply invaginate, the two oblong teguminal lobes joined obliquely; uncus trefoil-shaped tip reduced to a small, more or less rhomboid nipple; juxta U-shaped, tapered ventrally; valvae complex, intrasaccular flange transposed towards latero-ventral edge and sclerotized to form a trigger-shaped process; robust, spine-like setae at base of valva; saccular margin sharply angled at saccular mid-point; ventro-marginal setae distributed along length of outer margin of sacculus; valva with pronounced, elongate secondary outer lobe or process below costa; fleshy setose lobe and recurved/decumbent setal plume associated with terminus of costa; sharply hooked setal cluster prominent. Phallus moderately sclerotized; vesica with two large cornuti. Female genitalia - Unknown.
Unknown.
The specific epithet refers to the third of the three fates. Treated as a noun in apposition.
Unknown.
Northern Venezuela.
Given the phenomenon described by
The moths treated in this paper compise a range of geographically widespread and potentially localized cryptic species united by a range of synapomorphies. It is not possible to infer an unambiguous center of origin for Schacontia; members of both the typical medalba group and the ysticalis-themis group are distributed from Mexico to South America. While we note at least three trans-isthmian species (Schacontia chanesalis, Schacontia ysticalis, and Schacontia themis), several species - including the entire Schacontia nyx complex - are restricted to South America, while Schacontia rasa is known only from Mexico and the Caribbean. Among the more intriguing features of Schacontia that warrant further study is their gall-forming habits in association with capparaceous plants, and the enormous variation in size, which may be a function of indeterminate instar number.
There likely exist undiscovered Schacontia species, cryptic and otherwise. Schacontia chanesalis in particular may represent a complex of Mayrian species that are difficult to diagnose without more extensive molecular data. However, available data are such that obvious breaks in the continuum of variation are not obvious, and rather than allowing the weakly articulated epithet Schacontia replica to persist as valid we have elected to synonymize it, along with Schacontia pfeifferi. Although its presumptive range differs geographically from that of S. chanesalis, we saw little point in retaining Schacontia pfeifferi given a lack of apparent distinguishing characters. Although its presumptive range differs geographically from that of Schacontia chanesalis, we saw little point in retaining Schacontia pfeifferi given a lack of apparent distinguishing characters. We took a less conservative approach within the Schacontia nyx complex, nominating species on the basis of characters we suspect may be more labile than our limited collection of specimens suggests. We attribute the lack of phylogenetic decisiveness, particularly in the nyx complex, to homoplasy among characters associated with the male secondary sexual features.
By far the richest source of phylogenetic signal in our matrix are the male genitalia, accounting for almost half the characters included in our analysis. This is not unusual for species-level studies of Lepidoptera (or insects generally), and there may be a growing consensus that despite their likely being subjected to sexual selection and thus potentially evolving quite rapidly (Fisherian runaway), male genitalic characters nonetheless contain valuable information at multiple hierarchic levels (
In contrast, it is of particular relevance to the taxonomy of Schacontia that relatively little complex variation is observed in the female genitalia, particularly given that four of the five species described prior to this work - two of which are synomized herein - have female holotypes.
Apropos of species diagnosis, we recall that as compelling as are the raw diversity of secondary sexual characters and the demonstrations of their phylogenetic lability, there have been suggestions that the expression of such structures may be underlain by rather simple genetic systems. Following
We wish to thank J. A. Lewis (JAL) for numerous dissections, D. Adamski (DA) for dissections and preliminary character interpretation, S. Escher for illustrations, T. Litwak for assistance with figures, E. Munroe (deceased) for making his then in-progress work available to M.A. Solis, and Axel Hausmann, The Natural History Museum, Munich, Germany for his hospitality. John Rawlins of the Carnegie Museum of Natural History provided a significant loan of specimens from Bolivia and the Dominican Republic. James Hayden eagerly shared suggestions for outgroups and character codings relevant to the diagnosis of Schacontia, supplied the Florida record, and reviewed an earlier version of this paper. Scott Miller and M. Rosati supervised the handling of DNA barcode data for Schacontia themis and Schacontia rasa and we acknowledge the Biodiversity Institute of Ontario for generating the sequences. Support for collection of Costa Rican specimens by was provided by NSF BSR 83-07887 and BSR 86-10149 to D.H. Janzen, the Philadelphia Academy of Sciences, and the University of Pennsylvania. Eugene Munroe inspired the collection of many of these moths by Dan Janzen and Winnie Hallwachs as part of the “Moths of Costa Rica” project. Matthias Nuss and an anonymous reviewer provided careful and constructive comments. Don Lafontaine generously edited the manuscript format in post-review. In particular, we wish to thank V.O. Becker for providing material that formed the basis for several new species descriptions, and to S. Miller and V.O. Becker for making certain material from the British Virgin Islands available to this study. DNA barcode sequences were provided by the University of Guelph under the iBOL project funded by Genome Canada. Paul Goldstein was supported a Specific Cooperative Agreement, USDA with Charles Mitter, Dept. of Entomology, University of Maryland.
Matrix
| 0 10 | 20 | 30 | 40 | 50 | 60 | ||
| medalba | 0011011-01 | 10110-0001 | 0001101000 | 011210-000 | 1110--010- | -0-10-00-0 | -001 |
| chanesalis | 0011011-01 | 10110-0001 | 0001101000 | 011210-000 | 1100--010- | -0-10-0100 | -001 |
| umbra | 0011011-01 | 10110-0-01 | 0001101000 | 011210-000 | 1110--010- | -0-?0----- | ---1 |
| speciosa | 0022011-01 | 10110-0002 | 1102101000 | 011210-000 | 2100--000- | -1010-1101 | 0001 |
| ysticalis | 1122011-11 | 00110-0102 | 1102101111 | 011110-001 | 21010-0120 | 0101101101 | 0011 |
| themis | 1102011010 | 0101111102 | 1102101111 | 1111111111 | 22010-0120 | 0101111111 | 1111 |
| rasa | 1102011010 | 01010-0002 | 1102101111 | 0111111111 | 22010-0120 | 0101111101 | 1111 |
| nyx | 1102011110 | 0101100102 | 1102101111 | 1111110001 | 3201111121 | 1101111111 | 1111 |
| clotho | 1102011010 | 0101111102 | 1102101111 | 1111110001 | 3201111121 | 1111111101 | 1111 |
| lachesis | 1102011110 | 01011*1102 | 1102101111 | 1111110101 | 3201100111 | 1111111111 | 1111 |
| atropos | 1102011110 | 01010-0-02 | 1102101111 | 0111110001 | 3201100111 | 111111---- | ---1 |
| Glaphyria | 1100100010 | 10000-0111 | 0010010110 | 000000-000 | 00?0--000- | -0-0111?-1 | 1010 |
| Hellula | 1100100-01 | 00000-0110 | 0010010110 | 000000-000 | 00?0--000- | ?0-00-10-1 | 1010 |
| Eustixia | 1111000--1 | 00-00-0111 | 0101010110 | 000000-000 | 00?0--000- | -0-0101101 | 1000 |