Citation: Sánchez-Reyes UJ, Niño-Maldonado S, Jones RW (2014) Diversity and altitudinal distribution of Chrysomelidae (Coleoptera) in Peregrina Canyon, Tamaulipas, Mexico. ZooKeys 417: 103–132. doi: 10.3897/zookeys.417.7551
The Chrysomelidae (Coleoptera) is a highly speciose family that has been poorly studied at the regional level in Mexico. In the present study, we estimated species richness and diversity in oak-pine forest, Tamaulipan thorny scrub and in tropical deciduous forests in Peregrina Canyon within the Altas Cumbres Protected Area of the northeastern state of Tamaulipas, Mexico. Sampling of Chrysomelidae consisted of five sweep net samples (200 net sweeps) within each of three sites during four sample periods: early dry season, late dry season, early wet season, and late wet season. Species were identified and total numbers per species were recorded for each sample. A total of 2, 226 specimens were collected belonging to six subfamilies, 81 genera and 157 species of Chrysomelidae from the study area. Galerucinae was the most abundant subfamily with 1, 828 specimens, representing 82.1% of total abundance in the study area. Lower abundance was recorded in Cassidinae (8.5%), Eumolpinae (3.6%), Cryptocephalinae (2.2%), Chrysomelinae (2.2%), and finally Criocerinae (1.3%). The highest species richness was also presented in the subfamily Galerucinae with 49% of the total obtained species followed by Cassidinae (20%), Cryptocephalinae (9.7%), Eumolpinae (9.7%), Chrysomelinae (6.5%) and Criocerinae (5.2%). The most common species were Centralaphthona fulvipennis Jacoby (412 individuals), Centralaphthona diversa (Baly) (248), Margaridisa sp.1 (219), Acallepitrix sp.1 (134), Longitarsus sp.1 (104), Heterispa vinula (Erichson) (91), Epitrix sp.1 (84) and Chaetocnema sp.1 (72). Twenty-two species were doubletons (1.97% of total abundance) and 52 were singletons (2.33%). The estimated overall density value obtained was 0.0037 individuals/m2. The greatest abundance and density of individuals were recorded at the lowest elevation site. However, alpha diversity increased with increasing altitude. Similarity values were less than 50% among the three sites indicating that each site had distinct species assemblages of Chrysomelidae. The highest abundance was obtained during the late dry season, whereas diversity indices were highest during the early wet season. The present work represents the first report of the altitudinal variation in richness, abundance, and diversity of Chrysomelidae in Mexico. These results highlight the importance of conservation of this heterogeneous habitat and establish baseline data for Chrysomelidae richness and diversity for the region.
Chrysomelidae, leaf beetles, species richness, abundance, altitude, Northeast Mexico
Chrysomelidae is one of the largest families within the order Coleoptera, with over 35, 000 species described worldwide (
The great species richness of Chrysomelidae and their role as a phytophagous functional group make the Chrysomelidae a potentially useful indicator group for: 1) biodiversity of a region (
Recent climatic and environmental changes create an ecological imbalance that threatens biodiversity. It is vital that baseline data is available through faunistic inventories along elevational gradients to record and predict how organisms alter distributions and adapt to environmental changes (
The present study was conducted in the Cañon of the Peregrina within Altas Cumbres Protected Area (
The objectives of the present study were: 1) determine the species richness of Chrysomelidae in Peregrina Canyon, Tamaulipas, Mexico; 2) conduct the first site-specific evaluation of diversity for this taxon in northeast Mexico; and 3) analyze the variation of species richness, abundance and diversity of the family along an altitudinal gradient during different seasons within the study area.
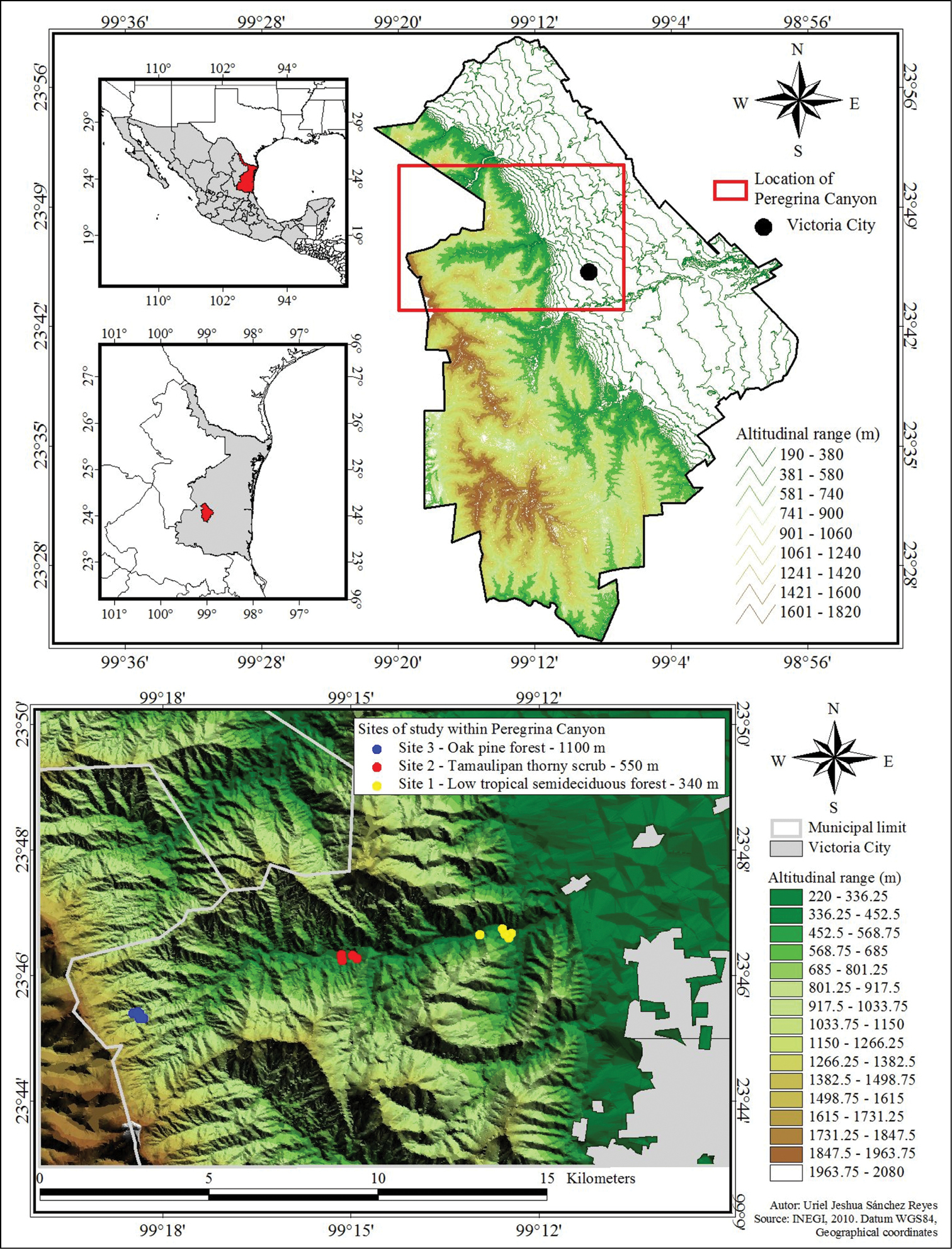
The Peregrina Canyon (Canyon San Felipe or Liberty), is located in the northwest portion of municipality of Victoria, Tamaulipas, along the San Felipe River (Figure 1). The area is located in the Sierra Madre Oriental and is part of Altas Cumbres Protected Area, considered a Special Zone subject to Ecological Conservation established by state decree in 1997 (
Location of Peregrina Canyon in Tamaulipas, Mexico, and location of sampling sites along study area.
Three sites were established within which five quadrants of 2500 m2 (50×50 m) were delineated in representative vegetation at each site. Site 1 had the lowest elevation at 340 m and consisted of low tropical semideciduous forest (23°45.30'N, 99°18.39'W). Site 2 was located at an intermediate altitude at 550 m where the plant community consisted of Tamaulipan thorny scrub (23°46.32'N, 99°14.96'W). Site 3 was the highest site at 1100 m with the vegetation composed of oak-pine forest (23°46.62'N, 99°12.55'W).
Sampling was conducted using a standard entomological sweep net of 40 cm diameter. Individual samples consisted of 200 sweeps of the shrub and herbaceous vegetation in each quadrant. The contents of the net were emptied into a 2000 cm3 plastic bag, adding 60% ethanol and an indelible label with corresponding data. Samples were collected at each site from 10:00 to 14:00 hrs. Five samples (one sample for each quadrant, 200 net sweeps) were taken within each of three sites (one site per day), at four different dates in each of the four seasons of the year (early dry season, EDR, December-February; late dry season, LDS, March-May; early wet season, EWS, June-August; and late wet season, LWS, September-November) between January and December 2009, for a total of 240 samples.
Processing of the samples was performed in the laboratory in the following manner. First, the contents of each plastic bag (sample) were placed in a plastic tray with water, and the more voluminous plant remains (wood fragments, branches, stems, leaves) were removed. A sieve ALSA (0.175 mm) was then used to filter the sample, and the reduced contents placed in a petri dish and observed under a stereoscopic microscope for extraction of all chrysomelid beetles. These were separated and mounted on paper points according to standard entomological technique. All specimens are stored in the collection of the Facultad de Ingeniería y Ciencias at the Universidad Autónoma de Tamaulipas, Ciudad Victoria, Tamaulipas, Mexico.
The identification of the specimens was performed using the available literature on Chrysomelidae (
Abundance was calculated using the number of individuals per species collected at each site, season and for the entire study area. Species abundance was divided into five categories: 1) very common (more than 70 individuals); 2) common (11 to 70); 3) rare (10 to three specimens); 4) doubletons (two specimens); and 5) singletons (one specimen only). As a measure of species richness, we used the number of species present throughout the Peregrina Canyon, in each of the three altitudinal strata analyzed, and in each season. To estimate the potential number of species (total, site, and season), the nonparametric estimators Chao 1 and Jackknife 1 were used. These estimators were chosen because: 1) we did not assume a previous abundance distribution model, 2) they are robust when calculating minimum estimate of species richness, 3) their use is recommended as a recurrent measure in analysis of biodiversity, 4) Chao 1 is based on abundance data, or singletons and doubletons, and Jackknife 1 (incidence) is based on uniques, or species found in only one sample (
After testing for normality of the data, we used the nonparametric Kruskall Wallis and Mann-Whitney tests to analyze the differences in abundance and number of species among the three sites and between different seasons (PAST version 1.94b,
Alpha diversity for the whole study area and by site and season was calculated using the Simpson diversity index (1/D) and the Shannon diversity index (H ‘) (
A total of 2, 226 specimens of Chrysomelidae were collected from 240 samples from May 2009 to April 2010, belonging to six subfamilies, 81 genera and 157 species (Table 1). Galerucinae was the most abundant subfamily with 1, 828 specimens, representing 82.1% of total abundance in the study area. Lower abundance was recorded in Cassidinae (8.5%), Eumolpinae (3.6%), Cryptocephalinae (2.2%), Chrysomelinae (2.2%), and finally Criocerinae (1.3%). The highest species richness was also presented in the subfamily Galerucinae with 49% of the total obtained species followed by Cassidinae (20%), Cryptocephalinae (9.7%), Eumolpinae (9.7%), Chrysomelinae (6.5%) and Criocerinae (5.2%).
Taxonomic list and abundance of Chrysomelidae by season and site in Peregrina Canyon, Tamaulipas, Mexico. N = Total abundance; 1 = Low tropical semideciduous forest, 340 m; 2 = Tamaulipan thorny scrub, 550 m; 3 = Oak-pine forest, 1100 m.
| Dry Season | Wet Season | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early (Dec-Feb) |
Late (Mar-May) |
Early (Jun-Aug) |
Late (Sep-Nov) |
N | |||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| CASSIDINAE Gyllenhal, 1813 | |||||||||||||
| Tribu Chalepini Weise, 1910 | |||||||||||||
| Anisostena pilatei (Baly, 1864) | 1 | 1 | 1 | 3 | |||||||||
| Brachycoryna pumila Guérin-Méneville, 1844 | 1 | 3 | 4 | 1 | 5 | 14 | |||||||
| Chalepusbellulus (Chapuis, 1877) | 5 | ||||||||||||
| Chalepus digressus Baly, 1885 | 1 | 1 | |||||||||||
| Euprionota aterrima Guérin-Méneville, 1844 | 1 | 1 | |||||||||||
| Glyphuroplata sp. 1 | 1 | 1 | |||||||||||
| Heterispavinula (Erichson, 1847) | 1 | 6 | 1 | 20 | 8 | 1 | 29 | 1 | 10 | 12 | 2 | 91 | |
| Octotoma championi Baly, 1885 | 2 | 2 | |||||||||||
| Octotoma intermedia Staines, 1989 | 1 | 1 | |||||||||||
| Sumitrosis inaequalis (Weber, 1801) | 2 | 2 | 1 | 5 | |||||||||
| Sumitrosis pallescens (Baly, 1885) | 1 | 1 | |||||||||||
| Sumitrosisrosea (Weber, 1801) | 1 | 1 | 1 | 3 | 2 | 8 | |||||||
| Sumitrosis sp. 1 | 1 | 1 | 2 | ||||||||||
| Sumitrosis sp. 2 | 1 | 1 | |||||||||||
| Xenochalepus (Neochalepus) chapuisi (Baly, 1885) | 1 | 1 | |||||||||||
| Xenochalepus (Xenochalepus) omogerus (Crotch, 1873) | 1 | 1 | 2 | ||||||||||
| Tribu Cassidini Gyllenhal, 1813 | |||||||||||||
| Charidotella bifossulata (Boheman, 1855) | 1 | 3 | 1 | 5 | |||||||||
| Charidotella (Chaerocassis) emarginata (Boheman, 1855) | 1 | 1 | |||||||||||
| Charidotella sexpunctata (Fabricius, 1781) | 3 | 1 | 4 | ||||||||||
| Charidotella tuberculata (Fabricius, 1775) | 2 | 2 | |||||||||||
| Charidotis auroguttata Boheman, 1855 | 1 | 1 | |||||||||||
| Tribu Cassidini Gyllenhal, 1813 | |||||||||||||
| Charidotellatuberculata (Fabricius, 1775) | 2 | 2 | |||||||||||
| Charidotis auroguttata Boheman, 1855 | 1 | 1 | |||||||||||
| Coptocycla (Psalidonota) texana (Schaeffer, 1933) | 2 | 2 | |||||||||||
| Microctenochirapunicea (Boheman, 1855) | 1 | 2 | 2 | 5 | |||||||||
| Microctenochira varicornis (Spaeth, 1926) | 1 | 1 | |||||||||||
| Microctenochiravivida (Boheman, 1855) | 1 | 1 | 2 | ||||||||||
| Helocassis crucipennis (Boheman, 1855) | 9 | 3 | 1 | 1 | 14 | ||||||||
| Helocassis testudinaria (Boheman, 1855) | 1 | 1 | 1 | 2 | 5 | ||||||||
| Tribu Mesomphaliini Hope, 1840 | |||||||||||||
| Chelymorpha pubescens Boheman, 1854 | 1 | 1 | |||||||||||
| Hilarocassis exclamationis (Linnaeus, 1767) | 1 | 1 | |||||||||||
| Ogdoecosta juvenca (Boheman, 1854) | 3 | 1 | 2 | 6 | |||||||||
| Tribu Ischyrosonychini Chapuis, 1875 | |||||||||||||
| Physonota alutacea Boheman, 1854 | 1 | 1 | |||||||||||
| CHRYSOMELINAE Latreille, 1802 | |||||||||||||
| Tribu Chrysomelini Latreille, 1802 | |||||||||||||
| Subtribu Doryphorina Motschulsky, 1860 | |||||||||||||
| Calligrapha fulvipes Stål, 1859 | 1 | 1 | |||||||||||
| Calligrapha sp. 1 | 1 | 1 | |||||||||||
| Calligrapha sp. 2 | 2 | 2 | |||||||||||
| Calligrapha suffriani Jacoby, 1882 | 1 | 1 | |||||||||||
| Labidomera suturella Chevrolat, 1844 | 1 | 3 | 3 | 7 | |||||||||
| Zygogramma piceicollis (Stål, 1859) | 1 | 1 | |||||||||||
| Subtribu Chrysomelina Latreille, 1802 | |||||||||||||
| Chrysomela texana (Schaeffer, 1919) | 2 | 3 | 5 | ||||||||||
| Phaedon cyanescens Stål, 1860 | 1 | 1 | |||||||||||
| Plagiodera semivittata Stål, 1860 | 3 | 3 | 1 | 1 | 2 | 2 | 12 | ||||||
| Plagiodera thymaloides Stål, 1860 | 1 | 2 | 5 | 1 | 9 | 18 | |||||||
| CRIOCERINAE Latreille, 1807 | |||||||||||||
| Tribu Lemini Heinze, 1962 | |||||||||||||
| Lema balteata LeConte, 1884 | 1 | 1 | |||||||||||
| Lema sp. 1 | 2 | 1 | 1 | 4 | |||||||||
| Neolema quadriguttata White, 1993 | 2 | 1 | 1 | 4 | |||||||||
| Neolema sp. 1 | 1 | 1 | |||||||||||
| Neolema sp. 2 | 1 | 1 | 2 | ||||||||||
| Neolema sp. 3 | 2 | 1 | 1 | 4 | |||||||||
| Oulema sp. 1 | 4 | 3 | 2 | 3 | 12 | ||||||||
| Oulema sp. 2 | 1 | 1 | |||||||||||
| CRYPTOCEPHALINAE Gyllenhal, 1813 | |||||||||||||
| Tribu Cryptocephalini Gyllenhal, 1813 | |||||||||||||
| Subtribu Cryptocephalina Gyllenhal, 1813 | |||||||||||||
| Cryptocephalus duryi Schaeffer, 1906 | 1 | 2 | 3 | ||||||||||
| Cryptocephalus sp. 1 | 1 | 1 | |||||||||||
| Cryptocephalusumbonatus Schaeffer, 1906 | 2 | 1 | 1 | 4 | |||||||||
| Diachus auratus (Fabricius, 1801) | 5 | 8 | 2 | 15 | |||||||||
| Subtribu Pachybrachina Chapuis, 1874 | |||||||||||||
| Pachybrachis sp. 1 | 1 | 1 | 2 | ||||||||||
| Pachybrachis sp. 2 | 1 | 1 | 2 | ||||||||||
| Pachybrachis sp. 3 | 1 | 1 | 1 | 1 | 4 | ||||||||
| Pachybrachis sp. 4 | 2 | 1 | 3 | ||||||||||
| Pachybrachis sp. 5 | 2 | 2 | |||||||||||
| Pachybrachis sp. 6 | 1 | 1 | |||||||||||
| Pachybrachis sp. 7 | 1 | 1 | 2 | ||||||||||
| Tribu Clytrini Lacordaire, 1848 | |||||||||||||
| Subtribu Clytrina Lacordaire, 1848 | |||||||||||||
| Anomoea rufifrons Chevrolat, 1837 | 1 | 4 | 5 | ||||||||||
| Subtribu Megalostomina Chapuis, 1874 | |||||||||||||
| Coscinoptera scapularis scapularis (Lacordaire, 1848) | 1 | 1 | |||||||||||
| Coscinopteravictoriana L. Medvedev, 2012 | 1 | 1 | |||||||||||
| Subtribu Babiina Chapuis, 1874 | |||||||||||||
| Babia tetraspilota LeConte, 1858 | 2 | 2 | |||||||||||
| Tribu Chlamisini Gressitt, 1946 | |||||||||||||
| Chlamisus texanus (Schaeffer, 1906) | 3 | 3 | |||||||||||
| Neochlamisus sp. 1 | 1 | 1 | |||||||||||
| EUMOLPINAE Hope, 1840 | |||||||||||||
| Tribu Eumolpini Hope, 1840 | |||||||||||||
| Brachypnoea sp. 1 | 9 | 9 | 18 | ||||||||||
| Brachypnoea sp. 2 | 9 | 2 | 2 | 1 | 14 | ||||||||
| Chalcophana cincta Harold, 1874 | 1 | 4 | 5 | ||||||||||
| Colaspis melancholica Jacoby, 1881 | 1 | 1 | |||||||||||
| Colaspis sp. 1 | 1 | 1 | |||||||||||
| Colaspistownsendi Bowditch, 1921 | 4 | 4 | |||||||||||
| Tymnes sp. 1 | 1 | 1 | 1 | 3 | |||||||||
| Zenocolaspis inconstans Bechyné, 1997 | 3 | 3 | |||||||||||
| Tribu Adoxini Baly, 1863 | |||||||||||||
| Fidia albovittata Lefèvre, 1877 | 1 | 1 | |||||||||||
| Xanthonia sp. 1 | 1 | 5 | 1 | 3 | 1 | 11 | |||||||
| Xanthonia sp. 2 | 3 | 3 | |||||||||||
| Xanthonia sp. 3 | 1 | 4 | 5 | ||||||||||
| Xanthonia sp. 4 | 1 | 7 | 8 | ||||||||||
| Xanthonia sp. 5 | 1 | 1 | 2 | ||||||||||
| Tribu Typophorini Chapuis, 1874 | |||||||||||||
| Typophorusnigritus (Fabricius, 1801) | 1 | 1 | |||||||||||
| GALERUCINAE Latreille, 1802 | |||||||||||||
| Tribu Alticini Newman, 1835 | |||||||||||||
| Acallepitrix sp. 1 | 2 | 35 | 8 | 82 | 1 | 2 | 3 | 1 | 134 | ||||
| Acallepitrix sp. 2 | 1 | 1 | 15 | 1 | 3 | 2 | 3 | 26 | |||||
| Acallepitrix sp. 3 | 3 | 3 | 1 | 2 | 3 | 2 | 5 | 4 | 2 | 25 | |||
| Acallepitrix sp. 4 | 4 | 4 | 8 | ||||||||||
| Acallepitrix sp. 5 | 1 | 1 | |||||||||||
| Acrocyum dorsalis Jacoby, 1885 | 1 | 1 | |||||||||||
| Alagoasa bipunctata (Chevrolat, 1834) | 1 | 1 | 1 | 3 | |||||||||
| Alagoasa decemguttatus (Fabricius, 1801) | 1 | 2 | 1 | 4 | 1 | 1 | 10 | ||||||
| Alagoasa sp. 1 | 1 | 4 | 5 | ||||||||||
| Asphaera abdominalis (Chevrolat, 1834) | 8 | 2 | 2 | 12 | |||||||||
| Asphaera sp. 1 | 13 | 1 | 14 | ||||||||||
| Asphaera sp. 2 | 1 | 1 | |||||||||||
| Asphaera sp. 3 | 1 | 1 | |||||||||||
| Asphaera sp. 4 | 1 | 1 | |||||||||||
| Blepharida rhois (Forster, 1771) | 1 | 1 | |||||||||||
| Centralaphthona diversa (Baly, 1877) | 6 | 80 | 6 | 1 | 52 | 30 | 14 | 33 | 25 | 1 | 248 | ||
| Centralaphthona fulvipennis (Jacoby, 1885) | 309 | 1 | 42 | 3 | 56 | 1 | 412 | ||||||
| Chaetocnema sp. 1 | 27 | 12 | 12 | 9 | 9 | 2 | 1 | 72 | |||||
| Chaetocnema sp. 2 | 1 | 1 | 1 | 5 | 8 | ||||||||
| Chaetocnema sp. 3 | 5 | 5 | 2 | 1 | 7 | 3 | 2 | 25 | |||||
| Derocrepis sp. 1 | 5 | 5 | |||||||||||
| Derocrepis sp. 2 | 2 | 2 | |||||||||||
| Disonycha antennata Jacoby, 1884 | 1 | 1 | |||||||||||
| Disonycha glabrata (Fabricius, 1781) | 5 | 1 | 1 | 1 | 8 | ||||||||
| Tribu Alticini cont | |||||||||||||
| Disonycha stenosticha Schaeffer, 1931 | 1 | 1 | 2 | ||||||||||
| Epitrix sp. 1 | 20 | 7 | 15 | 9 | 7 | 1 | 20 | 2 | 3 | 84 | |||
| Epitrix fasciata Blatchley, 1918 | 2 | 2 | 1 | 2 | 7 | ||||||||
| Epitrix sp. 3 | 2 | 2 | 1 | 7 | 12 | ||||||||
| Glenidion sp.1 | 1 | 1 | 2 | ||||||||||
| Heikertingerella sp. 1 | 1 | 1 | 2 | ||||||||||
| Heikertingerella sp. 2 | 4 | 4 | |||||||||||
| Heikertingerella variabilis (Jacoby, 1885) | 4 | 1 | 1 | 1 | 7 | ||||||||
| Longitarsus sp. 1 | 8 | 36 | 25 | 14 | 8 | 2 | 9 | 2 | 104 | ||||
| Longitarsus sp. 2 | 1 | 1 | |||||||||||
| Longitarsus sp. 3 | 1 | 1 | 2 | ||||||||||
| Longitarsus sp. 4 | 1 | 1 | |||||||||||
| Longitarsus sp. 5 | 1 | 2 | 8 | 8 | 2 | 21 | |||||||
| Lupraea sp. 1 | 1 | 13 | 18 | 32 | |||||||||
| Lupraea sp. 2 | 8 | 8 | |||||||||||
| Lupraea sp. 3 | 16 | 1 | 7 | 24 | |||||||||
| Lupraea sp. 4 | 26 | 6 | 1 | 33 | |||||||||
| Lysathia sp. 1 | 1 | 1 | |||||||||||
| Margaridisa sp. 1 | 52 | 2 | 3 | 61 | 27 | 37 | 5 | 29 | 3 | 219 | |||
| Margaridisa sp. 2 | 1 | 1 | 1 | 3 | 6 | ||||||||
| Monomacra sp. 1 | 5 | 4 | 2 | 5 | 3 | 2 | 5 | 3 | 4 | 33 | |||
| Monomacra sp. 2 | 6 | 6 | |||||||||||
| Monomacra sp. 3 | 1 | 1 | |||||||||||
| Omophoita cyanipennis octomaculata (Crotch, 1873) | 4 | 2 | 6 | ||||||||||
| Orthaltica sp. 1 | 5 | 5 | |||||||||||
| Orthaltica sp. 2 | 1 | 1 | |||||||||||
| Parchicola sp. 1 | 2 | 1 | 3 | ||||||||||
| Phyllotreta sp. 1 | 3 | 1 | 2 | 1 | 1 | 8 | |||||||
| Plectrotetra sp. 1 | 3 | 13 | 2 | 18 | |||||||||
| Scelidopsis rufofemorata Jacoby, 1888 | 1 | 1 | |||||||||||
| Tribu Alticini cont | |||||||||||||
| Sphaeronychusfulvus (Baly, 1879) | 1 | 1 | 2 | 1 | 5 | ||||||||
| Strabala sp. 1 | 1 | 1 | |||||||||||
| Syphrea sp. 1 | 2 | 10 | 8 | 1 | 1 | 22 | |||||||
| Syphrea sp. 2 | 1 | 2 | 6 | 1 | 1 | 1 | 1 | 13 | |||||
| Systena contigua Jacoby, 1884 | 1 | 19 | 7 | 27 | |||||||||
| Systena sp. 1 | 1 | 1 | |||||||||||
| Walterianella signata (Jacoby, 1886) | 2 | 1 | 3 | ||||||||||
| Tribu Galerucini Latreille, 1802 | |||||||||||||
| Grupo Coelomerites Chapuis, 1875 | |||||||||||||
| Coraiasubcyanescens (Schaeffer, 1906) | 1 | 1 | 2 | 1 | 5 | ||||||||
| Derospidea cyaneomaculata (Jacoby, 1886) | 1 | 1 | |||||||||||
| Trirhabda sp. 1 | 1 | 1 | |||||||||||
| Grupo Schematizites Chapuis, 1875 | |||||||||||||
| Ophraearugosa Jacoby, 1886 | 1 | 1 | 2 | ||||||||||
| Tribu Luperini Chapuis, 1875 | |||||||||||||
| Subtribu Diabroticina Chapuis, 1875 | |||||||||||||
| Grupo Diabroticites Chapuis, 1875 | |||||||||||||
| Acalymma vittatum (Fabricius, 1775) | 1 | 1 | 2 | ||||||||||
| Diabroticabalteata LeConte, 1865 | 1 | 1 | 1 | 3 | |||||||||
| Diabrotica porracea Harold, 1875 | 1 | 1 | |||||||||||
| Diabroticaunderwoodi Bowditch, 1911 | 1 | 7 | 2 | 10 | |||||||||
| Gynandrobrotica lepida (Say, 1835) | 5 | 1 | 13 | 9 | 5 | 1 | 4 | 1 | 1 | 40 | |||
| Grupo Cerotomites Chapuis, 1875 | |||||||||||||
| Cerotoma atrofasciata Jacoby, 1879 | 1 | 1 | |||||||||||
| Cerotoma ruficornis (Olivier, 1791) | 1 | 1 | |||||||||||
| Cyclotrypemafurcata (Olivier, 1808) | 3 | 4 | 7 | ||||||||||
| Neobrotica sexmaculata Jacoby, 1887 | 1 | 1 | |||||||||||
| Neobrotica tampicensis Blake, 1966 | 2 | 2 | |||||||||||
| Subtribu Luperina Chapuis, 1875 | |||||||||||||
| Grupo Monoleptites Chapuis, 1875 | |||||||||||||
| Calomicrus sp. 1 | 1 | 1 | |||||||||||
Eight species were categorized as “very common” in the Peregrina Canyon, each with greater than 70 specimens and accounted for 61.22% of the total abundance. These very common species were Centralaphthona fulvipennis Jacoby (412 individuals), Centralaphthona diversa (Baly) (248), Margaridisa sp.1 (219), Acallepitrix sp.1 (134), Longitarsus sp.1 (104), Heterispa vinula (Erichson) (91), Epitrix sp.1 (84) and Chaetocnema sp.1 (72). Twenty-five species were considered common, constituting 22.66% of the total number of chrysomelids. Fifty species were considered rare (263 specimens) by occupying 11.8% of the total abundance. Twenty-two species were doubletons (1.97% of total abundance) and 52 were singletons (2.33%). The estimated density value obtained was 0.0037 individuals/m2 (Table 2).
Richness, abundance and diversity parameters of Chrysomelidae in the Peregrina Canyon, Tamaulipas, Mexico. S obs = Observed richness; N = Abundance; Dst = Density; S est = Estimated richness; R2 = Clench model determination coefficient; 1/D = Simpson diversity index; H´= Shannon diversity index.
| Ecological Parameter | Site | Season | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Low tropical semideciduous forest | Tamaulipan thorny scrub | Oak-pine forest | Dry | Wet | ||||
| Early (Dec-Feb) |
Late (Mar-May) |
Early (Jun-Jul) | Late (Aug-Nov) |
|||||
| S obs |
85a | 96ab | 84b | 43c | 96a | 84b | 56bc | 157 |
| N |
1123a | 641b | 464c | 696c | 822a | 304b | 406bc | 2228 |
| Dst | 0.0056 | 0.0032 | 0.0023 | 0.0046 | 0.0054 | 0.002 | 0.0027 | 0.0037 |
| S est | ||||||||
| Chao 1 | 119.13 | 196.04 | 140.89 | 49.05 | 132 | 134.7 | 84.41 | 218.45 |
| Jackknife 1 | 126.48 | 150.31 | 123.5 | 55.78 | 137.3 | 130.22 | 84.52 | 216.75 |
| Clench | ||||||||
| R2 | 0.997 | 0.998 | 0.999 | 0.998 | 0.998 | 0.999 | 0.999 | 0.997 |
| S est | 128.49 | 171.80 | 133.91 | 57.70 | 143.46 | 175.59 | 94.85 | 212.41 |
| Slope | 0.368 | 0.532 | 0.395 | 0.187 | 0.539 | 0.729 | 0.385 | 0.178 |
| Diversity | ||||||||
| 1/D | 5.95a | 10.12b | 19.26c | 4.28d | 17.31a | 24.66b | 13.92c | 14.6 |
| H’ | 2.73a | 3.24b | 3.67c | 2.17d | 3.49a | 3.78b | 3.06c | 3.54 |
† Values with different letters within rows are significantly different using Kruskal-Wallis and Mann-Whitney Tests: abundance between sites, K=15.92, DF=2, p=0.0003; richness between sites, K=8.17, DF=2, p=0.0157; abundance between seasons, K=42.42, DF=3, p=0.000; richness between seasons, K=50.15, DF=3, p=0.000.
‡ Diversity values with different letters within rows are significantly different at p<0.05, using permutation and bootstrap tests in PAST program.
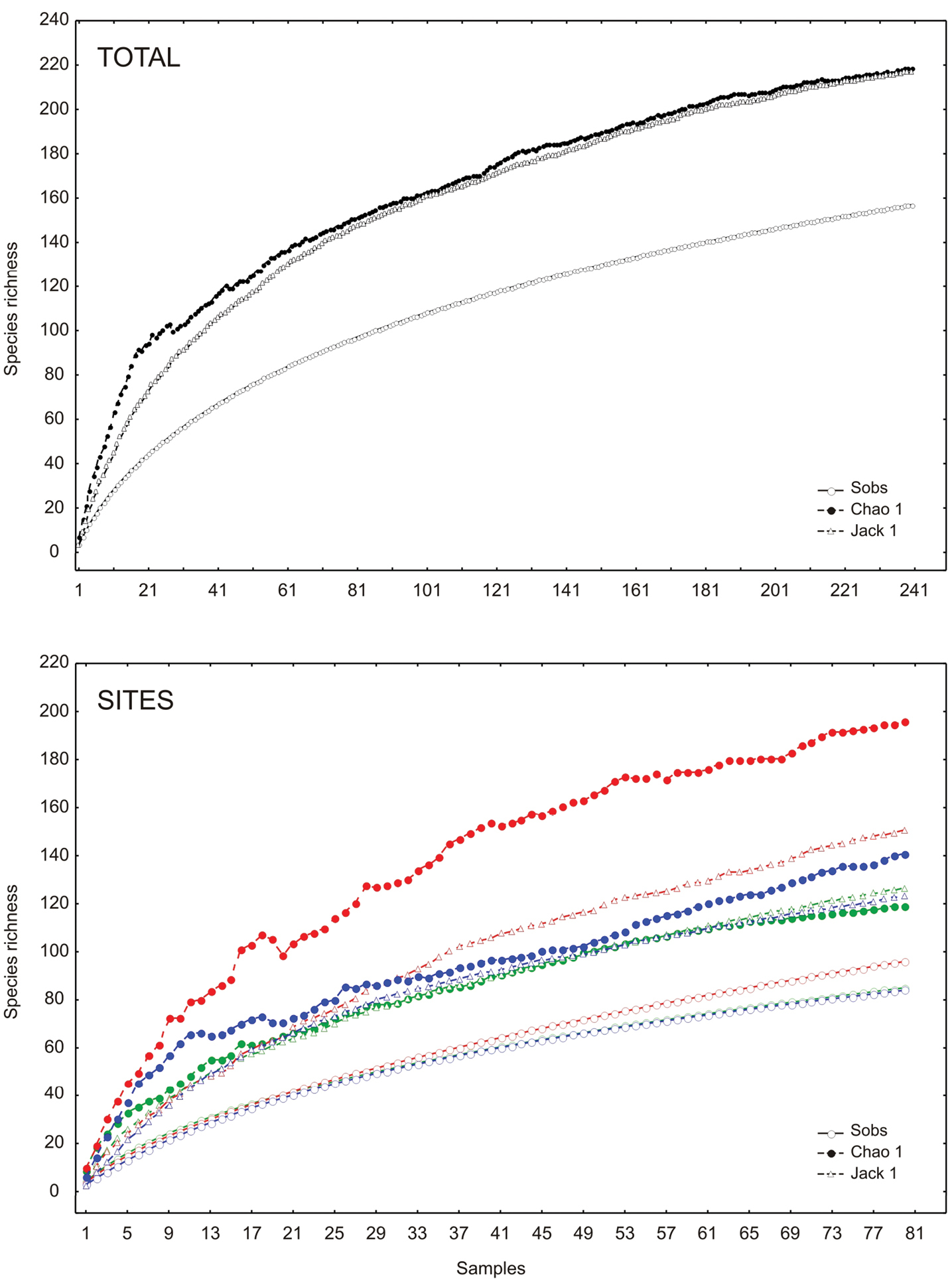
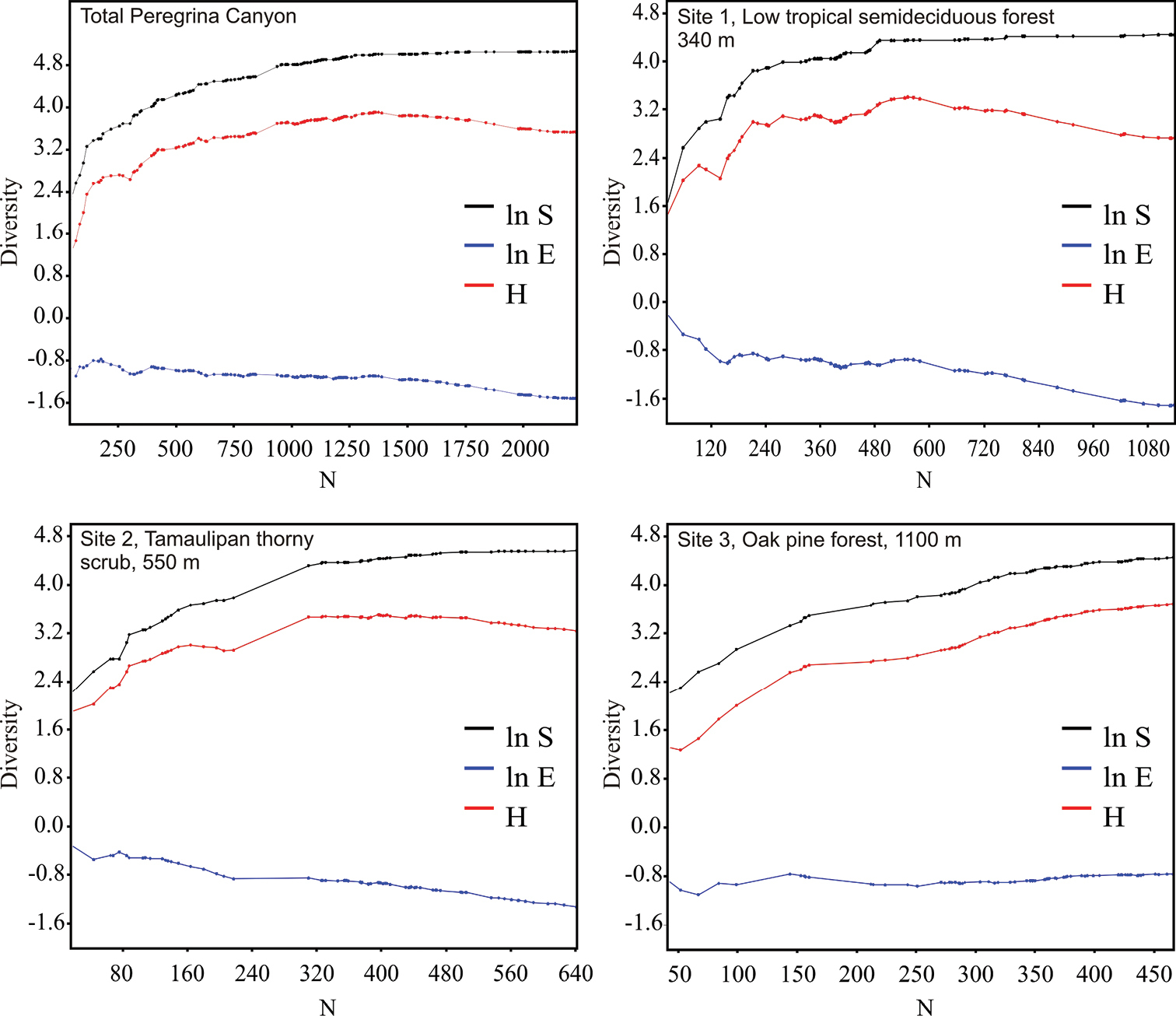
The richness estimators indicated that the total number of chrysomelid species in the study area was between 216 and 218 species (Table 2, Figure 2) suggesting that the observed total of 157 species represented 71.86 to 72.43% of the actual richness. The data showed a good fit to the Clench model (R2 = 0.99), with a registered proportion of species of 73.91% and a slope close to 0.1. Total diversity values of Chrysomelidae in Peregrina Canyon were 14.58 for the Simpson index and 3.53 for the Shannon index (Table 2). The SHE analysis shows that changes in Shannon diversity value are attributed to increase and stability of species richness curve (Figure 3).
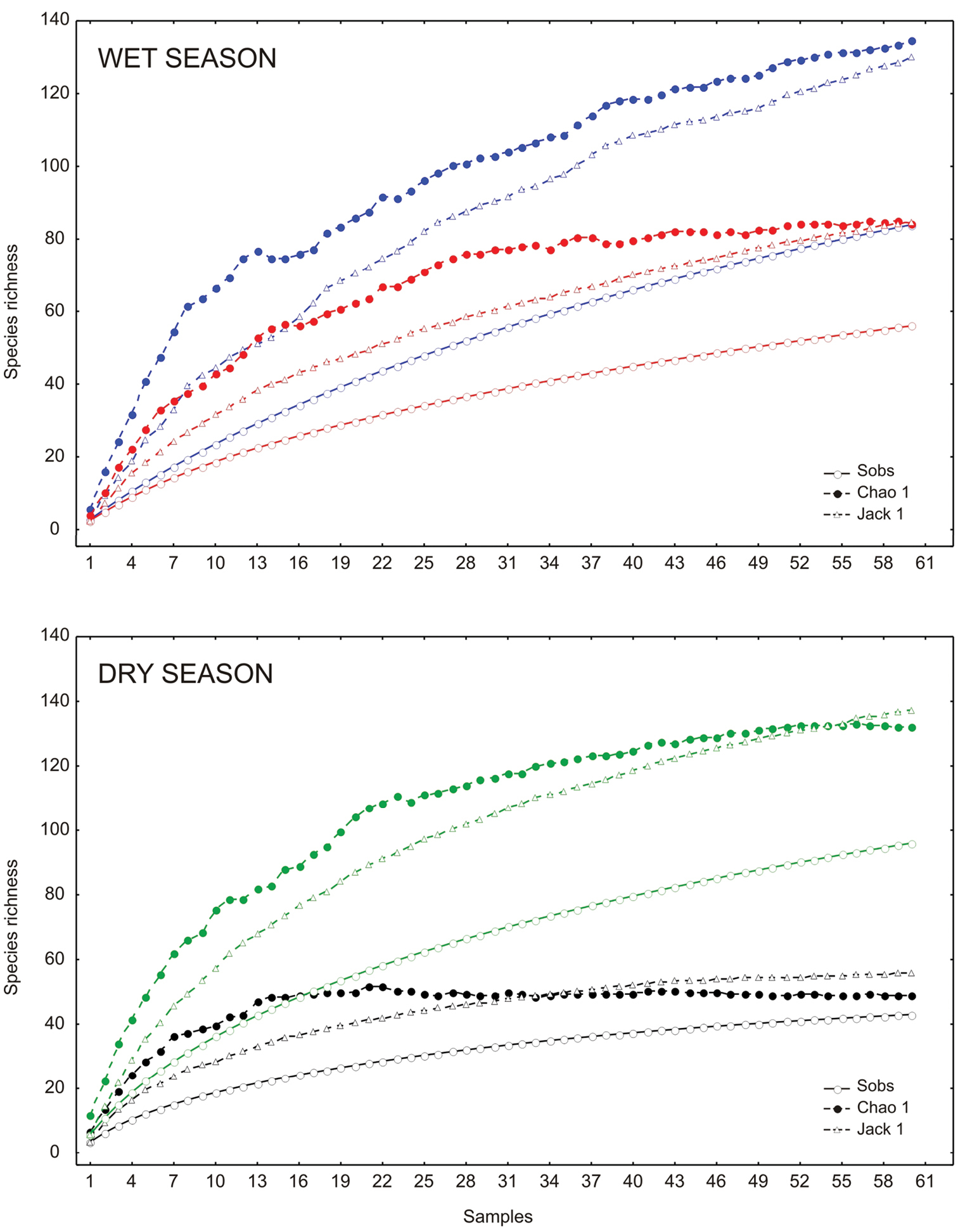
Species accumulation curves by altitudinal site in the Peregrina Canyon, Tamaulipas, Mexico. Upper graphic: accumulation curves for all study area. Lower graphic: site 1 (green color), site 2 (red color) and site 3 (blue color).
SHE analysis of diversity for the Peregrina Canyon and for each one of altitudinal sites. ln S natural logarithm of species richness; ln E natural logarithm of evenness; H diversity (Shannon index).
The abundance of chrysomelid beetles was significantly different among the three sites (Table 2). The greatest abundance and density (individuals per square meter) were recorded at the lowest elevation site, and decreased with increasing altitude (Table 2). The middle altitude site (Site 2) had the greatest number of species (Table 2). The number of species significantly differed only between the lowest and the highest altitudinal strata (Site 1 and Site 3; Table 2). In the low altitude site, 85 species were recorded which represented between 67.2 to 71.35% of the estimated richness (minimum and maximum) with the models used. In the second site, the number increased to 96 species (48.96 to 63.86% of the estimate) and at the highest site, 84 species were recorded (59.62 to 68.01% of the estimate) (Figure 2). A determination coefficient greater than 0.99 was obtained for all sites, indicating a good fit of the Clench model to the data obtained at each site, but the slope calculated was greater than 0.1 in all sites (Table 2).
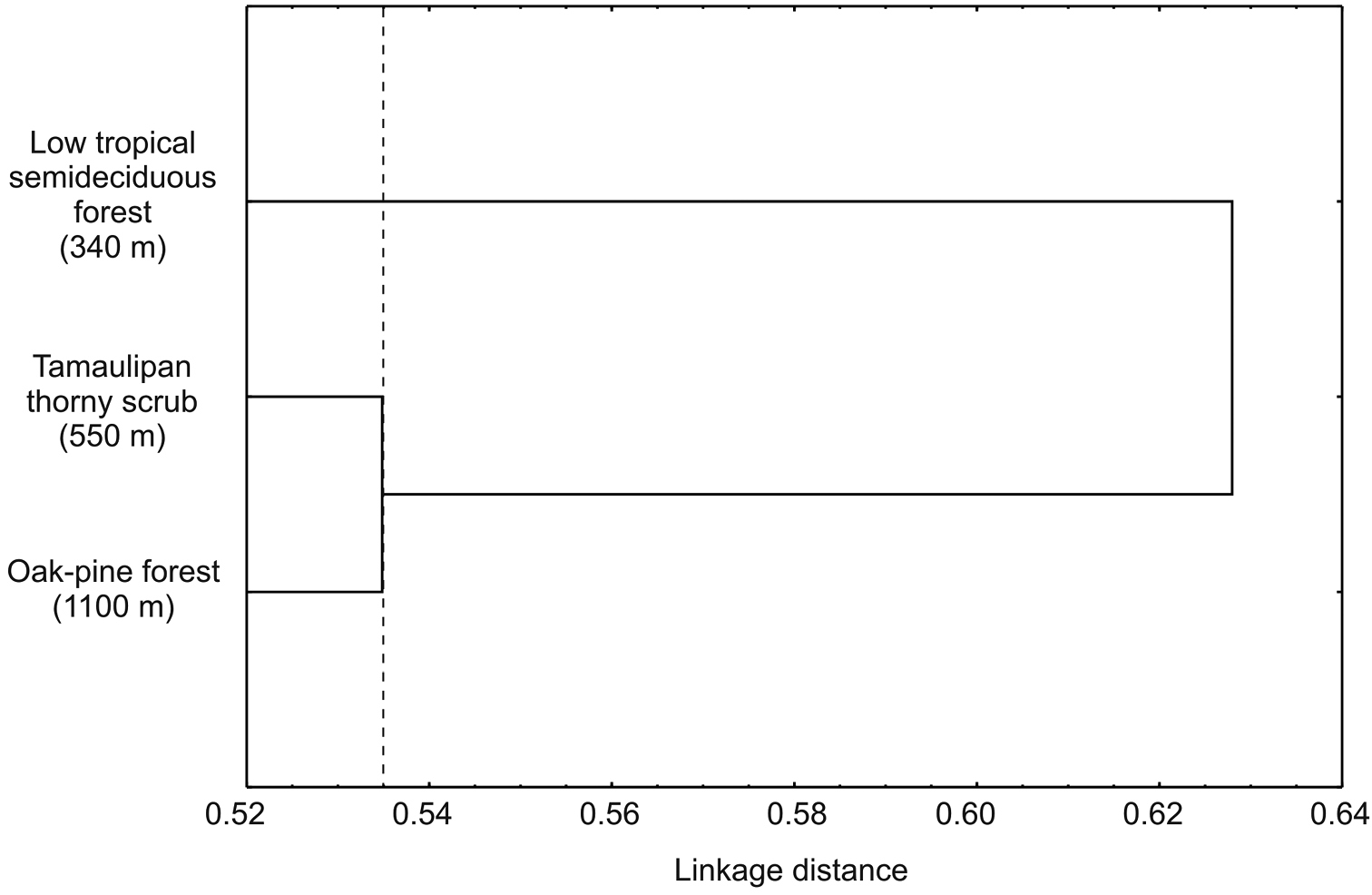
Alpha diversity at the three sites differed significantly (p < 0.05) with indices increasing progressively with increasing altitude (Table 2). Lower diversity values in both sites 1 and 2, were a result of a reduction in eveness and a more or less stable number of species with the increase of samples. In site 3, diversity increases as eveness remained constant and the number of species increased with sample numbers (Figure 3). Of the 157 species recorded in the Peregrina Canyon, 34 were distributed along the entire altitudinal gradient, 40 were recorded only in two sites, and 83 were unique to one of the three sites. Of these, 29 were exclusively from Site 1, 34 for Site 2, and 20 for Site 3 (Table 1). Similarity values were in all cases less than 50%; according to the cluster analysis, each of the three sites was an independent group, containing distinct species assemblages of Chrysomelidae (Figure 4).
Cluster analysis from sites in the Peregrina Canyon, Tamaulipas, Mexico.
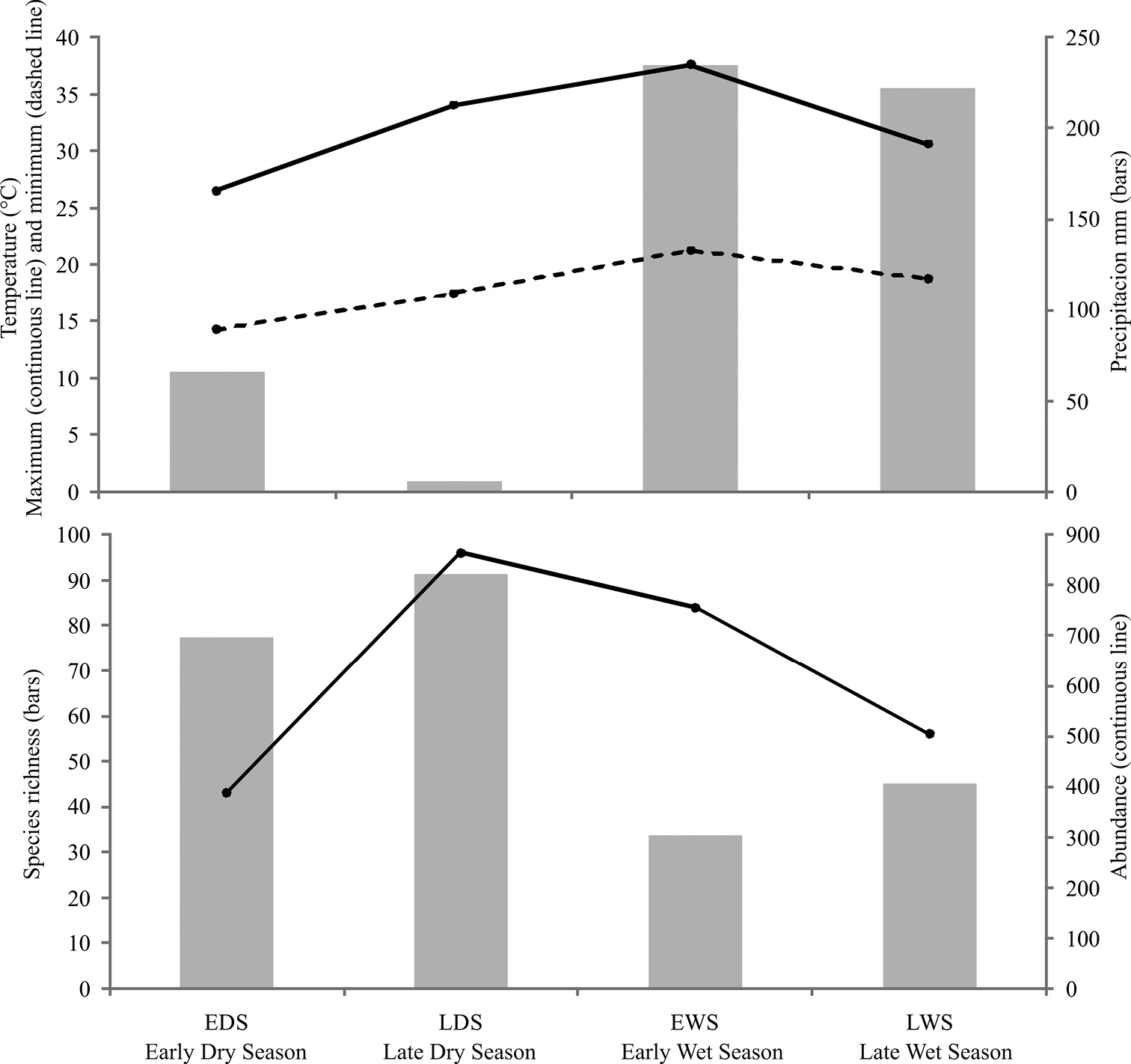
General abundance of Chrysomelidae was greater in the dry season than the wet season. Late wet season was not significantly different from early dry and wet seasons; the rest of comparisons between seasons were significantly different. The highest abundance was obtained during the late dry season, with 822 individuals. Fewer individuals were found during the early dry season (696 specimens), and late and early wet seasons, 406 and 302, respectively. Density for seasons followed the same pattern as the abundance, being late dry season the period with higher densities of chrysomelid beetles (Table 2). The number of species collected per season declined as the year progresses. During late dry season, 96 species were recorded, representing between 69.91 and 72.72% of the estimated richness for that season; 84 species were found in early wet season (62.36 to 64.5% of estimated richness), while the number decreases to 56 species in late wet season (66.25 to 66.34%) and 43 species in early dry season (77.08 to 87.66%) (Table 2; Figure 5). Determination coefficients based on the Clench model was higher than 0.99 for all seasons, while the slope values were above 0.1 (Table 2). Higher temperatures and precipitation were found within both wet seasons (Figure 6). High correlation values were present between temperature and richness, and between precipitation and abundance. Abundance was negatively correlated with precipitation, while species richness was positively correlated with maximum temperature. Other comparisons were not significant (Table 3).
Species accumulation curves by season in the Peregrina Canyon, Tamaulipas, Mexico. Upper graphic: Early wet season (blue color) and late wet season (red color). Lower graphic: Early dry season (black color) and late dry season (green color).
Variation of Chrysomelidae with precipitation and temperature during 2009 in Peregrina Canyon.
Spearman rank order correlations of abundance and species richness of Chrysomelidae with precipitation and temperature in Peregrina Canyon; marked (*) correlations are significant at p < 0.05.
| Abundance | Richness | |
|---|---|---|
| Precipitation | -0.769* | 0.112 |
| Max °C | 0.321 | 0.842* |
| Min °C | -0.066 | 0.587 |
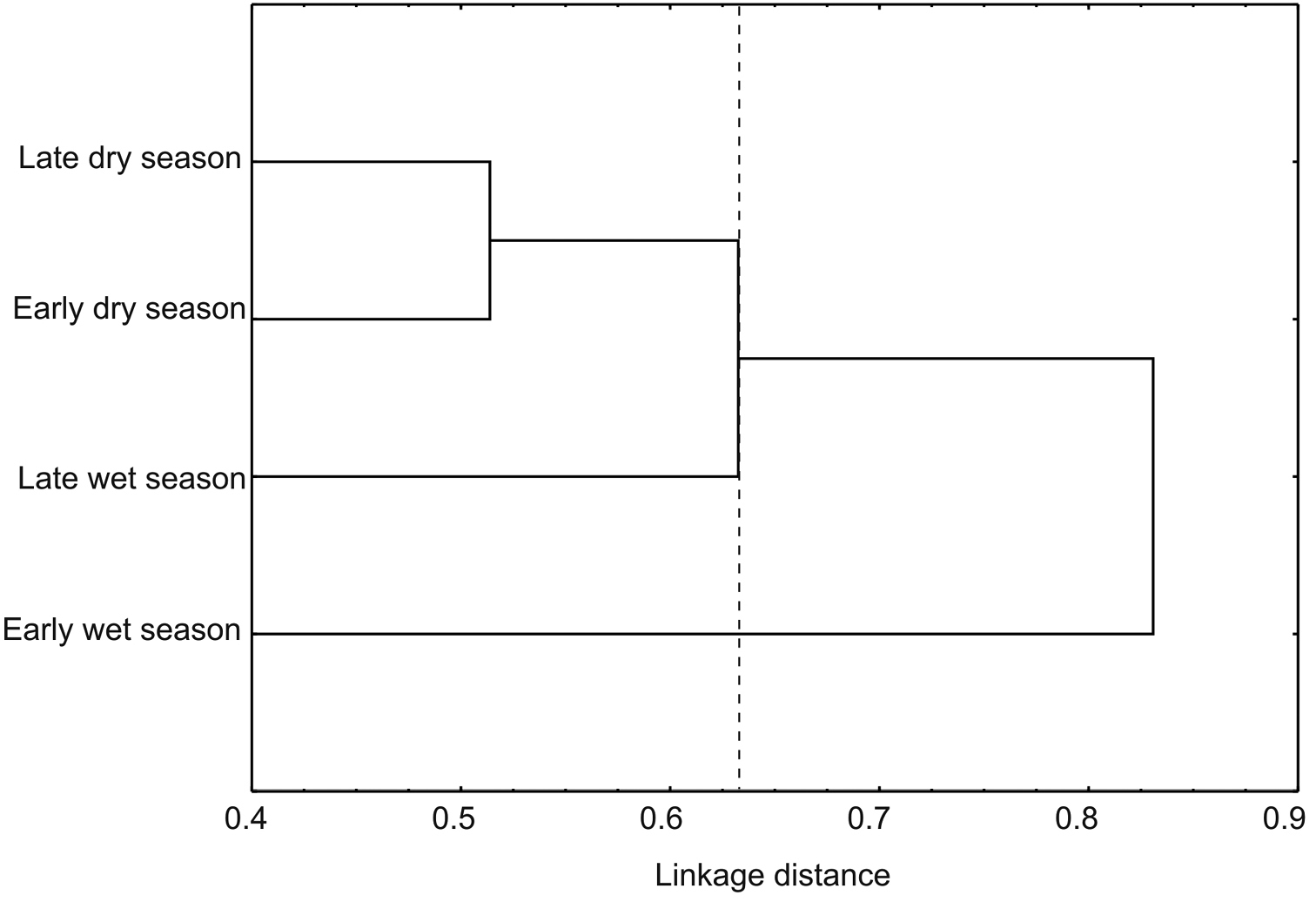
In contrast to abundance, the Shannon and Simpson indices indicated the highest diversity during the early wet season. Lower values of diversity occurred in late dry season, followed by late wet and early dry seasons. Based on diversity indices, all seasons were statistically different (p < 0.05) (Table 2). Reduction in diversity value at early dry season was originated by the drop in evenness with the increase of samples. The rest of year, evenness values, remained constant with the increase of samples in each season (Figure 7). Of the total species recorded in the annual period, only 13 were present throughout the year, and 23 were registered in three seasons, 37 in only two, and 84 were unique to a single season. From these exclusive species, 38 were recorded in late dry season, 26 in early wet season, 12 in late wet season, and only eight in early dry season (Table 1). Bray Curtis index established the greatest similarity between early and late dry seasons (45.1%), and in descending order were presented late wet and early dry seasons (38.8%), late dry and late wet seasons (37.9%), late dry and early wet seasons (36.3%), early and late wet seasons (35.6%) and early wet and early dry seasons (25.7%). The cluster analysis showed the formation of three groups according to the composition of species in each season: the first group consists of the species present in early and late dry seasons, the second group corresponds to late wet season species, and the last group was the early wet season species (Figure 8).
SHE analysis of diversity for each season in the Peregrina Canyon. ln S natural logarithm of species richness; ln E natural logarithm of evenness; H diversity (Shannon index).
Cluster analysis from seasons in the Peregrina Canyon, Tamaulipas, Mexico.
There are few studies of the richness and diversity of Chrysomelidae in Mexico with which the present study can be compared.
Using the latest higher classification scheme of
According to both non-parametrics estimators used, our data represent a sampling efficiency superior to 70%. Similarly, Clench model indicated a percentage superior to 70% and a slope close to 0.1, indicating both data accuracy and the reliability of the study (
Alpha diversity of Chrysomelidae in Peregrina Canyon was high, and represents one of the first known studies of site specific data for Mexico.
We present the first record of the altitudinal variation in richness, abundance, and diversity of Chrysomelidae in Mexico. In our study, greater species richness was found in the intermediate altitudes, which is a similar result to that found by
Decreasing abundance with increasing altitude has been observed in several studies with other insects, such as necrophilic entomofauna (
On a temporal scale, the Chrysomelidae community followed a markedly seasonal pattern, where the dry season was the most favorable for collection of this group in the study area (greatest abundance and species richness). This is a pattern that was also found in the subtropical region where the higher captures of insects occur in the dry season.
Results presented here can be partially explained by the indirect effect of precipitation and temperature on the abundance and species richness, respectively. In this study, abundance increases significantly as precipitation decreases, while species richness increases significantly with an increasing in the maximum temperature. Influence of high temperature present during the wet season has been observed in other studies with Chrysomelidae in tropical areas (
Although our data only considered a single canyon, beta diversity between sites at different altitudes was high. Similarity values obtained for all comparisons of sites were below 50%. Among the sites there was greater similarity of the first two altitudinal sites, with the lower similarity at the higher site. This pattern apparently reflects the greater difference between the tropical and temperate affinities of the vegetation and associated chrysomelids between the lower sites (Neotropical affinities) with the higher site (Nearctic affinities). Similarly, all comparisons between seasons had a similarity less than 50%, and a high number of exclusive species to each season. This pattern of low similarity indicates a significant turnover of species with altitude, which could be caused by the environmental heterogeneity of the area reflected in the different vegetal communities along the altitudinal gradient, which is observed in the three groups formed by the site cluster, and also the three groups (group 1: LWS and EDS; group 2: EWS; group 3: LDS) that were formed in seasonal cluster. However, we also found high values of alpha diversity for each of the sites and analyzed seasons, which suggests that biotic and abiotic factors present at each site and for each station generates unique conditions for certain species of chrysomelid beetles.
Our results highlight the importance of conservation of a heterogeneous habitat which can generate unique species compositions of highly diverse taxa, such as Chrysomelidae. Results presented here establish baseline data for Chrysomelidae richness and diversity for the region and can serve as a reference study for future work on the potential use of Chrysomelidae as indicator group of community diversity in natural areas. However, further studies are still needed to analyze the importance of environmental factors in the distribution and phenology of Chrysomelidae species and possible changes to expect due to climate change.
A total of 2, 226 specimens were collected belonging to six subfamilies, 81 genera and 157 species of Chrysomelidae from the study area. The greatest abundance and density of individuals were recorded at the lowest elevation site; however, alpha diversity increased with increasing altitude, and species richness was higher at intermediate altitude. Similarity values were less than 50% among the three sites indicating that each site had distinct species assemblages of Chrysomelidae.
The highest abundance and species richness was obtained during the late dry season, whereas diversity was higher during the early wet season. Geographical location of the study area plus different vegetal compositions from the three sites sampled could be the principal reason for the variation here found in Chrysomelidae communities with altitude and season. Also, precipitation and temperature may influence the Chrysomelidae community in study area; however, both abiotic factors affect directly the vegetal composition which is assumed to be the principal factor in determining leaf beetle species composition and abundance.
The present work is one of the first specific area studies of Chrysomelidae conducted in Mexico, in which both altitude and season are analyzed. The information presented here provides baseline data that allow for comparisons of the diversity and species richness of Chrysomelidae on a regional and national scale. This information could be used as an initial step to analyze the potential use of Chrysomelidae as an indicator group of biodiversity in Mexico.
The authors express their thanks to PROMEP for financial support to cover publishing fees. We wish to thank to Itzcóatl Martínez Sánchez, Javier Alejandro Obregón Zuñiga, Niver Tarqui, Lucas Hernández Hernández, Ivette de León, Pablo Ochoa, y Justo Sánchez for their help in fieldwork. Also, we thank the authorities responsible of the Ecological Park Los Troncones, for their assistance given to the authors for the realization of this study.