Citation: Ribera C, Elverici M, Kunt KB, Özkütük RS (2014) Typhlonesticus gocmeni sp. n., a new cave-dwelling blind spider species from the Aegean region of Turkey (Araneae, Nesticidae). ZooKeys 419: 87–102. doi: 10.3897/zookeys.419.5739
A new species of the troglobitic spider genus Typhlonesticus is described from specimens found in Keloğlan Cave (Denizli Province, Dodurgalar Town), Turkey. Typhlonesticus gocmeni sp. n. is described on the basis of both sexes; and its phylogenetic relationships with closely related European genera and species are discussed based on morphological and molecular data (the cox1, rrnL and H3 genes). Three new combinations are proposed: Typhlonesticus idriacus (Roewer, 1931), comb. n., Typhlonesticus morisii (Brignoli, 1975) comb. n. and Typhlonesticus obcaecatus (Simon, 1907), comb. n. all ex Nesticus.
Arachnida, taxonomy, description, caves, Anatolia, troglobiont
Nesticids are medium-sized spiders common in underground ecosystems in the northern Mediterranean basin and many of them exhibit all the typical troglobite characters: depigmentation, anophthalmia and appendage lengthening. The following four genera have been recorded from this region: Aituaria Esyunin & Efimik, 1998; Carpathonesticus Lehtinen & Saaristo, 1980; Nesticus Thorell, 1869 and Typhlonesticus Kulczyński, 1914, representing a total of 49 species. Two more genera occur in bordering regions: Canarionesticus Wunderlich, 1992, endemic to the Canarian archipelago and Nesticella Lehtinen & Saaristo, 1980, broadly spread throughout the Asian continent. Nesticella mogera (Yaginuma, 1972) was recorded from the southeast of Caucasus (
Most described species are well defined and illustrated, but the taxonomy of the group is not well established at the genus level, mainly in Carpathonesticus and Nesticus which show conspicous morphological variability in their genital organs, suggesting the existence of independent evolutionary lineages. This morphological variability was already pointed out by
The genus Typhlonesticus was described in 1914 and included two species, but a type species was not selected.
An extensive survey of caves in Anatolia during the last 10 years has provided a high number of morphospecies, one of which, from Keloğlan Cave in the Denizli Province, shows a clear morphological similarity with Typhlonesticus absoloni. The discovery of this new species of Typhlonesticus has also led us to review some of the described Mediterranean species that show morphological similarity with Typhlonesticus absoloni, such as Nesticus obcaecatus from the Spanish Pyrenees, Nesticus morisii from Italy and Nesticus idriacus from Austria and Italy. In order to check the phylogenetic relationships among these species a molecular phylogenetic analysis based on nuclear and mitochondrial gene sequences was performed.
The aim of this paper is to describe a new species belonging to the genus Typhlonesticus and to propose three new combinations of the above mentioned species.
Taxonomic sampling. Representatives of Mediterranean Nesticus, Carpathonesticus and Typhlonesticus were included in the analysis. We could not include representatives of the genus Aituaria, the easternmost Mediterranean nesticid genus whose range extends from southern Urals to the northwest of Caucasus, due to lack of suitable material for molecular analysis. Sequences from Canarionesticus quadridentatus Wunderlich, 1992 from Canary Islands were used to root the tree. We have also included sequences of Typhlonesticus absoloni, Nesticus idriacus, Nesticus morisii and Nesticus obcaecatus Simon, to check the phylogenetic relationships of these species (see Appendix 1 for localities and GenBank accession numbers).
Sample storage and DNA extraction. For DNA studies, live specimens were collected in the field, fixed in 96% or absolute ethanol and stored at 4 °C. Total genomic DNA was extracted from legs or from the prosoma of a single specimen using the QIamp® DNA Mini Kit (QIAGEN) following the manufacturer’s protocols. The approximate concentration and purity of the DNA obtained were verified using 1% agarose/TBE gel electrophoresis.
PCR amplification and sequencing. Partial fragments of two mitochondrial (cytochrome oxidase I: cox1 and 16S rRNA: rrnL) and one nuclear (Histone 3: H3) genes were selectively amplified and sequenced using the primers and conditions shown in Appendix 2. The PCR reaction mixture contained a final concentration of 0.2 μM of each primer, 0.2 mM of each dNTPs, 0.5 U Taq polymerase (Promega), with the supplied buffer, and 1.5–2.5 mM MgCl2 in a final volume of 25 μL. The PCR products were cycle-sequenced in both directions using the same PCR primers and the BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems) and analyzed using an ABI 3700 automated sequencer at the Serveis Científico-Tècnics of the Universitat de Barcelona.
Alignment, genetic distances and phylogenetic analyses. Raw sequences were edited and assembled with GENEIOUS v4.6.5 (
Maximum Likelihood (ML) analyses of the combined data matrix corresponding to the three sequenced genes were conducted using the online version of RAxML (
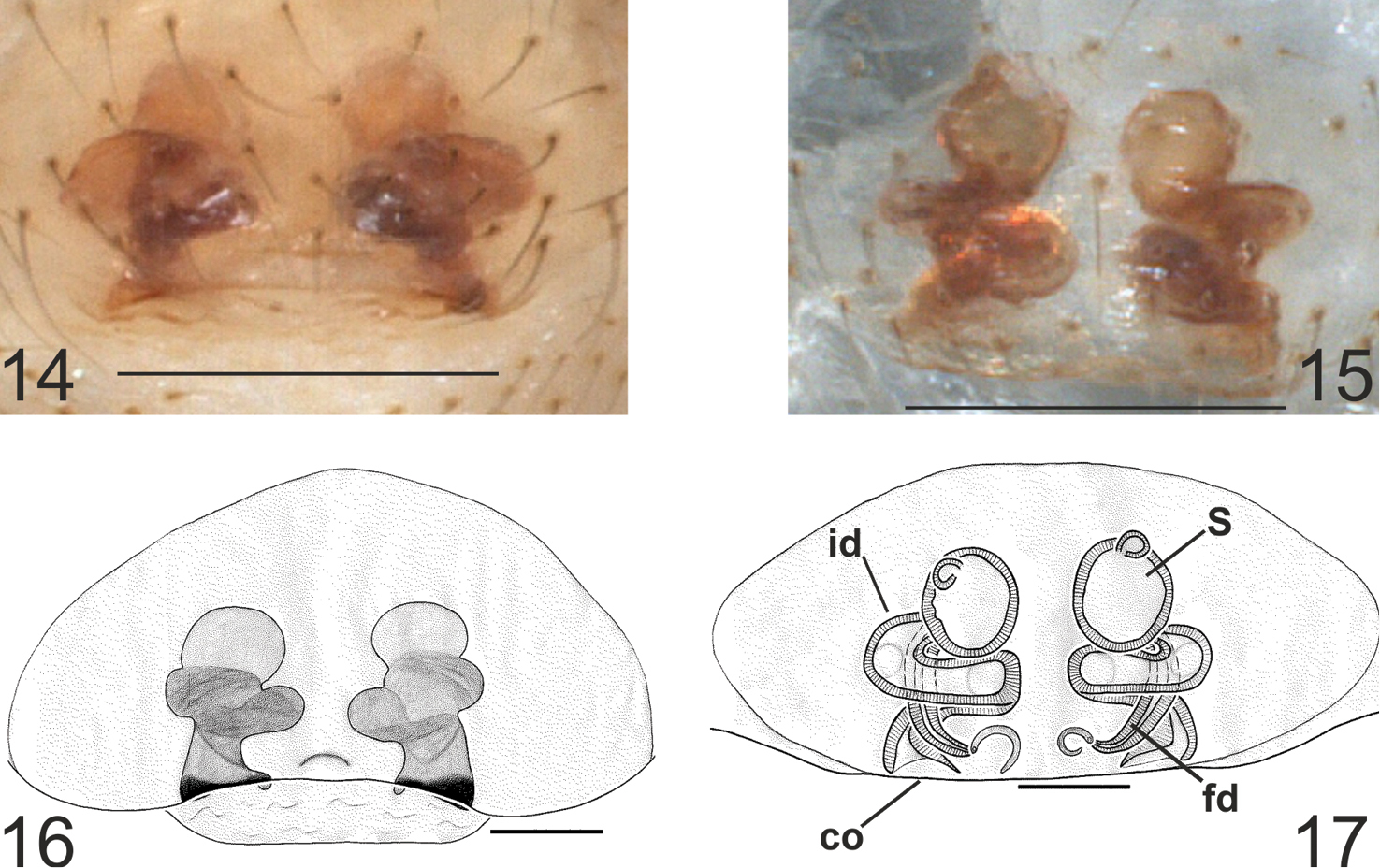
The following abbreviations are used in the text and figures: E = embolus, T = tegulum, ST = subtegulum, MA = median apophysis, TTA = theridioid tegular apophysis, p1 = process 1 of TTA, p2 = process 2 of TTA, P = paracymbium, vp = ventral process of paracymbium, dp = dorsal process of paracymbium, co = copulatory orifice, id = insemination duct, fd = fertilization duct, S = spermatheca, AUZM Anadolu University Zoological Museum (Eskişehir, Turkey), CRBA Centre de Recursos de Biodiversitat Animal de la Universitat de Barcelona (Spain). All measurements are in millimetres.
Specimens of Typhlonesticus gocmeni sp. n. were collected using hand aspirators and placed directly into 96% ethanol in the field. Body colour descriptions are based on digital images taken in the cave environment. Photography was performed using a Nikon D100 camera equipped with a Nikon 105mm f/2.8G ED-IF AF-S VR Micro-Nikkor lens and a Sigma EM-140 DG macro ring flash for Nikon SLR cameras.
The female vulva was removed and treated with 30% KOH prior to examination. After observation and drawings, the vulva was washed in distilled water and stored in 70% ethanol. The left male palp was drawn in all cases. We follow
Digital images of the palps and vulvae were taken with a Leica DFC295 digital camera attached to a Leica S8AP0 stereomicroscope, with 5–15 photographs taken in different focal planes and combined using image stacking software. Photographic images were edited using PHOTOSHOP CS2 and COREL-DRAW X3 was used to create the plates. For SEM micrographs, the male palps were dried at -30 °C and coated with a thin layer of gold using an Electron Microscopy sciences EMS 550X sputter coater. The materials were examined at an acceleration voltage of 12 kV using a ZEISS ULTRA PLUS Scanning Electron Microscope (University of Anadolu, Eskişehir, Turkey).
In addition of the new species we also examined the following material: Nesticus morisii (♀) from Sotterranei del Forte di Vernante, Vernante, Cuneo, Italy, 19.09.2007, leg. A. López-Pancorbo & M. Isaia; Nesticus idriacus (♂♀) from Grotte Pre Oreak, Nimis, Friuli. Italy, 15.09.2007, leg. A. López-Pancorbo; Nesticus obcaecatus (♂♀) from Cueva Del Molino de Aso, Boltaña, Prov. Huesca, Spain, 27.05.2004, leg. S. Carranza; and Typhlonesticus absoloni (♀) from Baba Tusha Cave, Trnovo, Virpazar Distr., Montenegro, 24.03.2006, leg. B. Petrov & S. Lazarov. Baba Tusha Cave is located about 10 km in a straight line from Grboćica pećina, in Krivośije (locus typicus of Typhlonesticus absoloni) and about 20 km away from Cetinsjska pećina, from which
Type species. Nesticus absoloni Kratochvíl, 1933; see
Holotype ♂ (AUZM) Denizli Province, Acıpayam District, Dodurgalar Town, Keloğlan Cave (37°23'14.74"N, 29°34'18.29"E), 10.07.2011, leg. M. Elverici. Paratypes 1 ♂ 1 ♀ 9 juveniles (AUZM), 1 ♂ 1 ♀ (CRBA) same data as holotype.
The specific name is given in honour of the prominent Turkish biologist, Prof. Dr. Bayram Göçmen (University of Ege, İzmir, Turkey). Noun in apposition.
Males of the new species differ from Typhlonesticus absoloni by the shape and length of the tegulum, the shape of p1 and p2 processes, the arrangement of the embolus and the shape and arrangement of the paracymbial apophyses, mainly the ventral one, which is erected as a thin spine in Typhlonesticus gocmeni sp. n., whereas in Typhlonesticus absoloni it consists of a curved lamella. Females differ from Typhlonesticus absoloni by the shape of the epigyne and the position of the spermathecae. The dimension and orientation of the insemination and fertilization ducts are also diagnostic. In Typhlonesticus gocmeni sp. n. the epigyne is scarcely sclerotized and the spermathecae and insemination ducts are visible through the tegument, moreover the spermathecae are nearly spherical and separated by a distance approximately equal to their diameter. In Typhlonesticus absoloni the epigyne is strongly sclerotized and the spermathecae are separated by almost twice their diameter. In Typhlonesticus absoloni the insemination and fertilization ducts are thicker and almost fill the entire genital area.
Coloration. Carapace whitish, slightly yellowish. Appendages and sternum slightly testaceous. Opisthosoma brownish, with many dark patches (Fig. 20). The specimens preserved in alcohol have a whitish opisthosoma, slightly greyer than the prosoma.
Prosoma. Carapace approximately circular in dorsal view. Cephalic region not differentiated from the rest of the carapace. Eyeless (Figs 18, 19).
Opisthosoma. Sub-elliptical in dorsal view.
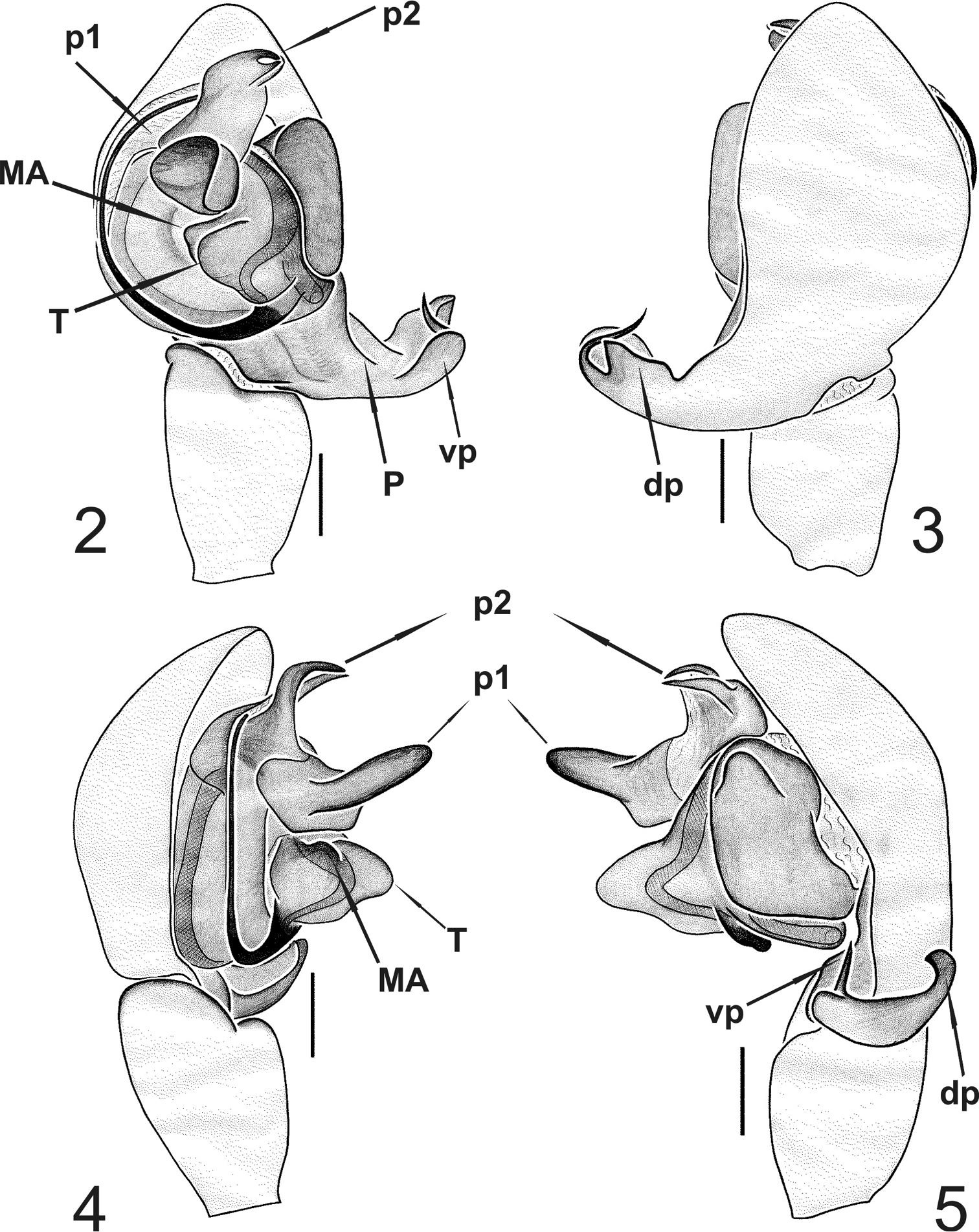
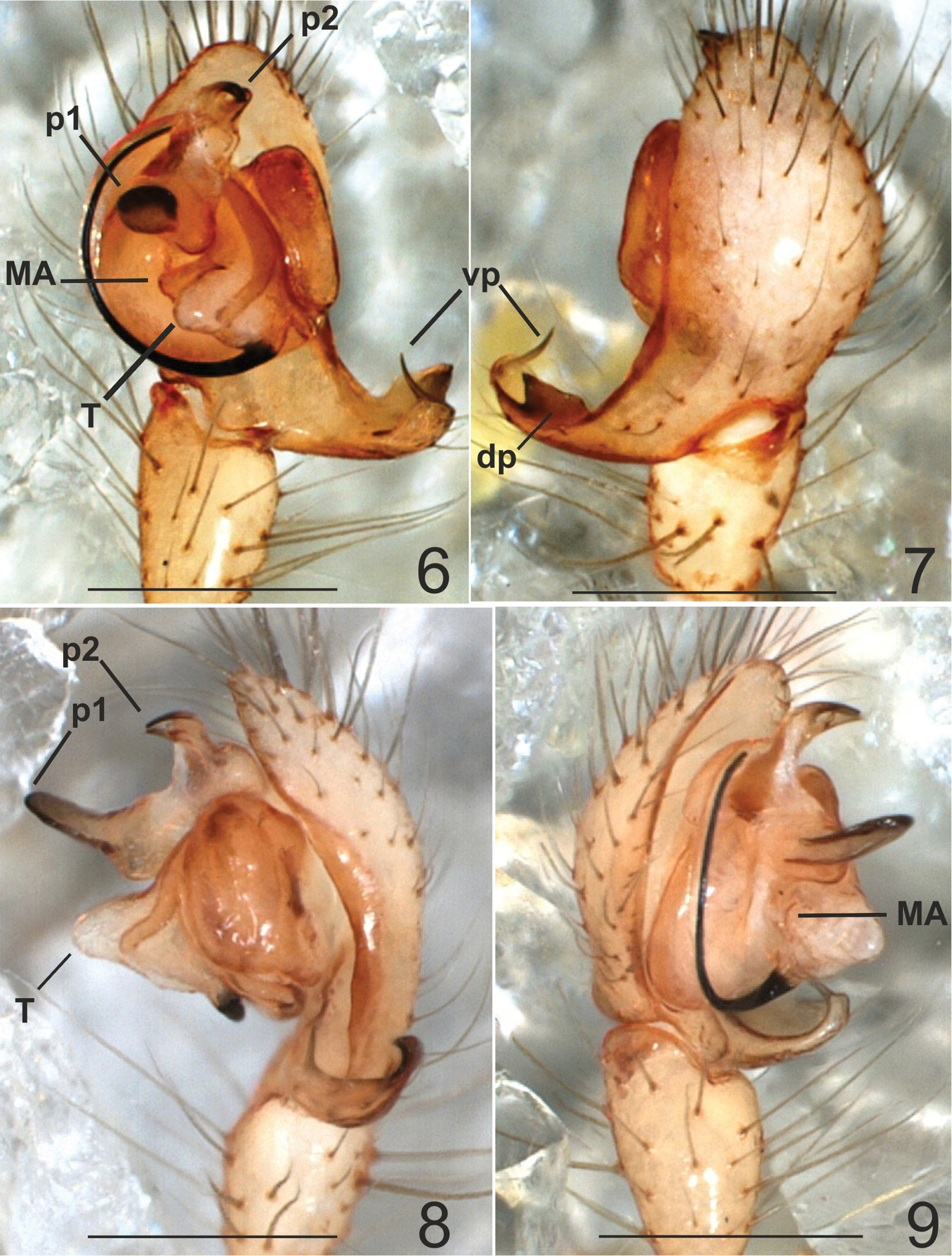
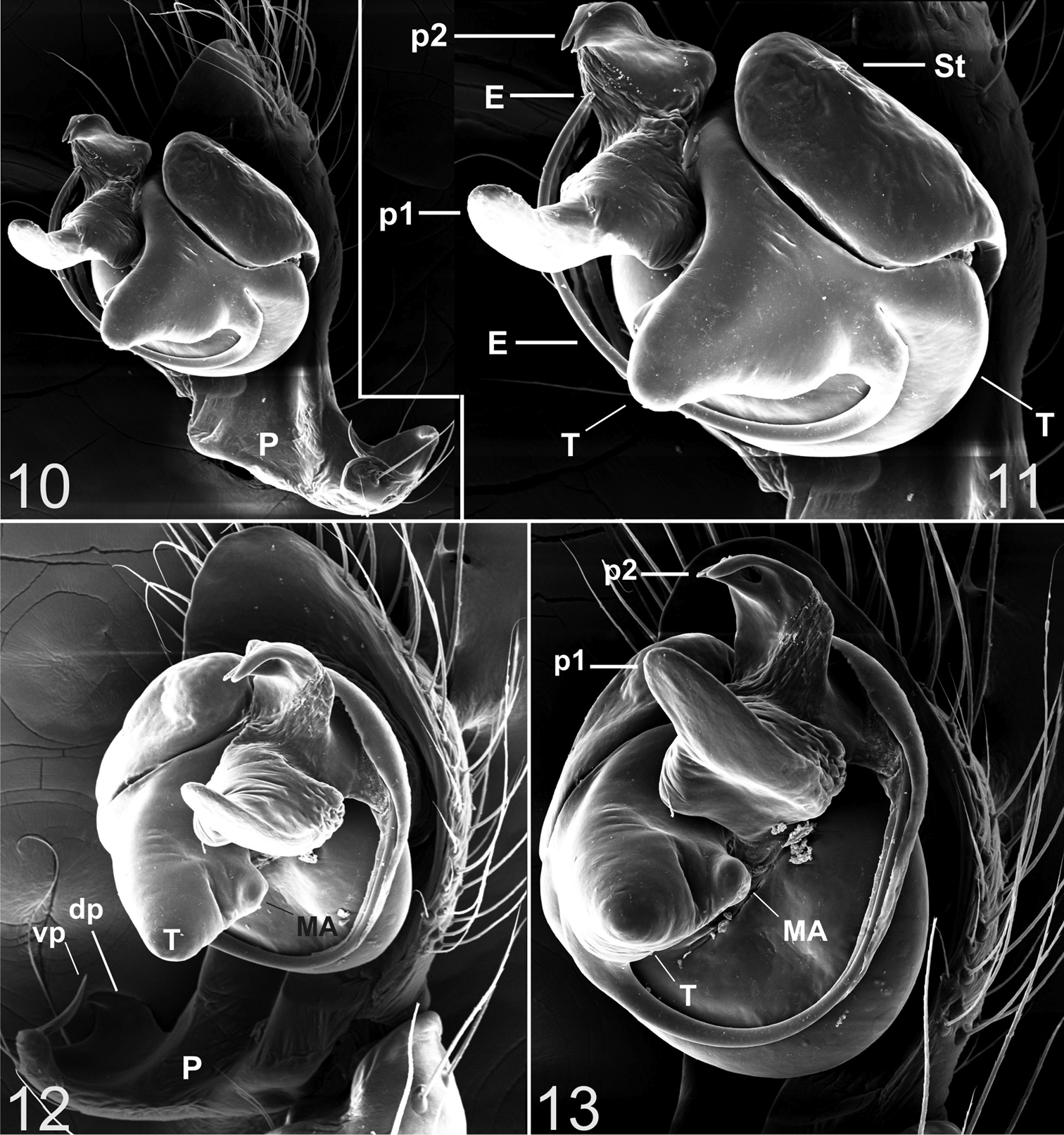
Appendages. prolateral margin of the chelicerae with 3 teeth, the central slightly longer. Male palp (Figs 2–13). Paracymbium short, dorsal and ventral processes scarcely developed. The ventral process consists of a short and flattened lamella, curved towards the apex and prolonged into a thin spine. The ventral one consisting of a short laminar apophysis, apically curved toward to the ventral side. Distal, paradistal and dorsomedian paracymbial apophyses absent (Figs 2–3 and 5–7). Tegulum very prominent, consisting of a ventrally directed triangular apophysis. Small inconspicuous median apophysis located behind the tegulum (Figs 2, 4, 6, 8, 12). TTA with two well developed processes (p1 and p2): p1 is saddle-shaped, longer than wide, slightly curved in the middle and directed ventrally; p2 is in an apical position and ends with two convergent apical hooks running as a conductor for the embolus (Figs 2, 4–6, 8, 11, 13). Embolus filamentous following a semicircular course towards the apex and bordering the tegulum.
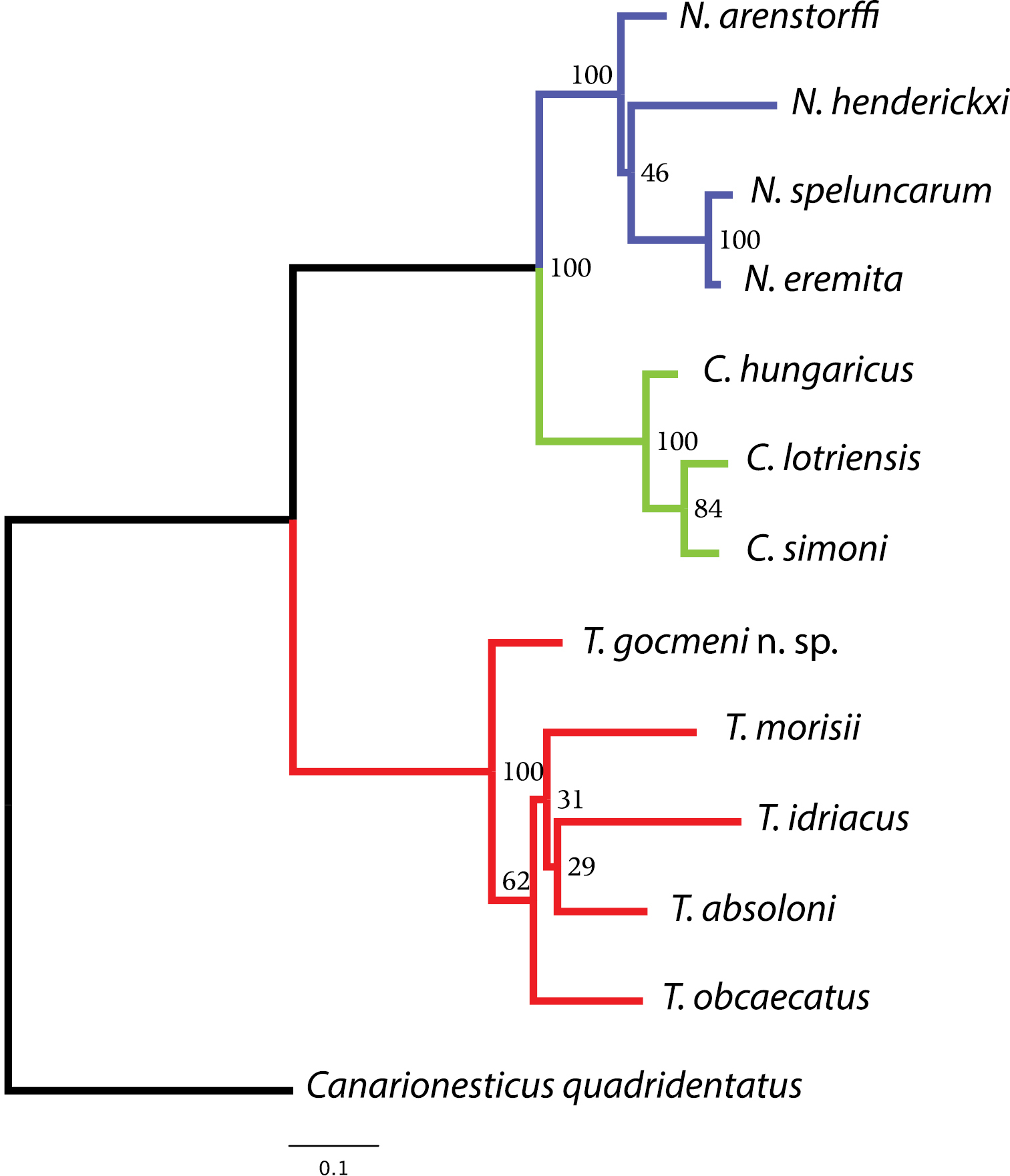
ML tree inferred using the concatenated dataset of cox1, rrnL mtDNA and H3 nuDNA gene fragments. Numbers next to nodes correspond to bootstrap support values. The tree was rooted using Canarionesticus quadridentatus from the Canary Islands.
Typhlonesticus gocmeni sp. n. male palp. 2 ventral view 3 dorsal view 4 prolateral view 5 retrolateral view. Abbreviations: T = tegulum, MA = median apophysis, p1 = process 1 of TTA, p2 = process 2 of TTA, P = paracymbium, vp = ventral process of paracymbium, dp = dorsal process of paracymbium. Scale bars 0.1 mm.
Typhlonesticus gocmeni sp. n. male palp. 6 ventral view 7 dorsal view 8 retrolateral view 9 prolateral view. Abbreviations: T = tegulum, MA = median apophysis, p1 = process 1 of TTA, p2 = process 2 of TTA, vp = ventral process of paracymbium, dp = dorsal process of paracymbium. Scale bars 0.5 mm.
Typhlonesticus gocmeni sp. n. male palp. 10 nearly retrolateral view 11 ditto 12 nearly ventral view 13 ventral view. Abbreviations: E = embolus, T = tegulum, St = subtegulum, MA = median apophysis, p1 = process 1 of TTA, p2 = process 2 of TTA, P = paracymbium, vp = ventral process of paracymbium, dp = dorsal process of paracymbium.
Measurements (holotype ♂): carapace length 1.15, width 0.88, opisthosoma length 1.60, width 0.84. Total length = 2.75.
| Leg | coxa | troc. | femur | patella | tibia | meta. | tarsus | total |
|---|---|---|---|---|---|---|---|---|
| I | 0.40 | 0.20 | 3.28 | 0.52 | 3.48 | 3.12 | 1.13 | 12.13 |
| II | 0.28 | 0.20 | 2.20 | 0.45 | 2.20 | 2.00 | 0.88 | 8.21 |
| III | 0.25 | 0.18 | 1.75 | 0.43 | 1.45 | 1.55 | 0.68 | 6.29 |
| IV | 0.30 | 0.20 | 2.42 | 0.40 | 2.15 | 1.95 | 0.89 | 8.31 |
All somatic characters as in male but slightly larger in size. Epigyne convex and prominent, without sclerotized plates (Fig. 14). The posterior edge is slightly sclerotized. Spermathecae and insemination ducts can be observed through the tegument. Vulva quite simple (Figs 15–17), consisting of two almost spherical spermathecae, insemination and fertilization ducts. Insemination duct coiled, forming two laps around the fertilization duct before reaching the spermatheca. Vulval pockets absent.
Typhlonesticus gocmeni sp. n. epigyne and vulva. 14 epigyne ventral view 15 vulva ventral view, 16 ditto 17 vulva dorsal view. Abbreviations: co = copulatory orifice, id = insemination duct, fd = fertilization duct, S = spermatheca. Scale bars 14–15 0.25 mm 16–17 0.1 mm.
Typhlonesticus gocmeni sp. n. 18–19 male, prosoma 18 dorsal view 19 frontal view 20 male in the web 21 female.
Measurements (paratype ♀): carapace length 1.20, width 1.00, opisthosoma length 2.04, width 1.28. Total length = 3.24.
| Leg | coxa | troc. | femur | patella | tibia | meta. | tarsus | total |
|---|---|---|---|---|---|---|---|---|
| I | 0.38 | 0.15 | 2.64 | 0.45 | 3.40 | 2.80 | 1.11 | 10.93 |
| II | 0.32 | 0.13 | 2.48 | 0.45 | 2.25 | 1.95 | 0.93 | 8.51 |
| III | 0.20 | 0.13 | 1.75 | 0.34 | 1.40 | 1.20 | 0.61 | 5.63 |
| IV | 0.37 | 0.14 | 2.52 | 0.42 | 2.36 | 1.88 | 0.97 | 8.66 |
Typhlonesticus gocmeni sp. n. is only known from the type locality. This new species was previously identified as Nesticus morisii by Aydın Topçu and collaborators (
Specimens, locality and sequences with corresponding GenBank accession numbers analyzed in the present study are listed in Appendix 1. The final concatenated dataset of the three partial genes sequences includes 13 terminals and 1807 aligned characters (cox1 = 1049, rrnL = 420 and H3 = 338). Primer fidelity across taxa was not always consistent in cox1, consequently some specimens have slightly truncated sequence lengths. Uncorrected cox1 genetic divergences among terminal taxa, and uncorrected genetic cox1 divergences within and between the analyzed genera are summarized in Appendices 3 and 4.
Figure 1 shows the ML tree inferred using the combined data matrix. The new species groups with Typhlonesticus absoloni, Nesticus morisii, Nesticus obcaecatus and Nesticus idriacus. These five species constitute a highly supported evolutionary lineage (bootstrap support = 100). The remaining species included in the analysis belong to the genera Nesticus and Carpathonesticus, which constitute independent and highly supported evolutionary lineages as well. Typhlonesticus gocmeni sp. n. occupies a basal position in the Typhlonesticus clade, and is the sister species of the European representatives. Within this lineage the evolutionary relationships of the species are poorly supported (low bootstrap supports).
The mean uncorrected p-distances of cox1 between and within taxa analyzed (Appendices 3–4) show high values. The mean p-distance between genera ranges from 11.29% (Nesticus versus Carpathonesticus) to 17.19 (Typhlonesticus versus Carpathonesticus). Also, the average evolutionary divergence within the representatives of the three Mediterranean genera analyzed ranges from 6.43% (Carpathonesticus) to 11.11% (Typhlonesticus).
This paper describes a new species belonging to the genus Typhlonesticus. The molecular phylogenetic analysis including representatives of Nesticus, Carpathonesticus and Typhlonesticus, all of them from the Mediterranean basin indicates that the new species lies with Typhlonesticus absoloni along with Nesticus morisii, Nesticus obcaecatus and Nesticus idriacus. These five species form a highly supported clade (bootstrap value = 100) suggesting that all of them constitute a well-defined evolutionary lineage. Accordingly, we propose the following new combinations:
Typhlonesticus idriacus (Roewer, 1931), comb. n., ex Nesticus
Typhlonesticus morisii (Brignoli, 1975), comb. n., ex Nesticus
Typhlonesticus obcaecatus (Simon, 1907), comb. n., ex Nesticus.
These new data increase significantly the distribution of the genus, which is spread throughout the northern Mediterranean, from the Iberian Peninsula to Turkey.
To date, the Typhlonesticus generic diagnosis has been based on a single species (
A very special trait of these species is that all of them have highly troglomorphic characters, such as the absence of eyes or reduced eye size and number (only six eyes in Typhlonesticus obcaecatus) and lack of body pigment. In addition, most of these species are known from a single or a small number of caves, and all of them have very narrow ranges. On other hand, the uncorrected genetic distances of cox1 between Typhlonesticus gocmeni n. sp., Typhlonesticus absoloni, Typhlonesticus morisii, Typhlonesticus obcaecatus and Typhlonesticus idriacus range between 10.03 to 12.19%. Assuming an average substitution rate for arthropod mitochondrial genes of between 2% (
We express our warmest gratitude to Meltem Altunöz Hatipoğlu (Turkey) for cooperating and assisting during our surveys to Keloğlan Cave. We also thank to Mr. Bülent Erdem (Turkey) for providing some literature. All drawings presented in this paper belong to Dr. Mykola Kovblyuk (Ukraine). The English of the final draft was kindly checked by Dr. David Penney (United Kingdom). This research was partially supported by CGL2004 05771/BOS and CGL2006-13374/BOS projects from the Ministerio de Educación y Ciencia, Spain).
Species included in the phylogenetic analysis and GenBank accession numbers for the cox1, rrnL and H3 partial sequences.
| Species | Locality | cox1 | rrnL | H3 |
|---|---|---|---|---|
| Typhlonesticus gocmeni sp. n. | Keloğlan Cave. Dodurgalar Town, Acıpayam District. Denizli Province. Turkey, (37°23'14.74"N, 29°34'18.29"E) | KF939310 | KF939307 | KF939313 |
| Typhlonesticus absoloni (Kratochvíl, 1933) | Baba Tusha Cv. Trnovo, Virpazar Distr., Montenegro, (42°17'25.1''N, 019°02'10.8''E / 350m) | KF417410 | KF417397 | KF417416 |
| Typhlonesticus idriacus (Roewer, 1931) | Grotte Pre Oreak, Nimis, Friuli. Italy | KF939312 | ||
| Typhlonesticus morisii (Brignoli, 1975) | Sotterranei del Forte di Vernante, Vernante, Cuneo, Italy | KF939311 | KF939308 | |
| Typhlonesticus obcaecatus (Simon, 1907) | Cueva del Molino de Aso, Boltaña, Prov. Huesca, Spain. | KF939309 | EU746437 | |
| Nesticus eremita Simon, 1879 | Pishurka Cave. (=Paganetijeva Pécina), Korchula, KorchulaIsl., Croatia. (42°57.568'N, 17°07.751'E / 58m) | KF417414 | KF417400 | |
| Nesticus speluncarum Pavesi, 1873 | Shpella e Dragoit, Gjirokastër, Albania | KF417405 | KF417421 | |
| Nesticus arenstorffi Kulczyński, 1914 | Chora Pecina Cave. Crni nugli, Dragalsko polje, Gorno krivoshije, Shelakov dol, Risan Distr. Montenegro. (42°35'36.5''N, 018°41'41.6''E / 750m) | KF417407 | KF417403 | KF417422 |
| Nesticus henderickxi Bosselaers, 1998 | Kournas Cave. Kournas. Crete | KF417409 | KF417404 | |
| Carpathonesticus hungaricus (Chyzer, 1894) | Pestera Cave. Liliecilor, Cheile Ampoitei Gorges, Romania. (46°08'21.7748"N, 23°23'39.8507"E) | KF417412 | KF417402 | KF417419 |
| Carpathonesticus lotriensis Weiss, 1983 | Unnamed Cave in Lotrioara Valley, Lotrului Mountains, Sibiu, Romania. (45°34'45.8319"N, 24°11'16.7493"E) | KF417413 | KF417399 | KF417418 |
| Carpathonesticus simoni (Fage, 1931) | Unnamed Cave in Bisbrita Gorges, Stogu-Vinturarita Mts., Romania. (45°11'42.2789"N, 024°02'03.2702"E / 491m) | KF417408 | KF417398 | KF417417 |
| Canarionesticus quadridentatus Wunderlich, 1992 | Cv. Felipe Reventón, Icod de los Vinos, Tenerife, Canary Islands. (28°21'00.7727"N, 016°42'17.1028"W / 595m) | KF417411 | KF417415 |
Primers and conditions used in the present study.
| Gene fragment | Primer | Or. | Sequence (5'-3') | PCR Conditions | Reference |
|---|---|---|---|---|---|
| cox1 | C1-J-1718 | F | GGAGGATTTGGAAATTGATTAGTTCC | 94°(1'); 94°(30"), 45°(30"), 72°(80") × 35; 72°(4') | |
| C1-N-2191 | R | CCCGGTAAAATTAAAATATAAACTTC | |||
| C1-J-2183 | F | CAACATTTATTTTGATTTTTTGG | 94°(1'); 94°(30"), 45°(30"), 72°(80") × 35; 72°(4') | ||
| C1-N-2776 | R | GGATAATCAGAATATCGTCGAGG | |||
| rrnL | LR-N-13398 | F | CGCCTGTTTATCAAAAACAT | 94°(1'); 94°(30"), 45°(35"), 72°(80") × 35; 72°(4') | |
| LR-J-12864 | R | CTCCGGTTTGAACTCAGATCA | |||
| H3 | H3a | F | ATGGCTCGTACCAAGCAGACVGC | 94°(1'); 94°(30"), 48°(30"), 72°(80") × 35; 72°(4') | |
| H3a | R | ATATCCTTRGGCATRATRGTGAC |
Uncorrected genetic distances of cox 1 gene between terminal taxa.
| Canarionesticus quadridentatus | ||||||||||||
| Typhlonesticus gocmeni sp. n | 0.1874 | |||||||||||
| Typhlonesticus obcaecatus | 0.2002 | 0.1003 | ||||||||||
| Typhlonesticus obsoloni | 0.1941 | 0.1090 | 0.0947 | |||||||||
| Typhlonesticus morisii | 0.1953 | 0.1178 | 0.1078 | 0.1078 | ||||||||
| Typhlonesticus idriacus | 0.2021 | 0.1219 | 0.1144 | 0.1194 | 0.1205 | |||||||
| Carpathonesticus simoni | 0.1798 | 0.1785 | 0.1639 | 0.1706 | 0.1898 | 0.1860 | ||||||
| Carpathonesticus lotriensis | 0.1791 | 0.1687 | 0.1604 | 0.1715 | 0.1847 | 0.1845 | 0.0471 | |||||
| Carpathonesticus hungaricus | 0.1763 | 0.1530 | 0.1531 | 0.1608 | 0.1705 | 0.1822 | 0.0697 | 0.0775 | ||||
| Nesticus arenstorffi | 0.1880 | 0.1476 | 0.1458 | 0.1601 | 0.1774 | 0.1791 | 0.1096 | 0.1093 | 0.1007 | |||
| Nesticus henderickxi | 0.1788 | 0.1504 | 0.1515 | 0.1548 | 0.1572 | 0.1798 | 0.1265 | 0.1202 | 0.1143 | 0.0905 | ||
| Nesticus speluncarum | 0.1657 | 0.1485 | 0, 1540 | 0.1582 | 0.1595 | 0.1694 | 0.1176 | 0.1075 | 0.1143 | 0.0791 | 0.0992 | |
| Nesticus eremita | 0.1723 | 0.1507 | 0.1508 | 0.1609 | 0.1700 | 0.1660 | 0.1140 | 0.1102 | 0.1105 | 0.0760 | 0.0925 | 0.0190 |
Average evolutionary divergence between groups (below diagonal), standard error (above diagonal), and average evolutionary divergence within groups (d) and standard error (SE) of cox 1 over sequence pairs.
| Canarionesticus | 0.0107 | 0.0106 | 0.0104 | d | SE | |
| Typhlonesticus | 0.1958 | 0.0104 | 0.0086 | 0.1111 | 0.0065 | |
| Carpathonesticus | 0.1784 | 0.1719 | 0.0091 | 0.0643 | 0.0083 | |
| Nesticus | 0.1762 | 0.1596 | 0.1129 | 0.0768 | 0.0059 |