Citation: Skvarla MJ, Fisher JR, Dowling APG (2014) A review of Cunaxidae (Acariformes, Trombidiformes): Histories and diagnoses of subfamilies and genera, keys to world species, and some new locality records. ZooKeys 418: 1–103. doi: 10.3897/zookeys.418.7629
Cunaxidae are predaceous mites found in a variety of habitats. This work provides comprehensive keys to world subfamilies, genera, and species. Diagnoses and historical reviews are provided for subfamilies and genera.
Cunaxa boneti, C. denmarki, C. exoterica, C. floridanus, C. lehmanae, C. lukoschusi, C. metzi, C. myabunderensis, C newyorkensis, C. rackae, C. reevesi, and C. reticulatus are moved to Rubroscirus and C. otiosus, C. valentis, and C. rasile are returned to Rubroscirus. Cunaxoides neopectinatus is moved to Pulaeus. Neocunaxoides pradhani and N. gilbertoi are transferred to Scutopalus. Pulaeus minutus and P. subterraneus are moved to Lupaeus. Pseudobonzia bakari, P. malookensis, and P. shamshadi are transferred to Neobonzia. Dactyloscirus bifidus is transferred to Armascirus.
Scirula papillata is reported from the Western Hemisphere for the first time. Armascirus ozarkensis, A. primigenius, and Dactyloscirus dolichosetosus are reported from new localities.
Identification, key, Bdelloidea, Prostigmata, Eupodina
Cunaxidae (Fig. 1) are common predatory mites that are present in forest systems, grasslands, agricultural fields, and anthropogenically disturbed areas. Surveys of mites in these habitats often report only family or generic-level identification. This is problematic because little is known about where cunaxid species occur, both regionally and in what habitats, and unfortunate because such reports are potentially very useful collectively if species were identified.
Examples of cunaxids in ethanol illustrating how they would appear while sorting. 1a Armascirus 1b Cunaxa 1c Pulaeus 1d Parabonzia 1e Coleoscirus 1f Neobonzia.
Part of the reason behind the lack of specific identification is the difficulty in reliably identifying cunaxids without extensive knowledge of the primary literature. Keys to cunaxid species are often regional, so of little use to researchers outside of that specific region, and scattered across countless journals. The last comprehensive attempt to present keys to world species was by
Biology. All cunaxids are thought to be opportunistic predators, though an undescribed Rubroscirus was observed to drink drops of honeydew in addition to feeding on live prey (
Both ambush and active hunting have evolved within the family, sometimes within the same subfamily. Within Cunaxinae, for instance, Armascirus and Dactyloscirus wait, sometimes for hours, to ambush prey (
Cunaxids occur in most terrestrial habitats, including soil and leaf litter (
While cunaxids are often often found on plants in agricultural settings, their effect on prey populations is unclear.
Cunaxids appear to be active year round.
Cunaxids have been reported to be found phoretically on bark beetles, though they were not identified to species (
Both sexual reproduction and thelytokous parthenogenesis have been reported in cunaxids (
Cunaxids spin silk, which is used for a variety of purposes. Cunaxatricha tarsospinosa produces a webbing around eggs laid on leaves, but not branches;
Biogeography. Cunaxids have been found on every continent except Antarctica. South Africa and the Philippines have the most well-documented cunaxid diversity – 68 and 57 species respectively – thanks to the efforts of Den Heyer and Corpuz-Raros (
The cunaxid fauna of Europe and North America north of Mexico fall between these extremes. Most reports have been sporadic and span more than a century, beginning with
The diagnoses and keys presented are based on published descriptions and examination of available type specimens. However, for many species the types were not available for examination. The accuracy of the keys is therefore dependent upon the accuracy of the published descriptions. This also influenced which characters were chosen for couplets. Often a character that is potentially useful and informative (such as the presence or absence of a cheliceral seta) was not reported in the original description. Thus, unlike previous keys, characters such as setal counts of leg segments were often preferred. This may prove to be problematic as extra setae are sometimes reported on leg segments; however, examination of multiple specimens in a population should help overcome this.
An effort is made to utilize terminology that is broadly applicable and well-accepted across mite taxa, despite conventions used among bdelloid researchers. Some terms widely used by bdelloid researchers are either inaccurate or outdated, and others are misleading. Therefore, we follow the suggestions outlined by
Subcapitulum. The part of the gnathosoma that bears the palps and chelicerae has been variously termed by researchers of Bdelloidea. One such term – hypostome – more properly refers to the area of the subcapitulum anterior to the oral opening (
Body segmentation. The terminology associated with the acariform idiosoma remains controversial. Classically, these regions have been most widely called the propodosoma and hysterosoma. However,
Phylogenetic analyses of large datasets that include molecular data has corroborated previous suspicions of the non-monophyly of “Acari” and provided substantial support for a clade that combines camel spiders with acariforms called Poecilophysidea (
Therefore, we continue with the suggestions of
Idiosomal setae. For hysterosomal setae, we follow the notation of
Therefore, we reject the suggestion of Den Heyer and
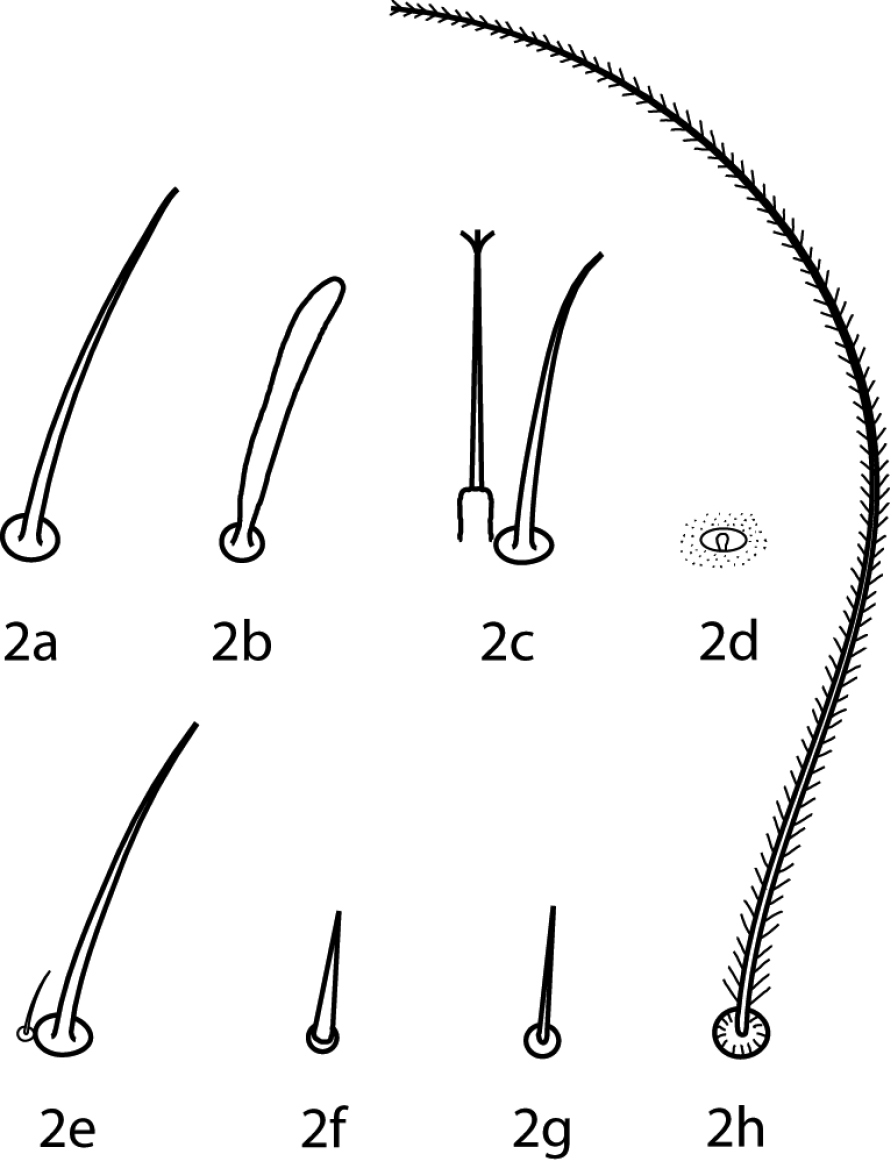
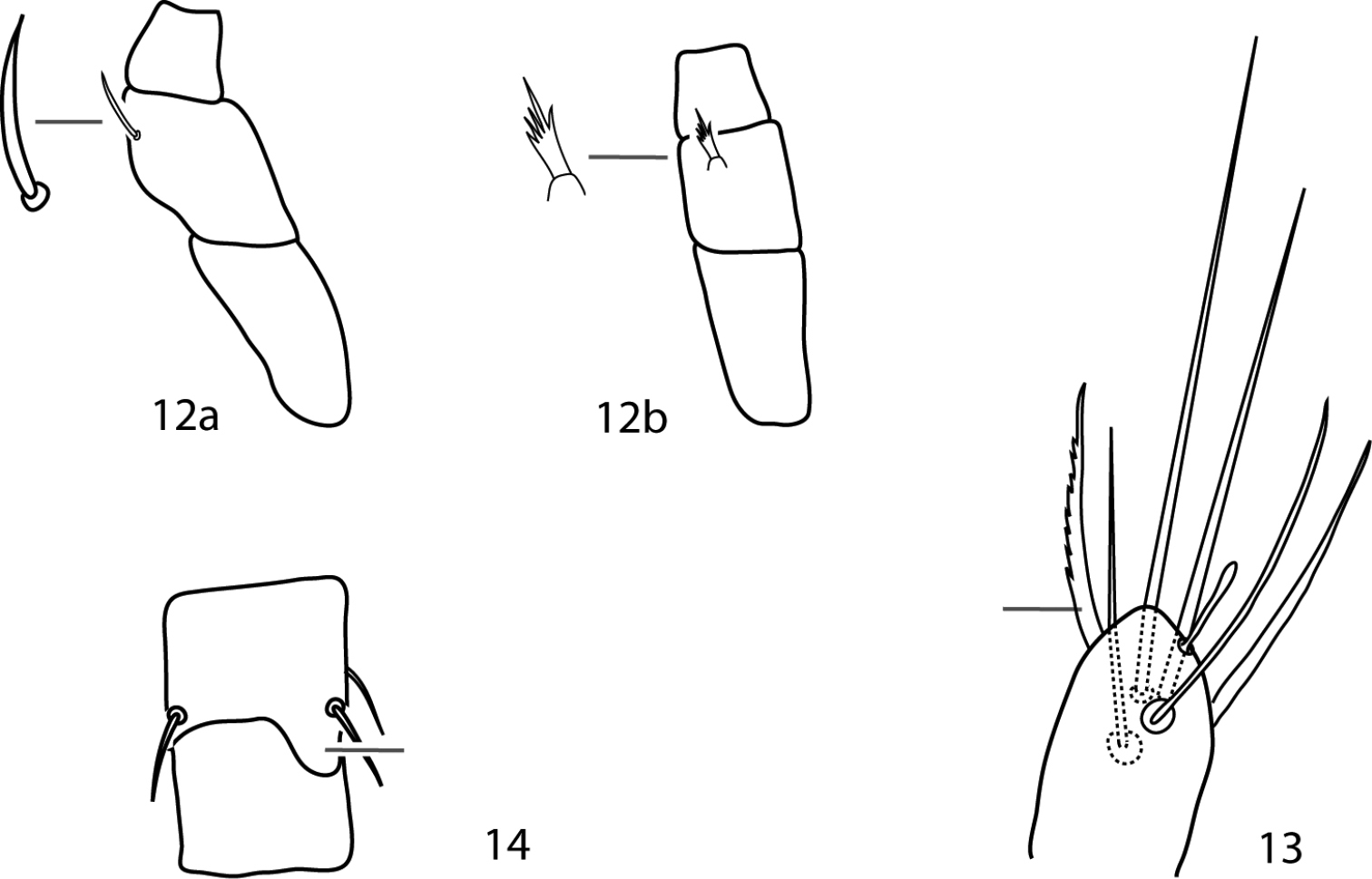
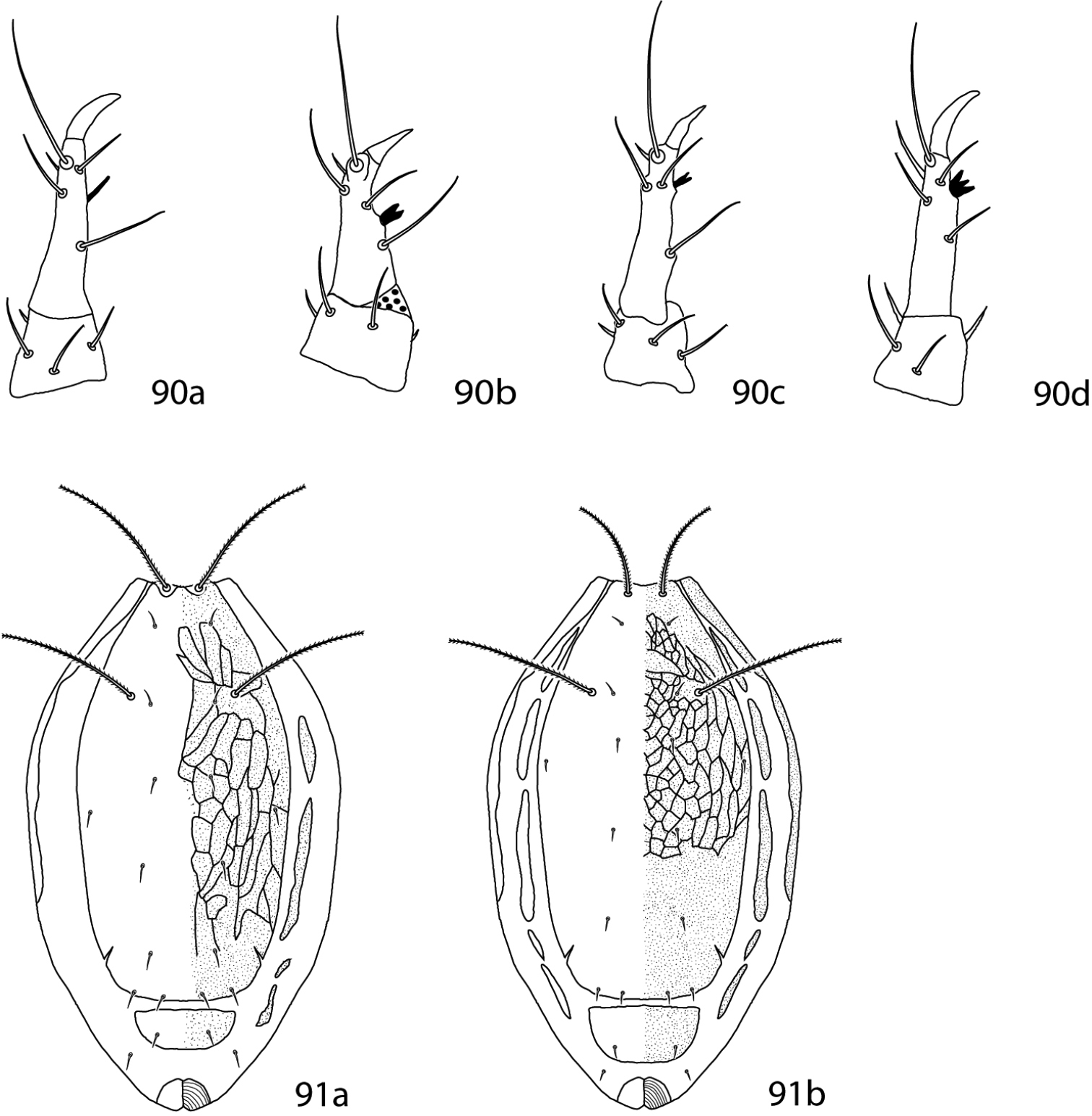
Abbreviations. The following abbreviations (Fig. 2) are used: attenuate solenidion (asl), blunt rod-like solenidion (bsl), famulus (fam)(=peg organ), microseta (mst), solenidion (s) (this is used only when a description does not specify what type of solenidion and may refer to any solenidion type), spine-like seta (spls), simple tactile seta (sts), trichobothrium (T). When setal types are not specified (e.g., coxae I–IV setal formula 5-5-4-3) it is assumed all setae are simple (sts).
Setal types. Relative sizes will vary within a given setal type 2a Attenuate solenidion (asl) 2b Blunt rod-like solenidion (bsl) 2c Elongate, tri-pronged famulus (fam), as seen in Dactyloscirus 2d Famulus (fam), as seen in the majority of cunaxids 2e Duplex setae - microseta (mst) and attenuate solenidion 2f Spine-like seta (spls) 2g Simple tactile seta (sts) 2h Trichobothrium (T).
Illustrations were produced using the methods outlined by
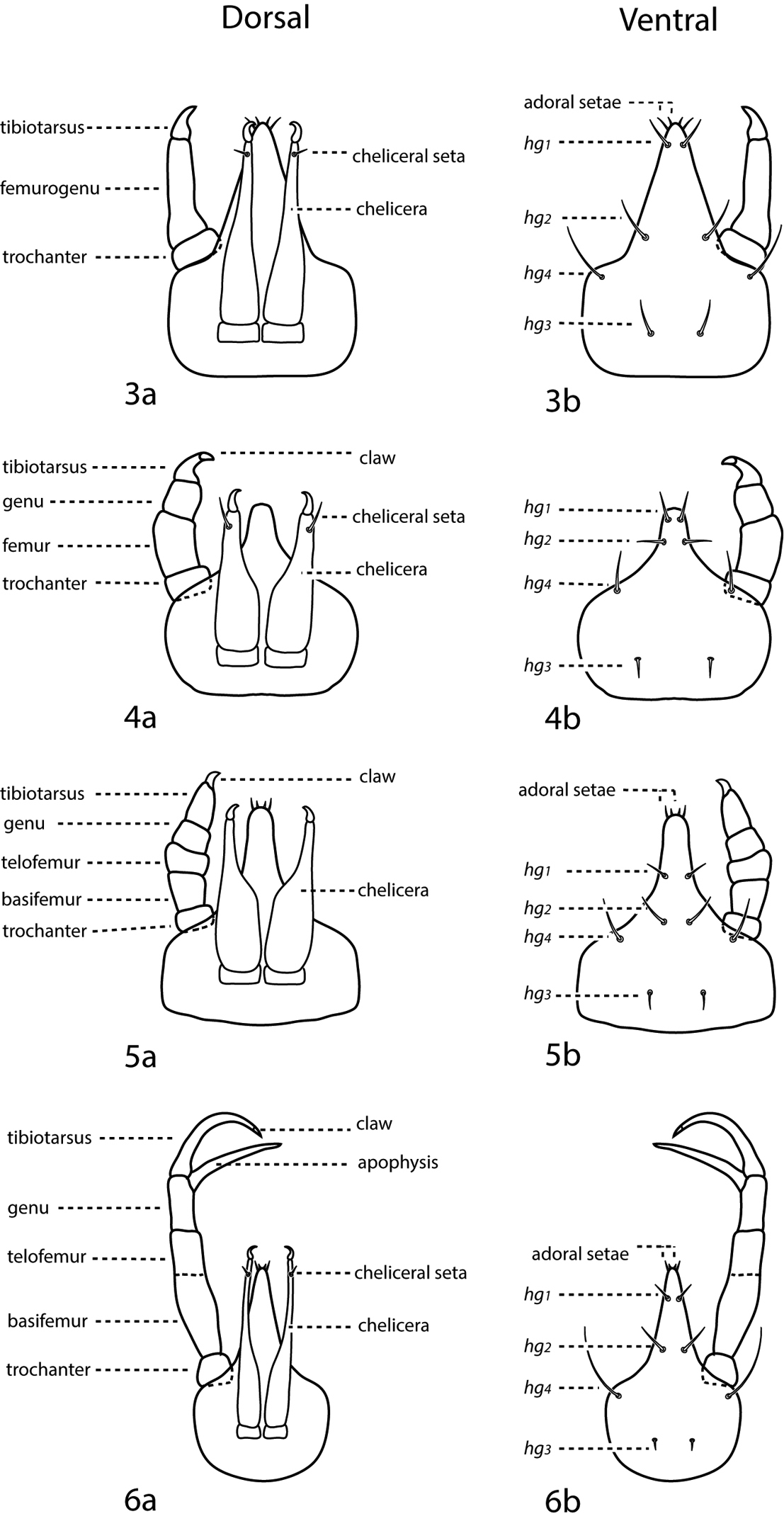
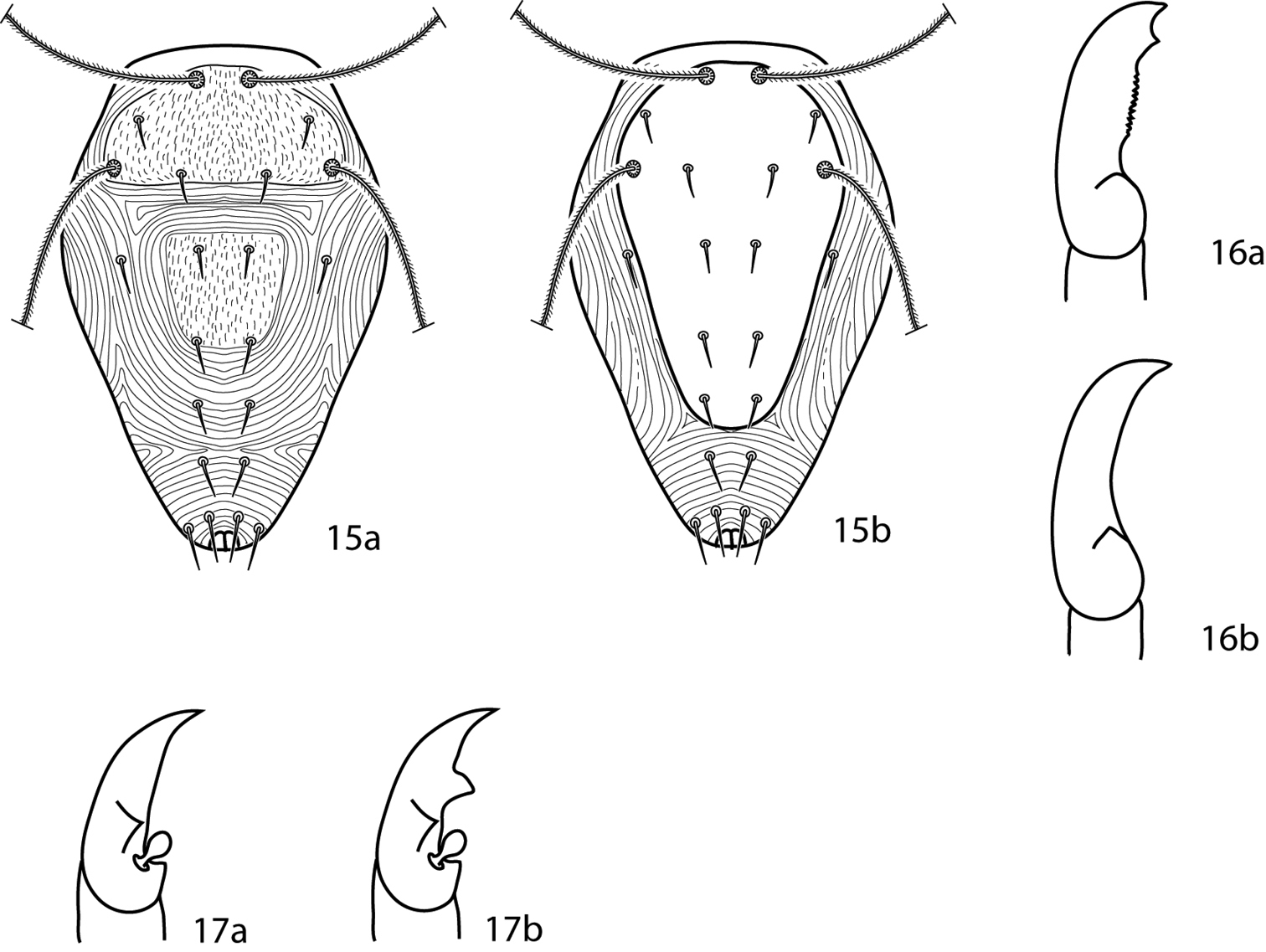
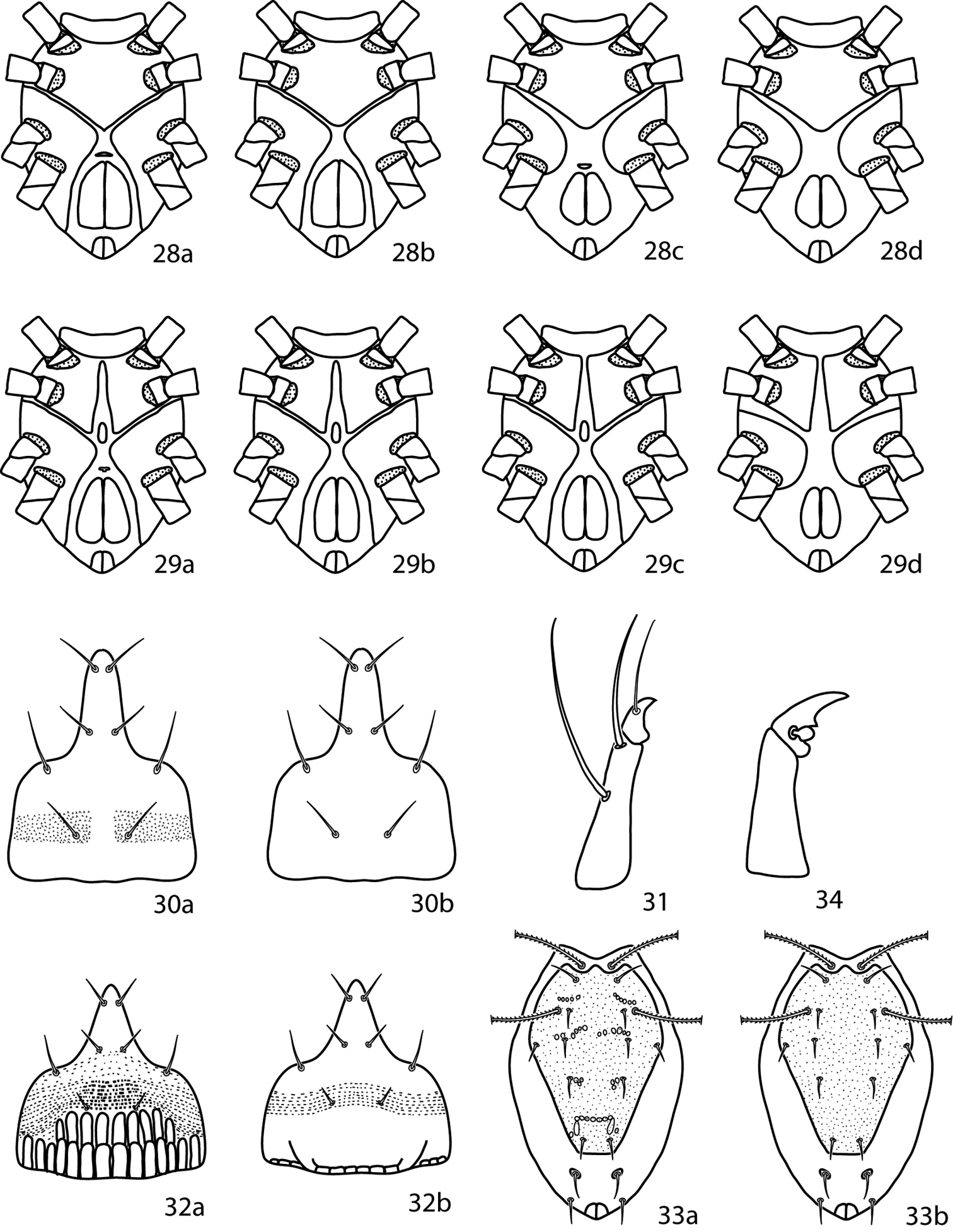
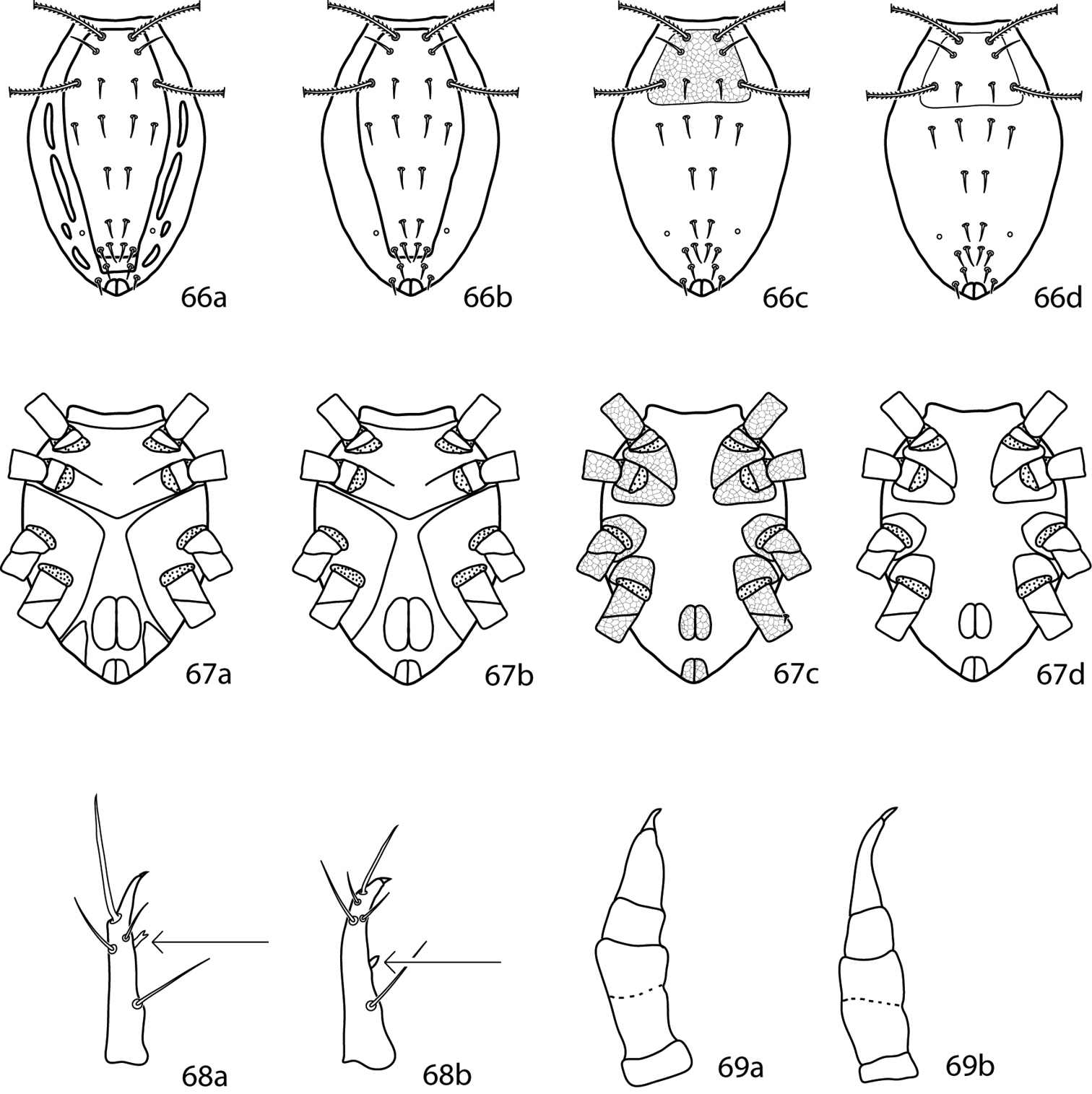
Gnathosoma (Figs 3–6). Pedipalps 3-, 4-, or 5-segmented and end in a strong claw (except in Pseudobonzia). They may be shorter than, equal to, or extend beyond the distal end of the subcapitulum. Femora of 5-segmented pedipalps divided into basi- and telofemora, though may be secondarily fused; a dark line often indicates the previous articulation (Fig. 5a, b illustrate a fully divided femur and Fig. 6a, b illustrate a secondarily fused femur. This is for illustration purposes only, i.e., cunaxids with long and short 5-segmented pedipalps may have either fully divided or secondarily fused femora). Telofemora and genua are uniquely fused in Allocunaxa, though the basifemoral/telofemoral articulation is present. Apophyses present or not on the telofemora, adjoining the genua and tibiotarsi, or on the tibiotarsi. Subcapitulum wedge-shaped and may be patterned with random dots or papillae, dots or papillae forming lines, a single row of cells on the posterior edge, or reticulations forming polygonal cells. Subcapitulum with up to 6 pairs of setae are present: hg1–4 and 2 pairs of adoral setae. Seta hg1 usually straight, but geniculate in Bonziinae and may be curved in Neoscirula; hg4 often longest pair of subcapitular setae. Chelicerae with or without seta near the cheliceral digit.
a. dorsal. b. ventral. 3 3-segmented pedipalp (Cunaxoidinae) 4 4-segmented pedipalp (Scirulinae) 5 5-segmented pedipalp that does not extend beyond the subcapitulum by more than the distal half of the genua (Bonziinae, Coleoscirinae, and Orangescirulinae) 6 5-segmented pedipalp that reaches beyond the subcapitulum by at least the distal half of the genua (Cunaxinae).
Idiosoma, dorsal (Fig. 7a). Idiosoma diamond-shaped. Dorsal proterosoma covered with a sclerotized shield that bears 2 pairs of setae (lps and mps) and 2 pairs of setose sensilla (at and pt); rarely one pair of setae or sensillae absent. Dorsal hysterosoma complemented with 0–2 large shields or plates and 0–4 pairs of platelets. These plates and platelets may capture one or more pairs of setae. Up to 8 pairs of dorsal hysterosomal setae present (c1–h1, c2, f2, and h2); h2 may occur ventrally. Setae may occur on small platelets that are barely larger than the setal socket. Integument not covered in shields, plates, or platelets is striated. Cupule im present, usually laterad and slightly posterior to e1. Dorsal idiosomal shields and plates smooth or patterned with random dots or papillae, dots or papillae forming lines, reticulations forming polygonal cells, or cells which form rows.
Generalized schematic of cunaxid idiosomal morphology. 7a Dorsal. 7b Ventral.
Idiosoma, ventral (Fig. 7b) Ventral idiosoma may be complemented with 1 or a few small platelets in addition to the coxae. Coxae fused to body and form plates. Coxae I–II are often fused in adults and may coalesce medially to form a sternal shield. Coxae III–IV are often fused in adults and may extend caudally beyond the genital plates. Each coxa complemented with 0–4 setae; in addition, extensive coxae or sternal shields may capture setae normally on the integument and therefore have more. Coxae may be plain or patterned with random dots or papillae, dots or papillae forming lines, or reticulations forming polygonal cells. Genital plates (sometimes called anal valves) present in adults and bear 3 (rarely) or 4 (usually) setae, except in Parabonzia which have up to 9 pairs of setae. 2 pairs of genital papillae visible underneath the plates. Anal plates (sometimes called anal valves) bear 1–2 setae (ps1-2). Setae ps2 may occur off the anal plates. Legs 6-segmented in larvae, 7-segmented in nymphs and adults. In adults these segments are coxa, trochanter, baifemur, telofemur, genu, tibia, and tarsus, however, the coxae are often treated separately from the other leg articles. Femora undivided in larvae. Trichobothrium present on leg tibia IV. Ambulacral claws present on either side of a 4-rayed empodium.
(modified from
| 1 | Pedipalpal telofemoral multi-branched seta present (except Parabonzia mindanensis) (Fig. 7a) | Bonziinae |
| – | Pedipalpal telofemoral multi-branched seta absent | 2 |
| 2 (1) | Pedipalps 3-segmented (Figs 3a, b) | Cunaxoidinae |
| – | Pedipalps 4-segmented (Figs 4a, b) | Scirulinae |
| – | Pedipalps 5-segmented (basi-and telofemora may be partially fused) (Figs 5a, b; 6a, b) | 3 |
| 3 (2) | Pedipalps extend beyond the subcapitulum by at most the distal half of the tibiae (Figs 5a, b) | 4 |
| – | Pedipalps extend beyond the subcapitulum by at least the distal half of the tibiae (Figs 6a, b) | Cunaxinae |
| 4 (3) | Trichobothrium on tibiae IV present; setae hg1 not geniculate; cheliceral seta usually present | Coleoscirinae |
| – | Trichobothrium on tibiae IV absent; setae hg1 geniculate; cheliceral seta absent | Orangescirulinae |
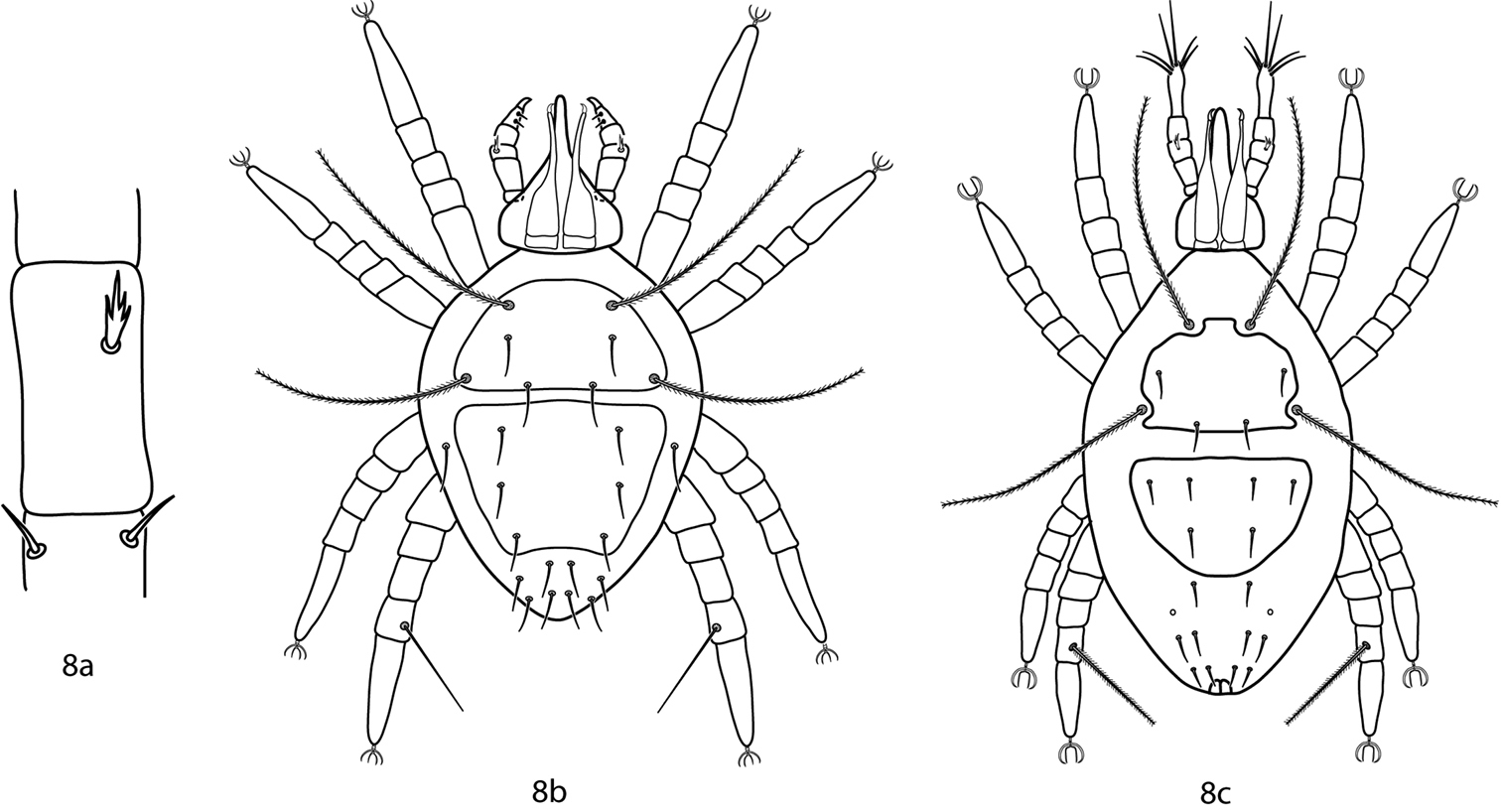
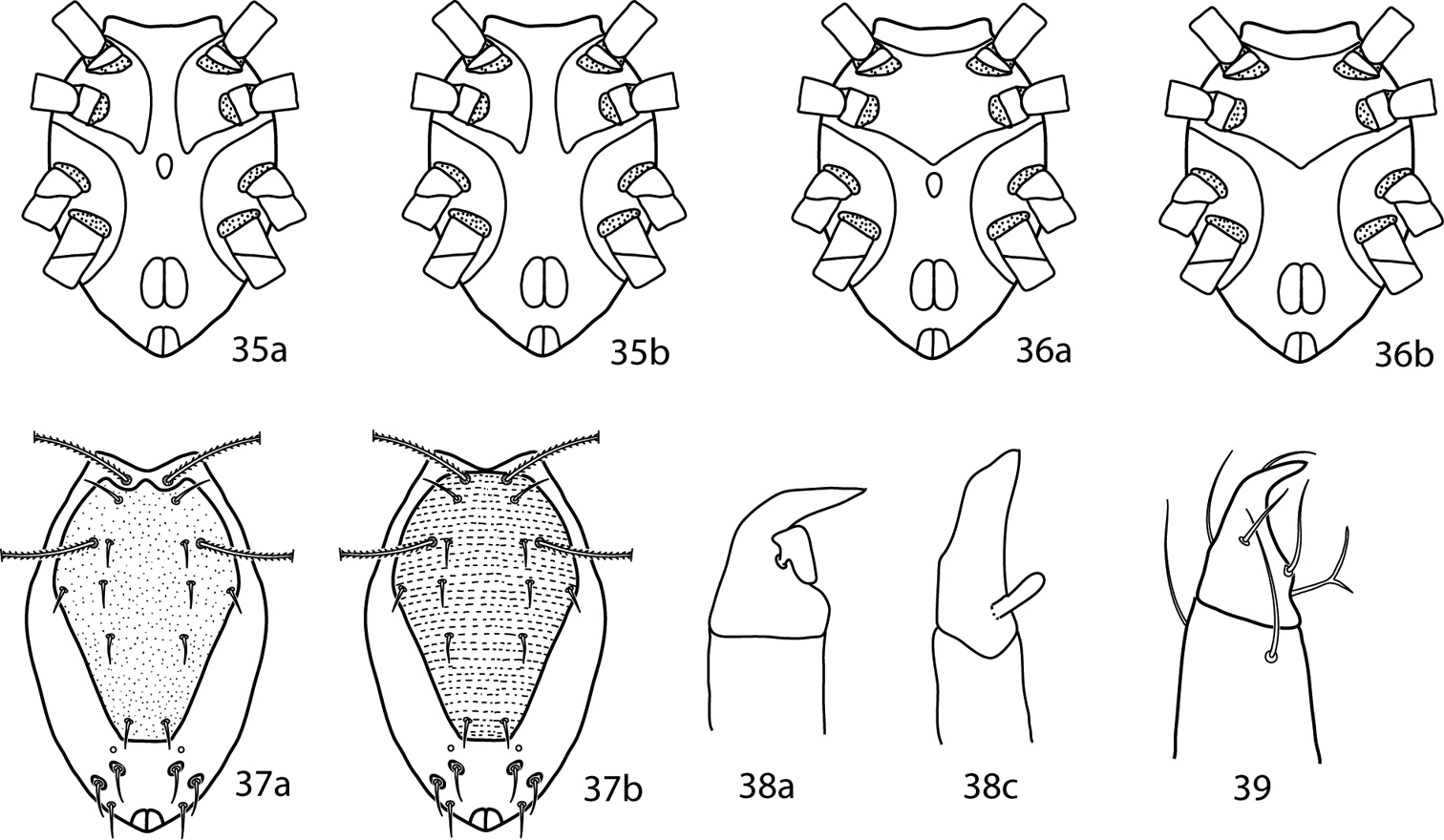
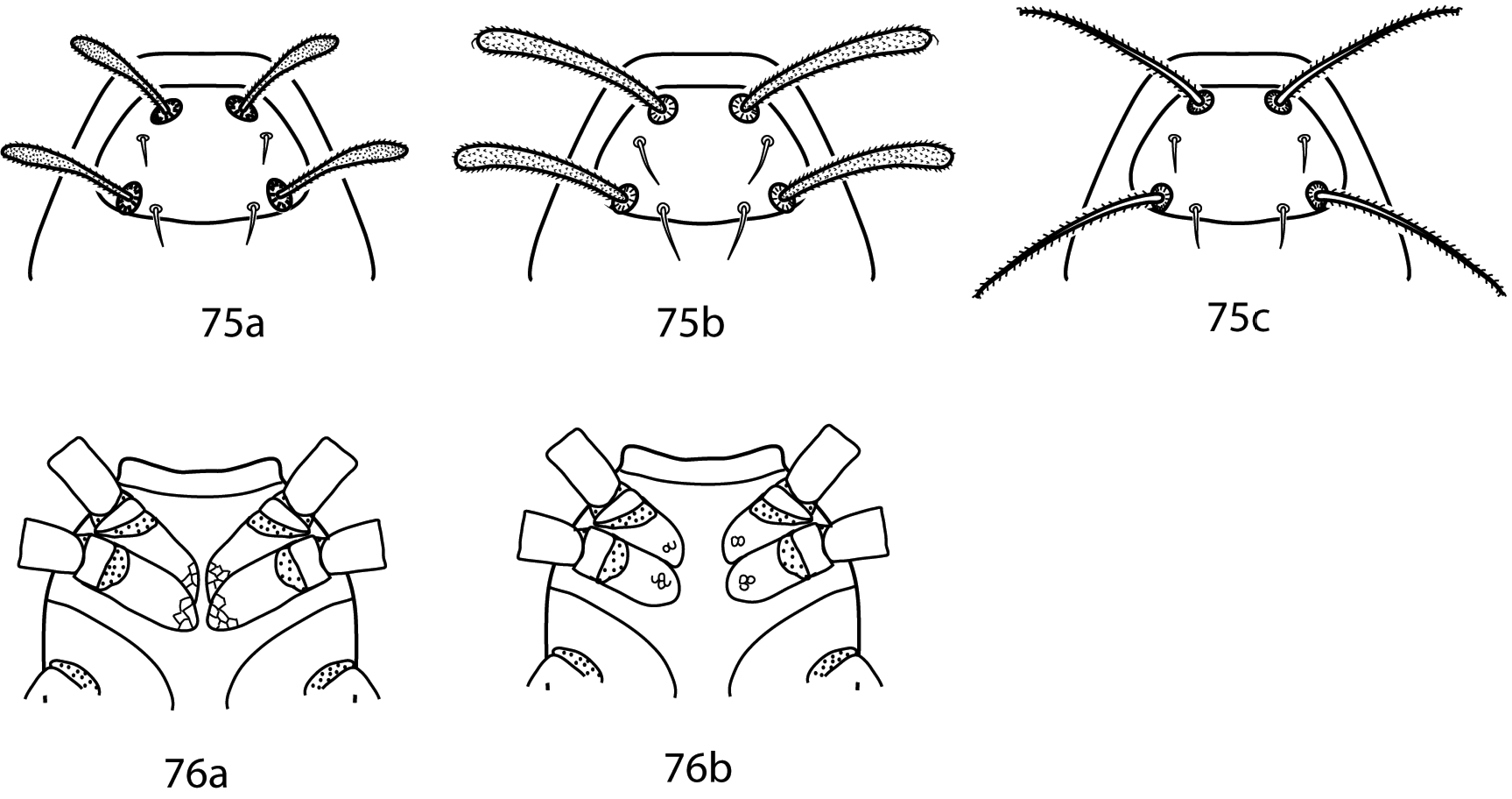
Gnathosoma. Pedipalps 5-segmented and reach beyond the subcapitulum by at most the distal half of the tibiae. Apophyses absent. A multi-branched seta present dorsally on the telofemora. Tibiotarsi terminate in a stout claw or two strong setae. 2 pairs of adoral setae present or absent. Subcapitulum with 4 pairs of setae (hg1–4) present in Bonzia; up to 6 pairs of subcapitular setae (hg1-4 + additional setae) present in Parabonzia.
Idiosoma, dorsal. Proterosoma bears a shield complemented with 2 pairs of setae (at and pt) and 2 pairs of setose sensillae (lps and mps). Dorsal hysterosoma may bear a shield; if a shield is present it may be complemented with a variable number of setae depending on the extent of the shield. Setae c1–h1, c2, f2 and h2 present and are smooth or spiculate. Cupule im present laterad and caudally of e1. Integument that does not bear shields or plates is striated.
Idiosoma, ventral. Coxae I–II fused or not and coxae III–IV fused or not. Genital plates bear 4–9 setae; 2 pairs of genital papillae visible underneath the plates. Up to 4 pairs of setae present on the anal plates. Up to 9 pairs of setae present on the integument between coxae II and the anal plates. Legs. Trichobothrium present on leg tibia IV. The ambulacral claws occur on either side of a 4-rayed empodium.
(modified from
| 1 | Pedipalp tibiotarsal claw present; 2 pedipalp tibiotarsal spine-like tubercles present (Fig. 8b); genital plates with 4 pairs of setae; internal genital setae absent | Bonzia Oudemans, 1927 |
| – | Pedipalp tibiotarsal claw absent; 2 pedipalp tibiotarsal spine-like tubercles absent (Fig. 8c); genital plates with 5–9 pairs of setae; internal genital setae present | Parabonzia Smiley, 1975 |
Bonziinae key illustrations. 8a Telofemoral branched seta present in Bonziinae 8b Bonzia 8c Parabonzia.
Gnathosoma. Pedipalps 5-segmented and reach beyond the subcapitulum by at most the distal half of the tibiae. Apophyses absent. A dorsal multi-branched seta present on the telofemora. The tibiotarsi terminate in a stout claw. 2 pairs of adoral setae present or absent. Subcapitulum with 4 pairs of setae (hg1–4) present. Setae hg1 are geniculate.
Idiosoma, dorsal. proterosoma bears a shield complemented with 2 pairs of setae (at and pt) and 2 pairs of setose sensillae (lps and mps). The dorsal hysterosoma bears a shield that may be complemented with a variable number of setae depending on the extent of the shield. Setae c1–h1, c2, f2 and h2 present, and are smooth or spiculate. Cupule im present laterad and caudally of e1. Integument that does not bear shields or plates is striated.
Coxae I–II fused and coxae III–IV fused. Genital plates bear 4 setae; 2 pairs of genital papillae visible underneath the plates. 4 pairs of setae present on the anal plates. Trichobothrium on leg tibia IV present. Ambulacral claws occur on either side of a 4-rayed empodium.
(modified from
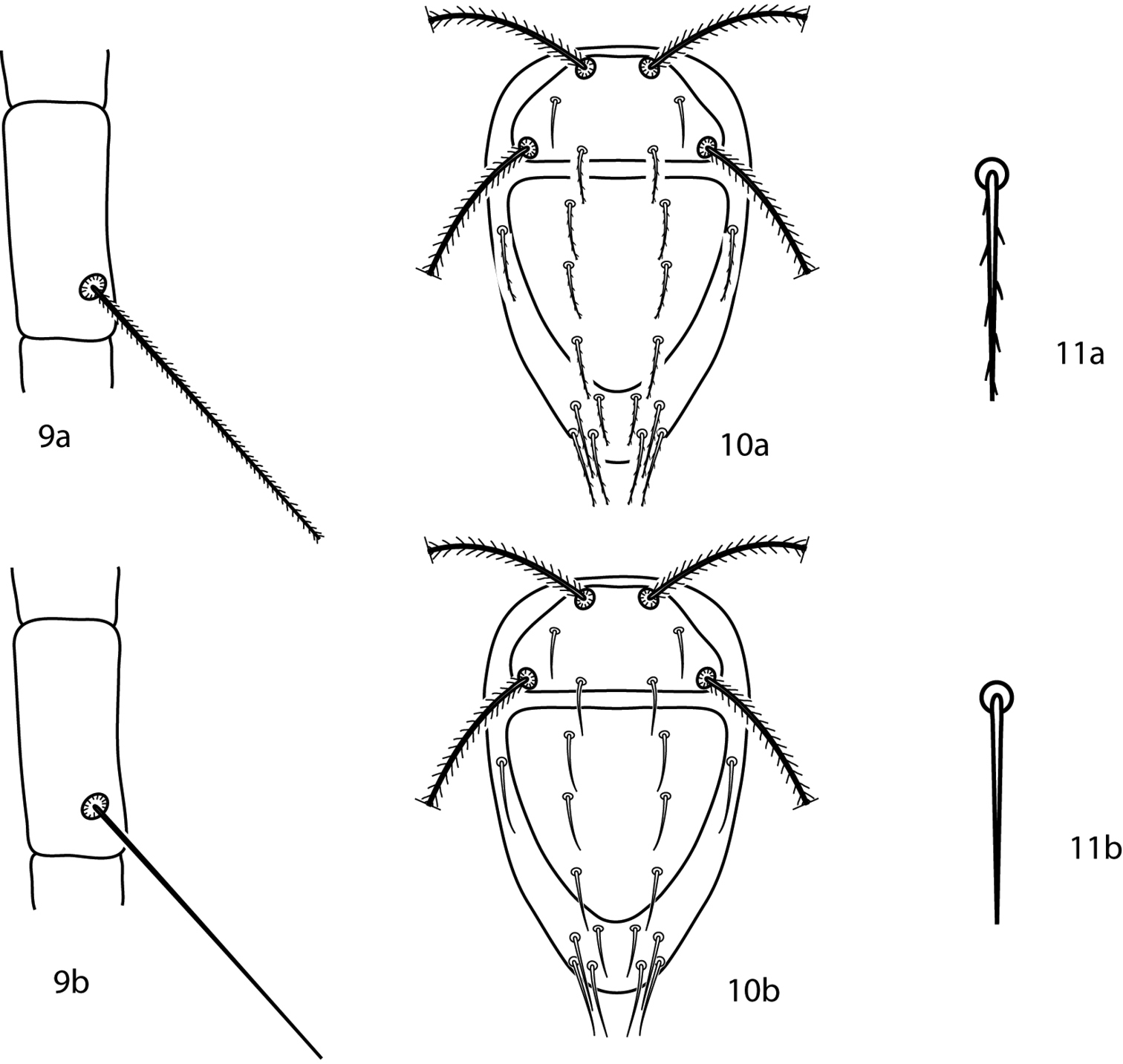
| 1 | Tibiae IV trichobothrium setose (Fig. 9a) | 2 |
| – | Tibiae IV trichobothrium smooth (Fig. 9b) | 3 |
| 2 (1) | Hysterosomal shield with 2 pairs of setae; Germany | Bonzia sphagnicola Willmann, 1939 |
| – | Hysterosomal shield with 3 pairs of setae; N. America, S. America, Europe (possibly cosmopolitan) | Bonzia halacaroides Oudemans, 1927 |
| 3 (1) | Dorsal setae spiculate (Figs 10a, 11a); New Zealand | Bonzia woodi Smiley, 1992 |
| – | Dorsal setae smooth (Figs 10b, 11b); USA: Virginia, Ozark Highlands | Bonzia yunkeri Smiley, 1992 |
Bonzia key illustrations. 9a Setose tibial trichobothrium 9b Smooth tibial trichobothrium 10a Spiculate dorsal setae 10b Smooth dorsal setae 11a Close up of a spiculate seta 11b Close up of a smooth seta.
Gnathosoma. Pedipalps 5-segmented and reach beyond the subcapitulum by at most the distal half of the tibiae. Apophyses absent. A multi-branched seta present dorsally on the telofemora. Tibiotarsi terminate in two strong setae. 2 pairs of adoral setae present or absent. Subcapitulum with up to 8 pairs of setae present.
Idiosoma, dorsal. Proterosoma bears a shield complemented with 2 pairs of setae (at and pt) and 2 pairs of setose sensillae (lps and mps). Dorsal hysterosoma may bear a shield; if a shield is present it may be complemented with a variable number of setae depending on the extent of the shield. Setae c1–h1, c2, f2 and h2 present and smooth. Cupule im is present laterad and caudally of e1. Integument that does not bear shields or plates is striated.
Idiosoma, ventral. Coxae I–II fused or not and coxae III–IV fused or not. Genital plates with up to 9 pairs of setae; 2 pairs of genital papillae visible underneath the plates. Up to 4 pairs of setae present on the anal plates. Up to 9 pairs of setae on the integument between coxae II and the anal plates. Legs. Trichobothrium on leg tibia IV present. The ambulacral claws occur on either side of a 4-rayed empodium.
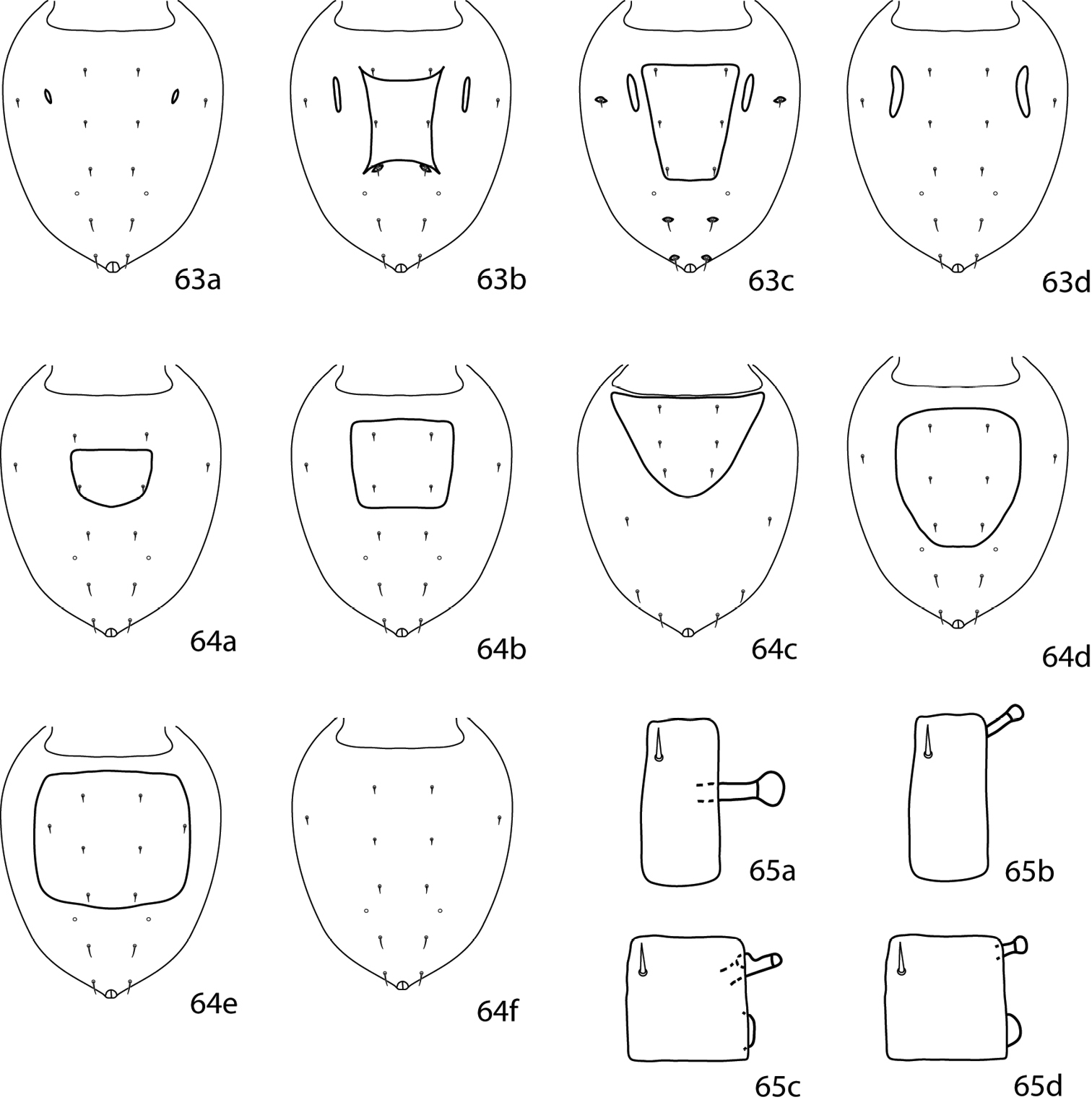
| 1 | 8–9 genital setae present | 2 |
| – | 6–7 genital setae present | 3 |
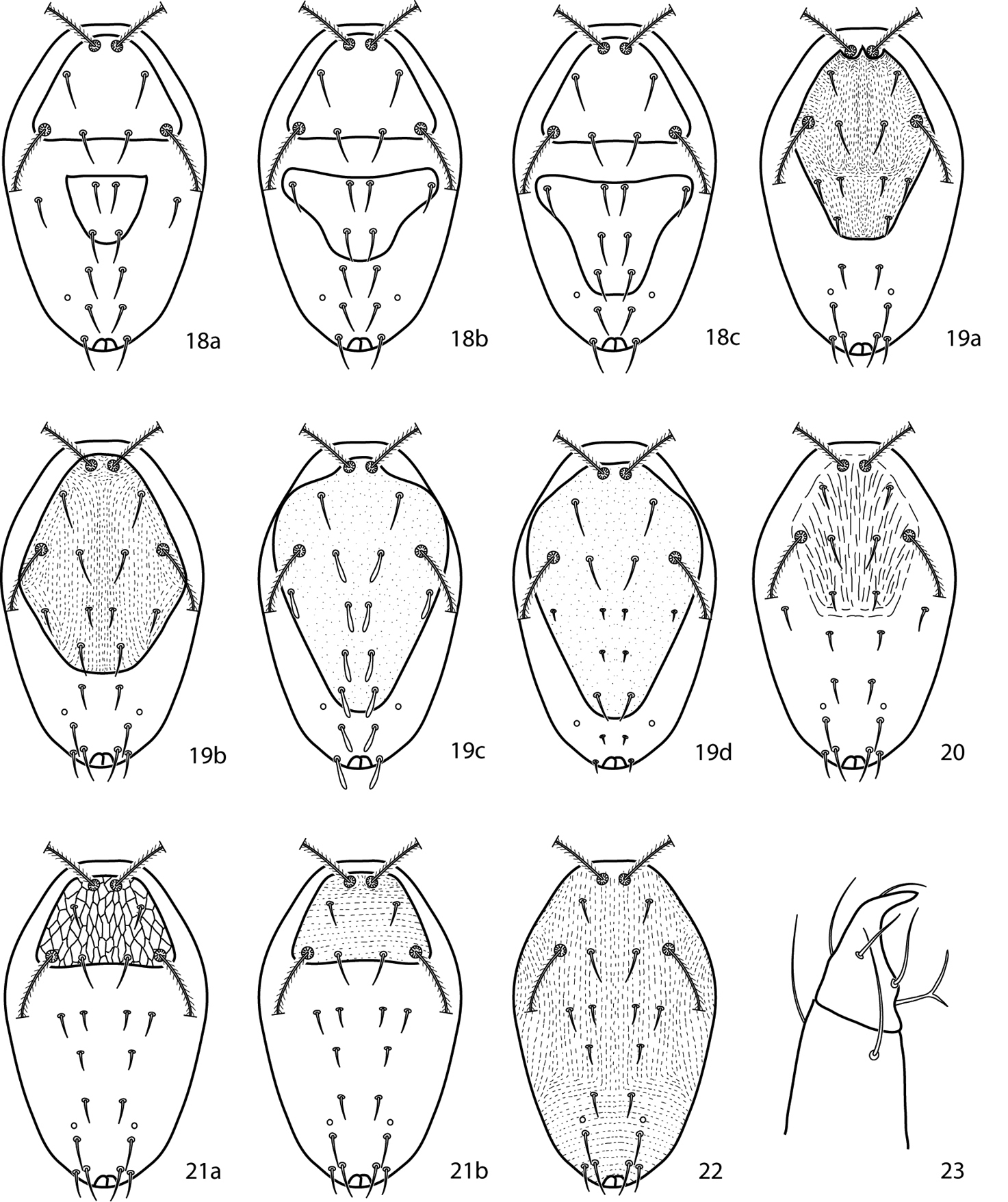
| 2 (1) | Pedipalpal telofemoral seta unbranched (Fig. 12a); Philippines, Mindanao Is | Parabonzia mindanensis Corpuz-Raros, 1996 |
| – | Pedipalpal telofemoral seta branched, with 4–5 tines (Fig. 12b); China: Hubei Province | Parabonzia zhangi Lin & Zhang, 2002 |
| 3 (1) | Hysterosomal shield with 3 pairs of setae | 4 |
| – | Hysterosomal shield with 4 pairs of setae | 6 |
| 4 (3) | Pedipalpal tibiotarsal sigmoid setae lightly barbed (Fig. 13); South Africa: West Transvaal | Parabonzia marthae (Den Heyer, 1975) |
| – | Pedipalpal tibiotarsal sigmoid setae smooth | 5 |
| 5 (4) | Large spur-like process present on femora III (Fig. 14); USA: Florida | Parabonzia mumai Smiley, 1992 |
| – | Large spur-like process absent on femora III; Ivory Coast | Parabonzia athiasae Den Heyer, 1977 |
| 6 (3) | Coxae I–IV setal formula 7-5-6-7 sts; basifemora I–IV setal formula 4-7-3-2 sts; China: Fujian | Parabonzia trioxys Lin & Zhang, 1998 |
| – | Coxae I–IV setal formula 6-6 (sometimes 7)-7-7 sts; basifemora I–IV setal formula 5-8-3-2 sts; USA, Russia | Parabonzia bdelliformis (Atyeo, 1958) |
Parabonzia key illustrations. 12a Unbranched pedipalp telofemoral seta 12b Multi-branched pedipalp telofemoral seta 13 Lightly barbed pedipalp tibiotarsal sigmoid seta 14 Spur-like process on femora III.
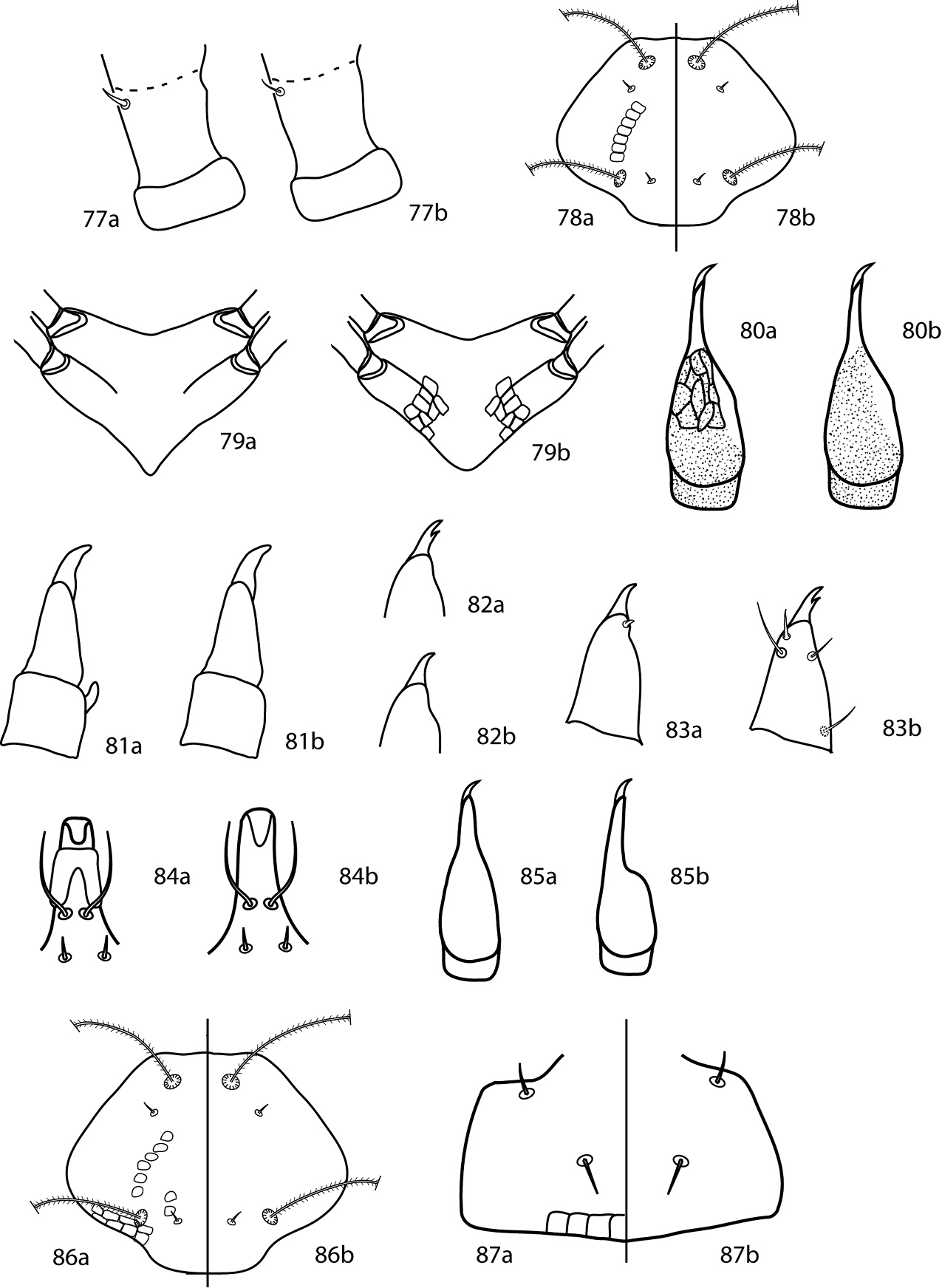
Gnathosoma. Pedipalps 3-segmented: a trochanter which lacks setae, fused femurogenu (femur + genu) which is complemented with 5 or 6 setae, and tibiotarsus (tibia + tarsus) which is complemented with 5 or 6 setae. Tibiotarsi may be complemented with a bladder- or bulb-like apophysis. Pedipalps do not reach beyond the subcapitulum by more than the distal half of the tibiotarsi. Chelicera with or without seta near the cheliceral digit. Subcapitulum with 4 pairs of setae (hg1–4) are present; setae hg4 is often the longest. 2 pairs of adoral setae are present or absent.
Idiosoma, dorsal. Female with proterosomal shield (absent in Cunaxoides ulcerosus) which is complemented with two pairs of setae (lps and mps) and two pairs of setose sensillae (at and pt) and may bear a hysterosomal plate complemented with a varying number of setae; when present the dorsal hysterosomal plate may be fused with the proterosomal shield. Dorsal plates well demarcated or not. Dorsal setae c1–h1 are present; c2, f2 and h2 may also be present. If f2 is present, f1 and f2 may be located together on a small platelet. Setae not on larger plates may be born on small platelets barely larger than the setal socket. Cupule im present laterad and posterior of e1. Integument that is not covered in shields or plates is striated
Idiosoma, ventral. Coxae of female vary in size, from being restricted to the trochantral bases to being extensive and nearly forming a holoventral shield. Coxae may or may not be well demarcated. Coxae I–II fused (usually) or not, coxae III–IV fused (usually) or not. Coxae I–II may coalesce medially to form a sternal shield. The genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath the plates. The anal plates bear one pair of setae (ps1); one pair of setae is present ventrally on the integument near the anal plates (either ps2 or pa). Cupule ih is present ventrally laterad the integumental setae associated with the anal plates. The integument that is not covered in shields or plates is striated. Legs. Tarsi never constricted apically so as to end in lobes. Trichobothrium on leg tibia IV present. Ambulacral claws are rippled and occur on either side of a 4-rayed empodium.
(modified from
| 1 | Pedipalpal tibiotarsi with 3 sts, 1 spls; New Zealand | Paracunaxoides Smiley, 1992 |
| – | Pedipalpal tibiotarsi with 5 or 6 sts, 0 spls | 2 |
| 2 (1) | Pedipalpal femurogenu with 5 setae; long setae ending in terminal bulb-like knob (very small) on tarsi III and IV present; telofemoral setal formula not 5-5-4-3; usually 6 setae on pedipalp tibiotarsus Cunaxoidini | 3 |
| – | Pedipalpal femurogenu with 6 setae; long setae ending in terminal bulb-like knob (very small) on tarsi III and IV absent; telofemoral setal formula 5-5-4-3; usually 5 setae on pedipalp tibiotarsus Pulaeini | 9 |
| 3 (2) | Femora I and II divided; setae f2 absent; trichobothrium on tibiae IV present or absent | 4 |
| – | Femora I and II not divided; setae f2 present; trichobothrium on tibiae IV absent | Denheyernaxoides Smiley, 1992 |
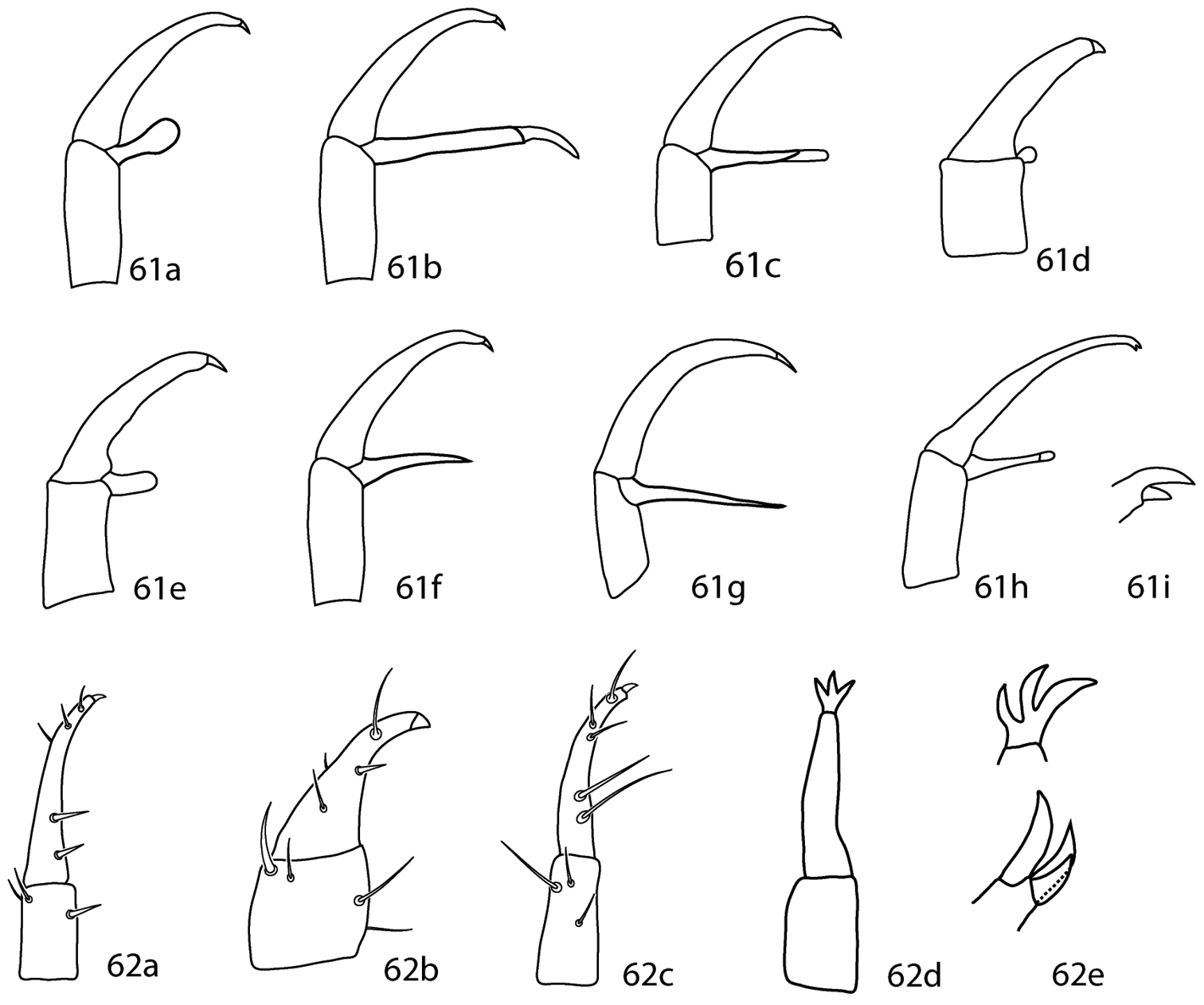
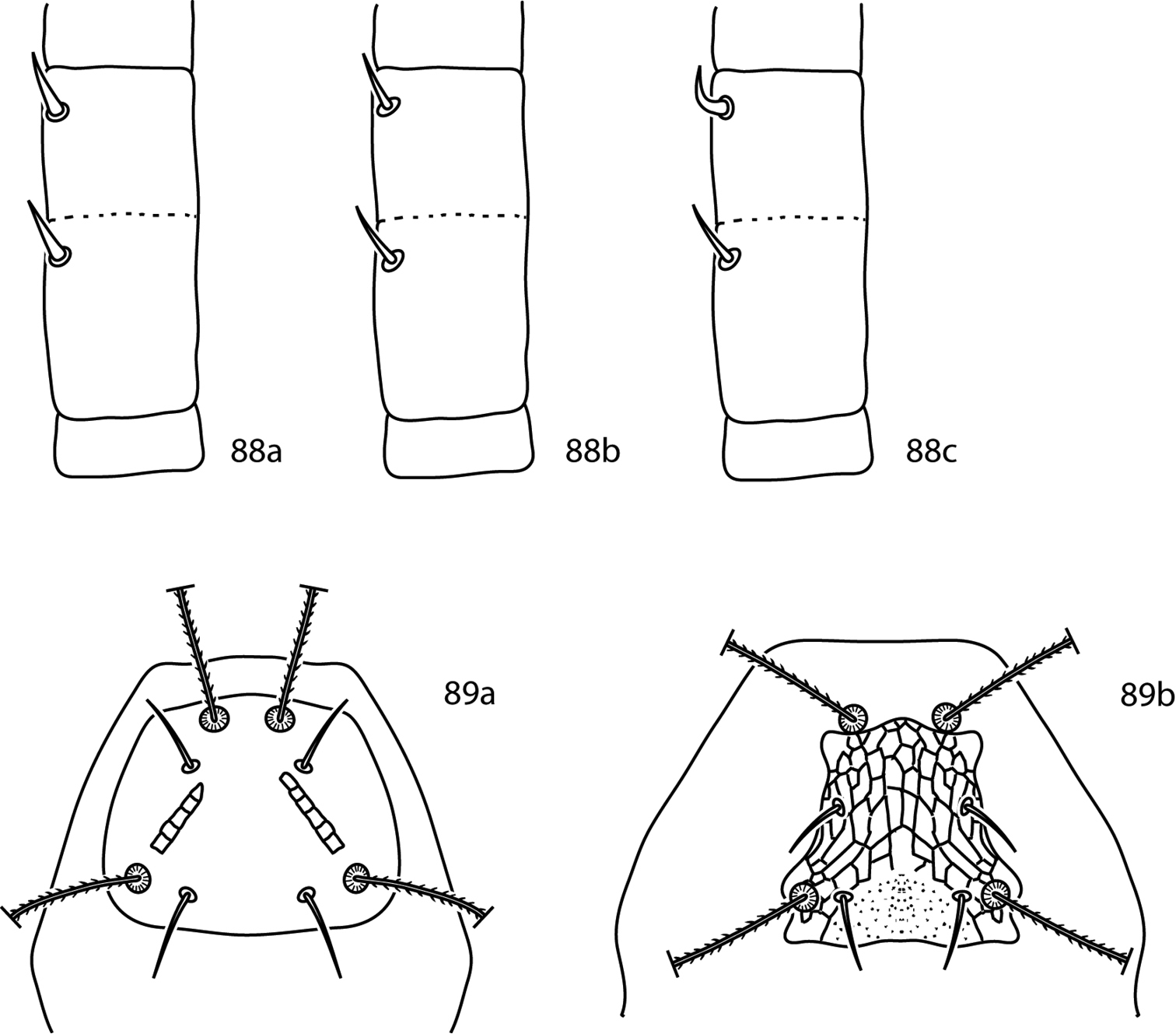
| 4 (3) | Dorsum with ill-defined weakly sclerotized dorsal plates (Fig. 15a); subterminal pointed process on pedipalp tibiotarsal claw present (Fig. 16a); small teeth (=serrated edge) on pedipalp tibiotarsal claw present (Fig. 16a); cheliceral setae absent | 5 |
| – | Dorsum with well-defined and sclerotized dorsal plates (Fig. 15b); subterminal pointed process on pedipalp tibiotarsal claw absent (Fig. 16b); small teeth on pedipalp tibiotarsal claw absent (Fig. 16b); cheliceral setae present | Scutopalus Den Heyer, 1979 |
| 5 (4) | Trichobothrium on tibiae IV present; famulus present, on distal portion of tarsus I | Cunaxoides |
| – | Trichobothrium on tibiae IV absent; famulus present or absent | 6 |
| 6 (5) | Tibiae III with 1 bsl, 3–5 sts; tibiae IV with 2 or 4 sts | 7 |
| – | Tibiae III with 1 lts, 4 sts; tibiae IV with 1 lsts, 4 sts | Dunaxeus Den Heyer & Castro, 2009 |
| 7 (6) | Tibiae III with 1 bsl, 3–5 sts; tibiae IV with 1 lts, 2 sts | Funaxopsis Den Heyer & Castro, 2009 |
| – | Tibiae III with 1 bsl, 5 sts; tibiae IV setal formula not as above | 8 |
| 8 (7) | Tibiae IV with 1 lsts, 4 sts; famulus present | Qunaxella Den Heyer & Castro, 2009 |
| – | Tibiae IV with 4 sts; famulus absent | Bunaxella Den Heyer & Castro, 2009 |
| 9 (2) | Setae f2 present; basifemora I–IV setal formula 4-6-3-1 or 4-6-3-2 | 10 |
| – | Setae f2 absent; basifemora I–IV setal formula 3-5-2-0 (rarely with 3-5-2-1) | Neocunaxoides Smiley, 1975 |
| 10 (9) | Basifemora I–IV setal formula 4-6-3-2; pedipalp tibiotarsus with one pointed process (ventral) (Fig. 17a); famulus on proximal half of tarsus I; tibiae I–II with non-striated blunt solenidia | Pulaeus Den Heyer, 1979 |
| – | Basifemora I–IV setal formula 4-6-3-1; pedipalp tibiotarsus with two pointed processes (1 ventral, 1 median) (Fig. 17b); famulus on distal half (subapical) of tarsus I; tibiae I–II with transversely striated blunt solenidia | Lupaeus Castro & Den Heyer, 2009 |
Cunaxoidinae key illustrations. Setae are removed from figures 16–17 for clarity 15a Idiosoma with poorly demarcated dorsal plates 15b Idiosoma with well demarcated dorsal plates 16a Pedipalp tibiotarsus with subapical process and small teeth present 16b Pedipalp tibiotarsus with subapical process and small teeth absent 17a Pedipalp tibiotarsus with a single pointed process 17b Pedipalp tibiotarsus with two pointed processes.
Gnathosoma. Pedipalps 3-segmented. Femurogenua are at least twice as long as wide and complemented with 5 setae. Tibiotarsi at least twice as long as wide and usually complemented with 6 setae. A small apophysis present basally and a pointed process occurs near the terminal tip; a ridge present between the apophysis and pointed process. Subcapitulum with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae) present; setae hg4 is often the longest. Chelicera without seta.
Idiosoma, dorsal. Proterosoma bears an ill-defined and weakly sclerotized shield which is complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). The dorsal hysterosoma may or may not bear a plate; if a plate is present it is ill-defined and weakly sclerotized, may be complemented with a variable number of setae, and may or may not be fused with the proterosomal shield. Setae c1–h1, c2, and h2 are present. Seta c2 plumose or fan-shaped. Cupule im is present laterad and posterior of e1. Integument that is not covered in shields or plates is striated.
Idiosoma, ventral. Coxae are weakly sclerotized and ill-defined; they can be recognized by possessing somewhat denser striations than the surrounding integument. Coxae I–II may be fused and may coalesce medially to form a sternal shield. Coxae III–IV fused or not. Each coxa complemented with 2-4 setae. Genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath the plates. Anal plates bear one pair of setae; one pair of setae is present ventrally on the integument near the anal plates. Up to 7 pairs of setae present on the integument between the coxal and genital plates. Cupule ih present ventrally laterad the integumental setae associated with the anal plates. Integument that is not covered in shields or plates is striated. Legs. Tarsi are never constricted apically so as to end in lobes. Depression for the famulus on tarsus I is absent. Tibia III complemented with 1 bsl, 5 sts. Tibia IV is complemented with 4 sts and lacks a trichobothrium. Ambulacral claws occur on either side of a 4-rayed empodium.
(modified from
| 1 | Basifemora I–IV with 3-3-3-0 sts; telofemora IV with 1 sts; dorsal setae fan-shaped, except for smooth f2 | Bunaxella quini (Den Heyer, 1981) |
| – | Basifemora I–IV with 4-4-3-1 sts; telofemora IV with 2 sts; dorsal setae plumose, except for h2 which may be plumose or smooth | 2 |
| 2 (1) | Setae h2 plumose | Bunaxella oribensis (Den Heyer, 1981) |
| – | Setae h2 smooth | Bunaxella zebedielensis (Den Heyer, 1981) |
Gnathosoma. Pedipalps 3-segmented. Femurogenua at least twice as long as wide and complemented with 5 setae. Tibiotarsi at least twice as long as wide and usually complemented with 6 setae. A small apophysis present basally and a pointed process present near the terminal tip; a ridge present between the apophysis and pointed process. Subcapitulum with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae) are present; setae hg4 longest. Chelicera without seta.
Idiosoma, dorsal. Proterosoma bears an ill-defined and weakly sclerotized shield which is complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). The dorsal hysterosoma may or may not bear a plate; if a plate is present it is ill-defined and weakly sclerotized, may be complemented with a variable number of setae, and may or may not be fused with the proterosomal shield. Setae c1–h1, c2, and h2 are present. Cupule im present laterad and posterior of e1. Integument that is not covered in shields or plates is striated.
Idiosoma, ventral. Coxae weakly sclerotized and ill-defined; they can be recognized by possessing somewhat denser striations than the surrounding integument. Coxae I–II may be fused and may coalesce medially to form a sternal shield. Coxae III–IV may be fused. Each coxa is complemented with 2-4 setae. Genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath the plates. Anal plates bear one pair of setae; one pair of setae present ventrally on the integument near the anal plates. Up to 7 pairs of setae present on the integument between the coxal and genital plates. Cupule ih present ventrally laterad the integumental setae associated with the anal plates. Integument that is not covered in shields or plates is striated. Legs. Tarsi never constricted apically so as to end in lobes. Trichobothrium present on leg tibia IV. Ambulacral claws are rippled and occur on either side of a 4-rayed empodium.
The following species have not been included because the original descriptions and subsequent papers describing them (
| 1 | Dorsal hysterosomal median plate present (may be fused with proterosomal shield or only suggested by cuticular pattern) (Figs 18a–c, 19a–d, 20) | 2 |
| – | Dorsal hysterosomal median plate absent (Figs 21a, b, 22) | 9 |
| 2 (1) | Hysterosomal median plate obvious, sclerotized (Figs 18a–d, 19a–c) | 3 |
| – | Hysterosomal median plate not be obvious or sclerotized, may only be suggested by cuticular pattern (Fig. 20) | 8 |
| 3 (2) | Hysterosomal median plate not complemented with setae; USA | Cunaxoides parvus (Ewing, 1917) |
| – | Hysterosomal median plate complemented with setae | 4 |
| 4 (3) | Hysterosomal median plate and proterosomal shield separate (Figs 18a–c) | 5 |
| – | Hysterosomal median plate and proterosomal shield fused (Figs 19a–d) | 6 |
| 5 (4) | Hysterosomal median plate complemented with c1, d1 (Fig. 18a); Canada, USA | Cunaxoides biscutum (Nesbitt, 1946) |
| – | Hysterosomal median plate complemented with c1, d1, c2 (Fig. 18b); Russia | Cunaxoides fidus Kuznetzov & Livshitz, 1979 |
| – | Hysterosomal median plate complemented with c1–e1, c2 (Fig. 18c); Russia | Cunaxoides longistriatus Kuznetzov & Livshitz (1979 |
| 6 (4) | Hysterosomal shield complemented with setae c1, d1, c2; (Figs 19a, b) | 7 |
| – | Hysterosomal shield complemented with setae c1- e1, c2; (Figs 19c) | Cunaxoides decastroae Den Heyer, 2013 |
| 7 (6) | Genua IV with 1 asl, 5 sts; striae between sci and c1 U-shaped (Fig. 19a); Greece | Cunaxoides paracroceus Sionti & Papadoulis, 2013 |
| – | Genua IV with 2 asl, 5 sts; striae between sci and c1 parallel (Fig. 19b); Europe | Cunaxoides croceus (Koch, 1838) |
| 8 (2) | Dorsal striae form one “shield-like” area, similar to fused proterosomal and hysterosomal shield (Fig. 23a); Poland | Cunaxoides kielczewskii Gupta & Ghosh, 1980 |
| – | Dorsal striae form two “shield-like” areas, similar to separate proterosomal and hysterosomal shields (Fig. 23b); Iran | Cunaxoides lootsi Den Heyer, 2013 |
| 9 (1) | Proterosomal shield present (Figs 21a, b) | 10 |
| – | Proterosomal shield absent (Fig. 22); Russia | Cunaxoides ulcerosus Kuznetzov & Livshitz (1979) |
| 10 (9) | Dorsal shield reticulated (Fig. 21a); Russia | Cunaxoides desertus Kuznetzov & Livshitz (1979) |
| – | Dorsal shield striated (Fig. 21b) | 11 |
| 11 (10) | Telofemora I–III setal formula 4-3-3; India | Cunaxoides nicobarensis Gupta & Ghosh, 1980 |
| – | Telofemora I–III setal formula 5-5-4 or 5-5-6 | 12 |
| 12 (11) | Telofemur III with 3 sts; Pakistan | Cunaxoides sialkotensis Bashir & Afzal, 2009 |
| – | Telofemur III with 4 sts | 13 |
| – | Telofemur III with 6 sts; Pakistan | Cunaxoides negans Bashir & Afzal, 2009 |
| 13 (12) | Basifemur I with 1 sts | 14 |
| – | Basifemur I with 2 sts; Pakistan | Cunaxoides daskaensis Bashir & Afzal, 2009 |
| 14 (13) | Basifemora II–IV setal formula 1-1-0; Pakistan | Cunaxoides trisetosis Bashir & Afzal, 2004 |
| – | Basifemora II–IV setal formula 4-2-0; Pakistan | Cunaxoides sargodhaensis Bashir, Afzal & Raza, 2007 |
Cunaxoides key illustrations. See key for explanations.
Canestrini (1885) described Eupalus brevirostris.
Gnathosoma. Pedipalps 3-segmented. Femurogenua at least twice as long as wide, complemented with 5 setae. Tibiotarsi at least twice as long as wide, usually complemented with 6 setae. A small apophysis occurs basally and a pointed process occurs near the terminal tip; a ridge runs between the apophysis and pointed process. Subcapitulum with 4 pairs of setae (hg1–4); setae hg4 often the longest. Adoral setae absent. Chelicera without seta.
Idiosoma, dorsal. Proterosoma lacks a shield, complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Dorsal hysterosoma lacks a plate. Setae c1–h1, c2, and f2, h2 present. Cupule im present laterad and posterior of e1. Integument not covered in shields or plates is striated.
Idiosoma, ventral. Coxae I–II connected by small apodemes. Coxae III–IV fused. Each coxa complemented with 2–4 setae. Genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath the plates. Anal plates bear 1 pair of setae; 1 pair of setae present ventrally on the integument near the anal plates. 5 pairs of setae present on the integument between the coxal and genital plates. Cupule ih present ventrally laterad the integumental setae associated with the anal plates. Integument not covered in shields or plates is striated. Legs. Femora I and II not divided. Trichobothrium on tibia IV absent. Tarsi never constricted apically so as to end in lobes. Ambulacral claws on either side of a 4-rayed empodium present.
| 1 | Coxa I with 1 sts; trochanters I–IV setal count 1-1-1-1; femora I-II setal count 2–2; gnathosoma with deep indention posterioventrally | Denheyernaxoides martini Smiley, 1992 |
| – | Coxa I with 3 sts; trochanters I–IV setal count 0-0-1-0; femora I-II setal count 4–5; gnathosoma with slight indention posterioventrally | Denheyernaxoides brevirostris (Canestrini 1885) |
Gnathosoma. Pedipalps 3-segmented. Femurogenua at least twice as long as wide, complemented with 5 setae. Tibiotarsi at least twice as long as wide, usually complemented with 6 setae. A small apophysis occurs basally and a pointed process occurs near the terminal tip; a ridge runs between the apophysis and pointed process. Subcapitulum with 4 pairs of setae (hg1–4 and 2 pairs of adoral setae); setae hg4 is often the longest. Chelicera without seta.
Idiosoma, dorsal. Proterosoma bears an ill-defined and weakly sclerotized shield which is complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Dorsal hysterosoma may or may not bear a plate; if a plate is present it is ill-defined and weakly sclerotized, may be complemented with a variable number of setae, and may or may not be fused with the proterosomal shield. Setae c1–h1, c2, and h2 are present. Cupule im is present laterad and posterior of e1. The integument that is not covered in shields or plates is striated.
Idiosoma, ventral. Coxae weakly sclerotized and ill-defined; they can be recognized by possessing somewhat denser striations than the surrounding integument. Coxae I–II may be fused and may coalesce medially to form a sternal shield. Coxae III–IV fused. Each coxa complemented with 2–4 setae. Genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath plates. Anal plates bear 1 pair of setae; 1 pair of setae present ventrally on the integument near the anal plates. Up to 7 pairs of setae present on the integument between the coxal and genital plates. Cupule ih present ventrally laterad the integumental setae associated with the anal plates. Integument not covered in shields or plates is striated. Legs. Tarsi never constricted apically so as to end in lobes. Tibia III complemented with 5 sts (4 short, 1 long). Tibia IV complemented with 5 sts (4 short, 1 long), and lacks a trichobothrium. Ambulacral claws on either side of a 4-rayed empodium present.
| 1 | Basifemora IV with 1 sts | Dunaxeus elongatus (Den Heyer, 1981) |
| – | Basifemora IV with 2 sts | 2 |
| 2 (1) | Famulus on tarsus I present | Dunaxeus capensis (Den Heyer, 1981) |
| – | Famulus on tarsus I absent | Dunaxeus duosetosus Den Heyer & Castro, 2009 |
Gnathosoma. Pedipalps 3-segmented. Femurogenua at least twice as long as wide, complemented with 5 setae. Tibiotarsi at least twice as long as wide, usually complemented with 6 setae. A small apophysis occurs basally and a pointed process occurs near the terminal tip; a ridge runs between the apophysis and pointed process. Subcapitulum with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae); setae hg4 is often longest. Chelicera without seta.
Idiosoma, dorsal. Proterosoma bears an ill-defined and weakly sclerotized shield complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Dorsal hysterosoma may or may not bear a plate; if plate present, it is ill-defined and weakly sclerotized, may be complemented with a variable number of setae, and may or may not be fused with the proterosomal shield. Setae c1–h1, c2, and h2 present. Cupule im present laterad and posterior e1. Integument not covered in shields or plates striated.
Idiosoma, ventral. Coxae weakly sclerotized and ill-defined; they can be recognized by possessing somewhat denser striations than the surrounding integument. Coxae I–II may be fused and may coalesce medially to form a sternal shield. Coxae III–IV may be fused. Each coxa complemented with 2–4 setae. Genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath the plates. Anal plates bear 1 pair of setae; 1 pair of setae present ventrally on the integument near the anal plates. Up to 7 pairs of setae present on the integument between the coxal and genital plates. Cupule ih present ventrally laterad integumental setae associated with the anal plates. Integument not covered in shields or plates striated. Legs. Tibia III complemented with 1 bsl and 3, 4, or 5 sts. Tibia IV complemented with 3 sts (2 short, 1 long) and lacks a trichobothrium. Tarsi never constricted apically so as to end in lobes. Ambulacral claws on either side of a 4-rayed empodium present.
(modified from
| 1 | Basifemora I–IV setal formula 3-3-3-1 sts; sci smooth | Funaxopsis visci (Den Heyer, 1981) |
| – | Basifemora I–IV setal formula 2-2-2-0 sts; sci finely setose | 2 |
| 2 (1) | Telofemora I–IV setal formula 4-3-1-1 sts; h1 smooth | Funaxopsis passerinae (Den Heyer, 1981) |
| – | Telofemora I–IV setal formula 4-4-3-1 sts; h1 finely setose | Funaxopsis vaneedeni (Den Heyer, 1981) |
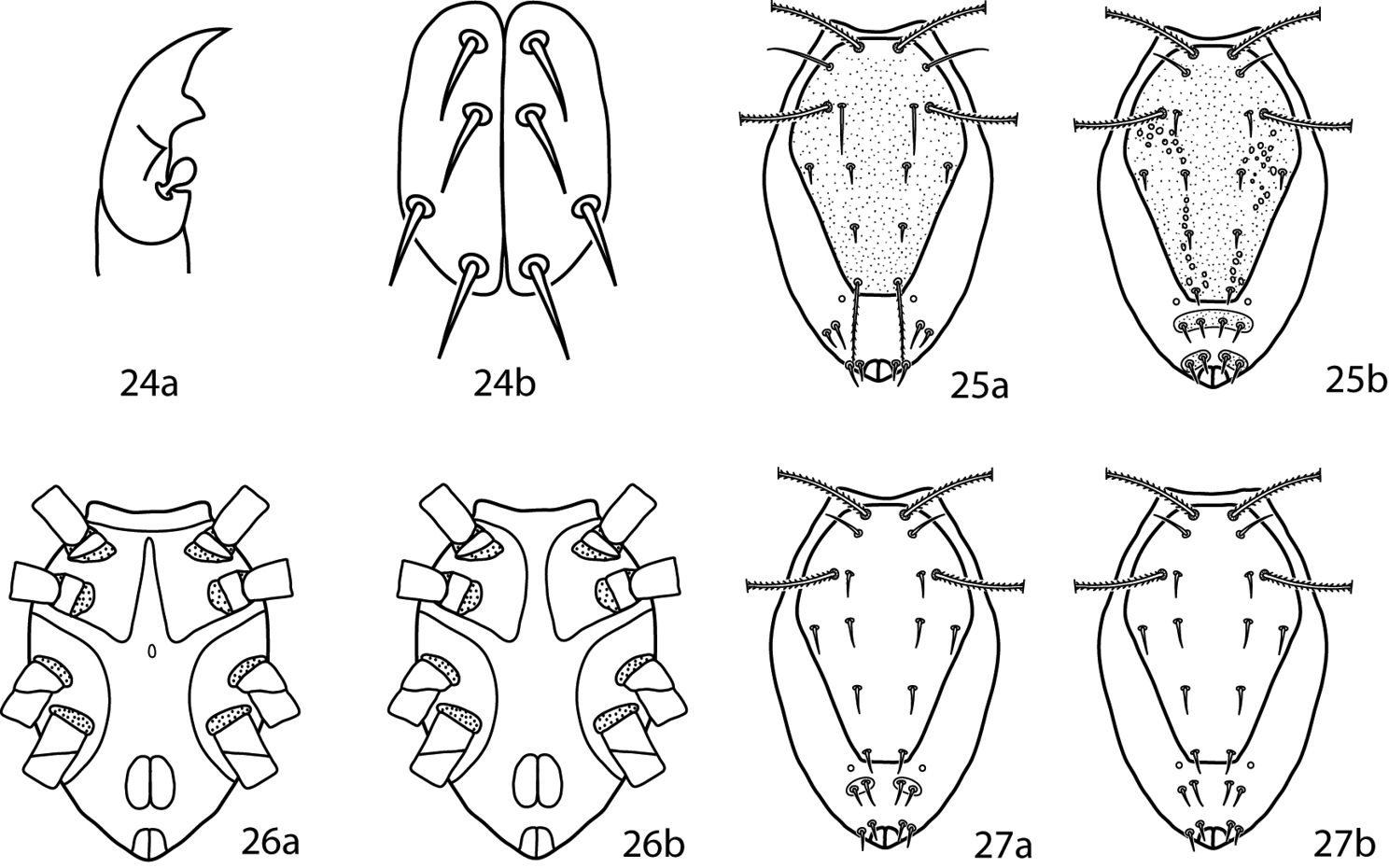
Gnathosoma. Pedipalps 3-segmented. Femurogenua at least twice as long as wide, complemented with 6 setae. Tibiotarsi at least twice as long as wide, usually complemented with 6 setae; they possess 2 or 3 pointed processes and may possess a bladder- or knob-like apophysis (Fig. 24a). Subcapitulum with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae); setae hg4 often the longest. Chelicera with seta present.
Lupaeus illustrations. 24a Pedipalp tibiotarsus 24b Genital setae not in a row, g3 out of line 25–27 Lupaeus key illustrations. Setae and cupules removed from figures 25a, b to increase clairity 25a Lupaeus longisetus, dorsal 25b Lupaeus polilloensis, dorsal 26a Ventral, small platelet present 26b Ventral, small platelet absent 27a Setae f1, f2 born on small platelets 27b Setae f1, f2 born on integument.
Idiosoma, dorsal. Proterosoma bears a well-sclerotized shield complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Dorsal hysterosoma bears a sclerotized plate that is variable in size and fused with the proterosomal shield; it may be complemented with a variable number of setae depending on the size of the plate. Setae c1–h1, c2, f2, and h2 present. Cupule im present laterad and posterior of e1. Integument not covered in shields or plates is striated.
Idiosoma, ventral. Coxae sclerotized and well-defined. Coxae I–II may be fused and may coalesce medially to form a sternal shield. Coxae III–IV may be fused. Each coxa complemented with 2–4 setae. Genital plates each bear 4 setae (g1–4). Setae g1, 2, 4 usually occur in a straight line near the midline and setae g3 occur near the edge of the genital plates (Fig. 24b). 2 pairs of genital papillae visible underneath the plates. Anal plates bear 1 pair of setae; 1 pair of setae present ventrally on the integument near the anal plates. Cupule ih present ventrally laterad; the integumental setae associated with the anal plates. Integument not covered in shields or plates striated. Legs. Tarsi never constricted apically so as to end in lobes. Trichobothrium on leg tibia IV present. Basifemora setal formula 4-6-3-1. Depression of the famulus occurs on distal half of tarsus I. Tibiae I–II possess striated blunt solenidia. Ambulacral claws rippled and occur on either side of a 4-rayed empodium.
Lupaeus longisetus is known only from the male and is not included in the key. It can be recognized by the following characters: small platelet between the edges of a divided sternal shield absent, basifemora I with 3 sts, and setae e1 elongate and barbed (Fig. 25a).
Lupaeus polilloensis is only known from the male and is not included in the key. It can be recoginized by the following characters: small platelet between the edges of a divided sternal shield absent; basifemora I–II setal formula 4-6; platelets complemented with setae f1, f2 with fused medially into one plate; and the dorsal shield densely granulate (Fig. 25b).
As suggested by
| 1 | Small platelet ventromedially between edges of divided sternal plate present (Fig. 26a); South Africa, Brazil | Lupaeus martini (Den Heyer, 1979) |
| – | Small platelet ventromedially between edges of divided sternal plate absent (Fig. 26b) | 2 |
| 2 (1) | Basifemora I with 4 sts | 3 |
| – | Basifemora I with 5 sts; Philippines | Lupaeus filipinus (Corpuz-Raros, 1996) |
| 3 (2) | Basifemora II with 4 sts; USA | Lupaeus minutus (Baker & Hoffmann, 1948) |
| – | Basifemora II with 5 sts | 4 |
| – | Basifemora II with 6 sts | 7 |
| 4 (3) | Setae f1 shorter than c1; Philippines | Lupaeus lenis (Corpuz-Raros, 1996) |
| – | Setae f1 the same length as c1 | Lupaeus lectus Castro & Den Heyer, 2009 |
| – | Setae f1 longer than c1, usually by at least 1.5 times | 5 |
| 5 (4) | Genua I with 9 total simple setae and solenidia; Philippines | Lupaeus dentatus (Corpuz-Raros, 1996) |
| – | Genua I with 7 total simple setae and solenidia | 6 |
| 6 (5) | Setae c1–e1 equal in length; Brazil | Lupaeus lobidorsalis Castro & Den Heyer, 2009 |
| – | Setae e1 one-fourth longer than c1, d1; Italy, USA | Lupaeus subterraneus (Berlese, 1916) |
| 7 (3) | Setae f1, f2 on platelets, which may be separate or fused medially (Fig. 27a) | 8 |
| – | Setae f1, f2 on integeument (Fig. 27b) | 11 |
| 8 (7) | Tibia II with 1 s, 5 sts | 9 |
| – | Tibia II with 2 s (1 asl, 1 bsl), 5 sts; Ukraine | Lupaeus valentinae Sergeyenko, 2011 |
| 9 (8) | Pedipalp tibiotarsus with 4 sts; Philippines | Lupaeus villacarlosae (Corpuz-Raros, 1996) |
| – | Pedipalp tibiotarsus with 5 sts | 10 |
| 10 (9) | Tarsus I with 3 asl, 2 terminal solenidion, 1 fam, 20 or 21 sts; tarsus IV with 14 sts | Lupaeus iranensis Den Heyer, 2013 |
| – | Tarsus I with 3 asl, 1 dorsodistal solenidion, 1 terminal solenidion, 1 fam, 22 sts; tarsus IV with 16 sts | Lupaeus sativae Den Heyer, 2013 |
| 11 (7) | Cheliceral seta not as long as width of cheliceral digit; China | Lupaeus platygnathus (Bu & Li, 1991) |
| – | Cheliceral seta longer than width of cheliceral digit; South Africa, Brazil | Lupaeus clarae (Den Heyer, 1979) |
Gnathosoma. Pedipalps 3-segmented. Femurogenua at least twice as long as wide, complemented with 6 setae. Tibiotarsi at least twice as long as wide and usually complemented with 6 setae. Tibiotarsi possess two or three knob-like apophyses, a single spur, or sometimes a flange-like seta. Subcapitulum with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae); setae hg4 often the longest. Chelicera with seta present.
Idiosoma, dorsal. Proterosoma bears a well-sclerotized shield which is complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Dorsal hysterosoma bears a sclerotized plate which is variable in size and fused with the proterosomal shield; it may be complemented with a variable number of setae depending on the size of the plate. Setae c1–h1, c2, and h2 present. Setae f2 absent. Cupule im present laterad and posterior of e1. The integument not covered in shields or plates is striated.
Idiosoma, ventral. Coxae sclerotized and well-defined. Coxae I–II may be fused and may coalesce medially for form a sternal shield. Coxae III–IV may be fused. Each coxa complemented with 2–4 setae. Genital plates each bear 4 setae (g1–4), which are usually in a straight now; 2 pairs of genital papillae visible underneath the plates. Anal plates bear one pair of setae; one pair of setae is present ventrally on the integument near the anal plates. Cupule ih present ventrally laterad the integumental setae associated with the anal plates. Integument not covered in shields or plates is striated. Legs. Tarsi never constricted apically so as to end in lobes. Trichobothrium on leg tibia IV present. Basifemora setal formula 3-5-2-0. Ambulacral claws rippled and occur on either side of a 4-rayed empodium.
Cunaxoides philippinensis (Corpuz-Raros, 2007) is regarded as belonging to Neocunaxoides because it has 6 seatae on the femurogenu and lacks setae f2. Neocunaxoides makapalus, Neocunaxoides philippinensis (Corpuz-Raros, 1996c), Neocunaxoides unguianalis, and Neocunaxoides rugosus are regarded as belonging to Scutopalus as they possess 5 sts on pedipalp femurogenu and extensive dorsal shields. They have therefore not been included in the following key.
Neocunaxoides biramus is not included in the key because it is only known from the male. It can be distinguished from all other Neocunaxoides, and indeed all described cunaxids, by the presence of a branched sci and 4 teeth on the lateral lips of the hypostome.
Neocunaxoides metwallyi is not included in the key as, despite the best efforts of the authors and the University of Arkansas Interlibrary Loan Department, the description could not be obtained.
We agree with and follow Castro and
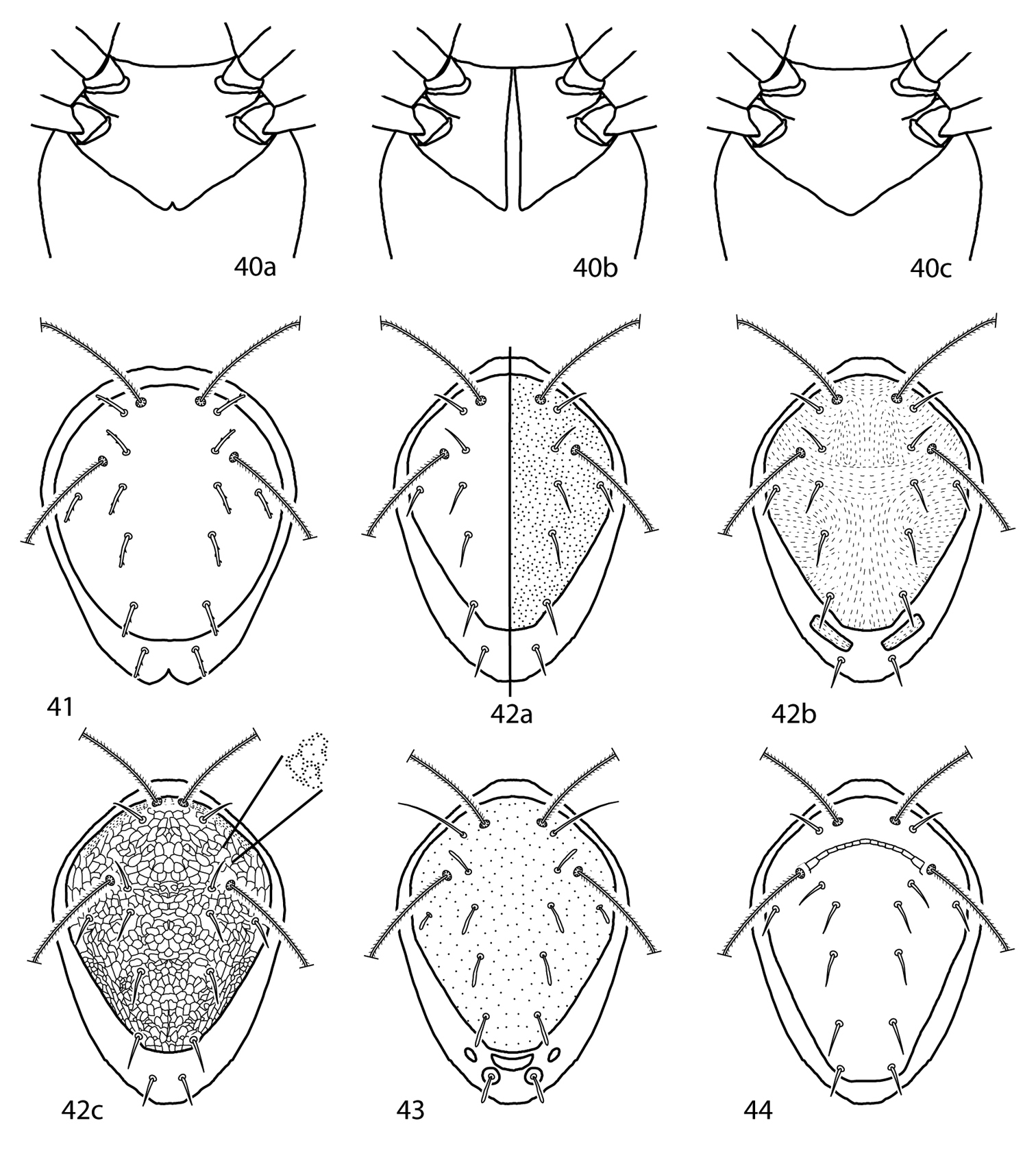
| 1 | Coxae I–II fused medially to form sternal shield (Figs 28a–d) | 2 |
| – | Coxae I–II not fused medially (may be connected anteromedially) (Figs 29a–d) | 6 |
| 2 (1) | Posterior edge of coxae IV extending beyond anterior edge of genital plates (Figs 28a, b) | 3 |
| – | Posterior edge of coxae IV not extending beyond anterior edge of genital plates (Figs 28c, d) | 5 |
| 3 (2) | Small platelet anteriomedially of genital plates present (Fig. 28a) | Neocunaxoides fani Lin, Zhang & Ji, 2001 |
| – | Small platelet anteriomedially of genital plates absent (Fig. 28b) | 4 |
| 4 (3) | Solid or broken band of papillae on ventral subcapitulum present (Fig. 30a); subcapitulum longer, length: width 1.75:1 | Neocunaxoides zuluensis Den Heyer, 1980 |
| – | Solid or broken band of papillae on ventral subcapitulum absent (Fig. 30b); subcapitulum shorter, length: width 1.25:1 | Neocunaxoides lajumensis Den Heyer, 1980 |
| 5 (2) | Hysterosomal plate present, fused with proterosomal shield, and bearing c1–e1, c2; small platelet anteriomedially of genital plates present (Fig. 28c) | Neocunaxoides boltoides Lin, Zhang & Ji, 2001 |
| – | Hysterosomal plate absent; small platelet anteriomedially of genital plates absent (Fig. 28d) | Neocunaxoides philippinensis (Corpuz-Raros, 2007) |
| 6 (1) | Median platelet between coxae II present (Figs 29a–c) | 7 |
| – | Median platelet between coxae II absent (Fig. 29d) | 13 |
| 7 (6) | Basifemora V with 1 sts | 8 |
| – | Basifemora V with 0 sts | 11 |
| 8 (7) | Basifemora I with 2 sts | Neocunaxoides biswasi Gupta & Chattopadhyay, 1978 |
| – | Basifemora I with 3 sts | 9 |
| 9 (8) | All setae on pedipalp of normal length, none extremely long | 10 |
| – | 2 setae on pedipalp femurogenu extremely long, nearly as long as segment; 1 distal pedipalp tibiotarsal setalong, longer than segment (Fig. 31) | Neocunaxoides mahabaeus Corpuz-Raros, 1996 |
| 10 (9) | Basal subcapitular polygonal pattern elongate (Fig. 32a); foveolae on dorsal shield present (Fig. 33a) | Neocunaxoides ornatus Corpuz-Raros & Gruèzo, 2007 |
| – | Basal subcapitular polygonal pattern not elongate (Fig. 32b); foveolae on dorsal shield absent (Fig. 33b) | Neocunaxoides grandis Corpuz-Raros, 1996 |
| 11 (7) | Small platelet anteriomedially of genital plates present (Fig. 29a) | Neocunaxoides ovatus Lin, Zhang & Ji, 2003 |
| – | Small platelet anteriomedially of genital plates absent (Fig. 29b, c) | 12 |
| 12 (11) | Coxae I connected anteromedially (Fig. 29b); mushroom-shaped seta on pedipalp tibiotarsi absent | Neocunaxoides rykei Den Heyer, 1980 |
| – | Coxae I not connected anteromedially (Fig. 29c); mushroom-shaped seta on pedipalp tibiotarsi present (Fig. 34) | Neocunaxoides andrei (Baker & Hoffmann, 1948) |
| 13 (6) | Femora I (basifemora I + telofemora I) with 6 setae | Neocunaxoides cerasoides Inayatullah & Shahid, 1989 |
| – | Femora I (basifemora I + telofemora I) with 9 setae | 15 |
| 14 (13) | Coxae I-IV setal formula 2-3-3-1; combined femora (basifemora + telofemora) II-IV setal formula 11-7-5 | Neocunaxoides dilato Inayatullah & Shahid, 1989 |
| – | Coxae I-IV setal formula 2-2-3-2; combined femora (basifemora + telofemora) II-IV setal formula 10-7-4 | Neocunaxoides kalamiensis Inayatullah & Shahid, 1989 |
Neocunaxoides key illustrations. See key for explanations of figures.
Gnathosoma. Pedipalps 3-segmented. Femerogenu complimented with 5 setae. Tibiotarsi at least twice as long as wide and complemented with 3 setae. Tibiotarsi possess a stout, spine-like apophysis. Subcapitulum with 4 pairs of setae (hg1–4); setae hg2–4 subequal. Adoral setae absent.
Idiosoma, dorsal. Proterosoma complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). A pair of oval shields formed by flat, bacillus-like striae present between the sensillae. Setae c1–h1, c2, and h2 present. Setae f2 absent. Integument not covered in shields or plates is striated.
Idiosoma, ventral. Coxae sclerotized and well-defined. Coxae I–II thinly connected. Coxae III–IV more broadly connected. Genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath the plates. Anal plates bear 1 pair of setae; 1 pair of setae present ventrally on the integument near the anal plates. Integument not covered in shields or plates is striated. Legs. Trichobothrium on tibia IV present.
Gnathosoma. Pedipalps 3-segmented. Femurogenua at least twice as long as wide, complemented with 6 setae. Tibiotarsi at least twice as long as wide, usually complemented with 6 setae, 1 pointed process, and may possess a bladder- or knob-like apophysis (Fig. 39a–c). Subcapitulum with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae); setae hg4 often the longest. Chelicera with seta present.
Pulaeus illustrations. 35 Genital setae in a row 36–39 Pulaeus key illustrations 36, 37 Venter, setae removed for clairity 36a Coxae I–II not coalesced medially, median platelet present 36b Coxae I–II not coalesced medially, median platelet absent 37a Coxae I–II coalesced medially, median platelet present 37b Coxae I–II coalesced medially, median platelet absent 38a Dorsal shield with punctures 38b Dorsal shield with broken striae 39a–c Pedipalp tibiotarsus 39a Tibiotarsus with elongate apophysis 39b Tibiotarsus with flat apophysis 39c Tibiotarsus with flange-like apophysis.
Idiosoma, dorsal. Proterosoma bears a well-sclerotized shield, complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Dorsal hysterosoma bears a sclerotized plate which is variable in size and fused with the proterosomal shield; it may be complemented with a variable number of setae depending on the size of the plate. Setae c1–h1, c2, f2, and h2 and present. Cupule im present laterad and posterior of e1. Integument not covered in shields or plates striated.
Idiosoma, ventral. Coxae sclerotized and well-defined. Coxae I–II may be fused and may coalesce medially to form a sternal shield. Coxae III–IV may be fused. Each coxa complemented with 2–4 setae. Genital plates each bear 4 setae (g1–4), which are usually in a straight row; 2 pairs of genital papillae visible underneath the plates. Anal plates bear one pair of setae; 1 pair of setae present ventrally on the integument near the anal plates. Cupule ih present ventrally laterad the integumental setae associated with the anal plates. The integument not covered in shields or plates striated. Legs. Tarsi never constricted apically so as to end in lobes. Trichobothrium on leg tibia IV present. Depression of the famulus occurs on proximal half of tarsus I. Tibiae I–II possess non-striated blunt solenidia. Ambulacral claws rippled and occur on either side of a 4-rayed empodium.
Pulaeus ardeola was not included in the key because the original text is in Cyrillic script and the illustrations do not provide enough characters to differentiate it from other species. Neocunaxoides cinctus is moved from Neocunaxoides to Pulaeus based on features given in the original description, namely that f2 is present and basifemora IV are complemented with 2 sts.
The following were species assigned to Pulaeus before Lupaeus was erected. The characters that divide the two genera are not given in the original species descriptions and types have not been viewed. These indeterminable species are therefore not included in either generic key, but instead characters are given for each species that will serve to identify them.
Pulaeus parapatzuarensis (Shiba, 1978) – This species has a divided sternal plate, lacks a sclerotized area anterior to the genital plates, and does not have f1, 2 located on platelets. In addition it has 6 pairs of setae on the integument between coxal and genital plates.
Pulaeus patzcuarensis (Baker & Hoffmann, 1948) – This species can be recognized by the sternal plates being connected anteriorly and divided in a v-shape posteriorly.
Pulaeus pseudominutus (Shiba, 1978) – Setae e1 being 3 times the length of c1 and d1 distinguishes this species.
Pulaeus payatopalpus (Corpuz-Raros, 1996) – The hypostome is 2/3 the length of the gnathosoma and the pedipalps are extremely long and slender, at least 8 times longer than wide. In addition the tibiotarsus is complemented with a seta that is longer than the segment.
Pulaeus zaherii (El-Bishlawy & Rakha, 1983) – This species can be recognized by the divided sternal plates, f1 being 4/5 the length of e1, and f1 being ½ the length of f2.
| 1 | Sternal plate divided medially (Fig. 35a, b) | 2 |
| – | Sternal plate not divided medially (Fig. 36a, b) | 23 |
| 2 (1) | Median platelet between coxae II–III present (Fig. 35a) | 3 |
| – | Median platelet between coxae II–III absent (Fig. 35b) | 7 |
| 3 (2) | Dorsal shield with surface smooth anteriorly and broken striae or lobes posteriorly; Ukraine | Pulaeus semistriatus Sergeyenko, 2011 |
| – | Dorsal shield with surface patterned (broken striae/lobes or dotted) on entire surface | 4 |
| 4 (3) | Dorsal shield patterend with dots; Pakistan | Pulaeus punctatus Bashir, Afzal & Akbar, 2005 |
| – | Dorsal shield patterned with broken striae/lobes | 5 |
| 5 (4) | Genua II with solenidia present | 6 |
| – | Genua II with solenidia absent; Pakistan | Pulaeus banksi Bashir & Afzal, 2009 |
| 6 (5) | Genua II with 1 asl, 5 sts; genua III with 2 asl, 5 sts; South Africa | Pulaeus glebulentus Den Heyer, 1979 |
| – | Genua II with 2 asl, 4 sts; genua III with 1 asl, 5 sts; Iran | Pulaeus razanensis Den Heyer, 2013 |
| 7 (2) | Setae f1 and f2 located on sclerotized platelets or shields | 8 |
| – | Setae f1 and f2 not located on sclerotized platelets or shields | 20 |
| 8 (7) | Pedipalp femurogenu at least 6 times as long as wide; Philippines | Pulaeus rimandoi Corpuz-Raros, 1996 |
| – | Pedipalp femurogenu at most 4 times as long as wide | 9 |
| 9 (8) | Genua II with 0 solenidia; Pakistan | 10 |
| – | Genua II with 1 solenidion | 12 |
| – | Genua II with 2 solenidia; Philippines | Pulaeus samarensis Corpuz-Raros, 2007 |
| – | Genua II with 3 solenidia | 17 |
| – | Genua II with 4 solenidia | 19 |
| 10 (9) | Genua I wth 2 bsl, 6 sts; tibia I with 1 bsl, 6 sts; Pakistan | Pulaeus ferventis Muhammad & Chaudhri, 1990 |
| – | Genua I with 2 asl, 3 bsl, 3 sts; tibia I with 1 bsl, 7 sts; Pakistan | Pulaeus erinaceus Muhammad & Chaudhri, 1991 |
| – | Genua I with 3 bsl, 6 sts; tibia I with 1 asl, 1 bsl, 6 sts; Pakistan | Pulaeus galumma Muhammad & Chaudhri, 1991 |
| – | Genua I with 4 asl, 4 sts; tibia I with 1 asl, 6 sts; Pakistan | Pulaeus walii Bashir & Afzal, 2009 |
| – | Genua I with 5 bsl, 4 sts; tibia I with 1 bsl, 6 sts; Pakistan | 11 |
| 11 (10) | Basifemora I–IV setal formula 5-5-4-3; Pakistan | P. silicula Muhammad & Chaudhri, 1991 |
| – | Basifemora I–IV setal formula 4-6-3-1; Pakistan | Pulaeus stultus Muhammad & Chaudhri, 1991 |
| 12 (9) | Basifemora I with solenidion present; telofemora I–IV setal formula 5-5-3-2; Pakistan | Pulaeus camar Muhammad & Chaudhri, 1991 |
| – | Basifemora I with solenidion absent; telofemora I–IV setal formula not as above | 13 |
| 13 (12) | Basifemora II with 5 (rarely 4) sts; Ukraine | Pulaeus leonidi Sergeyenko, 2011 |
| – | Basifemora II with 6 sts | 14 |
| 14 (13) | Genua II with solenidia present | 15 |
| – | Genua II with solenidia absent; Pakistan | Pulaeus akbari Bashir & Afzal, 2009 |
| 15 (14) | Genua II with 1 asl, 5 sts; Ukraine | Pulaeus maslovi Sergeyenko, 2011 |
| – | Genua II with 1 bsl, 6 sts | 16 |
| 16(15) | Genua III–IV setal formula 5 sts–5 sts; Pakistan | Pulaeus osculum Muhammad & Chaudhri, 1990 |
| – | Genua III–IV setal formula 5 sts–6 sts; Pakistan | Pulaeus haurio Muhammad & Chaudhri, 1991 |
| – | Genua III–IV setal formula 1 bsl, 4 sts–2 bsl, 4 sts; Pakistan | Pulaeus verno Muhammad & Chaudhri, 1990 |
| 17 (9) | Setae f1 and h1 approximately equal in length | 18 |
| – | Setae f1 approximately half the length as h1; China | Pulaeus musci Liang, 1985 |
| 18 (17) | Coxa IV with 2 sts; basifemora IV with 2 sts; Brazil | Pulaeus myrtaceus Castro & Den Heyer, 2009 |
| – | Coxa IV with 3 sts; basifemora IV with 1 sts; Pakistan | Pulaeus anjumi Bashir & Afzal, 2006 |
| 19 (9) | Dorsal shield with punctuations (Fig. 37a); Brazil | Pulaeus quadrisolenidius Castro & Den Heyer, 2009 |
| – | Dorsal shield with flat broken striae (Fig. 37b); USA | Pulaeus whartoni (Baker & Hoffmann, 1948 |
| 20 (7) | 4 pairs of setae on integument between coxal and genital plates | Pulaeus cinctus (Chaudhri, 1980) |
| – | 5 pairs of setae on integument between coxal and genital plates | 21 |
| – | 6 pairs of setae on integument between coxal and genital plates | 22 |
| 21 (20) | Coxae II with 2 sts; telofemora II with 5 sts; Pakistan | Pulaeus desitis Muhammad & Chaudhri, 1990 |
| – | Coxae II with 2 sts; telofemora II with 4 sts; Philippines | Pulaeus palawanensis Corpuz-Raros, 2007 |
| 22 (20) | Sensillum at approximately as long as sce; setae f1 approximately equal in length to h1 | Pulaeus cebuensis Corpuz-Raros, 2007 |
| – | Sensillum at longer than sce; setae f1 approximately 1.25 the length of h1 | Pulaeus franciscae Den Heyer, 1981 |
| 23 (1) | Ventral medial platelet present (Fig. 36a); dorsum punctuate (Fig. 37a); pedipalpal tibiotarsus with truncate, flange-like apophysis (Fig. 38a); USA | Pulaeus pectinatus Den Heyer, 1979 |
| – | Ventral medial platelet absent (Fig. 36b); dorsum striated (Fig. 37b); pedipalpal tibiotarsus with elongate apophysis (Fig. 38b) | 24 |
| 24 (23) | Posterior pedipalpal tibiotarsal seta bifurcate (Fig. 39) | Pulaeus neopectinatus (Shiba, 1978) |
| – | Posterior pedipalpal tibiotarsal seta not bifurcate | 25 |
| 25 (24) | Pedipalp femurogenua at most 4 times as long as wide; setae f1 and f2 approximately equal in length; USA | Pulaeus americanus (Baker & Hoffmann, 1948) |
| – | Pedipalp femurogenua at least 6 times as long as wide; setae f1 ¼ longer than f2; Pakistan | Pulaeus krama (Chaudhri, Akbar & Rasool 1979) |
Gnathosoma. Pedipalps 3-segmented. Femurogenu complimented with 5 sts. Tibiotarsi at least twice as long as wide and complemented with 5 sts, 1 asl. Subcapitulum with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae).
Idiosoma, dorsal. Proterosoma with weakly defined shield present which is complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Dorsal hysterosoma lacks a plate. Setae c1–h1, c2, and h2 present. Setae c1–f1 finely setose and c2, h1, and h2 smooth. Setae f2 absent. Integument not covered in shields or plates striated.
Idiosoma, ventral. Coxae weakly sclerotized and ill-defined. Coxae I–II fused. Coxae III–IV fused. Genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath the plates. Integument not covered in shields or plates striated. Legs. Basifemora I–IV setal formula 3-4-2-0 sts. Telofemora I–IV setal formula 4-4-3-3. Tibiae III with 1 bsl, 5 sts. Tibiae IV with 5 sts (4 short, 1 long).
Gnathosoma. Pedipalps 3-segmented. Femurogenu complimented with 5 sts. Tibiotarsi at least twice as long as wide and complemented with 5 sts, 1 asl. Subterminal pointed process on pedipalp tibiotarsal claw absent; small teeth on pedipalp tibiotarsal claw absent. Subcapitulum with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae). Chelicera without seta.
Idiosoma, dorsal. Proterosoma with a well-defined shield present, complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Dorsal hysterosoma with a well-defined plate fused to the proterosomal plate. Small platelets may be present laterad and posterior to the dorsal shield. Setae c1–h1, c2, and h2 present. Setae f2 absent. Integument not covered in shields or plates striated.
Idiosoma, ventral. Coxae well-sclerotized. Coxae I–II fused medially. Coxae III–IV fused. Genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath the plates. A small platelet may be present laterad the genital plate. Integument not covered in shields or plates striated. Legs. Basifemora I–IV setal formula 3-4-2-0 sts. Telofemora I–IV setal formula 5-5-4-3. Tibiae III with 1 bsl, 5 sts. Tibiae IV with 5 sts (4 short, 1 long).
(modified from
As suggested by
| 1 | Coxae I–II faintly or totally divided (Fig. 40a, b) | 2 |
| – | Coxae I–II fused medially (Fig. 40c) | 7 |
| 2 (1) | Coxae I–II faintly divided (Fig. 40a) | 3 |
| – | Coxae I–II totally divided (Fig. 40b) | 4 |
| 3 (2) | Sternal shield bearing 6 pairs of setae; setae c2 and mps simple; coxae II with 2 setae; basifemora I–IV setal formula 3-3-2-0; Greece | Scutopalus abiesae Sionti & Papadoulis, 2003 |
| – | Sternal shield bearing 5 pairs of setae; setae c2 and mps setose; coxae II with 1 setae; basifemora I–IV setal formula 2-2-2-1; South Africa | Scutopalus arboreus Den Heyer, 1979 |
| 4 (2) | At least 2 pairs of thick rod-like setae on the dorsum (Fig. 41); India | Scutopalus pradhani (Gupta & Ghosh, 1980) |
| – | Rod-like setae on dorsal shield absent | 5 |
| 5 (4) | Coxae II with 2 sts | 6 |
| – | Coxae II with 3 sts; Pakistan | Scutopalus gilbertoi (Bashir & Afzal, 2004) |
| 6 (4) | Setae f1 and h1 on small platelets; ratio c1: c2 2:1; genua I with 4 asl, 5 sts; genua II with 2 asl, 5 sts; South Africa | Scutopalus latisetosus Den Heyer, 1979 |
| – | Setae f1 and h1 on integument; ratio c1: c2 1:1; genua I with 3 asl, 5 sts; genua II with 1 asl, 5 sts; Greece | Scutopalus smolikensis Sionti & Papadoulis, 2003 |
| 7 (1) | Dorsal shield smooth and/or punctate (Fig. 42a) | 8 |
| – | Dorsal shield sparse granulate, rugose, or reticulate (Fig. 42b–d) | 12 |
| 8 (7) | Coxae II and IV with 2 setae | 9 |
| – | Coxae II and IV with 3 setae | 11 |
| 9 (8) | Setae mps, c1, c2, d1, e1, f1 clavate (Fig. 43); a small subscutum situated posterior to the dorsal shield present; Malaysia | Scutopalus clavatus (Shiba, 1978) |
| – | Setae mps, c1, c2, d1, e1, f1 setiform; a small subscutum situated posterior to the dorsal shield absent | 10 |
| 10 (9) | Setae f1 on dorsal shield; setae lps, mps, c1, c2, d1, e1, f1 set on tubercles (Fig. 44); area between pt more heavily sclerotized, forming ridges; Taiwan | Scutopalus osseus (Tseng, 1980) |
| – | Setae f1 on integument; setae lps, mps, c1, c2, d1, e1, f1 set normally; area between pt normally sclerotized, not forming ridges; Ukraine | Scutopalus trepidus (Kuznetzov & Livshitz, 1979) |
| 11 (8) | 4 pairs of hysterosomal setae around genital shield; long slender platelet laterad genital shield present; with a narrow transverse sclertie behind main shield; Philippines | Scutopalus philippinensis (Corpuz-Raros, 1996) |
| – | 3 pairs of hystersomal setae around genital shield; long slender platelet laterad genital shield absent; dorsal sclerites absent; Philippines | Scutopalus makapalus (Corpuz-Raros, 1996) |
| 12 (7) | 1 or more dorsal sclerites present (behind or laterad dorsal shield); dorsal shield rugose or reticulate (Fig. 42b, c); basifemora IV with 1 seta; pedipalpal tibiotarsus with 6 setae present and apophysis absent | 13 |
| – | Dorsal sclerites absent; dorsal shield sparsely granulate; basifemora IV with 2 setae; pedipalpal tibiotarsus with 5 setae and a rod-shaped dorsal apophysis present; Taiwan | Scutopalus unguianalis (Tseng, 1980) |
| 13 (12) | Dorsal shield rugose (Fig. 42b); setae f1 and h1 on integument; dorsal setae (except c2 and h2) distally rod-like (slightly clavate), with minute barbs; narrow transverse shield behind main dorsal shield present; Philippines | Scutopalus rugosus (Corpuz-Raros, 1996) |
| – | Dorsal shield reticulate (Fig. 42c); setae f1 and h1 on small platelets; dorsal setae (except c2 and h2) broad and serrate; sclerites laterad and behind dorsal shield present; Brazil | Scutopalus tomentosus Rocha, Skvarla & Ferla, 2013 |
Scutopalus key illustrations. 40a Coxae I–II faintly divided 40b Coxae I–II totally divided 41 Coxae I–II fused medially 42 Dorsal shield with thick, rod-like setae present 43 Dorsal shield smooth or punctate 44a Dorsal shield rugose 44b Dorsal shield reticulate 44c Dorsal shield sparsely granulate 45a Setae mps, c1, c2, d1, e1, f1 clavate 45b Setae mps, c1, c2, d1, e1, f1 setiform 46 Setae lps, mps, c1, c2, d1, e1, f1 set on tubercles.
This is a monobasic subfamily, with the single genus containing two described and one undescribed species. The subfamily and genus are therefore treated together.
Gnathosoma. Pedipalps 4-segmented and do not reach beyond the subcapitulum. A flange-like apophysis present on either the genua or tibiotarsi. Pedipalps end in a stout claw. Subcapitulum with 4 pairs of r setae (hg1-4).
Idiosoma, dorsal. Proterosoma covered in a plate which bears 4 pairs of setae: 2 pairs of simple setae (lps and mps) and 2 pairs of setose sensilla (at and pt). Dorsal hysterosoma may or may not be complemented with a plate. 6 dorsal setae, c1–h1, c2 present. Cupule im present.
Idiosoma, ventral. Coxae I– IV fused, resulting in a complete shield covering the ventral idiosoma. Genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. Cupule ih present. Anal plates bear 2 pairs of setae (ps1 and ps2); 1 pair of setae born on integument next to anal plates.
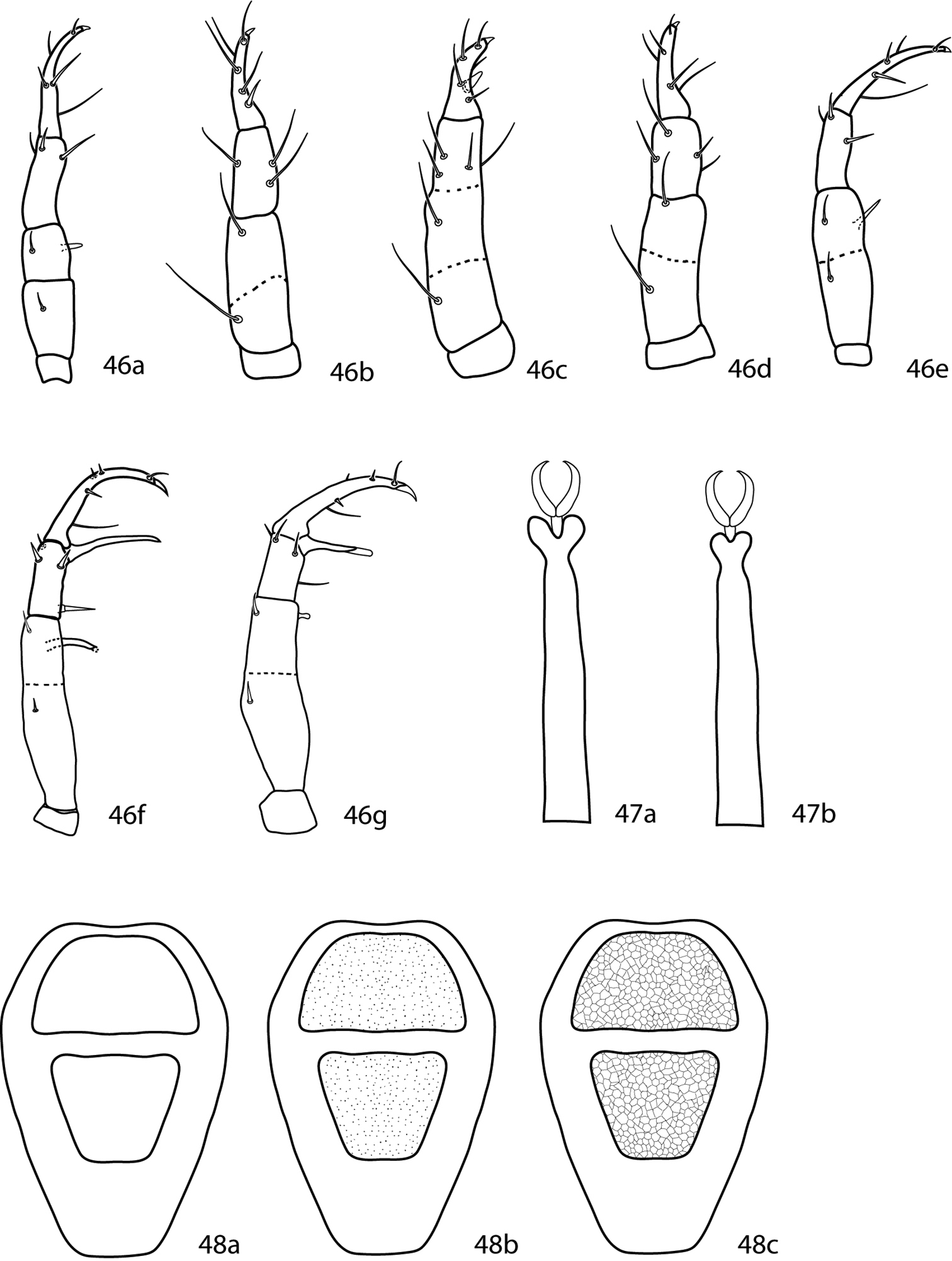
Gnathosoma. Pedipalps 5-segmented and extend beyond the subcapitulum by at least the distal half of the tibiae. Basifemora and telofemora fused but often dark line remains to indicate the division between the segments; telofemora and genua also fused in this manner in Allocunaxa. Apophyses may be present on the telofemora and between the genua and tibiotarsi. Tibiotarsi end in a strong claw. Chelicera with or without seta. Subcapitulum with up to 6 pairs of setae; setae hg1–4 always present, 2 pairs of adoral setae present or absent. Setae hg4 longest. In species with pedipalpal apophyses, the apophyses of the males shorter.
Idiosoma, dorsal. Female proterosoma bears a shield complemented with 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Dorsal hysterosoma may bear any combination of a median plate and lateral platelets (i.e., median plate and platelets absent, only median plate present, only lateral platelets present, or both median plate and lateral platelets present). Median plate, if present, may be complemented with 0–6 pairs of dorsal setae; lateral platelets, if present, may bear setae c2. Setae not born on plates or platelets may be born on tiny platelets barely larger than the setal socket. Integument that does not bear plates or platelets striated. Males differ in that the dorsal shields often more extensive and may be holodorsal.
Idiosoma, ventral. Coxae I–II fused or divided and may coalesce medially to form a sternal shield; coxae III–IV fused or divided and may extend caudally past the genital plates. Coxae each complemented 0–3 setae. Genital plates each bear 4 setae (g1–4); 2 pairs of genital papillae visible underneath the plates. Anal plates complemented with at least one pair of setae, ps1. Setae ps2 present or absent, either on the anal plates or on the integument adjacent to the anal plates. Setae h2 present ventrally on the integument adjacent to the anal plates. Cupule ih present laterad of h2. Integument that does not bear plates striated. Legs. Tarsi constricted apically so as to end in lobes. A trichobothrium on tibia IV present or absent.
(modified from
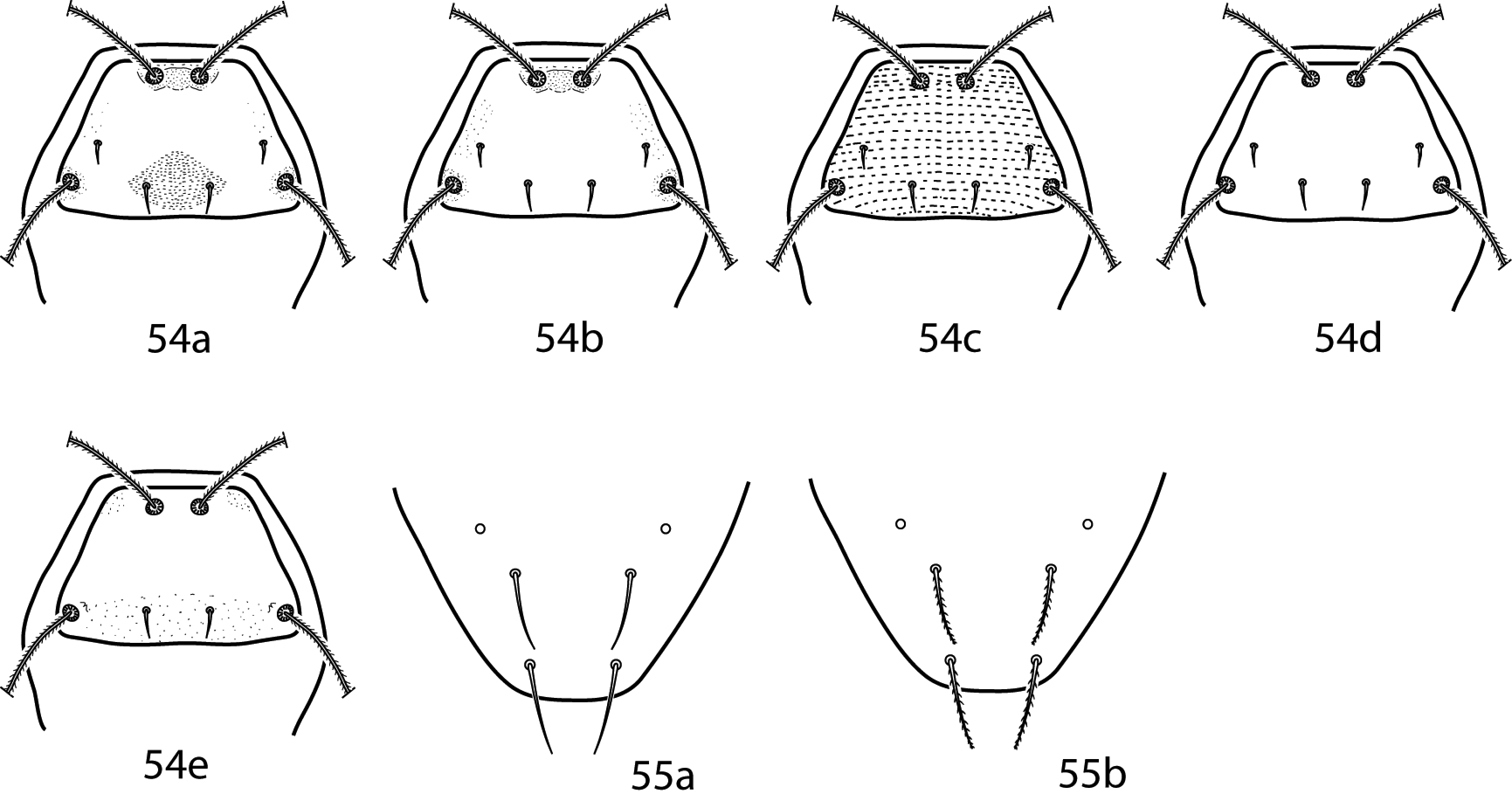
| 1 | Anal seta ps2 absent; pedipalp telofemora with dorsal simple seta (Figs 46a–e); tarsal lobes small to medium size (Fig. 47a); dorsal plates reticulated or not (Figs 48a–c) Cunaxini | 2 |
| – | Anal seta ps2 present; pedipalp telofemora with dorsal spine-like seta (Figs 46f, g); tarsal lobes medium to large size (Fig. 47b); dorsal plates always reticulated (Fig. 48c) Armascirini | 6 |
| 2 (1) | Dorsal plates never reticulated (Figs 48a, b); integumental striae smooth or lobed; coxae II–IV setal formula usually 1-3-2 (rarely 2-3-1) | Cunaxa Von Heyden, 1826 |
| – | Dorsal plates usually reticulated (Fig. 48c); integumental striae usually papillated; coxae II–IV setal formula usually 1-3-1 | 3 |
| 3 (2) | Pedipalpal telofemora with one or more apophyses (Fig. 46a); sensillae at and pt not densely pilose | Rubroscirus Den Heyer, 1979 |
| – | Pedipalpal telofemora without apophyses (Figs 46b–e); sensillae at and pt densely pilose | 4 |
| 4 (3) | Tibiae IV trichobothrium present | 5 |
| – | Tibiae IV trichobothrium absent | Cunaxatricha Castro & Den Heyer, 2008 |
| 5 | Articulation joint between pedipalpal telofemora and genua functional (Fig. 46b) | Riscus Den Heyer, 2006 |
| – | Articulation joint between pedipalpal telofemora and genua fused/non-functional (Fig. 46c) | Allocunaxa Den Heyer & Castro, 2008 |
| 6 (1) | Pedipalpal basifemora with simple seta (Fig. 46f); coxae II–IV setal formula usually 1-3-3 (male) or 2-3-3 (female); famulus normal; pedipalpal apophyses (when present) usually long in females and short in males, and with pointed apices (Fig. 46f) | Armascirus Den Heyer, 1978 |
| – | Pedipalpal basifemora with spine-like seta (Fig. 46g); coxae II–IV setal formula usually 3-3-3; famulus large, broad based with tri-pronged tip; pedipalpal apophyses (when present) usually equal length in females and males, and with bulbous apices (Fig. 46g) | Dactyloscirus Berlese, 1916 |
Cunaxinae key illustrations. 46 Pedipalps, dorsal 46a Rubroscirus 46b Riscus 46c Allocunaxa 46d Cunaxatricha 46e Cunaxa 46f Armascirus 46g Dactyloscirus. 47a, b. Distal end of tarsus 47a Armascirini, showing large tarsal lobes 47b Cunaxini, showing small to medium tarsal lobes 48a–c Idiosoma, dorsal. Setae and cupules have been removed for clairity. Shape of proterosomal plate and presence or absence, shape, and extent of hysterosomal plate(s) will differ between species 48a Plates smooth 48b Plates with dot-like pattern 48c Plates with reticulated pattern.
Gnathosoma. Pedipalps 5-segmented, end in a strong claw, and extend beyond the subcapitulum by at least the last segment. Pedipalpal apophyses absent. Basifemora complemented with a long simple seta and telofemora with a short simple seta; these two segments fused, although a line remains visible and they can thus be differentiated. Telofemora and genu nearly fused, although a line remains visible and they can thus be differentiated. Subcapitulum complemented with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae) and covered by integumental papillae.
Idiosoma, dorsal. Proterosoma with an ill-defined, weakly sclerotized shield that bears 2 pairs of setose sensillae (at and pt) and 2 pairs of simple setae (lps and mps). 7 pairs of setae, c1–2, d1–h1, present. Cupule im present, usually posteriolaterad of e1. Integument striated.
Idiosoma, ventral. Coxae I and II fused. Coxae III and IV fused. Genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. Integument between plates striated and bears 4 pairs of additional setae. Legs shorter than the body. Leg 4 longest. Famulus on tarsi I normally shaped. Tarsi constricted apically, resulting in large tarsal lobes. Trichobothrium on leg tibia IV present. Ambulacral claws on either side of a 4-rayed empodium present.
The first Armascirus was described by
Gnathosoma. Pedipalps 5-segmented, end in a strong claw, and extend beyond the subcapitulum by at least the last segment. Apophysis between the genua and tibiotarsi, which tapers to a point, usually present; this apophysis shorter in males than in females. Basifemora complemented with a simple seta; telofemora with a spine-like seta. These two segments fused, although a line remains visible and they can thus be differentiated. Subcapitulum complemented with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae). It can be covered by integumental papillae which are either randomly distributed or form a polygonal, reticulated pattern.
Idiosoma, dorsal. Female dorsal idiosoma with at least one sclerotized plate that bears 2 pairs of setose sensillae (at and pt) and 2 pairs of simple setae (lps and mps). 0–4 other major plates and platelets may also be present. All plates, if present, covered by integumental papillae that form a reticulated pattern. Integument between the plates is striated. 7 pairs of setae, c1–2, d1–h1, present. Each seta, when not on a major plate or platelet, surrounded by a minute platelet that is only slightly larger than the setal socket. Cupule im present, usually laterad or in the proximity of e1. Dorsal idiosoma of males is similar except a single large plate complemented with c1–2, d1–e1 present.
Idiosoma, ventral. Coxae reticulated in the same manner as the dorsal plates. Coxae I–II often fused; Coxae III–IV often fused. Setal formula of coxae I–IV in males 3-1-3-3 (including the paracoxal seta), in females 3-2-3-3 (including the paracoxal seta). Genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. Anal plates bear 1 pair of setae (ps1). 2 pairs of setae (ps2 and h2) associated with but do not occur on the anal plates. Cupule ih present in close proximity to h2. Integument between plates striated and bears 5–7 pairs of additional setae. The ventral idiosoma of males similar except the coxae are much more extensive. A sclerotized aedeagus is often visible in association with the genital plates. Legs comparatively long, at least ¾ the length, and often longer than the body. Famulus on tarsi I normally shaped. Tarsi are constricted apically, resulting in large tarsal lobes. Trichobothrium on leg tibia IV present. Ambulacral claws occur on either side of a 4-rayed empodium.
(modified from
Dactyloscirus bifidus Corpuz-Raros, 2008 is transferred to Armascirus as it posessess a spine-like seta on the pedipalpal basifemora.
Armascirus gojraensis and Armascirus sabrii appear to be nymphs based on the leg setal counts given in the original descriptions. Having not seen the type material, however, they are retained within the key. Caution should be exercised if these species are reached.
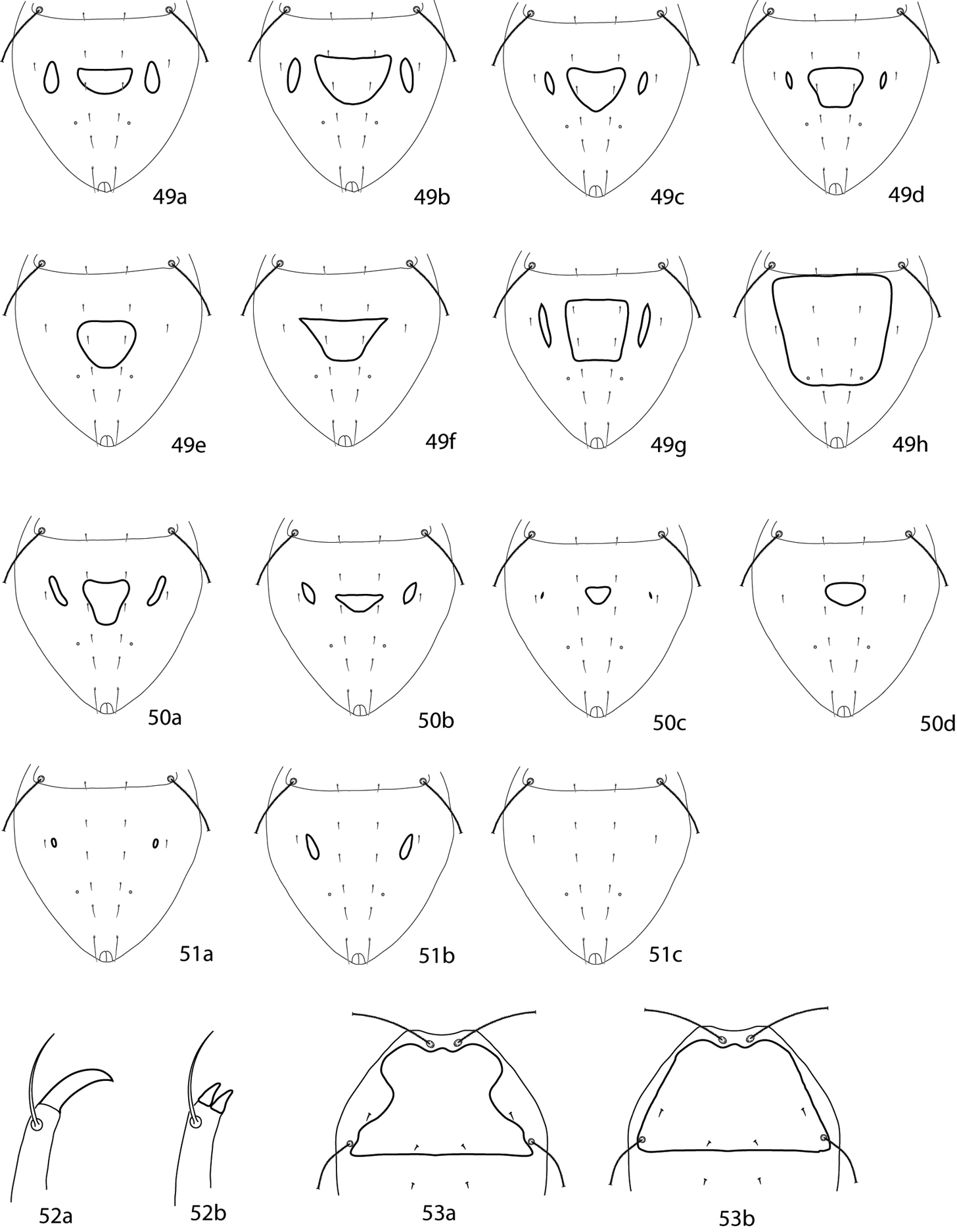
| 1 | Hysterosomal median shield present (Figs 49a–h, 50a–d) | 2 |
| – | Hysterosomal median shield absent (Figs 51a–c) | 30 |
| 2 (1) | Median shield complemented with setae, small or large (Figs 49a–h) | 3 |
| – | Median shield not complemented with setae, small (Figs 50a–d) | 22 |
| 3 (2) | One pair of setae (d1) on hysterosomal median shield (Figs 49a–f) | 4 |
| – | Two or more pairs of setae on hysterosomal median shield (Figs 49g–h) | 18 |
| 4 (3) | Lateral hysterosomal platelets present (Figs 49a–d) | 5 |
| – | Lateral hysterosomal platelets absent (Figs 49e, f) | 15 |
| 5 (4) | Setae c1 very short, the distance between the bases of c1–c1 20 times the length of c1; venter caudally from coxae II with 5 pairs of simple setae (excluding genital, coxal, and anal setae); Poland | Armascirus rafalskii (Michocka, 1987) |
| – | Setae c1 longer, the distance between the bases of c1–c1 less than 10 times the length of c1; venter caudally from coxae II with 6 or more pairs of simple setae (excluding genital, coxal, and anal setae) | 6 |
| 6 (5) | The distance between caudal parts of hysterosomal lateral platelets wider than the distance between their frontal parts (Figs 49a, b) | 7 |
| – | The distance between caudal parts of hysterosomal lateral platelets shorter than the distance between their frontal parts (Figs 49c, d) | 9 |
| 7 (6) | Lateral hysterosomal platelets equal to or longer than hysterosomal median shield (Fig. 49a); venter caudally from coxae II with 6 pairs of simple setae (excluding genital, coxal, and anal setae); Pakistan | Armascirus jasmina Bashir, Afzal & Khan, 2008 |
| – | Lateral hysterosomal platelets shorter than hysterosomal median shield (Fig. 49b); venter caudally from coxae II with 7 pairs of simple setae (excluding genital, coxal, and anal setae) | 8 |
| 8 (7) | Pedipalpal genua with 3 spls, 1 sts; important leg I–IV sts chaetotaxy: coxae 3-1-3-2, basifemora 4-5-3-1, genua 8-8-6-5, tibiae 5-6-6-6, tarsi 15-12-8-9; Pakistan | Armascirus akhtari Bashir, Afzal & Khan, 2008 |
| – | Pedipalpal genua with 3 spls; important leg I–IV sts chaetotaxy: coxae 3-2-3-3, basifemora 4-4-3-3, genua 8-4-6-7, tibiae 6-5-6-5, tarsi 11-10-9-7; Pakistan | Armascirus satianaensis Bashir & Afzal, 2005 |
| 9 (6) | Venter caudally from coxae II with 4 pairs of simple setae (excluding genital, coxal, and anal setae); Brazil | Armascirus bahiaensis Den Heyer & Castro, 2012 |
| – | Venter caudally from coxae II with 6 pairs of simple setae (excluding genital, coxal, and anal setae) | 10 |
| – | Venter caudally from coxae II with 7 pairs of simple setae (excluding genital, coxal, and anal setae) | 14 |
| – | Venter caudally from coxae II with 8 pairs of simple setae (excluding genital, coxal, and anal setae); South Africa | Armascirus albiziae Den Heyer, 1978 |
| 10 (9) | Tarsus I with more than 27 setae; tarsus II with at least 24 setae | 11 |
| – | Tarsus I with less than 25 setae; tarsus II with less than 23 setae | 12 |
| 11 (10) | Leg genua I with 4 bsl, 4 sts; genital valve with random dot-like lobes; tarsal sts chaetotaxy I–IV 29-25-23-22; Pakistan | Armascirus pluri Muhammad & Chaudhri, 1991 |
| – | Leg genua I with 2 asl, 4 bsl, 3 sts; genital valve longitudinal rows of dot-like lobes; tarsal sts chaetotaxy I–IV 29-24-22-21; Pakistan | Armascirus mactator Muhammad & Chaudhri, 1991 |
| 12 (10) | Pedipalpal telofemora with 1 apophysis, 2 spls; pedipalpal genua with 1 ap, 2 spls, 2 sts; South Africa | Armascirus huyssteeni Den Heyer, 1978 |
| – | Pedipalpal telofemora with 1 apophysis, 1 spls; pedipalpal genua with 1 ap, 3 spls, 1 sts | 13 |
| 13(12) | Genua II with 1 asl, 5 sts; genua IV with 2 asl, 5 sts; cosmopolitan | Armascirus taurus (Kramer, 1881) |
| – | Genua II with 1 asl, 6 sts; genua IV with 1 asl, 4 or 5 sts; USA | Armascirus primigenius Skvarla & Dowling, 2012 |
| 14 (9) | Median shield pointed caudally (Fig. 49c); Pakistan | Armascirus asghari Bashir & Afzal, 2005 |
| – | Median shield truncated caudally (Fig. 49d); Brazil | Armascirus brasiliensis Den Heyer & Castro, 2012 |
| 15 (4) | Hysterosomal median shield with a straight or concave frontal margin and with very acute anterior lateral corners (angle less than 45°) (Fig. 49e) | 16 |
| – | Hysterosomal median shield with convex frontal margin and with rounded anterior lateral corners (Fig. 49f) | 17 |
| 16 (15) | Pedipalpal genua with 1 ap, 2 spls, 1 sts; legs I–IV sts formulae (excluding solenidia): basifemora 1-2-1-0; telofemora 4-4-4-4; genua 6-7-5-6; h1 4 times the length of c1;hysterosomal shield width: length = 2.2:1; Pakistan | Armascirus sabrii Bashir, Afzal & Khan, 2008 |
| – | Pedipalpal genua with 1 ap, 3 spls, 1 sts; legs I–IV sts formulae (excluding solenidia): basifemora 2-2-1-1; telofemora 4-4-4-3; genua 8-6-6-6; h1 3 times the length of c1;hysterosomal shield width: length 1.5:1; Pakistan | Armascirus gojraensis Bashir, Afzal & Khan, 2008 |
| 17 (15) | Apophysis adjoining genu and tibiotarsus shorter than pedipalpal tibiotarsus; pedipalpal telofemoral apophyses three times longer than spine-like seta; distance between the bases of sci–sci 9 times the length of sci; Brazil, Mexico | Armascirus bison (Berlese, 1988) |
| – | Apophysis adjoining genu and tibiotarsus longer than pedipalpal tibiotarsus; pedipalpal telofemoral apophyses three times longer than spine-like seta; distance between the bases of sci–sci 5 times the length of sci; Pakistan | Armascirus fixus (Chaudhri, 1980) |
| 18 (3) | Hysterosomal median shield with 2 pairs of setae (c1, d1) (Fig. 49g) | 19 |
| – | Hysterosomal median shield with more than 3 pairs of setae (Fig. 49h) | 20 |
| 19 (18) | Pedipalpal telofemora with 2 ap, 1 spls; pedipalpal genua with 2 spls, 2 sts; venter caudally from coxae II with 6 pairs of simple setae (excluding genital, coxal, and anal setae); tarsi I–IV with 21-20-15-13 sts (excluding solenidia); the distance between bases of c1–c1 4 times the distance of h1–h1; distance between c1–c1 5 times the length of c1 | Armascirus anastosi Smiley, 1992 |
| – | Pedipalpal telofemora with 1 ap, 1 spls; pedipalpal genua with 3 spls, 1 sts; venter caudally from coxae II with 5 pairs of simple setae (excluding genital, coxal, and anal setae); tarsi I–IV with 19-13-13-13 sts (excluding solenidia); the distance between c1–c1 2 times the distance between h1–h1; the distance between c1–c1 4 times the length of c1 | Armascirus heryfordi Smiley, 1992 |
| 20 (18) | Apophysis adjacent to pedipalpal genua and tibiotarsi present | Armascirus multioculus Shiba, 1986 |
| – | Apophysis adjacent to pedipalpal genua and tibiotarsi absent | 21 |
| 21 (20) | 5 pairs of genital setae; pedipalp claw bifid (Fig. 52a); hysterosomal setae not serrate; Philippines | Armascirus apoensis Corpuz-Raros, 2008 |
| – | 4 pairs of genital setae; pedipalp claw entire, not bifid (Fig. 52b); hysterosomal setae serrate; Pakistan | Armascirus fuscus (Chaudhri, 1977) |
| 22 (2) | Lateral hysterosomal platelets present (Figs 50a–c) | 23 |
| – | Lateral hysterosomal platelets absent (Fig. 50d) | 27 |
| 23 (22) | Hysterosomal median shield width: length 1:1; venter caudally from coxae II with 6 or 7 pairs of sts (excluding genital and anal setae) | 24 |
| – | Hysterosomal median shield width: length 2:1; venter caudally from coxae II with 5 or 6 pairs of sts (excluding genital and anal setae) | 25 |
| 24 (23) | Hysterosomal platelets large, as long as median shield (Fig. 50a); venter caudally from coxae II with 7 sts; pedipalp telofemur with 1 apophysis | Armascirus cerris Kalúz, 2009 |
| – | Hysterosomal platelets about 1/3 the length of median shield; venter caudally from coxae II with 6 sts; pedipalp telofemur with 2 apophysis | Armascirus fendai Kalúz & Vrabec, 2013 |
| 25 (23) | Hysterosomal platelets as long as median shield (Fig. 50b) | 26 |
| – | Hysterosomal platelets ½ as long as median shield (Fig. 50c); Mexico, USA | Armascirus gimplei |
| 26 (25) | Hysterosomal plate concave on lateral edges (Fig. 53a); USA | Armascirus ozarkensis Skvarla & Dowling, 2012 |
| – | Hysterosomal plate not concave on lateral edges (Fig. 53b); Japan | Armascirus hastus Shiba, 1986 |
| 27 (22) | Apophysis on pedipalp telofemur extends to distal margin of segment; 2 pairs of ventral pregenital setae thickened and spiculate; f1 1/3 length of h1; Philippines | Armascirus makilingensis Corpuz-Raros, 1995 |
| – | Apophysis on pedipalp telofemur extends well beyond distal margin of segment; ventral pregenital setae not thickened and spiculate; f1 subequal to h1 | 28 |
| 28 (27) | Pedipalpal telofemora with 2 ap, 1 spls; the distance between the bases of c1–c1 two times the distance of d1–d1; South Africa | Armascirus limpopoensis Den Heyer, 1978 |
| – | Pedipalp telofemora with 1 ap, 1 spls; the distances between the bases of c1–c1 = d1–d1 | 29 |
| 29 (28) | Pedipalp tibiotarsus with 1 spls, 4 sts; USA | Armascirus harrisoni Smiley, 1992 |
| – | Pedipalp tibiotarsus with 1 spls, 3 sts; Canada | Armascirus bakeri (Smiley, 1992) |
| 30 (1) | Pedipalpal telofemoral apophyses long, reaching apical apophysis on pedipalpal genu; lateral platelets present | 31 |
| – | Pedipalpal telofemoral apophyses short, not reaching apical apophysis on pedipalpal genu; lateral platelets present or absent | 32 |
| 31 (30) | Pedipalpal basifemora with 1 subrectangular apophysis; pedipalp tibiotarsal spls 3 times the length of terminal claw; hysterosomal platelets small, equal in length to c2 (Fig. 51a); coxal chaetotaxy I–IV 3-2-3-3; South Africa | Armascirus lebowensis Den Heyer, 1978 |
| – | Pedipalpal basifemora without subrectangular apophysis; pedipalp tibiotarsal spls equal in length to terminal claw; hysterosomal platelets long, 2–3 times the length of c2 (Fig. 51b); coxal chaetotaxy I–V 3-1-3-1; USA | Armascirus campbelli (Smiley, 1992) |
| 32 (30) | Coxal setal count I–IV 3-2-3-3 | 33 |
| – | Coxal setal count I–IV 3-2-3-2 | 35 |
| – | Coxal setal count I–IV 3-3-3-3 | Armascirus bifidus (Corpuz-Raros, 2008) |
| 33 (32) | Pedipalpal telofemora with 1 apophysis, 2 spls, 1 sts; the distance between d1–d1 9 times the length of d1; pedipalpal genua with 2 spls, 1 sts; Slovakia | Armascirus cyaneus Kalúz, 2009 |
| – | Pedipalpal telofemora with 1 apophysis, 2 spls; the distance between d1–d1 4 times the length of d1; pedipalpal genua chaetotaxy not as above | 34 |
| 34 (33) | Hysterosomal platelets present (Fig. 51b); pedipalpal genua with 2 spls, 2 sts; basifemora with 5-5-4-2 sts; USA | Armascirus virginiensis Smiley, 1992 |
| – | Hysterosomal platelets absent (Fig. 51c); pedipalpal genua with 1 spls, 1 sts; basifemora with 6-6-4-2 sts; Philippines | Armascirus javanus Corpuz-Raros & Gruèzo, 2007 |
| 35 (32) | Pedipalpal telofemoral apophyses as long as width of telofemora; pedipalpal genu with 1 apophysis, 2 spls, 2 sts; USA | Armascirus pennsylvanicus Skvarla & Dowling, 2012 |
| – | Pedipalpal telofemoral apophyses only 1/3 width of telofemora; pedipalpal genu with 1 apophysis, 3 spls, 1 sts; Philippines | Armascirus garciai Corpuz-Raros, 1995 |
Armascirus key illustrations. 49–51 Dorsal idiosoma 49a–e Hysterosomal shield complemented with setae 50a–d Hysterosomal shield small, not complemented with setae 51a–c Hysterosomal shield absent 52a, b Pedipalp tibiotarsal claw 52a Single claw 52b Bifid claw 53a Hysterosomal plate concave on lateral edges 53b Hysterosomal plate not concave on lateral edges.
(modified from
| 1 | Venter with 5 or fewer pairs of setae, excluding genital, anal, and adanal setae; setal formula of coxae I–IV not as below; setal formula of basifemora I–IV not as below | 2 |
| – | Venter with 6 pairs of setae, excluding genital, anal, and adanal setae; setal formula of coxae I–IV 3-2-3-3; setal formula of basifemora I–IV 5-5-4-2; cosmopolitan | Armascirus taurus (Kramer, 1881) |
| 2 (1) | Setal formula of basifemora I–IV 5-5-4-1; Pakistan | Armascirus ebrius (Chaudhri, 1977) |
| – | Setal formula of basifemora I–IV not as above | 3 |
| 3 (2) | Coxae I–IV setal formula 3-1-3-3; papillae on circular region anterior to setae pt present; South Africa | Armascirus huyssteeni Den Heyer, 1978 |
| – | Coxae I–IV setal formula 3-2-3-3; papillae on circular region anterior to setae pt present or absent | 4 |
| 4 (3) | Setal formula of basifemora I–IV 5-4-3-0; papillae on circular region anterior to setae pt present; South Africa | Armascirus limpopoensis Den Heyer, 1978 |
| – | Setal formula of basifemora I–IV not as above; papillae on circular region anterior to setae pt absent; South africa | 5 |
| 5 (4) | Genua I with 3 asl, 5 sts; South Africa | Armascirus lebowensis Den Heyer, 1978 |
| – | Genua I with 2 asl, 1 mst, 5 sts; Ukraine | Armascirus masani Kalúz & Vrabec, 2013 |
Gnathosoma. Pedipalps–5-segmented and reach beyond the subcapitulum by at most the distal half of the tibiae. An apophysis on the telofemora present or absent. Dorsolateral setae on the basi- and telofemora simple. Stout spine-like setae on the genua and tibiotarsi present or absent. Tibiotarsi end in a strong claw. Subcapitulum with 6 pairs of setae: 2 pairs of adoral setae and 4 pairs of subcapitular setae (hg1–4). Subcapitulum smooth or patterned with random dots, but never reticulated.
Idiosoma, dorsal. Proterosoma bears a shield that is complemented with 2 pairs of setae (at and pt) and 2 pairs of setose sensillae (lps and mps). Dorsal hysterosoma may bear a shield; if a shield is present, it may bear up to 4 pairs of setae. Dorsal shields may be smooth or patterned with random dots, but never reticulated. Lateral platelets (as in Armascirus and Dactyloscirus) absent. Setae c1–h1, and c2 present. Setae not born on the median plate may be born on small platelets that are barely larger than the setal socket. Cupule im present laterad and caudally of e1. Integument not bearing the proterosomal shield and median plate (if present) striated. These striations smooth or lobed but never papillated.
Idiosoma, ventral. Coxae I–II may be fused and coxae III–IV may be fused. Coxae II–IV setal formula 1-3-2. Genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. Anal plates bear 1 pair of setae (ps1). 1 pair of setae (h2) associated with, but do not occur on, the anal plates. Cupule ih present in close proximity to h2. Integument between plates striated and bears up to 7 pairs of additional setae. Legs. Tarsi long and slender. Tarsi constricted distally but the tarsal lobes are small and not conspicuous as in Armascirus and Dactyloscirus. A trichobothrium on tibia IV present. Ambulacral claws on either side of a 4-rayed empodium present.
Cunaxa bochkovi is not included in the key because the original description is in Cyrillic and the illustration does not contain enough detail or diagnostic characteristics. Den Heyer (pers. comm., Jan. 13, 2014) indicated that Cunaxa setirostris var. plurisetosa and Cunaxa setirostris var. diversa were described in “Mihelčič, F. 1958” but did not have the entire citation and had not seen the original description. The authors have also not been able to locate such a publication after extensive searching and so have not included the taxa here.
As suggested by
Cunaxa nankanaensis Bashir, Afzal, Ashfaq, Raza, Kamran, 2011 is considered a junior synonym and junior homonym of Cunaxa nankanaensis Bashir & Afzal, 2009.
| 1 | Setae lps present (Figs 54a–d) | 2 |
| – | Setae lps absent (Fig. 54e) | Cunaxa anomala Khaustov & Kuznetzov, 1998 |
| 2 (1) | Setae at normal, nearly as long as pt | 3 |
| – | Setae at short and stubby, less than half the length of pt | Cunaxa anacardae Gupta, 1992 |
| 3 (2) | Basifemora I with 1 sts | 4 |
| – | Basifemora I with 2 sts | 5 |
| – | Basifemora I with 3 sts | 7 |
| – | Basifemora I with 4 sts | 14 |
| – | Basifemora I with 5 sts | 43 |
| 4 (3) | Basifemora I–IV setal formula 1-2-3-0; telofemora I–IV setal formula 2-2-4-3; India | Cunaxa prinia Gupta & Paul, 1985 |
| – | Basifemora I–IV setal formula 1-1-1-2; telofemora I–IV setal formula 2-2-1-1; India | Cunaxa magniferae Gupta, 1992 |
| 5 (3) | Basifemora II-IV setal formula 2-1-0 | 6 |
| – | Basifemora II-IV setal formula 3-3-1 | Cunaxa dotos Bashir & Afzal, 2009 |
| 6 (5) | Tibia II with 5 sts; Pakistan | Cunaxa mahmoodi Bashir & Afzal, 2009 |
| – | Tibia II with 7 sts; Pakistan | Cunaxa okaraensis |
| 7 (4) | Genua I with 3 solenidia | 8 |
| – | Genua I with 4 solenidia | 9 |
| 8 (7) | Genua II with 1 solenidion; setae f1, h1 smooth (Fig. 55a) | Cunaxa setirostris (Hermann, 1804) |
| – | Genua II with 2 solenidia; setae f1, h1 spiculate (Fig. 55b) | Cunaxa magoebaensis Den Heyer, 1979 |
| 9 (7) | Coxae I–IV setal formula 3-1-3-2 sts | 10 |
| – | Coxae I–IV setal formula 3-2-3-1 sts | Cunaxa eupatoriae Chinniah & Mohanasundaram, 2001 |
| 10 (9) | Dorsal setae short (c1–f1, c2: 7-10, h1: 17) | Cunaxa mercedesae Corpuz-Raros & Garcia, 1995 |
| – | Dorsal setae longer (19-40) | 11 |
| 11 (10) | Oval area formed by broken striae around setae sci present (Fig. 54a) | Cunaxa maculata Sergeyenko, 2009 |
| – | Oval area formed by broken striae around setae sci absent (Fig. 54b) | 12 |
| 12 (11) | Genua II proximal solenidion extremely short, its length subequal to the diameter of its alveolus; ventral surface of the coxal region of hypognathum smooth | Cunaxa guanotoleranta Sergeyenko, 2009 |
| – | Genua II proximal solenidion long, its length several times longer than the diameter of its alveolus; ventral surface of the coxal region of the hypognathum with numerous papillae | 13 |
| 13 (12) | Length of setae sci longer than half the distance between their bases; dorsal hysterosomal striae distinctly lobed (= with festoons) (Fig. 56a) | Cunaxa papuliphora Sergeyenko, 2009 |
| – | Length of setae sci shorter or equal to half the distance between their bases; dorsal hysterosomal striae smooth (Fig. 56b) | Cunaxa gordeevae Sergeyenko, 2009 |
| 14 (3) | Basifemora III with 2 sts | 15 |
| – | Basifemora III with 3 sts | 17 |
| – | Basifemora III with 4 sts | 41 |
| 15 (14) | Telofemoral apophysis uncinated (e.g., bent, hook-shaped) (Fig. 59a) | Cunaxa jatoiensis Bashir & Afzal, 2006 |
| – | Telofemoral apophysis straight, not uncinated | 16 |
| 16 (14) | Basifemora IV with 1 sts; cheliceral longitudinal striations present (Fig. 57a) | Cunaxa heterostriata Khaustov & Kuznetzov, 1998 |
| – | Basifemora IV with 0 sts; cheliceral longitudinal striations absent (Fig. 57b) | Cunaxa yaylensis Sergeyenko, 2009 |
| 17 (14) | Basifemora IV with 0 sts | Cunaxa violaphila Sergeyenko, 2009 |
| – | Basifemora IV with 1 sts | 18 |
| – | Basifemora IV with 2 sts | Cunaxa brevicrura Den Heyer, 1979 |
| – | Basifemora IV with 5 sts | Cunaxa meiringi Den Heyer, 1979 |
| 18 (17) | Median plate present (may be indistinctly defined) (Figs 58a–e) | 19 |
| – | Median plate absent (Fig. 58f) | 36 |
| 19 (18) | Telofemoral apophysis uncinated (e.g., bent, hook-shaped) (Fig. 59a) | 20 |
| – | Telofemoral apophysis present or absent; if present, not uncinated (Figs 59b–e) | 25 |
| 20 (19) | Setae c1 not on hysterosomal shield, on integument | 21 |
| – | Setae c1 on hysterosomal shield | 22 |
| 21 (20) | Tibiae I with 3 asl, 4 sts; Pakistan | Cunaxa clusus Bashir & Afzal, 2009 |
| – | Tibiae I with 2 asl, 4 sts; Pakistan | Cunaxa nankanaensis |
| 22 (20) | Setae f1 on hysterosomal shield | 23 |
| – | Setae f1 not on hysterosmal shield, on integument | 24 |
| 23 (22) | Tibia III with 5 sts | Cunaxa leuros Bashir, Afzal, Ashfaq, Akbar & Ali 2010 |
| – | Tibia III with 6 sts | Cunaxa rafiqi Bashir, Afzal, Ashfaq, Akbar & Ali 2010 |
| 24 (22) | Genua I with 2 asl, 5 sts | Cunaxa capreolus (Berlese, 1887) |
| – | Genua I with 3 asl, 3 sts; tibia I with 2 asl, 4 sts; Pakistan | Cunaxa pakpatanensis |
| – | Genua I with 3 asl, 4 sts; tibia I with 2 asl, 4 sts; Pakistan | Cunaxa bashiri Bashir & Afzal, 2009 |
| 25 (19) | Telofemoral apophysis truncated (Fig. 59b) | Cunaxa carina Den Heyer, 1979 |
| – | Telofemoral apophysis not truncated (Figs 59c–e) | 26 |
| 26 (25) | Line of small sharp spines on pedipalp tibiotarsi present (Fig. 60a) | Cunaxa dentata Sergeyenko, 2003 |
| – | Line of small sharp spines on pedipalp tibiotarsi absent (Fig. 60b) | 27 |
| 27 (26) | Median plate complemented with c2 (Figs 58a–d) | 28 |
| – | Median plate not complemented with c2 (Fig. 58e) | Cunaxa terrula Den Heyer, 1979 |
| 28 (27) | Median plate indistinctly defined (Fig. 58a) | 29 |
| – | Median plate distinctly defined (Fig. 58b–d) | 30 |
| 29 (28) | Setae f1, h1 smooth | Cunaxa romblonensis Corpuz-Raros & Garcia, 1995 |
| – | Setae f1, h1 finely setose | Cunaxa sordwanaensis Den Heyer, 1979 |
| 30 (28) | Median shield complemented with c1, d1, c2 (Fig. 58b) | Cunaxa sudakensis Khaustov & Kuznetzov, 1998 |
| – | Median shield complemented with c1–e1, c2 (Fig. 58c, d) | 31 |
| 31 (30) | Coxae IV with 1 sts | 32 |
| – | Coxae IV with 2 sts | 33 |
| 32 (31) | Broken striae that form cell-like structures on median shield present (Fig. 58c) | Cunaxa thailandicus Smiley, 1992 |
| – | Broken striae that form cell-like structures on median shield absent (Fig. 58d) | Cunaxa veracruzana Baker & Hoffmann, 1948 |
| 33 (31) | Setae c1 longer than all other dorsal setae | Cunaxa womersleyi Baker & Hoffmann, 1948 |
| – | Setae c1 not longer than all other dorsal setae | 34 |
| 34 (33) | Genua I–IV with 4-2-1-1 solenidia | Cunaxa lamberti Den Heyer, 1979 |
| – | Genua I–IV with 3-1-1-1 solenidia | 35 |
| 35 (34) | Setae c1–h1 approximately equal in length | Cunaxa hermanni Den Heyer, 1979 |
| – | Setae c1–e1 half as long as f1, h1 | Cunaxa thessalica Sionti & Papadoulis, 2003 |
| 36 (18) | Telofemoral apophysis uncinated (Fig. 59a) | 37 |
| – | Telofemoral apophysis not uncinated (Fig. 59b-e) | 38 |
| 37 (36) | Genua I–IV setal formula 1 asl, 6 sts-7-6-6; Philippines | Cunaxa pantabanganensis Corpuz-Raros & Garcia, 1995 |
| – | Genua I–IV setal formula 1 asl, 4 sts-5-6-6; Pakistan | Cunaxa lodhranensis Bashir & Afzal, 2009 |
| 38 (36) | Proterosomal shield striated (Fig. 54c) | 39 |
| – | Proterosomal shield smooth (Fig. 54d) | Cunaxa potchensis Den Heyer, 1979 |
| 39 (38) | Setae f1, h1 smooth (Fig. 55a) | 40 |
| – | Setae f1, h1 spiculate (Fig. 55b) | Cunaxa gazella (Ewing, 1913) |
| 40 (39) | Pedipalp telofemoral apophysis short and cone-like (Fig. 59c) | Cunaxa mageei Smiley, 1992 |
| – | Pedipalp telofemoral apophysis short and finger-like (Fig. 59d) | Cunaxa neogazella, Smiley, 1992 |
| 41 (14) | Median plate present (Fig. 58d); basifemora IV with 1 sts | Cunaxa luzonica Corpuz-Raros & Garcia, 1995 |
| – | Median plate absent (Fig. 58f); basifemora IV with 1 or 2 sts | 42 |
| 42 (41) | Basifemora IV with 1 sts | Cunaxa cogonae Corpuz-Raros & Garcia, 1995 |
| – | Basifemora IV with 2 sts | Cunaxa doxa Chaudhri, 1980 |
| 43 (3) | Basifemora III with 4 sts | Cunaxa evansi Smiley, 1992 |
| – | Basifemora III with 6 sts | Cunaxa grobleri Den Heyer, 1979 |
Cunaxa key illustrations. 54a–e Proterosomal shield, dorsal 54a Proterosomal shield with oval area formed by broken striae around pt present, mps present 54b Proterosomal shield with oval area formed by broken striae around pt absent, mps present 54c Proterosomal shield striated, mps present 54d Proterosomal shield smooth, mps present 54e Proterosomal shield with lps absent 55a Smooth f1, h1 55b Spiculate f1, h1.
Cunaxa key illustrations. 56a, b Integumental striations 57a Chelicera with longitudinal striations present 57a Chelicera with longitudinal striations absent 58a–f Examples of variation in the hysterosomal median plate 59a Pedipalp telofemoral apophysis uncinated 59b Pedipalp telofemoral apophysis truncated 59c Pedipalp telofemoral apophysis short and cone-like 59d Pedipalp telofemoral apophysis short and finger-like 59e Pedipalp telofemoral femoral apophysis long 60a Pedipalp tibiotarsus with small teeth present 60b Pedipalp tibiotarsus with small teeth absent.
Gnathosoma. Pedipalps 5-segmented and end in a strong claw. They extend beyond the subcapitulum by at least the last segment; apophyses absent. Basifemora complemented with a long simple seta; telofemora complemented with a short simple seta. These two segments fused, although a line remains visible and they can thus be differentiated. Subcapitulum complemented with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae). Setae hg4 located between hg2–3 instead of in the coxal region. Chelicera with seta present.
Idiosoma, dorsal. Female dorsal idiosoma bears a sclerotized shield that bears 2 pairs of setose sensillae (at and pt) and 2 pairs of simple setae (lps and mps). Idiosomal shield reticulated. 7 pairs of setae, c1–2, d1–h1, present. Cupule im present, usually posteriolaterad of e1. Integument striated.
Idiosoma, ventral. Coxae I and II fused, as are coxae III and IV. 6 pairs of setae present between and posterior to the coxae. Genital plates each bear 4 setae; 2 pairs of genital papillae not visible underneath the plates. Integument between plates striated and bears 4 pairs of additional setae. Legs shorter than the body. Leg 4 longest. Famulus on tarsi I normally shaped and set in a deep depression. Tarsi slightly constricted apically, resulting in small tarsal lobes. Basifemora and telofemora of legs I and II partially fused. A trichobothrium on leg tibia IV absent. Ambulacral claws on either side of a 4-rayed empodium present.
Gnathosoma. Pedipalps 5-segmented, extend beyond the subcapitulum by at least the last segment, and end in a strong claw. An apophysis between the genua and tibiotarsi usually present. This apophysis long or short and generally ends in a bulbous, hyaline tip; it can, however, end in a tapering point as in Armascirus. This apophysis approximately equal between males and females or shorter in males. Basifemora and telofemora complemented with spine-like setae; these two segments fused, although a line remains visible and they can thus be differentiated. Subcapitulum complemented with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae) and covered by integumental papillae that are either randomly distributed or form a polygonal, reticulated pattern.
Idiosoma, dorsal. Female dorsal idiosoma has at least one sclerotized plate that bears 2 pairs of setose sensillae (at and pt) and 2 pairs of simple setae (lps and mps). 0–4 other major plates and platelets present. All plates, if present, covered by integumental papillae that form a reticulated pattern. Integument between plates striated. 7 pairs of setae (c1–2, d1–h1) present. Each seta, when not on a major plate or platelet, surrounded by a minute platelet only slightly larger than the setal socket. Cupule im present, usually laterad or in the proximity of e1. Dorsal idiosoma of males similar except a single large plate complemented with c1–2, d1–e1 present.
Idiosoma, ventral. Coxae I and II often fused; coxae III and IV often fused. Setal formula for coxae I–IV 3-3-3-3 (including paracoxal seta). Genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. Anal plates bear 1 pair of setae (ps1). 2 pairs of setae (ps2 and h2) associated with, but do not occur on, anal plates. Cupule ih present in close proximity to h2. Integument between plates striated and bears 5–7 pairs of additional setae. Ventral idiosoma of males similar except the coxae much more extensive. A sclerotized aedeagus often visible in association with the genital plates. Legs comparatively short, generally not exceeding ¾ the length of the body. Famulus on tarsi I enlarged and ends in a tri-tipped prong. Tarsi constricted apically, resulting in large tarsal lobes. Trichobothrium on leg tibia IV present. Ambulacral claws occur on either side of a 4-rayed empodium.
(modified from
| 1 | Pedipalpal tibiotarsi and genua with adjoining apophyses present (Figs 61a–i) | 2 |
| – | Pedipalpal tibiotarsi and genua with adjoining apophyses absent (Figs 62a–d) | 21 |
| 2 (1) | Dorsal hysterosomal lateral platelets present (Figs 63a–d) | 3 |
| – | Dorsal hysterosomal lateral platelets absent (Figs 64a–f) | 15 |
| 3 (2) | Pedipalp telofemora with one or two apophyses (Figs 65a–c) | 4 |
| – | Pedipalp telofemora without an apophysis; distribution unknown | Dactyloscirus poppi Smiley, 1992 |
| 4 (3) | Pedipalpal telofemora with 1 apophysis (Figs 65a, b) | 5 |
| – | Pedipalpal telofemora with 2 apophyses: 1 basal, flattened and disc-shaped, 1 apical, short, thick and bulbous (Fig. 65c); South Africa | Dactyloscirus condylus Den Heyer, 1979 |
| 5 (4) | Lateral platelets inconspicuous, length less than 2 times the length of c1 or c2; cosmopolitan (Fig. 63a) | Dactyloscirus inermis (Trägårdh, 1905) |
| – | Lateral platelets large, length greater than 2 times the length of c1 or c2 (Figs 63b–d) | 6 |
| 6 (5) | Dorsal setae f1 and h1 equal in length; median shield present (Figs 63b, c) or absent (Fig. 63d) | 7 |
| – | Dorsal setae f1 shorter than h1; median shield absent (Fig. 63d) | 11 |
| 7 (6) | Apophysis adjoining pedipalpal genua and telofemora shorter than length of genu, blunt distally (Fig. 61a); median shield absent (Fig. 63d) | 8 |
| – | Apophysis adjoining pedipalpal genua and telofemora as long or longer than length of genu, blunt or pointed distally (Fig. 61c); median shield present or absent(Figs 63b, c) | 10 |
| 8 (7) | Median shield present | 9 |
| – | Median shield absent; Japan | Dactyloscirus mesonotus Shiba, 1986 |
| 9 (8) | Coxa IV with 2 sts; Pakistan | Dactyloscirus manzoori Bashir & Afzal, 2006 |
| – | Coxa IV with 3 sts; South Africa | Dactyloscirus dolichosetosus Den Heyer, 1979 |
| 10 (7) | Apophysis adjoining pedipalpal genua and telofemora pointed distally (Fig. 61b); pedipalp tibiotarsi with 4 sts; median shield complimented with setae c1, d1; e1 on small platelets (Fig. 63b); leg basifemora with 5-5-3-1 sts; Luzon I., Philippines | Dactyloscirus philippinensis Corpuz-Raros, 1995 |
| – | Apophysis adjoining pedipalpal genua and telofemora blunted distally (Fig. 61c); setae c1–e1 on median shield (Fig. 63c); pedipalp tibiotarsi with 5 sts; leg basifemora with 5-5-3-2 sts; Ozark Mountains, USA | Dactyloscirus pseudophilippinensis Skvarla & Dowling, 2012 |
| 11 (6) | Apophysis adjoining pedipalpal genua and telofemora inconspicuous: circular, minute and hyaline (Fig. 61d); Oahu I., Hawaiian Islands | Dactyloscirus hoffmannae Swift, 1996 |
| – | Apophysis adjoining pedipalpal genua and telofemora conspicuous, blunt apically (Fig. 61e) | 12 |
| 12 (11) | Coxa IV with 2 sts | 13 |
| – | Coxae IV with 3 sts | 14 |
| 13 (12) | Tibiae I with 1 asl, 4 sts; tibiae III with 1 asl, 5 sts | Dactyloscirus kahrorensis Bashir, Afzal & Akbar, 2006 |
| – | Tibiae I with 2 asl, 4 sts; tibiae III with 2 asl, 4 sts | Dactyloscirus imbecillus Bashir & Afzal, 2006 |
| 14 (12) | Genital setae g3 longest, 1.5–1.7 times the length of g2 and g4, more than 2 times the length of g1; Kauai I., Hawaiian Islands | Dactyloscirus smileyi Swift, 1996 |
| – | Genital setae g4 longest, 2 times the length of g1–3; Shanghai, China | Dactyloscirus humuli Liang, 1986 |
| 15 (2) | Dorsal hysterosomal median shield present (Figs 64a–e) | 16 |
| – | Dorsal hysterosomal median shield absent (Fig. 64f) | 18 |
| 16 (15) | Median shield complemented with c1, d1 (Fig. 64b); apophysis adjacent to pedipalpal genua and tibiotarsi blunt distally (Fig. 61c); Mexico, Philippines | Dactyloscirus mansoni Smiley, 1992 |
| – | Median shield complemented with c1–e1 (Figs 64c, d); apophysis adjacent to pedipalpal genua and tibiotarsi blunt or pointed distally | 18 |
| – | Median shield complemented with c1–e1, c2 (Fig. 64e); apophysis adjacent to pedipalpal genua and tibiotarsi pointed distally | Dactyloscirus illutus Inayatullah & Shahid, 1996 |
| 17 (18) | Apophysis adjacent to pedipalpal genua and tibiotarsi blunt distally (Fig. 61e); median shield triangular and nearly as wide as proterosomal shield (Fig. 64c); Bihar, India | Dactyloscirus pataliputraensis Gupta, 1981 |
| – | Apophysis adjacent to pedipalpal genua and tibiotarsi tapering and pointed distally (Fig. 61f); median shield subrectangular and not as wide as proterosomal shield (Fig. 64d); Mexico | Dactyloscirus johnstoni Smiley, 1992 |
| 18 (17) | Pedipalpal telofemora without apophysis (Fig. 61g); apophysis adjoining pedipalpal genua and telofemora longer than telofemora and tapering to a point; Sumatra, Indonesia | Dactyloscirus machairodus (Oudemans, 1922) |
| – | Pedipalpal telofemora with 1 or 2 apophyses (Figs 65a–d); apophysis adjoining pedipalpal genu and telofemur shorter than telofemora and with a bulbus tip (Fig. 61a, d) | 19 |
| 19 (18) | Pedipalpal telofemora with 1 apical apophysis (Figs 65a, b); apophysis adjoining genua and tibiotarsi larger (Fig. 61a) | 20 |
| – | Pedipalpal telofemora inner surface with 2 apophyses: 1 basal, flattened and disc-shaped, 1 apical, short, thick and bulbous (Fig. 65d); apophysis adjoining genua and tibiotarsi small, inconspicuous (Fig. 61d); Luzon I., Philippines | Dactyloscirus discocondylus Corpuz-Raros, 2008 |
| 20 (19) | Basal pair of adoral setae very long, more than 4 times the distal pair; pedipalp telofemoral apophysis about as long as width of segment (Fig. 65a); genital setae g4 twice as long as g1–g3; Luzon I., Philippines | Dactyloscirus rosarioae Corpuz-Raros, 1995 |
| – | Basal pair of adoral setae not unusually long, subequal to distal pair; pedipalp telofemoral apophysis short, less than width of segment (Fig. 65b); genital setae g4 only slightly longer than g1–g3; Luzon I., Philippines | Dactyloscirus agricolus, Corpuz-Raros, 1995 |
| 21 (1) | Median shield present (Figs 64d, e) | 22 |
| – | Median shield absent (Fig. 64f) | 23 |
| 22 (21) | Median shield complimented with c1–e1 (Fig. 64d); Europe, North and South America | Dactyloscirus eupaloides Berlese, 1916 |
| 23 (21) | Coxa I with 2 sts; Pakistan | Dactyloscirus bengalensis Gupta, 1992 |
| – | Coxa I with 3 sts | 24 |
| 24 (23) | Pedipalp tibiotarsal claw trifid (Fig. 62c, d); coxa II–IV setal formula 3-3-3 sts; Luzon I., Philippines | Dactyloscirus trifidus Corpus-Raros, 2008 |
| – | Pedipalp tibiotarsal claw entire, unbranched (Fig. 62a, b); coxa II–IV setal formula not as above | 25 |
| 25 (24) | Coxal setal formula II–IV 1-3-2 sts; Peshawar, Pakistan | Dactyloscirus orsi Inayatullah & Shahid, 1996 |
| – | Coxal setal formula II–IV 2-3-1 sts; Brazil | Dactyloscirus saopauloensis Den Heyer & Castro, 2012 |
Dactyloscirus key illustrations. 61a–h Pedipalp genu and tibiotarsus with adjoining apophysis present 61i Close up of bifid claw 62a–d Pedipalp genu and tibiotarsus with adjoining apophysis absent 62e Close up of trifid claw.
Dactyloscirus key illustrations. 63a–d Dorsal idiosoma, lateral hysterosomal platelets present 64a–f Dorsal idiosoma, lateral hysterosomal platelet absent 65a Pedipalp telofemur with one apophysis, which is about as long as the width of the telofemur 65b Pedipalp telofemur with one apophysis, which is shorter than the width of the telofemur 65c, d Pedipalp telofemur with two apophyses, one apical and one basal which is flattened and disc-shaped.
Gnathosoma. Pedipalps 5-segmented, extend beyond the subcapitulum by at least the last segment, and end in a strong claw; apophysis absent. Basifemora and telofemora complemented with simple setae; these two segments fused, although a line remains visible and they can thus be differentiated. Subcapitulum complemented with 6 pairs of setae (hg1–4 and 2 pairs of adoral setae). Setae hg3 and hg4 both near the coxal bases of the pedipalps.
Idiosoma, dorsal. Female dorsal idiosoma has a sclerotized plate that bears 2 pairs of setose sensillae (at and pt) and 2 pairs of simple setae (lps and mps). Idiosomal shield covered by integumental papillae that form a reticulated pattern. Hysterosoma lacks a plate and bears 7 pairs of setae (c1–2, d1–h1). Cupule im present, usually laterad or in the proximity of e1.
Idiosoma, ventral. Coxae ill-defined. Coxae I and II fused; coxae III and IV fused. Coxae I–IV setal formula 3-1-3-1 (including paracoxal seta). Genital plates each bear 4 setae. Anal plates bear 1 pair of setae (ps1). 2 pairs of setae (ps2 and h2) associated with, but do not occur on, the anal plates. Cupule ih present in close proximity to h2. Integument between plates striated and bears 5 pairs of additional setae. Legs. Ambulacral claws on either side of a 4-rayed empodium present.
(modified from
| 1 | Five pairs of genital setae | Riscus austroamericanus Den Heyer & Castro, 2008 |
| – | Four pairs of genital setae; tibiae IV with 1 T, 4 sts; tibiae II with {1 asl, 1 sts}, 4 sts | 2 |
| 2 (1) | Pedipalpal genu with 3 sts | 3 |
| – | Pedipalpal genu with 4 sts | Riscus bambusae (Gupta & Ghosh 1980) |
| 3 (2) | Pedipalpal tibiotarsus with 1 spls, 3 sts, 1 dorsoterminal solenidion | Riscus thailandensis Den Heyer, 2006 |
| – | Pedipalpal tibiotarsus with 5 sts, 1 dorsoterminal solenidion (original description states 6 sts present; one of these is assumed to be a solenidion here) | Riscus cynodonae (Gupta & Ghosh, 1980) |
Gnathosoma. Pedipalps 5-segmented and reach beyond the subcapitulum by at most the distal half of the tibiae. An apophysis on the telofemora present. Stout spine-like seta on the genua and tibiotarsi setae present or absent. Tibiotarsi end in a strong claw. Subcapitulum with 6 pairs of setae: 2 pairs of adoral setae and 4 pairs of subcapitular setae (hg1–4). Subcapitulum is reticulated.
Idiosoma, dorsal. Proterosoma bears a shield, complemented with 2 pairs of setose sensillae (at and pt) and 2 pairs of setae (lps and mps). Sensillae at and pt not as densely pilose as in Allocunaxa, Cunaxatricha, and Riscus. Proterosomal shield reticulated. Hysterosomal shield absent in females. Lateral platelets (as in Armascirus and Dactyloscirus) absent. Setae c1–h1, and c2 present. Cupule im present laterad and caudally of e1. Integument not bearing the l shield striated. Striations papillated, not smooth or lobed as in Cunaxa.
Idiosoma, ventral. Coxae I–II may be fused; coxae III–IV may be fused. Coxae II–IV setal formula 1-3-1. Genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. Anal plates bear 1 pair of setae (ps1). 1 pair of setae (h2) associated with, but do not occur on, the anal plates. Cupule ih present in close proximity to h2. Integument between plates striated and bears up to 7 pairs of additional setae. Legs. Tarsi long and slender, and constricted distally but tarsal lobes small and not conspicuous as in Armascirus and Dactyloscirus. A trichobothrium on tibia IV present. Ambulacral claws either side of a 4-rayed empodium present.
Rubroscirus is recognized as a valid genus. As suggested by
| 1 | Basifemora I with 3 sts | 2 |
| – | Basifemora I with 5 sts | Rubroscirus denmarki (Smiley, 1992) |
| 2 (1) | Basifemora III with 1 sts | 3 |
| – | Basifemora III with 2 sts; Pakistan | Rubroscirus reticulatus Bashir, Afzal & Ali, 2006 |
| 3 (2) | Basifemora IV with 1 sts | 4 |
| – | Basifemora IV with 2 sts; Mexico, Central America, USA | Rubroscirus boneti (Baker & Hoffmann, 1948) |
| 4 (3) | Coxae I with 2 sts; Taiwan | Rubroscirus exoterica (Tseng, 1980) |
| – | Coxae I with 3 sts | 5 |
| 5 (4) | Coxae II with 1 sts | 6 |
| – | Coxae II with 2 sts | 16 |
| 6 (5) | Coxae IV with 1 sts | 7 |
| – | Coxae IV with 2 sts | 12 |
| 7 (6) | Genua I with 1 asl, 5 sts; Ukraine | Rubroscirus khaustovi Sergeyenko, 2006 |
| – | Genua I with 2 asl, 4 or 6 sts | 8 |
| – | Genua I with 3 asl, 5 or 6 sts | 10 |
| – | Genua I with 3 asl, 1 bsl, 5 sts; Pakistan | Rubroscirus rasile Chaudhri, 1993 |
| 8 (7) | Genua I with 2 asl, 4 sts; genua IV with 1 asl, 5 sts | 9 |
| – | Genua I with 2 asl, 6 sts; genua IV with 2 asl, 5 sts; USA | Rubroscirus newyorkensis (Smiley, 1992) |
| 9 (8) | Genua II with 1 asl, 5 sts; China | Rubroscirus denheyeri Fan, 1992 |
| – | Genua II with 1 asl, 6 sts; Brazil | Rubroscirus nidorum Ferla & Rocha, 2012 |
| 10 (7) | Genua I with 5 sts; genua II with 1 asl, 5 sts; USA | Rubroscirus lehmanae (Smiley, 1992) |
| – | Genua I with 6 sts; genua II with 2 asl, 5 or 6 sts | 11 |
| 11 (10) | Genua II with 2 asl, 5 sts; Pakistan | Rubroscirus valentis Muhammad, Chaudhri & Akbar, 1989 |
| – | Genua II with 2 asl, 6 sts; Pakistan | Rubroscirus otiosus Muhammad & Chaudhri, 1993 |
| 12 (6) | Genua I with 7 sts; Phillipines | Rubroscirus viscayana Corpuz-Raros & Garcia, 1995 |
| – | Genua I with 2 asl, 5 or 6 sts | 13 |
| – | Genua I with 3 asl, 4 sts; China | Rubroscirus sinensis Fan, 1992 |
| – | Genua I with 4 asl, 5 sts; USA | Rubroscirus floridanus (Smiley, 1992) |
| 13 (12) | Genua I with 2 asl, 5 sts; genua II with 2 asl, 5 sts; genua IV with 1 asl, 5 sts | 14 |
| – | Genua I with 2 asl, 6 sts; genua II with 6 sts; genua IV with 6 sts; Philippines | Rubroscirus venusae Corpuz-Raros & Garcia, 1995 |
| 14 (13) | Genua III with 1 asl, 5 sts; setae c1, c2, d1, e1, f1, and h1 smooth | 15 |
| – | Genua III with 2 asl, 5 sts; setae c1, c2, d1, e1, f1, and h1 spiculate; Costa Rica | Rubroscirus rackae (Smiley, 1992) |
| 15 (14) | Minute thorn-like seta adjacent to median spine-like seta on pedipalp tibiotarsus present; New Zealand | Rubroscirus reevesi (Smiley, 1992) |
| – | Minute thorn-like seta adjacent to median spine-like seta on pedipalp tibiotarsus absent; USA | Rubroscirus metzi Smiley, 1992 |
| 16 (5) | Basifemora I with 1 asl, 5 sts; basifemora II with 1 asl, 5 sts; basifemora III with 1 asl, 5 sts; basifemora IV with 1 asl, 5 sts; South Africa | Rubroscirus africanus Den Heyer, 1979 |
| – | Basifemora I with 2 asl, 5 sts; basifemora II with 1 asl, 5 sts; basifemora III with 1 asl, 5 sts; basifemora IV with 2 asl, 5 sts | 17 |
| – | Basifemora I with 3 asl, 5 sts; basifemora II with 1 asl, 5 sts; basifemora III with 1 asl, 5 sts; basifemora IV with 1 asl, 5 sts; South Africa | Rubroscirus vestus Den Heyer, 1979 |
| – | Basifemora I with 4 asl, 5 sts; basifemora II with 2 asl, 5 sts; basifemora III with 2 asl, 5 sts; basifemora IV with 1 asl, 5 sts; South Africa | Rubroscirus rarus Den Heyer, 1979 |
| 17 (16) | Setae c1, c2, d1, e1, f1, and h1 smooth; India | Rubroscirus myabunderensis (Gupta & Ghosh, 1980) |
| – | Setae c1, c2, d1, e1, f1, and h1 spiculate; Australia, Cominican Republic | Rubroscirus lukoschusi (Smiley, 1992) |
Gnathosoma. Pedipalps 5-segmented and reach beyond the subcapitulum by at most the distal half of the tibiotarsi. Basifemora and telofemora fused but retain a dark line. Tibiotarsi usually complemented with a tubercle and a dorsodistal solenidion. Pedipalps end in a stout claw. Chelicera with seta present or absent. Subcapitulum bears 6 pairs of setae: 2 pairs of adoral setae and 4 pairs of subcapitular setae (hg1–4). Setae hg4 often longest.
Idiosoma, dorsal. Proterosoma covered in a shield which bears 4 pairs of setae: 2 pairs of simple setae (lps and mps) and 2 pairs of setose sensilla (at and pt). Dorsal hysterosoma median plate present or absent; if present this plate separate or fused to the proterosomal shield. Plates and shields smooth or variously covered with papillae that form reticulations. Up to 8 pairs of setae present on the dorsal hysterosoma (c1–f1, c2, f2, h2); if these setae do not occur on larger plates or shields they may be born on small platelets that are barely larger than the setal socket. Cupule im present, usually laterad or in the proximity of e1. Unsclerotized integument striated.
Idiosoma, ventral. Coxae I–II fused and may coalesce medially to form a single sternal plate. Each pair of coxae complemented with 3 pairs of setae; if they form an extensive sternal shield, setae normally born on the unsclerotized integument may be located on the shield. Coxae III–IV fused; they may be restricted to the trochantral bases or extend posteriorly beyond the genital plates. Each pair of coxae complemented with 3 pairs of setae; if the plates are extensive they may bear setae normally born on the unsclerotized integument. The genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. 1–8 pairs of setae present on the integument between coxae III and the genital plates. Anal plates complemented with 2 pairs of setae (ps1-2). Two pairs of setae (h2, pa) located on the integument near the anal plates. Cupule ih present in close proximity to h2. Legs shorter than idiosoma; they are never constricted apically so as to end in lobes. Trichobothrium on leg tibia IV present. Ambulacral claws on either side of a four-rayed empodium present.
(modified from
| 1 | Idiosomal plates well-developed and defined; hysterosomal shield present and fused to proterosomal plate (Fig. 66a, b); females and most males with coxae I–II fused medially into a sternal shield (Fig. 67a); apices of some solenidia, especially on tarsi I, swollen | 2 |
| – | Idiosomal plates poorly developed and sometimes ill-defined; hysterosomal plate absent (Fig. 66c, d); coxae I–II usually not fused medially and restricted to trochantral bases (Fig. 67b, c); solenidia on tarsi I and II usually cylindrical | 3 |
| 2 (1) | Idiosoma with 15 to 19 plates, including 4 pairs of dorsolateral plates (Fig. 66a); 2 dorsal plates; pedipalp tibiotarsal ventral tubercle often bifurcate (Fig. 68a) | Scutascirus |
| – | Idiosomal with no more than 8 plates; dorsolateral plates absent (Fig. 66b); females with only one dorsal plate but males with up to 3 dorsal plates; pedipalp tibiotarsal ventral tubercle not bifurcate, plain (Fig. 68b) | Coleoscirus Berlese, 1916 |
| 3 (1) | Pedipalp tibiotarsus short and nearly cone-like (Fig. 69a); cheliceral trochanters broad; ambulacral claws smooth | Neoscirula Den Heyer, 1977 |
| – | Pedipalp tibiotarsus long and usually narrow and S-shaped (Fig. 69b); cheliceral trochanters narrow; ambulacral claws rippled | 4 |
| 4 (3) | Subcuticular reticulated pattern present on proterosomal, coxal, and genital plates: usually very conspicuous, even proximal leg segments may possess such pattern (Fig. 67c) | Pseudobonzia Smiley, 1975 |
| – | Subcuticular reticulated pattern absent or restricted to the edge of coxae (Fig. 67d) | Neobonzia Smiley, 1992 |
Cunaxoidinae key illustrations. 66a–d Idiosoma, dorsal. Position of setae will vary between species. 67a–d Idiosoma, ventral 66a, 67a Generalized Scutascirus. Presence, position, and extent of lateral plates will vary between species 66b, 67b Generalized Coleoscirus 66c, 67c Generalized Pseudobonzia 66d, 67d Generalized Neobonzia 68a Scutascirus pedipalp tibiotarsus, arrow indicates bifurcate tubercle 68b Coleoscirus pedipalp tibiotarsus, arrow indicates plan tubercle 69a Neoscirula pedipalps with short, cone-like tibiotarsus 69b Pseudobonzia and Neobonzia pedipalps with elongate, s-shaped tibiotarsus.
Gnathosoma. Pedipalps 5-segmented; basifemora and telofemora fused but retain a dark line which indicates the presence of the joint. Pedipalps extend beyond the subcapitulum by at most the apical half of the tibiotarsi. Pedipalp tibiotarsal tubercle plain, not bifurcate as in Scutascirus. Subcapitulum bears 6 pairs of setae: 2 pairs of adoral setae and 4 pairs of subcapitular setae (hg1–4).
Idiosoma, dorsal. Dorsal idiosoma heavily sclerotized and the plates well-demarcated. A single dorsal shield present; it may range in size from terminating anteriorly to cupule im to being holodorsal. No papillated line or other marking indicates the separation of the proterosomal and hysterosomal shields. 2 pairs of setae and 2 pairs of setose sensillae present on the proterosomal. Setae c1–h1, c2, and f2 and cupule im present dorsally. Dorsolateral plates (such as present in Scutascirus) absent.
Idiosoma, ventral. Coxae I–II fused and coalesce medially to form a sternal shield which often has a prominent apex caudally. Sternal plate complemented with 5–7 pairs of setae. Coxae III–IV fused and may extend laterally and caudally past the genital plates. Genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. Anal plates bear two pairs of setae (ps1 and ps2). Seta h2 located ventrally near the anal plates. Cupule ih present in close proximity to h2. Legs shorter than the idiosoma, never constricted apically so as to end in lobes. The apices of solenidia, especially on tarsi I, may be swollen. Trichobothrium on leg tibia IV present. Ambulacral claws on either side of a four-rayed empodium present.
Males similar, except up to three shields or plates may occur on the dorsal idiosoma (that is the proterosomal shield may not be fused to a hysterosomal plate and up to two hysterosomal plates may be present) and coxae I–IV may be fused into a holoventral shield.
Coleoscirus brevicornis (Berlese) has been excluded from the key as the original publication (
Coleoscirus carex, Coleoscirus kifayati, and Coleoscirus mardi have been excluded from the key as the authors did not provide enough information in the original descriptions to include them.
Coleoscirus zaherii is not included in the key as, despite the best efforts of the authors and the University of Arkansas Interlibrary Loan Department, the description could not be obtained.
| 1 | Basifemora I with 4 setae | 2 |
| – | Basifemora I with 5 setae | 4 |
| 2 (1) | Basifemora II-IV setal formula 5-4-2 | 3 |
| – | Basifemora II-IV setal formula 6-4-2; Pakistan | Coleoscirus trudus Bashir, Afzal & Khan, 2006 |
| – | Basifemora II-IV setal formula 6-5-2; Pakistan | Coleoscirus afzali Bashir & Afzal, 2009 |
| 3 (2) | Telofemora I-IV setal formula 4-4-4-3; Pakistan | Coleoscirus baptus (Chaudhri, 1980) |
| – | Telofemora I-IV setal formula 4-5-4-3; Pakistan | Coleoscirus raviensis Bashir, Afzal & Khan, 2008 |
| 4 (1) | Basifemora II with 5 setae | 5 |
| – | Basifemora II with 6 setae | 12 |
| 5 (4) | Basifemora III with 4 setae | 6 |
| – | Basifemora III with 5 setae | 8 |
| 6 (5) | Basifemora IV with 2 setae | 7 |
| – | Basifemora IV with 3 setae; Java, South Africa | Coleoscirus halacaroides Berlese, 1916 |
| 7 (6) | Horizontal reticulations on dorsal shield present (Fig. 70); Taiwan | Coleoscirus horidula (Tseng, 1980) |
| – | Horizontal reticulations on dorsal shield absent; Taiwan | Coleoscirus monospinosus (Tseng, 1980) |
| 8 (5) | Basifemora I-IV setal formula 4-5-3-3; Argentina | Coleoscirus curtipalpus (Berlese, 1888) |
| – | Basifemora I-IV setal formula not as above | 9 |
| 9 (8) | Sternal shield bilobed posteriorly; Philippines | Coleoscirus barrioni Corpuz-Raros, 1996 |
| – | Sternal shield not bilobed posteriorly | 10 |
| 10 (9) | Extensive reticulations on gnathosoma present (Fig. 71); Philippines | Coleoscirus bakeri Corpuz-Raros, 1996 |
| – | Extensive reticulations on gnathosoma absent | 11 |
| 11 (10) | Hysterosomal shield present, complemented with c1-f1, c2, f2; Philippines | Coleoscirus philippinensis Corpuz-Raros, 1996 |
| – | Hysterosomal shield absent; Philippines | Coleoscirus intermedius Corpuz-Raros, 1996 |
| 12 (4) | Basifemora III with 4 setae | 13 |
| – | Basifemora III with 5 setae | 17 |
| – | Basifemora III with 6 setae | 20 |
| 13 (12) | Telofemora I-IV setal formula 4-4-4-3; USA, South Africa, Japan | Coleoscirus simplex (Ewing, 1917) |
| – | Telofemora I-IV setal formula 5-5-4-3 | 14 |
| 14 (13) | Setae f1, f2 born on soft integument | 15 |
| – | Setae f1, f2 born on dorsal shield; Pakistan | Coleoscirus tobaensis Bashir, Afzal & Khan, 2008 |
| 15 (14) | Sternal plate rounded posteriomedially (Figs 72a, b); South Africa | Coleoscirus tuberculatus Den Heyer, 1978 |
| – | Sternal plate truncated posteriomedially (Fig. 72c) | 16 |
| 16(15) | Light reticulation on dorsal shield present; dorsal shield evenly sclerotized (Fig. 73a); South Africa | Coleoscirus buartsus Den Heyer, 1980 |
| – | Light reticulation on dorsal shield absent; dorsal shield unevenly sclerotized (Fig. 73b); South Africa | Coleoscirus coatesi Den Heyer, 1980 |
| 17(12) | Sternal shield indented posteriomedially (Fig. 72a); Malaysia | Coleoscirus mizunoi (Shiba, 1978) |
| – | Sternal shield not indented posteriomedially (Fig. 72b) | 18 |
| 18 (17) | Setae f2 born on soft integument; Pakistan | Coleoscirus disparis Muhammad & Chaudhri, 1992 |
| – | Setae f2 born on dorsal shield | 19 |
| 19 (18) | Integumental dots on legs I-IV forming rows (Fig. 74a); Pakistan | Coleoscirus carnus Muhammad & Chaudhri, 1992 |
| – | Integumental dots on legs I-IV forming random (Fig. 74b); South Africa | Coleoscirus breslauensis Den Heyer, 1980 |
| 20 (12) | Basifemora IV with 2 setae; Philippines | Coleoscirus leytensis Corpuz-Raros, 1996 |
| – | Basifemora IV with 3 setae; Philippines | Coleoscirus dayamilocus Corpuz-Raros, 1996 |
Coleoscirus key illustrations. 70 Dorsal idiosomal shield with horizontal reticulations present 71 Gnathosoma with extensive reticulations present 72a Sternal plate rounded posteriomedially, indentation absent 72b Sternal plate rounded posteriomedially, indentation present 72c Sternal plate truncated posteriomedially 73a Dorsal idiosomal shield even sclerotized, light reticulation present 73b Dorsal idiosomal shield unevenly sclerotized, light reticulation absent 74a Integumental dots on legs forming rows 74b Integumental dots on legs random.
Gnathosoma. Pedipalps 5-segmented and reach beyond the subcapitulum by at most the distal half of the last segment. Simple setae present on the basi- and telofemora. Pedipalp tibiotarsi long and S-shaped (as opposed to short and cylindrical as in Neoscirula). Subcapitulum with 4 pairs of setae (hg1–4). 2 pairs of adoral setae present. Chelicera with seta usually present. Extensive reticulated pattern absent from the gnathosoma, though a row of single cells may be present caudally.
Idiosoma, dorsal. Plates lightly sclerotized and may not be well defined or demarcated. Proterosomal plate bears 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Extensive reticulated pattern absent, although a pair of rows of up to 6 cells may be present. Proterosomal plate may be covered with random dots or papillae. Hysterosomal plate absent. Setae c1–h1, and usually c2 and f2 present dorsally; h2 present or absent. Cupules im present laterad and sometimes caudally of e1. Integument striated.
Idiosoma, ventral. Coxae usually restricted to the trochantral bases, though sometimes coxae I–II may nearly touch medially. Coxae I–II fused. Coxae III–IV fused. All coxae lightly sclerotized and may be ill-defined. Extensive reticulated pattern absent from the coxae, though a row of cells or reticulated pattern may be present near the edges. Coxae may be covered with random dots or papillae. Coxae I–IV usually have the simple setal formula 3-3-3-3 (Neobonzia parilis is the exception with 2-2-3-2). Genital plates each bear 3–4 setae; 2 pairs of genital papillae visible underneath the plates. 2 pairs of setae (ps1–2) usually occur on the anal plates and 1 pair of setae (pa) occurs on the integument near the anal plates. However, at least one species (Neobonzia clavata) has 3 pairs of setae present on the anal plates and 0 pairs of setae on the integument. Cupules ih present ventrally near the anal plates. Legs. Tarsi never constricted apically so as to end in lobes. The apices of solenidia cylindrical, not swollen as in Coleoscirus and Scutascirus. Trichobothrium on leg tibia IV present. Ambulacral claws rippled and occur on either side of a 4-rayed empodium.
As suggested by
Neobonzia parvirostris (Berlese, 1910) is known only from the male and so is not included in the key. Neobonzia breviscuta (Luxton, 1982) is not included in the key as an insufficient number of characters are given in the original description.
| 1 | Sensilla at and pt clavate (Figs 75a, b) | 2 |
| – | Sensilla at and pt not clavate, normal (Fig. 75c) | 3 |
| 2 (1) | Sensilla at and pt short, length less than width of proterosomal plate (Fig. 75a); Philippines | Neobonzia clavata (Corpuz-Raros, 2008) |
| – | Sensilla at and pt long, length greater than width of proterosomal plate (Fig. 75b); Brazil | Neobonzia clava (Den Heyer & Castro, 2008) |
| 3 (1) | Coxae I–IV setal formula 2-2-3-2 sts; Pakistan | Neobonzia parilis (Chaudhri, 1977) |
| – | Coxae I–IV setal formula 3-3-3-3 sts | 4 |
| 4 (3) | Basifemora I with 2 sts | 5 |
| – | Basifemora I with 3 sts; Philippines | Neobonzia longispina (Corpuz-Raros & Garcia, 1996) |
| – | Basifemora I with 4 sts | 7 |
| – | Basifemora I with 5 sts | 12 |
| 5 (4) | Basifemora II–IV setal formula 2-2-1 sts; USA | Neobonzia moseri Smiley, 1992 |
| – | Basifemora II–IV setal formula 2-1-0 sts; Pakistan | Neobonzia malookensis (Bashir & Afzal, 2009) |
| – | Basifemora II–IV setal formula 3-3-1 sts | 6 |
| – | Basifemora II–IV setal formula 4-4-1 sts | Neobonzia bakari (Bashir & Afzal, 2009) |
| 6 (5) | Telofemora I–IV setal formula 4-6-4-2 sts; China | Neobonzia themedae (Den Heyer, 1977) |
| – | Telofemora I–IV setal formula 5-5-4-3 sts; South Africa | Neobonzia lootsi (Den Heyer, 1977) |
| 7 (4) | Basifemora II with 4 sts | 8 |
| – | Basifemora II with 5 sts | Pseudobonzia shamshadi (Bashir & Afzal, 2009) |
| – | Basifemora II with 6 sts | 10 |
| 8 (7) | Basifemora III–IV setal formula 4-2 sts | 9 |
| – | Basifemora III–IV setal formula 6-1 sts; New Zealand | Neobonzia newzealandicus (Smiley, 1992) |
| 9 (8) | Pedipalp tibiotarsal tubercle present; Brazil | Neobonzia moraesi (Den Heyer & Castro, 2008) |
| – | Pedipalp tibiotarsal tubercle absent; South Africa | Neobonzia saaymani (Den Heyer, 1977) |
| 10 (7) | Basifemora III–IV setal formula 3-0 sts; South Africa | Neobonzia nona (Den Heyer, 1977) |
| – | Basifemora III–IV setal formula 3-1 sts; South Africa | Neobonzia argillae (Den Heyer, 1977) |
| – | Basifemora III–IV setal formula 4-2 sts | 11 |
| 11 (10) | Setae lps and mps subequal; South Africa | Neobonzia smileyi (Den Heyer, 1980) |
| – | Setae lps about half as long as mps; USA | Neobonzia summersi (Smiley, 1992) |
| 12 (4) | Basifemora II with 5 sts | 13 |
| – | Basifemora II with 6 sts | 14 |
| 13 (12) | Coxae I–II nearly touching medially (Fig. 76a); USA, Austria | Neobonzia snowi (Baker & Hoffmann, 1948) |
| – | Coxae I–II widely separated medially (Fig. 76b); Philippines | Neobonzia gruezoi (Corpuz-Raros & Garcia, 1996) |
| 14 (12) | Basifemora III–IV with 5-2 sts | 15 |
| – | Basifemora III–IV with 6-2 sts; Pakistan | Neobonzia numida (Chaudhri, 1980) |
| 15 (14) | Setae g4 longest; posterior corners of proterosomal shield angled; China | Neobonzia shanghaiensis (Liang, 1980) |
| – | Setae g3 longest; posterior corners of proterosomal shield rounded; Russia | Neobonzia kuznetzovi (Sergeyenko, 2005) |
Neobonzia key illustrations 75a Sensilla at and pt clavate, short, length less than the width of the proterosomal shield 75b Sensilla at and pt clavate, long, length greater than the width of the proterosomal shield 75c Sensilla at and pt normal, not clavate 76a Coxae I–II nearly touching medially 76b Coxae I–II widely separated medially.
Gnathosoma. Pedipalps 5-segmented and end in a strong claw, which is complemented with a tooth in some species; they extend to the tip of the hypognathum or slightly beyond. Basifemur and telofemur are fused but retain the suture; each has a dorsolateral simple or spine-like seta. Pedipalp tibiotarsus short and cone-like. Subcapitulum with 4 pairs of setae (hg1–4). Seta hg1 longest and in some species bent at 90 degrees, though not geniculate as in Bonziinae. Adoral setae present or absent. Chelicera with seta present or absent.
Idiosoma, dorsal. Proterosomal shield weakly sclerotized and ill-defined, granulated or papillated; some species possess subcuticular reticulations.
Idiosoma, ventral. Coxae I–II separate or fused medially into a single sternal shield. Coxae III–IV contiguous on either side, restricted to area around trochantral bases. Dorsal cupules im present laterad to e1; ventral cupules ih present near h2, anal plates. Legs shorter than body. Tarsi never constricted apically so as to end in lobes. Apices of solenidia cylindrical, not swollen as in Coleoscirus and Scutascirus. Trichobothrium on leg tibia IV present. Ambulacral claws smooth and occur on either side of a 4-rayed empodium.
Neoscirula hoffmannae Mejía-Recamier & Palacios-Vargas, 2007 is excluded from the following key as it is only known from the male.
| 1 | Coxae I–II fused to form a sternal shield | 2 |
| – | Coxae I–II separated | 6 |
| 2 (1) | Cheliceral seta present | 3 |
| – | Cheliceral seta absent | 5 |
| 3 (2) | Pedipalp basifemoral dorsal seta spine-like (Fig. 77a); Luzon Is., Philippines | Neoscirula makilingica Corpuz-Raros, 1996 |
| – | Pedipalp basifemoral dorsal seta simple (Fig. 77b) | 4 |
| 4 (3) | Proterosomal shield with polygonal subcuticular sculpturing present (Fig. 78a); posteromedial portion of sternal shield V-shaped, polygonal subcuticular sculpturing absent (Fig. 79a); 6 pairs of setae between coxae III–IV (excluding genital setae); Luzon Is., Philippines | Neoscirula aspirasi Corpuz-Raros, 1996 |
| – | Proterosomal shield with polygonal subcuticular sculpturing absent (Fig. 78b); posteromedial portion of sternal shield rounded, polygonal subcuticular sculpturing present (Fig. 79b); 4 pairs of setae between coxae III–IV (excluding genital setae); Malaysia; Philippines | Neoscirula ogawai (Shiba, 1978) |
| 5 (2) | Chelicerae with dorsomedial reticulations present (Fig. 80a); genua II with 5 setae and 2 solenidia; genua IV with 5 setae and 1 solenidion; Interior Highlands, USA | Neoscirula reticulata Skvarla, 2011 |
| – | Chelicerae dorsomedial reticulations absent (Fig. 80b); genua II with 4 setae and 2 solenidia; genua IV with 4 setae and 1 solenidion; Jalisco, Mexico | Neoscirula baloghi Mejía-Recamier & Palacios-Vargas, 2007 |
| 6 (1) | Pedipalp genua hook-like apophysis present (Fig. 81a); South Africa | Neoscirula natalensis Den Heyer, 1977 |
| – | Pedipalp genua hook-like apophysis absent (Fig. 81b) | 7 |
| 7 (6) | Pedipalp tibiotarsal claw a tooth present, giving bifid appearance (Fig. 82a) | 8 |
| – | Pedipalp tibiotarsal claw a tooth absent (Fig. 82b) | 13 |
| 8 (7) | Cheliceral seta present; pedipalp tibiotarsal tubercle present (Fig. 83a) | 9 |
| – | Cheliceral seta absent; pedipalp tibiotarsal tubercle absent (Fig. 83b); São Paulo, Brazil | Neoscirula oliveirai Den Heyer & Castro, 2008 |
| 9 (8) | Basifemora II with 4 setae; telofemora I–II 4-4 setae; hypognathum with ventroapical shield-like process present (Fig. 84a); New Zealand; Philippines | Neoscirula luxtoni Smiley, 1992 |
| – | Basifemora II with 5 or 6 setae; telofemora I–II 5-5 setae; hypognathum with ventroapical shield-like process absent (Fig. 84b) | 10 |
| 10 (9) | Basifemora II with 5 setae | 11 |
| – | Basifemora II with 6 setae | 12 |
| 11 (10) | Basifemora I with 4 setae; telofemora III with 4 setae; 7 pairs of setae between coxae III–IV (excluding genital setae); Jalisco, Mexico | Neoscirula aliciae Mejía-Recamier & Palacios-Vargas, 2007 |
| – | Basifemora I with 5 setae; telofemora III with 3 setae; 5 pairs of setae between coxae III–IV (excluding genital setae); Luzon Is., Philippines | Neoscirula laboensis Corpuz-Raros, 2007 |
| 12 (10) | Chelicerae tapering gradually (Fig. 85a); Fujian, China | Neoscirula bidens Lin & Zhang, 1988 |
| – | Chelicerae tapering suddenly (Fig. 85b); São Paulo, Brazil | Neoscirula flechtmanni Den Heyer & Castro, 2008 |
| 13 (7) | Pedipalp basifemoral dorsal seta spine-like (Fig. 77a) | 14 |
| – | Pedipalp basifemoral dorsal seta simple (Fig. 77b) | 18 |
| 14 (13) | Telofemora I–II with 4-4 setae; New Zealand | Neoscirula proctorae Smiley, 1992 |
| – | Telofemora I–II with 5-5 setae | 15 |
| 15 (14) | Proterosomal shield with polygonal subcuticular sculpturing present (Fig. 86a); Fujian, China | Neoscirula saitoi |
| – | Proterosomal shield with polygonal subcuticular sculpturing absent (Fig. 86b) | 16 |
| 16 (15) | Cheliceral seta short, less than half the length of movable digit; South Africa | Neoscirula sevidi Den Heyer, 1977 |
| – | Cheliceral seta long, nearly as long or longer than movable digit | 17 |
| 17 (16) | Basifemora I–IV setal formula 5-5-4-3; Iran | Neoscirula sepasgosariani Den Heyer, 2011 |
| – | Basifemora I–IV setal formula 4-4-3-1; Brazil | Neoscirula queirozi Den Heyer & Castro, 2008 |
| 18 (13) | Coxae I–II with polygonal subcuticular sculpturing present (as in Fig. 79a) | 19 |
| – | Coxae I–II with polygonal subcuticular sculpturing absent (as in Fig. 79b) | 23 |
| 19 (18) | Proterosomal shield with polygonal subcuticular sculpturing present (Fig. 78a) | 20 |
| – | Proterosomal shield with polygonal subcuticular sculpturing absent (Fig. 78b) | 21 |
| 20 (19) | Basifemora II with 4 setae; telofemora I–II 4-4 setae; Maryland, USA | Neoscirula kenworthyi Smiley, 1992 |
| – | Basifemora II with 5 setae; telofemora I–II with 5-5 setae; Leyte Is., Philippines | Neoscirula taclobanensis Corpuz-Raros, 2007 |
| 21 (19) | Hypognathal setae hg1 more than two times as long as setae hg2–4; coxae II with 4 setae; Fujian, China | Neoscirula miaofengensis Lin & Zhang, 1988 |
| – | Hypognathal setae hg1 no more than two times as long as setae hg2–4; coxae II with 3 setae | 22 |
| 22 (21) | Chelicerae basally narrow, less than three times the width of the distal end; hypognathum narrow, nearly twice as long as wide; Uzbekistan | Neoscirula vitulus Barilo, 1991 |
| – | Chelicerae basally broad, four times the width of the distal end; hypognathum wide, nearly as wide as long; South Africa | Neoscirula delareyi Den Heyer, 1980 |
| 23 (18) | Proterosomal shield with polygonal subcuticular sculpturing present | 24 |
| – | Proterosomal shield with polygonal subcuticular sculpturing absent; Luzon Is., Philippines | Neoscirula imperata Corpuz-Raros, 1996 |
| 24 (23) | Subcapitulum with row of basal polygonal subcuticular sculpturing present (Fig. 87a); ventrally with 7 pairs of simple setae between coxae III–IV | 25 |
| – | Subcapitulum with row of basal polygonal subcuticular sculpturing absent (Fig. 87b); ventrally with 6 pairs of simple setae between coxae III–IV; Luzon Is., Philippines | Neoscirula abraensis Corpuz-Raros, 1996 |
| 25 (24) | Basifemora II with 4 setae; telofemora I–II with 4-4 setae; Western Transvaal, South Africa | Neoscirula theroni Den Heyer, 1977 |
| – | Basifemora II with 5 setae; telofemora I–II with 5-5 setae; Luzon Is., Philippines | Neoscirula puntiglupa Corpuz-Raros, 1996 |
Neoscirula key illustrations 77a Pedipalp basifemoral dorsal seta spine-like 77b Pedipalp basifemoral dorsal seta simple 78a Proterosomal shield with polygonal subcuticular sculpturing present 78b Proterosomal shield with polygonal subcuticular sculpturing absent 79a Sternal shield v-shaped posteriomedially, with polygonal subcuticular sculpturing absent 79b Sternal shield rounded posteriomedially, with polygonal subcuticular sculpturing present 80a Chelicera with dorsomedial reticulations present 80b Chelicera with dorsomedial reticulations absent 81a Pedipalp genua with hook-like apophysis present 81b Pedipalp genua with hook-like apophysis absent 82a Pedipalp tibiotarsal claw with tooth present 82b Pedipalp tibiotarsal claw with tooth absent 83a Pedipalp tibiotarsus with tubercle present 83b Pedipalp tibiotarsus with tubercle absent 84a Hypognathum with ventroapical shield-like process present 84b Hypognathum with ventroapical shield-like process absent 85a Chelicera tapering gradually 85b Chelicera tapering suddenly 86a Proterosomal shield with polygonal subcuticular sculpturing present 86b Proterosomal shield with polygonal subcuticular sculpturing absent 87a Subcapitulum with row of basal subcuticular sculpturing present 87b Subcapitulum with row of basal subcuticular sculpturing absent.
Gnathosoma. Pedipalps 5-segmented and reach beyond the subcapitulum by at most the distal half of the last segment. Simple or spine-like setae on the basi- and telofemora present. Pedipalp tibiotarsi long and S-shaped (as opposed to short and cylindrical as in Neoscirula). Subcapitulum with 4 pairs of setae (hg1–4). 2 pairs of adoral setae present. Chelicera with seta present (usually) or absent. Extensive reticulated pattern present on the gnathosoma.
Idiosoma, dorsal. Plates lightly sclerotized and not be well defined or demarcated. The proterosomal plate bears 2 pairs of setae (lps and mps) and 2 pairs of setose sensillae (at and pt). Extensive reticulated pattern present. Hysterosomal plate absent. Setae c1–h1 present; setae c2, f2, and h2 present or absent. Cupules im present laterad and caudally of e1. Integument striated.
Idiosoma, ventral. Coxae restricted to the trochantral bases. Coxae I–II fused. Coxae III–IV fused. All coxae lightly sclerotized and may be ill-defined. Coxae with extensive reticulated pattern. Coxae I–IV usually have setal formula 3-3-3-3. Genital plates each bear 3–4 setae; 2 pairs of genital papillae visible underneath the plates. 2 pairs of setae (ps1–2) occur on the anal plates and 1 pair of setae (pa) occurs on the integument near the anal plates. Cupules ih present ventrally near the anal plates. Legs. Basal leg podomeres with reticulated pattern present or absent. Tarsi never constricted apically so as to end in lobes. Apices of solenidia cylindrical, not swollen as in Coleoscirus and Scutascirus. Trichobothrium on leg tibia IV present. Ambulacral claws are rippled and occur on either side of a 4-rayed empodium.
(modified from Den Heyer and
| 1 | Pedipalp basifemora and telofemora with similar setae, either spine-like or simple (Fig. 88a, b); proterosomal shield conspicuously reticulated | 2 |
| – | Pedipalp basifemora with simple seta, pedipalp telofemora with spine-like seta (Fig. 88c); proterosomal shield not conspicuously reticulated; Mexico | Pseudobonzia delfinadobakerae Smiley, 1992 |
| 2 (1) | Pedipalp basifemora and telofemora with simple setae (Fig. 88a); setae f2 present or absent | 3 |
| – | Pedipalp basifemora and telofemora with spine-like setae (Fig. 88b); setae f2 present; Guam | Pseudobonzia yini Smiley, 1992 |
| 3 (2) | Setae f2 present | 4 |
| – | Setae f2 absent | 5 |
| 4 (3) | Proterosomal shield concave posteromedially (Fig. 89a); South Africa | Pseudobonzia neoreticulata Den Heyer, 1977 |
| – | Proterosomal shield straight posteromedially (Fig. 89b); USA | Pseudobonzia landwehri Smiley, 1992 |
| – | Proterosomal shield convex posteromedially (Fig. 89c); Pakistan | Pseudobonzia ashfaqi Bashir, Afzal & Akbar, 2008 |
| 5 (3) | Proximal leg podomeres reticulated; Malaysia | Pseudobonzia clathratus (Shiba, 1978) |
| – | Proximal leg podomeres not reticulated; USA | Pseudobonzia reticulata (Heryford, 1965) |
Pseudobonzia key illustrations 88a Pedipalp basifemur and telofemur with spine-like setae on both segments 88b Pedipalp basifemur and telofemur with simple setae on both segments 88c Pedipalp with simple seta on basifemur, spine-like seta on telofemur 89a Proterosomal plate convex posteriomedially 89b Proterosomal plate not convex posteriomedially.
Gnathosoma. Pedipalps 5-segmented and reach beyond the subcapitulum by at most the distal half of the tibiotarsi. Basifemora and telofemora fused but retain a dark line. The tibiotarsi complemented with a tubercle and a dorsodistal solenidion. Pedipalps end in a stout claw. Chelicera with seta present or absent. Subcapitulum bears 6 pairs of setae: 2 pairs of adoral setae and 4 pairs of subcapitular setae (hg1–4). Setae hg4 often the longest.
Idiosoma, dorsal. Proterosoma covered in a shield which bears 4 pairs of setae: 2 pairs of simple setae (lps and mps) and 2 pairs of setose sensilla (at and pt). Dorsal hysterosoma bears a median plate which is fused with the proterosomal shield and four pairs of lateral platelets. Plates and shields covered with papillae that form reticulations. 8 pairs of setae present on the dorsal hysterosoma (c1–f1, c2, f2, h2); these setae occur on the fused dorsal shield. Cupule im present, usually laterad or in the proximity of e1. Unsclerotized integument striated.
Idiosoma, ventral. Coxae I–II fused and coalesce medially to form a single sternal plate. Each pair of coxae complemented with 3 pairs of setae; if they form an extensive sternal shield setae normally born on the unsclerotized integument may be located on the shield. Coxae III–IV fused and extend posteriorly beyond the genital plates. Genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. 1–8 pairs of setae present on the integument between coxae III and the genital plates. Anal plates complemented with 2 pairs of setae (ps1-2). Two pairs of setae (h2, pa) located on the integument near the anal plates. Cupule ih present in close proximity to h2. Legs shorter than idiosoma. Tarsi never constricted apically so as to end in lobes. Trichobothrium on leg tibia IV present. Ambulacral claws on either side of a four-rayed empodium present.
Scutascirus tactus is not included in the following key as it is described only from the male.
| 1 | Tubercle on inner margin of pedipalp tibiotarsus not branched (Fig. 90a) | 2 |
| – | Tubercle on inner margin of pedipalp tibiotarsus bifurcate (Figs 90b, c) | 5 |
| – | Tubercle on inner margin of pedipalp tibiotarsus trifurcate (Fig. 90d); China | Scutascirus triangulum Lin, Zhang & Ji, 2001 |
| 2 (1) | Telofemora III-IV setal formula 4-3 | 5 |
| – | Telofemora III-IV setal formula 5-2; Philippines | Scutascirus contiguus Corpuz-Raros & Garcia, 1996 |
| 3 (2) | Genua II with 1 asl, 5 sts; dorsum with lateral scutella absent; Pakistan | Scutascirus pigrus Chaudhri, 1980 |
| – | Genua II with 2 asl, 1 bsl, 5 sts; dorsum with lateral scutella present; Malaysia | Scutascirus exasperatus (Shiba, 1978) |
| 4 (1) | Basifemora I–IV setal formula 4-6-4-2; Telofemora I–IV setal formula 5-5-4-3; 4 pairs of dorsolateral hysterosomal plates present (Fig. 91a) | 5 |
| – | Basifemora I–IV setal formula 5-5-4-3; Telofemora I–IV setal formula 5-5-5-2; 5 pairs of dorsolateral hysterosomal plates present (Fig. 91b); Luzon Is., Philippines | Scutascirus pentascutellus Corpuz-Raros & Garcia, 1996 |
| 5 (4) | Pedipalp with entire tibiotarsus projecting past entomalae; bifurcate tubercle positioned halfway along the length of the tibiotarsus (Fig. 90b); Brazil | Scutascirus braziliensis Den Heyer, 1978 |
| – | Pedipalp with distal 2/3 of tibiotarsus projecting past entomalae; bifurcate tubercle positioned on distal third of tibiotarsus (Fig. 90c); South Africa | Scutascirus polyscutosus Den Heyer, 1976 |
Scutascirus key illustrations. 90a (after
Gnathosoma. Pedipalps 5-segmented and reach beyond the subcapitulum by at most the distal half of the tibiotarsi. Basifemoral seta simple or spine-like. Telofemoral seta spine-like. Pedipalps end in a stout claw. Subcapitulum bears 6 pairs of setae: 2 pairs of adoral setae and 4 pairs of subcapitular setae (hg1–4). Setae hg1 long and bent.
Idiosoma, dorsal. Proterosoma covered in a shield which bears 4 pairs of setae: 2 pairs of simple setae (lps and mps) and 2 pairs of setose sensilla (at and pt). Dorsal hysterosoma median plate present, fused to proterosomal shield; 1 to 5 pairs of dorsolateral plates present. Plates and shields smooth or reticulated. Seven pairs of setae present on the dorsal hysterosoma (c1–f1, c2, h2). Unsclerotized integument striated.
Idiosoma, ventral. Coxae I–II fused, coxae III–IV fused; coxae may coalesce medially for form a sternal shield. Each pair of coxae complemented with 3 pairs of setae. The genital plates each bear 4 setae; 2 pairs of genital papillae visible underneath the plates. 4–9 pairs of setae present on the integument between coxae II and the genital plates. Anal plates complemented with 2 pairs of setae (ps1-2). Two pairs of setae (h2, pa) located on the integument near the anal plates. Cupule ih present in close proximity to h2. Legs shorter than idiosoma; they are never constricted apically so as to end in lobes. Trichobothrium on leg tibia IV present. Ambulacral claws on either side of a four-rayed empodium present.
(in part modified from
| 1 | Pedipalpal basifemora seta simple | Orangescirula filipina |
| – | Pedipalpal basifemora seta spine-like | 2 |
| 2 (1) | Dorsal shields with large subcuticular reticulations; 2 pairs of dorsolateral plates present | Orangescirula yongchuanensis |
| – | Dorsal shield with extremely small subcuticular reticulations; 5 pairs of dorsolateral plates present | Orangescirula kethleyi |
The specimens examined represent the first report of Scirula papillata from the Western Hemisphere. The specimens examined correspond to
Material examined(2 individuals on slides). 1 female adult (APGD 10-0424-008, #135719), ex deciduous leaf litter, USA, Arkansas, Washington Co, Devil’s Den State Park (35°46.817N, 94°14.750W), 24 April 2010, col. M. J. Skvarla ● 1 female adult (APGD 10-0826-003, # 135720), ex thick moss by creek near deciduous litter (maple, oak), USA, Pennsylvania, Somerset Co, Laurel Hill State Park, 1985’ elevation (40°00.963 N, 79°14.233 W), 26 August 2010, col. M. J. Skvarla.
The specimens examined expand the range of this species within the Interior Highlands and are a new state record for Missouri.
(2 individuals on slides). 1 adult female (APGD 11-1129-002), ex litter, USA, Arkansas, Bradley/Drew Co, Warren Prairie Natural Area, 21 June 2010, col. L. C. Thompson ● 1 adult female (APGD 10-0523-004), ex litter, USA, Missouri, Taney Co (36°41'11.98"N, 92°58'16.44"W), 23 May 2010, col. J. R. Fisher, D. M. Keeler.
The specimens examined significantly expand the range of this species within the United States. The Ouachita specimens correspond to
(3 individuals on slides). 1 adult female (APGD 13-0304-041, #131238), ex. Malaise trap in marsh, USA, Fairfax Co, George Washington Memorial Parkway, Dyke Marsh Wildlife Preserve, 11 April 2009, col. E. M. Barrows ● 2 adult females (APGD 12-0706-002, #135716), ex very dry oak.pine litter in small, rocky depression, USA, Arkansas, Polk Co, Ouachita National Forest, Black Fork Mountain Wilderness, Black Fork Trail (34°41.312'N, 94°18.691'W), 6 July 2012, col. M. J. Skvarla.
The specimens examined significantly expand the range of this species within the United States.
(3 individuals on slides). 2 adult females (APGD 12-1020-012, #135721), ex. deciduous litter (maple, sweet gum, poison ivy) in disturbed area, USA, Virginia, Fairfax Co, George Washington Memorial Parkway, Dyke Marsh Wildlife Preserve (38°46'25"N, 77°03'06"W), 22 October 2012, col. A. P. G. Dowling ● 1 adult female (JRF 12-1028-010, #135722), ex. dry mixed litter with little tree cover in recently (~5 years) cut pine stand with shrubby oaks, USA, Arkansas, Montgomery Co, Ouachita National Forest (34°23'56"N, 93°51'22"W), 28 October 2010, col. J. R. Fisher, D. M. Keeler.