Citation: Flores GE, Pizarro-Araya J (2014) Towards a revision of the South American genus Praocis Eschscholtz (Coleoptera, Tenebrionidae), with estimation of the diversity of each subgenus. In: Bouchard P, Smith AD (Eds) Proceedings of the Third International Tenebrionoidea Symposium, Arizona, USA, 2013. ZooKeys 415: 53–80. doi: 10.3897/zookeys.415.6656
A review of the subgenera of the South American genus Praocis Eschscholtz (Pimeliinae: Praociini) is presented. Praocis comprises 77 species and 8 subspecies arranged in nine subgenera distributed in arid lands from Central Peru and Bolivia to the Southern part of Patagonia in Chile and Argentina. For each subgenus of Praocis: Praocis Eschscholtz, Mesopraocis Flores & Pizarro-Araya, subgen. n., Anthrasomus Guérin-Méneville, Filotarsus Gay & Solier, Postpraocis Flores & Pizarro-Araya, subgen. n., Hemipraocis Flores & Pizarro-Araya, subgen. n., Orthogonoderes Gay & Solier, Praonoda Flores & Pizarro-Araya, subgen. n., and Praocida Flores & Pizarro-Araya, subgen. n., we present a diagnosis using new and constant characters of adult morphology such as clypeal configuration, length and proportion of antennomeres 9, 10 and 11, arrangement of apical tomentose sensory patches on antennomeres 10 and 11, anterior margin of prosternum, lateral margin of elytron, ventral surface of profemora, and shape of protibiae. An identification key for the nine subgenera of Praocis is presented. Type species are designated for the five new subgenera; for Mesopraocis: Praocis calderana Kulzer, for Postpraocis: Praocis pentachorda Burmeister, for Hemipraocis: Praocis sellata Berg, for Praonoda: Praocis bicarinata Burmeister, for Praocida: Praocis zischkai Kulzer, and for the previously described subgenus Orthogonoderes: Praocis subreticulata Gay & Solier. The current number of species and the estimated number of species to be described are presented. The distribution ranges of the subgenera, including new records from collections and recent expeditions, are given. Habitat preferences and a discussion of the biogeography of the genus are also presented.
Taxonomy, Pimeliinae, Praociini, Praocis, key, diversity, South America
The genus Praocis Eschscholtz belongs to the Praociini, an endemic Neotropical tribe of Pimeliinae with 151 species arranged in 15 genera (
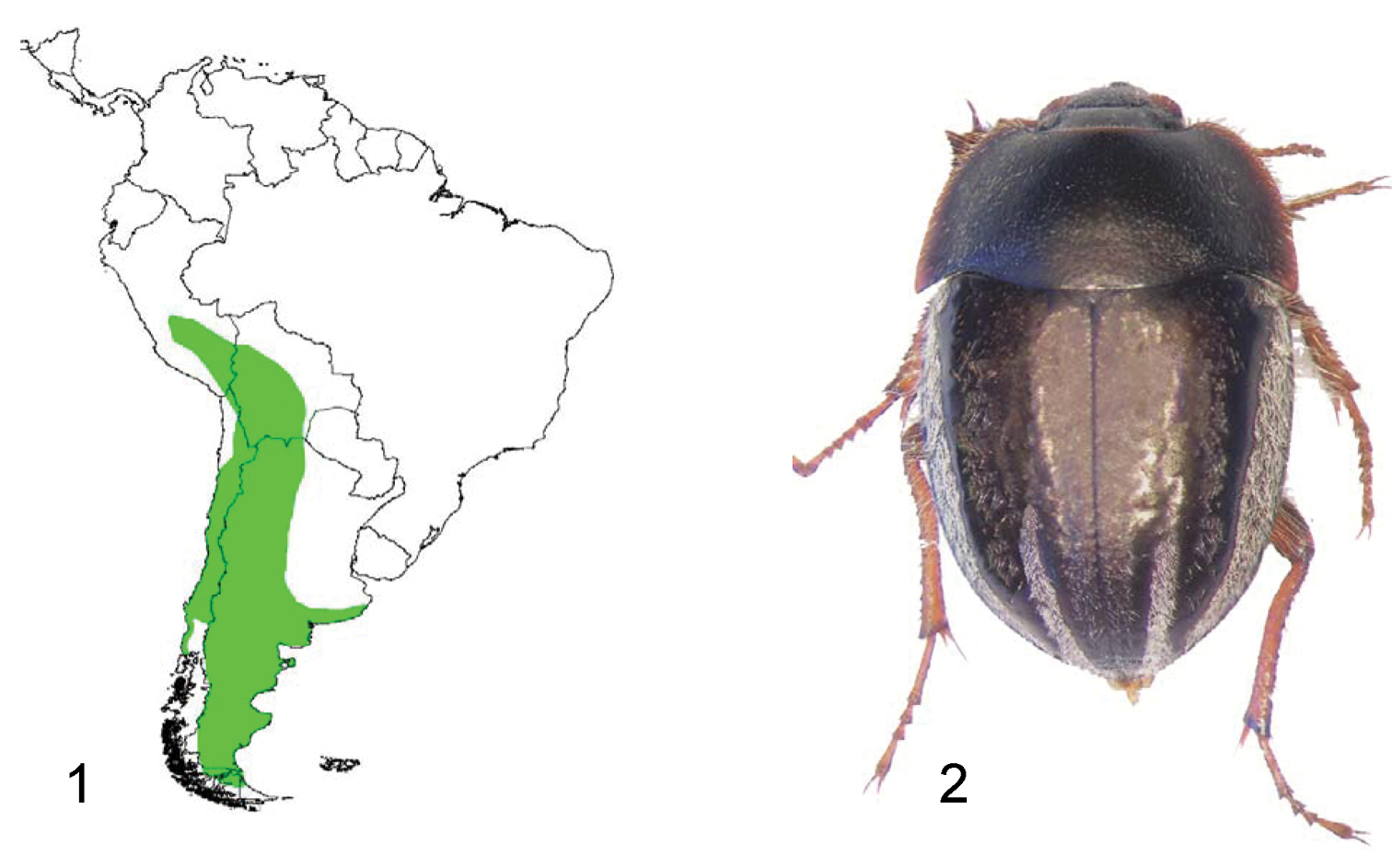
1 Distribution area of the whole genus Praocis 2 Dorsal view of Praocis (Praocis) bicentenario, holotype (previously published in
The last revision of Praocis was made by
The subgeneric classification of the genus was recently reviewed (
In the current study we report new constant characters to define each Praocis subgenus, such as shape of clypeus, frons and clypeal suture, length and proportion of antennomeres 9, 10 and 11, arrangement of apical tomentose sensory patches on antennomeres 10 and 11, and ventral surface of profemora. We also used the characters defined by Kulzer: shape of anterior margin of prosternum, posterior angles of pronotum, lateral margin of pronotum, lateral margin of elytron, shape of body and apical process of protibiae.
The objectives of this study are to present elements for a revision of the genus Praocis by incorporating new constant characters from external morphology to define each subgenus, to designate type species for some subgenera that remain unavailable, to estimate the diversity of each subgenus, to detail the geographic distribution and habitat of each subgenus and to report new distributional records for some subgenera.
Material examined. The present study is based on an examination of specimens deposited in the following collections (we follow
FMNH Field Museum of Natural History, Chicago, USA
IADIZA Instituto Argentino de Investigaciones de las Zonas Áridas, Mendoza, Argentina
LEULS Laboratorio de Entomología Ecológica, Universidad de La Serena, Chile
MACN Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina
MLPA Museo de La Plata, Buenos Aires, Argentina
MNHN Muséum National d’Histoire Naturelle, Paris, France
MNHUB Museum fur Naturkunde der Humboldt Universitat, Berlin, Germany
MNNC Museo Nacional de Historia Natural, Santiago, Chile
NHMB Natural History Museum, Basel, Switzerland
Type species. For the subgenera Praocis, Anthrasomus, and Filotarsus the type species were designated prior to this study. For Orthogonoderes Gay & Solier (in
The remaining five names of the subgenera proposed by
Characters. For each subgenus of Praocis we present a diagnosis using the following characters and character states:
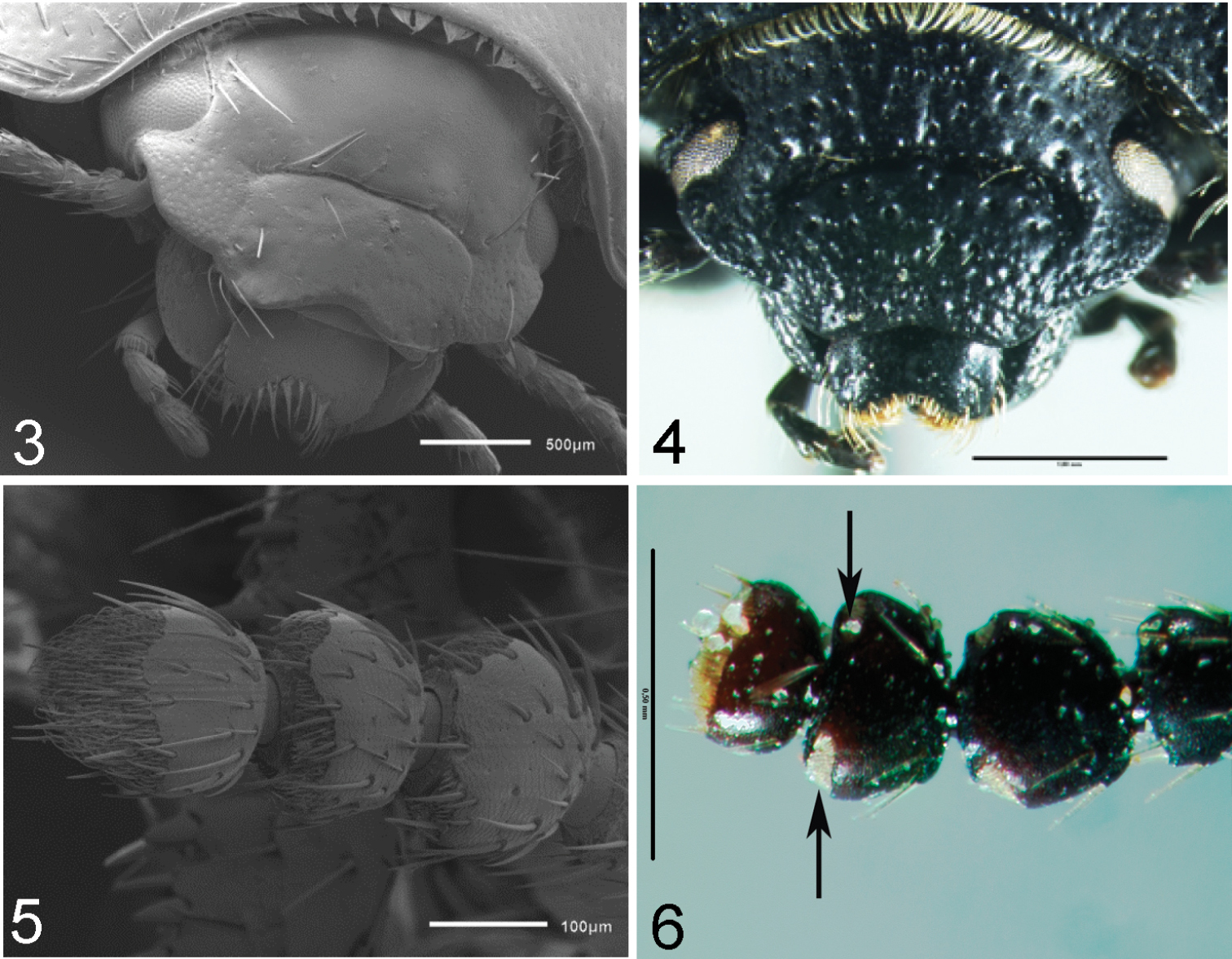
Clypeal configuration (characters 1–3). The anterior margin of clypeus, in most subgenera, extends beyond the lateral expansion of frons (Fig. 3); in some species of Filotarsus it appears at same level as lateral expansion of frons. The width of the anterior margin of the clypeus, in most subgenera, does not exceed half the interocular width (Fig. 3), while in some species of Filotarsus it is equal to interocular width. The clypeal suture shows two different states: as horizontal groove (Fig. 4), the clypeus being lower than frons; and as vertical groove, in this state the clypeus and frons are at the same level (Fig. 3).
3 Praocis (Praocis) subaenea, head in dorsal view 4 Praocis (Orthogonoderes) rotundata, head in frontal view 5 Praocis (Praocis) bicentenario, antennomeres 9–11 in dorsal view 6 Praocis (Orthogonoderes) rotundata, antennomeres 9–11 in dorsal view (Figs 3 and 5 scanning electron micrographs previously published in
Length and proportion of antennomeres 9, 10 and 11 (characters 4–5) are very variable among subgenera. Antennomere 9 can be longer than antennomere 10 (Fig. 5) or equal in length to antennomere 10. Antennomere 11 is in most subgenera longer than antennomere 10 (Fig. 5), in Orthogonoderes it is shorter than antennomere 10 (Fig. 6) and equal in length to antennomere 10 in Mesopraocis.
Arrangement of apical tomentose sensory patches on antennomeres 10 and 11 (characters 6–7) are also variable among the subgenera. The apical tomentose sensory patches on antennomere 10 are arranged in two areas subequal in size (Fig. 6), or in a dorsally continuous semicircle (Fig. 5). On antennomere 11, the apical tomentose sensory patches are located in a single area on the distal third (Fig. 6) or on the distal half of its surface (Fig. 5).
The anterior margin of the prosternum (character 8) presents two states: with a narrow sharp edge or lacking that edge. The lateral margin of the elytron (character 9) can be not defined, rounded, continuous between dorsal area of elytron and pseudopleuron, or well defined by a narrow, sharp carina-shaped edge or by a wide longitudinal, prominent edge. The ventral surface of the profemora (character 10) presents a row of setae on the anterior edge or lacks that row of setae. The shape of protibiae (character 11) varies between explanate, distal margin width exceeding 1/3 of protibial length, and not explanate, distal margin width equal to 1/4 of protibial length.
Distribution. With the distributional data published (
Estimation of the diversity of each subgenus. Based on the types of known species (deposited at FMNH, MACN, MLPA, MNHUB, MNHN, MNNC, and NHMB) and the keys of
Species list. Based on the last revision of the genus (
The species of Praocis can be recognized by having maxillary palps with last segment axe-shaped (apex twice as wide as base), antennomere 3 shorter than 4 + 5 combined, pronotum with anterior margin concave, width of posterior margin exceeding width of anterior margin, single lateral margin slender, expanded, remote from disc, and anterior angles rounded; mesosternum inclined forward, separated from prosternum; elytron with punctuate surface; apterous.
Praocis rufipes Eschscholtz, 1829, subsequent designation by
Clypeus with anterior margin extending beyond to lateral expansion of frons, width of anterior margin not exceeding half the interocular width, clypeal suture as a vertical groove, not covered by frons, clypeus and frons at same level; antennomere 10 wider than long, antennomere 9 longer than antennomere 10, antennomere 11 longer than antennomere 10; apical tomentose sensory patches on antennomere 10 in a dorsally continuous semicircle, on antennomere 11 on distal half; prosternum with a narrow edge on anterior margin; lateral margin of elytron well defined; ventral surface of profemora with a row of setae on anterior edge; protibiae explanate.
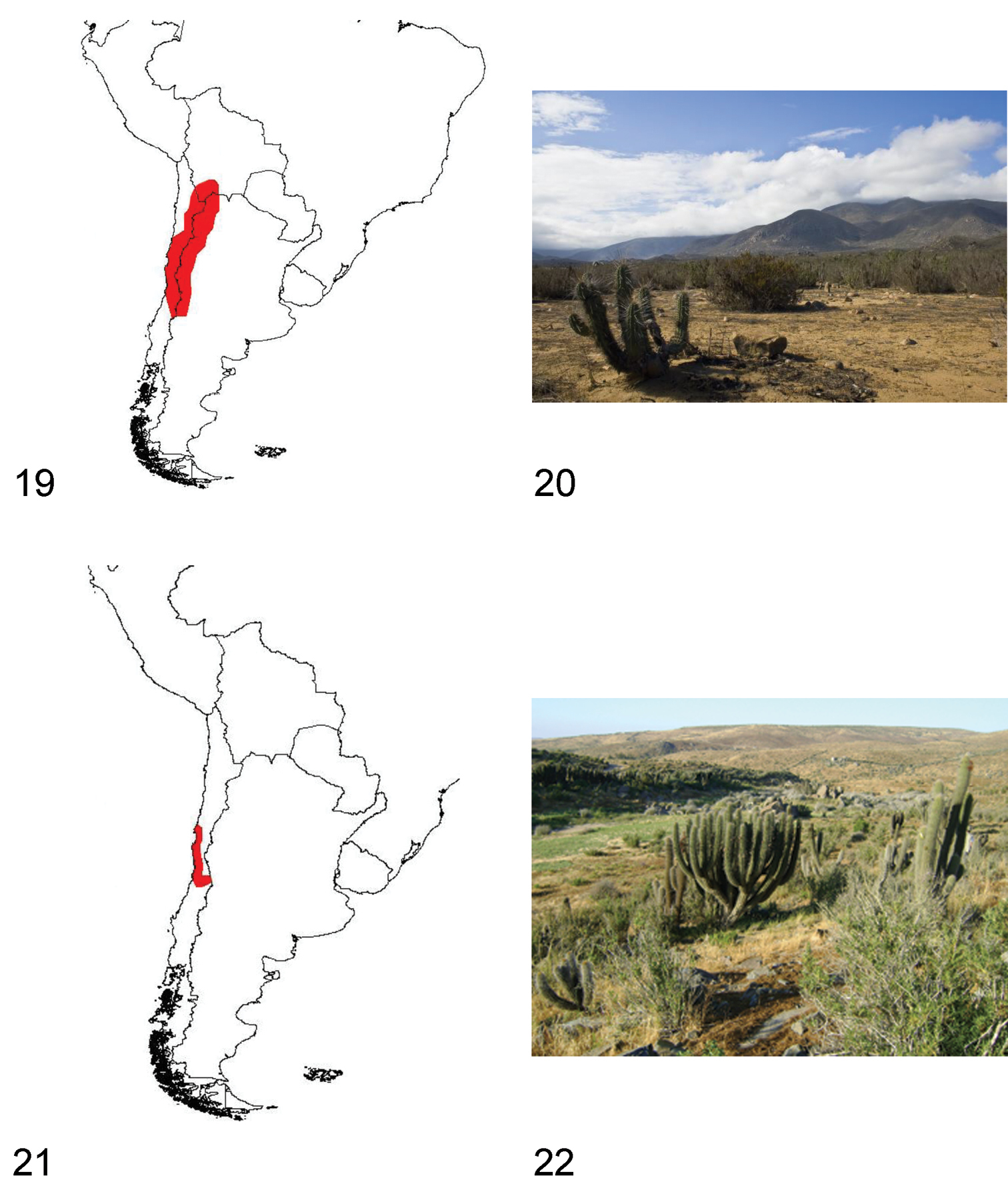
Species of Praocis s. str. are endemic to central and southern Chile and occur from 26°South (Quebrada el León, Atacama Region) to 42°South (Carelmapu, Los Lagos Region) in the biogeographic provinces of Atacama, Coquimbo, Santiago, Maule and Valdivian Forest (
We present new records for some Pacific islands. We recorded Praocis (Praocis) spinolai Gay & Solier for Damas (29°13'S, 71°31'W), Gaviota (29°15'S, 71°28'W) and Choros (29°15'S, 71°32'W) islands (
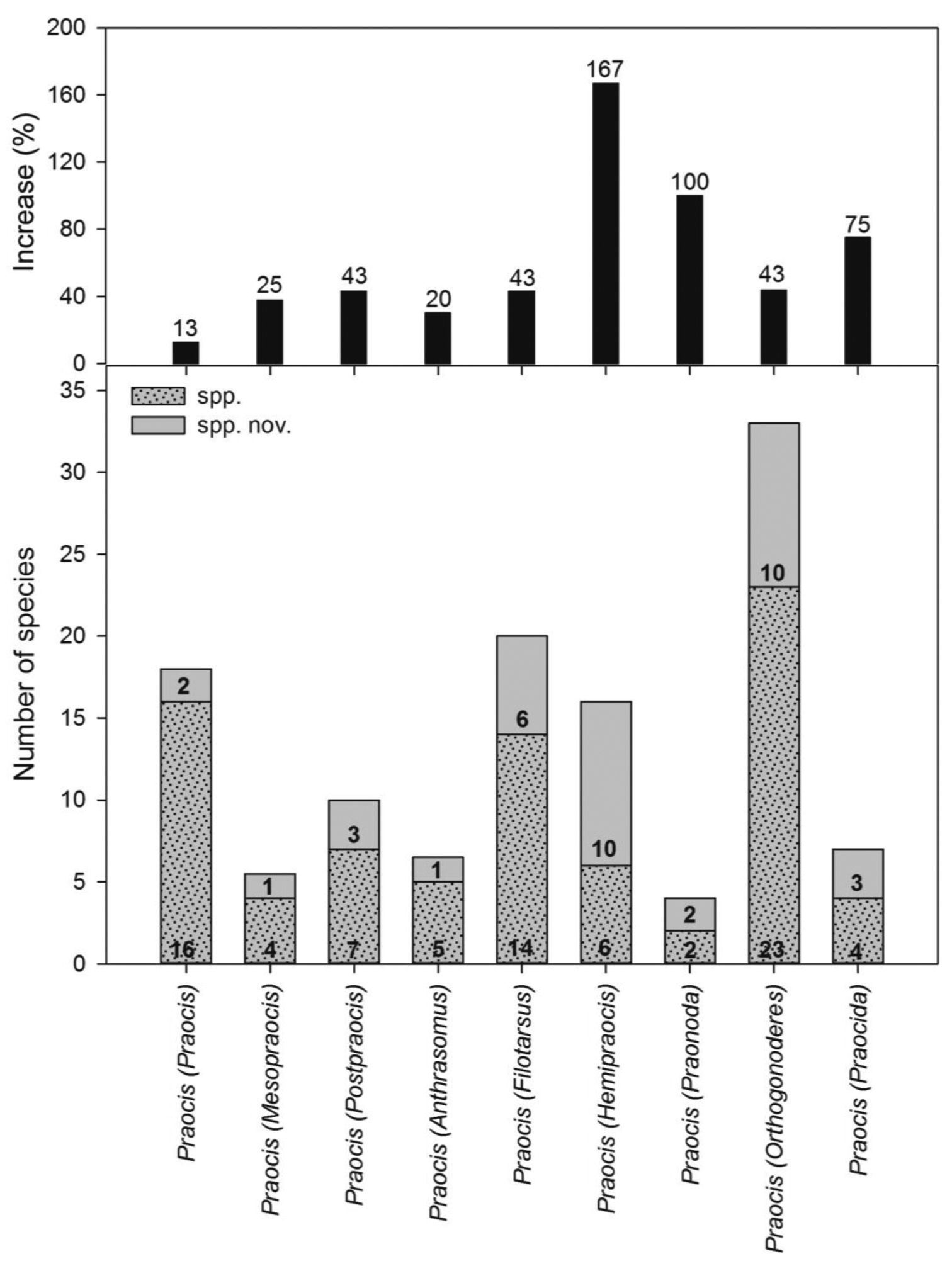
This subgenus contains 18 species of which 2 species were recently described (
The distribution range of the subgenus extends from sea level to an altitude of ~1300 m. Most species are distributed between the Huasco coastal desert and the coastal shrub steppe (
Praocis rufipes Eschscholtz, 1829 (= Sternodes mannerheimi Fischer, 1844, male, synonymy by
http://zoobank.org/C6C1EBD7-2CD9-4698-8D8A-A0823A43B03A
Figs 7, 17–18Praocis calderana Kulzer, 1958, present designation.
Clypeus with anterior margin extending beyond to lateral expansion of frons, width of anterior margin not exceeding half the interocular width, clypeal suture as a vertical groove, not covered by frons, clypeus and frons at same level; antennomere 10 wider than long, antennomere 9 of equal length to 10, antennomere 11 of equal length to 10; apical tomentose sensory patches on antennomere 10 in two areas subequal in size, on antennomere 11 on distal third; prosternum with a narrow edge on anterior margin; lateral margin of elytron not defined; ventral surface of profemora with a row of setae on anterior edge, protibiae very explanate.
Species of Praocis (Mesopraocis) are endemic to northern Chile and occur from 25°South (Paposo, Antofagasta Region) to 31°South (Caleta Limarí, Coquimbo Region) in the biogeographic provinces of Atacama and Coquimbo (
We present new records for some Pacific islands. We recorded the species Praocis (Mesopraocis) pilula Laporte and Praocis (Mesopraocis) flava Kulzer for Damas (29°13'S, 71°31'W) and Gaviota Islands (29°15'S, 71°28'W) (
This subgenus contains 4 species (
The distribution range of the subgenus extends from sea level to an altitude of ~1325 m. All Mesopraocis species are associated with coastal dunes stabilized with vegetation or paleodunes in the transitional coastal desert of Chile and have nocturnal habits, remaining during the day under stones or plants (
Praocis pilula Laporte, 1840 (= Coelus hirticollis Solier, 1840, synonymy by
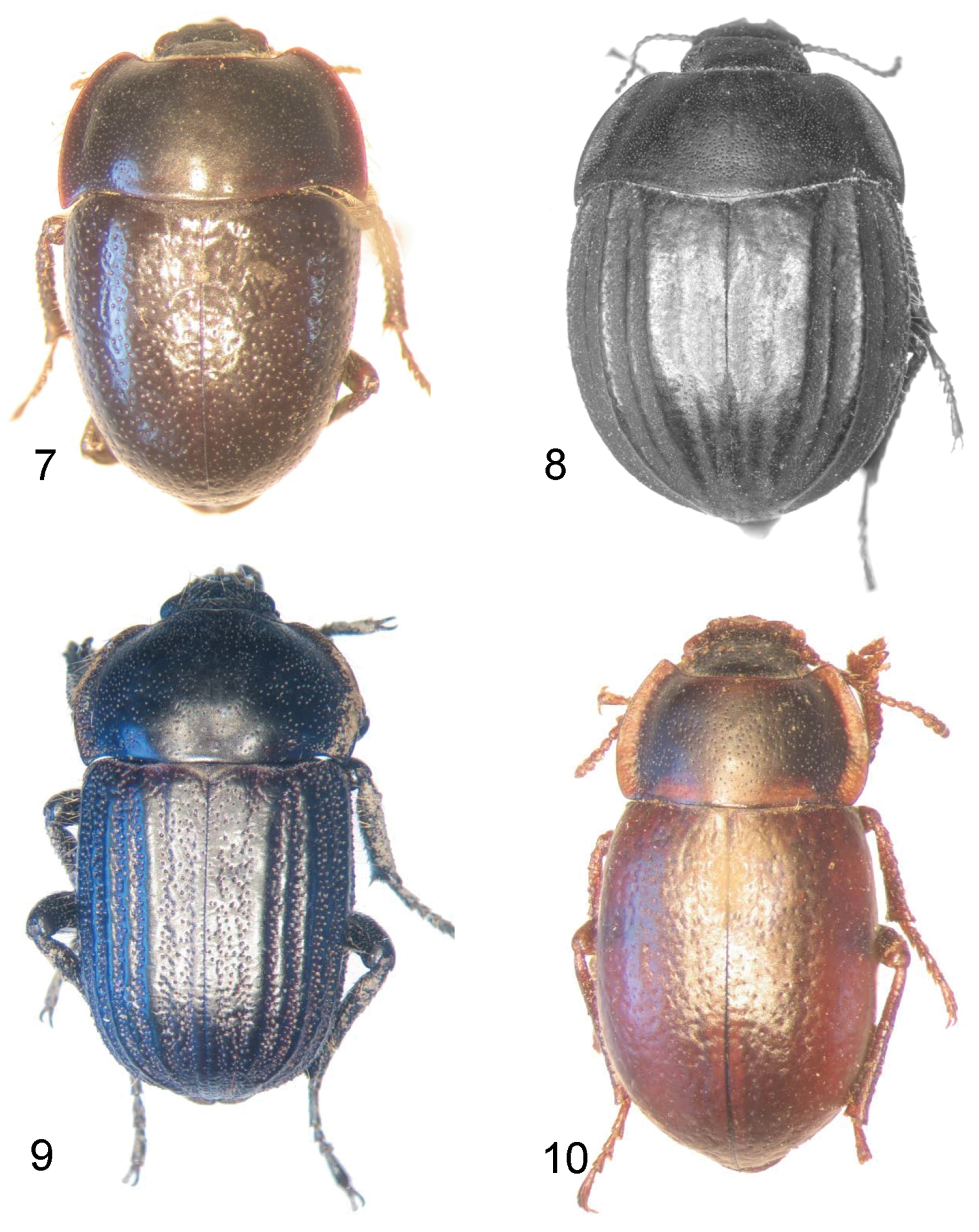
Dorsal view of Praocis species. 7 Praocis (Mesopraocis) calderana, paratype 8 Praocis (Postpraocis) pentachorda, lectotype (previously published in
http://zoobank.org/2EA923F4-48C6-4DA4-A0C7-A7E3B6714881
Figs 8, 19–20Praocis pentachorda Burmeister, 1875, present designation.
Clypeus with anterior margin extending beyond to lateral expansion of frons, width of anterior margin not exceeding half the interocular width, clypeal suture as a vertical groove, not covered by frons, clypeus and frons at same level; antennomere 10 wider than long, antennomere 9 longer than antennomere 10, antennomere 11 longer than antennomere 10; apical tomentose sensory patches on antennomere 10 in two areas subequal in size, on antennomere 11 on distal half; prosternum with a narrow edge on anterior margin; lateral margin of elytron well defined; ventral surface of profemora without a row of setae on anterior edge, protibiae not explanate.
Species of Praocis (Postpraocis) inhabit central and northern Chile and western and northern Argentina. They occur from 19°South (Termas de Enquelga, Colchane, Tarapacá Region, Chile) to 34°South in Chile (Rancagua) and 33°South in Argentina (Mendoza) in the biogeographic provinces of Atacama, Coquimbo, Santiago, Puna, Prepuna and Monte (
We present new records of Praocis (Postpraocis) pentachorda Burmeister for the Region Tarapacá of Chile and southern Bolivia and of Praocis (Postpraocis) curtisi Solier for the Pacific islands Damas (29°13'S, 71°31'W), Gaviota (29°15'S, 71°28'W) and Choros (29°15'S, 71°32'W) (
This subgenus contains 7 species/subspecies (
Species of Praocis (Postpraocis) have diurnal habits, remaining during the night under stones or plants. In central Chile they can be observed walking on coastal plains or in sandy places lying from sea level to an altitude of ~1300 m. In Argentina, northern Chile and Bolivia, they occur from 1600 m in high altitudinal valleys associated with the Andes mountain range to an altitude of 4200 m in the high Puna plateau, in sandy soils or clayey, poorly permeable soils (
Praocis curtisii Solier, 1851; Praocis costatula Gay & Solier in Solier, 1840 (= Praocis angulifera Philippi & Philippi, 1864, synonymy by
Anthrasomus chevrolatii Guérin-Méneville, 1834, monotypy.
Clypeus with anterior margin extending beyond to lateral expansion of frons, width of anterior margin not exceeding half the interocular width, clypeal suture as a horizontal groove covered by frons, clypeus lower than frons; antennomere 10 wider than long, antennomere 9 longer than antennomere 10, antennomere 11 longer than antennomere 10; apical tomentose sensory patches on antennomere 10 in two areas subequal in size, on antennomere 11 on distal half; prosternum with a narrow edge on anterior margin; lateral margin of elytron not defined; ventral surface of profemora without a row of setae on anterior edge, protibiae not explanate.
Species of Praocis (Anthrasomus) inhabit central Chile and occur from 28°South (Freirina, Atacama Region) to 33°South (San Fernando, Libertador General Bernardo O’Higgins Region) in the biogeographic provinces of Atacama, Coquimbo, and Santiago (
This subgenus contains 5 species/subspecies (
Species of Praocis (Anthrasomus) have nocturnal habits, remaining during the day under stones or plants in coastal plains, gullies, and transverse valleys in semiarid Chile. They occur from sea level to an altitude of 2800 m, in stony-clayey, poorly permeable soils (collection data FMNH, IADIZA, LEULS, and pers. obs.) (Fig. 22).
Praocis chevrolatii Guérin-Méneville, 1834 (= Praocis gayi Solier, 1840, synonymy by
Filotarsus tenuicornis Gay & Solier in Solier, 1840, monotypy and original designation by
Clypeus with anterior margin extending beyond to lateral expansion of frons or at same level as lateral expansion of frons, width of anterior margin not exceeding half the interocular width or width of anterior margin same as interocular width, clypeal suture as a vertical groove, not covered by frons, clypeus and frons at same level or clypeal suture as a horizontal groove not covered by frons, clypeus lower than frons; antennomere 9 longer than antennomere 10, antennomere 11 longer than antennomere 10; apical tomentose sensory patches on antennomere 10 in a dorsally continuous semicircle, on antennomere 11 on distal half; prosternum with a narrow edge on anterior margin; lateral margin of elytron not defined; ventral surface of profemora without a row of setae on anterior edge, protibiae explanate.
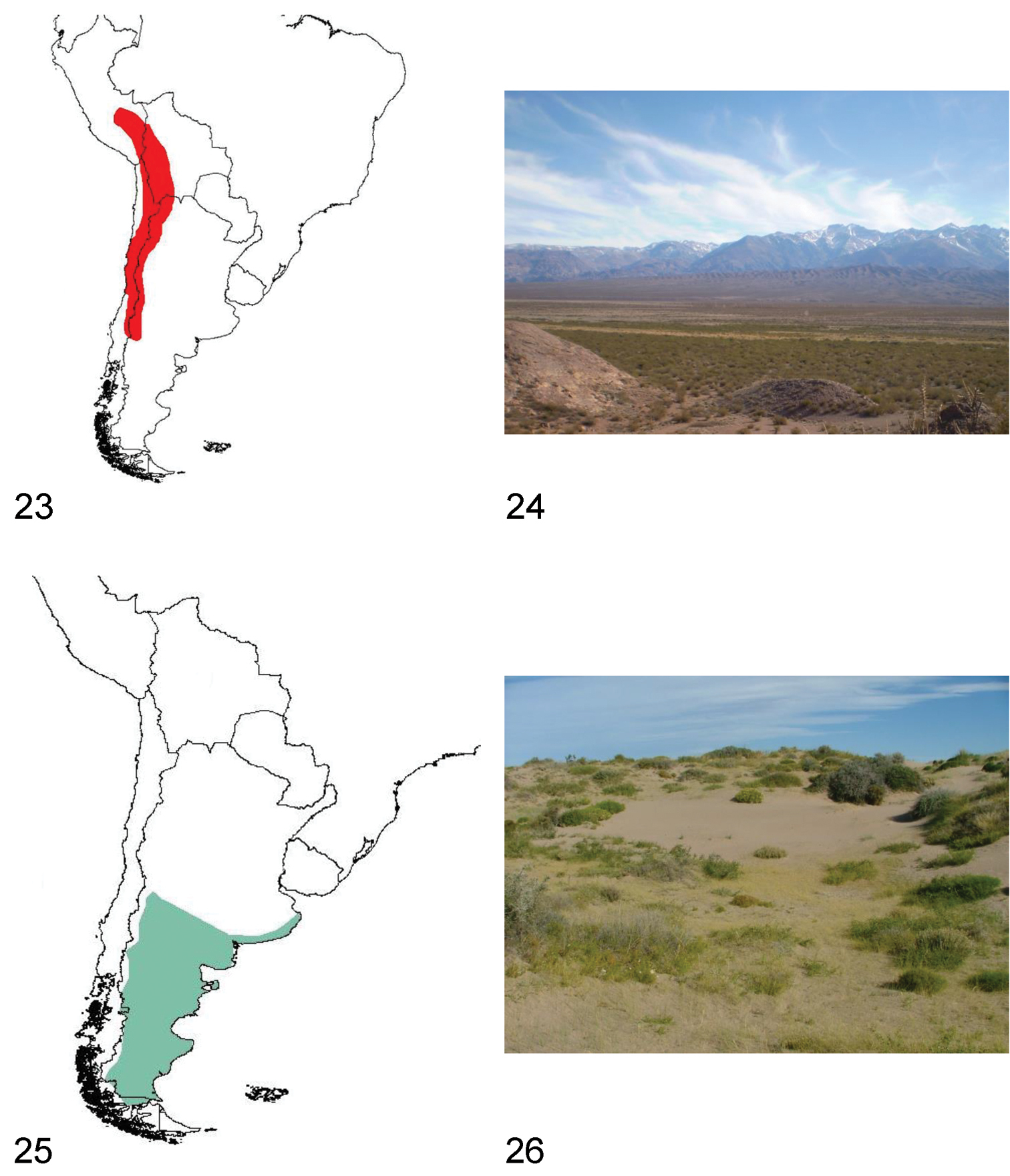
Species of Praocis (Filotarsus) inhabit central and northern Chile, western and northern Argentina, estern Bolivia and southern Peru. They occur from 12°South (Cuzco, Peru) to 39°South (Neuquén, Argentina) in the biogeographic provinces of Puna, Atacama, Coquimbo, Santiago, Prepuna, Monte, and Central Patagonia (
This subgenus contains 14 species (
Species of Praocis (Filotarsus) have nocturnal habits, remaining during the day under stones or plants. In central Chile they can be observed in gullies and Coastal and Andean mountain ranges from 400 m to an altitude of 2500 m. In Argentina, Bolivia, northern Chile and Peru they occur from 1600 m in high altitudinal valleys associated with the Andes mountain range to an altitude of 5200 m in the high Puna plateau, in clayey, poorly permeable soils (
Praocis tenuicornis Gay & Solier in Solier, 1840; Praocis castanea Germain, 1855; Praocis rufilabris Gay & Solier in Solier, 1840; Praocis uretai Kulzer, 1958 (= Praocis freyi Marcuzzi, 1977, synonymy by
http://zoobank.org/1EF65CCD-D4C2-4B9F-BB5B-7BB98170599D
Figs 11, 25–26Praocis sellata Berg, 1889, present designation.
Clypeus with anterior margin extending beyond to lateral expansion of frons, width of anterior margin not exceeding half the interocular width, clypeal suture as a horizontal groove not covered by frons, clypeus lower than frons; antennomere 9 longer than antennomere 10, antennomere 11 longer than antennomere 10; apical tomentose sensory patches on antennomere 10 in two areas subequal in size, on antennomere 11 on distal half; prosternum without a narrow edge on anterior margin; lateral margin of elytron well defined; ventral surface of profemora without a row of setae on anterior edge, protibiae explanate.
The species of Praocis (Hemipraocis) occur from central Argentina (southern Mendoza 36°S and coastal Buenos Aires 36°S), to southern Argentina and Chile (northern Magellan Strait 52°S), in the biogeographic provinces of Patagonia, Monte and Pampa (
We present a new record for the Peninsula Valdés in Argentina (
This subgenus contains 8 species/subspecies (
Species of Praocis (Hemipraocis) have diurnal and crepuscular habits, hiding during the night under shrubs, stones or buried in sand. They inhabit the Patagonian steppes and coastal Pampa from sea level to an altitude of 1700 m, in sandy soils or clayey, poorly permeable soils (
Praocis sellata Berg, 1889; Praocis sellata bergi Kulzer, 1958; Praocis sellata bruchi Kulzer, 1958 (= Praocis sellata topali Kaszab, 1964, synonymy by
Dorsal view of Praocis species. 11 Praocis (Hemipraocis) sellata peninsularis, holotype (reproduced from
http://zoobank.org/5D327D83-AAE6-4E40-B7BC-B338ADE4CA09
Figs 12, 27–28Praocis bicarinata Burmeister, 1875, present designation.
Clypeus with anterior margin extending beyond to lateral expansion of frons, width of anterior margin not exceeding half the interocular width, clypeal suture as a horizontal groove not covered by frons, clypeus lower than frons; antennomere 9 longer than antennomere 10, antennomere 11 longer than antennomere 10; apical tomentose sensory patches on antennomere 10 in two areas subequal in size, on antennomere 11 on distal half; prosternum without a narrow edge on anterior margin; lateral margin of elytron well defined; ventral surface of profemora without a row of setae on anterior edge, protibiae explanate.
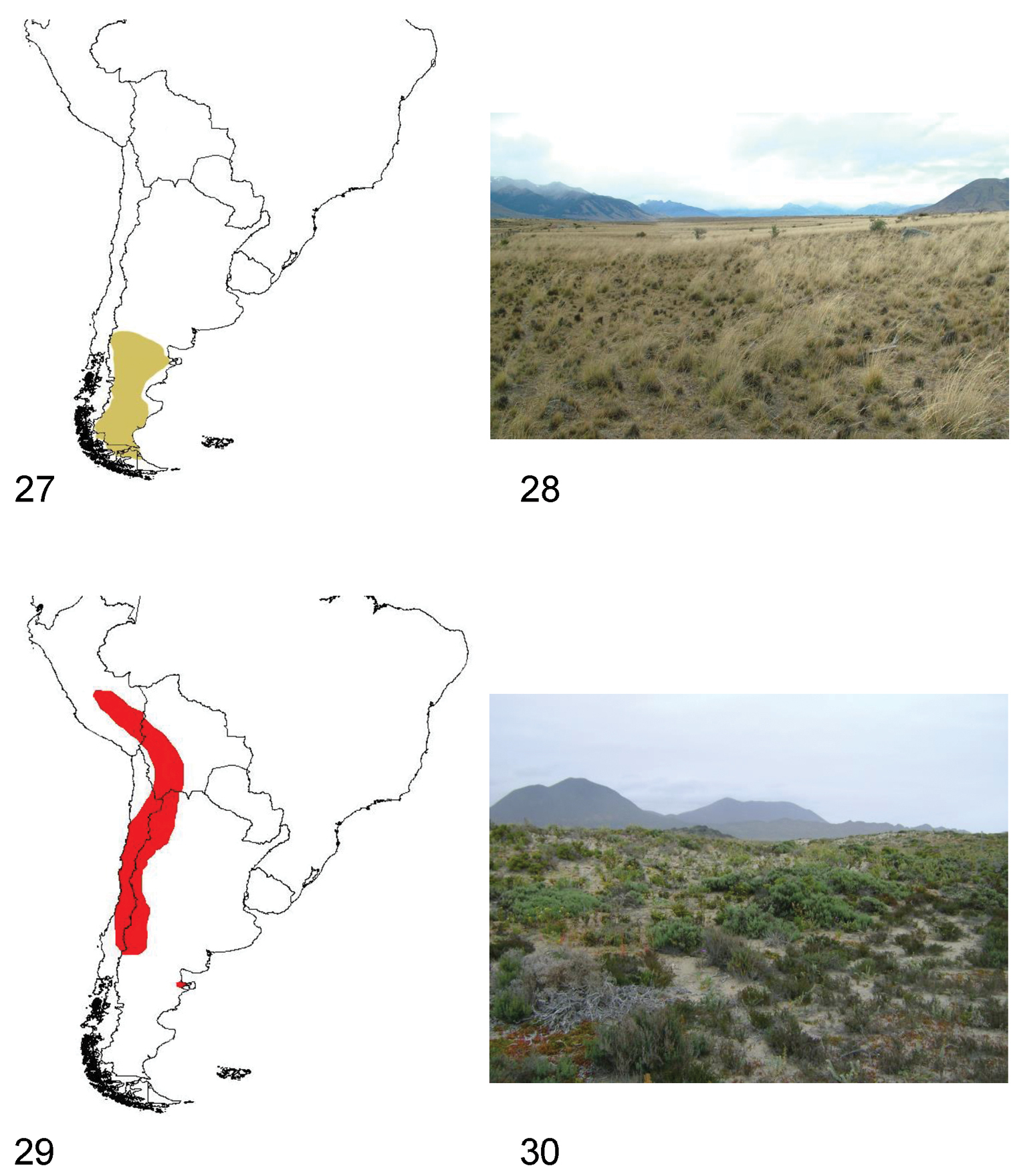
The species of Praocis (Praonoda) occur from Neuquén and Rio Negro provinces in Argentina (40°S) to northern Tierra del Fuego Island (52°30'S) with the species Praocis (Praonoda) bicarinata as the unique species of Praocis inhabiting Tierra del Fuego. They inhabit the biogeographic provinces of Patagonia and Monte (
This subgenus contains 2 species (
Species of Praocis (Praonoda) have diurnal and crepuscular habits, hiding during the night under shrubs or stones. They inhabit the Patagonian steppes from sea level to an altitude of 1250 m, in sandy soils or clayey, poorly permeable soils (collection data FMNH, IADIZA and pers. obs.) (Fig. 28).
Praocis bicarinata Burmeister, 1875 (= Praocis silphomorpha Fairmaire, 1883a, synonymy by
Praocis subreticulata Gay & Solier in Solier, 1840, present designation.
Clypeus with anterior margin extending beyond to lateral expansion of frons, width of anterior margin not exceeding half the interocular width, clypeal suture as a horizontal groove covered by frons, clypeus lower than frons; antennomere 9 longer than antennomere 10, antennomere 11 shorter than antennomere 10; apical tomentose sensory patches on antennomere 10 in two areas subequal in size, on antennomere 11 on distal third; prosternum without a narrow edge on anterior margin; lateral margin of elytron well defined; ventral surface of profemora without a row of setae on anterior edge, protibiae explanate.
Species of Praocis (Orthogonoderes) inhabit central and northern Chile, western and northern Argentina, western Bolivia and southern Peru. They occur from 12°South (Cuzco, Peru) to 38°South in Chile (Nahuelbuta) and 39°South in Argentina (Neuquén) in the biogeographic provinces of Puna, Atacama, Coquimbo, Santiago, Maule, Prepuna, Monte, and Central Patagonia (
We present a new record of Praocis argentina Kulzer for the Atlantic coast in Argentina, the isthmus of Peninsula Valdés, 42°30'S.
This subgenus contains 23 species (
Species of Praocis (Orthogonoderes) have diurnal and crepuscular habits, hiding during the night under shrubs or stones. In central Chile they can be observed in coastal dunes stabilized with vegetation or paleodunes, gullies, coastal plains, transverse valleys and Coastal and Andean mountain ranges from sea level to an altitude of 2700 m. In Argentina, Bolivia, Peru, and northern Chile, they occur from 1600 m high altitudinal valleys associated with the Andes mountain range to an altitude of 4200 m in the high Puna plateau, in sandy soils or in clayey, poorly permeable soils (
Praocis cribrata Gay & Solier in Solier, 1840; Praocis adspersa Germain, 1855; Praocis depressicollis Germain, 1855; Praocis ecostata Kulzer, 1958; Praocis subreticulata Gay & Solier in Solier, 1840; Praocis dentipes Germain, 1855; Praocis pleuroptera Gay & Solier in Solier, 1840 (= Praocis convexa Germain, 1855, synonymy by
http://zoobank.org/4B6EC138-1D93-4567-BB66-E3A6B1FC4593
Figs 14, 31–32Praocis zischkai Kulzer, 1958, present designation.
Clypeus with anterior margin extending beyond to lateral expansion of frons, width of anterior margin not exceeding half the interocular width, clypeal suture as a horizontal groove not covered by frons, clypeus lower than frons; antennomere 9 longer than antennomere 10, antennomere 11 longer than antennomere 10; apical tomentose sensory patches on antennomere 10 in two areas subequal in size, on antennomere 11 on distal third; prosternum without a narrow edge on anterior margin; lateral margin of elytron well defined; ventral surface of profemora without a row of setae on anterior edge, protibiae explanate.
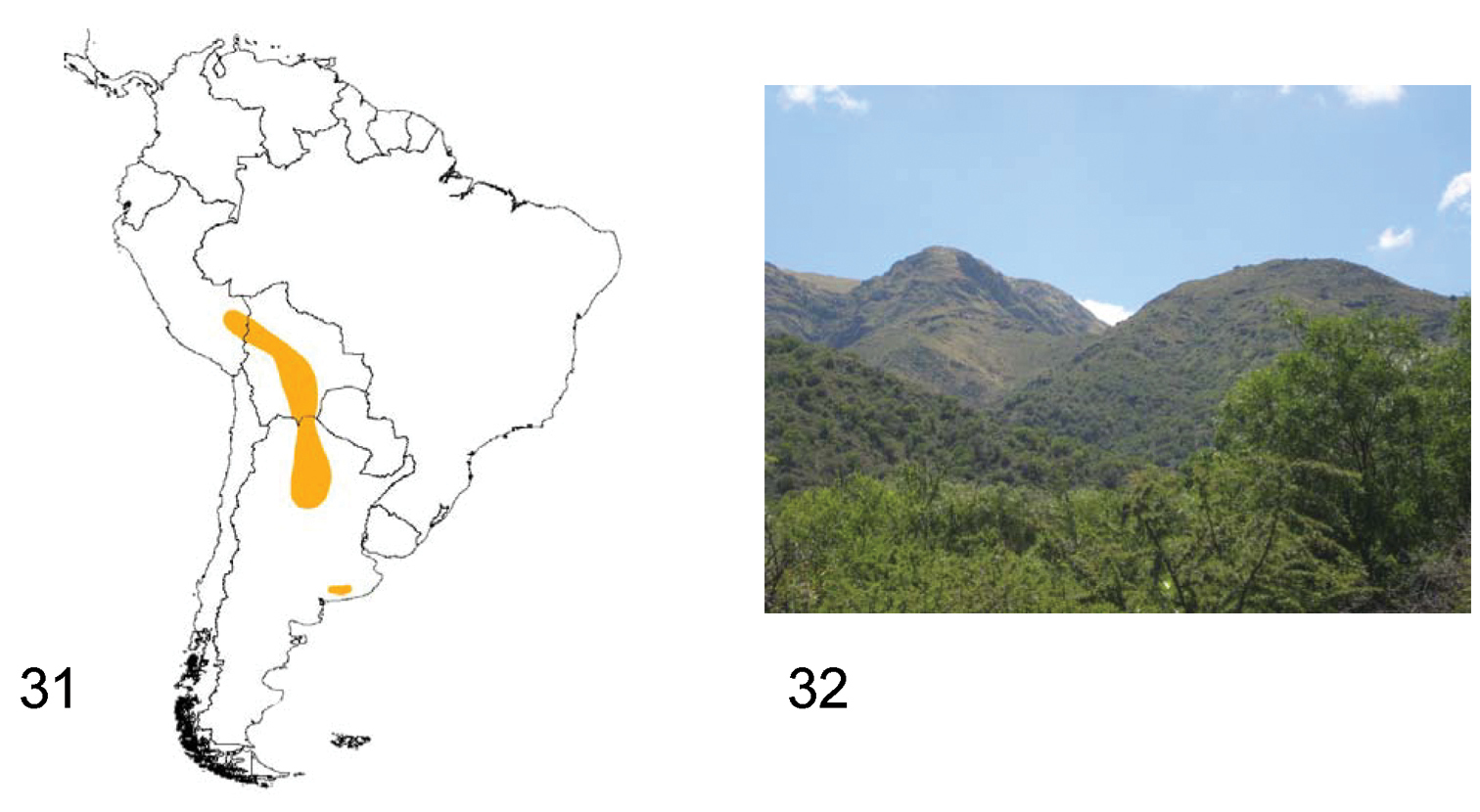
Species of Praocis (Praocida) inhabit southern Peru, central and southern Bolivia and northern Argentina. They occur from 12°South (Cuzco, Peru) to 31°South in Cordoba (northern Argentina), in the biogeographic provinces of Puna, Chaco, and Pampa (
We present a new record of Praocis (Praocida) teniucosta Kulzer for the mountains in South Buenos Aires province (38°S).
This subgenus contains 4 species (
Species of Praocis (Praocida) have nocturnal habits, hiding during the day under shrubs, stones or logs in clayey, poorly permeable soils. They occur from 1200 m in the Chacoan forest to an altitude of 4000 m in Puna (collection data FMNH, IADIZA and pers. obs.) (Fig. 32).
Praocis tenuicosta Kulzer, 1958; Praocis zischkai Kulzer, 1958; Praocis kuscheli Kulzer, 1958; Praocis montana Kulzer, 1958 (= Praocis baloghi Marcuzzi, 2001, synonymy by
Praocis pentagona Lacordaire, 1830; Praocis squalida Lacordaire, 1830; Praocis silphoides Lacordaire, 1830; Praocis spinipes Laporte, 1840; Praocis hirticollis Laporte, 1840. Type material belonging to these five species is missing (
| 1 | Anterior margin of prosternum with a narrow, sharp edge | 2 |
| – | Anterior margin of prosternum rounded, smooth, lacking edge | 6 |
| 2 | Lateral margin of elytron well defined by a sharp edge carina-shaped, narrow or broad (Figs 2, 8), dorsal area of elytron well differentiated from pseudopleuron | 3 |
| – | Lateral margin of elytron not defined, rounded (Figs 7, 9–10), surface continuous between dorsal area of elytron and pseudopleuron | 4 |
| 3 | Apical tomentose sensory patches on antennomere 10 arranged in a dorsally continuous semicircle (Fig. 3); ventral surface of profemora with a row of setae on anterior edge (Fig. 2) | Praocis Eschscholtz |
| – | Apical tomentose sensory patches on antennomere 10 arranged in two areas subequal in size (Fig. 4); ventral surface of profemora lacking a row of setae on anterior edge (Fig. 8) | Postpraocis Flores & Pizarro-Araya |
| 4 | Antennae very short, reaching only 1/4 of lateral margin of pronotum; antennomere 9 equal length as antennomere 10; antennomere 11 equal length as antennomere 10; apical tomentose sensory patches on antennomere 11 on distal third (Fig. 4); ventral surface of profemora with a row of setae on anterior edge (Fig. 7) | Mesopraocis Flores & Pizarro-Araya |
| – | Antennae long, reaching or surpassing the midpoint of lateral margin of pronotum; antennomere 9 longer than antennomere 10 (Fig. 3); antennomere 11 longer than antennomere 10 (Fig. 3); apical tomentose sensory patches on antennomere 11 on distal half (Fig. 3); ventral surface of profemora lacking a row of setae on anterior edge | 5 |
| 5 | Apical tomentose sensory patches on antennomere 10 arranged in two areas subequal in size (Fig. 4); dorsal area of elytron with 2 to 5 carinae; protibiae not explanate (Fig. 9) | Anthrasomus Guérin-Méneville |
| – | Apical tomentose sensory patches on antennomere 10 arranged in a dorsally continuous semicircle (Fig. 3); dorsal area of elytron lacking carinae; protibiae explanate (Fig. 10) | Filotarsus Gay & Solier |
| 6 | Apical tomentose sensory patches on antennomere 11 on distal half (Fig. 3) | 7 |
| – | Apical tomentose sensory patches on antennomere 11 on distal third (Fig. 4) | 8 |
| 7 | Body spherical, wide, rounded seen from above; lateral margin of elytra as a wide, prominent edge; lateral margin of pronotum with a row of long, black or golden setae (Fig. 11) | Hemipraocis Flores & Pizarro-Araya |
| – | Body elongate, narrow, subparallel seen from above, lateral margin of elytra as sharp edge carina-shaped; lateral margin of pronotum lacking a row of setae (Fig. 12) | Praonoda Flores & Pizarro-Araya |
| 8 | Antennomere 11 shorter than antennomere 10; clypeal suture as a horizontal groove covered by frons (Figs 4, 13) | Orthogonoderes Gay & Solier |
| – | Antennomere 11 longer than antennomere 10; clypeal suture as a horizontal groove not covered by frons (Figs 3, 14) | Praocida Flores & Pizarro-Araya |
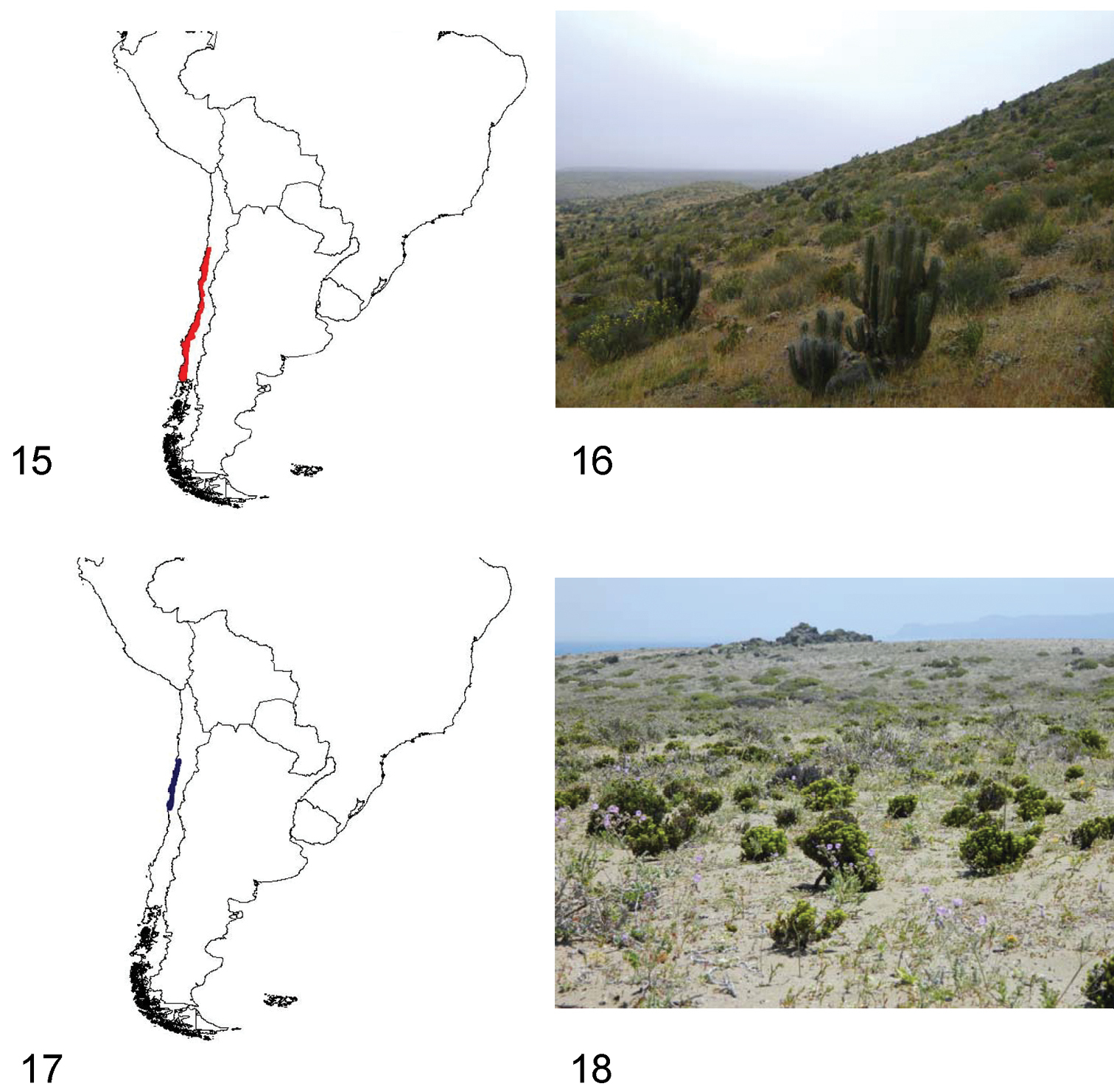
15 Distribution area of the subgenus Praocis (Praocis) 16 Punta de Choros (Coquimbo Region, Chile), habitat of Praocis (Praocis) spinolai 17 Distribution area of the subgenus Praocis (Mesopraocis) 18 Chañaral de Aceituno, Huasco (Atacama Region, Chile), habitat of Praocis (Mesopraocis) pilula.
19 Distribution area of the subgenus Praocis (Postpraocis) 20 Totoralillo Norte (Coquimbo Region, Chile), habitat of Praocis (Postpraocis) curtisii 21 Distribution area of the subgenus Praocis (Anthrasomus) 22 Socos (Coquimbo Region, Chile), habitat of Praocis (Anthrasomus) chevrolatii subcostata.
23 Distribution area of the subgenus Praocis (Filotarsus) 24 Uspallata Valley (Mendoza, Argentina), habitat of Praocis (Filotarsus) oblonga 25 Distribution area of the subgenus Praocis (Hemipraocis) 26 Peninsula Valdés (Chubut, Argentina), habitat of Praocis (Hemipraocis) sellata peninsularis.
27 Distribution area of the subgenus Praocis (Praonoda) 28 Southern Santa Cruz (Argentina), habitat of Praocis (Praonoda) bicarinata 29 Distribution area of the subgenus Praocis (Orthogonoderes) 30 Choros Bajos, (Coquimbo Region, Chile), habitat of Praocis (Orthogonoderes) chilensis.
31 Distribution area of the subgenus Praocis (Praocida) 32 Capilla del Monte (Córdoba, Argentina), habitat of Praocis (Praocida) teniucosta (Photo by Liliana Arguello).
Diversity of the subgenera of Praocis. Current number of species (dotted); number of species to be described or recently described (grey) and percentage of increasing of species for each subgenus (black).
Praocis currently contains 77 species and 8 subspecies (
A table was made with the character states used in the diagnoses (Table 1). This table summarizes the distribution of character states among the subgenera. It can be observed that each subgenus can be defined by a particular combination of these characters, stated in each diagnosis. For the characters here named 1-3, different species of Filotarsus present both the states found for each character, which are constant and well defined in all the species of the other subgenera, suggesting that in Filotarsus there are at least two groups of species which will be elucidated further by examining all the species of the subgenus and conducting a cladistic analysis of the group.
Characters studied and distribution of character states among the subgenera of Praocis.
| Characters | Character states | Subgenera | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Praocis s. str. | Mesopraocis | Postpraocis | Anthrasomus | Filotarsus | Hemipraocis | Praonoda | Orthogonoderes | Praocida | ||
| 1) Clypeus, anterior margin | A) extending beyond to lateral expansion of frons | × | × | × | × | × | × | × | × | × |
| B) at same level as lateral expansion of frons | × | |||||||||
| 2) Clypeus, width of anterior margin | A) not exceeding half the interocular width | × | × | × | × | × | × | × | × | × |
| B) same width as interocular distance | × | |||||||||
| 3) Clypeal suture | A) as horizontal groove | × | × | × | × | × | × | |||
| B) as vertical groove | × | × | × | × | ||||||
| 4) Antennomere 9 | A) longer than antennomere 10 | × | × | × | × | × | × | × | × | |
| B) equal in length to antennomere 10 | × | |||||||||
| 5) Antennomere 11 | A) Longer than antennomere 10 | × | × | × | × | × | × | × | ||
| B) shorter than antennomere 10 | × | |||||||||
| C) equal in length to antennomere 10 | × | |||||||||
| 6) Apical tomentose sensory patches on antennomere 10 | A) in two areas subequal in size | × | × | × | × | × | × | × | ||
| B) in a dorsally continuous semicircle | × | × | ||||||||
| 7) Apical tomentose sensory patches on antennomere 11 | A) on distal third | × | × | × | ||||||
| B) on distal half | × | × | × | × | × | × | ||||
| 8) Prosternum | A) with a narrow edge on anterior margin | × | × | × | × | × | ||||
| B) without edge on anterior margin | × | × | × | × | ||||||
| 9) Lateral margin of elytron | A) well defined | × | × | × | × | × | × | |||
| B) not defined | × | × | × | |||||||
| 10) Ventral surface of profemora | A) with a row of setae on anterior edge | × | × | |||||||
| B) without a row of setae on anterior edge | × | × | × | × | × | × | × | |||
| 11) Protibiae | A) explanate | × | × | × | × | × | × | × | ||
| B) not explanate | × | × | ||||||||
Some character states appear as unique for some subgenera such as antennomere 11 of equal length to 10 in Mesopraocis and antennomere 11 shorter than antennomere 10 in Orthogonoderes. One third of the characters analysed here are from the antennae, suggesting the importance of studying the length and proportion of antennomeres 9, 10 and 11 and the arrangement of the apical tomentose sensory patches on antennomeres 9, 10 and 11. Using these character states, we presented a preliminary identification key for the subgenera of Praocis.
The distribution of the whole genus Praocis is related to the arrangement of the Andes mountain range in southern South America. The Andes are the only high mountain chain in the continent, running along the Pacific coast of South America from Venezuela down South to Tierra del Fuego, extending over 8500 km and separating xeric habitats both eastward and westward (
We gratefully acknowledge to the organizators of the Third International Symposium on Tenebrionoidea 2013 (Arizona, USA) for the invitation to write this paper presented at the meeting; to the curators for the loan of material: Margaret Thayer, James Boone (FMNH), Sergio Roig-Juñent (IADIZA), Jorge Cepeda-Pizarro (LEULS), Arturo Roig-Alsina (MACN), Analía A. Lanteri, Nora Cabrera (MLPA), Claude Girard, Antoine Mantilleri (MNHN), Manfred Uhlig, Bernd Jaeger (MNHUB), Mario Elgueta D. (MNNC) and Eva Sprecher (NHMB); Margaret Thayer and Alfred Newton for their hospitality during the visit of GEF to FMNH; Nelly Horak for correction of the English language; Mariana Chani Posse and Aaron Smith for suggestions improving the manuscript; Remedios Marin for help with the artwork; two anonymous reviewers for suggestions for improving this paper and Patrice Bouchard (Editor) for his advise on nomenclatural questions. This study was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina), by a grant PIP 112-201101-00987 (CONICET, Argentina) (GEF) and DIULS-PR13121-VACDDI001 from DIULS (Universidad de La Serena) (JPA).