Citation: Valdez-Mondragón A, Mendoza JI, Francke OF (2014) First record of the mygalomorph spider family Paratropididae (Arachnida, Araneae) in North America with the description of a new species of Paratropis Simon from Mexico, and with new ultramorphological data for the family. ZooKeys 416: 1–21. doi: 10.3897/zookeys.416.7253
A new species of the genus Paratropis is described from North America: Paratropis tuxtlensis sp. n., from a tropical rainforest in Veracruz, Mexico. This is the fifth Paratropis and the tenth paratropidid species described and the first North American record of this Neotropical family. The species is described based on adult males and females, and juveniles. The juveniles show ontogenetic variation in the number of cuspules on the labium and endites, and in the number and position of leg trichobothria. This is the second Paratropis species, and the third paratropidid known from both sexes. The scanning electron photographs (SEM) reveal new morphological data and contribute to the knowledge of the family.
Mygalomorphae, Spider taxonomy, North America, Tropical rainforest
The spider family Paratropididae Simon, 1889 is currently composed of four genera and nine species, being one of the less known spider families in the infraorder Mygalomorphae (Platnick, 2014). The genus with the highest number of species is Paratropis Simon, 1889 with four species: Paratropis papilligera F. O. P.-Cambridge, 1896, Paratropis sanguinea Mello-Leitão, 1923; Paratropis scruposa Simon, 1889; and Paratopis seminermis Caporiacco, 1955. The genus Melloina Brignoli, 1985 is composed of three species: Melloina gracilis (Schenkel, 1953); Melloina rickwesti Raven, 1999; and Melloina santuario Bertani, 2013. The genera Anisaspis Simon, 1891 and Anisaspoides F. O. P.-Cambridge, 1896 have one species each: Anisaspis tuberculata Simon, 1891 and Anisaspoides gigantea F. O. P.-Cambridge, 1896. Three of the nine previously described species are known only from males (Melloina gracilis, Melloina santuario, and Paratropis papilligera); two are known from both male and female (Melloina santuario and Paratropis papilligera); and the remaining four are known only from females. Although some species, such as the recently described Melloina santuario have recorded juveniles, juveniles paratropidid specimens have never been described.
The majority of the species have a natural distribution in South America, principally in Brazil, with other species distributed in Venezuela and Peru. The northern-most records of the family Paratropididae are in Central America (Panama) and the West Indies (St. Vincent) (
In this work, we describe a new species of Paratropis from a tropical rainforest from Veracruz, Mexico, based on juveniles and adults. It is the second species for the genus, and the third species for the family Paratropididae for which adults of both sexes are known. Juveniles specimens for the family are described for the first time. New morphological characteristics, distribution records, and natural history observations are presented.
The specimens were collected manually and deposited in ethanol (80%). Samples for future molecular studies are cold-stored in vials with ethanol (96%). The general description of the species and terminology of the chaetotaxy follows
The specimens were examined, measured and photographed with a Nikon SMZ 645 stereoscope; measurements are in millimeters (mm). Female epigyna were dissected in ethanol (80%) and cleaned in KOH (10%) for 15 minutes. All structures photographed and drawn under the stereoscope were submerged first in gel alcohol (available commercially as a hand cleaner), the firm consistency of the gel allowing the immobilization and positioning of the structure. The structure suspended in the gel alcohol was then covered with liquid ethanol 80% to minimize diffraction during examination and photography. The morphological structures were dissected and cleaned (first with a needle and a fine paintbrush, then with an ultrasonic cleaner at 20–40 kHz to remove the soil particles encrusted on the exoskeleton); subsequently they were critical-point dried, and examined at low vacuum in a HITACHI S-2460N scanning electron microscope (SEM) to take the photomicrographs. All scale measurements on SEM photomicrographs are in microns. The map was done with ArcView GIS version 3.2 (
Paratropis scruposa Simon, 1889
The genus can be diagnosed with the following combination of characters (after
MEXICO: Veracruz: male holotype (CNAN-T0766) from Estación de Biología Tropical “Los Tuxtlas”, Universidad Nacional Autónoma de México (UNAM), Municipio San Andrés Tuxtla (18.58500°N, 95.07510°W, 1039 m), 10 November 2012; A. Valdez. O. Francke, G. Montiel, J. Cruz, R. Monjaraz Cols. Paratypes: 2 males (CNAN-T0768 and T0769), same data as holotype. 1 female (CNAN-T0822), same locality as holotype, 27 August 2005; A. Valdez. O. Francke, H. Montaño, M. Córdova, A. Jaimes Cols. 2 females (CNAN-T0767 and T0821) from 1 km SE of Díaz Ordaz, Municipio San Andrés Tuxtla (18.52775°N, 95.08691°W, 480 m), 14 June 2011; A. Valdez. O. Francke, C. Santibáñez, J. Cruz, R. Monjaraz, G. Contreras Cols.
MEXICO: Veracruz: 1 immature (CNAN), same data as holotype. 1 immature (CNAN), same locality as holotype, 11 January 2012; O. Francke, G. Montiel, J. Cruz, R. Monjaraz Cols.
Distinguished from Paratropis papilligera (the other species where the male is known) by the male palp with conical tibia (Fig. 14), in Paratropis papilligera the tibia is cylindrical (
Paratropis tuxtlensis sp. n. Male. 1 Carapace, dorsal view 2 Prosoma, ventral view, showing the sternum, labium, endites and chelicerae 3 Opisthosoma, dorsal view 4 Carapace, right lateral view 5–6 Ocular region, dorsal and lateral views, respectively 7 Endites and labium, ventral view 8 Spinnerets, ventral view (arrows indicate the PMS) 9 Left chelicerae, teeth on promargin (left) and retromargin (right) 10–13 Bulb and embolus, prolateral, retrolateral, dorsal, and ventral views respectively. Scales: 0.4 mm (Figures 5, 6), 0.5 mm (Figures 7, 9–13), 1 mm (Figure 8), 2 mm (Figures 1–4).
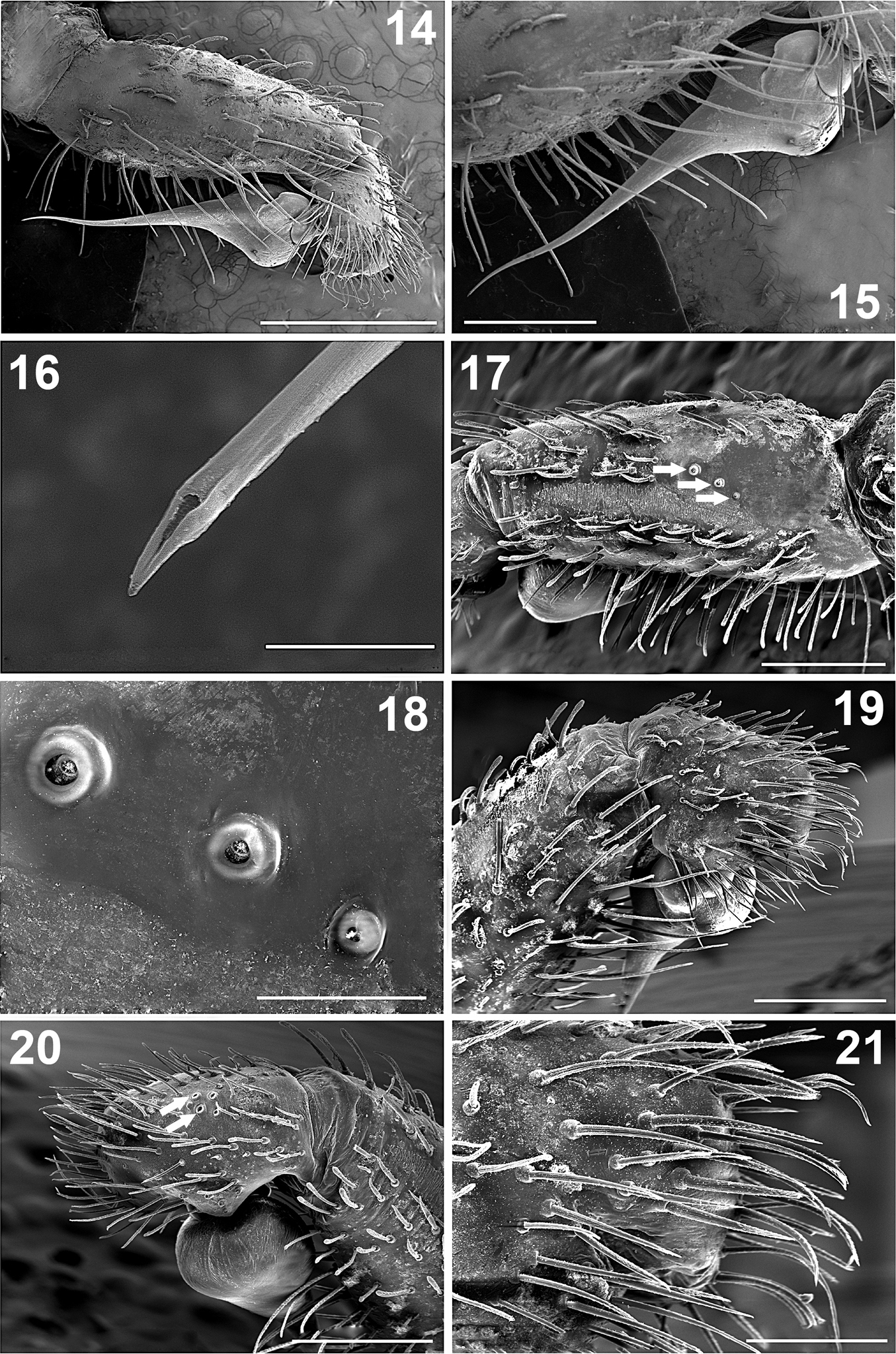
Paratropis tuxtlensis sp. n. Male 14 Left palp, prolateral view 15 Left palp, detail of bulb and embolus, prolateral view 16 Detail of the embolus opening, distal view 17 Left palp tibia, dorsal-retrolateral view (arrows indicate the trichobothria) 18 Detail of the trichobothria sockets on the palp tibia 19 Left palp, tibia and tarsus, prolateral view 20 Left palp, tibia and tarsus, retrolateral view (arrows indicate the trichobothria) 21 Detail of the setae on tarsus of left palp, prolateral view. Scales: 10 µm (Figure 16), 100 µm (Figure 18), 200 µm (Figure 21), 500 µm (Figures 15, 17, 19, 20), 1 mm (Figure 14).
Holotype male (CNAN-T0766). Body length 8.20 (not including chelicerae and spinnerets); chelicerae length 1.50; carapace length 4.90, width 4.00; opisthosoma length 5.20, width 3.70.
Coloration: The general coloration under alcohol is the same as soil particles encrusted on the body, which is pale brown (Figures 1–4, 6, 8). Chelicerae orange ventrally (Figures 2, 7, 9), becoming brown dorsally (Figures 1, 4), fangs of chelicerae dark reddish brown (Figure 2). The carapace has reddish coloration when the soil particles are cleaned. Sternum pale orange; endites and labium orange (Figure 2). Legs olive color when soil particles are cleaned, becoming paler on tibia, metatarsi, and tarsi. The opisthosoma was difficult to clean, even with longer time in the ultrasonic cleaner, and the coloration could be similar to the carapace. Spinnerets pale yellow.
Carapace: Orbiculate, concave posteriorly (Figure 1). Eye tubercle elevated; fovea shallow, slightly recurved, width 0.4, visible only when the carapace is cleaned of soil encrustations. All eyes well developed; in dorsal view anterior eye row slightly recurved, posterior eye row recurved. Eye sizes and interocular distances: AME 0.24; ALE 0.26; PME 0.16; PLE 0.26; AME–AME 0.08; AME–ALE 0.04; PME–PME 0.38; PME–PLE 0.05; ALE–PLE 0.08. Ocular tubercle raised: 0.74 length; 0.88 width; clypeus lacking (Figures 5, 6).
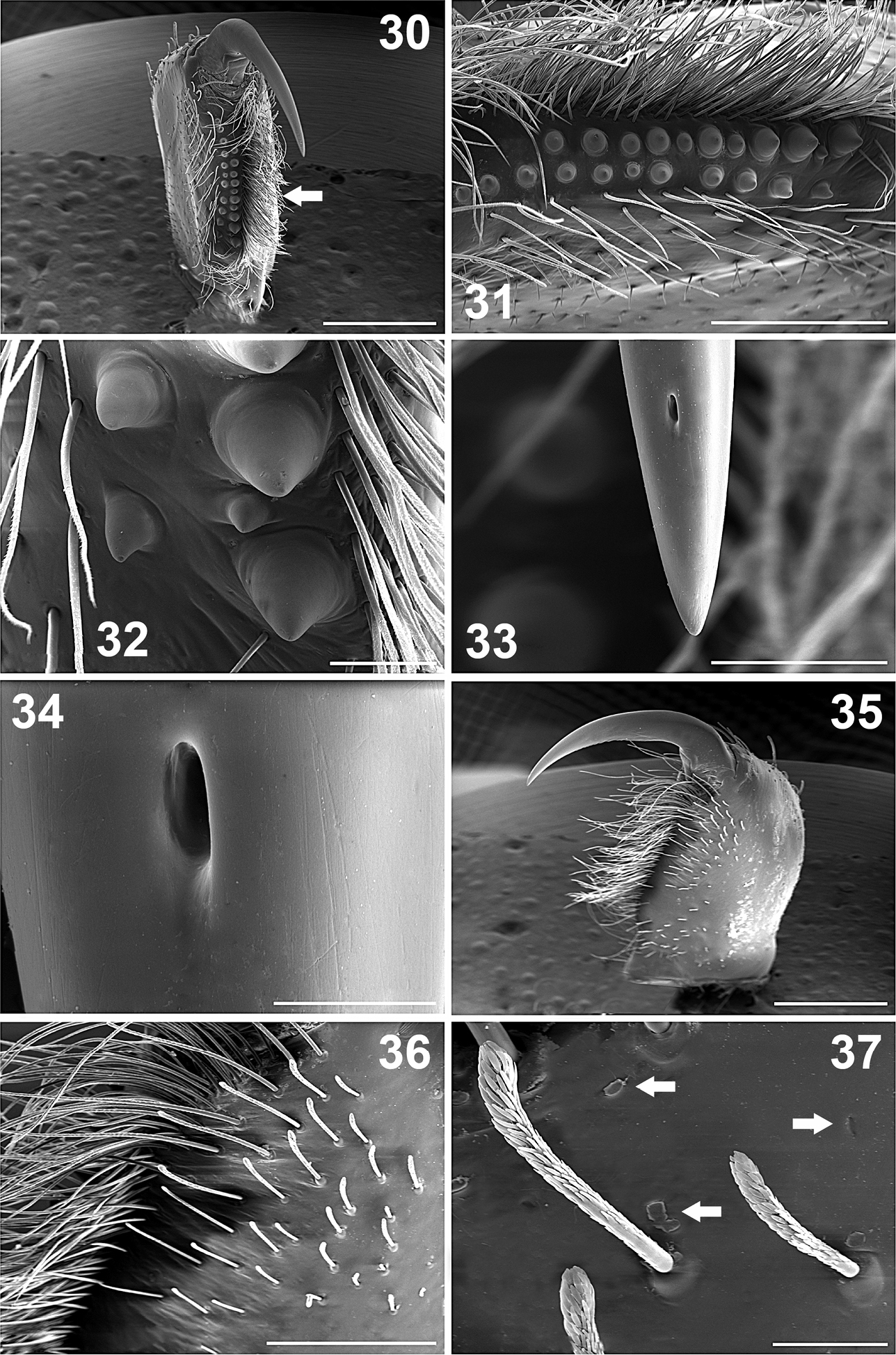
Palps: Bulb pyriform (Figures 10–13, 14, 15), spermatic duct visible through integument (Figures 10–13). Embolus very long and conical, filiform apically (Figures 10–13), with spermatic opening distally (Figure 16). Tarsus with two types of setae: (i) numerous long, scattered, slightly curved, acuminate setae (Figure 21); (ii) long, clubbed setae retrolaterally (Figure 20). Tarsus with four medial-dorsal trichobothria (arrows, Figure 20). Tibiae ventrally with numerous long, curved setae (Figures 14, 15); with five trichobothria, two medial-prolateral, and three medial-retrolateral (arrows Figures 17; 18, 46). Tibiae with long, clubbed setae pro- and retrolaterally (Figures 19, 20). Patellae with numerous, curved, barbed setae. Femora concave prolaterally, dorsally with few clubbed setae on distal part. Trochanters cylindrical, with clubbed setae dorsally and ventrally. All palps segments are covered with encrusted soil particles, except the bulbs, embolus (Figures 10–13), and prolateral region of femora and trochanters.
Chelicerae: Cheliceral furrow promargin and retromargin with short, wide, conical teeth, wider on retromargin than on promargin (Figures 9, 30–32); promargin with 11 teeth, retromargin with 9 teeth (Figure 9); on both margins the proximal teeth are wider and longer than distal teeth (Figures 9, 31, 32). Retromargin of chelicerae with numerous long, barbed setae (Figures 30, 31), more numerous and longer than on promargin (Figure 31). Retrolateral face with clubbed setae, curved distally (Figures 36, 37), becoming shorter mesally (Figures 35, 36). Fang with venom gland duct opening dorsal sub-distal (Figures 33, 34). Cuticle on retrolateral face of chelicerae with numerous glandular pores (arrows, Figures 37).
Endites: Longer than wide, with small conical projection anteriorly (Figures 2, 7, 22, 23). Prolaterally with numerous long, curved, barbed setae (Figures 22, 23), shorter proximally (Figures 22); ventrally with scattered, long, curved setae (Figures 22–24). Endites ventrally with numerous, scattered, finger-shaped cuspules; 42 cuspules on right endite and 40 on left one (Figures 7, 24, 25). Endites without pores ventrally (Figures 24, 25). Retrolateral area with small, spine-like setae (Figure 26). The cuticle is not encrusted with soil particles (Figures 2, 7).
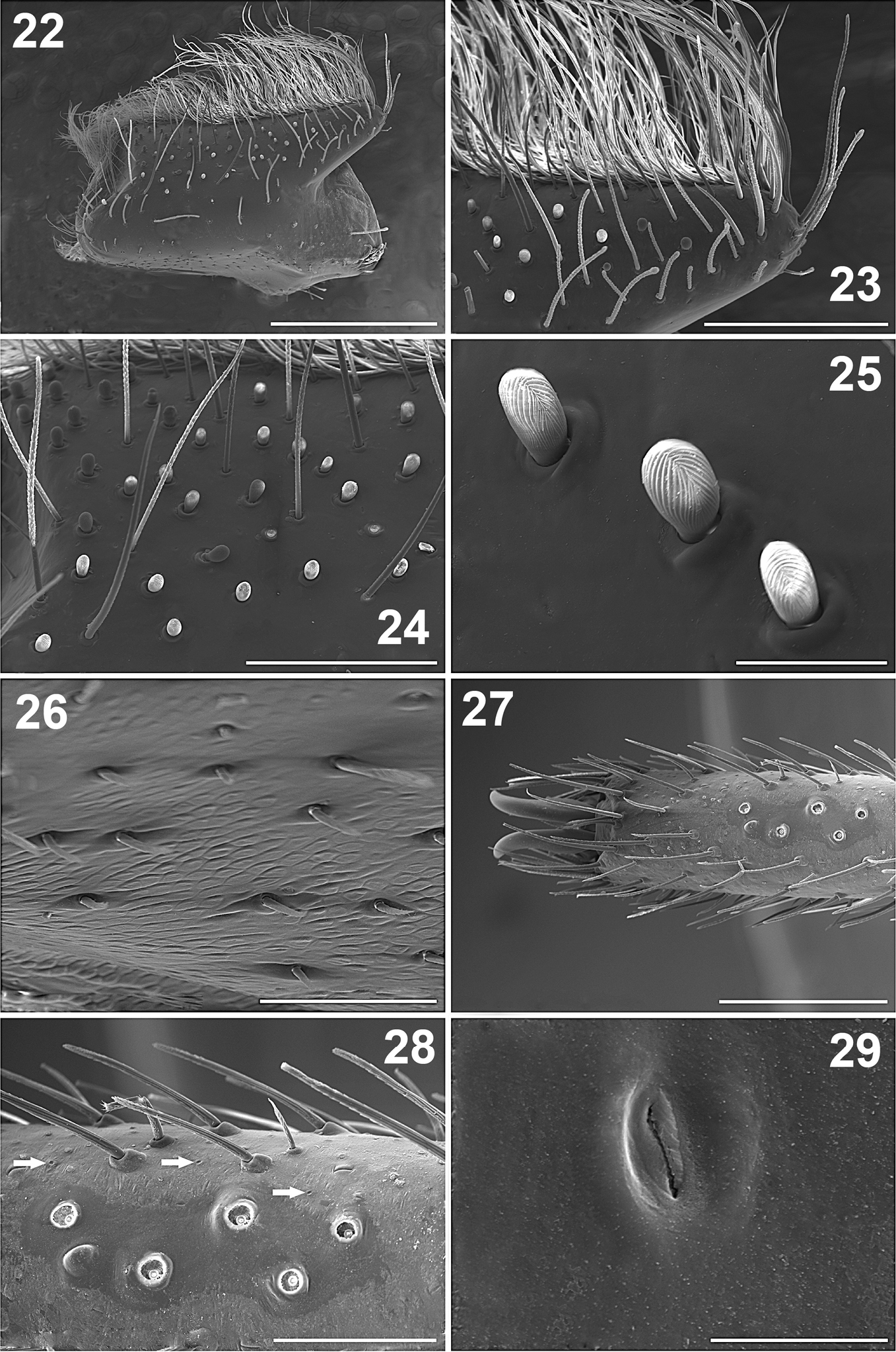
Paratropis tuxtlensis sp. n. Male. 22 Left endite, dorsal view 23 Left endite, apical detail 24 Left endite, detail of the setae 25 Detail of the finger-shaped cuspules on endite 26 Detail of the setae on retrolateral region of left endite 27 Left leg I, dorsal view of the tarsus 28 Left tarsus I, detail of the trichobothria sockets (arrows indicate the cuticular pores) 29 Detail of the glandular pore on the surface of the tarsus. Scales: 10 µm (Figure 29), 50 µm (Figure 25), 100 µm (Figure 26), 200 µm (Figure 28), 300 µm (Figure 24), 400 µm (Figure 23), 500 µm (Figure 27), 1 mm (Figure 22).
Paratropis tuxtlensis sp. n. Male. 30 Left chelicerae with extended fang, ventral view 31 Left chelicerae, teeth on promargin (lower line) and retromargin (upper line) 32 Detail of the chelicerae teeth 33 Left chelicerae, apical part of the fang 34 Detail of the venom gland duct opening on left fang of the chelicerae 35 Left chelicerae, retrolateral view 36 Setae on retrolateral part of left chelicerae 37 Detail of the clubbed setae on retrolateral region of chelicerae (left arrows indicate the glandular pores on the cuticle surface with secretion coming out at the moment of the microphotograph, right arrow indicates pore without secretion). Scales: 20 µm (Figure 34), 50 µm (Figure 37), 100 µm (Figures 32, 33), 400 µm (Figure 36), 500 µm (Figure 31), 1 mm (Figures 30, 35).
Labium: Trapezoidal, length 0.53, width 1.37, with 38 finger-shaped cuspules grouped on anterior part; anteriorly with several long, slightly curved setae (Figure 7); without pores on surface, not encrusted with soil particles. Labium merged to sternum, labium-sternal furrow shallow (Figures 2, 7).
Sternum: Circular, length 2.275, width 2.77, with few, scattered, long setae. Sigillae oval; third and fourth pairs hardly visible; fourth pair half its length from margin (Figure 2).
Legs: Length of legs and palp (femur, patella, tibia, metatarsus, tarsus, total): I: 5.60, 2.60, 3.75, 3.55, 1.95, 17.45. II: 3.85, 2.00, 2.70, 3.00, 1.55, 13.10. III: 3.40, 1.60, 2.25, 2.60, 1.60, 11.45. IV: 4.40, 2.00, 3.45, 3.70, 2.00, 15.55. Leg formula: 1-4-2-3. Palp: 2.00, 1.43, 1.65, -, 0.80, 5.88. Leg I longer and stouter than others, leg III shorter and thinner than others (Figure 58). Legs covered with curved, conical, barbed setae; in addition to clubbed setae (Figure 40); with numerous pores on cuticular surface (arrows, Figures 28, 40, 41), which are oval depressions with a longitudinal slit (Figure 29). Leg I without tibial spurs. Femora with long, clubbed setae. Metatarsi and tarsi with spinose setae ventrally, which are wider on legs III and IV. Tarsi with inconspicuous scopula (Figure 42), formed by small setae ending in a blunt tip (Figure 43).
Paratropis tuxtlensis sp. n. Male. 38 Left leg, trichobothria of metatarsus I, dorsal view 39 Left leg, setae on tibia I, retrolateral view 40 Detail of setae on tibiae I (arrows indicate the glandular pores) 41 Detail of the glandular pores on tibia I (arrow indicates pore with secretion) 42 Left leg, tarsus I, retrolateral view 43 Detail of the scopular setae on tarsus I 44 Claws of tarsus I, retrolateral view (arrow indicates the single long tooth on left claw) 45 Detail of the paired claws of tarsus I (arrow indicates the unpaired ventral claw). Scales: 40 µm (Figure 41), 100 µm (Figure 43), 200 µm (Figures 40, 45), 300 µm (Figure 44), 400 µm (Figure 38), 500 µm (Figures 39, 42).
Claws: Tarsi with long paired claws (Figures 42, 44, 45), which have just one long median tooth ventrally (arrow, Figure 44). Only tarsus I has the third, unpaired claw (arrow, Figure 45), tarsus I ventral-distally with some barbed setae, near to unpaired claw (Figure 45).
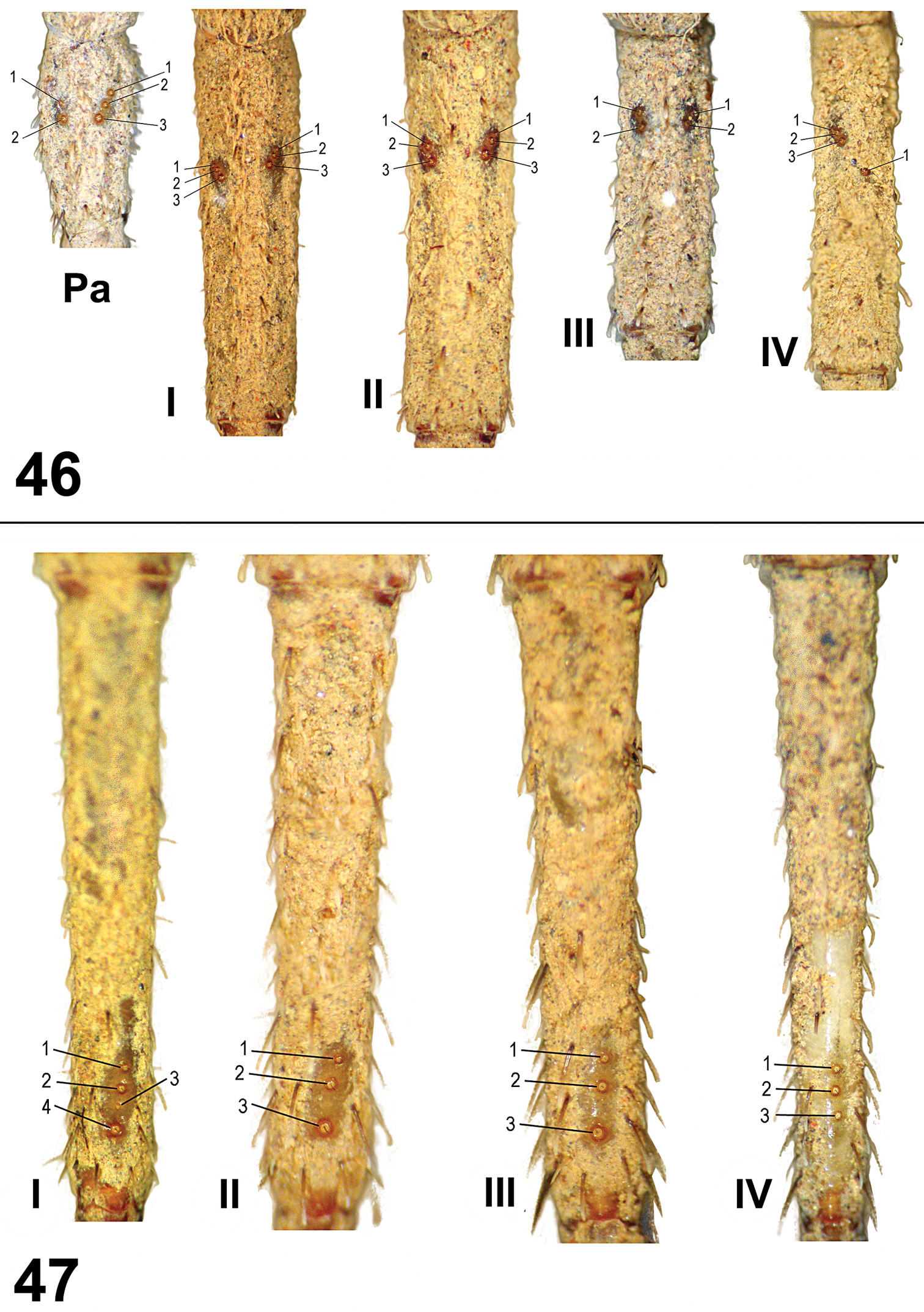
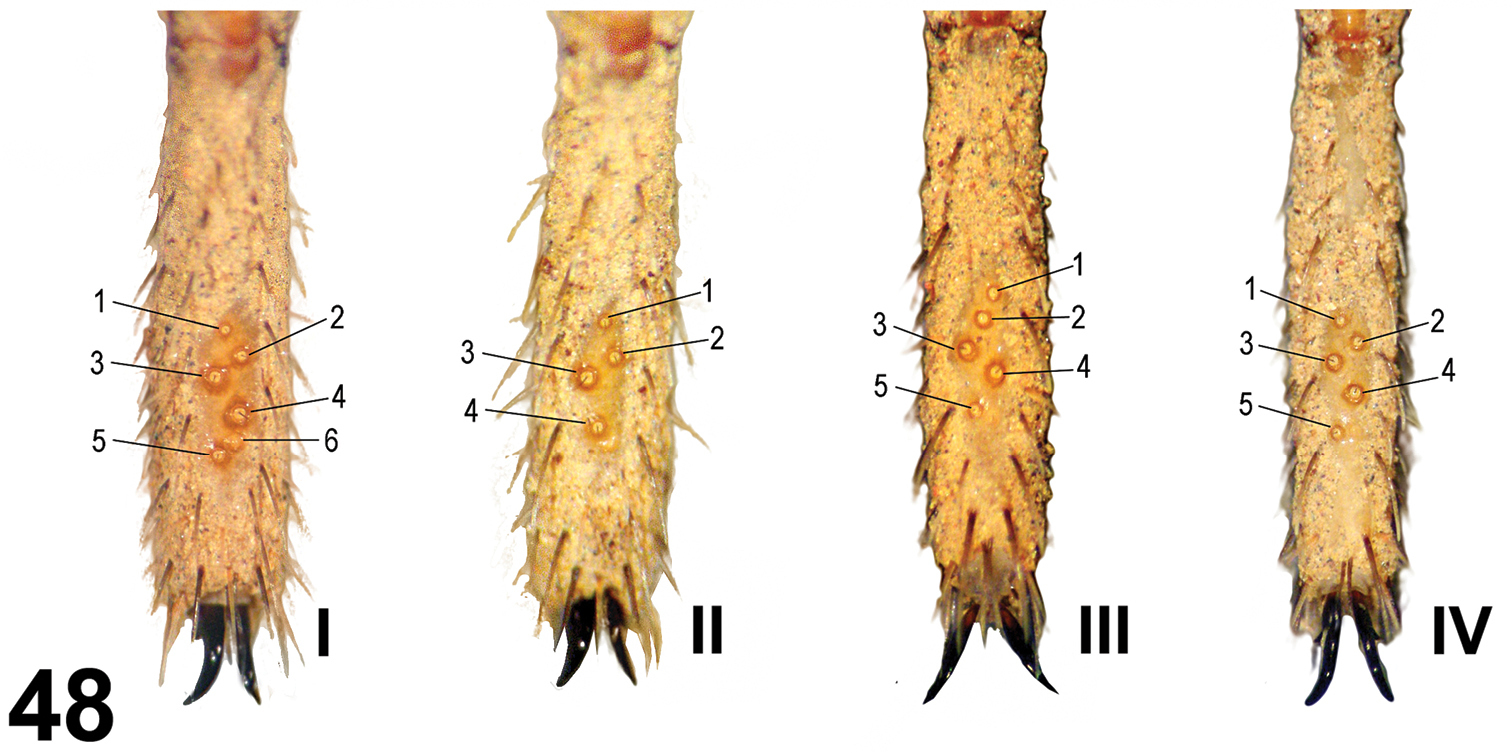
Leg trichobothria: Located on tibiae, metatarsi, and tarsi (Figures 46–48). Cuticle around the trichobothria without soil-particle encrustations (Figures 18, 28, 38, 46–48). Trichobothria sockets variable in size, basal-most smallest (Figures 18, 28, 38, 46–48). Dividing each leg segment into thirds (basal, median, apical), tibiae I has six trichobothria, three medial-prolateral, three medial-retrolateral (Figure 46). Tibia II has six trichobothria, three medial-prolateral, three medial-retrolateral (Figure 46). Tibia III has four trichobothria, two medial-prolateral, two medial-retrolateral (Figure 46). Tibia IV has four trichobothria, three medial-prolateral, one medial-retrolateral (Figure 46). All metatarsi have three trichobothria apical-dorsal (Figure 47). Finally, tarsus I has six trichobothria medial-dorsal, tarsus II has four medial-dorsal, tarsi III and IV have five trichobothria medial-dorsal (Figure 48).
Paratropis tuxtlensis sp. n. Trichobothria on male appendages 46 Trichobothria pattern on tibiae of palp (Pa) and legs I-IV 47 Trichobothria pattern on metatarsi I-IV.
Paratropis tuxtlensis sp. n. Trichobothria pattern on tarsi I-IV on male legs.
Chaetotaxy (left side): Metatarsus III 1v; IV 1v. The legs on males have numerous conical, barbed setae.
Opisthosoma: Oval, longer than wide (Figure 3), dorsally with eight longitudinal rows of clubbed setae, each row with eight setae. Opisthosoma completely covered with soil particles (Figure 3), genital gonopore not visible. Booklung openings oval, sclerotized.
Spinnerets: PMS considerably shorter than PLS (arrows Figure 8). First and second segments of PLS cylindrical, third segment finger-shaped distally (Figure 8). Measurements: PMS length 0.22, width 0.12, 0.10 apart. Segments of PLS (length): basal 0.70, middle 0.50, distal 0.90; midwidths PLS (width): basal 0.48, middle 0.46, distal 0.34.
Paratype female (CNAN-T0767). Body length 12.90 (not including chelicerae and spinnerets); chelicerae length 1.80; carapace length 6.00; width 5.70; opistosoma length 6.40, width 5.10.
Female similar to the male, differences: Coloration: Chelicerae, endites, labium, and sternum darker orange than the male (Figures 50, 55).
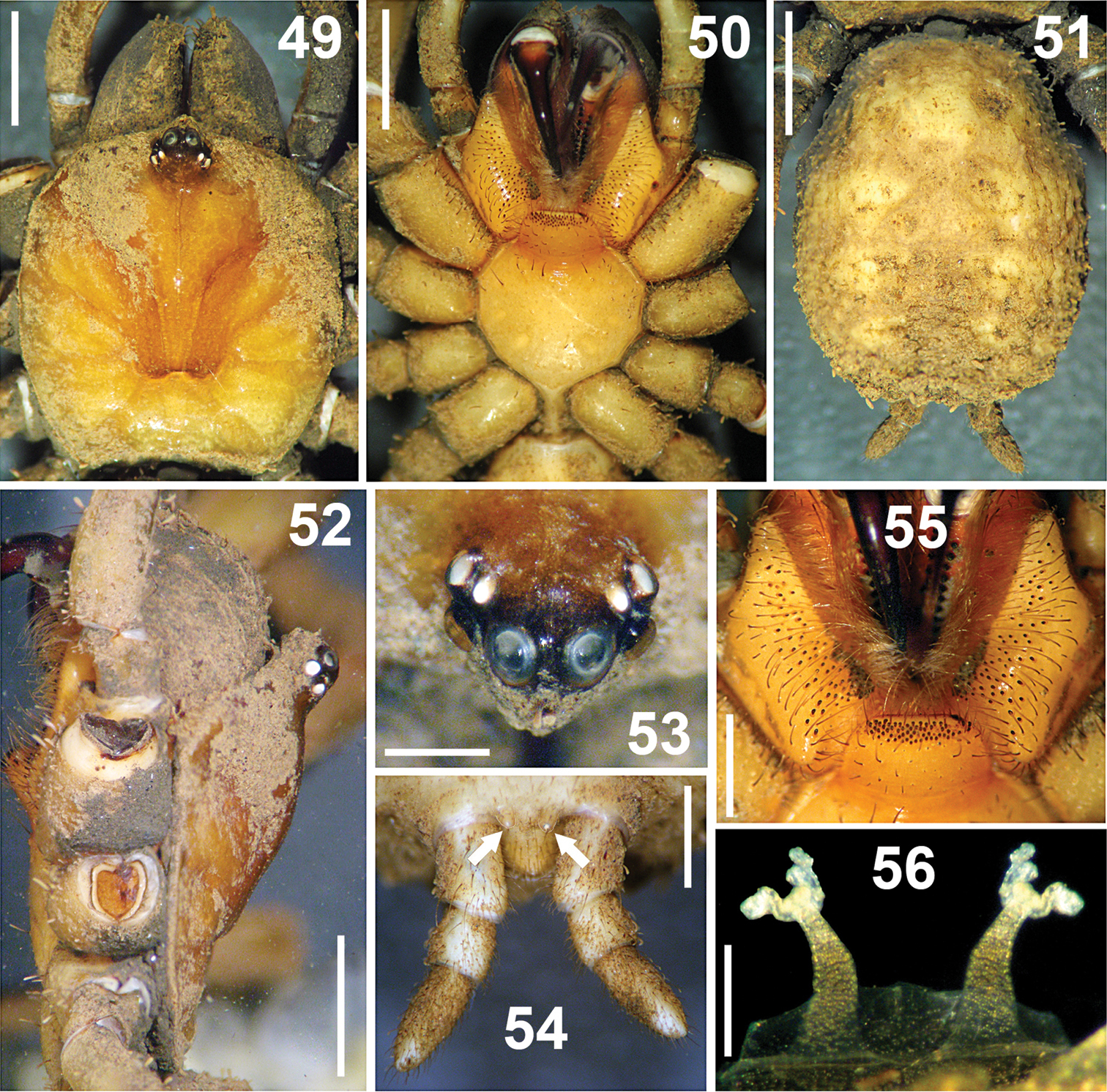
Paratropis tuxtlensis sp. n. Female. 49 Carapace, dorsal view 50 Prosoma, ventral view, showing the sternum, labium, endites and chelicerae 51 Opisthosoma, dorsal view 52 Carapace, left lateral view 53 Ocular region, dorsal view 54 Spinnerets, ventral view (arrows indicate the PMS) 55 Endites and labium, ventral view 56 Spermathecae, dorsal view. Scales: 0.25 mm (Figure 56), 0.6 mm (Figure 53), 1 mm (Figures 54, 55), 2 mm (Figures 49–52).
Carapace: Oblong-orbiculate (Figure 49). Caput elevated (Figure 52); fovea shallow, slightly recurved, 1.08 wide, visible only when carapace is cleaned of soil particle encrustations. Eye sizes and interocular distances: AME 0.35; ALE 0.25; PME 0.15; PLE 0.28; AME–AME 0.13; AME–ALE 0.05; PME–PME 0.55; PME–PLE 0.06; ALE–PLE 0.08. Ocular tubercle raised; length 1.08, width 1.13; clypeus lacking (Figure 53).
Palps: Thicker than on male. Tarsi with one distal, long, curved unpaired claw, which has just one tooth. Tarsi ventrally with spines; left tarsus with spines: 2+1, right tarsus with spines: 1+2+2. All palp segments covered with encrusted soil particles, except prolateral regions of femora and trochanters. Tibia with five trichobothria, two medial-prolateral, three medial-retrolateral. Tarsus with four medial-dorsal trichobothria.
Chelicerae: Fangs wider than on male (Figure 50). Chelicerae furrows with conical, wide, short teeth on promargin and retromargin. Right chelicera promargin with 13 teeth, retromargin with 11 teeth; left chelicera promargin with 12 teeth, retromargin with 11 teeth; teeth on retromargin wider than teeth on promargin; on both rows the proximal teeth are wider and longer than distal teeth.
Endites: Endites ventrally with numerous scattered, finger-shaped cuspules, 61 cuspules on right endite and 64 on left one (Figure 55).
Labium: Trapezoidal, length 1.02, width 1.58, with 69 cuspules grouped on anterior region; with several long and slightly curved setae on anterior part. Cuspules finger-shaped, as on endites. Labium merged to sternum, without glandular pores on surface, cuticle not encrusted with soil. Labium-sternal furrow shallow (Figure 55).
Sternum: Circular, length 2.5, width 3.05; with few scattered, long setae. Sigillae oval, third and fourth pairs hardly visible; fourth pair 3/4 times its length from margin.
Legs: Length of legs and palp (femur, patella, tibia, metatarsus, tarsus, total): I: 3.65, 2.65, 3.25, 2.70, 1.40, 13.65. II: 3.50, 2.05, 2.35, 2.50, 1.45, 11.85. III: 2.95, 1.85, 2.05, 2.30, 1.35, 10.50. IV: 4.00, 1.60, 3.10, 3.40, 1.75, 13.85. Leg formula: 4-1-2-3. Palp: 2.67, 1.67, 1.53, -, 1.90, 7.77. Metatarsi and tarsi with spinose setae ventrally, wider on legs III and IV; thicker and more visible than on male. Tarsi with inconspicuous scopula, composed of small setae ending in blunt tip.
Claws: Slightly longer than the male. Only the tarsi I and II with small, unpaired third claw (differing from the male, which lacks it in tarsus II, and from
Leg trichobothria: Located on tibiae, metatarsi, and tarsi. Cuticle around trichobothria not covered with encrusted soil particles. Trichobothrial socket size variable, smallest basally and apically. Tibia I has six trichobothria: three medial-prolateral, two medial-retrolateral, one medial-dorsal; tibia II has six trichobothria: three medial-prolateral, three medial-retrolateral; tibia III has six trichobothria: three medial-prolateral, three medial-retrolateral; tibia IV has five trichobothria: one medial-prolateral, one medial-dorsal, three medial-retrolateral; palpal tibia has six trichobothria: three medial-prolateral, three medial-retrolateral. Metatarsi I-III has three apical-dorsal trichobothria, metatarsus IV has four trichobothria: one medial-dorsal, three apical-dorsal. Tarsus I has nine medial-dorsal trichobothria; tarsus II has seven medial-dorsal trichobothria; tarsus III has six medial-dorsal trichobothria; tarsus IV has seven medial-dorsal trichobothria. Palpal tarsus has five medial dorsal trichobothria.
Chaetotaxy (left side): Metatarsus I 28v; II 6v; III 5v; IV 3v; tarsus I 14v; II 7v; III 3v; IV 1v; palp 7v. Conspicuous spines are more visible in females than in males.
Opisthosoma: Bigger than in male (Figure 51), genital operculum not visible due to encrusted soil particles.
Spermathecae: Two long, separated lobes, wider basally, slightly curved outwards from base; apically with paired sigmoid receptacles (Figure 56).
Spinnerets: PMS considerably shorter than PLS (arrows Figure 54), however PLS bigger than on male (Figure 54). First and second segments of PLS cylindrical, third segment finger-shaped distally. Measurements: PMS length 0.24, width 0.18, 0.24 apart; Segments of PLS (length): basal 0.90, middle 0.46, distal 1.00; midwidths PLS (width): basal 0.64, middle 0.58, distal 0.46 (Figure 54).
Males (N= 3), females (N= 3). There is no variation in male secondary sexual characteristics. It is difficult to determine if there is variation in coloration in males due to the soil particles encrusted on the cuticle. However, in the endites, labium, and sternum which do not have encrusted soil particles, there was no variation in coloration. Males: Carapace length 4.50–5.35 (x = 4.88), width 4.7–5.2 (x = 4.93). Tibia I length 3.90–4.50 (x = 4.25). Sternum length 2.25–2.50 (x = 2.35), width 2.55–2.85 (x = 2.70). Endites length 2.00–2.30 (x = 2.13). Cuspules: endites, male 1 (right/left) (42/40), male 2 (38/39), male 3 (54/48); labium, male 1 (39), male 2 (47), male 3 (32). Females: Carapace length 5.40–6.10 (x = 5.80), width 5.10–5.80 (x = 5.50). Tibia I length 3.65–4.00 (x = 3.81). Sternum length 2.40–2.85 (x = 2.68), width 3.00–3.30 (x = 3.16). Endites length 2.45–2.75 (x = 2.61). Cuspules: endites, female 1 (60/66), female 2 (75/77), female 3 (43/51); labium, female 1 (64), female 2 (68), female 3 (41).
Juveniles (N= 2) (two different instars): Body lengths 4.30 (#1), 5.10 (#2) (not including chelicerae and spinnerets); Carapace lengths 2.05, 2.25, widths 1.95, 2.37. Tibia I lengths 1.35, 1.55. Sternum lengths 1.10, 1.30, widths 1.40, 1.55. Endites lengths 0.86, 1.00. Cuspules: endites, juvenile #1 (right/left) (12/12), juvenile #2 (19/17); labium: juvenile 1 (14), juvenile 2 (15). Leg trichobothria: Juvenile #1: Tibia I (4 trichobothria) (2 medial-prolateral, 2 medial-retrolateral), tibia II (4) (2 medial-prolateral, 2 medial-retrolateral), tibia III (4) (2 medial-prolateral, 2 medial-retrolateral), tibia IV (3) (2 medial-prolateral, 1 medial-retrolateral). Metatarsus I (2) (1 medial-dorsal, 1 apical-dorsal), metatarsus II (2) (1 medial-dorsal, 1 apical-dorsal), metatarsus III (2) (1 medial-dorsal, 1 apical-dorsal), metatarsus IV (2) (apical-dorsal). All tarsi (2) (medial-dorsal). Juvenile #2: Tibia I (4) (2 medial-prolateral, 2 medial-retrolateral), tibia II (4) (2 medial-prolateral, 2 medial-retrolateral), tibia III (4) (2 medial-prolateral, 2 medial-retrolateral), tibia IV (3) (2 medial-prolateral, 1 medial-retrolateral). All metatarsus (2) (apical-dorsal). Tarsus I (4) (medial-dorsal), tarsi II-IV (3) (medial-dorsal). Palp trichobothria: Juvenile #1: Tibiae (3) (1 medial-prolateral, 2 medial-retrolateral), Tarsus (2) (medial-dorsal). Juvenile #2: Tibiae (3) (1 medial-prolateral, 2 medial-retrolateral), Tarsus (2) (medial-dorsal).
The specific name is an adjective and refers to the type locality: Estación de Biología Tropical “Los Tuxtlas”, Municipio San Andrés Tuxtla, Veracruz, Mexico.
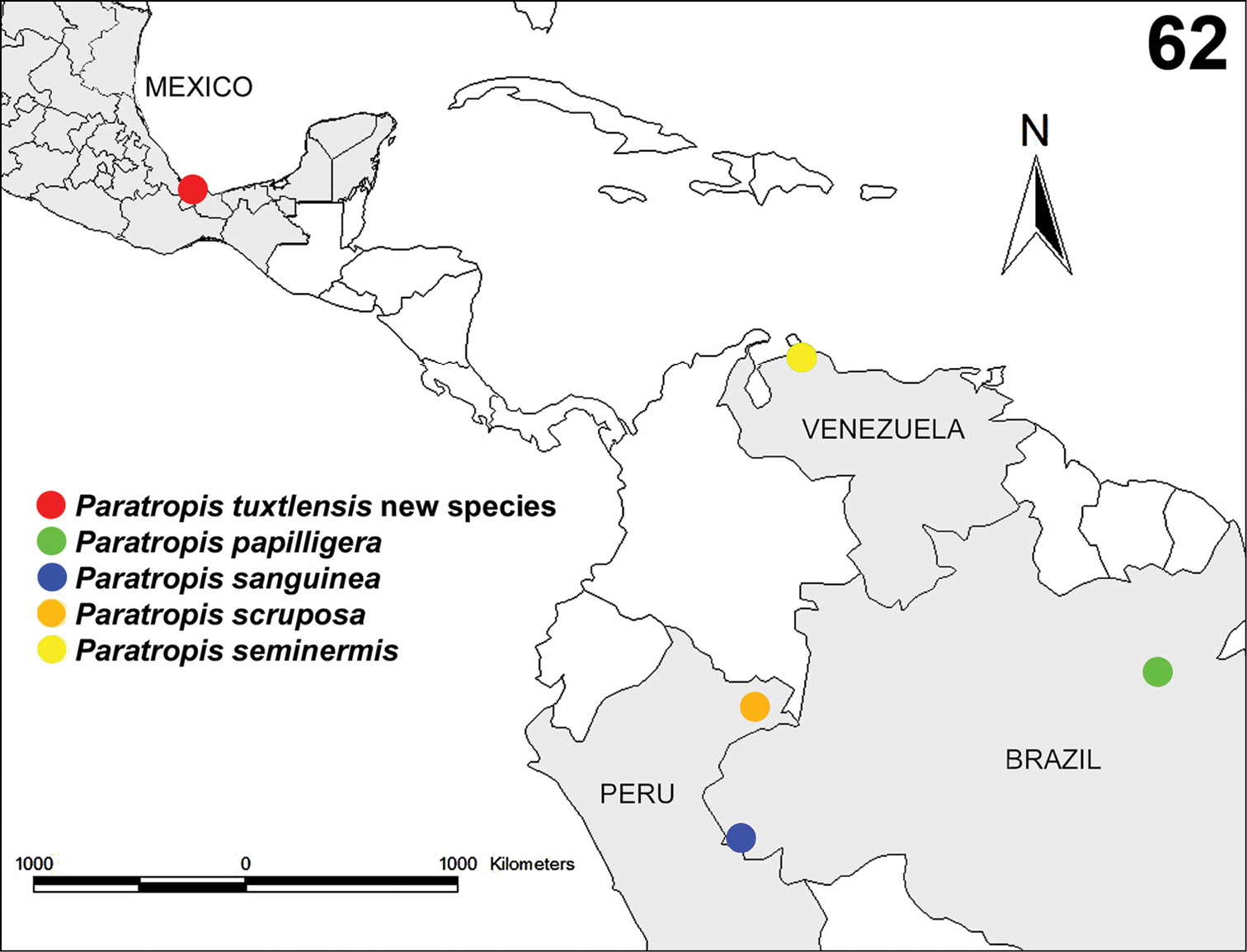
The species is known only from the region around the type locality in the Volcan San Martin Biosphere Reserve (Figure 62).
The specimens were collected in tropical rain-forest, under boulders on the ground (Figure 57). The holotype, two paratype males and one juvenile where collected near each other, within around 3 m2, in a zone with numerous small and big boulders on the ground. The specimens remained motionless when they were exposed by removing the rock that provided shelter, possibly as a defense mechanism because the soil particles encrusted on the body cuticle serves as camouflage with the moist ground (Figures 57–60). The type locality is at 1039 m elevation, and two adult females where collected nearby at 480 m.
Tropical rain-forest in the Estación de Biología Tropical “Los Tuxtlas”, Veracruz, Mexico. Habitat of Paratropis tuxtlensis new species, arrow indicates the microhabitat where the specimens were collected (under boulders).
Paratropis tuxtlensis sp. n. Photographs of live specimens, kept in the laboratory 58 Adult male 59 Immature specimen 60 Adult female 61 Adult female protecting her egg sac. Scales: 1 mm (Figure 59), 4 mm (Figure 58), 6 mm (Figure 60).
Known records of the species of the genus Paratropis: Paratropis tuxtlensis new species from Estación de Biología Tropical “Los Tuxtlas”, Veracruz, Mexico; Paratropis papilligera from Santarém, Pará, Brazil; Paratropis sanguinea from Alto-Jurúa, Amazonas, Brazil; Paratropis scruposa from Pebas, Loreto, Peru; and Paratopis seminermis from Santa Ana, Falcón, Venezuela.
Towards the end of spring (May 19, 2012), one paratypes female (CNAN-T0767) kept in captivity in the laboratory laid an egg sac (Figure 61). The female kept her palps and legs in contact with the egg sac constantly. Twenty-three spiderlings emerged 38 days after oviposition (July 26, 2012).
Although Paratropis papilligera was described by
The spider family Paratropididae Simon, 1889 was considered as being monophyletic and as the sister group of the family Theraphosidae Thorell, 1869 (Superfamily Theraphosoidea) based on morphological evidence by
The monophyly of the four genera that compose the family has never been tested. Even the monophyly of the subfamilies proposed by
We report the ontogenetic variation for Paratropididae juveniles for the first time. The number of the cuspules on labium and sternum is considerably lower in younger instars than on adults. We found two juveniles that we hypothesize belong to two different, not necessarily successive instars; the smallest had fewer cuspules on labium and endites than the larger juvenile and adults. It seems that the number of cuspules in each instar is incremented with growth, and the adult instars have the highest number. In addition, there is ontogenetic variation in the number of leg trichobothria in each instar, with fewest trichobothria on the younger instars and the highest number on the adults. Although we hypothesize that the juveniles belong to two different, not successive instars, based on size differences, both specimens had the same number of trichobothria on tibiae and metatarsi. However, there is allometric growth reflected in the trichobothria position on metatarsi: in the smallest juvenile one trichobothrium is located on the median third and the other one in the apical third; whereas in the larger juvenile both trichobothria are located in the apical third. Although on both juvenile specimens the trichobothria number on tibiae and metatarsi was the same, the number of trichobothria on the tarsi is different in the larger juvenile, having one more trichobothrium.
The genus Paratropis Simon, 1889 was diagnosed by
We thank M.Sc. Berenit Mendoza Gárfias for the help with the photomicrographs taken with the scanning electron microscope (SEM). To Dr. Norman I. Platnick for his help in the identification of the specimens. To the reviewers for the revision and comments on the manuscript. To students of the Colección Nacional de Arácnidos (CNAN) and Colección Nacional de Acaros (CNAC), Instituto de Biología, UNAM, for their help with the fieldwork. To the Estación de Biología Tropical “Los Tuxtlas”, Instituto de Biología, UNAM, for the help and facilities to work in the station. Thanks to the Instituto de Biología (IBUNAM), Posgrado en Ciencias Biológicas of the UNAM; the Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico; Dra.Virginia León Règagnon, leader of the Proyecto Biotas Tropicales, Red Temática Código de Barras, CONACYT; and the Instituto Bioclon, Mexico, for their financial support. The specimens were collected under Scientific Collector Permit FAUT-0175 from the Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), to Dr. Oscar F. Francke.