Citation: Sartori M (2014) Status of the enigmatic Oriental genus Rhithrogeniella Ulmer, 1939 (Ephemeroptera, Heptageniidae). ZooKeys 429: 47–61. doi: 10.3897/zookeys.429.8116
Based on historic collections and new material from Sumatra and Java, the species Rhithrogeniella ornata Ulmer, 1939, type species of the genus Rhithrogeniella, is reinvestigated. The nymph is described for the first time and is closely related to the continental Southeast Asian species Rhithrogeniella tonkinensis Soldán and Braasch, 1986. Rhithrogeniella belongs to the subfamily Ecdyonurinae, and is related to the genera Nixe Flowers, 1980 and/or Paracinygmula Bajkova, 1975 based on characters of the nymphal stage. Species described from Taiwan in the genus Nixe are transferred to the genus Rhithrogeniella: Rh. littoralis (Kang and Yang, 1994) comb. n., Rh. mitifica (Kang and Yang, 1994) comb. n. and Rh. obscura (Kang and Yang, 1994) comb. n.
Rhithrogeniella ornata, Rhithrogeniella tonkinensis, Nixe, Paracinygmula, new combinations, Sumatra, Java
Major advancement was made by
Two questions need to be resolved. Are
The type material of Rhithrogeniella ornata, deposited in the collection of the Zoological Museum of Hamburg University, Germany (ZMH) has been reinvestigated together with new material from Sumatra. It is now possible to provide the first description of the nymph of Rhithrogeniella ornata.
Material studied here is deposited in the following institutions:
Zoologisches Museum und Biozentrum Grindel, Hamburg, Germany [ZMH]
Musée cantonal de zoologie, Lausanne, Switzerland [MZL]
Lembaga Ilmu Pengetahuan Indonesia (Indonesian Institute of Sciences), Museum of Zoology, Bogor, Indonesia [LIPI] (Bogor was formerly known as Buitenzorg)
Drawings were made with the help of a camera lucida taken from stereomicroscope Leica DM 750 and pictures from microscope Zeiss Axioscop 2 or Visionary Digital Passport II. Final digital drawings were performed on Adobe Illustrator CS6. For scanning electronic microscope (SEM) pictures, the eggs were dehydrated, carbon coated, and observed under a LEO 1525 at 5.00kV; maxillae were dehydrated, critical point dried, and then platinum coated, and observed under a FEI Quanta 250 at 5.00kV. Final figs were assembled in Adobe Photoshop CS6.
Nymphs and adults were associated with the help of the egg structure (Fig. 5).
One male holotype, one female allotype: Indonesia, Java, Buitenzorg, VII 1932, Dr. Lieftinck leg. [ZMH]
Paratypes: 4 female subimagos, 1 male subimago: Indonesia, Java, Buitenzorg, Bellevue, caught at light, VII.1929, Prof. Thienemann leg. [ZMH]; 4 female imagos, 2 male subimagos: Indonesia, Sumatra, Padang, VII 1925, Prof. Fulmek leg. [ZMH]; 1 male subimago: Indonesia, Sumatra, Pangkalang, Kota baru, X 1925, Prof. Fulmek leg. [ZMH]
All specimens in ethanol, except fore- and hind legs, fore- and hind wings of the male subimago from Buitenzorg mounted on slide in Canada balsam.
Other material: 5 nymphs: Indonesia, Sumatra Barat, Sawahlunto, stream, 275m, 00°41.33'S, 100°46.72'E, (UN5), 10.XI.2011, M. Balke leg. [ZML]; 26 nymphs, of which two entirely mounted on microscopic slides: Indonesia, Sumatra Barat, Talawi, Ombilin River, 277m, 00°34.15'S, 100°43.54'E, (UN4), 8.XI.2011, M. Balke leg. [ZMH, MZL, LIPI]
Specimen completely faded; for color patterns see
Mesonotum with transverse suture; medial depression of furcasternum sub parallel anteriorly.
Foreleg with tarsi sub equal in length to the tibia, which is 1.25x longer than the femur. Tarsal composition: 2>3>4>5>1.
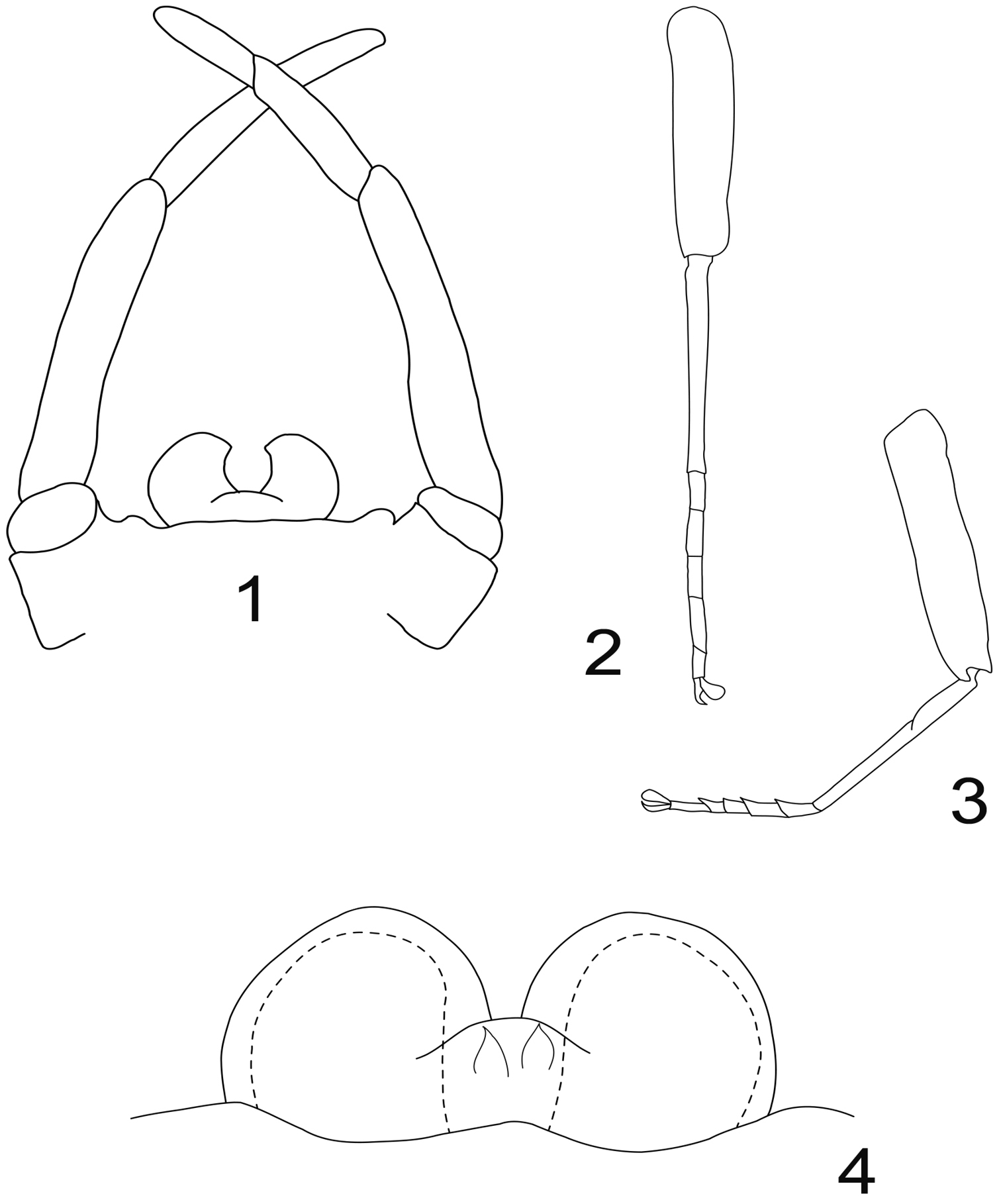
Genitalia (Fig. 1): margin of the styliger fig straight to slightly convex, with two small sub-lateral rounded processes; last gonopod segment ca 0.7× the length of the previous, both together ca 0.75× the length of the antepenultimate. Penis constituted of two kidney-shape lobes, separated by a “U” incision, i.e. the inner margin of each lobe is concave and slightly hooked near the apex. No lateral or median titillators, no apical spines visible.
Fore leg (Fig. 2) with femur ca 1.15x the length of tibia, which is subequal in length to tarsi. Tarsal composition 4≥2>3≥1>5.
Hind leg (Fig. 3) with femur ca 1.35x the length of tibia, which is ca 1.45× the length of tarsi. Tarsal composition 1=2=5>3≥4.
Genitalia (Fig. 4) with penis lobes rounded, ellipsoid, without any spine or titillators; in median position, a pair of membranous processes ending with a spine like sclerotization present in ventral view.
Thoracic structures similar to the male.
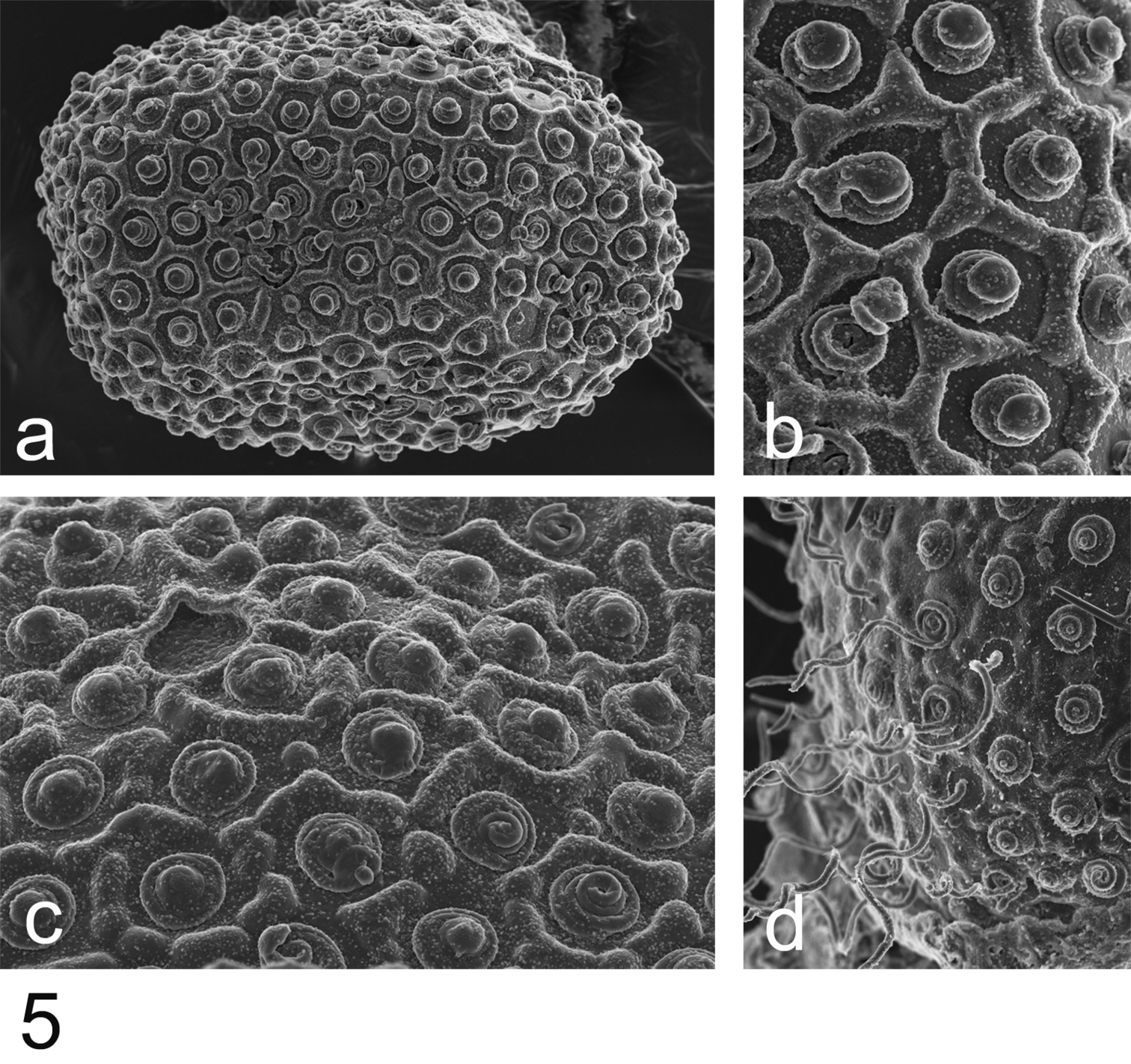
Eggs (Fig. 5): ovoid, ca 130 µm × 90 µm; chorion regularly covered with hexagonal mesh ridges, with KCT in-between, not larger at poles; micropyle rounded to slightly oval in equatorial area.
Size: Body length: up to 5.2 mm and 5.6 mm for male and female respectively; cerci and terminal filament subequal and ca ¾ the length of the body.
Coloration similar to Figs 6 and 7.
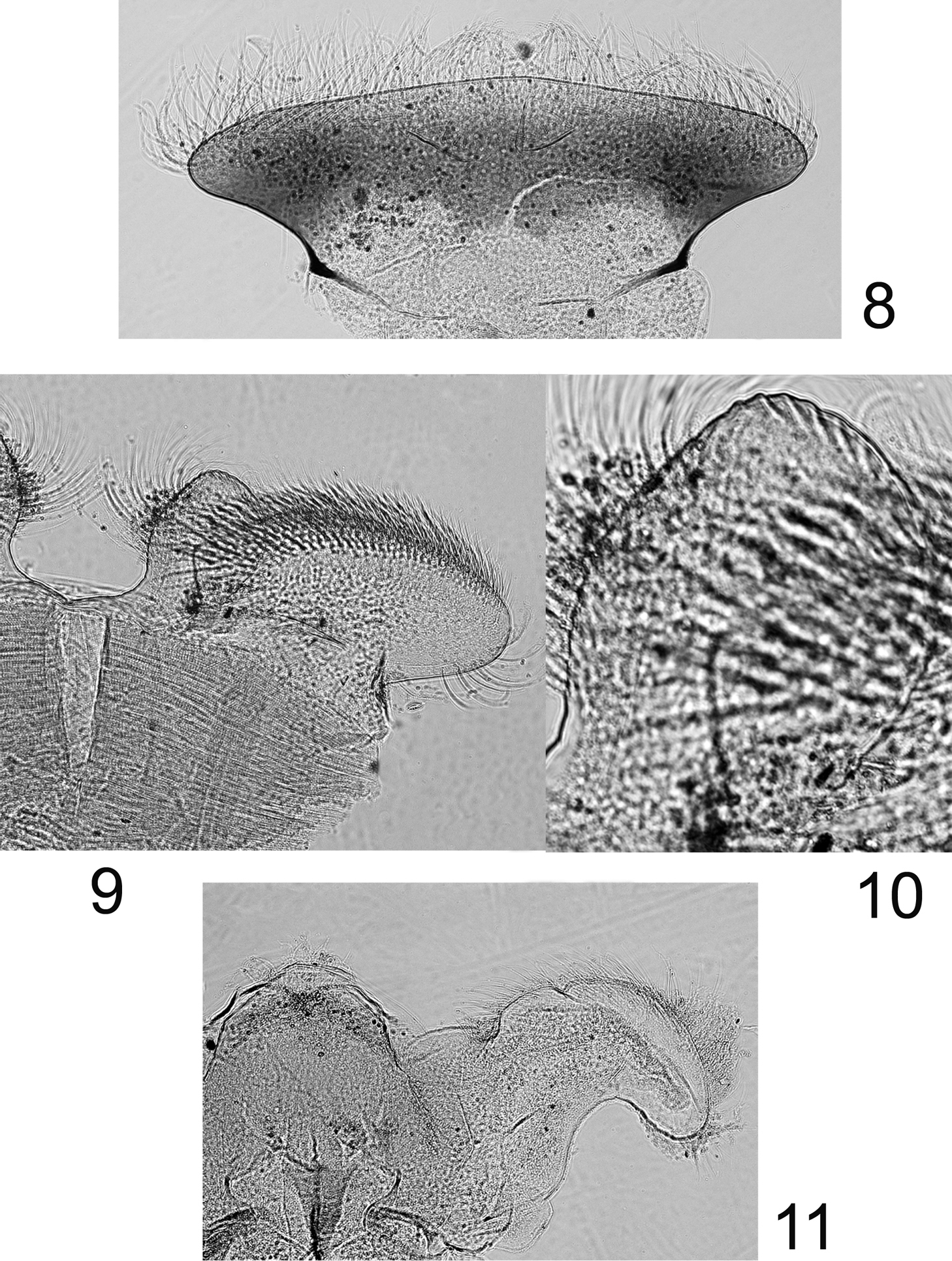
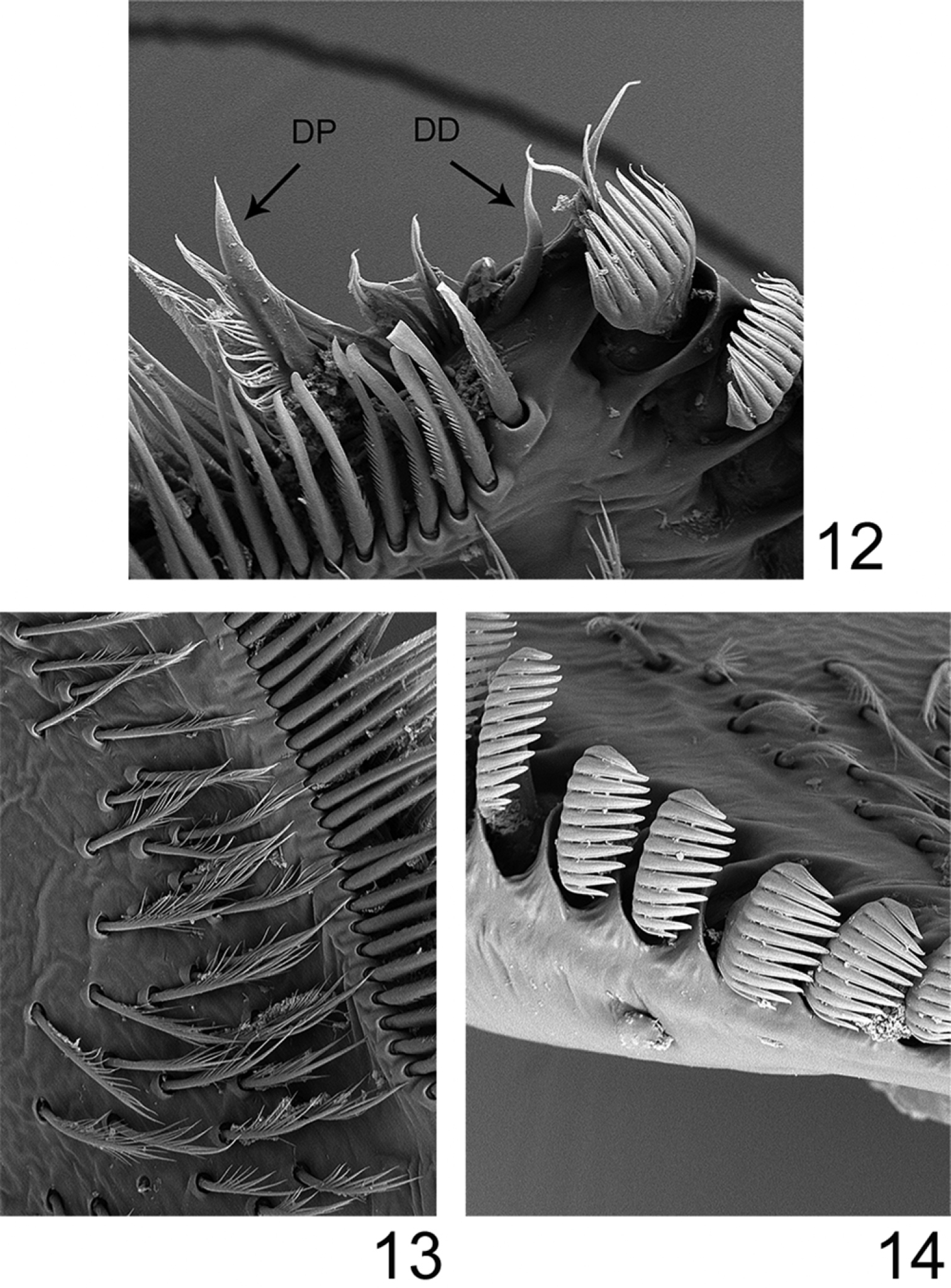
Labrum (Fig. 8) moderately expended laterally, ca 2.6× wider than long; lateral margins regularly rounded; no anteromedian emargination; dorsal face covered with long and thin setae anteriorly; ventral face with shorter and stout setae along the anterior margin. Mandibles covered with numerous long and thin setae on the outer margin; right mandible with outer incisor saw-like, inner one with a trifid apex with 2–3 pectinate setae below it, and 2–3 long and simple setae below the mola; left mandible with outer incisor saw-like, inner one with a bifid apex with 3–4 pectinate setae below it, and 3–4 long and simple setae below the mola. Maxillary palp three-segmented; first segment covered with thin setae on inner and outer margin; second segment with thin setae on the outer margin; third segment slightly pointed, only with long and thin setae. Maxillae with fimbriate scattered setae on the ventral surface (Fig. 13): 13–14 comb-shape setae on the crown of the galea, median ones with 10–11 teeth (Fig. 14); proximal dentiseta bifid, outer margin feathered; distal dentiseta simple, entire and unbranched (Fig. 12). Labium (Fig. 9) with glossae rhomboid, inner margin covered with long and thin setae, apex characteristic with scale-like margin (Fig. 10); paraglossae moderately expended laterally. Hypopharynx (Fig. 11) with rhomboid lingua bearing a tuft of short and thin setae at apex; superlinguae well developed and expended laterally with rounded apex and setae on the outer margin extended beyond the apex.
Pronotum moderately expended laterally. Foreleg with femur ca 2.6× longer than wide; outer margin covered with long and stout setae, becoming thinner near the apex; inner margin with only few spine-like setae on the distal third. Outer margin of tibia with very few thin and short setae (Fig. 15), inner margin with few spine-like setae in the middle; tarsi with only a few spine-like setae in the middle of the inner margin. Hind leg similar, except the spine-like setae on inner margin of the femur present on the whole margin; outer margin of tibia with a row of long and thin setae (Fig. 16) and inner margin with more numerous spine-like setae. Middle leg similar to hind leg, except spine-like setae on the inner margin of the femur only present on the distal half. Bristles on the upper face of femora variable in length, always with divergent margins and rounded apically (Fig. 17). Tarsal claw moderately hooked, bearing 4–6 teeth (Fig. 18). No supracoxal spurs present.
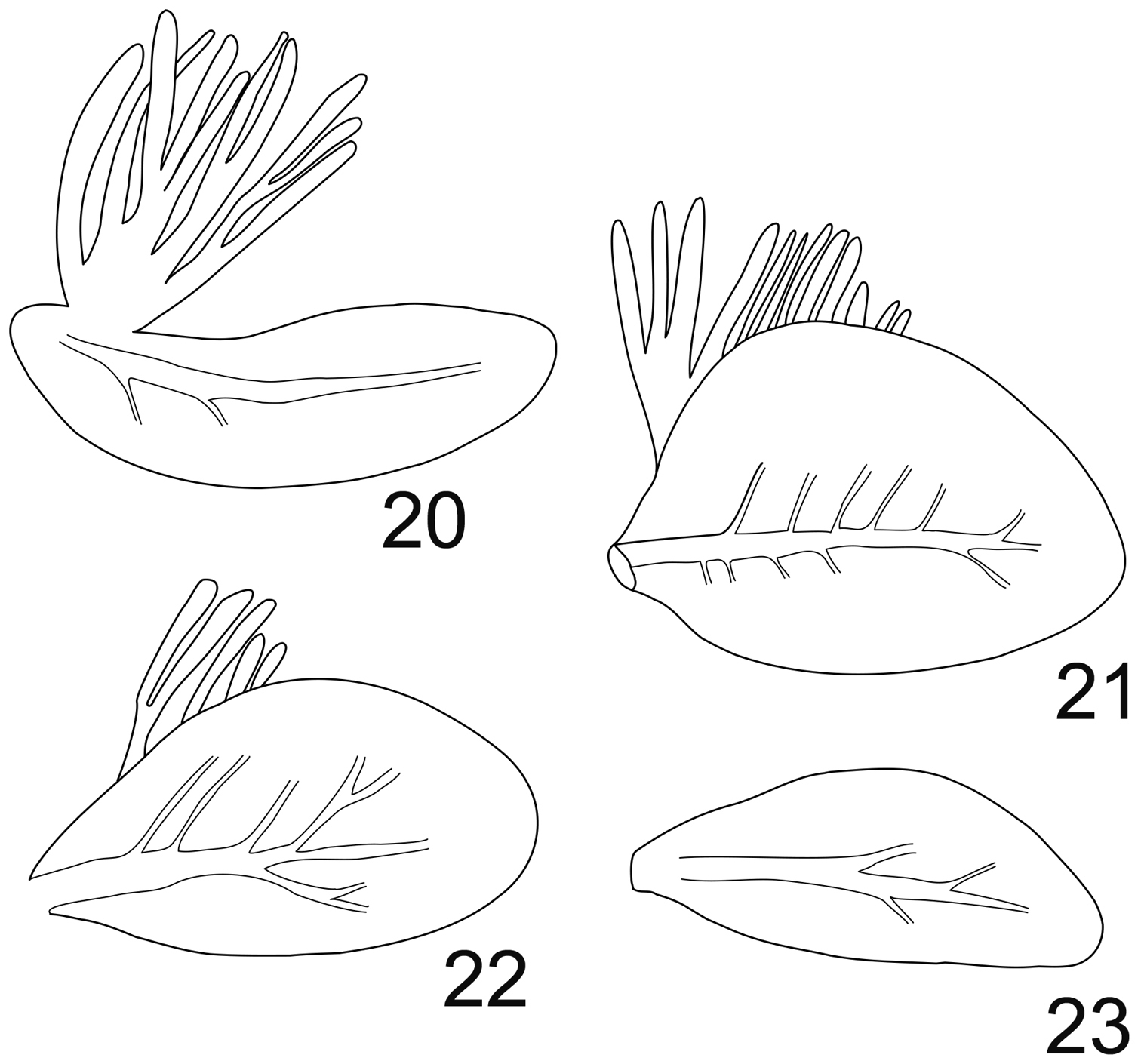
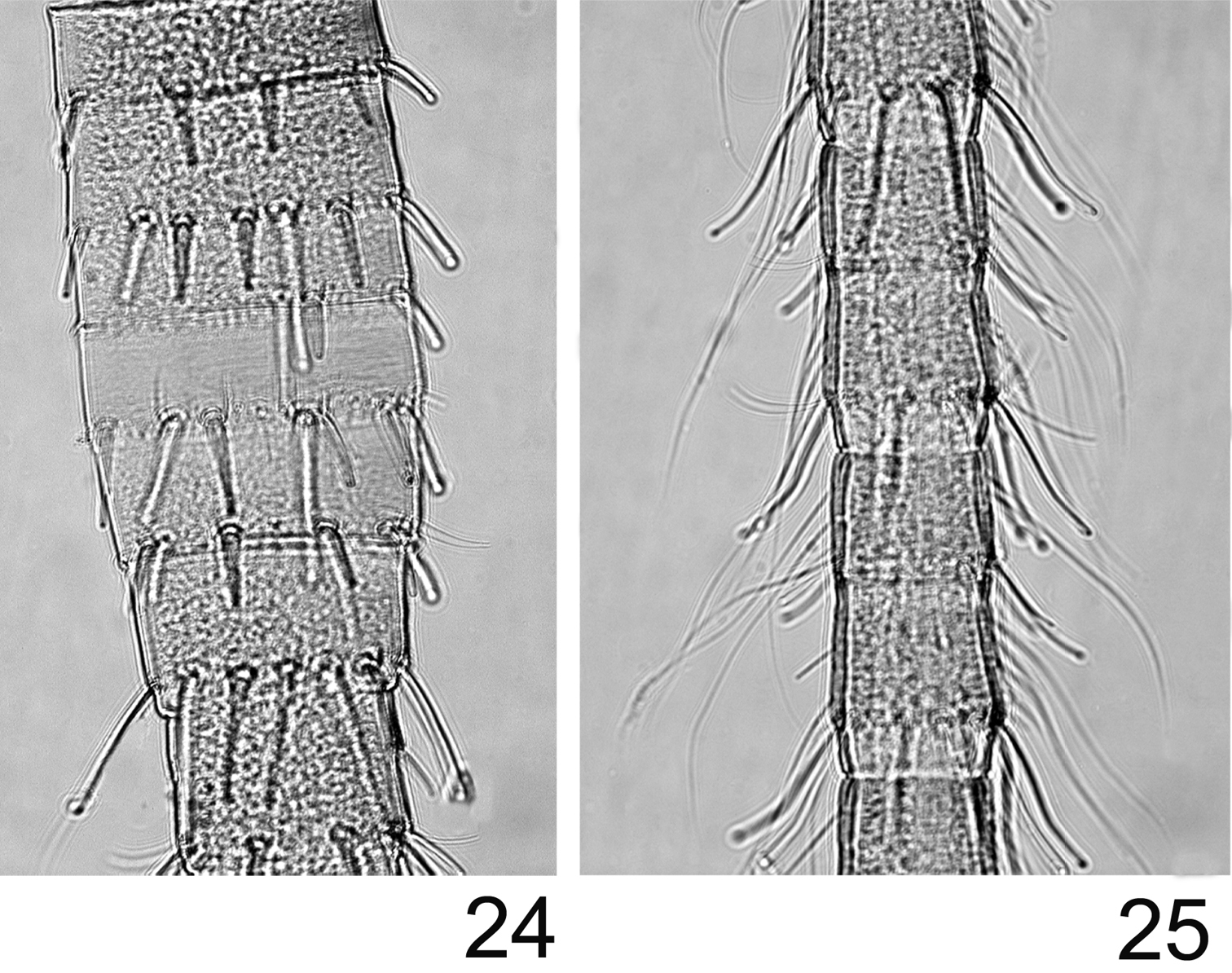
Abdomen with posterolateral extensions weakly developed, visible only on segments V–VIII. Gills present on abdominal segments I–VII. Gill I banana-shape (Fig. 20), with fibrillar part well developed, gill IV ca 1.5× longer than wide, strongly asymmetrical (Fig. 21), gill VI with well-developed fibrillar part, more elongated and slightly asymmetrical (Fig. 22), gill VII ca 2.5× longer than wide, without fibrillar part and slightly asymmetrical (Fig. 23). Posterior margin of abdominal terga with weakly developed spines of different size and shape (Fig. 19). Cerci and terminal filament with long and stout setae in whorls on the proximal part (Fig. 24), together with long and thin setae in the median and distal part (Fig. 25).
One specimen has been used for the study by
Rhithrogeniella ornata Ulmer, 1939. 1 Genitalia of the male imago (holotype) in ventral view 2 Foreleg of a male subimago (paratype) 3 Hindleg of a male subimago (paratype) 4 Penis lobes of a male subimago (paratype): plain line, cuticular structures of the subimago; dotted line, outline of the imago penis lobes.
Rhithrogeniella ornata Ulmer, 1939, SEM pictures of egg structures. 5a Egg extracted from a female subimago paratype from Padang, Sumatra 5b Details of the chorionic structure of a female nymph from Ombilin River, Sumatra 5c Details of the chorionic structure and micropyle of a female subimago paratype from Buitenzorg [Bogor], Java 5d chorionic surface of the female allotype from Buitenzorg [Bogor], Java.
Rhithrogeniella ornata Ulmer, 1939. 6 Male nymph 7 Female nymph with slight color variations.
Rhithrogeniella ornata Ulmer, 1939, nymphal mouthparts. 8 Labrum in dorsal view 9 Left glossae and paraglossae of the labium 10 Detail of the glossae from 9 11 Hypopharynx, ventral view lingua and left superlingua.
Rhithrogeniella ornata Ulmer, 1939, SEM pictures of the maxilla. 12 Dentisetae (DP: proximal dentiseta, DD: distal dentiseta) 13 Fimbriate setae on the ventral surface 14 Comb-shape setae on the crown of the galea-lacinia.
Rhithrogeniella ornata Ulmer, 1939. 15 Outer margin of the fore tibia 16 Outer margin of the hind tibia 17 Bristles on the dorsal surface of hind femur 18 Tarsal claw 19 Posterior margin of tergite V.
Rhithrogeniella ornata Ulmer, 1939. 20 Gill I 21 Gill IV 22 Gill VI 23 Gill VII.
Rhithrogeniella ornata Ulmer, 1939. 24 Proximal part of the terminal filament 25 Median part of the terminal filament.
The genitalia of the male imago differ slightly from those described by
Three species of Nixe known only from the nymphal stage are reported from Taiwan (
Rhithrogeniella littoralis (
Rhithrogeniella mitifica (
Rhithrogeniella obscura (
Nixe/Paracinygmula is therefore restricted to the Holarctic Realm, whereas Rhithrogeniella is Oriental, reported from Taiwan, continental Southeast Asia and from Java and Sumatra in the Sunda Islands. The genus is presently recorded neither from Borneo (
Based on the Bayesian majority-rule consensus tree reconstructed from the combined data set in
One remaining question concerns the presence or absence of titillators on Rhithrogeniella male genitalia. These structures are mentioned by
Rhithrogeniella ornata appears to be closely related to Rhithrogeniella tonkinensis, known from Vietnam and Thailand. It differs from the latter mainly by the ornamentation of the crown of the galea-lacinia, with 13–14 comb-shape setae, median ones with 10–11 teeth, whereas Rhithrogeniella tonkinensis bears only 10–11 comb-shape setae, median ones with 6–8 teeth. Additional nymphal characters, and egg chorionic structure are also very similar. Differences between subimagos of both species proposed by
Compared to the Taiwan species, Rhithrogeniella ornata can be easily separated from Rhithrogeniella littoralis and Rhithrogeniella obscura by the shape of the mandibles with inner and outer incisors subequal in length (inner incisor much shorter in Rhithrogeniella littoralis and Rhithrogeniella obscura), from Rhithrogeniella mitifica and Rhithrogeniella obscura, by the higher number of teeth on the comb-shape setae of the galea-lacinia (4–5 teeth only in Rhithrogeniella mitifica and Rhithrogeniella obscura vs 10–11 in Rhithrogeniella ornata), from Rhithrogeniella mitifica by the shape of the spines on the posterior margin of the tergites (pointed in Rhithrogeniella ornata vs tabular in Rhithrogeniella mitifica), and from Rhithrogeniella littoralis by the much more elongated gill VII.
Michael Balke (Munich) is warmly thanked for donation of important material from Sumatra. I address my sincere thanks to Kai Schütte, Hossein Rajaei (ZMH) and their colleagues for facilities provided during my stay in their museum. Technical help by Renate Walter (ZMH), Geneviève L’Eplattenier and Raphael Grand (MZL) with SEM preparation and pictures was much valued. My appreciation goes to Laurent Vuataz (MZL) for useful discussions about molecular reconstructions. Thanks also to Hossein Rajaei (ZMH) for his help with the color photographs of the nymphs. Comments by Janice Peters (Tallahassee) and an anonymous reviewer were very helpful.