Citation: Sartori M (2014) The species of Thalerosphyrus Eaton, 1881 (Insecta, Ephemeroptera, Heptageniidae, Ecdyonurinae) in Java and Sumatra, with some comments on the diversity of the genus in the Oriental Realm. ZooKeys 420: 19–39. doi: 10.3897/zookeys.420.7904
Three species belonging to the genus Thalerosphyrus Eaton, 1881 are reported from Java and Sumatra. The nymphs of Th. determinatus (Walker, 1853) from Java, Th. sinuosus (Navás, 1933) from Java and Sumatra and Th. lamuriensis Sartori, 2014 from Sumatra are redescribed. The egg morphology of the three species is also presented for the first time. A key to the nymphs is proposed. General considerations on the composition of the genus Thalerosphyrus in the Oriental Realm are given. The distribution of the genus is greatly expended, and currently ranges over the Himalaya and Sumbawa in the Sunda Islands.
Thalerosphyrus determinatus, Thalerosphyrus sinuosus, Thalerosphyrus lamuriensis, Ecdyonurus sumatranus, distribution, Bali, Sumbawa, nymph, eggs, SEM
The genus Thalerosphyrus was created by
The concept of the genus Thalerosphyrus is far from being clear, because the type material of the type species, Thalerosphyrus determinatus is in bad state, missing all legs but one as well as the abdomen (hence the genitalia) (
When revising Ulmer's collection in the Zoologisches Museum in Hamburg,
Within the ongoing revision of Ulmer's collection (
Original material studied here is deposited in the following institutions:
ZMH Zoologisches Museum und Biozentrum Grindel, Hamburg, Germany
MZL Musée cantonal de zoologie, Lausanne, Switzerland
LIPI Lembaga Ilmu Pengetahuan Indonesia (Indonesian Institute of Sciences), Museum of Zoology, Bogor, Indonesia.
In the absence of adequate life stages to link nymph and adults as previously proposed by
Ontogenetic stage association relies thus on the following assumptions; three nymphal forms present together with three different egg morphologies, one species found only on Java, one on Java and Sumatra and the latter only on Sumatra.
Drawings were made with the help of a camera lucida taken from stereomicroscope Leica DM 750 and pictures from microscope Zeiss Axioscop 2 or Visionary Digital Passport II. Final digital drawings were performed on Adobe Illustrator CS6. For scanning electronic microscope (SEM) pictures, the eggs were dehydrated, carbon coated, and observed under a LEO 1525 at 5.00 kV; maxillae were dehydrated, critical point dried, and then platinum coated, and observed under a FEI Quanta 250 at 5.00 kV. Final plates were assembled in Adobe Photoshop CS6.
Medium to large Heptageniidae (up to 20 mm) with contrasting color patterns.
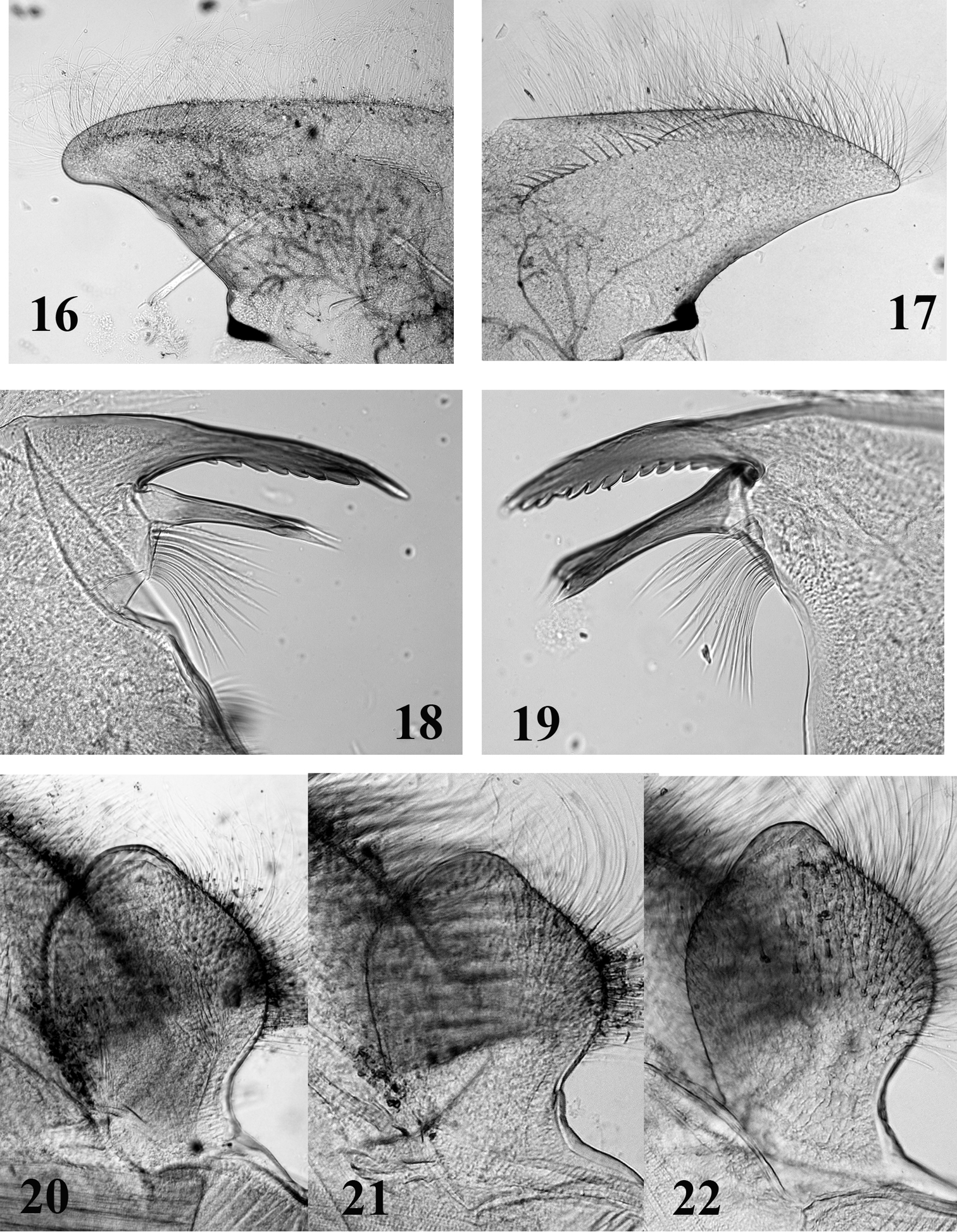
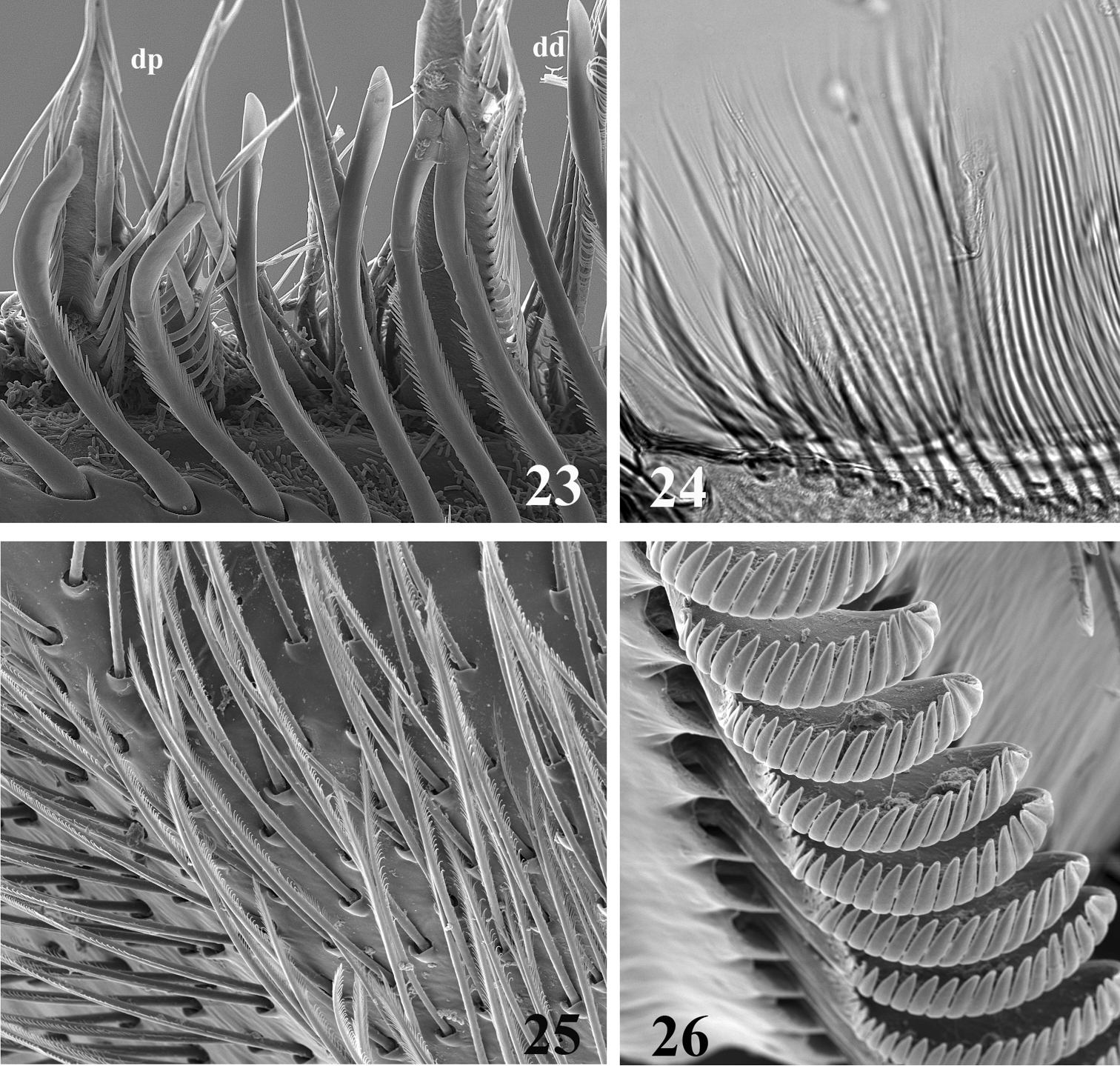
Head broad and thickened anteriorly (Figs 2, 5, 8); labrum (Figs 16–17) small, wider than long, without conspicuous median incision; mandibles (Figs 18–19) with outer margin covered with numerous thin setae, outer and inner incisors subequal in length, outer one saw-like on both sides, inner one trifid, left mandible with tuft of setae above mola; maxillae with 3-segmented palp, ventral surface of galea-lacinia covered with numerous long setae (Fig. 25), which appear entire in optical microscope, but are slightly feathered in SEM, crown of the galea-lacinia with 20–25 comb-shape setae, median ones bearing 12–17 teeth (Fig. 26), distal dentisetae bifid and fimbriate, as the proximal one (Figs 23–24); hypopharynx with robust lingua and well developed superlinguae bend backwards (Figs 27–28); labium with rhomboid glossae (Figs 20–22), paraglossae regularly curved, apex not bend backwards and moderately expended laterally.
Thalerosphyrus determinatus (Walker, 1853). 1 Habitus in dorsal view 2 Habitus in ventral view 3 Detail of abdominal segments VI–IX in ventral view.
Thalerosphyrus sinuosus (Navás, 1933). 4 Habitus in dorsal view 5 Habitus in ventral view 6 Detail of abdominal segments VI–IX in ventral view.
Thalerosphyrus lamuriensis Sartori, 2014. 7 Habitus in dorsal view 8 Habitus in ventral view 9 Detail of abdominal segments VI–IX in ventral view.
Thorax with pronotum slightly to greatly enlarged laterally; supracoxal spurs acute and well developed especially on mid- and hindlegs; femora rather similar between the three pairs of legs, row of stout and pointed bristles on inner and outer margins, no thin setae present; outer margin of fore tibia with few thin setae on proximal fourth, mid tibia with a row of thin setae on outer margin almost to tarsi, hind tibia (Figs 30, 32, 34) with two rows of thin setae, one on the outer margin, one in submarginal position, spine-like bristles absent or present.
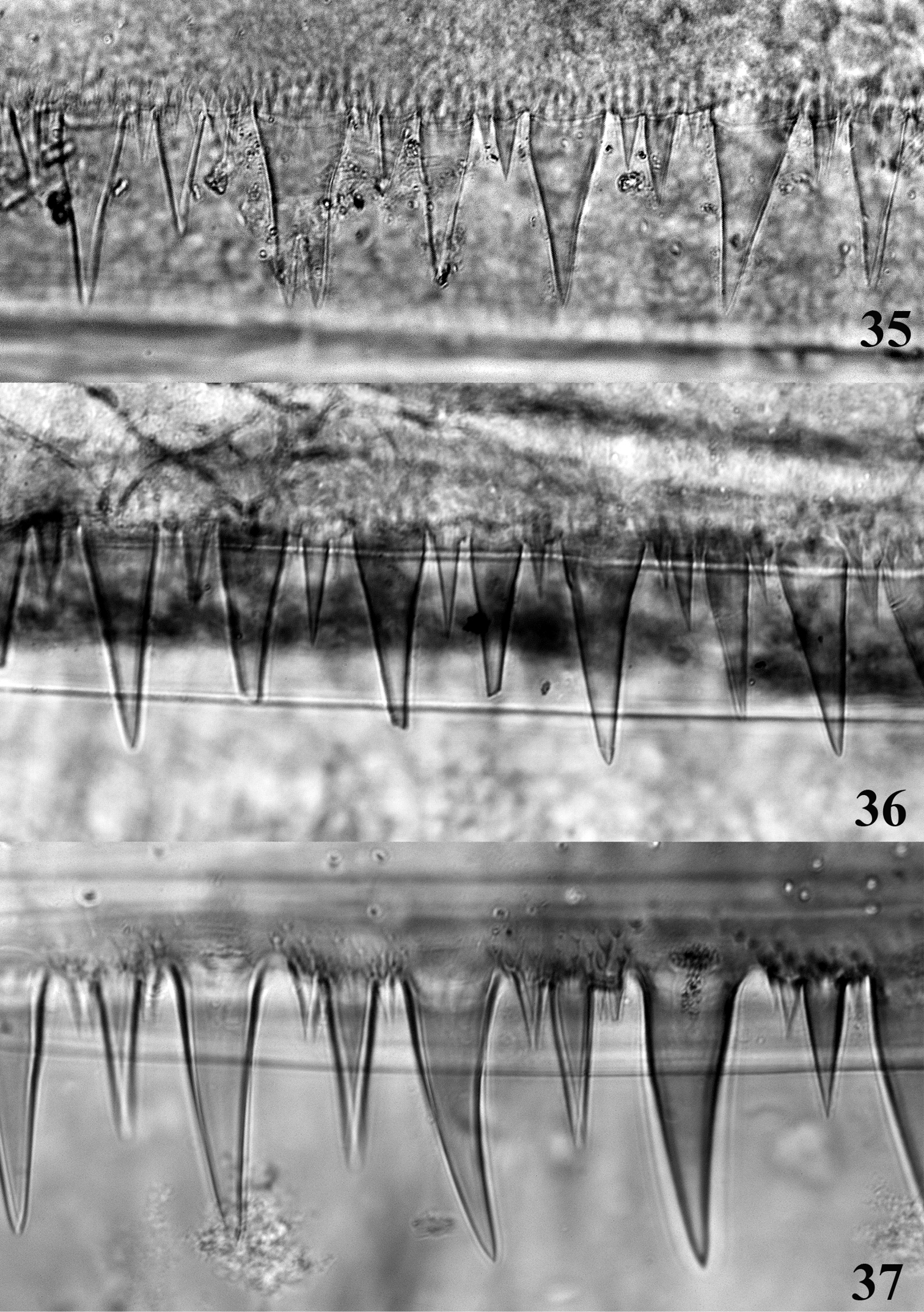
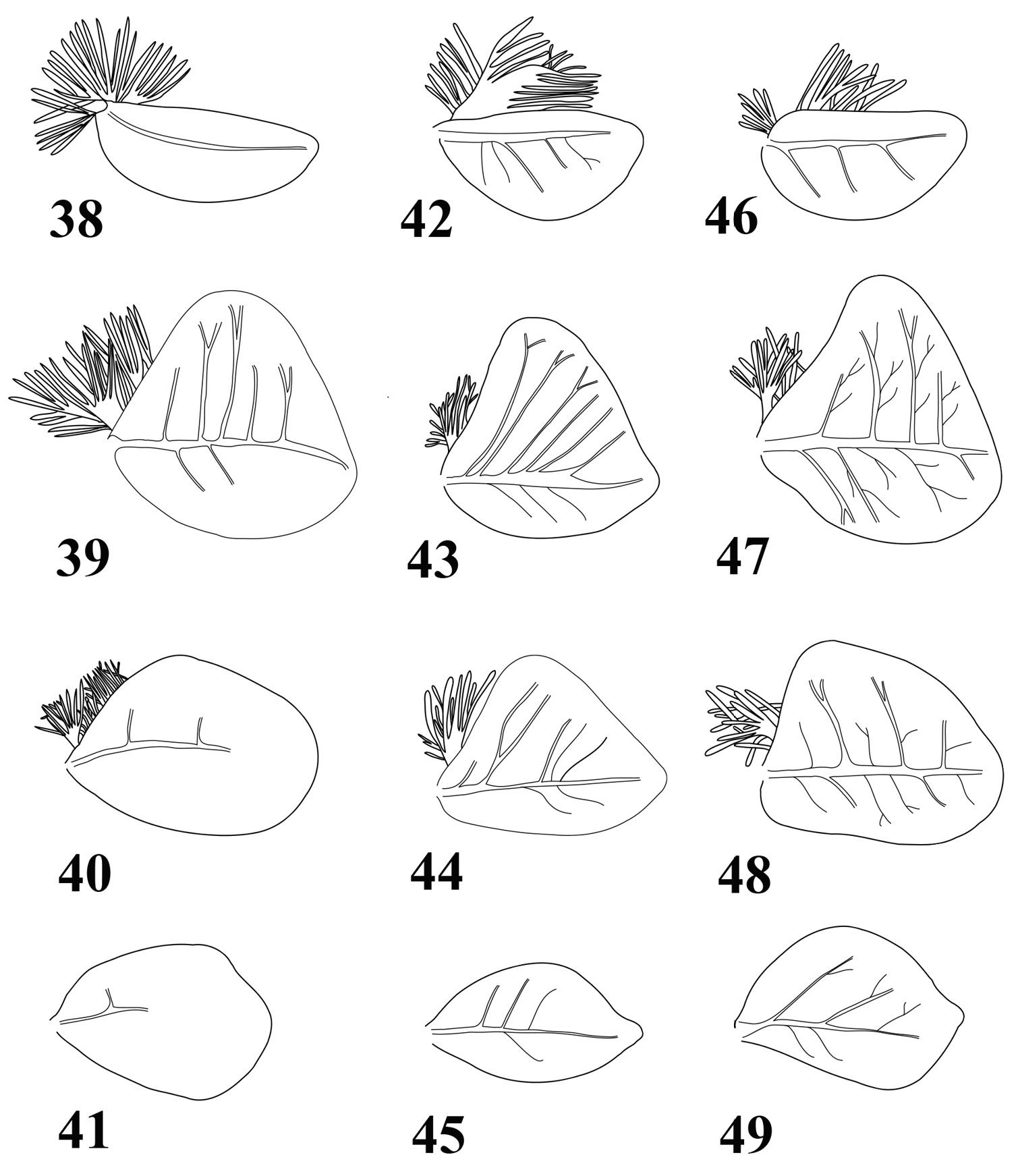
Abdomen with posterolateral projection generally greatly enlarged from segment III to VII or VIII (Figs 3, 6, 9); posterior margin of tergites (Figs 35–37) with large and pointed teeth, microdenticles present and generally numerous; all gills asymmetrical (Figs 38–49), gills I–VI with plate-like and extremely developed fibrillose parts, gill VII only plate-like; terminal filament well developed, cerci whitish with more or less enlarged brown bands; segments with whorls of stout and pointed setae.
The scattered setae on the ventral surface of the maxilla indicate clearly that Thalerosphyrus belong to the subfamily Ecdyonurinae. The presence of acute supracoxal spurs, the anterior margin of the head thickened and generally well developed posterolateral projections of the abdomen are discriminating characters according to
Thalerosphyrus determinatus (Walker, 1853): Java
Thalerosphyrus sinuosus (Navás, 1933): Java, Sumatra
Thalerosphyrus vietnamensis (Dang, 1967): Vietnam
Thalerosphyrus bishopi Braasch & Soldán, 1986: West-Malaysia
Thalerosphyrus flowersi Venkataraman & Sivamarakrishnan, 1987: South India
Thalerosphyrus lamuriensis Sartori, 2014: Sumatra
The species described by
The genus Thalerosphyrus is endemic to the Oriental Realm. It is known from India, through Southeast Asia (Thailand, Vietnam, West Malaysia), up to Sumbawa in the Sunda Islands (see below), suggesting, as for Rhithrogena (
2 nymphs, Java, Diengplateau, stream Seraju (D13), ca 1950 m a.s.l., 5.VI.1929, Prof. Thienemann leg. [ZMH]; 1 nymph entirely mounted on microscopic slide, Java, Gedeh Panggerango, Tjisarua, 1050 m, 10.VIII.1930, Dr. Lieftinck leg [ZMH]; 1nymph, Java, Java Barat Province, rocky stream at Cibodas (CL 2186), 1300 m, 3.XI.1985, J.T. & D.A. Polhemus leg [MZL]; 1 nymph, Bali, Baturiti, Desa Antapan, 815 m, 8°19.34'S, 115°11.61'E, 9.X.2009 (BLI005), M. Balke & D. Amran leg [MZL]; 1 nymph, Sumbawa, Nusa Tenggara Barat Province, Madsewu River, 2 km above Badindi, 61 km NW of Bima (CL 2174), 750 m, 20.X.1985, J.T. & D.A. Polhemus leg [MZL].
Eggs extracted from a female imago (caught together with a male imago) and identified by Ulmer as Thalerosphyrus determinatus: West Java, Tjibodas, Tjiwalen Bridge, 1400 m, 4.IX.1932, Dr Lieftinck leg [ZMH].
Body size: up to at least 14.5 mm (not full grown nymph).
Coloration pattern: see Figs 1–2.
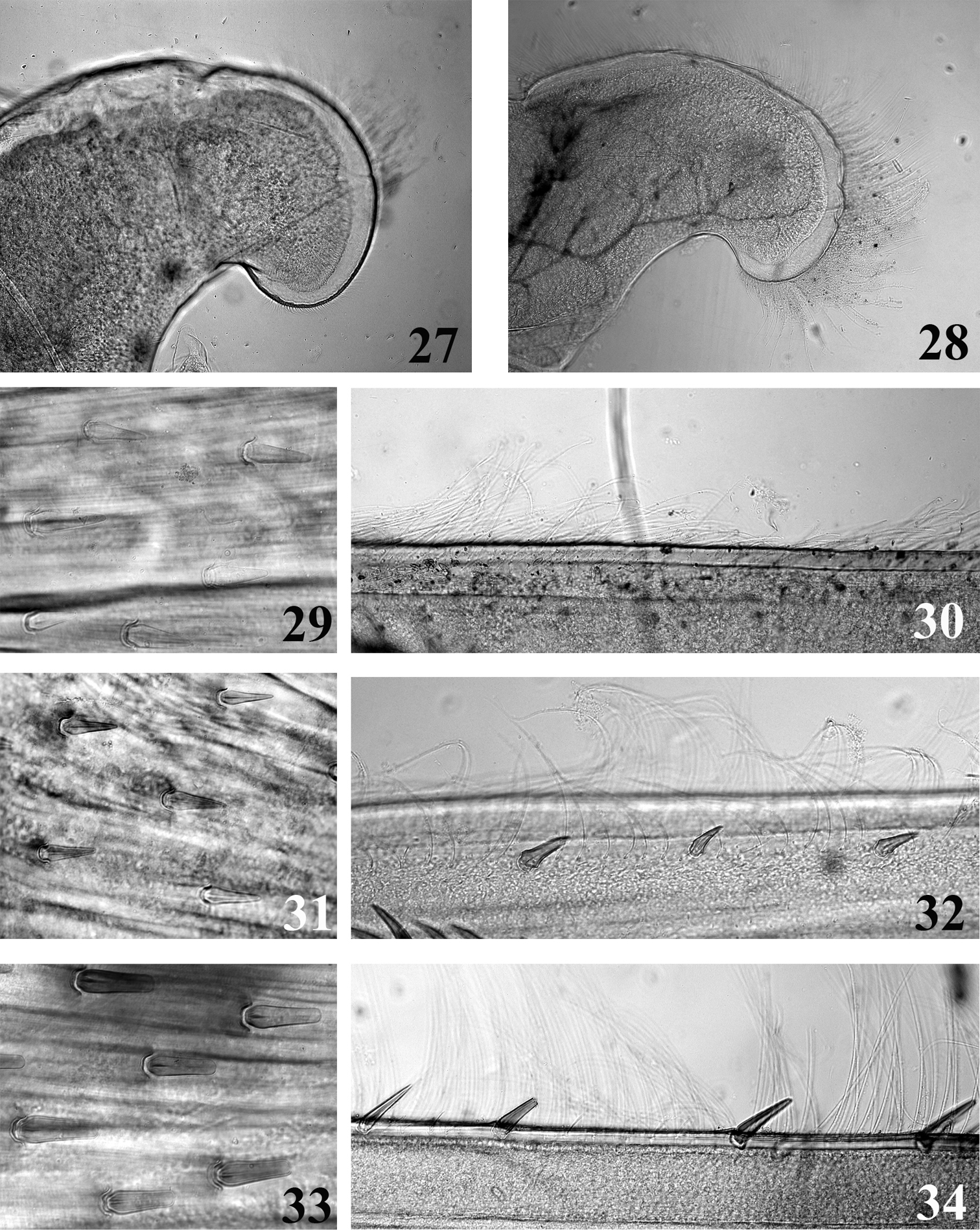
Head. Labrum moderately expended laterally, less than 4 times larger than long, with rounded apexes (as in Fig. 16); dorsal surface and anterior margin covered with long and thin setae; ventral surface with a median arch of less than 10 strong and pointed setae. Crown of the galea-lacinia of the maxillae composed of ca. 25 comb-shape setae, the median ones bearing 12–15 teeth. Right mandible with 5–6 bifid and fimbriate setae below the inner incisor and ca. 10 long simple and thin setae below the mola; left mandible with 8–9 simple and fimbriate setae below the inner incisor and ca. 9–10 long simple and thin setae below the mola. Hypopharynx with robust lingua bearing a tuft of small setae, superlinguae densely covered with long and thin setae replaced before the apex by very small setae up to the lower part of the superlinguae (Fig. 27). Labium with glossae rhomboid, slightly concave on their outer margin near apex (Fig. 20), dorsal surface with three stout setae and numerous thin and simple setae.
Thorax. Pronotum weakly expended laterally (Fig. 1). Femora with submarginal rows of pointed bristles on the inner and outer margins, increasing in numbers from the fore to the hind leg. Bristles on the upper face of hind femora arrow-shaped, clearly pointed (Fig. 29). Hind tibia (Fig. 30) without any bristles in outer marginal or submarginal position. Tarsal claw with 2–3 teeth.
Abdomen. Posterolateral expansions not developed on segments I–II, increasing in size from segment III to VII where it may reach the middle of segment VIII, shorter on segment VIII (Fig. 3) and comparable proportionally to those of segments V–VI. Gill I (Fig. 38) with elongated and rounded plate, ca 2.5× longer than wide; gill IV strongly asymmetrical (Fig. 39), wider than long, gill VI and VII oval and asymmetrical with obtuse apex (Figs 40–41). Posterior margin of tergites with irregular pointed teeth, and numerous microdenticles (Fig. 35). Cerci rather unicolor medium brown, some segments darker in the proximal half.
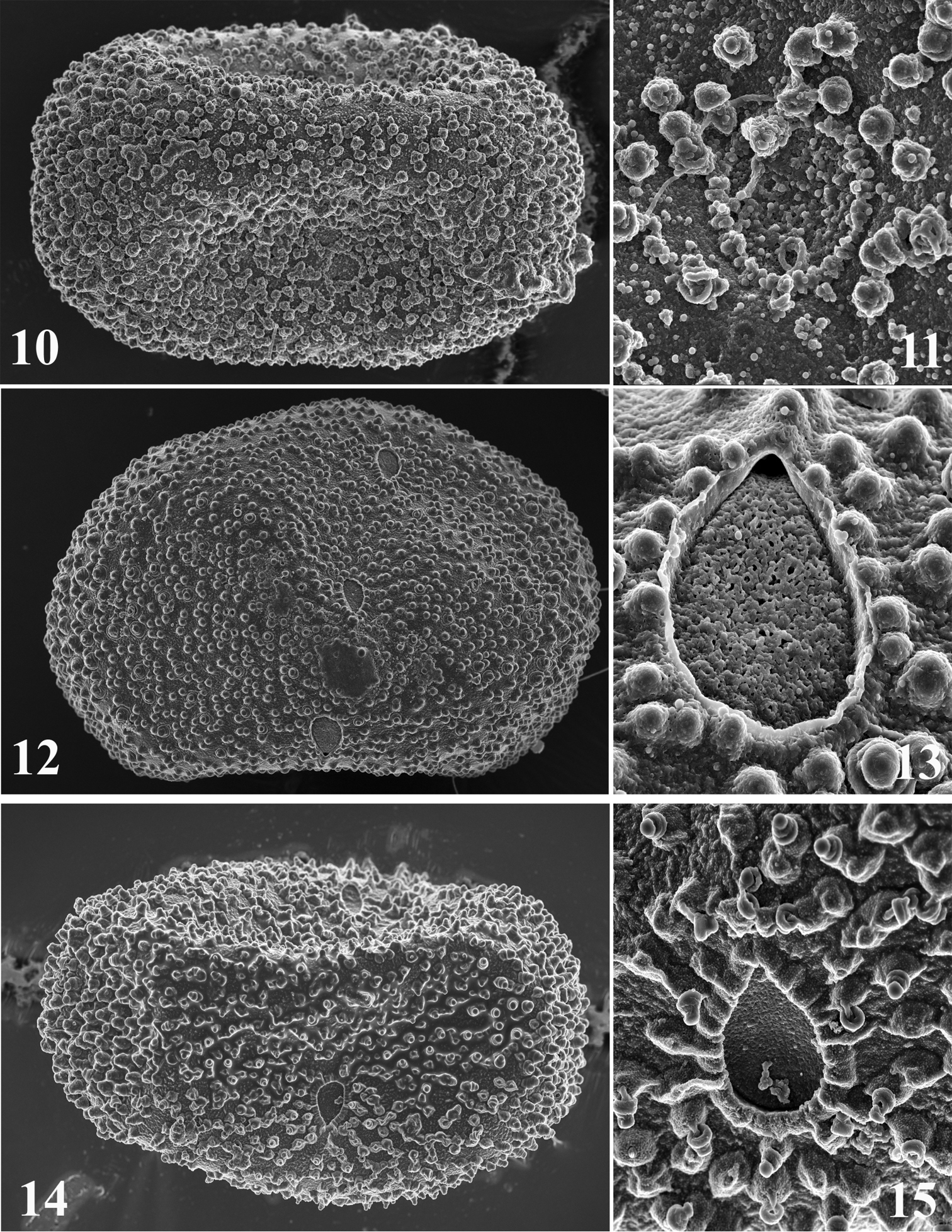
Size: ca 120 µm × 75 µm; chorion regularly covered by small KCT'S, (1.0–1.5 µm), a little bit larger at poles (Fig. 10), and by microgranules (< 0.3 µm); margin of micropyle irregular and formed by microgranules (Fig. 11).
Egg structure of Thalerosphyrus determinatus (10–11), Thalerosphyrus sinuosus (12–13), Thalerosphyrus lamuriensis (14–15). 10, 12, 14 Egg in toto 11, 13, 15 Details of the micropyle and chorionic structures.
The abdominal pattern of the nymph is the one which is the closest to the one of the male imago redescribed by
The species may be easily recognized from its relatives mainly by the weak posterolateral expansions of the abdomen, and the absence of bristles on the outer margin of the hind tibiae.
Thalerosphyrus determinatus as defined here is the less common species found in the investigated area. However it is reported from Bali and Sumbawa for the first time. The species is absent from Sumatra and seems to live in middle to high altitudes, based on the few available data.
4 nymphs, two partially mounted on two microscopic slides, Sumatra, Singkarak, stream at Subanpass (F20), 1000 m, 4.III.1929, Prof. Thienemann leg [ZMH]; 1 nymph, Sumatra, Tjurup, Kali Dzernih, forested stream (M9), 7.V.1929, Prof. Thienemann leg [ZMH]; 1 nymph, Sumatra, Ranau, stream in primary forest (R25c), 29.I.1929, Prof. Thienemann leg [ZMH]; 2 nymphs, one partially mounted on a microscopic slide, Java, Gurung Ungaran, XII. 1909, Jacobson leg [ZMH]; 1 nymph, Java, Kali Tjiwalen near Tjibodas, 1350 m, in mosses and dead leaves (FY7f), 10.VII.1929, Prof. Feuerborn leg [ZMH]; 1 nymph, West Java, stream in Tjibodas, under the “mountain garden” (FY14c), 15.VII.1929, Prof. Feuerborn leg [ZMH]. [All specimens sub. nom Thalerosphyrus determinatus det. Ulmer].
10 nymphs, Java Tengah, Wonosobo-Kertek village road, creek, 800 m, 7°21.68'S, 109°55.67'E, 10.X.2011 (JVA011), M. Balke leg. [LIPI, MZL]; 2 nymphs, Sumatra Barat, Sijunjung / Muara area, forest, 488 m, 00°40.10'S, 101°07.26'E, 10.XI.2011 (UN7), M. Balke leg [MZL]; 7 nymphs, one entirely mounted on a microscopic slide, Sumatra Barat, Universitas Andalas campus, forest stream, 360 m, 00°54.67'S, 100°28.38'E, 8.XI.2011 (UN1), M. Balke leg [MZL]; 2 nymphs, Sumatra Barat, Lubukbargalung, Lubuk Paraku River, 50 km south Solok, 420 m, 100°32.50'E, 0°56.75'S, 25.V.2010 (SU5), J.-M. Elouard leg [MZL].
Eggs extracted from a female imago: Java, Buitenzorg, 13.II.1932, Dr Lieftinck leg [ZMH], and from a female subimago: Western Sumatra, Danau di Atas, stream near the road, 1000–1100 m (FF20e), 16.III.1929, Prof. Feuerborn leg [ZMH] and identified by Ulmer as Thalerosphyrus sinuosus.
One specimen from Sumatra (SU5) and one from Java (JVA011) have been used for the study by
Body size: up to at least 10.5 mm (not full grown nymph).
Coloration pattern: see Figs 4–5.
Head. Labrum slightly expended laterally, ca 3.5 times larger than long, with rounded apexes (Fig. 16); dorsal surface and anterior margin covered with long and thin setae; ventral surface with a median arch of ca 10 strong and pointed setae. Crown of the galea-lacinia of the maxillae composed of ca 25 comb-shape setae, the median ones bearing 12–15 teeth (Fig. 26). Right mandible with 7–8 fimbriate setae below the inner incisor and ca. 5–6 long simple and thin setae below the mola; left mandible with 10–11 simple and fimbriate setae below the inner incisor and ca. 8–9 long simple and thin setae below the mola. Hypopharynx with robust lingua bearing a tuft of small setae, superlinguae densely covered with long and thin setae replaced before the apex by very small setae up to the lower part of the superlinguae. Labium with glossae rhomboid, slightly concave on their inner margin near apex (Fig. 21), dorsal surface with three stout setae and numerous thin and simple setae.
Mouthparts structure of Thalerosphyrus determinatus (20), Thalerosphyrus sinuosus (16, 21) and Thalerosphyrus lamuriensis (17, 18, 19, 22). 16–17 Hemi-labrum 18 Left mandible 19 Right mandible 20–22 Labial glossa.
SEM (23, 25, 26) and optic (24) pictures of maxillar structure. 23–24 Dentisetae of Thalerosphyrus lamuriensis dp: proximal dentisetae, dd distal dentisetae 25 Scattered setae on the ventral face of the galea-lacinia of Thalerosphyrus sinuosus 26 Comb-shape setae on the crown of the galea-lacinia of Thalerosphyrus sinuosus.
Thorax. Pronotum slightly expended laterally and posteriorly (Fig. 4). Femora with submarginal rows of pointed bristles on the inner and outer margins, increasing in numbers from the fore to the hind leg. Bristles on the upper face of hind femora arrow-shaped, clearly pointed (Fig. 31). Hind tibia with a row of 6–7 arrow-shaped bristles in submarginal position (Fig. 32). Tarsal claw with 2–3 teeth.
Mouthpart (27–28) and thoracic (29–34) structures of Thalerosphyrus determinatus (27, 29, 30), Thalerosphyrus sinuosus (31, 32) and Thalerosphyrus lamuriensis (28, 33, 34). 27–28 Apex of superlingua of hypopharynx 29, 31, 33 Bristles on the dorsal face of hind femur 30, 32, 34 Outer margin of hind tibia.
Abdomen. Posterolateral expansions not developed on segment I, weakly developed on segment II, strongly developed on segment III and increasing in size up to VII where they may be as long as segment VIII, shorter on segment VIII and smaller proportionally to those of segments III (Fig. 6). Gill I with elongated and rounded plate, less than two times longer than wide (Fig. 42); gill III–VI strongly asymmetrical, wider than long (Figs 43–44), gill VII oval and asymmetrical with inner concave margin near apex (Fig. 45). Posterior margin of tergites with irregular pointed teeth, and numerous microdenticles (Fig. 36). Cerci whitish in proximal part, with dark brown segment every two or three, distal part more uniformly medium brown.
Posterior margin of abdominal tergite IV. 35 Thalerosphyrus determinatus 36 Thalerosphyrus sinuosus 37 Thalerosphyrus lamuriensis.
Gills of Thalerosphyrus determinatus (38–41), Thalerosphyrus sinuosus (42–45) and Thalerosphyrus lamuriensis (46–49). 38, 42, 46 Gill I 39, 43, 47 Gill IV 40, 44, 48 Gill VI 41, 45, 49 Gill VII.
Size: ca 130–140 µm × 85–90 µm; chorion regularly covered by small KCT'S, (1.5–2.0 µm), a little bit larger at poles (Fig. 12), and by mesogranules (1.0 µm); margin of micropyle smooth and entire (Fig. 13).
The nymph mentioned here includes what
The eggs of Thalerosphyrus sinuosus differ from those of Thalerosphyrus determinatus by the margin of the micropyle and by the presence of mesogranules on the chorion.
Thalerosphyrus sinuosus is present on Java and Sumatra. We cannot confirm the occurrence of the species outside these two islands, although based on egg morphology, and some partial details of the nymph (
Besides the type material mentioned in
1 nymph, Sumatra, Singkarak, stream at Subanpass (F19), 1000 m, 4.III.1929, Prof. Thienemann leg [ZMH]; 3 nymph, one partially mouted on microscopic slide, Sumatra, Toba area, stream south of Balige (FT13), 8.IV.1929, Prof. Feuerborn leg [ZMH]; 2 nymphs, Sumatra, Toba area, Balige, stream at ca 1100 m (T13), 5.IV.1929, Prof. Feuerborn leg [ZMH] [All specimens sub. nom Thalerosphyrus determinatus det. Ulmer].
1 nymph, Sumatra Utara Province, swift stream 20 km East of Parlilitan (CL 2192), 1070 m, 10.XI.1985, J.T. & D.A. Polhemus leg [MZL]; 2 nymphs, Sumatra Barat, Tarusan, upstream Tarusan, 10 m, 100°29.84'E, 1°13.61'S, 24.V.2010 (SU3), J.-M. Elouard leg [MZL]; 2 nymphs, Sumatra Barat, Kotobarapak, upstream Kototbarapack, 100 m, 100°32.08'E, 1°13.78'S, 24.V.2010 (SU4), J.-M. Elouard leg [MZL]; 4 nymphs, Sumatra Barat, Lubukbargalung, Lubuk Paraku River, 50 km south Solok, 420 m, 100°32.50'E, 0°56.75'S, 25.V.2010 (SU5), J.-M. Elouard leg [MZL].
Eggs extracted from the mature female nymph mentioned above from Polhemus collected specimens.
Three specimens (SU3, SU4, SU5) have been used for the study by
Body size: up to 21 mm (full grown female nymph).
Coloration pattern: see Figs 7–8.
Head. Labrum greatly expended laterally, ca 4 times larger than long, with narrow and somewhat acute apexes (Fig. 17); dorsal surface and anterior margin covered with long and thin setae; ventral surface with a long median arch of ca. 20 strong and pointed setae ending close to the anterior margin. Crown of the galea-lacinia of the maxillae composed of ca. 20 comb-shape setae, the median ones bearing 12–14 teeth. Right mandible (Fig. 19) with 11–12 fimbriate setae below the inner incisor and 5 long simple and thin setae below the mola; left mandible (Fig. 18) with 8–9 fimbriate setae below the inner incisor and ca. 8–9 long simple and thin setae below the mola. Hypopharynx with robust lingua bearing a tuft of small setae, superlinguae densely covered with long and thin setae up to the lower part of the superlinguae (Fig. 28). Labium with glossae rhomboid, clearly concave on their inner and outer margins near apex (Fig. 22), dorsal surface with numerous stout setae and numerous thin and simple setae.
Thorax. Pronotum greatly expended laterally and posteriorly (Fig. 7). Femora with submarginal rows of pointed bristles on the inner and outer margins, only slightly increasing in numbers from the fore to the hind leg. Bristles on the upper face of hind femora with subparallel or slightly convergent margins, apex rounded or truncate (Fig. 33). Outer margin of hind tibia with a row of 12–15 pointed bristles in marginal or submarginal position (Fig. 34). Tarsal claw with 3–4 teeth.
Abdomen. Posterolateral expansions not developed on segments I–II, moderately developed on segment III and strongly increasing in size up to VIII where they may be longer than segment IX (Fig. 9). Gill I with asymmetrical elongated and rounded plate, less than two times longer than wide (Fig. 46); gill III–VI strongly asymmetrical, wider than long (Figs 47–48), gill VII oval and asymmetrical with slightly pointed apex (Fig. 49). Posterior margin of tergites with long and pointed teeth regularly alternating with two small ones, and few microdenticles (Fig. 37). Cerci whitish with 3–4 dark brown bands increasing in size towards the apex.
Size: ca 140–150 µm × 85–90 µm; chorion regularly covered by pedunculate KCT'S, (1.0–1.5 µm), a little bit larger at poles (Fig. 14), no micro- or mesogranules present; margin of micropyle edged, as formed by fused peduncles (Fig. 15).
A major surprise was to find nymphs of Thalerosphyrus lamuriensis among the material identified by
Thalerosphyrus lamuriensis possesses anyway far more characters in common with Thalerosphyrus determinatus and Thalerosphyrus sinuosus than the observed (although quite obvious) differences, and there is no reason on this basis to propose other generic rearrangement for Ecdyonuroides/g(1).
Eggs of Thalerosphyrus lamuriensis are very peculiar with pedunculate KCT'S, which distinguish them from the two other species.
Thalerosphyrus lamuriensis is the most abundant Thalerosphyrus species in Sumatra, and seems widespread throughout the island. In several places, it has been found together with Thalerosphyrus sinuosus.
| 1 | Posterolateral expansions on the abdomen greatly enlarged (Fig. 8), reaching their maximum on segment VIII; protonum greatly enlarged laterally (Fig. 7); bristles on the dorsal face of hind femora truncate or rounded at apex (Fig. 33); hypopharynx with outer margin of superlinguae evenly covered with long setae (Fig. 28) | Thalerosphyrus lamuriensis |
| – | Posterolateral expansions of the abdomen more or less developed, those of segment VIII always shorter than those of segment VII (Figs 3, 6); pronotum moderately enlarged laterally; bristles on the dorsal face of hind femora arrow-shaped (Figs 29, 31); hypopharynx with outer margin of superlinguae covered with long setae ending at apex by minute setae (Fig. 27) | 2 |
| 2 | Hind tibia with only two rows of thin setae (Fig. 30); posterolateral expansions of the abdomen weakly developed (Fig. 3); gill I more than 2.5 times longer than wide (Fig. 38) | Thalerosphyrus determinatus |
| – | Hind tibia with two rows of thin setae and a submarginal row of arrow-shape bristles (Fig. 32); posterolateral expansions of the abdomen strongly developed (Fig. 6); gill I less than 2 times longer than wide (Fig. 42) | Thalerosphyrus sinuosus |
The ZMH collections housed few male imagos of Thalerosphyrus, namely a single male of Thalerosphyrus determinatus and two of Thalerosphyrus sinuosus. These have been described in details by
I am indebted to the staff of the Zoologisches Museum Universität Hamburg (ZMH) for allowing me to study Ulmer's, collection, especially to Kai Schütte and Hossein Rajaei. My appreciation goes to Michael Balke (Munich) and Jean-Marc Elouard (Montpellier) for putting their collections at my disposal, and to Jeff Webb (Missoula, USA) for useful comments. Technical assistance with SEM pictures by Geneviève L’Eplattenier and Raphael Grand (MZL) and Renate Walter (ZMH) was essential and they are warmly thanked for their help.