Citation: Lackner T (2014) Revision of the genus Hemisaprinus Kryzhanovskij, 1976 (Coleoptera, Histeridae, Saprininae). ZooKeys 429: 101–130. doi: 10.3897/zookeys.429.7949
The monophyletic genus Hemisaprinus Kryzhanovskij in Kryzhanovskij & Reichardt, 1976 is revised herein. All three species Hemisaprinus subvirescens (Ménétries, 1832), H. lutshniki (Reichardt, 1941) and H. cyprius (Dahlgren, 1981) are found to be correctly assigned to the genus and their monophyly is supported by the synapomorphy of the presence of prosternal foveae. The three species are re-described and supplemented with colour photographs as well as SEM micrographs outlining their differences. Male genitalia drawing of H. subvirescens and H. lutshniki are provided and a key to the species is given. Hemisaprinus subvirescens (Ménétries, 1832) is newly reported from Armenia, Azerbaijan, Kyrgyzstan, Uzbekistan, Turkmenistan, Tajikistan, Jordan, Cyprus and Mongolia. The lectotypes and paralectotypes of the following species are designated herein: Saprinus foveisternus Schmidt, 1884, Saprinus syriacus Marseul, 1855 and Saprinus viridulus Marseul, 1855.
Coleoptera, Histeridae, Saprininae, Hemisaprinus, Palaearctic Region, taxonomic revision
The genus Hemisaprinus was established by Kryzhanovskij in
All dry-mounted specimens were relaxed in warm water for several hours or overnight, depending on the body size. After removal from original cards, the beetles were side-mounted on triangular points and observed under a Nikon 102 stereoscopic microscope with diffused light. Body structures were studied using methods described by
CAS Alexander Sokolov collection, Moscow, Russia;
CND Nicolas Dégallier collection, Paris, France;
MMBC Moravské Zemské Muzeum Brno, Czech Republic (P. Baňař);
MNHN Musém National d’Histoire Naturelle, Paris, France (A. Taghavian);
MNHUB Museum für Naturkunde, Humboldt- Universität, Berlin, Germany (B. Jaeger);
MZLU Museum of Zoology Lund, Lund, Sweden (C. Fägerström);
NCB Naturalis Biodiversity Centre, Leiden, Netherlands (B. Brugge);
TLAN Tomáš Lackner collection, temporarily housed at Naturalis Biodiversity Centre, Leiden, Netherlands;
ZIN Zoological Institute, Russian Academy of Sciences, St. Petersburg, Russia (B. Kataev).
Abbreviations. Abbreviations of morphological measurements follow
APW width between anterior angles of pronotum
EL length of elytron along elytral suture
EW maximum width between outer margins of elytra
PEL length between anterior angles of pronotum and apices of elytra
PPW width between posterior angles of pronotum.
Although Hemisaprinus has been recently diagnosed by
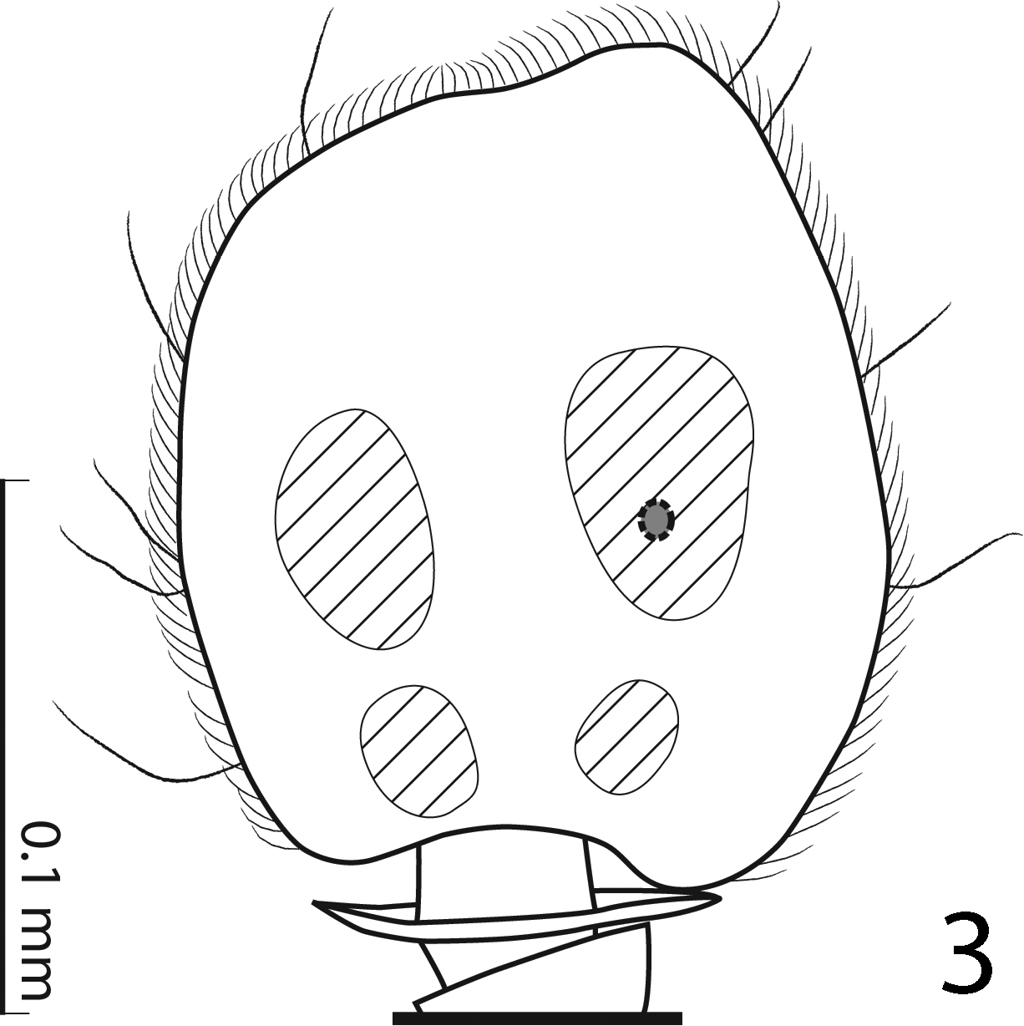
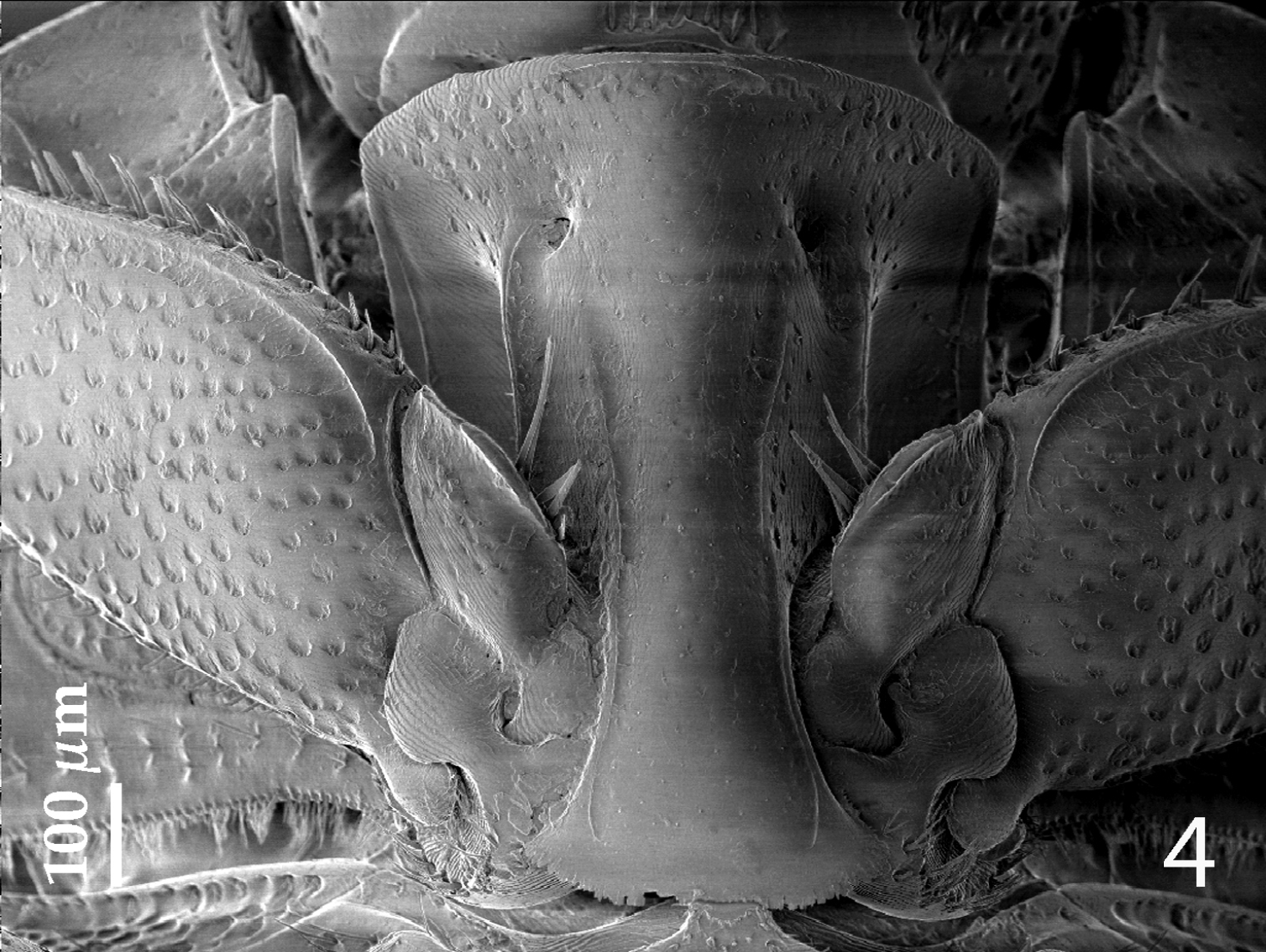
By the presence of prosternal foveae Hemisaprinus can be readily differentiated from members of the genus Saprinus, which it otherwise strongly resembles, by the absence of complete frontal stria as well as general appearance. The sensory structures of the antenna (Figs 3, 15) are typically Saprinus-like as well, with four oval sensory areas on ventral side of the club with a corresponding vesicle situated under internal distal sensory area. The reader is referred to the Key to the genera of the Palaearctic Saprininae by the author (
Hemisaprinus subvirescens is found chiefly on carcasses in arid regions while Hemisaprinus lutshniki is found in decomposing vegetable matter, and has not been found on carcasses so far (
This genus includes three described species: Hemisaprinus subvirescens (Ménétriés, 1832) known from Georgia, southern Russia, Kazakhstan, Turkey, Syria, Israel, Iran, Iraq, Afghanistan, Pakistan, Burma and China (
Russia, Caucasus.
Saprinus subvirescens: Holotype: spec., “subvirens / Mén. Cauc (written) / Salian (red label, printed) / Holotypus (red label, printed) / round golden label” (ZIN).
Saprinus foveisternus Schmidt, 1884: Lectotype (present designation): female, glued on a mounting point with the following labels: “Baku” (written); followed by: “foveisternus / mihi typ.” (written); followed by: “Type” (brick-red label, printed); followed by: “coll. J. Schmidt” (printed); followed by: “foveisternus / Schmidt” (double-margined, written label); followed by: “Saprinus / foveisternus / Coll. Schmidt-Bickhardt” (printed); followed by: “Saprinus / foveisternus / Schmidt, 1884 / LECTOTYPE / des.
Saprinus syriacus Marseul, 1855: Lectotype (present designation): male, with genitalia extracted, glued to the same mounting card as the specimen, right protibia broken off, glued next to specimen, right mid-leg and left hind leg missing, with the following labels: “90 / Saprinus / syriacus / Syrie m. ♂ / Laferté” (round yellow label, written); followed by: “Saprinus / syriacus m / Syria / 89” (yellow label, written); followed by: “Ti...further illegible text / 63” (tiny yellow label, written); followed by: “342” (orange label, written); followed by: “Schm. / 31” (written); followed by: “MUSEUM PARIS / COLL. / DE MARSEUL 1890” (printed); followed by: “TYPE” (red-printed label); followed by: “Sapr. subvires- / cens Men. / G. Dahlgren det” (printed-written); followed by: “Saprinus syriacus / Marseul, 1855 / LECTOTYPE 2014 / des. T. Lackner” (red label, written) (MNHN). This species has been described from unknown number of specimens and the lectotype designation fixes the identity of the species.
Saprinus viridulus Marseul, 1855: Lectotype (present designation): female, right metatarsus missing, with the following labels: small pink rectangular label, followed by: “89 / Saprinus / viridulus / Kurmaul / Deyr. Inde” (yellow, round label, written); followed by: “♀” (written); followed by: “MUSEUM PARIS / COLL. / DE MARSEUL 1890” (printed); followed by: “TYPE” (red-printed label); followed by: “Sapr. subnites- / cens Men. / G. Dahlgren det” (printed-written); followed by: “Saprinus viridulus / Marseul, 1855 / LECTOTYPE 2014 / des. T. Lackner” (red label, written) (MNHN). The species was described from unknown number of specimens and the lectotype designation fixes the identity of the species. Note that Dahlgren mistakenly identified it as Saprinus subnitescens Ménétriés (sic!). What he meant was Saprinus subvirescens, which was indeed described by Ménétriés, and not Saprinus subnitescens, which was in turn described by Bickhardt in 1909.
Israel: 2 ♂♂, Adullam, 17.v.2002, Y. Mandelik & V. Chikatunov lgt. (TLAN); 2 specs., Jerusalem, coll. Lange, no further data (MNHUB). Tajikistan: 5 ♂♂, Aruk Tau Mts., 20.iv.1978, A. Olexa lgt.; 1 ♂, Vachrobod, 8.vi.1966, A. Olexa leg.; 1 spec., Tigrovaya Balka, 2.–6.vi.1966, A. Olexa leg.; 1 ♂ Aruk-Tau (Garavuti), 29.iv.1978, M. Dvořák leg. (all exs. TLAN). 1 spec., Khujand, 21.iv.1921, Arkhangelskij leg.; 1 spec., Yagnob, Chichartob, 1892, Glasunov leg.; 4 specs., Pyanzh, from Khorod to Ishkashim, 6.vi.1928, Grishin leg.; 2 specs., Koktau Mts., near Kurgan-Tyube pass, 28.iv.1962, Guryeva leg.; 2 specs., Tian-Shan, Musart, vi.1894, Hauser leg.; 1 spec., Gandzhina, 15.iv.1966 (all exs. ZIN). 2 specs., Tian-Shan, Tekesthal, no further data (BMNH). 1 spec., Pyandzh Karatau ridge Mt. Astana 23.iv.1991, Gratchev leg.; 1 spec., Tigrovaya Balka reserve right side Vakhsh river 16.iv.1989, V. Gorbatovskiy leg. (all exs. CAS). Turkmenistan: 1 ♂, Turkmenistan, Firjuza, Ashghabad, 27.iv.1977, A. Olexa leg.; 1 spec., ibid, but 22.iv.1981, J. Strnad leg.; 7 ♂♂ & 1 ♀, Ashgabat, Nisa, 21.iv.1975, A. Olexa leg.; 1 ♂ Amurdarya-Kirki, 1.–5.v.1993, collector unknown; 1 ♂ & 1 spec. Kopet Dagh, near Firjuza, Vanovskij, 21.v.1991, Z. Kejval leg.; 1 ♂, Tekke, no further data; 1 ♂, Annau, Karakum, 21.iv.1981, A. Olexa leg.; 7 specs., Annau, 15.iv.1985, Kapler leg.; 1 spec., Firjuza, 18.iv.1988, Kafka leg.; 2 specs., Ashghabad, 14.iv.1988, S. Jákl leg.; 1 spec., ditto, but 29.iv.1991, R. Dunda leg.; 5 specs., Chuli, 12.-13.iv.1990, M. Kafka leg.; 4 specs., Tusly Kala, 11.iv.1990, R. Dunda leg. (all exs. TLAN). 1 spec., Kopet-Dagh Mts., further locality illegible, 15.x.1969, collector unknown, near Opimus burrow; 1 spec., Atrek River, Jacobson leg.; 1 spec., Chikishlyar, 30.–31.iv.1916, V. Ilin leg.; 2 specs., Kushka, 18.v.1936, Kreizberg leg.; 1 ♂ & 1 spec., Badkhyz, Penkhatchetpe, 6.iv.1971, Tikhomirova leg.; 2 ♂♂, Badkhyz, Kyzyl-Dzhar, 18.iv.1970, Tikhomirova leg.; 3 specs., Kelif, 18.iv.1988, Atamuradov leg.; 3 ♂♂ & 2 specs., Kara-Kala env., 19.v.1968, Tikhomirova leg.; 4 specs., Firjuza, 30.iii.1952, Kryzhanovskij leg.; 2 specs., Kopet-Dagh Mts., Geok-Tepe, Izgait, desert, 10.iv.1987, V.N. Prasopov leg.; 1 spec., Ashghabat, no further data; 3 specs., idem, but 23.iii.1903, G. Jacobson leg.; 7 specs., idem, but 19.iv.1929, sands, Vlasov leg.; 2 specs., idem, but 6.iv.1928; 7 specs., idem, but 6.vi.1925, Opanin leg.; 3 specs., idem, but 22.iii.1952, Romadina leg.; 8 specs., Repetek, 23.iii.1983, Krivoshatskij leg.; 1 spec., idem, but 17.iv.1914, Plavilstshikov leg.; 2 specs., idem, but 5.ii.1904, E. Fisher leg.; 1 spec., Germab, 12.x.1988, collector unknown; 2 specs., Murgab, no further data; 1 spec., Iolatanj, 1.iv.1927, Kizeritskij leg.; 4 specs., Tedzhen, 21.vi.1904, Arris leg.; 4 specs., Krasnovodsk (=Turkmenbashi), 28.iii.1919, B. Ilin leg.; 1 spec., Kopet-Dagh Mountains, 12 km S of Kyzyl-Arbat, 25.iv.1952, D. Stenberg leg.; 2 specs., Kara-Bogaz, 40 km N from Kyzyl-Arbat, 21.iv.1952, Sternberg leg.; 2 specs., 2–6 km N of Kara-Kala, 24.v.1952, Kryzhanovskij leg.; 1 spec., Sumbar river, 1894, Herz leg.; 1 spec., Firjuza env., 30.iii.1952, V. Ilichyov leg.; 1 spec., Annau, 12.v.1928, V. Gussakovskij leg.; 6 specs., Gyaurs, 3.iv.1984, Kh. Atamuradov leg.; 6 specs., Karabil, Shiram-Kuy, 22.iv.1984, Kh. Atamuradov leg. (all exs. ZIN). 1 spec., Amudaryinskiy reserve, Amudarya River, Nargiz island, 9–16.iv.1983, S. Alexeyev leg. (CAS). 1 spec., Badkhyz Penhancheshme, 6.iv.1971, Tikhomirova leg. (CND); 1 spec., Repetek, v. 1914, N. Plaviltshikov leg. (MNHN). Jordan: 1 ♀, Al Qatrana Saliya, 15.iv.2002, Wadi Mujib env., M. Snížek leg. (TLAN). Kazakhstan: 2 ♂♂ & 2 specs. Akkol, Jambul, 8.v.1979, A. Olexa leg.; 2 specs., ibid, but 10.v.1978, M. Dvořák leg.; 11 specs., Tjunja, Charyn River, 26.v.1994, collector unknown. (all exs. TLAN). 1 spec., Alma-Atinskaya oblast, Kuskuduk, 30.iv.1930, Kirschenbladt leg.; 1 spec., Alma-Atinskaya oblast, Karatalsk, 18.v.1930, Kirschenbladt leg.; 3 specs., SE Kazakhstan, Ilijskij, 22.viii.1911, Matissep leg.; 1 spec., Chelkar, 2.vi.1928, Olenev & Popov leg.; 1 spec., Aktyubinskaya oblast, Dzhilandy, 11.vi.1908, D. Borodin & B. Uvarov leg.; 1 spec., Ostashkino, Almatinka, 20.vii.1928, Shnitnikov leg.; 2 specs., Mogyl Daumchar on River Emba, Temirsk region, 30.v.1908, Borodin leg.; 2 specs., Astau-Sardy, banks of River Emba, Temirsk region, 28.v.1908, Borodin leg.; 1 spec., Ak-Buta mountains, Temir, 2.vi.1908, Borodin leg.; 9 specs., Dzhilandy, Uralskaya oblast', Temirskij uezd, 11.vi.1908, D. Borodin & B. Uvarov leg.; 20 specs., surroundings of lake Inder, 4.vi.1907, B. Uvarov leg.; 1 spec., idem, but 10.vi.1907, A. Borodin leg.; 1 spec., Karatal (= Ush-Tobe), 16.v.1930, Kirschenbladt leg. (all exs. ZIN). 4 specs., North slope Talass ridge. near Talass vill., iv. 1993, no collector (CAS). 1 spec., Kapchagay env., Ili River, 10.v.1993, A. Ogarkov leg.; (CAS). TURKEY: 1 ♂ Cappadocia, 7.–10.vii. 1983, Avanos env., A. Olexa leg.; 1 ♂, Eskishehir, 5.v.1969, C. Holzschuch leg.; 1 ♂, Demircili, 70 km W Silifke, 5.iv.1992, O. Kapler leg. (all exs. TLAN). 1 spec., Kars env., Kaladzhika, 1.v.1915, Olsufyev leg. (ZIN); 1 spec., Anatolia, 29.iii.1977, 10 km SE Serefli Kochisar, Tuz Gölü, 1. Orient Exkursion, Inst. f. Zool. Mainz, Prof. R. Kinzelbach leg. (MNHN). UZBEKISTAN: 1 ♂ & 2 ♀♀ & 1 spec., Tashkent, 22.iv.1972, A. Olexa leg.; 3 ♂♂ & 2 specs., Samarkand, Aman Kutan, 21.iv.1972, A. Olexa leg.; 1 spec., ibid, but 20.iv.1972; 1 ♀, Khamsa-Abad, Ferghana, 26.iv.1972, A. Olexa leg.; 1 spec., Chimgan (Tian-Shan), 2500 m, 17.vii.1979, M. Dvořák leg.; 1 spec., Ak-Tash (Tashkent), 30.iv.1978, M. Dvořák leg.; 2 specs., 200 km W of Tashkent, Kyzyl-Kum Desert, Chardara (Koksu), 3.–5.v.1990, J. Turna leg. (all exs. TLAN). 3 specs., Ursatevskaya (=Khavast), 19.v.1920, I. Ivanov leg., on the ground in steppe; 1 spec., upper Upalanga river, Gissar Mt. range, 1898, Willberg, leg.; 1 spec., Tashkent, behind the Salar canal, 28.iii.1920, Ivanov leg.; 1 spec., Tashkent env., 16.v.1920, Ivanov leg.; 1 spec., idem, but 11.vi.1909, V. Grekov leg.; 1 spec., Sansar, 1892, Glasunov leg.; 4 specs., Kalma-Tai, 1892, Glasunov leg.; 2 specs., Tamdy, 1892, Glasunov leg.; 2 specs., Dzhizak, 1892, Glasunov leg.; 5 specs., Jgam-Berdy, 1892, Glasunov leg.; 1 spec., Kschtut Artutch, 1892, Glasunov leg.; 43 specs., Khodjent env., Golodnaya step, 23.iv.1903, G. Jakobson leg.; 1 spec., Bukhara region, Guzar-Tengi, Khoram, 28.iv.1897, Kaznakov leg.; 1 spec., Kammashi, N of Guzar, Bukhara region, 15.iv.1931, Gussakovskij leg.; 9 specs., Kizilcha, Bukhara region, Guzar env., 23.iv.1926, Gerasimov leg. (all exs. ZIN). AZERBAIJAN: 1 spec., Kobystan, Baku, 16.v.1975, A. Olexa leg. (TLAN). 1 spec., Kyurdamyr, near Baku, 15.v.1923, Bezrukov leg.; 3 specs., Pirsaat valley, 6.vii.1907, collector unknown; 1 spec., Baladzhary near Baku, 5.iv.1927, Kirschenbladt leg.; 2 specs., Lenkoran region, Nova Andreevka, 3.v.1923, Bezrukov leg.; 1 spec., Baku region, Belosovar, 5.v.1923, Bezrukov leg.; 1 spec., Ganja, no date, Dr. Kolenati leg.; 2 specs., Baku env., 18.iv.1927, Kirschenbladt leg. (all exs. ZIN). KYRGYZSTAN: 1 ♂, Kashka-su, v. 1984, J. Palička leg. (TLAN). 8 exs., Przhevalsk, 7.v.1930, Titov leg. (ZIN). 1 spec., Tian-Shan, Musart, no further data (BMNH). 1 spec., Kungey Alatau ridge, Grigoryevskoye canyon, 2000 m, 12–22.vii.1993 A. Ogarkov leg. (CAS).
SYRIA: 1 spec., Syria, no further data (BMNH). 1 spec., Palmyra, 10.–15.v.1995, P. Kabátek leg. (TLAN); 2 specs., Tadmor, Palmyra, Turkish Bath, 12.iii.1977, 1. Orient Exkursion, Inst. f. Zool. Mainz, Prof. R. Kinzelbach leg. (MNHN). ARMENIA: 1 spec., Rozdan, viii. 1981, Kletečka leg. (TLAN). AFGHANISTAN: 7 exs., Nengrahar prov., Jalalabad, 560 m, 20.iv.1967, D. Povolný et coll. leg. (MMBC). 1 ♂ & 1 spec., Laghman prov., Shamakat, 900 m, 22.iv.1972, Kabakov leg.; 1 spec., idem, but river Shamakat, ca 1000m, 31.iii.1972, Kabakov leg.; 1 spec., Herat prov., Anardara, 1200 m, 30.iii.1971, Kabakov leg.; 1 ♂ + 4 specs., Lataband pass, 30 km E Kabul, 4.iv.1970, Kabakov leg.; 1 spec., 15 km W of Jalalabad, 700 m, 30.iv.1972, Kabakov leg.; 1 spec., Nuristan prov., Petch, 1500 m, 21.x.1971, Kabakov leg.; 2 ♂♂ & 2 specs., Nuristan prov., Dara-i-Petch, 1600 m, 21.v.1971, Kabakov leg.; 14 specs., Kabul, 21.iii.1970, Kabakov leg.; 1 ♂ & 6 specs., idem, but 1800 m, 20.iii.1970; 4 specs., idem, but 26.iii.1971; 1 spec., idem, but 7.vi.1973; 1 spec., idem, but 15.iv.1970; 1 spec., idem, but 9.iv.1971; 1 spec., idem, but 12.iii.1971, 1800 m; 6 specs., idem, but 19.iii.1971 (all exs. ZIN).; 1 spec., 46 km NO Jalalabad, 800 m, Sar Kardou, 25.v.1962, Dr. K. Lindberg leg.; 21 specs., Ghourmatch, between Gaiar & Dala Morghab, carcass of Herrison, 16.iv.1959, Dr. K. Lindberg leg.; 6 specs., Decht-Bazar, 27.vii.1962, Dr. K. Lindberg leg., 1 ♀, prov. Bamyan, dirt track from Lanjaw to Bissoude, 2800 m, 23.viii.1978, G. Ledoux leg. (all exs. MNHN). IRAQ: 1 spec., Mesopotamia, without further data (ZIN). 1 spec., Euph. [=Euphrat?], no further data (BMNH); 5 specs., Mosul, no further data (MNHUB). MONGOLIA: 1 spec., Mongolia bor., without further data. (ZIN). IRAN: 1 ♂, Teheran, without further data; 1 spec., Kerman, Sargad, 4.v.1901, N. Zarudnij leg.; 1 spec., idem, but 13.iii.1928, B. Kuznetsov leg. (all exs. ZIN). 1 spec., Kerman, 4.iii.1935, H.E.J. Biggs leg. (BMNH). 1 ♂, Evine (Tehran), no date, Petrovitz leg. (CND). GEORGIA: Tbilisi, 19.iv.1880, collector unknown (ZIN). RUSSIA: 2 specs., Dagestan, Petrovsk, 1.v.1925, Kirishechenko leg.; 1 spec., Sarepta, Bekker leg., no further data; 1 spec., idem, but no date or collector; 3 specs., Stavropol reg., Faust leg.; 1 spec., Stavropol region, Roguli, 1925, collector unknown; 1 spec., Astrakhan, no date, A. Semenov-Tian-Shanskij leg.; 1 spec., Samara, Dr. Bols leg.; 1 spec., Stavropol'skij kray, Mitrofanovskoe, iv. 1925, collector unknown; 6 specs., Selitrennoe, Yenot uezd, 10.vi.1910, Chernovin leg. (all exs. ZIN). 1 spec., South Russia, Kalmykiya, 10 km S Tchernozemelskiy vill., 15.iv.1982, A. Zamesov leg.; 1 spec., Astrakhan reg. near Lower Baskunchak vill., Mt. Bogdo, 43°07.880'N, 46°49.168'E, 23–25.v.2013, A. Shadenkov leg. (all exs. CAS). CYPRUS: 6 specs., Cyprus, Nicosia, 19.iii.[19]35, Th. Shiakides leg. in cow dung (BMNH). INDIA: 1 spec., India, no further data (BMNH). 1 spec., Uttaranchal state, Naini Tal distr., near Sathkol vill., 20–28.vi.2006, S. Saluk leg.; (CAS). PAKISTAN: 1 spec., 22.ii.1978, Gujranwala, S. Kinelski leg. (CND).
Although this species has been recently re-described by the author (
Body length: PEL: 2.25–3.00 mm; APW: 0.75–1.00 mm; PPW: 1.75–2.00 mm; EL: 1.50–1.90 mm; EW: 1.87–2.50 mm.
Body (Fig. 1) roundly oval, convex, cuticle pitch-black usually with greenish hue, shining, but older specimens can be completely dark without hue; legs, mouthparts and antennae dark brown; antennal club black.
Antennal scape (Fig. 2) not particularly thickened, with shallow sparse punctures and two short setae; club round, without visible articulation, entire surface with dense short sensilla intermingled with sparser longer erect sensilla; sensory structures of antennal club (Fig. 3) in form of four ovoid sensory areas on ventral side and one vesicle situated under internal distal margin.
Mouthparts: mandibles (Fig. 2) with rounded outer margin, laterally with deep dense punctures, moderately curved inwardly, mandibular apex pointed; sub-apical tooth obtuse, inconspicuous; labrum (for fig. see
Clypeus (Fig. 2) flat, constricted laterally, with coarse and dense punctures; frontal stria largely interrupted medially, for short distance prolonged onto clypeus, supraorbital stria well impressed, carinate; frontal disc (Fig. 2) with coarse and dense punctures; eyes convex, well visible from above.
Pronotal sides moderately (Fig. 1) narrowing anteriorly, apical angles obtuse, pronotal depressions vaguely impressed, almost absent, anterior incision for head shallow, almost straight in middle; marginal pronotal stria complete; pronotal disc laterally with longitudinal depression, with very coarse and dense punctures, punctures become finer and sparser medially; row of ovoid punctures present along pronotal base; pronotal hypomeron glabrous; scutellum small, but visible.
Elytral epipleuron with scattered fine punctures, area between marginal epipleural stria and elytral margin smooth; marginal epipleural stria fine, complete; marginal elytral stria straight, well impressed and slightly carinate, continued as weakened complete apical elytral stria; along marginal elytral stria a row of round dense punctures present. Humeral elytral stria weakly impressed on basal third; inner subhumeral stria present as short median fragment; all four dorsal elytral striae 1–4 weakly impressed, short, not reaching elytral half apically, in shallow punctures; fourth dorsal elytral stria basally vaguely connected with sutural elytral stria; sutural elytral stria well-impressed and complete, in deep punctures, apically connected with apical elytral stria; entire elytral disc with punctuation, punctures dense and coarse; along elytral margin, on elytral humeri and on interval between fourth dorsal and sutural elytral striae punctation weakens, extreme apex of elytra impunctate.
Propygidium and pygidium densely and coarsely punctate, punctures separated by about half their own diameter.
Anterior margin of median portion of prosternum (Fig. 4) almost straight; marginal prosternal stria present laterally and as a short anterior fragment; prosternal process concave, surface between carinal prosternal striae with scattered fine punctuation, laterally finely strigulate, punctures coarser and deeper; carinal prosternal striae well-impressed, on prosternal apophysis parallel, slightly divergent anteriorly, not connected apically; prosternal foveae deep; lateral prosternal striae carinate, sub-parallel, apically terminating in prosternal foveae.
Anterior margin of mesoventrite (for fig. see
Intercoxal disc of the first abdominal sternite laterally with incomplete stria; except for median part with coarse round punctures, becoming finer along posterior margin.
Protibia (for fig. see
Mesotibia slender, outer margin with two rows of short denticles; setae of outer row regular, dense, shorter than denticles; setae of intermedian row shorter and finer than those of outer row, regular; posterior mesotibial stria almost complete; anterior surface of mesotibia (for fig. see
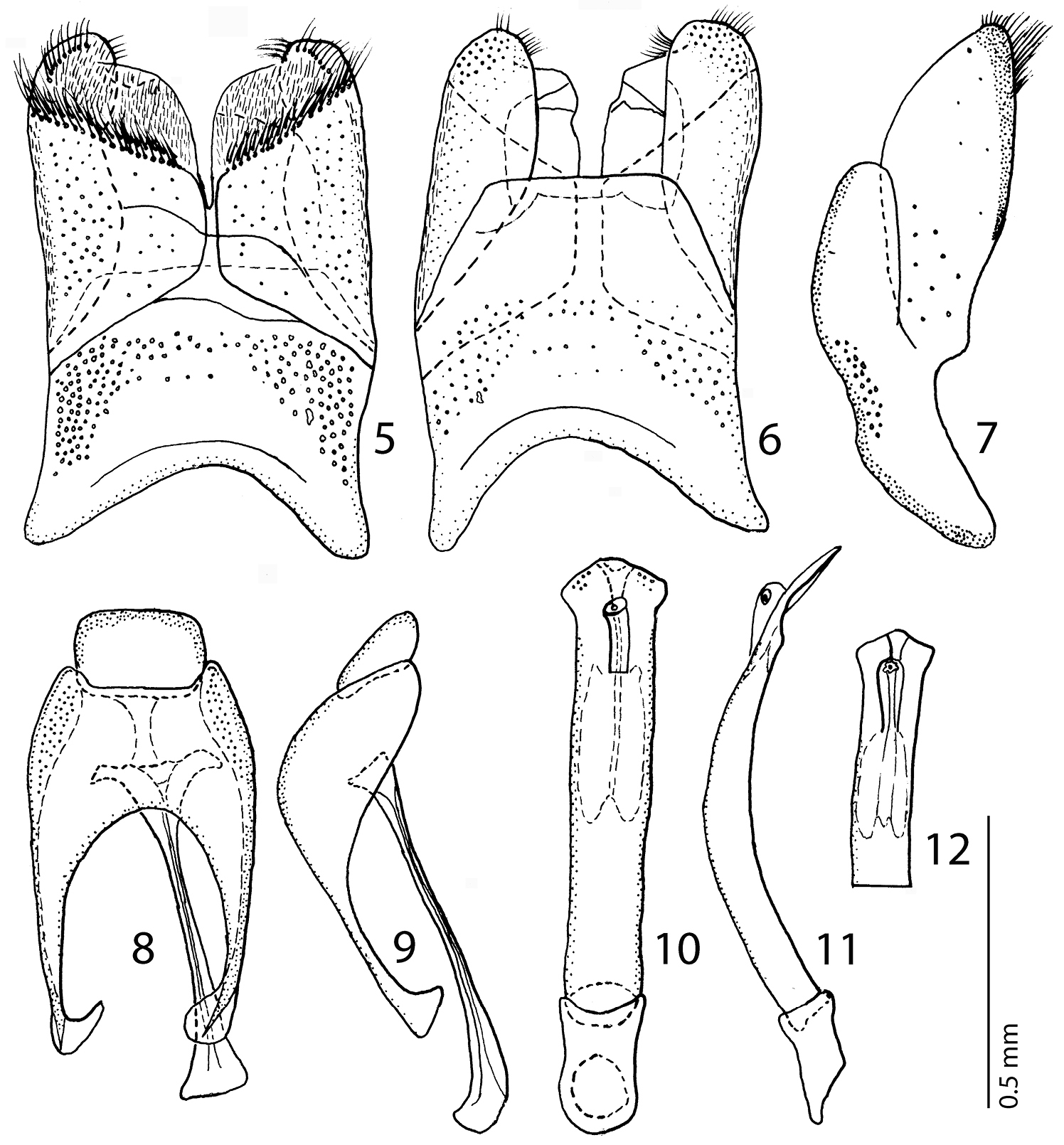
Male genitalia: Eighth sternite (Figs 5–6) widely separated medially, covered with pseudo-pores, apically with numerous close-set setae forming a conspicuous apical brush, velum with dense, much shorter and finer setae; on outer margin fringed with a single row of longer setae; eighth tergite (Fig. 6) apically straight; eighth tergite and eighth sternite fused laterally (Fig. 7). Ninth tergite (Figs 8–9) fused medially, laterally with pseudo-pores; spiculum gastrale (Fig. 8) almost parallel with apical end strongly, and basal end only slightly expanded. Aedeagus (Figs 10–12) slender; basal piece of aedeagus short, ratio of its length: length of parameres 1: 3.50; parameres fused almost along their apical three-fourths; aedeagus constricted apically, thence slightly dilated, curved ventrad (Fig. 11).
Hemisaprinus subvirescens (Ménétries, 1832) habitus.
Hemisaprinus subvirescens (Ménétries, 1832) head, dorsal view.
Hemisaprinus subvirescens (Ménétries, 1832) antennal club, showing the sensory structures.
Hemisaprinus subvirescens (Ménétries, 1832) prosternum.
5 Hemisaprinus subvirescens (Ménétries, 1832) 8th sternite and tergite, ventral view 6 ditto, dorsal view 7 ditto, lateral view 8 Hemisaprinus subvirescens (Ménétries, 1832) 9th + 10th tergites, dorsal view; spiculum gastrale, ventral view 9 Hemisaprinus subvirescens (Ménétries, 1832) 9th + 10th tergites, spiculum gastrale, lateral view 10 Hemisaprinus subvirescens (Ménétries, 1832) aedeagus, dorsal view 11 ditto, lateral view 12 Hemisaprinus subvirescens (Ménétries, 1832) apex of aedeagus, dorsal view.
Russia: Totskiy Rayon, near Samara.
Saprinus cribellatus ab. lutshniki: Lectotype, sex unidentified, left mesotarsus missing, with following labels: circle, gold label, followed by: “Totskij lag / Samarsk. g. / 26.iv. 1917” (hand-written label in Russian); followed by: “Saprinus cribell. / a. lutshniki nov. / A. Reichardt det.” (printed-written); followed by: “Lectotypus / Saprinus lutshniki Rchdt. / Kryzhanovskij det., 66” (red label, printed-written). Paratypes: 1 ♂, with following labels: “Saratov / N.L. Sacharov” (black-margined label, written-printed); followed by: “Paratypus” (red label, printed). 1 spec., with the following labels: “O.B. Don 15.iv.[1]912 / Persianovka / B. Kizeritskij” (printed-written); followed by: “Paratypus” (red label, printed). 1 ♀, with following labels: “G. Temir Ural Obl. / 15.iv.[19]07 / D. Borodin & V. Uvarov” (printed-written in Russian); followed by: “Paratypus” (red label, printed); followed by: “Zoological / Institute RAS / St. Petersburg” (yellow label, printed); followed by: “lutshniki” (yellow, pencil-written label) and: “09-063” (yellow, pencil-written label) added by myself. 2 specs., with following labels: “Peremezhnoe, / okr. Uralska / Lyubishev 1.v.[19]33” (hand-written label in Russian); followed by red label, printed: “Paratypus” (all exs. ZIN).
KAZAKHSTAN: 1 ♂, River Ural near Kharkin, 14.v.1951, L. Arnoldi leg. (NCB); 56 exs., Ural River, near Kharkin, 14.v.1951, L. Arnoldi, under desert plants Atraphaxys (Polygonaceae) (ZIN); 1 spec., Ganibek nat. reserve, 49°23' N, 46°47' E, 1.v.2003, O. Khrulyova leg. (CAS); 2 specs., Ural River, Kharkin, 14.v.1951, L. Arnoldi leg. (MNHN). RUSSIA: 1 ♂, Orenburskaya oblast, 3 km NW Pervomaiskij, Donguz, steppe, 1.v.–28.vi.2009, Kozminykh V.O. leg. (TLAN). 1 spec., Volgograd, ovrag (=ravine) of the Tsaritsa River, 23.iv.1986, Matveev leg.; 2 specs., Samarskaya gubernia, Nikolaevskij distr., Bostanyhoglo leg., 1917; 1 spec., Samara, no date, Dr. Volz leg.; 1 spec., Kalmytskaya ASSR, Priozernij rayon, Tugtyn, 11.v.1976, Iviliev leg.; 1 spec., Petrovsk-port, N. Caucasus, 4.v.1931, M. Ryabov leg.; 1 spec., Tverskaya obl., Pokhot'-Krugloe, Zubtsovskij uezd, 30.v.1925, collector unknown; 3 exs., Kuybyshevskaya obl., Pestravskij rayon, kolkhoz “Rodina”, 14–15.v.1960, collector unknown; 4 specs., idem, but selo “Mosty”, 14.v.1960, under Agropyron plants in a ditch (all exs. ZIN); 1 spec., Kuybyshiev distr., 14.v.1960, Alejnikova leg. (BMNH); 1 spec., Astrakhan reg., 10 km S Upper Baskunchak vill., "Shikli" sands, 4.v.1995, I. Melnik leg. (CAS); 2 specs. Astrakhan reg., Palass distr., N side Elton lake, right side Khara River, 20–31.v.2006, A. Matalin leg. (CAS).
Body length: PEL= 2.75–3.35 mm, APW= 1.00–1.25 mm, PPW= 2.00–2.35 mm, EW= 2.25–2.60 mm, EL= 1.90–2.20 mm. Body (Fig. 13) rectangular oval, convex, elytra widest at humeri; cuticle of elytra on impunctate ‘mirror’ dark brown to black, on punctate part reddish-brown, shining, pronotum dark, almost black; body ventrally dark brown to almost black; abdominal ventrites (except for first visible) rufescent; legs, mouthparts and antennae rufo-castaneous; antennal club somewhat darker.
Antennal scape (Fig. 14) slightly thickened, substrigulate, finely punctate, lower margin carinate, with few short setae; club (Figs 14, 15) round, pointed apically, without visible articulation, entire surface with dense short sensilla intermingled with sparser longer erect sensilla; sensory structures of antennal club in form of four ovoid sensory areas on ventral side (Fig. 15); vesicle(s) not examined.
Mouthparts: mandibles (Fig. 14) stout, densely punctate, mandibular apex pointed; sub-apical tooth of left mandible not examined; labrum convex, densely punctate, with slight median concavity interrupted by semi-globular convexity; labral pits deep, each with two well-sclerotized long setae; terminal labial palpomere elongated, about twice as long as pen-ultimate, its width about one-third its length; mentum sub-trapezoid, anterior margin medially with deep notch surrounded with sparse rather long setae, lateral margins with single row of sparse shorter ramose setae; cardo of maxilla with few short setae; stipes triangular, with three short setae; terminal maxillary palpomere elongated, pointed apically, about three times as long as pen-ultimate; its width about one-third its length.
Clypeus (Fig. 14) flat, gradually sloping down laterally, rugulose-lacunose; frontal stria broadly interrupted medially, for short distance prolonged onto clypeus, supraorbital stria well impressed, carinate; frontal disc (Fig. 14) very coarsely and densely punctate; eyes convex, well visible from above.
Pronotal sides (Fig. 13) on basal half moderately narrowing anteriorly, strongly narrowing on apical half; apical angles obtuse; median emargination for head shallow; pronotal depressions absent; marginal pronotal stria complete, somewhat weakened behind head; pronotal disc shining on most part, with sparse punctures separated by several times their diameter, laterally and behind head more coarse and dense punctures appear, punctures form a depressed band of confluent punctuation, between it and pronotal margin a narrow band with simple punctuation present; several rows of ovoid punctures present along pronotal base; pronotum with faint ante-scutellar depression; pronotal hypomeron asetose, in fine scattered punctures; scutellum well visible.
Elytral epipleura glabrous; marginal epipleural stria fine, complete; marginal elytral stria straight, well impressed and slightly carinate, continued as weakened complete apical elytral stria. Humeral elytral stria weakly impressed on basal fourth, doubled, surface between it and second dorsal elytral stria in longitudinal irregular strioles; inner subhumeral stria present as short median fragment; elytra with thin striae 1-4; striae with weak punctures within, except for first stria which is shorter than the others reaching approximately elytral half apically; fourth dorsal elytral stria basally connected with sutural elytral stria by broad arch; sutural elytral stria well-impressed and complete, fine punctures within, apically connected with apical elytral stria, between it and elytral suture a row of fine punctures present; elytral humeri and flanks almost impunctate, elytral disc along sutural elytral stria on apical two-fifths with dense, almost confluent punctation, forming longitudinal rugae; weakened punctuation slightly enters elytral intervals, apically punctuation weakens, leaving an impunctate band before extreme elytral apex; rest of elytral disk with large impunctate ‘mirror’, most prominent on 2-4 elytral intervals; this mirror occasionally bears fine scattered punctures, in most cases limited to second elytral interval.
Propygidium and pygidium densely and coarsely punctate, punctures separated by about half to their own diameter; interspaces with microsculpture.
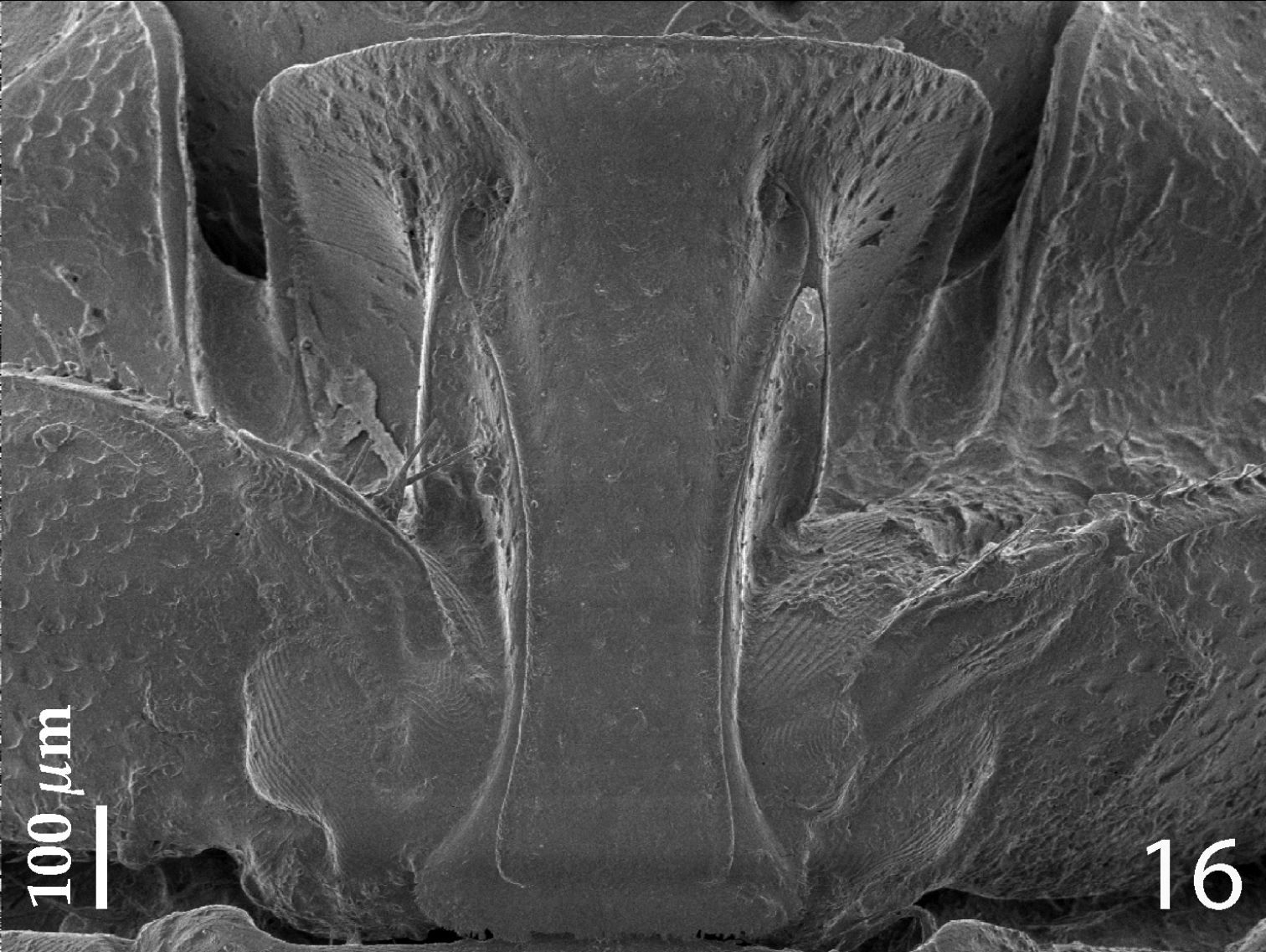
Anterior margin of median portion of prosternum (Fig. 16) rounded; marginal prosternal stria present laterally and as short anterior fragment; prosternal process on apical sixth distinctly elevated in respect to the remaining part; surface between carinal prosternal striae slightly convex, with scattered fine punctation, punctures surrounded by microsculpture; carinal prosternal striae well-impressed, parallel on prosternal apophysis, thence divergent anteriorly, terminating in deep and large prosternal foveae; lateral prosternal striae carinate, sub-parallel, apically terminating near the point where carinal prosternal striae enter prosternal foveae.
Anterior margin of mesoventrite broadly, but shallowly inwardly arcuate; discal marginal mesoventral stria well impressed, carinate; disc of mesoventrite with dense deep large punctures intermingled with much smaller microscopic punctuation; meso-metaventral sutural stria marked as straight row of punctures; intercoxal disc of metaventrite slightly convex with scattered microscopic punctures, becoming coarser and denser along basal margin; lateral metaventral stria well impressed, carinate, almost straight, shortened; lateral disc of metaventrite concave, with regular shallow large setigerous punctures; metepisternum with denser and coarser punctation, punctures almost confluent; fused metepimeron with somewhat sparser punctures; metepisternum + fused metepimeron with metepisternal stria.
Intercoxal disc of first abdominal ventrite incompletely striate laterally; on basal third with irregular scattered fine punctures separated by several times their own diameter; rest of first visible abdominal ventrite with scattered microscopic punctuation.
Protibia slightly dilated, outer margin apically with single low tooth topped by tiny denticle, in proximal direction three low triangular teeth topped by short rounded denticle appear, all three approximately of the same size, followed by another low tooth (occasionally bearing two tiny denticles), followed by a single tiny denticle growing out directly from outer margin of protibia; setae of outer row regular, rather short; protarsal groove rather deep; anterior protibial stria very shortened (absent?); setae of intermedian row situated on ridge delimiting proximal margin of protarsal groove; single tarsal denticle present near tarsal insertion; protibial spur short, bent, growing out from apical margin of protibia; apical margin of protibia posteriorly with three tiny denticles almost abutting each other; outer part of posterior surface obscurely variolate, punctate, separated from imbricate median part of posterior surface by vague boundary and row of short sclerotized setae; posterior protibial stria complete, bearing a row of fine sparse setae along its length, terminating in two tiny inner denticles; inner row of setae double, setae dense and short.
Mesotibia slender, outer margin with a single row of short denticles situated on low teeth; setae of outer row regular, sparse, about as long as denticles themselves; setae of intermedian row shorter and finer than those of outer row, regular; posterior mesotibial stria almost complete; anterior surface of mesotibia imbricate, with another row of approximately seven shorter denticles than those of outer row; anterior mesotibial stria complete, terminating in single tiny inner anterior denticle; mesotibial spur short; apical margin of mesotibia anteriorly with three short denticles; claws of apical tarsomere slightly bent, shorter than half its length; metatibia slenderer and longer than mesotibia, outer margin with approximately five short denticles situated on even lower teeth than those of mesotibia; apical-most tooth bearing two denticles; setae of outer row distinctly longer than denticles themselves; anterior face of metatibia punctate, with a row of approximately five tiny denticles; claws of apical-most metatarsomere longer than half of its length; metatibia otherwise similar to mesotibia.
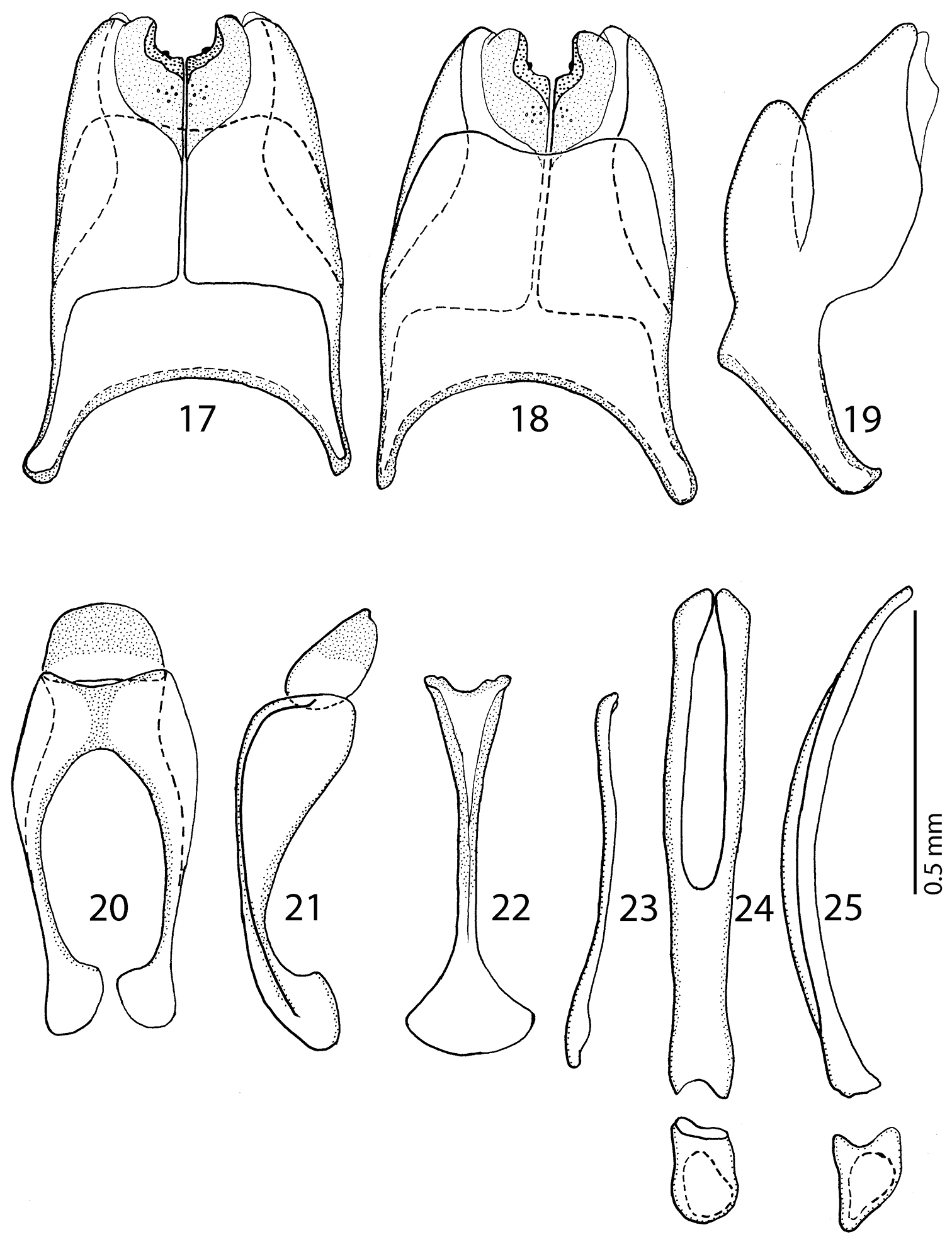
Male genitalia: Eighth sternite (Figs 17–18) longitudinally medially separated, apically with medially-sized velum covered with dense micro-pores and several larger pseudopores medially; eighth tergite inwardly arcuate; eighth tergite and sternite fused laterally (Fig. 19). Ninth tergite (Fig. 20) medially with strong longitudinal sclerotization, apically inwardly slightly arcuate; tenth tergite outwardly arcuate apically, basally slightly inwardly arcuate. Spiculum gastrale (Figs 22–23) basally strongly dilated, outwardly arcuate; apically slightly triangularly dilated, without typical inwardly-turned apical “tails”. Aedeagus (Figs 24–25) sub-parallel, parameres fused approximately on their apical halves, apex of aedeagus blunt. Basal piece of aedeagus rather short, ratio to parameres approximately 1:6; aedeagus curved laterally (Fig. 25).
This species is very similar to Hemisaprinus cyprius, differing from it chiefly by the presence of a second dorsal elytral stria, absent with Hemisaprinus cyprius and aciculate elytral punctuation, as well as shining pronotum (matt in cyprius).
Hemisaprinus lutshniki (Reichardt, 1941) paratype, habitus.
Hemisaprinus lutshniki (Reichardt, 1941) paratype, head, dorsal view.
Hemisaprinus lutshniki (Reichardt, 1941) paratype, antennal club, ventro-lateral view showing sensory structures of the antenna.
Hemisaprinus lutshniki (Reichardt, 1941) paratype, prosternum.
17 Hemisaprinus lutshniki (Reichardt, 1941) paratype, 8th sternite and tergite, ventral view 18 ditto, dorsal view 19 ditto, lateral view 20 Hemisaprinus lutshniki (Reichardt, 1941) paratype, 9th + 10th tergites, dorsal view 21 ditto, lateral view 22 Hemisaprinus lutshniki (Reichardt, 1941) paratype, spiculum gastrale, ventral view 23 ditto, lateral view 24 Hemisaprinus lutshniki (Reichardt, 1941) paratype, aedeagus, dorsal view 25 ditto, lateral view.
Cyprus, Kyrenia.
Saprinus cyprius: Holotype, ♀, side-mounted on triangular mounting point with left antennal club missing, female genitalia extracted, glued to another mounting label below the specimen, with the following labels: “Cypern, Kyrenia / 22/2 - 14/3 [19]62 / Th. Palm leg.” (printed); followed by: “HOLOTYPE / SAPRINUS / CYPRIUS / G. DAHLGREN / 25.1.1981” (written in black ink); followed by: “Zool. Mus. Lund Sweden / Type No. 2280: 1-2 / Histeridae” (printed-written); followed by: “MZLU / 2013 / 313” (green label, printed) (MZLU). Paratype, female, with following labels: “♀” (printed); followed by: “KYRENIA / CYPERN / 28.2.1962 / T. PALM LEG.” (written in black ink); followed by: “PARATYPE / SAPRINUS / CYPRIUS / G. DAHLGREN / 25.1.1981” (written in black ink); followed by: “Type No. / 2280:2” (printed-written); followed by: “MZLU / 2013 / 314” (green label, printed) (MZLU).
Body length: PEL: 3.00–3.05 mm; APW: 1.00–1.05 mm; PPW: 2.15–2.25 mm; EL: 1.85–2.10 mm; EW: 2.35–2.50 mm. Body (Fig. 26) roundly oval, convex, elytra widest at humeri; cuticle of elytra castaneous, shining, pronotum dark, almost black, matt; body ventrally dark brown to almost black; abdominal ventrites (except for first visible) rufescent; legs, mouthparts and antennae rufo-castaneous; antennal club somewhat darker.
Antennal scape (Fig. 27) slightly thickened, densely punctate, lower margin carinate, with few short setae; club round, pointed apically, without visible articulation, entire surface with dense short sensilla intermingled with sparser longer erect sensilla; sensory structures of antennal club in form of four ovoid sensory areas on ventral side; vesicle(s) not examined.
Mouthparts: mandibles with rounded outer margin, densely punctate, mandibular apex pointed; sub-apical tooth of left mandible not examined; labrum convex, densely punctate; labral pits deep, each with two well-sclerotized long setae; terminal labial palpomere elongated, about twice as long as pen-ultimate, its width about one-third its length; mentum sub-trapezoid, anterior margin medially with deep notch surrounded with sparse rather long setae, lateral margins with single row of sparse shorter ramose setae; cardo of maxilla with few short setae; stipes triangular, with three short setae; terminal maxillary palpomere elongated, pointed apically, about three times as long as pen-ultimate; its width about one-third its length.
Clypeus (Fig. 27) flat, gradually sloping down laterally, coarsely and densely punctate, punctures almost confluent; frontal stria largely interrupted medially, for short distance prolonged onto clypeus, supraorbital stria well impressed, carinate; frontal disc (Fig. 27) with coarse and dense punctures similar to those of clypeus, punctures in bottom with microsculpture; eyes convex, well visible from above.
Pronotal sides (Fig. 26) on basal half moderately narrowing anteriorly, strongly narrowing on apical half; apical angles obtuse; median emargination for head shallow; pronotal depressions absent; marginal pronotal stria complete, somewhat weakened behind head; pronotal disc matt due to very dense microsculpture, laterally with very coarse and dense punctures, separated by less than their own diameter, punctures become finer and sparser medially where they are separated by several times their diameter; several rows of ovoid punctures present along pronotal base; pronotum with ante-scutellar depression; pronotal hypomeron asetose, with fine scattered punctures; scutellum well visible.
Elytral epipleuron with scattered fine punctures; marginal epipleural stria fine, complete; marginal elytral stria straight, well impressed and slightly carinate, continued as weakened complete apical elytral stria. Humeral elytral stria weakly impressed on basal fourth, doubled, surface mesad from it with irregular longitudinal strioles; inner subhumeral stria present as short median fragment; elytra with thin, impunctate striae 1, 3-4 (stria 2 absent); striae stopping short of elytral half apically; fourth dorsal elytral stria basally connected with sutural elytral stria by broad arch; sutural elytral stria well-impressed and complete, fine punctures within, apically connected with apical elytral stria; elytral humeri and flanks almost impunctate, elytral disc along sutural elytral stria on apical 2/5 with fine regular punctuation, punctures aciculate, separated by about twice their own diameter, interspaces with very dense microsculpture, punctuation enters elytral intervals, reaching its climax along first dorsal elytral stria where it reaches elytral base, toward elytral apex microsculpture as well as punctuation weakens; extreme elytral apex impunctate.
Propygidium and pygidium densely and coarsely punctate, punctures separated by about half to their own diameter; interspaces with microsculpture.
Anterior margin of median portion of prosternum (Fig. 28) almost straight; marginal prosternal stria present laterally and as short anterior fragment; prosternal process between carinal prosternal striae slightly convex, surface between carinal prosternal striae with scattered fine punctuation, punctures surrounded by microsculpture; carinal prosternal striae well-impressed, parallel on prosternal apophysis, thence divergent anteriorly, terminating in deep and large prosternal foveae; lateral prosternal striae carinate, sub-parallel, apically terminating near the point where carinal prosternal striae enter prosternal foveae.
Anterior margin of mesoventrite (Fig. 28) broadly inwardly arcuate; discal marginal mesoventral stria well impressed, carinate; disc of mesoventrite with dense shallow large punctures intermingled with much smaller microscopic punctuation; meso-metaventral sutural stria marked as a straight row of punctures; intercoxal disc of metaventrite slightly convex with scattered microscopic punctures, becoming coarser and denser along basal margin; lateral metaventral stria well impressed, carinate, almost straight, shortened; lateral disc of metaventrite concave, with dense shallow large punctures; metepisternum with even denser and coarser punctation, punctures almost confluent; fused metepimeron with somewhat sparser punctures; metepisternum + fused metepimeron with metepisternal stria, which is almost unrecognizable under coarse punctuation.
Intercoxal disc of the first abdominal ventrite incompletely striate laterally; on basal third with irregular larger punctures separated by about their own to twice their diameter; rest of first visible abdominal ventrite with scattered microscopic punctuation.
Protibia slightly dilated, outer margin with four moderately large triangular teeth topped by short rounded denticle, diminishing in size in proximal direction, followed by three tiny denticles growing out directly from outer margin of protibia; setae of outer row regular, rather short; protarsal groove deep; anterior protibial stria shortened on basal half; setae of intermedian row not examined; two tarsal denticles present near tarsal insertion; protibial spur short, bent, growing out from apical margin of protibia; apical margin of protibia posteriorly with four tiny denticles almost abutting each other; outer part of posterior surface obscurely variolate, punctate, separated from glabrous median part of posterior surface by vague boundary and row of short sclerotized setae; posterior protibial stria complete, terminating in several tiny inner denticles; inner row of setae double, setae dense and short.
Mesotibia slender, outer margin with a single row of short denticles situated on low teeth; setae of outer row regular, sparse, longer than denticles; setae of intermedian row shorter and finer than those of outer row, regular; posterior mesotibial stria not examined; anterior surface of mesotibia glabrous, with another much sparser row of shorter denticles than those of outer row; anterior mesotibial stria complete, terminating in single tiny inner anterior denticle; mesotibial spur short; apical margin of mesotibia anteriorly with three short denticles; claws of apical tarsomere slightly bent, shorter than half its length; metatibia slenderer and longer than mesotibia, in all aspects similar to it, but denticles on outer margin much sparser, situated on even lower teeth than those of mesotibia; apical-most tooth bearing two denticles.
Male unavailable.
Hemisaprinus cyprius (Dahlgren, 1981) paratype, habitus.
Hemisaprinus cyprius (Dahlgren, 1981) paratype, head, dorsal view.
Hemisaprinus cyprius (Dahlgren, 1981) paratype, prosternum + mesoventrite.
| 1(2) | Almost entirely dark-brown to black species, dorsal cuticle often with slight greenish metallic hue (Fig. 1) carinal prosternal striae stopping short of prosternal foveae, lateral prosternal striae terminate in them (Fig. 4); widely distributed species | Hister subvirescens (Ménétries, 1832) |
| 2(1) | Usually bi-colored species: pronotum dark, almost black; elytra at least partly reddish-brown; dorsal cuticle without greenish hue, with slight to prominent bronze metallic tinge (Figs 13, 26); carinal prosternal striae terminate in prosternal foveae, lateral prosternal striae terminate near apices of carinal prosternal striae (Figs 16, 28). | |
| 3(4) | Second dorsal elytral stria absent; elytral ‘mirror’ impunctate, with bronze lustre; punctate part of the elytra with dense aciculate punctures and microsculpture; pronotum matt, medially almost impunctate (Fig. 26); species from Cyprus | Hemisaprinus cyprius (Dahlgren, 1981) |
| 4(3) | Second dorsal elytral stria present; elytral ‘mirror’ with sparse scattered punctures, with slight bronze lustre; punctures on punctate part of elytra less dense, not aciculate, microsculpture absent; pronotum wholly punctate (Fig. 13); species from southern Russia, west Siberia and Kazakhstan | Hemisaprinus lutshniki (Reichardt, 1941) |
Although
Hemisaprinus, although presumably related to Saprinus based on external as well as genitalic characters (Lackner, unpublished), is presumed to be monophyletic sharing the synapomorphy of present prosternal foveae. It contains three species that, on one hand, share the synapomorphy of the presence of prosternal foveae, on the other hand, however, the species differ in the arrangements of the two sets of prosternal striae. Carinal prosternal striae of Hister subvirescens do not enter the prosternal foveae; while the lateral prosternal striae do. In the case of the two other species (Hemisaprinus lutshniki and Hemisaprinus cyprius) the carinal prosternal striae do terminate in the prosternal foveae, while the lateral prosternal striae terminate near the apices of carinal prosternal striae. According to my recent studies on the morphology of the Saprininae, the configuration of the two sets of prosternal striae was found to be a rather variable character, even within one genus (and even within one species!) and I was unable to score this character unambiguously or parse it into discrete character states. Hence, I refrained from using this character in my phylogenetic studies (Lackner, unpublished) and do not use the different arrangements of the two sets of striae to further split Hemisaprinus.
On the other hand, a very similarly structured prosternal process, including the prosternal foveae is found among some members of the Nearctic and Neotropical subgenus Hesperosaprinus Wenzel, 1962 of the genus Euspilotus Lewis, 1907. The author is not familiar with most members of this species-rich subgenus (45 currently valid species,
All curators of the aforementioned institutes as well as proprietors of private collections that loaned me material or provided the data are being thanked. Special thanks to P. Schüle (Herrenberg, Germany) for his help with the translation from the German language and Alexander Moyseyko (Saint Petersburg, Russia), for his help with the deciphering and translation of the Russian labels of the specimens housed at ZIN, as well as all who gave me specimens. Thanks are also due to an anonymous reviewer and editor of the Histeroidea section for ZooKeys for their critical reading of the manuscript. This research received support from the SYNTHESYS Project http://www.synthesys.info/, which is financed by the European Community Research Infrastructure Action under the FP7 Integrating Activities Program.