Citation: Karanovic T, Kim K, Lee W (2014) Morphological and molecular affinities of two East Asian species of Stenhelia (Crustacea, Copepoda, Harpacticoida). ZooKeys 411: 105–143. doi: 10.3897/zookeys.411.7346
Definition of monophyletic supraspecific units in the harpacticoid subfamily Stenheliinae Brady, 1880 has been considered problematic and hindered by the lack of molecular or morphology based phylogenies, as well as by incomplete original descriptions of many species. Presence of a modified seta on the fifth leg endopod has been suggested recently as a synapomorphy of eight species comprising the redefined genus Stenhelia Boeck, 1865, although its presence was not known in S. pubescens Chislenko, 1978. We redescribe this species in detail here, based on our freshly collected topotypes from the Russian Far East. The other species redescribed in this paper was collected from the southern coast of South Korea and identified as the Chinese S. taiae Mu & Huys, 2002, which represents its second record ever and the first one in Korea. A fragment of the mtCOI gene was successfully PCR-amplified from two specimens of each species, which represents the first molecular data for this genus, and from additional 19 specimens belonging to six different species of other stenheliins from Korea and Russia. Reconstructed phylogenies confirm previously postulated monophyly of Stenhelia and polyphyly of the closely related genus Delavalia Brady, 1869. Average pairwise maximum likelihood distances between S. pubescens and S. taiae are only slightly above 10%, suggesting a very close relationship despite numerous newly discovered micro-morphological differences and despite macro-morphological similarities being probable plesiomorphies.
Miraciidae, Stenheliinae, marine, systematics, phylogeny, DNA barcoding
The subfamily Stenheliinae Brady, 1880 is currently recognised as one of three well-defined suprageneric groups within the second largest harpacticoid family Miraciidae Dana, 1846, beside the nominotypical subfamily and Diosaccinae Sars, 1906 (see
The most speciose and morphologically most diverse genus Delavalia is also taxonomically most problematic, and expectedly postulated to be either paraphyletic (
The genus Stenhelia was redefined recently by
Employing molecular techniques in addition to traditional morphological ones was one of the priorities of this study to aid in species delineation and reconstruction of their phylogenetic relationships. Recently, DNA-based species identification methods, referred to as “DNA barcoding”, have been widely employed to estimate levels of species diversity, with the 5’end of the mitochondrial cytochrome C oxidase subunit 1 gene (mtCOI) proposed as the “barcode” for all animal species (
All Korean samples for this study were taken at seventeen stations in Gwangyang Bay, on the South Coast of South Korea, on four occasions: 18 February 2012, 30 July 2012, 14 October 2012, and 18 November 2012 (see
Environmental conditions at 17 sampling stations in Gwangyang Bay, rescorded on 18 January 2006. Water temperature was measured on the surface. Granular analysis was conducted manually according to the protocol described by
| Station | Temperature (C) | pH | Sal. (ppt) | DO (mg/L) | Chlorophyl a | Cond. (mS/cm) | Granular analysis | Coordinates | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | ST | total | nano | gravel | sand | mud | ||||||
| St.01 | 5.9 | 7.0 | 8.1 | 33.3 | 11.5 | 4.6 | 2.2 | 32.8 | 0.0% | 9.8% | 90.2% | 34.913194°N, 127.600917°E |
| St.02 | 6.3 | 7.1 | 8.1 | 33.3 | 11.0 | 4.5 | 0.6 | 33.1 | 0.0% | 46.1% | 53.9% | 34.881861°N, 127.635083°E |
| St.03 | 5.1 | 7.0 | 7.9 | 33.4 | 12.5 | 5.2 | 2.9 | 32.8 | 1.9% | 37.0% | 63.0% | 34.884417°N, 127.664028°E |
| St.04 | 5.1 | 7.8 | 8.2 | 31.8 | 12.0 | 3.1 | 1.5 | 30.8 | 0.1% | 29.6% | 70.4% | 34.910722°N, 127.696806°E |
| St.05 | 6.0 | 7.3 | 7.3 | 33.4 | 10.8 | 8.9 | 8.9 | 32.9 | 0.0% | 19.7% | 80.3% | 34.852500°N, 127.684722°E |
| St.06 | 6.3 | 7.2 | 8.1 | 33.3 | 12.0 | 4.1 | 2.0 | 33.1 | 0.0% | 13.3% | 86.7% | 34.860861°N, 127.733417°E |
| St.07 | 6.4 | 8.3 | 8.2 | 33.4 | 12.3 | 6.7 | 1.4 | 33.3 | 0.0% | 13.7% | 86.3% | 34.897056°N, 127.757722°E |
| St.08 | 6.8 | 8.8 | 8.2 | 32.2 | 10.8 | 3.9 | 0.3 | 32.6 | 0.0% | 16.6% | 83.4% | 34.865417°N, 127.767222°E |
| St.09 | 5.9 | 7.3 | 7.5 | 27.1 | 12.9 | - | - | 27.2 | 0.0% | 25.4% | 74.6% | 34.951389°N, 127.734361°E |
| St.10 | 5.9 | 8.1 | 8.2 | 29.5 | 12.8 | 3.7 | 0.9 | 29.4 | 0.1% | 55.1% | 44.9% | 34.920944°N, 127.785528°E |
| St.11 | 7.7 | 8.1 | 7.9 | 33.4 | 10.1 | 0.5 | 0.4 | 34.4 | 0.0% | 31.0% | 69.0% | 34.924333°N, 127.852333°E |
| St.12 | 5.8 | 8.3 | 8.2 | 30.7 | 11.5 | 3.8 | 0.4 | 30.4 | 0.0% | 67.0% | 33.0% | 34.890139°N, 127.795111°E |
| St.13 | 6.6 | 9.2 | 8.1 | 33.2 | 11.5 | 5.6 | 1.5 | 33.3 | 0.4% | 73.3% | 26.7% | 34.852750°N, 127.791000°E |
| St.14 | 6.6 | 8.1 | 8.2 | 33.3 | 10.9 | 5.0 | 3.8 | 33.3 | 0.0% | 46.6% | 53.4% | 34.824222°N, 127.787750°E |
| St.15 | 6.9 | 7.7 | 8.2 | 33.6 | 10.8 | 3.2 | 1.1 | 33.9 | 0.3% | 60.5% | 39.5% | 34.797194°N, 127.786444°E |
| St.16 | 6.7 | 7.5 | 8.2 | 33.8 | 10.9 | 6.6 | 3.5 | 34.0 | 2.5% | 33.7% | 66.3% | 34.768889°N, 127.783806°E |
| St.17 | 6.2 | 7.7 | 8.2 | 33.8 | 10.5 | 4.4 | 1.6 | 33.5 | 0.0% | 37.0% | 63.0% | 34.743444°N, 127.778972°E |
Specimens were dissected and mounted on microscope slides in Faure’s medium (see
Morphological terminology follows
Specimens for molecular analysis were examined without dissection under a compound microscope (objective 63× dry) in propylene glycol, using a cavity well slide with a central depression. After examination they were returned to 99.9% ethanol. Before amplification whole specimens were transferred into distilled water for two hours for washing (to remove ethanol), and then minced with a small glass stick. DNA was extracted from whole specimens, except in one case when only one antennula was available, using the LaboPassTM extraction kit (COSMO Co. Ltd., Korea) and following the manufacturer’s protocols for fresh tissue, except that samples were incubated in the Proteinase K solution overnight, step five was skipped, and 60 instead of 200 μl of Buffer AE was added in the final step, to increase the density of DNA. Mitochondrial cytochrome oxidase subunit I (mtCOI) gene was amplified through polymerase chain reaction (PCR) using PCR premix (BiONEER Co.) in TaKaRa PCR thermal cycler (Takara Bio Inc., Otsu, Shiga, Japan). The amplification primers used were the ‘universal’ primers LCO1490 and HCO2198 (
List of copepod specimens for which mtCOI fragment was successfully amplified.
| Code | Species | Country | Station | Date | Bases | GenBank |
|---|---|---|---|---|---|---|
| 0330 | Itostenhelia golikovi | Russia | Posyet Bay | 06 May 2012 | 448 | KF524863 |
| 0433 | Itostenhelia golikovi | Russia | Posyet Bay | 06 May 2012 | 515 | KF524864 |
| 0631 | Itostenhelia golikovi | Russia | Posyet Bay | 06 May 2012 | 514 | KF524865 |
| 0734 | Itostenhelia golikovi | Russia | Posyet Bay | 06 May 2012 | 503 | KF524866 |
| 0832 | Itostenhelia golikovi | Russia | Posyet Bay | 06 May 2012 | 493 | KF524867 |
| 0176 | Itostenhelia polyhymnia | Korea | 10 | 30 Jul 2012 | 660 | KF524868 |
| 0273 | Itostenhelia polyhymnia | Korea | 10 | 30 Jul 2012 | 664 | KF524869 |
| 0271 | Itostenhelia polyhymnia L-form | Korea | 10 | 30 Jul 2012 | 278 | KF524883 |
| 8417 | Schizopera leptafurca | Australia | YYAC0016A | 20 Mar 2010 | 517 | JQ390578 |
| 0152 | Stenhelia pubescens | Russia | Posyet Bay | 06 May 2012 | 659 | KF524870 |
| 0254 | Stenhelia pubescens | Russia | Posyet Bay | 06 May 2012 | 647 | KF524871 |
| 0163 | Stenhelia taiae | Korea | 16 | 18 Nov 2012 | 558 | KF524884 |
| 0167 | Stenhelia taiae | Korea | 16 | 18 Nov 2012 | 662 | KF524885 |
| 0122 | Wellstenhelia calliope | Korea | 5 | 30 Jul 2012 | 576 | KF524872 |
| 0187 | Wellstenhelia clio | Korea | 10 | 30 Jul 2012 | 519 | KF524873 |
| 0113 | Wellstenhelia qingdaoensis | Korea | 15 | 18 Nov 2012 | 518 | KF524874 |
| 0143 | Willenstenhelia thalia | Korea | 10 | 30 Jul 2012 | 657 | KF524875 |
| 0146 | Willenstenhelia thalia | Korea | 10 | 18 Nov 2012 | 664 | KF524878 |
| 0241 | Willenstenhelia thalia | Korea | 10 | 30 Jul 2012 | 524 | KF524876 |
| 0245 | Willenstenhelia thalia | Korea | 10 | 18 Nov 2012 | 662 | KF524879 |
| 0342 | Willenstenhelia thalia | Korea | 10 | 30 Jul 2012 | 330 | KF524877 |
| 0348 | Willenstenhelia thalia | Korea | 10 | 18 Nov 2012 | 660 | KF524880 |
| 0444 | Willenstenhelia thalia | Korea | 10 | 18 Nov 2012 | 667 | KF524881 |
| 0547 | Willenstenhelia thalia | Korea | 10 | 18 Nov 2012 | 661 | KF524882 |
Obtained sequences were checked manually and aligned by ClustalW algorithm (
Stenhelia (Stenhelia) pubescens Chislenko, sp. n. –
Russia, Primorsky Krai, Sea of Japan, Posyet Bay, Minonosok inlet, benthic sands at 3-4 m depth, 42.609258°N, 130.861661°E.
Two females (one ovigerous) together on one SEM stub (collection number NIBRIV0000232715), one female dissected on one slide (collection number NIBRIV0000232716), one female in ethanol (collection number NIBRIV0000232717), and two ovigerous females destroyed for DNA sequences (GenBank accession nos. KF524870 & KF524871); all from type locality, 6 May 2012, leg. Y. Trebukhova.
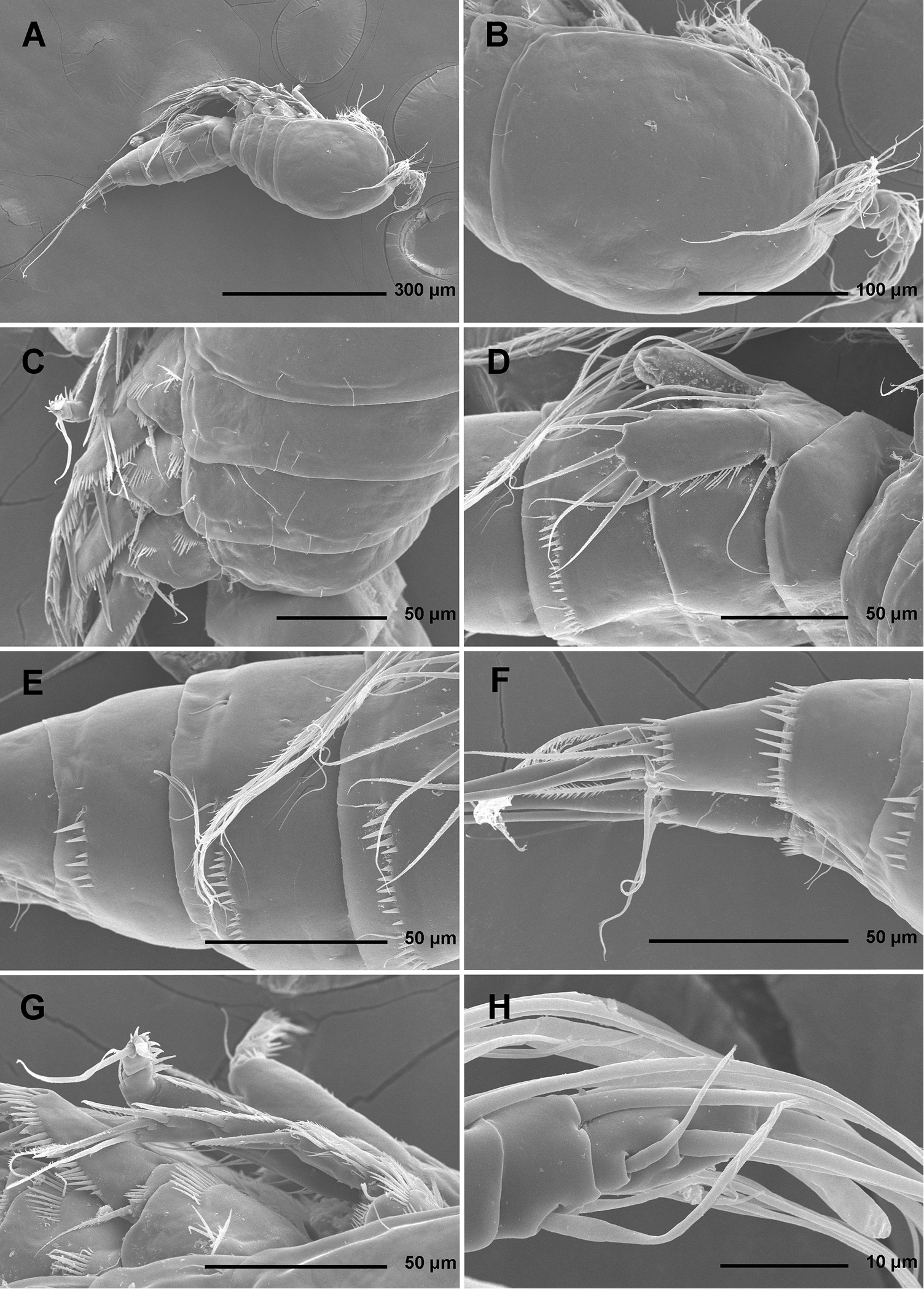
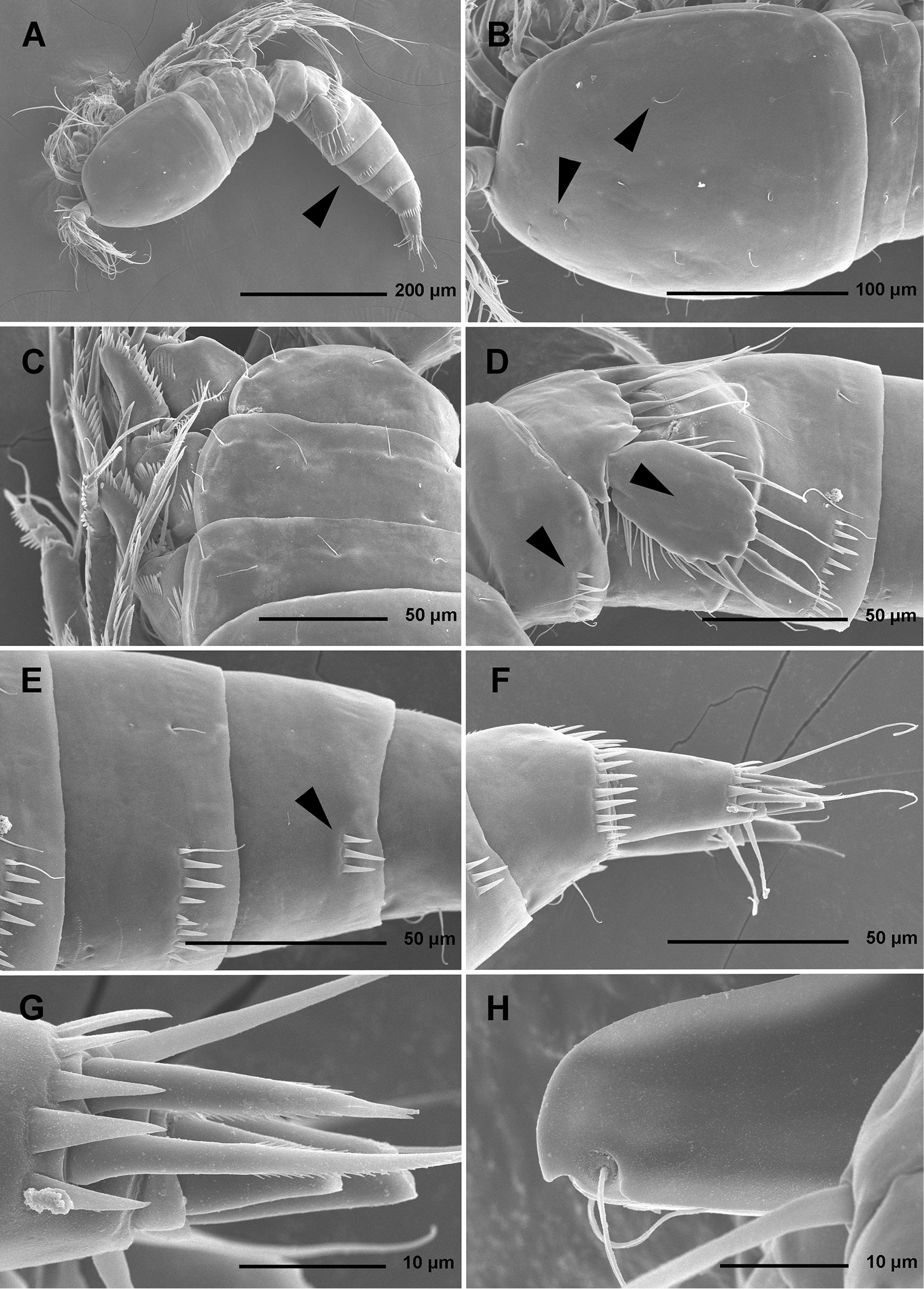
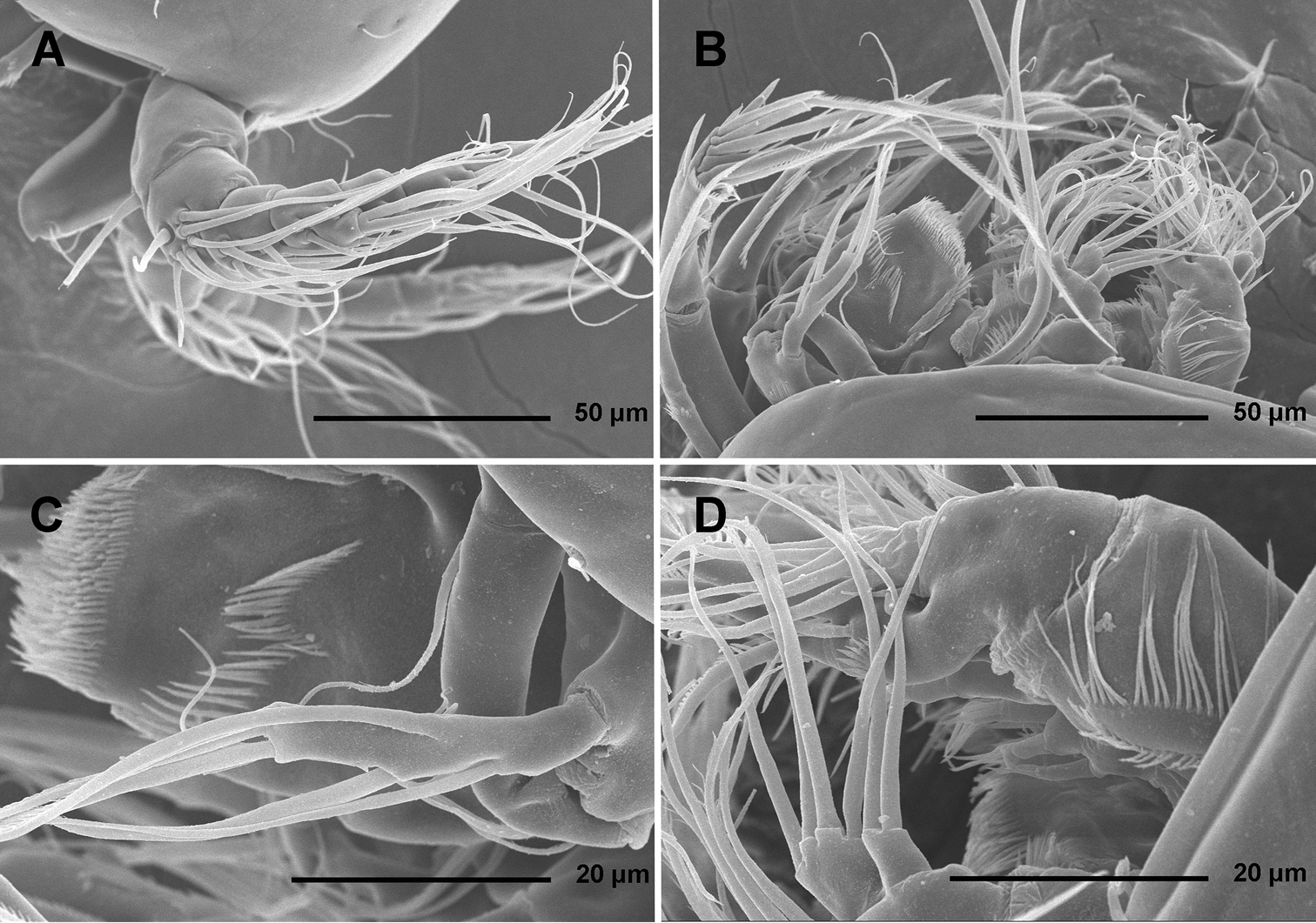
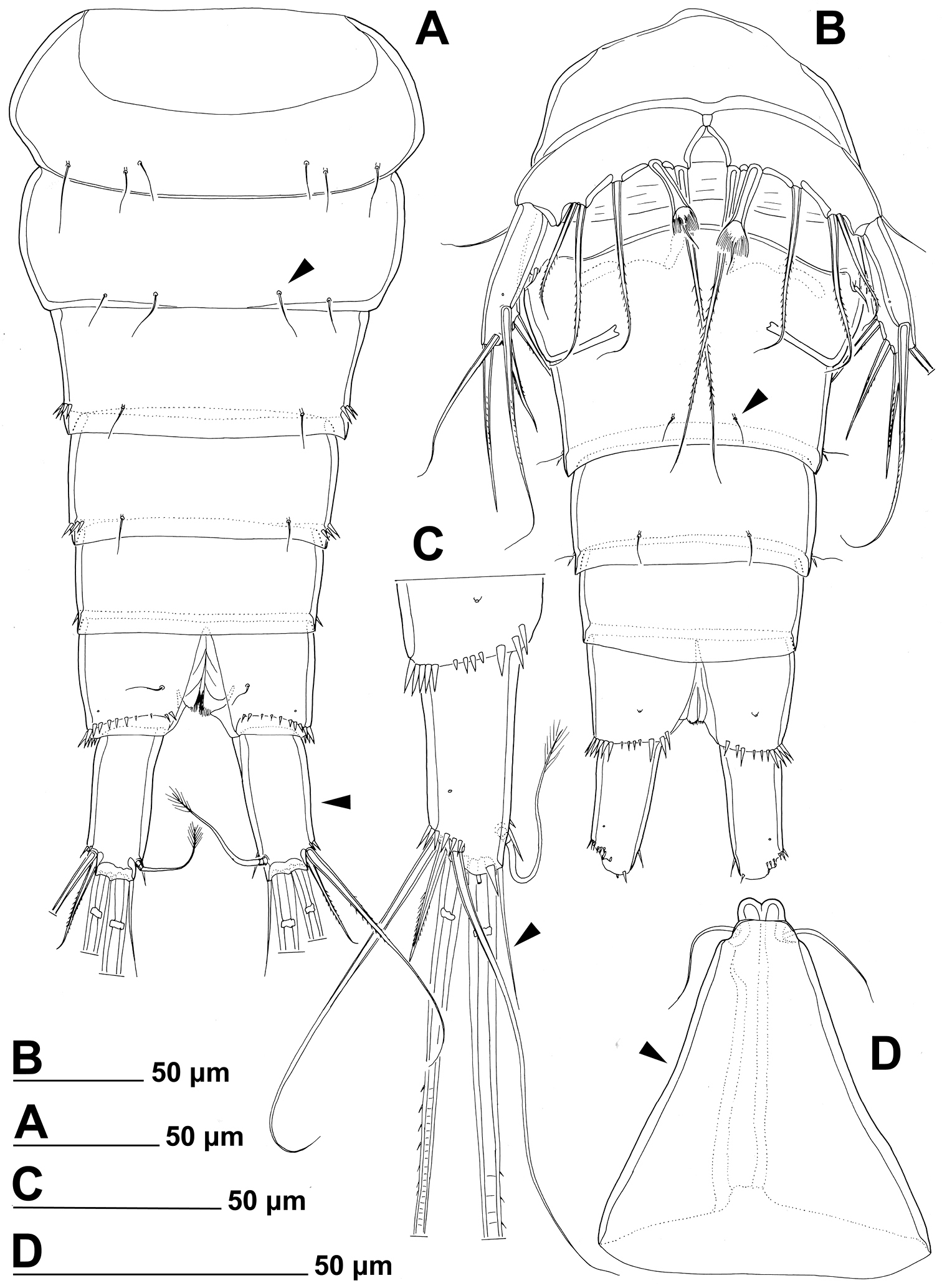
Total body length, measured from tip of rostrum to distal margin of caudal rami, from 558 to 583 μm (n = 6). Colour of preserved specimens yellowish; live specimens not observed. Nauplius eye not visible. Several filamentous bacterial colonies in various places, some resembling sensilla (see Fig. 1C). Prosome comprising cephalothorax with completely fused first pedigerous somite, and three free pedigerous somites; urosome comprising first urosomite (= fifth pedigerous somite), genital double-somite (fused genital and third urosomites) and three free urosomites (last one being anal somite). Short sclerotized joint between prosome and urosome only discernible on ventral side. Habitus (Figs 1A, 2A) robust, spindle shaped in dorsal view, widest at posterior end of cephalothorax and tapering posteriorly, boundary between prosome and urosome conspicuous; prosome/urosome length ratio about 1.2, but prosome much wider and more voluminous. Body length/width ratio about 2.9; cephalothorax 1.65 times as wide as genital double-somite. Free pedigerous somites without lateral or dorsal expansions, pleurons only partly covering coxae of legs in lateral view (Fig. 1C). Integument of all somites relatively weakly sclerotized, generally very smooth, without cuticular windows or pits. Hyaline fringe of all somites broad and smooth, except for fourth pedigerous somite with narrow fringe dorsally, and for anal somite without hyaline fringe. Surface ornamentation of somites and caudal rami consisting of three unpaired dorsal pores, 61 paired pores and sensilla, and posterior row of spinules on last four urosomites only.
Stenhelia pubescens Chislenko, 1978, scanning electron micrographs, female 1: A habitus, lateral B cephalothorax, lateral C free thoracic somites, lateral D fifth pedigerous somite and genital double-somite, lateral, with one spermatophore attached on ventral side E fourth and fifth urosomites, lateral F anal somite and caudal rami, lateral G first legs and proximal part of second and third legs, lateral H distal part of right antennula, dorsal.
Stenhelia pubescens Chislenko, 1978, scanning electron micrographs, ovigerous female 2: A habitus, ventral B rostrum and left antennula, ventral C mouth appendages, ventral D first leg, anterior E second, third, and fourth legs, anterior F exopod of fifth leg and sixth leg, ventral G anal somite and caudal rami, ventral H posterior part of left caudal ramus, ventral.
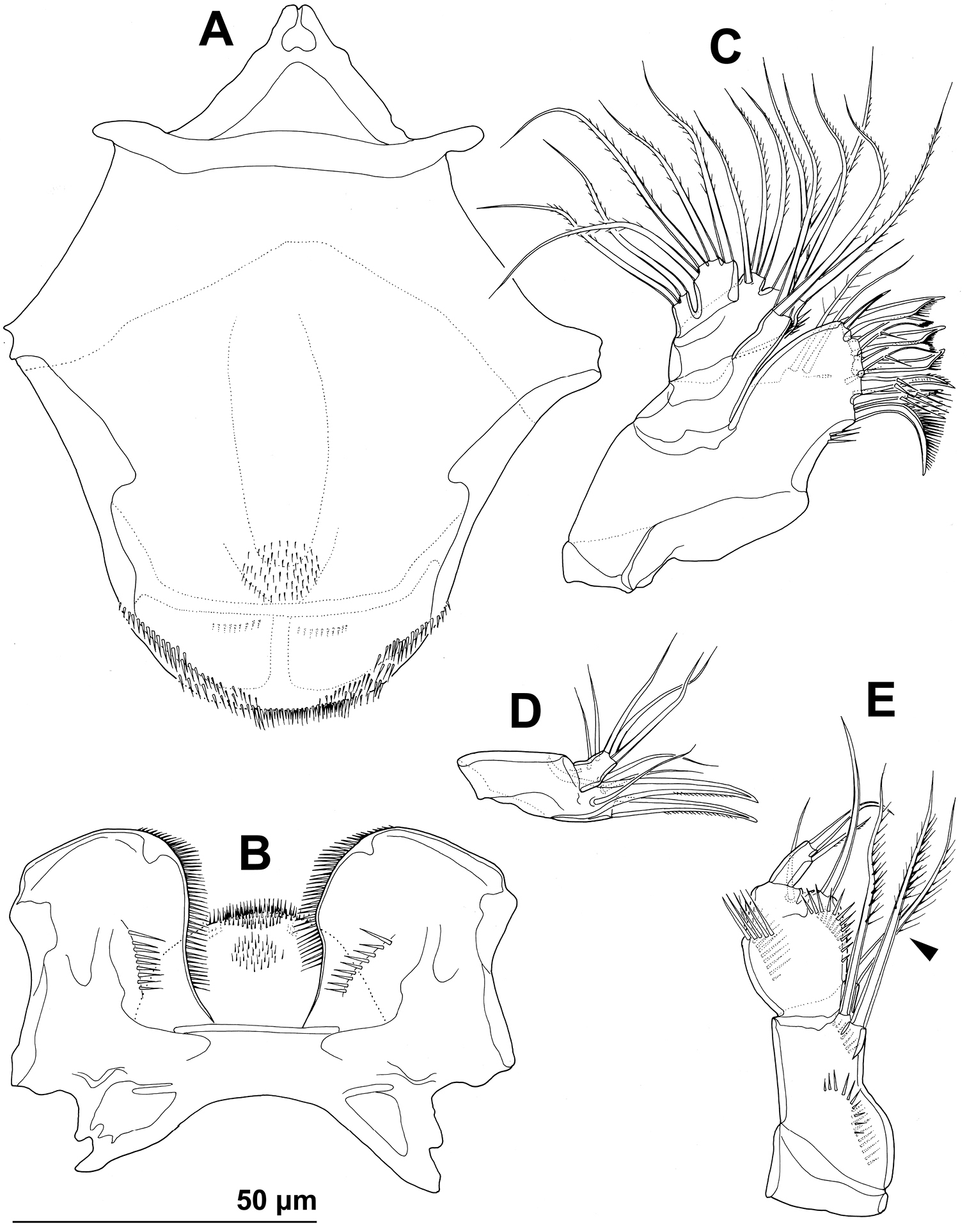
Rostrum (Figs 1B, 2B, 3C) large, trapezoidal, clearly demarcated at base, reaching midlength of second antennular segment, with bilobate tip, about as long as wide, with smooth dorsal surface and central keel on ventral surface, with two large lateral sensilla near tip inserted into deep recesses.
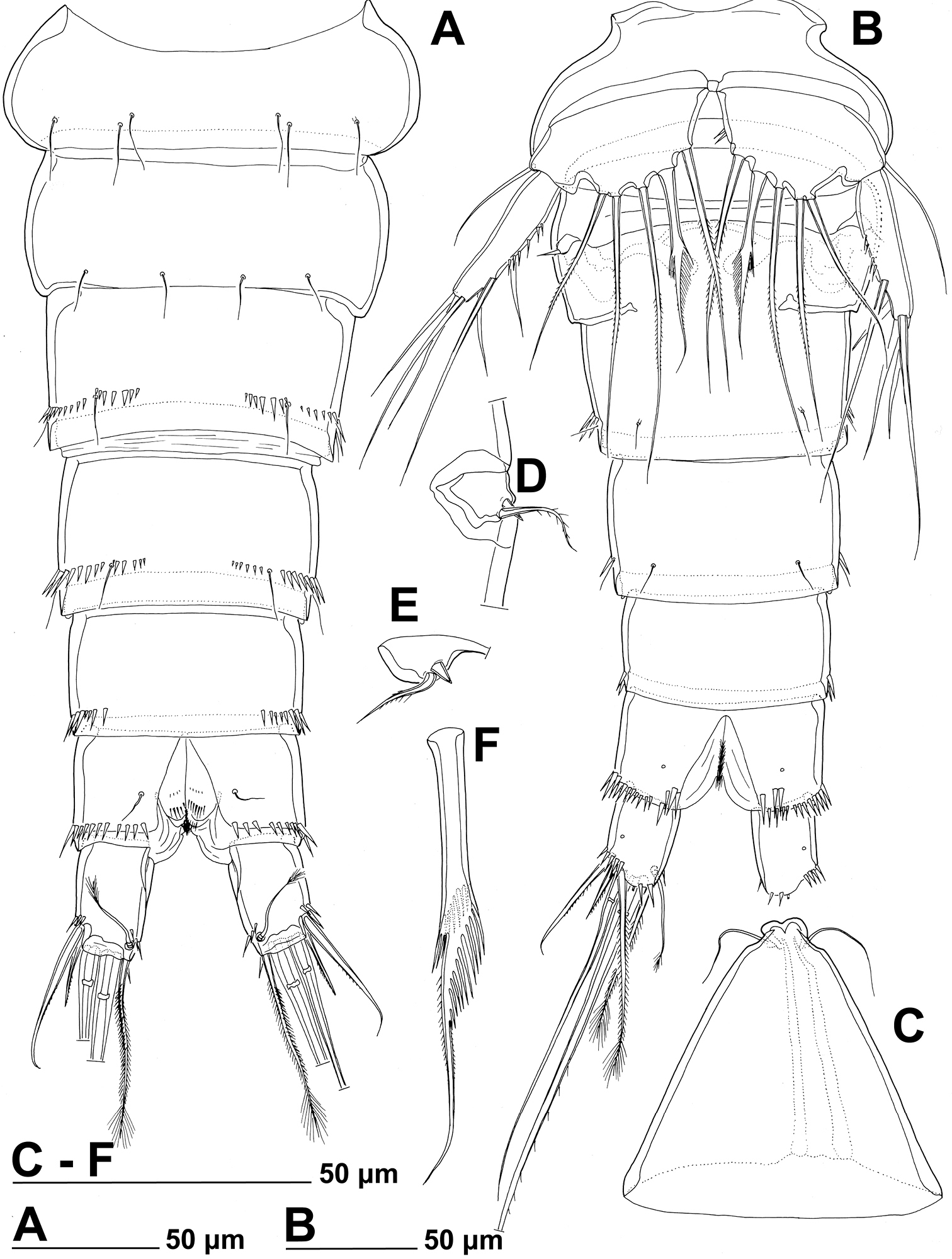
Stenhelia pubescens Chislenko, 1978, line drawings, female 3: A urosome, dorsal B urosome, ventral (armature on left caudal ramus omitted) C rostrum, dissected and compressed, dorsal D sixth leg, dorso-lateral E sixth leg, ventro-lateral F fifth leg second endopodal seta from inner side, anterior.
Cephalothorax (Figs 1B, 2A, B, D) tapering anteriorly in dorsal view, about as long as wide; comprising 35% of total body length. Surface of cephalothoracic shield with three pairs of small pores near antero-ventral corner between antennula and antenna (Fig. 2B), one dorsal unpaired pore in anterior half, and 25 pairs of long sensilla (Fig. 1B); of those only eight pairs of sensilla belonging to first pedigerous somite incorporated into cephalothorax (Figs 1B, 2D)
Pleuron of second pedigerous somite (first free) (Fig. 1C) with nearly rectangular lateral section, without pores but with seven pairs of large sensilla, two of them near lateral margin; serial homologies with sensilla on posterior part of cephalothorax (belonging to first pedigerous somite) difficult to define, except perhaps for anterior lateral sensilla and two other posterior pairs.
Pleuron of third pedigerous somite (Fig. 1C) somewhat shorter than that of second pedigerous somite and with slightly more rounded lateral section, but also with no pores and with seven pairs of large sensilla; recognising sensilla serially homologous to those on pleuron of second pedigerous somite easy for all seven pairs.
Pleuron of fourth pedigerous somite (Fig. 1C) much shorter and with more rounded lateral section than those of previous two somites, especially narrow in dorsal view, with only five pairs of large sensilla; serial homology of sensilla to those on two previous somites relatively difficult to establish, but probably two dorsal pairs homologous to two dorsalmost pairs on pleuron of third pedigerous somite and two lateral pairs homologous to those near lateral margin on two previous somites.
First urosomite (Figs 1D, 3A, B) about as long and as wide as fourth pedigerous somite but with wider hyaline fringe, with only three dorso-lateral pairs of long sensilla and no pores or spinules.
Genital double-somite (Figs 1D, 3A, B) about 1.2 times as wide as long (ventral view); completely fused ventrally but with deep suture indicating original segmentation between genital and third urosomites dorso-laterally, thus dividing double-somite into equally long halves; anterior half of genital double-somite 1.2 times as wide as posterior, inflated laterally; anterior part with one unpaired dorsal pore and two pairs of long dorsal sensilla; serially homologous sensilla of anterior part of double-somite and those of first urosomite not easy to establish; posterior part with three pairs of posterior sensilla (one dorsal, one lateral, and one ventral) and long row of posterior dorso-lateral spinules of various length; establishing serially homologous sensilla of posterior and anterior part of double-somite not easy; hyaline fringe wider than in first urosomite. Female genital complex (Fig. 3B) weakly sclerotized and hardly distinguishable from internal sutures and soft tissue, copulatory pores not exposed on surface but their position could be deduced from attached spermatophores (Fig. 1D); paired genital apertures situated ventro-laterally, close to anterior margin and covered by reduced sixth legs.
Third urosomite (Figs 1E, 3A, B) slightly narrower than posterior half of gential double-somite, but about as long and ornamented very similarly with three pairs of posterior sensilla and posterior row of spinules of various size, interrupted dorsally and ventrally; all sensilla with homologous pairs on posterior half of genital double-somite; hyaline fringe as wide as in genital double-somite.
Fourth urosomite (preanal) (Figs 1E, 3A, B) without sensilla or pores, only ornamentation posterior row of spinules with wider dorsal and ventral interruption than in previous two somites; hyaline fringe slightly narrower than in third urosomite.
Fifth urosomite (anal) (Figs 1F, 2G, 3A, B) clefted medially in posterior half, without anal operculum, with one pair of large dorsal sensilla, one pair of ventral pores, and posterior row of spinules at base of each caudal ramus; anal sinus with several diagonal rows of hair-like spinules on both sides of median cleft, widely open, with weakly sclerotised walls, and without chitinous projections.
Caudal rami (Figs 1F, 2G, H, 3A, B) short and slender, cylindrical, about as long as anal somite, 1.5 times as long as wide (dorsal view), slightly divergent, with space between them about one ramus width; armature consisting of seven setae (three lateral, one dorsal and three apical), all in posterior sixth of ramus length; ornamentation consisting of one ventral pore at midlenght, one posterior ventral tubular pore, several spinules at base of each lateral seta and at base of dorsal seta, and two large posterior ventral spinules at base of innermost apical seta. Dorsal seta slender, plumose at distal tip, inserted close to inner margin, about 1.2 times as long as caudal ramus, triarticulate at base (i.e. inserted on two pseudojoints). Lateral setae all bipinnate and uniarticulate; ventralmost one longest and most slender, with distal tuft of longer pinnules, inserted very close to distal margin, about 1.3 times as long as caudal ramus; dorsalmost one strongest, without distal tuft of long pinnules, about 0.8 times as long as ventralmost one, inserted slightly more anteriorly than ventralmost one, at about same level as dorsal seta; central one half as long as dorsalmost one, also strong, inserted at about same level, also without distal tuft of long pinnules. Inner apical seta only slightly shorter than ventralmost lateral seta but very similar in thickness and ornamentation, i.e. also with distal tuft of long pinnules. Principal apical setae not fused basally, both with breaking planes; middle apical seta much stronger and longer, about 2.2 times as long as outer apical one, bipinnate; outer apical seta smooth, about 3.8 times as long as caudal ramus.
Antennula (Figs 1H, 2B, 5A) eight-segmented, joined to cephalotholax with small triangular cuticular plate, about half as long as cephalothorax, with single short anterior row of spinules on first segment. Fourth segment sometimes with suture along caudal margin. Distal caudal corner of first segment not produced. Long aesthetasc on fourth segment slender, fused basally with adjacent large seta, and reaching beyond tip of appendage; slender short apical aesthetasc on eighth segment fused basally with two apical setae, forming apical acrothek. Setal formula: 1.11.9.6+ae.3.4.4.6+ae. All setae smooth, dorsalmost setae on second segment with breaking plane, two caudal setae on seventh segment and four caudal setae on eight segment biarticulate. Length ratio of antennular segments, measured along caudal margin, 1 : 0.4 : 0.3 : 0.4 : 0.3 : 0.4 : 0.4 : 0.5.
Antenna (Figs 2C, 5B, C) relatively short, composed of coxa, allobasis, one-segmented endopod and three-segmented exopod. Coxa short, with arched row of long posterior spinules. Allobasis with smaller or bigger suture marking ancestral division between basis and first endopodal segment, most robust segment of antenna, more than four times as long as coxa and about as long as endopod, widest at base and about 2.5 times as long as wide, with single unipinnate inner seta at about midlength and several longer and smaller spinules in proimal half. Endopod about as wide as distal part of allobasis, almost cylindrical, about 3.6 times as long as wide, with two surface frills subdistally, row of large spinules all along anterior margin, two lateral spines flanking two thin setae, apical armature consisting of seven pinnae setae (four strong, long, and geniculate, innermost one strong but short, and two short and slender); two caudalmost setae fused basally. Exopod long and slender, almost cylindrical, about as long as allobasis but only half as wide; armature formula 1.1.4 and length ratio of segments 1 : 0.3 : 1.1; proximal segment with transverse distal row of small anterior spinules, bearing a unipinnate seta close to distomedial corner; second segment unornamented, with a unipinnate setae at distomedial corner; distal segment with two parallel longitudinal anterior rows of small spinules joining at distal margin, with one bipinnae inner seta, at about first third of its length, and three apical slender (two smooth and one bipinnate).
Labrum (Fig. 2C) large and complex tri-dimensional structure, trapezoidal in anterior view, rigidly sclerotized, with relatively wide convex cutting edge, subapically and apically with several rows of short slender spinules, with one additional transverse row of small anterior spinules and another patch of small posterior spinules.
Paragnaths (Fig. 2C) also forming complex tri-dimensional structure, trilobate, with two ellipsoid anterior lobes and one central, much shorter posterior lobe, all lobes fused at base; anterior lobes with one long row of slender spines along inner margin and one additional and parallel row of stronger spinules on anterior surface; posterior (central) lobe similar in shape and ornamentation to distal part of labrum but much smaller.
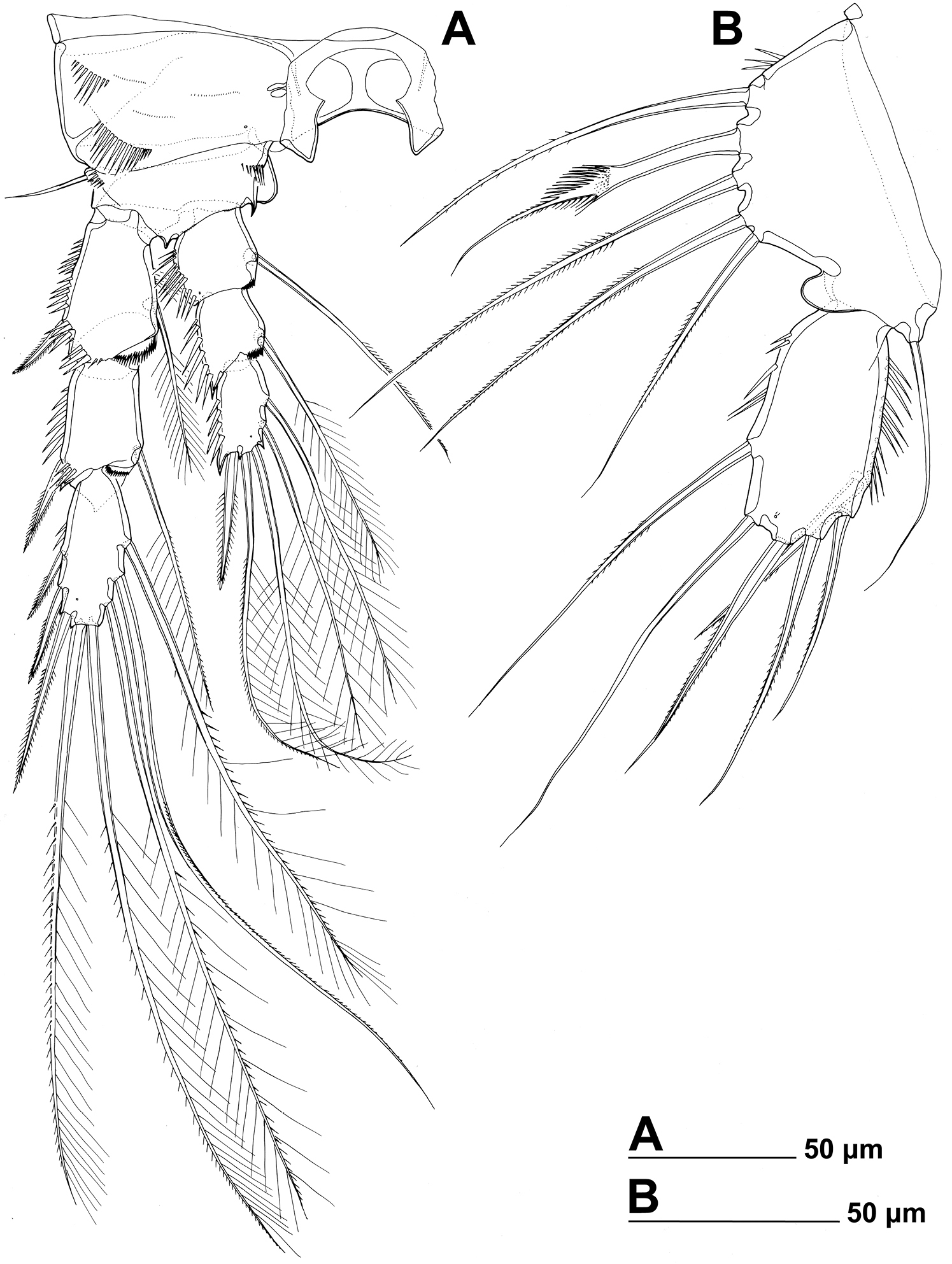
Mandibula (Figs 2C, 4A) with wide cutting edge on relatively short coxa, with three strong bicuspidate teeth ventrally, eight smaller unicuspidate teeth dorsally, and single unipinnate dorsalmost seta; seta fused basally to neighbouring tooth and twice as long as it; only ornamentation on coxa short row of six slender posterior spinules. Palp biramous, comprising basis, one-segmented exopod, and one-segmented endopod. Basis with somewhat inflated central part, about 2.5 times as long as wide, with three slender but pinnate distal outer setae, and with three transverse rows of strong spinules, distalmost one with strongest spinules. Exopod 0.6 times as long as basis and less than half as wide, narrowest medially, curved back towards coxa and almost parallel with basis, with three lateral and five apical setae; all lateral and three apical setae slender, two apical setae strong and geniculate, longer one of them almost four times as long as exopod; two apical setae unipinnate, all other exopodal setae smooth. Endopod 0.8 times as long as exopod, 3.8 times as long as wide, with one inner, three apical, and two outer slender setae; inner seta bipinnate, proximal outer and inner apical setae unipinnate, others smooth.
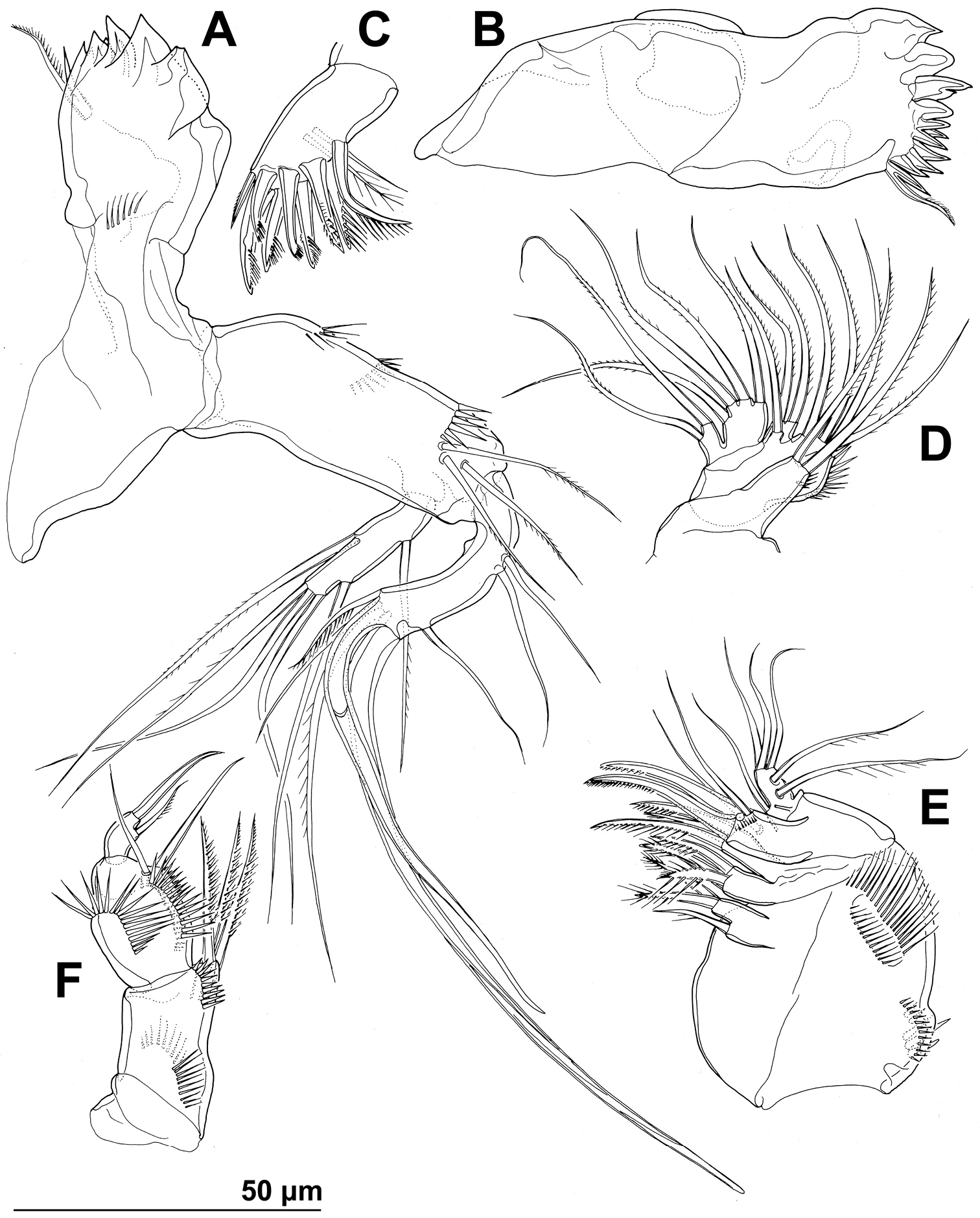
Stenhelia pubescens Chislenko, 1978, line drawings, female 3: A mandibula, posterior B mandibular coxa, anterior C maxillula, praecoxa arthrite, posterior D maxilular palp, posterior E maxilla, anterior F maxilliped, anterior.
Maxillula (Figs 2C, 4C, D) composed of praecoxa, coxa, basis, one-segmented endopod, and one-segmented exopod; endopod and exopod fused basally. Praecoxa large; arthrite rectangular, without spinules, with nine strong curved spines apically and subapically, all except ventralmost spine with dense tuft of distal spinules along convex margin; dorsalmost spine on praecoxal arthrite longest, ventralmost one shortest. Coxa with anterior arched row of short spinules, endite shorter than praecoxal arthrite, with three slender pinnate apical (on inner margin) setae. Basis wider and longer than coxa, with two endites, with dorsal row of strong spinules and three unipinnate setae on dorsal endite, and another three unipinnate setae on ventral endite. Endopod minute, rectangular, with four slender bipinnate apical setae. Exopod smaller than endopod, with two slender bipinnate apical setae.
Maxilla (Figs 2C, 4E) composed of large syncoxa, small basis and even smaller one-segmented endopod. Syncoxa with four rows of outer long spinules and with three endites; dorsal endite smallest, with one subapical and two apical strong pinnate setae; central and ventral endites slender, with three apical pinnate setae each, setae on ventral endite longest; two distal rows of spinules parallel on anterior surface, two proximal rows of spinules near outer margin, one on anterior, one on posterior surface, posterior distal surface smooth. Basis slightly larger than ventral endite of syncoxa, with anterior row of minute spinules, apically with two strong and geniculate, unipinnate spines, and two slender setae on ventral and posterior surfaces. Endopod much smaller than basis, twice as long as wide, with basal tubular pore, no spinules, with three lateral and three apical slender setae of similar length; two lateral setae unipinnate, others smooth.
Maxilliped (Figs 2C, 4F) prehensile, four-segmented, composed of coxa, basis, and two-segmented endopod. Coxa short, almost triangular, unarmed and unornamented. Basis largest and longest segment, about 1.8 times as long as wide and nearly five times as long as coxa, with one arched posterior row and two longitudinal anterior rows of slender spinules, with three strong unipinnate distomedial setae of about same length. First endopodal segment 0.8 times as long as basis but slightly wider, almost ovoid in shape, also with one posterior and two anterior rows of spinules but spinules much longer and stronger, with two smooth distomedial setae, one of them slightly longer and considerably stronger. Second endopodal segment minute, nearly rectangular, 1.6 times as long as wide, 0.4 times as long as first endopodal segment, unornamented, with apical strong prehensile smooth spine, and with subapical shorter and much more slender, unpinnate seta.
All swimming legs (Figs 1A, 2A) of similar size and long in comparison to body length, composed of small triangular and unarmed praecoxa, large rectangular and unarmed coxa, shorter and nearly pentagonal basis, slender three-segmented exopod, and slender three-segmented endopod; pair of legs joined by simple intercoxal sclerite.
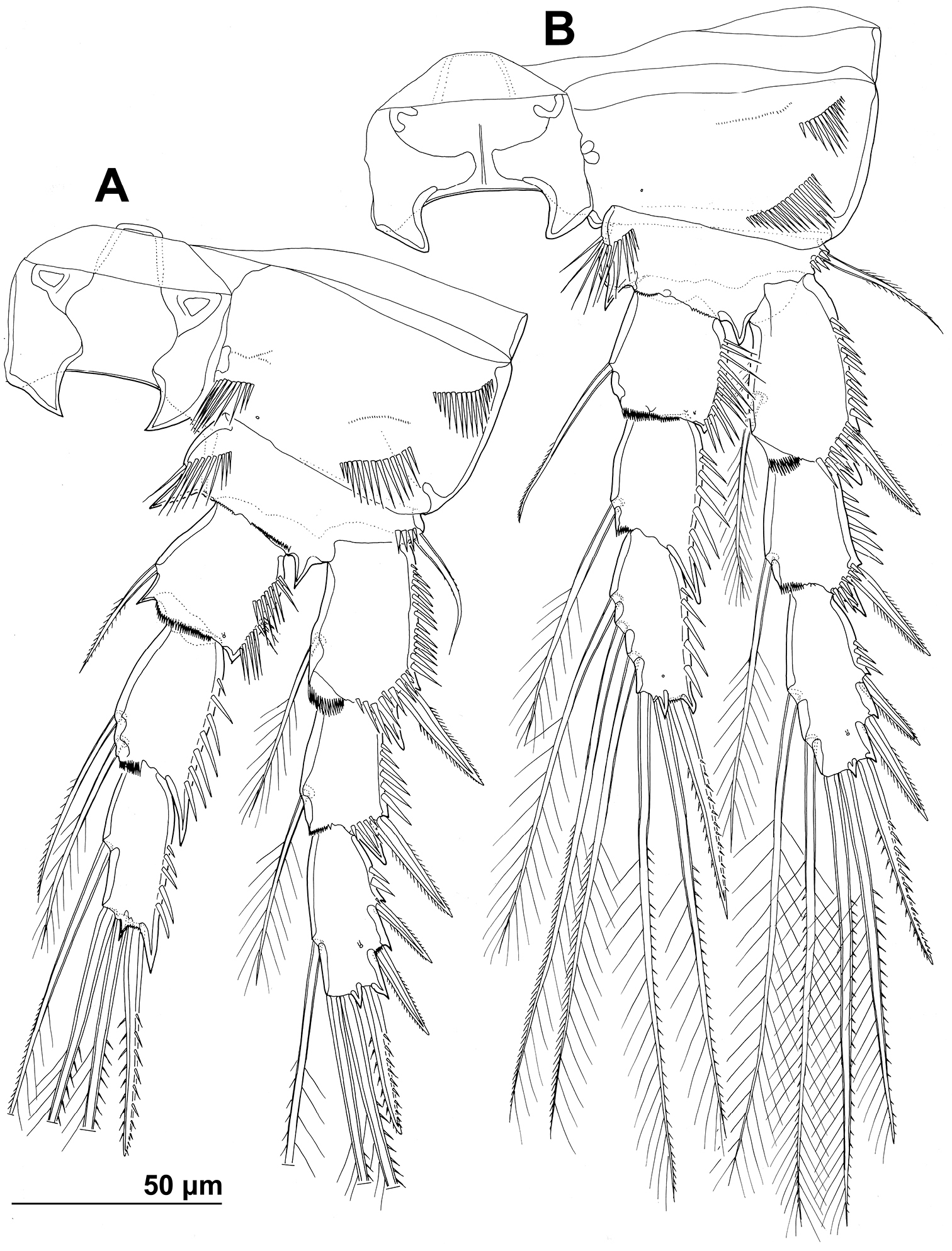
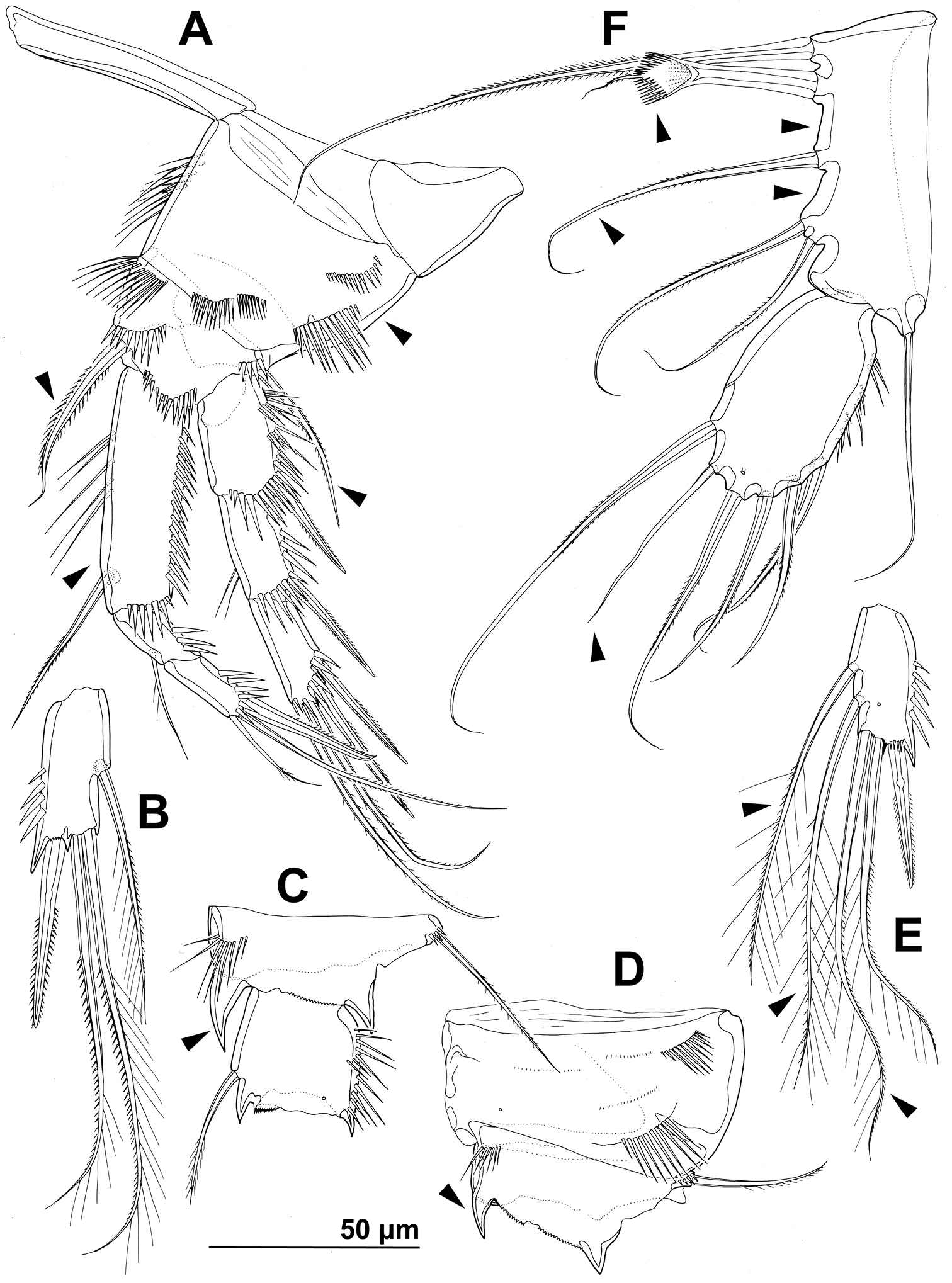
First leg (Figs 1G, 2D, 5D) with smooth and short intercoxal sclerite, its distal margin nearly straight. Praecoxa longer than wide, longer than intercoxal sclerite but shorter than coxa, unornamented. Coxa 1.8 times as wide as long, with longitudinal row of long and slender inner spinules, three transverse rows of shorter but stronger anterior spinules, and two short rows of even smaller posterior spinules. Basis with one long strong and finely bipinnate outer spine, one shorter but stronger bipinnate inner spine, and four transverse rows of large anterior spinules (one at base of each spine, one at base of endopod, and one on proximal inner corner; latter with longest spinules). Exopod with all segments of similar length, each about twice as long as wide and with strong outer spinules and subdistally on anterior surface; first segment with anterior pore near distal outer corner; second segment with slender inner spinules; first two segments with single strong and finely bipinnate distolateral spine; third segment with two strong and finely bipinnate outer spines and two slender and finely bipinnate apical setae; apical setae not prehensile; length ratio of elements on third segment, starting from outer margin, 1 : 1.4 : 2 : 2.4. Endopod three-segmented, prehensile, about 1.4 times as long as exopod; first endopodal segment about as long as entire exopod and 3.3 times as long as wide, with slender and long inner spinules, shorter and stronger outer and anterodistal spinules, with single bipinnate inner seta, the latter slender and about 0.4 times as long as segment; second segment small, rhomboidal, slightly longer than wide and only one sixth of first segment’s length, with several strong anterodistal spinules, and single slender and bipinnate inner seta; latter about 1.6 times as long as segment; third segment about 2.5 times as long as wide and 1.4 times as long as second segment, with several strong inner spinules and three smaller antero distal spinules, with one slender inner seta, one strong and long apical seta, and another shorter and stronger outer apical spine; apical spine 1.7 times as long as third segment, half as long as apical seta, and 1.5 times as long as inner seta on third segment; longest seta on exopod and endopod of about same length.
Stenhelia pubescens Chislenko, 1978, line drawings, female 3: A antennula, ventral B basis, endopod, and first exopodal segment of antenna, anterior C antennal exopod, anterior D first leg, anterior. Arrowhead indicates the presence of caudal suture on the fourth antennular segment.
Second leg (Figs 1G, 2E, 6A), intercoxal sclerite about as long as wide, unornamented, with two sharp and inwardly pointed distal processes. Praecoxa very short, unornamented. Coxa nearly 1.5 times as wide as long, with anterior pore near distomedial corner, three short rows of strong anterior spinules (one at distomedial corner, one near proximal outer corner, and one near distal outer corner), and two short rows of minute anterior spinules. Basis with nearly smooth (minute pinnules bearly visible), short and slender outer spine; inner distal corner produced into long and sharp process directed inwardly, another smaller distal process between exopod and endopod; with transverse row of long anterior spinules near inner margin, several smaller spinules ar base of outer spine, and discontinuous row of minute spinules at base of endopod. First exopodal segment widest, third segment slender and about 2.3 times as long as wide, 1.4 times as long as second segment, and about as long as first one; first and second segment with strong outer and anterodistal spinules and with distomedial frills, third segment with several outer strong spinules in proximal half and with anterior pore; first and second segments with single strong and finely bipinnate outer distal spine and slender bipinnate inner dista seta; third segment with three strong finely bipinnate outer spines, two apical strong bipinnate setae, and one slender bipinnate inner seta; inner apical seta on third segment longest, about 1.2 times as long as outer apical one, 2.4 times as long as third segment, and 2.7 times as long as outer distal spine; outer distal corner of first and second segment produced into small spiniform process. Endopod about as long as exopod; all segments of about same length, but progressively narrower from proximal to distal end, each with outer distal corner produced into strong spiniform process (first segment also with distomedial smaller process), and each with row of strong outer spinules, first two segments additionally with small distomedial frills, and first and third segments with anterior cuticular pore; armature consisting of single bipinnate inner seta on first segment, two pinnate slender inner setae on second segment, and one inner and three apical elements on third segment (probably outermost spine and two strong setae); seta on first segment about as long as segment, those on second segment about 1.4 times as long as segment, and those on third segment about twice as long as segment, except outer spine, which is about 1.4 times as long as segment. Two apical exopodal and endopodal setae each with shorter and stronger outer pinnules, inner setae on third exopodal and endopodal segments and proximal inner seta on second endopodal segment with shorter inner pinnules, all other bipinnate setae and spines with symmetrical pinnules.
Stenhelia pubescens Chislenko, 1978, line drawings, female 3: A second leg, anterior B third leg, anterior.
Third leg (Figs 2E, 6B) similar to second leg, except for slightly less sharp processes on intercoxal sclerite, absence on distomedial row of strong spinules on coxa, smaller spiniform distomedial process on basis, two inner setae on third exopodal segment, one inner seta on second endopodal segment, and three inner seta on third endopodal segment; proximal inner seta on third endopodal and exopodal segment with long pinnules on both sides, distal inner seta on third exopodal segment with short pinnules on inner margin in addition to long ones, other setae and spines as in second leg.
Fourth leg (Figs 2E, 7A) relatively similar to third leg, but with endopod only about 0.6 times as long as exopod, with slightly shorter distomedial process on basis, much longer seta on first endopodal segment, only two inner setae on third endopodal segment, and three inner setae on third exopodal segment; central inner seta on third exopodal segment spiniform and characteristically curved inwards; all setae on third exopodal segment proportionately longer than in second or third leg.
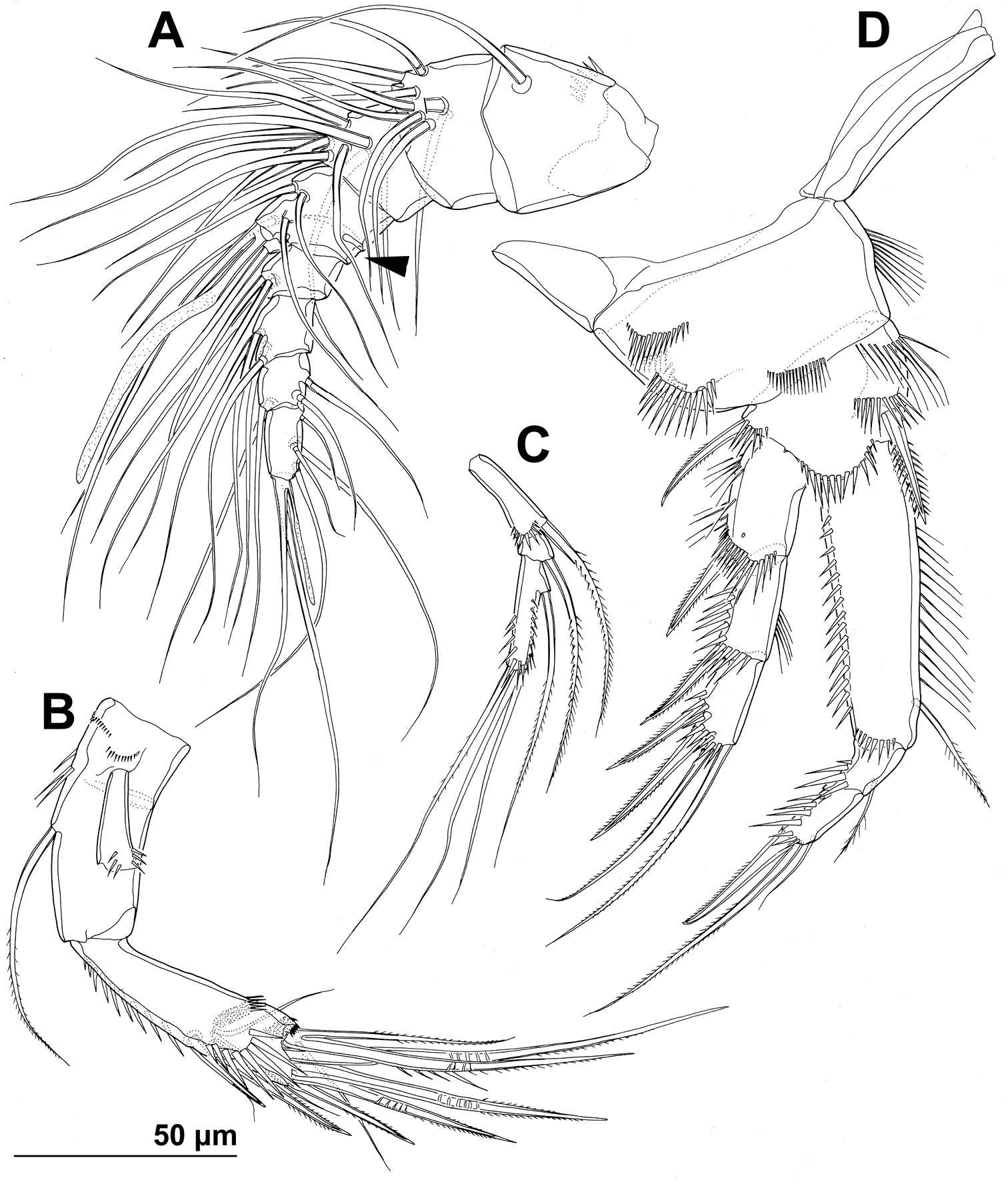
Stenhelia pubescens Chislenko, 1978, line drawings, female 3: A fourth leg, anterior B fifth leg, dissected and flattened, anterior.
Fifth leg (Figs 1D, 2F, 3B, F, 7B) composed of wide baseoendopod (fused basis and endopod) and much smaller and almost ovoid exopod, pair of legs joined by minute trapezoidal sclerite. Baseoendopod about 1.8 times as wide as long, more or less pentagonal, unornamented, with short and blunt process at base of exopod; outer basal seta slender and smooth, arising from short setophore, about 1.6 times as long as segment; endopodal lobe relatively narrow and short, more or less trapezoidal, not extending beyond proximal fifth of exopod, with five stout, bipinnate setae, their length ratio, starting from inner side, 1 : 0.8 : 1.2 : 1 : 0.8. Second endopodal seta from inner side with stout and smooth proximal half, characteristic transverse serrate comb near mid-length, and distal slender finely bipinnate whip; whip about as long as proximal part of seta. Exopod about 2.1 times as long as its maximum width, more or less ovoid, with narrower base than rest of it, with strong outer and inner spinules and single anterior pore close to distal margin, with six setae; innermost and second inner seta slender, others shorter and spiniform, second seta from inner side smooth, other setae bipinnate; length ratio of exopodal setae, starting from inner side, 1 : 1 : 1.4 : 1.4 : 0.6 : 0.6.
Sixth leg (Figs 2F, 3B, D, E) minute flap covering ventro-lateral genital aperture, mostly fused to somite, unornamented, with single short bipinnate seta near outer margin and one minute inner spine. Sixth legs seemingly joined on ventral side by fold-like suture which hides copulatory pores.
Most morphological features in examined topotypes were conservative, including the sensilla and pores pattern on somites, and length ratio of different armature on appendages. The only significant form of morphological variability, except for the body length, was presence/absence of caudal suture on the fourth antennular segment (compare Figs 2B and 5A; arrowed in Fig. 5A) and the size of suture on the antennar allobasis indicating remnants of ancestral arthroidal membrane (Fig. 5B). We redescribe this species in order to show some previously unreported characters, so they can be compared with those of Stenhelia taiae. Differences from the original description of
Stenhelia taiae sp. n. –
China, Bohai Sea, central region, sandy and muddy sediments at about 20 m depth, approximately 38.5°N, 120°E.
One female on one SEM stub (collection number NIBRIV0000232718), one female dissected on one slide (collection number NIBRIV0000232719), and two females destroyed for DNA sequences (GenBank accession nos. KF524885 & KF524884); all from South Korea, South Sea, Gwangyang Bay, sampling station 16, muddy sediments at about 10 m depth, 34.768889°N, 127.783806°E, 18 November 2012, leg. K. Kim.
Body length from 565 to 578 μm (n = 4). Body segmentation, colour, nauplius eye, hyaline fringes, integument thickness and surface appearence as in Stenhelia pubescens, including very smooth integument on all somites and their posterior frills. Most somite ornamentation also similar to Stenhelia pubescens, and homologous pores and sensilla easy to establish. Habitus (Fig. 8A) slightly less robust, with proportionately longer urosome (arrowed in Fig. 8A), prosome/urosome length ratio less than 1.1, body length/width ratio about 3.1, cephalothorax 1.6 times as wide as genital double-somite.
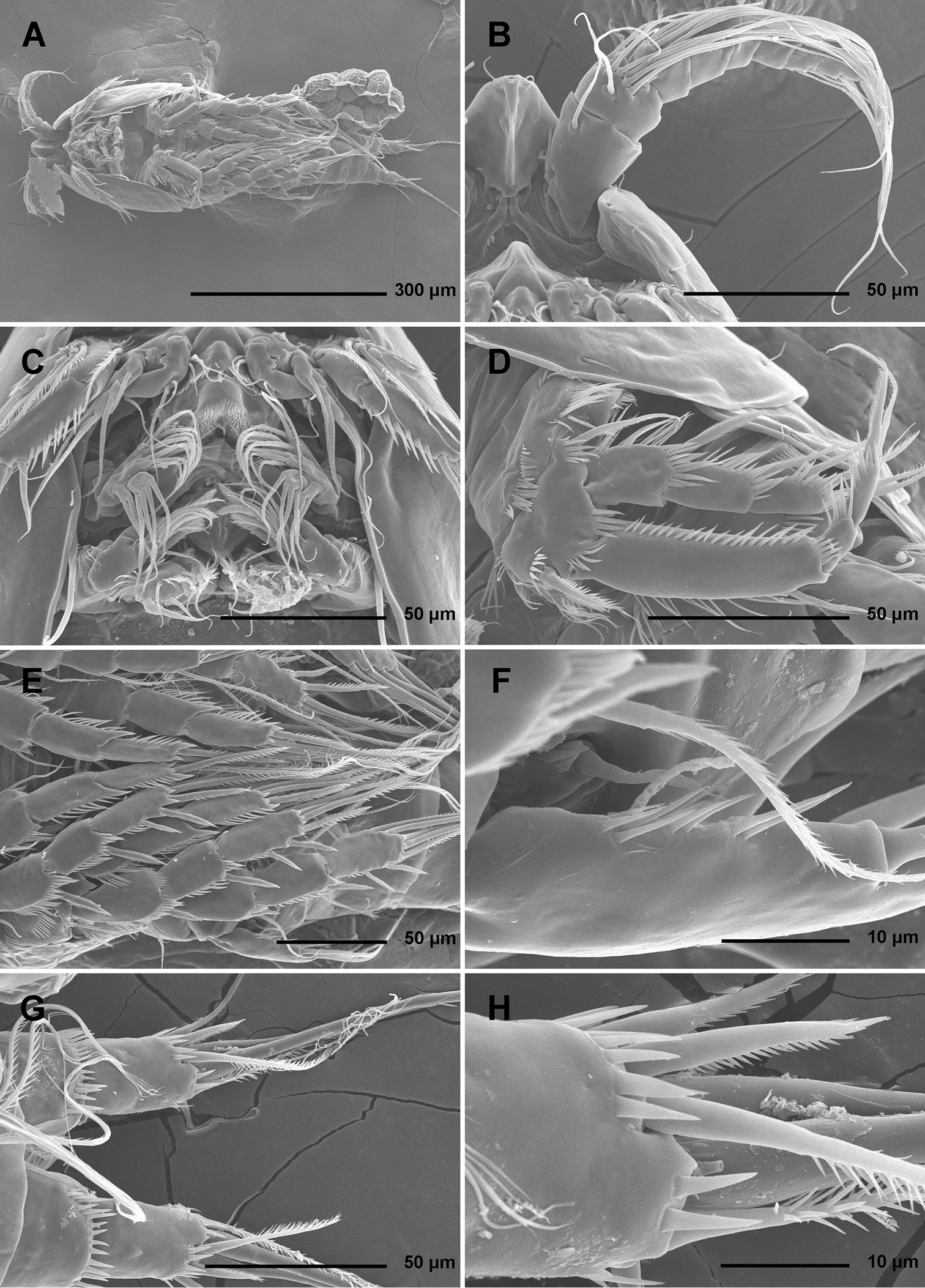
Stenhelia taiae Mu & Huys, 2002, scanning electron micrographs, female: A habitus, lateral B cephalothoracic shield, lateral C free thoracic somites, lateral D fifth pedigerous somite and genital double-somite, lateral E fourth and fifth urosomites, lateral F anal somite and caudal rami, lateral G posterior part of right caudal ramus, lateral H rostrum, lateral. Arrowheads indicate morphological characters different from those in Stenhelia pubescens Chislenko, 1978.
Rostrum (Figs 8H, 10D) slightly longer and narrower in dorsal view than in Stenhelia pubescens (arrowed in Fig. 10D).
Cephalothorax (Fig. 8B) about 0.9 times as long as wide; comprising about 30% of total body length, with posterior lateral corner slightly more rounded than in Stenhelia pubescens. Surface of cephalothoracic shield ornamented as in Stenhelia pubescens, except one anterior pair of lateral sensilla absent (arrowed in Fig. 8B) and one additional pair of anterior pores present (also arrowed in Fig. 8B).
Pleurons of second to fourth pedigerous somites (Fig. 8C) without any difference in shape or ornamentation from those in Stenhelia pubescens.
First urosomite (Figs 8D, 10A, B) with three pairs of long sensilla, as in Stenhelia pubescens, but with one additional short row of strong lateral spinules (arrowed in Fig. 8D).
Genital double-somite (Figs 8D, 10A, B) shape and most ornamentation as in Stenhelia pubescens, except anterior dorsal pair of sensilla more widely spaced (arrowed in Fig. 10A), posterior ventral pair of sensilla closer to each other (arrowed in Fig. 10B), and no spinules in between posterior dorsal pair of sensilla.
Third urosomite (Figs 8E, 10A, B) as in Stenhelia pubescens, except no spinules in between posterior dorsal pair of sensilla.
Fourth urosomite (Figs 8E, 10A, B) as in Stenhelia pubescens, except with fewer lateral spinules (arrowed in Fig. 8E).
Anal somite (Figs 8F, 10A, B) similar to that in Stenhelia pubescens, but additional pair of dorsal pores present, posterior spinules smaller and less dense, and medial cleft slightly narrower.
Caudal rami (Figs 8F, G, 10A, C), much longer than in Stenhelia pubescens (arrowed in Fig. 10A), about 1.3 times as long as anal somite, cylindrical, 2.1 times as long as wide (ventral view), slightly divergent, and with space between them about one ramus width; ornamentation and armature as in Stenhelia pubescens, except inner apical seta much shorter and smooth (arrowed in Fig. 10C), and ventralmost lateral seta smooth and slender; posteroventral tubular pore also present, but ventral pore at base of lateral setae situated at two thirds of ramus length, not at midlength.
Antennula (Fig. 9A), antenna (Fig. 9B), labrum (Figs 9C, 11A), paragnaths (Fig. 11B), mandibula (Fig. 9B, C), maxillula (Figs 9B, D, 11C), and maxilla (Figs 9D, 11D) as in Stenhelia pubescens.
Stenhelia taiae Mu & Huys, 2002, scanning electron micrographs, female: A rostrum and antennulae, lateral B antenna and mouth appendages, lateral C mandibular palp and labrum, lateral D maxilla and part of maxillular palp, lateral.
Stenhelia taiae Mu & Huys, 2002, line drawings, female: A urosome, dorsal B urosome, ventral (caudal rami armature omitted) C right caudal ramus, ventral D rostrum, dissected and compressed, dorsal. Arrowheads indicate morphological characters different from those in Stenhelia pubescens Chislenko, 1978.
Stenhelia taiae Mu & Huys, 2002, line drawings, female: A labrum, posterior B paragnaths, anterior C maxillula, posterior D maxillar basis and endopod, posterior E maxilliped, posterior. Arrowhead indicates morphological character different from that in Stenhelia pubescens Chislenko, 1978.
Maxilliped (Fig. 11E) as in Stenhelia pubescens, except basal setae proportionately longer (arrowed in Fig. 11E) and apical endopodal spine proportionately shorter.
First leg (Figs 8A, C, 12A) as in Stenhelia pubescens, except first exopodal segment proportionately shorter, both basal spines proportionately longer, and coxa without posterior spinules (all four arrowed in Fig. 12A).
Stenhelia taiae Mu & Huys, 2002, line drawings, female: A first leg, anterior B third endopodal segment of second leg, anterior C basis and first endopodal segment of third leg, anterior D coxa and basis of fourth leg, anterior E third endopodal segment of fourth leg, anterior F fifth leg, dissected and flattened, anterior. Arrowheads indicate morphological characters different from those in Stenhelia pubescens Chislenko, 1978.
Second leg (Figs 8A, C, 12B) as in Stenhelia pubescens.
Third leg (Figs 8A, C, 12C) as in Stenhelia pubescens, except distomedial basal process slightly larger (arrowed in Fig. 12C).
Fourth leg (Figs 8A, 12D, E) as in Stenhelia pubescens, except distomedial basal process larger (arrowed in Fig. 12D), both inner setae on third endopodal segment with additional short pinnules (arrowed in Fig. 12E), and inner apical seta on third endopodal segment with short outer pinnules (arrowed in Fig. 12E).
Fifth leg (Figs 8D, 12F) segmentation, general shape, number of armature elements, and most ornamentation as in Stenhelia pubescens, except exopod proportionately shorter (arrowed in Fig. 8D), second endopodal seta from inner side shorter (arrowed in Fig. 12F), second and third endopodal seta from inner side shorter (both arrowed in Fig. 12F), and spaces between central endopodal seta and two neighbouring setae significantly wider (both arrowed in Fig. 12F). Distal whip on second endopodal seta much shorter than in Stenhelia pubescens, only about 0.35 times as long as proximal stout part of seta (including transverse serrate comb). Length ratio of endopodal setae, starting from inner side, 1 : 0.4 : 0.6 : 0.5 : 0.4. Length ratio of exopodal setae, starting from inner side, 1 : 0.5 : 0.7 : 0.5 : 0.5 : 0.6.
Sixth leg (Fig. 10B) as in Stenhelia pubescens.
Most morphological features in the examined Korean specimens were extremely conservative, including the sensilla and pores pattern on somites, and length ratio of different armature on appendages. Except for the body length, the only other variable feature in the Korean population was the number of spinules on the inner margin of the fifth leg exopod (compare Figs 8D and 12F). We redescribe this species in order to show some previously unreported characters, so they can be compared with those of Stenhelia pubescens. Differences from the original description of
DNA was extracted and the mtCOI fragment successfully PCR-amplified from 23 stenheliin copepod specimens (Table 2), belonging to eight different morpho-species. All the sequences were translated into protein using MEGA and were shown to have no evidence of stop codons, ambiguities or insertions–deletions indicative of non-functional copies of mtCOI. BLAST analyses of GenBank revealed that the obtained sequences are copepod in origin and not contaminants, and one of the GenBank COI sequences (JQ390578.1) from the species Schizopera leptafurca Karanovic & Cooper, 2012 was included in our phylogenetic analyses.
Average pairwise distances between morpho-species were found to be very high, with the lowest divergence (7.1%) between the Korean Itostenhelia polyhymnia Karanovic & Kim, 2014 and the Russian Itostenhelia golikovi (Chislenko, 1978) (Table 3). Second (10.1%) and third (16.9%) lowest divergences were found between Stenhelia taiae and Stenhelia pubescens and between Stenhelia taiae and Willenstenhelia thalia Karanovic & Kim, 2014, while those between all other taxa were in excess of 17%. These high divergence values are generally indicative of distinct species by comparison with other crustaceans (
Average pairwise maximum likelihood distances (TN model) among mtCOI sequences between each morpho-species (lower diagonal) and within morho-species (diagonal).
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Wellstenhelia calliope | - | ||||||||
| 2. Itostenhelia polyhymnia | 0.271 | 0.000 | |||||||
| 3. Wellstenhelia qingdaoensis | 0.267 | 0.228 | - | ||||||
| 4. Wellstenhelia clio | 0.202 | 0.328 | 0.245 | - | |||||
| 5. Itostenhelia golikovi | 0.218 | 0.071 | 0.278 | 0.267 | 0.006 | ||||
| 6. Willenstenhelia thalia | 0.285 | 0.201 | 0.291 | 0.338 | 0.181 | 0.008 | |||
| 7. Schizopera leptafurca | 0.302 | 0.241 | 0.376 | 0.344 | 0.270 | 0.199 | - | ||
| 8. Stenhelia taiae | 0.317 | 0.193 | 0.342 | 0.240 | 0.170 | 0.169 | 0.245 | 0.000 | |
| 9. Stenhelia pubescens | 0.318 | 0.220 | 0.352 | 0.311 | 0.201 | 0.173 | 0.311 | 0.101 | 0.000 |
The highest divergences within morpho-taxa were those between eight specimens of Willenstenhelia thalia (0.8%), which all came from the same sampling station (St. 10), although collected on two separate occasions. Divergences between five specimens of Itostenhelia golikovi were about 0.6%. (Table 3). These are all indicative of intraspecific variability (
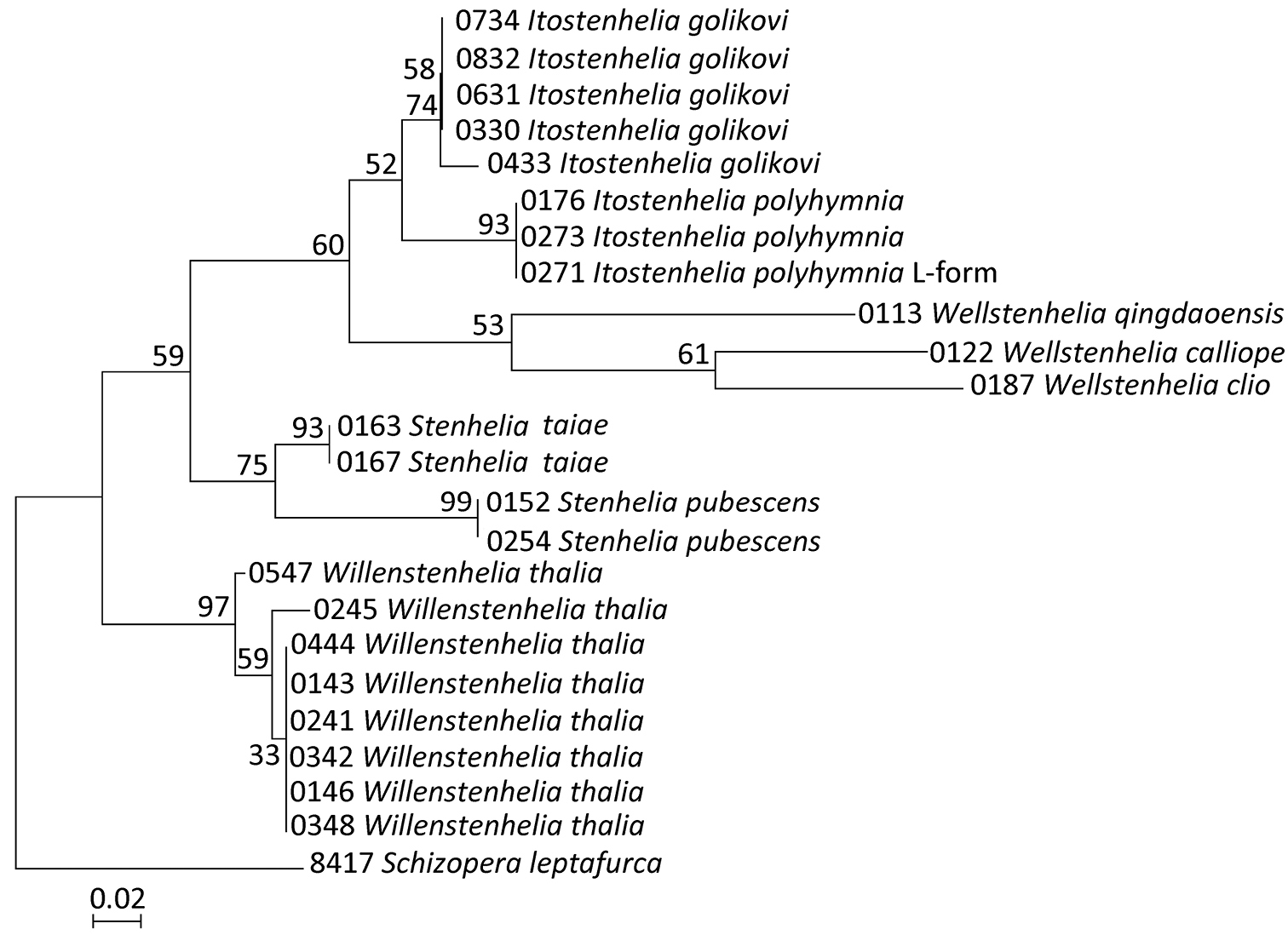
All analyses (Fig. 13) supported the presence of at least nine highly divergent lineages and all five of the multisample lineages were supported with high bootstrap values (>74% for ML). The tree topology in our NJ analysis was the same as in the ML analysis (Fig. 13), except the bootstrap values were generally slightly higher. Our MP analysis resulted in two equally parsimonious trees, each 61 steps long, and their consensus also had a very similar topology to our ML tree, except that bootstrap values were generally slightly lower; also the terminal clade in Willenstenhelia thalia was not supported in our MP analysis, nor was the sister relationship between Wellstenhelia calliope Karanovic & Kim, 2014 and Wellstenhelia clio Karanovic & Kim, 2014 (instead a sister relationship was suggested between Wellstenhelia qingdaoensis (Ma & Li, 2011) and Wellstenhelia clio, but the bootstrap value for this clade was only 39%). Our previous morphological analyses (see
Maximum likelihood (ML) tree based on mtCOI sequence data of 23 stenheliin specimens from Gwangyang Bay (South Korea) and Posyet Bay (Russia), constructed using MEGA v 5.0.3 and an HKY+G model of evolution, with numbers on the branches representing bootstrap values from 500 pseudoreplicates. The tree is rooted with Schizopera leptafurca Karanovic & Cooper, 2012 from Western Australia. The cladogram is drawn to scale and the specimen codes correspond to those in Table 2.
All basal nodes are supported only by moderate bootstrap values (between 52% and 75%), which could be explained by the low phylogenetic resolution of the mtCOI gene in basal nodes of the trees, possibly due to saturation at third codon positions (
Phylogenetic implications. Our phylogenetic analysis (Fig. 13) resulted in demonstrating a polyphyly of the genus Delavalia Brady, 1869, as postulated by
The smallest average divergence values in mtCOI gene (Table 3) were observed between two allopatric (Korea/Russia) species pairs: Itostenhelia polyhymnia/Itostenhelia golikovi and Stenhelia taiae/Stenhelia pubescens (7.1% and 10.1% respectively). Average divergence values between all sympatric Korean stenheliins were very high (all in excess of 16.9%), which suggests a long independent evolutionary history. This is also reflected in their numerous morphological differences (
Micro-characters in harpacticoid taxonomy.
In this study, one of our aims was to examine pores and sensilla pattern of the two closely related Stenhelia congeners. Differences involved not just relative positions of some pores and sensilla, but also a complete absence of some. Cephalothoracic shield has one sensilla pair less and one pore pair more in Stenhelia taiae than in Stenhelia pubescens (compare Figs 1B and 8B). Genital double-somite in Stenhelia taiae has the ventral posterior pair of sensilla less widely spaced and the dorsalmost anterior pair of sensilla more widely spaced than in Stenhelia pubescens (compare Figs 3A, B and 10A, B). Finally, the anal somite in Stenhelia pubescens lacks the dorsal pair of pores (compare Figs 3A and 10A). Differences between these two species in the cuticular pores and sensilla pattern are no fewer than differences in the more tradidionally used macro-morphological characters, such as the length of caudal rami (compare Figs 3A and 10A), shape and armature proportions of the fifth leg (Figs 7B and 12F), several differences in shape and ornamentation of the swimming legs (Figs 5D, 6B, 7A and 12A, C, D, E), and spinular ornamentation of the urosomites (Figs 1D, E and 8D, E). This is all very surprising given their relatively low divergence values in the mtCOI gene of only 10.1% (see Table 3).
Almost all pores and sensilla can be homologised in these two species without many problems, suggesting a potential use of these structures in future phylogenetic reconstructions of harpacticoid copepods. However, many more families would have to be studied before this could happen. Even so, these preliminary studies in three of the four largest harpacticoid families (
Discrepancies between original descriptions and redescriptions. Careful examination of our topotypes of Stenhelia pubescens revealed a number of morphological differences from the original description by
As for the differences between Korean and Chinese populations of Stenhelia taiae, they are all minor and some could possibly be contributed to geographic intraspecific variability. We did not examine the types of this species either, but the original drawings of
Morphology and phylogeny of Stenhelia. Major synapomorphy of the eigth species currently recognised as members of this genus, as redefiend by
This work was supported by grants from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment of the Republic of Korea (NIBR No. 2013-02-001), and from the Basic Science Research Programme of the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology of the Republic of Korea (2012R1A1A2005312). Scanning electron microscope was made available through Prof. Jin Hyun Jun (Eulji University, Seoul), and we also want to thank Mr. Junho Kim (Eulji University, Seoul) for the technical help provided. We are very grateful to Dr. Yulia Trebukhova (Institute of Marine Biology, Vladivostok) for collecting the samples of Stenhelia pubescens and Itostenhelia golikovi, as well as to Dr. Marina Malyutina (Institute of Marine Biology, Vladivostok) and Prof. Angelika Brandt (Zoological Museum, Hamburg) for their assistance in transporting these specimens. We also thank Dr. Kanghyun Lee and Ms. Eunkyoung Park for their help in the field and in the molecular lab respectively.