(C) 2013 Gary A. P. Gibson. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The extinct Eocene Baltic amber genus Propelma Trjapitzin 1963 is removed from synonymy under Eupelmus Dalman 1820 (Hymenoptera, Eupelmidae, Eupelminae) and treated as a valid genus within Neanastatinae Kalina 1984 based on examination of the holotype female of Propelma rohdendorfi Trjapitzin. Propelma rohdendorfi is redescribed, illustrated by photomacrographs, and compared to other described extant and extinct genera of Neanastatinae. Taxonomic, morphological and geological diversity of Neanastatinae relative to Eupelminae and Calosotinae is also discussed relative to potential age of the subfamily.
Eocene, fossil, Dominican amber
When Trjapitzin described Propelma he stated that it was similar to Metapelma
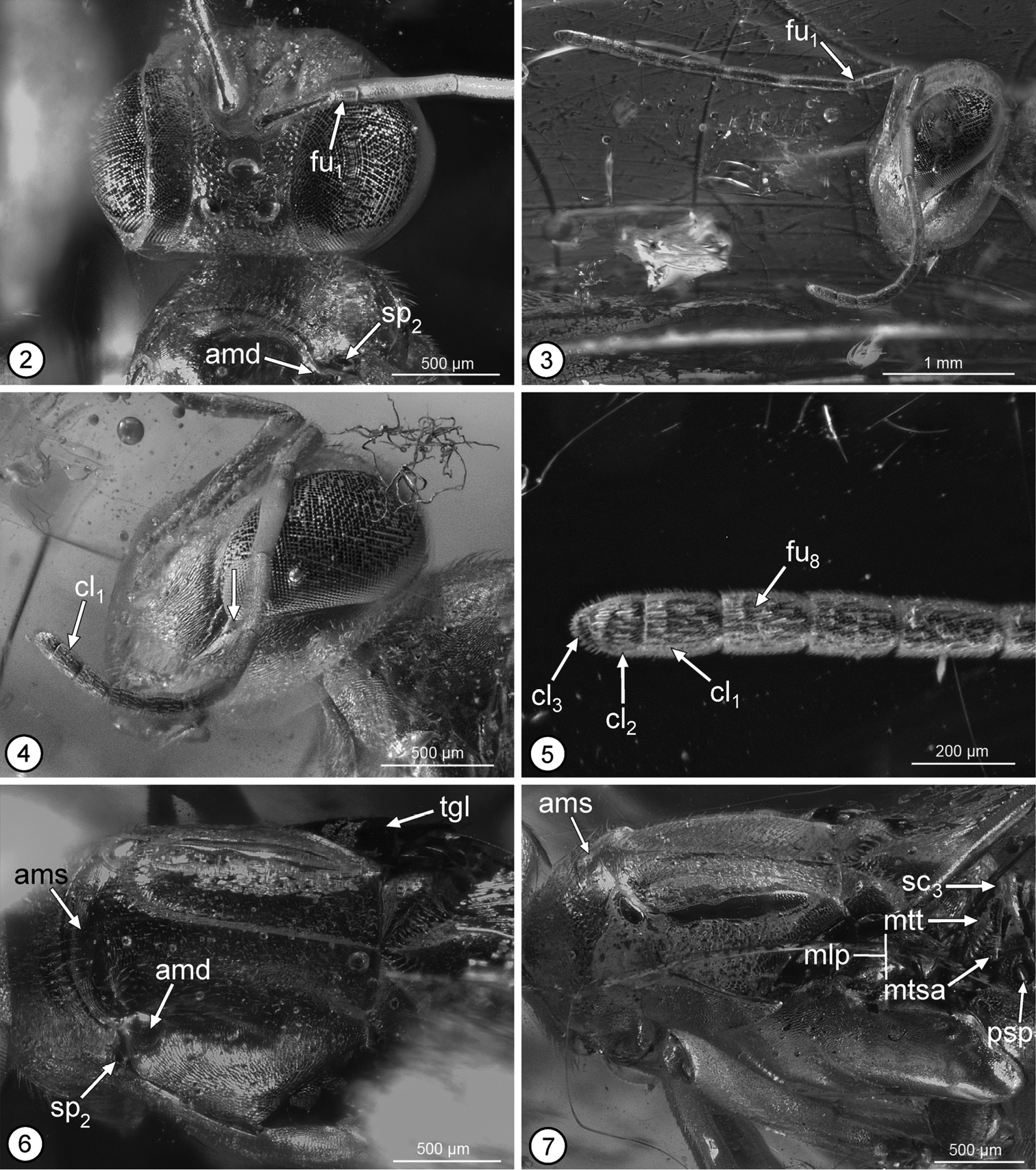
The description and photomacrographs are based on the holotype female of Propelma rohdendorfi [Holotype no. 364/360, Orlov Museum of Paleontology (formerly, Paleontological Institute of Russian Academy of Sciences), Moscow, Russia]. The holotype is in a mostly dark orange-colored block of amber (Fig. 1). It is complete except that an unknown length of the ovipositor sheaths are missing, as is most of the femur and tibia of the left hind leg and the apices of the femora and bases of the tibiae of the right middle and hind legs. The missing parts are because these projected beyond the sides of the polished amber block. The right side of the specimen, in particular, is clearly visible (Fig. 1), but artefacts prevent a direct ventral view of the mesosoma or the dorsal surface of the body beyond about the posterior angles of the axillae, and thickness of the amber prevents clear observation of the face. Images were taken with a Leica DFC 425C, 5 megapixel digital camera attached to a Leica Z16 APO macroscope. Serial images were combined using Zerene Stacker and digitally manipulated using Camera Raw and Adobe Photoshop 4 to enhance clarity. Images taken of the right side of the holotype for the plates of illustrations were flipped so that they face in the normal direction for specimen observation. All images except for Fig. 1, which illustrates color of the amber, are published in greyscale because this better facilitates differentiation of structures.
Propelma rhodendorfi, amber block bearing holotype female.
Terminology follows
Propelma Trjapitzin, stat. rev.
Propelma Trjapitzin, 1963: 89–91. Type species: Propelma rhodendorfi Trjapitzin, by original designation and monotypy. Synonymy under Eupelmus Dalman by
http://species-id.net/wiki/Propelma_rhodendorfi
Figs 1–13Female (Fig. 1). Length (anterior margin of head to posterior margin of syntergum in lateral view) = 7.9 mm [7.5]. Body mostly bright shiny orange (a reflection artefact, original color apparently mostly or entirely dark based on some regions of the body such as part of tegula (Fig. 6: tgl) and gastral tergites (Figs 12, 13)).
Head in frontal view almost as wide as high, with ventral margin of torulus in line with lower orbits and with convex, dorsally tapered interantennal region separating distinct scrobes over at least ventral half of scrobal depression (Fig. 2); scrobal depression inverted U-shaped with minimum distance between lateral margin and inner orbit about 0.4× maximum diameter of anterior ocellus, abruptly margined dorsolaterally to within about one maximum diameter of anterior ocellus where slight change in curvature differentiates more obscure dorsal margin from bare, similarly finely coriaceous, slightly concave region below anterior ocellus (Fig. 2) such that under some angles of view scrobal depression superficially appears to extend to ocellus; upper parascrobal region and frontovertex minutely coriaceous-granular with minute setiferous punctures; lower parascrobal region and gena more vertically coriaceous-alutaceous with short white setae similar to upper parascrobal region, frontovertex and interantennal region. Head in lateral view (Fig. 4) with vertex smoothly rounded into occiput; almost twice as high as maximum length at level of toruli; malar sulcus appearing bifurcate near lower orbit, delineating small triangular region below posteroventral orbit (Fig. 4: arrow) (see discussion); eye about 1.6× as high as wide, superficially bare, but with very short, sparse setae. Head in dorsal view with minimum distance between inner orbits about 0.3× width of head; anterior ocellus slightly transverse, with maximum diameter equal to distance between its outer margin and inner orbit, and slightly greater than maximum diameter of posterior ocellus (Fig. 2); POL: LOL: OOL: maximum diameter of anterior ocellus = 1.0: 0.8: 0.3: 1.0. Antenna (Figs 3, 4) with scape slightly widened distally, ventral margin straight; length of pedicel plus flagellum about 1.7× width of head; flagellum and clava slender, of similar width throughout (Fig. 3); length of scape = 4.0 (approximate); length of pedicel = 2.0; funicle 8-segmented, with fu1 longer than wide, but much shorter than pedicel or fu2 (Figs 2, 3), relative lengths of funiculars = 0.7: 2.7: 2.2: 1.6: 1.1: 1.0: 0.9: 0.9; clava 3-segmented (Fig. 5), length = 1.5, basal clavomere slightly longer than cl2+cl3, separated from cl2 by distinct transverse suture (Figs 4, 5), but cl3 as tiny apical micropilose sensory region delineated by extremely fine, sinuate suture such that under some angles clava superficially 2-segmented (Fig. 4) (relative length of clavomeres = 8:5:2); [length:width ratios of pedicel to clava = 30:9, 10:7, 40:8, 33:8, 23:8, 16:7, 15:8, 12:9, 12:9, 22:9]; flagellum with numerous multiporous plate sensilla in multiple rows per flagellomere and with very short, inconspicuous setae (Figs 4, 5).
Propelma rhodendorfi: 2 head, frontodorsal view 3 head and antennae, lateral view 4 head and right antenna, lateral view 5 apical three funiculars and clava 6 mesoscutum, dorsal view 7 mesosoma, dorsolateral view. See Methods for abbreviations for structural features (arrow on Fig. 4 points to triangular region differentiated by putatively bifurcate malar sulcus).
Pronotum uniformly sclerotized, dorsally convex in transverse plane and flat mediolongitudinally, hence without differentiated collar and neck (Fig. 7); more or less bell-shaped, sinuately narrowed anteriorly, with incurved posterior margin (Figs 2, 6) (holotype with pronotum rotated anteroventrally such that dorsal surface at obtuse angle relative to mesonotum (Fig. 7) and exposing convex, asetose, transverse anterior part of mesoscutum (Figs 6, 7: ams) between short lateral depression (Figs 2, 6: amd) posterior to each mesothoracic spiracle (Figs 2, 6: sp2), which accept dorsolateral angles of pronotum when this rotated horizontally in same plane as mesoscutum); finely coriaceous-granular to coriaceous-alutaceous with short black setae except for a line of longer setae along posterior margin. Mesoscutum slightly wider than long, with ridge-like medial elevation extending between exposed transverse anterior portion and transscutal articulation (Figs 6, 7), and with lateral lobes evenly convex; finely coriaceous, with short dark setae similar to pronotum. Mesoscutellar-axillar complex (Figs 6, 7, 9) with axillae transverse-triangular with contiguous inner angles, convex with abruptly angled, oblique, strongly crenulate posterior surfaces forming scutoscutellar sutures; mesoscutellum similarly convex as axillae, teardrop-shaped (cf.
Propelma rhodendorfi: 8 mesosoma, lateral view 9 mesoscutellar-axillar complex to base of metasoma, lateral view 10 fore wing 11 apex of mesotibia and mesotarsus 12 gaster, lateral view 13 Mt6 to apex of metasoma, lateral view. See Methods for abbreviations for structural features (arrows on Fig. 9 point to sulci on propodeal callus and metanotal scutellar arm).
Metasoma (Fig. 12) about 0.9× combined length of mesosoma and head; petiole (Fig. 9: ptl) a strongly transverse dorsal strip; in lateral view medial length of tergites from petiole to syntergum = 0.3: 5.2: 1.5: 2.0: 2.8: 3.1: 2.3: 0.8 [Mt2–syntergum = 43: 11: 16: 23: 29: 18: 3+4]; Mt2 to basal half of Mt6 comparatively sparsely setose with dark setae, but Mt7 and apical half of Mt6 (Fig. 13) more densely setose with longer dark setae, the setae longest in apical half of Mt7, and tergites dorsally very finely meshlike coriaceous; Mt7 with gastral spiracle (Fig. 13: gsp) cone-like protuberant anterolaterally; Mt8+Mt9 fused into syntergum (Fig. 13), but with transverse carina (Fig. 13: car) extending at least partly between cerci, the carina continuous along anterior and outer margins of cercus (Fig. 13: cer), with cercus at about mid-length of syntergum, and syntergum sparsely setose but with a few longer, more conspicuous dark setae along posteromesal margin; triangular membranous lobe (Fig. 13: mbl) extending from posterior margin of syntergum, but not extended into anal filament. Ovipositor sheaths tubular, conspicuously exerted, but of unknown length.
Propelma is assigned to Neanastatinae based on pronotal structure, mesopectus posteroventrally abutting the mesocoxal bases without a membranous region anterior to each coxa, and mesotarsal peg pattern, all of which are diagnostic of the subfamily (
Propelma keys to couplet 6 (Brevivula and Lambdobregma) using the key to genera of Neanastatinae in
The recognition of Propelma as a valid genus of Neanastatinae results in four extinct genera described from Baltic amber (Aspidopleura, Brevivula, Neanaperiallus and Propelma) and four extant genera, of which one (Lambdobregma) is restricted to the New World, one (Eopelma) is restricted to the Oriental region, one (Neanastatus) is Old World in distribution, and one (Metapelma) is more widely distributed throughout both the Old and New World. Metapelma is also the only extant genus with a described extinct species, Metapelma archetypon
I thank Ekaterina Sidorchuk (Russian Academy of Sciences, Moscow) for hand-carrying the holotype of Propelma rhodendorfi to and from the Museum of Palaeontology. I also thank Lisa Bearss for the images and plates that illustrate this work.