Citation: Golovatch SI, Geoffroy J-J, VandenSpiegel D (2014) Review of the millipede family Trichopolydesmidae in the Oriental realm (Diplopoda, Polydesmida), with descriptions of new genera and species. ZooKeys 414: 19–65. doi: 10.3897/zookeys.414.7671
In the Oriental Region, the large, basically Northern Hemisphere family Trichopolydesmidae is shown to currently comprise 18 genera and 43 species. Based mainly on gonopod structure, all of them, as well as the whole family, are (re)diagnosed, including five new genera and seven new species. These new taxa are keyed, also being the first to be described from Indochina in general and from Vietnam in particular: Aporodesmella gen. n., with three species: A. securiformis sp. n. (the type species), A. similis sp. n. and A. tergalis sp. n., as well as the following four monotypic genera: Deharvengius gen. n., with D. bedosae sp. n., Gonatodesmus gen. n., with G. communicans sp. n., Helicodesmus gen. n., with H. anichkini sp. n., and Monstrodesmus gen. n., with M. flagellifer sp. n. In addition, Cocacolaria hauseri Hoffman, 1987, hitherto known only from New Ireland Island, Papua New Guinea, is redescribed based on material from Vanuatu whence it is recorded for the first time. One of the new genera, Gonatodesmus gen. n., provides a kind of transition or evolutionary bridge between Trichopolydesmidae and Opisotretidae, thus reinforcing the assignment of these two families to the single superfamily Trichopolydesmoidea.
Diplopoda, Trichopolydesmoidea, taxonomy, new genera, new species, key, Vietnam, Vanuatu
The large, chiefly Northern Hemisphere millipede family Trichopolydesmidae has recently been rediagnosed and, together with the much smaller, Oriental and Papuan family Opisotretidae (
Because the present contribution focuses on the fauna of the Oriental realm alone, by default all tropical and subtropical Asian, plus the few known Papuan trichopolydesmids have hitherto been treated as fuhrmannodesmids. However, following the recent synonymization of Fuhrmannodesmidae and several other, smaller, Holarctic, Nearctic or Palaearctic families with Trichopolydesmidae (
Naturally, similar general trends can be surmised to have occurred in the “fuhrmannodesmids” of the Afrotropical and, especially, Oriental realms, which also support fairly diverse faunas of this family. Prompted by the need to identify and allocate several species from Vietnam and Vanuatu, the present paper is an attempt to review all Oriental Trichopolydesmidae, i.e. 36 species or subspecies from 13 genera, arranged in alphabetic order:
1. Assamodesmus lindbergi Manfredi, 1955, the type species of Assamodesmus Manfredi, 1954, by original designation, described from the Himalayas of Assam, northeastern India (
2. Coonoorophilus monstruosus Carl, 1932, the type species of Coonoorophilus Carl, 1932, by monotypy, described from a single gynandromorph (abnormal, with only one gonopod retained) from southern India (
3. Hingstonia beatae Golovatch, 1990, described from Nepal, Himalayas (
4. Hingstonia dorjulana Golovatch, 1988, described from Bhutan, Himalayas (
5. Hingstonia eremita Carl, 1935, the type species of Hingstonia Carl, 1935, by monotypy, described from Nepal, Himalaya (
6. Hingstonia falcata Golovatch, 1986, described from Nepal, Himalaya (
7. Hingstonia fittkaui Golovatch, 1990, described from Nepal, Himalaya (
8. Hingstonia gogonana Golovatch, 1988, described from Bhutan, Himalayas (
9. Hingstonia pahakholana Golovatch, 1990, described from Nepal, Himalayas (
10. Hingstonia pelelana Golovatch, 1988, described from Bhutan, Himalaya (
11. Hingstonia perarmata Golovatch, 1986, described from Nepal, Himalaya (
12. Hingstonia serrata Golovatch, 1987, described from Nepal, Himalayas (
13. Hingstonia sympatrica Golovatch, 1990, described from Nepal, Himalayas (
14. Hingstonia variata Golovatch, 1987, described from Nepal, Himalayas (
15. Hingstonia yeti Golovatch, 1988, described from Bhutan, Himalaya (
16. Kukkalodesmus exiguus Carl, 1932, the type species of Kukkalodesmus Carl, 1932, by monotypy, described from southern India (
17. Magidesmus affinis Golovatch, 1988, described from Bhutan, Himalayas (
18. Magidesmus bhutanensis Golovatch, 1988, the type species of Magidesmus Golovatch, 1988, by original designation, described from Bhutan, Himalayas (
19. Mastodesmus zehntneri Carl, 1911, the type species of Mastodesmus Carl, 1911, by monotypy, described from Java, Indonesia (
20. Nasodesmus cognatus (Humbert, 1865), originally described as Polydesmus cognatus Humbert, 1865, from Sri Lanka (
21. Ootacadesmus humilis Carl, 1932, the type species of Ootacadesmus Carl, 1932, by original designation, described from southern India (
22. Peronorchus parvicollis Attems, 1907, the type species of Peronorchus Attems, 1907, by monotypy, described from Buitenzorg (= Bogor), Java, Indonesia (
23. Pseudosphaeroparia cardamoni Carl, 1932, described from southern India (
24. Pseudosphaeroparia cavernicola Turk, 1945, described from a series of ♀ or juvenile syntypes from a cave near Dehra Dun, Uttar Pradesh, Himalayas of India (
25. Pseudosphaeroparia nilgirensis Carl, 1932, described from southern India (
26. Pseudosphaeroparia palnensis Carl, 1932, the type species of Pseudosphaeroparia Carl, 1932, by original designation, described from southern India (
27. Pseudosphaeroparia palnensis soror Carl, 1932, described from southern India (
28. Sholaphilus albidus Carl, 1932, the type species of Sholaphilus Carl, 1932, by monotypy (not by original designation, as mistakenly stated by
29. Sholaphilus asceticus Golovatch, 1986, described from Nepal, Himalayas (
30. Sholaphilus dalai Golovatch, 1986, described from Nepal, Himalayas (
31. Sholaphilus gompa Golovatch, 1990, described from Nepal, Himalayas (
32. Sholaphilus lama Golovatch, 1986, described from Nepal, Himalayas (
33. Sholaphilus martensi Golovatch, 1986, described from Nepal, Himalayas (
34. Sholaphilus monachus Golovatch, 1990, described from Nepal, Himalayas (
35. Topalodesmus communis Golovatch, 1988, the type species of Topalodesmus Golovatch, 1988, by original designation, described from the Himalayas of Darjeeling District, northern India (
We can also add here one more genus and species from the region concerned:
36. Cocacolaria hauseri Hoffman, 1987, the type species of Cocacolaria Hoffman, 1987, by original designation, described from New Ireland, Papua New Guinea by
The following taxa must be excluded from Trichopolydesmidae or remain unclassified:
1. Glenniea Turk, 1945, with six species from the Himalayas of India, Nepal and Bhutan (
2. Typhlopygmaeosoma hazeltonae Turk, 1972, the type species of Typhlopygmaeosoma Turk, 1972, described from a cave near Shimla (formerly Simla), Himachal Pradesh, northern India (
In addition, closer unidentified “Fuhrmannodesmidae”, provisionally referred to as Gen. sp. 1, Gen. sp. 2 and Gen. sp. 3, have been recorded in the Cat Tien National Park, southern Vietnam (
As one can see from the above list, only one species, Pseudosphaeroparia cavernicola, is known too poorly (
The main objective of the present paper is to address the generic classification of Trichopolydesmidae in the Oriental Region in order to identify and name a number of fresh samples from Vietnam and Melanesia.
Abbreviations used:
MNHN Muséum national d’Histoire naturelle, Paris, France
SEM Scanning electron microscopy
ZMUC Natural History Museum of Denmark, Copenhagen, Denmark
ZMUM Zoological Museum, State University of Moscow, Moscow, Russia
Most of the material treated below was taken by Louis Deharveng, Anne Bedos (MNHN) and/or their collaborators in Vietnam. Several samples derive from ZMUM, collected by Alexander Anichkin (Joint Russia-Vietnam Tropical Center, Ho Chi Minh City, Vietnam) in a national park in southern Vietnam.
SEM micrographs were taken using a JEOL JSM-6480LV scanning electron microscope. After examination, SEM material was removed from stubs and returned to alcohol, all such samples being kept in MNHN.
The course of the seminal groove was always checked and, if necessary, depicted using transmission light microscopy.
The following characters have been used for defining genera in Oriental Trichopolydesmidae (= “Fuhrmannodesmidae”). Note that there is nothing particular in the peripheral, non-gonopod features that would uniquely characterize the Trichopolydesmidae (
- Number of body segments
Like in most other families in Polydesmida, the number is 18, 19 or 20, largely being sex-characteristic. Thus, 20 segments in both sexes are known in most of the Oriental Trichopolydesmidae, 19 in both sexes only in Assamodesmus, Peronorchus, Aporodesmella gen. n., Gonatodesmus gen. n. and Helicodesmus gen. n., 19 in the ♂, but 20 in the ♀ in Cocacolaria, at least 19 in the ♂ of Coonoorophilus and Ootacadesmus. Deharvengius gen. n. is the sole genus in Trichopolydesmoidea which has only 18 segments in both sexes.
- Metatergal setae
All known species of Trichopolydesmidae show 3 transverse rows of tergal setae. The setation pattern is typical of the Polydesmidea, being 3+3 or more setae per row, only exceptionally 2+2 (Deharvengius gen. n.), plus 2 or 3 setae at each lateral margin. However, the setae themselves are typically ribbed, often helicoid, varying from long and sharp, like in Cocacolaria, Mastodesmus or Deharvengius gen. n., through short and simple, like in Nasodesmus or Ootacadesmus, to long and bacilliform, like in Assamodesmus, Deharvengius gen. n., Helicodesmus gen. n. or Gonatodesmus gen. n., or short and clavate, like in Magidesmus, Pseudosphaeroparia, Sholaphilus or some species of Aporodesmella gen. n. At least the bacilli- and claviform setae seem to always be modified, ribbed all along (e.g. Figs 11L, 13L, M, 16N, O). However, similar setae occur in some other families of Polydesmida such as Opisotretidae or Paradoxosomatidae (e.g.
- Metatergal sculpture
The pattern of metatergal sculpturing is typical of the Polydesmidea, i.e. 3 transverse rows of polygonal bosses or rounded tubercles, either with a more or less deep sulcus separating the first row from the two following ones or with clear sulci between all 3 rows. Each boss or tubercle is typically surmounted by a seta sometimes borne on a small knob. Among the Oriental Trichopolydesmidae, only very few species show truly distinct bosses or tubercles, e.g. Cocacolaria or Mastodesmus, whereas in the vast majority of cases the bosses are either flat or missing, and the transverse sulcus or sulci are largely superficial to wanting.
- Location and size of ozopores
Unlike species of Opisotretidae (
- Shape and size of paraterga
Variation in the degree of development of paraterga is considerable, ranging from fully wanting (some species of Aporodesmella gen. n.), through very poorly developed (nearly missing in Cocacolaria, Peronorchus or Mastodesmus) to evident or strong (Nasodesmus, Magidesmus, Deharvengius gen. n. or certain species of Aporodesmella gen. n.), usually varying between species in speciose genera. As in the other Polydesmida, paraterga tend to be slightly underdeveloped and set lower in ♀♀ and juveniles compared to ♂♂.
- Antennae
Normally, the antennae in Trichopolydesmoidea tend to be geniculate between antennomeres 5 and 6, these segments also usually bearing conspicuous apicodorsal groups of bacilliform sensilla. However, in a few Oriental Trichopolydesmidae, e.g. Cocacolaria or Mastodesmus, this geniculation is either feeble or totally wanting. Only exceptionally is ♂ antennomere 6 supplied with a conspicuous dorsoparabasal stump (some species of Aporodesmella gen. n.).
- ♂ head modifications
The ♂ vertex in Oriental Trichopolydesmidae, unlike that in some species from the Neotropical and Afrotropical realms (e.g.
- Legs
Variation in leg length and armament in Oriental Trichopolydesmidae is quite pronounced, ranging from rather short and stout, sometimes also supplied with special ventral trichomes in the ♂, e.g. in some Hingstonia, to long and slender, e.g. in most of Hingstonia species, but the bulk of species show medium-sized, moderately to considerably stout legs which are usually devoid of modified trichomes in the ♂ and thus fail to differ much between the sexes. This contradicts
- Gonopod structure
In the systematics of any subgroup of the order Polydesmida, the gonopods offer most of the characters deemed useful, if not crucial, for the discrimination of genera and species. This fully applies to Trichopolydesmidae as well.
The gonopods in Trichopolydesmidae appear to vary much more strongly than in the Opisotretidae, the sole other family of Trichopolydesmoidea, in agreement with the fact that Trichopolydesmidae is much larger and is widespread throughout the Northern Hemisphere. Moreover, a gonopod-based diagnosis of Trichopolydesmidae is still not entirely satisfactory (
Such a definition is basically correct, although a few Neotropical “fuhrmannodesmids” show no solenomere whatsoever (
Amongst the Oriental Trichopolydesmidae, like in the Neotropical ones (
Only the gonopods of Gonatodesmus gen. n. stand apart in showing a kind of transition to Opisotretidae. Indeed, the gonocoel is small, the telopodites are strongly exposed and well separated, their basal parts are held parallel to the main axis, whereas their distal parts, following a clear midway geniculation, are directed abruptly laterad. Moreover, this genus demonstrates a strongly developed, hairy pulvillus and a small accessory seminal chamber so characteristic of most species of Opisotretidae (and Polydesmidae).
- Vulva
No special studies have been conducted on the conformation of the vulva in “Fuhrmannodesmidae”.
Based on the above information, as well as facing the need to properly allocate several new genera and species described below, we propose the following new diagnosis of Trichopolydesmidae and a new classification of the family’s constituent Oriental genera.
A family of the superfamily Trichopolydesmoidea, suborder Polydesmidea with 18, 19 or 20 segments, sometimes varying between sexes. Body very small to small (ca 2–20 mm long). Tegument microalveolate, limbus mostly microspiculate. ♂ head with or without vertigial modifications. Antennae often geniculate between segments 5 and 6, antennomeres 5 and 6 each usually with a compact group of bacilliform sensilla apicodorsally, rarely 6th in ♂ with a dorsoparabasal stump. Metaterga usually with 3 regular, more rarely with more and/or irregular, transverse rows of sharp (= simple), bacilliform or clavate setae sometimes borne on knobs; side margin of paraterga incised or tuberculate, with 2 or 3 setae. Paraterga from absent to strongly developed, usually only slightly indented laterally, only exceptionally deeply trilobate (Trilobodesmus Golovatch & Mauriès, 2007). Pore formula usually normal: 5, 7, 9, 10, 12, 13, 15–17 (18, 19), but sometimes ozopores completely wanting, rarely unusually large, normally opening flush on dorsal or dorsolateral surface either near penultimate lateral incision or at caudal corner of paraterga. Legs rather short to long, ♂ ones often stouter and elongate, rarely modified, inflated, only sometimes with peculiar ventral setae, including sphaerotrichomes.
Gonopods with subglobose, medially fused coxae, these being rather small to quite large (with correspondingly large gonocoel), micropapillate and at most only slightly setose laterally, sometimes with a frontal (= anterior) process, each supporting a cannula medially (exception: Caucasodesmus Golovatch, 1987, in which there is no cannula) and a sack-shaped to elongate telopodite. The latter ranging from stout and short, deeply sunken inside a deep gonocoel, to slender and long, nearly fully exposed, strongly to modestly curved caudad or mesad, only exceptionally geniculate (distal half directed abruptly laterad: Gonatodesmus gen. n.) or perforating coxal wall with a prominent process (Schizotelopus Verhoeff, 1941), usually complex, with various processes or outgrowths, sometimes fringed; seminal groove normally terminating distally or apically on a separate branch or tooth (= solenomere), rarely on a tooth inside gonocoel, exceptionally absent (Caucasodesmus). Typically neither an accessory seminal chamber nor a hairy pulvillus (a few exceptions, e.g. Gonatodesmus gen. n.).
Trichopolydesmus Verhoeff, 1910.
Most of the above somatic and gonopod features of Trichopolydesmidae are in no way unique to the family, sometimes being also encountered, in various combinations, in the micropolydesmoid family Opisotretidae of the same superfamily Trichopolydesmoidea, as well as in certain macropolydesmoid members of the family Polydesmidae, superfamily Polydesmoidea (
The following 13 nominate Oriental genera of Trichopolydesmidae seem to be valid, and are arranged and defined below in alphabetic order.
19 segments (♂, ♀), pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga modest, 3 rows of bacilliform metatergal setae; gonopod telopodites subfalcate and clearly twisted, held parallel to main body axis, strongly exposed (gonocoel small); solenomere (sl) a long, simple, distal spine with an adjacent, distal, bipartite solenophore (sph) consisting of an apical spine and a massive velum.
Assamodesmus lindbergi Manfredi, 1955, by original designation.
This well-defined Himalayan genus remains monobasic.
19 (♂) or 20 (♀) segments, pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga poorly developed, 3 rows of sharp long metatergal setae, metatergal sculpture (bosses) unusually high; gonopod telopodites subfalcate, crossing mesally, very strongly exposed (gonocoel small); sl a small branchlet at midway along telopodite; sph distal to sl unipartite, large, bifid and rather simple.
Cocacolaria hauseri Hoffman, 1987, by original designation.
This genus remains monobasic, its sole constituent species being quite widespread in Melanesia. Cocacolaria hauseri is redescribed below, based on samples from Vanuatu.
19 segments (♂), ♀ unknown, much like Ootacadesmus (see below), but gonopods strongly resembling those of Sholaphilus.
Coonoorophilus monstruosus Carl, 1932, by monotypy.
This monobasic genus is retained only provisionally, because it combines the basic characters of Ootacadesmus, Kukkalodesmus, Pseudosphaeroparia and Sholaphilus. The gynandromorph ♂ holotype of Coonoorophilus monstruosus retained only a single gonopod.
20 segments (♂, ♀); pore formula normal, ozopores lying opposite 3rd incision, placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga keel-shaped, rather well developed, 3 rows of short, bacilliform to clavate setae on knobs; ♂ legs nearly normal, often with sphaerotrichomes; gonopod coxae from modest to large (gonocoel also from modest to deep), telopodites strongly exposed, usually massive, never fringed; sph(distal part of telopodite) complex, at least with one process or outgrowth, usually more; sl a rather long, simple, apical or subapical branch.
Hingstonia eremita Carl, 1935, by monotypy.
This relatively species-rich, well-defined, Himalayan genus mostly contains relatively large species (up to 20 mm long).
20 segments (♂, ♀); pore formula normal, ozopores lying opposite 2nd incision, placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga keel-shaped, rather well developed, 3 rows of short, bacilliform to clavate metatergal setae on knobs; ♂ femur 3 strongly enlarged; gonopod coxae not very large (gonocoel modest), telopodites strongly exposed; sph distal, complex, with 2 caudal lobes and a frontal process, sl a small, plumose, subapical branchlet.
Kukkalodesmus exiguus Carl, 1932, by monotypy.
This monotypic genus seems to be rather poorly defined versus Sholaphilus, Pseudosphaeroparia, Coonoorophilus and Ootacadesmus, especially given the strong variation in gonopod structure in the relatively species-rich genera Sholaphilus and Pseudosphaeroparia (see also above and below).
20 segments (♂), ♀ unknown; pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga well developed and set quite high, 3 rows of short clavate metatergal setae; gonopod coxae very large (gonocoel deep), telopodites deeply sunken and barely exposed, sac-shaped, hollow in the middle, apically somewhat fringed; a sph missing, sl a small denticle at bottom of this hollow.
Magidesmus bhutanensis Golovatch, 1988, by original designation.
This small Himalayan genus is well-defined.
20 segments (♂, ♀); pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga almost missing and set low, 3 rows of long, curved, simple metatergal setae on unusually prominent tubercles; gonopod coxae modest (gonocoel not very deep), telopodites bipartite in distal half, well exposed, very simple; sph a simple exomere branch, sl a long, simple, caudal spine branching off from distal half of telopodite.
Mastodesmus zehntneri Carl, 1911, by monotypy.
This monobasic genus strongly resembles Peronorchus and Nasodesmus, but seems to be sufficiently well defined.
20 segments (♂, ♀); pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga well developed, 3 rows of short simple metatergal setae; ♂ legs 3 strongly elongate, the femur very strongly enlarged; gonopod coxae not very large (gonocoel not too deep); telopodites strongly exposed, simple, unipartite, slightly curved mesad; sph (exomere) simple, strong, with a small process at base; sl a small, subapical, mesal branchlet.
Polydesmus cognatus Humbert, 1865, by original designation.
This monobasic genus strongly resembles Peronorchus and Mastodesmus, but seems to be sufficiently well defined.
19 segments (♂), ♀ unknown; pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga well developed, 3 rows of very short and simple metatergal setae; ♂ legs 3 clearly elongate, the femur very strongly enlarged; gonopod coxae very large (gonocoel deep); telopodites deeply sunken and only very little exposed, with a parabasal process; sph bipartite, with various apical outgrowths, including a lateral velum, sl a small subapical branchlet.
Ootacadesmus humilis Carl, 1932, by original designation.
This monotypic genus seems to be rather poorly defined versus Sholaphilus, Pseudosphaeroparia, Coonoorophilus and Kukkalodesmus (see also above and below).
19 segments (♂, ♀); pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified; paraterga very small, body nearly cylindrical, 3 rows of 3+3 or 4+4, rather long bacilliform metatergal setae (regardless of lateral setae on paraterga); gonopod coxae with gonocoel not deep; telopodites clearly exposed, without parabasal processes, moderately curved and held parallel to each other; sph bifid distally, with sl a rather long, flagelliform, apical branch lying in sph fork.
Peronorchus parvicollis Attems, 1907, by monotypy.
This genus strongly resembles Nasodesmus and Mastodesmus, but seems to be sufficiently well defined.
20 segments (♂, ♀); pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga modest to well developed, 3 rows of short clavate metatergal setae; gonopod coxae very large (gonocoel deep) to modest; telopodites from rather deeply sunken to rather clearly exposed, with or without parabasal processes; sph unipartite, with various outgrowths, sl a rather evident apical branch.
Pseudosphaeroparia palnensis Carl, 1932, by original designation.
This small genus is quite poorly defined versus Sholaphilus, Coonoorophilus, Ootacadesmus and Kukkalodesmus (see also above and below).
20 segments (♂, ♀); pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga modest to well developed, 3 rows of short clavate metatergal setae; gonopod coxae very large (gonocoel deep) to modest, sometimes with a strong frontal process; telopodites rather deeply sunken and usually only little exposed, but sometimes rather strongly exposed, with or without parabasal processes; sph uni- to only faintly bipartite, with various outgrowths, densely fringed, sl a small apical or subapical tooth or branchlet.
Sholaphilus albidus Carl, 1932, by monotypy.
Even though this rather small genus is quite poorly defined versus Ootacadesmus, Pseudosphaeroparia, Coonoorophilus and Kukkalodesmus (see above), several species of Sholaphilus from the Himalayas have been described.
20 segments (♂, ♀); pore formula normal, ozopores placed closer to lateral than to caudal margin of paratergite; ♂ vertex unmodified, paraterga modest, 3 rows of short simple/claviform metatergal setae; gonopod coxae massive, each with a prominent frontal process; gonocoel rather deep, but telopodites subfalcate and strongly exposed, tripartite, each with a strong, parabasal, unciform, caudomedial process, a short laterobasal stump-shaped exomere, and a prominent, elongate sph crowned with a small apical sl.
Topalodesmus communis Golovatch, 1988, by original designation.
This monobasic Himalayan genus is well-defined (see also below under Monstrodesmus gen. n.).
http://species-id.net/wiki/Cocacolaria_hauseri
Figs 1, 21 ♂, 7 ♀, 1 fragment (MNHN JC 354), Vanuatu, Espirito Santo Island, Natawa forest, small doline Arifos, 167.183167E, 15.2962S, sieved litter & Berlese extraction, 24.09.2006, leg. L. Deharveng & A. Bedos (SK06-24-24); 1 ♂ (SEM), same locality, date and collectors (SK06-24-22); 3 ♀ (MNHN JC 354), Espirito Santo Island, Boutmas, forest near entrance to Cave Fapon, 166.9648833E, 15.33101667S, 380 m a.s.l., litter, Berlese extraction, 08.09.2006, leg. L. Deharveng & C. Rahmadi (SK06-08-24).
Length of adults of both sexes 4–6 mm (♂, ♀), width of midbody pro- and metazonae ca 0.3 and 0.4 mm, respectively. Coloration in alcohol from uniformly pallid to light yellowish.
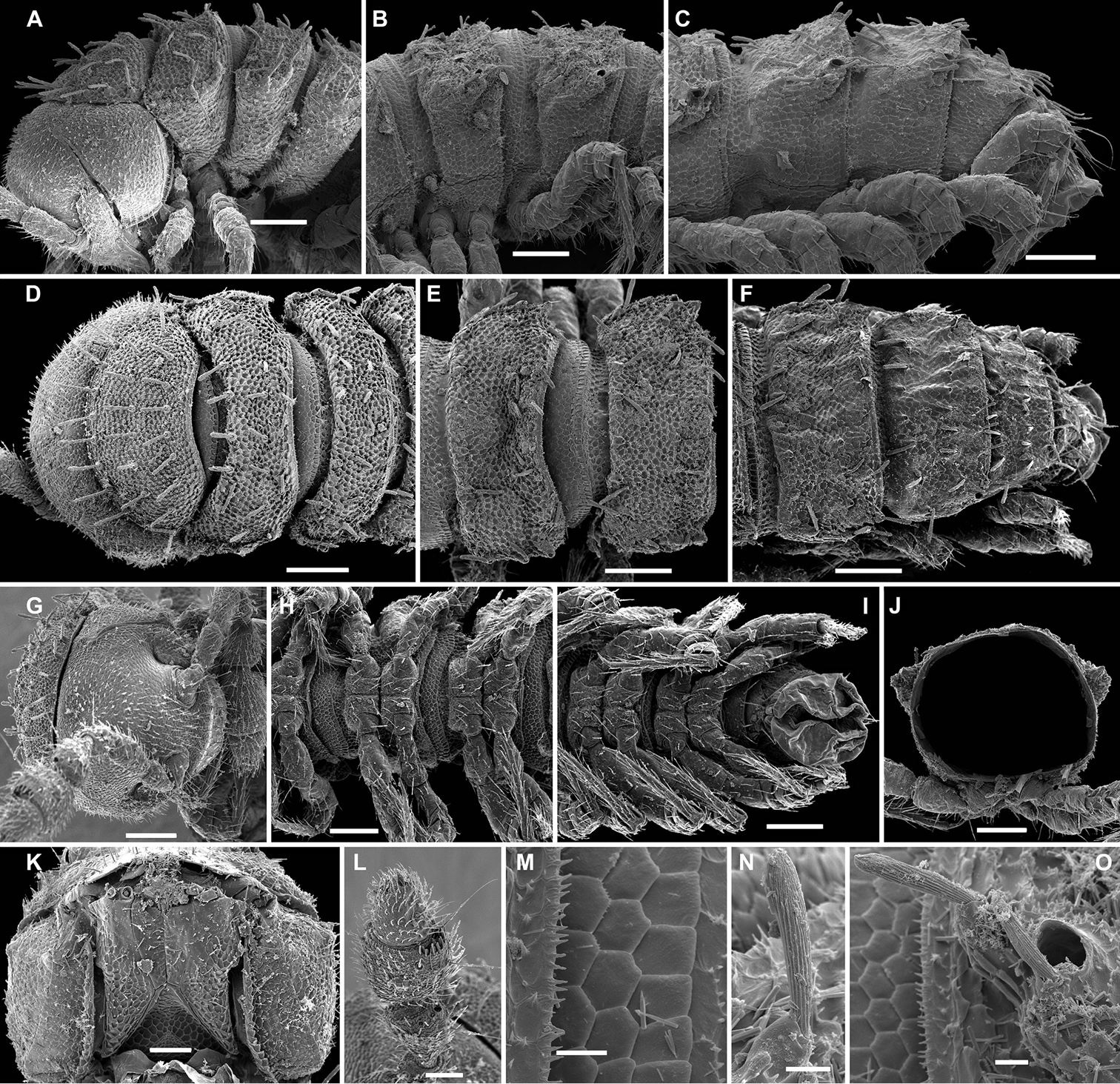
Body with 19 (♂) or 20 (♀) segments. Tegument mainly dull, at most slightly shining, texture very delicately alveolate. Head densely pilose throughout; epicranial suture superficial and thin (Fig. 1A); isthmus between antennae about 1.5 times as broad as diameter of antennal socket. Antennae short, reaching only behind (♂) or midway of collum (♀) when stretched dorsally, not geniculate, very clearly clavate, especially so due to largest antennomere 6, the latter with a small, but evident distodorsal group of bacilliform sensilla.
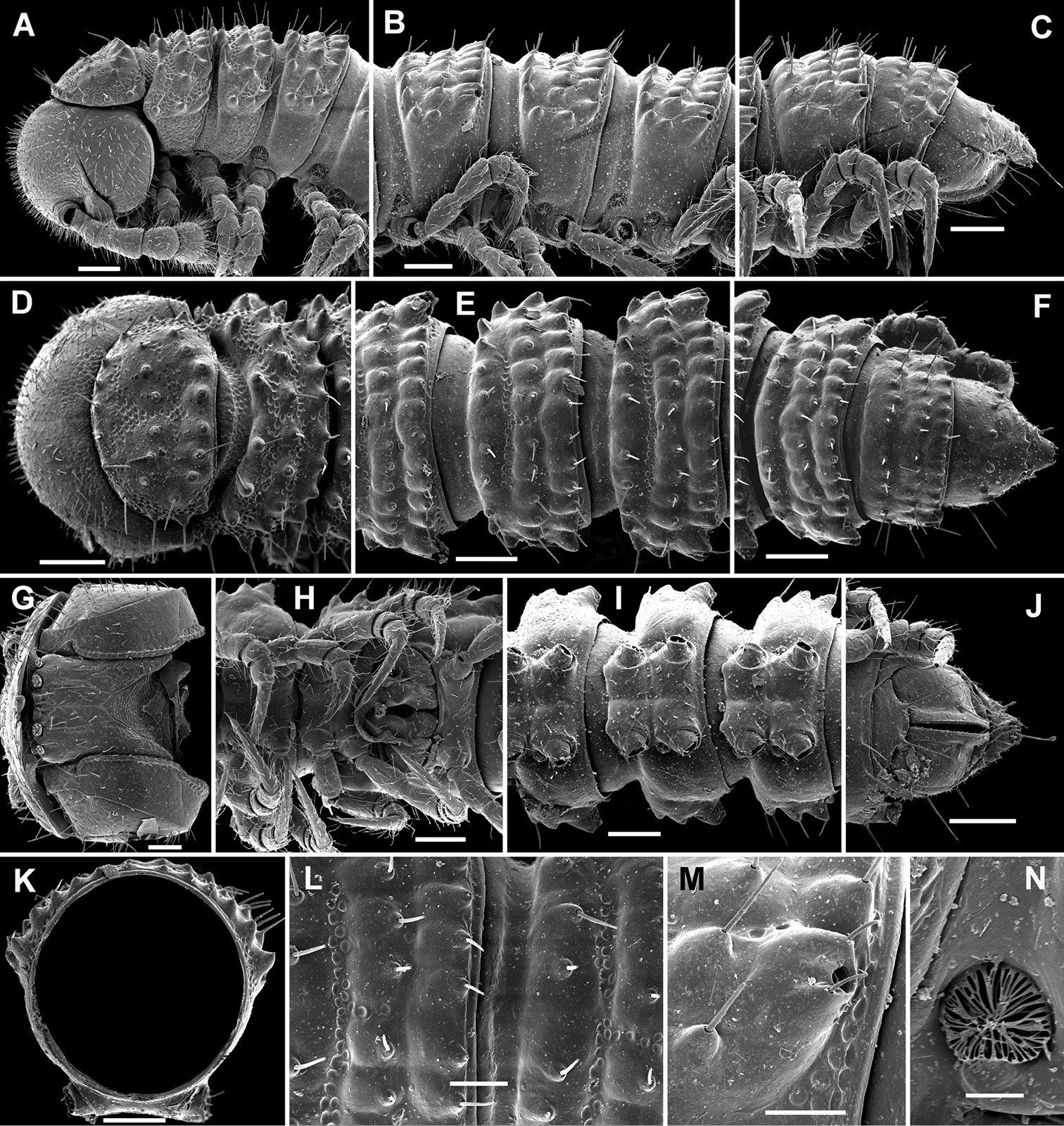
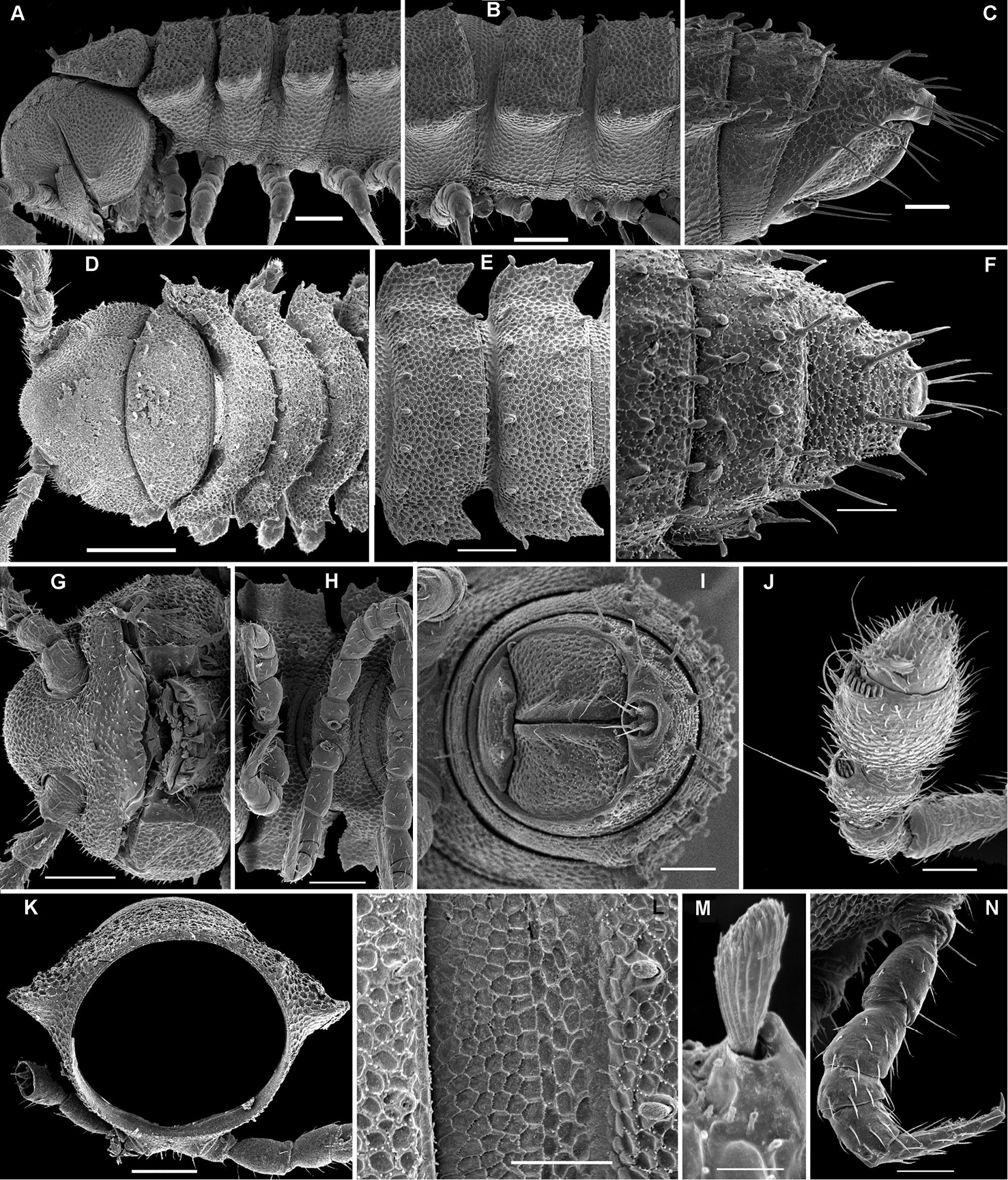
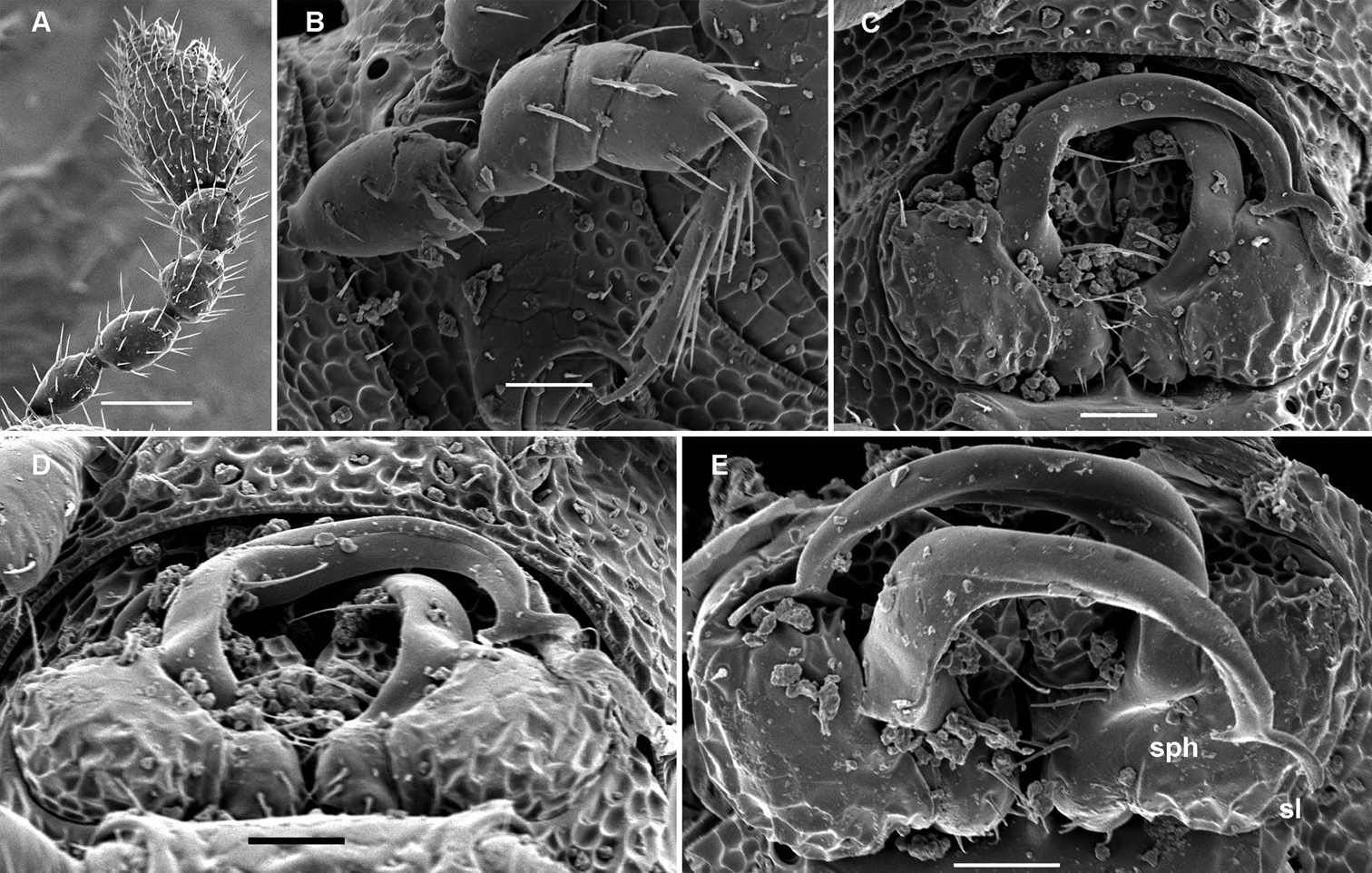
Cocacolaria hauseri Hoffman, 1987, ♂ from Natawa forest, Espirito Santo Island, Vanuatu; A, D anterior part of body, lateral and dorsal views, respectively B, E, I midbody segments, lateral, dorsal and ventral views, respectively C, F, J posterior part of body, lateral, dorsal and ventral views, respectively G head, ventral view H body segments 6 and 7, ventral view K cross-section of a midbody segment, caudal view L midbody tergal sculpture and setae, dorsal view M caudal part of a midbody pore-bearing paratergite, lateral view N midbody spiracle. Scale bars: A–F, H–K 0.1 mm; G, L, M 0.05 mm; N 0.02 mm.
Body moniliform, especially so in ♂ (Fig. 1D–F). In width, collum << head < segment 2 < 3 = 4 < 5(6) = 15 (♂, ♀), thereafter body gradually tapering towards telson. Paraterga very poorly developed, mainly represented by setigerous tubercles (Fig. 1A–F, H–K), starting from collum, set rather low (at about upper 1/3–1/2 of segment’s height), leaving a highly convex dorsum (Fig. 1A–C & K). Caudal corner of postcollum paraterga increasingly tuberculiform, mainly clearly rounded, not extending behind rear tergal margin. Pore formula normal; ozopores evident, round, lying dorsolaterally on top of caudolateral tubercle of paraterga very close to lateral margin, more remote from caudal margin (Fig. 1M). Collum subovoid, each following metatergum with 3 transverse rows of mostly long, pointed, very faintly ribbed, helicoid setae largely borne on 6+6 unusually high tubercles, these being especially prominent in last row (Fig. 1A–F). Stricture between pro- and metazonae deep, rather narrow and smooth. Limbus thin and entire, not microdenticulate. Pleurosternal carinae absent (Fig. 1A). Epiproct short, conical, directed caudoventrally; pre-apical papillae evident (Fig. 1C, F, J). Hypoproct nearly semi-circular (Fig. 1J), setiferous papillae at caudal corners very clear, stalk-shaped and well separated.
Sterna without modifications, broad, flat, poorly setose (Fig. 1H–J). Stigma openings flat, beset with dense and long filaments (Fig. 1N). Legs in ♀ and juveniles clearly shorter and slenderer, ca 1.0–1.1 times as long as body height, in ♂ somewhat incrassate and ca 1.3–1.4 times as long as body height (Figs 1B, 2A); tarsi longest; sphaerotrichomes missing (Fig. 2A). Each ♂ coxa 2 with a short, cylindrical, distoventral gonapophysis.
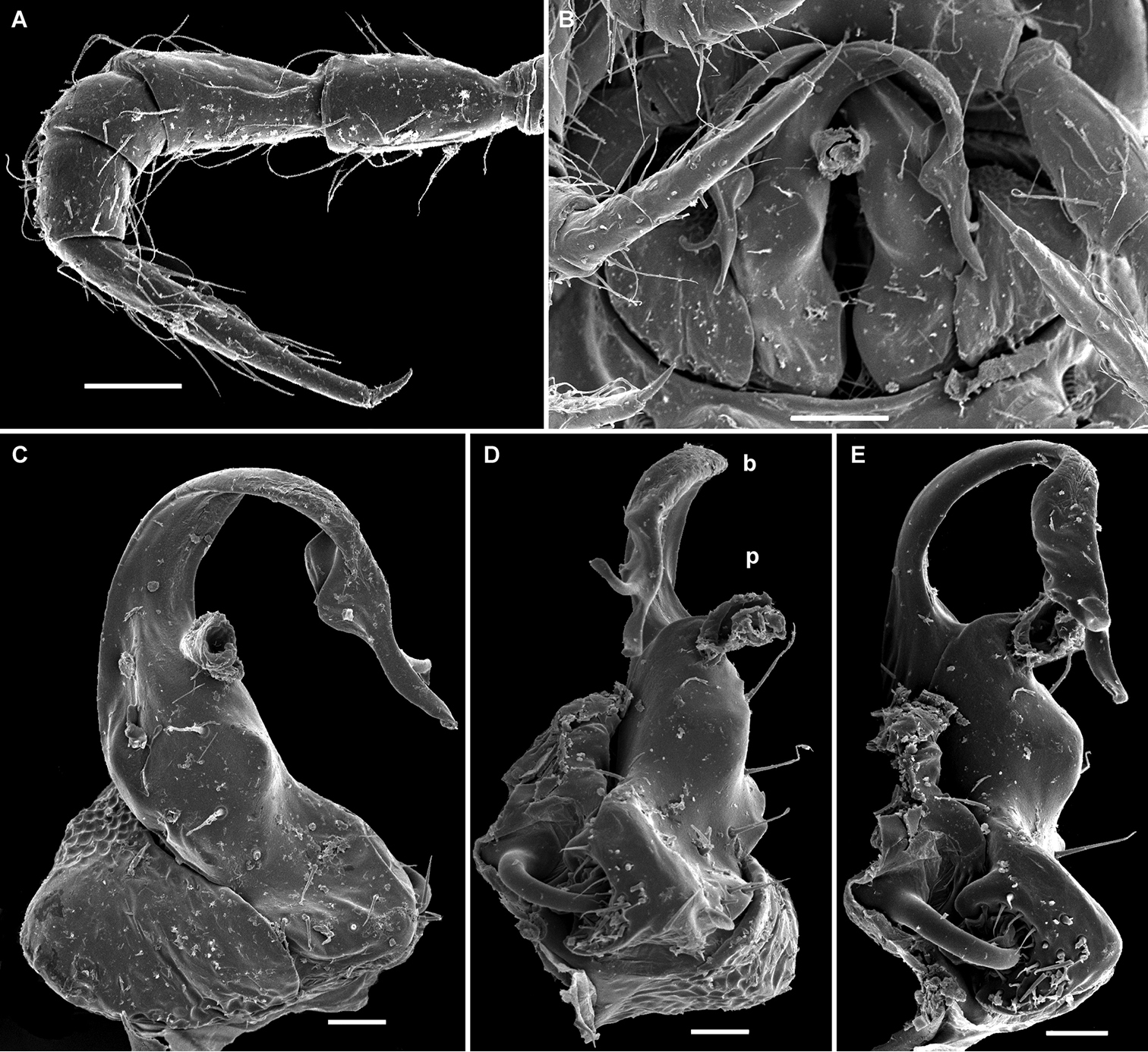
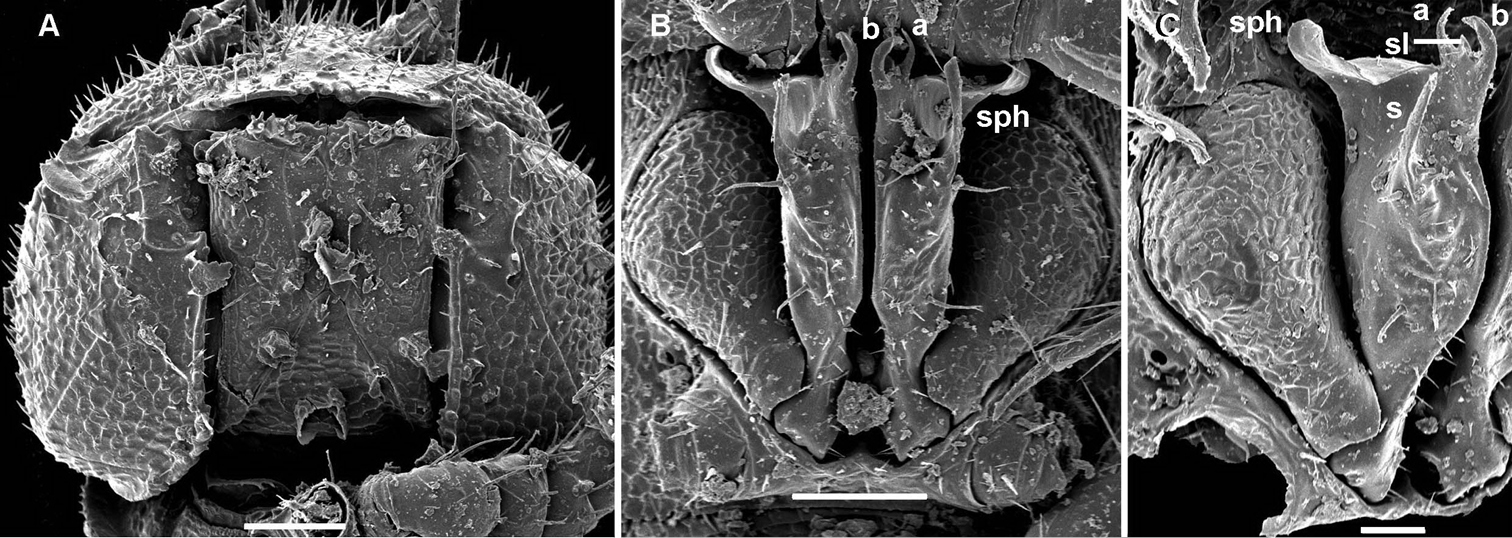
Cocacolaria hauseri Hoffman, 1987, ♂ from Natawa forest, Espirito Santo Island, Vanuatu; A midbody leg, lateral view B gonopod aperture with both gonopods in situ, ventral view C right gonopod, lateral view D, E left gonopod, caudomesal and mesal views, respectively. Scale bars: A, B 0.05 mm; C–E 0.02 mm. Designations of gonopod structures in text.
Gonopod aperture transversely oblong-oval, taking up most of ventral part of metazonite 7, its edges nowhere elevated (Figs 1H, 2B). Gonopods (Fig. 2B–E) with rather moderately large, globose, bare, ventrolaterally papillate, medially fused coxae carrying normal cannulae mesally. Telopodite strongly elongate and curved, subunciform, bipartite, directed mesad and crossing in situ, each at about midway with a short, mesal, calyciform process (p) (= solenomere) terminating a fully mesal seminal groove, with neither an accessory seminal chamber nor a hairy pulvillus. Main branch (b) (= solenophore) bifid and slightly expanded subterminally.
This species, originally described from a single ♂ taken in a cave in Lelet Plateau, New Ireland, Papua New Guinea (
The original description is quite detailed and nicely illustrated (
19 segments (♂, ♀); ozopores wanting; ♂ vertex modified or not; ♂ antennomere 6 sometimes with a conspicuous dorsoparabasal stump; paraterga absent to rather well developed, 3 rows of 3+3 or 4+4, short or long, bacilli- and/or claviform metatergal setae (regardless of lateral setae on or instead of paraterga); gonopod coxae with gonocoel not deep; telopodites clearly exposed, mostly or considerably represented by elongate prefemoral parts, at most only moderately curved, medially subcontiguous and held parallel to each other; acropodites, or solenophores (sph), lying distal to prefemoral parts shortened, only sometimes set off basally by a ventral sulcus, subtruncate, usually more or less coaxial with prefemoral parts, but sometimes extended laterad into a distinct process; solenomere (sl) subapical, rather long to vestigial.
To emphasize the lack of ozopores, feminine.
Aporodesmella securiformis sp. n., by present designation.
The new genus is unique among the Trichopolydesmoidea for its complete loss of ozopores. Among Oriental Trichopolydesmidae, Aporodesmella gen. n. seems to be especially similar to Peronorchus, sharing with it not only 19 body segments in both sexes, but also the gonopods demonstrating a modest gonocoel, elongate, clearly exposed and modestly curved telopodites which are (sub)contiguous medially and held parallel to each other (and to the main body axis), the solenophore being enlarged, more or less cup-shaped and membranous while the solenomere is a short subapical process or tooth. However, the prefemoral (= densely setose) part of the gonopod in Peronorchus is clearly shorter.
The following three new species are attributed to Aporodesmella gen. n.
http://zoobank.org/A08E5C4F-B6E8-493B-A832-6C383E1FC438
http://species-id.net/wiki/Aporodesmella_securiformis
Figs 3, 4Holotype ♂ (MNHN JC 355), Vietnam, Kien Giang Province, Kien Luong, Hon Chong, Nui Bai Voi, 104.618E, 10.218N, soil, Berlese extraction, 02.06.2008, leg. L. Deharveng & A. Bedos (Vn08-045).
Paratypes: 1 ♂, 4 ♀ (MNHN JC 355), 1 ♂, 1 ♀ (ZMUM ρ2346), 1 ♂ (SEM), same locality, together with holotype; 2 ♂, 1 ♀ (MNHN JC 355), 1 ♂ (SEM), same province, Kien Luong, Hon Chong, Nui Hang Tien, litter & soil, Berlese extraction, 02.06.2008, leg. Ly Ngoc Sam (Vn08-065); 3 ♂ (MNHN JC 355), 1 ♂ (SEM), same data (Vn08-066).
To emphasize the axe-shaped solenophore.
Differs from congeners except Aporodesmella similis sp. n. by the presence of a remarkable dorsoparabasal stump on ♂ antennomere 6, from Aporodesmella similis sp. n. and other congeners by a peculiar, unusually short, axe-shaped solenophore and a simple, lanceolate, shorter and stout solenomere.
Length of adults ca 2.8–2.9 mm (♂, ♀), up to 3.0 mm (♀), width of midbody pro- and metazonae 0.15 and 0.2 (♂, ♀), up to 0.2 and 0.25 mm (♀), respectively. Coloration in alcohol uniformly pallid, tegument translucent.
Body moniliform, with 19 segments (♂, ♀). Tegument mainly dull, at most slightly shining, texture very delicately alveolate and microgranulate. Head without modifications, densely pilose throughout except occiput; epicranial suture superficial and thin; genae squarish (Fig. 3A, D, G); gnathochilarium narrow, setae dense and short (Fig. 4A); isthmus between antennae about as broad as diameter of antennal socket (Fig. 3G). Antennae very short, reaching only behind collum (♂, ♀) when stretched dorsally, not geniculate, strongly clavate due to an abruptly and particularly enlarged antennomere 6, the latter with a usual, tight, distodorsal group of numerous bacilliform sensilla, but in ♂ also with a large, highly conspicuous, dorsoparabasal, rounded stump (s); antennomere 5 with a loose distodorsal group of only a few tiny sensilla, 7th with a tiny mid-dorsal knob (Figs 3G, J, 4A).
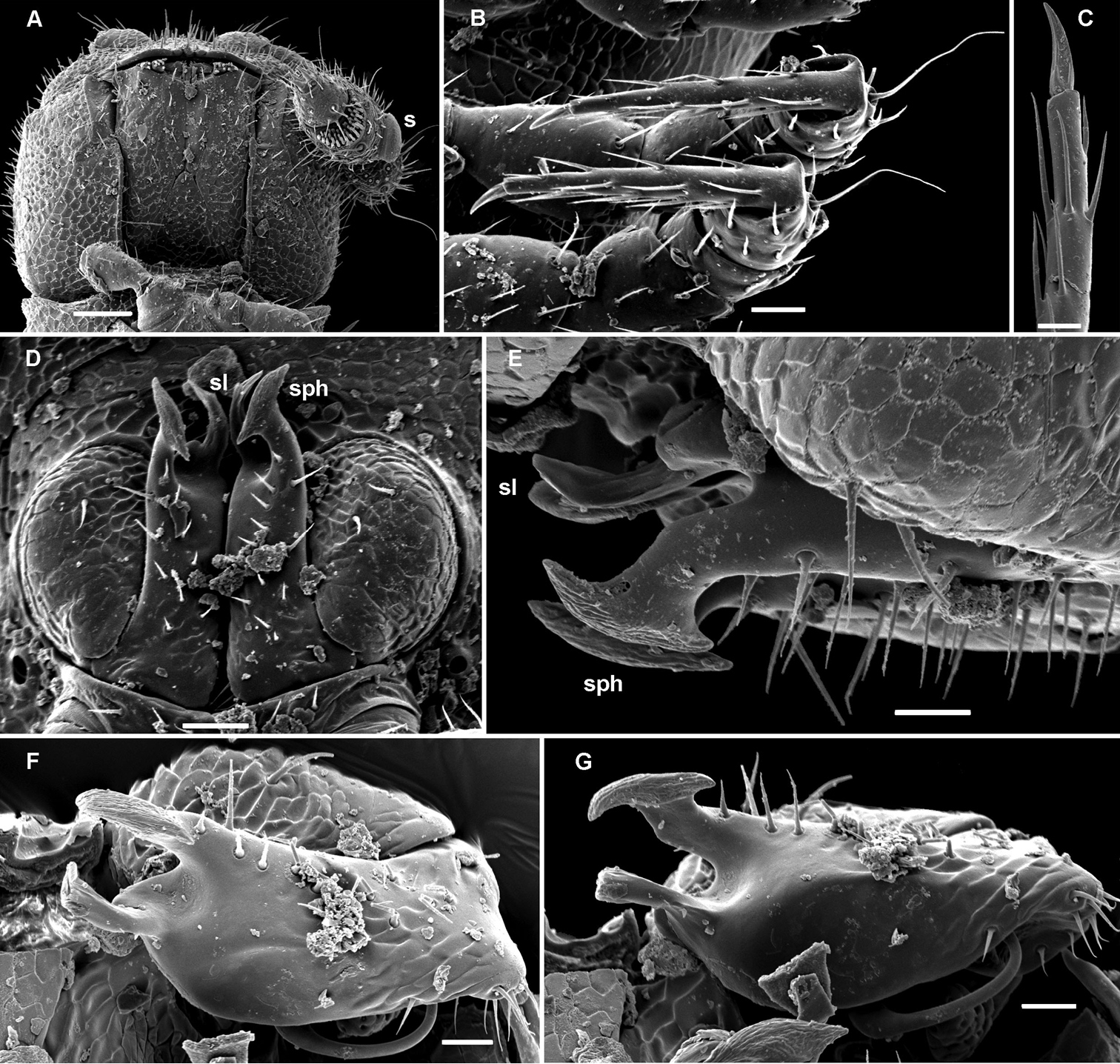
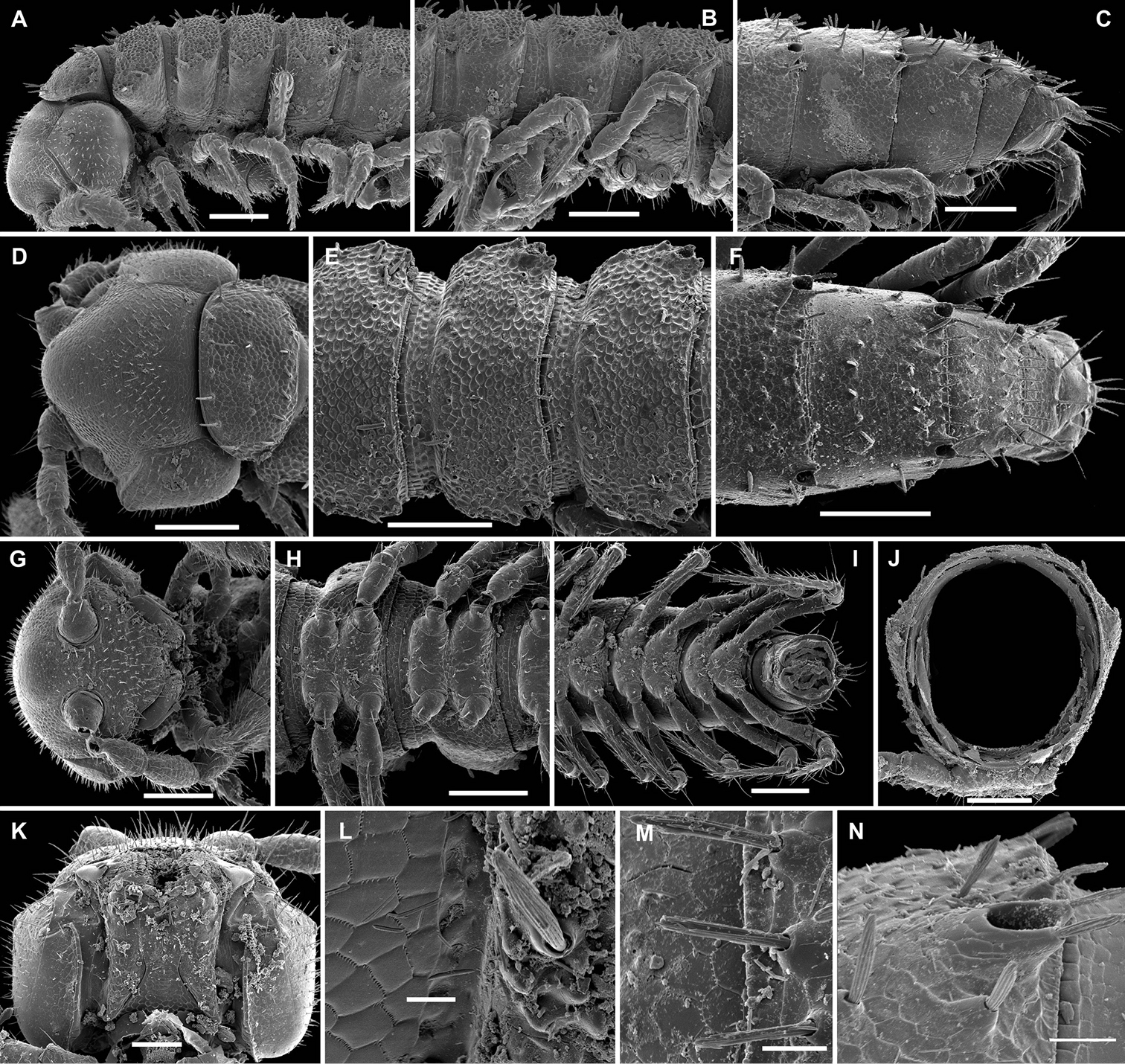
Aporodesmella securiformis sp. n., ♂ paratype from Nui Hang Tien; A, D, G anterior part of body, lateral, dorsal and ventral views, respectively B, E, H midbody segments, lateral, dorsal and ventral views, respectively C, F, I posterior part of body, lateral, dorsal and ventral views, respectively J antenna, dorsal view K tergal texture and setae, lateral view L tergal setae, limbus and stricture region, sublateral view M tergal seta, lateral view. Scale bars: A, D, H 0.1 mm; B, C, E–G, I, J 0.05 mm; K 0.02 mm; L 0.01 mm; M 0.005 mm. Designation of antennal structure in text.
Aporodesmella securiformis sp. n., ♂ paratype from Nui Hang Tien; A head, ventral view B midbody legs, ventral view C midbody tarsus and claw, lateral view D, E gonopod aperture and gonopods in situ, ventral and lateral views, respectively F, G left gonopod, subventral and ventromedial views, respectively. Scale bars: A 0.05 mm; B, D 0.02 mm; C, E–J 0.01 mm. Designations of gonopod structures in text.
In width, head > segment 2 > collum = segment 3 = 4 > 5(6) = 16 (♂, ♀), thereafter body gradually tapering towards telson (Fig. 3D–F). Paraterga wanting, metazonae subcylindrical, dorsum strongly convex (Fig. 3A–I). Ozopores totally absent (Fig. 3A, B, D–F, K). Collum subovoid, each following metatergum mostly with 4+4 rather long, slightly blunted, subclavate to subbacilliform, thickened and longitudinally ribbed setae arranged in 3 transverse regular rows and borne on minute knobs (Fig. 3A–F, K–M). Stricture between pro- and metazonae rather wide and shallow, scaly like rear part of prozonae. Limbus very fine and microcrenulate (Fig. 3L). Pleurosternal carinae thin lines (Fig. 3A, B, H). Epiproct short, conical, directed caudoventrally; pre-apical papillae small (Fig. 3C, F, I). Hypoproct subtrapeziform, caudal setigerous papillae evident and well separated (Fig. 3I).
Sterna without modifications, rather broad and sparsely setose (Fig. 3H). Legs short, ca 1.3–1.4 (♂) or 1.1–1.2 times as long as midbody height (♀); ♂ prefemora, femora, postfemora and tibiae clearly incrassate, tarsi longest, slender, sphaerotrichomes missing (Figs 3A, B, 4B), claws simple, slightly curved (Fig. 4B, C); ♂ coxae 2 with short, membranous, cylindrical gonapophyses (Fig. 3G).
Gonopod aperture transversely oblong-oval, slightly subcordate, taking up most of ventral part of metazonite 7 (Fig. 4D). Gonopods (Fig. 4D–G) simple, with globose, scaly, medially fused coxae carrying a few setae on ventral face and a normal cannula mesally. Telopodites nearly straight, mostly exposed, in situ held parallel to each other, contiguous medially, largely unipartite due to prominent, rather densely setose prefemoral parts, rather short and stout, only distal quarter distinctly divided into a peculiar, axe-shaped, lateral solenophore (sph) and a smaller, anteriorly lying, sublanceolate solenomere (sl) directed slightly laterad. Seminal groove running entirely mesally, terminating on top of sl.
http://zoobank.org/7DE6B4E3-4698-4D44-8B74-7E7B60BE07D0
http://species-id.net/wiki/Aporodesmella_similis
Figs 5, 6Holotype ♂ (MNHN JC 356), Vietnam, Kien Giang Province, Ha Tien, Nui Da Dung, valley, 104.477E, 10.4288N, secondary forest, litter, Berlese extraction, 29.11.2006, leg. L. Deharveng & A. Bedos (Vn06-102).
Paratypes: 1 ♂ (SEM), same locality, together with holotype; 1 ♂, 1 ♀ fragment (MNHN JC 356), 1 ♀ (SEM), same locality, soil, Berlese extraction, 05.12.2005, leg. L. Deharveng & A. Bedos (Vn05-107); 1 ♂ (ZMUM ρ2347), same locality, litter, Berlese extraction, 05.12.2005, leg. L. Deharveng & A. Bedos (Vn05-112).
To emphasize the particularly strong similarity to the previous new species.
Differs from all congeners, except Aporodesmella securiformis sp. n., in the presence of a peculiar, dorsoparabasal stump on ♂ antennomere 6, from Aporodesmella securiformis sp. n. in the shape of the solenophore and solenomere.
Length of adults ca 3.0 mm, width of midbody pro- and metazonae 0.2 and 0.25 mm (♂). Coloration in alcohol uniformly pallid, tegument nearly translucent.
Body moniliform, subcylindrical (Fig. 5A–J), with 19 segments (♂).
All characters as in Aporodesmella securiformis sp. n. (Figs 5, 6A), except as follows.
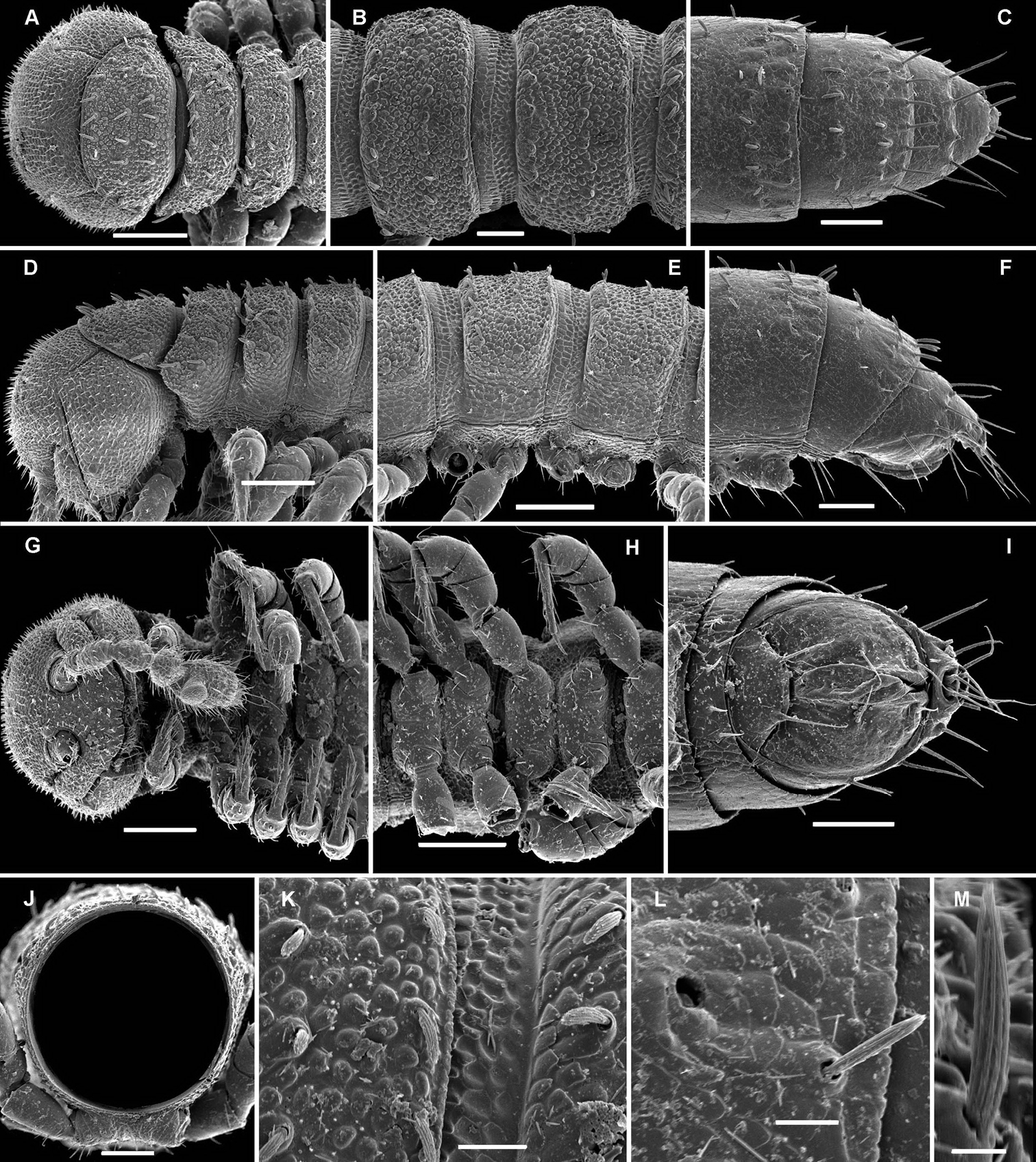
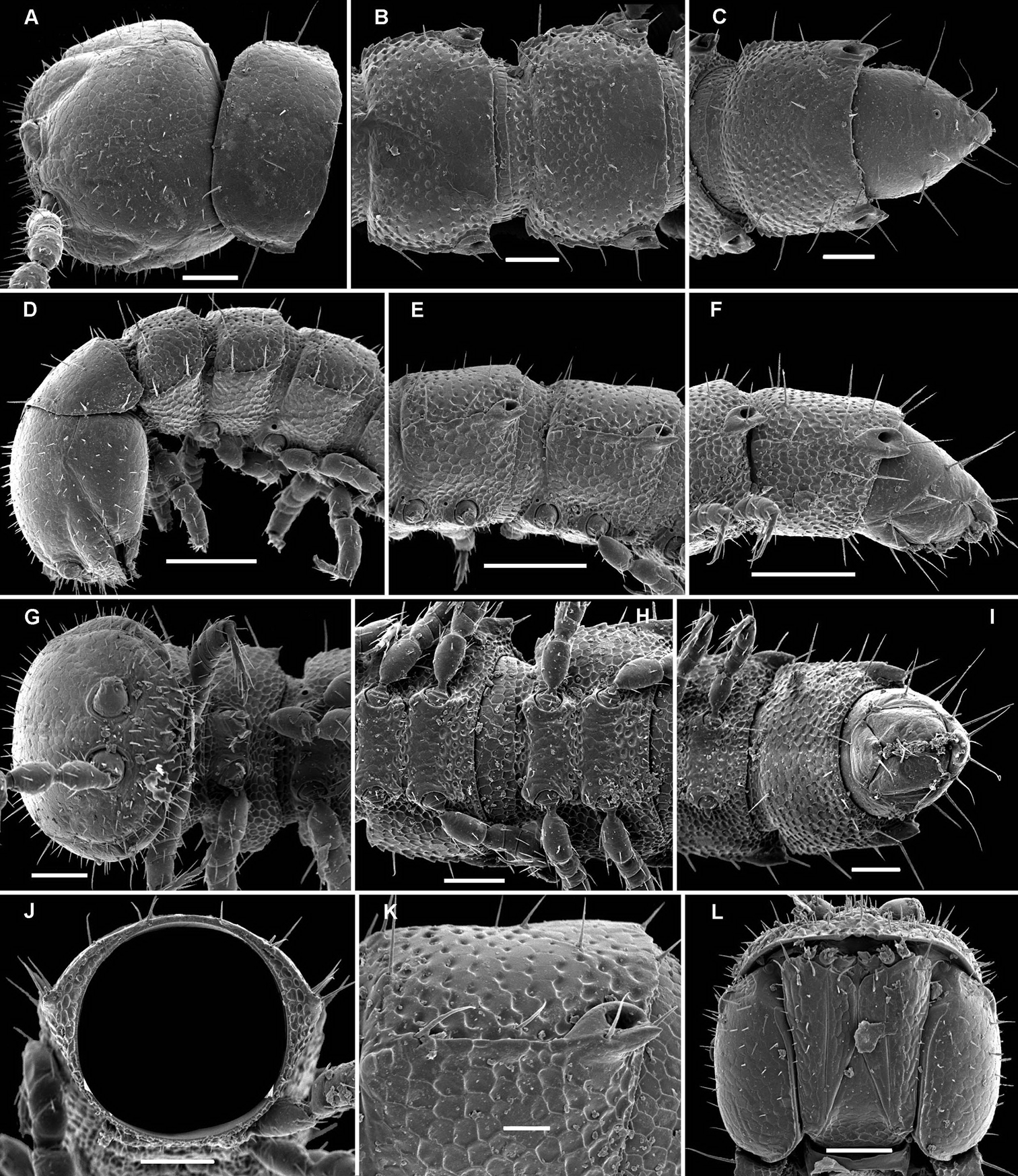
Aporodesmella similis sp. n., ♂ paratype; A, D, G anterior part of body, dorsal, lateral and ventral views, respectively B, E, H midbody segments, dorsal, lateral and ventral views, respectively C, F, I posterior part of body, dorsal, lateral and ventral views, respectively J cross-section of a midbody segment, caudal view K, L tergal setae, limbus and stricture region, subdorsal views M tergal seta, lateral view. Scale bars: A, D, E, G, H 0.1 mm; B, C, F, I, J 0.05 mm; K 0.02 mm; L 0.01 mm; M 0.005 mm.
Aporodesmella similis sp. n., ♂ paratype; A head, ventral view B gonopod aperture and both gonopods in situ, ventral view C right gonopod, ventrolateral view. Scale bars: A, B 0.05 mm; C 0.02 mm. Designations of gonopod structures in text.
Gonopods (Fig. 6B, C) more complex, especially due to an elaborate and subtruncate tip of a more elongate and slenderer telopodite. Solenophore branch (sph) long, membranous, somewhat spoon-shaped, directed laterad, with a long, caudolateral, similarly membranous, subspiniform process (s) starting at base of sph and remaining coaxial with a prominent prefemoral portion. Mesal part of telopodite tip divided into 2 small horns (a and b) bearing a very small, dentiform solenomere (sl) lying in front and in between.
http://zoobank.org/4B5EB78A-B952-4DB6-A5F1-F984EC2ADFFE
http://species-id.net/wiki/Aporodesmella_tergalis
Figs 7, 8Holotype ♂ (MNHN JC 357), Vietnam, Kien Giang Province, Kien Luong, Hon Chong, Nui Bai Voi, cirque du Français, 104.618799E, 10.218541N, litter, Berlese extraction, 23.08.2003, leg. L. Deharveng & A. Bedos (Vn0308-112).
Paratypes: 1 ♂, 1 ♀ (SEM), same locality, together with holotype.
To emphasize the well-developed paraterga.
Differs from congeners by well developed paraterga, short tergal setae, the ♂ also by the presence of a peculiar, central hump above the antennae, the laterally bent, beak-shaped solenophore and the absence of a solenomere.
Length of adults ca 4.0 mm, width of midbody pro- and metazonae 0.4 and 0.5 mm (♂). Coloration in alcohol uniformly pallid, tegument nearly translucent.
Body with 19 segments (♂). Tegument dull, texture delicately alveolate, a cerategument on metazonae well-developed (Fig. 8A). Head with an evident, round, very finely pilose, central hump above antennae (♂) (Fig. 7D, G), more roughly setose over remaining surface except occiput; epicranial suture superficial; genae squarish (Fig. 7A, D, G); gnathochilarium narrow, setae on lamellae linguales particularly strong (Fig. 8B); isthmus between antennae about 1.5 times as broad as diameter of antennal socket (Fig. 7G). Antennae very short, reaching only behind collum when stretched dorsally, not geniculate, strongly clavate due to an abruptly and particularly enlarged antennomere 6, the latter with a usual, tight, distodorsal group of numerous bacilliform sensilla, antennomere 5 with a smaller, but also compact, distodorsal group of only a few shorter sensilla, 7th with a tiny mid-dorsal knob (Fig. 7J).
Aporodesmella tergalis sp. n., ♂ paratype; A, D anterior part of body, lateral and dorsal views, respectively B, E midbody segments, lateral and dorsal views, respectively C, F, I posterior part of body, lateral, dorsal and caudal views, respectively G head, ventral view H legs 1 and 2, ventral view J antenna, sublateral view K cross-section of a midbody segment, caudal view L tergal setae, limbus and stricture region, subdorsal view M tergal seta, lateral view N midbody leg. Scale bars: D 0.2 mm; A, B, E, G, H, K 0.1 mm; C, F, I, J, L, N 0.05 mm; M 0.005 mm.
Aporodesmella tergalis sp. n., ♂ paratype; A cerategument and tergal setae, sublateral view B gnathochilarium, ventral view C gonopod aperture and both gonopods in situ, ventral view D, F left gonopod, subventral and submesal views, respectively E right gonopod, anteroventral view. Scale bars: B, C, E 0.05 mm; D, F 0.02 mm; A 0.01 mm. Designations of gonopod structures in text.
In width, head = collum < segment 3 = 4 < 2 < 5 = 16 (♂), thereafter body gradually tapering towards telson (Fig. 7D–F). Paraterga well-developed, set low (at about upper 1/3-1/2 of body height), mostly clearly declined (Fig. 7A–F, K), front edges moderately convex and forming a shoulder, caudal edges increasingly strongly concave caudad, lateral edges mostly straight, on each side with 3 setigerous, equidistant knobs; caudal corners mostly nearly sharp, lying within rear tergal margin until segment 10, thereafter increasingly well produced behind the margin (Fig. 7A–F). Ozopores totally absent (Figs 7A–F, I, 8A). Collum biconvex, nearly sharp laterally on both sides, with 3 transverse rows of short setae (Fig. 7D). Each following metatergum mostly with 3+3 short, slightly blunted, clavate, thickened and longitudinally ribbed setae arranged in 3 transverse regular rows and borne on minute knobs; sulci between the rows absent (Figs 7A–F, M, 8A). Stricture between pro- and metazonae rather shallow and narrow, scaly like rear part of prozonae (Fig. 7E, L). Limbus very fine, very delicately and sparsely microdenticulate (Fig. 7L). Pleurosternal carinae absent (Fig. 7A, B, H). Epiproct short, conical, truncate, directed caudoventrally; pre-apical papillae large (Fig. 7C, F, I). Hypoproct subtrapeziform, caudal setigerous papillae well-developed and clearly separated (Fig. 7I).
Sterna without modifications, rather broad and sparsely setose. Legs short, ca 1.2–1.3 times as long as midbody height (♂); prefemora, femora, postfemora and tibiae clearly incrassate, tarsi longest, slender, sphaerotrichomes missing; claws simple, slightly curved (Fig. 7N); ♂ coxae 2 with very short, membranous, cylindrical gonapophyses (Fig. 7H).
Gonopod aperture transversely oblong-oval, slightly subcordate, taking up most of ventral part of metazonite 7 (Fig. 8C). Gonopods (Fig. 8C–F) rather complex, with globose, microgranulate, medially fused coxae carrying a few setae on ventral face and a normal cannula mesally. Telopodites mostly exposed, in situ held parallel to each other, nearly contiguous medially, each unipartite, with a rather large, densely setose prefemoral part clearly set off from acropodite by an oblique ventral sulcus and a curved dorsal spine (k); acropodite divided into 2 subequally long parts, each also about equal in length to prefemoral portion; basal half of acropodite remaining coaxial with prefemoral portion, slightly broadened distad and carrying an evident orifice (o) of a fully mesally running seminal groove on a vestigial mesal solenomere (= tubercle), whereas distal half of acropodite, or solenophore (sph), acuminate, directed abruptly laterad, subflagelliform (b), carrying a parabasal apical tooth (t) and a conspicuous, curved, denticulate lamina (a) beginning from near o and turning around b on lateral side.
This species served elsewhere (
18 segments (♂, ♀); pore formula normal: 5, 7, 9, 10, 12, 13, 15–17; head modified in both sexes in being somewhat flattened dorsoventrally; paraterga very poorly developed, serrate/microdentate at lateral edge, with 3 rows of 2+2, long, simple setae (regardless of lateral setae); gonopod coxae with gonocoel not deep; telopodites clearly exposed, but lying tightly appressed and parallel to venter, strongly curved, semi-circular, unipartite, slender, directed mesad and strongly overlapping; prefemoral parts about half as long as telopodites, set off from acropodites neither by a sulcus nor a cingulum; acropodites with small solenophores (sph) lying basal to a spiniform, apical solenomere (sl). Seminal groove running mostly along ventral surface of subbasally obviously twisted acropodites.
To honour Louis Deharveng (MNHN), one of the principal collectors, masculine.
Deharvengius bedosae sp. n., by present designation.
In having only 18 body segments, this new genus is almost unique amongst the Trichopolydesmoidea. The same segment counts seem to solely concern Moojenodesmus pumilus Schubart, 1944, only one of the species of the rather small Neotropical genus Moojenodesmus Schubart, 1945, from the same family Trichopolydesmidae; Moojenodesmus pumilus is especially minute (< 2.5 mm long), and it seems to be parthenogenetic and quite widespread in Brazil (
Deharvengius gen. n. currently contains only one species.
http://zoobank.org/5C60DC06-021E-402F-8D03-871532155442
http://species-id.net/wiki/Deharvengius_bedosae
Figs 9, 10Holotype ♂ (MNHN JC 358), Vietnam, Kien Giang Province, Kien Luong, Hon Chong, Nui Bai Voi (cirque sud), 104.617E, 10.2199N, soil, Berlese extraction, 26.11.2008, leg. L. Deharveng & A. Bedos (Vn06-30).
Paratypes: 2 ♀, 4 juv. (subadults) (MNHN JC 358), same province, Kien Luong, Hon Chong, Nui Bai Voi (cirque sud), 104.617E, 10.2199N; soil, Berlese extraction, 02.06.2008, leg. L. Deharveng & A. Bedos (Vn08-055); 1 ♂, 2 ♀ (MNHN JC 358), 1 ♂ (ZMUM ρ2348), 1 ♂, 1 juv. (subadult) (SEM), same province, Hon Chong, Nui Khoe La, soil, Berlese extraction, 01.06.2008, leg. L. Deharveng & A. Bedos (Vn08-027).
To honour Anne Bedos (MNHN), one of the principal collectors.
Length of adults ca 3.0 mm, width of midbody pro- and metazonae 0.3 and 0.45 mm (♂, ♀). Coloration in alcohol uniformly pallid, tegument often nearly translucent.
Body with 18 segments (♂, ♀). Tegument dull, texture of metazonae very delicately punctate on dorsum and sterna, but alveolate laterally below paraterga; collum smooth (Fig. 9A–K). Head relatively sparsely and finely pilose, less convex than usual (♂, ♀); epicranial suture superficial; genae squarish (Fig. 9A, D, G, L); gnathochilarium narrow, sparsely and uniformly setose (Fig. 9L); isthmus between antennae about 0.8 times as broad as diameter of antennal socket (Fig. 9A, G). Antennae very short (Fig. 10A), reaching only behind head when stretched dorsally, not geniculate, strongly clavate due to an abruptly and particularly enlarged antennomere 6, the latter with a usual, tight, distodorsal group of rather numerous, bacilliform sensilla; antennomere 7 with a smaller distodorsal group of only a few shorter and curved sensilla in front of a tiny mid-dorsal knob.
Deharvengius bedosae sp. n., ♂ paratype from Nui Khoe La; A, D, G anterior part of body, dorsal, lateral and ventral views, respectively B, E, H midbody segments, dorsal, lateral and ventral views, respectively C, F, I posterior part of body, dorsal, lateral and ventral views, respectively J cross-section of a midbody segment, caudal view K poriferous midbody paratergite, tergal setae, tegument structure, limbus and stricture region L head, ventral view. Scale bars: D–F 0.1 mm; A–C, G–J, L 0.05 mm; K 0.02 mm.
Deharvengius bedosae sp. n., ♂ paratype from Nui Khoe La; A antenna, lateral view B midbody leg, lateral view C–E gonopod aperture and both gonopods in situ, ventral, ventrocaudal and anteroventral views, respectively. Scale bars: A 0.05 mm; B–E 0.02 mm Designations of gonopod structures in text.
Body moniliform, subcylindrical (Fig. 9A–J). In width, head > segments 5-15 > 2 > 3 = 4 > collum; body gradually tapering on segments 16-18 (Fig. 9A–I). Paraterga very poorly developed, starting from collum, set low (at about upper 1/3-1/2 of body height), mostly represented by vestigial, delicately serrate ridges and sharp caudal teeth, the latter being clearly enlarged and slightly produced behind rear tergal margin in poriferous segments (Fig. 9A–K). Ozopores evident, ovoid, dorsolateral, lying about equally close to caudal corner and lateral edge (Fig. 9B, C, E, F, K). Collum roundly subquadrate, with 3 transverse rows of long setae dorsally and 2 similar setae on paraterga (Fig. 9A). Each following metatergum mostly with 2+2 long and pointed setae arranged in 3 transverse regular rows and not borne on knobs; sulci between the rows absent (Fig. 9B–F). Stricture between pro- and metazonae rather deep and narrow, scaly like rear part of prozonae (Fig. 9B, E). Limbus very fine, very delicately and sparsely microdenticulate (Figs 9J, K, 10D). Pleurosternal carinae absent (Fig. 9D–F). Epiproct short, conical, truncate, directed caudoventrally; pre-apical papillae small (Fig. 9C, F, I). Hypoproct subtrapeziform, relatively high and narrow, caudal setigerous papillae large and moderately separated, with a faintly convex edge in between (Fig. 9I).
Sterna without modifications, rather broad and sparsely setose (Fig. 9G–I). Legs short, ca 1.2–1.3 (♂) or 1.0–1.1 (♀) times as long as midbody height; prefemora, femora, postfemora and tibiae clearly incrassate, especially so in ♂ (Fig. 10B), tarsi longest, slender, sphaerotrichomes missing; claws simple, slightly curved; ♂ coxae 2 with very short, membranous, cylindrical gonapophyses (Fig. 9G).
Gonopod aperture transversely oblong-oval, taking up most of ventral part of metazonite 7 (Fig. 10C–E). Gonopod coxae with gonocoel not deep; telopodites clearly exposed, but lying tightly appressed and parallel to venter, strongly curved, semi-circular, unipartite, slender, directed mesad and strongly overlapping; prefemoral parts about half as long as telopodites, set off from acropodites neither by a sulcus nor a cingulum; each acropodite with a small solenophore (sph) lying basal to a spiniform, apical solenomere (sl). Seminal groove running mostly along ventral (= lateral) surface of a subbasally obviously twisted acropodite.
19 segments (♂, ♀); pore formula normal: 5, 7, 9, 10, 12, 13, 15-18; ♂ head with a considerable, rounded, central hump above antennae; paraterga poorly developed, serrate/microdentate at lateral edge, with 3 rows of 3+3 or 4+4, long, bacilliform setae (regardless of lateral setae); gonopod coxae with gonocoel not deep; telopodites clearly exposed, but lying rather tightly appressed and mostly parallel to venter, unipartite, slender, abruptly geniculate and directed laterad distal to prefemoral parts, the latter about half as long as telopodites, held parallel to main axis, each set off from a twisted acropodite by a geniculation cingulum (c); acropodites elongate, near midway with a remarkably large hairy pulvillus (p) on top of a small accessory seminal chamber; neither a separate solenophore branch nor a solenomere. Seminal groove (sg) running mostly on mesal side, turning caudad (= ventrad) only beyond geniculation.
To emphasize the geniculated gonopod telopodite (γόνατο meaning “a knee” in Greek), masculine.
Gonatodesmus communicans sp. n., by present designation.
This new genus is highly disjunct in being the sole member of the family which shows a remarkable midway geniculation of the gonopod telopodite. The gonopod tip being directed laterad is not unique in Trichopolydesmidae, shared also with Aporodesmella tergalis sp. n. However, the entire distal, post-geniculation half of the gonopod in Gonatodesmus gen. n. is obviously twisted and strongly elongate, also showing a remarkably distinct hairy pulvillus on top of a small, but evident accessory seminal chamber. Only vestiges of the latter are occasionally observed in a couple of Euro-Mediterranean genera of Trichopolydesmidae (
http://zoobank.org/3B7B82CD-75D0-4444-9E99-4FB6C7741F60
http://species-id.net/wiki/Gonatodesmus_communicans
Figs 11, 12Holotype ♂ (MNHN JC 359), Vietnam, Dongnai Prov., Cat Tien National Park, 107°10'–107°34'E, 11°21'–11°48'N, lowland tropical forest, litter and topsoil, 01.06.2005, leg. A. Anichkin.
Paratypes: 5 ♂, 1 ♀ (MNHN JC 359), 9 ♂, 1 ♀, 1 juv. (ZMUM ρ2336–2339), 1 ♂ (SEM), 1 ♂ (ZMUC), same locality, together with holotype; 1 ♀ (MNHN JC 359), same locality, 15.07.2005, leg. A. Anichkin.
The species epithet refers to the gonopod conformation somewhat transitional between the families Trichopolydesmidae and Opisotretidae.
Length of adults ca 2.8-3.7 mm, width of midbody pro- and metazonae 0.2–0.25 and 0.28–0.4 mm (♂, ♀). Coloration in alcohol uniformly pallid, tegument often nearly translucent.
Body with 19 segments (♂, ♀). Tegument dull, texture of pro- and metazonae delicately alveolate to scaly, metaterga a little more roughly alveolate, only sterna mostly smooth (Fig. 11A–I). Head very densely and finely pilose; epicranial suture absent (♂) or superficial (♀); genae squarish (Fig. 11A, D, G, K); gnathochilarium narrow, sparsely and uniformly setose (Fig. 11K); isthmus between antennae about 1.2 times as broad as diameter of antennal socket (Fig. 11G). Antennae short (Figs 11A, G, 12A), reaching only behind collum when stretched dorsally, not geniculate, rather strongly clavate due to a particularly enlarged antennomere 6, the latter with a usual, tight, distodorsal group of numerous, bacilliform sensilla; a similar, but smaller, also distodorsal group of sensilla on antennomere 5; antennomere 7 with a smaller distodorsal group of only a few shorter and curved sensilla in front of a tiny mid-dorsal knob.
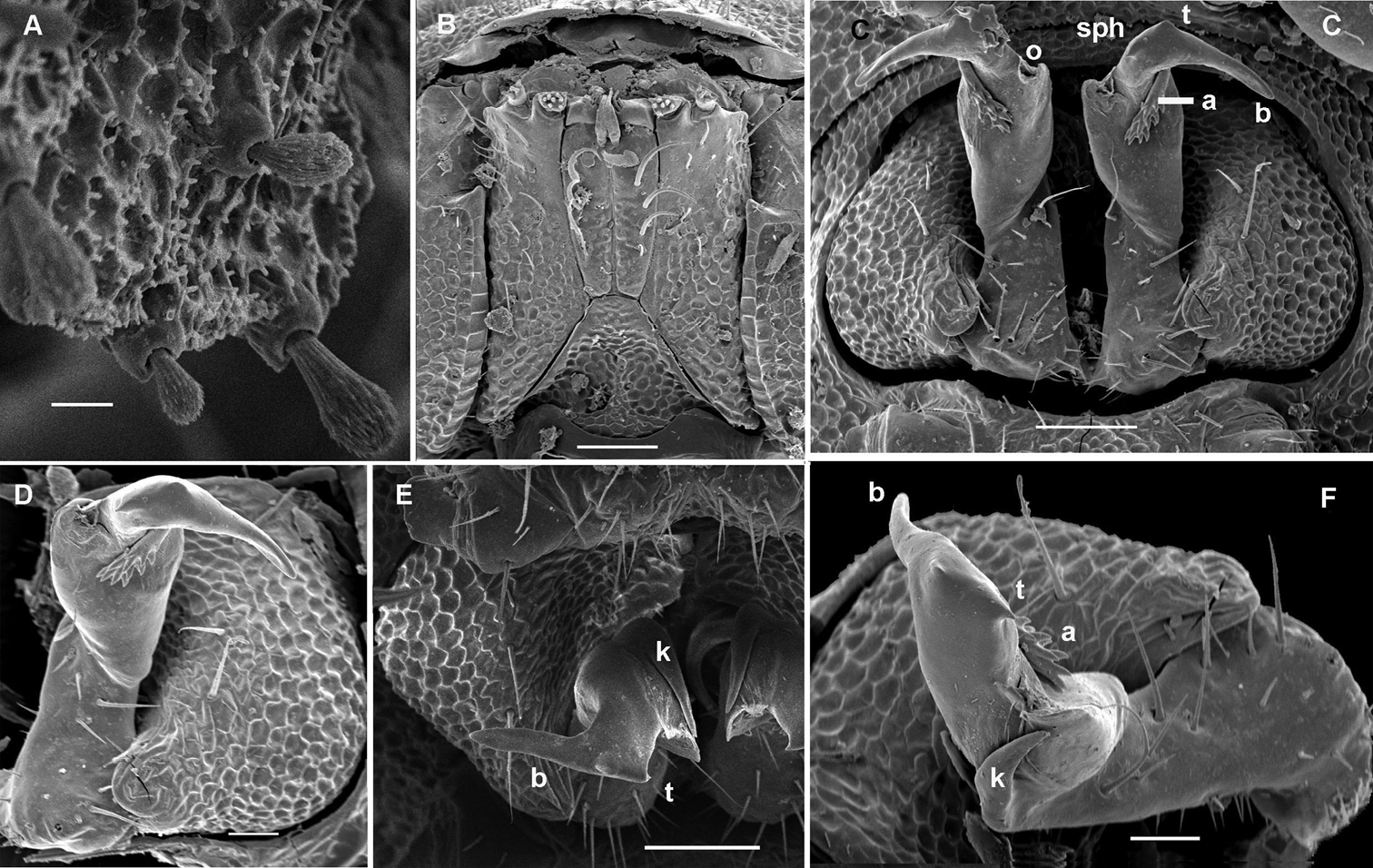
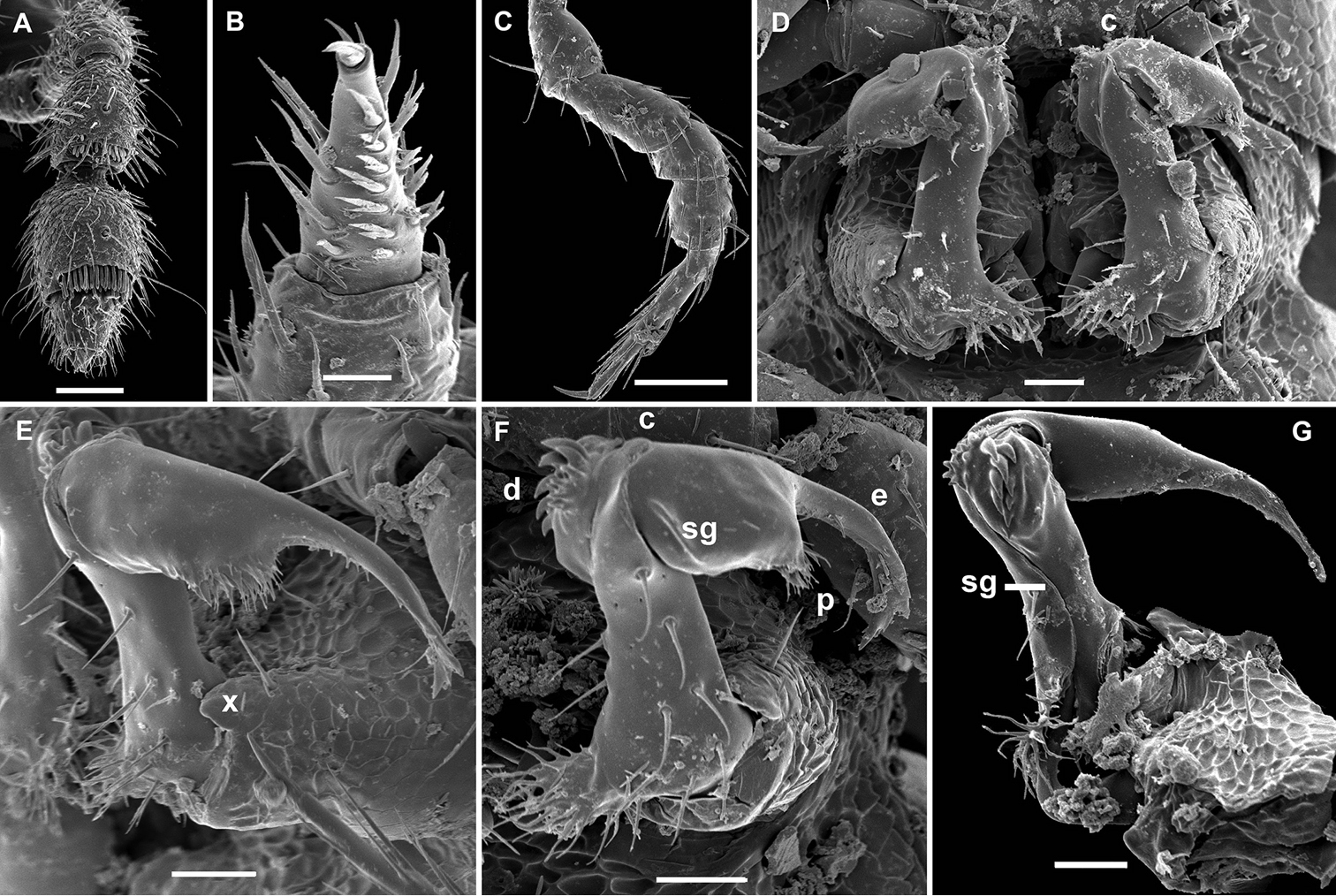
Gonatodesmus communicans sp. n., ♂ paratype; A, D, G anterior part of body, lateral, dorsal and ventral views, respectively B, E, H midbody segments, lateral, dorsal and ventral views, respectively C, F, I posterior part of body, lateral, dorsal and ventral views, respectively J cross-section of a midbody segment, caudal view K head, ventral view L, M poriferous midbody paratergite, tergal setae, tegument structure, limbus and stricture region N ozopore region of segment 16, subdorsal view. Scale bars: A–D, F–J 0.1 mm; E, K 0.05 mm; N 0.02 mm; L, M 0.01 mm.
Gonatodesmus communicans sp. n., ♂ paratype; A antennomeres 4-8, dorsal view B tarsus 1, ventral view C midbody leg, lateral view D both gonopods in situ, ventral view E–G left gonopod, anteroventral, ventral and submesal views, respectively. Scale bars: A, C 0.05 mm; D–G 0.02 mm; B 0.01 mm. Designations of gonopod structures in text.
In width, collum < segment 3 = 4 < 2 = 5 < 6–15 < head; body gradually tapering on segments 16–18 (Fig. 11A–I). Paraterga poorly developed, starting from collum, set rather low (at about upper 1/3 of body height, Fig. 11J), at most small ridges with 2–3 lateral, setigerous knobs, caudal corner never produced behind rear tergal margin even in poriferous segments (Fig. 11A–F, N). Ozopores evident, ovoid, dorsolateral, mostly lying closer to lateral edge, but in segments 15-18 slightly elevated and positioned closer to caudal edge between 2 subequal, setigerous stalks (Fig. 11N). Collum regularly rounded, transversely oblong-oval, with 3 transverse rows of 4+4, 2+2 and 3+3 rather long setae dorsally and 2 similar setae on paraterga (Fig. 11A, D). Each following metatergum with 3+3 or 4+4, similarly long, bacilliform, delicately ribbed setae arranged in 3 transverse regular rows and borne on knobs; sulci between the rows absent (Fig. 11A–F, L–N). Stricture between pro- and metazonae rather deep and narrow, microalveolate like metazonae (Fig. 11E, L, N). Limbus very fine, delicately and densely microcrenulate (Fig. 11E, N). Pleurosternal carinae absent (Fig. 11A–C). Epiproct short, conical, truncate, directed caudoventrally; pre-apical papillae very small (Fig. 11C, F). Hypoproct subtrapeziform, caudal setigerous papillae large and well separated, with a faintly convex edge in between (Fig. 11I).
Sterna without modifications, rather broad and sparsely setose (Fig. 11H, I). Legs short and stout, ca 1.2–1.3 (♂) or 1.0–1.1 (♀) times as long as midbody height; prefemora, femora, postfemora and tibiae clearly incrassate, especially so in ♂ (Fig. 12C), tarsi longest, slender, sphaerotrichomes missing; claws simple, slightly curved; ♂ tarsi 1 with peculiar, bi- or trifid setae ventrally (Fig. 12B); ♂ coxae 2 with very short, membranous, cylindrical gonapophyses (Fig. 11G).
Gonopod aperture transversely oblong-oval, taking up most of ventral part of metazonite 7 (Fig. 12D). Gonopod coxae (Fig. 12D–G) rather small (gonocoel not deep), sparsely setose and clearly micropapillate laterally, each with a subtriangular ventral projection (x); telopodites clearly exposed, but lying rather tightly appressed and parallel to venter, unipartite, slender, abruptly geniculate and directed laterad distal to prefemoral parts, the latter about half as long as telopodites, held parallel to main axis, each with an apicomedian field of strong curved spines (d), set off from a twisted acropodite by a strong geniculation cingulum (c); acropodite elongate, near midway with a remarkably large hairy pulvillus (p) on top of a small accessory seminal chamber; acropodite distal to pulvillus particularly slender, slightly curved caudad, subacuminate; neither a separate solenophore branch nor a solenomere. Seminal groove (sg) running mostly on mesal side, turning caudad (= ventrad) only beyond geniculation.
19 segments (♂, ♀); pore formula normal: 5, 7, 9, 10, 12, 13, 15–18; head without modifications; paraterga poorly developed, metaterga with 3 rows of 3+3 or, more rarely, 4+4, long, bacilliform setae (regardless of lateral setae); gonopod coxae with gonocoel not deep; telopodites rather clearly exposed and transverse, but stout and remarkably complex, very strongly twisted, partly fimbriate/plumose distally, with several outgrowths of varying shapes; prefemoral part about half the height of telopodite, demarcated on lateral (not medial!) side by an oblique seminal groove running further mesad onto a medioventral outgrowth of acropodite to terminate distally, with neither a solenomere nor an accessory seminal chamber, nor a pulvillus.
To emphasize the strongly helicoid gonopod telopodite, masculine.
Helicodesmus anichkini sp. n., by present designation.
This new genus is remarkable within Trichopolydesmidae in showing particularly strongly twisted gonopods, including their prefemoral parts, such that the seminal groove turns ca 180° around the highly complex transverse acropodite. Also noteworthy is the complete lack of a solenomere.
http://zoobank.org/981D48EE-2D59-4940-8954-4CA702DD2B5C
http://species-id.net/wiki/Helicodesmus_anichkini
Figs 13–15Holotype ♂ (MNHN JC 360), Vietnam, Dongnai Prov., Cat Tien National Park, 107°10'–107°34'E, 11°21'–11°48'N, lowland tropical forest, litter and topsoil, 01.06.2005, leg. A. Anichkin.
Paratypes: 2 ♂, 1 ♀ (ZMUM ρ2340, ρ2343), 1 ♂ (SEM), same locality, together with holotype; 1 ♂ (ZMUM ρ2342), same locality, 01.04.2005; 2 ♂ (MNHN JC 360), 1 ♂ (ZMUM ρ2341), same locality, 15.07.2005; 1 ♂, 1 ♀ (MNHN JC 360), 2 ♂ (ZMUM ρ2344, ρ2345), same locality, 23.11.2005; 1 ♂ (MNHN JC 360), same locality, 17.07.2005; 1 ♂ (ZMUC), same locality, 15.05.2005, all leg. A. Anichkin.
To honour Alexander E. Anichkin, who provided for study all millipede material he had collected in Cat Tien National Park, including three trichopolydesmids described here.
Length of adults ca 3.0-4.0 mm, width of midbody pro- and metazonae 0.28-0.32 and 0.4-0.45 mm (♂, ♀). Coloration in alcohol uniformly pallid, tegument often nearly translucent.
Body with 19 segments (♂, ♀). Tegument dull, texture of nearly entire body delicately alveolate to scaly, only sterna nearly smooth (Fig. 13A–I). Head very densely and finely pilose; epicranial suture highly superficial; genae squarish (Fig. 13A, D, G, K); gnathochilarium rather broad, sparsely and uniformly setose (Fig. 13K); isthmus between antennae about 1.2–1.3 times as broad as diameter of antennal socket (Fig. 13G, K). Antennae short (Fig. 13A, D, G), reaching only behind collum when stretched dorsally, not geniculate, rather strongly clavate due to a particularly enlarged antennomere 6, the latter with a usual, tight, distodorsal group of numerous, bacilliform sensilla; a similar, but smaller, also distodorsal group of sensilla on antennomere 5; antennomere 7 with a smaller distodorsal group of only a few shorter and curved sensilla in front of a tiny mid-dorsal knob.
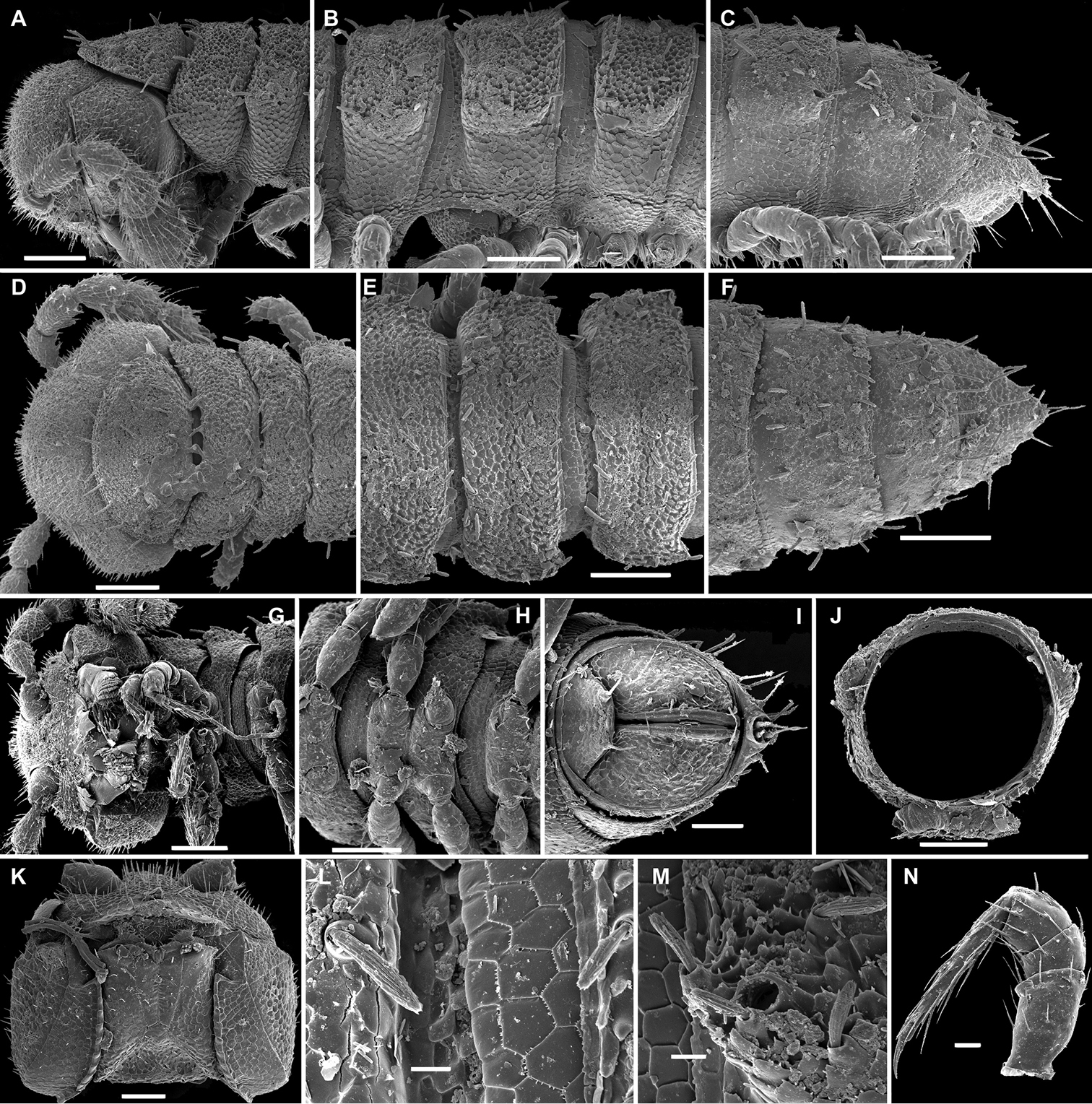
Helicodesmus anichkini sp. n., ♂ paratype; A, D, G anterior part of body, lateral, dorsal and ventral views, respectively B, E, H midbody segments, lateral, dorsal and ventral views, respectively C, F, I posterior part of body, lateral, dorsal and ventral views, respectively J cross-section of a midbody segment, caudal view K head, ventral view L tergal setae, tegument structure, limbus and stricture region M ozopore region of a midbody segment, sublateral view N midbody leg, lateral view. Scale bars: A–J 0.1 mm; K 0.05 mm; N 0.02 mm; L, M 0.01 mm.
In width, collum = segment 3 = 4 < 5 < 2 < 6–15 < head; body gradually tapering on segments 16-18 (Fig. 13D–F). Paraterga mostly moderately wide, starting from collum, set rather low (at about upper 1/3 of body height, Fig. 13J), at most small ridges with 2-3 lateral, setigerous knobs, absent from segment 18, caudal corner never produced behind rear tergal margin even in poriferous segments (Fig. 13A–G, M). Ozopores evident, ovoid, dorsolateral, mostly lying closer to lateral edge (Fig. 13M). Collum biconvex, sides (= paraterga) narrowly rounded, dorsal surface with 3 transverse rows of 5+5, 3+3 and 4+4 rather long setae dorsally and 2 similar setae on paraterga (Fig. 13A, D). Each following metatergum with 3+3 or, more rarely, 4+4, similarly long, bacilliform, delicately ribbed setae arranged in 3 transverse regular rows and borne on knobs; sulci between the rows absent (Fig. 13A–G, L, M). Stricture between pro- and metazonae rather deep and narrow, microalveolate like adjacent metazonae (Fig. 13E, L). Limbus very fine, delicately and densely microcrenulate (Fig. 13L, M). Pleurosternal carinae absent (Fig. 13A–C). Epiproct short, conical, truncate, directed caudoventrally; pre-apical papillae evident (Fig. 13C, F, I). Hypoproct subtrapeziform, caudal setigerous papillae moderate and well separated (Fig. 13I).
Sterna without modifications, rather broad and sparsely setose (Fig. 13G, H). Legs short and stout, ca 1.1–1.2 (♂) or 0.9–1.0 (♀) times as long as midbody height; prefemora, femora, postfemora and tibiae clearly incrassate, especially so in ♂ (Fig. 13N), tarsi longest, slender, sphaerotrichomes missing; claws simple, slightly curved; ♂ coxae 2 with very short, membranous, cylindrical gonapophyses (Fig. 13G).
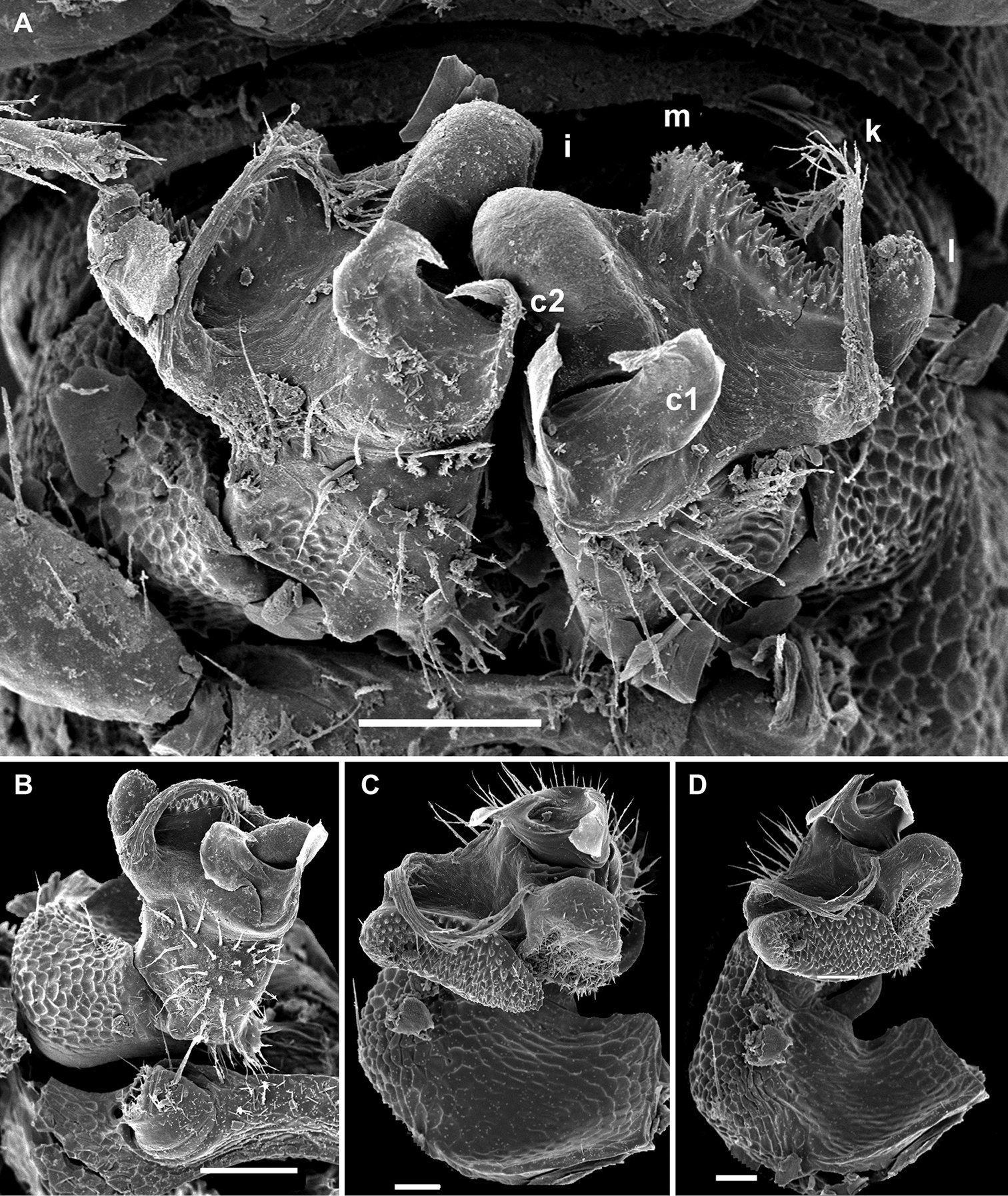
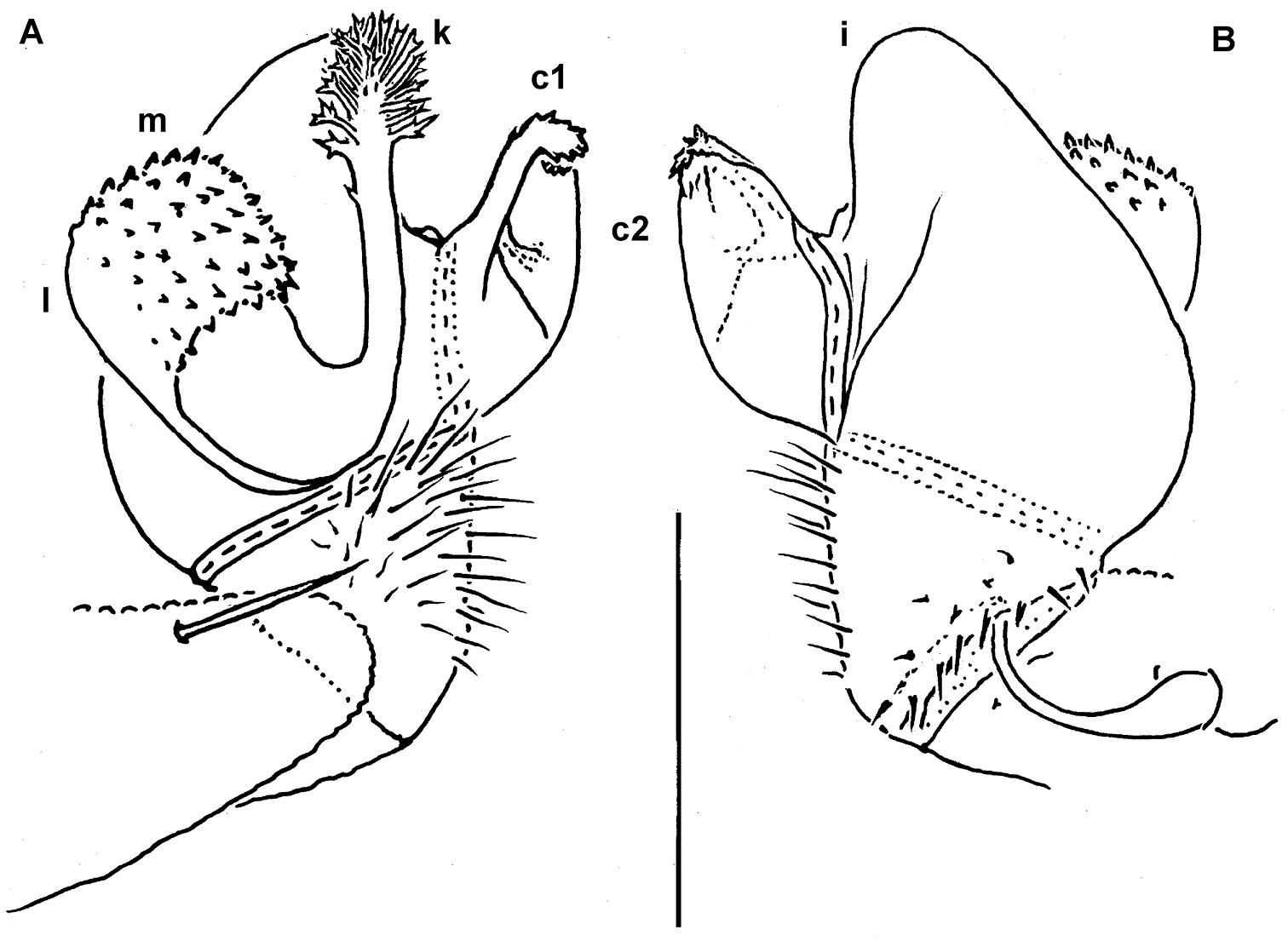
Gonopod aperture transversely oblong-oval, taking up most of ventral part of metazonite 7 (Fig. 14A). Gonopod coxae rather small (gonocoel not deep), sparsely setose and clearly micropapillate laterally (Figs 14, 15); telopodites quite well exposed and unusually transverse, but stout and remarkably complex, very strongly twisted, partly fimbriate(curved ventral projections c1 and c2) or plumose (a long lateral process k), with several outgrowths of varying shapes (a stump-shaped, rounded, mesal i and 2 indistinctly separated, densely microdentate, also rounded m and l); prefemoral part about half the height of telopodite, demarcated on lateral (not medial!) side by an oblique seminal groove running further mesad onto a medioventral outgrowth of acropodite to terminate distally at joint base of c1 and c2, with neither a solenomere nor an accessory seminal chamber, nor a pulvillus.
Helicodesmus anichkini sp. n., ♂ paratype; A both gonopods in situ, ventral view B–D right gonopod, anteroventral, ventrolateral and lateral views, respectively. Scale bars: A, B, D 0.05 mm; C 0.02 mm. Designations of gonopod structures in text.
Helicodesmus anichkini sp. n., ♂ paratype; A, B right gonopod, lateral and mesal views, respectively. Scale bar: 0.1 mm. Designations of gonopod structures in text.
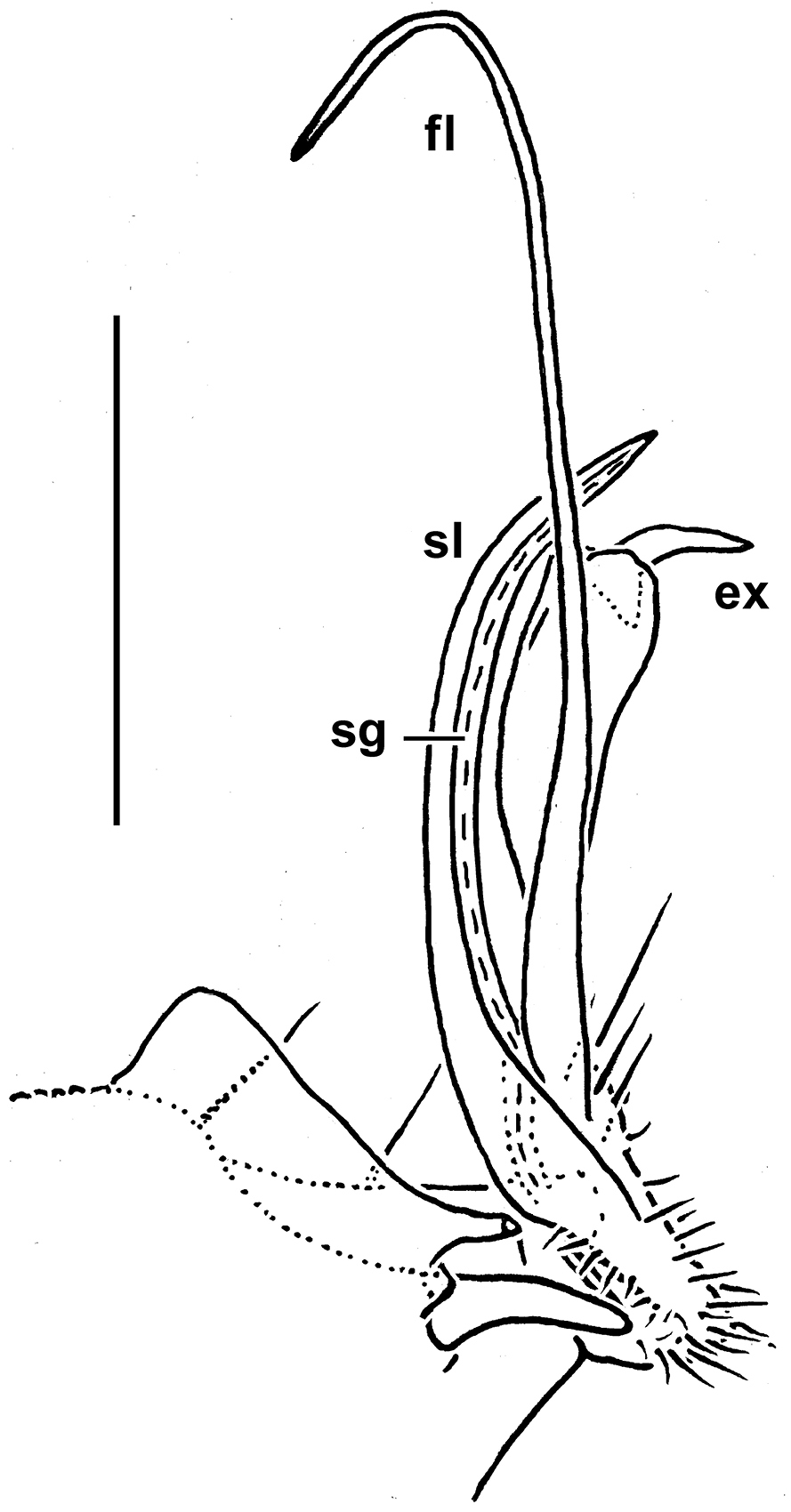
19 segments (♂), ♀ unknown; pore formula normal: 5, 7, 9, 10, 12, 13, 15–18; head without modifications; paraterga poorly developed, metaterga with 3 rows of 3+3 or, more rarely, 4+4, long, bacilliform setae (regardless of lateral setae); gonopod coxae large (gonocoel quite deep); telopodites rather well exposed, tripartite, without evidence of torsion, elongate, moderately curved caudad, subcontiguous medially and held parallel to each other; acropodites lying distal to prefemoral parts much longer than and coaxial with the latter; solenomere (sl), or endomere, a strong, simple, frontal branch about as long as a lateral exomere (ex); a very long, flagelliform, mesal branch (fl) at base of both sl and ex; seminal groove (sg) running entirely along mesal side of sl to terminate on top with neither an accessory seminal chamber nor a pulvillus.
To emphasize the monstrously long flagellum of the gonopod, masculine.
Monstrodesmus flagellifer sp. n., by present designation.
This new genus seems to be particularly similar to Topalodesmus Golovatch, 1988, monobasic, from the Himalayas of India (
http://zoobank.org/46A8E604-6546-481A-A138-25C47E2323A6
http://species-id.net/wiki/Monstrodesmus_flagellifer
Figs 16–18Holotype ♂ (MNHN JC 361), Vietnam, Dongnai Prov., Cat Tien National Park, 107°10'–107°34'E, 11°21'–11°48'N, lowland tropical forest, litter and topsoil, 01.06.2005, leg. A. Anichkin.
Paratype: 1 ♂ (SEM), same locality, together with holotype.
The species epithet, a noun in apposition, is to emphasize the remarkably long gonopod flagellum.
Length of adults ca 4.5 mm, width of midbody pro- and metazonae 0.33 and 0.4 mm (♂). Coloration in alcohol uniformly pallid, tegument often nearly translucent.
Body with 19 segments (♂). Tegument dull, texture of nearly entire body delicately alveolate to scaly, only sterna nearly smooth (Fig. 16A–I). Head very densely and finely pilose; epicranial suture highly superficial; genae squarish (Fig. 16A, D, G); gnathochilarium rather broad, sparsely and uniformly setose (Fig. 16K); isthmus between antennae about 1.2 times as broad as diameter of antennal socket (Fig. 16G). Antennae short (Fig. 16G, L), reaching only behind collum when stretched dorsally, not geniculate, rather strongly clavate due to a particularly enlarged antennomere 6, the latter with a usual, tight, distodorsal group of numerous, bacilliform sensilla; a similar, but smaller, also distodorsal group of sensilla on antennomere 5; antennomere 7 with a smaller distodorsal group of only a few shorter and curved sensilla in front of a tiny mid-dorsal knob.
Monstrodesmus flagellifer sp. n., ♂ paratype; A, D anterior body part, lateral and dorsal views, respectively B, E, H midbody segments, lateral, dorsal and ventral views, respectively C, F, I posterior body part, lateral, dorsal and ventral views, respectively G, K head, frontal and ventral views, respectively J cross-section of a midbody segment, caudal view L antennomeres 4-8, sublateral view; tergal seta M tegument structure, limbus and stricture region N tergal seta, lateral view O ozopore region of a midbody segment, sublateral view. Scale bars: A–J 0.1 mm; K, L 0.05 mm; M–O 0.01 mm.
In width, collum < segment 3 = 4 < head = 2 < 5-15; thereafter body gradually tapering (Fig. 16D–F). Paraterga mostly moderate, starting from collum, set rather low (at about upper 1/3 of body height, Fig. 16J), at most small ridges with 2-3 lateral, setigerous knobs, absent from segment 18, caudal corner never produced behind rear tergal margin even in poriferous segments (Fig. 16A–I, O). Ozopores evident, ovoid, dorsolateral, mostly lying closer to lateral edge (Fig. 16B, C, E, F, O). Collum subovoid, sides (= paraterga) well rounded, dorsal surface with 3 transverse rows of 4+4, 3+3 and 3+3 rather long setae dorsally and 2 similar setae on paraterga (Fig. 16A, D). Each following metatergum with 3+3 or, more rarely, 4+4, similarly long, bacilliform, delicately ribbed setae arranged in 3 transverse regular rows and borne on knobs; sulci between the rows superficial (Fig. 16A–F). Stricture between pro- and metazonae rather deep and narrow, microalveolate like adjacent metazonae (Fig. 16A–F, M, O). Limbus very fine, delicately and densely microspiculate (Fig. 16M, O). Pleurosternal carinae absent (Fig. 16A–C). Epiproct short, conical, truncate, directed caudoventrally; pre-apical papillae small (Fig. 16C, F, I). Hypoproct subtrapeziform, caudal setigerous papillae moderate and well separated (Fig. 16I).
Sterna without modifications, rather broad and sparsely setose (Fig. 16H, I). Legs short and stout, ca 1.1-1.2 times as long as midbody height (♂); prefemora, femora, postfemora and tibiae clearly incrassate (Fig. 17A), tarsi longest, slender, sphaerotrichomes missing; claws simple, slightly curved (Fig. 17B); coxae 2 with very short, membranous, cylindrical gonapophyses.
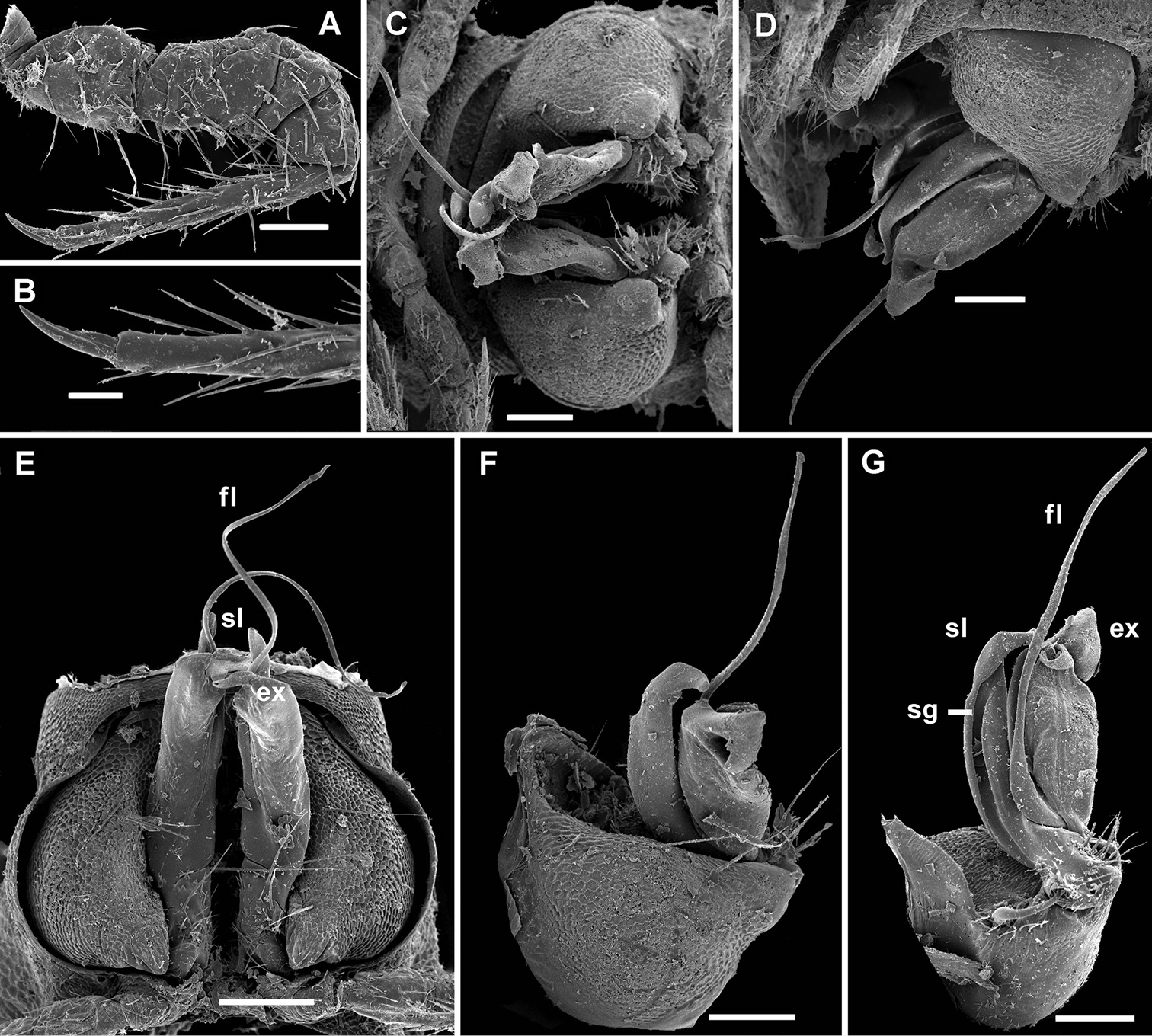
Monstrodesmus flagellifer sp. n., ♂ paratype; A midbody leg, lateral view B claw of a midbody leg C–E both gonopods in situ, ventral, lateral and ventrocaudal views, respectively F right gonopod, lateral view G left gonopod, mesal view. Scale bars: C–G 0.1 mm; A 0.05 mm; B 0.02 mm. Designations of gonopod structures in text.
Gonopod aperture transversely obcordate, taking up most of ventral part of metazonite 7 (Fig. 17C, E). Gonopod coxae large (gonocoel rather deep), with 2 long setae and clearly micropapillate laterally, but without any frontal outgrowths (Fig. 17C–G, 18); telopodites quite well exposed, not twisted, tripartite, elongate, moderately curved caudad, subcontiguous medially and held parallel to each other; acropodites lying distal to prefemoral parts much longer than and coaxial with the latter; solenomere (sl) (= endomere) a strong, simple, frontal branch about as long as an apically bifid lateral exomere (ex); an extremely long, flagelliform, mesal branch (fl) at base of both sl and ex; seminal groove (sg) running entirely along mesal side of sl to terminate on top with neither an accessory seminal chamber nor a pulvillus.
Monstrodesmus flagellifer sp. n., ♂ holotype, right gonopod, mesal view. Scale bar: 0.2 mm. Designations of gonopod structures in text.
Since brief diagnoses of all previously known Oriental trichopolydesmid genera are given above, and their scopes and statuses also considered, below we only present a key to the new genera and species, the first to be reported from Indochina in general and from Vietnam in particular.
| 1 | Ozopores totally missing (Figs 3A–F, 5A–F, 7A–F) | Aporodesmella gen. n., 2 |
| – | Pore formula normal | 4 |
| 2 | Paraterga strongly developed (Fig. 7A–F). ♂ head with an evident, round, very finely pilose, central hump above antennae (Fig. 7D, G). Antennomere 6 unmodified (Fig. 7J). Gonopods as in Fig. 8C–F | Aporodesmella tergalis sp. n. |
| – | Paraterga poorly developed to missing (Figs 3A–F, 5A–F). ♂ head unmodified. Antennomere 6 with a peculiar dorsoparabasal stump (s) (Figs 3J, 4A, 5G). Gonopods different | 3 |
| 3 | Solenophore (sph) unusually short and axe-shaped, solenomere (sl) simple and lanceolate (Fig. 4D–G) | Aporodesmella securiformis sp. n. |
| – | Gonopod tip more elaborate, both sph and sl different (Fig. 6B, C) | Aporodesmella similis sp. n. |
| 4 | ♂ head with an evident, round, central hump above antennae (Fig. 11A, D, G). Gonopod telopodites clearly geniculate due to a midway cingulum (c), distal half directed laterad, with an unusually strongly developed hairy pulvillus (p) (Figs 12D–G) | Gonatodesmus gen. n. (Gonatodesmus communicans sp. n.) |
| – | ♂ head devoid of a hump. Gonopod telopodites non-geniculate, at most strongly, but regularly curved, distal parts never directed laterad; a hairy pulvillus absent | 5 |
| 5 | Head in both sexes somewhat flattened dorsoventrally (Fig. 9A, D, G). Gonopod telopodites long and slender, semi-circular, in situ crossing medially, bifid subapically (Fig. 10C–E) | Deharvengius gen. n. (Deharvengius bedosae sp. n.) |
| – | Head clearly more convex dorsally. Gonopod telopodites far more elaborate | 6 |
| 6 | Gonopods especially complex, unusually strongly twisted, in situ clearly transverse, devoid of a solenomere (Figs 14, 15) | Helicodesmus gen. n. (Helicodesmus anichkini sp. n.) |
| – | Gonopod telopodites held parallel to main axis, each tripartite, including a remarkably long flagellum (fl) (Figs 17C–G, 18) | Monstrodesmus gen. n. (Monstrodesmus flagellifer sp. n.) |
As observed elsewhere (
Gonatodesmus gen. n., in which the gonopods are considerably elongate, geniculate at about midway while their distal halves are directed laterad, provides a kind of transition or evolutionary bridge between Trichopolydesmidae and Opisotretidae, thus reinforcing the assignment of these two families to the single superfamily Trichopolydesmoidea. This is in contrast to the much more frequent condition of the gonotelopodites as observed in Trichopolydesmidae and most other groups of Polydesmida, which either cross mesally or are held parallel to each other. Based on this example, the Opisotretidae might well be viewed as a direct and disjunct derivative of the more basal Trichopolydesmidae, with their gonotelopodite growing increasingly elongate and orientated laterally (
There can be no doubt that many more species of Trichopolydesmidae await discovery and description. Representatives of this family might seem to be rare, but it is very likely that they are seriously under-collected due to their very small size and perhaps also to seasonality. Thus, most of the adults have been taken during or shortly after the rainy season (May to November), when the animals are surface-active. During the dry season, like most other Diplopoda in lowland tropical forests of Vietnam, the very small trichopolydesmoids seem to be dormant (
This work only became possible through the support rendered to the first author by the Muséum national d’Histoire naturelle, Paris. We heartily thank Louis Deharveng and Anne Bedos (both MNHN) for the precious material they made available for study. Alexander Anichkin (Joint Russia-Vietnam Tropical Center, Ho Chi Minh City, Vietnam) kindly provided some more samples from Vietnam.
The material from Vanuatu was collected during the SANTO 2006 Expedition organized by MNHN, Pro Natura International (PNI), and Institut de Recherche pour le Développement (IRD), which operated under a permit granted to P. Bouchet by the Environment Unit of the Government of Vanuatu. The different trips to the Hon Chong area in Vietnam were partly funded by the Plan Pluri-Formation du MNHN (Etat et structure phylogénétique de la biodiversité actuelle et fossile, led by Philippe Janvier) in 2005, 2006 and 2008; the field work was carried out in collaboration with the Institute of Tropical Biology and the National University of Ho Chi Minh City.
Special thanks go to Robert Mesibov, Cathy Car and a third, anonymous reviewer who kindly provided very important critiques and suggestions to improve the paper.