Citation: Chakrabarty P, Prejean JA, Niemiller ML (2014) The Hoosier cavefish, a new and endangered species (Amblyopsidae, Amblyopsis) from the caves of southern Indiana. ZooKeys 412: 41–57. doi: 10.3897/zookeys.412.7245
We describe a new species of amblyopsid cavefish (Percopsiformes: Amblyopsidae) in the genus Amblyopsis from subterranean habitats of southern Indiana, USA. The Hoosier Cavefish, Amblyopsis hoosieri sp. n., is distinguished from A. spelaea, its only congener, based on genetic, geographic, and morphological evidence. Several morphological features distinguish the new species, including a much plumper, Bibendum-like wrinkled body with rounded fins, and the absence of a premature stop codon in the gene rhodopsin. This is the first new cavefish species described from the United States in 40 years and exemplifies how molecular data can alert us to the presence of otherwise cryptic biodiversity.
Cryptic diversity, GenSeq, new species, subterranean, taxonomy
The teleost family Amblyopsidae (order Percopsiformes) comprises North America’s largest clade of stygobiotic (obligate cave-dwelling) fishes and has long been of interest to evolutionary biologists and ecologists studying adaptations to extreme subterranean habitats (reviewed in
There has been a revival in amblyopsid systematics and taxonomy in recent years, as several studies have examined higher-level phylogenetic relationships as well as population level differentiation using molecular approaches (Niemiller and Fitzpatrick 2008, Dillman et al. 2011,
A recent study of Amblyopsis spelaea based on phylogeographic structure of one mitochondrial and four nuclear loci identified two evolutionary lineages associated with the modern Ohio River (
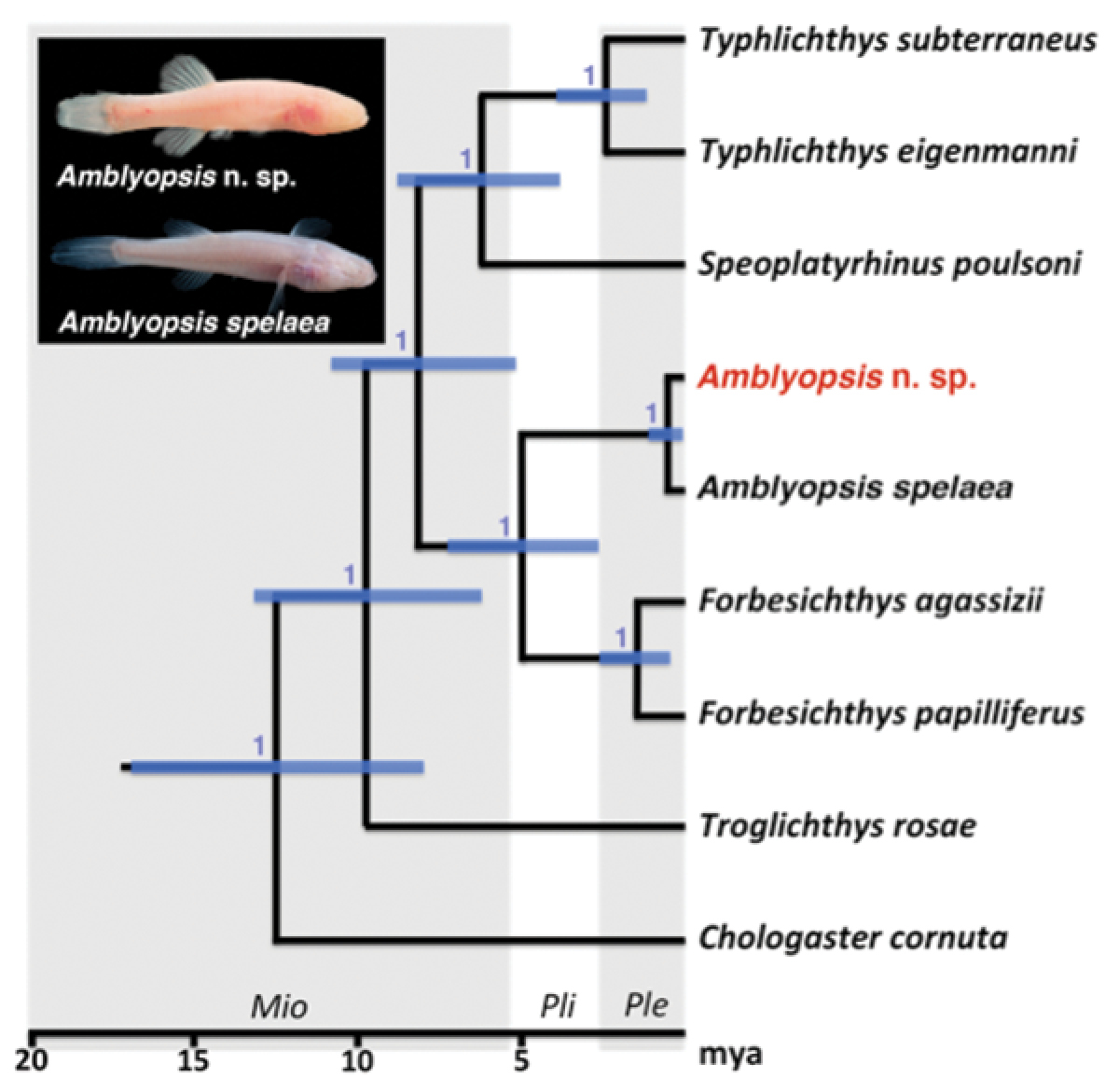
Phylogeny of Amblyopsidae. Modified from
These northern and southern lineages were recovered as reciprocally monophyletic for two of the nuclear loci, s7 and rhodopsin, and the mtDNA locus nd2. Little variation was exhibited at the nuclear loci rag1 and tbr. The gene tree estimated from the mitochondrial nd2 locus contained two strongly supported clades (Bayesian posterior probability > 0.95) separated by 27 mutational steps with observed average uncorrected pairwise genetic distances of 3.1% between these lineages. Observed uncorrected pairwise genetic distances were considerably lower for all nuclear loci; however, segregating variation was observed in s7 and rhodopsin. A single nucleotide substitution at s7 segregated between the northern and southern lineages, whereas three nucleotide substitutions segregated at rhodopsin. The differences in rhodopsin include a mutation that results in a premature stop codon in the open reading frame of all individuals sampled in the southern lineage that is absent from the northern lineage.
The results of
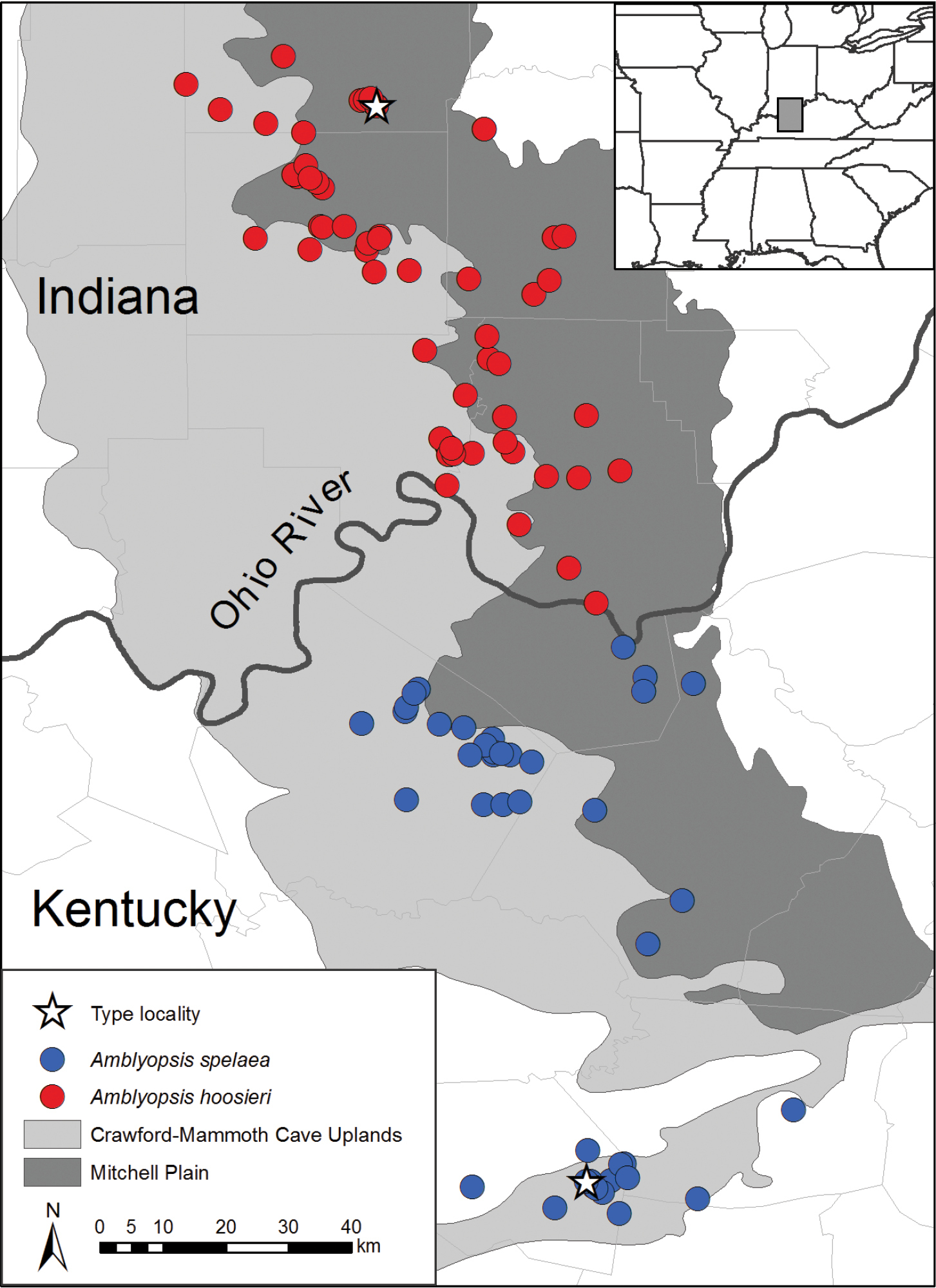
Distribution of Amblyopsis spp., Amblyopsis spelaea and Amblyopsis hoosieri, in the Mitchell Plain and Crawford-Mammoth Uplands of Indiana and Kentucky.
Institutional abbreviations are as follows: CAS (California Academy of Science); INHS (Illinois Natural History Survey); IU (Indiana University); LSUMZ (Louisiana State University Museum of Natural Science); UMMZ (University of Michigan Museum of Zoology), YPM (Yale Peabody Museum). Non-type materials examined include: Amblyopsis spelaea: INHS 50129 (n=1), 60573 (n=1); UMMZ 146991 (n=1); 179149 (n=1); YPM 25294 (n=8, n=1 cleared and stained). Materials examined for the new species are listed below as types. Cleared and stained specimens were prepared following modifications of the method outlined by
Radiographs were made for all specimens using a Faxitron x-ray cabinet. All meristics (numbers of fin rays and vertebrae) were counted using these radiographs.
Morphometric measurements, following
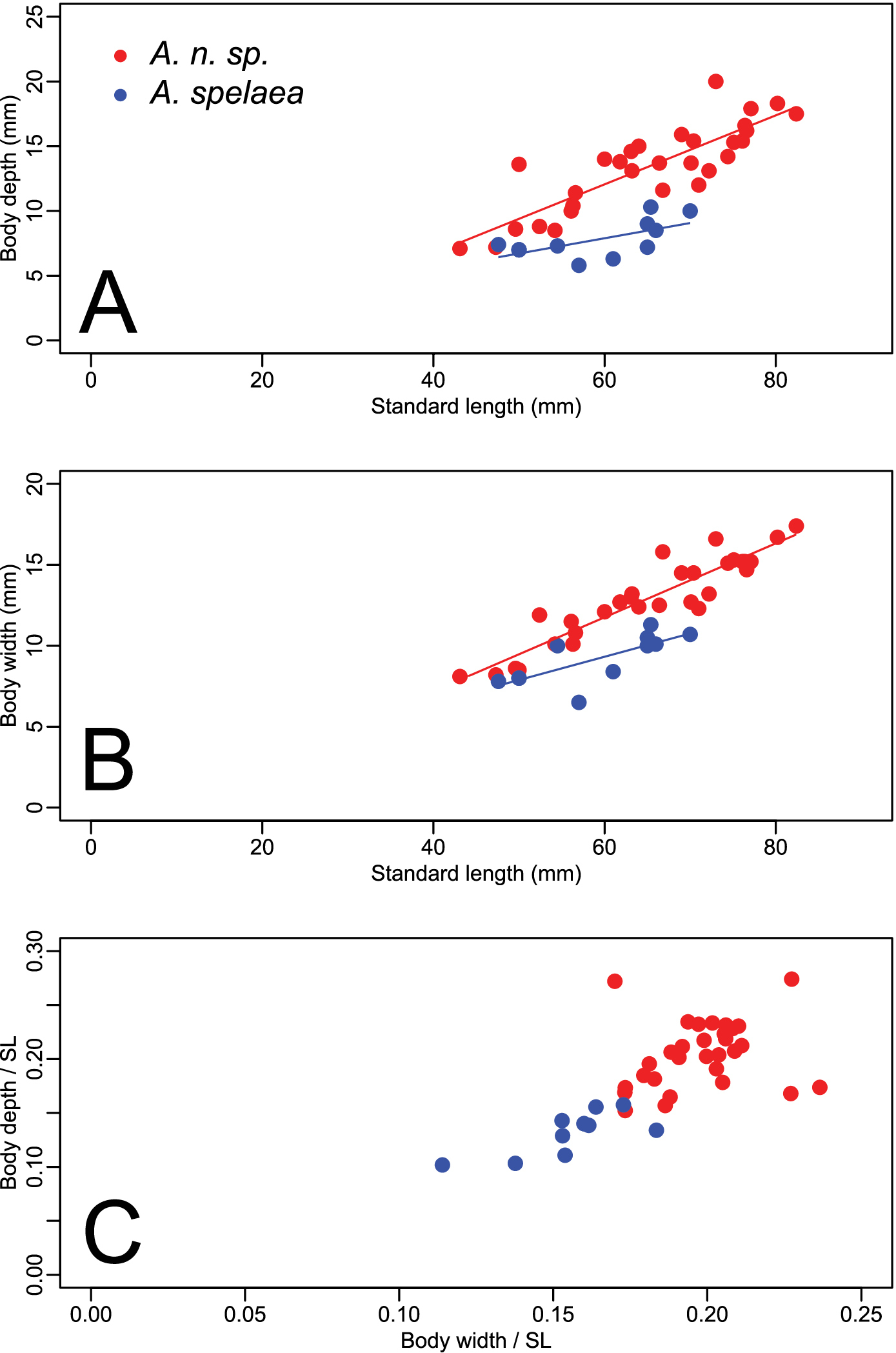
Forty-one specimens were examined, 30 from north of the Ohio River in Indiana, and 11 from south of the river in Kentucky. Specimens from Kentucky included the type locality of Amblyopsis spelaea, Mammoth Cave in Edmonson County. Figure 3 (A–C) shows the results of analyses of covariance (ANCOVA) comparing body depth and body width versus SL. Notably, Amblyopsis hoosieri has a deeper body compared to Amblyopsis spelaea (ANCOVA, p < 0.001; Fig. 3A, C). The body is also wider in Amblyopsis hoosieri compared to Amblyopsis spelaea (ANCOVA, p < 0.001; Fig. 3B, C). Except for juveniles (those under 50 mm), individuals of the new species are deeper and wider bodied than individuals of Amblyopsis spelaea (Fig. 3C). Given individuals of the same standard length, one would expect those of the new species to be much more robust.
Plots illustrating the relationships between body depth, body width and standard length (SL). Circles in red represent specimens of Amblyopsis hoosieri, sp. n., from north of the Ohio River in Indiana; triangles in blue represent Amblyopsis spelaea from south of the Ohio River in Kentucky. (a) body depth versus standard length, (b) body width versus standard length, and (c) and body depth versus body width as proportions of standard length.
http://zoobank.org/688BC3D5-7773-41E8-961F-67E3E4902BE2
http://species-id.net/wiki/Amblyopsis_hoosieri
Figures 4A, 5–7; Table 1Holotype. INHS 106675, Bronson’s Cave (White River Dr.) Spring Mill State Park, Lawrence County, Indiana, USA; 38°44', -86°25'; 9 December 1962, W.U. Bringham [formerly in lot 102504]
Paratypes. INHS 40424 (n=12 in ETOH; n=2 cleared and stained), Bronson’s Cave (White River Dr.) Spring Mill State Park, Lawrence County, Indiana, 5 April 1964, W.U. Brigham, G.W. Barlow & J. Mertz; INHS 60574 (n=1), Spring, (Lost River Dr.) Near West Baden, Orange County, Indiana, 18 January 1904, N.H. Haden; INHS 102504 (n=4), same data as holotype; LSUMZ 17419 (n=1), same as UMMZ 157175, formerly of that lot; LSUMZ 17420 (n=1), same data as UMMZ 65000, formerly of that lot; UMMZ 65000 (n=2), Twin Caves, near Mitchell, Lawrence County, Indiana, 17 May 1924, Hubbs & party; UMMZ 90379 (n=2), Sibert’s Well Cave, beside Wyandotte Cave, Indiana, 17 August 1930, P. Hickie; UMMZ 113550 (n=1), Lost River, Indiana, ca. 2 mi NE of Orangeville, September 1935, J.J. & W.P. Petravicz; UMMZ 114890 (n=2), Donaldson’s Cave, Spring Mills State Park, Lawrence County, 16 June 1934, A.E. Emerson; UMMZ 144604 (n=1), Stream in Sibert’s Well Cave, Wyandotte, Crawford County, Indiana, 01 October 1942, L. Hubricht; UMMZ 146992 (n=1), Stream in Sheep Cave, near Wyandotte, Crawford County, Indiana, 01 September 1939, , L. Hubricht; UMMZ 146994 (n=3), Stream in Bronson Cave, Spring Mill State Park, Lawrence County, Indiana, 2 September 1939, L Hubricht; UMMZ 157174 (n=1), “possibly” Donaldson farm caves near Indiana University, Indiana, C.H. Eigenmann; UMMZ 157175 (n=1), same as previous lot; UMMZ 157176 (n=1), same as previous lot; UMMZ 160944 (n=1), Twin Cave, Mitchell, Lawrence County, Indiana, 18 June 1924, F.N. Blanchard; YPM 25304 (n=2), Donaldson Cave, Spring Mill State Park, Lawrence County, Indiana, 21 December 2007, M.L. Niemiller et al.; YPM 25305 (n=1), Blue Springs Caverns, Lawrence County, Indiana, 21 December 2007, M.L. Niemiller et al. [Note tissue samples and published sequences are available from paratypes in YPM 25304 and 25305 (
Measurements and meristic counts of species of Amblyopsis. Standard length is in mm. Other measurements are percentages of standard length or head length. Values reported are means and ranges are in parentheses. For meristic counts, number of specimens with a count value is in parentheses.
| Character | Amblyopsis hoosieri (Indiana) | Amblyopsis spelaea (Kentucky) |
|---|---|---|
| N=30 | N=11 | |
| Standard length | 65.2 (43.1–82.4) | 59.2 (47.6–70.0) |
| Percentage of standard length | ||
| Head length | 34.1 (30.6–37.0) | 33.0 (30.0–37.2) |
| Body depth | 20.3 (15.2–27.2) | 13.2 (10–16.6) |
| Body width | 19.7 (17–23.7) | 15.5 (11.4–18.3) |
| Pectoral-fin length | 18.2 (11.9–24.0) | 24.0 (20.0–27.5) |
| Pelvic-fin length | 8.0 (5.6–9.7) | 8.4 (5.7–10.4) |
| Caudal-fin length | 19.9 (12.0–25.3) | 25.5 (19.1–29.4) |
| Dorsal-fin base | 12.0 (9.5–15.8) | 11.0 (7.5–12.8) |
| Anal-fin base | 10.8 (6.5–14.9) | 11.6 (6.6–14.0) |
| Caudal-peduncle length | 29.8 (27.0–34.7) | 30.0 (27.7–33.2) |
| Caudal-peduncle depth | 11.0 (9.4–12.9) | 9.7 (8.6–11.0) |
| Caudal-peduncle width | 6.1 (4.7–8.3) | 5.9 (4.9–6.7) |
| Predorsal length | 59.0 (50.6–64.1) | 60.3 (58.5–63.0) |
| Preanal length | 63.4 (58.6–67.2) | 59.6 (56.0–63.1) |
| Prepelvic length | 55.3 (50.5–59.7) | 51.8 (48.6–55.5) |
| Percentage of head length | ||
| Head width | 70.0 (51.5–82.4) | 65.0 (56.3–70.4) |
| Upper jaw length | 32.8 (27.2–37.5) | 33.4 (30.0–40.0) |
| Counts | ||
| Dorsal-fin rays | 11(1), 10(16), 9(11), 8(2) | 10(3), 9(8) |
| Anal-fin rays | 10(7), 9(21), 8(2) | 10(3), 9(8) |
| Vertebral Count | 30(16), 29(14) | 31(1), 30(9), 29(1) |
GenBank accession numbers for sequences from Amblyopsis hoosieri paratypes, which receive genseq-2 ranking in the classification of
| Gene | tbr1 | rag1 | s7 | nd2 | rhod |
|---|---|---|---|---|---|
| Specimen # | |||||
| YPM 25304.A | JX978106 | JX978036 | JX977966 | JX977896 | JX459497 |
| YPM 25304.B | JX978107 | JX978037 | JX977967 | JX977897 | JX459498 |
| YPM 25305 | JX978108 | JX978038 | JX977968 | JX977898 | JX459499 |
Amblyopsis hoosieri can be distinguished from its only congener, Amblyopsis spelaea, by having a more plump, fleshy and rounded body (versus sculpted and thin) with Bibendum-like wrinkles along myomeres (versus tight skin) and by having rounder pectoral fins (versus pointed; Figs 4–6). Additionally, the mechansensory papillae on the body and caudal fin are reduced in size and less elevated on the skin (versus conspicuous in Amblyopsis spelaea).
Comparative image of two similarly sized individuals of both species of Amblyopisis. Amblyopsis hoosieri, holotype, INHS 106675, 75.1 mm SL, Bronson’s Cave, Lawrence Co., Indiana (A); a specimen of Amblyopsis spelaea (YPM ICH 25294) of similar SL (67 mm SL) showing the more elongate and sculpted (versus plump) body, pointed fins, less prominent myomeres and more prominent papillae on the body (B).
Photograph of a paratype of Amblyopsis hoosieri in life, YPM ICH 25304, 60.7 mm SL. Photograph by M.L. Niemiller.
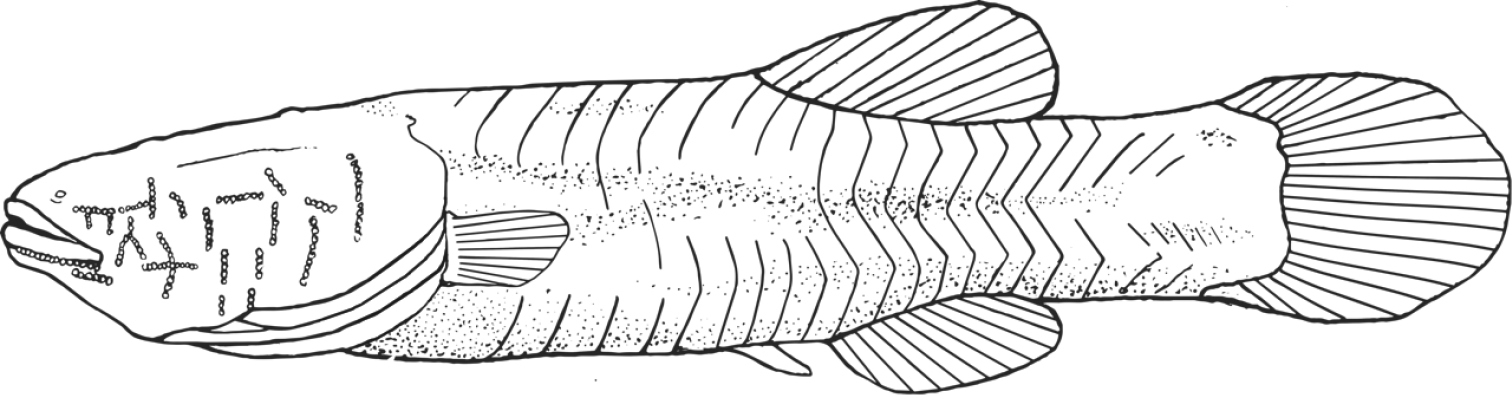
Illustration of Amblyopsis hoosieri based on the holotype, INHS 106675, 75.1 mm SL. Illustration by Nathan Coussou.
Average uncorrected pairwise genetic distance at the mitochondrial NADH dehydrogenase 2 (nd2) locus between Amblyopsis hoosieri and Amblyopsis spelaea is 3.1%, with 27 mutations separating the two species. Amblyopsis hoosieri and Amblyopsis spelaea can also be readily diagnosed using molecular data at the nuclear rhodopsin gene, a G-coupled photoreceptor expressed in the retina of the vertebrate eye. All rhodopsin sequences of Amblyopsis hoosieri code for the amino acid glutamine (Q) at position 184, whereas Amblyopsis spelaea possesses a point mutation that results in a premature stop codon at this position. In addition, Amblyopsis hoosieri rhodopsin codes for the amino acid valine (V) at position 254, whereas Amblyopsis spelaea codes for the amino acid phenylalanine (F). A single mutation in intron 1 of ribosomal protein S7 (s7) also distinguishes the two species.
Robust, blind (eye not developed, Fig. 7), unpigmented cavefish typically reaching between 60–80 mm in adult standard length. Head large (about ¼ body length) flat dorsally but broad; head widest part of body. Body widest at operculum, narrows to caudal fin. Body rectangular, dorsal and vertical profile of body nearly symmetrical; deepest point at dorsal-fin origin. Fleshy protuberance present anterior to dorsal-fin origin; similar protuberance at anal-fin origin. Body narrows posterior to dorsal- and anal-fin origins, narrowest point at midpoint of caudal peduncle. Body plump, wrinkly in appearance (as in Bibendum) prominent deep myomeres present. Deep groove on ventral side of body from operculum and anus to pelvic fin. Scales inconspicuous and cycloid.
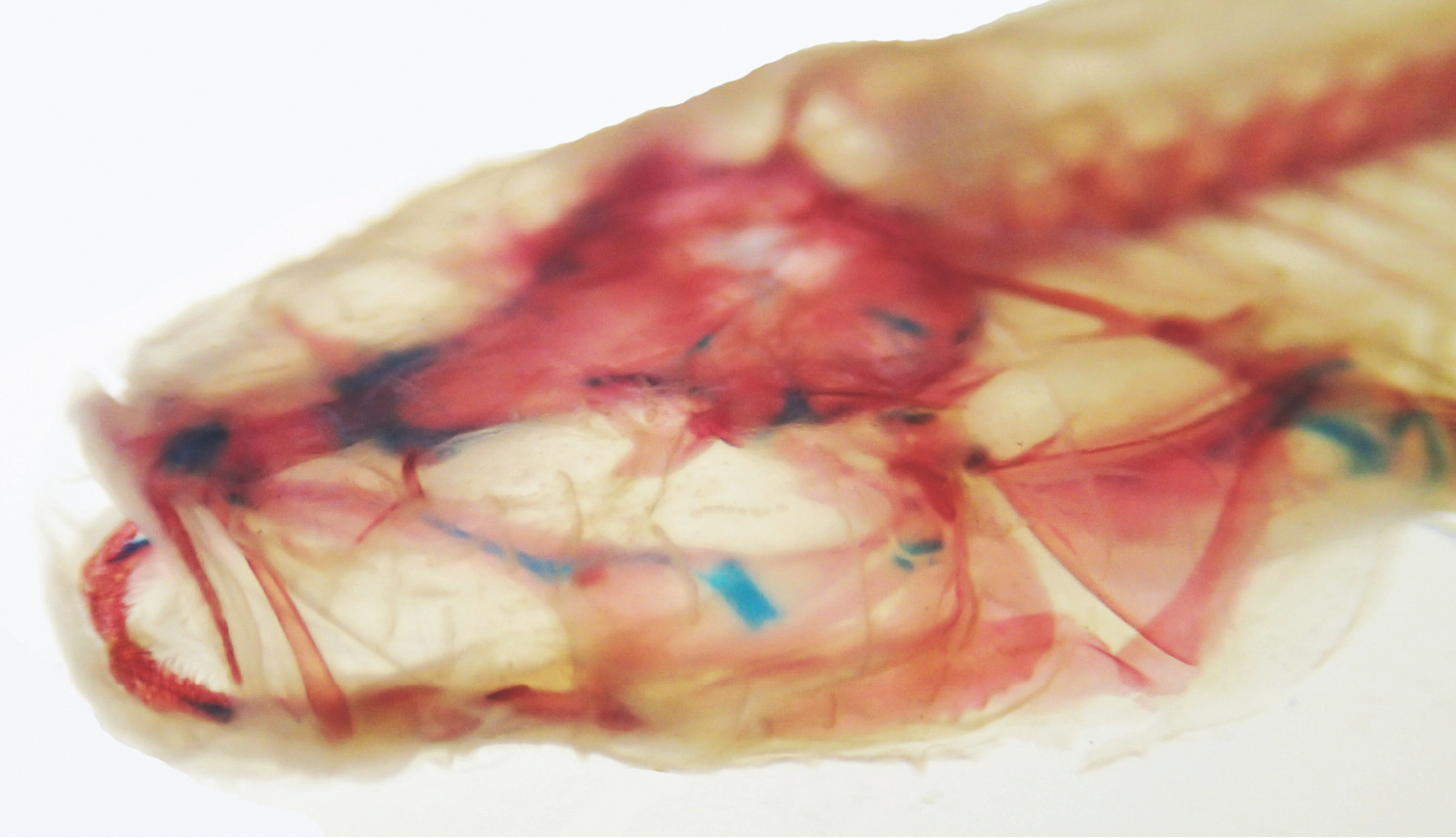
Cleared and stained image of the head of Amblyopsis hoosieri. Note lack of eye or a clearly defined bony orbit. Specimen is a paratype from INHS 40424, 71.3 mm SL.
Superficial mechanosensory neuromasts on papillae (
Anterior nares small, tube shaped; posterior nares slightly larger with small anterior flap, but otherwise circular. Lips somewhat thin and fleshy. Lower jaw slightly longer than upper jaw.
Vertical through dorsal-fin origin between more anterior pelvic-fin origin and more posterior anal-fin origin. Anal-fin and dorsal-fin insertions near same vertical plane. All fins relatively short and rounded. Anus located anteriorly on body, behind isthmus of united gill membranes (i.e., jugular). Caudal skeleton upturned and asymmetrical (externally appearing homocercal), with last half centrum (preural 1 + ural 1) including hypural (3-X; following
Branchiostegals six in number, robust and prominent. Papilliform flap at dorsal origin of operculum. Six or seven gill rakers on ceratobranchial of first gill arch. Rakers short, stubby and denticulated. Central and upper tooth plates also heavily denticulated.
Buccal teeth villiform, in three to five rows. Individual teeth unicuspid, slender and long; teeth deeply embedded in mouth so only top 1/3 visible. Teeth recumbent, particularly those on upper jaw. Palatine and vomerine teeth also present.
Body uniformly depigmented, including inside mouth. Body pinkish-white, reddish near gills, fins transparent. In alcohol, body color uniformly yellowish/beige, fins opaque yellow.
The specific epithet hoosieri is in reference to this species being from the state of Indiana. It is also a reference to Indiana University, where biologist Carl H. Eigenmann was a Professor of Zoology and studied blind cave vertebrates, including populations of Amblyopsis hoosieri in Lawrence County just to the south of Bloomington (
Amblyopsis hoosieri occurs in caves developed in carbonate rock of the Crawford-Mammoth Cave Uplands and Mitchell Plain in the South-Central karst region of Indiana (Fig. 2) within the area that remained ice free throughout the Pleistocene Epoch (
Amblyopsis hoosieri is found primarily in larger cave streams at or near the water table where it has been observed in pools with low flow at depths as shallow as 0.1 m to > 2 m deep. Amblyopsis cavefishes from Indiana have been found in association with silt-sand, gravel, cobble and bedrock substrates (
We describe a new species of North American cavefish, Amblyopsis hoosieri, that is distinguished from its sole congener Amblyopsis spelaea based on body and pectoral-fin shape and the absence of a stop codon in rhodopsin among other molecular and morphological features. In addition, the distributions of the two species of Amblyopsis are separated by the Ohio River, which has downcut through major cave-bearing rock strata and has subsequently isolated populations on the north (Amblyopsis hoosieri) and south (Amblyopsis spelaea) sides of the river. Cavefish diversity is certainly underestimated globally but perhaps particularly in North America: this is the first new cavefish species from the U.S. in 40 years.
Notably, previous cavefish researchers did not recognize this taxon as novel.
Molecular data have become an important tool to help identify cryptic or otherwise poorly recognized species level diversity, particularly among subterranean taxa (
Conservation status. Amblyopsis spelaea, and Amblyopsis hoosieri by extension, is considered endangered in Indiana because of presumed vulnerability to groundwater pollution and other perturbations of aquatic subterranean habitats. The species is considered “Endangered” (S1) in Indiana by
Potential threats to populations of Amblyopsis hoosieri are discussed in detail (for Amblyopsis spelaea) by
Doug Nelson and Bill Fink (University of Michigan Museum of Zoology), Greg Watkins-Colwell and Tom Near (Peabody Museum of Natural History, Yale University), Daniel B. Wylie and Chris A. Taylor (Illinois Natural History Survey) all provided specimens for this project. Jon Fong (California Academy of Science) graciously photographed what remains of the holotype of Typhlichthys wyandotte for us. Thanks to Caleb McMahan and Bill Ludt (LSU MNS) for assisting with loans, clearing and staining and for scientific advice related to the description.