(C) 2013 Rick Hochberg. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Lepidodasys is described from sublittoral sandy sediments off the Atlantic coast of Florida. Lepidodasys ligni sp. n. is a small species (≤ 450 µm) with a crossed-helical pattern of small, non-keeled, non-imbricated scales on the dorsal and lateral body surfaces, two columns of ventral, interciliary scales that form a herringbone pattern, and a series of anterior, lateral, dorsal and posterior adhesive tubes. Similar to Lepidodasys castoroides from the Faroe Islands, the new species possesses a caudal constriction that demarcates the posterior end containing the caudal organ. The frontal organ lies within the posterior constriction, which is heavily invested with somatic circular muscles. These muscles are also present throughout the trunk and represent a novel condition for species of Lepidodasys, which were previously considered to lack somatic circular muscles. Posterior of the caudal constriction is a large, barrel-shaped caudal organ that is wrapped in a series of interdigitating, spindle-shaped, incomplete circular muscle fibers. The caudal organ contains a sclerotized central canal, but the absence of distal cuticular endpieces distinguishes the new species from its morphologically similar congener, Lepidodasys castoroides.
Meiofauna, Florida, sublittoral, Capron Shoal, taxonomy, confocal
Marine gastrotrichs are common members of the meiobenthos in intertidal and subtidal sandy sediments worldwide, and recent studies of various waters around the Caribbean – from the southern Atlantic waters of Florida to Panama - have revealed a wealth of previously undescribed species (
While most species of Lepidodasys are identified based on the structure of their cuticle and the distribution and abundance of their adhesive tubes, two species are known to possess large reproductive organs that further differentiate them from the remaining members of the genus. Lepidodasys castoroides Clausen, 2004 possesses a large, barrel-shaped caudal organ with cuticular endpieces, and Lepidodasys tsushmainensis Lee & Chang, 2011 possesses a pyriform-shaped caudal organ without cuticular endpieces. During a recent sampling expedition to Capron Shoal, Florida, we discovered a previously undescribed species of Lepidodasys with a caudal organ reminiscent of the two aforementioned species. Herein, we describe this new species and provide detailed information on its caudal organ using f-actin staining and confocal laser scanning microscopy.
In June 2012, approximately 18 liters of sediment were collected via anchor dredge from 9 m depth at Capron Shoal, Florida (27°26’52” N, 80°13’81” W). Meiofauna were extracted from the sediments using the anesthetization-decantation techinique with 7% MgCl2 and a 53 µm mesh. Specimens were sorted with a Leica EZ4 stereomicroscope, transferred to a glass slide, and viewed with a Zeiss A1 Axioscope equipped with DIC (differential interference contrast) and a Sony Handycam digital camera. Measurements of all specimens were performed with an ocular micrometer, and the size and positions of various organs are described in terms of percentage body units: anterior (U00) to posterior (U100) is 100 units.
For phalloidin staining of f-actin, we fixed two specimens in 4% paraformaldehyde in 0.1M phosphate buffer saline (PBS, pH 7.3) for 1 h, rinsed them in 0.1M PBS + 1% Triton X-100 (PBT) for 1 hr, and then stained them in Alexa Fluor 488 Phalloidin for 1 hr (following the manufacturer’s protocol, Invitrogen). Stained specimens were then briefly rinsed in PBS and mounted directly in Fluoromount G on glass microscope slides with coverslips.
Phalloidin-stained specimens were examined on a Zeiss LSM 510 confocal microscope system at the Smithsonian Marine Station (Fort Pierce, Florida). Zeiss Zen 2009 software (Carl Zeiss Microimaging, Thornwood, NY) was used to collect a series of 0.25-0.4 mm optical sections with maximum intensity projection along the z-axis. Confocal images were saved as TIF files and rendered into 3-D images using Volocity software (Perkin Elmer). Carnoy V 2.0 (© 2001 Peter Schols) was used to measure various organs in the digital images.
One stained specimen was removed from Fluoromount G after examination and prepared for museum archival with the following procedure: 1 hr rinse with PBS followed by 1% OsO4 in 0.1M PBS for 10 min (to increase contrast); rinse in PBS for 1 hr; dehydrate in an ethanol series; transfer to propylene oxide for 30 min; embed in resin (epon) on a glass microscope slide and place in an oven at 60º C for 48 hrs. The type specimen is deposited in the National Museum of Natural History, Smithsonian Institution, Washington, DC.
Grain size was determined on a subsample of the sediments by drying them out in a 60º C oven for 24 hrs, and then sieving them with a Gilson SS-15 sieve shaker with mesh sizes 2 mm, 1 mm, 500 µm, 250 µm, 125 µm and 63 µm. Sieve fraction weights were entered into the program GRANPLOTS with line segment (
urn:lsid:zoobank.org:act:41A72CCB-BEAD-4207-BDA5-71F36B350C05
http://species-id.net/wiki/Lepidodasys_ligni
Figs 1–3Sediment from 9 m depth at Capron Shoal, Florida (27°26'52"N, 80°13'81"W), collected by Mr. Woody Lee. Granulometry as follows: Mean size 1.66 phi; SD 0.72 phi; skewness -0.95; and kurtosis 5.11. The sediments can be characterized as medium to fine grain sand with a large proportion of the sand fraction larger than the median (positive kurtosis).
Adult specimen, reproductively mature, 450 µm long; resin preparation: USNM # 1202682.
Digital video of two adult specimens submitted to the National Museum of Natural History, Smithsonian Institution, Washington, DC. Scale patterns and reproductive anatomy visible in videos.
Eleven specimens were examined alive. Digital video was captured of three specimens. Two of the eleven specimens were prepared for phalloidin staining. One of the phalloidin-stained specimens was prepared for archival.
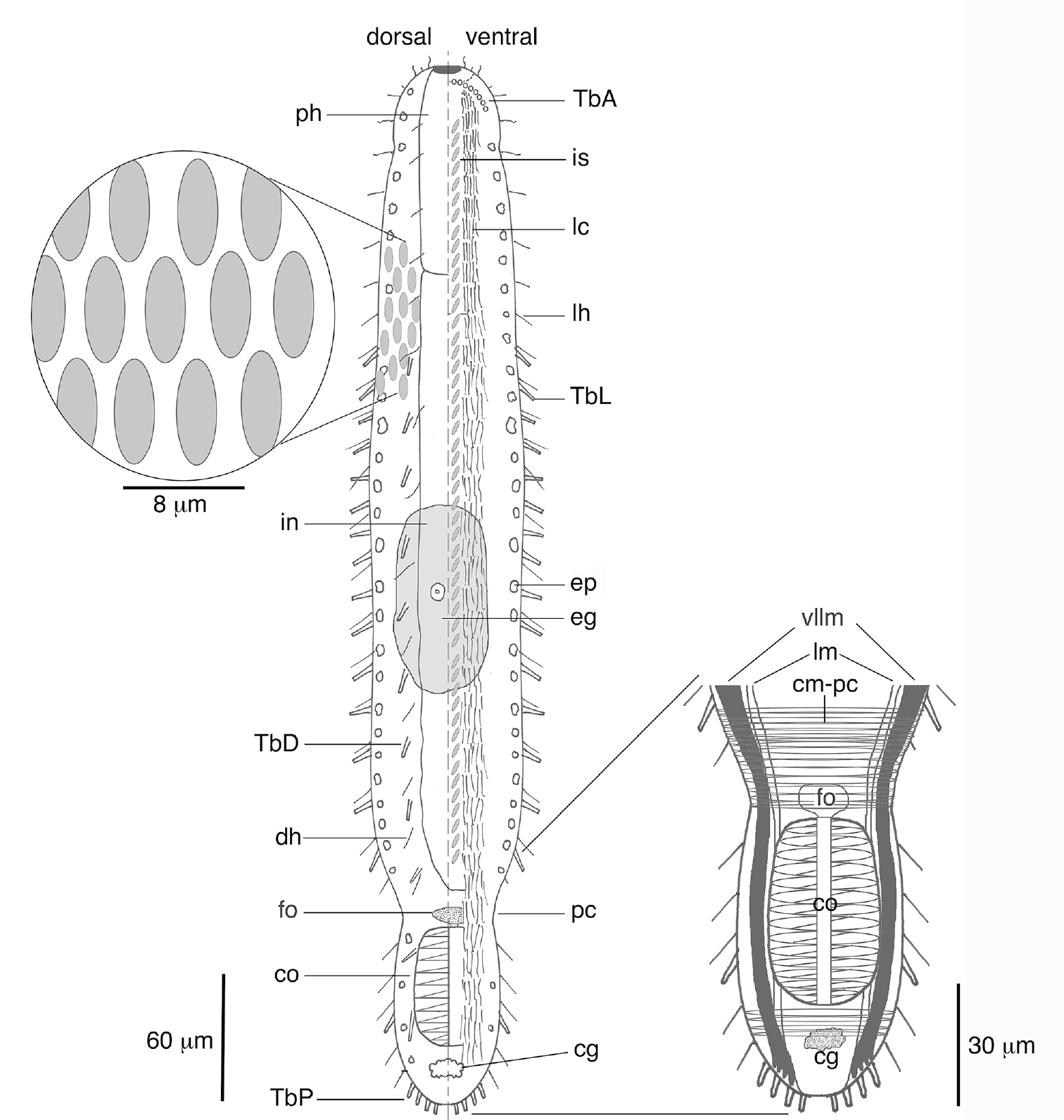
Lepidodasys with a strap-shaped adult body to 450 µm long. The body possesses two constrictions at approximately U06–07 and U82; the first constriction demarcates the approximate mid-region of the pharynx, and the second constriction demarcates the posterior region containing the caudal organ. Maximum body width at U06/pharyngeo-intestinal junction (PhIJ:U20)/midpoint of body (U50) is 32/44/50 µm, respectively. Pharynx to 90 µm long. Body covered with eye-shaped, non-imbricating scales arranged in a crossed-helical pattern across the dorsal and lateral surfaces; two columns of ventral interciliary scales present at midline in herringbone pattern. Up to eight anterior adhesive tubes (TbA) per side inserting directly on body surface and forming an arc toward the lateral body wall. Lateral adhesive tubes (TbL) present, up to 14 per side, beginning posterior of the PhIJ. Dorsal adhesive tubes (TbD) present, up to 11 per side, beginning at the PhIJ and extending to the caudal end. Eight posterior adhesive tubes (TbP) insert terminally on rounded caudal end. Frontal organ and large barrel-shaped caudal organ with strong circular muscles and inner canal present; ovaries and testes not observed.
The Latin ligni (wood) refers to Mr. William “Woody” Lee at the Smithsonian Marine Station at Fort Pierce, Florida, who assisted in the collection of the species and has been instrumental over the past decade in sublittoral collection of meiofauna for the first author (RH).
The description is based on the holotype (adult, 445 µm long; Fig. 2A), with ranges provided from specimens measured in vivo. Body strap-shaped with two notable constrictions at mid-pharynx (U06-07) and caudal end (~U82); first constriction demarcates the approximate mid-length of the pharynx and the second constriction demarcates the region of the accessory copulatory organs (Figs 1, 2A). Pharynx 90 mm long with no pharyngeal pores observed. Few sensory hairs to 8 µm long line the mouth. Stiff sensory hairs 8–15 µm are present along the ventrolateral, lateral and dorsolateral margins of the body. Ventral locomotory cilia present as two columns, each approximately 8 µm wide, that extend from ca. U05 to the posterior end (Fig. 1). Numerous small (2–5 µm) epidermal glands along the margins of the body.
Cuticular armature. A crossed-helical pattern of up to eight columns of elliptical scales extend across the dorsal body wall; the number of scales per column varies from 8-10 in the pharyngeal region to up to 12 in the trunk. At least three columns of elliptical scales extend around the lateral body margins and reach the ventral locomotory cilia (Fig. 1). All scales are similar in shape, lack a keel, and are approximately 7–8 µm long. None of the scales are imbricated. Two columns of ventral scales are present at the midline between the ciliary fields (Figs 1, 2C). The scales are relatively small, ca. 4–5 µm long, and oriented in a herringbone pattern in most specimens, with the anterior end of both scales farther apart from each other (ca. 5 µm) relative to their posterior ends (ca. 2–3 µm). While the herringbone pattern is evident in all specimens, some specimens had individual scales (not an entire column) oriented in a more parallel fashion (e.g., see Fig 2C); this may be evidence of individual variation in scale orientation. There was approximately 4–5 µm of space between each column and its adjacent ciliary field.
Adhesive tubes.Anterior adhesive tubes (TbA) distributed as a posteriorly curving arc of seven to eight tubes, 3–4 µm long, from the midline to the lateral body wall (Fig. 2C). Lateral adhesive tubes (TbL) absent from the pharyngeal region (Fig. 1). Fourteen to seventeen TbL present in the trunk region, 8–10 µm long, beginning at U28 and extending to the second body constriction. Tubes are spaced evenly down most of the trunk. A single tube is present in the demarcated region of the caudal organ, around U92. Approximately eleven evenly spaced dorsal adhesive tubes (TbD), 8–10 µm long, beginng at U28 and extending to ~U90. Eight posterior adhesive tubes (TbP), 9–10 µm long, distributed as four adhesive tubes per side on rounded caudum.
Digestive tract.Small terminal mouth to 5 µm wide (Fig. 2B). Pharynx to 20 µm wide and 90 µm long. Pharnygeo-intestinal junction at U20. No pharyngeal pores observed. Intestine narrow and tapering toward posterior end. Diatoms present in a single specimen. Anus at U78.
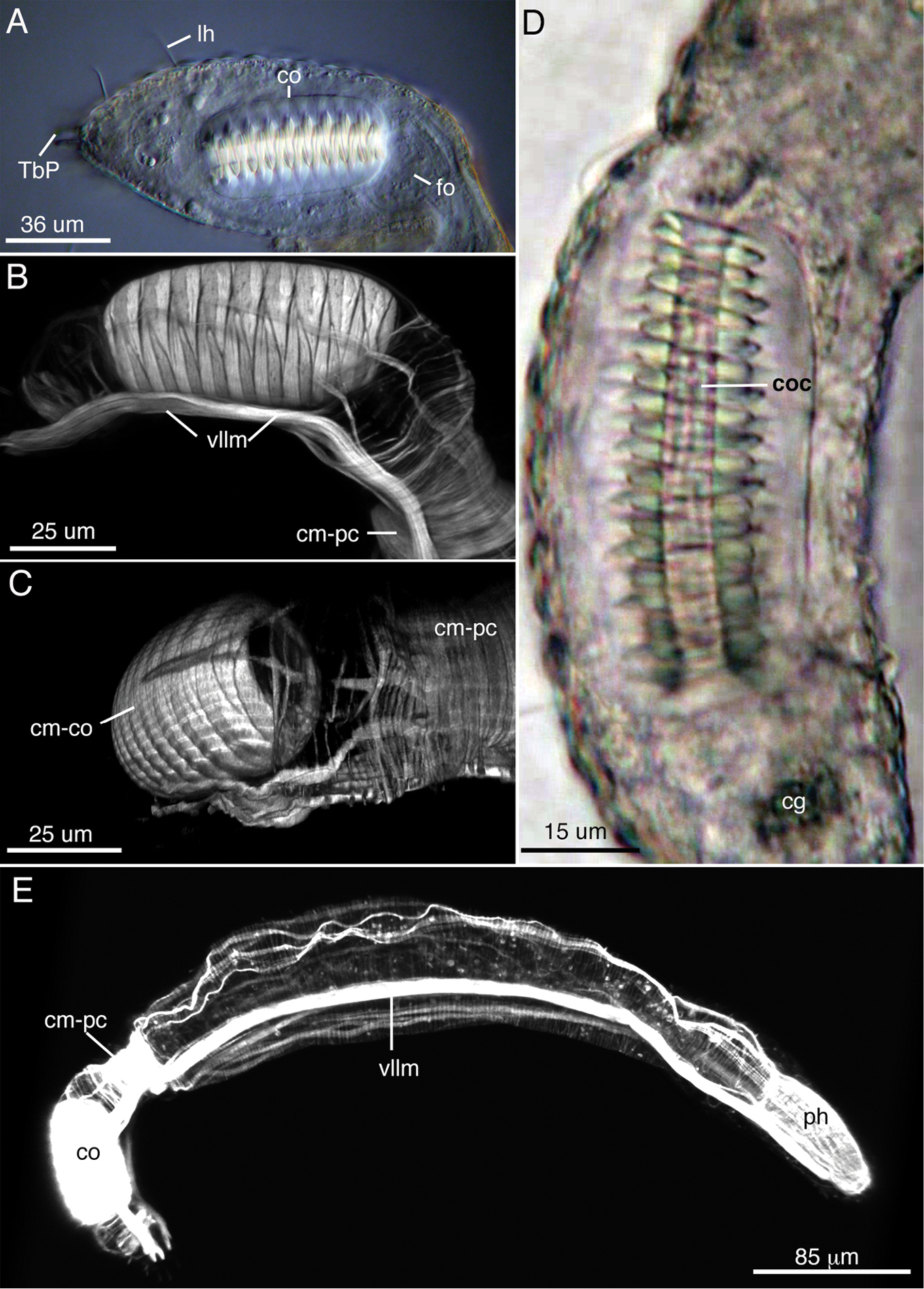
Reproductive system.Neither testes or ovaries observed. A single large egg, approximately 62 µm diameter, was present in one adult (holotype). A clear, sac-like organ interepreted as the frontal organ was present at U81–83; the organ was approximately 22 µm x 16 µm in diameter and in a single specimen appeared to contain filiform sperm. A caudal organ was present at the posterior end (U83–95), approximately 63 µm long x 30 µm wide (range: 50 µm–63 µm long, 24 µm–30 µm wide), hyaline in appearance with a straight central canal that extends along the organ’s anterior-posterior axis; the canal appears sclerotic (coc, Fig. 3D). The caudal organ is muscular. The muscle cells interdigitate to form spindle-shaped, incomplete circular fibers(Fig. 3B). A single gland, ca. 10 µm in diameter, is present posterior of the caudal organ (Fig. 3A, D).
Muscular system. Musculature present as circular, helicoidal and longitudinal bands. Pharynx strongly invested with splanchnic circular muscles and overlain by helicoidal bands and longitudinal muscles (Fig. 3E). The number of individual circular muscles and helicoidal bands could not be determined. In the trunk region, the helicoidal muscles extend to ca. U40 (Fig. 3E). Longitudinal muscles extend from the pharyngeal region to the caudum as three pairs of dorsal (dlm)/dorsolateral (dllm) bands (Fig. 3E). The ventrolateral longitudinal muscles (vllm) are the thickest muscles in the body and extend from ca. U03 to the caudal end (Fig. 3E). Three pairs of ventral longitudinal bands extend from the pharynx to the posterior end. All longitudinal muscles in the trunk region are surrounded by somatic circular muscles (scm) that form numerous, distinct rings down the length of the body (Fig. 3E). Beginning ca. U80-81, there is a large number (>30) of closely spaced somatic circular muscles that extend posteriorly for approximately 40 µm; the circular muscles demarcate the posterior region of the body (cm-pc) with the accessory sexual organs (Figs 1, 3B, C).
Schematic of Lepidodasys ligni sp. n. showing the crossed-helical scale pattern and a closeup of the musculature in the caudal region. Abbreviations: cg caudal gland; cm-pc circular musculature of the posterior constriction; co caudal organ; dh dorsal sensory hairs; eg mature egg; ep epidermal gland; fo frontal organ; in intestine; is interciliary scales; lc locomotory cilia; lh lateral sensory hairs; lm longitudinal muscle; pc posterior constriction; ph pharynx; TbA anterior adhesive tubes; TbD dorsal adhesive tubes; TbL lateral adhesive tubes; TbP posterior adhesive tubes; vllm ventrolateral longitudinal muscle.
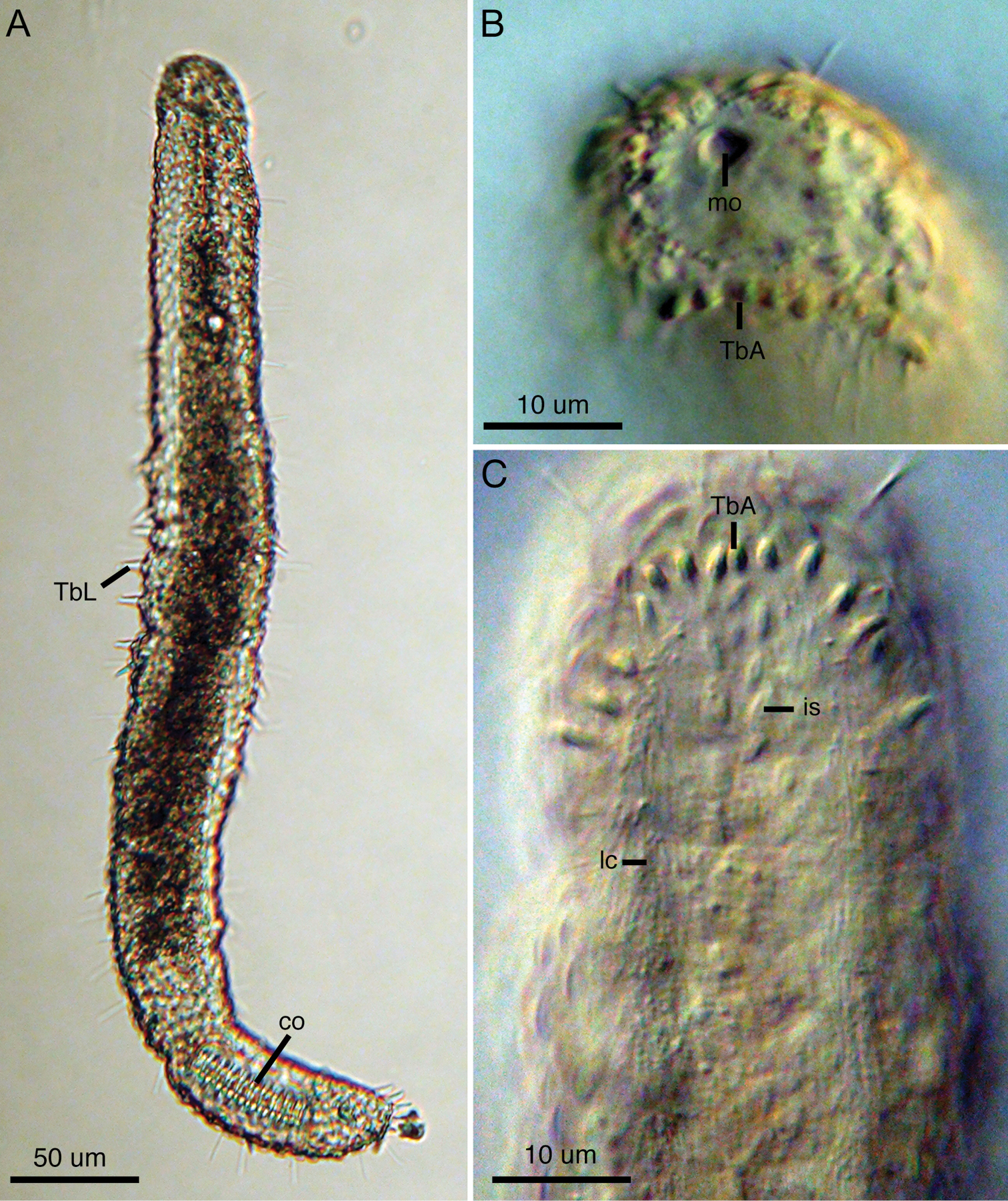
Light micrographs of Lepidodasys ligni sp. n. (holotype). A Dorsal view B Closeup of anterior end with differential interference contrast (DIC) C Ventral view of anterior end with DIC. Abbreviations: co caudal organ; is interciliary scales; lc locomotory cilia; mo mouth; TbA anterior adhesive tubes; TbL lateral adhesive tubes.
The reproductive and muscular systems of Lepidodasys ligni sp. n. A Differential interference contrast photograph of the posterior end showing the accessory reproductive organs. B, C Confocal images (47 × 0.35 µm optical sections) of the musculature of the posterior end in lateral (B) and dorsal (C) views D Closeup of the caudal organ with DIC microscopy E Lateral view of an entire specimen revealing the muscular system (73 × 0.4 µm optical sections). Abbreviations: cg caudal gland; cm-co circular muscles of the caudal organ; cm-pc circular muscles of the posterior constriction; co caudal organ; coc caudal organ canal; dlm dorsal longitudinal muscle; dllm dorsal lateral longitudinal muscle; fo frontal organ; hm helicoidal muscle (end position on midgut); lh lateral sensory hair; ph pharynx; TbP posterior adhesive tube; scm somatic circular muscles (thoughout trunk); vllm ventrolateral longitudinal muscle.
The genus Lepidodasys consists of eight described species and several undescribed specimens from marine waters across the globe (see
Species of Lepidodasys are most easily distinguished by their slow gliding movement and their heavily sculptured cuticle, which in general consists of rounded or elliptical scales across most of their body. The shape and distribution of the scales are important taxonomic characters for distinguishing the species. Of the eight described species, six species have a crossed helical pattern (parallel pattern sensu
Apart from scale pattern, scale shape is also an important taxonomic character, and the new species can easily be distinguished by the smooth (non-keeled) structure of its scales. The only other species with smooth scales are Lepidodasys castoroides, Lepidodasys laeviacus and Lepidodasys tsushimaenensis. Among these species, Lepidodasys ligni sp. n. is most similar to Lepidodasys castoroides and Lepidodasys tsushimaenensis in general body shape and the presence of a large caudal organ in the posterior body region. While all three species are similarly slender, Lepidodasys tsushimaenensis is much larger (to 730 µm) than the other two species (Lepidodasys castoroides to 475 µm and Lepidodasys ligni sp. n. to 450 µm) and lacks the notable indentation at the posterior end that demarcates the region of the accessory sexual organs. In fact, the caudal indentation gives both Lepidodasys castoroides and Lepidodasys ligni sp. n. the appearance of possessing a beaver-like tail, hence
In addition to our taxonomic findings, this study revealed novel information on the muscular system of the new species. In contrast to previous reports (e.g.,
The authors acknowledge the helpful comments of Dr. Alexander Kieneke and Dr. M. Antonio Todaro. The authors also thank the staff at the Smithsonian Marine Station in Fort Pierce, Florida for their assistance in collection. This research was funded by an award from the National Science Foundation (DEB 0918499) to Dr. Rick Hochberg. This is Smithsonian Marine Station contribution number 908.