(C) 2013 Nancy F. Mercado-Salas. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of the freshwater cyclopoid copepod genus Metacyclops Kiefer, 1927 is described from a single pond in northern Mexico, within the binational area known as the Chihuahuan Desert. This species belongs to a group of Metacyclops species with a 3443 spine formula of swimming legs. It is morphologically similar to Metacyclops lusitanus Lindberg, 1961 but differs from this and other congeners by having a unique combination of characters, including a caudal rami length/width proportion of 3.5–3.8, a innermost terminal seta slightly longer than the outermost terminal seta, intercoxal sclerites of legs 1-4 naked, a strong apical spine of the second endopodal segment of leg 1 and one row of 6-8 small spinules at the insertion of this spine. The finding of this species represents also the first record of the genus in Mexico and the third in North America, where only two other species, Metacyclops gracilis (Lilljeborg, 1853)and Metacyclops cushae Reid, 1991 have been hitherto reported. This is also the first continental record of a species of Metacyclops from an arid environment in the Americas. This species appears to be endemic to the Chihuahuan Desert, thus emphasizing the high endemicity of this area.
Arid environments, Cyclopoida, freshwater zooplankton, inland water crustaceans, copepod taxonomy
The freshwater copepod genus Metacyclops Kiefer, 1927 was revised by
Most of the surveys on the Mexican cyclopoid copepod fauna have dealt with the central and southern regions (
During the development of a project to estimate the diversity of cyclopoid copepods in arid and semi-arid regions of Mexico, zooplankton samples were collected between 1981 and 2009 in more than 500 water bodies from six states of Central and Northern Mexico (Aguascalientes, Chihuahua, Coahuila, Durango, San Luis Potosí, and Zacatecas). Samples were collected using a conical standard plankton net (250 mm diameter and 50 µm-mesh size) hauled near the shoreline of water bodies. The biological material was then fixed and preserved in 4% formalin solution. Copepods were sorted out from the original samples and then transferred to 70% ethanol with a drop of glycerine for long term preservation. Several female and male specimens of a cyclopoid copepod were collected from Coahuila in northern Mexico. These copepods were tentatively identified as Apocyclops panamensis (March, 1913). A second, closer examination of these specimens was performed in the laboratory and differences with respect to Apocyclops panamensis motivated a deeper analysis which revealed these specimens as members of Metacyclops. The taxonomically relevant characters of this genus were evaluated following
urn:lsid:zoobank.org:act:440E0C58-17CB-4A7F-A841-DC46403F02AB
http://species-id.net/wiki/Metacyclops_deserticus
Figures 1–5Holotype. Adult ♀, specimen dissected, mounted in glycerin sealed with Entellan (ECO-CH-Z-08585). Allotype. Adult ♂, dissected and mounted in glycerin and sealed with Entellan (ECO-CH-Z-08586). Paratypes.15 adult ♀♀ specimens, undissected, ethanol-preserved, vial (ECO-CH-Z-08587). Original plankton samples containing several additional specimens, and the SEM-processed specimens are deposited in the collection of M. Silva-Briano, Laboratorio de Ecología of the Universidad Autónoma de Aguascalientes, Mexico. Samples from the type locality were collected by Alejandro Maeda-Martínez in October 10, 1981.
Ephemeral pond at El Refugio bridge, Cerro Bola, Km 70, east of Torreón city, federal highway 40, Coahuila (25°35'02"N, 102°45'02"W).This pond is located in a desertic plain in the southwest margin of the ancient Laguna de Mayrán, a system which is part of the endorheic drainage of the Nazas and Parras rivers. The altitude of the type locality is about 1, 100 meters above sea level and the average annual precipitation in the area is 200 mm. At the moment of sampling the surface area of the pond was about 55 meters long and 25 meters wide, the water had an average depth of 50 cm and 80 cm at its deepest point.
The specific epithet makes reference to the arid habitat from which this species was collected. It was used to emphasize that it is the first American record from arid conditions.
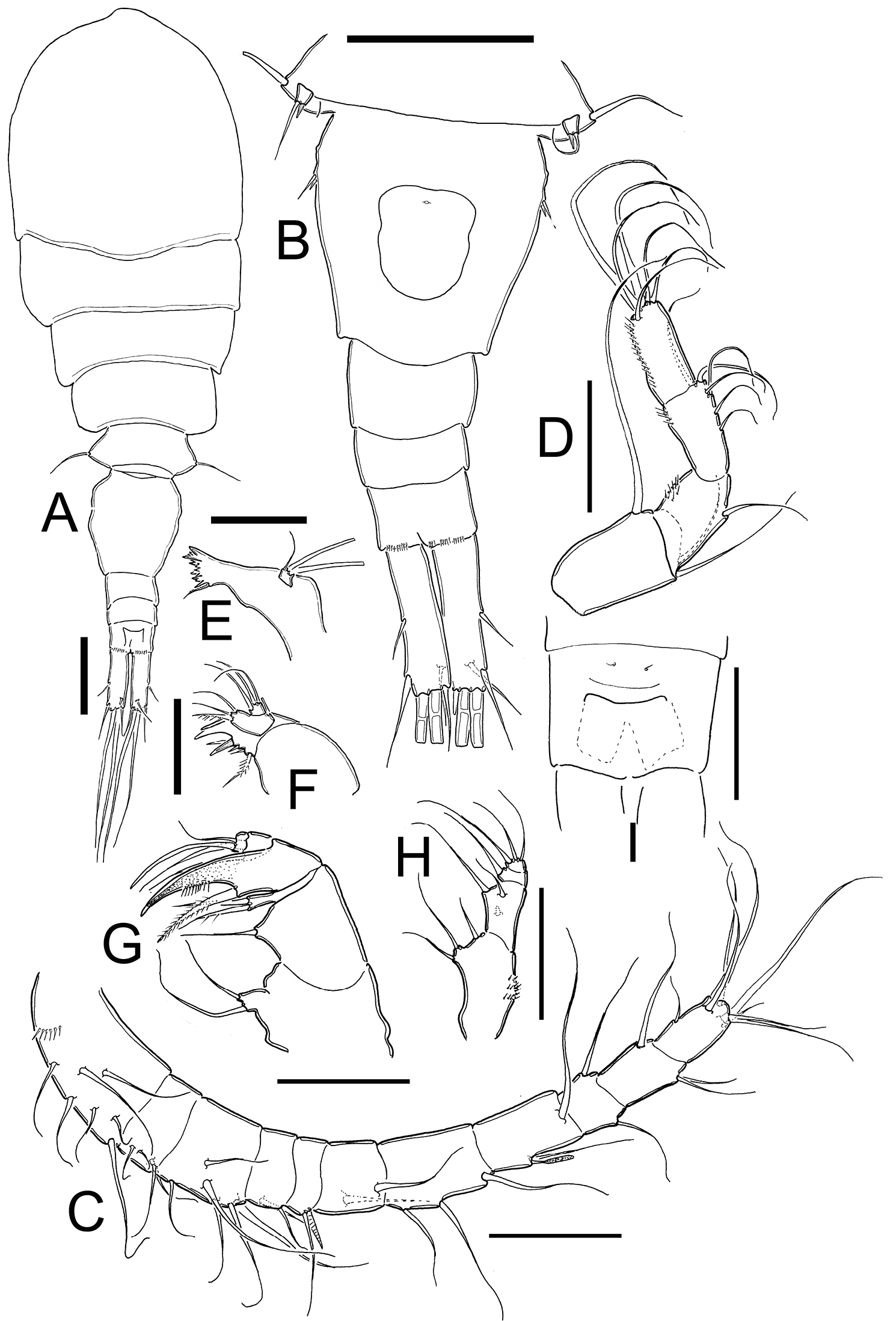
Female: Habitus as in Fig. 1A (dorsal view) and Fig. 4A (lateral view).Length of holotype 0.87 mm from anterior end of cephalothorax to posterior margin of caudal rami (range=0.72–0.87 mm; mean=0.80 mm; n=9). Body robust, cephalothorax relatively long, slightly expanded laterally at midlength of cephalosome in dorsal view; lateral margins of pedigers 3 and 4 straight, produced posteriorly. Cephalothorax length= 0.55 mm, representing 63% of total body length. Dorsal surface smooth, antennules not reaching distal margin of first pediger. Urosome (excluding caudal ramus) (Fig. 1B) representing 37% of body. Posterior margins of genital double-somite, free urosomites, and anal somite smooth both dorsally and ventrally. Relative length of each urosomite (proximal to distal) as: 65.4: 10.3: 10.3: 14.1=100. Genital double-somite (Fig. 5E) representing 17% of body length (excluding caudal rami), somite about 1.1 times longer than broad, with maximum width at proximal half; ventral and dorsal surfaces smooth. Anterior half of genital double-somite expanded laterally. Seminal receptacle with a reduced and narrow anterior part, posterior part rounded and expanding along the somite. Anal somite with distal rows of spines at insertion points of each caudal rami on ventral and dorsal margins. Anal operculum (Fig. 1I) slightly rounded and smooth.
Caudal ramus (Fig. 1B, 5F): Ramus representing 8.2% of total body length and 0.3 times as long as urosome. Length/width ratio= 3.5–3.8. Inner and outer margins smooth. Lateral caudal seta (II) inserted at 53% of total length of caudal rami. Outermost terminal seta (III) without ornamentation at point of insertion and 0.6–0.7 times as long as caudal ramus. Dorsal seta (VII) relatively short, 0.4–0.5 times as long as caudal ramus. Innermost terminal seta (VI) about 0.5 times as long as caudal ramus. Innermost terminal seta (VI) about 0.8–0.84 times outermost terminal seta (III). All terminal caudal setae plumose.
Antennule (Fig. 1C, 4A): 11-segmented in all specimens examined, armature per segment as follows (s=seta, sp= spine, ae=aesthetasc): 1(7s), 2(4s), 3(6s), 4(2s), 5(1s +1sp), 6(2s), 7(3s), 8(2s + 1ae), 9(2s), 10(3s), 11(7s). Antennule not reaching posterior margin of first thoracic somite.
Antenna (Fig. 1D, 4C): Four-segmented, basis without cuticular ornamentation, armed with long exopodal seta and two basipodal setae of different size, outer seta 1.6 times longer than inner seta. First endopodal segment with single outer seta and inner group of spinules. Second segment with 6 setae; inner margin with longitudinal row of spinules. Third endopodal segment with 6 terminal setae; inner margin with row of spinules.
Mandible (Fig. 1E): Gnathobase with 7 strongly chitinized teeth and dorsal seta armed with inner row of spinules. Palp reduced, with 2 long and 1 short setae, the later not reaching half-length of former two.
Maxillule (Fig. 1F): Precoxal arthrite with 3 strong chitinized claws and 2 spiniform setae on frontal side. Palp 2-segmented, proximal segment armed with 3 inner setae and outer exopodal seta. Distal segment of palp armed with 3 setae.
Maxilla (Fig. 1G): Precoxa and coxa not fused; precoxal endite armed with two strong biserially setulated setae. Coxal surface naked, proximal endite well developed, with two subequal apical setae. Claw-like distal endite well developed, with row of 6 spinules and basal seta. Endopodite 2-segmented, proximal segment with 2 robust setae, distal segment with single seta.
Maxilliped (Figs. 1H, 4D): Four-segmented. Syncoxa with 3 spiniform setae along inner margin: proximal one without ornamentation at insertion, middle one longest, more than twice as long as the other setae. Basis with 2 spiniform setae and transverse row of spines. Endopod reduced, 2-segmented, first segment with single lightly spinulate seta. Second endopodal segment armed with spiniform proximal seta and 2 slender setae.
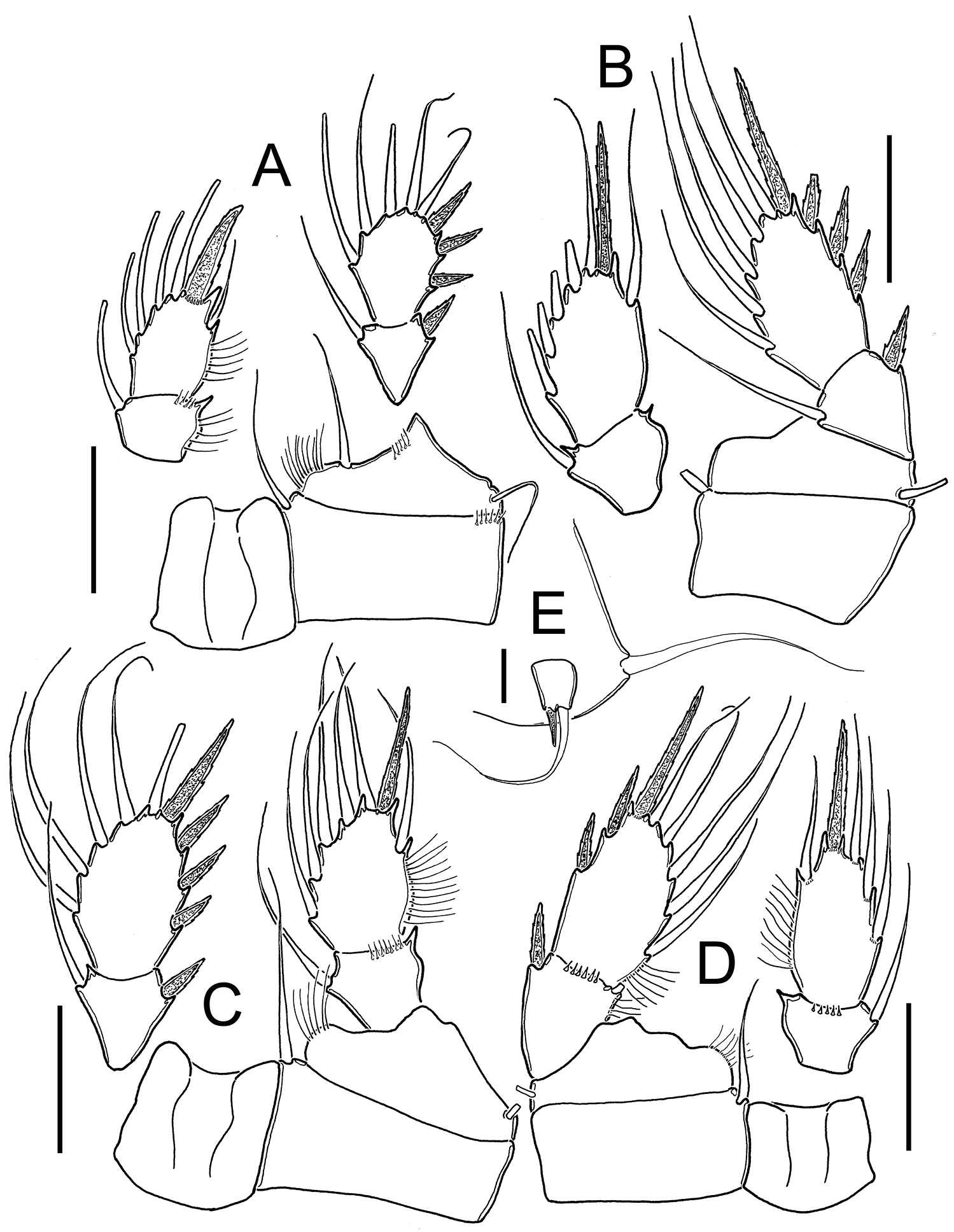
Legs P1-P4: with naked intercoxal sclerites, distal margins with rounded projections. All endopodal and exopodal setae slender and plumose. Armature formula of all swimming legs as in Table 1.
Leg 1(Fig. 2A, 5A): Coxa with inner seta and transverse row of 6 spinules on distal outer margin. Basis with inner row of short setae and long slender basipodal seta, reaching middle margin of second endopodal segment, row of hair-like setules along inner margin, row of 5 spines adjacent to insertion of endopodal ramus. Endopod slightly shorter than exopodite. Apical spine of second endopodal segment strong, slightly longer than segment, with spinules at insertion point.
Leg 2 (Fig. 2B): Coxa with inner seta . Basis with short slender seta on outer margin. Surface of coxa and basis smooth. Endopod slightly shorter than exopodite.
Leg 3 (Fig. 2C): Coxa with inner seta. Basis with outer seta. Surface of coxa and basis naked. Exopodite slightly longer than endopod.
Leg 4 (Figs. 2D, 5B): Coxa and basis as in legs 2-3. Endopod shorter than exopodite. Second endopod about two times longer than wide (1.9), with apical spine shorter than bearing segment(0.8 times as long as segment). Spinules at insertion of all elements of second endopodal segment. Second exopodal segment with 2 outer spines and 1 apical spine with small spinules at insertion point.
Leg 5 (Figs. 1B, 2E, 5C): Basal segment completely fused to somite, dorsal seta stout and plumose, about 1.4 times longer than outer seta of free segment. Free segment subrectangular, 1.2 times longer than wide, inner spine slightly shorter than bearing segment. Outer seta about 4 times longer than inner spine. Inner spine strong and smooth; outer seta plumose on distal half.
Leg 6 (Figs 1B, 5D): Represented by small, low plate near lateral margin of genital double somite. Leg armed with relatively long plumose seta, and with 2 short, subequal smooth spines.
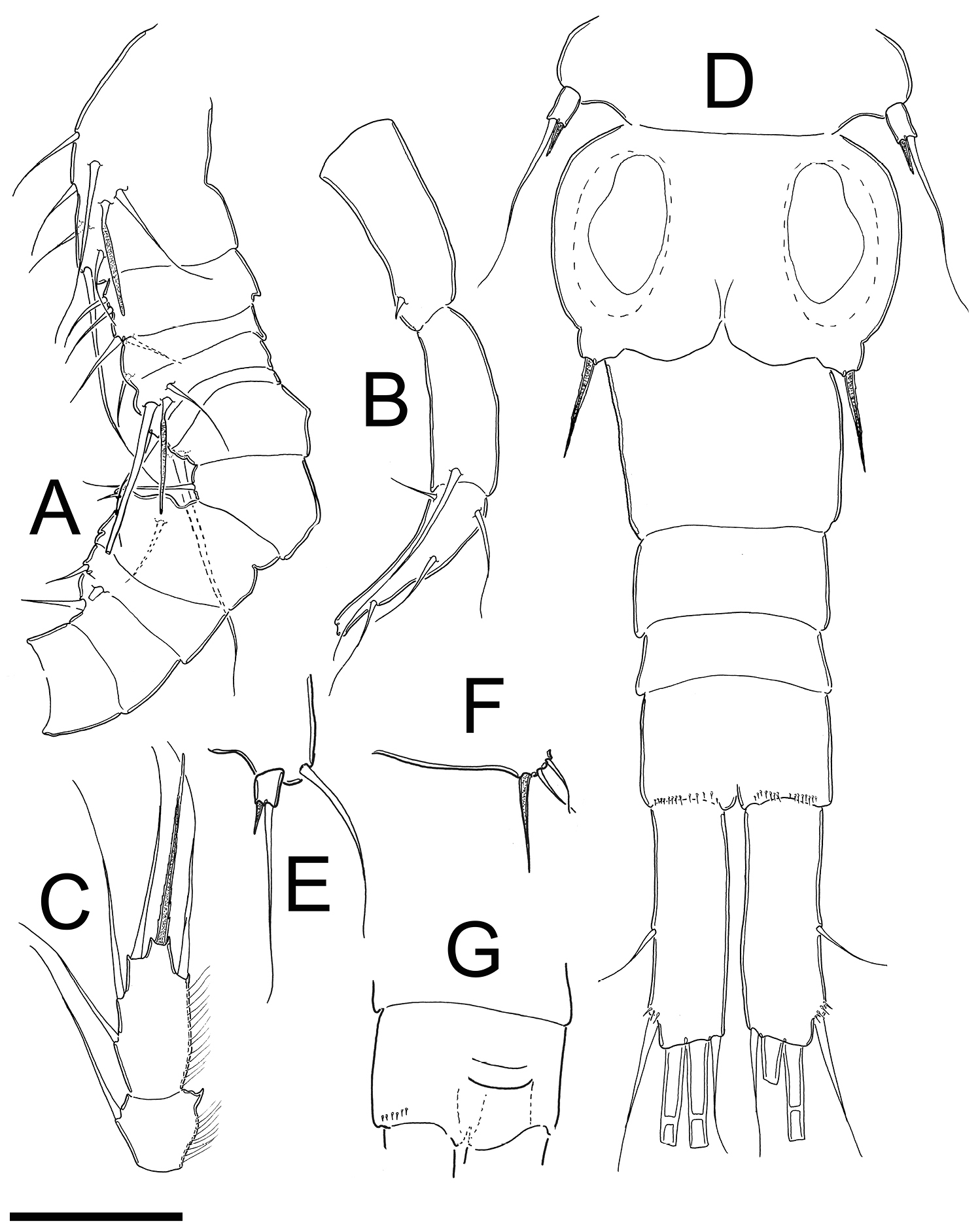
Male:Length of allotype 0.58 mm (excluding caudal ramus) (range=0.58–0.64 mm; mean= 0.61mm; n =2). Body slender than in female, cephalothorax relatively long, slightly expanded laterally at midlength of cephalosome in dorsal view; lateral margins of pedigers 3 and 4 straight, produced posteriorly. Cephalothorax length= 0.40 mm, representing 68% of total body length, dorsal surface smooth. Posterior margins of genital somite, free urosomites, and anal somite smooth ventrally (Fig. 3D) and dorsally. Ventral surface of anal somite smooth; distal ventral margin with rows of 13–15 spines at insertion point of caudal rami. Anal operculum (Fig. 3G) slightly rounded, smooth.
Caudal ramus (Fig. 3D): Length of ramus 0.07 mm. Length/width ratio= 3.1–3.2. Inner and outer margins smooth, unornamented. Lateral apical seta (II) inserted al 52.3% of total length of caudal ramus. Outermost terminal (III) seta with small spinules at insertion and 0.7–0.8 times as long as caudal ramus. Dorsal seta (VII) longer than in females; about 0.7 times as long as caudal ramus. Innermost terminal seta (VI) 0.5 times as long as caudal ramus and. Innermost terminal seta (III) slightly shorter than outermost terminal seta (VI), III/VI ratio 0.78–0.9. All terminal caudal setae plumose.
Antennule (Fig. 3A–B): 14-segmented, geniculate, armature of segments 12, 13 and 14 not seen clearly (they could have more setae). Armature per segment as follows (s=seta; sp= spine ae= aesthetasc): 1(7s+1ae), 2(4s), 3(1s), 4(2s+1ae), 5(1s), 6(1s), 7(1s), 8(1s+ 1sp), 9(2s), 10(1sp), 11(0), 12(1sp), 13(1s), 14(4s).
Antenna, mouthparts and legs 1–3 as in female.
Leg 4 (Fig. 3C): as in female except for relatively longer exopodite.
Leg 5 (Fig. 3E): Basal segment completely fused to somite, dorsal seta stout, as long as outer seta of free segment. Free segment subrectangular, 1.5 times longer than wide, spine as long as segment, outer seta about 5 times longer than inner spine. Inner spine strong, smooth; outer seta plumose on distal half.
Leg 6 (Fig. 3F): Represented by small, low plate near lateral margin of genital somite with relatively strong and long inner spine, two outer setae about the half of length of inner spine. Spine and setae smooth.
Metacyclops deserticus sp. n., female holotype from Coahuila, Mexico. A habitus, dorsal view B urosome, ventral view C antennule D antenna E mandible F maxillule G maxilla H maxilliped I anal operculum. Scales bars A–B= 100µm; C–I= 50 µm.
Metacyclops deserticus sp. n., female holotype from Coahuila, Mexico. A Leg 1 B Leg 2 C Leg 3 D Leg 4 E Leg 5. Scales bars A–D= 50 µm; E= 10 µm.
Metacyclops deserticus sp. n., male allotype from Coahuila, Mexico. A antennule (segments 1–11) B antennule (segments 12–14) C Endopod P4 D urosome E Leg 5 F Leg 6 G Anal operculum. Scales bars A–G = 50 µm.
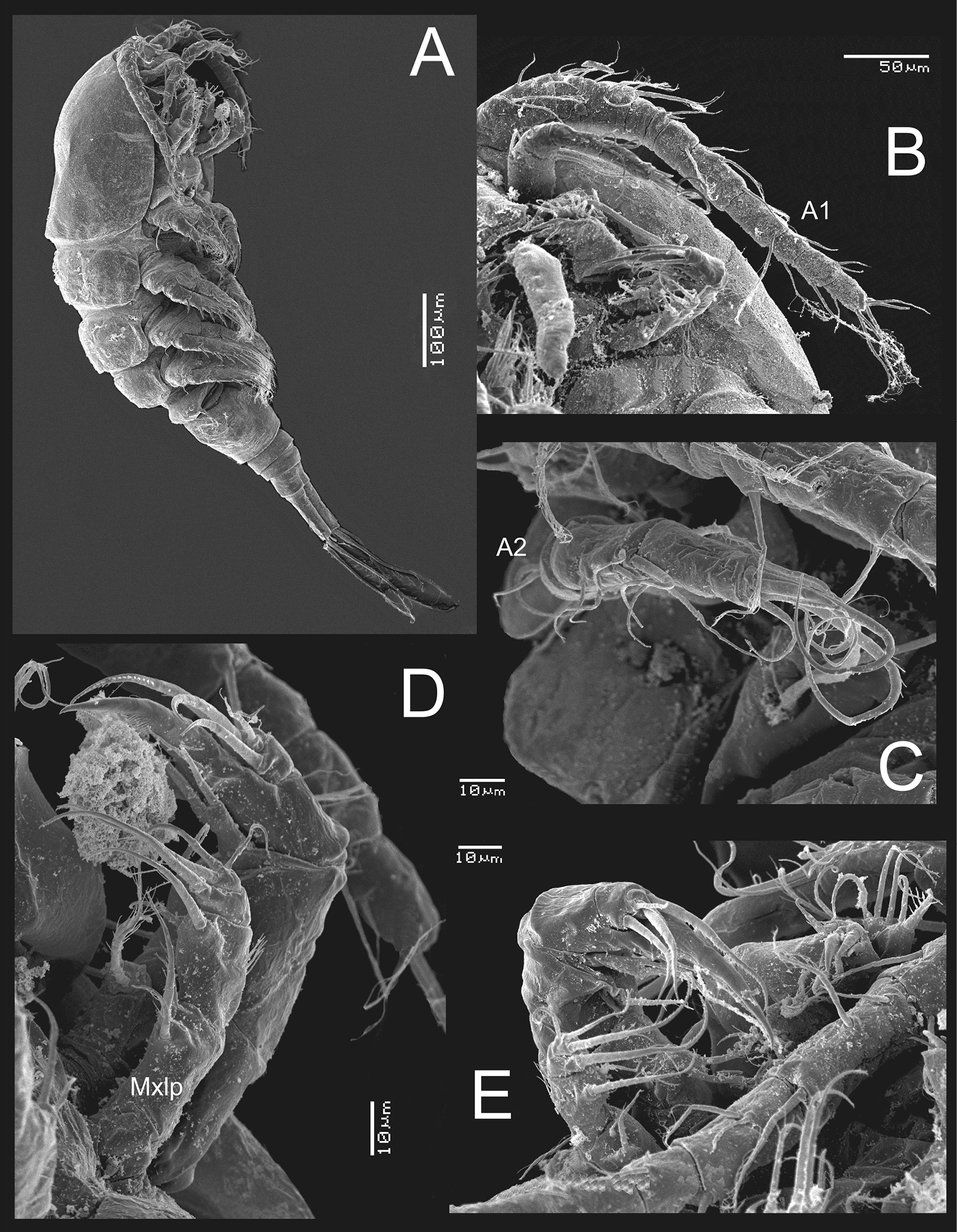
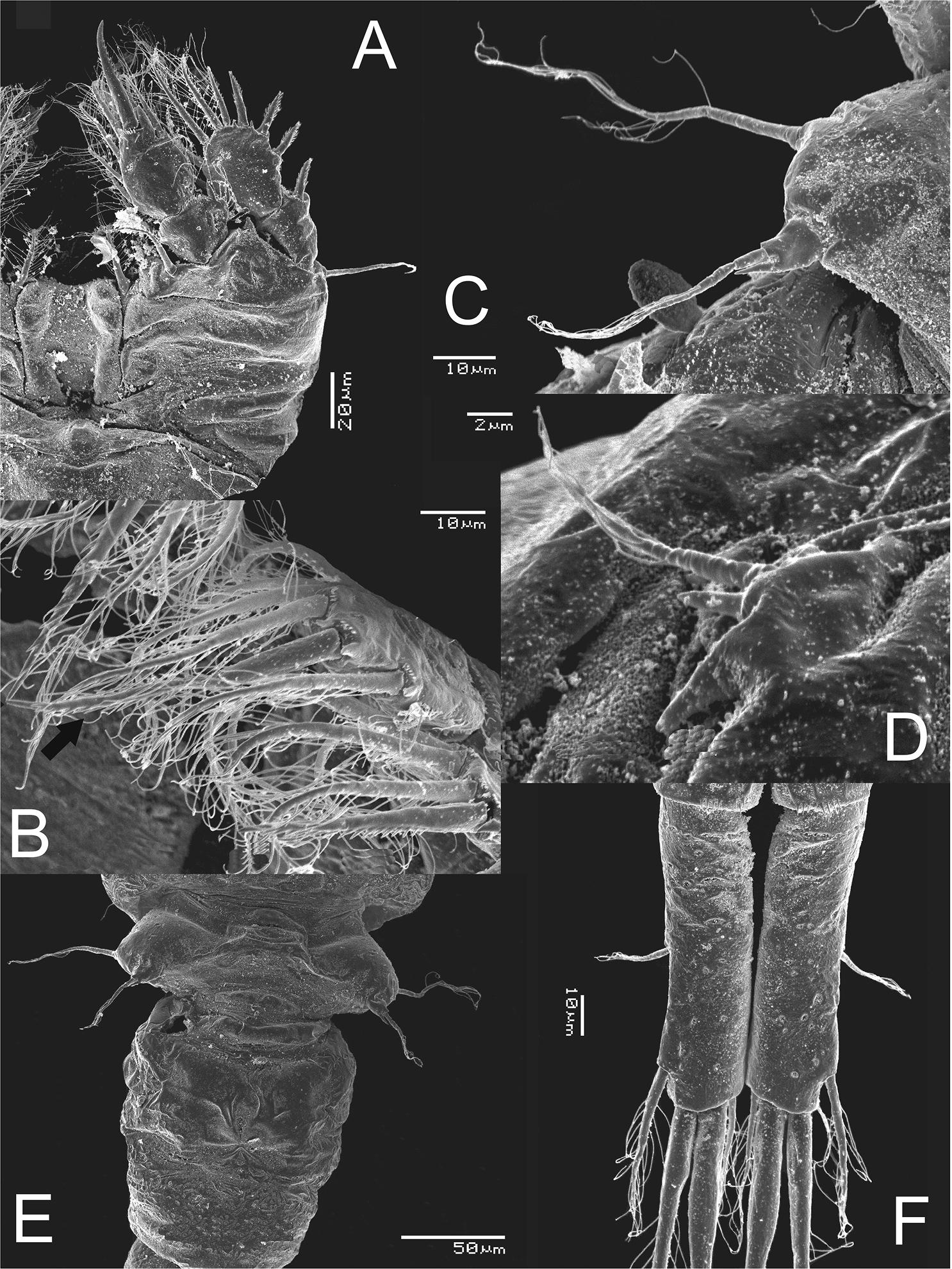
Metacyclops deserticus sp. n., SEM-processed female from Coahuila, Mexico. A habitus, lateral view B antennule C antenna D maxilliped (lateral view) E mouthparts.
Metacyclops deserticus sp. n., SEM-processed female from Coahuila, México. A leg 1 B endopodite 2 leg 4 C leg 5 D leg 6 E genital double somite, ventral view F caudal ramus, ventral.
Armature of swimming legs 1–4 (spines in Roman numerals, setae in Arabic) of Metacyclops deserticus sp. n. Sequence follows external to internal positions.
| coxa | basis | endopodite | exopodite | |
|---|---|---|---|---|
| leg 1 | 0-1 | 1-1 | 0-1;1-I-4 | I-1;III-5 |
| leg 2 | 0-1 | 1-0 | 0-1;1-I-5 | I-1;IV-5 |
| leg 3 | 0-1 | 1-0 | 0-1;1-I-5 | I-1;IV-5 |
| leg 4 | 0-1 | 1-0 | 0-1;1-I-3 | I-1; III-5 |
The only two other species of the genus known to occur in North America, Metacyclops cushae Reid, 1991 and Metacyclops gracilis (Lilljeborg, 1853), are easily distinguishable from the new species. The former species belongs to the “Group C” (
Following the comprehensive key to the known species of Metacyclops (
We also followed
Additional differences of the new species with respect to the American congeners include the length of the apical spine of the second endopodal segment of leg 1/length of segment ratio (1.2), vs. about 0.7 in Metacyclops curtispinosus and Metacyclops agnitus andabout 0.9 in Metacyclops subdolus, Metacyclops hannensis, and Metacyclops gasparoi. In Metacyclops deserticus sp. n., Metacyclops curtispinosus, Metacyclops subdolus, and Metacyclops gasparoi, the length of the basipodal seta of leg 1 exceeds the medial margin of the second endopodal segment of leg, whereas in Metacyclops hannensis it exceeds the total length of the endopodite and in Metacyclops agnitus it is absent. All these species have naked coxal sclerites of legs 1–4.
The new species shares a similar length/width ratio of the second endopodal segment of leg4 with Metacyclops curtispinosus, Metacyclops agnitus, Metacyclops pectiniatus, and Metacyclops subdolus (range= 1.9–2.1), thus differing from the range reported for Metacyclops hannensis (1.6–1.7), and Metacyclops gasparoi (3.3). There are additional differences in the length ratio of the apical spine of leg 4 second endopodal segment/length of segment; Metacyclops deserticus sp. n. shares with Metacyclops curtispinosus (a value of about 0.7) whereas this value is different in Metacyclops pectiniatus, Metacyclops subdolus and Metacyclops hannensis (0.9), Metacyclops agnitus (1.1) and Metacyclops gasparoi (1.4). The length ratio of external seta of leg 4 second endopodal segment/length of apical spine, is about 0.8 in Metacyclops deserticus sp. n. and Metacyclops curtispinosus, thus differing from Metacyclops gasparoi and Metacyclops hannensis (0.9–1.1), Metacyclops subdolus, Metacyclops pectiniatus and Metacyclops agnitus (1.2–1.3). An additional difference between these species is the shape of the inner margin of the leg 4 basis. In the new species but also in Metacyclops curtispinosus, Metacyclops agnitus, Metacyclops pectiniatus, and Metacyclops hannensis it is rounded vs. triangular- in Metacyclops subdolus and Metacyclops gasparoi.
In addition, the new species differs from its congeners in the length of the external seta of free segment of P5/ inner spine length ratio; in the new species, this ratio is 4.0, whereas it ranges between 2.9 and 3.1 in Metacyclops subdolus and between 5.0 and 5.7 in Metacyclops hannensis, Metacyclops pectiniatus and Metacyclops agnitus. In Metacyclops gasparoi this valueis 6.6 and in Metacyclops curtispinosus it is about 8.0. The proportion between inner spine/length of segment of P5 is a character that also differs among these species. In Metacyclops curtispinosus the ratio is 0.3, in the new species it is about 0.8, in Metacyclops hannensis and Metacyclops agnitus the spine is as long as the segment, in Metacyclops pectiniatus and Metacyclops gasparoi it is about 1.3, in Metacyclops subdolus 1.5 times. Also the length proportion of the seta of fifth leg fused to the segment/outer seta of free segment represents a character that differs between species, in Metacyclops gasparoi it is 0.6, Metacyclops hannensis and Metacyclops subdolus have a proportion ranging between 0.8 and 0.9. Metacyclops agnitus and Metacyclops curtispinosus shares similar values (1.1–1.2) and both Metacyclops pectiniatus and the new species have a length ratio close to 1.4 (
The length/width ratio of the caudal ramus also differs among these species, Metacyclops curtispinosus has relatively low value (2.4–2.8) that differs from those in Metacyclops agnitus, Metacyclops pectiniatus, and Metacyclops hannensis (3.2–3.4). Metacyclops subdolus has a wide range of variation (2.9–3.4). The new species has a relatively longer caudal ramus (3.5–3.8), but it is shorter than in Metacyclops gasparoi (5.5–5.7). Another valuable character is the length ratio of innermost terminal seta/outermost terminal seta; we found two main groups for this character. In the first one the innermost terminal seta is shorter than the outermost terminal seta; this character is present in Metacyclops agnitus (0.5), Metacyclops pectiniatus (0.6), Metacyclops hannensis (0.7), and inthe new species(0.8). In the second group the innermost terminal seta is longer than outermost terminal seta: Metacyclops curtispinosus (1.2), Metacyclops subdolus (1.5–1.7), and Metacyclops gasparoi (2.0). In addition, the length dorsal seta/length of caudal ramus ratio also separates two groups. In the first group, the dorsal seta is shorter than the ramus: Metacyclops deserticus sp. n. (0.4–0.5), Metacyclops curtispinosus and Metacyclops hannensis (0.7). In the second group the dorsal seta is longer than caudal ramus like in Metacyclops gasparoi, Metacyclops agnitus (1.1), and Metacyclops subdolus (1.8–2.1).
Metacyclops deserticus sp. n. from northern central Mexico represents the first new species of the genus Metacyclops described in this country and is also the third record for North America (
The arid areas of north-central Mexico were formed between the Late Oligocene and Middle Miocene (30-20 MYA), and were part of a general trend toward a greater aridity resulting from climate changes associated to the intense volcanic activity and tectonics that characterized the Cenozoic. The Rocky Mountains, the Mexican and Central-American Plateaus, and the sierras Madre were formed as result of tectonic activity during Cenozoic. The formation of the Sierra Madre Occidental and Sierra Madre Oriental during the Eocene and continuing until the middle Miocene provided a new barrier to the atmospheric flow. This barrier blocked the masses of warm, moist air from the Pacific Ocean and the Gulf of Mexico and caused a severe drought and desertification of the Mexican Plateau. The Mexican Plateau includes the states of Coahuila, Chihuahua, Zacatecas, Durango. The Miocene climate change segregated the species along latitudinal and longitudinal gradients, thus favoring radiation processes of some lineages (
The cyclopoid copepod fauna of arid areas of central-north of Mexico (Chihuahuan Desert) is currently represented by 39 species belonging to 12 genera. This binational zone is currently deemed as an area with a high endemicity; up to 20% of these species are endemic to these arid areas (
We gratefully acknowledge the support by Araceli Adabache, Laboratorio de Ecología, Universidad de Aguascalientes, for help and advice in the SEM processing and examination of the specimens. Also, we acknowledge the advice and comments by Dr. Janet W. Reid. A previous version of this contribution was greatly improved by constructive comments and suggestions from two anonymous reviewers. This work is part of the first author’s (NM-S) Master of Sciences thesis developed at El Colegio de la Frontera Sur (ECOSUR) and was derived from a project supported by the Mexican Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO-GT-034).