Citation: Prena J, Korotyaev B, Wang Z, Li Ren, Liu N, Zhang R (2014) A taxonomic revision of Limnobaris Bedel in the strict sense (Coleoptera, Curculionidae, Baridinae), with particular emphasis on the species found in China. ZooKeys 416: 41–66. doi: 10.3897/zookeys.416.7164
The genus name Limnobaris Bedel is applied in a restricted sense to baridine weevils with a covered pygidium and non-prominent, decussate mandibles which occur on sedges in the Palaearctic Region and immediately adjacent parts of tropical Southeast Asia. Calyptopygus Marshall and Pertorcus Voss are syn. n. of Limnobaris. Some species from Africa and the Americas are maintained provisionally in Limnobaris in the widest sense but will need to be transferred to other genera in future studies. A total of eleven species is recognized in Asia, two of which are widespread and occur also in the Western Palaearctic Region. Limnobaris martensi Korotyaev sp. n. is described from Nepal. Pertorcus tibialis basalis Voss is raised to species rank, as L. basalis (stat. prom.). New or reestablished synonyms are L. dolorosa (Goeze) (= L. jucunda Reitter, = L. koltzei Reitter), L. tibialis (Voss) (= Pertorcus tibialis pilifer Voss) and L. t-album (Linnaeus) (= L. bedeli Reitter, = Baridius crocopelmus Gyllenhal, = L. sahlbergi Reitter, = L. scutellaris Reitter, = Baris t-album sculpturata Faust). Calandra uniseriata Dufour is considered a junior synonym of Sitophilus oryzae (L.) (syn. n.). A key for identification and a distribution map are provided.
Weevil, sedge, distribution, life history, parasitoid, Palaearctic
Baridinae are hyperdiverse, oligophagous weevils with a worldwide distribution. Many are uniformly oblong-ovate and notoriously poor in taxonomically useful characters. A particular problem is that their higher classification is based largely on poorly known exotic species with aberrant shapes and bizarre character formations, while at the same time leaving the bulk of nondescript species in an unwieldy, phylogenetically meaningless disarray. The situation has escalated over the past century because regionally conducted studies increasingly failed to associate new collections and observations with previously published information. Even the relatively well-studied species of the temperate northern hemisphere are in need of much work (
The present study is concerned with an ill-defined complex of small, slender, usually black species associated with Cyperaceae (Fig. 1).
Live habitus images of Limnobaris species from China (including type species of Calyptopygus and Pertorcus). AB Limnobaris dolorosa (3.6 mm) on Carex and Scirpus in Changbaishan, Jilin Province C Limnobaris tibialis (2.8 mm) on Carex in Wuyishan, Fujian Province DE Pupa and adult (3.7 mm) of Limnobaris elliptica in/on Scirpus wichurai in Xiaozahe, Yunnan Province. Photos by Wang Zhiliang (A, B, D, E) and Ding Liang (C).
This study deals with the European and Asian species currently placed in the genera Limnobaris, Calyptopygus and Pertorcus, but ignores one nominal Limnobaris species from Africa and 26 from the Americas. We have examined most of them and concluded that the taxonomic problems are too complex to be treated here. Rather than adding to the confusion with incomplete and preliminary new generic placements, we decided to leave them provisionally in Limnobaris sensu lato.
Several species treated herein have been documented in great detail by
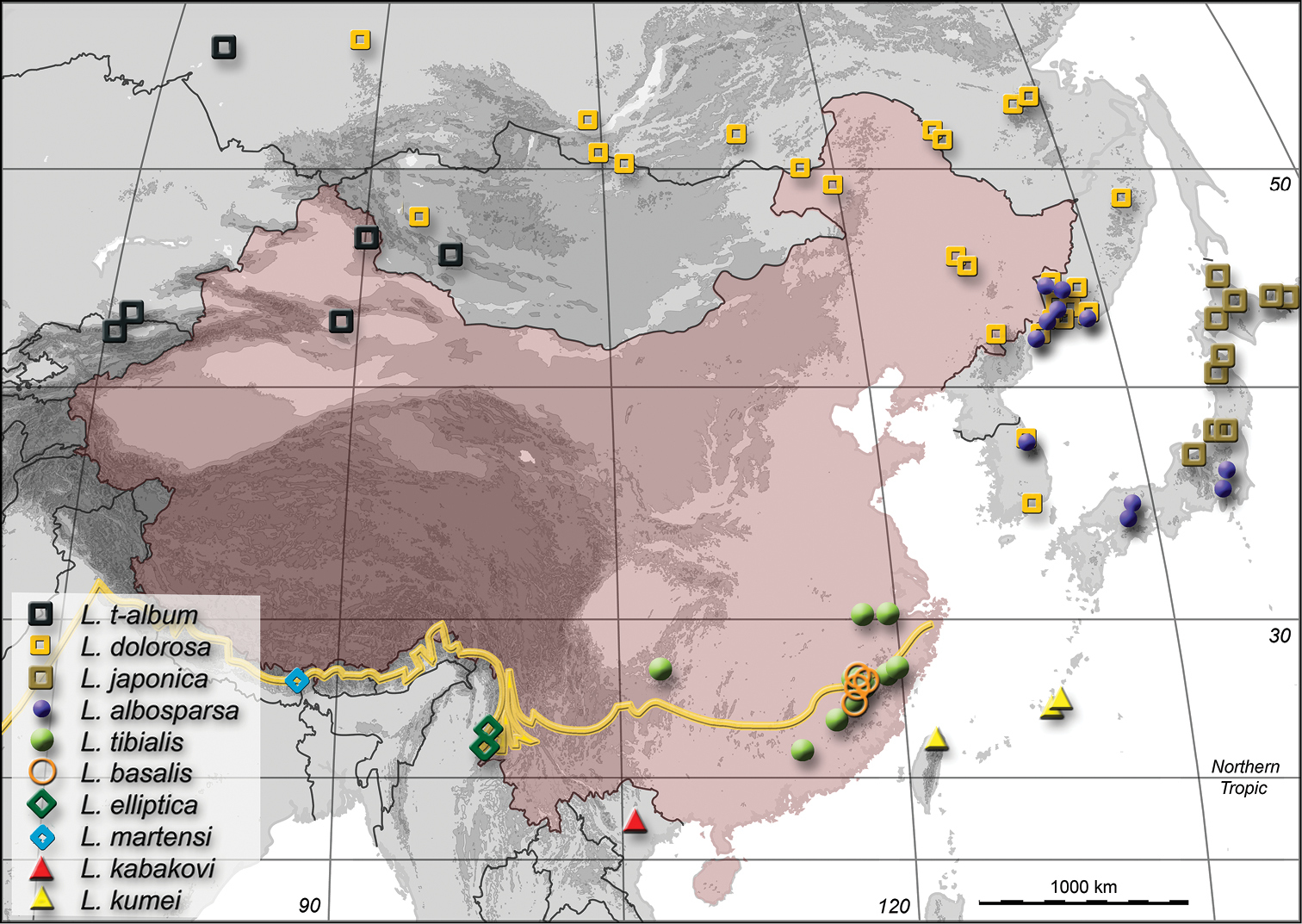
Because of our emphasis on Chinese fauna, we list under “Material examined” only specimens collected not further than 1000 km away from the Chinese mainland. Collecting trips were made by us to Fujian, Jilin and Yunnan in 2013 to obtain biological data and fresh specimens for morphological and future molecular comparison of regional populations. For the map in Fig. 13, we combined our records with collecting data published by
The present ambiguities in the usage of the name Limnobaris relate to two different issues. The first is associated with its application to species in the New World and tropical Africa, the second with the placement of very similar East Asian species in three different genera.
The taxonomy of the American and African species presently placed in Limnobaris is not the objective of this study. Their descriptions were based on
The chief problem in the current usage of the name Limnobaris in the Palaearctic Region is the paraphyly to Calyptopygus and Pertorcus.
The unresolved relationships of the Limnobaris complex in the wide sense affect approximately 25 genera worldwide, 160 currently valid species-group names and at least two family-group names. Most of these genera occur in the Americas and are placed in Zygobaridina Pierce, 1907, currently with Limnobaridina Casey, 1922 and Torcina Bondar, 1943 included as junior synonyms (
Calandra uniseriata Dufour, 1843 was described from the French Pyrenees. The author characterized briefly the genus and placed the species between Sphenophorus Schönherr and Sitophilus Schönherr in the modern sense. However,
Small size (2.7–3.2 mm) and scattered, squamiform, appressed setae on the elytron make Limnobaris albosparsa a distinctive species around the Eastern Sea (Sea of Japan). The aedeagus is identical to that of the more southern Limnobaris tibialis except for its somewhat wider base (Fig. 10 vs.
The species has been reported from Japan (Honshu), Russia (Khabarovsk and Primorsky krajs) and South Korea (
Nojima (in
RUSSIA. Primorsky Kraj: Golubiny Utes, 27.viii.1988 (ZIN 1); Kamen'-Rybonov 20 km NW, 26.vi.1974 (ZIN 3); Kamenushka, 12.vi.1989 (ZIN 1); Khanka Lake, 20 km W Spassk, 12.vi.1989 (ZIN 1); Provalovo, 12.ix.1982 (ZIN 2).
The Japanese Limnobaris babai is very similar to Limnobaris elliptica from Myanmar and South China. These two species can be distinguished from all others by the blunt ventromedian process on the male protibia and almost glabrous elytra. Limnobaris babai is on average larger than Limnobaris elliptica (3.5–4.5 mm vs. 3.2–3.8 mm), has shorter setae on the profemur, an apically rounded penis and the female protibia has a moderate ventromedian projection which is more subtle in Limnobaris elliptica. However, we have not compared specimens of the same size and these differences may not always hold. Limnobaris basalis, another morphologically similar species from Fujian, is smaller (2.3–3.1 mm) and has a longer rostrum.
The species occurs in Honshu and Kyushu, Japan (
Adult weevils have been collected from Carex sp. (
JAPAN. Saitama Pref., Urawa City, 23.v.1998 (JPPC 4).
Limnobaris basalis is a small species (2.3–3.1 mm) with white, squamiform setae on the elytral base. Males can be distinguished from the equally small Limnobaris albosparsa and Limnobaris tibialis by the shape and position of the ventral process on the protibia (Fig. 3). Abraded females of Limnobaris basalis and Limnobaris tibialis may be distinguished by body proportion (Limnobaris basalis is slightly stouter) and host association, Limnobaris basalis and Limnobaris albosparsa by allopatry. Other similar but allopatric species are Limnobaris babai (Japan) and Limnobaris elliptica (South China, Myanmar). They are larger, have smaller and fewer scales on the elytral base and a shorter rostrum.
The species is known from several sites in Fujian Province, China (Fig. 13).
The overwintered weevil appears on Scirpus in early April and feeds on the leaves. In late April, when the plant flowers in Fujian, we observed numerous specimens on the inflorescence. The larva develops in the culm, apparently most successfully in the basal, often submerged internode. Occupants of more distal internodes frequently were parasitized by an unidentified species of the Eupelmus (s. str.)urozonus Dalman group (Eupelmidae, Hymenoptera). Pupation starts in June and newly eclosed adults appear in July. In middle July 2013, we found mostly larvae, approximately one third pupae and one eclosed adult in the culms we dissected.
Pertorcus tibialis basalis is transferred here to Limnobaris and raised to full species rank based on plant association, molecular data and morphological details of the protibia, genitalia and vestiture.
CHINA. Fujian: 建阳坳头[?]田坵 [?tianqiu, Aotou, Jianyang], 25.iv.1965 (IZCAS 1); 建阳坳头三板桥 [Sanban Bridge, Aotou, Jianyang], 19.iv.1965, 26.iv.1965, 18.v.1991 (IZCAS 3); Daanyuan, Wuyi Mts., 24.–27.iv.2013 (BMNH 4, IZCAS 17, JPPC 11, ZIN 4), 16.–19.vii.2013 [larvae, pupae, 1 adult, parasites] (IZCAS, JPPC); Guadun, Wuyi Mts., 1.iv.1938 (PT), 2.iv.1938 (HT), 7.iv.1938 (4x), 8.iv.1938 (5x), 12.iv.1938 (2 PT), 15.iv.1938, 16.iv.1938, 19.iv.1938, 25.iv.1938 (5x), 27.iv.1938, 5.v.1938, 6.v.1938 (1 + 1 PT), 7.v.1938 (AKMB 25); 大竹栏 [Dazhulan], 4.vii.1965 (IZCAS 1); Guangze, 5.ix.1937 (AKMB 1); 建阳黄坑新历 [Xinli, Huangkeng, Jianyang], 25/27.v.1965 (IZCAS 2); 建阳黄坑 [Huangkeng, Jianyang], 10.–13.iv.1965 (IZCAS 4); 建阳将乐龙栖山 [Longqi Mts., Jiangle, Jianyang], 19.iv.1965, 3.vii.1965, 17.–19.v.1991 (6x), 1.viii.1991 (IZCAS 9); 梅花山双车村 [Shuangche Village, Meihua Mts.], 6.xi.2008 (IZCAS 1); 黄坑大竹篮先峰岭 [Xian Fengling, Huangkeng Dazhulan, Jianyang], 28.v.1960 (IZCAS 2).
Limnobaris dolorosa has characteristic ventrolateral vestiture of dense, squamiform setae, which occurs also in Limnobaris t-album and Limnobaris japonica. All three species can be distinguished from each other based on details of the male genitalia and vestiture. In addition, Limnobaris japonica is allopatric (see under this species for further details). The safest way to separate Limnobaris dolorosa from Limnobaris t-album is the short and wide penis (Figs 6, 7). Moreover, Limnobaris dolorosa has evenly dense vestiture on the entire flank, whereas Limnobaris t-album usually has less dense vestiture on the first ventrites and the metasternum.
Specimens with a dolorosa-type of aedeagus show regional variation in size, body shape, vestiture and surface sculpture.
The range of Limnobaris dolorosa extends from Western Europe (without Iberian Peninsula) to the Pacific coast (apparently without offshore islands, although
Because of taxonomic confusion with Limnobaris t-album, published life history data are unreliable and need verification. Specimens of the typical size range occur on Carex rostrata Stokes ex With. in Scotland (
CHINA. Heilongjiang: 哈尔滨 [Haerbin], 31.v.1943 (IZCAS 2); 虎林 [Hulin], 9.vi.1971 (IZCAS 3); 虎林虎头 [Hutou, Hulin], 9.vi.1971 (IZCAS 2); 二層甸子 [Ercengdianzi, =Yuquan], 15.vi.1941, 30.vi.1941 (3x) (IZCAS 4). Inner Mongolia: 海拉尔嵯岗 [Cuogang, Hailaer], 22.vi.1994 (IZCAS 1). Jilin: Changbai Mts., Dongwo, 8.–11.vi.2013 (IZCAS 11, JPPC 8). MONGOLIA. Dornod Aymag: Duro-Nur Lake [Dörgön núr], 15 km W Khukh-Nur Lake, 28.vi.1976 (ZIN 1). RUSSIA. Zabajkal’skij Kraj: Darasun, 28.vi.1975 (ZIN 2); Soktuj, 23.vi.1925 (ZIN 1). Irkutskaya Oblast: Irkutsk, vii.1994 (JPPC 1). Kemerovskaya Oblast: Tyazhin, 17.vi.1958 (IZCAS 1). Republic of Buryatia: Kjakhta District, NW Kiram, 27.vi.1999 (JPPC 1); Barun-Torej Lake, Ulza River, 29.vi.1925 (ZIN 2).
Limnobaris elliptica is very similar to Limnobaris babai from Japan. These two species can be distinguished from all others by the short, curved rostrum, the blunt ventromedian process on the male protibia and almost glabrous elytra. Limnobaris elliptica is on average somewhat smaller than Limnobaris babai (3.2–3.8 mm vs. 3.5–4.5 mm), has an apically pointed penis (rounded in Limnobaris babai) and at least the males have longer setae on the underside of the profemur. Limnobaris basalis, another similar species, has a longer rostrum, larger scales on the elytral base and occurs in Fujian. The Nepalese Limnobaris martensi is slenderer and almost entirely glabrous.
The species is known from three sites west of the Gaoligong Mountains in the border region between Myanmar and Yunnan Province, China (Fig. 13).
We found larvae, pupae and freshly eclosed adults inside flowering culms of Scirpus wichurai Böckeler in late September. A few specimens already had exited the plant and one was observed on a tall Scleria species, which does not appear to be a larval host. Many larvae were killed by three to five specimens of an apparently undescribed Entedon Dalman species (Eulophidae, Hymenoptera), a large and widespread genus known to parasitize beetle larvae (C. Hansson, in litt.). The weevil overwinters and the first specimens appear on the host plant in late March.
CHINA. Yunnan: 盈江伐木場 [Famuchang (logging headquarter), Yingjiang ], 1700 m, 13.iv.1980 (IZCAS 3); Xiaozahe, Tengchong County, 1800 m, 26.ix.2013 (IZCAS 4, JPPC 4, ZIN 2). MYANMAR. Kachin: Kambaiti [Kan Paik Ti], Myitkyina District, 2130 m, 28.iii.–3.iv.1934 (3x), 14.iv.1934 (BMNH 4).
Limnobaris japonica is the only species in Hokkaido, Honshu and the Kuril Islands that has dense lateroventral vestiture. Unlike Limnobaris dolorosa and Limnobaris t-album, the two other species with this trait, Limnobaris japonica has squamiform vestiture also on the prosternum. The penis is similarly elongate as in Limnobaris t-album but apically more gradually narrowed.
The species occurs in Hokkaido and Honshu (
Adult weevils have been collected from Carex thunbergii Steud. and unidentified Carex species (
JAPAN. Hokkaido, Fukushima-cho, Sengen, 13.vi.1998 (JPPC 2). RUSSIA. Sakhalin Oblast: Kunashir Island, Yushno-Kurilsk, 15.vi.1991 (ZIN 1).
Limnobaris kabakovi is a relatively large (4.1–4.6 mm) species with ventral projection on the male protibia and scattered, appressed, squamiform setae on the elytron. Similarly appressed elytral vestiture occurs also in Limnobaris albosparsa, but that species is smaller and occurs only around the Eastern Sea. At least the type series of Limnobaris kabakovi has brownish elytra.
The species is known only from the type locality, a mountain resort and national park in Northern Vietnam (Fig. 13). This is the most southern record for a species of Limnobaris.
Unknown.
VIETNAM. Tinh Vinh Phuc: Tam Dao, 25.ii.1962 (ZIN 5).
Limnobaris kumei is the only known species that has neither dense lateroventral vestiture nor a male protibial projection. Moreover, the rostrum is longer and sexually more dimorphic than in other congeners. Females have the antenna inserted in the basal half of the rostrum, a condition otherwise noticed only in Limnobaris albosparsa and Limnobaris tibialis.
The species is known from the Ryūkyū Islands (Okinawa) and Taiwan (Fig. 13).
Adult weevils were collected from unidentified Cyperaceae (
TAIWAN. [data not recorded] (BPBM 1).
Limnobaris martensi is a shiny, almost entirely glabrous species that has just a few inconspicuous setae on the ventral side and the legs (Fig. 2). The slightly bulging apical section of the tenth interstria is nearly smooth in Limnobaris martensi but more or less serrated in the other glabrous species Limnobaris babai and Limnobaris elliptica. Very slender, abraded Limnobaris tibialis can be distinguished from Limnobaris martensi by the sharply pointed, more basally inserted male protibial projection and apically round penis (Figs 8, 10).
Limnobaris martensi, dorsal and lateral view of holotype (L = 3.2 mm).
Rostrum as long as pronotum, weakly and evenly curved, cylindrical, parallel-sided in basal 2/3, slightly dilated to apex; depression before eyes very weak but distinct. Dorsal surface of rostrum evenly convex, with short striole at level of antennal insertion; shiny, with sparse minute round punctures. Sides of rostrum at base with denser and larger elongate punctures. Antennae inserted at 0.57 × length of rostrum from base, antennal scrobe shortly continued beyond base of antenna. Ventral margin of antennal scrobe merging with lateroventral edge of rostrum at half way to eye; dorsal margin of scrobe reaching eye. Scape of antenna slender, shortly widened at apex. First segment of funicle 1.5 × as long as wide, 2nd slightly longer than wide, 3rd weakly transverse, 4–7th moderately to strongly transverse. Base of club very broadly rounded, almost truncate, but clearly separated from broad 7th funicular segment, apex of club broadly rounded. Frons weakly convex, at anterior margin as broad as base of rostrum, slightly widened posteriad, shiny, with sparse small punctures. Vertex with reticulate microsculpture. Eyes large.
Pronotum 1.1 × as wide as long, parallel-sided in basal half, then weakly narrowed to shallow apical constriction. Base of pronotum feebly bisinuate. Disc weekly and evenly convex, sub-matt due to reticulate microsculpture, with rather sparse fine, somewhat angular, round or oblong punctures, separated usually by not less than own diameter, in some places by 2–3 × diameter. Median line without microreticulation and punctures. Scutellum shiny, nearly rectangular, feebly widened at base.
Elytra 1.8 × as long as wide, with well-pronounced humeri, parallel-sided in basal half, rather narrowly rounded at apex; sutural angle slightly sinuate. Disc flattened, with fairly abrupt declivity; preapical prominences very distinct. Striae deep and narrow, intervals flat, about 4 × as wide as striae, shiny, with 1 row of small punctures and much finer microreticulation than on pronotum. Intervals in many places distinctly impressed around sparse and inconspicuous punctures in striae.
Legs slender and fairly long. Fore tibia with large tooth slightly proximal of middle of inner surface, apex of tooth blunt (Fig. 4). 1st tarsite 1.5 × as long as wide, 2nd clearly transverse, rounded at sides, 3rd in fore tarsus 1.7 × as broad as 2nd. 5th tarsite slender, weakly widened toward apex, by one-half of its length projecting beyond lobes of 3rd tarsite. Length of claw 1.5 × width of claw-segment at apex. Penis as in Fig. 8, moderately bent ventrally at base and apex, basal apodemes shorter than in other species.
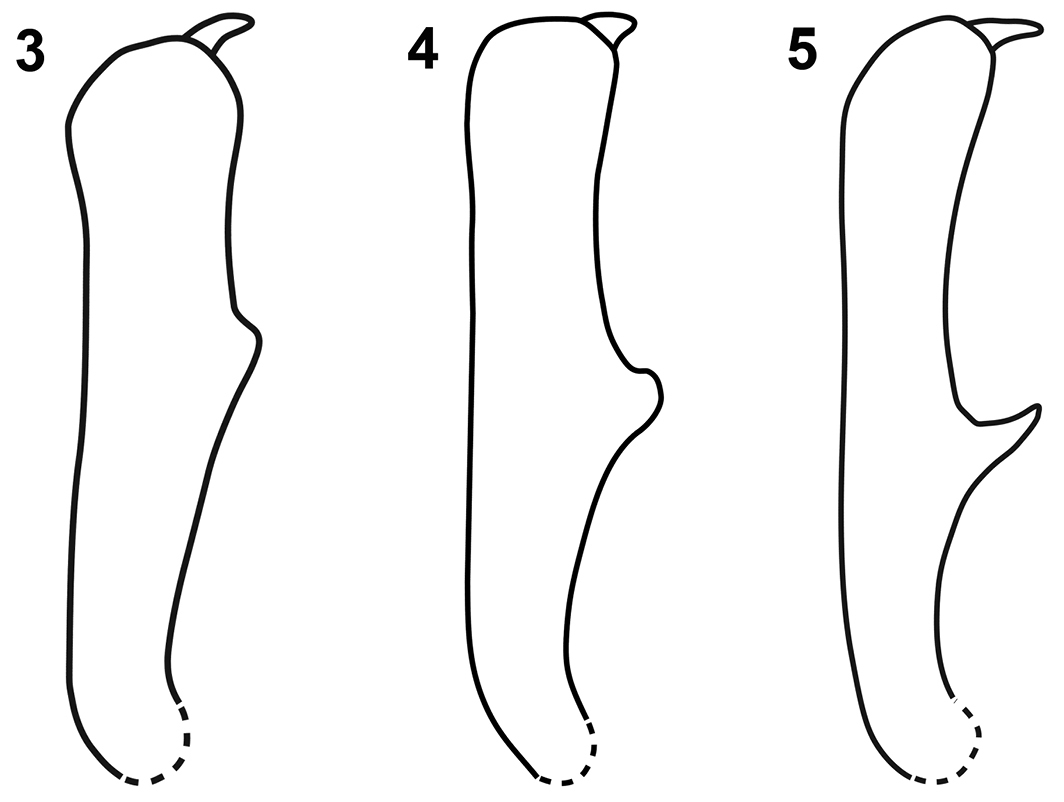
Male protibia of Limnobaris species. 3 Limnobaris basalis 4 Limnobaris martensi 5 Limnobaris tibialis.
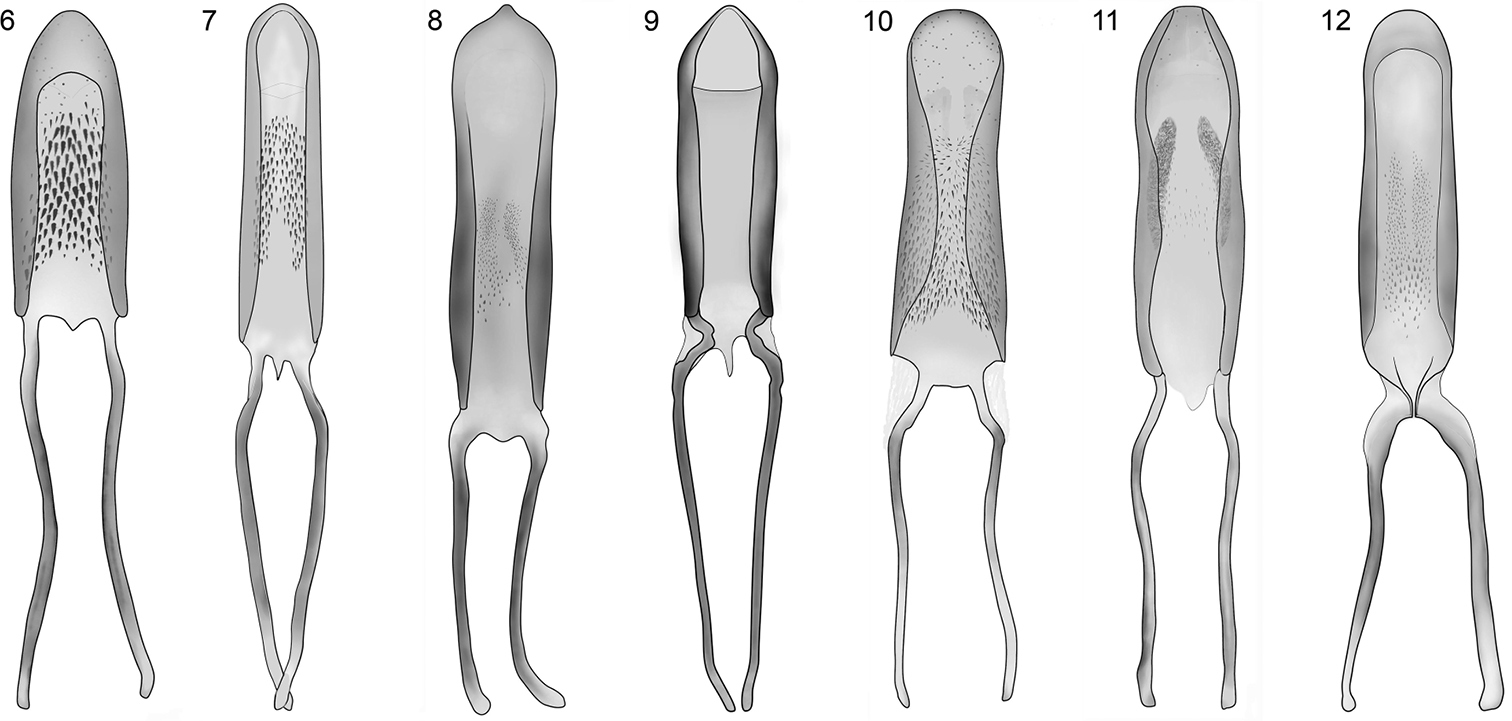
Penis of Limnobaris species, dorsal view. 6 Limnobaris dolorosa 7 Limnobaris t-album 8 Limnobaris martensi 9 Limnobaris elliptica 10 Limnobaris tibialis 11 Limnobaris basalis 12 Limnobaris kabakovi.
Body black; scape of antenna in basal 2/3 light brown, apical third of scape, 1st and base of 2nd segments of antennal funicle, and tarsi dark brown, humeral callus brownish. Upper side bare, legs with sparse short recumbent white setae, sides of abdomen with few inconspicuous setae.
Length of body 3.2 mm, width at shoulders 1.15 mm.
The only known specimen is from Eastern Nepal (Fig. 13).
Map of China and neighboring countries showing the distribution of ten of eleven Palaearctic Limnobaris species. See
Unknown.
Holotype male: Nepal, 272, Taplejung Distr., Kabeli Khola, N Yamputhin, S-Hang, 1700–2200 m, Kulturland/Busch, 5 Sep. 1983, J. Martens and B. Daams (SMNS).
The species is named after Dr. Jochen Martens (Mainz).
Limnobaris tibialis is a small species (2.1–3.1 mm) with sparse ventrolateral vestiture and erect setae on the elytron. Males have a sharply pointed, anteriorly directed process on the ventral edge of the protibia, which is located more basally than the blunt tooth of other species (Fig. 5). Limnobaris albosparsa, the only other species with a sharply pointed process, has a slightly wider penis basally, appressed rather than erect setae on the elytron and is allopatric. The number and width of white setae on the elytral interstriae vary considerably even in series taken at the same locality. Females may be confused with sympatrically occurring Limnobaris basalis, but the latter species has white setae crowded at the elytral base and is slightly less elongate. Most female Limnobaris tibialis can be distinguished by more basally inserted antennae.
The species is known from Southeast China (Anhui, Fujian, Guizhou, Jiangxi, Zhejiang) (Fig. 13).
In Northern Fujian, we found this species on several, ca. 20–30 cm tall Carex species. The larva develops in the basal, ca. 2 mm wide section of the culm. Pupation starts in July and newly eclosed adults appear in the same month.
CHINA. Anhui: Taipingshien, x.1932 (MCZ 30+). Fujian: Guadun, Wuyi Mts., 3.iv.1938 (PT of P. t. pilifer), 5.iv.1938 (HT + 2 PT of P. t. tibialis + 1), 8.iv.1938 (4x), 18.iv.1938 (1 PT of P. t. tibialis + 1 PT of P. t. pilifer), 20.iv.1938 (HT of P. t. pilifer), 5.v.1938 (2x), 26.v.1938 (PT of P. t. pilifer) (AKMB 15); ditto, 3.iii.1938 10.iv.1938 (PT of P. t. tibialis), 10.iv.1938 (PT of P. t. tibialis) (ZIUH 2); ditto, 7.iv.1938, 18.iv.1938 (2x) (CWOB 3); Changting, Hotien, 15.iv.1941 (BPBM 1), Changteh, Talungchan, 15.iv.1941 (BPBM 1); Daanyuan, Wuyi Mts., 24.–27.iv.2013 (BMNH 4, IZCAS 21, JPPC 12, ZIN 4), 16.–19.vii.2013 [larvae, pupae] (JPPC); 将乐龙栖山余家坪 [Yujiaping, Longxi Mts., Jiangle], 11.iv.1991 (IZCAS 2); 将乐龙栖山里山 [Lishan, Longxi Mts., Jiangle], 22. iv.1991 (IZCAS 1); 建阳黄坑桂林 [Guilin, Huangkeng, Jianyang], 11.iv.1960 (IZCAS 1); 建阳 [Jianyang], 10.v.1965 (IZCAS 1); 邵武铁洋 [Tieyang, Shaowu], 9.vi.1965, 28.vi.1965 (IZCAS 2); Shaowu, Wuyi Mts., 30.iv.1943 (BPBM 1). Guizhou: 遵义市绥阳县宽阔水自然保护区香广山村[Xiangguangshan village, Kuan Kuoshui Natural Reserve, Suiyang, Zunyi], 4.vi.2010 (IZCAS 1). Jiangxi: 九连山保护区 [Jiu Lianshan Natural Reserve], 29/30.ix.1979 (IZCAS 2). Zhejiang: 临安市西天目山禅院寺[Chanyuan Temple, West Tianmu Mts., Lin’an], 22.viii.2009 (IZCAS 2); 景宁 [Jingning], 28.vii.2010 (ZAFU 1); 庆元百山祖 [Baishanzu, Qingyuan], 16.v.1994 (IZCAS 2).
Diagnostic characters for the distinction of Limnobaris t-album and two other species with dense lateroventral vestiture are given under Limnobaris dolorosa.
The squamiform setae on the first two ventrites and the metasternum vary in size and density between local populations. Specimens with nearly imbricate setae have been recognized by some authors either as a subspecies or species.
Limnobaris t-album is widespread in the Palaearctic Region and apparently extends further north than the other common species, Limnobaris dolorosa. In fact, Limnobaris t-album is the only baridine weevil worldwide that reaches either of the polar circles. The most eastern records are from Mongolia (ca. 95°E), Xinjiang Province, China (ca. 90°E) and the adjacent Russian territory (
Because of taxonomic confusion with Limnobaris dolorosa, published life history data are unreliable and need verification. The adult occurs on Carex acutiformis Ehrh. between middle May and early July in Northern Germany (J. Prena, unpubl. data). Carex atherodes Spreng., Carex pseudocyperus L., Carex rostrata and Scirpus sylvaticus grew occasionally nearby but received no attention by the weevil. Reports from Carex rostrata, Carex vesicaria L. and the tall sedges Cladium mariscus and Schoenoplectus lacustris (i.e., Heyden in Brisout 1870,
CHINA. Xinjiang: 青河县达巴特新村 [Dabate, Qinghe], 7.vii.2009 (IZCAS 1); Turpan (HNHM 1). KAZAKHSTAN. Aulie-Ata [=Taraz] (HNHM 3); Wernyi [=Almaty] (HNHM 1, SFFM 6). KYRGYZSTAN. Bishkek (HNHM 2); Ketmen’tebe (HNHM 1); Tschu [Tschüi] River, Issyk-kul [Ysyk-Köl] (SDEI 1); Yssik-kul [Ysyk-Köl] (SNSD 3). MONGOLIA. Sargyn-Gobi, S Som. Sarga, Aimak Gobi Altai, 970 m, 18.–20.vi.1964 (MNKB 1). RUSSIA. Novosibirskaya Oblast: Kuibyshev Distr., Zonovo, 29.v.1961 (IZCAS 1). UZBEKISTAN. Jizzakh Prov.: Djizak [Jizzakh] (SNSD 1).
| 1 | Lateral parts of mesothorax, metathorax and abdomen densely covered with whitish or yellowish squamiform setae (Fig. 1); male protibia without ventromedian projection | 2 |
| – | Lateral parts of mesothorax, metathorax and first three ventrites with widely spaced slender to moderately wide setae, derm partially uncovered, remaining underside with sparse hairlike setae; male protibia with ventromedian projection except in Limnobaris kumei | 4 |
| 2 | Mesoscutellum with lateral portions squamose; prosternum with yellowish white, squamiform setae similar to those on meso- and metasternum; elytron with squamiform setae restricted to basal third of interstriae 3–6; Japan, Russia (Kuril Islands) | Limnobaris japonica |
| – | Mesoscutellum glabrous; prosternum with dingy white, slender setae dissimilar to those on meso- and metasternum; elytron with squamiform setae more evenly distributed if present; Palaearctic Region except Pacific offshore islands | 3 |
| 3 | Mesosternum, metasternum and abdominal ventrites laterally with wide, imbricate setae completely covering integument; male with ventrites 1–2 strongly depressed; penis shorter and wider (Fig. 6); transpalaearctic | Limnobaris dolorosa |
| – | Mesepisternum, mesepimeron and metepisternum with imbricate scales forming T-shaped pattern, adjacent sclerites (flanks of metasternum, often also first two ventrites) less densely covered with wide setae, leaving integument in between visible; male with ventrites 1–2 slightly depressed; penis longer and narrower (Fig. 7); Western and Central Palaearctic Region | Limnobaris t-album |
| 4 | Elytron with squamiform setae only at base or entirely glabrous | 5 |
| – | Elytron with squamiform setae scattered throughout (setae occasionally sparse or absent in abraded Limnobaris tibialis) | 8 |
| 5 | Squamiform setae at elytral base conspicuous, ca. as long as interstrial width; male ventrotibial process distally of mid-length of tibia (Fig. 3); total length 2.3–3.1 mm; East China (Fujian) | Limnobaris basalis |
| – | Squamiform setae at elytral base inconspicuous or entirely absent; male ventrotibial process at mid-length of tibia (Fig. 4); total length >3.1 mm; other distribution | 6 |
| 6 | Body very slender, elytra ca. 2.0 × as long as wide; apical margin of elytron smooth in dorsal view; Nepal | Limnobaris martensi |
| – | Body stouter, elytra <1.8 × as long as wide; apical margin of elytron serrate in dorsal view; more eastern distribution | 7 |
| 7 | Apical margin of elytron distinctly serrate in dorsal view; profemur of fresh specimens ventrally with curved setae longer than tarsal claw; body length 3.2–3.8 mm; penis triangularly narrowed and pointed apically; China (Yunnan), Myanmar | Limnobaris elliptica |
| – | Apical margin of elytron indistinctly serrate in dorsal view; profemur of fresh specimens ventrally with curved setae shorter than tarsal claw; penis rounded apically; body length 3.5–4.5 mm; Japan | Limnobaris babai |
| 8 | Rostrum long (male ca. 1.3 ×, female ca. 1.5 × as long as pronotum); funicular joint 7 elongate; male protibia without ventromedian projection; Ryūkyū Islands and Taiwan | Limnobaris kumei |
| – | Rostrum moderate (male <1.1 ×, female <1.3 × as long as pronotum); funicular joint 7 transverse; male protibia with ventromedian projection; other distribution | 9 |
| 9 | Elytron with erect squamiform setae; central East China (Anhui, Fujian, Guizhou, Jianxi, Zhejiang) | Limnobaris tibialis |
| – | Elytron with appressed squamiform setae; northern or southern East Asia | 10 |
| 10 | Body length <3.5 mm; rostrum at least as long as pronotum; southern continental Russian Far East, Korean Peninsula and Japan | Limnobaris albosparsa |
| – | Body length >4.0 mm; rostrum shorter than pronotum; Vietnam | Limnobaris kabakovi |
We sincerely thank Dirk Ahrens (Bonn), Max Barclay (London), Lutz Behne (Müncheberg), Zoltán Gyorgy (Budapest), Olaf Jäger (Dresden), Andrea Hastenpflug-Vesmanis (Frankfurt a. M.), Huang Junhao (Zhejiang), Shep Myers (Honolulu), Phil Perkins (Boston), Wolfgang Schawaller (Stuttgart), Joachim Willers (Berlin), Bert Viklund (Stockholm) and Hiraku Yoshitake (Tsukuba) for access to specimens and literature. Hélène Perrin (Paris) kindly searched for Calandra uniseriata Dufour. Gary Gibson (Ottawa) and Christer Hansson (Lund) identified the eupelmid and eulophid wasps, respectively. Ding Liang and Zhang Menglei (Beijing) participated in fieldwork and contributed biological observations, specimens and photos. Frank Köhler (Bornheim) provided additional photos. An Yuan (Beijing) transcribed label data and Yang Jiani (Beijing) determined geographic coordinates of collecting sites in China. Steve Davis (Lawrence, KS) checked the language and, together with an anonymous reviewer and subject editor Miguel Alonso-Zarazaga, commented on the manuscript. The first author was funded by a 1-year grant for senior international scientists awarded by the Chinese Academy of Sciences (2012T1S0025). The second author was supported by the Russian Foundation for Basic Research (Grant No 13-04-01002). Fieldwork was supported by NSFC programs 31210103909, 31172130 and J1210002. All of these contributions were important and much appreciated.