(C) 2013 Thanit Siriboon. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Three new species of the streptaxid snail genus Perrottetia are described from north and northeastern Thailand, Perrottetia aquilonaria sp. n., Perrottetia dermapyrrhosa sp. n. and Perrottetia phuphamanensis sp. n. Each species is endemic to a single or a few limestone mountain ranges. The species are characterized by the morphology of their genital organs, as well as by shell characters. Perrottetia aquilonaria sp. n. has a club shaped distal penis and large penial hooks are present and penial papillae cover almost the entire penial hook portion; adjacent areas possess low reticulated folds. Perrottetia dermapyrrhosa sp. n. has a long genital atrium and the penial sheath is about two-thirds of the penis length. Penial hooks are long, scattered and sunken into deep ovate hollows; vaginal hooks are present. Perrottetia phuphamanensis sp. n. has a rounded and protruded shell periphery. The aperture is subcircular, peristome is thick and the second parietal lamella is adjacent to the first parietal lamella; a basal lamella is the smaller than in the other Thai species.
Systematics, land snails, taxonomy, genitalia, predator
Terrestrial gastropods are primarily herbivores and only a few groups are carnivorous. Carnivorous snails usually feed on other snail species or on weak individuals of the same species; some feed on insect larvae or earthworms (
With 13 genera and about 130 nominal species, the second most diverse streptaxid fauna can be found in Southeast Asia (
The most prominent characters of Perrottetia are the sub-oblique heliciform shell, often with whorls coiling around an oblique axis. The last whorls do not descend below the preceding whorl, and short longitudinal furrows are present behind the apertural lip. Internally, the aperture possesses two parietal lamellae (
Our faunistic surveys throughout Thailand from 2008–2012 yielded rich collections of both, shells and live streptaxids, from north and northeast Thailand. Based on their distinctive shell characters, three new Perrottetia species are recognized. In addition to shell characters we examined the genitalia and radulae. Identifications were provisionally based on
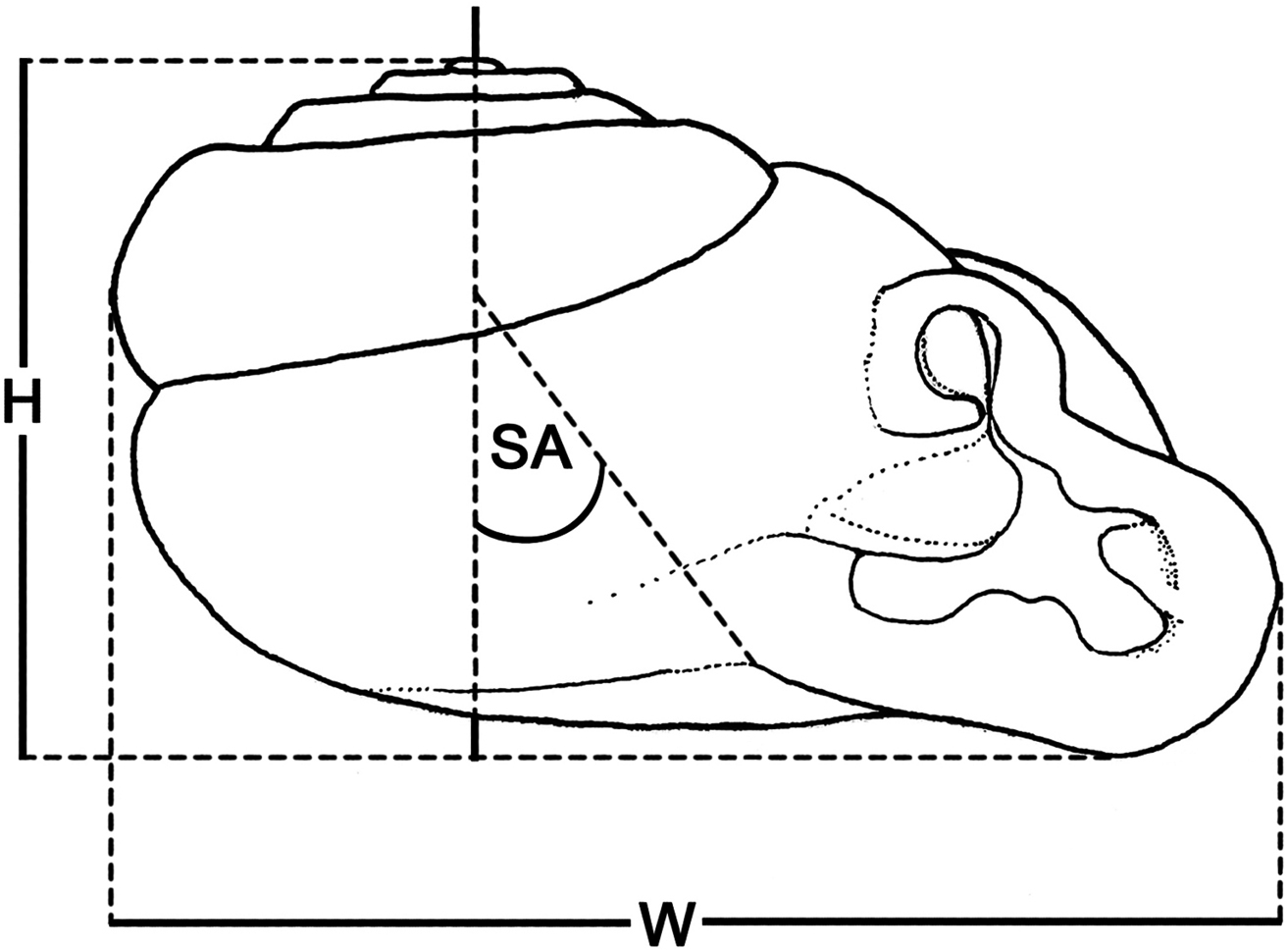
Schematic diagram illustrating methods for measuring specimens: H shell height SA shell angle W shell width.
The nomenclature of the shell apertural dentition follows that of
Material examined in this study is deposited in the following institutions: CUMZ, Chulalongkorn University Museum of Zoology, Bangkok; NHMUK, The Natural History Museum, London; SMF, Forschungsinstitut und Naturmuseum Senckenberg, Frankfurt am Main.
All descriptions of the new species are here attribute to the first and the fourth author, Siriboon and Panha, respectively.
http://species-id.net/wiki/Perrottetia
Helix peroteti Petit, 1841 by subsequent designation of
Theshell is oblique-heliciform, usually thin and opaque. Its surface is smooth and glossy but fine transverse ridges may be present. The embryonic shell is smooth. The 5–7 whorls increase regularly. The shell periphery is usually rounded and the last whorl does not descend below the preceding whorl but is parallel to the preceding suture. The outer wall of the last whorl generally possesses two short longitudinal furrows that correspond with internal apertural lamellae. The umbilicus is narrow and deep. The semi-ovate aperture has an expanded peristome with a reflexed lip. The apertural dentition consists of two parietal lamellae; palatal, basal and columellar lamellae are usually present; upper palatal and supracolumellar lamellae may also be present.
Living animals possess a yellowish to reddish reticulated skin. The brown digestive gland and the black kidney are visible through the transparent shell. The upper tentacles are longer than the lower pair with a black eye-spot on the tip of the fully extended tentacle; bright red or yellow retractor muscles show though the transparent skin. The foot is narrow, undivided, the tail short.
Genitalia with a long, slender penis; penial sheath short, about half of penis length; internal wall of introverted penis with black to brown penial hooks; vas deferens passes through a short section of penial sheath before connecting distally to penis; vagina and free oviduct short to long, vaginal hooks may be present; gametolytic duct and sac may not extend as far as albumin gland; seminal vesicle present with about the same length from vesicle to talon.
Perrottetia consists of 27 nominal species distributed across Southern Asia, southern China to northern Vietnam. So far, it was not recorded from Thailand (
The first description of the genital system of a member of the subfamily Streptaxinae Gray, 1860 was published by
urn:lsid:zoobank.org:act:029F7FDD-9A8A-4B36-A782-815BFA0D32EB
http://species-id.net/wiki/Perrottetia_dermapyrrhosa
Figs 2A, 3A–C, 4A–C, 5A–G, 6A, Table 1Holotype CUMZ 5001 (Fig. 3A). Measurement: shell height 6.1 mm, shell width 7.7 mm, and with 6 whorls. Paratypes NHMUK 20130062 (2 shells), SMF 341486 (1 shell), CUMZ 5002 (2 shells).
Wat Tam Namsrithong, Nong Kungsi, Kalasin, Thailand, 16°48'18.0"N, 103°16'42.5"E.
Living snails of A Perrottetia dermapyrrhosa sp. n.(paratype CUMZ 5002) from the type locality (shell width about 7 mm), and B Perrottetia aquilonaria sp. n. (paratype CUMZ 5004) from the type locality (shell width about 6 mm).
Shells of Perrottetia spp. A–C Perrottetia dermapyrrhosa sp. n. A holotype CUMZ 5001 B apertural dentition of the holotype CUMZ 5001, and C paratype CUMZ 5002 D–H Perrottetia aquilonaria sp. n. D holotype CUMZ 5003 E apertural dentition of the holotype CUMZ 5003 F paratype CUMZ 5004 G specimen from Tam Chiangdao, Chiangmai, CUMZ 5008 and H apertural dentition of the specimen from Tam Chiangdao, Chiangmai CUMZ 5008 I–K Perrottetia phuphamanensis sp. n. I holotype CUMZ 5011 J paratype CUMZ 5012, and K apertural dentition of the holotype CUMZ 5011 L Perrottetia gudei Fulton, 1915, syntype NHMUK 1919.12.31.51.
Perrottetia mabillei (Bavay and Dautzenberg, 1903) can be distinguished from Perrottetia dermapyrrhosa sp. n. by its lower spire with a distinct suture. The left periphery of the penultimate whorl is shouldered and does not extend beyond the diameter of the last whorl. The aperture is triangular and a supracolumellar lamella is absent. In comparison, Perrottetia peroteti (Petit, 1841) possesses a lower spire with a distinct suture, fine transverse ridges are present and a smaller basal lamella, while upper palatal and supracolumellar lamellae are absent. Perrottetia gudei (Fulton, 1915) (syntype Fig. 3L) differs from Perrottetia dermapyrrhosa sp. n. in its lower spire, the second parietal lamella being smaller and shorter than the first lamella and an upper palatal lamella that is usually present (
Perrottetia dermapyrrhosa sp. n. differs from Perrottetia aquilonaria sp. n. in its larger shell, which is less deviated from the vertical axis. A sinulus sensu
Shell oblique-heliciform, white and translucent; whorls 6, spire conical, suture distinct; shell surface glossy, with transverse ridges that diminish below the periphery; embryonic shell large, consisting of about 2 whorls with smooth surface, following whorls regularly expanding; shell periphery rounded, last whorl axially deflected; two deep and short longitudinal furrows present; umbilicus narrow (Fig. 3A); aperture subcircular, peristome discontinuous, thickened and expanded; apertural dentition with a large transverse first parietal lamella, with second parietal lamella adjoined at right angles; one upper palatal lamella, one small palatal lamella, one large basal lamella, one long subcolumellar lamella, one large strong columellar lamella and one small supracolumellar lamella (Fig. 3B).
Radula: Teeth arranged in anteriorly V-shaped rows, each row contains 29–31 teeth with formula (14-15)-1-(14-15); central tooth very small and triangular with a pointed cusp; lateral and marginal teeth undifferentiated, unicuspid and lanceolate; lateral teeth gradually reducing in length and size; outer teeth much smaller and shorter than inner teeth (Fig. 6A).
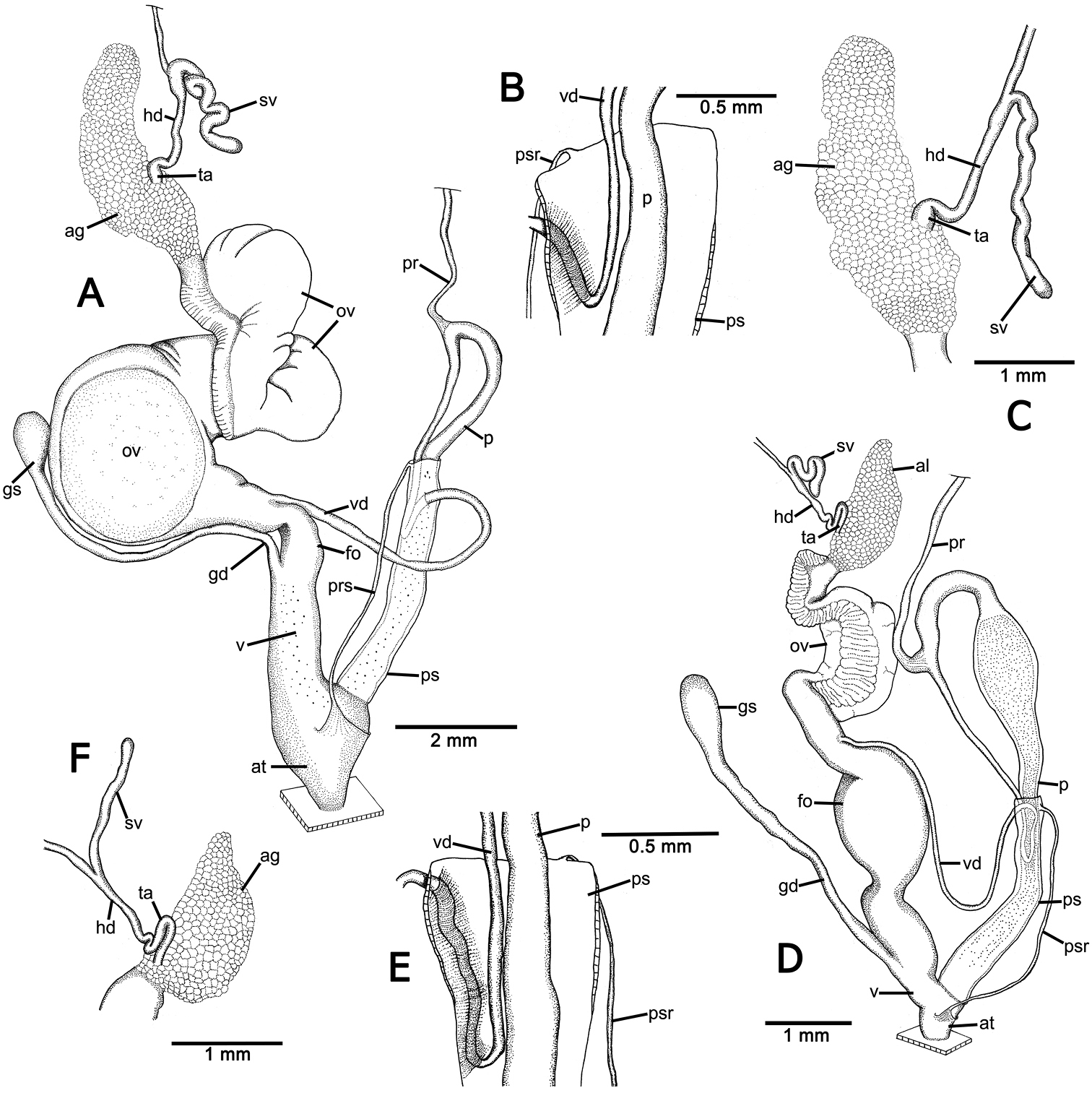
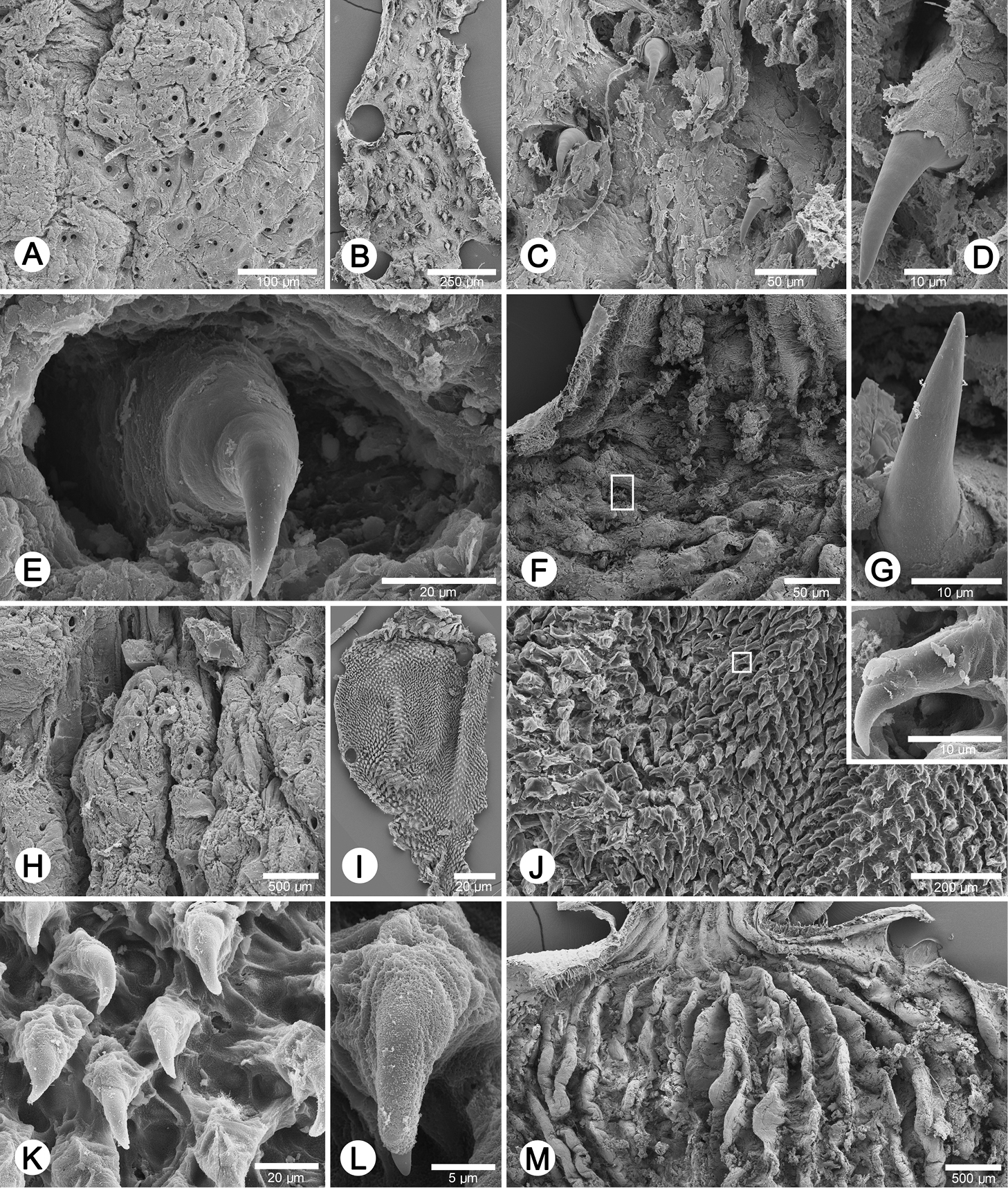
Genital organs: Atrium (at) long and slender; proximal penis (p) long, slender and with solid muscular penis sheath extending distally beyond penis sheath as a narrow tube; penial sheath (ps) reaching about two-thirds of total penis length, penial sheath retractor muscle very thin (psr), originating at atrium and inserting distally on penial sheath (Fig. 4A); vas deferens (vd) passes through about one-sixth of penial sheath length before entering into penis distally (Fig. 4B); penial retractor muscle (pr) thin and very long, inserting at penis and vas deferens junction; internal wall of atrium generally smooth with numerous pores (Fig. 5A); penial wall with scattered and pale brown penial hooks, about 3 hooks/200 µm2 (Fig. 5C), and hooks located on conical papillae surrounded by deep ovate hollows; penial hooks of small size (<0.04 mm in length), expanding at base, tip sharp and curved towards genital orifice (Fig. 5E); vagina (v) short, stout, about one third of total penis length; gametolytic duct (gd) a long tube not extending as far as albumin gland, gametolytic sac ovate (gs); free oviduct (fo) very short, oviduct (ov) enlarged and folded; prostate gland inconspicuous and bound to oviduct (Fig. 4A); talon (ta) small, very short and club shaped; hermaphroditic duct (hd) bearing long seminal vesicle (sv) about one and half times longer than the length from talon to branching point of seminal vesicle (Fig. 4C); vagina wall with a corrugated fold and pale brown vaginal hooks, about 8 hooks/200 µm2, hooks small (<0.03 mm in length) with pointed tip slightly curving away from genital orifice (Figs 5F, G).
Animal: Live specimens exhibit yellowish-red reticulated skin, and reddish tentacular retractor muscles are visible through the semi-transparent body (Fig. 2A).
The specific epithet “dermapyrrhosa” is derived from the Greek “derma” meaning “skin” and “pyrrhos” meaning “red or yellowish-red”.
This species is known only from the type locality, which is an isolated limestone hill reaching about 300 meters above mean sea level, and which is surrounded by the Korat Plateau.
Up to now, the only description of the reproductive system of a Perrottetia species was that of Perrottetia gudei from Vietnam in which the presence of streptaxid vaginal hooks were recorded for the first time, but without being figured (
urn:lsid:zoobank.org:act:2E376204-2D0F-4021-B795-399E33C8A677
http://species-id.net/wiki/Perrottetia_aquilonaris
Figs 2B, 3D–H, 4D–F, 5H–M, 6B, Table 1Holotype CUMZ 5003 (Fig. 3D). Measurement: height 3.9 mm, shell width 6.6 mm, and with 6 whorls. Paratypes NHMUK 20130064 (2 shells), SMF 341487 (1 shell), CUMZ 5004 (1 shell).
Tam Phra Bumpenboon, Phan, Chiangrai: CUMZ 5005. Wat Tam Pha Jaruey, Pa-daet, Chiangrai: CUMZ 5006. Tam Maesuai, Maesuai, Chiangrai: CUMZ 5007. Tam Chiangdao, Chiangmai: CUMZ 5008. Pha Chu, Nanoi, Nan: CUMZ 5009. Tam Pha Nangkoi, Rongkwang, Phrae: CUMZ 5010.
Wat Tam Pha Plong, Chiangdao, Chiangmai, Thailand, 19°24'7.3"N, 98°55'5.6"E.
Genitalia of Perrottetia spp. A–C Perrottetia dermapyrrhosa sp. n. (paratype CUMZ 5002) A reproductive system B insertion of vas deferens into penial sheath, and C details of hermaphroditic duct and seminal vesicle D–F Perrottetia aquilonaria sp. n. (paratype CUMZ 5004), D reproductive system E insertion of vas deferens into penis sheath, and F details of hermaphroditic duct and seminal vesicle. Abbreviations: ag, albumen gland; at, atrium; fo, free oviduct; gd, gametolytic duct; gs, gametolytic sac; hd, hermaphroditic duct; ov, oviduct; p, penis; pr, penial retractor muscle; ps, penial sheath; psr, penial sheath retractor muscle; sv, seminal vesicle; ta, talon; v, vagina; vd, vas deferens.
Internal sculpture of genitalia of Perrottetia spp. A–G Perrottetia dermapyrrhosa sp. n. (paratype CUMZ 5002) A details of atrial pore on the atrium surface B low magnification shows arrangement of penial hooks C high magnification of penial hooks D lateral view of penial hook E top view of penial hook situate inside hollow F arrangement of vaginal fold with hook in white square, and G lateral view of vaginal hook (from white square in F) H–M. Perrottetia aquilonaria sp. n. (paratype CUMZ 5004) H details of atrial pore on the atrium surface I low magnification shows dense arrangement of penial hooks J high magnification of penial hooks with (inset) lateral view of penial hook K arrangement of penial hooks L top view of penial hook, and M arrangement of vaginal folds without vaginal hook.
Perrottetia aquilonaris sp. n. can be distinguished from the similar south Indian species Perrottetia watsoni (Blanford, 1860) and Perrottetia beddomii (Blanford, 1899) by its smooth shell surface and the presence of a sinulus and a bifid columellar lamella. In comparison, Perrottetia beddomii possesses a supracolumellar lamella, while Perrottetia watsoni hasa second parietal lamella adjacent to the first parietal lamella (Blanford 1860, 1899,
Shell suboblique-heliciform, white and translucent; teleoconch with 6 whorls, spire convex, suture indistinct; shell surface glossy, with transverse ridges diminishing below the periphery; embryonic shell large, about 2½ whorls, with smooth surface; following whorls regularly expanding; shell periphery shouldered, in apertural view left periphery of the penultimate whorl extending beyond the diameter of the last whorl; last whorl axially deflected; two deep and short longitudinal furrows present; umbilicus narrow (Fig. 3D); aperture subcircular, peristome discontinuous, thickened and expanded, short sinulus present, sometimes with a longer and tapering sinulus (Fig. 3H); apertural dentition consisting of one strong first parietal lamella, a small second parietal lamella separated at right angles, one small upper palatal lamella, one large palatal lamella, one basal lamella and a bifid columellar lamella (Fig. 3E).
Radula: Teeth arranged in anteriorly V-shaped rows, each row containing 21–23 teeth with the formula (10-11)-1-(10-11); central tooth small, sharp, triangular with pointed cusp; lateral and marginal teeth undifferentiated, unicuspid and lanceolate; lateral teeth gradually reduced in length and size, outer teeth much smaller and shorter than inner teeth (Fig. 6B).
Genital organs: Atrium (at) short; penis tripartite, proximal part long and narrow, central section globular with a thick muscular wall, distal section again long and narrow; penial sheath (ps) thin, extends about half of total penis length; penial sheath retractor muscle (psr) very thin, originating at the atrium, inserting distally on penial sheath (Fig. 4D); vas deferens (vd) passes through about one-fifth of penial sheath length before entering into penis distally (Fig. 4E); penial retractor muscle (pr) thin and very long, inserting at penis and vas deferens junction; internal wall of atrium generally smooth with numerous pores (Fig. 5H); penial wall with scattered and pale brown penial hooks about 24 hooks/200 µm2; hooks located on papillae (pl), papillae separated by low reticulated folds; penial hooks of small size (< 0.02 mm in length), expanding at base, tip sharp and curved towards genital orifice (Figs 5J–L); vagina (v) short, stout, about a seventh of total penis length; gametolytic duct (gd) long but not extending as far as albumin gland; gametolytic sac ovate (gs); proximal free oviduct (fo) stout and distally enlarged; oviduct (ov) enlarged and folded; prostate gland inconspicuous and bound to oviduct (Fig. 4D); talon (ta) small, very short and club shaped; hermaphroditic duct (hd) bearing long seminal vesicle (sv) about one and half times longer than the length from talon to branching point of seminal vesicle (Fig. 4F); vaginal wall with parallel vaginal folds; vaginal hooks absent (Fig. 5M).
Animal: Live specimens exhibit yellowish reticulated skin, and pale yellowish tentacular retractor muscles are visible through the semi-transparent body (Fig. 2B).
The specific epithet is from the Latin “aquilonaris” meaning “north or northern”. It refers to the distribution range of this new species in northern Thailand.
This species is known from severallimestone areas in northern Thailand. The animals can be found in altitudes up to 200 meters above mean sea level.
Some variation has been observed in the sinulus and the bifid columellar lamella. Populations from Chiangmai and Chiangrai Provinces possess a longer and tapered sinulus (Figs 3G, H). Specimens collected between those two provinces have a shorter sinulus, and specimens from Chiangmai possess a large bifid columellar lamella (Fig. 3H).
urn:lsid:zoobank.org:act:4E8CC516-99E8-4652-9B2A-1796689E2456
http://species-id.net/wiki/Perrottetia_phuphamanensis
Figs 3I–K, Table 1Holotype CUMZ 5011 (Fig. 3I). Measurement: shell height 5.0 mm, shell width 6.9 mm, and with 6¼ whorls. Paratypes NHMUK 20130066 (2 shells), SMF 341488 (2 shells), CUMZ 5012 (14 shells).
Tam Kangkao, Phuphaman, Khonkaen: CUMZ 5013.
Phuphaman National Park, Phuphaman, Khonkaen, Thailand, 16°45'34.0"N, 101°57'50.3"E.
Radula morphology of A Perrottetia dermapyrrhosa sp. n. (paratype CUMZ 5002), and B Perrottetia aquilonaria sp. n. (paratype CUMZ 5004).
Perrottetia concinna (Blanford, 1880) differs from Perrottetia phuphamanensis sp. n. in its smaller shell, higher spire and more distinct suture. The left periphery of the penultimate whorl does not extend beyond the diameter of the last whorl, the aperture is semi-ovate, and a bifid columellar lamella is present. Perrottetia peroteti differs from this new species in its fine transverse ridge at the suture, the smaller second parietal lamella, and the absence of the upper palatal and supracolumellar lamellae.
Perrottetia gudei can be distinguished from Perrottetia phuphamanensis sp. n. by its lower spire, a stronger transverse ridge at the suture, its triangular aperture, the second parietal lamella not being adjacent to the first parietal lamella and the absence of a supracolumellar lamella. Distinguishing features from Perrottetia aquilonaria sp. n.are the smaller shell and the presence of a sinulus. In addition, the left periphery of the penultimate whorl does not extend beyond the diameter of the last whorl, the peristome is thinner, the second parietal lamella is not adjacent to the first parietal lamella, and a bifid columellar and supracolumellar lamellae are both absent.
Shell suboblique-heliciform, white and translucent; whorls 6¼, spire convex, suture distinct; shell surface glossy, with a reduced transverse ridge; embryonic shell large consisting of about 2½ whorls, with smooth surface; following whorls regularly expanding; shell periphery shouldered, last whorl axially deflected; two shallow and short longitudinal furrows present; umbilicus narrow (Fig. 3I); aperture subcircular, peristome discontinuous, very thick and slightly expanded; apertural dentition with a large first parietal lamella and with a second parietal lamella adjoining at right angles; one upper palatal lamella, one palatal lamella, one basal lamella, one large strong columellar lamella, one small supracolumellar lamella (sometimes absent) (Fig. 3K).
The specific epithet is derived from the type locality of this new species, the Phuphaman National Park, Khonkaen Province.
This species is known only from the type locality.
To date no living examples have been found.
Shell measurements of the three new Perrottetia species. Specimen collections and catalogue numbers indicated in parentheses.
| Species and locality and CUMZ nos | No. of specimens | Ranges, mean ± S.D. in mm of: | Number of whorls | |||
|---|---|---|---|---|---|---|
| Shell height | Shell width | H/W ratio | Shell angle | |||
| Perrottetia dermapyrrhosa sp. n. | ||||||
| Wat Tam Namsrithong, Nong Kungsi, Kalasin: (5001, 5002) | 7 | 5.4–6.6 6.2±0.39 |
7.4–8.1 7.7±0.26 |
0.7–0.9 0.8±0.06 |
14.2–28 21.1±5.01 |
6–6½ |
| Perrottetia aquilonaria sp. n. | ||||||
| Wat Tam Pha Plong, Chiangdao, Chiangmai: (5003, 5004) | 5 | 3.7–4.3 4.0±0.23 |
6.3–6.6 6.4±0.13 |
0.6–0.7 0.6±0.04 |
19.8–38.0 27.5±7.59 |
6 |
| Tam Phra Bumpenboon, Phan, Chiangrai: (5005) | 5 | 4.0–4.3 4.2±0.12 |
6.9–7.4 7.2±0.20 |
0.6–0.6 0.6±0.01 |
23.3–34.9 28.7±4.14 |
5½–6 |
| Wat Tam Pha Jaruey, Pa-daet, Chiangrai: (5006) | 6 | 2.9–3.4 3.2±0.17 |
5.9–6.3 6.1±0.15 |
0.5–0.6 0.5±0.02 |
26.4–31.8 29.7±2.26 |
5½–6 |
| Tam Maesuai, Maesuai, Chiangrai: (5007) | 13 | 3.9–4.7 4.3±0.27 |
6.5–7.5 7.1±0.34 |
0.5–0.7 0.6±0.05 |
19.3–37.3 24.6±5.07 |
6 |
| Km 93+200, Tam Chiangdao, Chiangmai: (5008) | 11 | 3.6–4.1 3.7±0.14 |
5.7–6.4 6.1±0.26 |
0.6–0.7 0.6±0.03 |
21.5–29.4 24.9±2.48 |
5½–6 |
| Pha Chu, Nanoi, Nan: (5009) | 21 | 3.4–4.4 3.9±0.31 |
6.0–6.9 6.4±0.26 |
0.5–0.7 0.6±0.05 |
15.6–30.7 21.7±3.98 |
5½–6 |
| Tam Pha Nangkoi, Rongkwang, Phrae: (5010) | 25 | 2.9–3.9 3.3±0.25 |
5.5–6.1 5.7±0.17 |
0.5–0.7 0.6±0.04 |
17.6–35.8 25.7±3.91 |
5½–6 |
| Perrottetia phuphamanensis sp. n. | ||||||
| Phuphaman National Park, Khonkaen: (5011, 5012) | 19 | 4.6–5.6 5.0±0.24 |
6.8–8.1 7.3±0.34 |
0.6–0.8 0.7±0.05 |
15.0–38.8 25.4±5.09 |
6–6½ |
| Tam Kangkao, Phuphaman, Khonkaen: (5013) | 11 | 4.9–5.5 5.1±0.20 |
6.9–7.7 7.2±0.28 |
0.7–0.8 0.7±0.04 |
16.8–31.1 22.6±4.20 |
6–6¼ |
The Streptaxidae were divided into 6 subfamilies and 3 new subfamilies by
Records in the literature show Perrottetia having a tropical distribution in South Asia, Southeast Asia and some parts of East Asia. There is a concentration of 11 species in the Western and Eastern Ghats of peninsular India and two species are recorded from Sri Lanka, one of which is endemic (
Anatomical studies of streptaxids that included internal anatomy were pioneered by
We gratefully extend our thanks to J. Ablett, A. Peiris, D. Raheem, D. Reid, A. Salvador, S. Williams and J. Taylor (NHM, London) for access to material, help in recognizing type material, and useful suggestions. We also thank A. Ball and T. Goral (NHM, London) for guidance and support on SEM and critical point drying.We are also indebted to the Animal Systematics Research Unit Members, Chulalongkorn University for field assistance. This project was funded by the Development and Promotion of Science and Technology talents project (DPST) to TS; The TRF Senior Scholar Grant (2012–2015) RTA 5580001 from the Thailand Research Fund (TRF); The National Research University Project (NRU) FW0646A-56.