(C) 2013 M. Antonio Todaro. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of marine Gastrotricha from Brazil is described and discussed. Ptychostomella lamelliphora sp. n. is one of the several new taxa that were found during an extensive survey of the gastrotrich fauna carried out in 2002 and 2003 along the coastline of the State of São Paulo. The new species is unique in that it possesses cuticular ornamentations in the form of plate-like structures (scales) along the lateral borders of the body and two massive clusters of densely packed adhesive tubes on the ventral surface, near the ano-genital opening. Both these features appear to be adaptations to challenge the high energy waters that characterize the species’ microhabitat: the coarse sublittoral sand in the channel between the mainland and the largest island in the State, Ihlabela. Additionally, a key to the described Ptychostomella species of the world is provided.
Gastrotrichs, Brazil, São Paulo, meiofauna, biodiversity, taxonomy, new species, key
The study is part of a larger research program aimed at shedding light on the diversity of marine invertebrates of the northern coasts of the State of São Paulo, Brazil (see
Survey along the northern coasts of the State of São Paulo took place between 20 April and 3 May 2002 and in September 2003. A general account on the visited locations are reported in
The description of the new species follows the convention of
Granulometric analysis of the substrata was carried out according to
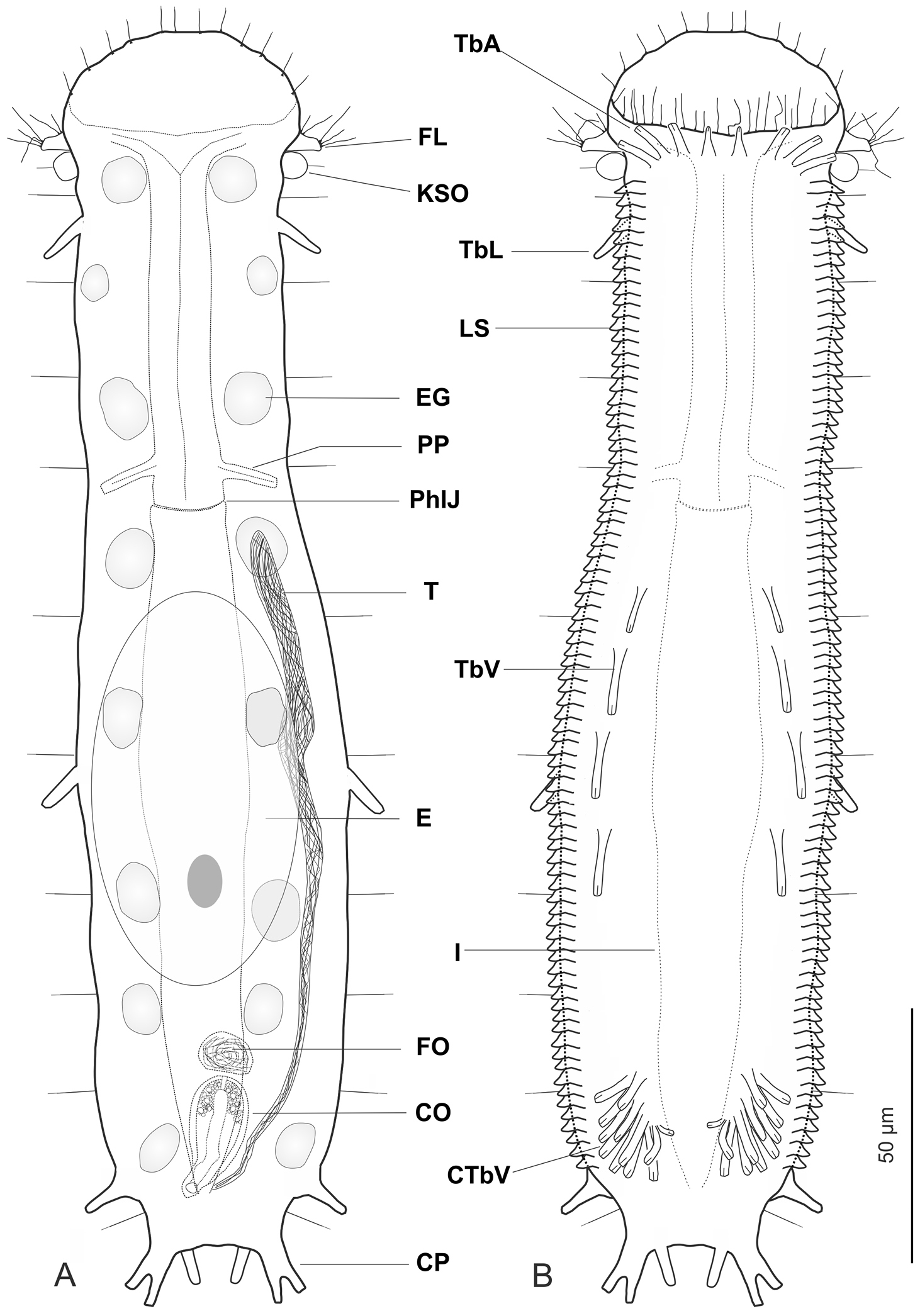
Abbreviations are as follows: PhIJ, pharyngeo-intestinal junction; TbA, adhesive tubes of the anterior series; TbL, adhesive tubes of the lateral series; TbV, adhesive tubes of the ventral series; TbP, adhesive tubes of the posterior series.
The rationale for the key to the ecological characteristics of the species, according to
Abundance of a species among other species of a sample - Rare, less than 1% of a sample; scarce, 3–5% of a samples; numerous, 10–20% of a sample (often a sub-dominant); prevalent, more than 30% of a sample (usually dominant or co-dominant).
urn:lsid:zoobank.org:act:05285351-37C4-4343-9864-74B4C240C293
http://species-id.net/wiki/Ptychostomella_lamelliphora
Figs 1–4Praia Grande on Ilhabela, State of São Paulo, Brazil (Lat. 23°51'S; Long. 45°25'W), at 3 m water depth in coarse (mean grain size, 0.51 mm), moderately well sorted (sorting, 0.85 mm) siliceous sand. Values of salinity, temperature and pH of the interstitial water at date of sampling 35.0‰, 23.2 °C and 7.90 respectively (Table 1).
Ptychostomella lamelliphora sp. n. schematic drawings. A Habitus as seen from the dorsal side showing the internal anatomy B Habitus as seen from the ventral side. CO caudal organ CP caudal pedicle CTbV cluster of ventral adhesive tubes E egg EG epidermal gland FL fleshy lobe FO frontal organ I intestine KSO Knob-like sensory organ LS lamellate scales PhIJ pharyngeo-intestinal junction Pp pharyngeal pores T testicle TbA anterior adhesive tubes TbL lateral adhesive tubes TbV ventral adhesive tubes.
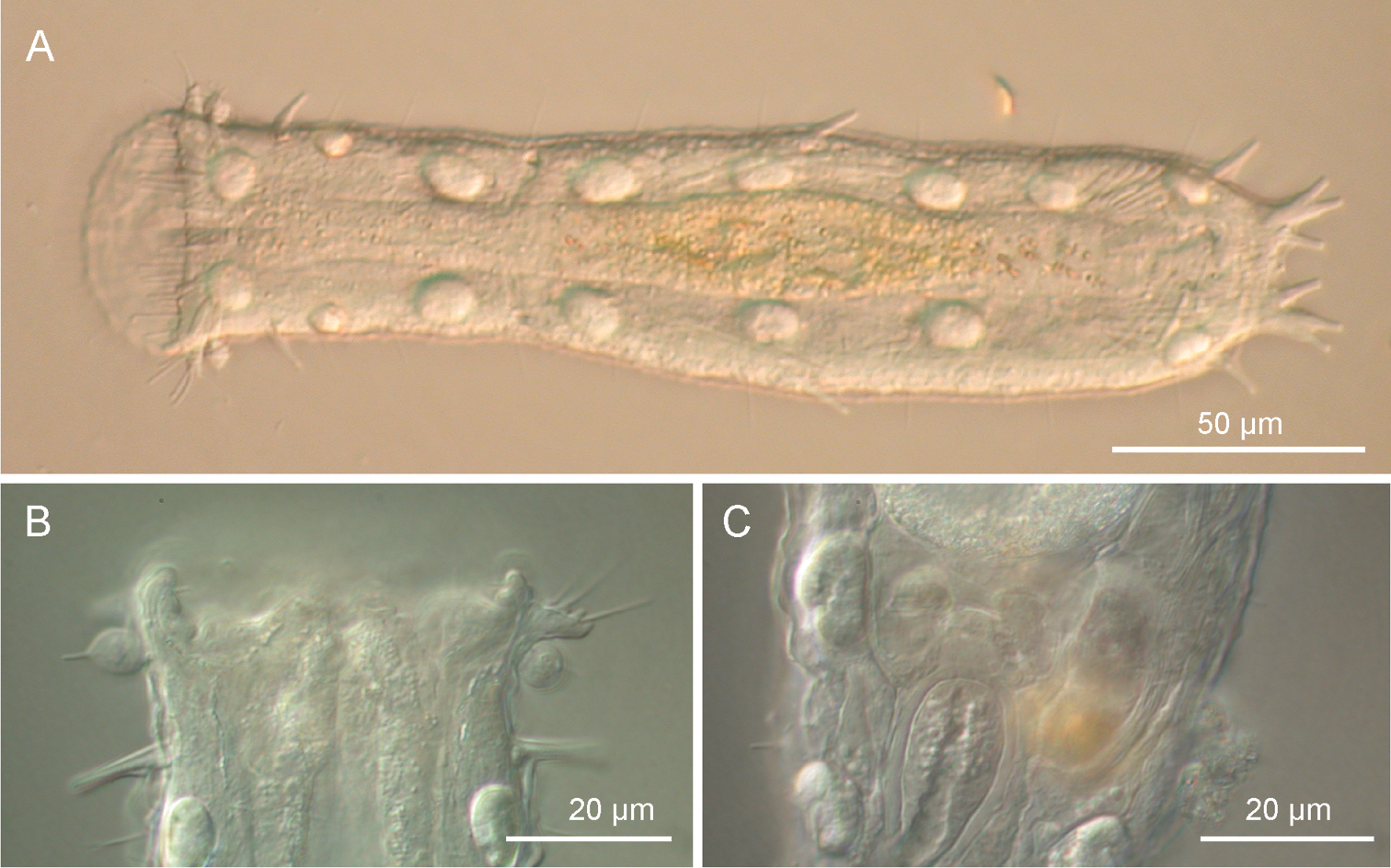
Ptychostomella lamelliphora sp. n. DIC photomicrographs. A habitus B close-up of the anterior region showing the knob-like sensory organs and the trapezoidal, fleshy lobes C Close-up of the posterior region of the trunk showing the caudal organ.
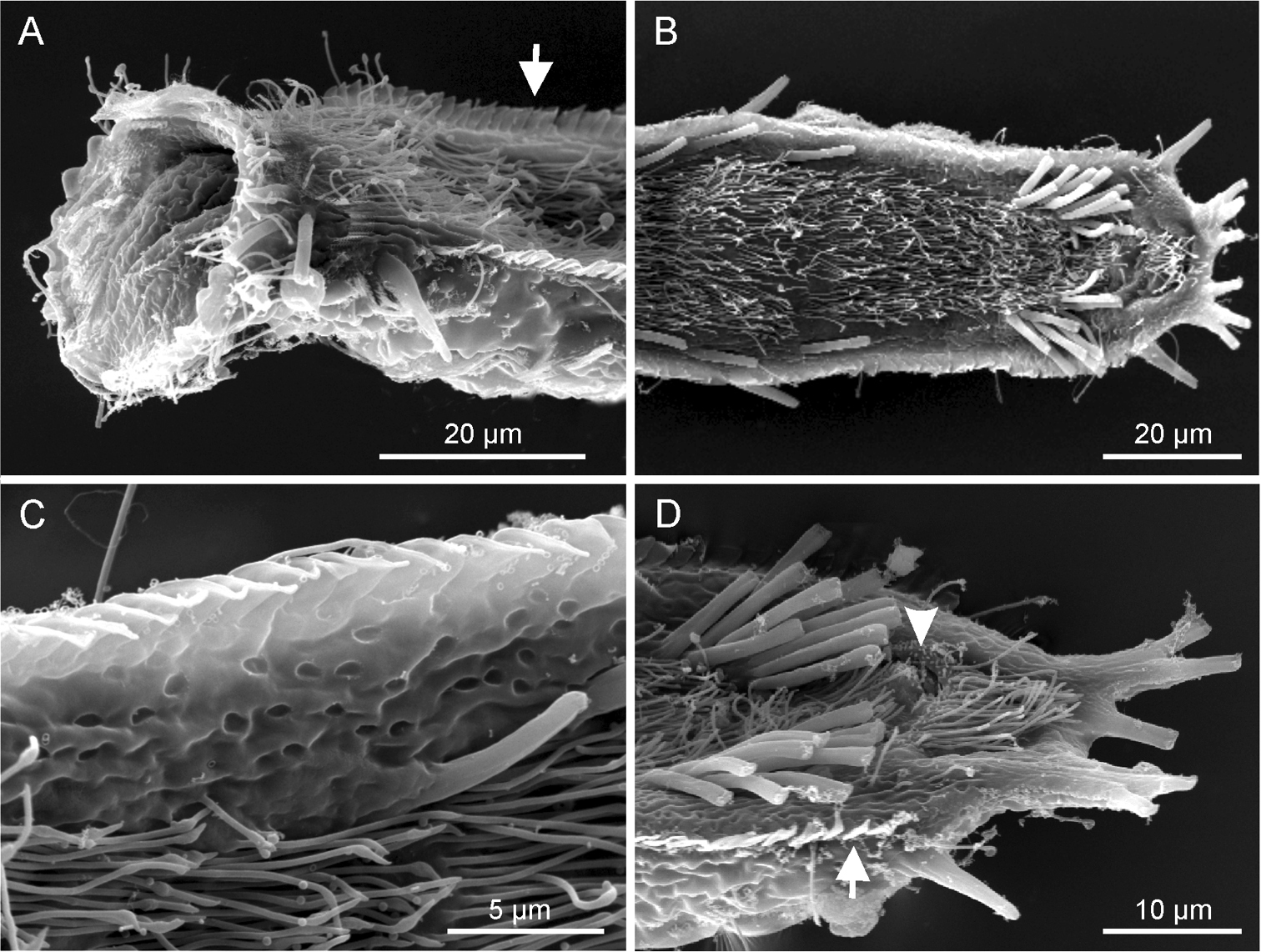
Ptychostomella lamelliphora sp. n. SEM photomicrographs ventral view. A close-up of the anterior region showing, among others, the plate-like-scales(arrow) B trunk region showing the locomotory ciliation and most of the tubular adhesive apparatus C close-up of the ventrolateral region of the trunk, showing the cuticle punctuated by shallow pits D close-up of the posterior region showing the two clusters of ventral adhesive tubes, the ano-genital opening (arrowhead) and the column of plate-like-scales.
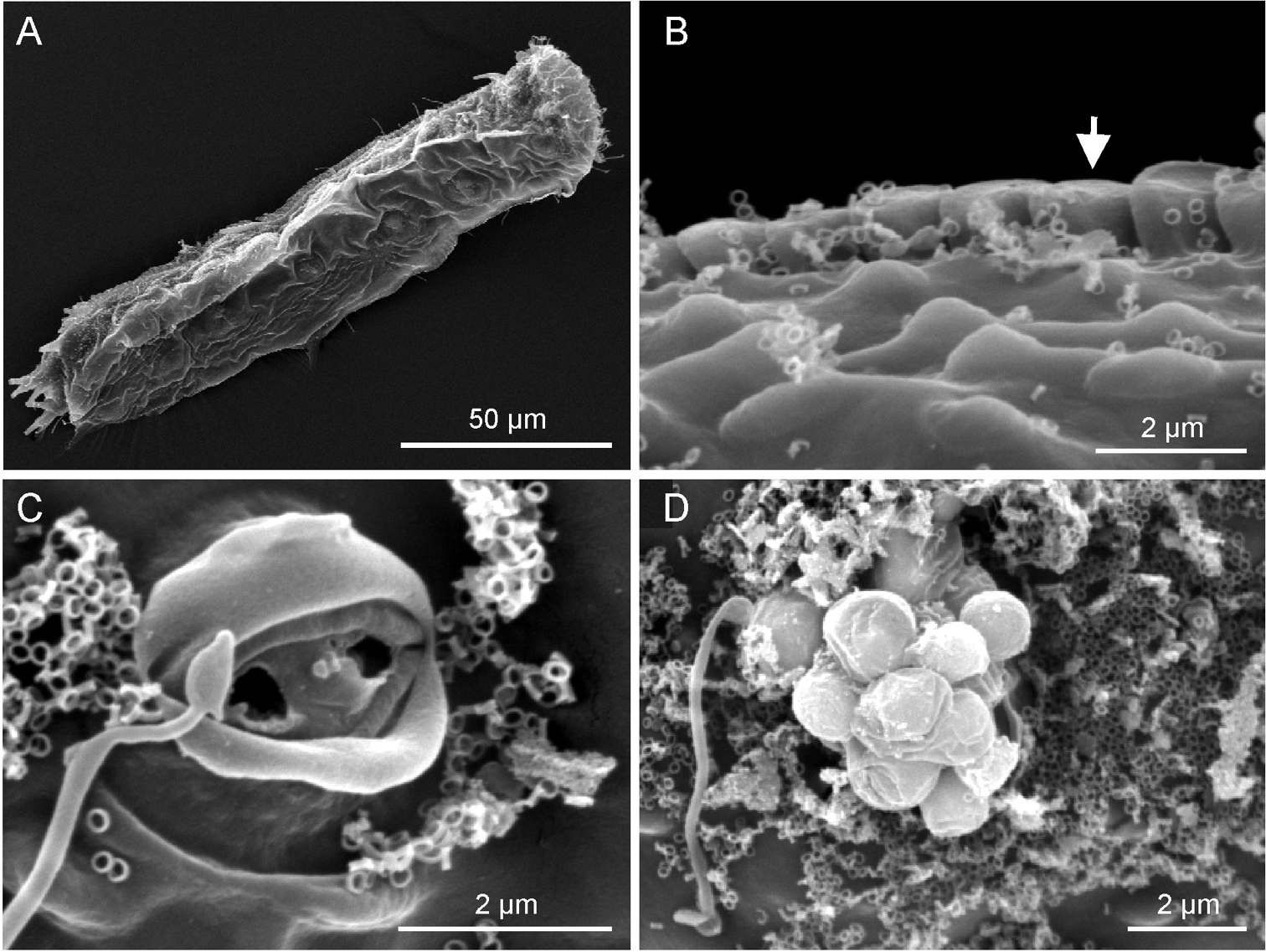
Ptychostomella lamelliphora sp. n.SEM photomicrographs, dorsal view. A habitus B close-up of the midtrunk region showing the plate-like scales (arrow) and the cuticle embossed with keel-like ornamentation C close-up of an epidermal gland pore D close-up of an epidermal gland secretion droplets.
Sampling locations along the São Sebastião channel in the State of São Paulo, Brazil; geographic coordinates, date of collection, water depth and physical, chemical characteristics of the water and granulometric characteristics of the sediment.
| Variable | Locality | |
|---|---|---|
| Praia Grande of Ihlabela | Beluga | |
| Geographic coordinates | 23°51'S, 45°25'W | 23°52'S, 45°26'W |
| Date of sampling | 30/04/2002 | 30/04/2002 |
| Salinity | 35.0 ‰ | 35.2 ‰ |
| Temperature | 23.2 (°C) | 22.4 (°C) |
| pH | 7.90 | 7.93 |
| Water Depth | 3.0 m | 6.0 m |
| Mean grain size and size class | 0.95 phi coarse sand |
0.55 phi coarse sand |
| Sorting and Sorting class |
0.60 phi, moderately well sorted |
0.46 phi well sorted |
| Skewness | 0.23 | 1.25 |
| Kurtosis | 2.12 | 5.40 |
Holotype, the adult specimen 250 µm long shown in Figure 2 (International Code of Zoological Nomenclature, Articles 73.1.1 and 73.1.4). After observation it was fixed in 95% ethanol and subsequently utilised for DNA extraction and 18S, 28S and CO I gene sequencing (GenBank accession number JF357643, JF357691 and JF432033 respectively, see
Eightadult specimens(including the holotype) collected by the author, five from the type locality and three from a Beluga a nearby location (see Table 1). Four specimens were observed alive and are not longer extant, while four were prepared for SEM survey and are kept in the meiofauna collection of the author (Ref. n. 2002-BR-01-02-05-06).
Frequency of occurrence: sparse, found only in sub-littoral sediment of two locations along the southern portion of the São Sebastião channel. Abundance: numerous in coarse sediment with little detritus of Praia Grande, scarce in coarse sediment rich in detritus of Beluga.
A Ptychostomella with an adult length to 250 µm; pharynx length to 74 µm, with pharyngeal pores at base. PhIJ at U37; body with almost parallel sides and short, bilobed caudum. Head bearing paired knob-like sensory organs and small, trapezoidal, fleshy lobes; eye spots missing; sensory hairs a few, forming lateral columns along the body, a fringe around the oral opening and loose tufts at the tip of the lobes; epidermal glands noticeable, eight per side, scattered along the length of the body. Cuticular covering generally smooth except for peculiar, subrectagular scales arranged in a column of on each ventrolateral side. Adhesive tubes: TbA, 4 per side, one slightly smaller, cone-shaped, in the middle at U9 and three lateral, rod-like, of equal size at U9-U10; TbL, 3 on each side, roughly of the same size, a small isolated one implanted anteriorly at U15, one in mid trunk region at U58 and one more robust near the base of the caudal lobes, at U90. TbV, up to 16 per side, four of the same size more or less evenly spaced, implanted along trunk region from U44 to U63, the remainder 10-12 forming a noticeable cluster at U83-U85. TbP, six in all, two medial and two on each of two paired caudal pedicles. Ventral locomotor cilia: a continuous field of transverse rows covering the entire surface except the ano-genital area. Reproductive system: testis on the right body side, caudal organ pyriform, frontal organ round filled with motile spermatozoa, a ripe egg dorsally in the mid-intestinal region.
The specific epithet lamelliphora (lamella, L, thin plateand phero, Gr., to bear)refers to the presence of the thin, plate-like scales along the ventrolateral body sides.
Description is mainly based on the holotypic specimen, 250 µm in total length. Pharynx 74 µm in length, measured from the posterior margin of the oral opening to the pharyngeo-intestinal junction, with pharyngeal pores near the base, at U34; pharyngeo-intestinal junction at U37. Head bearing paired knob-like sensory organs and small, trapezoidal, fleshy lobes; body robust, with side lines slightly widening to mid-trunk, then gradually narrowing to a short, bilobed caudum; widths of head\neck\trunk\caudal base 46\34\56\26 µm at U07\U25\U46\U93, respectively.
Oral hood slightly protruding anteriorly (to U08), with gently undulating borders. Sensory hair sparse, up to 7 µm in length, forming a fringe around the oral opening and loose tufts at the tip of the head’s lobes; a single hair emerges from each knob-like tentacle; other sensory hairs 9–14 µm in length form lateral and dorsolateral columns that are evenly spaced within columns but differ between columns. Eight pairs of noticeable epidermal glands are regularly spaced along the pharyngeal and intestinal region from U11 to U 87 with glands of the second and eighth pairs positioned somewhat more lateral; glands are round in shape and roughly of the same size (7–9 µm in diameter), except for the one of the second pairs, markedly smaller (4–6 µm). Each gland opens to the exterior via a well structured pore, the produced material is excreted in the form of small round droplets (see Figure 4C, D).
Cuticular armature. Body covering apparently smooth as typical of the genus, however on the ventrolateral sides, the cuticle generates subrectangular plates (scales), partially overlapping each other and tightly arranged in two columns running from U11 to U88. Scales, roughly of the same size (1.5–2.0 µm), protrude from the body and form bilateral structures that recall the lateral aerodynamic ‘mini-skirts’ of racing cars.
Adhesive tubes. TbA, 4 per side, inserting directly on the body surface at U9-U 10, one 4 µm in length medially and three 6–7 µm in length laterally. TbL, 3 per side (8–10 µm in length) inserting respectively at U15, U58 and U90. TbD, absent. TbV, up to 16 per side; 4 (8–11 µm in length) inserted singly along the intestinal region from U44 to U63 while the remaining 12 (4–11 µm in length) form an impressive cluster centered at U85 (cTbV; Figs 2A, 3B, D); tubes in the cluster originate singly and their number may slightly change from side to side. TbP, 3 per side, 2 (5–6 μm in length) at the end of each pedicle of the furcated caudum and the other one (6.5 μm in length) flanking each caudal pedicle medially.
Ventral ciliation. A continuous, dense field of cilia arranged in transverse rows that extend from the ventral border of the oral opening to the base of the caudal pedicles, being broadest at mid body and somewhat sparse in the ano-genital area at U90.
Reproductive system. testis on the right body side, caudal organ pyriform (10 × 21 μm), at U78; frontal organ bladder-like (10 μm in diameter) at U74.5; maturing eggs dorsal to the mid intestine.
Length of the 4 living specimens ranged from 204 to 250 μm (mean = 230 μm, SD = 18 μm) all of them were mature (i.e., showed at least the testicles filled with sperm). The SEM prepared adult specimens resulted of smaller size (range 144–183 μm) even though size of these specimens appeared not dissimilar from the others under the dissecting microscope; these measurements fall well below the 5.5% length reduction allowed for fixed specimens (cf.
The genus Ptychostomella was originally created to include small thaumastodermatid gastrotrichs whose body is enveloped by a smooth cuticle i.e., a cuticle that does not give rise to the typical scales and/or spines (e.g., ancres) found in other members of the family (
Adhesive tubes of the ventral series forming ‘feet’ or ‘clusters’ are not uncommon among members of the family Thaumastodermatidae (e.g., Tetranchyroderma and Pseudostomella) and they are present also in members of the genus Ptychostomella e.g., Ptychostomella bergensis Clausen, 1996 (
Adhesive and aptic structures are universally present among interstitial animals (
An alternative hypothesis could be that these structures play a role during reproduction e.g., used for sperm transfer or holding of the partner during cross fertilization. Only future TEM studies revealing ultrastructural difference between the ventral tubules clustering near the ano-genital opening and the genuine adhesive tubes (e.g., single tubes) could make the second hypothesis on this subject most plausible. A study of reproductive behaviour would also be revealing.
| 1 | dorsal surface smooth | 2 |
| – | other | 11 |
| 2 | lateral margins smooth | 3 |
| – | other | 10 |
| 3 | eyespots present | Ptychostomella ommatophora Remane, 1927 |
| – | other | 4 |
| 4 | head with knob-like or club-shaped sensory organs | 5 |
| – | other | 8 |
| 5 | head with paired club-shaped sensory organs | Ptychostomella helane Roszczak, 1939 |
| – | head with paired knob-like sensory organs | 6 |
| 6 | adhesive tubes between the caudal pedicles present | 7 |
| – | adhesive tubes between the caudal pedicles absent | Ptychostomella tyrrhenica Hummon, Todaro & Tongiorgi, 1993 |
| 7 | 4 (2 + 2) adhesive tubes between the caudal pedicles | Ptychostomella mediterranea Remane, 1927 |
| – | up to 10 (5 + 5) adhesive tubes between the caudal pedicles | Ptychostomella higginsi Clausen, 2004 |
| 8 | with some of the TbV forming a pair of clusters or ventral feet (4 + 4 tubes each) | Ptychostomella bergensis Clausen, 1996 |
| – | without cluster of TbV | 9 |
| 9 | TbV evenly space along the intestinal region | Ptychostomella jejuensis Lee, Hwang & Chang, 2009 |
| – | TbV gathered in the first third of the intestinal region | Ptychostomella pectinata Remane, 1926 |
| 10 | each lateral side bearing a column of rod-like papillae | Ptychostomella brachycephala (Levi, 1954) |
| – | each lateral side bearing a column of subrectangular scales | Ptychostomella lamelliphora n. sp. |
| 11 | cuticular covering bearing scale-like elevantions | Ptychostomella lepidota Clausen, 2000 |
| – | other | 12 |
| 12 | cuticular covering embossed with smooth hemispherical elevantions | Ptychostomella orientalis Lee & Chang, 2003 |
| – | dorsal surface with terrace-shaped cuticular protrusions on head and numerous papillae with sensory hair(s) | Ptychostomella papillata Lee & Chang 2003 |
This work was mainly supported by the State of São Paulo Research Foundation (FAPESP) within the BIOTA/FAPESP - The Biodiversity Virtual Institute Program. I’m grateful to Carlos Rocha for inviting me to perform the research. Many thanks to the staff of CEBIMar for the invaluable assistance received during the stay at the São Bebastião laboratory. Additional funding for the study was provided by the BIOTOME project (UNIMORE). Comments by R. Hochberg and an anonymous reviewer improved the readability of the article.